- 1Department of Gastroenterology, Shenzhen People’s Hospital, The Second Clinical Medical College, Jinan University, Shenzhen, Guangdong, China
- 2Department of General Medicine, Yantian District People’s Hospital, Shenzhen, Guangdong, China
- 3Department of Medical Administration, Huizhou Institute of Occupational Diseases Control and Prevention, Huizhou, Guangdong, China
- 4Department of Emergency, Shenzhen People’s Hospital (The Second Clinical Medical College, Jinan University, The First Affiliated Hospital, Southern University of Science and Technology), Shenzhen, Guangdong, China
- 5College of Rehabilitation Medicine, Jining Medical University, Jining, Shandong, China
- 6Department of Rehabilitation, Shenzhen People’s Hospital (The Second Clinical Medical College, Jinan University, The First Affiliated Hospital, Southern University of Science and Technology), Shenzhen, Guangdong, China
- 7Department of Child and Adolescent Psychiatry, Shenzhen Kangning Hospital, Shenzhen Mental Health Center, Shenzhen, Guangdong, China
- 8Department of Gastroenterology, Shenzhen People’s Hospital (The Second Clinical Medical College, Jinan University, The First Affiliated Hospital, Southern University of Science and Technology), Shenzhen, Guangdong, China
Breast milk-derived extracellular vesicles (MEVs) are natural nanocarriers characterized by their stability, biocompatibility, and low immunogenicity. These small, lipid bilayer-enclosed nanoparticles carry diverse bioactive molecules, including proteins, nucleic acids, and lipids, enabling them to facilitate inter-organismal communication. This review highlights the therapeutic potential of MEVs as innovative drug delivery systems, with a focus on their unique composition, functional properties, and mechanisms of action—from biogenesis and secretion to cellular uptake. We critically examine current methods for isolating and purifying MEVs, addressing challenges related to scalability, purity, cost, and standardization in industrial production. Furthermore, we discuss strategies to enhance the bioavailability and stability of MEVs for pharmaceutical applications. In conclusion, MEVs represent a scalable and cost-effective platform for therapeutic delivery, with significant potential in both nutritional and medicinal contexts. Future research should focus on optimizing production processes and advancing clinical translation to fully harness their capabilities.
1 Introduction
Extracellular vesicles (EVs) are lipid bilayer-enclosed particles released from cells that lack the ability to replicate independently (Vaiaki and Falasca, 2024). They carry proteins, lipids, and nucleic acids, facilitating intercellular communication and pathophysiology (Shen et al., 2022; Pishavar et al., 2022). Encased in a phospholipid bilayer, EVs resist degradation in harsh gastrointestinal tract conditions (Li et al., 2022a; Zhu et al., 2023). Furthermore, EVs have several advantages (Li et al., 2022a; Cheng et al., 2023; Durmaz et al., 2024). First, their high biocompatibility, low immunogenicity, and lack of toxicity in vivo make them promising drug delivery carriers. Second, their double-layered lipid membranes protect therapeutically relevant molecules in the gastrointestinal tract and enable long-term circulation in the body. Third, exosomal membranes can be artificially modified for targeted binding to specific organs and cells, making them promising therapeutic agents in clinical practice. Previously, we reviewed the biogenesis, characteristics, composition, functions, and drug delivery potential of EVs derived from plants, gut microbiota, mesenchymal stem cells, and immune cells in diagnosing and treating gastrointestinal diseases (Li et al., 2022a; Li et al., 2023a; Tian et al., 2023a; Tian et al., 2023b; Liu et al., 2023a; Li et al., 2022b).
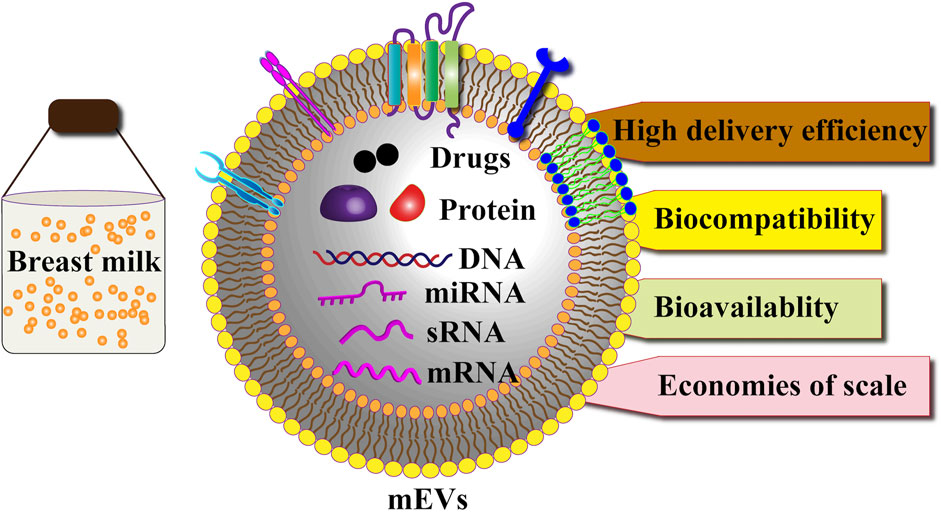
Mammalian milk, a heterogeneous fluid that provides nutrition to newborn mammals, contains several bioactive components, such as proteins, antibodies, and peptides that modulate the immune system modulation, promote cell growth, and exhibit antioxidant effects (Einerhand et al., 2022; Liu et al., 2021; Hobbs et al., 2021). Figure 1 shows the pathway of EVs from the breast tissue to milk. Recently, milk-derived EVs (MEVs) from sources such as humans, goat, and sheep have garnered significant attention for their therapeutic potential in human metabolism, immunology, and physiology. Moreover, MEVs have great potential as drug delivery vehicles and in imaging and therapeutic applications owing to their non-toxicity, high availability, low immunogenicity, and stability (Kim et al., 2023; Timofeeva et al., 2023; Tian et al., 2023c; Li et al., 2022c; Garcia-Martinez et al., 2022). This review explores MEVs, detailing their isolation, purification methods, and therapeutic applications, and summarizes their bioavailability, biocompatibility, and immunogenicity in drug delivery systems. Additionally, the challenges for industrializing of MEV production are highlighted.
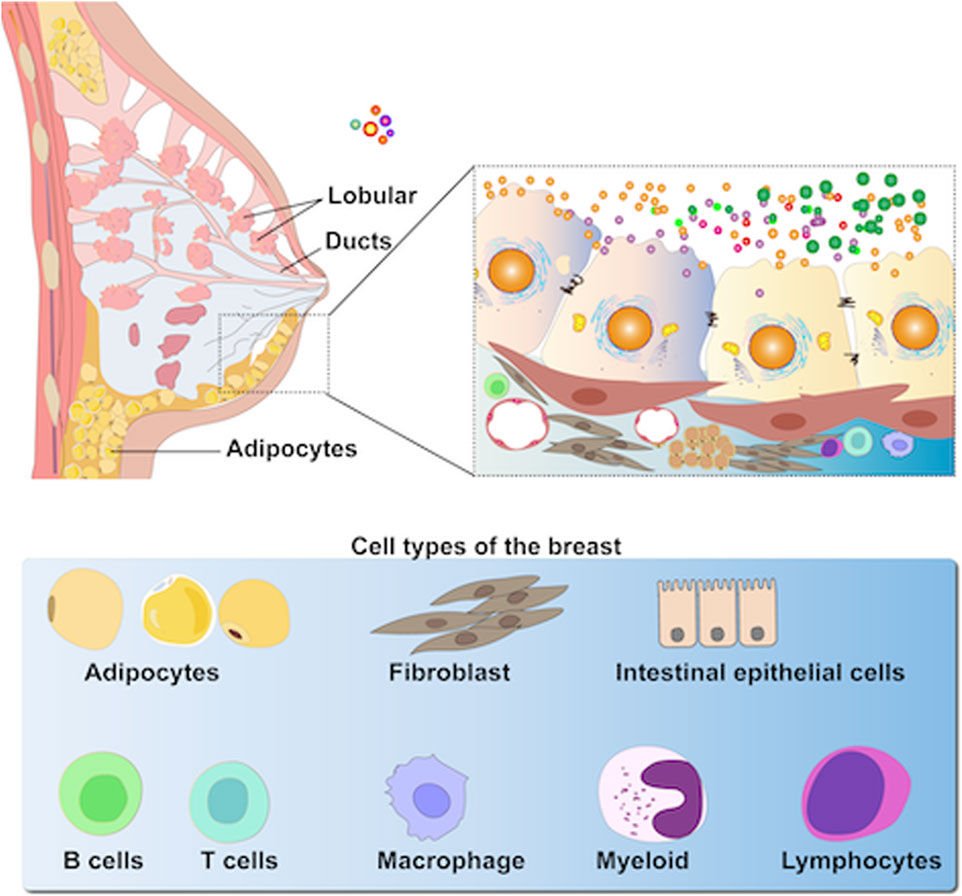
Figure 1. MEVs are highly heterogeneous as they are secreted primarily by epithelial cells within mammary alveoli and stored in the lumen of alveoli adjacent to these cells (including immune cells, stem cells, bacteria, and adipocytes, etc.).
2 Characteristics of MEVs
MEVs share common components with other EVs, including Alix, Flillin1, transmembrane proteins (CD9, CD63, and CD81), integrins, and cell adhesion molecules, while also containing unique proteins, such as testilin, Rab GTPase, and Tsg101, which regulate membrane fusion, interact with cytoskeletal proteins, and participate in endocytosis (van Herwijnen et al., 2016). Moreover, MEVs have specific markers for butyrin, lactase, and xanthine dehydrogenase, and offer unique advantages over other EVs (Vaswani et al., 2021). For instance, resistant glycoproteins (XDH, BTN, and MUC1) encapsulating MEVs and their surface proteins (FLOT1, ICAM1, ALIX, and EpCAM) enhance their resistance to pepsin and ensure stability in the gastrointestinal tract (Benmoussa et al., 2019). Furthermore, MEVs contain mRNAs, microRNAs (miRNAs), and DNAs transferred to offspring through breastfeeding, playing vital roles in infant development, including gastrointestinal structure and function, bone metabolism, endocrine regulation, and metabolism. Beyond their physiological roles, MEVs can cross the intestinal mucosal barrier and enter the bloodstream, enhancing the oral bioavailability of protein and several small-molecule drugs while reducing dosage and toxicity compared to cytotoxic anticancer drugs alone. This section explores the biogenesis and cargo of MEVs.
2.1 MEV biogenesis
EV biogenesis begins with endocytosis of invaginated endosomes from the plasma membrane, predominantly involving both endosomal sorting complex required for transport (ESCRT)-dependent and ESCRT-independent machinery (Figure 2). The detailed process of EV biogenesis is available in our previous reviews (Li et al., 2022a; Li et al., 2023a; Li et al., 2022b).
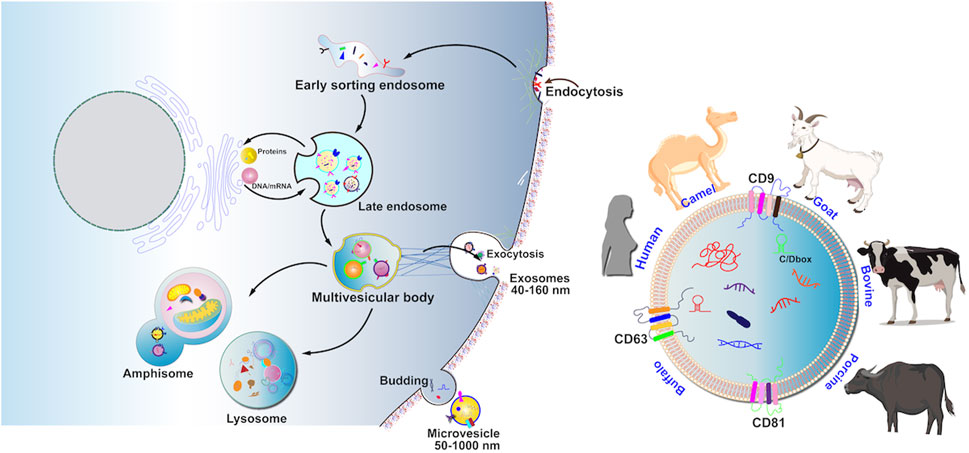
Figure 2. MEV production involves four processes including cargo sorting, MVB formation, maturation and transportation of MVB, and fusion of MVB with the cell membrane. Recent research-based articles reported on MEVVs in the animal kingdom, including cattle, humans, pigs, goats, donkeys, buffaloes, and camels.
2.2 Cargoes in MEVs
MEV cargoes primarily contain proteins, lipids, and nucleic acids, with variations depending on the origin of the MEV cells, individual differences, and physiological conditions. These cargoes of MEVs are delivered to recipient cells, where they carry out physiological functions and biological actions. This section reviews the functions and biological actions of MEV cargoes.
