Abstract
Objective:
Previous randomized controlled trials have shown good efficacy and safety of omadacycline in patients with infectious diseases, but relevant real-world evidence is still insufficient. This large-scale study aimed to explore the efficacy and safety of omadacycline for the treatment of infectious diseases in real-world conditions.
Methods:
This was a retrospective, real-world study. A total of 2587 patients with infectious diseases who received omadacycline treatment were enrolled. Clinical success was defined as resolution or significant improvement of infection-related signs and symptoms without the need for salvage antimicrobial therapy.
Results:
After omadacycline treatment, 81.6% of patients achieved clinical success. Multivariate logistic regression analysis suggested that older age [odds ratio (OR): 0.991, P = 0.025], history of any malignancy (OR: 0.630, P = 0.016), abnormal renal function (OR: 0.432, P = 0.003), mechanical ventilation (OR: 0.559, P = 0.013), intensive care unit (ICU) (OR: 0.328, P < 0.001), and longer length of stay (LOS) (OR: 0.941, P < 0.001) were independently related to a lower probability of achieving clinical success. However, longer days of omadacycline use were independently associated with a higher probability of achieving clinical success (OR: 1.121, P < 0.001). Regarding adverse events, the incidence of coagulation disorders, acute kidney injury, liver injury, and gastrointestinal reaction was 10.5%, 9.9%, 6.2%, and 5.3%, respectively.
Conclusion:
Omadacycline possesses favorable efficacy and safety for the treatment of infectious diseases. Baseline age, malignancy history, renal function, mechanical ventilation, ICU, LOS, and omadacycline use duration are strong predictors of clinical success.
1 Introduction
Infectious diseases caused by bacteria, such as pneumonia, urinary tract infections, and skin and skin-structure infections, constitute a profound global health burden (Spagnolo, 2024). The primary pharmacological agents for treating bacterial infections include beta-lactams, macrolides, fluoroquinolones, and tetracyclines (Sujith et al., 2024; Vaughn et al., 2024; Tiseo and Falcone, 2025). However, some pathogens, such as Enterococcus faecium, Acinetobacter baumannii, Klebsiella pneumoniae, and Staphylococcus aureus, are becoming increasingly resistant to conventional antimicrobial agents, leading to poor clinical outcomes (Ho et al., 2025; Aggarwal et al., 2024; Krishnaprasad and Kumar, 2024; Larkin, 2023). It is estimated that bacterial antimicrobial resistance contributed to 1.14 million deaths globally in 2021, and projections suggest that this number will rise to 1.91 million in 2050 (GBDAR, 2024). Therefore, exploring promising antibiotics for the treatment of infectious diseases is fundamental.
Omadacycline, a novel aminomethylcycline belonging to the tetracycline class, is effective against a broad range of Gram-positive and select Gram-negative pathogens, including resistance determinant-containing strains (Macone et al., 2014; Karvouniaris et al., 2023; Kounatidis et al., 2024). Currently, several studies have demonstrated favorable efficacy and safety profiles of omadacycline in treating infectious diseases, including community-acquired bacterial pneumonia and acute bacterial skin and skin-structure infections (Torres et al., 2021; O'Riordan et al., 2019a; Stets et al., 2019; O'Riordan et al., 2019b; Gao et al., 2024). However, the majority of evidence is derived from randomized controlled trials (Torres et al., 2021; O'Riordan et al., 2019a; Stets et al., 2019; O'Riordan et al., 2019b), which employed stringent patient selection criteria. Such strict inclusion criteria limit the extrapolation of these findings to routine clinical settings. Although two real-world studies have been performed (Gao et al., 2024; El Ghali et al., 2023), the sample size was small, with one enrolling 183 patients (Gao et al., 2024) and the other enrolling 75 patients (El Ghali et al., 2023), which restricts the statistical power.
Accordingly, we established a large retrospective cohort and aimed to explore the efficacy and safety of omadacycline for the treatment of infectious diseases in real-world conditions.
2 Methods
2.1 Study design and population
This was a retrospective, real-world study that consecutively included 2587 adult patients who were treated with intravenous omadacycline (loading dose: 0.2 g once daily or 0.1 g twice daily; maintenance dose: 0.1 g once daily) between April 2022 and December 2024. To justify the representativeness of our study population in real world conditions, our cohort was drawn from diverse hospital departments, including respiratory and critical care medicine, public health medicine, emergency, infectious diseases, and general practice. Moreover, it encompassed a wide spectrum of infection sites, including pulmonary infections, urinary infections, skin and soft tissue infections, and others. Patients were included if they were: 1) age ≥18 years; 2) had a clinically and/or microbiologically confirmed diagnosis of infectious diseases, including but not limited to pulmonary infection, urinary infection, or skin and soft tissue infection; 3) received omadacycline as part of their antimicrobial therapy (either as monotherapy or in combination with other antimicrobials); 4) had complete baseline clinical data; 5) had available information for efficacy or safety assessment. Exclusion criteria were as follows: 1) died within 48 h of starting omadacycline treatment; 2) early discontinuation for other reason (e.g., financial or administrative issues) within 48 h; 3) received omadacycline therapy for less than 72 h. Although this was a single-center study, the hospital serves as a major tertiary referral center that manages a broad range of infectious diseases, and the included cases reflect the diversity and complexity of real-world patients receiving omadacycline. The Ethical Committee approved this study with the approval number of IIT20250347, and the waiver of informed consent was also approved. To protect patient privacy, all clinical data were de-identified before analysis: personal identifiers were permanently deleted, and only study-relevant clinical/treatment variables were retained. De-identified data were stored on the hospital’s encrypted server, with access limited to authorized research team members. All data management followed the Declaration of Helsinki and local medical data privacy regulations.
2.2 Data collection and variables
Clinical characteristics were screened from the Electronic Medical Record. The specific variables were as follows: general data (e.g., demographic, admission year, department of hospitalization), disease history (e.g., history of surgery, history of any malignancy), comorbid conditions (e.g., hypertension, coronary heart disease (CHD), diabetes mellitus (DM), septic shock, abnormal liver function, abnormal renal function, intracranial hemorrhage), and treatment information (e.g., infection site, treatment type, omadacycline form, days of omadacycline use, concomitant use of other antibiotics, mechanical ventilation, intensive care unit (ICU), and length of stay (LOS)). Laboratory data before and after treatment were extracted, involving liver function, renal function, and coagulation parameters.
2.3 Study outcomes
The efficacy outcome was clinical success, defined as resolution or significant improvement of infection-related signs and symptoms without the need for salvage antimicrobial therapy (Gao et al., 2024). Assessment of clinical success was conducted during the time window spanning from 72 h after the first dose to 7 days after the final administration of omadacycline. Besides, liver injury, acute kidney injury, gastrointestinal reaction, and coagulation disorders were screened for safety analysis (Technology Committee on DP et al., 2023; National Clinical Research Center for Kidney D et al., 2023; Author anonymous, 2024). All laboratory measurements in this study were performed using fully automated, clinically validated analyzers that were routinely used in our hospital’s clinical laboratory, including the Mindray BC-7500 for complete blood count, the Sysmex CN-6000 for coagulation tests, and the Roche cobas 8000 for biochemical parameters.
