Abstract
Objective:
This study aimed to detect tirofiban-related adverse event (AE) signals using the FAERS and WHO-VigiAccess databases to support safer clinical use.
Methods:
All tirofiban-related AE reports were retrieved from FAERS (2004Q1 to 2024Q4) and WHO-VigiAccess (Retrieval date 2024.12.15). Disproportionality analyses were performed using ROR (Reporting Odds Ratio), PRR (Proportional Reporting Ratio), BCPNN (Bayesian Confidence Propagation Neural Network), and MGPS (Multi-item Gamma Poisson Shrinker) to detect potential drug-AE associations. Time - to - onset was assessed with Weibull distribution and Kaplan-Meier methods. Sensitivity analyses were performed according to reporter type, age group, and sex to assess the robustness of the findings.
Results:
A total of 2,421 reports from FAERS and 3,485 from WHO-VigiAccess were identified. Bleeding and thrombocytopenia were the most frequent AEs, consistent with drug labeling. Notably, 21 AE signals suggestive of possible associations not listed in the current drug label were observed, such as vascular stent thrombosis and cardiogenic death, which require further studies to verify their causal relationship with tirofiban. The mean onset time was 12 h, and 96.49% occurred within 1 month of exposure. Subgroup analyses showed that male patients exhibited a stronger signal for thrombocytopenia, whereas female patients had a higher risk of ischemic heart disease. Elderly patients (≥65 years) more frequently experienced hemoglobin decreased, while younger patients (<65years) had a higher risk of thrombosis in device.
Conclusion:
This study identified both known and potentially novel tirofiban-related AEs. The rapid onset, particularly of bleeding and thrombocytopenia, highlights the importance of early monitoring. Management strategies, such as dose adjustment, temporary discontinuation, or supportive treatment including platelet transfusion, may help mitigate severe complications. These findings provide real-world evidence to guide safer tirofiban use, although further studies are required to confirm causality.
1 Introduction
Tirofiban is a highly selective, short half-life, rapid-acting platelet surface glycoprotein IIb/IIIa (GPIIb/IIIa) receptor antagonist (Yang et al., 2019), which has played an important role in the treatment of cardiovascular diseases since it was approved for marketing by the U.S. Food and Drug Administration (FDA) in 1998 (Lazarovici et al., 2019). It mainly blocks the binding sites on platelets and fibrinogen by binding to the GPIIb/IIIa receptor on the platelet surface, thus effectively inhibiting platelet aggregation and reducing the incidence of acute thrombus reocclusion (Topol et al., 1999; Li et al., 2024; Dobrzycki et al., 2007). Currently, tirofiban is widely used in the treatment of acute angina or acute myocardial infarction to prevent the occurrence of cardiac ischemic events; or as a prophylactic drug for intracoronary plaque excision or coronary angioplasty in patients with acute coronary syndromes, to reduce the occurrence of cardiac ischemic complications associated with sudden occlusion of coronary arteries (Li et al., 2024; Dobrzycki et al., 2007; Zhou et al., 2017; Cannon et al., 2001; Zhao et al., 1999).
In recent years, tirofiban has demonstrated significant efficacy in the treatment of acute ischemic stroke (Sun et al., 2021; Shi et al., 2023; Wang M. et al., 2024; Sang et al., 2023a; Zhao et al., 2024). Although the overall safety profile appeared favorable, the incidence of symptomatic intracranial hemorrhage was slightly higher in the tirofiban group in the RESCUE-BT2 study (1.0%) (Zi et al., 2023). Despite these therapeutic advantages, the clinical use of tirofiban is associated with potential safety concerns. Common AEs include bleeding and thrombocytopenia, with severe cases potentially leading to life-threatening complications such as alveolar hemorrhage and intracranial hemorrhage (Zi et al., 2023; Guo et al., 2012). The incidence of tirofiban-induced thrombocytopenia ranges from 0.4% to 5.6%, and in severe cases, it may increase mortality risk (Wang and Zou, 2023; Yi et al., 2018). As the use of tirofiban becomes increasingly widespread, it is crucial to raise awareness of its safety, especially regarding adverse events not explicitly mentioned in the drug labeling.
The Food and Drug Administration’s Adverse Event Reporting System (FAERS) is one of the largest databases for post-marketing safety surveillance, recording real-world data that can be analyzed to detect potential drug risk signals. Meanwhile, WHO-VigiAccess hosts the world’s most extensive adverse events (AEs) database, encompassing data from regular AE reports submitted by pharmacovigilance centers in over 150 countries. Recently, a pharmacovigilance study based on the FAERS database (Guo Q et al., 2025) analyzed AEs associated with tirofiban and identified several disproportionality signals suggesting potential safety concerns. However, that study relied solely on FAERS data up to Q3 2024, which may limit the generalizability of its findings. So our study will, for the first time, systematically and comprehensively mine AE signals for tirofiban using data from both FAERS and WHO-VigiAccess. To the best of our knowledge, it could enhance the robustness of signal detection by integrating two independent international pharmacovigilance databases. The inclusion of WHO-VigiAccess allows cross-validation across different reporting systems, thereby reducing database-specific bias and improving the reliability of detected signals. Through this dual-database approach, we extend data coverage to Q4 2024 and identify additional potential safety signals not previously reported, offering a more comprehensive perspective on tirofiban’s post-marketing safety profile.
2 Methods
2.1 Data sources and data processing
The FAERS database is a globally recognized repository for AE reporting, mainly collecting AE data submitted by drug manufacturers, healthcare professionals, and consumers (Dhodapkar et al., 2022). FAERS data has been accessible to the public since the first quarter of 2004, and is updated and released quarterly in both ASCII and XML formats for download. For this study, the original ASCII packet was downloaded for data mining and statistical analysis, at https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html. A total of 22,375,298 cases were initially obtained. As the database relies on spontaneous reporting, which may include duplicate or withdrawn/deleted reports, this study adhered strictly to the data cleaning guidelines provided by the FDA.
The WHO Uppsala Monitoring Centre (UMC) is the WHO Collaborating Centre for International Drug Monitoring and has the world’s largest AE database. Since 1968, pharmacovigilance centers in more than 150 countries have partnered with UMC to regularly submit AE reporting data. In 2015, WHO launched VigiAccess to make AE reporting information from the VigiBase database available to the public at http://www.Vigiaccess.org/. The database is updated weekly. On December 15, 2024, we accessed the VigiAccess database to retrieve all publicly available drug data, which were subsequently exported into Excel for further analysis. A total of 24,152 drugs with baseline information and 24,127 drugs with AE information were obtained. The WHO information system collects data on age group, sex, year of reporting, and global continent.
AEs reported in both databases were coded using terminology from the Medical Dictionary of Regulatory Activities (MedDRA27.1). These coded terms were arranged in a hierarchy of 5 categories, both broad (system organ class [SOC]) and category-specific (e.g., preferred term [PT]).
2.2 Eligibility criteria
We obtained data from the FAERS database for a total of 84 quarters, ranging from the first quarter of 2004 (2004Q1) to the fourth quarter of 2024 (2024Q4). Additionally, on December 15, 2024, we accessed the WHO-VigiAccess database to retrieve the relevant AE report data. In these datasets, tirofiban was designated as a primary suspected (PS) drug. A Suspect product is defined as a drug or biologic that the reporter believes to be associated with the reported AE.
2.3 Statistical analysis
In this study, we employed a combination of disproportionality analysis methods to detect signals of AEs. The methods used include the Reporting Odds Ratio (ROR) (Sakaeda et al., 2013), Proportional Reporting Ratio (PRR) (Kelly et al., 2007), Bayesian Confidence Propagation Neural Network (BCPNN) (Bate et al., 1998), and Multi-item Gamma Poisson Shrinker (MGPS) (Szarfman et al., 2004). These methods are detailed in Supplementary Table S1. Both ROR and PRR are frequency-based methods known for their high sensitivity but lower specificity, while BCPNN and MGPS are Bayesian methods suitable for handling complex variables, though they tend to have lower sensitivity. Although there is no gold standard for signal detection methods, each approach has its own characteristics, with distinct advantages and limitations in terms of applicability and feasibility within databases. Therefore, We define a PT as a positive signal if it simultaneously meets the threshold criteria of all four methods and is present in both the FAERS and WHO-VigiAccess databases. Consistent detection of signals across multiple methods helps reduce the likelihood of false positives. The strength of the signal is indicated by the magnitude of the parameter values, and higher values suggest stronger signals. The specific formulas and criteria for these algorithms are provided in Supplementary Table S2. For statistical analysis, we used SAS 9.4 software and Excel 2019.
2.4 Time to onset analysis
To thoroughly evaluate the TTO characteristics, we utilized several statistical measures, including the median, interquartile range, minimum, maximum, and Weibull shape parameters (Kinoshita et al., 2020). The Kaplan-Meier method was used to illustrate the cumulative incidence of AEs associated with tirofiban (Wang Q. et al., 2024). Changes in the incidence of AE risk over time can be identified and predicted by the Weibull distribution test. This distribution is characterized by two parameters: the scale parameter (α), which determines the proportion or width of the distribution, and the shape parameter (β), which influences the curvature of the distribution. In our analysis of onset time, the shape parameter was crucial to predict the hazard of AEs over time, categorizing the outcome as early, random, or wear-out failure type. When β is less than 1 and its 95% confidence interval (CI) is also below 1, it suggests a decreasing risk of AEs over time, indicating an early failure pattern. Conversely, if β is approximately 1 and its 95% CI contains 1, the risk is estimated to remain constant over time, representing a random failure pattern. Finally, if β exceeds 1 and its 95% CI does not encompass 1, it implies an increasing risk over time, characteristic of a wear-out failure pattern (Sauzet et al., 2013; Liu et al., 2024).
2.5 Sensitivity analysis
To evaluate the robustness of the observed tirofiban-related AE signals, sensitivity analyses were performed stratified by reporter type, age, and sex. Disproportionality analyses (ROR, PRR, BCPNN, and MGPS) were conducted separately within subgroups of healthcare professionals and non-healthcare reporters to examine whether the reporting source influenced signal detection. For age- and gender-difference analyses, the association between AEs and demographic variables was assessed using the chi-square (χ2) test or Fisher’s exact test, depending on the data distribution and expected cell counts. A two-tailed P value <0.05 was considered statistically significant. All analyses were conducted using SAS 9.4. These analyses allowed us to determine whether the identified AE signals were consistent across different patient subgroups and reporting sources, enhancing the reliability and generalizability of the findings.
3 Result
3.1 General characteristics
In this study, a total of 55,357,463 AE reports involving 18,613,992 patients were obtained from the FAERS database after data cleaning and deduplication. Among these, 3,487 AE reports were reported with tirofiban as the primary suspected drug, involving 2,421 patients. Additionally, through the WHO-VigiAccess database, we extracted a total of 117,822,562 AE reports involving 46,480,843 patients, of which 6,770 AE reports were related to tirofiban, involving 3,694 patients. The detailed process of data collection, interpretation, and analysis is shown in Figure 1.
FIGURE 1
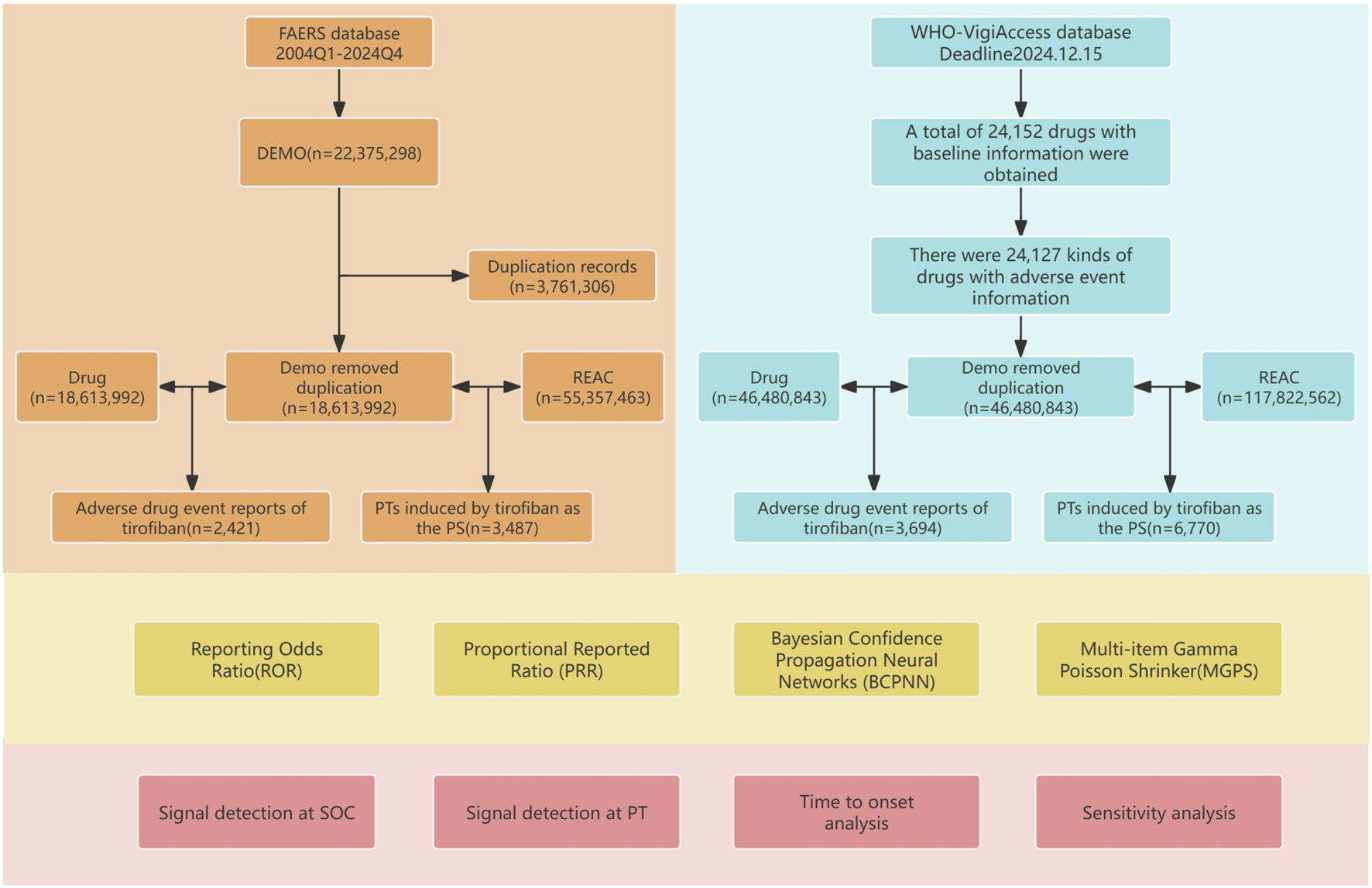
Flowchart of the study. The study consisted of data collection and cleaning, disproportionate analysis methods for calculating signal strength, and presentation of results.
