- Department of Psychology, College of Arts and Sciences, University of Alabama, Tuscaloosa, AL, United States
Objective: The current study examined the impact of the use of hormonal birth control, cannabis (CB), and alcohol on depression symptoms.
Study Design: Survey data from 3,320 college-aged women collected over a 2-year period. Depression symptoms were assessed using the PHQ-9.
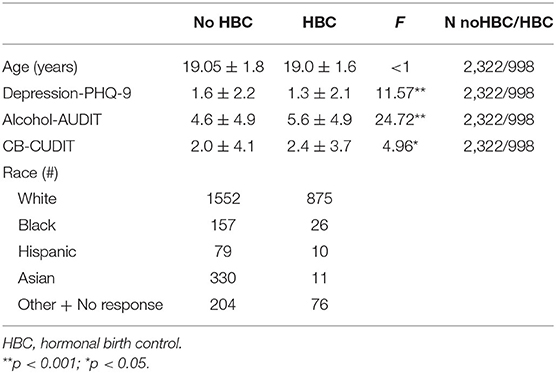
Results: Individuals taking hormonal birth control (N = 998; age = 19.1 ± 1.6 years) had lower overall depression scores than did those not taking birth control (N = 2,322; age = 19.1 ± 1.8 years) with 15.2% of those not taking hormonal birth control had depressive symptoms while 12.1% of those in the birth control group had depressive symptoms. Additionally, those taking hormonal birth control had higher scores on the alcohol and CB use assessment. A between-subjects ANOVA with depression score as the dependent variable found significant effects hormonal birth control use, CB and alcohol use, as well as a significant interaction between CB use and hormonal birth control use.
Conclusions: While there are some limitations (e.g., the between subjects design makes it such that there may be uncontrolled differences between groups), the results suggest that hormonal birth control use may help to reduce depressive symptoms.
Implications: More studies examining the impact of hormonal birth control and substance use on depression are required. The results suggest a potential interaction between CB and hormonal birth control use on depression symptoms that is not observed for alcohol. This implies that alcohol and CB may be linked to depression via different mechanisms.
Highlights
- Alcohol, cannabis and hormonal birth control use all associated with PHQ-9 depression score.
- Hormonal birth control use and cannabis user interact.
- There is no observed interaction between hormonal birth control and alcohol use.
Introduction
The college-aged, emerging adult developmental stage is one of great change that coincides with widespread alterations in synaptic connectivity and white matter myelination, including in the prefrontal cortex (1). The emerging adult developmental stage is one in which individuals may experience their first episode of psychiatric disorders such as depression and anxiety with 30% of college students reporting having depressive symptoms (2). Additionally, emerging adulthood is characterized by an increase in engagement in risk-taking behavior including the use of alcohol and cannabis (CB) (3, 4). The growing research on sex differences has also revealed significant sex differences in both depression prevalence and the effect of CB and alcohol use. In fact, clinical and preclinical studies show that females have greater vulnerability toward drug abuse at all stages of the addiction cycle including drug initiation, binging, withdrawal, and relapse (5, 6). Also, female substance users show an increase in sexual risk-taking (7); and an increased risk of depression and anxiety (8, 9). These sex differences in the effects of substance use and the incidence of depression are both thought to be influenced by sex hormones.
Not only is depression more prevalant in emerging adult women, the lifetime prevalence of depression is significantly higher in women compared to men (10–15). Sex hormones are a likely cause of many cases of depression in women (16, 17). For example, oestrogens have been shown to play a protective role in modulating serotonin which has important implications for mood disorders (18, 19). Furthermore, a subtype of depression, referred to as reproductive depression, occurs during hormonal change including during the premenstrual phase of the menstrual cycle, postnatally, and during the transition to men-pause (17, 20) suggesting a relationship between hormonal fluctuations and depression. It should be noted that even though these later in life hormonal changes have been linked to depression, a 2003 study by Kessler found that sex differences in depression prevalence is due primarily to differences at depression onset during adolescence and emerging adulthood, not to later life experiences demonstrating the importance of examining this emerging adulthood developmental period.
