- 1The Fifth Clinical Medical College, Guangzhou University of Chinese Medicine, Guangzhou, China
- 2Baishui Health Center, Qujing, China
- 3Guangzhou University of Chinese Medicine, Guangzhou, China
- 4Qujing Hospital of Traditional Chinese Medicine, Qujing, China
- 5Guangdong Second Traditional Chinese Medicine Hospital, Guangzhou, China
Background: Rheumatoid arthritis (RA) is a common systemic inflammatory autoimmune disease. The disease has a serious impact on mental health and requires more effective non-pharmacological interventions.
Objective: This study aims to systematically evaluate the effectiveness of patient education on psychological status and clinical outcomes in rheumatoid arthritis.
Methods: This systematic review and meta-analysis was conducted based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. PubMed, Cochrane Library, EMBASE database, and Web of Science database were screened for articles published until November 2, 2021. Randomized controlled trials (RCTs) of patient education for RA were included. Outcomes measures included pain, physical function, disease activity, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), anxiety, depression, Arthritis Self-Efficacy (pain, other symptoms, total), and General health. For each outcome, standardized mean differences or mean differences and 95% confidence intervals (CIs) were calculated.
Results: A total of 24 RCTs (n = 2,276) were included according to the inclusion and exclusion criteria. Meta-analysis revealed a statistically significant overall effect in favor of patient education for physical function [SMD = −0.52, 95% CI (−0.96, −0.08), I2 = 93%, P = 0.02], disease activity [SMD = −1.97, 95% CI (−3.24, −0.71), I2 = 97%, P = 0.002], ASE (pain) [SMD = −1.24, 95% CI (−2.05, −0.43), I2 = 95%, P = 0.003], ASE (other symptoms) [SMD = −0.25, 95% CI (−0.41, −0.09), I2 = 25%, P = 0.002], ASE (total) [SMD = −0.67, 95% CI (−1.30, −0.05), I2 = 90%, P = 0.03], and general health [SMD = −1.11, 95% CI (−1.36, −0.86), I2 = 96%, P < 0.00001]. No effects were found for anxiety [SMD = 0.17, 95% CI (−0.64, 0.98), I2 = 82%, P = 0.68], depression [SMD = −0.18, 95% CI (−0.52, 0.15), I2 = 52%, P = 0.28], pain [SMD = −0.37, 95% CI (−0.80, 0.05), I2 = 89%, P = 0.08], and CRP [SMD = −0.27, 95% CI (−0.57, 0.02), I2 = 0%, P = 0.07].
Conclusions: Patient education may be effective in improving clinical outcomes and psychological status in patients with rheumatoid arthritis. Considering the methodological limitations of the included RCTs, more high-quality and large-sample RCTs are needed to confirm this conclusion in the future.
Systematic Review Registration: http://www.crd.york.ac.uk/prospero, identifier: CRD42021250607.
Introduction
Rheumatoid arthritis (RA) is an autoimmune disease characterized by chronic inflammation, which can cause joint destruction, deformity, pain, and dysfunction (1, 2). This chronic disease has led to a decline in patient's physical function, quality of life, and workability, as well as an increase in medical expenses (3, 4), which brings a heavy burden to individuals and society (5). At present, RA has become one of the main global public health problems, affecting nearly 1% of the world's population (6). RA is clinically incurable, but antirheumatic drugs and biological agents can control symptoms and improve inflammation (7). However, the efficacy of drugs was affected by patients' adherence with medications (8), and oral medications have brought many adverse reactions to patients (9). Studies have found that about 12%-17% of patients have adverse drug events after discharge (10). Therefore, doctors and patients are often looking for more beneficial non-pharmacological interventions. In addition, some studies have found that 30 to 80% of patients with rheumatic and chronic musculoskeletal diseases do not adhere to treatment plans, that poor patient adherence to treatment can affect treatment outcomes, and that poor adherence is associated with reduced functioning and health-related quality of life (11–13). Therefore, strategies to improve patient adherence are critical to improving the effectiveness of clinical interventions (14). Several studies have made recommendations to increase patient adherence, such as patient-centredness, the inclusion of patients in treatment decisions, and patient participation in shared decision-making may be critical factors in improving adherence (13). Some studies suggest that individualized patient education improves patient adherence (15, 16).
Patient education is a low-cost intervention with no side effects, and it has been accepted by patients, family members, and medical workers. At present, the educational intervention has become an effective supplement to traditional medical treatment, which aims to support and help patients with RA to strengthen their life and health management (17). Previous studies have found that educational interventions can increase awareness of patients with RA about the disease and treatment methods, thereby improving their medication adherence (17, 18). Other studies have reported that educational intervention may have a positive effect on the control of disease activity (19, 20). At the same time, educational intervention can improve the health, pain, swollen joint count, tender joint count, and physical function of patients with RA (21). However, other studies have shown that the effect of educational intervention on disease control was not yet clear, the short-term and long-term effects may be inconsistent (22, 23).
According to previously reported studies, the effectiveness of patient education interventions is still controversial. It is necessary to conduct a systematic review and meta-analysis to evaluate its efficacy. Although some previous studies have summarized the effect of patient education on rheumatoid arthritis using a systematic review approach, this study did not perform a meta-analysis (24, 25). Another meta-analysis summarized the impact of patient education on rheumatoid arthritis, reporting outcomes including disability, tender joint count, depression, general health, and psychological status. The study found that patient education had a short-term effect on rheumatoid arthritis and no long-term effects. The literature included in this study was mainly published before 2001, which was published a long time ago (26). In recent years, due to the growing interest of researchers in patient education, many randomized controlled trials of patient education in the treatment of rheumatoid arthritis have been published. It is necessary to recapitulate and update this evidence based on the latest published literature. This meta-analysis aims to review and analyze the effectiveness of patient education in the treatment of RA. Several variables were compared, including pain, physical function, disease activity, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), anxiety, depression, Arthritis Self-Efficacy (pain, other symptoms, total), and general health.
Methods
This study was conducted according to the PRISMA guidelines and the recommendations of the Cochrane Collaboration (27). All analyses were based on previously published studies, and ethical approval was not required in this review. Systematic Review Registration: http://www.crd.york.ac.uk/prospero, identifier: CRD42021250607.
Search Strategy
We searched all clinical studies published in PubMed, Cochrane Library, Embase, and Web of Science database before November 2, 2021. Search terms such as the following were used: “Arthritis, Rheumatoid,” “Rheumatoid Arthritis,” “Education,” “Educational Activities,” “Training Programs,” “Workshops,” “Randomized Controlled Trial,” “Clinical Trial,” “Randomly,” “Randomised,” and “random allocation.” We conducted a detailed search for each database according to the search methods of the different databases. The Supplementary Appendix described the search strategy of each database in detail. Four researchers screened the retrieved documents according to the inclusion criteria and exclusion criteria to read the title, abstract, and full text. The disagreements between the four researchers were discussed with the fifth researcher until a consensus was reached.
Selection Criteria
Patients
This study included patients with RA, and the diagnostic criteria included diagnosis based on doctors (Physician diagnosed), or the diagnostic criteria of the American College of Rheumatology (ACR). There were no restrictions on the patients' age, gender, course of the disease, and where RA occurs.
Interventions
Patient education is defined as a planned and systematic educational activity aimed at improving the health of patients, such as providing disease-related information, health consultation, behavior guidance, behavior modification and advice (24, 28). These methods are aimed at improving the patient's experience of their disease, raising awareness of the disease, promoting the patient's healthy behavior, and improving the patient's ability to deal with the disease. These educational activities can be carried out verbally, in writing, or remotely (such as by telephone). Patient education for patients with rheumatoid arthritis mainly includes providing patients with rheumatoid arthritis with disease-related information, treatment methods, coping strategies for disease symptoms, suggestions and guidance on daily activities, and other activities to improve patients' disease knowledge and health behavior. These educational activities are planned and systematic. We also excluded studies that systematically taught patients to exercise or exercise therapy with the primary goal of exercise or increasing their exercise adherence. However, some suggestions for exercise methods can be used as part of the intervention component of patient education. There were no restrictions on the duration, frequency, and specific methods of educational intervention.
Comparisons
In this study, the types of interventions in the intervention and control group included education vs. usual care, education + usual care vs. usual care, education + conventional treatment vs. conventional treatment, and education vs. waiting list (no intervention). Conventional treatment includes other treatments that patients used before entering the study, such as medications or other routine treatments. No intervention mainly refers to that the control group patients were in the waiting list group and did not receive any intervention before the end of the study, while the same interventions as the intervention group were used after the end of the study.
Outcomes
For inclusion in this review, RCTs had to assess at least one outcome, and the outcome parameters in the respective studies had to be the primary outcomes:
1. Pain. Pain was measured using the visual analog scale (VAS), or Arthritis Impact Measurement Scales 2 (AIMS 2).
2. Physical function. Physical function was measured using the Health Assessment Questionnaire (HAQ), Arthritis Impact Measurement Scales 2 (AIMS2), or the Short-Form Health Survey (SF-36).
3. Disease activity. Disease activity was measured using the disease activity score 28 (DAS-28) or Ritchie Articular Index (RAI).
4. Erythrocyte sedimentation rate (ESR).
5. C-reactive protein (CRP).
6. Anxiety. Anxiety was measured using the Hospital Anxiety and Depression Scale (HADS) or State-Trait Anxiety Inventory (STAI).
7. Depression. Depression was measured using the Hospital Anxiety and Depression Scale (HADS), Beck Depression Inventory (BDI), or Center for Epidemiologic Studies Depression Scale (CES-D).
8. Arthritis Self-Efficacy (pain, other symptoms, total). ASE was measured using the Arthritis Self-Efficacy scale (ASE).
9. General health. General health was measured using the Short-Form Health Survey (SF-36), EuroQol Five Dimensions Questionnaire (EQ5D), or Arthritis Impact Measurement Scales 2 (AIMS2).
Studies Types
This study included only randomized controlled trials (RCTs). Observational studies, non-randomized controlled trials, and reviews were not included. The language of the included studies was limited to English.
Data Extraction and Quality Assessment
Four reviewers independently extracted study data from eligible studies according to a prespecified study protocol, including the characteristics of the researchers (e.g., name and country), patient characteristics (e.g., age, gender, and duration of disease), research characteristics (e.g., study design, publication years, sample size, the frequency and duration of intervention), and study outcomes. When the follow-up time was inconsistent between studies, we chose to include the final follow-up time. The disagreements between the four researchers were discussed with the fifth researcher until a consensus was reached.
Assessment of Risk of Bias in Included Studies
Two reviewers independently used the Cochrane risk-of-bias tool to assess the quality and risk of bias of the included studies, which included the following domains: selection bias (random sequence generation and allocation concealment), performance bias (blinding of participants and personnel), detection bias (blinding of outcome assessment), attrition bias (incomplete outcome data), reporting bias (selective reporting), and other bias (29). The evaluation results were examined by a third reviewer, and the disagreed evaluations were further discussed until a consensus was reached.
