- 1Changchun University of Chinese Medicine, Changchun, China
- 2The Jilin Province School-Enterprise Cooperation Technology Innovation Laboratory of Herbal Efficacy Evaluation Based on Zebrafish Model Organisms, Changchun University of Chinese Medicine, Changchun, China
- 3Jilin Provincial International Cooperation Key Laboratory of Traditional Medicine for Prevention and Treatment of Metabolic Diseases, Changchun University of Chinese Medicine, Changchun, China
- 4Wish Technology, Changchun, China
- 5Center for Food Evaluation, State Administration for Market Regulation, Beijing, China
- 6Institute of Botany, Academy of Sciences of Uzbekistan, Tashkent, Uzbekistan
- 7Institute of Agricultural Biotechnology, Jilin Academy of Agricultural Sciences (Northeast Agricultural Research Center of China), Changchun, China
Cardiovascular diseases (CVDs) are a major global health challenge, significantly impacting public health and healthcare systems. Ethnopharmacological remedies and botanical medicines are widely used for the prevention and treatment of CVDs due to their multi-component, multi-target properties. Understanding the mechanisms of these natural products is essential for developing safe and effective therapies. The zebrafish model, an emerging tool in experimental pharmacology, has been increasingly used to evaluate the pharmacological activities of natural products. This review focuses the use of zebrafish models to test the prevention and treatment effects of various CVDs, and to investigate the inhibition of hyperlipidemia, thrombosis, the progress of heart failure, and the promotion of angiogenesis and toxicity. Emphasizing its experimental advantages-including the transparency characteristics of the body, its applicability to the analysis of multiple samples, and its support for real-time monitoring-aims to reveal its potential value in combining traditional cognition with contemporary pharmacological testing capabilities. The literature summary provides strong evidence for the cognitive improvement of the zebrafish system in clarifying the efficacy of natural ingredients in the cardiovascular field.
1 Introduction
Cardiovascular diseases (CVDs) are a leading global cause of death, contributing significantly to morbidity and mortality worldwide (Bournele and Beis, 2016). These include various heart and blood vessel conditions such as coronary artery disease, heart failure, thrombosis, hyperlipidemia, atherosclerosis, and vascular damage (Flora and Nayak, 2019). CVDs treatment mainly involves pharmacological intervention and surgery (Li, 2024). Although these therapies can relieve symptoms, long-term use of drugs can bring the risk of drug dependence and adverse reactions.
Traditional medicine (ethnopharmacology) has a long history of use. With people’s increasing interest in traditional medicine, more and more research is exploring the potential of traditional therapies and their natural compounds in the treatment of CVDs (Zhong et al., 2024). Traditional medicine emphasizes the treatment of CVDs by improving blood circulation, enhancing overall energy, enhancing heart function, and reducing inflammation or toxins to restore body balance. In recent years, modern pharmacological research has gradually revealed the role of certain natural products in cardiovascular treatment. For example, phytochemicals such as tanshinone from Salvia miltiorrhiza Bunge [Lamiaceae; Salviae miltiorrhizae radix et rhizoma], notoginsenoside from Panax notoginseng (Burkill) F.H.Chen [Araliaceae; Notoginseng radix et rhizoma], and ginsenosides from Panax ginseng C.A.Mey. [Araliaceae; Ginseng radix et rhizoma] regulate cardiovascular function through multi-target and multi-pathway mechanisms (Fan W. X. et al., 2020; Zeng et al., 2023; Dabbaghi et al., 2024). Furthermore, the active components of these natural products have shown significant therapeutic effects in anti-inflammatory, antioxidant, lipid-lowering, and cardiovascular protection, and are widely used in the treatment of CVDs. Overall, as a vast repository of knowledge, traditional medicine holds significant value for cardiovascular disease research and warrants further investigation.
Preclinical studies of CVDs commonly use cell and mammalian models to evaluate the efficacy of therapeutic candidates (Camacho et al., 2016; Lippi et al., 2020). It is possible to replicate the pathological processes of many CVDs in vivo by using rats and mice as model organisms (Garbern et al., 2013). Cell models are easy to use, sensitive, and convenient for studying drug mechanisms. However, factors such as cost, trial duration, and the need for continuous, holistic in vivo assessment of pathological changes and drug safety and efficacy have made the zebrafish model a powerful tool for investigating how natural compounds regulate CVDs (Bournele and Beis, 2016).
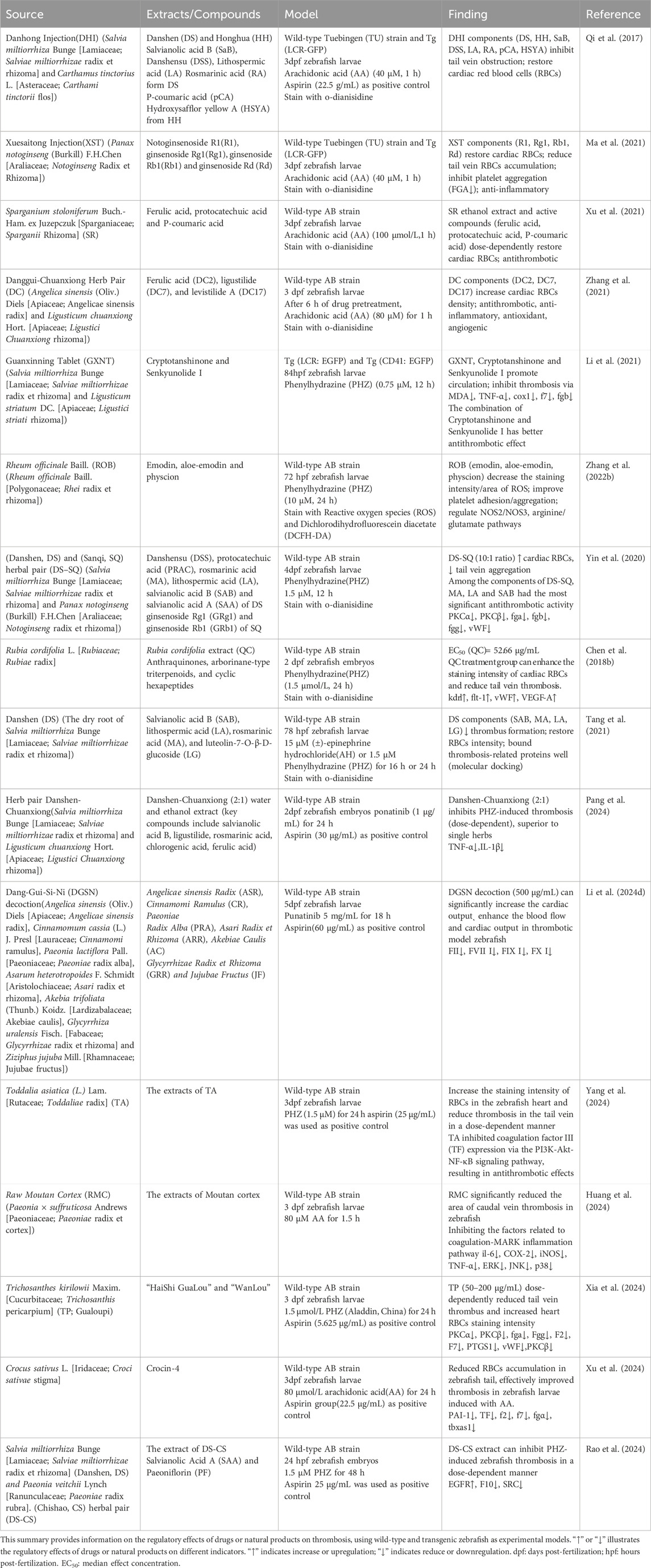
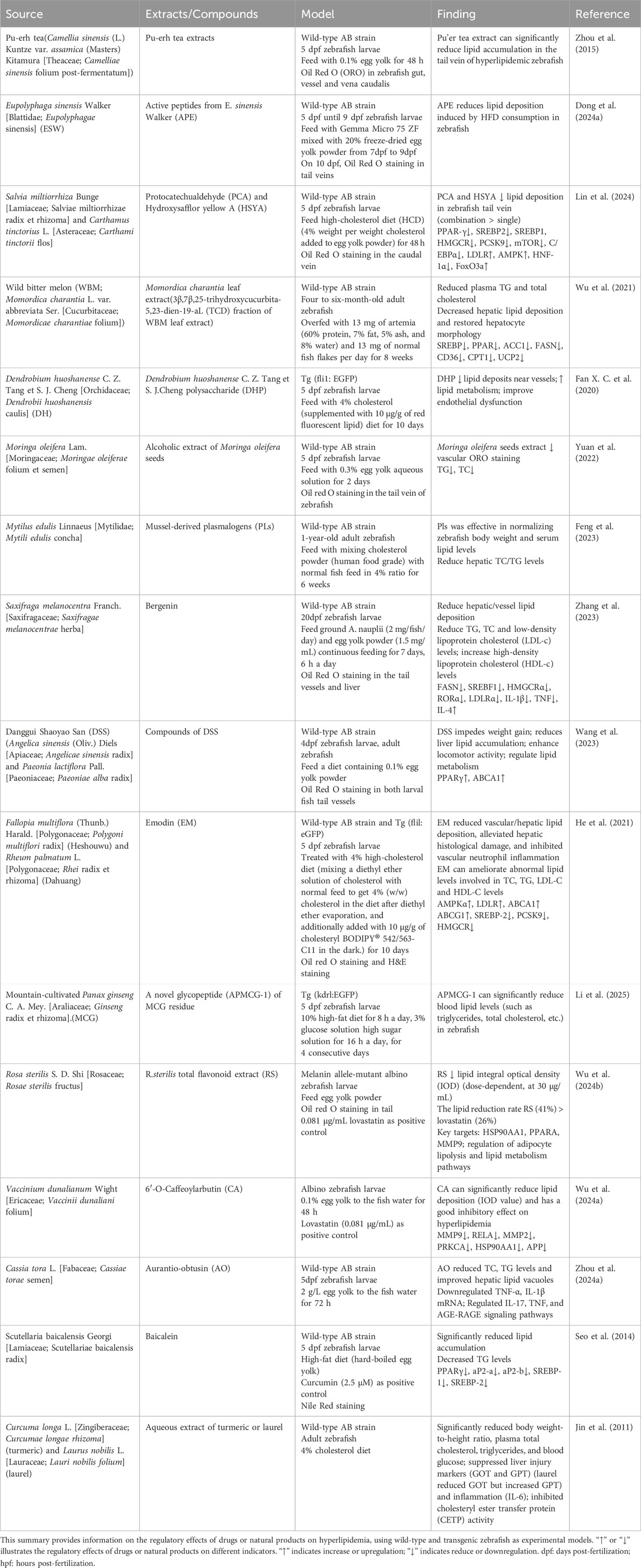
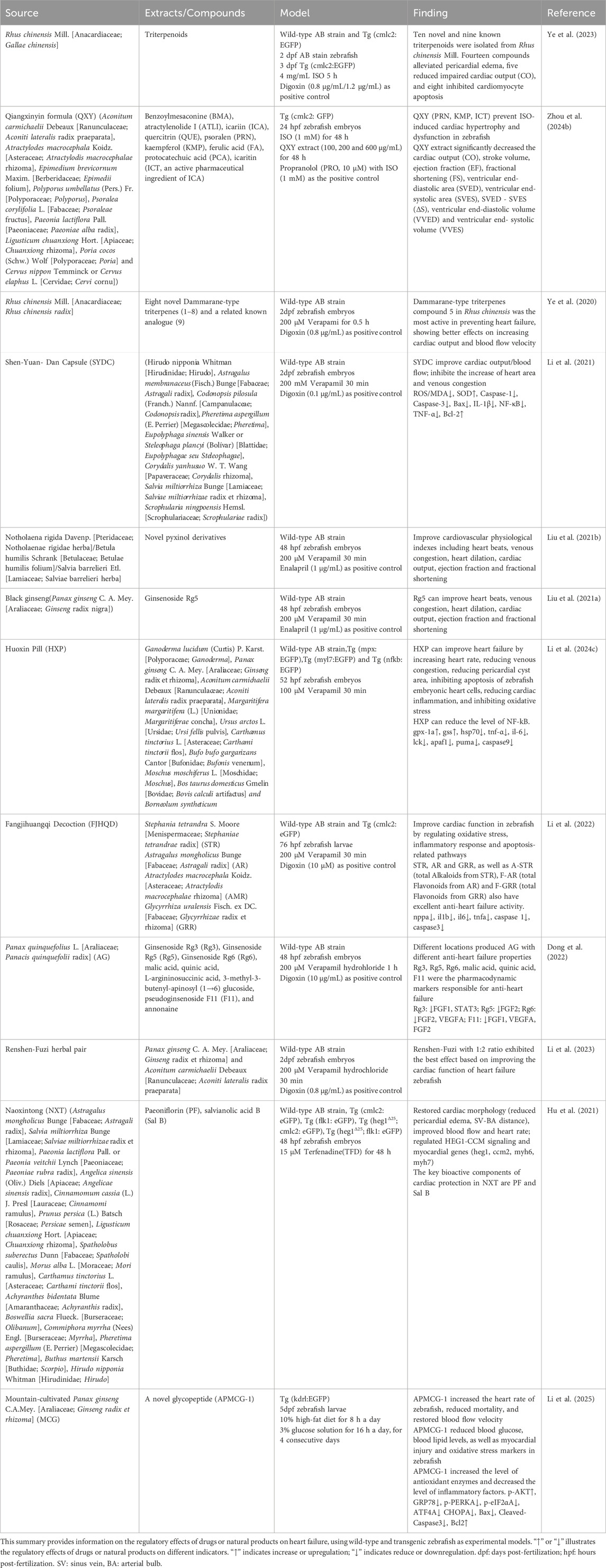
Zebrafish (Danio rerio) are excellent models for biological and pharmacological research because they are transparent at an early stage, can dynamically observe organs and cells, have low cost, high throughput, and short experimental cycles (Gao et al., 2017). Their cardiovascular system has many structural and functional similarities with mammals. Within 24 h after fertilization (hpf), the circulatory system, including the sinuses, atria, ventricles, and bulbous arteries, is visible under a microscope. Fluorescent transgenic zebrafish have further enhanced the ability to study the cardiovascular system in vivo. For example, the Tg (gata1:DsRed/kdrl:eGFP) double transgenic zebrafish express eGFP (green fluorescent protein) in vascular endothelial cells, while red blood cells express DsRed (red fluorescent protein). This zebrafish was imaged using a confocal microscope to produce a composite image of the cardiovascular system (Figure 1A), in which the red fluorescently labeled red blood cells are clearly visible in the blood vessel network, providing details of blood distribution and circulation. Simultaneously, the green fluorescence of the blood vessel network reveals the complex structure of the zebrafish cardiovascular system, including the aorta, veins, and caudal vein vascular plexus. This model provides a comprehensive view of red blood cell flow and cardiovascular architecture. It has been used to study tumor vasculature dynamics (Zhong et al., 2023). Additionally, Tg (myl7:DsRed) transgenic zebrafish express DsRed in cardiomyocytes, with bright red fluorescence outlining heart morphology and position, providing an important basis for evaluating the contractile and diastolic function of the heart (Figures 1B–D).
