- 1Department of Biomedical Sciences for Health, University of Milan, Milan, Italy
- 2Department of Pathophysiology and Transplantation, University of Milan, Milan, Italy
- 3Department of Neurosciences and Mental Health, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy
Major Depressive Disorder (MDD) is a severe psychiatric disorder characterized by selective impairments in mood regulation, cognition and behavior. Although it is well-known that antidepressants can effectively treat moderate to severe depression, the biochemical effects of these medications on white matter (WM) integrity are still unclear. Therefore, the aim of the study is to review the main scientific evidence on the differences in WM integrity in responders and non-responders to antidepressant medications. A record search was performed on three datasets (PubMed, Scopus and Web of Science) and ten records matched our inclusion criteria. Overall, the reviewed studies highlighted a good efficacy of antidepressants in MDD treatment. Furthermore, there were differences in WM integrity between responders and non-responders, mainly localized in cingulate cortices, hippocampus and corpus callosum, where the former group showed higher fractional anisotropy and lower axial diffusivity values. Modifications in WM integrity might be partially explained by branching and proliferation as well as neurogenesis of axonal fibers mediated by antidepressants, which in turn may have positively affected brain metabolism and increase the quantity of the serotonergic neurotransmitter within synaptic clefts. However, the reviewed studies suffer from some limitations, including the heterogeneity in treatment duration, antidepressant administration, medical posology, and psychiatric comorbidities. Therefore, future studies are needed to reduce confounding effects of antidepressant medications and to adopt longitudinal and multimodal approaches in order to better characterize the differences in WM integrity between responders and non-responders.
1 Introduction
Major depressive disorder (MDD) is a psychiatric disorder characterized by low mood, low self-esteem, and loss of interest in ordinary activities (1).
From a neuroimaging perspective, impairments in signal transduction caused by alteration in levels of neurotransmitters have been consistently reported in MDD (2, 3), especially in subcortical structures that are part of the limbic system and known to be involved in emotional codification and processing (4).
Among neurotransmitters, serotonin is one of the most involved due to its crucial role in mood regulation (5). Indeed, decreased levels of serotonin were observed in MDD (6), maybe due to deficits in the serotonin transporter (5-HTT) within raphe nuclei, at the level of brainstem (7), or within the amygdala and midbrain (8), as well as in the hippocampus, whose neurochemical disruption has been suggested to contribute to developing depressive and anxiety behaviors (9).
In order to rebalance the levels of serotonin in MDD, several pharmacological treatments were developed, including antidepressant medications, which aim to regulate the quantity of neurotransmitters within the brain cortex (10) and to rewire axonal fiber connections by exploiting neuroplasticity (11). Considering the individual tolerability and safety in administration, nowadays the two most used antidepressant drug classes are the Selective Serotonin Reuptake Inhibitors (SSRIs) and the Serotonin-Norepinephrine Reuptake Inhibitors (SNRIs) (12). SSRIs increase the amount of serotonin at the level of synaptic clefts by blocking the normal reuptake of the neurotransmitter (13), and their efficacy was tested against the most severe MDD diagnosis (14). The SNRIs, on the other hand, have a double action on both serotonin and noradrenaline by blocking their reuptake within the synaptic clefts and working similarly to cyclic antidepressants (15).
Beside neurotransmitter impairment, Diffusion Tensor Imaging (DTI) studies consistently reported widespread disruption within white matter (WM) tissue in MDD (16). DTI estimates the directionality of water molecules movement in each voxel and describes it with four diffusivity indices: fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD) and radial diffusivity (RD) (17). FA is a scalar value between 0 and 1 describing the degree of anisotropy within brain tissues; MD is an estimate of the average water movement inside the considered voxel; AD describes the diffusivity along the principal diffusion direction; and, finally, RD describes the diffusivity perpendicular to the main diffusion direction. In the field of DTI, tract-based spatial statistics (TBSS) and tractography are employed to analyze WM pathways in the brain. TBSS is a statistical method used for group-wise analysis of WM microstructure and it is based on the alignment of individual subjects’ DTI to a common space (18). On the other hand, tractography is a visualization technique that reconstructs and maps the pathways of WM tracts in the brain based on DTI data (19). In particular, in MDD structural disruptions within WM tissue are reflected by decreased FA mainly localized in the corpus callosum (CC), cingulum and uncinate fasciculus (20). Moreover, reduction of FA has been found correlating with severity of depressive symptomatology (21) and MDD duration (22), probably explaining the impairments in general cognitive functioning often observed in these patients according to anatomical disruptions (23).
Importantly, as demonstrated by several pieces of evidence, WM is very sensitive to external factors (24), such as pharmacological treatments. Indeed, drugs seem to have a great impact on axonal fibers by modifying their synaptic plasticity and structural organization (25, 26). Therefore, it is likely that during a treatment based on antidepressant medications WM undergoes neuroplasticity processes which may characterize a specific clinical outcome, as already demonstrated by functional connectivity evidence (27).
In this context, this review aims to collect evidence on WM integrity after antidepressant medications by focusing on diffusion differences of clinical outcomes, such as responders vs. non-responders and remitters vs. non-remitters, as they are paramount to describe different levels of success at the end of a pharmacological treatment (28). Responders are patients who show clinically significant improvements in their symptomatology, which is typically defined by a certain percentage reduction in symptom severity scores, often measured by standardized rating scales. On the other hand, remitters are patients who not only respond to medications but also achieve complete resolution of their symptomatology, returning to a clinical state comparable to healthy individuals.