Rating Quality of Evidence
The Grading of Recommendations, Assessment, Development and Evaluation (GRADE) system was used to evaluate the quality of evidence for each outcome. The strength of the evidence was categorized as high, moderate, low, or very low. Two reviewers independently used the GRADE system to assess the quality of evidence. The disagreements between the two researchers were discussed with the third researcher until a consensus was reached.
Statistical Analysis
We conducted this meta-analysis of the included literature by Review Manager 5.3 software (Cochrane Collaboration, Oxford, UK) and illustrated the results of data merging intuitively with a forest map. The mean differences (MDs), standard mean differences (SMDs), and 95% confidence intervals (CIs) were calculated by random-effects models or fixed-effects models. The heterogeneity between various studies was statistically analyzed by I2 and chi-square tests. Significant heterogeneity was indicated when I2 ≥ 50% or P < 0.1, and the random-effects models were used. When I2 < 50% or P > 0.1 showed no significant heterogeneity, the fixed-effects models were used. When there was heterogeneity among various studies, a subgroup analysis was conducted according to the type of interventions. We used meta-regression and sensitivity analysis to explore the sources of heterogeneity. In addition, publication bias was assessed using Egger's and Begg's tests (30). P-values < 0.05 were considered statistically significant.
Results
Study Selection
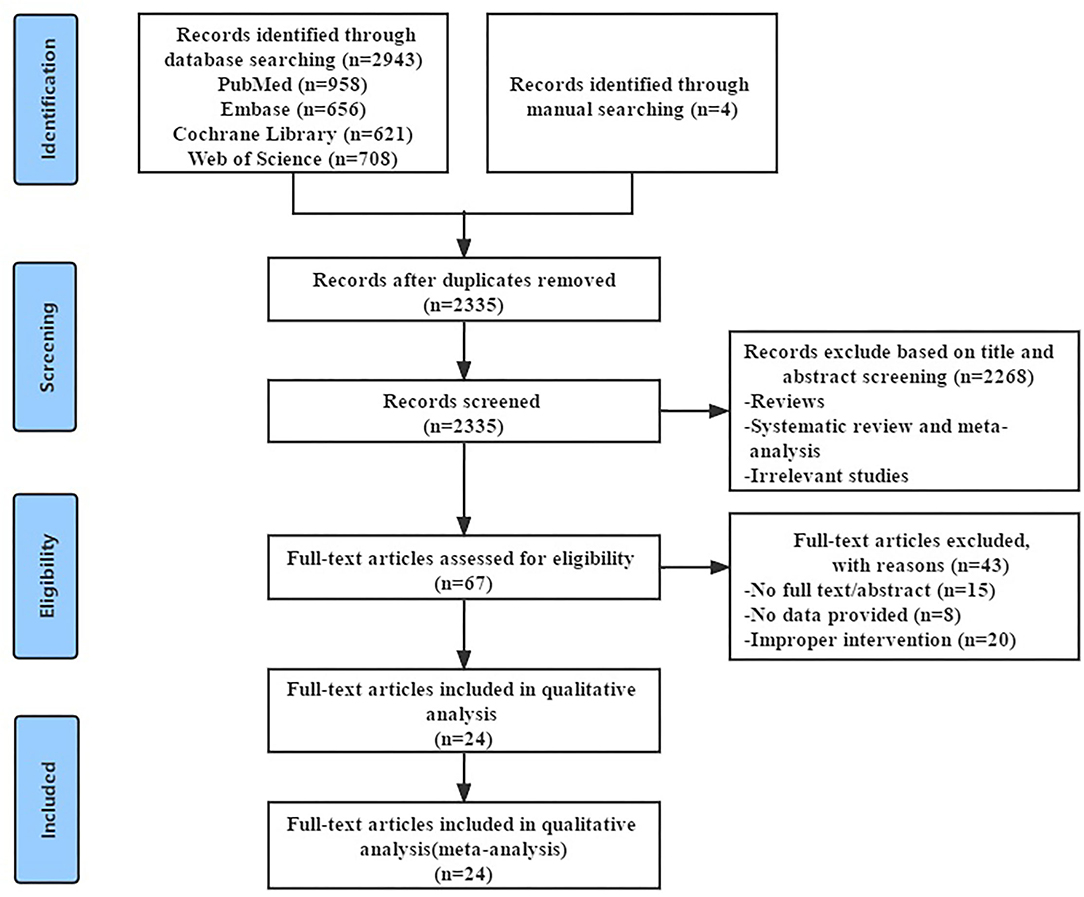
The literature search strategy was detailed in the Supplementary Appendix, and the screening process was summarized in Figure 1. We searched the four English databases (PubMed, Cochrane Library, Embase, and Web of Science database). Initially, we retrieved a total of 2,947 potentially relevant records and excluded 612 duplicate records. After reading the title and abstract, we excluded 2,268 obviously irrelevant records, leaving 67 research need to read the full text for further confirmation. After reading the full text, 43 studies that did not meet the inclusion criteria were excluded, 24 RCTs were retained. Finally, 24 RCTs (31–54) were included, with a total of 2,276 patients with RA.
Study Characteristics
Overview of Included Studies
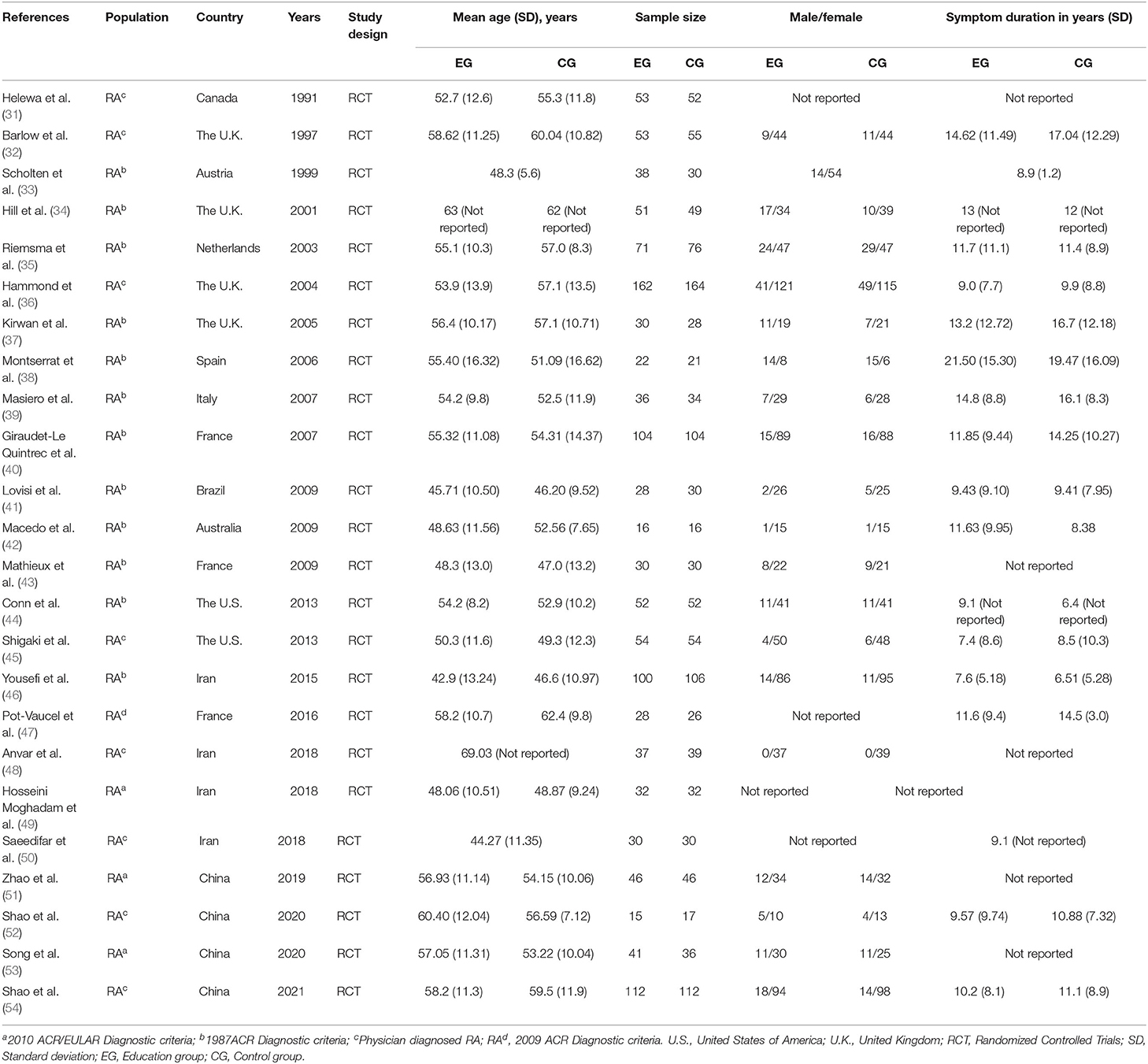
The study characteristics of the 24 RCTs can be observed in Table 1. These studies were published from 1991 to 2021. The 24 RCTs included were conducted in different countries, with two in the United States (44, 45), three in France (40, 43, 47), four in China (51–54), four in United Kingdom (32, 34, 36, 37), four in Iran (46, 48–50), one each in the Canada (31), Austria (33), Netherlands (35) Spain (38), Italy (39), Brazil (41), and Australia (42). Of the 24 studies included, 2,276 patients with RA were analyzed. The number of patients with RA in each study ranged from 32 to 258, with a total of 1,155 patients in the education group and 1,121 in the control group. Twenty-four studies reported the age of patients with RA. Their mean age ranged from 44.27 to 69.03 years. Eighteen studies reported the duration of symptoms, with the mean duration ranged from 7.05 to 20.48 years. Sixteen studies were based on the diagnosis of KOA based on clinical and radiographic features by a physician (33–35, 37–44, 46, 47, 49, 51, 53), while the remaining eight studies were based on the diagnosis criteria of the American College of Rheumatology (31, 32, 36, 45, 48, 50, 52, 54).
