- 1Department of Pharmacy, Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 2Department of Nephrology, Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
Introduction: Molnupiravir (MPV) is an oral, potent ribonucleoside analog that inhibits the replication of SARS-CoV-2 and is indicated for the treatment of adults with COVID-19. Patients with COVID-19 are at an elevated risk of adverse outcomes, underscoring the need for precise pharmacokinetic (PK) data to guide safe drug use. For MPV and its active metabolite, N4-hydroxycytidine (NHC), renal excretion is not the primary elimination pathway. Consequently, renal impairment has minimal impact on their PK profiles, and no dose adjustment is recommended for patients with mild to moderate renal impairment. However, there are no relevant pharmacokinetic studies in patients with severe renal insufficiency (eGFR<30 mL/min/1.73 m2) or patients requiring dialysis. This study aims to explore the plasma concentration of MPV in patients with severe renal insufficiency, providing a basis for rational clinical use of drugs.
Methods: Liquid chromatography-tandem mass spectrometry (LC-MS/MS) was used to detect the plasma concentrations of MPV and NHC. All analytes were extracted by protein precipitation using acetonitrile at a ratio equivalent to 3:1, and QMPV and QNHC were evaluated by calculating the ratio of plasma concentrations.
Results: A total of four patients with stage 4 or 5 chronic kidney disease (CKD) were enrolled in the study. All patients received a standard oral dose of molnupiravir (MPV), and plasma samples were collected 12 h post-administration (C12h) for analysis. Plasma concentrations of MPV itself were consistently low at 12 h post-dose. In contrast, the 12-h plasma concentration of N4-hydroxycytidine (NHC, the active metabolite of MPV) in patients with severe renal insufficiency (stage 4/5 CKD) was significantly higher than that in reference populations: compared with healthy subjects (NHC C12h: 16.7 ng/mL) and patients with mild to moderate renal impairment (NHC C12h: 31.1 ng/mL), the NHC C12h in the severe renal insufficiency group ranged from 43 to 1,600 ng/mL. Notably, the patient with stage 5 CKD exhibited the highest NHC plasma concentration (1,600 ng/mL) at 12 h post-dose, which remained at a persistently elevated level.
Conclusion: MPV was rapidly hydrolyzed to NHC in the body and maintained at a low level. The NHC is significantly higher than that of patients with mild to moderate symptoms, especially those with stage 5 chronic kidney disease. The blood drug concentration is equivalent to Cmax, which suggests that when used clinically in patients with uremia, the dosing interval should be adjusted to avoid drug accumulation and occurrence of AEs.
1 Introduction
Molnupiravir (MPV) is an oral antiviral drug originally developed for influenza but later evaluated as a treatment for COVID-19 (Jayk Bernal et al., 2022). MPV is a small-molecule ribonucleoside prodrug of N-hydroxycytidine (NHC), which is taken up by cells and phosphorylated to form the active ribonucleoside triphosphate (NHC-TP). NHC-TP is incorporated into SARS-CoV-2 RNA by the viral RNA polymerase, leading to the accumulation of errors in the viral genome, thereby inhibiting replication (Butler et al., 2023).
MPV is available for oral use only in 200 mg capsules. The existing recommended dose for moderate to severe COVID-19 patients is 800 mg (4 capsules) every 12 h with or without food for 5 days (Saravolatz et al., 2023). Studies have showed that MPV and NHC are not substrates, inhibitors, or inducers of multiple CYP enzymes, human P-glycoprotein (P-gp) or assessed transport proteins. Therefore, no drug interactions with MPV have been found (Saravolatz et al., 2023). What’s more, NHC is cleared via metabolism to cytidine and/or uridine through pyrimidine metabolic pathways, which was not significantly eliminated by renal clearance when evaluated in mild to moderate renal impairment (Syed, 2022). Thus, no dose adjustment is required in patients with mild renal impairment.