2.4 Statistics
The sample size was not calculated, and patients were consecutively included. Descriptive statistics were performed to describe the data. Continuous variables were summarized using medians with interquartile ranges (IQR). Between-group comparisons were conducted using the Mann-Whitney U test. The Wilcoxon signed-rank test was employed to compare paired data before and after omadacycline use, while unpaired data before and after omadacycline use were conducted via Mann-Whitney U test. Categorical variables were described as counts and corresponding percentages. Comparisons between categorical variables were assessed via the Chi-square test. Logistic regression analysis was conducted to explore the association between clinical characteristics and clinical success. Variables of general data, disease histories, comorbid conditions, and treatment information were included in a multivariate logistic regression model (forward step-wise method) to explore independent predictors of clinical success and adverse events. To minimize selection bias, we employed a consecutive enrollment strategy for all eligible patients. Measurement bias was minimized by using validated tools and pre-defined criteria for all assessments. Confounding was addressed by including the relevant covariates collected in multivariate regression analyses to adjust for potential baseline differences among patients. SPSS V.29.0 (IBM, United States of America) was used for data analysis. A P value <0.05 indicated statistical significance.
3 Results
3.1 Clinical features and treatment information
The median (IQR) age of patients was 66.0 (53.0–75.0) years. There were 1558 (60.2%) males and 1029 (39.8%) females. A total of 234 (9.0%) patients had a history of any malignancy. Regarding comorbidities, 781 (30.2%), 106 (4.1%), 342 (13.2%), 29 (1.1%), 266 (10.3%), 105 (4.1%), and 62 (2.4%) patients had hypertension, CHD, DM, septic shock, abnormal liver function, abnormal renal function, and intracranial hemorrhage, respectively. The detailed clinical features of patients are shown in Table 1.
TABLE 1
| Items | Patients (N = 2587) |
|---|---|
| General data | |
| Age (years), median (IQR) | 66.0 (53.0–75.0) |
| Sex, n (%) | |
| Male | 1558 (60.2) |
| Female | 1029 (39.8) |
| BMI (kg/m2), median (IQR) (n = 2123) | 22.0 (19.6–24.7) |
| Admission year, n (%) | |
| 2022 | 13 (0.5) |
| 2023 | 986 (38.1) |
| 2024 | 1588 (61.4) |
| Department of hospitalization, n (%) | |
| Respiratory and critical care medicine | 875 (33.8) |
| Public health medicine | 758 (29.3) |
| Emergency | 266 (10.3) |
| Infectious diseases | 204 (7.9) |
| General practice | 85 (3.3) |
| Others | 400 (15.5) |
| Disease histories | |
| History of surgery, n (%) | 1024 (39.6) |
| History of any malignancy, n (%) | 234 (9.0) |
| Comorbid conditions | |
| Hypertension, n (%) | 781 (30.2) |
| CHD, n (%) | 106 (4.1) |
| DM, n (%) | 342 (13.2) |
| Septic shock, n (%) | 29 (1.1) |
| Abnormal liver function, n (%) | 266 (10.3) |
| Abnormal renal function, n (%) | 105 (4.1) |
| Intracranial hemorrhage, n (%) | 62 (2.4) |
Clinical characteristics of patients receiving omadacycline treatment.
IQR, interquartile range; BMI, body mass index; CHD, coronary heart disease; DM, diabetes mellitus.
Regarding treatment-related information, the median (IQR) duration of omadacycline use was 8.0 (6.0–11.0) days. Mechanical ventilation was applied in 200 (7.7%) patients. Three hundred and sixty-three (14.0%) patients were admitted to the ICU. The median (IQR) LOS was 13.0 (9.0–19.0) days. The detailed treatment information is shown in Table 2. A total of 14.1% of patients used other antibiotics in combination, of which, β-lactam (35.3%) and carbapenems (27.4%) were the most common concomitant antibiotics. The detailed information on the concomitant antibiotics is shown in Supplementary Table S1.
TABLE 2
| Items | Patients (N = 2587) |
|---|---|
| Infection site, n (%) | |
| Pulmonary infection | 2183 (84.4) |
| Urinary infection | 74 (2.9) |
| Skin and soft tissue infection | 52 (2.0) |
| Others or unknown infection | 359 (13.9) |
| Treatment type, n (%) | |
| Targeted | 461 (17.8) |
| Empiric | 2126 (82.2) |
| Omadacycline form, n (%) | |
| Injection | 2587 (100.0) |
| Days of omadacycline use (days), median (IQR) | 8.0 (6.0–11.0) |
| Concomitant use of other antibiotics, n (%) | |
| No | 2222 (85.9) |
| Yes | 365 (14.1) |
| Mechanical ventilation, n (%) | |
| No | 2387 (92.3) |
| Yes | 200 (7.7) |
| ICU, n (%) | |
| No | 2224 (86.0) |
| Yes | 363 (14.0) |
| LOS (days), median (IQR) | 13.0 (9.0–19.0) |
Treatment information of patients receiving omadacycline treatment.
IQR, interquartile range; ICU, intensive care unit; LOS, length of stay.
3.2 Pathogens
A total of 463 patients were microbiologically confirmed infections, and 580 pathogens were detected. The leading pathogens identified were K. pneumoniae (18.6%), followed by Mycoplasma pneumoniae (14.3%), A. baumannii (13.8%), Pseudomonas aeruginosa (6.9%) and Stenotrophomonas maltophilia (6.0%). The detailed pathogen profile is shown in Supplementary Table S2.
3.3 Clinical success rate after omadacycline treatment
After omadacycline treatment, a total of 2,111 patients achieved resolution or significant improvement of infection-related signs and symptoms, and did not require salvage antimicrobial therapy, resulting in a clinical success rate of 81.6%. The other 476 (18.4%) patients failed to achieve clinical success (Figure 1). The above data suggested that the efficacy of omadacycline was acceptable for the treatment of infectious diseases.
FIGURE 1
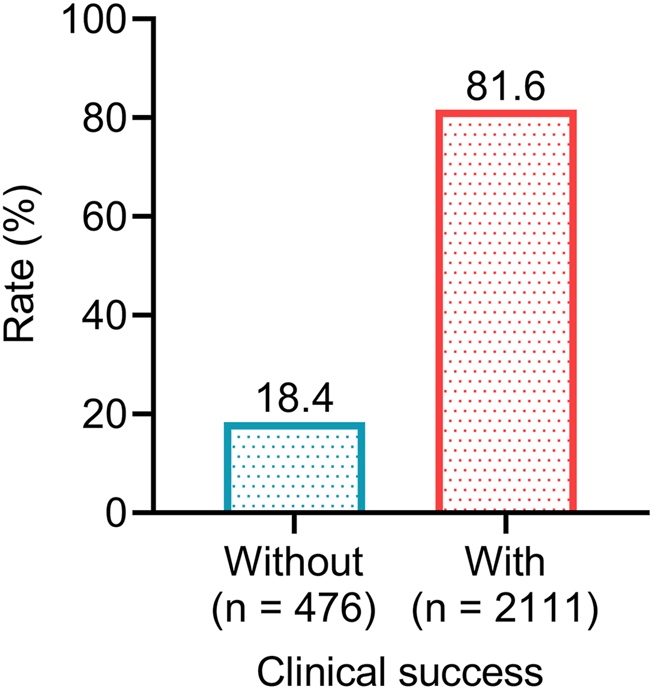
The proportions of patients achieving clinical success after omadacycline treatment.
3.4 Factors related to clinical success after omadacycline treatment
Regarding general data, younger age (P < 0.001), female sex (P < 0.001), and general practice department of hospitalization (P = 0.003) were associated with a higher rate of clinical success, which indicated that patients with the above characteristics might benefit more from omadacycline treatment. However, body mass index (BMI) and admission year were not associated with clinical success (both P > 0.05) (Figures 2A–E). Regarding disease histories and comorbid conditions, without a history of any malignancy (P = 0.014), without septic shock (P = 0.025), without abnormal renal function (P < 0.001), and without intracranial hemorrhage (P = 0.029) were related to a higher rate of clinical success. The above data suggested that patients without the major organ dysfunction and critical illness were more likely to achieve clinical success after omadacycline treatment. History of surgery and other comorbid conditions, including hypertension, CHD, DM, and abnormal liver function were not related to clinical success (all P > 0.05) (Figures 3A–I).
