- 1Sonde Health, Boston, MA, United States
- 2Neuroscience Graduate Program, Department of Health Sciences, McMaster University, Hamilton, ON, Canada
- 3St. Joseph’s Healthcare Hamilton, Hamilton, ON, Canada
- 4Department of Psychiatry, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil
- 5Cognitive Behavior Institute, Pittsburgh, PA, United States
- 6M3 Information, Rockville, MD, United States
Background: The utility of vocal biomarkers for mental health assessment has gained increasing attention. This study aims to further this line of research by introducing a novel vocal scoring system designed to provide mental fitness tracking insights to users in real-world settings.
Methods: A prospective cohort study with 104 outpatient psychiatric participants was conducted to validate the “Mental Fitness Vocal Biomarker” (MFVB) score. The MFVB score was derived from eight vocal features, selected based on literature review. Participants’ mental health symptom severity was assessed using the M3 Checklist, which serves as a transdiagnostic tool for measuring depression, anxiety, post-traumatic stress disorder, and bipolar symptoms.
Results: The MFVB demonstrated an ability to stratify individuals by their risk of elevated mental health symptom severity. Continuous observation enhanced the MFVB’s efficacy, with risk ratios improving from 1.53 (1.09-2.14, p=0.0138) for single 30-second voice samples to 2.00 (1.21-3.30, p=0.0068) for data aggregated over two weeks. A higher risk ratio of 8.50 (2.31-31.25, p=0.0013) was observed in participants who used the MFVB 5-6 times per week, underscoring the utility of frequent and continuous observation. Participant feedback confirmed the user-friendliness of the application and its perceived benefits.
Conclusions: The MFVB is a promising tool for objective mental health tracking in real-world conditions, with potential to be a cost-effective, scalable, and privacy-preserving adjunct to traditional psychiatric assessments. User feedback suggests that vocal biomarkers can offer personalized insights and support clinical therapy and other beneficial activities that are associated with improved mental health risks and outcomes.
1 Introduction
Associations between psychiatric conditions and alterations in vocal and speech characteristics are well documented in clinical practice and the domain of speech research (1, 2). These associations have prompted considerable research on methods that could quantify the presence or severity of such conditions using automated vocal analysis and modeling approaches (3–13). The interest in vocal biomarkers can be explained by several attractive characteristics: they are non-invasive, easy to obtain on consumer devices, allow for frequent or continuous monitoring, and can carry rich information about health and wellness. For example, a smartphone application could analyze changes in speech patterns over time to detect early signs of depressive states. Such vocal biomarker applications are considered among the promising digital approaches in psychiatry (14, 15). Moreover, “smart” personal devices equipped with microphones and signal processing capabilities (e.g., phones, speakers, TVs, vehicle cabins) offer convenient and scalable means of reaching large populations and could help people track their mental wellbeing in ways that go beyond simply logging moods and feelings (16, 17). Finally, because vocal biomarkers are predicated on the acoustic and prosodic features of speech—the qualities of sound rather than the content—they do not require speech recognition, presenting comparatively minimal privacy concerns.
Vocal feature selection for detection or monitoring of psychiatric conditions is complex. Common mood disorders like depression, anxiety, and stress are associated with numerous physiological or behavioral changes that impact vocal and speech production, for example: increased muscle tension leading to throat strain, fatigue, or tightness (18, 19), heightened vocal effort and frequent throat clearing (20), vocal cord dryness (21), certain medications prescribed for psychiatric conditions (22–25), dietary changes or sleep disturbances (26), alterations in muscle control or tension due to neurotransmitter imbalances or hormonal shifts (19), chronic inflammation or frequent infections of the vocal cords (27), changes in brain activity, connectivity or functioning (28, 29), psychomotor retardation leading to slowing of speech, increased pauses, and a flattening of prosody (30–34). Conversely, voice disorders may also create a predisposition for individuals to develop mental health issues. For instance, voice disorders can lead to social withdrawal, communication challenges, stigma, and an overall reduced quality of life. These consequences may give rise to feelings of loneliness, low self-esteem, stress, anxiety, and depression (35–38).
Studies such as those referenced in the first paragraph have addressed the vocal feature selection issue using machine learning approaches, with vocal feature values as inputs, diagnoses or symptom severity as training labels, and model scores as outputs. While some of these studies have showed high levels of performance, very few have been validated on new datasets. Most studies were also retrospective case-control designs, where data was obtained from research participants in controlled settings that did not interact with a digital application that could provide them with feedback regarding their mental wellbeing. Comparatively less work has been done to demonstrate technology that can both accurately measure and inform users about relevant vocal characteristics related to mental wellbeing outside of such controlled settings and then incorporate these into an application that provides results in a way that is understandable and informative to users. The present study aims to demonstrate that digital health tools can maintain strong user engagement and retention, overcoming typical barriers such as negative attitudes towards apps and wearables, lack of measurement agreement with gold standards, and missing evidence around efficacy (39). We do this by showing that a vocal biomarker-based journaling application can provide objective information regarding mental wellbeing that is calibrated against validated assessment tools, in a way that engages and benefits users, using previously identified vocal features relevant to a range of psychiatric conditions.
To test the potential for vocal biomarkers in real-time mental health tracking, this report presents a prospective cohort study with a predesigned vocal biomarker application that provides users with direct feedback. Our vocal feature selection was hypothesis-based, using accumulated evidence from published research, and visualize the results in a digital health app that study participants use on their personal smartphone. To simplify interpretation of results, these features are further aggregated into a single composite score which we call “Mental Fitness”. This terminology is intended to convey the non-medical nature of the score, avoid stigma associated with illness or disorders, and suggest an analogy with physical fitness – strength, resilience, and ability to rapidly recover from injury or setbacks. The “Mental Fitness” concept underscores the opportunity for individuals to engage in healthy behaviors that can proactively mitigate mental health risks, fostering well-being and mental resilience akin to how physical fitness promotes overall health and vitality.
In parallel to the success of tools that monitor and enhance physical activity, our focus on Mental Fitness aims to foster mental well-being and enhanced mental self-awareness through engagement and objective measurement. Much like the established benefits of activity self-monitoring for promoting physical health (40), we envision Mental Fitness tracking as a catalyst for fostering mental resilience. Providing users with this familiar fitness tracking framework could facilitate better understanding and improving of their mental well-being within an integrated digital ecosystem, extending the benefits seen in the physical health domain to empower individuals in actively managing their mental fitness.
The objective of our study is to evaluate whether a smartphone application, designed to capture Mental Fitness vocal biomarkers as part of day-to-day activities, produces results that align with established mental health assessments. Additionally, we aim to assess the application’s ability to maintain user engagement over a four-week period and to collect user feedback on its usability and effectiveness. We tested the approach in a clinical population receiving outpatient treatment for common mental disorders. The study was conducted at a single site (Cognitive Behavioral Institute, Pittsburgh PA), although a pilot phase also included St Joseph’s Healthcare Hamilton (Hamilton, ON).
2 Materials and methods
2.1 Mental fitness study app
Vocal biomarker analysis and visualization was accomplished using a smartphone app developed by Sonde Health, described in detail in the Supplementary Material. Briefly, the app implements a voice journaling capability where users can record 30 second voice samples in response to a range of predefined prompts or talk about their own thoughts and feelings. The response is automatically transcribed and stored in a journaling section, while the recorded audio is used for vocal biomarker analysis using methods similar to those described in OpenSMILE (41) and Praat (42). A “Mental Fitness” Vocal Biomarker (MFVB) score and its feature components are presented to the user after each journaling session and stored in a history section.
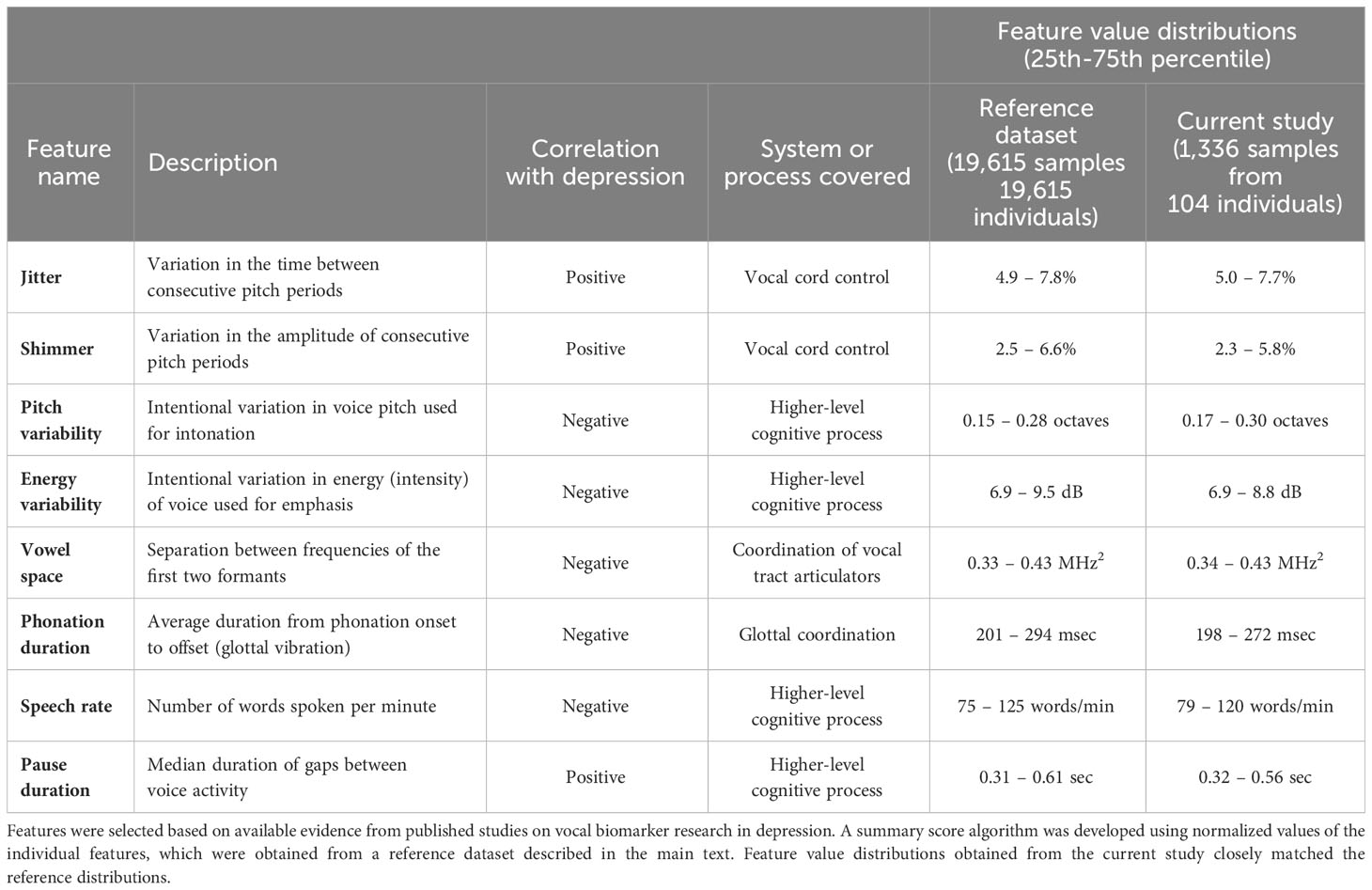
The MFVB score components were selected based on a literature review of well supported vocal features relevant to mental health, in particular depression. The features measure different systems involved in vocal production – briefly, they include jitter and shimmer (43–45), pitch variability (43, 45–48), energy variability (43, 49), vowel space (50, 51), phonation duration (11), speech rate (3, 45, 48, 52), and pause duration (3, 45, 48, 53, 54). More information on these features is provided in Table 1 and the Supplementary Material.
These features were used as inputs to the MFVB summary score using an averaging approach, resulting in an output range of 0-100 with higher scores indicating higher mental fitness (lower likelihood of elevated mental health symptoms). No machine learning models were used in any part of the MFVB score algorithm. Before averaging, the vocal feature values were normalized using distributions (see Table 1) calculated from a large proprietary digital biobank previously acquired through clinical studies across many hospital sites in India. To facilitate interpretation by the user, score categories indicating relatively high, medium, or low ranges were created using MFVB score distributions from the India-based reference population. The score ranges are “Excellent” (MFVB range: 80-100), “Good” (MFVB range: 70-79), and “Pay Attention” (MFVB range: 0-69). The MFVB result is presented to the user as the score and its associated label.
