- 1Division of Gastroenterology and Hepatology, Renji Hospital, Shanghai Jiao Tong University School of Medicine; NHC Key Laboratory of Digestive Diseases; Shanghai Research Center of Fatty Liver Disease, Shanghai, China
- 2Department of Hepatology, Hepatology Research Institute, The First Affiliated Hospital, Fujian Medical University, Fujian Clinical Research Center for Liver and Intestinal Diseases, Fuzhou, Fujian, China
- 3Department of General Surgery, Tianjin Medical University General Hospital, Tianjin, China
- 4Department of Medical Oncology, Clinical Oncology School of Fujian Medical University, Fujian Cancer Hospital, Fuzhou, Fujian, China
Antibody-drug conjugates (ADCs) are rapidly developing targeted cancer therapeutic agents that combine specific monoclonal antibodies with cytotoxic agents. Currently, 17 ADCs are approved for the global market for treating hematological and solid tumors with more than 100 ADCs are in phase III clinical trials. ADC-induced hepatotoxicity is a significant concern with unclear mechanisms, and the incidence of hepatic adverse events (AEs) varies across different ADCs. Most hepatic AEs are moderate; however, some ADCs can cause life threatening or fatal AEs. The management of hepatic AEs is limited and is mainly based on product labeling information and the recommendations of study investigators. Therefore, it is critical to raise awareness among oncologists regarding ADC-related hepatotoxicity, and collaboration between oncologists and hepatologists is recommended to provide effective support. This review is the first to focus the hepatotoxicity of ADC, provide an overview of approved ADCs, summarize the potential mechanisms underlying hepatotoxicity, discuss the hepatic toxicities reported in clinical trials and postmarketing studies, and integrate the current recommended management strategies. This article will serve as a valuable resource for medical practitioners in comprehending and managing ADC-related hepatotoxicity, while facilitating further considerations regarding the clinical application of these novel agents.
Introduction
Antibody-drug conjugates (ADCs), described as ‘biological missiles,’ are targeted cancer therapies consisting of a monoclonal antibody, a cytotoxic agent, and a chemical linker (Fu et al., 2022). Since the approval of gemtuzumab ozogamicin (GO) by the FDA, ADC development has rapidly advanced, with 17 ADCs approved and over 100 in phase III trials as of December 2024 (Ruan et al., 2024; Crescioli et al., 2025). With the expanding clinical application of ADCs across diverse tumors, increasing attention has been drawn to treatment-related toxicities, particularly hepatotoxicity.
ADC-related side effects influence multiple organs, including the liver, and have led to FDA black-box warnings (Figure 1). Hepatotoxicity accounts for a significant proportion of these complications and can lead to fatal outcome, with GO once being withdrawn from the market due to severe liver toxicity (Giles et al., 2001). This early experience highlighted that liver injury could become a major barrier to the safe use and further development of ADCs. A recent meta-analysis found that abnormal liver function accounted for >30% of all-grade adverse events (AEs) and 18.8% of grade ≥ 3 events, often requiring dose reductions or treatment discontinuation (Zhu et al., 2023). Thus, the rising use of ADCs underscores the need to better understand the mechanisms, clinical presentation and management of ADC-related hepatotoxicity.
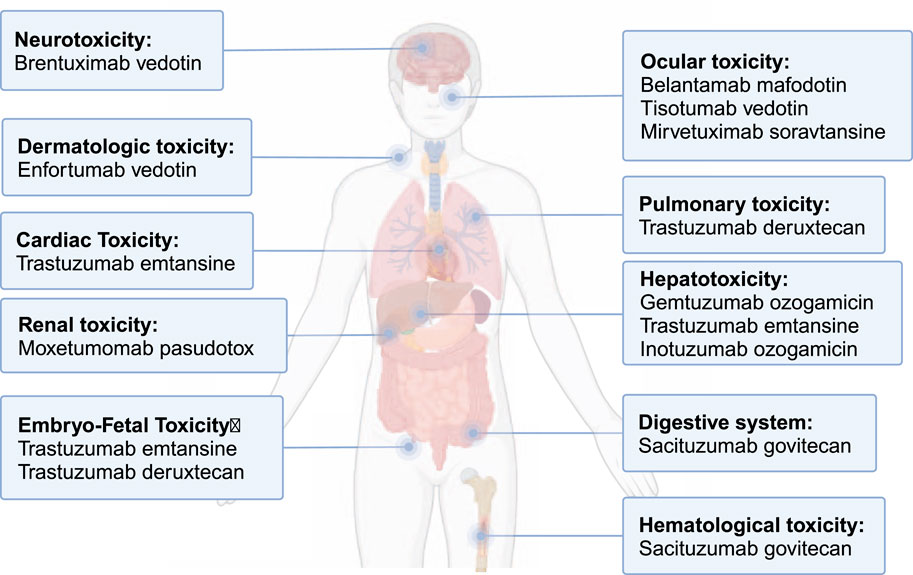
Figure 1. Toxicity received FDA black-box warnings among approved ADCs. This figure summarizes approved ADCs that carry black-box warnings due to serious adverse events, such as hepatotoxicity, neurotoxicity, and other organ toxicities, which highlight the importance of post-marketing surveillance and careful monitoring during ADC therapy.
Overview of ADCs
ADCs consist of three essential components: monoclonal antibody, cytotoxic drug (payload), and linker (Figure 2a). Each component plays a pivotal role in determining the indication, efficacy, and safety of ADCs (Jin et al., 2022). However, ADCs are not a homogeneous class of drugs—differences in linker stability (cleavable vs. non-cleavable), payload types (e.g., microtubule inhibitors, DNA-damaging agents), and mechanisms of action result in distinct pharmacokinetics and toxicity profiles. A detailed summary of the characteristics of the approved ADCs and some investigated ADCs in phase III trials is presented in Supplementary Tables A1, A2. Meanwhile, the field is rapidly evolving, with next-generation ADCs being developed to improve tumor selectivity, reduce off-target toxicity, and enhance payload delivery efficiency.
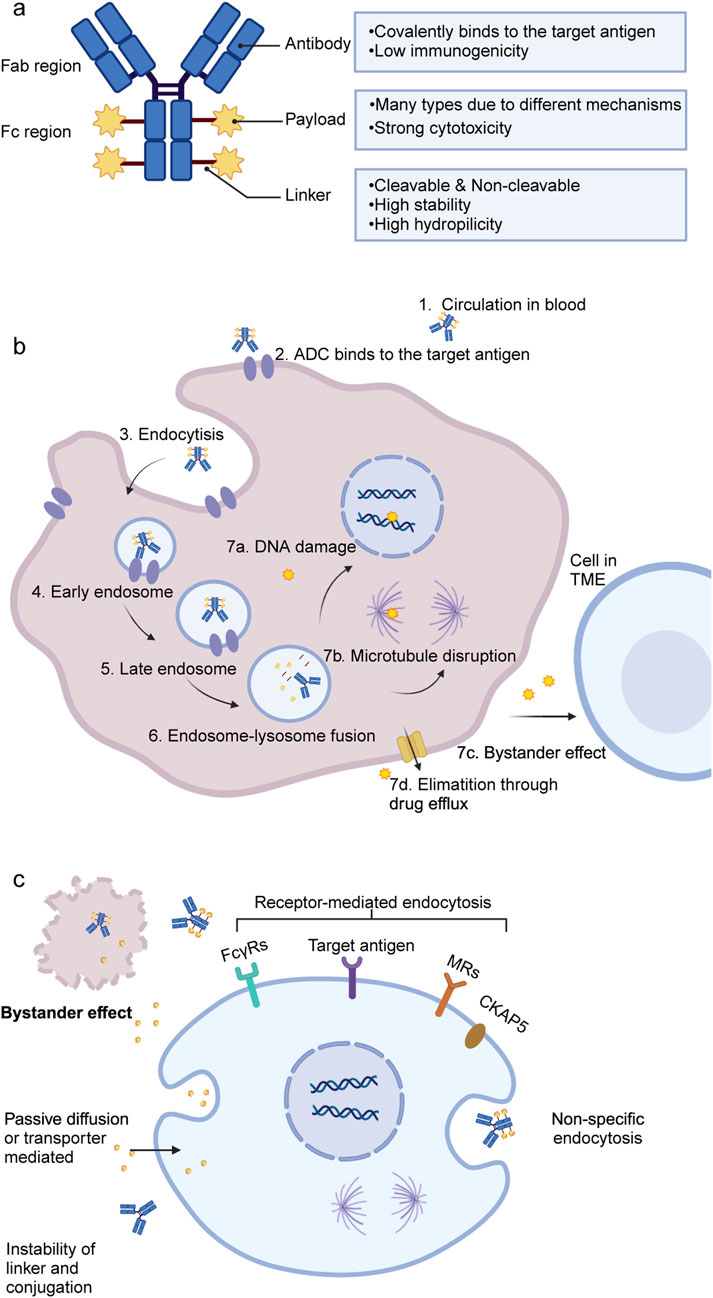
Figure 2. Structure and mechanisms of ADCs. (a) General structure of ADCs, consisting of a monoclonal antibody, a linker, and a cytotoxic payload. (b) Mechanism of action in target tumor cells: ADCs bind to specific antigens, undergo internalization, and release cytotoxic payloads that induce apoptosis through DNA or microtubule damage. (c) Potential mechanisms for ADC or free payload uptake in normal liver cells. ADC, antibody drug-conjugate; DNA, deoxyribonucleic acid; TME, tumor microenvironment; FcγRs, Fc gamma receptors; MRs, mannose receptors; CKAP5, cytoskeleton-associated protein 5.
Upon binding to its target antigen, ADC is internalized into the target cell via endocytosis, progressing through early and late endosomes, and ultimately fuses with lysosomes. Within the lysosomes, the cytotoxic payload is released, triggering apoptosis or cell death by inhibiting DNA synthesis or disrupting microtubule formation (Figure 2b) (Drago et al., 2021). Additionally, the bystander effect, in which released payloads diffuse into neighboring cells, further enhances ADC-mediated anticancer activity, provided that the payload is permeable or transmembrane (Staudacher and Brown, 2017).
Possible mechanism for ADC-related hepatotoxicity
Though ADCs could more precisely target intended tumor site than traditional anti-cancer therapy, research indicates that less than 10% of administered ADCs accumulate at the intended tumor site, while the majority remain in circulation, where they can be internalized by normal cells, potentially leading to toxicity (Huang et al., 2025; Liu et al., 2025). Due to the high perfusion of the liver and the abundant expression of endocytosis receptors, including FcγRIIb, liver sinusoidal endothelial cells (LSECs) are particularly susceptible to ADC-induced damage (Casi and Neri, 2015; Poisson et al., 2017). Both hepatocytes and Kupffer cells contribute to ADC disposition in the liver. Hepatocytes can internalize free or deconjugated cytotoxic payloads by passive diffusion or transporter-mediated uptake, while Kupffer cells and liver sinusoidal cells clear circulating ADCs and immune complexes via Fcγ receptors and lectin receptors (e.g., mannose receptor), processes that have been implicated in altered hepatic cytokine responses and liver injury in preclinical and clinical studies (Nguyen et al., 2023; Aoyama et al., 2022; Liu, 2018).
These cellular events provide a mechanistic basis for clinical manifestations such as serum ALT/AST elevation, cholestasis, sinusoidal obstruction syndrome (SOS), and in severe cases, hepatic failure. However, the precise mechanisms underlying ADC-related hepatotoxicity remain incompletely understood.
