- 1West China School of Pharmacy, Sichuan University, Chengdu, China
- 2State Key Laboratory of Southwestern Chinese Medicine Resources, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 3Xi’an DeNovo Hith Medical Technology Co., Ltd., Xi’an, China
- 4Department of Radiation Oncology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 5Key Laboratory of Drug-Targeting and Drug Delivery System of the Education Ministry and Sichuan Province, Sichuan Engineering Laboratory for Plant-Sourced Drug and Sichuan Research Center for Drug Precision Industrial Technology, Sichuan University, Chengdu, China
Mussel adhesive proteins (MAPs) are natural proteins derived from Mytilus edulis, renowned for their exceptional adhesive properties. These proteins, rich in 3,4-dihydroxyphenylalanine (DOPA) residues, enable mussels to adhere firmly to surfaces in challenging environments. Due to these unique biochemical and mechanical characteristics, MAPs have attracted significant attention in the biomedical field, offering promising applications in wound healing, drug delivery, tissue engineering, and cosmetics. Recombinant MAPs (rMAPs), in particular, hold great potential due to their enhanced properties, including antioxidant, anti-inflammatory, antibacterial, and cell-protective effects. They are increasingly being explored for their role in tissue repair, skin regeneration, and targeted drug delivery systems. Despite challenges in recombinant production, toxicity control, and underwater adhesion efficiency, ongoing advancements in genetic engineering and protein design are expanding the application prospects of rMAPs. This review explores the structure, pharmacological effects, and biomedical applications of MAPs, with a focus on the potential of rMAPs in precision medicine, drug delivery, and tissue regeneration, while highlighting the challenges and future directions for their development.
1 Introduction
Mussel adhesive proteins (MAPs) are a class of natural proteins with unique adhesive properties, widely found in Mytilus edulis. Their most prominent feature is the ability to firmly adhere to various surfaces in moist, salt-rich environments. This ability arises from the presence of 3,4-dihydroxyphenylalanine (DOPA) residues in their molecular structure, allowing MAPs to generate strong adhesive forces through multiple intermolecular interactions, such as oxidation crosslinking, charge interactions, metal-phenolic coordination, hydrogen bonding, and hydrophobic interactions (Waite, 2017; Liang et al., 2018). During mussels’ physiological processes, the adhesive proteins form gel-like aggregates in their foot via a special secretion mechanism, ensuring the protein’s adsorption and extension on surfaces under extreme conditions (such as low pH, low ionic strength, and high reducing environments), eventually solidifying into a robust adhesive layer (Yang B. et al., 2018). This characteristic is crucial not only for mussels’ ecological adaptation but also provides significant potential for biomedical applications.
It is interesting to notice that emerging natrual products exert various pharmacological effects, including anti-inflammation, antioxidation, cardioprotection, neuroprotection and anticancer effect (Shi et al., 2020; Li et al., 2021; Zou et al., 2021; Liu and Liu, 2018; Wang Y. et al., 2024). In recent decades, the unique biochemical and mechanical properties of MAPs have garnered extensive attention prospects in the biomedical field. Their ability to promote wound healing and tissue regeneration, as well as their potential use in drug delivery systems, tissue engineering, and immunotherapy, has gained increasing recognition (Guo et al., 2020; Quan et al., 2019). The bioactive properties of MAPs extend beyond their adhesive capabilities. Studies have shown that MAPs possess antioxidant, anti-inflammatory, antibacterial, and cell-protective properties, making them promising candidate materials for biomedical applications (Zhao et al., 2023). Furthermore, the biocompatibility and biodegradability of MAPs enhance their appeal as a safe and sustainable medical material (Zhang et al., 2020; Madhurakkat Perikamana et al., 2015).
In contrast to previous reviews that mainly focused on the structural chemistry and adhesion mechanisms of MAPs, this review aims to explore the basic structure and pharmacological characteristics of MAPs, with a focus on their current research applications in wound healing, drug delivery, tissue engineering, and cosmetics and skincare products. Furthermore, we systematically compare recombinant MAPs (rMAPs) with other natural biomaterials and catechol-based synthetic systems, highlighting their distinctive advantages and limitations in translational applications, providing theoretical support for future application studies.
2 Fundamental structure and modifications of MAPs
The unique molecular structure and amino acid sequence features of MAPs allow them to adhere to various surfaces underwater, adapting to tidal changes and the impact of waves. MAPs have a complex structure with high functionality, especially certain structural domains that are critical to their adhesive capabilities.
2.1 Molecular architecture of MAPs
The basic structural unit of MAPs consists mainly of multiple amino acid sequences with various post-translational modifications. Regions rich in tyrosine and glycine are considered to be the key functional areas of MAPs. Glycine, the smallest amino acid, often forms flexible connecting regions in the sequence of MAPs, which helps the protein adapt to changes in complex environments (Ou et al., 2020). Tyrosine, on the other hand, undergoes oxidation to form DOPA, playing a core role in the adhesion process. The high content of DOPA is a distinctive feature that sets MAPs apart from other adhesive proteins (Deepankumar et al., 2022).
In the structure of MAPs, several key structural domains play crucial roles. Particularly, the linker domain and the adhesive domain are responsible for the stability of the protein and its adhesion to the surface (Zeng et al., 2018). The linker domain is usually composed of sequences rich in glycine and proline, which can form helical or folded structural units. These amino acids improve the spatial stability of the protein and enhance its adhesive function by interacting with other proteins or surfaces, making the adhesion more durable. The adhesive domain, especially regions rich in tyrosine and DOPA, directly participate in the underwater adhesion process (Yoon et al., 2025; Shin et al., 2022). DOPA contains a phenolic hydroxyl group, a structure that allows it to form stable complexes with metal ions (such as iron and copper) and other molecules. It can also interact with surrounding molecules or surfaces through hydrogen bonding, thus enhancing adhesion (Kim et al., 2018).
In summary, the structural characteristics of MAPs, such as the regions rich in tyrosine, glycine, and DOPA, provide them with powerful adhesive properties in underwater environments (Figure 1). These structural units and functional domains work together through multiple mechanisms, including chemical bonds, metal ion coordination, hydrogen bonding, and hydrophobic interactions, ensuring that mussels can firmly attach to various surfaces. Through in-depth research on these adhesive properties and structural domains, we can not only better understand the biological adhesion mechanisms in nature but also provide theoretical and technical support for developing new types of biological adhesives and other high-performance materials.
2.2 Impact of structural modifications on adhesive properties
Traditional adhesives often lose their adhesive power due to the influence of water, whereas MAPs can maintain stable adhesion in water. Research has shown that the thin film formed by Mefp-1 (a type of MAPs) on a platinum surface exhibits good adhesive properties, and the formation and compactness of this film can be controlled under different potentials. Mefp-1 adsorbs to surfaces through both electrostatic and non-electrostatic interactions, effectively blocking electroactive sites, which allows it to retain strong adhesive strength in underwater environments (Zhang et al., 2017).
The structural modification of MAPs mainly involves improving their adhesion properties in aquatic environments by mimicking the adhesion mechanism of mussels. For example, CMC-DA hydrogels, which are carboxymethyl cellulose (CMC) molecules conjugated with dopamine (DA), have been developed. These hydrogels undergo enzyme cross-linking under the catalysis of horseradish peroxidase (HRP) and hydrogen peroxide (H2O2), significantly enhancing their adhesion strength (Zhong et al., 2019).
Moreover, the co-precipitation effect of MAPs provides them with broader adaptability. Studies have altered the ionic properties and pH conditions of polymers, creating non-charged, biodegradable polyester materials that can quickly form co-precipitates in water, exhibiting excellent underwater adhesive properties across a wide range of pH and ionic strengths (Narayanan et al., 2020). This design not only overcomes the sensitivity of traditional co-precipitation adhesives to changes in pH and salinity but also enhances their potential for application in complex environments.
MAPs are inherently insoluble in water. Through recombinant DNA technology, Pilakka Veedu A et al. successfully developed a mussel adhesive protein fusion protein that maintains high solubility and stability in aqueous solutions (Pilakka et al., 2023). For example, Foot Protein 1 (Fp1), a type of MAPs, when fused with the highly water-soluble ice-nucleation protein K (InaKC), results in a fusion protein that exhibits lower adhesive strength but better water solubility and stability. Once separated by protease cleavage sites, the fusion protein can regain its adhesive properties. This demonstrates that by altering the structure and fusing other proteins, the water solubility and adhesive properties of MAPs can be flexibly controlled to meet different application needs.
MAPs can also promote cell adhesion through interactions with cell surface receptors, a characteristic crucial for the design of tissue engineering and biomedical materials. By fusing MAPs with functional peptides derived from laminin, fusion proteins have been designed that significantly improve adhesion to A549 cells, further proving the potential of MAPs in cellular biomaterials (Wang et al., 2017).
Additionally, studies show that the secretion of MAPs is time-regulated, allowing their adhesive abilities to adjust according to environmental conditions at different time points. This regulatory mechanism optimizes the surface interactions of MAPs and facilitates the assembly of foot proteins (Petrone et al., 2015).
In conclusion, MAPs exhibit excellent performance in underwater adhesion and cell adhesion, among other fields. Their unique physicochemical properties, such as time-regulated secretion mechanisms and adjustable water solubility and adhesiveness, enable them to function effectively in various environments. These characteristics offer vast prospects for the application of MAPs, especially in replacing traditional adhesives and developing new biomaterials, making them of significant importance.
2.3 Comparative composition of MAPs across species and secretion conditions
MAPs constitute a heterogeneous family rather than a single molecule. Their amino-acid composition, sequence motifs, and post-translational modifications (PTMs) differ markedly among species and are further modulated by secretion and curing conditions (DeMartini et al., 2017). Comparative analyses across Mytilus, Perna, and Bathymodiolus species reveal systematic variations reflecting habitat and functional specialization (Rees et al., 2019). Intertidal Mytilus species exhibit high tyrosine and lysine contents, with efficient enzymatic conversion of tyrosine to DOPA, producing strong wet adhesion through multivalent catechol–metal coordination. In contrast, tropical or deep-sea mussels synthesize proteins richer in glycine, proline, and cysteine, imparting chain flexibility, coacervate formation, and redox buffering capacity. Consequently, DOPA/Lys-rich proteins enhance tissue binding and drug retention, low-complexity Gly/Pro-rich domains promote slow release, and Cys-rich variants improve oxidative stability and compatibility with sensitive biomolecules (Li and Zeng, 2016). MAPs also differ in motif architecture: DOPA-rich decapeptide repeats and Lys–Tyr–Gly (KYG) clusters concentrate catechol groups for adhesion; His-rich segments enable pH- or metal-responsive binding; and Cys motifs form disulfide networks for structural stabilization. These sequence features are fine-tuned by PTMs such as DOPA hydroxylation, phosphorylation, glycosylation, and controlled oxidation, which together regulate charge, hydrophilicity, and redox balance.
The secretion of MAPs during byssus formation is dynamically regulated by environmental cues (Priemel et al., 2020). Secretion begins in an acidic, reducing milieu that preserves DOPA in its catechol form, favoring surface adsorption, followed by exposure to oxygen and Fe3+ that drives oxidative curing and covalent cross-linking. This sequential release of adhesive, cohesive, and protective proteins produces a gradient from compliant interface to rigid plaque core (Valois et al., 2020). Variations in pH, redox potential, or metal ion concentration between species or experimental conditions thus alter the molecular mixture and ultimately affect mechanical and biological performance. The same protein framework can therefore yield distinct materials depending on environmental modulation, emphasizing the adaptive nature of MAP chemistry.
These compositional and environmental factors collectively shape pharmacological behavior. High DOPA/Lys content confers prolonged adhesion and residence at target tissues, low-complexity sequences support coacervate-based diffusion control, and Cys- or redox-active domains mitigate oxidative stress and inflammation. MAP systems enriched in catechol–metal motifs can further act as antimicrobial or stimuli-responsive coatings (Santonocito et al., 2019). Pharmacological efficacy thus arises not from a single residue but from the combinatorial interplay of motifs and PTMs regulated by secretion context. Recognizing this interspecies and condition-dependent diversity provides a mechanistic foundation for designing MAP-inspired biomaterials with tunable adhesion, controlled release, and improved biocompatibility.
2.4 Expansion of the MAP family: newly identified components and structural diversity
Recent transcriptomic and proteomic investigations have substantially broadened the MAP family beyond the classical Mfp-1–Mfp-6 set.
In Mytilus californianus foot glands, approximately fifteen previously unrecognized candidates (provisionally named Mcfp-7p to Mcfp-19p) were identified. These proteins share hallmark MAP features—including signal peptides, basic isoelectric points, and Lys/Gly/Tyr-rich compositions. Among them, Mcfp-7p and Mcfp-8p are short KYG (Lys–Tyr–Gly) repeat peptides, while Mcfp-10p–Mcfp-12p are longer sequences enriched in His/Gly or Cys residues. Their strong expression in both the phenol and accessory glands suggests roles in the early stages of plaque formation (DeMartini et al., 2017). In addition, preCOL-P, preCOL-D, and preCOL-NG are the main components of the inner core fibrils of the mussel foot filaments, and they are modified with glycine. Furthermore, there are the foot-specific filamentous matrix proteins PTMP (proximal filamentous matrix protein) and DTMP (distal filamentous matrix protein) (Xu S. et al., 2024).
The expanded catalog of MAPs fundamentally reshapes our understanding of how molecular diversity governs pharmacological performance and materials design. Each subclass provides distinct chemical functionalities that map to specific therapeutic outcomes: DOPA and Lys-rich proteins enhance surface adhesion and local residence; cysteine- and redox-regulating proteins protect biomolecules from oxidative stress. Together, these discoveries reveal that pharmacological efficacy arises from the synergistic interplay of multiple motifs rather than a single adhesive residue.
