- 1Department of Epidemiology, West Virginia University, Morgantown, WV, USA
- 2Center for the Study of Complementary and Alternative Therapies, University of Virginia Health System, Charlottesville, VA, USA
Alzheimer’s disease (AD) is a chronic, progressive, brain disorder that affects at least 5.3 million Americans at an estimated cost of $148 billion, figures that are expected to rise steeply in coming years. Despite decades of research, there is still no cure for AD, and effective therapies for preventing or slowing progression of cognitive decline in at-risk populations remain elusive. Although the etiology of AD remains uncertain, chronic stress, sleep deficits, and mood disturbance, conditions common in those with cognitive impairment, have been prospectively linked to the development and progression of both chronic illness and memory loss and are significant predictors of AD. Therapies such as meditation that specifically target these risk factors may thus hold promise for slowing and possibly preventing cognitive decline in those at risk. In this study, we briefly review the existing evidence regarding the potential utility of meditation as a therapeutic intervention for those with and at risk for AD, discuss possible mechanisms underlying the observed benefits of meditation, and outline directions for future research.
Alzheimer’s disease (AD), the most common form of dementia, is a chronic, progressive, brain disorder resulting in a loss of memory, reasoning, language skills, and the ability to care for one’s self (1). AD is the seventh leading cause of death in the US (2), affecting 5.2 million Americans at an estimated cost of $214 billion, figures that are expected to increase dramatically in the coming years (3, 4). AD affects quality of life for both the patient and the caregiver in profound ways. Many individuals with cognitive impairment become unable to engage in once loved activities that gave them a sense of purpose or pleasure (5). Behavioral and social skills may also deteriorate, which in turn, further increase risk for poor mental and physical health outcomes (6, 7). For example, neuropsychiatric symptoms are common in adults with AD (8–10), with up to 64% of AD patients experiencing disturbed sleep (11) and up to 87% suffering depressive symptoms (12, 13).
In most patients, AD develops slowly, often preceded years earlier by perceived and/or objective cognitive decline, offering a potential window for therapeutic intervention. First defined in the late 1990s and now widely recognized, mild cognitive impairment (MCI) is generally considered a transition phase between healthy cognitive aging and dementia (14, 15). Risk of progression to AD in those with MCI is very high, with an estimated 5–15% of those with MCI converting to AD each year (14–16), and up to 50% or more eventually progressing to AD (15). More recently, an even earlier harbinger of cognitive impairment, subjective cognitive impairment (SCI), has been associated with accelerated decline in cognitive function, an up to 4.5-fold increased risk for progression to MCI, and an approximately threefold elevated likelihood of developing AD (17–20). The annual conversion rate from SCI to MCI or dementia in otherwise healthy individuals has been estimated to be 7–10% (20, 21). While cognitive performance is only modestly reduced in those with MCI, and is in the normal range in those with SCI (22), recent research has shown cerebral spinal fluid markers of AD to be more common in adults with SCI and MCI, and to be associated with cognitive decline in those with MCI (23). In addition, prevalence of neuropsychiatric impairment is high in both these at-risk populations (8–10, 22), and, as discussed in more detail below, cognitive function appears strongly related, in a bidirectional manner, to anxiety, depression, and sleep disturbance (22, 24, 25). Up to 62% of MCI patients experience sleep disturbance (25, 26) and up to 83% suffer depressive symptoms (13), rates similar to those reported in AD (11, 12, 26). Similarly, adults with subjective memory complaints are significantly more likely to report symptoms of depression (19, 27) and anxiety (19). As detailed below, these mood and sleep disturbances lead, in turn, to accelerated cognitive decline and deterioration of both physical and mental health (12, 25, 28–31).
Chronic Stress, Sleep Impairment, Mood Disturbance, and Cognitive Decline: A Vicious Cycle
Chronic stress can contribute to a vicious cycle that may not only have deleterious effects on health and cognitive function (32), but ultimately increase risk for institutionalization (29, 30). The chronic stress that often characterizes the lives of those with cognitive impairment, as well as their caregivers, has been linked to adverse changes in sleep (30), mood (33, 34), and immunological function (33, 35), and elevated risk for metabolic syndrome, cardiovascular disease (CVD), and mortality (36, 37). Chronic psychological stress can have profound effects on memory and behavior in persons both with and without cognitive impairment, and has been prospectively linked to increased risk for incident MCI and dementia in older adults, and to accelerated cognitive decline (38–40). Chronic stress leads to deleterious neuroendocrine and associated inflammatory changes, to suppression of IGF-1 and other neuroprotective factors, and to impaired synaptic plasticity, suppressed neurogenesis, reduced neuronal survival, and other adverse morphological and functional changes in the hippocampus, prefrontal cortex, and other brain structures; all these changes can profoundly affect mood, sleep, memory, and learning (41–45). A large body of experimental, clinical, and epidemiological research has also implicated chronic stress and associated sympathoadrenal activation in the etiology of hypertension, obesity, dyslipidemia, and other components of the metabolic syndrome, and in the development and progression of CVD, type 2 diabetes, depression, and related chronic disorders (41, 46). These disorders have, in turn, been shown to predict cognitive dysfunction, and to increase risk for the development and progression of AD (47–54). Autonomic and hypothalamic pituitary adrenal (HPA) axis dysfunction has also been linked directly to cognitive decline, and to adverse changes in brain structure and function. For example, HPA axis activation, manifested by elevated cortisol levels, has been associated with hippocampal volume loss and memory impairment in non-demented elders (41, 55).
As noted above, depression and other mood disorders are common in those with and at risk for AD, including adults with MCI and SCI (12, 13, 28, 56). Depressive symptoms and other distressful states have also been linked to significantly increased risk for diabetes, CVD, stroke, and the metabolic syndrome (57–59), and are a significant contributor to the profound reductions in quality of life reported by those with cognitive impairment (12, 31). Anxiety and depressive symptoms are also significant predictors of cognitive decline and incident cognitive impairment (60, 61). Moreover, in those with MCI, behavioral and psychological symptoms, including anxiety, depression, irritability, and apathy, are strong predictors of progression to AD (28, 62). In addition, mood disturbance can contribute not only to impairment of memory, but also to sleep disturbance, HPA axis dysregulation, and autonomic dysfunction and related pro-inflammatory changes, thus helping to promote a vicious cycle of adverse physiologic, neuroendocrine, and psychosocial changes that foster the development and progression of AD, CVD, and related chronic conditions (41, 63, 64).
Sleep disruption, also common in cognitively impaired adults (26), likewise has negative effects on health, functioning, and quality of life, and is a major reason for institutionalization (25, 29, 30, 65, 66). Sleep deficits are known to impair cognitive function in healthy populations (67, 68), to accelerate cognitive decline (29, 30), and to predict incident MCI and dementia (25). In addition, sleep disturbances have been strongly associated, in a bidirectional manner, with mood disorders (69) and autonomic dysfunction (70, 71), and can promote glucose intolerance, pro-inflammatory changes, dyslipidemia, obesity, and hypertension (70, 72, 73). Sleep impairment has likewise been linked to increased risk for incident type 2 diabetes and for CVD morbidity and mortality (41, 72–74), disorders that have, in turn, been significantly and prospectively linked to AD (75–78). The association of impaired sleep to chronic illness and related risk factors appears strongly reciprocal (72, 79).
Alzheimer’s Disease and Cognitive Impairment: Need for New Prevention and Treatment Strategies
Despite decades of research, there is still no cure for AD. While a number of lifestyle factors have been linked to the subsequent development of this devastating disorder, effective therapies for preventing or slowing progression of AD in at-risk populations remain elusive (80), and there are no approved treatments for MCI or age-associated cognitive decline (22, 81). Given the high prevalence of chronic stress, sleep disturbance, and mood impairment in those with or at risk for cognitive impairment, the deleterious impact of these and related factors on health and cognitive function, interventions that specifically address these risk factors may hold promise not only for enhancing health and well-being, but also for slowing and possibly preventing cognitive decline in those at risk for AD. Of particular interest in this regard is meditation, an ancient mind–body practice that is gaining increasing favor throughout the western industrialized world as a means of reducing stress and improving both mental and physical well-being (82).