Recent studies further highlight the role of liver-resident macrophages and monocyte-derived macrophages in amplifying inflammation following immunotherapy, suggesting they may serve as potential therapeutic targets (Siwicki et al., 2021). This review synthesizes recent findings and proposes a classification framework for ADC-induced hepatotoxicity, categorizing it into on-target and off-target toxicity (Figure 2c). Figure 2c illustrates off-target hepatotoxicity by showing how hepatocytes, Kupffer cells and LSECs internalize ADCs or free payloads through non-specific or receptor-mediated mechanisms.
On-target toxicity
On-target toxicity occurs when ADCs bind to target antigens that are present in both tumor and normal liver cells, leading to hepatotoxicity. Maniecki et al. demonstrated that GO binds to normal cells via the CD33 receptor, causing sinusoidal liver toxicity (Maniecki et al., 2011). However, this does not explain the hepatotoxicity of inotuzumab ozogamicin (InO), which targets CD22, absent in normal liver cells (McDonald et al., 2019). Trastuzumab emtansine (T-DM1), a HER2-targeting ADC, is associated with significant hepatotoxicity, although other HER2-targeting ADCs show mild toxicity. This disparity highlights the complexity of on-target mechanisms. In conclusion, on-target toxicity accounts for only a small proportion of ADC-related hepatotoxicity, as target antigens are usually more highly expressed in tumor cells than in normal tissues.
Off-target toxicity
Off-target toxicity refers to adverse effects unrelated to the target antigen, and is considered the primary mechanism of ADC toxicity (Wei et al., 2024; Dumontet et al., 2023). Multiple mechanisms contribute to these off-target effects, including:
Instability of linker and conjugation
The instability of linkers and premature payload release in non-target tissues significantly impact the efficacy and safety of ADCs. A meta-analysis has shown that the type of toxicity in ADCs often correlates with the payload type. With calicheamicin-based ADCs (e.g., GO and InO) showing the strongest association with sinusoidal obstruction syndrome (SOS) and hepatotoxicity (Zhu et al., 2023).
Linkers in ADCs are classified as cleavable or non-cleavable based on the mechanism of cytotoxic drug release (McKertish and Kayser, 2021). Most marketed ADCs use cleavable linkers, which exploit differences between plasma and intracellular environments (Supplementary Table A1), while non-cleavable linkers, which require proteolytic degradation, are associated with reduced hepatotoxicity but may compromise efficacy, particularly for low-expression targets (Jin et al., 2022; Drago et al., 2021; Polson et al., 2009). Additionally, linker design influences the drug-to-antibody ratio (DAR), which affects efficacy, pharmacokinetics, and hepatotoxicity (López et al., 2023). Higher DARs increase the hydrophobicity and systemic exposure of ADCs, which can enhance their accumulation in non-target tissues such as the liver, thereby elevating the risk of hepatotoxicity (Lyon et al., 2015; Tan et al., 2025). Advances in linker technologies and conjugation methods will enable the development of more stable, homogeneous ADCs with improved efficacy and safety.
Receptor-mediated internalization
Off-target toxicity of ADCs can result from uptake by normal cells through specific receptors, such as Fc gamma receptors (FcγRs) and mannose receptors (MRs), which bind to the Fc region of the IgG antibody (Mahalingaiah et al., 2019). While FcγRs enhance ADC efficacy via antibody-mediated functions, they also contribute to toxicity, including sinusoidal obstruction syndrome (SOS) in liver sinusoids, especially via FcγRIIb on LSECs (Stewart et al., 2014; Taylor and Lindorfer, 2024). MR-mediated uptake of ADCs by non-target cells, particularly in the liver, may also contribute to hepatotoxicity, as observed with GO (Gorovits and Krinos-Fiorotti, 2013). MR is expressed on various cell types, including Kupffer cells and LSECs, and interacts with the Fc region of ADCs, facilitating their uptake and potentially exacerbating liver toxicity (Sancho and Reis, 2012; Linehan et al., 1999). Hepatocytes can internalize T-DM1 via cytoskeleton-associated protein 5, leading to mitochondrial dysfunction and apoptosis (Endo et al., 2018).
Non-specific endocytosis
Endocytosis, a key process for cellular internalization of nutrients and substances, includes phagocytosis and pinocytosis. The rate and mechanism of endocytosis vary across tissues and cell types, with immune cells like Kupffer and endothelial cells exhibiting particularly high rates (Mahalingaiah et al., 2019; Adler et al., 2018). In a retrospective analysis of clinical trials involving InO, McDonald et al. proposed that hepatic injury associated with InO might be linked to non-specific endocytosis by LSECs, although definitive experimental evidence remains lacking (McDonald et al., 2019).
Bystander effect
The bystander effect is a key mechanism in the off-target toxicity of ADCs. This phenomenon occurs following ADC degradation within a target cell when the free payload is released into the tumor microenvironment, owing to its membrane permeability, highly lipophilic nature, or as a consequence of target cell death. The released payload can subsequently diffuse into adjacent non-target cells, leading to unintended cytotoxicity (Theocharopoulos et al., 2024). While the bystander effect enhances the anticancer efficacy, it also increases the risk of hepatotoxicity, posing a significant challenge to clinical application.
Immunogenicity related hepatotoxicity
Immunogenicity is influenced by various factors such as drug structure, patient characteristics, dosage, administration frequency, and co-therapies, and is typically assessed via anti-drug antibodies (ADAs) (Carrasco-Triguero et al., 2019). Further investigation into ADC immunogenicity and its potential link to hepatotoxicity is needed, with ongoing evaluation during development and post-approval critical for ensuring safety and efficacy.
Epidemiology of hepatotoxicity-related AEs
Incidence
As there is no previous report on the overall incidence of hepatic AEs in ADC patients, this article integrates data from pivotal clinical trials (Supplementary Table A3) and postmarketing studies (Table 1), revealing significant variability in the incidence of hepatotoxicity-related AEs across ADCs. Notably, the severity of liver injury is typically classified using the Common Terminology Criteria for Adverse Events, but this system fails to precisely represent the clinical severity of hepatic toxicity and is less applicable than specialized grading systems, such as the Drug-Induced Liver Injury Network severity index, in evaluating drug-induced liver injury (DILI) (Atallah et al., 2023; European Association for the Study of the Liver, 2019; CTEP, 2017). Nevertheless, none of these grading systems is specifically designed to assess ADC-associated hepatotoxicity.
ADCs are discussed in the chronological order of their initial approval in the following.
Gemtuzumab ozogamicin
Gemtuzumab ozogamicin (GO) is a CD33-directed monoclonal antibody conjugated to the DNA-damaging agent calicheamicin via a cleavable linker with a DAR of 2–3 (Dumontet et al., 2023). Approved by the FDA in 2000, GO was withdrawn in 2010 due to liver toxicity and VOD. However, the 2012 phase 3 ALFA-0701 trial demonstrated that fractionated lower doses (3 mg/m2) improved outcomes in AML patients with manageable toxicity, leading to its reapproval by the FDA in 2017 for CD33-positive AML treatment in adults and children (Pawinska-Wasikowska et al., 2023).
Hepatotoxicity-related AEs are frequent in GO clinical trials and typically present as elevated liver function tests. Most cases were grade 1–2 in terms of severity (Lambert et al., 2019; Gamis et al., 2014; Döhner et al., 2023; Party CsOGR, 2025). However, VOD, also known as SOS, is a potentially life-threatening complication that warrants close attention. The ALFA-0701 and AAML0531 studies are pivotal phase 3 trials of combination therapies for AML in adult and pediatric populations, with the occurrence of VOD in 4.6% and 3.5% respectively (Lambert et al., 2019; Gamis et al., 2014). In ALFA-0701, the median time from the administration of GO to the onset of VOD was 9 days (range: 2–298 days) (Wyeth Pharmaceuticals LLC asoPI, 2021). Rapid weight gain is typically the earliest clinical manifestation of sinusoidal obstruction syndrome (Markides et al., 2025). Persistent thrombocytopenia is considered as an early indicator of VOD, and in the two aforementioned clinical trials, patients in the GO arm were significantly more likely to experience prolonged thrombocytopenia compared to the control arm, indicating, suggesting the possibility of undiagnosed early-stage VOD (Taylor and Lindorfer, 2024; Lambert et al., 2019; Gamis et al., 2014; Wyeth Pharmaceuticals LLC asoPI, 2021).
A meta-analysis of RCTs and retrospective studies found that GO-treated groups exhibited a consistently higher risk of hepatic AEs—including VOD and SOS—than non-GO groups, with higher GO doses correlating with increased VOD/SOS incidence (Xu et al., 2021). Case reports suggest that VOD typically occurs within the first cycle of GO treatment (often within 1 week) and is characterized by increased bilirubin and transaminase levels, hepatomegaly, ascites, and weight gain (Figure 3 Case 1) (Tack et al., 2001; Sievers et al., 2001; Saviola et al., 2003; Kurt et al., 2005; Lannoy et al., 2006). The diagnosis of VOD is mainly confirmed by computed tomography and liver biopsy.
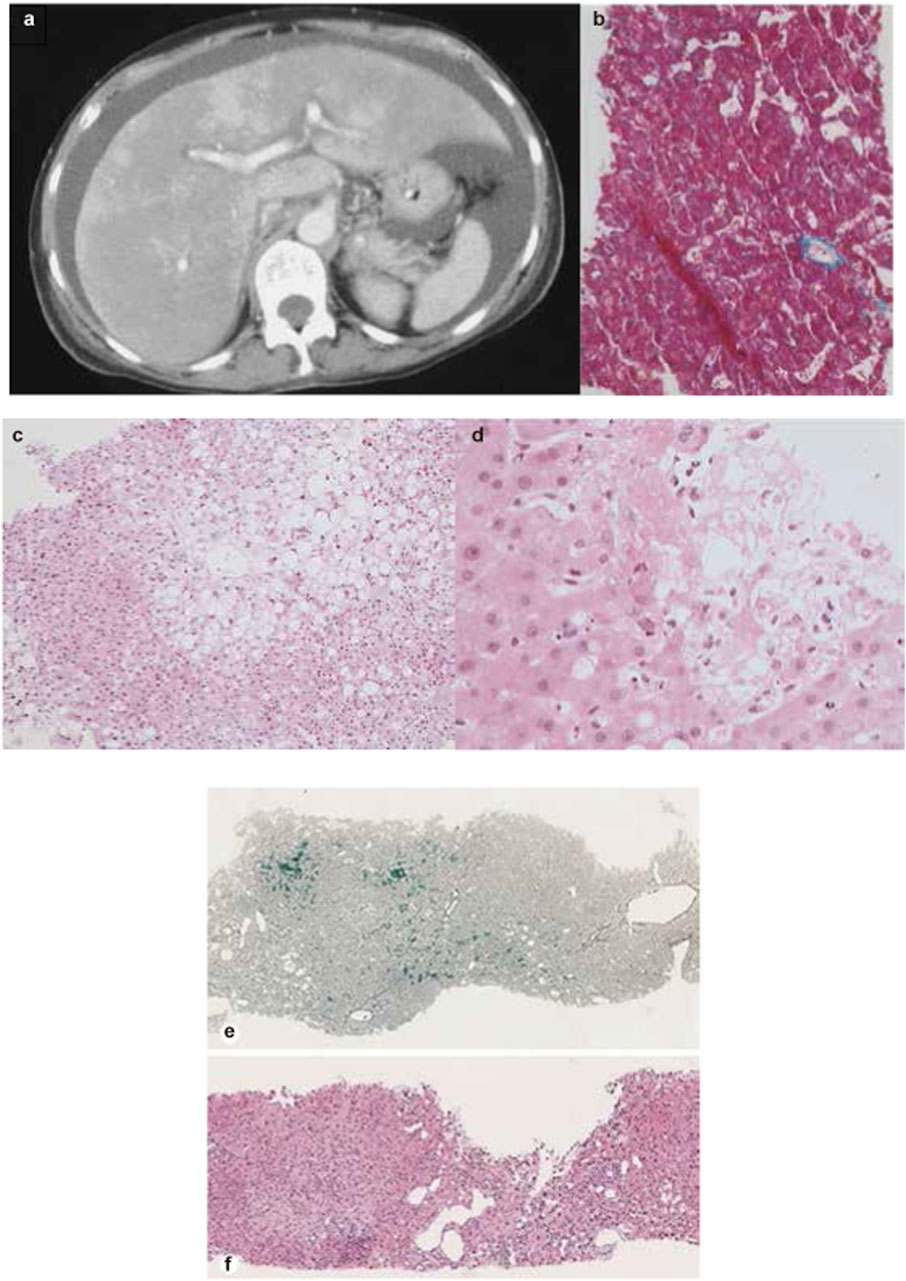
Figure 3. Representative imaging and liver biopsy findings of ADC-associated hepatotoxicity. (a) Abdominal CT scan showing massive ascites and heterogeneous hepatic enhancement consistent with veno-occlusive disease (VOD) after treatment with gemtuzumab ozogamicin. (b) Liver biopsy demonstrating sinusoidal congestion and perivenular hemorrhage compatible with early VOD. (c,d) Liver biopsy specimens from a patient treated with brentuximab vedotin, showing cholestatic liver injury and progressive hepatocellular changes at two different time points. (e,f) Liver biopsy from a patient treated with trastuzumab emtansine, showing features of nodular regenerative hyperplasia. (a, b) Reprinted with permission form Tack et al. (2001), Copyright © 2001 Macmillan Publishers Limited. (c, d) Reprinted from Neeman et al. (2019) with permission of the publisher (Taylor & Francis Ltd, http://www.tandfonline.com). (e, f) Reprinted with permission from Garrido et al. (2022), Copyright © 2022 Karger Publishers, Basel, Switzerland.