Previous studies have confirmed that MPV does not accumulate in CKD patients with COVID-19, and there is no increased risk of adverse events (AEs) in patients with CKD stage 4, 5 and end-stage kidney disease (Chen et al., 2024; Cho et al., 2023; Dufour et al., 2023). However, limited data exist on the concentration of MPV and NHC in patients with eGFR <30 mL/min/1.73 m2 and receiving dialysis. Our study focused on those patients with eGFR less than 30 mL/min/1.73 m2 or receiving dialysis to explore the impact of renal insufficiency on MPV pharmacokinetics.
2 Methods
2.1 Subjects
Eligible patients were are those with eGFR less than 30 mL/min/1.73 m2 or receiving dialysis and treated with MPV (800 mg). The pharmacokinetic parameters in healthy volunteers and patients without renal impairment were from the drug instructions.
2.2 Blood specimen collection and bioanalysis
Blood specimens were collected after 12 h after dosing on Day 5. Stock solutions were prepared from their respective reference standards in 50% acetonitrile to obtain a final concentration of 1 mg/mL and stored at −80 °C until use. Working solutions, containing varying concentrations of MPV and NHC, were prepared by diluting this stock solution in 50% acetonitrile to produce several concentrations which were then spiked into drug-free plasma. Using Ribavirin as internal standard and the internal standard (IS) working solutions for plasma were prepared in 50% acetonitrile.
Plasma working calibration standards of MPV and NHC were prepared from the several concentrations above by diluting in the appropriate volume of blank, drug-free plasma to produce a final concentration of 1.0, 5.0, 10.0, 50.0, 100, 500, 1,000 and 2,500 ng/mL.
Quality control (QC) samples were prepared from the MPV and NHC primary stock. These consisted of high QC (HQC: 2000 ng/mL of MPV and NHC), medium QC (MQC: 200 ng/mL of MPV and NHC), low QC (LQC: 2.0 ng/mL of MPV and NHC) and the lower limit of quantification (LLOQ: 1.0 ng/mL of MPV and NHC) for plasma.
