- 1Biochemistry, Pharmacology and Newborn Screening Unit, Central Laboratory of Analysis, IRCCS Istituto Giannina Gaslini, Genoa, Italy
- 2Pediatric Emergency Room and Emergency Medicine, IRCCS Istituto Giannina Gaslini, Genoa, Italy
- 3Pediatric Pain and Palliative Care Service, IRCCS Istituto Giannina Gaslini, Genoa, Italy
- 4Department of Internal Medicine, Pharmacology & Toxicology Unit, University of Genoa, Genoa, Italy
- 5Clinical Pharmacology Unit, Ente Ospedaliero Ospedali Galliera, Genoa, Italy
Accidental exposure to edible cannabis products in children is an increasing public health concern. The clinical presentation is often nonspecific, which can delay diagnosis and lead to inappropriate management. Toxicological screening is therefore essential for accurate diagnosis and appropriate treatment. We report the toxicokinetic (TK) profiles of delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) following unintentional ingestion of cannabis in two pediatric cases, aged 12 and 15 months, respectively. Plasma concentrations of THC and CBD were measured using a validated liquid chromatography–tandem mass spectrometry (LC-MS/MS) method. THC was consistently detected in all plasma samples collected (four per patient), with highest measured concentrations of 45.0 μg/L in Case 1 and 54.7 μg/L in Case 2. CBD was not detected in Case 1, whereas in Case 2 it was measurable only in the first plasma sample, at a concentration of 1.11 μg/L. Non-compartmental analysis (NCA) of the THC concentration–time data enabled calculation of the TK profiles in both cases. The elimination rate constant (kel) was 0.013 h−1 in Case 1 and 0.031 h−1 in Case 2, corresponding to an half-life (t½) of 52.5 and 21.7 h, respectively. Given the variability and unpredictability of THC/CBD TK monitoring drug levels over time is crucial for managing intoxications in children.
Introduction
The escalating accidental exposure of children to edible Cannabis (Cannabaceae family) products and passive smoke is becoming a significant concern (Richards et al., 2017; Vo et al., 2018). This issue includes instances of passive smoke inhalation, ingestion of various products made from different species of Cannabis plants, such as cannabis-infusions or cookie-like preparations, and the availability of unprotected or partially consumed hashish or joints (Richards et al., 2017). In many cases, ingestion occurs when young children encounter and consume cannabis-containing products (CCPs) that have been left unattended by adults (Richards et al., 2017; Vo et al., 2018). This type of exposure is often described as “exploratory,” as children naturally investigate unfamiliar objects by mouthing or ingesting them (Richards et al., 2017). Ingestion represents the most common route of intoxication in pediatric cases, with symptom onset typically occurring within 1.5–3.5 h, although delays up to 4–6 h have been reported. The most frequently observed clinical signs in children exposed to CCPs include lethargy, ataxia, hypotonia, mydriasis, tachycardia, and hypoventilation (Richards et al., 2017; Morini et al., 2018; Spadari et al., 2009; Gaulier et al., 2002; Carstairs et al., 2011; Macnab et al., 1989). These symptoms often persist for a prolonged duration, ranging from 6 to 8 h up to 24 h (Richards et al., 2017; Morini et al., 2018; Spadari et al., 2009; Gaulier et al., 2002; Carstairs et al., 2011; Macnab et al., 1989). Due to the nonspecific nature of these symptoms, Cannabis intoxication can be easily misdiagnosed, potentially leading to inappropriate interventions such as antibiotic therapy, unnecessary imaging, or invasive diagnostic procedures like lumbar puncture (Lavi et al., 2016). For this reason, toxicological screening is essential for accurate diagnosis and appropriate clinical management.
The two main components of Cannabis are 9-tetrahydrocannabinol (THC) and cannabidiol (CBD). Given the highly variable and unpredictable pharmacokinetics (PK) of THC and CBD, both in therapeutic contexts and intoxication, plasma level monitoring is strongly recommended (Cafaro et al., 2025; Pigliasco et al., 2024).
In this paper we describe the clinical presentation and corresponding plasma levels of THC and CBD following unintentional ingestion of CCPs in two pediatric patients.