Brentuximab vedotin
Brentuximab vedotin (BV) is a CD30-targeted ADC comprising an IgG1 antibody, the microtubule-disrupting agent MMAE, and a cleavable linker with a DAR of 4. It has been approved for treating Hodgkin lymphoma, anaplastic large cell lymphoma, and other CD30-expressing peripheral T-cell lymphomas (Dailymed, 2025a).
In clinical trials, the most commonly reported hepatic AE is increased transaminase levels, with incidence rates varying significantly from 10% to 52% across studies (Connors et al., 2018; Co et al., 2018). Postmarketing meta-analyses have failed to highlight significant hepatotoxicities (Chen et al., 2015; Gao et al., 2020). A case of fatal hepatotoxicity was reported in a patient receiving BV monotherapy, presenting with acute liver injury characterized by substantial increases in blood bilirubin and ALP levels (Dailymed, 2025a). Liver biopsy findings suggested cholestatic drug-induced liver injury, which clinicians attributed to BV (Figure 3 Case 2).
Trastuzumab emtansine
Trastuzumab emtansine (T-DM1) is a HER2-directed monoclonal antibody covalently linked to the microtubule-inhibitory agent DM1 via a non-cleavable linker with a DAR of 3.5. T-DM1 has been approved for the treatment of metastatic breast cancer and as an adjuvant therapy for early stage high-risk patients (Genentech, 2024).
Serious hepatic AEs, including three fatal cases (severe DILI and hepatic encephalopathy), have been reported in clinical trials involving T-DM1 monotherapy (Krop et al., 2017; Krop et al., 2012). In pivotal clinical trials, elevated transaminase levels were among the most frequently reported adverse events in patients receiving T-DM1, and were also a leading cause of dose reduction or treatment discontinuation (Genentech, 2024; Krop et al., 2017; von Minckwitz et al., 2019). In the KATHERINE trial, aminotransferase elevation was reported more frequently in the T-DM1 group than in the trastuzumab group (ALT increased, 23.1% vs. 5.7%; AST increased, 28.4% vs. 5.6%) (von Minckwitz et al., 2019). NRH, a rare hepatic condition that can lead to noncirrhotic portal hypertension and is associated with T-DM1, was identified in liver biopsy of 2/740 and 3/403 patients in the KATHERINE and TH3RESA studies, respectively (Krop et al., 2017; von Minckwitz et al., 2019). Liver biopsy of a representative NRH case associated with T-DM1 is shown in Figure 3 Case 3. Furthermore, one death attributed to hepatic encephalopathy related to T-DM1 was reported in the TH3RESA trial.
Inotuzumab ozogamicin
Inotuzumab ozogamicin (InO) comprises a CD22-directed monoclonal antibody conjugated to the DNA-damaging agent, calicheamicin, via a cleavable linker with a DAR of 2–3 (Dumontet et al., 2023). Both InO and GO utilize the same cytotoxic agent and carry FDA black-box warnings for hepatotoxicity, including the risk of fatal or life-threatening VOD (Wyeth Pharmaceuticals LLC asoPI, 2021; Dailymed, 2024).
Pivotal clinical trials have highlighted the significant hepatotoxicity associated with InO treatment. In the INO-VATE trial, treatment-emergent hepatotoxic events were more frequently observed in the InO arm compared to the standard therapy arm (51% vs. 34%). Additionally, sinusoidal obstruction syndrome (SOS) occurred in 22 out of 164 patients (13%) receiving InO, compared to only one case (<1%) in the standard care group (Kant et al., 2017). The median time from hematopoietic stem cell transplantation (HSCT) to SOS onset was 15 days ([IQR] 10–17). In the ITCC-059 study, which assessed the efficacy and safety of InO monotherapy in pediatric patients, SOS was reported in 8 out of 53 patients (15%) (Dailymed, 2024). Notably, no cases of hepatitis B virus (HBV) reactivation have been documented in patients with chronic HBV infection treated with InO (Mustafayev and Torres, 2022).
A retrospective study including 10 patients who underwent allogeneic hematopoietic cell transplantation before receiving InO found that three patients developed VOD, resulting in one death (Izumi et al., 2022). Postmarketing meta-analysis revealed that the pooled incidence of VOD/SOS in patients receiving InO was 8%, with a notably higher prevalence in pediatric patients (13%; 95% CI, 7%–21%) than in adults (6%; 95% CI, 3%–13%) (Li et al., 2021).
Polatuzumab vedotin
Polatuzumab vedotin (PV) is a CD79b-directed monoclonal antibody conjugated to the MMAE via a cleavable linker with a DAR of 3–4 (Dumontet et al., 2023). PV has been approved for use in combination therapy for the treatment of diffuse large B-cell lymphoma (DLBCL).
In pivotal clinical trials, PV demonstrated modest hepatotoxicity, primarily characterized by elevated ALT (20%–38%) and AST (13%–36%) levels (Genentech, 2025; Abrisqueta et al., 2024). In the Study GO29365, grade 3 and grade 4 transaminase elevations were reported in 1·9% of patients receiving PV in combination with bendamustine and rituximab (Genentech, 2025). Postmarketing retrospective studies have not identified any additional significant hepatotoxicities.
Enfortumab vedotin
Enfortumab vedotin (EV) is a Nectin-4-directed ADC consisting of an IgG1 monoclonal antibody conjugated to the MMAE via a cleavable linker with a DAR of 4, and has been approved for treating locally advanced or metastatic urothelial cancer in adults (Challita-Eid et al., 2016).
In clinical trials, skin reactions and peripheral neuropathy have garnered more attention than hepatic toxicity as the predominant severe treatment-related adverse events (AEs) associated with EV (Dailymed, 2025b; Powles et al., 2021; Astellas Pharma Inc Astellas Pharma Global Development IRP, 2025). The most frequent hepatic-related AEs of all grades were increased ALT and AST levels, with 5%–9% of patients experiencing grade ≥3 elevation (Dailymed, 2025b; Powles et al., 2021). No cases of VOD or NRH were reported in clinical trials. However, in the EV-301 trial, one patient receiving EV monotherapy died due to abnormal hepatic function (Powles et al., 2021). Postmarketing meta-analyses have not identified additional hepatotoxicities, and only 114 (3·66%) reported hepatobiliary disorders cases in FAERS to date (Zheng et al., 2024).
Trastuzumab deruxtecan
Trastuzumab deruxtecan (T-Dxd) is a HER2-directed ADC comprising a monoclonal antibody linked to the topoisomerase inhibitor deruxtecan via a cleavable linker with a DAR of 8, and has been approved for the treatment of adults with solid tumor, based on results from several pivotal clinical trials (Keam, 2020).
In clinical trials, the hepatotoxicity associated with T-Dxd is mild, primarily characterized by elevated transaminase levels, with an incidence of approximately 20% across all grades and is generally not associated with dose interruptions, except for one fatal liver failure related to T-Dxd in the DESTINY-CRC02 trial (Hurvitz et al., 2023; André et al., 2023; Goto et al., 2023; Van Cutsem et al., 2023; Raghav et al., 2024). Postmarketing meta-analysis failed to report further hepatic toxicities, and only a small percentage of hepatobiliary disorders cases have been documented in the FAERS database to date (Guo et al., 2022; FAERS, 2025a).
Sacituzumab govitecan
Sacituzumab govitecan (SG) is a TROP2-targeted ADC, comprising a monoclonal antibody conjugated to SN-38 via a hydrolyzable linker, with an average DAR of 7.6. It is approved for treating triple-negative breast cancer in adults and has received accelerated approval for urothelial cancer (Syed, 2020).
Existing clinical trial data suggest that the hepatotoxicity of SG is relatively mild, with liver-related AEs primarily consisting of grade 1–2 elevations in liver function tests (Bardia et al., 2019; Rugo et al., 2022; Rugo et al., 2023; Bardia et al., 2021; Xu B. et al., 2023). A pooled analysis of 1,063 patients exposed to SG identified increased ALP levels (28%) as one of the most common adverse reactions (Dailymed, 2025c). Importantly, no dose interruptions or drug-related deaths due to hepatic AEs were reported. Postmarketing analyses further characterized the hepatic safety profile. A recent pharmacovigilance study using the FAERS database found that, in addition to elevated transaminases, hepatobiliary toxicity associated with SG primarily manifests as acute cholecystitis and increased blood bilirubin levels, potentially linked to the drug’s excretion through the gallbladder (Liu et al., 2023; Ocean et al., 2017).
Belantamab mafodotin
Belantamab mafodotin (BM) contains a BCMA-directed monoclonal antibody linked to the microtubule inhibitor MMAF via a protease-resistant linker, with a DAR of 4, indicated for treating adults with multiple myeloma (Markham, 2020).
Hepatic AEs are minor in clinical trials and mostly manifest as abnormal liver function tests, whereas thrombocytopenia and keratopathy are more AEs of interest in the application (Dimopoulos et al., 2023; Lonial et al., 2020). In DREAMM-2 Study, the prevalence of all-grade AST elevation in patients receiving BM was 20%, with 2% experiencing ≥ grade 3, and all-grade ALP increased occurred in 8% patients (Nooka et al., 2023). No dose interruptions or drug-related deaths due to hepatotoxicity were reported in clinical trials. A postmarketing meta-analysis did not identify additional hepatic toxicities, and only a few cases of hepatobiliary disorders have been reported in the FAERS database to date (Musto and La Rocca, 2020).
Loncastuximab tesirine
Approved for treating DLBCL, loncastuximab tesirine (LT) is a CD19-directed ADC consisting of an anti-CD19 monoclonal antibody conjugated to cytotoxic alkylating agent via a cleavable linker with an average DAR of 2·3 (Lee, 2021).