FIGURE 2
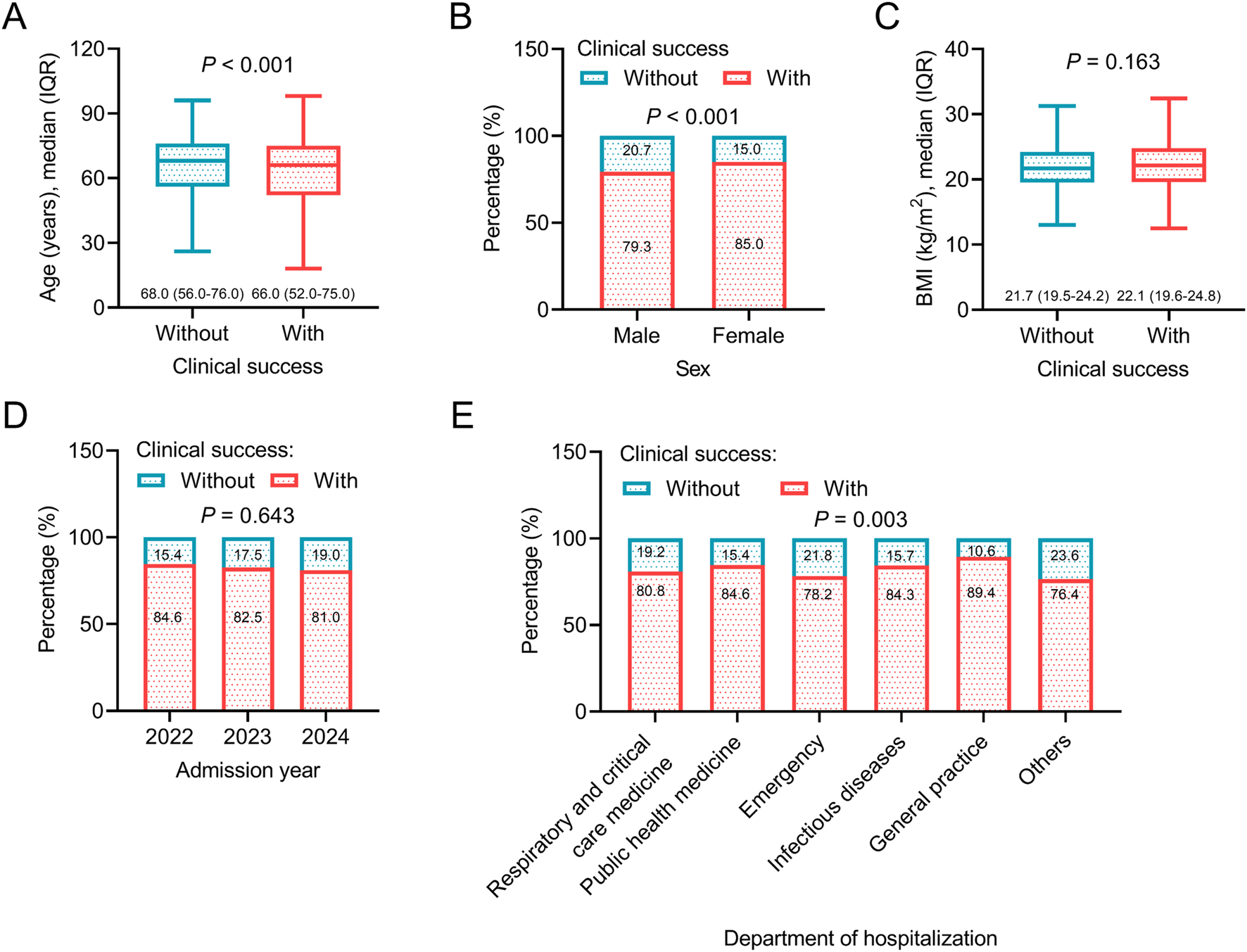
Association between general data and clinical success. Association of age (A), sex (B), BMI (C), admission year (D), and department of hospitalization (E) with clinical success in patients receiving omadacycline.
FIGURE 3
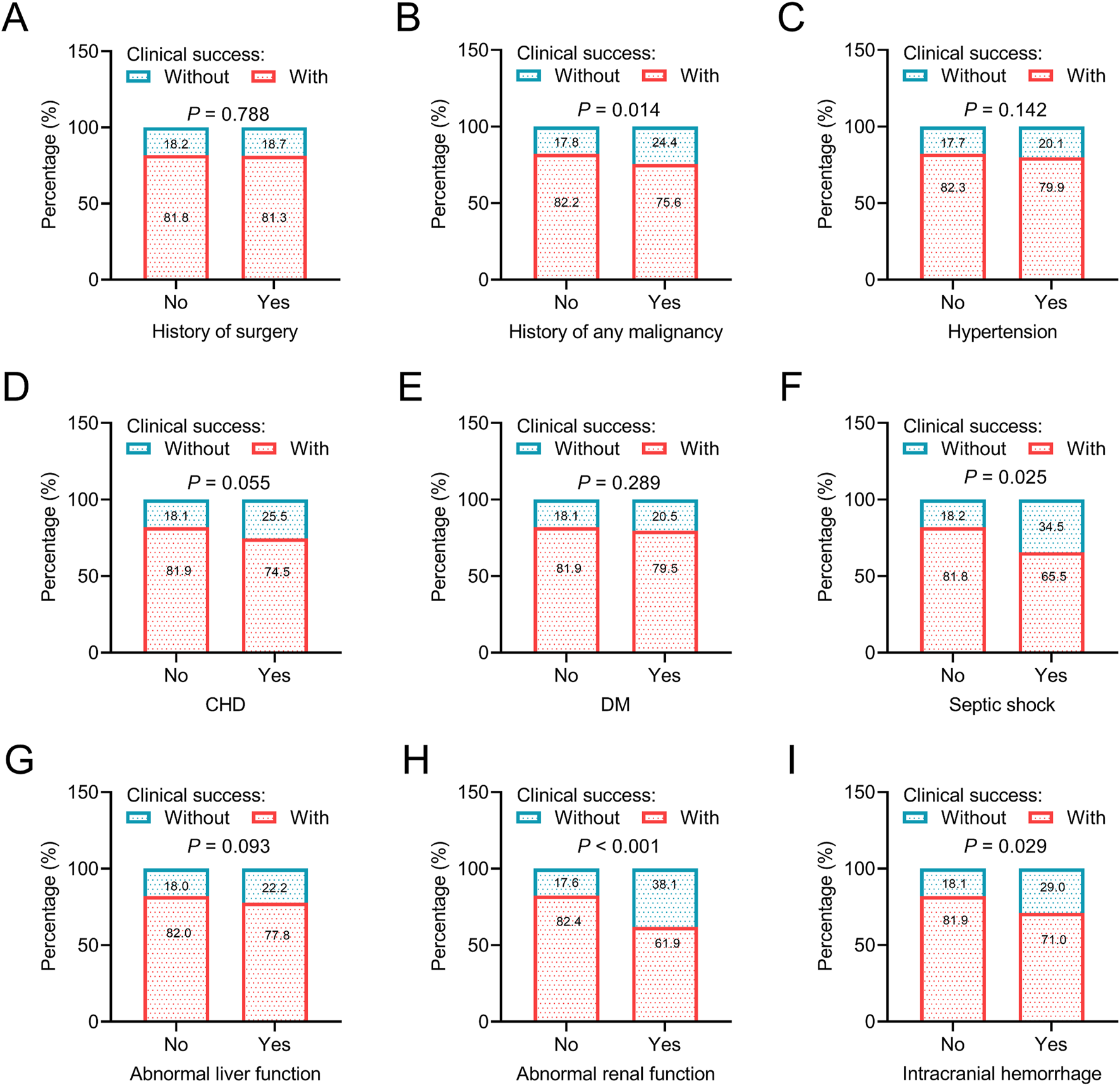
Association of disease histories and comorbid conditions with clinical success. Association of history of surgery (A), history of any malignancy (B), hypertension (C), CHD (D), DM (E), septic shoch (F), abnormal liver function (G), abnormal renal function (H), and intracranial hemorrhage (I) with clinical success in patients receiving omadacycline.
