- 1Department of Psychiatry, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, Kaohsiung, Taiwan
- 2School of Occupational Therapy, College of Medicine, National Taiwan University, Taipei, Taiwan
- 3Professional Animal-Assisted Therapy Association of Taiwan, Taipei, Taiwan
- 4Department of Nursing, School of Nursing, National Taipei University of Nursing and Health Sciences, Taipei, Taiwan
Objective: Animal-assisted therapy (AAT) has the potential to improve the symptomology, negative emotions, and level of well-being in older adults, as well as patients with mental illness. However, there remains limited evidence supporting the treatment efficacy of AAT in middle-aged and older adults with schizophrenia. Therefore, this study implemented a randomized controlled trial to assess the efficacy of a 12-week AAT psychological intervention with dogs for middle-aged and older patients with chronic schizophrenia in a clinical setting.
Method: Patients, age ≥ 40 years, with chronic schizophrenia were allocated randomly to either the AAT group or control group. Patients in the AAT group received an additional hour -long AAT session every week for 12 weeks. Patients in the control group received the usual treatment plus an hour long non-animal related intervention. All patients were assessed based on primary outcome measures before and after the 12-week intervention, including the Positive and Negative Syndrome Scale (PANSS), Depression Anxiety Stress Scales Assessment (DASS), and Chinese Happiness Inventory (CHI).
Results: Patients who received AAT had greater improvements in the PANSS and DASS-stress subscale scores than the control group (p < 0.05). The effect was small (success ratio different, SRD = 0.25) for the PANSS and the DASS-stress subscale (SRD = 0.15). There were no significant differences in the change scores of the CHI between the AAT and control groups (p = 0.461).
Conclusions: AAT seemed to be effective in reducing psychiatric symptoms and stress levels of middle-aged and older patients with schizophrenia. AAT could be considered as a useful adjunctive therapy to the usual treatment programs.
Introduction
Schizophrenia is a mental disability characterized by positive symptoms (e.g., delusions and hallucinations), negative symptoms (e.g., apathy and anhedonia) (1), and general psychopathology (e.g., depression and anxiety) (2–5). Schizophrenia has adverse impacts on major areas of life, such as work and interpersonal relations (1). Approximately 1% of population are affected by schizophrenia (6), of which 25% or more will soon be middle-aged and older individuals (7, 8). Schizophrenia is particularly challenging for this age group because these individuals tend to have more severe psychotic symptoms and poorer psychosocial function (9). About 60% of patients with schizophrenia living in the hospital are middle-aged and older (10), dying 10–15 years earlier than the general population (11). Their negative symptoms and cognitive impairment are significantly more severe than those in younger patients (12) and pose a significant challenge to psychiatric treatment (7).
Treatments for schizophrenia typically involve antipsychotic drugs and psychotherapy (13). However, the effectiveness of these treatment is questionable (14–17) because negative and cognitive symptoms often remain problematic (11, 18). Therefore, it is important to seek alternative psychosocial treatments to improve the psychotic symptoms of patients with schizophrenia.
Animal-Assisted Therapy (AAT) has recently garnered increased attention and been used as an adjunct to typical treatments and interventions for patients with mental illness, including schizophrenia (19, 20). AAT is a structured, planned, and goal-oriented therapeutic intervention involving interactions between a patient and an animal (typically a dog), along with a therapist and an animal handler (21, 22). Example AAT activities include taking care of a dog and playing with a dog (19, 20, 23–25). AAT is typically used to improve the symptoms, functioning (emotional, social, and cognitive), and quality of life for patients with mental illness (19, 20, 23, 26–30), In addition, it may be particularly helpful in the treatment of schizophrenia (22). Interacting with animals can also increase oxytocin levels, which has been shown to improve psychiatric symptoms (31, 32). Relaxing human–animal relations may help dampen negative emotions (20, 29). Therapy dogs can serve as emotional mediators to provide support and company (20, 33), and may increase patient quality of life and well-being (29).
Previous studies have used AAT to improve psychiatric symptoms, emotion, and quality of life in patient with schizophrenia, but there still remains insufficient significant evidence demonstrating its effectiveness. Some studies have revealed positive results, with improvements in positive symptoms, negative symptoms, and general psychopathology symptoms (including stress, anxiety, and depression) (20, 23, 25, 29, 34). However, other studies found no significant improvements in motivation (25), general psychopathology symptoms (23), and quality of life (20). Moreover, there were several methodological limitations in previous studies, including the lack of structural AAT programs, small sample sizes, lack of control groups, short durations of intervention, and limited professionals and animals involved in AAT sessions (20, 21). Few studies have targeted middle-aged and older patients with schizophrenia (21). Therefore, the evidence regarding the effectiveness of AAT in middle-aged and older patients with schizophrenia remains inconclusive and insufficient (20, 21). As such, this study sought to evaluate the effects of AAT for middle-aged and older schizophrenia patients on psychotic symptoms, negative emotions, and well-being.
Method
Participants
Participants were recruited from a psychiatric rehabilitation ward and the day-care ward of a medical center in Taiwan. Participants met the following criteria: (1) diagnosis of schizophrenia according to the fifth edition of Diagnostic and Statistical Manual of Mental Disorders, (2) age≥40 years, and (3) stable physical and psychological health conditions based on clinician assessment. Participants with the following criteria were excluded: (1) severe cognitive impairment (e.g., aphasia or inability to follow three-step directions), (2) animal allergies, (3) history of asthma, (4) coagulation disorders, (5) presenting symptoms of dog-related specific phobia, anxiety disorder, and obsessive-compulsive disorder, and (6) had participated in other clinical trials in the past 6 months.