2 Materials and methods
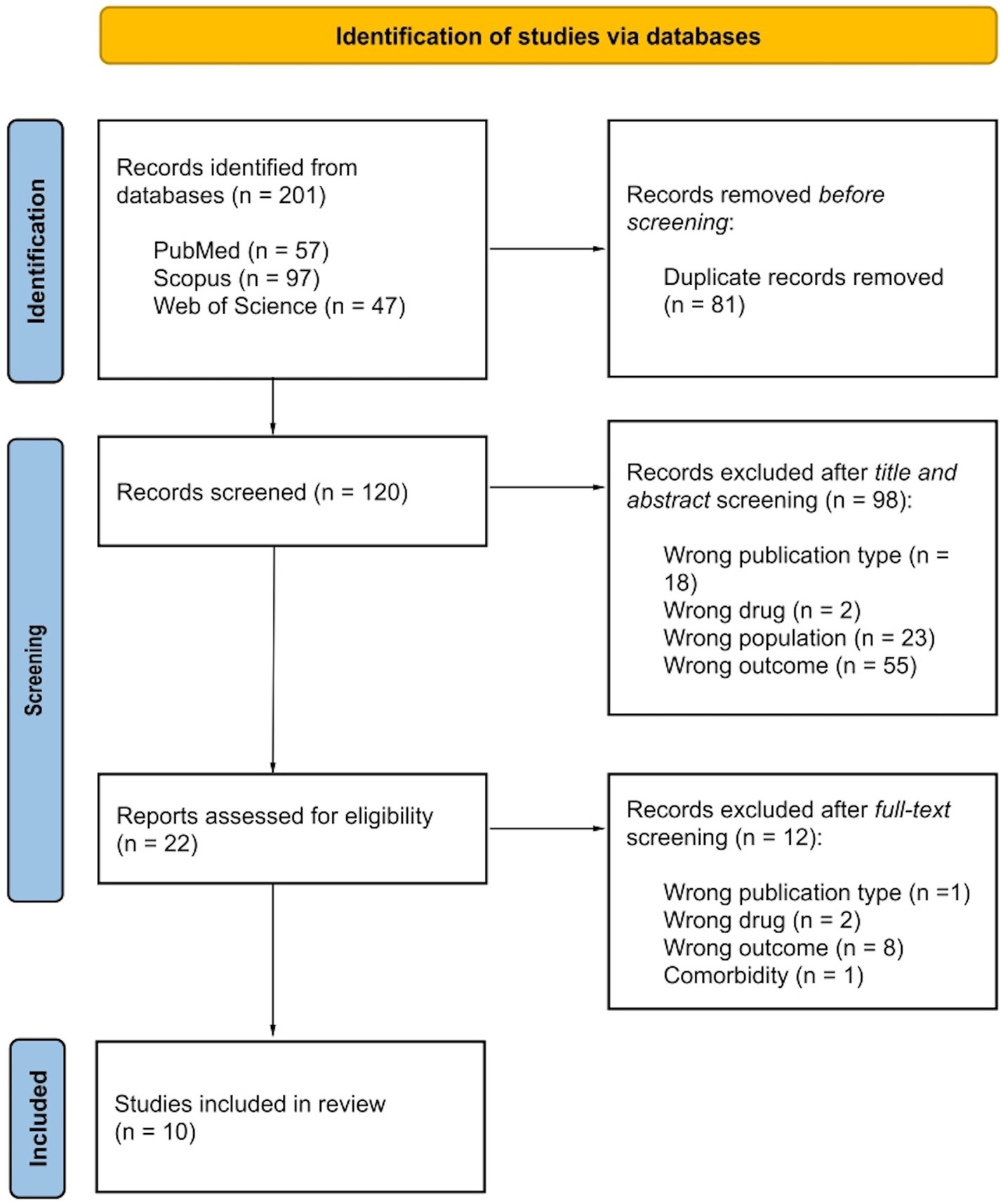
Record research was performed on three datasets: PubMed, Scopus and Web of Science. Combining Boolean operators the string used was the following: (Diffusion Tensor Imaging OR DTI) AND white matter AND antidepress* AND (Major Depressive Disorder OR MDD). The inclusion criteria were: (i) peer-reviewed original publication, (ii) English language, (iii) MDD diagnosis, (iv) DTI investigation of WM integrity after antidepressant medications by focusing on clinical outcomes, and (v) employment of at least one diffusion index (FA, MD, AD and/or RD) to describe WM integrity. Exclusion criteria were: (i) animal studies, (ii) current psychiatric and/or neurological comorbidities (excluding generalized anxiety disorder and social anxiety disorder), and (iii) no comparison between clinical outcomes. No limit was placed regarding antidepressant administration (e.g., type of drugs, duration of treatment, clinical efficacy) and type of clinical outcome comparisons (e.g., remitters vs. non-remitters, pre- vs. post-treatment individual conditions). Record research was performed on August 11th, 2023 with no temporal windows. In Figure 1 the record research is reported. 201 records were found, of which 120 were unique records. After title and abstract screening, 22 records were assessed for eligibility and only 10 were included in the review by matching inclusion and exclusion criteria. Table 1 provides records, extracted variables and main results.