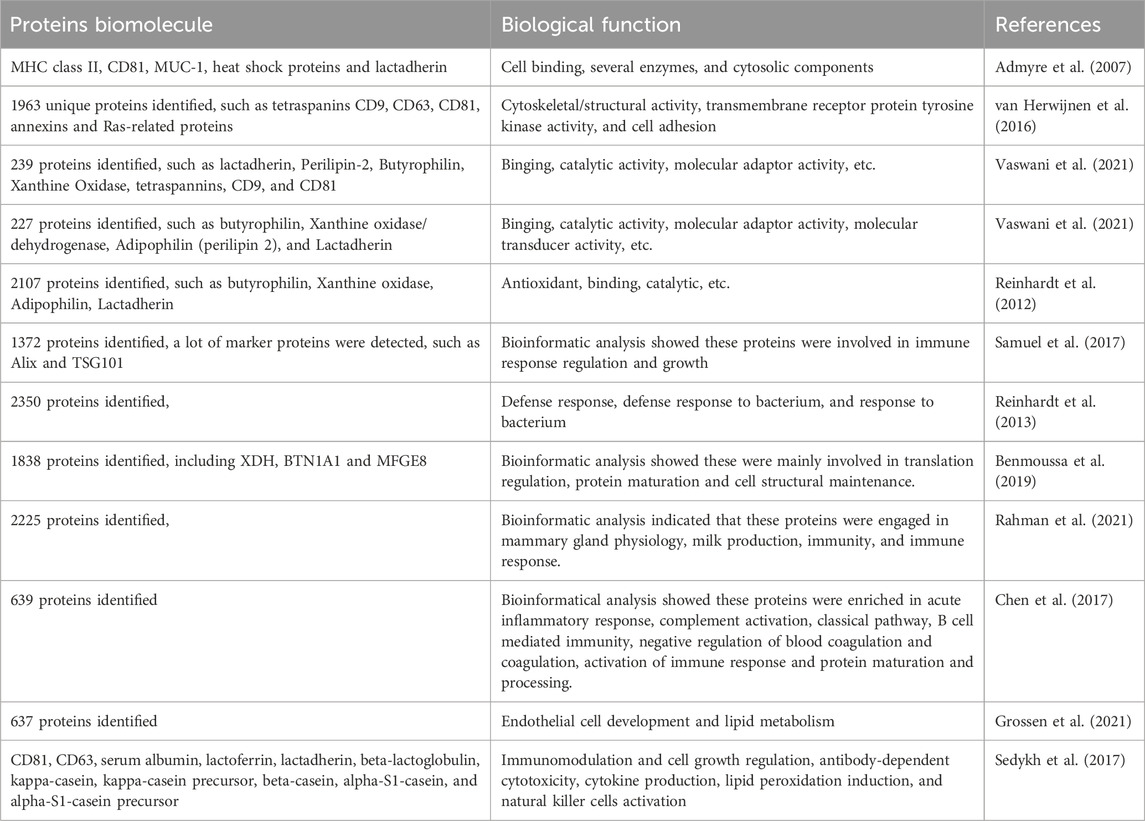
Proteome analysis of MEVs has been used to detect over 2000 proteins, which vary across different MEVs and proteomic methods. For example, more than 2000 proteins have been detected in bovine MEVs, whereas 1963 proteins have been identified in human MEVs (van Herwijnen et al., 2016; Reinhardt et al., 2012). MEV proteins are categorized into specific and functional proteins (Table 1). Specific proteins, initially identified in human and bovine MEVs, have not been detected in EVs derived from other biofluids or cell types and may serve as potential MEV biomarkers. Van Herwijnen et al identified 1963 proteins in human MEVs, of which 633 were exclusive to MEV. Bioinformatics analysis revealed that these proteins are significantly enriched in cytoskeletal/structural activity, transmembrane receptor protein tyrosine kinase activity, and cell adhesion (van Herwijnen et al., 2016). Admyre et al demonstrate that specific MEV proteins can inhibit anti-CD3-induced cytokine production in peripheral blood mononuclear cells and increase the number of Foxp3+CD4+CD25+ T regulatory cells. However, the specific proteins identified are currently limited. (Admyre et al., 2007). Moreover, bovine MEVs contain unique adhesion proteins such as intercellular cell adhesion molecule-1, surface receptors, and glycoproteins that play vital roles in signal transduction pathways and molecular (including drug) delivery (Pullan et al., 2022; Wehbe and Kreydiyyeh, 2022). MEVs also carry several functional proteins involved in cell growth, tissue regeneration, immune modulation, and drug delivery. However, few functional proteins have been identified, and this area requires further investigation.
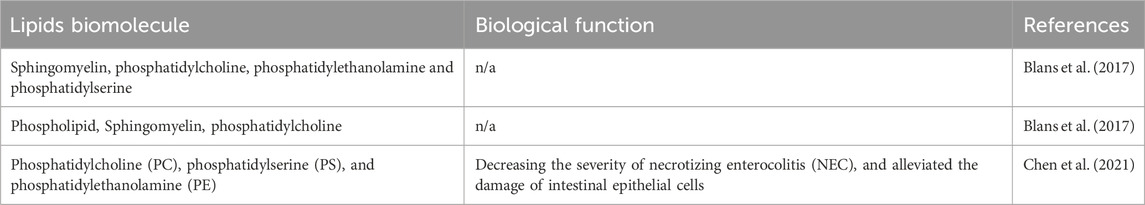
MEVs contain lipid molecules embedded in their membrane structure, including phosphatidylcholine, phosphatidylethanolamine, and phosphatidylserine, which maintain their structure and stability while facilitating cargo transport to recipient cells (Table 2) (Grossen et al., 2021). Moreover, bioactive lipids in MEVs are crucial for gastrointestinal health, supporting neonatal intestinal development, protecting the intestinal epithelium, and inhibiting intestinal inflammation. Studies on the lipid bioactivities of MEVs are relatively scarce. Lipidomic profiling has identified 395 lipids in human MEVs (Chen et al., 2021). Additionally, the top 50 lipids significantly decrease necrotizing enterocolitis (NEC) severity and alleviate intestinal epithelial cell damage by inhibiting the ERK/MAPK pathway activity (Chen et al., 2021). However, current technology faces challenges in isolating and identifying lipids from MEVs (Ong et al., 2021; Blans et al., 2017).
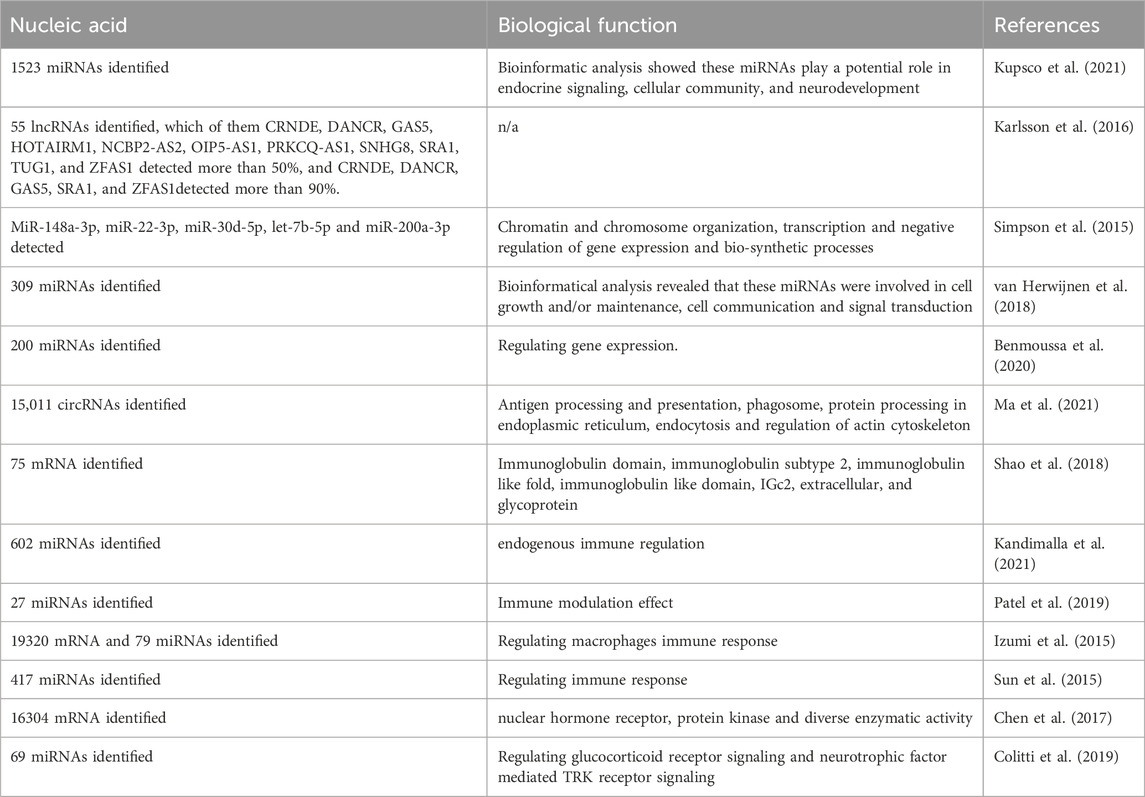
Besides their protein and lipid profiles, MEVs contain several nucleic acids, including mRNAs, microRNAs, and double-stranded DNAs. Studies show that these nucleic acids contribute to their anti-inflammatory and immunomodulatory activities (Table 3). Microarray analyses have revealed 19,320 mRNAs in bovine MEVs (Izumi et al., 2015). Moreover, bovine MEVs are taken up by human macrophages (THP-1 cells), promoting their differentiation through cargo mRNAs (Izumi et al., 2015). Another study identified 16,304 mRNAs, including 13,895 known and 2,409 novel mRNAs, in porcine MEVs (Chen et al., 2017). Moreover, bioinformatics analysis indicates that most of these mRNAs are mainly involved in binding activities such as nuclear hormone receptor and protein kinase functions and diverse enzymatic activities, including transcription coactivators, exonucleases, and small conjugating protein ligases (Chen et al., 2017). Ma et al found that miR-3168, enriched in breast MEVs, plays a crucial role in early neurodevelopment and neural stem cell differentiation in preterm infants (Ma et al., 2024). Gao et al discovered that miR-30a-5p, a key component of coat MEVs, ameliorates the intestinal epithelial cell-6 inflammatory response and it attenuates lipopolysaccharide (LPS)-induced intestinal inflammation (Gao et al., 2024). However, research on DNA function in MEVs is scarce. Table 3 highlights a few nucleic acids and their biological functions in MEVs, offering just a glimpse into this area of research. Therefore, future studies should explore this promising field.
2.3 The biological functions of MEVs
Beyond their established biological roles, MEVs exhibit emerging therapeutic functions. They mediate epigenetic regulation by delivering miRNAs (e.g., human milk miR-148a suppresses DNMT1 in colorectal cancer cells) and circRNAs (e.g., porcine milk circ-XPO4 enhances intestinal IgA via miR-221-5p inhibition) (Kosaka et al., 2010; Zhou et al., 2021; Zeng et al., 2021; Samuel et al., 2021; Melnik and Schmitz, 2017). MEVs also modulate metabolic homeostasis, as bovine milk exosomes restore short-chain fatty acids (acetate/butyrate) and L-arginine while downregulating pro-inflammatory lipids in colitis models (Du et al., 2023). Additionally, they reshape gut microbiota composition, increasing Akkermansia abundance and butyrate-producing bacteria (e.g., Lachnospiraceae) to reinforce intestinal barrier integrity (Tong et al., 2021; Du et al., 2022). Crucially, MEVs facilitate maternal-infant communication by transferring immune-related miRNAs (e.g., miR-320/375 in colostrum) and circRNAs that activate VEGF signaling to promote neonatal intestinal development (Kosaka et al., 2010; Zhou et al., 2021).
3 Isolation and purification of MEVs
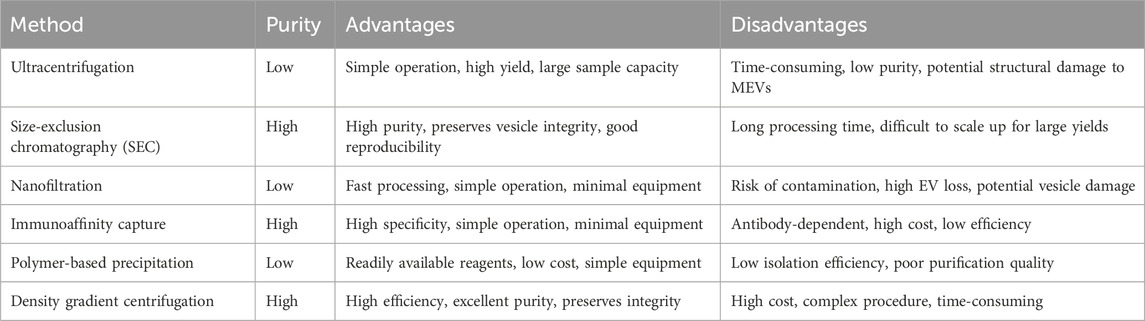
The complex composition of milk complicates MEV isolation and purification. Methods such as ultracentrifugation, size-exclusion chromatography, nanofiltration, immunoaffinity, and polymer-based precipitation are used to isolate and purify MEVs based on their different physicochemical properties. However, no standardized and efficient technique exists for this process. Table 4; Figure 3 present the advantages and disadvantages of various methods.
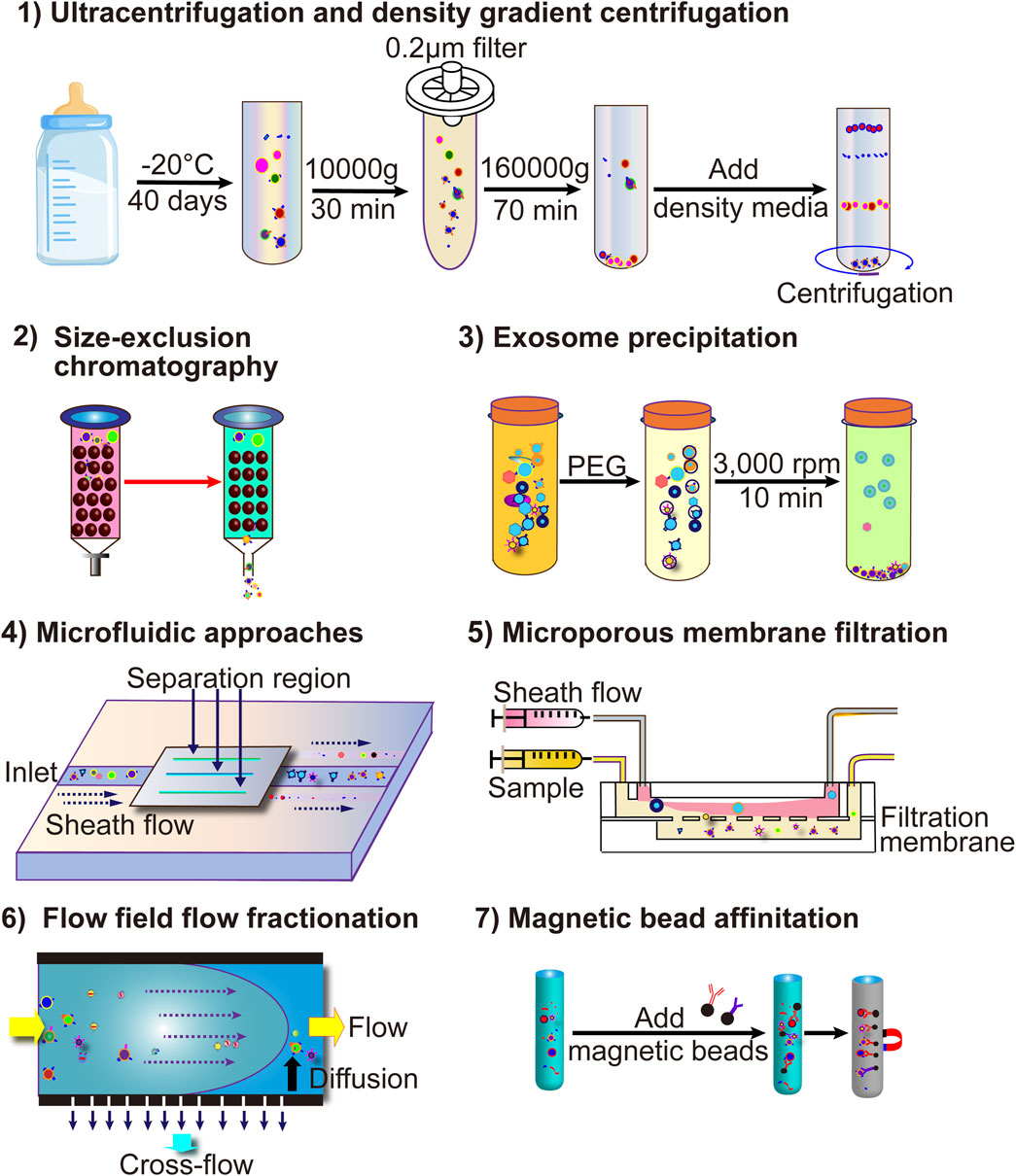
Figure 3. The current more common techniques for MEV isolation include ultracentrifugation, ultrafiltration, immunoaffinity capture, polymer co-precipitation, and microfluidic technology-based EV separation methods.
