- 1Department of Endocrinology and Metabology, The First Affiliated Hospital of Shandong First Medical University & Shandong Provincial Qianfoshan Hospital, Jinan, China
- 2Biomedical Sciences College (Shandong Medical Biotechnology Research Center), NHC Key Laboratory of Biotechnology Drugs, Shandong First Medical University (Shandong Academy of Medical Sciences), Jinan, China
- 3College of Traditional Chinese Medicine, Shandong University of Traditional Chinese Medicine, Jinan, China
Gypenosides (Gyps), a group of dammarane triterpene saponins that are primarily from Gynostemma pentaphyllum, have been identified as promising natural compounds with a diverse array of potent pharmacological activities. In the past 2 decades, a growing body of evidence has demonstrated that Gyps are crucial for the regulation of metabolic homeostasis, the reduction of oxidative stress and inflammation, the protection of the cardiovascular and hepatic systems, and the exhibition of anti-cancer potential. However, obstacles such as limited oral bioavailability, a lack of standardized extracts, and insufficient clinical data restrict the translational potential of Gyps. Recent developments in the pharmacological effects of Gyps, such as the biological characteristics of Gynostemma pentaphyllum and the pharmacokinetic and toxicological properties of Gyps, are summarized in this review. We examine the current research limitations and prospective directions for Gyps as potential therapeutic drugs or functional supplements.
1 Introduction
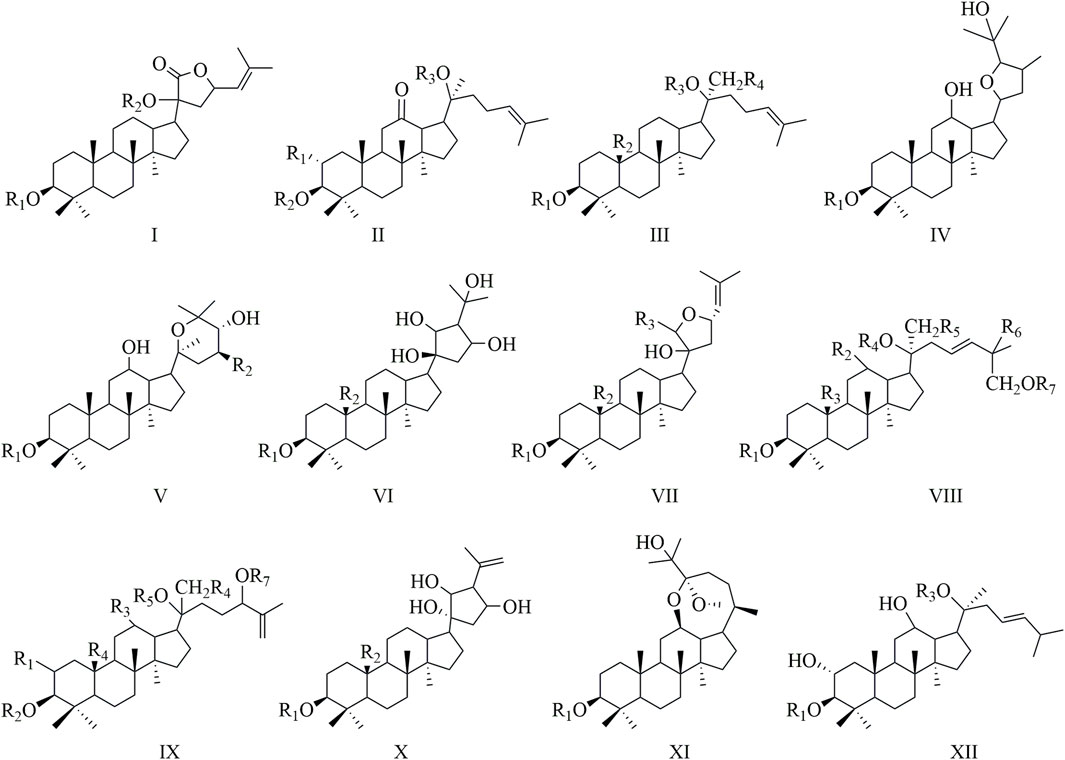
Gynostemma pentaphyllum (Thunb.) Makino, or Jiao-Gu-Lan, belongs to the Cucurbitaceae family and has been traditionally employed in East Asian medical practices. The plant’s remarkable adaptability and health advantages have led to its widespread cultivation throughout various regions of China (Liang et al., 2025). The plant contains a diverse array of bioactive metabolites, such as saponins, flavonoids, terpenes, and polysaccharides (Wang et al., 2020b; Chen et al., 2023). The primary active metabolites, referred to as Gyps, are noteworthy for their substantial pharmacological properties, encompassing antioxidant, anti-inflammatory, anticancer, cardiovascular-protecting, and immunomodulatory effects. This highlights their significant therapeutic potential (Pang et al., 2017; Qi et al., 2021; Weng et al., 2021). A taxonomic categorization of Gyps has been conducted, resulting in the identification of 12 separate categories based on their unique structural features (Zheng et al., 2018) (Figure 1). To date, more than 300 distinct Gyps have been found and characterized by Gynostemma pentaphyllum (Nguyen et al., 2021).
This review aims to summarize and evaluate recent advancements in research on the therapeutic potential of Gyps, emphasizing their roles in antioxidant, anti-inflammatory, anticancer, and cardiovascular protective mechanisms, among others. Numerous studies have demonstrated that Gyps exhibit significant antioxidant capabilities (Liu et al., 2025). Gyps effectively alleviate oxidative damage by regulating oxidative stress responses and inhibiting the nuclear factor kappa-B (NF-κB) signaling pathway. In vitro studies indicate that Gyps enhance the expression of intracellular antioxidant enzymes by upregulating the nuclear factor erythroid 2-related factor 2 (Nrf2) signaling pathway, leading to efficient free radical scavenging and diminished cellular damage (Chen et al., 2022; Ping et al., 2024). Considerable emphasis has been directed towards the therapeutic potential of Gyps in oncological treatment; evidence indicates that Gyps induce apoptosis in cancer cells while inflicting minimal harm to normal cells (Xiao et al., 2024). Furthermore, investigations utilizing animal models have shown that Gyps significantly mitigate symptoms associated with inflammation (Wang et al., 2017b).
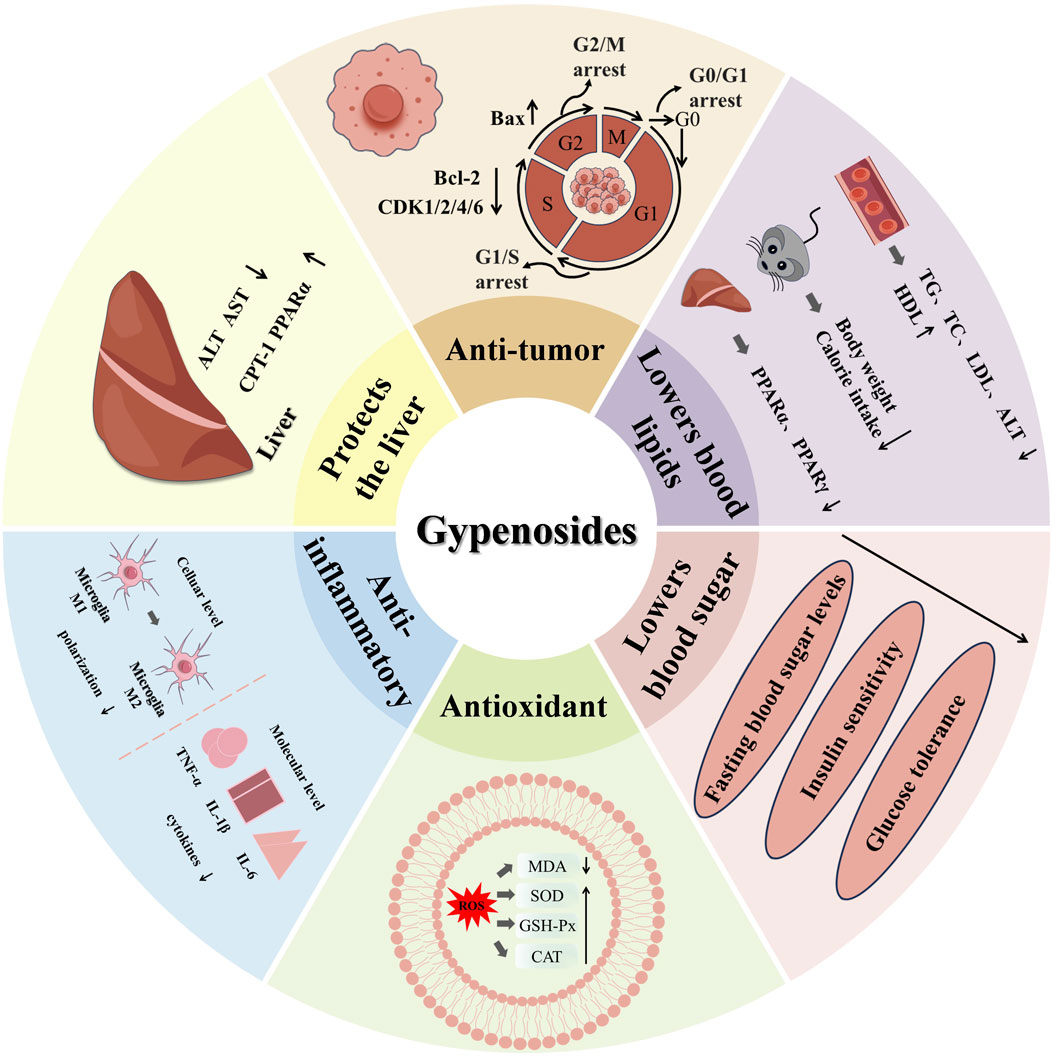
Gyps have demonstrated significant cardioprotective qualities in the context of cardiovascular diseases. Gyps are crucial in the prevention and treatment of several cardiovascular diseases by modulating lipid metabolism, reducing blood pressure, enhancing endothelial function, and preventing atherosclerosis. Studies utilizing animal models have demonstrated that prolonged administration of Gyps extract significantly reduces serum cholesterol levels, improves cardiac function, and lowers the incidence of atherosclerosis (Gao et al., 2022). As well, Gyps have been documented to possess hepatoprotective (Song et al., 2017), neuroprotective (Xing et al., 2020), antiviral (Zhao et al., 2022), and other functions (Figure 2).
However, human clinical trials on pure Gyps are extremely limited. A 12-week study in 80 Korean subjects showed that daily supplementation with 450 mg of heat-processed Gynostemma pentaphyllum extract significantly reduced abdominal fat area, body weight, and percentage body fat without adverse effects (Park et al., 2014). Similarly, a 4-week trial (450 mg/day) in 16 healthy men found that Gynostemma pentaphyllum significantly enhanced exercise performance by activating the AMPK pathway (Nayyar et al., 2023). In terms of mental health, anxiety symptoms and stress hormone levels were improved in 72 chronically stressed adults after an 8-week intervention with Gynostemma pentaphyllum 400 mg/d (Choi et al., 2019). In addition, two separate studies on 100 adults confirmed the anti-fatigue (Ahn et al., 2023) and hair health-promoting effects (Lee et al., 2025) of Gynostemma pentaphyllum extract. The small sample size, short research period, and single population continue to limit the application potential of the existing evidence. More rigorous large-scale clinical trials are needed to verify it in the future.
Gyps are natural herbal metabolites of considerable scientific significance and exceptional developmental potential. This review summarizes recent advancements in the pharmacological characteristics, pharmacokinetics, and toxicological profiles of Gyps, as well as the biological qualities of Gynostemma pentaphyllum. It provides a comprehensive examination of the antioxidant mechanisms of Gyps, explores their potential therapeutic uses, and aims to establish a scientific foundation for the development of novel therapeutic strategies.
2 Biological characteristics of Gynostemma pentaphyllum
2.1 Botanical characteristics of Gynostemma pentaphyllum
Gynostemma pentaphyllum is a herbaceous vine belonging to the Cucurbitaceae family. The slender, branching stems are sulcate and bear leaves usually consisting of five to seven leaflets (range: 3–9). The core leaflet measures 3–12 cm in length and 1.5–4 cm in width, whilst the lateral leaflets are relatively smaller. The species is dioecious; male flowers are organized in a slender, extensively branching panicle, sometimes pubescent at the base and occasionally featuring tiny leaflets. Flowers possess a slender pedicel of 1–4 mm in length. The fruit is a sticky, globose berry that is 5–6 mm across and turns black when it's ready. Its surface is smooth and hairless. Each fruit comprises two inverted seeds that are ovate-cordate, roughly 4 mm in width, featuring a blunt apex, cordate base, flattened profile, and two papillate projections. Flowering transpires from March to November, whereas fruiting occurs from April to December (Yang et al., 2019b).