Design
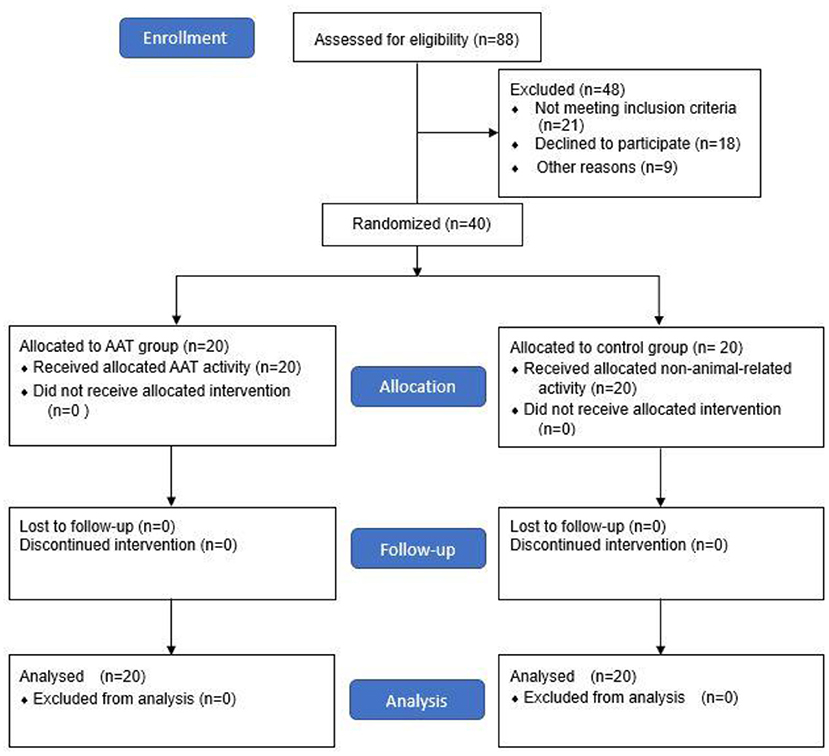
This study implemented a randomized controlled trial (RCT) with parallel-group design and pre-post measurements. Because of the different functional characteristics of patients in day-care and rehabilitation wards, stratification was performed in order to control for confounding variables. Forty patients who met the inclusion criteria were recruited and randomly assigned to the AAT group (intervention group) and control group, with 20 participants in each group (Figure 1). To ensure allocation concealment, participants were randomized by an external clinic using sequentially numbered, opaque sealed envelopes. Stratified randomization was carried out with an online randomizer (www.randomiser.com). Previous studies suggested that group size was best kept small for AAT sessions to ensure quality and safety of treatment; accordingly, participants in the AAT group were further divided into two small groups (groups A and B) to attend the AAT session at the same time. There were no differences in treatment between groups A and B.
Procedure
Participants in both groups received their usual treatment programs, consisting of nursing interventions, pharmacotherapy, occupational therapy, psychotherapy, sociotherapy, and recreational activities. The AAT group received an additional hour of AAT session for 12 weeks. The control group received a non-animal-related nursing intervention and occupational therapy session of usual treatment programs instead. All participants were assessed 1 week prior to and after the 12-week program by one psychiatric physician and one occupational therapist.
Measures
Positive and Negative Syndrome Scale (PANSS)
The PANSS is widely used to assess the symptom severity of patients with schizophrenia in clinical trials (2–4). The scale comprises 30 items across 3 subscales: the positive subscale (seven items), negative subscale (seven items), and general psychopathology subscale (16 items). Each item is scored on a 7-point scale based on the level of psychopathology present, from 1 (absent) to 7 (extreme). The total score ranges from 30 to 210, with higher scores indicating greater overall symptom severity (35). The validity and reliability of the Chinese Mandarin version of the PANSS has been established in a previous study (36). A minimum total change score of 10.4 was accepted as having responded to treatment (37). The minimum change score for each subscale was assessed based on the total score percentage of each subscale (positive: 2.4, negative: 2.4, general psychopathology: 5.6).
Chinese Happiness Inventory (CHI)
The 20-item version of the CHI is a self-report measure of subjective wellbeing for those following the Chinese culture (38, 39). The CHI comprises items with relation to achievement at work, downward social comparisons, peace of mind, optimism, social commitment, positive affect, sense of control, physical fitness, and satisfaction with self in the Chinese society (39, 40). Each item is scored on a 4-point scale from 1 to 4, corresponding to a different level of happiness (37). The total score ranges from 20 to 80, with higher scores indicating greater happiness (37). The CHI has high reliability (41).
Depression, Anxiety Stress Scales-21 (DASS-21)
The DASS-21 is a self-report questionnaire used to assess symptoms of depression, anxiety, and stress (42). The DASS-21 comprises 21 items, each describing a negative emotional symptom the participant experienced in the past week (42). Each item is scored on a 4-point scale from 0 (never) to 3 (almost always). Scores of depression, anxiety, and stress are calculated by summing the relevant items. Higher scores indicate more severe negative emotional symptoms. The DASS-21 is a well-established instrument with sufficient reliability and validity (42). The minimal detectable change of total score and each subscale of anxiety, depression, and stress were 7.8, 2.4, 2.2, and 3.2 respectively (43).
Interventions
Personnel
Each AAT session was conducted by an animal-assisted therapist, an occupational therapist, and a dog-handler pair (breeder). Prior to the commencement of the study, the animal-assisted therapist and occupational therapist cooperated to design the intervention protocol to match the needs of the patients. The AAT sessions were primarily led by the animal-assisted therapist, with the help of the occupational therapist as the co-leader, who helped encourage patients to participate. The breeder's job was to instruct their therapy dog to follow the directions of the therapist. All members were sufficiently qualified to effectively carry out the session.
