- 1Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 2The Affiliated Traditional Chinese Medicine Hospital, Southwest Medical University, Luzhou, China
Myocardial ischemia-reperfusion injury (MIRI) remains a major clinical challenge, with oxidative stress as a key driver. Despite extensive preclinical promise, antioxidant therapies have consistently failed in clinical translation. This critical review deconstructs this translational gap, which stems from the nuanced complexity of redox biology, inappropriate therapeutic timing, and patient heterogeneity. We argue that overcoming these hurdles requires a paradigm shift from broad antioxidant supplementation to precision medicine. This approach involves enhancing endogenous defense mechanisms, leveraging targeted drug delivery, and developing multi-modal strategies. Ultimately, integrating dynamic biomarkers, multi-omics, and artificial intelligence to tailor treatments to individual patient profiles holds the key to finally fulfilling the promise of effectively managing MIRI.
1 Introduction
Myocardial ischemia (MI) is a critical global health concern with high prevalence and mortality (Sayed et al., 2025). The global burden of ischemic heart disease is projected to rise dramatically, remaining the leading cause of cardiovascular death and accounting for a projected 20 million deaths by 2050 (Chong et al., 2025). MI is defined as the inadequate blood supply to the myocardium, which, if severe and prolonged, can result in myocardial infarction (Aslam et al., 2025). Thus, sophisticated and targeted therapeutic strategies are imperative to mitigate this disease burden.
Oxidative stress represents a fundamental pathophysiological mechanism implicated in myocardial ischemia-reperfusion injury (MIRI) (Luo et al., 2023). This pivotal process is marked by a dynamic and often overwhelming increase in reactive oxygen species (ROS). While ROS generation is modest during the ischemic phase due to oxygen deprivation (Sen et al., 2024), a substantial and rapid “burst” occurs upon the sudden reintroduction of oxygen during reperfusion (Xiang et al., 2021; Dhalla et al., 2022; Guo et al., 2024). Principal contributors to this oxidative burst include dysfunctional mitochondria during reoxygenation, activated NADPH oxidases, and the infiltration of inflammatory cells (Liu N. et al., 2021). The excessive generation of ROS catalyzes a cascade of deleterious events within cardiomyocytes and the surrounding tissue. The oxidative burst is instrumental in initiating inflammatory pathways, notably the activation of the NLRP3 inflammasome, which subsequently results in the secretion of potent pro-inflammatory cytokines such as IL-1β and IL-18 (Chen et al., 2025). These oxidative and inflammatory signals interact synergistically, contributing to irreversible myocardial damage characterized by lipid peroxidation, protein oxidation, DNA damage, and various forms of cell death. This cascade ultimately impairs cardiac function and leads to adverse clinical outcomes (Yu et al., 2021; Duan D. et al., 2023). Consequently, a comprehensive understanding of oxidative stress and its downstream effects is essential for the development of effective therapeutic strategies aimed at mitigating MIRI and improving patient prognosis.
In recent decades, a myriad of antioxidant strategies, ranging from direct exogenous supplementation to modulation of endogenous defense mechanisms, have been extensively investigated to alleviate MIRI. While preclinical studies have consistently demonstrated promising cardioprotective effects, the clinical application of numerous antioxidant therapies has, paradoxically, produced largely inconsistent and often disappointing outcomes, leading to an ‘unmet promise’ in this critical field (Lonn et al., 2005; Rodrigo et al., 2014). This discrepancy highlights substantial challenges, including poor drug delivery, suboptimal timing, patient heterogeneity, and the nuanced dual roles of ROS.
This review seeks to conduct a critical examination of the complex role that oxidative stress plays in the pathogenesis of MI and MIRI, moving beyond a mere summary of existing knowledge. We will analytically synthesize both current and emerging therapeutic strategies, with a particular focus on deconstructing the reasons behind their limited clinical success. Crucially, we aim to delineate a forward-looking ‘path towards precision medicine’ within this vital field, particularly by leveraging insights from individual patient oxidative stress profiles, robust biomarker development, and advanced technological integration to tailor treatment strategies and ultimately improve patient prognosis.
2 Pathophysiological mechanisms of oxidative stress in MIRI
2.1 Concept and dynamic changes of oxidative stress in MIRI
Oxidative stress is fundamentally defined as an imbalance between the production of ROS and the capacity of the body’s antioxidant defense systems to neutralize them (Figure 1) (Jaganjac et al., 2022). ROS are highly reactive oxygen-derived molecules (e.g., superoxide anion •O2−, hydroxyl radical •OH, hydrogen peroxide H2O2). Although these species are generated during normal cellular metabolism and participate in essential physiological processes like signal transduction and gene expression at low concentrations (Krishnamurthy et al., 2024), their excessive accumulation or insufficient scavenging leads to oxidative stress, causing damage to vital biomolecules and cellular structures.
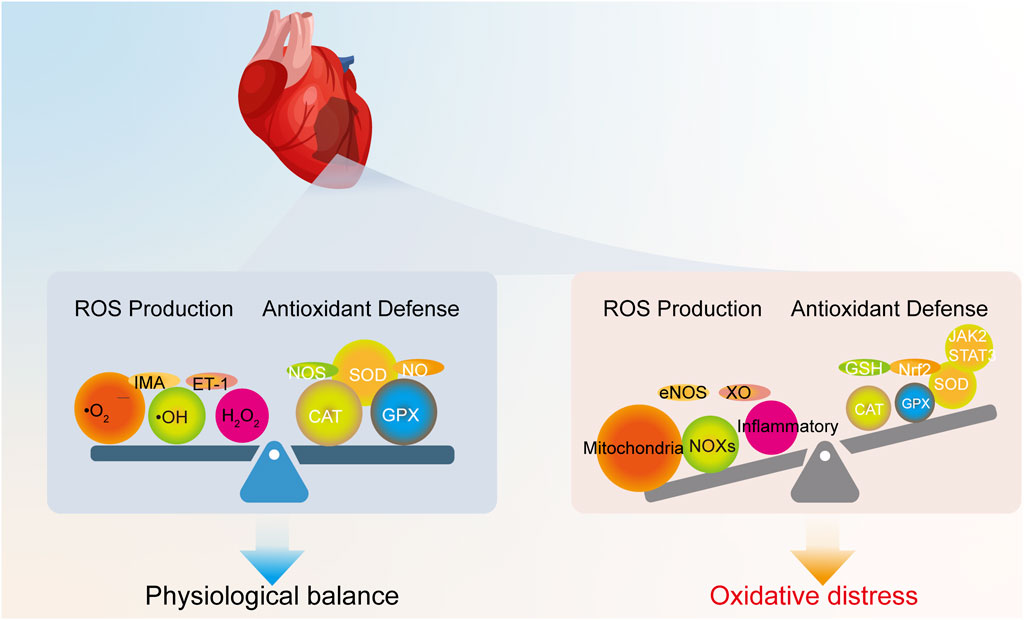
Figure 1. Physiological redox balance versus oxidative stress in the heart. Under physiological conditions, ROS generated from normal metabolism are neutralized by endogenous antioxidant systems. In contrast, oxidative stress occurs when excessive ROS production from various sources overwhelms these defense mechanisms.
In the specific pathological context of MIRI, oxidative stress becomes particularly aggressive and dynamic. The generation of ROS in the myocardium is dynamic, undergoing significant changes throughout the phases of ischemia and reperfusion (Alizadehasl et al., 2019), as summarized in Figure 2. During the ischemic phase, severe hypoxia and nutrient deprivation impair mitochondrial function, leading to a modest increase in ROS production due to limited electron leakage from the dysfunctional mitochondrial electron transport chain (ETC.) (Sen et al., 2024). However, the lack of oxygen and substrates generally keeps ROS generation relatively lower compared to the subsequent phase.

Figure 2. Timeline of pathological events in MIRI. The progression from ischemia (low ROS) to a massive oxidative burst upon reperfusion, which triggers downstream inflammation, cell death, and adverse remodeling.
The most damaging oxidative insult occurs upon reperfusion, where the sudden reintroduction of oxygen paradoxically triggers a massive burst of ROS and reactive nitrogen species (RNS). This concept, widely known as the “oxidative burst,” was foundationally established in seminal studies demonstrating that post-ischemic tissue injury is driven by oxygen-derived free radicals generated upon reoxygenation (Granger et al., 1981; Epstein and McCord, 1985) and has since been extensively characterized (Guo et al., 2024). This burst is a critical mediator of reperfusion injury, overwhelming the compromised antioxidant defense systems and causing widespread cellular damage. This dramatic increase is exacerbated by factors unique to the reperfused myocardium, including calcium overload, rapid restoration of mitochondrial electron transport, activation of enzymes like NADPH oxidase and xanthine oxidase, and the infiltration of inflammatory cells that release a wide array of oxidants (Liu N. et al., 2021; Quarato et al., 2022) (Figure 3).

Figure 3. The pathophysiological cascade of MIRI. Ischemic triggers lead to a reperfusion-induced oxidative burst. This overwhelms endogenous defenses, causing direct molecular damage and activating downstream inflammation, cell death, and signaling alterations, which collectively drive myocardial injury.
2.1.1 Mitochondrial dysfunction and reoxygenation
Mitochondria are the primary site of oxygen consumption and a major source of ROS in the heart. During ischemia, mitochondrial, ETC., components become damaged and partially reduced (Sen et al., 2024). Upon reperfusion, the sudden availability of oxygen allows rapid, but often inefficient, electron flow through the damaged, ETC. This leads to significant electron leakage, particularly from Complexes I and III, which directly reduces oxygen to superoxide anion (•O2−) (Xue et al., 2020; Liu N. et al., 2021).
A key mechanism of mitochondrial ROS production upon reperfusion is reverse electron transport (RET) at Complex I. During ischemia, succinate accumulates significantly within the mitochondrial matrix. Upon reperfusion, this accumulated succinate is rapidly oxidized by succinate dehydrogenase (SDH, Complex II), leading to a highly reduced coenzyme Q (CoQ) pool. Subsequently, driven by a high mitochondrial membrane potential (ΔΨm) and the reduced CoQ pool, electrons are “pushed back” through Complex I, where they react with molecular oxygen, leading to a burst of superoxide anion production (•O2−) (Chouchani et al., 2014). The re-energization of the mitochondria with substrates like succinate can lead to a high proton-motive force, driving electrons backward through Complex I, which then reduces oxygen to superoxide at a high rate (Casey et al., 2025). Furthermore, ischemia-induced mitochondrial calcium overload and permeability transition pore (mPTP) opening exacerbate mitochondrial dysfunction and ROS production (Griffiths and Halestrap, 1993; Quarato et al., 2022).
2.1.2 Calcium overload
Elevated intracellular and mitochondrial calcium levels, a hallmark of MIRI, contribute to ROS generation through multiple mechanisms. Calcium activates proteases that convert XDH to XO, disrupts mitochondrial function leading to increased electron leakage, and can activate other ROS-producing enzymes (Huang et al., 2020).
2.1.3 Xanthine oxidase
During ischemia, ATP is degraded to hypoxanthine and xanthine due to energy depletion. The enzyme xanthine dehydrogenase (XDH), normally producing NADH, is converted to its oxidase form (XO) through calcium-activated proteases or oxidative modification (Kusano et al., 2023). Upon reperfusion, with the reintroduction of oxygen, XO utilizes the accumulated hypoxanthine/xanthine and oxygen to produce large amounts of uric acid and •O2− (and subsequently H2O2) (Liu N. et al., 2021). This pathway is a significant contributor to the early reperfusion oxidative burst.
2.1.4 NADPH oxidases
NADPH Oxidases (NOXs) are multi-subunit enzymes that generate •O2− from oxygen and NADPH (Kračun et al., 2025). Various NOX isoforms (e.g., NOX2, NOX4) are expressed in cardiomyocytes, endothelial cells, and fibroblasts. Their activity is significantly upregulated during both ischemia and, more prominently, reperfusion in response to stimuli like angiotensin II, cytokines, and mechanical stress (Quarato et al., 2022). Notably, the infiltration and activation of inflammatory cells (neutrophils, macrophages) during reperfusion introduce a substantial source of ROS via their highly active phagocytic NOX (NOX2) system (Li et al., 2025).
2.1.5 Endothelial nitric oxide synthase (eNOS) uncoupling
Endothelial cells produce nitric oxide (NO•) via eNOS, which is crucial for vasodilation and cardioprotection. However, under conditions of oxidative stress and substrate/cofactor (like tetrahydrobiopterin, BH4) depletion during ischemia/reperfusion, eNOS can become “uncoupled.” In this state, it produces •O2− instead of NO•. The reaction between simultaneously produced NO• and •O2− rapidly forms the highly reactive and damaging reactive nitrogen species (RNS), peroxynitrite (ONOO−) (Liu N. et al., 2021).
2.1.6 Inflammatory cell infiltration and activation
As mentioned regarding NOX, the recruitment and activation of neutrophils and macrophages to the injured myocardium during reperfusion is a major source of sustained ROS production. These cells release a barrage of oxidants as part of their immune response, contributing significantly to tissue damage and the inflammatory cascade (Zhang H. et al., 2021; Li et al., 2025).
In addition to these major sources, the endoplasmic reticulum can contribute to the ROS burden (Gong et al., 2025). The accumulation of misfolded proteins during ischemia triggers the unfolded protein response (UPR). While initially adaptive, prolonged or severe ER stress leads to excessive ROS production, further exacerbating cellular injury (Zhang et al., 2025). The major cellular sources contributing to this ROS burst are summarized in Figure 4.

Figure 4. Primary sources of ROS in cardiomyocytes during MIRI. Key sources include mitochondria, NADPH oxidases, uncoupled eNOS, xanthine oxidase, ER stress, and inflammatory cells.
2.2 Dual roles of ROS: from physiological signaling to the critical pitfall of redox hormesis
The role of ROS in MIRI is not a simple dichotomy of beneficial signaling versus pathological damage; it is a complex, concentration-dependent phenomenon that is fundamental to understanding therapeutic failures. At low, physiological concentrations, ROS function as essential second messengers in a process known as redox signaling. Species like H2O2 and NO• participate in vital cellular cascades that regulate cell growth, differentiation, and survival by reversibly oxidizing specific protein targets, thereby maintaining cellular homeostasis (Gammella et al., 2016; Krishnamurthy et al., 2024). In stark contrast, the massive and uncontrolled “oxidative burst” during reperfusion overwhelms these delicate regulatory systems, causing widespread, indiscriminate damage to lipids, proteins, and nucleic acids, which directly drives myocardial injury (Fedoreyeva, 2024).
