- 1Department of Pharmacy, Universitas Muhammadiyah A.R. Fachruddin, Tangerang, Banten, Indonesia
- 2Department of Pharmacy, Sekolah Tinggi Ilmu Kesehatan ISFI Banjarmasin, Banjarmasin, Indonesia
- 3Deraya Center for Scientific Research, Deraya University, New Minia, Egypt
- 4Data Science, Evidence-Based and Clinical Research Laboratory, Department of Health, Social, and Clinical Pharmacy, College of Pharmacy, Chung-Ang University, Seoul, Republic of Korea
- 5Jurusan Keperawatan, Fakultas Kedokteran dan Ilmu Kesehatan, Universitas Lambung Mangkurat, Banjarmasin, Indonesia
- 6Guangzhou HC Pharmaceutical Co., Ltd, Guangzhou, China
- 7Master Pharmaceutical of Science, Faculty of Pharmacy, Universitas Gadjah Mada, Yogyakarta, Indonesia
1 Introduction
In 2020, breast cancer was estimated to reach 2.3 million cases (new cases), with the impact of deaths occurring in the same year of 685,000 (Arnold et al., 2022). The total number of new cancer cases in women is approximately 9.2 million. Breast cancer accounts for approximately 2.3 million cases, representing approximately 24%–25% of all cancers in women. Breast cancer cases with high incidence rates are observed in Australia/New Zealand, Western Europe, North America, Central/South Asia, and parts of Africa. The highest breast cancer mortality rates are observed in Melanesia, Africa, and the Caribbean. New cases of breast cancer are expected to continue to increase, with a significant increase anticipated by 2050, resulting in a 38% increase (3.2 million new breast cancer cases) and causing 1.1 million breast cancer-related deaths annually (Kim et al., 2025).
Chemotherapy is the first-line treatment for breast cancer (Wang and Wu, 2023). Treatment regimens using this class of drugs have a better response profile and can prevent breast cancer (Gao et al., 2020). Treatment regimens using this class of drugs have a better response profile and can prevent breast cancer development (Davodabadi et al., 2023; Gu et al., 2025). A patient’s quality of life is affected by the side effects of chemotherapy, including neurotoxicity, gastrointestinal disturbances, cardiotoxicity, and immunosuppression (Brianna and Lee, 2023; Davodabadi et al., 2023; Kuderer et al., 2022; Was et al., 2022). However, the problem of chemotherapy drug toxicity remains a challenge, with suboptimal drug concentrations at the tumor site and significant systemic toxicity (Davodabadi et al., 2023; Raavé et al., 2018). Furthermore, the discovery and development of methods to reduce the limitations of breast cancer drugs require large investments and time (McIntosh et al., 2023; Michaeli et al., 2024). One solution to overcome this problem is to provide breast cancer drug repurposing that is effective, has minimal toxicity, and has low research costs.
A potential source of drugs that can be used as anti-breast cancer agents is bacterial secondary metabolites, such as S. peucetius var. Caesius, which produces doxorubicin (Bano et al., 2024; Rawat et al., 2021). Secondary metabolites derived from bacteria and their analogs have been repurposed as anti-breast cancer agents, including salinomycin, doxycycline, and tigecycline (Chen et al., 2022; Tang et al., 2017; Wang H. et al., 2021; Yu et al., 2024). Recent findings related to the therapeutic effects of antibiotics against breast cancer include those of benzimidazole derivatives and their derivatives (Lee et al., 2023). A benzimidazole derivative (BMPE) and prebiotic bacterial levan (LevAE) have been associated with triple-negative breast cancer (TNBC) in a 4T1-cell syngeneic mouse model (Shawky et al., 2025).
The high toxicity of antibiotic-derived breast cancer drugs should be addressed using drug repurposing approaches that explore other agents (Aggarwal et al., 2021; Kumbhar et al., 2022; Pfab et al., 2021). Currently, promising anticancer agents are those derived from natural depsipeptides isolated from E. terrae using an isolation chip (iChip). This technology produces new active metabolites with characteristics and activities that require further exploration (Chabib et al., 2025; Gauthier et al., 2025; Lodhi et al., 2018; Perrier et al., 2024). Antibiotics in this class include teixobactin and clovibactin, which are useful for treating infections caused by microbial resistance (Adeiza, 2024; Gunjal et al., 2020).
2 Natural depsipeptide characteristic
Natural depsipeptides, currently being developed as antibiotics, have the potential to be developed into anti-breast cancer agents. Natural depsipeptides, such as teixobactin and clovibactin, are a class of drugs that have peptide (amide) and ester bonds in their structure, particularly in their macrocyclic or linear structures. The structure of compounds in this class offers advantages, making them promising candidates for use as antibiotics against resistant bacteria owing to their ability to resist enzymatic degradation. Fragments that play a role in the structure of natural depsipeptides against enzymatic degradation are non-standard amino acids and structural motifs that have not been recognized or are not standard from existing structures. The stability of this drug against enzymatic degradation, as reported in previous studies, is strengthened by the presence of a bond between the d-amino acid and residues in the structure that possess macrocyclic properties (Chabib et al., 2025; Gunjal et al., 2020; Karas et al., 2020).
This class of drugs was first isolated from E. terrae bacteria, which were previously difficult to cultivate using traditional laboratory techniques. This class of drugs, including teixobactin and clovibactin, can only be cultured using methods known by several similar terms, including uncultured soil technology (UST), in situ incubation, and isolation chip (iChip) (Chabib et al., 2025). The presence of three d-amino acids and an l-allo-enduracididine residue distinguishes teixobactin from other natural peptides. The composition of teixobactin is believed to play a significant role in the action of this class of antibiotics against Gram-positive pathogens, such as methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococci. Its mechanism of action involves binding to lipids II and III, which affects bacterial cell wall synthesis. This mechanism causes significant bacterial cell death (Giltrap et al., 2016; Gunjal et al., 2020; Hussein et al., 2020; Karas et al., 2020).
