- 1Menninger Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX, United States
- 2Department of Neurology and Psychiatry, Sapienza University of Rome, Rome, Italy
- 3Centro Lucio Bini, Rome, Italy
- 4Azienda Ospedaliera Universitaria Policlinico Umberto I, Sapienza School of Medicine and Dentistry, Sapienza University of Rome, Rome, Italy
- 5NESMOS Department, Faculty of Medicine and Psychology, Sapienza University of Rome, Sant'Andrea University Hospital, Rome, Italy
- 6Institute of Psychiatry, Università Cattolica del Sacro Cuore, Rome, Italy
- 7Department of Psychiatry, Fondazione Policlinico Universitario “Agostino Gemelli” IRCCS, Rome, Italy
Background: There are different ways to define stabilization and currently, the main standpoint regards it as no-depression/no-mania. Furthermore, each person is physiologically different from childhood to adulthood, and in old age, thus the meaning of stabilization should take into account both growth and maturity. We aimed to review systematically studies focusing on mood stabilization in all phases of bipolar disorder (BD) and across all life phases, including pregnancy and the perinatal period, which is still a different phase in women's life cycles.
Methods: We carried out a PubMed search focusing on studies of bipolar disorder treated with drugs and aimed at stabilization with the following search strategy stabiliz*[ti] OR stabilis*[ti] OR stable[ti] OR stability[ti]) AND mood[ti] AND bipolar. In conducting our review, we followed the PRISMA statement. Agreement on inclusion was reached by consensus of all authors through a Delphi rounds procedure.
Results: The above search strategy produced 509 records on January 25, 2020. Of them, 58 fitted our inclusion criteria and were discussed. The eligible studies spanned from September 1983 to July 6, 2019.
Conclusions: No clear-cut indications could be drawn due to a number of limitations involving sample inconsistency and different methods of assessing mood stabilization. The evidence collected so far does not allow recommended treatments for Adolescents, pregnant or perinatal women, and aged patients. However, adults, not within these groups, better focused upon. For their manic/mixed phases, second generation antipsychotic drugs may be useful in the short-to-medium run, alone or combined with mood stabilizers (MSs). However, MSs, and especially lithium, continue to be pivotal in chronic treatment. Bipolar depression should rely on MSs, but an antidepressant may be added on and can prove to be helpful. However, there are concerns with the tendency of antidepressants to induce the opposite polarity or mood instability, rendering the need for concurrent MS prescription mandatory.
Introduction
The treatment of bipolar disorder (BD) is currently unsatisfactory. Despite the good results obtained in the treatment of its acute manic phase, this may also be the result of the natural course of the disorder. The challenge would be to obtain a clinical response that maintains patients euthymic, without mood swings, and for a sustained time-period. The latter is unfortunately an unmet need, because few patients manage to stay in treatment for a sufficient time to be declared as remitted. In fact, 40% (1) to 60% (2) of patients discontinue lithium after 5-7 years, and despite good adherence, some 13% of patients who were responders for five years, become resistant to lithium treatment after 10 years (3). BD has its onset usually in late adolescence and early adulthood, less often in later adulthood or advanced age, and seldom during childhood (4), with each range of age at onset displaying a normal-like distribution (5). Since it runs a cyclic course, with manic and depressive episodes and with relatively asymptomatic intervals, and is a biologically heterogeneous entity with precise neural (6–9) and peripheral correlates (10), BD needs to be treated according to subtype and for the entire life span (11). Drug treatment of BD is further complicated not only by the side effects that keep patients away from treatment, but also by long-term drug-induced alterations that prompt doctors to stop or switch to other drugs. For example, there is much concern about the long-term nephrotoxicity of lithium (12–14), but other mood stabilizers (MSs) are not devoid of dangerous side effects (15). Hence, drug treatment of BD has to be tailor-cut to patient's individual needs (16).
Different concepts are included in what we mean by mood stabilization. Mood is normally swaying between what is not mania and not depression, something we call euthymia, but is not a flat line. Consequently, an optimal MS should keep the patient within this range. However, hypomania, which is subsyndromal with respect to mania, is not an acceptable state, since it is often linked to BD-II type and is likely to be followed by bipolar depression, which is a clinical state most unpleasant to the patients and their doctors. It is generally accepted that to be called a MS, a drug must relieve at least one phase of BD and not to cause the opposite. However, this simplistic definition would include antidepressant (ADs) and antipsychotic drugs (APs) as well, not causing respectively (hypo)mania or depression, adding much to the confusion. There is much debate about how much ADs trigger manic switches and how some second-generation APs are endowed with antidepressant action and currently indicated by the US Food and Drug Administration (FDA) for depression (for example, lurasidone and brexpiprazole). This prompted Nassir Ghaemi (17) to develop an elaborate concept of MS, proposing that a MS should be conservatively defined as “an agent with efficacy in two of the three phases of bipolar illness (acute depression, acute mania, prophylaxis)”. In this definition, prophylaxis is meant as a protection from the occurrence of either manic or depressive episodes. This definition excludes all APs and leaves lithium, carbamazepine, valproate, and lamotrigine. Terence Ketter's proposal many years later (18), retained much of the essence of Ghaemi's proposed definition, by stating “any treatment that is effective in any phase of bipolar disorder (an America‐centric approach would be to say FDA‐approved for any phase of bipolar disorder) but not active at dopamine receptors (thus excluding antipsychotics)”. He should have added directly at dopamine receptors, since lithium and other MSs indirectly affect dopaminergic transmission (19–21). Both these definitions are acceptable and the drugs they envisage as MSs are the ones we will here consider. The identification of a neurochemical signature of mood stabilization, like a decreased glutamate-to-gamma aminobutyric acid ratio or genetic markers such as the GAD1 rs1978340 allele A (22), would greatly aid and steer the future pharmacological treatment strategies. However, their adoption in treatment models of BD should await confirmation by future studies (and the identification of other markers as well is to be expected.
The onset of BD during infancy is an extremely rare event. However, in many instances, it develops during adolescence. The latter is a period of rapid physiological changes and adaptation, with the brain in continuous maturation. It is accepted that the brain does not conclude its developmental trajectory before the 24th year of life (23). Any action of a drug at this stage might affect further development, hence particular caution is mandatory in facing cases of adolescent BD. Furthermore, the brain in the two genders matures differently, both in normal (24) and BD adolescents (25), thus forcing treating clinicians to personalize their interventions by taking into account multiple factors, including gender, and substance use that could arrest a normal maturational process in the neurobiological interplay between the “inbuilt” underlying disorder and the “acquired” substance use disorder (26). The gender concern comes to the fore when women become pregnant. During this particular phase of life, the hormonal turmoil that occurs during gestation and the post-partum makes the woman vulnerable to psychiatric events, including later first occurrences or recurrences of BD (27, 28). The old age comes with a decay of functioning bodily systems, including the brain, so the clinical expression of BD and the organism's response to drugs are consequently affected. Generally, dose adjustments of the same types of medications are sufficient to deal with BD in the elderly.
Aim of the Review
To identify drug treatment patterns for BD stabilization across different phases of life, we conducted a systematic review with keywords focusing on mood stabilization and bipolar disorder. The studies emerging from database search were subsequently subdivided according to the age range involved or the special condition (pregnancy or postpartum).
Methods
We conducted our review according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement (29). We search the PubMed database using the following strategy: stabiliz*[ti] OR stabilis*[ti] OR stable[ti] OR stability[ti]) AND mood[ti] AND bipolar. Papers were individually searched for adherence to our inclusion criteria. Retrieved relevant papers, comprising reviews and meta-analyses, were searched in their reference lists for providing additional papers with adequate research data and meeting our inclusion criteria. These were: double blind, placebo or comparator-controlled trials, open-label trials and naturalistic studies investigating the efficacy/effectiveness of drugs given either as monotherapy or add-on medication and belonging to drug classes like MSs, ADs, first generation APs (FGAs), second-generation APs (SGAs), anticonvulsant benzodiazepines (BDZs), or nonconventional mood-stabilizing medications (e.g., allopurinol) in reducing manic, hypomanic, mixed, or depressive symptoms and/or preventing the occurrence of new mood episodes in patients with BD.
Exclusion criteria were: reviews and meta-analyses, animal and in vitro studies, unfocused studies, i.e., studies with nonclinical outcomes not reporting efficacy data, editorials and opinion papers, like letters to the editor with no data or comments on other literature, case reports and case series with no reliable statistics, studies not focusing on BD or including disorders other than BD without separately reporting on BD, congress/conference abstracts, studies lacking clinical data, studies focusing on pharmacokinetics, surveys, studies of registries, papers reporting on data originally published by others. When a study was extended and used the same sample on which results were previously reported, we eliminated the first report and kept the paper with the larger sample, provided that quality of reporting was maintained. We accurately avoided to include studies referring to the same patient sample.
Inclusion and exclusion were based on consensus among all authors; unanimity was required for both and was achieved through Delphi rounds. Two rounds were sufficient to reach complete agreement among authors.