2.2 Bioactive metabolites
Gynostemma pentaphyllum comprises various bioactive metabolites, including saponins, polysaccharides, and flavonoids. Saponins are the most extensively studied metabolites, demonstrating a wide range of pharmacological activities (Su et al., 2021). Gyps were categorized chemically using cucurbitane-type triterpene skeletons. Its fundamental structure is the cucurbitane core of C-20β-H and C-21-CH3, which undergoes C-20 hydroxylation and C-3 glycosylation to produce various saponins (Gypenoside VII, for instance, is 3-O-β-D-glucose). Gypenoside XVII (C-20β-OH), for instance, is the most prevalent active metabolite in Gynostemma pentaphyllum, and the antioxidant activity is strongly correlated with its backbone structure. Gypenoside III, IV, VIII, and XII exhibit structural analogies to ginsenosides Rb1, Rb3, Rd, and F2, respectively. This significant chemical similarity indicates the possibility of comparable pharmacological effects (Zhang et al., 2021c; Chen et al., 2023). Ginsenoside, for instance, has been extensively investigated in the context of cognitive function enhancement and anti-aging, with a particular emphasis on the regulation of the NMDA receptor and SIRT1 pathway (Lou et al., 2021; Chu et al., 2025). In clinical practice, Rg3, a representative metabolite of ginsenoside, has been employed as a tumor adjuvant drug (Li et al., 2016b).
Polysaccharides constitute a significant category of bioactive metabolites. Research shows that Gynostemma polysaccharides (GPP) are primarily heteropolysaccharides made up of different monosaccharides, with galactose identified as the most prevalent unit (Ji et al., 2018; Wang et al., 2019). Flavonoids, primarily in the form of flavonols and their glycosidic derivatives, represent a notable category of phytochemicals in this plant (Zhao et al., 2024). Additionally, trace amounts of sterols and organic acids have been identified (Li et al., 2016a).
2.3 Nomenclature of Gynostemma pentaphyllum
Gynostemma pentaphyllum is a traditional Chinese medicinal botanical drug obtained mostly from the dried aerial parts of the plant. This plant is widespread in China, South Korea, Japan, and other Southeast Asian areas and is considered a form of “South Asian ginseng” in China (Zhang et al., 2022). The leaves, roots, and stems of Gynostemma pentaphyllum are utilized as botanical drugs and in the production of various food items, such as tea, drinks, and biscuits; they are extensively incorporated into daily life (Wang et al., 2020b). Comprehensive pharmacodynamic research demonstrates that Gynostemma pentaphyllum possesses various advantageous pharmacological effects, including hypoglycemic, hypolipidemic, anti-cancer, anti-inflammatory, cardioprotective, and neuroprotective capabilities (He et al., 2019; Li et al., 2023d; Jo et al., 2024).
2.4 Extraction methods and quality control
Conventional techniques employed to extract the active metabolites of Gynostemma pentaphyllum encompass solvent-comparison extraction, ultrasonic-assisted extraction, microwave-assisted enzymatic extraction, and microwave-ultrasonic dual-assisted extraction (Ji et al., 2018; Li et al., 2022a). Methods for the qualitative or quantitative assessment of Gyps encompass thin-layer chromatography, UV spectrophotometry, and high-performance liquid chromatography, among others (Li et al., 2023b).
Gynostemma pentaphyllum has not been incorporated into the national pharmacopoeia but is cited in particular regional standards. In 2021, Wen et al. (Xiuping et al., 2021) utilized the testing procedures specified in the general principles of the Chinese Pharmacopoeia (2020 Edition) to assess the quality of Gynostemma pentaphyllum. The stipulated parameters mandate that the water content must not surpass 12.0%, the total ash content must not exceed 16.0%, the acid-insoluble ash must remain under 3.0%, and the water-soluble extract content, as ascertained by the hot immersion method, must be at least 25.0%.
3 Pharmacological activity of gypenosides
3.1 Antioxidant activity
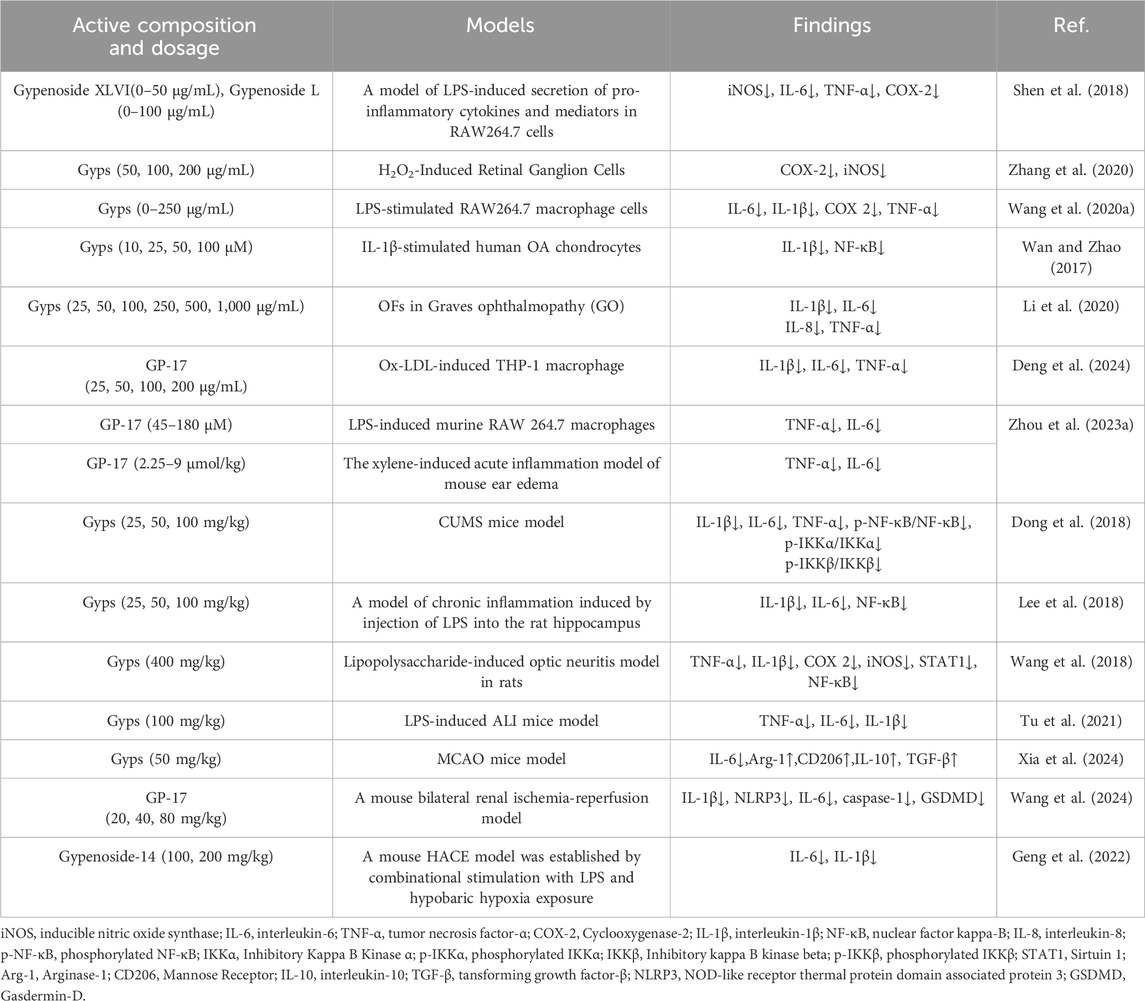
Reactive oxygen species (ROS) are generated by cellular metabolism and external influences. An imbalance between ROS formation and antioxidant defense results in oxidative stress (Hajam et al., 2022). Current research indicates substantial antioxidant capabilities in Gyps. Tables 1, 2 elucidate this activity as recognized at the cellular and organismal levels. Gypenoside XLIX, a principal active metabolite, mitigates oxidative damage caused by several sources. The method entails the suppression of ROS levels while enhancing catalase (CAT), glutathione (GSH), and total antioxidant capacity (T-AOC) (Gao et al., 2022; Xu et al., 2024). Gypenoside XVII (GP-17) similarly provides a protective effect against oxidative damage by decreasing malondialdehyde (MDA) levels in the blood and augmenting the activity of antioxidant enzymes such as superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and CAT (Yang et al., 2017). Furthermore, in murine asthma models, gypenoside A markedly increases diminished GSH levels while simultaneously decreasing MDA levels, so validating its effectiveness as an antioxidant (Huang et al., 2022a). In rats with atherosclerosis produced by high-fat milk and vitamin D3, the monascus-gypenoside mixture dramatically enhances peroxisome proliferator-activated receptor α and carnitine palmitoyltransferase 1. Consequently, research indicates elevated activities of SOD and CAT in hepatic tissue, accompanied by reduced serum levels of ROS and MDA. A comparative analysis indicates that HG exhibits more antioxidant capacity and improved anti-atherosclerotic efficiency compared to simvastatin (Gou et al., 2018). Additionally, the research indicates that Gyps markedly enhances Nrf2 protein levels, thereby augmenting the downstream expression of heme oxygenase-1 (HO-1) (Ying et al., 2018). These findings indicate that Gyps may confer protection against radiation-induced oxidative damage through the modulation of the Nrf2 antioxidant signaling pathway.
Gyps are vital in combating cellular oxidative stress, especially in ocular cells, where oxidative stress is a major contributor to retinopathy pathogenesis. Gyps has been shown to greatly reduce oxidative stress generated by hydrogen peroxide (H2O2) in orbital fibroblasts. This reduction is associated with decreased expression of apoptosis-related mRNA and autophagy activation proteins, as well as increased Nrf2/ERK/HO-1 pathway proteins and SOD (Ma et al., 2022). Moreover, Alhasani et al. (2018) discovered that Gyps protects human retinal pigment epithelial cells (RPE) against oxidative damage, which is associated with Nrf2 pathway activation. This method may provide treatment pathways for retinal disorders. Gyps increases antioxidant capacity by increasing Nrf2/ARE and HO-1 expression, inhibits inflammation by downregulating iNOS and COX-2, and reduces apoptosis via the endogenous mitochondrial pathway, protecting retinal ganglion cells (RGCs) from H2O2-induced damage (Zhang et al., 2020). Recent research shows Gyps protects osteoblasts from oxidative damage caused by H2O2, in addition to protecting ocular cells. This effect is due to NOX4 downregulation and involves the NOX/BMP/Smad signaling pathway (Yanping et al., 2020). Gyps also decrease oxidative stress generated by oxidized low-density lipoprotein (oxLDL) in RPE cells (Biswas et al., 2020). In particular, GP-17 inhibits ROS and MDA formation while increasing SOD, GSH-Px, and CAT levels in oxLDL-injured human umbilical vein endothelial cells (HUVECs). This chemical activates the ERα-mediated PI3K/Akt pathway, increasing Nrf2 and HO-1 levels and enhancing antioxidant enzymes. OxLDL may reduce HUVEC death by lowering the Bax/Bcl-2 ratio and controlling activated caspase-3—mechanisms previously implicated in retinopathy research (Yang et al., 2017). In addition, Li et al. (Alhasani et al., 2020; Li et al., 2021) discovered that Gyps therapy reduces ROS production, upregulates antioxidant genes (SOD1, SOD2, GPx1, GCLM, NQO1, Nrf2), and increases antioxidant enzyme activity and GSH levels in rpgrip1 mutant zebrafish.
While current research has robustly established that Gyps and its monomers exhibit extensive antioxidant properties in cellular and animal models and has preliminarily indicated that Gyps function via critical pathways like Nrf2, significant issues persist in preclinical investigations, including inadequate mechanistic exploration, reliance on singular experimental models, and a deficiency in comprehensive pharmacodynamic and safety assessments. Figure 3 illustrates the pharmacological mechanism of Gyps antioxidant.