3 Pharmacological effects of MAPs
In recent years, MAPs have received widespread attention for their pharmacological effects in areas such as anti-inflammatory, antibacterial, wound healing, and tumor immunotherapy. Researchers have found that MAPs can improve the process of skin tissue repair, reduce inflammation, and promote wound healing, offering new perspectives for clinical treatment.
3.1 Anti-inflammatory effects and prevention of hyperpigmentation
MAPs have shown anti-inflammatory effects in the application of skin inflammation and wound healing. Studies have shown that rMAPs r-fp-151-V can effectively inhibit the expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) in mouse macrophages stimulated by lipopolysaccharide (LPS), thereby reducing the production of nitric oxide (NO). Furthermore, when applied to keratinocytes after ultraviolet (UV) exposure, r-fp-151-VT significantly reduced the expression of iNOS and COX-2 and decreased the production of inflammatory cytokines such as IL-1β, IL-6, and TNF-α. This result indicates that MAPs have particularly significant anti-inflammatory effects on skin cells, especially in the treatment of UV-induced skin inflammation, where it demonstrates strong efficacy (Ahn et al., 2019).
MAPs have also shown potential in preventing and treating hyperpigmentation. Post-inflammatory hyperpigmentation (PIH) is a common complication after skin inflammation, especially more pronounced in individuals with darker skin tones. Research has found that MAPs can significantly reduce the likelihood of PIH occurring when used as a wound dressing. MAPs were found to be equally effective in reducing pigmentation compared to the potent steroid drug fluticasone propionate. Specifically, after treatment with MAPs, skin samples exhibited significantly lower melanin indices and surface differences in pigmentometer and confocal microscopy examinations, indicating its promising efficacy in inhibiting hyperpigmentation (Liu et al., 2020).
3.2 Powerful antibacterial effects
In addition to its anti-inflammatory and anti-pigmentation effects, MAPs also exhibit antibacterial activity. Research shows that MAPs, by binding with functional peptides (MAP-FPs), not only inhibit the growth of Gram-negative bacteria but also rapidly kill the bacteria. In in vitro experiments, MAP-FP peptides can kill various Gram-negative bacteria, such as Escherichia coli and Salmonella, within just 10 min. More importantly, these MAP-FP peptides maintain good stability and low cytotoxicity under high-temperature conditions, demonstrating their high safety for practical applications (Kim DY. et al., 2024).
The antibacterial properties of MAPs also provide new directions for their development as wound dressing materials. With their strong adhesiveness and antibacterial activity, MAPs can help wounds heal quickly and prevent bacterial infections, especially showing great potential in controlling drug-resistant bacterial strains. This offers a novel biomaterial option for wound healing and skin treatment (Fichman et al., 2021).
MAPs have also demonstrated performance in antibacterial coatings for orthopedic implants. They can firmly bind to the surfaces of implants, such as titanium alloys, and provide controlled drug release capabilities. This coating uses pH-dependent metal coordination to release antibiotics in the acidic environment caused by infection. Specifically, the antibacterial coating of MAPs can quickly release antibiotics in response to changes in bacterial concentration, showing excellent antibacterial effects, especially in early and late infections after implantation (Choi et al., 2024). This feature is of great clinical significance in preventing and treating complications of orthopedic implants, particularly prosthetic failure caused by bacterial infections.
3.3 MAPs in tumor immunotherapy
The application of MAPs in tumor immunotherapy has also made significant progress. In cancer treatment, immune checkpoint blockade (ICB) therapy has shown great potential; however, current ICB therapies are often accompanied by serious systemic autoimmune reactions and other side effects. To address this issue, Joo KI et al. developed an immune bio-glue (imuGlue) local drug delivery platform based on MAPs. This platform utilizes the underwater adhesion properties of MAPs to retain anti-PD-L1 drugs at the tumor site and enable on-demand drug release in response to changes in the tumor microenvironment. Studies have shown that imuGlue significantly enhances antitumor effects in triple-negative breast cancer and melanoma models while reducing systemic toxicity (Joo et al., 2020). This local release mechanism effectively prevents the rapid diffusion of anti-PD-L1 drugs in the body, alleviating their systemic toxicity, thus providing a new therapeutic strategy for cancer immunotherapy.
Additionally, MAPs have been applied in developing new tumor immunotherapy strategies, particularly in overcoming the immunosuppressive tumor microenvironment. By combining immune enhancers and photosensitizers with the MAP carrier, precise drug co-delivery has been achieved, which not only promotes tumor photothermal ablation but also expands the variety of tumor antigens. This enhances the persistence and effectiveness of local immunotherapy (He et al., 2025). This MAP-based drug delivery system can modulate the tumor microenvironment, overcome immune suppression, and enhance antitumor immune responses, further advancing the research into combination immunotherapy.
Figure 2 shows the pharmacological effects of MAPs in anti-inflammatory, antibacterial, and tumor immunotherapy.
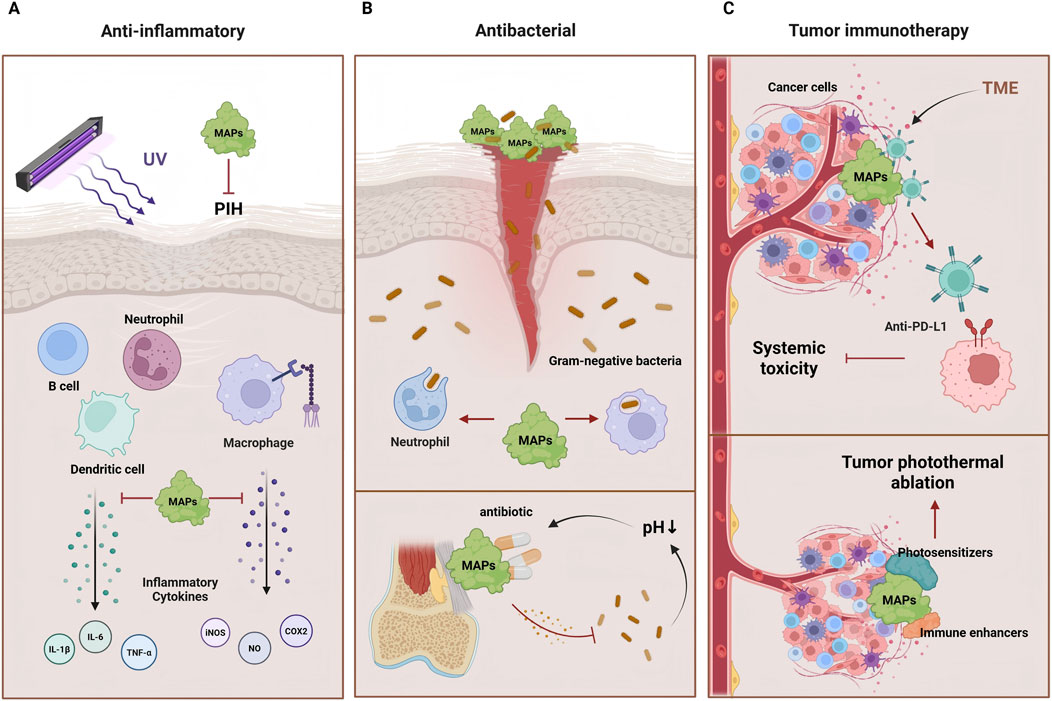
Figure 2. The pharmacological effects of MAPs. (A) MAPs can inhibit the occurrence of skin inflammation and PIH induced by LPS and UV radiation by suppressing inflammatory cytokines. (B) MAPs not only exhibit activity in inhibiting the growth of Gram-negative bacteria but can also release antibiotics in the acidic environment induced by infections, preventing bacterial infections. (C) MAPs can retain anti-PD-L1 drugs at the tumor site and release the drugs on demand in response to changes in TME. Furthermore, by combining immune enhancers and photosensitizers into MAP carrier, tumor photothermal ablation is promoted, enhancing the efficacy of immunotherapy.
4 Applications of MAPs in the biomedical field
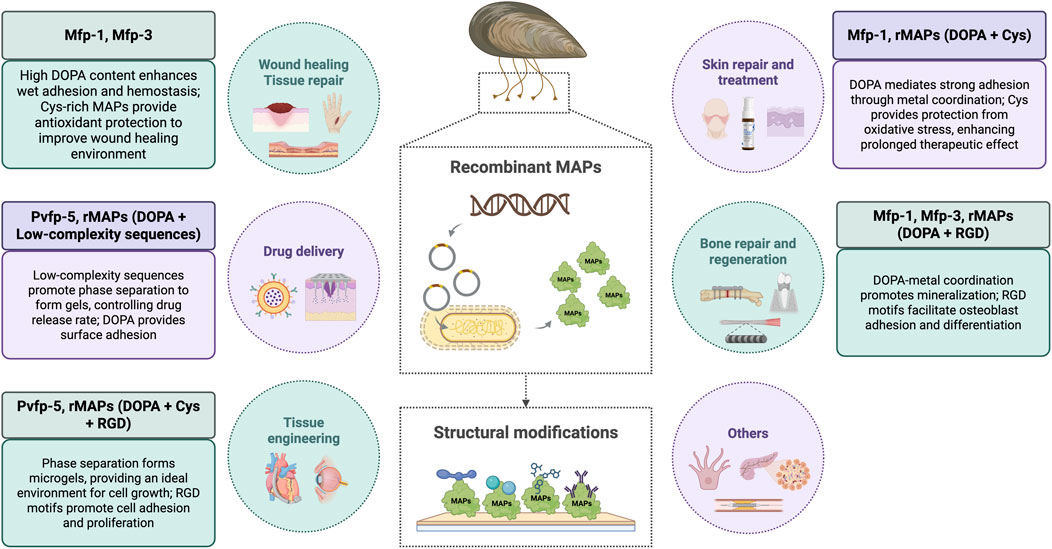
Native mussel adhesive proteins (MAPs), particularly Mfp-1, Mfp-3, and Mfp-5, have been extensively studied as natural templates for bioadhesive development. These proteins play distinct roles in the mussel byssus and have also been directly employed in biology and materials fields.
Mfp-1, a high-molecular-weight protein rich in DOPA and lysine residues (Lin et al., 2007), is primarily responsible for forming protective and corrosion-resistant films on metal and oxide surfaces. Studies have demonstrated that Mfp-1 can self-assemble into stable, oxidation-resistant coatings that improve surface hydrophilicity and biocompatibility, suggesting potential use in biomedical implants and antifouling coatings (Baskaran et al., 2025).
Native Mfp-3 coatings have been shown to enhance cell attachment and spreading on polymer and glass surfaces, while Mfp-5 displays superior adhesion strength under seawater-mimicking conditions. These native MAP-based coatings have also been applied to modify tissue scaffolds, promoting osteoblast and fibroblast adhesion at cell–material interfaces. Proteins with highest levels of DOPA, such as Mefp-3 (20 mol.%) and Mefp-5 (30 mol.%), exhibit wet adhesion to both inorganic and organic substrates through multiple catechol-mediated interactions. By integrating Mfp-3 and Mfp-5 peptide motifs into elastin-like polypeptides, adhesive hydrogels with tunable mechanical properties, adhesion, and excellent cytocompatibility were developed, providing a novel strategy for designing bioinspired adhesive materials for biomedical applications (Park et al., 2019; Li Z. et al., 2025).
Also MAPs have been applied as surface coatings for implants to enhance osseointegration, as adjuncts in guided bone regeneration to improve osteoinductivity (Kwan et al., 2023). Their unique adhesive properties and biocompatibility make them crucial for wound healing, skin repair, and trauma care (Santonocito et al., 2019).
4.1 Recombinant mussel adhesive proteins
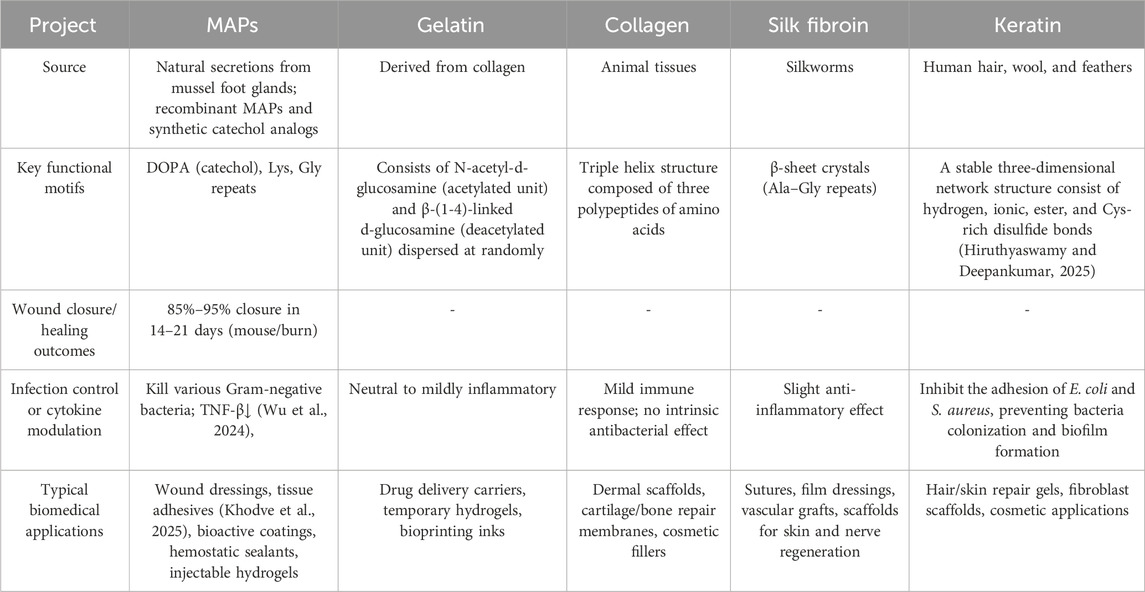
Currently, the acquisition of MAP mainly involves directly extracting the natural adhesive protein components from the foot glands of mussels. The natural sources are rather limited, the production process is complex, the cost is high, and it is prone to solidification, which greatly restricts the application of mussel adhesive protein. To break through this production bottleneck, recombinant mussel adhesive proteins (recombinant Mussel Adhesive Proteins, rMAPs) have emerged. rMAPs are proteins expressed and produced in microbial hosts such as Escherichia coli through genetic engineering, and they have similar amino acid sequences and functional characteristics to natural MAPs (Kaushik et al., 2015). The similarities and differences between natural and recombinant mussel adhesive proteins are shown in Table 1.