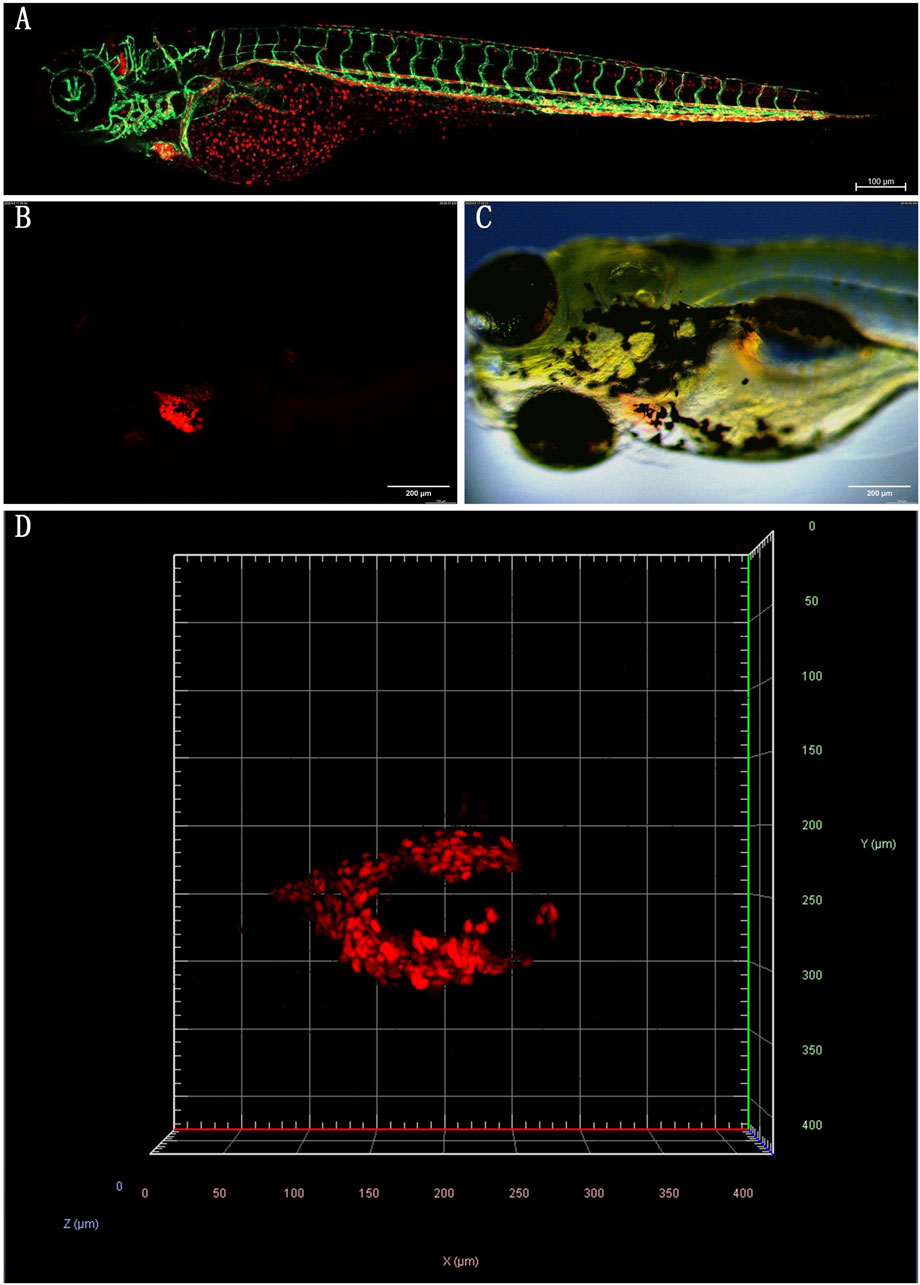
Figure 1. (A) Tg (gata1:DsRed/kdrl:eGFP) double transgenic zebrafish (2dpf): Confocal images of red fluorescence in blood cells and green fluorescence in vasculature are merged. (B) Tg (myl7:DsRed) transgenic zebrafish (4dpf) was observed under a fluorescence microscope. (C) The merge images of Tg (myl7:DsRed) transgenic zebrafish under bright and fluorescence field. (D) Confocal microscopy image of the heart of Tg (myl7:DsRed) transgenic zebrafish. Head is to the left. (All images in this figure are original images, taken by the author in the laboratory of Changchun University of Chinese Medicine using Leica fluorescence microscope and confocal microscope. Zebrafish were anesthetized with MS-222, fixed in low-melting agarose gel, and immobilized under the microscope. Images were taken under fluorescence and bright-field conditions, and the captured images were merged using Visio software for final processing).
This paper reviews the application of the zebrafish model in studying the effects and mechanisms of natural products on cardiovascular health over the past two decades, spanning from 2002 to 2025. Literature searches were conducted across multiple databases, including PubMed, Web of Science, and Google Scholar, using keywords such as “zebrafish,” “cardiovascular disease,” “natural medicine,” and “pharmacological activity.” Studies were included if they utilized zebrafish models to investigate the effects of natural compounds or extracts on CVDs—such as heart failure, hyperlipidemia, and thrombosis—provided they featured clear experimental designs and reliable data. Publications that did not employ zebrafish models, involved synthetic compounds, or presented incomplete data were excluded. We comprehensively reviewed zebrafish models assessing the effects of natural products on cardiovascular function and pathology (Figure 2). Additionally, this study aims to encourage researchers to focus on zebrafish models, promoting the development and utilization of effective models to identify candidate compounds from phytomedicines and natural products for modulating CVDs, ultimately advancing drug and health product development.
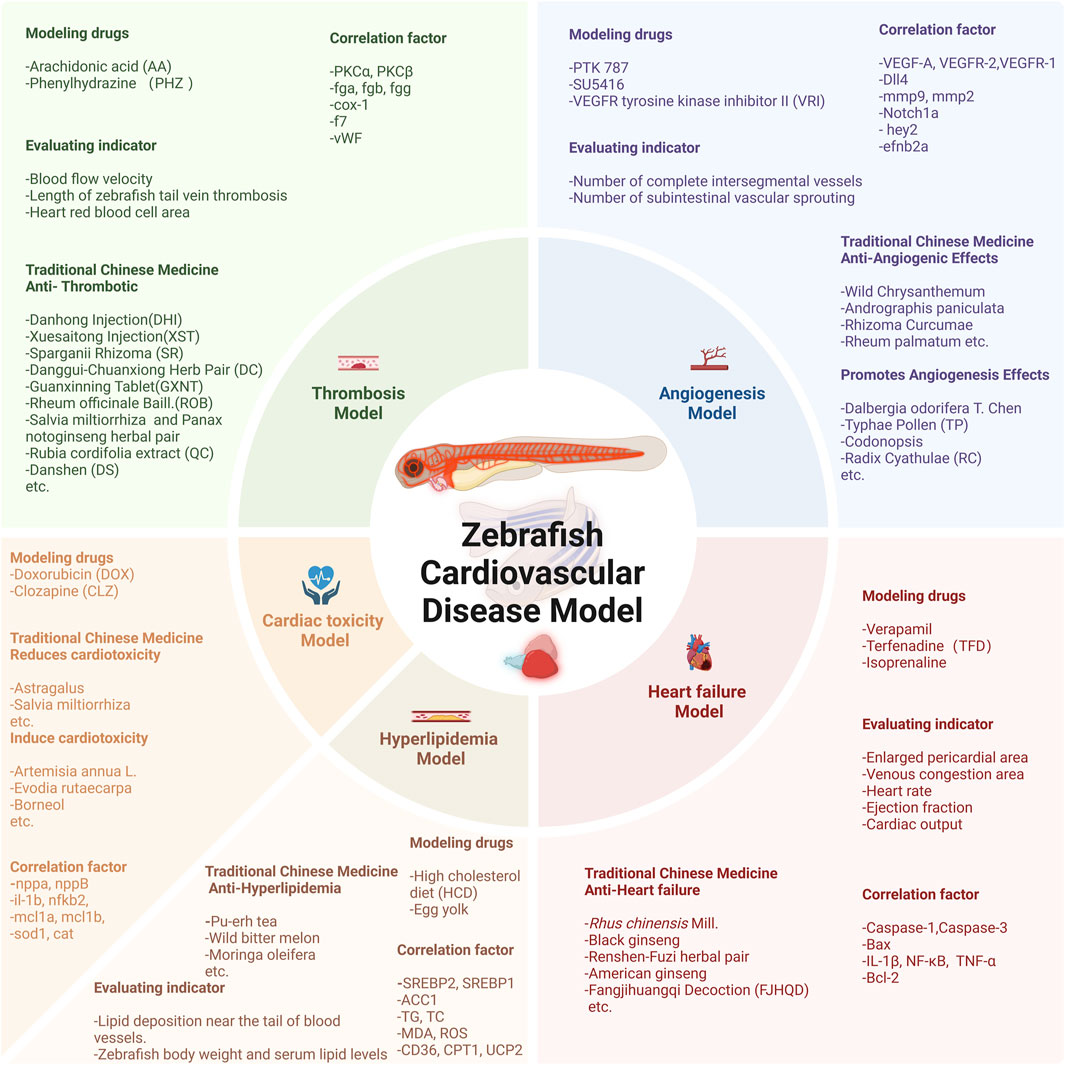
Figure 2. Overview of the zebrafish CVDs model and the natural products with therapeutic effects. This figure was originally created by the author using BioRender (https://biorender.com) and does not reproduce any published material.
2 Evaluation of natural product bioactivities in various zebrafish CVDs models
2.1 Thrombosis model
Thrombotic disorders impact human health by affecting tissues and organs. Disrupted downstream circulation due to embolism and thrombosis can lead to ischemia and necrosis, contributing to numerous significant cardiovascular and cerebrovascular diseases (Zhang et al., 2020). The coagulation cascade promotes fibrinogenesis through the sequential activation of coagulation components. The fibrinogen α chain gene (FGA) is involved in fibrin formation, coagulation factor VII (F7) mediates the extrinsic coagulation pathway, and cyclooxygenase 1 (COX1) catalyzes the conversion of arachidonic acid into thromboxane A2, which activates platelets and promotes thrombosis. These genes and their products play critical roles in disease progression (Ishikawa et al., 2007; Hellmann et al., 2015). The zebrafish model shares similar hemostatic and thrombotic mechanisms with mammals, as it contains the same key proteins involved in platelet adhesion, activation, aggregation, and release. Moreover, zebrafish coagulation factors and platelet receptors respond to clinically used antiplatelet and anticoagulant drugs (Weyand and Shavit, 2014). Thus, zebrafish models can be effectively used to study thrombus formation in CVDs.
The developmental stage of zebrafish larvae features a closed circulatory system, comprising the heart, dorsal aorta (DA), dorsal longitudinal anastomotic vessel (DLAV), and caudal vein (CV), which together form a complete blood circulation circuit (Figure 3). Thrombosis in the zebrafish tail vein reduces the number of cardiac red blood cells (RBCs). Most studies utilize o-dianisidine to stain wild-type AB zebrafish. Tail vein thrombosis in zebrafish is inversely correlated with cardiac RBCs intensity, with the length and area of the thrombus used to assess its severity (Xu et al., 2021). The model is considered successful when image analysis and statistical data show a reduction in RBCs following medication intervention. Some studies have employed transgenic zebrafish strains, such as Tg (LCR-GFP), to observe blood flow and blood flow velocity under a fluorescent microscope, using these metrics to evaluate thrombosis severity (Qi et al., 2017; Li J. et al., 2020).
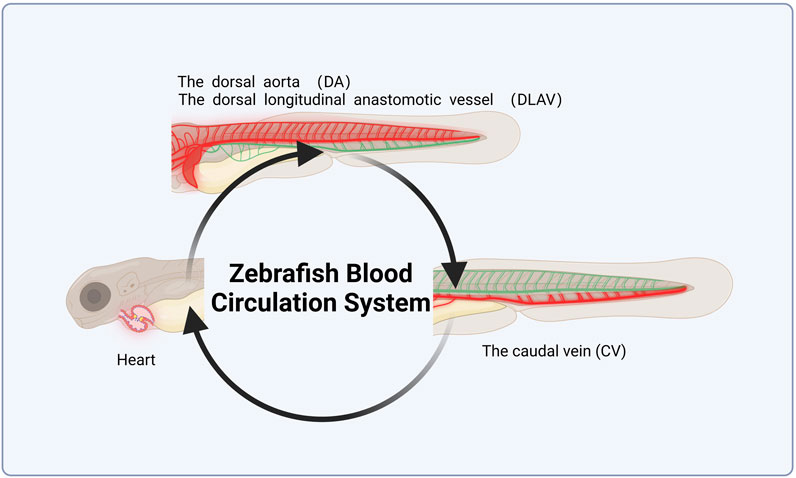
Figure 3. Schematic diagram of zebrafish blood circulation system. This figure was originally created by the author using BioRender (https://biorender.com) and does not reproduce any published material.
Arachidonic acid (AA) and phenylhydrazine (PHZ) are commonly used to model thrombosis in zebrafish. AA activates platelets through the generation of TXA2, which binds to fibrinogen via GPIIb/IIIa receptors, promoting platelet aggregation and thrombosis in zebrafish (Warner et al., 2011). Table 1 summarizes the natural products with antithrombotic activity studied in the AA-induced zebrafish model. For instance, salvianolic acid B, danshensu, lithospermic acid, rosmarinic acid, P-coumaric acid, and hydroxysafflor yellow A—key active metabolites in danhong Injection, a traditional Chinese medicine—alleviate blood flow obstruction in the zebrafish tail vein and restore heart RBCs count (Qi et al., 2017). Furthermore, the primary bioactive metabolites of Xuesaitong Injection, including notoginsenoside R1, ginsenosides Rg1, Rb1, and Rd, downregulate coagulation-related genes such as fga and ptgs2b, reducing inflammation. This approach prevents thrombus formation by inhibiting platelet aggregation, restoring cardiac RBCs density, and reducing abnormal erythrocyte accumulation in the caudal vein (Ma et al., 2021). Additionally, Danggui-Chuanxiong Herb Pair (DC), comprising Angelica sinensis (Oliv.) Diels [Apiaceae; Angelicae sinensis radix] and Ligusticum chuanxiong Hort. [Apiaceae; Ligustici Chuanxiong rhizoma], enhances myocardial erythrocyte density and suppresses AA-induced thrombosis (Pang et al., 2024). A network pharmacology study identified 24 DC-derived antithrombotic compounds and 89 targets, mainly involved in inflammatory pathways, angiogenesis and hormone regulation (Zhang et al., 2021). Moreover, ferulic acid, protocatechuic acid, and P-coumaric acid derived from the ethanol extract of Sparganium stoloniferum Buch.-Ham. ex Juzepczuk [Sparganiaceae; Sparganii Rhizoma] have been shown to promote cardiac erythrocyte recovery in a dose-dependent manner (Xu et al., 2021).
On the other hand, PHZ increases thrombin production, exonerates phosphatidylserine in the RBCs membrane, and produces superoxide radicals, which oxidize thrombin. PHZ also induces inflammation, causes endothelial dysfunction, and promotes a state of hypercoagulation, which ultimately leads to thrombosis (Zhu et al., 2016). Pharmacological therapies targeting these mechanisms have been explored. A PHZ-induced zebrafish thrombosis model demonstrated the synergistic antithrombotic effects of cryptotanshinone and senkyunolide I (Li J. et al., 2020). Cryptotanshinone inhibits cox1 and TNF-α, while ligustrinone I targets f7 and fibrinogen β chain (fgb) expression in the extrinsic coagulation pathway. These compounds collectively enhance RBCs and platelet circulation, reduce peripheral blood flow restriction, and modulate endogenous platelet activation and coagulation cascades. Furthermore, the TCM formula Dang-Gui-Si-Ni Decoction can regulate a variety of coagulation factors and has anticoagulant effects. It increases the cardiac output and blood flow rate of zebrafish, while significantly reducing the expression of coagulation factor II (FII), VII (FVII), IX (FIX) and X (FX) RNA in vivo (Li Y. et al., 2024). When evaluating the yolk sac thrombosis area in zebrafish larvae, the P. notoginseng-Danshen herbal pair (DS-SQ) exhibited the most potent antithrombotic activity (Yin et al., 2020). Several studies have found that compounds such as luteolin-7-O-β-D-glucoside, rosmarinic acid, salvianolic acid B, and lithospermic acid bind strongly to zebrafish thrombosis proteins. These active compounds significantly reduce PHZ-induced tail vein thrombosis and enhance cardiac RBCs levels (Tang et al., 2021). The aqueous extract of Rheum officinale Baill (ROB) mitigates thrombus formation, oxidative stress, and platelet adhesion by upregulating nitric oxide synthase 3 (NOS3) expression and modulating arginine biosynthesis pathways (Zhang Y. R. et al., 2022). Lastly, the extract of Toddalia asiatica (L.) Lam. [Rutaceae; Toddaliae radix] demonstrates notable antithrombotic effects in zebrafish through regulation of the PI3K-Akt-NF-κB signaling pathway (Yang et al., 2024).
While these models excel at high-throughput screening and allow real-time visualization of thrombus formation, the absence of platelets structurally identical to those in mammals—combined with differences in coagulation factor composition—requires cautious interpretation when extrapolating to human thrombotic diseases. We recommend that promising candidates identified in zebrafish should undergo validation in rodent models before clinical consideration.