2.2 Study design
This study was designed and reported in accordance with STARD (55), and aimed to demonstrate that MFVB-based mental health symptom severity information could be obtained in consumer-grade products from users that are not guided or trained by a coordinator or clinician.
2.2.1 Study site, recruitment and participant instructions
Participants were recruited from Cognitive Behavior Institute (Pittsburgh, PA), a provider of mental health counseling to people with a range of mental disorders, including depression, anxiety, trauma and stress, obsessive compulsive disorder, and others. The study was advertised via an email campaign that included all patients with either a recent or upcoming counseling appointment. The advertisement included a link to an online consent form where patients were able to review study details, including potential benefits and risk, incentives, and participant expectations. Patients that wished to participate could provide electronic consent and leave a contact email address, which was used by the study team to email a study instruction document.
Study instructions included details on how to install and set up the Mental Fitness app and how to get best results from the recordings. An online video with explanation of how to properly use the app and interpret the results was also made available. Product and study support was available to participants via email if needed, although few participants required assistance.
2.2.2 Eligibility criteria
Patients treated at the study site were eligible for the study if they had at least one clinician-verified symptom of depression according to DSM-5 (56), 14 years or older, and have English as their first language or have conversational proficiency in English as judged from the audio recordings. Participants must own a smartphone device and willing to install and use the Mental Fitness app.
Potential participants were excluded if having a diagnosis of severe cognitive disability precluding informed consent, diagnosis of dementia, schizophrenia, or a speech disorder, or use of certain medications. Although most medications are not formally evaluated for effects on voice and speech, psychiatric, metabolic or other physiologic side effects can occur (22–25). In the context of this study, a short list of psychiatric medication that may have such side effects was excluded, including first-generation antipsychotics (chlorpromazine, haloperidol, loxapine, thioridazine), two or more second-generation antipsychotics (amisulpride, aripiprazole, clozapine, iloperidone, lurasidone, olanzapine, paliperidone, quetiapine, risperidone, ziprasidone, or use of high-dose benzodiazepines (diazepine>=30 mg/day, clonazepam>=2 mg/day, lorazepam>=4 mg/day, alprazolam>=2 mg/day). Additional exclusion criteria were substance abuse, defined as any of the following behaviors in the prior 12 months: 5+ alcoholic drinks in a single day, use of prescription drugs for non-medical use, use of illicit drugs, to avoid potential confounding effects on vocal production (57, 58).
Because participants were recruited via email advertisements, we relied on participant self-report in the onboarding survey to verify the exclusion criteria, in particular the criteria involving medical diagnoses and medication use. Language ability and speech disorders were subjectively judged from manual quality control by trained staff on the voice recordings from the study app (noting participants with excessively poor articulation, stuttering, excessively hoarse or breathy voice, fragmented speech, irregular prosody or disfluencies). Any participant who met any of the exclusion criteria was not removed from the study, but their data was omitted from the analysis cohort (described in the Statistical section below).
2.2.3 Study schedule and incentive structure
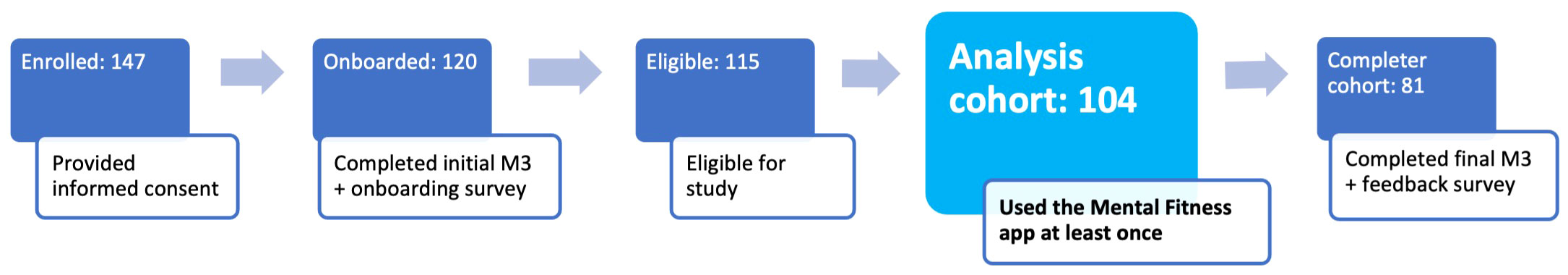
The study flow is further illustrated in Figure 1. Consent and onboarding questionnaires are hosted online through the SurveyLex platform (59). Completion of the onboarding survey was used to define Day 1 for each participant. At the end of week 4, participants received a second online questionnaire as their final study activity. Participants were informed that the study app was intended for daily use in voice journaling, although app use was self-guided.
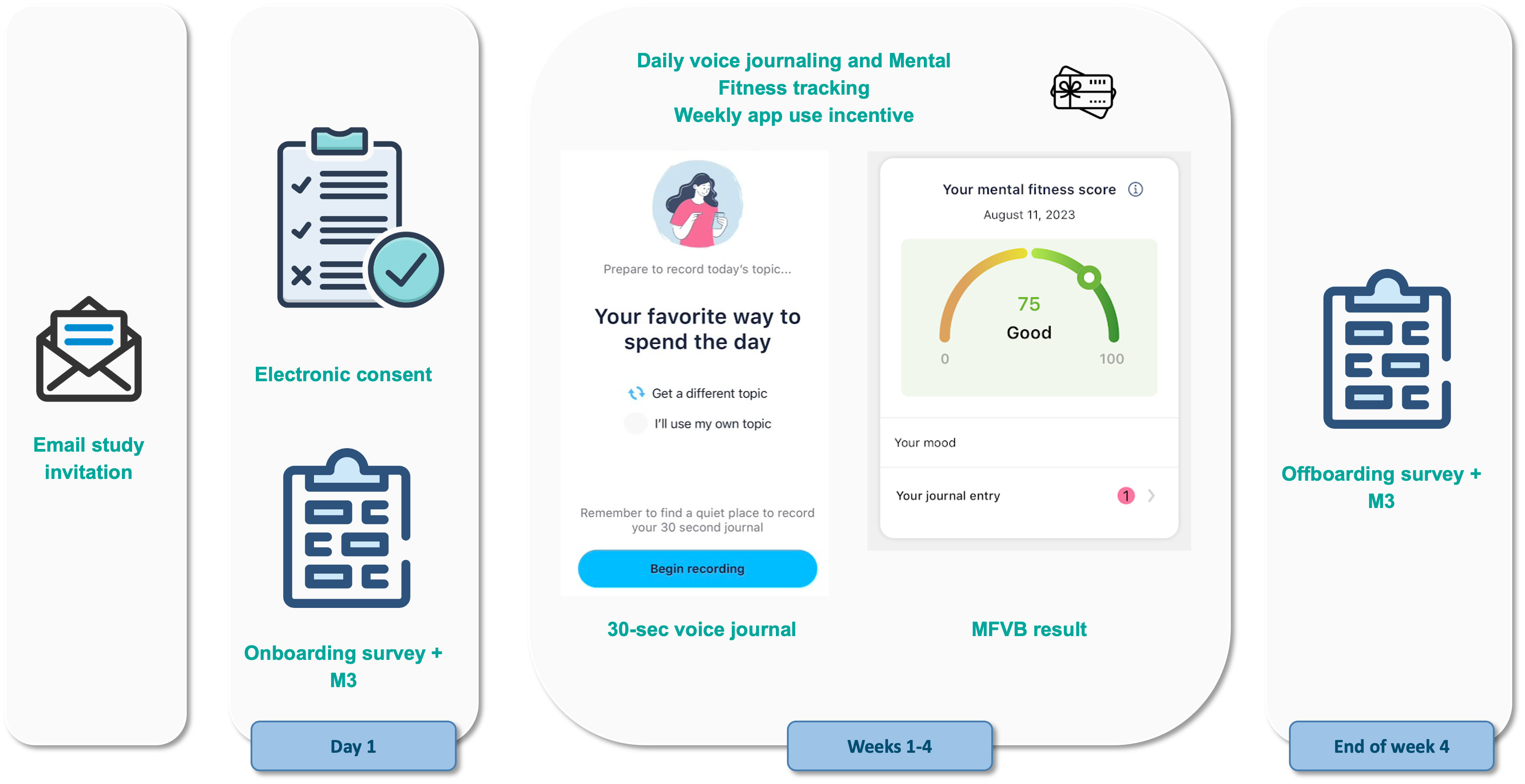
Figure 1 Study design and participant flow. Patients under treatment for mental health disorders at the study site receive an email invitation for the study with a link to the online consent form. After consenting and providing contact information, they receive study instructions containing a link to the onboarding survey and guidance for how to use the Mental Fitness study app. Participants use the study app for voice journaling and Mental Fitness tracking on their own for 4 weeks and receive weekly gift card incentives if they use the app at least 4 times per week. At the end of the study, they receive an offboarding survey.
Participants received a $50 Amazon gift card upon completion of the onboarding and also the final questionnaire. To incentive app use, a $15 gift card was provided on a weekly basis to participants that used the app at least 4 times during the preceding week. Participants that did not meet this requirement did not receive any reminders to promote app usage during the study. App usage was monitored by the study team using dashboards hosted on the Sonde Health infrastructure.
The 4-week per-participant duration of the study was chosen to support the study goals by allowing participants sufficient time to use the app so that they could provide meaningful feedback. It also provides a reasonable amount of time to study engagement and retention. Finally, it provides the opportunity to obtain two self-reported mental health questionnaires with sufficient separation in time, each of which can be associated with vocal biomarker results from voice recordings conducted around the time of the questionnaires.
2.3 Assessments and data collection
2.3.1 Voice recordings
Voice recordings were collected through the Sonde Mental Fitness app on participants personal smartphone device. These were 30-second recordings of responses to predefined randomized prompts or the participant’s own topic. The voice journals were transcribed and logged in the app for the user, but transcriptions were not part of the study data set and not considered in any analysis. Acoustic features were extracted from the voice recordings and used to calculate MFVB scores and categories (see Supplementary Material). The number of voice recordings per participant varied as app use was self-directed.
2.3.2 Questionnaires and mental health assessment
SurveyLex, an web-based platform for research (59), was used to collected various questionnaires:
● Onboarding questionnaire including demographic (gender, age, race, ethnicity), medical (excluded medications, other medication use, diagnosed medical conditions) and behavior (smoking, vaping, substance abuse, use of mental health focused apps) questions.
● M3 Checklist, collected once at onboarding and once at the end of the study in week 4.
● App use and level of understanding using 5-item Likert response scales (completely disagree, somewhat disagree, neither agree nor disagree, somewhat agree, completely agree): liking to use the study app, understanding of the results, helpfulness of the results, helpfulness as addition to treatment, and desire to keep using the study app after the end of the study.
● Free text responses were obtained to questions about how the study app was used and how it was or was not helpful and the best and worst aspects about the app and the study.
The M3 Checklist was used to gather patient-reported mental health symptoms and includes 27 questions related to depression, anxiety, post-traumatic stress disorder (PTSD) and bipolar disorder, as well as questions related to substance abuse and impact of symptoms on work, school, social, and home life. The M3 has been validated vs. MINI mental states diagnoses and compared with existing screening instruments for mental disorders (60, 61). We used the M3 due to the convenience of covering multiple mental disorders in one instrument and the ability to assess mental health symptom severity using established score ranges (normal, mild, moderate, severe) for overall transdiagnostic mental health as well for each of the 4 subdomains.
The M3 Checklist results include a score and symptom severity category, including normal, mild, moderate and severe; these scores and categories are provided for overall mental health and in each of the four categories of depression, anxiety, post-traumatic stress disorder (PTSD), and bipolar. For analysis purposes in the study, severity categories of moderate and severe will be referred to as “elevated” vs. normal and mild as “lower”.
2.3.3 Medical record data
The following data was collected from patient medical records at the study site (with participant permission granted through the informed consent process):
● Diagnosis from treating clinician, reflecting the primary focus of treatment at the time of the study. Typically, this also reflects the condition having the most significant impact on the participant’s mental health, if other conditions are present.
● Treatment duration at the study site at the time of onboarding.
● Treatment elsewhere immediately prior to treatment the study site.
● Date and results of most recent PHQ-9 administered as part of the participant’s treatment.
