- 1The National Clinical Research Center for Mental Disorders & Beijing Key Laboratory of Mental Disorders, Beijing Anding Hospital, Capital Medical University, Beijing, China
- 2Advanced Innovation Center for Human Brain Protection, Capital Medical University, Beijing, China
Objectives: To investigate the altered intrinsic brain activity (IBA) in patients suffering from late-life depression (LLD) using a percent amplitude of fluctuation (PerAF) method.
Methods: In total, fifty patients with LLD and 40 non-depressed controls (NCs) were recruited for the present research. Participants underwent the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) test and resting-state functional MRI (rs-fMRI) scans. The RBANS test consists of 12 sub-tests that contribute to a total score and index scores across the following five domains: immediate memory, visuospatial/constructional, language, attention, and delayed memory. The PerAF method was used for data analysis to detect changes in neural activity in the relevant brain regions. A receiver operating characteristic (ROC) curve was conducted to evaluate the ability of the RBANS test and proposed the PerAF method in distinguishing the two groups. The relationships between altered IBA and neuropsychologic deficits were determined by the Pearson correlation analysis.
Results: A significant difference existed in RBANS total score, immediate memory, visuospatial/constructional, language, attention, and delayed memory between groups (P < 0.05). Compared with the NCs group, the LLD group demonstrated decreased PerAF differences in the bilateral superior frontal gyrus, orbital part (Frontal_Sup_Orb), and bilateral anterior cingulate cortex (ACC). The PerAF method and RBANS test exhibited an excellent discriminatory power with the area under curve (AUC) values in distinguishing the two groups. In addition, the attention score of the RBANS test positively correlated with the PerAF values of the bilateral Frontal_Sup_Orb and bilateral ACC.
Conclusion: The changes of PerAF in the bilateral Frontal_Sup_Orb and bilateral ACC are related to an increased risk of developing LLD. Moreover, the PerAF method could be used as an underlying sensitivity biomarker to identify the psychiatric disorder.
Introduction
Depression after 60–65 is generally called late-life depression (LLD), affecting 4%−10% of the elderly (1, 2). Compared with the depression observed in the younger generation, LLD is often related to aging-associated neurodegeneration, cognitive impairment, or somatic complaints, increasing the risk for dementia, disability, and mortality (3). Exploring changes in the functional and structural connectivity in brain networks in association with emotional and cognitive symptoms may contribute to understanding the neural mechanisms underlying LLD, whereas the specific brain regions engaged with such changes are still unclear.
Recently, the modern brain neuroimaging techniques have developed rapidly. Functional magnetic resonance imaging (fMRI) has appeared as a popular technology because of its being non-invasive and does not require exposure to radioactive tracers, and provides new insights into the pathophysiology of depression. According to the collection status, fMRI is divided into task-state fMRI (ts-fMRI) and resting-state fMRI (rs-fMRI). In terms of the ts-fMRI study, hypoactivation of the anterior cingulate cortex (ACC) in the elderly patients experienced multiple depressive episodes in a verbal fluency task (4). However, another study did not observe the abnormal activation of ACC in response to an explicit sequence learning task, and they discovered diminished activation in the dorsolateral pre-frontal cortex (DLPFC) bilaterally while increased activation in the right caudate and putamen (5). Confounds associated with illness chronicity, such as the number of episodes and prolonged exposure to antidepressants, may be inconsistent across studies (6). Compared with the studies including patients with a long course of depression, few studies have investigated first-episode, treatment-naive patients with LLD. The study of the first-episode, drug-naive patients with LLD may be significant for elucidating the core pathogenesis of this illness. In addition, another issue pertains to the task-related functional neuroimaging studies that require patients to follow complicated cognitive tasks, and thus, the performance may confound the results (7).
Rs-fMRI has been considered a feasible and widely accepted method since the study of Biswal et al. (8). They first reported that the spontaneous low-frequency (0.01-0.08 Hz) fluctuations were closely associated with the intrinsic brain activity (IBA) and physiological meaning. IBA indicates sustained neural activity, which affects brain functions (9). In rs-fMRI studies of depression, most of the current work has focused on the major depressive disorder (MDD), regional brain activity in the frontal, temporal, occipital, and cerebellar lobes, and also in the thalamus and insula, displays reduced local synchronization among patients with MDD (10–12). The amplitude of low-frequency fluctuations (ALFFs), fractional ALFF (fALFF), and regional homogeneity (ReHo) constitute three major rs-fMRI approaches in testing IBA (13, 14). An rs-fMRI study using ALFF to investigate first-episode, drug-naive patients with LLD revealed that compared with the control group, the left superior temporal gyrus activation increased while the activation of the bilateral superior frontal gyrus decreased (15). However, another study using ReHo demonstrated that the left Crus I of the cerebellum increased while the activation of the right precuneus decreased (16). The population criteria of the two studies aforementioned are similar, and the inconsistent results may be related to the fact that ALFF and ReHo methods are susceptible to high-frequency physiological cardiac and respiratory noises (17). As a novel approach, percent amplitude of fluctuation (PerAF) exhibits optimal performance in-degree centrality, ALFF, and regional homogeneity (18). Thus, the PerAF approach makes it possible to enhance sensitivity and lower bias while dealing with the IBA changes related to LLD. However, there is still a lack of research on LLD.
Research demonstrates that the severity of depressive symptomatology among patients with LLD contributes to neurocognitive decline (19), while the latest cross-sectional and longitudinal surveys have not recognized any prominent correlation between depression symptoms with cognitive impairments (20–22). Therefore, we hypothesized that compared with non-depressed controls, widespread IBA alternations occurred in patients with LLD. These changes were associated with depression symptoms or cognitive impairment, providing a good understanding of the neurobiological mechanisms that underlay LLD.
Materials and Methods
Participants
The present research recruited 50 patients suffering from LLD and 40 non-depressed controls (NCs) between February 2021 and October 2021. All the participants presented informed consent. The study protocols gained approval from the institutional review board of the Beijing Anding Hospital. All the subjects were 60–75 years old and right-handed, first episode and no previous treatment with psychotropic drugs, confirmed by a certified geriatric psychiatrist through Axis I major depressive episode according to the 5th edition of Diagnostic and Statistical Manual of Mental Disorders (DSM-V) through the diagnostic interview (23, 24). Besides, no cases were diagnosed with additional Axis I major psychiatric disorders, except for anxiety disorders. Patients enrolled in the study had the least 17-item Hamilton depression rating scale (HAMD) (17). In addition, the participants were all requested to get the least Mini-Mental State Examination (MMSE) scale scores of 24 (excluding the presence of dementia) (25). Patients conforming to the following standards were excluded, including major neurocognitive decline, major head trauma history, Parkinson's disease, stroke, serious cardiovascular, respiratory, immune, and other systemic diseases.