Meditation: Health Effects and Therapeutic Promise
Meditation has been broadly defined as “an intentional and self-regulated focusing of attention, whose purpose is to relax and calm the mind and body” (83). The studies included in this mini-review encompass a wide variety of meditative techniques, including mantra, mindfulness, and Kundalini meditation practices, among others. As indicated in recent systematic reviews by our group and other investigators, and by the growing body of original research on the health effects of meditation, there is mounting evidence that even brief meditative practices (5 days–8 weeks) may improve neuropsychological, metabolic, and clinical profiles in a range of populations (82, 84–87). For example, studies have shown meditation to reduce perceived stress (85, 88–90), anxiety (85, 88–91), and depressive symptoms (89–92), enhance quality of life (87, 92), decrease sleep disturbance (90, 93), improve several domains of cognition (90, 94, 95), reduce sympathetic activation and enhance cardiovagal tone (96–99), both acutely and long term in clinical as well as non-clinical populations (84, 100). A growing body of research also suggests that meditation promotes beneficial changes in CNS dopaminergic and other neurochemical systems (101, 102), and increases blood flow, oxygen delivery, and glucose utilization in specific regions of the brain associated with mood elevation, memory, and attentional processing, including the hippocampus, prefrontal cortex, and anterior cingulate gyrus (91, 95, 103–106). Long-term meditation practice has also been associated with cortical thickening and increased gray matter volume in brain regions involved in attentional performance, sensory processing, and interoception (103, 107, 108), apparently offsetting typical age-related cortical thinning and gray matter loss (108). In addition, recent research suggests that meditation programs can enhance immune response (109) and clinical outcomes (82, 85), and reduce blood pressure (85, 90, 100, 110), insulin resistance and glucose intolerance (97, 111), oxidative stress (84, 112), inflammation (93), and other related risk indices (84, 85). While research in cognitively impaired populations remains limited, findings from previous observational studies (113, 114) and two recent small clinical trials (90, 91, 95, 105) suggest that meditation practice may reduce stress, anxiety, depression, and blood pressure; improve cognition; promote beneficial changes in brain structure and function; and improve health outcomes in adults with memory disorders.
In addition to its many reported health benefits, meditation carries numerous practical advantages as a therapeutic intervention and health promotion method. It is a simple, economical, non-invasive therapy that is easy to learn and can be practiced even by very elderly, ill, or disabled individuals, including those suffering from cognitive impairment (82, 90, 95, 115). Requiring no special equipment and little in the way of professional personnel, meditation is a practice that is relatively easy to maintain at no cost, with several studies indicating excellent long-term adherence (84, 116, 117). Meditation practice typically brings immediate positive benefits, including feelings of relaxation and tranquility, and even short-term (5 day–6 week) meditation programs have been shown to result in significant improvement in mood, sleep, and other distressful symptoms (82, 84, 97, 110), helping to encourage continued practice. Several studies indicate that meditation programs may also reduce health care costs in both clinical and non-clinical populations (82, 118).
Potential Underlying Mechanisms
Although the mechanisms underlying the putative beneficial effects of meditation on cognitive, psychological, and physical health are not yet well understood, the observed changes likely occur through at least four pathways (41, 46, 84, 85, 101, 119). First, by reducing activation and reactivity of the sympathoadrenal system and the HPA axis and promoting feelings of well-being, meditation may alleviate the effects of stress, enhance sleep and mood, and foster multiple positive downstream effects on cognition, neuroendocrine status, neurological and metabolic function and related inflammatory responses (Figure 1, pathway 1). Second, meditation may enhance parasympathetic output, possibly via direct vagal stimulation, and thereby shift the autonomic nervous system balance from primarily sympathetic to parasympathetic, leading to positive changes in cardiovagal function, in mood, sleep, and energy state, and in related neuroendocrine, metabolic, and inflammatory responses, in turn, reducing risk for depression and cognitive decline (Figure 1, pathway 2). Third, findings of recent neuroimaging and neurophysiological studies (98, 106, 120) suggest that meditation, by selectively activating specific neurochemical systems and brain structures associated with positive mood, attention, and memory, may likewise promote beneficial changes in sympathetic/parasympathetic balance, in neurological structure and function, in affect and memory, and in related metabolic and inflammatory responses (Figure 1, pathway 3). Finally, findings of a recent study in dementia caregivers (121) suggest that meditation may also, by directly or indirectly stimulating increased telomerase activity, help promote telomere maintenance and buffer the effects of stress-induced cellular aging, thereby helping to preserve immune function and possibly reduce neuronal loss and other degenerative changes associated with aging and cognitive decline (122, 123). As discussed below, reductions in telomerase activity and telomere length have been linked to stress, depression, sleep loss, and cognitive impairment (99, 124–135) and shown to predict cognitive decline in both clinical and non-clinical populations (136, 137). Likewise, recent research in healthy adults (138–140), lonely older adults (141), and depressed dementia caregivers (142, 143) suggest that meditation may also buffer or reverse multiple stress-related changes in specific gene expression pathways implicated in the development and progression of AD, including those regulating oxidative stress, inflammation, cellular aging, and other factors contributing to impaired brain structure and function, and ultimately, to cognitive decline (144–150).
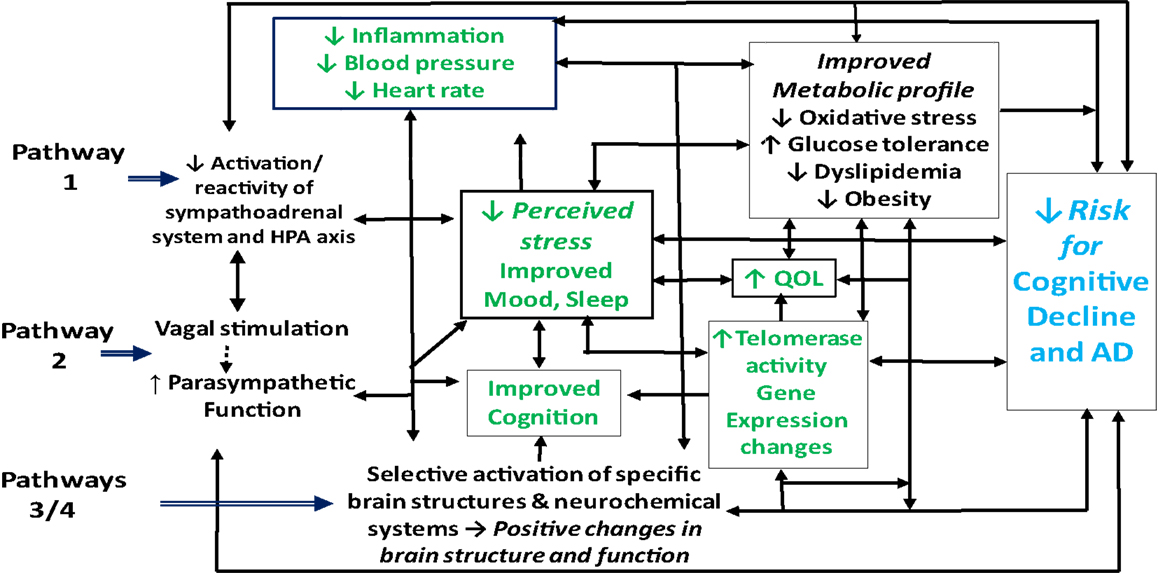
Figure 1. Possible pathways by which meditation may improve health outcomes in adults with cognitive impairment.