In terms of treatment-related information, targeted treatment type (P < 0.001), longer days of omadacycline use (P < 0.001), without concomitant use of other antibiotics (P = 0.021), without mechanical ventilation (P < 0.001), not admitting in ICU (P < 0.001), and shorter LOS (P < 0.001) were related to a higher rate of clinical success, which suggested that adequate treatment course and less severe clinical status at baseline might be determinants for achieving clinical success after omadacycline treatment. However, the infection sites were not related to clinical success (all P > 0.05) (Figures 4A–G).
FIGURE 4
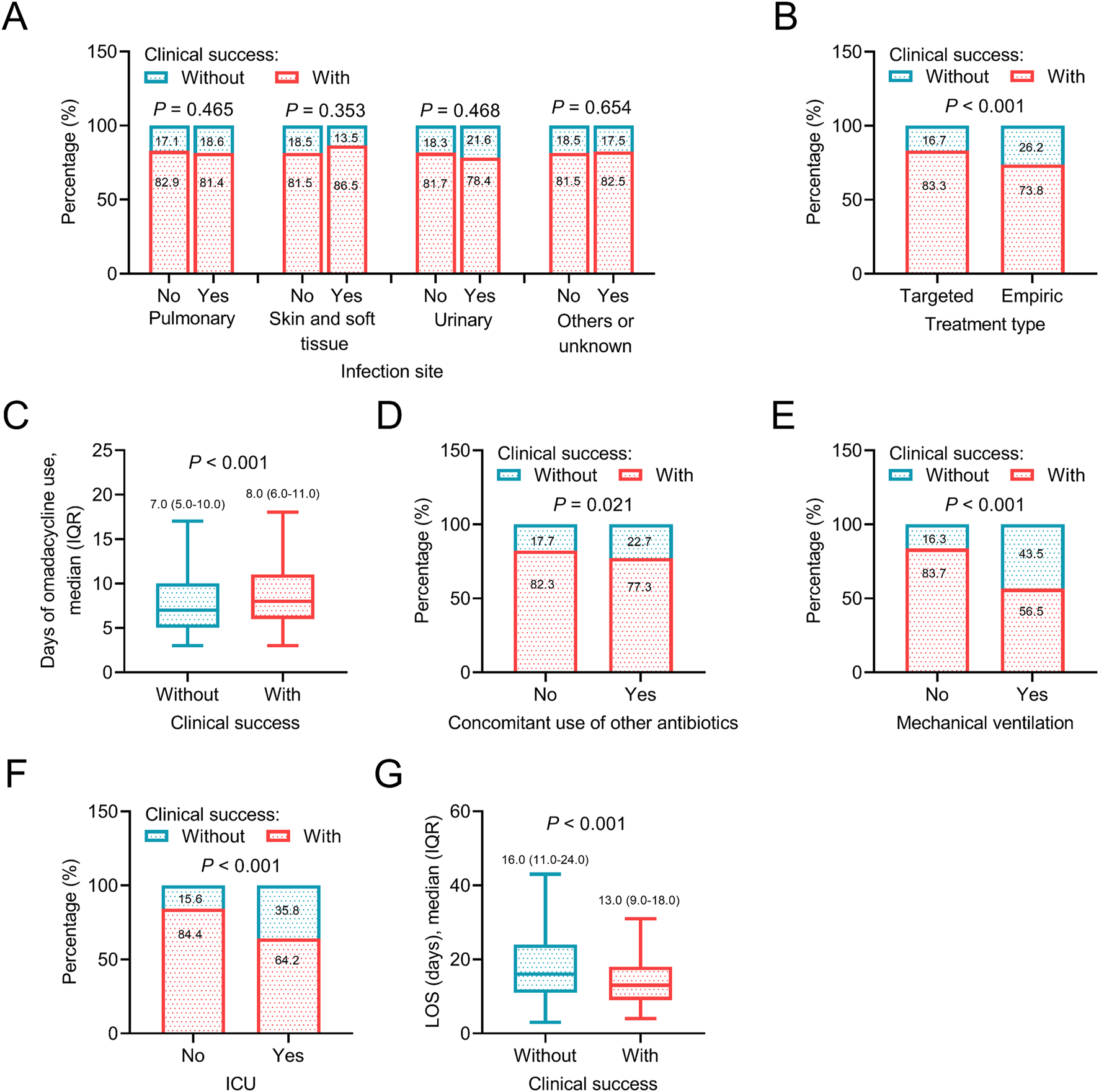
Association between treatment information and clinical success. Association of infection site (A), treatment type (B), days of omadacycline use (C), concomitant use of other antibiotics (D), mechanical ventilation (E), ICU (F), and LOS (G) with clinical success in patients receiving omadacycline.
3.5 Multivariate logistic regression analysis for clinical success after omadacycline treatment
According to the multivariate logistic regression analysis, older age [odds ratio (OR): 0.991, P = 0.018], history of any malignancy (OR: 0.587, P = 0.004), abnormal renal function (OR: 0.423, P = 0.002), targeted treatment type (OR: 0.725, P = 0.035) mechanical ventilation (OR: 0.419, P < 0.001), and ICU (OR: 0.403, P < 0.001) were independently related to a lower probability of achieving clinical success. The above factors might serve as crucial predictors of clinical success after omadacycline treatment. Other factors, including concomitant use of other antibiotics, were not independently related to clinical success (Table 3).
TABLE 3
| Factors | P value | OR | 95% CI | |
|---|---|---|---|---|
| Lower | Upper | |||
| Age (per year) | 0.018 | 0.991 | 0.984 | 0.998 |
| History of any malignancy (yes vs. no) | 0.004 | 0.587 | 0.409 | 0.843 |
| Abnormal renal function (yes vs. no) | 0.002 | 0.423 | 0.243 | 0.738 |
| Treatment type (targeted vs. empiric) | 0.035 | 0.725 | 0.537 | 0.978 |
| Mechanical ventilation (yes vs. no) | <0.001 | 0.419 | 0.270 | 0.650 |
| ICU (yes vs. no) | <0.001 | 0.403 | 0.287 | 0.566 |
Multivariate logistic regression analysis on clinical success.
OR, odds ratio; CI, confidence interval; ICU, intensive care unit; LOS, length of stay. Identifier information, disease histories, comorbid conditions, and treatment information were input into the model. Days of omadacycline use and length of stay were not put in the model due to causal inversion problem. A forward step-wise multivariate logistic regression analysis was conducted.
3.6 The change in blood routine examination parameters after omadacycline treatment
In the unpaired analysis, after omadacycline treatment, improvements in blood routine examination parameters were noted (all P < 0.001). The paired analysis showed that after omadacycline treatment, white blood cell (WBC), hemoglobin, neutrophil, and C-reactive protein (CRP) were decreased, while platelet (PLT) and eosinophils were increased (all P < 0.001) (Table 4). The above findings indicated a significant resolution of inflammation following omadacycline treatment.