3.1 Ultracentrifugation
Ultracentrifugation, a common technique for isolating MEVs, involves differential centrifugation (low-speed centrifugation combined with high-speed centrifugation) of milk samples to remove cell debris and free proteins, yielding purified MEVs (Thery et al., 2006). Although ultracentrifugation is effective for obtaining large quantities, it is time-consuming, highly non-specific, and damages MEVs (Table 4) (Zhong et al., 2021).
3.2 Size-exclusion chromatography (SEC)
SEC is an important technique for isolating MEVs based on the pore size of the stationary phase relative to EV size (Sidhom et al., 2020). As milk samples containing EVs pass through the gel column, substances smaller than the pore size are trapped, while others are removed (Sidhom et al., 2020). SEC offers several advantages for MEV isolation and purification, including high purity, integrity, and repeatability (Sidhom et al., 2020; Jia et al., 2022). However, its long running time and limited scalability are drawbacks (Table 4) (Sidhom et al., 2020; Jia et al., 2022).
3.3 Nanofiltration
Nanofiltration, commonly performed to isolate and purify MEVs based on EV size using the same principle as SEC, (Haraszti et al., 2018), offers several advantages, such as shorter running time, easy operation, and simple equipment. However, it is prone to contamination, EV loss, and damage (Table 4) (Haraszti et al., 2018).
3.4 Immunoaffinity
Immunoaffinity is a technique used to isolate and purify MEVs based on the interaction between EV surface membrane antigens and monoclonal antibodies. Specific markers such as CD9, CD63, CD81, HSP70, and TSG101 are present on EV surfaces (Thery et al., 2002; Mathivanan and Simpson, 2009; Li Y. et al., 2023; Vaswani et al., 2019; Ross et al., 2021). Therefore, specific antibodies, including anti-CD9, anti-CD63, anti-CD81, anti-HSP70, and anti-TSG10, employ immunoaffinity activity and capture targeted EVs (Sedykh et al., 2022). This method offers high specificity, ease of operation, and simple equipment. However, it is limited by marker dependence, high cost, and low efficiency (Table 4) (Shao et al., 2018).
3.5 Polymer-based precipitation
Polymer-based precipitation, a commonly used method for extracting MEVs, reduces EV solubility by interacting with water molecules, causing precipitation (Kandimalla et al., 2021a). Compared to other isolation methods, it is easy to use, inexpensive, and requires simple equipment. However, it has low isolation efficiency and poorly purifies MEVs (Table 4) (Patel et al., 2019; Szatanek et al., 2017).
3.6 Density gradient centrifugation
Density gradient centrifugation enriches EVs based on the sedimentation coefficients of different substances (Hata et al., 2010). We previously reported that sucrose gradient centrifugation effectively extracts plant-derived EV nanoparticles (Zhu et al., 2023). This method offers greater efficiency, higher purity, and better integrity than the other techniques (Szatanek et al., 2015). However, it is expensive, complex, and time-consuming (Table 4) (Szatanek et al., 2015).
Various techniques for isolating and purifying MEVs, based on different principles, have distinct advantages and disadvantages, making standardization difficult. Combining SEC with ultracentrifugation can enhance EV enrichment, purity, and integrity (Vaswani et al., 2017). We anticipate that simpler and more efficient methods or various commercial separation kits will be developed for broader clinical applications.
3.7 Recent advances in scalable isolation
Recent protocol optimizations demonstrate promising pathways toward GMP-compatible manufacturing of MEVs. Enzymatic pretreatment with microbial rennet (0.5% vol/vol, 37°C, 20 min) effectively eliminates casein contaminants while preserving vesicular integrity, coupled with dual centrifugation (3,000×g) for lipid removal and pre-processing freezing at −80°C to enhance purity. Subsequent isolation via differential centrifugation—scalable to tangential flow filtration—yields MEVs suitable for therapeutic applications. Critical quality assessment requires nanoparticle tracking analysis for dimensional profiling, ExoView SP-IRIS for tetraspanin validation, and Western blot for residual casein detection (Medel-Martinez et al., 2024). Various techniques for isolating and purifying MEVs, based on different principles, have distinct advantages and disadvantages, making standardization difficult. Combining SEC with ultracentrifugation can enhance EV enrichment, purity, and integrity (Medel-Martinez et al., 2024).
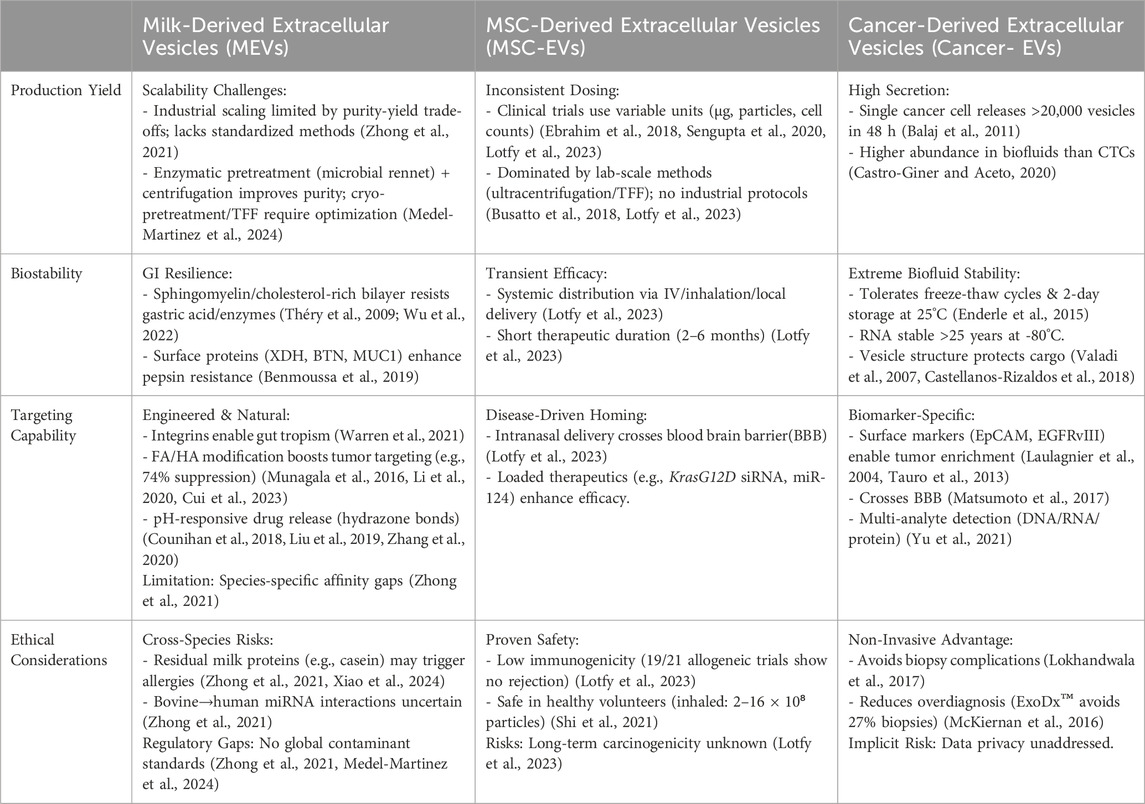
Current challenges in the scalable production of MEVs involve multifaceted obstacles. Industrial requirements for purity and yield fundamentally diverge from laboratory research paradigms, where the absence of standardized separation/purification methods remains the primary technical bottleneck for clinical translation—existing technologies struggle to simultaneously ensure exosome preparation homogeneity and optimized purification efficiency (Zhong et al., 2021). Further complicating the landscape are regulatory and commercialization barriers: undefined safety thresholds for interspecies applications (e.g., bovine-to-human), lack of global contaminant standards, and quality control complexities arising from source-dependent heterogeneity, compounded by inadequate regulatory frameworks for commercialization. Additionally, shortages of skilled professionals in industrial-scale exosome processing exacerbate these challenges (Zhong et al., 2021). To address these constraints, an integrated approach is essential: advancing localized implementation of ISEV characterization guidelines to establish technical benchmarks, strengthening regulatory collaboration to define safety and contaminant limits, developing scalable separation platforms (e.g., tangential flow filtration kits), and implementing specialized training programs (Medel-Martinez et al., 2024). This comprehensive strategy will systematically bridge the gap between laboratory research and industrial-scale manufacturing, thus enabling a sustainable pathway toward clinical adoption (Medel-Martinez et al., 2024). Table 5 systematically compares key characteristics of MEVs with other widely studied extracellular vesicles—mesenchymal stem cell-derived EVs and cancer-derived EVs—highlighting critical challenges in scalability, stability, targeting capability, and ethical considerations for clinical translation.
4 Interactions of MEVs with the gut microbiome
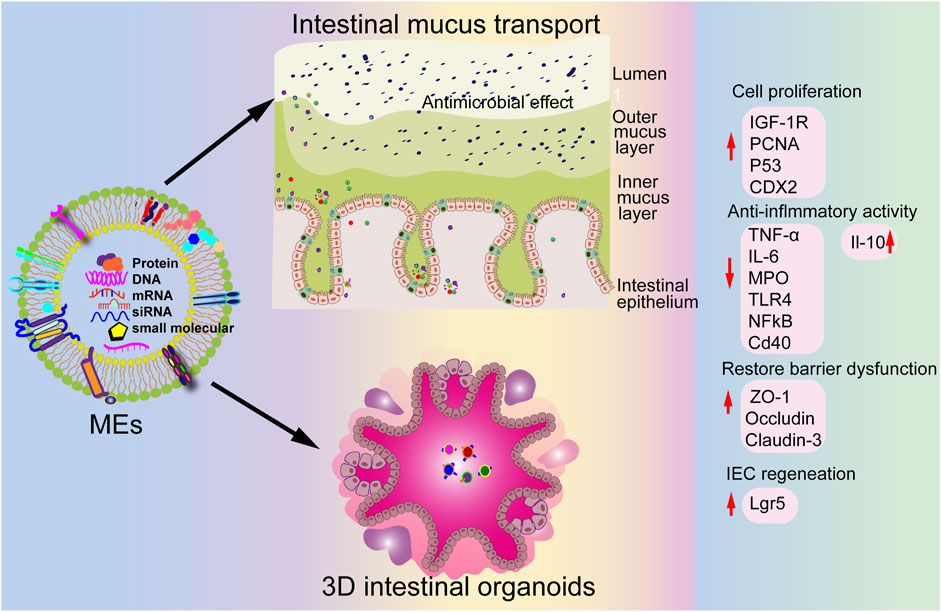
The gut microbiome comprises more than 100 trillion commensal microorganisms, including bacteria, archaea, fungi, and protozoa, residing in the human gastrointestinal tract (Ley et al., 2006). It plays an essential role in gastrointestinal mucosal function and structure, host protection against pathogens, and maintenance of overall human health (Maynard et al., 2012). Imbalances in the gut microbiota are associated with diseases such as inflammatory bowel disease (IBD), rheumatoid arthritis, diabetes, and obesity in humans (Maynard et al., 2012). Portulaca oleracea L-derived EV-like nanoparticles promote microbiota balance and increase probiotic Lactobacillus reuteri proliferation in dextran sulfate sodium salt (DSS)-induced colitis, thereby alleviating colitis (Zhu et al., 2023). Additionally, MEVs play a crucial role in gut microbiota communication, with miR-21, miR-497, and miR-166a MEV cargoes surviving the gastrointestinal tract to target yegH, ptsG, and rpoC, promoting the growth of Escherichia coli and Lactobacillus plantarum in vivo (Yu et al., 2019). Moreover, Holstein cow MEVs restore Enterorhabdus and unclassified_Bacteroidia levels while increasing the probiotic Akkermansia abundance, contributing to reshaping the gut microbiota in DSS-induced colitis (Figure 4) (Tong et al., 2021). In addition, oral administration of bovine MEVs increases the abundance of several beneficial gut microbes, including Ruminococcaceae, Lachnospiraceae, and Akkermansia_muciniphila, and suppresses pro-inflammatory bacteria levels such as Proteobacteria in osteoarthritis, alleviating cartilage degeneration, enhancing matrix synthesis, and reducing cartilage-degrading enzymes (Liu et al., 2023b). These findings suggest that EVs may protect and maintain gut microbiota balance and offer a promising strategy for treating of human diseases.
5 Biological activities of MEVs
Studies show that MEVs contain several bioactive components that affect breastfeeding mothers, infants, and even adults who consume milk (Zempleni et al., 2017). MEVs are stable under harsh gastrointestinal conditions, protecting their cargoes for physiological activity (Rahman et al., 2019; Pieters et al., 2015; Izumi et al., 2012). Additionally, they exhibit excellent biocompatibility, being taken up by various cell types, including intestinal epithelial cells (IECs), macrophages, and vascular endothelial cells, and can cross the blood-brain barrier (Del Pozo-Acebo et al., 2021; Wolf et al., 2015; Prasadani et al., 2024; Liu et al., 2024; Zeng et al., 2019). Thus, MEVs can reach various tissues and perform diverse physiological functions.
