- 1The First Hospital of Hunan University of Chinese Medicine, Changsha, Hunan, China
- 2Sichuan Academy of Chinese Medicine Sciences, Chengdu, Sichuan, China
Objective: This study analyzed severe cutaneous adverse reactions (SCARs) linked to anti-osteoporosis drugs using FDA Adverse Event Reporting System (FAERS) data and characterized implicated drugs and clinical features through a literature review.
Methods: A retrospective disproportionality analysis of SCAR reports from FAERS (2004–2024) utilized signal detection metrics, including reporting odds ratio (ROR), proportional reporting ratio (PRR), and Bayesian confidence propagation neural network (BCPNN). A structured literature search across PubMed, Web of Science, and Scopus gathered case reports of SCARs induced by anti-osteoporosis drugs.
Results: Of 77,789 SCAR reports, 399 (0.51%) involved anti-osteoporosis drugs, mainly affecting female patients (76.25%) with a median age of 69 years. Denosumab (24%), alendronate (23.25%), and zoledronic acid (17.13%) were most frequently reported. Significant signals included risedronic acid with erythema multiforme [ROR = 9.06; PRR = 9.03; information component (IC) = 3.17], zoledronic acid with cutaneous vasculitis (ROR = 3.15; PRR = 3.15; IC = 1.65), and alendronic acid with Stevens–Johnson syndrome (SJS) (ROR = 4.03; PRR = 4.02; IC = 2.00). The literature review (33 cases) confirmed a median symptom onset of 22 days, with treatments often involving corticosteroids and supportive care.
Conclusion: Anti-osteoporosis drugs, notably bisphosphonates and strontium ranelate, are rarely linked to SCARs but may cause serious consequences. Increased clinical awareness, pre-treatment risk evaluation, and vigilant monitoring are essential for at-risk patients.
1 Introduction
Severe cutaneous adverse reactions (SCARs) are a group of rare but potentially fatal T cell-mediated type IV hypersensitivity reactions, encompassing Stevens–Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), drug reaction with eosinophilia and systemic symptoms (DRESS), and acute generalized exanthematous pustulosis (AGEP) (Temp et al., 2022). Despite their low overall incidence—ranging from 0.4 to 1.2 cases per million annually—the associated morbidity and mortality are substantial, particularly in TEN, where fatality rates may reach 48%, compared to 2%–6% in DRESS and approximately 4% in SJS (2, 3). Early diagnosis and prompt withdrawal of the suspected causative drug are critical determinants of clinical outcomes. Although extensive pharmacovigilance research has elucidated SCARs associated with antiepileptic drugs (Wei et al., 2023), immune checkpoint inhibitors (Li et al., 2024), and antifungal agents (Shan et al., 2025), limited systematic pharmacovigilance analysis has been conducted on anti-osteoporosis drugs.
Anti-osteoporosis drugs are generally regarded as having an overall good tolerance profile (Varenna et al., 2013). However, rare reports have documented severe cutaneous adverse reactions associated with anti-osteoporosis drugs. In particular, bisphosphonates are linked to SJS and TEN (Barrera et al., 2005), while strontium ranelate is associated with DRESS (Kolitz et al., 2021) and TEN (Yang et al., 2014). Previous reviews, such as Musette et al. (2011), have highlighted rare cutaneous adverse reactions associated with anti-osteoporosis drugs, particularly bisphosphonates and strontium ranelate. These exceptionally rare but severe dermatological toxicities underscore the urgent need for pharmacovigilance studies to evaluate adverse reactions to anti-osteoporosis drugs, particularly rare SCARs, as an increasing number of novel agents enter clinical use.
Real-world pharmacovigilance using spontaneous reporting systems, such as the FDA Adverse Event Reporting System (FAERS), provides a valuable tool for detecting potential safety signals related to rare adverse drug reactions, despite limitations such as voluntary reporting, potential biases, incomplete data, and the lack of causality assessment. These constraints necessitate cautious interpretation to avoid the misattribution of false-positive signals (Morris et al., 2024). Disproportionality analysis tools—such as the reporting odds ratio (ROR) and proportional reporting ratio (PRR)—have proven effective in quantifying drug–event associations and prioritizing high-risk agents (Fusaroli et al., 2024a). This method has successfully characterized SCAR signals across various therapeutic classes, revealing, for instance, that certain antifungals [e.g., fluconazole: ROR 9.50 (Shan et al., 2025)] and immunotherapies [e.g., pembrolizumab: ROR 4.93 (Zhu et al., 2021)] exhibit strong associations with SCARs.
In light of the existing knowledge gaps surrounding anti-osteoporosis drug-induced SCARs, this study aimed to (1) characterize SCARs related to commonly prescribed anti-osteoporosis drugs using FAERS data from 2004 to 2024; (2) compare SCAR signal intensities across drug subclasses; and (3) summarize demographic, clinical, and prognostic patterns through a literature review. The findings will inform risk stratification strategies and contribute to safer, more personalized management of osteoporosis therapy.
2 Methods
2.1 Data source
The FAERS, a globally recognized spontaneous reporting database, was used in this study. FAERS data are anonymized and updated on a quarterly basis. Raw data were retrieved using the OpenVigil 2.1 platform, a third-party tool designed for standardized data processing, widely used in pharmacovigilance for data extraction, mining, and analysis.
2.2 Identification of anti-osteoporosis drugs and adverse events
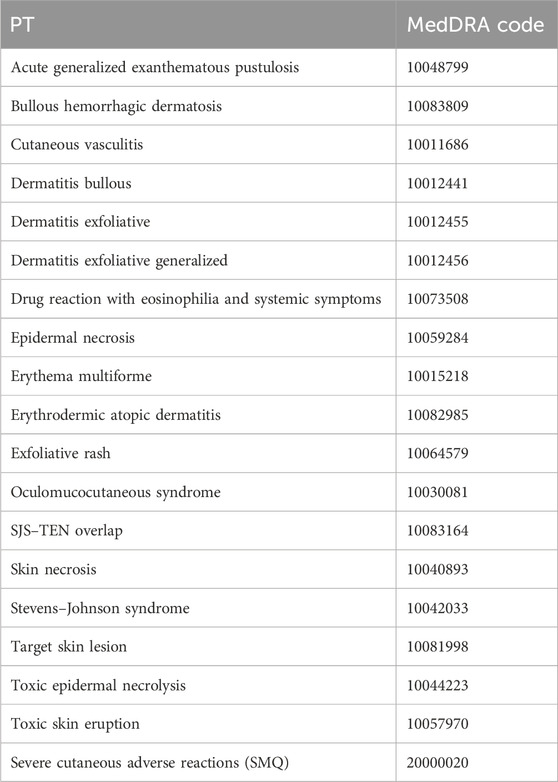
Anti-osteoporosis drugs were selected based on the World Health Organization’s Anatomical Therapeutic Chemical (ATC) classification system, initially identifying 27 drugs. To address potential confounders such as polypharmacy and comorbidities, drugs were included if they were indicated for osteoporosis treatment and designated as the “primary suspect” in FAERS reports, resulting in the selection of 12 drugs: etidronic acid (M05BA01), pamidronic acid (M05BA03), alendronic acid (M05BA04), ibandronic acid (M05BA06), risedronic acid (M05BA07), zoledronic acid (M05BA08), denosumab (M05BX04), romosozumab (M05BX06), raloxifene (G03XC01), estradiol (G03CA03), teriparatide (H05AA02), and abaloparatide (H05AA04). Exclusion criteria included drugs not primarily indicated for osteoporosis or those reported as secondary suspects or concomitant medications. Adverse events were limited to SCARs, identified using a narrow Standardized MedDRA Query (SMQ) search (MedDRA version 23.1, SMQ code: 20000020), encompassing 18 preferred terms (PTs), including SJS, TEN, DRESS, and AGEP; details are presented in Table 1. Cases lacking sufficient data (e.g., missing drug or event details) or not meeting the SMQ criteria were excluded. The study covers reports from 1 January 2004 to 31 December 2024.
