- 1Department of Cardiothoracic Surgery, The Alfred Hospital, Melbourne, VIC, Australia
- 2Department of Surgery, School of Clinical Sciences at Monash Health, Monash University, Melbourne, VIC, Australia
- 3Department of Cardiothoracic Surgery, Monash Health, Melbourne, VIC, Australia
Background: Depression is common in the cardiac surgery population. This contemporary narrative review aims to explore the main pathophysiological disturbances underpinning depression specifically within the cardiac surgery population. The common non-pharmacological and pharmacological management strategies used to manage depression within the cardiac surgery patient population are also explored.
Methods: A total of 1291 articles were identified through Ovid Medline and Embase. The findings from 39 studies were included for qualitative analysis in this narrative review.
Results: Depression is associated with several pathophysiological and behavioral factors which increase the likelihood of developing coronary heart disease which may ultimately require surgical intervention. The main pathophysiological factors contributing to depression are well characterized and include autonomic nervous system dysregulation, excessive inflammation and disruption of the hypothalamic–pituitary–adrenal axis. There are also several behavioral factors in depressed patients associated with the development of coronary heart disease including poor diet, insufficient exercise, poor compliance with medications and reduced adherence to cardiac rehabilitation. The common preventative and management modalities used for depression following cardiac surgery include preoperative and peri-operative education, cardiac rehabilitation, cognitive behavioral therapy, religion/prayer/spirituality, biobehavioral feedback, anti-depressant medications, and statins.
Conclusion: This contemporary review explores the pathophysiological mechanisms leading to depression following cardiac surgery and the current management modalities. Further studies on the preventative and management strategies for postoperative depression in the cardiac surgery patient population are warranted.
1. Introduction
Cardiovascular disease (CVD) is a leading cause of mortality, accounting for over 18 million deaths globally in 2019 (1). As a management modality, over 2 million cardiac surgeries are performed globally per annum (2). Common examples of cardiac surgery include coronary artery bypass graft (CABG), valve replacements and heart transplantation. Given the aging population, patients who undergo cardiac surgery are more likely to be older and possess significant medical comorbidities such as hypertension and diabetes mellitus (3). Cardiac surgery intends to offer definitive management for persistent cardiovascular disease which is refractory to medical management. Successful cardiac surgery significantly improves quality of life, which in turn improves psychological outcomes. Depression is a mood disorder with a lifetime prevalence between 2 and 21% (4). Within the cardiac surgery population, the prevalence of depression is significantly higher. The prevalence of pre-operative depression ranges from 20 to 47% while postoperative depression affects 23–61% of patients following cardiac surgery (5–11). The disparity in prevalence between pre- and postoperative depression may be attributed to differences in modalities used to detect depression (different questionnaires vs. psychiatric interviews), differences in parameters used to define depression and differences in post-operative follow-up. Moreover, patients may display somatic symptoms such as fatigue and sleep disturbances which can be difficult to discern from depression (12). Both pre-operative and post-operative depression are underpinned by the same pathophysiological mechanisms and are associated with poor clinical outcomes. Psychiatric assessment should occur pre-operatively and post-operatively. Prior to operating on a patient with pre-operative depression, it may be worthwhile offering additional counseling or support. During the postoperative period, the patient’s psychiatric state should be monitored. Reductions in depression symptoms are expected postoperatively and associated with improvements in quality of life. Conversely, increases in postoperative depression may be attributed to the occurrence of complications or major adverse cardiovascular events (MACE) (13). Hence, there is significant interest in understanding the underlying pathophysiology of depression following cardiac surgery and investigating effective preventative and management modalities within this population.
1.1. Risk factors for depression
Risk factors for postoperative depression following cardiac surgery include female gender (5, 14, 15), younger age (16), previous depressive episodes or family history of depression (17) and history of pre-operative depression (18, 19). Social factors such as lower educational levels, lower levels of social support or social isolation also increase the risk of postoperative depression. Social support during the first month following surgery may reduce the likelihood of postoperative depression and impairments in activities of daily living (ADL) at 6 months (20). Naturally, emergency surgery and an extended length of hospital stay contribute to the likelihood of developing postoperative depression (15, 21).
Risk factors for developing preoperative depression are similar to the above, but uniquely include dyspnea upon exertion and at rest (contributing to a higher NYHA classification) (8, 22) and previous myocardial infarction (22). The identification of these contributory factors may allow early recognition of vulnerable patients and early referral for psychiatric assessment.
1.2. Screening questionnaires for depression in cardiac surgery patients
The following questionnaires are commonly utilized in depression studies involving cardiac surgery patients.
1.2.1. Patient Health Questionnaire 9
The Patient Health Questionnaire 9 (PHQ-9) is a nine-item self-administered questionnaire used to assess depressive symptoms over the previous 2 weeks using the Diagnostic and Statistical Manual of Mental Disorders 5th Edition (DSM-5) criteria. The PHQ-9 assesses affective, cognitive, and somatic symptoms. Four items are related to somatic symptoms (sleeping difficulties, fatigue, reduced appetite and psychomotor agitation/retardation) (23). The PHQ-9 has high specificity, but low sensitivity for detecting depression in patients with CVD (24, 25). McManus et al. reported a 54% sensitivity and 90% specificity for detecting depression when using a score of ≥10 as the cut-off. This questionnaire may be administered over telephone/telehealth to reliably assess for depressive symptoms (26).
In 2008, the American Heart Association recommends screening for pre-operative depression in patients with coronary heart disease (CHD) with the PHQ-2 or PHQ-9 (preferred). Patients with a positive PHQ-2 screen are asked to complete a PHQ-9 questionnaire, whereas those with a positive PHQ-9 questionnaire should be referred for psychiatric evaluation (27). Patients with a positive PHQ-9 depression screen were at increased risk of MACE at 6 months (OR: 2.16, 95% CI: 0.98–4.74) and five times more likely to receive anti-depressant medication (28). Stenman et al. demonstrated screening with the PHQ-9 in cardiac surgery patients was practically feasible and economically viable, with 64% of elective patients completing the questionnaire prior to surgery (29). Similarly, Gorini et al. reported over an 80% completion rate of the PHQ-9 for pre-operative screening (30). To improve the response rate, the PHQ-9 may be included in the preadmission screen.
1.2.2. Centre for Epidemiological Study of Depression scale
The Centre for Epidemiological Study of Depression (CES-D) is a 20-item self-administered questionnaire which measures common depressive symptoms through a 4-point Likert scale. It comprises an amalgamation of previously validated depression questionnaires. The CES-D has a high internal consistency, as evidenced by a Cronbach’s alpha score of 0.85 (31). For each question, a score of 0 represents minimal symptoms, and a score of 4 represents depressive symptoms most of the time (32). A score greater than 16 indicates clinically significant depression. Uniquely, the CES-D includes several items which assesses interpersonal problems. However, the DSM-5 does not include interpersonal problems within their assessment of depression and interpersonal problems are more likely to occur in psychopathologies such as social anxiety (33).
1.2.3. Beck Depression Inventory
The Beck Depression Inventory (BDI) is a 21-item questionnaire which assesses depressive symptoms over the past 2 weeks. Each answer is recorded on a scale of 0–3, with higher scores indicating more severe depressive symptoms. Studies commonly define depressive symptoms by a score of greater than 10 or greater than 14 (34, 35). A cognitive-affective subscale may be created by adding up the scores from the first 13 items. Conversely, a somatic subscale may be produced by summing the scores from the remaining 8 items (36). Used in over 2,000 studies, the BDI has high internal consistency evidenced by a Cronbach α of 0.82 in a non-psychiatric population (37). The BDI is available and validated in numerous other languages. The BDI is copyrighted, and payment is required to access the forms, which may lead to accessibility issues in resource poor nations (35).
1.2.4. Hospital Anxiety and Depression Scale
The Hospital Anxiety and Depression Scale (HADS) is a 14-question survey, with 7 questions pertaining to depression and anxiety, respectively. Each question is scored between 0 and 3. For the depression subscale, a score less than 8 generally indicates no depression, and a score greater than 11 associated with likely depression (38). The HADS predominantly focuses on cognitive and psychological symptoms rather than somatic features to reduce the potential compounder of somatic features during a hospital admission (39). The HADS was initially designed for the inpatient setting, but can reliably detect anxiety and depression in the primary care and general population (40).
1.2.5. Cardiac Depression Scale
Hare and Davis developed the Cardiac Depression Scale (CDS) specifically for cardiac patients in Australia to account for the range of depressive symptoms, including adjustment disorder with depressed mood. The CDS is a 26-item questionnaire with a Likert scale rating system ranging from 1 to 7, with a score of 1 representing “strongly disagree” while a score of 7 represents “strongly agree.” Higher total scores represent more severe depressive symptoms (41). A CDS cut-off score equal to or greater than 95 provided 97% sensitivity and 85% specificity (42). Findings from the CDS have correlated strongly with the BDI (r = 0.73) (41, 43).
1.2.6. Limitations
Self-administered depression screening questionnaires should not replace structured clinical interviews. Several questionnaires, such as the BDI, are restricted by copyright, which may limit access to certain institutions. Additionally, the depression questionnaires should be validated in various languages for different cultures to determine appropriate cut-offs for a positive result. Furthermore, stigma surrounding depression in different cultures may subconsciously influence an individual’s responses to the questionnaires. Finally, studies use different parameter points to define the presence and severity of depression. This makes it difficult to conduct meta-analyses to pool the data for cardiac surgery patients.
1.3. Clinical implications of depression
Both pre-operative and post-operative depression with poor clinical outcomes. Psychiatric assessment should occur pre-operatively and post-operatively.
Depression is an independent risk factor for the development of cardiac disease. The risk of death due to cardiovascular disease (CVD) is two times greater in depressed patients (44). The depressed state is associated with poor nutritional choices, limited exercise, tobacco use, and reduce compliance to medications (45–48). This may exacerbate cardiac disease, resulting in the patient requiring surgical intervention. Prior to operating on a patient with pre-operative depression, it may be worthwhile offering additional counseling or support. Preoperative depression is associated with poor functional status (49) poorer quality of life (50), a longer length of hospital stay (p < 0.001) (10) and increased level of postoperative pain (51). These patients are less likely to return to work in both a fulltime (OR: 9.43, CI: 3.15–28.21) or part time capacity (OR: 5.44, 95% CI: 1.60–18.53) (52) and are more likely to be re-hospitalized for a cardiovascular cause at 6 months postoperatively (X2 = 4.24, p < 0.04) (53). Pre-operative depression is also associated with increased mortality following CABG and valve surgery (54–57).
During the postoperative period, the psychiatric state should be monitored. Improvements in physical functioning and quality of life are expected postoperatively and would likely be associated with improvements in depression symptoms. Postoperative depression is associated with higher pain levels up to weeks post discharge (34), lack of functional improvement in patients 6 months post-surgery (58, 59), two fold increased risk of re-admission (60) and substantial risk of atherosclerotic progression (OR 1.50, 95% CI 1.08 to 2.10, p = 0.02) (61). Postoperative depression is also associated with increased mortality following coronary artery bypass grafting (CABG) (62). Overall, postoperative depression is an increased 10-year mortality rate following cardiac surgery (HR: 1.8, p = 0.04) (44).
2. Methods
A literature search using OVID Medline and Embase was performed for studies included in this narrative review. Keywords for depression include depression; pre-operative depression, post-operative depression, depress*. The following keywords for cardiac surgery were included: cardiac surgery; heart surgery; cardiac operation; cardiothoracic surgery; coronary artery bypass graft; CABG; revascularisation surgery; valve replacement and valve repair. Limits included English language, human studies and adults only.
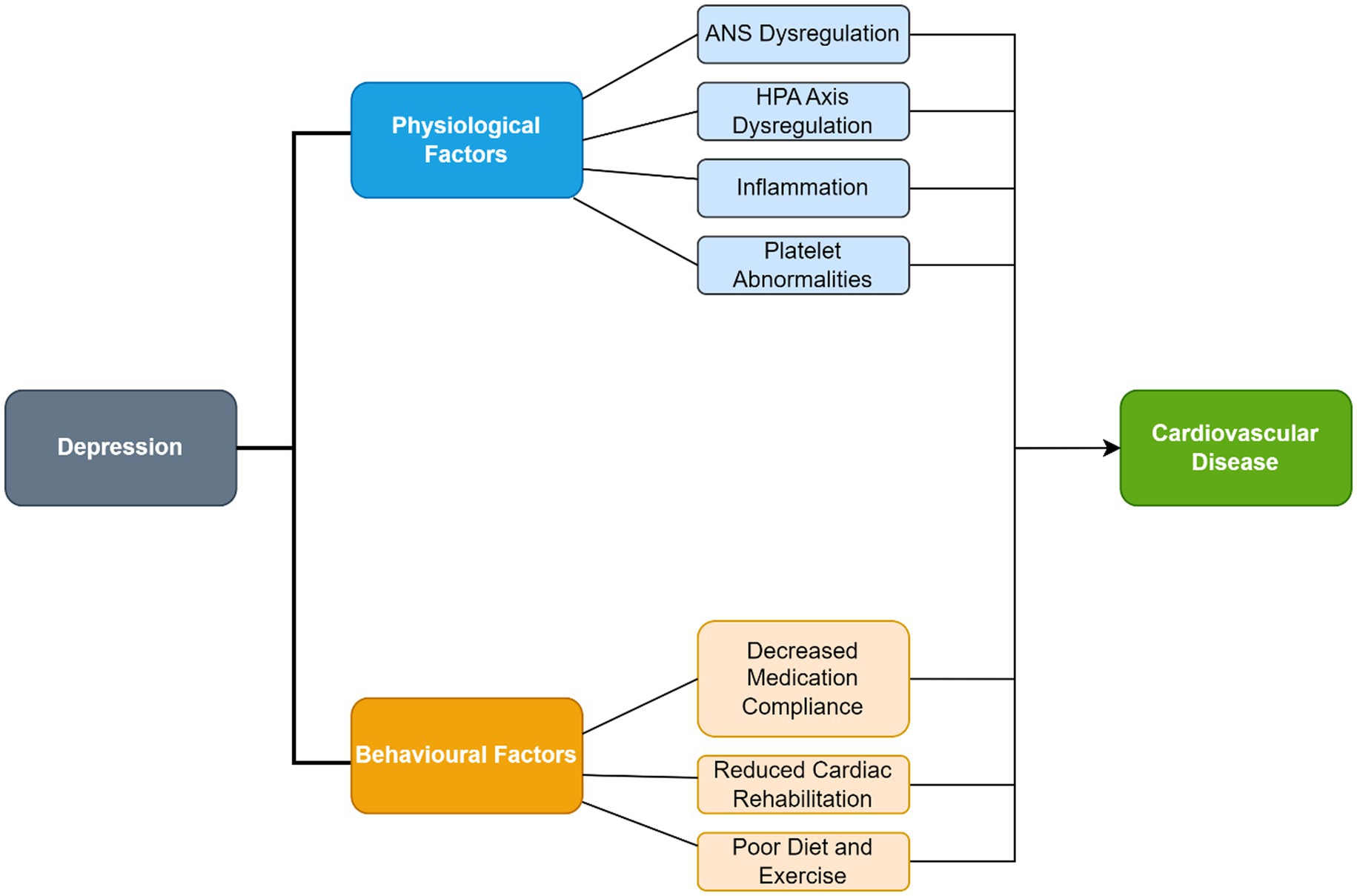
The identification and selection of studies is depicted in Figure 1. Study designs of interest included observational cohort studies and randomized controlled trials. Thirty-nine studies were qualitatively assessed (heart rate variability: 5, inflammation: 4, hypothalamic adrenal axis dysregulation: 2, education: 5, cardiac rehabilitation: 3, cognitive behavioral therapy: 4, prayer: 3, biobehavioral feedback: 1, antidepressants: 7, statins: 2, alternative care: 3). Background information was obtained through references from the search-strategy, and from references within studies.
3. Pathophysiology
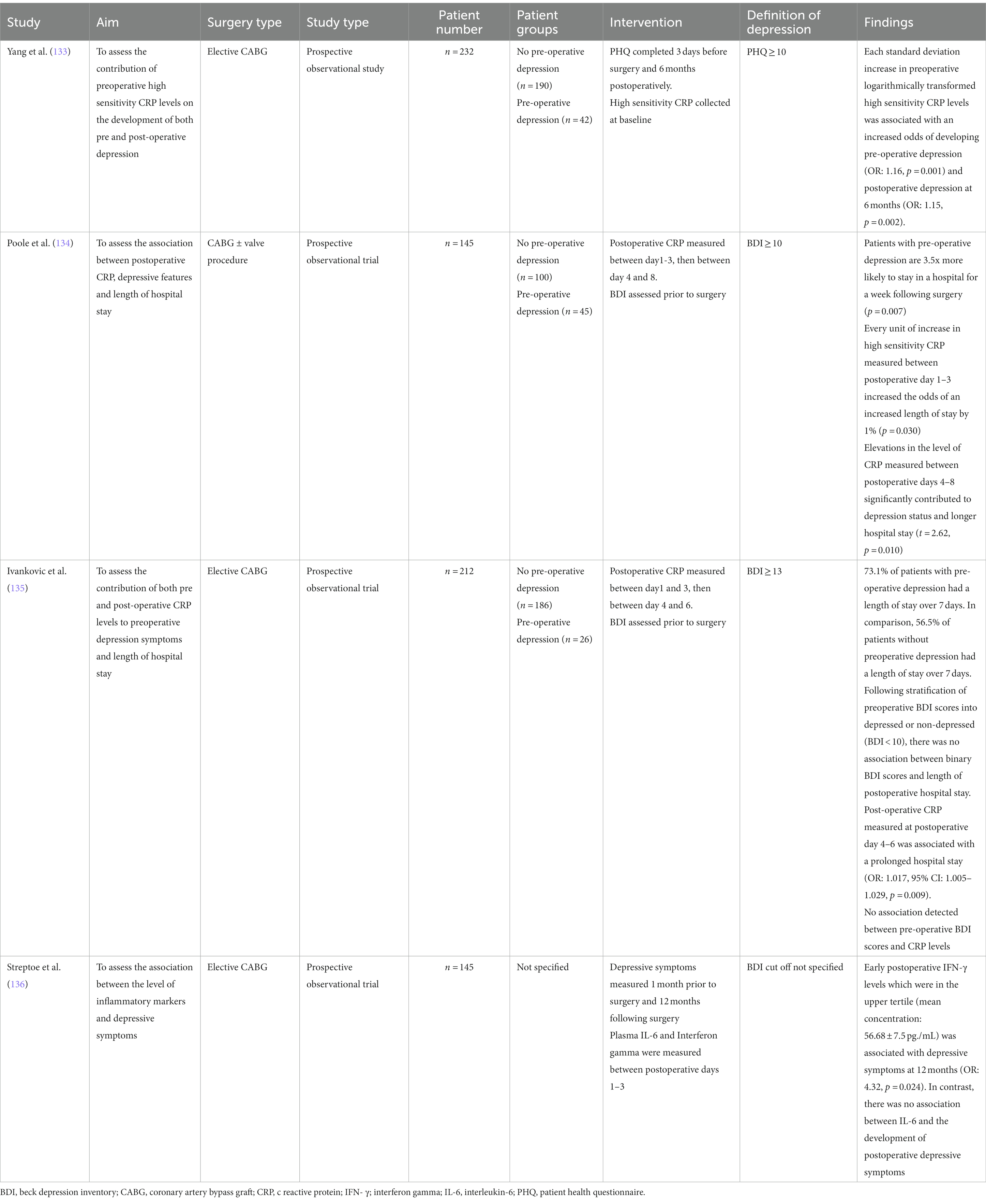
The pathophysiology underlying depression in CHD patients includes autonomic nervous system (ANS) dysregulation, inflammation and hypothalamic pituitary adrenal (HPA) axis dysregulation. Surgical trauma may also contribute to postoperative depression through the pathophysiological mechanisms mentioned above. The depressed state may contribute to behavioral factors which may exacerbate CHD. These behavioral and pathophysiological factors are summarized in Figure 2.
