- 1Department of Pharmacy, The Affiliated Hospital of Xuzhou Medical University, Xuzhou, Jiangsu, China
- 2Department of Pharmacy, Southeast University Affiliated Xuzhou Central Hospital, Xuzhou, Jiangsu, China
- 3Department of Cardiology, The Affiliated Hospital of Xuzhou Medical University, Xuzhou, Jiangsu, China
- 4School of Public Health, Fudan University, Shanghai, China
- 5Department of Clinical Pharmacy and Pharmacy Administration, School of Pharmacy, Fudan University, Shanghai, China
Objective: To evaluate the cost-utility of different dosing regimens of PCSK9 inhibitors, added to statin therapy, in patients with hypercholesterolemia or at high cardiovascular risk in China.
Methods: A Markov cohort multistate-transition model was developed from the perspective of the Chinese healthcare system, with a 1-year cycle and lifetime horizon. Treatment effects were derived from a network meta-analysis. Costs, utilities, and mortality data were obtained from published literature and national databases. Both costs and outcomes were discounted at a rate of 5% annually. The primary outcome was the incremental cost-utility ratio (ICUR). The willingness-to-pay (WTP) threshold was set at 1–3 times China’s 2024 per capita gross domestic product. One-way, probabilistic, and scenario sensitivity analyses were performed to test model robustness.
Results: In the base-case analysis, evolocumab 140 mg every 2 weeks (Q2W) was the most cost-effective option, dominating all other active regimens. Compared with statin therapy alone, it generated incremental costs of $11,109.27 and a quality-adjusted life year (QALY) gain of 0.42. The resulting ICUR was $26,217.47 per QALY, which is below the WTP threshold of $39,875.0 per QALY. Although less favorable than evolocumab, alirocumab 75 mg Q2W and tafolecimab 150 mg Q2W were also cost-effective versus statins alone, with ICURs of $34,279.73 and $34,002.10 per QALY, respectively. All other regimens were dominated, and inclisiran showed the least favorable cost-utility profile (ICUR $113,800.14 per QALY). Sensitivity analyses identified the discount rate as the key driver of uncertainty, with ICURs for evolocumab 140 mg Q2W versus statins alone ranging from $15,903.46 to $34,573.62 per QALY. Probabilistic sensitivity analysis showed a 98.9% probability of evolocumab 140 mg Q2W being cost-effective versus statins alone.
Conclusion: At the current negotiated price, evolocumab 140 mg Q2W is the most cost-effective PCSK9 inhibitor regimen for Chinese patients with hypercholesterolemia or at high cardiovascular risk when added to statin therapy. Alirocumab 75 mg Q2W and tafolecimab 150 mg Q2W also represent cost-effective alternatives. These findings provide important evidence to support clinical decision-making and optimize resource allocation in China.
1 Introduction
Cardiovascular disease (CVD) remains the leading chronic non-communicable disease worldwide. In China, atherosclerotic cardiovascular diseases (ASCVD), including ischemic heart disease and ischemic stroke, are the primary causes of death in both urban and rural populations, accounting for over 40% of total mortality (National Center for Cardiovascular Diseases, 2022). Despite advances in prevention and treatment, the burden of ASCVD in China continues to rise (Zhao et al., 2019), underscoring the urgent need for more effective interventions.
Extensive epidemiological, genetic, and clinical evidence has firmly established low-density lipoprotein cholesterol (LDL-C) as a causal risk factor for ASCVD (Ference et al., 2017). However, while the prevalence of dyslipidemia among Chinese adults has increased substantially (Song et al., 2019), awareness, treatment, and control rates remain low (Zhang et al., 2018). This mismatch between disease burden and lipid control highlights the need for more effective lipid-lowering strategies to improve cardiovascular outcomes.
Statins remain the cornerstone of LDL-C-lowering therapy, but many Chinese patients fail to achieve LDL-C targets even with high-intensity regimens. This reflects both lower statin tolerability and a higher incidence of adverse effects among Chinese patients (Gitt et al., 2016; HPS2-THRIVE Collaborative Group, 2013). Moreover, doubling the statin dose only yields an additional ∼6% LDL-C reduction while increasing the risk of adverse effects, including liver dysfunction, myopathy, and new-onset diabetes (Stroes et al., 2015; Maki et al., 2014). As a result, Chinese guidelines recommend standard or moderate-intensity rather than high-intensity statin therapy.
The advent of proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors has transformed lipid management. By inhibiting PCSK9-mediated degradation of LDL receptors (LDLR), these therapies increase LDL-C clearance, lowering LDL-C levels by 50%–70% and reducing the risk of major adverse cardiovascular events (MACE) when added to statins (Robinson et al., 2015; Ference et al., 2017). Approved agents include monoclonal antibodies (evolocumab and alirocumab) and small interfering RNA (siRNA) agents such as inclisiran.
Landmark trials have confirmed the efficacy of PCSK9 inhibitors in both LDL-C reduction and cardiovascular risk mitigation (Sabatine et al., 2017; Schwartz et al., 2018). Evolocumab, the first approved agent, lowers LDL-C by 59% on top of statins, reduces cardiovascular events by 20%, and achieves a 66% LDL-C reduction in Asian populations (Sabatine et al., 2017; Keech et al., 2021). Over a long-term follow-up, alirocumab consistently reduced LDL-C by nearly 50% and significantly lowered MACE risk (Goodman et al., 2023). Tafolecimab, China’s first domestically developed PCSK9 monoclonal antibody, offers flexible dosing every 2, 4, or 6 weeks to improve treatment adherence (Liu et al., 2025). The CREDIT phase III trials showed that tafolecimab plus statins reduced LDL-C by up to 70% and lipoprotein(a) [Lp(a)] by 50% (Huo et al., 2023; Chai et al., 2023; Qi et al., 2023). Inclisiran, a siRNA therapy, achieves comparable LDL-C reductions to monoclonal antibodies with a single injection maintaining efficacy for up to 6 months (Fitzgerald et al., 2017), offering significant adherence advantages. However, it has not yet been included in the 2025 National Reimbursement Drug List (NRDL), limiting patient access in China.
Despite their clinical benefits, the high costs of PCSK9 inhibitors raise concerns about economic sustainability, especially in resource-limited settings. As new PCSK9 inhibitors and dosing regimens emerge, clinicians face increasing complexity in balancing efficacy, safety, adherence, and cost-effectiveness when selecting optimal therapies. To date, no study has comprehensively compared the cost-utility of different PCSK9 inhibitor regimens within the Chinese healthcare system.
To address these challenges, we developed a Markov cohort state-transition model to compare the cost-utility of various PCSK9 inhibitor regimens combined with statins for Chinese patients with hypercholesterolemia or at high cardiovascular risk. Our aim was to provide robust evidence to inform clinical decision-making, optimize healthcare resource allocation, and support policy development in China.
2 Methods
This study was presented per the Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) checklist (Supplementary Table S1) (Husereau et al., 2022).
2.1 Model structure
A Markov cohort state-transition model was established to compare the cost-effectiveness of statins in combination with PCSK9 inhibitors versus statins alone for patients with hypercholesterolemia. The model structure was adapted from previously published economic evaluation frameworks of lipid-lowering therapies (Wan et al., 2023; Xie et al., 2023; Wan et al., 2025), and was constructed in TreeAge Pro Healthcare Version 2022 (TreeAge Software, LLC., Williamstown, MA, United States).
The model consisted of 11 mutually exclusive health states to capture the long-term occurrence, progression, and outcomes of cardiovascular events (CVEs). Patients entered the model in the baseline state of being alive without CVD and could either remain in this state or experience a first myocardial infarction (MI) or ischemic stroke (IS). After the first event, patients transitioned into the corresponding post-event state (post-MI or post-IS) and could experience recurrent CVEs, including second or subsequent MI (MI 2+) or IS (IS 2+). In each health state, patients were at risk of transitioning to death, which was classified as either cardiovascular (CV)-related death or non-CV-related death, both considered irreversible absorbing states. See Figure 1 for detailed information.
