- 1Department of Pharmacy Administration and Clinical Pharmacy, School of Pharmaceutical Sciences, Peking University, Beijing, China
- 2Department of Population Medicine, Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston, MA, United States
- 3International Research Center for Medicinal Administration, Peking University, Beijing, China
Objective: We aimed to compare the efficacy, acceptability, and tolerability of vortioxetine in the treatment of Major Depressive Disorder (MDD) in adults.
Method: We searched PubMed, Embase, Web of Science, Cochrane Central Register of Controlled Clinical Trials (CENTRAL), and www.ClinicalTrials.gov for randomized controlled trials that examined vortioxetine vs. placebo or other antidepressants for the treatment of MDD from database inception to August 30, 2021, using keywords Vortioxetine, Brintellix, Trintellix, LuAA21004, major depressive disorder, mood disorder, affective disorder, and MDD. We identified 789 publications after removing duplicates. After screening, 20 eligible randomized controlled trials were identified, of which 19 were included in the final meta-analysis. We included adults (aged 18 years and older) with a primary diagnosis of MDD. Two review authors independently selected the studies and extracted data. We extracted data on study characteristics, participant characteristics, intervention details and outcome measures in terms of efficacy, acceptability, and tolerability. Analyses were performed using random-effects models, and outcomes were pooled as risk ratios (RRs) and standardized mean differences (SMDs).
Results: In total, 20 studies (8,547 participants) met the inclusion criteria. Vortioxetine outperformed the placebo in efficacy outcomes, including response (RR 1.35, 95% CI 1.23–1.48; P < 0.001), remission (RR 1.33, 95% CI 1.17–1.52; P < 0.001), and cognitive function (SMD 0.34, 95% CI 0.16–0.52; P = 0.0003). Compared with the serotonin noradrenaline reuptake inhibitors (SNRIs), vortioxetine had better tolerability (RR 0.90, 95% CI 0.86–0.94; P < 0.001) but no significant difference in response (RR 0.91, 95%CI 0.82–1.00; P = 0.06) or remission (RR: 0.99, 95% CI 0.81–1.20, P = 0.88). Vortioxetine had no difference in response (RR 1.08, 95% CI 0.88–1.32; P = 0.46), remission (RR 1.00, 95% CI 0.41–2.44; P = 1.00) comparing with selective serotonin reuptake inhibitors (SSRIs).
Conclusions: Vortioxetine is more advantageous over placebo in treating MDD among adults, but no significant difference compared to SNRIs and SSRIs in general.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42021278355, identifier: CRD42021278355.
Introduction
Major depressive disorder (MDD) is characterized by depressed mood, loss of pleasure, low energy, and feelings of low self-worth (1). According to the World Health Organization, MDD is the most prevalent psychiatric disorder globally (2), affecting more than 350 million individuals worldwide (3). MDD reduced the patients' quality of life (4), lowered social productivity (5), increased their suicide attempts (6), and burdened their financial status (7), resulting in significant and far-reaching adverse medical and social consequences.
Various pharmacological and psychological interventions have been used to treat MDD. Major pharmacotherapies include selective serotonin reuptake inhibitors (SSRIs), serotonin noradrenaline reuptake inhibitors (SNRIs), conventional tricyclic antidepressants (TCAs), monoamine oxidase inhibitors (MAOIs), and other antidepressant agents. Most guidelines by far recommend SSRIs as the first-line treatment due to their minor adverse effects (8, 9). Though antidepressants approved for treating MDD is increasing, only 50% of patients have sufficient response to treatment with adequate duration (10), about 40% of patients who achieved remission are at risk of relapse within a year (11, 12). Therefore, discovering antidepressants with superior effectiveness and tolerability is in urgent clinical need in treating MDD (13).
Vortioxetine is a novel antidepressant with multimodal activities that was first licensed by the United States' Food and Drug Administration (FDA) to treat MDD in adults in 2013 (14–16), and has a distinct pharmacological profile from current treatment options by both directly modulating 5-HT receptors and inhibiting the serotonin transporter (14, 15). The clinical trials demonstrated significantly improved Montgomery-Åsberg Depression Scale (MADRS) scores and increased in response rate in those with depression who were administered vortioxetine compared with placebo (5, 17–19), and have also shown a favorable tolerability profile and improved cognitive dysfunction (20). With a growing number of studies focusing on the vortioxetine treatment of MDD in recent years, this study aimed to conduct a systematic review to assemble the efficacy, acceptability, and tolerability of vortioxetine in treating MDD compared to placebo, SNRIs and SSRIs.
Method
Search Strategy and Selection Criteria
For this systematic review and meta-analysis, we searched PubMed, Embase, Web of Science, and the Cochrane Central Register of Controlled Clinical Trials (CENTRAL) for studies published from database inception to August 30, 2021. To identify ongoing or unpublished studies, we searched ClinicalTrials.gov, using keywords “Vortioxetine,” “Brintellix,” “Trintellix,” “LuAA21004,” “major depressive disorder,” “mood disorder,” “affective disorder,” and “MDD.” Manual searches were conducted in the reference sections of the published meta-analyses and reviews to identify potentially relevant articles. We imposed no language, publication status, or publication type restrictions.
Protocol and Registration
This study was registered on PROSPERO (CRD42021278355) and conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses recommendations (21).
Types of Studies
Only RCTs were included in this review.
Types of Participants
We included adults (aged 18 years and older) with a primary diagnosis of MDD according to the standard operationalized diagnostic criteria.
Types of Interventions
Studies with vortioxetine as monotherapy vs. any comparator intervention were included. Identified comparisons include placebo and other antidepressants, such as SSRIs, SNRIs, TCAs, MAOIs, and other antidepressant agents. We combined various dosages of the same comparator into a single group.
Types of Outcome Measures
The efficacy outcome includes response (≥50% reduction on an observer-rated depression scale), remission [MADRS ≤ 10, Hamilton Depression Rating Scale (HAM-D) total score ≤ 7], tolerability (total number of participants experiencing at least one adverse event), acceptability (dropout due to adverse events) and cognitive function. For response or remission rates, we gave preference to MADRS outcomes over HAM-D. Other validated depression scales are also acceptable if neither was reported (8).
Study Selection
Two independent reviewers screened the titles and abstracts using the systematic review program, obtained relevant studies in full, and determined separately whether the study met the inclusion criteria (with an average kappa of 0.89). Any disagreements between reviewers were resolved via discussion to achieve consensus, and a third reviewer consulted if the two initial reviewers could not reach a consensus.
Data Extraction
Two reviewers' extracted data into a pre-piloted, standardized data extraction form in Microsoft Excel 2019, detailing the sample size, mean age, diagnostic criteria, interventions, comparisons, the dose of administration, and outcomes of each study. We contacted the authors for additional information when data were not reported in full. Data were extracted as a single study when we identified multiple associated publications. For randomized controlled trials, we assessed the risk of bias using the Cochrane Risk of Bias Tool to generate allocation sequence, allocation concealment, masking of study personnel and participants, masking of outcome assessor, attrition bias, and selective outcome reporting bias. According to the criteria set out in the Cochrane Handbook for Systematic Reviews of Interventions (22), each trial was classified into low, unclear, or high risk. At least two independent reviewers selected the studies and assessed the risk of bias.