3 Results
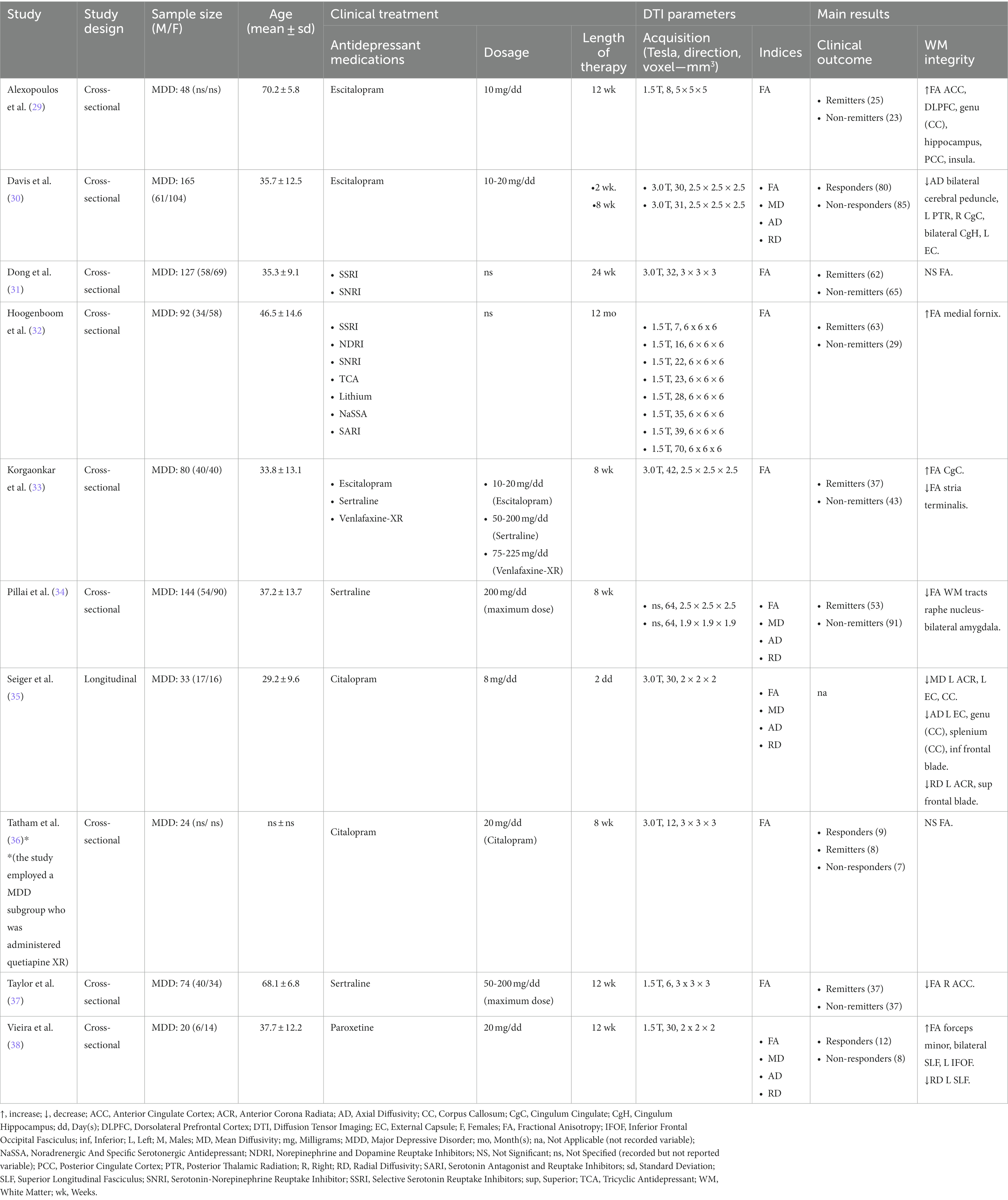
All studies employed SSRIs and SNRIs for MDD treatment. Beside SSRIs and SNRIs, Hoogenboom et al. (32) investigated the combined effects of several antidepressants, including norepinephrine and dopamine reuptake inhibitors, tricyclic antidepressants, lithium, noradrenergic and specific serotonergic antidepressants, serotonin antagonist and reuptake inhibitors. The majority of the studies adopted a pharmacological treatment lasting in total between 8 and 12 weeks, except for three studies that considered either a more extended (24 weeks and 12 months) (31, 32) or shorter (2 days) (35) temporal window. Out of ten studies, only four investigated MD, AD and RD other than FA (30, 34, 35, 38). Finally, with regards to DTI analysis, only five studies employed TBSS (31, 33, 35, 36, 38), one tractography (34), and the remaining ones a voxel-based approach (29, 30, 32, 37).
3.1 Single-drug treatment
Six studies administered a single-drug treatment and investigated WM integrity of MDD patients by considering both remission and response as clinical outcomes (29, 30, 34, 35, 37, 38).
Alexopoulos et al. (29) administered 10 milligrams per day (mg/dd) of escitalopram to 48 MDD elderly patients for 12 weeks. At the end of treatment, 25 patients were remitters and 23 were non-remitters. The authors found that compared to non-remitters, remitters showed higher FA in several WM regions, including anterior cingulate cortex (ACC) and posterior cingulate cortex, dorsolateral prefrontal cortex, genu of CC, hippocampus, and insula.
Similarly, Davis et al. (30) investigated the effect of escitalopram (10-20 mg/dd) on WM and clinical outcome in 165 MDD patients after 2 and 8 weeks of treatment. Interestingly, the authors found that the most significant differences between 85 responders and 80 non-responders were observed after 8 weeks of treatment. Specifically, compared to non-responders, responders had lower AD in bilateral cerebral peduncle, left posterior thalamic radiation, right cingulum cingulate (CgC), bilateral cingulum hippocampus, and left external capsule (EC). No significant differences were observed for FA, MD and RD.
On the other hand, Pillai et al. (34) explored the effect of sertraline on WM integrity and clinical outcome in 144 MDD patients from Establishing Moderators and Biosignatures of Antidepressant Response in Clinical Care (EMBARC) dataset. After an 8 weeks treatment with a maximum dosage of 200 mg/dd, 53 patients were remitters and 91 were non-remitters. Compared to non-remitters, remitters showed lower FA in WM tracts connecting the raphe nucleus and bilateral amygdala without, though, showing any significant difference in MD, AD and RD.
These results were only partially confirmed by Taylor et al. (37), who designed a similar study with 74 MDD patients by focusing only on FA and following 12 weeks of treatment based on sertraline (50–200 mg/dd). Among 37 remitters, lower FA was found within the right ACC in comparison with the 37 non-remitters.