Cognitive Assessment
This study performed a cognitive assessment with the Repeatable Battery in the Assessment of Neuropsychological Status (RBANS) by well-trained clinicians (26). It contains 12 standardized cognitive tests categorized into the 5 following fields: visuospatial/constructional (line orientation and figure copy), immediate memory (story memory and list learning), attention (digit symbol coding and digit span), language (semantic fluency and picture naming), and delayed memory (list recognition, list recall, figure recall, and story recall). A higher RBANS score suggests superior cognitive performance. Former research has proved RBANS as a helpful screener to evaluate cognitive impairments in psychiatric patients (27).
Rs-fMRI Protocol and Data Analysis
Each participant was scanned using the Siemens 3T scanner (Siemens, Erlangen, Germany), and the head was tightly fastened using foam pads and straps to avoid motion. This study initially captured images in T1 image (excluding intracranial organic diseases preliminary, such as tumor-like lesion or absence), and then the rs-fMRI was made (around 7 min). When collecting resting-state fMRI data, each participant was asked to relax with eyes closed, lie still, and keep awake. The T1 images was gathered with the T1-weighted sagittal 3D magnetization-prepared rapid gradient echo (MPRAGE) sequence: TE = 1.85 ms; TR = 2530 ms; FOV = 256 × 256 mm2; FA = 9°; voxel size = 1.0 × 1.0 × 1.0 mm3; thickness = 1.0 mm and matrix size = 256 × 256. Then, images were gathered in resting-state axially with the echo-planar imaging (EPI) sequence under the following parameters, echo time (TE) = 30 ms; repetition time (TR) = 2,000 ms; field of view (FOV) = 256 × 256 mm2; flip angle (FA) = 90°; axial slices = 33; matrix size = 64 × 64; slice thickness = 3.5 mm, voxel size = 3.1 × 3.1 × 3.5 mm3; and a total of 200 time points.
We classified and studied functional images with MRIcro software (www.MRIcro.com). Data were pre-processed with RESTplus V1.2 (http://www.restfmri.net) toolbox based on the MATLAB R2018b platform (28). Initially, the first ten functional volumes were excluded to acquire balanced measurement signals. Then, it was followed by form transformation (DICOM to NIFTI), slice timing, correction of head motion, Montreal Neurological Institute (MNI) space normalization (using T1 image unified segmentation), and re-sample data at 3 × 3 × 3 mm3 resolution, smoothing (full-width Gaussian kernel = 6 × 6 × 6 mm3), and also linear detrending and filtering (0.01–0.08 Hz) (29). In total, 11 of them were excluded due to head movement over 3-mm translocation or over 3° rotation toward each direction in scanning (5 in the LLD group and 6 in the NCs group). This study referenced Friston's 24 head-motion parameters as covariates in regressing the head motion effects. Linear regression was adopted for removing covariates for white matter, global mean signal, cerebrospinal fluid signal, and head motion. In addition, the PerAF approach indicates the ratio of frequency-domain in blood oxygen level-dependent signal in the resting-state to average signal strength for a specific period. Following the pre-processing, the PerAF method was computed (30).
where “X” represents the signal intensity of the time point, “n” refers to the total number of time points of the time course, and “μ” represents the mean value of the time course.
Statistical Analysis
In this study, statistical analyses were conducted with SPSS 26.0 (SPSS, Chicago, IL, USA). The independent descriptive variables (age) were expressed as mean ± SD, whereas categorical variables (sex) were expressed as counts and percentages. Comparison for continuous variables was conducted by performing an independent t-test, and comparison for categorical variables was made with chi-square or Mann–Whitney U test. Statistical test differences between the LLD and NCs groups showed statistical significance with P-values < 0.05. The ‘Statistical Analysis' module of the RESTplus V1.2 toolbox was adopted to compare the average PerAF values of the LLD group and the NCs group. To maintain the balance between the two groups. Some covariates, such as age, gender, and education level, were regressed. For the preliminary comparison results of the average PerAF values between the two groups, the multiple-comparison correction based on the Gaussian random field theory (GRF, two-tailed, cluster-wise p < 0.05, voxel-wise p < 0.001) was further conducted (30). Previous studies reported that, compared with the Bonferroni correction and false discovery rate (FDR), GRF could reduce the false-positive rate and improve statistical power by utilizing the spatial information of fMRI data (31). Subsequently, the “Viewer” module displayed the brain regions with different perAF values between the two groups. Then, the brain regions with different PerAF values were further made into masks, and the PerAF values of altered brain regions of patients with LLD were extracted using the “Extract ROl Signals” module in RESTplus V1.2 toolbox for the next analysis. Here, we adopted the receiver operating characteristic (ROC) curves for identifying the presented PerAF method's ability to distinguish the two groups compared with the RBANS test. The correlations of the PerAF values for the region of interests (ROIs) with clinical symptoms (HAMD-17 and RBANS score) were assessed through Pearson correlation analysis.
Results
Demographics and Neuropsychologic Data
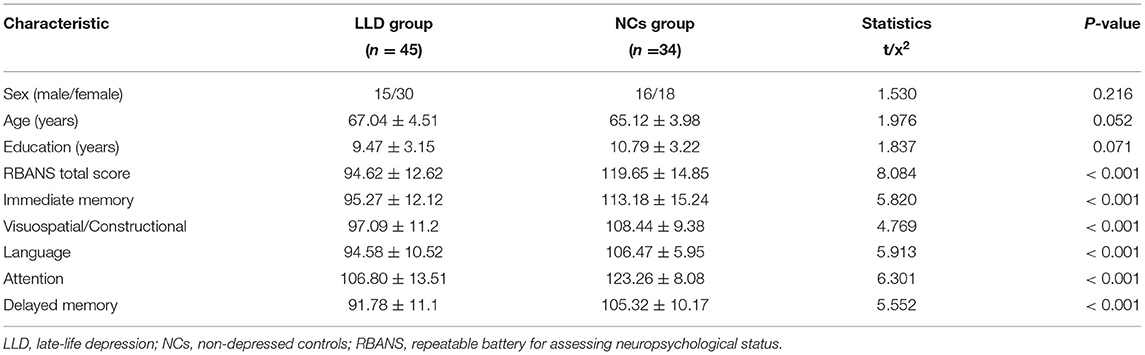
Upon removing patients with head motion above 3° rotation or 3 mm translocation (5 in the LLD group, 3 men and 2 women; 6 in the NCs group, 2 men and 4 women), 45 in the LLD group and 34 in the NCs group were eventually recruited for the current research. Patients with the LLD and NCs were aged 67.04 ± 4.51 and 65.12 ± 3.98, respectively. No differences in age, gender, and educational level could be observed. However, differences in the RBANS total score were observed in immediate memory, visuospatial/constructional, language, attention, and delayed memory between groups (P < 0.05). More details are illustrated in Table 1.