Intervention Characteristics and Outcome Measures
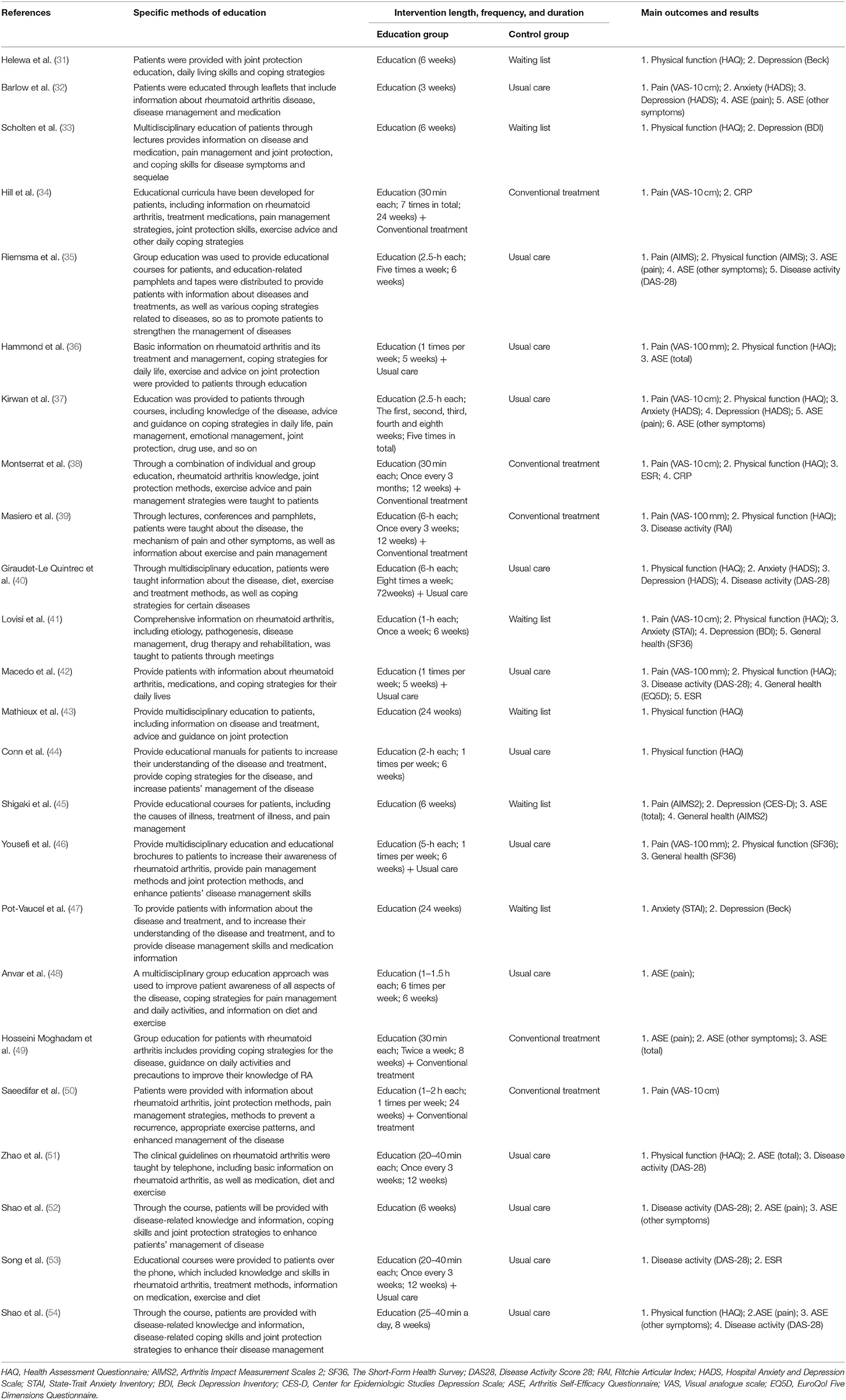
Table 2 shows the characteristics of interventions in these 24 RCTs, including the specific methods of education, duration of intervention, and outcomes. To compare interventions between the education group and the control group, eight studies used education vs. usual care (32, 35, 37, 44, 48, 51, 52, 54), and five studies used education + usual care vs. usual care (36, 40, 42, 46, 53), five studies used education + conventional treatment vs. conventional treatment (34, 38, 39, 49, 50), and six studies used education vs. waiting list (no intervention) (31, 33, 41, 43, 45, 47). The outcomes of these 24 RCTs include pain, physical function, disease activity, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), anxiety, depression, ASE (pain), ASE (other symptoms), ASE(total), and general health. Twelve studies that assessed pain used VAS (10 cm) scores (32, 34, 37, 38, 41, 50),VAS (100 mm) scores (36, 39, 42, 46), and AIMS2 scores (35, 45), respectively. Fifteen studies assessed physical function using HAQ (31, 33, 36–44, 51, 54), SF-36 (46) and AIMS2 (35), respectively. Eight studies assessed disease activity using DAS-28 (35, 40, 42, 51–54) and RAI (39), respectively. Five studies assessed the anxiety using HADS (32, 37, 40) and STAI (41, 47), respectively. Five studies assessed the depression using HADS (32, 37, 40), BDI (31, 33, 41, 47), and CES-D (45), respectively. Ten studies assessed ASE using Arthritis Self-Efficacy Questionnaire (32, 35–37, 45, 48, 49, 51, 52, 54). Four studies assessed the general health using SF-36 (41, 46), AIMS2 (45) and EQ5D (42), respectively.
Risk of Bias
The details of the risk of bias for each study can be observed in Figure 2. Sixteen studies reported specific randomization methods (31, 34, 36–38, 40–42, 44, 46, 50–54), eight RCTs did not specify the specific randomization method (32, 33, 35, 39, 43, 45, 47, 48). The allocation concealment of nine RCTs remained unclear (32, 33, 35, 38, 43, 45, 47, 48, 52). The performance bias of five RCTs was judged to be high (41, 42, 50, 51, 53), and nine RCTs was judged to be unclear (32, 33, 35, 37, 43–45, 47, 48). The detection bias of one RCT was judged to be high (53), and fourteen RCTs remained unclear (32, 33, 35, 37–40, 43–46, 49, 50, 52). Two RCT was judged to be high in incomplete outcome data (attrition bias) (36, 46), and two RCTs remained unclear (32, 48). The other bias of the thirteen studies was unclear (32, 33, 35, 39, 42, 43, 45–48, 50, 52, 53). Among the twenty-four RCTs included, only three RCTs had a low risk of publication bias (31, 34, 54).
Quality of Evidence
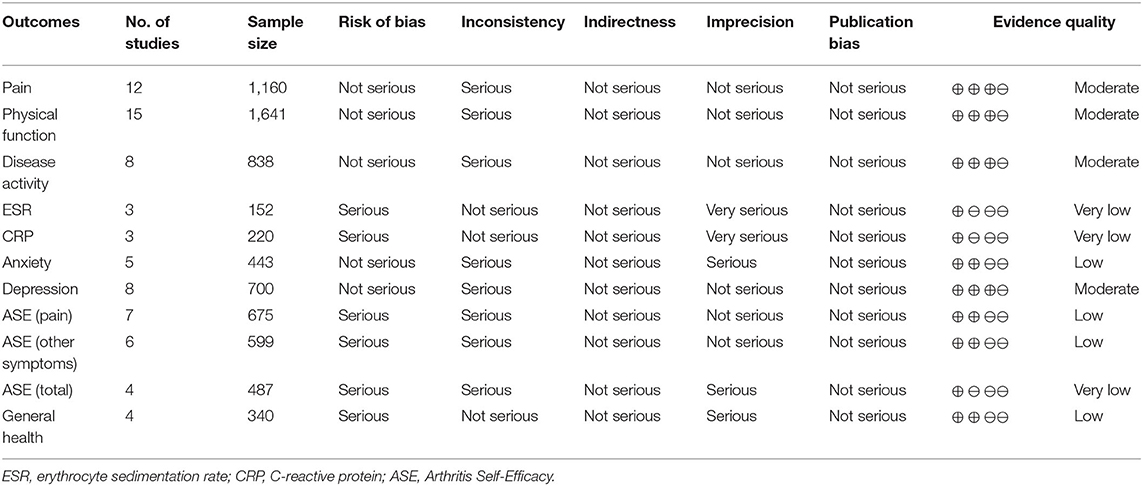
The results of the quality of evidence assessed by the GRADE system can be found in Table 3. The quality of evidence for pain, physical function, disease activity, and depression was classified as moderate. The quality of evidence for anxiety, ASE (pain), ASE (other symptoms), and general health was classified as low. The quality of evidence for ESR, CRP, and ASE (total) was classified as very low.
Assessment of Overall Effect Size
Pain
Twelve studies evaluated pain and included 1,160 participants. Twelve studies that assessed pain used VAS (10 cm) scores (32, 34, 37, 38, 41, 50), VAS (100 mm) scores (36, 39, 42, 46), and AIMS2 scores (35, 45), respectively. The lower the VAS score, and AIMS2 score, the less painful. Of the 12 RCTs, five studies reported that patient education improved pain in patients with RA (P < 0.05) (38, 42, 46, 50), while the other seven studies reported that patient education did not improve pain in patients with RA (P > 0.05) (32, 34–37, 39, 41, 45). Four RCTs was not included in the meta-analysis (35, 38, 41, 42). Eight of the twelve RCTs were included in the meta-analysis (32, 34, 36, 37, 39, 45, 46, 50). The pooled results showed no significant improvement in pain in the education group compared to the control group [SMD = −0.37, 95% CI (−0.80, 0.05), I2 = 89%, P = 0.08]. When education was compared with usual care, the subgroup analysis showed no significant difference in pain improvement in the education group [SMD = 0.07, 95% CI (−0.29, 0.42), I2= 13%, P = 0.71]. Similarly, when education + usual care was compared with usual care alone, subgroup analysis showed no difference in pain improvement in the education group [SMD = −0.78, 95% CI (−2.27, 0.71), I2 = 98%, P = 0.30]. In addition, when education was compared with waiting list (no intervention), subgroup analysis showed no difference in pain improvement in the education group [SMD = −0.08, 95% CI (−0.50, 0.34), P = 0.72]. However, when education + conventional treatment was compared with conventional treatment alone, subgroup analysis showed a significant difference in pain improvement in the education group [SMD = −0.48, 95% CI (−0.82, −0.14), I2 = 40%, P = 0.006] (Figure 3).