A slightly different characteristic of teixobactin is its clovibactin compound, also known as Novo29. Clovibactin is the second compound after teixobactin that can be isolated from E. terrae using the iChip method. Clovibactin contains an eight-residue depsipeptide consisting of the rare amino acid hydroxy asparagine in a macrolactone ring. The structure of clovibactin, a macrolactone, enhances its antibacterial activity by binding to water and anions (Krumberger et al., 2023). The antibacterial activity of clovibactin against Gram-positive pathogens is similar to that of teixobactin, as it binds to the pyrophosphate groups of several essential peptidoglycan precursors (C55-PP, lipid II, and lipid III), thereby inhibiting bacterial cell wall synthesis (Adeiza, 2024; Shukla et al., 2023).
Modifications to natural depsipeptide compounds can enhance their antibacterial activities. Research has been conducted on teixobactin compounds, including modifications to the macrocyclic ring and N-terminal hydrophobic tail. This modification was performed by replacing l-allo-enduracidine with L-lysine. This modification can enhance the activity of these peptides against methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococci (VRE) (Morris et al., 2022; Parmar et al., 2018; Zong et al., 2019; 2018). Increased clovibactin activity was also observed in this study. Clovibactin modifications that can increase its antibacterial activity occur in the d-hydroxy asparagine fragment, followed by d-threonine, leucine, and cyclohexylalanine modifications (Adeiza, 2024; Bashir et al., 2024; Brunicardi et al., 2025; Shukla et al., 2022). Findings related to this modification can serve as the basis for modifying the structure of teixobactin or clovibactin into analogs that are effective as anti-breast cancer agents, with the aim of increasing efficacy and reducing toxicity.
3 Natural depsipeptide group as an anticancer agent
Teixobactin and clovibactin contain a cyclic tetra-depsipeptide bound to a depsipeptide, and four isoleucine residues are critical components of these compounds (Fiers et al., 2017; Matheson et al., 2019; Parmar et al., 2023). Compounds containing cyclic depsipeptides, such as Sansalvamide A exhibit anti-breast cancer activity in vitro in MDA-MB-231 cells (Trinidad-Calderón et al., 2023). These compounds inhibit histone deacetylase (HDACs) enzymes, including the Food and Drug Administration (FDA)-approved compounds romidepsin and vorinostat (El Omari et al., 2023; Lian et al., 2023; Parveen et al., 2023). Isoleucine in teixobactin is associated with anticancer activity by activating mTORC1. This activation slows cancer cell growth (Wang et al., 2023). However, these associations remain theoretical and should be approached with caution until validated by biochemical or cellular research. Further in vitro methodologies or simulations utilizing artificial intelligence may elucidate whether depsipeptides interact with HDAC or mTORC1 molecular targets.
The 13-membered macrolactone ring or cyclic tetra-depsipeptide contained in teixobactin and clovibactin has been previously studied and is closely related to their anticancer activity, including antimigratory, cytostatic, cytotoxic, and antiproliferative effects (Magpusao et al., 2010). Pharmacophores from this group can enhance antibacterial and anticancer activities by possessing amphipathic properties on hydrophilic and hydrophobic surfaces (Aghamiri et al., 2021; Gunjal et al., 2020; Shukla et al., 2022; Yang et al., 2020; 2017; 2016).
The potential of teixobactin as a breast cancer drug is supported by its good toxicity data. Teixobactin does not cause damage to human cells or eukaryotes. This mechanism is supported by data showing that teixobactin damages only lipid II-containing bacterial membranes (Matheson et al., 2019; Shukla et al., 2022). Clovibactin, which targets lipid II, has the same potential for low toxicity in both host and human cells (Sierra-Hernandez et al., 2025).
4 Future perspectives and research directions
While current research predominantly focuses on antibacterial activity, the distinctive capacity of these depsipeptides to target conserved molecular motifs and disrupt membrane-associated processes indicates their potential for anticancer applications. Numerous anticancer agents utilize analogous mechanisms, such as targeting the cell membrane integrity or biosynthetic pathways. Nevertheless, direct evidence or studies on teixobactin or clovibactin in cancer models have not been documented in the literature (Gunjal et al., 2020; Ling et al., 2015; Piddock et al., 2024; Shukla et al., 2023; 2020).
Structural modifications, including ongoing research on analogs and structure-activity relationships, may result in the development of derivatives with anticancer properties (Gunjal et al., 2020; Shukla et al., 2020). Mechanistic exploration necessitates Further studies are needed to determine whether the membrane-targeting actions of these depsipeptides can be effectively utilized against cancer cells. Drug discovery platforms, such as iChip technology and the investigation of uncultured bacteria, present new opportunities for identifying novel compounds with potential anticancer activity (Ling et al., 2015; Piddock et al., 2024).
5 AI-driven approaches
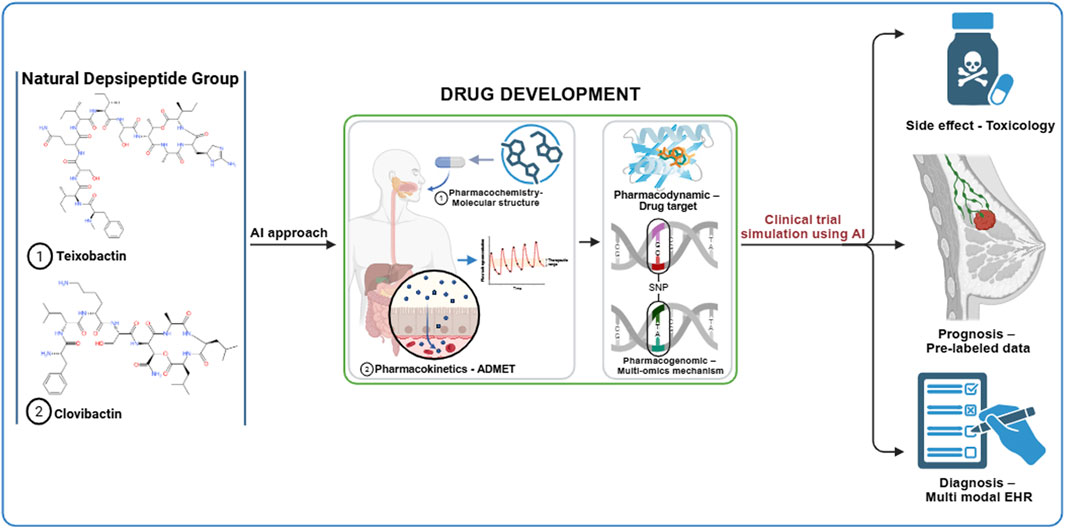
The drug repurposing of natural depsipeptide compounds, such as teixobactin and clovibactin, can be accelerated using an artificial intelligence (AI) approach at each stage of preclinical and clinical trials. The initial development of the AI approach involves creating large datasets that can be utilized for machine learning (ML) or deep learning (DL) analysis. The data required in the early stages include genomic, transcriptomic, and clinical databases. The expected results of the initial development are gene expression profiles, molecular pathways, and drug-target interactions (Bhinder et al., 2021; Issa et al., 2020; Tanoli et al., 2021; You et al., 2022; Zhou et al., 2023). Table 1 lists the AI tools relevant to the repurposing of anti-breast cancer drugs within the natural depsipeptide category. The graphical abstract outlines the AI-based drug repurposing process using natural depsipeptides as therapeutic agents for breast cancer. By combining rigorous hypothesis-driven research with advanced AI methodologies, future studies can more accurately determine whether depsipeptides, such as teixobactin and clovibactin, can evolve from antibacterial discoveries to validated anticancer applications.
