- 1Department of Psychiatry and Psychotherapy, Otto-von-Guericke University of Magdeburg, Magdeburg, Germany
- 2Department of Psychiatry, Social Psychiatry and Psychotherapy, Hanover Medical School, Hanover, Germany
- 3Department of Psychiatry, Paracelsus Medical University, Nuremberg, Germany
- 4Department of Psychiatry and Psychotherapy, University of Cologne, Cologne, Germany
- 5German Centre for Neurodegenerative Diseases, Magdeburg, Germany
Objective: Epigenetic mechanisms have been described in several mental disorders, such as mood disorders, anxiety disorders and schizophrenia. However, less is known about the influence of epigenetic mechanisms with regard to personality disorders (PD). Therefore, we conducted a literature review on existing original data with regards to epigenetic peculiarities in connection with personality disorders.
Methods: Systematic literature review using PRISMA guidelines. Search was performed via NCBI PubMed by keywords and their combinations. Used search terms included “epigenetic,” “methylation,” “acetylation” plus designations of specified personality traits and disorders according to DSM-IV.
Results: Search yielded in total 345 publications, 257 thereof with psychiatric topic, 72 on personality disorder or traits, 43 of which were in humans and epigenetic, 23 thereof were original studies. Lastly, 23 original publications fulfilled the intended search criteria and were included. Those are 13 studies on gene methylation pattern with aggressive, antisocial and impulsive traits, 9 with borderline personality disorder (BPD), and 2 with antisocial personality disorder (ASPD). The results of these studies showed significant associations of PD with methylation aberrances in system-wide genes and suggest evidence for epigenetic processes in the development of personality traits and personality disorders. Environmental factors, of which childhood trauma showed a high impact, interfered with many neurofunctional genes. Methylation alterations in ASPD and BPD repeatedly affected HTR2A, HTR3A, NR3C1, and MAOA genes.
Summary: Epigenetic studies in PD seem to be a useful approach to elucidate the interaction of co-working risk factors in the pathogenesis of personality traits and disorders. However, the complexity of pathogenesis leads to divergent results and impedes an explicit interpretation. Differing methylation patterns within the selected PD could indicate subgroups which would benefit from patient-oriented therapeutic adjustments. They might play a major role in the future design and observation of early therapeutic intervention and thus could help to prevent severe dysfunctional conduct or full-blown personality disorder in risk subjects.
Introduction
The epigenetic view on genes presumably associated with psychiatric disorders is gaining increasing academic interest and enables auxiliary insights in the pathogenesis of a particular disease. Severe psychiatric axis-I disorders like major depressive disorder (MDD) are currently widely investigated at the epigenetic level, and results account for a substantial pathogenetic impact of gene epigenetic modifications.
Epigenetic mechanisms in general function to homeostatically control gene accessibility and transcriptional functionality. Thus, gene function can be organized in both a highly programmed and life-enduring, but also in an environmentally-responsive way (1). The transcriptome as the immediate representation of genomic activity is regulated by (i) control of gene access for the transcriptional machinery through chromatin condensation and histone modification such as (de-)acetylation, (de-)phosphorylation, sumoylation, (ii) non-coding and microRNA that influences chromatin formation, as well as RNA translation and degradation, and (iii) covalent DNA changes by methylation of the cytosine nucleotide, which hampers transcriptase accessibility to the methylated region, and which can activate enzymes that interact in silencing the specific gene [reviewed in (2)].
Whereas epigenetic patterns stably determine mitosis-persistent cell differentiation as a precondition for embryonal development without changing DNA sequence, the epigenome also represents a dynamic adaption to environmental conditions. There is growing evidence in the last two decades that early life experience can affect long standing somatic and mental health trajectories in animals and humans by influencing the epigenetic pattern and thus affects structure and accessibility of the genome. Studies in rodents demonstrated the decisive impact of maternal care and early social adversity on the offspring's development and adult phenotypes. Early life adversity (ELA) in rats led to increased hippocampal glucocorticoid receptor (GR) expression, disturbed hypothalamus-pituitary-adrenal (HPA) axis functionality, and changed DNA methylation of the GR gene (NR3C1) in the hippocampus (3), of the brain-derived neurotropic factor gene (BDNF) promoter in the prefrontal cortex (4) and of the Arginine Vasopressin gene (AVP) (5). In humans, ELA was associated with decreased GR mRNA expression and NR3C1 hypermethylation in post-mortem human brains (6). Hitherto findings indicate that epigenetic patterns found in context with ELA in animals and humans are not restricted to suggestive disease-associated functional genes but are spread genome-wide (7, 8), and are not stringently tissue-specific (9), since peripheral blood cells (PBC), especially T-cell lymphocytes, were shown to reflect epigenetic patterns similar to neuronal cells in culture and in brain tissue (9–11).
The translation of environmental signals into epigenetic information can be triggered by neuronal activity that initiates intracellular pathways such as cAMP signaling mediated histone acetylation or that influences and interacts with other epigenetic processes (12–14). Additionally, activity of the AVP promoter is regulated by the methyl-CpG-binding protein 2 (MeCp2), which is phosphorylated and activated by depolarization of hypothalamic neurons and in turn moderates demethylation of the BDNF promoter (5, 15, 16). In sum, although many details of molecular mechanisms remain unknown, the present insights substantiate and refine the idea how environmental signals might be translated into intracellular information and molecular memory.
Currently, a considerable number of studies explore epigenetic changes in association with behavior or affect difficulties like aggression or fear in human and animal subjects, particularly in connection with disturbances of the serotonergic system that meanwhile is well-known to be crucial in early brain development. The objective of this work was to review the current original publications on epigenetic modifications associated with personality disorders (PD) in humans.
Methods
Literature search was performed as a systematic review according the Preferred Recording Items for Systematic Reviews and Meta-Analyses (PRISMA-P) guidelines (17). We based our search on the PubMed Central database of the U.S. National Institutes of Health's National Library of Medicine (NIH/NLM) using terms oriented on the Medical Subject Headings (MeSH) of the NCBI Library.
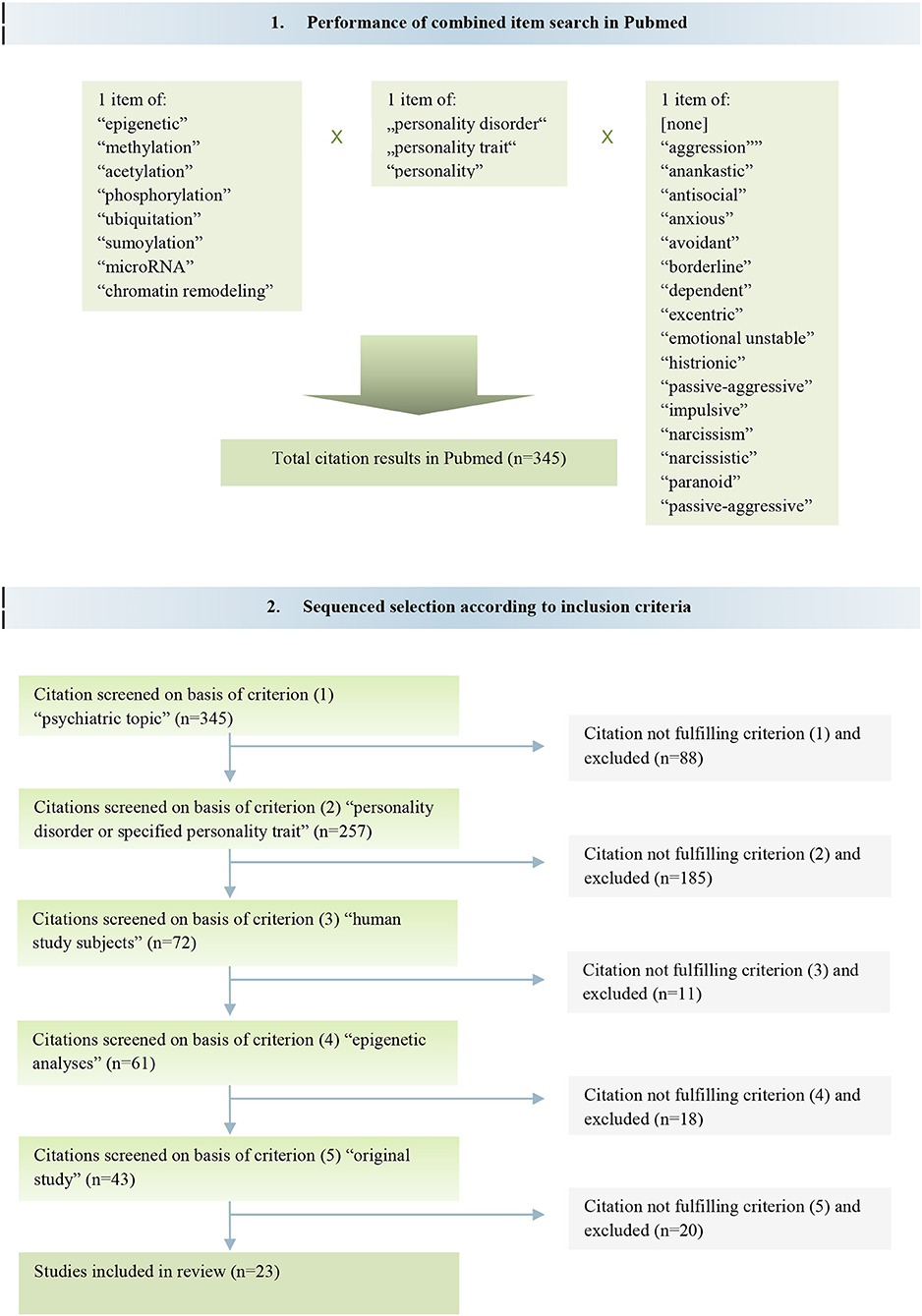
For the search keywords were inserted in a double or triple combination to yield comprehensive hits. The following keywords were utilized: “personality,” “personality disorder,” “personality trait,” each of them combined with “epigenetic,” “methylation,” “acetylation,” “phosphorylation,” “ubiquitation,” “sumoylation,” “microRNA,” “chromatin” and “chromatin remodeling,” respectively, as well as with one of the keywords “aggression,” “anankastic,” “antisocial,” “anxious,” “avoidant,” “borderline,” “dependent,” “eccentric,” “emotionally unstable,” “histrionic,” “passive-aggressive,” “impulsive,” “narcissism,” “narcissistic,” “paranoid,” “schizoid,” and “schizotypal,” respectively. The search included publications until May 15th 2018.