Results
Our search produced 509 records on January 25, 2020. Authors identified 3 duplicates, which were excluded; hence, the pooled records amounted to 506. Excluded were: 158 reviews, 72 animal studies, 62 unfocused studies, 60 opinion/editorial papers, 28 case reports/case series, 28 studies with inadequate design, 22 in vitro (nonanimal) studies, 5 studies on mixed samples which did not provide data for subgroups affected by BD (identified as lumping), 4 congress/conference abstracts without complete data, 3 studies not focusing on BD samples, identified as no BD, 2 studies lacking clinical data, 1 study on pharmacokinetics, 1 survey, 1 registry study, 1 study which reported data originally published by others (sample overlap). Therefore, the final number of studies included in this review was 58. The results of our search is shown as a PRISMA flowchart in Figure 1 with the reasons of exclusion. Detailed, study per study information about inclusion/exclusion is provided in the supplement. The search of reference lists of reviews yielded no further articles.
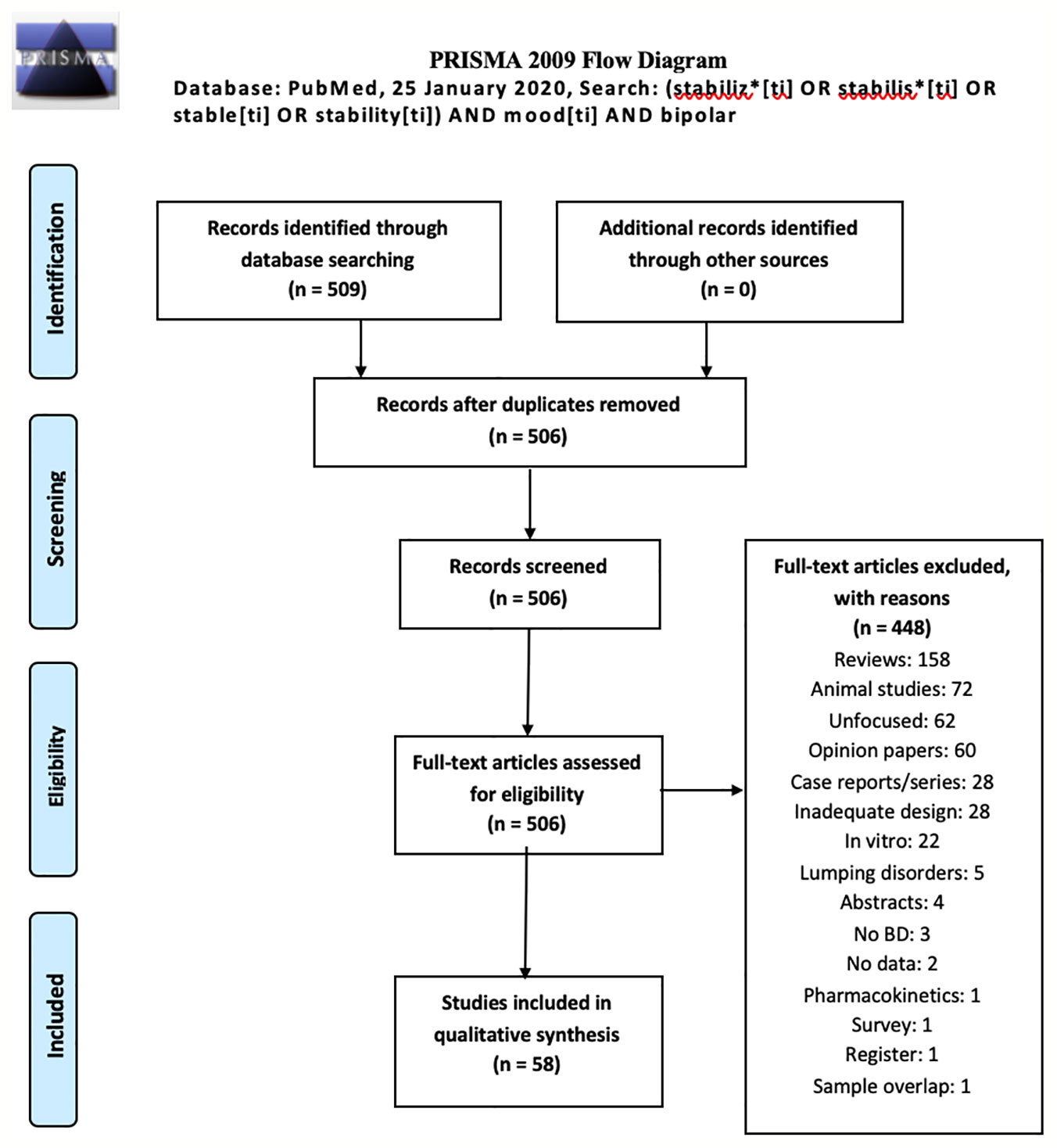
Figure 1 PRISMA flowchart of our review's results (30).
Included studies are summarized in Table 1. They spanned from September 1983 to July 6, 2019, while the complete output spanned from April 1970 to December 19, 2019. We split our included records as childhood and adolescence (N=3), adulthood (N=52), old age (N=2), and pregnancy/perinatal period (N=1). One study was conducted on both elderly and adult patients. Since all nonadult trials were few, we applied a further distinction on studies of adults, subdivided into acute phase (N=31), single drug (N=3), add-on (N=25) or mixed (N=3), and long-term with survival curves (Kaplan-Meier) (N=10). All articles were in English, in spite of the fact that non-English language was not an exclusionary criterion.
Discussion
Our aim in writing this review was to clarify which drug treatments achieve stabilization in the various phases of BD and across which age ranges or physiological conditions, like pregnancy and motherhood. Ideally, drug treatment strategies should have been tested phase-specifically in each age; however, studies were not sufficiently numerous for the childhood, older age and pregnancy, so the major focus will be on adulthood. Furthermore, rather than stabilization, the focus of most studies was on symptom improvement, so a reduction in depressive symptoms during the depressive phase and the reduction of excitement symptoms during manic/hypomanic phases are taken per se as stabilization, which sensu strictu are not.
Adulthood
In this review, most results regarded adult patients in various phases of their disorder, so we will expose our findings according to the phase and the type of pharmacological treatment employed.
Manic/Hypomanic/Mixed
In a multicenter naturalistic study, Perugi et al. (84) investigated rates of remission and improvement in mood symptoms and functioning in manic patients treated with MSs and/or APs: Remission rates were 82% in 4 weeks, with Young Mania Rating Scale (YMRS) and Clinical Global Impressions-Bipolar (CGI-BP) mania scores rapidly decreasing. However, the authors did not identify any factors that were associated with remission. Although the extent of the occurrence of mixed states in BD is debated, it is estimated to be around 30% (89). In these cases, treatment should comprise judicious polytherapy (90).
Add-on SGAs
Risperidone
Acute treatment with add-on risperidone to MS (lithium [Li+], valproate and carbamazepine) has been investigated in five studies, which include one naturalistic (39), two placebo-controlled (42, 47), and two-open label studies (36, 51). Add-on risperidone (4 mg/day) has proven to be superior to placebo in decreasing manic/mixed symptoms as shown by greater reduction of YMRS and Clinical Global Impressions (CGI) scores. In two separate open-label studies, add-on risperidone (3-4 mg/day) showed similar rates of response after 4 and 6 weeks (74% and 70% respectively), while euthymia after 6 months was present in 73% of patients. Add-on risperidone (2-6 mg/day) to MS failed to show superior antimanic effects than add-on haloperidol (range: 4-12 mg/day) in one study (42), whereas in another study of inpatients, it showed to be superior to add-on SGAs in reducing mania at discharge (39). In the same study, no differences in effectiveness/efficacy were found as compared with add-on olanzapine (5-20 mg/day).
Olanzapine
Acute effectiveness/efficacy of olanzapine was investigated in one double-blind, placebo-controlled trial (43). Add-on olanzapine treatment (5-20 mg/day) to patients previously treated with MSs (Li+ or valproate) was superior to placebo in reducing YMRS scores and was associated with higher rates of remission. Furthermore, olanzapine monotherapy was similarly effective independently from whether the patients had failed or succeeded in the past to respond to another MS for mania.
Quetiapine
Effectiveness of add-on quetiapine (mean 267.9 ± 105.4 mg/day) to a MS in reducing manic/mixed symptoms was investigated by one open-label trial (53). After four weeks, add-on quetiapine reduced both manic and depressive symptoms, as demonstrated by the reduction of both YMRS and Hamilton Depression Rating Scale (HDRS) scores.
Mood-stabilizers
Add-on MS (Li+, carbamazepine, and valproate) to olanzapine (5-20 mg) in manic/mixed patients was associated with greater remission rates (61% vs. 95%) than olanzapine monotherapy (69). On the other hand, Goldberg et al. (32) showed that patients with Li+ or AE monotherapy had similar response rates and duration of remission, whereas time to remission in cases of combined therapy is somehow longer. This outcome may be related to the fact that people receiving combined treatment added the antiepileptic after an ineffective Li+ trial. After starting the combined therapy, time to remission was similar to the monotherapy group, i.e., 2-3 weeks.