Table 1. A comparison of the similarities and differences between natural and recombinant mussel adhesive proteins.
Despite this, the development of rMAPs still faces a core challenge: how to efficiently and accurately introduce its key active groups - DOP - into the recombinant protein. DOPA is the chemical basis that endows mussel adhesion protein with strong wet adhesion ability. Currently, the recombinant mussel adhesion protein produced through the Escherichia coli expression system requires tyrosinase modification to convert tyrosine residues into 3,4-dihydroxyphenylalanine (DOPA), but it still faces low DOPA conversion rate and low modification efficiency, making it difficult to obtain the adhesion properties similar to those of the naturally extracted MAP. To overcome this problem, researchers have developed various optimization strategies. Adjusting the synthesis pathways or co-expressing tyrosyl-tRNA synthetase (TyrRS), which recognizes DOPA, can significantly improve the incorporation rate of DOPA, thereby enhancing its adhesive properties (Jeong et al., 2020). Moreover, the catalytic action of tyrosinase can directly convert tyrosine into DOPA without additional cofactors. Researchers have constructed functionalized tyrosinase to further improve the modification efficiency of DOPA and developed tyrosinases that remain stable under neutral and alkaline conditions. The application of these enzymes not only increases the yield of rMAPs but also enhances their potential in skin repair and tissue engineering (Kim S. et al., 2021).
Beyond traditional genetic engineering methods, another innovative direction involves synthesizing polymers that mimic the structure and function of MAPs. Studies have shown that by polymerizing monomers containing repetitive peptide segments via ring-opening polymerization reactions, synthetic polymers resembling the structure of natural MAPs can be prepared. These polymers exhibit superior adhesive performance compared to natural MAPs on certain surfaces, and this strategy holds potential for various biomedical applications, such as the fixation of living cells (Berger et al., 2022).
An important research direction for rMAPs is the development of composite co-precipitates based on MAPs. By combining rMAPs with charged macromolecules to form complex co-precipitates, these composite materials demonstrate excellent adhesive properties in underwater environments. These materials have potential applications in biomedical and tissue engineering, particularly as a new bio-adhesion solution for non-invasive surgical repairs (Kim HJ. et al., 2017).
Compared to natural MAPs, rMAPs offer significant advantages in terms of production cost and application scope. Mfp-151, obtained through recombinant DNA technology by tandem expression of Mfp-1 and Mfp-5 proteins found in mussels, represent a typical structure of natural MAPs (Yu et al., 2013; Lu et al., 2013). The rMAPs obtained through this method possess structural characteristics similar to those of extracted MAPs, including DOPA and lysine residues. These structural features grant rMAPs excellent adhesive properties and biocompatibility. The DOPA group enhances the adhesive performance of the product, while the lysine group, with its positive charge and susceptibility to oxidation, promotes cell migration and wound healing. Mfp-151, through its unique physical properties, can not only form a protective biofilm over wounds but also create a microscopic discontinuous layer on the wound surface, facilitating tissue repair and protection.
With the continuous progress in production technologies, including improving DOPA incorporation rates, enhancing tyrosinase catalytic efficiency, and developing synthetic polymers, rMAPs will exhibit broad application prospects in the biomedical field as materials with excellent biocompatibility and adhesive properties. This makes them a critical research direction in biomedical materials (Horsch et al., 2018; Xue et al., 2025a).
Although rMAPs exhibit remarkable adhesive and biological characteristics, it is also important to distinguish their advantages from those of purely catechol-based synthetic systems. Synthetic materials such as polydopamine (PDA) coatings and catechol-grafted polymers share the same DOPA/catechol chemistry and have been widely applied as versatile, cost-effective, and scalable surface modifiers (Omidian and Wilson, 2024).
The primary strength of synthetic catechol systems lies in their superior practicality and scalability. Polydopamine’s “dip-coating” process is extremely straightforward (Saraf et al., 2024), while catechol-grafted polymers allow for tunable mechanical properties and compatibility with standard industrial processes, making them more competitive for large-scale, cost-sensitive applications. In contrast, rMAPs offer molecular precision and programmability. Their key advantage lies in the ability to integrate specific functional domains via genetic engineering, enabling more specific and tougher adhesion (Xue et al., 2025a), enhancing anti-inflammatory and antimicrobial properties (Kim S. et al., 2024) and so on, with greater potential in fields demanding high biocompatibility, such as biomedicine.
Therefore, the choice depends on the application scenario. rMAPs offers unique solutions for high-value precision biomedical applications, while the synthetic catechol system is more practical and feasible in general fields that require large-scale and economical surface treatment.
4.2 Wound healing and tissue repair
rMAPs exhibit adhesive properties, anti-inflammatory, antibacterial, antioxidant effects, and promotes cell adhesion, which can accelerate the wound healing process and is widely used in the development of various wound healing materials (Dai et al., 2024). For example, studies have shown that nanofiber scaffolds made by mixing rMAPs with polycaprolactone (PCL) demonstrate accelerated regeneration in a rat skin wound healing model. These nanofiber scaffolds not only provide good mechanical strength and a cell-friendly growth environment but also effectively adsorb growth factors, promote the growth of keratinocytes, and thus enhance wound healing (Kim BJ. et al., 2017).
In addition to nanofiber scaffolds, rMAPs are also applied in other types of biorepair materials. Researchers have developed self-healing materials using the adhesive and biocompatible properties of rMAPs, such as a polyurethane-urea self-healing material based on rMAPs and aromatic disulfide bonds. This material can efficiently self-heal at room temperature and possesses strong water resistance and excellent mechanical properties, demonstrating broad potential for various biomedical applications (Liu et al., 2021). This material is particularly suitable for making wound dressings and trauma repair products, effectively working in moist environments.
rMAPs can also accelerate wound healing, especially when treating skin infections and burns. When combined with other natural materials like guar gum (CG), rMAPs can be used to create hydrogels with self-healing, antibacterial, anti-inflammatory, and cell-proliferating properties. These composite hydrogels not only possess excellent mechanical strength and biocompatibility but also effectively promote wound healing and reduce necrosis progression at burn sites, thereby avoiding severe local inflammatory reactions (Cui et al., 2020; Han et al., 2024).
Another important application of rMAPs is in wound hemostasis and bioadhesive fields. Traditional hemostatic agents often suffer from insufficient adhesion and instability, while nanofiber dressings based on rMAPs can form stable physical barriers at the wound site, effectively promoting platelet aggregation and blood clotting (Wang Z. et al., 2024). For example, a double-sided nanofiber dressing composed of rMAPs and silk fibroin (SF) can promote hemostasis through the inner layer of rMAPs, while the outer SF layer provides physical protection, reducing the risk of wound infection. This dressing not only possesses biodegradability but also provides long-term protection in the body without causing a long-term burden on tissues (Lee et al., 2024).
To better understand the unique advantages of MAPs in comparison to other commonly used bioadhesives, we have summarized key properties such as wound closure rates, infection control, cytokine profiles, and adhesion metrics. Table 2 presents a comparative analysis of MAPs and traditional bioadhesives like collagen, gelatin, and silk fibroin, highlighting the distinctive benefits of MAPs, particularly their superior performance in moist environments and their ability to modulate the immune response. This comparison emphasizes the multifaceted pharmacological effects of MAPs, which arise from the synergistic interplay of multiple motifs rather than a single residue.
In conclusion, rMAPs have demonstrated unique biomedical value in wound healing and tissue repair applications. Their excellent biological adhesion, biocompatibility and biodegradability make rMAPs an ideal material for wound repair, as they effectively promote wound healing, haemostasis and antibacterial effects, as well as tissue repair.
4.3 Skin repair and treatment
rMAPs have anti-inflammatory properties that help alleviate skin itching and other discomforts, showing significant potential in the treatment of various skin diseases. For example, in scar repair after deep skin wounds, abnormal collagen fiber remodeling is one of the key factors that lead to poor healing. rMAPs, by forming a collagen-targeting adhesive with specific glycosaminoglycans (GAGs), can specifically bind to type I collagen and regulate the rate and extent of collagen fiber generation, effectively promoting wound regeneration and collagen synthesis (Jeon et al., 2017). Additionally, this collagen-targeting adhesive improves the structure and function of skin tissue by restoring normal collagen fiber architecture, exhibiting a good scar inhibition effect.
Moreover, rMAPs have shown unique therapeutic effects in treating inflammatory skin diseases such as facial erythema and rosacea. Due to the complex etiology of rosacea, existing treatments often have limited efficacy. However, rMAPs, delivered via microneedles, can improve the skin’s immune response, reduce inflammatory infiltration, and restore abnormal neurovascular regulation. In a clinical trial, after microneedle treatment with rMAPs, patients showed significant relief from erythema, flushing, and capillary dilation symptoms, with no noticeable adverse reactions during the treatment (Luo et al., 2024). Similarly, rMAPs have also shown positive clinical effects in treating sensitive skin (SS). When delivered via microneedles, rMAPs improve symptoms such as dryness, tightness, and flaking. During treatment, patients’ skin reactions were effectively alleviated, with no significant side effects or relapse, demonstrating good safety and high patient satisfaction (Dong et al., 2023).
With continued research, more mature products based on rMAPs have successfully been launched and applied in clinical practice. Currently, there are various skin repair products on the market that use rMAPs as a base, such as rMAP wound healing dressings and rMAP cream dressings. These products have been clinically validated, especially in treating non-chronic wounds, where rMAPs have played a significant role. For instance, Zhanyan recombinant mussel adhesive protein, a rMAP-based product, is suitable for treating skin wounds post-laser, photorejuvenation, dermatitis, eczema, sensitive skin, acne, etc., helping repair and protect these superficial wounds.
Laser and photorejuvenation technologies are commonly used to treat pigmentary disorders, skin aging, and scars, and the superficial wounds formed during treatment typically require rapid repair. rMAPs can provide protection for these wounds, promote epidermal regeneration, and reduce postoperative discomfort (Fu et al., 2019). Chemical peeling, which causes superficial skin damage through substances like fruit acids, can also be alleviated by rMAPs, which helps reduce redness, swelling, and accelerate recovery (Lee et al., 2019).
Overall, the role of rMAPs in skin repair and treatment has been extensively researched and applied. The launch of mature products marks the transition of MAP from laboratory research to clinical practice, with broad market prospects.
4.4 Bone repair and regeneration
The role of rMAPs in bone repair and regeneration has gradually gained attention, especially in promoting bone tissue regeneration and improving the stability of medical implants (Yang K. et al., 2024). MAP coatings can support the attachment and proliferation of human keratinocytes and chondrocytes, indicating that MAPs provide a good growth environment for cells during the bone repair process (Ivarsson et al., 2021).
In recent years, researchers have used bioengineering methods to combine MAPs with other bioactive molecules, successfully creating various surface coatings and scaffolding materials with bone-promoting functions. For example, when MAPs are mixed with gelatin and loaded onto the surface of nano-titanium tubes, they can promote osteogenic differentiation and bone formation by activating the FAK-PI3K-MAPKs-Wnt/β-catenin signaling pathway (Kim JE. et al., 2021). MAPs play a role in bone repair by promoting the adhesion of osteoblasts and activating FAK, thereby regulating the PI3K/Akt signaling pathway. This not only enhances the proliferation and survival of osteoblasts, but also stabilizes β-catenin and promotes its nuclear translocation. Together with the Wnt signaling pathway, they form a synergistic effect, jointly promoting osteoblast differentiation and extracellular matrix remodeling, thereby accelerating the bone integration process around the implant. This pharmacological mechanism has been fully verified in in vitro cell experiments and in vivo rat model experiments in the literature, providing a scientific basis for the application of MAPs in the surface modification of dental implants. By combining MAPs with silica nanoparticles (SiNPs) through the fusion of R5 peptides, a micro-rough structure can be formed on the surface of titanium alloy implants, effectively promoting the proliferation and differentiation of pre-osteoblasts, improving implant stability and long-term effectiveness (Jo et al., 2017).
Furthermore, when MAPs are combined with hyaluronic acid (HA), the resulting co-precipitates significantly enhance the blood resistance and mechanical properties of bone graft materials, thereby promoting bone regeneration. This material can effectively improve osteoconductivity and accelerate the regeneration of bone tissue while maintaining the stability of bone grafts (Kim et al., 2016).
In the application of guided bone regeneration (GBR), MAP-loaded collagen membranes also show good results. Experimental studies have found that MAPs effectively function as barrier membranes, supporting bone regeneration and promoting the formation of new bone. Compared to traditional cyanoacrylate (CA)-loaded collagen membranes, MAP-loaded collagen membranes demonstrate better biocompatibility and fewer inflammatory responses, indicating their promising application in bone repair (Song et al., 2018).
rMAPs can also be combined with multifunctional bioactive peptides to improve bone regeneration outcomes. Research shows that materials containing MAPs and multifunctional bioactive peptides can enhance cell aggregation and mineral deposition in the bone healing process by promoting macrophage migration (Lee et al., 2023).