3.1. Dysregulation of the autonomic nervous system
3.1.1. Overview of heart rate variability
Heart rate may be controlled through sympathetic and parasympathetic mechanisms. Sympathetic autonomic activation increases the heart rate, while parasympathetic activation reduces the heart rate (63). Heart rate variability (HRV) refers to the oscillation in time intervals between heart beats (64). HRV is predominantly recorded using electrocardiography (ECG) and physiological monitors such as the Nexus 10 BioTrace equipment. Generally, during the recording process, patients are asked to keep their eyes open and keep their wrists still. Occasionally, the investigator will read pleasant travel excerpts to keep patients relaxed. This standardized process has been previously shown to mimic normal waking states of arousal (65). Moreover, patients are usually excluded from studies if they are not in sinus rhythm, given the increased difficulty in determining HRV (66).
Physiologically, HRV reflects the overall balance between the sympathetic and parasympathetic systems on the cardiovascular system (67, 68). A higher HRV is generally indicative of a well-functioning autonomic nervous system that is responsive to physiological and psychological stressors. Conversely, a low HRV may represent excessive sympathetic nervous system activation, or inadequate vagal tone (64). Additionally, a low HRV may predict acute cardiac complications and sudden cardiac death in patients with acute myocardial infarction and major pulmonary resections (69, 70).
HRV may be calculated using the time domain, or the frequency domain (64). The time domain measurements of HRV quantify the time between heart beats. HRV may be calculated as the standard deviation of the mean R-R or N-N interval (SDNN) on ECG in milliseconds. The SDNN accounts for cyclical components responsible for HRV such as respiration and blood pressure fluctuations (71). Additionally, HRV may also be calculated as the root of the mean square differences in the N-N intervals (rMSSD). This parameter represents the short-term heart rate variability and acts as an index of vagal outflow (72). Power spectral analysis can quantify the contribution of autonomic cardiac regulation to HRV using the knowledge of frequency and power (the energy signal found within a frequency band) (73). Very Low Frequency (VLF) power is defined as <0.04 Hz, Low Frequency (LF) power is between 0.04–0.15 Hz and High Frequency (HF) power is between 0.15–0.40 Hz HF power represents parasympathetic activity, whereas LF power represents sympathetic and some parasympathetic activity (64). Thus, a ratio of LF / HF (ms2/ms2) assesses overall autonomic balance.
An increase in the LF/HF ratio suggests sympathetic dominance, whereas a decrease reflects a parasympathetic dominance (74, 75). Autonomic dysregulation may result in electrical instability of myocytes and predispose patients to arrhythmias, myocardial ischemia, and sudden cardiac death (76, 77). Depressed patients exhibit features of ANS dysregulation through elevations in plasma and urinary catecholamines such as noradrenaline, decreased HRV and an elevated basal HR compared to non-depressed patients (78, 79). Consequently, there is a likely link between CHD, depression and ANS dysregulation.
3.1.2. Depression reduces vagal outflow following cardiac surgery
Depression affects the sympathovagal balance following cardiac surgery, specifically with reduced vagal outflow. Studies assessing the pathophysiology of depression in the cardiac surgery population are summarized in Table 1.
Table 1. Studies assessing the association between autonomic dysregulation and depression in the cardiac surgery population.
Dao et al. proposed autonomic cardiovascular dysregulation as a potential mechanism in the development of depression following cardiac surgery (80). ANS dysregulation was defined as a high basal HR, low HRV and high levels of plasma norepinephrine. The depressed + CABG group had a significantly lower HRV and higher basal HR compared to the other groups, but there were no differences in the level of plasma norepinephrine. This lack of difference may be reflective of the autonomic state of the arm rather than systemically, as blood was taken from the cubital fossa. Additionally, patients with depression + CABG had a longer length of stay compared to non-depressed patients. This suggests that autonomic dysregulation resulting from depression may negatively affect CABG outcomes.
Within the cardiac surgery population, ANS dysregulation is driven by an attenuated vagal response. In a study of 33 patients, Patron et al. demonstrated a reduction in HRV as calculated by SDNN in patients with depression (CES-D greater than16) following cardiac surgery (17.5 ms vs. 36.7 ms, p = 0.02) (81). Depressed cardiac surgery patients exhibited an attenuated vagal response, as demonstrated by a reduced high-frequency power and subsequently an increased LF/HF ratio (Depressed: 3.6 vs. non-Depressed: 0.9, p = 0.08). This suggests reduced vagal outflow, rather than sympathetic hyperactivity, is the corresponding factor between depression and CHD in the cardiac surgery population. Anxiety may also be associated with a reduction in HRV (85). However, Patron et al. demonstrated HRV reductions in depressed patients following cardiac surgery was independent of anxiety, using the STAI Y1 and STAI Y2 tests.
The mechanisms underlying a reduction in HRV in depressed patients following cardiac surgery is poorly understood. Patron et al. assessed whether emotional regulation strategies such as cognitive reappraisal or emotional suppression contributed to autonomic dysfunction in depressed patients following cardiac surgery. Cognitive reappraisal refers to the reframing of an emotion-provoking situation to a neutral thought (86). Conversely, emotional suppression strategies refer to the self-inhibition of negative and intrusive thoughts (87). Within this study, depressed patients were more likely to use emotional suppression strategies, and this coping mechanism was associated with autonomic dysfunction following cardiac surgery (82). Additionally, Patron et al. examined the effect of pleasant, neutral or unpleasant emotional imagery on depressed patients following cardiac surgery. They found depressive patients had increased vagal withdrawal in response to unpleasant emotional imagery, as measured by high-frequency power (p = 0.003) (83). These results complement the literature, and suggest depressive patients demonstrate mood congruent bias and are more likely to react negatively to negative stimuli (88, 89).
Gentili et al. developed a regression model using HRV features in time and frequency, as extracted from five-minutely ECG recordings to predict cardiac surgery patients’ CES-D score, and consequently the presence and severity of depressive symptoms. The model could predict the CES-D score in all 31 patients, with a variance of 89.93%. Additionally, they could discriminate whether patients were depressed or non-depressed with 86.75% accuracy (84). This model should be tested, and validated in a larger population. If successful, it may be feasible to collect HRV measurements automatically at the bedside and use this parameter as a screening tool to detect patients with depression. This would be a useful tool in hospitals where psychological evaluation of patients following cardiac surgery is unavailable.
3.2. Inflammation
3.2.1. Link between inflammation and depression in cardiac surgery patients
There is a bidirectional relationship between inflammation and depression (90). In the cardiac surgery population, inflammation drives the pathophysiology of the underlying CHD but may also manifest because of cardiac surgery itself. This pro-inflammatory state may affect serotonergic neurotransmission through the Kynurenine pathway and contribute to depression (91). Pro-inflammatory cytokines within the peripheries may enter the brain by crossing the blood–brain barrier, or indirectly facilitate activation of microglia within the brain. Hence, excessive peripheral inflammation may contribute to neuroinflammation and potentially to depression (92, 93). On the contrary, depression may contribute to inflammation through the upregulation of pro-inflammatory cytokines and acceleration of the atherosclerotic process (94).
3.2.1.1. Inflammatory nature of coronary heart disease
Atherosclerosis is a chronic inflammatory process which leads to the buildup of plaque within the arterial wall (95). Atherosclerosis is the main pathophysiological contributor to CHD. The pathogenesis of atherosclerosis involves the following key steps: endothelial damage and the formation of foam cells, fatty streaks, intermediate lesions, atheroma and finally, atherosclerotic plaques (96, 97).
Firstly, endothelial insults may be caused by reactive oxygen species, turbulent blood flow (particularly at arterial branch points), hyperglycemia and hyperlipidemia. Low-density lipoproteins may penetrate the injury site and migrate into the tunica intima (98). This triggers a pro-inflammatory process which upregulates the expression of cell-adhesion molecules such as VCAM-1 upon the endothelial surface (99). Monocytes adhere to the endothelial surface and migrate into the subendothelial spaces. Subsequently, the macrophages phagocytose oxidized low-density lipoprotein to form foam cells. The accumulation of foam cells leads to fatty streaks, which may remain stable and ultimately form atherosclerotic plaques or regress (100).
3.2.1.2. Association between inflammation and cardiac surgery
During cardiac surgery, factors which may contribute to a systemic inflammatory response include surgical trauma, blood surface interactions with the cardiopulmonary bypass circuitry, endotoxemia, and ischemic reperfusion injuries. These initiating factors trigger a series of responses from the complement system, neutrophils, cytokines, the coagulation cascade, and the vascular endothelium which result in a systemic inflammatory response. In addition, excessive inflammation may cause organ dysfunction (101–103).
3.2.1.3. The kynurenine hypothesis of depression
Tryptophan is an essential amino acid with two predominant fates – conversion into kynurenine or 5-hydroxytryptamine (serotonin) (104, 105). Over 95% of consumed dietary tryptophan undergoes degradation through the kynurenine pathway (106). The conversion of tryptophan into kynurenine is catalyzed by hepatic tryptophan 2,3-dioxygenase (TDO) and extra-hepatic Indolamine 2,3-dioxygenase (IDO) (107).
From here, kynurenine has three possible fates. Firstly, within microglia, kynurenine may be consumed to facilitate the formation of 3-hydoxykynurenine and its metabolites 3-hydroxyanthranilic acid and quinolinic acid. Quinolinic acid is a glutamate N-methyl-D-aspartate (NMDA) agonist which has excitotoxic and neurotoxic properties associated with depression (108–110). Secondly, also within microglia, kynurenine may also be converted to anthranilic acid, which may be implicated in depression or schizophrenia (111–113). Thirdly, kynurenine may be consumed within the skeletal muscles or peripheries to form kynurenic acid. Kynurenic acid has neuroprotective, antidepressant and anticonvulsant properties, by acting as a non-competitive NMDA receptor blocker (114, 115).
In stressful and pro-inflammatory states, tryptophan is preferentially converted to kynurenine. This is represented by an elevated plasma kynurenine to tryptophan ratio (K/T ratio) (116). An elevated K/T ratio has been observed in CHD depressive patients (117). Perturbations in the metabolism of tryptophan have also been associated with metabolic syndrome risk factors, such as hypertension, obesity and dyslipidemia (91). Inflammation suppresses intrahepatic TDO, while extrahepatic IDO is expressed. Hence, inflammation is associated with an increased production of neurotoxic substrates from the kynurenine pathway which may contribute to depression (118). Moreover, the shunting of tryptophan down the kynurenine pathway depletes serotonin levels, supporting the monoamine hypothesis of depression whereby low serotonin levels contribute to low mood (119). Consequently, there is significant interest in examining inflammation as a pathophysiological mechanism in driving depression in cardiac surgery patients.
3.2.2. Commonly assessed inflammatory markers
Psychological and psychosocial stressors facilitate the release of pro-inflammatory cytokines (120). Magnocellular neurons are sensitive to stress and neuroendocrine changes and facilitate the release of pro-inflammatory cytokines into the general circulation via the neurohypophysis (121). Studies have primarily assessed whether there is a relationship between the peripheral concentration of pro-inflammatory markers such as c-reactive protein (CRP), interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) and the development of depression. Elevated inflammatory markers may also attenuate the response to antidepressant therapy (122, 123).
CRP is an acute phase reactant which increases during inflammation (124, 125). CRP is an established cardiovascular risk factor, with higher levels associated with poorer cardiovascular outcomes (126). A large proportion of studies assessing the association between inflammation and depression use CRP as their marker of inflammation. IL-6 is a multifunctional cytokine involved in immunological processes such as hematopoiesis, immune modulation and acute inflammation (127, 128). It is produced by numerous cells including macrophages, neutrophils, and lymphocytes (129). Given its multisystem involvement, IL-6 has been previously suggested to play a large role in the pathophysiology of depression (130). Excessive levels of IL-6 may affect the HPA axis and may be associated with left ventricular dysfunction and poor outcomes following open cardiac surgery (131, 132). Studies of inflammation and depression in the cardiac surgery population are summarized in Table 2.
3.2.3. Inflammatory markers may predict depression following cardiac surgery
Studies assess different inflammatory markers on the development of depression, making it difficult to draw definitive conclusions about their effect. Measuring the levels of CRP may identify patients at risk of developing depression following cardiac surgery. Yang et al. reported that for an increase in each standard deviation of logarithmically transformed high sensitivity pre-operative CRP, there was an increased odds of developing depression pre-operatively (OR: 1.16, p = 0.001) and at 6 months post-operatively (OR: 1.15, p = 0.002). This relationship was maintained even after adjusting for confounding variables and risk factors such as gender, education level, medications such as statins, and occurrence of major cardiac adverse events (133). Similarly, Poole et al. demonstrated that elevations in the level of CRP measured between postoperative days 4–8 significantly contributed to the depression status and longer hospital stay (t = 2.62, p = 0.010) (134). In contrast, Ivankovic et al. did not demonstrate a relationship between preoperative depression status and postoperative CRP levels (135).
Additionally, Streptoe et al. reported that early postoperative interferon γ (IFN-γ) levels which were in the upper tertile (mean concentration: 56.68 7.5 pg./mL) was associated with depressive symptoms at 12 months (OR: 4.32, p = 0.024) (136). In contrast, there was no association between IL-6 and the development of postoperative depressive symptoms. Previous studies have demonstrated a correlation between the level of IL-6 and development of depressive symptoms (130, 137). IFN-γ is directly involved in the induction of indoleamine 2,3-dioxygenase, which is involved in the catabolism of tryptophan to products such as kynurenine (138). Elevated concentrations of kynurenine increase the likelihood of deleterious downstream effects as mentioned previously.
3.2.4. Relationship between depression, inflammatory markers, and length of hospital stay
3.2.4.1. Association between depression and length of hospital stay
The association between depression status and length of hospital stay following cardiac surgery is unclear. Length of hospital stay is a common proxy measure of acute physical recovery (139). Poole et al. reported that patients with elevated preoperative depressive symptoms (BDI greater than 10) prior to CABG were significantly more likely to stay in the hospital for longer than a week, compared to non-depressed patients (OR 3.51, 95% CI: 1.415–8.693, p = 0.007) (134). This relationship is likely mediated by an elevation in the level of CRP measured between postoperative days 4–8 (t = 2.62, p = 0.010). These results suggest pre-operative depression may promote excessive inflammation and lead to poorer outcomes and extended hospital stays. On the contrary, Ivankovic et al. did not detect a significant association between elevated preoperative depression scores (BDI greater than 13) and extended postoperative length of stay (greater than 7 days) (135). Additionally, within the same study, Ivankovic and colleagues subsequently stratified the patients’ preoperative BDI scores into depressed or non-depressed by applying the same cutoff as Poole et al. (Non-depressed: BDI less than 10; depressed: BDI greater than 10). Even with this filter, Ivankovic did not detect a significant association between binary BDI scores, and the length of postoperative hospital stay. The average EuroSCORE in Poole et al., study was 4.21 ± 2.79, while the median in Ivankovic et al.’s study was 1.1 with a range between 0.7 and 2.0. This may have contributed to the differences in postoperative recovery and length of hospital stay.
3.3. Disruption of the hypothalamic pituitary adrenal axis
3.3.1. Overview of the HPA axis
Consisting of the hypothalamus, pituitary gland and adrenal gland, the HPA axis is responsible for regulating the mammalian stress response, immunity, metabolic functioning, neurogenesis, neuronal survival and the emotional appraisal of events (140). Stressful stimuli trigger the production of corticotrophin-releasing hormone (CRH) and arginine vasopressin (AVP) by the paraventricular nucleus of the hypothalamus (141). CRH is a 41 amino acid peptide found within the central nervous system (142). Conversely, AVP is a cyclic nonapeptide with two forms, with one responsible for blood pressure regulation and the other responsible for the stress response (143). Following the binding of CRH to the CRH receptors within the anterior pituitary, adrenocorticotrophic hormone (ACTH) is released. Subsequently, ACTH will travel via the blood stream to act on the adrenal gland receptors to facilitate the release of glucocorticoids such as cortisol, mineralocorticoids such as aldosterone and androgens such as testosterone (144).
The HPA axis is regulated through a negative feedback mechanism. High concentrations of cortisol inhibit the further release of CRH and ACTH from the hypothalamus and pituitary gland, respectively, (145). Rapid increases in the concentration of cortisol also inhibit the HPA axis (146). On the contrary, glucocorticoids have been demonstrated to increase the concentration of CRH in limbic regions such as amygdala (147). Elevated expression of CRH within the limbic region has been associated with depressive symptoms (148, 149).
3.3.2. Dysregulation of the HPA axis in depression
Dysregulation of the HPA axis is commonly seen in MDD. Firstly, chronic emotional stress and MDD is associated with elevated cortisol secretion (150, 151). Normally, the HPA axis reacts appropriately through negative feedback mechanisms and reduce excess cortisol secretion. However, with HPA axis dysregulation, the negative feedback loop is impaired by reduced sensitivity of glucocorticoid receptors. This leads to hyperactivity of the HPA axis and further secretion of cortisol (152, 153). Cortisol hypersecretion is associated with depression following cardiac surgery (154). Poole et al. demonstrated a steeper change in cortisol concentration throughout the day (steeper cortisol slope) measured at 2 months post CABG is associated with a reduced odds of developing depression (defined as BDI greater than 10) at 12 months following surgery (OR: 0.661, 95% CI: 0.437–0.998, p = 0.049) (155).
Elevated CRH concentrations are observed in MDD, but not in psychopathologies such as schizophrenia and bipolar disorder (156, 157). Upon resolution of MDD, the CRH levels appear to normalize (158). Additionally, in response to exogenous CRH administration, there is a reduction in ACTH secretion (159–161). This may be attributed to the downregulation of CRH receptors within the hypothalamus. Disturbances in the HPA axis may lead to structural changes to the effectors of this axis. Hypersecretion of CRH due to impairment of the negative feedback loop may lead to pituitary hypertrophy (162, 163). Hippocampal changes in response to MDD have been an area of interest given this structure contains a high concentration of glucocorticoid receptors. A reduction in hippocampal volume is associated with the chronicity of disease, the severity and frequency of MDD episodes (164, 165). Reports of alterations to the size of the amygdala in MDD has been conflicting (166–168). HPA axis hyperactivity is associated with the development of classic cardiovascular risk factors including hypertension, dyslipidemia, impaired glucose tolerance and truncal obesity (169, 170). Hence, HPA axis dysregulation is a likely link between depression and cardiovascular disease.
3.3.3. Effect of dexamethasone on depression following cardiac surgery
Dexamethasone is a glucocorticoid receptor agonist with anti-inflammatory properties. Prolonged use is associated with neuropsychiatric deficits such as postoperative cognitive dysfunction, depressive symptoms and mania (171). Studies of dexamethasone in the cardiac surgery population are summarized in Table 3. Kok et al. assessed the effectiveness of administering a single intraoperative dose of intravenous dexamethasone, a glucocorticoid receptor agonist, on depression and post-traumatic stress disorder (PTSD) following cardiac surgery (172). Compared to the placebo, a single intraoperative dose of dexamethasone (1 mg/kg of bodyweight up to a maximum of 100 mg) did not affect the overall prevalence of depression following cardiac surgery. The overall rates of PTSD were similar between the groups. However, dexamethasone demonstrated long-lasting protective effects against the development of depression and PTSD in women. Six women who received dexamethasone developed depression, while 20 women who received placebo developed depression (p < 0.003). Similarly, 4 women who received dexamethasone developed PTSD, while 16 women who received placebo developed PTSD (p < 0.004). Women are more likely to be affected by HPA axis dysregulation and to have a higher basal cortisol concentration (174, 175). Additionally, gender may differentially affect HPA axis activation and glucocorticoid sensitivity, which in turn modulates the pro-inflammatory cytokine production (176, 177).