Finally, Seiger et al. (35) and Vieira et al. (38) investigated the effects of citalopram and paroxetine, respectively, on WM integrity in MDD patients. Seiger et al. (35) analyzed the short-term effects of citalopram according to a 2-days treatment with a dosage of 8 mg/dd. In 33 MDD patients the authors evaluated FA, MD, AD and RD pre- and post-treatment and found lower MD in left anterior corona radiata (ACR), left EC and CC; lower AD in left EC, genu and splenium of CC, inferior frontal blade; lower RD in left ACR and superior frontal blade. Finally, Vieira et al. (38) recruited 20 MDD patients who underwent paroxetine treatment for 12 weeks. The WM integrity was compared between 12 responders and 8 non-responders. In responders, higher FA was localized in forceps minor, bilateral superior longitudinal fasciculus (SLF) and left inferior fronto-occipital fasciculus whereas reduced RD was localized in left SLF.
3.2 Multiple-drug treatment
Four studies employed antidepressant medications based on administration of multiple drugs in order to investigate the clinical outcomes related to WM integrity among MDD patients (31–33, 36).
In a sample of 127 MDD patients, Dong et al. (31) investigated FA differences between remitters and non-remitters after administration of several SSRIs and SNRIs for 24 weeks. At the end of treatment, patients who achieved remission were 62, while those who did not were 65. However, no significant differences in FA were observed between remitters and non-remitters.
In contrast, Hoogenboom et al. (32) carried out a legacy-data study on 92 MDD patients. Authors retrieved clinical records of patients who had been recruited from 1999 to 2009 and had been administered different antidepressants for a period of almost 24 months. The only significant result found by authors was the higher FA in medial fornix in 63 remitters compared to 29 non-remitters.
Also, Korgaonkar et al. (33) administered two SSRIs, escitalopram and sertraline, and an SNRI, venlafaxine-XR, to 80 MDD patients for 8 weeks. The dosage was different for each drug: 10-20 mg/dd, for escitalopram; 50-200 mg/dd, for sertraline; 75-225 mg/dd, for venlafaxine-XR. Similar to Dong et al. (31) and Hoogenboom et al. (32), the authors investigated only FA in 37 remitters and 43 remitters and reported that the former group had higher FA in CgC and lower FA in stria terminalis compared to the latter group.
Finally, Tatham et al. (36) administered citalopram (20 mg/dd) to 24 MDD patients, at the end of which the authors examined WM integrity through FA. Between 9 responders, 7 non-responders and 8 remitters no statistical difference was detected in FA values.
4 Discussion
The present study reviewed the literature on WM integrity after antidepressant medications by comparing the clinical outcomes of MDD patients. Overall, the results showed that MDD patients who achieved remission or responded to the pharmacological treatment, showed a difference in DTI indices in respect to non-remitters and non-responders, mainly localized in cingulate cortices, hippocampus and CC.
Overall, considering the efficacy of antidepressant medications, almost half of reviewed studies demonstrated a good rate of clinical outcome (29, 32, 36–38), in line with previous evidence reported by the literature (39). However, almost all the reviewed studies employed a cross-sectional design and, therefore, it is not possible to state whether the differences in WM integrity are due to the effect of medications or were already in place before administering antidepressants. Only Seiger et al. (35) designed a longitudinal study showing partial recovery effects on WM integrity before and after pharmacological treatment, ultimately suggesting that antidepressants may have a normalizing effect on axonal fiber organization.
As described by the reviewed studies the main effects of antidepressant medications were found within cingulate cortices, where, in line with literature, an overall increase of FA occurs in MDD patients at the end of pharmacological treatment (40). Specifically, the reviewed studies found a difference in WM integrity in the cingulum between remitters and non-remitters (29, 33), and between responders and non-responders (30), with the formers showing higher FA and lower AD, respectively. The cingulum belongs to the limbic system and creates anatomical connections with thalamus, hypothalamus and brainstem nuclei (41). Its functional role is related to emotional codification which characterizes depressive symptomatology (42) and, therefore, it is not surprising that in MDD it is one of the most disrupted structures (43). A lower FA in the cingulum might be associated with dysfunctions in connectivity patterns (44) due to either a reduction (45) or a demyelination (46) of axonal fibers. Moreover, abnormal connectivity in cingulate cortices determines an altered activation in frontal regions (47, 48), which correlated with the severity of MDD (49, 50). The specific effects of antidepressant medications on cingulate cortices might be explained by structural modifications within axonal fibers related to proliferation and branching processes (51). This would be coherent to higher WM integrity in responders and may reflect both an improvement in brain metabolism and an increase of serotonergic neurotransmitter within synaptic clefts (52, 53).
Similarly, at the end of SSRIs treatment two reviewed studies (29, 30) also reported higher FA and lower AD within the hippocampus in remitters and responders compared to non-remitters and non-responders. Hippocampus is a key region known to be involved in the pathophysiology of MDD whose abnormal neurogenesis has always been identified as a distinctive trait of depressive symptomatology (54). Hippocampal neurogenesis involves the normal growth of axonal fibers (55) and has been revealed to be sensitive to stress (56) and brain-induced neurotrophic effects (57). Delay or stop of neurogenesis within hippocampal cortices has important consequences on brain metabolism (58), volumetric dimensions (59) and synaptic organization (60). Therefore, by considering hippocampal neurogenesis as clinical target for MDD treatment (54), the higher FA observed in remitters and responders after antidepressant medications might be due to the reactivation of axonal fiber growth which may reverse the atrophy process (61) and up-regulate the neurotrophin signaling pathways (62). In other words, pharmacological treatment based on SSRIs seem to restore 5-HTT deficits (63, 64) by mediating the pro-proliferate effect of antidepressant response (65) and guaranteeing prolonged outcomes even after the total attenuation of depressive symptomatology (66).