Statistical Analysis
We used Review Manager (version 5.3; Cochrane Collaboration, Oxford, United Kingdom) and the STATA version 15 for all analysis. Continuous outcomes were pooled as standardized mean differences (SMDs). Dichotomous data were pooled as risk ratios (RRs). We contacted the authors for additional data when the reported information was insufficient to calculate the mean and the standard deviation. Heterogeneity between studies was assessed using I2 values. Thresholds for the interpretation of heterogeneity were consistent with those of the Cochrane Collaboration [I2 values of 0%−40% might not be important; 30%−60% may represent moderate heterogeneity; 50%−90% may represent substantial heterogeneity; and 75%−100% had high levels of heterogeneity; Higgins and Green, (23)]. A fixed-effects model was used if heterogeneity was moderate or less; otherwise, a random-effects model was applied. For all analysis, statistical significance was set at P < 0.05. We performed sensitivity analysis by excluding studies judged at high risk of bias.
Evaluation of Publication Bias
Publication bias was assessed by visually inspecting funnel plots and Egger's linear regression intercept in Stata [Egger et al. (24)] in our meta-analysis. Significant publication bias was defined as a P-value <0.1.
Results
Study Selection
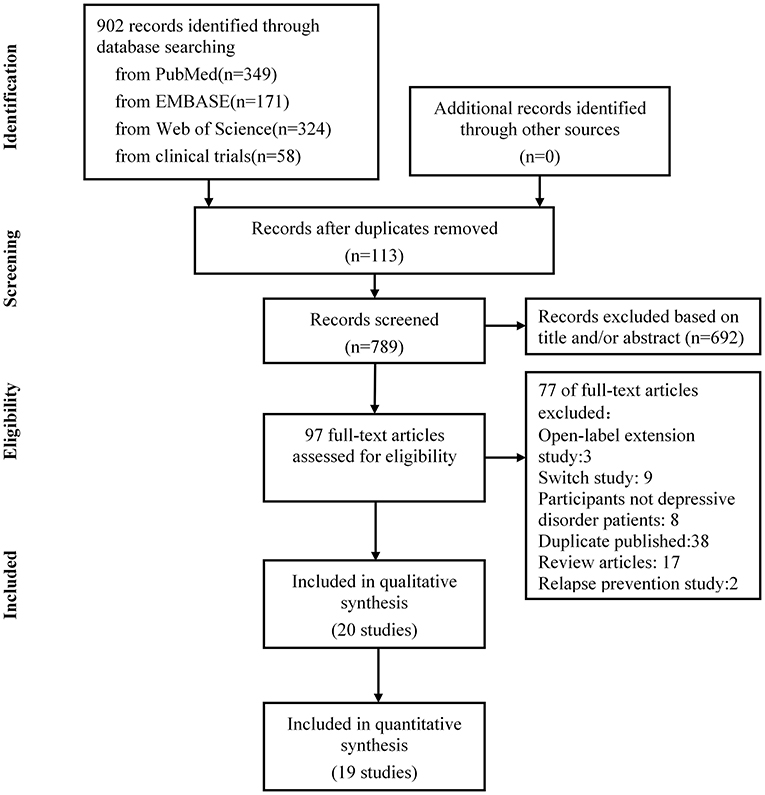
The literature search was updated on September 2021. Nine hundred and two publications were identified through electronic search, manual search, author contact, and trial registries. We retrieved 97 full-text articles for detailed examination, excluded 77 articles that did not meet the eligibility criteria, and removed 38 publications reporting the same trials. Further, we excluded eight studies that did not meet our inclusion criteria for depressive disorder patients, two studies of relapse prevention, 17 reported review articles, three open-label extension studies, and nine trials with inadequate response. After screening, 20 eligible randomized controlled trials were identified, of which 19 were included in the final meta-analysis. The PRISMA flowchart is presented in Figure 1.
Description of Studies
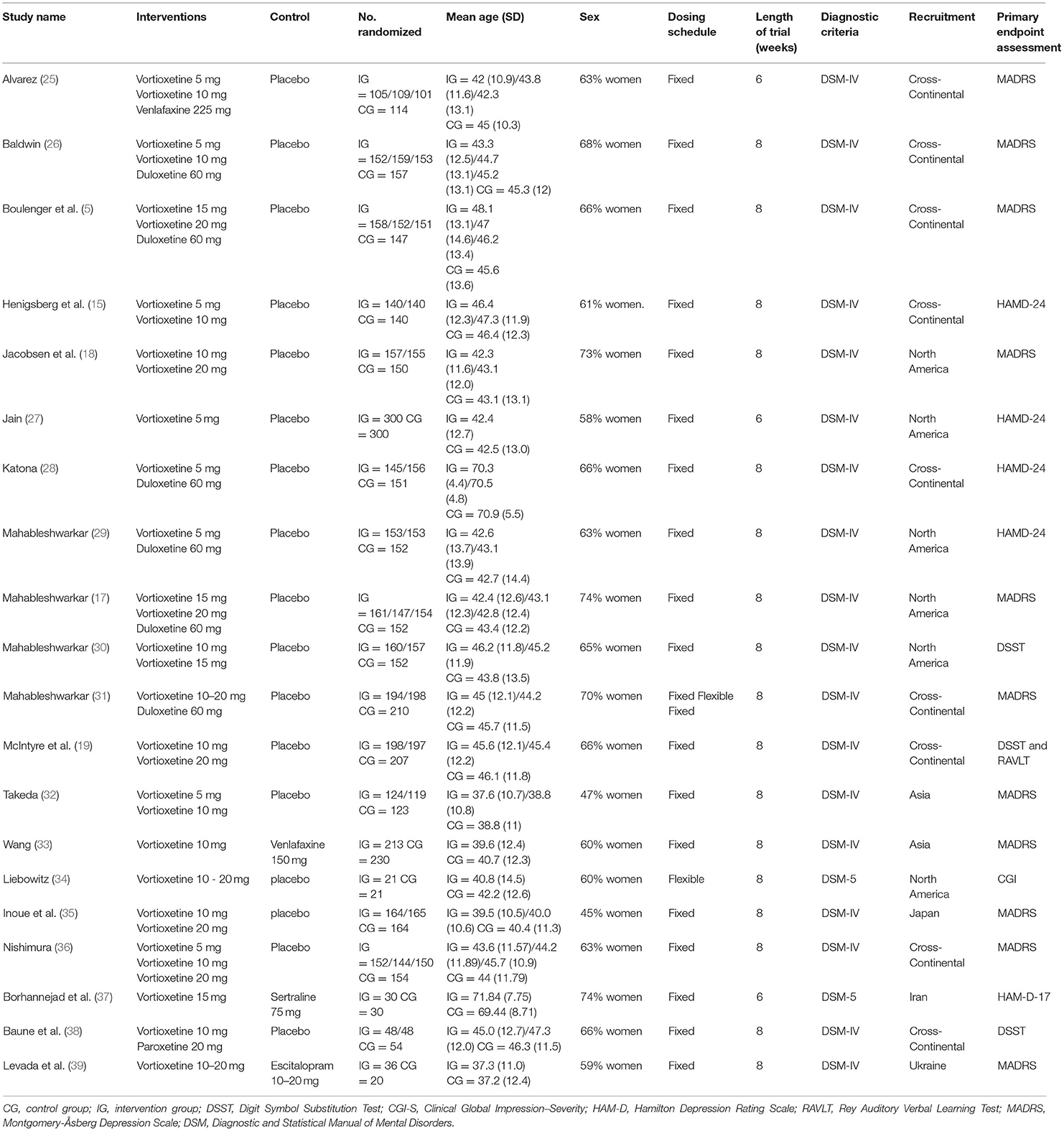
Table 1 summarized the characteristics of each included randomized controlled trial. Overall, this study comprised 8,547 participants, of which 4,598 were allocated to vortioxetine, 2,538 to the placebo, 98 to SSRIs, 1,313 to SNRIs. Among the included trials, eight were triple-armed with vortioxetine, an active comparison, and a placebo; 12 were double-armed, including nine placebo-controlled and three active comparisons trials. Nine trials compared vortioxetine with placebo only; two compared the efficacy of vortioxetine with that of SSRIs; eight compared vortioxetine with SNRIs. The vortioxetine dosage ranged from 5 to 20 mg/day in the studies. Three studies lasted 6 weeks, and 17 studies lasted 8 weeks.