Telomeres, Inflammation, Cognitive Decline, and Chronic Stress: Possible Benefits of Meditation
An emerging body of literature suggests that both inflammation and telomere maintenance may be important factors in the pathogenesis of AD. Telomeres are DNA–protein complexes that protect the ends of chromosomes, and are essential for maintaining chromosomal integrity during replication (127, 151). The cellular enzyme telomerase acts to repair and replace the genetic telomeric material lost, helping to counteract the telomere shortening that occurs with age. There is growing evidence linking both shorter telomere length and lower telomerase activity with reduced survival and several age-related diseases (127, 151, 152), including AD (152–156) and suggesting that these telomeric alterations may mediate the degenerative changes associated with these conditions (131, 151, 157). Telomere shortening has been linked to cognitive impairment in several non-clinical populations (124–126) and shown in prospective studies to predict cognitive decline in post-stroke patients (136) and older community-dwelling women (137). Moreover, recent studies suggest that several lifestyle and environmental factors are important determinants of both telomere length and telomerase activity (127, 131, 152). Notably, these factors include chronic stress, depression, and impaired sleep, now emerging as powerful predictors of accelerated telomere shortening and reduced telomerase activity (99, 127–135). As indicated above, these deleterious changes may be buffered by meditative practices (99, 121, 158).
Telomere degradation may also be accelerated by chronic inflammation (156, 159, 160) which is, in turn, thought to be an important mechanism underlying cognitive decline and the pathogenesis of AD (54). Systemic inflammatory markers, including IL-6, TNF-alpha, and hsCRP, have been linked to loss of cerebral volume, and large population-based studies have consistently shown high blood levels of these inflammatory indices, to predict cognitive decline (54). Elevated inflammatory markers are also strongly associated, in a bidirectional manner, to chronic psychological stress, mood disturbance, sleep loss, and other distressful states (41, 72, 161–165). Emerging evidence suggests that meditative practices can not only reduce stress, improve sleep, and enhance mood, but may also decrease indices of systemic inflammation (121, 166, 167).
Genomic Changes in Cognitive Impairment, Stress, and Potential Benefits of Meditation
Genomic changes characterizing the inception and progression of AD is an active area of investigation (168–170). To date, over 180 genes distributed across the human genome have been directly or indirectly implicated in the pathogenesis of AD (168). Transcriptional profiling of blood mononuclear cells by microarray in those with AD have identified 19 upregulated and 136 downregulated genes (170, 171). While gene expression profiling in those with or at risk for AD is an emerging and still rapidly developing field of inquiry, recent studies suggest that multiple changes in gene expression may be important in AD progression (145, 147–150). These include alterations related to pro-inflammatory pathways (145), immune function (146), synaptic function (150), and regulation of apoptosis (146), oxidative stress (144) and other pathways (147, 148, 171); several of these changes have been linked to chronic psychological stress (139, 140, 144). Exploratory research in depressed dementia caregivers (142) and lonely older adults (141), as well as in healthy adults (138–140), suggests that the practice of meditation may lead to multiple beneficial changes in gene expression and may buffer or reverse adverse stress-related changes in transcriptional profiles. These include favorable alterations in several pathways linked to cognitive impairment, including those regulating cellular metabolism and aging, oxidative stress, immune function, inflammation, DNA repair, cell-cycle control, and apoptosis (138–143). However, while these preliminary findings suggest that meditative practices may help prevent, mitigate, or even reverse specific genomic changes implicated in cognitive decline, studies regarding the effects of meditation or other mind-body therapies on gene expression profiles in adults with memory loss are lacking.
Summary
In brief, meditation may offer considerable promise as a safe and cost-effective intervention for reducing stress and for improving cognition, mood, sleep, and related outcomes in adults with or at risk for cognitive impairment. However, despite the apparent therapeutic potential of meditation for these populations, research remains sparse, and interpretation of existing studies is limited by small sample sizes, selection bias, and/or lack of appropriate control groups. Clearly, larger, rigorous randomized controlled trials are needed to establish the efficacy of meditation for improving cognitive function, stress, mood, sleep and related neuropsychosocial and physiological outcomes in adults with cognitive impairment, and to examine the long-term effects of meditation on cognitive decline and on the inception and progression of AD. Also needed are high-quality studies to assess the potential cost-effectiveness of meditation as a therapeutic intervention for those with or at risk for cognitive impairment, and to investigate potential underlying mechanisms, including changes in inflammatory markers, brain structure and function, cellular aging, and gene expression. If future studies show meditation to be effective in reducing stress and improving cognition and related outcomes in adults at risk for AD, it may offer a novel, safe, and low-cost approach to preventing or slowing cognitive decline in this population, and ultimately help reduce the significant health and economic burden associated with AD.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was made possible in part by the West Virginia University Health Sciences Center, the National Institutes of Health National Center for Complementary and Alternative Medicine, and the Alzheimer’s Research and Prevention Foundation (ARPF). The contents are solely the responsibility of the authors and do not represent the official views of the authors’ academic institution, the National Institutes of Health, or the ARPF.
References
1. Gauthier S. Should we encourage the use of high-dose vitamin E in persons with memory complaints as a preventive strategy against Alzheimer’s disease? J Psychiatry Neurosci (2000) 25(4):394.
2. National Center for Health Statistics (2004). Available from: http://www.cdc.gov/nchs/fastats/deaths.htm
3. Alzheimer’s Association. 2014 Alzheimer’s Disease Facts and Figures. Alzheimers Dement (2014) 10(2):1–75.
4. Khalsa DS. Mindfulness effects on caregiver stress: should we expect more? J Altern Complement Med (2010) 16(10):1025–6. doi:10.1089/acm.2010.0431
5. Logsdon RG, Teri L. The Pleasant Events Schedule-AD: psychometric properties and relationship to depression and cognition in Alzheimer’s disease patients. Gerontologist (1997) 37(1):40–5. doi:10.1093/geront/37.1.40
6. Teri L. Behavior and caregiver burden: behavioral problems in patients with Alzheimer disease and its association with caregiver distress. Alzheimer Dis Assoc Disord (1997) 11(4):S35–8.