PerAF Differences
As shown in between-group statistical maps, by contrast to the NCs group, the LLD group showed decreased PerAF differences in the bilateral superior frontal gyrus, orbital part (Frontal_Sup_Orb) [Brodmann area (BA) 11], and bilateral anterior cingulate cortex (ACC, BA24) (Table 2, Figure 1A). Figure 1B displayed the mean PerAF signal value of altered brain regions between the two groups.
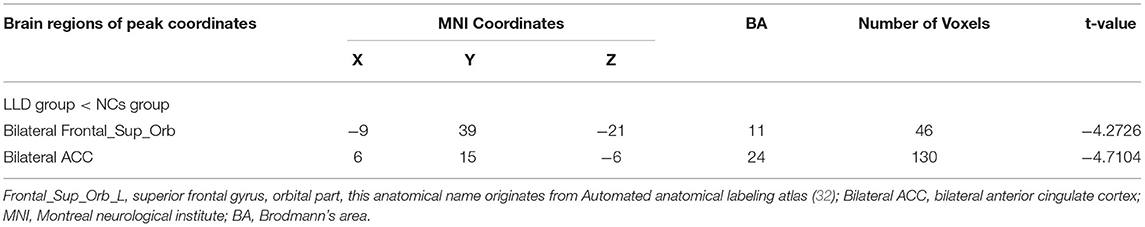
Table 2. Brain regions with significantly different PerAF values of the LLD group and the NCs group.
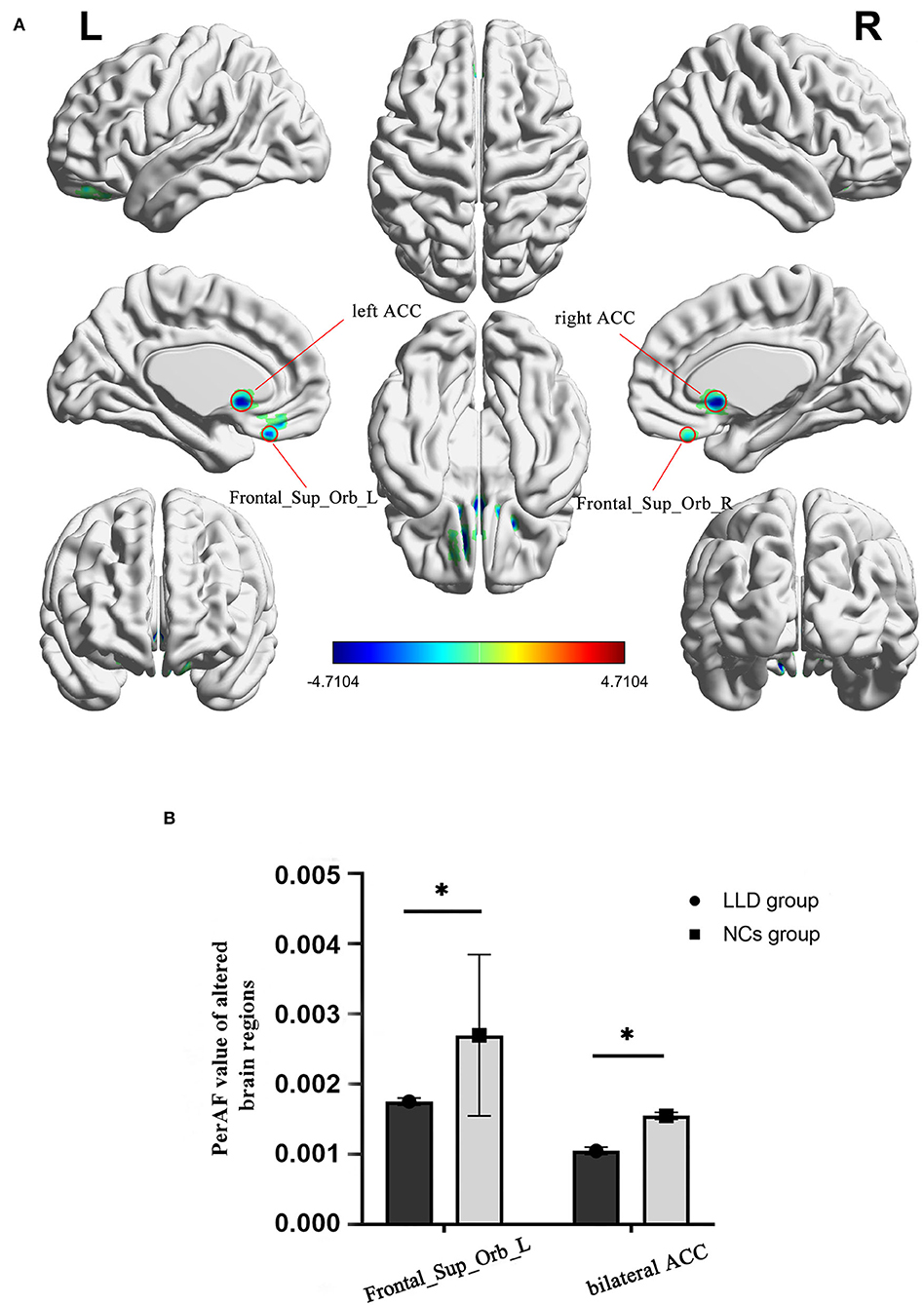
Figure 1. Altered brain regions in PerAF of the LLD group and the NCs group. (A) A comprehensive view; Blue color, decreased PerAF areas R, right; L, left; PerAF, percent amplitude of fluctuation; by contrast to the NCs group, the LLD group showed decreased PerAF differences in the bilateral superior frontal gyrus, orbital part (Frontal_Sup_Orb) [Brodmann area (BA) 11] and bilateral anterior cingulate cortex (ACC, BA24). (B) The mean PerAF signal value of bilateral Frontal_Sup_Orb and bilateral ACC between the two groups. Bilateral Frontal_Sup_Orb, bilateral superior frontal gyrus, orbital part; bilateral ACC, bilateral anterior cingulate cortex. *indicates that the difference between groups is statistically significant.