Finally, some reviewed studies (29, 35, 38) found higher FA and lower AD in CC between responders and non-responders at the end of antidepressant medications. The CC has been consistently found disrupted in MDD patients, especially the genu, where a decrease of FA, probably connected with a reduction of axonal fibers (67), has been largely reported (68, 69). According to WM neuroanatomy, the axonal fibers originating from genu constitute forceps minor, a well-known clinical biomarker for MDD treatment (38), and reach the frontal regions (70). Therefore, decreased FA in genu of MDD patients may be linked to functional deactivation of those networks which are involved in emotional codification within the frontal cortex (71). As for cingulate cortices, also for CC, the effects of antidepressant medications on WM integrity might be explained by axonal proliferation and branching, which in turn determine higher FA in responders (72).
However, in order to correctly interpret these results, some study limitations should be considered. First, reviewed studies did not employ specific analysis to investigate the impact of each antidepressant in multiple-drug treatments. Second, the effects of different medical dosage on WM integrity were not quantified. Third, the heterogeneity of length of treatment and the extraction of only one DTI index (FA) may not allow a clear interpretation on the effect of antidepressant medications on WM tissue. Fourth, some of the reviewed studies often recruited individuals with comorbid anxiety disorders which did not allow to generalize the observed findings to just MDD patients. Fifth, another limitation associated with inclusion and exclusion criteria can be identified in the choice of selecting only English language publications which could provide a biased assessment of a topic, and can lead to biased results in literature reviews. Finally, we have not conducted a full systematic review therefore our work has intrinsic limits mostly related to deep search (e.g.: bibliography screening) and critical evaluation (e.g.: quality bias assessment) of papers as well as retrieval of missing variables of interest.
In conclusion, from the reviewed studies it emerged that at the end of pharmacological treatment MDD patients who achieved remission or responded to antidepressant medications, showed higher WM integrity mainly localized in cingulate cortices, hippocampus and CC. This would ultimately suggest that antidepressants may indeed have neurotrophic effects on selective brain regions, which may be therefore considered putative clinical biomarkers for MDD treatment. However, future research is warranted for at least two reasons. First, it is necessary to consider the confounding effects of external factors related to antidepressant medications and of overlapping comorbidities related to anxiety disorders. Second, it becomes essential to employ more refined neuroimaging techniques to better characterize neurobiological processes underlying the modifications in WM integrity. This would increase our understanding on the etiology of MDD and will allow the identification of more personalized clinical treatments for depressive patients.
Author contributions
GV: Writing – original draft. LS: Writing – review & editing. CP: Writing – review & editing. PB: Supervision, Writing – review & editing. GD: Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by Fondazione Cariplo under Award Number 2019-3415 to PB.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5, vol. 5. Washington, DC: American Psychiatric Association (2013).
2. Duman, RS . Pathophysiology of depression: the concept of synaptic plasticity. Eur Psychiatry. (2002) 17 Suppl 3:306–10. doi: 10.1016/S0924-9338(02)00654-5
3. Schmidt, HD, Shelton, RC, and Duman, RS. Functional biomarkers of depression: diagnosis, treatment, and pathophysiology. Neuropsychopharmacology. (2011) 36:2375–94. doi: 10.1038/npp.2011.151
4. Belzung, C, Willner, P, and Philippot, P. Depression: from psychopathology to pathophysiology. Curr Opin Neurobiol. (2015) 30:24–30. doi: 10.1016/j.conb.2014.08.013
5. Owens, MJ, and Nemeroff, CB. Role of serotonin in the pathophysiology of depression: focus on the serotonin transporter. Clin Chem. (1994) 40:288–95. doi: 10.1093/clinchem/40.2.288
6. Kayabaşı, Y, Güneş, B, and Erbaş, O. Serotonin receptors and depression. J Exp Basic Med Sci. (2021) 2:240–6. doi: 10.5606/jebms.2021.75662
7. Bartlett, EA, Zanderigo, F, Shieh, D, Miller, J, Hurley, P, Rubin-Falcone, H, et al. Serotonin transporter binding in major depressive disorder: impact of serotonin system anatomy. Mol Psychiatry. (2022) 27:3417–24. doi: 10.1038/s41380-022-01578-8
8. Parsey, RV, Hastings, RS, Oquendo, MA, Huang, YY, Simpson, N, Arcement, J, et al. Lower serotonin transporter binding potential in the human brain during major depressive episodes. Am J Psychiatr. (2006) 163:52–8. doi: 10.1176/appi.ajp.163.1.52
9. Lee, JH, Kim, HJ, Kim, JG, Ryu, V, Kim, BT, Kang, DW, et al. Depressive behaviors and decreased expression of serotonin reuptake transporter in rats that experienced neonatal maternal separation. Neurosci Res. (2007) 58:32–9. doi: 10.1016/j.neures.2007.01.008
10. Kirsch, I, Deacon, BJ, Huedo-Medina, TB, Scoboria, A, Moore, TJ, and Johnson, BT. Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration. PLoS Med. (2008) 5:e45. doi: 10.1371/journal.pmed.0050045