In addition to sex hormones, substance use has also been associated with depression (21) with one-third of people with depression meeting criteria for an alcohol use disorder (22). CB use, particularly heavy use, has also been linked to psychiatric disorders (23, 24). For example, a meta- analysis of longitudinal studies examining the relationship between CB use and depression found a moderate association between heavy CB use (defined as at least weekly use) and increased risk of developing depression (23). There is also an association between alcohol use disorder and depression (25) with some suggestion of a causal link with increasing alcohol use resulting in increased risk of depression (26). Interestingly, relationships between sex hormones and substance use have also been observed. For example, there is evidence of increased alcohol (27) and CB (28) use during the premenstrual phase suggesting a link between substance use, depression and fluctuating sex hormones. There is also a growing literature that demonstrates that estrogens interact with the endocannabinoid system (29–32). Together, these previous studies suggest a potential interaction between circulating sex hormones and substance use that has implications for depressive symptoms.
Given the link between hormonal cycling and depressive symptoms, one hypothesis is that the use of hormonal birth control (HBC) reduces depressive symptoms by interfering with hormonal cycling. The potential neuroprotective properties of progesterone (33, 34) may contribute to the protective factor of HBC. Although the role of progesterone is still debated (35), studies have shown that metabolites of progesterone, particularly allopregnanolone, may be associated with the severity of depressive symptoms (36–38). Recent studies have also reported a link between neuro-inflammation and depression (39, 40). Progesterone has been shown to have anti-inflammatory properties (41). For example, Gallagher et al. (42) found that females have a longer length of recovery after concussion than males despite the same peak symptom severity. Among females, hormonal contraceptive use was associated with lower symptom severity. The explanation for this finding put forward by Wunderle et al. (43) is that women on hormonal contraceptives who have regulated progesterone levels are protected from the sudden drop in progesterone, providing them some protection against the inflammatory response from head injury. Taken together, previous studies motivate the examination of the effect of HBC use on depressive symptoms.
Given that changes in hormones have been associated with depression and substance use, it may be expected that HBC, which disrupts the cycling of sex hormones, will result in reductions in depressive symptoms. However, results from previous studies examining this relationship have been mixed. For example, a study examining the Finnish population-based Health 2000 study data found that the longer the HBC use the lower the risk of major depressive disorder (44) supporting the hypothesis that HBC use reduces depressive symptoms. However, other studies have found the reverse with a study examining the data from the National Prescription Register and the Psychiatric Central Research Register in Denmark reporting HBC use, particularly in adolescents, resulted in increased use of antidepressants (45). The differences in participant demographics (e.g., age, substance use history) may contribute to differences across studies. The goal of this preliminary study is explore the relationship between HBC use, substance use (alcohol and CB) and depressive symptoms in a group of emerging adult women.
Methods
Participants
Three thousand three hundred twenty females completed the series of survey questionnaires, as part of an introductory psychology course at Indiana University for course credit. The study was conducted in a span of 2 years (2015–2017). Inclusion criteria included being enrolled in the introductory psychology course. Conditional exclusion criterion for the final analysis was reporting a non-binary gender identification or identifying as male. Participants gave informed consent as approved by Indiana University's Institutional Review Board for the protection of human subjects.
Measures
A computer-based, online survey with questions regarding current medications including birth control was administered. The following assessments were included in the survey:
Patient Health Questionnaire (PHQ): Depression
The PHQ was employed for the assessment of panic disorder, other anxiety disorders, and depressive disorders (46–48). Depression was assessed using the depression module of the PHQ [PHQ-9; (49)]. Each of the nine PHQ-9 depression items describes one symptom corresponding to one of the nine Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition diagnostic. The scoring protocol used was the one prescribed by the assessment. The score range is zero to 27. There were three classifications: major depressive syndrome, other depressive syndrome, none. For both major and other depressive syndrome, participants had to state that they have “little interest or pleasure in doing things” or “feeling down, depressed, or hopeless” more than half of the days in the past 2 weeks. In addition, for major depressive syndrome an additional five or more questions had to be answered in this manner and for other depressive syndrome 2–4 questions.
Alcohol Use Disorders Identification Test (AUDIT)
The AUDIT (50) is a 10-item screening tool developed by the World Health Organization (WHO) to assess alcohol consumption, drinking behaviors, and alcohol-related problems. The scores range from zero to 40. The scores are divided into three categories—safe, hazardous and dependent. A score of eight or more indicate hazardous drinking and a score of 13 or more in women (15 or more in men) indicate possible alcohol use disorder (dependent).
Cannabis Use Disorders Identification Test-Revised (CUDIT)
The CUDIT is a brief, 8-item screening measure (51). It is a valid measure for the identification of likely cases of DSM-5 cannabis use disorder (CUD) and is a screening tool to identify problematic CB use. The scores range from zero to 32. The scores are divided into three categories—safe, hazardous, and disordered. A score of eight or more indicates hazardous use and 12 or more indicate possible CUD.