ROC Curve Analysis
Receiver operating characteristic curve analysis and further diagnostic analysis were performed to evaluate the ability of the RBANS test and PerAF method in distinguishing the two groups, and higher area under curve (AUC) value indicated higher diagnostic accuracy. As for the RBANS test, AUC value for RBANS total score reached 0.899 (P < 0.001; 95% CI: 0.834–0.964, sensitivity: 0.794, specificity: 0.844), and AUC values of immediate memory, visuospatial/constructional, language, attention, and delayed memory were 0.809 (P < 0.001; 95% CI: 0.711–0.908, sensitivity: 0.676, specificity: 0.844), 0.781 (P < 0.001; 95% CI: 0.679–0.883, sensitivity: 0.794, specificity: 0.689), 0.852 (P < 0.001; 95% CI: 0.770–0.935, sensitivity: 0.912, specificity: 0.711) 0.866 (P < 0.001; 95% CI: 0.788–0.945, sensitivity: 0.882, specificity: 0.711), and 0.818 (P < 0.001; 95% CI: 0.725–0.911, sensitivity: 0.618, specificity: 0.889) (Figure 2A, Table 3), respectively. As a comparison, AUC value for bilateral Frontal_Sup_Orb reached 0.770 (P < 0.001; 95% CI: 0.667 −0.873, sensitivity: 0.559, specificity: 0.867), and AUC value of the bilateral ACC was 0.782 (P < 0.001; 95% CI: 0.676–0.887, sensitivity: 0.676, specificity: 0.867) (Figure 2B, Table 3).
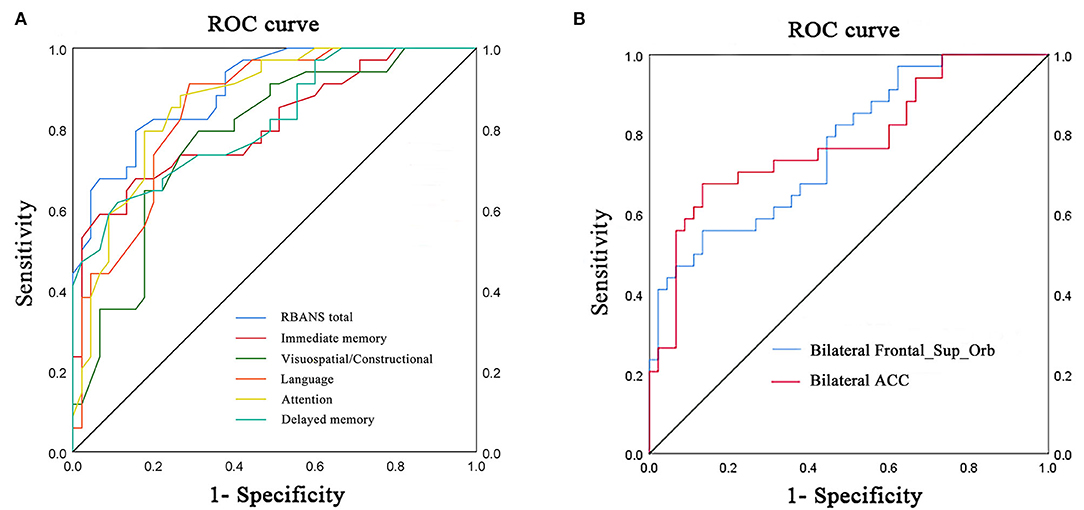
Figure 2. ROC curve analysis of RBANS test and PerAF values of altered brain regions in distinguishing LLD group from NCs group. (A) ROC curve analysis of RBANS total, immediate memory, visuospatial/constructional, language, attention, and delayed memory. (B) ROC curve analysis of bilateral Frontal_Sup_Orb and bilateral ACC. ROC, receiver operating characteristic; bilateral Frontal_Sup_Orb, bilateral superior frontal gyrus, orbital part; bilateral ACC, bilateral anterior cingulate cortex.
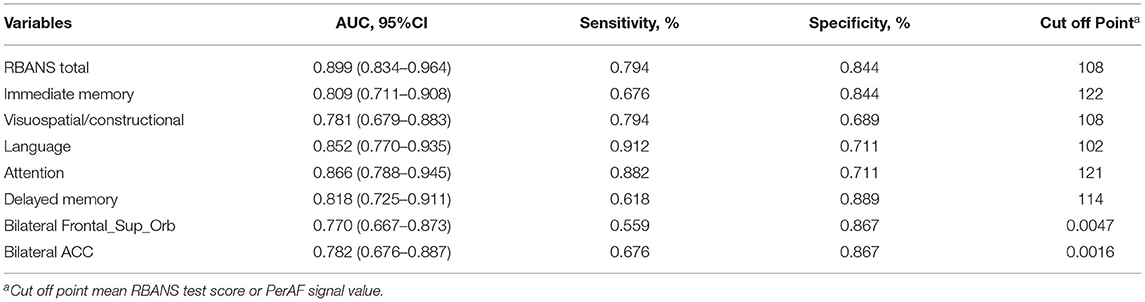
Table 3. ROC curve analysis of RNANS test and PerAF values of altered brain regions in distinguishing the LLD group from the NCs group.
Correlation Analysis
Linear correlation analysis only observed the positive correlation between the attention score of RBANS test and the bilateral Frontal_Sup_Orb (r = 0.344, p = 0.021, n = 45, Figure 3A) and bilateral ACC (r = 0.313, p = 0.036, n = 45, Figure 3B) in the LLD group, respectively. It is worth noting that the current correlation results did not passed the multiple-comparison correction.
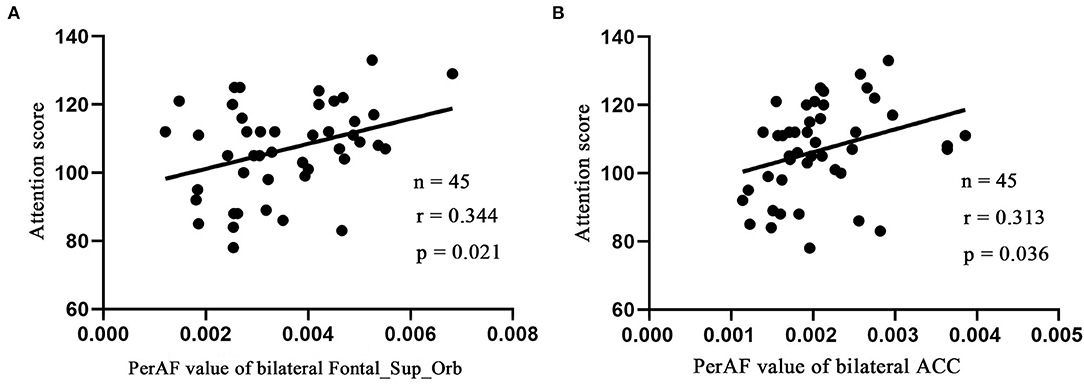
Figure 3. Pearson's correlation. Correlations were between attention score and bilateral Frontal_Sup_Orb (A), bilateral ACC (B). Bilateral Frontal_Sup_Orb, superior frontal gyrus, orbital part; bilateral ACC, bilateral anterior cingulate cortex.