2.3 Data processing and signal detection criteria
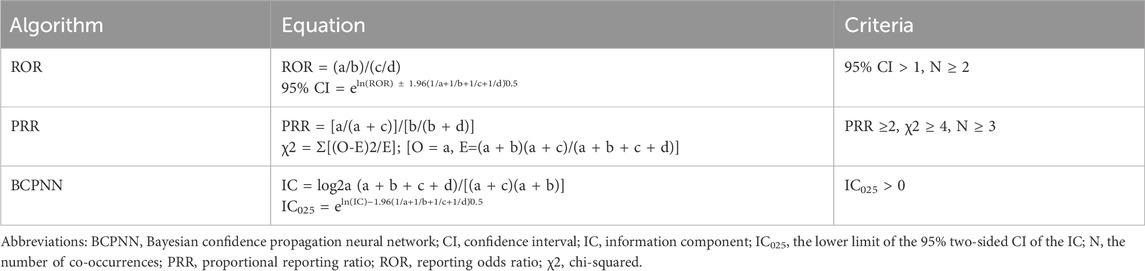
This study adheres to the Reporting of A Disproportionality Analysis for Pharmacovigilance (READUS-PV) guideline to ensure transparent and comprehensive reporting of disproportionality analyses (Fusaroli et al., 2024b). Key elements include the following: (1) a clear definition of the study population and data source (FAERS, 2004–2024, accessed via OpenVigil 2.1); (2) specification of case and non-case selection criteria, including primary suspect drugs and narrow SMQ for SCARs; (3) ensuring reliable detection of significant signals by considering the sample size, with positive signals identified based on the multiple disproportionality metrics according to the following criteria: a minimum of three reported cases (N ≥ 3); ROR ≥2 with the lower bound of the 95% confidence interval (CI) exceeding 1; N ≥ 3, PRR ≥2, and χ2 ≥ 4; and information component (IC) > 1 and IC025 > 0; and (4) ensuring data integrity and avoiding overestimation of signals by identifying and removing duplicate reports in the FAERS database using a systematic approach. First, multiple versions of the same report (e.g., follow-up reports) were identified using the unique case ID, which includes a suffix indicating follow-up numbers, and only the most recent version of each report was retained. Subsequently, potential duplicates were manually reviewed by cross-referencing key data fields, including patient demographics (age and sex), event date, drug name, adverse event, and reporter country. Reports with identical or highly similar data across these fields were consolidated to retain only one record per unique case. (5) Data were extracted from the dataset, including the year of the report, patient demographics (gender, age, and nationality), and clinical outcomes. Continuous variables were reported as the means ± standard deviations, and categorical variables were expressed as percentages. All signal detection metrics (ROR, PRR, and IC) are reported to two decimal places for consistency, unless specified otherwise. The formulas used for these calculations are presented in Tables 2, 3.
2.4 Review of published cases
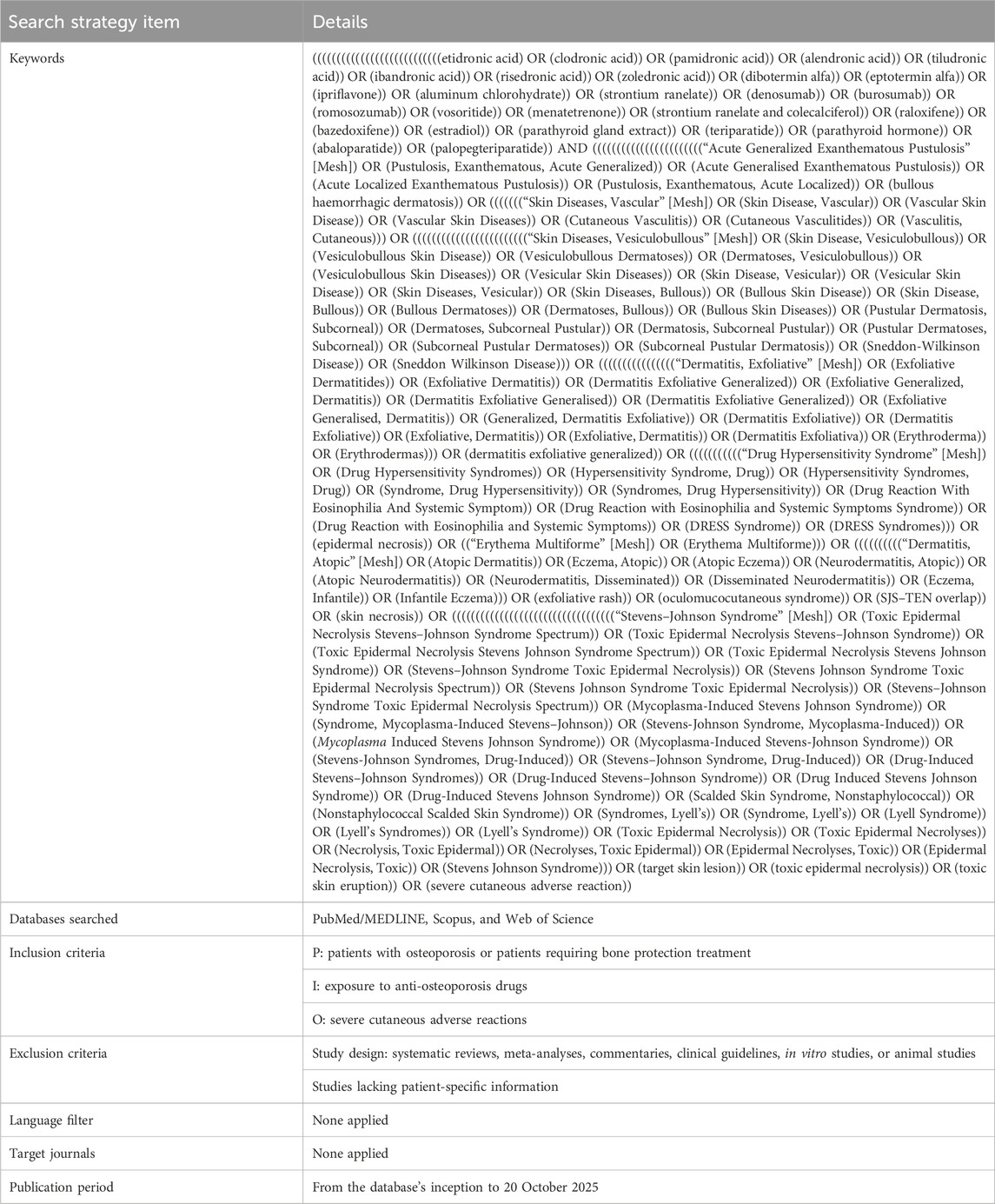
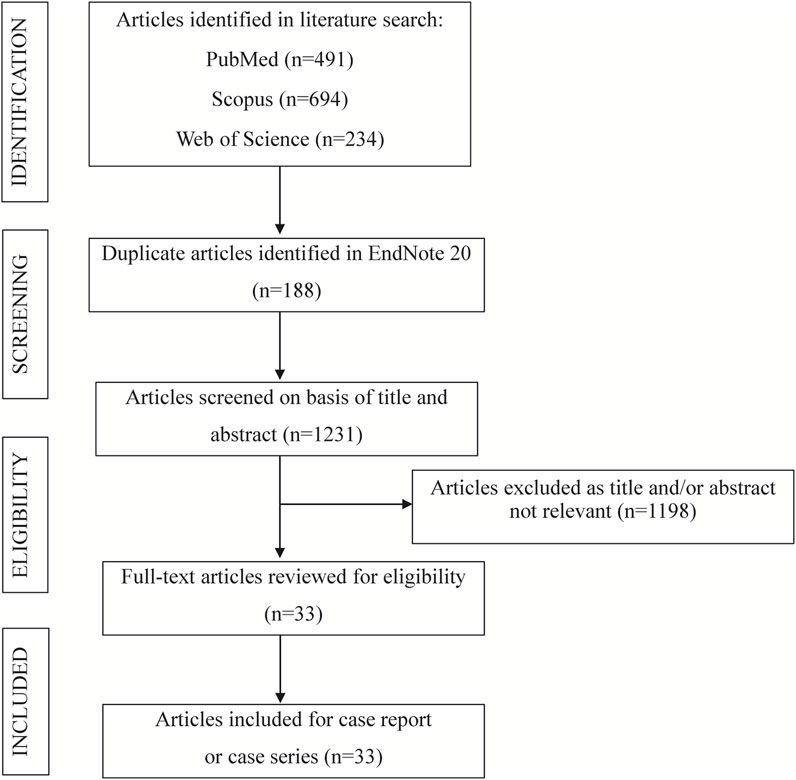
The systematic literature review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines to ensure methodological rigor. A structured search was conducted across PubMed, Web of Science, and Scopus from inception to 20 October 2025. Details of the search strategy used in the case review are presented in Table 4. Studies were eligible for inclusion if they satisfied the following criteria: the publication was a case report or case series (Temp et al., 2022); the study described SCARs associated with anti-osteoporosis drugs, identifying 18 SCARs-SMQ preferred terms, including SJS, TEN, DRESS, and AGEP (Hsu et al., 2016); and detailed patient and ADR data were reported, with the full text accessible (Liang et al., 2024). Studies were excluded