Case presentation
Case 1
A 1-year-old female patient was transferred from a peripheral hospital due to drowsiness and a suspected critical episode. The episode was described as crying followed by stiffening and shaking of the limbs, lasting a few seconds. In the days leading up to admission, she had been generally well, with only a mild cough and rhinitis, and no fever or other significant symptoms. The patient’s parents denied any history of trauma or accidental drug ingestion. Upon physical examination, the patient weighed 10 kg, had a Glasgow Coma Scale (GCS) score of 8, a heart rate of 174 bpm, respiratory rate of 15 breaths/min, and oxygen saturation (SaO2) of 96%, Poisoning Severity Score (PSS) (Persson et al., 1998) of 3. She was unconscious, responding only to painful stimuli, with noted mydriasis, absent photomotor reflexes, and generalized hypotonia. Cardiothoracic and abdominal examinations were unremarkable, with no meningeal signs. Hydration status was normal, and capillary refill time was less than 2 s. Endorectal administration of 5 mg of diazepam (Micropam®, 5 mg/2.5 mL, rectal solution) did not provide any benefit. Laboratory tests, including blood chemistry and venous pH, were within normal limits. An urgent brain cranial computed tomography (CT) scan showed no abnormalities. Intravenous hydration was initiated with normal saline and 5% dextrose at 40 mL/h, later increased to 70 mL/h. Urine toxicology screening was positive for THC, with results confirmed by both the initial Quidel Triage TOX immunoassay (94600; Quidel Cardiovascular Inc., San Diego, United States) and subsequent LC-MS/MS analysis.
Case 2
Male, 15 months old, transferred from a peripheral hospital due to a state of unconsciousness that arose after an afternoon nap, a doubtful critical episode treated with midazolam and levetiracetam without benefit. In the previous days, regular wellbeing. No trauma reported, accidental intake of drugs and/or other substances denied. On physical examination, the child weighed 12 kg, with a GCS score of 3, a heart rate of 100 bpm, a respiratory rate of 16 breaths/min, and a SaO2 of 98%, PSS of 3 (Persson et al., 1998). He was unconscious but responsive to painful stimuli, with fixed mydriasis, absent pupillary reflexes, trismus, and nuchal rigidity. The patient also presented with a flexed posture and more pronounced hyposthenia on the right side. Capillary refill time was less than 2 s, while cardiothoracic and abdominal examinations were normal. Initial investigations, including blood tests, brain CT, EEG, and ECG, showed no abnormalities. An immunometric urine toxicology screening conducted at the peripheral hospital, which focused solely on drugs of abuse, returned a positive result for benzodiazepines, consistent with the medications administered upon admission. The subsequent brain magnetic resonance imaging was also negative. Intravenous hydration with normal saline and 5% dextrose was initiated at 50 mL/h. The patient received ceftriaxone and acyclovir after consultation with an infectious disease specialist for suspected meningoencephalitis. The patient was transferred to the intensive care unit (ICU), where a lumbar puncture was performed to investigate suspected encephalitis, which returned negative results. A toxicological assessment was subsequently performed under chain of custody. The initial urine toxicology screening, conducted using the Quidel Triage TOX immunoassay (94600; Quidel Cardiovascular Inc., San Diego, United States), tested positive for THC. This finding was confirmed by a follow-up analysis using LC-MS/MS.