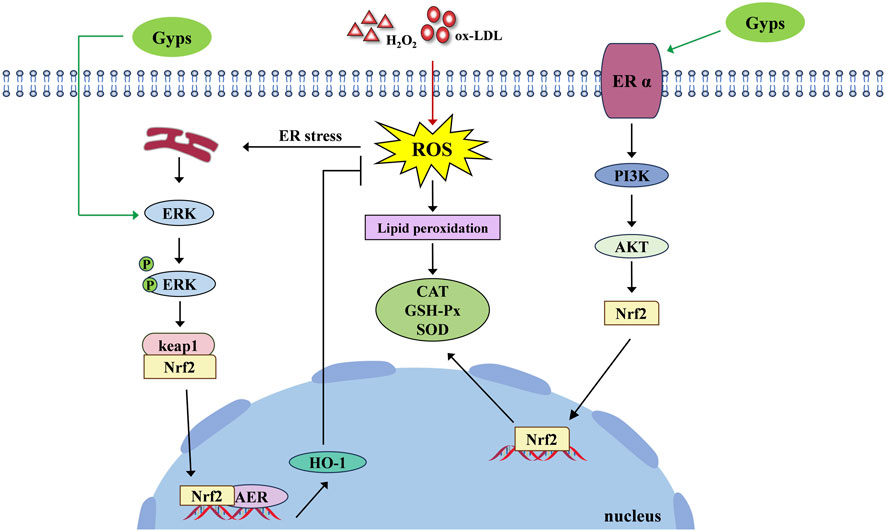
Figure 3. Pharmacological mechanism of the antioxidant activity of Gyps. ER α, estrogen receptor alpha; PI3K, phosphoinositide 3-kinases; AKT, protein kinase B.
3.2 Hypoglycemic activity
Diabetes is among the most prevalent and serious chronic diseases worldwide, characterized by a complex pathogenesis (Chou et al., 2023). GP-75, a natural PPARγ agonist, demonstrates pleiotropic antidiabetic effects. In db/db mice models, it significantly reduces fasting blood glucose through time- and dose-dependent mechanisms while enhancing glucose tolerance, insulin sensitivity, and lipid metabolism (Meng et al., 2022). Mechanistic studies reveal that GP-75 activates the PPARγ/Akt/GLUT4 signaling pathway, which enhances cerebral glucose uptake. This action concurrently improves glycemic control (reduced HbA1c, normalized insulin levels) and reverses cognitive impairment (Meng et al., 2023). Regarding pancreatic pathology, gypenoside A ameliorates high-fat diet (HFD)-induced β-cell dysfunction by suppressing miR-150-3p expression, augmenting insulin production, and inhibiting β-cell apoptosis (Li, 2022). Combination therapy employing low-dose berberine, Gyps, and biphenyl diester (BGB) demonstrates synergistic efficacy in mitigating hyperglycemia in T2DM murine models (Zhang et al., 2021a). Gyps attenuate diabetic cardiomyopathy progression by inhibiting ROS-mediated NLRP3 inflammasome activation (Zhang et al., 2018). Collectively, preclinical evidence establishes Gyps as multi-target therapeutic agents that regulate glucose homeostasis, preserve pancreatic function, and prevent diabetic complications. Figure 4 schematically summarizes these hypoglycemic mechanisms.
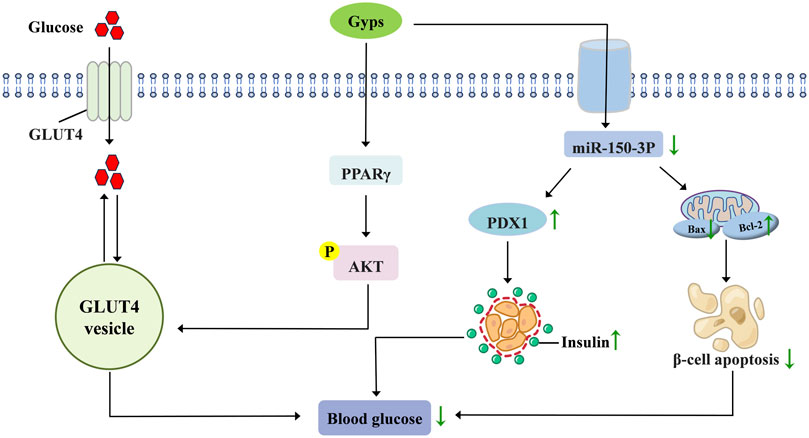
Figure 4. Pharmacological mechanisms of Gyps in lowering blood glucose. GLUT4, glucose transporter type 4; PPARγ, peroxisome proliferator-activated receptor γ; PDX1, pancreatic and duodenal homeobox 1.
3.3 Hypolipidemic activity
Dyslipidemia is a prevalent group of diseases characterized by elevated levels of atherosclerotic lipids or lipoproteins in plasma or impaired function of anti-atherosclerotic lipids or lipoproteins (Xiang et al., 2022). Gypenoside XIII effectively inhibits hepatocyte lipogenesis and significantly improves hepatic lipid metabolism by blocking fatty acid absorption, accelerating triglyceride breakdown, and reducing hepatic lipid accumulation (Cheng et al., 2024). Concurrently, gypenoside LVI demonstrates regulatory advantages in cholesterol homeostasis, potentially serving as an adjunctive therapy to statins for hypercholesterolemia management (Wang et al., 2021). Thermally processed Gyps activate the SREBP/ACC/PPAR/LXRα signaling axis, reducing serum total cholesterol (TC), triglycerides (TG), and low-density lipoprotein cholesterol (LDL-C) levels while diminishing hepatic lipid accumulation in hyperlipidemic mice (Xie et al., 2023). In obesity models, Gyps reduces body weight and upregulates hepatic PPARα/γ expression (Xie et al., 2022). Mechanistically, Gyps ameliorates high-fat diet (HFD)-induced dyslipidemia by promoting cholesterol-to-bile acid conversion and decreasing the cholic acid/chenodeoxycholic acid (CA/CDCA) ratio (Lu et al., 2018). Lee et al., (2019) confirmed that Gynostemma pentaphyllum extract (GPE) alleviates HFD-induced hyperglycemia and hyperlipidemia in C57BL/6N mice through enhanced AMPK phosphorylation and suppressed lipogenesis without observable toxicity. In terms of intervention for atherosclerosis, Gyps can not only inhibit plaque formation by regulating the PI3K/Akt/bad pathway but also improve vascular disease by lowering the levels of serum adhesion molecules such as ICAM-1, VCAM-1, and MCP-1 (Song et al., 2020; Huang et al., 2022b). While current research has established the potential of Gyps in modulating lipid metabolism and combating atherosclerosis, mechanistic investigations primarily focus on phenotypic associations and lack direct evidence of the regulation of core targets.
3.4 Antineoplastic activity
It has been demonstrated that Gyps and their bioactive monomers exhibit significant antitumor activity across a range of cancer types. This provides a theoretical foundation for their development as novel anticancer agents. In lung cancer, both gypenoside L and gypenoside LI have been observed to effectively suppress the proliferation of A549 cells, albeit through distinct mechanisms: gypenoside L primarily induces G0/G1 phase arrest, whereas gypenoside LI triggers G2/M phase arrest (Xing et al., 2018). Recent evidence further suggests that the antitumor effect of Gyps in lung cancer may involve activation of the MAPK14/STAT3 pathway (Qi et al., 2022). In models of breast cancer, gypenoside LI has been observed to promote apoptosis via the upregulation of Bax and the downregulation of PARP-1/Bcl-2 (Zu et al., 2021). In addition, gypenoside I has been shown to inhibit proliferation by suppressing the AKT/GSK3β/β-catenin signaling pathway (Tan et al., 2022).
In renal cancer, gypenoside L and LI have inhibitory effects on cell viability, with Gyps particularly triggering apoptosis via activation of the PI3K/Akt/mTOR pathway (Liu et al., 2022; Liu et al., 2021a). The experiment has shown that gypenoside L inhibits the proliferation of hepatocellular and esophageal carcinoma cells by causing cellular senescence while also augmenting the antineoplastic efficacy of cisplatin and 5-fluorouracil (Ma et al., 2019). Moreover, in melanoma, gypenoside LI has been demonstrated to inhibit tumor development via microRNA-128-3p-mediated cell cycle arrest (Zu et al., 2020). In vitro studies further validated the extensive anti-tumor efficacy of Gyps, demonstrating inhibitory effects on several malignant neoplasms, including bladder cancer (Li et al., 2022b), hepatocellular carcinoma (Xiao et al., 2023), and oral squamous cell carcinoma (Lu et al., 2017).
Although existing studies have confirmed the antiproliferative effects of Gyps in various cancers, they have generally overlooked the influence of the tumor immune microenvironment. Furthermore, the drug concentrations used (10–100 μM) significantly exceed clinically achievable blood concentrations. Future research should focus on exploring the regulatory mechanisms of Gyps in the immune microenvironment within pharmacologically relevant concentration ranges to enhance the persuasiveness of its clinical translation.
3.5 Anti-inflammatory activity
Inflammation constitutes a complex pathophysiological response orchestrated by diverse pro-inflammatory cytokines and mediators. In vitro studies confirm that Gyps extracts enriched with gypenoside XLVI and gypenoside L variably inhibit pro-inflammatory cytokine secretion, demonstrating subtype-specific anti-inflammatory activities among saponin metabolites (Shen et al., 2018). Furthermore, Gyps significantly downregulate mRNA expression of pro-inflammatory mediators (IL-6, IL-1β, COX-2, TNF-α), reduce IL-6 and TNF-α protein levels, and suppress nitric oxide (NO) production, collectively mediating anti-inflammatory effects (Wan and Zhao, 2017; Li et al., 2020; Wang et al., 2020a; Zhang et al., 2020). Notably, GP-17 induces macrophage polarization toward the M2 phenotype and effectively suppresses inflammatory responses in THP-1 macrophage-derived foam cells (Deng et al., 2024). This metabolite exhibits significantly superior anti-inflammatory potency compared to its precursor saponin Rb1 (Zhou et al., 2023a), establishing GP-17 as a promising therapeutic molecule for inflammation modulation.
Under chronic unpredictable mild stress (CUMS) conditions, 7-day Gyps treatment reduced depressive behaviors and hippocampal proinflammatory cytokines (IL-1β, IL-6, TNF-α) in mice (Dong et al., 2018). Gyps significantly suppresses proinflammatory mediators (IL-1β, IL-6, NF-κB) in LPS-induced neuroinflammation and anxiety models, demonstrating therapeutic potential for neuroinflammation-associated anxiety behaviors (Lee et al., 2018). It attenuates optic neuritis in LPS-challenged rats through NF-κB/STAT pathway inhibition (Wang et al., 2018). Oral administration prevents excessive TNF-α/IL-1β production and reduces pathological damage in acute lung injury models (Tu et al., 2021), potentially via STAT-3/HIF-1α and TLR-4/NF-κB/HIF-1α pathway modulation (Xia et al., 2024). Furthermore, GP-17 blocks NLRP3 inflammasome activation and subsequent pyroptosis (Wang et al., 2024). The novel saponin GP-14 specifically inhibits serum IL-6/IL-1β elevation (minimal effect on TNF-α) and exhibits neuroprotective effects against neuroinflammation in high-altitude cerebral edema (HACE) models (Geng et al., 2022). The experimental details of the anti-inflammatory pharmacological activity of Gyps are presented in Table 3.