Genetic polymorphisms in the glucocorticoid receptor may significantly influence an individual’s susceptibility to develop depression and dictate their therapeutic response to antidepressant therapy (178, 179). Kok et al. assessed five common, single nucleotide polymorphisms on the glucocorticoid receptor including: rs41423247, rs10052957, rs6189, rs6195, and rs6198. They not identify polymorphisms which conferred protection against depression following cardiac surgery. On the contrary, three single nucleotide polymorphisms in the glucocorticoid receptor were required for dexamethasone to exert its protective effects against PTSD, including the rs41423247, rs10052957, and the rs6189 polymorphisms (173). The glucocorticoid receptor single nucleotide polymorphism rs6189 has been associated with reduced glucocorticoid receptor sensitivity and a faster response to treatment in MDD patients (180, 181). Thus, it is unclear why the protective effects of dexamethasone did not interact with this glucocorticoid receptor in the study. This is the largest study to date examining the genetic variability of the HPA axis in response to the administration of intraoperative dexamethasone in the cardiac surgery population. Future studies should also assess the concentration of CRH, ACTH and serum/salivary cortisol to gain a holistic view of HPA axis functioning.
4. Preventative and management strategies
The preventative and management strategies for depression following cardiac surgery are summarized in Figure 3. Management strategies may be divided into pharmacological or non-pharmacological strategies.
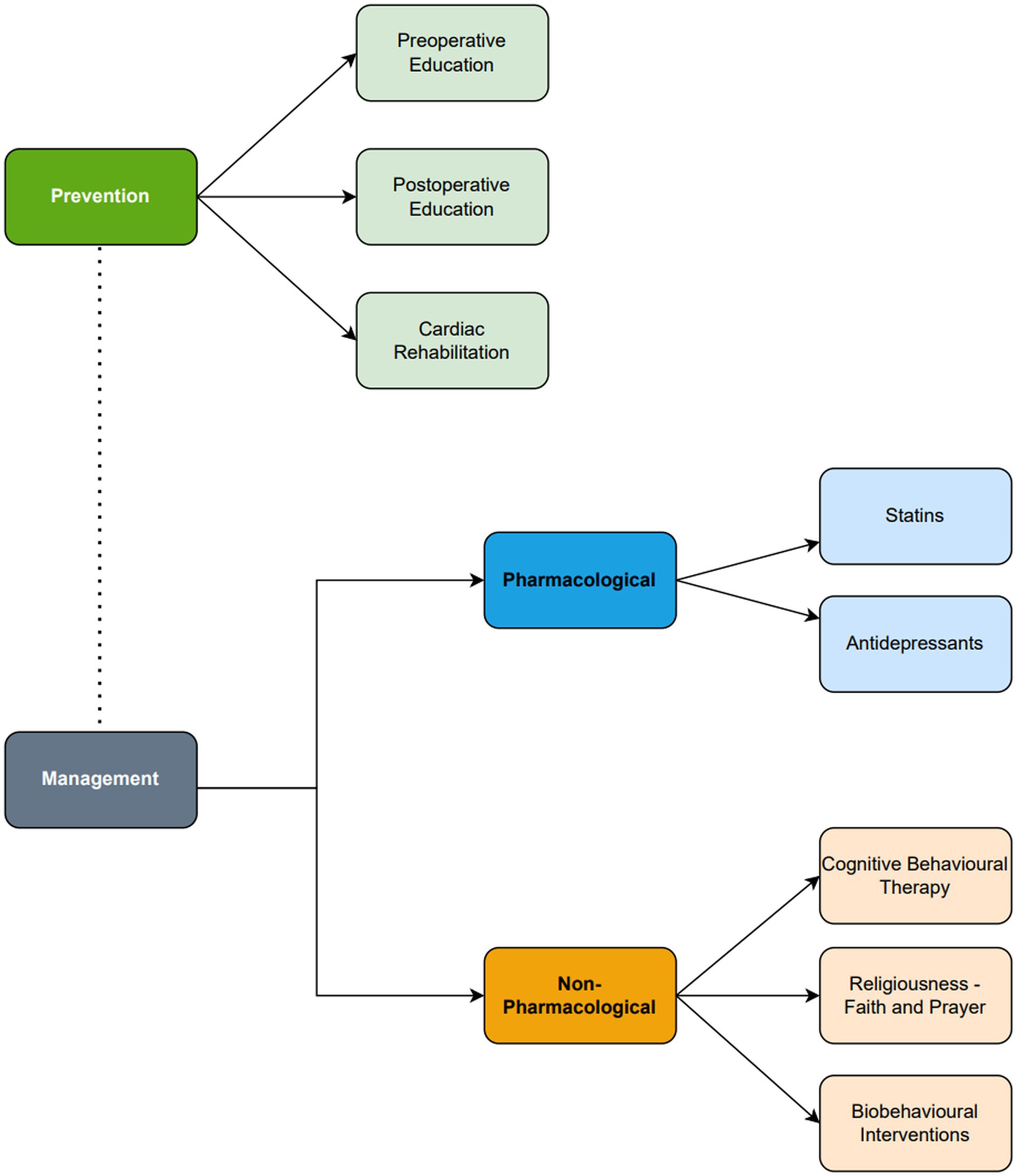
Figure 3. Summary diagram of the prevention and management of depression following cardiac surgery. Antidepressant and cognitive behavioral therapy are the core management for depression following cardiac surgery. Statins, religiousness/spirituality and biobehavioral interventions are emerging adjuncts which should only be considered in clinical practice following further evidence.
4.1. Preventative strategies
4.1.1. Preoperative education
Preoperative education is effective in reducing depressive symptoms following cardiac surgery. Education may be delivered at the bedside, in a group, via phone call, or even through social media platforms. Long wait times for surgery, particularly in the elective setting, contributes to mental distress (182, 183). Preoperatively, patients should be informed of the surgical process and risks involved with the procedure, required investigations, secondary prevention through risk factor modification as well as any other concerns or expectations. Patients should also be informed of whether surgical access will be achieved via median sternotomy or through minimally invasive methods (e.g., lateral thoracotomy). Access via median sternotomy is known to cause anxiety and addressing this concern may reduce the risk of anxiety and depression (184).
Preoperative education should also involve psychological support and setting health and recovery expectations (185). Conversely, postoperative education should focus on the recovery and rehabilitation process. Centers should provide both preoperative and postoperative education to reduce depression and anxiety. The results of educational interventions in the cardiac surgery setting are discussed below.
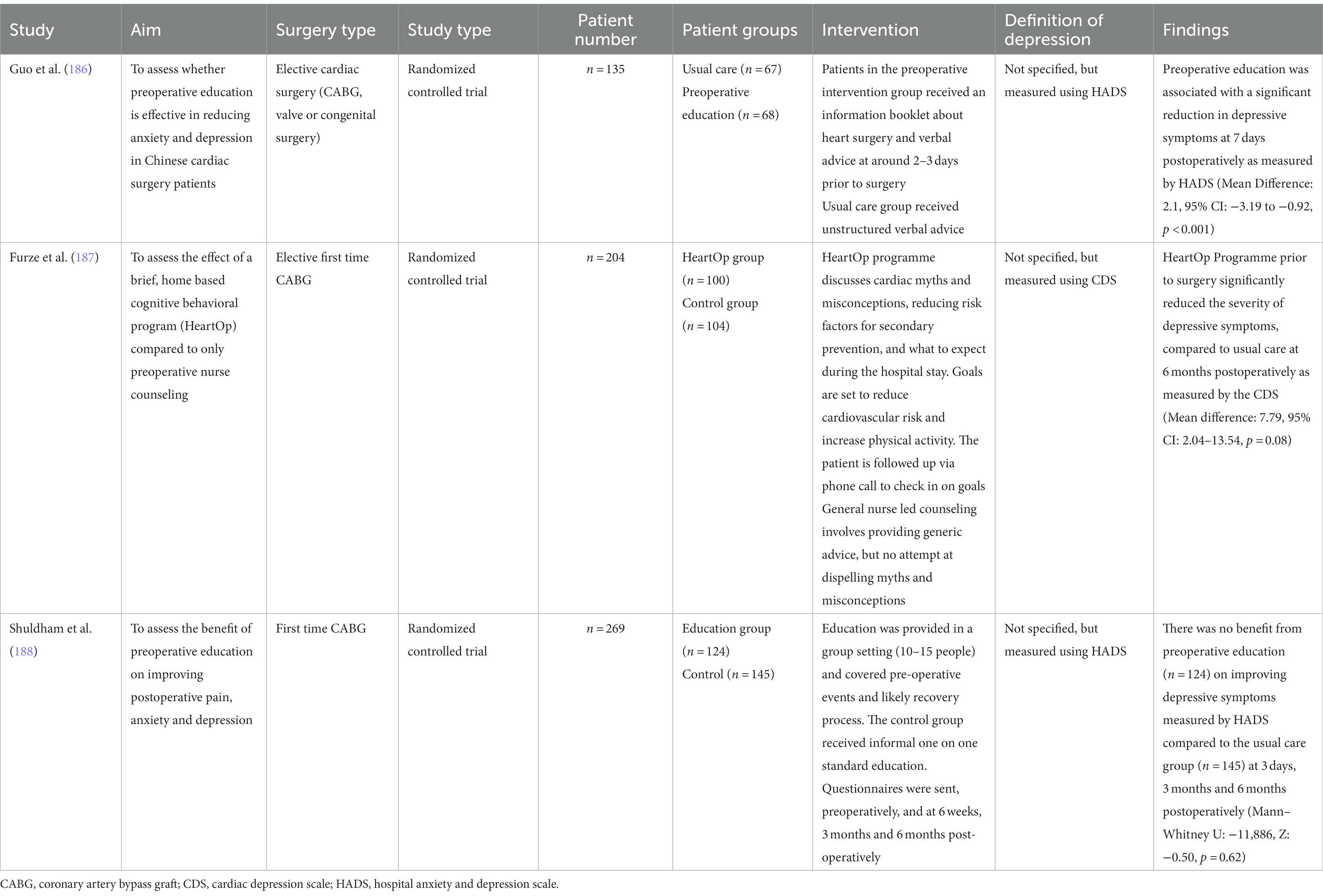
4.1.1.1. Preoperative education alone
Three studies were identified which examined preoperative education alone (Table 4). Two of the studies supported preoperative education in reducing depressive symptoms. In an RCT of a Chinese population, Guo et al. reported that compared to usual care, preoperative education was associated with a significant reduction in depressive symptoms at 7 days postoperatively as measured by HADS (Mean Difference: 2.1, 95% CI: −3.19 to −0.92, p < 0.001) (186). Their preoperative education intervention involved the distribution of a flyer and an accompanying explanation of what to expect from preadmission through to surgery and postoperative recovery. In comparison, the usual care group received general advice from the surgeon and anesthetist the day prior to their surgery. Preoperative education may have benefitted this population for several reasons. Firstly, patients undergoing cardiac surgery in several Chinese hospitals are admitted 1 week pre-operatively, contributing to the build-up of anxiety and depressive symptoms. Secondly, Chinese cardiac patients tend to be less informed about the finer details of their procedure. A qualitative analysis revealed surgeons generally discussed the severity of cardiac disease with the patient, but not the risks of surgery (which they limited to the patients’ family members) (189). However, surgeons should discuss risks with patients, as one reported “knowledge is comforting, with the more [they] knew, the less anxious [they] became” (190). Patients undergoing cardiac surgery should be placed in nearby beds to allow patients to support each other.
Additionally, Furze et al. demonstrated that a nurse-led educational and cognitive behavioral intervention (HeartOp Programme) prior to surgery significantly reduced the severity of depressive symptoms, compared to usual care at 6 months postoperatively as measured by the CDS (Mean difference: 7.79, 95% CI: 2.04–13.54, p = 0.08) (187). Both groups initially received a 1-h interview in the outpatient clinic, followed by phone calls 1, 3, and 6 weeks later and then monthly communication until the procedure. In the HeartOp group, nursing staff addressed common cardiac misconceptions, discussed risk factor alterations for secondary prevention and expectations within the hospital setting. They also facilitated goal setting for risk factor reduction. The program appears to be economical, costing £288.83 per quality adjusted life year. Common myths and goal setting was not addressed in the usual care group.
Conversely, Shuldham et al. did not demonstrate a benefit from preoperative education (n = 124) on improving depressive symptoms measured by HADS compared to the usual care group (n = 145) at 3 days, 3 months and 6 months postoperatively (Mann–Whitney U: –11,886, Z: −0.50, p = 0.62) (188). Education was delivered through a 4-h group session with 10–15 patients and involved discussion of the pre/post-operative period and rehabilitation. In contrast, the usual care group received informal verbal advice at the time of admission. The lack of benefit is likely attributed to an optimal standard of preoperative education already provided by the hospital. On the other hand, shy and reserved patients may not have sufficiently engaged in group discussions despite having the opportunity. Future studies should also compare pre-operative education in a group setting compared to a regular patient consultation.
4.1.1.2. Combination of pre-operative and post-operative education
In a Turkish population, Yaman Aktas et al. demonstrated preoperative and discharge education (n = 33) was effective in reducing HADS scores compared to usual care (n = 33) at 10 days and 4 weeks post-discharge following cardiac surgery (F = 19.23, p < 0.01) (191). Patients in the education group were provided one preoperative and four postoperative educational sessions. Preoperative education involved an informational pamphlet, an explanation of CHD, an overview of CABG and what to expect postoperatively. Postoperative education was commenced on postoperative day 3 to account for drowsiness and weakness immediately following surgery. Postoperative education involved an explanation of the recovery process, rehabilitation exercises, activities to avoid and when to re-present to hospital. The average education time per patient was 113.3 min, which appears justified given the reduction in depressive symptoms.
4.1.2. Cardiac rehabilitation
Cardiac rehabilitation programs are focused on restoring a patient’s physical capacity following surgery, decreasing the likelihood of further cardiac events, and improving psychological wellbeing. Patients who develop postoperative depression while attending cardiac rehabilitation are commonly affected by comorbidities such as poor lifestyle choices, diabetes, chronic pain, or angina (192). These patients are more resistant to improvements in mental health. Future cardiac rehabilitation programs should aim to address these comorbidities.
Cardiac rehabilitation is effective in reducing depressive symptoms following cardiac surgery (Table 5) (193–195). The cardiac rehabilitation interventions have been variable in focus as well as timing. Hojskov et al. conducted early physical rehabilitation and psychoeducation following CABG over 4 weeks. The physical intervention consisted of deep breathing exercises, peak flow spirometry, walking, neck/shoulder and cycling exercises, whereas psychoeducation was provided over four face-to-face consultations with a focus on mindfulness (193). On the other hand, Ma et al. conducted a 12-month intervention involving not only physical exercise guidance, but also counseling on CAD-related health education, risk factor controls strategies and psychological monitoring (194). Cardiac rehabilitation has also been shown to be effective in reducing depressive symptoms even if the patient completes it from home (195). This significantly increases the accessibility of services, particularly for patients with transport or logistical difficulties when attending outpatient appointments.
4.2. Non-pharmacological management
4.2.1. Cognitive behavioral therapy
Cognitive behavioral therapy (CBT) is effective, evidence-based psychotherapy for the management of psychopathologies such as depression and anxiety (196). CBT has several advantages. Firstly, the efficacy of CBT rivals that of anti-depressants in managing depression. Secondly, the concurrent use of CBT with antidepressants enhances the pharmacological effect of the medication and improves adherence to medications and rehabilitation plans (197, 198). Thirdly, the anti-depressive effect of CBT may last longer in comparison to anti-depressants and prevent relapse (199). Moreover, early initiation of CBT may halt the progression of new cases of depression. Persistent depressive symptoms are associated with a reduced chance of recovery. For example, the chance for recovery within the next 6 weeks if a patient has had depressive symptoms for 3 and 23 weeks is 40% and 5%, respectively, (200). CBT is limited by the necessity for patients to take ownership of their management, significant time commitments, travel requirements to clinic and limited focus on social networks (201, 202).
CBT sessions generally last an hour and run over several weeks. Within these sessions, trained mental health professionals will assist patients to identify automatic negative thoughts. They also aim to challenge and restructure negative thinking patterns and cognitive distortions with a goal to improve affect. In subsequent sessions, strategies aimed at maintaining psychological wellbeing are explored (203, 204). Following cardiac surgery, patients generally experience an improvement in psychological symptoms as their symptoms and quality of life significantly improve. However, postoperative pain, complications and slower than expected recovery may demotivate patients (205). This may also contribute to dysfunctional thoughts, loss of self-efficacy and fear of progression with rehabilitation exercises. CBT may directly address these cognitive distortions. Despite the clearly documented benefit of CBT in managing depression, there have been few studies exploring the efficacy of CBT in the cardiac surgery population (Table 6).
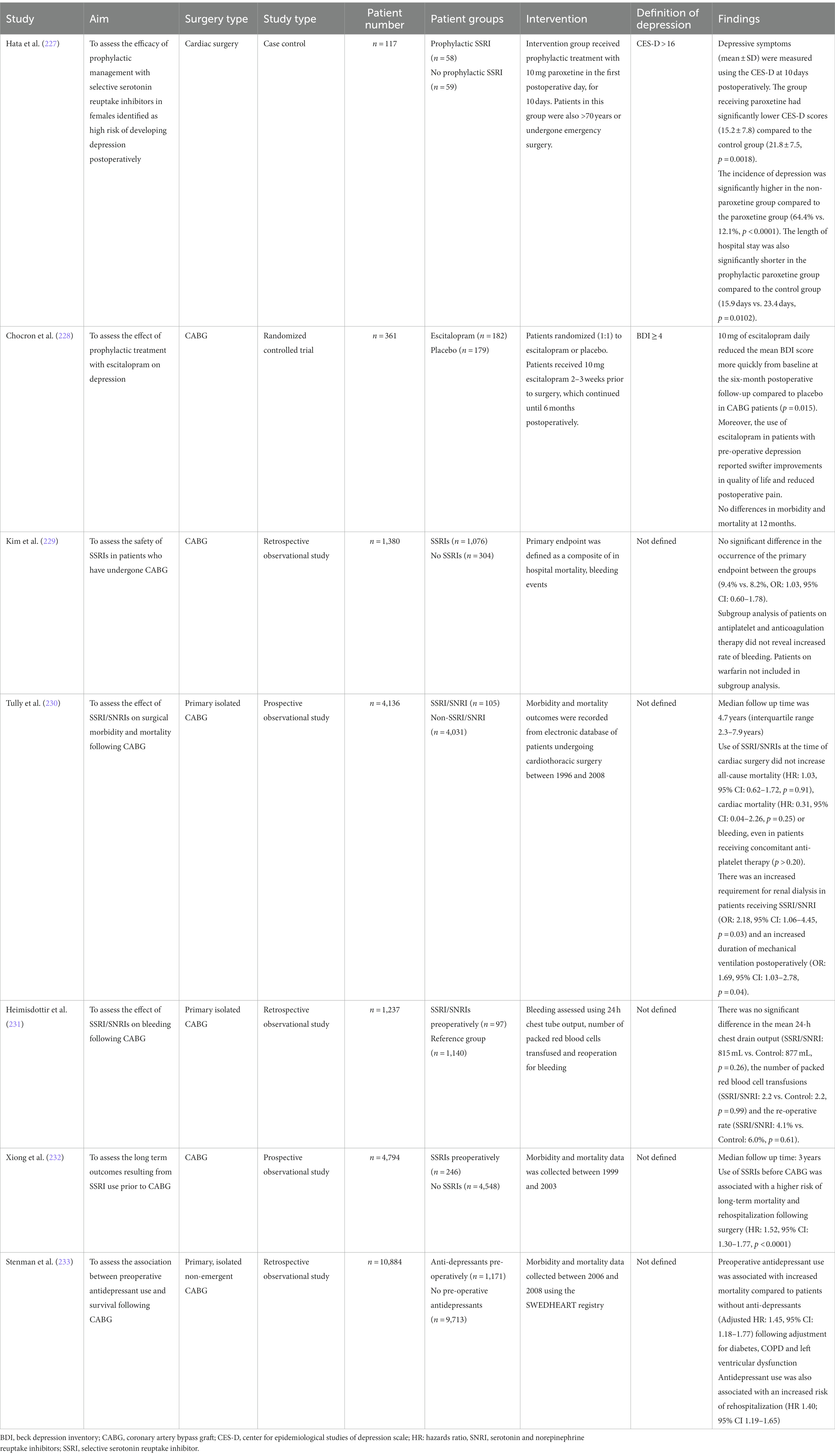
Standard delivery of CBT over a 12 week period appears to be effective at inducing sustained remission of depression following CABG compared to supportive stress management or usual care (χ2 = 11.95, p = 0.003) (206). To overcome the large time commitment required by traditional CBT models, a brief form of CBT such as the ‘Managing Anxiety and Depression Using Education and Skills’ (MADES) model have been proposed. For example, in the study by Dao et al., patients would attend 2 sessions preoperatively and 2 sessions within the first postoperative week. Compared to the usual care group, patients undergoing MADES had a shorter hospital stay (7.9 days ±2.6 days vs. 9.2 ± 3.5 days, p = 0.049) and demonstrated reduced depressive symptoms (207). However, the MADES model requires validation in a larger cardiac surgery population prior to widespread use. Furthermore, CBT has been shown to be effective in reducing depressive symptoms following cardiac surgery even when delivered at a patient’s home by nurses. This significantly eliminates accessibility and travel barriers required for patients to attend outpatient clinics (208). However, it should be noted that within this study, the number of females were under-represented within the CBT. This is a significant limitation as females have been shown to be more resistant to the effects of CBT (209). Lastly, a 9 month long CBT intervention following CABG has also been shown to be effective in improving the overall adaptability of the autonomic system. However, CBT did not improve vagal outflow (69).