based on the following criteria: failure to meet the specified study type (Temp et al., 2022); reporting of duplicate cases (Hsu et al., 2016); classification as secondary literature (Liang et al., 2024); unavailability of full text or absence of patient-specific information (Wei et al., 2023); and systematic reviews, meta-analyses, commentaries, clinical guidelines, in vitro studies, or animal studies (Li et al., 2024). Study selection was performed independently by two reviewers, with discrepancies resolved through consensus to ensure methodological rigor. For each case, the following variables were extracted: patient demographic characteristics, including country of origin, age, and sex (Temp et al., 2022); details of the anti-osteoporosis drugs associated with cutaneous toxicity, encompassing the generic name, therapeutic indication, clinical presentation of the cutaneous ADR, time to onset of the ADR, histopathological findings from skin biopsies, implemented interventions, clinical outcomes, and time to resolution (Hsu et al., 2016). A PRISMA flow diagram (Figure 1) illustrates the study selection process.
3 Results
3.1 Descriptive analysis of SCAR cases
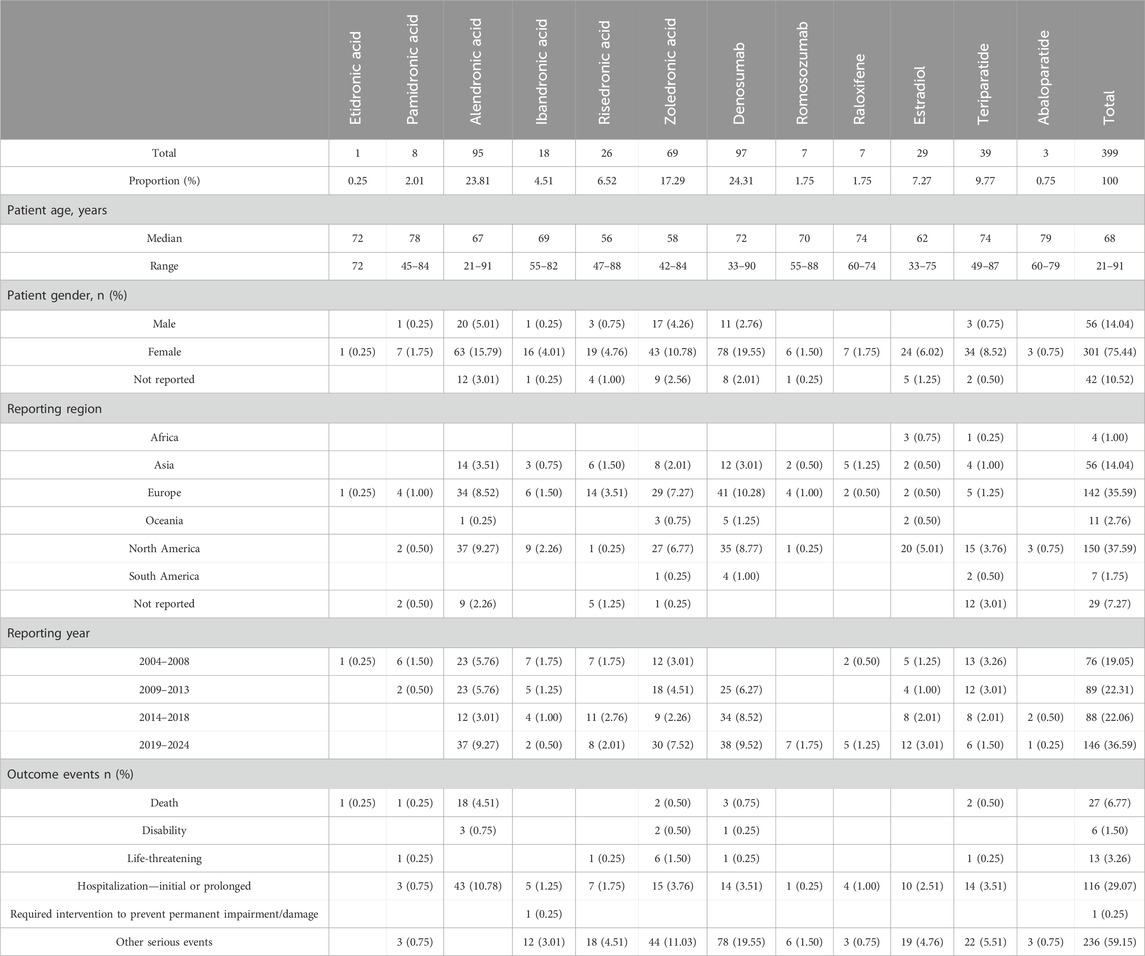
From 1 January 2004 to 31 December 2024, the FAERS database yielded 77,789 SCAR-related reports, of which 399 (0.51%) were associated with anti-osteoporosis drugs as the primary suspect. Predominantly affecting female patients (76.3%), these cases had a median patient age of 68 years (interquartile range: 21–91 years), with the ≥67-year age group comprising the largest proportion (40.1%). Geographically, North America contributed the highest number of reports (150 cases, 37.6%), followed by Europe (35.6%) and Asia (14.0%), reflecting regional variations in reporting practices. Temporally, SCAR reports exhibited a steady increase, with 146 cases (36.6%) recorded from 2019 to 2024, surpassing earlier periods. Severe outcomes were notable, with hospitalization reported in 29.1% of cases and mortality in 6.8% (27 cases). Alendronate-associated SCARs demonstrated the highest hospitalization rate (10.8%), followed by zoledronate (3.8%) and denosumab (3.5%). Detailed demographic and clinical characteristics are presented in Table 5.
3.2 Identification and distribution of suspected culprit drugs
The analysis encompassed 12 anti-osteoporosis drugs, classified according to the World Health Organization’s Anatomical Therapeutic Chemical (ATC) system: etidronate, pamidronate, alendronate, ibandronate, risedronate, zoledronate, denosumab, romosozumab, raloxifene, estradiol, teriparatide, and abaloparatide. Denosumab (24.3%), alendronate (23.8%), and zoledronate (17.3%) emerged as the most frequently associated agents. Among SCAR subtypes, erythema multiforme (65 cases, 16.3%), skin necrosis (50 cases, 12.5%), cutaneous vasculitis (43 cases, 10.8%), Stevens–Johnson syndrome (42 cases, 10.5%), and bullous dermatitis (39 cases, 9.8%) accounted for the majority of reported PTs, as delineated in Table 4.