In total, the search yielded 345 different articles. We secondly perused the gained articles by reviewing their titles, abstracts and full texts in order to identify the proper articles matching to our literal research question. Therefore, studies were sequentially selected if they met the criteria (1) psychiatric topic [n = 257], (2) personality disorder or specified personality trait [n = 72 of (1)], (3) human study subjects [n = 61 of (2)], (4) epigenetic analyses [n = 43 of (3)], and (5) original study [n = 23 of (4)]. Following these selection criteria, it remained 23 articles according to the intended objective of this review (Figure 1).
Concomitantly, we used the PubMed search function of f1000prime (Faculty of 1000 Limited, London, UK). Herewith we found one further expedient study that met all of the described inclusion criteria (18). Finally, 24 original studies were included in this review.
Results
Among the included studies, 13 explored the epigenetic influence on PT (two on impulsiveness, six on antisocial traits, seven on aggression) and 11 studied epigenetic differences in personality disorders (two in antisocial personality disorder (ASPD), 9 in borderline personality disorder (BPD)).
Size, constitution of study groups, and the number of investigated genes varied between the studies and ranged from single gene assays to genome-wide methylation analyses (GWA) with implications for the statistical power.
Personality Disorders
Antisocial Personality Disorder
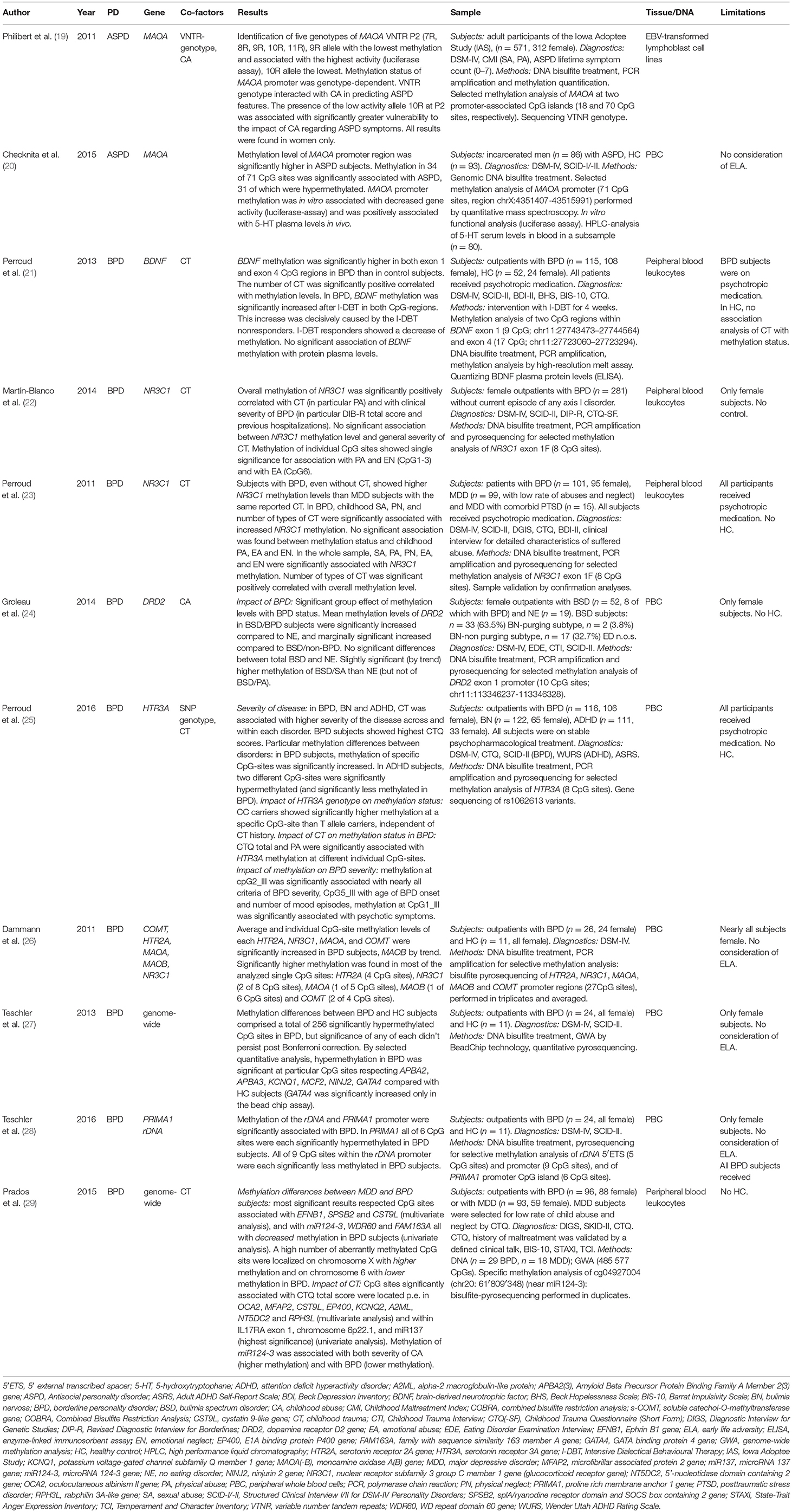
With regards on antisocial PD, only methylation of the monoamine oxidase A gene (MAOA) has been examined. The two studies differed decisively with respect to study design and methylation results.
Philibert et al. considered the known variable nucleotide repeat (VTNR) region of MAOA and introduced a new VTNR region upstream of the transcriptional start site (TSS) of the gene (MAOA VTNR P2). They found a genotype-dependent methylation level and gene activity, but only in females (19). Within a total of well characterized 571 subjects (312 female) of the Iowa Adoption Study (IAS) they measured ASPD lifetime symptom counts in a linear mode according to DSM-IV criteria. Methylation patterns were analyzed at two promoter-associated CpG islands of MAOA in DNA extracted from EBV transformed lymphoblast cell lines from peripheral blood. Sequence analyses of the VTNR P2 revealed five genotypes with each seven to 11 eleven repeats (7R, 8R, 9R, 10R, 11R), of which the 9R genotype showed the lowest methylation in homozygous females, and the greatest gene activity in the functional analysis via luciferase essay. Presence of the low activity allele 10R was associated with higher vulnerability to the harming effects of childhood sexual and physical abuse and it accounted significantly for variances in symptom severity of ASPD in women. In male subjects no significant effect of the P2 genotype on MAOA methylation status was found.
In contrast, in a population of incarcerated men (n = 86) fulfilling the DSM-IV criteria for ASPD, Checknita et al. found a significant overall hypermethylation of the MAOA promoter region in the ASPD group compared with healthy controls (n = 93) with significant differences in methylation levels at 34 of 71 distinct MAOA promoter CpG sites. In their analysis, they did not consider symptom severity or childhood adversity. Methylation of MAOA promoter was associated with decreased gene activity (luciferase-assay) and positively correlated with 5-hydroxytryptophane levels in blood, thus suggesting functional relevance (20).
Borderline Personality Disorder
With respect to epigenetic modifications in BPD, different genes were suggested to be involved during individual development as well as in phenotypic characteristics of the disorder (Table 1). Apart from genome wide analyses (GWA), the main focus of theory-driven epigenetic studies was in gene regions coding for BDNF, glucocorticoid receptor (NR3C1), dopamine and serotonin receptors, MAOA, and catechol-O-methyltransferase (COMT). Most of the studies that focused on targeted genes considered a history of an early child trauma as a confounding factor in their analyses. The diversity in control group definition in the individual studies reflects the struggle for a suitable study design that allows for isolating disorder-specific characteristics. Methylation aberrations were mostly evaluated with diagnostic, but also with predictive concerns (21).