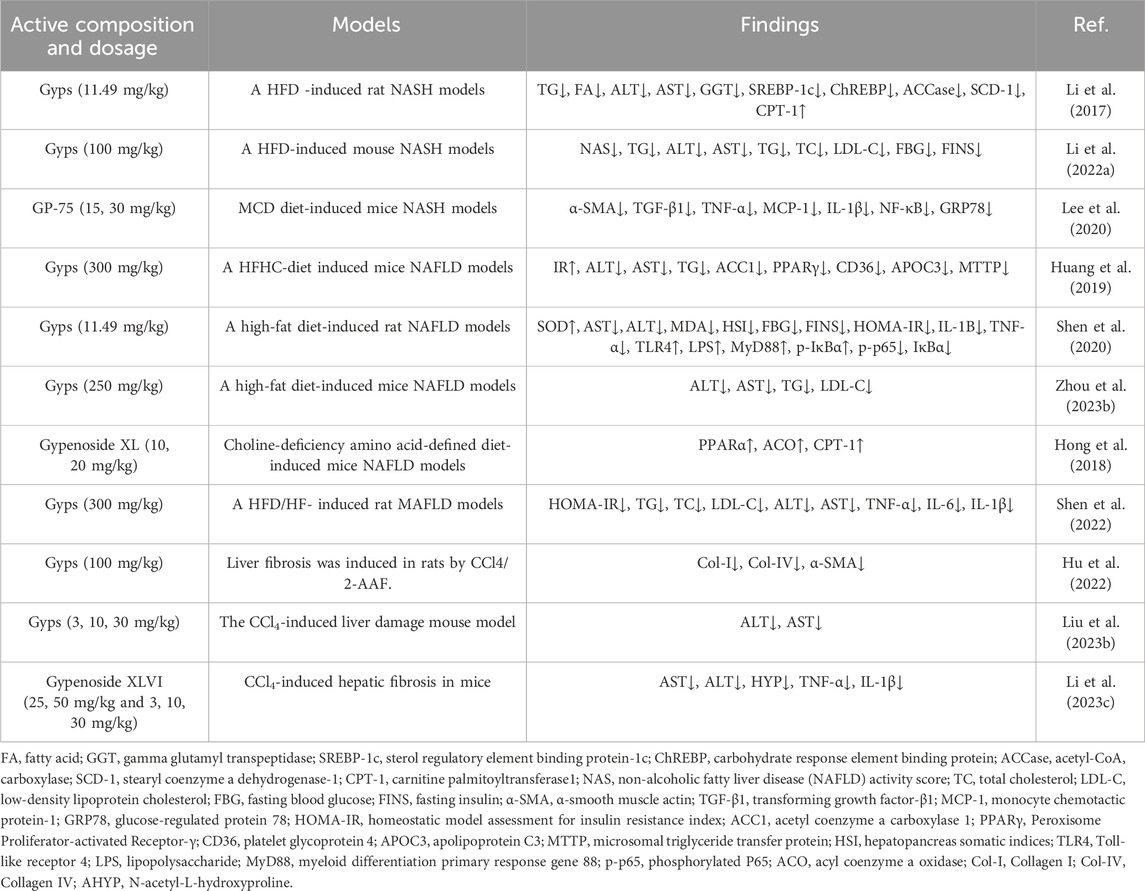
3.6 Hepatoprotective activity
Non-alcoholic fatty liver disease (NAFLD) represents one of the most prevalent hepatic disorders globally. Without appropriate intervention, it progresses through increasingly severe stages, including non-alcoholic steatohepatitis (NASH), hepatic fibrosis, and ultimately hepatocellular carcinoma (Rong et al., 2023). Gyps modulate the pathological progression of NAFLD through multi-target mechanisms. In NASH, they significantly reduce hepatic triglyceride and free fatty acid accumulation while improving serum activities of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and γ-glutamyltransferase (GGT) (Li et al., 2017; Li et al., 2022a). Gypenoside LXXV specifically attenuates methionine-choline deficient (MCD) diet-induced liver injury, lipid deposition, and macrophage activation (Lee et al., 2020). Against simple steatosis (NAFL), Gyps modulates gut microbiota composition (Huang et al., 2019), inhibits the LPS/Toll-like receptor 4 (TLR4)-mediated inflammatory cascade (Shen et al., 2020), and dually regulates lipid metabolism by suppressing fatty acid/cholesterol synthesis while promoting β-oxidation (Zhou et al., 2023b). Particularly, gypenoside XL alleviates hepatocyte injury through upregulation of peroxisome proliferator-activated receptor α (PPARα) protein expression (Hong et al., 2018). During progression to metabolic dysfunction-associated fatty liver disease (MAFLD), Gyps activates the AMP-activated protein kinase (AMPK) pathway to suppress TLR4/NF-κB signaling, concurrently improving insulin resistance, dyslipidemia (reduced total cholesterol, triglycerides, and LDL-C), and intestinal barrier integrity (Shen et al., 2022). In fibrotic stages, Gyps reduces carbon tetrachloride (CCl4)-induced collagen deposition and hydroxyproline content (Hu et al., 2022; Liu et al., 2023b), with gypenoside XLVI demonstrating antifibrotic efficacy in acute/chronic liver injury models (Li et al., 2023c). Notably, gypenoside XLIX ameliorates hepatic steatosis via epigenetic regulation of long non-coding RNA RPARP-AS1 (Liu et al., 2023a) and exhibits cross-disease protection by inhibiting duck hepatitis A virus type 1 (DHAV-1) replication and virus-induced hepatocyte apoptosis (Du et al., 2019). A recent study has indicated that the C3 deglycosylated metabolite of gypenoside XLVI inhibits collagen deposition in liver fibrosis by regulating the AMPK/p300/Smad3 axis in the TGF-β signaling pathway and shows a significant hepatoprotective effect in a mouse model of liver injury (Wang et al., 2025). The experimental details of the hepatoprotective pharmacological activity of Gyps are presented in Table 4.
3.7 Neuroprotection
Neurodegenerative diseases are chronic progressive disorders characterized by neurodegeneration and neurological dysfunction (Xu et al., 2022). Research has demonstrated that Gyps protects visual function by inhibiting demyelination and axonal degeneration in experimental optic neuritis and neurodegenerative models (Zhang et al., 2017). As demonstrated by Xing et al. (2020), the discovery of novel dammarane saponins, comprising three newly identified structural metabolites along with gypenoside LVII, has shown protective activity against H2O2-induced oxidative damage in SH-SY5Y neuroblastoma cells. Subsequent research has confirmed that four neodammarane saponins provide concentration-dependent neuroprotection against H2O2 toxicity without causing cell death in A549 or HepG2 lines (Zhai et al., 2021). Mechanistically, Gynostemma pentaphyllum ethanol extract (GP-EX) inhibits α-synuclein-induced dopaminergic neuron death in A53T transgenic mice via ERK1/2-BadSer112-JNK1/2 axis regulation (Park et al., 2020). In MPTP-induced Parkinson’s models, Gyps (50 mg/kg) ameliorates memory deficits while restoring tyrosine hydroxylase-positive cells and dopamine levels in substantia nigra/striatum and reactivating hippocampal ERK1/2-CREB phosphorylation (Zhao et al., 2017). Gyps has been shown to provide a substantial neuroprotective impact against hypoxia-induced damage to PC12 cells and to improve the hypoxia tolerance of C57 BL/6 mice when supplied orally (Wang et al., 2022a). In addition, Geng et al., (2022b) discovered that GP-14 pretreatment reduced hypoxia insult to PC12 cell viability and death by activating the AKT and ERK signaling pathways. Studies have indicated that GP-17 can prevent neuronal apoptosis and inflammation, increase functional recovery, and protect against spinal cord damage. This impact is due to upregulation of miR-21, which reactivates the PTEN/AKT/mTOR pathway (Sun et al., 2021). According to a recent study (Lei et al., 2023), gypenoside IX inhibits Aβ synthesis via Akt/GSK-3β signaling, preventing cognitive deterioration.
3.8 Other pharmacological activities
Gyps demonstrate renal protective effects and antagonize ischemia-reperfusion (I/R) injury through distinct mechanisms. For renal pathology, they inhibit PI3K/AKT signaling via miR-378a-5p upregulation, thereby reducing TGF-β1-induced fibrosis (Zhang et al., 2024). Gypenoside XLIX directly suppresses TGF-β/Smad3 transduction to attenuate collagen deposition, with its PLGA nanoformulation significantly enhancing antifibrotic efficacy (Liu et al., 2021b). Against I/R injury, this monomer activates cellular survival pathways by disrupting IGFBP7/IGF1R binding, consequently inhibiting renal tubular cell apoptosis and inflammation (Yang et al., 2021). In myocardial protection, gypenoside A mitigates cardiac injury through AMPK pathway activation and miR-143-3p downregulation (Chang et al., 2020), while GP-17 co-activates PI3K/AKT and p38 MAPK pathways to suppress endoplasmic reticulum stress and mitochondrial dysfunction, thereby enhancing functional recovery (Yu et al., 2021).
4 Pharmacokinetics of gypenosides
Gyps face significant challenges in oral administration, mainly stemming from poor absorption and low bioavailability, attributed to their high molecular weight, increased polarity, and limited lipid solubility. Studies indicate that the oral bioavailability of total Gyps is approximately 1.2% (Xu et al., 2017; Wang et al., 2022b). A notable absorption pathway involves the metabolism mediated by gut microbiota, which converts Gyps into aglycone forms that possess reduced polarity and enhanced lipid solubility, facilitating absorption across intestinal membranes (Han et al., 2023). The liver is essential for Gyps metabolism and distribution, with evidence showing that bile acid metabolism is regulated by FXR receptor-mediated signaling, which provides hepatoprotective effects (Xie et al., 2020). A validated UPLC-MS/MS method was utilized to quantify gypenoside A and XLIX in rat plasma, revealing notably short half-lives and low bioavailability (0.90% and 0.14%, respectively) (He et al., 2022). Gypenoside A demonstrates rapid absorption, detectable in plasma within 15 min post-administration, achieving peak concentration at 0.75 h, followed by rapid elimination (Hu et al., 2020). Deglycosylation serves as the main metabolic pathway for gypenoside LVI, correlating with reduced intestinal absorption (Chen et al., 2015). Following intravenous injection, GP-17 exhibits rapid elimination, characterized by a half-life of under 2 h. In contrast, oral administration results in swift absorption, achieving peak concentration at 0.19 h. The rapid absorption is likely due to high polarity and low lipid solubility, contributing to its low oral bioavailability of 1.87% (Wang et al., 2017a).
Gyps is rapidly degraded and metabolized in vivo, particularly after oral administration, necessitating large or frequent doses. To address this, a tumor-targeted Gyps nanodrug delivery system was developed with 57.64% encapsulation efficiency. However, in artificial intestinal fluid (pH 6.8), its encapsulation efficiency was significantly reduced compared to controls. The SYL3C-Lipo@Gyps-MSN formulation showed minimal drug release (<10%) within 2.5 h, followed by sustained release, enhancing tumor-site drug accumulation and demonstrating suitability for oncological applications (Lai et al., 2024). Incorporating sodium glycocholate (SGC) into nanostructured lipid carriers (NLCs) improved Gyps delivery, achieving 74.22% encapsulation efficiency and 4.89% drug loading (Gyps-SGC-NLCs). In vitro, Gyps-SGC-NLCs exhibited sustained release over 48 h, surpassing the Gyps physical mixture (32.2% cumulative release), and increased bioavailability 8.5-fold compared to Gyps powder (Yang et al., 2019a). Encapsulating gypenoside A in mPEG-PLGA nanoparticles yielded 84.4% encapsulation efficiency and 4.02% drug loading, with prolonged release kinetics and enhanced bioavailability versus free gypenoside A (Chen et al., 2024). Similarly, PLGA nanoparticles loaded with gypenoside XLIX achieved 82.4% encapsulation efficiency and 9.04% drug loading, enabling kidney-targeted delivery and sustained release (Liu et al., 2021b).
5 Toxicology of gypenosides
Current evidence suggests that human safety data for Gyps are limited to extracted preparations (Shaito et al., 2020). However, there is a lack of hepatotoxicity studies of pure Gyps, and a safe dose range has not been established. Chiranthanut et al., (2013) conducted a study in which rats received 240 mg/kg of Gynostemma pentaphyllum aqueous extract via oral gavage for 30 days, resulting in no observed toxicity or mortality. In a comparable study, the daily administration of 5,000 mg/kg of a standardized extract for 90 days demonstrated no observable toxicity. The findings validate the safety of Gyps, even at higher doses. Long-term studies in Wistar rats demonstrated no toxicity or mortality following 6 months of daily administration of 750 mg/kg Gynostemma pentaphyllum water extract. No significant changes were observed in body weight, hematological parameters (such as white blood cell count and hemoglobin), hepatic and renal function, or urinalysis outcomes at a dosage of 500 mg/kg/day over the same period. Histopathological examination of key organs (heart, liver, spleen, lungs, and kidneys) revealed no lesions, thereby supporting the safety of extended use (Attawish et al., 2004). Gyps may influence dyslipidemia through the modulation of gut microbiota metabolites, specifically trimethylamine (TMA) and trimethylamine-N-oxide (TMAO); however, the potential chronic risks associated with these metabolites require further investigation (Zhang et al., 2021b). In murine models, a 6-week oral administration of gypenoside L improved exercise capacity and demonstrated anti-fatigue effects (Kim et al., 2022). A 12-week randomized, double-blind, placebo-controlled trial demonstrated that a gypenoside L-containing extract alleviates exercise-induced fatigue without adverse effects, underscoring its therapeutic potential (Ahn et al., 2023). Acute (0.8 g/kg) and subacute (10–50 mg/kg) toxicity studies of GP-75 in mice demonstrated no instances of mortality or significant toxicity (Wu et al., 2024). In randomized controlled trials, the incidence of adverse events of Gyps was substantially lower than that of conventional lipid-lowering drugs, as indicated by the most recent systematic review. Additionally, no serious adverse reactions were observed after continuous use of Gyps for more than 8 weeks (Dai et al., 2022). Gyps demonstrate a favorable safety profile at recommended doses; however, the risks linked to long-term use necessitate further research.