2.2 Hyperlipidemia model
Hyperlipidemia, a chronic disturbance in lipid metabolism, elevates the risk of atherosclerosis, coronary heart disease, and other CVDs (Bozkurt et al., 2016). It involves the complex regulation of several pathways and key molecules. For example, blood lipid balance is controlled by the activation of the SREBP and PPAR pathways. Acetyl-CoA carboxylase 1 (ACC1) plays a vital role in fatty acid synthesis, which affects plasma lipid levels. Triglycerides (TG) and cholesterol (TC) are the main components of blood lipids and are direct indicators of lipid metabolism. These pathways regulate intracellular lipid synthesis, fatty acid metabolism, cholesterol transport, and inflammation. In addition, lipid metabolism is regulated by factors such as cd36, CPT1, and UCP2, which regulate energy metabolism, fatty acid transport, and β-oxidation.
Zebrafish have similarities with humans in lipid metabolism and have significant homology in apolipoproteins (Otis et al., 2015). High-density lipoprotein (HDL), low-density lipoprotein (LDL), and very low-density lipoprotein (VLDL) have been identified in zebrafish plasma (Stoletov et al., 2009). Therefore, zebrafish is a valuable model for studying the pathophysiology of hyperlipidemia and testing potential therapies. In hyperlipidemia studies using natural products, zebrafish models are usually induced by a high-fat diet (Table 2). For example, feeding 0.1% egg yolk of zebrafish for 48 h induces hyperlipidemia, and treatment with Pu’er tea extract significantly reduces blood lipid levels (Zhou et al., 2015). Another study using a 4% cholesterol diet and red fluorescent lipids (10 μg/g) in 5-day-old Tg (fli1:EGFP) zebrafish demonstrated that Dendrobium huoshanense polysaccharide (DHP) reduced vascular lipid deposition, improved lipid metabolism, and alleviated oxidative stress and inflammation by enhancing SOD activity, inhibiting neutrophil recruitment, and decreasing TC/TG/MDA/ROS levels (Fan X. C. et al., 2020). Additionally, a hyperlipidemia model using ground Artemia nauplii and egg yolk powder confirmed Bergenin’s effectiveness in reducing hepatic and vascular lipid accumulation in high-fat-fed larvae (Zhang et al., 2023).
Due to the successful establishment and reliable drug response of zebrafish hyperlipidemia models, researchers have been able to investigate the lipid-lowering mechanisms of natural substances. Protocatechualdehyde (PCA) and Hydroxysafflor yellow A (HSYA), either alone or in combination, reduce lipid deposition in zebrafish tail veins and alleviate hyperlipidemia-induced liver damage (Lin et al., 2024). Active peptides from E. sinensis Walker (APE) suppress high-fat diet (HFD)-induced lipid accumulation in zebrafish (Dong P. P. et al., 2024). The wild bitter melon (WBM) (Momordica charantia L. var. abbreviata Ser. [Cucurbitaceae; Momordicae charantiae folium]) leaf extract metabolite 3β,7β,25-trihydroxycucurbita-5,23-dien-19-al (TCD) inhibits adipocyte differentiation and hypertrophy, improves hepatocyte morphology, downregulates lipid metabolism genes (CD36, CPT1, UCP2), reduces ACC1 levels, and modulates the SREBP/PPAR pathway, thereby alleviating hepatic lipid accumulation and hyperlipidemia (Wu et al., 2021). Additionally, Moringa oleifera Lam. [Moringaceae; Moringae oleiferae folium] alcoholic extract reduces triglyceride (TG) and cholesterol (TC) levels and decreases Oil Red O staining in hyperlipidemic zebrafish tail vasculature (Yuan et al., 2022). Emodin mitigates fat accumulation in blood vessels and liver, improves liver histology, and inhibits vascular neutrophil inflammation (He et al., 2021). Eleutheroside B, the main active compound of Eleutherococcus senticosus (Rupr. et Maxim.) Maxim.) [Araliaceae; Acanthopanacis senticosi radix et rhizoma], reduces TG, TC, and LDL-C levels, increases HDL-C, and improves glucose and lipid metabolic disorders (Dong X. et al., 2024). Aqueous extracts of Curcuma longa L. [Zingiberaceae; Curcumae longae rhizoma] and Laurus nobilis L. [Lauraceae; Lauri nobilis folium] reduce plasma cholesterol and triglycerides by inhibiting CETP activity and improve obesity, hyperglycemia, and inflammation (Jin et al., 2011). Baicalein decreases lipid accumulation and triglyceride levels in zebrafish by downregulating adipogenic genes such as PPARγ, adipose protein 2 (aP2), and SREBP (Seo et al., 2014). Danggui Shaoyao San reduces hepatic lipid deposition in hyperlipidemic zebrafish, enhances motor activity, and regulates lipid metabolism (Wang et al., 2023). Collectively, these studies demonstrate the lipid-lowering effects of natural products and the widespread application of the zebrafish model in hyperlipidemia drug screening and evaluation. Further details are provided in Table 2. The zebrafish model holds significant value in screening lipid-lowering metabolites. However, differences in lipoprotein metabolism between zebrafish and mammals may affect the translational relevance of lipid metabolism findings. To improve translational value, future studies should complement simple lipid staining with quantitative lipoprotein analysis, plasma and tissue lipidomics, and tracking of triglyceride and cholesterol metabolism. Combining these data with mammalian validation will help better elucidate therapeutic mechanisms and enhance the predictive relevance of zebrafish hyperlipidemia screening.
2.3 Heart failure model
Heart failure (HF) is characterized by cardiac hypertrophy, venous congestion, bradycardia, and reduced cardiac output. Zebrafish HF models are simple, cost-effective, and allow easy monitoring of heart function in embryos (Narumanchi et al., 2021). Zebrafish embryonic heart development begins at 5 hpf, and by 48 hpf, the atrial and ventricular structures are fully formed with a stable heart rate, enabling transparent cardiac visualization under a microscope. HF models can be induced through genetic manipulation or drug treatments (e.g., isoproterenol, aristolochic acid, verapamil) (Dong et al., 2022). After exposure to these modeling agents at 48 hpf, the embryo exhibits morphological changes, such as cardiac hypertrophy and venous congestion, as well as functional changes in heart rate and hemodynamics, which can be observed through a microscope (Singleman and Holtzman, 2012; Zhu et al., 2018). In successfully modeled HF zebrafish, the therapeutic efficacy was evaluated by monitoring morphological improvements and functional changes. The recent investigation of natural products using the zebrafish HF model is summarized in Table 3.
Studies demonstrate that dammarane-type triterpenes isolated from Rhus chinensis Mill. [Anacardiaceae; R. chinensis radix] roots increase cardiac output, blood flow velocity, and reduce pericardial edema and cardiomyocyte apoptosis in zebrafish. Subsequent investigations further isolated other dammarane-type triterpenoids from the same plant source, confirming their cardioprotective effects in zebrafish models (Ye et al., 2020; Ye et al., 2023). Ginsenosides Rg3, Rg5, Rg6, malic acid, quinic acid, and pseudoginsenoside F11 from Panax quinquefolius L. [Araliaceae; Panacis quinquefolii radix] exhibit differential effects on verapamil hydrochloride-induced HF in zebrafish. Specifically, Rg3, Rg6, quinic acid, and pseudoginsenoside F11 relieve pericardial dilation, venous congestion and abnormal SV-BA interval, while Rg5 and malic acid mainly reduce venous congestion (Dong et al., 2022). The cardioprotective effect of Rg5 may be due to its regulation of various metabolic pathways, including arachidonic acid metabolism, D-glutamate metabolism, phenylalanine metabolism, tricarboxylic acid cycle, glycerophospholipid metabolism, purine metabolism, steroid biosynthesis, and linoleic acid metabolism (Liu et al., 2021a). Moreover, a novel glycopeptide (APMCG-1) extracted from mountain-cultivated P. ginseng C.A.Mey. [Araliaceae; Ginseng radix et rhizoma] can increase zebrafish heart rate, restore blood flow velocity, reduce blood glucose and lipid levels, alleviate myocardial injury and oxidative stress, while increasing antioxidant enzyme levels, decreasing inflammatory factors, and mitigating HF in type 2 diabetic zebrafish (Li et al., 2025). The zebrafish HF model is also employed for screening Chinese medicine formulations. For example, P. ginseng C.A.Mey. [Araliaceae; Ginseng radix et rhizoma] and Aconitum carmichaelii Debeaux [Ranunculaceae; Aconiti lateralis radix praeparata], in a 1:2 dosage ratio, significantly increase cardiac output and blood flow velocity, while effectively inhibiting pericardial dilatation and venous congestion (Li et al., 2023). This paradigm now extends to a broader range of natural products and TCM formula research (Table 3). Examples include Shen-Yuan-Dan Capsule, Huoxin Pill (Li et al., 2024c), Fangjihuangqi Decoction (Li et al., 2022b), and Naoxintong Decoction (Hu et al., 2021). These formulations have been shown to improve HF by enhancing cardiac function, and their mechanisms of action have been scientifically validated. For instance, Shen-Yuan-Dan Capsule ameliorates verapamil-induced cardiac dysfunction by modulating oxidative stress and inflammatory markers such as MDA, ROS, SOD, Caspase-1, Bax, and IL-1β (Li et al., 2021).
Current zebrafish heart failure research relies largely on verapamil-induced models, which are convenient, rapid, and reproducible. However, this model mainly reflects calcium channel blockade and does not capture the multifactorial causes of human heart failure, limiting its translational relevance. Future studies should use diverse models, such as genetic cardiomyopathy models and ischemia-reperfusion injury (Zou et al., 2019), to better mimic human disease and provide a more comprehensive evaluation of therapeutics.
2.4 Angiogenesis model
Angiogenesis involves the growth and formation of new vascular networks from pre-existing vessels, regulated by VEGF/VEGFR signaling (Ramjiawan et al., 2017), and includes processes such as sprouting, endothelial cell migration, proliferation, and lumen formation. Zebrafish have become a widely used model for vascular research due to their genetic similarity to humans and embryonic transparency (Hung et al., 2012). Commonly used transgenic strains, such as Tg (fli1a:EGFP) and Tg (Flk1:GFP) label vascular endothelial cells with green fluorescent protein, enabling real-time visualization of vascular dynamics. Pharmaceutical regulatory effects on zebrafish vasculature are assessed using subintestinal vessels (SIVs) sprouting assays and intersegmental vessels (ISVs) damage tests (Fan et al., 2017). Vascular injury models are induced using compounds such as SU5402 (FGFR antagonist), PTK787 (VEGFR2 inhibitor), and vascular endothelial growth factor (VEGF) receptor tyrosine kinase inhibitor II (VRI) (Tal et al., 2014; Zhang et al., 2014). In zebrafish embryonic development, angiogenesis begins 20 hpf (Hong et al., 2008) with its activity peaking between 24 and 72 hpf. Angiogenesis can be assessed by counting fluorescently tagged ISVs (Camus et al., 2012).
First, in TCM, botanicals and natural substances promote zebrafish SIVs production, correct ISVs deficits and other phenotypic improvements, and modulate angiogenesis-related targets or pathways to treat angiogenic diseases (Li et al., 2022a). The main process involves VEGF, PI3K-Akt, and MAPK. As shown in Table 4, Dalbergia odorifera T.C.Chen [Fabaceae; Dalbergiae odoriferae lignum] extract upregulates vascular endothelial growth factor receptors (VEGFRs, including kdr, kdrl, and flt-1) to repair VRI-induced ISVs damage in zebrafish (Fan et al., 2017). Litospermic acid, the active metabolite of S. miltiorrhiza Bunge [Lamiaceae; Salviae miltiorrhizae radix et rhizoma] Bge (Liang et al., 2024), and notoginseng saponins (Hong et al., 2008), promote angiogenesis by regulating VEGF-KDR/Flk-1 and VEGF, PI3K-Akt signaling pathways, respectively. Notably, different Chinese botanical medicine ingredients have diverse characteristics. For example, Ilexsaponin A1, the main ingredient of Ilex pubescens Hook. and Arn. [Aquifoliaceae; I. pubescens radix], modulates Akt/mTOR, MAPK/ERK and Src-FAK signaling pathways to reduce VRI-induced vascular dysfunction (Li J. J. et al., 2017), while Perilla frutescens (L.) Britton [Lamiaceae; Perillae frutescentis folium] volatile oil and perillaldehyde activate the p-ERK1/2/ERK1/2 pathway and upregulate the Bcl-2/Bax ratio to increase angiogenesis in a sunitinib-induced zebrafish vascular damage model (Zhou et al., 2021). Many studies have revealed that Gastrodia elata Blume [Orchidaceae; Gastrodiae rhizoma] and its polysaccharide and non-polysaccharide metabolites induce angiogenesis in a dose-dependent manner (Liu et al., 2020). Achyranthes bidentata Blume [Amaranthaceae; Achyranthis bidentatae radix] and Cyathula officinalis K.C.Kuan [Amaranthaceae; Cyathulae radix] extracts increase endothelial cell migration and SIVs sprouting (Zhou et al., 2017), while Carthamus tinctorius L. [Asteraceae; Carthami tinctorii flos] (Honghua) extracts exert pro-angiogenic effects via upregulating vascular development-associated genes like IGF1, NRP2, and VEGFR3 (Zhou et al., 2014). Aucubin from Eucommia ulmoides Oliv. [Eucommiaceae; Eucommiae cortex] can alleviate VRI-induced suppression of vascular-related genes (flt-1, kdrl, ang-1/2, etc.) in zebrafish embryos and restore ISVs integrity (He et al., 2023). Additionally, other natural products, including ferulic acid, curculigoside, deoxycholic acid, ursodeoxycholic acid, 1-beta-hydroxyalantolactone, and cinobufotalin, have also been shown to promote angiogenesis (Zhu et al., 2023).
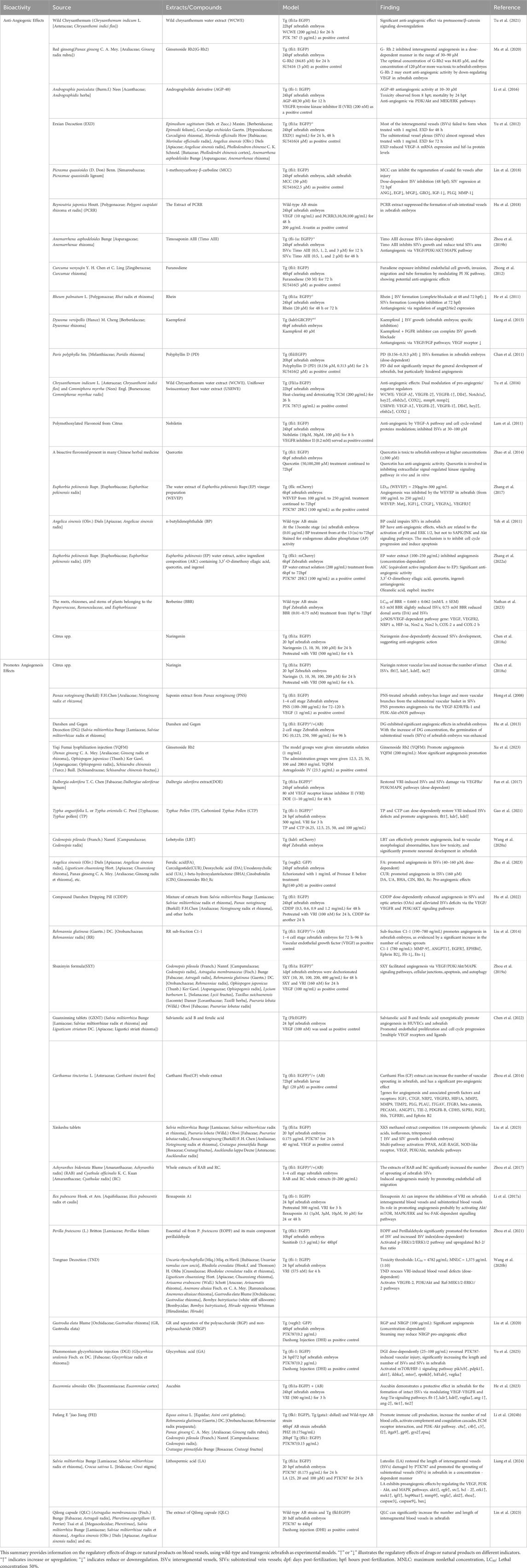
Table 4. Evaluation of natural products for angiogenic and anti-angiogenic effects using zebrafish models.