MAPs are also used in the surface modification of titanium alloys to enhance their clinical application in dentistry. By combining with titanium nano-network structures (TNS), MAPs significantly improve cell attachment and proliferation and promote the expression of bone-related genes. In in vivo experiments, titanium alloys with MAP coatings promoted new bone growth at the bone-implant interface, further verifying their potential in bone regeneration (Yin et al., 2019).
Overall, rMAPs play a multifaceted role in bone repair and regeneration, promoting osteoblast function and improving implant biocompatibility and stability. By combining with other bioactive molecules and materials, MAPs provide innovative solutions for bone repair, particularly in the clinical applications of dental and orthopedic treatments (Jo et al., 2023; Yun et al., 2025).
4.5 Tissue engineering
The application of rMAPs in tissue engineering has been increasingly recognized, particularly in areas such as biological adhesion, regenerative medicine, and material surface functionalization. rMAPs can effectively promote the adhesion of biomaterials to tissues through their underwater adhesion properties, demonstrating outstanding performance in improving the biocompatibility and biological functionality of biomaterials (Fang et al., 2017).
In cardiovascular implants, MAPs can enhance cell proliferation and migration by fusing with growth factors (such as VEGF) and extracellular matrix proteins (such as fibronectin). Through this biofunctionalization approach, MAPs help improve cardiac remodeling and myocardial protection, particularly in the repair following myocardial infarction, significantly extending the retention time of therapeutic peptides and promoting stable adhesion between the therapeutic patch and the host myocardium through their natural strong adhesive properties (Lim et al., 2021). Therefore, MAPs are widely used in surface modification of vascular stents. By combining MAPs with other bioactive molecules (such as NO-generating compounds and endothelial progenitor cell-targeting peptides), they can effectively prevent thrombosis, smooth muscle cell proliferation, and promote the recruitment and proliferation of endothelial progenitor cells, thereby reducing the occurrence of restenosis in stents (Yang Z. et al., 2020). This research shows that MAPs not only play a role in biological adhesion but also improve the clinical outcomes of stents by regulating cellular responses (Wang et al., 2020).
Moreover, rMAPs have become ideal materials for wound healing (Yao et al., 2022). Traditional suturing and stapling methods may cause significant tissue damage and inflammatory responses, especially in the repair of internal organ wounds. MAPs offer a new approach to address these issues. For instance, by developing a bilayer microneedle patch based on MAPs, researchers have achieved superior wound sealing capability, effectively preventing leakage and fluid infiltration in internal organ wounds (Jeon et al., 2019). This innovative bioadhesive material not only exhibits excellent adhesion but also provides reliable repair effects in dynamic environments, making it suitable for both external and internal tissue wound healing.
Unlike traditional synthetic adhesives, rMAPs also perform exceptionally well in underwater adhesion. Copolymers based on MAPs, such as poly(catechol-styrene), exhibit underwater adhesion strengths that even surpass the natural adhesion ability of live mussels (North et al., 2017). This adhesion property is particularly significant in saline environments, offering new solutions for underwater wound repair and medical devices that need to function in moist or submerged environments. For example, MAP-based water-incompatible bioadhesives have been successfully used to close urethral and vesicovaginal fistulas, demonstrating excellent adhesion and durability in wet environments. This feature makes them a potential repair material for urological and other internal surgeries (Kim HJ. et al., 2015; Kim HJ. et al., 2021).
In ophthalmic surgeries, researchers have successfully achieved sutureless amniotic membrane transplantation using the FixLight protein bioadhesive developed from MAPs. This adhesive can rapidly solidify and has good biocompatibility with ocular tissues, greatly simplifying the surgical process and reducing postoperative complications (M et al., 2021).
Finally, rMAPs also show broad prospects in the repair of internal organs and the implantation of medical devices. By adjusting the physical properties and biodegradability of the adhesives, customized bioadhesive patches can provide strong underwater adhesion and controllable degradation properties to meet the specific needs of different organs, thereby playing a role in various biomedical applications (Yang et al., 2024b).
MAPs can be used to construct thermoresponsive tissue adhesive hydrogels to promote soft tissue regeneration. By combining MAPs with poly(N-isopropylacrylamide) (PNIPAM), an injectable hydrogel system was prepared that activates and enhances tissue adhesion and moisture retention at body temperature. Compared to the use of PNIPAM gel alone, MAP-PNIPAM hydrogels exhibit significantly stronger tissue adhesion and better hydration, thus improving the effectiveness of adipose tissue engineering, such as cell survival rates and integration with host tissues (Jeon et al., 2020).
In summary, the role of MAPs in tissue engineering is not limited to their underwater adhesion properties but also involves promoting cell growth and tissue repair through their interaction with biomolecules. They offer new insights into the functionalization of biomaterial surfaces, with wide applications in regenerative medicine, cardiovascular therapy, and tissue engineering (Hu et al., 2024).
4.6 Targeted drug delivery and therapeutic applications
Due to the ability of rMAPs to firmly bind to biological tissues through DOPA residues, their application in drug delivery systems has become a highly promising option. A drug delivery system based on MAPs can form a coordination complex with iron (III) by modifying the DOPA residues, enabling drug release in acidic environments and demonstrating excellent pH responsiveness. This property gives it a significant advantage in anticancer drug delivery, especially in local and targeted therapies. Drug-loaded polymeric nanoparticles modified with MAPs can effectively enter cancer cells via the cell membrane and release drugs in the low pH microenvironment, enhancing local drug accumulation and reducing systemic toxicity (Kim BJ. et al., 2015).
The excellent adhesion and biocompatibility of rMAPs make them an ideal choice for the preparation of functionalized nanoparticles. MAPs can react with dopamine to form polydopamine (PDA) nanoparticles, which exhibit good near-infrared absorption and photothermal effects, making them suitable for use as a targeted drug delivery platform in photothermal therapy for cancer (Zhang et al., 2018; Chen et al., 2020). Mussel adhesive protein-modified polyacrylic acid nanoparticles can form micro-needle patches with strong adhesive properties, offering excellent biocompatibility and enabling continuous drug release and long-term targeted therapeutic effects (Yang et al., 2024c). These nanoparticles effectively inhibit tumor growth, and after a single administration, the drug remains at therapeutic concentrations in the tumor site (Yang et al., 2025; Jeong et al., 2025). This sustained release effect makes MAPs a highly promising tool in cancer therapy.
A spray-based drug delivery system utilizing MAPs has also been proposed as an innovative cancer treatment strategy (Feng et al., 2024). By spraying MAP nanoparticles loaded with chemotherapy drugs directly onto the tumor surface, the drug residence time on the tumor surface can be increased, effectively inhibiting tumor growth and significantly improving treatment efficacy while reducing adverse reactions (Jeong et al., 2018).
Furthermore, MAPs have made significant progress in nerve regeneration applications. By combining MAPs with extracellular matrix (ECM) peptides, electrospun nanofiber conduits were fabricated, which not only enhanced the adhesion and proliferation of nerve cells but also promoted neuronal regeneration through contact guidance. This multidimensional strategy incorporating MAPs provides a new direction for nerve injury repair, particularly in nerve grafting and nerve conduit design, with great potential for application (Cheong et al., 2019).
rMAPs also play an important role in stem cell delivery after myocardial infarction. Drug carrier systems APICLS prepared using MAPs not only improve stem cell encapsulation efficiency and survival rates but also promote the integration of cells with damaged myocardial tissue, enhancing cell persistence and paracrine effects, thereby aiding in heart repair and regeneration (Park et al., 2020).
The applications of rMAPs demonstrate their diversity and powerful functionality in drug delivery and targeted therapy. Through modification and combination with other substances, rMAPs can achieve more precise and sustained drug release, enhance drug efficacy, and reduce systemic toxicity (Xu Z. et al., 2024). Whether in microneedle systems for cancer treatment or in nerve repair applications, MAPs, with their unique adhesive properties and biocompatibility, offer multiple potential therapeutic approaches and drive innovation in biomedical materials (Liu et al., 2024).
4.7 Other applications
rMAPs have shown significant effects in promoting angiogenesis, especially in enhancing endothelial cell repair. After vascular injury, repair work is often challenging because the original vascular structure cannot be fully restored. In recent years, therapeutic angiogenesis has been considered a promising strategy; however, there is a lack of effective methods to simulate the in vivo angiogenesis process. To address this, Park TY et al. developed a novel therapeutic angiogenesis platform based on MAPs. Through their high adhesion and strong stickiness at lesion sites, rMAPs can release angiogenesis-promoting growth factors in a controlled, targeted manner, thus facilitating vascular repair. By working synergistically with platelet-derived growth factor (PDGF) and vascular endothelial growth factor (VEGF), this platform successfully achieved effective angiogenesis in vivo, demonstrating its great potential for treating ischemic diseases (Park et al., 2021).
Endothelial cells, as the primary constituents of blood vessels, are crucial for vascular repair. Research has shown that complexes formed by MAPs and exogenous VE-cadherin extracellular domains can effectively promote the adhesion of endothelial cells and endothelial progenitor cells, accelerating the vascular repair process. Specifically, this complex significantly increased the adhesion of endothelial cells and progenitor cells in vitro and, through interaction with VE-cadherin, promoted the aggregation and functional recovery of endothelial cells (Yang D. et al., 2018).
In clinical applications, vascular stents often need to accelerate the endothelialization process to reduce the risk of thrombosis. Studies have shown that MAP-coated stents quickly attract endothelial progenitor cells after implantation, forming a smooth endothelial layer within a short period. This reduces platelet adhesion and the formation of neointima, providing strong support for the biocompatibility and long-term efficacy of vascular stents (Yang D. et al., 2020).
rMAPs are also widely used in surface modification of biodegradable materials. For example, a composite film formed by MAPs and chitosan can effectively control the degradation rate of magnesium alloys in body fluids. This composite film not only enhances the corrosion resistance of magnesium alloys but also significantly improves their biocompatibility, providing a safer guarantee for the use of medical magnesium alloys (Jiang et al., 2016).
Moreover, MAPs have shown important applications in diabetes management. In the development of continuous glucose monitoring systems, researchers utilized the biocompatibility of MAPs to firmly immobilize glucose oxidase onto microneedle electrodes via electrochemical oxidation methods, achieving highly durable and selective glucose monitoring. Experimental verification showed that this MAP-based monitoring system exhibited performance comparable to commercially available systems, with high clinical application potential (Kim et al., 2019).
Finally, the anti-thrombotic properties of MAPs have led to their widespread use in blood compatibility modifications. Studies have found that MAPs can effectively inhibit platelet adhesion and activation, thus reducing the risk of blood clotting. By forming complexes with polysaccharides, MAPs not only enhanced their anti-platelet adhesion effects but also improved the biocompatibility of blood-contacting materials by reducing plasma protein adsorption (da Câmara et al., 2020).
In summary, rMAPs have shown extensive application potential in vascular repair, tissue regeneration, medical device modification, and more (Figure 3). As research progresses, the potential of MAPs as a biomaterial will be further explored, providing more innovative therapeutic solutions for modern medicine.
5 Challenges and development directions of rMAPs
MAPs are considered ideal underwater adhesives, but they face several challenges, including recombinant protein production, toxicity control, and underwater adhesion efficiency. However, with the continuous development of new strategies, such as genetic engineering, light-activated technologies, and hybrid protein design, the application prospects of MAPs are becoming broader, providing significant technological support and innovation opportunities for biomedical repair, underwater engineering, and wound healing.
5.1 Current challenges facing rMAPs
MAPs have great potential as biomaterials due to their excellent adhesive properties. However, their application still faces many challenges, especially in terms of high-efficiency expression, functionalization, and use in wet environments.
Firstly, the natural yield of MAPs is relatively low, which limits their widespread use in practical applications. Although recombinant technology can increase production efficiency, due to the complexity of their expression systems and purification processes, particularly during large-scale production, balancing yield and cost remains a challenge. To address this issue, research has proposed improvement strategies through structural analysis. By altering the position and quantity of tyrosine, mutant proteins with enhanced expression levels, adhesive ability, and solubility have been successfully constructed (Xue et al., 2025b). Meanwhile, the CRISPR/Cas gene editing technology has been applied to optimize microbial expression systems, aiming to increase the expression level and solubility of recombinant proteins, thereby enhancing production. For instance, the CRISPR/Cas system was employed in engineered Escherichia coli, successfully improving the expression level and solubility of the target protein (Feser et al., 2023).
Secondly, although recombinant technology can provide a large amount of adhesive proteins, the resulting rMAPs often lack the complex three-dimensional structure and functional characteristics possessed by natural MAPs, leading to lower adhesive strength and stability. Some studies have enhanced adhesion performance using nanoparticle-based adhesives. Experiments have shown that the addition of metal ions can increase the cohesion between MAPs, thereby improving their adhesion (Baskaran et al., 2023).
Furthermore, the performance of rMAPs under different environmental conditions (such as varying pH, temperature, and humidity) may not be as stable as that of natural MAPs, limiting their application in complex environments. Specifically, rapid adhesion in underwater and wet environments remains a significant challenge. Hauf et al. adopted a strategy based on genetic code expansion, designing efficient aminoacyl-tRNA synthetases (aaRSs) and introducing photoactivatable atypical amino acids (such as ortho-nitrobenzyl DOPA, ONB-DOPA) into rMAPs. This innovative strategy enabled the MAPs to equip multiple ONB-DOPA molecules at specific sites, which were activated by ultraviolet light, significantly enhancing their adhesion in wet environments, providing a new direction for the recombinant production of underwater adhesives (Hauf et al., 2017). Additionally, stabilizers such as trifluoroethanol (TFE) and urea can maintain the unfolded state of proteins and promote their aggregation, forming strong underwater adhesives. This method reveals a universal principle for converting ordinary proteins into strong underwater adhesives, showcasing the rich potential of proteins from biological resources (Liu et al., 2023). It can also be optimized through AI-assisted algorithms, which can predict the amino acid substitutions that can enhance the adhesion strength, stability and overall performance of rMAPs. AI helps identify the modifications that improve the functional characteristics of rMAPs, making them more stable and effective in biomedical environments (such as drug delivery and tissue engineering) (Jiang et al., 2025).