6 Conclusions and views
Gyps, the principal bioactive metabolites of Gynostemma pentaphyllum, have garnered significant interest owing to their diverse pharmacological actions, including antioxidant, hypoglycemic, anti-tumor, hepatoprotective, and neuroprotective effects. Despite numerous preclinical studies validating their therapeutic promise, three significant hurdles impede clinical translation. Primarily, the majority of saponin monomers have low oral bioavailability and an ambiguous active state in vivo, attributable to inadequate membrane permeability and intricate metabolic processes. The second issue is the absence of a defined extraction procedure, leading to irregular metabolite composition; third, variations in plant sources and processing methods result in fluctuations of active compounds. Future study should concentrate on creating novel delivery strategies to enhance bioavailability, instituting standardized quality control measures, and methodically comparing the compositional attributes of saponins from various sources. This will establish the groundwork for achieving the clinical transformation of Gyps and their advancement as lead compounds or adjunct medicinal agents.
Author contributions
XL: Investigation, Writing – original draft. YC: Writing – review and editing. RW: Writing – review and editing. BC: Writing – review and editing. TD: Writing – review and editing. JH: Writing – review and editing. MY: Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Natural Science Foundation of China [No. 82004212]; the Project of Shandong Traditional Chinese Medicine Plan [No. M-2022259]; and the Coconstruction Project of the National Administration of Traditional Chinese Medicine [No. GZY-KJS-SD-2023-084].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahn, Y., Lee, H. S., Lee, S. H., Joa, K. L., Lim, C. Y., Ahn, Y. J., et al. (2023). Effects of gypenoside L-containing Gynostemma pentaphyllum extract on fatigue and physical performance: a double-blind, placebo-controlled, randomized trial. Phytother. Res. 37 (7), 3069–3082. doi:10.1002/ptr.7801
Alhasani, R. H., Biswas, L., Tohari, A. M., Zhou, X. Z., Reilly, J., He, J. F., et al. (2018). Gypenosides protect retinal pigment epithelium cells from oxidative stress. Food Chem. Toxicol. 112, 76–85. doi:10.1016/j.fct.2017.12.037
Alhasani, R. H., Zhou, X. Z., Biswas, L., Li, X., Reilly, J., Zeng, Z. H., et al. (2020). Gypenosides attenuate retinal degeneration in a zebrafish retinitis pigmentosa model. Exp. Eye Res. 201, 108291. doi:10.1016/j.exer.2020.108291
Attawish, A., Chivapat, S., Phadungpat, S., Bansiddhi, J., Techadamrongsin, Y., Mitrijit, O., et al. (2004). Chronic toxicity of Gynostemma pentaphyllum. Fitoterapia 75 (6), 539–551. doi:10.1016/j.fitote.2004.04.010
Biswas, L., Zeng, Z. H., Graham, A., and Shu, X. H. (2020). Gypenosides mediate cholesterol efflux and suppress oxidized LDL induced inflammation in retinal pigment epithelium cells. Exp. Eye Res. 191, 107931. doi:10.1016/j.exer.2020.107931
Chang, L. P., Shi, R., Wang, X. J., and Bao, Y. (2020). Gypenoside A protects ischemia/reperfusion injuries by suppressing miR-143-3p level via the activation of AMPK/Foxo1 pathway. Biofactors 46 (3), 432–440. doi:10.1002/biof.1601
Chen, D. J., Hu, H. G., Xing, S. F., Liu, H. M., and Piao, X. L. (2015). Metabolite profiling of gypenoside LVI in rat after oral and intravenous administration. Archives Pharmacal Res. 38 (6), 1157–1167. doi:10.1007/s12272-014-0506-2
Chen, S., Liu, X., Zhao, H., Cheng, N., Sun, J., and Cao, W. (2022). Beneficial effects of Gynostemma pentaphyllum honey paste on obesity via counteracting oxidative stress and inflammation: an exploration of functional food developed from two independent foods rich in saponins and phenolics. Food Res. Int. 157, 111483. doi:10.1016/j.foodres.2022.111483
Chen, X. B., Yao, C. L., Hou, J. R., Nie, M., Li, Y., Wei, W. L., et al. (2023). Systematical characterization of gypenosides in Gynostemma pentaphyllum and the chemical composition variation of different origins. J. Pharm. Biomed. Anal. 232, 115328. doi:10.1016/j.jpba.2023.115328
Chen, Q., Qiu, F. S., Xie, W., Yu, W. Y., Su, Z. A., Qin, G. M., et al. (2024). Gypenoside A-loaded mPEG-PLGA nanoparticles ameliorate high-glucose-induced retinal microvasculopathy by inhibiting ferroptosis, 1873–3476. (Electronic)).
Cheng, S. C., Liou, C. J., Wu, Y. X., Yeh, K. W., Chen, L. C., and Huang, W. C. (2024). Gypenoside XIII regulates lipid metabolism in HepG2 hepatocytes and ameliorates nonalcoholic steatohepatitis in mice. Kaohsiung J. Med. Sci. 40 (3), 280–290. doi:10.1002/kjm2.12795
Chiranthanut, N., Teekachunhatean, S., Panthong, A., Khonsung, P., Kanjanapothi, D., and Lertprasertsuk, N. (2013). Toxicity evaluation of standardized extract of Gynostemma pentaphyllum makino. J. Ethnopharmacol. 149 (1), 228–234. doi:10.1016/j.jep.2013.06.027
Choi, E. K., Won, Y. H., Kim, S. Y., Noh, S. O., Park, S. H., Jung, S. J., et al. (2019). Supplementation with extract of Gynostemma pentaphyllum leaves reduces anxiety in healthy subjects with chronic psychological stress: a randomized, double-blind, placebo-controlled clinical trial. Phytomedicine 52, 198–205. doi:10.1016/j.phymed.2018.05.002
Chou, C. Y., Hsu, D. Y., and Chou, C. H. (2023). Predicting the onset of diabetes with machine learning methods. J. Personalized Med. 13 (3), 406. doi:10.3390/jpm13030406
Chu, H., Sun, N., Tang, Q., Yu, X., Li, L., and Chang, H. (2025). Deciphering the polypharmacology of Dingzhi Xiaowan against comorbid depression: integrated metabolomics of brain tissue and network pharmacology analysis in chronic restraint stress (CRS)-LPS model. J. Ethnopharmacol. 349, 119960. doi:10.1016/j.jep.2025.119960
Dai, N., Zhao, F. F., Fang, M., Pu, F. L., Kong, L. Y., and Liu, J. P. (2022). Gynostemma pentaphyllum for dyslipidemia: a systematic review of randomized controlled trials. Front. Pharmacol. 13, 917521. doi:10.3389/fphar.2022.917521
Deng, W. Y., Zhou, C. L., and Zeng, M. Y. (2024). Gypenoside XVII inhibits ox-LDL-induced macrophage inflammatory responses and promotes cholesterol efflux through activating the miR-182-5p/HDAC9 signaling pathway. J. Ethnopharmacol. 319, 117070. doi:10.1016/j.jep.2023.117070
Dong, S. Q., Zhang, Q. P., Zhu, J. X., Chen, M., Li, C. F., Liu, Q., et al. (2018). Gypenosides reverses depressive behavior via inhibiting hippocampal neuroinflammation. Biomed. & Pharmacother. 106, 1153–1160. doi:10.1016/j.biopha.2018.07.040
Du, H. X., Bai, J. Y., Wang, J. L., He, M., Xiong, W., Yuan, W. J., et al. (2019). Assessment of the hepatocyte protective effects of gypenoside and its phosphorylated derivative against DHAV-1 infection on duck embryonic hepatocytes. Bmc Veterinary Res. 15, 134. doi:10.1186/s12917-019-1891-z
Gao, M., Heng, X., Jin, J., and Chu, W. (2022). Gypenoside XLIX ameliorate high-fat diet-induced atherosclerosis via regulating intestinal microbiota, alleviating inflammatory response and restraining oxidative stress in ApoE(-/-) mice. Pharm. (Basel) 15 (9), 1056. doi:10.3390/ph15091056
Geng, Y. A., Yang, J. L., Cheng, X., Han, Y., Yan, F., Wang, C. B., et al. (2022). A bioactive gypenoside (GP-14) alleviates neuroinflammation and blood brain barrier (BBB) disruption by inhibiting the NF-κB signaling pathway in a mouse high-altitude cerebral edema (HACE) model. Int. Immunopharmacol. 107, 108675. doi:10.1016/j.intimp.2022.108675
Geng, Y. N., Zhao, M., Yang, J. L., Cheng, X., Han, Y., Wang, C. B., et al. (2022b). GP-14 protects against severe hypoxia-induced neuronal injury through the AKT and ERK pathways and its induced transcriptome profiling alteration. Toxicol. Appl. Pharmacol. 448, 116092. doi:10.1016/j.taap.2022.116092
Gou, S. H., Liu, B. J., Han, X. F., Wang, L., Zhong, C., Liang, S., et al. (2018). Anti-atherosclerotic effect of fermentum rubrum and Gynostemma pentaphyllum mixture in high-fat emulsion- and vitamin D3-induced atherosclerotic rats. J. Chin. Med. Assoc. 81 (5), 398–408. doi:10.1016/j.jcma.2017.08.018
Hajam, Y. A., Rani, R., Ganie, S. Y., Sheikh, T. A., Javaid, D., Qadri, S. S., et al. (2022). Oxidative stress in human pathology and aging: molecular mechanisms and perspectives. Cells 11 (3), 552. doi:10.3390/cells11030552
Han, B., Li, J. N., Lv, X. Y., Chen, M. Y., Du, D. F., Li, F., et al. (2023). Research progress on metabolic transformation of traditional Chinese medicinesaponins by intestinal flora. Chin. Traditional Botanical drugal Drugs 54 (20), 6922–6932. doi:10.7501/j.issn.0253-2670.2023.20.035