Gabapentin (900-1200 mg/day) add-on treatment to antimanic drugs (Li+, valproate, and risperidone) was effective in rapidly reducing HDRS and Bech-Rafaelsen Mania Scale (BRMaS) scores after 1 week. In the following month, BRMaS scores stabilized, whereas HDRS scores continued to decrease (34). On the other hand, topiramate (50-400 mg/day) added on a MS (Li+, valproate) or an AP failed to show superior efficacy than placebo add on after 12-weeks (56).
Other
Add-on memantine in not stabilized BD is related to 47.5% and 52.5% rates of remission after 6 and 12 months (70), whereas sedatives (mainly BDZs) added on lamotrigine monotherapy (100-200 mg/day) in patients with either manic or depressive episode, were associated with higher rates of stabilization than adding other psychotropic drugs (mainly SGAs, ADs, and MSs) (59). Add-on nutritional supplements, like vitamins and minerals, proved able to reduce in some patients both manic and depressive symptoms (38), whereas allopurinol was not superior to placebo in reducing manic symptoms (76). However, allopurinol also improved YMRS scores in its double-blind study and with a greater effect size than what vitamins and chelated minerals were able to achieve in the open study.
Summarizing the evidence of studies treating acute episodes of mania or mixed, adding one SGA to a MS seems the best strategy to stabilize mood. The evidence of the antimanic effect of SGAs are most prominent for risperidone, a bit less for olanzapine and quetiapine. There are no differences between olanzapine and risperidone or valproate and Li+ as regards their antimanic effect. The evidence of differences between SGAs and FGAs as for their antimanic effect is at least poor and conflicting.
Depression
Add-on SGAs
Two double-blind placebo-controlled trials evaluated the effectiveness of aripiprazole (67) and quetiapine (83) as add-on treatments for bipolar depression. Quante et al. (67) failed to demonstrate superiority of augmentation therapy with aripiprazole (10-30 mg/day) as compared to placebo in patients treated with citalopram (40 mg/day) and a MS. Conversely, Garriga et al., showed that add-on quetiapine-extended release (300-600 mg/day) to a MS (Li+, valproate, or carbamazepine) was superior to placebo in improving subthreshold depressive symptoms after 6 weeks, and also in improving functioning after 12 weeks.
MSs
Hantouche et al. (54) assessed the characteristics of poor vs. good responders to add-on MSs (Li+, carbamazepine, and valproate) treatment to ADs in major depressive disorder patients with depression who met Angst's criteria for lifetime presence of subtle hypomanic and cyclothymic features, i.e., patients that the authors consider as belonging to the bipolar spectrum. Poor responders were prescribed a MS later than good responders, suggesting that MS augmentation should be undertaken without delay.
Valproate Monotherapy
Two studies investigated the effectiveness of valproate monotherapy in relieving depressive symptoms (41, 68). Valproate was associated with a 63% response after one year in patients with BD-II. Valproate monotherapy was also superior to placebo in improving HDRS scores after 3, 4, 5, and 6 weeks. However, differently from Winsberg et al. (41), such difference in Muzina et al. (68) was mainly driven by data regarding the subgroup affected by BD-I.
Lamotrigine
Two studies investigated the effectiveness/efficacy of lamotrigine in BD depression, either as monotherapy (65), or as add-on treatment (66). After 16 weeks, lamotrigine monotherapy (200-400 mg/day) increased YMRS scores by more than 4 points in 35% of patients, and such increase was predicted by the number of manic/hypomanic/mixed episodes in the preceding year. lamotrigine add-on treatment (145.5 ± 113.2 mg/day) to a MS (Li+ or valproate) or APs (quetiapine, olanzapine, or ziprasidone) reduced CGI-BP-S scores after 4 and 12 weeks. Such scores remained significantly lower during the following year, indicating successful stabilization.
Topiramate
Effectiveness of add-on topiramate (50-100 mg/day) in reducing both manic and depressive symptoms and in inducing response was compared with add-on bupropion (100-400 mg/day) to a MS (Li+ or valproate) and SGAs. Add-on treatments with either topiramate or bupropion were able to induce similar response rates (56% vs. 59%, respectively), within a similar time lag (2-4 weeks). Reductions in YMRS, HDRS, and CGI-I scores were also similar.
ADs
Bottlender et al. (37) evaluated the impact of AD treatment on the incidence of switches from depression to mania/hypomania in 158 BD-I patients with depression. Rates of switches were 25%, with higher risks for patients taking tricyclic antidepressants (TCAs) and lower for those on combined AD+MS treatment.
Add-on paroxetine
Three studies evaluated the effectiveness/efficacy of paroxetine in reducing depressive symptoms and rates of switch. Young et al. (35) compared the effectiveness/switch rates of either add-on paroxetine (36 mg/day) or additional MS (Li+, 1300 mg/day or valproate 1200 mg/day) to stable MS treatment. Both add-on treatments were associated with significant reductions in HDRS scores after 6 weeks, with no significant YMRS score increases.
Vieta et al. (45) compared add-on treatment with paroxetine (32.3 mg ± 11.2) or venlafaxine (179.2mg ± 91.0) to a MS (Li+, valproate, or carbamazepine) and investigated response, remission, and switch rates. After 6 weeks, similar proportions of responders (paroxetine+MS: 50%; venlafaxine+MS: 59%) and similar remission rates (paroxetine+MS: 37%; venlafaxine+MS: 41%) were found. Venlafaxine showed higher, even though not significantly so, rates of remission (48% vs. 43% with paroxetine). Nevertheless, the authors concluded that acute add-on treatment with venlafaxine raises concerns due to the higher rates of switch, although rates did not differ significantly, but only numerically (13% with venlafaxine, 3% with paroxetine). Authors stressed the need to replicate their preliminary findings, but no follow-up ensued.
Venlafaxine, Sertraline, and Bupropion
Amsterdam et al. (77) showed superiority of venlafaxine monotherapy over placebo in BD-II patients as concerns response rates at the 12-week endpoint (67.7% vs. 34.4%, respectively). Two randomized trials investigated the effectiveness/switch rates of add-on venlafaxine, sertraline or bupropion to MSs (Li+, valproate, or carbamazepine). Post et al. (40) reported a 37% response rate after 10 weeks, with 14% of switches into mania/hypomania, On the other hand, Leverich et al. (57) showed that after 10 weeks, response rates were 48.7%. However, response rates dropped to 32.5% after excluding patients who had a switch. Switch rate to full (hypo)mania was 19.3%, with higher rates for venlafaxine and lowest for bupropion. Both studies showed that AD augmentation is not likely to yield a high rate of sustained AD response without a switch.
Escitalopram
The study of Parker et al. (58) showed superiority of 10 mg of escitalopram monotherapy over placebo in a double-blind crossover study lasting 9 months, in reducing symptom severity and percent days impaired in a small sample of 10 drug-naïve patients with BD-II and monthly mood episodes.
Other
Goldberg et al. (49) evaluated the effectiveness of add-on pramipexole (1.0-2.5 mg/day) to MSs (Li+, valproate, or carbamazepine) in improving HDRS and CGI-S scores. After 6 weeks, pramipexole was superior to placebo in reducing depressive symptoms (pramipexole+MS was followed by more than 50% drop in HDRS scores compared to 20% in the placebo+MS; furthermore, it was associated with lower CGI-S scores (2.7 ± 1.4) than placebo+MS (4.4 ± 1.3). On the other hand, add-on therapy with agomelatine (25-50 mg) to a MS (Li+ and valproate) was not superior to add-on treatment with placebo in reducing depression after8 weeks (80). Goldberg et al. (71) found moderate antidepressant effect of nefazodone (300-600 mg/day) added on a MS (Li+, lamotrigine, valproate or carbamazepine) or an AP (clozapine).
Concluding, in the acute treatment of depression, adding ADs on ongoing MS treatment is effective in improving mood symptoms but it is also related to an increase in switch rates, specifically in BD-I or mixed samples. The evidence points to higher switch rates during add-on treatment with venlafaxine, a drug that inhibits the reuptake of both norepinephrine and serotonin, or TCAs, a group of drugs that are effective in blocking both transporters similarly to venlafaxine, than with selective serotonin reuptake inhibitors (SSRIs) or bupropion, which blocks the reuptake of norepinephrine and dopamine, and leaves the serotonin transporter almost unaffected. Risk of switch seems intermediate for SSRIs and lower with bupropion. ADs are effective in the short-term treatment of BD-II, even in monotherapy, but switch rates are not clearly evaluated across studies. Monotherapy with valproate and lamotrigine showed also short-term effectiveness, like topiramate and quetiapine-extended release add.