7. Lu YF, Haase JE. Experience and perspectives of caregivers of spouse with mild cognitive impairment. Curr Alzheimer Res (2009) 6(4):384–91. doi:10.2174/156720509788929309
8. Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA (2002) 288(12):1475–83. doi:10.1001/jama.288.12.1475
9. Rosenberg PB, Mielke MM, Appleby B, Oh E, Leoutsakos JM, Lyketsos CG. Neuropsychiatric symptoms in MCI subtypes: the importance of executive dysfunction. Int J Geriatr Psychiatry (2011) 26(4):364–72. doi:10.1002/gps.2535
10. Ryu S-H, Lee K-J. Neuropsychiatric symptoms in patients with mild cognitive impairment and mild Alzheimer’s disease in the community-dwelling elderly in Korea. Alzheimers Dementia (2008) 4(Suppl 1):T450. doi:10.1016/j.jalz.2008.05.1343
11. Rongve A, Boeve BF, Aarsland D. Frequency and correlates of caregiver-reported sleep disturbances in a sample of persons with early dementia. J Am Geriatr Soc (2010) 58(3):480–6. doi:10.1111/j.1532-5415.2010.02733.x
12. Winter Y, Korchounov A, Zhukova TV, Bertschi NE. Depression in elderly patients with Alzheimer dementia or vascular dementia and its influence on their quality of life. J Neurosci Rural Pract (2011) 2(1):27–32. doi:10.4103/0976-3147.80087
13. Panza F, Frisardi V, Capurso C, D’Introno A, Colacicco AM, Imbimbo BP, et al. Late-life depression, mild cognitive impairment, and dementia: possible continuum? Am J Geriatr Psychiatry (2010) 18(2):98–116. doi:10.1097/JGP.0b013e3181b0fa13
15. DeCarli C. Mild cognitive impairment: prevalence, prognosis, aetiology, and treatment. Lancet Neurol (2003) 2(1):15–21. doi:10.1016/S1474-4422(03)00262-X
16. Mitchell AJ, Shiri-Feshki M. Rate of progression of mild cognitive impairment to dementia – meta-analysis of 41 robust inception cohort studies. Acta Psychiatr Scand (2009) 119(4):252–65. doi:10.1111/j.1600-0447.2008.01326.x
17. Reisberg B, Shulman MB, Torossian C, Leng L, Zhu W. Outcome over seven years of healthy adults with and without subjective cognitive impairment. Alzheimers Dementia (2010) 6(1):11–24. doi:10.1016/j.jalz.2009.10.002
18. Loewenstein D, Greig M, Schinka J, Barker W, Shen Q, Potter E, et al. An investigation of PreMCI: subtypes and longitudinal outcomes. Alzheimers Dement (2012) 8(3):172–9. doi:10.1016/j.jalz.2011.03.002
19. Reisberg B, Gauthier S. Current evidence for subjective cognitive impairment (SCI) as the pre-mild cognitive impairment (MCI) stage of subsequently manifest Alzheimer’s disease. Int Psychogeriatr (2008) 20(1):1–16. doi:10.1017/S1041610207006412
20. Jessen F, Wolfsgruber S, Wiese B, Bickeld H, Mösch E, Kaduszkiewicz H, et al. AD dementia risk in late MCI, in early MCI, and in subjective memory impairment. Alzheimer Dement (2014) 10(1):76–83. doi:10.1016/j.jalz.2012.09.017
21. Gauthier S, Reisberg B, Zaudig M, Petersen RC, Ritchie K, Broich K, et al. Mild cognitive impairment. Lancet (2006) 367(9518):1262–70. doi:10.1016/S0140-6736(06)68542-5
22. Reisberg B, Prichep L, Mosconi L, John ER, Glodzik-Sobanska L, Boksay I, et al. The pre-mild cognitive impairment, subjective cognitive impairment stage of Alzheimer’s disease. Alzheimers Dementia (2008) 4(Suppl 1):S98–108. doi:10.1016/j.jalz.2007.11.017
23. Visser PJ, Verhey F, Knol DL, Scheltens P, Wahlund LO, Freund-Levi Y, et al. Prevalence and prognostic value of CSF markers of Alzheimer’s disease pathology in patients with subjective cognitive impairment or mild cognitive impairment in the DESCRIPA study: a prospective cohort study. Lancet Neurol (2009) 8(7):619–27. doi:10.1016/S1474-4422(09)70139-5
24. Woods DL, Phillips LR, Martin JL. Biological basis for sleep disturbance and behavioral symptoms in dementia: a biobehavioral model. Res Gerontol Nurs (2011) 4(4):281–93. doi:10.3928/19404921-20110302-01
25. Beaulieu-Bonneau S, Hudon C. Sleep disturbances in older adults with mild cognitive impairment. Int Psychogeriatr (2009) 21(4):654–66. doi:10.1017/S1041610209009120
26. Guarnieri B, Adorni F, Musicco M, Appollonio I, Bonanni E, Caffarra P, et al. Prevalence of sleep disturbances in mild cognitive impairment and dementing disorders: a Multicenter Italian Clinical Cross-Sectional Study on 431 patients. Dement Geriatr Cogn Disord (2012) 33(1):50–8. doi:10.1159/000335363
27. Schofield PW, Marder M, Dooneief G, Jacobs DM, Sano M, Stern Y. Association of subjective memory complaints with subsequent cognitive decline in community-dwelling elderly individuals with baseline cognitive impairment. Am J Psychiatry (1997) 154(5):609–15.
28. Palmer K, Berger AK, Monastero R, Winblad B, Backman L, Fratiglioni L. Predictors of progression from mild cognitive impairment to Alzheimer disease. Neurology (2008) 68(19):1596–602. doi:10.1212/01.wnl.0000260968.92345.3f
29. Lee DR, Thomas AJ. Sleep in dementia and caregiving – assessment and treatment implications: a review. Int Psychogeriatr (2011) 23(2):190–201. doi:10.1017/S1041610210001894
30. McCurry SM, Logsdon RG, Teri L, Vitiello MV. Sleep disturbances in caregivers of persons with dementia: contributing factors and treatment implications. Sleep Med Rev (2007) 11(2):143–53. doi:10.1016/j.smrv.2006.09.002
31. Valimaki TH, Vehvilainen-Julkunen KM, Pietila AM, Pirttila TA. Caregiver depression is associated with a low sense of coherence and health-related quality of life. Aging Ment Health (2009) 13(6):799–807. doi:10.1080/13607860903046487
32. Reagan LP, Grillo CA, Piroli GG. The As and Ds of stress: metabolic, morphological and behavioral consequences. Eur J Pharmacol (2008) 585(1):64–75. doi:10.1016/j.ejphar.2008.02.050
33. Schulz R, Martire LM. Family caregiving of persons with dementia: prevalence, health effects, and support strategies. Am J Geriatr Psychiatry (2004) 12(3):240–9. doi:10.1176/appi.ajgp.12.3.240
34. Alspaugh ME, Stephens MA, Townsend AL, Zarit SH, Greene R. Longitudinal patterns of risk for depression in dementia caregivers: objective and subjective primary stress as predictors. Psychol Aging (1999) 14(1):34–43. doi:10.1037/0882-7974.14.1.34
35. Lovell B, Wetherell MA. The cost of caregiving: endocrine and immune implications in elderly and non elderly caregivers. Neurosci Biobehav Rev (2011) 35(6):1342–52. doi:10.1016/j.neubiorev.2011.02.007
36. Dimsdale JE. Psychological stress and cardiovascular disease. J Am Coll Cardiol (2008) 51(13):1237–46. doi:10.1016/j.jacc.2007.12.024
37. Vitaliano PP, Scanlan JM, Zhang J, Savage MV, Hirsch IB, Siegler IC. A path model of chronic stress, the metabolic syndrome, and coronary heart disease. Psychosom Med (2002) 64(3):418–35.
38. Wilson RS, Arnold SE, Schneider JA, Kelly JF, Tang Y, Bennett DA. Chronic psychological distress and risk of Alzheimer’s disease in old age. Neuroepidemiology (2006) 27(3):143–53. doi:10.1159/000095761
39. Wilson RS, Schneider JA, Boyle PA, Arnold SE, Tang Y, Bennett DA. Chronic distress and incidence of mild cognitive impairment. Neurology (2007) 68(24):2085–92. doi:10.1212/01.wnl.0000264930.97061.82
40. Wilson RS, Arnold SE, Schneider JA, Li Y, Bennett DA. Chronic distress, age-related neuropathology, and late-life dementia. Psychosom Med (2007) 69(1):47–53. doi:10.1097/01.psy.0000250264.25017.21
41. Innes KE, Vincent HK, Taylor AG. Chronic stress and insulin resistance-related indices of cardiovascular disease risk, part I: neurophysiological responses and pathological sequelae. Altern Ther Health Med (2007) 13(4):46–52.
42. Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci (2002) 3(6):453–62.
43. Lucassen PJ, Heine VM, Muller MB, van der Beek EM, Wiegant VM, De Kloet ER, et al. Stress, depression and hippocampal apoptosis. CNS Neurol Disord Drug Targets (2006) 5(5):531–46. doi:10.2174/187152706778559273
44. Swaab DF, Bao AM, Lucassen PJ. The stress system in the human brain in depression and neurodegeneration. Ageing Res Rev (2005) 4(2):141–94. doi:10.1016/j.arr.2005.03.003
45. Lucassen PJ, Meerlo P, Naylor AS, van Dam AM, Dayer AG, Fuchs E, et al. Regulation of adult neurogenesis by stress, sleep disruption, exercise and inflammation: implications for depression and antidepressant action. Eur Neuropsychopharmacol (2010) 20(1):1–17. doi:10.1016/j.euroneuro.2009.08.003
46. Innes KE, Vincent HK, Taylor AG. Chronic stress and insulin resistance-related indices of cardiovascular disease risk, part 2: a potential role for mind-body therapies. Altern Ther Health Med (2007) 13(5):44–51.