The clinical characteristics of tirofiban-related AEs are summarized in Table 1. Excluding reports with missing demographic data, AEs were more frequently reported in older adults and males across both databases. In reports with clealy specified gender,15.49% of AEs in the FAERS occurred in males and 11.24% in females. A total of 73.28% of all reports lacked gender information and were therefore excluded from the gender-based distribution analysis. In the WHO-VigiAccess, 61.37% of AEs occurred in males and 28.64% in females, while 9.99% of the total reports lacked gender information. Notably, in WHO-VigiAccess, the male to female ratio was approximately 2:1. With respect to age distribution, the highest proportion of AEs was observed among patients aged ≥65 years (12.64% in FAERS, 47.03% in WHO-VigiAccess), followed by those aged 45–64 years (7.19% in FAERS, 32.89% in WHO-VigiAccess). Temporal analysis of AE reporting, as shown in Figure 2, indicates that the FAERS database recorded the highest number of reports in 2019 (n = 1,134, 46.84%). In contrast, AE reports in the WHO-VigiAccess database were primarily concentrated in two periods: 2000–2006 and 2019–2024.
TABLE 1
| Characteristics | FAERS(n = 2,421) | WHO-VigiAccess(n = 3,694) | ||
|---|---|---|---|---|
| N | % | N | % | |
| Sex | ||||
| Female | 272 | 11.24 | 1058 | 28.64 |
| Male | 375 | 15.49 | 2267 | 61.37 |
| Unknown | 1774 | 73.28 | 369 | 9.99 |
| Age (years) | ||||
| <18 | 1 | 0.04 | 6 | 0.27 |
| 18–44 | 35 | 1.45 | 158 | 4.28 |
| 45–64 | 174 | 7.19 | 1215 | 32.89 |
| ≥65 | 306 | 12.64 | 1737 | 47.03 |
| Unknown | 1905 | 78.69 | 577 | 15.62 |
| Continent | ||||
| Asia | 1498 | 61.88 | 1511 | 40.9 |
| Americas | 190 | 7.85 | 1162 | 31.46 |
| Europe | 136 | 5.62 | 898 | 24.31 |
| Africa | 57 | 2.35 | 25 | 0.68 |
| Oceania | 14 | 0.58 | 98 | 2.65 |
| Unknown | 526 | 21.73 | - | - |
Characteristics of AE reports related to tirofiban in both the FAERS and WHO-VigiAccess databases.
FIGURE 2
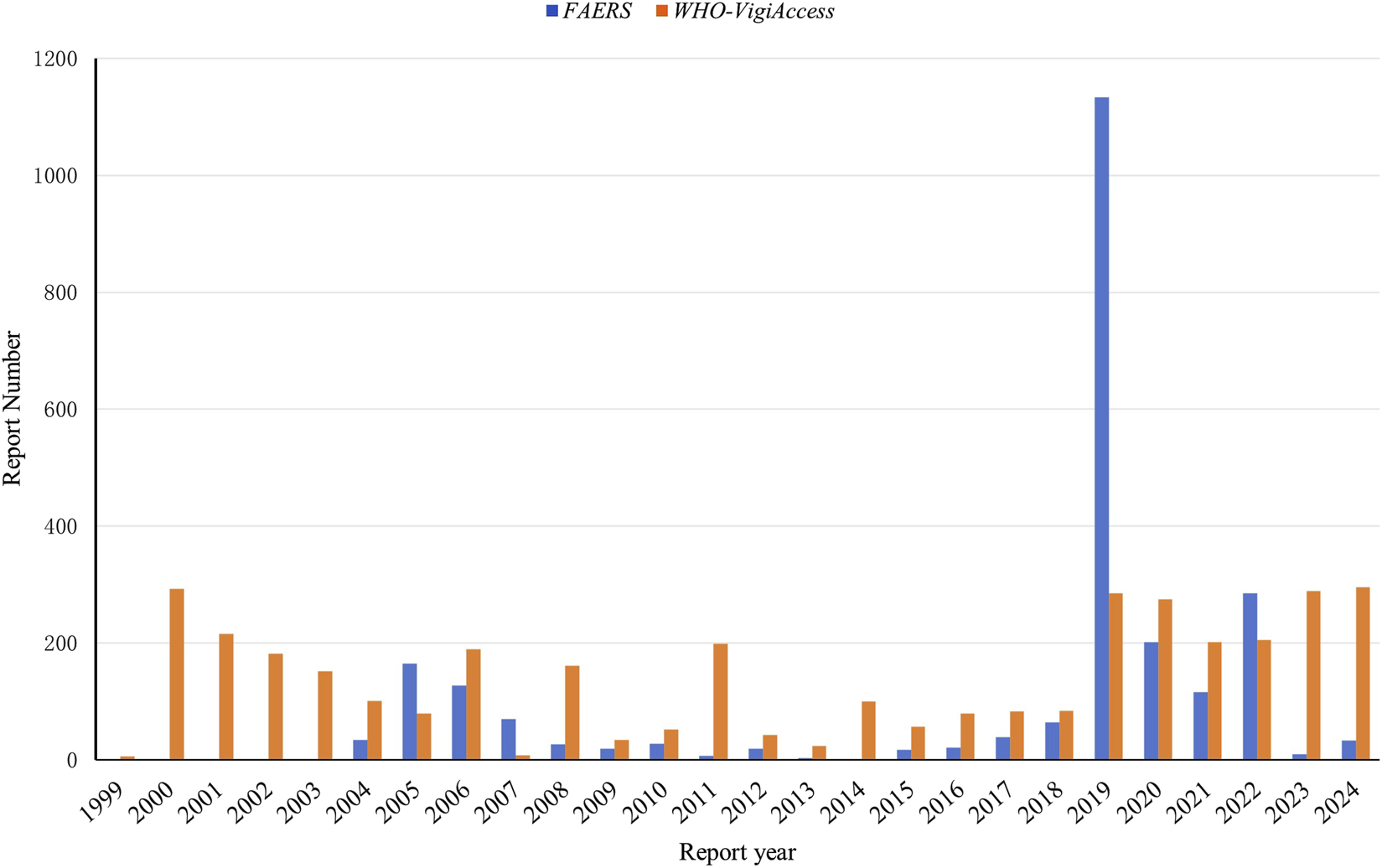
Annual distribution of AE reports involving tirofiban in the FAERS and WHO-VigiAccess databases.
In the FAERS database, the majority of AE reports were submitted by healthcare professionals, including physicians (61.75%), pharmacists (12.85%), and other health professionals (19.70%), collectively accounting for 94.3% of all reports. Among reporting countries, China contributed the largest number of AE reports (n = 1,242, 51.30%), followed by the United States (n = 379, 15.65%). Regarding the reported indications, antiplatelet therapy was the most frequently cited (n = 1,430, 59.07%), followed by ischemic stroke (n = 220, 9.09%). A substantial proportion of patients experienced serious outcomes (n = 2,115, 87.36%), with death (n = 339, 14.00%), life-threatening events (n = 290, 11.98%), and hospitalizations (n = 242, 10.00%). These data are detailed in Supplementary Figure S1 and Supplementary Table S3. It is important to note that corresponding data on reporter identity, indication, and outcome are not available in the WHO-VigiAccess database.
3.2 Signal detection at the SOC level
AEs was categorized according to the System Organ Class (SOC) system. The number of PTs under each SOC was ranked in descending order. Tirofiban-related AEs were distributed across 24 SOCs in the FAERS dataset and 25 SOCs in the WHO-VigiAccess dataset, as detailed in Supplementary Tables S4, S5. Among these, 23 SOCs were shared between the two databases, as summarized in Table 2. Notably, three SOCs emerged as key contributors in both databases based on the disproportionality analysis. These included cardiac disorders (n = 862 in FAERS, n = 497 in WHO-VigiAccess, vascular disorders (n = 547 in FAERS, n = 648 in WHO-VigiAccess), and blood and lymphatic system disorders (n = 293 in FAERS, n = 1,569 in WHO-VigiAccess). In the FAERS database, cardiac disorders represented the most frequently reported and most strongly signaled SOC. In contrast, in the WHO-VigiAccess database, blood and lymphatic system disorders were the most prevalent and prominently signaled SOC.
TABLE 2
| System organ Class (SOC) | FAERS | WHO-VigiAccess | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Case reports | ROR (95% CI) | PRR (χ2) | IC (IC025) | EBGM (EBGM05) | Case reports | ROR (95% CI) | PRR (χ2) | IC (IC025) | EBGM (EBGM05) | |
| Cardiac disordersa | 862 | 12.18 (11.28,13.16) | 9.42 (6656.83) | 3.23 (3.11) | 9.41 (8.72) | 497 | 3.26 (2.98,3.57) | 3.09 (721.32) | 1.63 (1.49) | 3.09 (2.82) |
| Vascular disordersa | 547 | 8.55 (7.80,9.36) | 7.36 (3071.13) | 2.88 (2.73) | 7.36 (6.72) | 648 | 4.86 (4.48,5.27) | 4.49 (1794.99) | 2.17 (2.04) | 4.49 (4.14) |
| Nervous system disorders | 414 | 1.45 (1.31,1.61) | 1.40 (51.79) | 0.49 (0.33) | 1.40 (1.26) | 493 | 0.69 (0.63,0.75) | 0.71 (64.53) | −0.49 (-0.63) | 0.71 (0.65) |
| General disorders and administration site conditions | 386 | 0.59 (0.53,0.65) | 0.63 (98.31) | −0.66 (-0.81) | 0.63 (0.57) | 722 | 0.47 (0.43,0.51) | 0.53 (387.51) | −0.93 (-1.04) | 0.53 (0.49) |
| Blood and lymphatic system disordersa | 293 | 5.34 (4.74,6.02) | 4.98 (947.09) | 2.32 (2.12) | 4.98 (4.42) | 1569 | 13.37 (12.63,14.14) | 10.50 (13783.0) | 3.39 (3.30) | 10.49 (9.92) |
| Respiratory, thoracic and mediastinal disorders | 225 | 1.40 (1.22,1.60) | 1.37 (23.72) | 0.46 (0.25) | 1.37 (1.20) | 404 | 1.38 (1.25,1.53) | 1.36 (39.71) | 0.44 (0.29) | 1.36 (1.23) |
| Investigations | 183 | 0.85 (0.73,0.98) | 0.86 (4.78) | −0.23 (-0.44) | 0.86 (0.74) | 442 | 1.04 (0.94,1.14) | 1.03 (0.52) | 0.05 (-0.09) | 1.03 (0.94) |
| Injury, poisoning and procedural complications | 153 | 0.40 (0.34,0.46) | 0.42 (135.32) | −1.25 (-1.48) | 0.42 (0.36) | 264 | 0.59 (0.52,0.67) | 0.61 (71.10) | −0.72 (-0.90) | 0.61 (0.54) |
| Gastrointestinal disorders | 144 | 0.46 (0.39,0.55) | 0.49 (85.62) | −1.04 (-1.28) | 0.49 (0.41) | 838 | 1.28 (1.19,1.38) | 1.25 (45.58) | 0.32 (0.21) | 1.25 (1.16) |
| Surgical and medical procedures | 64 | 1.37 (1.07,1.75) | 1.36 (6.13) | 0.44 (0.07) | 1.36 (1.06) | 21 | 0.38 (0.25,0.58) | 0.38 (21.10) | −1.39 (-1.96) | 0.38 (0.25) |
| Renal and urinary disorders | 52 | 0.78 (0.59,1.03) | 0.78 (3.16) | −0.35 (-0.74) | 0.78 (0.60) | 262 | 2.47 (2.19,2.80) | 2.42 (221.11) | 1.27 (1.08) | 2.42 (2.14) |
| Skin and subcutaneous tissue disorders | 39 | 0.20 (0.14,0.27) | 0.21 (124.92) | −2.27 (-2.70) | 0.21 (0.15) | 329 | 0.53 (0.47,0.59) | 0.55 (132.61) | −0.86 (-1.02) | 0.55 (0.49) |
| Infections and infestations | 36 | 0.19 (0.14,0.26) | 0.20 (124.68) | −2.35 (-2.79) | 0.20 (0.14) | 35 | 0.12 (0.09,0.17) | 0.13 (214.48) | −2.95 (-3.40) | 0.13 (0.09) |
| Product issues | 22 | 0.38 (0.25,0.58) | 0.39 (21.56) | −1.36 (-1.93) | 0.39 (0.26) | 8 | 0.13 (0.07,0.26) | 0.13 (46.28) | −2.93 (-3.74) | 0.13 (0.07) |
| Eye disorders | 15 | 0.21 (0.13,0.35) | 0.22 (43.62) | −2.21 (-2.86) | 0.22 (0.13) | 48 | 0.41 (0.31,0.54) | 0.41 (41.31) | −1.28 (-1.68) | 0.41 (0.31) |
| Musculoskeletal and connective tissue disorders | 12 | 0.06 (0.04,0.11) | 0.07 (165.74) | −3.91 (-4.60) | 0.07 (0.04) | 47 | 0.13 (0.09,0.17) | 0.13 (283.58) | −2.92 (-3.31) | 0.13 (0.10) |
| Immune system disorders | 11 | 0.28 (0.16,0.51) | 0.29 (19.81) | −1.81 (-2.55) | 0.29 (0.16) | 63 | 0.74 (0.58,0.95) | 0.74 (5.80) | −0.43 (-0.79) | 0.74 (0.58) |
| Hepatobiliary disorders | 9 | 0.28 (0.15,0.54) | 0.28 (16.69) | −1.83 (-2.64) | 0.28 (0.15) | 12 | 0.22 (0.13,0.39) | 0.22 (32.88) | −2.17 (-2.88) | 0.22 (0.13) |
| Psychiatric disorders | 8 | 0.04 (0.02,0.08) | 0.04 (191.24) | −4.62 (-5.42) | 0.04 (0.02) | 35 | 0.10 (0.07,0.15) | 0.11 (267.69) | −3.20 (-3.64) | 0.11 (0.08) |
| Metabolism and nutrition disorders | 3 | 0.04 (0.01,0.12) | 0.04 (71.27) | −4.66 (-5.70) | 0.04 (0.01) | 15 | 0.12 (0.07,0.20) | 0.12 (94.67) | −3.01 (-3.65) | 0.12 (0.07) |
| Reproductive system and breast disorders | 3 | 0.10 (0.03,0.30) | 0.10 (25.46) | −3.37 (-4.44) | 0.10 (0.03) | 7 | 0.10 (0.05,0.20) | 0.10 (59.53) | −3.37 (-4.21) | 0.10 (0.05) |
| Ear and labyrinth disorders | 2 | 0.13 (0.03,0.53) | 0.13 (11.43) | −2.92 (-4.09) | 0.13 (0.03) | 7 | 0.19 (0.09,0.40) | 0.19 (23.98) | −2.38 (-3.25) | 0.19 (0.09) |
| Neoplasms benign, malignant and unspecified (incl cysts and polyps) | 2 | 0.02 (0.01,0.09) | 0.02 (89.78) | −5.51 (-6.61) | 0.02 (0.01) | 2 | 0.02 (0.01,0.08) | 0.02 (91.80) | −5.56 (-6.66) | 0.02 (0.01) |
Overlap of tirofiban-related AEs by SOC across FAERS and WHO-VigiAccess databases.