Statistical Analysis
A between subjects ANOVA with PHQ-9 score as the dependent variable with birth control use, CUDIT category, AUDIT category and was performed using SAS version 9.4. Planned t-test were performed to examine simple effects using the Satterwaite approximation. Participants with missing data cells were removed for a given analysis.
Results
Demographics
The 998 participants who reported using some form of hormonal birth control used a variety of oral contraceptives including progestin only and combination estrogen/progestin pills (see Table 1 for demographic data); 132 of these participants did not know the type or brand of oral contraception they used. In addition, a small percentage used other forms of hormonal birth control −7 used NuvaRing, 11 Depo-Provera shots, and 3 IUDs.
Group Differences
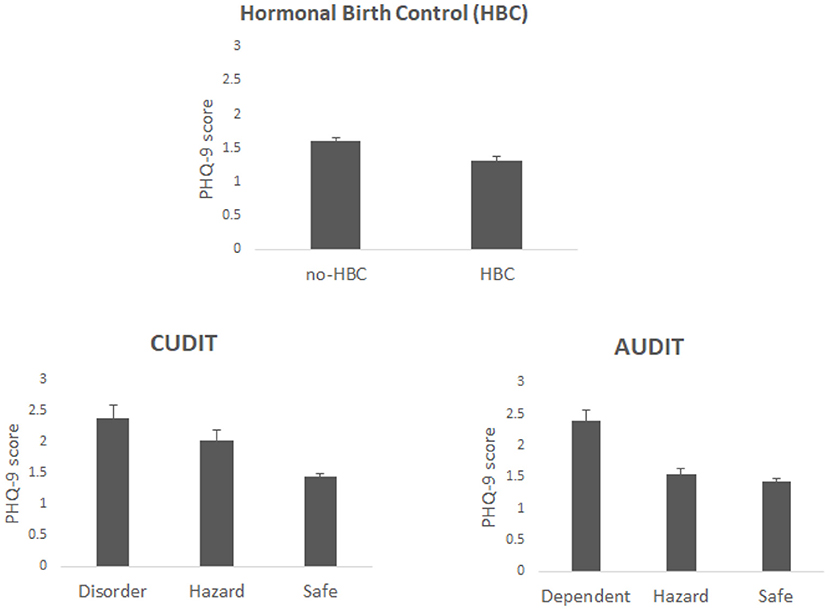
The group demographics are found in Table 1. The results revealed that individuals taking HBC had lower overall PHQ-9 scores than did those not taking HBC [F(1, 3302) = 12.45, p = 0.0004, η2 = 0.0037], see Figure 1. Also, when examining individuals who were classified by the PHQ-9 as having depressive symptoms (i.e., classified with either major or other depressive symptoms), 15.2% (9.9% classified as major depression) of non-HBC users had depressive symptoms while 12.1% (7.6% classified as major depression) of those in the birth control group had depressive symptoms.
PHQ-9 score significantly varied with CUDIT classification with it being lowest for individuals classified as safe (with low scores) and highest for those classified as disordered (highest scores) [F(2, 3302) = 16.97, p <0.001, η2 = 0.01], see Figure 1. A similar finding was observed for AUDIT [F(2, 3302) = 16.31, p < 0.001, η2 = 0.0096]. Additionally, there was an interaction between HBC use and CUDIT [F(2, 3302) = 6.02, p = 0.0025, η2 = 0.0035]. Planned contrasts showed that for the CUDIT safe group PHQ-9 score was higher for non-HBC user than users (t = 4.3, p < 0.0001) but for the hazard group the reverse was found (t = 2.06, p = 0.041) see Figure 2; there was no significant difference for the disordered group (t = 1.09, p = 0.28). The interaction between HBC use and AUDIT was not significant (F <1; planned contrasts: safe—t = 2.66, p = 0.0079; hazard—t = 2.61, p = 0.0094; dependent—t = 1.42, p = 0.16). The 3-way interaction between HBC use, CUDIT and AUDIT was also significant [F(4, 3302) = 3.24, p = 0.012, η2 = 0.0038].