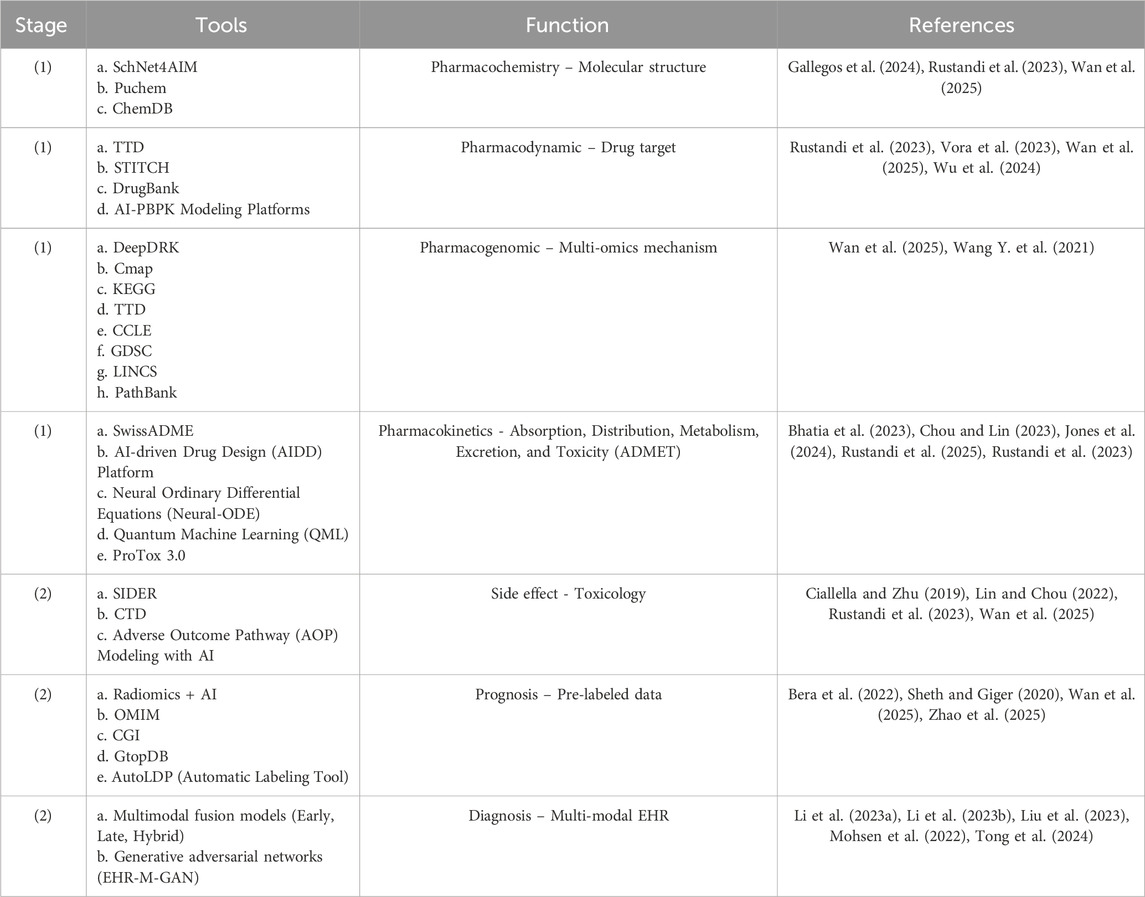
Table 1. AI drug repurposing tools for natural depsipeptides as breast cancer agents. (1) Drug development and (2) Clinical trial simulation.
6 Concluding remark
Breast cancer remains a global health challenge, with increasing incidence and mortality rates, necessitating the development of novel, effective, and low-toxicity therapeutics. The repurposing of natural depsipeptides, such as teixobactin and clovibactin, originally isolated from E. terrae using the iChip technique, offers a promising avenue for breast cancer therapy. These compounds exhibit unique structural properties, including macrocyclic depsipeptide motifs and non-standard amino acids, which confer resistance to enzymatic degradation and enhance their biological activity.
Owing to their structural similarities, teixobactin and clovibactin are suggested to have anti-breast cancer effects, potentially through the inhibition of histone deacetylases (HDAC) and modulation of the mechanistic target of rapamycin complex 1 (mTORC1) pathway. Figure 1 depicts the proposed mechanism of action of the depsipeptide group, although these conceptual relationships require experimental validation. The integration of natural product discovery, AI-driven drug repurposing, and precision oncology has positioned natural depsipeptides as a groundbreaking therapeutic approach for breast cancer. Future research should focus on translational validation and combinatorial strategies to enhance clinical effectiveness. By harnessing advanced biotechnology and computational tools, this approach has the potential to revolutionize the standard of care for patients with breast cancer worldwide.