SOCs, identified in both databases by all four disproportionality methods simultaneously.
ROR, reporting odds ratio; CI, confidence interval; PRR, proportional reporting ratio; IC, information component; IC025, the lower limit of 95% CI, of the IC; EBGM, empirical Bayesian geometric mean; EBGM05, the lower limit of 95% CI, of EBGM.
3.3 Distribution of AEs at the PT level
We further analyzed tirofiban-related AEs at the PT level. In the FAERS database, 95 PTs met all four disproportionality algorithm criteria simultaneously (Supplementary Table S6), while in the WHO-VigiAccess database, 147 PTs met the same criteria (Supplementary Table S7). A total of 63 PTs were identified in both datasets (Supplementary Table S8, in Figure 3). Among these overlapping PTs, commonly reported events included hemorrhage, thrombocytopenia, hematoma, petechiae and hemoptysis, align closely with the known adverse reactions listed in the tirofiban drug label. These 63 shared PTs were ranked by the frequency of reports in the FAERS database. The 30 most frequently reported PTs are presented in Table 3. The top five PTs by reporting frequency were: hemorrhage (n = 370 in FAERS, n = 223 in WHO-VigiAccess), thrombocytopenia (n = 248 in FAERS, n = 1,391 in WHO-VigiAccess), myocardial infarction (n = 207 in FAERS, n = 89 in WHO-VigiAccess), angina pectoris (n = 181 in FAERS, n = 26 in WHO-VigiAccess), and intracranial hemorrhage (n = 176 in FAERS, n = 77 in WHO-VigiAccess). In addition, the PTs were ranked based on signal strength using EBGM values in the FAERS database. The top 30 PTs with the highest EBGM signals are shown in Table 4. The three strongest signals were observed for vascular access site hemorrhage (6 cases, EBGM = 835.55 in FAERS, 4 cases, EBGM = 351.59 in WHO-VigiAccess),vascular stent thrombosis (55 cases, EBGM = 479.75 in FAERS, 10 cases, EBGM = 97.55 in WHO-VigiAccess), cardiac death (31 cases, EBGM = 437.46 in FAERS, 20 cases, EBGM = 283.22 in WHO-VigiAccess).
FIGURE 3
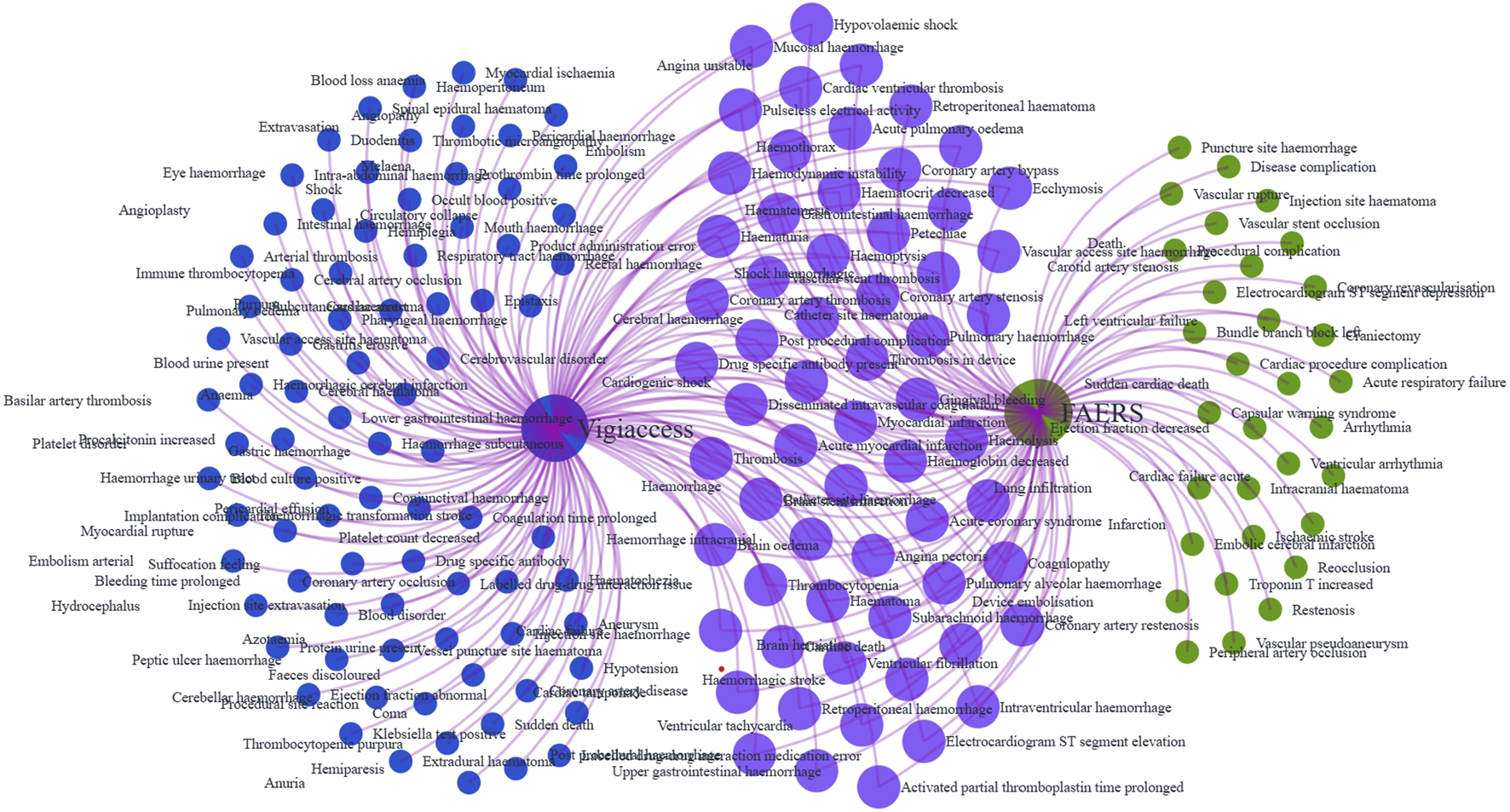
Venn diagram of tirofiban-related AE signals at the PT level identified in the FAERS and WHO-VigiAccess databases.
TABLE 3
| Preferred terms | FAERS | WHO-VigiAccess | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Case report | ROR (95% CI) | PRR (χ2) | IC (IC025) | EBGM (EBGM05) | Case report | ROR (95% CI) | PRR (χ2) | IC (IC025) | EBGM (EBGM05) | |
| Haemorrhage | 370 | 71.12 (63.84,79.23) | 63.68 (22774.5) | 5.99 (5.60) | 63.43 (56.94) | 223 | 28.48 (24.92,32.55) | 27.58 (5709.44) | 4.78 (4.43) | 27.53 (24.09) |
| Thrombocytopenia | 248 | 42.79 (37.60,48.70) | 39.82 (9379.40) | 5.31 (4.91) | 39.72 (34.91) | 1391 | 89.61 (84.47,95.06) | 71.40 (96442.6) | 6.15 (6.00) | 71.11 (67.04) |
| Myocardial infarctiona | 207 | 20.95 (18.20,24.11) | 19.76 (3693.82) | 4.30 (3.97) | 19.74 (17.15) | 89 | 7.49 (6.08,9.24) | 7.41 (493.92) | 2.89 (2.48) | 7.40 (6.01) |
| Angina pectorisa | 181 | 113.77 (97.91,132.19) | 107.91 (19051.5) | 6.74 (5.86) | 107.19 (92.25) | 26 | 8.73 (5.94,12.83) | 8.70 (177.13) | 3.12 (2.20) | 8.69 (5.91) |
| Haemorrhage intracranial | 176 | 211.77 (181.81,246.66) | 201.13 (34618.0) | 7.63 (6.33) | 198.63 (170.53) | 77 | 56.01 (44.73,70.14) | 55.39 (4099.89) | 5.79 (4.70) | 55.21 (44.09) |
| Cerebral haemorrhage | 96 | 48.33 (39.44,59.21) | 47.02 (4314.07) | 5.55 (4.69) | 46.89 (38.27) | 105 | 29.47 (24.30,35.74) | 29.03 (2838.33) | 4.86 (4.24) | 28.98 (23.90) |
| Cardiac failurea | 80 | 17.95 (14.38,22.41) | 17.56 (1249.56) | 4.13 (3.54) | 17.54 (14.05) | 25 | 4.72 (3.19,6.99) | 4.71 (73.02) | 2.23 (1.47) | 4.71 (3.18) |
| Vascular stent thrombosisa | 55 | 502.58 (383.49,658.66) | 494.67 (26278.9) | 8.91 (5.26) | 479.75 (366.07) | 10 | 98.24 (52.74,182.99) | 98.10 (955.71) | 6.61 (2.45) | 97.55 (52.37) |
| Cardiogenic shocka | 38 | 49.84 (36.18,68.64) | 49.30 (1793.13) | 5.62 (3.99) | 49.15 (35.69) | 30 | 37.88 (26.45,54.24) | 37.72 (1070.11) | 5.23 (3.59) | 37.64 (26.28) |
| Haemoglobin decreased | 32 | 5.43 (3.84,7.70) | 5.39 (114.64) | 2.43 (1.75) | 5.39 (3.81) | 80 | 10.12 (8.12,12.62) | 10.01 (649.45) | 3.32 (2.85) | 10.01 (8.03) |
| Cardiac deatha | 31 | 453.85 (317.10,649.56) | 449.82 (13500.3) | 8.77 (4.38) | 437.46 (305.65) | 20 | 288.73 (185.49,449.45) | 287.88 (5624.77) | 8.15 (3.66) | 283.22 (181.94) |
| Gastrointestinal haemorrhage | 29 | 5.89 (4.09,8.49) | 5.85 (116.69) | 2.55 (1.80) | 5.85 (4.06) | 138 | 16.28 (13.76,19.28) | 15.97 (1937.50) | 4.00 (3.60) | 15.96 (13.48) |
| Acute myocardial infarctiona | 25 | 14.43 (9.73,21.38) | 14.33 (309.89) | 3.84 (2.67) | 14.32 (9.66) | 17 | 8.68 (5.39,13.97) | 8.66 (115.11) | 3.11 (1.92) | 8.65 (5.38) |
| Coronary artery stenosisa | 24 | 80.28 (53.68,120.06) | 79.74 (1856.83) | 6.31 (3.68) | 79.34 (53.05) | 10 | 37.80 (20.31,70.33) | 37.74 (356.92) | 5.24 (2.25) | 37.66 (20.24) |
| Haematoma | 24 | 15.94 (10.66,23.81) | 15.83 (333.32) | 3.98 (2.73) | 15.82 (10.59) | 147 | 40.25 (34.18,47.41) | 39.40 (5491.92) | 5.30 (4.72) | 39.31 (33.38) |
| Haematuria | 23 | 11.62 (7.71,17.51) | 11.55 (221.57) | 3.53 (2.41) | 11.54 (7.66) | 190 | 44.02 (38.10,50.86) | 42.81 (7745.08) | 5.42 (4.92) | 42.71 (36.97) |
| Haemoptysis | 20 | 12.55 (8.09,19.49) | 12.49 (211.30) | 3.64 (2.38) | 12.48 (8.04) | 88 | 36.81 (29.82,45.43) | 36.34 (3019.10) | 5.18 (4.39) | 36.27 (29.38) |
| Pulmonary alveolar haemorrhage | 19 | 62.68 (39.90,98.47) | 62.34 (1142.43) | 5.96 (3.29) | 62.10 (39.53) | 20 | 62.36 (40.17,96.79) | 62.17 (1199.58) | 5.95 (3.36) | 61.96 (39.91) |
| Thrombosisa | 18 | 3.91 (2.46,6.21) | 3.90 (38.78) | 1.96 (1.09) | 3.89 (2.45) | 25 | 4.23 (2.86,6.26) | 4.22 (61.39) | 2.08 (1.34) | 4.22 (2.85) |
| Thrombosis in device | 18 | 90.34 (56.78,143.75) | 89.88 (1573.28) | 6.48 (3.32) | 89.38 (56.17) | 4 | 20.49 (7.68,54.65) | 20.48 (74.03) | 4.35 (0.77) | 20.46 (7.67) |
| Embolisma | 17 | 35.39 (21.97,57.03) | 35.23 (564.15) | 5.14 (2.92) | 35.15 (21.81) | 13 | 17.43 (10.12,30.05) | 17.40 (200.80) | 4.12 (2.23) | 17.39 (10.09) |
| Catheter site haemorrhage | 15 | 110.15 (66.22,183.23) | 109.68 (1604.32) | 6.77 (3.09) | 108.93 (65.49) | 15 | 92.80 (55.84,154.23) | 92.60 (1352.00) | 6.53 (3.06) | 92.12 (55.43) |
| Petechiae | 14 | 23.96 (14.17,40.51) | 23.86 (306.27) | 4.57 (2.49) | 23.83 (14.09) | 37 | 17.75 (12.85,24.52) | 17.66 (581.07) | 4.14 (3.15) | 17.64 (12.77) |
| Ventricular fibrillationa | 14 | 22.04 (13.04,37.27) | 21.96 (279.72) | 4.45 (2.45) | 21.93 (12.97) | 17 | 17.56 (10.91,28.28) | 17.52 (264.60) | 4.13 (2.51) | 17.50 (10.87) |
| Post procedural complication | 14 | 12.61 (7.46,21.31) | 12.56 (148.90) | 3.65 (2.08) | 12.55 (7.42) | 11 | 11.04 (6.11,19.95) | 11.02 (100.19) | 3.46 (1.75) | 11.02 (6.10) |