5.1 Intestinal health
Numerous studies show that MEVs are crucial for maintaining intestinal health. Martin et al indicate that MEVs protect IECs against H2O2-induced oxidative stress (Martin et al., 2018). Moreover, MEVs promote tight-junction proteins ZO-1, claudin-1, and occludin levels in vitro and increase goblet cell numbers in vivo, helping to alleviate NEC (He S. et al., 2021; Li et al., 2019). Similarly, Chiba et al reveal that MEVs increase ZO-1 expression by inhibiting the expression of key cellular stress gene REDD1 in vitro (C et al., 2023). Additionally, MEVs enhance intestinal epithelial cell viability and promote their growth (Hock et al., 2017).
5.2 Bone and muscle health
Bone health relies on the balance between osteoblasts (cells that form bone) and osteoclasts (cells that resorb bone). Disruptions in this balance can lead to bone diseases such as osteoporosis. Previous studies indicate that MEVs promote osteogenesis and inhibit osteoclastogenesis, enhancing osteoblast proliferation and differentiation in vitro and in vivo (Yun et al., 2020). These findings suggest that MEVs may stimulate bone formation, prevent osteoporosis (Yun et al., 2020), and inhibit osteoclast differentiation, thereby reducing bone resorption (Kim et al., 2023; Melnik et al., 2021). These findings suggest that MEVs may play a vital role in maintaining bone density and strength by modulating the activity of bone-forming and bone-resorbing cells (Kim et al., 2023; Melnik et al., 2021). These findings suggest that MEVs may play a vital role in maintaining bone density and strength by modulating the activity of bone-forming and bone-resorbing cells (Kim et al., 2023; Melnik et al., 2021).
Muscle maintenance and growth depend on a balance between protein synthesis and degradation. MEVs support muscle health by promoting myogenesis and tissue formation, particularly in conditions such as sarcopenia and age-related muscle loss. MEVs contain growth factors and proteins that stimulate muscle protein synthesis, inhibit its degradation, and promote muscle hypertrophy while preventing muscle wasting (Parry et al., 2019). In addition, MEVs enhance muscle cell proliferation and development by activating the AKT/mTOR pathway and myogenic regulatory factors (Meng et al., 2023). Therefore, MEVs may offer a novel dietary approach to combat muscle degeneration and support overall muscle health.
5.3 Anti-inflammatory and antioxidant activities
Chronic inflammation contributes to the development of several diseases, such as IBD (Rubin et al., 2012; Fernandes et al., 2024; Yan and Shao, 2024). MEVs may help reduce inflammation by inhibiting the expression of pro-inflammatory cytokines such as interleukin-6 and tumor necrosis factor-alpha, thereby alleviating inflammatory response (Cui et al., 2023). Additionally, MEVs reduce pro-inflammatory cytokine levels and attenuate LPS-induced inflammation in macrophages (Matic and Dia, 2022).
Oxidative stress results from an imbalance between reactive oxygen species (ROS) production and antioxidant defenses, contributing to various chronic diseases (Schieber and Chandel, 2014; Korovesis et al., 2023). MEVs have antioxidant properties owing to their antioxidant substance cargoes, such as superoxide dismutase and glutathione peroxidase, which neutralize ROS and reduce oxidative stress (Feng et al., 2021). Moreover, MEVs decrease ROS production and mitigate oxidative stress by inhibiting NOX2 expression and ROS production (Rashidi et al., 2022). They also activate the Nrf2 pathway, enhancing antioxidant gene expression and cellular defenses against oxidative damage (Wang et al., 2021).
5.4 Neuronal development and brain health
MEVs have recently gained attention for their potential role in neuronal development and brain health. They cross the blood-brain barrier, offer neuroprotection, and enhance cognitive function, making them promising for maintaining brain health and treating neurological disorders (Zhou et al., 2022). MEVs promote neural stem cell differentiation and proliferation and support neuronal development by regulating genes critical for neurogenesis and synaptic plasticity (Jiang et al., 2021). MEVs exhibit neuroprotective effects by neutralizing ROS and downregulating pro-inflammatory cytokines (Zempleni et al., 2019). They also support cognitive function and overall brain health by contributing phospholipids and sphingolipids to neuronal membrane integrity and facilitating neurotransmission, which is crucial for cognitive processes (Wang et al., 2024). Additionally, MEVs enhance synaptic plasticity, a key mechanism underlying learning and memory, and regulate synaptic strength by targeting specific synaptic proteins (Wang et al., 2024).
5.5 Immunity
Studies show that MEVs play crucial roles in immune regulation by modulating T and B cell differentiation, suppressing inflammatory cytokine production in macrophages (Zhang et al., 2019), boosting macrophage phagocytosis, and promoting dendritic cell maturation, thereby enhancing innate immune responses (He Y. et al., 2021). Furthermore, MEVs carry antigens to immune cells to activate antigen-specific T cells and influence adaptive immunity (Komine-Aizawa et al., 2020). They also promote the proliferation and function of regulatory T cells, exerting a key role in maintaining immune tolerance and preventing autoimmune diseases (Munagala et al., 2016).
5.6 Immunogenicity and safety profile of MEVs
MEVs demonstrate inherently low immunogenicity and favorable biocompatibility, positioning them as promising drug delivery vehicles (Zhong et al., 2021; Cui et al., 2023). Critical evidence reveals that MEVs evade significant immune activation across administration routes: repeated intravenous injections in mice (up to 6 mg/kg) induce no systemic anaphylaxis or histamine elevation, while chronic oral dosing in rats (300 μg/kg/day for 21 days) maintains serum IgG/IgM levels comparable to controls. (Xiao et al., 2024). This immunological inertness extends to cellular interactions, where MEVs are efficiently internalized by macrophages at concentrations up to 200 μg/mL without cytotoxicity. Systemic safety assessments further confirm the absence of hepatic/renal toxicity, hematological abnormalities, or pathological changes in major organs (Somiya et al., 2018). Notably, zwitterionic modifications (e.g., DSPE-Hyd-PMPC functionalization) enhance this intrinsic safety by mitigating immunogenicity risks associated with conventional PEGylation (Xiao et al., 2024). Despite these advantages, translational challenges persist, including potential allergenicity from residual milk proteins (e.g., casein) in susceptible populations and species-specific immune response variations that warrant human-relevant validation (Xiao et al., 2024; Somiya et al., 2018). Collectively, the robust safety profile of MEVs supports their clinical translation, though ultra-purification protocols and engineered surface designs remain essential for therapeutic applications.
6 Milk EVs in drug delivery
Small molecules, proteins, and oligonucleotides offer promising treatments for various diseases; however, their poor bioavailability, non-targeted accumulation, and instability limit their widespread application. MEVs, with their high bioavailability, non-toxicity, low immunogenicity, and stability, are gaining attention as potential drug carriers.
6.1 Oral delivery advantages of MEVs
MEVs exhibit exceptional gastrointestinal stability due to their unique structural composition. The sphingomyelin- and cholesterol-rich phospholipid bilayer membrane confers inherent resistance against gastric acid and digestive enzyme degradation (Munagala et al., 2016; Théry et al., 2009). Experimentally, orally administered MEVs maintain structural integrity while traversing the intestinal barrier and selectively targeting gut cells (Wu et al., 2022; Vashisht et al., 2017). This stability enables effective oral delivery of therapeutic cargo, as evidenced by MEVs-loaded insulin and curcumin retaining bioactivity and significantly enhancing oral bioavailability (Wu et al., 2022; Carobolante et al., 2020). MEVs further demonstrate natural tropism for intestinal tissues through surface proteins (e.g., integrins), facilitating mucus penetration and cellular uptake (Warren et al., 2021). Critically, MEVs exhibit no systemic toxicity, with distribution studies confirming prolonged GI retention after oral administration versus hepatic/renal accumulation following intravenous injection (Samuel et al., 2021; Tong et al., 2021).
6.2 Significant advances in therapeutic delivery
Substantial progress has been achieved in utilizing MEVs as nanocarriers for chemotherapeutic agents and nucleic acid-based therapies. Since 2016, bovine MEVs have been successfully engineered to encapsulate diverse chemotherapeutics including curcumin, withaferin A (WFA), anthocyanins, paclitaxel (PAC), and doxorubicin. (Vashisht et al., 2017; Warren et al., 2021; Agrawal et al., 2017; Luo et al., 2021).Critically, orally delivered MEVs exhibit exceptional resistance to digestive degradation and enhance intestinal permeability—validated in Caco-2 monolayer models. (Vashisht et al., 2017). These vesicles significantly improve drug stability; for instance, curcumin-loaded MEVs demonstrate markedly higher stability in PBS compared to free drug formulations (Vashisht et al., 2017). In vivo efficacy is evidenced by WFA-loaded MEVs significantly suppressing lung tumor xenograft growth (Munagala et al., 2016), while PAC-loaded MEVs reduce hepatorenal/systemic toxicity and immunogenicity relative to intravenous administration (Agrawal et al., 2017).
Beyond small-molecule delivery, MEVs effectively transport nucleic acid therapeutics. β-Catenin siRNA loaded via lipofection mediates potent gene knockdown in vitro versus scrambled controls (Aqil et al., 2019), while *hsa-miR148a-3p*-enriched MEVs achieve functional delivery in HepG2 and Caco-2 cells, with microarray analyses confirming their utility as miRNA nanocarriers (Del Pozo-Acebo et al., 2021).
6.3 Cargo loading
Munagala et al first reported that MEVs, as drug delivery systems, load small drug molecule compounds, including curcumin, withaferin, anthocyanidins, paclitaxel (PAC), and docetaxel, targeting lung and breast cancer cells while enhancing anticancer and anti-inflammatory effects (Munagala et al., 2016). MEVs containing isobavachalcone and polymyxin B effectively combat pathogenic bacteria (Xu et al., 2024), eliminating 99% of multidrug-resistant bacterial pathogens and nearly 100% microbial inhibition in animal models (Xu et al., 2024). Resveratrol (RSV), a natural polyphenolic phytoalexin, exhibits antidiabetic, anti-inflammatory, anticancer, wound healing, and antioxidant effects; however, its poor solubility and stability limit its clinical application (Summerlin et al., 2015; Monika and Sardana, 2017). MEVs loaded with RSV enhance its oral bioavailability and effectively reduce inflammation in experimental colitis (Esfahani et al., 2024). miRNAs, endogenous small non-coding RNA molecules, regulate gene expression post-transcriptionally (Bartel, 2009). and offer potential avenues for treating human diseases (Kazemi Shariat Panahi et al., 2024). However, instability and rapid degradation present challenges (Kazemi Shariat Panahi et al., 2024). Meng et al report that MEVs containing miR-146a (MEVs-miR-146a) suppress myocardial tissue apoptosis reduce inflammatory factor expression, and improve cardiac function by inhibiting the IRAK1/TRAF6/NF-κB signaling pathway after myocardial ischemia-reperfusion injury (MIRI), suggesting a promising strategy for MIRI treatment (Meng et al., 2024). MEVs can deliver oral chemotherapeutic drugs, enhancing their efficacy and reducing toxicity. For instance, MEVs carrying PAC significantly reduced liver, renal, and systemic toxicity while inhibiting the growth of human lung tumor xenografts in nude mice, compared to PAC treatment alone (Agrawal et al., 2017). Therefore, MEVs are promising natural drug vehicles for proteins, drugs, and nucleic acids in disease treatment.
6.4 MEV delivery methods
Various strategies, including co-incubation, electroporation, and freeze-thaw cycles, have been used recently to load therapeutic molecules into MEVs. Co-incubation, the simplest and most effective passive drug-loading strategy, allows the loading of photosensitizer chlorin e6, which is crucial for precision treatment of deep solid tumors (Guo et al., 2023). Moreover, drugs such as PAC, curcumin, and docetaxel can be loaded through co-incubation in an appropriate buffer. However, co-incubation has the disadvantage of low load efficiency.
Electroporation utilizes electrical current to disrupt the EV phospholipid bilayer, forming temporary pores through which small molecules can enter. After this process, the membrane integrity of MEVs is restored. Electroporation is generally used to load siRNAs or miRNAs, such as miR-146a and miR-31-5p (Warren et al., 2021; Meng et al., 2024; Yan et al., 2022). However, electroporation may cause membrane instability, RNA aggregation, and low loading efficiency (Luan et al., 2017). This method often causes MEV aggregation and has low loading efficiency (S et al., 2016).
Mechanical sonication involves using shear force to disrupt MEV membrane integrity, allowing drug molecules to diffuse into the MEVs during membrane deformation (Luan et al., 2017). Sonication is primarily used for loading proteins and hydrophobic drugs (Tian et al., 2023c). This method has a better loading efficiency than that of other methods (Tian et al., 2023c). However, its disadvantages include time wastage and MEV degradation (Luan et al., 2017). Figure 5 shows the exogenous method for loading various therapeutics into MEVs.
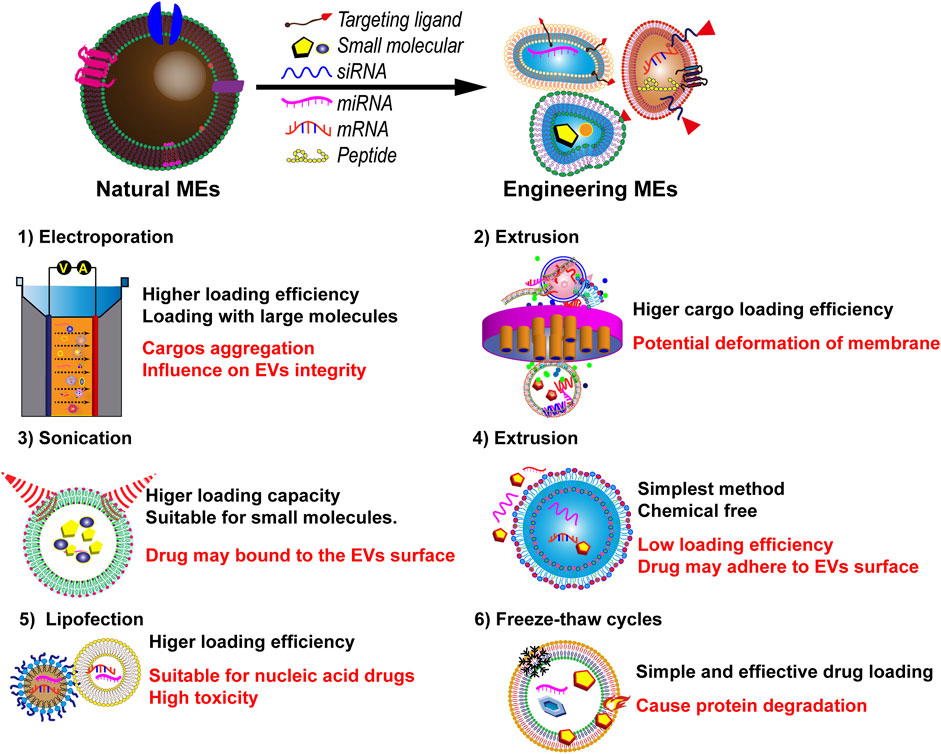
Figure 5. Various current techniques for loading therapeutics into MEVs, and a comparison of their advantages and disadvantages.