On the other hand, the equilibrium between anti-angiogenic and pro-angiogenic processes maintains physiological homeostasis (Sajib et al., 2018). Tumor malignancy is closely associated with pathological angiogenesis (Kondo et al., 2002; Mantovani, 2010), which makes angiogenesis inhibition a critical target for cancer therapy and anti-tumor drug development (Viallard and Larrivée, 2017). In addition to promoting angiogenesis, certain natural products exhibit anti-angiogenic effects. For instance, Polygonum cuspidatum Siebold and Zucc. [Polygonaceae; Polygoni cuspidati rhizoma et radix] extract inhibits angiogenesis by suppressing VEGF-induced endothelial cell migration and proliferation (Hu et al., 2018). Timosaponin AIII, isolated from Anemarrhena asphodeloides Bunge [Asparagaceae; Anemarrhenae rhizoma], suppresses angiogenesis by directly inhibiting the VEGF/PI3K/AKT/MAPK signaling cascade (Zhou et al., 2019b). Moreover, the andrographolide derivative AGP-40 disrupts vascularization by inhibiting PI3K/Akt and MEK/ERK pathways (Li et al., 2016), while furanodiene derived from C. longa L. [Zingiberaceae; Curcumae longae rhizoma] reduces endothelial cell growth, invasion, and angiogenesis via modulation of the PI3K pathway (Zhong et al., 2012). Chrysanthemum indicum L. [Asteraceae; Chrysanthemi indici flos] and Commiphora myrrha (Nees) Engl. [Burseraceae; Commiphorae myrrhae radix] inhibit angiogenesis by differentially regulating VEGFRs, the Notch pathway, Dll4, hey2, and matrix-degrading enzymes (MMP2/9, COX2) in zebrafish (Tu et al., 2016; Tu et al., 2021). Additionally, 1-methoxycarbony-β-carboline from Picrasma quassioides (D.Don) Benn. [Simaroubaceae; Picrasmae radix] suppresses angiogenesis by downregulating autocrine proteins such as ANG and bFGF (Lin et al., 2018). Furthermore, Dysosma versipellis (Hance) M. Cheng [Berberidaceae; Dysosmae rhizoma] (Liang et al., 2015), polyphyllin D (Chan et al., 2011), and nobiletin (Lam et al., 2011) inhibit angiogenesis by blocking VEGF/FGF signaling or endothelial differentiation. Notably, nobiletin prevents new vessel formation without affecting existing vasculature. Other compounds, including rhein, quercetin, berberine, and active constituents of Euphorbia pekinensis Rupr. [Euphorbiaceae; Euphorbiae pekinensis radix] (e.g., 3,3′-O-dimethoxy ellagic acid), also inhibit angiogenesis through Src/FAK pathway modulation, highlighting the multi-target advantages of natural products in controlling abnormal vascular proliferation (He et al., 2011; Zhao et al., 2014; Zhang W. T. et al., 2022; Nathan et al., 2023).
Some Chinese medicines exhibit bidirectional vascular regulation through distinct active ingredients. Panax ginseng C.A.Mey. [Araliaceae; Ginseng Radix et Rhizoma] is an example, as its numerous ginsenosides have opposing effects on angiogenesis (Sengupta et al., 2004). Specifically, Rg1, Rb3, and Rc show pro-angiogenic activity in zebrafish models (Leung et al., 2006; Zhu et al., 2023), while Rb1 and Rg3 demonstrate anti-angiogenic activity (Yue et al., 2006; Leung et al., 2007). Processed red ginseng-derived ginsenoside Rh2 (G-Rh2) dose-dependently inhibits intersegmental angiogenesis at concentrations ranging from 30 to 90 μM (Ma et al., 2020). These opposite effects may be associated with structural differences in the sugar chains and hydroxyl substituents among various ginsenosides. In addition, changes in drug dosage may influence their pro-angiogenic and anti-angiogenic activities, exhibiting a characteristic dose-dependent bidirectional regulatory effect (Sengupta et al., 2004). Similarly, A. sinensis (Oliv.) Diels [Apiaceae; Angelicae sinensis radix]’s volatile component, n-butylidenephthalide, suppresses angiogenesis, contrasting with the pro-angiogenic effects of its aqueous extract (Yeh et al., 2011). These discrepancies highlight the importance of distinguishing extraction methods and compound polarity when interpreting opposite pharmacological outcomes. Naringin from citrus fruits restores vascular loss in zebrafish, whereas its aglycone, naringenin, exhibits anti-angiogenic potential (Chen L. et al., 2018), further illustrating that subtle structural variations can reverse angiogenic activity among natural products.
Alongside the aforementioned individual medicinal components, Chinese herbal compound prescriptions and Chinese patent medicines also exert regulatory influence on angiogenesis. Erxian Decoction inhibits angiogenesis in zebrafish by downregulating VEGF-A and HIF-1α (Yu et al., 2012). Tongnao Decoction activates the vascular endothelial growth factor receptor-2 (VEGFR-2), phosphoinositide 3-kinase (PI3K), protein kinase B (Akt), and Raf-MEK1/2-ERK1/2 signaling pathways to repair VRI-induced damage to zebrafish ISVs, SIVs, and central arteries (CtAs) (Wang J. L. et al., 2020). Both Compound Danshen Dripping Pill (Hu et al., 2022) and Shuxinyin Formula (Zhou et al., 2019a) regulate the VEGF/PI3K/Akt/MAPK signaling pathway to enhance angiogenic potential. Additionally, the Shuxinyin Formula is also involved in the regulation of cell junctions and autophagy. Xinkeshu tablets integrate multiple pathways, including PPAR, AGE-RAGE, and VEGF, to promote angiogenesis (Liu et al., 2023). High dosages of Danshen Gegen decoction increased SIVs germination (Hu et al., 2013). Among licorice-derived preparations (such as magnesium isoglycyrrhizic acid injection (MII), diammonium glycyrrhizic acid injection (DGI), compound glycyrrhizic acid tablets (CGT)), DGI exhibits the strongest activity and can increase the length and number of zebrafish ISVs and SIVs in a concentration-dependent manner (Yu et al., 2025). Furthermore, the synergistic effects of salvianolic acid B and ferulic acid in GXNT tablets promote angiogenesis by upregulating VEGF receptor expression (Chen et al., 2022), while the active constituents of Yiqi Fumai Lyophilization Injection (YQFM) outperform ginsenoside Rb2 (Xu et al., 2023) (Other similar functional research is listed in Table 4)
Zebrafish have unique advantages in studying both pro-angiogenic and anti-angiogenic effects, particularly in investigating these dual effects within the same model system. This capability is of significant importance in cardiovascular disease research, especially in the regulation of angiogenesis, where it holds promise for simultaneously inhibiting abnormal vessel formation while preventing excessive vascular regression. However, many plant metabolites and natural products show biphasic dose-response patterns, which can result in incorrect conclusions if only one concentration is tested (Zhu et al., 2016; Caprifico et al., 2025). Therefore, future studies should carefully examine the dose-response relationships and the timing of angiogenic effects to enhance therapeutic targeting.
2.5 Cardiotoxicity model
Zebrafish embryos have been used to screen a variety of toxicants, particularly for cardiotoxicity testing. Table 5 provides a summary of recent studies that use zebrafish to investigate natural product-mediated or -induced cardiotoxicity.
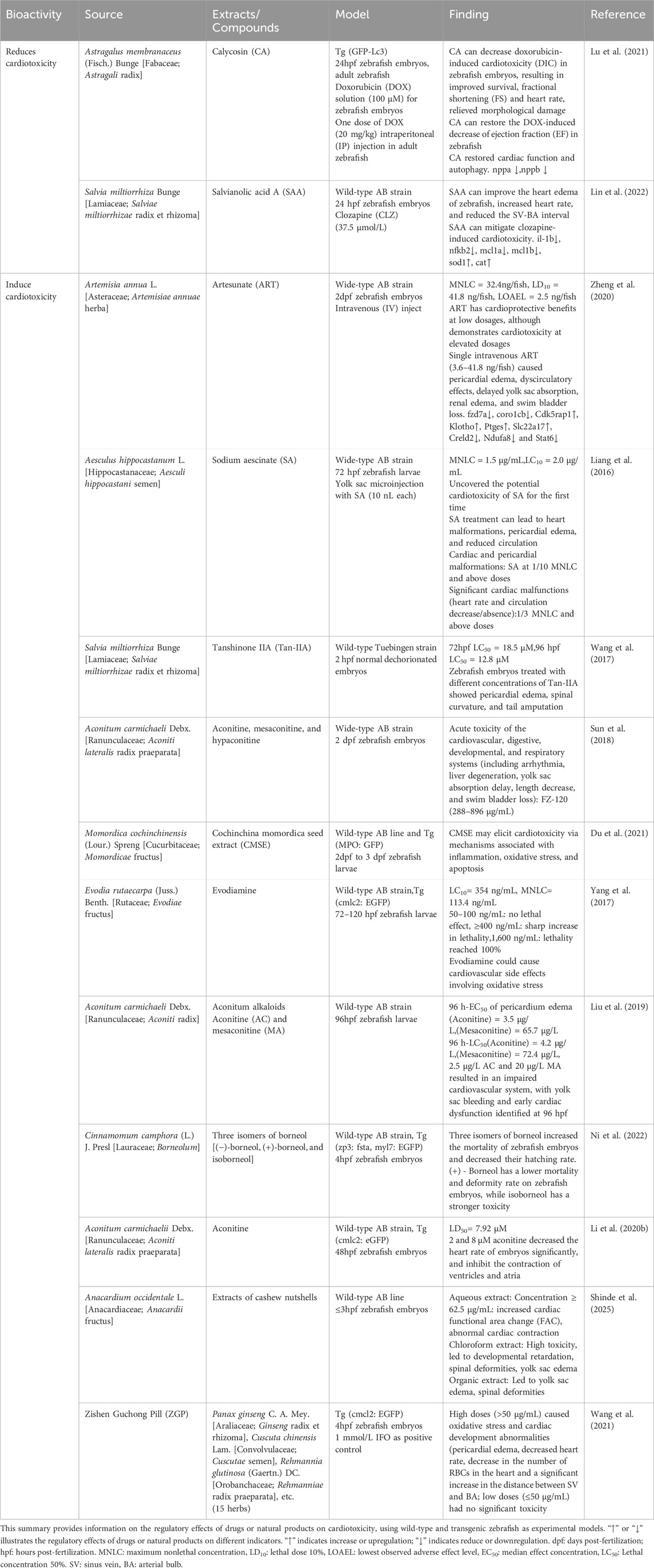
Table 5. Evaluation of natural products for cardiotoxicity and cardioprotective effects using zebrafish models.
A study induced cardiotoxicity in adult zebrafish Tg (GFP-Lc3) by injecting doxorubicin (DOX) and subsequently treated the zebrafish with Astragalus membranaceus (Fisch.) Bunge [Fabaceae; Astragali radix] extract. The results showed that A. membranaceus (Fisch.) Bunge [Fabaceae; Astragali radix] extract significantly reduced heart damage in zebrafish, improved cardiac function, restored autophagy, and significantly decreased the expression of two pathological markers of cardiac remodeling, natriuretic peptide A (nppa) and natriuretic peptide B (nppB) (Lu et al., 2021). Additionally, salvianolic acid A can counteract clozapine-induced cardiotoxicity, improve zebrafish cardiac edema, increase heart rate, and shorten the SV-BA interval. Salvianolic acid A also downregulates inflammatory response-related genes IL-1b, NFKB2, mCl1a, and mCl1b, and upregulates the expression of SOD1 and CAT (Lin et al., 2022).
In addition to the traditional Chinese medicines mentioned above that can improve cardiotoxicity, numerous studies have shown that some natural products can also induce cardiotoxicity. For instance, experiments involving the microinjection of sodium aescinate (SA) extracted from Aesculus hippocastanum L. [Sapindaceae; Aesculi hippocastani semen] into zebrafish yolk sacs have demonstrated that SA treatment can lead to cardiac malformations, pericardial edema, and reduced blood circulation (Liang et al., 2016). Next, zebrafish embryos treated with different concentrations of Tan-IIA exhibited pericardial edema (Wang et al., 2017). In addition, the component FZ-120 of A. carmichaelii Debeaux [Ranunculaceae; Aconiti lateralis radix praeparata] radix could induce arrhythmias in zebrafish at a dose range of 288–896 μg/mL (Sun et al., 2018). Components of A. carmichaelii Debx. [Ranunculaceae; Aconiti lateralis radix praeparata], such as aconitine and meaconitine, may cause defects in the cardiovascular system and significantly reduce the heart rate of zebrafish embryos (Liu et al., 2019; Li M. T. et al., 2020). Moreover, Momordica cochinchinensis (Lour.) Spreng. [Cucurbitaceae; Momordicae cochinchinensis semen] extract may induce cardiotoxicity by triggering apoptosis, oxidative stress, and inflammatory pathways. Evodiamine may result in oxidative stress-related cardiovascular adverse effects (Yang et al., 2017). High concentrations of cashew nut shell aqueous extract induce cardiac contractile dysfunction in zebrafish embryos. The TCM formulation Zishen Guchong Pill exhibits considerable harmful effects on the heart development of zebrafish at elevated concentrations, likely associated with the activation of oxidative stress and apoptotic signaling pathw ays triggered by Zishen Guchong Pill (Wang et al., 2021). Furthermore, artesunate (ART), the active substance in Artemisia annua L. [Asteraceae; Artemisiae annuae herba], has different effects on cardiotoxicity at different doses. Research indicates that when ART is intravenously injected into 2dpf zebrafish larvae, it exhibits cardiotoxicity at high doses but has a cardioprotective effect at low concentrations. This bidirectional regulatory effect may be closely related to the different expression of genes such as Cdk5rap1, Klotho, Ptges, Slc22a17, Creld 2, Ndufa 8 and Stat6 under ART regulation (Zheng et al., 2020).
Zebrafish are an excellent tool for cardiotoxicity screening, capable of rapidly assessing heart rate, contractility, and structural abnormalities. However, their remarkable cardiac regenerative capacity may mask chronic toxicity effects that would emerge in mammals with limited regenerative potential (González-Rosa et al., 2017). Therefore, compounds exhibiting promising or unclear cardiotoxicity profiles in zebrafish should be further evaluated in mammalian models to ensure accurate risk assessment and safety, providing more reliable data for clinical applications.
3 Discussion and perspective
Cardiovascular disease has long been a major public health concern, posing a serious threat to human health. Its origins can be traced back to ancient medical practices. Heart-related illnesses are documented in the medical texts of many ancient civilizations. For example, the concepts of the heart and blood vessels appear in ancient Egyptian and Greek medicine (Loukas et al., 2016). Additionally, traditional Chinese medical classics, such as the Huangdi Neijing (Yellow Emperor’s Inner Canon), describe symptoms like “palpitations” and “chest impediment,” attributing their causes primarily to visceral dysfunction and disturbances in the flow of Qi and blood. Therefore, in traditional therapies, the primary function of natural products in improving CVDs is to promote blood circulation and remove blood stasis. Furthermore, the function of the heart is closely linked to the circulation of Qi and blood in the body, making Qi-tonifying Chinese medicines commonly used in the treatment of CVDs (Li D. X. et al., 2024; Si and Yu, 2024). Here, “Qi” can be understood as a vital life energy or vitality that helps the body’s various parts function properly. Additionally, traditional medicines are used to eliminate excess heat and toxins from the body. These medicines are commonly applied to treat cardiovascular issues caused by inflammation, internal heat, or harmful substances (Wang et al., 2022). In modern pharmacology, these natural products also demonstrate effects in regulating blood lipids and lowering blood pressure (Li Y. P. et al., 2017).