In addition to the material- and structure-oriented challenges of recombinant MAPs discussed above, it is also important to consider the limitations that arise when MAPs are explored as therapeutic agents themselves. Recombinant MAPs share the same chemical and structural complexity as natural MAPs, which may cause issues related to enzymatic degradation, oxidation, and limited systemic stability. Their large molecular size and high hydrophilicity make targeted delivery difficult, while their potential immunogenicity has not been fully evaluated. There is a lack of long-term safety and immunogenicity data. While rMAPs have good biocompatibility and biodegradability, systematic animal and clinical studies are still scarce, leaving uncertainties about chronic inflammatory responses and degradation products (Xue et al., 2025b). For example, the oxidation of phenolic compounds catalyzed by tyrosinase can generate bisphenols, quinones, and highly polymerized melanins, which may cause biocompatibility issues in some applications, particularly in biomedical fields (Axambayeva et al., 2018). Some applications of rMAPs during curing processes may release hydrogen peroxide, which could lead to localized cytotoxicity and affect immune responses around tissues. Therefore, when developing bioadhesives containing MAPs, it is essential to carefully monitor and control the generation of these byproducts to ensure biocompatibility for specific applications (Meng et al., 2017).
Meanwhile, when compared with clinically approved tissue adhesives, rMAPs show both advantages and shortcomings. Fibrin glue offers rapid hemostasis and biodegradability but limited mechanical strength. PEG-based hydrogels are tunable and biocompatible yet less adhesive under wet conditions. Cyanoacrylate adhesives provide strong adhesion and fast curing but cause local cytotoxicity and tissue irritation. In contrast, rMAPs balance bioadhesion and biocompatibility but currently lack the scalability and regulatory maturity for clinical adoption.
Moreover, large-scale production, batch variability, and regulatory classification as biologics may further complicate clinical translation. To overcome these hurdles, researchers and manufacturers can collaborate closely with regulatory bodies to establish clear guidelines for the approval of rMAPs. Early-stage clinical trials should prioritize evaluating the biocompatibility, toxicity, and long-term stability of rMAPs. This collaborative approach will help streamline the regulatory approval process and facilitate the clinical translation of rMAPs into therapeutic use (Jiang et al., 2025). Addressing these pharmacological and translational barriers will be crucial for realizing the full therapeutic potential of MAPs beyond their material-based applications.
5.2 Future research directions of rMAPs
The future research directions of rMAPs mainly focus on improving their production efficiency, functional modification, and expanding their applications across multiple fields. Researchers aim to optimize the production processes of rMAPs, improving expression systems and purification methods to reduce costs and increase yields. Simultaneously, functional modifications through genetic engineering will enhance their stability and adhesive strength in extreme environments.
Exploring the combination of rMAPs with nanomaterials, drug carriers, and other components to develop multifunctional composite materials for use in medical, environmental, and smart material fields is also a promising avenue. For example, researchers have developed a composite material based on MAPs and graphene oxide (GO), synthesized through a green aqueous solution. This composite material not only exhibits high tensile strength and elongation but also demonstrates extraordinary toughness, surpassing many existing composite materials (Kim et al., 2020). This green synthesis strategy avoids the use of organic solvents and surfactants, resulting in a lower environmental impact and highlighting the potential applications of MAPs in high-performance materials.
In precision medicine, the unique adhesive properties of rMAPs can be utilized to develop personalized biomaterials, such as drug delivery systems targeting specific lesions. Through genetic engineering and synthetic biology techniques, researchers can introduce specific functional groups into the structure of rMAPs, enabling them to target and treat tumors, infectious diseases, and other diseased tissues in precision medicine.
The future research of rMAPs not only holds significant medical and biotechnological applications but also provides inspiration for research on other materials. The unique properties of rMAPs, such as efficient underwater adhesion, good biocompatibility, and adjustability, offer valuable experience for developing new high-performance materials.
Currently, traditional surgical tissue adhesives fail to meet the ideal requirements for biological adhesives due to their insufficient adhesive strength in moist conditions and higher toxicity. To address this issue, researchers have developed a novel light-activated biological adhesive, LAMBA, based on mussel adhesion mechanisms and insect di-tyrosine crosslinking chemistry (Jeon et al., 2015). This adhesive exhibits much stronger adhesion on moist tissues than commercially available fibrin glues and has good biocompatibility. By regulating crosslinking reactions through light exposure, LAMBA can effectively close wounds on demand and promote wound healing, showing broad medical application potential, especially in seamless wound closure and visceral tissue repair.
To address fast adhesion in moist environments, catechol-based adhesives inspired by MAPs have attracted widespread attention (Ni et al., 2024). Studies have shown that catechol compounds with long-chain fatty groups can be used to prepare thin, optically transparent underwater adhesive strips. These adhesive strips are rapidly activated in water and possess strong adhesive strength. Moreover, Wei W et al. have successfully enhanced adhesion by designing synthetic peptides mimicking MAPs and utilizing their co-precipitation properties with water-repellent surfaces (Wei et al., 2016).
Although powerful underwater adhesives are highly attractive for biomedical and underwater repair applications, their success in practical use still faces challenges such as underwater curing, surface adhesion, and the balance between cohesion and adhesion forces (Kim E. et al., 2021). Researchers have designed a novel hybrid protein combining the amyloid protein’s zipper structure, spider silk’s flexible sequence, and the DOPA sequence of MAPs. This hybrid protein can self-assemble into semi-crystalline hydrogels, exhibiting extremely high strength and toughness, and shows underwater adhesion on various surfaces (such as plastics, tendons, and skin). Selective de-adhesion can be achieved through oxidation or iron chelation treatments. This study provides new ideas for the design and synthesis of multifunctional hybrid protein materials and shows potential for wide applications.
Finally, exploring the pharmacological effects and mechanisms of rMAPs is also a key direction for future research. In addition to traditional adhesive applications, rMAPs may also exhibit bioactivity, such as antibacterial, anti-inflammatory, or tissue repair-promoting effects. By deeply studying the mechanisms of rMAPs’ action and integrating cell biology and molecular biology techniques, their potential applications in wound healing, tissue engineering, and even immunotherapy could be uncovered. Therefore, future research should not only focus on the physicochemical properties of rMAPs but also emphasize the exploration of their biological functions and the mechanism analysis, unlocking more application scenarios.
In summary, rMAPs have broad application prospects in fields such as precision medicine and smart drug delivery. Genetic engineering and synthetic biology will provide strong technical support for their development, driving innovation in future medical, material science, and other applications (Lee et al., 2016).
6 Conclusion
MAPs, as a type of natural underwater adhesive molecules, exhibit tremendous application potential in various fields such as biomedicine, drug development, and material science due to their exceptional adhesive properties, biocompatibility, and environmental adaptability (Hollingshead et al., 2022; Sun et al., 2020). In particular, MAPs have become ideal carrier materials in drug delivery and tissue engineering due to their unique properties. With in-depth research into their pharmacological effects and mechanisms, the clinical application prospects of MAPs are increasingly broad, especially showing significant potential in precision medicine, wound repair, and biosensor fields (Aich et al., 2018).
The pharmacological effects of MAPs primarily lie in their excellent bio-adhesion ability and multifunctionality. Through the covalent bonding of amino acids such as tyrosine and lysine, MAPs form strong and stable adhesive forces, enabling them to remain attached stably in underwater environments (Burke et al., 2016). Meanwhile, MAPs exhibit low immunogenicity, allowing for long-term use in vivo without causing immune rejection, which contributes to their high biocompatibility. These characteristics provide MAPs with unique advantages in drug delivery systems. MAPs not only enhance the solubility and stability of drugs but also improve drug targeting, increase bioavailability, reduce side effects, and enable sustained and controlled drug release (Talebian et al., 2020). Therefore, MAPs have shown great potential in targeted drug delivery, cancer therapy, and vaccine carriers.
However, further research is needed to strengthen the safety assessment and long-term effects of MAPs in clinical applications. Although they exhibit good biocompatibility, their long-term biological safety in large-scale clinical applications still requires in-depth study (Jiao et al., 2024). Particularly in scenarios involving long-term implantation, such as tissue repair and wound healing, it is crucial to ensure that their degradation products are non-toxic and do not cause immune reactions (Yun et al., 2024).
Natural products, as valuable sources of pharmacologically active molecules, have long provided inspiration for modern drug discovery and biomaterial design (Sun et al., 2024; Han et al., 2025; Li X. et al., 2025; M’barek et al., 2025). The study of MAP exemplifies how nature’s molecular architectures can serve as blueprints for next-generation therapeutic and bioadhesive innovations.
In conclusion, rMAPs hold enormous application potential in drug development and biomedical fields. With technological advancements, particularly in genetic engineering and synthetic biology, rMAPs are expected to overcome current research bottlenecks and be applied in more clinical settings, such as personalized drug delivery systems, wound repair, and cancer treatment. Despite facing some challenges, with ongoing research, the multifunctionality and wide applicability of MAPs will make them an important material in biomedicine, driving the development of precision medicine and novel treatment methods.
Author contributions
JA: Writing – original draft, Conceptualization, Data curation, Visualization. ZH: Conceptualization, Data curation, Visualization, Writing – review and editing. JL: Data curation, Writing – review and editing. QB: Writing – review and editing. SH: Data curation, Writing – review and editing. CP: Data curation, Funding acquisition, Writing – review and editing. FP: Conceptualization, Funding acquisition, Supervision, Writing – review and editing.
Funding
The authors declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (No. 82574683), Project of Science and Technology Department of Sichuan Province (Nos. 2023NSFSC1928, 2023NSFSC1992), Project of State Administration of Traditional Chinese Medicine of China (No. ZYYCXTD-D-202209), Project of Sichuan Provincial Administration of Traditional Chinese Medicine (Nos. 2022C001, 2024ZD02), the Open Research Fund of Chengdu University of Traditional Chinese Medicine Key Laboratory of Systematic Research of Distinctive Chinese Medicine Resources in Southwest China (SKLTCM202305), the Fundamental Research Funds for the Central Universities, and Sichuan University Interdisciplinary Innovation Fund.
