Abstract
The processed Buthus martensii Karsch scorpions, commonly called Quanxie or Scorpio, have been a valuable traditional medicine for over a millennium, with documented use dating back to 935-960 AD. Traditionally employed in treating rheumatism, epilepsy, stroke, and chronic pain, Scorpio contains diverse chemical constituents, including venom, steroid derivatives, alkaloids, amino acids, and nucleosides. Modern pharmacological studies have identified active components, particularly venom and proteins, that exhibit analgesic, antitumor, antiepileptic, and antithrombotic properties. However, these same active ingredients can also induce neurotoxicity and autonomic dysfunction, with overdose leading to such adverse effects. Consequently, numerous processing methods have emerged to mitigate toxicity while preserving pharmacological activity. Despite these advances, key research gaps persist: modern studies overly rely on isolated venom components (with insufficient attention to multi-constituent interactions in processed whole scorpions), high-quality clinical trials are lacking in the functional research on active ingredients, and the mechanisms underlying processing-induced detoxification remain unclear.
1 Introduction
Processed scorpion Buthus martensii Karsch is a traditional animal-based medicine in Asia. The conventional processing method for scorpion medicinal material involves boiling live scorpions in salt water until their bodies become stiff, after which they are shade-dried (Commission, 2025). In this review, Scorpio refers to the dried body of the processed B. martensii scorpion. According to many ancient medical books in China, Scorpio has a medicinal history of more than 1,000 years (Zhang et al., 2024). The earliest record of Scorpio can be traced back to “Shu Bencao” (AD 935–960). Subsequent works, such as “Kaibao Bencao” (AD 973–974), “Bencao Tujing” (AD 1061), “Compendium of Materia Medica” (AD 1552–1,578) and “Benjing Fengyuan” (AD 1695), further recorded the medicinal properties (Zhang et al., 2024). There are 2,119 prescriptions containing Scorpio in the Dictionary of Traditional Chinese Medicine Formulas, and 34 kinds of preparations containing Scorpio are also included in the Chinese Pharmacopoeia (2025), such as Dianxianping tablets, Kangshuan Zaizao pills, and Tongbi capsules, which suggests a medicinal effect in treating stroke, epilepsy, rheumatism, hernia, chancre, and tetanus (Ma, 2019; Commission, 2025). South Korea, India, and countries in Africa also widely use Scorpio as a medicinal material (Yang et al., 2020). In modern medicine, Scorpio has also been used to treat gastric cancer, liver cancer, prostate cancer, and other tumors, as well as skin diseases and bronchial asthma (Shi and Guo, 2023), far exceeding the curative effects recorded in the Pharmacopoeia, which is closely related to advances in modern pharmacological research. Research indicates that Scorpio exhibits significant pharmacological effects including antitumor, antiepileptic, anticonvulsant, anticoagulant, and antibacterial properties (Wali et al., 2019). Additionally, they possess various biological activities such as analgesic and anti-inflammatory effects, growth promotion, and immune enhancement (Yang et al., 2020; Liu et al., 2021a). It is hypothesized that their pharmacological activities are primarily attributed to bioactive components like scorpion venom, steroidal derivatives, and alkaloids (Yang et al., 2020). Furthermore, in regions like Shandong and Henan Provinces, Scorpio is regarded as exceptional delicacy served at banquets, demonstrating high culinary value (Yang et al., 2020). This edible significance correlates with their rich nutritional composition containing amino acids, peptides, lipids, calcium, magnesium, and abundant trace elements (Song et al., 2024). It was found that Scorpio not only has obvious pharmacological effects, but also causes many adverse reactions such as abnormal liver function, cardiovascular system reactions, kidney injury, gastrointestinal reactions, and neurotoxic reactions (Yang et al., 2020). To mitigate its associated toxicity beyond dosage control, various processing methods–including boiling, mint-processing, licorice rinsing, and wine washing–are employed to reduce toxicity while enhancing therapeutic efficacy (Zhang et al., 2024). Nevertheless, the precise mechanisms underlying these effects remain poorly understood. At present, researchers have accumulated a large amount of research results on the chemical composition, pharmacological action, and toxicity of Scorpio. Based on this, this paper summarizes the above aspects in order to provide important reference materials for the in-depth application and development of Scorpio.
A comprehensive literature search covering the period from 1991 to 2024 was conducted using multiple electronic databases, including PubMed, Web of Science, Scienceing, Google Scholar, Elsevier, CNKI, WanFang, and WeiPu. The search terms included “Buthus martensii Karsch”, “Chinese Scorpion”, “Quanxie”, “traditional uses”, “chemical composition”, “pharmacology”, “toxicology”, and “processing”. In addition, Bopu Think Tank (https://www.bopuyun.com/) and Bolan Yishu (https://www.imedbooks.com/) provide information related to the Chinese Pharmacopoeia (2025 edition) and traditional Chinese medicine herbs. Studies with unclear experimental methods, insufficient sample sizes, or unvalidated outcome measures were excluded for quality filtering. Taxonomic identification of the studied scorpion species (Buthus martensii Karsch) was validated using the following authoritative resources: World Spider Catalog (integrated with Scorpion taxonomy, Version 2024 https://wsc.nmbe.ch/).
2 Traditional uses
The use of Scorpio in Chinese Traditional Medicine has a long history and is recorded in multiple classical Chinese medical texts. Scorpio was first documented as a medicinal substance in the Shu Bencao, although its efficacy and indications were not detailed therein (Ma, 2019). During the Song Dynasty, Kaibao Bencao began to document the therapeutic uses of Scorpio (Lu, 1995). Subsequent medical works in other historical periods successively added new records of its applications. It was not until the Qing Dynasty that a summary of its traditional uses was compiled, including dissipating blood heat, eliminating wind-dampness, resolving sores and ulcers, and serving as a channel-guiding agent (Ma, 2019). The traditional uses and initial documentation period of Scorpio are summarized in Table 1.
TABLE 1
| Period | Reference classics | Traditional uses | Modern pharmacological correlates |
|---|---|---|---|
| Song dynasty | Kaibao Bencao (Lu, 1995) | Stroke, hemiplegia, facial deviation (wry mouth and eyes) | Anticoagulant, Antithrombotic, Immunoregulation |
| Spasms or convulsions | Anti-epilepsy, Anticonvulsant | ||
| Wind-toxin induced urticaria/rashes (engdu yinzhen) | Antibiosis, Antiviral, Immunoregulation | ||
| Bencao Yanyi (Kou, 1990) | Infantile convulsions (jingfeng) | Anticonvulsant | |
| Baoqing Bencao Zhezhong (Chen, 1991) | Resolve phlegm and dissipate nodules (huatan sanjie) | Antitumor, Immunoregulation | |
| Gui Ren Fang (Tang, 2011) | Deafness due to kidney deficiency | Immunoregulation | |
| Jin and Yuan dynasty |
-
- |
Hernial pain, rheumatic pain | Analgesic, Anti-inflammatory |
| Gynecological disorders (such as leucorrhea) | Immunoregulation, Antibiosis | ||
| Ming dynasty | Compendium of materia Medica (Li, 1998) | External applications for hemorrhoids and ulcers | Anti-inflammatory, Antibiosis |
| Localized treatments for disorders of the five senses and dermatology | Immunoregulation, Antibiosis |
Traditional uses and initial documentation period of Scorpio.
3 Chemical composition
The study of chemical constituents is the premise and foundation for clarifying the scientific connotation of traditional Chinese medicine in treating diseases (Li et al., 2023). The Chinese Scorpio, as an important animal-based traditional medicine, is very complicated in chemical composition. At present, many components such as protein peptides, steroid derivatives, alkaloids, fatty acids and amino acids have been found in scorpions.
3.1 Scorpion venom
Scorpion venom, secreted by B. martensii scorpion, can cause paralysis and even death. It has a complex composition, consisting of protein and non-protein components (Zhang et al., 2024). The protein portion is categorized into enzymes and peptides. To date, hyaluronidase, phospholipase A2, gelatinase, acetylcholinesterase, and nucleosidase have been found in scorpion venom (Yang et al., 2020). Peptides include protease inhibitors, bradykinin-potentiating peptides, histamine releasing factors, small molecular peptides, etc., among which small molecular peptides are also called scorpion toxins or neurotoxin peptides (Zhang et al., 2024). Scorpion venom toxins consist of 20–80 amino acids, contain 3-4 pairs of disulfide bonds, and have molecular weights ranging from 6,000 to 9,000 Da (Yang et al., 2020). These toxins can exert pharmacological effects by acting on corresponding receptor sites or ion channels (K+, Ca2+, Na+, Cl−), and are the main active components responsible for Scorpio’s pharmacological effects (Chen et al., 2001; Zheng et al., 2024).
According to the bond connections, scorpion toxins can be divided into disulfide-bridged peptides (DBPs) and non-disulfide-bridged peptides (NDBPs) (Almaaytah and Albalas, 2014; Rincón-Cortés et al., 2019). The main function of disulfide bonds in DBPs is to maintain the stability of scorpion toxins and exhibit various neurotoxic and cytotoxic effects (Zhang and Wang, 2007). With the in-depth study, it has been found that they can specifically act on ion channels (Na+, K+, Ca2+, Cl−) on the membrane to play antibacterial, anticancer, anti-inflammatory, and immunomodulatory roles (Cyril et al., 2002; Almaaytah and Albalas, 2014). NDBPs have pharmacological effects such as antibacterial, bradykinin enhancement, hemolysis, and immunomodulation (Vega et al., 2010). Information on the types and pharmacological effects of scorpion venom peptides is shown in Table 2.
TABLE 2
| Types of scorpion venom peptide | Polypeptide name | Sequences | Pharmacological action | References |
|---|---|---|---|---|
| Targeted Na+ channels | CvIV4 | MNYFILILVAALLILDVNCKKDGYPVEHSGCKYTCWKNEYCDK VCKDLKGEGGYCYINLTCWCTGLPDNVPLKTNQRCNGKRK |
Analgesic | Yang et al. (2020) |
| MKTxⅢ | MNYLIVISFALLLMTGVESGRDAYIAKKENCTYFCALNPYCND LCTKNGAKSGYCQWAGRYGNACWCIDLPDKVPIRIPGPCIGR |