Perroud et al. studied methylation status of the BDNF gene in peripheral blood leucocytes and its modulation by a focused therapeutic intervention with intensive dialectical behavior therapy (I-DBT) comprising 4 weeks in outpatients with BPD (n = 115). Contrasted with healthy subjects, CpG-rich regions in exon 1 and exon 4 were significantly more highly methylated in BPD subjects before the therapeutic intervention. The number of different types of childhood trauma (CT) according the Childhood Trauma Questionnaire (CTQ) correlated positively with the mean methylation percentage of both CpG-regions. After intensive DBT, methylation status of the considered CpG sites was significantly increased in BPD patients. This effect could be traced back to the non-responders, whereas the responders showed a methylation decrease. However, the comparison of BDNF methylation status with peripheral serum protein levels of BDNF revealed no significant association (21).
Regarding studies on NR3C1 methylation and PD, two studies focused on exon 1F promoter, which is functionally crucial. In a cohort of BPD outpatients (n = 281) Martin-Blanco et al. (22) found a significant positive correlation between overall NR3C1 exon 1F methylation level of peripheral blood leucocytes and clinical severity. Exon 1F methylation was further significantly associated with childhood physical abuse. Individual CpG sites were associated with particular subscores of CTQ.
Perroud et al. (23) considered that a current severe mental illness might have epigenetic implications per se and could confound analyses that aim to isolating methylation characteristics specific for BPD. Therefore, in a comparison of subjects with BPD (n = 101) to those with MDD (n = 99) their results showed higher overall NR3C1 exon 1F methylation levels in BPD than in MDD subjects in peripheral blood leucocytes. Further, methylation was associated with CT scaled by the CTQ, and correlated with childhood sexual, physical and emotional abuse, and physical and emotional neglect, respectively, and the number of these types of CT. NR3C1 exon 1F hypermethylation in subjects with BPD was still significant when corrected for childhood maltreatment.
Regarding monoamine receptor genes, within a large-scaled study on subjects with bulimia spectrum disorders (BSD) with and without comorbid BPD Groleau et al. (24) found significant but marginally increased methylation of the dopamine D2 receptor gene (DRD2) exon 1 promoter region in whole peripheral blood cell (PBC) DNA of subjects with BSD and BPD compared with that of subjects with BSD only.
Methylation of the serotonin receptor 3A gene (5HTR3A) was found by Perroud et al. (25) to be correlated to clinical severity of BPD and other psychiatric disorders. The authors compared PBC DNA methylation levels of eight CpG sites within the 5HT3A gene in subjects with BPD (n = 116), attention deficit hyperactivity disorder (ADHD) (n = 111) and bipolar disorder (BD) (n = 122). They also considered single nucleotide variants (SNP) of the gene and CT history as additional factors and explored associations with methylation levels of the particular CpG sites.
Methylation levels between the patient groups differed significantly in most of the CpG sites and showed a distinct pattern of hyper- and hypomethylation in the several disorders in selected CpG sites. For BPD, subjects showed the highest scores in the CTQ and the highest methylation level between the patient groups. CT was associated with mean methylation status, and CTQ total score and physical abuse each with different selected CpG sites. CT was further associated with higher severity of disease. Carrying the CC-allele was significantly associated with methylation at one specific CpG site independent of CT (in all disorders).
Dammann et al. (26) analyzed five neuropsychiatric genes assumed to be of significance for psychopathological phenotype, in particular genes coding for soluble catechol-O-methyltransferase (s-COMT), serotonin receptor 2A (HTR2A), NR3C1, and X-chromosomal MAOA and MAOB. Methylation levels of each gene promoter were quantified in PBC DNA of individuals with BPD (n = 26, 24 female) and in healthy controls (n = 11, all female). In comparison to healthy controls, quantitative DNA methylation analysis showed significant elevated overall methylation levels in BPD subjects within HTR2A, NR3C1, and s-COMT. Gene methylation of MAOA and MAOB could only be analyzed in female subjects, and methylation of MAOA was significantly higher in BPD (of MAOB only by trend). Considering all 27 individual CpG sites, across the five genes that were investigated, average methylation level across all quantified regions was significantly higher in BPD patients compared to controls. Implications of attendant data as trauma history of the subjects weren't presented as part of the study, but it was noted that aberrant methylation had not been associated with traumatic experience in appropriate statistical tests.
Meanwhile epigenetic assays include genome-wide association studies (GWA) that indicate specific CpG sites of aberrant methylation levels across the whole genomic DNA and facilitate comprehensive aspects within epigenetic evaluations.
In extension to the aforementioned study, Teschler et al. (27) performed a GWA in PBC DNA between female BPD (n = 24) and HC (n = 11) subjects. Results showed a total of 256 significantly hypermethylated CpG sites in BPD, but significance of any of each didn't persist post Bonferroni correction. The research group selected seven hypermethylated genes for validation analyses and could endorse increased methylation in five of the genes, with specified CpG sites related to the amyloid beta (A4) precursor protein-binding family A member 2 (APBA2) and member 3 (APBA3) genes, potassium voltage-gated channel KQT-like subfamily member 1 gene (KCNQ1), MCF2 cell line derived transforming sequence gene (MCF2) and the ninjurin 2 gene (NINJ2). GATA binding protein 4 (GATA4) and holocarboxylase synthetase (HLCS) genes showed increased methylation in BPD in the GWA, but not in the validation analysis.
Methylation studies of the ribosomal RNA gene (rDNA) promoter, 5′ external transcribed spacer gene (5′ETS) and of the proline rich membrane anchor 1 gene (PRIMA1) promoter in PBC DNA of female subjects with BPD (n = 24) and HC (n = 11) revealed significantly less methylation of the rDNA promoter region in BPD compared with HC subjects, and hypomethylation of the 5′ETS in BPD by trend. PRIMA1 showed a higher methylation in BPD subjects (28).
Another GWA with PBC DNA was performed by Prados et al. (29) on BPD subjects affected with high levels of childhood adversity (n = 96) and subjects with MDD and a history of low levels of childhood adversity (n = 93). Uni- and multivariate analyses revealed significant methylation differences in a significant number of particular CpG sites associated with BPD compared with MDD subjects or related to childhood maltreatment, respectively. Contrasting BPD with MDD subjects, most significant results of multivariate analyses resulted in significantly different methylated CpG sites located e.g., within the gene coding for a ligand of Eph-related receptor tyrosine kinases (EFBN1), closely to the gene coding for a suppressor of cytokine signaling (SOCS) family member (SPSB2) and near the gene coding for a protein similar to mouse cystatin 9 (CST9L). Univariate analyses detected hypomethylated CpG sites in BPD i.e., near the encoding region of microRNA 124 (miR124-3), which targets several genes that have been described to be correlated with BPD (including NR3C1), near the gene coding for the WD repeat domain 60 (WD60), and a CpG site within the gene of the family with sequence similarity 163 member A (FAM163A). Many of the significantly hypermethylated sites were found on chromosome X. Targeting on CT, results of multivariate analyses with the CTQ revealed strong associations with loci within or near the genes of human homolog of the mouse p (pink-eyes dilution) (OCA2), microfibrillar-associated protein 2 (MFAP2), CST9L, E1A binding protein p400 (EP400), KCNQ2, alpha-2-macroglobulin-like 1 (A2ML1), 5′-nucleotide domain containing 2 (NT5DC2) and rabphilin 3A-like (RPH3AL). Univariate analyses yielded most significant alterations located within the gene coding for the interleukin 17 receptor A (IL17RA), in an intergenic region on chromosome 6p22.1, and closed by miR124-3 and miR137. Methylation of miR124-3 was associated with both severity of childhood adversity (higher methylation) and with BPD (lower methylation).
In summary, large part of the studies in PD focused on single genes, including MAOA in ASPD, and BDNF, NR3C1, DRD2, and HTR3A in BPD, or on a set of theory-driven suggestive genes (26). They revealed significant methylation differences in blood cell DNA of subjects with the respective PD. Within all independent gene-targeted studies, NR3C1 hypermethylation was most frequently and consistently found to be associated with BPD; therefore, current results most strongly indicate NR3C1 to be implicated in BPD. NR3C1 further was the only gene that was as well affected in one of the GWA (29), however this was mediated by methylation differences of miR124-3 which targets, among other genes, on NR3C1. In contrast, GWA studies, as performed by Teschler and Prados, found large number of genes that were differentially methylated in BPD, indicating a system-wide involvement in PD including genes associated with immune-response, cell-signaling or transcription control (27–29). The transcriptional relevance of the respective methylation differences was only verified by two authors. Thus, Checknita et al. found an indirect negative association of MAOA promoter hypermethylation with 5-HT serum level in vitro (20). Otherwise, Perroud et al. (21) didn't found an association of BDNF promoter methylation and the peripheral protein serum level of BDNF. All studies were based on DNA extracted from PBC, in most cases from whole blood cells, partly from only leucocytes (21–23), or selective from lymphocytes (19). However, the studies particularly differ in their design of study subjects and controls, and at least in the consideration of environmental factors that have impact on epigenetic modulation like early childhood adversity, which impedes a well-defined interpretation in most of the studies (Table 1, col. “Limitations”).