Long-Term Studies
APs
Two retrospective naturalistic studies investigated rates of relapse over a 1-year period in patients with BD (59, 78) treated with MS monotherapy, MS+SGAs and MS+FGAs, and reported conflicting results. Rehospitalization rates have been reported not to differ after a 1-year follow-up (Patel et al., 2006) or to be lower in patients receiving MS+SGAs, compared to MS monotherapy and MS+FGAs (78). Differences in sample characteristics [BD-I in Patel et al. [2006] and BD-I/BD-II mixed sample in Hochman et al. (78)] or type of SGA used might have played a role in such discrepancy. In partial agreement with Patel et al. (55), Tournier et al. (88), found similar treatment discontinuation rates, i.e., > 60% across the aforementioned three groups during a 1-year period, with slightly, but not significantly lower rates in the MS+SGAs combined group than the other two. Bernardo Dell'Osso et al. (64) investigated relapse rates after over 2 years in patients with early, middle, and late onset of BD, and found that MS treatment (Li+ or valproate+SGAs) are more effective in preventing depressive episodes in those patients with an early BD onset.
Olanzapine, Risperidone, and Quietiapine
Two placebo-controlled trials (51, 80) and one naturalistic study (63) investigated the effectiveness in relapse prevention of olanzapine, risperidone and quetiapine. Tohen et al. (41) found that patients on combined olanzapine (5-20 mg)/MS (Li+, 954.6-1174.7 mg/day or valproate, 1060.4-1512 mg/day) treatment had a longer mean time to symptomatic relapse into mania or depression then patients receiving MS+placebo (163 and 42 days, respectively). The effectiveness of add-on olanzapine was superior than add-on placebo or add-on risperidone in increasing the time of syndromic relapse during the short- (24 weeks), but not in the long-term (81). Altamura et al. (63) investigated rates of relapse over 4 years in patients treated with Li+ or valproate or lamotrigine or quetiapine as monotherapy or a combination of quetiapine to either Li+ or valproate. Patients with a combined treatment (quetiapine+Li+ and quetiapine+valproate) showed higher rates of euthymia (80% and 78.3%, respectively) than those with quetiapine alone (29.3%), Li+ alone (46.2%) and lamotrigine alone (41.9%). Patients with Li++quetiapine and Li++valproate did not relapse for longer times (41.4 and 39.2 months, respectively) than patients on quetiapine (24.9 months) and valproate (26.3 months) alone. Only patients with Li++quetiapine did not relapse for significantly longer times than Li+ alone (33.1 months). Furthermore, patients with Li+ monotherapy showed smaller relapse rates than those with quetiapine monotherapy.
MSs
DePaulo et al. (31) investigated self-reported mood stability in patients with BD on long-term lithium therapy and found greater ratings of absence of mood swings than HCs. Ahn et al. (82) found that treatment response rates did not differ among patients with add-on Li+ to SGAs (quetiapine, olanzapine, risperidone, aripiprazole, paliperidone, clozapine, amisulpride), or other MSs (lamotrigine and carbamazepine) as compared to those receiving add-on treatment with valproate. On the other hand, Savas et al. (60) found that adjunctive therapy with MSs (Li+, valproate, carbamazepine or lamotrigine) to SGAs (risperidone, olanzapine or quetiapine) was not superior in preventing relapses as compared with SGAs alone over a 6 month-period.
Mean time of relapse after MS discontinuation was investigated by Sharma et al. (74) in a sample of Indian patients. Mean time to relapse was 10 months, and all relapses were manic, thus replicating existing data in samples belonging to Western countries. Steardo et al. (87) showed that impaired glucose metabolism was associated to poor long-term response to MSs (Li, valproate, lamotrigine, and carbamazepine) and APs. On the other hand, Henry et al. (46) showed that anxiety was a predictor of poor long-term (2 years) response to AEs, but not to Li+.
One open label, placebo-controlled trial (65) tested the effectiveness of lamotrigine monotherapy (100-200 mg/day) in reducing switch rates over 6 months. The authors found no differences between lamotrigine and placebo in percentage or hazard ratio for a medical intervention due to the onset of a mood episode. However, patients on lamotrigine monotherapy had consistently higher survival estimates than patients on placebo. Furthermore, YMRS scores at screening and presence of ≥3 manic/hypomanic/mixed episodes in the preceding year significantly increased the hazard ratio for a mood episode. The authors concluded that emergent manic or hypomanic features appear to be driven by the pre-existing or historical burden of mania features, rather than the use of lamotrigine.
ADs
Amsterdam et al. (77) found no difference in relapse rates over 6 months between patients with BD-II treated with venlafaxine or with lithium monotherapy. Two studies evaluated manic switch rates over one year of add-on bupropion, venlafaxine or sertraline to MS in patients who had responded in the past to AD augmentation (40, 57). In both studies, switch rates were higher than 30% (33% and 36%). AD switch rates were not significantly different among the three ADs; however, the threshold/subthreshold switch ratio was lowest with bupropion (1.2), intermediate with sertraline (1.65), and highest with venlafaxine (3.75). The long-term administration of 25-50 mg/day of the strong melatonin MT1 and MT2 receptor agonist and moderate serotonin 5-HT2C and 5-HT2B receptor antagonist, agomelatine, as an add-on to a MS, did not result in different switch rates compared to placebo added on a MS (80). However, also the response rates were similar in the two groups, raising questions about the antidepressant potency of agomelatine.
Other
Norris et al. (72) investigated the long-term effectiveness of add-on ramelteon (8 mg/day), a sleep inducer which shares with agomelatine the strong melatonin MT1 and MT2 receptor agonist activity, and is also endowed with weak MT3 and 5-HT2B activity, to standard medications (including APs, MSs, ADs, and stimulants) in stabilized patients with BD over a 6-month period. As compared with placebo, patients with add-on ramelteon showed lower rates of relapse into any mood episode than placebo.
Studies focusing on stabilization of adult patients make the greatest part of those included in this review. As far, results appear to be inconsistent if not conflicting, but there is weak evidence supporting either the addition of a SGA to MS or using a SGA alone, which both confer mood stabilization that is superior to that obtained using MSs alone, at least in the medium term. For timeframes extending over six months, results are more conflicting. However, evidence supporting the effectiveness of combined therapy in reducing relapse is stronger than the one supporting the superiority of the use of MS or SGA alone. Li+ seems not to be superior to valproate in stabilizing mood as an add-on treatment. Add-on ADs to MSs are related with higher switch rates. If this holds true for BD-I or mixed samples, there is a week, preliminary evidence that this might not be true for BD-II.
Children/Adolescents
Tramontina et al. (61) showed that the switch to a monotherapy with topiramate (150 mg/day) in youth (11-17 years) with BD, previously treated with MSs (Li+, valproate) or SGAs (risperidone), was associated to both reduction of YMRS scores and weight loss after 4 weeks.
Chen et al. (73) retrospectively investigated relapse rates after 12 months of treatment with either MSs (Li+, valproate, or carbamazepine) or SGAs (risperidone, aripiprazole or quetiapine). Patients who initiated MSs and SGAs had a comparable risk of psychiatric hospital admission; however, patients who initiated on SGAs were less likely to discontinue treatment and less likely to receive treatment augmentation. Hence, the authors concluded that in youths with BD, SGAs might be more effective and better tolerated than traditional MSs as a maintenance treatment. Conversely, Hafeman et al. (86) investigated suicide attempts and suicidal ideation, rates of threshold and subthreshold depression, (hypo)mania, psychosocial functioning, hospitalization, aggression, and substance use disorders in patients receiving Li+ or medications other than Li+ (AEs, FGAs and SGAs), regardless of other psychotropic medications, for more than 75% of the 10-year follow up. They found that Li+-treated youths were less likely to have lifetime anxiety, and less likely to be on ADs. Youth on Li+ had half as many suicide attempts, fewer depressive symptoms, psychosocial impairment due to illness, and less aggression than those not treated with Li+.
Elderly
Sanderson et al. (33) compared length of stay and symptom improvement in elderly inpatients receiving monotherapy with Li+, valproate, or carbamazepine and found no significant differences across the groups. Tournier et al. (88) investigated rates of treatment discontinuation, switch, adjunctive medication, hospitalization, suicide attempt, and death over a 1-year period in patients treated with either MS (Li+, valproate, carbamazepine, and lamotrigine), SGAs (risperidone, aripiprazole, quetiapine, and olanzapine) or a combination of the two classes. Treatment failure was higher in those receiving SGAs than MSs. Addition of another drug was less frequent in those taking SGAs than in those taking MSs, while early discontinuation, psychiatric hospitalizations and death occurred more frequent in patients who were prescribed SGAs. The authors concluded that, in older patients, SGAs are less effective and fail more often than MSs. Mortality was particularly high in SGA-treated elderly patients, either as a monotherapy or in combination with MSs.