The animal-assisted therapists, therapy dogs, and breeders have been certified by the Professional Animal-Assisted Therapy Association of Taiwan; and the therapy dogs, including Corgi, Labrador Retriever, Maltese, and Shiba Inu, passed the therapy dog test to ensures that they could remain calm in difficult, distracting, and stressful situations. The occupational therapists specialized in psychiatric rehabilitation and were familiar with the global functions of the patients.
Animal-Assisted Therapy
The primary goal of the AAT was to improve the negative symptoms (blunted affect, emotional withdrawal, social withdrawal, lack of spontaneity, and flow of conversation) and general psychopathology symptoms (anxiety, depression, uncooperativeness, disorientation, and poor attention) of patients. Secondary goals were aimed at improving positive symptoms (conceptual disorganization, suspiciousness, persecution, and hostility) and patient well-being.
The AAT sessions were conduct in a spacious and quiet classroom, with the participants seated in a semicircle. The animal assisted therapist, occupational therapist, and therapy dog were positioned in front of the participants. The dog approached the participants in turn, and each participant walked the dog around the classroom.
Each AAT session was carried out according to a similar overall structure: 15-min warm-up, 45-min therapeutic activities, and 5-min feedback. In the warm-up, the animal assisted therapist started by greeting each participant, introduced the therapy dog, reviewed the contents of the last session, and oriented participants to the therapeutic activities.
There were four types of therapeutic activities carried out to achieve the therapeutic goal: activity for positive emotion, social activity, cognitive activity, and physical activity. Activities aimed at positive emotion included touching the dog, singing a song, massaging the dog, playing with the dog (ball, loop, game), and artistic creation (dot art). Social activity involved introducing, greeting, praising, thanking, helping, talking, making appropriate physical and eye contact, and cooperating in the games with each other and the dog. Cognitive activity included questions and answers, training the dog, orienting the content of activity, playing a cognitive game (puzzle, triangle, and memory card), and writing a worksheet. Physical activity involved walking, handling, feeding, grooming, dressing, and doing exercises with the dog. Each activity was performed for three sessions with gradually increasing levels of difficulty.
Therapists gave feedback on what the group did during the therapeutic activities, asked how they felt with the dog, and previewed the content of the next session.
Data Analysis
Statistical analyses were conducted using Statistical Package for Social Sciences (SPSS, version 25.0; Chicago, IL, USA). Data from groups A and B of the AAT condition were analyzed collectively. Categorical variables (e.g., sex and level of education) were converted to percentages and compared using the chi-square test. Given the small sample size, the Shapiro–Wilk test was used to test the normality of the data. As the data fit a non-normal distribution, we used the non-parametric statistics. The continuous variables of the outcome measures (PANSS, DASS, and CHI scores) are presented as the median and interquartile range (IQR) and compared with the Mann-Whitney U-test. All tests were two-tailed with a probability ≤ 0.05 (p ≤ 0.05) considered reflective of significance. Additionally, the effect size of success rate difference (SRD) (e.g., treatment group success rate–control group success rate) were calculated for all significant findings (44), with values of 0.11–0.27, 0.28–0.43, and >0.43 categorized as small, moderate, and large effect size, respectively (45).
Ethics
The study was approved by the local ethics committee of the Chang Gung Medical Foundation Institutional Review Board (No. 202000549B0C601). All participants provided written informed consent. The study was registered at ClinicalTrials.gov (Identifier: NCT04476836).
Results
Sample Characteristics
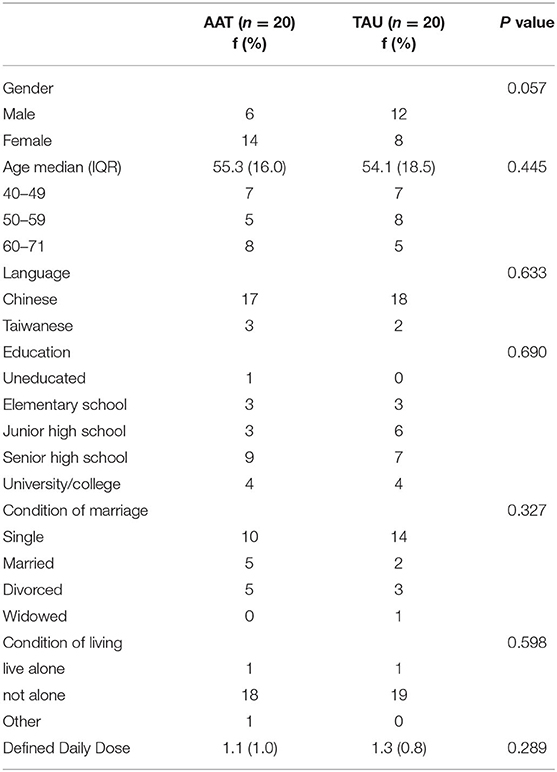
A final sample of 40 participants (20 per group) was analyzed (Figure 1). No participants dropped out. There were no significant differences between the groups at baseline regarding age, sex, language, level of education, marital status, and living condition (Table 1). At pre-test, there were no significant differences between the AAT and control groups in the PANSS scores (p = 0.340), DASS (p = 0.659), and CHI (p = 0.659).
PANSS Scores
The change in the total PANSS score of the AAT group revealed a significant improvement compared to control group (p = 0.001). Moreover, the positive, negative, and general psychopathology subscale revealed a significant between-group difference (p < 0.001). The AAT group had less psychotic symptoms after the intervention than the control group. The change score in negative subscale presented a large effect size (SRD = 0.5), and the change score for total score, positive subscale, and general psychopathology subscale revealed a small effect size (SRD = 0.15–0.25).