47. Hellstrom HR. The altered homeostatic theory: a hypothesis proposed to be useful in understanding and preventing ischemic heart disease, hypertension, and diabetes – including reducing the risk of age and atherosclerosis. Med Hypotheses (2007) 68(2):415–33. doi:10.1016/j.mehy.2006.05.031
48. Lee PY, Yun AJ, Bazar KA. Conditions of aging as manifestations of sympathetic bias unmasked by loss of parasympathetic function. Med Hypotheses (2004) 62(6):868–70. doi:10.1016/j.mehy.2003.11.024
49. Raber J. Detrimental effects of chronic hypothalamic-pituitary-adrenal axis activation. From obesity to memory deficits. Mol Neurobiol (1998) 18(1):1–22. doi:10.1007/BF02741457
50. Starr VL, Convit A. Diabetes, sugar-coated but harmful to the brain. Curr Opin Pharmacol (2007) 7(6):638–42. doi:10.1016/j.coph.2007.10.007
52. Martins IJ, Hone E, Foster JK, Sunram-Lea SI, Gnjec A, Fuller SJ, et al. Apolipoprotein E, cholesterol metabolism, diabetes, and the convergence of risk factors for Alzheimer’s disease and cardiovascular disease. Mol Psychiatry (2006) 11(8):721–36. doi:10.1038/sj.mp.4001854
53. Gottfries CG, Balldin J, Blennow K, Brane G, Karlsson I, Regland B, et al. Regulation of the hypothalamic-pituitary-adrenal axis in dementia disorders. Ann N Y Acad Sci (1994) 746:336–43. doi:10.1111/j.1749-6632.1994.tb39253.x
54. Gorelick PB. Role of inflammation in cognitive impairment: results of observational epidemiological studies and clinical trials. Innate Inflam Stroke (2010) 1207:155–62. doi:10.1111/j.1749-6632.2010.05726.x
55. McEwen BS. Sex, stress and the hippocampus: allostasis, allostatic load and the aging process. Neurobiol Aging (2002) 23(5):921–39. doi:10.1016/S0197-4580(02)00027-1
56. Steffens DC, Otey E, Alexopoulos GS, Butters MA, Cuthbert B, Ganguli M, et al. Perspectives on depression, mild cognitive impairment, and cognitive decline. Arch Gen Psychiatry (2006) 63(2):130–8. doi:10.1001/archpsyc.63.2.130
57. Clarke DM, Currie KC. Depression, anxiety and their relationship with chronic diseases: a review of the epidemiology, risk and treatment evidence. Med J Aust (2009) 190(7 Suppl):S54–60.
58. Cohen BE, Panguluri P, Na B, Whooley MA. Psychological risk factors and the metabolic syndrome in patients with coronary heart disease: findings from the Heart and Soul Study. Psychiatry Res (2010) 175(1/2):133–7. doi:10.1016/j.psychres.2009.02.004
59. Luppino FS, de Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BW, et al. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry (2010) 67(3):220–9. doi:10.1001/archgenpsychiatry.2010.2
60. Chodosh J, Kado DM, Seeman TE, Karlamangla AS. Depressive symptoms as a predictor of cognitive decline: MacArthur studies of successful aging. Am J Geriatr Psychiatry (2007) 15(5):406–15. doi:10.1097/01.JGP.0b013e31802c0c63
61. Beaudreau SA, O’Hara R. Late-life anxiety and cognitive impairment: a review. Am J Geriatr Psychiatry (2008) 16(10):790–803. doi:10.1097/JGP.0b013e31817945c3
62. Copeland MP, Daly E, Hines V, Mastromauro C, Zaitchik D, Gunther J, et al. Psychiatric symptomatology and prodromal Alzheimer’s disease. Alzheimer Dis Assoc Disord (2003) 17(1):1–8. doi:10.1097/00002093-200301000-00001
63. Leonard BE. Inflammation, depression and dementia: are they connected? Neurochem Res (2007) 32(10):1749–56. doi:10.1007/s11064-007-9385-y
64. McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev (2007) 87(3):873–904. doi:10.1152/physrev.00041.2006
65. Boeve BF. Update on the diagnosis and management of sleep disturbances in dementia. Sleep Med Clin (2008) 3(3):347–60. doi:10.1016/j.jsmc.2008.04.010
66. Dauvilliers Y. Insomnia in patients with neurodegenerative conditions. Sleep Med (2007) 8:S27–34. doi:10.1016/S1389-9457(08)70006-6
67. Cole CS, Richards KC. Sleep and cognition in people with Alzheimer’s disease. Issues Ment Health Nurs (2005) 26(7):687–98. doi:10.1080/01612840591008258
68. Xu L, Jiang CQ, Lam TH, Liu B, Jin YL, Zhu T, et al. Short or long sleep duration is associated with memory impairment in older Chinese: the Guangzhou Biobank Cohort Study. Sleep (2011) 34(5):575–80.
69. Peterson MJ, Benca RM. Sleep in mood disorders. Sleep Med Clin (2008) 3:231–49. doi:10.1016/j.jsmc.2008.01.009
70. McEwen BS. Sleep deprivation as a neurobiologic and physiologic stressor: allostasis and allostatic load. Metabolism (2006) 55(10 Suppl 2):S20–3. doi:10.1016/j.metabol.2006.07.008
71. Innes KE, Selfe TK, Taylor AG. Menopause, the metabolic syndrome, and mind-body therapies. Menopause (2008) 15(5):1005–13. doi:10.1097/01.gme.0b013e318166904e
72. Miller MA, Cappuccio FP. Inflammation, sleep, obesity and cardiovascular disease. Curr Vasc Pharmacol (2007) 5(2):93–102. doi:10.2174/157016107780368280
73. Suarez EC. Self-reported symptoms of sleep disturbance and inflammation, coagulation, insulin resistance and psychosocial distress: evidence for gender disparity. Brain Behav Immunity (2008) 22(6):960–8. doi:10.1016/j.bbi.2008.01.011
74. Trenell MI, Marshall NS, Rogers NL. Sleep and metabolic control: waking to a problem? Clin Exp Pharmacol Physiol (2007) 34(1–2):1–9. doi:10.1111/j.1440-1681.2007.04541.x
75. Biessels GJ, Kappelle LJ. Utrecht Diabetic Encephalopathy Study G. Increased risk of Alzheimer’s disease in Type II diabetes: insulin resistance of the brain or insulin-induced amyloid pathology? Biochem Soc Trans (2005) 33(Pt 5):1041–4. doi:10.1042/BST20051041
76. Whitmer RA. Type 2 diabetes and risk of cognitive impairment and dementia. Curr Neurol Neurosci Rep (2007) 7(5):373–80. doi:10.1007/s11910-007-0058-7
77. Newman AB, Fitzpatrick AL, Lopez O, Jackson S, Lyketsos C, Jagust W, et al. Dementia and Alzheimer’s disease incidence in relationship to cardiovascular disease in the Cardiovascular Health Study cohort. J Am Geriatr Soc (2005) 53(7):1101–7. doi:10.1111/j.1532-5415.2005.53360.x
78. Rosano C, Newman AB. Cardiovascular disease and risk of Alzheimer’s disease. Neurol Res (2006) 28(6):612–20. doi:10.1179/016164106X130407
79. Foley D, Ancoli-Israel S, Britz P, Walsh J. Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey. J Psychosom Res (2004) 56(5):497–502. doi:10.1016/j.jpsychores.2004.02.010
80. Apostolova LG, Thompson PM. Mapping progressive brain structural changes in early Alzheimer’s disease and mild cognitive impairment. Neuropsychologia (2006) 46(6):1597–612. doi:10.1016/j.neuropsychologia.2007.10.026
81. Corey-Bloom J. Treatment trials in aging and mild cognitive impairment. Curr Top Behav Neurosci (2012) 10:347–56. doi:10.1007/7854_2011_153
82. Bonadonna R. Meditation’s impact on chronic illness. Holist Nurs Pract (2003) 17(6):309–19. doi:10.1097/00004650-200311000-00006
83. Dorland WAN. Dorland’s Illustrated Medical Dictionary. 32nd ed. Philadelphia: Saunders (2012). 2147 p.
84. Innes KE, Bourguignon C, Taylor AG. Risk indices associated with the insulin resistance syndrome, cardiovascular disease, and possible protection with yoga: a systematic review. J Am Board Fam Pract (2005) 18(6):491–519. doi:10.3122/jabfm.18.6.491
85. Schneider RH, Walton KG, Salerno JW, Nidich SI. Cardiovascular disease prevention and health promotion with the transcendental meditation program and Maharishi consciousness-based health care. Ethn Dis (2006) 16(3 Suppl 4):S4–15.