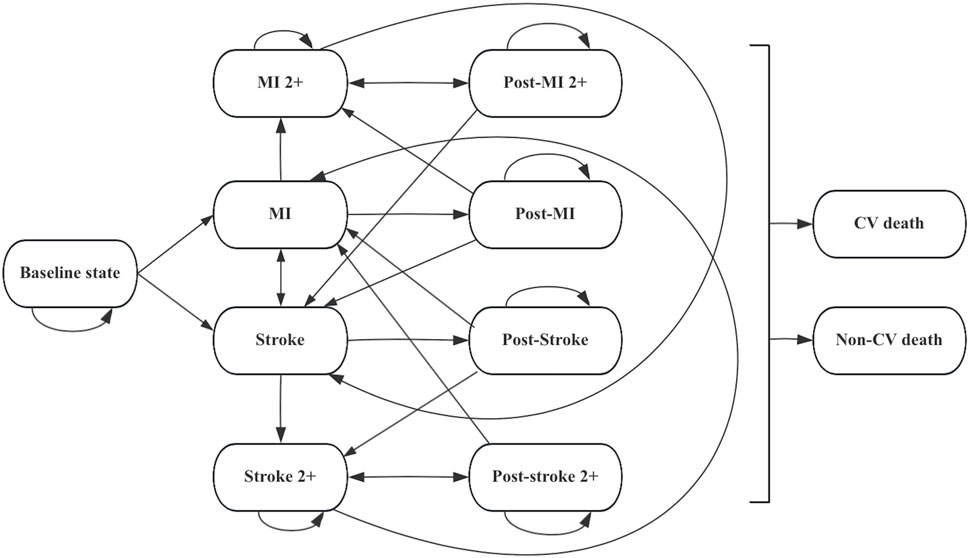
Figure 1. Markov model diagram for hypercholesterolemia patients. Abbreviations: CV cardiovascular, MI myocardial infarction.
The model applied a 1-year cycle length and a 30-year lifetime horizon to approximate lifetime cardiovascular risk. It was assumed that only one CV event could occur per cycle, but patients could experience multiple recurrent events across the entire time horizon.
2.2 Patient population
This study population was defined based on a network meta-analysis that evaluated the efficacy and safety of PCSK9 inhibitors in patients with hypercholesterolemia or at high cardiovascular risk whose LDL-C levels remained inadequately controlled despite standard lipid-lowering therapy. This network meta-analysis included 26 randomized controlled trials with a total of 16,510 participants. Across trials, the mean age varied from 49.0 to 66.1 years (mean age: 60.6 years), the proportion of female participants ranged between 17.6% and 57.3%, and the mean baseline LDL-C level was 3.13 mmol/L (Liu et al., 2024) (Supplementary Table S2).
All patients were assumed to receive high-intensity statin therapy (atorvastatin 40–80 mg/day or rosuvastatin 20 mg/day, administered orally once daily) as background lipid-lowering treatment. The intervention group received statins in combination with one of the PCSK9 inhibitor regimens, comprising 11 dosing strategies across four agents: alirocumab (75 mg every 2 weeks, 150 mg every 2 weeks, 300 mg every month), evolocumab (140 mg every 2 weeks, 420 mg every month), tafolecimab (150 mg every 2 weeks, 450 mg every 4 weeks, 600 mg every 6 weeks), and inclisiran (300 mg on day 1, day 90, and every 6 months thereafter). The control group received statin therapy alone without the addition of a PCSK9 inhibitor.
2.3 Clinical event probabilities and intervention effects
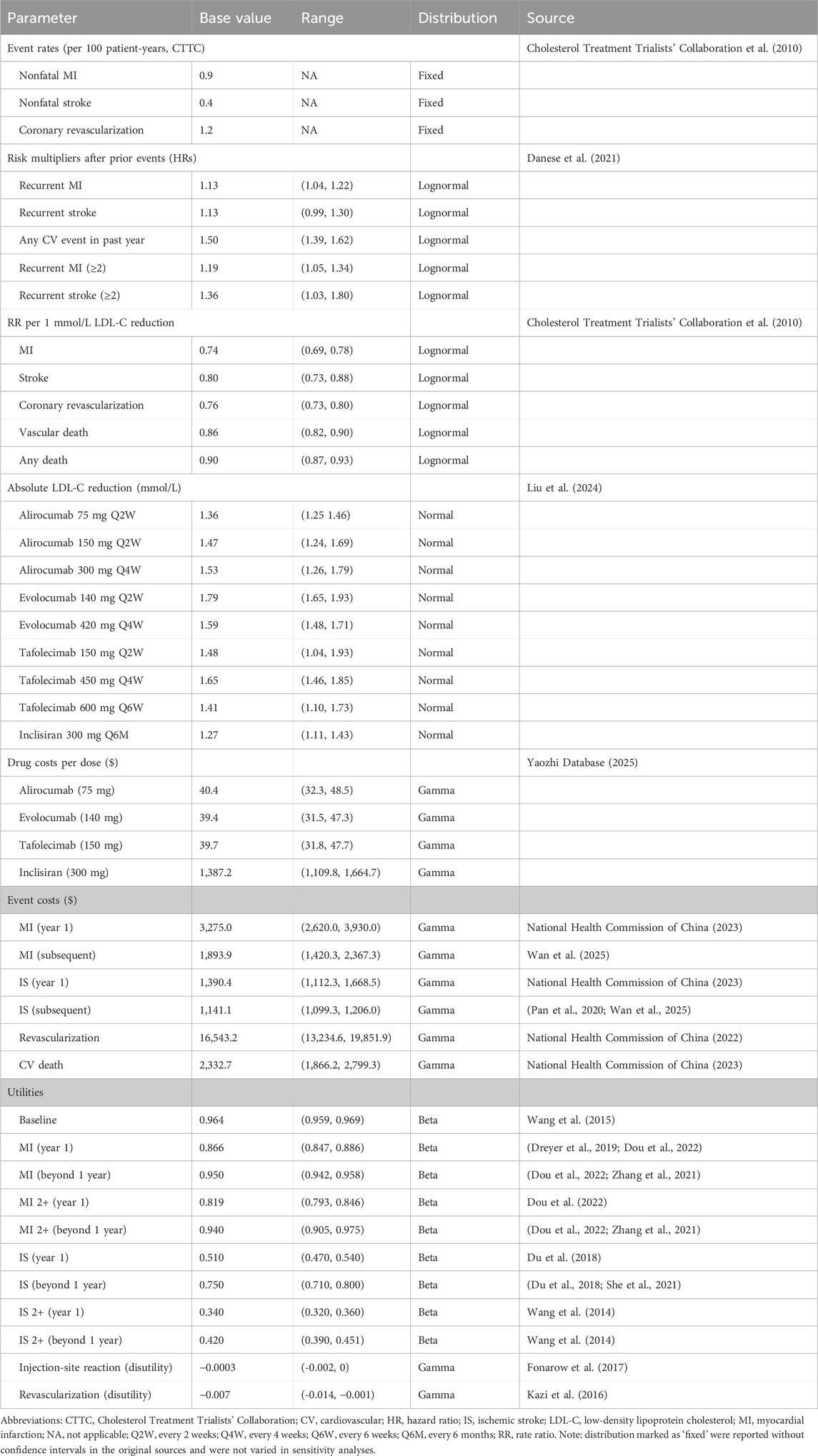
Baseline CV event rates were derived from the statin arm of the Cholesterol Treatment Trialists’ Collaboration (CTTC) meta-analysis (Cholesterol Treatment Trialists' Collaboration et al., 2010). To reflect our target population, we adjusted these rates for age and LDL-C levels using the following equation (Equation 1) (Danese et al., 2021):
where
Transition probabilities were estimated based on the CTTC meta-analysis, which links LDL-C reduction to CV risk reduction (Cholesterol Treatment Trialists' Collaboration et al., 2010), an approach widely adopted in previous cost-effectiveness studies of PCSK9 inhibitors (Xie et al., 2023; Fonarow et al., 2017; Wan et al., 2025). To capture the increased risks following a prior CV event, we applied risk multipliers from the re-estimated REACH model (Table 1) (Danese et al., 2021). The LDL-C–lowering effects of PCSK9 inhibitors, in addition to standard statin therapy, were derived from a network meta-analysis comparing multiple PCSK9 inhibitor dosing regimens with placebo added to statins. All regimens showed significant LDL-C reductions (Table 1) (Liu et al., 2024). Based on CTTC findings, each 1.0 mmol/L (38.7 mg/dL) LDL-C reduction was associated with a 26% lower risk of MI, 20% lower risk of stroke, 14% lower risk of vascular death, and 24% lower risk of coronary revascularization (Cholesterol Treatment Trialists' Collaboration et al., 2010; Cholesterol Treatment Trialists’ Collaboration et al., 2015). Event rates after treatment were estimated using Equation 2:
where
Finally, event-specific annual rates were converted to transition probabilities using the standard exponential formula (Equation 3) (Gidwani and Russell, 2020):
where P represents the annual transition probability, r is the annual event rate, and t is the cycle length (1 year).
All calculated transition probabilities between health states are provided in Supplementary Table S3.