Toxicokinetic analysis
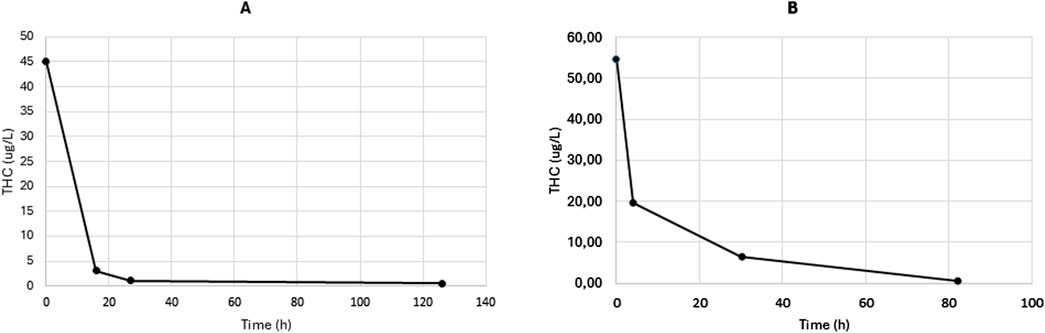
Blood samples for TK analysis were collected by venipuncture into K3EDTA tubes (four samples at random time per patient). Plasma was separated by centrifugation at 4,000 g for 5 min and stored at −20 °C until analysis. Quantification of THC and CBD plasma concentrations was performed at the Giannina Gaslini Institute (Genoa, Italy) using a previously validated liquid chromatography–tandem mass spectrometry (LC-MS/MS) method, as described by our group (Barco et al., 2018). The 8-point calibration curve ranged from 0.2 to 300 μg/L for both THC and CBD. The THC concentration–time profiles for both patients are shown in Figure 1 (panel A: Case 1; panel B: Case 2). In Case 1, the ingestion of CCP was estimated to have occurred approximately 7 h prior to the first blood sample (THC concentration: 45.0 μg/L). In Case 2, ingestion was presumed to have occurred approximately 10 h before the first sample was drawn (THC concentration: 54.7 μg/L). CBD was undetectable in all samples from Case 1, while it was measurable only in the first plasma sample from Case 2 (CBD concentration: 1.11 μg/L).
TK parameters were determined using non-compartmental analysis (NCA) of the concentration–time data, performed with Phoenix WinNonlin Professional Edition v8.4 (Certara, France Sarl). The elimination rate constant (kel) of THC was calculated by log-linear regression of the terminal phase of the plasma concentration–time curve. Because the exact time of ingestion was unknown in both cases, the first concentration point was considered as time zero and excluded from the regression analysis. The estimated THC kel values were 0.013 h−1 for Case 1 and 0.031 h−1 for Case 2, corresponding to terminal half-lives (t½) of 52.5 and 21.7 h, respectively. Plasma concentrations and toxicokinetic parameters of Δ9-THC and CBD in the two cases are summarized in Table 1.
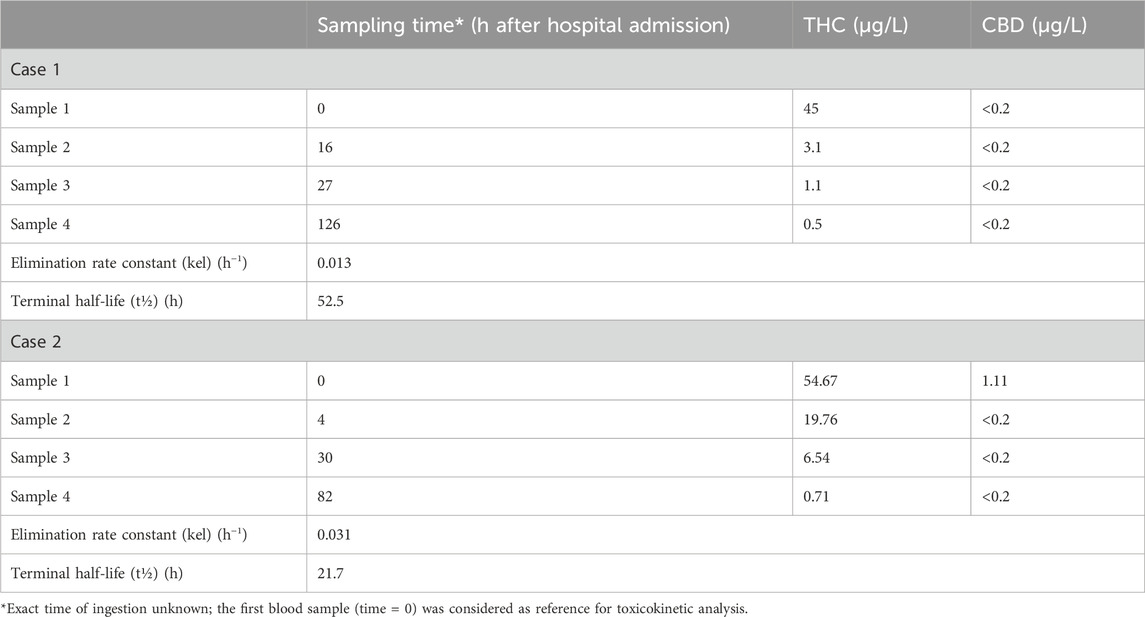
Table 1. Plasma concentrations and toxicokinetic parameters of Δ9-THC and CBD in the two pediatric intoxication cases.