The application of natural products in the treatment of CVDs has been confirmed by modern pharmacological studies. For example, S. miltiorrhiza Bunge [Lamiaceae; Salviae miltiorrhizae radix et rhizoma] and P. notoginseng (Burkill) F.H.Chen [Araliaceae; Notoginseng radix et rhizoma] are commonly used to promote blood circulation and prevent thrombosis. Studies have shown that their active ingredients, such as tanshinone, salvianolic acid B, notoginsenoside R1, Rg1, and Rb1, exert multi-target protective effects. These compounds improve blood circulation, exhibit anti-thrombotic activity, and demonstrate therapeutic effects on conditions such as HF and other CVDs (Yin et al., 2020; Ma et al., 2021). As a representative herbal medicine for nourishing qi and nourishing the heart, ginseng plays its role by enhancing heart function and restoring the flow of qi in the body. Modern studies have found that ginsenosides can improve cardiac blood flow and heart rate, and when used in combination with Aconitum carmichaeli Debx. [Ranunculaceae; Aconiti lateralis radix praeparata], they enhance cardiac output (Li et al., 2025). Scutellaria baicalensis Georgi [Lamiaceae; Scutellariae radix] and C. longa L. [Zingiberaceae; Curcumae longae rhizoma] are widely used in the treatment of CVDs due to their heat-clearing, detoxifying, lipid-lowering, and anti-inflammatory effects (Jin et al., 2011; Seo et al., 2014; Zhang et al., 2025). In addition, Senna tora (L.) Roxb. [Fabaceae; Semen cassiae torae] (Juemingzi), traditionally known for its heat-clearing and detoxifying properties, has also shown significant lipid-lowering effects in zebrafish studies, particularly in reducing plasma cholesterol levels (Zhou C. et al., 2024). By combining traditional medicine with modern pharmacological research, the zebrafish model has become an effective tool to validate the therapeutic mechanisms of natural products, further advancing the modernization of traditional Chinese medicine in the treatment of CVDs.
The analysis of various natural products reveals that they have a certain inherent consistency in the regulation of cardiovascular function. Although these active compounds are derived from different plant sources, they often act in similar ways to exert cardiovascular benefits. Studies have shown that many natural compounds regulate common key signaling pathways—especially by activating the VEGF/PI3K/Akt pathway, reducing oxidative stress and inhibiting inflammation-thereby promoting blood vessel repair, improving endothelial function and enhancing cardiomyocyte survival (Liu et al., 2021; Zhong et al., 2024). This convergence of mechanisms reflects the coordinated regulation of the signal network by natural products at the system level.
Based on the above understanding, this article reviews the combination of zebrafish cardiovascular disease model and natural product research, focusing on thrombosis, angiogenesis, HF, hyperlipidemia and cardiotoxicity and other conditions. In thrombosis studies (Table 1), most experiments use arachidonic acid-induced models, and the concentration of natural products is usually 1–100 µg/mL. It is worth noting that TCM formulas usually show pharmacological activity at higher concentrations than a single compound, which indicates that there may be synergistic effects between multiple ingredients. In hyperlipidemia studies (Table 2), models rich in cholesterol and egg yolk feeding are the most common. Natural products generally reduce cholesterol and triglyceride levels by about 30%–50%, which is confirmed by oily red O staining of vascular lipid deposits. Comparative analyses identified several multitarget compounds—such as salvianolic acid B and ginsenosides—with antithrombotic, cardioprotective, and proangiogenic effects (Qi et al., 2017; Chen et al., 2022; Ma et al., 2021; Dong et al., 2022), indicating that single-pathway evaluations may underestimate their therapeutic potential. Methodologically, most studies relied heavily on larvae 48–72 h post-fertilization, suggesting future work could explore adult zebrafish models or different developmental stages.
Compared to traditional animal models, zebrafish offer advantages such as high reproductive capacity, low cost, transparent embryos, and ease of real-time imaging, making them ideal for high-throughput drug screening and genetic research. With their genome highly homologous to that of humans, combined with advanced gene-editing techniques, zebrafish provide unique conditions for functional gene validation and the study of drug mechanisms. From an ethical perspective, zebrafish are increasingly preferred over rodent models because of their reduced sentience, simpler nervous system, and lower ethical burden in experimental use. Their use also aligns with the 3Rs principles (Replacement, Reduction, and Refinement), promoting more ethical and efficient research. These advantages make zebrafish an attractive option for early-stage pharmacological studies, particularly in natural product screening (Cassar et al., 2020). However, zebrafish have limitations for clinical translation: their heart has only one atrium and ventricle, lacking mammalian pulmonary circulation, and drugs are absorbed passively through water, unlike human administration. Zebrafish have only one atrium and one ventricle, which changes the pressure and flow pattern of the blood, resulting in the mixing of oxygenated and deoxygenated blood. Despite this, zebrafish and mammals still have high similarities in cardiac contraction, electrical conduction, and many basic signaling pathways (Genge et al., 2016; Nguyen et al., 2008). Because zebrafish do not have pulmonary circulation, they cannot simulate diseases such as pulmonary hypertension or right heart failure, which are important parts of human cardiovascular disease. Moreover, we emphasize that zebrafish are not a simple replacement for mammalian models, but rather a research system with complementary advantages, playing a unique role in high-throughput screening, real-time imaging, and complex natural product studies. However, mammalian models are closer to humans in terms of anatomy, physiology, and pharmacokinetics (Chahardehi et al., 2020). Therefore, the zebrafish model is more suitable for early-stage drug efficacy screening and mechanism studies, while preclinical validation still requires the use of mammalian models. Combining these two approaches enables more comprehensive and efficient cardiovascular research.
Zebrafish cardiovascular studies show notable methodological differences, particularly in the choice of strain, dosing strategies, and measurement approaches. The AB and TU wild-type strains are most commonly used for general toxicity and efficacy testing (van de Venter et al., 2020), while transgenic lines such as Tg (fli1:EGFP) and Tg (myl7:DsRed) provide valuable imaging advantages (Choe et al., 2021). However, these transgenic models may display physiological differences from wild-type fish, making it important for researchers to clearly report strain-specific details. Inconsistent dose selection, often without pharmacokinetic justification or complete dose–response analysis, remains a key limitation (Cortemeglia and Beitinger, 2005; Holden and Brown, 2018). Future research should aim to quantify exposure levels, ensure chemical stability, and conduct negative controls. Differences in endpoints such as red blood cell counts, pericardial area, and blood flow velocity further impact comparability. Moreover, reproducibility can be affected by environmental and biological factors, including temperature, circadian rhythm, developmental stage, and clutch-to-clutch variability. Addressing these issues through standardized experimental protocols and transparent reporting will greatly enhance the reliability and translational relevance of zebrafish-based cardiovascular research.
It is worth noting that the excellent heart regeneration ability of zebrafish represents an innovative tool for natural product research. However, there are still challenges in TCM research, especially the underutilization of this regenerative ability. As an ideal model for heart regeneration research, zebrafish has developed a series of advanced technologies that provide strong support for mechanical analysis. For example, tools such as gene editing enabling precise knockout or overexpression of regeneration-related genes), optogenetics (which regulates cardiomyocyte proliferation) (Baillie et al., 2021), and single-cell sequencing (enabling the analysis of regenerative responses in cardiomyocyte subsets) are instrumental in supporting mechanistic studies (Luz et al., 2025). Despite the establishment of cardiac regeneration models in developmental biology for drug screening, literature over the past decade shows minimal application of these models in TCM or natural product research. While modern medicine uses zebrafish models to elucidate key pathways (e.g., the VEGFC-EMILIN2A-CXCL8A axis) (El-Sammak et al., 2022), TCM research still relies on traditional indicators, such as heart rate and pericardial edema, failing to fully leverage advanced technologies to uncover the mechanisms of natural products.
In addition, the application of the zebrafish model has extended to multiple fields, including inflammation, injury repair, and regeneration. For example, in the Dextran sulfate sodium salt (DSS)-induced zebrafish inflammatory bowel disease (IBD) model, Chrysanthemum morifolium Ramat. [Asteraceae; Chrysanthemi caulis et folium] extract exerts anti-inflammatory and antioxidant effects by inhibiting inflammatory factors such as IL-1. In a spinal cord injury model, feeding fish food containing fig extract accelerates swimming function recovery and promotes spinal cord regeneration (Motohashi et al., 2025). In the caudal fin regeneration model, Rehmannia glutinosa (Gaertn.) DC. [Orobanchaceae; Rehmanniae radix praeparata] extract significantly promotes tail fin regeneration (Chen et al., 2024). These studies have shown that zebrafish not only has important value in cardiovascular pharmacology, but also provides strong support for the application of natural products in other disease fields. Since natural product extracts often exhibit overall regulatory effects, it is difficult to fully reflect their pharmacological activity with a single indicator. Integrating multiple omics techniques, such as transcriptomics and metabolomics, can help identify key regulatory genes. For example, combined transcriptomic-metabolomic analysis has demonstrated how Achillea alpina L. [Asteraceae; Achilleae herba] essential oil modulates amino acid metabolism and inflammatory pathways to exert anti-inflammatory effects (Cheng et al., 2025). This approach can advance cardiovascular research by enabling a systematic analysis of the multi-target molecular networks in TCM and natural products, revealing their connections to key nodes in cardiovascular pathophysiology. Integrating zebrafish’s heart regeneration abilities with natural product research can uncover potential molecular targets in TCM formulas or compounds and deepen the understanding of their therapeutic effects. With the development of multi-omics and real-time imaging technologies, zebrafish are poised to become a key platform for cardiac regeneration research in TCM, aiding in the discovery of new therapeutic strategies and drug candidates. Future studies should combine multi-omics data with zebrafish high-throughput screening to create a “component-target-pathway” framework and explore natural products’ unique regulatory mechanisms in heart regeneration.
In short, natural products have shown strong potential in the treatment of CVDs, including managing blood lipids, promoting angiogenesis, and fighting atherosclerosis and HF, usually through multi-target mechanisms. The zebrafish model has the advantages of high-throughput screening and similar to human disease pathways. It is a key tool for exploring its efficacy and mechanism, and provides new insights for the prevention, treatment and drug development of CVDs.
Author contributions
YiW: Data curation, Formal Analysis, Investigation, Visualization, Writing – original draft. XM: Data curation, Formal Analysis, Investigation, Writing – review and editing. XS: Data curation, Visualization, Writing – review and editing. LW: Data curation, Formal Analysis, Writing – review and editing. XHM: Funding acquisition, Resources, Writing – review and editing. XD: Writing – review and editing. ZY: Funding acquisition, Writing – review and editing. KT: Writing – review and editing. YuW: Conceptualization, Funding acquisition, Supervision, Writing – review and editing. MH: Conceptualization, Funding acquisition, Supervision, Writing – review and editing. MS: Conceptualization, Funding acquisition, Supervision, Writing – review and editing.
Funding
The authors declare that financial support was received for the research and/or publication of this article. This research was financially supported by the Jilin Provincial Development and Reform Commission (No. 2023C028-1); the Pilotscale Selection Project of Colleges and Universities in Changchun City (No. 24GXYSZZ10); the Ministry of Human Resources and Social Security of the People’s Republic of China High-Level Talent Project (No. 030102070 and 030102071); the Scientific and Technological Developing Project of Jilin Province (No. YDZJ202502CXJD079); and the Biological Breeding-Major Projects (No. 2023ZD04062).
Acknowledgements
The authors thank Zhongfeng Chen, Jianan Wang and Qizhong Lian provided the technical guidance.
Conflict of interest
Author XHM was employed by Wish Technology.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Baillie, J. S., Stoyek, M. R., and Quinn, T. A. (2021). Seeing the light: the use of zebrafish for optogenetic studies of the heart. Front. physiol. 12. doi:10.3389/fphys.2021.748570
Bournele, D., and Beis, D. (2016). Zebrafish models of cardiovascular disease. Heart fail. Rev. 21, 803–813. doi:10.1007/s10741-016-9579-y
Bozkurt, B., Aguilar, D., Deswal, A., Dunbar, S. B., Francis, G. S., Horwich, T., et al. (2016). Contributory risk and management of comorbidities of hypertension, obesity, diabetes mellitus, hyperlipidemia, and metabolic syndrome in chronic heart failure: a scientific statement from the American heart association. Circulation 134 (23), e535–e578. doi:10.1161/cir.0000000000000450
Camacho, P., Fan, H. M., Liu, Z. M., and He, J. Q. (2016). Small Mammalian animal models of heart disease. Am. J. Cardiovasc. Dis. 6, 70–80.