2.4 Mortality
Age-specific all-cause mortality rates were derived from the 2023 China Health Statistics Yearbook (National Health Commission of China, 2023), representing baseline mortality in the general Chinese population (Supplementary Table S4). CV mortality was extracted from the same source, and non-CV mortality was calculated by subtracting CV mortality from all-cause mortality. To reflect the increased risk of death after a cardiovascular event, patients with an MI or IS in the previous year were assigned a relative risk of CV death of 1.31 (95% CI: 1.15–1.49) (Wilson et al., 2012). All mortality rates were then converted into transition probabilities using Equation 3.
2.5 Costs and utilities
We conducted the analysis from the perspective of the Chinese healthcare system, considering only direct medical costs. These included hospitalization expenses (e.g., surgical, examination, and nursing fees) and medication costs, while direct non-medical costs, such as accommodation and transportation, were excluded. Hospitalization costs were obtained from the 2023 China Health Statistics Yearbook (National Health Commission of China, 2023). Long-term post-discharge treatment costs for myocardial infarction were derived from basic health insurance data (Wang et al., 2018; Xiong et al., 2018). Stroke subsequent annual costs were estimated from the expenses of secondary preventive treatments (Pan et al., 2020; Wan et al., 2025). Due to data limitations, we assumed the cost of CV deaths equaled the average hospitalization costs for acute MI and stroke in Chinese public hospitals (National Health Commission of China, 2023), whereas non-CV deaths were considered cost-free and independent of treatment regimens, consistent with previous studies (Xie et al., 2023; Fonarow et al., 2017). As in previous models (Wan et al., 2023; Xie et al., 2023), revascularization (RV) was considered a procedure incurring treatment costs but was not modeled as a separate health state. Outpatient follow-up and monitoring costs (e.g., lipid panels, physician visits) were not included, as these are similar between treatment groups and comprehensive national data are limited.
We sourced drug acquisition costs from publicly available Chinese databases. Unit prices for PCSK9 inhibitors were retrieved from the China Yaozhi website, while statin prices were based on the highest bid prices from the Chinese centralized procurement platform. Using the median daily cost of atorvastatin and rosuvastatin, we calculated an annual statin cost of USD 846.1 per patient (Supplementary Table S5). All costs were converted to 2024 U.S. dollars (USD) using an exchange rate of 1 USD = 7.20 Chinese Yuan (CNY) and adjusted for inflation using the Consumer Price Index (CPI) from the World Bank (https://data.worldbank.org/indicator/PA.NUS.FCRF) (Turner et al., 2019).
We derived health state utility values from published studies of Chinese populations, primarily measured using the Chinese version of the European Quality of Life 5-Dimensional Three-Level Scale (EQ-5D-3L) and its associated scoring algorithm. We incorporated temporary disutilities associated with RV or injection-related discomfort, based on previous literature (Wan et al., 2023; Xie et al., 2023; Xi et al., 2023).
Following the 2020 China Guidelines for Pharmacoeconomic Evaluations (Liu, 2020), we applied 5% annual discount rate to both costs and health outcomes and applied half-cycle corrections to all health states and costs, accounting for transitions occurring, on average, midway through each model cycle.
2.6 Outcomes
The primary outcome was the incremental cost-utility ratio (ICUR), defined as the additional cost per quality-adjusted life year (QALY) gained with PCSK9 inhibitors versus statin therapy alone. Health outcomes were expressed as QALYs, with incremental QALYs (ΔQALYs) and costs (ΔCosts) calculated as differences between treatment arms. ICUR was computed as ΔCosts/ΔQALYs. ICURs were calculated only among non-dominated strategies. A strategy was deemed dominated if it was more costly and less effective than an alternative, or subject to extended dominance if its ICUR exceeded that of a more effective option.
The willingness-to-pay (WTP) threshold was set at 1–3 times the 2024 national gross domestic product (GDP) per capita ($13,291.7–$39,875.0 per QALY) (Liu, 2020; Griffiths et al., 2015), which is established by the World Health Organization for cost-effectiveness and China Guidelines for Pharmacoeconomic Evaluations (Liu, 2020; World Health Organization, 2025). Cost-effectiveness was categorized as: (1) ICUR <1 × GDP/QALY, highly cost-effective; (2) ICUR 1–3 × GDP/QALY, cost-effective; and (3) ICUR >3 × GDP/QALY, not cost-effective.
To further explore value-based pricing, a price threshold analysis was performed to estimate the maximum price at which PCSK9 inhibitors would be considered cost-effective under the specified WTP threshold.
2.7 Sensitivity analyses
Deterministic and probabilistic sensitivity analyses were performed to evaluate the robustness of the model results. In the one-way sensitivity analysis, key parameters—including treatment costs, utility values, efficacy estimates, and RRs for cardiovascular events—were varied individually within their 95% confidence intervals (CIs), or by ±20% of base-case values when CIs were unavailable. The annual discount rate was varied between 0% and 8%. Results were presented as tornado diagrams.
For the probabilistic sensitivity analysis (PSA), 1,000 Monte Carlo simulations were performed to account for joint parameter uncertainty. Standard probability distributions were assigned: gamma for costs, lognormal for RRs, normal for mean LDL-C reduction, and beta for transition probabilities and utility values (Table 1). PSA results were illustrated using scatter plots and cost-effectiveness acceptability curves (CEACs).
Scenario analyses were also conducted to assess the impact of treatment duration, with alternative time horizons ranging from 5 to 30 years.
2.8 Model validation
The model was adapted from previously published and validated economic evaluation frameworks for lipid-lowering therapies. Therefore, it underwent validation for face and internal validity, but not independent external validation against specific trial data.
Face validity was established through consultations with clinical pharmacists and health economists, who reviewed the model structure, assumptions, input parameters, and outputs to ensure clinical relevance and logical consistency. Internal validity was verified by comprehensive checks of parameter sources, formula integrity, and computational logic. Independent cross-checking by another researcher confirmed the accuracy of the model’s implementation and results.
3 Results
3.1 Base-case analysis
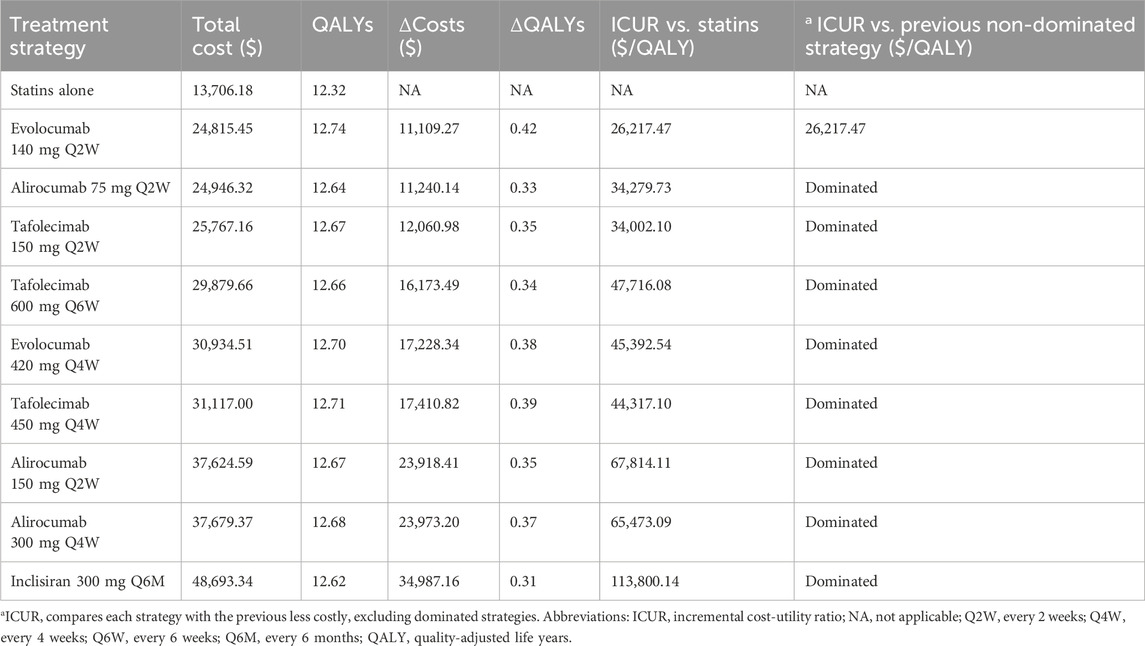
In the base case, using a network meta-analysis of the hypercholesterolemia population with a mean baseline LDL-C of 3.13 mmol/L, statin therapy alone was the least costly option, with a mean lifetime cost of $13,706.18 and effectiveness of 12.32 QALYs. Among active comparators, evolocumab 140 mg every 2 weeks (Q2W) was the only non-dominated option. It yields a mean lifetime cost of $24,815.45 and effectiveness of 12.74 QALYs, corresponding to incremental costs of $11,109.27 and a QALY gain of 0.42 versus statins alone. The resulting ICUR was $26,217.47 per QALY gained, which is well within the WTP threshold of 1–3× GDP per QALY ($39,875/QALY), indicating cost-effectiveness (Table 2). All other regimens, including various doses of alirocumab (75–300 mg), tafolecimab (150–600 mg), evolocumab 420 mg every 4 weeks (Q4W), and inclisiran 300 mg every 6 months (Q6M), were absolutely dominated, showing lower QALY gains at higher costs compared with evolocumab 140 mg Q2W.