Further complicating this picture is the crucial concept of redox hormesis, which reveals that the biological response to ROS follows a non-linear, often U-shaped, curve. This model posits that while both very low and very high levels of ROS can be detrimental, a moderate and transient increase in ROS can be profoundly protective (Wang et al., 2014). This mild oxidative challenge acts as a trigger, activating powerful endogenous defense mechanisms, most notably the Nrf2 pathway. This process effectively pre-conditions the cell to withstand subsequent, more severe insults, a mechanism that mirrors the protective effects of ischemic preconditioning (Wu et al., 2023). Therefore, a moderate ROS signal is not an early sign of damage but a critical catalyst for cellular resilience.
This non-linear relationship explains why non-specific, high-dose antioxidant therapies have persistently failed in clinical trials. Aiming for complete ROS eradication, these strategies disrupt this vital hormetic balance and may inadvertently cause harm. By indiscriminately scavenging all ROS, these therapies likely blunt the essential, moderate signaling required to activate the heart’s own powerful intrinsic defense pathways. In doing so, they may render the myocardium more vulnerable, providing a stark example of a therapeutic strategy that, while mechanistically logical on the surface, fails to account for the underlying biological complexity. This critical therapeutic pitfall underscores why future strategies must shift from broad ROS elimination to the nuanced restoration of redox homeostasis.
2.3 Dysregulation and failure of endogenous antioxidant defense mechanisms
Under physiological conditions, the myocardium maintains redox homeostasis through a robust endogenous antioxidant defense system, comprising enzymatic components like SOD, Catalase (CAT), and Glutathione Peroxidases (GPxs), as well as non-enzymatic antioxidants such as glutathione (GSH) and thioredoxin (Kumar et al., 2022). These systems are crucial for neutralizing physiological levels of ROS and preventing oxidative damage. However, during MIRI, this crucial defense system is severely overwhelmed and dysregulated, rendering it incapable of counteracting the massive oxidative burst (Jiang et al., 2023).
This multifaceted dysregulation involves the rapid depletion and inactivation of key antioxidants and enzymes due to excessive ROS production (Karaduleva et al., 2016; Korshunov and Imlay, 2024). Furthermore, factors like calcium overload can interfere with the function of antioxidant enzymes, and epigenetic modifications can negatively impact the expression of antioxidant genes. Crucially, key regulatory signaling pathways that normally bolster antioxidant defenses are also disrupted or insufficiently activated to cope with the overwhelming stress. The Nuclear factor erythroid 2-related factor 2 (Nrf2) pathway, a master regulator of antioxidant gene expression (Shen et al., 2019), is activated by oxidative stress as a protective feedback mechanism (Itoh et al., 1997; 1999). Similarly, the JAK2/STAT3 pathway is known to promote cardiomyocyte survival and upregulate anti-apoptotic and antioxidant proteins in response to stress (Xiong et al., 2021). Upon activation, JAK2 phosphorylates STAT3, leading to STAT3 dimerization, translocation to the nucleus, and binding to target gene promoters. In the context of MIRI, JAK2/STAT3 activation is often considered a protective response in the acute phase, promoting cardiomyocyte survival by upregulating anti-apoptotic proteins (like Bcl-xL and Mcl-1) and antioxidant enzymes (Liu P. et al., 2021; Xiong et al., 2021). While Nrf2 and JAK2/STAT3 are indeed activated as protective responses during MIRI, the overwhelming and dynamic nature of the oxidative insult often impairs their full protective capacity or overwhelms the defense mechanisms they regulate, leading to a functional failure in restoring redox balance. This failure of the endogenous defense system to restore redox balance is a critical step that allows oxidative stress to exert its full pathological effects.
In summary, the overwhelming production of ROS during MIRI, coupled with the depletion, inactivation, and dysregulation of the intrinsic antioxidant defense mechanisms and their regulatory pathways, leads to a state of severe redox imbalance. This failure to effectively neutralize oxidants is a prerequisite for the subsequent cascade of damaging downstream events that drive myocardial injury.
2.4 Oxidative stress-mediated downstream pathological consequences
With the endogenous antioxidant defense system overwhelmed and dysregulated (as discussed in Section 2.3), the excessive and unchecked accumulation of ROS during MIRI triggers direct and widespread damage at the molecular level. This molecular carnage manifests as: (i) lipid peroxidation, which disrupts membrane integrity and is a key driver of ferroptosis (Yu et al., 2021; Fujii and Imai, 2024); (ii) protein oxidation, leading to conformational changes and loss of function in enzymes and structural proteins (Li Y. et al., 2022; Duong et al., 2024); and (iii) DNA/RNA damage, which can activate cell death pathways (Seixas et al., 2021; Cardinale et al., 2022). Collectively, this widespread molecular damage serves as the direct trigger for the major downstream pathological consequences of inflammation and cell death.
2.4.1 Inflammation
Oxidative stress is a potent trigger and amplifier of the inflammatory response in the ischemic and reperfused myocardium. Unchecked ROS can directly activate key pro-inflammatory transcription factors, such as Nuclear Factor-kappa B (NF-κB) and Activator Protein 1 (Yusuf et al., 2025). Activation of NF-κB, for instance, promotes the transcription of genes encoding a wide array of pro-inflammatory mediators, including cytokines (e.g., TNF-α, IL-1β, IL-6, IL-18) and chemokines (e.g., MCP-1, IL-8) (Liu et al., 2020). These secreted factors attract and activate inflammatory cells, such as neutrophils and macrophages, to the site of injury (Liang et al., 2025). Furthermore, oxidative stress is a critical activator of inflammasomes, particularly the NLRP3 inflammasome (Jomova et al., 2023), leading to the release of potent pro-inflammatory cytokines that drive sterile inflammation and contribute significantly to tissue damage (Chen et al., 2025). The interplay between unchecked oxidative stress and inflammation forms a vicious cycle, amplifying injury (Zhang H. et al., 2021; Li et al., 2025).
2.4.2 Cell death
Oxidative stress is a major inducer of cardiomyocyte death in MIRI, contributing significantly to the loss of functional myocardium. Multiple forms of cell death are involved, including apoptosis, necrosis, and ferroptosis. Excessive ROS can trigger the mitochondrial pathway of apoptosis by disrupting mitochondrial membrane potential and releasing pro-apoptotic factors (Luis-García et al., 2021; Wolf et al., 2022). Oxidative stress also contributes to necrosis by damaging cell membranes through lipid peroxidation and exacerbating calcium dysregulation, leading to cell swelling and lysis (Munoz et al., 2017; Xue et al., 2020; Fujii and Imai, 2024). Increasingly recognized, ferroptosis is a distinct form of regulated cell death driven by iron accumulation and lethal lipid peroxidation (Yu et al., 2021; Fujii and Imai, 2024). Oxidative stress plays a central role by initiating lipid peroxidation chains and inhibiting the activity of glutathione peroxidase 4 (GPX4), a key enzyme preventing ferroptosis (Xu et al., 2021).
2.4.3 Signaling pathway alterations
Beyond activating inflammatory and cell death cascades, overwhelming oxidative stress pathologically alters numerous other intracellular signaling pathways crucial for myocardial function and survival. The MAPK pathways (including p38, JNK, and ERK) are highly sensitive to oxidative stress and play diverse, often detrimental, roles in MIRI, mediating inflammation, apoptosis, and maladaptive stress responses (Xu et al., 2015). The Phosphoinositide 3-Kinase (PI3K)/Akt pathway, while typically pro-survival, can be complexly modulated by pathological oxidative stress, impacting cardiomyocyte viability and metabolism in ways that may not always be protective (Lu et al., 2023). While Nrf2 and JAK2/STAT3 have protective roles, severe oxidative stress can also lead to their dysregulation or interaction with other pathways in ways that contribute to later-stage remodeling or inflammation (Mahdiani et al., 2022; Ye et al., 2024). These widespread alterations in signaling networks contribute to the overall pathological phenotype of the injured myocardium.
In summary, the failure of the endogenous antioxidant defense system allows the massive oxidative insult to trigger a complex array of downstream pathological consequences, including robust inflammatory responses, multiple forms of cardiomyocyte death, and widespread disruption of vital signaling networks. These interconnected events collectively contribute to the extent of myocardial damage and ultimately determine functional recovery.
3 Therapeutic strategies targeting oxidative stress
3.1 Exogenous antioxidant supplementation
Direct administration of exogenous antioxidants, while intuitively appealing, is a significant component of the ‘unmet promise’ in MIRI. Despite extensive preclinical support, clinical trials of these agents have yielded largely inconsistent and disappointing outcomes (summarized in Table 1), highlighting fundamental translational challenges.
Vitamins C and E, acting as water-soluble (C) and lipid-soluble (E) antioxidants respectively to scavenge ROS and protect membranes (Kalogerakou and Antoniadou, 2024; Alberts et al., 2025), have shown mixed clinical results. Large trials of high-dose Vitamin E, such as the HOPE trial, failed to show cardiovascular benefit and even suggested potential harm, possibly due to complex metabolism, potential pro-oxidant effects in chronic settings, or insufficient targeting to acute injury (Lonn et al., 2005). Conversely, smaller studies exploring acute, peri-procedural administration, such as intravenous Vitamin C combined with E pre-PCI [PREVEC trial (Rodrigo et al., 2014)] or intracoronary Vitamin E during surgery (Canbaz et al., 2003), showed some positive signals regarding reduced infarct size or injury markers, underscoring the importance of timing and local delivery. Indeed, the failure of long-term, high-dose Vitamin E in the HOPE trial offers a classic clinical example of disregarding the principles of redox hormesis discussed in Section 2.2. Instead of restoring balance, this non-specific scavenging strategy likely blunted essential, low-level ROS signals required for activating endogenous protective pathways, paradoxically leaving the myocardium more vulnerable.
N-acetylcysteine (NAC), a glutathione precursor that enhances endogenous defense and acts as a direct scavenger (Orhan et al., 2006; Pasupathy et al., 2017), has demonstrated more consistent acute benefits. Phase II trials showed reduced injury markers during surgery (Orhan et al., 2006) and, notably, a significant reduction in infarct size and improved myocardial salvage when combined with nitrates in acute STEMI patients undergoing primary PCI [NACIAM trial (Pasupathy et al., 2017)], suggesting potential utility in the acute setting, possibly by enhancing endogenous defenses. However, its broader clinical utility remains unproven, with inconsistent results across studies likely stemming from fundamental strategic flaws. Firstly, the delivery method: existing trials have universally relied on systemic (oral or IV) administration, a route likely too slow and diffuse to counter the rapid oxidative burst in the reperfusing heart, while a more direct intracoronary approach has never been tested. Secondly, an emerging safety concern: NAC’s long-held safety profile has been challenged by preclinical evidence that it can paradoxically promote tumor angiogenesis (Wang T. et al., 2023). These unaddressed issues of suboptimal delivery and potential off-target toxicity provide a compelling explanation for NAC’s translational failure to date. The case of NAC encapsulates the core challenges of MIRI therapy: while its mechanism of enhancing endogenous defenses is conceptually superior to direct scavenging (as argued in Section 2.3), its clinical potential is ultimately nullified by the formidable practical hurdles of delivery and timing required to counteract the rapid and localized oxidative burst (Section 2.1).
While the mitochondrial antioxidant Coenzyme Q10 (CoQ10) showed promise in the Q-SYMBIO trial (Mortensen et al., 2014), its clinical translation is severely constrained by profound pharmacokinetic hurdles. The combination of poor oral bioavailability and a saturable absorption mechanism makes it exceptionally difficult for standard formulations to achieve and sustain therapeutic plasma concentrations reliably. This fundamental delivery challenge is a key factor behind the inconsistent clinical outcomes reported for CoQ10 and underscores the critical necessity of advanced formulations, such as nano-emulsions or the use of ubiquinol, to unlock its true therapeutic potential.
Edaravone, a synthetic free radical scavenger, showed potential by reducing reperfusion injury markers in a Phase II acute MI clinical trial (Tsujita et al., 2006). However, despite this early promise and supportive preclinical data, edaravone has failed to become a standard therapy for myocardial reperfusion injury. Its clinical adoption is significantly hampered by practical limitations, including an intravenous-only administration route unsuitable for long-term or outpatient care and a narrow therapeutic window. Furthermore, its current regulatory approval is restricted to neurological conditions like ALS, creating substantial off-label barriers. Crucially, its efficacy in large-scale cardiovascular outcome trials remains unproven, leaving it as a theoretically interesting but clinically unvalidated option in an era dominated by well-established, evidence-backed reperfusion strategies.
Conversely, Melatonin, investigated for its potential antioxidant effects, failed to demonstrate significant improvement in outcomes for STEMI patients undergoing PCI in the MARI trial (Dominguez-Rodriguez et al., 2017), highlighting that effectiveness varies among antioxidants and depends on their specific properties and targets.
The overall mixed clinical results for exogenous antioxidants underscore significant challenges in translating preclinical success, largely contributing to their “unmet promise.” These hurdles include poor pharmacokinetics and bioavailability, leading to insufficient concentrations at the injury site, and a critical lack of specificity. Many exogenous antioxidants fail to selectively target the most relevant oxidants or critical cellular compartments (e.g., mitochondria) where ROS are primarily generated. This non-specific action can also interfere with the physiological signaling roles of ROS (as discussed in Section 2.2), potentially leading to unintended adverse effects or a lack of targeted efficacy. Furthermore, inappropriate dosing or timing relative to the dynamic MIRI process, coupled with inherent patient heterogeneity, further complicates their clinical utility (Lonn et al., 2005; Dominguez-Rodriguez et al., 2017). These limitations collectively emphasize the urgent need for more targeted and sophisticated approaches.
Beyond these, a vast number of natural compounds have demonstrated preclinical antioxidant and cardioprotective potential in MIRI models. These include resveratrol (Shahcheraghi et al., 2023), curcumin (Cox et al., 2022), quercetin (Bellavite et al., 2023), and epigallocatechin gallate (Mohd Sabri et al., 2022), which often act through multiple mechanisms including direct scavenging and activation of pathways like Nrf2. However, their translation is hampered by poor bioavailability and a lack of robust clinical trials in the acute MIRI setting.
3.2 Enhancing endogenous antioxidant defense
Recognizing the limitations of directly scavenging the massive and dynamic oxidative burst with exogenous antioxidants, enhancing the heart’s intrinsic antioxidant defense mechanisms represents a potentially more effective and sophisticated strategy, directly addressing the functional failure of these systems during MIRI. The Nrf2 pathway is the master regulator of cellular antioxidant and detoxification responses, making it a prime target for bolstering endogenous protection against oxidative stress in MIRI. The Nrf2 pathway operates via a Keap1-mediated mechanism where oxidative stress triggers Nrf2 release, allowing its nuclear translocation and binding to antioxidant response elements (Shen et al., 2019). This upregulates a wide array of protective genes, including enzymatic antioxidants (e.g., HO-1, NQO1) and components of GSH synthesis, significantly enhancing cellular capacity to neutralize oxidants. Numerous preclinical studies with pharmacological Nrf2 activators (e.g., sulforaphane, naringenin, curcumin) have demonstrated significant cardioprotection in MIRI models, attenuating injury, reducing infarct size, and decreasing oxidative stress, inflammation, and cell death (Wang R. et al., 2023; Cui et al., 2025). These benefits are often linked to enhanced antioxidant capacity and potential crosstalk with other protective pathways (Liu P. et al., 2021; Xu et al., 2021; Wang et al., 2022). Despite compelling preclinical evidence, clinical translation of Nrf2 activators for MIRI remains in its infancy, with a significant lack of published trial data specifically in this acute setting (summarized in Table 1). While an ongoing Phase II trial is evaluating sulforaphane (NCT05408559), results are not yet available, highlighting the limited clinical evidence.