● Initial (start of treatment) and most recent depressive symptoms noted by the treating clinician.
2.3.4 Data linking
Data were linked across platforms by matching patient name and email as reported in the informed consent document to medical records at the study site, survey responses, and study app account information.
2.4 Endpoints
2.4.1 Primary endpoint
The primary endpoint is the Relative Risk Ratio (RR Ratio) based on relative risk (RR) estimates for elevated overall M3 symptom severity in MFVB ranges of 0-69 (“pay attention”) vs. 80-100 (“excellent”). RR is calculated as the prevalence of elevated symptom severity within a specified MFVB range divided by the analogous prevalence for the reference cohort, which will in general be the analysis cohort (defined in the Statistical section below), unless otherwise specified. An RR Ratio of 1.0 indicates that the MFVB scores have no ability to differentiate mental symptom severity risk. The primary endpoint is also computed for the four mental health domains of the M3 Checklist and also for subgroups based on demographic, clinical, and engagement-related factors1.
RR Ratio based on relative risk was selected as the primary endpoint instead of other measures like accuracy, sensitivity, specificity, positive/negative predictive value as these other measures are more typically applied to diagnostic or screening instruments, which is not consistent with the intended use of the MFVB scores. Rather than providing a prediction about the likely absence or presence of mental health symptoms with a binary outcome, RR provides a more nuanced way to convey increasing or decreasing likelihood of elevated symptoms.
RR Ratios will be reported with 95% confidence intervals, derived using the standard error of the natural logarithm of the RR Ratio, and back-transformed to the original scale. Statistical significance will be assumed if the confidence interval does not include the null value of 1.0, with corresponding two-tailed p value less than 0.05.
2.4.2 Secondary endpoints
Secondary endpoints are the engagement levels with the study app, measured as number of app sessions in study week 1, 2, 3 and 4; as well as retention in week 4, defined as the percentage of participants that use the study app at least once in week 4. Engagement groups were defined based on total app sessions: high engagement was defined as 16 or more sessions, medium engagement as 8-15 sessions, low engagement as fewer than 8 sessions.
The criteria for defining engagement groups were based on the app’s incentive structure and were aimed to differentiate consistent from intermittent usage patterns. High engagement, requiring near-daily app interaction, aligns with the incentive of consistent weekly usage (four or more sessions per week). Medium engagement represents a moderate interaction frequency, roughly once every 2-3 days. Low engagement indicates sporadic use, insufficient for app use incentive and potentially less conducive to habit formation; this group is anticipated to have suboptimal outcomes.
Secondary endpoints also include participant feedback, including the multiple choice questions regarding usability and helpfulness of the app and free-text responses, which will be summarized by theme. Both engagement and feedback will be assessed in the same subgroups as mentioned in the primary endpoint description to uncover potential variations in performance or feasibility of the tool within these subgroups.
2.5 Statistical considerations
2.5.1 Cohort definitions
The following cohorts are defined:
● Enrolled cohort: all participants that provided informed consent.
● Onboarded cohort: subset of the enrolled cohort which completed the onboarding questionnaire at the start of the study.
● Analysis cohort: subset of the onboarded cohort which completed at least 1 Mental Fitness app voice recording session within a period starting 2 weeks before onboarding and ending 2 weeks after the final study survey in week 4 and did not meet any of the exclusion criteria. This cohort allows comparison between MFVB results and mental symptoms per the study’s primary endpoint as well as the engagement analysis in the secondary endpoints.
● Completer cohort: subset of the analysis cohort which also completed the final study survey in week 4. This cohort allows assessment of the feedback responses per the study’s secondary endpoints.
The 2-week time window mentioned in the definition of the analysis cohort was used because some participants used the study app before completing the onboarding questionnaire (or after the offboarding questionnaire). As described below, a 2-week window is used to associate study app voice recordings to M3 surveys.
2.5.2 Statistical analyses
The association between MFVB scores and M3 results was conducted in two ways, illustrated in Figure 2:
1. Closest-MFVB: The single MFVB result that was nearest in time (before or after) to the completion of the M3, but not more than 2 weeks before or after.
2. Time-weighted MFVB: Using all MFVB scores that were available from 2 weeks before to 2 weeks after the M3 was completed. Each MFVB result was weighted as function of its temporal proximity to the M3 with a linear decreasing function of absolute time separation (weighting of 1 for no separation, weighting of 0 for 2 weeks separation). After the initial weights were thus assigned, they were all scaled by a constant so that the weights would sum to 1.
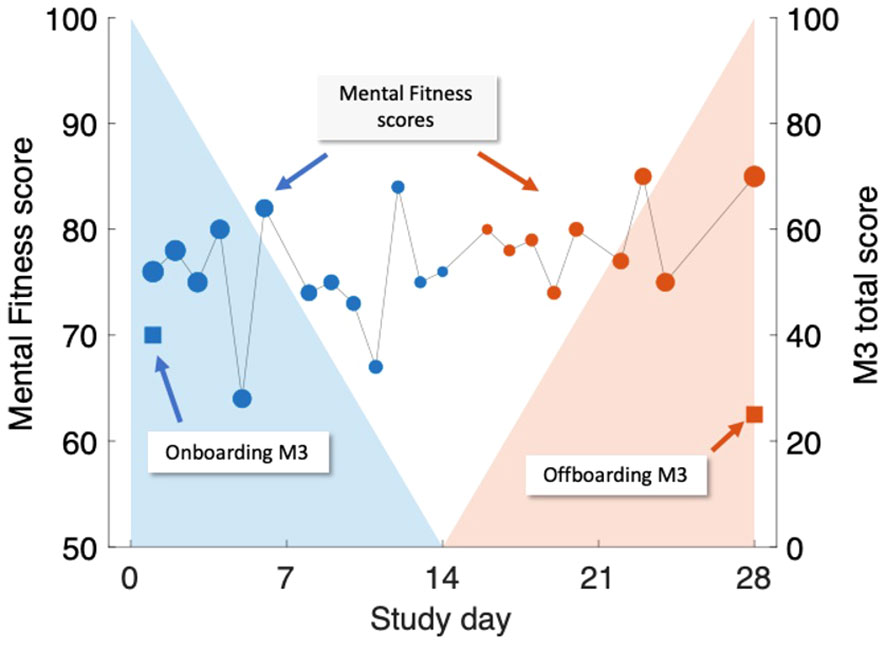
Figure 2 Illustration of time-weighted method to link M3 assessments (right-hand scale, square symbols) to Mental Fitness Vocal Biomarker (MFVB) scores (left-hand scale, round symbols). M3 assessments are obtained on Day 1 (blue) and Day 28 (red) and are each paired with weighted averages of MFVB scores obtained in a period from 2 weeks prior to 2 weeks after the M3 timepoint. Weighting emphasizes MFVB scores closer to the M3 time point, indicated by the size of the MFVB symbol and the decreasing shading applied around each M3 time point. Weights are normalized to sum to 1. Note that in this example, the M3 total score declines over time, paired with a positive trending MFVB score, as intended (recreation of actual participant data).
The rationale and interpretation for the closest-MFVB approach is that this demonstrates the association between a single voice recording analysis to the M3 result, which is useful for one-time or screening-like approaches.
The time-weighted approach evaluates whether the association between MFVB scores and M3 results can be enhanced through repeated app usage over time. This is particularly relevant for scenarios like fitness tracking, where users engage with the application repeatedly. The chosen 2-week window aligns with the M3 survey’s duration, mirroring similar time frames in mental health questionnaires like PHQ-9 and GAD-7. This window encourages participants to consider symptoms over time, reducing the risk of momentary biases during assessments. Aggregating vocal characteristics over a 2-week period enhances alignment with survey results, offering a more comprehensive evaluation compared to a single time point measure.
The implementation of decaying weights in the time-weighted MFVB approach introduces a recency bias, assuming that M3 responses are more influenced by recent feelings and moods during the 2-week window. The linear weighting function was selected for its simplicity in incorporating this recency bias into the MFVB aggregation. Associating MFVB scores from both before and after the M3 questionnaire is suitable under the assumption that symptoms change gradually, allowing mental states after the M3 completion to remain correlated with the result for some duration. This ensures that vocal characteristics during this time window remain pertinent to the mental health assessment.
Engagement was assessed descriptively in each of the engagement groups (high, medium, low) as the number and percentage of participants in each group, as well as the average number of app sessions in each of the 4 study weeks and retention in week 4 (percentage of participants with 1 or more app session in week 4).
2.5.3 Handling of missing data
In defining the analysis cohort, which comprises individuals who completed the M3 assessment at onboarding and provided at least one MFVB result, we ensure that all participants contribute to the calculation of the primary endpoint. Nonetheless, there may be instances where participants do not complete the offboarding questionnaire, resulting in a lack of week-4 M3 assessment data. No imputation methods will be used for the primary endpoint calculation; instead, we acknowledge this limitation and will conduct a sub-analysis using only the onboarding M3 assessment. This will help determine any potential bias introduced by missing offboarding assessments.
For participants who do not complete the offboarding questionnaire, their feedback on the study app will also be absent. We do not have a strategy for imputing this feedback data; the implications of such missing data will be considered and discussed in relation to our findings if it affects a large number of participants.
Variability in the number of MFVB results across participants is expected due to differing levels of engagement with the study app. Since engagement is a key variable of interest, we plan to conduct a subgroup analysis to determine if alignment between MFVB and M3 assessments is influenced by engagement.
Medical record data (psychiatric diagnosis, time in treatment, etc.) are retrieved directly by the study team, and no missing data in this area is anticipated.
2.6 Ethical considerations
The study was conducted in accordance with the principles of Good Clinical Practice as described by the study designs and controls previously. Furthermore, the research protocol was reviewed and approved by WCG IRB (Protocol #20220961) prior to study initiation, and the Hamilton Integrated Research Ethics Board (Project #14494) for the pilot phase at St Joseph’s Healthcare Hamilton. All participants provided electronic informed consent in the online SurveyLex platform before participating in the study. The study app and databases were hosted on Amazon Web Services cloud servers with various security mechanisms in place, described in more detail in the Supplementary Material. Participants were made aware in the informed consent information about financial incentives in the form of electronic gift vouchers upon completion of onboarding and final questionnaire ($50 in each case) and $15 for any of the 4 study weeks if they used the study app at least 4 times during that week. Total potential incentive amount was $160. Incentives were provided solely based on the completion of these study activities and were not contingent on specific outcomes.
3 Results
3.1 Enrollment
The participant cohorts are illustrated in Figure 3: a total of 147 participants from the study site provided electronic informed consent on the web application between May and August 2023. All these participants were emailed study instructions and a link to the onboarding questionnaire, which was completed by 120 participants. Of these, 115 participants met eligibility criteria (exclusions were all due to unallowed medication use only), and of those 104 participants used the study app at least once during the study. This set of 104 participants forms the analysis cohort and will be used to report results, unless otherwise indicated. The offboarding survey at the end of week 4 was completed by 81 participants, forming the completer cohort.
3.2 Demographics and treatment factors
Demographic and treatment factors of the analysis cohort are provided in Table 2. The analysis cohort was majority female (73%); skewed to younger adults with proportion in age ranges below 30, 30-39 and over 40 of 41%, 27% and 32% (age range 16-80); mostly white (93%) and non-Hispanic (99%). Most participants have been in longer-term treatment (66% for >1 or 2 years) and were not treated elsewhere prior to their current therapy (75%). Most participants (73%) reported using prescription medications for their mental health treatment (main reported medications being fluoxetine, sertraline, escitalopram, bupropion, lamotrigine, buspirone, lisdexamfetamine, methylphenidate, venlafaxine, and duloxetine). About half of the participants (47%) reported one or more diagnosed health conditions, including obesity (19%), asthma (15%), hypertension (12%), diabetes (3%) and other conditions (19%).