6.5 Targeted modification of MEVs
MEVs exhibit considerable promise as oral drug carriers owing to their cross-species tolerance and capacity to traverse gastrointestinal barriers (Zhong et al., 2021). Their evolutionarily conserved surface proteins (e.g., CD9, CD63) facilitate uptake by human intestinal cells, enabling therapeutic efficacy in preclinical models (Kalluri and LeBleu, 2020). However, inherent species disparities may introduce functional variations: differential affinities of bovine exosomal ligands for human receptors could impact cellular internalization, while interspecies divergence in miRNA-mRNA interactions might attenuate regulatory functions (Zhong et al., 2021). Biodistribution patterns—characterized by GI retention after oral administration versus hepatic/renal accumulation following intravenous delivery—may exhibit cross-species variability due to distinct immune clearance (Agrawal et al., 2017; Jang et al., 2021). To overcome these limitations and further enhance targeting specificity, significant advances have been made in bioengineering MEVs. The core technologies primarily involve ligand functionalization employing the versatile post-insertion technique. This strategy entails pre-conjugating the targeting molecule (e.g., folic acid (FA), hyaluronic acid (HA), targeting peptides, or antibodies) to a phospholipid anchor molecule, such as DSPE-PEG2000 or phosphatidylethanolamine (PE), followed by its hydrophobic insertion into the MEVs’ lipid bilayer. This achieves a mild, efficient, and non-destructive modification (Cui et al., 2023; Jang et al., 2023).
Functionalization of MEVs with tumor-specific ligands has demonstrated substantial improvements in active targeting. For instance, FA modification leverages the overexpression of folate receptors on certain cancer cells. Orally administered MEVs co-loaded with wheat germ agglutinin (WGA) and FA (Exo-WGA-FA) significantly increased the tumor suppression rate from 50% to 74% (*p* = 0.016) in a lung cancer model, mediated by FA receptor-specific internalization (Munagala et al., 2016). Similarly, HA coating utilizes the binding between HA and the CD44 receptor overexpressed on tumor cells. HA-DSPE-PEG2000 conjugates, formed via amide condensation (validated by NMR and FTIR), are anchored onto the MEVs surface, significantly enhancing the enrichment and therapeutic efficacy of payloads like miR-204 or doxorubicin within tumor tissues (Cui et al., 2023; Cui et al., 2022). Furthermore, peptide display (e.g., the tumor-penetrating peptide iRGD), antibody conjugation (targeting ubiquitous EV membrane proteins like CD63/CD9 or disease-specific antigens), and aptamer modification (e.g., EpCAM aptamer) have also been effectively employed to reprogram the tropism of MEVs towards specific pathological sites (Zhong et al., 2021).
Collectively, these engineering strategies not only improve the accumulation of MEVs at disease sites but also enhance drug delivery efficiency through receptor-mediated endocytosis, while largely preserving their inherent low immunogenicity. They establish a robust technical foundation for the clinical translation of targeted therapies based on engineered milk exosomes (Cui et al., 2023; Jang et al., 2023). To address these challenges, engineering strategies such as surface modification with targeting peptides (e.g., iRGD) or hybrid vesicle systems represent viable approaches to bridge species-specific gaps, as discussed below (Carobolante et al., 2020).
Recent modifications to MEV surfaces enhance drug delivery targeting. While folate receptors are minimally expressed in normal tissues, they are overexpressed in some cancer cells, such as non-small cell lung cancer cells and lung adenocarcinoma cells (Shi et al., 2015; Kandimalla et al., 2021b). Therefore, folic acid-conjugated MEVs can be used to precisely target tumor sites in clinical applications. For instance, folic acid-modified MEVs loaded with apherin A and PAC significantly suppress tumor cell growth compared to that of non-functionalized surface MEVs while reducing system toxicity (Munagala et al., 2016; Kandimalla et al., 2021b). The hyaluronic acid receptor CD44 is significantly overexpressed in pancreatic, lung, ovarian, and breast cancers (Naor et al., 2002). Hyaluronic acid-modified MEVs loaded with doxorubicin specifically target CD44-overexpressing cancer cells, enhancing anticancer sensitivity (Li et al., 2020). Additionally, the slightly acidic pH (6.5–7.4) of many solid tumors offers an alternative target for clinical treatment (Counihan et al., 2018). In such environments, the imine bond, sensitive to pH below 6.8, degrades rapidly (Liu et al., 2019). This property enables hydrazone-bound chemotherapeutic drugs conjugated to MEV membranes to release drugs at tumor sites under acidic conditions (Zhang et al., 2020).
Commercial translation of this technology is exemplified by PureTech Health (founded 2001), which leverages MEVs within its preclinical Discovery platform for oral delivery of biologics (e.g., antisense oligonucleotides) and complex small molecules targeting rheumatoid arthritis, diabetes, autoimmune disorders, and oncology (Munagala et al., 2016; Agrawal et al., 2017; Aqil et al., 2019). Key translational advantages include low-cost scalability through abundant milk sources and reduced immunogenicity inherent to bovine-derived vesicles (Munagala et al., 2016; Agrawal et al., 2017; Aqil et al., 2019).
7 Conclusion and future perspectives
This study reviewed the characteristics, isolation, purification, and biological activities of MEVs. Notably, MEVs possess unique advantages of stability, low immunogenicity, biocompatibility, and excellent biofilm penetration. As drug carriers, MEVs offer a promising strategy for treating diseases such as IBD and cancer. Furthermore, their ability to undergo specific modifications significantly enhances therapeutic efficacy and reduces side effects, especially in cancer treatment.
However, the clinical application of MEVs faces several challenges. First, industrial production remains underdeveloped, as most research is limited to laboratory settings, necessitating further efforts to establish standardized production platforms. Second, few clinical trials have evaluated the pharmacological effects and pharmacokinetics of MEV-based drug delivery, highlighting the need for more robust evidence to support their widespread clinical use. Third, storage conditions significantly impact the quantity, purity, and biological activity of MEVs, yet no consensus exists on optimal storage practices to preserve their integrity and functionality, warranting further functional studies. Fourth, the low drug-loading efficacy of MEVs necessitates the development of more effective methods.
MEVs represent a promising natural nanocarrier for drug delivery and therapeutic applications. Their stability, biocompatibility, and low immunogenicity make them ideal candidates for targeted therapies, particularly in oral delivery and disease treatment. However, challenges in industrial production and clinical application, such as inconsistent isolation methods, safety concerns, and limited clinical data, must be addressed. Future research should focus on optimizing isolation and purification techniques, developing efficient oral delivery systems, and enhancing the targeting and stability of MEVs through functional modifications. Large-scale clinical trials and comprehensive safety assessments will be critical for translating MEVs from the laboratory to clinical practice. Through interdisciplinary collaboration and technological innovation, MEVs hold the potential to revolutionize disease treatment and health management, offering novel solutions in both nutritional and medical fields.
8 Plain language summary
• Mammalian milk is a rich source of extracellular vesicles (EVs).
• Milk-derived EVs are used to load small molecules for therapeutic purposes.
• MEVs with targeted ligand function are used for tissue/organ-targeted therapy.
Author contributions
CK: Conceptualization, Data curation, Visualization, Writing – original draft, Writing – review and editing. L-bH: Formal Analysis, Visualization, Writing – original draft. M-fY: Investigation, Supervision, Writing – review and editing. N-nY: Formal Analysis, Writing – review and editing. YZ: Methodology, Writing – review and editing. C-mT: Software, Writing – review and editing. Y-hW: Supervision, Writing – review and editing. D-rW: Supervision, Writing – review and editing. R-yS: Supervision, Writing – review and editing. Y-jL: Supervision, Writing – review and editing. JY: Funding acquisition, Resources, Writing – review and editing. L-sW: Funding acquisition, Resources, Writing – review and editing. D-fL: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Science and Technology Innovation Committee of Shenzhen (JCYJ2024083175903005, JCYJ20210324113613035, and YCYJ2022530151810024). Guangdong Basic and Applied Basic Research Foundation (Grant Nos. 2023A1515011936 and 2024A1515011554), Sanming Project of Medicine in Shenzhen (No. SZSM202311025), and Provincial Natural Science Foundation (ZR2019MH100).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
MEVs, Milk-derived extracellular vesicles; EVs, extracellular vesicles; miRNA, microRNA; ESCRT, endosomal sorting complex required for transport; PBMC, peripheral blood monoculear cell; ICAM-1, intercellular cell adhesion molecule-1; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PS, phosphatidylserine; NECv, necrotizing enterocolitis; NSCs, neural stem cells; IEC-6, intestinal epithelial cell-6; LPS, Lipopolysaccharides; SEC, size exclusion chromatography; IBD, inflammatory bowel disease; PELNs, Portulaca oleracea L-derived EV-like nanoparticles; DSS, dextran sulfate sodium salt; OA, osteoarthritis; IECs, intestinal epithelial cells; NEC, necrotizing enterocolitis; REDD1, regulated in development and DNA damage response 1; MRFs, myogenic regulatory factors; IL-6, interleukin-6; LPS, lipopolysaccharide; ROS, reactive oxygen species; SOD, superoxide dismutase; GPx, glutathione peroxidase; Tregs, regulatory T cells; IS, isobavachalcone; PB, polymyxin B; IP-MEVs, MEVs comprising isobavachalcone and polymyxin B; MDR, multidrug-resistant; RSV, Resveratrol; MEVs-miR-146a, MEVs containing miR-146a; MIRI, myocardial ischemia-reperfusion injury; PAC, paclitaxel.
References
Admyre, C., Johansson, S. M., Qazi, K. R., Filén, J. J., Lahesmaa, R., Norman, M., et al. (2007). Exosomes with immune modulatory features are present in human breast milk. J. Immunol. 179, 1969–1978. doi:10.4049/jimmunol.179.3.1969
Agrawal, A. K., Aqil, F., Jeyabalan, J., Spencer, W. A., Beck, J., Gachuki, B. W., et al. (2017). Milk-derived exosomes for oral delivery of paclitaxel. Nanomedicine 13, 1627–1636. doi:10.1016/j.nano.2017.03.001
Aqil, F., Munagala, R., Jeyabalan, J., Agrawal, A. K., Kyakulaga, A. H., Wilcher, S. A., et al. (2019). Milk exosomes - natural nanoparticles for siRNA delivery. Cancer Lett. 449, 186–195. doi:10.1016/j.canlet.2019.02.011
Balaj, L., Lessard, R., Dai, L., Cho, Y. J., Pomeroy, S. L., Breakefield, X. O., et al. (2011). Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat. Commun. 2, 180, doi:10.1038/ncomms1180
Bartel, D. P. (2009). MicroRNAs: target recognition and regulatory functions. Cell. 136, 215–233. doi:10.1016/j.cell.2009.01.002
Benmoussa, A., Gotti, C., Bourassa, S., Gilbert, C., and Provost, P. (2019). Identification of protein markers for extracellular vesicle (EV) subsets in cow's milk. J. Proteomics 192, 78–88. doi:10.1016/j.jprot.2018.08.010
Benmoussa, A., Laugier, J., Beauparlant, C. J., Lambert, M., Droit, A., and Provost, P. (2020). Complexity of the microRNA transcriptome of cow milk and milk-derived extracellular vesicles isolated via differential ultracentrifugation. J. Dairy Sci. 103 (1), 16-29.
Blans, K., Hansen, M. S., Sorensen, L. V., Hvam, M. L., Howard, K. A., Möller, A., et al. (2017). Pellet-free isolation of human and bovine milk extracellular vesicles by size-exclusion chromatography. J. Extracell. Vesicles 6, 1294340. doi:10.1080/20013078.2017.1294340
Busatto, S., Vilanilam, G., Ticer, T., Lin, W. L., Dickson, D. W., Shapiro, S., et al. (2018). Tangential flow filtration for highly efficient concentration of extracellular vesicles from large volumes of fluid. Cells 7 (12), 273. doi:10.3390/cells7120273
Carobolante, G., Mantaj, J., Ferrari, E., and Vllasaliu, D. (2020). Cow milk and intestinal epithelial cell-derived extracellular vesicles as systems for enhancing oral drug delivery. Pharmaceutics 12, 226. doi:10.3390/pharmaceutics12030226
Castellanos-Rizaldos, E., Grimm, D. G., Tadigotla, V., Hurley, J., Healy, J., Neal, P. L., et al. (2018). Exosome-based detection of egfr t790m in plasma from non-small cell lung cancer patients. Clin. Cancer Res. 24 (12), 2944-2950. doi:10.1158/1078-0432.CCR-17-3369
Castro-Giner, F., and Aceto, N. (2020). Tracking cancer progression: from circulating tumor cells to metastasis. Genome. Med. 12 (1), 31. doi:10.1186/s13073-020-00728-3
Chen, T., Xi, Q. Y., Sun, J. J., Ye, R. S., Cheng, X., Sun, R. P., et al. (2017). Revelation of mRNAs and proteins in porcine milk exosomes by transcriptomic and proteomic analysis. BMC Vet. Res. 13, 101. doi:10.1186/s12917-017-1021-8
Chen, W., Chen, X., Qian, Y., Wang, X., Zhou, Y., Yan, X., et al. (2021). Lipidomic profiling of human milk derived exosomes and their emerging roles in the prevention of necrotizing enterocolitis. Mol. Nutr. Food Res. 65, e2000845. doi:10.1002/mnfr.202000845
Cheng, X., Xie, Q., and Sun, Y. (2023). Advances in nanomaterial-based targeted drug delivery systems. Front. Bioeng. Biotechnol. 11, 1177151. doi:10.3389/fbioe.2023.1177151
Chiba, T., and Maeda, T. (2023). Human milk exosomes induce ZO-1 expression via inhibition of REDD1 expression in human intestinal epithelial cells. Biol. Pharm. Bull. 46, 893–897. doi:10.1248/bpb.b22-00880
Colitti, M., Sgorlon, S., Licastro, D., and Stefanon, B. (2019). Differential expression of miRNAs in milk exosomes of cows subjected to group relocation. Res. Vet. Sci. 122, 148-155.