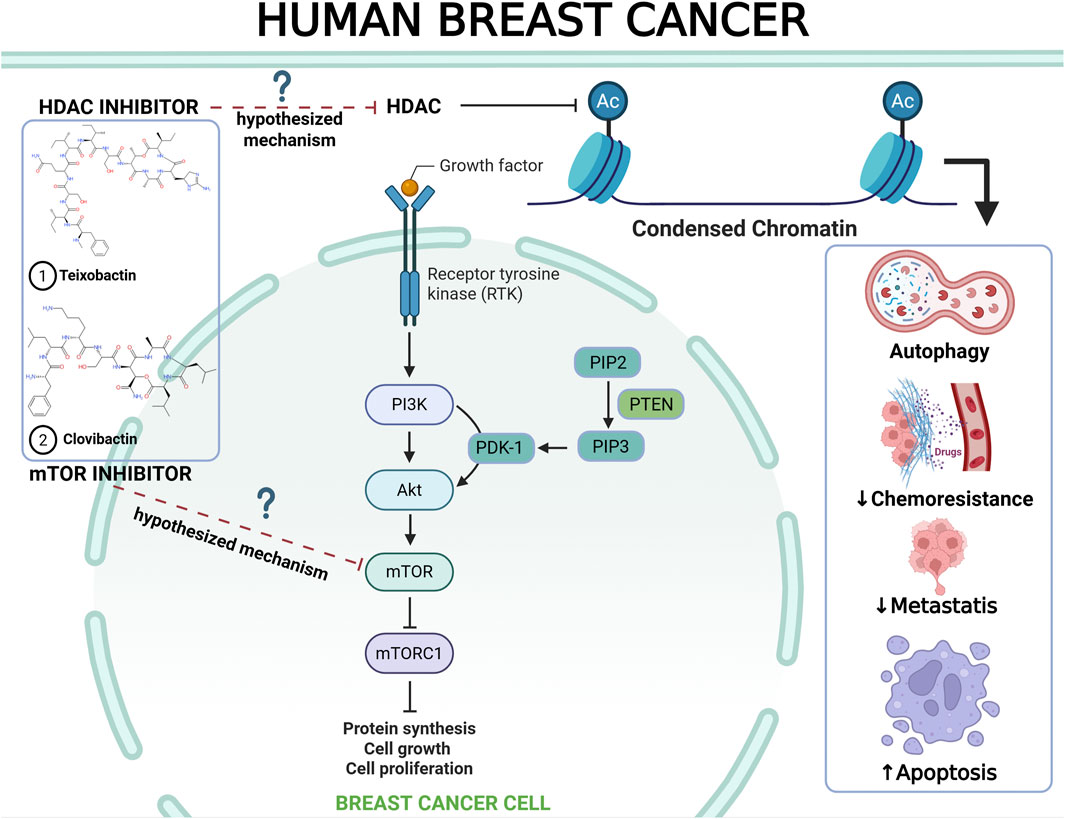
Figure 1. Proposed mechanistic hypothesis of natural depsipeptides (teixobactin and clovibactin) for anti-breast cancer therapy (Chilamakuri and Agarwal, 2022; El Omari et al., 2023; Khan et al., 2019; Parveen et al., 2023; Zucchetti et al., 2019).
7 Limitations and hypothesis context
The proposed connection between teixobactin and clovibactin and their potential anticancer effects remain speculative and require further investigation. These ideas stem from the well-documented antibacterial properties of these substances and their structural resemblance to the known HDAC inhibitors. Therefore, this hypothesis should be approached with caution until supported by direct biochemical or cellular evidence. In conclusion, the proposed anticancer mechanisms for teixobactin and clovibactin should be regarded as theoretical and model-based extrapolations derived from their structural characteristics and antibacterial actions. These initial hypotheses offer a conceptual framework to guide future mechanistic research rather than providing definitive mechanistic assertions.
Author contributions
NF: Funding acquisition, Writing – original draft. TR: Conceptualization, Methodology, Supervision, Visualization, Writing – original draft, Writing – review and editing. UA: Funding acquisition, Project administration, Resources, Writing – original draft. LR: Funding acquisition, Investigation, Writing – review and editing. Ma’sum: Funding acquisition, Investigation, Writing – review and editing. YE: Formal Analysis, Validation, Writing – review and editing. IP: Formal Analysis, Writing – review and editing. Nordin: Formal Analysis, Validation, Writing – review and editing. AM: Data curation, Software, Writing – review and editing. AR: Formal Analysis, Validation, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Publication funding was provided by the Department of Pharmacy, Universitas Muhammadiyah A.R. Fachruddin, Tigaraksa, Tangerang, Banten, Indonesia. This work was funded by the Program Hilirisasi Riset Prioritas skema SINERGI dari Direktorat Jenderal Riset dan Pengembangan (Kementerian Pendidikan Tinggi, Sains, dan Teknologi), Republic of Indonesia, under Grant: Master Contract No. 303/SPK/C.C4/PPK.DHK/IX/2025 and Supplementary Contract No. 192/LL11/KM/2025.
Acknowledgments
Umbrella project: Downstreaming of iChip and CGDT Devices at Pilot Scale to the National Biopharmaceutical Industry to Enhance the Discovery of New Antibiotics from Kalimantan Peat Soil/Hilirisasi Perangkat Ichip dan CGDT Skala Pilot ke Industri Biofarmasi Nasional Untuk Meningkatkan Penemuan Antibiotik Baru Dari Tanah Gambut Kalimantan.