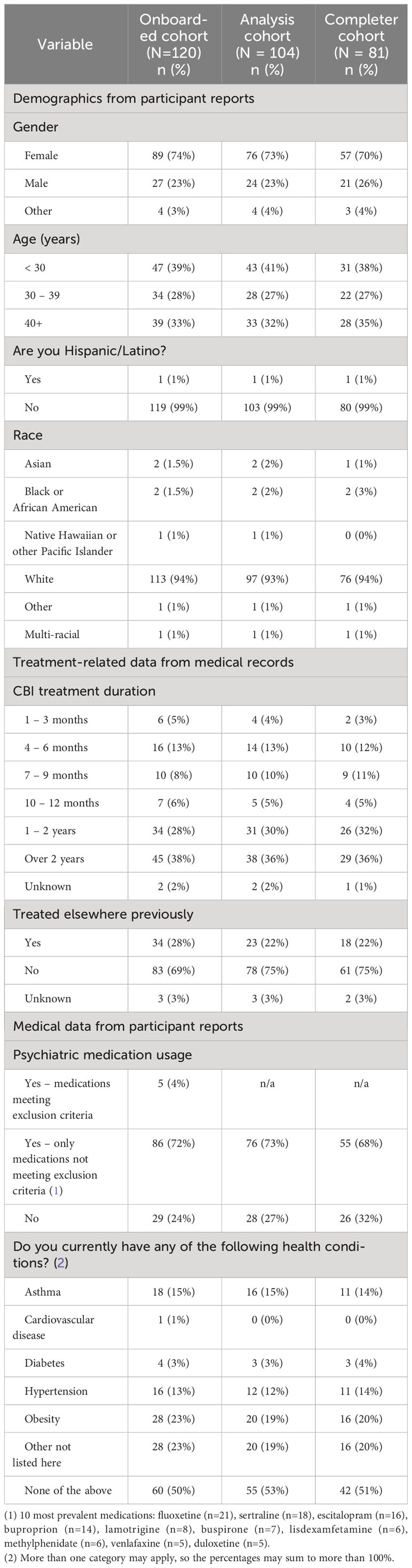
Table 2 Demographics, treatment, and medical characteristics of the study participants. Information was reported by participants, except treatment information which was obtained from medical records.
3.3 Clinical presentation
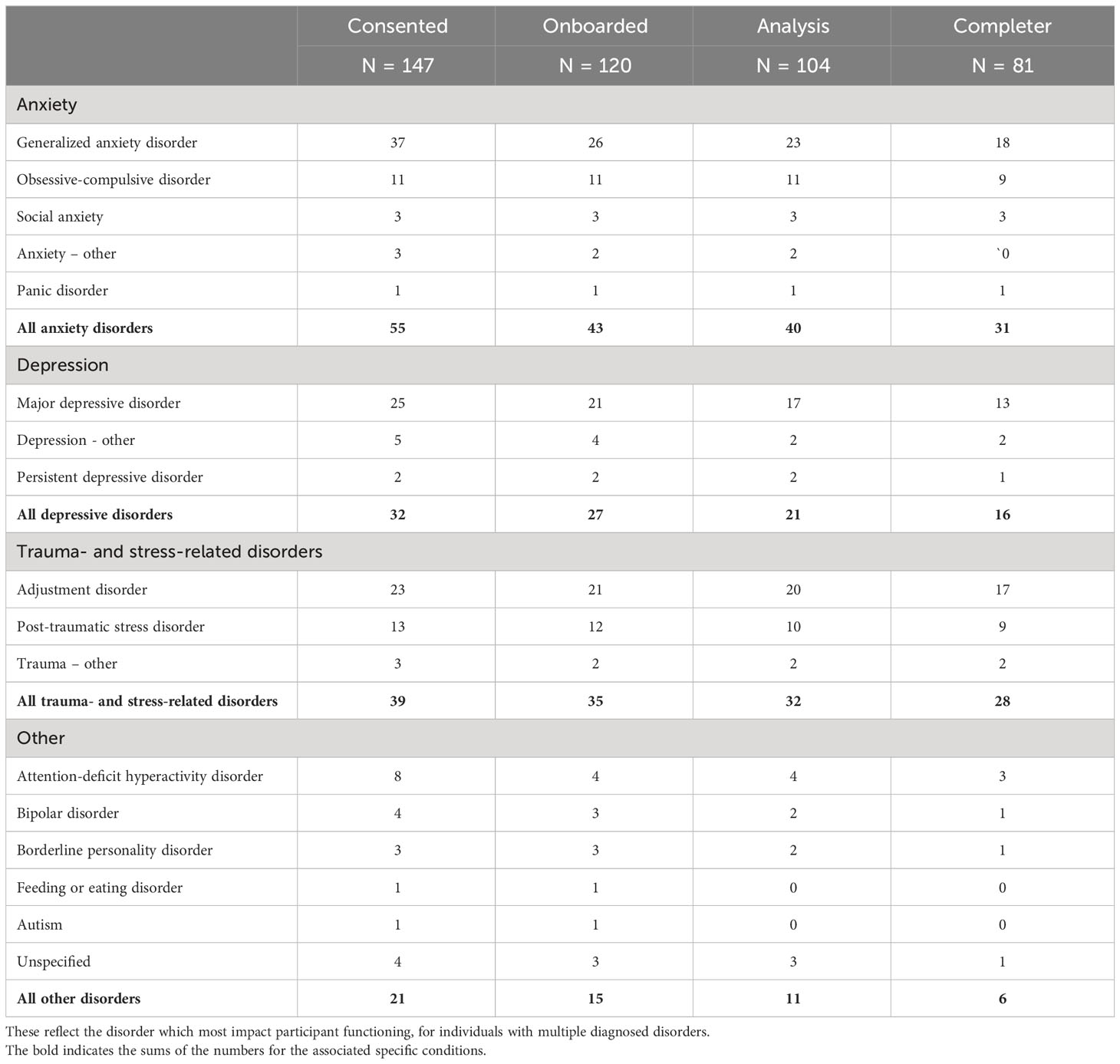
Psychiatric diagnoses were extracted from participant medical records at the study site and grouped into high-level categories as indicated in Table 3. Anxiety-related diagnoses were the most common (38% of the analysis cohort), followed by trauma and stress-related disorders (31%), and depression disorders (20%). The most prevalent specific diagnoses were generalized anxiety disorder (22% of the analysis cohort), major depressive disorder (16%), adjustment disorder (19%), obsessive-compulsive disorder (11%), and post-traumatic stress disorder (PTSD, 10%). Various other conditions were also present in smaller numbers, e.g., attention-deficit hyperactivity disorder, bipolar disorder, and borderline personality disorder.
Among consented participants that failed to onboard onto the study, a relatively large number included participants with diagnoses of generalized anxiety disorder (11, or 30% of the consented group with this diagnosis). The relative proportion of diagnoses in the consented, onboarded, analysis and completer cohorts were otherwise similar. Most participants in the analysis cohort (80%) also completed the offboarding questionnaire (completer cohort).
Table 4 offers insights into mental health symptom severity through M3 Checklists, encompassing 104 participants and 185 M3 assessments (5 M3 assessments could not be linked to any participant and were excluded from analysis). Results are presented at onboarding for both the analysis and completer cohorts, allowing for direct comparisons with offboarding outcomes available exclusively from the completer cohort. At onboarding, the analysis cohort presented the following severity distribution: normal (0%), mild (31%), moderate (48%), and severe (21%). The cumulative prevalence of elevated (moderate-to-severe) symptoms was 69%. Examining mental health categories, elevated symptoms were prevalent in 53% for depression, 30% for anxiety, 27% for PTSD, and 11% for bipolar.
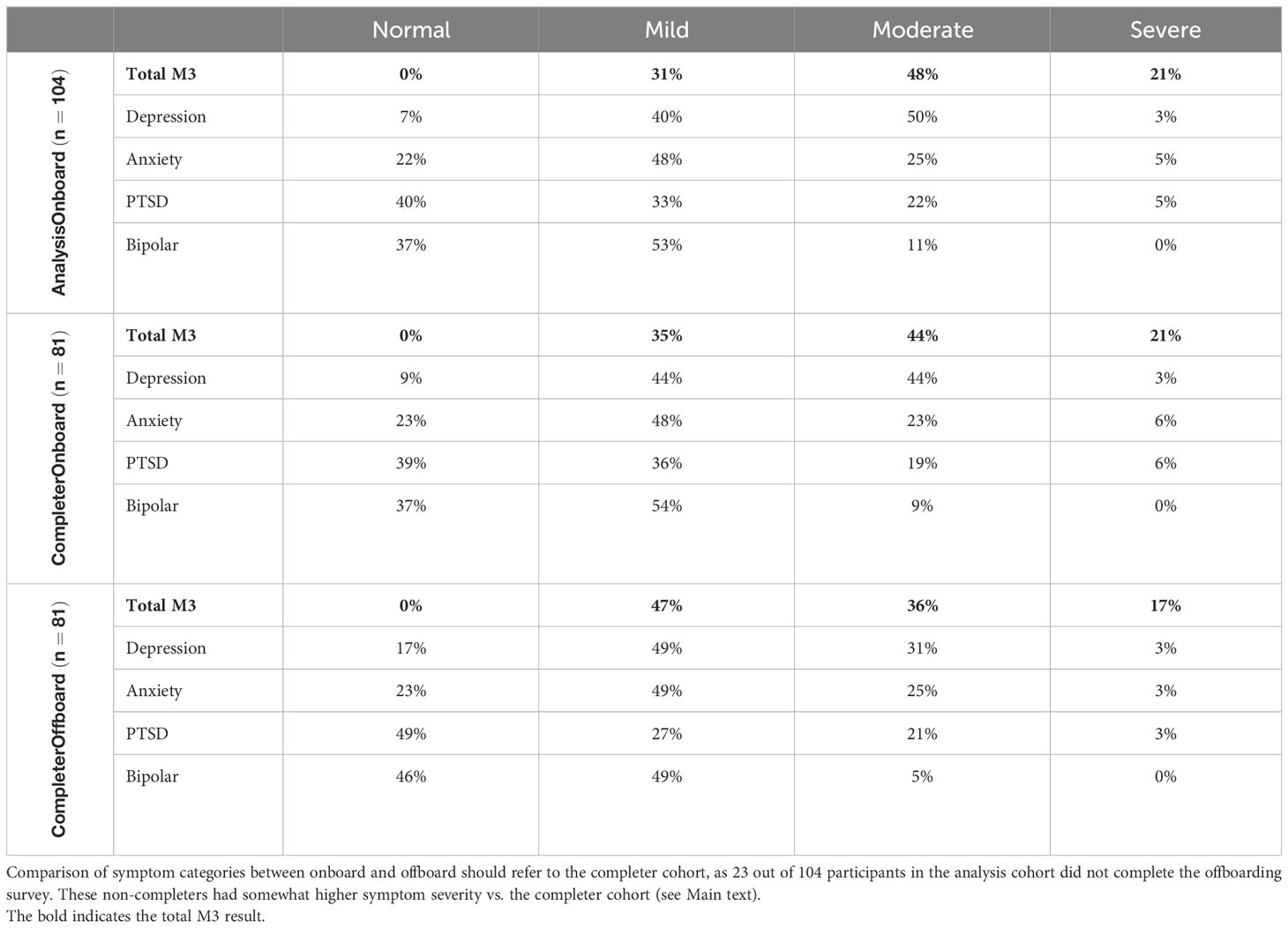
Table 4 Mental health symptom severity prevalence reported using the M3 Checklist at onboarding (analysis and completer cohorts) and offboarding.
Symptom severity at onboarding was correlated with the likelihood to complete the offboarding survey: completers vs. non-completer (participants from the analysis cohort that are not included in the completer cohort) demonstrated the following elevated symptom severity distribution: Total (65% vs. 81%), depression (47% vs. 71%), anxiety (29% vs. 33%), PTSD (27% vs. 29%), and bipolar (9% vs. 14%).
Symptom severity reported in the offboarding survey at the end of week 4 indicates a notable reduction in elevated symptom severity within the completer cohort over the study period. The prevalence decreased from 65% at onboarding to 53% at offboarding, reflecting a 13% reduction—suggestive of the potential impact of treatment provided at the study site. These improvements were also noted on functional impairment outcomes of the M3: 25-30% of participants reported improvements in functioning at work or school and relationships with friends or family. These numbers rose to 35-40% for those participants that reported significant impairment in functioning on their initial M3.
3.4 Voice sample recordings
Participants conducted 1,336 app sessions with voice recordings during their 4-week study period, resulting in an average 12.8 sessions per participant, or 3.2 per week – nearly exactly once every other day. Additional detail regarding app use will be provided in the Participant Engagement section below. Vocal feature distributions from the study data set were compared with those from the reference dataset that was used to develop the MFVB scoring algorithm and were found to be in close alignment (see Table 1).
