- 1 College of Traditional Chinese Medicine, Changchun University of Chinese Medicine, Changchun, China
- 2 College of Basic Medicine, Changchun University of Chinese Medicine, Changchun, China
- 3 College of Nursing, Changchun University of Chinese Medicine, Changchun, China
Cerebrovascular neurological disorders, especially high-mortality and disabling stroke subtypes such as ischemic stroke and hemorrhagic stroke, have become a major global health issue. In addition to conventional treatments, the role of herbal medicines and their active ingredients in the prevention and treatment of cerebrovascular and nervous system diseases has received increasing attention in recent years. Among them, the primary active ingredient of Panax notoginseng is Panax Notoginseng Saponins (PNS), has become a research hotspot due to its diverse pharmacological activities. Existing evidence suggests that PNS exhibits various effects including anti-inflammatory, antioxidant, anti-apoptotic, immune regulation, neuroprotection, blood sugar and lipid lowering, and cardiovascular protection. This review systematically searches multiple databases for literature related to PNS and cerebrovascular neurological disorders, focusing on summarizing the role of PNS in specific diseases such as ischemic stroke, hemorrhagic stroke, and neurodegenerative disorders, exploring its pharmacokinetic characteristics, main mechanisms of action, and clinical application prospects, aiming to provide a theoretical basis for the in-depth research and development of PNS in cerebrovascular neurological disorders.
1 Introduction
The incidence of neurological diseases is increasing year by year. These diseases are caused by many endogenous (genetic, metabolic, immune, etc.) and exogenous factors (trauma, environment, lifestyle, etc.) (Mehndiratta and Aggarwal, 2021), mainly neurodegenerative and cerebrovascular diseases, which are the second leading cause of death worldwide in addition to cardiovascular diseases, including stroke Alzheimer’s disease (AD), Parkinson’s disease, and depression are the most common neurological disorders, and their pathogenesis is complex, there is no effective cure or prevention strategy, and the efficacy and side effects of related therapeutic drugs change with the prolongation of the disease. As an effective multi-targeted therapy, traditional Chinese medicine, as an effective multi-targeted therapy, plays an important role in treating symptoms and slowing disease progression.
Panax notoginseng is the dried root and rhizome of the plant of the Araliaceae, which has the effects of stopping bleeding and dispersing blood stasis, subduing swellings and relieving pain, and is one of the most widely used Chinese herbs in China and other countries mainly for the treatment of cardiovascular and cerebral vascular diseases, such as coronary artery disease, atherosclerosis and cerebral infarction (Li et al., 2020). At present, more than 200 compounds have been isolated from Panax notoginseng, mainly including saponins, flavonoids and polysaccharides, etc. PNS is a class of chemical mixtures containing different dammarane-type saponins extracted from Panax notoginseng, including PNS (R1-R6) and PNS (VII); different parts of Panax notoginseng (roots, stems, leaves, and flowers) contain different saponins, such as Ginsenoside (Rb1, Re, Rg1, Rg2, Rh1) (Zhou et al., 2017; Sun et al., 2023), and currently there are more than 180 PNS isolated and identified from various parts of Panax notoginseng, based on the structure of the saponins, the saponins can be classified into five main types, the most important of which are the protopanaxadiol type (PPD) and protopanaxatriol type (PPT) (Yang et al., 2014; Wang et al., 2016), and the content of saponins contained in different parts of the plant varies (Wei et al., 2020), and the type and amount of saponins change with the age, growth environment, and tissue type of Panax notoginseng. In addition to neurological aspects, PNS can play an important role in inhibiting inflammation, oxidative stress, apoptosis, and immunomodulation (Ling et al., 2024). It also plays an important role in the treatment of cardiovascular, diabetes, liver disease, gastrointestinal tract, bone metabolism regulation, anticancer and renal disease (Liu et al., 2020), and possesses a wide range of activities such as haemostasis, pro-angiogenesis, immunomodulation, anti-inflammation, anti-tumor, antioxidant, etc (Li W. et al., 2025). For example, PNS can further exert anti-obesity effects by reducing lipid synthesis, inhibiting adipogenesis, promoting browning of white adipose tissue, increasing energy expenditure and improving insulin sensitivity (Zhang et al., 2020).
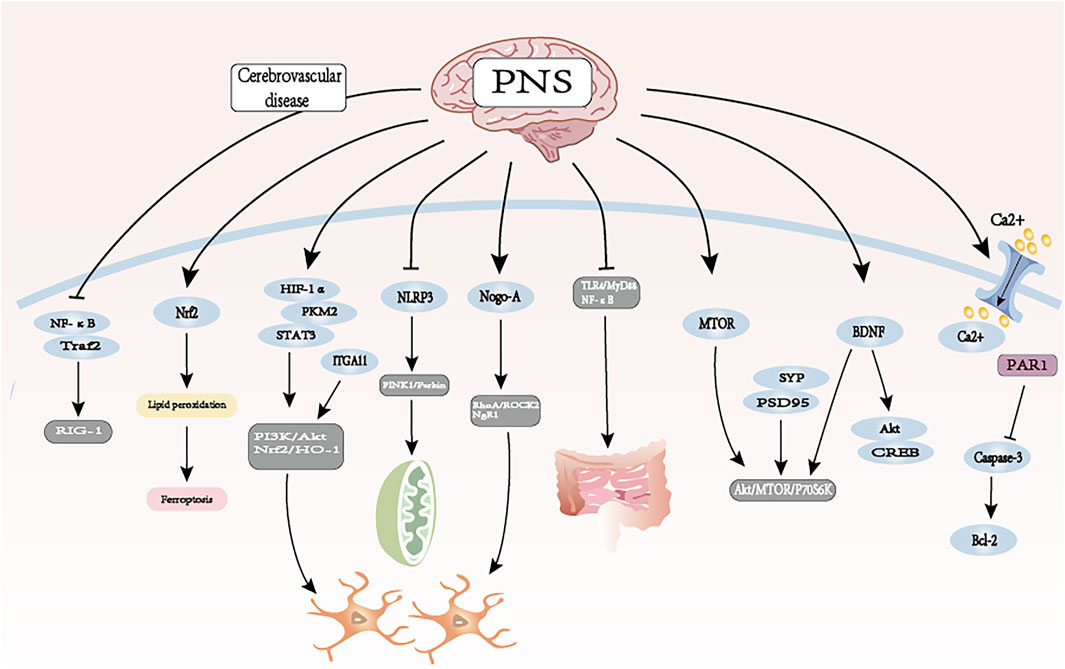
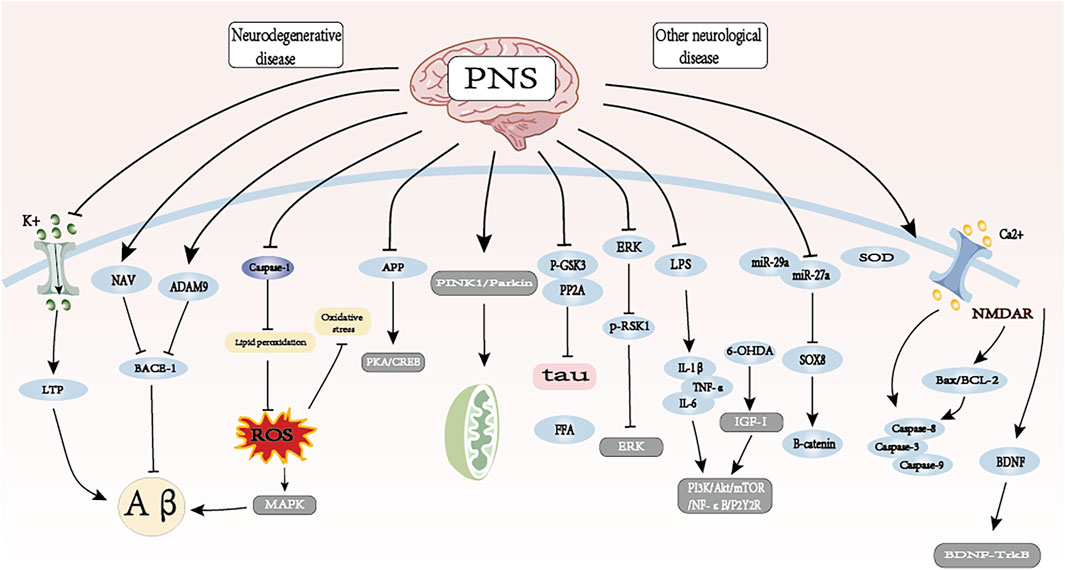
As a novel potential therapeutic agent, herbal monomers play a unique role in the treatment of various aspects such as cancer, cardiovascular and cerebrovascular diseases (Li Z. et al., 2025). Nevertheless, besides exerting different pharmacological activities in the treatment of cerebrovascular and neurological diseases, PNS can still have some problems in the treatment of other systemic diseases and their complications, such as toxic reactions to some osteoporosis, cancer, diabetes and some drugs (Xu et al., 2019). Therefore, this paper provides a comprehensive summary of the mechanism application of PNS in cerebrovascular neurological diseases, reviewing the chemical structure, pharmacokinetics, pharmacological activity and mechanism of action in different diseases, neurotoxicity, etc., and analyzing its mechanism of action and therapeutic potential in the process of treatment, so as to improve the reference for further promotion of PNS research and application in experimental clinics (see Figure 1).

Figure 1. Mechanisms of PNS in the treatment of cerebrovascular neurological disorders. Partly reprinted with permission from the official website maisanqi.com.
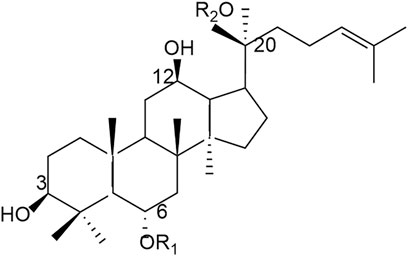
As one of the main chemical components of Panax notoginseng, PNS is a dammarane-type tetracyclic triterpenoid saponin, which is mainly classified into dammarane-type 20 (S)- PPD and 20 (S)- PPT according to the hydroxyl groups attached to the glycosides (see Figure 2).
2 Search strategy and selection criteria
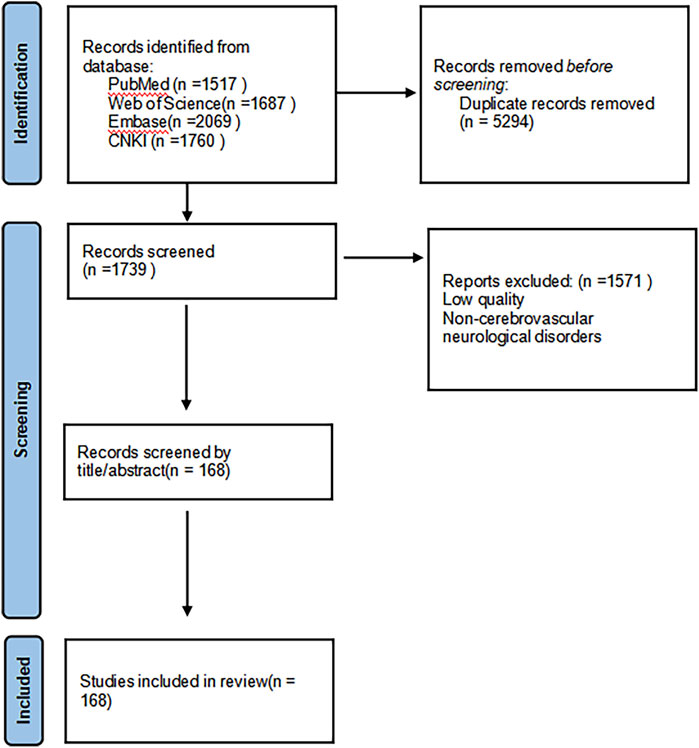
In order to comprehensively review the pharmacological effects of PNS and its therapeutic potential for cerebrovascular neurological disorders, we systematically searched multiple Chinese and English databases (including Web of Science, PubMed, Embase, and CNKI), with a time frame from the inception of each database to 31 May 2025, and supplemented the references of relevant literature through manual searches. The search strategy employed a wide range of keywords, including general terms such as “panax notoginseng saponins,” “panax notoginseng,” “mechanism of action,” “pharmacological effects,” as well as specific disease names such as “ischemic stroke,” “hemorrhagic stroke,” “Alzheimer’s disease”, “Parkinson’s disease,” and “pharmacokinetics” to ensure coverage of all aspects of its molecular mechanisms and therapeutic applications. This study established predefined inclusion criteria, primarily incorporating peer-reviewed research articles, reviews, and clinical trial reports published in English, to provide a comprehensive overview of its pharmacological characteristics; literature that did not directly relate to its pharmacological effects or was of poor quality was excluded. Detailed information about the included studies is provided in Figure 3 (see Figure 3).
3 Pharmacokinetics of PNS
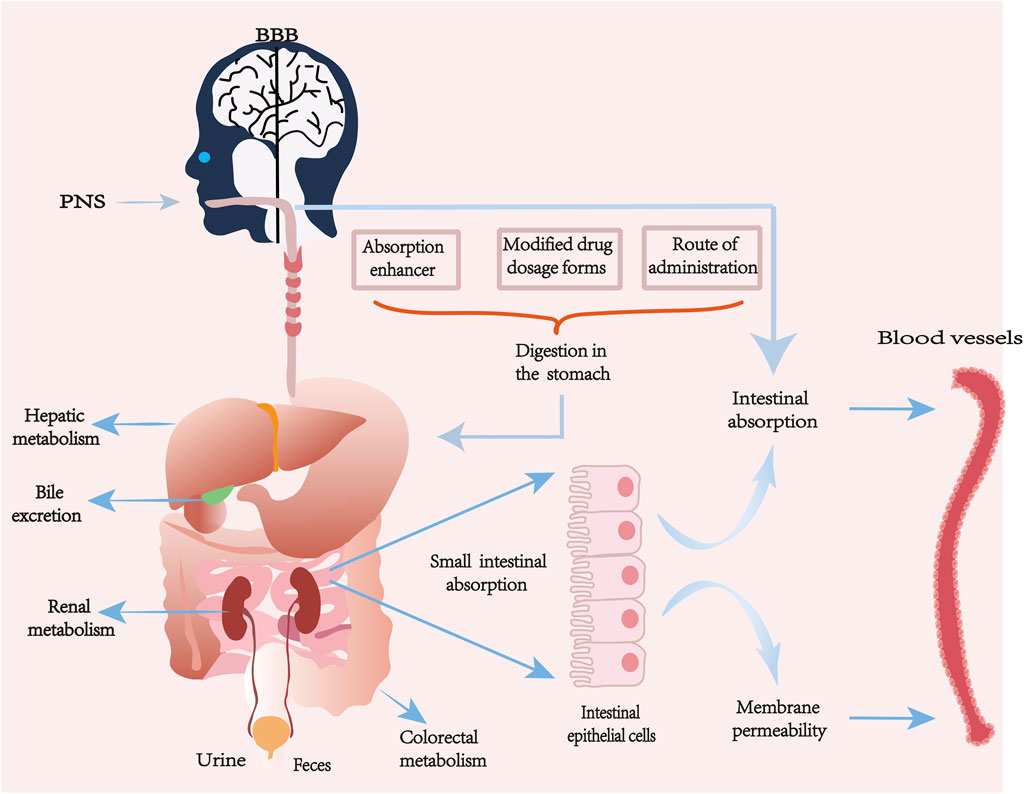
The pharmacokinetic process of PNS in vivo varies from component to component, and its absorption, distribution, metabolism and excretion processes are also affected by many factors. Some natural major saponins will be released by sugar groups or dehydrated to become secondary saponins during the processing and heating of Panax notoginseng. Not only the active ingredients will be changed, but also the transformation of the compound bases makes the pharmacological effects and pharmacokinetics change (Xiong et al., 2017; Peng et al., 2018). PNS has good water solubility, low membrane permeability and high molecular weight are the main factors affecting poor bioavailability. About 90% of drug absorption mainly occurs in the small intestine, the intestinal epithelium as the main site of drug absorption, its surface mucus layer and epithelial cell membrane fluidity will have an impact on drug permeability, and the monomeric saponins in PNS are mostly large molecule water-soluble components, and when ingested orally or by gavage, these components mainly rely on the cellular bypass passive transport mechanism to be absorbed, due to the intestinal epithelial cell membrane has a due to the lipophilic nature of the intestinal epithelial cell membrane, this can prevent hydrophilic drugs from being absorbed through the transcellular pathway. In addition, PNS is easily hydrolyzed or metabolized by gut bacteria, which further affects its bioavailability. After entering the bloodstream, its distribution shows tissue specificity, but overall it is characterized by a high plasma protein binding rate and a limited volume of tissue distribution. The main metabolic pathway is mediated by hepatic cytochrome P450 enzyme system (especially CYP3A and CYP1A2) which involves hydrolysis and deglycosylation, producing active or inactive metabolites. Ultimately, the parent drug and its metabolites are excreted through the kidneys in urine, slowly eliminated from serum and tissues, with some being excreted via bile in feces, resulting in a relatively short elimination half-life (Masaoka et al., 2006; Hao et al., 2010; Kim, 2018; Li et al., 2019; Miao et al., 2022; Fu et al., 2023).