| Gingival bleeding | 13 | 16.78 (9.73,28.94) | 16.72 (192.02) | 4.06 (2.20) | 16.71 (9.69) | 157 | 105.86 (90.32,124.07) | 103.43 (15834.9) | 6.68 (5.73) | 102.82 (87.73) |
| Retroperitoneal haemorrhage | 13 | 88.48 (51.25,152.76) | 88.15 (1114.00) | 6.45 (2.83) | 87.67 (50.78) | 40 | 129.26 (94.62,176.58) | 128.50 (5023.60) | 7.00 (4.51) | 127.57 (93.38) |
| Haematocrit decreased | 11 | 9.37 (5.18,16.93) | 9.34 (81.91) | 3.22 (1.63) | 9.34 (5.16) | 32 | 19.31 (13.64,27.33) | 19.22 (552.24) | 4.26 (3.13) | 19.20 (13.56) |
| Drug specific antibody presenta | 10 | 27.71 (14.89,51.58) | 27.64 (256.31) | 4.79 (2.14) | 27.59 (14.82) | 8 | 22.67 (11.33,45.36) | 22.64 (165.28) | 4.50 (1.77) | 22.61 (11.30) |
| Pulmonary haemorrhage | 10 | 21.86 (11.74,40.67) | 21.80 (198.18) | 4.44 (2.04) | 21.77 (11.70) | 50 | 81.10 (61.36,107.18) | 80.51 (3908.31) | 6.32 (4.57) | 80.14 (60.64) |
Top 30 PTs in both databases ranked by FAERS reporting frequency (descending order).
Not mentioned in the drug label.
TABLE 4
| Preferred terms | FAERS | WHO-VigiAccess | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Case report | ROR (95% CI) | PRR (χ2) | IC (IC025) | EBGM (EBGM05) | Case report | ROR (95% CI) | PRR (χ2) | IC (IC025) | EBGM (EBGM05) | |
| Vascular access site haemorrhage | 6 | 883.43 (388.02,2011.38) | 881.91 (5001.61) | 9.71 (1.67) | 835.55 (366.99) | 4 | 359.03 (133.36,966.56) | 358.82 (133.36,965.43) | 8.46 (1.00) | 351.59 (130.60) |
| Vascular stent thrombosisa | 55 | 502.58 (383.49,658.66) | 494.67 (26278.9) | 8.91 (5.26) | 479.75 (366.07) | 10 | 98.24 (52.74,182.99) | 98.10 (52.71,182.56) | 6.61 (2.45) | 97.55 (52.37) |
| Cardiac deatha | 31 | 453.85 (317.10,649.56) | 449.82 (13500.3) | 8.77 (4.38) | 437.46 (305.65) | 20 | 288.73 (185.49,449.45) | 287.88 (185.18,447.55) | 8.15 (3.66) | 283.22 (181.94) |
| Catheter site haematoma | 5 | 308.08 (127.07,746.94) | 307.64 (1499.19) | 8.24 (1.37) | 301.81 (124.49) | 5 | 237.27 (98.14,573.64) | 237.09 (98.13,572.85) | 7.87 (1.37) | 233.92 (96.75) |
| Haemorrhage intracranial | 176 | 211.77 (181.81,246.66) | 201.13 (34618.0) | 7.63 (6.33) | 198.63 (170.53) | 77 | 56.01 (44.73,70.14) | 55.39 (44.34,69.18) | 5.79 (4.70) | 55.21 (44.09) |
| Catheter site haemorrhage | 15 | 110.15 (66.22,183.23) | 109.68 (1604.32) | 6.77 (3.09) | 108.93 (65.49) | 15 | 92.80 (55.84,154.23) | 92.60 (55.78,153.72) | 6.53 (3.06) | 92.12 (55.43) |
| Angina pectorisa | 181 | 113.77 (97.91,132.19) | 107.91 (19051.5) | 6.74 (5.86) | 107.19 (92.25) | 26 | 8.73 (5.94,12.83) | 8.70 (5.93,12.77) | 3.12 (2.20) | 8.69 (5.91) |
| Coronary artery restenosisa | 4 | 106.84 (39.94,285.77) | 106.72 (416.12) | 6.73 (0.97) | 106.01 (39.63) | 3 | 79.14 (25.45,246.07) | 79.10 (25.45,245.84) | 6.30 (0.50) | 78.75 (25.33) |
| Thrombosis in devicea | 18 | 90.34 (56.78,143.75) | 89.88 (1573.28) | 6.48 (3.32) | 89.38 (56.17) | 4 | 20.49 (7.68,54.65) | 20.48 (7.68,54.58) | 4.35 (0.77) | 20.46 (7.67) |
| Retroperitoneal haemorrhage | 13 | 88.48 (51.25,152.76) | 88.15 (1114.00) | 6.45 (2.83) | 87.67 (50.78) | 40 | 129.26 (94.62,176.58) | 128.50 (94.24,175.23) | 7.00 (4.51) | 127.57 (93.38) |
| Coronary artery stenosisa | 24 | 80.28 (53.68,120.06) | 79.74 (1856.83) | 6.31 (3.68) | 79.34 (53.05) | 10 | 37.80 (20.31,70.33) | 37.74 (20.30,70.16) | 5.24 (2.25) | 37.66 (20.24) |
| Coronary artery thrombosisa | 8 | 66.54 (33.20,133.35) | 66.39 (513.08) | 6.05 (2.04) | 66.11 (32.99) | 10 | 57.61 (30.95,107.24) | 57.53 (30.94,106.98) | 5.84 (2.36) | 57.34 (30.81) |
| Haemorrhage | 370 | 71.12 (63.84,79.23) | 63.68 (22774.5) | 5.99 (5.60) | 63.43 (56.94) | 223 | 28.48 (24.92,32.55) | 27.58 (24.23,31.38) | 4.78 (4.43) | 27.53 (24.09) |
| Pulmonary alveolar haemorrhage | 19 | 62.68 (39.90,98.47) | 62.34 (1142.43) | 5.96 (3.29) | 62.10 (39.53) | 20 | 62.36 (40.17,96.79) | 62.17 (40.11,96.38) | 5.95 (3.36) | 61.96 (39.91) |
| Cardiogenic shocka | 38 | 49.84 (36.18,68.64) | 49.30 (1793.13) | 5.62 (3.99) | 49.15 (35.69) | 30 | 37.88 (26.45,54.24) | 37.72 (26.38,53.92) | 5.23 (3.59) | 37.64 (26.28) |
| Brain stem infarctiona | 3 | 47.95 (15.43,149.00) | 47.91 (137.38) | 5.58 (0.47) | 47.77 (15.37) | 3 | 32.79 (10.56,101.80) | 32.77 (10.56,101.70) | 5.03 (0.43) | 32.71 (10.54) |
| Cerebral haemorrhage | 96 | 48.33 (39.44,59.21) | 47.02 (4314.07) | 5.55 (4.69) | 46.89 (38.27) | 105 | 29.47 (24.30,35.74) | 29.03 (24.01,35.10) | 4.86 (4.24) | 28.98 (23.90) |
| Mucosal haemorrhage | 3 | 45.44 (14.62,141.18) | 45.40 (129.89) | 5.50 (0.46) | 45.27 (14.57) | 7 | 81.62 (38.83,171.58) | 81.54 (38.82,171.27) | 6.34 (1.86) | 81.16 (38.61) |
| Thrombocytopenia | 248 | 42.79 (37.60,48.70) | 39.82 (9379.40) | 5.31 (4.91) | 39.72 (34.91) | 1391 | 89.61 (84.47,95.06) | 71.40 (68.13,74.84) | 6.15 (6.00) | 71.11 (67.04) |
| Cardiac ventricular thrombosisa | 9 | 36.09 (18.75,69.47) | 36.00 (305.55) | 5.17 (2.08) | 35.92 (18.66) | 8 | 27.93 (13.95,55.91) | 27.90 (13.95,55.80) | 4.80 (1.84) | 27.86 (13.92) |
| Embolisma | 17 | 35.39 (21.97,57.03) | 35.23 (564.15) | 5.14 (2.92) | 35.15 (21.81) | 13 | 17.43 (10.12,30.05) | 17.40 (10.11,29.96) | 4.12 (2.23) | 17.39 (10.09) |
| Pulseless electrical activitya | 8 | 28.69 (14.32,57.44) | 28.62 (212.89) | 4.84 (1.85) | 28.57 (14.27) | 5 | 16.21 (6.74,38.98) | 16.20 (6.74,38.93) | 4.02 (1.02) | 16.19 (6.73) |
| Drug specific antibody presenta | 10 | 27.71 (14.89,51.58) | 27.64 (256.31) | 4.79 (2.14) | 27.59 (14.82) | 8 | 22.67 (11.33,45.36) | 22.64 (11.32,45.28) | 4.50 (1.77) | 22.61 (11.30) |
| Brain herniationa | 4 | 23.92 (8.96,63.80) | 23.89 (87.60) | 4.58 (0.81) | 23.85 (8.94) | 6 | 34.39 (15.43,76.63) | 34.36 (15.43,76.51) | 5.10 (1.48) | 34.29 (15.39) |
| Petechiae | 14 | 23.96 (14.17,40.51) | 23.86 (306.27) | 4.57 (2.49) | 23.83 (14.09) | 37 | 17.75 (12.85,24.52) | 17.66 (12.80,24.36) | 4.14 (3.15) | 17.64 (12.77) |
| Retroperitoneal haematoma | 3 | 23.65 (7.62,73.44) | 23.63 (64.94) | 4.56 (0.38) | 23.60 (7.60) | 3 | 15.23 (4.91,47.26) | 15.23 (4.91,47.22) | 3.93 (0.30) | 15.21 (4.90) |
| Cardiac tamponadea | 6 | 22.09 (9.91,49.24) | 22.06 (120.46) | 4.46 (1.37) | 22.03 (9.88) | 17 | 60.62 (37.63,97.66) | 60.48 (37.59,97.30) | 5.91 (3.13) | 60.27 (37.41) |
| Ventricular fibrillationa | 14 | 22.04 (13.04,37.27) | 21.96 (279.72) | 4.45 (2.45) | 21.93 (12.97) | 17 | 17.56 (10.91,28.28) | 17.52 (10.90,28.17) | 4.13 (2.51) | 17.50 (10.87) |
| Pulmonary haemorrhage | 10 | 21.86 (11.74,40.67) | 21.80 (198.18) | 4.44 (2.04) | 21.77 (11.70) | 50 | 81.10 (61.36,107.18) | 80.51 (61.04,106.18) | 6.32 (4.57) | 80.14 (60.64) |
| Electrocardiogram ST segment elevationa | 4 | 20.68 (7.75,55.16) | 20.66 (74.73) | 4.37 (0.77) | 20.63 (7.73) | 4 | 13.92 (5.22,37.13) | 13.92 (5.22,37.08) | 3.80 (0.67) | 13.91 (5.22) |
Top 30 PTs with the strongest AE signals in both databases ranked by ROR in the FAERS database (Descending Order).