3.5 Vocal biomarker correlation with mental health symptom severity
3.5.1 Total symptom severity
Out of 185 M3 assessments, 177 were included in analysis of the primary endpoint due to having 1 more associated MFVB results within the 2-week time window. The prevalence or risk of elevated mental health symptom severity across this dataset was 62% (110 out of 177), defining the Relative Risk (RR) level of 1.00.
Visual representation of the alignment between MFVB score categories and total symptom scores from the M3 assessment is shown in Figure 4, using the closest-MFVB and time-weighted MFVB approach. Both approaches indicate that M3 scores and thus symptom severity distributions increase from MFVB categories of “Excellent” to “Good” to “Pay Attention”, as intended.
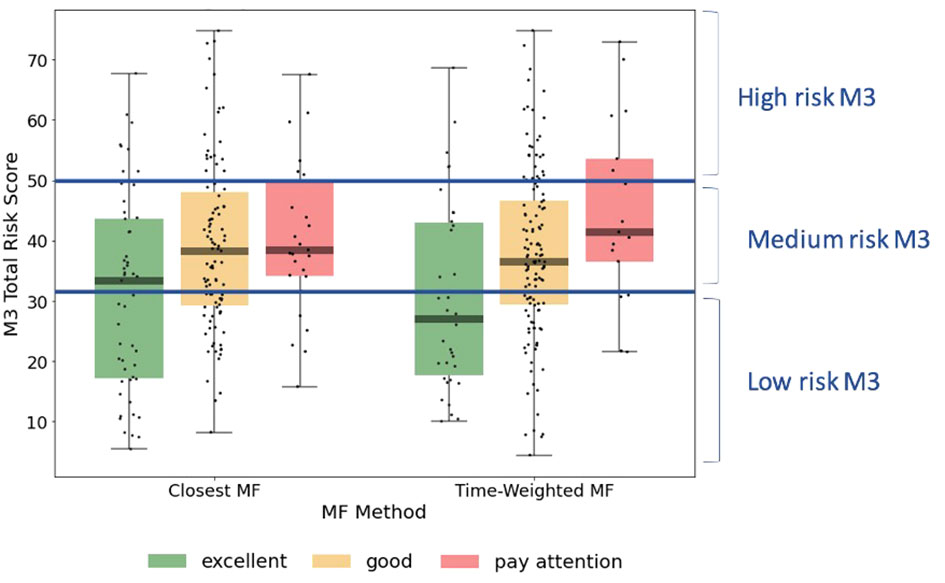
Figure 4 M3 total symptom score distributions by MFVB score category, indicating the correlation between vocal analysis results and participant reported mental health symptom severity. The left-hand side of the panel associated the MFVB result closest in time to the M3, whereas the right-hand side of the panel uses a time-weighted average of MFVB results within 2 weeks of the M3 (see Methods). Results in Table 5 are calculated on the same data as displayed here.
RR was determined within MFVB score categories of Excellent, Good, and Pay Attention. Table 5 outlines the RR estimates and 95% confidence intervals (omitted here for brevity) for these MFVB score categories as 0.82, 1.02, and 1.25 when using the closest-MFVB method and 0.62, 1.07, and 1.23 when using the time-weighted method.
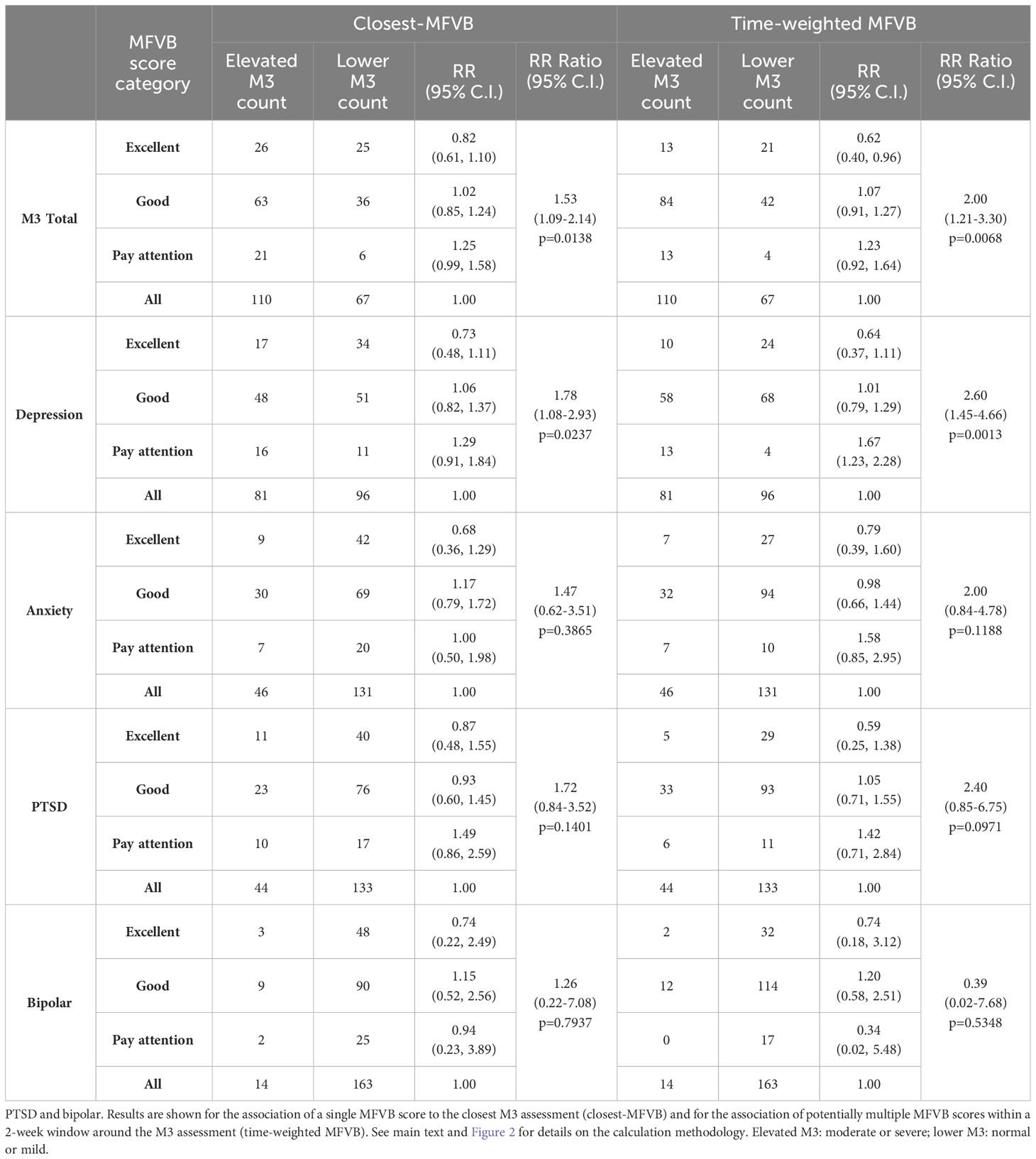
Table 5 Relative Risk (RR) and RR Ratio for MFVB score categories compared to the analysis cohort (“all”) for total mental health symptoms and symptom categories of depression, anxiety.
Notably, both methods show that the “Excellent” and “Pay Attention” MFVB categories reflect RR below and above 1.00 respectively, as originally intended, as well as an RR near 1 for the MFVB score category of “Good”. The RR Ratios between Pay Attention and Excellent MFVB score categories for these two methods are statistically significant at 1.53 (1.09-2.14, p=0.0138) and 2.00 (1.21-3.30, p=0.0068), respectively, meeting the primary endpoint of the study. A principal effect of the time-weighted versus the closest-MFVB method is an increased distinction in RR away from 1, presumably benefiting from “multiple looks” at the MFVB results across the 2-week aggregation period.
Taken together, these results confirm the primary objective of the study in that the MFVB results provide users with objective information about mental health symptom severity in alignment with clinically validated reference instruments. The Relative Risk Ratios capture to what extent users with MFVB scores in the “Pay Attention” range are more likely to experience elevated mental symptoms vs. users in the MFVB score range of “Excellent”.
3.5.2 Symptom severity for depression, anxiety, PTSD and bipolar
The same principles observed for the total M3 can be extended to the subcategories for depression, anxiety, PTSD, and bipolar (Table 5). MFVB efficacy for depression is more pronounced compared to total symptoms: this heightened distinction may be attributed to the selection of vocal features, which were primarily chosen for their established association with depression. RR Ratios for closest and time-weighted methods are both statistically significant at 1.78 (1.08-2.93, p=0.0237) and 2.60 (1.45-4.66, p=0.0013), respectively. Results for anxiety and PTSD indicate consistent RR trends despite wider confidence intervals and non-significant RR Ratio due to a lower prevalence of elevated symptoms in these categories. It is particularly challenging to accurately assess RR for bipolar due to the limited total number of reported cases of elevated symptom severity (only 14 M3 results fall into this category). Additionally, hypomanic patients may manifest a mixture of vocal qualities, some of which are normally associated with strong mental health.
3.6 Participant engagement
As indicated in Table 6, participants engaged with the study app an average of 12.8 total times over the course of the 4-week study period, with 70% of users remaining active in week 4. These average usage patterns can be further examined by categorizing engagement into three levels, as described in the Methods section.
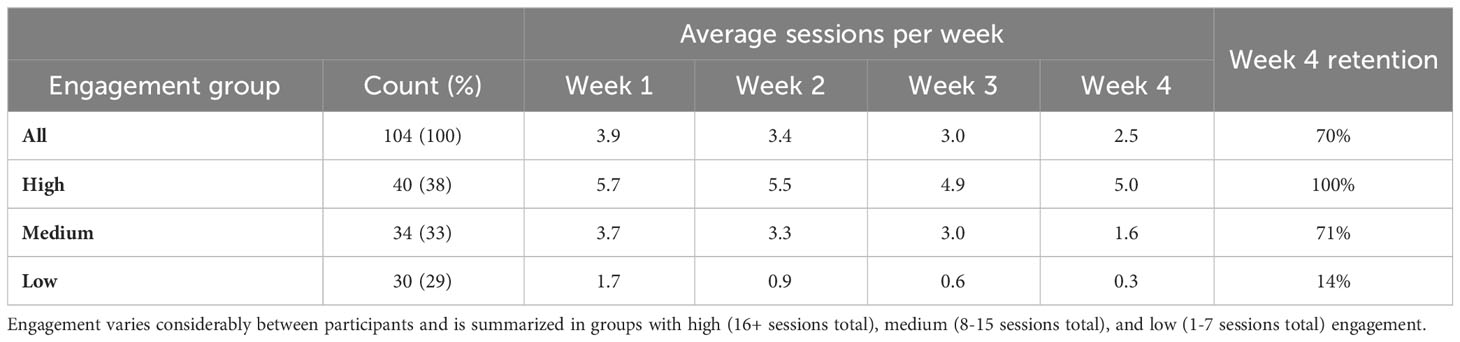
Table 6 Participant engagement during the 4-week study period, measured as average weekly sessions and retention in week 4.
The high engagement category included 38% of participants, with a consistent 5-6 app sessions each week, 100% retention in week 4 and an average of 21.1 sessions in total (approximately 10 minutes of voice recordings over the study period). The medium engagement group (8-15 sessions) comprised 33% of participants. This group typically utilized the app 3-4 times per week with a gradual decrease over time, had 71% retention rate in week 4, and an average of 11.6 sessions overall. The remaining 29% of participants exhibited low engagement and had minimal app usage, even in the initial study week; only 14% of these users were retained in week 4, with an average of 3.5 total app uses. The engagement groups thus differed both on total app usage but also persistence of use over time.
To find potential reasons for these very different levels of engagement, cohorts defined by the engagement groups were constructed and assessed for variations in demographics, health characteristics, and clinical presentation. Statistical significance was assessed in each case using chi-square test and 5% significance level. The only statistically significant factor was age (p=0.0131), with older age groups displaying higher levels of engagement. The oldest age group (40+ years) comprised 45% of the high engagement group, 26% of the medium engagement group, and 20% of the low engagement. While the middle age group (30-39 years) trended similarly, for the youngest age group (<30 years) the analogous proportions were 20%, 53%, and 57%, revealing an opposite trend.