Conflict of interest
Author ZH was employed by Xi’an DeNovo Hith Medical Technology Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahn, J. M., Lee, J. S., Um, S. G., Rho, B. S., Lee, K. B., Park, S. G., et al. (2019). Mussel adhesive protein-conjugated vitronectin (fp-151-VT) induces anti-inflammatory activity on LPS-stimulated macrophages and UVB-irradiated keratinocytes. Immunol. Invest. 48 (3), 242–254. doi:10.1080/08820139.2018.1506476
Aich, P., An, J., Yang, B., Ko, Y. H., Kim, J., Murray, J., et al. (2018). Self-assembled adhesive biomaterials formed by a genetically designed fusion protein. Chem. Commun. (Camb) 54 (89), 12642–12645. doi:10.1039/c8cc07475e
Axambayeva, A. S., Zhaparova, L. R., Shagyrova, Z. S., Ramankulov, E. M., and Shustov, A. V. (2018). Unusual stability of a recombinant Verrucomicrobium spinosum tyrosinase to denaturing agents and its use for a production of a protein with adhesive properties. Appl. Biochem. Biotechnol. 185 (3), 736–754. doi:10.1007/s12010-017-2686-y
Baskaran, N., Wang, Y. C., Tan, R. J., Chung, R. J., and Wei, Y. (2023). Overcoming the yield challenge of mussel foot proteins: enhancing adhesion through metal ion-incorporated nanoparticles. Colloids Surf. B Biointerfaces 229, 113479. doi:10.1016/j.colsurfb.2023.113479
Baskaran, P., Muthiah, B., and Uthirapathy, V. (2025). A systematic review on biomaterials and their recent progress in biomedical applications: bone tissue engineering. Rev. Inorg. Chem. 45 (4), 747–781. doi:10.1515/revic-2024-0062
Berger, O., Battistella, C., Chen, Y., Oktawiec, J., Siwicka, Z. E., Tullman-Ercek, D., et al. (2022). Mussel adhesive-inspired proteomimetic polymer. J. Am. Chem. Soc. 144 (10), 4383–4392. doi:10.1021/jacs.1c10936
Burke, K. A., Roberts, D. C., and Kaplan, D. L. (2016). Silk fibroin aqueous-based adhesives inspired by mussel adhesive proteins. Biomacromolecules 17 (1), 237–245. doi:10.1021/acs.biomac.5b01330
Chen, Y., Feng, X., Zhao, Y., Zhao, X., and Zhang, X. (2020). Mussel-inspired polydopamine coating enhances the intracutaneous drug delivery from nanostructured lipid carriers dependently on a follicular pathway. Mol. Pharm. 17 (4), 1215–1225. doi:10.1021/acs.molpharmaceut.9b01240
Cheong, H., Kim, J., Kim, B. J., Kim, E., Park, H. Y., Choi, B. H., et al. (2019). Multi-dimensional bioinspired tactics using an engineered mussel protein glue-based nanofiber conduit for accelerated functional nerve regeneration. Acta Biomater. 90, 87–99. doi:10.1016/j.actbio.2019.04.018
Choi, H. S., Yun, J., Jeong, Y., Jo, Y. K., and Cha, H. J. (2024). Self-controllable proteinic antibacterial coating with bacteria-triggered antibiotic release for prevention of periprosthetic infection. Biomaterials 305, 122457. doi:10.1016/j.biomaterials.2023.122457
Cui, R., Chen, F., Zhao, Y., Huang, W., and Liu, C. (2020). A novel injectable starch-based tissue adhesive for hemostasis. J. Mater Chem. B 8 (36), 8282–8293. doi:10.1039/d0tb01562h
da Câmara, P. C. F., Madruga, L. Y. C., Sabino, R. M., Vlcek, J., Balaban, R. C., Popat, K. C., et al. (2020). Polyelectrolyte multilayers containing a tannin derivative polyphenol improve blood compatibility through interactions with platelets and serum proteins. Mater Sci. Eng. C Mater Biol. Appl. 112, 110919. doi:10.1016/j.msec.2020.110919
Dai, Q., Liu, H., Gao, C., Sun, W., Lu, C., Zhang, Y., et al. (2024). Advances in mussel adhesion proteins and mussel-inspired material electrospun nanofibers for their application in wound repair. ACS Biomater. Sci. Eng. 10 (10), 6097–6119. doi:10.1021/acsbiomaterials.4c01378
Deepankumar, K., Guo, Q., Mohanram, H., Lim, J., Mu, Y., Pervushin, K., et al. (2022). Liquid-liquid phase separation of the green mussel adhesive protein Pvfp-5 is regulated by the post-translated dopa amino acid. Adv. Mater 34 (25), e2103828. doi:10.1002/adma.202103828
DeMartini, D. G., Errico, J. M., Sjoestroem, S., Fenster, A., and Waite, J. H. (2017). A cohort of new adhesive proteins identified from transcriptomic analysis of mussel foot glands. J. R. Soc. Interface 14 (131), 20170151. doi:10.1098/rsif.2017.0151
Dong, R., Jin, Q., Zhi, J., Luo, Y., Yuan, J., Pi, L., et al. (2023). Mussel adhesive protein treatment delivered by microneedling for sensitive skin: a clinical study. J. Cosmet. Dermatol 22 (6), 1835–1843. doi:10.1111/jocd.15645
Fang, H., Li, Q. L., Han, M., Mei, M. L., and Chu, C. H. (2017). Anti-proteolytic property and bonding durability of mussel adhesive protein-modified dentin adhesive interface. Dent. Mater 33 (10), 1075–1083. doi:10.1016/j.dental.2017.07.008
Feng, X., Shi, Y., Zhang, Y., Lei, F., Ren, R., and Tang, X. (2024). Opportunities and challenges for inhalable nanomedicine formulations in respiratory diseases: a review. Int. J. Nanomedicine 19, 1509–1538. doi:10.2147/IJN.S446919
Feser, C. J., Williams, J. M., Lammers, D. T., Bingham, J. R., Eckert, M. J., Tolar, J., et al. (2023). Engineering human cells expressing CRISPR/Cas9-Synergistic activation mediators for recombinant protein production. Int. J. Mol. Sci. 24 (10), 8468. doi:10.3390/ijms24108468
Fichman, G., Andrews, C., Patel, N. L., and Schneider, J. P. (2021). Antibacterial gel coatings inspired by the cryptic function of a mussel byssal peptide. Adv. Mater 33 (40), e2103677. doi:10.1002/adma.202103677
Fu, X., Dong, J., Wang, S., Yan, M., and Yao, M. (2019). Advances in the treatment of traumatic scars with laser, intense pulsed light, radiofrequency, and ultrasound. Burns Trauma 7, 1. doi:10.1186/s41038-018-0141-0
Guo, Q., Chen, J., Wang, J., Zeng, H., and Yu, J. (2020). Recent progress in synthesis and application of mussel-inspired adhesives. Nanoscale 12 (3), 1307–1324. doi:10.1039/c9nr09780e
Han, M., Li, S., Fan, H., An, J., Peng, C., and Peng, F. (2024). Regulated cell death in glioma: promising targets for natural small-molecule compounds. Front. Oncol. 14, 1273841. doi:10.3389/fonc.2024.1273841
Han, M., Yang, J., Chen, P., Li, S., Tang, H., Fan, H., et al. (2025). Isocucurbitacin B inhibits gliomas through the promotion of anoikis by targeting caveolin 1. Cancer Lett. 629, 217873. doi:10.1016/j.canlet.2025.217873
Hauf, M., Richter, F., Schneider, T., Faidt, T., Martins, B. M., Baumann, T., et al. (2017). Photoactivatable mussel-based underwater adhesive proteins by an expanded genetic code. Chembiochem. 18 (18), 1819–1823. doi:10.1002/cbic.201700327
He, Y., Sun, H., Bao, H., Hou, J., Zhou, Q., Wu, F., et al. (2025). A natural adhesive-based nanomedicine initiates photothermal-directed in situ immunotherapy with durability and maintenance. Biomaterials 312, 122751. doi:10.1016/j.biomaterials.2024.122751
Hiruthyaswamy, S. P., and Deepankumar, K. (2025). Suckerin based biomaterials for wound healing: a comparative review with natural protein-based biomaterials. Mater. Adv. 6 (4), 1262–1277. doi:10.1039/d4ma01005a
Hollingshead, S., Siebert, H., Wilker, J. J., and Liu, J. C. (2022). Cytocompatibility of a mussel-inspired poly(lactic acid)-based adhesive. J. Biomed. Mater Res. A 110 (1), 43–51. doi:10.1002/jbm.a.37264
Horsch, J., Wilke, P., Pretzler, M., Seuss, M., Melnyk, I., Remmler, D., et al. (2018). Polymerizing like mussels do: toward synthetic mussel foot proteins and resistant glues. Angew. Chem. Int. Ed. Engl. 57 (48), 15728–15732. doi:10.1002/anie.201809587
Hu, O., Lu, M., Cai, M., Liu, J., Qiu, X., Guo, C. F., et al. (2024). Mussel-bioinspired lignin adhesive for wearable bioelectrodes. Adv. Mater 36 (38), e2407129. doi:10.1002/adma.202407129
Ivarsson, M., Prenkert, M., Cheema, A., Wretenberg, P., and Andjelkov, N. (2021). Mussel adhesive protein as a promising alternative to fibrin for scaffold fixation during cartilage repair surgery. Cartilage 13 (2_Suppl. l), 663s–671s. doi:10.1177/1947603519887319
Jeon, E. Y., Hwang, B. H., Yang, Y. J., Kim, B. J., Choi, B. H., Jung, G. Y., et al. (2015). Rapidly light-activated surgical protein glue inspired by mussel adhesion and insect structural crosslinking. Biomaterials 67, 11–19. doi:10.1016/j.biomaterials.2015.07.014
Jeon, E. Y., Choi, B. H., Jung, D., Hwang, B. H., and Cha, H. J. (2017). Natural healing-inspired collagen-targeting surgical protein glue for accelerated scarless skin regeneration. Biomaterials 134, 154–165. doi:10.1016/j.biomaterials.2017.04.041
Jeon, E. Y., Lee, J., Kim, B. J., Joo, K. I., Kim, K. H., Lim, G., et al. (2019). Bio-inspired swellable hydrogel-forming double-layered adhesive microneedle protein patch for regenerative internal/external surgical closure. Biomaterials 222, 119439. doi:10.1016/j.biomaterials.2019.119439
Jeon, E. Y., Joo, K. I., and Cha, H. J. (2020). Body temperature-activated protein-based injectable adhesive hydrogel incorporated with decellularized adipose extracellular matrix for tissue-specific regenerative stem cell therapy. Acta Biomater. 114, 244–255. doi:10.1016/j.actbio.2020.07.033
Jeong, Y., Jo, Y. K., Kim, B. J., Yang, B., Joo, K. I., and Cha, H. J. (2018). Sprayable adhesive nanotherapeutics: mussel-protein-based nanoparticles for highly efficient locoregional cancer therapy. ACS Nano 12 (9), 8909–8919. doi:10.1021/acsnano.8b04533
Jeong, Y. S., Yang, B., Yang, B., Shin, M., Seong, J., Cha, H. J., et al. (2020). Enhanced production of Dopa-incorporated mussel adhesive protein using engineered translational machineries. Biotechnol. Bioeng. 117 (7), 1961–1969. doi:10.1002/bit.27339
Jeong, Y., Shim, Y. S., Jo, Y. K., and Cha, H. J. (2025). Redox-activatable inhalable mucoadhesive proteinic nanotherapeutics for targeted treatment of lung cancer. Biomaterials 316, 123004. doi:10.1016/j.biomaterials.2024.123004
Jiang, P. L., Hou, R. Q., Chen, C. D., Sun, L., Dong, S. G., Pan, J. S., et al. (2016). Controllable degradation of medical magnesium by electrodeposited composite films of mussel adhesive protein (Mefp-1) and chitosan. J. Colloid Interface Sci. 478, 246–255. doi:10.1016/j.jcis.2016.06.001
Jiang, P., Dai, Y., Hou, Y., Stein, J., Lin, S. S., Zhou, C., et al. (2025). Artificial intelligence-assisted design, synthesis and analysis of smart biomaterials. BMEMat.n/a (n/a), e70004. doi:10.1002/bmm2.70004
Jiao, X. F., Pu, L., Lan, S., Li, H., Zeng, L., Wang, H., et al. (2024). Adverse drug reaction signal detection methods in spontaneous reporting system: a systematic review. Pharmacoepidemiol Drug Saf. 33 (3), e5768. doi:10.1002/pds.5768
Jo, Y. K., Choi, B. H., Kim, C. S., and Cha, H. J. (2017). Diatom-inspired silica nanostructure coatings with controllable microroughness using an engineered mussel protein glue to accelerate bone growth on titanium-based implants. Adv. Mater. 29 (46), 1704906. doi:10.1002/adma.201704906
Jo, Y. K., Choi, B. H., Zhou, C., Jun, S. H., and Cha, H. J. (2023). Cell recognitive bioadhesive-based osteogenic barrier coating with localized delivery of bone morphogenetic protein-2 for accelerated guided bone regeneration. Bioeng. Transl. Med. 8 (3), e10493. doi:10.1002/btm2.10493
Joo, K. I., Jeong, Y., Hwang, S. M., Shin, M., Lee, J., Sharma, G., et al. (2020). Harnessing the bioresponsive adhesion of immuno-bioglue for enhanced local immune checkpoint blockade therapy. Biomaterials 263, 120380. doi:10.1016/j.biomaterials.2020.120380
Kaushik, N. K., Kaushik, N., Pardeshi, S., Sharma, J. G., Lee, S. H., and Choi, E. H. (2015). Biomedical and clinical importance of mussel-inspired polymers and materials. Mar. Drugs 13 (11), 6792–6817. doi:10.3390/md13116792
Khodve, G., Banerjee, S., Kumari, M., Ravichandiran, V., Banerjee, S., and Roy, S. (2025). Exploration of biomaterial and stem cell-based strategies for promoting neuronal regeneration and creating engineered 3D in-vitro disease models. J. Transl. Med. 23 (1), 1197. doi:10.1186/s12967-025-07266-9
Kim, H. J., Choi, B. H., Jun, S. H., and Cha, H. J. (2016). Sandcastle worm-inspired blood-resistant bone graft binder using a sticky mussel protein for augmented in vivo bone regeneration. Adv. Healthc. Mater 5 (24), 3191–3202. doi:10.1002/adhm.201601169
Kim, H. J., Hwang, B. H., Lim, S., Choi, B. H., Kang, S. H., and Cha, H. J. (2015). Mussel adhesion-employed water-immiscible fluid bioadhesive for urinary fistula sealing. Biomaterials 72, 104–111. doi:10.1016/j.biomaterials.2015.08.055