Not mentioned in the drug label.
In addition, besides the common AEs listed in the tirofiban drug insert, we also identified 21 suspected AEs not mentioned in the drug label as shown in Supplementary Table S8, including myocardial infarction (207 cases, EBGM = 19.74 in FAERS,89 cases, EBGM = 7.4 in WHO-VigiAccess), angina pectoris (181 cases, EBGM = 107.19 in FAERS,26 cases, EBGM = 8.69 in WHO-VigiAccess), cardiac failure (80 cases, EBGM = 17.54 in FAERS, 25 cases, EBGM = 4.71 in WHO-VigiAccess), vascular stent thrombosis (55 cases, EBGM = 479.75 in FAERS, 10 cases, EBGM = 97.55 in WHO-VigiAccess), cardiogenic shock (38 cases, EBGM = 49.15 in FAERS, 30 cases, EBGM = 37.64 in WHO-VigiAccess), cardiac death (31 cases, EBGM = 437.46 in FAERS, 20cases, EBGM = 283.22 in WHO-VigiAccess), acute myocardial infarction (25 cases, EBGM = 14.32 in FAERS, 17 cases, EBGM = 8.65 in WHO-VigiAccess), coronary artery stenosis (24 cases, EBGM = 79.34 in FAERS, 10 cases, EBGM = 37.66 in WHO-VigiAccess), thrombosis (18 cases, EBGM = 3.89 in FAERS, 25 cases, EBGM = 4.22 in WHO-VigiAccess), embolism (17 cases, EBGM = 35.15 in FAERS, 13 cases, EBGM = 17.39 in WHO-VigiAccess), ventricular fibrillation (14 cases, EBGM = 21.93 in FAERS, 17 cases, EBGM = 17.50 in WHO-VigiAccess), drug specific antibody present (10 cases, EBGM = 27.59 in FAERS, 8 cases, EBGM = 22.61 in WHO-VigiAccess), coronary artery restenosis (4 cases, EBGM = 106.01 in FAERS, 3 cases, EBGM = 78.75 in WHO-VigiAccess),thrombosis in device (18 cases, EBGM = 89.38 in FAERS, 4 cases, EBGM = 20.46 in WHO-VigiAccess),coronary artery thrombosis (8 cases, EBGM = 66.11 in FAERS, 10 cases, EBGM = 57.34 in WHO-VigiAccess), brain stem infarction (3 cases, EBGM = 47.77 in FAERS, 3 cases, EBGM = 32.71 in WHO-VigiAccess), cardiac ventricular thrombosis (9 cases, EBGM = 35.92 in FAERS, 8 cases, EBGM = 27.86 in WHO-VigiAccess), pulseless electrical activity (8 cases, EBGM = 28.57 in FAERS, 5 cases, EBGM = 16.19 in WHO-VigiAccess), brain herniation (4 cases, EBGM = 23.85 in FAERS, 6 cases, EBGM = 34.29 in WHO-VigiAccess), cardiac tamponade (6 cases, EBGM = 22.03 in FAERS, 17 cases, EBGM = 60.27 in WHO-VigiAccess) and electrocardiogram ST segment elevation (4 cases, EBGM = 20.63 in FAERS, 4 cases, EBGM = 13.91 in WHO-VigiAccess).
3.4 Time to onset of tirofiban-related AEs
In the FAERS database, the onset time of tirofiban-related AEs was recorded after excluding missing data or outliers. A total of 342 AEs reported the time of onset, with the majority occurring within 0–30 days (330 cases, 96.49%) (Figure 4A). The median onset time was 0.5 days (12 h) (IQR 0–2 days). For the top three most frequent PTs, the median onset time for hemorrhage and intracranial hemorrhage was very brief, at 0.00 days (IQR, 0.00,0.00), while the median onset time for thrombocytopenia was 1.00 days (IQR, 0.00,1.00) (Table 5; Figure 4B). Data on onset time are not available in the WHO-VigiAccess database. Figure 4B shows the cumulative incidence curves for all AEs, including thrombocytopenia.
FIGURE 4
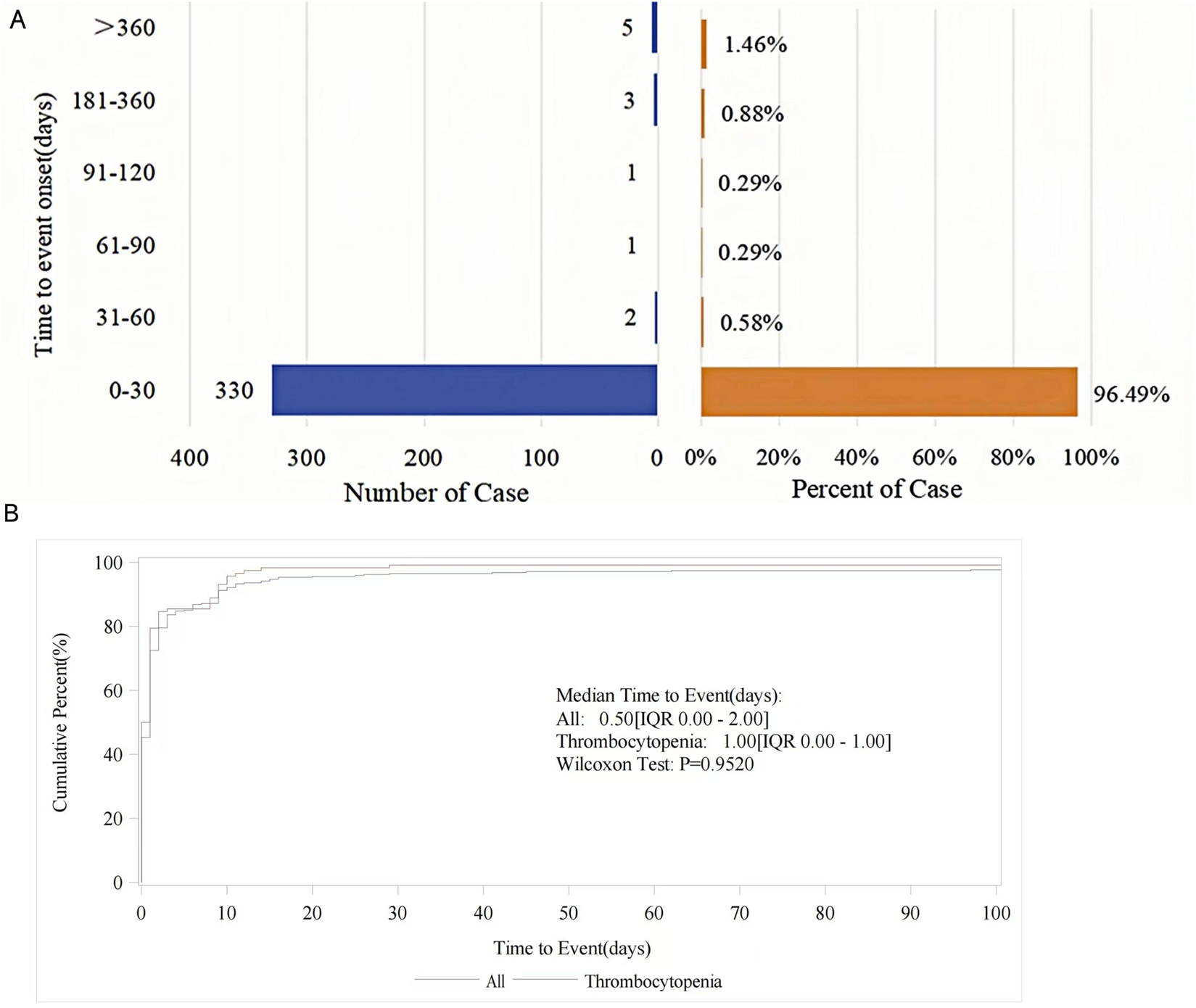
(A) Distribution of AE onset time in reported cases. (B) Cumulative incidence curves for tirofiban-related AEs.
TABLE 5
| PT | Available cases (n) | Time to event (day,median (Q1,Q3)) | Weibull distribution | Failure type | |
|---|---|---|---|---|---|
| α(95%) | β(95%) | ||||
| Haemorrhage | 16 | 0.00 (0.00,0.00) | 13.599 (2.845,64.996) | 0.935 (0.295,2.970 | Random failure |
| Thrombocytopenia | 117 | 1.00 (0.00,1.00) | 4.193 (2.707,6.495) | 0.597 (0.513,0.694) | Early failure |
| Haemorrhage intracraniala | 5 | 0.00 (0.00,0.00) | - | - | - |
| Overall | 342 | 0.50 (0.00,2.00) | 7.772 (5.335,11.324) | 0.424 (0.388,0.464) | Early failure |
Weibull distribution and failure types for the top 3 most frequent PTs and total onset time of AEs.
Relatively small number of cases, not analyzed by Weibull distribution. IQR, interquartile range.
Furthermore, the Weibull distribution test for TTO revealed that the lower bounds of the 95% CI for the shape parameter (β) were less than 1 for both thrombocytopenia and total cases, suggesting indicating an early failure type. This indicates a gradual decline in the probability of AE over time. In contrast, bleeding events were identified as having a random failure type.
3.5 Sensitivity analysis
In the stratified analysis based on reporter type within the FAERS database (presented in Supplementary Table S9), we found that the AE signals identified from healthcare professional reports were largely consistent with those derived from all reporters. The five most frequently reported PTs remained hemorrhage (367 cases, EBGM = 69.41), thrombocytopenia (220 cases, EBGM = 22.57), myocardial infarction (204 cases, EBGM = 26.21), angina pectoris (179 cases, EBGM = 105.58), and intracranial hemorrhage (175 cases, EBGM = 134.22).
In the gender-difference analysis (Supplementary Table S10), we observed that thrombocytopenia showed a significantly stronger signal in male patients than female patients (ROR 0.47, 95% CI 0.33–0.69, P < 0.001). In contrast, female patients appeared to have a higher likelihood of experiencing angina pectoris (ROR 2.72, 95% CI 1.43–5.15, P = 0.0015), chest pain (ROR 4.46, 95% CI 1.20–16.52, P = 0.0316) and coronary artery stenosis (ROR 3.64, 95% CI 1.50–8.82, P = 0.0023). However, for the PTs about bleeding, including haemorrhage, cerebral haemorrhage, pulmonary alveolar haemorrhage, gastrointestinal haemorrhage, and haemoglobin decreased, we found no statistically significant differences between different gender (P > 0.05).
The results of the age-stratified analysis are shown in Table 6. For most AEs, such as thrombocytopenia, myocardial infarction, and death, we did not observe statistically significant differences between patients aged <65 years and those aged ≥65 years (P > 0.05). However, two notable heterogeneous signals were identified: thrombosis in device showed a significantly stronger signal in younger patients (ROR = 3.53, 95% CI 1.08–11.52, P = 0.0259), whereas hemoglobin decreased was reported significantly more frequently in older patients (ROR = 0.37, 95% CI 0.15–0.90, P = 0.0221).
TABLE 6
| Preferred term (PT) | <65 years (N) | ≥65 years (N) | ROR (95% CI) | χ2 | P |
|---|---|---|---|---|---|
| Thrombocytopenia | 47 | 86 | 0.83 (0.57,1.21) | 0.9211 | 0.3372 |
| Myocardial infarction | 18 | 28 | 1.00 (0.55,1.82) | 0.0001 | 0.9916 |
| Haemoglobin decreased | 6 | 25 | 0.37 (0.15,0.90) | 5.2400 | 0.0221 |
| Cardiogenic shock | 11 | 17 | 1.00 (0.47,2.16) | 0.0001 | 0.9929 |
| Death | 8 | 17 | 0.73 (0.31,1.69) | 0.5543 | 0.4566 |
| Coronary artery stenosis | 10 | 13 | 1.20 (0.52,2.75) | 0.1792 | 0.6721 |
| Acute myocardial infarction | 9 | 14 | 1.00 (0.43,2.32) | 0.0001 | 0.9941 |
| Haemorrhage | 6 | 17 | 0.54 (0.21,1.38) | 1.6880 | 0.1939 |
| Cerebral haemorrhage | 4 | 14 | 0.44 (0.14,1.34) | 2.2062 | 0.1375 |
| Pulmonary alveolar haemorrhage | 5 | 11 | 0.70 (0.24,2.03) | 0.4296 | 0.5122 |
| Haematoma | 4 | 12 | 0.51 (0.16,1.60) | 1.3700 | 0.2418 |
| Ventricular fibrillation | 6 | 8 | 1.16 (0.40,3.38) | 0.0792 | 0.7784 |
| Platelet count decreased | 4 | 10 | 0.62 (0.19,1.98) | 0.6708 | 0.4128 |
| Hypotension | 8 | 6 | 2.08 (0.72,6.04) | 1.9091 | 0.1671 |
| Cardiac failure | 4 | 9 | 0.69 (0.21,2.24) | 0.3917 | 0.5314 |
| Post procedural complication | 5 | 8 | 0.97 (0.32,2.98) | 0.0030 | 0.9560 |
| Thrombosis in device | 9 | 4 | 3.53 (1.08,11.52) | 4.9632 | 0.0259 |
| Haemoptysis | 3 | 10 | 0.46 (0.13,1.69) | 1.4319 | 0.2315 |
| Chest pain | 3 | 9 | 0.51 (0.14,1.91) | 1.0246 | 0.4744a |
| Petechiae | 2 | 10 | 0.31 (0.07,1.14) | 2.5794 | 0.1905a |
| Cardiac arrest | 5 | 6 | 1.29 (0.39,4.26) | 0.1817 | 0.9075a |
| Gastrointestinal haemorrhage | 3 | 8 | 0.58 (0.15,2.19) | 0.6622 | 0.6144a |
| Anaemia | 3 | 7 | 0.66 (0.17,2.57) | 0.3580 | 0.7846a |
| Vascular stent thrombosis | 6 | 4 | 2.34 (0.66,8.33) | 1.8274 | 0.3045a |
| Pyrexia | 3 | 7 | 0.66 (0.17,2.57) | 0.3580 | 0.7846a |
| Drug ineffective | 3 | 7 | 0.66 (0.17,2.57) | 0.3580 | 0.7846a |
| Drug specific antibody present | 3 | 7 | 0.66 (0.17,2.57) | 0.3580 | 0.7846a |
| Ejection fraction decreased | 3 | 7 | 0.66 (0.17,2.57) | 0.3580 | 0.7846a |
| Haematocrit decreased | 2 | 8 | 0.39 (0.08,1.82) | 1.5585 | 0.3558a |
| Haemorrhage intracranial | 4 | 3 | 2.08 (0.46,9.31) | 0.9498 | 0.5575a |
Age-stratified analysis of specific PTs associated with tirofiban in the FAERS database.