Personality Traits
Most original publications on methylation and personality traits were found with antisocial or aggressive features in connection with epigenetic alterations associated with the functionality of the serotonergic system or the hypothalamic–pituitary–adrenal (HPA-) axis functionality, which is the main neuroendocrine system for regulation of stress reaction and adaptation. Accordingly, theory-driven studies examined genes associated with these systems, such as the serotonin transporter gene (SLC6A4), dopamine and serotonin receptor genes (DRD1, HTR1B, HTR1D, HTR3A), MAOA, and NR3C1 promoter exon 1F. With respect to its role in socio-affective perception and processing, some studies exist that pertain to antisocial behavior and oxytocin and oxytocin receptor genes (OXT, OXTR). Further, the role of cytokines and other factors were considered.
Antisocial Traits
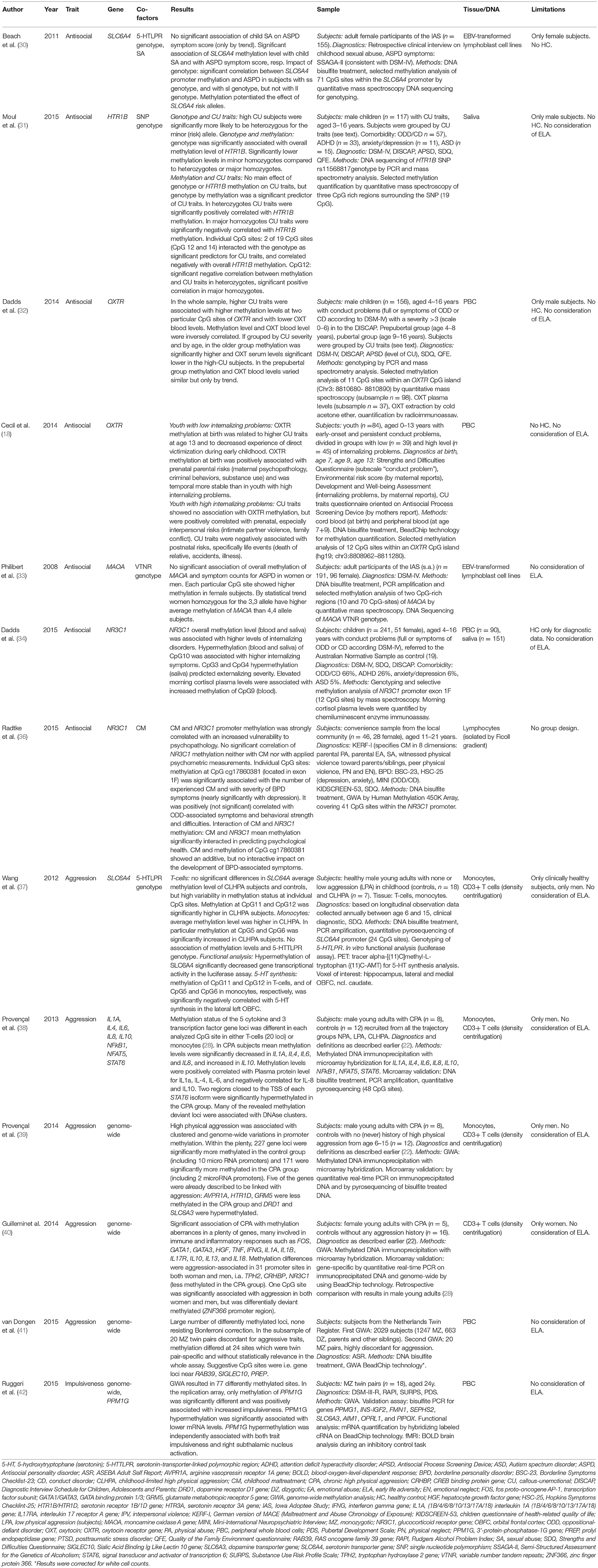
Consistent with a large body of literature that implicates the serotonergic system in the regulation of anxiety, aggression, and stress response, epigenetic alterations in the serotonin transporter gene (SLC6A4) were found to be related to a history of child sexual abuse as well as to antisocial behavior in adulthood by Beach et al. (30). The authors performed methylation analyses on the participant's peripheral blood lymphocyte DNA that was EBV transformed into lymphoblast cell lines. Participants (n = 155, all female) were gradually diagnosed by means of symptom score of ASPD. Child sexual abuse was highly associated with mean methylation level and methylation was highly significantly associated with symptoms of ASPD, thereby playing a modulating role to develop antisocial traits after childhood sexual abuse. Interestingly, influence of SLC6A4 methylation on ASPD severity was impacted by genotype, since association of methylation with the ss and the sl genotype of SLC6A4 was significant, but not with the ll genotype. These results suggest an aggravating effect of methylation in SLC6A4 risk (s) alleles for ASPD, further, methylation was increasingly associated with ASPD in carriers with greater number of s alleles.
Moul et al. found antisocial traits being associated with genetic and epigenetic modulation of the rs11568817 SNP in the HTR1B promoter region (31). In an investigation with boys (n = 117) exhibiting callous-unemotional (CU) traits and antisocial behavior problems, and grouped by high and less strong CU traits, they found lower methylation levels of HTTR1B in saliva cell DNA in the high CU trait group. In turn, methylation was decisively moderated by rs11568817 SNP genotype and carried by two exclusive CpG sites (CpG12 and CpG14), which individual methylation levels were negatively associated with overall methylation levels in this gene region. The authors assume two ways of risk for high CU traits, first, carrier of the risk (minor) allele (s) with low levels of methylation at CpG sites 12 and 14 (what was associated with high overall promoter methylation) and second, carrier without risk allele but with high methylation at CpG 12 and CpG14 (what was associated with low overall methylation) and high expression of HTR1B (31).
In the same sample of participants stratified by CU score level and by age, the aforementioned research group analyzed methylation characteristics of the ocytocin receptor gene (OXTR) in PBC DNA. They revealed high CU traits being associated with increased methylation at two of the six analyzed CpG loci within the OXTR promoter and being correlated with decreased oxytocin blood levels. Yet, when divided in age group, these findings reached significance only in the older group (age 9–16 years), not in the prepubertal children (age 4–8 years) (here only by trend). Similarly, oxytocin serum levels significantly were negatively correlated with gene methylation level in the older boys (32).
Further, in a prospective, longitudinal study, Cecil et al. (18) studied 84 youth with early-onset and persistent conduct problems with regard to early life risks and to methylation changes of OXTR in PBC DNA. Clinical surveys took place at birth, age 7, 9, and 13 years, and collected an environmental risk score (kind and time of risk factor), diagnosis of conduct problems, CU traits and internalizing problems. Equally, methylation analyses were performed longitudinally with sampling at birth (cord blood), and at age 7 and 9 years (peripheral blood). Finally, for evaluation subjects were divided by severity of internalizing problems, collected by maternal reports at each study time.
Results for youth with low internalizing problems revealed that OXTR hypermethylation at birth was significant related to higher CU traits at age 13. OXTR methylation at birth further was associated with decreased experience of victimization during early childhood, specifically direct victimization. The only environmental risk factor associated with OXTR methylation at birth was a prenatal parental risk i.e., maternal psychopathology, criminal behaviors, and substance use. Within the youth with high internalizing problems, CU traits showed no significant association with OXTR gene methylation, but were positively correlated with prenatal, specifically interpersonal, risks like intimate partner violence or family conflicts. Interestingly, CU traits were negatively correlated with postnatal risks, specifically life events as the death of a relative, an accident or illness. These different correlation results are suggestive for discriminative pathways in the development of CU traits, the one epigenetically co-determined, and the other influenced by different environmental factors. If associated with OXTR hypermethylation at birth, CU traits may protect for internalizing problems and for experienced victimization, as discussed by the authors as a possible evocative correlation (18).
In view of ASPD symptoms, Philibert et al. (33) explored the MAOA VTNR polymorphism genotype and methylation status in EBV transformed lymphoblast cell lines with respect to lifetime symptom severity of ASPD and substance use disorder as defined as alcohol or nicotine dependence in a collective of 191 subjects (96 female). Methylation level was consistently higher in the female subjects than in males at each analyzed CpG site. There was only a statistical trend for women homozygous for the 3,3 allele showing higher average methylation than for the 4,4 allele. The research revealed no association between symptom counts for ASPD and methylation levels in neither men nor women (33).
With attention to multifaceted features in subjects with early-onset behavior problems as i.e., higher irritability, anxiety, impulsivity, possibly associated with an impaired hypothalamic-pituitary-adrenal (HPA) function, Dadds et al. (34) examined the methylation patterns of the NR3C1 promoter exon 1F in whole blood and saliva cell DNA and the morning plasma cortisol levels within a study collective (n = 241, 51 female) with full criteria or features of conduct disorder (CD) or oppositional defiant disorder (ODD) in contrast to an Australian normative sample as control (35). Psychopathologic symptoms were scaled into more internalizing disorders in contrast to features of externalizing disorders like CU traits or conduct problems. Overall methylation levels of NR3C1 exon 1 in blood and saliva DNA probes were associated with higher levels of internalizing disorders. Distinctly, CpG10 showed an association of increased methylation with higher scores of internalizing symptoms. In contrast, methylation levels of CpG3 and CpG4 in saliva DNA probes predicted externalizing severity (34). Peripheral morning cortisol plasma levels were associated with hypermethylation of CpG9 in PBC DNA only.