Kim, B. J., Cheong, H., Hwang, B. H., and Cha, H. J. (2015). Mussel-inspired protein nanoparticles containing Iron(III)-DOPA complexes for pH-Responsive drug delivery. Angew. Chem. Int. Ed. Engl. 54 (25), 7318–7322. doi:10.1002/anie.201501748
Kim, H. J., Yang, B., Park, T. Y., Lim, S., and Cha, H. J. (2017). Complex coacervates based on recombinant mussel adhesive proteins: their characterization and applications. Soft Matter 13 (42), 7704–7716. doi:10.1039/c7sm01735a
Kim, B. J., Cheong, H., Choi, E. S., Yun, S. H., Choi, B. H., Park, K. S., et al. (2017). Accelerated skin wound healing using electrospun nanofibrous mats blended with mussel adhesive protein and polycaprolactone. J. Biomed. Mater Res. A 105 (1), 218–225. doi:10.1002/jbm.a.35903
Kim, E., Dai, B., Qiao, J. B., Li, W., Fortner, J. D., and Zhang, F. (2018). Microbially synthesized repeats of mussel foot protein display enhanced underwater adhesion. ACS Appl. Mater Interfaces 10 (49), 43003–43012. doi:10.1021/acsami.8b14890
Kim, K. B., Choi, H., Jung, H. J., Oh, Y. J., Cho, C. H., Min, J. H., et al. (2019). Mussel-inspired enzyme immobilization and dual real-time compensation algorithms for durable and accurate continuous glucose monitoring. Biosens. Bioelectron. 143, 111622. doi:10.1016/j.bios.2019.111622
Kim, E., Qin, X., Qiao, J. B., Zeng, Q., Fortner, J. D., and Zhang, F. (2020). Graphene oxide/mussel foot protein composites for high-strength and ultra-tough thin films. Sci. Rep. 10 (1), 19082. doi:10.1038/s41598-020-76004-6
Kim, S., Bae, G., Shin, M., Kang, E., Park, T. Y., Choi, Y. S., et al. (2021). Oriented in situ immobilization of a functional tyrosinase on microcrystalline cellulose effectively incorporates DOPA residues in bioengineered mussel adhesive protein. Biotechnol. J. 16 (12), e2100216. doi:10.1002/biot.202100216
Kim, J. E., Takanche, J. S., Jang, S., and Yi, H. K. (2021). Mussel adhesive protein blended with gelatin loaded into nanotube titanium dental implants enhances osseointegration. Drug Deliv. Transl. Res. 11 (3), 956–965. doi:10.1007/s13346-020-00807-3
Kim, H. J., Pyun, J. H., Park, T. Y., Yoon, S. G., Maeng, S. W., Choi, H. S., et al. (2021). Preclinical evaluation of a regenerative immiscible bioglue for vesico-vaginal fistula. Acta Biomater. 125, 183–196. doi:10.1016/j.actbio.2021.02.037
Kim, E., Jeon, J., Zhu, Y., Hoppe, E. D., Jun, Y. S., Genin, G. M., et al. (2021). A biosynthetic hybrid spidroin-amyloid-mussel foot protein for underwater adhesion on diverse surfaces. ACS Appl. Mater Interfaces 13 (41), 48457–48468. doi:10.1021/acsami.1c14182
Kim, D. Y., Oh, Y. B., Park, J. S., Min, Y. H., and Park, M. C. (2024). Anti-microbial activities of mussel-derived recombinant proteins against gram-negative bacteria. Antibiot. (Basel) 13 (3), 239. doi:10.3390/antibiotics13030239
Kim, S., Kim, N. H., Khaleel, Z. H., Sa, D. H., Choi, D., Ga, S., et al. (2024). Mussel-inspired recombinant adhesive protein-based functionalization for consistent and effective antimicrobial treatment in chronic inflammatory skin diseases. Adv. Ther. 7 (4), 2300353. doi:10.1002/adtp.202300353
Kwan, J. C., Dondani, J., Iyer, J., Muaddi, H. A., Nguyen, T. T., and Tran, S. D. (2023). Biomimicry and 3D-Printing of mussel adhesive proteins for regeneration of the periodontium—A review. Biomimetics 8 (1), 78. doi:10.3390/biomimetics8010078
Lee, Y. B., Shin, Y. M., Kim, E. M., Lee, J. Y., Lim, J., Kwon, S. K., et al. (2016). Mussel adhesive protein inspired coatings on temperature-responsive hydrogels for cell sheet engineering. J. Mater Chem. B 4 (36), 6012–6022. doi:10.1039/c6tb01057a
Lee, K. C., Wambier, C. G., Soon, S. L., Sterling, J. B., Landau, M., Rullan, P., et al. (2019). Basic chemical peeling: superficial and medium-depth peels. J. Am. Acad. Dermatol 81 (2), 313–324. doi:10.1016/j.jaad.2018.10.079
Lee, M. S., Jeon, J., Park, S., Lim, J., and Yang, H. S. (2023). Rationally designed bioactive milk-derived protein scaffolds enhanced new bone formation. Bioact. Mater 20, 368–380. doi:10.1016/j.bioactmat.2022.05.028
Lee, J., Kim, E., Kim, K. J., Rhie, J. W., Joo, K. I., and Cha, H. J. (2024). Protective topical dual-sided nanofibrous hemostatic dressing using mussel and silk proteins with multifunctionality of hemostasis and anti-bacterial infiltration. Small 20 (18), e2308833. doi:10.1002/smll.202308833
Li, L., and Zeng, H. (2016). Marine mussel adhesion and bio-inspired wet adhesives. Biotribology 5, 44–51. doi:10.1016/j.biotri.2015.09.004
Li, J., Zhang, Z., Wang, L., Jiang, L., Qin, Z., Zhao, Y., et al. (2021). Maresin 1 attenuates lipopolysaccharide-induced acute kidney injury via inhibiting NOX4/ROS/NF-κB pathway. Front. Pharmacol. 12, 782660. doi:10.3389/fphar.2021.782660
Li, Z., Wang, X., Tasich, K., Hike, D., Schumacher, J. G., Zhou, Q., et al. (2025). Eupatilin unveiled: an in-depth exploration of research advancements and clinical therapeutic prospects. J. Transl. Intern. Med. 13 (2), 104–117. doi:10.1515/jtim-2025-0016
Li, X., Luo, Z., Hu, T., Ou, D., Guo, R., Li, C., et al. (2025). Exploring the anti-inflammatory effects of total flavonoids from L. gracile on LPS-induced inflammation: an integrated approach combining network pharmacology, molecular docking, and experimental validation. Int. Immunopharmacol. 161, 115027. doi:10.1016/j.intimp.2025.115027
Liang, C., Ye, Z., Xue, B., Zeng, L., Wu, W., Zhong, C., et al. (2018). Self-assembled nanofibers for strong underwater adhesion: the trick of barnacles. ACS Appl. Mater Interfaces 10 (30), 25017–25025. doi:10.1021/acsami.8b04752
Lim, S., Park, T. Y., Jeon, E. Y., Joo, K. I., and Cha, H. J. (2021). Double-layered adhesive microneedle bandage based on biofunctionalized mussel protein for cardiac tissue regeneration. Biomaterials 278, 121171. doi:10.1016/j.biomaterials.2021.121171
Lin, Q., Gourdon, D., Sun, C., Holten-Andersen, N., Anderson, T. H., Waite, J. H., et al. (2007). Adhesion mechanisms of the mussel foot proteins mfp-1 and mfp-3. Proc. Natl. Acad. Sci. U. S. A. 104 (10), 3782–3786. doi:10.1073/pnas.0607852104
Liu, H. J., and Liu, B. (2018). Inhibition of MicroRNA-23 contributes to the isoflurane-mediated cardioprotection against oxidative stress. Cardiovasc Toxicol. 18 (5), 450–458. doi:10.1007/s12012-018-9455-1
Liu, Z., Jiang, M., Zhao, J., Wang, Q., Zhang, C., Gao, M., et al. (2020). Efficacy of a wound-dressing biomaterial on prevention of postinflammatory hyperpigmentation after suction blister epidermal grafting in stable vitiligo patients: a controlled assessor-blinded clinical study with in vitro bioactivity investigation. Arch. Dermatol Res. 312 (9), 635–645. doi:10.1007/s00403-020-02049-2
Liu, Y., Zheng, J., Zhang, X., Du, Y., Li, K., Yu, G., et al. (2021). Mussel-inspired and aromatic disulfide-mediated polyurea-urethane with rapid self-healing performance and water-resistance. J. Colloid Interface Sci. 593, 105–115. doi:10.1016/j.jcis.2021.03.003
Liu, Y., Li, K., Tian, J., Gao, A., Tian, L., Su, H., et al. (2023). Synthesis of robust underwater glues from common proteins via unfolding-aggregating strategy. Nat. Commun. 14 (1), 5145. doi:10.1038/s41467-023-40856-z
Liu, J., Cabral, H., and Mi, P. (2024). Nanocarriers address intracellular barriers for efficient drug delivery, overcoming drug resistance, subcellular targeting and controlled release. Adv. Drug Deliv. Rev. 207, 115239. doi:10.1016/j.addr.2024.115239
Lu, Q., Danner, E., Waite, J. H., Israelachvili, J. N., Zeng, H., and Hwang, D. S. (2013). Adhesion of mussel foot proteins to different substrate surfaces. J. R. Soc. Interface 10 (79), 20120759. doi:10.1098/rsif.2012.0759
Luo, Y., Nan, M., Dong, R., Jin, Q., Yuan, J., Zhi, J., et al. (2024). Rosacea treatment with mussel adhesive protein delivered via microneedling: in vivo and clinical studies. J. Cosmet. Dermatol 23 (5), 1654–1662. doi:10.1111/jocd.16190
Maeng, S. W., Park, T. Y., Min, J. S., Jin, L., Joo, K. I., Park, W. C., et al. (2021). Sutureless transplantation of amniotic membrane using a visible light-curable protein bioadhesive for ocular surface reconstruction. Adv. Healthc. Mater 10 (13), e2100100. doi:10.1002/adhm.202100100
Madhurakkat Perikamana, S. K., Lee, J., Lee, Y. B., Shin, Y. M., Lee, E. J., Mikos, A. G., et al. (2015). Materials from mussel-inspired chemistry for cell and tissue engineering applications. Biomacromolecules 16 (9), 2541–2555. doi:10.1021/acs.biomac.5b00852
Meng, H., Liu, Y., and Lee, B. P. (2017). Model polymer system for investigating the generation of hydrogen peroxide and its biological responses during the crosslinking of mussel adhesive moiety. Acta Biomater. 48, 144–156. doi:10.1016/j.actbio.2016.10.016
M’barek, L., Jin, A., Pan, Y., Li, S., Li, H., Xiong, Y., et al. (2025). A similar effect of fibrinogen on efficacy and safety of tenecteplase versus alteplase in acute ischemic cerebrovascular events (TRACE II) trial. J. Transl. Intern. Med. 13 (3), 295–303. doi:10.1515/jtim-2025-0010
Narayanan, A., Menefee, J. R., Liu, Q., Dhinojwala, A., and Joy, A. (2020). Lower critical solution temperature-driven self-coacervation of nonionic polyester underwater adhesives. ACS Nano 14 (7), 8359–8367. doi:10.1021/acsnano.0c02396
Ni, P., Chen, Y., Wan, K., Cheng, Y., Fang, Y., Weng, Y., et al. (2024). Mussel foot protein-inspired adhesive tapes with tunable underwater adhesion. ACS Appl. Mater Interfaces 16 (34), 45550–45562. doi:10.1021/acsami.4c09709
North, M. A., Del Grosso, C. A., and Wilker, J. J. (2017). High strength underwater bonding with polymer mimics of mussel adhesive proteins. ACS Appl. Mater Interfaces 9 (8), 7866–7872. doi:10.1021/acsami.7b00270
Omidian, H., and Wilson, R. L. (2024). Polydopamine applications in biomedicine and environmental science. Mater. (Basel) 17 (16), 3916. doi:10.3390/ma17163916
Ou, X., Xue, B., Lao, Y., Wutthinitikornkit, Y., Tian, R., Zou, A., et al. (2020). Structure and sequence features of mussel adhesive protein lead to its salt-tolerant adhesion ability. Sci. Adv. 6 (39), eabb7620. doi:10.1126/sciadv.abb7620
Park, B. M., Luo, J., and Sun, F. (2019). Enzymatic assembly of adhesive molecular networks with sequence-dependent mechanical properties inspired by mussel foot proteins11Electronic supplementary information (ESI) available: materials and methods, amino acid sequences, raw rheological data. Polym. Chem. 10 (7), 823–826. doi:10.1039/c8py01581c
Park, T. Y., Oh, J. M., Cho, J. S., Sim, S. B., Lee, J., and Cha, H. J. (2020). Stem cell-loaded adhesive immiscible liquid for regeneration of myocardial infarction. J. Control Release 321, 602–615. doi:10.1016/j.jconrel.2020.02.047
Park, T. Y., Maeng, S. W., Jeon, E. Y., Joo, K. I., and Cha, H. J. (2021). Adhesive protein-based angiogenesis-mimicking spatiotemporal sequential release of angiogenic factors for functional regenerative medicine. Biomaterials 272, 120774. doi:10.1016/j.biomaterials.2021.120774
Petrone, L., Kumar, A., Sutanto, C. N., Patil, N. J., Kannan, S., Palaniappan, A., et al. (2015). Mussel adhesion is dictated by time-regulated secretion and molecular conformation of mussel adhesive proteins. Nat. Commun. 6, 8737. doi:10.1038/ncomms9737
Pilakka, V. A., Nakashima, K., Shiga, H., Sato, T., Godigamuwa, K., Hiroyoshi, N., et al. (2023). Functional modification of mussel adhesive protein to control solubility and adhesion property. J. Biosci. Bioeng. 136 (2), 87–93. doi:10.1016/j.jbiosc.2023.05.002
Priemel, T., Palia, R., Babych, M., Thibodeaux, C. J., Bourgault, S., and Harrington, M. J. (2020). Compartmentalized processing of catechols during mussel byssus fabrication determines the destiny of DOPA. Proc. Natl. Acad. Sci. U. S. A. 117 (14), 7613–7621. doi:10.1073/pnas.1919712117
Quan, W. Y., Hu, Z., Liu, H. Z., Ouyang, Q. Q., Zhang, D. Y., Li, S. D., et al. (2019). Mussel-inspired catechol-functionalized hydrogels and their medical applications. Molecules 24 (14), 2586. doi:10.3390/molecules24142586
Rees, D. J., Hanifi, A., Obille, A., Alexander, R., and Sone, E. D. (2019). Fingerprinting of Proteins that Mediate Quagga Mussel Adhesion using a de novo Assembled Foot Transcriptome. Sci. Rep. 9 (1), 6305. doi:10.1038/s41598-019-41976-7