Key challenges hindering translation include identifying activators with optimal safety and pharmacokinetic profiles that allow for precise, selective, and temporal activation of Nrf2. This is paramount to maximize cardioprotection while minimizing potential off-target effects. Specifically, while Nrf2 activation is protective in acute MIRI, its sustained or non-selective overactivation may promote cancer cell proliferation and drug resistance by regulating the expression of antioxidant genes, thereby helping cancer cells resist oxidative damage caused by chemotherapy drugs and enhancing their survival capabilities (Nezu and Suzuki, 2021; Nguyen et al., 2025). Furthermore, determining the effective dose and therapeutic window, the lack of reliable biomarkers to confirm Nrf2 activation and predict response, and the need for better patient selection are also critical. This translational challenge highlights the inherent risks of manipulating master regulators. Potential off-target effects, such as promoting cancer, serve as a crucial reminder that even protective pathways have context-dependent effects, echoing the delicate balance of redox signaling versus distress laid out in Section 2.2.
Beyond Nrf2, the JAK2/STAT3 signaling pathway represents another crucial component of the heart’s endogenous defense against MIRI. As discussed in Section 2.3, this pathway is influenced by oxidative stress and plays a protective role in the acute phase by promoting cardiomyocyte survival and upregulating anti-apoptotic and antioxidant proteins (Liu P. et al., 2021; Xiong et al., 2021). Preclinical studies suggest that activation of the JAK2/STAT3 pathway contributes to the cardioprotective effects of various agents (Mahdiani et al., 2022), and some compounds may exert their benefits partly through this mechanism (Xiong et al., 2021). This positions JAK2/STAT3 modulation as a potential strategy for enhancing endogenous cardioprotection against oxidative damage. Despite this preclinical promise, clinical translation specifically targeting JAK2/STAT3-mediated oxidative stress as the primary therapeutic strategy in acute myocardial ischemia is notably lacking, highlighting the need for more focused clinical investigation. Therefore, realizing the significant theoretical promise of enhancing endogenous antioxidant defense via pathways like Nrf2 and JAK2/STAT3 requires extensive further research, particularly well-designed clinical trials incorporating biomarkers to monitor pathway activation and patient response.
3.3 Strategies targeting downstream pathological consequences of oxidative stress
Given that oxidative stress initiates and exacerbates a cascade of downstream pathological events, therapeutic strategies can also focus on interrupting these consequences, even if the primary oxidative insult is not fully neutralized.
3.3.1 Anti-inflammatory agents
Given that oxidative stress is a powerful trigger for inflammation, forming a vicious cycle of tissue damage, targeting the inflammatory cascade is a rational, albeit indirect, strategy to mitigate the downstream consequences of the initial oxidative insult. Targeting the inflammatory response represents a crucial therapeutic avenue. Various anti-inflammatory agents have been investigated, broadly categorized into agents with general anti-inflammatory effects and those targeting specific mediators or pathways.
3.3.1.1 Broad anti-inflammatories
Encompass agents that exert relatively non-specific anti-inflammatory actions. Examples include nonsteroidal anti-inflammatory drugs (NSAIDs) and corticosteroids. While these drugs can reduce inflammation, their clinical utility in acute MI is significantly hampered by substantial side effects, such as increased cardiovascular risk (particularly with certain NSAIDs) and systemic immunosuppression (with corticosteroids) (Ju et al., 2023). These agents often lack specificity for the precise inflammatory pathways driving MIRI, leading to a poor risk-benefit profile and inconsistent efficacy in clinical trials for this acute setting. Colchicine, which inhibits microtubule polymerization and inflammasome activation, can also be considered in this category due to its multifaceted anti-inflammatory actions, although its mechanism involves specific targets like the NLRP3 inflammasome, a key mediator of oxidative stress-induced inflammation. Notably, low-dose colchicine has demonstrated significant clinical benefits in reducing cardiovascular events in patients with chronic coronary disease and after acute MI in trials like LoDoCo2 and COLCOT, respectively (Tardif et al., 2019; Nidorf et al., 2020). This suggests that modulating inflammation, even through agents with broader mechanisms like inflammasome inhibition, can be clinically effective. However, despite some success with agents like colchicine, the general class of broad anti-inflammatories often lacks specificity for the precise inflammatory pathways driving MIRI, leading to a poor risk-benefit profile and inconsistent efficacy in clinical trials for this acute setting. Given these limitations, strategies targeting specific inflammatory mediators are considered a more promising therapeutic approach.
3.3.1.2 Targeting inflammatory mediators/pathways
More targeted approaches focus on specific cytokines or signaling pathways activated by oxidative stress and inflammation. Examples include inhibitors of IL-1β, IL-6, or components of the NF-κB pathway. Natural compounds like midazolam, vitamin B12, metformin, Cangrelor, and hesperidin have also shown anti-inflammatory effects in preclinical MIRI models, often by modulating macrophage polarization or inhibiting inflammatory pathways like NF-κB (Huang et al., 2020; Xu et al., 2024). Clinical trials evaluating anti-inflammatory agents in MIRI have yielded mixed but overall more encouraging results compared to broad antioxidant supplementation (summarized in Table 1).
3.3.1.3 Targeting interleukins
Tocilizumab, an IL-6 receptor inhibitor, showed a significantly higher myocardial salvage index but no significant difference in final infarct volume compared to placebo (Broch et al., 2021). This suggests potential benefit in limiting early damage but highlights the complexity of translating salvage to reduced final infarct size.
Anakinra, an IL-1 receptor antagonist, demonstrated a significant reduction in 1-year death or new-onset heart failure in STEMI patients (Abbate et al., 2022). This positive outcome suggests that targeting the IL-1 pathway, a key downstream effector of NLRP3 inflammasome activation (which is triggered by oxidative stress), can improve long-term clinical outcomes.
Canakinumab, an interleukin-1β inhibitor, in patients with prior MI and elevated CRP, provided strong evidence for a significant reduction in the risk of recurrent MI, stroke, and cardiovascular death (Ridker et al., 2017). While not specifically in the acute MIRI setting, this trial strongly supports the concept that targeting inflammation, particularly the IL-1β pathway, is beneficial in secondary prevention of atherosclerotic cardiovascular events, which are linked to chronic oxidative stress and inflammation.
However, not all anti-inflammatory strategies have succeeded. The CIRT trial found that low-dose methotrexate did not reduce the risk of cardiovascular events (Ge et al., 2024), and the SOLID-TIMI 52 trial with darapladib (an Lp-PLA2 inhibitor) showed no reduction in major coronary events after acute coronary syndrome (O’Donoghue et al., 2014).
The varied success of anti-inflammatory agents suggests that targeting specific inflammatory pathways (like IL-1β or IL-6) or using agents with broad but relevant mechanisms (like colchicine affecting inflammasomes and neutrophil function) may be more effective than general immunosuppression (methotrexate) or targeting single enzymes (darapladib). The timing and duration of anti-inflammatory therapy relative to the dynamic inflammatory response in MIRI are also likely critical factors. Furthermore, patient selection based on inflammatory biomarkers (like CRP in CANTOS) might be important for identifying responders. Collectively, the varied success within this class powerfully illustrates that merely being ‘anti-inflammatory’ is insufficient. The positive outcomes of agents like canakinumab and colchicine suggest that effective strategies must precisely target the key nodes—such as the IL-1β pathway or the NLRP3 inflammasome—that directly perpetuate the vicious cycle between oxidative stress and inflammation detailed in Section 2.4.1. The failure of broader or less specific agents, therefore, reinforces the central theme that therapeutic success in MIRI demands a highly targeted approach that addresses the specific downstream consequences of the initial oxidative insult.
3.3.2 Anti-cell death agents
In addition to fueling a damaging inflammatory cycle, unchecked oxidative stress directly triggers multiple cell death pathways. Therefore, preventing cardiomyocyte death is paramount for preserving myocardial function after MIRI. Initially, strategies targeted apoptosis, as oxidative stress is a known trigger of the mitochondrial apoptotic pathway. However, clinical trials specifically designed to test direct anti-apoptotic agents in acute MIRI have been largely disappointing (summarized in Table 1).
A precise understanding of these failures requires acknowledging the complexity of cell death in MIRI. It has been shown that the predominant form of cell death following reperfusion is necrosis, whereas apoptosis accounts for only a small percentage of the overall cell death. This dominance of necrosis may explain why anti-apoptotic therapies have shown limited success; for instance, neither the mitochondria-targeting peptide Bendavia nor erythropoietin demonstrated significant benefits in clinical trials (Minamino et al., 2018; Hortmann et al., 2019). Consequently, regulators of the mitochondrial permeability transition pore (mPTP), considered a key driver of necrosis, became the next major therapeutic target.
Cyclosporine A (CsA) was introduced as an mPTP inhibitor, acting indirectly by inhibiting cyclophilin D (CypD) to prevent pore opening. While early, smaller trials showed promise by reducing infarct size (Piot et al., 2008), the larger CIRCUS trial subsequently failed to demonstrate improved clinical outcomes (Cung et al., 2015). This translational failure is likely multifactorial, stemming from CsA’s indirect mechanism, which may permit pore opening under high stimulation, confounding off-target effects like calcineurin inhibition, and the reality that, much like the challenge facing anti-apoptotic strategies, MIRI activates multiple redundant cell death programs (such as the increasingly recognized ferroptosis), making the inhibition of mPTP-mediated necrosis alone likely insufficient to salvage the myocardium (Zhou et al., 2020). The consistent clinical failure of agents targeting a single mode of cell death provides compelling clinical validation for the mechanistic principle outlined in Section 2.4.2: MIRI triggers an interconnected web of cell death programs, suggesting future strategies may need to be multi-modal or target more upstream events.
3.4 Clinically successful multi-targeted agents with pleiotropic antioxidant effects
Beyond strategies primarily focused on directly modulating oxidative stress or its immediate downstream consequences, certain established or emerging cardioprotective agents have demonstrated clinical success in MIRI, at least in part, through mechanisms that involve mitigating oxidative stress alongside other pathways. Their success may offer valuable insights into overcoming the ‘unmet promise’ of single-target antioxidant therapies. These agents often possess multi-targeted actions, addressing several facets of MIRI pathophysiology simultaneously.
3.4.1 SGLT2 inhibitors
Sodium-glucose co-transporter 2 (SGLT2) inhibitors, initially developed for diabetes management, have demonstrated remarkable cardiovascular benefits, including reduced hospitalization for heart failure and cardiovascular death, even in patients without diabetes. While their primary mechanism involves inhibiting glucose reabsorption in the kidney, their cardioprotective effects in MIRI are thought to be mediated by multiple pathways, including a significant contribution from reducing oxidative stress and inflammation (Pham et al., 2024; Armillotta et al., 2025). These antioxidant effects are thought to be mediated through a dual mechanism: indirectly, by improving systemic metabolism and cardiac energy efficiency (Usman et al., 2024), and more directly, through the inhibition of myocardial NOX2 expression and the activation of endogenous antioxidant pathways such as Nrf2(Preda et al., 2024).
Preclinical studies have shown SGLT2 inhibitors can decrease ROS production, enhance antioxidant defense, and suppress inflammatory responses in the myocardium (Harris et al., 2024; Pham et al., 2024; Armillotta et al., 2025). Clinical evidence, as summarized in Table 1, includes studies showing that Empagliflozin improved oxidative stress markers and NYHA functional class in T2DM patients with HFrEF (Eshraghi et al., 2025), and Dapagliflozin reduced the number of AHREs and improved oxidative stress/mitochondrial function in the clinical AF subgroup of patients with CIEDs (Nantsupawat et al., 2024). Dapagliflozin also improved endothelial function and reduced oxidative stress in patients with early-stage T2DM (Shigiyama et al., 2017) and improved endothelial function compared to glibenclamide in T2DM patients with subclinical atherosclerosis (Sposito et al., 2021). Another study explored Empagliflozin’s effects on myocardial glucose uptake and blood flow (Lauritsen et al., 2021). While large outcome trials were not specifically focused on acute MIRI, these studies in Table 1 provide clinical evidence of SGLT2 inhibitors influencing oxidative stress and related parameters in various patient populations.
3.4.2 Statins
HMG-CoA reductase inhibitors (statins) are cornerstone therapies for preventing and treating atherosclerotic cardiovascular disease. While their primary mechanism is cholesterol lowering, statins also possess pleiotropic effects, including anti-inflammatory and antioxidant properties (Iqbal et al., 2019). Statins can reduce oxidative stress by inhibiting NOX activity, enhancing eNOS function, and upregulating antioxidant enzymes. These effects contribute to improved endothelial function, reduced inflammation, and potentially direct cardioprotection in ischemic conditions (Chen W.-H. et al., 2024). Clinical trials, as shown in Table 1, have investigated the effects of statins on oxidative stress markers in various patient populations. For instance, Atorvastatin and Rosuvastatin significantly improved oxidative stress markers and lipid profiles in hyperlipidemic patients (Tonu et al., 2019). Atorvastatin also improved microvascular reactivity and reduced the oxidative stress marker malondialdehyde in patients with HFpEF (Iacovelli et al., 2024), and Simvastatin significantly decreased protein carbonylation (an oxidative stress marker) in COPD patients. While Atorvastatin had modest effects on oxidative/nitrosative stress biomarkers in the PREVENT trial involving patients receiving anthracyclines (Makhlin et al., 2024), these studies collectively support the concept that statins influence redox balance. Additionally, Icosapent ethyl, listed under this category in Table 1, modulated vascular regenerative cells and reduced oxidative stress in progenitor cells in patients with hypertriglyceridemia (Bakbak et al., 2024). These rapid pleiotropic effects, including their influence on redox balance, contribute to their standard use in the acute MI setting (Wu et al., 2024).