However, when compared individually to statins alone, the ICURs for alirocumab 75 mg Q2W, and tafolecimab 150 mg Q2W were all below 3× GDP/QALY ($39,875/QALY), indicating cost-effectiveness. Notably, inclisiran showed the least favorable profile, with a lifetime cost of $48,693.34 and 12.62 QALYs, resulting in an ICUR of $113,800.14 per QALY gained versus statins alone.
3.2 Sensitivity analyses
The one-way sensitivity analysis confirmed the robustness of base-case results when individual parameters were varied. As shown in Figure 2, the parameters with the greatest impact on the ICUR of evolocumab 140 mg Q2W versus statins alone were discount rate (ICUR range: $15,903.46–$34,573.62/QALY), the effect of LDL-C reduction on all-cause death ($21,234.78–$34,696.08/QALY), and the cost of evolocumab per dose ($20,322.14–$32,112.78/QALY). Despite these variations, all ICURs remained below the WTP threshold of 3× GDP per QALY ($39,875/QALY).
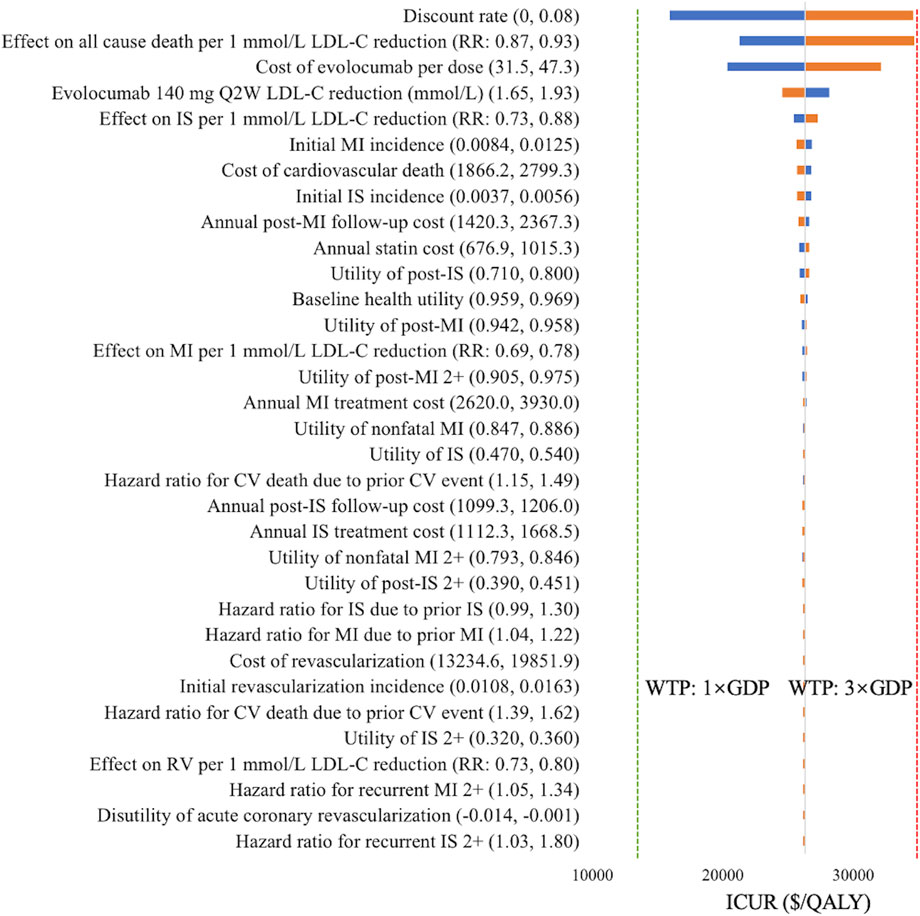
Figure 2. Tornado diagram of evolocumab 140 mg Q2W versus statin therapy alone. Blue bars indicate ICURs at the lower parameter values, and yellow bars indicate ICURs at the upper parameter values. The green dotted line denotes the WTP threshold of one time GDP per capita ($13,291.7/QALY), and the red dotted line denotes the WTP threshold of three times GDP per capita ($39,875/QALY). Abbreviations: CV, cardiovascular; GDP, gross domestic product; ICUR, incremental cost-utility ratio; IS, ischemic stroke; LDL-C, low-density lipoprotein cholesterol; MI, myocardial infarction; Q2W, every 2 weeks; QALY, quality-adjusted life years; RR, rate ratio; RV, revascularization; WTP, willingness-to-pay.
For the PSA, we performed 1,000 Monte-Carlo simulations based on the specified ranges and distributions of model parameters. As shown in Figure 3, the majority of simulations fell below the WTP threshold of 3× GDP per QALY ($39,875/QALY), indicating that adding evolocumab Q2W to statins has a 98.9% probability of being cost-effective.
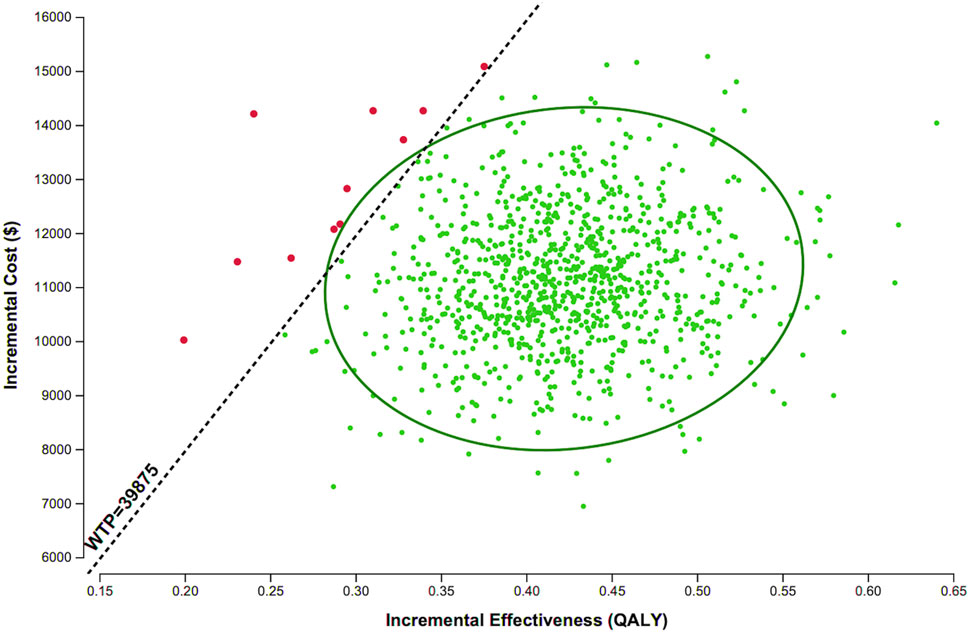
Figure 3. Scatter plot of evolocumab 140 mg Q2W versus statin therapy alone. Each point represents the corresponding incremental costs and QALYs. Abbreviations: QALY, quality-adjusted life years; WTP, willingness-to-pay.
Figure 4 presents the CEAC for various PCSK9 inhibitor regimens versus statin therapy alone. At the WTP threshold of 3× GDP per QALY, evolocumab Q2W had a 90.3% probability of being cost-effective, compared with 7.6% for tafolecimab Q2W, 1.3% for alirocumab 75 mg Q2W, 0.8% for statin therapy alone, and 0% for all other treatments.