Physical Function
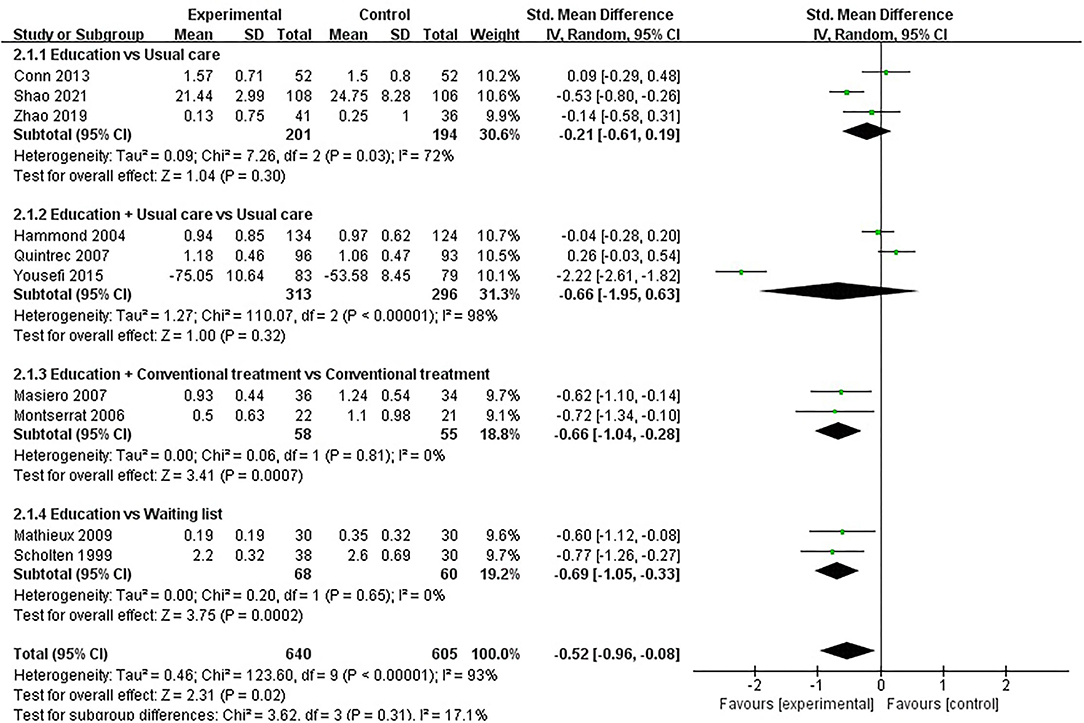
Physical function was assessed in fifteen studies involving 1,641 participants. Fifteen studies assessed physical function using HAQ (31, 33, 36–44, 51, 54), SF-36 (46) and AIMS2 (35), respectively. The lower the HAQ score and AIMS2 score, the better the physical function, while the higher the SF-36 score, the better the physical function. Of the fifteen RCTs, six studies reported that patient education improved physical function in patients with RA (P < 0.05) (33, 38, 39, 43, 46, 54), while the other nine studies reported that patient education did not improve physical function in patients with RA (P > 0.05) (31, 35–37, 40–42, 44, 51). Five RCTs was not included in the meta-analysis (31, 35, 37, 41, 42). Ten of the 15 RCTs were included in the meta-analysis (33, 36, 38–40, 43, 44, 46, 51, 54). Pooled results showed a significant improvement in physical function in the education group compared to the control group [SMD = −0.52, 95% CI (−0.96, −0.08), I2 = 93%, P = 0.02]. When education was compared with usual care, the subgroup analysis showed no significant difference in physical function improvement in the education group [SMD = −0.21, 95% CI (−0.61, 0.19), I2= 72%, P = 0.30]. Similarly, when education + usual care was compared with usual care alone, subgroup analysis showed no difference in physical function improvement in the education group [SMD = −0.66, 95% CI (−1.95, 0.63), I2 = 98%, P = 0.32]. However, when education was compared with waiting list (no intervention), subgroup analysis showed a significant difference in physical function improvement in the education group [SMD = −0.69, 95% CI (−1.05, −0.33), I2 = 0%, P = 0.0002]. In addition, when education + conventional treatment was compared with conventional treatment alone, subgroup analysis showed a significant difference in physical function improvement in the education group [SMD = −0.66, 95% CI (−1.04, −0.28), I2 = 0%, P = 0.0007] (Figure 4).
Disease Activity
Eight studies assessed disease activity, involving a total of 838 participants. Eight studies assessed disease activity using DAS-28 (35, 40, 42, 51–54) and RAI (39), respectively. The lower the DAS-28 score and RAI score, the better the disease activity. Of the eight RCTs, four studies reported that patient education improved disease activity in patients with RA (P < 0.05) (35, 38, 51, 53), while the other four studies reported that patient education did not improve disease activity in patients with RA (P > 0.05) (40, 42, 52, 54). Three RCTs was not included in the meta-analysis (35, 40, 42). Five of the eight RCTs were included in the meta-analysis (39, 51–54). Pooled results showed a significant improvement in disease activity in the education group compared to the control group [SMD = −1.97, 95% CI (−3.24, −0.71), I2 = 97%, P = 0.002]. When education was compared with usual care, subgroup analysis showed that the education group had significantly improved disease activity [SMD = −0.26, 95% CI (−0.48, −0.04), I2 = 0%, P = 0.02]. Similarly, when education + usual care was compared with usual care alone, subgroup analysis showed that the education group had significantly improved disease activity [SMD = −0.52, 95% CI (−0.98, −0.07), P = 0.03]. In addition, when education + conventional treatment was compared with conventional treatment alone, subgroup analysis showed a significant difference in disease activity improvement in the education group [SMD = −12.54, 95% CI (−14.73, −10.35), P < 0.00001] (Figure 5).
ESR
ESR were reported in three RCTs (38, 42, 53) with a total of 152 patients with RA. Three RCTs was not included in the meta-analysis (38, 42, 53). In three RCTs, the comparisons involved (1) education + conventional treatment was compared with conventional treatment alone, and (2) education + usual care was compared with usual care alone. When education + conventional treatment was compared with conventional treatment alone, the results showed that there was difference in improved ESR (P < 0.05). However, when education + usual care was compared with usual care alone, the results showed that there was no difference in improved ESR (P > 0.05).
CRP
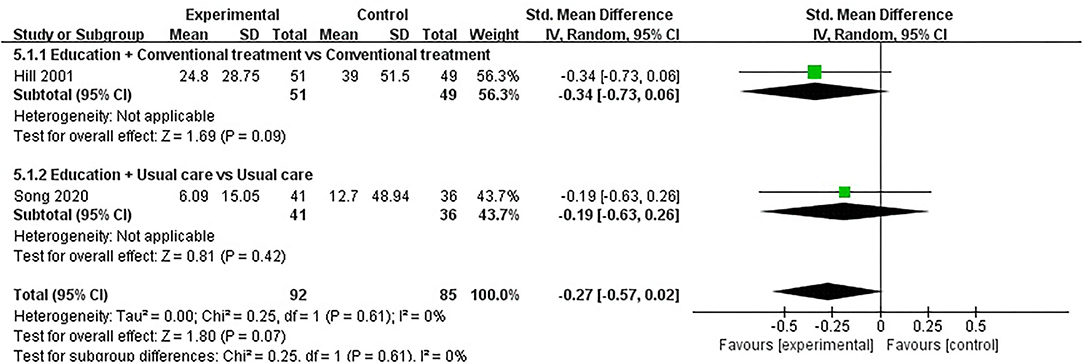
Three studies assessed CRP and included a total of 220 participants. Of the three RCTs, three studies reported that patient education did not improve CRP in patients with RA (P > 0.05) (34, 38, 53). One RCT was not included in the meta-analysis (38). Two of the three RCTs were included in the meta-analysis (34, 53). The pooled results showed no significant improvement in CRP in the education group compared to the control group [SMD = −0.27, 95% CI (−0.57, 0.02), I2 = 0%, P = 0.07]. When education + conventional treatment was compared with conventional treatment alone, the subgroup analysis revealed that the education group showed no significant difference in improved CRP [SMD = −0.34, 95% CI (−0.73, 0.06), P = 0.09]. Similarly, when education was compared with usual care, subgroup analysis also showed no statistical difference in CRP improvement between the education group and usual care [SMD = −0.19, 95% CI (−0.63, 0.26), P = 0.42] (Figure 6).
Anxiety
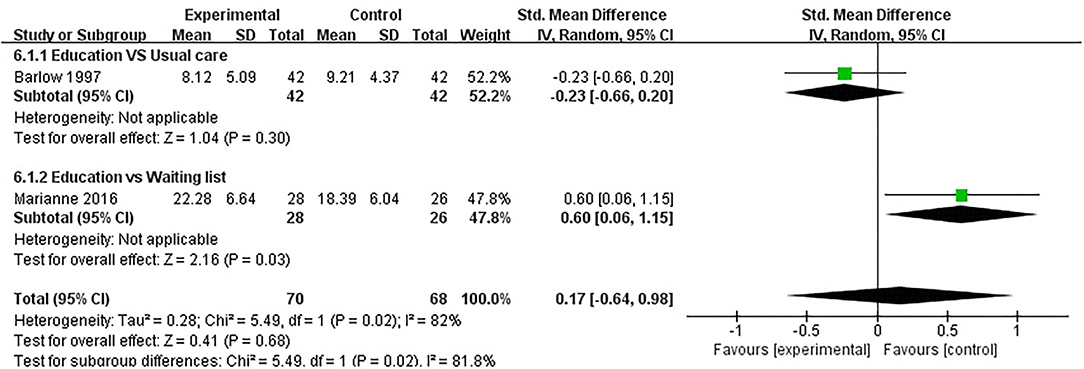
Anxiety was evaluated in five studies, and 443 participants were included. Five studies assessed anxiety using HADS (32, 37, 40) and STAI (41, 47), respectively. The lower the HADS score and STAI score, the better the anxiety. Of the five RCTs, five studies reported that patient education did not improve anxiety in patients with RA (P > 0.05) (32, 37, 40, 41, 47). Three RCTs was not included in the meta-analysis (37, 40, 41). Two of the five RCTs were included in the meta-analysis (32, 47). The pooled results showed no significant improvement in anxiety in the education group compared to the control group [SMD = 0.17, 95% CI (−0.64, 0.98), I2 = 82%, P = 0.68]. When education was compared with usual care, the subgroup analysis showed no significant improvement in the anxiety of the education group [SMD = −0.23, 95% CI (−0.66, 0.20), P = 0.30]. However, when education was compared with waiting list (no intervention), the subgroup analysis revealed that the control group showed a significant difference in improved anxiety [SMD = 0.60, 95% CI (0.06, 1.15), P = 0.03] (Figure 7).