3.4.3 Nitrates
Organic nitrates like nitroglycerin are vasodilators widely used in ischemic heart disease to improve myocardial oxygen supply by dilating coronary arteries. Beyond vasodilation, nitrates can also exert antioxidant effects, for instance, by scavenging free radicals or improving eNOS function (Zhu et al., 2021). Clinical studies listed in Table 1, primarily focusing on dietary nitrate/nitrite supplementation, have shown effects such as subtly shifting redox balance towards a less pro-oxidative profile and decreasing classical monocytes in hypertensive patients, significantly lowering daily systolic BP and increasing nitrate/nitrite markers when combined with vitamin C in healthy young adults (Lbban et al., 2024), and increasing VO2max and improving skeletal muscle oxidative capacity in T2DM (Turner et al., 2022). A study protocol is also listed investigating dietary nitrate effects on cardiovascular performance, endothelial function, and oxidative stress during and after exercise in postmenopausal women with hypertension (Benjamim et al., 2023). These studies suggest that modulating the nitrate-nitrite-NO pathway, including through dietary intake, can influence redox status, although clinical trials with organic nitrates specifically assessing antioxidant effects in acute MIRI are less represented in Table 1.
These multi-targeted agents highlight that effective cardioprotection in MIRI may require simultaneously addressing multiple pathological pathways, including oxidative stress, inflammation, and metabolic dysfunction. While their primary indications and mechanisms may vary (Figure 5), their ability to favorably influence the redox balance, as supported by the clinical studies on biomarkers and surrogate endpoints listed in Table 1, contributes to their overall beneficial effects. The clinical evidence for some of these agents (like SGLT2 inhibitors and statins) in improving cardiovascular outcomes is strong, supporting the concept that modulating pathways intertwined with oxidative stress is a viable therapeutic approach. However, further research is needed to fully elucidate the contribution of their antioxidant effects to clinical outcomes in acute MIRI and to explore their optimal use in this specific context. The clinical evidence for the diverse strategies discussed above, ranging from direct antioxidants to multi-targeted agents, is summarized in Table 1, which illustrates the varied outcomes and highlights the ongoing translational challenges.

Figure 5. Mechanisms of therapeutic agents targeting oxidative stress in MIRI. Strategies include: (1) direct ROS scavenging (e.g., Vitamins C, E); (2) inhibiting ROS/RNS sources (e.g., Statins, Nitrates); (3) targeting downstream inflammation (e.g., Canakinumab, Colchicine); and (4) enhancing endogenous defenses (e.g., Sulforaphane).
3.5 Overcoming delivery barriers with advanced therapeutic systems
A significant challenge contributing to the ‘unmet promise’ of many promising preclinical antioxidant and other oxidative stress-modulating agents is the inefficient and non-targeted delivery of these compounds to the injured myocardium (Borrelli et al., 2021). Systemic administration often results in low drug concentrations at the site of injury, rapid clearance, and potential off-target effects. Novel drug delivery systems offer powerful solutions to overcome these limitations by enhancing drug bioavailability, improving targeting specificity to the ischemic/reperfused tissue, and controlling drug release kinetics (Shi et al., 2022).
3.5.1 Nanoparticle-based delivery
Nanocarriers, such as liposomes, polymeric nanoparticles, and micelles, are at the forefront of MIRI therapy. They can encapsulate a wide array of therapeutic agents, including natural compounds (e.g., Notoginsenoside R1), small molecule inhibitors, and gene therapies targeting oxidative stress pathways (Li H. et al., 2022; Ji et al., 2023). Nanoparticles protect their cargo from degradation, improve solubility, and favorably alter pharmacokinetics. A key strategy involves designing systems responsive to the MIRI microenvironment, such as nanoparticles that release their payload in response to high levels of ROS (Chang et al., 2024; Zuo et al., 2025).
By engineering their surface properties, nanoparticles can be designed for passive accumulation in the injured myocardium or active targeting of specific cell types. This targeted delivery significantly increases local drug concentration while minimizing systemic toxicity. For instance, engineered neutrophil membrane-mimicking nanocomposites have shown significant efficacy by leveraging integrin-mediated targeting (Jiang et al., 2025). Similarly, biomimetic systems like exosomes and nanozymes enhance therapeutic efficacy through their inherent biocompatibility (Jin et al., 2024; Geng et al., 2025).
3.5.2 Cell-based and exosome delivery
Utilizing cells, such as mesenchymal stem cells (MSCs) embedded within biomaterial patches, as carriers for therapeutic factors is another promising approach (Sharma et al., 2022). These cells can home to the injury site, providing sustained local delivery of antioxidant and regenerative support.
Building on this, exosome-based systems are emerging as a superior solution, capitalizing on their role as natural nanocarriers with low immunogenicity (Yuan et al., 2018; Chi et al., 2025). Exosomes can deliver protective cargo, like ncRNAs (e.g., miR-126), to directly mitigate oxidative stress (Wang et al., 2019). The future of this field lies in engineering these vesicles for enhanced efficacy. Advanced strategies include modifying their surface with cardiac-targeting peptides to improve homing and delivering highly advanced cargo, such as CRISPR-Cas9 ribonucleoproteins for precise cardiac genome editing (Mun et al., 2024).
3.5.3 Targeting ligands and advanced biomaterials
To further enhance precision, attaching targeting ligands (e.g., peptides, antibodies) to nanocarriers has proven highly effective. Examples include using CD11b antibodies to target inflammatory cells or folic acid to bind activated macrophages at the infarct site (Ma et al., 2025).
Injectable hydrogels and advanced biomaterials offer another dimension of control, providing a localized depot for the sustained release of therapeutics. These systems can be designed to be “paintable” for easy application on the epicardial surface or as microneedle patches for sequential, multi-drug delivery aligned with the pathological phases of MIRI (Lee et al., 2024; He et al., 2025). Injectable microporous scaffolds can also facilitate tissue ingrowth while releasing anti-fibrotic and pro-angiogenic drugs (Fang et al., 2020). These advanced systems ensure that therapeutic agents reach the myocardium at sufficient concentrations and for an adequate duration, representing a critical step in translating promising science into effective treatments.
3.5.4 The ‘Bench-to-Bedside’ translational gap
While these advanced delivery systems show immense preclinical promise, their clinical translation faces significant hurdles. The primary challenge lies in manufacturing and quality control. Reproducing complex, multi-component systems—such as hybrid cell membrane-coated nanoparticles or hydrogels with precisely tuned degradation and sequential drug release profiles (He et al., 2025)—at a clinical scale under Good Manufacturing Practice (GMP) standards is a formidable task. Ensuring batch-to-batch consistency in particle size, drug loading efficiency, surface chemistry, and release kinetics is critical for predictable therapeutic outcomes and regulatory approval.
Furthermore, immunogenicity remains a critical safety concern. While endogenous carriers like extracellular vesicles or biomimetic coatings are designed to minimize immune recognition, the introduction of targeting ligands, synthetic polymers, or residual manufacturing components can provoke unintended inflammatory or foreign body responses, potentially neutralizing the therapeutic effect or causing adverse events. Predictive computational tools are emerging to de-risk these platforms, but thorough in vivo assessment is indispensable (Cun et al., 2025). Finally, navigating the regulatory landscape for these combination products (drug/biologic + device) is inherently complex, requiring extensive data on long-term stability, biodistribution, and the ultimate fate and toxicity of all system components. Overcoming these practical barriers is the final, critical step toward translating these innovative delivery strategies into meaningful clinical benefit for MIRI.
4 Challenges, future directions, and the promise of precision medicine
4.1 Key challenges in targeting oxidative stress for MIRI treatment
Despite compelling preclinical evidence highlighting the central role of oxidative stress in MIRI pathophysiology, translating these findings into effective clinical therapies has proven remarkably challenging, largely contributing to the ‘unmet promise’ observed thus far. A primary hurdle lies in the inherent complexity and dynamic nature of oxidative stress itself. It involves a diverse array of ROS species generated from multiple sources with distinct reactivities and temporal patterns throughout ischemia and reperfusion. This complexity makes identifying and specifically targeting the most relevant pathways within this vast network difficult, as targeting a single oxidant or source may be insufficient and could potentially interfere with physiological redox signaling, given ROS’s crucial dual roles as signaling molecules (as elaborated in Section 2.2) (Cheng et al., 2024). Indeed, non-selective or excessive scavenging of ROS can disrupt this delicate redox balance, potentially leading to unintended adverse effects by impairing vital physiological signaling pathways, as exemplified by their essential roles in processes like neuronal antioxidant responses, embryonic development, and reproductive function (Gualtieri et al., 2021; Liu L. et al., 2021).
Further challenges relate to the practical aspects of therapeutic intervention. Achieving sufficient and sustained drug concentrations specifically at the site of myocardial injury remains a major obstacle for systemically administered agents due to poor pharmacokinetics, rapid clearance, and lack of specific targeting to affected cells (Duan X. et al., 2023). Moreover, the dynamic nature of oxidative stress dictates a potentially narrow and stage-dependent therapeutic window, making the determination of optimal timing for intervention crucial yet challenging in the clinical setting (Fasnacht et al., 2022).
Significant patient heterogeneity in baseline oxidative stress levels, comorbidities, and genetic predispositions further complicates a one-size-fits-all approach, influencing individual responses to targeted therapies (Zou et al., 2024). A critical bottleneck remains the lack of robust biomarkers to assess myocardial redox status, predict therapeutic response, or stratify patients (Vukašinović et al., 2023). These factors, combined with the complexity of designing clinical trials that adequately account for heterogeneity, timing, and appropriate endpoints, contribute to the difficulty in demonstrating clinical efficacy. Furthermore, oxidative stress is intertwined with other pathologies like inflammation and metabolic disturbances, suggesting that targeting it in isolation may be insufficient (Hernández-Ruiz et al., 2025). Addressing these fundamental challenges is essential for successful clinical translation.
4.2 Oxidative stress in different MI subtypes and special populations
The role and impact of oxidative stress in MI vary significantly depending on the specific clinical presentation of MI and the presence of underlying comorbidities, underscoring the fundamental need for tailored therapeutic strategies and a precision medicine approach. In acute MI, particularly reperfused STEMI, the dominant feature is a massive oxidative burst upon reperfusion, superimposed on ischemic stress (Yazdi et al., 2023). While core ROS generation mechanisms are similar in STEMI and NSTEMI, the severity, duration of ischemia, and nature of reperfusion influence the magnitude and kinetics of this burst. In contrast, chronic ischemic heart disease involves prolonged, low-grade oxidative stress contributing to maladaptive remodeling and fibrosis (Gallo et al., 2024; Loffredo and Carnevale, 2024), with ROS sources potentially more linked to chronic inflammation and metabolic alterations, suggesting different therapeutic targets than acute scavenging.
Furthermore, the presence of comorbidities profoundly alters the baseline redox state and inflammatory milieu, influencing MIRI severity and therapeutic response. Diabetic patients exhibit elevated chronic oxidative stress and inflammation (Li et al., 2025), exacerbating MIRI injury with larger infarcts and poorer outcomes (Sztolsztener et al., 2022), potentially due to hyperactive ROS sources and impaired defenses. Chronic hypertension is also associated with increased vascular and myocardial oxidative stress (Loffredo and Carnevale, 2024), priming the heart for more severe ischemic injury. Similarly, patients with pre-existing heart failure (Aimo et al., 2020; Gallo et al., 2024) or those who are aging (Zhang J. et al., 2021) often have elevated chronic oxidative stress and impaired defenses, increasing their vulnerability to acute ischemic insults and reducing recovery capacity.
Understanding these distinct oxidative stress profiles and responses across different MI subtypes and patient populations is critical for moving beyond a generalized approach. The variability introduced by factors like the type of ischemia, diabetes, hypertension, heart failure, and aging necessitates patient stratification and the development of personalized therapeutic strategies. Future approaches should integrate individual risk factors, comorbidities, and potentially biomarkers of oxidative stress to guide the selection and timing of interventions, ultimately aiming to improve outcomes based on each patient’s unique oxidative stress landscape (Vornic et al., 2024).
4.3 Future research directions
Addressing the challenges in translating oxidative stress research into effective MIRI therapies requires concerted future efforts focused on refining mechanistic understanding and developing innovative, targeted interventions. Moving beyond broad antioxidant approaches, research must prioritize more precise target identification and validation within the complex oxidative stress network. This includes investigating the specific roles of different NOX isoforms in distinct cell types, developing strategies to regulate mitochondrial ROS production without compromising function (Romo-González et al., 2023), identifying critical nodes in oxidative stress-sensitive signaling pathways, and exploring the potential of modulating miRNAs involved in redox regulation to achieve highly specific redox modulation (Carbonell and Gomes, 2020). A modular overview of these strategies is presented in Figure 6.

Figure 6. A precision medicine framework for targeting oxidative stress in MIRI. This framework integrates therapeutic strategies targeting inflammation and cell death with multi-targeted agents. The choice of strategy is guided by precision medicine tools (biomarkers, multi-omics, AI) to address the specific pathological drivers of oxidative stress.
A critical need is the development and validation of robust, dynamic, and specific biomarkers for oxidative stress in MIRI. These biomarkers should reflect real-time redox status, predict therapeutic response, and enable patient stratification. Identifying biomarkers that reflect the activity of specific ROS-generating enzymes or pathway activation is also crucial. Such biomarkers must be validated in large clinical cohorts to determine their utility for early diagnosis, risk stratification, predicting therapeutic response, and monitoring efficacy (Rosenblatt et al., 2025). Furthermore, advancing and clinically translating novel drug delivery systems with enhanced targeting capabilities to the injured myocardium or specific cell types is essential to achieve sufficient local drug concentrations for precise redox modulation.
Given the complex and interconnected nature of oxidative stress and its downstream effects, exploring multi-target combination therapy regimens may be more effective than single-target approaches, aiming for synergistic effects on the ‘oxidative storm’ and its consequences. Research should investigate rational combinations of agents targeting different aspects of oxidative stress or combining them with established MIRI treatments. Integrating multi-omics data and utilizing artificial intelligence can provide deeper mechanistic insights and facilitate personalized approaches by identifying individual variations and optimizing treatment strategies. Ultimately, designing more targeted and effective clinical trials based on improved understanding, utilizing novel delivery systems, incorporating validated biomarkers, and focusing on specific patient subgroups will be critical for successfully translating research findings into improved outcomes for MIRI patients.
4.4 The promise of precision medicine based on oxidative stress
The inherent heterogeneity of MIRI patients underscores the critical need for a paradigm shift from a ‘one-size-fits-all’ approach to precision medicine, a path now being paved by tangible technological advancements. At the forefront of this revolution is the integration of artificial intelligence (AI) and machine learning (ML), which are transforming the rapid and accurate stratification of patients. Landmark studies demonstrate that AI-enhanced electrocardiogram analysis can identify high-risk occlusion myocardial infarction even without classic ST-segment elevation, outperforming both clinicians and existing commercial systems (Al-Zaiti et al., 2023; Lee et al., 2025). This diagnostic power extends to biomarkers. For instance, algorithms like CoDE-ACS and ARTEMIS personalize troponin interpretation by integrating clinical features. This approach has more than doubled the efficiency of safely ruling out MI in the emergency department compared to standard guidelines (Doudesis et al., 2023; Toprak et al., 2024). Furthermore, AI-driven analysis of cardiac imaging, from quantifying patient-specific coronary plaque risk on CT angiography to using “ultrasomics” to characterize myocardial texture on echocardiograms, is providing deeper, more predictive insights than ever before, establishing a robust foundation for personalized risk assessment (Miller et al., 2024; Jamthikar et al., 2025).