Analgesic | Cyril et al. (2002) | |
| BmK Ⅰ1 | - | Analgesic | Cyril et al. (2002) | |
| BmK Ⅰ4 | - | Analgesic | Shao et al. (2007a) | |
| BmK Ⅰ6 | - | Analgesic | Shao et al. (2007a) | |
| BmK I | VRDAYIAKPHNCVYECARNEYCNDLCTKNGAKSGYCQW VGKYGNGCWCIELPDNNVPIRVPGKCH |
Pro-epilepsy | Xiao et al. (2022) | |
| BmK IT1 | KKNGYAVDSSGKVSECLLNNYCNNICTKVYYATSGYCC LLSCYCFGLDDDKAVLKIKDATKSYCDVQIIG |
Anti-epilepsy and Anticonvulsant | Zhao et al. (2008) | |
| BmK IT2 | DGYIKGKSGCRVACLIGNQGCLKDCRAYGASYGYCW TWGLACWCEGLPDNKTWKSESNTCG |
Anti-epilepsy and Anticonvulsant | Zhao et al. (2008) | |
| BmK IT-AP | KKNGYAVDSSGKVAECLFNNYCNNECTKVYYAD KGYCCLLKCYCFGLADDKPVLDIWDSTKNYCDVQIIDLS |
Analgesic | Shao et al. (2007a) | |
| BmK M10 | MNYLVMISFALLLMKGVESVRDAYIAKPENCVYECGITQD CNKLCTENGAESGYCQWGGKYGNACWCIKLPDSVPIRVPGKCQR |
Antithrombotic | Cyril et al. (2002) | |
| MKTxI | GRDAYIADSENCTYTCALNPYCNDLCTKNGAKSGY CQWAGRYGNACWCIDLPDKVPIRISGSCR |
Nitrate energy reaction | Cyril et al. (2002) | |
| Bukatoxin | VRDGYIADDKNCAYFCGRNAYCDEECIINGAESGYC QQAGVYGNACWCYKLPDKVPIRVSGECQQ |
Analgesic | Cyril et al. (2002) | |
| Css4 | MNSLLMITACLALVGTVWAKEGYLVNSYTGCKFECFKLGDNDYCL RECRQQYGKGSGGYCYAFGCWCTHLYEQAVVWPLPNKTCNGK |
Analgesic | Liu et al. (2016) | |
| BmK AngP1 | KKNGYAVDSSGKVAE | Analgesic | Shao et al. (2007a) | |
| BmK Ang M1 | MNYLVMISFA LLLMKGVESV RDAYIAKPEN CVYECGITQD CNKLCTENGA ESGYCQWGGK YGNACWCIKLPDSVPIRVPGKCQR |
Analgesic, Anti-Inflammatory | Shao et al. (2007a) | |
| BmK IT3 | DGYIRGSNGCKISCLWGNEGCNKECKGFGAYYGYCWTW GLACWCBGLPDDKTWKSESNTCG |
Kill insects | Cyril et al. (2002) | |
| BmK IT4 | DGYIRGSNGCKISCLWGNEGCNKECKGFGAYYGY CWTWGLACWCBGLPDDKTWKSESNTCG |
Kill insects | Cyril et al. (2002) | |
| BmK IT5 | DGYIKRHDGCKVTCLINDNYCDTECKREGGSYG YCYSVGFACWCEGLPDDKAWKSETNTCD |
Kill insects | Cyril et al. (2002) | |
| BmK d IT-AP3 | - | Analgesic, Anti-Epilepsy and Anticonvulsant | Shao et al. (2007a) | |
| BmK AS1 | DNGYLLDKYTGCKIWCVINNESCNSECKLRRGNYGYC YFWKLACYCEGAPKSELWAYETNKCNKGM |
Analgesic, Anti-Inflammatory | Cyril et al. (2002) | |
| BmK AS | DNGYLLDKYTGCKVWCVINNESCNSECKIRGGYY GYCYFWKLACFCQGARKSELWNYNTNKCNGKL |
Anti-inflammatory, Analgesic, and Anticonvulsant | Zhao et al. (2011a), Liu et al. (2012) | |
| BmK ANEP | MKLSLLLVISASMLIDGLVNADGYIRGSNGCKVSCLWGNDGCNKEC RAYGASYGYCWTWGLACWCEGLPDDKTWKSESNTCGGKK |
Analgesic | Lu et al. (2022), Song et al. (2011) | |
| BmK AGAP | VRDGYIADDKNCAYFCGRNAYCDDECKKNGAES GYCQWAGVYGNACWCYKLPDKVPIRVPGKCNGG |
Analgesic, Antitumor | Zhao et al., 2011b; Liu (2013),Li et al. (2016),Xu et al. (2017) | |
| BmNal-3SS2 | - | Analgesic | Cui et al. (2017) | |
| BmK bpp | FRFGSFLKKVWKSKLAKKLRSKGKQLLKDYANKVLNGPEEEAAAPAE | Antibiosis | Cyril et al. (2002) | |
| BmK BTx | MMKLVLFGIIVILFSLIGSIHGISGNYPLNPYGGYYYCTILGENEYCK KICRIHGVRYGYCYDSACWCETLKDEDVSVWNAVKKHCKNPYL |
Analgesic | Cui et al. (2017) | |
| BmK AEP | DGYIRGSNGCKVSCLLGNEGCNKECRAYGASYGY CWTYKLACWCBGLPDDKTWKSESNTCG |
Anti-epilepsy | Wang et al. (2010a) | |
| BmK αIV | MNYLVFFSLALLLMTGVESVRDGYIADDKNCAYFCGRNAYCDDEC KKKGAESGYCQWAGVYGNACWCYKLPDKVPIRVPGRCNGG |
Pro-epilepsy | Xiao et al. (2022) | |
| BmK NT1 | - | Neurotoxicity | He et al. (2017) | |
| BmK M2 | VRDAYIAKPHNCVYECARNEYCNNLCTKNGAKS GYCQWSGKYGNGCWCIELPDNVPIRVPGKCH |
Neurotoxicity | He et al. (2000) | |
| BmK M7 | VRDGYIALPHNCAYGCLNNEYCNNLCTKDGAKI GYCNIVGKYGNACWCIQLPDNVPIRVPGRCHPA |
Cardiotoxicity | Guan et al. (2002) | |
| Targeted K+ Channels | BmKTX | VGINVKCKHSGQCLKPCKDAGMRFGKCINGKCDCTPK | Analgesic, Immunoregulation | Yang et al. (2020) |
| BmKKx2 | RPTDIKCSASYQCFPVCKSRFGKTNGRCVNGLCDCF | Antitumor | Jian et al. (2013) | |
| BmKTT-1 | QKDCSLPVDTGRGKGWFLRYYYNKNSKTCES FIYGGVGGNKNNFLNIENCCKICKAKNC |
Inhibit protease activity | Chen et al. (2012) | |
| BmKTT-2 | VDCTLPSDTGRCKAYFIRYFYNQKAGECQ KFVYGGCEGNSNNFLTKSDCCKQCSPGKC |
Inhibit protease activity | Chen et al. (2012) | |
| BmKTT-3 | SINCRLPPERGPCRGNITKYYYHNESRTCRT FSYGGCEGNSNNFRNRHYCMKYCARKRH |
Inhibit protease activity | Chen et al. (2012) | |
| BmTX1 | MKISFLLLALVICSIGWSEAQFTDVKCTGS KQCWPVCKQMFGKPNGKCMNGKCRCYS |
Kill insects | Cyril et al. (2002) | |
| BmTX2 | MKISFLLLLAIVICSIGWTEAQFTNVSCSAS SQCWPVCKKLFGTYRGKCMNSKCRCYS |
- | Cyril et al. (2002) | |
| BmTX3B | MKIFSILLVALIICSISICTEAFGLIDVKCFAS SECWTACKKVTGSGQGKCQNNQCRCY |
- | Cyril et al. (2002) | |
| BmTXK-β | MMKQQFFLFLAVIVMISSVIEAGRGKEIMKNIKEKLTEVKDKMKHS WNKLTSMSEYACPVIEKWCEDHCAAKKAIGKCEDTECKCLKLRK |
Antibiosis | Almeida et al. (2013) | |
| BmP01 | ATCEDCPEHCATQNARAKCDNDKCVCEPK | - | Cyril et al. (2002) | |
| BmP02 | VGCEECPMHCKGKNAKPTCDDGVCNCNV | - | Cyril et al. (2002) | |
| BmP03 | VGCEECPMHCKGKNANPTCDDGVCNCNV | - | Cyril et al. (2002) | |
| BmP05 | AVCNLKRCQLSCRSLGLLGKCIGDKCECVKH | - | Cyril et al. (2002) | |
| IbTX | QFTDVDCSVSKECWSVCKDLFGVDRGKCMGKKCRCYQ | Anti-inflammatory | Yang et al. (2020) | |
| BmSKTx1 | MKIFFAILLILAVCSMAIWTVNGTPFAIKCA TNADCSRKCPGNPPCRNGFCACT |
- | Cyril et al. (2002) | |
| LmKTT | MKISFVLLLTLFICSIGWSEARPTDIKCSASYQ CFPVCKSRFGKTNGRCVNGLCDCF |
Immunoregulation | Yang et al. (2020) | |
| Kbot21 | AACYSSDCRVKCRAMGFSSGKCIDSKCKCYK | Immunoregulation | Yang et al. (2020) | |
| Anuroctoxin | QKECTGPQHCTNFCRKNKCTHGKCMNRKCKCFNCK | Immunoregulation | Yang et al. (2020) | |
| Urotoxin | MNAKLIYLLLVVTTMMLTFDTTQAGDIKC SGTRQCWGPCKKQTTCTNSKCMNGKCKCYGCVG |
Immunoregulation | Yang et al. (2020) | |
| Maurotoxin | VSCTGSKDCYAPCRKQTGCPNAKCINKSCKCYGC | Immunoregulation | Yang et al. (2020) | |
| HeTx204 | GTVYVFLLLLAFGIFTDISNACSEQMDDEDSYE VEKRGNACIEVCLQHTGNPAECDKPCDK |
Immunoregulation | Yang et al. (2020) | |
| OcyKTx2 | IRCQGSNQCYGHCREKTGCMNGKCINRVCKCYGC | Immunoregulation | Yang et al. (2020) | |
| ImKTx10 | - | Immunoregulation | Zhang et al. (2022b) | |
| ImKTx58 | - | Immunoregulation | Zhang et al. (2022b) | |
| ImKTx88 | - | Immunoregulation | Zhang et al. (2022b) | |
| ImKTx104 | MNFWVTFIRLIVVLSIVFAFQIAVAKA ACVTHEDCTLLCYDTIGTCVDGKCKCM |
Immunoregulation | Yang et al. (2020) | |
| ADWX-1 | VGINVKCKHSRQCLKPCKDAGMRFGKCTNGKCHCTPX | Immunosuppression | Cyril et al. (2002) | |
| BmTxKS1 | MNRLTTIILMLIVINVIMDDISESKVAAGIVCK VCKIICGMQGKKVNICKAPIKCKCKKG |
- | Cyril et al. (2002) | |
| BmTxKS2 | MTYAILIIVSLLLISDRISNVVDKYCSENPLD CNEHCLKTKNQIGICHGANGNEKCSCMES |
Antibiosis | Cyril et al. (2002) | |
| CoTX1 | MEGIAKITLILLFLFVTMHTFANWNTEAAVCVY RTCDKDCKRRGYRSGKCINNACKCYPYGK |
Neurotoxicity | Yang et al. (2020) | |
| Agitoxin1 | GVPINVKCTGSPQCLKPCKDAGMRFGKCINGKCHCTPK | Neural regulation | Yang et al. (2020) | |
| Agitoxin2 | GVPINVSCTGSPQCIKPCKDAGMRFGKCMNRKCHCTPK | Neural regulation | Yang et al. (2020) | |
| Targeted Ca2+ Channels | Kurtoxin | KIDGYPVDYWNCKRICWYNNKYCNDLCKGLK ADSGYCWGWTLSCYCQGLPDNARIKRSGRCRA |
Neural regulation | Yang et al. (2020) |
| Martentoxin | MKIFSILLVALIICSISICTEAFGLIDVKCFAS SECWTACKKVTGSGQGKCQNNQCRCY |
Neural regulation | Yang et al. (2020) | |
| BmK-YA | MIFHQFYSILILCLIFPNQVVQSDKERQDWIPSDYGGYMNPAGRSD EERQDWIPSDYGGHMNPAGRSDEERQDWIPSDYGGHMNPAGRSN EERQDWIPSDYGGYMNPAGRSDEERQDWIPSDYGGHMNPAGRSN EERQDWIPSDYGGYMNPAGRSDEERQDWIPSDYGGHMNPAGRSD EERQDWIPSDYGGYMNPAGRSD |
Analgesic | Yang et al. (2020) | |
| Targeted Cl− Channels | Bm-12 | CGPCFTTDANMARKCRECCGGIGKCFGPQCLCNRT | - | Wu et al. (2000) |
| Chlorotoxin | MKFLYGIVFIALFLTVMFATQTDGCGPCFTTDANMA RKCRECCGGIGKCFGPQCLCNRI |
Cell proliferation promotion | Yang et al. (2020) | |
| Bs-Tx7 | - | Cell proliferation promotion | Yang et al. (2020) | |
| BmK AGP-SYPU1 | GRDAYIAQNYNCVYHCFRDDYCNGLCTENGADS GYCYLAGKYGHACWCINLPDDKPIRIPGKCHRR |
Analgesic | Meng et al. (2015) | |
| BmK AGP-SYPU2 | MNYMVIISLALLVMTGVESVKDGYIADDRNCPYFCGRNAYCDGEC KKNRAESGYCQWASKYGNACWCYKLPDDARIMKPGRCNGG |
Cell proliferation promotion Analgesic | Zhang et al. (2011), Yang et al. (2020) | |
| BmKn2 | FIGAIARLLSKIF | Anti-cancer, Antibiosis | Cao et al. (2012), Satitmanwiwat et al. (2016) | |
| BmK CT | CGPCFTTDANMARKCRECCGGIGKCFGPQCLCNRI | Anti-cancer | Li et al. (2019) |
Scorpion venom peptides contained in B. martensii scorpion and their pharmacological action.
Protein peptides in scorpion venom, as the main chemical components, have been widely studied at present. Transcriptomic and proteomic analyses have been applied to detect the potential toxins of scorpions from different habitats and instars (Gao et al., 2021; Guo et al., 2024). However, it is still unknown whether these substances remain in Scorpio used as traditional Chinese medicine materials. Proteomic analysis was used to comprehensively characterize the functional toxins in Scorpio. A total of 66 heat-resistant potassium channel-regulating toxins were identified, and various degraded toxin fragments were also detected, indicating that their relative thermal instability may reduce toxicity (Yang et al., 2019).
3.2 Steroid derivatives
Steroidal compounds are widely distributed in nature. Currently, 6 steroidal compounds have been isolated from B. martensii, including cardenolides and cholesterol derivatives. Pharmacological activity studies have shown that cardenolides exhibit antibacterial, cardiotonic, and antitumor activities, among others; cholesterol derivatives also possess activities such as antibacterial, antitumor, antiviral, and anti-inflammatory effects (Gao, 2012; Gao et al., 2014). These steroidal compounds are hypothesized to be the material basis for the pharmacological activities of Scorpio, such as its antitumor and anti-inflammatory effects.
3.2.1 Cardiac glycosides
To date, two specific cardiac glycosides with lactone alcohol structures have been identified from Scorpio: 2β-,22-dihydroxy,3-acetoxyl,20-methoxy-cardenolidol (C28H45O7) (Gao, 2012) and 3β-acetoxyl,2,14,22-trihydroxy,19-hydroxymethyl,9a,5β,14β-card-20 (22)enolide (C25H36O8) (showed in Figure 1) (Gao et al., 2014).
FIGURE 1
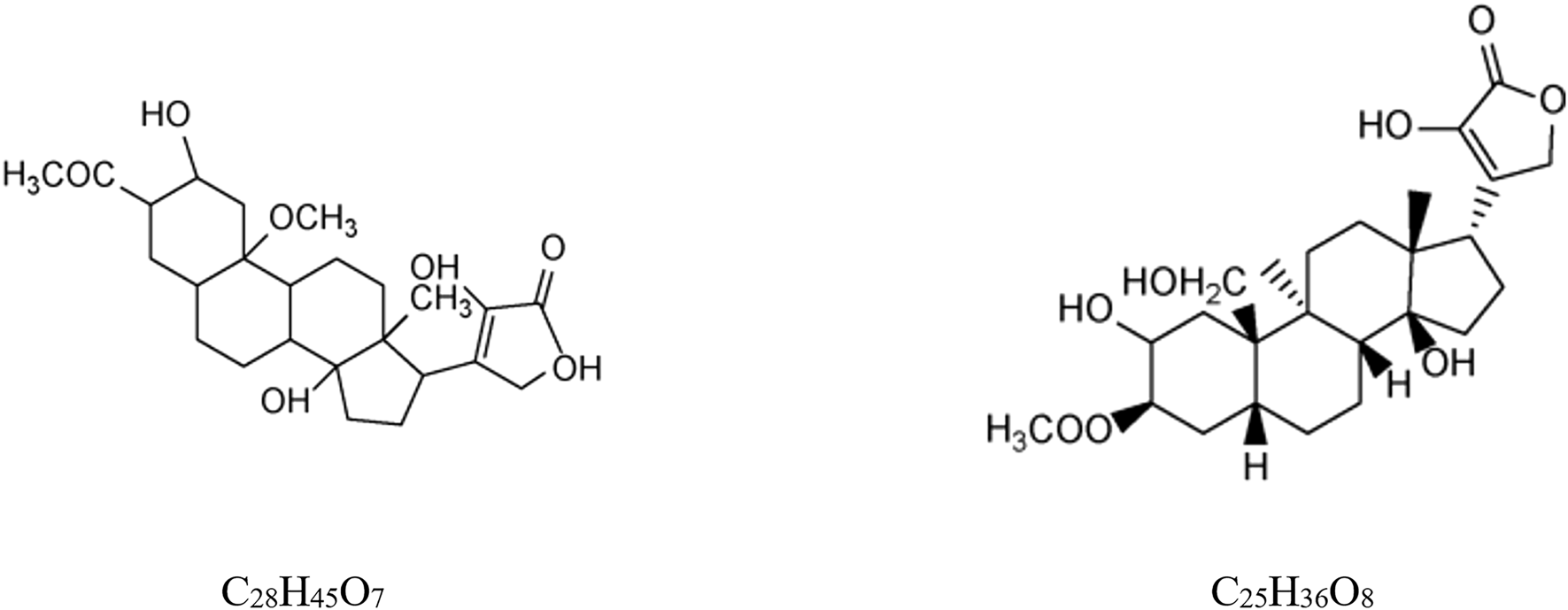
Structures of cardiac glycosides from B. martensii
3.2.2 Cholesterol and its analogues
The initial identification of cholesterol in B. martensii scorpion was reported in 2012 (Gao, 2012). Subsequent phytochemical investigations have led to the isolation and characterization of four distinct sterol compounds from B. martensii, whose detailed chemical structures are systematically presented in Table 3 and Figure 2.
TABLE 3
| No. | Compound | Molecular formula | Molecular weight | Reference |
|---|---|---|---|---|
| 1 | Cholesterol | C27H46O | 386.7 | Gao (2012) |
| 2 | Cholest-4-en-3-one | C27H44O | 383.0 | Ai (2017) |
| 3 | (−)22E,24-3-cholestone-4,22 (23)-diene-25-alcohol | C27H43O2 | 427.0 | Liu (2018) |
| 4 | (−)5(6),22(23)-Cholestadien-3β-acetoxy ester | C29H47O2 | 494.0 | Liu (2018) |
Cholesterol components in B. martensii.