Pregnancy
Viguera et al. (62) studied 89 pregnant women with polytherapy (including MSs, ADs, APs) for BD who 1) used at least one MS (Li+, valproate, carbamazepine, gabapentin, lamotrigine) or AP (olanzapine and quetiapine) at conception and continued treatment for more than 12 weeks; 2) discontinued MSs during the 6 months preceding the conception and for the following 12 weeks. Pregnant women were followed up each trimester and at 6, 12, 24, and 52 weeks postpartum to ascertain recurrence of mania, hypomania (lasting ≥1 week), major depression, or a mixed state, and current treatments. The authors found that 70.8% of women experienced ≥1 episode during pregnancy. Risk of recurrence was 2.3 times higher in those who discontinued treatment than in those who continued (85.5% vs. 37.0%, respectively). Discontinuers spent >40% of pregnancy in an illness episode, vs. 8.8% of pregnancy of women continuing on MS. Median time to first recurrence was 9.0 weeks for discontinuers and >40 weeks for continuers. Those who abruptly or rapidly discontinued MS treatment (< 14 days) had 50% risk of recurrence within 2 weeks, whereas gradual discontinuers (> 14 days) required 22 weeks to reach 50% risk of recurrence. Treatment-related risk factors, besides MS discontinuation, included polytherapy with two or more psychotropic drugs, use of ADs, primary MS other than Li+, and previous switch from depression to mania/hypomania during past AD treatment.
Final considerations
Stabilizing treatments through the lifespan differ. In youth, SGAs are more tolerated and effective than MS in stabilizing mood (73). However, Li+ remains the cornerstone of mood stabilization as seeb in pediatric populations with BD, as it protects from impulsive acts and suicidal behavior (86). Furthermore, Li+ is also important for dimensions related to impulsive behavior and mood dysregulation, which are often encountered in such population (91). In adults, the use of add-on SGAs to MS in the treatment of manic/mixed state is still important, at least in the first half year of treatment. The combined treatment seems to confer greater mood stabilization. There is also preliminary evidence for greater effectiveness of some SGAs, like olanzapine, quetiapine, and risperidone, compared to MS monotherapy, but confirmatory studies are needed. In the elderly, the use of SGAs is contraindicated because of the impact on health and higher risk of death (all APs have a warning for increased risk of stroke in the elderly). Henceforth, the ratio of SGA/MS use varies across the lifespan, being highest during youth (frequent use for longer times of SGAs), intermediate in adult life (combined therapy), and low in the elderly (greater use of MSs).
Limitations
We based our conclusions on findings of sometimes underpowered studies, conducted with no double blinding, and often conducted on small samples. There is temporal discontinuity in the included studies, in that earlier years are less densely represented than recent years, and this might have affected the relative quality of the included studies. However, we found that most pre-millennial studies to be of high quality in both design and performance whereas not all recent trends in article standards resulted in improved data. The ways in which mood stabilization was considered and measured differed among studies. Only one study asked participants to rate their mood on a continuous visual scale, most others measured it as a reduction in HDRS or YMRS scores. This affected the evaluation of the stabilizing effect of the drug tested. Generally, we could not meta-analyze the eligible studies due to their extreme methodological differences in both design and assessment of outcomes; for example, about half were open-label and the other half double-blind. Furthermore, many were sponsored by the pharmaceutical industry, raising concerns that they could be biased in some sense. Risk of bias was high in most studies. Another limitation was that we did not assess the effects of physical therapies, like electroconvulsive therapy, deep and repetitive transcranial magnetic stimulation, and direct current transcranial stimulation, that may play a part in BD patients' treatment (92-94), but this would go beyond the scope of this review.
Summarizing, the indications for different treatments across the lifespan in BD are not supported by sufficient evidence, but appear nevertheless to differ. This is due to the dearth of studies carried out heretofore. The need for the future is for studies following the same methodology and adopting a consensus definition of stabilization.
Conclusions
Mood stabilization is currently achieved at suboptimal levels. The evidence gathered heretofore is quite insufficient to propose treatment recommendations for cAdolescents, pregnant women, and elderly people. Regarding adults, in manic/mixed phases AP drugs, especially SGAs, have shown usefulness in acute to medium term treatment, especially in combination with MSs. The latter, especially Li+, is still the mainstay of chronic treatment, even though there is increasing evidence supporting the superiority of long-term combined therapy. Depressive phases of BD benefit from MS and quetiapine treatment, and there is some concern with the switch-inducing potential of some ADs, but less with others. The use of ADs in bipolar depression is safer when the AD is prescribed along with a MS.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.
Author Contributions
GS and AS designed the review. All authors were involved in selection of eligible material and in Delphi rounds to reach consensus. AS and GK wrote the introduction, methods and results, designed the search strategy, gathered eligible material, and supervised the writing of the paper along with LJ and GS. GS, AK, DJ, and LD wrote the discussion. GK and AS wrote the limitations and conclusions. All authors approved the final form of the document. AS and AK equally contributed to the writing of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgements
We wish to thank the Librarians of the Library of the Faculty of Medicine and Psychology of the Sapienza University of Rome Ms. Mimma Ariano, Ms. Felicia Proietti, Ms. Ales Casciaro, Ms. Teresa Prioreschi, and Ms. Susanna Rospo for providing us precious bibliographic material.
Abbreviations
AAQ, Anger Attacks Questionnaire; AD(s), antidepressant drug(s); AE(s), anticonvulsant(s), antiepileptic(s); AP(s), antipsychotic(s); APT, adjunctive personalized treatment; ARP, aripiprazole; BD, bipolar disorder; -I, type I; -II, type II; BL, baseline; BPRS, Brief Psychiatric Rating Scale; BRMaS, Bech-Rafaelsen Mania Scale; CARS-M, Clinician-Administered Rating Scale for Mania; CBZ, carbamazepine; CGI, Clinical Global Impressions; -S, severity; -I, improvement; CGI-BP, Clinical Global Impressions Overall Scale for Bipolar Disorder; CLZ, clozapine; DB, double-blind; EQ-5D, the quality of life questionnaire in relation to health status “Euroqol”; FAST, Functioning Assessment Short Test; FGA(s), first-generation antipsychotic drug(s); GAF, Global Assessment of Functioning; GAS, Global Assessment Scale; GPT, gabapentin; HAL, haloperidol; HARS, Hamilton Anxiety Rating Scale; HDRS, Hamilton Depression Rating Scale (-N, item number specifying version); HCs, healthy controls; HDRS, Hamilton Depression Rating Scale; HR, hazard ratio; IDS, Inventory of Depressive Symptomatology; LAM, lamotrigine; LSEQ, Leeds Sleep Evaluation Questionnaire; Li+, lithium; MADRS, Montgomery-Åsberg Depression Rating Scale; MDD, major depressive disorder; MS, mood stabilizer; NOS, not otherwise specified; n.s., not significant; OLZ, olanzapine; OPT, optimized personalized treatment; PANSS, Positive And Negative Syndrome Scale; pts., patients; QLESQ, Quality of Life Enjoyment and Satisfaction Questionnaire Short Form, QTP, quetiapine (-XR, extended-release), RISP, risperidone; SABT, schizoaffective-bipolar type; SCZ, schizophrenia spectrum disorders; SGA(s), second-generation antipsychotic drug(s); SUD, substance use disorder; TCAs, tricyclic antidepressants; TOOL, the TOlerability and quality Of Life; TPX, topiramate; TRD, treatment-resistant depression; VPA, valproate; YMRS, Young Mania Rating Scale; yrs, years; ZIPR: ziprasidone; , mean; ♂, male; ♀, female; ±, standard deviation; , titrated up to or went from to; ↑, increase(d); ↓, decrease(d), drop.