For the study outcomes, 19 studies provided the response and remission rates. All but two studies reported tolerability and acceptability. Seventeen studies reported dropouts owing to adverse effects. Eleven studies used MADRS to measure the outcome, four used the HAM-D-24, three used the Digit Symbol Substitution Test (DSST), others used the Clinical Global Impression–Severity (CGI-S), the HAM-D-17, and the Rey Auditory Verbal Learning Test (RAVLT) as the outcome measure.
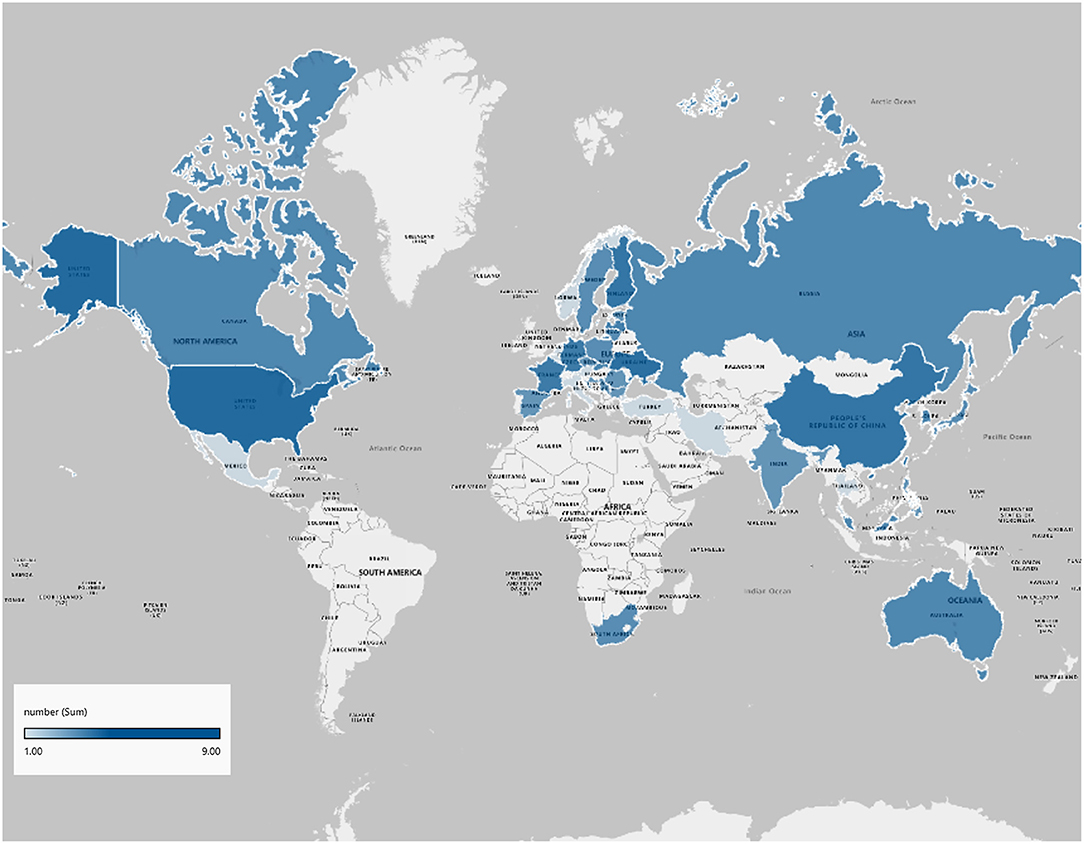
Regarding the representativeness of participants, nine studies were multinational across continents and nine were multicenter studies conducting in a single nation in our analysis and two (10%) were conducted at a single center. Figure 2 shows an overview of the countries where participants were recruited.
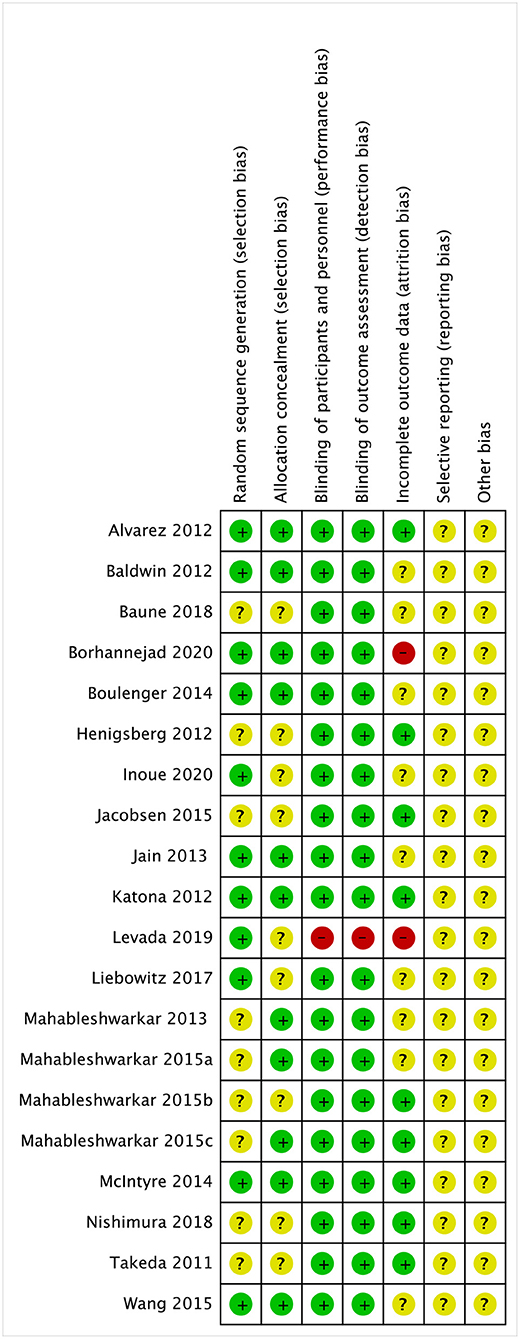
Graphical representations of the risk of bias assessment are shown in Figure 3. Briefly, nine studies (45%) of the randomized controlled trials did not report adequate randomization sequence generation and concealment, while the 19 studies (95%) were at low risk of bias for masking of participants, personnel, and outcome assessors.