He, Y., Hu, Z. Y., Li, A. R., Zhu, Z. Z., Yang, N., Ying, Z. X., et al. (2019). Recent advances in biotransformation of saponins. Molecules 24 (13), 2365. doi:10.3390/molecules24132365
He, Y., Liang, Q. S., Luo, L., He, Y. F., Huang, X. L., and Wen, C. C. (2022). Determination of gypenoside A and gypenoside XLIX in rat plasma by UPLC-MS/MS and applied to the pharmacokinetics and bioavailability. Int. J. Anal. Chem. 2022, 6734408. doi:10.1155/2022/6734408
Hong, M., Cai, Z., Song, L., Liu, Y. Q., Wang, Q., and Feng, X. F. (2018). Gynostemma pentaphyllum attenuates the progression of nonalcoholic fatty liver disease in mice: a biomedical investigation integrated with In silico assay. Evidence-Based Complementary Altern. Med. 2018, 8384631. doi:10.1155/2018/8384631
Hu, X. H., Sun, W. J., Gao, X., and Wang, X. J. (2020). Pharmacokinetics of gypenoside A in glanxinning soft capsules in rats. Mod. Chin. Med. 22 (7), 1066–1071. doi:10.13313/j.issn.1673-4890.20190905009
Hu, Y. H., He, X. L., Zhou, X. X., Liang, Y., Fu, Y. D., Zhang, L. Z., et al. (2022). Gypenosides ameliorate ductular reaction and liver fibrosis via inhibition of hedgehog signaling. Front. Pharmacol. 13, 1033103. doi:10.3389/fphar.2022.1033103
Huang, X. Q., Chen, W. F., Yan, C. S., Yang, R. Z., Chen, Q. Y., Xu, H. Z., et al. (2019). Gypenosides improve the intestinal microbiota of non-alcoholic fatty liver in mice and alleviate its progression. Biomed. & Pharmacother. 118, 109258. doi:10.1016/j.biopha.2019.109258
Huang, W. C., Wu, S. J., Yeh, K. W., and Liou, C. J. (2022a). Gypenoside A from Gynostemma pentaphyllum attenuates airway inflammation and Th2 cell activities in a murine asthma model. Int. J. Mol. Sci. 23 (14), 7699. doi:10.3390/ijms23147699
Huang, Y. P., Wang, Y. S., Liu, Y. Y., Jiang, C. H., Wang, J., Jiang, X. Y., et al. (2022b). Chemical characterization and atherosclerosis alleviation effects of gypenosides from Gynostemma pentaphyllum through ameliorating endothelial dysfunction via the PCSK9/LOX-1 pathway. J. Agric. Food Chem. 70, 11944–11957. doi:10.1021/acs.jafc.2c02681
Ji, X., Shen, Y., and Guo, X. (2018). Isolation, structures, and bioactivities of the polysaccharides from Gynostemma pentaphyllum (thunb.) makino: a review. Biomed. Res. Int. 2018, 6285134. doi:10.1155/2018/6285134
Jo, H. G., Baek, C. Y., Hwang, Y., Baek, E., Park, C., Song, H. S., et al. (2024). Investigating the anti-Inflammatory, analgesic, and chondroprotective effects of Gynostemma pentaphyllum (thunb.) makino in osteoarthritis: an in vitro and in vivo study. Int. J. Mol. Sci. 25 (17), 9594. doi:10.3390/ijms25179594
Kim, Y. H., Jung, J. I., Jeon, Y. E., Kim, S. M., Hong, S. H., Kim, T. Y., et al. (2022). Gynostemma pentaphyllum extract and its active component gypenoside L improve the exercise performance of treadmill-trained mice. Nutr. Res. Pract. 16 (3), 298–313. doi:10.4162/nrp.2022.16.3.298
Lai, Z. Q., Bai, F. C., Pu, T., Li, J., Wu, L. N., Zhou, Z. T., et al. (2024). Tumor-targeted gypenoside nanodrug delivery system with double protective layers. J. Cancer Res. Ther. 20 (2), 684–694. doi:10.4103/jcrt.jcrt_134_23
Lee, B., Shim, I., Lee, H., and Hahm, D. H. (2018). Gypenosides attenuate lipopolysaccharide-induced neuroinflammation and anxiety-like behaviors in rats. Animal Cells Syst. 22 (5), 305–316. doi:10.1080/19768354.2018.1517825
Lee, H. S., Lim, S. M., Jung, J. I., Kim, S. M., Lee, J. K., Kim, Y. H., et al. (2019). Gynostemma pentaphyllum extract ameliorates high-fat diet-induced obesity in C57BL/6N mice by upregulating SIRT1. Nutrients 11 (10), 2475. doi:10.3390/nu11102475
Lee, J. H., Oh, J. Y., Kim, S. H., Oh, I. J., Lee, Y. H., Lee, K. W., et al. (2020). Pharmaceutical efficacy of gypenoside LXXV on non-alcoholic steatohepatitis (NASH). Biomolecules 10 (10), 1426. doi:10.3390/biom10101426
Lee, J., Jin, Y., Zhang, X., Kim, M., Koh, A., Zhou, S., et al. (2025). Therapeutic potential of Gynostemma pentaphyllum extract for hair health enhancement: a randomized, double-blind, placebo-controlled clinical trial. Nutrients 17 (5), 767. doi:10.3390/nu17050767
Lei, L., Luo, Y., Kang, D. K., Yang, F. M., Meng, D. L., Wang, J. Z., et al. (2023). Gypenoside IX restores Akt/GSK-3β pathway and alleviates Alzheimer's disease-like neuropathology and cognitive deficits. Aging-Us 15 (23), 14172–14191. doi:10.18632/aging.205295
Li, Y. (2022). Gypenoside A attenuates dysfunction of pancreatic β cells by activating PDX1 signal transduction via the inhibition of miR-150-3p both in vivo and in vitro. J. Biochem. Mol. Toxicol. 36 (4), e23004. doi:10.1002/jbt.23004
Li, Y., Lin, W., Huang, J., Xie, Y., and Ma, W. (2016a). Anti-cancer effects of Gynostemma pentaphyllum (thunb.) Makino (Jiaogulan). Chin. Med. 11, 43. doi:10.1186/s13020-016-0114-9
Li, Y., Wang, Y., Niu, K., Chen, X., Xia, L., Lu, D., et al. (2016b). Clinical benefit from EGFR-TKI plus ginsenoside Rg3 in patients with advanced non-small cell lung cancer harboring EGFR active mutation. Oncotarget 7 (43), 70535–70545. doi:10.18632/oncotarget.12059
Li, H. S., Ying, H., Hu, A. R., Hu, Y. R., and Li, D. Z. (2017). Therapeutic effect of gypenosides on nonalcoholic steatohepatitis via regulating hepatic lipogenesis and fatty acid oxidation. Biol. & Pharm. Bull. 40 (5), 650–657. doi:10.1248/bpb.b16-00942
Li, H. Y., Ma, C., Liu, W., He, J. F., and Li, K. J. (2020). Gypenosides protect orbital fibroblasts in graves ophthalmopathy via anti-inflammation and anti-fibrosis effects. Investigative Ophthalmol. & Vis. Sci. 61 (5), 64. doi:10.1167/iovs.61.5.64
Li, X., Alhasani, R. H., Cao, Y. Q., Zhou, X. Z., He, Z. M., Zeng, Z. H., et al. (2021). Gypenosides alleviate cone cell death in a zebrafish model of Retinitis pigmentosa. Antioxidants 10 (7), 1050. doi:10.3390/antiox10071050
Li, H. S., Xi, Y. F., Liu, H. L., and Xin, X. (2022a). Gypenosides ameliorate high-fat diet-induced non-alcoholic steatohepatitis via farnesoid X receptor activation. Front. Nutr. 9, 914079. doi:10.3389/fnut.2022.914079
Li, X. M., Liu, H., Lv, C. C., Du, J., Lian, F. C., Zhang, S. Y., et al. (2022b). Gypenoside-induced apoptosis via the PI3K/AKT/mTOR signaling pathway in bladder cancer. Biomed Res. Int. 2022, 9304552. doi:10.1155/2022/9304552
Li, C. Y., Ma, Y., Zhi, X. L., and Peng, G. P. (2023a). Optimization of ultrasonic assisted membrane strategy for saponins from Gynostemma Pentaphyllum with response surface methodology. Food Sci. Biotechnol. 32 (3), 319–328. doi:10.1007/s10068-022-01196-8
Li, G., Lu, P. X., Liang, H. Z., Zheng, W., Chen, X. J., Zhang, J., et al. (2023b). An effective and high-throughput sample preparation method involving demalonylation followed by an ultrahigh-performance liquid chromatography-charged aerosol detector for analyzing gypenoside XLIX and gypenoside A in Gynostemma longipes. J. Pharm. Biomed. Analysis 230, 115393. doi:10.1016/j.jpba.2023.115393
Li, H., Wang, H. H., Yang, A. P., Xue, M. Z., Wang, J. Y., Lv, Q., et al. (2023c). Gypenosides synergistically reduce the extracellular matrix of hepatic stellate cells and ameliorate hepatic fibrosis in mice. Molecules 28 (14), 5448. doi:10.3390/molecules28145448
Li, Y., Ouyang, Q., Li, X., Alolgal, R. N., Fan, Y., Sun, Y., et al. (2023d). The role of Gynostemma pentaphyllum in regulating hyperlipidemia. Am. J. Chin. Med. 51 (4), 953–978. doi:10.1142/S0192415X23500441
Liang, H. Z., Wang, M. Y., Yang, G., Li, G., Zhang, J., Yao, L., et al. (2025). Untargeted qualitative and targeted quantitative analysis of saponins reveal differential chemotypes of Gynostemma pentaphyllum and G. longipes from different geographical origins. Food Chem. 468, 142412. doi:10.1016/j.foodchem.2024.142412
Liu, H., Li, X. M., Duan, Y., Xie, J. B., and Piao, X. L. (2021a). Mechanism of gypenosides of Gynostemma pentaphyllum inducing apoptosis of renal cell carcinoma by PI3K/AKT/mTOR pathway. J. Ethnopharmacol. 271, 113907. doi:10.1016/j.jep.2021.113907
Liu, Q. X., Chen, X. H., Kan, M., Yang, J., Gong, Q., Jin, R., et al. (2021b). Gypenoside XLIX loaded nanoparticles targeting therapy for renal fibrosis and its mechanism. Eur. J. Pharmacol. 910, 174501. doi:10.1016/j.ejphar.2021.174501
Liu, H., Li, X. M., Xie, J. B., Lv, C. C., Lian, F. C., Zhang, S. Y., et al. (2022). Gypenoside L and gypenoside LI inhibit proliferation in renal cell carcinoma via regulation of the MAPK and arachidonic acid metabolism pathways. Front. Pharmacol. 13, 820639. doi:10.3389/fphar.2022.820639
Liu, F. M., Wei, Q., Liang, Y. D., Yang, Q. M., Huang, C. X., Huang, Q. J., et al. (2023a). Effects of Gypenoside XLIX on fatty liver cell gene expression in vitro: a genome-wide analysis. Am. J. Transl. Res. 15 (2), 834–846.