Discussion
The current work performed a novel and more feasible method, namely, PerAF, which was also helpful in lowering the impact of BOLD signal strength, aiming to investigate the IBA in patients suffering from LLD. The findings indicated that the PerAF values of bilateral Frontal_Sup_Orb and bilateral ACC were significantly lower than those of the NCs. The RBANS test and PerAF values in the aforementioned altered brain regions exhibited a quite good discriminatory power with the AUC values in distinguishing the two groups.
Compared with the NCs group, patients with LLD exhibited poor-cognitive performance in all domains such as in the previous studies (33). Many hypotheses are present on the pathogenesis of LLD, one of which is depression executive dysfunction syndrome (DED). The main features of DED include decreased pleasure, intellectual disability, and lack of insight (34). In some cognitive tests, compared with the normal elderly, patients suffering from DED tend to score lower, such as language fluency, reaction inhibition, novelty problem solving, cognitive flexibility, and working memory, which are consistent with the destruction frontal-subcortical functional network. The disorder of subcortical structure, such as ACC, insula, and hippocampus, often occurs in patients with LLD, leading to abnormal IBA and projection to the pre-frontal cortex (PFC) (35).
Most researchers have demonstrated a declined metabolism within pre-frontal, parietal and temporal cortices and cingulate regions among patients suffering from major depression disorder (MDD) (36). According to the literature, frontal regions (ACC included) exert a key role in MDD-related ReHo pathophysiology. Similarly, our study also discovered that the IBA detected by the proposed PerAF method in bilateral Frontal_Sup_Orb and bilateral ACC of patients with LLD was decreased in contrast to the NCs group. Former research showed that, relative to controls, IBA of superior frontal gyrus among patients suffering from remitted geriatric depression was mitigated (37), and interestingly, the patients incorporated in our research suffered from moderate to severe depressive disorder. It suggests that the decreased IBA superior frontal gyrus does not recover with the relief of depression, which may be an inherent attribute of IBA in some patients suffering from LLD. However, these two studies are all cross-sectional designs.
Moreover, the current finding needs further support from large-scale longitudinal research. In addition, the ROC curve showed that the PerAF method was lower than the RBANS test in discriminatory power for differentiating the two groups. Indeed, the RBANS test, including immediate memory, visuospatial/constructional, language, attention, and delayed memory, is commonly used in the neuropsychological tests, it makes judgments based on the symptomatic characteristics of LLD, and the heterogeneity of the results varies greatly. The advantage of the PerAF method is that it is based on hemodynamics at the resting state. The results are objective and repeatable. From the observed perspective, it could be considered that bilateral Frontal_Sup_Orb and bilateral ACC both exhibited a quite good discriminatory power in differentiating the two groups, indicating that the PerAF method was potentially a neuroimaging indicator in identifying the individuals with depression among the elderly.
From the perspective of anatomical labeling atlas, Frontal_Sup_Orb is a part of the orbital frontal gyrus (OFC), which belongs to Brodmann area 11. Guo et al. (10) discovered that compared with the control group, the activation of the left superior temporal gyrus increased, while the activation of bilateral superior frontal gyrus decreased among first-episode, drug-naive patients with LLD (15). Similarly, an rs-fMRI study of late-life subthreshold depression (StD) revealed that, compared with the controls, subjects with StD displayed a lower ReHo value in the right OFC (38). OFC is considered to play a key role in the pathophysiology of depression (39). A study demonstrated that patients with LLD exhibited smaller OFC volumes and positively correlated with white-matter lesion volume (40). A study exhibited that compared with patients with LLD without odor identification (OI) impairment and normal controls, patients with LLD with OI impairment exhibited increased functional connectivity (FC) between the left OFC and left calcarine gyrus, between the left OFC and right lingual gyrus, between the right OFC and right rectus gyrus (41). Odor identification (OI) impairment increases the risk of Alzheimer's disease in patients with LLD (42). Hypoactivity in the superior frontal gyrus may partially result in non-response to initial antidepressants in patients with LLD (43). Reports on OFC indicate that abnormal resting-state activity in Frontal_Sup_Orb or OFC may be related to the persistence of depressive symptoms in some patients with LLD, which should also arouse the attention of the elderly with StD.
The ACC connects structurally and functionally with various brain areas, including the lateral pre-frontal cortex, OFC, parietal cortex, amygdala, superior temporal gyrus (STG), nucleus accumbens, hypothalamus, insula, raphe nucleus, and hippocampus (44, 45). In addition, the ACC is thought to play a crucial role in allocating attentional resources in situations of conflicting cognitive and emotional demands (46). One study revealed that patients with LLD in the depressed and remitted phases showed significantly smaller gray matter volume in the left ACC and left posterior STG than healthy subjects (47). In addition, they also discovered that remitted patients with LLD showed lower functional ACC-pSTG connectivity than healthy subjects and positively correlated with lower global function during remission. Liu et al. (48) applied a novel analytical method, named coherence-based regional homogeneity (Cohe–ReHo), to assess regional IBA during the resting state in 15 first-episode, treatment-naive patients with LLD and demonstrated that, compared with the healthy controls, the LLD group showed significantly decreased Cohe–ReHo in right ACC (48). Thus, our finding that decreased PerAF in the bilateral ACC is compatible with these previous studies. These findings suggest that functional alteration in ACC is deeply involved in depression.
Executive function processes, such as focusing attention, organizing, and strategizing, are representative symptoms of LLD, fully demonstrated to be maintained by frontotemporal brain regions (49). The superior frontal gyrus is conventionally considered the frontal eye field. However, functional research has revealed the major contribution of this region to executive function, working memory, and attention (50, 51). As a major function of cognition, attention is essential to perception, language, and memory. Individuals with MDD exhibited impairment in emotion and attention control. The regulation of emotion is vital for adaptive behavior in a social environment. The different strategies may be adopted to achieve successful emotion regulation, ranging from attentional control (e.g., distraction) to cognitive change (e.g., reappraisal). A study revealed that the OFC is involved in distinguishing presently relevant from previously relevant information and was activated for reappraisal only (52, 53).