Effects of Interventions
We presented the meta-analysis results by grouping the comparators into three categories: placebo, SNRIs, and SSRIs.
Vortioxetine vs. Placebo
Sixteen studies with 6755 participants provided data comparing vortioxetine vs. placebo.
Response
Vortioxetine had a significantly higher response rate than placebo (RR 1.35, 95% CI = 1.23–1.48; P < 0.001; I2 = 53%; Figure 4A).
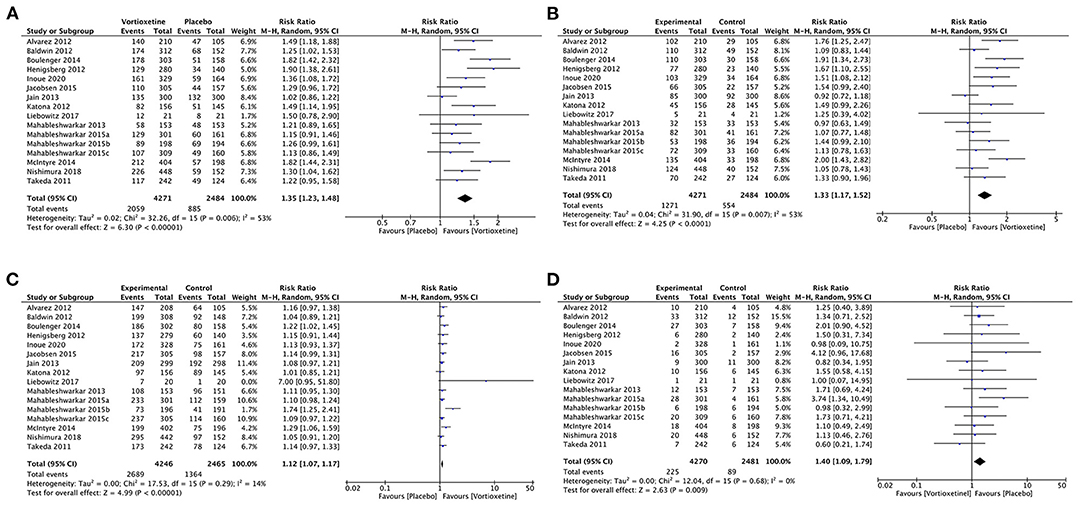
Figure 4. Forest plot of comparison on vortioxetine vs. placebo (A) Response, (B) remission, (C) tolerability, and (D) acceptability.
Remission
Vortioxetine was associated with a higher remission rate than the placebo (RR 1.33, 95% CI = 1.17–1.52; P < 0.001; I2 = 53%; Figure 4B).
Tolerability
Patients using vortioxetine experienced more adverse effects than the placebo (RR 1.12, 95% CI = 1.07–1.17; P < 0.001; I2 = 14%; Figure 4C).
Acceptability
Vortioxetine was associated with a higher dropout rate due to adverse events than the placebo (RR 1.40, 95% CI = 1.09–1.79; P = 0.009; I2 = 0%; Figure 4D).
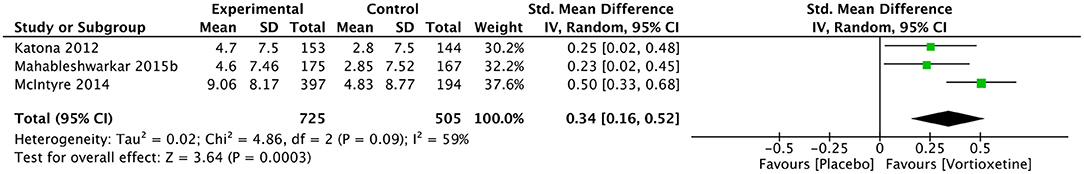
Cognitive Function
Vortioxetine significantly improved DSST score than the placebo (SMD 0.34, 95% CI = 0.16–0.52; P = 0.0003; I2 = 59%; Figure 5).
Vortioxetine vs. SNRIs
Eight studies including 3,159 participants provided data comparing vortioxetine to SNRIs.
Response
Vortioxetine had no significant difference in response rate compared with SNRIs in general (RR 0.91, 95% CI = 0.82–1.00; P = 0.06; I2 = 61%). Specifically, as shown in Figure 6A, based on the results of six studies (n = 2,392), the response rates were significantly lower for vortioxetine than duloxetine; the pooled RR was 0.86, (95% CI = 0.79–0.94; P = 0.001; I2 = 28%), while two studies (n = 767) showed that there was no difference in response rates compared to venlafaxine (RR 1.03, 95% CI = 0.85–1.25; P = 0.73; I2 = 69%; Figure 6A).
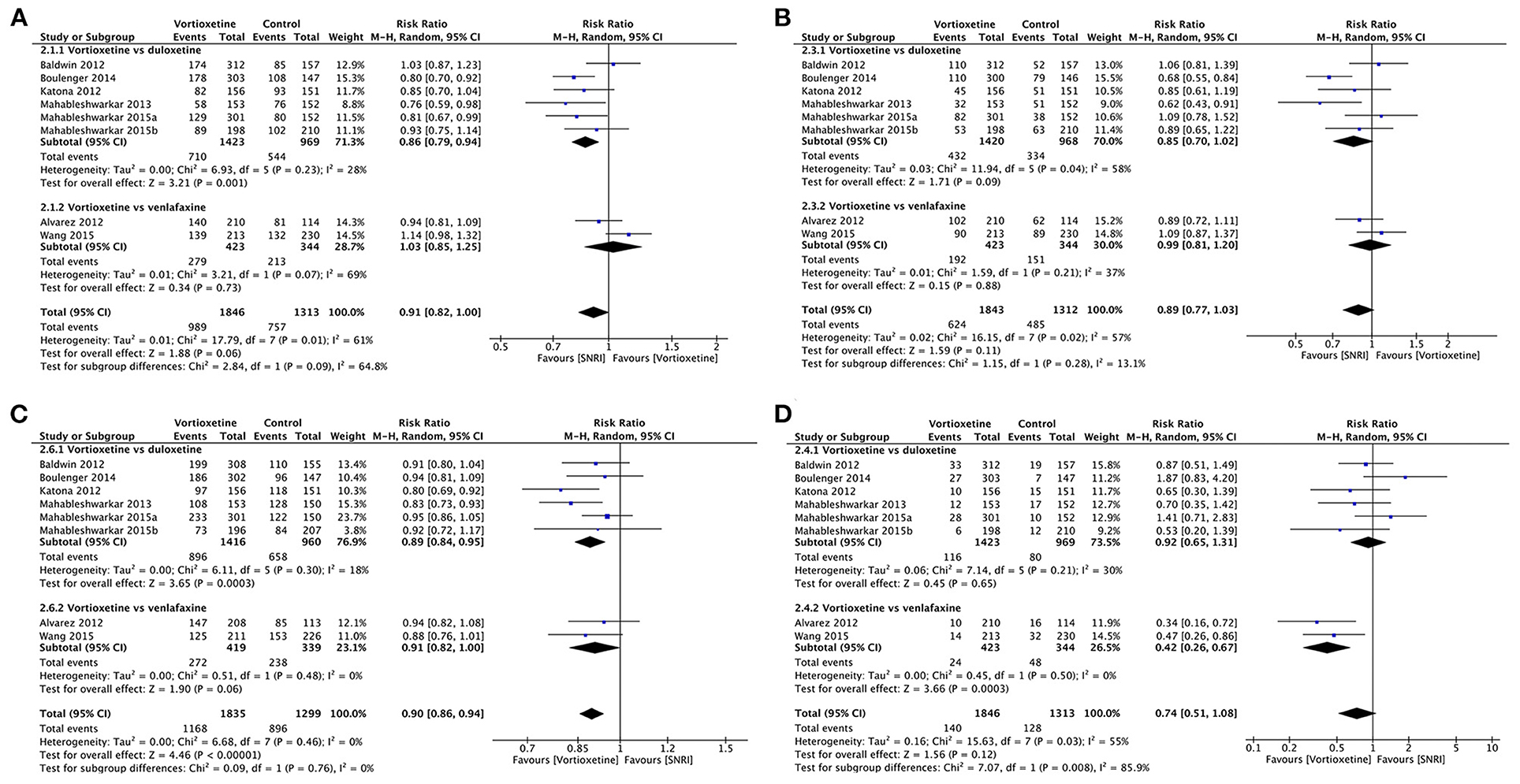
Figure 6. Forest plot of comparison on vortioxetine vs. SNRIs (A) Response, (B) remission, (C) tolerability, and (D) acceptability.