In clinical trials, the hepatotoxicity associated with LT was primarily characterized by abnormal hepatic function. Unlike other ADCs, GGT elevation was more frequently observed in patients treated with LT. The phase 2 LOTIS-2 trial, which evaluated the LT monotherapy in patients with relapsed or refractory DLBCL, reported GGT elevations in 41% and 17% of patients for all grades and ≥ Grade 3, respectively. GGT elevation was also the most common treatment-emergent AE leading to treatment discontinuation. However, subsequent analyses did not identify any long-term liver toxicity of any grade in patients with GGT elevation (Caimi et al., 2021; Caimi et al., 2024). Postmarketing data have not revealed any additional hepatic safety concerns.
Disitamab vedotin
Disitamab vedotin is an HER2-directed ADC consisting of a monoclonal antibody linked to the MMAE via a cleavable linker with a DAR of 4, and has been approved for treating urothelial and gastric cancers in China (DeeksVedotin, 2021).
Liver function abnormalities are among the most common hepatic AEs observed in clinical trials with the incidence of 11.6%-43·2%, predominantly grade 1–2. A pooled safety analysis of 414 patients treated with disitamab vedotin showed increased transaminase levels were the most frequent adverse event, occurring in 55.8% of patients across all grades, mainly grades 1–2. Transaminase increase led to dose delays in 5.1% and dose modifications in 2.3% of patients (RemeGen, 2025). No hepatotoxicity-related deaths were reported (Sheng et al., 2021; Peng et al., 2021). Real-world studies also demonstrated mild hepatotoxicity, predominantly characterized by reversible and manageable transaminase elevations (Wang and Xia, 2023; Zhou et al., 2023; Chen et al., 2023; Nie et al., 2023).
Tisotumab vedotin
Tisotumab vedotin is a tissue factor (TF)-directed ADC consisting of an anti-TF monoclonal antibody conjugated to MMAE via a cleavable linker, with a DAR of 4, and received FDA approval for treating adult patients with recurrent or metastatic cervical cancer in April 2024 (Administration FaD, 2025).
Existing clinical trials demonstrated that the hepatotoxicity associated with tisotumab vedotin is moderate and primarily manifests as liver function abnormalities. Most hepatotoxicity-related adverse events were grade 1–2, and no hepatotoxicity-related deaths were reported (Da ilymed, 2025d; Party SIR, 2025; de Bono et al., 2019). A postmarketing meta-analysis found no additional hepatic toxicities, with only nine cases of abnormal liver investigations and three cases of hepatobiliary disorders reported in FAERS to date (Mokresh et al., 2024; FAERS, 2025b).
Mirvetuximab soravtansine
Mirvetuximab soravtansine (MIRV) is a folate receptor alpha (FRα)-directed ADC consisting of a monoclonal antibody conjugated to the anti-tubulin agent DM4 via a cleavable linker, with an average DAR of 3.3–5. It is indicated for the treatment of adult patients with FRα-positive, platinum-resistant epithelial ovarian, fallopian tube, or peritoneal cancer (Heo, 2023).
Compared to significant ocular toxicity which carries a black-box warning from the FDA, available clinical trial data indicate that the hepatotoxicity of MIRV is relatively minor (Dailymed, 2025e). Hepatic adverse events are primarily characterized by abnormal liver function, with an incidence of less than 20%, mostly of grade 1–2 severity. The only hepatic AE leading to dose reductions or delays was three (1%) cases of increased AST in the FORWARD I Study (Moore et al., 2021). No hepatotoxicity-related deaths were reported. An integrated safety analysis of 682 MIRV-treated patients across four clinical studies demonstrated low hepatotoxicity, with 16% and 13% of patients experiencing ALT increased and AST increased (all grade), respectively, and less than 1% of cases reaching grade ≥ 3 severity (Moore et al., 2024). Two initial meta-analyses confirmed a low hepatotoxicity profile, with only 12 hepatotoxicity-related cases reported in FAERS (Xu K. et al., 2023; Wang et al., 2024; FAERS, 2025c). However, further studies are needed to fully assess the drug’s toxicity given its recent approval.
Management
Most hepatic AEs associated with ADCs are mild and reversible, and can generally be managed through treatment interruption or dosage modification. Given the absence of formal guidelines or consensus on managing hepatic AEs in patients treated with ADCs, we have compiled the available recommendations from product labeling and study investigators (Figure 4).
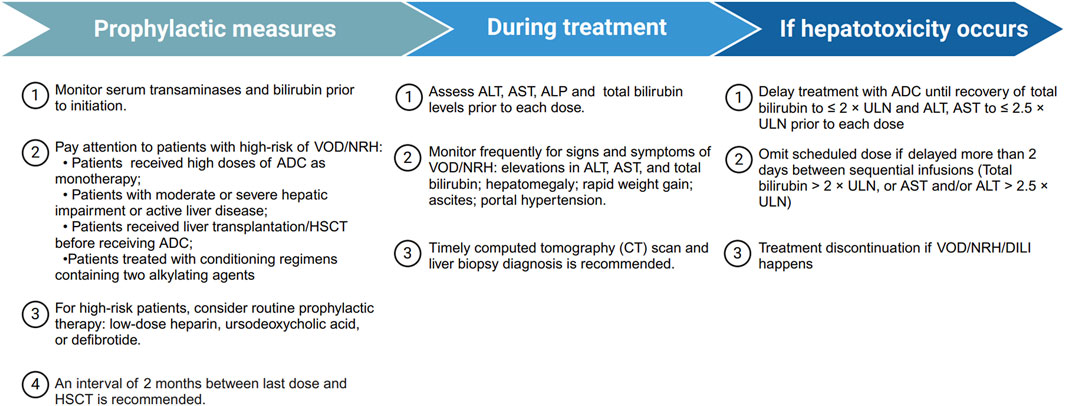
Figure 4. Summary of management recommendations for ADC-related hepatotoxicity. Overview of prophylactic measures, monitoring strategies during treatment, and management steps if hepatotoxicity occurs, including treatment delay, dose omission, or discontinuation in severe cases. ADC, antibody drug-conjugate; ALT, alanine aminotransferase; ALP, alkaline phosphatase; AST, aspartate aminotransferase; DILI, drug-induced liver injury; GO, gemtuzumab ozogamicin; HSCT, hematopoietic stem-cell transplantation; NRH, nodular regenerative hyperplasia; ULN, upper limit of normal; VOD, veno-occlusive disease.
Diagnostic approach and early detection
The diagnostic approach for ADC-induced hepatotoxicity generally parallels that of conventional DILI, but certain ADCs present distinct hepatic patterns, such as SOS associated with calicheamicin-based ADCs (Gamis et al., 2014; Dailymed, 2024). Early recognition is critical, as timely withdrawal of the offending agent is the most effective intervention.
A thorough evaluation should include a detailed drug exposure history, temporal relationship between ADC administration and onset of liver test abnormalities, and exclusion of alternative causes such as viral hepatitis, autoimmune liver diseases, or hepatic metastasis. Baseline and serial liver biochemical assessments are recommended during ADC therapy, including ALT, AST, ALP, and TBIL.
In clinical practice, monitoring should be performed at baseline and prior to each treatment cycle, or more frequently in patients with preexisting liver dysfunction or those receiving hepatotoxic combination regimens. Acute DILI should be suspected when ALT ≥ 5× ULN, ALP ≥ 2× ULN, or ALT ≥ 3× ULN with TBL ≥ 2× ULN, consistent with international criteria (European Association for the Study of the Liver, 2019; Fettiplace et al., 2024). Imaging and, when necessary, liver histopathology can aid differential diagnosis and severity assessment.
Management during treatment
Dose adjustment and discontinuation criteria
Currently, there are no universally accepted or ADC-specific guidelines for the management of hepatotoxicity. In clinical practice, management strategies are generally adapted from conventional DILI principles, and uniform criteria for dose modification or treatment discontinuation are lacking (Dailymed, 2025a; Genentech, 2025).
Supportive and pharmacologic interventions
At present, no specific antidote is available for ADC-induced hepatotoxicity. Supportive care remains the cornerstone of management and should be individualized based on the pattern and severity of liver injury. Hepatoprotective agents may be considered for acute hepatocellular or mixed-type injury, although robust clinical evidence supporting their efficacy is limited. Anticholestatic therapies, such as ursodeoxycholic acid, may be beneficial in patients presenting with cholestatic injury, while defibrotide, approved for the treatment of SOS, has shown potential benefit when administered early (Stutz et al., 2022; Larue et al., 2025).
Incorporating clinical pharmacists into multidisciplinary care teams can further optimize drug selection, minimize drug–drug interactions, and assist in individualized re-challenge decisions.
Prevention and monitoring
Preventive strategies focus on baseline risk stratification, early recognition, and careful monitoring throughout treatment. All patients should undergo baseline liver function and viral hepatitis screening prior to ADC initiation. Particular attention should be given to high-risk patients, including those who receive high doses of ADCs as monotherapy, patients with moderate or severe hepatic impairment or active liver disease, or those who have undergone liver transplantation or HSCT before ADC treatment. In these cases, routine prophylactic therapy with low-dose heparin, ursodeoxycholic acid, or defibrotide may be considered. For patients undergoing HSCT, a minimum interval of 2 months between the last ADC dose and transplantation is recommended (Kantarjian et al., 2019).
Routine monitoring every 2–3 weeks or before each ADC cycle is recommended, with closer surveillance for those with elevated baseline liver enzymes or concomitant hepatotoxic therapies. Prompt hepatology consultation is advised if ALT/AST exceeds 5× ULN or if clinical symptoms (jaundice, fatigue, right upper quadrant pain) develop. Re-challenge decisions should be individualized based on clinical recovery and benefit–risk assessment.
Future perspective
The field of ADC therapeutics has seen rapid development in recent years, with 17 ADCs currently approved and numerous candidates in clinical trials. Despite considerable advances, hepatotoxicity continues to be a major obstacle, restricting the widespread use of ADCs.
Future research should focus on identifying both patient-specific and drug-specific risk factors for ADC-induced hepatotoxicity, including genetic predispositions, comorbidities, and drug characteristics such as payloads and linkers. To achieve this, integrative approaches combining multi-omics profiling, genome-wide CRISPR screens, and single-cell RNA sequencing could be employed for diagnostic precision, leading to more specific target assessment and patient stratification (Zhou et al., 2025; Chen et al., 2025).
The establishment of reliable biomarkers for early detection and monitoring is also a key research priority. Circulating microRNAs, extracellular vesicles, and metabolomic signatures hold promise as potential biomarkers, which could be developed and validated through prospective clinical cohorts and pharmacovigilance databases. These tools would enable timely intervention, minimize treatment interruptions, and ultimately improve patient outcomes (Ruan et al., 2024).
A critical gap in the field is the limited understanding of the epidemiology, natural history, and clinical characteristics of hepatotoxicity associated with specific ADCs. Future studies should adopt multicenter, longitudinal registry designs to determine incidence, risk factors, and long-term hepatic outcomes, as well as to evaluate the reversibility and chronic sequelae of liver injury. Such efforts would also facilitate the differentiation of ADC-related hepatotoxicity from other etiologies and inform evidence-based management strategies.
Multidisciplinary collaboration between oncologists, hepatologists, pharmacologists, and data scientists would also benefit the development and usage of ADC. Integration of artificial intelligence–driven modeling with real-world evidence could help predict patient risk and optimize dosing strategies. Moreover, the emergence of novel molecular targets enables the development of ADCs and similar targeted therapies with enhanced patient specificity and safety (Wang et al., 2026). In addition, greater emphasis should be placed on patient-centered outcomes, such as health-related quality of life, long-term hepatic function, and post-treatment recovery to align future research with the principles of precision and patient-centric oncology.