Yates’ continuity-corrected chi-square test. Bold values indicate statistical significance (P < 0.05) unless otherwise specified.
Abbreviations: FAERS, the U.S., Food and Drug Administration’s Adverse Event Reporting System; PT, preferred term; ROR, reporting odds ratio; CI, confidence interval. χ2:chi-square.
4 Discussion
In our study, AE signals associated with tirofiban were identified from two databases and were found to involve 23 SOCs and 63 PTs, primarily concentrated in the SOCs of cardiac disorders, vascular disorders, and blood and lymphatic system disorders. The top 3 most frequently reported AEs were hemorrhage, thrombocytopenia, and intracranial hemorrhage, which are consistent with the AEs listed in the drug prescribing information, thereby supporting the reliability of our findings. In addition, this study identified 21 AEs that were not mentioned in the official drug label, including myocardial infarction, angina pectoris, cardiac failure, vascular stent thrombosis, cardiogenic shock, cardiac death, acute myocardial infarction, coronary artery stenosis, thrombosis, embolism, ventricular fibrillation, drug specific antibody present, coronary artery restenosis, thrombosis in device, coronary artery thrombosis, brain stem infarction, cardiac ventricular thrombosis, pulseless electrical activity, brain herniation, cardiac tamponade and electrocardiogram ST segment elevation.
4.1 Baseline information description
This study systematically analyzed the baseline characteristics of tirofiban-associated AEs reported in the FAERS and WHO-VigiAccess databases, revealing notable differences between the two data sources. Both databases showed a higher proportion of AEs reported in elderly and male patients. These findings align with existing literature, which reports a higher prevalence of cardiovascular diseases, particularly among older patients, with a greater incidence in males compared to females (Roth et al., 2021; Mensah et al., 2023). Our data analysis supports these epidemiological trends. As shown in Figure 2, a marked increase in the number of tirofiban-related adverse event reports was observed in 2019. This phenomenon may be related to the increased use of the drug (e.g., its exploratory application in ischemic stroke, such as in the RESCUE BT randomized clinical trial), which may have heightened clinical attention and temporarily increased reporting rates. It may also reflect an improvement in adverse event reporting awareness during this period.
Among the AE reports in the FAERS database, a significant proportion (94.3%) were submitted by healthcare professionals, including physicians, pharmacists, and other health professionals. This suggests that the FAERS database is a reliable source of AE reporting. In terms of geographic distribution, China accounted for the highest number of reports. This may be attributed to several factors, including a larger population of drug users, greater overall population size, racial disparities, a higher willingness to report, and the broader indications for tirofiban, all contributing to its more widespread use in this region. Regarding reported indications, the majority of AEs were associated with antiplatelet therapy, which aligns with its primary indication. Ischemic stroke followed as the second most reported indication. This finding is consistent with recent studies demonstrating the significant efficacy and safety of tirofiban in the treatment of acute ischemic stroke (Sun et al., 2021; Zi et al., 2023). These findings suggest that, while tirofiban is effective in acute clinical settings, its use necessitates careful patient selection, monitoring, and management to mitigate risks.
4.2 SOC for which both databases satisfy the thresholds
In both databases, we identified 23 organ systems commonly affected by tirofiban-related AEs. Among these, significant SOCs that met all four disproportionality analysis criteria included cardiac disorders, vascular and lymphovascular disorders, and hematologic and lymphatic disorders. Notably, cardiac disorders were the most frequently reported and highest signaling SOC in the FAERS database, while blood and lymphatic system disorders were the most common and highest signaling SOC in the WHO-VigiAccess database. These findings highlight the significant risk of cardiovascular and hematologic complications associated with tirofiban, consistent with the drug’s known mechanism as an antiplatelet agent.
4.2.1 AEs related to cardiac disorders
Based on the drug label for tirofiban, hemopericardium is a commonly reported AE associated with cardiac disorders. However, our study identified that the most frequently reported PTs related to cardiac disorders among the top 30 AEs were myocardial infarction, angina pectoris, cardiac failure, cardiogenic shock, acute myocardial infarction, coronary artery stenosis, and ventricular fibrillation. Notably, myocardial infarction, angina pectoris, acute myocardial infarction, and coronary artery stenosis are recognized indications for tirofiban (De et al., 2009; Valgimigli et al., 2010; Deng et al., 2021; Zhao et al., 1999; PRISM-PLUS Study Investigators, 1998), which may introduce bias at the SOC level in the analysis of cardiac disorders. Cardiac failure may also represent a comorbidity of the patients’ underlying conditions rather than a direct effect of tirofiban. For instance, myocardial infarction is primarily caused by rupture of atherosclerotic plaques in the coronary arteries, leading to platelet aggregation and thrombosis. The subsequent occlusion of coronary vessels results in myocardial ischemia, which can cause interstitial fibrosis, progressive ventricular dilation, and ultimately heart failure, thereby compromising patients’ quality of life and safety (Zhuo et al., 2022). Similarly, cardiogenic shock and ventricular fibrillation may be complications secondary to the underlying disease or its treatment. There have been case reports of tirofiban being used in patients with acute myocardial infarction complicated by cardiogenic shock (Knezević et al., 2008; Canji et al., 2010). Moreover, a randomized, prospective, open-label trial indicated that tirofiban, when used in addition to unfractionated heparin (UFH), could prevent platelet depletion and preserve platelet function in patients with cardiogenic shock and acute kidney injury requiring continuous renal replacement therapy (CRRT) (Link et al., 2008). Based on the data from both databases and disproportionality analysis, cardiac-related adverse events such as cardiac failure, cardiogenic shock, and ventricular fibrillation showed strong disproportionality signals. However, these findings should be interpreted with caution, as the statistical association observed in spontaneous reporting systems does not establish a causal relationship. The presence of underlying cardiovascular conditions and concomitant therapies may contribute to these signals. Therefore, these events should be considered potential safety signals rather than confirmed adverse drug reactions, and further clinical and mechanistic studies are warranted to clarify their clinical relevance.
4.2.2 AEs related to vascular disorders
Tirofiban treatment may lead to a range of vascular disorders, including bleeding and hematoma at various anatomical sites, with a higher incidence of hemorrhagic events when used in combination with heparin. Typically, bleeding events associated with tirofiban are mild, whereas severe cases, such as intracranial hemorrhage and retroperitoneal bleeding, are exceedingly rare. In addition to detecting signals of bleeding and intracranial hemorrhage, our study also identified thrombotic and embolic events, which are not mentioned in the drug label. From the pharmacological perspective, a clinical study reported a 10.5% incidence of minor bleeding with tirofiban, whereas the incidence of major bleeding, as defined by the thrombolysis in myocardial infarction (TIMI) criteria, was 1.4% (Huynh et al., 2003). For intracranial hemorrhage, in an analysis evaluating the safety outcomes of tirofiban in acute ischemic stroke treatment, the incidence of symptomatic intracranial hemorrhage (sICH) within 7 days was 0.6%, and any bleeding occurred in 4.0% of patients (Han et al., 2022). Moreover, intracranial hemorrhage has been reported in multiple studies following the use of tirofiban (Ihn et al., 2011; Liu et al., 2022; Zhang et al., 2020; De Almeida Monteiro et al., 2025), with fatal intracranial hemorrhage in some studies (relative risk = 3.59) (Liu et al., 2022). A recent phase III, multicenter, double-blind, randomized, placebo-controlled trial showed that the incidence of intracranial hemorrhage associated with tirofiban was relatively low (1.7%), although still higher than that observed in the placebo group (Chunrong et al., 2025). There are case reports of tirofiban-induced alveolar hemorrhage (Sebastian et al., 2024). The RESCUE BT study reported that older patients, had more severe neurological deficits, or had cardioembolic stroke were at a higher risk of developing sICH following intravenous tirofiban administration (Sang et al., 2023b). In addition, haemorrhage associated with tirofiban often occurs at arterial access sites used in cardiac catheterization procedures. It is recommended to minimize the use of invasive or potentially traumatic procedures, such as arterial and venous puncture, intramuscular injection, and nasotracheal intubation. Apart from drug-related factors, bleeding is also influenced by patient specific factors, such as advanced age, sex (particularly small-statured females), and comorbidities, including renal impairment, a history of prior bleeding, or malignancy (Chang et al., 2024; Sameer et al., 2009). Consequently, bleeding risk assessment should be performed before tirofiban administration, and patients should be closely monitored throughout treatment.
Regarding other vascular AEs, such as thrombosis and embolism, current evidence suggests that pre-procedural intravenous administration of tirofiban can reduce the risk of acute in-stent thrombosis during stenting procedures in patients with symptomatic high-grade intracranial atherosclerotic stenosis (Zhang et al., 2024). Some studies have reported that in intra-arterial strategies using tirofiban for the treatment of acute ischemic stroke, there is a risk of reocclusion following intra-arterial thrombolysis (Kim et al., 2012). A study showed that in-stent thrombosis occurred at an annual rate of 3% within 0.6 days–3 years post-implantation (Daemen et al., 2007). According to the FDA, various factors contribute to the formation of in-stent thrombosis, including the implantation of drug-eluting stents in patients with complex lesions (e.g., bifurcations, lesions requiring overlapping stents, or lesions associated with acute myocardial infarction), as well as conditions such as renal insufficiency or diabetes, which can lead to a higher incidence of stent thrombosis compared to implantation for approved indications. The FDA also notes a small increase in risk (ranging from <1% to approximately 5%) due to premature discontinuation of antiplatelet therapy, which remains an independent risk factor for thrombosis. Notably, thrombosis can occur even years after stent implantation (Farb and Boam, 2007). The high signal intensity for these AEs could be explained by the lack of symptom relief or the worsening of symptoms despite treatment with medications such as tirofiban, which may lead to clinical misdiagnosis. Bleeding from vascular access, hematoma at the cannulation site, and intracranial hemorrhage are well-established AEs of tirofiban use, aligning with those documented in the drug’s prescribing information. Based on the data from the FAERS and WHO-VigiAccess databases and the proportional imbalance analysis, these signals were statistically significant. However, while the statistical association is clear, the physiological relevance of these findings remains uncertain. Given the strength of these signals, it is essential to maintain close monitoring of patients for these AEs during the clinical course of tirofiban therapy.
4.2.3 AEs related to blood and lymphatic system disorders
Our study identified Blood and lymphatic system disorders as a significant SOC for tirofiban associated AEs. Among these, thrombocytopenia emerged as the most prominent signal, although its underlying mechanism remains unclear. Muhammad (Rawala et al., 2020) reported a case of a 69-year-old female who received tirofiban before undergoing percutaneous coronary intervention. On the second day post-procedure, the patient developed ecchymosis and mild epistaxis. Further evaluation revealed a sharp decline in platelet count from 224 × 109/L to 2 × 109/L. Following a hematology consultation, the patient was treated with corticosteroids and transfused with two units of platelets. One week later, her platelet count increased to 442 × 109/L. The thrombocytopenia was considered to be associated with tirofiban. Thrombocytopenia is defined as a platelet count of less than 100 × 109/L or a reduction of more than 50% from baseline. The potential mechanisms underlying tirofiban-induced thrombocytopenia are as follows. Tirofiban may induce structural changes in platelet surface glycoprotein receptors, resulting in the formation of new antigenic determinants. These determinants can be recognized by antibodies to platelet glycoproteins in the blood. If cross-reactivity occurs between pre-existing antibodies and the new antigenic determinants, the platelets become coated with immunoglobulins and are cleared from circulation. Alternatively, these antigenic complexes may be recognized by the liver, leading to platelet sequestration and removal from circulation (Wang and Zou, 2023; Merlini et al., 2004; Rikken et al., 2023). The reported incidence of tirofiban-induced thrombocytopenia ranges from 0.4% to 5.6%, with severe cases potentially leading to increased mortality risk (Wang and Zou, 2023; Yi et al., 2018). Bleeding is typically the only symptom of thrombocytopenia, and upon confirmation of thrombocytopenia, it is recommended to discontinue tirofiban immediately. Subsequent adjustments to the use of medications such as aspirin, clopidogrel, and heparin should be based on whether bleeding-related complications are present. Platelet transfusion is required when the platelet count falls below 10 ×109/L or in cases of severe bleeding. Consequently, routine blood testing, including platelet counts, hemoglobin levels, and erythrocyte counts, is recommended for all patients before administration, 6 h after the loading dose, and daily thereafter.
4.3 Other AE signals
In our analysis, we also detected unexpected and significant disproportionality signals for adverse events such as cerebral hemorrhage, cardiogenic shock, cardiac death, ventricular fibrillation, and the presence of drug-specific antibodies. All such novel and unforeseen adverse events warrant further investigation in future studies, and clinicians should remain vigilant during tirofiban administration in clinical practice.