FIGURE 2
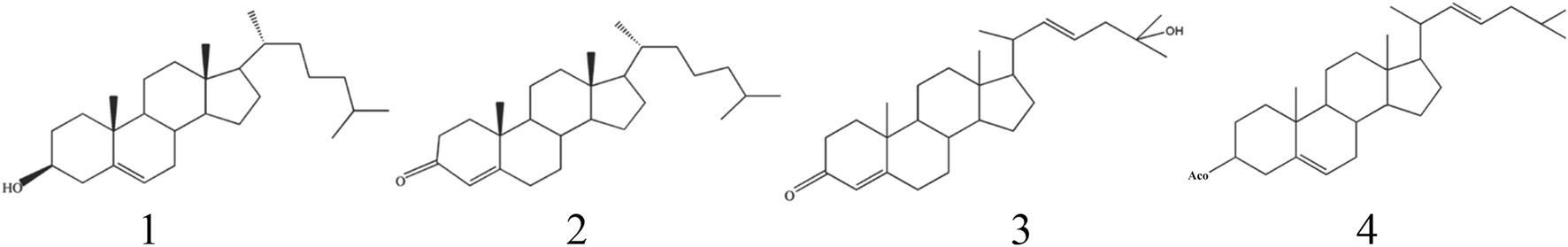
Structures of cholesterol components from B. martensii.
3.3 Alkaloids
Alkaloids represent a diverse class of naturally occurring nitrogen-containing organic compounds with significant pharmacological potential. Extensive phytochemical investigations have identified several alkaloids in B. martensii, including 1-stearyl- glycerol-3-phosphorylcholine, trigonelline, martensine A and martensine B, whose structural details are systematically presented in Table 4 and Figure 3.
TABLE 4
| No. | Compound | Molecular formula | Molecular weight | Reference |
|---|---|---|---|---|
| 5 | 1-stearyl- glycerol-3-phosphorylcholine | C26H55NO7P | 524.4 | Ai (2017) |
| 6 | trigonelline | C7H8NO2 | 138.0 | Ai (2017) |
| 7 | martensine A | C12H18N4O2 | 250.2 | Fan (2020) |
| 8 | martensine B | C11H18N5O | 236.2 | Fan (2020) |
| 9 | harmanyl-β-D-glucopyranoside | C18H21 N2O6 | 361.4 | Kim (2013) |
Alkaloid components in B. martensii.
FIGURE 3
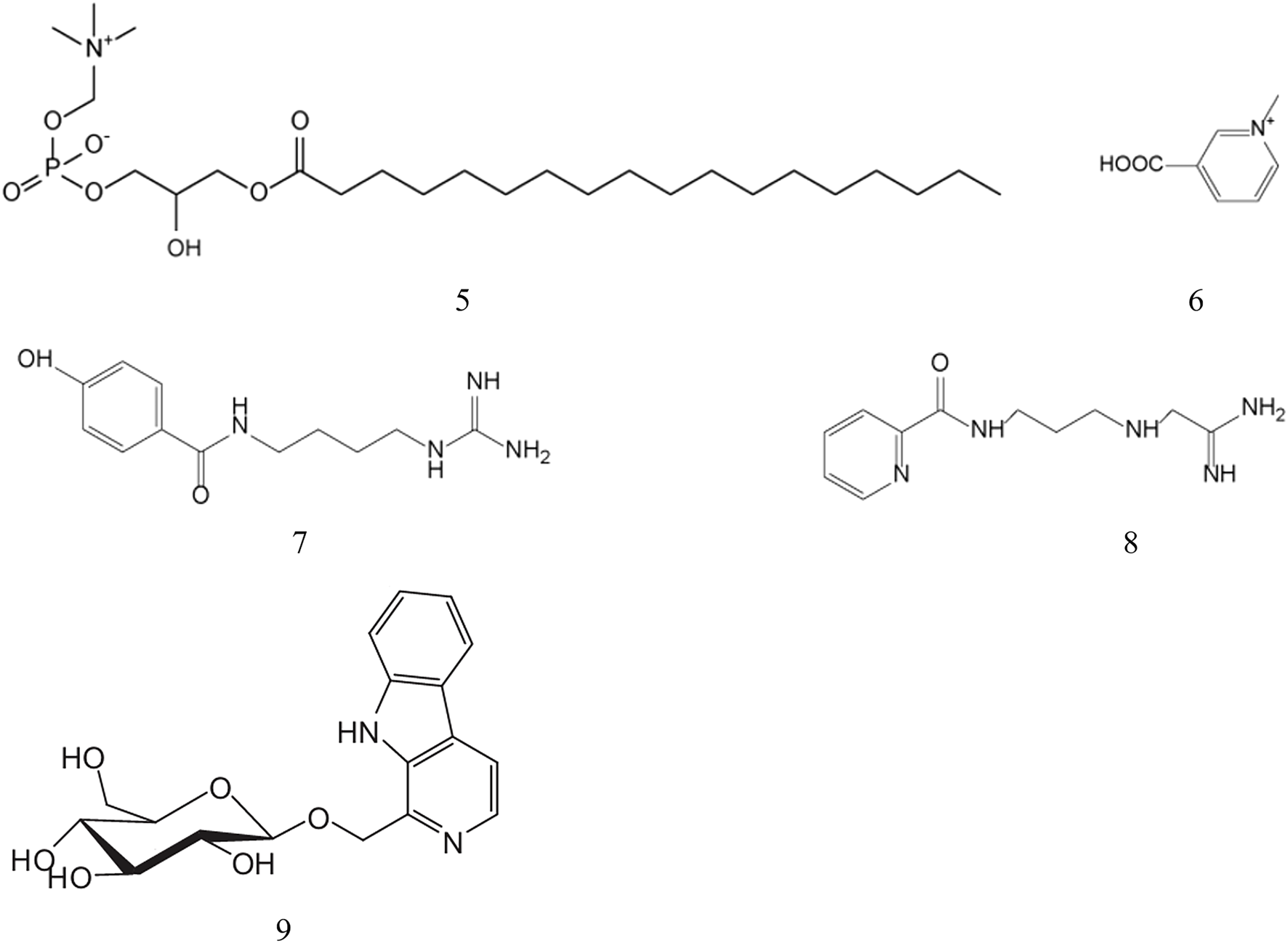
Structures of alkaloids from B. martensii.
Pharmacological studies have demonstrated that scorpion-derived alkaloids exhibit a broad spectrum of bioactivities, including antimicrobial, antitumor, anti-aging, hypoglycemic, hypolipidemic, neuroprotective, and cognitive-enhancing properties (Kamble and Bodhankar, 2013; Pravalika et al., 2018; Zeng et al., 2021). Notably, martensine A and martensine B have shown remarkable propidium iodide substitution capacity, suggesting their potential therapeutic application in Alzheimer’s disease treatment (Fan, 2020). Furthermore, the isolation of harmanyl-β-D-glucopyranoside, a novel β-carboline glucopyranoside from B. martensii, has revealed significant hypoglycemic activity, highlighting the therapeutic potential of scorpion alkaloids in diabetes management.
3.4 Odor components
The characteristic fishy odor of Scorpio is primarily attributed to their volatile organic compounds, including aldehydes, terpenoids, and aromatic compounds with distinct olfactory properties (Yang et al., 2020).
In 2012, a groundbreaking discovery revealed the presence of a unique nitro aldehyde structure in scorpions, specifically identified as 6-hydroxy-1-ene-tetrahydro-pyrimidine aldehyde, representing a hydroxyl-containing alkanal (Gao, 2012). Terpenoids, characterized by their structural composition of two or more isoprene units, were further investigated in scorpions. Notably (−)4-(2′-iso-octanoic acid)-6-hydroxy-1-methyl cyclohexene, a carboxyl-containing terpene, was first isolated from scorpions in 2018 (Liu, 2018). The structural details are presented in Table 5; Figure 4. Through advanced analytical techniques, researchers have employed headspace solid-phase microextraction (HS-SPME) coupled with gas chromatography-mass spectrometry (GC-MS) to comprehensively profile the volatile components of scorpions. A total of 43 components were separated and 42 components were identified, including aldehydes and ketones (11 species), terpenoids (8 species), and aromatics (4 species). The main chemical constituents are benzaldehyde (19.03%), phenol (13.22%), 2-pentylfuran (7.42%) and cubeba olefine (7.31%) (Zhou et al., 2018). In order to remove the fishy smell, doctors in past dynasties fried scorpion and glutinous rice together or prepared them with vinegar to absorb and mask the unpleasant odor-causing substances (Du et al., 2024).
TABLE 5
| No. | Compound | Molecular formula | Molecular weight | Reference |
|---|---|---|---|---|
| 10 | 6-hydroxy-1-ene-tetrahydro-pyrimidine aldehyde | C5H8N2O2 | 129 | Gao (2012) |
| 11 | (−)4-(2′-iso-octanoic acid)-6-hydroxy-1-methyl cyclohexene | C15H25O3 | 253 | Liu (2018) |
Odor components in Scorpio.
FIGURE 4
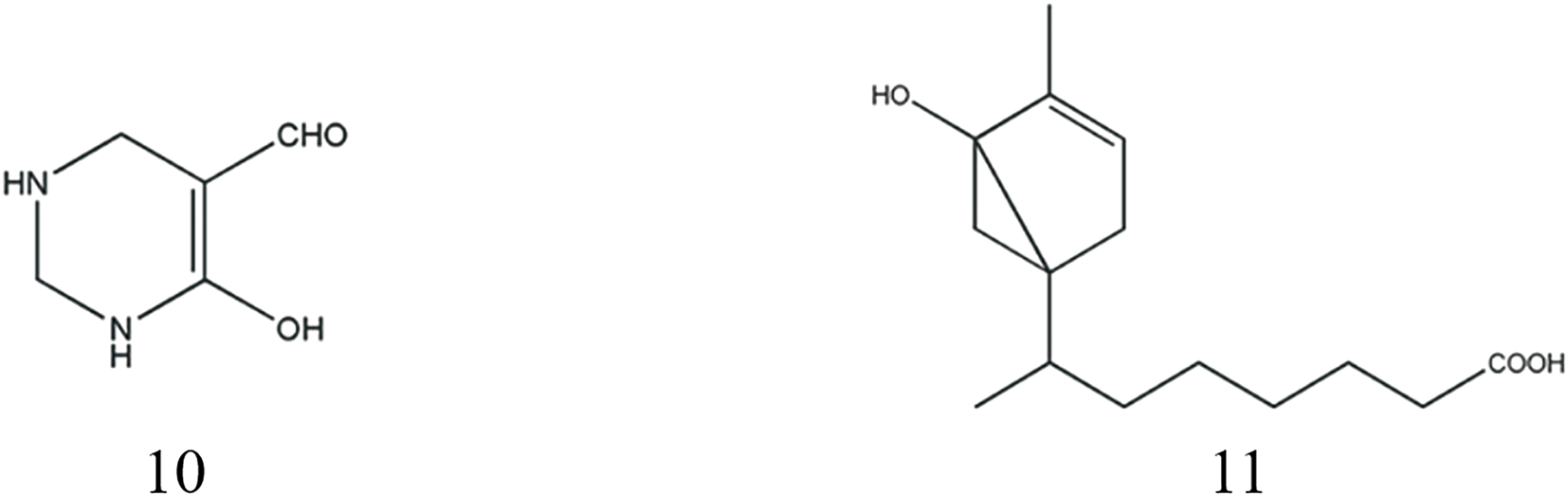
Structures of Odor components from Scorpio.
3.5 Nutritional ingredients
Scorpio represents a valuable source of essential nutrients, comprising amino acids, lipids, and mineral elements that are crucial for maintaining human physiological functions and promoting overall health (Yang et al., 2020).
3.5.1 Amino acids
As fundamental building blocks of proteins, amino acids play a pivotal role in sustaining vital biological processes. Extensive biochemical analyses have revealed that scorpions contain a diverse array of amino acids, with more than 17 distinct types identified, including 6 essential amino acids (Li et al., 2007). Notably, the amino acid profile of scorpions exhibits significant variation depending on geographical origin and processing methodologies. This variability in both quantitative composition and qualitative distribution of amino acids has been established as a reliable biochemical marker for assessing the quality of scorpion-derived products (Li et al., 2007; Liang et al., 2014).
3.5.2 Lipids
Lipids are essential substances for the human body to store and supply energy, including lipids (phospholipids) and oils. The phospholipid composition of Scorpio has been extensively characterized, with particular attention to lecithin components that feature hydrophilic phosphorylcholine groups (Ai, 2017). Specifically, scorpions contain a unique phospholipid identified as 1-stearyl-glycero-3-phosphocholine, characterized by its long-chain fatty acyl group structure. In addition, scorpion oil contains many unsaturated fatty acids such as palmitic acid, stearic acid, oleic acid, linoleic acid, linolenic acid, and behenic acid (Uchiyama et al., 2010; Ferreira et al., 2015).
3.5.3 Other ingredients
Nucleosides represent fundamental biomolecules that play crucial roles in cellular metabolism and the maintenance of vital biological processes. Scorpio has been found to contain a diverse array of nucleosides, including uracil, hypoxanthine, xanthine, guanosine, and adenosine (Zhang et al., 2024). Notably, the concentration profile of these nucleoside components exhibits significant variation depending on the extraction methodology employed, with different decoction techniques resulting in markedly distinct nucleoside compositions in the final extract (Tian et al., 2013). In addition to nucleosides, scorpions are a rich source of essential minerals, particularly calcium (Ca) and magnesium (Mg), along with various trace elements that are vital for human physiological functions. These inorganic nutrients are predominantly concentrated in the abdominal region of scorpions (Ma and Lin, 2000). Interestingly, comparative analyses have revealed that female scorpions contain higher concentrations of Ca and Mg compared to their male counterparts. This sexual dimorphism in mineral content has been proposed as a potential biomarker for quality assessment in the evaluation of premium-grade scorpion specimens (Ma and Lin, 2000).
Functional active components in scorpions have been described, but how many effective substances are retained in processed scorpion medicinal materials? Integrated transcriptomics–proteomics–metabolomics analysis can be used to track the formation patterns of degraded scorpion venom peptide fragments and other small molecules after processing, thereby enriching the research on the material basis of Scorpio.
4 Pharmacological effects
Scorpio has a long history of medicinal use. It is an important medicinal material for treating spasms and convulsions, and has the functions of detoxifying and dredging collaterals (Ma, 2019). Modern research shows that Scorpio has many pharmacological activities, such as anti-epilepsy, anticonvulsant, anti-tumor, bacteriostatic, anticoagulant, and antithrombotic effects (Wali et al., 2019; Yang et al., 2020) (Figure 5).