Collectively, these advances will contribute to mitigating ADC-related adverse effects, enhancing therapeutic safety and efficacy, and ultimately paving the way toward safer, more personalized antibody–drug conjugate therapies.
Author contributions
YD: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft. YZ: Project administration, Software, Validation, Visualization, Writing – original draft. XYL: Formal Analysis, Resources, Validation, Visualization, Writing – original draft. MM: Data curation, Supervision, Validation, Writing – review and editing. MY: Data curation, Formal Analysis, Software, Validation, Writing – original draft. SH: Supervision, Validation, Writing – review and editing. JT: Supervision, Validation, Visualization, Writing – review and editing. WZ: Software, Supervision, Validation, Writing – review and editing. XHL: Software, Supervision, Validation, Writing – review and editing. YM: Conceptualization, Funding acquisition, Resources, Supervision, Writing – review and editing.
Funding
The authors declare that financial support was received for the research and/or publication of this article. This work was supported by the National Key R&D Program of China (2022YFC3502101); the National Natural Science Foundation of China (NSFC 82270619, NSFC 82470621).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1694436/full#supplementary-material
References
Abrisqueta, P., González-Barca, E., Panizo, C., Pérez, J. M. A., Miall, F., Bastos-Oreiro, M., et al. (2024). Polatuzumab vedotin plus rituximab and lenalidomide in patients with relapsed or refractory diffuse large B-cell lymphoma: a cohort of a multicentre, single-arm, phase 1b/2 study. Lancet Haematol. 11 (2), e136–e146. doi:10.1016/s2352-3026(23)00345-9
Adler, M., Mayo, A., Zhou, X., Franklin, R. A., Jacox, J. B., Medzhitov, R., et al. (2018). Endocytosis as a stabilizing mechanism for tissue homeostasis. Proc. Natl. Acad. Sci. U. S. A. 115 (8), E1926–e1935. doi:10.1073/pnas.1714377115
Administration FaD (2025). FDA approves tisotumab vedotin-tftv for recurrent or metastatic cervical cancer. Available online at: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-tisotumab-vedotin-tftv-recurrent-or-metastatic-cervical-cancer (Accessed November 30, 2024).
Administration USFD (2025). FDA adverse events reporting system (FAERS) public dashboard. Available online at: https://fis.fda.gov/sense/app/95239e26-e0be-42d9-a960-9a5f7f1c25ee/sheet/7a47a261-d58b-4203-a8aa-6d3021737452/state/analysis.
André, F., Hee Park, Y., Kim, S. B., Takano, T., Im, S. A., Borges, G., et al. (2023). Trastuzumab deruxtecan versus treatment of physician's choice in patients with HER2-positive metastatic breast cancer (DESTINY-Breast02): a randomised, open-label, multicentre, phase 3 trial. Lancet 401 (10390), 1773–1785. doi:10.1016/s0140-6736(23)00725-0
Aoyama, M., Tada, M., Yokoo, H., Demizu, Y., and Ishii-Watabe, A. (2022). Fcγ receptor-dependent internalization and off-target cytotoxicity of antibody-drug conjugate aggregates. Pharm. Res. 39 (1), 89–103. doi:10.1007/s11095-021-03158-x
Astellas Pharma Inc Astellas Pharma Global Development IRP (2025). A study to evaluate enfortumab vedotin versus (vs) chemotherapy in subjects with previously treated locally advanced or metastatic urothelial cancer (EV-301). Available online at: https://clinicaltrials.gov/study/NCT03474107?term=EV-301&limit=10&rank=1 (Accessed November 26, 2024).
Atallah, E., Welsh, S. J., O'Carrigan, B., Oshaughnessy, A., Dolapo, I., Kerr, A. S., et al. (2023). Incidence, risk factors and outcomes of checkpoint inhibitor-induced liver injury: a 10-year real-world retrospective cohort study. JHEP Rep. 5 (10), 100851. doi:10.1016/j.jhepr.2023.100851
Bardia, A., Mayer, I. A., Vahdat, L. T., Tolaney, S. M., Isakoff, S. J., Diamond, J. R., et al. (2019). Sacituzumab govitecan-hziy in refractory metastatic triple-negative breast cancer. N. Engl. J. Med. 380 (8), 741–751. doi:10.1056/NEJMoa1814213
Bardia, A., Hurvitz, S. A., Tolaney, S. M., Loirat, D., Punie, K., Oliveira, M., et al. (2021). Sacituzumab govitecan in metastatic triple-negative breast cancer. N. Engl. J. Med. 384 (16), 1529–1541. doi:10.1056/NEJMoa2028485
Brivio, E., Chantrain, C. F., Gruber, T. A., Thano, A., Rialland, F., Contet, A., et al. (2021). Inotuzumab ozogamicin in infants and young children with relapsed or refractory acute lymphoblastic leukaemia: a case series. Br. J. Haematol. 193 (6), 1172–1177. doi:10.1111/bjh.17333
Caimi, P. F., Ai, W., Alderuccio, J. P., Ardeshna, K. M., Hamadani, M., Hess, B., et al. (2021). Loncastuximab tesirine in relapsed or refractory diffuse large B-cell lymphoma (LOTIS-2): a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 22 (6), 790–800. doi:10.1016/s1470-2045(21)00139-x
Caimi, P. F., Ai, W. Z., Alderuccio, J. P., Ardeshna, K. M., Hamadani, M., Hess, B., et al. (2024). Loncastuximab tesirine in relapsed/refractory diffuse large B-cell lymphoma: long-term efficacy and safety from the phase II LOTIS-2 study. Haematologica 109 (4), 1184–1193. doi:10.3324/haematol.2023.283459
Carrasco-Triguero, M., Dere, R. C., Milojic-Blair, M., Saad, O. M., Nazzal, D., Hong, K., et al. (2019). Immunogenicity of antibody-drug conjugates: observations across 8 molecules in 11 clinical trials. Bioanalysis 11 (17), 1555–1568. doi:10.4155/bio-2018-0259
Casi, G., and Neri, D. (2015). Antibody-drug conjugates and small molecule-drug conjugates: opportunities and challenges for the development of selective anticancer cytotoxic agents. J. Med. Chem. 58 (22), 8751–8761. doi:10.1021/acs.jmedchem.5b00457
Challita-Eid, P. M., Satpayev, D., Yang, P., An, Z., Morrison, K., Shostak, Y., et al. (2016). Enfortumab vedotin antibody-drug conjugate targeting Nectin-4 is a highly potent therapeutic agent in multiple preclinical cancer models. Cancer Res. 76 (10), 3003–3013. doi:10.1158/0008-5472.Can-15-1313
Chen, R., Wang, F., Zhang, H., and Chen, B. (2015). Brentuximab vedotin for treatment of relapsed or refractory malignant lymphoma: results of a systematic review and meta-analysis of prospective studies. Drug Des. Devel Ther. 9, 2277–2283. doi:10.2147/dddt.S83592
Chen, M., Yao, K., Cao, M., Liu, H., Xue, C., Qin, T., et al. (2023). HER2-targeting antibody-drug conjugate RC48 alone or in combination with immunotherapy for locally advanced or metastatic urothelial carcinoma: a multicenter, real-world study. Cancer Immunol. Immunother. 72 (7), 2309–2318. doi:10.1007/s00262-023-03419-1
Chen, B., Zheng, X., Wu, J., Chen, G., Yu, J., Xu, Y., et al. (2025). Antibody-drug conjugates in cancer therapy: current landscape, challenges, and future directions. Mol. Cancer 24 (1), 279. doi:10.1186/s12943-025-02489-2
Cole, P. D., McCarten, K. M., Pei, Q., Spira, M., Metzger, M. L., Drachtman, R. A., et al. (2018). Brentuximab vedotin with gemcitabine for paediatric and young adult patients with relapsed or refractory hodgkin's lymphoma (AHOD1221): a Children's oncology group, multicentre single-arm, phase 1-2 trial. Lancet Oncol. 19 (9), 1229–1238. doi:10.1016/s1470-2045(18)30426-1
Cobert, A. M., Helms, C., Larck, C., and Moore, D. C. (2020). Risk of hepatotoxicity with trastuzumab emtansine in breast cancer patients: a systematic review and meta-analysis. Ther. Adv. Drug Saf. 11, 2042098620915058. doi:10.1177/2042098620915058
Connors, J. M., Jurczak, W., Straus, D. J., Ansell, S. M., Kim, W. S., Gallamini, A., et al. (2018). Brentuximab vedotin with chemotherapy for stage III or IV hodgkin's lymphoma. N. Engl. J. Med. 378 (4), 331–344. doi:10.1056/NEJMoa1708984
Crescioli, S., Kaplon, H., Wang, L., Visweswaraiah, J., Kapoor, V., and Reichert, J. M. (2025). Antibodies to watch in 2025. MAbs 17 (1), 2443538. doi:10.1080/19420862.2024.2443538
CTEP (2017). Institute NCINC. Available online at: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf (Accessed November 24, 2024).
Dailymed (2025d). INC. S. TIVDAK- tisotumab vedotin injection, powder, for solution. Available online at: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c9fe3f32-4219-466e-acb9-3f609b4f4df1 (Accessed November 24, 2024).
Dacoregio, M. I., Michelon, I., Ernesto do Rego Castro, C., Cezar Aquino de Moraes, F., Rossato de Almeida, G., Ravani, L. V., et al. (2024). Safety profile of sacituzumab govitecan in patients with breast cancer: a systematic review and meta-analysis. Breast 79, 103853. doi:10.1016/j.breast.2024.103853
Dailymed (2024). Wyeth pharmaceuticals LLC asoPI. BESPONSA-inotuzumab ozogamicin injection, powder, lyophilized, for solution. Available online at: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=cc7014b1-c775-411d-b374-8113248b4077 (Accessed November 24, 2024).
Dailymed (2025a). INC. S. ADCETRIS- brentuximab vedotin injection, powder, lyophilized, for solution. Available online at: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3904f8dd-1aef-3490-e48f-bd55f32ed67f (Accessed November 25, 2024).
Dailymed (2025b). INC S. PADCEV EJFV- enfortumab vedotin injection, powder, lyophilized, for solution. Available online at: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b5631d3e-4604-4363-8f20-11dfc5a4a8ed (Accessed November 27, 2024).
Dailymed (2025c). Gilead sciences I. TRODELVY- sacituzumab govitecan powder, for solution. Available online at: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=57a597d2-03f0-472e-b148-016d7169169d (Accessed November 27, 2024).