Depression
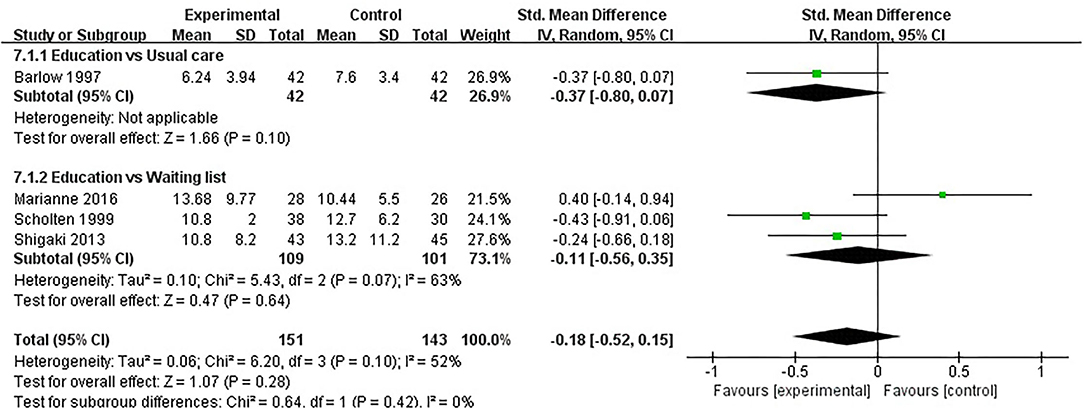
Depression was evaluated in eight studies, and 700 participants were included. Eight studies assessed depression using HADS (32, 37, 40), BDI (31, 33, 41, 47), or CES-D (45), respectively. The lower the HADS score, BDI score and CES-D score, the better the depression. Of the eight RCTs, eight studies reported that patient education did not improve depression in patients with RA (P > 0.05) (31–33, 37, 40, 41, 45, 47). Four RCTs was not included in the meta-analysis (31, 37, 40, 41). Four of the eight RCTs were included in the meta-analysis (32, 33, 45, 47). The pooled results showed no significant improvement in depression in the education group compared to the control group [SMD = −0.18, 95% CI (−0.52, 0.15), I2 = 52%, P = 0.28]. When education was compared with usual care, the subgroup analysis showed no significant improvement in the depression of the education group [SMD = −0.37, 95% CI (−0.80, 0.07), P = 0.10]. In addition, When education was compared with waiting list (no intervention), the subgroup analysis showed that there was no significant difference in improved depression [SMD = −0.11, 95% CI (−0.56, 0.35), I2 = 63%, P = 0.64] (Figure 8).
ASE (Pain)
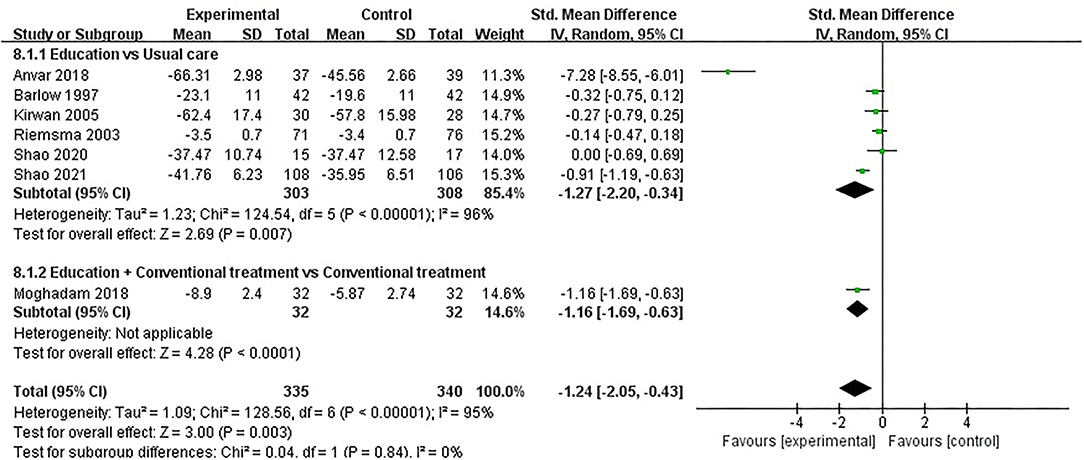
ASE (pain) was evaluated in seven studies involving a total of 675 participants. Seven studies assessed ASE (pain) using the ASE scale (32, 35, 37, 48, 49, 52, 54). Of the seven RCTs, three studies reported that patient education improved ASE (pain) in patients with RA (P < 0.05) (48, 49, 54), while the other four studies reported that patient education did not improve ASE (pain) in patients with RA (P > 0.05) (32, 35, 37, 52). Seven RCTs were included in the meta-analysis (32, 35, 37, 48, 49, 52, 54). Pooled results showed a significant improvement in ASE(pain) in the education group compared to the control group [SMD = −1.24, 95% CI (−2.05, −0.43), I2 = 95%, P = 0.003]. When education was compared with usual care, the subgroup analysis showed a significant improvement in ASE (pain) in the education group [SMD = −1.27, 95% CI (−2.20, −0.34), I2 = 96%, P = 0.007]. Similarly, when education + conventional treatment was compared with conventional treatment alone, subgroup analysis showed a significant improvement in ASE (pain) [SMD = −1.16, 95% CI (−1.69, −0.63), P < 0.0001] (Figure 9).
ASE (Other Symptoms)
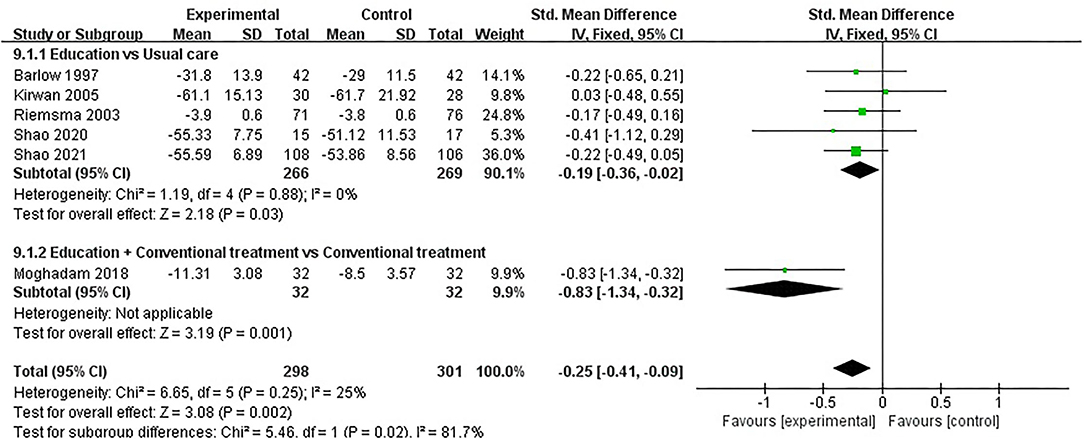
ASE (other symptoms) was evaluated in six studies involving a total of 599 participants. Six studies assessed ASE (other symptoms) using the ASE scale (32, 35, 37, 49, 52, 54). Of the six RCTs, one studies reported that patient education improved ASE (other symptoms) in patients with RA (P < 0.05) (49), while the other five studies reported that patient education did not improve ASE (other symptoms) in patients with RA (P > 0.05) (32, 35, 37, 52, 54). Six RCTs were included in the meta-analysis (32, 35, 37, 49, 52, 54). Pooled results showed a significant improvement in ASE (other symptoms) in the education group compared to the control group [SMD = −0.25, 95% CI (−0.41, −0.09), I2 = 25%, P = 0.002]. When education was compared with usual care, the subgroup analysis showed a significant improvement in ASE (other symptoms) in the education group [SMD = −0.19, 95% CI (−0.36, −0.02), I2 = 0%, P = 0.03]. Similarly, when education + conventional treatment was compared with conventional treatment alone, subgroup analysis showed a significant improvement in ASE (other symptoms) [SMD = −0.83, 95% CI (−1.34, −0.32), P = 0.001] (Figure 10).
ASE (Total)
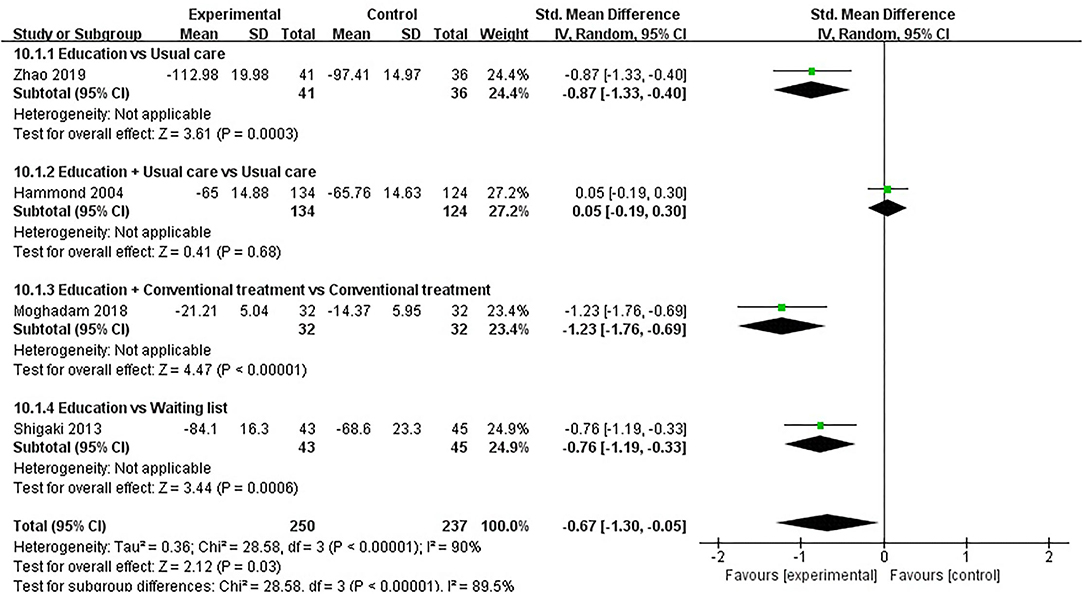
ASE (total) was evaluated in four studies involving a total of 487 participants. Four studies assessed ASE (total) using the ASE scale (36, 45, 49, 51). Of the four RCTs, three studies reported that patient education improved ASE (total) in patients with RA (P < 0.05) (45, 49, 51), while the other study reported that patient education did not improve ASE (total) in patients with RA (P > 0.05) (36). Four RCTs were included in the meta-analysis (36, 45, 49, 51). Pooled results showed a significant improvement in ASE (total) in the education group compared to the control group [SMD = −0.67, 95% CI (−1.30, −0.05), I2 = 90%, P = 0.03]. When education was compared with usual care, the subgroup analysis showed a significant improvement in ASE (total) in the education group [SMD = −0.87, 95% CI (−1.33, −0.40), P = 0.0003]. When education + conventional treatment was compared with conventional treatment alone, the subgroup analysis showed a significant improvement in ASE (total) in the education group [SMD = −1.23, 95% CI (−1.76, −0.69), P < 0.00001]. Similarly, When education was compared waiting list (no intervention), the subgroup analysis showed a significant improvement in ASE (total) in the education group [SMD = −0.76, 95% CI (−1.19, −0.33), P = 0.0006]. However, when education + usual care was compared with usual care alone, the subgroup analysis revealed that the education group showed no significant difference in improved ASE (total) [SMD = −0.05, 95% CI (−0.30, 0.19), P = 0.68] (Figure 11).