Camus, S., Quevedo, C., Menéndez, S., Paramonov, I., Stouten, P. F., Janssen, R. A., et al. (2012). Identification of phosphorylase kinase as a novel therapeutic target through high-throughput screening for anti-angiogenesis compounds in zebrafish. Oncogene 31 (39), 4333–4342. doi:10.1038/onc.2011.594
Caprifico, A. E., Calabrese, G., Tornese, R., Montefusco, A., Placì, R., Semeraro, T., et al. (2025). Pomegranate extracts as dual regulators of angiogenesis: a systematic review of preclinical evidence in cancer and chronic wound healing. Mol. Nutr. Food Res. 69 (11), e70060. doi:10.1002/mnfr.70060
Cassar, S., Adatto, I., Freeman, J. L., Gamse, J. T., Iturria, I., Lawrence, C., et al. (2020). Use of zebrafish in drug discovery toxicology. Chem. Res. Toxicol. 33 (1), 95–118. doi:10.1021/acs.chemrestox.9b00335
Chahardehi, A. M., Arsad, H., and Lim, V. (2020). Zebrafish as a successful animal model for screening toxicity of medicinal plants. Plants (Basel). 9 (10), 1345. doi:10.3390/plants9101345
Chan, J. Y. W., Koon, J. C. M., Liu, X. Z., Detmar, M., Yu, B., Kong, S. K., et al. (2011). Polyphyllin D, a steroidal saponin from Paris polyphylla, inhibits endothelial cell functions in vitro and angiogenesis in zebrafish embryos in vivo. J. Ethnopharmacol. 137, 64–69. doi:10.1016/j.jep.2011.04.021
Chen, L. M., Yang, B. R., Tang, B. Q., Gong, G. Y., Kam, H. T., Gao, C., et al. (2018a). Differential angiogenic activities of naringin and naringenin in zebrafish in vivo and human umbilical vein endothelial cells in vitro. J. Funct. Foods 49, 369–377. doi:10.1016/j.jff.2018.08.010
Chen, Y., Chen, P. D., Bao, B. H., Shan, M. Q., Zhang, K. C., Cheng, F. F., et al. (2018b). Anti-thrombotic and pro-angiogenic effects of Rubia cordifolia extract in zebrafish. J. Ethnopharmacol. 219, 152–160. doi:10.1016/j.jep.2017.11.005
Chen, J., Wang, Y. C., Wang, S. F., Zhao, X. P., Zhao, L., and Wang, Y. (2022). Salvianolic acid B and ferulic acid synergistically promote angiogenesis in HUVECs and zebrafish via regulating VEGF signaling. J. Ethnopharmacol. 283, 114667. doi:10.1016/j.jep.2021.114667
Chen, F. Y., Pu, S. M., Tian, L., Zhang, H., Zhou, H. X., Yan, Y. J., et al. (2024). Radix rehmanniae praeparata promoted zebrafish fin regeneration through aryl hydrocarbon receptor-dependent autophagy. J. Ethnopharmacol. 331, 118272. doi:10.1016/j.jep.2024.118272
Cheng, J., Fu, Y., Meng, X., Tang, G., Li, L., Yusupov, Z., et al. (2025). Investigation of anti-inflammatory effect of essential oil extracted from achillea alpina L. through multi-omics analysis in zebrafish tail fin amputation model. J. Ethnopharmacol. 344. doi:10.1016/j.jep.2025.119519
Choe, C. P., Choi, S.-Y., Kee, Y., Kim, M. J., Kim, S.-H., Lee, Y., et al. (2021). Transgenic fluorescent zebrafish lines that have revolutionized biomedical research. Laboratory Animal Res. 37 (1), 26. doi:10.1186/s42826-021-00103-2
Cortemeglia, C., and Beitinger, T. L. (2005). Temperature tolerances of wild-type and red transgenic zebra danios. Trans. Am. Fish. Soc. 134 (6), 1431–1437. doi:10.1577/T04-197.1
Dabbaghi, M. M., Roudi, H. S., Safaei, R., Rahimi, V. B., Fadaei, M. R., and Askari, V. R. (2024). Unveiling the mechanism of protective effects of tanshinone as a new fighter against cardiovascular diseases: a systematic review. Cardiovasc. Toxicol. 24, 1467–1509. doi:10.1007/s12012-024-09921-x
Dong, R., Zhang, Y. G., Chen, S. J., Wang, H., Hu, K. Q., Zhao, H. X., et al. (2022). Identification of key pharmacodynamic markers of American ginseng against heart failure based on metabolomics and zebrafish model. Front. Pharmacol. 13, 909084. doi:10.3389/fphar.2022.909084
Dong, P. P., Wang, H., Li, Y. N., Yu, J. Y., Liu, X., Wang, Y. L., et al. (2024a). Active peptides from Eupolyphaga sinensis walker attenuates experimental hyperlipidemia by regulating the gut microbiota and biomarkers in rats with dyslipidemia. Biomed. Pharmacother. 170, 116064. doi:10.1016/j.biopha.2023.116064
Dong, X., Chen, Q., Chi, W., Qiu, Z., and Qiu, Y. (2024b). A metabolomics study of the effects of eleutheroside B on glucose and lipid metabolism in a zebrafish diabetes model. Mol. Basel, Switz. 29 (7), 1545. doi:10.3390/molecules29071545
Du, Z. C., Xia, Z. S., Huang, Y. F., Peng, Y., Cao, B. B., Li, C. Q., et al. (2021). Cardiotoxicity induced by cochinchina momordica seed extract in zebrafish. J. Appl. Toxicol. 41, 1222–1231. doi:10.1002/jat.4108
El-Sammak, H., Yang, B. S., Chen, W., Marín-Juez, R., Stainier, D. Y. R., et al. (2022). A vegfc-emilin2a-cxcl8a signaling axis required for zebrafish cardiac regeneration. Circ. Res. 130 (7), 1014–1029. doi:10.1161/circresaha.121.319929
Fan, Z. M., Wang, D. Y., Yang, J. M., Lin, Z. X., Lin, Y. X., Yang, A. L., et al. (2017). Dalbergia odorifera extract promotes angiogenesis through upregulation of VEGFRs and PI3K/MAPK signaling pathways. J. Ethnopharmacol. 204, 132–141. doi:10.1016/j.jep.2017.04.006
Fan, W. X., Huang, Y. L., Zheng, H., Li, S. Q., Li, Z. H., Yuan, L., et al. (2020). Ginsenosides for the treatment of metabolic syndrome and cardiovascular diseases: pharmacology and mechanisms. Biomed. Pharmacother. 132, 110915. doi:10.1016/j.biopha.2020.110915
Fan, X. C., Han, J. C., Zhu, L. J., Chen, Z. P., Li, J. J., Gu, Y., et al. (2020). Protective Activities of Dendrobium huoshanense C. Z. Tang et S. J. Cheng Polysaccharide against High-Cholesterol Diet-Induced Atherosclerosis in Zebrafish. Oxid. Med. Cell. Longev. 2020, 8365056–10. doi:10.1155/2020/8365056
Feng, J. L., Chen, X., Wang, S. T., Zhang, J., Wang, Q. C., Guo, S. Y., et al. (2023). Transcriptomics integrated with metabolomics reveals the ameliorating effect of mussel-derived plasmalogens on high-fat diet-induced hyperlipidemia in zebrafish. Food Funct. 14, 3641–3658. doi:10.1039/d3fo00063j
Flora, G. D., and Nayak, M. K. (2019). A brief review of cardiovascular diseases, associated risk factors and current treatment regimes. Curr. Pharm. Des. 25, 4063–4084. doi:10.2174/1381612825666190925163827
Gao, X. Y., Li, K., Jiang, L. L., He, M. F., Pu, C. H., Kang, D. Z., et al. (2017). Developmental toxicity of auranofin in zebrafish embryos. J. Appl. Toxicol. 37, 602–610. doi:10.1002/jat.3410
Gao, M. L., Ge, Z. P., Deng, R., Bao, B. H., Yao, W. F., Cao, Y. D., et al. (2021). Evaluation of VEGF mediated pro-angiogenic and hemostatic effects and chemical marker investigation for typhae Pollen and its processed product. J. Ethnopharmacol. 268, 113591. doi:10.1016/j.jep.2020.113591
Garbern, J. C., Mummery, C. L., and Lee, R. T. (2013). Model systems for cardiovascular regenerative biology. Cold Spring Harb. Perspect. Med. 3, a014019. doi:10.1101/cshperspect.a014019
Genge, C. E., Lin, E., Lee, L., Sheng, X., Rayani, K., Gunawan, M., et al. (2016). The zebrafish heart as a model of mammalian cardiac function. Rev. Physiol. Biochem. Pharmacol. 171, 99–136. doi:10.1007/112_2016_5
González-Rosa, J. M., Burns, C. E., and Burns, C. G. (2017). Zebrafish heart regeneration: 15 years of discoveries. Regen. (Oxf) 4 (3), 105–123. doi:10.1002/reg2.83
He, Z. H., Zhou, R., He, M. F., Lau, C. B. S., Yue, G. G. L., Ge, W., et al. (2011). Anti-angiogenic effect and mechanism of rhein from rhizoma Rhei. Phytomedicine 18, 470–478. doi:10.1016/j.phymed.2010.10.006
He, L. F., Wang, C., Zhang, Y. F., Guo, C. C., Wan, Y., and Li, Y. X. (2021). Effect of emodin on hyperlipidemia and hepatic lipid metabolism in zebrafish larvae fed a high-cholesterol diet. Chem. Biodivers. 19, e202100675. doi:10.1002/cbdv.202100675
He, Y. L., Kam, H., Wu, X., Chen, Q., and Lee, S. M. Y. (2023). Dual effect of aucubin on promoting VEGFR2 mediated angiogenesis and reducing RANKL-induced bone resorption. Chin. Med. 18, 108. doi:10.1186/s13020-023-00786-w
Hellmann, J., Tang, Y. N., Zhang, M. J., Hai, T., Bhatnagar, A., Srivastava, S., et al. (2015). Atf3 negatively regulates Ptgs2/Cox2 expression during acute inflammation. Prostagl. Other Lipid Mediat 116–117, 49–56. doi:10.1016/j.prostaglandins.2015.01.001
Holden, L. A., and Brown, K. H. (2018). Baseline mRNA expression differs widely between common laboratory strains of zebrafish. Sci. Rep. 8 (1), 4780. doi:10.1038/s41598-018-23129-4
Hong, S. J., Wan, J. B., Zhang, Y., Hu, G., Lin, H. C., Seto, S. W., et al. (2008). Angiogenic effect of saponin extract from Panax notoginseng on HUVECs in vitro and zebrafish in vivo. Phytother. Res. 23, 677–686. doi:10.1002/ptr.2705
Hu, F., Chan, J. Y., Koon, C. M., and Fung, K. P. (2013). Angiogenic effects of Danshen and Gegen decoction on human endothelial cells and zebrafish embryos. Am. J. Chin. Med. 41, 887–900. doi:10.1142/s0192415x13500596
Hu, W. H., Chan, G. K. L., Lou, J. S., Wu, Q. Y., Wang, H. Y., Duan, R., et al. (2018). The extract of Polygoni Cuspidati Rhizoma et Radix suppresses the vascular endothelial growth factor-induced angiogenesis. Phytomedicine 42, 135–143. doi:10.1016/j.phymed.2018.03.029
Hu, M. Y., Liu, P. R., Lu, S. X., Wang, Z. H., Lyu, Z. J., Liu, H. K., et al. (2021). Myocardial protective effect and transcriptome profiling of Naoxintong on cardiomyopathy in zebrafish. Chin. Med. 16, 119. doi:10.1186/s13020-021-00532-0
Hu, Y. X., You, H. M., Ren, C. Z., Hu, B. W., Zhang, L. J., Zhang, Y. D., et al. (2022). Proangiogenesis effects of compound danshen dripping pills in zebrafish. BMC Complement. Med. Ther. 22, 112. doi:10.1186/s12906-022-03589-y
Huang, Y. T., Chen, Q. R., Pan, W. J., Zhang, Y., Li, J. S., Xue, X. Y., et al. (2024). Moutan cortex exerts blood-activating and anti-inflammatory effects by regulating coagulation-inflammation cascades pathway in cells, rats and zebrafish. J. Ethnopharmacol. 320, 117398. doi:10.1016/j.jep.2023.117398
Hung, M. W., Zhang, Z. J., Li, S., Lei, B., Yuan, S., Cui, G. Z., et al. (2012). From omics to drug metabolism and high content screen of natural product in zebrafish: a new model for discovery of neuroactive compound. Evid. Based Complement. Altern. Med. 2012, 605303. doi:10.1155/2012/605303
Ishikawa, T. O., Griffin, K. J. P., Banerjee, U., and Herschman, H. R. (2007). The zebrafish genome contains two inducible, functional cyclooxygenase-2 genes. Biochem. Biophys. Res. Commun. 352, 181–187. doi:10.1016/j.bbrc.2006.11.007
Jin, S., Hong, J. H., Jung, S. H., and Cho, K. H. (2011). Turmeric and laurel aqueous extracts exhibit in vitro anti-atherosclerotic activity and in vivo hypolipidemic effects in a zebrafish model. J. Med. Food 14 (3), 247–256. doi:10.1089/jmf.2009.1389
Kondo, T., Ohta, T., Igura, K., Hara, Y., and Kaji, K. (2002). Tea catechins inhibit angiogenesis in vitro, measured by human endothelial cell growth, migration and tube formation, through inhibition of VEGF receptor binding. Cancer Lett. 180 (2), 139–144. doi:10.1016/s0304-3835(02)00007-1
Lam, K. H., Alex, D., Lam, I. K., Tsui, S. K. W., Yang, Z. F., and Lee, S. M. Y. (2011). Nobiletin, a polymethoxylated flavonoid from citrus, shows anti-angiogenic activity in a zebrafish in vivo model and HUVEC in vitro model. J. Cell. Biochem. 112, 3313–3321. doi:10.1002/jcb.23257
Leung, K. W., Pon, Y. L., Wong, R. N., and Wong, A. S. (2006). Ginsenoside-Rg1 induces vascular endothelial growth factor expression through the glucocorticoid receptor-related phosphatidylinositol 3-kinase/Akt and beta-catenin/T-cell factor-dependent pathway in human endothelial cells. J. Biol. Chem. 281 (47), 36280–36288. doi:10.1074/jbc.M606698200
Leung, K. W., Cheung, L. W., Pon, Y. L., Wong, R. N., Mak, N. K., Fan, T. P., et al. (2007). Ginsenoside Rb1 inhibits tube-like structure formation of endothelial cells by regulating pigment epithelium-derived factor through the oestrogen beta receptor. Br. J. Pharmacol. 152 (2), 207–215. doi:10.1038/sj.bjp.0707359
Li, X. Y. (2024). The treatment of cardiovascular disease. Theor. Nat. Sci. 71, 144–150. doi:10.54254/2753-8818/2024.LA18889
Li, J. J., Peng, Y. R., Li, S., Sun, Y. C., Chan, J. Y. W., Cui, G. Z., et al. (2016). Anti-angiogenic activity of a new andrographolide derivative in zebrafish and HUVECs. Eur. J. Pharmacol. 789, 344–353. doi:10.1016/j.ejphar.2016.07.043
Li, J. J., Zhang, J. M., Zou, L., Lee, S. M., Yang, C., Seto, S. W., et al. (2017a). Pro-angiogenic effects of ilexsaponin A1 on human umbilical vein endothelial cells in vitro and zebrafish in vivo. Phytomedicine 36, 229–237. doi:10.1016/j.phymed.2017.10.006
Li, Y. P., Wang, X. L., and Shen, Z. J. (2017b). Traditional Chinese medicine for lipid metabolism disorders. Am. J. Transl. Res. 9, 2038–2049.
Li, J., Liu, H., Yang, Z. Z., Yu, Q. Q., Zhao, L., and Wang, Y. (2020a). Synergistic effects of cryptotanshinone and senkyunolide I in guanxinning tablet against endogenous thrombus formation in zebrafish. Front. Pharmacol. 11, 622787. doi:10.3389/fphar.2020.622787
Li, M. T., Xie, X. F., Chen, H. M., Xiong, Q. Y., Tong, R. S., Peng, C., et al. (2020b). Aconitine induces cardiotoxicity through regulation of calcium signaling pathway in zebrafish embryos and in H9c2 cells. J. Appl. Toxicol. 40, 780–793. doi:10.1002/jat.3943
Li, S. N., Liu, H. X., Li, Y., Qin, X. M., Li, M. J., Shang, J. J., et al. (2021). Shen-yuan-dan capsule attenuates verapamil-induced zebrafish heart failure and exerts antiapoptotic and anti-inflammatory effects via reactive oxygen species–induced NF-κB pathway. Front. Pharmacol. 12, 626515. doi:10.3389/fphar.2021.626515
Li, Y., Liu, X. J., Su, S. L., Yan, H., Guo, S., Qian, D. W., et al. (2022). Evaluation of anti-inflammatory and antioxidant effectsof chrysanthemum stem and leaf extract on zebrafish inflammatory bowel disease model. Molecules 27, 2114. doi:10.3390/molecules27072114
Li, J., Li, R., Wu, X., Zheng, C., Shiu, P. H., Rangsinth, P., et al. (2022a). An update on the potential application of herbal medicine in promoting angiogenesis. Front. Pharmacol. 13, 928817. doi:10.3389/fphar.2022.928817
Li, J., Zhu, Y., Zhao, X. P., Zhao, L., Wang, Y., and Yang, Z. Z. (2022b). Screening of anti-heart failure active compounds from fangjihuangqi decoction in verapamil-induced zebrafish model by anti-heart failure index approach. Front. Pharmacol. 13, 999950. doi:10.3389/fphar.2022.999950
Li, C. J., Zhai, R. R., Zhu, X. Y., Guo, Z. F., and Yang, H. (2023). Discovery of effective combination from renshen-fuzi herbal pair against heart failure by spectrum-effect relationship analysis and zebrafish models. J. Ethnopharmacol. 317, 116832. doi:10.1016/j.jep.2023.116832