4.2.2. Religiousness, faith, and prayer
The lead up to cardiac surgery induces anxiety in many patients. Patients may cope using active or maladaptive coping strategies. Active coping strategies include behavioral or cognitive strategies. Behavioral coping strategies include actions to improve a situation, while cognitive strategies involve mental activities such as changing one’s perspective on the situation, and positive reappraisal (210). Maladaptive coping strategies include actions which do not attempt to improve the situation, but rather ignore it or make it worse.
Religion is an unrecognized psychosocial factor and coping mechanism which may affect recovery following cardiac surgery (211). Religiousness refers to the belief in religious doctrines or the involvement in religious practices such as attending services or prayer (212). Religious involvement is postulated to improve postoperative recovery. Attending religious services is associated with increased social support, leading to greater connection with congregation members. Secondly, regular attendance of religious services may influence cognitive appraisal processes, which may therefore modulate immunological, autonomic and neuroendocrine activity (213).
The effect of religion and prayer on health outcomes in patients following cardiac surgery is not well established (Table 7). Contrada et al. reported stronger religious beliefs were associated with reduced postoperative complications and a shorter length of hospital stay. However, when postoperative complications were controlled for, there was no relationship between religion and length of stay (211). Religious involvement was also associated with an improvement in depressive symptoms. However, attendance at religious events was associated with poorer recovery and increased length of hospital stay. This may be attributed to whether a patient has a positive or negative religious coping style. Negative religious coping styles are seen in patients with religious struggles, and who have an insecure relationship with their god. Individuals with spiritual conflicts report poorer wellbeing scores, including depression (216).
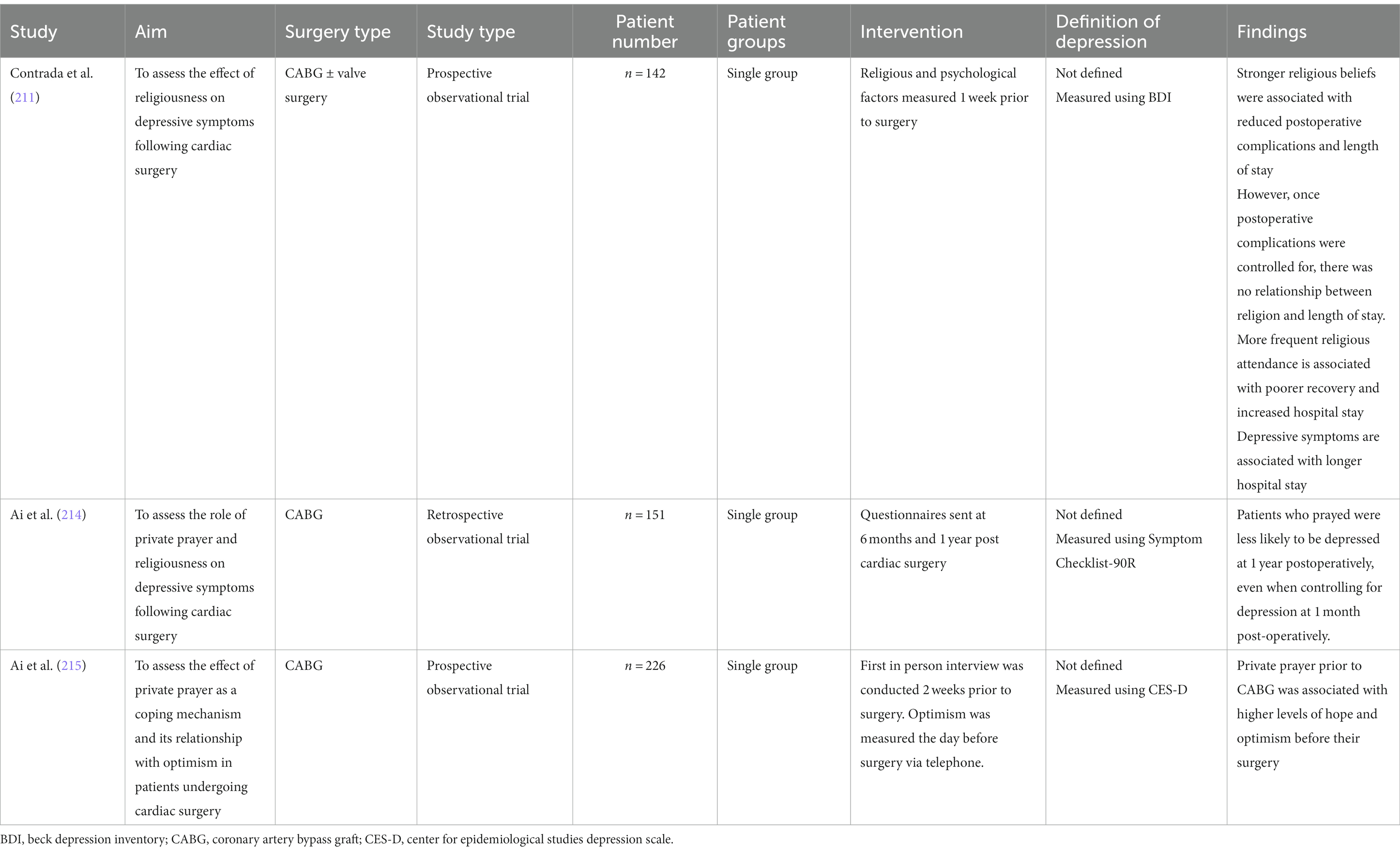
Table 7. Studies assessing the effect of religion on depressive features in patients undergoing cardiac surgery.
Ai and colleagues postulated that the use of prayer when faced with a medical issue draws allows an individual to draw upon one’s inner spirituality, and does not necessarily relate to specific religion or faith (214). Particularly in spiritual patients, the use of prayer may indicate a survival instinct and may also reduce anxiety (217). Patients who engaged in private prayer postoperatively had a lower level of distress 1 year following surgery (214). Ai et al. reported that private prayer prior to CABG was associated with higher levels of hope and optimism before their surgery (215). In contrast, Contrada and colleagues did not find an association between prayer and health outcomes or quality of life (211). They documented the frequency of prayer, but not the intent behind it. Clinicians should recognize that spirituality, religion, and prayer may be effective methods of coping. Spiritual guidance and services from a hospital chaplain or pastor should be offered to all patients.
4.2.3. Postoperative education alone
Postoperative education has been uniquely delivered via an online format. Ma et al. compared an internet-based education and rehabilitation program (n = 70) against standard care (n = 70) for patients following CABG (218). They designed an educational rehabilitation program on the social media platform WeChat, the main communication modality in China (219). A 12-month intervention delivered by nurses involved health education, rehabilitation guidance, exercise supervision and psychological care. Health education involved the delivery of weekly videos on post-CABG management/recovery and modification of cardiovascular risk factors. Videos demonstrating rehabilitation exercises were created. Additionally, patients received fortnightly calls from nurses to discuss their psychological wellbeing. In comparison, the usual care group received once-off verbal advice prior to discharge and a call every month to discuss further rehabilitation guidance. At 12 months postoperatively, the HADS score was significantly lower in the WeChat group compared to the usual care group (5.2 ± 2.5 vs. 6.1 ± 3.1, p = 0.048). Internet-based rehabilitation programs are more convenient for patients and are not limited by travel requirements. These interventions are cost-effective and patient queries are promptly answered. It is important to monitor adherence to medication or rehabilitation, as internet-based education may result in reduced patient engagement.
4.2.4. Biobehavioral interventions
Respiratory sinus arrythmia refers to the rhythmic increases and decreases in heart rate associated with respiration. Given respiratory sinus arrythmia is largely driven by vagal outflow and the pathophysiology of depression involves autonomic dysfunction, it reasonable to target this underlying mechanism. Patients in the biofeedback group may be trained to breathe abdominally and at a slower rate to synchronize their heart rate and abdominal breathing.
Despite limited data, Patron et al. found that biofeedback training to increase respiratory sinus arrythmia appears to be effective in decreasing depressive symptoms following cardiac surgery (220). However, given no follow up studies were conducted, it is unknown whether the improvements in depressive symptoms are long lasting. Additionally, it is also unclear whether depressive symptoms are reduced due to an improvement in autonomic regulation, or by another pathway. No other recent studies have been identified to examine the effect of biobehavioral interventions on depression following cardiac surgery.
4.3. Pharmacological management
4.3.1. Antidepressants
The management of moderate to severe depression may involve psychological or pharmacotherapy. Psychotherapy has been shown to be equivalent pharmacotherapy in managing depression. Over 50% of patients do not respond to their initially prescribed antidepressant, and over 30% of patients do not respond to subsequent management (221). The most used antidepressants include selective serotonin reuptake inhibitors (SSRIs), serotonin and noradrenaline reuptake Inhibitors (SNRIs) and tricyclic antidepressants (222). For cardiac surgery patients, SSRIs and SNRIs are most commonly used (12). Examples of SSRIs include escitalopram, citalopram, fluoxetine, paroxetine and sertraline (222). Tricyclic antidepressants are largely avoided given their cardiotoxic nature (223).
The main aim of antidepressant therapy is to reduce psychological and physical symptoms and improve their functional capacity. Antidepressants should be used in conjunction with psychotherapies (224). SSRIs competitively inhibit the presynaptic uptake of serotonin, consequently increasing the level of serotonin within the brain (222). Moreover, they have also been reported to attenuate autonomic dysfunction through improvements via HRV and have anti-inflammatory effects (225). Recent evidence suggests that anti-depressants may exert cardioprotective properties the attenuation of platelet function by interfering with serotonin uptake within platelets. This may reduce the development and progression of atherosclerotic lesions, and thus reduce the risk of CHD (226).
4.3.1.1. Efficacy
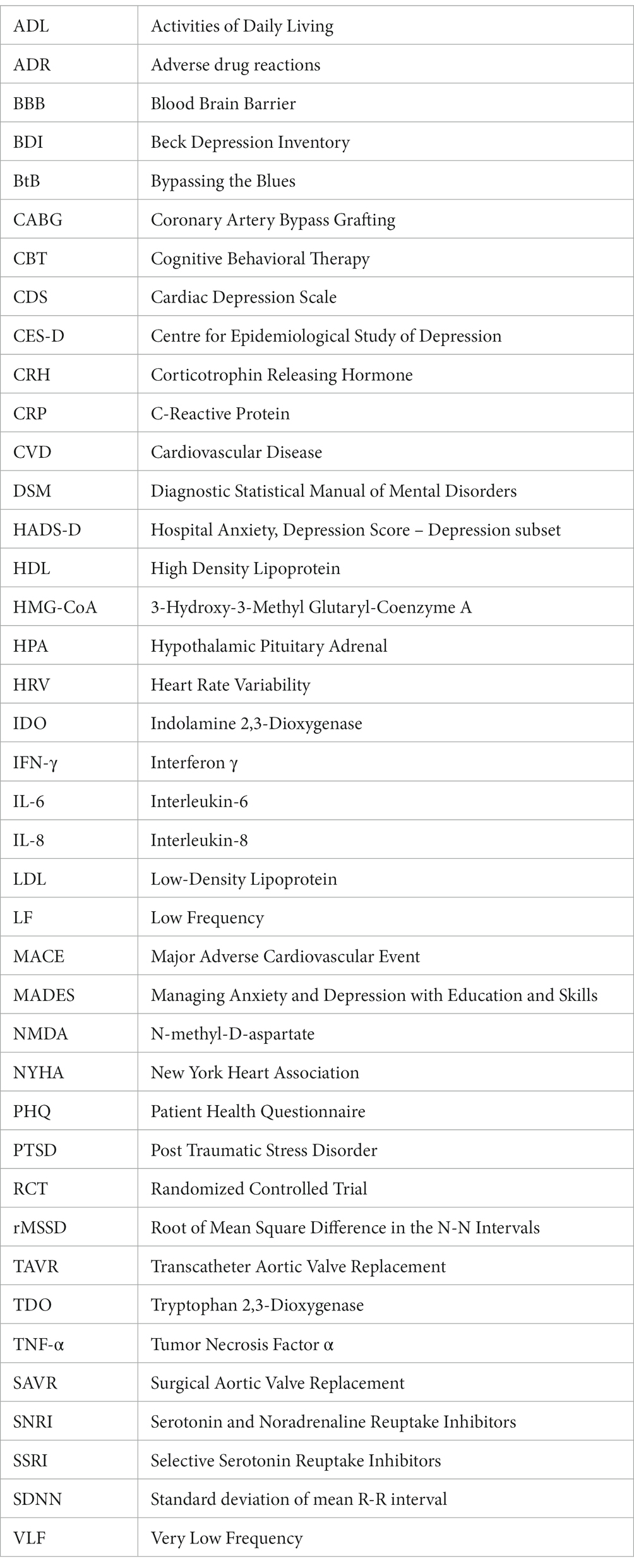
Despite limited evidence, SSRIs appear to be efficacious in reducing depression following cardiac surgery (Table 8) (227, 228). Prophylactic treatment with 10 mg of Paroxetine for 10 days in individuals identified to be at high risk of postoperative depression (older than 70 years and underwent emergency surgery) was associated with significantly lower CES-D scores (15.2 ± 7.8) compared to the control group (21.8 ± 7.5, p = 0.0018). The incidence of depression was significantly higher in the non-paroxetine group compared to the paroxetine group (64.4% vs. 12.1%, p < 0.0001) (227). Similarly, prophylactic treatment with 10 mg of escitalopram daily for 6 months was effective in reducing the mean BDI score from baseline compared to placebo in CABG patients (p = 0.015) (228). Moreover, the use of escitalopram in patients with pre-operative depression reported swifter improvements in quality of life and reduced postoperative pain. It should be noted that escitalopram was imitated starting 2–3 weeks prior to surgery to account for the delay period before the beneficial effects of SSRIs become clinically apparent (234).
4.3.1.2. Safety profile
Studies of the safety profile of antidepressants in the cardiac surgery population have mainly involved assessment of morbidity, mortality, and the risk of bleeding.
Mortality: The evidence describing the effect of SSRI/SNRIs on mortality in patients following cardiac surgery has been conflicting. Tully et al. reported that use of SSRI/SNRIs at the time of cardiac surgery did not increase all-cause mortality (HR: 1.03, 95% CI: 0.62–1.72, p = 0.91) or cardiac mortality (HR: 0.31, 95% CI: 0.04–2.26, p = 0.25) (230). Chocron et al. also did not report a difference in mortality between patients who were taking 10 mg of escitalopram and the control group (228). Similarly, Kim et al. reported no significant difference in the composite endpoint of hospital mortality and bleeding in patients who used antidepressants (9.4% vs. 8.2%, OR: 1.03, 95% CI: 0.60–1.78) (229). A systematic review comprising 162,001 patients (with 9,751 using SSRIs) revealed that the use of SSRIs pre-operatively or postoperatively did not increase 30-day hospital mortality or long-term mortality (235).
Conversely, Xiong et al. reported that use of SSRIs before CABG was associated with a higher risk of long-term mortality and rehospitalization following surgery (HR: 1.52, 95% CI: 1.30–1.77, p < 0.0001) (232). Notably, patients in the SSRI group were more likely to be affected by diabetes, dyslipidemia, hypertension, cerebrovascular disease, peripheral vascular disease, and a family history of coronary artery disease. These metabolic syndrome risk factors may have contributed to the poorer health of the patients in the SSRI group. Additionally, Xiong and colleagues did not adjust for potential covariates such as renal dysfunction and left ventricular dysfunction, factors which may significantly contribute to the increased mortality. Moreover, they only reported 5.1% of patients used SSRIs prior to surgery, while the prevalence of preoperative depression in CABG is estimated to be approximately 20% (12). This means patients may be underdiagnosed and undertreated, which inherently lead to poorer outcomes. Additionally, Stenman et al. also observed that preoperative antidepressant use was associated with increased mortality (HR: 1.45, 95% CI: 1.18–1.77) following adjustment for diabetes, COPD and ventricular dysfunction (233). This is concerning and warrants further investigation into the safety of SSRIs in the cardiac surgery population.
Bleeding: Concerns have been raised regarding the potential bleeding risk of SSRIs/SNRIs. Bleeding risk is particularly heightened in a cardiothoracic surgery population given they are at increased risk of rhythm disorders and are likely already on anti-platelet or anti-coagulants. SSRIs do not appear to increase bleeding risk in the vulnerable cardiac surgery population. Kim et al. performed a sub-analysis within their study which demonstrated no increased bleeding risk in patients already on anti-platelets or direct oral anticoagulants (229). However, patients on warfarin were not included in this sub-analysis, warranting future investigations into this common cardiac surgery demographic. Similarly, Tully et al. did not report increased bleeding risk from SSRIs, even in patients receiving anti-platelet therapy (p > 0.20) (230). Lastly, Heimisdottir et al. demonstrated that there was no significant difference in the mean 24-h chest drain output (SSRI/SNRI: 815 mL vs. Control: 877 mL, p = 0.26), the number of packed red blood cell transfusions (SSRI/SNRI: 2.2 vs. Control: 2.2, p = 0.99) and the re-operative rate (SSRI/SNRI: 4.1% vs. Control: 6.0%, p = 0.61) (231).
Other adverse reactions: The rate of adverse drug reactions to SSRIs/SNRIs appears to be low. Some examples of more common adverse reactions include diarrhea, nausea, vomiting, constipation, shivering and peripheral neuropathy (228). Notably, Tully and colleagues observed an increased requirement for renal dialysis in patients receiving SSRI/SNRI (OR: 2.18, 95% CI: 1.06–4.45, p = 0.03) and an increased duration of mechanical ventilation postoperatively (OR: 1.69, 95% CI: 1.03–2.78, p = 0.04) (230). However, these are novel results and should be interpreted with caution. SSRIs, especially citalopram, have also been associated with QTc prolongation. Rarely, they are associated with ventricular arrythmias and Torsade’s- de-Pointes. Consequently, the United States Food and Drug Administration do not recommend dosing citalopram higher than 40 mg/day (223).
4.3.2. Statins
Statins are predominantly used to manage hypercholesterolemia, a modifiable risk factor which strongly contributes to CHD (236). Mechanistically, statins reduce cholesterol biosynthesis within hepatocytes through competitive inhibition of 3-hydroxy-3-methyl glutaryl- coenzyme A (HMG-CoA) reductase (237). This results in an increased hepatic uptake of cholesterol from the bloodstream, reduced concentration of total cholesterol, and slightly increased concentration of high-density lipoprotein (HDL). Statins have also been associated with a myriad of pleiotropic effects such as anti-inflammatory, anti-sclerotic, antioxidant and anti-depressant activity (238).
The brain is a metabolically demanding and lipophilic organ which is highly susceptible to reactive oxygen species and oxidative stress (239). Statins, particularly atorvastatin, can act as an antioxidant to counteract the imbalance of reactive oxygen species, preventing further neuronal damage (240). Additionally, statins have been demonstrated to regulate glutamate excitotoxicity, another potential contributor to the pathophysiological mechanism of depression (240). Interestingly, the antidepressant effects of statins are also dependent on serotonergic modulation. However, this mechanism is unclear.