Other factors, including male gender (p=0.1273), absence of prescription medication use in mental health treatment (p=0.3157), lower symptom severity (p=0.4425, comparing completer and non-completer participants), and having a psychiatric diagnosis other than anxiety-related, exhibited positive trends toward increased engagement, although statistical significance was not reached. Notably, age and these non-significant factors, which trended positively with higher engagement, were also linked to lower symptom severity. This suggests that symptom severity may be a primary determinant of engagement, with higher symptom levels associated with reduced engagement and vice versa. This aligns with the expectation that individuals experiencing more severe symptoms might be less engaged.
3.7 Participant feedback
3.7.1 Level of agreement with statements about MFVB tool and score characteristics
Participant agreement with five statements presented in the offboarding questionnaire at the conclusion of week 4 is summarized in Table 7. This questionnaire was completed by 80 participants, 77% of the analysis cohort. The high response rate reduces potential responder bias in the response analysis.
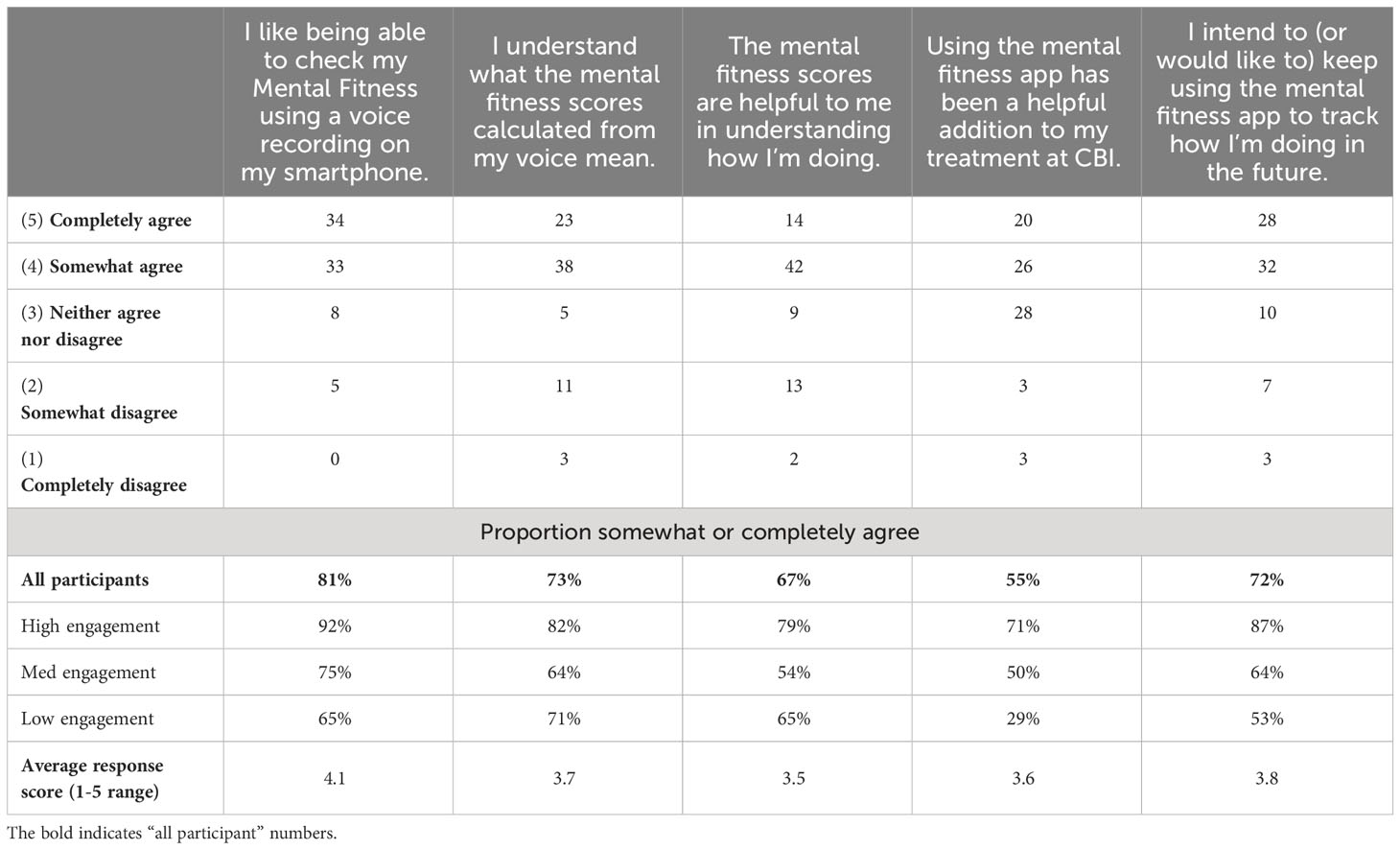
Table 7 Participant level of agreement with statements in the feedback portion of the offboarding survey; 80 participants included (completer cohort, except 1 participant that did not provide feedback responses).
In general, a substantial majority of participants expressed partial or full agreement with all statements. The highest level of agreement (81%) was in response to the statement expressing contentment with the capability to assess Mental Fitness through voice recordings on their smartphone, underscoring the favorable reception of this activity and application among participants. The lowest level of agreement (55%) was noted in response to the statement asserting that the Mental Fitness app constituted a beneficial adjunct to their treatment at CBI. Here, a relatively large proportion of participants indicated a neutral opinion. The remaining three statements (pertaining to comprehension of MFVB scores, the perceived utility of MFVB scores for self-assessment, and the intention to continue using the Mental Fitness app in the future) received partial or full agreement from approximately 70% of participants.
Participant responses to the statements were also analyzed based on level of engagement to determine if satisfaction correlated with usage (Table 7). Results showed that participants in the high engagement group agreed more frequently, with 82% somewhat or completely agreeing on average across all statements. Using the same calculation, the medium engagement group had a 61% agreement rate, while the low engagement group had a 57% rate. Interestingly, the statement about understanding the MFVB scores showed a modest 9% difference between the high and low engagement groups, indicating that level of understanding of the scores wasn’t a significant factor driving app usage. Conversely, the statement about the app being a helpful addition to treatment at CBI had the most substantial differences between engagement groups, ranging from 71% agreement in the high engagement group to 50% in the medium engagement group and 29% in the low engagement group. This suggests that the perceived value of the app as an addition to treatment was closely tied to consistent app usage. Overall, engagement and satisfaction were strongly correlated.
3.7.2 Open response feedback
The offboarding survey at the end of week 4 also included several free response questions where participants could provide more detail about how they used the app, whether it was a helpful addition to their treatment at the study site, and what was perceived as the best and worst things about the study app. The combined response set from these questions provided a rich source of insight on how participants used the app and what sources of benefits they perceived, with approximately 40% of participants reported making some change in their behavior or lifestyle as a result of using the MFVB tool and approximately 30% mentioned perceived benefits to their wellbeing. The following sections highlight key themes in participant responses to the questions, selected to best illustrate the types of preferences and behaviors mentioned by participants.
3.7.2.1 How the app was used
Participants were asked to “Briefly explain how you’ve used the mental fitness app and how it has (or has not) been helpful to you”. Participants reported a variety of uses, with some describing the app as akin to a therapeutic self-reflection exercise, while others found it challenging to use consistently due to issues with notifications and varying levels of engagement. Many noted that the app helped them track and reflect on their mental health, recognize patterns, and provided a structured daily check-in. However, some participants questioned the accuracy of the MFVB assessment, as it did not always align with their self-assessment. The time constraint for recordings (30 seconds) was a concern for several participants, who desired a longer recording option.
3.7.2.2 Complement to clinical treatment
Although neither the app nor the study was integrated with treatment at the study site, participants were also asked to “please briefly explain how the mental fitness app has (or has not) been a helpful addition to your treatment at CBI”. This question was intended to reveal whether participants would develop their own ways to complement their treatment and how it might be most helpful in a treatment context.
Feedback themes partially overlapped with responses to the previous question, as several participants noted that it helped them reflect, self-assess, and maintain a routine. Some mentioned that it offered a moment for self-reflection and set a positive tone for the day or helped them track their emotions when they didn’t have therapy appointments. However, others found it challenging to remember daily use or believed that it didn’t significantly contribute to their treatment. A few participants mentioned discrepancies in the MFVB score, which didn’t always align with their self-assessment. While some found it beneficial for voicing their thoughts and feelings, others felt it did not align with the specific needs of their therapy.
3.7.2.3 Best and worst things
Participants valued the app’s convenience and ease of use, highlighting its quickness and ability to track emotions over time. They appreciated the score system for visualizing feelings and patterns. The prompts, speech-to-text journaling, and the reminder for positivity were well-received. The app served as a bridge between therapy sessions, offering structured daily check-ins and journaling. Participants also found the tips and score breakdowns into the 8 vocal features helpful, providing a consistent and reassuring external perspective on their emotions.
On the other hand, participants encountered various challenges and limitations with the app, including difficulties in consistently remembering to use it, technical issues such as app glitches and notifications, and the need to find a quiet place to record. The fixed 30-second recording time posed a constraint, leading to desires for greater self-expression flexibility. Repetitive prompts caused some users to lose interest, while doubts about the accuracy of mood interpretation based on voice recordings emerged. Some concerns were raised about privacy and potential data mining.
3.8 Vocal biomarker correlation: subgroup analyses
3.8.1 Gender and age
With reference to Table 8, RR patterns using the time-weighted MFVB approach for total symptom severity appear to indicate better performance in males vs. females as the RR Ratio is higher (5.00 vs. 1.94). However, confidence intervals for males are wide due to relatively fewer participants, and the data does not provide conclusive evidence for a difference. Still, differences in vocal changes that may impact MFVB effectiveness cannot be ruled out, because gender differences in depressive symptom profiles have been described for other non-voice related behaviors (62).
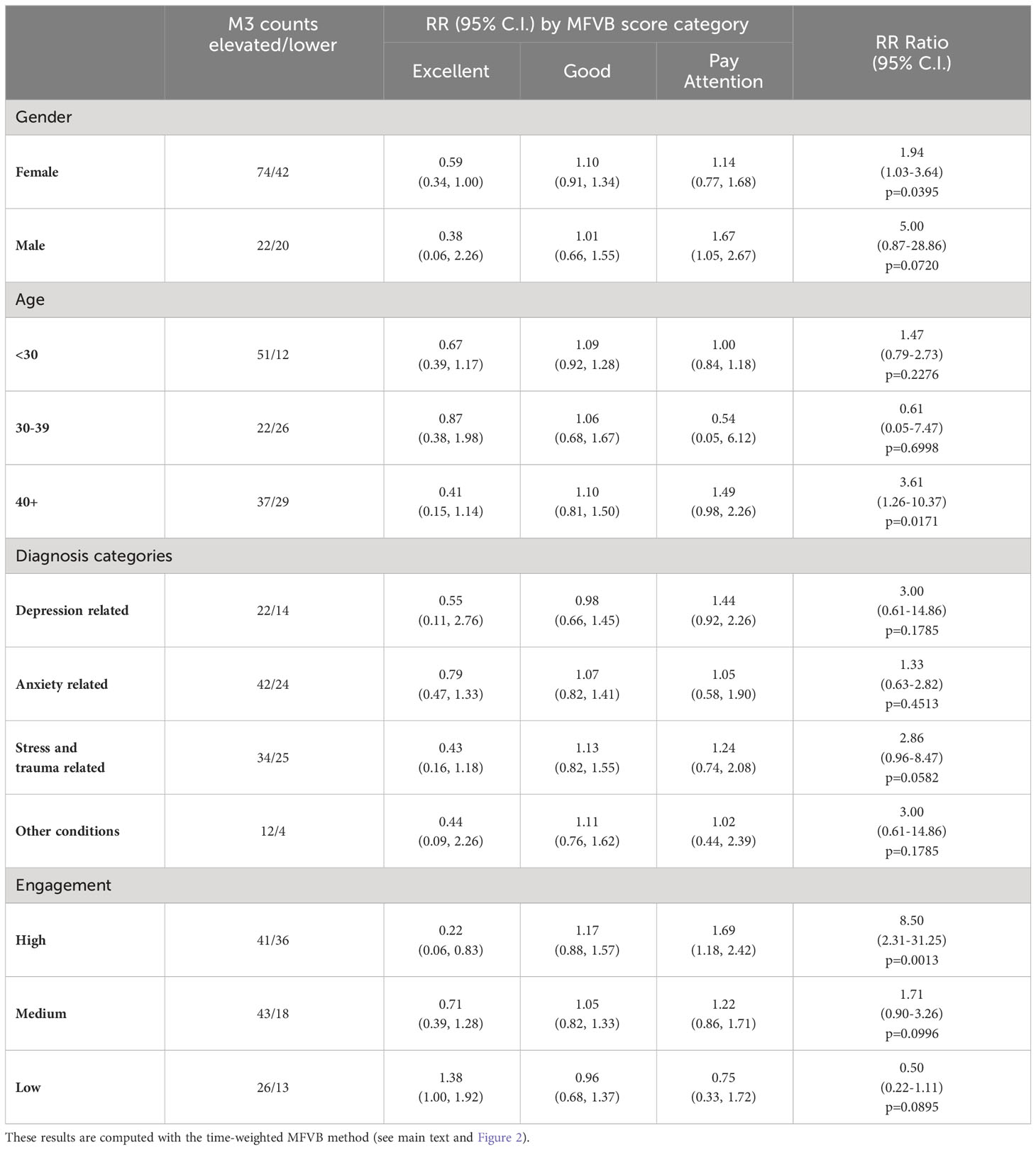
Table 8 Relative Risk (RR) and RR Ratio for MFVB score categories by selected subgrouping variables.