References
1. Nilsson A, Axelsson R. Factors associated with discontinuation of long-term lithium treatment. Acta Psychiatr Scand (1989) 80(3):221–30. doi: 10.1111/j.1600-0447.1989.tb01331.x
2. Schumann C, Lenz G, Berghöfer A, Müller-Oerlinghausen B. Non-adherence with long-term prophylaxis: a 6-year naturalistic follow-up study of affectively ill patients. Psychiatry Res (1999) 89(3):247–57. doi: 10.1016/s0165-1781(99)00108-0
3. Maj M, Pirozzi R, Magliano L. Late non-response to lithium prophylaxis in bipolar patients: prevalence and predictors. J Affect Disord (1996) 39(1):39–42. doi: 10.1016/0165-0327(96)00018-3
4. Larsson S, Lorentzen S, Mork E, Barrett EA, Steen NE, Lagerberg TV, et al. Age at onset of bipolar disorder in a Norwegian catchment area sample. J Affect Disord (2010) 124(1-2):174–7. doi: 10.1016/j.jad.2009.10.031
5. Nowrouzi B, McIntyre RS, MacQueen G, Kennedy SH, Kennedy JL, Ravindran A, et al. Admixture analysis of age at onset in first episode bipolar disorder. J Affect Disord (2016) 201:88–94. doi: 10.1016/j.jad.2016.04.006
6. Glahn DC, Bearden CE, Barguil M, Barrett J, Reichenberg A, Bowden CL, et al. The neurocognitive signature of psychotic bipolar disorder. Biol Psychiatry (2007) 62(8):910–6. doi: 10.1016/j.biopsych.2007.02.001
7. Kupferschmidt DA, Zakzanis KK. Toward a functional neuroanatomical signature of bipolar disorder: quantitative evidence from the neuroimaging literature. Psychiatry Res (2011) 193(2):71–9. doi: 10.1016/j.pscychresns.2011.02.011
8. Kotzalidis GD, Rapinesi C, Savoja V, Cuomo I, Simonetti A, Ambrosi E, et al. Neurobiological Evidence for the Primacy of Mania Hypothesis. Curr Neuropharmacol (2017) 15(3):339–52. doi: 10.2174/1570159X14666160708231216
9. Janiri D, Sani G, De Rossi P, Piras F, Banaj N, Ciullo V, et al. Hippocampal subfield volumes and childhood trauma in bipolar disorders. J Affect Disord (2019) 253:35–43. doi: 10.1016/j.jad.2019.04.071
10. Padmos RC, Hillegers MH, Knijff EM, Vonk R, Bouvy A, Staal FJ, et al. A discriminating messenger RNA signature for bipolar disorder formed by an aberrant expression of inflammatory genes in monocytes. Arch Gen Psychiatry (2008) 65(4):395–407. doi: 10.1001/archpsyc.65.4.395
11. Sani G, Perugi G, Tondo L. Treatment of bipolar disorder in a lifetime perspective: is lithium still the best choice? Clin Drug Investig (2017) 37(8):713–27. doi: 10.1007/s40261-017-0531-2
12. Hetmar O, Brun C, Ladefoged J, Larsen S, Bolwig TG. Long-term effects of lithium on the kidney: functional-morphological correlations. J Psychiatr Res (1989) 23(3-4):285–97. doi: 10.1016/0022-3956(89)90034-4
13. Bendz H, Aurell M, Balldin J, Mathé AA, Sjödin I. Kidney damage in long-term lithium patients: a cross-sectional study of patients with 15 years or more on lithium. Nephrol Dial Transplant (1994) 9(9):1250–4. doi: 10.1093/ndt/9.9.1250
14. Tondo L, Abramowicz M, Alda M, Bauer M, Bocchetta A, Bolzani L, et al. Long-term lithium treatment in bipolar disorder: effects on glomerular filtration rate and other metabolic parameters. Int J Bipolar Disord (2017) 5(1):27. doi: 10.1186/s40345-017-0096-2
15. Keränen T, Sivenius J. Side effects of carbamazepine, valproate and clonazepam during long-term treatment of epilepsy. Acta Neurol Scand (1983) 68(Suppl. 97):69–80. doi: 10.1111/j.1600-0404.1983.tb01536.x
16. Perugi G, De Rossi P, Fagiolini A, Girardi P, Maina G, Sani G, et al. Personalized and precision medicine as informants for treatment management of bipolar disorder. Int Clin Psychopharmacol (2019) 34(4):189–205. doi: 10.1097/YIC.0000000000000260
17. Ghaemi SN. On defining ‘mood stabilizer'. Bipolar Disord (2001) 3(3):154–8. doi: 10.1034/j.1399-5618.2001.030304.x
18. Ketter TA. Definition of the term “mood stabilizer”. Bipolar Disord (2018) 20(1):74–5. doi: 10.1111/bdi.12579
19. Scatton B, Fage D, Oblin A, Zivkovic B, Arbilla S, Langer SZ, et al. Influence of GABA mimetics and lithium on biochemical manifestations of striatal dopamine target cell hypersensitivity. Psychopharmacology (1985) 85-87(Suppl. 2):39–45. doi: 10.1007/978-3-642-70140-5_5
20. Del' Guidice T, Beaulieu JM. Selective disruption of dopamine D2-receptors/beta-arrestin2 signaling by mood stabilizers. J Recept Signal Transduct Res (2015) 35(3):224–32. doi: 10.3109/10799893.2015.1072976
21. Fortin SM, Chartoff EH, Roitman MF. The aversive agent lithium chloride suppresses phasic dopamine release through central GLP-1 receptors. Neuropsychopharmacology. (2016) 41(3):906–15. doi: 10.1038/npp.2015.220
22. Scotti-Muzzi E, Chile T, Moreno R, Pastorello BF, da Costa Leite C, Henning A, et al. ACC Glu/GABA ratio is decreased in euthymic bipolar disorder I patients: possible in vivo neurometabolite explanation for mood stabilization. Eur Arch Psychiatry Clin Neurosci (2020). doi: 10.1007/s00406-020-01096-0
23. Hochberg Z, Konner M. Emerging adulthood, a pre-adult life-history stage. Front Endocrinol (2020) 10:918. doi: 10.3389/fendo.2019.00918
24. Ingalhalikar M, Smith A, Parker D, Satterthwaite TD, Elliott MA, Ruparel K, et al. Sex differences in the structural connectome of the human brain. Proc Natl Acad Sci U S A (2014) 111(2):823–8. doi: 10.1073/pnas.1316909110
25. Mitchell RH, Metcalfe AW, Islam AH, Toma S, Patel R, Fiksenbaum L, et al. Sex differences in brain structure among adolescents with bipolar disorder. Bipolar Disord (2018) 448–58. doi: 10.1111/bdi.12663
26. Janiri D, Di Nicola M, Martinotti G, Janiri L. Who's the leader, mania or depression? Predominant polarity and alcohol/polysubstance use in bipolar disorders. Curr Neuropharmacol (2017) 15(3):409–16. doi: 10.2174/1570159X14666160607101400
27. Sharma V. Relationship of bipolar disorder with psychiatric comorbidity in the postpartum period-a scoping review. Arch Womens Ment Health (2018) 21(2):141–7. doi: 10.1007/s00737-017-0782-1
28. Tebeka S, Le Strat Y, Dubertret C. Is parity status associated with bipolar disorder clinical features, severity or evolution? J Affect Disord (2018) 225:201–6. doi: 10.1016/j.jad.2017.08.042
29. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. (2009) 339:b2535. doi: 10.1136/bmj.b2535
30. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med (2009) 6(7):e1000097. doi: 10.1371/journal.pmed.1000097
31. DePaulo JR Jr, Correa EI, Folstein MF. Does lithium stabilize mood? Biol Psychiatry (1983) 18(9):1093–7. doi: 10.1016/0006-3223/83/0900-1093
32. Goldberg JF, Garno JL, Leon AC, Kocsis JH, Portera L. Rapid titration of mood stabilizers predicts remission from mixed or pure mania in bipolar patients. J Clin Psychiatry (1998) 59(4):151–8. doi: 10.4088/JCP.v59n0402
33. Sanderson DR. Use of mood stabilizers by hospitalized geriatric patients with bipolar disorder. Psychiatr Serv (1998) 49(9):1145–7. doi: 10.1176/ps.49.9.1145
34. Sokolski KN, Green C, Maris DE, DeMet EM. Gabapentin as an adjunct to standard mood stabilizers in out patients with mixed bipolar symptomatology. Ann Clin Psychiatry (1999) 11(4):217–22. doi: 10.1023/a:1022361412956
35. Young LT, Joffe RT, Robb JC, MacQueen GM, Marriott M, Patelis-Siotis I. Double-blind comparison of addition of a second mood stabilizer versus an antidepressant to an initial mood stabilizer for treatment of patients with bipolar depression. Am J Psychiatry (2000) 157(1):124–6. doi: 10.1176/ajp.157.1.124
36. Benabarre A, Vieta E, Colom F, Martínez A, Reinares M, Corbella B. Treatment of mixed mania with risperidone and mood stabilizers. Can J Psychiatry (2001) 46(9):866–7. doi: 10.1177/070674370104600925
37. Bottlender R, Rudolf D, Strauss A, Möller HJ. Mood-stabilisers reduce the risk of developing antidepressant-induced maniform states in acute treatment of bipolar I depressed patients. J Affect Disord (2001) 63(1-3):79–83. doi: 10.1016/s0165-0327(00)00172-5
38. Kaplan BJ, Simpson JS, Ferre RC, Gorman CP, McMullen DM, Crawford SG. Effective mood stabilization with a chelated mineral supplement: an open-label trial in bipolar disorder. J Clin Psychiatry (2001) 62(12):936–44. doi: 10.4088/jcp.v62n1204