FIGURE 5
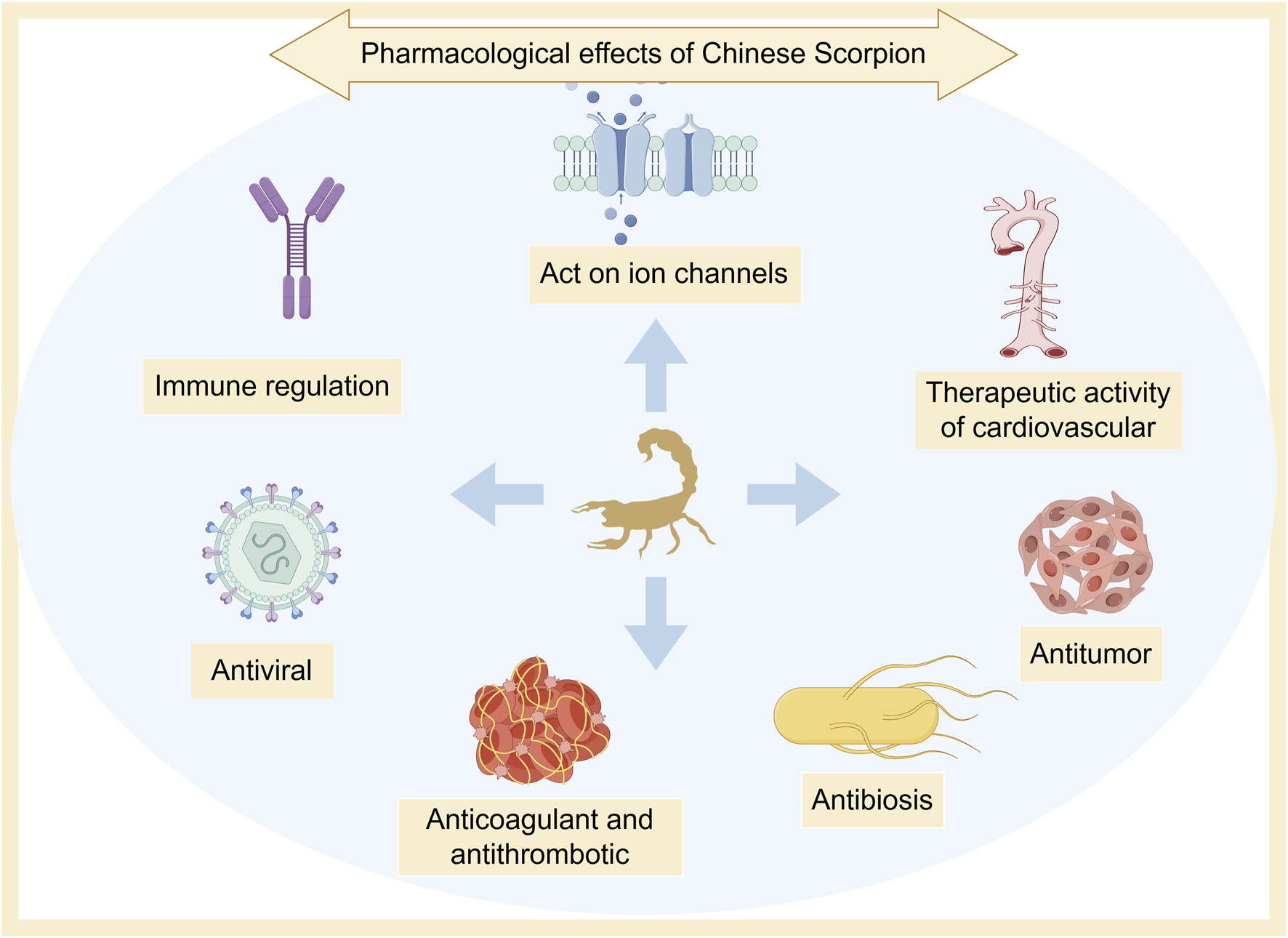
Pharmacological effects of Scorpio.
4.1 Anticancer activity
Although great progress has been made in cancer treatment, the morbidity and mortality are still increasing. Therefore, it is still urgent to develop new and effective anticancer drugs. The crude peptide extracts and peptide monomers from the venom of B. martensii scorpions show anti-cancer effects, which indicates that the venom is a good source for finding new anti-cancer drugs (Wang and Ji, 2005). A large number of studies have confirmed that scorpion venom polypeptides have shown good effects in the treatment of liver cancer, lung cancer, glioma, brain tumor, breast cancer, melanoma, prostate cancer and esophageal cancer (Fu et al., 2007; Mishal et al., 2013). The mechanisms of anti-tumor activity are summarized in Table 6 and Figure 6.
TABLE 6
| Name | Effective concentration | Cellular/Animal model | Mode of action | Reference |
|---|---|---|---|---|
| PESV | 50–100 mg/L | Human bladder carcinoma T24 cells | Upregulate pro-apoptosis protein Bax and downregulate anti-apoptosis protein Bcl-2 | Hou et al. (2013) |
| 40 mg/kg | Lewis lung carcinomas | Reduce the expression of DII4 and Notch1 | Sun et al. (2011) | |
| 10–40 mg/L | Pancreatic cancer MIA-paca-2 cells | Inhibit the secretion of MMP-9 and upa, reduce FN levels, upregulate the expression of E-Cadherin protein | Zhang et al. (2009) | |
| 10 mg/kg, 40 mg/kg | H22 Hepatocellular Carcinoma Subcutaneously Implanted Tumor Model | Increase the expression of PTEN and decrease the expression of PI3K、P-Akt、COX-2、HIF-1α and VEGF-A | Sui et al. (2014) | |
| 10 mg/kg, 40 mg/kg | H22 Hepatocellular Carcinoma Subcutaneously Implanted Tumor Model | Increase the proportion of NK cells infiltrating tumor tissues, restore NK cell cytotoxic activity | Han et al. (2016) | |
| 25–200 mg/L | Non-small cell lung cancer cell line A549 cells | Increase the expression of PTEN and decrease the expression of HIF-1α and VEGF, arrest the cell cycle in the G0/G1 phase | Wang et al. (2012) | |
| 10 mg/kg, 20 mg/kg | Human SKOV3 Nude Mouse Xenograft Tumor Model | Inhibit the expression of PI3 K and P-Akt and increase PTEN in the microenvironment of tumors | Song et al. (2012b) | |
| SVCIII | 29 μg/mL and 39.6 μg/mL | Human leukemia THP-1cells and Jurkat cells | Induce cell cycle arrest in the G1 phase and inhibit the activation of NF-κB | Song et al. (2012a) |
| rBmk AGAP | 10–30 μM | Human gliomas SHG-44 cells | Inhibit the expression of cell cycle regulatory proteins CDK2, CDK6, and p-RB, arrest the cell cycle in the G1 phase, and interfere with the p-AKT, NF-kb, BCL-2, and MAPK signaling pathways | Zhao et al. (2011b) |
| 15–60 μM | Human breast cancer MDA-MB-231 and MCF-7 cells | Decrease the expression of Oct4, Sox2, N-cadherin, Snail, and increase the expression of E-cadherin. | KAMPO (2019) | |
| 1.0 mg/kg | S-180 fibrosarcoma model | Inhibit the growth of solid tumors and prolong the survival days | Liu et al. (2003) | |
| rBmk CTa | 0.28 μM | Human glioma SHG-44 cells | Inhibit the proliferation | Fu et al. (2007) |
| Bmk CT | 0.6–2.4 μM | Rat glioma C6 cells | Inhibit the invasion and migration by antagonizing MMP-2 | Fu et al. (2011) |
The anti-tumor activity of B. martensii scorpions.
FIGURE 6
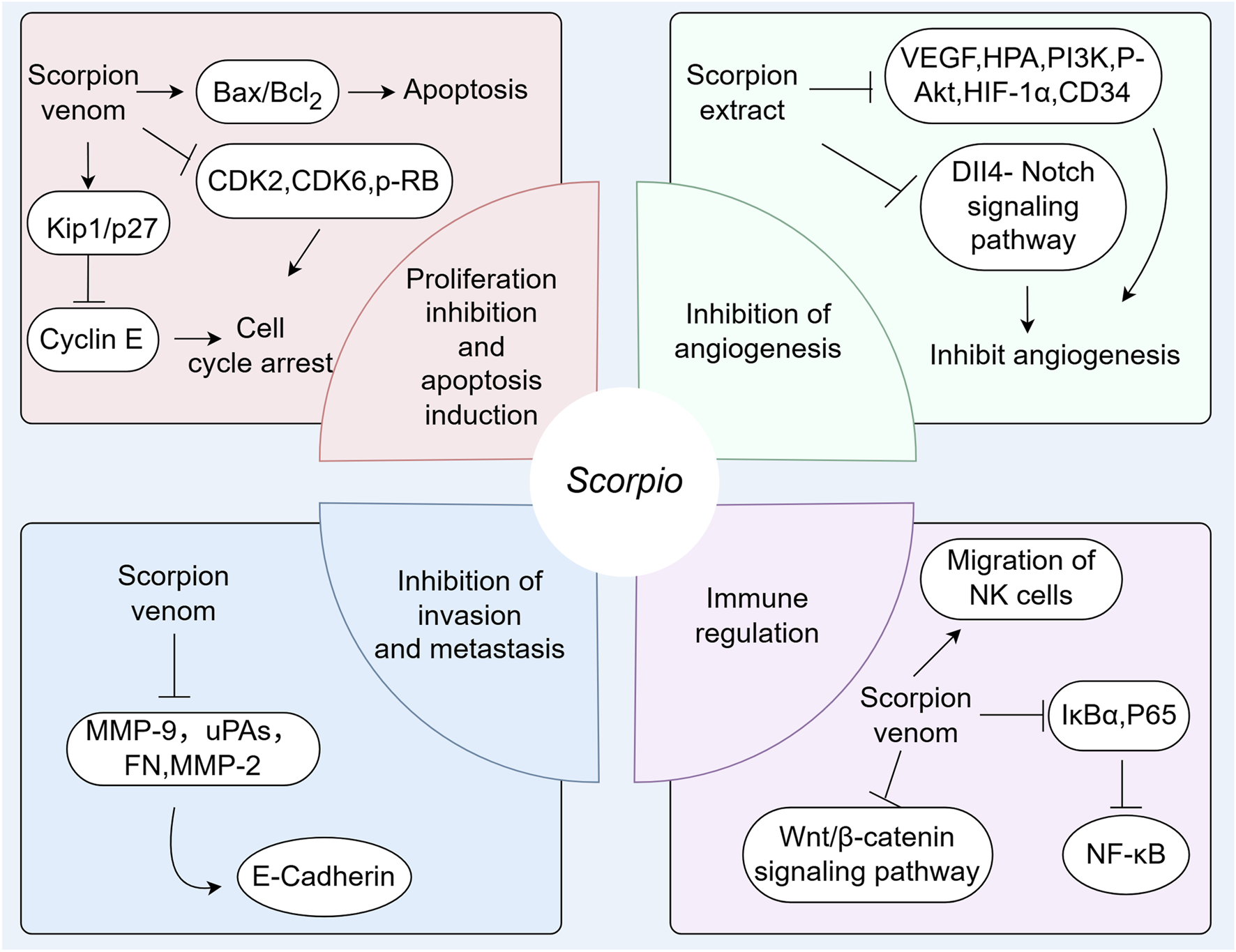
Mechanisms of Scorpio’s anti-tumor activity.
It has been found that scorpion venom polypeptides can inhibit the proliferation of many cancer cell lines. The main mechanisms are to induce cancer cell apoptosis, cell cycle arrest, inhibition of angiogenesis, invasion and migration, and immune regulation. Vascular endothelial growth factor (VEGF), epidermal growth factor receptor (EGFR), and basic fibroblast growth factor (bFGF) are highly expressed in various tumors and are considered important signal transducers in angiogenesis, involving the MAPK (Mitogen-Activated Protein Kinase), PI3K (Phosphoinositide 3-kinase)/Akt, and other signaling pathways (Sui et al., 2014). Many research results suggest that scorpion extract can inhibit the expression of VEGF, HPA, PI3K, P-Akt, HIF-1α, and CD34 in the microenvironment of liver cancer, thereby inhibiting angiogenesis (Wang et al., 2010b; Sui et al., 2014). The invasion and metastasis of tumor cells constitute one of the primary factors contributing to disease progression and deterioration. Currently, numerous studies focus on processes such as epithelial-mesenchymal transition (EMT) and the matrix metalloproteinase family (MMPs). Following EMT, epithelial cells undergo a series of cellular transformations ultimately resulting in basement membrane degradation and extracellular matrix remodeling (Patrick Henriet and Hervé Emonard, 2019). The MMP family is extensive and capable of degrading various types of collagen and adhesion proteins, thereby playing a crucial role in tumor cell adhesion, invasion, and migration (Patrick Henriet and Hervé Emonard, 2019). Tumor cells evade immune recognition and attack through complex mechanisms, allowing unchecked growth. Blocking this immune escape and remobilizing the immune system is a key anti-tumor strategy (Uzhachenko et al., 2025).
PESV (polypeptide extract from scorpion venom) is a group of 50–60 amino acids long peptides extracted from B. martensii crude venom. And it exhibits inhibitory effects on a variety of cancer cells. In addition to inducing tumor cell apoptosis, arresting the cell cycle, and inhibiting angiogenesis, PESV significantly suppress liver metastasis of pancreatic cancer with an inhibition rate exceeding 70%, which is markedly superior to the anti-angiogenic drug TNP-470 (Cui et al., 2009; Zhang et al., 2009). BmK AGAP is a long-chain toxin isolated from B. martensii venom, which contains 66 amino acid residues and 4 pairs of disulfide bridges (Zeng et al., 2000; Liu et al., 2002). Voltage-gated Na+ ion channel Nav1.5 is overexpressed in breast cancer and is associated with tumor progression (Brackenbury, 2012). BmK AGAP downregulates PTX3 expression by inhibiting or binding to Nav1.5, thereby reducing the activation of the NF-κB and Wnt/β-catenin signaling pathways as well as the production of the inflammatory factor TNF-α(Kampo et al., 2019). This demonstrates the potential of BmK AGAP in the treatment of inflammation-induced cancers. Recombinant BmK AGAP is more effective for tumor cells and less harmful for healthy cells than cyclophosphamide in S-180 fibrosarcoma model (Liu et al., 2003). In summary, both natural polypeptides and recombinant polypeptides exhibit great potential in antitumor applications. Meanwhile, the high expression efficiency and high yield of recombinant polypeptides give them certain advantages in the research and development of new drugs.
4.2 Analgesic activity
Studies have shown that using the whole body or tails of Scorpio in the treatment of somatic pain, visceral pain, neuralgia, and cancerous pain yields good therapeutic effects (Liu and Ma, 1993). The hot plate tail-flick test and acetic acid-induced writhing test were used to detect the analgesic effects of scorpion bodies and tails, respectively, and the results indicated that both exhibit significant analgesic effects (Liu and Ma, 1993). Scorpio can exert an analgesic effect on rats with bone cancer pain by inhibiting bone destruction and suppressing the activation of spinal astrocytes and microglia (Yu et al., 2020).Since analgesia is the main pharmacological effect of Scorpio, in recent years, many scholars have been committed to isolating and purifying single-component analgesic active peptides from scorpion venom, the main active component of Scorpio. The comparison between the peptides with analgesic activity and the positive control drug is summarized in Table 7.