Analysis by age was hampered by the fact that the middle age group had only one MFVB score in the “Pay Attention” range, and the estimated RR Ratio at 0.61 for this age category has very wide confidence intervals (0.05-7.47, p=0.2276). The younger and older age groups were somewhat better balanced in MFVB scores, and both had RR Ratios consistent with the population at 1.47 and 3.61, the latter being statistically significant.
3.8.2 Clinical diagnosis
Subgrouping by clinical diagnosis revealed the most consistent relationship between MFVB score category and RR for depression and stress- and trauma-related conditions, although neither reached statistical significance. For conditions in the anxiety and “other” categories, RR for MFVB scores of “Excellent” and “Good” were as expected (RR<1.0 and ~1.0, respectively) but showed little increase in the “Pay Attention” range.
Note that the analysis reported here using total M3 score stratified by clinical diagnosis differs from the previously reported analysis by mental health symptom type (Sec. 3.5.2, Table 5), where RR estimates were reported on the entire analysis cohort (all clinical diagnoses combined) separated by symptom type. That said, both analyses indicate best performance in depression and stress- and trauma-related categories, followed by anxiety.
While such correspondence in MFVB performance measured either as a function of clinical diagnosis or symptom type appears reasonable on grounds that patients with a given diagnosis would be expected to have the strongest symptoms within that condition, other factors that may impact MFVB effectiveness—such as engagement with study procedures, proper use of the study app, overall severity of symptoms or ability to provide accurate self-reported information—could differ between these subgroups.
3.8.3 Engagement level
Subgrouping by engagement level reveals that for the high engagement group the RR estimates are considerably more pronounced than for the overall study sample. This subgroup has an RR Ratio of 8.50 (2.31-31.25, p=0.0013). For the medium engagement subgroup a less pronounced RR Ratio of 1.71 (0.90-3.26, p=0.0996) is observed, and finally in the low engagement subgroup an RR Ratio <1.0 is observed but with wide confidence intervals. This subgroup is characterized by higher symptom burden and fewer M3 scores in the normal-to-mild range, posing limitations on accurate RR estimation.
To investigate whether the marked RR improvements with increasing engagement were due to having more MFVB scores available for association with M3 results during the 2-week aggregation window or other factors, RR estimates and RR Ratios were calculated as function of engagement group using the closest-MFVB approach, which uses a single MFVB score only with each M3 result. This approach should diminish the potential advantage of more MFVB scores in the high engagement subgroup. Indeed, this yielded lower RR Ratios of 2.5, 1.3, and 0.90 for the high, medium and low engagement subgroups, suggesting the important contribution of aggregating MFVB results over time.
To further balance the comparison between engagement groups, the closest-MFVB analysis was conducted again using the onboarding M3 only, as the low engagement group was comprised of a large proportion of non-completers which did not provide responses to the offboarding survey at the end of week 4. Isolating the analysis to the onboarding M3 only (which was provided by all participants) will further equalize the data available for association of MFVB results to M3 assessments. This yielded an additional decrease in RR Ratios to 1.5, 1.2, and 0.79 for the high, medium, and low engagement subgroups, respectively. This further solidifies the notion that aggregation of MFVB results over time is the main driver for improving effectiveness of vocal analysis.
4 Discussion
4.1 Vocal biomarker scores stratify participants into mental health symptom severity categories
This study marks a significant step forward in the practical application of vocal biomarker technology through the use of a smartphone-based tool within a prospective cohort. By analyzing 30-second free speech voice recordings, the tool provides participants with immediate quantitative feedback on their mental health symptom severity. The correlation between the Mental Fitness Vocal Biomarker (MFVB) scores and established mental health assessments was not only statistically significant but also meaningful for participants. The tool maintained participant engagement over the duration of the 4-week study, with participants expressing mostly favorable opinions and noting various tangible benefits. This research overcomes previous limitations in the field by successfully operationalizing voice-related feature analysis into a user-friendly technology and testing in a longitudinal study, a combined approach that has not been documented in published literature to date.
A few recent studies share some similarities with our work. One recent smartphone app-based speech analysis approach used semantic analysis to screen for depression and anxiety in an older demographic, requiring 5-minute voice recordings, which were then compared with PHQ-8 and GAD-7 scales for validation (63). While this study demonstrated good performance, it differed from our methodology by not utilizing acoustic features and providing only binary outcomes, limiting its utility as a personalized mental wellness resource. In contrast, our approach requires substantially less recording time by focusing on acoustic and prosodic features. A uniquely long-term (6-month) study in bipolar patients tested whether a machine-learning based vocal biomarker approach could make use of repeated observations over time to identify euthymia, hypomania, and manic episodes in participants via weekly telephone interactions (64), and reported good performance for differentiating hypomania and depression from euthymia. As we found in our study, continuous observations over time proved essential, especially for bipolar disorder, characterized by fluctuating mood over time. Our study not only reaffirms the value of continuous data collection but also expands on it by providing immediate feedback to users, enhancing the application’s role in regular mental health self-assessment and management.
Our method, averaging normalized values of eight vocal features from a 30-second speech sample (see Supplementary Material), facilitates measurement of moderate-to-severe (elevated) mental health symptom severity in terms of Relative Risk (RR), laying a foundation for application-specific products that offer objective and quantitative mental fitness information. RR offers comparative understanding of the likelihood of elevated symptom severities being present within a range of Mental Fitness Vocal Biomarker (MFVB) scores compared to an overall population average.
The use of RR instead of accuracy or sensitivity and specificity, typically employed as outcome measures in vocal biomarker studies, is appropriate given the likely intended uses, which would not primarily involve the identification or differentiation of mental health conditions. The underlying vocal features were selected based on reported correlations with depression but do not have well-supported threshold values that separate clinical categories. Interpretation of MFVB score in terms of RR avoids potentially incorrect interpretation by users that a high (or low) MFVB score rules out (or in) the presence of a mental health condition, whereas the risk concept more appropriately conveys a change in likelihood of underlying symptoms.
We found that MFVB scores also stratified symptom severities within subdomains of depression, anxiety, and PTSD. The RR Ratio was greatest for depression, perhaps explained by the fact the underlying vocal features were primarily selected based on pre-existing evidence for that condition. What is unique about the current study is the transdiagnostic participant sample, applying a uniform vocal processing approach to demonstrate MFVB efficacy for multiple and potentially comorbid mental health conditions. Another differentiating feature of our work is the inclusion of diagnosed individuals only, without healthy controls, aiming to differentiate symptom severity rather than diagnosis status.
4.2 Optimal vocal biomarker performance requires frequent observation over time
We employed two methods for linking MFVB scores to M3 results: one associating a single MFVB score closest in time to the M3, and the other averaging all MFVB results within 2 weeks before or after the M3 (applying weights to diminish the contributions of more distant MFVB scores). Both approaches affirm that MFVB scores provide information about symptom severity that is aligned with validated mental health assessments. Notably, the time-weighted method exhibits superior risk stratification ability, capitalizing on the accumulation of vocal information over time and aligning more closely with the M3 time window. This distinction, pointing toward the significance of longitudinal observations, is not merely an accidental statistical outcome but an expected behavior. Analogous to understanding a region’s climate, reliable insights into overall mental fitness—the backdrop against which day-to-day mood fluctuations occur—require frequent observation over time. Vocal biomarker tools are inherently well positioned to gather and refine insights over time, emphasizing the need for future studies and product designs to leverage this intrinsic quality.
Our results confirmed the utility of frequent measurement over time in another way, by revealing significantly enhanced RR estimates in participants that were more highly engaged with the study app. We were able to determine that the enhancement was primarily the result of more frequent “observation” (voice sample recordings). This is good news, because it implies that improvements in MFVB performance can be achieved by boosting engagement for users that would otherwise have low engagement levels. Achieving this goal will require research into strategies that can increase engagement, perhaps tailored to specific user characteristics. An alternative option is to develop passive voice monitoring tools that can continuously observe the user’s voice in the background, once consent has been provided. This would remove the engagement element completely and allow a maximum of observation to occur for all users.
4.3 Participant engagement factors
As we found above, achieving and sustaining high engagement is essential for many health apps, but often proves challenging in practice. Although the duration of this study is relatively brief, the observed engagement levels are promising. This is evident from the proportion of participants engaged, which combined the mid and high engagement groups, reaching 71%, and the persistence of that engagement, with an observed 87% retention in week 4 averaged across these two groups.
These metrics compare favorably to most other app-based or mHealth studies (65, 66). Study design factors that have previously been found to promote retention that were present in this study are the recruitment method (study invitation sent from participant’s clinical treatment organization), financial compensation for participation, and having a relevant condition to the app design (mental health concerns). Aside from study design factors, a potentially important factor was delivering real-time useful information (MFVB scores) back to users through the study app, a characteristic not shared by many other digital health apps (65, 66). The combination of insight and engagement that this approach fosters could be a natural way to enhance the perceived value and success of other digital therapeutic products.
Although participants were offered a financial incentive of $15 per week to encourage app usage of 4 or more times weekly, the incentive did not drive high levels of engagement among all participants. The proportion of participants receiving gift cards in each week was, not surprisingly, correlated with engagement level. That proportion was about 80% in the high engagement group, about 40% in the medium engagement group, and near zero in the low engagement group. Further, a potential small positive effect on app usage in weeks where a gift card was not received for the prior week was observed for participants in the low and medium engagement groups.
Engagement was negatively correlated with mental health symptom severity, both for total symptoms and within symptom categories. In particular, the prevalence of elevated depressive symptoms was relatively high in the low engagement subgroup, consistent with previous findings on barriers to engagement with digital mental health interventions (67). Individuals experiencing more severe symptoms may find it challenging to engage consistently with the study app due to the potential impact of their mental health condition on motivation, energy levels, and overall well-being. Higher symptom severity often correlates with increased psychological distress and reduced functional capacity, making it difficult for individuals to sustain regular and active participation in the study.
Engagement was positively correlated with age, an effect that has been observed previously in digital health app studies (65, 66); although it is contrary to conventional expectations that it is more difficult to engage older populations with digital tools. In this study, age was also negatively correlated with symptom severity; it is therefore possible that age and symptom severity both reinforced the observed engagement trends.
Varying engagement levels observed in this study underscore the importance of tailoring the user experience to individual needs. Although MFVB scores demonstrate efficacy across a spectrum of mental symptom categories and severities, a uniform solution approach may not optimally engage and thus fall short for users facing particular mental health challenges. Better understanding and addressing the engagement barriers would help to create solutions that are not only more accessible and inclusive but also resonate strongly with those who may stand to benefit the most.
4.4 Vocal biomarker perception and benefits as a function of engagement
Comments made by participants from the high engagement group indicate a strong commitment to the Mental Fitness app, appreciating the daily check-ins and addition to their daily routines. They found the app beneficial for tracking their mental wellbeing and for self-expression. Participants in the medium engagement group displayed more mixed sentiments, with concerns about notifications and scoring accuracy affecting their overall engagement. This group also indicated the lowest level of agreement with the statement that the MFVB scores are helpful in understanding how they are doing. The low engagement group participants exhibited the least enthusiasm in their feedback, with limited app use due to forgetfulness or perceived inconvenience, although some participants still acknowledged potential benefits from more frequent use.