39. Miller DS, Yatham LN, Lam RW. Comparative efficacy of typical and atypical antipsychotics as add-on therapy to mood stabilizers in the treatment of acute mania. J Clin Psychiatry (2001) 62(12):975–80. doi: 10.4088/jcp.v62n1210
40. Post RM, Altshuler LL, Frye MA, Suppes T, Rush AJ, Keck PE Jr, et al. Rate of switch in bipolar patients prospectively treated with second-generation antidepressants as augmentation to mood stabilizers. Bipolar Disord (2001) 3(5):259–65. doi: 10.1034/j.1399-5618.2001.30505.x
41. Winsberg ME, DeGolia SG, Strong CM, Ketter TA. Divalproex therapy in medication-naïve and mood-stabilizer-naïve bipolar II depression. J Affect Disord (2001), 67(1–3207–12. doi: 10.1016/s0165-0327(01)00434-7
42. Sachs GS, Grossman F, Ghaemi SN, Okamoto A, Bowden CL. Combination of a mood stabilizer with risperidone or haloperidol for treatment of acute mania: a double-blind, placebo-controlled comparison of efficacy and safety. Am J Psychiatry (2002) 159(7):1146–54. doi: 10.1176/appi.ajp.159.7.1146
43. Baker RW, Goldberg JF, Tohen M, Milton DR, Stauffer VL, Schuh LM. The impact of response to previous mood stabilizer therapy on response to olanzapine versus placebo for acute mania. Bipolar Disord (2002) 4(1):43–9. doi: 10.1034/j.1399-5618.2002.40103.x
44. McIntyre RS, Mancini DA, McCann S, Srinivasan J, Sagman D, Kennedy SH. Topiramate versus bupropion SR when added to mood stabilizer therapy for the depressive phase of bipolar disorder: a preliminary single-blind study. Bipolar Disord (2002) 4(3):207–13. doi: 10.1034/j.1399-5618.2002.01189.x
45. Vieta E, Martinez-Arán A, Goikolea JM, Torrent C, Colom F, Benabarre A, et al. A randomized trial comparing paroxetine and venlafaxine in the treatment of bipolar depressed patients taking mood stabilizers. J Clin Psychiatry (2002) 63(6):508–12. doi: 10.4088/jcp.v63n0607
46. Henry C, Van den Bulke D, Bellivier F, Etain B, Rouillon F, Leboyer M. Anxiety disorders in 318 bipolar patients: prevalence and impact on illness severity and response to mood stabilizer. J Clin Psychiatry (2003) 64(3):331–5. doi: 10.4088/JCP.v64n0316
47. Yatham LN, Grossman F, Augustyns I, Vieta E, Ravindran A. Mood stabilisers plus risperidone or placebo in the treatment of acute mania. International, double-blind, randomised controlled trial. Br J Psychiatry (2003) 182(2):141–7. doi: 10.1192/bjp.182.2.141
48. Bowden CL, Myers JE, Grossman F, Xie Y. Risperidone in combination with mood stabilizers: a 10-week continuation phase study in bipolar I disorder. J Clin Psychiatry (2004) 65(5):707–14. doi: 10.4088/jcp.v65n0518
49. Goldberg JF, Burdick KE, Endick CJ. Preliminary randomized, double-blind, placebo-controlled trial of pramipexole added to mood stabilizers for treatment-resistant bipolar depression. Am J Psychiatry (2004) 161(3):564–6. doi: 10.1176/appi.ajp.161.3.564
50. Mammen OK, Pilkonis PA, Chengappa KN, Kupfer DJ. Anger attacks in bipolar depression: predictors and response to citalopram added to mood stabilizers. J Clin Psychiatry (2004) 65(5):627–33. doi: 10.4088/JCP.v65n0506
51. Tohen M, Chengappa KN, Suppes T, Baker RW, Zarate CA, Bowden CL, et al. Relapse prevention in bipolar I disorder: 18-month comparison of olanzapine plus mood stabiliser v. mood stabiliser alone. Br J Psychiatry (2004) 184:337–45. doi: 10.1192/bjp.184.4.337
52. Bahk WM, Yoon JS, Kim YH, Lee YH, Lee C, Kim KS, et al. Risperidone in combination with mood stabilizers for acute mania: a multicentre, open study. Int Clin Psychopharmacol (2004a) 19(5):299–303. doi: 10.1097/01.yic.0000138821.85744.20
53. Bahk WM, Yoon BH, Lee KU, Chae JH. Combination of mood stabilizers with quetiapine for treatment of acute bipolar disorder: an open label study. Hum Psychopharmacol (2004b) 19(3):181–5. doi: 10.1002/hup.577
54. Hantouche EG, Akiskal HS, Lancrenon S, Chatenêt-Duchêne L. Mood stabilizer augmentation in apparently “unipolar” MDD: predictors of response in the naturalistic French national EPIDEP study. J Affect Disord (2005) 84(2-3):243–9. doi: 10.1016/j.jad.2004.01.006
55. Patel NC, Crismon ML, Pondrom M. Rehospitalization rates of patients with bipolar disorder discharged on a mood stabilizer versus a mood stabilizer plus an atypical or typical antipsychotic. J Behav Health Serv Res (2005) 32(4):438–45. doi: 10.1007/bf02384203
56. Chengappa RKN, Schwarzman LK, Hulihan JF, Xiang J, Rosenthal NR. Clinical Affairs Product Support Study-168 Investigators. Adjunctive topiramate therapy in patients receiving a mood stabilizer for bipolar I disorder: a randomized, placebo-controlled trial. J Clin Psychiatry (2006) 67(11):1698–706. doi: 10.4088/jcp.v67n1105
57. Leverich GS, Altshuler LL, Frye MA, Suppes T, McElroy SL, Keck PE Jr, et al. Risk of switch in mood polarity to hypomania or mania in patients with bipolar depression during acute and continuation trials of venlafaxine, sertraline, and bupropion as adjuncts to mood stabilizers. Am J Psychiatry (2006) 163(2):232–9. doi: 10.1176/appi.ajp.163.2.232
58. Parker G, Tully L, Olley A, Hadzi-Pavlovic D. SSRIs as mood stabilizers for Bipolar II Disorder? A proof of concept study. J Affect Disord (2006) 92(2-3):205–14. doi: 10.1016/j.jad.2006.01.024
59. Spaulding T, Westlund R, Thomason C, White R, Dann R, Thompson T. Adjunctive treatment for mood stabilization of patients with bipolar I disorder treated with lamotrigine. CNS Spectr (2006) 11(9):711–6; quiz 719. doi: 10.1017/s1092852900014802
60. Savas HA, Yumru M, Kaya MC, Selek S. Atypical antipsychotics as “mood stabilizers”: a retrospective chart review. Prog Neuropsychopharmacol Biol Psychiatry (2007) 31(5):1064–7. doi: 10.1016/j.pnpbp.2007.03.007
61. Tramontina S, Zeni CP, Pheula G, Rohde LA. Topiramate in adolescents with juvenile bipolar disorder presenting weight gain due to atypical antipsychotics or mood stabilizers: an open clinical trial. J Child Adolesc Psychopharmacol (2007) 17(1):129–34. doi: 10.1089/cap.2006.0024
62. Viguera AC, Whitfield T, Baldessarini RJ, Newport DJ, Stowe Z, Reminick A, et al. Risk of recurrence in women with bipolar disorder during pregnancy: prospective study of mood stabilizer discontinuation. Am J Psychiatry (2007) 164(12):1817–24; quiz 1923. doi: 10.1176/appi.ajp.2007.06101639
63. Altamura AC, Mundo E, Dell'Osso B, Tacchini G, Buoli M, Calabrese JR. Quetiapine and classical mood stabilizers in the long-term treatment of Bipolar Disorder: a 4-year follow-up naturalistic study. J Affect Disord (2008) 110(1-2):135–41. doi: 10.1016/j.jad.2008.01.017
64. Dell'Osso B, Buoli M, Riundi R, D'Urso N, Pozzoli S, Bassetti R, et al. Clinical characteristics and long-term response to mood stabilizers in patients with bipolar disorder and different age at onset. Neuropsychiatr Dis Treat (2009) 5:399–404. doi: 10.2147/NDT.S5970
65. Goldberg JF, Calabrese JR, Saville BR, Frye MA, Ketter TA, Suppes T, et al. Mood stabilization and destabilization during acute and continuation phase treatment for bipolar I disorder with lamotrigine or placebo. J Clin Psychiatry (2009) 70(9):1273–80. doi: 10.4088/JCP.08m04381
66. Chang JS, Moon E, Cha B, Ha K. Adjunctive lamotrigine therapy for patients with bipolar II depression partially responsive to mood stabilizers. Prog Neuropsychopharmacol Biol Psychiatry (2010) 34(7):1322–6. doi: 10.1016/j.pnpbp.2010.07.020
67. Quante A, Zeugmann S, Luborzewski A, Schommer N, Langosch J, Born C, et al. Aripiprazole as adjunct to a mood stabilizer and citalopram in bipolar depression: a randomized placebo-controlled pilot study. Hum Psychopharmacol (2010) 25(2):126–32. doi: 10.1002/hup.1096
68. Muzina DJ, Gao K, Kemp DE, Khalife S, Ganocy SJ, Chan PK, et al. Acute efficacy of divalproex sodium versus placebo in mood stabilizer-naive bipolar I or II depression: a double-blind, randomized, placebo-controlled trial. J Clin Psychiatry (2011) 72(6):813–9. doi: 10.4088/JCP.09m05570gre