Remission
There was no significant difference in the remission rates between vortioxetine and venlafaxine (RR: 0.99, 95% CI = 0.81–1.20, P = 0.88; I2 = 37%), vortioxetine and duloxetine (RR 0.85, 95% CI = 0.70–1.02; P = 0.09; I2 = 58%), or vortioxetine and SNRIs in general (RR 0.89, 95% CI = 0.77–1.03; P = 0.11; I2 = 57%; Figure 6B).
Tolerability
Participants treated with vortioxetine experienced fewer adverse effects than those treated with SNRIs (RR 0.90, 95% CI = 0.86–0.94; P < 0.001; I2 = 0%). In subgroup analyses, vortioxetine treatment had fewer adverse effects than duloxetine (RR 0.89, 95% CI = 0.84–0.95; P < 0.001; I2 = 18%), but no significant difference is observed between vortioxetine and venlafaxine (RR 0.91, 95% CI = 0.82–1.00; P = 0.06; I2 = 0%; Figure 6C).
Acceptability
Vortioxetine had no significant difference in dropout rate due to adverse events comparing with SNRIs in general (RR 0.74, 95% CI = 0.51–1.08; P = 0.12, I2 = 55%). Specifically, there was no statistically significant difference between vortioxetine and duloxetine in total dropout rates due to adverse events (RR 0.92, 95% CI = 0.65–1.31; P = 0.65; I2 = 30%), but vortioxetine is superior when compared with venlafaxine (RR 0.42, 95% CI = 0.26–0.67; P < 0.001; I2 = 0%; Figure 6D).
Vortioxetine vs. SSRIs
Two studies included 126 participants that provided efficacy and acceptability data comparing vortioxetine and SSRIs.
Vortioxetine had no difference in response rate (RR 1.08, 95% CI = 0.88–1.32; P = 0.46; I2 = 0%), remission rate (RR 1.00, 95% CI = 0.41–2.44; P = 1.00; I2 = 65%) or acceptability (RR 0.78, 95% CI = 0.35–1.74; P = 0.55; I2 = 0%) comparing with SSRIs (Figure 7).
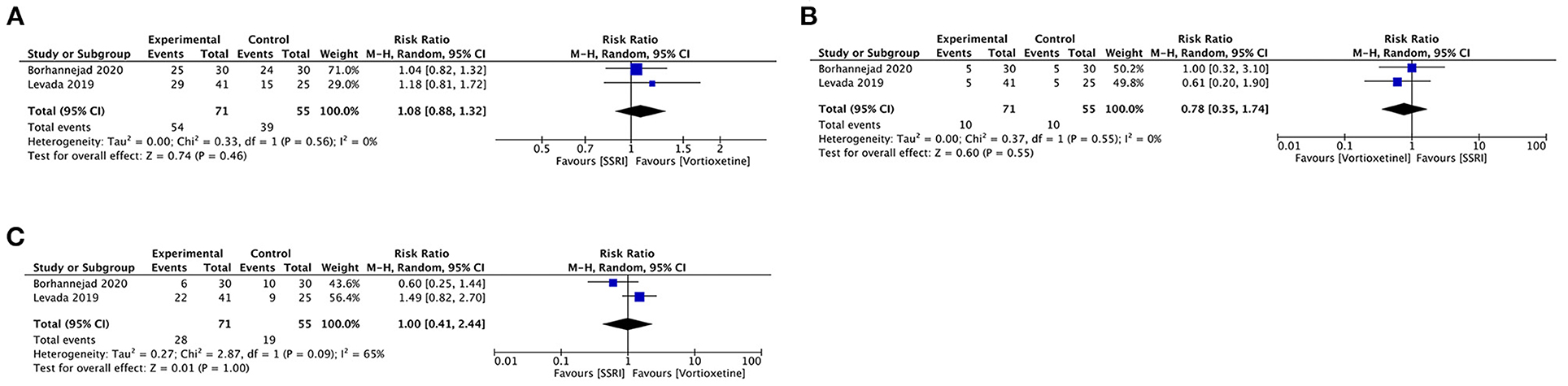
Figure 7. Forest plot of comparison on vortioxetine vs. SSRIs (A) Response, (B) remission, and (C) acceptability.
Sensitivity Analysis
Effect estimates were consistent with overall summary effect estimates for outcomes when contributing data excluded low quality studies.
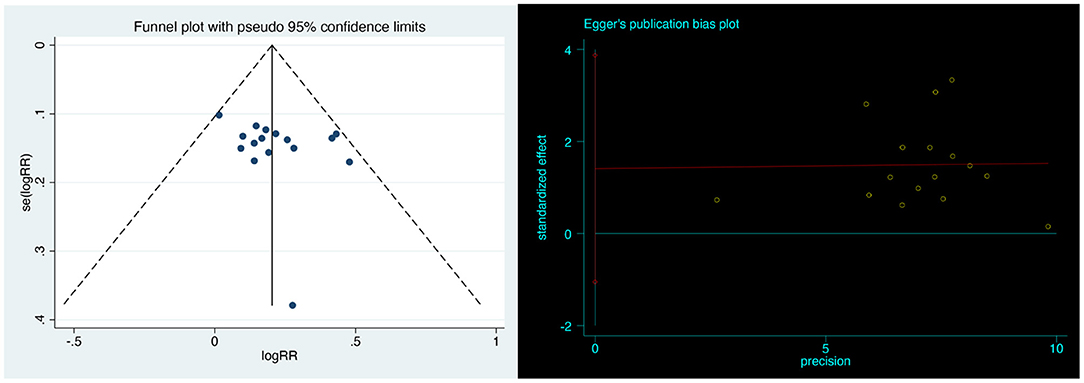
Publication Bias
Visual inspection of funnel plots revealed no obvious publication bias. Egger's linear regression test further justified that the publication bias was non-significant (P = 0.239; Figure 8).