Counihan, J. L., Grossman, E. A., and Nomura, D. K. (2018). Cancer metabolism: current understanding and therapies. Chem. Rev. 118, 6893–6923. doi:10.1021/acs.chemrev.7b00775
Cui, W., Tie, S., Guo, M., Qiao, F., Tan, M., and Su, W. (2022). Engineering milk-derived exosome for enhancing cellular astaxanthin delivery. J. Agric. Food Chem. 70, 10794–10806. doi:10.1021/acs.jafc.2c03683
Cui, Z., Amevor, F. K., Zhao, X., Mou, C., Pang, J., Peng, X., et al. (2023). Potential therapeutic effects of milk-derived exosomes on intestinal diseases. J. Nanobiotechnology 21, 496. doi:10.1186/s12951-023-02176-8
Del Pozo-Acebo, L., Hazas, M. L. L., Tomé-Carneiro, J., Gil-Cabrerizo, P., San-Cristobal, R., Busto, R., et al. (2021). Bovine milk-derived exosomes as a drug delivery vehicle for miRNA-Based therapy. Int. J. Mol. Sci. 22, 1105. doi:10.3390/ijms22031105
Du, C., Quan, S., Zhao, Y., Nan, X., Chen, R., Tang, X., et al. (2023). Bovine milk-derived extracellular vesicles prevent gut inflammation by regulating lipid and amino acid metabolism. Food Funct. 14, 2212–2222. doi:10.1039/d2fo03975c
Du, C., Wang, K., Zhao, Y., Nan, X., Chen, R., Quan, S., et al. (2022). Supplementation with milk-derived extracellular vesicles shapes the gut microbiota and regulates the transcriptomic landscape in experimental colitis. Nutrients 14, 1808. doi:10.3390/nu14091808
Durmaz, E., Dribika, L., Kutnyanszky, M., and Mead, B. (2024). Utilizing extracellular vesicles as a drug delivery system in glaucoma and RGC degeneration. J. Control Release 372, 209–220. doi:10.1016/j.jconrel.2024.06.029
Ebrahim, N., Mostafa, O., El Dosoky, R. E., Ahmed, I. A., Saad, A. S., Mostafa, A., et al. (2018). Human mesenchymal stem cell-derived extracellular vesicles/estrogen combined therapy safely ameliorates experimentally induced intrauterine adhesions in a female rat model. Stem Cell Res. Ther. 9 (1), 175. doi:10.1186/s13287-018-0924-z
Einerhand, A. W. C., van Loo-Bouwman, C. A., Weiss, G. A., Wang, C., Ba, G., Fan, Q., et al. (2022). Can lactoferrin, a natural mammalian milk protein, assist in the battle against COVID-19? Nutrients 14, 5274. doi:10.3390/nu14245274
Enderle, D., Spiel, A., Coticchia, C. M., Berghoff, E., Mueller, R., Schlumpberger, M., et al. (2015). Characterization of RNA from exosomes and other extracellular vesicles isolated by a novel spin column-based method. PLoS One 10 (8), e0136133. doi:10.1371/journal.pone.0136133
Esfahani, S. K., Dehghani, S., Hosseinzadeh, H., Abnous, K., Taghdisi, S. M., Ramezani, M., et al. (2024). An exosomal approach for oral delivery of resveratrol: implications for inflammatory bowel disease treatment in rat model. Life Sci. 346, 122638. doi:10.1016/j.lfs.2024.122638
Feng, X., Chen, X., Zheng, X., Zhu, H., Qi, Q., Liu, S., et al. (2021). Latest trend of milk derived exosomes: cargos, functions, and applications. Front. Nutr. 8, 747294. doi:10.3389/fnut.2021.747294
Fernandes, Q., Inchakalody, V. P., Bedhiafi, T., Mestiri, S., Taib, N., Uddin, S., et al. (2024). Chronic inflammation and cancer; the two sides of a coin. Life Sci. 338, 122390. doi:10.1016/j.lfs.2023.122390
Gao, F., Wu, S., Zhang, K., Xu, Z., and Quan, F. (2024). Goat milk exosomal microRNAs alleviate LPS-Induced intestinal inflammation in mice. Int. J. Biol. Macromol. 268, 131698. doi:10.1016/j.ijbiomac.2024.131698
Garcia-Martinez, J., Perez-Castillo, I. M., Salto, R., López-Pedrosa, J. M., Rueda, R., and Girón, M. D. (2022). Beneficial effects of bovine milk exosomes in metabolic interorgan cross-talk. Nutrients 14, 1442. doi:10.3390/nu14071442
Grossen, P., Portmann, M., Koller, E., Duschmalé, M., Minz, T., Sewing, S., et al. (2021). Evaluation of bovine milk extracellular vesicles for the delivery of locked nucleic acid antisense oligonucleotides. Eur. J. Pharm. Biopharm. 158, 198–210. doi:10.1016/j.ejpb.2020.11.012
Guo, R., Jiang, D., Gai, Y., Qian, R., Zhu, Z., Gao, Y., et al. (2023). Chlorin e6-loaded goat milk-derived extracellular vesicles for cerenkov luminescence-induced photodynamic therapy. Eur. J. Nucl. Med. Mol. Imaging 50, 508–524. doi:10.1007/s00259-022-05978-4
Haraszti, R. A., Miller, R., Stoppato, M., Sere, Y. Y., Coles, A., Didiot, M. C., et al. (2018). Exosomes produced from 3D cultures of MSCs by tangential flow filtration show higher yield and improved activity. Mol. Ther. 26, 2838–2847. doi:10.1016/j.ymthe.2018.09.015
Hata, T., Murakami, K., Nakatani, H., Yamamoto, Y., Matsuda, T., and Aoki, N. (2010). Isolation of bovine milk-derived microvesicles carrying mRNAs and microRNAs. Biochem. Biophys. Res. Commun. 396, 528–533. doi:10.1016/j.bbrc.2010.04.135
He, S., Liu, G., and Zhu, X. (2021a). Human breast milk-derived exosomes May help maintain intestinal epithelial barrier integrity. Pediatr. Res. 90, 366–372. doi:10.1038/s41390-021-01449-y
He, Y., He, Z., Leone, S., and Liu, S. (2021b). Milk exosomes transfer oligosaccharides into macrophages to modulate immunity and attenuate adherent-invasive E. coli (AIEC) infection. Nutrients 13, 3198. doi:10.3390/nu13093198
Hobbs, M., Jahan, M., Ghorashi, S. A., and Wang, B. (2021). Current perspective of sialylated milk oligosaccharides in mammalian milk: implications for brain and gut health of newborns. Foods 10, 473. doi:10.3390/foods10020473
Hock, A., Miyake, H., Li, B., Lee, C., Ermini, L., Koike, Y., et al. (2017). Breast milk-derived exosomes promote intestinal epithelial cell growth. J. Pediatr. Surg. 52, 755–759. doi:10.1016/j.jpedsurg.2017.01.032
Izumi, H., Kosaka, N., Shimizu, T., Sekine, K., Ochiya, T., and Takase, M. (2012). Bovine milk contains microRNA and messenger RNA that are stable under degradative conditions. J. Dairy Sci. 95, 4831–4841. doi:10.3168/jds.2012-5489
Izumi, H., Tsuda, M., Sato, Y., Kosaka, N., Ochiya, T., Iwamoto, H., et al. (2015). Bovine milk exosomes contain microRNA and mRNA and are taken up by human macrophages. J. Dairy Sci. 98, 2920–2933. doi:10.3168/jds.2014-9076
Jang, H., Kim, H., Kim, E. H., Han, G., Jang, Y., Kim, Y., et al. (2023). Post-insertion technique to introduce targeting moieties in milk exosomes for targeted drug delivery. Biomater. Res. 27, 124. doi:10.1186/s40824-023-00456-w
Jang, S. C., Economides, K. D., Moniz, R. J., Sia, C. L., Lewis, N., McCoy, C., et al. (2021). ExoSTING, an extracellular vesicle loaded with STING agonists, promotes tumor immune surveillance. Commun. Biol. 4, 497. doi:10.1038/s42003-021-02004-5
Jia, Y., Yu, L., Ma, T., Xu, W., Qian, H., Sun, Y., et al. (2022). Small extracellular vesicles isolation and separation: current techniques, pending questions and clinical applications. Theranostics 12, 6548–6575. doi:10.7150/thno.74305
Jiang, X., You, L., Zhang, Z., Cui, X., Zhong, H., Sun, X., et al. (2021). Biological properties of milk-derived extracellular vesicles and their physiological functions in infant. Front. Cell. Dev. Biol. 9, 693534. doi:10.3389/fcell.2021.693534
Kalluri, R., and LeBleu, V. S. (2020). The biology, function, and biomedical applications of exosomes. Science 367, eaau6977. doi:10.1126/science.aau6977
Kandimalla, R., Aqil, F., Alhakeem, S. S., Jeyabalan, J., Tyagi, N., Agrawal, A., et al. (2021b). Targeted oral delivery of paclitaxel using colostrum-derived exosomes. Cancers (Basel) 13, 3700. doi:10.3390/cancers13153700
Kandimalla, R., Aqil, F., Tyagi, N., and Gupta, R. (2021a). Milk exosomes: a biogenic nanocarrier for small molecules and macromolecules to combat cancer. Am. J. Reprod. Immunol. 85, e13349. doi:10.1111/aji.13349
Karlsson, O., Rodosthenous, R. S., Jara, C., Brennan, K. J., Wright, R. O., Baccarelli, A. A., et al. (2016). Detection of long non-coding RNAs in human breastmilk extracellular vesicles: Implications for early child development. Epigenetics 11 (10), 721-729.
Kazemi Shariat Panahi, H., Dehhaghi, M., Guillemin, G. J., Peng, W., Aghbashlo, M., and Tabatabaei, M. (2024). Targeting microRNAs as a promising anti-cancer therapeutic strategy against traffic-related air pollution-mediated lung cancer. Cancer Metastasis Rev. 43, 657–672. doi:10.1007/s10555-023-10142-x
Kim, N. H., Kim, J., Lee, J. Y., Bae, H. A., and Kim, C. Y. (2023). Application of milk exosomes for musculoskeletal health: talking points in recent outcomes. Nutrients 15, 4645. doi:10.3390/nu15214645
Komine-Aizawa, S., Ito, S., Aizawa, S., Namiki, T., and Hayakawa, S. (2020). Cow milk exosomes activate NK cells and γδT cells in human PBMCs in vitro. Immunol. Med. 43, 161–170. doi:10.1080/25785826.2020.1791400
Korovesis, D., Rubio-Tomas, T., and Tavernarakis, N. (2023). Oxidative Stress in Age-Related Neurodegenerative Diseases: An Overview of Recent Tools and Findings. Antioxidants (Basel) 12, 131. doi:10.3390/antiox12010131
Kosaka, N., Izumi, H., Sekine, K., and Ochiya, T. (2010). microRNA as a new immune-regulatory agent in breast milk. Silence 1, 7. doi:10.1186/1758-907X-1-7
Kupsco, A., Prada, D., Valvi, D., Hu, L., Petersen, M. S., Coull, B., et al. (2021). Human milk extracellular vesicle miRNA expression and associations with maternal characteristics in a population-based cohort from the Faroe Islands. Sci. Rep. 11 (1), 5840.