11. Rădulescu, I, Drăgoi, AM, Trifu, SC, and Cristea, MB. Neuroplasticity and depression: rewiring the brain's networks through pharmacological therapy. Exp Ther Med. (2021) 22:1–8. doi: 10.3892/etm.2021.10565
12. Spina, E, Santoro, V, and D'Arrigo, C. Clinically relevant pharmacokinetic drug interactions with second-generation antidepressants: an update. Clin Ther. (2008) 30:1206–27. doi: 10.1016/S0149-2918(08)80047-1
13. Hirschfeld, RM . Efficacy of SSRIs and newer antidepressants in severe depression: comparison with TCAs. J Clin Psychiatry. (1999) 60:326–35. doi: 10.4088/JCP.v60n0511
14. Fournier, JC, DeRubeis, RJ, Hollon, SD, Dimidjian, S, Amsterdam, JD, Shelton, RC, et al. Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA. (2010) 303:47–53. doi: 10.1001/jama.2009.1943
15. Cashman, JR, and Ghirmai, S. Inhibition of serotonin and norepinephrine reuptake and inhibition of phosphodiesterase by multi-target inhibitors as potential agents for depression. Bioorg Med Chem. (2009) 17:6890–7. doi: 10.1016/j.bmc.2009.08.025
16. Chen, G, Hu, X, Li, L, Huang, X, Lui, S, Kuang, W, et al. Disorganization of white matter architecture in major depressive disorder: a meta-analysis of diffusion tensor imaging with tract-based spatial statistics. Sci Rep. (2016) 6:21825. doi: 10.1038/srep21825
17. Basser, PJ, Mattiello, J, and LeBihan, D. MR diffusion tensor spectroscopy and imaging. Biophys J. (1994) 66:259–67. doi: 10.1016/S0006-3495(94)80775-1
18. Smith, SM, Jenkinson, M, Johansen-Berg, H, Rueckert, D, Nichols, TE, Mackay, CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage. (2006) 31:1487–505. doi: 10.1016/j.neuroimage.2006.02.024
19. Basser, PJ, Pajevic, S, Pierpaoli, C, Duda, J, and Aldroubi, A. In vivo fiber tractography using DT-MRI data. Magn Reson Med. (2000) 44:625–32. doi: 10.1002/1522-2594(200010)44:4<625::AID-MRM17>3.0.CO;2-O
20. He, E, Liu, M, Gong, S, Fu, X, Han, Y, and Deng, F. White matter alterations in depressive disorder. Front Immunol. (2022) 13:826812. doi: 10.3389/fimmu.2022.826812
21. Chen, G, Guo, Y, Zhu, H, Kuang, W, Bi, F, Ai, H, et al. Intrinsic disruption of white matter microarchitecture in first-episode, drug-naive major depressive disorder: a voxel-based meta-analysis of diffusion tensor imaging. Prog Neuro-Psychopharmacol Biol Psychiatry. (2017) 76:179–87. doi: 10.1016/j.pnpbp.2017.03.011
22. Abe, O, Yamasue, H, Kasai, K, Yamada, H, Aoki, S, Inoue, H, et al. Voxel-based analyses of gray/white matter volume and diffusion tensor data in major depression. Psychiatry Res Neuroimaging. (2010) 181:64–70. doi: 10.1016/j.pscychresns.2009.07.007
23. Meinert, S, Nowack, N, Grotegerd, D, Repple, J, Winter, NR, Abheiden, I, et al. Association of brain white matter microstructure with cognitive performance in major depressive disorder and healthy controls: a diffusion-tensor imaging study. Mol Psychiatry. (2022) 27:1103–10. doi: 10.1038/s41380-021-01330-8
24. Sampaio-Baptista, C, and Johansen-Berg, H. White matter plasticity in the adult brain. Neuron. (2017) 96:1239–51. doi: 10.1016/j.neuron.2017.11.026
25. Gerdeman, GL, Partridge, JG, Lupica, CR, and Lovinger, DM. It could be habit forming: drugs of abuse and striatal synaptic plasticity. Trends Neurosci. (2003) 26:184–92. doi: 10.1016/S0166-2236(03)00065-1
26. Lüscher, C, and Malenka, RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. (2011) 69:650–63. doi: 10.1016/j.neuron.2011.01.017
27. Qin, J, Liu, H, Wei, M, Zhao, K, Chen, J, Zhu, J, et al. Reconfiguration of hub-level community structure in depressions: a follow-up study via diffusion tensor imaging. J Affect Disord. (2017) 207:305–12. doi: 10.1016/j.jad.2016.09.048
28. Trivedi, MH, Corey-Lisle, PK, Guo, Z, Lennox, RD, Pikalov, A, and Kim, E. Remission, response without remission, and nonresponse in major depressive disorder: impact on functioning. Int Clin Psychopharmacol. (2009) 24:133–8. doi: 10.1097/YIC.0b013e3283277614
29. Alexopoulos, GS, Murphy, CF, Gunning-Dixon, FM, Latoussakis, V, Kanellopoulos, D, Klimstra, S, et al. Microstructural white matter abnormalities and remission of geriatric depression. Am J Psychiatr. (2008) 165:238–44. doi: 10.1176/appi.ajp.2007.07050744
30. Davis, AD, Hassel, S, Arnott, SR, Harris, J, Lam, RW, Milev, R, et al. White matter indices of medication response in major depression: a diffusion tensor imaging study. Biol Psychiatry Cogn Neurosci Neuroimaging. (2019) 4:913–24. doi: 10.1016/j.bpsc.2019.05.016
31. Dong, Q, Liu, J, Zeng, L, Fan, Y, Lu, X, Sun, J, et al. State-independent microstructural white matter abnormalities in major depressive disorder. Front Psych. (2020) 11:431. doi: 10.3389/fpsyt.2020.00431
32. Hoogenboom, WS, Perlis, RH, Smoller, JW, Zeng-Treitler, Q, Gainer, VS, Murphy, SN, et al. Limbic system white matter microstructure and long-term treatment outcome in major depressive disorder: a diffusion tensor imaging study using legacy data. World J Biol Psychiatry. (2014) 15:122–34. doi: 10.3109/15622975.2012.669499
33. Korgaonkar, MS, Williams, LM, Song, YJ, Usherwood, T, and Grieve, SM. Diffusion tensor imaging predictors of treatment outcomes in major depressive disorder. Br J Psychiatry. (2014) 205:321–8. doi: 10.1192/bjp.bp.113.140376
34. Pillai, RL, Huang, C, LaBella, A, Zhang, M, Yang, J, Trivedi, M, et al. Examining raphe-amygdala structural connectivity as a biological predictor of SSRI response. J Affect Disord. (2019) 256:8–16. doi: 10.1016/j.jad.2019.05.055