Additionally, Radtke et al. (36) investigated the mutual influences of childhood maltreatment history, methylation of the NR3C1 promoter in peripheral blood lymphocytes and psychopathological load in subjects of a convenience sample (n = 46, 28 female). Their findings didn't indicate a significant correlation between average methylation of the NR3C1 promoter region with childhood maltreatment severity or with scores of the collected psychometric data. Though, by single analyses they depicted two of 41 selected CpG sites that interacted with childhood maltreatment and vulnerability to psychopathology. One of the two mentioned CpG site was located in exon 1F promoter and its methylation was highly significantly correlated with the number of experienced childhood adversities according to the CTQ as well as with BPD symptoms. With performing a linear model the authors revealed an independently strong effect of both childhood maltreatment and methylation status of this CpG site on the development of BPD-associated symptoms, and they found an additive effect of both of these factors (36).
In summary, studies on epigenetic associations with antisocial traits all focused on single genes. Each one study revealed significant methylation modifications in the SLC6A4 promoter (30) and in HTR1B (31), both in interaction with genotype variants of the concerned gene. Two studies focused on OXTR, with different results. In the first study, high CU traits were associated with OXTR hypermethylation, which was correlated with lower serum OXT level (32). In another, longitudinally performed study, OXTR hypermethylation at birth was associated with higher CU traits at prepubertal age in youth with low internalizing problems, but OXTR methylation was not associated with CU traits in youth with high internalizing problems, in which they were positively correlated with prenatal interpersonal risks and negatively with negative life events (18). This suggests a different (epi-)genetic pathogenesis of CU traits. In contrast, mean methylation of MAOA was not associated with ASPD symptom scores (33), nor was it significantly related to the MAOA VTNR genotypes. However, differentiated results exist in the two independent studies with NR3C1. Therefore, hypermethylation of distinct CpG-Sites within NR3C1 promoter exon 1F, but not overall methylation, was associated with externalizing symptoms (34). This is consistent with the results of another study, in which NR3C1 promoter exon 1F methylation showed no correlation with psychometric scores, but in consideration with childhood maltreatment both factors interacted in predicting vulnerability to psychopathology (36). Interestingly, NR3C1 was most investigated in BPD (-> Borderline Personality Disorder). Only two studies considered ELA as an independent factor (30, 36). All studies fail to provide a healthy control group; most of the studies are restricted to only male or female study subjects. Only one study analyzed the transcriptional relevance of the detected methylation alterations by means of the protein level (32). Hence, the represented studies in antisocial and BPD-associated personality traits yet cannot provide a base for general conclusions.
Aggression
Within a 21 year longitudinal study on early onset aggression the research group Wang et al. specified children by severity and persistence of aggressive behavior during their age of 6–15 years and established four states of childhood aggression trajectories: the first consisted in subjects with no physical aggression at any time points (no physical aggression, NPA), a second in subjects with low to moderate aggression that declined between age 10–15 (low physical aggression, LPA), the third in subjects with high rates of aggression that subsequently declined (childhood-limited high physical aggression, CLHPA) and finally in subjects with consistently high physical aggression until age 15 (chronic high physical aggression, CPA).
Among the described study cohort, Wang et al. (37) studied SLC6A4 methylation in comparison of healthy male young adult subjects with childhood-limited high physical aggression (CLHPA, n = 7) and those with no and low physical aggression in childhood, combining the latter as a control group (n = 18). They analyzed DNA extracted from isolated peripheral monocytes and in T cells. Not in T cells but in monocytes the authors found a significantly higher average methylation level across all CpG sites and the authors suggest monocytes to be more reliably in their association study. However, functional analysis in a luciferase assay showed significantly decreased SLC6A4 gene transcriptional activity for both T cells and monocytes in the CLHPA group. Regarding particular CpG sites individually, methylation at CpG11 and CpG12 were highly positively correlated and significantly higher in the CLHPA group. Individual methylation at CpG5 and CpG6 were highly correlated and higher in the CLHPA subjects. No association was found between mean methylation levels of SLC6A4 with mRNA level, and with the existing serotonin-transporter-linked polymorphic region (5-HTTLPR) genotype. Amendatory, in a cranial PET scan, the authors observed a significant negative association between methylation levels at CpG 11 and CpG12 in T cells or at CpG5 and CpG6 in monocytes, respectively, and in vivo 5-HT synthesis in the lateral left and right orbitofrontal cortex (OBFC), indicating long-term functional effects in vivo. The authors discuss their results with caution due to the small sample size (37). In connection with the aforementioned study, Provençal et al. (38) compared male adult subjects with chronic high physical aggression (CPA, n = 8) with subjects of a conjoint cohort of the other three above defined aggression groups (37) which had developed normal aggression trajectories in adulthood (n = 12). They surveyed the methylation status in extracted DNA from peripheral monocytes and T cells with a comprehensive microarray encompassing the entire genomic region including the cytokines IL1A, IL6, IL8, IL4, and IL10 and the transcription factors nuclear factor kappa B subunit 1 (NFkB1), nuclear factor of activated T cells 5 (NFAT5), signal transducer and activator of transcription 6 (STAT6) in both study groups. The results showed significant methylation differences concerning each of the analyzed cytokine gene loci. In CPA subjects overall methylation levels were significantly decreased in IL1A, IL4, IL6, and IL8, and were significantly increased in IL10. Methylation was positively correlated with serum protein levels of IL1A (significant), IL4, and IL-6, and was negatively correlated for IL-8 and IL-10, respectively. In regard to the transcription factors, two regions closely located to the TSS of each STAT6 isoform were significantly higher methylated in the CPA group, which was significantly associated with lower transcription of IL4. Next, Provençal et al. conducted a GWA (39) in connection with CPA by comparing male young adult subjects with early development of CPA (n = 8) with subjects without any history of high physical aggression in childhood (NPA, n = 16) according to the former study definition (37, 38). They analyzed DNA from peripheral T cells and found early onset CPA being associated with clustered and genome-wide methylation differences at 900 sites within 448 distinct gene promoters. The detected genes comprised several that have been associated with aggression earlier: arginine vasopressin receptor 1A (AVPR1A), serotonin receptor 1D (HTR1D) and glutamate metabotropic receptor 5 (GRM5) genes with less methylation in the CPA group, and the dopamine receptor D1 gene (DRD1) and SLC6A3 with higher methylation in the CPA group. In total, most functional categories of genes with different methylation in CPA included behavior (hyperactivity), metabolic and neurological diseases, inflammatory response (chemotaxis and phagocytes), cellular growth and proliferation, and gene expression (transcription factors, signal transducer as STAT6). Concerned specific canonical pathways included cytokine signaling and G-protein coupled receptor signaling.
In a further GWA Guillemin et al. studied methylation aberrancies in DNA from isolated peripheral T-cells in female subjects with CPA trajectories since childhood (n = 5) compared to women without any aggression history (n = 16) (40). A total of 917 probes corresponding to 430 distinct gene promoters were differentially methylated, of which many are involved in immune and inflammatory responses such as FOS, GATA1, GATA3, hepatocyte growth factor (HGF), TNF, IFNG, IL1A, IL1B, IL10, IL13, IL17R, and IL18. Thereof, some had previously been shown to be associated with aggressive behavior, including Tryptophan hydroxylase 2 (TPH2), NR3C1, and Corticotrophin-releasing hormone-binding protein (CRHBP), all of them were significantly less methylated in the CPA subjects.
In a second step, the researchers compared their results with their previous data of a GWA in male subjects out of the same underlying longitudinal study by Provençal et al. (39). Methylation levels of 31 gene promoters emerged to associate with physical aggression in both women and men and therewith constituted a significant overlap, including TPH2, CRHBP, and NR3C1. In the region within the zinc finger protein 366 gene (ZNF366) promoter, aggression-associated methylation was differentially directed in both women and men (40).
Van Dongen et al. (41) studied methylation differences with respect to aggressive behavior in 40 monozygotic twins (20 pairs) highly discordant for aggressive traits. They performed a GWA with PBC DNA and received about 24 methylation sites with high methylation difference, yet differences were generally twin pair-specific; thus, statistically no genome-wide significant methylation differences were identified in this sample.