Accurate stratification must be coupled with a deep understanding of an individual’s unique biological landscape, a feat now achievable through multi-omics and novel biomarkers. This approach moves beyond simply diagnosing an MI to uncovering its specific pathophysiological drivers. For example, untargeted plasma metabolomics can reveal distinct metabolic signatures, such as elevated acylcarnitines, that point directly to the mitochondrial dysfunction and impaired fatty acid oxidation that fuel the oxidative burst in MIRI (Lv et al., 2024). Clinically accessible markers like the stress-hyperglycemia ratio (SHR) further reflect this interplay between systemic metabolic disturbance and oxidative stress, offering powerful prognostic value (Lucci et al., 2025; Song et al., 2025). Critically, integrating genomic and molecular data has uncovered fundamental mechanisms of patient heterogeneity, such as the discovery that a diurnal BMAL1-HIF2A complex regulates myocardial vulnerability to injury, providing a molecular rationale for the long-observed circadian patterns of MI severity and opening the door to “chronopharmacology”—timing interventions for maximum efficacy (Ruan et al., 2025).
Ultimately, personalized diagnosis and phenotyping are only effective if they guide targeted interventions that overcome the historical barriers of drug delivery and non-specificity. Here, precision medicine offers powerful solutions. The emergence of “theranostic” agents, such as the near-infrared dye IR-780, exemplifies this progress; this molecule not only selectively accumulates in ischemic cardiomyocytes for precise imaging but also exerts a direct protective effect by inducing a mitochondrial “quiescent state” that mitigates calcium overload and ROS production (Chen Z. et al., 2024). Concurrently, advanced delivery systems are being engineered to conquer the delivery hurdle. Technologies like ultrasound-targeted microbubble destruction (UTMD) enable the cardiac-specific delivery of gene therapies, enhancing the efficacy of safe, clinically relevant drug doses that would otherwise be insufficient (Wang S. et al., 2023). This synergistic integration—using AI for rapid stratification, multi-omics for deep phenotyping, and targeted systems for precise treatment—forms the definitive framework for translating our understanding of oxidative stress into meaningful clinical benefit and finally fulfilling the ‘unmet promise’ of MIRI therapy.
5 Conclusion
MIRI remains a critical clinical challenge, largely driven by oxidative stress. This review has highlighted that in MIRI, excessive ROS production overwhelms the heart’s endogenous defenses, leading to unchecked oxidative damage and severe downstream consequences. While therapeutic strategies targeting oxidative stress are crucial, their clinical translation has largely represented an ‘unmet promise’ due to the complexity of redox biology, delivery challenges, and patient heterogeneity. To overcome these limitations, we advocate for a paradigm shift towards precision medicine. This involves moving beyond simplistic exogenous scavenging to focus on enhancing intrinsic defense systems (e.g., Nrf2, JAK2/STAT3), leveraging novel drug delivery systems for targeted intervention, and exploring multi-target therapies. Ultimately, integrating robust biomarkers, multi-omics, and AI is the most promising path to tailor therapies to individual redox profiles, finally fulfilling the promise of antioxidant strategies and improving MIRI outcomes.
Author contributions
HC: Conceptualization, Data curation, Writing – original draft, Writing – review and editing. YT: Writing – review and editing. PR: Data curation, Writing – original draft. WW: Supervision, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Science and Technology Development Fund of the Affiliated Hospital of Chengdu University of Traditional Chinese Medicine (Project Y2024080) and the Qi Huang Scholar Program [No. [2022]256] from the National Administration of Traditional Chinese Medicine.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abbate, A., Wohlford, G. F., Del Buono, M. G., Chiabrando, J. G., Markley, R., Turlington, J., et al. (2022). Interleukin-1 blockade with anakinra and heart failure following ST-segment elevation myocardial infarction: results from a pooled analysis of the VCUART clinical trials. Eur. Heart J. - Cardiovasc. Pharmacother. 8, 503–510. doi:10.1093/ehjcvp/pvab075
Abdel Hamid, D. Z., Nienaa, Y. A., and Mostafa, T. M. (2022). Alpha-lipoic acid improved anemia, erythropoietin resistance, maintained glycemic control, and reduced cardiovascular risk in diabetic patients on hemodialysis: a multi-center prospective randomized controlled study. Eur. Rev. Med. Pharmacol. Sci. 26, 2313–2329. doi:10.26355/eurrev_202204_28461
Aimo, A., Castiglione, V., Borrelli, C., Saccaro, L. F., Franzini, M., Masi, S., et al. (2020). Oxidative stress and inflammation in the evolution of heart failure: from pathophysiology to therapeutic strategies. Eur. J. Prev. Cardiol. 27, 494–510. doi:10.1177/2047487319870344
Al-Zaiti, S. S., Martin-Gill, C., Zègre-Hemsey, J. K., Bouzid, Z., Faramand, Z., Alrawashdeh, M. O., et al. (2023). Machine learning for ECG diagnosis and risk stratification of occlusion myocardial infarction. Nat. Med. 29, 1804–1813. doi:10.1038/s41591-023-02396-3
Alberts, A., Moldoveanu, E.-T., Niculescu, A.-G., and Grumezescu, A. M. (2025). Vitamin C: a comprehensive review of its role in health, disease prevention, and therapeutic potential. Molecules 30, 748. doi:10.3390/molecules30030748
Alehagen, U., Johansson, P., Svensson, E., Aaseth, J., and Alexander, J. (2022). Improved cardiovascular health by supplementation with selenium and coenzyme Q10: applying structural equation modelling (SEM) to clinical outcomes and biomarkers to explore underlying mechanisms in a prospective randomized double-blind placebo-controlled intervention project in Sweden. Eur. J. Nutr. 61, 3135–3148. doi:10.1007/s00394-022-02876-1
Alizadehasl, A., Alavi, M. S., Alavi, M. S., and Roohbakhsh, A. (2019). TRPA1 as a promising target in ischemia/reperfusion: a comprehensive review. Iran. J. Basic Med. Sci. 27, 270–278. doi:10.22038/IJBMS.2023.74590.16198
Armillotta, M., Angeli, F., Paolisso, P., Belmonte, M., Raschi, E., Di Dalmazi, G., et al. (2025). Cardiovascular therapeutic targets of sodium-glucose co-transporter 2 (SGLT2) inhibitors beyond heart failure. Pharmacol. Ther. 270, 108861. doi:10.1016/j.pharmthera.2025.108861
Aslam, H., Albaqami, F., Rehman, N. U., and Shah, F. A. (2025). Carvacrol attenuated myocardial infarction through NLRP3-mediated pyroptosis and mTOR/Nrf2/PPARγ-dependent autophagic signaling. Toxicol. Appl. Pharmacol. 498, 117281. doi:10.1016/j.taap.2025.117281
Bakbak, E., Krishnaraj, A., Bhatt, D. L., Quan, A., Park, B., Bakbak, A. I., et al. (2024). Icosapent ethyl modulates circulating vascular regenerative cell content: the IPE-PREVENTION CardioLink-14 trial. Med 5, 718–734.e4. doi:10.1016/j.medj.2024.03.009
Bellavite, P., Fazio, S., and Affuso, F. (2023). A descriptive review of the action mechanisms of berberine, quercetin and silymarin on insulin resistance/hyperinsulinemia and cardiovascular prevention. Molecules 28, 4491. doi:10.3390/molecules28114491
Benjamim, C. J. R., Sousa, Y. B. A., Porto, A. A., de Moraes Pontes, Y. M., Tavares, S. S., da Silva Rodrigues, G., et al. (2023). Nitrate-rich beet juice intake on cardiovascular performance in response to exercise in postmenopausal women with arterial hypertension: study protocol for a randomized controlled trial. Trials 24, 94. doi:10.1186/s13063-023-07117-2
Borrelli, M. A., Turnquist, H. R., and Little, S. R. (2021). Biologics and their delivery systems: trends in myocardial infarction. Adv. Drug Deliv. Rev. 173, 181–215. doi:10.1016/j.addr.2021.03.014
Broch, K., Anstensrud, A. K., Woxholt, S., Sharma, K., Tøllefsen, I. M., Bendz, B., et al. (2021). Randomized trial of Interleukin-6 receptor inhibition in patients with acute ST-Segment elevation myocardial infarction. J. Am. Coll. Cardiol. 77, 1845–1855. doi:10.1016/j.jacc.2021.02.049
Canbaz, S., Duran, E., Ege, T., Sunar, H., Cikirikcioglu, M., and Acipayam, M. (2003). The effects of intracoronary administration of vitamin E on myocardial ischemia-reperfusion injury during coronary artery surgery. Thorac. Cardiovasc. Surg. 51, 57–61. doi:10.1055/s-2003-38983
Carbonell, T., and Gomes, A. V. (2020). MicroRNAs in the regulation of cellular redox status and its implications in myocardial ischemia-reperfusion injury. Redox Biol. 36, 101607. doi:10.1016/j.redox.2020.101607
Cardinale, A., Saladini, S., Lupacchini, L., Ruspantini, I., De Dominicis, C., Papale, M., et al. (2022). DNA repair protein DNA-PK protects PC12 cells from oxidative stress-induced apoptosis involving AKT phosphorylation. Mol. Biol. Rep. 49, 1089–1101. doi:10.1007/s11033-021-06934-5
Casey, A. M., Ryan, D. G., Prag, H. A., Chowdhury, S. R., Marques, E., Turner, K., et al. (2025). Pro-inflammatory macrophages produce mitochondria-derived superoxide by reverse electron transport at complex I that regulates IL-1β release during NLRP3 inflammasome activation. Nat. Metab. 7, 493–507. doi:10.1038/s42255-025-01224-x
Chang, R., Han, B., Ben Mabrouk, A., and Hasegawa, U. (2024). Controlled dissociation of polymeric micelles in response to oxidative stress. Biomacromolecules 25, 1162–1170. doi:10.1021/acs.biomac.3c01156
Chen, W.-H., Chen, C.-H., Hsu, M.-C., Chang, R.-W., Wang, C.-H., and Lee, T.-S. (2024a). Advances in the molecular mechanisms of statins in regulating endothelial nitric oxide bioavailability: interlocking biology between eNOS activity and L-arginine metabolism. Biomed. Pharmacother. 171, 116192. doi:10.1016/j.biopha.2024.116192
Chen, Z., Tan, X., Jin, T., Wang, Y., Dai, L., Shen, G., et al. (2024b). Pharmaceutical manipulation of mitochondrial F0F1-ATP synthase enables imaging and protection of myocardial ischemia/reperfusion injury through stress-induced selective enrichment. Adv. Sci. 11, 2307880. doi:10.1002/advs.202307880
Chen, S., Zhu, L., Fang, X., Appiah, C., Ji, Y., Chen, Z., et al. (2025). Alloferon mitigates LPS-induced endometritis by attenuating the NLRP3/CASP1/IL-1β/IL-18 signaling Cascade. Inflammation 48, 730–746. doi:10.1007/s10753-024-02083-6
Cheng, P.-F., Yuan-He, , Ge, M.-M., Ye, D.-W., Chen, J.-P., and Wang, J.-X. (2024). Targeting the main sources of reactive oxygen species production: possible therapeutic implications in chronic pain. Curr. Neuropharmacol. 22, 1960–1985. doi:10.2174/1570159X22999231024140544
Chi, W., He, Y., Chen, S., Guo, L., Yuan, Y., Li, R., et al. (2025). Advances in research on biomaterials and stem cell/exosome-based strategies in the treatment of traumatic brain injury. Acta Pharm. Sin. B 15, 3511–3544. doi:10.1016/j.apsb.2025.05.010
Choi, H. K., Neogi, T., Stamp, L. K., Terkeltaub, R., and Dalbeth, N. (2021). Reassessing the cardiovascular safety of febuxostat: implications of the febuxostat versus allopurinol streamlined trial. Arthritis and Rheumatology 73, 721–724. doi:10.1002/art.41638
Chong, B., Jayabaskaran, J., Jauhari, S. M., Chan, S. P., Goh, R., Kueh, M. T. W., et al. (2025). Global burden of cardiovascular diseases: projections from 2025 to 2050. Eur. J. Prev. Cardiol. 32, 1001–1015. doi:10.1093/eurjpc/zwae281
Chouchani, E. T., Pell, V. R., Gaude, E., Aksentijević, D., Sundier, S. Y., Robb, E. L., et al. (2014). Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 515, 431–435. doi:10.1038/nature13909
Cox, F. F., Misiou, A., Vierkant, A., Ale-Agha, N., Grandoch, M., Haendeler, J., et al. (2022). Protective effects of curcumin in cardiovascular diseases—impact on oxidative stress and mitochondria. Cells 11, 342. doi:10.3390/cells11030342
Cui, J., Li, H., Zhang, T., Lin, F., Chen, M., Zhang, G., et al. (2025). Research progress on the mechanism of curcumin anti-oxidative stress based on signaling pathway. F. Phar 16, 1548073. doi:10.3389/fphar.2025.1548073
Cun, Y., Ding, H., Mao, T., Wang, Y., Wang, C., Li, J., et al. (2025). SITA: predicting site-specific immunogenicity for therapeutic antibodies. J. Pharm. Anal. 15, 101316. doi:10.1016/j.jpha.2025.101316
Cung, T.-T., Morel, O., Cayla, G., Rioufol, G., Garcia-Dorado, D., Angoulvant, D., et al. (2015). Cyclosporine before PCI in patients with acute myocardial infarction. N. Engl. J. Med. 373, 1021–1031. doi:10.1056/NEJMoa1505489