Discussion
The two cases presented highlight the importance of considering Cannabis intoxication in the differential diagnosis of infants presenting with unexplained neurological symptoms, particularly in the absence of fever or signs of infection. As Cannabis use becomes increasingly widespread and accidental pediatric exposures rise, emergency physicians and pediatricians must remain vigilant in recognizing the potential for THC intoxication in young children.
Both infants in our cases exhibited central nervous system depression, a hallmark feature of Cannabis intoxication, yet their initial workups included considerations of infectious and metabolic causes. This underscores a critical issue: the potential for unnecessary and invasive investigations, such as lumbar punctures, CT scans, or empirical antiviral and antibiotic therapies, when a toxicological cause is overlooked. These interventions not only increase healthcare costs and resource utilization but also expose pediatric patients to unnecessary risks, including radiation exposure and medication side effects.
Routine toxicology screening should be strongly considered in infants and young children presenting with altered mental status, hypotonia, or excessive sleepiness in the absence of fever or other clear signs of infection, as it can prevent unnecessary interventions and facilitate timely and appropriate clinical management.
Rapid urine drug screening represents a non-invasive, cost-effective, and timely diagnostic tool that can prevent unnecessary investigations and accelerate appropriate clinical management. A positive toxicology result facilitates early diagnosis, supports the prompt initiation of supportive care, and provides reassurance to families. It also enables the activation of targeted social interventions to prevent future exposures.
Importantly, a positive screening result should be viewed as a clinical “red flag” warranting more specific and accurate confirmatory testing, such as second-tier toxicological analysis and, when available, therapeutic drug monitoring (TDM) using LC-MS/MS. This stepwise diagnostic strategy ensures precise identification of the substance involved, more accurate assessment of the degree of exposure, and ultimately more informed and effective clinical decision-making.
In our two pediatric intoxication cases, the observed plasma peak concentrations (45 μg/L and 54.7 μg/L, respectively) were markedly higher than the mean Cmax values previously reported in pediatric patients treated with standardized cannabis preparations for refractory epilepsy, analyzed using the same LC–MS/MS methodology (Gherzi et al., 2020). In that cohort, administered THC doses ranged from 6.6 mg to 33.0 mg, yielding proportionally lower plasma concentrations. A comparable dose–Cmax relationship has been reported in adults by Pellesi et al. (2018), who observed mean Cmax values of approximately 3 μg/L following ingestion of 50 mg THC edibles, consistent with the findings of Vandrey et al. (2017).
Conversely, the peak THC concentrations observed in our two cases are consistent with those described by Guidet et al. (2020), who reported plasma THC levels ranging from 4.4 to 127 ng/mL in 10 infants admitted for accidental cannabis intoxication. These similarities further support the clinical plausibility of our toxicokinetic findings and underline the marked variability in systemic THC exposure following unintentional ingestion in young children.
With regard to elimination kinetics, the estimated half-lives (52.5 h and 21.7 h) indicate markedly prolonged THC persistence compared with adult data, where half-lives typically range from 20 to 30 h. When compared with both our pediatric reference data and available adult studies, this prolongation may suggest a dose-dependent kinetic behavior in acute intoxication settings.
Due to limited information on the time interval between ingestion and blood collection, it was not possible to reliably assess the relationship between plasma THC concentrations and the severity of clinical manifestations. However, all subsequent blood samples were collected at defined intervals relative to the patients’ arrival at the hospital, providing a consistent temporal framework for toxicokinetic interpretation.
It should be noted that urine THC concentrations primarily reflect prior exposure and do not predict current impairment (Fitzgerald et al., 2023). Similarly, while the toxicokinetic data obtained in these pediatric cases provide valuable information on the timing and extent of exposure, they cannot be directly translated into an assessment of functional impairment. The toxicokinetic evaluation is further limited by the small number of samples (four per patient) and the inclusion of only two cases, which precludes broader generalization of the findings.