General Health
General health was evaluated in four studies involving a total of 340 participants. Four studies assessed general health using SF-36 (41, 46), EQ5D (42), or AIMS2 (45), respectively. The lower the AIMS2 score, the better the general health, and the higher the SF-36 and EQ5D, the better the general health. Of the four RCTs, two studies reported that patient education improved general health in patients with RA (P < 0.05) (42, 46), while the other two studies reported that patient education did not improve general health in patients with RA (P > 0.05) (41, 45). One RCT was not included in the meta-analysis (42). Three of the four RCTs were included in the meta-analysis (41, 45, 46). Pooled results showed a significant improvement in general health in the education group compared to the control group [SMD = −1.11, 95% CI (−1.36, −0.86), I2 = 96%, P < 0.00001]. When education was compared waiting list (no intervention), the subgroup analysis showed a significant improvement in general health in the education group [SMD = −0.37, 95% CI (−0.69, −0.04), I2 = 0%, P = 0.03]. Similarly, when education + usual care was compared with usual care alone, subgroup analysis showed a significant improvement in general health [SMD = −2.17, 95% CI (−2.55, −1.78), P < 0.00001] (Figure 12).
Publication Bias
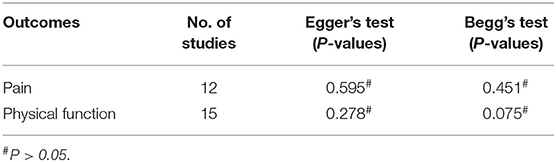
When the meta-analysis includes more than ten studies, the possibility of publication bias should be reported (55). We used the Egger's and Begg's test to assess the publication bias of each outcome (30). The evaluation results showed that there was no publication bias in the outcomes (Table 4).
Meta-Regression Analysis and Sensitivity Analysis
In this study, we evaluated a total of 10 outcomes. The outcomes with high heterogeneity were pain, physical function, disease activity, anxiety, depression, ASE (pain), ASE (total), and general health. We used meta-regression analysis and sensitivity analysis to explore the sources of heterogeneity. Results of meta-regression analysis and sensitivity analysis were presented in Supplementary Appendix.
Discussion
We conducted this review to evaluate evidence for the benefits of patient education interventions in people with RA. The outcomes include pain, physical function, disease activity, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), anxiety, depression, ASE (pain, other symptoms, total), and general health. The results of this meta-analysis suggest that patient education may be beneficial for improving physical function, disease activity, increasing ASE (pain), ASE (other symptoms), and ASE (total), and general health in patients with RA. However, there was no significant effect on anxiety, depression, pain, and CRP in patients with RA.
This study shows that patient education is generally beneficial for rheumatoid arthritis. Based on our previous work, we expected that patient education would contribute to clinical outcomes and psychological status in rheumatoid arthritis. Several previous studies have explored the impact of patient education on diseases such as low back pain and rheumatoid arthritis (26, 56). These studies found that patient education improved pain and general health in patients with acute low back pain, and improved general health, mental health, and depression in rheumatoid arthritis but did not significantly improve anxiety or disease activity. Our study found that patient education can help improve clinical outcomes in rheumatoid arthritis, such as physical function, disease activity, increasing ASE (pain), ASE (other symptoms), and ASE (total), and general health. The positive effect of patient education may be closely related to the active participation of patients, and it is very important for patients to adhere to rheumatoid arthritis-related interventions. However, our study found that patient education did not significantly improve anxiety, depression, pain, and CRP in patients with rheumatoid arthritis, and further research in this part is needed in the future. Overall, our results are in line with our prior expectations.
RA is a chronic inflammatory joint disease that can occur at any age, causing disability, loss of workability, and even death in patients with RA (57). Many patients with RA continue to experience joint pain, deformities, disability, and poor quality of life despite professional treatment (24). There are some interventions that can enhance the effectiveness of RA, and patient education is one of them, this may be related to patient participation and shared decision making. It can help patients understand RA and strengthen the management of the disease (58, 59). Patient education has been defined as “any set of planned educational activities designed to improve patients' health behaviors and health status” (24). EULAR recommends that patient education should be part of the treatment of RA (17). Studies have shown that patients may play an important role in the disease management of RA (39), but this important role may require active participation and shared decision making by patients, and patients must contribute actively to adhere to interventions for their RA. Health education for patients with RA can have a positive impact on the perception of pain and the management of the disease, and they transform the knowledge of the disease and the methods of preventing pain into changes in health behavior, which not only reduces the symptoms of pain and disability but also it will also improve body function (39, 60, 61). Antirheumatoid medications and biological agents can effectively control the symptoms and inflammatory response of RA, but the effectiveness of the medications is severely limited by patient adherence (8), and patient education can enhance the adherence of patients with RA and improve the effectiveness of medications (53), this may have a positive effect on improving their pain, physical function and reducing inflammatory cytokines (such as ESR, CRP, and RF). For patients with RA, self-efficacy is an important influencing factor to comply with health advice and health outcomes (62). The study found that the self-efficacy of patients with RA is significantly related to their health status (63, 64) and that patient education has a positive impact on clinical efficacy through self-efficacy (65). In addition, the effect of patient education on patients with RA was also affected by the education content. Some education content includes guiding patients to take family exercise, and family exercise has been proved to promote the maintenance of muscle strength, increase handgrip strength and flexibility (66–68), which can further improve the physical function of patients with RA. Clinical studies have shown that patient education was closely related to patients' mental health, especially in anxiety and depression, and patient education can improve patients' anxiety and depression (32, 69). The symptoms of RA are the result of the interaction between the body and the mind, and the symptoms of RA and psychological factors are closely related (69). Health education for patients with RA can increase the understanding of the disease and its treatment methods, which is beneficial to improve the anxiety and depression of patients with RA, and may also have a significant impact on the control of the disease. The study found that long-term behavior change of patients with RA through education can have a positive impact on disease control and also significantly reduce the disease activity of RA (19). Other studies have found that patient education may not have a direct impact on disease activity but maybe in an indirect way (17).
Patient education is increasing of interest to healthcare workers as a complementary intervention. Patient education has been recognized by many clinicians and is widely used in clinical practice. Some medical, orthopedic, and other diseases use patient education as an intervention, such as knee osteoarthritis, cancer, stroke, etc. (70–72). However, the mechanisms by which patient education works are complex and integrative. Some studies have found that patient education can reduce patient pain and function, and the mechanism by which patient education affects health outcomes may be related to self-efficacy (73). Patient education can improve patient self-efficacy, and some information, including exercise and health management, can promote healthy behavior changes (70, 73). Increased patient awareness of the disease can promote patient self-motivation, change their behavior, enhance disease management further, as well as increase patient adherence (74). In addition, patient education can improve psychosocial support, which contributes to disease management to a certain extent and promotes changes in patient behavior (75). Several studies suggest that patient education interventions should not be limited to treatment adherence alone but should also focus on the patient's mental health, which is valuable by combining psychosocial factors, clinical outcomes, and self-reported adherence (75, 76). Patient education is a long-term ongoing process and should be implemented with periodic assessment of its benefits and updating its interventions as necessary to accommodate changes in the patient's disease (76). The effectiveness of patient education may be affected by multiple factors, such as the patient's learning ability, literacy level, cultural environment, etc. Therefore, careful and comprehensive consideration should be given to developing interventions for patient education (77). In addition, some studies suggest that effective patient education should encourage patients to enhance self-efficacy and self-management, improve patient satisfaction, and effectively promote collaboration and communication between patients and professionals (38, 78–80).
Limitations
This systematic review and meta-analysis had several limitations. Firstly, although all the included RCTs were randomized, it was not clear whether some included RCTs had a bias in allocation concealment and bias in performance bias (blinding of participants and personnel) and detection bias (blinding of outcome assessment). Therefore, the quality of evidence for outcomes had been reduced to low levels. Secondly, the sample size included in the study was small. Although a small sample size can be used for meta-analysis, the results might be biased, and the conclusions drawn should be considered preliminary (81). Thirdly, the RCTs varied in terms of their patient populations (such as disease duration), comparative treatments (specific methods, the length of intervention time, and frequency of interventions), and outcome measures. Therefore, the results of the meta-analysis showed that there was a high degree of heterogeneity. However, due to the small number of included RCTs and the fact that some RCTs did not specifically report these differences, we could not conduct a subgroup analysis to check whether these factors had an impact on the outcome of the study.
Implications for Further Research and Practice
Considering that patient education is an intervention without side effects and helps to improve the curative effect of rheumatoid arthritis and control the development of rheumatoid arthritis, it is recommended that patient education be used as a treatment strategy for rheumatoid arthritis. The use of patient education as an intervention for RA is of great significance for reducing the medical cost of RA, especially in developing countries. In addition, this study also provides data for future clinical research on RA. However, due to the low level of evidence in this study and the high heterogeneity of the study, more high-quality RCTs should be conducted in the future to verify these conclusions. We acknowledge the difficulty of conducting RCTs in this study, but some methods can make future study designs more comprehensive and rigorous. Firstly, the implementation of RCTs on patient education in the future should strictly follow the Consolidated Standards of Reporting Trials (CONSORT) statement to improve the quality of research (82). Secondly, researchers should complete registration at a standard clinical study center before starting a clinical study, and complete study protocols should be published to reduce publication bias (83). Finally, clinical studies should extend the duration of follow-up and increase the frequency of follow-up to assess the long-term efficacy of interventions.
Patient education is a broad-based intervention that may serve as a foundational intervention for many diseases. However, patient education is effective on clinical outcomes remains inconclusive. Studies have found that it is difficult to compare the effects of patient education on different chronic diseases and also among different types of arthritis (84). Therefore, we are only studying rheumatoid arthritis and not simultaneously studying and comparing larger diseases, such as rheumatic diseases, osteoarthritis, or other chronic musculoskeletal diseases. In the future, our research should be extended to other arthritis or chronic musculoskeletal diseases to explore further the impact of patient education on other arthritic or chronic musculoskeletal diseases. At the same time, patient education can be considered as a disease-based intervention, combining patient education with other interventions.
Conclusion
Patient education may be effective in improving clinical outcomes and psychological status in patients with rheumatoid arthritis. Considering the methodological limitations of the included RCTs, more high-quality and large-sample RCTs are needed to confirm this conclusion in the future.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
ZGW, WGL, and XMX designed the study. ZGW, YZ, YW, and RZ conducted a literature search and screening and extracted data from the literature. XLY, ZHC, CCL, and JYL checked the extracted data. ZGW, ZBW, and ZXY conducted the statistical analysis. ZGW, YZ, YW, and RZ wrote the first draft. WGL and XMX corrected the manuscript. ZXY did the language editing. WGL and XMX supervised the conduct of the study. All authors have read and approved the final submitted version.