In contrast, the attentional control condition is characterized by orienting attention away from the emotional stimulus to a cognitive task, the commitment of resources to the processing of this task, and the detection of potential conflicts between task processing and emotional activation. The dorsal portion of the ACC has been widely discussed as a major node in the attentional control network, for the monitoring of conflict between opposing activations (54). Loeffler et al. (55) thought that attention control and emotion regulation are conceptually similar and might share common mechanisms (55), which indicate that emotional disturbances among patients suffering from LLD can be ameliorated through interventions with target attention control.
In this study, the attention score of the RBANS test is positively correlated with the PerAF values in Frontal_Sup_Orb_L and bilateral ACC. During the cognitive assessment of the RBANS test, the Attention test contains two subtests, respectively, Digit Span Test and Symbol-Digit Coding Test. The former can reflect working memory capacity to a certain extent, while the latter is closely related to psychomotor speed and cognitive flexibility (56, 57), which also reflects the inherent complexity and hierarchy of advanced cognitive function. Attention, interference control, and working memory are essential components of executive function. Executive dysfunction is one of the representative symptoms of patients with LLD. Therefore, it can be speculated that in future intervention studies, if bilateral Frontal_Sup_Orb and bilateral ACC can be used as therapeutic targets, it may be conducive to improving the executive function of LLD.
However, the research has some limitations. First, the participants all come from or around Beijing, near our hospital, which decides the small sample size. Second, adequate attention must be paid to potential confounders, such as diabetes and hypertension, often co-morbid with LLD. Personality and special skills also affect resting-state hemodynamic fluctuations, thus, requiring further evaluation and control in future research. Third, changes in cognitive impairment severity result in confounding bias though average MMSE scores of the two groups are all >24. Finally, research has demonstrated that the neuroendocrine stress and subjective reactions resulting from MRI involvement affect brain functioning, which relates to adverse reactions, such as dizziness, phosphenes, and head ringing (58). To solve the existing problem, a several-minute habituation time is recommended for the participants by the scanner in MRI research before the actual examination, particularly, for female participants (59).
Conclusion
Notwithstanding the limitations mentioned earlier, the research showed that changes of PerAF in bilateral Frontal_Sup_Orb and bilateral ACC are related to an increased risk of developing LLD. Moreover, the PerAF method could be used as an underlying sensitivity biomarker to identify the psychiatric disorders.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Beijing Anding Hospital Affiliated to Capital Medical University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
CL and WP conceived and designed the research protocol. CL completed the data analyses. DZ and PM assisted with neuropsychological assessment and data processing. YR and XM checked the rs-fMRI data and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The present work was supported by Beijing Municipal Science & Technology Commission (No. Z191100006619105).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sjöberg L, Karlsson B, Atti AR, Skoog I, Fratiglioni L, Wang HX. Prevalence of depression: comparisons of different depression definitions in population-based samples of older adults. J Affect Disord. (2017) 221:123–31. doi: 10.1016/j.jad.2017.06.011
2. Li H, Lin X, Liu L, Su S, Zhu X, Zheng Y, et al. Disruption of the structural and functional connectivity of the frontoparietal network underlies symptomatic anxiety in late-life depression. NeuroImage Clin. (2020) 28:102398. doi: 10.1016/j.nicl.2020.102398
3. Manning K, Wang L, Steffens D. Recent advances in the use of imaging in psychiatry: functional magnetic resonance imaging of large-scale brain networks in late-life depression. F1000Res. (2019) 8:1366. doi: 10.12688/f1000research.17399.1
4. Takami H, Okamoto Y, Yamashita H, Okada G, Yamawaki S. Attenuated anterior cingulate activation during a verbal fluency task in elderly patients with a history of multiple-episode depression. Am J Geriatr Psychiatry. (2007) 15:594–603. doi: 10.1097/01.JGP.0b013e31802ea919
5. Aizenstein HJ, Butters MA, Figurski JL, Stenger VA, Reynolds CF. third, Carter CS. Prefrontal and striatal activation during sequence learning in geriatric depression. Biol Psychiatry. (2005) 58:290–96. doi: 10.1016/j.biopsych.2005.04.023
6. Zou K, Deng W, Li T, Zhang B, Jiang L, Huang C, et al. Changes of brain morphometry in first-episode, drug-naïve, non-late-life adult patients with major depression: an optimized voxel-based morphometry study. Biol Psychiatry. (2010) 67:186–88. doi: 10.1016/j.biopsych.2009.09.014
7. Lebreton M, Bavard S, Daunizeau J, Palminteri S. Assessing inter-individual differences with task-related functional neuroimaging. Nat Hum Behav. (2019) 3:897–905. doi: 10.1038/s41562-019-0681-8
8. Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. (1995) 34:537–41. doi: 10.1002/mrm.1910340409
9. Dai Z, He Y. Disrupted structural and functional brain connectomes in mild cognitive impairment and Alzheimer's disease. Neurosci Bull. (2014) 30:217–32. doi: 10.1007/s12264-013-1421-0
10. Guo WB, Sun XL, Liu L, Xu Q, Wu RR, Liu Z N, et al. Disrupted regional homogeneity in treatment-resistant depression: a resting-state fMRI study. Prog Neuropsychopharmacol Biol Psychiatry. (2011) 35:1297–302. doi: 10.1016/j.pnpbp.2011.02.006