Total MPV and NHC concentrations were determined by using a quality control-validated method based on LC-MS/MS (6460 LC/TQ, Agilent Corp.). Control of hardware and processing of data were performed utilizing Mass Hunter Workstation (Agilent, American). 10 μL IS solution was spiked into 100 μL plasma samples. All the analytes were extracted by protein precipitation using acetonitrile at an equivalent 3:1 ratio as used to extract and stable the clinical samples. MPV diffusion (QMPV) and NHC diffusion (QNHC) were assessed by calculating the ratio of plasma concentrations.
3 Results
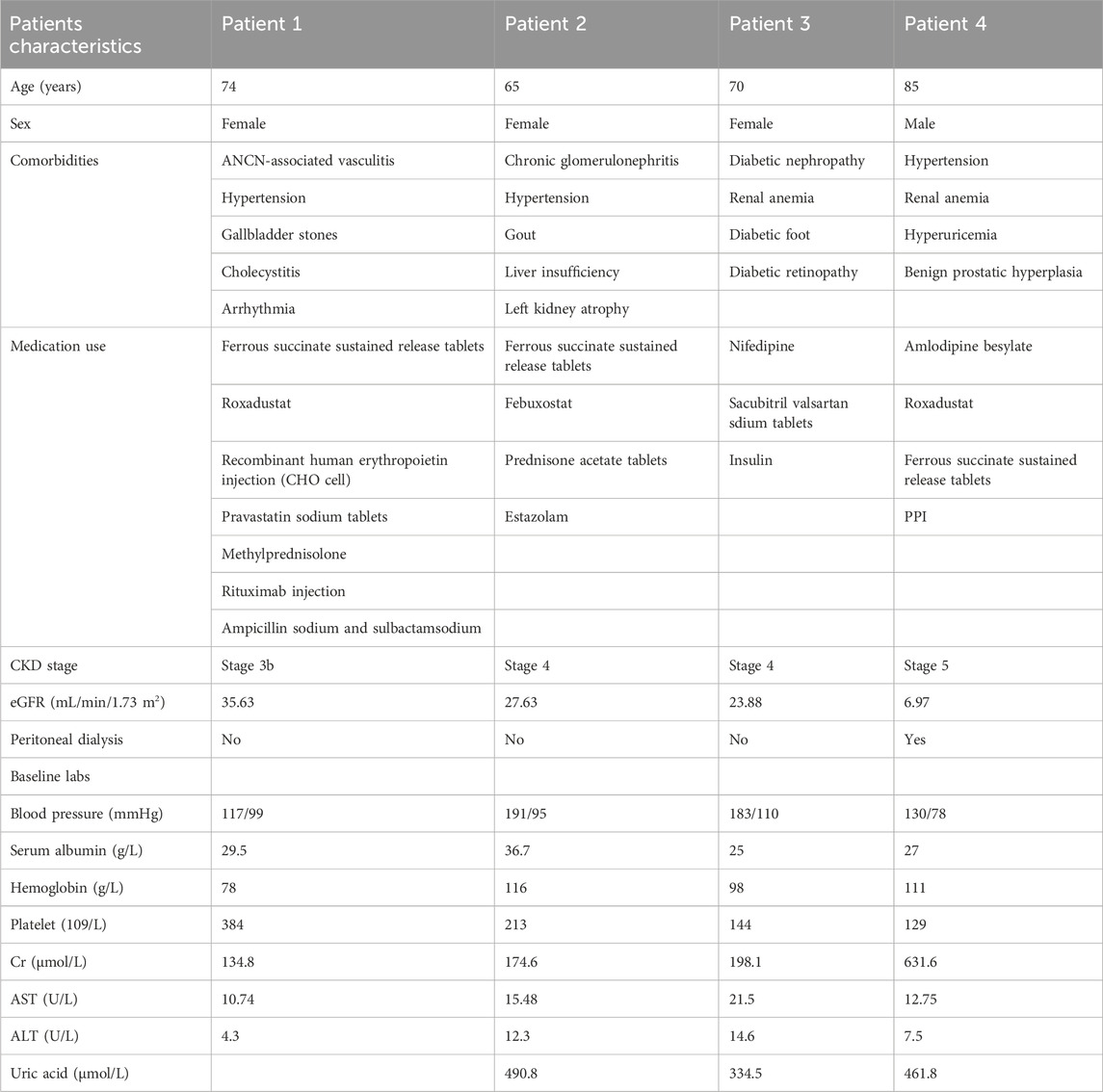
A total of four patients were included in this study, and the basic characteristics were showed in Table 1. The included patients were diagnosed with chronic kidney disease (CKD) and combined with other diseases. One patient had CKD G3 (eGFR, 32 mL/min/1.73 m2), and two had CKD G4 (eGFR, 26 and 22 mL/min/1.73 m2) (Table 1). Patients four had CKD G5 (eGFR, 6 mL/min/1.73 m2) and was under peritoneal dialysis. Patient 1’s eGFR was close to 30 and showed significant differences before and after medication, therefore was also included in the study. They all received MPV at a dosage of 800 mg twice daily for 5 days (given after hemodialysis on dialysis day) for mild-to-moderate COVID-19.
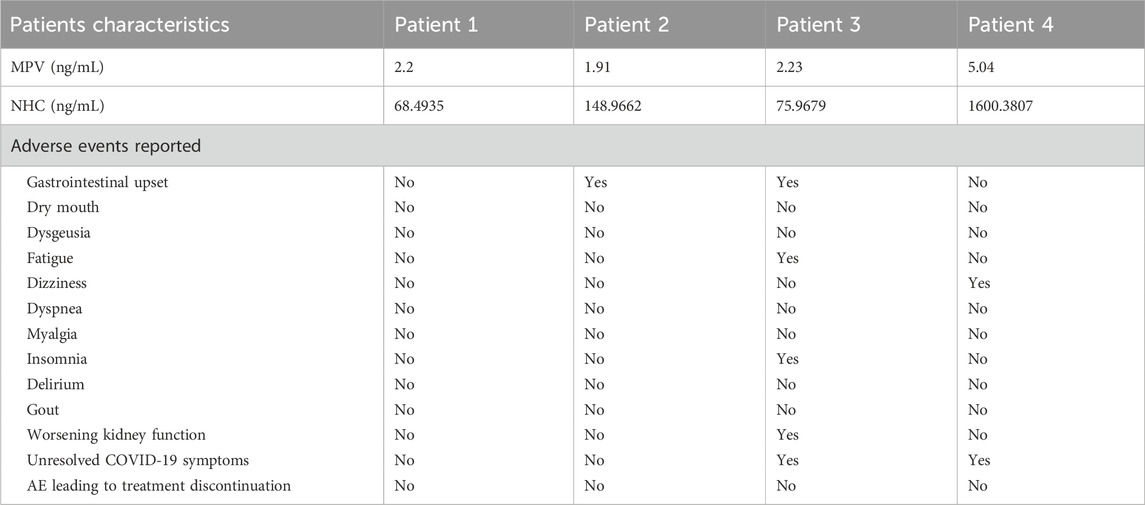
The results showed that the concentration of MPV was very low in the blood samples of patients, which rapidly degraded into NHC after oral administration. Notably, patients with low eGFR (<30 mL/min/1.73 m2) value exhibited higher NHC concentration than subjects with normal kidney function (C12h: 31.1 ng/mL) and healthy volunteers (C12h: 16.7 ng/mL). Especially, patients 4 (CKD G5, eGFR, 6.97 mL/min/1.73 m2), who was under peritoneal dialysis, had the highest NHC concentration (1600.4 ng/mL) (Table 2).
4 Discussion
To our knowledge, the higher the blood concentration of a drug, the greater the intensity of the drug’s effect. When it is higher than the minimum concentration that produces side effects, toxic side effects may occur. If it is lower than the minimum concentration that produces a therapeutic effect, concentrations may be ineffective or may lead to resistance. In this study, patient two developed sudden hepatic insufficiency and had gastrointestinal upset, which is the common adverse event (AE to MPV, including nausea and vomiting. MPV does not affect liver function, and