Different formulation types and delivery modes are different for the pharmacokinetic properties of PNS in vivo (Pang et al., 2017). The solubility and absorption of different types of saponins can be improved by adding some absorption enhancers and improving the different dosage forms of the drug, such as nanoformulations, microemulsions, enteric dissolution, etc., or by applying nano-delivery systems to increase the permeability and to combat the gastrointestinal degradation, which can largely improve the bioavailability of PNS (Ruan et al., 2010; Li Y. et al., 2018; Balusamy et al., 2023; Wang et al., 2023). Some studies have shown that the use of bioadhesive materials can improve the bioavailability of PNS in compound danshen formulations to some extent compared with other dosage forms (Chen et al., 2018); the relative bioavailability of PNS bioadhesive tablets prepared using chitosan with the main components R1, Rg1, and Rb1 was increased to 204.53% compared with ordinary tablets, respectively, 152.73%, and 150.50% (Feng et al., 2011). The lyophilization process can effectively improve the stability of PNS-loaded transfer bodies (PNS-TFSs) without affecting their transdermal absorption properties and promote faster drug absorption (Lu et al., 2024). Xuesaitong, as one of the main preparations of Panax notoginseng freeze-dried extract, has been found that the saponins in the freeze-dried Xuesaitong preparation have a high potential for drug interactions mediated by organic anion-transporting polypeptide (OATP) 1B, which can enhance its bioavailability (Pintusophon et al., 2019). The use of enteric formulations, which can release most or all of the drug at a site in the drug intestine, increases drug stability and delays the time of intestinal absorption of the drug.
Interaction with other drugs may affect their metabolism and efficacy. Ice tablet acts as an absorption enhancer, and ice tablet significantly increases the permeability of the active ingredients of PNS classes at certain molar ratios, and the degree of oral absorption of prescriptions containing ice tablet (molar ratio 1:27) was approximately three times that of the control compared to prescriptions containing no ice tablet (Kim et al., 2021). Borneol (BO) may enhance absorption and affect the distribution and metabolism of other ingredients in combination with PNS 1:1 (PNS 75 mg/kg; BO 75 mg/kg) ratio (Mei et al., 2024). A study showed that when aspirin and PNS were used together, the apparent permeability coefficient value increased significantly, and the combination of aspirin and salicylic acid was able to interfere with the function of tight junction proteins, which led to the enlargement of cellular gaps, and ultimately facilitated the uptake of drugs by PNS (Tian et al., 2018). Nanoemulsions: water-in-oil (W/O) or oil-in-water (O/W) droplets stabilized by amphiphilic surfactants, for hydrophilic macro-ingredients such as PNS, which are poorly absorbed, W/O-type nanoemulsions surfactants improve membrane fluidity and enhance the degree of transmembrane uptake, which in turn improves bioavailability (Singh et al., 2017).
In addition to oral, gavage, intravenous, and nano-delivery methods of drug administration, there are also various non-gastrointestinal mucosal delivery methods, such as nasal administration, pulmonary inhalation, and transdermal administration; these methods are all beneficial for the absorption of PNS. Nasal administration is a direct route for delivering drugs to the brain, avoiding the Blood-brain barrier (BBB), gastrointestinal degradation, and the first-pass effect in the liver (Guo et al., 2014). Through investigating the tissue distribution of drug administration in the rat’s nasal cavity and intravenous administration, the study found that after nasal administration of PNS solution, the concentration of Rg1 in the brain was significantly higher than that after intravenous injection. A major challenge currently faced in PNS research is the lack of dosage standardization; the sources of PNS extracts, the ratio of saponins, purity, and formulation types vary across different studies, making it very difficult to directly compare their pharmacokinetic parameters and efficacy. Future research urgently needs to standardize and quantify the core active components of PNS to enhance comparability across different studies.
The pharmacokinetics of PNS is complex, with low absorption and bioavailability. To improve the related efficacy of PNS, it is necessary to conduct in-depth research on formulation selection, administration routes, and absorption mechanisms. Continuous exploration of novel drug delivery systems is required to address the quality control and dosage standardization issues of PNS formulations, ensuring that their clinical efficacy is reproducible and assessable, and increasing their bioavailability (see Figure 4; Table 1).
4 Pharmacological effects and mechanisms of PNS
PNS has multiple pharmacological activities, including anti-inflammatory, antioxidant, anti-apoptotic, anti-tumor, immunomodulation, improvement of microcirculation, and promotion of nerve regeneration.
4.1 Anti-inflammatory
PNS-like components have good anti-inflammatory effects, mainly exerting neuroprotective effects by modulating inflammatory signaling pathways, inhibiting microglia/astrocyte over-activation, and reducing the release of pro-inflammatory factors. Studies have shown that the inflammatory response of the central nervous system is closely linked to the activation of glial cells. Different types of saponins inhibit inflammatory mediators and cytokines from multiple pathways and attenuate the resulting waterfall cascade effect (Ruan et al., 2020).
PNS inhibits lipopolysaccharide (LPS) and interferon-gamma-induced microglial cell activation, reduces serum production of tumor necrosis factor-
The anti-inflammatory mechanism exerted by PNS is to play a regulatory role on inflammatory cells and inflammatory mediators through multi-target and multi-pathway synergism, which can be used to start from the classical inflammatory pathways, such as NF-κB, and the modulation of immune cell polarization and function, and to deeply study the potential therapeutic value of these properties in cardiovascular and cerebrovascular diseases, neurodegenerative diseases, and autoimmune diseases.
4.2 Antioxidant
Oxidative stress is a series of reactions caused by the accumulation of oxygen free radicals in the body due to the dysregulation of the body’s production and scavenging of oxygen free radicals caused by various external stimuli. Associated with increased production of reactive oxygen species and decreased effectiveness of the antioxidant system (Sies et al., 2017), oxidative stress leads to aberrant signaling in cells thus leading to a range of pathological responses. PNS can further play a role in protecting cerebrovascular and neuronal cells through multiple mechanisms such as direct scavenging of free radicals, enhancement of antioxidant enzyme activity, activation of the Nrf2 pathway, and protection of mitochondrial function.
PNS has a certain antioxidant activity capacity (Xiang et al., 2020), and is able to protect astrocytes from H2O2-induced injury by activating the antioxidant system, as well as alleviating the damage in SH-SY5Y cells under oxygen glucose deprivation/reoxygenation (OGD/R) conditions. In addition, it can help the brain resist damage caused by oxidative stress in neurological diseases by activating the PI3K/Akt/Nrf2 antioxidant signaling pathway (Zhou N. et al., 2014; Hu et al., 2018). When intracellular Ca2+concentration is elevated, energy metabolism is reduced, which catalyzes the activation of hypoxanthine production, PNS can inhibit the elevation of hypoxanthine, inhibit the production of free radicals, and also reduce the production of reductive coenzyme II-NADPH oxidase (Meng et al., 2014); on superoxide dismutase (SOD), enhance glutathione peroxidase (GSH-PX) activity and reducing malondialdehyde (MDA) and nitric oxide levels in brain tissue as a means of scavenging free radicals. The protective properties could also be enhanced by increasing the activities of antioxidant enzymes, which significantly increased the activities of antioxidant enzymes (including SOD, GSH-PX, and CAT) in SAMP8 mice and B16 melanoma cells by activating the nuclear factor erythroid 2-related factor 2 (Nrf2)/heme oxygenase-1 (HO-1) signaling pathway, and simultaneously decreased the oxidative damage markers, 8-hydroxylated deoxyguanosine (8-OHdG) levels.
In addition, the cellular antioxidant defense system was further enhanced by up-regulating the expression of mitochondrial uncoupling proteins 4 and 5, which effectively protected the properties of neuronal cells (Huang J. L. et al., 2017; Peiran et al., 2017). PNS inhibited endoplasmic reticulum stress in PC12 cells by inducing the thioredoxin-1 (Trx-1) response to the H2O2-induced oxidative damage exhibited a strong protective effect (Zeng et al., 2015).
4.3 Anti-apoptosis
Apoptosis is a cell death process caused by various factors inside and outside the body triggering the pre-existing death program in the cell, which has an important physiological regulatory mechanism in regulating the development of the organism, cell renewal and differentiation. Apoptosis occurs in addition to necrosis during ischemia, hypoxia, and ischemia-reperfusion, mainly through endogenous and exogenous pathways.
PNS inhibits aberrant apoptosis and autophagy in hippocampal neurons of learning- and memory-impaired mice, which is associated with reactivation of phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin signaling. It has been found that Rb1 possesses anti-apoptotic properties and reduces oxidative stress damage (Kim et al., 2012), When investigating the protective effect of Rb1 on PC12 cells, it was found that Rb1 was effective in increasing cell viability, exerting neuroprotection and avoiding apoptosis in the presence of H2O2 induced neurotoxicity and leading to cell death (Zeng et al., 2015). In addition, PNS can reduce neuronal apoptosis after cerebral hemorrhage in mice by inhibiting the JNK signaling pathway and down-regulating the protein expression levels of mitochondrial cytochrome C, Caspase-9 and Caspase-3 in the brain tissues (Li et al., 2009). The Caspase family and the Bal-2 family of proteins, as apoptosis research The main core object of apoptosis research, in most cases neuronal apoptosis occurs mainly through the endogenous pathway. PNS can reduce the expression of the key protease caspase-3 by inhibiting the transcription of caspase-3 mRNA and the cleavage activation of caspase-3 p20 protein in rat cerebral hemorrhage foci and peri-focal area and thus reduce the occurrence of neuronal apoptosis in the ischemia-reperfusion period.
Brain-derived neurotrophic factor (BDNF) can inhibit apoptosis by regulating the expression of Bal-2 and Bax proteins, etc. PNS can promote the expression of BDNF, which enhances the endogenous repair mechanism after cerebral ischemia, and helps to improve the neurological deficits in rats, reduce the scope of cerebral infarction, and reduce neuronal apoptosis. In addition, PNS also promotes the transcription of Bcl-2 mRNA and the expression of Bcl-2 protein in cerebral hemorrhage foci and around foci. It reduces neuronal apoptosis by enhancing the expression of anti-apoptotic genes. PNS exerts anti-apoptotic effects by reducing the occurrence of necrotic apoptosis in OGD/R brain microvascular endothelial cells through inhibition of the RIP1-RIP3-MLK signaling pathway and attenuating mitochondrial damage (Hu et al., 2022).
4.4 Anti-tumor
PNS exhibits certain inhibitory activity against various types of tumors. In the field of the central nervous system, neurological tumors are one of the most lethal forms of cancer in the United States. Gliomas account for approximately one-third of all tumors affecting the central nervous system and brain, with glioblastoma multiforme (GBM) being the most common primary malignant tumor in the central nervous system (Miller et al., 2021; Berger et al., 2022). PNS can inhibit the protein expression of p-AKT and p-mTOR in U251 and U87 cells, further inhibiting the PI3K/Akt/mTOR signaling pathway to regulate GBM proliferation, migration, and apoptosis (Sami and Karsy, 2013). PNS Rh2 can induce cancer cells to revert to non-cancerous cells, induce apoptosis in various tumor cells such as mouse glioma C6Bu-1 cells, and inhibit the growth and differentiation of mouse melanoma B16 cells.
In general, PNS has clear broad-spectrum antitumor effects. PNS exerts its antitumor effects by directly inhibiting the proliferation and migration of tumor cells, and can also prevent the deterioration of pathological processes by promoting cell apoptosis. In addition, it can be used as a sensitizer in combination with traditional antitumor drugs to reverse tumor cell resistance.
4.5 Immunomodulation
PNS regulates the expression of cellular immune inflammatory factors through multi-targets and multi-pathways, and has the effect of reducing oxidative stress and alleviating inflammation. In addition to PNS, flavonoids and other substances in Panax notoginseng have a deep impact on immune regulation at multiple levels and play important roles in various types of inflammation (Yang C. et al., 2024).
After gavage of purified Panax notoginseng root saponin and leaf saponin for 7 consecutive days, PNS was able to increase the rate of erythrocyte complement C3b receptor flowering, suggesting that it has the effect of enhancing the immune function of erythrocytes in the body. PNS can regulate the level of cytokine secretion, optimize the distribution of peripheral blood T-lymphocyte subpopulations, and promote the secretion of cytokines (e.g., IL-2) that have a protective role, and at the same time, inhibit the production of harmful cytokines, so as to play an immunomodulatory role. PNS also showed a slight hemolytic activity against mouse ovalbumin-specific IgG2b antibody in ovalbumin-induced immunized mice, and had a significant adjuvant effect on ovalbumin-specific antibody and cellular responses in mice (Qin et al., 2006). PNS has a regulatory role in immunomodulation through multiple pathways on inflammatory cells and mediators, and is important for immunomodulation. PNS exerts regulatory effects on inflammatory cells and mediators through various pathways, and has an important impact on immunomodulation.
4.6 Improvement of microcirculation
PNS can significantly improve microcirculation of brain tissue, its main mechanism of action is to increase blood flow to the brain by dilating blood vessels, thus improving blood supply to brain tissue; at the same time, it can also inhibit platelet aggregation, reduce the possibility of thrombosis, further improve microcirculation; regulate the function of the vascular endothelium, and reduce the damage of vascular endothelium, which is a good way to improve the effects on a series of cerebral vascular diseases, such as ischemia and hypoxia. It has a good improvement effect on a series of cerebrovascular diseases such as ischemia.
4.6.1 Regulation of vascular endothelial function
Vascular endothelial growth factor (VEGF) is a protein that binds specifically to vascular endothelial cells and has the ability to promote the proliferation and migration of vascular endothelial cells, increase vascular permeability, and promote angiogenesis. It was found that Rg1 could regulate VEGF secretion from human umbilical vein endothelial cells through activation of PI3K/Akt and β-catenin/T-cell factor signaling pathways, thus protecting the vascular endothelium (Leung et al., 2006). When vascular endothelial cells are damaged, endothelial cell dysfunction occurs, which seriously affects the physiological and pathological processes such as vascular tone regulation, hemostasis and thrombosis, and vascular chronic inflammation, etc. PNS is able to regulate endothelial cell dysfunction in multiple ways, and it has a significant vascular endothelial protective effect. PI3K/Akt signaling pathway, inhibit Protein kinase C, and protect endothelial cell function (Lan et al., 2011). Rg1 can inhibit the mitochondrial apoptotic cascade, downregulate the expression of HIF1
PNS also has a role in promoting angiogenesis, which is the stimulation of endothelial cells to form new blood vessels, and is potentially therapeutic for cerebrovascular diseases such as chronic stroke (Giacca and Zacchigna, 2012). Can be shown to be pro-angiogenic by activating the VEGF-KDR/Flk-1 and PI3K-Akt-eNOS signaling pathways in vivo and in vitro (Yang B. R. et al., 2016). R1 also promotes the proliferation, migration, and tube formation of human umbilical vein endothelial cells, and activates the Ang2/Tie2 signaling pathway to promote angiogenesis (Zhong et al., 2020). By regulating the function of vascular endothelial cells, PNS are able to reduce the damage of vascular endothelial cells and maintain the integrity of blood vessels.
4.6.2 Hemostasis and antithrombosis
PNS exhibits a highly dependent bidirectional regulatory effect on platelet function (promoting and inhibiting platelet aggregation), capable of exerting hemostatic and anti-thrombotic effects. PNS has the effect of promoting platelet aggregation, which can enhance platelet aggregation in a concentration-dependent manner. Among the saponins that show platelet aggregation effects, PNSFt1 is the most effective; Ft1 enhances platelet aggregation by activating the P2Y12 receptor-mediated signaling network, showing a dose-dependent synergistic effect with adenosine diphosphate (ADP). The rate of platelet aggregation increases with the dosage of Ft1, further inhibiting the production of cAMP, activating the phosphorylation of downstream PI3K and Akt in rat platelets through the P2Y12 signaling pathway, thus promoting platelet aggregation, making it the most effective coagulant in vitro (Gao et al., 2014). Ft1 can also enhance the thrombin-induced PLCγ2-IP3/DAG-[Ca2+]/PKC-TXA2 signaling pathway, promoting platelet aggregation to exert hemostatic effects (Liu Y. et al., 2019).