11. Peng DH, Jiang KD, Fang YR, Xu YF, Shen T, Long XY, et al. Decreased regional homogeneity in major depression as revealed by resting-state functional magnetic resonance imaging. Chin Med J (Engl). (2011) 124:369–73. doi: 10.3760/cma.j.issn.0366-6999.2011.03.009
12. Yao X, Yin Z, Liu F, Wei S, Zhou Y, Jiang X, et al. Shared and distinct regional homogeneity changes in bipolar and unipolar depression. Neurosci Lett. (2018) 673:28–32. doi: 10.1016/j.neulet.2018.02.033
13. Dai XJ, Liu CL, Zhou RL, Gong HH, Wu B, Gao L, et al. Long-term total sleep deprivation decreases the default spontaneous activity and connectivity pattern in healthy male subjects: a resting-state fMRI study. Neuropsychiatr Dis Treat. (2015) 11:761–72. doi: 10.2147/NDT.S78335
14. Zou QH, Zhu CZ, Yang Y, Zuo XN, Long XY, Cao QJ, et al. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J Neurosci Methods. (2008) 172:137–41. doi: 10.1016/j.jneumeth.2008.04.012
15. Guo WB, Liu F, Xun GL, Hu MR, Guo XF, Xiao CQ, et al. Reversal alterations of amplitude of low-frequency fluctuations in early and late onset, first-episode, drug-naive depression. Prog Neuropsychopharmacol Biol Psychiatry. (2013) 40:153–59. doi: 10.1016/j.pnpbp.2012.08.014
16. Chen J D, Liu F, Xun GL, Chen HF, Hu M R, Guo XF, et al. Early and late onset, first-episode, treatment-naive depression: same clinical symptoms, different regional neural activities. J Affect Disord. (2012) 143:56–63. doi: 10.1016/j.jad.2012.05.025
17. Jia XZ, Sun JW Ji GJ, Liao W, Lv YT, Wang J, et al. Percent amplitude of fluctuation: A simple measure for resting-state fMRI signal at single voxel level. PloS ONE. (2020) 15:e0227021. doi: 10.1371/journal.pone.0227021
18. Zhao N, Yuan LX, Jia XZ, Zhou XF, Deng XP, He HJ, et al. Intra- and inter-scanner reliability of voxel-wise whole-brain analytic metrics for resting state fMRI. Front Neuroinform. (2018) 12:54. doi: 10.3389/fninf.2018.00054
19. O'Shea DM, Fieo RA, Hamilton JL, Zahodne LB, Manly JJ, Stern Y. Examining the association between late-life depressive symptoms, cognitive function, and brain volumes in the context of cognitive reserve. Int J Geriatr Psychiatry. (2015) 30:614–22. doi: 10.1002/gps.4192
20. Huang CM, Fan YT, Lee SH, Liu HL, Chen YL, Lin C, et al. Cognitive reserve-mediated neural modulation of emotional control and regulation in people with late-life depression. Soc Cogn Affect Neurosci. (2019) 14:849–60. doi: 10.1093/scan/nsz054
21. Ganguli M, Du Y, Dodge HH, Ratcliff GG, Chang CC. Depressive symptoms and cognitive decline in late life: a prospective epidemiological study. Arch Gen Psychiatry. (2006) 63:153–60. doi: 10.1001/archpsyc.63.2.153
22. Becker JT, Chang YF, Lopez OL, Dew MA, Sweet RA, Barnes D, et al. Depressed mood is not a risk factor for incident dementia in a community-based cohort. Am J Geriatr Psychiatry. (2009) 17:653–63. doi: 10.1097/JGP.0b013e3181aad1fe
23. American Psychiatric Association. The Diagnostic and Statistical Manual of Mental Disorders, 5th Edn. Washington: American Psychiatric Association. (2013).
24. Mendes-Silva AP, Vieira ELM, Xavier G, Barroso LSS, Bertola L, Martins EAR. Telomere shortening in late-life depression: a potential marker of depression severity. Brain Behav. (2021) 11:e2255. doi: 10.1002/brb3.2255
25. Bohr IJ, Kenny E, Blamire A, O'Brien JT, Thomas AJ, Richardson J, et al. Resting-state functional connectivity in late-life depression: higher global connectivity and more long distance connections. Front Psychiatry. (2012) 3:116. doi: 10.3389/fpsyt.2012.00116
26. Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. (1998) 20:310–19. doi: 10.1076/jcen.20.3.310.823
27. Bailie JM, King LC, Kinney D, Nitch SR. The relationship between self-reported neuropsychological risk factors and RBANS test performance among forensically committed psychiatric inpatients. Appl Neuropsychol Adult. (2012) 19:279–86. doi: 10.1080/09084282.2012.670146
28. Jia XZ, Wang J, Sun HY, Zhang H, Zang YF. RESTplus: an improved toolkit for resting-state functional magnetic resonance imaging data processing. Sci Bull. (2019) 64:953–54. doi: 10.1016/j.scib.2019.05.008
29. Chen LC Li X, Shen L. Self-limited focal epilepsy decreased regional brain activity in sensorimotor areas. Acta Neurol Scand. (2021) 143:188–94. doi: 10.1111/ane.13350
30. Zheng Y, Xie Y, Qi M, Zhang L, Wang W, Zhang W, et al. Ginkgo biloba extract is comparable with donepezil in improving functional recovery in Alzheimer's disease: results from a multilevel characterized study based on clinical features and resting-state functional magnetic resonance imaging. Front Pharmacol. (2021) 12:721216. doi: 10.3389/fphar.2021.721216
31. Gong W, Wan L, Lu W, Ma L, Cheng F, Cheng W, et al. Statistical testing and power analysis for brain-wide association study. Med Image Anal. (2018) 47:15–30. doi: 10.1016/j.media.2018.03.014
32. Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. (2002) 15:273–89. doi: 10.1006/nimg.2001.0978
33. Riddle M, Potter GG, McQuoid DR, Steffens DC, Beyer JL, Taylor WD. Longitudinal cognitive outcomes of clinical phenotypes of late-life depression. Am J Geriatr Psychiatry. (2017) 25:1123–34. doi: 10.1016/j.jagp.2017.03.016
34. Rapp MA, Dahlman K, Sano M, Grossman HT, Haroutunian V, Gorman JM. Neuropsychological differences between late-onset and recurrent geriatric major depression. Am J Psychiatry. (2005) 162:691–98. doi: 10.1176/appi.ajp.162.4.691
35. Alexopoulos GS, Murphy CF, Gunning-Dixon FM, Glatt CE, Latoussakis V, Kelly RE, et al. Serotonin transporter polymorphisms, microstructural white matter abnormalities and remission of geriatric depression. J Affect Disord. (2009) 119:132–41. doi: 10.1016/j.jad.2009.03.004
36. Greicius MD, Flores BH, Menon V, Glover G H, Solvason HB, Kenna H, et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. (2007) 62:429–37. doi: 10.1016/j.biopsych.2006.09.020