Dhalla, N. S., Elimban, V., Bartekova, M., and Adameova, A. (2022). Involvement of oxidative stress in the development of subcellular defects and heart disease. Biomedicines 10, 393. doi:10.3390/biomedicines10020393
Dominguez-Rodriguez, A., Abreu-Gonzalez, P., de la Torre-Hernandez, J. M., Gonzalez-Gonzalez, J., Garcia-Camarero, T., Consuegra-Sanchez, L., et al. (2017). Effect of intravenous and intracoronary melatonin as an adjunct to primary percutaneous coronary intervention for acute ST-elevation myocardial infarction: results of the melatonin adjunct in the acute myocaRdial infarction treated with angioplasty trial. J. Pineal Res. 62, e12374. doi:10.1111/jpi.12374
Doudesis, D., Lee, K. K., Boeddinghaus, J., Bularga, A., Ferry, A. V., Tuck, C., et al. (2023). Machine learning for diagnosis of myocardial infarction using cardiac troponin concentrations. Nat. Med. 29, 1201–1210. doi:10.1038/s41591-023-02325-4
Duan, D., Fan, T., Zhang, L., Li, L., Wang, H., Guo, M., et al. (2023a). The correlation between cardiac oxidative stress and inflammatory cytokine response following myocardial infarction. Clin. Appl. Thromb. Hemost. 29, 10760296231211907. doi:10.1177/10760296231211907
Duan, X., Hu, M., Yang, L., Zhang, S., Wang, B., Li, T., et al. (2023b). IRG1 prevents excessive inflammatory responses and cardiac dysfunction after myocardial injury. Biochem. Pharmacol. 213, 115614. doi:10.1016/j.bcp.2023.115614
Dunning, B. J., Bourgonje, A. R., Bulthuis, M. L. C., Alexander, J., Aaseth, J. O., Larsson, A., et al. (2023). Selenium and coenzyme Q10 improve the systemic redox status while reducing cardiovascular mortality in elderly population-based individuals. Free Radic. Biol. Med. 204, 207–214. doi:10.1016/j.freeradbiomed.2023.04.024
Duong, L. D., West, J. D., and Morano, K. A. (2024). Redox regulation of proteostasis. J. Biol. Chem. 300, 107977. doi:10.1016/j.jbc.2024.107977
Epstein, F. H., and McCord, J. M. (1985). Oxygen-derived free radicals in postischemic tissue injury. NEJM 312, 159–163. doi:10.1056/NEJM198501173120305
Eshraghi, A., Khalesi, S., Amini, K., Salleh, F. H., Sharifikia, M., Hajmiri, M. S., et al. (2025). Empagliflozin ameliorates the oxidative stress profile in type 2 diabetic patients with heart failure and reduced ejection fraction: results of a randomized, double-blind, placebo-controlled study. RRCT 20, 167–179. doi:10.2174/0115748871323540241212060946
Fang, J., Koh, J., Fang, Q., Qiu, H., Archang, M. M., Hasani-Sadrabadi, M. M., et al. (2020). Injectable drug-releasing microporous annealed particle scaffolds for treating myocardial infarction. Adv. Funct. Mat. 30, 2004307. doi:10.1002/adfm.202004307
Fasnacht, M., Gallo, S., Sharma, P., Himmelstoß, M., Limbach, P. A., Willi, J., et al. (2022). Dynamic 23S rRNA modification ho5C2501 benefits Escherichia coli under oxidative stress. Nucleic Acids Res. 50, 473–489. doi:10.1093/nar/gkab1224
Fedoreyeva, L. I. (2024). ROS as signaling molecules to initiate the process of plant acclimatization to abiotic stress. Int. J. Mol. Sci. 25, 11820. doi:10.3390/ijms252111820
Fejes, R., Pilat, N., Lutnik, M., Weisshaar, S., Weijler, A. M., Krüger, K., et al. (2024). Effects of increased nitrate intake from beetroot juice on blood markers of oxidative stress and inflammation in older adults with hypertension. Free Radic. Biol. Med. 222, 519–530. doi:10.1016/j.freeradbiomed.2024.07.004
Fujii, J., and Imai, H. (2024). Oxidative metabolism as a cause of lipid peroxidation in the execution of ferroptosis. Int. J. Mol. Sci. 25, 7544. doi:10.3390/ijms25147544
Gallo, G., Rubattu, S., and Volpe, M. (2024). Mitochondrial dysfunction in heart failure: from pathophysiological mechanisms to therapeutic opportunities. Int. J. Mol. Sci. 25, 2667. doi:10.3390/ijms25052667
Gammella, E., Recalcati, S., and Cairo, G. (2016). Dual role of ROS as signal and stress agents: iron tips the balance in favor of toxic effects. Oxid. Med. Cell. Longev. 2016, 8629024. doi:10.1155/2016/8629024
Ge, T., Ning, B., Wu, Y., Chen, X., Qi, H., Wang, H., et al. (2024). MicroRNA-specific therapeutic targets and biomarkers of apoptosis following myocardial ischemia–reperfusion injury. Mol. Cell. Biochem. 479, 2499–2521. doi:10.1007/s11010-023-04876-z
Geng, J.-X., Lu, Y.-F., Zhou, J.-N., Huang, B., and Qin, Y. (2025). Exosome technology: a novel and effective drug delivery system in the field of cancer therapy. World J. Gastrointest. Oncol. 17, 101857. doi:10.4251/wjgo.v17.i3.101857
Gong, Y., Lu, X., Wang, X., Wang, Y., Shen, Z., Gao, Y., et al. (2025). Mitochondrial tumor suppressor 1A attenuates myocardial infarction injury by maintaining the coupling between mitochondria and endoplasmic reticulum. Circulation 152, 183–201. doi:10.1161/CIRCULATIONAHA.124.069737
Granger, D. N., Rutili, G., and McCord, J. M. (1981). Superoxide radicals in feline intestinal ischemia. Gastroenterology 81, 22–29. doi:10.1016/0016-5085(81)90648-X
Griffiths, E. J., and Halestrap, A. P. (1993). Protection by cyclosporin A of Ischemia/reperfusion-induced damage in isolated rat hearts. J. Mol. Cell. Cardiol. 25, 1461–1469. doi:10.1006/jmcc.1993.1162
Gualtieri, R., Kalthur, G., Barbato, V., Longobardi, S., Di Rella, F., Adiga, S. K., et al. (2021). Sperm oxidative stress during in vitro manipulation and its effects on sperm function and embryo development. Antioxidants 10, 1025. doi:10.3390/antiox10071025
Guo, Z., Tian, Y., Gao, J., Zhou, B., Zhou, X., Chang, X., et al. (2024). Enhancement of mitochondrial homeostasis: a novel approach to attenuate hypoxic myocardial injury. Int. J. Med. Sci. 21, 2897–2911. doi:10.7150/ijms.103986
Harris, D. D., Sabe, S. A., Xu, C. M., Sabra, M., Broadwin, M., Malhotra, A., et al. (2024). Sodium-glucose co-transporter 2 inhibitor canagliflozin modulates myocardial metabolism and inflammation in a swine model for chronic myocardial ischemia. Surgery 175, 265–270. doi:10.1016/j.surg.2023.09.043
He, F., Andrabi, S. M., Shi, H., Son, Y., Qiu, H., Xie, J., et al. (2025). Sequential delivery of cardioactive drugs via microcapped microneedle patches for improved heart function in post myocardial infarction rats. Acta Biomater. 192, 235–247. doi:10.1016/j.actbio.2024.12.009
Hernández-Ruiz, R. G., Olivares-Ochoa, X. C., Salinas-Varela, Y., Guajardo-Espinoza, D., Roldán-Flores, L. G., Rivera-Leon, E. A., et al. (2025). Phenolic compounds and anthocyanins in legumes and their impact on inflammation, oxidative stress, and metabolism: comprehensive review. Molecules 30, 174. doi:10.3390/molecules30010174
Hortmann, M., Robinson, S., Mohr, M., Mauler, M., Stallmann, D., Reinöhl, J., et al. (2019). The mitochondria-targeting peptide elamipretide diminishes circulating HtrA2 in ST-segment elevation myocardial infarction. Eur. Heart J. Acute Cardiovasc. Care 8, 695–702. doi:10.1177/2048872617710789
Huang, K., Que, J., Hu, Z., Yu, Y., Zhou, Y., Wang, L., et al. (2020). Metformin suppresses inflammation and apoptosis of myocardiocytes by inhibiting autophagy in a model of ischemia-reperfusion injury. Int J Bio Sci 16, 2559–2579. doi:10.7150/ijbs.40823
Iacovelli, J. J., Alpenglow, J. K., Ratchford, S. M., Craig, J. C., Simmons, J. M., Zhao, J., et al. (2024). Statin therapy improves locomotor muscle microvascular reactivity in patients with heart failure with preserved ejection fraction. Am. J. Physiol. Heart Circ. Physiol. 327, H859–H865. doi:10.1152/ajpheart.00427.2024
Iqbal, R., Akhtar, M. S., Hassan, M. Q., Jairajpuri, Z., Akhtar, M., and Najmi, A. K. (2019). Pitavastatin ameliorates myocardial damage by preventing inflammation and collagen deposition via reduced free radical generation in isoproterenol-induced cardiomyopathy. Clin. Exp. Hypertens. 41, 434–443. doi:10.1080/10641963.2018.1501059
Itoh, K., Chiba, T., Takahashi, S., Ishii, T., Igarashi, K., Katoh, Y., et al. (1997). An Nrf2/Small maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 236, 313–322. doi:10.1006/bbrc.1997.6943
Itoh, K., Wakabayashi, N., Katoh, Y., Ishii, T., Igarashi, K., Engel, J. D., et al. (1999). Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 13, 76–86. doi:10.1101/gad.13.1.76
Jaganjac, M., Milkovic, L., Zarkovic, N., and Zarkovic, K. (2022). Oxidative stress and regeneration. Free Radic. Biol. Med. 181, 154–165. doi:10.1016/j.freeradbiomed.2022.02.004
Jamthikar, A. D., Hathaway, Q. A., Maganti, K., Hamirani, Y., Bokhari, S., Yanamala, N., et al. (2025). Ultrasonic texture analysis for predicting acute myocardial infarction. JACC Cardiovasc. Imaging, S1936878X25003705. doi:10.1016/j.jcmg.2025.06.018
Ji, X., Meng, Y., Wang, Q., Tong, T., Liu, Z., Lin, J., et al. (2023). Cysteine-based redox-responsive nanoparticles for fibroblast-targeted drug delivery in the treatment of myocardial infarction. ACS Nano 17, 5421–5434. doi:10.1021/acsnano.2c10042
Jiang, J., Ni, L., Zhang, X., Gokulnath, P., Vulugundam, G., Li, G., et al. (2023). Moderate-intensity exercise maintains redox homeostasis for cardiovascular health. Adv. Biol. 7, e2200204. doi:10.1002/adbi.202200204
Jiang, Y., Jiang, R., Xia, Z., Guo, M., Fu, Y., Wang, X., et al. (2025). Engineered neutrophil membrane-camouflaged nanocomplexes for targeted siRNA delivery against myocardial ischemia reperfusion injury. J. Nanobiotechnol. 23, 134. doi:10.1186/s12951-025-03172-w
Jie, K. E., van der Putten, K., Wesseling, S., Joles, J. A., Bergevoet, M. W., Pepers-de Kort, F., et al. (2023). Short-term erythropoietin treatment does not substantially modulate monocyte transcriptomes of patients with combined heart and renal failure. Plosone 7, e41339. doi:10.1371/journal.pone.0041339
Jin, Y., Cai, D., Mo, L., Jing, G., Zeng, L., Cheng, H., et al. (2024). Multifunctional nanogel loaded with cerium oxide nanozyme and CX3CL1 protein: targeted immunomodulation and retinal protection in uveitis rat model. Biomaterials 309, 122617. doi:10.1016/j.biomaterials.2024.122617
Jomova, K., Raptova, R., Alomar, S. Y., Alwasel, S. H., Nepovimova, E., Kuca, K., et al. (2023). Reactive oxygen species, toxicity, oxidative stress, and antioxidants: chronic diseases and aging. Arch. Toxicol. 97, 2499–2574. doi:10.1007/s00204-023-03562-9
Ju, Z., Shang, Z., Mahmud, T., Fang, J., Liu, Y., Pan, Q., et al. (2023). Synthesis and anti-inflammatory activity of the natural cyclooxygenase-2 inhibitor axinelline A and its analogues. J. Nat. Prod. 86, 958–965. doi:10.1021/acs.jnatprod.2c01153
Kalogerakou, T., and Antoniadou, M. (2024). The role of dietary antioxidants, food supplements and functional foods for energy enhancement in healthcare professionals. Antioxidants 13, 1508. doi:10.3390/antiox13121508
Karaduleva, E. V., Mubarakshina, E. K., Sharapov, M. G., Volkova, A. E., Pimenov, O.Yu., Ravin, V. K., et al. (2016). Cardioprotective effect of modified peroxiredoxins in retrograde perfusion of isolated rat heart under conditions of oxidative stress. Bull. Exp. Biol. Med. 160, 639–642. doi:10.1007/s10517-016-3237-1
Korshunov, S., and Imlay, J. A. (2024). Antioxidants are ineffective at quenching reactive oxygen species inside bacteria and should not be used to diagnose oxidative stress. Mol. Microbiol. 122, 113–128. doi:10.1111/mmi.15286
Kračun, D., Lopes, L. R., Cifuentes-Pagano, E., and Pagano, P. J. (2025). NADPH oxidases: redox regulation of cell homeostasis and disease. Physiol. Rev. 105, 1291–1428. doi:10.1152/physrev.00034.2023
Krishnamurthy, H. K., Pereira, M., Rajavelu, I., Jayaraman, V., Krishna, K., Wang, T., et al. (2024). Oxidative stress: fundamentals and advances in quantification techniques. Front. Chem. 12, 1470458. doi:10.3389/fchem.2024.1470458
Kruk, A., Ząbczyk, M., Natorska, J., and Undas, A. (2025). Statin treatment reduces protein carbonylation in patients with COPD: a randomized controlled study. Eur. J. Clin. Invest. 55, e70009. doi:10.1111/eci.70009
Kumar, S., Saxena, J., Srivastava, V. K., Kaushik, S., Singh, H., Abo-El-Sooud, K., et al. (2022). The interplay of oxidative stress and ROS scavenging: antioxidants as a therapeutic potential in sepsis. Nato. Adv. Sci. Inst. Se. 10, 1575. doi:10.3390/vaccines10101575
Kusano, T., Nishino, T., Okamoto, K., Hille, R., and Nishino, T. (2023). The mechanism and significance of the conversion of xanthine dehydrogenase to xanthine oxidase in mammalian secretory gland cells. Redox Biol. 59, 102573. doi:10.1016/j.redox.2022.102573