3.3 SCAR signal detection
Disproportionality analyses, using ROR, PRR, and Bayesian confidence propagation neural network (BCPNN), identified significant SCAR signals for several anti-osteoporosis drugs. Signals were deemed statistically significant when meeting predefined thresholds: N ≥ 3, ROR ≥2 (lower 95% CI > 1), PRR ≥2 (χ2 ≥ 4), and IC > 1 (IC025 > 0). Notable signals included risedronate with erythema multiforme (n = 16; ROR 9.06, 95% CI 5.54–14.81; PRR 9.03; IC 3.17), zoledronate with cutaneous vasculitis (n = 26; ROR 3.15, 95% CI 2.14–4.64; PRR 3.15; IC 1.65), alendronate with SJS (n = 22; ROR 4.03, 95% CI 2.65–6.13; PRR 4.02; IC 2.00), pamidronate with SJS (n = 3; ROR 4.64, 95% CI 1.50–14.41; PRR 4.64; IC 2.21), and raloxifene with erythema multiforme (n = 6; ROR 2.73, 95% CI 1.23–6.08; PRR 2.73; IC 1.45). No significant signals were detected for denosumab, romosozumab, or teriparatide across evaluated PTs. Comprehensive signal detection results are summarized in Figure 2.
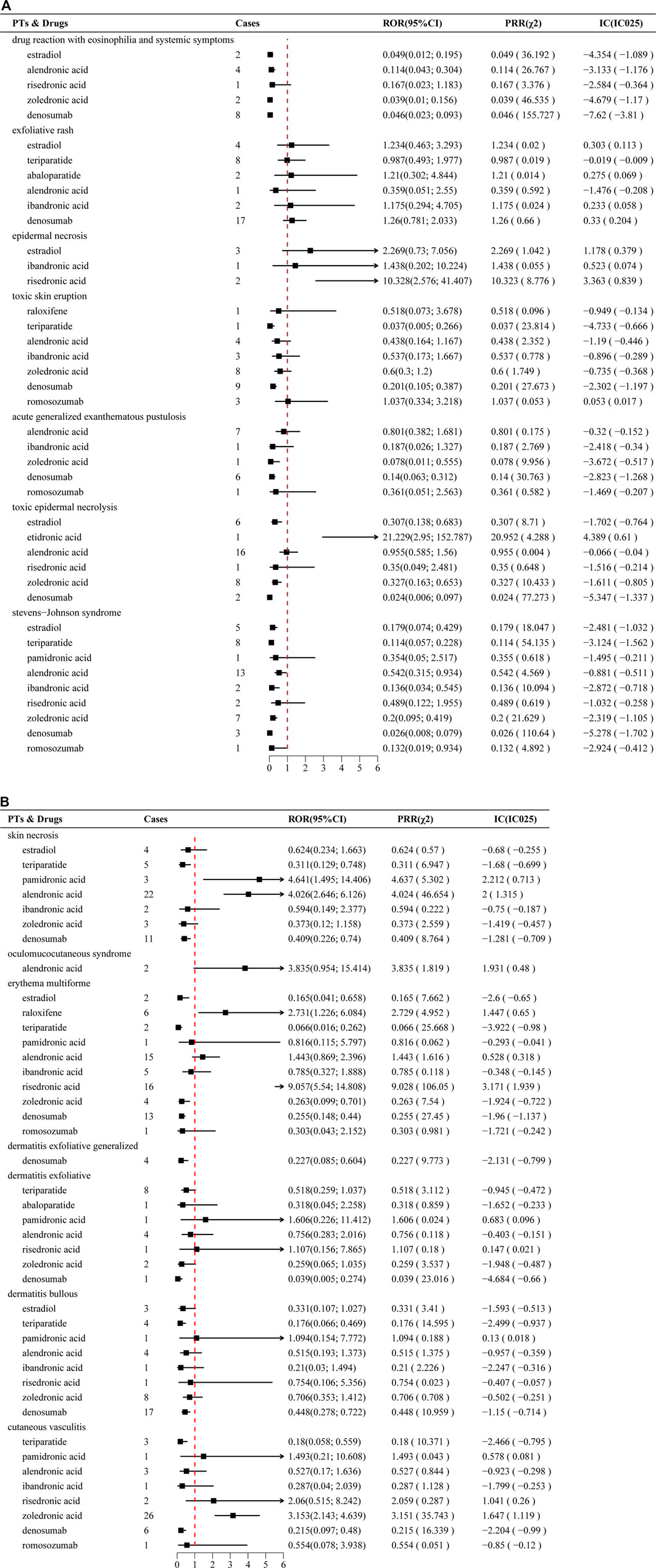
Figure 2. (A) Disproportionately reported adverse events of narrow-scope PTs in the SMQ classification of SCARs for anti-osteoporosis drugs in the FAERS database. (B) Continuation of Figure 2A.
3.4 Case report review
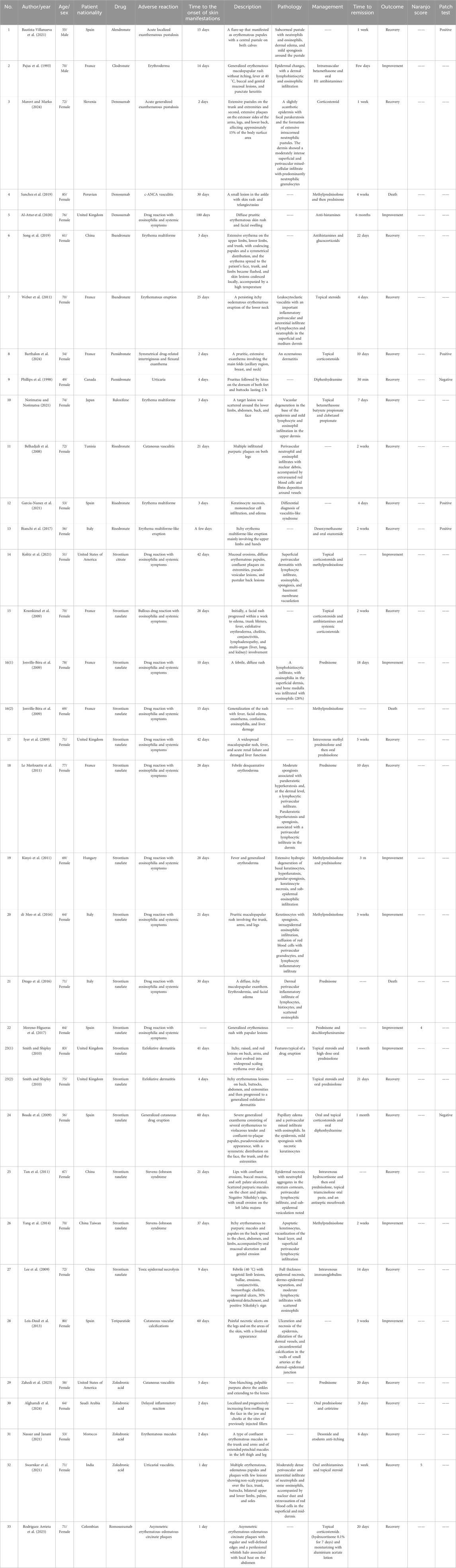
A systematic literature review identified 33 case reports involving 35 patients with SCARs attributed to anti-osteoporosis drugs. The median patient age was 67 years (range: 49–85 years), with a pronounced female predominance (94%). From the perspective of drug distribution, among the 35 adverse reactions, strontium ranelate was the most frequently associated agent (n = 16, 50%), followed by bisphosphonates (n = 13; e.g., alendronate and clodronate) and denosumab (n = 3), with raloxifene, teriparatide, and romosozumab each involved in one case. Clinical manifestations included DRESS (11 cases, 10 linked to strontium ranelate and 1 to denosumab), SJS (2 cases, both strontium ranelate), and TEN (1 case, strontium ranelate). The median onset time for SCARs was 22 days (range: 2–180 days). Therapeutic interventions predominantly involved systemic corticosteroids, topical corticosteroids, oral antihistamines, intravenous immunoglobulin, and supportive care. Clinical outcomes were favorable in most cases, with 62% (n = 22) achieving full recovery and 28% (n = 10) showing improvement; however, two strontium ranelate-associated DRESS cases (Drago et al., 2016; Jonville-Béra et al., 2009) and one denosumab-associated c-ANCA vasculitis (Sanchez et al., 2019) resulted in death. It should be noted that this case review has heterogeneity; among the 35 patients, only 21 underwent skin pathological biopsy, while 2 cases underwent Naranjo assessment, and 6 cases included patch testing. It limits the ability to definitively attribute causality in some reports. Detailed case characteristics are presented in Table 6.