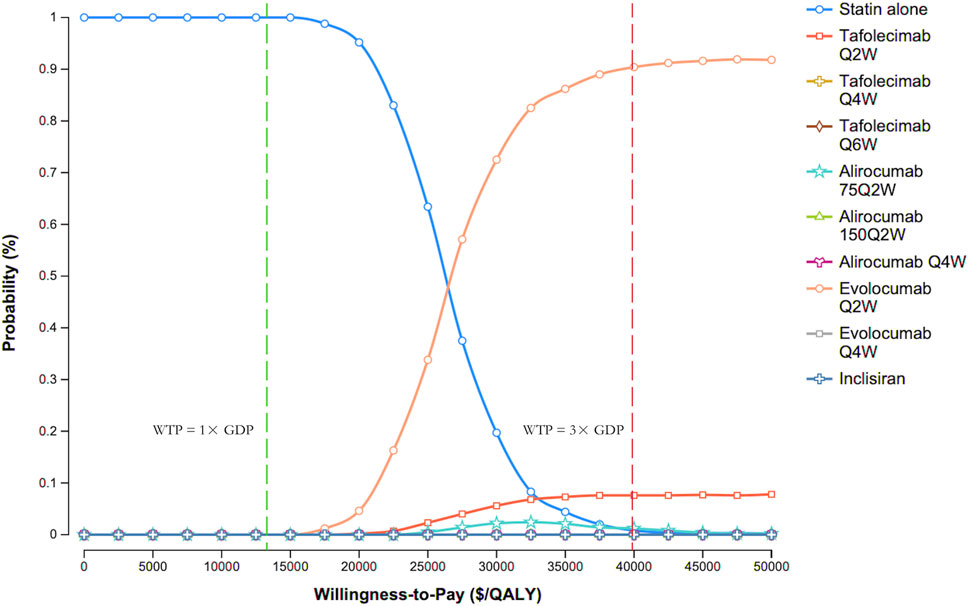
Figure 4. Cost-effectiveness acceptability curves. Abbreviations: GDP, gross domestic product; Q2W, every 2 weeks; Q4W, every 4 weeks; Q6W, every 6 weeks; WTP, willingness-to-pay.
3.3 Scenario analysis
A scenario analysis varying the time horizon from 5 to 30 years showed progressively lower ICURs for evolocumab versus statin therapy alone: $220,505.85/QALY (5 years) to $26,217.47/QALY (30 years) (Table 3). At the WTP threshold of $39,875/QALY, evolocumab 140 mg Q2W was not cost-effective for horizons ≤20 years but became cost-effective at 25 and 30 years, indicating that longer horizons significantly improve its economic value under current pricing. Similar trends were observed for other PCSK9 inhibitor regimens (Supplementary Table S6).
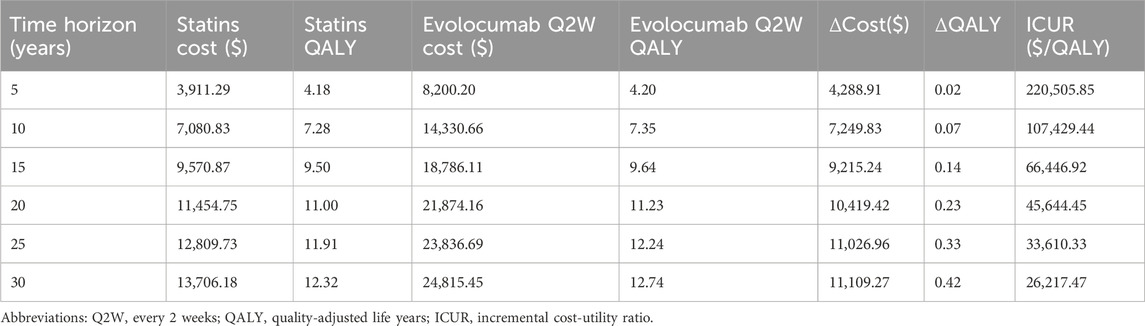
Table 3. Scenario analysis of evolocumab 140 mg Q2W vs. statins across different treatment durations.
3.4 Price threshold analysis
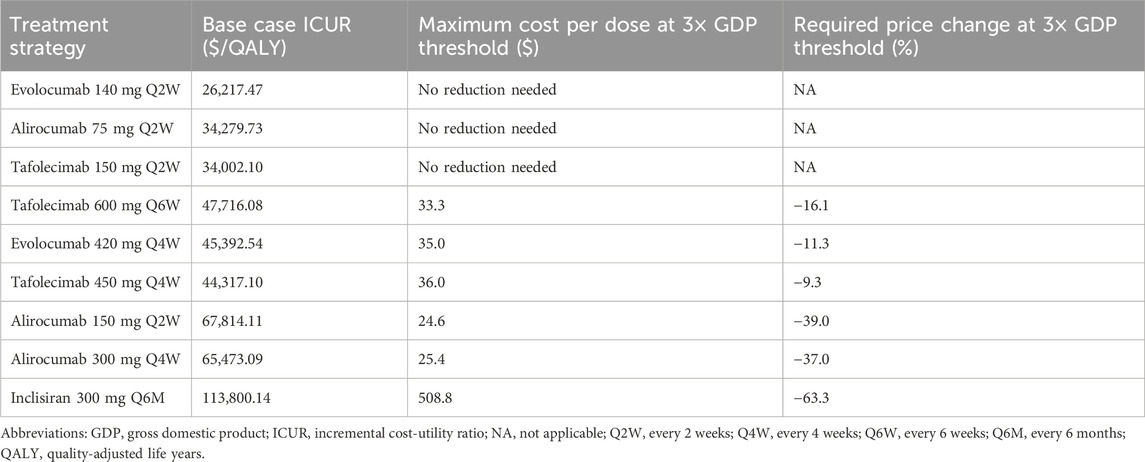
At current prices, evolocumab 140 mg Q2W, alirocumab 75 mg Q2W, and tafolecimab 150 mg Q2W were already cost-effective compared with statin therapy alone, requiring no price reduction. In contrast, regimens such as tafolecimab 600 mg every 6 weeks (Q6W), evolocumab 420 mg Q4W, and alirocumab 150 mg Q2W would need moderate price reductions of 9%–39% to reach cost-effectiveness. Inclisiran 300 mg Q6M, which exhibited the highest ICUR in the base-case analysis, would require a substantial price reduction of approximately 63% to meet the 3× GDP threshold (Table 4).
4 Discussion
This study evaluated the cost-utility of PCSK9 inhibitors (evolocumab, alirocumab, tafolecimab, and inclisiran) as adjuncts to statin therapy in Chinese patients with hypercholesterolemia or at high cardiovascular risk, using a Markov cohort multistate-transition model informed by LDL-C reductions from a network meta-analysis. To our knowledge, this is the first comprehensive pharmacoeconomic comparison of all PCSK9 inhibitors currently marketed in China from the perspective of the Chinese healthcare system, evaluating primary prevention in hypercholesterolemia.
In the base-case analyses, evolocumab 140 mg Q2W was the most cost-effective regimen, with an ICUR of $26,217.47/QALY versus statin therapy alone, well below the Chinese WTP threshold ($39,875/QALY). While evolocumab dominates other regimens, alirocumab 75 mg Q2W and tafolecimab 150 mg Q2W were also cost-effective compared with statin therapy alone under current negotiated prices.
Previous pharmacoeconomic evaluations of PCSK9 inhibitors in China, including studies of evolocumab (Wan et al., 2023; Xie et al., 2023; Xi et al., 2023; Liang et al., 2021b), alirocumab (Liang et al., 2021a), tafolecimab (Wan et al., 2025), and inclisiran (Zhou et al., 2024), predominantly adopted the healthcare system perspective, with one also incorporating the patient perspective (Wan et al., 2023). All employed Markov cohort state-transition models, varying in health states and model structure.
Before evolocumab’s inclusion in the National Drug List (2021) at CNY 1,298 ($180.3) per dose, results were mixed: it was not cost-effective in post-MI patients (Liang et al., 2021b) but cost-effective in acute coronary syndrome, particularly for very high-risk ASCVD patients (Xi et al., 2023), likely due to methodological differences. After national price negotiations reduced the price to CNY 283.8 ($39.4) per dose, evolocumab plus statins became cost-effective (Xie et al., 2023), consistent with our findings.
Similarly, alirocumab was initially not cost-effective at CNY 1,982 ($275.3)/dose, requiring an 88% price reduction to meet the WTP threshold (Liang et al., 2021a). At its current reimbursed price of $40.4 per dose, alirocumab 75 mg Q2W is cost-effective versus statins alone (ICUR $34,279.73/QALY).
Tafolecimab, a domestic PCSK9 inhibitor with flexible dosing options, was approved by the National Medical Products Administration (NMPA) in 2023 and added to the national medical insurance list in 2024. Although less cost-effective than evolocumab, tafolecimab 150 mg Q2W remained cost-effective versus statins alone at its current price of $39.7 per dose, similar to a prior threshold analysis (Wan et al., 2025).