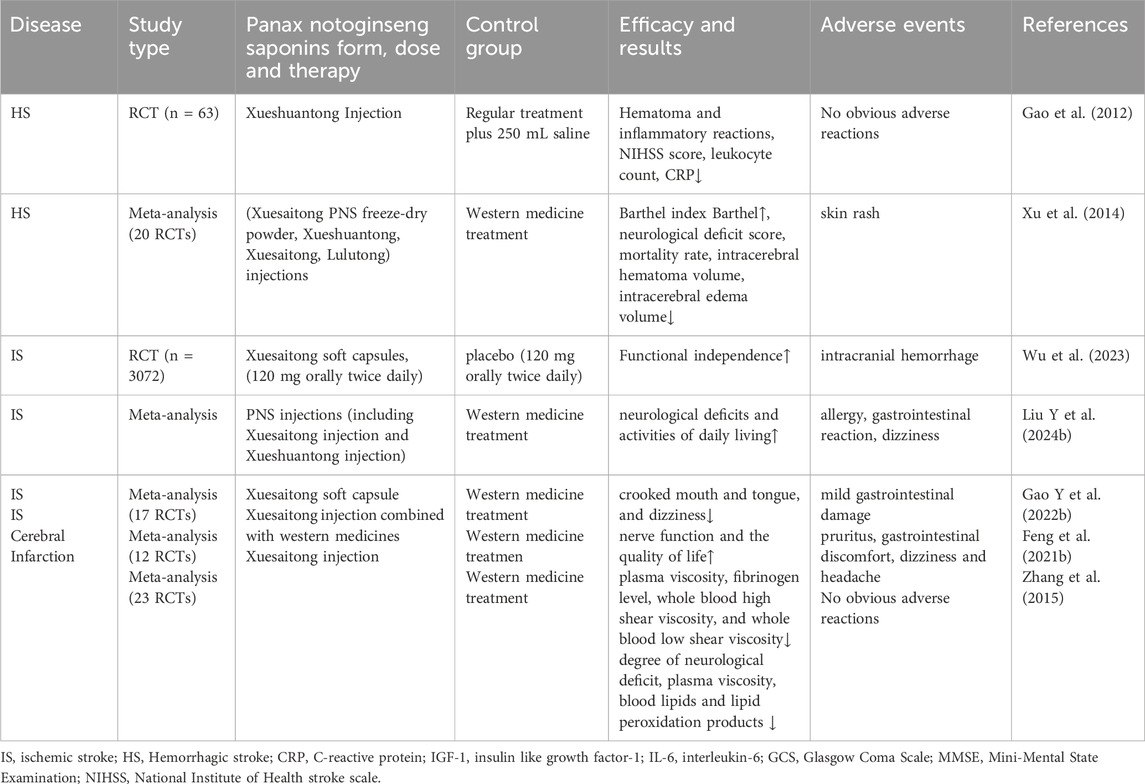
PNS also has the effect of inhibiting platelet aggregation, significantly suppressing collagen and ADP-induced platelet aggregation, inhibiting the reduction of platelets and the increase of fibrin degradation products, free calcium, TXA2, etc., inhibiting the thrombin-induced conversion from fibrinogen to fibrin, activating urokinase, promoting the dissolution of fibrin, improving blood flow, thus playing a role in anti-thrombosis (Wu et al., 2023). Pharmacological studies have found that PNS can inhibit thrombin-induced platelet aggregation by activating the PPAR-γ pathway and its downstream PI3K/Akt/eNOS pathway, effectively improving the hypercoagulable state in the body to reduce thrombosis (Shen et al., 2017). Rg1 can inhibit abnormal platelet activation by suppressing the ERK signaling pathway, thereby reducing arterial thrombosis induced by Fe Cl3 in mice (Zhou Q. et al., 2014). In a study on a rat model of cerebral ischemia with thrombosis in multiple sites such as cerebral artery occlusion, after PNS intervention, the levels of free fatty acids (FFA), adenosine triphosphate, phosphocreatine, and Ca2+ in rat brain tissue were significantly downregulated, and the area of cerebral ischemic infarction was significantly reduced, indicating that PNS has a good effect in alleviating cerebral thrombosis (Wu et al., 2022).
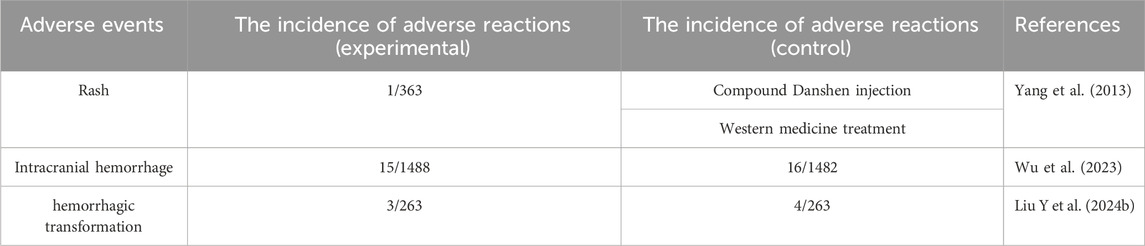
PNS’s hemostatic and antithrombotic effects on platelets are bidirectional, reflecting its multi-component and multi-target characteristics in different contexts. However, the antiplatelet and profibrinolytic effects of PNS may have additive effects with aspirin, clopidogrel, warfarin, or novel oral anticoagulants, increasing the risk of bleeding, allergies, gastrointestinal reactions, and other adverse responses. Analysis using a fixed-effects model for the occurrence of hemorrhagic transformation indicated that there was no significant difference in the incidence of hemorrhagic transformation between the groups (RR: 0.62; 95% CI: 0.34 to 1.14; p = 0.13, I2 = 0%) (Liu Y. et al., 2024). Based on the current high-level evidence, for patients with clear indications, the overall therapeutic net benefit of PNS outweighs its manageable risks. Its use should be guided by evidence of its anticoagulant and circulation-improving effects in pathological conditions, with full awareness of the potential risks when used in combination with standard anticoagulant therapy, to achieve safe and effective rational medication.
4.7 Promoting nerve regeneration
PNS plays an important role in the regulation of the nervous system, and its main components have the ability to protect neuroactivity, excite the brain center, promote blood circulation in the brain, enhance memory, improve cognitive impairment, and regulate neurotransmitters to promote nerve regeneration (Liu S. Z. et al., 2019).
As a BDNF, PNS can increase the expression of BDNF to upregulate Akt/CREB to restore the mechanism of neurological function and promote neurogenesis and oligodendrogliogenesis after ischemic stroke (Zhu et al., 2021a). As a potential novel neuroprotective agent, it can reduce neurological deficits after traumatic brain injury (TBI), ameliorate neuronal apoptosis and promote neuronal regeneration in TBI by inhibiting the ERK signaling pathway (Pei et al., 2023). PNS also upregulates nerve growth factor (NGF) and BDNF in spinal cord transected rats, providing neuroprotection, promoting axon growth, and improve hindlimb motor function (Wang et al., 2015). It can also attenuate bupivacaine-induced neurotoxicity by activating the Jak1/Stat3 pathway and triggering the upregulation of Mcl1 to rescue apoptosis and promote neuronal survival; and attenuate hepta flurane-induced neuronal cell damage by modulating the AKT signaling pathway to protect the neuronal cells and promote nerve regeneration (Yang et al., 2017; Yang et al., 2024c).
5 Effects on cerebrovascular system
PNS has effects on the cerebrovascular system, such as increasing cerebrovascular blood volume, anti-atherosclerosis, decreasing platelet surface activity, inhibiting platelet activation, adhesion and aggregation, promoting vascular neovascularization and repair, and anti-thrombosis. It can play a role in protecting the cerebrovascular system by improving cerebral ischemia-reperfusion injury, decreasing the permeability of blood-brain barrier, improving the function of cerebral blood flow, promoting neural remodeling and synaptic reconstruction, and improving the neurological deficits (see Figure 5; Table 2).
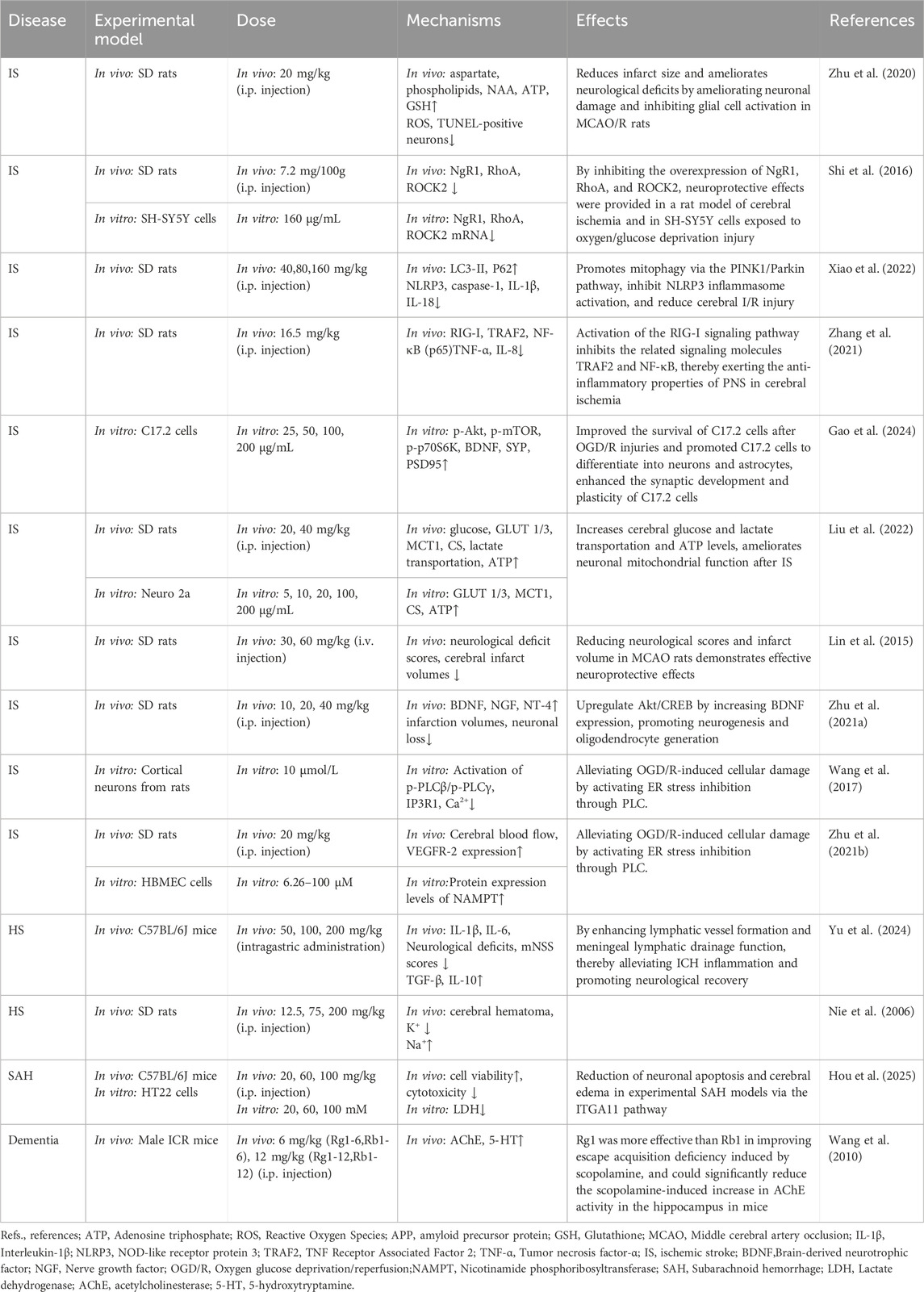
Table 2. Summary of the mechanisms and effects of panax notoginseng saponins on cerebrovascular diseases.
5.1 Anti-ischemic stroke (IS) effects
IS is an acute cerebrovascular disease in which cerebral blood flow is interrupted leading to necrosis of brain tissue, and the core mechanism is local cerebral ischemia and secondary injury (Hu et al., 2017). If cerebral ischemia is incompletely blocked and then returns to normal, cerebral ischemia reperfusion injury (CIRI) is triggered, and PNS has a significant neuroprotective effect on focal CIRI in rats (Yang P. F. et al., 2016; Li H. et al., 2018). PNS induces a variety of pharmacological effects in a multiscale mechanism of the pathophysiology of cerebral ischemia including anti-inflammatory activity, reduced oxidative stress, anti-apoptosis, inhibition of amino acid excitotoxicity, reduction of intracellular calcium overload, protection of mitochondria, repair of the blood-brain barrier and promotion of cell regeneration (Wang et al., 2021b). Compared with hemodialysis, ischemia-reperfusion injury is the most important part of ischemic stroke progression, and improvement of BBB injury is important to reduce ischemia-reperfusion injury (Feng et al., 2021a; Yang et al., 2022).
A systematic review and meta-analysis on the effect of PNS on acute ischemic stroke (AIS) indicates that treatment with Xuesaitong and PNS injection not only improves daily living activities, alleviated neurological deficits, improved cerebral blood flow, and attenuated CIRI, but also was well tolerated and reduced the incidence of adverse events (Wang et al., 2021a; Shi et al., 2023; Liu Y. et al., 2024). In a rat model of focal middle cerebral artery embolism (MCAO), PNS significantly ameliorated focal CIRI by decreasing the volume of cerebral infarction and inhibiting the release of the inflammatory factors IL-1β and TNF-α (Sun et al., 2020). PNS attenuated the extent of BBB destruction by cerebral ischemia/reperfusion (I/R) in the MCAO model, and reduced the extent of middle cerebral artery blockage in rats. Focal cerebral ischemia/reperfusion and reduce the water content of brain tissue and the area of cerebral infarction, and improve its neurobehavioral function and pathological characteristics (Luo et al., 2019; Dong et al., 2022). It can also reduce infarct area and improve neurological deficits by regulating brain small molecule metabolism to protect the brain from CIRI, ameliorating neuronal injury in middle cerebral artery embolization/reperfusion (MCAO/R) rats and inhibiting glial cell activation (Zhu et al., 2020).
Multiple saponins isolated from the aqueous extract of Panax quinquefolium exerted neuroprotective effects on damaged human neuroblastoma (SH-SY5Y) cells (Liu et al., 2018), Suppressing the overexpression of NgR1/RhoA/ROCK2 by modulating the myelin-associated inhibitory molecule, Nogo-A, in vivo and in vitro in the rat model of cerebral ischemia and in the rat model of exposure to OGD/R injury in the SH-SY5Y cell model providing neuroprotection (Shi et al., 2016). PNS also attenuates CIRI in rats by inhibiting the activation of NLRP3 inflammatory vesicles and promotes mitochondrial autophagy through the PINK1/Parkin pathway (Xiao et al., 2022). Neuronal energy failure during the acute phase of focal cerebral ischemia can also be circumvented by ameliorating mitochondrial impairment (Liu et al., 2022).
Inhibition of microglia activation and reduction of inflammatory response in the CNS is essential to reduce brain damage caused by IS as seen in the results of a PNS on microglia inflammatory response in cerebral ischemia (Duan et al., 2024). PNS can protect ischemically injured brain cells by activating the PI3K/Akt and Nrf2 signaling pathways, and it can also inhibit the downregulation of HIF-1α/PKM2/STAT3 signaling in microglial cells to reduce inflammation induced by microglial cell activation. PNS can also activate the RIG-I signaling pathway, and inhibit the related signaling molecules, TNF receptor associated factor 2 (TRAF2) and NF-κB, which are essential for the reduction of IS-induced brain injury. (NF-κB) exerts the anti-inflammatory properties of PNS in cerebral ischemia (Zhang et al., 2021; Gao J. et al., 2022). Activation of the Nrf2 signaling pathway attenuates inflammation, regulates the expression of iron overload and lipid peroxidation-related proteins and the activity of antioxidant enzymes as a means of inhibiting iron death and attenuating CIRI (Wang L. L. et al., 2024); it also inhibits the TLR4/MyD88/NF-κB signaling pathway, and inhibits cerebral ischemia through the microbiome-gut-brain axis. -gut-brain axis to inhibit the stimulation of astrocytes and microglia in the ischemic zone of the brain, restoring the structure of the BBB and attenuating CIRI (Zhang et al., 2024). In addition, PNS promotes the differentiation of cells expressing immature neuroblasts in the olfactory bulb after ischemia and reperfusion, and promotes neuronal regeneration (He et al., 2015). In another study, PNS stimulated C17.2 neural stem cell (NSC) differentiation and neuronal synapse development through mTOR signaling against OGD/R injury, demonstrating the neural differentiation-inducing properties of PNS in mouse C17.2 NSCs after OGD/R injury with the involvement of Akt/mTOR/p70S6K signaling pathway (Gao et al., 2024).
Rg1, as one of the major components of PNS, significantly reduces the volume of cerebral infarction and alleviates neurological dysfunction after I/R injury, and exhibits multiple mechanisms of physiological activity against cerebral ischemia and reperfusion injury. Antioxidant activity and related apoptosis through Akt, Nrf2/HO-1, PPARγ/HO-1, extracellular regulated protein kinase (ERK), p38 and c-Jun N-terminal kinase (JNK) pathway (or mitochondrial apoptosis pathway) and c-Caspase-3/ROCK1/MLC pathway; through MAPK pathway (JNK1/2 +), ERK1/2 and PPARγ/HO-1; and through MAPK pathway (JNK1/2 +), ERK1/2 and PPARγ/HO-1. ERK1/2 and PPARγ/HO-1 pathways), endoplasmic reticulum stress (ERS), anti-inflammatory and immunostimulation-associated activities of apoptosis or necrosis induced by high mobility group protein 1 (HMGB1) TLR2/4/9 and receptor for end products of advanced glycosylation (RAGE) pathways, and activation of NF-κB; Anti-inflammatory and immunostimulatory responses to apoptosis or necrosis; neuronal cell cycle, proliferation, differentiation, and regeneration; and regulation of energy metabolism and cellular ATP levels, BBB permeability, excitatory amino acids, and other processes, including NGF activation, excitotoxicity, and Ca2+ excess influx into neurons, which are mechanisms that play significant neuroprotective roles against brain ischemic injury (Lin et al., 2015; Xie et al., 2018b).