Conflict of interest
Author AM was employed by Guangzhou HC Pharmaceutical Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adeiza, S. S. (2024). Clovibactin and staphylococcus aureus: a new weapon against resistant strains. GMS Hyg. Infect. Control 19, Doc46. doi:10.3205/dgkh000501
Aggarwal, S., Verma, S. S., Aggarwal, S., and Gupta, S. C. (2021). Drug repurposing for breast cancer therapy: old weapon for new battle. Semin. Cancer Biol. 68, 8–20. doi:10.1016/j.semcancer.2019.09.012
Aghamiri, S., Zandsalimi, F., Raee, P., Abdollahifar, M. A., Tan, S. C., Low, T. Y., et al. (2021). Antimicrobial peptides as potential therapeutics for breast cancer. Pharmacol. Res. 171, 105777. doi:10.1016/j.phrs.2021.105777
Arnold, M., Morgan, E., Rumgay, H., Mafra, A., Singh, D., Laversanne, M., et al. (2022). Current and future burden of breast cancer: global statistics for 2020 and 2040. Breast 66, 15–23. doi:10.1016/j.breast.2022.08.010
Bano, N., Parveen, S., Saeed, M., Siddiqui, S., Abohassan, M., and Mir, S. S. (2024). Drug repurposing of selected antibiotics: an emerging approach in cancer drug discovery. ACS Omega 9, 26762–26779. doi:10.1021/acsomega.4c00617
Bashir, A., Fattani, B., and Syed, S. H. (2024). A novel antibiotic, clovibactin, holds promise in the battle against superbugs. J. Pak Med. Assoc. 74, 1579. doi:10.47391/JPMA.11043
Bera, K., Braman, N., Gupta, A., Velcheti, V., and Madabhushi, A. (2022). Predicting cancer outcomes with radiomics and artificial intelligence in radiology. Nat. Rev. Clin. Oncol. 19, 132–146. doi:10.1038/s41571-021-00560-7
Bhatia, A. S., Saggi, M. K., and Kais, S. (2023). Quantum machine learning predicting ADME-tox properties in drug discovery. J. Chem. Inf. Model 63, 6476–6486. doi:10.1021/acs.jcim.3c01079
Bhinder, B., Gilvary, C., Madhukar, N., and Elemento, O. (2021). Artificial intelligence in cancer research and precision medicine. Cancer Discov. 11 (4), 900–915. doi:10.1158/2159-8290.CD-21-0090
Brianna, B., and Lee, S. H. (2023). Chemotherapy: how to reduce its adverse effects while maintaining the potency? Med. Oncol. 40, 88. doi:10.1007/s12032-023-01954-6
Brunicardi, J. E. H., Small, J. J., Padilla, M. S. T. L., Carrera Plancarte, J. I., and Nowick, J. S. (2025). Potent analogues of clovibactin from commercially available amino acid building blocks. J. Org. Chem. 90, 2132–2136. doi:10.1021/acs.joc.4c02828
Chabib, L., Rustandi, T., Fawwazi, M. H. A. F., Kumalasari, E., Lestari, D. A., Amalia, S. P., et al. (2025). Harnessing iChip technology for novel antibiotic discovery from peat soil microbiomes to combat antimicrobial resistance. Front. Microbiol. 16, 1530273. doi:10.3389/fmicb.2025.1530273
Chen, Y. F., Yang, Y. N., Chu, H. R., Huang, T. Y., Wang, S. H., Chen, H. Y., et al. (2022). Role of integrin αvβ3 in doxycycline-induced anti-proliferation in breast cancer cells. Front. Cell Dev. Biol. 10, 829788. doi:10.3389/fcell.2022.829788
Chilamakuri, R., and Agarwal, S. (2022). Dual targeting of PI3K and HDAC by CUDC-907 inhibits pediatric neuroblastoma growth. Cancers (Basel) 14, 1067. doi:10.3390/cancers14041067
Chou, W. C., and Lin, Z. (2023). Machine learning and artificial intelligence in physiologically based pharmacokinetic modeling. Toxicol. Sci. 191, 1–14. doi:10.1093/toxsci/kfac101
Ciallella, H. L., and Zhu, H. (2019). Advancing computational toxicology in the big data era by artificial intelligence: data-Driven and mechanism-driven modeling for chemical toxicity. Chem. Res. Toxicol. 32, 536–547. doi:10.1021/acs.chemrestox.8b00393
Davodabadi, F., Sajjadi, S. F., Sarhadi, M., Mirghasemi, S., Nadali Hezaveh, M., Khosravi, S., et al. (2023). Cancer chemotherapy resistance: mechanisms and recent breakthrough in targeted drug delivery. Eur. J. Pharmacol. 958, 176013. doi:10.1016/j.ejphar.2023.176013
El Omari, N., Lee, L. H., Bakrim, S., Makeen, H. A., Alhazmi, H. A., Mohan, S., et al. (2023). Molecular mechanistic pathways underlying the anticancer therapeutic efficiency of romidepsin. Biomed. Pharmacother. 164, 114774. doi:10.1016/j.biopha.2023.114774
Fiers, W. D., Craighead, M., and Singh, I. (2017). Teixobactin and its analogues: a new hope in antibiotic discovery. ACS Infect. Dis. 3, 688–690. doi:10.1021/acsinfecdis.7b00108
Gallegos, M., Vassilev-Galindo, V., Poltavsky, I., Martín Pendás, Á., and Tkatchenko, A. (2024). Explainable chemical artificial intelligence from accurate machine learning of real-space chemical descriptors. Nat. Commun. 15, 4345. doi:10.1038/s41467-024-48567-9
Gao, Y., Shang, Q., Li, W., Guo, W., Stojadinovic, A., Mannion, C., et al. (2020). Antibiotics for cancer treatment: a double-edged sword. J. Cancer 11, 5135–5149. doi:10.7150/jca.47470
Gauthier, L. K., Foster, A., Wagner, B. D., and Kirby, C. W. (2025). Isolation of soil microorganisms using iChip technology. J. Vis. Exp. 2025-January. doi:10.3791/67426
Giltrap, A. M., Dowman, L. J., Nagalingam, G., Ochoa, J. L., Linington, R. G., Britton, W. J., et al. (2016). Total synthesis of teixobactin. Org. Lett. 18, 2788–2791. doi:10.1021/acs.orglett.6b01324
Gu, Y., Yang, R., Zhang, Y., Guo, M., Takehiro, K., Zhan, M., et al. (2025). Molecular mechanisms and therapeutic strategies in overcoming chemotherapy resistance in cancer. Mol. Biomed. 6, 2. doi:10.1186/s43556-024-00239-2