Liu, Y., Yang, Y. T., Wang, H. H., Li, H., Lv, Q., Wang, X. C., et al. (2023b). Dammarane-type triterpenoid saponins isolated from Gynostemma pentaphyllum ameliorate liver fibrosis via agonizing PP2Cα and inhibiting deposition of extracellular matrix. Chin. J. Nat. Med. 21 (8), 599–609. doi:10.1016/S1875-5364(23)60395-4
Liu, Y., Li, Y., Li, J., Rao, H., Sun, J., Xiu, J., et al. (2025). Gypenosides alleviate oxidative stress in the hippocampus, promote mitophagy, and mitigate depressive-like behaviors induced by CUMS via SIRT1. J. Ethnopharmacol. 337 (Pt 2), 118823. doi:10.1016/j.jep.2024.118823
Lou, T., Huang, Q., Su, H., Zhao, D., and Li, X. (2021). Targeting sirtuin 1 signaling pathway by ginsenosides. J. Ethnopharmacol. 268, 113657. doi:10.1016/j.jep.2020.113657
Lu, K. W., Ma, Y. S., Yu, F. S., Huang, Y. P., Chu, Y. L., Wu, R. S. C., et al. (2017). Gypenosides induce cell death and alter gene expression in human oral cancer HSC-3 cells. Exp. Ther. Med. 14 (3), 2469–2476. doi:10.3892/etm.2017.4840
Lu, Y. L., Du, Y. M., Qin, L., Wu, D., Wang, W., Ling, L., et al. (2018). Gypenosides altered hepatic bile acids homeostasis in mice treated with high fat diet. Evidence-Based Complementary Altern. Med. 2018, 8098059. doi:10.1155/2018/8098059
Ma, J. X., Hu, X. P., Liao, C. H., Xiao, H. T., Zhu, Q. C., Li, Y., et al. (2019). Gypenoside L inhibits proliferation of liver and esophageal cancer cells by inducing senescence. Molecules 24 (6), 1054. doi:10.3390/molecules24061054
Ma, C., Li, H. Y., Liu, W., Lu, S. W., Li, X., Chen, J. Y., et al. (2022). Therapeutic effect of gypenosides on antioxidant stress injury in orbital fibroblasts of graves' orbitopathy. J. Immunol. Res. 2022, 4432584. doi:10.1155/2022/4432584
Meng, X., Zhang, Y., Li, Z., Hu, J., Zhang, D., Cao, W., et al. (2022). A novel natural PPARγ agonist, Gypenoside LXXV, ameliorates cognitive deficits by enhancing brain glucose uptake via the activation of Akt/GLUT4 signaling in db/db mice. Phytother. Res. 36 (4), 1770–1784. doi:10.1002/ptr.7413
Meng, X. B., Zhang, Y., Li, Z. Y., Ma, G. X., Zhang, X. J., Zhang, D., et al. (2023). Increasing brain glucose uptake by Gypenoside LXXV ameliorates cognitive deficits in a mouse model of diabetic Alzheimer's disease. Phytotherapy Res. 37 (2), 611–626. doi:10.1002/ptr.7639
Nayyar, D., Yan, X., Xu, G., Shi, M., Garnham, A. P., Mathai, M. L., et al. (2023). Gynostemma pentaphyllum increases exercise performance and alters mitochondrial respiration and AMPK in healthy males. Nutrients 15 (22), 4721. doi:10.3390/nu15224721
Nguyen, N. H., Ha, T. K. Q., Yang, J. L., Pham, H. T. T., and Oh, W. K. (2021). Triterpenoids from the genus Gynostemma: chemistry and pharmacological activities. J. Ethnopharmacol. 268, 113574. doi:10.1016/j.jep.2020.113574
Pang, M., Fang, Y., Chen, S., Zhu, X., Shan, C., Su, J., et al. (2017). Gypenosides inhibits Xanthine oxidoreductase and ameliorates urate excretion in hyperuricemic rats induced by high cholesterol and high fat food (lipid emulsion). Med. Sci. Monit. 23, 1129–1140. doi:10.12659/msm.903217
Park, S. H., Huh, T. L., Kim, S. Y., Oh, M. R., Tirupathi Pichiah, P. B., Chae, S. W., et al. (2014). Antiobesity effect of Gynostemma pentaphyllum extract (actiponin): a randomized, double-blind, placebo-controlled trial. Obes. (Silver Spring) 22 (1), 63–71. doi:10.1002/oby.20539
Park, H. J., Zhao, T. T., Kim, S. H., Lee, C. K., Hwang, B. Y., Lee, K. E., et al. (2020). Ethanol extract from Gynostemma pentaphyllum ameliorates dopaminergic neuronal cell death in transgenic mice expressing mutant A53T human alpha-synuclein. Neural Regen. Res. 15 (2), 361–368. doi:10.4103/1673-5374.265557
Ping, K., Yang, R., Chen, H., Xie, S., Li, M., Xiang, Y., et al. (2024). Gypenoside XLIX alleviates intestinal injury by inhibiting sepsis-induced inflammation, oxidative stress, apoptosis, and autophagy. Chem. Biol. Interact. 397, 111077. doi:10.1016/j.cbi.2024.111077
Qi, Y. S., Xie, J. B., Xie, P., Duan, Y., Ling, Y. Q., Gu, Y. L., et al. (2021). Uncovering the anti-NSCLC effects and mechanisms of gypenosides by metabolomics and network pharmacology analysis. J. Ethnopharmacol. 281, 114506. doi:10.1016/j.jep.2021.114506
Qi, Y. S., Xiao, M. Y., Xie, P., Xie, J. B., Guo, M., Li, F. F., et al. (2022). Comprehensive serum metabolomics and network analysis to reveal the mechanism of gypenosides in treating lung cancer and enhancing the pharmacological effects of cisplatin. Front. Pharmacol. 13, 1070948. doi:10.3389/fphar.2022.1070948
Rong, L., Zou, J. Y., Ran, W., Qi, X. H., Chen, Y. K., Cui, H. J., et al. (2023). Advancements in the treatment of non-alcoholic fatty liver disease (NAFLD). Front. Endocrinol. 13, 1087260. doi:10.3389/fendo.2022.1087260
Shaito, A., Thuan, D. T. B., Phu, H. T., Nguyen, T. H. D., Hasan, H., Halabi, S., et al. (2020). Herbal medicine for cardiovascular diseases: efficacy, mechanisms, and safety. Front. Pharmacol. 11, 422. doi:10.3389/fphar.2020.00422
Shen, C. Y., Jiang, J. G., Shi, M. M., Yang, H. L., Wei, H., and Zhu, W. (2018). Comparison of the effects and inhibitory pathways of the constituents from Gynostemma pentaphyllum against LPS-induced inflammatory response. J. Agric. Food Chem. 66 (43), 11337–11346. doi:10.1021/acs.jafc.8b03903
Shen, S. H., Wang, K. E., Zhi, Y. H., Shen, W., and Huang, L. Q. (2020). Gypenosides improves nonalcoholic fatty liver disease induced by high-fat diet induced through regulating LPS/TLR4 signaling pathway. Cell Cycle 19 (22), 3042–3053. doi:10.1080/15384101.2020.1829800
Shen, S. H., Wang, K. E., Zhi, Y. H., and Dong, Y. (2022). Gypenosides counteract hepatic steatosis and intestinal barrier injury in rats with metabolic associated fatty liver disease by modulating the adenosine monophosphate activated protein kinase and toll-like receptor 4/nuclear factor kappa B pathways. Pharm. Biol. 60 (1), 1949–1959. doi:10.1080/13880209.2022.2126503
Song, Y. N., Dong, S., Wei, B., Liu, P., Zhang, Y. Y., and Su, S. B. (2017). Metabolomic mechanisms of gypenoside against liver fibrosis in rats: an integrative analysis of proteomics and metabolomics data. PLoS One 12 (3), e0173598. doi:10.1371/journal.pone.0173598
Song, N., Jia, L. Q., Cao, H. M., Ma, Y. X., Chen, N., Chen, S., et al. (2020). Gypenoside inhibits endothelial cell apoptosis in atherosclerosis by modulating mitochondria through PI3K/Akt/Bad pathway. Biomed Res. Int. 2020, 2819658. doi:10.1155/2020/2819658
Su, C., Li, N., Ren, R., Wang, Y., Su, X., Lu, F., et al. (2021). Progress in the medicinal value, bioactive compounds, and pharmacological activities of Gynostemma pentaphyllum. Molecules 26 (20), 6249. doi:10.3390/molecules26206249
Sun, T. Y., Duan, L. Y., Li, J. J., Guo, H. Y., and Xiong, M. Y. (2021). Gypenoside XVII protects against spinal cord injury in mice by regulating the microRNA-21-mediated PTEN/AKT/mTOR pathway. Int. J. Mol. Med. 48 (2), 146. doi:10.3892/ijmm.2021.4979
Tan, H., Li, M., Han, L., Zhao, Y., and Zhang, X. (2022). Gypensapogenin I suppresses cell proliferation in triple-negative breast cancer Via triggering the closure of AKT/GSK3β/β-Catenin and Notch-1 signaling pathways. J. Agric. Food Chem. 70 (17), 5438–5449. doi:10.1021/acs.jafc.2c02512
Tu, Q., Zhu, Y. B., Yuan, Y., Guo, L., Liu, L., Yao, L. F., et al. (2021). Gypenosides inhibit inflammatory response and apoptosis of endothelial and epithelial cells in LPS-induced ALI: a study based on bioinformatic analysis and in vivo/vitro experiments. Drug Des. Dev. Ther. 15, 289–303. doi:10.2147/Dddt.S286297
Wan, Z. H., and Zhao, Q. (2017). Gypenoside inhibits interleukin-1-induced inflammatory response in human osteoarthritis chondrocytes. J. Biochem. Mol. Toxicol. 31 (9), e21926. doi:10.1002/jbt.21926
Wang, C., Gu, D. D., Wang, Y., and Han, X. C. (2017a). pharmacokinetics of gypenoside XV in rats. China J. Chin. Materia Medica 42 (04), 783–788. doi:10.19540/j.cnki.cjcmm.2017.0023
Wang, X., Yang, L., Yang, L., Xing, F., Yang, H., Qin, L., et al. (2017b). Gypenoside IX suppresses p38 MAPK/Akt/NFκB signaling pathway activation and inflammatory responses in astrocytes stimulated by proinflammatory mediators. Inflammation 40 (6), 2137–2150. doi:10.1007/s10753-017-0654-x
Wang, F., Dang, Y. L., Wang, J., Zhou, T., and Zhu, Y. (2018). Gypenosides attenuate lipopolysaccharide-induced optic neuritis in rats. Acta Histochem. 120 (4), 340–346. doi:10.1016/j.acthis.2018.03.003
Wang, Z., Zhao, X., Liu, X., Lu, W., Jia, S., Hong, T., et al. (2019). Anti-diabetic activity evaluation of a polysaccharide extracted from Gynostemma pentaphyllum. Int. J. Biol. Macromol. 126, 209–214. doi:10.1016/j.ijbiomac.2018.12.231
Wang, B., Li, M., Gao, H., Sun, X. J., Gao, B. Y., Zhang, Y. Q., et al. (2020a). Chemical composition of tetraploid Gynostemma pentaphyllum gypenosides and their suppression on inflammatory response by NF-κB/MAPKs/AP-1 signaling pathways. Food Sci. & Nutr. 8 (2), 1197–1207. doi:10.1002/fsn3.1407
Wang, X., Li, D., Guo, X., Zhang, Q., Liao, X., Cao, Z., et al. (2020b). ComMS(n)DB-An automatable strategy to identify compounds from MS data sets (identification of gypenosides as an example). J. Agric. Food Chem. 68 (41), 11368–11388. doi:10.1021/acs.jafc.0c03693
Wang, J., Wang, Y. S., Huang, Y. P., Jiang, C. H., Gao, M., Zheng, X., et al. (2021). Gypenoside LVI improves hepatic LDL uptake by decreasing PCSK9 and upregulating LDLR expression. Phytomedicine 91, 153688. doi:10.1016/j.phymed.2021.153688
Wang, C. B., Zhao, M., Wang, J., Shi, J. T., Wang, W. F., Zhang, Y., et al. (2022a). Gypenosides (GPs) alleviates hypoxia-induced injury in PC12 cells and enhances tolerance to anoxia in C57BL/6 mice. J. Food Biochem. 46 (12), e14448. doi:10.1111/jfbc.14448
Wang, L., Yang, B., Niu, Y. Q., Xu, X., Tian, C. W., Zhang, T. J., et al. (2022b). Research progress on absorption and transport mechanism of tetracyclic triterpenes in traditional Chinese medicine. Drug Eval. Res. 45 (1), 162–170. doi:10.7501/j.issn.1674-6376.2022.01.020
Wang, J. R., Yu, Y. L., Zhang, H. R., Li, L., Wang, J., Su, S. J., et al. (2024). Gypenoside XVII attenuates renal ischemia-reperfusion injury by inhibiting endoplasmic reticulum stress and NLRP3 inflammasome-triggered pyroptosis. Eur. J. Pharmacol. 962, 176187. doi:10.1016/j.ejphar.2023.176187
Wang, H., Chen, C., Xue, M., Zhang, Y., Chen, K., Sun, B., et al. (2025). Identification and mechanism of hepatoprotective saponins and endogenous metabolites in the sweet variant of Gynostemma pentaphyllum. Bioorg Chem. 165, 108996. doi:10.1016/j.bioorg.2025.108996
Weng, X., Lou, Y. Y., Wang, Y. S., Huang, Y. P., Zhang, J., Yin, Z. Q., et al. (2021). New dammarane-type glycosides from Gynostemma pentaphyllum and their lipid-lowering activity. Bioorg Chem. 111, 104843. doi:10.1016/j.bioorg.2021.104843