Lauritsen, K. M., Nielsen, B. R. R., Tolbod, L. P., Johannsen, M., Hansen, J., Hansen, T. K., et al. (2021). SGLT2 inhibition does not affect myocardial fatty acid oxidation or uptake, but reduces myocardial glucose uptake and blood flow in individuals with type 2 diabetes: a randomized double-blind, placebo-controlled crossover trial. Diabetes 70, 800–808. doi:10.2337/db20-0921
Lbban, E., Macey, A., Rundle, J., Ashor, A., Idris, I., and Siervo, M. (2024). Effects of dietary nitrate and vitamin C co-ingestion on blood pressure and hand-grip strength in young adults. Int. J. Vitam. Nutr. Res. 94, 342–353. doi:10.1024/0300-9831/a000799
Lee, M., Kim, Y. S., Park, J., Choe, G., Lee, S., Kang, B. G., et al. (2024). A paintable and adhesive hydrogel cardiac patch with sustained release of ANGPTL4 for infarcted heart repair. Bioact. Mater. 31, 395–407. doi:10.1016/j.bioactmat.2023.08.020
Lee, M. S., Shin, T. G., Lee, Y., Kim, D. H., Choi, S. H., Cho, H., et al. (2025). Artificial intelligence applied to electrocardiogram to rule out acute myocardial infarction: the ROMIAE multicentre study. Eur. Heart J. 46, 1917–1929. doi:10.1093/eurheartj/ehaf004
Li, H., Zhu, J., Xu, Y., Mou, F., Shan, X., Wang, Q., et al. (2022a). Notoginsenoside R1-loaded mesoporous silica nanoparticles targeting the site of injury through inflammatory cells improves heart repair after myocardial infarction. Redox Biol. 54, 102384. doi:10.1016/j.redox.2022.102384
Li, Y., Li, S., and Wu, H. (2022b). Ubiquitination-proteasome system (UPS) and autophagy two main protein degradation machineries in response to cell stress. Cells 11, 851. doi:10.3390/cells11050851
Li, Y., Chen, T., Cheang, I., Liu, P., Zhao, L., He, X., et al. (2025). Macrophage A2aR alleviates LPS-induced vascular endothelial injury and inflammation via inhibiting M1Polarisation and oxidative stress. J. Cell. Mol. Med. 29, e70458. doi:10.1111/jcmm.70458
Liang, M., Li, W.-K., Xie, X.-X., Lai, B.-C., Zhao, J.-J., Yu, K.-W., et al. (2025). OAS1 induces endothelial dysfunction and promotes monocyte adhesion through the NFκB pathway in atherosclerosis. Arch. Biochem. Biophys. 763, 110222. doi:10.1016/j.abb.2024.110222
Liu, Y., Zhang, Z., Li, W., and Tian, S. (2020). PECAM1 combines with CXCR4 to trigger inflammatory cell infiltration and pulpitis progression through activating the NF-κB signaling pathway. Front. Cell Dev. Biol. 8, 593653. doi:10.3389/fcell.2020.593653
Liu, L., Xia, G., Li, P., Wang, Y., and Zhao, Q. (2021a). Sirt-1 regulates physiological process and exerts protective effects against oxidative stress. Biomed. Res. Int. 2021, 5542545. doi:10.1155/2021/5542545
Liu, N., Xu, H., Sun, Q., Yu, X., Chen, W., Wei, H., et al. (2021b). The role of oxidative stress in hyperuricemia and xanthine oxidoreductase (XOR) inhibitors. Oxid. Med. Cell. Longev. 2021, 1470380. doi:10.1155/2021/1470380
Liu, P., Li, J., Liu, M., Zhang, M., Xue, Y., Zhang, Y., et al. (2021c). Hesperetin modulates the Sirt1/Nrf2 signaling pathway in counteracting myocardial ischemia through suppression of oxidative stress, inflammation, and apoptosis. Biomed. Pharmacother. 139, 111552. doi:10.1016/j.biopha.2021.111552
Loffredo, L., and Carnevale, R. (2024). Oxidative stress: the hidden catalyst fueling atherosclerosis and cardiovascular disease. Antioxidants 13, 1089. doi:10.3390/antiox13091089
Lonn, E., Bosch, J., Yusuf, S., Sheridan, P., Pogue, J., Arnold, J. M. O., et al. (2005). Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. JAMA 293, 1338–1347. doi:10.1001/jama.293.11.1338
Lu, H., Gong, J., Zhang, T., Jiang, Z., Dong, W., Dai, J., et al. (2023). Leonurine pretreatment protects the heart from myocardial ischemia-reperfusion injury. Exp. Biol. Med. 248, 1566–1578. doi:10.1177/15353702231198066
Lucci, C., Marongiu, A., Genovese, S., Mazza, M., Cosentino, N., and Marenzi, G. (2025). Hypoglycemia and glycemic variability in acute myocardial infarction: the lesser-known aspects of glycemic control. Cardiovasc. Diabetol. 24, 309. doi:10.1186/s12933-025-02862-x
Luis-García, E. R., Becerril, C., Salgado-Aguayo, A., Aparicio-Trejo, O. E., Romero, Y., Flores-Soto, E., et al. (2021). Mitochondrial dysfunction and alterations in mitochondrial permeability transition pore (mPTP) contribute to apoptosis resistance in idiopathic pulmonary fibrosis fibroblasts. Int. J. Mol. Sci. 22, 7870. doi:10.3390/ijms22157870
Luo, Q., Sun, W., Li, Z., Sun, J., Xiao, Y., Zhang, J., et al. (2023). Biomaterials-mediated targeted therapeutics of myocardial ischemia-reperfusion injury. Biomaterials 303, 122368. doi:10.1016/j.biomaterials.2023.122368
Lv, J., Pan, C., Cai, Y., Han, X., Wang, C., Ma, J., et al. (2024). Plasma metabolomics reveals the shared and distinct metabolic disturbances associated with cardiovascular events in coronary artery disease. Nat. Commun. 15, 5729. doi:10.1038/s41467-024-50125-2
Ma, Z., Li, M., Guo, R., Tian, Y., Zheng, Y., Huang, B., et al. (2025). Treating myocardial infarction via a nano-ultrasonic contrast agent-mediated high-efficiency drug delivery system targeting macrophages. Sci. Adv. 11, eadp7126. doi:10.1126/sciadv.adp7126
Mahdiani, S., Omidkhoda, N., Rezaee, R., Heidari, S., and Karimi, G. (2022). Induction of JAK2/STAT3 pathway contributes to protective effects of different therapeutics against myocardial ischemia/reperfusion. Biomed. Pharmacother. 155, 113751. doi:10.1016/j.biopha.2022.113751
Makhlin, I., Demissei, B. G., D’Agostino, R., Hundley, W. G., Baleanu-Gogonea, C., Wilcox, N. S., et al. (2024). Statins do not significantly affect oxidative nitrosative stress biomarkers in the PREVENT randomized clinical trial. Clin. Cancer Res. 30, 2370–2376. doi:10.1158/1078-0432.CCR-23-3952
Miller, R. J. H., Manral, N., Lin, A., Shanbhag, A., Park, C., Kwiecinski, J., et al. (2024). Patient-specific myocardial infarction risk thresholds from AI-enabled coronary plaque analysis. Circ-cardiovasc. Imag. 17, e016958. doi:10.1161/CIRCIMAGING.124.016958
Minamino, T., Higo, S., Araki, R., Hikoso, S., Nakatani, D., Suzuki, H., et al. (2018). Low-dose erythropoietin in patients with ST-segment elevation myocardial infarction (EPO-AMI-II)― A randomized controlled clinical trial. Circ. J. 82, 1083–1091. doi:10.1253/circj.CJ-17-0889
Mohd Sabri, N. A., Lee, S.-K., Murugan, D. D., and Ling, W. C. (2022). Epigallocatechin gallate (EGCG) alleviates vascular dysfunction in angiotensin II-infused hypertensive mice by modulating oxidative stress and eNOS. Sci. Rep. 12, 17633. doi:10.1038/s41598-022-21107-5
Mortensen, S. A., Rosenfeldt, F., Kumar, A., Dolliner, P., Filipiak, K. J., Pella, D., et al. (2014). The effect of coenzyme Q10 on morbidity and mortality in chronic heart failure: results from Q-SYMBIO: a randomized double-blind trial. JACC Heart Fail. 2, 641–649. doi:10.1016/j.jchf.2014.06.008
Mun, D., Kang, J.-Y., Kim, H., Yun, N., and Joung, B. (2024). Small extracellular vesicle-mediated CRISPR-Cas9 RNP delivery for cardiac-specific genome editing. J. Control. Release 370, 798–810. doi:10.1016/j.jconrel.2024.05.023
Munoz, F. M., Zhang, F., Islas-Robles, A., Lau, S. S., and Monks, T. J. (2017). From the cover: ROS-induced store-operated Ca2+ entry coupled to PARP-1 hyperactivation is independent of PARG activity in necrotic cell death. Toxicol. Sci. 158, 444–453. doi:10.1093/toxsci/kfx106
Nantsupawat, T., Apaijai, N., Phrommintikul, A., Prasertwitayakij, N., Chattipakorn, S. C., Chattipakorn, N., et al. (2024). Effects of sodium-glucose cotransporter-2 inhibitor on atrial high-rate episodes in patients with cardiovascular implantable electronic device: a randomized controlled trial. Sci. Rep. 14, 27649. doi:10.1038/s41598-024-74631-x
Nezu, M., and Suzuki, N. (2021). Nrf2 activation for kidney disease treatment—a mixed blessing? Kidney Int. 99, 20–22. doi:10.1016/j.kint.2020.08.033
Nguyen, M. H., Le, N. T. H., Nguyen, B. Q. H., Nguyen, M. T. T., Do, T. N. V., Le, T. H., et al. (2025). In vitro and in silico hybrid approach to unveil triterpenoids from Helicteres hirsuta leaves as potential compounds for inhibiting Nrf2. RSC Adv. 15, 1915–1923. doi:10.1039/D4RA07646J
Nidorf, S. M., Fiolet, A. T. L., Mosterd, A., Eikelboom, J. W., Schut, A., Opstal, T. S. J., et al. (2020). Colchicine in patients with chronic coronary disease. NEJM 383, 1838–1847. doi:10.1056/NEJMoa2021372
Orhan, G., Yapici, N., Yuksel, M., Sargin, M., Şenay, Ş., Yalçin, A. S., et al. (2006). Effects of N-acetylcysteine on myocardial ischemia–reperfusion injury in bypass surgery. Heart Vessels 21, 42–47. doi:10.1007/s00380-005-0873-1
O’Donoghue, M. L., Braunwald, E., White, H. D., Steen, D. P., Lukas, M. A., Tarka, E., et al. (2014). Effect of darapladib on major coronary events after an acute coronary syndrome: the SOLID-TIMI 52 randomized clinical trial. JAMA 312, 1006–1015. doi:10.1001/jama.2014.11061
Pasupathy, S., Tavella, R., Grover, S., Raman, B., Procter, N. E. K., Du, Y. T., et al. (2017). Early use of N-acetylcysteine with nitrate therapy in patients undergoing primary percutaneous coronary intervention for st-segment–elevation myocardial infarction reduces myocardial infarct size (the NACIAM Trial [N-acetylcysteine in acute myocardial infarction]). Circulation 136, 894–903. doi:10.1161/CIRCULATIONAHA.117.027575
Pham, L. T. T., Mangmool, S., and Parichatikanond, W. (2024). Sodium-glucose cotransporter 2 (SGLT2) inhibitors: guardians against mitochondrial dysfunction and endoplasmic reticulum stress in heart diseases. ACS Pharmacol. Transl. Sci. 7, 3279–3298. doi:10.1021/acsptsci.4c00240
Piot, C., Croisille, P., Staat, P., Thibault, H., Rioufol, G., Mewton, N., et al. (2008). Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N. Engl. J. Med. 25, 473–481. doi:10.1056/NEJMoa071142
Preda, A., Montecucco, F., Carbone, F., Camici, G. G., Lüscher, T. F., Kraler, S., et al. (2024). SGLT2 inhibitors: from glucose-lowering to cardiovascular benefits. Cardiovasc. Res. 120, 443–460. doi:10.1093/cvr/cvae047
Quarato, G., Llambi, F., Guy, C. S., Min, J., Actis, M., Sun, H., et al. (2022). Ca2+-mediated mitochondrial inner membrane permeabilization induces cell death independently of Bax and Bak. Cell Death Differ. 29, 1318–1334. doi:10.1038/s41418-022-01025-9
Ridker, P. M., Everett, B. M., Thuren, T., MacFadyen, J. G., Chang, W. H., Ballantyne, C., et al. (2017). Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 377, 1119–1131. doi:10.1056/NEJMoa1707914
Ridker, P. M., Everett, B. M., Pradhan, A., MacFadyen, J. G., Solomon, D. H., Zaharris, E., et al. (2019). Low-dose methotrexate for the prevention of atherosclerotic events. NEJM 380, 752–762. doi:10.1056/NEJMoa1809798
Rodrigo, R., Hasson, D., Prieto, J. C., Dussaillant, G., Ramos, C., León, L., et al. (2014). The effectiveness of antioxidant vitamins C and E in reducing myocardial infarct size in patients subjected to percutaneous coronary angioplasty (PREVEC trial): study protocol for a pilot randomized double-blind controlled trial. Trials 15, 192. doi:10.1186/1745-6215-15-192
Romo-González, M., Ijurko, C., Alonso, M. T., Gómez de Cedrón, M., Ramirez de Molina, A., Soriano, M. E., et al. (2023). NOX2 and NOX4 control mitochondrial function in chronic myeloid leukaemia. Free Radic. Biol. Med. 198, 92–108. doi:10.1016/j.freeradbiomed.2023.02.005
Rosenblatt, K. P., Zhang, Z., Doss, R., Gurnani, P. P., Grobman, W. A., Silver, R. M., et al. (2025). A multisite study to develop and validate first trimester, circulating microparticle biomarkers for tiered risk stratification of spontaneous preterm birth in nulliparas. Am. J. Obstet. Gynecol. 232, 319.e1–319.e21. doi:10.1016/j.ajog.2024.05.032
Ruan, W., Li, T., Bang, I. H., Lee, J., Deng, W., Ma, X., et al. (2025). BMAL1–HIF2A heterodimer modulates circadian variations of myocardial injury. Nature 641, 1017–1028. doi:10.1038/s41586-025-08898-z
Sayed, A., Michos, E. D., Navar, A. M., Virani, S. S., Brewer, L. C., and Manson, J. E. (2025). Global sociodemographic disparities in ischemic heart disease mortality according to sex, 1980 to 2021. Circ Cardiovasc. Qual. Outcomes 18, e011648. doi:10.1161/CIRCOUTCOMES.124.011648
Seixas, A. F., Quendera, A. P., Sousa, J. P., Silva, A. F. Q., Arraiano, C. M., and Andrade, J. M. (2021). Bacterial response to oxidative stress and RNA oxidation. Front. Genet. 12, 821535. doi:10.3389/fgene.2021.821535