DASS Scores
The change scores of stress subscale in DASS revealed a significant between-group difference (p = 0.012), subjects in AAT group had less stress after the intervention compared to the control group. The change score for stress subscale revealed a small effect size (SRD = 0.15). Moreover, a decreasing trend in anxiety and depression were observed in the AAT group.
CHI Scores
There were no statistically significant differences in the change score of CHI between the AAT and control groups (p = 0.461). However, there was an increasing trend of change scores in the AAT group, with higher well-being in the posttest (see Table 2).
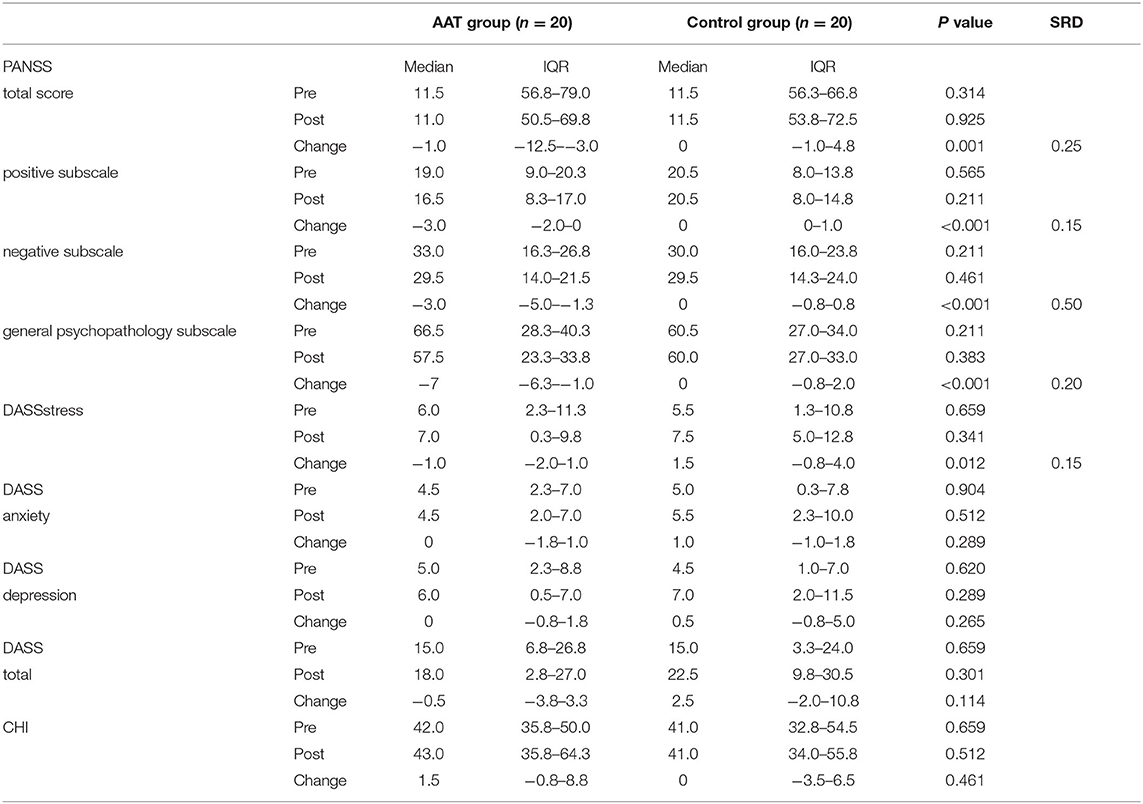
Table 2. Median and interquartile range of PANSS, DASS, and CHI change scores between pre-test and post-test for the AAT group and control group.
Discussion
Compared to the control group, the AAT group showed more significant improvements in negative symptoms, with a large effect size between the two groups. A previous study reported that participants given AAT showed a greater improvement in hedonic tone than the controls, with no significant effect on avolition. However, there was a trend toward improvement in avolition (25). Other studies demonstrated that participants received AAT showed significant improvements in negative symptoms after the intervention, but there were no significant differences than control groups (20, 23). It might be possibly due to the small sample size in previous studies (21 and 24, respectively) (20, 23). Besides, no study assessed the effect size between groups, and only one study has found a large effect size within the AAT group (20). Our study recruited relatively larger sample size, and the results revealed that AAT may improve the negative symptom of patients with schizophrenia.
Improvements in negative symptoms for the AAT group may be underpinned by three core mechanisms. First, therapy dogs acted as social catalysts or mediators to increase social interactions with therapist and patients (20, 46). Therapy dogs have been shown to increase verbal interactions (47), initiation, and participation in longer conversations in the older adults (48). Therefore, AAT improved negative symptoms including poor rapport, lack of spontaneity, and flow of conversation. Second, therapy dogs provided companionship and emotional support in the context of the AAT activities (49), leading to improvements in the apathetic social and emotional withdrawal, and blunted affect. Third, animals are known to help people release oxytocin (31, 32, 50), thereby reducing negative symptoms for patients with schizophrenia (51, 52). However, current evidence remains insufficient and these mechanisms warrant further investigation.
The other core finding was that of the significant effects on positive symptoms and general psychopathology with small effect size between the two groups. A previous study has revealed similar results and supports our finding (53). However, another study presented different results, wherein AAT showed no significant improvements in positive symptom and general psychopathology compared to controls (20, 23). As such, compared to previous results (20, 23), in our study, the AAT group improved more substantially in positive symptoms and general psychopathology. This may be due to our therapist ensured that participants felt well-oriented and that the sessions were realistic during the AAT sessions, clarifying participant conversations. This was not clearly mentioned in previous work. Thus, our study showed that AAT has more significant effects on positive symptoms (such as hallucinations, and conceptual disorganization). In addition, our activities included exercise in AAT, possibly helping to improve general psychiatric symptoms (such as tension, posturing, motor retardation, and impulse control).