Discussion
This comprehensive meta-analysis focused on the efficacy, acceptability, tolerability and cognitive function of vortioxetine as monotherapy among adult patients with MDD. We found significant advantages in the efficacy, tolerability and cognitive function of vortioxetine compared to the placebo, aligning with the existing literature. However, vortioxetine has no significant advantage in efficacy compared with SNRIs and SSRIs, but it dominates SNRIs in terms of tolerability.
Vortioxetine has been approved in more than 80 countries worldwide, with efficacy supported by numerous randomized controlled trials (35, 40, 41). Similar to previous studies, we found that vortioxetine treatment significantly improved patients' chances of response and remission rates. However, the vortioxetine in individual trials showed a nonconsistency benefit of treatment vs. placebo. It is noteworthy that 20 mg/day of vortioxetine did not outperform placebo in the United States. Possible reasons for difference may be related to disparities in ethnicity, geographic location, and the ability to detect efficacy in clinical trials (42).
Impairment in cognitive function is increasingly recognized as a core deficit in patients with MDD. Some residual symptoms, including impairments in executive function and attention often persist independently even after remission of depressive symptoms (43–45). Our evidence supports that vortioxetine has a statistically significant effect on the cognitive functions in comparison with the placebo. Vortioxetine is the only FDA-approved pharmacological agent for the treatment of MDD that specifically targets cognitive dysfunction (46), and increasing evidence has demonstrated the clinical benefits of vortioxetine on cognitive function. Vortioxetine was statistically more efficacious on cognitive function than escitalopram, nortriptyline, SSRIs and TCAs (38) and their impact independent of depressive symptoms (47), and the superiority compared with the placebo in speed of processing, verbal learning, and memory (17). Further research is needed to understand the relationship between the differential effects on cognition and unique pharmacological profile.
In this study, there was no difference in terms of efficacy between vortioxetine and SNRIs, and the results of the subgroup analysis showed that the response rate of vortioxetine might be lower than that of duloxetine and no different from venlafaxine, which is inconsistent with the results from previous meta-analysis which show the superior efficacy of venlafaxine over duloxetine (48). One explanation for this is that the pooled result might be biased because it is based only on a small number of studies. However, We also revealed that vortioxetine may have advantages over SNRIs in terms of tolerability profile, which was confirmed by the results reported in other head-to-head studies (20, 49, 50), and vortioxetine has generally been associated with a lower prevalence of adverse events compared with duloxetine and extended-release venlafaxine. Tolerability has been used as an indirect index of drug effectiveness (51); therefore, the better tolerability profile of vortioxetine may increase the probability of patient adherence and remission.
In the comparison with SSRIs, we found no significant differences in the efficacy and acceptability of vortioxetine. SSRIs are usually recommended as first-line treatment for MDD. Therefore, direct comparisons of these antidepressants may help to better guide the clinical application of vortioxetine. However, the current quantitative results are based only on two clinical trials with small sample sizes. A randomized trial of older patients with MDD showed no significant differences in the efficacy or safety of vortioxetine vs. sertraline at week 6 of the trial (37). However, compared to escitalopram, vortioxetine treatment improved overall cognitive performance, which led to higher response and remission rates (39). The evidence on the effect of SSRIs may need to be evaluated in additional high-quality clinical trials.
Several published systematic reviews and meta-analyses yielded evidence of efficacy for vortioxetine in depression. One narrative review gave an overview of the vortioxetine studies and reported results from 10 RCTs in adults with depression without pooling the results (49). Two other meta-analyses focused on specific doses of vortioxetine 5 mg or 10 mg compared to placebo (52, 53). Koesters et al. (54) conducted a comprehensive review of the effects of vortioxetine in the treatment of MDD in adults, but included RCTs only up to 2016. The present work is the most comprehensive published to date focusing on the efficacy of vortioxetine as monotherapy for MDD. Our study, however, is subject to several limitations. First, differences in the patient baseline characteristics, disease severity, and dose of vortioxetine among the identified clinical trials may cause the high heterogeneity of the results. Second, due to the limited availability of original trials, our pooled results might not provide sufficient evidence in comparison to other antidepressants in terms of cognitive function, as well as also not allowing for corresponding subgroups analysis. Third, our evidence applying the DSST as a single cognitive test comparison means that the comparison and pooling of data was limited in the analysis. Therefore, future research could improve the standardization of cognitive testing by combining self-reports and objective cognitive tests. Finally, most of the clinical trials included in this meta-analysis were funded by pharmaceutical companies, so the underlying conflicts of interest may influence the results.
Conclusions
In conclusion, our findings indicated that vortioxetine for the treatment of MDD in adults has significant advantages over placebo in terms of efficacy, tolerability, and cognitive function. In comparison to SNRIs and SSRIs in terms of efficacy, there was no statistically significant difference for vortioxetine, which may be similarly effective but has advantages over the SNRIs in terms of tolerability. Further studies including head-to-head comparisons with SNRIs and SSRIs are needed to supplement and verify the efficacy to define the role of vortioxetine in the treatment of depression.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author Contributions
XZ designed the study and registered on PROSPERO, searched the online databases, assessed the risk of bias of included studies, carried out the initial statistical analysis, drafted the initial manuscript, and revised the manuscript. CL searched the online databases, assessed the risk of bias of included studies, reviewed, and revised the manuscript. XH searched the online databases, evaluated all searched citations, and extracted the primary data. YC searched the online databases, evaluated all searched citations, analyzed the data, and extracted the data. XN and LS designed this study, reviewed, and revised the manuscript. All authors approved the final version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Uher R, Payne JL, Pavlova B, Perlis RH. Major depressive disorder in DSM-5: implications for clinical practice and research of changes from DSM-IV. Depress Anxiety. (2014) 31:459–71. doi: 10.1002/da.22217
2. Trivedi MH, Lin EHB, Katon WJ. Consensus recommendations for improving adherence, self-management, and outcomes in patients with depression. CNS Spectr. (2007) 12:1–27.
3. Lim GY, Tam WW, Lu Y, Ho CS, Zhang MW, Ho RC. Prevalence of depression in the community from 30 countries between 1994 and 2014. Sci Rep. (2018) 8:2861. doi: 10.1038/s41598-018-21243-x
4. Greenberg PE, Fournier A-A, Sisitsky T, Pike CT, Kessler RC. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry. (2015) 76:155–62. doi: 10.4088/JCP.14m09298
5. Boulenger J-P, Loft H, Olsen CK. Efficacy and safety of vortioxetine (Lu AA21004), 15 and 20 mg/day: a randomized, double-blind, placebo-controlled, duloxetine-referenced study in the acute treatment of adult patients with major depressive disorder. Int Clin Psychopharmacol. (2014) 29:138–49. doi: 10.1097/YIC.0000000000000018
6. Daly EJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Gaynes BN, Warden D, et al. Health-related quality of life in depression: a STAR*D report. Ann Clin Psychiatry. (2010) 22:43–55.