Inclisiran was the least favorable option, with the highest lifetime costs and lowest QALYs among the therapies assessed. Despite price reductions to CNY 9,988 ($1,387) per dose, its ICUR remains above the WTP threshold, consistent with prior studies estimating it would only be cost-effective with over 88% price reduction (Zhou et al., 2024). Similar international findings highlight the high price sensitivity of inclisiran (Kam et al., 2020; Galactionova et al., 2022; Desai et al., 2022).
All PCSK9 inhibitors improved QALYs relative to statins alone (Jena et al., 2016; Kazi et al., 2016), supporting broader population health benefits. Reductions in cardiovascular events, hospitalizations, and revascularizations contributed to a favorable cost-utility. Under current pricing in China, evolocumab is the most economically attractive option, with alirocumab and tafolecimab as viable alternatives, while further price adjustments would be needed to justify inclisiran in routine practice.
Because most trials have relatively short follow-up for hard outcomes, our model projected lifetime cardiovascular benefits by linking LDL-C reductions to event risk, supported by CTTC meta-analyses (Cholesterol Treatment Trialists' Collaboration et al., 2010; Cholesterol Treatment Trialists’ Collaboration et al., 2015; Cholesterol Treatment Trialists’ Collaboration, 2019). Mendelian randomization studies reinforce this approach, showing comparable reductions in coronary mortality for lifelong LDL-C lowering via 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase and PCSK9 pathways (Ference et al., 2016). Using this framework, we translated each regimen’s LDL-C reduction into proportional changes in MACE to estimate QALYs and costs. This approach aligns with prior economic evaluations of PCSK9 inhibitors that also projected outcomes from LDL-C reductions (Fonarow et al., 2017; Xie et al., 2023; Kazi et al., 2016; Gandra et al., 2016). LDL-C anchored models, by relying on a validated dose-response relationship, provide more stable estimates across settings and allow indirect comparisons and long-term extrapolation (Korman and Wisloff, 2018). In contrast, some models rely on trial-reported hazard ratios or country-specific risk equations (Villa et al., 2020), but their outcomes are more sensitive to trial populations, event definitions, and assumptions about cardiovascular mortality effects, thereby limiting generalizability (Fonarow et al., 2017).
Scenario analyses indicated that extending the time horizon substantially improved the cost-utility of evolocumab. The ICUR declined markedly with longer treatment durations, from $220,505.85/QALY at 5 years to $45,644.45/QALY at 20 years (approximately the average life expectancy in China) and further to $26,217.47/QALY at 30 years, covering nearly the entire population lifespan. This trend is consistent with the economic principles of chronic disease management, where the benefits of effective therapies accumulate over time. By delaying disease progression and reducing the incidence of major adverse cardiovascular events (e.g., myocardial infarction and stroke), long-term treatment can generate considerable health gains and offset significant costs (Mohiuddin et al., 2024). These events are not only associated with high direct medical expenditures but also with substantial losses in health-related quality of life. Consequently, sustained therapy reduces both the clinical and economic burden of disease, as reflected in the growing QALY gains and the declining ICURs observed in our analysis. Furthermore, the discount rate applied in the model influenced long-term cost-effectiveness estimates. When early clinical benefits persist and downstream costs plateau, a long-term perspective becomes essential to fully capture the economic value of high-cost preventive therapies (Hultkrantz, 2021).
This study has several limitations that should be acknowledged. First, the model’s clinical efficacy inputs were derived from a network meta-analysis with relatively short follow-up, while long-term benefits were extrapolated over a lifetime. However, if LDL-C–lowering effects or cardiovascular risk reductions attenuate over time, the actual cost-effectiveness of PCSK9 inhibitors could be over- or underestimated. Moreover, in the absence of head-to-head trials comparing different PCSK9 inhibitors and dosing regimens, efficacy estimates relied on indirect evidence, introducing additional uncertainty. Second, the relationship between LDL-C reduction and CV risk reduction was based on the CTTC meta-analysis. If the real-world CV risk reduction associated with PCSK9 inhibitors differs from this estimate, the cost-effectiveness results may be biased. Third, due to the lack of real-world data in Chinese patients, baseline CV event rates and many transition probabilities were drawn from international literature, potentially limiting the generalizability of the findings to the Chinese healthcare setting. Fourth, this analysis was conducted from the Chinese healthcare system perspective and included only direct medical costs. Exclusion of indirect costs and broader societal benefits, such as productivity gains from improved long-term outcomes, may underestimate the full economic value of PCSK9 inhibitors. Finally, the model assumed full adherence throughout the time horizon, whereas real-world treatment persistence is typically lower. Although some studies report variable adherence and persistence of PCSK9 inhibitors (Svensson et al., 2024; Gargiulo et al., 2023), their relatively short follow-up does not capture long-term patterns. Consequently, persistence and adherence were not included in the base-case analysis, which may have led to overestimated QALY gains and cost-utility.
5 Conclusion
This study provides the first comprehensive pharmacoeconomic evaluation of all currently available PCSK9 inhibitors in China. Using a Markov multistate-transition model, we found that evolocumab 140 mg Q2W emerged as the most cost-effective regimen for patients with hypercholesterolemia from the Chinese healthcare perspective, with ICUR well below the willingness-to-pay threshold. Alirocumab 75 mg Q2W and tafolecimab 150 mg Q2W were also identified as cost-effective alternatives. These results offer valuable evidence to guide clinical decision-making and optimize healthcare resource allocation.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participant’s legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
DH: Writing – review and editing, Conceptualization, Investigation. XD: Writing – review and editing, Writing – original draft, Data curation, Conceptualization. MF: Writing – review and editing, Investigation. XZ: Methodology, Writing – review and editing, Validation. QH: Writing – review and editing. TW: Supervision, Writing – review and editing. ZF: Writing – original draft, Investigation, Software, Visualization, Funding acquisition, Methodology, Writing – review and editing, Formal Analysis, Validation, Data curation, Conceptualization.
Funding
The authors declare that financial support was received for the research and/or publication of this article. This research was supported by the Affiliated Hospital of Xuzhou Medical University (No. 2023ZY01), Pharmaceutical Research Project of Xuzhou Municipal Health Commission (No. XWYXKY202302), and Jiangsu Research Hospital Association for Precision Medication (No. SYHKJ-JY-2025-16).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1708701/full#supplementary-material
References
Chai, M., He, Y., Zhao, W., Han, X., Zhao, G., Ma, X., et al. (2023). Efficacy and safety of tafolecimab in Chinese patients with heterozygous familial hypercholesterolemia: a randomized, double-blind, placebo-controlled phase 3 trial (Credit-2). BMC Med. 21, 77. doi:10.1186/s12916-023-02797-8
Cholesterol Treatment Trialists' Collaboration (2019). Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomised controlled trials. Lancet 393, 407–415. doi:10.1016/S0140-6736(18)31942-1
Cholesterol Treatment Trialists' Collaboration, Baigent, C., Blackwell, L., Emberson, J., Holland, L. E., Reith, C., et al. (2010). Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 376, 1670–1681. doi:10.1016/S0140-6736(10)61350-5
Cholesterol Treatment Trialists' Collaboration, Fulcher, J., O'connell, R., Voysey, M., Emberson, J., Blackwell, L., et al. (2015). Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet 385, 1397–1405. doi:10.1016/S0140-6736(14)61368-4
Danese, M. D., Pemberton-Ross, P., Catterick, D., and Villa, G. (2021). Estimation of the increased risk associated with recurrent events or polyvascular atherosclerotic cardiovascular disease in the United Kingdom. Eur. J. Prev. Cardiol. 28, 335–343. doi:10.1177/2047487319899212
Desai, N. R., Campbell, C., Electricwala, B., Petrou, M., Trueman, D., Woodcock, F., et al. (2022). Cost effectiveness of inclisiran in atherosclerotic cardiovascular patients with elevated low-density lipoprotein cholesterol despite statin use: a threshold analysis. Am. J. Cardiovasc Drugs 22, 545–556. doi:10.1007/s40256-022-00534-9
Dou, L., Mao, Z., Fu, Q., Chen, G., and Li, S. (2022). Health-related quality of life and its influencing factors in patients with coronary heart disease in China. Patient Prefer Adherence 16, 781–795. doi:10.2147/PPA.S347681
Dreyer, R. P., Zheng, X., Xu, X., Liu, S., Li, J., Ding, Q., et al. (2019). Sex differences in health outcomes at one year following acute myocardial infarction: a report from the China patient-centered evaluative assessment of cardiac events prospective acute myocardial infarction study. Eur. Heart J. Acute Cardiovasc Care 8, 273–282. doi:10.1177/2048872618803726
Du, X. D., Zhu, P., Li, M. E., Wang, J., Meng, H. D., and Zhu, C. R. (2018). Health utility of patients with stroke measured by Eq-5d and Sf-6d. Sichuan Da Xue Xue Bao Yi Xue Ban. 49, 252–257.