TABLE 7
| Name | Model | Dose- inhibition efficiency | Reference | |
|---|---|---|---|---|
| Peptide | Positive control | |||
| Bmk AEP | Acetic acid-induced writhing pain model | 0.75 mg/kg-50% | 0.2 mg/kg-70.7% | Cao (2004) |
| BmKBTx | Acetic acid-induced writhing pain model | Slightly less analgesic than morphine | Cui et al. (2017) | |
| BmNaL-3SS2 | Acetic acid-induced writhing pain model | Exhibit greater analgesic effects than morphine | Cui et al. (2017) | |
| BmK AGAP | Acetic acid-induced writhing pain model and hot plate test | 1.0 mg/kg-60% | 1.5 mg/kg-35% | Liu et al. (2003) |
| BmK AGP-SYPU1 | Acetic acid-induced writhing pain model | 1.0 mg/kg-68.8% | 1.0 mg/kg-65.2% | Yu et al. (2011) |
| BmK AGP-SYPU2 | Acetic acid-induced writhing pain model and hot plate test | 1.42 mg/kg-50% | 1.5 mg/kg-- | Shao et al. (2014) |
| BmK IT-AP | Acetic acid-induced writhing pain model | 1 mg/mL-54.4% | 80 mg/mL- 62.4% | Xiong et al. (1999) |
| BmK IT1 | Acetic acid-induced writhing pain model | 0.7 mg/kg-50% | 0.2 mg/kg-70.7% | Cao (2004) |
| BmK AngP1 | Acetic acid-induced writhing pain model | 5 μg/g-43.0% | 80 μg/g-62.4% | Guan et al. (2001b) |
| BmK dITAP3 | Acetic acid-induced writhing pain model | 5 μg/g-44.8% | - | Guan et al. (2001a) |
| BmK Ang M1 | Acetic acid-induced writhing pain model | 0.39 mg/kg-50% | 0.25 mg/kg-78.3% | Cao (2004) |
| BmK AS | Acetic acid-induced writhing pain model and hot plate test | 1.42 mg/kg-50% | 2.0 mg/kg-80% | Shao et al. (2007b) |
Peptides with analgesic activity from B. martensii scorpions.
Voltage-gated sodium channels (VGSCs) have been identified as crucial components in the molecular mechanisms underlying pain signaling pathways. Among the various VGSC subtypes, Nav1.3, Nav1.7, and Nav1.8 have been specifically implicated in nociceptive processing and pain pathogenesis (Li et al., 2019). It has been demonstrated that blocking these sodium channels significantly attenuates or completely abolishes pain responses. BmNaL-3SS2 and BmKBTx, two additional toxins identified from the cDNA library of B. martensii venom glands, have been shown to exhibit analgesic activity through selective inhibition of Nav1.7 channels (Cui et al., 2017). BmK AS, a β-toxin, was shown to dose-dependently inhibit sodium currents without affecting potassium currents or Ca2+ influx, indicating its specific targeting of VGSCs(Liu et al., 2008). In contrast to the aforementioned sodium channel-targeting peptides, BmK-YA specifically interacts with opioid receptors, demonstrating binding affinity for μ-, δ-, and κ-opioid receptor subtypes (Yan et al., 2012). Notably, BmK-YA exhibits a 72-fold lower EC50 value at μ-opioid receptors compared to morphine, suggesting a potentially reduced risk of receptor-mediated adverse effects (Yan et al., 2012).
At present, the commonly used analgesic drugs in clinical practice are opioid analgesics and non-steroidal anti-inflammatory drugs, which often lead to dependence and addiction. Active components from Scorpio and scorpion venom have strong inhibitory effects on various acute and chronic pains, with most exhibiting greater efficacy than morphine, and they are non-addictive. With further development of the analgesic components in scorpion venom, it is expected that new analgesic drugs with non-addictive properties, long-lasting effects, and high safety will be developed and widely used in clinical practice in the future, benefiting patients.
4.3 Anti-epilepsy activity
Accumulating evidence has demonstrated that venom-derived peptides from B. martensii exhibit high specificity toward VGSCs, positioning them as promising candidates for anti-epileptic therapeutics (Xiao et al., 2022). In preclinical studies, the anti-epileptic peptide AEP (28 mg/kg) significantly suppressed seizures induced by coriolide lactone and cephaloridine in rat models, and its efficacy outperformed that of diazepam. Meanwhile, AEP showed no adverse effects on cardiovascular parameters such as heart rate, blood pressure, or electrocardiographic profiles (Zhou et al., 1989). Further investigation into the anticonvulsant properties of BmK IT2 revealed its therapeutic potential in a pilocarpine-induced status epilepticus (SE) model. Administration of BmK IT2 markedly prolonged the latency to SE onset, attenuated SE severity, and suppressed hippocampal c-Fos expression during epileptogenesis, suggesting its role in modulating neuronal hyperexcitability (Zhao et al., 2008). Notably, BmK AS, a peptide targeting receptor site 4 of VGSCs, demonstrated potent inhibition of pentylenetetrazole (PTZ)-induced seizures (Zhao et al., 2011a). This selective interaction highlights BmK AS as a novel molecular probe for elucidating the pathophysiological role of specific sodium channel subtypes in epilepsy. Additionally, this interaction underscores BmK AS’s translational value in developing subtype-selective antiepileptic drugs.
4.4 Antibiosis activity
Scorpio exhibits broad-spectrum antibacterial activity and exerts a certain inhibitory effect on both Gram-positive and Gram-negative bacteria, primarily mediated by bioactive components including peptides, steroids, and quinones. Peptides with antibacterial activity are summarized in Table 8, including BmK bpp, BmKn2, and BmK AS. Mechanistic studies reveal that BmKn2 rapidly lyses Staphylococcus aureus by binding to lipoteichoic acid (LTA) on the bacterial surface, disrupting membrane integrity, and degrading the cell wall (Cao et al., 2012).
TABLE 8
| Name | Bacteria | Minimum inhibitory concentrations (mics) | Reference |
|---|---|---|---|
| BmK bpp | Gram-negative bacteria | 2.3–68.2 μM | Zeng et al. (2012) |
| Bacillus subtilis, Listeria monocytogenes, Micrococcus luteus, Enterococcus faecalis | 24.4 μM, 5.7 μM, 20.3 μM, 57.1 μM | ||
| BmKn2 | penicillin-resistant Staphylococcus aureus (PRSA), methicillin-resistant S. aureus (MRSA) | 0.6–50 μg/mL | Cao et al. (2012) |
| BmK AS | S. aureus, Salmonella typhi, Escherichia coli, Pseudomonas aeruginosa | 4.5–10 μM | Ji et al. (1999), Shao et al. (2008) |
Peptides with antibacterial activity from B. martensii scorpions.
Furthermore, cardiac glycosides derived from scorpion venom exhibit notable antimicrobial properties. A structurally unique cardiac glycoside, identified as 3β-acetoxy-2,14,22-trihydroxy-19-hydroxymethyl-9α,5β,14β-card-20 (22)-enolide, demonstrated significant inhibitory activity against the Gram-positive bacterium Bacillus subtilis, with a MIC of 15 μg/mL (Gao et al., 2014). These findings underscore the diversity of antimicrobial components in scorpion venom and their potential as novel therapeutic agents against drug-resistant infections.
4.5 Anticoagulant and antithrombotic activity
Recent studies have demonstrated that scorpion venom peptides exhibit potent anticoagulant and antithrombotic activities. In murine thrombosis models and zebrafish thrombosis assays, peptides such as BmK-M10 and BmKITc showed significant antithrombotic effects, with BmKITc exhibiting superior efficacy in a concentration-dependent manner (Sun et al., 2021). In vitro platelet aggregation assays revealed that scorpion venom peptides (SVPs) significantly inhibited thrombin-induced platelet aggregation in rabbits in a dose-dependent manner (Lv et al., 1995). Further investigations into the effects of scorpion venom anticoagulant peptides (SVAPs) on hemorheology demonstrated that SVAPs reduced plasma viscosity (PV), fibrinogen (Fib) levels, and erythrocyte aggregation while enhancing erythrocyte deformability. These findings suggest that SVAPs improve blood fluidity by lowering whole blood viscosity (BV), which may contribute to their antithrombotic and fibrinolytic mechanisms (Song et al., 2002). Additionally, scorpion-derived bioactive peptides have been shown to enhance mesenteric microcirculation in rats by increasing capillary blood flow and vascular diameter, thereby preventing thrombus formation (Tian and Zheng, 2004). Mechanistic studies indicate that scorpion venom fibrinolytic peptides upregulate prostacyclin (6-keto-PGF1α) and nitric oxide (NO) levels while modulating the activity of plasminogen activator inhibitor-1 (PAI-1) and tissue plasminogen activator (tPA). These effects protect endothelial cells under hypoxic conditions and enhance their anticoagulant and fibrinolytic functions (Wang and Wu, 2010). Furthermore, scorpion venom peptides stimulate NO secretion in endothelial cells, leading to elevated intracellular cyclic guanosine monophosphate (cGMP) levels, which inhibit platelet aggregation. Concurrently, they suppress thrombin-induced increases in intracellular calcium concentration, further attenuating prothrombotic pathways (Tian and Zheng, 2004).
4.6 Antiviral activity
According to the Chinese Pharmacopoeia, Scorpio is traditionally described as possessing therapeutic properties to “neutralize toxins and dissipate pathogenic accumulations.” Modern pharmacological studies have further revealed its broad-spectrum antiviral activity against the influenza virus, dengue virus (DENV), hepatitis C virus (HCV), herpes simplex virus (HSV), and human immunodeficiency virus (HIV) (Andrea et al., 2022). Notably, the scorpion venom-derived peptide HP-1090 exhibits potent anti-HCV effects by disrupting the viral capsid structure and suppressing genomic replication, with a half-maximal inhibitory concentration (IC50) of 7.62 μg/mL (Yan et al., 2011). Another antiviral peptide, AVP, targets the HIV-1 envelope glycoprotein gp120, competitively inhibiting its interaction with the CD4 receptor to block viral entry into host cells (da Mata et al., 2017). Beyond direct viral neutralization, AVP modulates host immune responses, potentially interfering with multiple stages of the viral life cycle (Andrea et al., 2022). Additionally, aqueous extracts from lyophilized scorpion venom demonstrate dose-dependent inhibition of HSV, respiratory syncytial virus (RSV), and enterovirus 71 (EV71), with optimal inhibitory concentrations of 2 mg/mL, 20 μg/mL, and 2 mg/mL, respectively (Zhang et al., 2019).
4.7 Therapeutic activity of cardiovascular diseases
Martentoxin, a 37-amino-acid peptide belonging to the α-KTx (alpha-potassium channel toxin) toxin family, is purified from the venom of B. martensii. This neuroactive peptide significantly attenuates tumor necrosis factor-α (TNF-α)-induced NO overproduction through dual mechanisms: inhibition of iNOS (inducible nitric oxide synthase) activity and blockade of calcium-activated potassium channels (Busse et al., 1991; Shi et al., 2008; Wang et al., 2013). Pathological NO overproduction is critically involved in multiple disease processes, including gram-negative bacterial sepsis, cardiac dysfunction, and ischemia-reperfusion injury in cerebral and cardiovascular systems (Silvia Llorens, 2003). These findings establish Martentoxin as a potential therapeutic agent for managing NO-mediated pathological conditions. Bumarsin, a 72-amino-acid polypeptide isolated from BmK venom, demonstrates potent inhibition of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, achieving 32% suppression of enzyme activity at a concentration of 0.6 mM(Chai et al., 2012). HMG-CoA reductase inhibitors are clinically established for managing hypercholesterolemia, a major risk factor for atherosclerosis and associated cardiovascular disorders. Notably, Bumarsin exhibits superior pharmacological efficacy compared to simvastatin, a first-line statin drug. While simvastatin achieves only 35% HMG-CoA reductase inhibition at 10 mM concentration, Bumarsin demonstrates comparable inhibition (32%) at 60-fold lower concentration (0.6 mM) (Chai et al., 2012). Moreover, Bumarsin modulates cholesterol homeostasis through upregulation of key regulatory proteins, including apolipoproteins (Apo-A1, Apo-E, Apo-CI/II/III) and the steroidogenic acute regulatory protein (StAR) (Chai et al., 2012). These findings collectively highlight Scorpio’s multifactorial mechanism of action and its clinical potential as a novel therapeutic agent for hypercholesterolemia management.
4.8 Other activities
Alzheimer’s disease (AD), a neurodegenerative disorder characterized by progressive cognitive decline, is closely associated with acetylcholine deficiency. Acetylcholinesterase (AchE) and butyrylcholinesterase (BchE), key enzymes catalyzing acetylcholine hydrolysis, represent critical therapeutic targets (Zhang et al., 2022a). Guanidine alkaloids (martensine A/B) isolated from Scorpio exhibit dual inhibition of AchE and BchE, potentially through interactions with catalytic and peripheral anion sites. These alkaloids also demonstrate bio metal chelation capacity (Cu2+, Fe2+, Zn2+, Al3+), suggesting multi-target therapeutic potential for AD (Liu et al., 2021b). Furthermore, a novel β-carbonyl saccharide alkaloid (Harmonyl-β-D-glucopyranoside) from Scorpio non-competitively inhibits α-glucosidase (IC50 = 24 μM), showing promise for metabolic disorder management (Kim, 2013). In cancer therapeutics, polypeptide PESV synergizes with driamycin to reverse multidrug resistance in leukemia K562/A02 stem cells by downregulating P-gp, BCRP, MDR1, and PI3K/NF-κB pathway components (Yang et al., 2016). Additionally, the neuroprotective scorpion venom heat-resistant peptide (SVHRP) exhibits neuroprotective effects by reducing neurological deficit scores, inhibiting edema formation, decreasing infarct volume and neuronal loss, and protecting primary neurons against oxygen-glucose deprivation/reoxygenation (OGD/R)-induced injury (Wang et al., 2020).
In summary, Scorpio exerts a wide range of pharmacological effects, and research on the material basis for exerting these effects focuses primarily on the active peptides of scorpion toxins. However, it must be clearly stated that the vast majority of these findings are based on in vitro models and animal experiments, lacking validation from human clinical trials. This limitation hinders our progress in translating these promising active substances into clinical drugs. BmK AGAP, a potential candidate molecule, has demonstrated heterologous expression and maintained its pharmacological effects while increasing its yield. In the future, it may be employed as a monotherapy drug to systematically promote the pre-clinical and clinical development that meets the standards. In light of the prevalent challenges associated with peptide drugs, including their limited membrane permeability and instability in vivo, there is an imperative to explore the potential of nanotechnology, such as liposomes and polymer nanoparticles, for the encapsulation of these peptides. This approach aims to enhance their bioavailability, target specificity, and controlled release, thereby facilitating their clinical success.
5 Toxicity and side effects
Research has demonstrated that although Scorpio exhibits significant pharmacological efficacy, its use at high doses may induce multiple adverse reactions. When investigating the analgesic effects of Centipede-Scorpion Powder in mice, the serum levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in the low- and medium-dose groups (20 and 50 mg/kg) showed no significant differences compared to the control group. However, in the high-dose group (80 mg/kg), both ALT and AST levels were significantly elevated, indicating increased permeability of hepatocyte membranes and suggesting mild hepatic injury at this dosage (Xu et al., 2018). Modern pharmacological studies have confirmed that the toxic components of Scorpio primarily originate from its venom, which can readily induce multi-system toxicity including neurotoxicity, cardiovascular damage, urinary system injury, and respiratory depression (Ma, 2019). The toxicological effects of scorpion venom predominantly stem from their interaction with VGSCs. BmK NT1 enhances sodium influx in cerebellar granule cells (CGCs) and inhibits the fast inactivation of VGSCs, mimicking the pharmacological behavior of α-scorpion toxins. Neurotoxicity induced by α-scorpion toxins via VGSC activation is attributed to intracellular Ca2+ overload mediated through NMDA receptor-dependent pathways and Na+/Ca2+ exchanger mechanisms (He et al., 2017). Notably, BmK M7, an α-scorpion toxin, exerts cardiotoxic effects by binding to cardiac Na+ channels and altering their electrophysiological properties (Guan et al., 2002). BmK I, another α-scorpion toxin comprising 64 amino acid residues, has been identified as specifically binding to receptor Site-3 and prolong the inactivation phase of VGSCs(Possani et al., 2010). Intrahippocampal administration of BmK I elevated c-Fos expression and caused significant morphological changes in the hippocampus, leading to neuronal loss in distinct hippocampal subregions and inducing convulsive behaviors in rats (Bai et al., 2006). It is important to note that quantitative toxicokinetic parameters (such as LD50 (median lethal dose) and therapeutic index) are lacking for most isolated scorpion venom components. Establishing quantitative thresholds for ‘safe’ exposure levels of these toxic constituents is therefore essential for ensuring clinical safety.