Li, D. X., Li, X. Y., Zhang, X. N., Chen, J. Y., Wang, Z. P., Yu, Z. L., et al. (2024a). Geniposide for treating atherosclerotic cardiovascular disease: a systematic review on its biological characteristics, pharmacology, pharmacokinetics, and toxicology. Chin. Med. 19, 111. doi:10.1186/s13020-024-00981-3
Li, X., Xu, X., Dong, Y., Fan, S. S., Ren, X. Y., Zheng, Y., et al. (2024b). Fufang e’jiao Jiang’s effect on immunity, hematopoiesis, and angiogenesis via a systematic “compound-effect-target” analysis. Food Sci. Hum. Well 13, 2813–2832. doi:10.26599/fshw.2022.9250228
Li, X., Zeng, L., Qu, Z., and Zhang, F. (2024c). Huoxin pill protects verapamil-induced zebrafish heart failure through inhibition of oxidative stress-triggered inflammation and apoptosis. Heliyon 10, e23402. doi:10.1016/j.heliyon.2023.e23402
Li, Y., Ren, T. T., Liu, S. S., Zhang, L., Yi, H., Li, C., et al. (2024d). Fingerprint analysis of dang-gui-si-ni decoction and its anticoagulant activity in vivo-in vitro. J. Ethnopharmacol. 325, 117890. doi:10.1016/j.jep.2024.117890
Li, Z. R., Zhou, D. Y., Wu, T. C., Lee, H., Zheng, F., Dai, Y. L., et al. (2025). A novel glycopeptide from mountain-cultivated ginseng residue protects type 2 diabetic symptoms-induced heart failure. J. Ethnopharmacol. 336, 118723. doi:10.1016/j.jep.2024.118723
Liang, F., Han, Y. X., Gao, H., Xin, S. C., Chen, S. D., Wang, N., et al. (2015). Kaempferol identified by zebrafish assay and fine fractionations strategy from Dysosma versipellis inhibits angiogenesis through VEGF and FGF pathways. Sci. Rep. 5, 14468. doi:10.1038/srep14468
Liang, J. F., Jin, W. D., Li, H. W., Liu, H. C., Huang, Y. F., Shan, X. W., et al. (2016). In vivo cardiotoxicity induced by sodium aescinate in zebrafish larvae. Molecules 21, 190. doi:10.3390/molecules21030190
Liang, Q. X., Zhang, H. Z., Han, C., Chen, X. Q., Zhang, Y., He, Q. X., et al. (2024). Lithospermic acid promotes angiogenesis in zebrafish and HUVECs by regulating the VEGF/PI3K-Akt/MAPK signaling pathways. J. Funct. Foods 115, 106121. doi:10.1016/j.jff.2024.106121
Lin, Q. H., Qu, W., Xu, J., Feng, F., and He, M. F. (2018). 1-Methoxycarbony-β-carboline from Picrasma quassioides exerts anti-angiogenic properties in HUVECs in vitro and zebrafish embryos in vivo. Chin. J. Nat. Med. 16, 599–609. doi:10.1016/s1875-5364(18)30097-9
Lin, S. H., Mo, C. L., Yan, L. Y., Zhang, F., Liu, X., Ma, H. L., et al. (2022). Protective effects of salvianolic acid A on clozapine-induced cardiotoxicity in zebrafish. J. Appl. Toxicol. 42, 1978–1985. doi:10.1002/jat.4368
Lin, S. H., Liu, X., Sun, A. N., Liang, H. L., Li, Z., Ye, S. Y., et al. (2023). Qilong capsule alleviates ponatinib-induced ischemic stroke in a zebrafish model by regulating coagulation, inflammation and apoptosis. J. Ethnopharmacol. 314, 116397. doi:10.1016/j.jep.2023.116397
Lin, B. Y., Wan, H. F., Yang, J. H., Yu, L., Zhou, H. F., and Wan, H. T. (2024). Lipid regulation of protocatechualdehyde and hydroxysafflor yellow A via AMPK/SREBP2/PCSK9/LDLR signaling pathway in hyperlipidemic zebrafish. Heliyon 10, e24908. doi:10.1016/j.heliyon.2024.e24908
Lippi, M., Stadiotti, I., Pompilio, G., and Sommariva, E. (2020). Human cell modeling for cardiovascular diseases. Int. J. Mol. Sci. 21, 6388. doi:10.3390/ijms21176388
Liu, C. L., Kwok, H. F., Cheng, L., Ko, C. H., Wong, C. W., Ho, T. W., et al. (2014). Molecular mechanisms of angiogenesis effect of active sub-fraction from root of Rehmannia glutinosa by zebrafish sprout angiogenesis-guided fractionation. J. Ethnopharmacol. 151, 565–575. doi:10.1016/j.jep.2013.11.019
Liu, F., Han, X., Li, N., Liu, K., and Kang, W. J. (2019). Aconitum alkaloids induce cardiotoxicity and apoptosis in embryonic zebrafish by influencing the expression of cardiovascular relative genes. Toxicol. Lett. 305, 10–18. doi:10.1016/j.toxlet.2019.01.002
Liu, M., Zhao, L., Han, L. W., Li, H. N., Shi, Y. P., Cui, J., et al. (2020). Discovery and identification of proangiogenic chemical markers from gastrodiae rhizoma based on zebrafish model and metabolomics approach. Phytochem. Anal. 31, 835–845. doi:10.1002/pca.2949
Liu, J., Xu, P., Liu, D., Wang, R., Cui, S., Zhang, Q., et al. (2021). TCM regulates PI3K/Akt signal pathway to intervene atherosclerotic cardiovascular disease. Evid. Based Complement. Altern. Med. 2021, 4854755. doi:10.1155/2021/4854755
Liu, J. L., Liu, Y. H., Lin, H. Q., Zhou, B. S., Yu, H., Li, L., et al. (2021a). The effect of ginsenoside Rg5, isolated from black ginseng, on heart failure in zebrafish based on untargeted metabolomics. J. Funct. Foods 76, 104325. doi:10.1016/j.jff.2020.104325
Liu, J. L., Liu, Y. H., Yu, H., Zhang, Y., Hsu, A. C. Y., Zhang, M. M., et al. (2021b). Design, synthesis and biological evaluation of novel pyxinol derivatives with anti-heart failure activity. Biomed. Pharmacother. 133, 111050. doi:10.1016/j.biopha.2020.111050
Liu, Q., Zhang, H. Z., An, Y., Zhang, Y., He, Q. X., Liu, K. C., et al. (2023). Xinkeshu tablets promote angiogenesis in zebrafish embryos and human umbilical vein endothelial cells through multiple signaling pathways. J. Ethnopharmacol. 314, 116636. doi:10.1016/j.jep.2023.116636
Loukas, M., Youssef, P., Gielecki, J., Walocha, J., Natsis, K., and Tubbs, R. S. (2016). History of cardiac anatomy: a comprehensive review from the egyptians to today. Clin. Anat. 29 (3), 270–284. doi:10.1002/ca.22705
Lu, X. G., Lu, L. H., Gao, L., Wang, Y., and Wang, W. (2021). Calycosin attenuates doxorubicin-induced cardiotoxicity via autophagy regulation in zebrafish models. Biomed. Pharmacother. 137, 111375. doi:10.1016/j.biopha.2021.111375
Luz, R. B. D. S., Paula, A. G. P., Czaikovski, A. P., Nunes, B. S. F., De Lima, J. D., Paredes, L. C., et al. (2025). Macrophages and cardiac lesion in zebrafish: what can single-cell RNA sequencing reveal?. Front. cardiovasc. med. 12. doi:10.3389/fcvm.2025.1570582
Ma, L. M., Qi, Y. X., Wang, W. J., and Zhong, Q. Y. (2020). Anti-angiogenic effect of ginsenoside Rh2 by downregulation of VEGF in a zebrafish model. Pak. J. Zool. 53, 111–118. doi:10.17582/journal.pjz/20190918070925
Ma, X. W., Chen, Y. Y., Jiang, S. M., and Zhao, X. P. (2021). A bioassay-based approach for the batch-to-batch consistency evaluation of xuesaitong injection on a zebrafish thrombosis model. Front. Pharmacol. 12, 623533. doi:10.3389/fphar.2021.623533
Mantovani, A. (2010). Molecular pathways linking inflammation and cancer. Curr. Mol. Med. 10 (4), 369–373. doi:10.2174/156652410791316968
Motohashi, H., Sugita, S., Hosokawa, Y., Hasumura, T., Meguro, S., Ota, N., et al. (2025). Novel nerve regeneration assessment method using adult zebrafish with crush spinal cord injury. J. Comp. Physiol. A 211, 185–197. doi:10.1007/s00359-024-01723-4
Narumanchi, S., Wang, H., Perttunen, S., Tikkanen, I., Lakkisto, P., and Paavola, J. (2021). Zebrafish heart failure models. Front. Cell Dev. Biol. 9, 662583. doi:10.3389/fcell.2021.662583
Nathan, J., Shameera, R., Devarajan, N., and Perumal, E. (2023). Role of berberine on angiogenesis and blood flow hemodynamics using zebrafish model. J. Appl. Toxicol. 44, 165–174. doi:10.1002/jat.4529
Nguyen, C. T., Lu, Q., Wang, Y., and Chen, J. N. (2008). Zebrafish as a model for cardiovascular development and disease. Drug Discov. Today Dis. Models 5 (3), 135–140. doi:10.1016/j.ddmod.2009.02.003
Ni, X., Yin, X. Y., Qi, C., Liu, C. Y., Chen, H., Zhou, Y. N., et al. (2022). Cardiotoxicity of (−)-borneol, (+)-borneol, and isoborneol in zebrafish embryos is associated with Na+/K+-ATPase and Ca2+-ATPase inhibition. J. Appl. Toxicol. 43, 373–386. doi:10.1002/jat.4388
Otis, J. P., Zeituni, E. M., Thierer, J. H., Anderson, J. L., Brown, A. C., Boehm, E. D., et al. (2015). Zebrafish as a model for apolipoprotein biology: comprehensive expression analysis and a role for ApoA-IV in regulating food intake. Dis. Model Mech. 8 (3), 295–309. doi:10.1242/dmm.018754
Pang, H. Q., Guo, J. X., Yang, Y., Xu, L., Wang, J., Yang, F., et al. (2024). Elucidating the chemical interaction effects of herb pair danshen-chuanxiong and its anti-ischemic stroke activities evaluation. J. Ethnopharmacol. 318, 117058. doi:10.1016/j.jep.2023.117058
Qi, Y. Q., Zhao, X. P., Liu, H., Wang, Y. M., Zhao, C., Zhao, T., et al. (2017). Identification of a quality marker (Q-Marker) of danhong injection by the zebrafish thrombosis model. Molecules 22, 1443. doi:10.3390/molecules22091443
Ramjiawan, R. R., Griffioen, A. W., and Duda, D. G (2017). Anti-angiogenesis for cancer revisited: Is there a role for combinations with immunotherapy?. Angiogenesis 20 (2), 185–204. doi:10.1007/s10456-017-9552-y
Rao, C., Hu, R. X., Hu, Y. X., Jiang, Y., Zou, X., Tang, H. L., et al. (2024). Theoretical exploring of potential mechanisms of antithrombotic ingredients in danshen-chishao herb-pair by network pharmacological study, molecular docking and zebrafish models. Chin. Med. 19, 100. doi:10.1186/s13020-024-00970-6
Sajib, S., Zahra, F. T., Lionakis, M. S., German, N. A., and Mikelis, C. M. (2018). Mechanisms of angiogenesis in microbe-regulated inflammatory and neoplastic conditions. Angiogenesis 21 (1), 1–14. doi:10.1007/s10456-017-9583-4
Sengupta, S., Toh, S. A., Sellers, L. A., Skepper, J. N., Koolwijk, P., Leung, H. W., et al. (2004). Modulating angiogenesis: the yin and the yang in ginseng. Circulation 110 (10), 1219–1225. doi:10.1161/01.Cir.0000140676.88412.Cf
Seo, M. J., Choi, H. S., Jeon, H. J., Woo, M. S., and Lee, B. Y. (2014). Baicalein inhibits lipid accumulation by regulating early adipogenesis and m-TOR signaling. Food Chem. Toxicol. 67, 57–64. doi:10.1016/j.fct.2014.02.009
Shinde, T., Peddakolmi, S., Patil, A., Bagwe, A. D., and Sharma, B. B. (2025). Assessment of embryotoxicity and teratogenic effects of Anacardium occidentale L. cashew nutshell extracts on zebrafish (Danio rerio) embryos. Toxicol. Environ. Chem. 107, 21–42. doi:10.1080/02772248.2024.2436365
Si, L. J., and Yu, L. (2024). Pharmacological mechanisms by which baicalin ameliorates cardiovascular disease. Front. Pharmacol. 15, 1415971. doi:10.3389/fphar.2024.1415971
Singleman, C., and Holtzman, N. G. (2012). Analysis of postembryonic heart development and maturation in the zebrafish, Danio rerio. Dev. Dyn. 241 (12), 1993–2004. doi:10.1002/dvdy.23882
Stoletov, K., Fang, L., Choi, S. H., Hartvigsen, K., Hansen, L. F., Hall, C., et al. (2009). Vascular lipid accumulation, lipoprotein oxidation, and macrophage lipid uptake in hypercholesterolemic zebrafish. Circ. Res. 104 (8), 952–960. doi:10.1161/circresaha.108.189803
Sun, W., Yan, B., Wang, R. R., Liu, F. C., Hu, Z. Y., Zhou, L., et al. (2018). In vivo acute toxicity of detoxified fuzi (lateral root of Aconitum carmichaeli) after a traditional detoxification process. EXCLI J. 17, 889–899. doi:10.17179/excli2018-1607
Tal, T. L., Mccollum, C. W., Harris, P. S., Olin, J., Kleinstreuer, N., Wood, C. E., et al. (2014). Immediate and long-term consequences of vascular toxicity during zebrafish development. Reprod. Toxicol. 48, 51–61. doi:10.1016/j.reprotox.2014.05.014
Tang, H. L., Qin, N. Y., Rao, C., Zhu, J. H., Wang, H. Q., and Hu, G. (2021). Screening of potential anti-thrombotic ingredients from Salvia miltiorrhiza in zebrafish and by molecular docking. Molecules 26, 6807. doi:10.3390/molecules26226807
Tu, X., Deng, Y. P., Chen, J., Hu, Q., He, C. S., Jordan, J. B., et al. (2016). Screening study on the anti-angiogenic effects of traditional Chinese medicine – part I: heat-clearing and detoxicating TCM. J. Ethnopharmacol. 194, 280–287. doi:10.1016/j.jep.2016.09.010
Tu, X., Wang, H. B., Huang, Q., Cai, Y., Deng, Y. P., Yong, Z., et al. (2021). Screening study on the anti-angiogenic effects of traditional Chinese medicine - part II: wild chrysanthemum. J. Cancer 12, 124–133. doi:10.7150/jca.52971
Van De Venter, M., Didloff, J., Reddy, S., Swanepoel, B., Govender, S., Dambuza, N. S., et al. (2020). Wild-type zebrafish (Danio rerio) larvae as a vertebrate model for diabetes and comorbidities: a review. Anim. (Basel) 11 (1), 54. doi:10.3390/ani11010054
Viallard, C., and Larrivée, B. (2017). Tumor angiogenesis and vascular normalization: alternative therapeutic targets. Angiogenesis 20 (4), 409–426. doi:10.1007/s10456-017-9562-9
Wang, T., Wang, C. X., Wu, Q., Zheng, K. D., Chen, J. J., Lan, Y. T., et al. (2017). Evaluation of tanshinone IIA developmental toxicity in zebrafish embryos. Molecules 22, 660. doi:10.3390/molecules22040660
Wang, C. N., Hui, J., Zhu, X. H., Cui, S. Y., Cui, Z. M., and Xu, D. W. (2020a). Lobetyolin efficiently promotes angiogenesis and neuronal development in transgenic zebrafish. Nat. Prod. Commun. 15, 1934578X20937174–7. doi:10.1177/1934578x20937174
Wang, J. L., Zhang, X. H., Xu, X. Y., Zhu, Q., Yao, B. B., Liang, S., et al. (2020b). Pro-angiogenic activity of tongnao decoction on HUVECs in vitro and zebrafish in vivo. J. Ethnopharmacol. 254, 112737. doi:10.1016/j.jep.2020.112737
Wang, J. Z., Mo, C. L., Tu, P. F., Ning, N., Liu, X., Lin, S. H., et al. (2021). Developmental toxicity of zishen guchong pill on the early life stages of Zebrafish. Phytomed. Plus 1, 100088. doi:10.1016/j.phyplu.2021.100088
Wang, D., Yu, X., Huang, K., Tang, J. Y., Wei, X. Q., Pu, H. Y., et al. (2022). Common anti-inflammatory effects of heat-clearing and toxin-removing Chinese medicines on diverse cardiovascular diseases. China J. Chin. Mater. Med. 47 (20), 5418–5423. doi:10.19540/j.cnki.cjcmm.20220601.701
Wang, Y. K., Pan, Y., Hou, M. R., Luo, R., He, J. W., Lin, F., et al. (2023). Danggui shaoyao san ameliorates the lipid metabolism via the PPAR signaling pathway in a Danio rerio (zebrafish) model of hyperlipidemia. Biomed. Pharmacother. 168, 115736. doi:10.1016/j.biopha.2023.115736
Warner, T. D., Nylander, S., and Whatling, C. (2011). Anti-platelet therapy: cyclo-oxygenase inhibition and the use of aspirin with particular regard to dual anti-platelet therapy. Br. J. Clin. Pharmacol. 72, 619–633. doi:10.1111/j.1365-2125.2011.03943.x
Weyand, A. C., and Shavit, J. A. (2014). Zebrafish as a model system for the study of hemostasis and thrombosis. Curr. Opin. Hematol. 21, 418–422. doi:10.1097/moh.0000000000000075