Subsuming the main results of studies on aggressive traits, four of the five studies derived from the same study group. Two studies were theory-driven and gene-targeted. Their results indicate increased methylation of SLC6A4 in healthy men with a history of CLHPA with implications for the transcriptional activity in vitro, and showed associations with brain 5-HT in the left lateral OBFC (37). A more comprehensive genome region analysis including IL1A, IL4, IL6, IL8, IL10, and transcription factors NFkB1, NFAT5, and STAT6 revealed significant methylation differences in men with CPA in transcriptional relevant sites near or within each of the investigated genes. However, correlations with interleukin serum plasma levels only were significant for IL1A. Many of the identified methylation aberrances concerned sites outlying the intrinsic gene sequences, underlining the relevancy of methylation analyses that are not restricted to targeted gene sequences (38). A complementary study in women with CPA revealed similar results for immune and inflammatory genes (40). GWA results confirmed genome-wide involvement of methylation differences with respect to aggressive traits in men (39). The mentioned studies were performed in a small sample size of 8 (men) and 5 (women) subjects, respectively, in the aggression group. None of the studies considered the experience of childhood trauma.
Overall, the depicted studies confirm the need of genome-wide methylation assays, or at least a focus on a comprehensive set of functional associated genes. Future studies should consider childhood trauma, and specify an applicable control group.
Impulsiveness
By performing a GWA Ruggeri et al. (42) studied methylation aberrancies in PBC DNA of monozygotic twin pairs (n = 18) discordant for alcohol use disorder. The authors received 77 differentially methylated regions associated with 62 genes. Replication of these findings in a microarray only revealed significant methylation changings at one CpG site in the 3′UTR of 3′-protein-phosphatase-1G gene (PPM1G). Hypermethylation in PPM1G was positively correlated with lower mRNA levels of PPM1G and significantly associated with early escalation of alcohol use, as well as with increased impulsiveness. Further analyses evidenced that PPM1G hypermethylation was independently associated with both trait impulsiveness and right subthalamic nucleus activation, presumptively due to an increased effort to carry out control inhibition. Since PPM1G is assembled with five SNP's, the authors performed regression analyses which could rule out significant influences of genotype variations on PPM1G methylation. Further, no correlation was found for the SNP's with the trait impulsiveness (42).
Studies on personality traits are summarized in Table 2.
Discussion
The objective of this review was to summarize the first complete number of current original publications on human personality disorders and personality traits in connection with epigenetic modifications and to discuss the results as to pathogenetic impact and treatment-relevant insights. Remarkably, literature search yielded no single study of epigenetic evaluations in narcissistic, histrionic, anankastic, avoidant, dependent, eccentric, paranoid, schizoid and schizotypal personality aspects. Neither there was any result with any keyword on histone modification, nor results with studies on microRNA, but only on methylation aberrancies. Aggression and antisocial traits were prevalently explored, possibly preferred since these behavioral characteristics have a high genetic loading [e.g., (43)].
Most of the theory-driven studies focussed on gene loci involved in the serotonergic, dopaminergic or noradrenergic neurotransmitter system, e.g., serotonin receptor and transporter, dopamine receptor, and MAOA. Further crucial interest was in epigenetic modulation of the functionality of neurotrophic factors, the HPA-axis circuit, and the oxytocinergic system. This seems consequent, since these gene products are constitutive for brain function. Thusly, MAOA, a X-chromosomal encoded enzyme, is responsible for the oxidative breakdown of monoamine neurotransmitters, that is in the brain specifically serotonin, epinephrine, norepinephrine, and dopamine. Mutations in the MAOA gene with impaired gene activity lead to excess of serotonin and norepinephrine which is associated with disturbed control of an affected subject's impulsivity and aggression (NIH, Genetics Home Reference). BDNF is involved in growth, maturation and maintenance of nerve cells and plays a crucial role in building up synapses and in synaptic plasticity. Polymorphisms in the BDNF gene are associated with an increased risk of psychiatric disorders like bipolar disorder, anxiety, and eating disorders. However, results from genome-wide association studies refer to epigenetic alterations of genes that, besides neurofunctional genes, include genomic regions affecting genes involved in inflammation, cell-signaling, metabolism, and genes coding for proteins of the transcriptional machinery themselves (27, 29, 39–42). Methylation differences therefore represent a complex pattern that precludes feasible functional verification tests of the concerned gene loci, as it complicates a conclusive interpretation. The here reviewed GWA studies all, except for Prados et al., lack the consideration of additional factors possibly influencing the epigenetic configuration, especially early childhood experiences, which is also of main interest in understanding the development of a PD. For example, early life adversity was associated with methylation of SLC6A4 that in turn showed association to brain structure and function (44). In contrast, most of the studies on PD and single gene analyses respected childhood trauma as a confounding factor in their epigenetic analyses. According to previous data, these gene targeted studies confirmed the relevance of the serotonergic system for affective regulation and revealed methylation aberrances of the serotonin transporter (SLC6A4) and receptor genes (5HTR1B, 5HTR2A, and 5HTR3A) to be linked with antisocial traits (30, 31) and BPD (25, 26). As well for DRD2, methylation status was shown to be associated with BPD (24). Based on its effect on the pathway of each serotonin, dopamine and norepinephrine, epigenetic changes of MAOA congruently were found to be associated with antisocial personality traits, ASPD and BPD (19, 20, 26, 33). Studies also indicate a significant role of NR3C1 promoter exon 1 methylation status in antisocial traits, as well as, in combination with early life adversity, in BPD (22, 26). Indeed, with the insight in a genome-wide involvement of epigenetic modified gene loci, the validity of the studies on particular genes today seems to be restricted.
Aside from a general association of epigenetic changes in personality disorders and traits, the study results highly differ regarding the particular gene sites of epigenetic differences. One major reason could be the lack or the incomparability of the respective control group in each study. Another methodical deficit is the inconstant consideration of environmental factors, particularly early child adversity. Epigenetically vulnerable influences further include exposure to intimate partner violence during pregnancy as well as mood, smoking or diet habits of the mother during pregnancy, and stress of the subject as a fetus or in its early life (45–49). Notably, in many studies, the sample size is very small and might impact the results.
All studies used extracted DNA from peripheral blood cells for methylation analyses, albeit different cell fractions, preferably whole blood cells, leucocytes, lymphocytes, T cells. There is evidence that peripheral mononuclear cells, particularly lymphocytes, show similar epigenetic pattern with brain tissue [i.e., (9)], and the use of peripheral blood cells has gained acceptance for epigenetic studies of neuropsychiatric disorders. But results also indicate differences of epigenetic patterns between blood leucocyte lines (37, 38) which also could account for discrepancies within the studies. Future studies should consider cell type and preparation from whole blood, since cell stress can cause epigenetic changes in the cells (50).
High requirements in the technical methods and in an expedient study design make the disentangling of personality-specific methylation patterns a sensitive challenge, especially since there might be more than one way to (epi-)genetically forward the development of a PD. This also might be a reason for the diversity of the final clinical epiphenotypes of these disorders. Conversely, the variety of pathogenesis and of the individually developed clinical shapes impedes the attribution of possibly identified genetic or epigenetic aberrances to a specific personality trait or disorder.
If the functional attribution of epigenetic aberrations to personality disorders is so highly impeded, which benefit can we gain in doing so?
The possibility of epigenetic subtyping helps to assess the individual pathophysiologic condition as well as to improve deductive therapeutic approaches. The identification of someone's epigenetic risk profile could initiate efforts to establish standards for intensified clinical watching in afflicted children, and methods for early parental coaching or for premature intervention in order to prevent further development of a full-blown clinical picture of a PD. As impressively shown by Cecil et al., divergent epigenetic conditions within psychopathologically similar subjects can elicit different implications for the individual treatment requirements (18).
As in other mental disorders, epigenetic patterns can help to predict medical response. Thus, in depression, selected methylation aberrations could predict different therapy response to escitalopram and between escitalopram and nortriptyline (51, 52). In BPD, methylation levels of APBA3 and MCF2 were predictive for psychotherapy outcome (53).
In cancer, advanced therapy today comprises epigenetic drugs targeting i.e., on silenced tumor suppressing genes. Due to the plurality and individual diversity of involved genes in psychiatric disorders, the use of demethylating drugs cannot be targeted to distinct genes. Adverse effects with general hypomethylation might conflict with the aimed benefits. But there is evidence that methyldonor components as valproic or folic acid have positive therapeutic effects in mental disorders by themselves as well as by increasing the effect of fluoxetine (54–57).
Aside from their primary neurobiological effect, psychotropic drugs can further exert direct epigenetic effects. Studies on antidepressants and antipsychotic drugs i.e., evidenced their possibility to modulate the epigenome by acetylation of gene-associated histones, by methylation changes in dopamine pathways (58), by increasing expression of DNA-methyltransferases, and by inducing chromatin remodeling (58, 59). Methylation aberrations also can be remodulated by psychopharmacological treatment or psychotherapy (60, 61).
These merely exemplary findings suggest a high dynamic in epigenetic processes in mental disorders and their course. The interaction of psychotropic drugs and other therapeutic interventions with epigenetic remodeling seems still understudied. Finally, the fact of this interaction then could be utilized as an intraindividual control of lasting therapy response.
Implications and Future Options
The pathophysiological principle of gene-environment-interaction not only explains the obvious differences in the severity or combination of a person's personality traits but further implicates the existence of genetic and epigenetic risk and protective factors during the formation of the ultimate personality structure. Hitherto epigenetic studies underline the impact of early life adversity on the multifactorial pathway to PD. They reveal several relevant gene loci that are epigenetically affected in PD but differ in between the studies. This can partially be explained by the multifactorial and multi-step genesis of each PD, leading to different pathogenetic subtypes.