patient two also took febuxostat, which affects liver functions. After discontinuing febuxostat and using hepatoprotective drugs, the patient’s liver function gradually recovered. Notably, patient three had gastrointestinal upset, fatigue, worsening kidney function, and unresolved COVID-19 symptoms. Due to the ineffectiveness of MPV, it was switched to Paxlovid (Nirmatrelvir/Ritonavir). Although the concentration of NHC was 50 times higher than that of subjects with normal kidney function, patient four still had COVID-19 infection and then took Paxlovid after the ineffectiveness of MPV. And patient four also reported AE of dizziness.
While the primary elimination route of NHC may be hepatic metabolism, significant accumulation of NHC is still observed in patients with advanced CKD. Beyond renal excretion, several key factors may contribute to the altered pharmacokinetics (PK) of NHC in this population, as elaborated below: First, the uremic state in advanced CKD can profoundly impair the function of drug-metabolizing enzymes and transporters. Uremic toxins can directly inhibit the activity of cytochrome P450 enzymes and uridine diphosphate-glucuronosyltransferases (UGTs)—families of enzymes likely involved in the metabolic clearance of NHC. Thus, the efficiency of its non-renal elimination (e.g., hepatic and intestinal clearance) can be substantially reduced. Second, advanced CKD is closely associated with marked gut microbiota dysbiosis and impaired intestinal barrier integrity. These pathological changes can perturb the enterohepatic circulation of drugs and their metabolites. A plausible mechanism is that NHC or its conjugated metabolites are excreted into the bile, then deconjugated by dysregulated gut flora in the intestinal lumen, and subsequently reabsorbed into the systemic circulation. This cycle of biliary excretion, deconjugation, and reabsorption leads to prolonged systemic exposure to NHC and eventual drug accumulation. The metabolic disturbances in advanced CKD are widespread and affect core biochemical pathways. Uremia leads to the retention of a wide array of nitrogenous waste products. Some of these may be structural analogs of endogenous pyrimidines (e.g., orotic acid, uracil).