37. Yuan Y, Zhang Z, Bai F., Yu H, Shi Y, Qian Y, et al. Abnormal neural activity in the patients with remitted geriatric depression: a resting-state functional magnetic resonance imaging study. J Affect Disord. (2008) 111:145–52. doi: 10.1016/j.jad.2008.02.016
38. Ma Z, Li R, Yu J, He Y, Li J. Alterations in regional homogeneity of spontaneous brain activity in late-life subthreshold depression. PLoS ONE. (2013) 8:e53148. doi: 10.1371/journal.pone.0053148
39. Saleh A, Potter GG, McQuoid DR, Boyd B, Turner R, MacFall JR, et al. Effects of early life stress on depression, cognitive performance and brain morphology. Psychol Med. (2017) 47:171–81. doi: 10.1017/S0033291716002403
40. Taylor WD, Macfall JR, Payne ME, McQuoid DR, Steffens DC, Provenzale JM, et al. Orbitofrontal cortex volume in late life depression: influence of hyperintense lesions and genetic polymorphisms. Psychol Med. (2007) 37:1763–73. doi: 10.1017/S0033291707000128
41. Yang M, Chen B, Zhong X, Zhou H, Mai N, Zhang M, et al. Disrupted olfactory functional connectivity in patients with late-life depression. J Affect Disord. (2022) 306:174–81. doi: 10.1016/j.jad.2022.03.014
42. Chen B, Zhong X, Mai N, Peng Q, Wu Z, Ouyang C, et al. Cognitive impairment and structural abnormalities in late life depression with olfactory identification impairment: an Alzheimer's disease-like pattern. Int J Neuropsychopharmacol. (2018) 21:640–48. doi; 10.1093/ijnp/pyy016. doi: 10.1093/ijnp/pyy016
43. Voshaar RC, Kapur N, Bickley H, Williams A, Purandare N. Suicide in later life: a comparison between cases with early-onset and late-onset depression. J Affect Disord. (2011) 132:185–91. doi: 10.1016/j.jad.2011.02.008
44. Margulies D S, Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham M P. Mapping the functional connectivity of anterior cingulate cortex. Neuroimage. (2007) 37:579–88. doi: 10.1016/j.neuroimage.2007.05.019
45. Ikuta T, Matsuo K, Harada K, Nakashima M, Hobara T, Higuchi N, et al. Disconnectivity between dorsal raphe nucleus and posterior cingulate cortex in later life Ddpression. Front Aging Neurosci. (2017) 9:236. doi: 10.3389/fnagi.2017.00236
46. Gasquoine PG. Localization of function in anterior cingulate cortex: from psychosurgery to functional neuroimaging. Neurosci Biobehav Rev. (2013) 37:340–48. doi: 10.1016/j.neubiorev.2013.01.002
47. Harada K, Ikuta T, Nakashima M, Watanuki T, Hirotsu M, Matsubara T, et al. Altered connectivity of the anterior cingulate and the posterior superior temporal gyrus in a longitudinal study of later-life depression. Front Aging Neurosci. (2018) 10:31. doi: 10.3389/fnagi.2018.00031
48. Liu F, Hu M, Wang S, Guo W, Zhao J, Li J, et al. Abnormal regional spontaneous neural activity in first-episode, treatment-naive patients with late-life depression: a resting-state fMRI study. Prog Neuropsychopharmacol Biol Psychiatry. (2012) 39:326–31. doi: 10.1016/j.pnpbp.2012.07.004
49. Nyhus E, Barceló F. The Wisconsin card sorting test and the cognitive assessment of prefrontal executive functions: a critical update. Brain Cogn. (2009) 71:437–51. doi: 10.1016/j.bandc.2009.03.005
50. du Boisgueheneuc F, Levy R, Volle E, Seassau M, Duffau H, Kinkingnehun S, et al. Functions of the left superior frontal gyrus in humans: a lesion study. Brain. (2006) (129):3315–28. doi: 10.1093/brain/awl244
51. Hadanny A, Daniel-Kotovsky M, Suzin G., Boussi-Gross R, Catalogna M, Dagan K, et al. Cognitive enhancement of healthy older adults using hyperbaric oxygen: a randomized controlled trial. Aging. (2020) 12:13740–61.
52. Schnider A, Valenza N, Morand S, Michel CM. Early cortical distinction between memories that pertain to ongoing reality and memories that don't. Cereb Cortex. (2002) 12:54–61. doi: 10.1093/cercor/12.1.54
53. Kanske P, Heissler J, Schönfelder S, Bongers A, Wessa M. How to regulate emotion? Neural networks for reappraisal and distraction. Cereb Cortex. (2011) 21:1379–88. doi; 10.1093/cercor/bhq216. doi: 10.1093/cercor/bhq216
54. Botvinick MM. Conflict monitoring and decision making: reconciling two perspectives on anterior cingulate function. Cogn Affect Behav Neurosci. (2007) 7:356–66. doi: 10.3758/CABN.7.4.356
55. Loeffler LAK, Satterthwaite TD, Habel U, Schneider F, Radke S, Derntl B. Attention control and its emotion-specific association with cognitive emotion regulation in depression. Brain Imaging Behav. (2019) 13:1766–79. doi: 10.1007/s11682-019-00174-9
56. Klojčnik M, Kavcic V, Bakracevic Vukman K. Relationship of depression with executive functions and visuospatial memory in elderly. Int J Aging Hum Dev. (2017) 85:490–503. doi: 10.1177/0091415017712186
57. Iverson GL, Brooks BL, Ashton VL, Johnson LG, Gualtieri CT. Does familiarity with computers affect computerized neuropsychological test performance? J Clin Exp Neuropsychol. (2009) 31:594–604. doi: 10.1080/13803390802372125
58. Mutschler I, Wieckhorst B, Meyer AH, Schweizer T, Klarhöfer M, Wilhelm FH, et al. Who gets afraid in the MRI-scanner? Neurogenetics of state-anxiety changes during an fMRI experiment. Neurosci lett. (2014) 583:81–6. doi: 10.1016/j.neulet.2014.09.021
Keywords: intrinsic brain activity, late-life depression, percent amplitude of fluctuation, receiver operating characteristic, biomarker
Citation: Liu C, Pan W, Zhu D, Mao P, Ren Y and Ma X (2022) Altered Intrinsic Brain Activity in Patients With Late-Life Depression: A Resting-State Functional MRI Study. Front. Psychiatry 13:894646. doi: 10.3389/fpsyt.2022.894646
Received: 12 March 2022; Accepted: 25 April 2022;
Published: 23 May 2022.
Edited by:
Lirong Liang, Capital Medical University, ChinaCopyright © 2022 Liu, Pan, Zhu, Mao, Ren and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanping Ren, cmVueWFucGluZ0BjY211LmVkdS5jbg==; Xin Ma, bWF4aW5hbmRpbmdAY2NtdS5lZHUuY24=
 Chaomeng Liu
Chaomeng Liu Weigang Pan1
Weigang Pan1 Xin Ma
Xin Ma