In addition to clinical and toxicokinetic considerations, preventive measures such as caregiver education and safer drug packaging may contribute to reducing the risk of accidental intoxications and to guiding appropriate social and child protection interventions (Amirshahi et al., 2019; Zwiebel et al., 2025). In conclusion, THC intoxication should be included in the differential diagnosis of any infant presenting with unexplained neurological symptoms, particularly in afebrile cases. Implementing early toxicological screening in such scenarios can prevent unnecessary invasive procedures, reduce the misuse of antimicrobials, and improve patient outcomes through appropriate and timely diagnosis.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The study was conducted in accordance with the ethical standards of the institutional and national research committees and with the 1975 Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients’ guardians for publication of the clinical histories. Clinical and laboratory data were collected from medical records as part of routine clinical care, and all information was anonymized prior to analysis.
Author contributions
AC: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Visualization, Writing – original draft, Writing – review and editing. FP: Investigation, Methodology, Writing – review and editing. SB: Software, Visualization, Writing – review and editing. IN: Resources, Writing – review and editing. EP: Writing – review and editing. LM: Writing – review and editing. SM: Data curation, Resources, Writing – review and editing. RB: Writing – review and editing. CD: Resources, Writing – review and editing. FM: Data curation, Formal Analysis, Writing – original draft, Writing – review and editing. GC: Conceptualization, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study has been funded by the Italian Ministry of Health, RC2024 and RC2025.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Amirshahi, M. M., Moss, M. J., Smith, S. W., Nelson, L. S., and Stolbach, A. I. (2019). ACMT position statement: addressing pediatric cannabis exposure. J. Med. Toxicol. 15 (3), 212–214. doi:10.1007/S13181-019-00708-Z
Barco, S., Fucile, C., Manfredini, L., De Grandis, E., Gherzi, M., Martelli, A., et al. (2018). A UHPLC-MS/MS method for the quantification of Δ9-tetrahydrocannabinol and cannabidiol in decoctions and in plasma samples for therapeutic monitoring of medical cannabis. Bioanalysis 10 (24), 2003–2014. doi:10.4155/bio-2018-0184
Cafaro, A., Riva, A., Pigliasco, F., Barco, S., Manca, V., Stella Vari, M., et al. (2025). Long-term plasma monitoring of THC and CBD in pediatric drug-resistant epilepsy: implications for cannabidiol therapy with epidyolex®. Epilepsia Open 10 (5), 1699–1704. doi:10.1002/EPI4.70112
Carstairs, S. D., Fujinaka, M. K., Keeney, G. E., and Ly, B. T. (2011). Prolonged coma in a child due to hashish ingestion with quantitation of THC metabolites in urine. J. Emerg. Med. 41 (3), e69–e71. doi:10.1016/J.JEMERMED.2010.05.032
Fitzgerald, R. L., Umlauf, A., Hubbard, J. A., Hoffman, M. A., Sobolesky, P. M., Ellis, S. E., et al. (2023). Driving under the influence of cannabis: impact of combining toxicology testing with field sobriety tests. Clin. Chem. 69 (7), 724–733. doi:10.1093/CLINCHEM/HVAD054
Gaulier, J. M., Tonnay, V., Benkemoun, P., Comet-Didierjean, P., Gury, D., and Lachâtre, G. (2002). Acute cannabis intoxication in a 10-month-old infant. Arch. Pediatr. 9 (10), 1112–1113. doi:10.1016/S0929-693X(02)00057-X
Gherzi, M., Milano, G., Fucile, C., Calevo, M. G., Mancardi, M. M., Nobili, L., et al. (2020). Safety and pharmacokinetics of medical cannabis preparation in a monocentric series of young patients with drug resistant epilepsy. Complement. Ther. Med. 51 (April), 102402. doi:10.1016/j.ctim.2020.102402