Furthermore, as number of samples in past studies was small (20, 23), it remains difficult to determine the effects on positive and general psychopathology symptoms. The benefit of AAT on general positive symptoms and the psychopathology of schizophrenia is worth further investigating. Finally, our study provides initial evidence that AAT may slightly improve positive and general psychopathology symptoms of patients with schizophrenia.
Our findings revealed a significant decrease of stress in the AAT group than in the control group, alongside a small effect size between the two groups. A previous study demonstrated that their AAT group revealed a significant decrease in cortisol, considered as a decrease in stress, but mentioned no comparison between groups (20). Other AAT studies demonstrated stress reduction in patients with post-traumatic stress disorder (54, 55) and dementia (56). Reduced stress-related hormonal responses in patients after AAT may be explained by three core mechanisms. First, the relaxing human–animal bond acted via the adrenal gland and other corticosteroids, the release of oxytocin, dopamine, and endorphins, which may reduce arterial pressure and cardiorespiratory rates, thus leading to decrease stress (20, 29). Second, our AAT session incorporated singing, massaging, artistic creation, and exercises which may help patients reduce stress, as confirmed by previous studies (57–60). As such, our study provided initial evidence that AAT may slightly decrease stress in patients with schizophrenia.
Our study revealed no significant difference between the two groups, although there was a trend toward decreased anxiety and depression in the AAT group. Some previous studies demonstrated similar results. In these studies, reduced anxiety was twice as great (34) and there was a significant decrease in depression in patients with schizophrenia after AAT (29), but no significant difference between the two groups (29, 34), consistent with our finding. However, another study found that neither the AAT nor the control group showed significant improvements in anxiety and depression (20). This study provided limited information regarding anxiety and depression scores because some patients could not fully understand all items' meanings (20). Therefore, it is difficult to compare the difference in results to ours.
Our study revealed that AAT had limited effectiveness in anxiety and depression, possibly because the pre-test levels of depression and anxiety subscales in our participants were mild to normal (61). As such, they had less depression and anxiety, meaning that the AAT had limited effectiveness. In the future, schizophrenic patients with moderate to extremely severe depression and anxiety can be recruited to further assess the effectiveness of AAT on depression and anxiety.
An increasing trend in well-being could be observed for the AAT group, but there was no significant difference between the two groups. Our study is the first to present the effects on well-being among patients with schizophrenia. A previous study showed that participants with mental illness reported the experience as enjoyable and interesting at the end of the AAT (29), partially supporting our findings. A few studies showed indirect evidence that participants with schizophrenia and dementia experienced a better quality of life after the AAT (19, 20, 62), which may further improve their sense of well-being (63). One reason for the improvement in their well-being may be that in company of dogs, participants experienced more love and support (64, 65), sharing feeling with the dogs. However, the effect and mechanism of AAT on well-being warrant further investigation.
There are three strengths to our study. First, our study used the trans-disciplinary approach enlisting both of animal-assisted therapist and occupational therapist, based on their unique knowledge and skills, together determine the therapy that would most benefit patients. Second, the AAT activity was structured and diversified, including activity for positive emotions, social activity, cognitive activity, and physical activity to achieve therapeutic goals. Lastly, there had been few controlled studies of AAT in the community other than in a hospital setting, and in our study no participants dropped out, thereby ensuring the feasibility and high adherence to the AAT for hospitalized psychiatric patients.
There are also a number of limitations that should be mentioned. Owing to the nature of the intervention, it was not possible to blind the participants and therapist to the allocations. Moreover, our study presented only short-term effects; and future research could extend the time of follow-up to ensure the long-term effects of AAT. Finally, we suggest using larger sample sizes and collecting biomarkers in further studies to minimize research bias.
Conclusion
Animal assisted therapy can be effective at reducing of psychopathology symptoms and stress in middle-age and older adults with schizophrenia, particularly improving negative symptoms. In addition, there may also be improvements in anxiety, depress and well-being. Therefore, AAT represents a potential adjunct therapy for patients with schizophrenia. However, future higher quality research is required in order to better understand the mechanisms underpinning AAT and studies assessing biomarkers are needed.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Chang Gung Medical Foundation Institutional Review Board. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
T-TC, C-FH, and C-RC conceived, designed, and conducted this study. T-TC, M-LC, and C-RC conducted intervention. W-TT and C-FH contributed in the statistical analysis and interpretation. T-TC, T-LH, and C-RC drafted the manuscript. All authors approved this manuscript.
Funding
This trial was supported by grant from the Kaohsiung Chang Gung Memorial Hospital Research Grant (CORPG8K0081) to C-RC, T-TC, and C-FH. The funders of the study bore no role in design and conduct of the study, nor in data collection, data analysis and interpretation of the data. The founders had no role in preparation, review, or approval of the manuscript. There was no industry support.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank to the patients for participating. We thank Shu-Fang Tsai, Wen-Chun Mao from the Professional Animal-Assisted Therapy Association of Taiwan and Jeng-Yen Wu from department of nursing of Kaohsiung Chang Gung Memorial Hospital for supporting this study.