Gunjal, V. B., Thakare, R., Chopra, S., and Reddy, D. S. (2020). Teixobactin: a paving stone toward a new class of antibiotics? J. Med. Chem. 63, 12171–12195. doi:10.1021/acs.jmedchem.0c00173
Hussein, M., Karas, J. A., Schneider-Futschik, E. K., Chen, F., Swarbrick, J., Paulin, O. K. A., et al. (2020). The killing mechanism of teixobactin against methicillin-resistant staphylococcus aureus: an untargeted metabolomics study. mSystems 5, e00077-20. doi:10.1128/msystems.00077-20
Issa, N., Stathias, V., Schürer, S., and Dakshanamurthy, S. (2020). Machine and deep learning approaches for cancer drug repurposing. Semin. Cancer Biol. 68, 132–142. doi:10.1016/j.semcancer.2019.12.011
Jones, J., Clark, R. D., Lawless, M. S., Miller, D. W., and Waldman, M. (2024). The AI-driven drug design (AIDD) platform: an interactive multi-parameter optimization system integrating molecular evolution with physiologically based pharmacokinetic simulations. J. Comput. Aided Mol. Des. 38, 14. doi:10.1007/s10822-024-00552-6
Karas, J. A., Chen, F., Schneider-Futschik, E. K., Kang, Z., Hussein, M., Swarbrick, J., et al. (2020). Synthesis and structure−activity relationships of teixobactin. Ann. N. Y. Acad. Sci. 1459, 86–105. doi:10.1111/nyas.14282
Khan, M. A., Jain, V. K., Rizwanullah, M., Ahmad, J., and Jain, K. (2019). PI3K/AKT/mTOR pathway inhibitors in triple-negative breast cancer: a review on drug discovery and future challenges. Drug Discov. Today 24, 2181–2191. doi:10.1016/j.drudis.2019.09.001
Kim, J., Harper, A., McCormack, V., Sung, H., Houssami, N., Morgan, E., et al. (2025). Global patterns and trends in breast cancer incidence and mortality across 185 countries. Nat. Med. 31, 1154–1162. doi:10.1038/s41591-025-03502-3
Krumberger, M., Li, X., Kreutzer, A. G., Peoples, A. J., Nitti, A. G., Cunningham, A. M., et al. (2023). Synthesis and stereochemical determination of the peptide antibiotic Novo29. J. Org. Chem. 88, 2214–2220. doi:10.1021/acs.joc.2c02648
Kuderer, N. M., Desai, A., Lustberg, M. B., and Lyman, G. H. (2022). Mitigating acute chemotherapy-associated adverse events in patients with cancer. Nat. Rev. Clin. Oncol. 19, 681–697. doi:10.1038/s41571-022-00685-3
Kumbhar, P., Kole, K., Khadake, V., Marale, P., Manjappa, A., Nadaf, S., et al. (2022). Nanoparticulate drugs and vaccines: breakthroughs and bottlenecks of repurposing in breast cancer. J. Control. Release 349, 812–830. doi:10.1016/j.jconrel.2022.07.039
Lee, Y. T., Tan, Y. J., and Oon, C. E. (2023). Benzimidazole and its derivatives as cancer therapeutics: the potential role from traditional to precision medicine. Acta Pharm. Sin. B 13, 478–497. doi:10.1016/j.apsb.2022.09.010
Li, J., Han, X., Qin, Y., Tan, F., Chen, Y., Wang, Z., et al. (2023a). Artificial intelligence accelerates multi-modal biomedical process: a survey. Neurocomputing 558, 126720. doi:10.1016/j.neucom.2023.126720
Li, J., Cairns, B. J., Li, J., and Zhu, T. (2023b). Generating synthetic mixed-type longitudinal electronic health records for artificial intelligent applications. NPJ Digit. Med. 6, 98. doi:10.1038/s41746-023-00834-7
Lian, B., Chen, X., and Shen, K. (2023). Inhibition of histone deacetylases attenuates tumor progression and improves immunotherapy in breast cancer. Front. Immunol. 14, 1164514. doi:10.3389/fimmu.2023.1164514
Lin, Z., and Chou, W. C. (2022). Machine learning and artificial intelligence in toxicological sciences. Toxicol. Sci. 189, 7–19. doi:10.1093/toxsci/kfac075
Ling, L. L., Schneider, T., Peoples, A. J., Spoering, A. L., Engels, I., Conlon, B. P., et al. (2015). A new antibiotic kills pathogens without detectable resistance. Nature 517, 455–459. doi:10.1038/nature14098
Liu, S., Liang, W., Huang, P., Chen, D., He, Q., Ning, Z., et al. (2023). Multi-modal analysis for accurate prediction of preoperative stage and indications of optimal treatment in gastric cancer. Radiol. Med. 128, 509–519. doi:10.1007/s11547-023-01625-6
Lodhi, A. F., Zhang, Y., Adil, M., and Deng, Y. (2018). Antibiotic discovery: combining isolation chip (iChip) technology and co-culture technique. Appl. Microbiol. Biotechnol. 102, 7333–7341. doi:10.1007/s00253-018-9193-0
Magpusao, A. N., Desmond, R. T., Billings, K. J., Fenteany, G., and Peczuh, M. W. (2010). Synthesis and evaluation of antimigratory and antiproliferative activities of lipid-linked [13]-macro-dilactones. Bioorg Med. Chem. Lett. 20, 5472–5476. doi:10.1016/j.bmcl.2010.07.083
Matheson, E., Jin, K., and Li, X. (2019). Establishing the structure-activity relationship of teixobactin. Chin. Chem. Lett. 30, 1468–1480. doi:10.1016/j.cclet.2019.07.004
McIntosh, S. A., Alam, F., Adams, L., Boon, I. S., Callaghan, J., Conti, I., et al. (2023). Global funding for cancer research between 2016 and 2020: a content analysis of public and philanthropic investments. Lancet Oncol. 24, 636–645. doi:10.1016/S1470-2045(23)00182-1
Michaeli, J. C., Michaeli, T., Trapani, D., Albers, S., Dannehl, D., Würstlein, R., et al. (2024). Breast cancer drugs: FDA approval, development time, efficacy, clinical benefits, innovation, trials, endpoints, quality of life, value, and price. Breast Cancer 31, 1144–1155. doi:10.1007/s12282-024-01634-x
Mohsen, F., Ali, H., El Hajj, N., and Shah, Z. (2022). Artificial intelligence-based methods for fusion of electronic health records and imaging data. Sci. Rep. 12, 17981. doi:10.1038/s41598-022-22514-4