4 Discussion
To our knowledge, this study represents the first comprehensive pharmacovigilance analysis of SCARs associated with anti-osteoporosis drugs using the FAERS database, complemented by a systematic review of published case reports. Using FAERS data from 2004 to 2024, we identified 399 cases of SCARs associated with anti-osteoporosis drugs, representing approximately 0.001% of the 30,390,978 adverse event reports for these drugs in the database. This suggests that SCARs associated with anti-osteoporosis drugs are rare adverse drug reactions. Even though signals derived from FAERS reflect statistical associations rather than definitive causal relationships and SCARs linked to anti-osteoporosis drugs are rare, their clinical significance remains substantial given the potential for severe clinical outcomes.
Demographically, our study found that 76.3% of SCAR cases occurred in female patients, with a median age of 68 years, which corresponds to 94% female patients and a median age of 67 years in the case review. A real-world study also confirmed that the incidence of SCARs was higher among female patients than in male patients, with the majority of cases occurring in the 61–70-year age group (Li et al., 2023). The prevalence of osteoporosis in elderly women represents a key contributing factor. Additionally, age-related decreases in hepatic and renal function, extended drug elimination half-lives, and heightened drug sensitivity collectively increase the propensity for elderly patients to develop adverse drug reactions (Woo et al., 2020). The high hospitalization rate (29.07%) and mortality rate (6.77%) associated with anti-osteoporosis drug-induced SCARs highlight their clinical severity, particularly for alendronic acid (10.78% hospitalization rate). These outcomes may be exacerbated by polypharmacy and comorbidities in elderly patients, which can complicate SCAR management. The case review further revealed that systemic corticosteroids and supportive care were the most common interventions, with recovery or improvement in 90% of cases, although two strontium ranelate-related DRESS cases (Drago et al., 2016; Jonville-Béra et al., 2009) and one denosumab-associated c-ANCA vasculitis (Sanchez et al., 2019) resulted in death (Drago et al., 2016; Kinyó et al., 2011).
Disproportionality analysis revealed significant signals for severe cutaneous toxicity associated with bisphosphonates, underscoring their potential for SCARs. Risedronic acid showed a strong association with erythema multiforme (ROR 9.06), while pamidronic acid and alendronic acid were associated with SJS, with RORs of 4.64 and 4.03, respectively. A pharmacovigilance study involving 13,164 patients in England reported a rare case of risedronate-associated SJS (8). Risedronate-induced erythema multiforme-like eruption (Bianchi et al., 2017) and erythema multiforme (Garcia-Nunez et al., 2021) were reported. Bautista-Villanueva et al. (2021) first reported a case of alendronate-induced acute localized exanthematous pustulosis. de Arruda et al. (2017) reported a case of erythema multiforme associated with alendronic acid. Zoledronic acid-induced erythematous macules (Nassar and Janani, 2021), urticarial vasculitis (Swarnkar et al., 2021), and cutaneous vasculitis (Zahedi et al., 2023) have also been documented. Consistent with prior findings, Norimatsu and Norimatsu (2021) reported the only known case, to date, of a 74-year-old female patient who developed erythema multiforme minor while taking raloxifene. In addition, in a single-center, randomized, double-blind, placebo-controlled study named TEMP, the incidence of etidronate-related hypersensitivity dermatological reactions was 2.7% (1/37) (Kranenburg et al., 2018). These findings emphasize the critical need for vigilance regarding severe cutaneous toxicities associated with bisphosphonate use.
In contrast, no significant signals were observed for denosumab, romosozumab, or teriparatide, potentially due to lower reporting rates or differing immunopathogenic mechanisms. Notably, documented cases have linked teriparatide to multiple pruritic erythematous papules (Chu and Kim, 2016) and cutaneous vascular calcification (Leis-Dosil et al., 2013). Moreover, a recent case was reported of a 71-year-old Colombian woman who developed SCARs, characterized by two asymmetric erythematous–edematous circinate plaques, on the day of romosozumab injection, leading to discontinuation of the treatment (Rodriguez Arrieta et al., 2025). Additionally, denosumab is also one of the most frequently reported drugs. In a large-scale clinical trial involving over 7,800 postmenopausal women with osteoporosis, the incidence of denosumab-related cutaneous adverse events, such as dermatitis, eczema, and rashes, was reported at 10.8% (Cummings et al., 2009). In the FREEDOM trial, serious adverse cutaneous infections, in particular, cellulitis and erysipelas, were observed in 12 (0.3%) participants receiving denosumab (Saag et al., 2018). In our retrospective analysis of clinical cases, we identified instances in which denosumab was associated with the development of AGEP (Marovt and Marko, 2024) and DRESS (Al-Attar et al., 2020). The evidence suggests that although denosumab, romosozumab, and teriparatide are generally safe and well-tolerated biologic agents, these findings highlight the potential for rare yet serious SCARs, warranting careful monitoring and prompt clinical management.
Notably, despite multiple reported cases of strontium ranelate-related SJS (Yang et al., 2014; Tan et al., 2011), TEN (39), DRESS (Kolitz et al., 2021; Drago et al., 2016; Jonville-Béra et al., 2009; Kinyó et al., 2011; Le Merlouette et al., 2011; di Meo et al., 2016; Moreno-Higueras et al., 2017; Iyer et al., 2009; Kramkimel et al., 2009), exfoliative dermatitis (Smith and Shipley, 2010), and generalized cutaneous drug eruption (Boada et al., 2009), among 199 patients with adverse drug reactions to strontium ranelate in France, DRESS accounted for the majority of cutaneous adverse events (19/51 cutaneous AEs) and occurred predominantly in women with a median age of 74 years (range: 58–87 years). The median time to the onset from the initiation of strontium ranelate treatment was 35 days (range: 23–365 days), while one patient died due to fulminant hepatitis associated with DRESS (Jonville-Bera and Autret-Leca, 2011). However, discrepancies were noted, particularly the high prevalence of strontium ranelate-associated SCARs (50% of cases) in the literature, which were absent in FAERS due to its non-approval in the U.S. This highlights the complementary role of case reports in capturing adverse events for drugs not widely reported in spontaneous reporting systems.