TABLE 4
| Items | Before medication | After medication | P value | ||
|---|---|---|---|---|---|
| n (%) | Values | n (%) | Values | ||
| Unpaired | |||||
| WBC (×109/L), median (IQR) | 2495 (96.4) | 8.5 (5.9–11.7) | 2537 (98.1) | 6.7 (4.9–9.1) | <0.001 |
| Hemoglobin (g/L), median (IQR) | 2490 (96.3) | 115.0 (98.0–130.0) | 2544 (98.3) | 110.0 (91.0–125.0) | <0.001 |
| PLT (×109/L), median (IQR) | 2511 (97.1) | 201.0 (135.0–271.0) | 2543 (98.3) | 253.0 (174.0–342.0) | <0.001 |
| Neutrophil (%), median (IQR) | 2497 (96.5) | 80.9 (72.6–87.5) | 2537 (98.1) | 70.3 (59.4–80.8) | <0.001 |
| Eosinophils (×109/L), median (IQR) | 2473 (95.6) | 0.03 (0.00–0.11) | 2527 (97.7) | 0.11 (0.04–0.20) | <0.001 |
| CRP (mg/L), median (IQR) | 2465 (95.3) | 66.9 (24.4–122.3) | 2457 (95.0) | 11.6 (4.3–40.8) | <0.001 |
| Paired | |||||
| WBC (×109/L), median (IQR) | 2454 (94.9) | 8.5 (5.9–11.7) | 2454 (94.9) | 6.7 (4.9–9.1) | <0.001 |
| Hemoglobin (g/L), median (IQR) | 2454 (94.9) | 115.0 (98.0–130.0) | 2454 (94.9) | 110.0 (92.0–125.0) | <0.001 |
| PLT (×109/L), median (IQR) | 2475 (95.7) | 201.0 (135.0–271.0) | 2475 (95.7) | 253.0 (175.0–341.0) | <0.001 |
| Neutrophil (%), median (IQR) | 2456 (94.9) | 81.0 (72.7–87.6) | 2456 (94.9) | 70.2 (59.2–80.8) | <0.001 |
| Eosinophils (×109/L), median (IQR) | 2430 (93.9) | 0.03 (0.00–0.11) | 2430 (93.9) | 0.11 (0.04–0.20) | <0.001 |
| CRP (mg/L), median (IQR) | 2355 (91.0) | 68.0 (25.1–123.5) | 2355 (91.0) | 11.5 (4.3–40.1) | <0.001 |
Parameters of blood routine examination.
WBC, white blood cell; IQR, interquartile range; PLT, platelet; CRP, C-reactive protein.
3.7 The change in liver function, kidney function, and coagulation function parameters after omadacycline treatment
In the unpaired analysis, most liver function, kidney function, and coagulation function parameters were numerically improved after omadacycline treatment (all P < 0.05), except for alkaline phosphatase (ALP) (P = 0.340) and D-dimer (P = 0.062). According to the paired analysis, liver function parameters, including aspartate aminotransferase (AST), total bilirubin (TBIL), and lactate dehydrogenase (LDH), were decreased, but alanine aminotransferase (ALT) was increased after omadacycline treatment (all P < 0.001). Regarding kidney function, creatinine (Cr), urea, and uric acid (UA) were decreased after omadacycline treatment (all P < 0.01). With respect to coagulation function, D-dimer, prothrombin time, and fibrinogen were reduced after omadacycline treatment (all P < 0.001) (Table 5). The above findings indicated that liver function, kidney function, and coagulation function were improved after omadacycline treatment.
TABLE 5
| Items | Before medication | After medication | P value | ||
|---|---|---|---|---|---|
| n (%) | Values | n (%) | Values | ||
| Unpaired | |||||
| ALT (U/L), median (IQR) | 1996 (77.2) | 25.0 (14.2–49.6) | 2413 (93.3) | 31.0 (18.1–56.0) | <0.001 |
| AST (U/L), median (IQR) | 1999 (77.3) | 30.0 (20.0–54.8) | 2411 (93.2) | 29.6 (20.3–44.8) | 0.017 |
| TBIL (μmol/L), median (IQR) | 1991 (77.0) | 10.0 (6.8–15.3) | 2400 (92.8) | 7.8 (5.4–11.6) | <0.001 |
| GGT (U/L), median (IQR) | 1872 (72.4) | 34.3 (20.0–74.0) | 2272 (87.8) | 40.3 (23.0–81.6) | <0.001 |
| ALP (U/L), median (IQR) | 1896 (73.3) | 85.5 (66.8–118.2) | 2296 (88.8) | 87.1 (66.8–119.9) | 0.340 |
| Cr (μmol/L), median (IQR) | 1996 (77.2) | 76.5 (60.1–103.0) | 2383 (92.1) | 69.9 (56.0–92.3) | <0.001 |
| Urea (mmol/L), median (IQR) | 1969 (76.1) | 5.8 (4.1–9.0) | 2338 (90.4) | 5.3 (3.8–7.7) | <0.001 |
| UA (μmol/L), median (IQR) | 1972 (76.2) | 252.0 (179.0–355.0) | 2359 (91.2) | 240.0 (171.3–322.0) | <0.001 |
| LDH (U/L), median (IQR) | 1605 (62.0) | 275.7 (219.0–371.8) | 1829 (70.7) | 253.3 (202.4–333.7) | <0.001 |
| D-dimer (mg/L), median (IQR) | 1894 (73.2) | 1.2 (0.5–3.0) | 1784 (69.0) | 1.1 (0.5–2.8) | 0.062 |
| PT (s), median (IQR) | 1932 (74.7) | 12.8 (11.9–13.9) | 1846 (71.4) | 12.3 (11.4–13.4) | <0.001 |
| Fibrinogen (g/L), median (IQR) | 1920 (74.2) | 4.8 (3.5–6.1) | 1821 (70.4) | 3.8 (2.9–4.8) | <0.001 |
| Paired | |||||
| ALT (U/L), median (IQR) | 1888 (73.0) | 25.5 (14.3–52.2) | 1888 (73.0) | 30.8 (18.0–56.0) | <0.001 |
| AST (U/L), median (IQR) | 1888 (73.0) | 31.0 (20.0–56.2) | 1888 (73.0) | 30.6 (21.0–47.0) | <0.001 |
| TBIL (μmol/L), median (IQR) | 1875 (72.5) | 10.1 (6.9–15.6) | 1875 (72.5) | 7.8 (5.4–11.9) | <0.001 |
| GGT (U/L), median (IQR) | 1711 (66.1) | 35.0 (20.0–76.8) | 1711 (66.1) | 41.0 (23.1–83.0) | 0.187 |
| ALP (U/L), median (IQR) | 1738 (67.2) | 86.0 (67.0–119.0) | 1738 (67.2) | 88.4 (67.2–121.4) | 0.404 |
| Cr (μmol/L), median (IQR) | 1869 (72.2) | 76.8 (60.6–104.6) | 1869 (72.2) | 70.5 (55.8–95.2) | <0.001 |
| Urea (mmol/L), median (IQR) | 1826 (70.6) | 5.9 (4.1–9.2) | 1826 (70.6) | 5.6 (3.9–8.2) | <0.001 |
| UA (μmol/L), median (IQR) | 1829 (70.7) | 252.3 (180.0–356.5) | 1829 (70.7) | 233.9 (164.6–319.2) | <0.001 |
| LDH (U/L), median (IQR) | 1189 (46.0) | 281.4 (222.6–391.0) | 1189 (46.0) | 257.4 (201.9–341.6) | <0.001 |
| D-dimer (mg/L), median (IQR) | 1411 (54.5) | 1.5 (0.6–3.7) | 1411 (54.5) | 1.2 (0.5–3.0) | <0.001 |
| PT (s), median (IQR) | 1461 (56.5) | 12.9 (12.0–14.1) | 1461 (56.5) | 12.4 (11.5–13.5) | <0.001 |
| Fibrinogen (g/L), median (IQR) | 1438 (55.6) | 4.7 (3.5–6.0) | 1438 (55.6) | 3.8 (2.9–4.8) | <0.001 |
Parameters of liver function, kidney function, and coagulation function.