Dailymed (2025e). ImmunoGen IRP. ELAHERE-mirvetuximab soravtansine injection, solution. Available online at: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=00c424b5-6ccd-48ab-9e88-1986451120e2 (Accessed November 30, 2024).
de Bono, J. S., Concin, N., Hong, D. S., Thistlethwaite, F. C., Machiels, J. P., Arkenau, H. T., et al. (2019). Tisotumab vedotin in patients with advanced or metastatic solid tumours (InnovaTV 201): a first-in-human, multicentre, phase 1-2 trial. Lancet Oncol. 20 (3), 383–393. doi:10.1016/s1470-2045(18)30859-3
DeeksVedotin, E. D. D. (2021). Disitamab vedotin: first approval. Drugs 81 (16), 1929–1935. doi:10.1007/s40265-021-01614-x
Dimopoulos, M. A., Hungria, V. T. M., Radinoff, A., Delimpasi, S., Mikala, G., Masszi, T., et al. (2023). Efficacy and safety of single-agent belantamab mafodotin versus pomalidomide plus low-dose dexamethasone in patients with relapsed or refractory multiple myeloma (DREAMM-3): a phase 3, open-label, randomised study. Lancet Haematol. 10 (10), e801–e812. doi:10.1016/s2352-3026(23)00243-0
Döhner, H., Weber, D., Krzykalla, J., Fiedler, W., Kühn, M. W. M., Schroeder, T., et al. (2023). Intensive chemotherapy with or without gemtuzumab ozogamicin in patients with NPM1-mutated acute myeloid leukaemia (AMLSG 09-09): a randomised, open-label, multicentre, phase 3 trial. Lancet Haematol. 10 (7), e495–e509. doi:10.1016/s2352-3026(23)00089-3
Drago, J. Z., Modi, S., and Chandarlapaty, S. (2021). Unlocking the potential of antibody-drug conjugates for cancer therapy. Nat. Rev. Clin. Oncol. 18 (6), 327–344. doi:10.1038/s41571-021-00470-8
Dumontet, C., Reichert, J. M., Senter, P. D., Lambert, J. M., and Beck, A. (2023). Antibody-drug conjugates come of age in oncology. Nat. Rev. Drug Discov. 22 (8), 641–661. doi:10.1038/s41573-023-00709-2
Endo, Y., Takeda, K., Mohan, N., Shen, Y., Jiang, J., Rotstein, D., et al. (2018). Payload of T-DM1 binds to cell surface cytoskeleton-associated protein 5 to mediate cytotoxicity of hepatocytes. Oncotarget 9 (98), 37200–37215. doi:10.18632/oncotarget.26461
European Association for the Study of the Liver (2019). EASL clinical practice guidelines: drug-induced liver injury. J. Hepatol. 70 (6), 1222–1261. doi:10.1016/j.jhep.2019.02.014
FAERS (2025a). Trastuzumab deruxtecan. Available online at: https://fis.fda.gov/sense/app/95239e26-e0be-42d9-a960-9a5f7f1c25ee/sheet/45beeb74-30ab-46be-8267-5756582633b4/state/analysis (Accessed November 28, 2024).
FAERS (2025b). Tisotumab vedotin. Available online at: https://fis.fda.gov/sense/app/95239e26-e0be-42d9-a960-9a5f7f1c25ee/sheet/45beeb74-30ab-46be-8267-5756582633b4/state/analysis (Accessed November 28, 2024).
FAERS (2025c). Mirvetuximab soravtansine. Available online at: https://fis.fda.gov/sense/app/95239e26-e0be-42d9-a960-9a5f7f1c25ee/sheet/45beeb74-30ab-46be-8267-5756582633b4/state/analysis (Accessed November 27, 2024).
Fettiplace, A., Marcinak, J., Merz, M., Zhang, H. T., Kikuchi, L., Regev, A., et al. (2024). Review article: recommendations for detection, assessment and management of suspected drug-induced liver injury during clinical trials in oncology patients. Aliment. Pharmacol. Ther. 60 (10), 1293–1307. doi:10.1111/apt.18271
Fischer, D., Toenges, R., Kiil, K., Michalik, S., Thalhammer, A., Bug, G., et al. (2024). Liver failure after treatment with inotuzumab and polychemotherapy including PEG-asparaginase in a patient with relapsed Philadelphia chromosome-negative acute lymphoblastic leukemia. Ann. Hematol. 103 (2), 489–498. doi:10.1007/s00277-023-05495-w
Fu, Z., Li, S., Han, S., Shi, C., and Zhang, Y. (2022). Antibody drug conjugate: the “biological missile” for targeted cancer therapy. Signal Transduct. Target Ther. 7 (1), 93. doi:10.1038/s41392-022-00947-7
Gamis, A. S., Alonzo, T. A., Meshinchi, S., Sung, L., Gerbing, R. B., Raimondi, S. C., et al. (2014). Gemtuzumab ozogamicin in children and adolescents with de novo acute myeloid leukemia improves event-free survival by reducing relapse risk: results from the randomized phase III Children’s Oncology Group trial AAML0531. J. Clin. Oncol. 32 (27), 3021–3032. doi:10.1200/jco.2014.55.3628
Gao, S., Zhang, M., Wu, K., Zhu, J., He, Z., Li, J., et al. (2020). Risk of adverse events in lymphoma patients treated with brentuximab vedotin: a systematic review and meta-analysis. Expert Opin. Drug Saf. 19 (5), 617–623. doi:10.1080/14740338.2020.1718103
Garrido, I., Magalhães, A., Lopes, J., and Macedo, G. (2022). Trastuzumab emtansine-induced nodular regenerative hyperplasia: is dose reduction enough as a preventable measure? Dig. Dis. 40 (6), 787–792. doi:10.1159/000521933
Genentech (2024). KADCYLA-ado-trastuzumab emtansine injection, powder, lyophilized, for solution. Available online at: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=23f3c1f4-0fc8-4804-a9e3-04cf25dd302e (Accessed November 24, 2024).
Genentech (2025). I. POLIVY- polatuzumab vedotin injection, powder, lyophilized, for solution. Available online at: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=20a16ab2-f338-4abb-9dcd-254bd949a2bc (Accessed November 27, 2024).
Giles, F. J., Kantarjian, H. M., Kornblau, S. M., Thomas, D. A., Garcia-Manero, G., Waddelow, T. A., et al. (2001). Mylotarg (gemtuzumab ozogamicin) therapy is associated with hepatic venoocclusive disease in patients who have not received stem cell transplantation. Cancer 92 (2), 406–413. doi:10.1002/1097-0142(20010715)92:2<406::aid-cncr1336>3.0.co;2-u
Gorovits, B., and Krinos-Fiorotti, C. (2013). Proposed mechanism of off-target toxicity for antibody-drug conjugates driven by mannose receptor uptake. Cancer Immunol. Immunother. 62 (2), 217–223. doi:10.1007/s00262-012-1369-3
Goto, K., Goto, Y., Kubo, T., Ninomiya, K., Kim, S. W., Planchard, D., et al. (2023). Trastuzumab deruxtecan in patients with HER2-Mutant metastatic non-small-cell lung cancer: primary results from the randomized, phase II DESTINY-Lung02 trial. J. Clin. Oncol. 41 (31), 4852–4863. doi:10.1200/jco.23.01361
Guo, Z., Ding, Y., Wang, M., Liu, J., Zhai, Q., and Du, Q. (2022). Safety of trastuzumab deruxtecan: a meta-analysis and pharmacovigilance study. J. Clin. Pharm. Ther. 47 (11), 1837–1844. doi:10.1111/jcpt.13777
Heo, Y. A. (2023). Mirvetuximab soravtansine: first approval. Drugs 83 (3), 265–273. doi:10.1007/s40265-023-01834-3
Huang, W., Li, L., Zhou, Y., Yang, Q., Mixdorf, J. C., Barnhart, T. E., et al. (2025). Preclinical evaluation of zirconium-89 labeled anti-Trop2 antibody-drug conjugate (trodelvy) for imaging in gastric cancer and triple-negative breast cancer. Eur. J. Nucl. Med. Mol. Imaging 52 (7), 2369–2383. doi:10.1007/s00259-025-07106-4
Hurvitz, S. A., Hegg, R., Chung, W. P., Im, S. A., Jacot, W., Ganju, V., et al. (2023). Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial. Lancet 401 (10371), 105–117. doi:10.1016/s0140-6736(22)02420-5
Izumi, A., Tachibana, T., Ando, T., Tanaka, M., Kanamori, H., and Nakajima, H. (2022). A case series of patients treated with inotuzumab ozogamicin for acute lymphoblastic leukemia relapsed after allogeneic hematopoietic cell transplantation. Int. J. Hematol. 115 (1), 69–76. doi:10.1007/s12185-021-03217-4
Jin, Y., Schladetsch, M. A., Huang, X., Balunas, M. J., and Wiemer, A. J. (2022). Stepping forward in antibody-drug conjugate development. Pharmacol. Ther. 229, 107917. doi:10.1016/j.pharmthera.2021.107917
Kantarjian, H. M., DeAngelo, D. J., Advani, A. S., Stelljes, M., Kebriaei, P., Cassaday, R. D., et al. (2017). Hepatic adverse event profile of inotuzumab ozogamicin in adult patients with relapsed or refractory acute lymphoblastic leukaemia: results from the open-label, randomised, phase 3 INO-VATE study. Lancet Haematol. 4 (8), e387–e398. doi:10.1016/s2352-3026(17)30103-5
Kantarjian, H. M., DeAngelo, D. J., Stelljes, M., Liedtke, M., Stock, W., Gökbuget, N., et al. (2019). Inotuzumab ozogamicin versus standard of care in relapsed or refractory acute lymphoblastic leukemia: final report and long-term survival follow-up from the randomized, phase 3 INO-VATE study. Cancer 125 (14), 2474–2487. doi:10.1002/cncr.32116
Keam, S. J. (2020). Trastuzumab deruxtecan: first approval. Drugs 80 (5), 501–508. doi:10.1007/s40265-020-01281-4
Krop, I. E., LoRusso, P., Miller, K. D., Modi, S., Yardley, D., Rodriguez, G., et al. (2012). A phase II study of trastuzumab emtansine in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer who were previously treated with trastuzumab, lapatinib, an anthracycline, a taxane, and capecitabine. J. Clin. Oncol. 30 (26), 3234–3241. doi:10.1200/jco.2011.40.5902
Krop, I. E., Kim, S. B., Martin, A. G., LoRusso, P. M., Ferrero, J. M., Badovinac-Crnjevic, T., et al. (2017). Trastuzumab emtansine versus treatment of physician's choice in patients with previously treated HER2-positive metastatic breast cancer (TH3RESA): final overall survival results from a randomised open-label phase 3 trial. Lancet Oncol. 18 (6), 743–754. doi:10.1016/s1470-2045(17)30313-3
Kurt, M., Shorbagi, A., Altundag, K., Elkiran, T., Güllü, I., and Kansu, E. (2005). Possible association between budd-chiari syndrome and gemtuzumab ozogamicin treatment in a patient with refractory acute myelogenous leukemia. Am. J. Hematol. 80 (3), 213–215. doi:10.1002/ajh.20432
Lambert, J., Pautas, C., Terré, C., Raffoux, E., Turlure, P., Caillot, D., et al. (2019). Gemtuzumab ozogamicin for de novo acute myeloid leukemia: final efficacy and safety updates from the open-label, phase III ALFA-0701 trial. Haematologica 104 (1), 113–119. doi:10.3324/haematol.2018.188888
Lannoy, D., Decaudin, B., Grozieux de Laguérenne, A., Barrier, F., Pignon, J. M., Wetterwald, M., et al. (2006). Gemtuzumab ozogamicin-induced sinusoidal obstructive syndrome treated with defibrotide: a case report. J. Clin. Pharm. Ther. 31 (4), 389–392. doi:10.1111/j.1365-2710.2006.00742.x
Larue, M., Malard, F., Alaskar, A. S., Aljurf, M., Arat, M., Balsat, M., et al. (2025). Management of liver sinusoidal obstruction syndrome/veno-occlusive disease in adults: a 2025 perspective from an international expert group. Bone Marrow Transpl. 60 (7), 1002–1008. doi:10.1038/s41409-025-02598-y
Lee, A. (2021). Loncastuximab tesirine: first approval. Drugs 81 (10), 1229–1233. doi:10.1007/s40265-021-01550-w