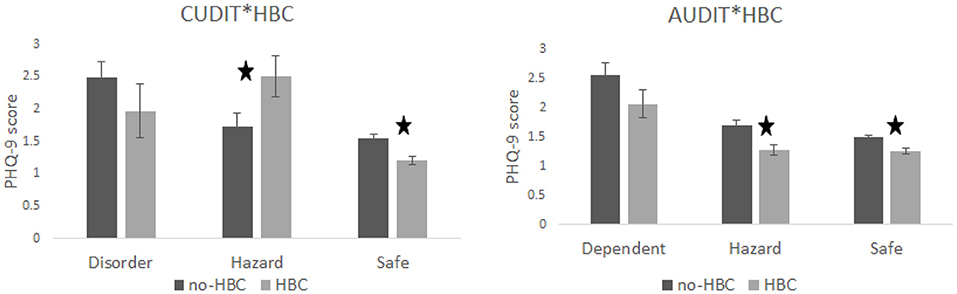
Figure 2. Shows the simple effects. For CUDIT group differences in PHQ-9 scores were observed for the safe and hazard groups with the safe group showing that non-HBC users had higher scores than HBC users. The opposite was true for the CUDIT hazard category, which led to a cross-over interaction. For AUDIT, the safe and hazard group showed significant effects with the non-HBC group having higher PHQ-9 scores than users.
Additionally, HBC users had higher scores on the AUDIT and CUDIT [AUDIT: F(1, 3319) = 25.15, p < 0.0001, η2 = 0.0075; CUDIT: F(1, 3319) = 4.71, p = 0.03; η2 = 0.0014]. In fact, the reported frequency of CB use as indicated on the CUDIT was higher [F(1, 3319) = 21.39, p < 0.0001; η2 = 0.0064] for the HBC user group.
Discussion
The primary goal of this preliminary study was to explore the relationship between HBC use, substance use and depression in emerging adult women. The results do show a relationship such that those who take HBC have lower PHQ-9 scores and fewer individuals with scores categorized as major depressive symptoms than those who do not take HBC. Substance use also varied with PHQ-9 score such that individuals with high PHQ-9 scores also had higher AUDIT and CUDIT scores. However, it appears that only CB use interacted with HBC use. The results indicate that the CUDIT safe group shows that individuals using HBC have lower depression scores than the non-HBC group, the hazard group showed the reverse with the HBC users having higher PHQ-9 scores; there was no significant difference for the disorder group.
The relationship between depression and sex hormones is a complex one. The current study suggests that taking HBC reduces depressive symptoms as measured by the PHQ-9, at least in emerging adult women. Previous studies have found similar effects (44, 52). However, other studies have failed to find such a benefit in the use of HBC (45). One explanation is that individuals who do not tolerate HBC well, likely due to the negative impact on mood, stop its use. Those individuals may have a progesterone intolerance, which may cause these adverse effects on mood (53). Support for this explanation can be found in a recent study in which 16 year olds were found to show an adverse relationship between HBC use and depression; however, this relationship was not found at older ages (54) suggesting that those 16 year olds with adverse reactions stopped taking HBC or adapted to it.
Several studies have shown a relationship between substance use, both alcohol and CB, and depression (21). In fact, recent studies have shown an increase in use of both substances during the premenstrual phase when progesterone is rapidly decreasing (27, 28). This phase of the menstrual cycle has also been linked to increased depressive symptoms (17). Together this suggests a potential relationship between sex hormones, particularly progesterone, depression and substance use. The results presented in the current study provide further support for these previous findings in that alcohol, CB and HBC use all predicted PHQ-9 with increased substance use correlating with increased depressive symptoms. It should be noted that this increase in symptoms with increasing use is in spite of finding that women on HBC have higher AUDIT and CUDIT scores and they have lower PHQ-9 scores.
Interestingly, the relationship between HBC and depression does not appear to be affected by alcohol use. Individuals using HBC had lower PHQ-9 scores regardless of AUDIT category, although for the disordered group the difference was not significant. Progesterone has been found to have neuroprotective properties (33, 34). Recently it has been suggested that progesterone reduces neuroinflammation (55). Alcohol has been shown to cause neuroinflammation (41) and damage to regions related to emotional regulation (56). As a result, the stabilizing effect of progesterone in hormonal birth control may potentially have the side effect of reducing the impact of alcohol use on depression by reducing the inflammatory effect of alcohol. Further research is necessary to test this hypothesis.