Epigenetic analyses in connection with PD represent a complex, but suitable amendment in the elucidation of personality development, and pose as valuable diagnostic step in the specification of an individual's premorbid risks, and for the development of individually tailored therapeutic strategies. They might play a valuable role in the future design and observation of early and personalized therapeutic intervention and thus could help to prevent the unfolding in severe dysfunctional conduct or personality disorder in risk subjects.
Author Contributions
DG conducted the full literature search process, read all found articles, wrote the manuscript. KK critically overworked focus and manuscript. TH and HF provided expert advice in epigenetics. JK and TF reviewed the manuscript and collaborated in the interpretation of the results and for the discussion.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Szyf M, McGowan P, Meaney M. The social environment and the epigenome. Environ Mol Mutagen. (2008) 49:46–60. doi: 10.1002/em.20357
2. Szyf M, Bick J. DNA methylation: a mechanism for embedding early life experiences in the genome. Child Dev. (2013) 84:49–57. doi: 10.1111/j.1467-8624.2012.01793.x
3. Weaver IC, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, et al. Epigenetic programming by maternal behavior. Nat Neurosci. (2004) 7:847–54. doi: 10.1038/nn1276
4. Roth TL, Lubin FD, Funk AJ, Sweatt JD. Lasting epigenetic influence of early-life adversity on the BDNF gene. Biol Psychiatry (2009) 65:760–9. doi: 10.1016/j.biopsych.2008.11.028
5. Murgatroyd C, Patchev AV, Wu Y, Micale V, Bockmuhl Y, Fischer D, et al. Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nat Neurosci. (2009) 12:1559–66. doi: 10.1038/nn.2436
6. McGowan PO, Sasaki A, D'Alessio AC, Dymov S, Labonte B, Szyf M, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. (2009) 12:342–8. doi: 10.1038/nn.2270
7. McGowan PO, Suderman M, Sasaki A, Huang TC, Hallett M, Meaney MJ, et al. Broad epigenetic signature of maternal care in the brain of adult rats. PLoS ONE (2011) 6:e14739. doi: 10.1371/journal.pone.0014739
8. Suderman M, McGowan PO, Sasaki A, Huang TC, Hallett M, Meaney MJ, et al. Conserved epigenetic sensitivity to early life experience in the rat and human hippocampus. Proc Natl Acad Sci USA. (2012) 109(Suppl. 2):17266–72. doi: 10.1073/pnas.1121260109
9. Provençal N, Suderman MJ, Guillemin C, Massart R, Ruggiero A, Wang D, et al. The signature of maternal rearing in the methylome in rhesus macaque prefrontal cortex and T cells. J Neurosci. (2012) 32:15626–42. doi: 10.1523/JNEUROSCI.1470-12.2012
10. Klengel T, Mehta D, Anacker C, Rex-Haffner M, Pruessner JC, Pariante CM, et al. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nat Neurosci. (2013) 16:33–41. doi: 10.1038/nn.3275
11. Auta J1, Smith RC, Dong E, Tueting P, Sershen H, Boules S, et al. DNA-methylation gene network dysregulation in peripheral blood lymphocytes of schizophrenia patients. Schizophr Res. (2013) 150:312–8. doi: 10.1016/j.schres.2013.07.030
12. Yuan LW, Gambee JE. Histone acetylation by p300 is involved in CREB-mediated transcription on chromatin. Biochim Biophys Acta (2001) 1541:161–9. doi: 10.1016/S0167-4889(01)00141-0
13. Weaver IC, Hellstrom IC, Brown SE, Andrews SD, Dymov S, Diorio J, et al. The methylated-DNA binding protein MBD2 enhances NGFI-A (egr-1)-mediated transcriptional activation of the glucocorticoid receptor. Philos Trans R Soc Lond B Biol Sci. (2014) 369:20130513. doi: 10.1098/rstb.2013.0513
14. Weaver IC, D'Alessio AC, Brown SE, Hellstrom IC, Dymov S, Sharma S, et al. The transcription factor nerve growth factor-inducible protein a mediates epigenetic programming: altering epigenetic marks by immediate-early genes. J Neurosci. (2007) 27:1756–68. doi: 10.1523/JNEUROSCI.4164-06.2007
15. Zhou Z, Hong EJ, Cohen S, Zhao WN, Ho HY, Schmidt L, et al. Brain-specific phosphorylation of MeCP2 regulates activity-dependent Bdnf transcription, dendritic growth, and spine maturation. Neuron (2006) 52:255–69. doi: 10.1016/j.neuron.2006.09.037
16. Chen WG, Chang Q, Lin Y, Meissner A, West AE, Griffith EC, et al. Derepression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. Science (2003) 302:885–9. doi: 10.1126/science.1086446
17. Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ (2015) 349:g7647. doi: 10.1136/bmj.g7647
18. Cecil CA, Lysenko LJ, Jaffee SR, Pingault JB, Smith RG, Relton CL, et al. Environmental risk, oxytocin receptor gene (OXTR) methylation and youth callous-unemotional traits: a 13-year longitudinal study. Mol Psychiatry (2014) 19:1071–7. doi: 10.1038/mp.2014.95
19. Philibert RA, Wernett P, Plume J, Packer H, Brody GH, Beach SR. Gene environment interactions with a novel variable monoamine oxidase A transcriptional enhancer are associated with antisocial personality disorder. Biol Psychol. (2011) 87:366–71. doi: 10.1016/j.biopsycho.2011.04.007
20. Checknita D, Maussion G, Labonté B, Comai S, Tremblay RE, Vitaro F, et al. Monoamine oxidase A gene promoter methylation and transcriptional downregulation in an offender population with antisocial personality disorder. Br J Psychiatry (2015) 206:216–22. doi: 10.1192/bjp.bp.114.144964
21. Perroud N, Salzmann A, Prada P, Nicastro R, Hoeppli ME, Furrer S, et al. Response to psychotherapy in borderline personality disorder and methylation status of the BDNF gene. Transl Psychiatry (2013) 3:e207. doi: 10.1038/tp.2012.140
22. Martín-Blanco A, Ferrer M, Soler J, Salazar J, Vega D, Andión O, et al. Association between methylation of the glucocorticoid receptor gene, childhood maltreatment, and clinical severity in borderline personality disorder. J Psychiatr Res. (2014) 57:34–40. doi: 10.1016/j.jpsychires.2014.06.011
23. Perroud N, Paoloni-Giacobino A, Prada P, Olié E, Salzmann A, Nicastro R, et al. Increased methylation of glucocorticoid receptor gene (NR3C1) in adults with a history of childhood maltreatment: a link with the severity and type of trauma. Transl Psychiatry (2011) 1:e59. doi: 10.1038/tp.2011.60
24. Groleau P, Joober R, Israel M, Zeramdini N, DeGuzman R, Steiger H. Methylation of the dopamine D2 receptor (DRD2) gene promoter in women with a bulimia-spectrum disorder: associations with borderline personality disorder and exposure to childhood abuse. J Psychiatr Res. (2014) 48:121–7. doi: 10.1016/j.jpsychires.2013.10.003
25. Perroud N, Zewdie S, Stenz L, Adouan W, Bavamian S, Prada P, et al. Methylation of serotonin receptor 3A in ADHD, Borderline Personality, and bipolar disorders: link with severity of the disorders and childhood maltreatment. Depress Anxiety (2016) 33:45–55. doi: 10.1002/da.22406
26. Dammann G, Teschler S, Haag T, Altmüller F, Tuczek F, Dammann RH. Increased DNA methylation of neuropsychiatric genes occurs in borderline personality disorder. Epigenetics (2011) 6:1454–62. doi: 10.4161/epi.6.12.18363
27. Teschler S, Bartkuhn M, Künzel N, Schmidt C, Kiehl S, Dammann G, et al. Aberrant methylation of gene associated CpG sites occurs in borderline personality disorder. PLoS ONE (2013) 8:e84180. doi: 10.1371/journal.pone.0084180
28. Teschler S, Gotthardt J, Dammann G, Dammann RH. Aberrant DNA Methylation of rDNA and PRIMA1 in borderline personality Disorder. Int J Mol Sci. (2016) 17:E67. doi: 10.3390/ijms17010067
29. Prados J, Stenz L, Courtet P, Prada P, Nicastro R, Adouan W, et al. Borderline personality disorder and childhood maltreatment: a genome-wide methylation analysis. Genes Brain Behav. (2015) 14:177–88. doi: 10.1111/gbb.12197
30. Beach SR, Brody GH, Todorov AA, Gunter TD, Philibert RA. Methylation at 5HTT mediates the impact of child sex abuse on women's antisocial behavior: an examination of the Iowa adoptee sample. Psychosom Med. (2011) 73:83–7. doi: 10.1097/PSY.0b013e3181fdd074