Ference, B. A., Robinson, J. G., Brook, R. D., Catapano, A. L., Chapman, M. J., Neff, D. R., et al. (2016). Variation in PCSK9 and HMGCR and risk of cardiovascular disease and diabetes. N. Engl. J. Med. 375, 2144–2153. doi:10.1056/NEJMoa1604304
Ference, B. A., Ginsberg, H. N., Graham, I., Ray, K. K., Packard, C. J., Bruckert, E., et al. (2017). Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European atherosclerosis society consensus panel. Eur. Heart J. 38, 2459–2472. doi:10.1093/eurheartj/ehx144
Fitzgerald, K., White, S., Borodovsky, A., Bettencourt, B. R., Strahs, A., Clausen, V., et al. (2017). A highly durable RNAi therapeutic inhibitor of PCSK9. N. Engl. J. Med. 376, 41–51. doi:10.1056/NEJMoa1609243
Fonarow, G. C., Keech, A. C., Pedersen, T. R., Giugliano, R. P., Sever, P. S., Lindgren, P., et al. (2017). Cost-effectiveness of evolocumab therapy for reducing cardiovascular events in patients with atherosclerotic cardiovascular disease. JAMA Cardiol. 2, 1069–1078. doi:10.1001/jamacardio.2017.2762
Galactionova, K., Salari, P., Mattli, R., Rachamin, Y., Meier, R., and Schwenkglenks, M. (2022). Cost-effectiveness, burden of disease and budget impact of inclisiran: dynamic cohort modelling of a real-world population with cardiovascular disease. Pharmacoeconomics 40, 791–806. doi:10.1007/s40273-022-01152-8
Gandra, S. R., Villa, G., Fonarow, G. C., Lothgren, M., Lindgren, P., Somaratne, R., et al. (2016). Cost-effectiveness of LDL-C lowering with evolocumab in patients with high cardiovascular risk in the United States. Clin. Cardiol. 39, 313–320. doi:10.1002/clc.22535
Gargiulo, P., Basile, C., Cesaro, A., Marzano, F., Buonocore, D., Asile, G., et al. (2023). Efficacy, safety, adherence and persistence of PCSK9 inhibitors in clinical practice: A single country, multicenter, observational study (AT-TARGET-IT). Atherosclerosis 366, 32–39. doi:10.1016/j.atherosclerosis.2023.01.001
Gidwani, R., and Russell, L. B. (2020). Estimating transition probabilities from published evidence: a tutorial for decision modelers. Pharmacoeconomics 38, 1153–1164. doi:10.1007/s40273-020-00937-z
Gitt, A. K., Lautsch, D., Ferrieres, J., Kastelein, J., Drexel, H., Horack, M., et al. (2016). Low-density lipoprotein cholesterol in a global cohort of 57,885 statin-treated patients. Atherosclerosis 255, 200–209. doi:10.1016/j.atherosclerosis.2016.09.004
Goodman, S. G., Steg, P. G., Poulouin, Y., Bhatt, D. L., Bittner, V. A., Diaz, R., et al. (2023). Long-term efficacy, safety, and tolerability of alirocumab in 8242 patients eligible for 3 to 5 years of placebo-controlled observation in the ODYSSEY outcomes trial. J. Am. Heart Assoc. 12, e029216. doi:10.1161/JAHA.122.029216
Griffiths, M., Maruszczak, M., and Kusel, J. (2015). The WHO-choice cost-effectiveness threshold: a country-level analysis of changes over time. Value Health 18, A88. doi:10.1016/j.jval.2015.03.517
HPS2-THRIVE Collaborative Group (2013). HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur. Heart J. 34, 1279–1291. doi:10.1093/eurheartj/eht055
Hultkrantz, L. (2021). Discounting in economic evaluation of healthcare interventions: what about the risk term? Eur. J. Health Econ. 22, 357–363. doi:10.1007/s10198-020-01257-x
Huo, Y., Chen, B., Lian, Q., Wang, S., Liu, L., Lu, D., et al. (2023). Tafolecimab in Chinese patients with non-familial hypercholesterolemia (CREDIT-1): a 48-week randomized, double-blind, placebo-controlled phase 3 trial. Lancet Reg. Health West Pac 41, 100907. doi:10.1016/j.lanwpc.2023.100907
Husereau, D., Drummond, M., Augustovski, F., De Bekker-Grob, E., Briggs, A. H., Carswell, C., et al. (2022). Consolidated health economic evaluation reporting standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. Value Health 25, 3–9. doi:10.1016/j.jval.2021.11.1351
Jena, A. B., Blumenthal, D. M., Stevens, W., Chou, J. W., Ton, T. G., and Goldman, D. P. (2016). Value of improved lipid control in patients at high risk for adverse cardiac events. Am. J. Manag. Care 22, e199–e207.
Kam, N., Perera, K., Zomer, E., Liew, D., and Ademi, Z. (2020). Inclisiran as adjunct lipid-lowering therapy for patients with cardiovascular disease: a cost-effectiveness analysis. Pharmacoeconomics 38, 1007–1020. doi:10.1007/s40273-020-00948-w
Kazi, D. S., Moran, A. E., Coxson, P. G., Penko, J., Ollendorf, D. A., Pearson, S. D., et al. (2016). Cost-effectiveness of PCSK9 inhibitor therapy in patients with heterozygous familial hypercholesterolemia or atherosclerotic cardiovascular disease. JAMA 316, 743–753. doi:10.1001/jama.2016.11004
Keech, A. C., Oyama, K., Sever, P. S., Tang, M., Murphy, S. A., Hirayama, A., et al. (2021). Efficacy and safety of long-term evolocumab use among Asian subjects-a subgroup analysis of the further cardiovascular outcomes research with PCSK9 inhibition in subjects with elevated risk (FOURIER) trial. Circ. J. 85, 2063–2070. doi:10.1253/circj.CJ-20-1051
Korman, M., and Wisloff, T. (2018). Modelling the cost-effectiveness of PCSK9 inhibitors vs. ezetimibe through LDL-C reductions in a Norwegian setting. Eur. Heart J. Cardiovasc Pharmacother. 4, 15–22. doi:10.1093/ehjcvp/pvx010
Liang, Z., Chen, Q., Wei, R., Ma, C., Zhang, X., Chen, X., et al. (2021a). Cost-effectiveness of alirocumab for the secondary prevention of cardiovascular events after myocardial infarction in the Chinese setting. Front. Pharmacol. 12, 648244. doi:10.3389/fphar.2021.648244
Liang, Z., Chen, Q., Yang, F., Yan, X., Zhang, X., Chen, X., et al. (2021b). Cost-effectiveness of evolocumab therapy for myocardial infarction: the Chinese healthcare perspective. Cardiovasc Drugs Ther. 35, 775–785. doi:10.1007/s10557-020-07079-6
Liu, G. (2020). China guidelines for pharmacoeconomic evaluations Chinese-English version. Beijing, China.
Liu, D., Zhang, J., Zhang, X., Jiang, F., Wu, Y., Yang, B., et al. (2024). The efficacy and safety of proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors combined with statins in patients with hypercholesterolemia: a network meta-analysis. Front. Cardiovasc Med. 11, 1454918. doi:10.3389/fcvm.2024.1454918
Liu, H. H., Li, S., and Li, J. J. (2025). Tafolecimab, a third monoclonal antibody for PCSK9 as the novel lipid-lowering drug around the world: a narrative review. Drugs 85, 627–642. doi:10.1007/s40265-025-02167-z
Maki, K. C., Ridker, P. M., Brown, W. V., Grundy, S. M., and Sattar, N.The Diabetes Subpanel of the National Lipid Association Expert Panel (2014). An assessment by the statin diabetes safety task force: 2014 update. J. Clin. Lipidol. 8, S17–S29. doi:10.1016/j.jacl.2014.02.012
Mohiuddin, S. G., Ward, M. E., Hollingworth, W., Watson, J. C., Whiting, P. F., and Thom, H. H. Z. (2024). Cost-effectiveness of routine monitoring of long-term conditions in primary care: informing decision modelling with a systematic review in hypertension, type 2 diabetes and chronic kidney disease. Pharmacoecon Open 8, 359–371. doi:10.1007/s41669-024-00473-y
National Center for Cardiovascular Diseases (2022). Cardiovascular health and disease report in China 2021. Beijing, China: Science Press.
National Health Commission of China (2022). China health statistical yearbook 2022. Available online at: http://www.nhc.gov.cn/ (Accessed July 30, 2025).