Lee, J., Yoon, J. H., Kwag, D., Lee, J. H., Kim, T. Y., Min, G. J., et al. (2021). Hepatic venoocclusive disease/sinusoidal obstruction syndrome with normal portal vein flow mimicking aggravated chronic hepatic GVHD following inotuzumab ozogamicin salvage therapy: a case report of pathologic-radiologic discrepancy. Ther. Adv. Hematol. 12, 20406207211066176. doi:10.1177/20406207211066176
Lepelley, M., Allouchery, M., Long, J., Boucherle, D., Ranchoup, Y., Le Marc'Hadour, F., et al. (2018). Nodular regenerative hyperplasia induced by trastuzumab emtansine: role of emtansine? Ann. Hepatol. 17 (6), 1067–1071. doi:10.5604/01.3001.0012.7207
Li, X., Xu, S. N., Qin, D. B., Tan, Y., Gong, Q., and Chen, J. P. (2014). Effect of adding gemtuzumab ozogamicin to induction chemotherapy for newly diagnosed acute myeloid leukemia: a meta-analysis of prospective randomized phase III trials. Ann. Oncol. 25 (2), 455–461. doi:10.1093/annonc/mdt566
Li, X., Zhou, M., Qi, J., and Han, Y. (2021). Efficacy and safety of inotuzumab ozogamicin (CMC-544) for the treatment of relapsed/refractory acute lymphoblastic leukemia and non-hodgkin lymphoma: a systematic review and meta-analysis. Clin. Lymphoma Myeloma Leuk. 21 (3), e227–e247. doi:10.1016/j.clml.2020.12.008
Linehan, S. A., Martínez-Pomares, L., Stahl, P. D., and Gordon, S. (1999). Mannose receptor and its putative ligands in normal murine lymphoid and nonlymphoid organs: in situ expression of mannose receptor by selected macrophages, endothelial cells, perivascular microglia, and mesangial cells, but not dendritic cells. J. Exp. Med. 189 (12), 1961–1972. doi:10.1084/jem.189.12.1961
Liu, L. (2018). Pharmacokinetics of monoclonal antibodies and Fc-fusion proteins. Protein Cell 9 (1), 15–32. doi:10.1007/s13238-017-0408-4
Liu, K., Li, Y. H., Zhang, X., Su, L., Li, J. H., Shi, H. Y., et al. (2022). Incidence and risk of severe adverse events associated with trastuzumab emtansine (T-DM1) in the treatment of breast cancer: an up-to-date systematic review and meta-analysis of randomized controlled clinical trials. Expert Rev. Clin. Pharmacol. 15 (11), 1343–1350. doi:10.1080/17512433.2022.2121704
Liu, W., Du, Q., Guo, Z., Ye, X., and Liu, J. (2023). Post-marketing safety surveillance of sacituzumab govitecan: an observational, pharmacovigilance study leveraging FAERS database. Front. Pharmacol. 14, 1283247. doi:10.3389/fphar.2023.1283247
Liu, Y., Wang, X., Zhang, N., He, S., Zhang, J., Xu, X., et al. (2025). Utility of (131)I-HLX58-Der for the precision treatment: evaluation of a preclinical radio-antibody-drug-conjugate approach in mouse models. Int. J. Nanomedicine 20, 723–739. doi:10.2147/ijn.S501689
Lonial, S., Lee, H. C., Badros, A., Trudel, S., Nooka, A. K., Chari, A., et al. (2020). Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): a two-arm, randomised, open-label, phase 2 study. Lancet Oncol. 21 (2), 207–221. doi:10.1016/s1470-2045(19)30788-0
López, D. S A., Díaz-Tejeiro, C., Poyatos-Racionero, E., Nieto-Jiménez, C., Paniagua-Herranz, L., Sanvicente, A., et al. (2023). Considerations for the design of antibody drug conjugates (ADCs) for clinical development: lessons learned. J. Hematol. Oncol. 16 (1), 118. doi:10.1186/s13045-023-01519-0
Lyon, R. P., Bovee, T. D., Doronina, S. O., Burke, P. J., Hunter, J. H., Neff-LaFord, H. D., et al. (2015). Reducing hydrophobicity of homogeneous antibody-drug conjugates improves pharmacokinetics and therapeutic index. Nat. Biotechnol. 33 (7), 733–735. doi:10.1038/nbt.3212
Mahalingaiah, P. K., Ciurlionis, R., Durbin, K. R., Yeager, R. L., Philip, B. K., Bawa, B., et al. (2019). Potential mechanisms of target-independent uptake and toxicity of antibody-drug conjugates. Pharmacol. Ther. 200, 110–125. doi:10.1016/j.pharmthera.2019.04.008
Maniecki, M. B., Hasle, H., Bendix, K., and Møller, H. J. (2011). Is hepatotoxicity in patients treated with gemtuzumabozogamicin due to specific targeting of hepatocytes? Leuk. Res. 35 (6), e84–e86. doi:10.1016/j.leukres.2011.01.025
Markham, A. (2020). Belantamab mafodotin: first approval. Drugs 80 (15), 1607–1613. doi:10.1007/s40265-020-01404-x
Markides, D. M., Hita, A. G., Merlin, J., Reyes-Gibby, C., and Yeung, S. J. (2025). Antibody-drug conjugates: the toxicities and adverse effects that emergency physicians must know. Ann. Emerg. Med. 85 (3), 214–229. doi:10.1016/j.annemergmed.2024.10.015
McDonald, G. B., Freston, J. W., Boyer, J. L., and DeLeve, L. D. (2019). Liver complications following treatment of hematologic malignancy with Anti-CD22-Calicheamicin (inotuzumab ozogamicin). Hepatology 69 (2), 831–844. doi:10.1002/hep.30222
McKertish, C. M., and Kayser, V. (2021). Advances and limitations of antibody drug conjugates for cancer. Biomedicines 9 (8), 872. doi:10.3390/biomedicines9080872
Mokresh, M. E., Alomari, O., Varda, A., Akdag, G., and Odabas, H. (2024). Safety and efficacy of tisotumab vedotin with cervical cancers: a systematic review and meta-analysis. J. Obstet. Gynaecol. Res. 50 (12), 2195–2210. doi:10.1111/jog.16126
Moore, K. N., Oza, A. M., Colombo, N., Oaknin, A., Scambia, G., Lorusso, D., et al. (2021). Phase III, randomized trial of mirvetuximab soravtansine versus chemotherapy in patients with platinum-resistant ovarian cancer: primary analysis of FORWARD I. Ann. Oncol. 32 (6), 757–765. doi:10.1016/j.annonc.2021.02.017
Moore, K. N., Lorusso, D., Oaknin, A., Oza, A., Colombo, N., Van Gorp, T., et al. (2024). Safety and tolerability of mirvetuximab soravtansine monotherapy for folate receptor alpha-expressing recurrent ovarian cancer: an integrated safety summary. Gynecol. Oncol. 191, 249–258. doi:10.1016/j.ygyno.2024.10.013
Mustafayev, K., and Torres, H. (2022). Hepatitis B virus and hepatitis C virus reactivation in cancer patients receiving novel anticancer therapies. Clin. Microbiol. Infect. 28 (10), 1321–1327. doi:10.1016/j.cmi.2022.02.042
Musto, P., and La Rocca, F. (2020). Monoclonal antibodies in relapsed/refractory myeloma: updated evidence from clinical trials, real-life studies, and meta-analyses. Expert Rev. Hematol. 13 (4), 331–349. doi:10.1080/17474086.2020.1740084
Neeman, J., Friedman, A., and McKendrick, J. (2019). Acute liver injury leading to death in the setting of brentuximab vedotin monotherapy. Leuk. Lymphoma 60 (9), 2283–2286. doi:10.1080/10428194.2019.1579321
Nguyen, T. D., Bordeau, B. M., and Balthasar, J. P. (2023). Mechanisms of ADC toxicity and strategies to increase ADC tolerability. Cancers (Basel) 15 (3), 713. doi:10.3390/cancers15030713
Nie, C., Xu, W., Guo, Y., Gao, X., Lv, H., Chen, B., et al. (2023). Immune checkpoint inhibitors enhanced the antitumor efficacy of disitamab vedotin for patients with HER2-positive or HER2-low advanced or metastatic gastric cancer: a multicenter real-world study. BMC Cancer 23 (1), 1239. doi:10.1186/s12885-023-11735-z
Nooka, A. K., Cohen, A. D., Lee, H. C., Badros, A., Suvannasankha, A., Callander, N., et al. (2023). Single-agent belantamab mafodotin in patients with relapsed/refractory multiple myeloma: final analysis of the DREAMM-2 trial. Cancer 129 (23), 3746–3760. doi:10.1002/cncr.34987
O'Sullivan, C. C., Higgins, A. S., Alkurashi, A. K., Ahluwalia, V., Taraba, J. L., McKie, P. M., et al. (2024). Hepatopulmonary syndrome associated with long-term use of ado-trastuzumab emtansine (T-DM1) for treatment of HER2-positive metastatic breast cancer - a case report and series. Front. Oncol. 14, 1434492. doi:10.3389/fonc.2024.1434492
Ocean, A. J., Starodub, A. N., Bardia, A., Vahdat, L. T., Isakoff, S. J., Guarino, M., et al. (2017). Sacituzumab govitecan (IMMU-132), an anti-Trop-2-SN-38 antibody-drug conjugate for the treatment of diverse epithelial cancers: safety and pharmacokinetics. Cancer 123 (19), 3843–3854. doi:10.1002/cncr.30789
Party CsOGR (2025). Combination chemotherapy with or without gemtuzumab in treating young patients with newly diagnosed acute myeloid leukemia. Available online at: https://clinicaltrials.gov/study/NCT00372593?term=AAML0531&limit=10&rank=2#study-plan (Accessed November 28, 2024).
Party SIR (2025). A trial of tisotumab vedotin in cervical cancer. Available online at: https://clinicaltrials.gov/study/NCT03438396?term=innovaTV%20204%E2%80%A0&limit=10&rank=1 (Accessed November 24, 2024).
Pawinska-Wasikowska, K., Czogala, M., Skoczen, S., Surman, M., Rygielska, M., Ksiazek, T., et al. (2023). Gemtuzumab ozogamicin for relapsed or primary refractory acute myeloid leukemia in children-the Polish pediatric leukemia and lymphoma study group experience. Front. Immunol. 14, 1268993. doi:10.3389/fimmu.2023.1268993
Peng, Z., Liu, T., Wei, J., Wang, A., He, Y., Yang, L., et al. (2021). Efficacy and safety of a novel anti-HER2 therapeutic antibody RC48 in patients with HER2-overexpressing, locally advanced or metastatic gastric or gastroesophageal junction cancer: a single-arm phase II study. Cancer Commun. (Lond). 41 (11), 1173–1182. doi:10.1002/cac2.12214
Poisson, J., Lemoinne, S., Boulanger, C., Durand, F., Moreau, R., Valla, D., et al. (2017). Liver sinusoidal endothelial cells: physiology and role in liver diseases. J. Hepatol. 66 (1), 212–227. doi:10.1016/j.jhep.2016.07.009
Polson, A. G., Calemine-Fenaux, J., Chan, P., Chang, W., Christensen, E., Clark, S., et al. (2009). Antibody-drug conjugates for the treatment of non-hodgkin's lymphoma: target and linker-drug selection. Cancer Res. 69 (6), 2358–2364. doi:10.1158/0008-5472.Can-08-2250
Powles, T., Rosenberg, J. E., Sonpavde, G. P., Loriot, Y., Durán, I., Lee, J. L., et al. (2021). Enfortumab vedotin in previously treated advanced urothelial carcinoma. N. Engl. J. Med. 384 (12), 1125–1135. doi:10.1056/NEJMoa2035807
Raghav, K., Siena, S., Takashima, A., Kato, T., Van den Eynde, M., Pietrantonio, F., et al. (2024). Trastuzumab deruxtecan in patients with HER2-positive advanced colorectal cancer (DESTINY-CRC02): primary results from a multicentre, randomised, phase 2 trial. Lancet Oncol. 25 (9), 1147–1162. doi:10.1016/s1470-2045(24)00380-2
RemeGen (2025). Disitamab vedotin for injection. Available online at: https://www.cde.org.cn/main/xxgk/postmarketpage?acceptidCODE=3b64008bb6186c26d19a65daf56bdd97 (Accessed November 27, 2024).