ALT, alanine aminotransferase; IQR, interquartile range; AST, aspartate aminotransferase; TBIL, total bilirubin; GGT, gamma-glutamyl transferase; ALP, alkaline phosphatase; Cr, creatinine; CKD-EPI, chronic kidney disease epidemiology collaboration; UA, uric acid; LDH, lactate dehydrogenase; PT, prothrombin time.
3.8 Safety
The most common adverse event was coagulation disorders (10.5%), followed by acute kidney injury (9.9%), liver injury (6.2%), and gastrointestinal reaction (5.3%). Most liver injury was grade I-II (5.9%), with only 0.3% being grade III. For acute kidney injury, the incidence was 5.5% for grade I-II and 4.4% for grade III (Table 6). These safety data suggested that omadacycline possessed a tolerable safety profile in real-world practice.
TABLE 6
| Items | Total | Grade I | Grade II | Grade III |
|---|---|---|---|---|
| Liver injury, n (%) | 160 (6.2) | 127 (4.9) | 25 (1.0) | 8 (0.3) |
| Acute kidney injury, n (%) | 256 (9.9) | 67 (2.6) | 75 (2.9) | 114 (4.4) |
| Coagulation disorders, n (%) | 271 (10.5) | (−) | (−) | (−) |
| Gastrointestinal reaction, n (%) | 138 (5.3) | (−) | (−) | (−) |
Adverse events.
3.9 Multivariate logistic regression analysis for adverse events after omadacycline treatment
Older age (OR: 1.020, P < 0.001), abnormal liver function (OR: 2.030, P < 0.001), abnormal renal function (OR: 6.628, P < 0.001), targeted treatment type (OR: 1.374, P = 0.013), mechanical ventilation (OR: 2.265, P < 0.001), and ICU (OR: 3.458, P < 0.001) were independently related to a higher risk of adverse events. The above factors might serve as crucial predictors of adverse events after omadacycline treatment. Other factors, including concomitant use of other antibiotics, were not independently related to adverse events (Table 7).
TABLE 7
| Factors | P value | OR | 95% CI | |
|---|---|---|---|---|
| Lower | Upper | |||
| Age (per year) | <0.001 | 1.020 | 1.014 | 1.026 |
| Abnormal liver function (yes vs. no) | <0.001 | 2.030 | 1.468 | 2.806 |
| Abnormal renal function (yes vs. no) | <0.001 | 6.628 | 3.198 | 13.737 |
| Treatment type (targeted vs. empiric) | 0.013 | 1.374 | 1.069 | 1.766 |
| Mechanical ventilation (yes vs. no) | <0.001 | 2.265 | 1.416 | 3.623 |
| ICU (yes vs. no) | <0.001 | 3.458 | 2.463 | 4.854 |
Multivariate logistic regression analysis on adverse events.
OR, odds ratio; CI, confidence interval; ICU, intensive care unit. Identifier information, disease histories, comorbid conditions, and treatment information (excepted for ‘days of omadacycline use’ and ‘length of stay’) were input into the model. A forward step-wise multivariate logistic regression analysis was conducted.
4 Discussion
Previous in vitro experiments have disclosed that omadacycline is active against various bacteria and antibiotic-resistant bacteria (Nicklas et al., 2022; Heine et al., 2024; Waites et al., 2022; Li et al., 2023; Skinner et al., 2023). Clinically, omadacycline demonstrates favorable efficacy for the treatment of diverse infectious diseases (Torres et al., 2021; O'Riordan et al., 2019a; Stets et al., 2019; O'Riordan et al., 2019b; Gao et al., 2024). For instance, two randomized controlled trials reported that omadacycline achieved a clinical success rate of 87.6%–88.4% in patients with community-acquired bacterial pneumonia (Torres et al., 2021; Stets et al., 2019). The other two previous randomized controlled trials indicated that the clinical success rate ranged from 84.0% to 86.1% after omadacycline treatment in patients with acute bacterial skin and skin-structure infections (O'Riordan et al., 2019a; O'Riordan et al., 2019b). Regarding patients with urinary tract infection, a phase 1b study found that the clinical success rate on 5–9 days after the last omadacycline dosing was 84.0% (Overcash et al., 2019). In this study, we found that after omadacycline treatment, 81.6% of patients with infectious diseases achieved clinical success. Our findings were in line with the previous trials, which suggested that omadacycline could be considered as a potent antibiotic for treating patients with infectious diseases. In real-world conditions, a previous study found that the clinical success rate was 71.0% after omadacycline treatment in patients with infectious diseases (Gao et al., 2024). Another study reported that 80.0% of patients with infectious diseases achieved clinical success after omadacycline treatment (El Ghali et al., 2023). The clinical success rate after omadacycline treatment seemed slightly different between our study and previous real-world studies. We speculated that the disparities might be attributed to variations in patients’ baseline clinical characteristics and treatment information. Overall, these collective data support that omadacycline had a good ability to relieve infection-related signs and symptoms, even under complex real-world clinical conditions.
According to a previous study, several factors were related to clinical success after omadacycline treatment in patients with infectious diseases, including moderate to severe liver function impairment, admission to the respiratory department, and omadacycline treatment duration (Gao et al., 2024). In this study, we performed a multivariate logistic regression analysis and discovered several independent factors related to clinical success after omadacycline treatment. (Spagnolo, 2024). Older age was independently related to a lower probability of clinical success. This finding suggested that younger patients might benefit more from omadacycline treatment, which may be related to their better immune function (Liu et al., 2023). (Sujith et al., 2024) History of any malignancy was independently related to a lower probability of clinical success. A potential reason might be that malignancies and their treatments might impair patients’ immune function, weakening the ability to fight infections (Lehrnbecher et al., 2008), thereby reducing the likelihood of clinical success after omadacycline treatment. (Vaughn et al., 2024). Abnormal renal function was independently related to a lower probability of clinical success after omadacycline treatment. A possible explanation would be that renal dysfunction often associates with immune impairment (Bumbea et al., 2024), which might weaken the body’s ability to clear bacteria, thereby preventing clinical success. (Tiseo and Falcone, 2025). Mechanical ventilation and ICU were independently associated with a lower probability of clinical success. This finding revealed that patients with severe disease conditions were more prone to treatment failure. Therefore, individualized treatment strategies and close monitoring are warranted for these patients.