National Health Commission of China (2023). China health statistical yearbook 2023. Available online at: http://www.nhc.gov.cn/ (Accessed May 13, 2025).
Pan, Y., Zhang, L., Li, Z., Meng, X., Wang, Y., Li, H., et al. (2020). Cost-effectiveness of a multifaceted quality improvement intervention for acute ischemic stroke in China. Stroke 51, 1265–1271. doi:10.1161/STROKEAHA.119.027980
Qi, L., Liu, D., Qu, Y., Chen, B., Meng, H., Zhu, L., et al. (2023). Tafolecimab in Chinese patients with hypercholesterolemia (CREDIT-4): a randomized, double-blind, placebo-controlled phase 3 trial. JACC Asia 3, 636–645. doi:10.1016/j.jacasi.2023.04.011
Robinson, J. G., Farnier, M., Krempf, M., Bergeron, J., Luc, G., Averna, M., et al. (2015). Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N. Engl. J. Med. 372, 1489–1499. doi:10.1056/NEJMoa1501031
Sabatine, M. S., Giugliano, R. P., Keech, A. C., Honarpour, N., Wiviott, S. D., Murphy, S. A., et al. (2017). Evolocumab and clinical outcomes in patients with cardiovascular disease. N. Engl. J. Med. 376, 1713–1722. doi:10.1056/NEJMoa1615664
Schwartz, G. G., Steg, P. G., Szarek, M., Bhatt, D. L., Bittner, V. A., Diaz, R., et al. (2018). Alirocumab and cardiovascular outcomes after acute coronary syndrome. N. Engl. J. Med. 379, 2097–2107. doi:10.1056/NEJMoa1801174
Svensson, M. K., James, S., Ravn-Fischer, A., Villa, G., Schalin, L., Cars, T., et al. (2024). A retrospective nationwide analysis of evolocumab use in Sweden and its effect on low-density lipoprotein cholesterol levels. Ups. J. Med. Sci. 129. doi:10.48101/ujms.v129.9618
She, R., Yan, Z., Hao, Y., Zhang, Z., Du, Y., Liang, Y., et al. (2021). Health-related quality of life after first-ever acute ischemic stroke: associations with cardiovascular health metrics. Qual. Life Res. 30, 2907–2917. doi:10.1007/s11136-021-02853-x
Song, P. K., Man, Q. Q., Li, H., Pang, S. J., Jia, S. S., Li, Y. Q., et al. (2019). Trends in lipids level and dyslipidemia among Chinese adults, 2002-2015. Biomed. Environ. Sci. 32, 559–570. doi:10.3967/bes2019.074
Stroes, E. S., Thompson, P. D., Corsini, A., Vladutiu, G. D., Raal, F. J., Ray, K. K., et al. (2015). Statin-associated muscle symptoms: impact on statin therapy-European atherosclerosis society consensus panel statement on assessment, aetiology and management. Eur. Heart J. 36, 1012–1022. doi:10.1093/eurheartj/ehv043
Turner, H. C., Lauer, J. A., Tran, B. X., Teerawattananon, Y., and Jit, M. (2019). Adjusting for inflation and currency changes within health economic studies. Value Health 22, 1026–1032. doi:10.1016/j.jval.2019.03.021
Villa, G., Postma, M., Heeg, B., Kroi, F., Sidelnikov, E., Gomez Montero, M., et al. (2020). PCV57 a systematic literature review of cost-utility analyses of PCSK9 inhibitors. Value Health 23, S497. doi:10.1016/j.jval.2020.08.548
Wan, Y., Liu, J., Zhan, X., Zhang, Y., and You, R. (2023). Methodology and results of cost-effectiveness of LDL-C lowering with evolocumab in patients with acute myocardial infarction in China. Cost. Eff. Resour. Alloc. 21, 93. doi:10.1186/s12962-023-00501-4
Wan, Y., Liu, J., Zhan, X., Zhang, Y., and You, R. (2025). Cost-effectiveness and price threshold analysis of tafolecimab in Chinese patients with elevated LDL cholesterol despite statin therapy. Am. J. Cardiovasc Drugs 25, 547–561. doi:10.1007/s40256-025-00733-0
Wang, Y. L., Pan, Y. S., Zhao, X. Q., Wang, D., Johnston, S. C., Liu, L. P., et al. (2014). Recurrent stroke was associated with poor quality of life in patients with transient ischemic attack or minor stroke: finding from the CHANCE trial. CNS Neurosci. Ther. 20, 1029–1035. doi:10.1111/cns.12329
Wang, L., Wu, Y. Q., Tang, X., Li, N., He, L., Cao, Y., et al. (2015). Profile and correlates of health-related quality of life in Chinese patients with coronary heart disease. Chin. Med. J. Engl. 128, 1853–1861. doi:10.4103/0366-6999.160486
Wang, Y., Liu, J., and Wu, J. (2018). Study on medical resource use and direct medical costs during secondary prevention of acute myocardial infarction in urban workers in Tianjin. Chin. J. Health Stat. 35, 61–64.
Wilson, P. W., D'agostino, R. Sr., Bhatt, D. L., Eagle, K., Pencina, M. J., Smith, S. C., et al. (2012). An international model to predict recurrent cardiovascular disease. Am. J. Med. 125, 695–703 e1. doi:10.1016/j.amjmed.2012.01.014
World Health Organization (2025). Cost effectiveness and strategic planning (WHO-Choice) Available online at: http://www.who.int/choice/cost-effectiveness/en/ [Accessed August 26 2025].
Xi, X., Wang, X., Xie, W., Jia, Y., Sanchez, S. Z., Martinez, L., et al. (2023). Comparison of evolocumab and ezetimibe, both combined with statin therapy, for patients with recent acute coronary syndrome: a cost-effectiveness analysis from the Chinese healthcare perspective. Cardiovasc Drugs Ther. 37, 905–916. doi:10.1007/s10557-021-07276-x
Xie, W., Song, Y., Qin, X., and Jin, P. (2023). Cost-effectiveness of evolocumab in adult patients with atherosclerotic cardiovascular disease from Chinese healthcare perspective. Adv. Ther. 40, 489–503. doi:10.1007/s12325-022-02372-2
Xiong, X., Zhang, Z., Ren, J., Zhang, J., Pan, X., Zhang, L., et al. (2018). Impact of universal medical insurance system on the accessibility of medical service supply and affordability of patients in China. PLoS One 13, e0193273. doi:10.1371/journal.pone.0193273
Yaozhi Database (2025). Yaozhi database. Available online at: https://www.yaozh.com/ (Accessed May 13, 2025).
Zhang, M., Deng, Q., Wang, L., Huang, Z., Zhou, M., Li, Y., et al. (2018). Prevalence of dyslipidemia and achievement of low-density lipoprotein cholesterol targets in Chinese adults: a nationally representative survey of 163,641 adults. Int. J. Cardiol. 260, 196–203. doi:10.1016/j.ijcard.2017.12.069
Zhang, M., Chen, P., Zhang, Y., Su, X., Chen, J., Xu, B., et al. (2021). Predictors of quality of life in patients with myocardial infarction combined with dyslipidemia. Front. Public Health 9, 713480. doi:10.3389/fpubh.2021.713480
Zhao, D., Liu, J., Wang, M., Zhang, X., and Zhou, M. (2019). Epidemiology of cardiovascular disease in China: current features and implications. Nat. Rev. Cardiol. 16, 203–212. doi:10.1038/s41569-018-0119-4
Keywords: PCSK9 inhibitors, hypercholesterolemia, cost-utility, pharmacoeconomics, Markov model, China
Citation: Hu D, Deng X, Feng M, Zhang X, He Q, Wang T and Feng Z (2025) Cost-utility analysis of PCSK9 inhibitors for hypercholesterolemia: a Chinese healthcare perspective. Front. Pharmacol. 16:1708701. doi: 10.3389/fphar.2025.1708701
Received: 19 September 2025; Accepted: 07 November 2025;
Published: 19 November 2025.
Edited by:
George Gourzoulidis, Health Through Evidence, GreeceReviewed by:
Nouran Omar El Said, Future University in Egypt, EgyptKapil Kapoor, AdventHealth, United States
Copyright © 2025 Hu, Deng, Feng, Zhang, He, Wang and Feng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tao Wang, bWlzc3d0MjAxMUAxMjYuY29t; Zhen Feng, NzM1NTAyOTI2QHFxLmNvbQ==
†These authors have contributed equally to this work
 Dandan Hu
Dandan Hu Xu Deng
Xu Deng Mengwen Feng
Mengwen Feng Xinyue Zhang4
Xinyue Zhang4 Qingfeng He
Qingfeng He Tao Wang
Tao Wang Zhen Feng
Zhen Feng