Morris, M. A., Vallmitjana, A., Grein, F., Schneider, T., Arts, M., Jones, C. R., et al. (2022). Visualizing the mode of action and supramolecular assembly of teixobactin analogues in Bacillus subtilis. Chem. Sci. 13, 7747–7754. doi:10.1039/d2sc01388f
Parmar, A., Lakshminarayanan, R., Iyer, A., Mayandi, V., Leng Goh, E. T., Lloyd, D. G., et al. (2018). Design and syntheses of highly potent teixobactin analogues against Staphylococcus aureus, methicillin-resistant Staphylococcus aureus (MRSA), and vancomycin-resistant enterococci (VRE) in vitro and in vivo. J. Med. Chem. 61, 2009–2017. doi:10.1021/acs.jmedchem.7b01634
Parmar, A., Lakshminarayanan, R., Iyer, A., Goh, E. T. L., To, T. Y., Yam, J. K. H., et al. (2023). Development of teixobactin analogues containing hydrophobic, non-proteogenic amino acids that are highly potent against multidrug-resistant bacteria and biofilms. Eur. J. Med. Chem. 261, 115853. doi:10.1016/j.ejmech.2023.115853
Parveen, R., Harihar, D., and Chatterji, B. P. (2023). Recent histone deacetylase inhibitors in cancer therapy. Cancer 129, 3372–3380. doi:10.1002/cncr.34974
Perrier, F., Morice, J., Gueulle, S., Géry, A., Riboulet-Bisson, E., Garon, D., et al. (2024). Assessing normandy soil microbial diversity for antibacterial activities using traditional culture and iChip methods. Microorganisms 12, 2422. doi:10.3390/microorganisms12122422
Pfab, C., Schnobrich, L., Eldnasoury, S., Gessner, A., and El-Najjar, N. (2021). Repurposing of antimicrobial agents for cancer therapy: what do we know? Cancers (Basel) 13, 3193. doi:10.3390/cancers13133193
Piddock, L. J. V., Malpani, R., and Hennessy, A. (2024). Challenges and opportunities with antibiotic discovery and exploratory research. ACS Infect. Dis. 10, 2445–2447. doi:10.1021/acsinfecdis.4c00530
Raavé, R., van Kuppevelt, T. H., and Daamen, W. F. (2018). Chemotherapeutic drug delivery by tumoral extracellular matrix targeting. J. Control. Release 274, 1–8. doi:10.1016/j.jconrel.2018.01.029
Rawat, P. S., Jaiswal, A., Khurana, A., Bhatti, J. S., and Navik, U. (2021). Doxorubicin-induced cardiotoxicity: an update on the molecular mechanism and novel therapeutic strategies for effective management. Biomed. Pharmacother. 139, 111708. doi:10.1016/j.biopha.2021.111708
Rustandi, T., Prihandiwati, E., Nugroho, F., Hayati, F., Afriani, N., Alfian, R., et al. (2023). Application of artificial intelligence in the development of jamu “traditional Indonesian medicine” as a more effective drug. Front. Artif. Intell. 6, 1274975. doi:10.3389/frai.2023.1274975
Rustandi, T., Mahmud Yumassik, A., Julian Eka Putri Nugraha, E., Riko Nugroho, M., Yasir, M., and Kamalia, K. (2025). Antioxidant and anticancer activities of Spatholobus littoralis stem extract: an in vitro and in silico computational investigation. J.Food Pharm.Sci 13, 10–24. doi:10.22146/jfps.16727
Shawky, H., Fayed, D. B., Abd El-Karim, S. S., Rezk, H., Esawy, M. A., and Farrag, E. K. (2025). Immunotherapeutic effects of de novo benzimidazole derivative and prebiotic bacterial levan against triple-negative breast tumors by harnessing the immune landscape to intercept the oncogenic transcriptome. Int. J. Biol. Macromol. 289, 138844. doi:10.1016/j.ijbiomac.2024.138844
Sheth, D., and Giger, M. L. (2020). Artificial intelligence in the interpretation of breast cancer on MRI. J. Magnetic Reson. Imaging 51, 1310–1324. doi:10.1002/jmri.26878
Shukla, R., Medeiros-Silva, J., Parmar, A., Vermeulen, B. J. A., Das, S., Paioni, A. L., et al. (2020). Mode of action of teixobactins in cellular membranes. Nat. Commun. 11, 2848. doi:10.1038/s41467-020-16600-2
Shukla, R., Lavore, F., Maity, S., Derks, M. G. N., Jones, C. R., Vermeulen, B. J. A., et al. (2022). Teixobactin kills bacteria by a two-pronged attack on the cell envelope. Nature 608, 390–396. doi:10.1038/s41586-022-05019-y
Shukla, R., Peoples, A. J., Ludwig, K. C., Maity, S., Derks, M. G. N., De Benedetti, S., et al. (2023). An antibiotic from an uncultured bacterium binds to an immutable target. Cell 186, 4059–4073.e27. doi:10.1016/j.cell.2023.07.038
Sierra-Hernandez, O., Saurith-Coronell, O., Rodríguez-Macías, J., Márquez, E., Mora, J. R., Paz, J. L., et al. (2025). In silico identification of potential clovibactin-like antibiotics binding to unique cell wall precursors in diverse gram-positive bacterial strains. Int. J. Mol. Sci. 26, 1724. doi:10.3390/ijms26041724
Tang, X., Wang, X., Zhao, Y. Y., Curtis, J. M., and Brindley, D. N. (2017). Doxycycline attenuates breast cancer related inflammation by decreasing plasma lysophosphatidate concentrations and inhibiting NF-ΚB activation. Mol. Cancer 16, 36. doi:10.1186/s12943-017-0607-x
Tanoli, Z., Vähä-Koskela, M., and Aittokallio, T. (2021). Artificial intelligence, machine learning, and drug repurposing in cancer. Expert Opin. Drug Discov. 16, 977–989. doi:10.1080/17460441.2021.1883585
Tong, L., Shi, W., Isgut, M., Zhong, Y., Lais, P., Gloster, L., et al. (2024). Integrating multi-omics data with EHR for precision medicine using advanced artificial intelligence. IEEE Rev. Biomed. Eng. 17, 80–97. doi:10.1109/RBME.2023.3324264
Trinidad-Calderón, P. A., Varela-Chinchilla, C. D., and García-Lara, S. (2023). Depsipeptides targeting tumor cells: milestones from in vitro to clinical trials. Molecules. doi:10.3390/molecules28020670