Laulagnier, K., Motta, C., Hamdi, S., Roy, S., Fauvelle, F., Pageaux, J. F., et al. (2004). Mast cell- and dendritic cell-derived exosomes display a specific lipid composition and an unusual membrane organization. Biochem. J. 380 (Pt 1), 161-171. doi:10.1042/BJ20031594
Ley, R. E., Peterson, D. A., and Gordon, J. I. (2006). Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 124, 837–848. doi:10.1016/j.cell.2006.02.017
Li, B., Hock, A., Wu, R. Y., Minich, A., Botts, S. R., Lee, C., et al. (2019). Bovine milk-derived exosomes enhance goblet cell activity and prevent the development of experimental necrotizing enterocolitis. PLoS One 14, e0211431. doi:10.1371/journal.pone.0211431
Li, D., Yao, S., Zhou, Z., Shi, J., Huang, Z., and Wu, Z. (2020). Hyaluronan decoration of milk exosomes directs tumor-specific delivery of doxorubicin. Carbohydr. Res. 493, 108032. doi:10.1016/j.carres.2020.108032
Li, D. F., Tang, Q., Yang, M. F., Xu, H. M., Zhu, M. Z., Zhang, Y., et al. (2023a). Plant-derived exosomal nanoparticles: potential therapeutic for inflammatory bowel disease. Nanoscale Adv. 5, 3575–3588. doi:10.1039/d3na00093a
Li, D. F., Yang, M. F., Xu, H. M., Zhu, M. Z., Zhang, Y., Tian, C. M., et al. (2022a). Nanoparticles for oral delivery: targeted therapy for inflammatory bowel disease. J. Mater Chem. B 10, 5853–5872. doi:10.1039/d2tb01190e
Li, D. F., Yang, M. F., Xu, J., Xu, H. M., Zhu, M. Z., Liang, Y. J., et al. (2022b). Extracellular Vesicles: The Next Generation Theranostic Nanomedicine for Inflammatory Bowel Disease. Int. J. Nanomedicine 17, 3893–3911. doi:10.2147/IJN.S370784
Li, X., Su, L., Zhang, X., Chen, Q., Wang, Y., Shen, Z., et al. (2022c). Recent Advances on the Function and Purification of Milk Exosomes: A Review. Front. Nutr. 9, 871346. doi:10.3389/fnut.2022.871346
Li, Y., Xing, L., Wang, L., Liu, X., Wu, L., Ni, M., et al. (2023b). Milk-derived exosomes as a promising vehicle for oral delivery of hydrophilic biomacromolecule drugs. Asian J. Pharm. Sci. 18, 100797. doi:10.1016/j.ajps.2023.100797
Liu, N., Feng, G., Zhang, X., Hu, Q., Sun, S., Sun, J., et al. (2021). The Functional Role of Lactoferrin in Intestine Mucosal Immune System and Inflammatory Bowel Disease. Front. Nutr. 8, 759507. doi:10.3389/fnut.2021.759507
Liu, Q., Hao, H., Li, J., Zheng, T., Yao, Y., Tian, X., et al. (2023b). Oral Administration of Bovine Milk-Derived Extracellular Vesicles Attenuates Cartilage Degeneration via Modulating Gut Microbiota in DMM-Induced Mice. Nutrients 15, 747. doi:10.3390/nu15030747
Liu, Q., Li, D., Pan, X., and Liang, Y. (2023a). Targeted therapy using engineered extracellular vesicles: principles and strategies for membrane modification. J. Nanobiotechnology 21, 334. doi:10.1186/s12951-023-02081-0
Liu, S., Luo, X., Liu, S., Xu, P., Wang, J., and Hu, Y. (2019). Acetazolamide-Loaded pH-Responsive Nanoparticles Alleviating Tumor Acidosis to Enhance Chemotherapy Effects. Macromol. Biosci. 19, e1800366. doi:10.1002/mabi.201800366
Liu, W., Du, C., Nan, L., Li, C., Wang, H., Fan, Y., et al. (2024). The Difference of Milk-Derived Extracellular Vesicles from Cow Colostrum and Mature Milk on miRNAs Expression and Protecting Intestinal Epithelial Cells against Lipopolysaccharide Damage. Int. J. Mol. Sci. 25, 3880. doi:10.3390/ijms25073880
Lotfy, A., AboQuella, N. M., and Wang, H. (2023). Mesenchymal stromal/stem cell (MSC)-derived exosomes in clinical trials. Stem Cell Res. Ther. 14 (1), 66. doi:10.1186/s13287-023-03287-7
Lokhandwala, T., Bittoni, M. A., Dann, R. A., D'Souza, A. O., Johnson, M., Nagy, R. J., et al. (2017). Costs of diagnostic assessment for lung cancer: a medicare claims analysis. Clin. Lung Cancer 18 (1), e27-e34. doi:10.1016/j.cllc.2016.07.006
Luan, X., Sansanaphongpricha, K., Myers, I., Chen, H., Yuan, H., and Sun, D. (2017). Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol. Sin. 38, 754–763. doi:10.1038/aps.2017.12
Luo, S., Sun, X., Huang, M., Ma, Q., Du, L., and Cui, Y. (2021). Enhanced Neuroprotective Effects of Epicatechin Gallate Encapsulated by Bovine Milk-Derived Exosomes against Parkinson's Disease through Antiapoptosis and Antimitophagy. J. Agric. Food Chem. 69, 5134–5143. doi:10.1021/acs.jafc.0c07658
Ma, L., Huo, Y., Tang, Q., Wang, X., Wang, W., Wu, D., et al. (2024). Human Breast Milk Exosomal miRNAs are Influenced by Premature Delivery and Affect Neurodevelopment. Mol. Nutr. Food Res. 68, e2300113. doi:10.1002/mnfr.202300113
Ma, S., Niu, M., Hao, Z., Liu, M., Tong, C., and Zhao, X. (2021). Selective packaged circular RNAs in milk extracellular vesicles during Staphylococcus aureus infection may have potential against bacterial infection. RNA Biol. 18 (5), 818-831.
Martin, C., Patel, M., Williams, S., Arora, H., Brawner, K., and Sims, B. (2018). Human breast milk-derived exosomes attenuate cell death in intestinal epithelial cells. Innate Immun. 24, 278–284. doi:10.1177/1753425918785715
Mathivanan, S., and Simpson, R. J. (2009). ExoCarta: A compendium of exosomal proteins and RNA. Proteomics 9, 4997–5000. doi:10.1002/pmic.200900351
Matic, S., and Dia, V. P. (2022). Bovine milk exosomes affected proliferation of macrophages under hypoxia. Curr. Res. Food Sci. 5, 2108–2113. doi:10.1016/j.crfs.2022.11.002
Matsumoto, J., Stewart, T., Banks, W. A., and Zhang, J. (2017). The transport mechanism of extracellular vesicles at the blood-brain barrier. Curr. Pharm. Des. 23 (40), 6206-6214. doi:10.2174/1381612823666170913164738
Maynard, C. L., Elson, C. O., Hatton, R. D., and Weaver, C. T. (2012). Reciprocal interactions of the intestinal microbiota and immune system. Nature 489, 231–241. doi:10.1038/nature11551
McKiernan, J., Donovan, M. J., O'Neill, V., Bentink, S., Noerholm, M., Belzer, S., et al. (2016). A novel urine exosome gene expression assay to predict high-grade prostate cancer at initial biopsy. JAMA Oncol. 2 (7), 882-889. doi:10.1001/jamaoncol.2016.0097
Medel-Martinez, A., Redrado-Osta, A., Crespo-Barreda, A., Sancho-Albero, M., Sánchez, L., Sebastián, V., et al. (2024). Isolation and Characterization of Milk Exosomes for Use in Advanced Therapies. Biomolecules 14, 810. doi:10.3390/biom14070810
Melnik, B. C., and Schmitz, G. (2017). MicroRNAs: Milk's epigenetic regulators. Best. Pract. Res. Clin. Endocrinol. Metab. 31, 427–442. doi:10.1016/j.beem.2017.10.003
Melnik, B. C., Stremmel, W., Weiskirchen, R., John, S. M., and Schmitz, G. (2021). Exosome-Derived MicroRNAs of Human Milk and Their Effects on Infant Health and Development. Biomolecules 11, 851. doi:10.3390/biom11060851
Meng, W. T., Zhu, J., Wang, Y. C., Shao, C. l., Li, X. Y., Lu, P. P., et al. (2024). Targeting delivery of miR-146a via IMTP modified milk exosomes exerted cardioprotective effects by inhibiting NF-κB signaling pathway after myocardial ischemia-reperfusion injury. J. Nanobiotechnology 22, 382. doi:10.1186/s12951-024-02631-0
Meng, Z., Zhou, D., Lv, D., Gan, Q., Liao, Y., Peng, Z., et al. (2023). Human milk extracellular vesicles enhance muscle growth and physical performance of immature mice associating with Akt/mTOR/p70s6k signaling pathway. J. Nanobiotechnology 21, 304. doi:10.1186/s12951-023-02043-6
Monika, G. R., and Sardana, S. (2017). Research Problems Associated with Resveratrol (trans-3, 5, 4'- trihydroxystilbene; RSV) and Various Strategies to Overcome those Problems. Curr. Drug Deliv. 14, 364–376. https://www.frontiersin.org.cn/authors-proof-support/#QA53
Munagala, R., Aqil, F., Jeyabalan, J., and Gupta, R. C. (2016). Bovine milk-derived exosomes for drug delivery. Cancer Lett. 371, 48–61. doi:10.1016/j.canlet.2015.10.020
Naor, D., Nedvetzki, S., Golan, I., Melnik, L., and Faitelson, Y. (2002). CD44 in cancer. Crit. Rev. Clin. Lab. Sci. 39, 527–579. doi:10.1080/10408360290795574
Ong, S. L., Blenkiron, C., Haines, S., Acevedo-Fani, A., Leite, J. A. S., Zempleni, J., et al. (2021). Ruminant Milk-Derived Extracellular Vesicles: A Nutritional and Therapeutic Opportunity? Nutrients 13, 2505. doi:10.3390/nu13082505
Parry, H. A., Mobley, C. B., Mumford, P. W., Romero, M. A., Haun, C. T., Zhang, Y., et al. (2019). Bovine Milk Extracellular Vesicles (EVs) Modification Elicits Skeletal Muscle Growth in Rats. Front. Physiol. 10, 436. doi:10.3389/fphys.2019.00436
Patel, G. K., Khan, M. A., Zubair, H., Srivastava, S. K., Khushman, M., Singh, S., et al. (2019). Comparative analysis of exosome isolation methods using culture supernatant for optimum yield, purity and downstream applications. Sci. Rep. 9, 5335. doi:10.1038/s41598-019-41800-2
Pieters, B. C., Arntz, O. J., Bennink, M. B., Broeren, M. G. A., van Caam, A. P. M., Koenders, M. I., et al. (2015). Commercial cow milk contains physically stable extracellular vesicles expressing immunoregulatory TGF-β. PLoS One 10, e0121123. doi:10.1371/journal.pone.0121123
Pishavar, E., Trentini, M., Zanotti, F., Camponogara, F., Tiengo, E., Zanolla, I., et al. (2022). Exosomes as Neurological Nanosized Machines. ACS Nanosci. Au 2, 284–296. doi:10.1021/acsnanoscienceau.1c00062
Prasadani, M., Kodithuwakku, S., Pennarossa, G., Fazeli, A., and Brevini, T. A. L. (2024). Therapeutic Potential of Bovine Milk-Derived Extracellular Vesicles. Int. J. Mol. Sci. 25, 5543. doi:10.3390/ijms25105543
Pullan, J., Dailey, K., Bhallamudi, S., Feng, L., Alhalhooly, L., Froberg, J., et al. (2022). Modified Bovine Milk Exosomes for Doxorubicin Delivery to Triple-Negative Breast Cancer Cells. ACS Appl. Bio Mater 5, 2163–2175. doi:10.1021/acsabm.2c00015
Rahman, M. M., Shimizu, K., Yamauchi, M., Takase, H., Ugawa, S., Okada, A., et al. (2019). Acidification effects on isolation of extracellular vesicles from bovine milk. PLoS One 14, e0222613. doi:10.1371/journal.pone.0222613
Rahman, M. M., Takashima, S., Kamatari, Y. O., Shimizu, K., Okada, A., and Inoshima, Y. (2021). Comprehensive proteomic analysis revealed a large number of newly identified proteins in the small extracellular vesicles of milk from late-stage lactating cows. Animals (Basel) 11 (9).
Rashidi, M., Bijari, S., Khazaei, A. H., Shojaei-Ghahrizjani, F., and Rezakhani, L. (2022). The role of milk-derived exosomes in the treatment of diseases. Front. Genet. 13, 1009338. doi:10.3389/fgene.2022.1009338
Reinhardt, T. A., Lippolis, J. D., Nonnecke, B. J., and Sacco, R. E. (2012). Bovine milk exosome proteome. J. Proteomics 75, 1486–1492. doi:10.1016/j.jprot.2011.11.017
Reinhardt, T. A., Sacco, R. E., Nonnecke, B. J., and Lippolis, J. D. (2013). Bovine milk proteome: quantitative changes in normal milk exosomes, milk fat globule membranes and whey proteomes resulting from Staphylococcus aureus mastitis. J. Proteomics 82, 141-154.
Ross, M., Atalla, H., Karrow, N., and Mallard, B. A. (2021). The bioactivity of colostrum and milk exosomes of high, average, and low immune responder cows on human intestinal epithelial cells. J. Dairy Sci. 104, 2499–2510. doi:10.3168/jds.2020-18405
Rubin, D. C., Shaker, A., and Levin, M. S. (2012). Chronic intestinal inflammation: inflammatory bowel disease and colitis-associated colon cancer. Front. Immunol. 3, 107. doi:10.3389/fimmu.2012.00107
Samuel, M., Fonseka, P., Sanwlani, R., Gangoda, L., Chee, S. H., Keerthikumar, S., et al. (2021). Oral administration of bovine milk-derived extracellular vesicles induces senescence in the primary tumor but accelerates cancer metastasis. Nat. Commun. 12, 3950. doi:10.1038/s41467-021-24273-8
Samuel, M., Chisanga, D., Liem, M., Keerthikumar, S., Anand, S., Ang, C. S., et al. (2017). Bovine milk-derived exosomes from colostrum are enriched with proteins implicated in immune response and growth. Sci. Rep. 7 (1), 5933.
Sato, Y. T., Umezaki, K., Sawada, S., Mukai, S. a., Sasaki, Y., Harada, N., et al. (2016). Engineering hybrid exosomes by membrane fusion with liposomes. Sci. Rep. 6, 21933. doi:10.1038/srep21933
Schieber, M., and Chandel, N. S. (2014). ROS function in redox signaling and oxidative stress. Curr. Biol. 24, R453–R462. doi:10.1016/j.cub.2014.03.034
Sedykh, S. E., Purvinsh, L. V., Burkova, E. E., Dmitrenok, P. S., Ryabchikova, E. I., and Nevinsky, G. A. (2022). Analysis of Proteins and Peptides of Highly Purified CD9(+) and CD63(+) Horse Milk Exosomes Isolated by Affinity Chromatography. Int. J. Mol. Sci. 23, 16106. doi:10.3390/ijms232416106
Sedykh, S. E., Purvinish, L. V., Monogarov, A. S., Burkova, E. E., Grigor'eva, A. E., Bulgakov, D. V., et al. (2017). Purified horse milk exosomes contain an unpredictable small number of major proteins. Biochim. Open 4, 61–72. doi:10.1016/j.biopen.2017.02.004
Sengupta, V., Sengupta, S., Lazo, A., Woods, P., Nolan, A., and Bremer, N. (2020). Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19. Stem Cells Dev. 29 (12), 747-754. doi:10.1089/scd.2020.0080
Shao, H., Im, H., Castro, C. M., Breakefield, X., Weissleder, R., and Lee, H. (2018). New Technologies for Analysis of Extracellular Vesicles. Chem. Rev. 118, 1917–1950. doi:10.1021/acs.chemrev.7b00534
Shen, Q., Huang, Z., Yao, J., and Jin, Y. (2022). Extracellular vesicles-mediated interaction within intestinal microenvironment in inflammatory bowel disease. J. Adv. Res. 37, 221–233. doi:10.1016/j.jare.2021.07.002
Shi, H., Guo, J., Li, C., and Wang, Z. (2015). A current review of folate receptor alpha as a potential tumor target in non-small-cell lung cancer. Drug Des. Devel. Ther. 9, 4989–4996. doi:10.2147/DDDT.S90670
Shi, M. M., Yang, Q. Y., Monsel, A., Yan, J. Y., Dai, C. X., Zhao, J. Y., et al. (2021). Preclinical efficacy and clinical safety of clinical-grade nebulized allogenic adipose mesenchymal stromal cells-derived extracellular vesicles. J. Extracell Vesicles 10 (10), e12134. doi:10.1002/jev2.12134
Sidhom, K., Obi, P. O., and Saleem, A. (2020). A Review of Exosomal Isolation Methods: Is Size Exclusion Chromatography the Best Option? Int. J. Mol. Sci. 21, 6466. doi:10.3390/ijms21186466
Simpson, M. R., Brede, G., Johansen, J., Johnsen, R., Storro, O., Saetrom, P., et al. (2015). Human breast milk miRNA, maternal probiotic supplementation and atopic dermatitis in offspring. PLoS One 10 (12), e0143496.