35. Seiger, R, Gryglewski, G, Klöbl, M, Kautzky, A, Godbersen, GM, Rischka, L, et al. The influence of acute SSRI administration on white matter microstructure in patients suffering from major depressive disorder and healthy controls. Int J Neuropsychopharmacol. (2021) 24:542–50. doi: 10.1093/ijnp/pyab008
36. Tatham, EL, Hall, GB, Clark, D, Foster, J, and Ramasubbu, R. The 5-HTTLPR and BDNF polymorphisms moderate the association between uncinate fasciculus connectivity and antidepressants treatment response in major depression. Eur Arch Psychiatry Clin Neurosci. (2017) 267:135–47. doi: 10.1007/s00406-016-0702-9
37. Taylor, WD, Kuchibhatla, M, Payne, ME, MacFall, JR, Sheline, YI, Krishnan, KR, et al. Frontal white matter anisotropy and antidepressant remission in late-life depression. PLoS One. (2008) 3:e3267. doi: 10.1371/journal.pone.0003267
38. Vieira, R, Coelho, A, Reis, J, Portugal-Nunes, C, Magalhães, R, Ferreira, S, et al. White matter microstructure alterations associated with paroxetine treatment response in major depression. Front Behav Neurosci. (2021) 15:693109. doi: 10.3389/fnbeh.2021.693109
39. Cohen, SE, Zantvoord, JB, Wezenberg, BN, Bockting, CL, and van Wingen, GA. Magnetic resonance imaging for individual prediction of treatment response in major depressive disorder: a systematic review and meta-analysis. Transl Psychiatry. (2021) 11:168. doi: 10.1038/s41398-021-01286-x
40. Chi, KF, Korgaonkar, M, and Grieve, SM. Imaging predictors of remission to anti-depressant medications in major depressive disorder. J Affect Disord. (2015) 186:134–44. doi: 10.1016/j.jad.2015.07.002
41. Tekin, S, and Cummings, JL. Frontal–subcortical neuronal circuits and clinical neuropsychiatry: an update. J Psychosom Res. (2002) 53:647–54. doi: 10.1016/S0022-3999(02)00428-2
42. Drevets, WC, Savitz, J, and Trimble, M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. (2008) 13:663–81. doi: 10.1017/S1092852900013754
43. Versace, A, Almeida, JR, Quevedo, K, Thompson, WK, Terwilliger, RA, Hassel, S, et al. Right orbitofrontal corticolimbic and left corticocortical white matter connectivity differentiate bipolar and unipolar depression. Biol Psychiatry. (2010) 68:560–7. doi: 10.1016/j.biopsych.2010.04.036
44. Nugent, AC, Farmer, C, Evans, JW, Snider, SL, Banerjee, D, and Zarate, CA Jr. Multimodal imaging reveals a complex pattern of dysfunction in corticolimbic pathways in major depressive disorder. Hum Brain Mapp. (2019) 40:3940–50. doi: 10.1002/hbm.24679
45. Bhatia, KD, Henderson, LA, Hsu, E, and Yim, M. Reduced integrity of the uncinate fasciculus and cingulum in depression: a stem-by-stem analysis. J Affect Disord. (2018) 235:220–8. doi: 10.1016/j.jad.2018.04.055
46. Kieseppä, T, Eerola, M, Mäntylä, R, Neuvonen, T, Poutanen, VP, Luoma, K, et al. Major depressive disorder and white matter abnormalities: a diffusion tensor imaging study with tract-based spatial statistics. J Affect Disord. (2010) 120:240–4. doi: 10.1016/j.jad.2009.04.023
47. Peng, X, Wu, X, Gong, R, Yang, R, Wang, X, Zhu, W, et al. Sub-regional anterior cingulate cortex functional connectivity revealed default network subsystem dysfunction in patients with major depressive disorder. Psychol Med. (2021) 51:1687–95. doi: 10.1017/S0033291720000434
48. Rolls, ET, Cheng, W, Gong, W, Qiu, J, Zhou, C, Zhang, J, et al. Functional connectivity of the anterior cingulate cortex in depression and in health. Cereb Cortex. (2019) 29:3617–30. doi: 10.1093/cercor/bhy236
49. Philippi, CL, Motzkin, JC, Pujara, MS, and Koenigs, M. Subclinical depression severity is associated with distinct patterns of functional connectivity for subregions of anterior cingulate cortex. J Psychiatr Res. (2015) 71:103–11. doi: 10.1016/j.jpsychires.2015.10.005
50. Wu, H, Sun, H, Xu, J, Wu, Y, Wang, C, Xiao, J, et al. Changed hub and corresponding functional connectivity of subgenual anterior cingulate cortex in major depressive disorder. Front Neuroanat. (2016) 10:120. doi: 10.3389/fnana.2016.00120
51. Zatorre, RJ, Fields, RD, and Johansen-Berg, H. Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nat Neurosci. (2012) 15:528–36. doi: 10.1038/nn.3045
52. Buchsbaum, MS, Joseph, W, Siegel, BV, Hackett, E, Trenary, M, Abel, L, et al. Effect of sertraline on regional metabolic rate in patients with affective disorder. Biol Psychiatry. (1997) 41:15–22. doi: 10.1016/S0006-3223(96)00097-2
53. Mayberg, HS, Liotti, M, Brannan, SK, McGinnis, S, Mahurin, RK, Jerabek, PA, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatr. (1999) 156:675–82. doi: 10.1176/ajp.156.5.675
54. Eisch, AJ, and Petrik, D. Depression and hippocampal neurogenesis: a road to remission? Science. (2012) 338:72–5. doi: 10.1126/science.1222941
55. Toda, T, Parylak, SL, Linker, SB, and Gage, FH. The role of adult hippocampal neurogenesis in brain health and disease. Mol Psychiatry. (2019) 24:67–87. doi: 10.1038/s41380-018-0036-2
56. Snyder, JS, Soumier, A, Brewer, M, Pickel, J, and Cameron, HA. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. (2011) 476:458–61. doi: 10.1038/nature10287
57. Hanson, ND, Owens, MJ, and Nemeroff, CB. Depression, antidepressants, and neurogenesis: a critical reappraisal. Neuropsychopharmacology. (2011) 36:2589–602. doi: 10.1038/npp.2011.220
58. Malberg, JE . Implications of adult hippocampal neurogenesis in antidepressant action. J Psychiatry Neurosci. (2004) 29:196–205.