Ruan, D. Y., Wu, H. X., Meng, Q., and Xu, R. H. (2024). Development of antibody-drug conjugates in cancer: overview and prospects. Cancer Commun. (Lond) 44 (1), 3–22. doi:10.1002/cac2.12517
Rugo, H. S., Bardia, A., Marmé, F., Cortes, J., Schmid, P., Loirat, D., et al. (2022). Sacituzumab govitecan in hormone receptor-Positive/Human epidermal growth factor receptor 2-Negative metastatic breast cancer. J. Clin. Oncol. 40 (29), 3365–3376. doi:10.1200/jco.22.01002
Rugo, H. S., Bardia, A., Marmé, F., Cortés, J., Schmid, P., Loirat, D., et al. (2023). Overall survival with sacituzumab govitecan in hormone receptor-positive and human epidermal growth factor receptor 2-negative metastatic breast cancer (TROPiCS-02): a randomised, open-label, multicentre, phase 3 trial. Lancet 402 (10411), 1423–1433. doi:10.1016/s0140-6736(23)01245-x
Sancho, D., and Reis, e S. C. (2012). Signaling by myeloid C-type lectin receptors in immunity and homeostasis. Annu. Rev. Immunol. 30, 491–529. doi:10.1146/annurev-immunol-031210-101352
Saviola, A., Luppi, M., Potenza, L., Morselli, M., Ferrari, A., Riva, G., et al. (2003). Late occurrence of hepatic veno-occlusive disease following gemtuzumab ozogamicin: successful treatment with defibrotide. Br. J. Haematol. 123 (4), 752–753. doi:10.1046/j.1365-2141.2003.04667.x
Sheng, X., Yan, X., Wang, L., Shi, Y., Yao, X., Luo, H., et al. (2021). Open-label, multicenter, phase II study of RC48-ADC, a HER2-Targeting antibody-drug conjugate, in patients with locally advanced or metastatic urothelial carcinoma. Clin. Cancer Res. 27 (1), 43–51. doi:10.1158/1078-0432.Ccr-20-2488
Sievers, E. L., Larson, R. A., Stadtmauer, E. A., Estey, E., Löwenberg, B., Dombret, H., et al. (2001). Efficacy and safety of gemtuzumab ozogamicin in patients with CD33-positive acute myeloid leukemia in first relapse. J. Clin. Oncol. 19 (13), 3244–3254. doi:10.1200/jco.2001.19.13.3244
Siwicki, M., Gort-Freitas, N. A., Messemaker, M., Bill, R., Gungabeesoon, J., Engblom, C., et al. (2021). Resident kupffer cells and neutrophils drive liver toxicity in cancer immunotherapy. Sci. Immunol. 6 (61), eabi7083. doi:10.1126/sciimmunol.abi7083
Staudacher, A. H., and Brown, M. P. (2017). Antibody drug conjugates and bystander killing: is antigen-dependent internalisation required? Br. J. Cancer 117 (12), 1736–1742. doi:10.1038/bjc.2017.367
Stewart, R., Hammond, S. A., Oberst, M., and Wilkinson, R. W. (2014). The role of Fc gamma receptors in the activity of immunomodulatory antibodies for cancer. J. Immunother. Cancer 2 (1), 29. doi:10.1186/s40425-014-0029-x
Stutz, L., Halter, J. P., Heim, D., Passweg, J. R., and Medinger, M. (2022). Low incidence of hepatic sinusoidal obstruction syndrome/veno-occlusive disease in adults undergoing allogenic stem cell transplantation with prophylactic ursodiol and low-dose heparin. Bone Marrow Transpl. 57 (3), 391–398. doi:10.1038/s41409-021-01546-w
Syed, Y. Y. (2020). Sacituzumab govitecan: first approval. Drugs 80 (10), 1019–1025. doi:10.1007/s40265-020-01337-5
Tack, D. K., Letendre, L., Kamath, P. S., and Tefferi, A. (2001). Development of hepatic veno-occlusive disease after mylotarg infusion for relapsed acute myeloid leukemia. Bone Marrow Transpl. 28 (9), 895–897. doi:10.1038/sj.bmt.1703242
Tan, H. N., Morcillo, M. A., Lopez, J., Minchom, A., Sharp, A., Paschalis, A., et al. (2025). Treatment-related adverse events of antibody drug-conjugates in clinical trials. J. Hematol. Oncol. 18 (1), 71. doi:10.1186/s13045-025-01720-3
Taylor, R. P., and Lindorfer, M. A. (2024). Antibody-drug conjugate adverse effects can be understood and addressed based on immune complex clearance mechanisms. Blood 144 (2), 137–144. doi:10.1182/blood.2024024442
Theocharopoulos, C., Ziogas, I. A., Douligeris, C. C., Efstathiou, A., Kolorizos, E., Ziogas, D. C., et al. (2024). Antibody-drug conjugates for hepato-pancreato-biliary malignancies: “magic bullets” to the rescue? Cancer Treat. Rev. 129, 102806. doi:10.1016/j.ctrv.2024.102806
Van Cutsem, E., di Bartolomeo, M., Smyth, E., Chau, I., Park, H., Siena, S., et al. (2023). Trastuzumab deruxtecan in patients in the USA and Europe with HER2-positive advanced gastric or gastroesophageal junction cancer with disease progression on or after a trastuzumab-containing regimen (DESTINY-Gastric02): primary and updated analyses from a single-arm, phase 2 study. Lancet Oncol. 24 (7), 744–756. doi:10.1016/s1470-2045(23)00215-2
von Minckwitz, G., Huang, C. S., Mano, M. S., Loibl, S., Mamounas, E. P., Untch, M., et al. (2019). Trastuzumab emtansine for residual invasive HER2-Positive breast cancer. N. Engl. J. Med. 380 (7), 617–628. doi:10.1056/NEJMoa1814017
Wang, P., and Xia, L. (2023). RC48-ADC treatment for patients with HER2-expressing locally advanced or metastatic solid tumors: a real-world study. BMC Cancer 23 (1), 1083. doi:10.1186/s12885-023-11593-9
Wang, L., Shi, G., Zhao, G., He, W., Cen, Z., and Xu, F. (2023). Efficacy and safety of enfortumab vedotin in the treatment of advanced urothelial carcinoma: a systematic review and meta-analysis. Anticancer Drugs 34 (4), 473–478. doi:10.1097/cad.0000000000001449
Wang, Y., Liu, L., Jin, X., and Yu, Y. (2024). Efficacy and safety of mirvetuximab soravtansine in recurrent ovarian cancer with FRa positive expression: a systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 194, 104230. doi:10.1016/j.critrevonc.2023.104230
Wang, J., Liu, H., Ran, X., Tan, W., and Liu, Y. (2026). Amplifying tumor recognition and drug susceptibility by multivalent ApDC assembly to combat with tumor cell heterogeneity. Biomaterials 324, 123502. doi:10.1016/j.biomaterials.2025.123502
Wei, Q., Li, P., Yang, T., Zhu, J., Sun, L., Zhang, Z., et al. (2024). The promise and challenges of combination therapies with antibody-drug conjugates in solid tumors. J. Hematol. Oncol. 17 (1), 1. doi:10.1186/s13045-023-01509-2
Wyeth Pharmaceuticals LLC asoPI (2021). MYLOTARG-gemtuzumab ozogamicin injection, powder, lyophilized, for solution. Available online at: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=32fd2bb2-1cfa-4250-feb8-d7956c794e05 (Accessed November 24, 2024).
Xu, Q., He, S., and Yu, L. (2021). Clinical benefits and safety of gemtuzumab ozogamicin in treating acute Myeloid leukemia in various subgroups: an updated systematic review, Meta-analysis, and network meta-analysis. Front. Immunol. 12, 683595. doi:10.3389/fimmu.2021.683595
Xu, K., Wang, T., Pan, S., and He, J. (2023a). The efficacy and toxicity of mirvetuximab soravtansine, a novel antibody-drug conjugate, in the treatment of advanced or recurrent ovarian cancer: a meta-analysis. Expert Rev. Clin. Pharmacol. 16 (11), 1141–1152. doi:10.1080/17512433.2023.2262673
Xu, B., Ma, F., Wang, T., Wang, S., Tong, Z., Li, W., et al. (2023b). A phase IIb, single arm, multicenter trial of sacituzumab govitecan in Chinese patients with metastatic triple-negative breast cancer who received at least two prior treatments. Int. J. Cancer 152 (10), 2134–2144. doi:10.1002/ijc.34424
Xu, Z., Huang, D., Liu, Q., Liu, S., Liu, J., Wang, H., et al. (2025). A real-world pharmacovigilance analysis of the FDA adverse event reporting system events for polatuzumab vedotin. Expert Opin. Drug Saf. 24 (1), 49–57. doi:10.1080/14740338.2024.2348572
Zheng, F., Du, Y., Yuan, Y., Wang, Z., Li, S., Xiong, S., et al. (2024). Risk analysis of enfortumab vedotin: a real-world approach based on the FAERS database. Heliyon 10 (18), e37544. doi:10.1016/j.heliyon.2024.e37544
Zhou, Y. X., Wang, J. L., Mu, X. L., Zhu, Y. J., Chen, Y., and Liu, J. Y. (2023). Efficacy and safety analysis of a HER2-targeting antibody-drug conjugate combined with immune checkpoint inhibitors in solid tumors: a real-world study. Aging (Albany NY) 15 (24), 15473–15488. doi:10.18632/aging.205382
Zhou, K., Liu, X., and Zhu, H. (2025). Overcoming resistance to antibody-drug conjugates: from mechanistic insights to cutting-edge strategies. J. Hematol. Oncol. 18 (1), 96. doi:10.1186/s13045-025-01752-9
Keywords: antibody-drug conjugate, clinical trial, adverse event, hepatotoxicity, immunotherapy
Citation: Dong Y, Zhi Y, Li X, Ma M, Ye M, Huang S, Tang J, Zhong W, Lei X and Mao Y (2025) Navigating hepatotoxicity of antibody-drug conjugates: from mechanistic insights to clinical and postmarketing evidence. Front. Pharmacol. 16:1694436. doi: 10.3389/fphar.2025.1694436
Received: 28 August 2025; Accepted: 11 November 2025;
Published: 05 December 2025.
Edited by:
Zhiyu Zhang, Fourth Affiliated Hospital of China Medical University, ChinaReviewed by:
Michelle Teo, UCSI University, MalaysiaToshiaki Takakura, Wakayama Medical University, Japan
Copyright © 2025 Dong, Zhi, Li, Ma, Ye, Huang, Tang, Zhong, Lei and Mao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yimin Mao, bWFveW0xMTk2OEAxNjMuY29t
†These authors have contributed equally to this work
 Yinuo Dong1†
Yinuo Dong1† Minyan Ye
Minyan Ye Sha Huang
Sha Huang Yimin Mao
Yimin Mao