References
1. Tandon R, Gaebel W, Barch DM, Bustillo J, Gur RE, Heckers S, et al. Definition and description of schizophrenia in the DSM-5. Schizophr Res. (2013) 150:3–10. doi: 10.1016/j.schres.2013.05.028
2. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. (1987) 13:261–76. doi: 10.1093/schbul/13.2.261
3. Kay SR, Opler LA, Lindenmayer JP. Reliability and validity of the positive and negative syndrome scale for schizophrenics. Psychiatry Res. (1988) 23:99–110. doi: 10.1016/0165-1781(88)90038-8
4. Müller MJ, Wetzel H. Improvement of inter-rater reliability of PANSS items and subscales by a standardized rater training. Acta Psychiatr Scand. (1998) 98:135–9. doi: 10.1111/j.1600-0447.1998.tb10055.x
5. Leucht S, Davis JM. Schizophrenia, primary negative symptoms, and soft outcomes in psychiatry. Lancet. (2017) 389:1077–8. doi: 10.1016/S0140-6736(17)30181-2
6. Akira S, Solomon HS. Schizophrenia: diverse approaches to a complex disease. Science. (2002) 296:692–5. doi: 10.1126/science.1070532
7. Smart EL, Brown L, Palmier-Claus J, Raphael J, Berry K. A systematic review of the effects of psychosocial interventions on social functioning for middle-aged and older-aged adults with severe mental illness. Int J Geriatr Psychiatry. (2020) 35:449–62. doi: 10.1002/gps.5264
8. Cohen CI, Vahia I, Reyes P, Diwan S, Bankole AO, Palekar N, et al. Focus on geriatric psychiatry: schizophrenia in later life: clinical symptoms and social well-being. Psychiatr Serv. (2008) 59:232–4. doi: 10.1176/ps.2008.59.3.232
9. Elbaz-Haddad M, Savaya R. Effectiveness of a psychosocial intervention model for persons with chronic psychiatric disorders in long-term hospitalization. Eval Rev. (2011) 35:379–98. doi: 10.1177/0193841X11406080
10. Nakamura R, Asami T, Yoshimi A, Kato D, Fujita E, Takaishi M, et al. Clinical and brain structural effects of the Illness Management and Recovery program in middle-aged and older patients with schizophrenia. Psychiatry Clin Neurosci. (2019) 73:731–7. doi: 10.1111/pcn.12919
11. Labbate LA, Fava M, Rosenbaum JF, Arana GW editors. Handbook of psychiatric drug therapy, 6th edn. Philadelphia: Lippincott Williams and Wilkins. (2010)
12. Davidson M, Harvey PD, Powchik P, Parrella M, White L, Knobler HY, et al. Severity of symptoms in chronically institutionalized geriatric schizophrenic patients. Am J Psychiatry. (1995) 152:197–207. doi: 10.1176/ajp.152.2.197
13. Owen MJ, Sawa A, Mortensen PB. Schizophrenia. Lancet. (2016) 388:86–97. doi: 10.1016/S0140-6736(15)01121-6
14. Nasrallah H, Tandon R, Keshavan M. Beyond the facts in schizophrenia: closing the gaps in diagnosis, pathophysiology, and treatment. Epidemiol Psychiatr Sci. (2011) 20:317–27. doi: 10.1017/S204579601100062X
15. Girdler SJ, Confino JE, Woesner ME. Exercise as a Treatment for Schizophrenia: A Review. Psychopharmacol Bull. (2019) 49:56–69.
16. Palmer BW, Heaton SC, Jeste DV. Older Patients With Schizophrenia: Challenges in the Coming Decades. Psychiatr Serv. (1999) 50:1178–83. doi: 10.1176/ps.50.9.1178
17. Faulkner G, Cohn T, Remington G, Irving H. Body mass index, waist circumference and quality of life in individuals with schizophrenia. Schizophr Res. (2006) 90:174–8. doi: 10.1016/j.schres.2006.10.009
18. Howes OH, Kaar SJ. Antipsychotic drugs: challenges and future directions. World psychiatry. (2018) 17:170–1. doi: 10.1002/wps.20522
19. Barak Y, Savorai O, Mavashev S, Beni A. Animal-assisted therapy for elderly schizophrenic patients: a one-year controlled trial. Am J Geriatr Psychiatry. (2001) 9:439–42. doi: 10.1097/00019442-200111000-00013
20. Calvo P, Fortuny JR, Guzmán S, Macías C, Bowen J, García ML, et al. Animal assisted therapy (AAT) program as a useful adjunct to conventional psychosocial rehabilitation for patients with schizophrenia: results of a small-scale randomized controlled trial. Front Psychol. (2016) 7:631. doi: 10.3389/fpsyg.2016.00631
21. Hawkins EL, Hawkins RD, Dennis M, Williams JM, Lawrie SM. Animal-assisted therapy for schizophrenia and related disorders: A systematic review. J Psychiatr Res. (2019) 115:51–60. doi: 10.1016/j.jpsychires.2019.05.013
22. Nimer J, Lundahl B. Animal-assisted therapy: A meta-analysis. Anthrozoös. (2007) 20:225–38. doi.org/10.2752/089279307X224773 doi: 10.2752/089279307X224773
23. Villalta-Gil V, Roca M, Gonzalez N, Domenec E, Cuca, Escanilla A, et al. Dog-assisted therapy in the treatment of chronic schizophrenia inpatients. Anthrozoös. (2009) 22:149–59. doi.org/10.2752/175303709X434176 doi: 10.2752/175303709X434176