Vora, L. K., Gholap, A. D., Jetha, K., Thakur, R. R. S., Solanki, H. K., and Chavda, V. P. (2023). Artificial intelligence in pharmaceutical technology and drug delivery design. Pharmaceutics 15, 1916. doi:10.3390/pharmaceutics15071916
Wan, Z., Sun, X., Li, Y., Chu, T., Hao, X., Cao, Y., et al. (2025). Applications of artificial intelligence in drug repurposing. Adv. Sci. 12, e2411325. doi:10.1002/advs.202411325
Wang, J., and Wu, S. G. (2023). Breast cancer: an overview of current therapeutic strategies, challenge, and perspectives. Breast Cancer Targets Ther. 15, 721–730. doi:10.2147/BCTT.S432526
Wang, H., Zhang, H., Zhu, Y., Wu, Z., Cui, C., and Cai, F. (2021). Anticancer mechanisms of salinomycin in breast cancer and its clinical applications. Front. Oncol. 11, 654428. doi:10.3389/fonc.2021.654428
Wang, Y., Yang, Y., Chen, S., and Wang, J. (2021). Deepdrk: a deep learning framework for drug repurposing through kernel-based multi-omics integration. Brief. Bioinform 22, bbab048. doi:10.1093/bib/bbab048
Wang, H., Chen, S., Kang, W., Ding, B., Cui, S., Zhou, L., et al. (2023). High dose isoleucine stabilizes nuclear PTEN to suppress the proliferation of lung cancer. Discov. Oncol. 14, 25. doi:10.1007/s12672-023-00634-1
Was, H., Borkowska, A., Bagues, A., Tu, L., Liu, J. Y. H., Lu, Z., et al. (2022). Mechanisms of chemotherapy-induced neurotoxicity. Front. Pharmacol. 13, 750507. doi:10.3389/fphar.2022.750507
Wu, K., Li, X., Zhou, Z., Zhao, Y., Su, M., Cheng, Z., et al. (2024). Predicting pharmacodynamic effects through early drug discovery with artificial intelligence-physiologically based pharmacokinetic (AI-PBPK) modelling. Front. Pharmacol. 15, 1330855. doi:10.3389/fphar.2024.1330855
Yang, H., Chen, K. H., and Nowick, J. S. (2016). Elucidation of the teixobactin pharmacophore. ACS Chem. Biol. 11, 1823–1826. doi:10.1021/acschembio.6b00295
Yang, H., Du Bois, D. R., Ziller, J. W., and Nowick, J. S. (2017). X-ray crystallographic structure of a teixobactin analogue reveals key interactions of the teixobactin pharmacophore. Chem. Commun. 53, 2772–2775. doi:10.1039/C7CC00783C
Yang, H., Pishenko, A. V., Li, X., and Nowick, J. S. (2020). Design, synthesis, and study of lactam and ring-expanded analogues of teixobactin. J. Org. Chem. 85, 1331–1339. doi:10.1021/acs.joc.9b02631
You, Y., Lai, X., Pan, Y., Zheng, H., Vera, J., Liu, S., et al. (2022). Artificial intelligence in cancer target identification and drug discovery. Signal Transduct. Target Ther. 7, 156. doi:10.1038/s41392-022-00994-0
Yu, H., Tang, L., and Liu, S. (2024). Abstract PO3-25-12: tigecycline-Induced metabolic reprogramming and cytokine modulation suppress the characteristics of breast cancer cells. Cancer Res. 84, PO3–12. doi:10.1158/1538-7445.SABCS23-PO3-25-12
Zhao, Y., Ye, H., Yang, J., Yao, S., Lv, M., Chen, Z., et al. (2025). AutoLDP: an accurate and efficient artificial intelligence-based tool for automatic labeling of digital pathology. EngMedicine 2, 100060. doi:10.1016/j.engmed.2025.100060
Zhou, H., Liu, H., Yu, Y., Yuan, X., and Xiao, L. (2023). Informatics on drug repurposing for breast cancer. Drug Des. Devel Ther. 17, 1933–1943. doi:10.2147/DDDT.S417563
Zong, Y., Sun, X., Gao, H., Meyer, K. J., Lewis, K., and Rao, Y. (2018). Developing equipotent teixobactin analogues against drug-resistant bacteria and discovering a hydrophobic interaction between lipid II and teixobactin. J. Med. Chem. 61, 3409–3421. doi:10.1021/acs.jmedchem.7b01241
Zong, Y., Fang, F., Meyer, K. J., Wang, L., Ni, Z., Gao, H., et al. (2019). Gram-scale total synthesis of teixobactin promoting binding mode study and discovery of more potent antibiotics. Nat. Commun. 10, 3268. doi:10.1038/s41467-019-11211-y
Keywords: artificial intelligence, antibiotics, clovibactin, computational pharmacy, isolation chip, in situ culturing, microbial cultivation techniques, teixobactin
Citation: Fhatonah N, Rustandi T, Abdelmohsen UR, Rasydy LOA, Ma’sum , Elghanam Y, Pashar I, Nordin , Mahal A and Riski A (2025) Drug repurposing of natural depsipeptide from Eleftheria terrae isolated via iChip for anti-breast cancer therapy. Front. Pharmacol. 16:1694322. doi: 10.3389/fphar.2025.1694322
Received: 28 August 2025; Accepted: 14 October 2025;
Published: 27 October 2025.
Edited by:
Roberto Paganelli, YDA, Institute for Advanced Biologic Therapies, ItalyReviewed by:
Tao Liu, Guangdong Pharmaceutical University, ChinaCopyright © 2025 Fhatonah, Rustandi, Abdelmohsen, Rasydy, Ma’sum, Elghanam, Pashar, Nordin, Mahal and Riski. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nuriyatul Fhatonah, bnVyaXlhdHVsZmhhdG9uYWgwMjA3QGdtYWlsLmNvbQ==; Tedi Rustandi, dGVkaXJ1c3RhbmRpMjZAZ21haWwuY29t
†ORCID: Nuriyatul Fhatonah, orcid.org/0009-0004-6548-8331; Tedi Rustandi, orcid.org/0000-0002-5322-4476; Usama Ramadan Abdelmohsen, orcid.org/0000-0002-1014-6922; La Ode Akbar Rasydy, orcid.org/0000-0003-4774-654X; Ma’sum, orcid.org/0009-0002-9190-2925; Imran Pashar, orcid.org/0009-0009-1430-7293; Nordin, orcid.org/0009-0000-5836-5414; Ahmed Mahal, orcid.org/0000-0002-6977-3752
‡These authors have contributed equally to this work and share first authorship
 Nuriyatul Fhatonah1*†‡
Nuriyatul Fhatonah1*†‡ Tedi Rustandi
Tedi Rustandi Usama Ramadan Abdelmohsen
Usama Ramadan Abdelmohsen Ahmed Mahal
Ahmed Mahal