Funding
This work was supported by Guangdong Provincial Science and Technology Innovation Strategy Special Fund (2021B1111610007) and Natural Science Foundation of Guangdong Province (2021A1515011545).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2022.848427/full#supplementary-material
References
1. Sparks Jeffrey A. Rheumatoid arthritis. Ann Intern Med. (2019) 170: ITC1–16. doi: 10.7326/AITC201901010
2. Smolen JS, Aletaha D, Barton A, Burmester GR, Emery P, Firestein GS, et al. Rheumatoid arthritis. Nat Rev Dis Primers. (2018) 4:18001. doi: 10.1038/nrdp.2018.1
3. Kitas GD, Gabriel S E. Cardiovascular disease in rheumatoid arthritis: state of the art and future perspectives. Ann Rheum Dis. (2011) 70:8–14. doi: 10.1136/ard.2010.142133
4. Sokka T, Kautiainen H, Pincus T, Verstappen SMM, Aggarwal A, Alten R, et al. Work disability remains a major problem in rheumatoid arthritis in the 2000s: data from 32 countries in the QUEST-RA study. Arthritis Res Ther. (2010) 12:R42. doi: 10.1186/ar2951
5. Cross M, Smith E, Hoy D, Carmona L, Wolfe F, Vos T, et al. The global burden of rheumatoid arthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. (2014) 73:1316–22. doi: 10.1136/annrheumdis-2013-204627
6. Myasoedova E, Davis J, Matteson EL, Crowson C S. Is the epidemiology of rheumatoid arthritis changing? Results from a population-based incidence study, 1985-2014. Ann Rheum Dis. (2020) 79:440–4. doi: 10.1136/annrheumdis-2019-216694
7. Smolen JS, Landewé R, Bijlsma J, Burmester G, Chatzidionysiou K, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. (2017) 76:960–77. doi: 10.1136/annrheumdis-2016-210715
8. Scheiman-Elazary A, Duan L, Shourt C, Agrawal H, Ellashof D, Cameron-Hay M, et al. The rate of adherence to antiarthritis medications and associated factors among patients with rheumatoid arthritis: a systematic literature review and meta-analysis. J Rheumatol. (2016) 43:512–23. doi: 10.3899/jrheum.141371
9. Dolati S, Sadreddini S, Rostamzadeh D, Ahmadi M, Jadidi-Niaragh F, Yousefi M. Utilization of nanoparticle technology in rheumatoid arthritis treatment. Biomed Pharmacother. (2016) 80:30–41. doi: 10.1016/j.biopha.2016.03.004
10. Cohen MJ, Shaykevich S, Cawthon C, Kripalani S, Paasche-Orlow MK, Schnipper J L. Predictors of medication adherence postdischarge: the impact of patient age, insurance status, and prior adherence. J Hosp Med. (2012) 7:470–5. doi: 10.1002/jhm.1940
11. van den Bemt BJ, Zwikker HE, van den Ende CH. Medication adherence in patients with rheumatoid arthritis: a critical appraisal of the existing literature. Expert Rev Clin Immunol. (2012) 8:337–51. doi: 10.1586/eci.12.23
12. Achaval SD, Suarez-Almazor ME. Treatment adherence to disease-modifying antirheumatic drugs in patients with rheumatoid arthritis and systemic lupus erythematosus. Int J Clin Rheumtol. (2010) 5:313–26. doi: 10.2217/ijr.10.15
13. Ritschl V, Stamm TA, Aletaha D, Bijlsma JWJ, Böhm P, Dragoi RG, et al. 2020 EULAR points to consider for the prevention, screening, assessment and management of non-adherence to treatment in people with rheumatic and musculoskeletal diseases for use in clinical practice. Ann Rheum Dis. (2020) 18:707–13. doi: 10.1136/annrheumdis-2020-218986
14. Birgitta N, Cecilia F, Ingrid D, Gunnar B, Lundberg IE, Dufour AB, et al. An outsourced health-enhancing physical activity programme for people with rheumatoid arthritis: exploration of adherence and response. Rheumatology. (2015) 6:1065–073. doi: 10.1093/rheumatology/keu444
15. Bech B, Primdahl J, van Tubergen A, Voshaar M, Zangi HA, Barbosa L, et al. 2018 update of the EULAR recommendations for the role of the nurse in the management of chronic inflammatory arthritis. Ann Rheum Dis. (2020) 79:61–8. doi: 10.1136/annrheumdis-2019-215458
16. M'imunya JM, Kredo T, Volmink J. Patient education and counselling for promoting adherence to treatment for tuberculosis. Cochrane Database Syst Rev. (2012) 5:CD006591. doi: 10.1002/14651858.CD006591.pub2
17. Zangi HA, Ndosi M, Adams J, Andersen L, Bode C, Boström C, et al. European League Against Rheumatism (EULAR). EULAR recommendations for patient education for people with inflammatory arthritis. Ann Rheum Dis. (2015) 74:954–62. doi: 10.1136/annrheumdis-2014-206807
18. Aliha JM, Asgari M, Khayeri F, Ramazani M, Farajzadegan Z, Javaheri J. Group education and nurse-telephone follow-up effects on blood glucose control and adherence to treatment in type 2 diabetes patients. Int J Prev Med. (2013) 4:797–802.
19. Miedany YE, Gaafary ME, Arousy NE, Ahmed I, Youssef S, Palmer D. Arthritis education: the integration of patient-reported outcome measures and patient self-management. Clin Exp Rheumatol. (2012) 30:899–904.
20. Ravindran V, Jadhav R. The effect of rheumatoid arthritis disease education on adherence to medications and followup in Kerala, India. J Rheumatol. (2013) 40:1460–1. doi: 10.3899/jrheum.130350
21. Lorig KR, Mazonson PD, Holman H R. Evidence suggesting that health education for self-management in patients with chronic arthritis has sustained health benefits while reducing health care costs. Arthritis Rheum. (1993) 36:439–46. doi: 10.1002/art.1780360403
22. Galo JS, Mehat P, Rai SK, Avina-Zubieta A, Vera M A D. What are the effects of medication adherence interventions in rheumatic diseases: a systematic review. Ann Rheum Dis. (2016) 75:667–73. doi: 10.1136/annrheumdis-2014-206593
23. Shadick NA, Zibit MJ, Iannaccone CK, Thrower R, Sowell NF, Weinblatt ME, et al. A development and feasibility study of a peer support telephone program in rheumatoid arthritis. J Clin Rheumatol. (2018) 24:346–9. doi: 10.1097/RHU.0000000000000661
24. Riemsma RP, Taal E, Kirwan JR, Rasker J J. Systematic review of rheumatoid arthritis patient education. Arthritis Rheum. (2004) 51:1045–59. doi: 10.1002/art.20823
25. Carandang K, Pyatak EA, Vigen CL. Systematic review of educational interventions for rheumatoid arthritis. Am J Occup Ther. (2016) 70:7006290020p1–12. doi: 10.5014/ajot.2016.021386
26. Riemsma RP, Kirwan JR, Taal E, Rasker JJ. Patient education for adults with rheumatoid arthritis. Cochrane Database Syst Rev. (2003) CD003688. doi: 10.1002/14651858.CD003688
27. Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. (2015) 350:g7647. doi: 10.1136/bmj.g7647
29. Tarsilla M. Cochrane handbook for systematic reviews of interventions. J Multidisc Eval. (2010) 6:142–8.
30. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
31. Helewa A, Goldsmith CH, Lee P, Bombardier C, Hanes B, Smythe HA, et al. Effects of occupational therapy home service on patients with rheumatoid arthritis. Lancet. (1991) 337:1453–6. doi: 10.1016/0140-6736(91)93138-Y
32. Barlow JH, Wright CC. Knowledge in patients with rheumatoid arthritis: a longer term follow-up of a randomized controlled study of patient education leaflets. Br J Rheumatol. (1998) 37:373–6. doi: 10.1093/rheumatology/37.4.373
33. Scholten C, Brodowicz T, Graninger W, Gardavsky I, Pils K, Pesau B, et al. Persistent functional and social benefit 5 years after a multidisciplinary arthritis training program. Arch Phys Med Rehabil. (1999) 80:1282–7. doi: 10.1016/S0003-9993(99)90030-8
34. Hill J, Bird H, Johnson S. Effect of patient education on adherence to drug treatment for rheumatoid arthritis: a randomised controlled trial. Ann Rheum Dis. (2001) 60:869–75.
35. Riemsma RP, Taal E, Rasker J J. Group education for patients with rheumatoid arthritis and their partners. Arthritis Rheum. (2003) 49:556–66. doi: 10.1002/art.11207
36. Hammond A, Young A, Kidao R. A randomised controlled trial of occupational therapy for people with early rheumatoid arthritis. Ann Rheum Dis. (2004) 63:23–30. doi: 10.1136/ard.2002.001511
37. Kirwan JR, Hewlett S, Cockshott Z, Barrett J. Clinical and psychological outcomes of patient education in rheumatoid arthritis. Musculoskel Care. (2005) 3:1–16. doi: 10.1002/msc.21
38. Núñez M, Núñez E, Yoldi C, Quintó L, Hernández MV, Muñoz-Gómez J. Health-related quality of life in rheumatoid arthritis: therapeutic education plus pharmacological treatment versus pharmacological treatment only. Rheumatol Int. (2006) 26:752–7. doi: 10.1007/s00296-005-0071-6
39. Masiero S, Boniolo A, Wassermann L, Machiedo H, Volante D, Punzi L. Effects of an educational-behavioral joint protection program on people with moderate to severe rheumatoid arthritis: a randomized controlled trial. Clin Rheumatol. (2007) 26:2043–50. doi: 10.1007/s10067-007-0615-0
40. Giraudet-Le Quintrec JS, Mayoux-Benhamou A, Ravaud P, Champion K, Dernis E, Zerkak D, et al. Effect of a collective educational program for patients with rheumatoid arthritis: a prospective 12-month randomized controlled trial. J Rheumatol. (2007) 34:1684–91.
41. Lovisi Neto BE, Jennings F, Barros Ohashi C, Silva PG, Natour J. Evaluation of the efficacy of an educational program for rheumatoid arthritis patients. Clin Exp Rheumatol. (2009) 27:28–34.