86. Innes KE, Selfe TK, Vishnu A. Mind-body therapies for menopausal symptoms: a systematic review. Maturitas (2010) 66(2):135–49. doi:10.1016/j.maturitas.2010.01.016
87. Selfe TK, Innes KE. Mind-body therapies and osteoarthritis of the knee. Curr Rheumatol Rev (2009) 5(4):204–11. doi:10.2174/157339709790192512
88. Waelde LC, Thompson L, Gallagher-Thompson D. A pilot study of a yoga and meditation intervention for dementia caregiver stress. J Clin Psychol (2004) 60(6):677–87. doi:10.1002/jclp.10259
89. Lane JD, Seskevich JE, Pieper CF. Brief meditation training can improve perceived stress and negative mood. Altern Ther Health Med (2007) 13(1):38–44.
90. Innes KE, Selfe TK, Brown C, Rose K, Thompson-Heisterman A. The effects of meditation on perceived stress and related indices of psychological status and sympathetic activation in persons with Alzheimer’s disease and their caregivers: a pilot study. Evid Based Complement Alternat Med (2012) 2012:927509. doi:10.1155/2012/927509
91. Moss A, Wintering N, Roggenkamp H, Khalsa DS, Waldman MR, Monti D, et al. Effects of an eight week meditation program on mood and anxiety in patients with memory loss. J Altern Complement Med (2012) 18(1):48–53. doi:10.1089/acm.2011.0051
92. Jayadevappa R, Johnson JC, Bloom BS, Nidich S, Desai S, Chhatre S, et al. Effectiveness of transcendental meditation on functional capacity and quality of life of African Americans with congestive heart failure: a randomized control study. Ethn Dis (2007) 17(1):72–7.
93. Carlson LE, Speca M, Patel KD, Goodey E. Mindfulness-based stress reduction in relation to quality of life, mood, symptoms of stress, and immune parameters in breast and prostate cancer outpatients. Psychosom Med (2003) 65(4):571–81. doi:10.1097/01.PSY.0000074003.35911.41
94. Sharma VK, Das S, Mondal S, Goswami U, Gandhi A. Effect of Sahaj Yoga on neuro-cognitive functions in patients suffering from major depression. Indian J Physiol Pharmacol (2006) 50(4):375–83.
95. Khalsa DS, Newberg A. Kirtan Kriya meditation: a promising technique for enhancing cognition in memory-impaired older adults. In: Hartman-Stein PE, Rue AL editors. Enhancing Cognitive Fitness in Adults: A Guide to the Use and Development of Community-Based Programs. New York: Springer (2011). p. 419–31.
96. Curiati JA, Bocchi E, Freire JO, Arantes AC, Braga M, Garcia Y, et al. Meditation reduces sympathetic activation and improves the quality of life in elderly patients with optimally treated heart failure: a prospective randomized study. J Altern Complement Med (2005) 11(3):465–72. doi:10.1089/acm.2005.11.465
97. Paul-Labrador M, Polk D, Dwyer JH, Velasquez I, Nidich S, Rainforth M, et al. Effects of a randomized controlled trial of transcendental meditation on components of the metabolic syndrome in subjects with coronary heart disease. Arch Intern Med (2006) 166(11):1218–24. doi:10.1001/archinte.166.11.1218
98. Rubia K. The neurobiology of meditation and its clinical effectiveness in psychiatric disorders. Biol Psychol (2009) 82(1):1–11. doi:10.1016/j.biopsycho.2009.04.003
99. Epel E, Daubenmier J, Moskowitz JT, Folkman S, Blackburn E. Can meditation slow rate of cellular aging? Cognitive stress, mindfulness, and telomeres. Ann N Y Acad Sci (2009) 1172:34–53. doi:10.1111/j.1749-6632.2009.04414.x
100. Manikonda JP, Stork S, Togel S, Lobmuller A, Grunberg I, Bedel S, et al. Contemplative meditation reduces ambulatory blood pressure and stress-induced hypertension: a randomized pilot trial. J Hum Hypertens (2008) 22(2):138–40. doi:10.1038/sj.jhh.1002275
101. Newberg AB, Iversen J. The neural basis of the complex mental task of meditation: neurotransmitter and neurochemical considerations. Med Hypotheses (2003) 61(2):282–91. doi:10.1016/S0306-9877(03)00175-0
102. Kjaer TW, Bertelsen C, Piccini P, Brooks D, Alving J, Lou HC. Increased dopamine tone during meditation-induced change of consciousness. Brain Res Cogn Brain Res (2002) 13(2):255–9. doi:10.1016/S0926-6410(01)00106-9
103. Lazar SW, Kerr CE, Wasserman RH, Gray JR, Greve DN, Treadway MT, et al. Meditation experience is associated with increased cortical thickness. Neuroreport (2005) 16(17):1893–7. doi:10.1097/01.wnr.0000186598.66243.19
104. Gutman SA, Schindler VP. The neurological basis of occupation. Occup Ther Int (2007) 14(2):71–85. doi:10.1002/oti.225
105. Newberg AB, Wintering N, Khalsa DS, Roggenkamp H, Waldman MR. Meditation effects on cognitive function and cerebral blood flow in subjects with memory loss: a preliminary study. J Alzheimers Dis (2010) 20(2):517–26. doi:10.3233/JAD-2010-1391
106. Newberg AB, Wintering N, Waldman MR, Amen D, Khalsa DS, Alavi A. Cerebral blood flow differences between long-term meditators and non-meditators. Conscious Cogn (2010) 19(4):899–905. doi:10.1016/j.concog.2010.05.003
107. Srinivasan N, Baijal S. Concentrative meditation enhances preattentive processing: a mismatch negativity study. Neuroreport (2007) 18(16):1709–12. doi:10.1097/WNR.0b013e3282f0d2d8
108. Pagnoni G, Cekic M. Age effects on gray matter volume and attentional performance in Zen meditation. Neurobiol Aging (2007) 28(10):1623–7. doi:10.1016/j.neurobiolaging.2007.06.008
109. Tang YY, Ma Y, Wang J, Fan Y, Feng S, Lu Q, et al. Short-term meditation training improves attention and self-regulation. Proc Natl Acad Sci U S A (2007) 104(43):17152–6. doi:10.1073/pnas.0707678104
110. Rainforth MV, Schneider RH, Nidich SI, Gaylord-King C, Salerno JW, Anderson JW. Stress reduction programs in patients with elevated blood pressure: a systematic review and meta-analysis. Curr Hypertens Rep (2007) 9(6):520–8. doi:10.1007/s11906-007-0094-3
111. Rosenzweig S, Reibel DK, Greeson JM, Edman JS, Jasser SA, McMearty KD, et al. Mindfulness-based stress reduction is associated with improved glycemic control in type 2 diabetes mellitus: a pilot study. Altern Ther Health Med (2007) 13(5):36–8.
112. Sinha S, Singh SN, Monga YP, Ray US. Improvement of glutathione and total antioxidant status with yoga. J Altern Complement Med (2007) 13(10):1085–90. doi:10.1089/acm.2007.0567
113. McBee L. Mindfulness-Based Elder Care. A CAM Model for Frail Elders and Their Caregivers. New York: Springer Publishing Company (2008).
114. Lantz MS, Buchalter EN, McBee L. The Wellness Group: a novel intervention for coping with disruptive behavior among [corrected] elderly nursing home residents [erratum appears in Gerontologist 1997 Oct; 37(5):687]. Gerontologist (1997) 37(4):551–6.