59. Malykhin, NV, Carter, R, Seres, P, and Coupland, NJ. Structural changes in the hippocampus in major depressive disorder: contributions of disease and treatment. J Psychiatry Neurosci. (2010) 35:337–43. doi: 10.1503/jpn.100002
60. Serafini, G, Hayley, S, Pompili, M, Dwivedi, Y, Brahmachari, G, Girardi, P, et al. Hippocampal neurogenesis, neurotrophic factors and depression: possible therapeutic targets? CNS Neurol Disord Drug Targets. (2014) 13:1708–21.
61. Warner-Schmidt, JL, and Duman, RS. Hippocampal neurogenesis: opposing effects of stress and antidepressant treatment. Hippocampus. (2006) 16:239–49. doi: 10.1002/hipo.20156
62. D'Sa, C, and Duman, RS. Antidepressants and neuroplasticity. Bipolar Disord. (2002) 4:183–94. doi: 10.1034/j.1399-5618.2002.01203.x
63. Mahar, I, Bambico, FR, Mechawar, N, and Nobrega, JN. Stress, serotonin, and hippocampal neurogenesis in relation to depression and antidepressant effects. Neurosci Biobehav Rev. (2014) 38:173–92. doi: 10.1016/j.neubiorev.2013.11.009
64. Moncrieff, J, Cooper, RE, Stockmann, T, Amendola, S, Hengartner, MP, and Horowitz, MA. The serotonin theory of depression: a systematic umbrella review of the evidence. Mol Psychiatry. (2023) 28:3243–3256. doi: 10.1038/s41380-022-01661-0
65. Alenina, N, and Klempin, F. The role of serotonin in adult hippocampal neurogenesis. Behav Brain Res. (2015) 277:49–57. doi: 10.1016/j.bbr.2014.07.038
66. Kato, M, Hori, H, Inoue, T, Iga, J, Iwata, M, Inagaki, T, et al. Discontinuation of antidepressants after remission with antidepressant medication in major depressive disorder: a systematic review and meta-analysis. Mol Psychiatry. (2021) 26:118–33. doi: 10.1038/s41380-020-0843-0
67. Lee, S, Pyun, SB, Choi, KW, and Tae, WS. Shape and volumetric differences in the corpus callosum between patients with major depressive disorder and healthy controls. Psychiatry Investig. (2020) 17:941–50. doi: 10.30773/pi.2020.0157
68. Wise, T, Radua, J, Nortje, G, Cleare, AJ, Young, AH, and Arnone, D. Voxel-based meta-analytical evidence of structural disconnectivity in major depression and bipolar disorder. Biol Psychiatry. (2016) 79:293–302. doi: 10.1016/j.biopsych.2015.03.004
69. Yamada, S, Takahashi, S, Ukai, S, Tsuji, T, Iwatani, J, Tsuda, K, et al. Microstructural abnormalities in anterior callosal fibers and their relationship with cognitive function in major depressive disorder and bipolar disorder: a tract-specific analysis study. J Affect Disord. (2015) 174:542–8. doi: 10.1016/j.jad.2014.12.022
70. Goldstein, A., Covington, B. P., Mahabadi, N., and Mesfin, F. B. (2017). Neuroanatomy, corpus callosum.
71. Helm, K, Viol, K, Weiger, TM, Tass, PA, Grefkes, C, Del Monte, D, et al. Neuronal connectivity in major depressive disorder: a systematic review. Neuropsychiatr Dis Treat. (2018) 14:2715–37. doi: 10.2147/NDT.S170989
Keywords: major depressive disorder, antidepressant medications, diffusion tensor imaging, white matter, translational psychiatry
Citation: Videtta G, Squarcina L, Prunas C, Brambilla P and Delvecchio G (2024) White matter integrity and medication response to antidepressants in major depressive disorder: a review of the literature. Front. Psychiatry. 14:1335706. doi: 10.3389/fpsyt.2023.1335706
Edited by:
Stefanie Hassel, University of Calgary, CanadaReviewed by:
Darren William Roddy, Trinity College Dublin, IrelandCopyright © 2024 Videtta, Squarcina, Prunas, Brambilla and Delvecchio. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giuseppe Delvecchio, Z2l1c2VwcGUuZGVsdmVjY2hpb0Bwb2xpY2xpbmljby5taS5pdA==
 Giovanni Videtta
Giovanni Videtta Letizia Squarcina
Letizia Squarcina Cecilia Prunas3
Cecilia Prunas3 Paolo Brambilla
Paolo Brambilla Giuseppe Delvecchio
Giuseppe Delvecchio