7. Walker ER, McGee RE, Druss BG. Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiatry. (2015) 72:334–41. doi: 10.1001/jamapsychiatry.2014.2502
8. Kennedy SH, Lam RW, Cohen NL, Ravindran AV. Clinical guidelines for the treatment of depressive disorders. IV. Medications and other biological treatments. Can J Psychiatry. (2001) 46(Suppl 1):38S−58S. doi: 10.1177/0706743716659418
9. Qaseem A, Barry MJ, Kansagara D. Nonpharmacologic versus pharmacologic treatment of adult patients with major depressive disorder: a clinical practice guideline from the American College of Physicians. Ann Intern Med. (2016) 164:350–9. doi: 10.7326/M15-2570
10. Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. (2006) 163:28–40. doi: 10.1176/appi.ajp.163.1.28
11. Rouillon F, Gorwood P. The use of lithium to augment antidepressant medication. J Clin Psychiatry. (1998) 59:32–9.
12. Fava M, Davidson KG. Definition and epidemiology of treatment-resistant depression. Psychiatr Clin North Am. (1996) 19:179–200. doi: 10.1016/S0193-953X(05)70283-5
13. Schechter LE, Ring RH, Beyer CE, Hughes ZA, Khawaja X, Malberg JE, et al. Innovative approaches for the development of antidepressant drugs: current and future strategies. NeuroRx. (2005) 2:590–611. doi: 10.1602/neurorx.2.4.590
14. Westrich L, Pehrson A, Zhong H, Nielsen S, Fredericksen K, Stensbøl T, et al. In vitro and in vivo effects of the multimodal antidepressant vortioxetine (Lu AA21004) at human and rat targets. Int J Psychiatry Clin Pract. (2012) 5:47. doi: 10.1177/0269881116667710
15. Henigsberg N, Mahableshwarkar AR, Jacobsen P, Chen Y, Thase ME. A randomized, double-blind, placebo-controlled 8-week trial of the efficacy and tolerability of multiple doses of Lu AA21004 in adults with major depressive disorder. J Clin Psychiatry. (2012) 73:953–9. doi: 10.4088/JCP.11m07470
16. Harris JD, Quatman CE, Manring MM, Siston RA, Flanigan DC. How to write a systematic review. Am J Sports Med. (2014) 42:2761–8. doi: 10.1177/0363546513497567
17. Mahableshwarkar AR, Jacobsen PL, Chen Y, Serenko M, Trivedi MH A. randomized, double-blind, duloxetine-referenced study comparing efficacy and tolerability of 2 fixed doses of vortioxetine in the acute treatment of adults with MDD. Psychopharmacology. (2015) 232:2061–70. doi: 10.1007/s00213-014-3839-0
18. Jacobsen PL, Mahableshwarkar AR, Serenko M, Chan S, Trivedi MH. A randomized, double-blind, placebo-controlled study of the efficacy and safety of vortioxetine 10 mg and 20 mg in adults with major depressive disorder. J Clin Psychiatry. (2015) 76:575–82. doi: 10.4088/JCP.14m09335
19. McIntyre RS, Lophaven S, Olsen CK A. randomized, double-blind, placebo-controlled study of vortioxetine on cognitive function in depressed adults. Int J Neuropsychopharmacol. (2014) 17:1557–67. doi: 10.1017/S1461145714000546
20. Baldwin DS, Chrones L, Florea I, Nielsen R, Nomikos GG, Palo W, et al. The safety and tolerability of vortioxetine: analysis of data from randomized placebo-controlled trials and open-label extension studies. J Psychopharmacol. (2016) 30:242–52. doi: 10.1177/0269881116628440
21. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
22. van Tulder M, Furlan A, Bombardier C, Bouter L. Updated method guidelines for systematic reviews in the cochrane collaboration back review group. Spine. (2003) 28:1290–9. doi: 10.1097/01.BRS.0000065484.95996.AF
23. Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane, AB: The Cochrane Collaboration Updated method guidelines for systematic reviews in the cochrane collaboration back review group (2011).
24. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
25. Alvarez E, Perez V, Dragheim M, Loft H, Artigas F. A double-blind, randomized, placebo-controlled, active reference study of Lu AA21004 in patients with major depressive disorder. Int J Neuropsychopharmacol. (2012) 15:589–600. doi: 10.1017/S1461145711001027
26. Baldwin DS, Loft H, Dragheim M. A randomised, double-blind, placebo controlled, duloxetine-referenced, fixed-dose study of three dosages of Lu AA21004 in acute treatment of major depressive disorder (MDD). Eur Neuropsychopharmacol. (2012) 22:482–91. doi: 10.1016/j.euroneuro.2011.11.008
27. Jain R, Mahableshwarkar AR, Jacobsen PL, Chen Y, Thase ME. A randomized, double-blind, placebo-controlled 6-wk trial of the efficacy and tolerability of 5 mg vortioxetine in adults with major depressive disorder. Int J Neuropsychopharmacol. (2013) 16:313–21. doi: 10.1017/S1461145712000727
28. Katona C, Hansen T, Olsen CK. A randomized, double-blind, placebo-controlled, duloxetine-referenced, fixed-dose study comparing the efficacy and safety of Lu AA21004 in elderly patients with major depressive disorder. Int Clin Psychopharmacol. (2012) 27:215–23. doi: 10.1097/YIC.0b013e3283542457
29. Mahableshwarkar AR, Jacobsen PL, Chen Y. A randomized, double-blind trial of 2.5 mg and 5 mg vortioxetine (Lu AA21004) versus placebo for 8 weeks in adults with major depressive disorder. Curr Med Res Opin. (2013) 29:217–26. doi: 10.1185/03007995.2012.761600
30. Mahableshwarkar AR, Zajecka J, Jacobson W, Chen Y, Keefe RS. A randomized, placebo-controlled, active-reference, double-blind, flexible-dose study of the efficacy of vortioxetine on cognitive function in major depressive disorder. Neuropsychopharmacology. (2015) 40:2025–37. doi: 10.1038/npp.2015.52
31. Mahableshwarkar AR, Jacobsen PL, Serenko M, Chen Y, Trivedi MH. A randomized, double-blind, placebo-controlled study of the efficacy and safety of 2 doses of vortioxetine in adults with major depressive disorder. Psychiatry Clin Neurosci. (2015) 76:583–91. doi: 10.4088/JCP.14m09337
32. Takeda. A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, Phase III Study to Assess the Efficacy and Safety of Lu AA21004 in Patients with Major Depressive Disorder. U. S. National Library of Medicine (2011). Available online at: https://clinicaltrials.gov/ct2/show/NCT01355081
33. Wang G, Gislum M, Filippov G, Montgomery S. Comparison of vortioxetine versus venlafaxine XR in adults in Asia with major depressive disorder: a randomized, double-blind study. Curr Med Res Opin. (2015) 31:785–94. doi: 10.1185/03007995.2015.1014028