After CIRI, R1 promotes angiogenesis and improves energy metabolism mainly through antioxidant, anti-apoptotic and anti-inflammatory mechanisms (Tong et al., 2019). R1 intervenes in the degradation and redistribution of tight junctions through the Caveolin-1/MMP2/9 pathway and attenuates BBB permeability, cerebral infarct volume, and neurological impairments in acute cerebral ischemic rats (Liu et al., 2021). Increasing cerebral glucose and lactate transport and ATP levels and improving mitochondrial dysfunction improves cerebral energy metabolism to circumvent neuronal energy failure caused by acute ischemic stroke (Liu et al., 2022). R1 upregulates Akt/CREB by increasing the expression of BDNF, and restores neurological mechanisms through the participation of Akt/mTOR/p70S6K signaling pathway. R1 promotes oligodendrogenesis after ischemic stroke by increasing the expression of BDNF, thereby improving CIRI and long-term neurological recovery after ischemic stroke (Zhu et al., 2021a). R1 inhibits neuronal apoptosis and the expression of endoplasmic reticulum stress-associated pro-apoptotic proteins, promotes neural stem cell proliferation and differentiation, and protects neurons, endothelial cells, and astrocytes in ischemic stroke to mitigate brain injury. cells as a way to attenuate brain damage (Wang et al., 2017; Yang et al., 2020).
PNS has multi-scale pharmacological effects and good safety profile, which can effectively reduce the volume of cerebral infarction, improve neurological deficits and alleviate reperfusion injury. At the same time, clinical evidence supports that it has a good safety and tolerability in the treatment of AIS, which can improve the recovery of patients’ neurological function and serious adverse events are rare.
5.2 Anti-hemorrhagic stroke (HS) effects
HS is the most fatal form of stroke and mainly includes cerebral hemorrhage and subarachnoid hemorrhage (SAH) (Ohashi et al., 2023). The underlying cause of neurological impairment is the hematoma itself and the secondary damage around it. Brain edema, inflammatory response, apoptosis, ischemia of the tissues around the hematoma, and oxygen free radicals induced by cerebral hemorrhagic injury can enter the brain parenchyma through the damaged BBB and further exacerbate brain tissue damage (Chen et al., 2022).
PNS can significantly improve edema and hematoma in patients with ICH, and hematoma resorption and neurological function can be significantly improved in the treatment of ICH with Xueshuantong injections (175 mg/d) for 2 weeks (Gao et al., 2012). In an analysis, ICH patients treated with PNS demonstrated better outcomes than untreated ICH patients in a number of areas, including effective rate, neurological deficit score, intracranial hematoma volume, intracranial edema volume, and Barthel index (Xu et al., 2014). As the drainage system of the brain, the meningeal lymphatic system can also reduce hematoma volume, improve neurological inflammation, and enhance neurological recovery by enhancing lymphatic vessel formation and meningeal lymphatic drainage function (Yu et al., 2024). PNS significantly reduced neurological deficits in an experimental SAH model by inhibiting the expression level of ITGA11, activating the PI3K-Akt pathway to reduce neuronal apoptosis and cerebral edema, facilitating the absorption of intracranial hematomas after hemorrhage, improving the peripheral ischemic area blood flow, and decreasing the apoptosis of peripheral neurons, and thus exerting neuroprotective effects (Hou et al., 2025).
The formation of cerebral edema after cerebral hemorrhage is related to thrombin, and it is found that the expression of prothrombin receptor 1 (PAR1) in brain tissue after cerebral hemorrhage has a positive correlation with apoptosis and the emergence of cerebral edema, and thrombin gradually released from the hematoma may aggravate the cerebral injury by continuously activating PAR1 (Moller et al., 2000). PNS can prolong the clotting time of plasminogen and thrombin, and reduce the activation of platelet thrombosis. clotting time, reduce platelet activation, reduce apoptosis by inhibiting the transcription of Caspase-3 mRNA and the activation of Caspase-3 protein cleavage, and promote the transcription and protein expression of Bcl-2 gene to promote the survival of neurons and repair of damage in the brain after cerebral hemorrhage (Qureshi et al., 2003).
PNS may increase brain damage by up-regulating basic fibroblast growth factor (BFGF), which is the most important factor in the development of cerebral hemorrhage. PNS can promote neuronal survival and injury repair after cerebral hemorrhage by up-regulating the expression of BFGF, laminin, the transcription of anti-apoptotic gene Bcl-2 mRNA and the expression of Bcl-2 protein, up-regulating the expression of the rat forebrain excitatory amino acid receptor AMPA receptor subunit GluR2, the cerebral hemorrhage peripheral microtubule-associated protein-2, the growth-associated protein-43, and X-linked apoptosis inhibitory protein XIAP, as well as down-regulating the expression of the XIAP protein, downregulate the expression of rat forebrain excitatory amino acid receptors NR1, NR2A, NR2B, aquaporin-4, ICAM-1, and TNF-
PNS has shown significant neuroprotective and repair effects through multi-target mechanisms, including accelerating intracranial hematoma resorption, reducing cerebral edema, improving neurological deficits, and inhibiting secondary damage. PNS can effectively improve clinical outcomes and multiple functional indicators by enhancing meningeal lymphatic drainage, inhibiting inflammatory responses, regulating apoptosis-related pathways (such as inhibiting Caspase-3 and upregulating Bcl-2), protecting the integrity of the blood-brain barrier, and promoting nerve remodeling.
5.3 PNS and vascular dementia
VD is the most common form of dementia after neurodegenerative dementia. Oxidative stress, neuroinflammation, neurotransmitter system and mitochondrial dysfunction, lipid metabolism disorders, and changes in growth factors are all important factors that exacerbate the pathological process of dementia (Du et al., 2017).
Rg1 significantly reduces MDA, IL-β, TNF-α levels in the brain tissue of VD rats, and increases SOD activity in brain tissue, indicating that ginsenoside Rg1 has antioxidant and anti-inflammatory effects. It can reduce oxidative stress levels and inflammatory responses in the brain tissue of VD rats, potentially by inhibiting apoptosis of hippocampal neurons in VD rats through the activation of the MEK5/ERK5 signaling pathway, and improving inflammatory and oxidative damage in hippocampal tissue. Rg1 and Rb1 can increase ACh levels in the hippocampus and both can inhibit the reduction of 5-hydroxytryptamine (5-HT) induced by scopolamine, thereby improving dementia, enhancing memory, and exerting cognitive-enhancing effects (Wang et al., 2010). By regulating the Notch signaling pathway to activate the nicotinamide phosphoribosyltransferase-nicotinamide adenine dinucleotide-Sirtuin 1 (NAMPT-NAD+-SIRT1) cascade reaction, this approach promotes angiogenesis following stroke-induced ischemia, thereby improving vascular cognitive impairment and dementia (Zhu et al., 2021b).
PNS significantly improves cognitive function through antioxidant, anti-apoptotic, cholinergic nervous system regulation, and neuroplasticity regulation, demonstrating significant anti-dementia effects and providing new avenues for the treatment of diseases accompanied by neurological dysfunction (Wang et al., 2010).
6 Effects of PNS on neurological disorders
Modern pharmacological studies have found that PNS has good preventive and therapeutic effects on cerebral nervous system diseases such as AD, Parkinson’s disease, depression, and other neurological diseases (Su et al., 2014). It can inhibit neuronal apoptosis through antioxidant, anti-apoptotic, and anti-neuroinflammatory activities, promote synaptic plasticity and nerve regeneration, and protect damaged neurons, which can slow down and prevent the occurrence of neurological disorders, and it is an effective and safe natural drug for the treatment of neurological diseases (Qu et al., 2020) (see Figure 6; Table 3).
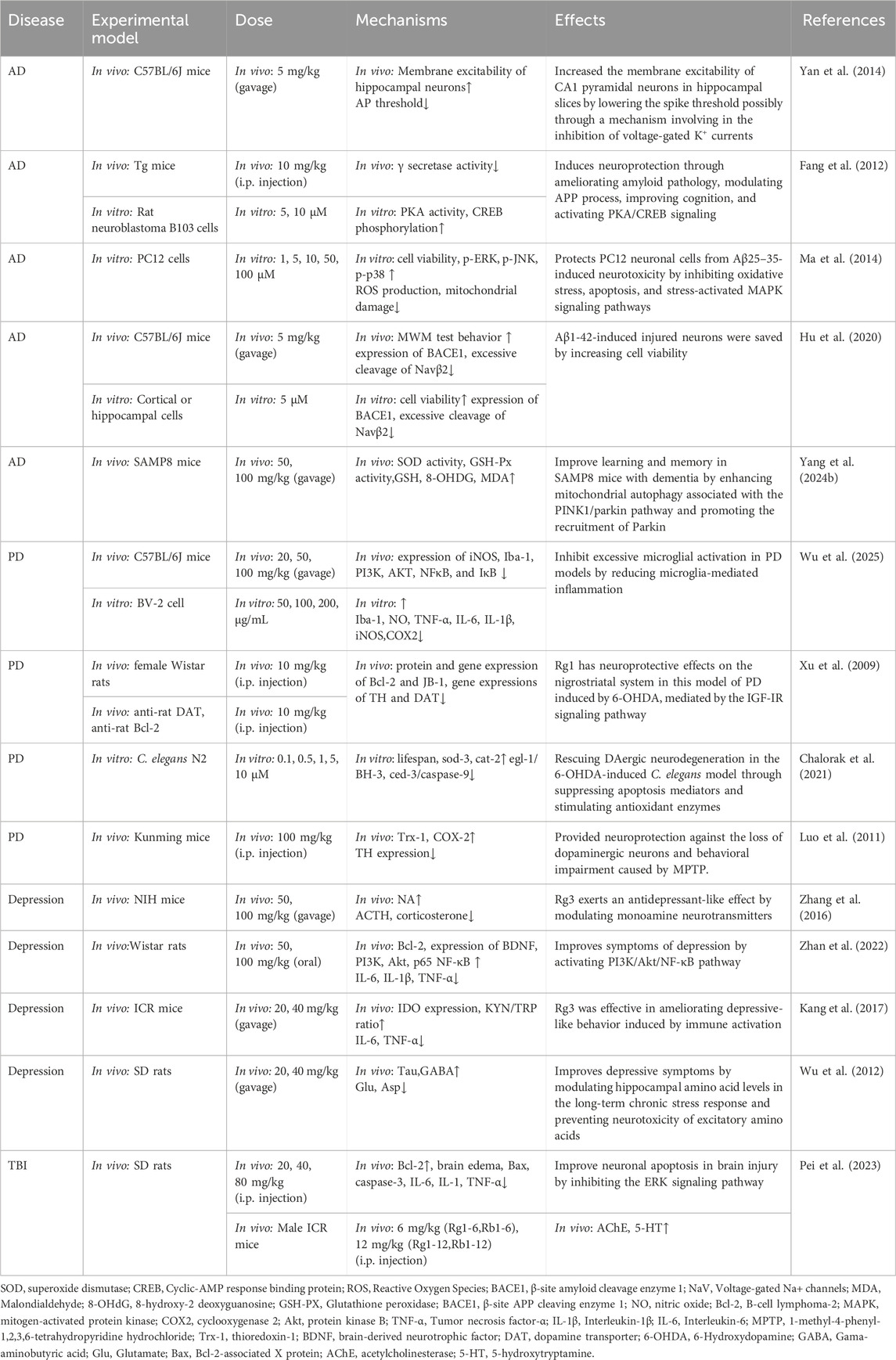
Table 3. Summary of the mechanisms and effects of panax notoginseng saponins on neurological disorders.
6.1 PNS and Alzheimer’s disease
AD is a chronic neurodegenerative disease characterized by progressive cognitive and memory deficits, as characterized by massive death and loss of central cholinergic neurons, hyperphosphorylation of Tau proteins to form neurofibrillary tangles and deposition of β-amyloid (Aβ), which leads to damage to cholinergic nerve cells, decreased levels of choline acetyltransferase (ChAT) and decreased acetylcholine transmitter synthesis is reduced (Busche and Hyman, 2020). PNS has been shown to be advantageous in ameliorating neurological disorders such as Alzheimer’s, improving learning and memory deficits (Liu S. et al., 2024). Its treatment mainly targets Aβ production deposition, Tau protein phosphorylation and cholinergic nervous system.
PNS can regulate the expression of amyloid beta precursor protein (APP) gene in the brain at the transcriptional level, activate PKA/CREB signaling, promote APP to shear with α-secretase and inhibit γ-secretase activity (Fang et al., 2012), upregulate the expression of ADAM9 mRNA, downregulate the expression of β-secretase (BACE1) protein in the brain, and reduce Aβ generation, and improve the learning and memory ability of rapid aging model mice (Huang et al., 2014). R1 intervention in APP/PS1 double transgenic AD model mice, by inhibiting K+ channel activity may contribute to the reduction of spiking thresholds, promote the increase of LTP and ultimately regulate neuronal excitability, which can improve their learning and memory ability, and have a certain restorative effect on degenerative neuropathy (Yan et al., 2014). Rb1 inhibits ROS production, increases Bcl-2/Bax and inhibits caspase-3 activity, and maintains the balance of oxidative stress in Aβ-induced neuronal cells to exert a neuroprotective effect (Xie et al., 2010; Shekhar et al., 2018). R1 also increases Aβ25-35cell viability in cultured PC12 neurons, reduces oxidative damage, restores mitochondrial membrane potential and activates the MAPK signaling pathway to counteract the effects of Aβ (Ma et al., 2014). Studies have shown that elevated levels of Nav1.1α are associated with cognitive deficits in mice (Corbett et al., 2013), R1 induced inhibition of BACE1 activity by altering the number and/or distribution of voltage-gated sodium channel (Nav) members, modulating the enzymatic cleavage of Navβ2 to regulate sodium currents, correcting the aberrant distribution of Nav1.1α, ameliorating abnormal neuronal overexcitation and memory deficits, promoting neuronal repair and cognitive improvement in AD mice (Hu et al., 2020).
The pathogenesis of AD is associated with the dephosphorylation of phosphatases such as glycogen synthase kinase-3 and protein phosphatase-2A (PP2A). Rb1 was found to protect against aluminum-induced neurotoxicity by preventing Tau protein phosphorylation through the regulation of p-GSK3 and PP2A levels (Zhao et al., 2013). PNS can also protect and improve the function of the central cholinergic system by improving the quantity and quality of cellular survival, and by increasing the content and activity of the enzyme ChAT through the improvement and repair of damaged neurons. The central cholinergic system.
In vitro and in vivo experiments have demonstrated that PNS can alleviate diseases caused by AD risk factors by modulating HPA axis disorders, lowering excess free FFA levels, improving insulin resistance, and promoting vascular wall thickening to reduce Aβ accumulation and Tau hyperphosphorylation in the brain (Wu et al., 2024). PNS enhances the PINK1/Parkin pathway, promotes mitophagy in the hippocampus, reduces brain oxidative stress in SAMP8 mice, and increases PC12 cell viability, elevating LC3II/I protein levels while decreasing p62 protein and OPTN, thereby alleviating neuronal damage in AD (Jiang et al., 2022; Yang et al., 2024b). PNS can alleviate the disease caused by AD risk factors through anti-inflammatory and anti-cellular effects, as well as through Tau excess phosphorylation. PNS can reduce glutamate metabolism dysfunction induced by the chemokine CC motif ligand 2, inhibit oxidative stress induced by the over-activation of the N-methyl-D-aspartate receptor, lower the Bax/BCL-2 ratio and inhibit apoptosis by reducing the expression of caspases-3, 8, and 9, and thus alleviate cognitive deficits in rats (Zhou et al., 2020).
R1 can alleviate impaired learning and memory in SD mice by modulating the melatonin receptor type 1A-mediated PI3K/Akt/mTOR signaling pathway to reduce excessive autophagy and apoptosis in hippocampal neurons (Huang G. et al., 2017; Cao et al., 2021). R2 is able to inhibit apoptosis through the miR-27a/SOX8/β-catenin axis expression, attenuate Aβ25-35-triggered neuronal apoptosis and inflammation, and inhibit cortical neuronal apoptosis and attenuate inflammation in AD rats as a way to enhance cognitive function and improve AD symptoms in AD rats (Hu et al., 2021); It can also prevent isoflurane-induced learning and cognitive impairments by promoting miR-29a expression and preventing inflammatory responses (Wang et al., 2022).
In conclusion, Aβ is a key initiator of the AD pathological cascade. PNS promotes mitochondrial autophagy, restores mitochondrial membrane potential, and reduces oxidative stress damage in the hippocampus through PINK1/Parkin and MAPK signaling pathways; regulates excessive autophagy and apoptosis in hippocampal neurons via PI3K/Akt/mTOR signaling pathways; and downregulates BACE1 protein expression in the brain through PKA/CREB signaling. BACE1 protein expression as a way to reduce Aβ production. PNS also prevents Tau protein phosphorylation by regulating p-GSK3 and PP2A. PNS plays a critical role in AD-induced neurological dysfunction by inhibiting neuroinflammation, oxidative stress, and regulating mitochondrial autophagy and apoptosis, thereby reducing Aβ production and preventing Tau protein phosphorylation.