Scorpio undergoes multiple preparatory methods—such as boiling (in plain water or saltwater), mint processing, licorice rinsing, and alcohol rinsing—prior to clinical application, aimed at mitigating toxicity while preserving pharmacological activity (Zhang et al., 2024). Adjuvants are also used to achieve the same goal. For example, Radix Glycyrrhizae and honey, as sweet-natured harmonizing agents, mitigate the violent toxicity of scorpion venom; mint (dispelling wind and detoxifying) and glutinous rice (moistening dryness) enhance its wind-dispelling and analgesic effects; and vinegar processing simultaneously masks odors, neutralizes toxins, and directs the medicinal properties toward the liver channel via its sour nature, thereby doubling the efficacy of vinegar-processed scorpion in resolving masses, freeing collateral channels, and alleviating pain (Ma, 2019; Zhang et al., 2024). Because chitosan has a good adsorption performance for trace heavy metal ions such as lead, the subsequent processing of scorpions with chitosan significantly lowers heavy metal content in processed scorpions (Xiang, 2014). Overall, proper processing methods significantly reduce toxicity, while maximising the retention of pharmacological activity. However, research on the mechanisms underlying this detoxification remains limited, and the patterns of component changes and toxicity variations before and after processing are not yet fully understood. Future studies could integrate the analysis of Scorpio’s active components with its toxicity, employing correlation analysis to elucidate the scientific basis for detoxification through processing.
6 Conclusion and future perspectives
This study reviews the core research advances on the Chinese medicinal scorpion (B. martensii) based on extant literature. Traditional applications trace back to its first documented medicinal value in the Shu Ben Cao, with the Kaibao Bencao explicitly noting its use in treating spasms and convulsions. As demonstrated in the Qing Dynasty summaries, the efficacy of this treatment in dispersing blood heat and expelling wind-dampness is well-established, constituting a significant contribution to the long-standing and systematic medicinal history. The chemical constituents of the venom are centered on scorpion venom peptides as the core active substances, supplemented by steroidal derivatives, alkaloids, amino acids, and other components, collectively forming the material basis for its pharmacological activity. The pharmacological effects span anticancer, analgesic, antiepileptic, antibacterial, anticoagulant, and antithrombotic activities. Scorpion venom peptides (e.g., BmK AGAP, PESV) have been shown to possess significant therapeutic potential through mechanisms such as ion channel regulation and apoptosis induction. However, it should be noted that high-dose administration of Scorpio and peptides may induce adverse reactions, including liver damage and neurotoxicity. Whilst processing methodologies such as boiling and licorice rinsing have been demonstrated to reduce toxicity, the underlying mechanisms remain unclear.
With a history of use in traditional Chinese medicine for over a millennium, B. martensii has been studied for its potential pharmacological applications, though further research is needed to validate its therapeutic efficacy. Firstly, there is a paucity of clinical evidence, as all pharmacological activities are based solely on in vitro or animal experiments, lacking human clinical trial data. Secondly, key mechanisms remain unelucidated, with no molecular mechanisms for processing and detoxification identified and no established quality control standards. Thirdly, there is a disconnect between component research and clinical application. Clinical use relies on processed scorpions, which are ground into powder and mixed with other medicinal materials before being administered orally to patients. However, modern reductionist studies focus on isolated scorpion venom components, without thoroughly exploring the significant differences between the effects of pure peptides and those of processed crude medicinal materials.
In the field of Traditional Chinese Medicine, future research on Scorpio should prioritize “clinical translation” as the central focus. This approach aims to address the discrepancy between “modern studies focusing on isolated scorpion venom components” and “clinically used processed scorpions.” To this end, researchers should employ interdisciplinary technologies to overcome existing limitations, thereby facilitating progress in this area of study. Omics technologies need to advance from the current preliminary exploration of scorpion venom components using transcriptomics and proteomics to an integrated “transcriptomics-proteomics-metabolomics” analysis (Gao et al., 2021). This not only involves excavating toxin genes specifically expressed in scorpion venom gland cells to optimize the expression of recombinant peptides, but also requires analyzing the interactions between scorpion venom components and other constituents (such as steroids and alkaloids). It is essential to clarify the differential mechanisms of component synergy and pharmacodynamic effects between pure peptides and crude, processed medicines. In the field of structural biology, it is imperative to elucidate the intricate structures of scorpion venom peptides bound to their targets (e.g., Nav1.7, μ-opioid receptors) (Yan et al., 2012; Cui et al., 2017). This provides a foundation for peptide modification (e.g., reducing the cardiotoxicity of BmK M7) (Guan et al., 2002). Concurrently, research should be conducted on the target network involved in the multi-component co-action, in conjunction with the compositional characteristics of processed scorpions. In addition to enhancing the druggability of scorpion venom peptides, contemporary drug delivery systems must be engineered to preserve the synergistic advantages of multiple components when crude, processed medicines are employed (Yang et al., 2024).
Although this review summarizes a large amount of research on Scorpio, there are some limitations. Firstly, our literature search was limited to English and Chinese databases (PubMed, CNKI, etc.), and we acknowledged that this may have excluded relevant studies published in other languages or regional databases—potentially narrowing the scope of evidence synthesized. Secondly, we implemented a pragmatic quality filter during literature selection—excluding studies with unclear experimental methods, insufficient sample sizes, or unvalidated outcome measures, and most of the included studies reported positive outcomes, which may reflect publication bias. Thirdly, this review did not fully incorporate international guidelines relevant to processed B. martensii research, which may limit the international applicability of its findings.
Statements
Author contributions
M-HL: Writing – original draft, Writing – review and editing. J-JZ: Investigation, Resources, Writing – review and editing. QL: Investigation, Resources, Writing – review and editing. Z-NQ: Investigation, Resources, Writing – review and editing. QZ: Investigation, Resources, Writing – review and editing. LZ: Investigation, Resources, Writing – review and editing. Y-PD: Writing – review and editing. D-HS: Conceptualization, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. We appreciate the support from the following projects: Shandong Provincial Natural Science Foundation (ZR2025QC1727), Shandong Province Traditional Chinese Medicine Science & Technology Project (M20242207, Z-2022042), National Heritage Studio for Veteran TCM Pharmacists, NA-TCM ([2024]255 and [2025]181), Construction Project of Heritage Base for TCM Processing Techniques, NA-TCM ([2022]185), Seventh Batch National Mentorship Program for TCM Expertise Inheritance, NA-TCM ([2022]76)).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. During the preparation of this work, the authors used Deep-seek in order to improve language. After using this tool, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
- AChE
Acetylcholinesterase
- AD
Alzheimer’s disease
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- BChE
Butyrylcholinesterase
- bFGF
Basic fibroblast growth factor
- B. martensii
Buthus martensii Karsch
- BV
Blood viscosity
- CGCs
Cerebellar granule cells
- cGMP
Cyclic guanosine monophosphate
- CNKI
China national knowledge infrastructure
- DBPs
Disulfide-bridged peptides
- DENV
Dengue virus
- DNA
Deoxyribonucleic acid
- EGFR
Epidermal growth factor receptor
- EMT
Epithelial-mesenchymal transition
- EV71
Enterovirus 71
- FN
Fibronectin
- GC-MS
Gas chromatography-mass spectrometry
- HCV
Hepatitis C virus
- HIV
Human immunodeficiency virus
- HMG-CoA
Hydroxy methylglutaryl coenzyme A
- HS-SPME
Headspace solid-phase microextraction
- HSV
Herpes simplex virus
- IC50
Half maximal inhibitory concentration
- LTA
Lipoteichoic acid
- MAPK
Mitogen-activated protein kinase
- MICs
Minimum inhibitory concentrations
- MMPs
Matrix metalloproteinase
- MRSA
Methicillin-resistant Staphylococcus aureus
- NDBPs
Non-disulfide-bridged peptides
- NF-κB
Nuclear factor kappa-B
- NK
Natural killer
- NO
Nitric oxide
- PAI-1
Plasminogen activator inhibitor-1
- PESV
Polypeptide extract from scorpion venom
- PI3K
Phosphoinositide 3-kinase
- PRSA
Penicillin-resistant Staphylococcus aureus
- PTZ
Pentylenetetrazole
- PV
Plasma viscosity
- RSV
Respiratory syncytial virus
- SVPs
Scorpion venom peptides
- TNF-α
Tumor necrosis factor α
- tPA
Tissue plasminogen activator
- uPA
Urokinase-type plasminogen activator
- VEGF
Vascular endothelial growth factor
- VGSCs
Voltage-gated sodium channels
References
1
Ai S. Y. (2017). Studies on the chemical constituents of buthus martensii and zanthoxylum nitidum. Tianjin, China: Tianjin University of Technology.
2
Almaaytah A. Albalas Q. (2014). Scorpion venom peptides with no disulfide bridges: a review. Peptides51, 35–45. 10.1016/j.peptides.2013.10.021
3
Almeida D. D. Torres T. M. Barbosa E. G. Lima J. P. M. S. Fernandes-Pedrosa M. D. F. (2013). Molecular approaches for structural characterization of a new potassium channel blocker from Tityus stigmurus venom: cDNA cloning, homology modeling, dynamic simulations and docking. Biochem. Biophysical Res. Commun.430 (1), 113–118. 10.1016/j.bbrc.2012.11.044
4
Andrea R. C. Alonso B. M. Antonio R. E. Angélica V. N. (2022). Antimicrobial activity developed by scorpion venoms and its peptide component. Toxins14 (11), 740. 10.3390/toxins14110740
5
Bai Z. T. Zhao R. Zhang X. Y. Chen J. Liu T. Ji Y. H. (2006). The epileptic seizures induced by BmK I, a modulator of sodium channels. Exp. Neurol.197 (1), 167–176. 10.1016/j.expneurol.2005.09.006
6
Brackenbury W. J. (2012). Voltage-gated sodium channels and metastatic disease. Channels6 (5), 352–361. 10.4161/chan.21910
7
Busse R. Lückhoff A. Mülsch A. (1991). Cellular mechanisms controlling EDRF/NO formation in endothelial cells. Arch. für Kreislaufforsch.86 (Suppl. 2), 7–16. 10.1007/978-3-642-72461-9_2
8
Cao Z. Y. (2004). Studies on Buthus martensi toxins: purification,identifcation, characterization and gene expression. Beijing, China: Chinese Academy of Medical Sciences and Peking Union Medical College.
9
Cao L. Dai C. Li Z. Fan Z. Li W. Wu Y. et al (2012). Antibacterial activity and mechanism of a scorpion venom peptide derivative in vitro and in vivo. PLoS ONE7 (7), e40135. 10.1371/journal.pone.0040135
10
Chai S. C. Armugam A. Strong P. N. Jeyaseelan K. (2012). Charaterization of bumarsin, a 3-hydroxy-3-methylglutaryl-coenzyme reductase inhibitor from Mesobuthus martensii Karsch venom. Toxicon60 (3), 272–279. 10.1016/j.toxicon.2012.04.352
11
Chen Y. (1991). Baoqing bencao zhezhong.
12
Chen B. Wang C. Ji Y. (2001). Scorpion BmK venom induces nociceptive response of rats by plantar injection. Neurotoxicology Teratol.23 (6), 675–679. 10.1016/s0892-0362(01)00174-x
13
Chen Z. Y. Hu Y. T. Yang W. S. He Y. W. Feng J. Wang B. et al (2012). Hg1, novel peptide inhibitor specific for Kv1.3 channels from first scorpion kunitz-type potassium channel toxin family. J. Biol. Chem.287 (17), 13813–13821. 10.1074/jbc.M112.343996
14
Commission C. P. (2025). Chinese pharmacopoeia (2025) volume I. Beijing: China Medical Science Press.
15
Cui Y. Z. Jia Q. Wang Z. P. wang Z. X. Zhang Y. Y. Zhang W. D. et al (2009). Polypeptide extract from scorpion venom inhibits liver metastasis in pancreatic cancer. J. Shandong Univ.47 (03), 20–22+29.
16
Cui Y. Zhang J. Zhao M. Zhao Y. Hu X. Wang X. et al (2017). Recombinant expression, functional characterization of two scorpion venom toxins with three disulfide bridges from the Chinese scorpion Buthus martensii Karsch. Protein Peptide Lett.24 (3), 235–240. 10.2174/0929866524666170117142404
17
Cyril G. Chi C. W. Tytgat J. (2002). An overview of toxins and genes from the venom of the Asian scorpion Buthus martensi Karsch. Toxicon40 (9), 1239–1258. 10.1016/s0041-0101(02)00142-3
18
da Mata é.C. G. Mour?O C. B. F. Rangel M. Schwartz E. F. (2017). Antiviral activity of animal venom peptides and related compounds. J. Venom. Animals Toxins Incl. Trop. Dis.23 (1), 3. 10.1186/s40409-016-0089-0
19
Du Q. Y. Qin C. C. Zhang Y. M. Sun M. L. Ma X. J. Li C. et al (2024). Textual research on the processing history and research progress of modern processing of scorpio. Chin. Tradit. Pat. Med.46 (02), 542–548.
20
Fan J. J. (2020). Studies on the active ingredients of diospyros and buthus martensii. Tianjin, China: Tianjin University of Technology.
21
Ferreira C. F. Bernardi J. R. Silva D. C. d. Couto-Pereira N. d.S. Mota C. d.S. Krolow R. et al (2015). Mitochondrial and oxidative stress aspects in hippocampus of rats submitted to dietary n-3 polyunsaturated fatty acid deficiency after exposure to early stress. Neurochem. Res.40 (9), 1870–1881. 10.1007/s11064-015-1679-x
22
Fu Y. J. Yin L. T. Liang A. H. Zhang C. F. Wei W. Chai B. F. et al (2007). Therapeutic potential of chlorotoxin-like neurotoxin from the Chinese scorpion for human gliomas. Neurosci. Lett.412 (1), 62–67. 10.1016/j.neulet.2006.10.056
23
Fu Y. J. An N. Chan K. G. Wu Y. B. Zheng S. H. Liang A. H. (2011). A model of BmK CT in inhibiting glioma cell migration via matrix metalloproteinase-2 from experimental and molecular dynamics simulation study. Biotechnol. Lett.33 (7), 1309–1317. 10.1007/s10529-011-0587-7
24
Gao T. (2012). Based on the class Arachnida arthropod animal medicinal value and antibacterial activity investigation. Luoyang, China: Henan University of Science and Technology.