24. Kovacs Z, Kis R, Rozsa S, Rozsa L. Animal-assisted therapy for middle-aged schizophrenic patients living in a social institution. A pilot study Clin Rehabil. (2004) 18:483–6. doi: 10.1191/0269215504cr765oa
25. Nathans-Barel I, Feldman P, Berger B, Modai I, Silver H. Animal-assisted therapy ameliorates anhedonia in schizophrenia patients. A controlled pilot study Psychother Psychosom. (2005) 74:31–5. doi: 10.1159/000082024
26. McCabe BW, Baun MM, Speich D, Agrawal S. resident dog in the alzheimer's special care unit. West J Nurs Res. (2002) 24:684–96. doi: 10.1177/019394502320555421
27. Richeson NE. Effects of animal-assisted therapy on agitated behaviors and social interactions of older adults with dementia. Am J Alzheimers Dis Other Demen. (2003) 18:353–8. doi: 10.1177/153331750301800610
28. Kramer SC, Friedmann E, Bernstein PL. Comparison of the effect of human interaction, animal-assisted therapy, and AIBO-assisted therapy on long-term care residents with dementia. Anthrozoös. (2009) 22:43–57. doi: 10.2752/175303708X390464
29. Moretti F, De Ronchi D, Bernabei V, Marchetti L, Ferrari B, Forlani C, et al. Pet therapy in elderly patients with mental illness. Psychogeriatrics. (2011) 11:125–9. doi: 10.1111/j.1479-8301.2010.00329.x
30. Trujillo KC, Kuo GT, Hull ML, Ingram AE, Thurstone CC. Engaging Adolescents: Animal Assisted Therapy for Adolescents with Psychiatric and Substance Use Disorders. J Child Fam Stud. (2020) 29:307–14. doi: 10.1007/s10826-019-01590-7
31. Pedersen I, Nordaunet T, Martinsen EW, Berget B, Braastad BO. Farm animal-assisted intervention: relationship between work and contact with farm animals and change in depression, anxiety, and self-efficacy among persons with clinical depression. Issues Ment Health Nurs. (2011) 32:493–500. doi: 10.3109/01612840.2011.566982
32. Odendaal JS, Meintjes RA. Neurophysiological correlates of affiliative behavior between humans and dogs. Vet J. (2003) 165:296–301. doi: 10.1016/S1090-0233(02)00237-X
33. Wilson CC, Netting FE. Companion animals and the elderly: a state-of-the-art summary. J Am Vet Med Assoc. (1983) 183:1425–9.
34. Barker SB, Dawson KS. The effects of animal-assisted therapy on anxiety ratings of hospitalized psychiatric patients. Psychiatr Serv. (1998) 49:797–801. doi: 10.1176/ps.49.6.797
35. Opler MGA, Yavorsky C, Daniel DG. Positive and negative syndrome scale (panss) training: challenges, solutions, and future directions. Innov Clin Neurosci. (2017) 14:77–81.
36. Cheng J. Positive and Negative Syndrome Scale (PANSS): establishment and reliability study of a Mandarin Chinese language version. Chin Psychiatry. (1996) 10:251–8.
37. McMichael AJ, Rolison JJ, Boeri M, Kane JPM, O'Neill FA, Kee F. How do psychiatrists apply the minimum clinically important difference to assess patient responses to treatment? MDM Policy Pract. (2016) 1:2381468316678855. doi: 10.1177/2381468316678855
38. Lu L, Shih JB. Personality and happiness: Is mental health a mediator? Pers Individ Dif . (1997) 22:249–56. doi.org/10.1016/S0191-8869(96)00187-0 doi: 10.1016/S0191-8869(96)00187-0
39. Lu L, Lin YY. Family roles and happiness in adulthood. Pers Individ Dif . (1998) 25:195–207. doi: 10.1016/S0191-8869(98)00009-9
40. Chiang H-H, Lin L, Lee T. Psychometric integrity of the Chinese Happiness Inventory among retired older people in Taiwan. Geriatr Gerontol Int. (2015) 16:865–72. doi: 10.1111/ggi.12568
41. Lu L, Gilmour R, Kao SF, Weng TH, Hu CH, Chern JG, et al. Two ways to achieve happiness: When the East meets the West. Pers Individ Dif . (2001) 30:1161–74. doi: 10.1016/S0191-8869(00)00100-8
42. Antony MM, Bieling PJ, Cox BJ, Enns MW, Swinson RP. Psychometric properties of the 42-item and 21-item versions of the Depression Anxiety Stress Scales in clinical groups and a community sample. Psychol Assessment. (1998) 10:176–81. doi: 10.1037/1040-3590.10.2.176
43. Silva HAd, Passos MHPD, Oliveira VMAd, Palmeira AC, Pitangui ACR, Araújo RC. Short version of the Depression Anxiety Stress Scale-21: is it valid for Brazilian adolescents? Einstein (São Paulo). (2016) 14:486–93. doi: 10.1590/s1679-45082016ao3732
44. Cliff N. Dominance statistics: ordinal analyses to answer ordinal questions. Psychol Bull. (1993) 114:494–509. doi: 10.1037/0033-2909.114.3.494
45. Kraemer HC, Kupfer DJ. Size of treatment effects and their importance to clinical research and practice. Biol Psychiatry. (2006) 59:990–6. doi: 10.1016/j.biopsych.2005.09.014
46. McNicholas J, Collis G. “Animals as social supports: Insights for understanding animal-assisted therapy.” In: A. H. Fine, editors. Handbook on animal-assisted therapy. New York, NY: Academic Press. (2006) p.49–71.