Santonocito, R., Venturella, F., Dal Piaz, F., Morando, M. A., Provenzano, A., Rao, E., et al. (2019). Recombinant mussel protein Pvfp-5β: a potential tissue bioadhesive. J. Biol. Chem. 294 (34), 12826–12835. doi:10.1074/jbc.RA119.009531
Saraf, M., Prateek, R. R., Balasubramaniam, B., Thakur, V. K., and Gupta, R. K. (2024). Polydopamine-enabled biomimetic surface engineering of materials: new insights and promising applications. Adv. Mater. Interfaces 11 (6), 2300670. doi:10.1002/admi.202300670
Shi, M., Guo, F., Liao, D., Huang, R., Feng, Y., Zeng, X., et al. (2020). Pharmacological inhibition of fatty acid-binding protein 4 alleviated kidney inflammation and fibrosis in hyperuricemic nephropathy. Eur. J. Pharmacol. 887, 173570. doi:10.1016/j.ejphar.2020.173570
Shin, M., Yoon, T., Yang, B., and Cha, H. J. (2022). Thiol-rich fp-6 controls the tautomer equilibrium of oxidized dopa in interfacial mussel foot proteins. Langmuir 38 (11), 3446–3452. doi:10.1021/acs.langmuir.1c03239
Song, W. K., Kang, J. H., Cha, J. K., Lee, J. S., Paik, J. W., Jung, U. W., et al. (2018). Biomimetic characteristics of mussel adhesive protein-loaded collagen membrane in guided bone regeneration of rabbit calvarial defects. J. Periodontal Implant Sci. 48 (5), 305–316. doi:10.5051/jpis.2018.48.5.305
Sun, J., Su, J., Ma, C., Göstl, R., Herrmann, A., Liu, K., et al. (2020). Fabrication and mechanical properties of engineered protein-based adhesives and fibers. Adv. Mater. 32 (6), e1906360. doi:10.1002/adma.201906360
Sun, X., Lin, Y., Zhong, X., Fan, C., Liu, Z., Chen, X., et al. (2024). Alendronate-functionalized polymeric micelles target icaritin to bone for mitigating osteoporosis in a rat model. J. Control. Release 376, 37–51. doi:10.1016/j.jconrel.2024.10.002
Talebian, S., Shim, I. K., Kim, S. C., Spinks, G. M., Vine, K. L., and Foroughi, J. (2020). Coaxial mussel-inspired biofibers: making of a robust and efficacious depot for cancer drug delivery. J. Mater Chem. B 8 (23), 5064–5079. doi:10.1039/d0tb00052c
Valois, E., Mirshafian, R., and Waite, J. H. (2020). Phase-dependent redox insulation in mussel adhesion. Sci. Adv. 6 (23), eaaz6486. doi:10.1126/sciadv.aaz6486
Waite, J. H. (2017). Mussel adhesion - essential footwork. J. Exp. Biol. 220 (Pt 4), 517–530. doi:10.1242/jeb.134056
Wang, K., Ji, L., and Hua, Z. (2017). Functional peptides from Laminin-1 improve the cell adhesion capacity of recombinant mussel adhesive protein. Protein Pept. Lett. 24 (4), 348–352. doi:10.2174/0929866524666170123142225
Wang, Y., Lan, H., Yin, T., Zhang, X., Huang, J., Fu, H., et al. (2020). Covalent immobilization of biomolecules on stent materials through mussel adhesive protein coating to form biofunctional films. Mater Sci. Eng. C Mater Biol. Appl. 106, 110187. doi:10.1016/j.msec.2019.110187
Wang, Y., An, J., Zhou, J., Chang, L., Zhang, Q., and Peng, F. (2024). Hydroxysafflor yellow A: a natural pigment with potential anticancer therapeutic effect. Front. Pharmacol. 15, 1495393. doi:10.3389/fphar.2024.1495393
Wang, Z., Liu, J., Zheng, Y., Zhang, B., Hu, Y., Wu, Y., et al. (2024). Copper ion-inspired dual controllable drug release hydrogels for wound management: driven by hydrogen bonds. Small 20 (34), e2401152. doi:10.1002/smll.202401152
Wei, W., Petrone, L., Tan, Y., Cai, H., Israelachvili, J. N., Miserez, A., et al. (2016). An underwater surface-drying peptide inspired by a mussel adhesive protein. Adv. Funct. Mater 26 (20), 3496–3507. doi:10.1002/adfm.201600210
Wu, Y., Li, F., Gong, Y., Wan, X., and Zhou, L.-M. (2024). Protective effects of recombined mussel adhesive protein against AD skin inflammation in mice. Cosmetics 11 (4), 134. doi:10.3390/cosmetics11040134
Xu, S., Kang, M., Xin, X., Liang, J., Xiao, H., Lu, Y., et al. (2024). Design principles and application research of mussel-inspired materials: a review. J. Environ. Chem. Eng. 12 (1), 111655. doi:10.1016/j.jece.2023.111655
Xu, Z., Zhou, H., Li, T., Yi, Q., Thakur, A., Zhang, K., et al. (2024). Application of biomimetic nanovaccines in cancer immunotherapy: a useful strategy to help combat immunotherapy resistance. Drug Resist Updat 75, 101098. doi:10.1016/j.drup.2024.101098
Xue, R., Wang, J., Huang, W., Qiu, Y., Xu, H., Wang, R., et al. (2025a). Efficient production of recombinant hybrid mussel proteins with improved adhesion. Int. J. Biol. Macromol. 289, 139937. doi:10.1016/j.ijbiomac.2025.139937
Xue, R., Zhang, M., Zhang, C., Zhang, J. Z. H., Xu, H., Wang, R., et al. (2025b). Molecular simulations guiding recombinant mussel protein with enhanced applicable properties for adhesive materials. Int. J. Biol. Macromol. 307 (Pt 2), 141988. doi:10.1016/j.ijbiomac.2025.141988
Yang, B., Jin, S., Park, Y., Jung, Y. M., and Cha, H. J. (2018). Coacervation of interfacial adhesive proteins for initial mussel adhesion to a wet surface. Small 14 (52), e1803377. doi:10.1002/smll.201803377
Yang, D., Qiu, J., Xu, N., Zhao, Y., Li, T., Ma, Q., et al. (2018). Mussel adhesive protein fused with VE-cadherin domain specifically triggers endothelial cell adhesion. J. Mater Chem. B 6 (24), 4151–4163. doi:10.1039/c8tb00526e
Yang, Z., Zhao, X., Hao, R., Tu, Q., Tian, X., Xiao, Y., et al. (2020). Bioclickable and mussel adhesive peptide mimics for engineering vascular stent surfaces. Proc. Natl. Acad. Sci. U. S. A. 117 (28), 16127–16137. doi:10.1073/pnas.2003732117
Yang, D., Yan, W., Qiu, J., Huang, Y., Li, T., Wang, Y., et al. (2020). Mussel adhesive protein fused with VE-cadherin extracellular domain promotes endothelial-cell tight junctions and in vivo endothelization recovery of vascular stent. J. Biomed. Mater Res. B Appl. Biomater. 108 (1), 94–103. doi:10.1002/jbm.b.34369
Yang, K., Wu, Z., Zhang, K., Weir, M. D., Xu, H. H. K., Cheng, L., et al. (2024a). Unlocking the potential of stimuli-responsive biomaterials for bone regeneration. Front. Pharmacol. 15, 1437457. doi:10.3389/fphar.2024.1437457
Yang, J. W., Song, K. I., Lee, J., Park, S., Huh, H., Choi, G., et al. (2024b). A customizable proteinic bioadhesive patch with water-switchable underwater adhesiveness, adjustable biodegradability, and modifiable stretchability for healing diverse internal wounds. Adv. Mater 36 (13), e2310338. doi:10.1002/adma.202310338
Yang, J. W., Lee, J., Song, K. I., Park, D., and Cha, H. J. (2024c). Acrylated adhesive proteinic microneedle patch for local drug delivery and stable device implantation. J. Control Release 371, 193–203. doi:10.1016/j.jconrel.2024.05.038
Yang, J. W., Yoon, T., Kim, H., Joo, K. I., and Cha, H. J. (2025). Acrylated bioengineered mussel protein-based adhesive nanoparticles for locoregional and sustained drug delivery. ACS Biomater. Sci. Eng. 11 (6), 3523–3532. doi:10.1021/acsbiomaterials.5c00390
Yao, L., Wang, X., Xue, R., Xu, H., Wang, R., Zhang, L., et al. (2022). Comparative analysis of mussel foot protein 3B co-expressed with tyrosinases provides a potential adhesive biomaterial. Int. J. Biol. Macromol. 195, 229–236. doi:10.1016/j.ijbiomac.2021.11.208
Yin, D., Komasa, S., Yoshimine, S., Sekino, T., and Okazaki, J. (2019). Effect of mussel adhesive protein coating on osteogenesis in vitro and osteointegration in vivo to alkali-treated titanium with nanonetwork structures. Int. J. Nanomedicine 14, 3831–3843. doi:10.2147/IJN.S206313
Yoon, T., Shin, M., Yang, B., Kim, H. J., Lim, S., and Cha, H. J. (2025). Junctional role of anionic domain of mussel foot protein type 4 in underwater mussel adhesion. Biomacromolecules 26 (2), 1161–1170. doi:10.1021/acs.biomac.4c01506
Yu, J., Kan, Y., Rapp, M., Danner, E., Wei, W., Das, S., et al. (2013). Adaptive hydrophobic and hydrophilic interactions of mussel foot proteins with organic thin films. Proc. Natl. Acad. Sci. U. S. A. 110 (39), 15680–15685. doi:10.1073/pnas.1315015110
Yun, J., Nam, I. H., Lee, H., Jo, Y. K., Lee, H., Jun, S. H., et al. (2024). In situ photo-crosslinkable protein bioadhesive for bone graft fixation. J. Dent. Res. 103 (4), 409–418. doi:10.1177/00220345231224709
Yun, J., Woo, H. T., Lee, S., and Cha, H. J. (2025). Visible light-induced simultaneous bioactive amorphous calcium phosphate mineralization and in situ crosslinking of coacervate-based injectable underwater adhesive hydrogels for enhanced bone regeneration. Biomaterials 315, 122948. doi:10.1016/j.biomaterials.2024.122948
Zeng, Z., Wang, H., Morsi, Y., and Mo, X. (2018). Synthesis and characterization of incorporating mussel mimetic moieties into photoactive hydrogel adhesive. Colloids Surf. B Biointerfaces 161, 94–102. doi:10.1016/j.colsurfb.2017.10.041
Zhang, F., Xie, G., and Pan, J. (2017). Tunable adsorption and film formation of mussel adhesive protein by potential control. Langmuir 33 (35), 8749–8756. doi:10.1021/acs.langmuir.6b04125
Zhang, D. Y., Zheng, Y., Zhang, H., Sun, J. H., Tan, C. P., He, L., et al. (2018). Delivery of phosphorescent anticancer Iridium(III) complexes by polydopamine nanoparticles for targeted combined photothermal-chemotherapy and thermal/photoacoustic/lifetime imaging. Adv. Sci. (Weinh) 5 (10), 1800581. doi:10.1002/advs.201800581
Zhang, C., Wu, B., Zhou, Y., Zhou, F., Liu, W., and Wang, Z. (2020). Mussel-inspired hydrogels: from design principles to promising applications. Chem. Soc. Rev. 49 (11), 3605–3637. doi:10.1039/c9cs00849g
Zhao, Y., Kang, J., Cui, Y., Ji, S., Nian, R., Yu, W., et al. (2023). Mechanically tunable, antibacterial and bioactive mussel adhesive protein/hyaluronic acid coacervates as bioadhesives. Int. J. Biol. Macromol. 247, 125773. doi:10.1016/j.ijbiomac.2023.125773
Zhong, Y., Wang, J., Yuan, Z., Wang, Y., Xi, Z., Li, L., et al. (2019). A mussel-inspired carboxymethyl cellulose hydrogel with enhanced adhesiveness through enzymatic crosslinking. Colloids Surf. B Biointerfaces 179, 462–469. doi:10.1016/j.colsurfb.2019.03.044
Zou, X., Yang, X. J., Gan, Y. M., Liu, D. L., Chen, C., Duan, W., et al. (2021). Neuroprotective effect of phthalide derivative CD21 against ischemic brain injury:involvement of MSR1 mediated DAMP peroxiredoxin1 clearance and TLR4 signaling inhibition. J. Neuroimmune Pharmacol. 16 (2), 306–317. doi:10.1007/s11481-020-09911-0
Glossary
aaRSs Aminoacyl-tRNA synthetases
CMC carboxymethyl cellulose
CA cyanoacrylate
COX-2 cyclooxygenase-2
DA dopamine
ECM extracellular matrix
Fp1 foot protein 1
GAGs glycosaminoglycans
GO graphene oxide
CG guar gum
GBR guided bone regeneration
HRP horseradish peroxidase
HA hyaluronic acid
InaKC ice-nucleation protein K
imuGlue immune bio-glue
ICB immune checkpoint blockade
iNOS inducible nitric oxide synthase
LPS lipopolysaccharide
MAPs mussel adhesive proteins
PDGF platelet-derived growth factor
PNIPAM poly(N-isopropylacrylamide)
PCL polycaprolactone
PDA polydopamine
PIH post-inflammatory hyperpigmentation
rMAPs recombinant mussel adhesive proteins
SS sensitive skin
SiNPs silica nanoparticles
SF silk fibroin
TNS titanium nano-network structures
TFE trifluoroethanol
TyrRS tyrosyl-tRNA synthetase
UV ultraviolet
VEGF vascular endothelial growth factor
Keywords: mussel adhesive proteins, adhesion, biomedical applications, drug delivery system, tissue engineering
Citation: An J, Hou Z, Li J, Bi Q, Huang S, Peng C and Peng F (2025) Pharmacological effects and application prospects of mussel adhesive proteins. Front. Pharmacol. 16:1704475. doi: 10.3389/fphar.2025.1704475
Received: 13 September 2025; Accepted: 24 November 2025;
Published: 09 December 2025.
Edited by:
Yong Ho Kim, Sungkyunkwan University, Republic of KoreaReviewed by:
Aliakbar Jafari, The University of Mississippi School of Engineering, United StatesYang Wei, National Taipei University of Technology, Taiwan
Jaecheol Lee, Sungkyunkwan University, Republic of Korea
Copyright © 2025 An, Hou, Li, Bi, Huang, Peng and Peng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shile Huang, aHVhbmdzbGVAMTI2LmNvbQ==; Cheng Peng, cGVuZ2NoZW5nY2hlbmdkdUAxMjYuY29t; Fu Peng, cGVuZ2ZAc2N1LmVkdS5jbg==
 Junsha An
Junsha An Zengmiao Hou3
Zengmiao Hou3 Jiaqi Li
Jiaqi Li Cheng Peng
Cheng Peng Fu Peng
Fu Peng