25
Gao J. Yin W. Gao T. Deng R. Li X. (2014). Two bioactive compounds from the Chinese scorpion Buthus martensii Karsch. Nat. Prod. Res.28 (10), 698–703. 10.1080/14786419.2013.873433
26
Gao S. Liang H. Shou Z. Yao Y. Lv Y. Shang J. et al (2021). De novo transcriptomic and proteomic analysis and potential toxin screening of Mesobuthus martensii samples from four different provinces. J. Ethnopharmacol.265, 113268. 10.1016/j.jep.2020.113268
27
Guan R. Wang C. G. Wang M. Wang D. C. (2001a). A depressant insect toxin with a novel analgesic effect from scorpion Buthus martensii Karsch. Biochimica biophysica acta1549 (1), 9–18. 10.1016/s0167-4838(01)00241-2
28
Guan R. J. Wang M. Wang D. Wang D. C. (2001b). A new insect neurotoxin AngP1 with analgesic effect from the scorpion Buthus martensii Karsch: purification and characterization. J. Peptide Res.58 (1), 27–35. 10.1034/j.1399-3011.2001.00869.x
29
Guan R. J. He X. L. Miao W. Ye X. Li G. P. Wang D. C. (2002). Purification, crystallization and initial structural solution of a new alpha-like toxin with cardiac toxicity from scorpion Buthus martensii Karsch. Protein Peptide Lett.9 (5), 441–449. 10.2174/0929866023408562
30
Guo Y. Zhu W. Yuan P. Huang X. Lu S. Cao Z. et al (2024). Similar neurotoxin expression profiles of traditional Chinese scorpion medicine material between juvenile and adult Mesobuthus martensii scorpions revealed by multiple strategic proteomics. J. Ethnopharmacol.332, 118338. 10.1016/j.jep.2024.118338
31
Han C. Wang Z. X. Jia Q. Wang Z. P. Zhang Y. Y. Zhang Y. et al (2016). Regulatory mechanism of PESV on tumor-infiltrating natural killer cells in liver orthotopic transplantation tumor. Chin. J. Immunol.32 (03), 390–395+400.
32
He X. L. Deng J. P. Wang M. Zhang Y. Wang D. C. (2000). Structure of a new neurotoxin from the scorpion Buthus martensii Karsch at 1.76 A. Acta Crystallogr. Sect. D. Biol. Crystallogr.56 (Pt 1), 25–33. 10.1107/s0907444999014614
33
He Y. W. Zou X. H. Li X. C. Chen J. Jin L. Zhang F. et al (2017). Activation of sodium channels by α-scorpion toxin, BmK NT1, produced neurotoxicity in cerebellar granule cells: an association with intracellular Ca2+ overloading. Archives Toxicol.91 (2), 935–948. 10.1007/s00204-016-1755-2
34
Hou Y. Long J. R. Zhang P. Long B. Chen X. B. Dong Z. Q. (2013). PESV inhibited proliferation of human bladder carcinoma T24 cells. Tianjin Med. J.41 (03), 204–207.
35
Ji Y. H. Li Y. J. Zhang J. W. Song B. L. Yamaki T. Mochizuki T. et al (1999). Covalent structures of BmK AS and BmK AS-1, two novel bioactive polypeptides purified from Chinese scorpion Buthus martensi Karsch. Toxicon Official J. Int. Soc. Toxinology37 (3), 519–536. 10.1016/s0041-0101(98)00190-1
36
Jian M. Youtian H. Mingxiong G. Zan H. Wenxin L. Yingliang W. et al (2013). hERG potassium channel blockage by scorpion toxin BmKKx2 enhances erythroid differentiation of human leukemia cells K562. Plos One8 (12), e84903. 10.1371/journal.pone.0084903
37
Kamble H. V. Bodhankar S. L. (2013). Antihyperglycemic activity of trigonelline and sitagliptin in nicotinamide-streptozotocin induced diabetes in wistar rats. Biomed. Aging Pathology3 (3), 125–130. 10.1016/j.biomag.2013.05.006
38
Kampo S. (2019). The effects of scorpion analgesic peptide, BmK-AGAPadded to lidocaine on nerve blocks and the antitumoreffect ofBmK AGAP on breast cancer in vitro and in vivo. Dalian, China: Dalian Medical University.
39
Kampo S. Ahmmed B. Zhou T. Owusu L. Anabah T. W. Doudou N. R. et al (2019). Scorpion venom analgesic peptide, BmK AGAP inhibits stemness, and epithelial-mesenchymal transition by down-regulating PTX3 in breast cancer. Front. Oncol.9, 21. 10.3389/fonc.2019.00021
40
Kim S. D. (2013). α-Glucosidase inhibitor from Buthus martensi Karsch. Food Chem.136 (2), 297–300. 10.1016/j.foodchem.2012.08.063
41
Kou Z. S. (1990). Bencao yanyi. Beijing, ChinaPeople's Medical Publishing House.
42
Li S. Z. (1998). Compendium of materia medica. Beijing, China: China Press of Chinese Medicine.
43
Li Z. H. Wu L. M. Yao Y. X. Wang Q. K. (2007). Comparison of amino acid contents in different products of Cervus nippon temminck velvet antler. Amino Acids Biotic Resour.29 (3), 16–18.
44
Li C. L. Liu X. F. Li G. X. Ban M. Q. Chen J. Z. Cui Y. et al (2016). Antinociceptive effects of AGAP, a recombinant neurotoxic polypeptide: possible involvement of the tetrodotoxin-resistant sodium channels in small dorsal root ganglia neurons. Front. Pharmacol.7, 496. 10.3389/fphar.2016.00496
45
Li Z. J. Hu P. Wu W. L. Wang Y. (2019). Peptides with therapeutic potential in the venom of the scorpion Buthus martensii Karsch. Peptides115, 43–50. 10.1016/j.peptides.2019.02.009
46
Li P. Zhang H. R. Zhang Y. Y. Zhan L. M. Chen J. X. (2023). New model and application of traditional Chinese medicine target research based on global chemical and biological profile inference. Chin. Traditional Herb. Drugs54 (06), 1986–1997.
47
Liang K. An R. You L. S. Wang X. H. Wang Z. T. (2014). Determination of amino acids in scorpio by HPLC with pre-column derivatization. Chin. J. New Drugs23 (06), 716–720.
48
Liu H. Q. (2018). Research on the antibacterial chemical composition from pheretima asperfillm E.Perrier and Buthus martensi kirsch. Luoyang, China: Henan University of Science and Technology.
49
Liu C. M. Ma S. H. (1993). Study on the analgesic effect of whole scorpion. J. Shenyang Pharm. Univ.137.
50
Liu Y. F. Hu J. Zhang J. H. Wang S. L. Wu C. F. (2002). Isolation, purification, and n-terminal partial sequence of an antitumor peptide from the venom of the Chinese scorpionbuthus martensiikarsch. Prep. Biochem. Biotechnol.32 (4), 317–327. 10.1081/PB-120015456
51
Liu Y.-F. Ma R.-L. Wang S.-L. Duan Z.-Y. Zhang J.-H. Wu L.-J. et al (2003). Expression of an antitumor–analgesic peptide from the venom of Chinese scorpion Buthus martensii Karsch in Escherichia coli. Protein Expr. Purif.27 (2), 253–258. 10.1016/s1046-5928(02)00609-5
52
Liu T. Pang X. Y. Jiang F. Bai Z. T. Ji Y. H. (2008). Anti-nociceptive effects induced by intrathecal injection of BmK AS, a polypeptide from the venom of chinese-scorpion Buthus martensi Karsch, in rat formalin test. J. Ethnopharmacol.117 (2), 332–338. 10.1016/j.jep.2008.02.003
53
Liu Z. R. Tao J. Dong B. Q. Ding G. Cheng Z. J. He H. Q. et al (2012). Pharmacological kinetics of BmK AS, a sodium channel site 4-specific modulator on Nav1.3. Neurosci. Bull.28 (3), 209–221. 10.1007/s12264-012-1234-6
54
Liu S. L. Liu S. L. Ju W. Z. Li C. Y. Cao P. (2013). Analgesic-antitumor peptide induces apoptosis and inhibits the proliferation of SW480 human colon cancer cells. Oncol. Lett.5 (2), 483–488. 10.3892/ol.2012.1049
55
Liu X. Yuan C. H. Yang Q. H. Bai Z. T. (2016). Pain induction and analgesia mechanisms of scorpion toxins targeting to ion channels. Chin. Bull. Life Sci.28 (01), 64–69. 10.13376/j.cbls/2016008
56
Liu Y. Li Y. Zhu Y. Zhang L. Ji J. Gui M. et al (2021a). Study of anti-inflammatory and analgesic activity of scorpion toxins DKK-SP1/2 from scorpion Buthus martensii Karsch (BmK). Toxins13 (7), 498. 10.3390/toxins13070498
57
Liu Y. M. Fan J. J. Wang L. N. (2021b). Discovery of guanidine derivatives from Buthus martensii Karsch with metal-binding and cholinesterase inhibition properties. Molecules26 (21), 6737. 10.3390/molecules26216737
58
Lu D. X. (1995). Kaibao bencao. Hefei, China: Anhui Science Technology Publishing House.
59
Lu W. Cheng X. Chen J. Chen Y. Cao P. Liu J. et al (2022). A Buthus martensii Karsch scorpion sting targets Nav1.7 in mice and mimics a phenotype of human chronic pain. Pain163 (2), e202–e214. 10.1097/j.pain.0000000000002397
60
Lv X. R. Jiang B. Gao B. B. Bai L. (1995). Inhibitory effect of scorpion venom peptide on platelet aggregation in rabbits. J. Shandong Second Med. Univ. (03), 175–176+218.
61
Ma X. M. (2019). Research of the using rules of scorpio based on toxicity-efficacy-syndrome relevance. Ji'nan, China: Shandong University of Traditional Chinese Medicine.
62
Ma X. W. Lin J. M. (2000). Comparative analysis of the content of macro - and micro-elements in Male and female scorpion. Guangdong Trace Elem. Sci. (07), 54–58. 10.16755/j.cnki.issn.1006-446x.2000.07.016
63
Meng X. Xu Y. Zhao M. Wang F. Ma Y. Jin Y. et al (2015). The functional property changes of muscular Na(v)1.4 and cardiac Na(v)1.5 induced by scorpion toxin BmK AGP-SYPU1 mutants Y42F and Y5F. Biochemistry54 (19), 2988–2996. 10.1021/acs.biochem.5b00067
64
Mishal R. Tahir H. M. Zafar K. Arshad M. (2013). Anti-cancerous applications of scorpion venom. Asian J. Pharm. Biol. Res.4 (5), 356–360.
65
Patrick Henriet A. Hervé Emonard B. (2019). Matrix metalloproteinase-2: not (just) a “hero” of the past. Biochimie166, 223–232. 10.1016/j.biochi.2019.07.019
66
Possani L. D. Becerril B. Delepierre M. Tytgat J. (2010). Scorpion toxins specific for Na+-channels. Febs J.264 (2), 287–300. 10.1046/j.1432-1327.1999.00625.x
67
Pravalika K. Sarmah D. Kaur H. Vats K. Saraf J. Wanve M. et al (2018). Trigonelline therapy confers neuroprotection by reduced glutathione mediated myeloperoxidase expression in animal model of ischemic stroke. Life Sci.216 (2019), 49–58. 10.1016/j.lfs.2018.11.014
68
Rincón-Cortés C. A. Olamendi-Portugal T. Carcamo-Noriega E. N. Santillán E. G. Possani L. D. Reyes-Montaño E. A. et al (2019). Structural and functional characterization of toxic peptides purified from the venom of the Colombian scorpion tityus macrochirus. Toxicon169, 5–11. 10.1016/j.toxicon.2019.07.013
69
Satitmanwiwat S. Changsangfa C. Khanuengthong A. Promthep K. Roytrakul S. Arpornsuwan T. et al (2016). The scorpion venom peptide BmKn2 induces apoptosis in cancerous but not in normal human oral cells. Biomed. Pharmacother.84, 1042–1050. 10.1016/j.biopha.2016.10.041
70
Shao J. Zhang R. Ge X. Yang B. Zhang J. (2007a). Analgesic peptides in Buthus martensii Karsch: a traditional Chinese animal medicine the analgesic peptides in scorpion venoms. Asian J. Traditional Med.2 (2), 45–50.
71
Shao J. H. Kang N. Liu Y. F. Song S. Wu C. F. Zhang J. H. (2007b). Purification and characterization of an analgesic peptide from Buthus martensii Karsch. Biomed. Chromatogr. BMC21 (12), 1266–1271. 10.1002/bmc.882
72
Shao J. H. Wang Y. Q. Wu X. Y. Jiang R. Zhang R. Wu C. F. et al (2008). Cloning, expression, and pharmacological activity of BmK AS, an active peptide from scorpion Buthus martensii Karsch. Biotechnol. Lett.30 (1), 23–29. 10.1007/s10529-007-9499-y
73
Shao J.-H. Cui Y. Zhao M.-Y. Wu C.-F. Liu Y.-F. Zhang J.-H. (2014). Purification, characterization, and bioactivity of a new analgesic-antitumor peptide from Chinese scorpion Buthus martensii Karsch. Peptides53, 89–96. 10.1016/j.peptides.2013.10.023
74
Shi X. H. Guo H. W. (2023). Explore the usage and dosage of scorpion based on literature. J. Zhejiang Chin. Med. Univ.47 (05), 563–568. 10.16466/j.issn1005-5509.2023.05.020
75
Shi J. He H. Q. Zhao R. Duan Y. H. Chen J. Chen Y. et al (2008). Inhibition of martentoxin on neuronal BK channel subtype (alpha+beta4): implications for a novel interaction model. Biophysical J.94 (9), 3706–3713. 10.1529/biophysj.107.122150
76
Silvia Llorens E. N. Nava E. (2003). Cardiovascular diseases and the nitric oxide pathway. Curr. Vasc. Pharmacol.1 (3), 335–346. 10.2174/1570161033476637
77
Song Y. M. Li X. K. Wang X. L. Li N. (2002). Effects of scorpion venom active peptides on blood rheology in rabbits. Chin. J. Hemorheol. (03), 185–187.
78
Song Y. B. Huang T. T. Lai L. L. Zhou J. Zhang J. H. (2011). Expression of anti-neuroexcitation peptide III of scorpion Buthus martensii Karsch BmK ANEP III in plants. Mol. Biol.45 (6), 872–878. 10.1134/s0026893311060124
79
Song X. F. Zhang G. J. Sun A. P. Guo J. Q. Tian Z. W. Wang H. et al (2012a). Scorpion venom component III inhibits cell proliferation by modulating NF-κB activation in human leukemia cells. Exp. Ther. Med.4 (1), 146–150. 10.3892/etm.2012.548
80
Song X. Y. Wang Z. P. Jia Q. Wang Z. X. Ning Y. N. Sun X. J. et al (2012b). Inhibitory effect of polypeptide extracts from scorpion venom on proliferation of ovarian cancer SKOV3 cells. Chin. J. New Drugs21 (03), 257–261.