Wu, S. M., Huang, C., Chen, Y. R., Huang, H. C., Huang, W. C., and Lai, Y. H. (2021). Momordica charantia leaf extract reduces hepatic lipid accumulation and diet-induced dyslipidemia in zebrafish through lipogenesis and beta-oxidation. J. Funct. Foods 87, 104857. doi:10.1016/j.jff.2021.104857
Wu, B. X., Li, C. R., Kan, H., Zhang, Y. J., Rao, X. P., Liu, Y., et al. (2024a). Hypolipidemic and antithrombotic effect of 6'-O-caffeoylarbutin from Vaccinium dunalianum based on zebrafish model, network pharmacology, and molecular docking. Molecules 29, 780. doi:10.3390/molecules29040780
Wu, B. X., Li, C. R., Luo, X. L., Kan, H., Li, Y. H., Zhang, Y. J., et al. (2024b). Identification of key hypolipidemic components and exploration of the potential mechanism of total flavonoids from Rosa sterilis based on network pharmacology, molecular docking, and zebrafish experiment. Curr. Issues Mol. Biol. 46, 5131–5146. doi:10.3390/cimb46060308
Xia, K. R., Zhang, X. Y., Zhang, H. Q., Su, K. L., Shang, E. X., Xiao, Q. L., et al. (2024). Network pharmacology analysis and experimental verification of the antithrombotic active compounds of trichosanthis pericarpium (gualoupi) in treating coronary heart disease. J. Ethnopharmacol. 329, 118158. doi:10.1016/j.jep.2024.118158
Xu, N., Sun, R., Shi, Y. P., Han, L. W., and Shi, H. Y. (2021). Discovery and identification of quality markers of sparganii rhizoma based on zebrafish thrombosis model. Chin. Herb. Med. 13, 389–395. doi:10.1016/j.chmed.2021.04.015
Xu, Q., Li, D., Wan, M., Zhang, Y., Li, Z., and Ju, A. (2023). An online CMC–LC/MS method for screening angiogenesis promoting components in yiqi fumai lyophilization injection (YQFM). Nat. Prod. Commun. 18, 1934578X231182209–10. doi:10.1177/1934578x231182209
Xu, G., Xu, P. H., Wang, N., Qi, W. M., Pu, Y. X., Kang, N. N., et al. (2024). Rare crocins ameliorate thrombus in zebrafish larvae by regulating lipid accumulation and clotting factors. Fitoterapia 179, 106278. doi:10.1016/j.fitote.2024.106278
Yang, W., Ma, L., Li, S., Cui, K., Lei, L., and Ye, Z. (2017). Evaluation of the cardiotoxicity of evodiamine in vitro and in vivo. Molecules 22, 943. doi:10.3390/molecules22060943
Yang, S., Zhao, M., Feng, Y., Zhang, X., Li, Q., Jiang, W., et al. (2024). Exploring the molecular mechanism of toddalia asiatica (L.) lam on the treatment of thrombosis based on zebrafish models, network pharmacology and experimental verification. Fitoterapia 179, 106224. doi:10.1016/j.fitote.2024.106224
Ye, M., Xu, W., He, D. Q., Wu, X., Lai, W. F., Zhang, X. Q., et al. (2020). Dammarane-type triterpenoids from the roots of Rhus chinensis and their preventive effects on zebrafish heart failure and thrombosis. J. Nat. Prod. 83, 362–373. doi:10.1021/acs.jnatprod.9b00857
Ye, M., Ruan, L. J., Huang, L. N., Zheng, H. Y., Xu, W., Xu, W., et al. (2023). Triterpenoids with diverse skeletons from the roots of Rhus chinensis and their protective effects on isoproterenol-induced heart failure in zebrafish. Phytochemistry 213, 113749. doi:10.1016/j.phytochem.2023.113749
Yeh, J. C., Cindrova-Davies, T., Belleri, M., Morbidelli, L., Miller, N., Cho, C. W., et al. (2011). The natural compound n-butylidenephthalide derived from the volatile oil of radix Angelica sinensis inhibits angiogenesis in vitro and in vivo. Angiogenesis 14, 187–197. doi:10.1007/s10456-011-9202-8
Yin, S. J., Luo, Y. Q., Zhao, C. P., Chen, H., Zhong, Z. F., Wang, S., et al. (2020). Antithrombotic effect and action mechanism of Salvia miltiorrhiza and Panax notoginseng herbal pair on the zebrafish. Chin. Med. 15, 35. doi:10.1186/s13020-020-00316-y
Yu, X. B., Tong, Y., Kwok, H. F., Sze, S. C. W., Zhong, L. D., Lau, C. B. S., et al. (2012). Anti-angiogenic activity of erxian decoction, a traditional Chinese herbal formula, in zebrafish. Biol. Pharm. Bull. 35, 2119–2127. doi:10.1248/bpb.b12-00130
Yu, S. Q., Yu, J. H., Sun, F. Z., Zhang, X. M., Sheng, W. L., Liu, K. C., et al. (2025). Diammonium glycyrrhizinate injection promotes zebrafish angiogenesis through the mTOR/HIF-1 signaling pathway. bioRxiv. doi:10.1101/2025.02.19.639026
Yuan, T., Wen, X. Y., Chen, Y., Deng, Y., Li, H. Z., and Huang, Y. (2022). Study on the hypolipidemic effect of Moringa oleifera seeds based on zebrafish hyperlipidemia model. Asian Plant Res. J. 10, 65–70. doi:10.9734/aprj/2022/v10i1184
Yue, P. Y., Wong, D. Y., Wu, P. K., Leung, P. Y., Mak, N. K., Yeung, H. W., et al. (2006). The angiosuppressive effects of 20(R)- ginsenoside Rg3. Biochem. Pharmacol. 72 (4), 437–445. doi:10.1016/j.bcp.2006.04.034
Zeng, J. J., Shi, H. Q., Ren, F. F., Zhao, X. S., Chen, Q. Y., Wang, D. J., et al. (2023). Notoginsenoside R1 protects against myocardial ischemia/reperfusion injury in mice via suppressing TAK1-JNK/p38 signaling. Acta Pharmacol. Sin. 44, 1366–1379. doi:10.1038/s41401-023-01057-y
Zhang, P., Deng, Y., Gui, Y., Chen, Z., Liu, C., and Zhang, D. (2020). Research review of antithrombotic mechanism of function. The Medical Forum. 24 (25), 3682–3685. doi:10.19435/j.1672-1721.2020.25.075
Zhang, Z.-R., Li, J.-H., Li, S., Liu, A., Hoi, M. P. M., Tian, H.-Y., et al. (2014). In Vivo angiogenesis screening and mechanism of action of novel tanshinone derivatives produced by one-pot combinatorial modification of natural tanshinone mixture from salvia miltiorrhiza. PLoS One 9, e100416. doi:10.1371/journal.pone.0100416
Zhang, W. T., Liu, B., Feng, Y. R., Liu, J., Ma, Z. Q., Zheng, J., et al. (2017). Anti-angiogenic activity of water extract from Euphorbia pekinensis rupr. J. Ethnopharmacol. 206, 337–346. doi:10.1016/j.jep.2017.05.033
Zhang, M. Q., Li, P. H., Zhang, S. S., Zhang, X. M., Wang, L. Z., Zhang, Y., et al. (2021). Study on the mechanism of the danggui–chuanxiong herb pair on treating thrombus through network pharmacology and zebrafish models. ACS Omega 6, 14677–14691. doi:10.1021/acsomega.1c01847
Zhang, W. T., Feng, Y. R., Ni, L., Liang, W. J., Li, X. R., and Lin, R. C. (2022a). Screening and identification of euphorbiae pekinensis rupr. Anti-angiogenic multi-components with UPLC-QTOF-MS in zebrafish. J. Pharm. Biomed. Anal. 207, 114396. doi:10.1016/j.jpba.2021.114396
Zhang, Y. R., Liu, Y. R., Tang, Z. S., Song, Z. X., Zhang, J. W., Chang, B. J., et al. (2022b). Rheum officinale baill. Treats zebrafish embryo thrombosis by regulating NOS3 expression in the arginine biosynthesis pathway. Phytomedicine 99, 153967. doi:10.1016/j.phymed.2022.153967
Zhang, L., Tong, Y. Y., Fang, Y., Pei, J. J., Wang, Q. L., and Li, G. (2023). Exploring the hypolipidemic effects of bergenin from Saxifraga melanocentra franch: mechanistic insights and potential for hyperlipidemia treatment. Lipids Health Dis. 22, 203. doi:10.1186/s12944-023-01973-2
Zhang, Z., Zhang, X., Yang, Y., Wang, H. Y., Yang, X. J., Xuan, L. Y., et al. (2025). Baicalein protects against heart failure by improving mitochondrial dysfunction and regulating endoplasmic reticulum stress to reduce apoptosis in vitro and in vivo. Int. J. Immunopathol. Pharmacol. 39, 3946320251315800–3946320251315815. doi:10.1177/03946320251315800
Zhao, D. X., Qin, C. J., Fan, X. H., Li, Y. C., and Gu, B. H. (2014). Inhibitory effects of quercetin on angiogenesis in larval zebrafish and human umbilical vein endothelial cells. Eur. J. Pharmacol. 723, 360–367. doi:10.1016/j.ejphar.2013.10.069
Zheng, C. R., Shan, L. T., Tong, P. J., and Efferth, T. (2020). Cardiotoxicity and cardioprotection by artesunate in larval zebrafish. Dose Response 18, 1559325819897180–13. doi:10.1177/1559325819897180
Zhong, Z. F., Hoi, P. M., Wu, G. S., Xu, Z. T., Tan, W., Chen, X. P., et al. (2012). Anti-angiogenic effect of furanodiene on HUVECs in vitro and on zebrafish in vivo. J. Ethnopharmacol. 141, 721–727. doi:10.1016/j.jep.2011.08.052
Zhong, J., Xiao, C. X., Chen, Q., Pan, X. Y., Xu, T. T., Wang, Y. Y., et al. (2023). Zebrafish functional xenograft vasculature platform identifies PF-502 as a durable vasculature normalization drug. iScience 26, 107734. doi:10.1016/j.isci.2023.107734
Zhong, L. F., Tan, X. Y., Yang, W. H., Li, P. S., Ye, L. B., Luo, Q., et al. (2024). Bioactive matters based on natural product for cardiovascular diseases. Smart Mater. Med. 5, 542–565. doi:10.1016/j.smaim.2024.11.001
Zhou, X., Siu, W. S., Fung, C. H., Cheng, L., Wong, C. W., Zhang, C., et al. (2014). Pro-angiogenic effects of carthami flos whole extract in human microvascular endothelial cells in vitro and in zebrafish in vivo. Phytomedicine 21, 1256–1263. doi:10.1016/j.phymed.2014.06.010
Zhou, J., Xu, Y. Q., Guo, S. Y., and Li, C. Q. (2015). Rapid analysis of hypolipidemic drugs in a live zebrafish assay. J. Pharmacol. Toxicol. Methods 72, 47–52. doi:10.1016/j.vascn.2014.12.002
Zhou, X., Siu, W. S., Zhang, C., Liu, C. L., Cheng, L., Kwok, H. F., et al. (2017). Whole extracts of Radix achyranthis bidentatae and Radix cyathulae promote angiogenesis in human umbilical vein endothelial cells in vitro and in zebrafish in vivo. Exp. Ther. Med. 13, 1032–1038. doi:10.3892/etm.2017.4053
Zhou, Z. Y., Xiao, Y., Zhao, W. R., Zhang, J., Shi, W. T., Ma, Z. L., et al. (2019a). Pro-angiogenesis effect and transcriptome profile of shuxinyin formula in zebrafish. Phytomedicine 65, 153083. doi:10.1016/j.phymed.2019.153083
Zhou, Z. Y., Zhao, W. R., Xiao, Y., Zhou, X. M., Huang, C., Shi, W. T., et al. (2019b). Antiangiogenesis effect of timosaponin AIII on HUVECs in vitro and zebrafish embryos in vivo. Acta Pharmacol. Sin. 41, 260–269. doi:10.1038/s41401-019-0291-z
Zhou, F., Dai, O., Peng, C., Xiong, L., Ao, H., Liu, F., et al. (2021). Pro-angiogenic effects of essential oil from Perilla frutescens and its main component (perillaldehyde) on zebrafish embryos and human umbilical vein endothelial cells. Drug Des. devel. Ther. 15, 4985–4999. doi:10.2147/dddt.S336826
Zhou, C., Peng, L. P., Dong, Y. D., Ma, M. J., Zhou, M. L., Li, Q., et al. (2024a). Network pharmacology study of cassiae semen in treatment of hyperlipidemia and experimental validation in zebrafish. Acad. J. Shanghai Uni. Tradit. Chin. Med. 38, 71–80. doi:10.16306/j.1008
Zhou, Z. Y., Ma, J., Zhao, W. R., Shi, W. T., Zhang, J., Hu, Y. Y., et al. (2024b). Qiangxinyin formula protects against isoproterenol-induced cardiac hypertrophy. Phytomedicine 130, 155717. doi:10.1016/j.phymed.2024.155717
Zhu, D., Wang, S., Lawless, J., He, J., and Zheng, Z. (2016). Dose dependent dual effect of baicalin and herb huang qin extract on angiogenesis. PLoS One 11 (11), e0167125. doi:10.1371/journal.pone.0167125
Zhu, X. Y., Wu, S. Q., Guo, S. Y., Yang, H., Xia, B., Li, P., et al. (2018). A zebrafish heart failure model for assessing therapeutic agents. Zebrafish 15 (3), 243–253. doi:10.1089/zeb.2017.1546
Zhu, Y., Yang, H., Han, L., Mervin, L. H., Hosseini-Gerami, L., Li, P., et al. (2023). In silico prediction and biological assessment of novel angiogenesis modulators from traditional Chinese medicine. Front. Pharmacol. 14, 1116081. doi:10.3389/fphar.2023.1116081
Zou, X., Liu, Q., Guo, S., Zhu, J., Han, J., Xia, Z., et al. (2019). A novel zebrafish larvae hypoxia/reoxygenation model for assessing myocardial ischemia/reperfusion injury. Zebrafish 16 (5), 434–442. doi:10.1089/zeb.2018.1722
Glossary
AA arachidonic acid
ACC1 acetyl-CoA carboxylase 1
BA arterial bulb
BAX BCL2-associated X protein
Bcl-2 B-cell lymphoma-2
CD36 cluster of differentiation 36
CO cardiac output
COX/cox cyclooxygenase
CPT1 carnitine palmityl transferase Ⅰ
CtAs central arteries
CV caudal vein
CVDs cardiovascular diseases
DA dorsal aorta
DLAV dorsal longitudinal anastomotic artery
DOX doxorubicin
Dpf days post-fertilization
EC50 median effect concentration
EF ejection fraction
F7 coagulation factor VII
FAC functional area change
FGA/fga fibrinogen α chain gene
FGB/fgb fibrinogen β chain
FII coagulation factor II
FIX coagulation factor IX
FS fractional shortening
FVII coagulation factor VII
FX coagulation factor X
GPⅡb/Ⅲa glycoprotein IIb/IIIa
HDL high-density lipoprotein
HF heart failure
hpf hours post-fertilization
IL interleukin
ISVs intersegmental vessels
JNK c-Jun N-terminal kinase
Kdrl kinase insert domain receptor like
LC50 lethal concentration 50%
LD10 lethal dose 10%
LDL low-density lipoprotein
LOAEL lowest observed adverse effect level
MARK mitogen-activated protein kinase
MDA malondialdehyde
mmp9 matrix metalloprotein 9
MNLC maximum nonlethal concentration
mTOR mammalian target of rapamycin
NF-κB nuclear factor kappa-B
NOS3 nitric oxide synthase 3
Notch1a notch receptor 1a
nppA natriuretic peptide A
nppB natriuretic peptide B
OAs optic arteries
PHZ phenylhydrazine
PI3K-Akt phosphatidylinositol-3-kinase/protein kinase B signaling pathway
PPAR peroxisome proliferator-activated receptor
ptgs2b prostaglandin-endoperoxide synthase 2 variant B
RBCs red blood cells
SIVs subintestinal vessels
SOD superoxide dismutase
Src-FAK steroid receptor coactivator/focal adhesion kinase pathway
SREBP sterol-regulatory element binding proteins
Stat6 signal transducer and the activator of the transcription 6
SV sinus vein
TC total cholesterol
TCM traditional Chinese medicine
TF coagulation factor III
TG triglyceride
TNF-α tumour necrosis factor-alpha
TXA2 thromboxane A2
UCP2 uncoupling protein 2
VEGF vascular endothelial growth factor
VLDL very low-density lipoprotein
VRI VEGF receptor tyrosine kinase inhibitor II
Keywords: natural products, cardiovascular diseases, zebrafish, bioactivity, ethnopharmacology
Citation: Wang Y, Meng X, Song X, Wang L, Meng X, Du X, Yusupov Z, Tojibaev K, Wang Y, He M and Sun M (2025) Evaluation of natural products bioactivity in cardiovascular diseases utilizing zebrafish models: a comprehensive review. Front. Pharmacol. 16:1713417. doi: 10.3389/fphar.2025.1713417
Received: 26 September 2025; Accepted: 31 October 2025;
Published: 27 November 2025.
Edited by:
Michał Tomczyk, Medical University of Bialystok, PolandReviewed by:
Venkata Ramireddy Narala, Yogi Vemana University, IndiaNaga Malleswara Rao Nakka, Mayo Clinic Florida, United States
Copyright © 2025 Wang, Meng, Song, Wang, Meng, Du, Yusupov, Tojibaev, Wang, He and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yunpeng Wang, d2FuZ3lwYmlvQDE2My5jb20=; Min He, aGVtaW5AY2N1Y20uZWR1LmNu; Mengmeng Sun, c3VubW1AY2N1Y20uZWR1LmNu
 Yiming Wang1,2,3
Yiming Wang1,2,3 Ziyoviddin Yusupov
Ziyoviddin Yusupov Min He
Min He Mengmeng Sun
Mengmeng Sun