In this study, there seems a connection between high MHC concentration and AEs in MPV-treated CKD and dialysis patients. However, the level of blood drug concentration is not directly proportional to the occurrence of AEs. Because patients also took other drugs at the same time, we cannot rule out AEs caused by other medications. Even high blood drug concentration cannot relieve COVID-19 symptoms. But we still detect excessively high blood drug concentration and pay close attention to AEs to promptly adjust medication intervals and doses.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Name of the ethic committee: Institutional Review Board of Shanghai General Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YW: Writing – original draft, Data curation. QC: Writing – original draft. XL: Writing – original draft. LZ: Formal Analysis, Data curation, Writing – review and editing. MC: Project administration, Writing – review and editing. SW: Methodology, Writing – review and editing. MY: Conceptualization, Writing – review and editing. GF: Methodology, Supervision, Writing – review and editing. QF: Conceptualization, Supervision, Writing – review and editing.
Funding
The authors declare that financial support was received for the research and/or publication of this article. The work was supported in part by funding from the National Natural Science Foundation of China (Grant numbers: 82070754) and Shanghai Pujiang Program (Grant number: 22PJD061).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Butler, C. C., Hobbs, F. D. R., Gbinigie, O. A., Rahman, N. M., Hayward, G., Richards, D. B., et al. (2023). Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial. Lancet 401 (10373), 281–293. doi:10.1016/S0140-6736(22)02597-1
Chen, C. C., Huang, C. Y., Wu, J. Y., Liu, M. Y., Chuang, M. H., Liu, T. H., et al. (2024). Clinical effectiveness of oral antiviral agents for treating non-hospitalized COVID-19 patients with chronic kidney disease. Expert Rev. Anti Infect. Ther. 22 (8), 705–712. doi:10.1080/14787210.2024.2334052
Cho, W. J., Harden, D., Moreno, D., Dinulos, J. E., Hanna, P. E., Wang, Q., et al. (2023). Oral antiviral therapies for COVID-19 in patients with advanced chronic kidney disease or kidney failure. Nephrol. Dial. Transpl. 38 (8), 1912–1914. doi:10.1093/ndt/gfad058
Dufour, I., Devresse, A., Scohy, A., Briquet, C., Georgery, H., Delaey, P., et al. (2023). Safety and efficiency of molnupiravir for COVID-19 patients with advanced chronic kidney disease. Kidney Res. Clin. Pract. 42 (2), 275–278. doi:10.23876/j.krcp.22.194
Jayk Bernal, A., Gomes, D. S. M. M., Musungaie, D. B., Kovalchuk, E., Gonzalez, A., Delos Reyes, V., et al. (2022). Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients. N. Engl. J. Med. 386 (6), 509–520. doi:10.1056/NEJMoa2116044
Saravolatz, L. D., Depcinski, S., and Sharma, M. (2023). Molnupiravir and nirmatrelvir-ritonavir: oral coronavirus disease 2019 antiviral drugs. Clin. Infect. Dis. 76 (1), 165–171. doi:10.1093/cid/ciac180
Keywords: molnupiravir, pharmacokinetic evaluation, chronic kidney disease, COVID-19, N4-hydroxycytidine
Citation: Wang Y, Chen Q, Liu X, Zhang L, Chen M, Wang S, Yang M, Fan G and Fan Q (2025) In vivo pharmacokinetic evaluation of molnupiravir in patients with severe chronic kidney disease. Front. Pharmacol. 16:1696197. doi: 10.3389/fphar.2025.1696197
Received: 31 August 2025; Accepted: 07 November 2025;
Published: 18 November 2025.
Edited by:
Krishna M. Boini, University of Houston, United StatesReviewed by:
Anna Whatling (nee Reznichenko), AstraZeneca R&D, SwedenAli Sarıdaş, Prof. Dr. Cemil Tascioglu City Hospital, Türkiye
Copyright © 2025 Wang, Chen, Liu, Zhang, Chen, Wang, Yang, Fan and Fan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Man Yang, eWFuZ21hbjUxMTNAMTI2LmNvbQ==; Guorong Fan, Z3VvcmZhbkAxNjMuY29t; Qiuling Fan, Y211ZnFsQDE2My5jb20=
†These authors have contributed equally to this work
 Yuanyuan Wang
Yuanyuan Wang Qiong Chen
Qiong Chen Xuan Liu2†
Xuan Liu2† Guorong Fan
Guorong Fan Qiuling Fan
Qiuling Fan