115. Lindberg DA. Integrative review of research related to meditation, spirituality, and the elderly. Geriatr Nurs (2005) 26(6):372–7. doi:10.1016/j.gerinurse.2005.09.013
116. Pradhan EK, Baumgarten M, Langenberg P, Handwerger B, Gilpin AK, Magyari T, et al. Effect of mindfulness-based stress reduction in rheumatoid arthritis patients. Arthritis Rheum (2007) 57(7):1134–42. doi:10.1002/art.23010
117. Sephton SE, Salmon P, Weissbecker I, Ulmer C, Floyd A, Hoover K, et al. Mindfulness meditation alleviates depressive symptoms in women with fibromyalgia: results of a randomized clinical trial. Arthritis Rheumatism (2007) 57(1):77–85. doi:10.1002/art.22478
118. Walton KG, Schneider RH, Salerno JW, Nidich SI. Psychosocial stress and cardiovascular disease. Part 3: clinical and policy implications of research on the transcendental meditation program. Behav Med (2005) 30(4):173–83. doi:10.3200/BMED.30.4.173-184
119. Allen NB, Chambers R, Knight W. Melbourne Academic Mindfulness Interest G. Mindfulness-based psychotherapies: a review of conceptual foundations, empirical evidence and practical considerations. Aust N Z J Psychiatry (2006) 40(4):285–94. doi:10.1111/j.1440-1614.2006.01794.x
120. Wang DJJ, Rao HY, Korczykowski M, Wintering N, Pluta J, Khalsa DS, et al. Cerebral blood flow changes associated with different meditation practices and perceived depth of meditation. Psychiatry Res Neuroimaging (2011) 191(1):60–7. doi:10.1016/j.pscychresns.2010.09.011
121. Lavretsky H, Epel ES, Siddarth P, Nazarian N, Cyr NS, Khalsa DS, et al. A pilot study of yogic meditation for family dementia caregivers with depressive symptoms: effects on mental health, cognition, and telomerase activity. Int J Geriatr Psychiatry (2013) 28(1):57–65. doi:10.1002/gps.3790
122. Andrews NP, Fujii H, Goronzy JJ, Weyand CM. Telomeres and immunological diseases of aging. Gerontology (2010) 56(4):390–403. doi:10.1159/000268620
123. Franco S, Blasco MA, Siedlak SL, Harris PL, Moreira PI, Perry G, et al. Telomeres and telomerase in Alzheimer’s disease: epiphenomena or a new focus for therapeutic strategy? Alzheimers Dementia (2006) 2(3):164–8. doi:10.1016/j.jalz.2006.03.001
124. Canela A, Vera E, Klatt P, Blasco MA. High-throughput telomere length quantification by FISH and its application to human population studies. Proc Natl Acad Sci U S A (2007) 104(13):5300–5. doi:10.1073/pnas.0609367104
125. Grodstein F, van Oijen M, Irizarry MC, Rosas HD, Hyman BT, Growdon JH, et al. Shorter telomeres may mark early risk of dementia: preliminary analysis of 62 participants from the nurses’ health study. PLoS One (2008) 3(2):e1590. doi:10.1371/journal.pone.0001590
126. Valdes AM, Deary IJ, Gardner J, Kimura M, Lu X, Spector TD, et al. Leukocyte telomere length is associated with cognitive performance in healthy women. Neurobiol Aging (2010) 31(6):986–92. doi:10.1016/j.neurobiolaging.2008.07.012
127. Lin J, Epel E, Blackburn E. Telomeres and lifestyle factors: roles in cellular aging. Mutat Res (2012) 730(1–2):85–9. doi:10.1016/j.mrfmmm.2011.08.003
128. Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A (2004) 101(49):17312–5. doi:10.1073/pnas.0407162101
129. Epel ES, Lin J, Wilhelm FH, Wolkowitz OM, Cawthon R, Adler NE, et al. Cell aging in relation to stress arousal and cardiovascular disease risk factors. Psychoneuroendocrinology (2006) 31(3):277–87. doi:10.1016/j.psyneuen.2005.08.011
130. Damjanovic AK, Yang YH, Glaser R, Kiecolt-Glaser JK, Nguyen H, Laskowski B, et al. Accelerated telomere erosion is associated with a declining immune function of caregivers of Alzheimer’s disease patients. J Immunol (2007) 179(6):4249–54.
131. Shammas MA. Telomeres, lifestyle, cancer, and aging. Curr Opin Clin Nutr Metab Care (2011) 14(1):28–34. doi:10.1097/MCO.0b013e32834121b1
132. Barcelo A, Pierola J, Lopez-Escribano H, de la Pena M, Soriano JB, Alonso-Fernandez A, et al. Telomere shortening in sleep apnea syndrome. Respir Med (2010) 104(8):1225–9. doi:10.1016/j.rmed.2010.03.025
133. Prather AA, Puterman E, Lin J, O’Donovan A, Krauss J, Tomiyama AJ, et al. Shorter leukocyte telomere length in midlife women with poor sleep quality. J Aging Res (2011) 2011:721390. doi:10.4061/2011/721390
134. Elvsashagen T, Vera E, Boen E, Bratlie J, Andreassen OA, Josefsen D, et al. The load of short telomeres is increased and associated with lifetime number of depressive episodes in bipolar II disorder. J Affect Disord (2011) 135(1–3):43–50. doi:10.1016/j.jad.2011.08.006
135. Simon NM, Smoller JW, McNamara KL, Maser RS, Zalta AK, Pollack MH, et al. Telomere shortening and mood disorders: preliminary support for a chronic stress model of accelerated aging. Biol Psychiatry (2006) 60(5):432–5. doi:10.1016/j.biopsych.2006.02.004
136. Martin-Ruiz C, Dickinson HO, Keys B, Rowan E, Kenny RA, Von Zglinicki T. Telomere length predicts poststroke mortality, dementia, and cognitive decline. Ann Neurol (2006) 60(2):174–80. doi:10.1002/ana.20869
137. Yaffe K, Lindquist K, Kluse M, Cawthon R, Harris T, Hsueh WC, et al. Telomere length and cognitive function in community-dwelling elders: findings from the health ABC study. Neurobiol Aging (2011) 32(11):2055–60. doi:10.1016/j.neurobiolaging.2009.12.006
138. Ravnik-Glavac M, Hrasovec S, Bon J, Dreo J, Glavac D. Genome-wide expression changes in a higher state of consciousness. Conscious Cogn (2012) 21(3):1322–44. doi:10.1016/j.concog.2012.06.003
139. Dusek JA, Otu HH, Wohlhueter AL, Bhasin M, Zerbini LF, Joseph MG, et al. Genomic counter-stress changes induced by the relaxation response. PLoS One (2008) 3(7):e2576. doi:10.1371/journal.pone.0002576
140. Sharma H, Datta P, Singh A, Sen S, Bhardwaj NK, Kochupillai V, et al. Gene expression profiling in practitioners of Sudarshan Kriya. J Psychosom Res (2008) 64(2):213–8. doi:10.1016/j.jpsychores.2007.07.003
141. Creswell JD, Irwin MR, Burklund LJ, Lieberman MD, Arevalo JM, Ma J, et al. Mindfulness-based stress reduction training reduces loneliness and pro-inflammatory gene expression in older adults: a small randomized controlled trial. Brain Behav Immun (2012) 26(7):1095–101. doi:10.1016/j.bbi.2012.07.006
142. Black DS, Cole SW, Irwin MR, Breen E, St Cyr NM, Nazarian N, et al. Yogic meditation reverses NF-kappaB and IRF-related transcriptome dynamics in leukocytes of family dementia caregivers in a randomized controlled trial. Psychoneuroendocrinology (2013) 38(3):348–55. doi:10.1016/j.psyneuen.2012.06.011
143. Saatcioglu F. Regulation of gene expression by yoga, meditation and related practices: a review of recent studies. Asian J Psychiatr (2013) 6(1):74–7. doi:10.1016/j.ajp.2012.10.002
144. Takimoto-Ohnishia E, Ohnishia J, Murakamia K. Mind–body medicine: effect of the mind on gene expression. Personal Med Univ (2012) 1(1):2–6. doi:10.1016/j.pmu.2012.05.001
145. Cotman CW. The role of neurotrophins in brain aging: a perspective in honor of Regino Perez-Polo. Neurochem Res (2005) 30(6–7):877–81. doi:10.1007/s11064-005-6960-y
146. Kalman J, Kitajka K, Pakaski M, Zvara A, Juhasz A, Vincze G, et al. Gene expression profile analysis of lymphocytes from Alzheimer’s patients. Psychiatr Genet (2005) 15(1):1–6. doi:10.1097/00041444-200503000-00001
147. Twine NA, Janitz K, Wilkins MR, Janitz M. Whole transcriptome sequencing reveals gene expression and splicing differences in brain regions affected by Alzheimer’s disease. PLoS One (2011) 6(1):e16266. doi:10.1371/journal.pone.0016266
148. Katsel P, Li C, Haroutunian V. Gene expression alterations in the sphingolipid metabolism pathways during progression of dementia and Alzheimer’s disease: a shift toward ceramide accumulation at the earliest recognizable stages of Alzheimer’s disease? Neurochem Res (2007) 32(4–5):845–56. doi:10.1007/s11064-007-9297-x
149. Rivera EJ, Goldin A, Fulmer N, Tavares R, Wands JR, de la Monte SM. Insulin and insulin-like growth factor expression and function deteriorate with progression of Alzheimer’s disease: link to brain reductions in acetylcholine. J Alzheimers Dis (2005) 8(3):247–68.