SCARs are classified as delayed-type, T-cell-mediated type IV hypersensitivity responses, with their pathophysiological mechanisms yet to be fully understood. The median onset time of 21 days (range: 2–60 days) in our case review aligns with the delayed nature of type IV hypersensitivity reactions. In our case review, histopathological analysis of SCARs linked to anti-osteoporosis drugs revealed distinct patterns. Alendronate-associated acute localized exanthematous pustulosis showed neutrophil and eosinophil infiltration in the epidermis, with dermal edema and mild epidermal disruption, indicating a pustular reaction (Bautista-Villanueva et al., 2021). Clodronate-associated erythroderma displayed diffuse dermal inflammation with lymphocytes, histiocytes, and eosinophils, suggesting a hypersensitivity reaction affecting both skin layers (Pajus et al., 1993). Strontium ranelate-associated DRESS exhibited severe hypersensitivity features, including eosinophilic infiltration, epidermal spongiosis, keratinocyte necrosis, and basal layer degeneration, reflecting systemic immune activation (Kolitz et al., 2021; Drago et al., 2016; Jonville-Béra et al., 2009; Kinyó et al., 2011; Le Merlouette et al., 2011). Strontium ranelate-linked SJS revealed extensive epidermal necrosis, apoptosis, and subepidermal vesiculation, typical of SJS (Yang et al., 2014; Tan et al., 2011). Similarly, strontium ranelate-associated TEN showed full-thickness epidermal necrosis and dermo-epidermal separation with lymphocytic and eosinophilic infiltrates (Lee et al., 2009). Denosumab-associated AGEP featured robust neutrophilic inflammation, intracorneal pustules, parakeratosis, and mixed dermal infiltrates, consistent with AGEP’s profile (Marovt and Marko, 2024). In SJS/TEN, cytotoxic mediators such as granulysin and perforin from CD8+ T cells drive keratinocyte apoptosis and necrosis (Hasegawa and Abe, 2024). In DRESS and AGEP, Th2-driven cytokines (IL-4, IL-5, and IL-13) promote eosinophilic responses and systemic inflammation (Ramirez et al., 2023; Chen et al., 2023). Human leukocyte antigen (HLA) molecules likely present drug antigens, triggering T-cell activation, while cytokine dysregulation (IL-6, IL-8, and TNF-α) amplifies inflammation (Deschaseaux et al., 2011). Drug metabolism and genetic predispositions, such as HLA alleles, may enhance susceptibility by forming immunogenic complexes or reactive metabolites (Deshpande et al., 2021). Research has found that strontium ranelate-related SJS/TEN is significantly associated with HLA-A*33:03 and HLA-B*58:01 (Lee et al., 2016). The diverse histopathological and clinical presentations underscore the need for prompt drug withdrawal, anti-inflammatory therapies, and supportive care to mitigate severe outcomes, including secondary infections such as sepsis, which contribute to morbidity and mortality.
The systematic literature review included only 32 case reports (34 patients), which represents a significant limitation due to the small sample size. This restricted number of cases may not fully reflect the diversity of SCAR presentations or the broader clinical context of anti-osteoporosis drug-induced cutaneous reactions. The scarcity of published cases likely stems from the rarity of SCARs, underreporting, or limited recognition of these events in clinical practice (Hung et al., 2024), particularly for newer agents such as romosozumab. This limitation underscores the need for larger, prospective studies or registries to better characterize the incidence and clinical patterns of SCARs associated with anti-osteoporosis drugs. Furthermore, incomplete reporting of histopathological findings or standardized causality tools (e.g., the Naranjo scale) in some case reports may introduce uncertainty in attributing SCARs to specific anti-osteoporosis drugs.
The FAERS database is vital for post-market medication safety monitoring, identifying potential drug-related risks, including rare adverse events not detected in clinical trials. However, limitations such as reporting bias, underdocumentation, duplicate entries, and incomplete records, especially in older adults with multiple chronic conditions, hinder its effectiveness. These issues limit drug–drug interaction detection and the robustness of findings, particularly with few case reports. In our study, the limited number of case reports restricted the validation of rare adverse event signals as low reporting may reflect underdocumentation rather than true incidence. We integrated FAERS data with detailed case reports, combining FAERS’s broad, population-level signals with clinical case reports. This approach maximizes the reliability of our findings regarding rare events. However, FAERS signals indicate statistical associations, not causality, increasing the risk of false-positive results. These limitations highlight the need for cautious interpretation and validation through clinical studies or complementary data sources. In addition, our study focused on primary suspect drugs, excluding combination regimens. Future research should explore interactions between anti-osteoporosis drugs and concomitant medications.
5 Conclusion
In conclusion, this study provides the first comprehensive pharmacovigilance analysis of SCARs associated with anti-osteoporosis drugs, identifying significant signals for risedronic acid, zoledronic acid, and alendronic acid. These findings, supported by a systematic case review, highlight the need for heightened clinical vigilance, particularly in elderly female patients. Clinicians should assess patient-specific risk factors, such as HLA profiles and polypharmacy, before initiating therapy and monitor for cutaneous reactions during the first 2–8 weeks. Future research should focus on elucidating the immunopathogenic mechanisms of these reactions and evaluating the impact of combination therapies to further optimize patient safety.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material; further inquiries can be directed to the corresponding author.
Author contributions
J-WL: Writing – original draft, Writing – review and editing. X-DL: Writing – original draft, Writing – review and editing. BD: Writing – oiginal draft, Writing – review and editing.
Funding
The authors declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Al-Attar, M., De Santis, M., and Massarotti, M. (2020). DRESS syndrome in response to denosumab: first documented case report. Bone Rep. 12, 100239. doi:10.1016/j.bonr.2019.100239
Alghamdi, N. J., Almuhaidib, S. R., Alharbi, A. S., Aljalfan, A. A. N., and Al-Husain, K. M. (2024). Delayed inflammatory reaction to hyaluronic acid dermal filler following zoledronic acid administration: a case report. Clin. Cosmet. Investig. Dermatol 17, 1347–1350. doi:10.2147/CCID.S458750
de Arruda, J. A., Silva, P., Amaral, M. B., Cotta, F., Avendanho, R., and Mesquita, R. (2017). Erythema multiforme induced by alendronate sodium in a geriatric patient: a case report and review of the literature. J. Clin. Exp. Dent. 9 (7), e929–e933. doi:10.4317/jced.53653
Barrera, B. A., Wilton, L., Harris, S., and Shakir, S. A. (2005). Prescription-event monitoring study on 13,164 patients prescribed risedronate in primary care in England. Osteoporos. Int. 16 (12), 1989–1998. doi:10.1007/s00198-005-1986-1