Guidet, C., Gregoire, M., Le Dreau, A., Vrignaud, B., Deslandes, G., and Monteil-Ganière, C. (2020). Cannabis intoxication after accidental ingestion in infants: urine and plasma concentrations of Δ-9-tetrahydrocannabinol (THC), THC-COOH and 11-OH-THC in 10 patients. Clin. Toxicol. (Phila). 58 (5), 421–423. doi:10.1080/15563650.2019.1655569
Lavi, E., Rekhtman, D., Berkun, Y., and Wexler, I. (2016). Sudden onset unexplained encephalopathy in infants: think of cannabis intoxication. Eur. J. Pediatr. 175 (3), 417–420. doi:10.1007/S00431-015-2639-9
Macnab, A., Anderson, E., and Susak, L. (1989). Ingestion of cannabis: a cause of coma in children. Pediatr. Emerg. Care 5 (4), 238–239. doi:10.1097/00006565-198912000-00010
Morini, L., Quaiotti, J., Moretti, M., Osculati, A. M. M., Tajana, L., Groppi, A., et al. (2018). Delta-9-tetrahydrocannabinolic acid A (THC-A) in urine of a 15-month-old child: a case report. Forensic Sci. Int. 286, 208–212. doi:10.1016/J.FORSCIINT.2018.03.020
Pellesi, L., Licata, M., Verri, P., Vandelli, D., Palazzoli, F., Marchesi, F., et al. (2018). Pharmacokinetics and tolerability of oral cannabis preparations in patients with medication overuse headache (MOH)—a pilot study. Eur. J. Clin. Pharmacol. 74 (11), 1427–1436. doi:10.1007/s00228-018-2516-3
Persson, H. E., Sjöberg, G. K., Haines, J. A., and De Garbino, J. P. (1998). Poisoning severity score. Grading of acute poisoning. J. Toxicol. Clin. Toxicol. 36 (3), 205–213. doi:10.3109/15563659809028940
Pigliasco, F., Cafaro, A., Barco, S., Stella, M., Mattioli, F., Riva, A., et al. (2024). Innovative LC-MS/MS method for therapeutic drug monitoring of fenfluramine and cannabidiol in the plasma of pediatric patients with epilepsy. J. Pharm. Biomed. Anal. 245, 116174. doi:10.1016/j.jpba.2024.116174
Richards, J. R., Smith, N. E., and Moulin, A. K. (2017). Unintentional cannabis ingestion in children: a systematic review. J. Pediatr. 190, 142–152. doi:10.1016/J.JPEDS.2017.07.005
Spadari, M., Glaizal, M., Tichadou, L., Blanc, I., Drouet, G., Aymard, I., et al. (2009). Accidental cannabis poisoning in children: experience of the marseille poison center. Presse Med. 38 (11), 1563–1567. doi:10.1016/J.LPM.2009.03.020
Vandrey, R., Herrmann, E. S., Mitchell, J. M., Bigelow, G. E., Flegel, R., LoDico, C., et al. (2017). Pharmacokinetic profile of oral cannabis in humans: blood and oral fluid disposition and relation to pharmacodynamic outcomes. J. Anal. Toxicol. 41 (2), 83–99. doi:10.1093/JAT/BKX012
Vo, K. T., Horng, H., Li, K., Wu, A. H. B., Lynch, K. L., et al. (2018). Cannabis intoxication case series: the dangers of edibles containing tetrahydrocannabinol. Ann. Emerg. Med. 71 (3), 306–313. doi:10.1016/J.ANNEMERGMED.2017.09.008
Keywords: cannabis, intoxication, children, pharmacokinetics, toxicokinetic
Citation: Cafaro A, Pigliasco F, Barco S, Negro I, Piccotti E, Manfredini L, Mahameed S, Bandettini R, Debbia C, Mattioli F and Cangemi G (2025) Accidental cannabis intoxication in two young children: clinical presentation and toxicokinetics - a case series. Front. Pharmacol. 16:1695194. doi: 10.3389/fphar.2025.1695194
Received: 29 August 2025; Accepted: 23 October 2025;
Published: 11 November 2025.
Edited by:
Karel Allegaert, KU Leuven, BelgiumReviewed by:
Jahan Marcu, Northeastern University, United StatesIlan Matok, Hebrew University of Jerusalem, Israel
Copyright © 2025 Cafaro, Pigliasco, Barco, Negro, Piccotti, Manfredini, Mahameed, Bandettini, Debbia, Mattioli and Cangemi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sebastiano Barco, c2ViYXN0aWFub2JhcmNvQGdhc2xpbmkub3Jn
†These authors have contributed equally to this work
 Alessia Cafaro
Alessia Cafaro Federica Pigliasco
Federica Pigliasco Sebastiano Barco
Sebastiano Barco Ilaria Negro2
Ilaria Negro2 Carla Debbia
Carla Debbia Francesca Mattioli
Francesca Mattioli Giuliana Cangemi
Giuliana Cangemi