150. Pasinetti GM, Ho L. From cDNA microarrays to high-throughput proteomics. Implications in the search for preventive initiatives to slow the clinical progression of Alzheimer’s disease dementia. Restor Neurol Neurosci (2001) 18(2–3):137–42.
151. Zanni GR, Wick JY. Telomeres: unlocking the mystery of cell division and aging. Consult Pharm (2011) 26(2):78–90. doi:10.4140/TCP.n.2011.78
152. Kananen L, Surakka I, Pirkola S, Suvisaari J, Lonnqvist J, Peltonen L, et al. Childhood adversities are associated with shorter telomere length at adult age both in individuals with an anxiety disorder and controls. PLoS One (2010) 5(5):e10826. doi:10.1371/journal.pone.0010826
153. Panossian LA, Porter VR, Valenzuela HF, Zhu X, Reback E, Masterman D, et al. Telomere shortening in T cells correlates with Alzheimer’s disease status. Neurobiol Aging (2003) 24(1):77–84. doi:10.1016/S0197-4580(02)00043-X
154. Thomas P, Callaghan NJO, Fenech M. Telomere length in white blood cells, buccal cells and brain tissue and its variation with ageing and Alzheimer’s disease. Mech Ageing Dev (2008) 129(4):183–90. doi:10.1016/j.mad.2007.12.004
155. Lukens JN, Van Deerlin V, Clark CM, Xie SX, Johnson FB. Comparisons of telomere lengths in peripheral blood and cerebellum in Alzheimer’s disease. Alzheimers Dement (2009) 5(6):463–9. doi:10.1016/j.jalz.2009.05.666
156. Bekaert S, De Meyer T, Rietzschel ER, De Buyzere ML, De Bacquer D, Langlois M, et al. Telomere length and cardiovascular risk factors in a middle-aged population free of overt cardiovascular disease. Aging Cell (2007) 6(5):639–47. doi:10.1111/j.1474-9726.2007.00321.x
157. Armanios M, Alder JK, Parry EM, Karim B, Strong MA, Greider CW. Short telomeres are sufficient to cause the degenerative defects associated with aging. Am J Hum Genet (2009) 85(6):823–32. doi:10.1016/j.ajhg.2009.10.028
158. Ornish D, Lin J, Daubenmier J, Weidner G, Epel E, Kemp C, et al. Increased telomerase activity and comprehensive lifestyle changes: a pilot study. Lancet Oncol (2008) 9(11):1048–57. doi:10.1016/S1470-2045(08)70234-1
159. Kaszubowska L. Telomere shortening and ageing of the immune system. J Physiol Pharmacol (2008) 59:169–86.
160. Carrero J, Stenvinkel P, Fellstrom B, Qureshi AR, Lamb K, Heimburger O, et al. Telomere attrition is associated with inflammation, low fetuin-A levels and high mortality in prevalent haemodialysis patients. J Intern Med (2008) 263(3):302–12. doi:10.1111/j.1365-2796.2007.01890.x
161. Dinan TG. Inflammatory markers in depression. Curr Opin Psychiatry (2009) 22(1):32–6. doi:10.1097/YCO.0b013e328315a561
162. Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med (2009) 71(2):171–86. doi:10.1097/PSY.0b013e3181907c1b
163. Motivala SJ, Sarfatti A, Olmos L, Irwin MR. Inflammatory markers and sleep disturbance in major depression. Psychosom Med (2005) 67(2):187–94. doi:10.1097/01.psy.0000149259.72488.09
164. von Kanel R, Dimsdale JE, Ancoli-Israel S, Mills PJ, Patterson TL, McKibbin CL, et al. Poor sleep is associated with higher plasma proinflammatory cytokine interleukin-6 and procoagulant marker fibrin D-dimer in older caregivers of people with Alzheimer’s disease. J Am Geriatr Soc (2006) 54(3):431–7. doi:10.1111/j.1532-5415.2005.00642.x
165. Gouin JP. Chronic stress, immune dysregulation, and health. Am J Lifestyle Med (2011) 5(6):476–85. doi:10.1177/1559827610395467
166. Pace TW, Negi LT, Adame DD, Cole SP, Sivilli TI, Brown TD, et al. Effect of compassion meditation on neuroendocrine, innate immune and behavioral responses to psychosocial stress. Psychoneuroendocrinology (2009) 34(1):87–98. doi:10.1016/j.psyneuen.2008.08.011
167. Pullen PR, Nagamia SH, Mehta PK, Thompson WR, Benardot D, Hammoud R, et al. Effects of yoga on inflammation and exercise capacity in patients with chronic heart failure. J Card Fail (2008) 14(5):407–13. doi:10.1016/j.cardfail.2007.12.007
168. Cacabelos R. Genomic characterization of Alzheimer’s disease and genotype-related phenotypic analysis of biological markers in dementia. Pharmacogenomics (2004) 5(8):1049–105. doi:10.1517/14622416.5.8.1049
169. Grunblatt E, Bartl J, Zehetmayer S, Ringel TM, Bauer P, Riederer P, et al. Gene expression as peripheral biomarkers for sporadic Alzheimer’s disease. J Alzheimers Dis (2009) 16(3):627–34. doi:10.3233/JAD-2009-0996
170. Cedazo-Minguez A, Winblad B. Biomarkers for Alzheimer’s disease and other forms of dementia: clinical needs, limitations and future aspects. Exp Gerontol (2010) 45(1):5–14. doi:10.1016/j.exger.2009.09.008
Keywords: cognitive impairment, meditation, mind–body therapies, mood, sleep, stress, cellular aging, epigenetics
Citation: Innes KE and Selfe TK (2014) Meditation as a therapeutic intervention for adults at risk for Alzheimer’s disease – potential benefits and underlying mechanisms. Front. Psychiatry 5:40. doi: 10.3389/fpsyt.2014.00040
Received: 19 November 2013; Accepted: 31 March 2014;
Published online: 23 April 2014.
Edited by:
Shirley Telles, Patanjali Research Foundation, IndiaReviewed by:
Peter Kirsch, Zentralinstitut für Seelische Gesundheit, GermanySalahadin Abdi, BIDMC, USA
Copyright: © 2014 Innes and Selfe. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kim E. Innes, Department of Epidemiology, School of Public Health, Health Sciences Center, West Virginia University, PO Box 9190, Morgantown, WV 26506, USA e-mail:a2lubmVzQGhzYy53dnUuZWR1
 Kim E. Innes
Kim E. Innes Terry Kit Selfe
Terry Kit Selfe