6.2 PNS and Parkinson’s disease (PD)
PD is a neurodegenerative disorder in which the balance between dopamine and acetylcholine is dysregulated due to degenerative changes in dopamine (DA) neurons in the dense nigrostriatal region of the midbrain (Marino et al., 2020). Associated with multiple factors such as aging, genetic susceptibility and environmental exposure (Gao and Hong, 2011).
PNS may attenuate microglia-mediated neuroinflammation through the P2Y2R/PI3K/AKT/NF-κB signaling pathway, inhibit the production of inflammatory markers, such as IL-1β, IL-6, and TNF-α, in LPS-stimulated BV-2 cells, and attenuate behavioral deficits and excessive microglial activation in PD model mice, thereby reducing the progression of PD (Wu et al., 2025). Rg3 has the ability to ameliorate dopamine neuron neurodegeneration in a 6-hydroxydopamine (6-OHDA)-induced PD model through inhibition of apoptotic mediators and stimulation of antioxidant enzymes (Chalorak et al., 2021). Mitochondrial dysfunction remains a key mechanism in a variety of neurodegenerative diseases, in which autophagy receptors are recruited by the ubiquitin kinase PINK1 to induce mitochondrial autophagy. PINK1 and PARKIN-related autophagy processes are able to modulate neurodegeneration and neuroinflammation by removing dysfunctional mitochondria, controlling mtDNA release, or promoting neuroprotective and anti-inflammatory manifestations (Lazarou et al., 2015; Quinn et al., 2020; Olagunju et al., 2023).
Rg1 can exert a neuroprotective effect on 6-OHDA induced dopaminergic neurons in the substantia nigra striata damaged dopaminergic neurons in a rat model of PD through the insulin-like growth factor-I receptor (IGF-I) signaling pathway (Xu et al., 2009). Ginsenoside triol saponin (PTS) from Panax notoginseng inhibited 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced neurotoxicity in PD mice, and further suppressed MPTP-induced neuronal death in the substantia nigra compacta by increasing Trx-1 expression, inhibiting cyclooxygenase-2 overexpression, and inhibiting mitochondrial-mediated apoptosis, and then could effectively PD (Luo et al., 2011).
Studies have shown that dopamine neurons lack degeneration, neuroinflammation, mitochondrial dysfunction and oxidative stress are the characteristics of its pathogenesis, and PNS can alleviate dopamine neuron damage and apoptosis through P2Y2R/PI3K/AKT/NF-κB and IGF-I signaling pathways by exerting anti-inflammatory and antioxidant effects, as well as through the autophagy process mediated by PINK1 and PARKIN to effectively ameliorate PD. effectively improve PD.
6.3 PNS and depression
Depression is a psychoaffective disorder characterized by persistent low mood, loss of interest, and impairment of cognitive function (Dean and Keshavan, 2017). Current evidence suggests that the onset of depression may be associated with reduced neurotransmitter secretion (Jeon and Kim, 2016). In PNS, saponins R1, Rg1, Rb1, Rc, and Rb3 are important representative components of antidepressants (Cui et al., 2012), which may be involved in the regulation of neurotransmitter mechanisms (5-HT, DA, and NE), modulation of gamma-aminobutyric acid (GABA) neurotransmission, the glutamatergic system, the HPA axis, BDNF, and their intracellular signaling in the CNS pathways produce neuronal protection and regulate nerve cell activity and secretion, thus exerting antidepressant and anxiolytic effects (Xie et al., 2018a).
PNS modulates calmodulin kinase channels (Ca2+/CaM/CaMK), reduces internal concentrations of Ca2+in neuronal cells, and promotes the release of the neurotransmitters 5-HT, NE, and DA to reduce depressive behavior (Albert and Fiori, 2014; Zhang et al., 2016). It has been shown that neuroinflammation is a key pathological mechanism contributing to depressive-like behaviors (O'Connor et al., 2009), High levels of pro-inflammatory cytokines (IL- 6, IL-1β, TNF-
In an antidepressant-like activity assay between ginsenoside Rb3 and its four deglycosylated derivatives, Rg3, Rh2, Compound K (C-K), and PPD, only C-K and Rg3, which are the active deglycosylated derivatives of Rb3, exerted antidepressant-like effects in mice in the forced-swimming test (FST) and the tail-suspension test (TST), and in particular Rg3 was more effective in restoring brain monocorticolysis than Rb3. Rb3 is more effective in restoring brain monoamine neurotransmitter 5-HT, decreasing dopamine levels, increasing norepinephrine levels, etc. exerting antidepressant-like effects (Zhang et al., 2016). Rg3 significantly reduces LPS-induced plasma levels of IL-6 and TNF-α, restores systemic homeostasis of tryptophan-kynurenine metabolism, and effectively ameliorates immune-activation-induced depressive-like behavior (Kang et al., 2017).
PNS can improve depressive-like behavior by multifaceted modulation of monoamine neurotransmitters, neuroinflammation, activation of the BDNF-TrkB signaling pathway, inhibition of oxidative stress, and regulation of the HPA axis.
6.4 PNS and traumatic brain injury (TBI)
TBI is a severe traumatic brain injury consisting of both primary and secondary damage; the primary damage is irreversible, while the secondary damage consists of a series of physiological and molecular biological changes such as oxidative stress, inflammatory response, mitochondrial apoptosis, oxygen free radical production, and disruption of the BBB (Jiang et al., 2019).
In a rat TBI model, R1 administration (intraperitoneal injection of 40 mg/kg) reduced the expression levels of ERK and p-RSK1, improved neuronal apoptosis in brain injury by inhibiting the ERK signaling pathway, reduced neurological deficits after TBI, and inhibited the expression of pro-inflammatory factors (Pei et al., 2023). Cerebral edema, as one of the serious secondary pathological changes after TBI, can lead to increased intracranial pressure and corresponding decreased cerebral perfusion, which is further aggravated leading to brain tissue injury (Lutton et al., 2017). PNS can improve microcirculation around the ischemic area of hematoma, promote the absorption of hematoma, and slow down and inhibit the development of cerebral edema. When using PNS to treat cerebral edema, the use of PNS in the early stage of cerebral hemorrhage or TBI may aggravate cerebral edema and increase neurological deficit scores, so PNS should be used with caution in treating patients with large numbers of cerebral hemorrhages in the early stage of cerebral hemorrhage or TBI (Nie et al., 2006).
7 Single-agent of PNS clinical application in neurological disorders
Currently, PNS is approved for clinical use in China by the China Drug Administration in various dosage forms. PNS injections are mainly used to treat neurological diseases such as cerebral hemorrhage and cerebral ischemia (see Table 4).
Clinical research evidence indicates that single-agent preparations of PNS (mainly injections or soft capsules of Xuesaitong and Xueshuantong) show positive effects in the treatment of hemorrhagic stroke, ischemic stroke, and related cerebral infarction. Multiple randomized controlled trials and meta-analyses have shown that their application can effectively improve patients’ neurological deficit scores, activities of daily living (Barthel index), promote hematoma absorption, reduce cerebral edema, and improve hemorheological indices. Overall, single-agent treatment with PNS has significant clinical efficacy and good safety in cerebrovascular diseases (especially ischemic and hemorrhagic strokes), providing strong evidence for its widespread application.
8 Treatment of neurotoxicity
Neurotoxicity refers to damage to the structure or function of the nervous system caused by exogenous chemicals or biological factors, including excessive production of free radicals and decreased activity of antioxidant enzymes; inflammatory factors inducing neuronal damage and death; apoptosis; and imbalance of calcium ions and neurotransmitters. This in turn affects neuronal, glial cell, synaptic transmission or overall neural circuit function.
In the study of H2O2 induced neurotoxicity and led to PC12 cell death, Rb1 was able to effectively increase cell viability and inhibit apoptosis, thus protecting PC12 cells from toxic effects (Zeng et al., 2015). In addition, Rb1 can prevent Tau protein phosphorylation and thus aluminum-induced neurotoxicity by regulating the levels of p-GSK3 and PP2A, further exerting neuroprotective effects (Zhao et al., 2013). It can also block spatial and cognitive deficits induced by acrylamide administration, attenuate acrylamide-induced neurotoxicity by up-regulating PC12 cells and regulating autophagy by Trx-1 (Wang et al., 2020). R1 can rescue apoptosis and attenuate bupivacaine-induced neurotoxicity by activating Jak1/Stat3 pathway and triggering upregulation of Mcl1. Induced neurotoxicity and reduce neuronal damage (Yang et al., 2024c). R1 can block the downregulation of Bcl-2 and the upregulation of Bax, which can inhibit the increase of intracellular free Ca2+, reduce the overproduction of intracellular ROS, and prevent the depolarization of the mitochondrial membrane potential, thereby protecting the cortical neuronal cells against neurotoxicity caused by glutamate (Glu) exposure in mice (Gu et al., 2009). PNS attenuates sevoflurane-induced neuronal cytotoxicity, protects neuronal cells, and promotes neuronal regeneration by modulating the AKT signaling pathway (Yang et al., 2017). PTS from Panax notoginseng inhibits MPTP induced neurotoxicity in PD mice, attenuates neurotoxicity and nourishes nerves through multi-pharmacological properties, such as antioxidant, inhibition of inflammatory response, and mitochondria-mediated apoptosis (Luo et al., 2011).
In conclusion, in the face of different types of neurotoxicity, PNS can regulate neuronal cells through multiple mechanisms and multiple cell signaling pathways. Therefore, future studies need more experiments to evaluate the potential efficacy and safety of PNS in clinical use for the treatment of neurological disorders.
9 Adverse events
Side effects of PNS are relatively rare in clinical practice. In a systematic evaluation, adverse reactions associated with Xuesaitong in combination with conventional medications included only 0.27% rash (1/363), and no serious adverse events were reported (Yang et al., 2013). In a randomized clinical trial of efficacy and safety, the incidence of serious adverse events in the PNS (Xuesaitong soft capsules) group at 3 months was 1.0% (15 of 1488 patients), and other secondary safety outcomes, including symptomatic intracranial hemorrhage (0.1%), all-cause mortality (0.1%), and adverse events (2.8%) were not reported (Wu et al., 2023). In a systematic evaluation and meta-analysis of the results of intravenous thrombolysis after the use of PNS injection for acute ischemic stroke, seven studies reported the occurrence of adverse events and five reported the occurrence of hemorrhagic transformation, with two studies reporting no adverse events and three reporting specific adverse events (n = 527), which were mild and self-limiting and consisted mainly of allergies, gastrointestinal reactions, and Dizziness (Liu Y. et al., 2024). In another study of the efficacy and safety of PNS in patients with acute ischemic stroke, the incidence of adverse events was very low, with nausea, dizziness, and skin irritation being the most commonly reported adverse events. In a study of the effect of age on the efficacy and safety of PNS for acute ischemic stroke, no symptomatic intracranial hemorrhage or all-cause mortality was observed, regardless of age. In summary, PNS has shown good safety in combination with conventional treatment (see Table 5).
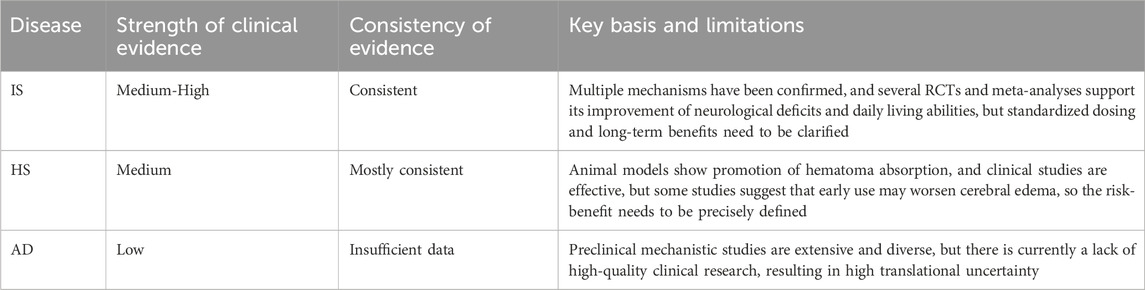
Regarding the strength and consistency of clinical evidence for PNS in different cerebrovascular and neurological diseases, the clinical application evidence was integrated, analyzed, and systematically evaluated, thereby revealing the current credibility of PNS in its research field and the direction for future studies (see Table 6).
Precise drug development should target specific potential components (such as the procoagulant factor Ft1); further clarify their pharmacokinetic-pharmacodynamic relationships in secondary prevention of acute ischemic stroke; evaluate their safety in real-world settings in combination with standard antithrombotic drugs; and develop innovative formulation strategies aimed at enhancing blood-brain barrier delivery efficiency.
10 Conclusion
PNS, as the main active ingredient of Panax notoginseng, has multi-target and multi-pathway neuroprotective effects in the treatment of cerebrovascular and neurological diseases, and its biological activity mainly exerts cerebrovascular and neurological protective effects through anti-inflammatory, antioxidant, anti-apoptosis, immunomodulation, etc. to improve microcirculation, promote nerve regeneration, and inhibit protein aggregation, etc. Many clinical studies have provided corresponding results for the use of PNS in the treatment of cerebrovascular-like and neurodegenerative diseases, etc. Many clinical studies have provided corresponding evidence for the treatment of PNS in cerebrovascular and neurodegenerative diseases, confirming that PNS is a potential neuroprotective agent and can be used as an adjunctive treatment for cerebrovascular and neurodegenerative diseases. However, the low oral bioavailability and limited penetration of BBB during drug delivery require further optimization of drug delivery strategies and strengthening of clinical translational research.
Author contributions
BW: Conceptualization, Data curation, Writing – review and editing, Writing – original draft. XG: Writing – review and editing, Visualization. YZ: Visualization, Writing – review and editing. YX: Writing – review and editing, Visualization. TC: Writing – review and editing, Visualization. ZS: Visualization, Writing – review and editing. YY: Visualization, Writing – review and editing. PL: Writing – review and editing, Supervision.