Wu, W. J., Qu, X., Hu, C. X., Zhu, X. P., Wan, M. Q., Zhou, Y. F., et al. (2024). Gypenoside LXXV alleviates colitis by reprograming macrophage polarization via the glucocorticoid receptor pathway. J. Agric. Food Chem. 72 (37), 20444–20457. doi:10.1021/acs.jafc.4c04784
Xia, X., Chen, J., Ren, H., Zhou, C., Zhang, Q., Cheng, H., et al. (2024). Gypenoside pretreatment alleviates the cerebral ischemia injury via inhibiting the microglia-mediated neuroinflammation. Mol. Neurobiol. 61 (2), 1140–1156. doi:10.1007/s12035-023-03624-0
Xiang, Q. Y., Tian, F., Xu, J., Du, X., Zhang, S. L., and Liu, L. (2022). New insight into dyslipidemia-induced cellular senescence in atherosclerosis. Biol. Rev. 97 (5), 1844–1867. doi:10.1111/brv.12866
Xiao, M. Y., Li, F. F., Xie, P., Qi, Y. S., Xie, J. B., Pei, W. J., et al. (2023). Gypenosides suppress hepatocellular carcinoma cells by blocking cholesterol biosynthesis through inhibition of MVA pathway enzyme HMGCS1. Chemico-Biological Interact. 383, 110674. doi:10.1016/j.cbi.2023.110674
Xiao, M. Y., Pei, W. J., Li, S., Li, F. F., Xie, P., Luo, H. T., et al. (2024). Gypenoside L inhibits hepatocellular carcinoma by targeting the SREBP2-HMGCS1 axis and enhancing immune response. Bioorg Chem. 150, 107539. doi:10.1016/j.bioorg.2024.107539
Xie, Z. F., Jiang, H. W., Liu, W., Zhang, X. W., Chen, D. K., Sun, S. M., et al. (2020). The triterpenoid sapogenin (2α-OH-Protopanoxadiol) ameliorates metabolic syndrome via the intestinal FXR/GLP-1 axis through gut microbiota remodelling. Cell Death & Dis. 11 (9), 770. doi:10.1038/s41419-020-02974-0
Xie, P., Guo, M., Xie, J. B., Xiao, M. Y., Qi, Y. S., Duan, Y., et al. (2022). Effects of heat-processed Gynostemma pentaphyllum on high-fat diet-fed mice of obesity and functional analysis on network pharmacology and molecular docking strategy. J. Ethnopharmacol. 294, 115335. doi:10.1016/j.jep.2022.115335
Xie, P., Xie, J. B., Xiao, M. Y., Guo, M., Qi, Y. S., Li, F. F., et al. (2023). Liver lipidomics analysis reveals the anti-obesity and lipid-lowering effects of gypnosides from heat-processed Gynostemma pentaphyllum in high-fat diet fed mice. Phytomedicine 115, 154834. doi:10.1016/j.phymed.2023.154834
Xing, S. F., Liu, L. H., Zu, M. L., Ding, X. F., Cui, W. Y., Chang, T., et al. (2018). The inhibitory effect of gypenoside stereoisomers, gypenoside L and gypenoside LI, isolated from Gynostemma pentaphyllum on the growth of human lung cancer A549 cells. J. Ethnopharmacol. 219, 161–172. doi:10.1016/j.jep.2018.03.012
Xing, S. F., Lin, M., Wang, Y. R., Chang, T., Cui, W. Y., and Piao, X. L. (2020). Novel dammarane-type saponins from Gynostemma pentaphyllum and their neuroprotective effect. Nat. Prod. Res. 34 (5), 651–658. doi:10.1080/14786419.2018.1495638
Xiuping, W., Fuxian, Z., Zeyu, L., Yi, H., Hongmin, Y., Xiaofen, L., et al. (2021). Analysis of moisture, ash and extract contents of Gynostemma pentaphyllum from different origins based on quality control. Guangdong Agric. Sci. 48 (05), 132–140. doi:10.16768/j.issn.1004-874X.2021.05.017
Xu, L. Y., Yang, Z. H., and Sun, X. B. (2017). Research overview of pharmacokinetic study of dammarane-type ginsenoside. Chin. J. Exp. Traditional Med. Formula 23 (1), 220–227. doi:10.13422/j.cnki.syfjx.2017010220
Xu, X. L., Li, S., Zhang, R., and Le, W. D. (2022). Neuroprotective effects of naturally sourced bioactive polysaccharides: an update. Neural Regen. Res. 17 (9), 1907–1912. doi:10.4103/1673-5374.335142
Xu, B. S., Yang, R. R., Qiang, J. C., Xu, X. H., Zhou, M. Y., Ji, X. M., et al. (2024). Gypenoside XLIX attenuates sepsis-induced splenic injury through inhibiting inflammation and oxidative stress. Int. Immunopharmacol. 127, 111420. doi:10.1016/j.intimp.2023.111420
Yang, K., Zhang, H., Luo, Y., Zhang, J., Wang, M., Liao, P., et al. (2017). Gypenoside XVII prevents atherosclerosis by attenuating endothelial apoptosis and oxidative stress: insight into the ERα-Mediated PI3K/Akt pathway. Int. J. Mol. Sci. 18 (2), 77. doi:10.3390/ijms18020077
Yang, G., Wu, F., Chen, M., Jin, J., Wang, R., and Yuan, Y. (2019a). Formulation design, characterization, and in vitro and in vivo evaluation of nanostructured lipid carriers containing a bile salt for oral delivery of gypenosides, 1178–2013. (Electronic)).
Yang, Q., Liu, S., Han, X., Ma, J., Deng, W., Wang, X., et al. (2019b). Integrated transcriptome and miRNA analysis uncovers molecular regulators of aerial stem-to-rhizome transition in the medical herb Gynostemma pentaphyllum. BMC Genomics 20 (1), 865. doi:10.1186/s12864-019-6250-8
Yang, Q., Zang, H. M., Xing, T., Zhang, S. F., Li, C., Zhang, Y., et al. (2021). Gypenoside XLIX protects against acute kidney injury by suppressing IGFBP7/IGF1R-mediated programmed cell death and inflammation. cell death Inflamm Phytomedicine 85. 153541. doi:10.1016/j.phymed.2021.153541
Yanping, L., Jiachun, H., Tongying, C., Jiangtao, M., Haiwei, G., Yuedong, W., et al. (2020). Mechanism by which gypenosides alleviate oxidative stress injury induced by H2O2 in rat osteoblasts. Chin. J. Tissue Eng. Res. 24 (23), 3649–3653. doi:10.3969/j.issn.2095-4344.2725
Ying, N., Mei-na, Z., Jin-rui, C., Jian, C., and Wei, Z. (2018). Protection and mechanism of gypenosides on the oxidative injury induced by irradiation in mice. Cent. South Pharm. 16 (07), 935–938. doi:10.7539/j.issn.1672-2981.2018.07.009
Yu, Y., Wang, M., Chen, R., Sun, X., Sun, G., and Sun, X. (2021). Gypenoside XVII protects against myocardial ischemia and reperfusion injury by inhibiting ER stress-induced mitochondrial injury. J. Ginseng Res. 45 (6), 642–653. doi:10.1016/j.jgr.2019.09.003
Zhai, X. F., Zu, M. L., Wang, Y. R., Cui, W. Y., Duan, Y., Yang, C., et al. (2021). Protective effects of four new saponins from Gynostemma pentaphyllum against hydrogen peroxide-induced neurotoxicity in SH-SY5Y cells. Bioorg. Chem. 106, 104470. doi:10.1016/j.bioorg.2020.104470
Zhang, H. K., Ye, Y., Zhao, Z. N., Li, K. J., Du, Y., Hu, Q. M., et al. (2017). Neuroprotective effects of gypenosides in experimental autoimmune optic neuritis. Int. J. Ophthalmol. 10 (4), 541–549. doi:10.18240/ijo.2017.04.07
Zhang, H. L., Chen, X., Zong, B. B., Yuan, H. M., Wang, Z. Z., Wei, Y. X., et al. (2018). Gypenosides improve diabetic cardiomyopathy by inhibiting ROS-mediated NLRP3 inflammasome activation. J. Cell. Mol. Med. 22 (9), 4437–4448. doi:10.1111/jcmm.13743
Zhang, H. K., Ye, Y., Li, K. J., Zhao, Z. N., and He, J. F. (2020). Gypenosides prevent H2O2-Induced retinal ganglion cell apoptosis by concurrently suppressing the neuronal oxidative stress and inflammatory response. J. Mol. Neurosci. 70 (4), 618–630. doi:10.1007/s12031-019-01468-9
Zhang, M., Li, J., Guo, X., Wang, X., Shi, D., Cui, L., et al. (2021a). Co-administration of berberine/gypenosides/bifendate ameliorates metabolic disturbance but not memory impairment in type 2 diabetic mice. Heliyon 7 (1), e06004. doi:10.1016/j.heliyon.2021.e06004
Zhang, Q., Guo, X. M., Xie, C., Cao, Z. L., Wang, X., Liu, L., et al. (2021b). Unraveling the metabolic pathway of choline-TMA-TMAO: effects of gypenosides and implications for the therapy of TMAO related diseases. Pharmacol. Res. 173, 105884. doi:10.1016/j.phrs.2021.105884
Zhang, Y., Shi, G., Luo, Z., Wang, J., Wu, S., Zhang, X., et al. (2021c). Activity components from Gynostemma pentaphyllum for preventing hepatic fibrosis and of its molecular targets by network pharmacology approach. Molecules 26 (10), 3006. doi:10.3390/molecules26103006
Zhang, H. X., Wang, Z. Z., and Du, Z. Z. (2022). Sensory-guided isolation and identification of new sweet-tasting dammarane-type saponins from Jiaogulan (Gynostemma pentaphyllum) botanical drugal tea. Food Chem. 388, 132981. doi:10.1016/j.foodchem.2022.132981
Zhang, L., Wang, X. T., He, S. S., Zhang, F., and Li, Y. (2024). Gypenosides suppress fibrosis of the renal NRK-49F cells by targeting miR-378a-5p through the PI3K/AKT signaling pathway. J. Ethnopharmacol. 318, 116466. doi:10.1016/j.jep.2023.116917
Zhao, T. T., Kim, K. S., Shin, K. S., Park, H. J., Kim, H. J., Lee, K. E., et al. (2017). Gypenosides ameliorate memory deficits in MPTP-lesioned mouse model of Parkinson's disease treated with L-DOPA. Bmc Complementary Altern. Med. 17, 449. doi:10.1186/s12906-017-1959-x
Zhao, H., Jiao, W., Xiu, Y., Zhou, K., Zhong, P., Wang, N., et al. (2022). Enzymatic biotransformation of gypenoside XLIX into gylongiposide I and their antiviral roles against enterovirus 71 in vitro. Molecules 27 (13), 4094. doi:10.3390/molecules27134094
Zhao, X., Ge, W., and Miao, Z. (2024). Integrative metabolomic and transcriptomic analyses reveals the accumulation patterns of key metabolites associated with flavonoids and terpenoids of Gynostemma pentaphyllum (Thunb.) Makino. Sci. Rep. 14 (1), 8644. doi:10.1038/s41598-024-57716-5
Zheng, Y., Li, N., Zheng, Z. Z., Chen, L. H., Tong, Q. X., Ming, Y. L., et al. (2018). Advances in research on bioactivity and biotransformation of gypenoside. Food Sci. 39 (13), 324–333. doi:10.7506/spkx1002-6630-201813048
Zhou, K. L., Zhang, Y. Y., Zhou, Y. K., Xu, M. H., and Yu, S. S. (2023a). Production of gypenoside XVII from ginsenoside Rb1 by enzymatic transformation and their anti-inflammatory activity in vitro and in vivo. Molecules 28 (19), 7001. doi:10.3390/molecules28197001
Zhou, T., Cao, L., Du, Y., Qin, L., Lu, Y., Zhang, Q., et al. (2023b). Gypenosides ameliorate high-fat diet-induced nonalcoholic fatty liver disease in mice by regulating lipid metabolism. PeerJ 11, e15225. doi:10.7717/peerj.15225
Zu, M. L., Piao, X. L., Gao, J. M., Xing, S. F., and Liu, L. H. (2020). Monomer gypenoside LI from Gynostemma pentaphyllum inhibits cell proliferation and upregulates expression of miR-128-3p in melanoma cells. J. Biochem. Mol. Toxicol. 34 (5), e22460. doi:10.1002/jbt.22460
Keywords: Gypenosides, pharmacological activity, antioxidant activity, pharmacokinetics, toxicology
Citation: Li X, Chen Y, Wang R, Cao B, Deng T, Han J and Yang M (2025) Gypenosides, a promising phytochemical triterpenoid: research progress on its pharmacological activity and mechanism. Front. Pharmacol. 16:1705946. doi: 10.3389/fphar.2025.1705946
Received: 15 September 2025; Accepted: 09 October 2025;
Published: 16 October 2025.
Edited by:
Javier Echeverria, University of Santiago, ChileReviewed by:
Ramoji Kosuru, Versiti Blood Research Institute, United StatesYinan Zhang, Nanjing University of Chinese Medicine, China
Copyright © 2025 Li, Chen, Wang, Cao, Deng, Han and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meina Yang, eWFuZ18yMDI1MDRAMTI2LmNvbQ==
 Xue Li1,2
Xue Li1,2 Ruyu Wang
Ruyu Wang Baorui Cao
Baorui Cao Tingting Deng
Tingting Deng Jinxiang Han
Jinxiang Han Meina Yang
Meina Yang