31. Moul C, Dobson-Stone C, Brennan J, Hawes DJ, Dadds MR. Serotonin 1B receptor gene (HTR1B) methylation as a risk factor for callous-unemotional traits in antisocial boys. PLoS ONE (2015) 10:e0126903. doi: 10.1371/journal.pone.0126903
32. Dadds MR, Moul C, Cauchi A, Dobson-Stone C, Hawes DJ, Brennan J, et al. Methylation of the oxytocin receptor gene and oxytocin blood levels in the development of psychopathy. Dev Psychopathol. (2014) 26:33–40. doi: 10.1017/S0954579413000497
33. Philibert RA, Gunter TD, Beach SR, Brody GH, Madan A. MAOA methylation is associated with nicotine and alcohol dependence in women. Neuropsychiatr Genet. (2008) 147B:565–70. doi: 10.1002/ajmg.b.30778
34. Dadds MR, Moul C, Hawes DJ, Mendoza Diaz A, Brennan J. Individual differences in childhood behavior disorders associated with epigenetic modulation of the cortisol receptor gene. Child Dev. (2015) 86:1311–20. doi: 10.1111/cdev.12391
35. Mellor D. Normative data for the strengths and difficulties questionnaire in Australia. Aust Psychol. (2005) 40:215–22. doi: 10.1080/00050060500243475
36. Radtke KM, Schauer M, Gunter HM, Ruf-Leuschner M, Sill J, Meyer A, et al. Epigenetic modifications of the glucocorticoid receptor gene are associated with the vulnerability to psychopathology in childhood maltreatment. Transl Psychiatry (2015) 5:e571. doi: 10.1038/tp.2015.63
37. Wang D, Szyf M, Benkelfat C, Provençal N, Turecki G, Caramaschi D, et al. Peripheral SLC6A4 DNA methylation is associated with in vivo measures of human brain serotonin synthesis and childhood physical aggression. PLoS ONE (2012) 7:e39501. doi: 10.1371/journal.pone.0039501
38. Provençal N, Suderman MJ, Caramaschi D, Wang D, Hallett M, Vitaro F, et al. Differential DNA methylation regions in cytokine and transcription factor genomic loci associate with childhood physical aggression. PLoS ONE (2013) 8:e71691. doi: 10.1371/journal.pone.0071691
39. Provençal N, Suderman MJ, Guillemin C, Vitaro F, Côté SM, Hallett M, et al. Association of childhood chronic physical aggression with a DNA methylation signature in adult human T cells. PLoS ONE (2014) 9:e89839. doi: 10.1371/journal.pone.0089839
40. Guillemin C, Provençal N, Suderman M, Côté SM, Vitaro F, Hallett M, et al. DNA methylation signature of childhood chronic physical aggression in T cells of both men and women. PLoS ONE (2014) 9:e86822. doi: 10.1371/journal.pone.0086822
41. Van Dongen J, Nivard MG, Baselmans BM, Zilhão NR, Ligthart L, BIOS Consortium, et al. Epigenome-wide association study of aggressive behavior. Twin Res Hum Genet. (2015) 18:686–98. doi: 10.1017/thg.2015.74
42. Ruggeri B, Nymberg C, Vuoksimaa E, Lourdusamy A, Wong CP, Carvalho FM, et al. Association of protein phosphatase PPM1G with alcohol use disorder and brain activity during behavioral control in a genome-wide methylation analysis. Am J Psychiatry (2015) 172:543–52. doi: 10.1176/appi.ajp.2014.14030382
43. Viding E, Blair RJR, Moffitt TE, Plomin R. Evidence for substantial genetic risk for psychopathy in 7-years-olds. J Child Psychol Psychiatry (2005) 46:592–7. doi: 10.1111/j.1469-7610.2004.00393.x
44. Frodl T, Szyf M, Carballedo A, Ly V, Dymov S, Vaisheva F, et al. DNA methylation of the serotonin transporter gene (SLC6A4) is associated with brain function involved in processing emotional stimuli. J Psychiatry Neurosci. (2015) 40:296–305. doi: 10.1503/jpn.140180
45. Reik W. Stability and flexibility of epigenetic gene regulation in mammalian development. Nature (2007) 447:425–32. doi: 10.1038/nature05918
46. Feil R, Fraga MF. Epigenetics and the environment: emerging patterns and implications. Nat Rev Genet (2012) 13:97–109. doi: 10.1038/nrg3142
47. Radtke KM, Ruf M, Gunter HM, Dohrmann K, Schauer M, Meyer A, et al. Transgenerational impact of intimate partner violence on methylation in the promoter of the glucocorticoid receptor. Transl Psychiatry (2011) 1:e21. doi: 10.1038/tp.2011.21
48. Zeilinger S, Kühnel B, Klopp N, Baurecht H, Kleinschmidt A, Gieger C, et al. Tobacco smoking leads to extensive genome-wide changes in DNA methylation. PLoS ONE (2013) 8:e63812. doi: 10.1371/journal.pone.0063812
49. McGowan PO, Meaney MJ, Szyf M. Diet and the epigenetic (re)programming of phenotypic differences in behavior. Brain Res. (2008) 1237:12–24. doi: 10.1016/j.brainres.2008.07.074
50. Philibert R, Caspers K, Beach SRH, Bakermans-Kranenburg MJ, van Ijzendoorn MH. Reply to: epstein-barr virus transformed DNA as a source of false positive findings in methylation studies of psychiatric conditions. Biol Psychiatry (2011) 70:e27–8. doi: 10.1016/j.biopsych.2011.02.029
51. Domschke K, Tidow N, Schwarte K, Deckert J, Lesch KP, Arolt V, et al. Serotonin transporter gene hypomethylation predicts impaired antidepressant treatment response. Int J Neuropsychopharmacol. (2014) 17:1167–76. doi: 10.1017/S146114571400039X
52. Uher R, Huezo-Diaz P, Perroud N, Smith R, Rietschel M, Mors O, et al. Genetic predictors of response to antidepressants in the GENDEP project. Pharmacogenomics J. (2009) 9:225–33. doi: 10.1038/tpj.2009.12
53. Knoblich N, Gundel F, Brückmann C, Becker-Sadzio J, Frischholz C, Nieratschker V. DNA methylation of APBA3 and MCF2 in borderline personality disorder: potential biomarkers for response to psychotherapy. Eur Neuropsychopharmacol. (2018) 28:252–63. doi: 10.1016/j.euroneuro.2017.12.010
54. Morris DW, Trivedi MH, Rush AJ. Folate and unipolar depression. J Altern Complement Med. (2008)14:277–85. doi: 10.1016/j.jad.2011.04.032
55. Farah A. The role of L-methylfolate in depressive disorders. CNS Spectr. (2009) 14:2–7. doi: 10.1017/S1092852900003473
56. Coppen A, Bailey J. Enhancement of the antidepressant action of fluoxetine by folic acid: a randomised, placebo controlled trial. J Affect Disord. (2000) 60:121–30. doi: 10.1016/S0165-0327(00)00153-1
57. Godfrey PS, Toone BK, Carney MW, Flynn TG, Bottiglieri T, Laundy M, et al. Enhancement of recovery from psychiatric illness by methylfolate. Lancet (1990) 336:392–5. doi: 10.1016/0140-6736(90)91942-4
58. Swathy B, Banerjee M. Understanding epigenetics of schizophrenia in the backdrop of its antipsychotic drug therapy. Epigenomics (2017) 9:721–36. doi: 10.2217/epi-2016-0106
59. Zimmermann N, Zschocke J, Perisic T, Yu S, Holsboer F, Rein T. Antidepressants inhibit DNA methyltransferase 1 through reducing G9a levels. Biochem J. (2012) 448:93–102. doi: 10.1042/BJ20120674
60. Vialou V, Feng J, Robison AJ, Nestler EJ. Epigenetic mechanisms of depression and antidepressants action. Ann Rev Pharmacol Toxicol. (2013) 53:59–87. doi: 10.1146/annurev-pharmtox-010611-134540
Keywords: epigenetic aberrations, personality disorder, personality trait, early childhood adversity, aggression, antisocial, molecular pathobiology, personalized therapy
Citation: Gescher DM, Kahl KG, Hillemacher T, Frieling H, Kuhn J and Frodl T (2018) Epigenetics in Personality Disorders: Today's Insights. Front. Psychiatry 9:579. doi: 10.3389/fpsyt.2018.00579
Received: 05 July 2018; Accepted: 23 October 2018;
Published: 19 November 2018.
Edited by:
Brisa S. Fernandes, University of Toronto, CanadaReviewed by:
Gabriel R. Fries, University of Texas Health Science Center at Houston, United StatesKurt Leroy Hoffman, Autonomous University of Tlaxcala, Mexico
Copyright © 2018 Gescher, Kahl, Hillemacher, Frieling, Kuhn and Frodl. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dorothee Maria Gescher, ZG9yb3RoZWUuZ2VzY2hlckBtZWQub3ZndS5kZQ==
†These authors share first authorship
 Dorothee Maria Gescher
Dorothee Maria Gescher Kai G. Kahl
Kai G. Kahl Thomas Hillemacher
Thomas Hillemacher Helge Frieling
Helge Frieling Jens Kuhn
Jens Kuhn Thomas Frodl1,5
Thomas Frodl1,5