42. Macedo AM, Oakley SP, Panayi GS, Kirkham B W. Functional and work outcomes improve in patients with rheumatoid arthritis who receive targeted, comprehensive occupational therapy. Arthritis Rheum. (2009) 61:1522–30. doi: 10.1002/art.24563
43. Mathieux R, Marotte H, Battistini L, Sarrazin A, Berthier M, Miossec P. Early occupational therapy programme increases hand grip strength at 3 months: results from a randomised, blind, controlled study in early rheumatoid arthritis. Ann Rheum Dis. (2009) 68:400–3. doi: 10.1136/ard.2008.094532
44. Conn DL, Pan Y, Easley KA, Comeau DL, Carlone JP, Culler SD, et al. The effect of the arthritis self-management program on outcome in african americans with rheumatoid arthritis served by a public hospital. Clin Rheumatol. (2013) 32:49–59. doi: 10.1007/s10067-012-2090-5
45. Shigaki CL, Smarr KL, Siva C, Ge B, Musser D, Johnson R. RAHelp: an online intervention for individuals with rheumatoid arthritis. Arthritis Care Res. (2013) 65:1573–81. doi: 10.1002/acr.22042
46. Yousefi H, Chopra A, Farrokhseresht R, Sarmukaddam S, Noghabi FA, Bedekar N, et al. Epidemiological evaluation quality of life in patients suffering from early rheumatoid arthritis: a pragmatic, prospective, randomized, blind allocation controlled of a modular program group intervention. Epidemiol Health. (2015) 37:e2015048. doi: 10.4178/epih/e2015048
47. Pot-Vaucel M, Aubert MP, Guillot P, Glémarec J, Berthelot JM, Le Goff B, et al. Randomised study versus control group of customised therapeutic education for patients in follow-up for rheumatoid arthritis. Joint Bone Spine. (2016) 83:199–6. doi: 10.1016/j.jbspin.2015.05.017
48. Anvar N, Matlabi H, Safaiyan A, Allahverdipour H, Kolahi S. Effectiveness of self-management program on arthritis symptoms among older women: a randomized controlled trial study. Health Care Women Int. (2018) 39:1326–39. doi: 10.1080/07399332.2018.1438438
49. Hosseini Moghadam M, Jahanbin I, Nazarinia M A. The effect of educational program on self-efficacy of women with rheumatoid arthritis: a randomized controlled clinical trial. Int J Community Based Nurs Midwifery. (2018) 6:12–20.
50. Saeedifar ES, Memarian R, Fatahi S, Ghelichkhani F. Use of the Orem self-care model on pain relief in women with rheumatoid arthritis: a randomized trial. Electron Phys. (2018) 10:6884–91. doi: 10.19082/6884
51. Zhao SP, Chen H. Effectiveness of health education by telephone follow-up on self-efficacy among discharged patients with rheumatoid arthritis: a randomised control trial. J Clin Nurs. (2019) 28:3840–7. doi: 10.1111/jocn.15002
52. Shao J H., Yu K H., Chen S H. Feasibility and acceptability of a self-management program for patients with rheumatoid arthritis. Orthop Nurs. (2020) 39:238–45. doi: 10.1097/NOR.0000000000000676
53. Song Y, Reifsnider E, Zhao S, Xie X, Chen H. A randomized controlled trial of the Effects of a telehealth educational intervention on medication adherence and disease activity in rheumatoid arthritis patients. J Adv Nurs. (2020) 76:1172–81. doi: 10.1111/jan.14319
54. Shao JH, Yu KH, Chen S H. Effectiveness of a self-management program for joint protection and physical activity in patients with rheumatoid arthritis: a randomized controlled trial. Int J Nurs Stud. (2021) 116:103752. doi: 10.1016/j.ijnurstu.2020.103752
55. Sterne JAC, Sutton AJ, Ioannidis JPA, Terrin Norma, Jones David R, Lau Joseph, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. (2011) 343:d4002. doi: 10.1136/bmj.d4002
56. Engers A, Jellema P, Wensing M, van der Windt DA, Grol R, van Tulder MW. Individual patient education for low back pain. Cochrane Database Syst Rev. (2008) 2008:CD004057. doi: 10.1002/14651858.CD004057.pub3
57. Aletaha D, Smolen J S. Diagnosis and management of rheumatoid arthritis: a review. JAMA. (2018) 320:1360–72. doi: 10.1001/jama.2018.13103
58. Kirwan J R. Patient education in rheumatoid arthritis. Curr Opin Rheumatol. (1990) 2:336–9. doi: 10.1097/00002281-199002020-00015
59. Taal E, Rasker JJ, Wiegman O. Patient education and self-management in the rheumatic diseases: a self-efficacy approach. Arthritis Care Res. (1996) 9:229–38.
60. Neuberger GB, Smith KV, Black SO, Hassanein R. Promoting self-care in clients with arthritis. Arthritis Care Res. (1993) 6:141–8. doi: 10.1002/art.1790060306
61. Taal E, Rasker JJ, Wiegman O. Group education for rheumatoid arthritis patients. Semin Arthritis Rheumat. (1997) 26:805–16. doi: 10.1016/S0049-0172(97)80024-8
62. Taal E, Seydel ER, Rasker JJ, Wiegman O. Psychosocial aspects of rheumatic diseases: introduction. Pat Educ Counsel. (1993) 20:55–61. doi: 10.1016/0738-3991(93)90121-C
63. Keefe FJ, Van Horn Y. Cognitive-behavioral treatment of rheumatoid arthritis pain: maintaining treatment gains. Arthritis Care Res. (1993) 6:213–22. doi: 10.1002/art.1790060408
64. Schiaffino KM, Revenson TA, Gibofsky A. Assessing the impact of self-efficacy beliefs on adaptation to rheumatoid arthritis. Arthritis Care Res. (1991) 4:150–7. doi: 10.1002/art.1790040404
65. Lorig K, Seleznick M, Lubeck D, Ung E, Chastain R L., Holman H R. The beneficial outcomes of the arthritis self-management course are not adequately explained by behavior change. Arthritis Rheum. (1989) 32:91–5. doi: 10.1002/anr.1780320116
66. Mccubbin J A. Resistance exercise training for persons with arthritis. Rheum Dis Clin N Am. (1990) 16:931–43. doi: 10.1016/S0889-857X(21)00916-9
67. Brighton SW, Lubbe JE, van der Merwe C A. The effect of a long-term exercise programme on the rheumatoid hand. Br J Rheumatol. (1993) 32:392–5. doi: 10.1093/rheumatology/32.5.392
68. Hoenig H, Groff G, Pratt K, Goldberg E, Franck W. A randomized controlled trial of home exercise on the rheumatoid hand. J Rheumatol. (1993) 20:785–9.
69. Barsky AJ, Ahern DK, Orav EJ, Nestoriuc Y, Liang Ma H, Berman IT, et al. A randomized trial of three psychosocial treatments for the symptoms of rheumatoid arthritis. Semin Arthritis Rheum. (2010) 40:222–32. doi: 10.1016/j.semarthrit.2010.04.001
70. Taglietti M, Facci LM, Trelha CS, de Melo FC, da Silva DW, Sawczuk G, et al. Effectiveness of aquatic exercises compared to patient-education on health status in individuals with knee osteoarthritis: a randomized controlled trial. Clin Rehabil. (2018) 32:766–76. doi: 10.1177/0269215517754240
71. Reif K, de Vries U, Petermann F, Görres S. A patient education program is effective in reducing cancer-related fatigue: a multi-centre randomised two-group waiting-list controlled intervention trial. Eur J Oncol Nurs. (2013) 17:204–13. doi: 10.1016/j.ejon.2012.07.002
72. Chu YM, Choi KS. Effectiveness of patient education in acute stroke: a comparison between a customised computer system and a pictorial information booklet. BMJ Health Care Inform. (2020) 27:e100144. doi: 10.1136/bmjhci-2020-100144
73. Pajares F. Overview of Social Cognitive Theory and Self-Efficacy Atlanta: Division of Educational Studies. Atlanta, GA: Emory College of Arts and Sciences, Emory University (2002).
74. Elder J, Ayala G Harris S. Theories and intervention approaches to health-behaviour change in primary care. Am J Prev Med. (1999) 4:275–84. doi: 10.1016/S0749-3797(99)00094-X
75. Welch JL, Thomas-Hawkins C. Psycho-educational strategies to promote fluid adherence in adult hemodialysis patients: a review of intervention studies. Int J Nurs Stud. (2005) 42:597–608. doi: 10.1016/j.ijnurstu.2004.09.015
76. Idier L, Untas A, Koleck M, Chauveau P, Rascle N. Assessment and effects of Therapeutic Patient Education for patients in hemodialysis: a systematic review. Int J Nurs Stud. (2011) 48:1570–86. doi: 10.1016/j.ijnurstu.2011.08.006
77. Marcus C. Strategies for improving the quality of verbal patient and family education: a review of the literature and creation of the EDUCATE model. Health Psychol Behav Med. (2014) 2:482–95. doi: 10.1080/21642850.2014.900450
78. Schrieber L, Colley M. Patient education. Best Pract Res Clin Rheumatol. (2004) 18:465–76. doi: 10.1016/j.berh.2004.03.008
79. Ndosi M, Johnson D, Young T, Hardware B, Hill J, Hale C, et al. Effects of needs-based patient education on self-efficacy and health outcomes in people with rheumatoid arthritis: a multicentre, single blind, randomised controlled trial. Ann Rheum Dis. (2016) 75:1126–32. doi: 10.1136/annrheumdis-2014-207171
80. Hoving C, Visser A, Mullen PD, van den Borne B. A history of patient education by health professionals in Europe and North America: from authority to shared decision making education. Patient Educ Couns. (2010) 78:275–81. doi: 10.1016/j.pec.2010.01.015
81. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed. Chichester: John Wiley & Sons (2019).
82. Boutron I, Moher D, Altman D, Schulz K, Ravaud P, CONSORT Group. Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. ACP J Club. (2008) 148:295–309. doi: 10.7326/0003-4819-148-4-200802190-00008
83. Wu Z, Wang Y, Ye X, Chen Z, Zhou R, Ye Z, et al. Myofascial release for chronic low back pain: a systematic review and meta-analysis. Front Med. (2021) 8:697986. doi: 10.3389/fmed.2021.697986
Keywords: patient education, psychotherapy, rheumatoid arthritis, meta-analysis, systematic review
Citation: Wu Z, Zhu Y, Wang Y, Zhou R, Ye X, Chen Z, Li C, Li J, Ye Z, Wang Z, Liu W and Xu X (2022) The Effects of Patient Education on Psychological Status and Clinical Outcomes in Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Front. Psychiatry 13:848427. doi: 10.3389/fpsyt.2022.848427
Received: 05 January 2022; Accepted: 10 February 2022;
Published: 17 March 2022.
Edited by:
Peng Wang, Anhui Medical University, ChinaReviewed by:
Rikke Helene Moe, Diakonhjemmet Hospital, NorwayValentin Ritschl, Medical University of Vienna, Austria
Copyright © 2022 Wu, Zhu, Wang, Zhou, Ye, Chen, Li, Li, Ye, Wang, Liu and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuemeng Xu, eHV4dWVtZW5nQDE2My5jb20=; Wengang Liu, OTE0MTk0MDc5QHFxLmNvbQ==
†These authors have contributed equally to this work
 Zugui Wu
Zugui Wu Yue Zhu2†
Yue Zhu2† Rui Zhou
Rui Zhou