47. Fick KM. The influence of an animal on social interactions of nursing home residents in a group setting. Am J Occup Ther. (1993) 47:529–34. doi: 10.5014/ajot.47.6.529
48. Bernstein PL, Friedmann E, Malaspina A. Animal-assisted therapy enhances resident social interaction and initiation in long-term care facilities. Anthrozoös. (2000) 13:213–24. doi: 10.2752/089279300786999743
49. Lai NM, Chang SMW, Ng SS, Tan SL, Chaiyakunapruk N, Stanaway F. Animal-assisted therapy for dementia. Cochrane Database Syst Rev. (2019):CD013243. doi: 10.1002/14651858.CD013243
50. Mitsui S, Yamamoto M, Nagasawa M, Mogi K, Kikusui T, Ohtani N, et al. Urinary oxytocin as a noninvasive biomarker of positive emotion in dogs. Horm Behav. (2011) 60:239–43. doi: 10.1016/j.yhbeh.2011.05.012
51. Ettinger U, Hurlemann R, Chan RCK. Oxytocin and schizophrenia spectrum disorders. Curr Top Behav Neurosci. (2018) 35:515–27. doi: 10.1007/7854_2017_27
52. Pedersen CA, Gibson CM, Rau SW, Salimi K, Smedley KL, Casey RL, et al. Intranasal oxytocin reduces psychotic symptoms and improves Theory of Mind and social perception in schizophrenia. Schizophr Res. (2011) 132:50–3. doi: 10.1016/j.schres.2011.07.027
53. Chu C-I, Liu C-Y, Sun C-T, Lin J. The effect of animal-assisted activity on inpatients with schizophrenia. J Psychosoc Nurs Ment Health Serv. (2009) 47:42–8. doi: 10.3928/02793695-20091103-96
54. Bergen-Cico D, Smith Y, Wolford K, Gooley C, Hannon K, Woodruff R, et al. Dog ownership and training reduces post-traumatic stress symptoms and increases self-compassion among veterans: results of a longitudinal control study. J Altern Complement Med. (2018) 24:1166–75. doi: 10.1089/acm.2018.0179
55. Krause-Parello CA, Sarni S, Padden E. Military veterans and canine assistance for post-traumatic stress disorder: A narrative review of the literature. Nurse Educ Today. (2016) 47:43–50. doi: 10.1016/j.nedt.2016.04.020
56. Menna LF, Santaniello A, Gerardi F, Sansone M, Di Maggio A, Di Palma A, et al. Efficacy of animal-assisted therapy adapted to reality orientation therapy: measurement of salivary cortisol. Psychogeriatrics. (2019) 19:510–2. doi: 10.1111/psyg.12418
57. Witusik A, Pietras T. Music therapy as a complementary form of therapy for mental disorders. Pol Merkur Lekarski. (2019) 47:240–3.
58. Field T, Hernandez-Reif M, Diego M, Schanberg S, Kuhn C. Cortisol decreases and serotonin and dopamine increase following massage therapy. Int J Neurosci. (2005) 115:1397–413. doi: 10.1080/00207450590956459
59. Sandak B, Huss E, Sarid O, Harel D. Computational Paradigm to Elucidate the Effects of Arts-Based Approaches and Interventions: Individual and Collective Emerging Behaviors in Artwork Construction. PLoS ONE. (2015) 10:e0126467. doi: 10.1371/journal.pone.0126467
60. Firth J, Rosenbaum S, Stubbs B, Gorczynski P, Yung AR, Vancampfort D. Motivating factors and barriers toward exercise in severe mental illness: a systematic review and meta-analysis. Psychol Med. (2016) 46:2869–81. doi: 10.1017/S0033291716001732
61. Tran TD, Tran T, Fisher J. Validation of the depression anxiety stress scales (DASS) 21 as a screening instrument for depression and anxiety in a rural community-based cohort of northern Vietnamese women. BMC Psychiatry. (2013) 13:24. doi: 10.1186/1471-244X-13-24
62. Olsen C, Pedersen I, Bergland A, Enders-Slegers MJ, Patil G, Ihlebaek C. Effect of animal-assisted interventions on depression, agitation and quality of life in nursing home residents suffering from cognitive impairment or dementia: a cluster randomized controlled trial. Int J Geriatr Psychiatry. (2016) 31:1312–21. doi: 10.1002/gps.4436
63. Iinuma T, Arai Y, Takayama M, Takayama M, Abe Y, Osawa Y, et al. Satisfaction with dietary life affects oral health-related quality of life and subjective well-being in very elderly people. J Oral Sci. (2017) 59:207–13. doi: 10.2334/josnusd.16-0414
64. Dell CA, Chalmers D, Gillett J, Rohr B, Nickel C, Campbell L, et al. PAWSing Student Stress: A Pilot Evaluation Study of the St. John Ambulance Therapy Dog Program on Three University Campuses in Canada. Can J Couns Psychotherapy. (2015) 49:332.
Keywords: animal assisted therapy (AAT), aging, schizophrenia, negative symptom, Adjunct therapy
Citation: Chen T-T, Hsieh T-L, Chen M-L, Tseng W-T, Hung C-F and Chen C-R (2021) Animal-Assisted Therapy in Middle-Aged and Older Patients With Schizophrenia: A Randomized Controlled Trial. Front. Psychiatry 12:713623. doi: 10.3389/fpsyt.2021.713623
Received: 23 May 2021; Accepted: 07 July 2021;
Published: 03 August 2021.
Edited by:
Swaran Singh, University of Warwick, United KingdomReviewed by:
Massimo Tusconi, University of Cagliari, ItalyMonika Mak, Pomeranian Medical University, Poland
Copyright © 2021 Chen, Hsieh, Chen, Tseng, Hung and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chyi-Rong Chen, Y2NyNzc2QGNnbWgub3JnLnR3
 Tzu-Ting Chen
Tzu-Ting Chen Ton-Lin Hsieh
Ton-Lin Hsieh Mei-Li Chen
Mei-Li Chen Wei-Ting Tseng
Wei-Ting Tseng Chi-Fa Hung1
Chi-Fa Hung1 Chyi-Rong Chen
Chyi-Rong Chen