Omadacycline possesses a good safety profile for the treatment of infectious diseases according to previous studies (Torres et al., 2021; O'Riordan et al., 2019a; Stets et al., 2019; O'Riordan et al., 2019b; Mingora et al., 2023). In this study, we found that inflammation-related parameters were improved after omadacycline treatment. This finding was supported by previous studies, which indicated that omadacycline possessed anti-inflammatory activities (Gomi et al., 2025; Sanders and Beringer, 2024). Apart from inflammation, we also found that most of the liver, kidney, and coagulation function-related parameters were improved after omadacycline treatment. However, we noted that ALT was elevated after omadacycline treatment in patients with infectious diseases. According to previous studies, increased ALT was a common phenomenon after omadacycline treatment (Torres et al., 2021; O'Riordan et al., 2019a; Stets et al., 2019; O'Riordan et al., 2019b). Additionally, the median ALT after omadacycline treatment was still within the normal range. Thus, this slight ALT elevation might not indicate a potential hepatotoxicity risk of omadacycline. It should be clarified that while our study observed statistically significant changes in multiple laboratory parameters after omadacycline treatment, the clinical relevance of many of these changes was limited. Previous studies reported that gastrointestinal reaction was a common adverse event after omadacycline treatment, with an incidence of 10.2%–28.0% in patients with infectious diseases (Stets et al., 2019; O'Riordan et al., 2019b; El Ghali et al., 2023). In this study, we found that the incidence of gastrointestinal reaction was only 5.3% in patients with infectious diseases receiving omadacycline. Other adverse events included coagulation disorders, acute kidney injury, and liver injury. Notably, coagulation disorders were relatively common after omadacycline treatment, with an incidence of 10.5%. A potential reason might be that tetracycline could suppress the activity of plasma prothrombin, which might further contribute to the occurrence of coagulation disorders (Cui et al., 2019). However, most cases of coagulation disorders in this study were mild (asymptomatic) based on our clinical observations, and no patients required special treatments. Regarding liver injury and acute kidney injury, most cases were mild to moderate. Overall, our study provided real-world evidence that omadacycline was safe and tolerable for the treatment of infectious diseases.
5 Limitations
Several limitations existed in this study. (Spagnolo, 2024). Selection bias and information bias might exist in this retrospective study, both of which could impair the representativeness of the patient population. Additionally, the completeness and accuracy of data could also be constrained by the quality of medical records. (Sujith et al., 2024). This study only included patients receiving omadacycline treatment and did not set a control group, such as patients treated with other antibiotics, which prevented us from comparing the efficacy and safety of omadacycline with other alternative antimicrobial agents. Therefore, the comparative advantages of omadacycline in treating infectious diseases should be further explored by subsequent studies. (Vaughn et al., 2024). This study focused on Chinese patients with infectious diseases. Therefore, the generalizability of our findings to patients from other regions was restricted. (Tiseo and Falcone, 2025). The definition of clinical success depended on the subjective judgement of clinicians, which might introduce evaluation bias. (Ho et al., 2025). This study lacked minimum inhibitory concentration (MIC) data for omadacycline against the isolated pathogens. As a real-world retrospective study, microbiological susceptibility testing for omadacycline was not routinely performed for all patients, which limited our ability to correlate clinical outcomes with in vitro susceptibility profiles. (Aggarwal et al., 2024). This study provided the incidence of adverse events after omadacycline treatment. However, it was difficult to distinguish whether these adverse events were caused by omadacycline due to retrospective design. Therefore, the incidence of treatment-related adverse events should be further explored by further prospective studies. (Krishnaprasad and Kumar, 2024). This study lacked resistance profiles for all pathogens because a substantial number of pathogens were detected by nucleic acid tests (without culture), which precluded conventional antimicrobial susceptibility testing. Lacking this data prevented us from exploring the efficacy of omadacycline in patients with infections caused by pathogens with a high level of resistance. (Larkin, 2023). The prespecified outcome of pathogen eradication rate was not reported in the final analysis. This was because a follow-up microbiological test to confirm pathogen clearance was not routinely performed for a substantial portion of the patients. The lack of this systematic follow-up data made it statistically unreliable to calculate the eradication rate.
6 Conclusion
In conclusion, our findings from a real-world cohort of 2,587 Chinese patients suggest that omadacycline possesses favorable efficacy and safety for the treatment of infectious diseases. Further studies incorporating control groups and diverse geographic populations are required to support the wide clinical application of omadacycline in patients with infectious diseases.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by The Ethical Committee of The First Affiliated Hospital, Jiangxi Medical College, Nanchang University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because this is a retrospective study.
Author contributions
JnL: Data curation, Formal Analysis, Investigation, Writing – original draft. JyL: Data curation, Formal Analysis, Investigation, Visualization, Writing – original draft. HL: Formal Analysis, Investigation, Methodology, Resources, Writing – original draft. JP: Data curation, Formal Analysis, Methodology, Validation, Writing – original draft. SC: Investigation, Methodology, Writing – original draft. JmL: Conceptualization, Funding acquisition, Resources, Supervision, Validation, Writing – original draft, Writing – review and editing. AO: Conceptualization, Funding acquisition, Supervision, Validation, Writing – review and editing.
Funding
The authors declare that financial support was received for the research and/or publication of this article. This study was supported by National Natural Science Foundation of China (No. 82360735) and Natural Science Foundation of Jiangxi Province (No. 20232BAB206137).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1706601/full#supplementary-material
References
1
Aggarwal R. Mahajan P. Pandiya S. Bajaj A. Verma S. K. Yadav P. et al (2024). Antibiotic resistance: a global crisis, problems and solutions. Crit. Rev. Microbiol.50 (5), 896–921. 10.1080/1040841X.2024.2313024
2
Author anonymous. (2024). Coagulation disorder diagnosis guidelines: T/CMEAS 019-2024[S].
3
Bumbea V. I. Bumbea H. Vladareanu A. M. (2024). Immune dysfunction in patients with end stage kidney disease; immunosenescence - Review. Rom. J. Intern Med.62 (1), 12–19. 10.2478/rjim-2023-0030
4
Cui N. Cai H. Li Z. Lu Y. Wang G. Lu A. (2019). Tigecycline-induced coagulopathy: a literature review. Int. J. Clin. Pharm.41 (6), 1408–1413. 10.1007/s11096-019-00912-5
5
El Ghali A. Morrisette T. Alosaimy S. Lucas K. Tupayachi-Ortiz M. G. Vemula R. et al (2023). Long-term evaluation of clinical success and safety of omadacycline in nontuberculous mycobacteria infections: a retrospective, multicenter cohort of real-world health outcomes. Antimicrob. Agents Chemother.67 (10), e0082423. 10.1128/aac.00824-23