The relationship between CB use and HBC was different from that between alcohol use and HBC. Individuals classified as hazard users who also use HBC had significantly higher PHQ-9 scores than hazardous CB users who did not use HBC. The underlying mechanism that accounts for this interaction is unknown. However, CB and alcohol have different mechanisms of action on brain and potentially interact differently with sex hormones. In a clinical trial, progesterone was found to reduce cannabis withdrawal symptoms in individuals with cannabis use disorder (57). Pre-clinical studies have found that estrogens interact with the endocannabinoid system. For example, estradiol regulates cannabinoid receptor density (58), transcription (30) and signal transduction (32). Additionally, the endocannabinoid system is affected by hormonal cycling (29). Also, there are several studies that link depression and CB use; with many individuals with depression, anxiety and psychosis using CB to self-medicate (59, 60). This may be associated with a relationship between regions linked to emotional regulation such as the medial prefrontal cortex and the hippocampus, both of which have a high density of CB1 receptors potentially making them the locus of the relationship between CB and depression. Furthermore, CB has not been found to cause neuroinflammation but instead may help to reduce it (61). Again, the current study suggests that the mechanism of action for CB is different from that of alcohol.
Limitations
The results presented should be interpreted with some caution, as there are limitations to the study. First, it may be difficult to collect sensitive data such as questions about substance use, particularly CB due to it being illegal in many states. However, the online nature of the study was expected to increase compliance. Second, the study is correlational; therefore, no causal inferences can be made. The study is designed to help direct future research and the associations observed have done just that. Third, this is a between subjects design and there could be other, uncontrolled, differences between groups. As mentioned above, individuals who do not tolerate HBC due to its effect on mood may discontinue use. Therefore, our birth control group is likely biased. Also, given that the women on birth control also have higher AUDIT and CUDIT scores, there are likely personality differences between groups that were not controlled. These two possibilities of sources of groups differences are in addition to potential cultural differences (e.g., progressive vs. traditional) which may make some behaviors more socially accepted. Another limitation of the study is a lack of detailed information about a history of contraception use and premenstrual dysphoric disorder both of which may influence results. A final major limitation is that the assessment of depression and substance use was performed using self-report surveys and not in-depth interviews. Therefore, the categorizations used are not clinical ones. Although there are a number of limitations of this preliminary study that make it difficult to determine the mechanism by which HBC may interact with depressive symptoms or substance use, the results do indicate that there is indeed a relationship between these factors. The results also guide future research and strongly support longitudinal, within-subject studies to systematically examine the relationships between substance use, hormonal birth control, and depression.
Conclusions
The current study found that those females taking HBC have lower PHQ-9 scores, fewer depressive symptoms, and have higher AUDIT and CUDIT scores. Additionally, the results replicate previous findings in that alcohol and CB use were both found to be associated with PHQ-9 score. Finally, we report that CB use may interact with HBC differently than alcohol use. Together the results presented provide significant motivation to further explore the relationship between CB use, depression and circulating sex hormones in females.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Indiana University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Funding
This publication was funded by the Indiana Clinical and Translational Sciences Institute, funded in part by grant # UL1TR002529 from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award.