81
Song Y. J. Zou H. C. Weng X. Ju J. H. (2024). Research progress on chemical constituents and pharmacological activities of small molecule compounds in scorpions. China J. Chin. Materia Medica49 (03), 661–670. 10.19540/j.cnki.cjcmm.20231212.201
82
Sui W. W. Zhang W. D. Wu L. C. Wang Z. P. Zhang Y. Y. Wang Z. X. et al (2014). Inhibitory mechanism of polypeptide from scorpion venom combined with 5-fluorouacil on angiogenesis of H22 hepatoma. Chin. Traditional Herb. Drugs45 (03), 392–397.
83
Sun X. J. Zhang Y. Y. Jia Q. Wang Z. P. Wang Z. X. Zhang W. D. (2011). Effect of polypeptide extract from scorpion venom(PESV) with chemotherapy inhibited angiogenesis of lewis lung carcinomas. China J. Chin. Materia Medica36 (12), 1644–1649.
84
Sun S. D. Wang Q. Zhang D. W. Zhang W. T. Fu H. Z. Yuan D. et al (2021). Purification and antithrombotic activity of two scorpion venom peptides. J. Shenyang Pharm. Univ.38 (01), 45–53. 10.14066/j.cnki.cn21-1349/r.2019.0489
85
Tang S. W. (2011). Zhenglei bencao. Beijing: China Medical Science and Technology Press.
86
Tian Z. G. Zheng Y. (2004). Effects of scorpion toxins on the cardiovascular system. China J. Chin. Materia Medica (07), 25–27.
87
Tian X. R. Fu T. M. Guo L. W. (2013). HPLC simultaneous determination of five nucleoside compounds in extracts of Buthus martensii by different extraction technologies. Chin. J. Exp. Traditional Med. Formulae19 (08), 13–16.
88
Uchiyama K. Nakamura M. Odahara S. Koido S. Tajiri H. Shiraishi H. et al (2010). N-3 polyunsaturated fatty acid diet therapy for patients with inflammatory bowel disease. Inflamm. Bowel Dis.16 (10), 1696–1707. 10.1002/ibd.21251
89
Uzhachenko R. V. Ochoa S. G. Kanagasabai T. Rajakaruna H. Thounaojam M. C. Aquino M. T. P. d. et al (2025). CD8+ T–NK cell crosstalk establishes preemptive immunosurveillance to eliminate antigen–escape tumors. Front. Immunol.16, 1593913. 10.3389/fimmu.2025.1593913
90
Vega R. C. R. d.l. Schwartz E. F. Possani L. D. (2010). Mining on scorpion venom biodiversity. Toxicon56 (7), 1155–1161. 10.1016/j.toxicon.2009.11.010
91
Wali A. Wubulikasimu A. Mirzaakhmedov S. Gao Y. Omar A. Arken A. et al (2019). Optimization of scorpion protein extraction and characterization of the proteins' functional properties. Molecules24 (22), 4103. 10.3390/molecules24224103
92
Wang W. X. Ji Y. H. (2005). Scorpion venom induces glioma cell apoptosis in vivo and inhibits glioma tumor growth in vitro. J. Neuro-Oncology73 (1), 1–7. 10.1007/s11060-004-4205-6
93
Wang Q. Y. Wu F. J. (2010). Effect of pretreatment with scorpion venom fibrinolytic active peptide on anticoagulant and fibrinolytic function of hypoxic vascular endothelial cells. J. Li-shizhen Traditional Chin. Med.21 (08), 2081–2082.
94
Wang C. G. He X. L. Shao F. Liu W. Ling M. H. Wang D. C. et al (2010a). Molecular characterization of an anti-epilepsy peptide from the scorpion Buthus martensi Karsch. Febs J.268 (8), 2480–2485. 10.1046/j.1432-1327.2001.02132.x
95
Wang Z. P. Zhang W. D. Wu L. C. Jia Q. Wang Z. X. Zhang Y. Y. et al (2010b). Inhibitive effect of polypeptide extract from scorpion venom on repopulation in H22 tumor cell during chemotherapy. China J. Chin. Materia Medica35 (01), 108–113. 10.4268/cjcmm20100123
96
Wang X. H. Wang Z. P. Zhang Y. Y. Jia Q. Wang Z. X. Zhang J. et al (2012). Mechanisms for inhibition effects of polypeptide extract from scorpion venom(PESV) on proliferation of A549 cell lines in vitro. China J. Chin. Materia Medica37 (11), 1620–1623.
97
Wang J. Qian W. Zhu Q. Chen J. Huan F. Gao R. et al (2013). Martentoxin, a large-conductance Ca∼(2+)-activated K∼+ channel inhibitor, attenuated TNF-α-induced nitric oxide release by human umbilical vein endothelial cells. J. Biomed. Res.000 (005), 386–393.
98
Wang X. G. Zhu D. D. Na L. Huang Y. L. Wang Y. Z. Zhang T. et al (2020). Scorpion venom heat-resistant peptide is neuroprotective against cerebral ischemia-reperfusion injury in association with the NMDA-MAPK pathway. Neurosci. Bull.36 (3), 243–253. 10.1007/s12264-019-00425-1
99
Wu J. J. Dai L. Lan Z. D. Chi C. W. (2000). The gene cloning and sequencing of Bm-12, a Chlorotoxin-like peptide from the scorpion Buthus martensi Karsch. Toxicon38 (5), 661–668. 10.1016/s0041-0101(99)00181-6
100
Xiang A. (2014). Comparative study on the quality of scorpion before and after processing. Inn. Mong. J. Traditional Chin. Med.33 (23), 66–67+51. 10.16040/j.cnki.cn15-1101.2014.23.068
101
Xiao Q. Zhang Z. P. Hou Y. B. Qu D. X. Tang L. L. Chen L. J. et al (2022). Anti-Epileptic/pro-epileptic effects of sodium channel modulators from Buthus martensii Karsch. Acta Physiol. Sin.74 (04), 621–632. 10.13294/j.aps.2022.0064
102
Xiong Y. M. Lan Z. D. Wang M. Liu B. Liu X. Q. Fei H. et al (1999). Molecular characterization of a new excitatory insect neurotoxin with an analgesic effect on mice from the scorpion Buthus martensi Karsch. Toxicon37 (8), 1165–1180. 10.1016/s0041-0101(98)00253-0
103
Xu Y. Meng X. Hou X. Sun J. Kong X. Sun Y. et al (2017). A mutant of the BmK antitumor-analgesic peptide exhibits reduced inhibition to hNav1.4 and hNav1.5 channels while retaining analgesic activity. J. Biol. Chem. (M117), 792697.
104
Xu L. S. Feng Q. L. Zhang X. P. Yao M. (2018). Experimental research on analgesic effect and side-effects of centipede-scorpion powder in mice. Chin. J. General Pract.16 (03), 346–348. 10.16766/j.cnki.issn.1674-4152.000096
105
Yan R. Zhao Z. He Y. Wu L. Cai D. Hong W. et al (2011). A new natural alpha-helical peptide from the venom of the scorpion Heterometrus petersii kills HCV. Peptides Int. J.1, 32.
106
Yan Z. Junyan X. Zhiwei W. Xiuli Z. Xinmiao L. Olivier C. et al (2012). BmK-YA, an enkephalin-like peptide in scorpion venom. PLoS ONE7 (7), e40417-. 10.1371/journal.pone.0040417
107
Yang X. D. Shi Z. X. Yao F. Yan L. X. Li D. G. Yang W. H. et al (2016). Effect of PESV on expression levels of MDR1mRNA and P-gp in leukemic stem cells. Acta Chin. Med. Pharmacol.44 (01), 36–40. 10.19664/j.cnki.1002-2392.2016.01.013
108
Yang F. Wang D. Tong Y. Qin C. Yang L. Yu F. et al (2019). Thermostable potassium channel-inhibiting neurotoxins in processed scorpion medicinal material revealed by proteomic analysis: implications of its pharmaceutical basis in traditional Chinese medicine. J. Proteomics206, 103435. 10.1016/j.jprot.2019.103435
109
Yang Z. X. Ji L. L. Liu H. Bian S. J. Xu G. J. Lv S. W. (2020). Research progress the study of chemical constituents and pharmacological effects of scorpion. Cent. South Pharm.18 (09), 1523–1529.
110
Yang S. Lan J. Li Z. Li M. Wu Y. Sun L. et al (2024). Bufonis venenum extract loaded novel cholesterol-free liposome for the treatment of hepatocellular carcinoma. Front. Pharmacol.15, 1486742. 10.3389/fphar.2024.1486742
111
Yu W. Li W. Yong C. Yong-Bo S. Yan-Feng L. Rong Z. et al (2011). Purification, characterization and functional expression of a new peptide with an analgesic effect from Chinese scorpion Buthus martensii Karsch (BmK AGP-SYPU1). Biomed. Chromatogr.25(7),801–807. 10.1002/bmc.1519
112
Yu J. C. Luo Y. Y. Jin H. D. Lv X. X. Zhou T. T. Yabasin Iddrisu B. et al (2020). Scorpion alleviates bone cancer pain through inhibition of bone destruction and glia activation. Mol. pain16, 1744806920909993. 10.1177/1744806920909993
113
Zeng X. C. Li W. X. Zhu S. Y. Peng F. Jiang D. H. Yang F. H. et al (2000). Cloning and characterization of the cDNA sequences of two venom peptides from Chinese scorpion Buthus martensii Karsch (BmK). Toxicon38 (7), 893–899. 10.1016/s0041-0101(99)00192-0
114
Zeng X. C. Wang S. Nie Y. Zhang L. Luo X. (2012). Characterization of BmKbpp, a multifunctional peptide from the Chinese scorpion Mesobuthus martensii karsch: gaining insight into a new mechanism for the functional diversification of scorpion venom peptides. Peptides33 (1), 44–51. 10.1016/j.peptides.2011.11.012
115
Zeng W. Y. Tan L. Han C. Zheng Z. Y. Wu G. S. Luo H. R. et al (2021). Trigonelline extends the lifespan of C. elegans and delays the progression of age-related diseases by activating AMPK, DAF-16, and HSF-1. Hindawi Ltd.2021 (1), 7656834. 10.1155/2021/7656834
116
Zhang H. S. Wang J. J. (2007). Research progress on pharmacological effects of scorpio in traditional Chinese medicine. J. Emerg. Traditional Chin. Med. (02), 224–226.
117
Zhang L. B. Zhang W. D. Wang Z. X. Zhang Y. Y. Wang Z. P. Jia Q. (2009). The inhibitive effect of polypeptide extract from sorpion venom (PESV) on invasion and metastasis of pancreatic cancer cells in vitro and its primary mechanism. Chin. Pharmacol. Bull.25 (06), 820–824.
118
Zhang R. Yang Z. Liu Y. F. Cui Y. Zhang J. H. (2011). Purification, characterization and cDNA cloning of an analgesic peptide from the Chinese scorpionButhus martensiikarsch (BmK AGP-SYPU2). Mol. Biol.45 (6), 879–885. 10.1134/s0026893311060203
119
Zhang R. Mao H. X. Sang X. Liu J. H. Zhang X. X. Wang J. H. et al (2019). Comparative study on chemical composition and antiviral effect of water extracts of wild scorpion and cultured scorpion. Contemporary chemical industry. Contemp. Chem. Ind.48 (06), 1158–1161. 10.13840/j.cnki.cn21-1457/tq.2019.06.012
120
Zhang H. H. Wang Y. Y. Wang Y. Q. Li X. L. Wang S. Z. Wang Z. (2022a). Recent advance on carbamate-based cholinesterase inhibitors as potential multifunctional agents against alzheimer's disease. Eur. J. Med. Chem.240, 114606. 10.1016/j.ejmech.2022.114606
121
Zhang X. Zhao Q. Yang F. Lan Z. Li Y. Xiao M. et al (2022b). Mechanisms underlying the inhibition of KV1.3 channel by scorpion toxin ImKTX58. Mol. Pharmacol.102 (3), 150–160. 10.1124/molpharm.121.000480
122
Zhang K. Y. Zhang Y. Q. Yang C. M. Fu J. Li Y. Jiang Q. et al (2024). Research progress on processing history evolution, chemical constituents, and pharmacological action of scorpio. China J. Chin. Materia Medica49 (04), 868–883. 10.19540/j.cnki.cjcmm.20230814.202
123
Zhao R. Zhang X. Y. Yang J. Weng C. C. Jiang L. L. Zhang J. W. et al (2008). Anticonvulsant effect of BmK IT2, a sodium channel-specific neurotoxin, in rat models of epilepsy. Br. J. Pharmacol. Chemother.154 (5), 1116–1124. 10.1038/bjp.2008.156
124
Zhao R. Weng C. C. Feng Q. Chen L. Zhang X. Y. Zhu H. Y. et al (2011a). Anticonvulsant activity of BmK AS, a sodium channel site 4-specific modulator. Epilepsy Behav.20 (2), 267–276. 10.1016/j.yebeh.2010.12.006
125
Zhao Y. Cai X. Ye T. Huo J. Liu C. Zhang S. et al (2011b). Analgesic-antitumor peptide inhibits proliferation and migration of SHG-44 human malignant glioma cells. J. Cell. Biochem.112 (9), 2424–2434. 10.1002/jcb.23166
126
Zheng Y. Wen Q. Huang Y. Guo D. (2024). The significant therapeutic effects of Chinese scorpion: modern scientific exploration of ion channels. Pharmaceuticals17 (12), 1735. 10.3390/ph17121735
127
Zhou X. H. Yang D. Zhang J. H. Liu C. M. Lei K. J. (1989). Purification and N-terminal partial sequence of anti-epilepsy peptide from venom of the scorpion Buthus martensii karsch. Biochem. J.257 (2), 509–517. 10.1042/bj2570509
128
Zhou B. S. Yang N. Li Y. M. Wang Y. R. Liu J. P. Li P. Y. (2018). Detection of chemical composition of volatile components of scorpio by HS-SPME-GC-MS. Special Wild Econ. Animal Plant40 (02), 52–55. 10.16720/j.cnki.tcyj.2018.02.011
Summary
Keywords
Buthus martensii Karsch, traditional uses, chemical constituents, pharmacological effects, toxicity
Citation
Liu M-H, Zhu J-J, Lu Q, Qu Z-N, Zhou Q, Zhang L, Dai Y-P and Shi D-H (2025) Scorpion (Buthus martensii Karsch) in Chinese medicine: a review of traditional uses, chemical constituents, pharmacology, and toxicology. Front. Pharmacol. 16:1702650. doi: 10.3389/fphar.2025.1702650
Received
10 September 2025
Revised
12 October 2025
Accepted
28 October 2025
Published
21 November 2025
Volume
16 - 2025
Edited by
Ahmed Abdeen, Benha University, Egypt
Reviewed by
Filipa Pinto-Ribeiro, University of Minho, Portugal
Ali Hafez El-Far, Damanhour University, Egypt
Mohamed El-Sherbiny, Almaarefa University, Saudi Arabia
Samah Ibrahim, Cairo University, Egypt
Afaf Abdelkader, Benha University, Egypt
Updates
Copyright
© 2025 Liu, Zhu, Lu, Qu, Zhou, Zhang, Dai and Shi.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan-Peng Dai, daiyanpeng1027@163.com; Dian-Hua Shi, shidianhua81@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.