6
Gao W. Yang J. Zhang X. Tian L. Xu D. Xu S. et al (2024). Omadacycline for diverse infections in China: a real-world analysis of efficacy and safety. Infect. Dis. Ther.13 (12), 2509–2526. 10.1007/s40121-024-01065-3
7
Gbdar C. (2024). Global burden of bacterial antimicrobial resistance 1990-2021: a systematic analysis with forecasts to 2050. Lancet404 (10459), 1199–1226. 10.1016/S0140-6736(24)01867-1
8
Gomi S. Price E. Burgoyne H. Faozia S. Katahira E. McIndoo E. et al (2025). Omadacycline exhibits anti-inflammatory properties and improves survival in a murine model of post-influenza MRSA pneumonia. Antimicrob. Agents Chemother.69 (9), e0046925. 10.1128/aac.00469-25
9
Heine H. S. Drusano G. Purcell B. K. Anastasiou D. Tanaka S. K. Serio A. W. (2024). Omadacycline is active in vitro and in vivo against ciprofloxacin-resistant Bacillus anthracis. Antimicrob. Agents Chemother.68 (9), e0059524. 10.1128/aac.00595-24
10
Ho C. S. Wong C. T. H. Aung T. T. Lakshminarayanan R. Mehta J. S. Rauz S. et al (2025). Antimicrobial resistance: a concise update. Lancet Microbe6 (1), 100947. 10.1016/j.lanmic.2024.07.010
11
Karvouniaris M. Almyroudi M. P. Abdul-Aziz M. H. Blot S. Paramythiotou E. Tsigou E. et al (2023). Novel antimicrobial agents for gram-negative pathogens. Antibiot. (Basel)12 (4), 761. 10.3390/antibiotics12040761
12
Kounatidis D. Dalamaga M. Grivakou E. Karampela I. Koufopoulos P. Dalopoulos V. et al (2024). Third-generation tetracyclines: current knowledge and therapeutic potential. Biomolecules14 (7), 783. 10.3390/biom14070783
13
Krishnaprasad V. H. Kumar S. (2024). Antimicrobial resistance: an ultimate challenge for 21st century scientists, healthcare professionals, and policymakers to save future generations. J. Med. Chem.67 (18), 15927–15930. 10.1021/acs.jmedchem.4c02002
14
Larkin H. (2023). Increasing antimicrobial resistance poses global threat, WHO says. JAMA329 (3), 200. 10.1001/jama.2022.23552
15
Lehrnbecher T. Koehl U. Wittekindt B. Bochennek K. Tramsen L. Klingebiel T. et al (2008). Changes in host defence induced by malignancies and antineoplastic treatment: implication for immunotherapeutic strategies. Lancet Oncol.9 (3), 269–278. 10.1016/S1470-2045(08)70071-8
16
Li A. He S. Li J. Zhang Z. Li B. Chu H. (2023). Omadacycline, eravacycline, and tigecycline express anti-mycobacterium abscessus activity in vitro. Microbiol. Spectr.11 (3), e0071823. 10.1128/spectrum.00718-23
17
Liu Z. Liang Q. Ren Y. Guo C. Ge X. Wang L. et al (2023). Immunosenescence: molecular mechanisms and diseases. Signal Transduct. Target Ther.8 (1), 200. 10.1038/s41392-023-01451-2
18
Macone A. B. Caruso B. K. Leahy R. G. Donatelli J. Weir S. Draper M. P. et al (2014). In vitro and in vivo antibacterial activities of omadacycline, a novel aminomethylcycline. Antimicrob. Agents Chemother.58 (2), 1127–1135. 10.1128/AAC.01242-13
19
Mingora C. M. Bullington W. Faasuamalie P. E. Levin A. Porter G. Stadnick R. et al (2023). Long-term safety and tolerability of omadacycline for the treatment of Mycobacterium abscessus infections. Open Forum Infect. Dis.10 (7), ofad335. 10.1093/ofid/ofad335
20
National Clinical Research Center for Kidney D, Chinese Nephrologist Association EGoAKIGChinese Nephrologist Association, Expert Group on AKI Guidelines (2023). Chinese clinical practice guideline for acute kidney injury. Zhonghua Yi Xue Za Zhi103 (42), 3332–3366. 10.3760/cma.j.cn112137-20230802-00133
21
Nicklas D. A. Maggioncalda E. C. Story-Roller E. Eichelman B. Tabor C. Serio A. W. et al (2022). Potency of omadacycline against mycobacteroides abscessus clinical isolates in vitro and in a mouse model of pulmonary infection. Antimicrob. Agents Chemother.66 (1), e0170421. 10.1128/AAC.01704-21
22
O'Riordan W. Cardenas C. Shin E. Sirbu A. Garrity-Ryan L. Das A. F. et al (2019a). Once-daily oral omadacycline versus twice-daily oral linezolid for acute bacterial skin and skin structure infections (OASIS-2): a phase 3, double-blind, multicentre, randomised, controlled, non-inferiority trial. Lancet Infect. Dis.19 (10), 1080–1090. 10.1016/S1473-3099(19)30275-0
23
O'Riordan W. Green S. Overcash J. S. Puljiz I. Metallidis S. Gardovskis J. et al (2019b). Omadacycline for acute bacterial skin and skin-structure infections. N. Engl. J. Med.380 (6), 528–538. 10.1056/NEJMoa1800170
24
Overcash J. S. Bhiwandi P. Garrity-Ryan L. Steenbergen J. Bai S. Chitra S. et al (2019). Pharmacokinetics, safety, and clinical outcomes of omadacycline in women with cystitis: results from a phase 1b study. Antimicrob. Agents Chemother.63 (5), e02083-18. 10.1128/AAC.02083-18
25
Sanders M. Beringer P. (2024). Immunomodulatory activity of omadacycline in vitro and in a murine model of acute lung injury. mSphere9 (11), e0067124. 10.1128/msphere.00671-24
26
Skinner A. M. Petrella L. A. Cheknis A. Johnson S. (2023). Antimicrobial susceptibility of Clostridioides difficile to omadacycline and comparator antimicrobials. J. Antimicrob. Chemother.78 (7), 1779–1784. 10.1093/jac/dkad170
27
Spagnolo A. M. (2024). Bacterial infections: surveillance, prevention and control. Pathogens13 (2), 181. 10.3390/pathogens13020181
28
Stets R. Popescu M. Gonong J. R. Mitha I. Nseir W. Madej A. et al (2019). Omadacycline for community-acquired bacterial pneumonia. N. Engl. J. Med.380 (6), 517–527. 10.1056/NEJMoa1800201
29
Sujith S. Solomon A. P. Rayappan J. B. B. (2024). Comprehensive insights into UTIs: from pathophysiology to precision diagnosis and management. Front. Cell Infect. Microbiol.14, 1402941. 10.3389/fcimb.2024.1402941
30
Technology Committee on DP, Management CMBA, Study Group of Drug-Induced Liver Disease CMAftSoLDStudy Group of Drug-Induced Liver Disease, Chinese Medical Association for the Study of Liver Diseases (2023). Chinese guideline for diagnosis and management of drug-induced liver injury (2023 version). Zhonghua Gan Zang Bing Za Zhi31 (4), 355–384. 10.3760/cma.j.cn501113-20230419-00176-1
31
Tiseo G. Falcone M. (2025). The future approach for the management of acute bacterial skin and skin structure infections. Curr. Opin. Infect. Dis.38 (2), 128–135. 10.1097/QCO.0000000000001092
32
Torres A. Garrity-Ryan L. Kirsch C. Steenbergen J. N. Eckburg P. B. Das A. F. et al (2021). Omadacycline vs moxifloxacin in adults with community-acquired bacterial pneumonia. Int. J. Infect. Dis.104, 501–509. 10.1016/j.ijid.2021.01.032
33
Vaughn V. M. Dickson R. P. Horowitz J. K. Flanders S. A. (2024). Community-acquired pneumonia: a review. JAMA332 (15), 1282–1295. 10.1001/jama.2024.14796
34
Waites K. B. Crabb D. M. Atkinson T. P. Geisler W. M. Xiao L. (2022). Omadacycline is highly active in vitro against Mycoplasma genitalium. Microbiol. Spectr.10 (6), e0365422. 10.1128/spectrum.03654-22
Summary
Keywords
infectious diseases, omadacycline, efficacy, safety, predictors
Citation
Liu J, Liu J, Li H, Pang J, Chen S, Liu J and Ouyang A (2025) Efficacy and safety of omadacycline for the treatment of infectious diseases: a real-world, retrospective study of 2587 patients. Front. Pharmacol. 16:1706601. doi: 10.3389/fphar.2025.1706601
Received
16 September 2025
Revised
27 October 2025
Accepted
31 October 2025
Published
18 November 2025
Volume
16 - 2025
Edited by
pier Maria Fornasari, Regenhealthsolutions, Italy
Reviewed by
Natalia Lazareva, I.M. Sechenov First Moscow State Medical University, Russia
Lia Amalia, Bandung Institute of Technology, Indonesia
Updates
Copyright
© 2025 Liu, Liu, Li, Pang, Chen, Liu and Ouyang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aijun Ouyang, Aijun_Ouyang@163.com; Jianming Liu, liujianming390@163.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.