Funding
The authors declare that financial support was received for the research and/or publication of this article. This work was supported by the Natural Science Foundation of Jilin Province (No. YDZJ202401133ZYTS), the National Traditional Chinese Medicine Clinical Excellence Talent Advanced Study Program (National Administration of TCM Personnel and Education Letter [2022] No. 239).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Correction note
A correction has been made to this article. Details can be found at: 10.3389/fphar.2025.1750932.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Albert, P. R., and Fiori, L. M. (2014). Transcriptional dys-regulation in anxiety and major depression: 5-HT1A gene promoter architecture as a therapeutic opportunity. Curr. Pharm. Des. 20 (23), 3738–3750. doi:10.2174/13816128113196660740
Balusamy, S. R., Perumalsamy, H., Huq, M. A., Yoon, T. H., Mijakovic, I., Thangavelu, L., et al. (2023). A comprehensive and systemic review of ginseng-based nanomaterials: synthesis, targeted delivery, and biomedical applications. Med. Res. Rev. 43 (5), 1374–1410. doi:10.1002/med.21953
Beamer, C. A., and Shepherd, D. M. (2012). Inhibition of TLR ligand- and interferon gamma-induced murine microglial activation by Panax notoginseng. J. Neuroimmune Pharmacol. 7 (2), 465–476. doi:10.1007/s11481-011-9333-0
Berger, T. R., Wen, P. Y., Lang-Orsini, M., and Chukwueke, U. N. (2022). World health organization 2021 classification of central nervous system tumors and implications for therapy for adult-type gliomas: a review. JAMA Oncol. 8 (10), 1493–1501. doi:10.1001/jamaoncol.2022.2844
Busche, M. A., and Hyman, B. T. (2020). Synergy between amyloid-beta and tau in Alzheimer's disease. Nat. Neurosci. 23 (10), 1183–1193. doi:10.1038/s41593-020-0687-6
Cao, Y., Li, Q., Zhou, A., Ke, Z., Chen, S., Li, M., et al. (2021). Notoginsenoside R1 reverses abnormal autophagy in hippocampal neurons of mice with sleep deprivation through melatonin receptor 1A. Front. Pharmacol. 12, 719313. doi:10.3389/fphar.2021.719313
Chalorak, P., Sanguanphun, T., Limboonreung, T., and Meemon, K. (2021). Neurorescue effects of frondoside A and ginsenoside Rg3 in C. elegans model of Parkinson's disease. Molecules 26 (16), 4843. doi:10.3390/molecules26164843
Chen, X. N., Li, D. Q., Zhao, M. D., Yu, G. Y., Du, S. Y., Lu, Y., et al. (2018). Pharmacokinetics of Panax notoginseng saponins in adhesive and normal preparation of Fufang Danshen. Eur. J. Drug Metab. Pharmacokinet. 43 (2), 215–225. doi:10.1007/s13318-017-0433-y
Chen, S., Li, L., Peng, C., Bian, C., Ocak, P. E., Zhang, J. H., et al. (2022). Targeting oxidative stress and inflammatory response for blood-brain barrier protection in intracerebral hemorrhage. Antioxid. Redox Signal 37 (1-3), 115–134. doi:10.1089/ars.2021.0072
Corbett, B. F., Leiser, S. C., Ling, H. P., Nagy, R., Breysse, N., Zhang, X., et al. (2013). Sodium channel cleavage is associated with aberrant neuronal activity and cognitive deficits in a mouse model of Alzheimer's disease. J. Neurosci. 33 (16), 7020–7026. doi:10.1523/JNEUROSCI.2325-12.2013
Cui, J., Jiang, L., and Xiang, H. (2012). Ginsenoside Rb3 exerts antidepressant-like effects in several animal models. J. Psychopharmacol. 26 (5), 697–713. doi:10.1177/0269881111415735
Dean, J., and Keshavan, M. (2017). The neurobiology of depression: an integrated view. Asian J. Psychiatr. 27, 101–111. doi:10.1016/j.ajp.2017.01.025
Dong, C., Li, J., Zhao, M., Chen, L., Zhai, X., Song, L., et al. (2022). Pharmacological effect of Panax notoginseng saponins on cerebral ischemia in animal models. Biomed. Res. Int. 2022, 4281483. doi:10.1155/2022/4281483
Du, S. Q., Wang, X. R., Xiao, L. Y., Tu, J. F., Zhu, W., He, T., et al. (2017). Molecular mechanisms of vascular dementia: what can be learned from animal models of chronic cerebral hypoperfusion? Mol. Neurobiol. 54 (5), 3670–3682. doi:10.1007/s12035-016-9915-1
Duan, Z., Jia, W., Wang, J., Xu, D., Yang, Y., Qi, Z., et al. (2024). Exploring the mechanism of Panax notoginseng saponin in inhibiting the inflammatory response of microglia in cerebral ischemia based on network pharmacology. Acta Biochim. Biophys. Sin. (Shanghai) 56 (10), 1566–1570. doi:10.3724/abbs.2024114
Fang, F., Chen, X., Huang, T., Lue, L. F., Luddy, J. S., and Yan, S. S. (2012). Multi-faced neuroprotective effects of Ginsenoside Rg1 in an Alzheimer mouse model. Biochim. Biophys. Acta 1822 (2), 286–292. doi:10.1016/j.bbadis.2011.10.004
Feng, H., Chen, W., and Zhu, C. (2011). Pharmacokinetics study of bio-adhesive tablet of Panax notoginseng saponins. Int. Arch. Med. 4 (1), 18. doi:10.1186/1755-7682-4-18
Feng, L., Han, F., Zhou, L., Wu, S., Du, Y., Zhang, D., et al. (2021a). Efficacy and safety of Panax Notoginseng saponins (Xueshuantong) in patients with acute ischemic stroke (EXPECT) trial: rationale and design. Front. Pharmacol. 12, 648921. doi:10.3389/fphar.2021.648921
Feng, L., Wu, X. J., Cao, T., and Wu, B. (2021b). The efficacy and safety of Xuesaitong injection combined with western medicines in the treatment of ischemic stroke: an updated systematic review and meta-analysis. Ann. Palliat. Med. 10 (9), 9523–9534. doi:10.21037/apm-21-1828
Fu, X., Chen, K., Li, Z., Fan, H., Xu, B., Liu, M., et al. (2023). Pharmacokinetics and oral bioavailability of Panax Notoginseng saponins administered to rats using a validated UPLC-MS/MS method. J. Agric. Food Chem. 71 (1), 469–479. doi:10.1021/acs.jafc.2c06312
Gao, H. M., and Hong, J. S. (2011). Gene-environment interactions: key to unraveling the mystery of Parkinson's disease. Prog. Neurobiol. 94 (1), 1–19. doi:10.1016/j.pneurobio.2011.03.005
Gao, L., Zhao, H., Liu, Q., Song, J., Xu, C., Liu, P., et al. (2012). Improvement of hematoma absorption and neurological function in patients with acute intracerebral hemorrhage treated with Xueshuantong. J. Neurol. Sci. 323 (1-2), 236–240. doi:10.1016/j.jns.2012.09.028
Gao, B., Huang, L., Liu, H., Wu, H., Zhang, E., Yang, L., et al. (2014). Platelet P2Y₁₂ receptors are involved in the haemostatic effect of notoginsenoside Ft1, a saponin isolated from Panax notoginseng. Br. J. Pharmacol. 171 (1), 214–223. doi:10.1111/bph.12435
Gao, J., Yao, M., Zhang, W., Yang, B., Yuan, G., Liu, J. X., et al. (2022). Panax notoginseng saponins alleviates inflammation induced by microglial activation and protects against ischemic brain injury via inhibiting HIF-1α/PKM2/STAT3 signaling. Biomed. Pharmacother. 155, 113479. doi:10.1016/j.biopha.2022.113479
Gao, Y., Chen, Z., Li, X., Cai, Y., Gao, F., Tang, Y., et al. (2022). The efficacy and safety of the Xuesaitong soft capsule in the treatment of patients with ischemic stroke: systematic review and meta-analysis. Ann. Palliat. Med. 11 (8), 2695–2708. doi:10.21037/apm-22-748
Gao, J., Yao, M., Zhang, Y., Jiang, Y., and Liu, J. (2024). Panax notoginseng saponins stimulates the differentiation and neurite development of C17.2 neural stem cells against OGD/R injuries via mTOR signaling. Biomed. Pharmacother. 172, 116260. doi:10.1016/j.biopha.2024.116260
Giacca, M., and Zacchigna, S. (2012). VEGF gene therapy: therapeutic angiogenesis in the clinic and beyond. Gene Ther. 19 (6), 622–629. doi:10.1038/gt.2012.17
Gu, B., Nakamichi, N., Zhang, W. S., Nakamura, Y., Kambe, Y., Fukumori, R., et al. (2009). Possible protection by notoginsenoside R1 against glutamate neurotoxicity mediated by N-methyl-D-aspartate receptors composed of an NR1/NR2B subunit assembly. J. Neurosci. Res. 87 (9), 2145–2156. doi:10.1002/jnr.22021
Guo, Q., Li, P., Wang, Z., Cheng, Y., Wu, H., Yang, B., et al. (2014). Brain distribution pharmacokinetics and integrated pharmacokinetics of Panax Notoginsenoside R1, Ginsenosides Rg1, Rb1, re and Rd in rats after intranasal administration of Panax Notoginseng Saponins assessed by UPLC/MS/MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 969, 264–271. doi:10.1016/j.jchromb.2014.08.034
Hao, H., Lai, L., Zheng, C., Wang, Q., Yu, G., Zhou, X., et al. (2010). Microsomal cytochrome p450-mediated metabolism of protopanaxatriol ginsenosides: metabolite profile, reaction phenotyping, and structure-metabolism relationship. Drug Metab. Dispos. 38 (10), 1731–1739. doi:10.1124/dmd.110.033845
He, X., Deng, F. J., Ge, J. W., Yan, X. X., Pan, A. H., and Li, Z. Y. (2015). Effects of total saponins of Panax notoginseng on immature neuroblasts in the adult olfactory bulb following global cerebral ischemia/reperfusion. Neural Regen. Res. 10 (9), 1450–1456. doi:10.4103/1673-5374.165514
Hou, Y., Zhang, L., Ma, W., and Jiang, Y. (2025). NGR1 reduces neuronal apoptosis through regulation of ITGA11 following subarachnoid hemorrhage. Mol. Med. Rep. 31 (3), 67. doi:10.3892/mmr.2025.13432
Hu, X., De Silva, T. M., Chen, J., and Faraci, F. M. (2017). Cerebral vascular disease and neurovascular injury in ischemic stroke. Circ. Res. 120 (3), 449–471. doi:10.1161/CIRCRESAHA.116.308427
Hu, S., Wu, Y., Zhao, B., Hu, H., Zhu, B., Sun, Z., et al. (2018). Panax notoginseng saponins protect cerebral microvascular endothelial cells against oxygen-glucose Deprivation/reperfusion-induced barrier dysfunction via activation of PI3K/Akt/Nrf2 antioxidant signaling pathway. Molecules 23 (11), 2781. doi:10.3390/molecules23112781
Hu, T., Li, S., Liang, W. Q., Li, S. S., Lu, M. N., Chen, B., et al. (2020). Notoginsenoside R1-Induced neuronal repair in models of alzheimer disease is associated with an alteration in neuronal hyperexcitability, which is regulated by nav. Front. Cell Neurosci. 14, 280. doi:10.3389/fncel.2020.00280
Hu, Y., Wu, L., Jiang, L., Liang, N., Zhu, X., He, Q., et al. (2021). Notoginsenoside R2 reduces Aβ25-35-induced neuronal apoptosis and inflammation via miR-27a/SOX8/β-catenin axis. Hum. Exp. Toxicol. 40 (12_Suppl. l), S347–S358. doi:10.1177/09603271211041996
Hu, Y., Lei, H., Zhang, S., Ma, J., Kang, S., Wan, L., et al. (2022). Panax notoginseng saponins protect brain microvascular endothelial cells against oxygen-glucose Deprivation/resupply-induced necroptosis via suppression of RIP1-RIP3-MLKL signaling pathway. Neurochem. Res. 47 (11), 3261–3271. doi:10.1007/s11064-022-03675-0
Huang, J., Wu, D., Wang, J., Li, F., Lu, L., Gao, Y., et al. (2014). Effects of Panax notoginseng saponin on α, β, and γ secretase involved in Aβ deposition in SAMP8 mice. Neuroreport 25 (2), 89–93. doi:10.1097/WNR.0000000000000048
Huang, G., Zou, B., Lv, J., Li, T., Huai, G., Xiang, S., et al. (2017). Notoginsenoside R1 attenuates glucose-induced podocyte injury via the inhibition of apoptosis and the activation of autophagy through the PI3K/Akt/mTOR signaling pathway. Int. J. Mol. Med. 39 (3), 559–568. doi:10.3892/ijmm.2017.2864
Huang, J. L., Jing, X., Tian, X., Qin, M. C., Xu, Z. H., Wu, D. P., et al. (2017). Neuroprotective properties of Panax notoginseng saponins via preventing oxidative stress injury in SAMP8 mice. Evid. Based Complement. Altern. Med. 2017, 8713561. doi:10.1155/2017/8713561
Jeon, S. W., and Kim, Y. K. (2016). Neuroinflammation and cytokine abnormality in major depression: cause or consequence in that illness? World J. Psychiatry 6 (3), 283–293. doi:10.5498/wjp.v6.i3.283
Jiang, B., Xiong, Z., Yang, J., Wang, W., Wang, Y., Hu, Z. L., et al. (2012). Antidepressant-like effects of ginsenoside Rg1 are due to activation of the BDNF signalling pathway and neurogenesis in the hippocampus. Br. J. Pharmacol. 166 (6), 1872–1887. doi:10.1111/j.1476-5381.2012.01902.x
Jiang, J. Y., Gao, G. Y., Feng, J. F., Mao, Q., Chen, L. G., Yang, X. F., et al. (2019). Traumatic brain injury in China. Lancet Neurol. 18 (3), 286–295. doi:10.1016/S1474-4422(18)30469-1
Jiang, Y., Li, H., Huang, P., Li, S., Li, B., Huo, L., et al. (2022). Panax notoginseng saponins protect PC12 cells against Aβ induced injury via promoting parkin-mediated mitophagy. J. Ethnopharmacol. 285, 114859. doi:10.1016/j.jep.2021.114859
Kang, A., Xie, T., Zhu, D., Shan, J., Di, L., and Zheng, X. (2017). Suppressive effect of ginsenoside Rg3 against lipopolysaccharide-induced depression-like behavior and neuroinflammation in mice. J. Agric. Food Chem. 65 (32), 6861–6869. doi:10.1021/acs.jafc.7b02386
Kim, D. H. (2018). Gut microbiota-mediated pharmacokinetics of ginseng saponins. J. Ginseng Res. 42 (3), 255–263. doi:10.1016/j.jgr.2017.04.011
Kim, S., Na, J. Y., Song, K. B., Choi, D. S., Kim, J. H., Kwon, Y. B., et al. (2012). Protective effect of ginsenoside Rb1 on hydrogen peroxide-induced oxidative stress in Rat articular chondrocytes. J. Ginseng Res. 36 (2), 161–168. doi:10.5142/jgr.2012.36.2.161
Kim, S., Kim, J. H., Seok, S. H., and Park, E. S. (2021). Enhanced permeability and oral absorption of Panax notoginseng saponins by borneol. J. Of Drug Deliv. Sci. And Technol. 66, 102819. doi:10.1016/j.jddst.2021.102819
Lan, T. H., Xu, Z. W., Wang, Z., Wu, Y. L., Wu, W. K., and Tan, H. M. (2011). Ginsenoside Rb1 prevents homocysteine-induced endothelial dysfunction via PI3K/Akt activation and PKC inhibition. Biochem. Pharmacol. 82 (2), 148–155. doi:10.1016/j.bcp.2011.04.001
Lazarou, M., Sliter, D. A., Kane, L. A., Sarraf, S. A., Wang, C., Burman, J. L., et al. (2015). The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature 524 (7565), 309–314. doi:10.1038/nature14893
Leung, K. W., Pon, Y. L., Wong, R. N., and Wong, A. S. (2006). Ginsenoside-Rg1 induces vascular endothelial growth factor expression through the glucocorticoid receptor-related phosphatidylinositol 3-kinase/Akt and beta-catenin/T-cell factor-dependent pathway in human endothelial cells. J. Biol. Chem. 281 (47), 36280–36288. doi:10.1074/jbc.M606698200
Li, H., Deng, C. Q., Chen, B. Y., Zhang, S. P., Liang, Y., and Luo, X. G. (2009). Total saponins of Panax notoginseng modulate the expression of caspases and attenuate apoptosis in rats following focal cerebral ischemia-reperfusion. J. Ethnopharmacol. 121 (3), 412–418. doi:10.1016/j.jep.2008.10.042
Li, H., Xiao, J., Li, X., Chen, H., Kang, D., Shao, Y., et al. (2018). Low cerebral exposure cannot hinder the neuroprotective effects of panax notoginsenosides. Drug Metab. Dispos. 46 (1), 53–65. doi:10.1124/dmd.117.078436
Li, Y., Yang, D., and Zhu, C. (2018). Impact of sodium N-[8-(2-Hydroxybenzoyl)amino]-caprylate on intestinal permeability for notoginsenoside R1 and salvianolic acids in Caco-2 cells transport and rat pharmacokinetics. Molecules 23 (11), 2990. doi:10.3390/molecules23112990
Li, Y., Wang, H., Wang, R., Lu, X., Wang, Y., Duan, M., et al. (2019). Pharmacokinetics, tissue distribution and excretion of saponins after intravenous administration of ShenMai injection in rats. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 1128, 121777. doi:10.1016/j.jchromb.2019.121777
Li, Q., Yuan, M., Li, X., Li, J., Xu, M., Wei, D., et al. (2020). New dammarane-type triterpenoid saponins from Panax notoginseng saponins. J. Ginseng Res. 44 (5), 673–679. doi:10.1016/j.jgr.2018.12.001
Li, W., Shi, H., and Wu, X. (2025). A narrative review of Panax notoginseng: unique saponins and their pharmacological activities. J. Ginseng Res. 49 (2), 118–133. doi:10.1016/j.jgr.2024.12.005
Li, Z., Peng, X., Zhu, X., Spanos, M., and Wu, L. (2025). Traditional Chinese medicine monomers are potential candidate drugs for cancer-induced cardiac Cachexia. Pharmacology 110 (1), 49–61. doi:10.1159/000540915
Lin, M., Sun, W., Gong, W., Ding, Y., Zhuang, Y., and Hou, Q. (2015). Ginsenoside Rg1 protects against transient focal cerebral ischemic injury and suppresses its systemic metabolic changes in cerabral injury rats. Acta Pharm. Sin. B 5 (3), 277–284. doi:10.1016/j.apsb.2015.02.001
Ling, G., Zhang, M., Chen, C., Wang, Y., Gao, Q., Li, J., et al. (2024). Progress of Ginsenoside Rb1 in neurological disorders. Front. Pharmacol. 15, 1280792. doi:10.3389/fphar.2024.1280792