Barthalon, E., Dupire, G., Calugareanu, A., and Said, B. B. (2024). Pamidronate-induced SDRIFE confirmed by skin testing without cross reactivity to zoledronate. Eur. J. Dermatol 34 (3), 304. doi:10.1684/ejd.2024.4699
Bautista-Villanueva, S., Galleani, C., Barranco, R., Bellón, T., Blanco, M., and García-Moguel, I. (2021). Acute localized exanthematous pustulosis due to alendronate. J. Investig. Allergol. Clin. Immunol. 32 (1), 69–70. doi:10.18176/jiaci.0709
Belhadjali, H., Slim, R., Aouam, K., Youssef, M., and Zili, J. (2008). Cutaneous vasculitis induced by risedronate. Allergy 63 (10), 1405. doi:10.1111/j.1398-9995.2008.01836.x
Bianchi, L., Hansel, K., Romita, P., Foti, C., and Stingeni, L. (2017). Erythema multiforme-like eruption induced by risedronate. Contact Dermat. 77 (5), 348–349. doi:10.1111/cod.12839
Boada, A., Carrascosa, J. M., Leal, L., and Ferrándiz, C. (2009). Generalized cutaneous drug eruption due to strontium ranelate. J. Eur. Acad. Dermatol Venereol. 23 (3), 321–322. doi:10.1111/j.1468-3083.2008.02856.x
Chen, C. B., Hung, W. K., Wang, C. W., Lee, C. C., Hung, S. I., and Chung, W. H. (2023). Advances in understanding of the pathogenesis and therapeutic implications of drug reaction with eosinophilia and systemic symptoms: an updated review. Front. Med. (Lausanne) 10, 1187937. doi:10.3389/fmed.2023.1187937
Chu, H., and Kim, D. S. (2016). Eczematous dermatitis due to subcutaneous teriparatide injection. Endocrine 52 (2), 395–396. doi:10.1007/s12020-015-0817-1
Cummings, S. R., San Martin, J., McClung, M. R., Siris, E. S., Eastell, R., Reid, I. R., et al. (2009). Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N. Engl. J. Med. 361 (8), 756–765. doi:10.1056/NEJMoa0809493
Deschaseaux, F., Delgado, D., Pistoia, V., Giuliani, M., Morandi, F., and Durrbach, A. (2011). HLA-G in organ transplantation: towards clinical applications. Cell Mol. Life Sci. 68 (3), 397–404. doi:10.1007/s00018-010-0581-6
Deshpande, P., Hertzman, R. J., Palubinsky, A. M., Giles, J. B., Karnes, J. H., Gibson, A., et al. (2021). Immunopharmacogenomics: mechanisms of HLA-associated drug reactions. Clin. Pharmacol. Ther. 110 (3), 607–615. doi:10.1002/cpt.2343
Drago, F., Cogorno, L., Broccolo, F., Ciccarese, G., and Parodi, A. (2016). A fatal case of DRESS induced by strontium ranelate associated with HHV-7 reactivation. Osteoporos. Int. 27 (3), 1261–1264. doi:10.1007/s00198-015-3384-7
Fusaroli, M., Raschi, E., Poluzzi, E., and Hauben, M. (2024a). The evolving role of disproportionality analysis in pharmacovigilance. Expert Opin. Drug Saf. 23 (8), 981–994. doi:10.1080/14740338.2024.2368817
Fusaroli, M., Salvo, F., Begaud, B., AlShammari, T. M., Bate, A., Battini, V., et al. (2024b). The REporting of A disproportionality analysis for DrUg safety signal detection using individual case safety reports in PharmacoVigilance (READUS-PV): explanation and elaboration. Drug Saf. 47 (6), 585–599. doi:10.1007/s40264-024-01423-7
Garcia-Nunez, I., Algaba-Marmol, M. A., and Castro-Cost, C. (2021). Erythema multiforme after intake of risedronate: a cross-reactivity study. J. Investig. Allergol. Clin. Immunol. 31 (5), 457–458. doi:10.18176/jiaci.0668
Hasegawa, A., and Abe, R. (2024). Stevens-johnson syndrome and toxic epidermal necrolysis: updates in pathophysiology and management. Chin. Med. J. Engl. 137 (19), 2294–2307. doi:10.1097/CM9.0000000000003250
Hsu, D. Y., Brieva, J., Silverberg, N. B., and Silverberg, J. I. (2016). Morbidity and mortality of Stevens-Johnson syndrome and toxic epidermal necrolysis in United States adults. J. Invest Dermatol 136 (7), 1387–1397. doi:10.1016/j.jid.2016.03.023
Hung, S.-I., Mockenhaupt, M., Blumenthal, K. G., Abe, R., Ueta, M., Ingen-Housz-Oro, S., et al. (2024). Severe cutaneous adverse reactions. Nat. Rev. Dis. Prim. 10 (1), 30. doi:10.1038/s41572-024-00514-0
Iyer, D., Buggy, Y., O'Reilly, K., and Searle, M. (2009). Strontium ranelate as a cause of acute renal failure and dress syndrome. Nephrol. Carlt. 14 (6), 624. doi:10.1111/j.1440-1797.2009.01125.x
Jonville-Bera, A. P., and Autret-Leca, E. (2011). Adverse drug reactions of strontium ranelate(Protelos) in France. Presse Med. 40 (10), e453–e462. doi:10.1016/j.lpm.2011.07.010
Jonville-Béra, A. P., Crickx, B., Aaron, L., Hartingh, I., and Autret-Leca, E. (2009). Strontium ranelate-induced DRESS syndrome: first two case reports. Allergy 64 (4), 658–659. doi:10.1111/j.1398-9995.2009.01940.x
Kinyó, Á., Belso, N., Nagy, N., Pálvölgyi, A., Nagy, I., Korom, I., et al. (2011). Strontium ranelate-induced DRESS syndrome with persistent autoimmune hepatitis. Acta Derm. Venereol. 91 (2), 205–206. doi:10.2340/00015555-1014
Kolitz, E., McKesey, J., Kwan, E., Gill, J. G., and Mauskar, M. (2021). Strontium citrate associated drug reaction with eosinophilia and systemic symptoms syndrome with granulomatous dermatitis. JAAD Case Rep. 10, 85–88. doi:10.1016/j.jdcr.2021.02.002
Kramkimel, N., Sibon, C., Le Beller, C., Saiag, P., and Mahé, E. (2009). Bullous DRESS in a patient on strontium ranelate. Clin. Exp. Dermatol 34 (7), e349–e350. doi:10.1111/j.1365-2230.2009.03302.x
Kranenburg, G., de Jong, P. A., Bartstra, J. W., Lagerweij, S. J., Lam, M. G., Ossewaarde-van Norel, J., et al. (2018). Etidronate for prevention of ectopic mineralization in patients with pseudoxanthoma elasticum. J. Am. Coll. Cardiol. 71 (10), 1117–1126. doi:10.1016/j.jacc.2017.12.062
Lee, H. Y., Lie, D., Lim, K. S., Thirumoorthy, T., and Pang, S. M. (2009). Strontium ranelate-induced toxic epidermal necrolysis in a patient with post-menopausal osteoporosis. Osteoporos. Int. 20 (1), 161–162. doi:10.1007/s00198-008-0677-0
Lee, H. Y., Shen, M. X., Lim, Y. L., Tay, Y. K., Chan, M. M., Pang, S. M., et al. (2016). Increased risk of strontium ranelate-related SJS/TEN is associated with HLA. Osteoporos. Int. 27 (8), 2577–2583. doi:10.1007/s00198-016-3568-9
Leis-Dosil, V. M., Rubio-Flores, C., Ruiz-Bravo Burguillos, E., and Díaz-Díaz, R. M. (2013). Cutaneous vascular calcifications secondary to treatment with teriparatide. Actas Dermosifiliogr. 104 (1), 87–88. doi:10.1016/j.ad.2012.01.023
Li, D., Gou, J., Zhu, J., Zhang, T., Liu, F., Zhang, D., et al. (2023). Severe cutaneous adverse reactions to drugs: a real-world pharmacovigilance study using the FDA adverse event reporting system database. Front. Pharmacol. 14, 1117391. doi:10.3389/fphar.2023.1117391
Li, C., Li, Z., Sun, Q., Xiang, Y., and Liu, A. (2024). Severe cutaneous adverse reactions associated with immune checkpoint inhibitors therapy and anti-VEGF combination therapy: a real-world study of the FDA adverse event reporting system. Expert Opin. Drug Saf. 23 (6), 777–784. doi:10.1080/14740338.2023.2251381
Liang, C., An, P., Zhang, Y., Liu, X., and Zhang, B. (2024). Fatal outcome related to drug reaction with eosinophilia and systemic symptoms: a disproportionality analysis of FAERS database and a systematic review of cases. Front. Immunol. 15, 1490334. doi:10.3389/fimmu.2024.1490334
Marovt, M., and Marko, P. B. (2024). Denosumab-induced acute generalized exanthematous pustulosis. Acta Derm. Venereol. 104, adv40430. doi:10.2340/actadv.v104.40430
di Meo, N., Gubertini, N., Crocè, L., Tiribelli, C., and Trevisan, G. (2016). DRESS syndrome with autoimmune hepatitis from strontium ranelate. Cutis 97 (5), E22–E26.