Statins may be classified as lipophilic or hydrophilic. Examples of lipophilic statins include atorvastatin, simvastatin and fluvastatin. On the contrary, examples of hydrophilic statins include pravastatin and rosuvastatin (241). Generally, lipophilic statins readily penetrate the blood brain barrier (BBB). Notably, simvastatin can pass through the BBB at least six times more easily compared to atorvastatin. Simvastatin’s stronger ability to penetrate the BBB may explain the result of studies where simvastatin was found to protect against the onset of Alzheimer’s Dementia compared to other statins such as lovastatin (242). Statins are well tolerated and a safe drug. The common adverse drug reactions (over 1% incidence) include myalgia, gastrointestinal symptoms, sleep disturbances and transient elevations in liver function tests. More significant drug reactions include rhabdomyolysis (particularly if taken with other cytochrome p450 inhibitors), hepatic dysfunction and peripheral neuropathy. There are few drug–drug interactions with statins (222).
4.3.2.1. Anti-inflammatory effect of statins
As previously discussed, depression may result in a pro-inflammatory state, which contributes to CHD. In turn, CHD and cardiac surgery itself is associated with a potentially a deleterious systemic inflammatory response which may contribute to neuroinflammation (101). Studies have demonstrated the anti-inflammatory properties of statins, even in the cardiac surgery population. Statins may indirectly attenuate the pro-inflammatory state through the reduction in LDL cholesterol (243). Chello et al. reported administration of atorvastatin (20 mg daily, n = 20) for 3 weeks in patients undergoing on-pump CABG resulted in significantly lower levels of IL-6 and IL-8 at four, and 24 h postoperatively (p = 0.02) compared to placebo (n = 20). They also observed a reduction in neutrophil CD18/CD11b expression at 4 h (p = 0.004) and 24 h (p = 0.01) postoperatively in the atorvastatin group compared to placebo. Chello and colleagues correlated this reduction in pro-inflammatory activity to reduced neutrophil adhesion in the saphenous vein endothelium (244). Similarly, a 2003 study by Chello and colleagues demonstrated an attenuation in neutrophil CD11b and endothelial P-selectin expression in the simvastatin group, compared with non-responders to simvastatin and the control group following on-pump CABG. This study supports the anti-inflammatory role of statins through a nitric oxide-mediated mechanism. Patients received statin therapy for a minimum of 3 months within this study to maximize the bioavailability of nitric oxide, but the average duration of treatment was not specified (245).
Moreover, the use of statins is associated with a reduction in CRP levels (246, 247). In a study comparing the effect of varying doses of atorvastatin (20 mg daily for 5 days, 80 mg for 4 days followed by 40 mg/day for a total of 5 days or no atorvastatin), the highest dose of atorvastatin was associated with a significant reduction in the CRP level (mean ± standard error of the mean). The CRP level in group B was 13,545 ± 959.9 mg/L.h (95% CIL 11,476 mg/L.h – 15,604 mg/L.h), whereas the CRP level in group A was 17,085 ± 858.4 mg/L.h (p = 0.01) (248).
4.3.2.2. Efficacy of statins in preventing or reducing depression
Statins should only be used as adjuncts for depression when further evidence regarding their efficacy emerges. From preliminary evidence, statins appear to prevent and improve depressive symptoms in the cardiac surgery population (Table 9). Stafford and Berk demonstrated the use of statin therapy commenced upon discharge was associated with a 79% reduction in the likelihood of developing MDD at 9 months post-operatively (95% CI: 0.052–0.876, p = 0.032) (249). Patients were started on either atorvastatin (n = 114), simvastatin (n = 29) or pravastatin (n = 14). The study was limited by an unclear adherence rate to the statins at 9 months. Hence, it is unclear how much the statins contributed to the reduced likelihood of depression. Notably, the patient cohort also consisted of patients who were hospitalized for CABG and percutaneous transluminal angioplasty or myocardial infarction. Despite the inclusion of three different presentations, the authors treated these patients as one homogenous group given evidence suggests CHD is both an etiological and prognostic factor for depression (251). Additionally, Abbasi et al. demonstrated that after 6 weeks, patients receiving simvastatin (20 mg daily) experienced a reduction in depressive symptoms (p = 0.026) (250). While the average Hamilton Depression Rating Scale score was lower in the simvastatin group (4.95 ± 3.98) compared to the atorvastatin group (8.56 ± 6.50), there was no significant difference between the groups. Future studies should have a larger sample size to compare simvastatin against placebo, as well confirm the potential superiority of simvastatin to other statins in preventing or managing depression following cardiac surgery.

Table 9. Studies assessing the efficacy of statins in preventing or reducing depression in cardiac surgery patients.
4.4. Alternative models of care
Collaborative Care is an emerging model of healthcare whereby a health professional, most commonly a nurse, acts as a case manager and facilitates communication between the patient and multi-disciplinary team. Based on Wagner’s Chronic Care Model, the case manager acts under the supervision of a primary care physician to educate patients about their medical condition, actively listens to their concerns and treatment preferences, offers evidence-based management advice, liaises with the multi-disciplinary team and proactively monitors a patients’ response to treatment (252).
The Bypassing the Blues (BtB) trial was an 8 month, RCT funded by the National Institute of Health (US) to assess the potential role of collaborative care in the management of depression following cardiac surgery (253, 254). Patients were allocated to either the usual care group (n = 152) or collaborative care group (n = 150). Collaborative care patients were contacted by case managers to receive psychoeducation and discuss preferred treatment options. Subsequently, treatment plans were formulated and reviewed by psychiatrists or family physicians. At the 8-month follow up, the proportion of patients with greater than a 50% reduction in depressive symptoms from baseline as measured by the Hamilton Depression Rating Scale were significantly higher in the collaborative care group (n = 75/150, 50%) compared to the usual care group (n = 46/152, 29.6%, p < 0.01). These results highlight the potential for collaborative care to facilitate the management of depression in cardiac surgery patients.
Post-hoc analysis of the BtB trial demonstrated that the benefits of collaborative care were not associated with adjustments in antidepressant medications through the 8-month period (p = 0.06) (255). Thus, the benefit of collaborative care is likely to be derived from additional time and rapport built with the care manager, rather than medication alterations. Through a 12-month cost-effectiveness study, collaborative care was associated with a median saving of $2068 US compared to the usual care group (256). However, this did not reach significance (p = 0.30). Given the potential healthcare savings and cost-effectiveness of collaborative care, primary care physicians should be involved in these emerging treatment approaches for managing depression following cardiac surgery.
5. Future directions
Additional validation studies should be conducted for the commonly used depression questionnaires within the cardiac surgery population. This would allow for the establishment of definitive cut-offs for depression within this population. Further studies should also focus on whether somatic symptoms following cardiac surgery significantly overestimate the severity of depression, and hence whether different questionnaires need to consider this issue. Additionally, given anxiety is highly comorbid with depression, these two mood states should be assessed concurrently rather than separately.
There are limited studies assessing the pathophysiological mechanisms leading to postoperative depression in cardiac surgery patients. Further research into autonomic dysregulation and the mechanism between postoperative depression and cardiac surgery should be explored. Studies assessing HRV and autonomic dysregulation should control for respiratory rate and sinus arrhythmias, given they may affect HRV readings (257). HRV is not routinely collected in clinical practice. Institutions should consider using HRV biofeedback tools to track HRV and aim to normalize vagal outflow. Elevated levels of CRP are associated with postoperative depression and predicts the length of hospital stay. Studies should also assess the levels of other inflammatory markers such as IL-6, TNF-α and their association with postoperative depression. However, little is known about how this inflammatory state translates into postoperative depression. There should be increased examination of HPA dysregulation and the association with postoperative depression in cardiac surgery patients. Metabolic and genomic studies into this area may provide insight into the underlying mechanisms of HPA dysregulation and the association with depression. Studies should assess the concentration of CRH, ACTH and cortisol specifically in cardiac surgery patients to gain a holistic view of HPA axis functioning.
Future studies should assess the ideal duration and format of CBT (i.e., clinic vs. telephone based) to manage depression postoperatively. Given the costs associated with CBT, studies should attempt to identify which postoperative patients should undergo CBT. Very little is known about the mechanism of statins in reducing depressive symptoms, and thus, studies should also compare one statin at a time. For example, one statin should be compared to placebo, or another type of statin. Studies involving pharmacological agents should also include adherence rates, and how long patients were taking the medications for.
6. Conclusion
The understanding of the bidirectional relationship between depression and cardiac disease is limited. Depression is associated with several pathophysiological and behavioral factors which increase the likelihood of developing CHD. In addition, these factors may also contribute to postoperative depression. Additional studies are needed to elucidate these possible mechanisms, particularly within the cardiac surgery population who are highly susceptible to postoperative depression and a better understanding of such will further inform future prevention and management strategies.
Author contributions
TV and JS conceptualized and designed the study. TV collected the data, analyzed the data, and produced the first draft of the manuscript. All authors contributed to subsequent drafts of the manuscript, including editing, and refining of the final manuscript and approved the final version of the manuscript for submission.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor MA declared a past co-authorship with the author JS.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Roth, GA, Mensah, GA, Johnson, CO, Addolorato, G, Ammirati, E, Baddour, LM, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J Am Coll Cardiol. (2020) 76:2982–3021. doi: 10.1016/j.jacc.2020.11.010
2. Zilla, P, Yacoub, M, Zühlke, L, Beyersdorf, F, Sliwa, K, Khubulava, G, et al. Global unmet needs in cardiac surgery. Glob Heart. (2018) 13:293–303. doi: 10.1016/j.gheart.2018.08.002
3. Natarajan, A, Samadian, S, and Clark, S. Coronary artery bypass surgery in elderly people. Postgrad Med J. (2007) 83:154–8. doi: 10.1136/pgmj.2006.049742
4. Gutiérrez-Rojas, L, Porras-Segovia, A, Dunne, H, Andrade-González, N, and Cervilla, JA. Prevalence and correlates of major depressive disorder: a systematic review. Braz J Psychiatry. (2020) 42:657–72. doi: 10.1590/1516-4446-2020-0650
5. Burker, EJ, Blumenthal, JA, Feldman, M, Burnett, R, White, W, Smith, LR, et al. Depression in male and female patients undergoing cardiac surgery. Br J Clin Psychol. (1995) 34:119–28. doi: 10.1111/j.2044-8260.1995.tb01444.x
6. McKhann, GM, Borowicz, LM, Goldsborough, MA, Enger, C, and Selnes, OA. Depression and cognitive decline after coronary artery bypass grafting. Lancet. (1997) 349:1282–4.
7. Khatri, P, Babyak, M, Clancy, C, Davis, R, Croughwell, N, Newman, M, et al. Perception of cognitive function in older adults following coronary artery bypass surgery. Health Psychol. (1999) 18:301–6. doi: 10.1037/0278-6133.18.3.301
8. Pirraglia, PA, Peterson, JC, Williams-Russo, P, Gorkin, L, and Charlson, ME. Depressive symptomatology in coronary artery bypass graft surgery patients. Int J Geriatr Psychiatry. (1999) 14:668–80. doi: 10.1002/(SICI)1099-1166(199908)14:8<668::AID-GPS988>3.0.CO;2-9
9. Wang, X-s, Mei, Y-q, Li, A-p, Ji, Q, Sun, Y-f, Zhu, C, et al. Depression before and after operation in patients undergoing coronary artery bypass grafting and the effect thereof on quality of life. Zhonghua Yi Xue Za Zhi. (2008) 88:3283–6. Available at: https://europepmc.org/article/med/19159556.
10. Beresnevaite, M, Benetis, R, Taylor, GJ, Jureniene, K, Kinduris, S, and Barauskiene, V. Depression predicts perioperative outcomes following coronary artery bypass graft surgery. Scand Cardiovasc J. (2010) 44:289–94. doi: 10.3109/14017431.2010.490593
11. Korbmacher, B, Ulbrich, S, Dalyanoglu, H, Lichtenberg, A, Schipke, JD, Franz, M, et al. Perioperative and long-term development of anxiety and depression in CABG patients. Thorac Cardiovasc Surg. (2013) 61:676–81. doi: 10.1055/s-0032-1333326
12. Tully, PJ. Psychological depression and cardiac surgery: a comprehensive review. J Extra Corpor Technol. (2012) 44:224–32. doi: 10.1051/ject/201244224
13. Peterson, JC, Charlson, ME, Williams-Russo, P, Krieger, KH, Pirraglia, PA, Meyers, BS, et al. New postoperative depressive symptoms and long-term cardiac outcomes after coronary artery bypass surgery. Am J Geriatr Psychiatry. (2002) 10:192–8. doi: 10.1097/00019442-200203000-00010
14. Doering, LV, Magsarili, MC, Howitt, LY, and Cowan, MJ. Clinical depression in women after cardiac surgery. J Cardiovasc Nurs. (2006) 21:132–1. doi: 10.1097/00005082-200603000-00010
15. Hata, M, Yagi, Y, Sezai, A, Niino, T, Yoda, M, Wakui, S, et al. Risk analysis for depression and patient prognosis after open heart surgery. Circ J. (2006) 70:389–92. doi: 10.1253/circj.70.389
16. Krannich, J-HA, Weyers, P, Lueger, S, Herzog, M, Bohrer, T, and Elert, O. Presence of depression and anxiety before and after coronary artery bypass graft surgery and their relationship to age. BMC Psychiatry. (2007) 7:47. doi: 10.1186/1471-244X-7-47
17. Li, A-p, Hu, D-y, Zhao, Z, Ma, W-l, Zhang, X, Mei, Y-q, et al. Depression before and after operation in coronary artery bypass grafting patients. Zhonghua Yi Xue Za Zhi. (2006) 86:2188–91. Available at: https://europepmc.org/article/med/17064504.
18. Timberlake, N, Klinger, L, Smith, P, Venn, G, Treasure, T, Harrison, M, et al. Incidence and patterns of depression following coronary artery bypass graft surgery. J Psychosom Res. (1997) 43:197–207. doi: 10.1016/S0022-3999(96)00002-5
19. McKenzie, LH, Simpson, J, and Stewart, M. A systematic review of pre-operative predictors of post-operative depression and anxiety in individuals who have undergone coronary artery bypass graft surgery. Psychol Health Med. (2010) 15:74–93. doi: 10.1080/13548500903483486
20. Oxman, TE, and Hull, JG. Social support, depression, and activities of daily living in older heart surgery patients. J Gerontol B Psychol Sci Soc Sci. (1997) 52B:P1–P14. doi: 10.1093/geronb/52B.1.P1
21. Horne, D, Kehler, S, Kaoukis, G, Hiebert, B, Garcia, E, Duhamel, TA, et al. Depression before and after cardiac surgery: do all patients respond the same? J Thorac Cardiovasc Surg. (2013) 145:1400–6. doi: 10.1016/j.jtcvs.2012.11.011
22. Dunkel, A, Kendel, F, Lehmkuhl, E, Babitsch, B, Oertelt-Prigione, S, Hetzer, R, et al. Predictors of preoperative depressive risk in patients undergoing coronary artery bypass graft surgery. Clin Res Cardiol. (2009) 98:643–50. doi: 10.1007/s00392-009-0050-0
23. Kroenke, K, and Spitzer, RL. The PHQ-9: a new depression diagnostic and severity measure. Psychiatr Ann. (2002) 32:509–15. doi: 10.3928/0048-5713-20020901-06
24. McManus, D, Pipkin, SS, and Whooley, MA. Screening for depression in patients with coronary heart disease (data from the heart and soul study). Am J Cardiol. (2005) 96:1076–81. doi: 10.1016/j.amjcard.2005.06.037
25. Thombs, BD, Ziegelstein, RC, and Whooley, MA. Optimizing detection of major depression among patients with coronary artery disease using the patient health questionnaire: data from the heart and soul study. J Gen Intern Med. (2008) 23:2014–7. doi: 10.1007/s11606-008-0802-y
26. Pinto-Meza, A, Serrano-Blanco, A, Penarrubia, MT, Blanco, E, and Haro, JM. Assessing depression in primary care with the PHQ-9: can it be carried out over the telephone? J Gen Intern Med. (2005) 20:738–42. doi: 10.1111/j.1525-1497.2005.0144.x
27. Lichtman, JH, Bigger, JT Jr, Blumenthal, JA, Frasure-Smith, N, Kaufmann, PG, Fo, Lespérance, et al. Depression and coronary heart disease: recommendations for screening, referral, and treatment: a science advisory from the American Heart Association prevention Committee of the Council on cardiovascular nursing, council on clinical Cardiology, council on epidemiology and prevention, and interdisciplinary council on quality of care and outcomes research: endorsed by the American Psychiatric Association. Circulation (2008);118(17):1768–75. doi: 10.1161/CIRCULATIONAHA.108.190769
28. Tully, PJ, Baumeister, H, Bennetts, JS, Rice, GD, and Baker, RA. Depression screening after cardiac surgery: a six month longitudinal follow up for cardiac events, hospital readmissions, quality of life and mental health. Int J Cardiol. (2016) 206:44–50. doi: 10.1016/j.ijcard.2016.01.015
29. Stenman, M, and Sartipy, U. Depression screening in cardiac surgery patients. Heart Lung Circ. (2019) 28:953–8. doi: 10.1016/j.hlc.2018.04.298
30. Gorini, A, Giuliani, M, Raggio, L, Barbieri, S, and Tremoli, E. Depressive and anxiety symptoms screening in cardiac inpatients: a virtuous Italian approach to Psychocardiology. Int J Environ Res Public Health. (2020) 17:5007. doi: 10.3390/ijerph17145007
31. Chabrol, H, Montovany, A, Chouicha, K, and Duconge, E. Study of the CES-D on a sample of 1,953 adolescent students. L’Encéphale. (2002) 28:429–32. Available at: https://europepmc.org/article/med/12386544.
