- 1Department of Clinical Pharmacy, Wuhan Children’s Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Department of Pediatrics, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 3Department of Pediatrics, Maternal and Child Hospital of Hubei Province, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 4Department of Pathology, Wuhan Children’s Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 5Department of Pharmacy, Wuhan Fourth Hospital, Puai Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 6Department of Pharmacy, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
A Correction on
Astragaloside IV alleviates tacrolimus-induced chronic nephrotoxicity via p62-Keap1-Nrf2 pathway
by Gao P, Du X, Liu L, Xu H, Liu M, Guan X and Zhang C (2021). Front. Pharmacol. 11:610102. doi: 10.3389/fphar.2020.610102
In the published article there were mistakes in Figures 3D, 6E as published. In Figure 3D, the image of Tac group (the second group) was misused. In Figure 6E, the Western blot of p62 was misused. The corrected Figures 3, 6 appear below.
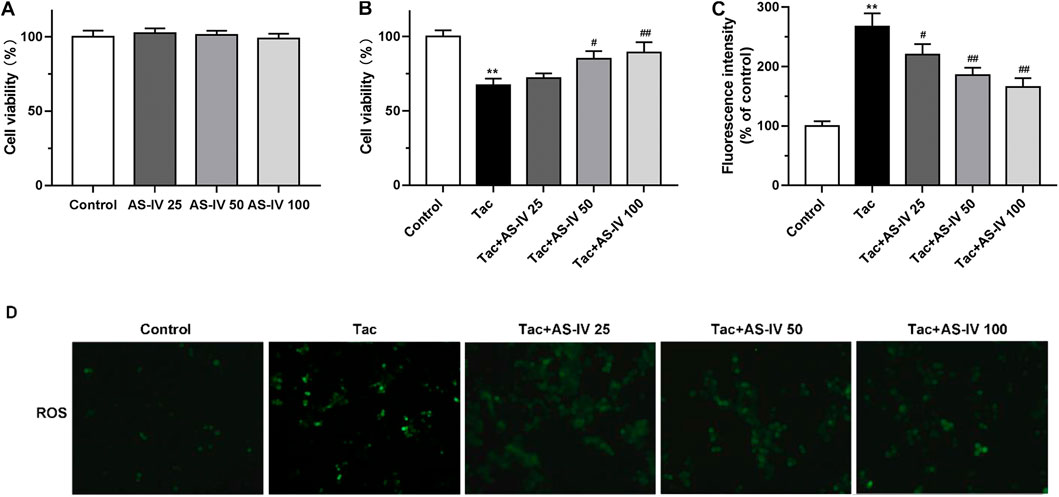
Figure 3. The cell protective and ROS scavenge effect of Astragaloside IV in HK-2 cells (A–B) HK-2 cells were treated with Astragaloside IV (25, 50 or 100 μM) ± tacrolimus (15 μM) for 24h, and then the cell viability was measured using Cell Counting Kit-8 assay. The results were calculated from three independent experiments (C–D) The levels of intracellular ROS were detected with 2′, 7′-dichlorodihydrofluorescein diacetate (H2DCFDA) assay. Results were calculated by the intensity of eight fields from three independent experiments. **p < 0.01 vs. the Control group; #p < 0.05 and ##p < 0.01 vs. the Tac group.
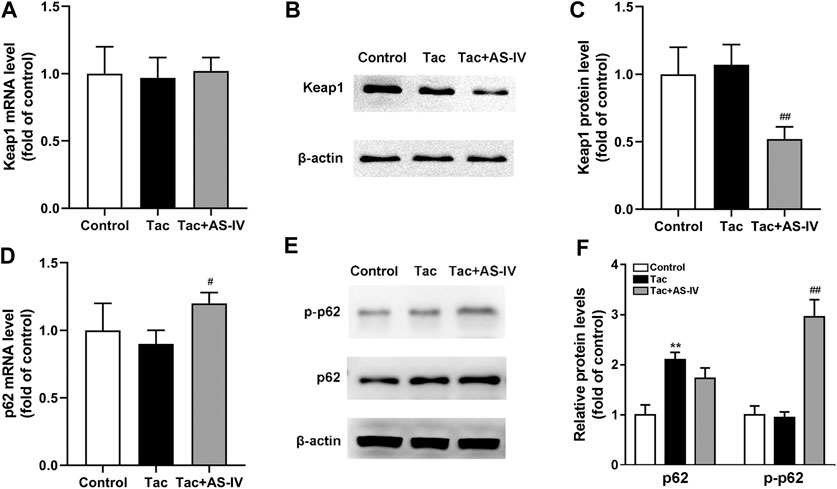
Figure 6. Astragaloside IV increased Keap1 degradation and p62 phosphorylation in vivo. Mice were treated with tacrolimus ± Astragaloside IV for 4 weeks, and then renal tissues were taken to evaluate the effects of Astragaloside IV on the Keap1 mRNA levels (n = 8) (A), the Keap1 protein levels (n = 6) (B)–(C), the p62 mRNA levels (n = 8) (D) and the p62 as well as phosphorylated p62 protein levels (n = 6) (E–F). **p < 0.01 vs. the Control group; #p < 0.05 and ##p < 0.01 vs. the Tac group.
The original article has been updated.
Generative AI statement
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Keywords: astragaloside IV, tacrolimus, chronic nephrotoxicity, p62-Keap1-Nrf2 pathway, oxidative stress
Citation: Gao P, Du X, Liu L, Xu H, Liu M, Guan X and Zhang C (2025) Correction: Astragaloside IV alleviates tacrolimus-induced chronic nephrotoxicity via p62-Keap1-Nrf2 pathway. Front. Pharmacol. 16:1710116. doi: 10.3389/fphar.2025.1710116
Received: 21 September 2025; Accepted: 02 October 2025;
Published: 27 October 2025.
Edited and reviewed by:
Giuseppe Remuzzi, Istituto di Ricerche Farmacologiche Mario Negri IRCCS, ItalyCopyright © 2025 Gao, Du, Liu, Xu, Liu, Guan and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinlei Guan, eGlubGVpZ3VhbkAxNjMuY29t; Chengliang Zhang, Y2x6aGFuZ0B0amgudGptdS5lZHUuY24=
†These authors share first authorship
 Ping Gao
Ping Gao Xiaoyi Du2,3†
Xiaoyi Du2,3† Xinlei Guan
Xinlei Guan Chengliang Zhang
Chengliang Zhang