Le Merlouette, M., Adamski, H., Dinulescu, M., Le Gall, F., Colin, F., Grimaud, H., et al. (2011). Strontium ranelate-induced DRESS syndrome. Ann. Dermatol Venereol. 138 (2), 124–128. doi:10.1016/j.annder.2010.11.006
Moreno-Higueras, M., Callejas-Rubio, J. L., and Gallo-Padilla, L. (2017). Dress syndrome and bilateral panuveitis caused by strontium ranelate. Med. Clin. Barc. 149 (7), 317–318. doi:10.1016/j.medcli.2017.05.031
Morris, R., Ali, R., and Cheng, F. (2024). Drug repurposing using FDA adverse event reporting system (FAERS) database. Curr. Drug Targets 25 (7), 454–464. doi:10.2174/0113894501290296240327081624
Musette, P., Kaufman, J. M., Rizzoli, R., Cacoub, P., Brandi, M. L., and Reginster, J. Y. (2011). Cutaneous side effects of antiosteoporosis treatments. Ther. Adv. Musculoskelet. Dis. 3 (1), 31–41. doi:10.1177/1759720X10387202
Nassar, K., and Janani, S. (2021). Diffuse adverse cutaneous reactions induced by zoledronic acid administration: a case report: eruptions cutanées diffuses induites par l'administration de l'acide zolédronique. Osteoporos. Int. 32 (12), 2583–2586. doi:10.1007/s00198-021-06021-2
Norimatsu, Y., and Norimatsu, Y. (2021). First report of erythema multiforme minor caused by raloxifene hydrochloride. Case Rep. Dermatol 13 (3), 445–449. doi:10.1159/000519029
Pajus, I., Lestang, P., Lioté, F., and Dryll, A. (1993). Erythroderma after clodronate treatment. BMJ 307 (6902), 484. doi:10.1136/bmj.307.6902.484
Phillips, E., Knowles, S., Weber, E., and Shear, N. H. (1998). Skin reactions associated with bisphosphonates: a report of 3 cases and an approach to management. J. Allergy Clin. Immunol. 102 (4 Pt 1), 697–698. doi:10.1016/s0091-6749(98)70291-x
Ramirez, G. A., Ripa, M., Burastero, S., Benanti, G., Bagnasco, D., Nannipieri, S., et al. (2023). Drug reaction with eosinophilia and systemic symptoms (DRESS): focus on the pathophysiological and diagnostic role of viruses. Microorganisms 11 (2), 346. doi:10.3390/microorganisms11020346
Rodriguez Arrieta, L. A., Rueda Galvis, P. A., Rueda Galvis, M. V., Pantoja Meneses, S. A., Yépes Rodriguez, J. M., and Suarez Contreras, F. J. (2025). When skin reactions interrupt bone therapy: severe cutaneous adverse reaction to romosozumab leading to treatment discontinuation. Eur. J. Case Rep. Intern Med. 12 (9), 05719. doi:10.12890/2025_05719
Saag, K. G., Wagman, R. B., Geusens, P., Adachi, J. D., Messina, O. D., Emkey, R., et al. (2018). Denosumab versus risedronate in glucocorticoid-induced osteoporosis: a multicentre, randomised, double-blind, active-controlled, double-dummy, non-inferiority study. Lancet Diabetes Endocrinol. 6 (6), 445–454. doi:10.1016/s2213-8587(18)30075-5
Sanchez, A., Lozier, M., Adkinson, B. C., and Ilaiwy, A. (2019). c-ANCA vasculitis after initiation of denosumab. BMJ Case Rep. 12 (3), e228336. doi:10.1136/bcr-2018-228336
Shan, H., Wei, C., Zhang, J., and Wu, B. (2025). Severe cutaneous adverse reactions associated with antifungal agents: a pharmacovigilance analysis based on the FDA adverse event reporting system (FAERS) database. Expert Opin. Drug Saf., 1–8. doi:10.1080/14740338.2024.2438744
Smith, E. V., and Shipley, D. R. (2010). Severe exfoliative dermatitis caused by strontium ranelate: two cases of a new drug reaction. Age Ageing 39 (3), 401–403. doi:10.1093/ageing/afq026
Song, X., Guo, Y., and Zhao, H. (2019). Erythema multiforme caused by ibandronate sodium: a rare case report. Dermatol Ther. 32 (4), e12984. doi:10.1111/dth.12984
Swarnkar, B., Biswal, S., Agarwal, S., and Gupta, S. (2021). Zoledronate induced urticarial vasculitis. Dermatol Ther. 34 (6), e15164. doi:10.1111/dth.15164
Tan, K. W., Wang, Y. S., and Tay, Y. K. (2011). Stevens-johnson syndrome due to strontium ranelate. Ann. Acad. Med. Singap 40 (11), 510–511. doi:10.47102/annals-acadmedsg.v40n11p510
Tempark, T., John, S., Rerknimitr, P., Satapornpong, P., and Sukasem, C. (2022). Drug-induced severe cutaneous adverse reactions: insights into clinical presentation, immunopathogenesis, diagnostic methods, treatment, and pharmacogenomics. Front. Pharmacol. 13, 832048. doi:10.3389/fphar.2022.832048
Varenna, M., Bertoldo, F., Di Monaco, M., Giusti, A., Martini, G., Rossini, M., et al. (2013). Safety profile of drugs used in the treatment of osteoporosis: a systematical review of the literature. Reumatismo 65 (4), 143–166. doi:10.4081/reumatismo.2013.143
Weber, I., Olaiwan, A., Bonte, I., Artigou, C., Pras-Landre, V., Moguelet, P., et al. (2011). Adverse cutaneous drug reactions to ibandronate. Eur. J. Dermatol 21 (4), 591–594. doi:10.1684/ejd.2011.1386
Wei, C., Zhang, J., Yin, W., Jiang, A., Liu, Y., and Wu, B. (2023). A real-world pharmacovigilance study of severe cutaneous adverse reactions associated with antiepileptic drug combination therapy: data mining of FDA adverse event reporting system. Expert Opin. Drug Saf. 22 (6), 509–515. doi:10.1080/14740338.2023.2147506
Woo, S. D., Yoon, J., Doo, G. E., Park, Y., Lee, Y., Lee, S. H., et al. (2020). Common causes and characteristics of adverse drug reactions in older adults: a retrospective study. BMC Pharmacol. Toxicol. 21 (1), 87. doi:10.1186/s40360-020-00464-9
Yang, C. Y., Chen, C. H., Wang, H. Y., Hsiao, H. L., Hsiao, Y. H., and Chung, W. H. (2014). Strontium ranelate related Stevens-Johnson syndrome: a case report. Osteoporos. Int. 25 (6), 1813–1816. doi:10.1007/s00198-014-2688-3
Zahedi, B., Wallace, Z. S., Côté, M. M., and Yu, E. W. (2023). An unexpected case of cutaneous vasculitis following zoledronic acid infusion. JCEM Case Rep. 1 (4), luad085. doi:10.1210/jcemcr/luad085
Zhu, J., Chen, G., He, Z., Zheng, Y., Gao, S., Li, J., et al. (2021). Stevens-johnson syndrome/toxic epidermal necrolysis in patients treated with immune checkpoint inhibitors: a safety analysis of clinical trials and FDA pharmacovigilance database. EClinicalMedicine 37, 100951. doi:10.1016/j.eclinm.2021.100951
Keywords: anti-osteoporosis drugs, severe cutaneous adverse reactions, pharmacovigilance, case review, adverse event reporting system
Citation: Li J-W, Lei X-D and Dai B (2025) Severe cutaneous adverse reactions to anti-osteoporosis drugs: a real-world pharmacovigilance study using the FDA Adverse Event Reporting System database and a review of published cases. Front. Pharmacol. 16:1707885. doi: 10.3389/fphar.2025.1707885
Received: 18 September 2025; Accepted: 06 November 2025;
Published: 26 November 2025.
Edited by:
Ingrid Fricke-Galindo, Instituto Nacional de Enfermedades Respiratorias-México (INER), MexicoReviewed by:
Bernd Rosenkranz, Fundisa African Academy of Medicines Development, South AfricaGhizal Fatima, ERA’s Lucknow Medical College, India
Copyright © 2025 Li, Lei and Dai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bing Dai, MTA5MjM0MTM2MUBxcS5jb20=
 Jun-Wei Li
Jun-Wei Li Xin-Dong Lei2
Xin-Dong Lei2