Liu, X. Y., Wang, S., Li, C. J., Ma, J., Chen, F. Y., Peng, Y., et al. (2018). Dammarane-type saponins from the leaves of Panax notoginseng and their neuroprotective effects on damaged SH-SY5Y cells. Phytochemistry 145, 10–17. doi:10.1016/j.phytochem.2017.09.020
Liu, S. Z., Cheng, W., Shao, J. W., Gu, Y. F., Zhu, Y. Y., Dong, Q. J., et al. (2019). Notoginseng Saponin Rg1 prevents cognitive impairment through modulating APP processing in Aβ1-42-injected rats. Curr. Med. Sci. 39 (2), 196–203. doi:10.1007/s11596-019-2019-1
Liu, Y., Liu, T., Zhao, J., He, T., Chen, H., Wang, J., et al. (2019). Phospholipase Cγ2 signalling contributes to the haemostatic effect of Notoginsenoside Ft1. J. Pharm. Pharmacol. 71 (5), 878–886. doi:10.1111/jphp.13057
Liu, H., Yang, J., Yang, W., Hu, S., Wu, Y., Zhao, B., et al. (2020). Focus on notoginsenoside R1 in metabolism and prevention against human diseases. Drug Des. Devel Ther. 14, 551–565. doi:10.2147/DDDT.S240511
Liu, B., Li, Y., Han, Y., Wang, S., Yang, H., Zhao, Y., et al. (2021). Notoginsenoside R1 intervenes degradation and redistribution of tight junctions to ameliorate blood-brain barrier permeability by Caveolin-1/MMP2/9 pathway after acute ischemic stroke. Phytomedicine 90, 153660. doi:10.1016/j.phymed.2021.153660
Liu, B., Zhao, T., Li, Y., Han, Y., Xu, Y., Yang, H., et al. (2022). Notoginsenoside R1 ameliorates mitochondrial dysfunction to circumvent neuronal energy failure in acute phase of focal cerebral ischemia. Phytother. Res. 36 (5), 2223–2235. doi:10.1002/ptr.7450
Liu, S., Wang, M., Xiao, H., Ye, J., Cao, L., Li, W., et al. (2024). Advancements in research on the effects of panax notoginseng saponin constituents in ameliorating learning and memory disorders. Heliyon 10 (7), e28581. doi:10.1016/j.heliyon.2024.e28581
Liu, Y., Niu, P., Ji, H., Chen, Z., Zhai, J., Jin, X., et al. (2024). The use of Panax notoginseng saponins injections after intravenous thrombolysis in acute ischemic stroke: a systematic review and meta-analysis. Front. Pharmacol. 15, 1376025. doi:10.3389/fphar.2024.1376025
Lu, Y., Cheng, B., Shan, Y., Zhou, S., Xu, C., Fei, Y., et al. (2024). Lyophilization enhances the stability of Panax notoginseng total saponins-loaded transfersomes without adverse effects on ex vivo/in vivo skin permeation. Int. J. Pharm. 649, 123668. doi:10.1016/j.ijpharm.2023.123668
Luo, F. C., Wang, S. D., Qi, L., Song, J. Y., Lv, T., and Bai, J. (2011). Protective effect of panaxatriol saponins extracted from Panax notoginseng against MPTP-induced neurotoxicity in vivo. J. Ethnopharmacol. 133 (2), 448–453. doi:10.1016/j.jep.2010.10.017
Luo, L., Kang, J., He, Q., Qi, Y., Chen, X., Wang, S., et al. (2019). A NMR-based metabonomics approach to determine protective effect of a combination of multiple components derived from Naodesheng on ischemic stroke rats. Molecules 24 (9), 1831. doi:10.3390/molecules24091831
Lutton, E. M., Razmpour, R., Andrews, A. M., Cannella, L. A., Son, Y. J., Shuvaev, V. V., et al. (2017). Acute administration of catalase targeted to ICAM-1 attenuates neuropathology in experimental traumatic brain injury. Sci. Rep. 7 (1), 3846. doi:10.1038/s41598-017-03309-4
Ma, B., Meng, X., Wang, J., Sun, J., Ren, X., Qin, M., et al. (2014). Notoginsenoside R1 attenuates amyloid-beta-induced damage in neurons by inhibiting reactive oxygen species and modulating MAPK activation. Int. Immunopharmacol. 22 (1), 151–159. doi:10.1016/j.intimp.2014.06.018
Marino, B. L. B., de Souza, L. R., Sousa, K. P. A., Ferreira, J. V., Padilha, E. C., da Silva, C., et al. (2020). Parkinson's disease: a review from pathophysiology to treatment. Mini Rev. Med. Chem. 20 (9), 754–767. doi:10.2174/1389557519666191104110908
Masaoka, Y., Tanaka, Y., Kataoka, M., Sakuma, S., and Yamashita, S. (2006). Site of drug absorption after oral administration: assessment of membrane permeability and luminal concentration of drugs in each segment of gastrointestinal tract. Eur. J. Pharm. Sci. 29 (3-4), 240–250. doi:10.1016/j.ejps.2006.06.004
Mehndiratta, M. M., and Aggarwal, V. (2021). Neurological disorders in India: past, present, and next steps. Lancet Glob. Health 9 (8), e1043–e1044. doi:10.1016/S2214-109X(21)00214-X
Mei, Y., Chen, Y., Zhang, H., Fan, W., Liu, L., Wang, Z., et al. (2024). Borneol acts as an adjuvant agent to enhance the oral absorption of Panax notoginseng saponins in rats: effect of optical configuration and compatibility ratios. J. Ethnopharmacol. 331, 118331. doi:10.1016/j.jep.2024.118331
Meng, X., Wang, M., Wang, X., Sun, G., Ye, J., Xu, H., et al. (2014). Suppression of NADPH oxidase- and mitochondrion-derived superoxide by Notoginsenoside R1 protects against cerebral ischemia-reperfusion injury through estrogen receptor-dependent activation of Akt/Nrf2 pathways. Free Radic. Res. 48 (7), 823–838. doi:10.3109/10715762.2014.911853
Miao, L., Yang, Y., Li, Z., Fang, Z., Zhang, Y., and Han, C. C. (2022). Ginsenoside Rb2: a review of pharmacokinetics and pharmacological effects. J. Ginseng Res. 46 (2), 206–213. doi:10.1016/j.jgr.2021.11.007
Miller, K. D., Ostrom, Q. T., Kruchko, C., Patil, N., Tihan, T., Cioffi, G., et al. (2021). Brain and other central nervous system tumor statistics, 2021. CA Cancer J. Clin. 71 (5), 381–406. doi:10.3322/caac.21693
Moller, T., Hanisch, U. K., and Ransom, B. R. (2000). Thrombin-induced activation of cultured rodent microglia. J. Neurochem. 75 (4), 1539–1547. doi:10.1046/j.1471-4159.2000.0751539.x
Nie, Y. X., Wang, D., and Zhang, X. (2006). Effect of panax notoginseng saponins injection on brain edema in intracerebral hemorrhage rats. Zhongguo Zhong Xi Yi Jie He Za Zhi 26 (10), 922–925.
O'Connor, J. C., Lawson, M. A., Andre, C., Moreau, M., Lestage, J., Castanon, N., et al. (2009). Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol. Psychiatry 14 (5), 511–522. doi:10.1038/sj.mp.4002148
Ohashi, S. N., DeLong, J. H., Kozberg, M. G., Mazur-Hart, D. J., van Veluw, S. J., Alkayed, N. J., et al. (2023). Role of inflammatory processes in hemorrhagic stroke. Stroke 54 (2), 605–619. doi:10.1161/STROKEAHA.122.037155
Olagunju, A. S., Ahammad, F., Alagbe, A. A., Otenaike, T. A., Teibo, J. O., Mohammad, F., et al. (2023). Mitochondrial dysfunction: a notable contributor to the progression of Alzheimer's and Parkinson's disease. Heliyon 9 (3), e14387. doi:10.1016/j.heliyon.2023.e14387
Pang, H. H., Li, M. Y., Wang, Y., Tang, M. K., Ma, C. H., and Huang, J. M. (2017). Effect of compatible herbs on the pharmacokinetics of effective components of Panax notoginseng in Fufang Xueshuantong Capsule. J. Zhejiang Univ. Sci. B 18 (4), 343–352. doi:10.1631/jzus.B1600235
Pei, X., Zhang, L., Liu, D., Wu, Y., Li, X., Cao, Y., et al. (2023). Notoginsenoside R1 attenuates brain injury in rats with traumatic brain injury: possible mediation of apoptosis via ERK1/2 signaling pathway. PLoS One 18 (12), e0295903. doi:10.1371/journal.pone.0295903
Peiran, L., Ying, L., Mingzhuo, Z., Ye, Y., and Xiuming, C. (2017). The development of a Panax notoginseng medicinal liquor processing technology using the response surface method and a study of its antioxidant activity and its effects on mouse melanoma B16 cells. Food Funct. 8 (11), 4251–4264. doi:10.1039/c7fo00880e
Peng, M., Yi, Y. X., Zhang, T., Ding, Y., and Le, J. (2018). Stereoisomers of saponins in Panax notoginseng (Sanqi): a review. Front. Pharmacol. 9, 188. doi:10.3389/fphar.2018.00188
Pintusophon, S., Niu, W., Duan, X. N., Olaleye, O. E., Huang, Y. H., Wang, F. Q., et al. (2019). Intravenous formulation of Panax notoginseng root extract: human pharmacokinetics of ginsenosides and potential for perpetrating drug interactions. Acta Pharmacol. Sin. 40 (10), 1351–1363. doi:10.1038/s41401-019-0273-1
Qin, F., Ye, Y. P., and Sun, H. X. (2006). Haemolytic activity and adjuvant effect of notoginsenoside K from the roots of Panax notoginseng. Chem. Biodivers. 3 (10), 1144–1152. doi:10.1002/cbdv.200690116
Qu, J., Xu, N., Zhang, J., Geng, X., and Zhang, R. (2020). Panax notoginseng saponins and their applications in nervous system disorders: a narrative review. Ann. Transl. Med. 8 (22), 1525. doi:10.21037/atm-20-6909
Quinn, P. M. J., Moreira, P. I., Ambrosio, A. F., and Alves, C. H. (2020). PINK1/PARKIN signalling in neurodegeneration and neuroinflammation. Acta Neuropathol. Commun. 8 (1), 189. doi:10.1186/s40478-020-01062-w
Qureshi, A. I., Suri, M. F., Ostrow, P. T., Kim, S. H., Ali, Z., Shatla, A. A., et al. (2003). Apoptosis as a form of cell death in intracerebral hemorrhage. Neurosurgery 52 (5), 1041–1048. ; discussion 1047-1048. doi:10.1093/neurosurgery/52.5.1041
Ruan, J. Q., Leong, W. I., Yan, R., and Wang, Y. T. (2010). Characterization of metabolism and in vitro permeability study of notoginsenoside R1 from Radix notoginseng. J. Agric. Food Chem. 58 (9), 5770–5776. doi:10.1021/jf1005885
Ruan, J., Zhang, Y., Zhao, W., Sun, F., Han, L., Yu, H., et al. (2020). New 12,23-Epoxydammarane type saponins obtained from Panax notoginseng leaves and their anti-inflammatory activity. Molecules 25 (17), 3784. doi:10.3390/molecules25173784
Sami, A., and Karsy, M. (2013). Targeting the PI3K/AKT/mTOR signaling pathway in glioblastoma: novel therapeutic agents and advances in understanding. Tumour Biol. 34 (4), 1991–2002. doi:10.1007/s13277-013-0800-5
Shekhar, S., Yadav, Y., Singh, A. P., Pradhan, R., Desai, G. R., Dey, A. B., et al. (2018). Neuroprotection by ethanolic extract of Syzygium aromaticum in Alzheimer's disease like pathology via maintaining oxidative balance through SIRT1 pathway. Exp. Gerontol. 110, 277–283. doi:10.1016/j.exger.2018.06.026
Shen, Q., Li, J., Zhang, C., Wang, P., Mohammed, A., Ni, S., et al. (2017). Panax notoginseng saponins reduce high-risk factors for thrombosis through peroxisome proliferator-activated receptor -gamma pathway. Biomed. Pharmacother. 96, 1163–1169. doi:10.1016/j.biopha.2017.11.106
Shi, X., Yu, W., Yang, T., Liu, W., Zhao, Y., Sun, Y., et al. (2016). Panax notoginseng saponins provide neuroprotection by regulating NgR1/RhoA/ROCK2 pathway expression, in vitro and in vivo. J. Ethnopharmacol. 190, 301–312. doi:10.1016/j.jep.2016.06.017
Shi, X., Yu, W., Liu, L., Liu, W., Zhang, X., Yang, T., et al. (2017). Panax notoginseng saponins administration modulates pro-/anti-inflammatory factor expression and improves neurologic outcome following permanent MCAO in rats. Metab. Brain Dis. 32 (1), 221–233. doi:10.1007/s11011-016-9901-3
Shi, X., Feng, L., Li, Y., Qin, M., Li, T., Cheng, Z., et al. (2023). Efficacy and safety of Panax notoginseng saponins (Xuesaitong) for patients with acute ischemic stroke: a systematic review and meta-analysis of randomized controlled trials. Front. Pharmacol. 14, 1280559. doi:10.3389/fphar.2023.1280559
Sies, H., Berndt, C., and Jones, D. P. (2017). Oxidative stress. Annu. Rev. Biochem. 86, 715–748. doi:10.1146/annurev-biochem-061516-045037
Singh, Y., Meher, J. G., Raval, K., Khan, F. A., Chaurasia, M., Jain, N. K., et al. (2017). Nanoemulsion: concepts, development and applications in drug delivery. J. Control Release 252, 28–49. doi:10.1016/j.jconrel.2017.03.008
Su, P., Wang, L., Du, S. J., Xin, W. F., and Zhang, W. S. (2014). Advance in studies of Panax notoginseng saponins on pharmacological mechanism of nervous system disease. Zhongguo Zhong Yao Za Zhi 39 (23), 4516–4521.
Su, P., Du, S., Li, H., Li, Z., Xin, W., and Zhang, W. (2016). Notoginsenoside R1 inhibits oxidized low-density lipoprotein induced inflammatory cytokines production in human endothelial EA.hy926 cells. Eur. J. Pharmacol. 770, 9–15. doi:10.1016/j.ejphar.2015.11.040
Sun, T., Wang, P., Deng, T., Tao, X., Li, B., and Xu, Y. (2020). Effect of Panax notoginseng saponins on focal cerebral ischemia-reperfusion in rat models: a meta-analysis. Front. Pharmacol. 11, 572304. doi:10.3389/fphar.2020.572304
Sun, X., Deng, H., Shu, T., Xu, M., Su, L., and Li, H. (2023). Study on chemical constituents of Panax notoginseng leaves. Molecules 28 (5), 2194. doi:10.3390/molecules28052194
Tian, Z., Pang, H., Zhang, Q., Du, S., Lu, Y., Zhang, L., et al. (2018). Effect of aspirin on the pharmacokinetics and absorption of panax notoginseng saponins. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 1074-1075, 25–33. doi:10.1016/j.jchromb.2017.12.033
Tong, Q., Zhu, P. C., Zhuang, Z., Deng, L. H., Wang, Z. H., Zeng, H., et al. (2019). Notoginsenoside R1 for organs Ischemia/Reperfusion Injury: a preclinical systematic review. Front. Pharmacol. 10, 1204. doi:10.3389/fphar.2019.01204
Wang, Q., Sun, L. H., Jia, W., Liu, X. M., Dang, H. X., Mai, W. L., et al. (2010). Comparison of ginsenosides Rg1 and Rb1 for their effects on improving scopolamine-induced learning and memory impairment in mice. Phytother. Res. 24 (12), 1748–1754. doi:10.1002/ptr.3130
Wang, B., Li, Y., Li, X. P., and Li, Y. (2015). Panax notoginseng saponins improve recovery after spinal cord transection by upregulating neurotrophic factors. Neural Regen. Res. 10 (8), 1317–1320. doi:10.4103/1673-5374.162766
Wang, T., Guo, R., Zhou, G., Zhou, X., Kou, Z., Sui, F., et al. (2016). Traditional uses, botany, phytochemistry, pharmacology and toxicology of Panax notoginseng (Burk.) F.H. Chen: a review. J. Ethnopharmacol. 188, 234–258. doi:10.1016/j.jep.2016.05.005
Wang, Y., Tu, L., Li, Y., Chen, D., Liu, Z., Hu, X., et al. (2017). Notoginsenoside R1 alleviates oxygen-glucose deprivation/reoxygenation injury by suppressing endoplasmic reticulum calcium release via PLC. Sci. Rep. 7 (1), 16226. doi:10.1038/s41598-017-16373-7
Wang, W., Huang, L., Thomas, E. R., Hu, Y., Zeng, F., and Li, X. (2020). Notoginsenoside R1 protects against the acrylamide-induced neurotoxicity via upregulating Trx-1-Mediated ITGAV expression: involvement of autophagy. Front. Pharmacol. 11, 559046. doi:10.3389/fphar.2020.559046
Wang, L. D., Xu, Z. M., Liang, X., Qiu, W. R., Liu, S. J., Dai, L. L., et al. (2021a). Overview of systematic reviews of Panax notoginseng saponins in treatment of acute cerebral infarction. Zhongguo Zhong Yao Za Zhi 46 (12), 2963–2971. doi:10.19540/j.cnki.cjcmm.20210325.501
Wang, L. D., Xu, Z. M., Liang, X., Qiu, W. R., Liu, S. J., Dai, L. L., et al. (2021b). Systematic review and meta-analysis on randomized controlled trials on efficacy and safety of Panax notoginseng saponins in treatment of acute ischemic stroke. Evid. Based Complement. Altern. Med. 2021, 4694076. doi:10.1155/2021/4694076
Wang, M., Liu, H., Xu, L., Li, M., and Zhao, M. (2022). The protective effect of notoginsenoside R1 on isoflurane-induced neurological impairment in the rats via regulating miR-29a expression and neuroinflammation. Neuroimmunomodulation 29 (1), 70–76. doi:10.1159/000518215
Wang, Y., Shang, Y., Tang, F., Qiu, K., Wei, X., and Wang, Z. (2023). Self-double-emulsifying drug delivery system enteric-coated capsules: a novel approach to improve oral bioavailability and anti-inflammatory activity of Panax notoginseng saponins. AAPS PharmSciTech 24 (4), 90. doi:10.1208/s12249-023-02549-0
Wang, L. L., Kang, M. L., Liu, C. W., Liu, L., and Tang, B. (2024). Panax notoginseng saponins activate nuclear factor erythroid 2-Related factor 2 to inhibit ferroptosis and attenuate inflammatory injury in cerebral ischemia-reperfusion. Am. J. Chin. Med. 52 (3), 821–839. doi:10.1142/S0192415X24500332
Wang, Y., Sun, X., Xie, Y., Du, A., Chen, M., Lai, S., et al. (2024). Panax notoginseng saponins alleviate diabetic retinopathy by inhibiting retinal inflammation: association with the NF-κB signaling pathway. J. Ethnopharmacol. 319 (Pt 1), 117135. doi:10.1016/j.jep.2023.117135
Wei, G., Yang, F., Wei, F., Zhang, L., Gao, Y., Qian, J., et al. (2020). Metabolomes and transcriptomes revealed the saponin distribution in root tissues of Panax quinquefolius and Panax notoginseng. J. Ginseng Res. 44 (6), 757–769. doi:10.1016/j.jgr.2019.05.009
Wu, H. F., Zhu, C. H., and Guo, J. Y. (2012). Effect of ginsenoside Rg1 on behaviors and hippocampal amino acids in depressive-like rats. Zhongguo Zhong Yao Za Zhi 37 (20), 3117–3121.