Somiya, M., Yoshioka, Y., and Ochiya, T. (2018). Biocompatibility of highly purified bovine milk-derived extracellular vesicles. J. Extracell. Vesicles 7, 1440132. doi:10.1080/20013078.2018.1440132
Summerlin, N., Soo, E., Thakur, S., Qu, Z., Jambhrunkar, S., and Popat, A. (2015). Resveratrol nanoformulations: challenges and opportunities. Int. J. Pharm. 479, 282–290. doi:10.1016/j.ijpharm.2015.01.003
Sun, J., Aswath, K., Schroeder, S. G., Lippolis, J. D., Reinhardt, T. A., and Sonstegard, T. S. (2015). MicroRNA expression profiles of bovine milk exosomes in response to Staphylococcus aureus infection. BMC Genomics 16, 806.
Szatanek, R., Baj-Krzyworzeka, M., Zimoch, J., Lekka, M., Siedlar, M., and Baran, J. (2017). The Methods of Choice for Extracellular Vesicles (EVs) Characterization. Int. J. Mol. Sci. 18, 1153. doi:10.3390/ijms18061153
Szatanek, R., Baran, J., Siedlar, M., and Baj-Krzyworzeka, M. (2015). Isolation of extracellular vesicles: Determining the correct approach (Review). Int. J. Mol. Med. 36, 11–17. doi:10.3892/ijmm.2015.2194
Tauro, B. J., Greening, D. W., Mathias, R. A., Mathivanan, S., Ji, H., and Simpson, R. J. (2013). Two distinct populations of exosomes are released from LIM1863 colon carcinoma cell-derived organoids. Mol. Cell Proteomics 12 (3), 587-598. doi:10.1074/mcp.M112.021303
Théry, C., Amigorena, S., Raposo, G., and Clayton, A. (2006). Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell. Biol. 30. Chapter 3:Unit 3 22. doi:10.1002/0471143030.cb0322s30
Théry, C., Ostrowski, M., and Segura, E. (2009). Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 9, 581–593. doi:10.1038/nri2567
Théry, C., Zitvogel, L., and Amigorena, S. (2002). Exosomes: composition, biogenesis and function. Nat. Rev. Immunol. 2, 569–579. doi:10.1038/nri855
Tian, C. M., Yang, M. F., Xu, H. M., Zhu, M. Z., Zhang, Y., Yao, J., et al. (2023a). Emerging role of bacterial outer membrane vesicle in gastrointestinal tract. Gut Pathog. 15, 20. doi:10.1186/s13099-023-00543-2
Tian, C. M., Yang, M. F., Xu, H. M., Zhu, M. Z., Zhang, Y., Yao, J., et al. (2023b). Mesenchymal Stem Cell-derived Exosomes: Novel Therapeutic Approach for Inflammatory Bowel Diseases. Stem Cells Int. 2023, 4245704. doi:10.1155/2023/4245704
Tian, M. Y., Hao, D. X., Liu, Y., He, J., Zhao, Z. H., Guo, T. Y., et al. (2023c). Milk exosomes: an oral drug delivery system with great application potential. Food Funct. 14, 1320–1337. doi:10.1039/d2fo02013k
Timofeeva, A. M., Paramonik, A. P., Sedykh, S. S., and Nevinsky, G. A. (2023). Milk Exosomes: Next-Generation Agents for Delivery of Anticancer Drugs and Therapeutic Nucleic Acids. Int. J. Mol. Sci. 24, 10194. doi:10.3390/ijms241210194
Tong, L., Hao, H., Zhang, Z., Lv, Y., Liang, X., Liu, Q., et al. (2021). Milk-derived extracellular vesicles alleviate ulcerative colitis by regulating the gut immunity and reshaping the gut microbiota. Theranostics 11, 8570–8586. doi:10.7150/thno.62046
Vaiaki, E. M., and Falasca, M. (2024). Comparative analysis of the minimal information for studies of extracellular vesicles guidelines: Advancements and implications for extracellular vesicle research. Semin. Cancer Biol. 101, 12–24. doi:10.1016/j.semcancer.2024.04.002
Valadi, H., Ekström, K., Bossios, A., Sjöstrand, M., Lee, J. J., and Lötvall, J. O. (2007). Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 9 (6), 654-659. doi:10.1038/ncb1596
van Herwijnen, M. J., Zonneveld, M. I., Goerdayal, S., Nolte-'t Hoen, E. N. M., Garssen, J., Stahl, B., et al. (2016). Comprehensive Proteomic Analysis of Human Milk-derived Extracellular Vesicles Unveils a Novel Functional Proteome Distinct from Other Milk Components. Mol. Cell. Proteomics 15, 3412–3423. doi:10.1074/mcp.M116.060426
van Herwijnen, M. J. C., Driedonks, T. A. P., Snoek, B. L., Kroon, A. M. T., Kleinjan, M., Jorritsma, R., et al. (2018). Abundantly present miRNAs in milk-derived extracellular vesicles are conserved between mammals. Front. Nutr. 5, 81.
Vashisht, M., Rani, P., Onteru, S. K., and Singh, D. (2017). Curcumin Encapsulated in Milk Exosomes Resists Human Digestion and Possesses Enhanced Intestinal Permeability in Vitro. Appl. Biochem. Biotechnol. 183, 993–1007. doi:10.1007/s12010-017-2478-4
Vaswani, K., Koh, Y. Q., Almughlliq, F. B., Peiris, H. N., and Mitchell, M. D. (2017). A method for the isolation and enrichment of purified bovine milk exosomes. Reprod. Biol. 17, 341–348. doi:10.1016/j.repbio.2017.09.007
Vaswani, K., Mitchell, M. D., Holland, O. J., Qin Koh, Y., Hill, R. J., Harb, T., et al. (2019). A Method for the Isolation of Exosomes from Human and Bovine Milk. J. Nutr. Metab. 2019, 5764740. doi:10.1155/2019/5764740
Vaswani, K. M., Peiris, H., Qin Koh, Y., Hill, R. J., Harb, T., Arachchige, B. J., et al. (2021). A complete proteomic profile of human and bovine milk exosomes by liquid chromatography mass spectrometry. Expert Rev. Proteomics 18, 719–735. doi:10.1080/14789450.2021.1980389
Wang, L., Shi, Z., Wang, X., Mu, S., Xu, X., Shen, L., et al. (2021). Protective effects of bovine milk exosomes against oxidative stress in IEC-6 cells. Eur. J. Nutr. 60, 317–327. doi:10.1007/s00394-020-02242-z
Wang, Y., Liu, Q., Liu, Y., Qiao, W., Zhao, J., Cao, H., et al. (2024). Advances in the composition, efficacy, and mimicking of human milk phospholipids. Food Funct. 15, 6254–6273. doi:10.1039/d4fo00539b
Warren, M. R., Zhang, C., Vedadghavami, A., Bokvist, K., Dhal, P. K., and Bajpayee, A. G. (2021). Milk exosomes with enhanced mucus penetrability for oral delivery of siRNA. Biomater. Sci. 9, 4260–4277. doi:10.1039/d0bm01497d
Wehbe, Z., and Kreydiyyeh, S. (2022). Cow's milk may be delivering potentially harmful undetected cargoes to humans. Is it time to reconsider dairy recommendations? Nutr. Rev. 80, 874–888. doi:10.1093/nutrit/nuab046
Wolf, T., Baier, S. R., and Zempleni, J. (2015). The Intestinal Transport of Bovine Milk Exosomes Is Mediated by Endocytosis in Human Colon Carcinoma Caco-2 Cells and Rat Small Intestinal IEC-6 Cells. J. Nutr. 145, 2201–2206. doi:10.3945/jn.115.218586
Wu, L., Wang, L., Liu, X., Bai, Y., Wu, R., Li, X., et al. (2022). Milk-derived exosomes exhibit versatile effects for improved oral drug delivery. Acta Pharm. Sin. B 12, 2029–2042. doi:10.1016/j.apsb.2021.12.015
Xiao, P., Wang, H., Liu, H., Yuan, H., Guo, C., Feng, Y., et al. (2024). Milk Exosome-Liposome Hybrid Vesicles with Self-Adapting Surface Properties Overcome the Sequential Absorption Barriers for Oral Delivery of Peptides. ACS Nano 18, 21091–21111. doi:10.1021/acsnano.4c02560
Xu, Q., Yang, S., Zhang, K., Liu, Y., Li, L., and Qu, S. (2024). Enhanced antibacterial activity of bovine milk exosome-based drug formulation against bacterial pathogens. Food Chem. 447, 139034. doi:10.1016/j.foodchem.2024.139034
Yan, C., Chen, J., Wang, C., Yuan, M., Kang, Y., Wu, Z., et al. (2022). Milk exosomes-mediated miR-31-5p delivery accelerates diabetic wound healing through promoting angiogenesis. Drug Deliv. 29, 214–228. doi:10.1080/10717544.2021.2023699
Yan, Z., and Shao, T. (2024). Chronic Inflammation in Chronic Kidney Disease. Nephron 148, 143–151. doi:10.1159/000534447
Yu, W., Hurley, J., Roberts, D., Chakrabortty, S. K., Enderle, D., Noerholm, M., et al. (2021). Exosome-based liquid biopsies in cancer: opportunities and challenges. Ann. Oncol. 32 (4), 466-477. doi:10.1016/j.annonc.2021.01.074
Yu, S., Zhao, Z., Xu, X., Li, M., and Li, P. (2019). Characterization of three different types of extracellular vesicles and their impact on bacterial growth. Food Chem. 272, 372–378. doi:10.1016/j.foodchem.2018.08.059
Yun, B., Maburutse, B. E., Kang, M., Park, M. R., Park, D. J., Kim, Y., et al. (2020). Short communication: Dietary bovine milk-derived exosomes improve bone health in an osteoporosis-induced mouse model. J. Dairy Sci. 103, 7752–7760. doi:10.3168/jds.2019-17501
Zempleni, J., Aguilar-Lozano, A., Sadri, M., Sukreet, S., Manca, S., Wu, D., et al. (2017). Biological Activities of Extracellular Vesicles and Their Cargos from Bovine and Human Milk in Humans and Implications for Infants. J. Nutr. 147, 3–10. doi:10.3945/jn.116.238949
Zempleni, J., Sukreet, S., Zhou, F., Wu, D., and Mutai, E. (2019). Milk-Derived Exosomes and Metabolic Regulation. Annu. Rev. Anim. Biosci. 7, 245–262. doi:10.1146/annurev-animal-020518-115300
Zeng, B., Chen, T., Xie, M. Y., Luo, J. Y., He, J. J., Xi, Q. Y., et al. (2019). Exploration of long noncoding RNA in bovine milk exosomes and their stability during digestion in vitro. J. Dairy Sci. 102, 6726–6737. doi:10.3168/jds.2019-16257
Zeng, B., Wang, H., Luo, J., Xie, M., Zhao, Z., Chen, X., et al. (2021). Porcine Milk-Derived Small Extracellular Vesicles Promote Intestinal Immunoglobulin Production through pIgR. Anim. (Basel) 11, 1522. doi:10.3390/ani11061522
Zhang, Q., Xiao, Q., Yin, H., Xia, C., Pu, Y., He, Z., et al. (2020). Milk-exosome based pH/light sensitive drug system to enhance anticancer activity against oral squamous cell carcinoma. RSC Adv. 10, 28314–28323. doi:10.1039/d0ra05630h
Zhang, Y., Liu, Y., Liu, H., and Tang, W. H. (2019). Exosomes: biogenesis, biologic function and clinical potential. Cell. Biosci. 9, 19. doi:10.1186/s13578-019-0282-2
Zhong, J., Xia, B., Shan, S., Zheng, A., Zhang, S., Chen, J., et al. (2021). High-quality milk exosomes as oral drug delivery system. Biomaterials 277, 121126. doi:10.1016/j.biomaterials.2021.121126
Zhou, F., Ebea, P., Mutai, E., Wang, H., Sukreet, S., Navazesh, S., et al. (2022). Small Extracellular Vesicles in Milk Cross the Blood-Brain Barrier in Murine Cerebral Cortex Endothelial Cells and Promote Dendritic Complexity in the Hippocampus and Brain Function in C57BL/6J Mice. Front. Nutr. 9, 838543. doi:10.3389/fnut.2022.838543
Zhou, Y., Yu, Z., Wang, X., Chen, W., Liu, Y., Zhang, Y., et al. (2021). Exosomal circRNAs contribute to intestinal development via the VEGF signalling pathway in human term and preterm colostrum. Aging (Albany NY) 13, 11218–11233. doi:10.18632/aging.202806
Keywords: milk, extracellular vesicles, targeted therapy, exosomes, drug delivery
Citation: Kong C, Huang L-b, Yang M-f, Yue N-n, Zhang Y, Tian C-m, Wang Y-h, Wei D-r, Shi R-y, Liang Y-j, Yao J, Wang L-s and Li D-f (2025) Milk-derived extracellular vesicles: nature’s nanocarriers for drug delivery and therapeutics. Front. Pharmacol. 16:1595891. doi: 10.3389/fphar.2025.1595891
Received: 31 March 2025; Accepted: 19 June 2025;
Published: 06 August 2025.
Edited by:
Kehao Zhao, Yantai University, ChinaReviewed by:
Barathan Muttiah, University of Malaya, MalaysiaKrisztian Kvell, University of Pécs, Hungary
Copyright © 2025 Kong, Huang, Yang, Yue, Zhang, Tian, Wang, Wei, Shi, Liang, Yao, Wang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dao-ru Wei, d2VpZHJAMTI2LmNvbQ==; Rui-yue Shi, cnVpeXVlc2hpQDEyNi5jb20=; Yu-jie Liang, bGlhbmd5amllQDEyNi5jb20=; Jun Yao, eWpfMTEwOEAxMjYuY29t; De-feng Li, bGRmODMwNzEyQDE2My5jb20=; Li-sheng Wang, d2FuZ2xzc3pybXl5QDE2My5jb20=
†These authors have contributed equally to this work
 Chen Kong1†
Chen Kong1† Mei-feng Yang
Mei-feng Yang Yuan Zhang
Yuan Zhang Cheng-mei Tian
Cheng-mei Tian Yu-jie Liang
Yu-jie Liang Li-sheng Wang
Li-sheng Wang De-feng Li
De-feng Li