32. Radloff, LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. (1977) 1:385–401. doi: 10.1177/014662167700100306
33. Jiang, L, Wang, Y, Zhang, Y, Li, R, Wu, H, Li, C, et al. The reliability and validity of the Center for Epidemiologic Studies Depression Scale (CES-D) for Chinese university students. Front Psych. (2019) 10:315. doi: 10.3389/fpsyt.2019.00315
34. Doering, LV, Chen, B, McGuire, A, Bodan, RC, and Irwin, MR. Persistent depressive symptoms and pain after cardiac surgery. Psychosom Med. (2014) 76:437–44. doi: 10.1097/PSY.0000000000000074
35. Jackson-Koku, G. Beck depression inventory. Occup Med. (2016) 66:174–5. doi: 10.1093/occmed/kqv087
36. Vingerhoets, G. Perioperative anxiety and depression in open-heart surgery. Psychosomatics. (1998) 39:30–7. doi: 10.1016/S0033-3182(98)71378-7
37. Richter, P, Werner, J, Heerlein, A, Kraus, A, and Sauer, H. On the validity of the Beck depression inventory. Psychopathology. (1998) 31:160–8. doi: 10.1159/000066239
38. Zigmond, AS, and Snaith, RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. (1983) 67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x
39. Johnston, M, Pollard, B, and Hennessey, P. Construct validation of the hospital anxiety and depression scale with clinical populations. J Psychosom Res. (2000) 48:579–84. doi: 10.1016/S0022-3999(00)00102-1
40. Bjelland, I, Dahl, AA, Haug, TT, and Neckelmann, D. The validity of the hospital anxiety and depression scale. An updated literature review. J Psychosom Res. (2002) 52:69–77. doi: 10.1016/S0022-3999(01)00296-3
41. Hare, DL, and Davis, CR. Cardiac depression scale: validation of a new depression scale for cardiac patients. J Psychosom Res. (1996) 40:379–86. doi: 10.1016/0022-3999(95)00612-5
42. Shi, WY, Stewart, AG, and Hare, DL. Major depression in cardiac patients is accurately assessed using the cardiac depression scale. Psychother Psychosom. (2010) 79:391. doi: 10.1159/000320897
43. Birks, Y, Roebuck, A, and Thompson, DR. A validation study of the cardiac depression scale (CDS) in a UK population. Br J Health Psychol. (2004) 9:15–24. doi: 10.1348/135910704322778696
44. Connerney, I, Sloan, RP, Shapiro, PA, Bagiella, E, and Seckman, C. Depression is associated with increased mortality 10 years after coronary artery bypass surgery. Psychosom Med. (2010) 72:874–81. doi: 10.1097/PSY.0b013e3181f65fc1
45. Carney, RM, Freedland, KE, Rich, MW, and Jaffe, AS. Depression as a risk factor for cardiac events in established coronary heart disease: a review of possible mechanisms. Ann Behav Med. (1995) 17:142–9. doi: 10.1007/BF02895063
46. Gehi, A, Haas, D, Pipkin, S, and Whooley, MA. Depression and medication adherence in outpatients with coronary heart disease: findings from the heart and soul study. Arch Intern Med. (2005) 165:2508–13. doi: 10.1001/archinte.165.21.2508
47. Swardfager, W, Herrmann, N, Marzolini, S, Saleem, M, Farber, SB, Kiss, A, et al. Major depressive disorder predicts completion, adherence, and outcomes in cardiac rehabilitation: a prospective cohort study of 195 patients with coronary artery disease. J Clin Psychiatry. (2010) 71:11022. doi: 10.4088/JCP.09m05810blu
48. John, U, Meyer, C, Rumpf, H-J, and Hapke, U. Self-efficacy to refrain from smoking predicted by major depression and nicotine dependence. Addict Behav. (2004) 29:857–66. doi: 10.1016/j.addbeh.2004.02.053
49. Mallik, S, Krumholz, HM, Lin, ZQ, Kasl, SV, Mattera, JA, Roumains, SA, et al. Patients with depressive symptoms have lower health status benefits after coronary artery bypass surgery. Circulation. (2005) 111:271–7. doi: 10.1161/01.CIR.0000152102.29293.D7
50. Goyal, TM, Idler, EL, Krause, TJ, and Contrada, RJ. Quality of life following cardiac surgery: impact of the severity and course of depressive symptoms. Psychosom Med. (2005) 67:759–65. doi: 10.1097/01.psy.0000174046.40566.80
51. Gohari, J, Grosman-Rimon, L, Arazi, M, Caspi-Avissar, N, Granot, D, Gleitman, S, et al. Clinical factors and pre-surgical depression scores predict pain intensity in cardiac surgery patients. BMC Anesthesiol. (2022) 22:204. doi: 10.1186/s12871-022-01740-3
52. Soderman, E, Lisspers, J, and Sundin, O. Depression as a predictor of return to work in patients with coronary artery disease. Soc Sci Med. (2003) 56:193–202. doi: 10.1016/S0277-9536(02)00024-2
53. Burg, MM, Benedetto, MC, Rosenberg, R, and Soufer, R. Presurgical depression predicts medical morbidity 6 months after coronary artery bypass graft surgery. Psychosom Med. (2003) 65:111–8. doi: 10.1097/01.PSY.0000038940.33335.09
54. Ho, PM, Masoudi, FA, Spertus, JA, Peterson, PN, Shroyer, AL, McCarthy, M Jr, et al. Depression predicts mortality following cardiac valve surgery. Ann Thorac Surg. (2005) 79:1255–9. doi: 10.1016/j.athoracsur.2004.09.047
55. Stenman, M, Holzmann, MJ, and Sartipy, U. Relation of major depression to survival after coronary artery bypass grafting. Am J Cardiol. (2014) 114:698–703. doi: 10.1016/j.amjcard.2014.05.058
56. Stenman, M, Holzmann, MJ, and Sartipy, U. Association between preoperative depression and long-term survival following coronary artery bypass surgery - a systematic review and meta-analysis. Int J Cardiol. (2016) 222:462–6. doi: 10.1016/j.ijcard.2016.07.216
57. Flaherty, LB, Wood, T, Cheng, A, and Khan, AR. Pre-existing psychological depression confers increased risk of adverse cardiovascular outcomes following cardiac surgery: a systematic review and meta-analysis. J Thorac Cardiovasc Surg. (2017) 154:1578–86.e1. doi: 10.1016/j.jtcvs.2017.06.052
58. Lee, GA. Determinants of quality of life five years after coronary artery bypass graft surgery. Heart Lung. (2009) 38:91–9. doi: 10.1016/j.hrtlng.2008.04.003
59. McKenzie, LH, Simpson, J, and Stewart, M. The impact of depression on activities of daily living skills in individuals who have undergone coronary artery bypass graft surgery. Psychol Health Med. (2009) 14:641–53. doi: 10.1080/13548500903254234
60. Tully, PJ, Baker, RA, Turnbull, D, and Winefield, H. The role of depression and anxiety symptoms in hospital readmissions after cardiac surgery. J Behav Med. (2008) 31:281–90. doi: 10.1007/s10865-008-9153-8
61. Wellenius, GA, Mukamal, KJ, Kulshreshtha, A, Asonganyi, S, and Mittleman, MA. Depressive symptoms and the risk of atherosclerotic progression among patients with coronary artery bypass grafts. Circulation. (2008) 117:2313–9. doi: 10.1161/CIRCULATIONAHA.107.741058
62. Blumenthal, JA, Lett, HS, Babyak, MA, White, W, Smith, PK, Mark, DB, et al. Depression as a risk factor for mortality after coronary artery bypass surgery. Lancet. (2003) 362:604–9. doi: 10.1016/S0140-6736(03)14190-6
63. Von Borell, E, Langbein, J, Després, G, Hansen, S, Leterrier, C, Marchant-Forde, J, et al. Heart rate variability as a measure of autonomic regulation of cardiac activity for assessing stress and welfare in farm animals—a review. Physiol Behav. (2007) 92:293–316. doi: 10.1016/j.physbeh.2007.01.007
64. Shaffer, F, and Ginsberg, JP. An overview of heart rate variability metrics and norms. Front Public Health. (2017) 5:258. doi: 10.3389/fpubh.2017.00258
65. Lehrer, PM, Vaschillo, E, and Vaschillo, B. Resonant frequency biofeedback training to increase cardiac variability: rationale and manual for training. Appl Psychophysiol Biofeedback. (2000) 25:177–91. doi: 10.1023/A:1009554825745
66. Wiklund, U, Hörnsten, R, Karlsson, M, Suhr, OB, and Jensen, SM. Abnormal heart rate variability and subtle atrial arrhythmia in patients with familial amyloidotic polyneuropathy. Ann Noninvasive Electrocardiol. (2008) 13:249–56. doi: 10.1111/j.1542-474X.2008.00228.x
67. Goldberger, JJ, Challapalli, S, Tung, R, Parker, MA, and Kadish, AH. Relationship of heart rate variability to parasympathetic effect. Circulation. (2001) 103:1977–83. doi: 10.1161/01.CIR.103.15.1977
68. Laborde, S, Mosley, E, and Thayer, JF. Heart rate variability and cardiac vagal tone in psychophysiological research–recommendations for experiment planning, data analysis, and data reporting. Front Psychol. (2017) 8:213. doi: 10.3389/fpsyg.2017.00213
69. Beresnevaite, M, Benetis, R, Taylor, GJ, Rasinskiene, S, Stankus, A, and Kinduris, S. Impact of a cognitive behavioral intervention on health-related quality of life and general heart rate variability in patients following cardiac surgery: an effectiveness study. Psychosomatics. (2016) 57:605–15. doi: 10.1016/j.psym.2016.04.004
70. Ong, MEH, Goh, K, Fook-Chong, S, Haaland, B, Wai, KL, Koh, ZX, et al. Heart rate variability risk score for prediction of acute cardiac complications in ED patients with chest pain. Am J Emerg Med. (2013) 31:1201–7. doi: 10.1016/j.ajem.2013.05.005
71. Kleiger, RE, Stein, PK, and Bigger, JT Jr. Heart rate variability: measurement and clinical utility. Ann Noninvasive Electrocardiol. (2005) 10:88–101. doi: 10.1111/j.1542-474X.2005.10101.x
72. Kleiger, RE, Stein, PK, Bosner, MS, and Rottman, JN. Time domain measurements of heart rate variability. Cardiol Clin. (1992) 10:487–98. doi: 10.1016/S0733-8651(18)30230-3
73. Kamath, MV, and Fallen, EL. Power spectral analysis of heart rate variability: a noninvasive signature of cardiac autonomic function. Crit Rev Biomed Eng. (1993) 21:245–311.
74. Malliani, A, Lombardi, F, and Pagani, M. Power spectrum analysis of heart rate variability: a tool to explore neural regulatory mechanisms. Br Heart J. (1994) 71:1. doi: 10.1136/hrt.71.1.1
75. Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. (1996) 93:1043–65.
76. Schwartz, PJ, and Vanoli, E. Cardiac arrhythmias elicited by interaction between acute myocardial ischemia and sympathetic hyperactivity: a new experimental model for the study of antiarrhythmic drugs. J Cardiovasc Pharmacol. (1981) 3:1251–9. doi: 10.1097/00005344-198111000-00012
77. Carney, RM, Freedland, KE, Miller, GE, and Jaffe, AS. Depression as a risk factor for cardiac mortality and morbidity: a review of potential mechanisms. J Psychosom Res. (2002) 53:897–902. doi: 10.1016/S0022-3999(02)00311-2
78. Hughes, JW, Watkins, L, Blumenthal, JA, Kuhn, C, and Sherwood, A. Depression and anxiety symptoms are related to increased 24-hour urinary norepinephrine excretion among healthy middle-aged women. J Psychosom Res. (2004) 57:353–8. doi: 10.1016/S0022-3999(04)00064-9
79. Carney, RM, Saunders, RD, Freedland, KE, Stein, P, Rich, MW, and Jaffe, AS. Association of depression with reduced heart rate variability in coronary artery disease. Am J Cardiol. (1995) 76:562–4. doi: 10.1016/S0002-9149(99)80155-6
80. Dao, TK, Youssef, NA, Gopaldas, RR, Chu, D, Bakaeen, F, Wear, E, et al. Autonomic cardiovascular dysregulation as a potential mechanism underlying depression and coronary artery bypass grafting surgery outcomes. J Cardiothorac Surg. (2010) 5:36. doi: 10.1186/1749-8090-5-36
81. Patron, E, Messerotti Benvenuti, S, Favretto, G, Valfre, C, Bonfa, C, Gasparotto, R, et al. Association between depression and heart rate variability in patients after cardiac surgery: a pilot study. J Psychosom Res. (2012) 73:42–6. doi: 10.1016/j.jpsychores.2012.04.013
82. Patron, E, Messerotti Benvenuti, S, Favretto, G, Gasparotto, R, and Palomba, D. Depression and reduced heart rate variability after cardiac surgery: the mediating role of emotion regulation. Auton Neurosci. (2014) 180:53–8. doi: 10.1016/j.autneu.2013.11.004
83. Patron, E, Messerotti Benvenuti, S, Favretto, G, Gasparotto, R, and Palomba, D. Depression is associated with increased vagal withdrawal during unpleasant emotional imagery after cardiac surgery. Auton Neurosci. (2015) 189:75–82. doi: 10.1016/j.autneu.2015.02.002
84. Gentili, C, Messerotti Benvenuti, S, Palomba, D, Greco, A, Scilingo, EP, and Valenza, G. Assessing mood symptoms through heartbeat dynamics: an HRV study on cardiosurgical patients. J Psychiatr Res. (2017) 95:179–88. doi: 10.1016/j.jpsychires.2017.08.018
85. Chalmers, JA, Quintana, DS, Abbott, MJ-A, and Kemp, AH. Anxiety disorders are associated with reduced heart rate variability: a meta-analysis. Front Psych. (2014) 5:80. doi: 10.3389/fpsyt.2014.00080
86. McRae, K, Ciesielski, B, and Gross, JJ. Unpacking cognitive reappraisal: goals, tactics, and outcomes. Emotion. (2012) 12:250. doi: 10.1037/a0026351
87. Cutuli, D. Cognitive reappraisal and expressive suppression strategies role in the emotion regulation: an overview on their modulatory effects and neural correlates. Front Syst Neurosci. (2014):175. doi: 10.3389/fnsys.2014.00175
88. Eizenman, M, Lawrence, HY, Grupp, L, Eizenman, E, Ellenbogen, M, Gemar, M, et al. A naturalistic visual scanning approach to assess selective attention in major depressive disorder. Psychiatry Res. (2003) 118:117–28. doi: 10.1016/S0165-1781(03)00068-4
89. Erickson, K, Drevets, WC, Clark, L, Cannon, DM, Bain, EE, Zarate, CA Jr, et al. Mood-congruent bias in affective go/no-go performance of unmedicated patients with major depressive disorder. Am J Psychiatr. (2005) 162:2171–3. doi: 10.1176/appi.ajp.162.11.2171
90. Beurel, E, Toups, M, and Nemeroff, CB. The bidirectional relationship of depression and inflammation: double trouble. Neuron. (2020) 107:234–56. doi: 10.1016/j.neuron.2020.06.002
91. Baumgartner, R, Forteza, MJ, and Ketelhuth, DF. The interplay between cytokines and the kynurenine pathway in inflammation and atherosclerosis. Cytokine. (2019) 122:154148. doi: 10.1016/j.cyto.2017.09.004
92. Kempuraj, D, Thangavel, R, Selvakumar, GP, Zaheer, S, Ahmed, ME, Raikwar, SP, et al. Brain and peripheral atypical inflammatory mediators potentiate neuroinflammation and neurodegeneration. Front Cell Neurosci. (2017) 11:216. doi: 10.3389/fncel.2017.00216
93. Penninx, BWJH. Depression and cardiovascular disease: epidemiological evidence on their linking mechanisms. Neurosci Biobehav Rev. (2017) 74:277–86. doi: 10.1016/j.neubiorev.2016.07.003
94. Baumgartner, R, Berg, M, Matic, L, Polyzos, K, Forteza, M, Hjorth, SA, et al. Evidence that a deviation in the kynurenine pathway aggravates atherosclerotic disease in humans. J Intern Med. (2021) 289:53–68. doi: 10.1111/joim.13142
95. Sakakura, K, Nakano, M, Otsuka, F, Ladich, E, Kolodgie, FD, and Virmani, R. Pathophysiology of atherosclerosis plaque progression. Heart Lung Circ. (2013) 22:399–411. doi: 10.1016/j.hlc.2013.03.001
96. Jebari-Benslaiman, S, Galicia-García, U, Larrea-Sebal, A, Olaetxea, JR, Alloza, I, Vandenbroeck, K, et al. Pathophysiology of atherosclerosis. Int J Mol Sci. (2022) 23:3346. doi: 10.3390/ijms23063346
97. Vlodaver, Z, and Edwards, JE. Pathology of coronary atherosclerosis. Prog Cardiovasc Dis. (1971) 14:256–74. doi: 10.1016/0033-0620(71)90023-5
98. Hermida, N, and Balligand, J-L. Low-density lipoprotein-cholesterol-induced endothelial dysfunction and oxidative stress: the role of statins. Antioxid Redox Signal. (2014) 20:1216–37. doi: 10.1089/ars.2013.5537
99. Cybulsky, MI, Iiyama, K, Li, H, Zhu, S, Chen, M, Iiyama, M, et al. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J Clin Invest. (2001) 107:1255–62. doi: 10.1172/JCI11871
100. Koskinas, KC, Chatzizisis, YS, Baker, AB, Edelman, ER, Stone, PH, and Feldman, CL. The role of low endothelial shear stress in the conversion of atherosclerotic lesions from stable to unstable plaque. Curr Opin Cardiol. (2009) 24:580–90. doi: 10.1097/HCO.0b013e328331630b
101. Vu, T, Fricke, TA, and Smith, JA. A review of alkaline phosphatase in preventing systemic inflammation after cardiac surgery. Eur J Respir Med. (2021) 3:187–99. doi: 10.31488/EJRM.115
102. Warltier, DC, Laffey, JG, Boylan, JF, and Cheng, DC. The systemic inflammatory response to cardiac surgery: implications for the anesthesiologist. J Am Soc Anesthesiol. (2002) 97:215–52. doi: 10.1097/00000542-200207000-00030
103. Scott, DA, Evered, LA, and Silbert, BS. Cardiac surgery, the brain, and inflammation. J Extra Corpor Technol. (2014) 46:15. doi: 10.1051/ject/201446015
104. Savitz, J. The kynurenine pathway: a finger in every pie. Mol Psychiatry. (2020) 25:131–47. doi: 10.1038/s41380-019-0414-4
105. Chen, Y, and Guillemin, GJ. Kynurenine pathway metabolites in humans: disease and healthy states. Int J Tryptoph Res. (2009) 2:IJTR.S2097. doi: 10.4137/IJTR.S2097
106. Badawy, AA-B. Hypothesis kynurenic and quinolinic acids: the main players of the kynurenine pathway and opponents in inflammatory disease. Med Hypotheses. (2018) 118:129–38. doi: 10.1016/j.mehy.2018.06.021
107. Mp, H, Saito, K, Crowley, J, Davis, L, Demitrack, M, Der, M, et al. Quinolinic acid and kynurenine pathway metabolism in inflammatory and non-inflammatory neurological disease. Brain. (1992) 115:1249–73. doi: 10.1093/brain/115.5.1249
108. Hestad, K, Alexander, J, Rootwelt, H, and Aaseth, JO. The role of tryptophan dysmetabolism and quinolinic acid in depressive and neurodegenerative diseases. Biomol Ther. (2022) 12:998. doi: 10.3390/biom12070998
109. Steiner, J, Walter, M, Gos, T, Guillemin, GJ, Bernstein, H-G, Sarnyai, Z, et al. Severe depression is associated with increased microglial quinolinic acid in subregions of the anterior cingulate gyrus: evidence for an immune-modulated glutamatergic neurotransmission? J Neuroinflammation. (2011) 8:1–9. doi: 10.1186/1742-2094-8-94
110. Meier, TB, Drevets, WC, Wurfel, BE, Ford, BN, Morris, HM, Victor, TA, et al. Relationship between neurotoxic kynurenine metabolites and reductions in right medial prefrontal cortical thickness in major depressive disorder. Brain Behav Immun. (2016) 53:39–48. doi: 10.1016/j.bbi.2015.11.003
111. Pawlowski, T, Pawlak, D, Inglot, M, Zalewska, M, Marciniak, D, Bugajska, J, et al. The role of anthranilic acid in the increase of depressive symptoms and major depressive disorder during treatment for hepatitis C with pegylated interferon-α2a and oral ribavirin. J Psychiatry Neurosci. (2021) 46:E166–75. doi: 10.1503/jpn.190139