Author Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
I would like to thank Jesse G. Grantz, Kelsie Brooks, and Arianna Gutierrez for the assistance with data collection.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2022.772412/full#supplementary-material
References
1. Lubman DI, Cheetham A, Yücel M. Cannabis and adolescent brain development. Pharmacol Ther. (2015) 148:1–16. doi: 10.1016/j.pharmthera.2014.11.009
2. Ibrahim AK, Kelly SJ, Adams CE, Glazebrook C. A systematic review of studies of depression prevalence in university students. J Psychiatr Res. (2013) 47:391–400. doi: 10.1016/j.jpsychires.2012.11.015
3. Jun HJ, Sacco P, Bright C, Cunningham-Williams RM. Gender differences in the relationship between depression, antisocial behavior, alcohol use, and gambling during emerging adulthood. Int J Ment Health Addict. (2019) 17:1328–39. doi: 10.1007/s11469-018-0048-9
4. Sussman S, Arnett JJ. Emerging adulthood: developmental period facilitative of the addictions. Eval Health Prof. (2014) 37:147–55. doi: 10.1177/0163278714521812
5. Calakos KC, Bhatt S, Foster DW, Cosgrove KP. Mechanisms underlying sex differences in cannabis use. Curr Addict Rep. (2017) 4:439–53. doi: 10.1007/s40429-017-0174-7
6. Anker JJ, Carroll ME. Females are more vulnerable to drug abuse than males: evidence from preclinical studies and the role of ovarian hormones. In: Biological Basis of Sex Differences in Psychopharmacology. Berlin; Heidelberg: Springer. (2010). p. 73–96. doi: 10.1007/7854_2010_93
7. Hallfors DD, Waller MW, Bauer D, Ford CA, Halpern CT. Which comes first in adolescence-sex and drugs or depression? Am J Prevent Med. (2005) 29:163–70. doi: 10.1016/j.amepre.2005.06.002
8. Patton GC, Coffey C, Carlin JB, Degenhardt L, Lynskey M, Hall W. Cannabis use and mental health in young people: cohort study. BMJ. (2002) 325:1195–8. doi: 10.1136/bmj.325.7374.1195
9. Zhu H, Wu LT. Sex Differences in Cannabis Use Disorder Diagnosis Involved Hospitalizations in the United States. J Addict Med. (2017) 11:357. doi: 10.1097/ADM.0000000000000330
11. Piccinelli M, Wilkinson G. Gender differences in depression: critical review. Br J Psychiatr. (2000) 177:486–92. doi: 10.1192/bjp.177.6.486
12. Desai HD, Jann MW. Women's health series major depression in women: a review of the literature. J Am Pharm Assoc. (2000) 40:525–37. doi: 10.1016/S1086-5802(15)30400-9
13. Kuehner C. Gender differences in unipolar depression: an update of epidemiological findings and possible explanations. Acta Psychiatr Scand. (2003) 108:163–74. doi: 10.1034/j.1600-0447.2003.00204.x
14. Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. (2013) 382:1575–86. doi: 10.1016/S0140-6736(13)61611-6
15. Kessler RC. Epidemiology of women and depression. J Affect Disord. (2003) 74:5–13. doi: 10.1016/S0165-0327(02)00426-3
16. Serati M, Redaelli M, Buoli M, Altamura AC. Perinatal major depression biomarkers: a systematic review. J Affect Disord. (2016) 193:391–404. doi: 10.1016/j.jad.2016.01.027
17. Studd JW, Savvas M, Watson N. Reproductive depression and the response to hormone therapy. In: Sex Steroids' Effects on Brain, Heart and Vessels. Cham: Springer (2019). p. 125–33. doi: 10.1007/978-3-030-11355-1_8
18. Chien CP, Cheng TA. Depression in Taiwan: epidemiological survey utilizing CES-D. Seishin shinkeigaku zasshi. (1985) 87:335–8.
19. Lokuge S, Frey BN, Foster JA, Soares CN, Steiner M. Depression in women: windows of vulnerability and new insights into the link between estrogen and serotonin. J Clin Psychiatry. (2011) 72:1563–69. doi: 10.4088/JCP.11com07089
20. Studd J, Nappi RE. Reproductive depression. Gynecol Endocrinol. (2012) 28:42–5. doi: 10.3109/09513590.2012.651932
21. Swendsen JD, Merikangas KR. The comorbidity of depression and substance use disorders. Clin Psychol Rev. (2000) 20:173–89. doi: 10.1016/S0272-7358(99)00026-4
22. Davis L, Uezato A, Newell JM, Frazier E. Major depression and comorbid substance use disorders. Curr Opin Psychiatr. (2008) 21:14–8. doi: 10.1097/YCO.0b013e3282f32408
23. Lev-Ran S, Roerecke M, Le Foll B, George TP, McKenzie K, Rehm J. The association between cannabis use and depression: a systematic review and meta-analysis of longitudinal studies. Psychol Med. (2014) 44:797–810. doi: 10.1017/S0033291713001438
24. Moore TH, Zammit S, Lingford-Hughes A, Barnes TR. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. (2007) 370:319–28. doi: 10.1016/S0140-6736(07)61162-3