69. Katagiri H, Takita Y, Tohen M, Higuchi T, Kanba S, Takahashi M. Safety and efficacy of olanzapine monotherapy and olanzapine with a mood stabilizer in 18-week treatment of manic/mixed episodes for Japanese patients with bipolar I disorder. Curr Med Res Opin (2012) 28(5):701–13. doi: 10.1185/03007995.2012.666961
70. Koukopoulos A, Serra G, Koukopoulos AE, Reginaldi D, Serra G. The sustained mood-stabilizing effect of memantine in the management of treatment resistant bipolar disorders: findings from a 12-month naturalistic trial. J Affect Disord (2012) 136(1-2):163–6. doi: 10.1016/j.jad.2011.09.040
71. Goldberg JF. A preliminary open trial of nefazodone added to mood stabilizers for bipolar depression. J Affect Disord (2013) 144(1-2):176–8. doi: 10.1016/j.jad.2012.04.037
72. Norris ER, Burke K, Correll JR, Zemanek KJ, Lerman J, Primelo RA, et al. A double-blind, randomized, placebo-controlled trial of adjunctive ramelteon for the treatment of insomnia and mood stability in patients with euthymic bipolar disorder. J Affect Disord (2013) 144(1-2):141–7. doi: 10.1016/j.jad.2012.06.023
73. Chen H, Mehta S, Aparasu R, Patel A, Ochoa-Perez M. Comparative effectiveness of monotherapy with mood stabilizers versus second generation (atypical) antipsychotics for the treatment of bipolar disorder in children and adolescents. Pharmacoepidemiol Drug Saf (2014) 23(3):299–308. doi: 10.1002/pds.3568
74. Sharma PS, Kongasseri S, Praharaj SK. Outcome of mood stabilizer discontinuation in bipolar disorder after 5 years of euthymia. J Clin Psychopharmacol (2014) 34(4):504–7. doi: 10.1097/JCP.0000000000000160
75. Viktorin A, Lichtenstein P, Thase ME, Larsson H, Lundholm C, Magnusson PK, et al. The risk of switch to mania in patients with bipolar disorder during treatment with an antidepressant alone and in combination with a mood stabilizer. Am J Psychiatry (2014) 171(10):1067–73. doi: 10.1176/appi.ajp.2014.13111501
76. Weiser M, Burshtein S, Gershon AA, Marian G, Vlad N, Grecu IG, et al. Allopurinol for mania: a randomized trial of allopurinol versus placebo as add-on treatment to mood stabilizers and/or antipsychotic agents in manic patients with bipolar disorder. Bipolar Disord (2014) 16(4):441–7. doi: 10.1111/bdi.12202
77. Amsterdam JD, Lorenzo-Luaces L, Soeller I, Li SQ, Mao JJ, DeRubeis RJ. Safety and effectiveness of continuation antidepressant versus mood stabilizer monotherapy for relapse-prevention of bipolar II depression: A randomized, double-blind, parallel-group, prospective study. J Affect Disord (2015) 185:31–7. doi: 10.1016/j.jad.2015.05.070
78. Hochman E, Krivoy A, Schaffer A, Weizman A, Valevski A. Antipsychotic adjunctive therapy to mood stabilizers and 1-year rehospitalization rates in bipolar disorder: A cohort study. Bipolar Disord (2016) 18(8):684–91. doi: 10.1111/bdi.12459
79. Shansis FM, Reche M, Capp E. Evaluating response to mood stabilizers in patients with mixed depression: A study of agreement between three different mania rating scales and a depression rating scale. J Affect Disord (2016) 197:1–7. doi: 10.1016/j.jad.2016.02.064
80. Yatham LN, Vieta E, Goodwin GM, Bourin M, de Bodinat C, Laredo J, et al. Agomelatine or placebo as adjunctive therapy to a mood stabiliser in bipolar I depression: randomised double-blind placebo-controlled trial. Br J Psychiatry (2016a) 208(1):78–86. doi: 10.1192/bjp.bp.114.147587
81. Yatham LN, Beaulieu S, Schaffer A, Kauer-Sant'Anna M, Kapczinski F, Lafer B, et al. Optimal duration of risperidone or olanzapine adjunctive therapy to mood stabilizer following remission of a manic episode: A CANMAT randomized double-blind trial. Mol Psychiatry (2016b) 21(8):1050–6. doi: 10.1038/mp.2015.158
82. Ahn SW, Baek JH, Yang SY, Kim Y, Cho Y, Choi Y, et al. Long-term response to mood stabilizer treatment and its clinical correlates in patients with bipolar disorders: a retrospective observational study. Int J Bipolar Disord (2017) 5(1):24. doi: 10.1186/s40345-017-0093-5
83. Garriga M, Solé E, González-Pinto A, Selva-Vera G, Arranz B, Amann BL, et al. Efficacy of quetiapine XR vs. placebo as concomitant treatment to mood stabilizers in the control of subthreshold symptoms of bipolar disorder: Results from a pilot, randomized controlled trial. Eur Neuropsychopharmacol (2017) 27(10):959–69. doi: 10.1016/j.euroneuro.2017.08.429
84. Perugi G, Vannucchi G, Barbuti M, Maccariello G, De Bartolomeis A, Fagiolini A, et al. Outcome and predictors of remission in bipolar-I patients experiencing manic episode and treated with oral antipsychotics and/or mood stabilizers: a prospective observational study in Italy. Int Clin Psychopharmacol (2018) 33(3):131–9. doi: 10.1097/YIC.0000000000000211
85. Bauer M, Glenn T, Alda M, Bauer R, Grof P, Marsh W, et al. Trajectories of adherence to mood stabilizers in patients with bipolar disorder. Int J Bipolar Disord (2019) 7(1):19. doi: 10.1186/s40345-019-0154-z
86. Hafeman DM, Rooks B, Merranko J, Liao F, Gill MK, Goldstein TR, et al. Lithium versus other mood stabilizing medications in a longitudinal study of bipolar youth. J Am Acad Child Adolesc Psychiatry (2019), S0890–8567(19)31399-1. doi: 10.1016/j.jaac.2019.06.013
87. Steardo L, Fabrazzo M, Sampogna G, Monteleone AM, D'Agostino G, Monteleone P, et al. Impaired glucose metabolism in bipolar patients and response to mood stabilizer treatments. J Affect Disord (2019) 245:174–9. doi: 10.1016/j.jad.2018.10.360
88. Tournier M, Neumann A, Pambrun E, Weill A, Chaffiol JP, Alla F, et al. Conventional mood stabilizers and/or second-generation antipsychotic drugs in bipolar disorders: A population-based comparison of risk of treatment failure. J Affect Disord (2019) 257:412–20. doi: 10.1016/j.jad.2019.07.054
89. Tavormina G. Bipolar disorders and bipolarity: the notion of the “mixity”. Psychiatr Danub (2019) 31(Suppl 3):S434–7.
90. Tavormina G. An approach to treat bipolar disorders mixed states. Psychiatr Danub (2016) 28(Suppl-1):9–12.
91. Etain B, Lajnef M, Henry C, Aubin V, Azorin JM, Bellivier F, et al. FACE-BD Clinical Coordinating Center (FondaMental Foundation); FACE-BD Data Coordinating Center (FondaMental Foundation); FACE-BD Clinical Sites and Principal Collaborators in France. Childhood trauma, dimensions of psychopathology and the clinical expression of bipolar disorders: A pathway analysis. J Psychiatr Res (2017) 95:37–45. doi: 10.1016/j.jpsychires.2017.07.013
93. Rapinesi C, Serata D, Del Casale A, Simonetti A, Milioni M, Mazzarini L, et al. Successful and rapid response to electroconvulsive therapy of a suicidal patient with comorbid bipolar I disorder and histrionic personality disorder. J ECT (2012) 28(1):57–8. doi: 10.1097/YCT.0b013e3182218c8c
94. Rapinesi C, Del Casale A, Di Pietro S, Ferri VR, Piacentino D, Sani G, et al. Add-on high frequency deep transcranial magnetic stimulation (dTMS) to bilateral prefrontal cortex reduces cocaine craving in patients with cocaine use disorder. Neurosci Lett (2016) 629:(43–47.
Keywords: mood, stabilization, antidepressant drugs, antipsychotic drugs, lithium, mood stabilizers
Citation: Simonetti A, Koukopoulos AE, Kotzalidis GD, Janiri D, De Chiara L, Janiri L and Sani G (2020) Stabilization Beyond Mood: Stabilizing Patients With Bipolar Disorder in the Various Phases of Life. Front. Psychiatry 11:247. doi: 10.3389/fpsyt.2020.00247
Received: 27 January 2020; Accepted: 13 March 2020;
Published: 27 April 2020.
Edited by:
Andreas Reif, University Hospital Frankfurt, GermanyReviewed by:
Sarah Kittel-Schneider, University Hospital Würzburg, GermanyGiuseppe Tavormina, Independent Researcher, Provaglio d'Iseo, Italy
Copyright © 2020 Simonetti, Koukopoulos, Kotzalidis, Janiri, De Chiara, Janiri and Sani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gabriele Sani, Z2FicmllbGUuc2FuaUB1bmljYXR0Lml0
†These authors have contributed equally to this work
 Alessio Simonetti1,2,3†
Alessio Simonetti1,2,3† Georgios D. Kotzalidis
Georgios D. Kotzalidis Luigi Janiri
Luigi Janiri Gabriele Sani
Gabriele Sani