112. Steiner, J, Dobrowolny, H, Guest, PC, Bernstein, H-G, Fuchs, D, Roeser, J, et al. Plasma anthranilic acid and leptin levels predict HAM-D scores in depressed women. Int J Tryptoph Res. (2021) 14:11786469211016474. doi: 10.1177/11786469211016474
113. Darlington, LG, Forrest, CM, Mackay, GM, Smith, RA, Smith, AJ, Stoy, N, et al. On the biological importance of the 3-hydroxyanthranilic acid: anthranilic acid ratio. Int J Tryptoph Res. (2010) 3:IJTR.S4282. doi: 10.4137/IJTR.S4282
114. Liu, H, Ding, L, Zhang, H, Mellor, D, Wu, H, Zhao, D, et al. The metabolic factor kynurenic acid of kynurenine pathway predicts major depressive disorder. Front Psych. (2018) 9:552. doi: 10.3389/fpsyt.2018.00552
115. Olajossy, M, Olajossy, B, Wnuk, S, Potembska, E, and Urbanska, E. Blood serum concentrations of kynurenic acid in patients diagnosed with recurrent depressive disorder, depression in bipolar disorder, and schizoaffective disorder treated with electroconvulsive therapy. Psychiatr Pol. (2017) 51:455. doi: 10.12740/PP/61584
116. Schröcksnadel, K, Wirleitner, B, Winkler, C, and Fuchs, D. Monitoring tryptophan metabolism in chronic immune activation. Clin Chim Acta. (2006) 364:82–90. doi: 10.1016/j.cca.2005.06.013
117. Wirleitner, B, Rudzite, V, Neurauter, G, Murr, C, Kalnins, U, Erglis, A, et al. Immune activation and degradation of tryptophan in coronary heart disease. Eur J Clin Investig. (2003) 33:550–4. doi: 10.1046/j.1365-2362.2003.01186.x
118. Ogyu, K, Kubo, K, Noda, Y, Iwata, Y, Tsugawa, S, Omura, Y, et al. Kynurenine pathway in depression: a systematic review and meta-analysis. Neurosci Biobehav Rev. (2018) 90:16–25. doi: 10.1016/j.neubiorev.2018.03.023
119. Heninger, G, Delgado, P, and Charney, D. The revised monoamine theory of depression: a modulatory role for monoamines, based on new findings from monoamine depletion experiments in humans. Pharmacopsychiatry. (1996) 29:2–11. doi: 10.1055/s-2007-979535
120. Izawa, S, Sugaya, N, Kimura, K, Ogawa, N, Yamada, KC, Shirotsuki, K, et al. An increase in salivary interleukin-6 level following acute psychosocial stress and its biological correlates in healthy young adults. Biol Psychol. (2013) 94:249–54. doi: 10.1016/j.biopsycho.2013.06.006
121. Jankord, R, Zhang, R, Flak, JN, Solomon, MB, Albertz, J, and Herman, JP. Stress activation of IL-6 neurons in the hypothalamus. Am J Phys Regul Integr Comp Phys. (2010) 299:R343–51. doi: 10.1152/ajpregu.00131.2010
122. Liu, JJ, Wei, YB, Strawbridge, R, Bao, Y, Chang, S, Shi, L, et al. Peripheral cytokine levels and response to antidepressant treatment in depression: a systematic review and meta-analysis. Mol Psychiatry. (2020) 25:339–50. doi: 10.1038/s41380-019-0474-5
123. Schmidt, MF, Kirkby, FC, and Lichtblau, N. Inflammation and immune regulation as potential drug targets in antidepressant treatment. Curr Neuropharmacol. (2016) 14:674–87. doi: 10.2174/1570159X14666160115130414
124. Shrivastava, AK, Singh, HV, Raizada, A, and Singh, SK. C-reactive protein, inflammation and coronary heart disease. Egypt Heart J. (2015) 67:89–97. doi: 10.1016/j.ehj.2014.11.005
125. Steel, DM, and Whitehead, AS. The major acute phase reactants: C-reactive protein, serum amyloid P component and serum amyloid a protein. Immunol Today. (1994) 15:81–8. doi: 10.1016/0167-5699(94)90138-4
126. Lagrand, WK, Visser, CA, Hermens, WT, Niessen, HW, Verheugt, FW, Wolbink, G-J, et al. C-reactive protein as a cardiovascular risk factor: more than an epiphenomenon? Circulation. (1999) 100:96–102. doi: 10.1161/01.CIR.100.1.96
127. Mihara, M, Hashizume, M, Yoshida, H, Suzuki, M, and Shiina, M. IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clin Sci. (2011) 122:143–59. doi: 10.1042/CS20110340
128. Kimura, A, and Kishimoto, T. IL-6: regulator of Treg/Th17 balance. Eur J Immunol. (2010) 40:1830–5. doi: 10.1002/eji.201040391
129. Tanaka, T, Narazaki, M, and Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. (2014) 6:a016295. doi: 10.1101/cshperspect.a016295
130. Zorrilla, EP, Luborsky, L, McKay, JR, Rosenthal, R, Houldin, A, Tax, A, et al. The relationship of depression and stressors to immunological assays: a meta-analytic review. Brain Behav Immun. (2001) 15:199–226. doi: 10.1006/brbi.2000.0597
131. Birner, CM, Ulucan, C, Fredersdorf, S, Rihm, M, Löwel, H, Stritzke, J, et al. Head-to-head comparison of BNP and IL-6 as markers of clinical and experimental heart failure: superiority of BNP. Cytokine. (2007) 40:89–97. doi: 10.1016/j.cyto.2007.08.009
132. Rothenburger, M, Soeparwata, R, Deng, MC, Schmid, C, Berendes, E, Tjan, T, et al. Prediction of clinical outcome after cardiac surgery: the role of cytokines, endotoxin, and anti-endotoxin core antibodies. Shock. (2001) 16:44–50. doi: 10.1097/00024382-200116001-00009
133. Yang, L, Wang, J, Zhang, L, Hou, J, Yuan, X, Hu, S, et al. Preoperative high-sensitivity C-reactive protein predicts depression in patients undergoing coronary artery bypass surgery: a single-center prospective observational study. J Thorac Cardiovasc Surg. (2012) 144:500–5. doi: 10.1016/j.jtcvs.2012.01.034
134. Poole, L, Kidd, T, Leigh, E, Ronaldson, A, Jahangiri, M, and Steptoe, A. Depression, C-reactive protein and length of post-operative hospital stay in coronary artery bypass graft surgery patients. Brain Behav Immun. (2014) 37:115–21. doi: 10.1016/j.bbi.2013.11.008
135. Ivankovic, S, Coric, V, Paic, F, Mihaljevic Peles, A, Svagusa, T, Kalamar, V, et al. The Association of Preoperative Depression, and C-reactive protein levels with a postoperative length of stay in patients undergoing coronary artery bypass grafting. Appl Sci. (2022) 12:10201. doi: 10.3390/app122010201
136. Steptoe, A, Poole, L, Ronaldson, A, Kidd, T, Leigh, E, and Jahangiri, M. Depression 1 year after CABG is predicted by acute inflammatory responses. J Am Coll Cardiol. (2015) 65:1710–1. doi: 10.1016/j.jacc.2014.12.068
137. Howren, MB, Lamkin, DM, and Suls, J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. (2009) 71:171–86. doi: 10.1097/PSY.0b013e3181907c1b
138. Myint, AM, Bondy, B, Baghai, TC, Eser, D, Nothdurfter, C, Schüle, C, et al. Tryptophan metabolism and immunogenetics in major depression: a role for interferon-γ gene. Brain Behav Immun. (2013) 31:128–33. doi: 10.1016/j.bbi.2013.04.003
139. Hannan, EL, Racz, MJ, Walford, G, Ryan, TJ, Isom, OW, Bennett, E, et al. Predictors of readmission for complications of coronary artery bypass graft surgery. JAMA. (2003) 290:773–80. doi: 10.1001/jama.290.6.773
140. Pariante, CM, and Lightman, SL. The HPA axis in major depression: classical theories and new developments. Trends Neurosci. (2008) 31:464–8. doi: 10.1016/j.tins.2008.06.006
141. Tsigos, C, and Chrousos, GP. Hypothalamic–pituitary–adrenal axis, neuroendocrine factors and stress. J Psychosom Res. (2002) 53:865–71. doi: 10.1016/S0022-3999(02)00429-4
142. Dautzenberg, FM, Kilpatrick, GJ, Hauger, RL, and Moreau, J-L. Molecular biology of the CRH receptors—in the mood. Peptides. (2001) 22:753–60. doi: 10.1016/S0196-9781(01)00388-6
143. Paranjape, SB, and Thibonnier, M. Development and therapeutic indications of orally-active non-peptide vasopressin receptor antagonists. Expert opinion on investigational drugs (2001) 10:825–34.
144. Papadimitriou, A, and Priftis, KN. Regulation of the hypothalamic-pituitary-adrenal axis. Neuroimmunomodulation. (2009) 16:265–71. doi: 10.1159/000216184
145. Gjerstad, JK, Lightman, SL, and Spiga, F. Role of glucocorticoid negative feedback in the regulation of HPA axis pulsatility. Stress. (2018) 21:403–16. doi: 10.1080/10253890.2018.1470238
146. Varghese, FP, and Brown, ES. The hypothalamic-pituitary-adrenal axis in major depressive disorder: a brief primer for primary care physicians. Prim Care Companion J Clin Psychiatry. (2001) 3:151. doi: 10.4088/pcc.v03n0401
147. Hand, GA, Hewitt, CB, Fulk, LJ, Stock, HS, Carson, JA, Davis, JM, et al. Differential release of corticotropin-releasing hormone (CRH) in the amygdala during different types of stressors. Brain Res (2002);949(1–2)–30. doi: 10.1016/S0006-8993(02)02972-4
148. Refojo, D, and Holsboer, F. CRH signaling: molecular specificity for drug targeting in the CNS. Ann N Y Acad Sci. (2009) 1179:106–19. doi: 10.1111/j.1749-6632.2009.04983.x
149. Keck, ME, and Holsboer, F. Hyperactivity of CRH neuronal circuits as a target for therapeutic interventions in affective disorders. Peptides. (2001) 22:835–44. doi: 10.1016/S0196-9781(01)00398-9
150. Stokes, PE. The potential role of excessive cortisol induced by HPA hyperfunction in the pathogenesis of depression. Eur Neuropsychopharmacol. (1995) 5:77–82. doi: 10.1016/0924-977X(95)00039-R
151. Owens, M, Herbert, J, Jones, PB, Sahakian, BJ, Wilkinson, PO, Dunn, VJ, et al. Elevated morning cortisol is a stratified population-level biomarker for major depression in boys only with high depressive symptoms. Proc Natl Acad Sci U S A. (2014) 111:3638–43. doi: 10.1073/pnas.1318786111
152. Juruena, MF, Cleare, AJ, and Pariante, CM. The hypothalamic pituitary adrenal axis, glucocorticoid receptor function and relevance to depression. Braz J Psychiatry. (2004) 26:189–201. doi: 10.1590/S1516-44462004000300009
153. Kazmierski, J, Banys, A, Latek, J, Bourke, J, and Jaszewski, R. Cortisol levels and neuropsychiatric diagnosis as markers of postoperative delirium: a prospective cohort study. Crit Care. (2013) 17:R38. doi: 10.1186/cc12548
154. Naber, D, Schmidt-Habelmann, P, Bullinger, M, Neff, A, Buchler, A, and Dietzfelbinger, A. Serum cortisol correlates with depression score after open-heart surgery. Lancet. (1983) 1:1052–3. doi: 10.1016/s0140-6736(83)92683-1
155. Poole, L, Kidd, T, Ronaldson, A, Leigh, E, Jahangiri, M, and Steptoe, A. Depression 12-months after coronary artery bypass graft is predicted by cortisol slope over the day. Psychoneuroendocrinology. (2016) 71:155–8. doi: 10.1016/j.psyneuen.2016.05.025
156. Nemeroff, CB, Widerlöv, E, Bissette, G, Walleus, H, Karlsson, I, Eklund, K, et al. Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science. (1984) 226:1342–4. doi: 10.1126/science.6334362
157. Banki, CM, Karmacsi, L, Bissette, G, and Nemeroff, CB. CSF corticotropin-releasing hormone and somatostatin in major depression: response to antidepressant treatment and relapse. Eur Neuropsychopharmacol. (1992) 2:107–13. doi: 10.1016/0924-977X(92)90019-5
158. Nemeroff, CB, Bissette, G, Akil, H, and Fink, M. Neuropeptide concentrations in the cerebrospinal fluid of depressed patients treated with electroconvulsive therapy: corticotrophin-releasing factor, β-endorphin and somatostatin. Br J Psychiatry. (1991) 158:59–63. doi: 10.1192/bjp.158.1.59
159. Lesch, K-P, Laux, G, Schulte, HM, Pfüller, H, and Beckmann, H. Corticotropin and cortisol response to human CRH as a probe for HPA system integrity in major depressive disorder. Psychiatry Res. (1988) 24:25–34. doi: 10.1016/0165-1781(88)90136-9
160. Holsboer, F, Von Bardeleben, U, Buller, R, Heuser, I, and Steiger, A. Stimulation response to corticotropin-releasing hormone (CRH) in patients with depression, alcoholism and panic disorder. Horm Metab Res Suppl. (1987) 16:80–8.
161. von Bardeleben, U, Stalla, GK, Müller, OA, and Holsboer, F. Blunting of ACTH response to human CRH in depressed patients is avoided by metyrapone pretreatment. Biol Psychiatry. (1988) 24:782–6. doi: 10.1016/0006-3223(88)90254-5
162. Plotsky, PM, Owens, MJ, and Nemeroff, CB. Psychoneuroendocrinology of depression: hypothalamic-pituitary-adrenal axis. Psychiatr Clin N Am. (1998) 21:293–307. doi: 10.1016/S0193-953X(05)70006-X
163. Krishnan, KRR, Doraiswamy, PM, Lurie, SN, Figiel, GS, Husain, MM, Boyko, OB, et al. Pituitary size in depression. J Clin Endocrinol Metabol. (1991) 72:256–9. doi: 10.1210/jcem-72-2-256
164. Eker, C, and Gonul, AS. Volumetric MRI studies of the hippocampus in major depressive disorder: meanings of inconsistency and directions for future research. World J Biol Psychiatry. (2010) 11:19–35. doi: 10.3109/15622970902737998
165. Nolan, M, Roman, E, Nasa, A, Levins, KJ, O’Hanlon, E, O’Keane, V, et al. Hippocampal and amygdalar volume changes in major depressive disorder: a targeted review and focus on stress. Chronic Stress. (2020) 4:2470547020944553. doi: 10.1177/2470547020944553
166. Frodl, T, Meisenzahl, E, Zetzsche, T, Bottlender, R, Born, C, Groll, C, et al. Enlargement of the amygdala in patients with a first episode of major depression. Biol Psychiatry. (2002) 51:708–14. doi: 10.1016/S0006-3223(01)01359-2
167. Lange, C, and Irle, E. Enlarged amygdala volume and reduced hippocampal volume in young women with major depression. Psychol Med. (2004) 34:1059–64. doi: 10.1017/S0033291703001806
168. Kronenberg, G, van Elst, LT, Regen, F, Deuschle, M, Heuser, I, and Colla, M. Reduced amygdala volume in newly admitted psychiatric in-patients with unipolar major depression. J Psychiatr Res. (2009) 43:1112–7. doi: 10.1016/j.jpsychires.2009.03.007
169. Jokinen, J, and Nordström, P. HPA axis hyperactivity and cardiovascular mortality in mood disorder inpatients. J Affect Disord. (2009) 116:88–92. doi: 10.1016/j.jad.2008.10.025
170. Ra, R, and Björntorp, P. The hypothalamic–pituitary–adrenal axis activity as a predictor of cardiovascular disease, type 2 diabetes and stroke. J Intern Med. (2000) 247:188–97. doi: 10.1046/j.1365-2796.2000.00603.x
171. Marques, AH, Silverman, MN, and Sternberg, EM. Glucocorticoid dysregulations and their clinical correlates: from receptors to therapeutics. Ann N Y Acad Sci. (2009) 1179:1–18. doi: 10.1111/j.1749-6632.2009.04987.x
172. Kok, L, Hillegers, MH, Veldhuijzen, DS, Cornelisse, S, Nierich, AP, van der Maaten, JM, et al. The effect of dexamethasone on symptoms of posttraumatic stress disorder and depression after cardiac surgery and intensive care admission: longitudinal follow-up of a randomized controlled trial. Crit Care Med. (2016) 44:512–20. doi: 10.1097/CCM.0000000000001419
173. Kok, L, Hillegers, MH, Veldhuijzen, DS, Boks, MP, Dieleman, JM, van Dijk, D, et al. Genetic variation in the glucocorticoid receptor and psychopathology after dexamethasone administration in cardiac surgery patients. J Psychiatr Res. (2018) 103:167–72. doi: 10.1016/j.jpsychires.2018.05.015
174. de Rezende, MG, Garcia-Leal, C, de Figueiredo, FP, de Carvalho, CR, Spanghero, MS, Barbieri, MA, et al. Altered functioning of the HPA axis in depressed postpartum women. J Affect Disord. (2016) 193:249–56. doi: 10.1016/j.jad.2015.12.065
175. Seeman, TE, Singer, B, Wilkinson, CW, and McEwen, B. Gender differences in age-related changes in HPA axis reactivity. Psychoneuroendocrinology. (2001) 26:225–40. doi: 10.1016/S0306-4530(00)00043-3
176. Rohleder, N, Schommer, NC, Hellhammer, DH, Engel, R, and Kirschbaum, C. Sex differences in glucocorticoid sensitivity of proinflammatory cytokine production after psychosocial stress. Psychosom Med. (2001) 63:966–72. doi: 10.1097/00006842-200111000-00016
177. Wegner, A, Benson, S, Rebernik, L, Spreitzer, I, Jäger, M, Schedlowski, M, et al. Sex differences in the pro-inflammatory cytokine response to endotoxin unfold in vivo but not ex vivo in healthy humans. Innate Immun. (2017) 23:432–9. doi: 10.1177/1753425917707026
178. Bet, PM, Penninx, BW, Bochdanovits, Z, Uitterlinden, AG, Beekman, AT, van Schoor, NM, et al. Glucocorticoid receptor gene polymorphisms and childhood adversity are associated with depression: new evidence for a gene–environment interaction. Am J Med Genet B Neuropsychiatr Genet. (2009) 150:660–9. doi: 10.1002/ajmg.b.30886
179. Claes, S. Glucocorticoid receptor polymorphisms in major depression. Ann N Y Acad Sci. (2009) 1179:216–28. doi: 10.1111/j.1749-6632.2009.05012.x
180. van Rossum, EF, Koper, JW, Huizenga, NA, Uitterlinden, AG, Janssen, JA, Brinkmann, AO, et al. A polymorphism in the glucocorticoid receptor gene, which decreases sensitivity to glucocorticoids in vivo, is associated with low insulin and cholesterol levels. Diabetes. (2002) 51:3128–34. doi: 10.2337/diabetes.51.10.3128
181. van Rossum, EF, Binder, EB, Majer, M, Koper, JW, Ising, M, Modell, S, et al. Polymorphisms of the glucocorticoid receptor gene and major depression. Biol Psychiatry. (2006) 59:681–8. doi: 10.1016/j.biopsych.2006.02.007
182. Oudhoff, JP, Timmermans, DR, Knol, DL, Bijnen, AB, and van der Wal, G. Waiting for elective general surgery: impact on health related quality of life and psychosocial consequences. BMC Public Health. (2007) 7:164. doi: 10.1186/1471-2458-7-164
183. Gagliardi, AR, Yip, CYY, Irish, J, Wright, FC, Rubin, B, Ross, H, et al. The psychological burden of waiting for procedures and patient-centred strategies that could support the mental health of wait-listed patients and caregivers during the COVID-19 pandemic: a scoping review. Health Expect. (2021) 24:978–90. doi: 10.1111/hex.13241
184. Gao, Q, Mok, H-P, Zhang, H-Y, Qiu, H-L, Liu, J, Chen, Z-R, et al. Inflammatory indicator levels in patients undergoing aortic valve replacement via median sternotomy with preoperative anxiety and postoperative complications: a prospective cohort study. J Int Med Res. (2021) 49:0300060520977417. doi: 10.1177/0300060520977417
185. Ng, SX, Wang, W, Shen, Q, Toh, ZA, and He, HG. The effectiveness of preoperative education interventions on improving perioperative outcomes of adult patients undergoing cardiac surgery: a systematic review and meta-analysis. Eur J Cardiovasc Nurs. (2022) 21:521–36. doi: 10.1093/eurjcn/zvab123
186. Guo, P, East, L, and Arthur, A. A preoperative education intervention to reduce anxiety and improve recovery among Chinese cardiac patients: a randomized controlled trial. Int J Nurs Stud. (2012) 49:129–37. doi: 10.1016/j.ijnurstu.2011.08.008