4.4 TTO analysis
Understanding the timing of AE occurrence is crucial for early detection and intervention, as it helps identify specific risk windows and facilitates the prevention or early diagnosis of AEs. TTO analysis indicated that the majority of AEs occurred within 1 month after tirofiban administration, with relatively few events reported thereafter. Notably, bleeding and thrombocytopenia tended to manifest early after dosing, with median onset times of 0 days and 1 day, respectively, followed by a sharp decline in the incidence of AEs. Thrombocytopenia induced by tirofiban has been reported in the literature to typically occur within 24 h of treatment initiation, which is consistent with our findings (Merlini et al., 2004). However, in certain cases, delayed-onset thrombocytopenia may develop as late as 10 days after treatment (Clofent-Sanchez et al., 2007; Bosco et al., 2005). Additionally, we found that the AEs of tirofiban predominantly occurred within 30 days after administration. The Weibull distribution model showed that thrombocytopenia follows an early failure pattern, indicating a higher risk early in treatment, which diminishes over time. In contrast, bleeding events showed a random failure pattern, suggesting a consistent risk throughout therapy, regardless of treatment duration. Although these results may not reflect the actual TTO of a given population in clinical trials, our findings are close to those of previous studies. These results emphasize the need for early monitoring of thrombocytopenia, particularly in the first days of treatment, while ongoing vigilance is required for bleeding events across the entire treatment course. These findings underscore the importance of early monitoring for thrombocytopenia, particularly on the first day of treatment. Given that the median time to bleeding onset was 0 days, clinicians should closely observe patients for any signs of bleeding from the very beginning of therapy, including catheter-related bleeding. Special attention should be paid to severe events such as intracranial or cerebral hemorrhage.
4.5 Sensitivity analysis
The results of the reporter-stratified analysis appeared to be consistent with those of the overall AE analysis, which strengthens the reliability of our conclusions. The sex-stratified analysis showed that the signal for thrombocytopenia was significantly stronger among male patients. This may be related to the higher incidence of cardiovascular diseases in elderly men (Roth et al., 2021; Mensah et al., 2023), who are often prescribed multiple medications, thereby increasing the risk of drug-induced thrombocytopenia (Inna et al., 2018). In contrast, female patients exhibited a significantly higher risk of ischemic heart diseases (such as angina pectoris and coronary artery stenosis). This finding aligns with the established understanding of sex differences in coronary artery disease. Female patients are more prone to microvascular dysfunction and non-obstructive coronary artery disease, and their symptoms often present as atypical chest pain, which may lead to underdiagnosis or misdiagnosis in routine clinical settings (Noel et al., 2017).
The age-stratified analysis indicated that the overall safety profile of tirofiban was generally consistent across age groups, with most risk signals remaining stable between patients aged <65 and ≥65 years. This further supports the robustness of our conclusions. However, two notable heterogeneous signals warrant further investigation. Thrombosis in device showed a higher risk in younger patients, which may be associated with differences in the types of interventional procedures performed, device duration, or more active platelet physiology in this population. Additionally, hemoglobin decreased was reported more frequently among elderly patients, which likely reflects their higher baseline burden of comorbidities and greater frequency of clinical monitoring rather than a truelly drug-related effect. Moreover, the prevalence of anemia itself increases with age, often associated with chronic kidney disease, malnutrition, or chronic inflammatory conditions. Most cases in the elderly are mild (hemoglobin 10–12 g/dL) and rise progressively with age (Domenica and Irene, 2018). These findings suggest that, in clinical practice, particular attention should be paid to device-related thrombosis in younger patients, while regular monitoring of hemoglobin levels is recommended for elderly patients.
4.6 Clinical implications
This dual-database pharmacovigilance study offers several key insights for the safe use of tirofiban. The predominance of hemorrhage, thrombocytopenia, and intracranial bleeding confirms that bleeding and platelet complications remain its main safety concerns. According to our findings, thrombocytopenia associated with tirofiban typically occurs within the first 24 h after drug administration. In most cases, platelet counts gradually return to normal after discontinuation. Therefore, we recommend routine complete blood count monitoring before tirofiban administration and again at 6 h after the loading dose, including platelet count, hemoglobin, and hematocrit, followed by daily reassessment whenever possible during treatment. If thrombocytopenia is confirmed to be related to tirofiban, clinicians should discontinue the drug promptly. In patients with elderly, renal impairment or others at high risk of bleeding, dose adjustment should be considered. For example, in patients with a creatinine clearance ≤60 mL/min, a 50% of maintenance dose reduction is generally advised (Robert et al., 2023). If oral antiplatelet therapy is required as a bridging strategy after tirofiban use, imaging examinations should be repeated to rule out bleeding. In cases of minor bleeding (Bleeding Academic Research Consortium [BARC] types 1–2), discontinuation of therapy is not usually necessary; instead, close observation, symptomatic management, and treatment of the underlying condition are recommended (Chinese Stroke Society et al., 2019). For instance, proton pump inhibitors can be used for gastrointestinal bleeding. In the event of major bleeding, immediate discontinuation of tirofiban and prompt supportive management are advised, such as platelet transfusion.
4.7 Limitations
Several limitations should be acknowledged. First, the voluntary nature of reporting to both the FAERS and WHO-VigiAccess databases complicates the accurate estimation of AE incidence and prevalence, leading to underreporting. To address this limitation, alternative strategies have been explored, including linking spontaneous reporting data with external resources such as prescribing or dispensing databases. This approach can provide a more accurate context for background dose exposure patterns and improve the interpretation of safety signals (Okoli et al., 2021; Mokbel et al., 2022). Furthermore, the WHO-VigiAccess database lacks essential information, including reporter details, drug indications, outcomes, and TTO, making it difficult to validate some of these factors. Second, while this study used a more stringent selection process by focusing on PTs that were common across both databases and met four specific criteria, this may have led to the exclusion of certain new signals. Finally, due to the inherent limitations of the disproportionate analysis method, it is not possible to infer a causal relationship between the drug and the AE; it only provides an estimate of signal strength, which is statistically significant. Moreover, given the potential regional differences, the conclusions should be interpreted with caution. Therefore, prospective clinical trials are essential to establish definitive causal links.
5 Conclusion
In conclusion, this study provides a systematic and comprehensive analysis of tirofiban-related AEs by utilizing disproportionality analysis on data from the FAERS and WHO-VigiAccess databases. The AEs detected were largely consistent with those reported in the drug insert. Clinicians should be aware of potential off-label AEs, such as cardiac death and cerebral hemorrhage, and monitor patients closely during the first 1–2 days post-administration. Furthermore, we analyzed the median time to onset of these AEs, offering clinicians and pharmacists valuable insights to refine dosing strategies and enhance safety monitoring practices when using tirofiban. Despite the valuable insights provided, the exploratory nature of this study necessitates further research to establish causal relationships between these AEs and tirofiban.
Statements
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
JL: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Methodology, Resources, Supervision, Writing – original draft, Writing – review and editing. FW: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Writing – original draft. LiZ: Investigation, Software, Writing – review and editing. LeZ: Data curation, Formal Analysis, Writing – review and editing. KJ: Data curation, Formal Analysis, Writing – review and editing. YZ: Supervision, Writing – review and editing.
Funding
The authors declare that financial support was received for the research and/or publication of this article. This study was funded by the Guangdong Provincial Hospital Pharmacy Research Fund (grant No. 2024YYYX101).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1713141/full#supplementary-material
References
1
Bate A. Lindquist M. Edwards I. R. Olsson S. Orre R. Lansner A. et al (1998). A Bayesian neural network method for adverse drug reaction signal generation. Eur. J. Clin. Pharmacol.54 (4), 315–321. 10.1007/s002280050466
2
Bosco A. Kidson-Gerber G. Dunkley S. (2005). Delayed tirofiban-induced thrombocytopenia: two case reports. J. Thromb. Haemost.3 (5), 1109–1110. 10.1111/j.1538-7836.2005.01296.x
3
Canji T. Jovelić A. Srdanović I. Petrović M. Panić G. Dodić S. (2010). Effects of tirofiban and percutaneous coronary intervention in an old patient with acute myocardial infarction and cardiogenic shock. Med. Pregl.63 (1-2), 117–122. 10.2298/mpns1002117c
4
Cannon C. P. Weintraub W. S. Demopoulos L. A. Vicari R. Frey M. J. Lakkis N. et al (2001). Comparison of early invasive and conservative strategies in patients with unstable coronary syndromes treated with the glycoprotein IIb/IIIa inhibitor tirofiban. N. Engl. J. Med.344 (25), 1879–1887. 10.1056/NEJM200106213442501
5
Chang L. Fengli Li. Liyuan C. Jiacheng H. Hongfei S. Thanh N. N. et al (2024). Effects of tirofiban on large vessel occlusion stroke are modified by etiology and renal function. Ann. Clin. Transl. Neurol.11 (3), 618–628. 10.1002/acn3.51982
6
Chinese Stroke Society (2019). Chinese stroke society neurointervention committee, Chinese Preventive Medicine association stroke Prevention and Control Professional Committee Intervention Group. Expert consensus on the clinical application of tirofiban in atherosclerotic cerebrovascular disease. Chin. J. Stroke10, 1034–1044. 10.3969/j.issn.1673-5765.2019.10.013
7
Chunrong T. Tianlong L. Tao C. Jie L. Zongliang Li. Youquan R. et al (2025). Early tirofiban infusion after intravenous thrombolysis for stroke. N. Engl. J. Med.393 (12), 1191–1201. 10.1056/NEJMoa2503678
8
Clofent-Sanchez G. Harizi H. Nurden A. Coste P. Jais C. Nurden P. (2007). A case of profound and prolonged tirofiban-induced thrombocytopenia and its correction by intravenous immunoglobulin G. J. Thromb. Haemost.5 (5), 1068–1070. 10.1111/j.1538-7836.2007.02440.x
9
Daemen J. Wenaweser P. Tsuchida K. Abrecht L. Vaina S. Morger C. et al (2007). Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: data from a large two-institutional cohort study. Lancet369 (9562), 667–678. 10.1016/S0140-6736(07)60314-6
10
De Almeida Monteiro G. Leite M. Gonçalves O. R. Ferreira M. Y. Mutarelli A. Marinheiro G. et al (2025). Efficacy and safety of intravenous tirofiban combined with reperfusion therapy versus reperfusion therapy alone in acute ischemic stroke: a meta-analysis of randomized controlled trials. J. Thromb. Thrombolysis58 (4), 526–537. 10.1007/s11239-025-03094-2
11
De L. G. Navarese E. Marino P. (2009). Risk profile and benefits from Gp IIb-IIIa inhibitors among patients with ST-segment elevation myocardial infarction treated with primary angioplasty: a meta-regression analysis of randomized trials. Eur. Heart J.30 (22), 2705–2713. 10.1093/eurheartj/ehp118
12
Deng L. Jia H. Z. Li M. C. Zhu W. (2021). Comparison of the effect of ticagrelor combined with tirofiban versus clopidogrel combined with tirofiban on inflammation response and prognosis of patients with unstable angina pectoris in long term follow-up. Kaohsiung J. Med. Sci.37 (11), 1010–1015. 10.1002/kjm2.12421
13
Dhodapkar M. M. Sh i X. Ramachandran R. Chen E. M. Wallach J. D. Ross J. S. (2022). Characterization and corroboration of safety signals identified from the US food and Drug Administration adverse event reporting system, 2008-19: cross sectional study. BMJ379, e071752. 10.1136/bmj-2022-071752
14
Dobrzycki S. Kralisz P. Nowak K. Prokopczuk P. Kochman W. Korecki J. et al (2007). Transfer with GP IIb/IIIa inhibitor tirofiban for primary percutaneous coronary intervention vs. on-site thrombolysis in patients with ST-elevation myocardial infarction (STEMI): a randomized open-label study for patients admitted to community hospitals. Eur. Heart J.28 (20), 2438–2448. 10.1093/eurheartj/ehm369
15
Domenica M. Cappellini C. Irene M. (2018). Anemia in clinical practice-definition and classification: does hemoglobin change with aging?Semin. Hematol.52 (4), 261–269. 10.1053/j.seminhematol.2015.07.006
16
Farb A. Boam A. B. (2007). Stent thrombosis redux--the FDA perspective. N. Engl. J. Med.356 (10), 984–987. 10.1056/NEJMp068304
17
Guo J. Xu M. Xi Y. (2012). Tirofiban-induced diffuse alveolar hemorrhage: after primary angioplasty. Tex Heart Inst. J.39 (1), 99–103. Available online at: https://pmc.ncbi.nlm.nih.gov/articles/PMC3298901.