25. Grant BF, Harford TC. Comorbidity between DSM-IV alcohol use disorders and major depression: results of a national survey. Drug Alcohol Depend. (1995) 39:197–206. doi: 10.1016/0376-8716(95)01160-4
26. Boden JM, Fergusson DM. Alcohol and depression. Addiction. (2011) 106:906–14. doi: 10.1111/j.1360-0443.2010.03351.x
27. Dozier BL, Stull CA, Baker EJ, Ford MM, Jensen JP, Finn DA, et al. Chronic ethanol drinking increases during the luteal menstrual cycle phase in rhesus monkeys: implication of progesterone and related neurosteroids. Psychopharmacology. (2019) 236:1817–28. doi: 10.1007/s00213-019-5168-9
28. Hanzal N, Joyce KM, Tibbo PG, Stewart SH. A pilot daily diary study of changes in stress and cannabis use quantity across the menstrual cycle. Cannabis. (2019) 2:120–34. doi: 10.26828/cannabis.2019.02.002
29. Bradshaw HB, Rimmerman N, Krey JF, Walker JM. Sex and hormonal cycle differences in rat brain levels of pain-related cannabimimetic lipid mediators. Am J Physiol Regulatory Integr Comparat Physiol. (2006) 291:R349–58. doi: 10.1152/ajpregu.00933.2005
30. Gonzalez S, Bisogno T, Wenger T, Manzanares J, Milone A, Berrendero F, et al. Sex steroid influence on cannabinoid CB1 receptor mRNA and endocannabinoid levels in the anterior pituitary gland. Biochem Biophys Res Commun. (2000) 270:260–6. doi: 10.1006/bbrc.2000.2406
31. Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Arch Intern Med. (1998) 158:1789–95. doi: 10.1001/archinte.158.16.1789
32. Mize AL, Alper RH. Rapid uncoupling of serotonin-1A receptors in rat hippocampus by 17β-estradiol in vitro requires protein kinases A and C. Neuroendocrinology. (2002) 76:339–47. doi: 10.1159/000067583
33. Cai W, Zhu Y, Furuya K, Li Z, Sokabe M, Chen L. Two different molecular mechanisms underlying progesterone neuroprotection against ischemic brain damage. Neuropharmacology. (2008) 55:127–38. doi: 10.1016/j.neuropharm.2008.04.023
34. Stein DG. Progesterone exerts neuroprotective effects after brain injury. Brain Res Rev. (2008) 57:386–97. doi: 10.1016/j.brainresrev.2007.06.012
35. Sundström-Poromaa I, Erika C, Rachael S, Eileen L. Progesterone-friend or foe? Front Neuroendocrinol. (2020) 59:100856. doi: 10.1016/j.yfrne.2020.100856
36. Morgan ML, Rapkin AJ, Biggio G, Serra M, Pisu MG, Rasgon N. Neuroactive steroids after estrogen exposure in depressed postmenopausal women treated with sertraline and asymptomatic postmenopausal women. Arch Women's Mental Health. (2010) 13:91–8. doi: 10.1007/s00737-009-0106-1
37. Miller KK. Neuroactive steroids and depression. In: The Massachusetts General Hospital Guide to Depression. Cham: Humana Press (2019). p. 147–151. doi: 10.1007/978-3-319-97241-1_11
38. Ströhle A, Romeo E, Hermann B, Pasini A, Spalletta G, Di Michele F, et al. Concentrations of 3α-reduced neuroactive steroids and their precursors in plasma of patients with major depression and after clinical recovery. Biol Psychiatry. (1999) 45:274–7. doi: 10.1016/S0006-3223(98)00328-X
39. Woelfer M, Kasties V, Kahlfuss S, Walter M. The role of depressive subtypes within the neuroinflammation hypothesis of major depressive disorder. Neuroscience. (2019) 403:93–110. doi: 10.1016/j.neuroscience.2018.03.034
40. Brites D, Fernandes A. Neuroinflammation and depression: microglia activation, extracellular microvesicles and microRNA dysregulation. Front Cell Neurosci. (2015) 9:476. doi: 10.3389/fncel.2015.00476
41. Alfonso-Loeches S, Pascual-Lucas M, Blanco AM, Sanchez-Vera I, Guerri C. Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage. J Neurosci. (2010) 30:8285–95. doi: 10.1523/JNEUROSCI.0976-10.2010
42. Gallagher V, Kramer N, Abbott K, Alexander J, Breiter H, Herrold A, et al. The effects of sex differences and hormonal contraception on outcomes after collegiate sports-related concussion. J Neurotr. (2018) 35:1242–7. doi: 10.1089/neu.2017.5453
43. Wunderle MK, Hoeger KM, Wasserman ME, Bazarian JJ. Menstrual phase as predictor of outcome after mild traumatic brain injury in women. J Head Trauma Rehabil. (2014) 29:E1. doi: 10.1097/HTR.0000000000000006
44. Toffol E, Heikinheimo O, Koponen P, Luoto R, Partonen T. Hormonal contraception and mental health: results of a population-based study. Hum Reprod. (2011) 26:3085–93. doi: 10.1093/humrep/der269