187. Furze, G, Dumville, JC, Miles, JNV, Irvine, K, Thompson, DR, and Lewin, RJP. “Prehabilitation” prior to CABG surgery improves physical functioning and depression. Int J Cardiol. (2009) 132:51–8. doi: 10.1016/j.ijcard.2008.06.001
188. Shuldham, CM, Fleming, S, and Goodman, H. The impact of pre-operative education on recovery following coronary artery bypass surgery. A randomized controlled clinical trial. Eur Heart J. (2002) 23:666–74. doi: 10.1053/euhj.2001.2897
189. Guo, P, East, L, and Arthur, A. Thinking outside the black box: the importance of context in understanding the impact of a preoperative education nursing intervention among Chinese cardiac patients. Patient Educ Couns. (2014) 95:365–70. doi: 10.1016/j.pec.2014.03.001
190. Bolton, V, and Brittain, M. Patient information provision: its effect on patient anxiety and the role of health information services and libraries. Health Libr Rev. (1994) 11:117–32. doi: 10.1046/j.1365-2532.1994.1120117.x
191. Yaman Aktas, Y, Gok Ugur, H, and Orak, OS. Discharge education intervention to reduce anxiety and depression in cardiac surgery patients: a randomized controlled study. J Perianesth Nurs. (2020) 35:185–92. doi: 10.1016/j.jopan.2019.08.012
192. Sever, S, Doherty, P, Golder, S, and Harrison, AS. Is improvement in depression in patients attending cardiac rehabilitation with new-onset depressive symptoms determined by patient characteristics? Open heart. (2020) 7:e001264. doi: 10.1136/openhrt-2020-001264
193. Hojskov, IE, Moons, P, Egerod, I, Olsen, PS, Thygesen, LC, Hansen, NV, et al. Early physical and psycho-educational rehabilitation in patients with coronary artery bypass grafting: a randomized controlled trial. J Rehabil Med. (2019) 51:136–43. doi: 10.2340/16501977-2499
194. Ma, L, Deng, L, and Yu, H. The effects of a comprehensive rehabilitation and intensive education program on anxiety, depression, quality of life, and major adverse cardiac and cerebrovascular events in unprotected left main coronary artery disease patients who underwent coronary artery bypass grafting. Ir J Med Sci. (2020) 189:477–88. doi: 10.1007/s11845-019-02129-x
195. Takroni, MA, Thow, M, Ellis, B, and Seenan, C. Home-based versus outpatient-based cardiac rehabilitation post-coronary artery bypass graft surgery: a randomized controlled trial. J Cardiovasc Nurs. (2022) 37:274–80. doi: 10.1097/JCN.0000000000000763
196. Cuijpers, P, Smit, F, Bohlmeijer, E, Hollon, SD, and Andersson, G. Efficacy of cognitive–behavioural therapy and other psychological treatments for adult depression: meta-analytic study of publication bias. Br J Psychiatry. (2010) 196:173–8. doi: 10.1192/bjp.bp.109.066001
197. Wetherell, JL, Petkus, AJ, White, KS, Nguyen, H, Kornblith, S, Andreescu, C, et al. Antidepressant medication augmented with cognitive-behavioral therapy for generalized anxiety disorder in older adults. Am J Psychiatr. (2013) 170:782–9. doi: 10.1176/appi.ajp.2013.12081104
198. Clarke, G, Debar, L, Lynch, F, Powell, J, Gale, J, O’Connor, E, et al. A randomized effectiveness trial of brief cognitive-behavioral therapy for depressed adolescents receiving antidepressant medication. J Am Acad Child Adolesc Psychiatry. (2005) 44:888–98. doi: 10.1016/S0890-8567(09)62194-8
199. DeRubeis, RJ, Gelfand, LA, Tang, TZ, and Simons, AD. Medications versus cognitive behavior therapy for severely depressed outpatients: mega-analysis of four randomized comparisons. Am J Psychiatr. (1999) 156:1007–13. doi: 10.1176/ajp.156.7.1007
200. Patten, SB. A major depression prognosis calculator based on episode duration. Clin Pract Epidemiol Ment Health. (2006) 2:1–8. doi: 10.1186/1745-0179-2-13
201. Gaudiano, BA. Cognitive-behavioural therapies: achievements and challenges. Evid Based Ment Health. (2008) 11:5–7. doi: 10.1136/ebmh.11.1.5
202. Goldberg, C. Cognitive-behavioral therapy for panic: effectiveness and limitations. Psychiatry Q. (1998) 69:23–44. doi: 10.1023/A:1022181206728
203. Gautam, M, Tripathi, A, Deshmukh, D, and Gaur, M. Cognitive behavioral therapy for depression. Indian J Psychiatry. (2020) 62:S223. doi: 10.4103/psychiatry.IndianJPsychiatry_772_19
204. Kertz, SJ, Koran, J, Stevens, KT, and Björgvinsson, T. Repetitive negative thinking predicts depression and anxiety symptom improvement during brief cognitive behavioral therapy. Behav Res Ther. (2015) 68:54–63. doi: 10.1016/j.brat.2015.03.006
205. Ghoneim, MM, and O’Hara, MW. Depression and postoperative complications: an overview. BMC Surg. (2016) 16:5. doi: 10.1186/s12893-016-0120-y
206. Freedland, KE, Skala, JA, Carney, RM, Rubin, EH, Lustman, PJ, Dávila-Román, VG, et al. Treatment of depression after coronary artery bypass surgery: a randomized controlled trial. Arch Gen Psychiatry. (2009) 66:387–96. doi: 10.1001/archgenpsychiatry.2009.7
207. Dao, TK, Youssef, NA, Armsworth, M, Wear, E, Papathopoulos, KN, and Gopaldas, R. Randomized controlled trial of brief cognitive behavioral intervention for depression and anxiety symptoms preoperatively in patients undergoing coronary artery bypass graft surgery. J Thorac Cardiovasc Surg. (2011) 142:e109–15. doi: 10.1016/j.jtcvs.2011.02.046
208. Doering, LV, Chen, B, Cross Bodan, R, Magsarili, MC, Nyamathi, A, and Irwin, MR. Early cognitive behavioral therapy for depression after cardiac surgery. J Cardiovasc Nurs. (2013) 28:370–9. doi: 10.1097/JCN.0b013e31824d967d
209. Berkman, LF, Blumenthal, J, Burg, M, Carney, RM, Catellier, D, Cowan, MJ, et al. Effects of treating depression and low perceived social support on clinical events after myocardial infarction the enhancing recovery in coronary heart disease patients (ENRICHD) randomized trial. JAMA. (2003) 289:3106–16. doi: 10.1001/jama.289.23.3106
210. Thompson, RJ, Mata, J, Jaeggi, SM, Buschkuehl, M, Jonides, J, and Gotlib, IH. Maladaptive coping, adaptive coping, and depressive symptoms: variations across age and depressive state. Behav Res Ther. (2010) 48:459–66. doi: 10.1016/j.brat.2010.01.007
211. Contrada, RJ, Goyal, TM, Cather, C, Rafalson, L, Idler, EL, and Krause, TJ. Psychosocial factors in outcomes of heart surgery: the impact of religious involvement and depressive symptoms. Health Psychol. (2004) 23:227–38. doi: 10.1037/0278-6133.23.3.227
212. Zinnbauer, BJ, and Pargament, KI. Religiousness and spirituality In: RF Paloutzian and CL Park, editors. Handbook of the psychology of religion and spirituality. New York, NY: The Guilford Press (2005). 21–42.
213. Denson, TF, Spanovic, M, and Miller, N. Cognitive appraisals and emotions predict cortisol and immune responses: a meta-analysis of acute laboratory social stressors and emotion inductions. Psychol Bull. (2009) 135:823. doi: 10.1037/a0016909
214. Ai, AL, Dunkle, RE, Peterson, C, and Bolling, SF. The role of private prayer in psychological recovery among midlife and aged patients following cardiac surgery. Gerontologist. (1998) 38:591–601. doi: 10.1093/geront/38.5.591
215. Ai, AL, Peterson, C, Bolling, SF, and Koenig, H. Private prayer and optimism in middle-aged and older patients awaiting cardiac surgery. Gerontologist. (2002) 42:70–81. doi: 10.1093/geront/42.1.70
216. Park, CL, Holt, CL, Le, D, Christie, J, and Williams, BR. Positive and negative religious coping styles as prospective predictors of well-being in African Americans. Psycholog Relig Spiritual. (2018) 10:318–26. doi: 10.1037/rel0000124
217. Carvalho, CC, Chaves, ECL, Iunes, DH, Simão, TP, Grasselli, CSM, and Braga, CG. Effectiveness of prayer in reducing anxiety in cancer patients. Rev Esc Enferm USP. (2014) 48:684–90. doi: 10.1590/S0080-623420140000400016
218. Ma, C, Wang, B, Zhao, X, Fu, F, Zheng, L, Li, G, et al. WeChat-based education and rehabilitation program in unprotected left main coronary artery disease patients after coronary artery bypass grafting: an effective approach in reducing anxiety, depression, loss to follow-up, and improving quality of life. Bra J Med Biol Res. (2021) 54:e10370. doi: 10.1590/1414-431x202010370
219. Zhang, X, Wen, D, Liang, J, and Lei, J. How the public uses social media WeChat to obtain health information in China: a survey study. BMC Med Inform Decis Mak. (2017) 17:71–9. doi: 10.1186/s12911-017-0470-0
220. Patron, E, Messerotti Benvenuti, S, Favretto, G, Valfre, C, Bonfa, C, Gasparotto, R, et al. Biofeedback assisted control of respiratory sinus arrhythmia as a biobehavioral intervention for depressive symptoms in patients after cardiac surgery: a preliminary study. Appl Psychophysiol Biofeedback. (2013) 38:1–9. doi: 10.1007/s10484-012-9202-5
221. Rush, AJ, Trivedi, MH, Wisniewski, SR, Stewart, JW, Nierenberg, AA, Thase, ME, et al. Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. N Engl J Med. (2006) 354:1231–42. doi: 10.1056/NEJMoa052963
223. Kahl, KG, Stapel, B, and Correll, CU. Psychological and psychopharmacological interventions in psychocardiology. Front Psychiatry. (2022) 13:831359. doi: 10.3389/fpsyt.2022.831359
224. David, D, and Gourion, D. Antidepressant and tolerance: determinants and management of major side effects. Encephale. (2016) 42:553–61. doi: 10.1016/j.encep.2016.05.006
225. Kemp, AH, and Quintana, DS. The relationship between mental and physical health: insights from the study of heart rate variability. Int J Psychophysiol. (2013) 89:288–96. doi: 10.1016/j.ijpsycho.2013.06.018
226. Yekehtaz, H, Farokhnia, M, and Akhondzadeh, S. Cardiovascular considerations in antidepressant therapy: an evidence-based review. J Tehran Heart Cent. (2013) 8:169–76. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4434967/.
227. Hata, M, Yagi, Y, Sezai, A, Yoshitake, I, Wakui, S, Takasaka, A, et al. Efficacy of prophylactic treatment with selective serotonin reuptake inhibitors for depression after open-heart surgery. Surg Today. (2011) 41:791–4. doi: 10.1007/s00595-010-4357-2
228. Chocron, S, Vandel, P, Durst, C, Laluc, F, Kaili, D, Chocron, M, et al. Antidepressant therapy in patients undergoing coronary artery bypass grafting: the MOTIV-CABG trial. Ann Thorac Surg. (2013) 95:1609–18. doi: 10.1016/j.athoracsur.2013.02.035
229. Kim, DH, Daskalakis, C, Whellan, DJ, Whitman, IR, Hohmann, S, Medvedev, S, et al. Safety of selective serotonin reuptake inhibitor in adults undergoing coronary artery bypass grafting. Am J Cardiol. (2009) 103:1391–5. doi: 10.1016/j.amjcard.2009.01.348
230. Tully, PJ, Cardinal, T, Bennetts, JS, and Baker, RA. Selective serotonin reuptake inhibitors, venlafaxine and duloxetine are associated with in hospital morbidity but not bleeding or late mortality after coronary artery bypass graft surgery. Heart Lung Circ. (2012) 21:206–14. doi: 10.1016/j.hlc.2011.12.002
231. Heimisdottir, AA, Enger, E, Morelli, S, Johannesdottir, H, Helgadottir, S, Sigursson, E, et al. Use of serotonin reuptake inhibitors is not associated with increased bleeding after CABG. Gen Thorac Cardiovasc Surg. (2020) 68:1312–8. doi: 10.1007/s11748-020-01353-y
232. Xiong, GL, Jiang, W, Clare, R, Shaw, LK, Smith, PK, Mahaffey, KW, et al. Prognosis of patients taking selective serotonin reuptake inhibitors before coronary artery bypass grafting. Am J Cardiol. (2006) 98:42–7. doi: 10.1016/j.amjcard.2006.01.051
233. Stenman, M, Holzmann, MJ, and Sartipy, U. Antidepressant use before coronary artery bypass surgery is associated with long-term mortality. Int J Cardiol. (2013) 167:2958–62. doi: 10.1016/j.ijcard.2012.08.010
234. Machado-Vieira, R, Baumann, J, Wheeler-Castillo, C, Latov, D, Henter, ID, Salvadore, G, et al. The timing of antidepressant effects: a comparison of diverse pharmacological and somatic treatments. Pharmaceuticals. (2010) 3:19–41. doi: 10.3390/ph3010019
235. Sepehripour, AH, Eckersley, M, Jiskani, A, Casula, R, and Athanasiou, T. Selective serotonin reuptake inhibitor use and outcomes following cardiac surgery-a systematic review. J Thorac Dis. (2018) 10:1112–20. doi: 10.21037/jtd.2018.01.69
236. Wilt, TJ, Bloomfield, HE, MacDonald, R, Nelson, D, Rutks, I, Ho, M, et al. Effectiveness of statin therapy in adults with coronary heart disease. Arch Intern Med. (2004) 164:1427–36. doi: 10.1001/archinte.164.13.1427
237. Stancu, C, and Sima, A. Statins: mechanism of action and effects. J Cell Mol Med. (2001) 5:378–87. doi: 10.1111/j.1582-4934.2001.tb00172.x
238. Köhler-Forsberg, O, Otte, C, Gold, SM, and Østergaard, SD. Statins in the treatment of depression: hype or hope? Pharmacol Ther. (2020) 215:107625. doi: 10.1016/j.pharmthera.2020.107625
239. Bajpai, A, Verma, AK, Srivastava, M, and Srivastava, R. Oxidative stress and major depression. J Clin Diagn Res. (2014) 8:CC04–7. doi: 10.7860/JCDR/2014/10258.5292
240. Ludka, FK, Dal-Cim, T, Binder, LB, Constantino, LC, Massari, C, and Tasca, CI. Atorvastatin and fluoxetine prevent oxidative stress and mitochondrial dysfunction evoked by glutamate toxicity in hippocampal slices. Mol Neurobiol. (2017) 54:3149–61. doi: 10.1007/s12035-016-9882-6
241. Climent, E, Benaiges, D, and Pedro-Botet, J. Hydrophilic or lipophilic statins? Front Cardiovasc Med. (2021) 8:687585. doi: 10.3389/fcvm.2021.687585
242. Wolozin, B, Wang, SW, Li, N-C, Lee, A, Lee, TA, and Kazis, LE. Simvastatin is associated with a reduced incidence of dementia and Parkinson’s disease. BMC Med. (2007) 5:20. doi: 10.1186/1741-7015-5-20
243. Kim, S-W, Kang, H-J, Jhon, M, Kim, J-W, Lee, J-Y, Walker, AJ, et al. Statins and inflammation: new therapeutic opportunities in psychiatry. Front Psych. (2019) 10:103. doi: 10.3389/fpsyt.2019.00103
244. Chello, M, Patti, G, Candura, D, Mastrobuoni, S, Di Sciascio, G, Agrò, F, et al. Effects of atorvastatin on systemic inflammatory response after coronary bypass surgery. Crit Care Med. (2006) 34:660–7. doi: 10.1097/01.CCM.0000201407.89977.EA
245. Chello, M, Mastroroberto, P, Patti, G, D’Ambrosio, A, Morichetti, MC, Di Sciascio, G, et al. Simvastatin attenuates leucocyte-endothelial interactions after coronary revascularisation with cardiopulmonary bypass. Heart. (2003) 89:538–43. doi: 10.1136/heart.89.5.538
246. Holm, T, Andreassen, AK, Ueland, T, Kjekshus, J, Frøland, SS, Kjekshus, E, et al. Effect of pravastatin on plasma markers of inflammation and peripheral endothelial function in male heart transplant recipients. Am J Cardiol. (2001) 87:815–8. doi: 10.1016/S0002-9149(00)01516-2
247. Sodha, NR, and Sellke, FW. The effect of statins on perioperative inflammation in cardiac and thoracic surgery. J Thorac Cardiovasc Surg. (2015) 149:1495–501. doi: 10.1016/j.jtcvs.2015.02.005
248. Krivoy, N, Adler, Z, Saloma, R, Hawadie, A, and Azzam, ZS. Targeting C-reactive protein levels using high-dose atorvastatin before coronary artery bypass graft surgery. Exp Clin Cardiol. (2008) 13:171–4. Available at: https://pubmed.ncbi.nlm.nih.gov/19343161/.
249. Stafford, L, and Berk, M. The use of statins after a cardiac intervention is associated with reduced risk of subsequent depression: proof of concept for the inflammatory and oxidative hypotheses of depression? J Clin Psychiatry. (2011) 72:1229–35. doi: 10.4088/JCP.09m05825blu
250. Abbasi, SH, Mohammadinejad, P, Shahmansouri, N, Salehiomran, A, Beglar, AA, Zeinoddini, A, et al. Simvastatin versus atorvastatin for improving mild to moderate depression in post-coronary artery bypass graft patients: a double-blind, placebo-controlled, randomized trial. J Affect Disord. (2015) 183:149–55. doi: 10.1016/j.jad.2015.04.049
251. Nicholson, A, Kuper, H, and Hemingway, H. Depression as an aetiologic and prognostic factor in coronary heart disease: a meta-analysis of 6362 events among 146 538 participants in 54 observational studies. Eur Heart J. (2006) 27:2763–74. doi: 10.1093/eurheartj/ehl338
252. Coleman, K, Austin, BT, Brach, C, and Wagner, EH. Evidence on the chronic care model in the new millennium. Health Aff. (2009) 28:75–85. doi: 10.1377/hlthaff.28.1.75
253. Rollman, BL, Belnap, BH, LeMenager, MS, Mazumdar, S, Schulberg, HC, and Reynolds, CF 3rd. The bypassing the blues treatment protocol: stepped collaborative care for treating post-CABG depression. Psychosom Med. (2009) 71:217–30. doi: 10.1097/PSY.0b013e3181970c1c
254. Rollman, BL, and Belnap, BH. The bypassing the blues trial: collaborative care for post-CABG depression and implications for future research. Cleve Clin J Med. (2011) 78:S4–S12. doi: 10.3949/ccjm.78.s1.01
255. Meyer, T, Belnap, BH, Herrmann-Lingen, C, He, F, Mazumdar, S, and Rollman, BL. Benefits of collaborative care for post-CABG depression are not related to adjustments in antidepressant pharmacotherapy. J Psychosom Res. (2014) 76:28–33. doi: 10.1016/j.jpsychores.2013.10.017
256. Donohue, JM, Belnap, BH, Men, A, He, F, Roberts, MS, Schulberg, HC, et al. Twelve-month cost-effectiveness of telephone-delivered collaborative care for treating depression following CABG surgery: a randomized controlled trial. Gen Hosp Psychiatry. (2014) 36:453–9. doi: 10.1016/j.genhosppsych.2014.05.012
257. Ritz, T, and Dahme, B. Implementation and interpretation of respiratory sinus arrhythmia measures in psychosomatic medicine: practice against better evidence? Psychosom Med. (2006) 68:617–27. doi: 10.1097/01.psy.0000228010.96408.ed
Glossary
Keywords: postoperative depression, cardiac surgery, autonomic nervous system dysregulation, hypothalamic pituitary adrenal axis dysregulation, inflammation, cognitive behavioral therapy, antidepressant, statins
Citation: Vu T and Smith JA (2023) The pathophysiology and management of depression in cardiac surgery patients. Front. Psychiatry. 14:1195028. doi: 10.3389/fpsyt.2023.1195028
Edited by:
Marlies Elizabeth Alvarenga, Institute of Health and Wellbeing, AustraliaReviewed by:
Changwei Wei, Capital Medical University, ChinaKai G. Kahl, Hannover Medical School, Germany
Copyright © 2023 Vu and Smith. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Julian A. Smith, anVsaWFuLnNtaXRoQG1vbmFzaC5lZHU=
 Tony Vu
Tony Vu Julian A. Smith
Julian A. Smith