34. Liebowitz MR, Careri J, Blatt K, Draine A, Morita J, Moran M, et al. Vortioxetine versus placebo in major depressive disorder comorbid with social anxiety disorder. Depress Anxiety. (2017) 34:1164–72. doi: 10.1002/da.22702
35. Inoue T, Sasai K, Kitagawa T, Nishimura A, Inada I. Randomized, double-blind, placebo-controlled study to assess the efficacy and safety of vortioxetine in Japanese patients with major depressive disorder. Psychiatry Clin Neurosci. (2020) 74:140–8. doi: 10.1111/pcn.12956
36. Nishimura A, Aritomi Y, Sasai K, Kitagawa T, Mahableshwarkar AR. Randomized, double-blind, placebo-controlled 8-week trial of the efficacy, safety, and tolerability of 5, 10, and 20 mg/day vortioxetine in adults with major depressive disorder. Psychiatry Clin Neurosci. (2018) 72:64–72. doi: 10.1111/pcn.12565
37. Borhannejad F, Shariati B, Naderi S, Shalbafan M, Mortezaei A, Sahebolzamani E, et al. Comparison of vortioxetine and sertraline for treatment of major depressive disorder in elderly patients: a double-blind randomized trial. J Clin Pharm Ther. (2020) 45:804–11. doi: 10.1111/jcpt.13177
38. Baune BT, Sluth LB, Olsen CK. The effects of vortioxetine on cognitive performance in working patients with major depressive disorder: a short-term, randomized, double-blind, exploratory study. J Affect Disord. (2018) 229:421–8. doi: 10.1016/j.jad.2017.12.056
39. Levada OA, Troyan AS. Cognitive-functional relationships in major depressive disorder: Crucial data from a Ukrainian open-label study of vortioxetine versus escitalopram. J Affect Disord. (2019) 250:114–22. doi: 10.1016/j.jad.2019.03.040
40. Thase ME, Mahableshwarkar AR, Dragheim M, Loft H, Vieta E. A meta-analysis of randomized, placebo-controlled trials of vortioxetine for the treatment of major depressive disorder in adults. Eur Neuropsychopharmacol. (2016) 26:979–93. doi: 10.1016/j.euroneuro.2016.03.007
41. Zheng J, Wang Z, Li E. The efficacy and safety of 10 mg/day vortioxetine compared to placebo for adult major depressive disorder: a meta-analysis. Afr Health Sci. (2019) 19:1716–26. doi: 10.4314/ahs.v19i1.48
42. Khin NA, Chen Y-F, Yang Y, Yang P, Laughren TP. Exploratory analyses of efficacy data from major depressive disorder trials submitted to the US Food and Drug Administration in support of new drug applications. J Clin Psychiatry. (2011) 72:464–72. doi: 10.4088/JCP.10m06191
43. Hasselbalch BJ, Knorr U, Kessing LV. Cognitive impairment in the remitted state of unipolar depressive disorder: a systematic review. J Affect Disord. (2011) 134:20–31. doi: 10.1016/j.jad.2010.11.011
44. McDermott LM, Ebmeier KP A. meta-analysis of depression severity and cognitive function. J Affect Disord. (2009) 119:1–8. doi: 10.1016/j.jad.2009.04.022
45. McClintock SM, Husain MM, Greer TL, Cullum CM. Association between depression severity and neurocognitive function in major depressive disorder: a review and synthesis. Neuropsychology. (2010) 24:9–34. doi: 10.1037/a0017336
46. Lundbeck. FDA updates Trintellix R (vortioxetine) label to include data showing improvement in processing speed, an important aspect of cognitive function in acute major depressive disorder (MDD). (2018). Available online at: https://www.globenewswire.com/news-release/2018/05/02/1495434/0/en/FDA-updates-Trintellix-vortioxetine-label-to-include-data-showing-improvement-in-processing-speed-an-important-aspect-of-cognitive-function-in-acute-Major-Depressive-Disorder-MDD.html (accessed August 30, 2021).
47. McIntyre RS, Harrison J, Loft H, Jacobson W, Olsen CK. The effects of vortioxetine on cognitive function in patients with major depressive disorder: a meta-analysis of three randomized controlled trials. Int J Neuropsychopharmacol. (2016) 19:pyw055. doi: 10.1093/ijnp/pyw055
48. Schueler YB, Koesters M, Wieseler B, Grouven U, Kromp M, Kerekes MF, et al. A systematic review of duloxetine and venlafaxine in major depression, including unpublished data. Acta Psychiatr Scand. (2011) 123:247–65. doi: 10.1111/j.1600-0447.2010.01599.x
49. Citrome L. Vortioxetine for major depressive disorder: a systematic review of the efficacy and safety profile for this newly approved antidepressant - what is the number needed to treat, number needed to harm and likelihood to be helped or harmed? Int J Clin Pract. (2014) 68:60–82. doi: 10.1111/ijcp.12350
50. Coleman KA, Xavier VY, Palmer TL, Meaney JV, Radalj LM, Canny LM. An indirect comparison of the efficacy and safety of desvenlafaxine and venlafaxine using placebo as the common comparator. CNS Spectr. (2012) 17:131–41. doi: 10.1017/S1092852912000648
51. Mahil SK, Ezejimofor MC, Exton LS, Manounah L, Burden AD, Coates LC, et al. Comparing the efficacy and tolerability of biologic therapies in psoriasis: an updated network meta-analysis. Br J Dermatol. (2020) 183:638–49. doi: 10.1111/bjd.19325
52. Fu J, Chen Y. The eficacy and safety of 5 mg/d Vortioxetine compared to placebo for major depressive disorder: a meta-analysis. Psychopharmacology. (2015) 232:7–16. doi: 10.1007/s00213-014-3633-z
53. Li G, Wang X, Ma D. The efficacy and safety of 10 mg vortioxetine in the treatment of major depressive disorder: a meta-analysis of randomized controlled trials. Neuropsychiatr Dis Treat. (2016) 12:523–31. doi: 10.2147/NDT.S103173
Keywords: vortioxetine, major depressive disorder, adults, randomized controlled trials, meta-analysis
Citation: Zhang X, Cai Y, Hu X, Lu CY, Nie X and Shi L (2022) Systematic Review and Meta-Analysis of Vortioxetine for the Treatment of Major Depressive Disorder in Adults. Front. Psychiatry 13:922648. doi: 10.3389/fpsyt.2022.922648
Received: 19 April 2022; Accepted: 24 May 2022;
Published: 24 June 2022.
Edited by:
Mirko Manchia, University of Cagliari, ItalyReviewed by:
Reiji Yoshimura, University of Occupational and Environmental Health Japan, JapanMarco Di Nicola, Agostino Gemelli University Polyclinic (IRCCS), Italy
Copyright © 2022 Zhang, Cai, Hu, Lu, Nie and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoyan Nie, bmlleHlAcGt1LmVkdS5jbg==
 Xinyan Zhang1
Xinyan Zhang1 Xiaoyan Nie
Xiaoyan Nie