Sen, B., Benoit, B., and Brand, M. D. (2024). Hypoxia decreases mitochondrial ROS production in cells. Free Radic. Biol. Med. 224, 1–8. doi:10.1016/j.freeradbiomed.2024.08.016
Shahcheraghi, S. H., Salemi, F., Small, S., Syed, S., Salari, F., Alam, W., et al. (2023). Resveratrol regulates inflammation and improves oxidative stress via Nrf2 signaling pathway: therapeutic and biotechnological prospects. Phytother. Res. 37, 1590–1605. doi:10.1002/ptr.7754
Sharma, V., Manhas, A., Gupta, S., Dikshit, M., Jagavelu, K., and Verma, R. S. (2022). Fabrication, characterization and in vivo assessment of cardiogel loaded chitosan patch for myocardial regeneration. Int. J. Biol. Macromol. 222, 3045–3056. doi:10.1016/j.ijbiomac.2022.10.079
Shen, Y., Liu, X., Shi, J., and Wu, X. (2019). Involvement of Nrf2 in myocardial ischemia and reperfusion injury. Int. J. Biol. Macromol. 125, 496–502. doi:10.1016/j.ijbiomac.2018.11.190
Shi, H., Huang, Z., Xu, T., Sun, A., and Ge, J. (2022). New diagnostic and therapeutic strategies for myocardial infarction via nanomaterials. eBioMedicine 78, 103968. doi:10.1016/j.ebiom.2022.103968
Shigiyama, F., Kumashiro, N., Miyagi, M., Ikehara, K., Kanda, E., Uchino, H., et al. (2017). Effectiveness of dapagliflozin on vascular endothelial function and glycemic control in patients with early-stage type 2 diabetes mellitus: DEFENCE study. Cardiovasc. Diabetol. 16, 84. doi:10.1186/s12933-017-0564-0
Song, G., Liu, X., Lu, Z., Guan, J., Chen, X., Li, Y., et al. (2025). Relationship between stress hyperglycaemic ratio (SHR) and critical illness: a systematic review. Cardiovasc. Diabetol. 24, 188. doi:10.1186/s12933-025-02751-3
Sposito, A. C., Breder, I., Soares, A. A. S., Kimura-Medorima, S. T., Munhoz, D. B., Cintra, R. M. R., et al. (2021). Dapagliflozin effect on endothelial dysfunction in diabetic patients with atherosclerotic disease: a randomized active-controlled trial. Cardiovasc. Diabetol. 20, 74. doi:10.1186/s12933-021-01264-z
Sztolsztener, K., Hodun, K., and Chabowski, A. (2022). α-lipoic acid ameliorates inflammation state and oxidative stress by reducing the content of bioactive lipid derivatives in the left ventricle of rats fed a high-fat diet. Biochimica Biophysica Acta (BBA) - Mol. Basis Dis. 1868, 166440. doi:10.1016/j.bbadis.2022.166440
Tardif, J.-C., Kouz, S., Waters, D. D., Bertrand, O. F., Diaz, R., Maggioni, A. P., et al. (2019). Efficacy and safety of low-dose colchicine after myocardial infarction. N. Engl. J. Med. 381, 2497–2505. doi:10.1056/NEJMoa1912388
Theruvath, T. P., and Lemasters, J. J. (2008). Cyclosporine in acute myocardial infarction. NEJM 359, 2286–2289. doi:10.1056/NEJMc081824
Tonu, S. H., Dewan, J. F., Akhter, N., Haque, M. E., and Wahab, M. A. (2019). Antioxidatant effect of atorvastatin and rosuvastatin in hyperlipidemic patients: a randomized controlled trial. J. Armed Forces Med. Coll. 14, 54–58. doi:10.3329/jafmc.v14i1.42723
Toprak, B., Solleder, H., Di Carluccio, E., Greenslade, J. H., Parsonage, W. A., Schulz, K., et al. (2024). Diagnostic accuracy of a machine learning algorithm using point-of-care high-sensitivity cardiac troponin I for rapid rule-out of myocardial infarction: a retrospective study. Lancet Digital Health 6, e729–e738. doi:10.1016/S2589-7500(24)00191-2
Tsujita, K., Shimomura, H., Kaikita, K., Kawano, H., Hokamaki, J., Nagayoshi, Y., et al. (2006). Long-term efficacy of edaravone in patients with acute myocardial infarction. Circ. J. 70, 832–837. doi:10.1253/circj.70.832
Turner, K. D., Kronemberger, A., Bae, D., Bock, J. M., Hughes, W. E., Ueda, K., et al. (2022). Effects of combined inorganic nitrate and nitrite supplementation on cardiorespiratory fitness and skeletal muscle oxidative capacity in type 2 diabetes: a pilot randomized controlled trial. Nutrients 14, 4479. doi:10.3390/nu14214479
Usman, M. S., Bhatt, D. L., Hameed, I., Anker, S. D., Cheng, A. Y. Y., Hernandez, A. F., et al. (2024). Effect of SGLT2 inhibitors on heart failure outcomes and cardiovascular death across the cardiometabolic disease spectrum: a systematic review and meta-analysis. Lancet Diabetes and Endocrinol. 12, 447–461. doi:10.1016/S2213-8587(24)00102-5
Vornic, I., Buciu, V., Furau, C. G., Gaje, P. N., Ceausu, R. A., Dumitru, C.-S., et al. (2024). Oxidative stress and placental pathogenesis: a contemporary overview of potential biomarkers and emerging therapeutics. Int. J. Mol. Sci. 25, 12195. doi:10.3390/ijms252212195
Vukašinović, A., Klisic, A., Ostanek, B., Kafedžić, S., Zdravković, M., Ilić, I., et al. (2023). Redox status and telomere–telomerase system biomarkers in patients with acute myocardial infarction using a principal component analysis: is there a link? Int. J. Mol. Sci. 24, 14308. doi:10.3390/ijms241814308
Wang, Z.-H., Liu, J.-L., Wu, L., Yu, Z., and Yang, H.-T. (2014). Concentration-dependent wrestling between detrimental and protective effects of H2O2 during myocardial ischemia/reperfusion. Cell Death Dis. 5, e1297. doi:10.1038/cddis.2014.267
Wang, W., Zheng, Y., Wang, M., Yan, M., Jiang, J., and Li, Z. (2019). Exosomes derived miR-126 attenuates oxidative stress and apoptosis from ischemia and reperfusion injury by targeting ERRFI1. Gene 690, 75–80. doi:10.1016/j.gene.2018.12.044
Wang, Z., Yao, M., Jiang, L., Wang, L., Yang, Y., Wang, Q., et al. (2022). Dexmedetomidine attenuates myocardial ischemia/reperfusion-induced ferroptosis via AMPK/GSK-3β/Nrf2 axis. Biomed. Pharmacother. 154, 113572. doi:10.1016/j.biopha.2022.113572
Wang, R., Dong, S., Xia, R., Sun, M., Sun, Y., Ren, H., et al. (2023a). Kinsenoside mitigates myocardial ischemia/reperfusion-induced ferroptosis via activation of the Akt/Nrf2/HO-1 pathway. Eur. J. Pharmacol. 956, 175985. doi:10.1016/j.ejphar.2023.175985
Wang, S., Chen, K., Wang, Y., Wang, Z., Li, Z., Guo, J., et al. (2023b). Cardiac-targeted delivery of nuclear receptor RORα via ultrasound targeted microbubble destruction optimizes the benefits of regular dose of melatonin on sepsis-induced cardiomyopathy. Biomater. Res. 27, 41. doi:10.1186/s40824-023-00377-8
Wang, T., Dong, Y., Huang, Z., Zhang, G., Zhao, Y., Yao, H., et al. (2023c). Antioxidants stimulate BACH1-dependent tumor angiogenesis. J. Clin. Invest. 133, e169671. doi:10.1172/JCI169671
Wolf, P., Schoeniger, A., and Edlich, F. (2022). Pro-apoptotic complexes of BAX and BAK on the outer mitochondrial membrane. Biochimica Biophysica Acta (BBA) - Mol. Cell Res. 1869, 119317. doi:10.1016/j.bbamcr.2022.119317
Wu, L., Li, Z., and Yao, Y. (2023). Hydrogen peroxide preconditioning is of dual role in cardiac ischemia/reperfusion. Eur. J. Pharmacol. 947, 175684. doi:10.1016/j.ejphar.2023.175684
Wu, H.-P., Yang, F.-C., Lin, H.-D., Cai, C.-Z., Chuang, M.-J., Chiang, K. F., et al. (2024). Association between statin therapy and long-term clinical outcomes in patients with stable coronary disease undergoing percutaneous coronary intervention. Sci. Rep. 14, 12674. doi:10.1038/s41598-024-63598-4
Xiang, M., Lu, Y., Xin, L., Gao, J., Shang, C., Jiang, Z., et al. (2021). Role of oxidative stress in reperfusion following myocardial ischemia and its treatments. Oxid. Med. Cell. Longev. 2021, 6614009. doi:10.1155/2021/6614009
Xiong, J., Yu, D., Li, W., and Wang, X. (2021). Rhein activates janus protein tyrosine Kinase2/Signal transducer and transcription activation Factor3 reduces mitochondrial oxidative damage caused by myocardial ischemia/reperfusion Injury. J. Biomater. Tissue Eng. 11, 89–98. doi:10.1166/jbt.2021.2525
Xu, J., Qin, X., Cai, X., Yang, L., Xing, Y., Li, J., et al. (2015). Mitochondrial JNK activation triggers autophagy and apoptosis and aggravates myocardial injury following ischemia/reperfusion. Biochimica Biophysica Acta (BBA) - Mol. Basis Dis. 1852, 262–270. doi:10.1016/j.bbadis.2014.05.012
Xu, S., Wu, B., Zhong, B., Lin, L., Ding, Y., Jin, X., et al. (2021). Naringenin alleviates myocardial ischemia/reperfusion injury by regulating the nuclear factor-erythroid factor 2-related factor 2 (Nrf2)/System xc-/glutathione peroxidase 4 (GPX4) axis to inhibit ferroptosis. Bioengineered 12, 10924–10934. doi:10.1080/21655979.2021.1995994
Xu, H., Chen, Y., Xie, P., Lei, T., Liu, K., Liu, X., et al. (2024). Remimazolam attenuates myocardial ischemia-reperfusion injury by inhibiting the NF-ĸB pathway of macrophage inflammation. Eur. J. Pharmacol. 965, 176276. doi:10.1016/j.ejphar.2023.176276
Xue, Y., Jin, W., Xue, Y., Zhang, Y., Wang, H., Zhang, Y., et al. (2020). Safranal, an active constituent of saffron, ameliorates myocardial ischemia via reduction of oxidative stress and regulation of Ca2+ homeostasis. J. Pharmacol. Sci. 143, 156–164. doi:10.1016/j.jphs.2020.03.005
Yazdi, A., Shirmohammadi, K., Parvaneh, E., Entezari-Maleki, T., Hosseini, S. K., Ranjbar, A., et al. (2023). Effects of coenzyme Q10 supplementation on oxidative stress biomarkers following reperfusion in STEMI patients undergoing primary percutaneous coronary intervention. J. Cardiovasc Thorac. Res. 15, 250–261. doi:10.34172/jcvtr.2023.31817
Ye, Y., Xie, X., Bi, Y., Liu, Q., Weng, X., Qiu, L., et al. (2024). Naoqing formula alleviates acute ischaemic stroke-induced ferroptosis via activating Nrf2/xCT/GPX4 pathway. F. Phar 15, 1525456. doi:10.3389/fphar.2024.1525456
Yu, Y., Yan, Y., Niu, F., Wang, Y., Chen, X., Su, G., et al. (2021). Ferroptosis: a cell death connecting oxidative stress, inflammation and cardiovascular diseases. Cell Death Discov. 7, 193. doi:10.1038/s41420-021-00579-w
Yuan, L., Dai, X., Fu, H., Sui, D., Lin, L., Yang, L., et al. (2018). Vaspin protects rats against myocardial ischemia/reperfusion injury (MIRI) through the TLR4/NF-κB signaling pathway. Eur. J. Pharmacol. 835, 132–139. doi:10.1016/j.ejphar.2018.07.052
Yusuf, J. A., Akanbi, S. T., Olorunlowu, D. R., Opoola, E. K., Ogunlade, E. E., Kayode, E. A., et al. (2025). Molecular mechanism underlying stress response and adaptation. Prog. Brain Res. 291, 81–108. doi:10.1016/bs.pbr.2025.01.005
Zhang, H., Liu, Y., Cao, X., Wang, W., Cui, X., Yang, X., et al. (2021a). Nrf2 promotes inflammation in early myocardial ischemia-reperfusion via recruitment and activation of macrophages. Front. Immunol. 12, 763760. doi:10.3389/fimmu.2021.763760
Zhang, J., Xue, X., Qiao, Y., Li, D., Wei, Q., Zhang, F., et al. (2021b). Astragaloside IV extends Lifespan of Caenorhabditis elegansby improving age-related functional declines and triggering antioxidant responses. Rejuvenation Res. 24, 120–130. doi:10.1089/rej.2020.2312
Zhang, C., Lan, X., Wang, Q., Zheng, Y., Cheng, J., Han, J., et al. (2025). Decoding ischemic stroke: perspectives on the endoplasmic reticulum, mitochondria, and their crosstalk. Redox Biol. 82, 103622. doi:10.1016/j.redox.2025.103622
Zhou, X., Qu, Y., Gan, G., Zhu, S., Huang, Y., Liu, Y., et al. (2020). Cyclosporine A plus ischemic postconditioning improves neurological function in rats after cardiac resuscitation. Neurocrit. Care 32, 812–821. doi:10.1007/s12028-019-00849-7
Zhu, D., Hou, J., Qian, M., Jin, D., Hao, T., Pan, Y., et al. (2021). Nitrate-functionalized patch confers cardioprotection and improves heart repair after myocardial infarction via local nitric oxide delivery. Nat. Commun. 12, 4501. doi:10.1038/s41467-021-24804-3
Zou, L., Zhu, D., Gong, M., and Yu, J. (2024). The influence of genetic predisposition to oxidative stress on painful diabetic peripheral neuropathy: a Mendelian randomization study. Cell. Mol. Biol. 70, 168–173. doi:10.14715/cmb/2024.70.3.25
Keywords: myocardial ischemia, oxidative stress, ischemia-reperfusion injury, endogenous antioxidant defense, translational challenges, redox signaling, therapeutic strategies, precision medicine
Citation: Chen H, Tang Y, Ren P and Wu W (2025) The unmet promise: a critical review of antioxidant strategies in myocardial ischemia-reperfusion injury and the path towards precision medicine. Front. Pharmacol. 16:1693441. doi: 10.3389/fphar.2025.1693441
Received: 27 August 2025; Accepted: 23 October 2025;
Published: 10 November 2025.
Edited by:
Guoliang Meng, Nantong University, ChinaReviewed by:
Sini Sunny, University of Alabama at Birmingham, United StatesLan Wu, Shanghai University of Medicine and Health Sciences, China
Maria Aslam, University of Lahore, Pakistan
Copyright © 2025 Chen, Tang, Ren and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenbin Wu, d3diMTIwMUB2aXAuc2luYS5jb20=
†These authors have contributed equally to this work
 Hao Chen
Hao Chen Yutao Tang
Yutao Tang Pan Ren
Pan Ren Wenbin Wu
Wenbin Wu