45. Skovlund CW, Mørch LS, Kessing LV, Lidegaard Ø. Association of hormonal contraception with depression. JAMA Psychiatry. (2016) 73:1154–62. doi: 10.1001/jamapsychiatry.2016.2387
46. Spitzer RL, Kroenke K, Williams JB. Patient Health Questionnaire Primary Care Study Group. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. JAMA. (1999) 282:1737–44. doi: 10.1001/jama.282.18.1737
47. Kroenke K, Spitzer RL. The PHQ-9: a new depression diagnostic and severity measure. Psychiatric Annals. (2002) 32:509–15. doi: 10.3928/0048-5713-20020901-06
48. Spitzer RL, Williams JB, Kroenke K, Hornyak R, McMurray J. Patient Health Questionnaire Obstetrics-Gynecology Study Group. Validity and utility of the PRIME-MD patient health questionnaire in assessment of 3000 obstetric-gynecologic patients: the PRIME-MD Patient Health questionnaire obstetrics-gynecology study. Am J Obstetr Gynecol. (2000) 183:759–69. doi: 10.1067/mob.2000.106580
49. Kroenke K, Spitzer RL, Williams JB, Löwe B. The patient health questionnaire somatic, anxiety, and depressive symptom scales: a systematic review. Gen Hosp Psychiatry. (2010) 32:345–59. doi: 10.1016/j.genhosppsych.2010.03.006
50. Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption–II. Addiction. (1993) 88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x
51. Adamson SJ, Kay-Lambkin FJ, Baker AL, Lewin TJ, Thornton L, Kelly BJ, et al. An improved brief measure of cannabis misuse: the Cannabis Use Disorders Identification Test-Revised (CUDIT-R). Drug Alcohol Depend. (2010) 110:137–43. doi: 10.1016/j.drugalcdep.2010.02.017
52. Keyes KM, Cheslack-Postava K, Westhoff C, Heim CM, Haloossim M, Walsh K, et al. Association of hormonal contraceptive use with reduced levels of depressive symptoms: a national study of sexually active women in the United States. Am J Epidemiol. (2013) 178:1378–88. doi: 10.1093/aje/kwt188
53. Panay N, Studd J. Progestogen intolerance and compliance with hormone replacement therapy in menopausal women. Hum Reprod Update. (1997) 3:159–71. doi: 10.1093/humupd/3.2.159
54. de Wit AE, Booij SH, Giltay EJ, Joffe H, Schoevers RA, Oldehinkel AJ. Association of use of oral contraceptives with depressive symptoms among adolescents and young women. JAMA Psychiatry. (2020) 77:52–59. doi: 10.1001/jamapsychiatry.2019.2838
55. Webster KM, Wright DK, Sun M, Semple BD, Ozturk E, Stein DG, et al. Progesterone treatment reduces neuroinflammation, oxidative stress and brain damage and improves long-term outcomes in a rat model of repeated mild traumatic brain injury. J Neuroinflamm. (2015) 12:238. doi: 10.1186/s12974-015-0457-7
56. Harper C. The neuropathology of alcohol-specific brain damage or does alcohol damage the brain? J Neuropathol Exp Neurol. (1998) 57:101–10. doi: 10.1097/00005072-199802000-00001
57. Sherman BJ, Caruso MA, McRae-Clark AL. Exogenous progesterone for cannabis withdrawal in women: Feasibility trial of a novel multimodal methodology. Pharmacol Biochem Behavior. (2019) 179:22–6. doi: 10.1016/j.pbb.2019.01.008
58. Rodriguez de Fonseca F, Cebeira M, Ramos JA, Martín M, FernándezRuiz JJ. Cannabinoid receptors in rat brain areas: Sexual differences, fluctuations during estrous cycle and changes afer gonadectomy and sex steroid replacement. Life Sci. (1993) 54:159–70. doi: 10.1016/0024-3205(94)00585-0
59. Hazekamp A, Pappas G. Self-medication with cannabis. In: Pertwee RG, editor. Handbook of Cannabis. Oxford: Oxford University Press (2014). p. 319–38. doi: 10.1093/acprof:oso/9780199662685.003.0017
60. Mané A, Fernández-Expósito M, Bergé D, Gómez-Pérez L, Sabaté A, Toll A, et al. Relationship between cannabis and psychosis: Reasons for use and associated clinical variables. Psychiatry Res. (2015) 229:70–4. doi: 10.1016/j.psychres.2015.07.070
Keywords: depression, birth control, alcohol, cannabis, hormones
Citation: Newman SD (2022) Association Between Hormonal Birth Control, Substance Use, and Depression. Front. Psychiatry 13:772412. doi: 10.3389/fpsyt.2022.772412
Received: 10 September 2021; Accepted: 14 January 2022;
Published: 08 February 2022.
Edited by:
Yuan-Pang Wang, University of São Paulo, BrazilReviewed by:
Daniel Stjepanović, The University of Queensland, AustraliaMassimiliano Buoli, University of Milan, Italy
Copyright © 2022 Newman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sharlene D. Newman, c2RuZXdtYW5AdWEuZWR1
 Sharlene D. Newman
Sharlene D. Newman