Wu, Y., Wang, W., Kou, N., Wang, M., Yang, L., Miao, Y., et al. (2022). Panax notoginseng saponins combined with dual antiplatelet drugs potentiates anti-thrombotic effect with alleviated gastric injury in A carotid artery thrombosis rat model. J. Stroke Cerebrovasc. Dis. 31 (8), 106597. doi:10.1016/j.jstrokecerebrovasdis.2022.106597
Wu, L., Song, H., Zhang, C., Wang, A., Zhang, B., Xiong, C., et al. (2023). Efficacy and safety of Panax notoginseng saponins in the treatment of adults with ischemic stroke in China: a randomized clinical trial. JAMA Netw. Open 6 (6), e2317574. doi:10.1001/jamanetworkopen.2023.17574
Wu, J. J., Zhang, L., Liu, D., Xia, J., Yang, Y., Tang, F., et al. (2024). Ginsenoside Rg1, lights up the way for the potential prevention of Alzheimer's disease due to its therapeutic effects on the drug-controllable risk factors of Alzheimer's disease. J. Ethnopharmacol. 318 (Pt B), 116955. doi:10.1016/j.jep.2023.116955
Wu, H., Ni, C., Zhang, Y., Song, Y., Liu, L., Huang, F., et al. (2025). Stem-leaf saponins of Panax notoginseng attenuate experimental Parkinson's disease progression in mice by inhibiting microglia-mediated neuroinflammation via P2Y2R/PI3K/AKT/NFκB signaling pathway. Chin. J. Nat. Med. 23 (1), 43–53. doi:10.1016/S1875-5364(25)60809-0
Xiang, C. P., Zhou, R., Zhang, Y., Zhang, J. J., and Yang, H. J. (2020). Research progress on saponins in Panax notoginseng and their molecular mechanism of anti-cerebral ischemia. Zhongguo Zhong Yao Za Zhi 45 (13), 3045–3054. doi:10.19540/j.cnki.cjcmm.20200302.403
Xiao, Q., Kang, Z., Liu, C., and Tang, B. (2022). Panax notoginseng saponins attenuate cerebral ischemia-reperfusion injury via mitophagy-induced inhibition of NLRP3 inflammasome in rats. Front. Biosci. Landmark Ed. 27 (11), 300. doi:10.31083/j.fbl2711300
Xie, X., Wang, H. T., Li, C. L., Gao, X. H., Ding, J. L., Zhao, H. H., et al. (2010). Ginsenoside Rb1 protects PC12 cells against beta-amyloid-induced cell injury. Mol. Med. Rep. 3 (4), 635–639. doi:10.3892/mmr_00000308
Xie, W., Meng, X., Zhai, Y., Zhou, P., Ye, T., Wang, Z., et al. (2018a). Panax notoginseng saponins: a review of its mechanisms of antidepressant or anxiolytic effects and network analysis on phytochemistry and pharmacology. Molecules 23 (4), 940. doi:10.3390/molecules23040940
Xie, W., Zhou, P., Sun, Y., Meng, X., Dai, Z., Sun, G., et al. (2018b). Protective effects and target network analysis of ginsenoside Rg1 in cerebral ischemia and reperfusion injury: a comprehensive overview of experimental studies. Cells 7 (12), 270. doi:10.3390/cells7120270
Xiong, Y., Chen, L., Hu, Y., and Cui, X. (2017). Uncovering active constituents responsible for different activities of raw and steamed Panax notoginseng roots. Front. Pharmacol. 8, 745. doi:10.3389/fphar.2017.00745
Xu, L., Chen, W. F., and Wong, M. S. (2009). Ginsenoside Rg1 protects dopaminergic neurons in a rat model of Parkinson's disease through the IGF-I receptor signalling pathway. Br. J. Pharmacol. 158 (3), 738–748. doi:10.1111/j.1476-5381.2009.00361.x
Xu, D., Huang, P., Yu, Z., Xing, D. H., Ouyang, S., and Xing, G. (2014). Efficacy and safety of Panax notoginseng saponin therapy for acute intracerebral hemorrhage, meta-analysis, and mini review of potential mechanisms of action. Front. Neurol. 5, 274. doi:10.3389/fneur.2014.00274
Xu, C., Wang, W., Wang, B., Zhang, T., Cui, X., Pu, Y., et al. (2019). Analytical methods and biological activities of Panax notoginseng saponins: recent trends. J. Ethnopharmacol. 236, 443–465. doi:10.1016/j.jep.2019.02.035
Yan, J., Liu, Q., Dou, Y., Hsieh, Y., Liu, Y., Tao, R., et al. (2013). Activating glucocorticoid receptor-ERK signaling pathway contributes to ginsenoside Rg1 protection against beta-amyloid peptide-induced human endothelial cells apoptosis. J. Ethnopharmacol. 147 (2), 456–466. doi:10.1016/j.jep.2013.03.039
Yan, S., Li, Z., Li, H., Arancio, O., and Zhang, W. (2014). Notoginsenoside R1 increases neuronal excitability and ameliorates synaptic and memory dysfunction following amyloid elevation. Sci. Rep. 4, 6352. doi:10.1038/srep06352
Yang, X., Xiong, X., Wang, H., Yang, G., and Wang, J. (2013). Xuesaitong soft capsule (Chinese patent medicine) for the treatment of unstable angina pectoris: a meta-analysis and systematic review. Evid. Based Complement. Altern. Med. 2013, 948319. doi:10.1155/2013/948319
Yang, W. Z., Hu, Y., Wu, W. Y., Ye, M., and Guo, D. A. (2014). Saponins in the genus Panax L. (Araliaceae): a systematic review of their chemical diversity. Phytochemistry 106, 7–24. doi:10.1016/j.phytochem.2014.07.012
Yang, B. R., Hong, S. J., Lee, S. M., Cong, W. H., Wan, J. B., Zhang, Z. R., et al. (2016). Pro-angiogenic activity of notoginsenoside R1 in human umbilical vein endothelial cells in vitro and in a chemical-induced blood vessel loss model of zebrafish in vivo. Chin. J. Integr. Med. 22 (6), 420–429. doi:10.1007/s11655-014-1954-8
Yang, P. F., Song, X. Y., and Chen, N. H. (2016). Advances in pharmacological studies of Panax notoginseng saponins on brain ischemia-reperfusion injury. Yao Xue Xue Bao 51 (7), 1039–1046.
Yang, X., Yang, S., Hong, C., Yu, W., and Guonian, W. (2017). Panax notoginseng saponins attenuates sevoflurane-induced nerve cell injury by modulating AKT signaling pathway. Mol. Med. Rep. 16 (5), 7829–7834. doi:10.3892/mmr.2017.7519
Yang, F., Ma, Q., Matsabisa, M. G., Chabalala, H., Braga, F. C., and Tang, M. (2020). Panax notoginseng for cerebral ischemia: a systematic review. Am. J. Chin. Med. 48 (6), 1331–1351. doi:10.1142/S0192415X20500652
Yang, R., Shen, Y. J., Chen, M., Zhao, J. Y., Chen, S. H., Zhang, W., et al. (2022). Quercetin attenuates ischemia reperfusion injury by protecting the blood-brain barrier through Sirt1 in MCAO rats. J. Asian Nat. Prod. Res. 24 (3), 278–289. doi:10.1080/10286020.2021.1949302
Yang, C., Qu, L., Wang, R., Wang, F., Yang, Z., and Xiao, F. (2024a). Multi-layered effects of Panax notoginseng on immune system. Pharmacol. Res. 204, 107203. doi:10.1016/j.phrs.2024.107203
Yang, Y., Chen, W., Lin, Z., Wu, Y., Li, Y., and Xia, X. (2024b). Panax notoginseng saponins prevent dementia and oxidative stress in brains of SAMP8 mice by enhancing mitophagy. BMC Complement. Med. Ther. 24 (1), 144. doi:10.1186/s12906-024-04403-7
Yang, Y., Wu, J., Feng, S., Yu, H., Liu, C., and Wang, S. (2024c). Notoginsenoside R1 attenuates bupivacaine induced neurotoxicity by activating Jak1/Stat3/Mcl1 pathway. Toxicology 503, 153740. doi:10.1016/j.tox.2024.153740
Young, J. J., Bruno, D., and Pomara, N. (2014). A review of the relationship between proinflammatory cytokines and major depressive disorder. J. Affect Disord. 169, 15–20. doi:10.1016/j.jad.2014.07.032
Yu, Z., Yang, X. Y., Cai, Y. Q., Hu, E., Li, T., Zhu, W. X., et al. (2024). Panax notoginseng saponins promotes the meningeal lymphatic system-mediated hematoma absorption in intracerebral hemorrhage. Phytomedicine 135, 156149. doi:10.1016/j.phymed.2024.156149
Zeng, X. S., Jia, J. J., and Ma, L. F. (2015). Gensenoside Rb1 protects rat PC12 cells from oxidative stress-induced endoplasmic reticulum stress: the involvement of thioredoxin-1. Mol. Cell Biochem. 410 (1-2), 239–246. doi:10.1007/s11010-015-2557-1
Zhai, Y., Meng, X., Luo, Y., Wu, Y., Ye, T., Zhou, P., et al. (2018). Notoginsenoside R1 ameliorates diabetic encephalopathy by activating the Nrf2 pathway and inhibiting NLRP3 inflammasome activation. Oncotarget 9 (10), 9344–9363. doi:10.18632/oncotarget.24295
Zhan, Q., Wu, Y., and Liu, L. (2022). Effects of notoginsenoside R1 on attenuating depressive behavior induced by chronic stress in rats through induction of PI3K/AKT/NF-κB pathway. Drug Dev. Res. 83 (1), 97–104. doi:10.1002/ddr.21847
Zhang, X., Wu, J., and Zhang, B. (2015). Xuesaitong injection as one adjuvant treatment of acute cerebral infarction: a systematic review and meta-analysis. BMC Complement. Altern. Med. 15, 36. doi:10.1186/s12906-015-0560-4
Zhang, H., Li, Z., Zhou, Z., Yang, H., Zhong, Z., and Lou, C. (2016). Antidepressant-like effects of ginsenosides: a comparison of ginsenoside Rb3 and its four deglycosylated derivatives, Rg3, Rh2, compound K, and 20(S)-protopanaxadiol in mice models of despair. Pharmacol. Biochem. Behav. 140, 17–26. doi:10.1016/j.pbb.2015.10.018
Zhang, J., Zheng, Q., Lu, H., Jin, F., Li, Y., Bi, F., et al. (2019). Notoginsenoside R1 protects human keratinocytes HaCaT from LPS-induced inflammatory injury by downregulation of Myd88. Int. J. Immunopathol. Pharmacol. 33, 2058738419857550. doi:10.1177/2058738419857550
Zhang, X., Zhang, B., Zhang, C., Sun, G., and Sun, X. (2020). Effect of Panax notoginseng saponins and major anti-obesity components on weight loss. Front. Pharmacol. 11, 601751. doi:10.3389/fphar.2020.601751
Zhang, C., Zhang, S., Wang, L., Kang, S., Ma, J., Liu, S., et al. (2021). The RIG-I signal pathway mediated Panax notoginseng saponin anti-inflammatory effect in ischemia stroke. Evid. Based Complement. Altern. Med. 2021, 8878428. doi:10.1155/2021/8878428
Zhang, S., Chen, Q., Jin, M., Ren, J., Sun, X., Zhang, Z., et al. (2024). Notoginsenoside R1 alleviates cerebral ischemia/reperfusion injury by inhibiting the TLR4/MyD88/NF-κB signaling pathway through microbiota-gut-brain axis. Phytomedicine 128, 155530. doi:10.1016/j.phymed.2024.155530
Zhao, H. H., Di, J., Liu, W. S., Liu, H. L., Lai, H., and Lu, Y. L. (2013). Involvement of GSK3 and PP2A in ginsenoside Rb1's attenuation of aluminum-induced tau hyperphosphorylation. Behav. Brain Res. 241, 228–234. doi:10.1016/j.bbr.2012.11.037
Zhong, J., Lu, W., Zhang, J., Huang, M., Lyu, W., Ye, G., et al. (2020). Notoginsenoside R1 activates the Ang2/Tie2 pathway to promote angiogenesis. Phytomedicine 78, 153302. doi:10.1016/j.phymed.2020.153302
Zhou, N., Tang, Y., Keep, R. F., Ma, X., and Xiang, J. (2014). Antioxidative effects of Panax notoginseng saponins in brain cells. Phytomedicine 21 (10), 1189–1195. doi:10.1016/j.phymed.2014.05.004
Zhou, Q., Jiang, L., Xu, C., Luo, D., Zeng, C., Liu, P., et al. (2014). Ginsenoside Rg1 inhibits platelet activation and arterial thrombosis. Thromb. Res. 133 (1), 57–65. doi:10.1016/j.thromres.2013.10.032
Zhou, X., Chen, L. L., Xie, R. F., Lam, W., Zhang, Z. J., Jiang, Z. L., et al. (2017). Chemosynthesis pathway and bioactivities comparison of saponins in radix and flower of Panax notoginseng (Burk.) F.H. Chen. J. Ethnopharmacol. 201, 56–72. doi:10.1016/j.jep.2016.11.008
Zhou, Y. J., Chen, J. M., Sapkota, K., Long, J. Y., Liao, Y. J., Jiang, J. J., et al. (2020). Pananx notoginseng saponins attenuate CCL2-induced cognitive deficits in rats via anti-inflammation and anti-apoptosis effects that involve suppressing over-activation of NMDA receptors. Biomed. Pharmacother. 127, 110139. doi:10.1016/j.biopha.2020.110139
Zhu, T., Wang, L., Tian, F., Zhao, X., Pu, X. P., Sun, G. B., et al. (2020). Anti-ischemia/reperfusion injury effects of notoginsenoside R1 on small molecule metabolism in rat brain after ischemic stroke as visualized by MALDI-MS imaging. Biomed. Pharmacother. 129, 110470. doi:10.1016/j.biopha.2020.110470
Zhu, T., Wang, L., Xie, W., Meng, X., Feng, Y., Sun, G., et al. (2021a). Notoginsenoside R1 improves cerebral Ischemia/Reperfusion injury by promoting neurogenesis via the BDNF/Akt/CREB pathway. Front. Pharmacol. 12, 615998. doi:10.3389/fphar.2021.615998
Keywords: Panax notoginseng saponins, cerebrovascular, neurological diseases, pharmacologiceffects, mechanism of action
Citation: Wang B, Gao X, Zhang Y, Xiao Y, Chen T, Shi Z, Yuan Y and Li P (2025) The role of Panax notoginseng saponins in cerebrovascular neurological disorders: an overview of mechanisms and functions. Front. Pharmacol. 16:1693253. doi: 10.3389/fphar.2025.1693253
Received: 26 August 2025; Accepted: 27 October 2025;
Published: 13 November 2025; Corrected: 03 December 2025.
Edited by:
Lei Chen, Guangdong Ocean University, ChinaReviewed by:
Juexian Song, Capital Medical University, ChinaYingyi Zheng, Guangdong Provincial Hospital of Traditional Chinese Medicine, China
Copyright © 2025 Wang, Gao, Zhang, Xiao, Chen, Shi, Yuan and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ping Li, bGlwaW5nQGNjdWNtLmVkdS5jbg==
 Bin Wang
Bin Wang Xu Gao2
Xu Gao2 Yali Zhang
Yali Zhang Yuhua Xiao
Yuhua Xiao Zhouying Shi
Zhouying Shi Ping Li
Ping Li