18
Guo Q. Li X. Zhao C. Li Y. Mao Y. Zhai C. et al (2025). A disproportionality analysis of real-world adverse events associated with Tirofiban from the FAERS database. Sci. Rep.15 (1), 32043. 10.1038/s41598-025-15964-z
19
Han B. Ma T. Liu Z. Wu Y. Tan W. Sun S. et al (2022). Efficacy and safety of Tirofiban in clinical patients with acute ischemic stroke. Front. Neurol.12, 785836. 10.3389/fneur.2021.785836
20
Huynh T. Theroux P. Snapinn S. Wan Y. Montreal General Hospital, Montreal, Quebec, Canada. thao.huynh@muhc.mcgill.ca (2003). Effect of platelet glycoprotein IIb/IIIa receptor blockade with tirofiban on adverse cardiac events in women with unstable angina/non-ST-elevation myocardial infarction (PRISM-PLUS study). Am. Heart J.146 (4), 668–673. 10.1016/S0002-8703(03)00255-2
21
Ihn Y. K. Sung J. H. Kim B. S. (2011). Intravenous glycoprotein IIb/IIIa inhibitor (Tirofiban) followed by low-dose intra-arterial urokinase and mechanical thrombolysis for the treatment of acute stroke. Neuroradiol. J.24 (6), 907–913. 10.1177/197140091102400614
22
Inna T. Ron H. Manuel M. (2018). Hemostasis and thrombosis in the oldest old. Semin. Thromb. Hemost.44 (7), 624–631. 10.1055/s-0038-1657779
23
Kelly W. N. Arellano F. M. Barnes J. Bergman U. Edwards R. I. Fernandez A. M. et al (2007). Guidelines for submitting adverse event reports for publication. Drug Saf.30 (5), 367–373. 10.2165/00002018-200730050-00001
24
Kim J. W. Jeon P. Kim G. M. Bang O. Y. Byun H. S. Kim K. H. (2012). Local intraarterial tirofiban after formation of anterograde flow in patients with acute ischemic stroke: preliminary experience and short term follow-up results. Clin. Neurol. Neurosurg.114 (10), 1316–1319. 10.1016/j.clineuro.2012.04.022
25
Kinoshita S. Hosomi K. Yokoyama S. Takada M. (2020). Time-to-onset analysis of amiodarone-associated thyroid dysfunction. J. Clin. Pharm. Ther.45 (1), 65–71. 10.1111/jcpt.13024
26
Knezević B. Nikolić G. Dragnić S. Musić L. Bosković A. (2008). Successful treatment of cardiogenic shock by stenting of the left main coronary artery in acute myocardial infarction. Vojnosanit. Pregl.65 (10), 769–773. 10.2298/vsp0810769k
27
Lazarovici P. Marcinkiewicz C. Lelkes P. I. (2019). From snake venom's disintegrins and C-Type lectins to anti-platelet drugs. Toxins (Basel).11 (5), 303. 10.3390/toxins11050303
28
Li X. Guo X. Zhang N. Chang Y. Sun Y. (2024). Effects of different doses of tirofiban combined with dual antiplatelet drugs on platelet indices, vascular endothelial function, and major adverse cardiovascular events in patients with acute ST-segment elevated myocardial infarction undergoing percutaneous coronary intervention. Platelets35 (1), 2402301. 10.1080/09537104.2024.2402301
29
Link A. Girndt M. Selejan S. Rbah R. Böhm M. (2008). Tirofiban preserves platelet loss during continuous renal replacement therapy in a randomised prospective open-blinded pilot study. Crit. Care12 (4), R111. 10.1186/cc6998
30
Liu J. Yang Y. Liu H. (2022). Efficacy outcomes and safety measures of intravenous tirofiban or eptifibatide for patients with acute ischemic stroke: a systematic review and meta-analysis of prospective studies. J. Thromb. Thrombolysis53 (4), 898–910. 10.1007/s11239-021-02584-3
31
Liu Q. Cui Z. Deng C. Yang C. Shi T. (2024). A real-world pharmacovigilance analysis of adverse events associated with irbesartan using the FAERS and JADER databases. Front. Pharmacol.15, 1485190. 10.3389/fphar.2024.1485190
32
Mensah G. A. Fuster V. Roth G. A. (2023). A heart-healthy and stroke-free world: using data to inform global action. J. Am. Coll. Cardiol.82 (25), 2343–2349. 10.1016/j.jacc.2023.11.003
33
Merlini P. A. Rossi M. Menozzi A. Buratti S. Brennan D. M. Moliterno D. J. et al (2004). Thrombocytopenia caused by abciximab or tirofiban and its association with clinical outcome in patients undergoing coronary stenting. Circulation109 (18), 2203–2206. 10.1161/01.CIR.0000127867.41621.85
34
Mokbel K. Daniels R. Weedon M. N. Jackson L. (2022). A comparative safety analysis of medicines based on the UK pharmacovigilance and general practice prescribing data in England. Vivo36 (2), 780–800. 10.21873/invivo.12765
35
Noel C. Merz. B. Carl J. P. Mary N. W. Jerome L. F. (2017). Ischemia and no obstructive coronary artery disease (INOCA): developing evidence-based therapies and research agenda for the next decade. Circulation135 (11), 1075–1092. 10.1161/CIRCULATIONAHA.116.024534
36
Okoli G. N. Myles P. Murray-Thomas T. Shepherd H. Wong I. C. K. Edwards D. (2021). Use of primary care data in research and pharmacovigilance: eight scenarios where prescription data are absent. Drug Saf.44 (10), 1033–1040. 10.1007/s40264-021-01093-9
37
PRISM-PLUS Study Investigators (1998). Inhibition of the platelet glycoprotein IIb/IIIa receptor with tirofiban in unstable angina and non-Q-wave myocardial infarction. N. Engl. J. Med.338 (21), 1488–1497. 10.1056/NEJM199805213382102
38
Rawala M. S. Ahmed A. S. Posina K. Sundaram V. (2020). Tirofiban induced thrombocytopenia: a rare but severe adverse effect. J. Community Hosp. Intern Med. Perspect.10 (2), 171–173. 10.1080/20009666.2020.1747783
39
Rikken S. A. O. F. van 't Hof A. W. J. Ten Berg J. M. Kereiakes D. J. Coller B. S. (2023). Critical analysis of thrombocytopenia associated with glycoprotein IIb/IIIa inhibitors and potential role of zalunfiban, a novel small molecule glycoprotein inhibitor, in understanding the mechanism(s). J. Am. Heart Assoc.12 (24), e031855. 10.1161/JAHA.123.031855
40
Robert A. B. Xavier R. Coughlan. J. J. Emanuele B. Alaide C. Marc J. C. et al (2023). 2023 ESC guidelines for the management of acute coronary syndromes. Eur. Heart J.12 (38), 3720–3826. 10.1093/eurheartj/ehad191
41
Roth G. A. Mensah G. A. Johnson C. O. Addolorato G. Ammirati E. Baddour L. M. et al (2021). Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J. Am. Coll. Cardiol.76 (25), 2982–3021. 10.1016/j.jacc.2020.11.010
42
Sakaeda T. Tamon A. Kadoyama K. Okuno Y. (2013). Data mining of the public version of the FDA adverse Event Reporting System. Int. J. Med. Sci.10 (7), 796–803. 10.7150/ijms.6048
43
Sameer K. M. Andrew D. F. Jason B. L. John A. H. John A. S. Sunil V. R. et al (2009). Bleeding in patients undergoing percutaneous coronary intervention: the development of a clinical risk algorithm from the National cardiovascular data Registry. Circ. Cardiovasc Interv.2 (3), 222–229. 10.1161/CIRCINTERVENTIONS.108.846741
44
Sang H. Huang J. Jiang B. Guo Q. Nguyen T. N. Abdalkader M. et al (2023a). Association between intravenous tirofiban and intracranial hemorrhage in acute large vessel occlusion stroke: insight from the RESCUE BT randomized placebo-controlled trial. J. Neurol.270 (4), 2246–2255. 10.1007/s00415-023-11579-4
45
Sang H. Xie D. Tian Y. Nguyen T. N. Saver J. L. Nogueira R. G. et al (2023b). Association of tirofiban with functional outcomes after thrombectomy in acute ischemic stroke due to intracranial atherosclerotic disease. Neurology100 (19), e1996–e2006. 10.1212/WNL.0000000000207194
46
Sauzet O. Carvajal A. Escudero A. Molokhia M. Cornelius V. R. (2013). Illustration of the weibull shape parameter signal detection tool using electronic healthcare record data. Drug Saf.36 (10), 995–1006. 10.1007/s40264-013-0061-7
47
Sebastian B. Ganesan V. Sebastian P. (2024). Tirofiban-induced diffuse alveolar haemorrhage. BMJ Case Rep.17 (8), e256335. 10.1136/bcr-2023-256335
48
Shi H. Hou M. M. Ren G. He Z. F. Liu X. L. Li X. Y. et al (2023). Tirofiban for acute ischemic stroke patients receiving intravenous thrombolysis: a systematic review and meta-analysis. Cerebrovasc. Dis.52 (5), 587–596. 10.1159/000527861
49
Sun Y. Zheng H. Liu H. Jiang Z. Ren Y. Huang X. et al (2021). Effect of Tirofiban on new cerebral microbleeds after mechanical thrombectomy in patients with acute ischemic stroke. Rev. Assoc. Med. Bras.67 (11), 1564–1569. 10.1590/1806-9282.20210563
50
Szarfman A. Tonning J. M. Doraiswamy P. M. (2004). Pharmacovigilance in the 21st century: new systematic tools for an old problem. Pharmacotherapy24 (9), 1099–1104. 10.1592/phco.24.13.1099.38090
51
Topol E. J. Byzova T. V. Plow E. F. (1999). Platelet GPIIb-IIIa blockers. Lancet353 (9148), 227–231. 10.1016/S0140-6736(98)11086-3
52
Valgimigli M. Biondi-Zoccai G. Tebaldi M. van't Hof A. W. Campo G. Hamm C. et al (2010). Tirofiban as adjunctive therapy for acute coronary syndromes and percutaneous coronary intervention: a meta-analysis of randomized trials. Eur. Heart J.31 (1), 35–49. 10.1093/eurheartj/ehp376
53
Wang J. Zou D. (2023). Tirofiban-induced thrombocytopenia. Ann. Med.55 (1), 2233425. 10.1080/07853890.2023.2233425
54
Wang M. Li J. Zhang L. Li N. Li X. Wang P. (2024). The efficacy and safety of continuous intravenous tirofiban for acute ischemic stroke patients treated by endovascular therapy: a meta-analysis. Front. Neurol.15, 1286079. 10.3389/fneur.2024.1286079
55
Wang Q. Zhou Q. Du Z. Lu R. Jiang Y. Zhu H. (2024). Clinical safety of daridorexant in insomnia treatment: analysis of FDA adverse event reports. J. Affect Disord.362, 552–559. 10.1016/j.jad.2024.07.034
56
Yang M. Huo X. Miao Z. Wang Y. (2019). Platelet glycoprotein IIb/IIIa receptor inhibitor tirofiban in acute ischemic stroke. Drugs79 (5), 515–529. 10.1007/s40265-019-01078-0
57
Yi Y. H. Yin W. J. Gu Z. C. Fang W. J. Li D. Y. Hu C. et al (2018). A simple clinical pre-procedure risk model for predicting thrombocytopenia associated with periprocedural use of Tirofiban in patients undergoing percutaneous coronary intervention. Front. Pharmacol.9, 1456. 10.3389/fphar.2018.01456
58
Zhang P. Guo Y. Shen J. Li H. Wang R. Wang Y. et al (2020). Efficacy and safety of Tirofiban therapy in patients receiving endovascular treatment after large vessel ischaemic stroke: a systematic review and meta-analysis. J. Clin. Neurosci.80, 112–120. 10.1016/j.jocn.2020.07.040
59
Zhang J. Huang X. Wang G. Wang X. Zhang T. Wang D. et al (2024). Efficacy and safety of Tirofiban before stenting for symptomatic intracranial atherosclerotic stenosis: a randomized clinical trial. Neurology102 (7), e209217. 10.1212/WNL.0000000000209217
60
Zhao X. Q. Théroux P. Snapinn S. M. Sax F. L. (1999). Intracoronary thrombus and platelet glycoprotein IIb/IIIa receptor blockade with tirofiban in unstable angina or non-Q-wave myocardial infarction. Angiographic results from the PRISM-PLUS trial (platelet receptor inhibition for ischemic syndrome management in patients limited by unstable signs and symptoms). PRISM-PLUS investigators. Circulation.100 (15), 1609–1615. 10.1161/01.cir.100.15.1609
61
Zhao W. Li S. Li C. Wu C. Wang J. Xing L. et al (2024). Effects of tirofiban on neurological deterioration in patients with acute ischemic stroke: a randomized clinical trial. JAMA Neurol.81 (6), 594–602. 10.1001/jamaneurol.2024.0868
62
Zhou X. Wu X. Sun H. Li J. (2017). Efficacy and safety of eptifibatide versus tirofiban in acute coronary syndrome patients: a systematic review and meta-analysis. J. Evid. Based Med.10 (2), 136–144. 10.1111/jebm.12253
63
Zhuo M. F. Zhang K. L. Shen X. B. Lin W. C. Hu B. Cai H. P. et al (2022). Postoperative adverse cardiac events in acute myocardial infarction with high thrombus load and best time for stent implantation. World J. Clin. Cases10 (7), 2106–2114. 10.12998/wjcc.v10.i7.2106
64
Zi W. Song J. Kong W. Huang J. Guo C. He W. et al (2023). Tirofiban for stroke without large or medium-sized vessel occlusion. N. Engl. J. Med.388 (22), 2025–2036. 10.1056/NEJMoa2214299
Summary
Keywords
tirofiban, adverse events (AEs), FAERS database, WHO-VigiAccess database, disproportionality analysis
Citation
Li J, Wang F, Zhong L, Zhang L, Ji K and Zheng Y (2025) Real-world safety of tirofiban: a disproportionality analysis using data from FAERS and WHO-VigiAccess. Front. Pharmacol. 16:1713141. doi: 10.3389/fphar.2025.1713141
Received
25 September 2025
Revised
12 November 2025
Accepted
26 November 2025
Published
11 December 2025
Volume
16 - 2025
Edited by
Christos Kontogiorgis, Democritus University of Thrace, Greece
Reviewed by
Kinan Mokbel, University of Exeter, United Kingdom
Xihui Yu, Second Affiliated Hospital of Shantou University Medical College, China
Updates
Copyright
© 2025 Li, Wang, Zhong, Zhang, Ji and Zheng.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jia Li, lijia37@mail.sysu.edu.cn
†These authors have contributed equally to this work.
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.