Although the Mental Fitness app nor the study were positioned as supplement to mental health treatment, about half of the study participants organically discovered meaningful ways to incorporate the app into their treatment regimens, considering that 55% of participants agreed that it was a helpful addition to their treatment. Participants that frequently used the app said that they generally found the app to be a valuable addition to their treatment as a tool for self-reflection, providing reminders to relax and a way to practice the skills they worked on in therapy. It helped them focus on positive thoughts and stay in a mental health routine. Less engaged users also appreciate the tool as a platform to voice thoughts and feelings but did not perceive it as contributing to their treatment as much, either because they did not use it as frequently or because they viewed it more as a mental exercise.
We may conclude that engagement with digital health tools like the MFVB app is strongly influenced by perceived relevance of features to the individual user’s needs and preferences, consistent with previous findings (65, 66). On the other hand, the feedback has also indicated user-driven discovery of value beyond the initial app design intentions, which can lead to strong engagement and satisfaction.
4.5 Limitations
4.5.1 Study population
The main limitations of the present study are related to the study population, which was an outpatient psychiatric sample with limited demographic diversity in race and ethnicity. Although a large age range was represented (16–80), most participants were young adults to early middle age. All were native or fluent English speakers and clients of a mental health care provider based in Pittsburgh, PA. On the other hand, the MFVB scoring algorithm was developed based on vocal feature analysis from a large Indian outpatient population speaking 5 different Indian languages, which would suggest that our results are not particularly sensitive to linguistic, geographic, or cultural differences. Reliance on acoustic features vs. linguistic analysis is likely a major contributing factor to this robustness. Given the marked difference in the development and validation cohorts, we hypothesize that many of the findings in this report would generalize to other populations as well.
The study implemented certain exclusion criteria that might have influenced the vocal attributes of the analysis cohort. However, the applied exclusions pertained exclusively to the use of prohibited medications, impacting a small subset of potential participants (n=5). Note that the use of other medications was permitted, with most participants (n=76) reporting such use. Given the limited scope and number of exclusions, their effect on the principal outcomes of the study is likely minimal.
The clinical population included in this study may exhibit certain characteristics that would be different in a more general population. For example, the study participants are actively engaged in mental health treatment and volunteered for the study, which suggests a certain level of motivation to engage with tools intended for mental fitness tracking. They were also financially incentivized to use the app. Other populations may exhibit different interests and uses for the tool studied here. We also found that those participants with more severe symptoms, in particular those related to depression, were on average less engaged relative to the participants with milder symptoms. These participants with milder symptoms may share many characteristics with a portion of the general population, given estimated levels of depression and anxiety in United States in the 30-35% range (68).
4.5.2 Study app functionality and user interface
Our findings regarding engagement levels and participant feedback are influenced both by the MFVB scoring algorithm and the overall app experience. Because our MFVB tool was presented as a voice-to-text journaling app, many participants reported valuing the combination of recording thoughts and moods, self-reflection, and tracking their wellbeing through the MFVB scores. Users also valued other aspects including notifications to help build habits, tips for healthy activities and behaviors, etc. This points to the need to consider the entire product experience and user journey in the context of digital health products like vocal biomarker assessment. Because this app was built primarily as demonstration and limited research tool, the positive reception and usage levels would likely further increase in an application more optimized for user experience and customization.
4.5.3 Lack of diagnostic specificity
Our results demonstrate a general ability of MFVB score categories to differentiate mental health symptom severity levels, but do not distinguish what type of symptoms these may be (e.g., depression, anxiety, PTSD, or bipolar). The eight acoustic and prosodic features that underlie the MFVB score algorithm (see Supplementary Material) capture enough voice acoustic changes that accompany the presence of these conditions that the resulting MFVB score is generally responsive transdiagnostically, serving as a kind of “mental wellbeing thermometer”. Rather than viewing the lack of diagnostic specificity as a limitation, it is a valuable feature if the intended use is to provide broad mental symptom severity information to users rather than serve as a diagnostic tool. Its broad applicability and possible use cases can accelerate validation and adoption versus tools that are more narrowly focused.
The 8 vocal features that underlie the MFVB score may still provide information that could differentiate among common mood disorders, although no existing work is known to the authors that has demonstrated such capability. In a vocal biomarker study of people with depression, bipolar, schizophrenia and healthy controls, it was found that it was possible to train classification models to differentiate the disordered participants from healthy controls, but not depression from bipolar (69). Schizophrenia could be differentiated from depression and bipolar but falls outside the scope of common mood disorders that is being targeted with our Mental Fitness approach.
4.6 Potential applications and future work
4.6.1 Application examples
While specific applications may require additional studies and validation, the positive findings reported here suggest several potential use cases of MFVB tools. The most obvious might be the one in which this study was conducted: mental health treatment, where patients can benefit from objective insights into their mental wellbeing over time. The information may also be useful to providers, allowing a complementary source of insight and, through a non-fatiguing assessment at relatively greater time resolution, filling temporal gaps between treatment sessions. Additionally, even users who are not actively undergoing mental health treatment may benefit from monitoring their mental fitness. Further exploration could involve integrating MFVB scores into workplace wellness programs, offering employers a tool to support employee mental health. This technology could be integrated into digital health platforms and wearables, extending the reach of these offerings and offering a more holistic view of well-being that includes mental aspects as well as traditional fitness measures such as step count, sleep, and cardiovascular metrics. Another avenue for exploration is the development of prognostic outcomes that could be used, for example, in primary care to identify those individuals most likely to be diagnosed with a mental disorder upon referral to a specialist. The potential extends to services targeting adolescents and college-age populations, considering that approximately 20% of our study participants belonged to this age range, with results consistent with the overall study cohort.
Because the MFVB algorithm is by design intended to broadly measure mental health symptom severity and is not trained or tuned to a specific outcome, its adoption across potentially multiple use cases could be relatively faster vs. a new and unique tool for each scenario. As the aforementioned “mental wellbeing thermometer”, the MFVB approach would function as a standard component integrated into a range of platforms designed for different applications.
4.6.2 Vocal biomarkers in the background
While we have shown that the active journaling component of the MFVB approach is valued by many users, some did not use it and others forgot to use the app consistently or found it difficult to incorporate into their daily life. Sonde Health has already developed passive measurement capabilities to analyze user voice in the background, which allows the MFVB results to be provided without active user engagement. The technology runs on edge devices rather than hosted on cloud platforms, eliminating the need for recording or transmitting voice data. This not only addresses privacy concerns but also aligns with evolving data protection standards. Passive monitoring also includes user voice identification to ensure that analysis pertains solely to the intended user, further enhancing confidentiality of the mental fitness solutions. Future studies will incorporate such passive measurement capabilities to address engagement and privacy challenges.
4.6.3 Optimizing processing and scoring algorithms
This study did not seek to optimize MFVB scoring algorithms to maximize risk stratification abilities; therefore, a relatively simple combination of pre-selected voice acoustic features was used for scoring. The current algorithm’s simplicity likely contributed to its robustness from the calibration data set (outpatient clinics across regions in India) to this validation cohort. Additionally, this study’s positive results are an encouraging foundation for future algorithm development, including machine learning or artificial intelligence approaches, to improve alignment of MFVB scores and mental health symptoms. The study app also did not include any voice sample quality control mechanisms to reject recordings with high background noise, which, if present, could negatively impact score accuracy. Including such control mechanisms may further enhance MFVB score performance.
4.6.4 Personalized change detection
The MFVB scoring algorithm in the study app, derived from cross-sectional data, assigns scores and category labels based on population vocal feature distributions, limiting direct applicability to individual users due to voice variations and smartphone device differences across users. While participants found tracking MFVB changes over time useful, determining the clinical significance of individual score changes was not possible. Longitudinal studies, assessing concurrent symptom and vocal changes, are needed. The study’s 4-week duration was inadequate to capture meaningful mental condition changes for most participants; for instance, 74% of participants did not change in symptom severity category, perhaps because most participants had been in treatment for an extended period of time already (66% >1 year, Table 2). A 12-week extension phase of the study aims to assess individual changes and potentially inform personalized scoring algorithms and will be reported in future communications.
4.6.5 Contributing to mental wellbeing
Given the positive experiences participants reported with the MFVB tool, it would be of interest to study if and how mental fitness tracking might improve mental wellbeing, akin to the well-establish impact that step-count monitoring has on physical activity and its attendant physical health benefits (40). Via the study feedback, approximately 40% of participants reported making some change in their behavior or lifestyle as a result of using the MFVB tool and approximately 30% mentioned perceived benefits to their wellbeing – while neither of these themes were specifically included as questions in the survey. These are remarkable statistics for a basic smartphone app that merits follow-up studies designed to measure the potential impact of an MFVB tool as health intervention.
5 Conclusions
We have demonstrated that a Mental Fitness Vocal Biomarker (MFVB) scoring algorithm, using pre-selected vocal features reported in the literature, incorporated into a smartphone voice journaling application can indicate increased or decreased risk for elevated mental health symptom severity using a single 30-second voice sample. This risk stratification improves by aggregating recordings over a 2-week period and is particularly effective for users who frequently engage with the tool. Similar performance was observed within mental symptom categories of depression, anxiety, and PTSD. Most study participants were consistently engaged and enjoyed using the tool, finding the MFVB scores helpful in understanding how they were doing. Participants mentioned benefits such as creating helpful daily routines, therapeutic benefits from self-expression, and valuing the fact that they could contrast their self-perception with an objective assessment.
While the MFVB tool is not intended to diagnose or treat mental health conditions, these findings provide a robust initial foundation upon which to further explore its potential in personalized wellness tracking, which has so far not yet been able to extend measurement of physical health to mental wellbeing. Its functional appeal, consistent use, and positive reception highlight its significant potential to benefit users. The key insight from this work – aggregating MFVB scores over time and integrating information amid day-to-day mood variations – is an important factor in attaining optimal performance levels. Vocal biomarker-based wellness tools are well-suited for continuous observation, engaging users to cultivate healthy habits and behaviors, ultimately contributing to improved wellbeing.
Data availability statement
The datasets presented in this article are not readily available because a data use agreement with Sonde Health is required, which will be granted upon reasonable request. Audio recordings will not be made available to safeguard participant privacy. Requests to access the datasets should be directed to Erik Larsen, elarsen@sondehealth.com.
Ethics statement
The studies involving humans were approved by WCG IRB (Western Copernicus Group). The studies were conducted in accordance with the local legislation and institutional requirements. Electronic informed consent for participation in this study was provided by participants aged 18 and over, and by the participants' legal guardians if under 18 years of age.
Author contributions
EL: Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Writing – original draft. OM: Data curation, Investigation, Visualization, Writing – original draft. XS: Data curation, Formal Analysis, Visualization, Writing – review & editing. DJ: Methodology, Writing – review & editing. DW: Funding acquisition, Investigation, Writing – review & editing. FK: Supervision, Writing – review & editing. LV: Data curation, Project administration, Resources, Writing – review & editing. GH: Conceptualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The pilot phase of the study at St Joseph’s Healthcare Hamilton was partially supported through the Mitacs Accelerate International -from Canada program (ref: IT29278).
Acknowledgments
The authors wish to acknowledge Krushnakant Mardikar and Mrityunjay Samanta from gslab (Pune, Maharashtra, India) for significant contributions to data analysis and signal processing methods.
Conflict of interest
EL, OM, XS, and DJ receive employment income and hold equity from Sonde Health, the developer of the Mental Fitness study app. GH is Chief Medical Officer of M3 Information.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2024.1342835/full#supplementary-material
Footnotes
- ^ The RR Ratio as defined in this paragraph is equivalent to the risk ratio between the “Pay Attention” and “Excellent” categories, as the reference group used in the relative risk calculation factors out when calculating this ratio. However, in some subgroup analyses the contingency tables include zeroes that would cause computational problems, and in those cases a value of 0.5 is added to all cells. This is done separately for the calculation of relative risk by MFVB category and risk ratio between the aforementioned MFVB categories. This results in a few instances where the ratio of reported relative risks values for “Pay Attention” vs. “Excellent” appear numerically different from the reported RR Ratio values (which are calculated with a different contingency table).
References
1. Low DM, Bentley KH, Ghosh SS. Automated assessment of psychiatric disorders using speech: A systematic review. Laryngoscope Investig Otolaryngol. (2020) 5:96–116. doi: 10.1002/lio2.354
2. Fagherazzi G, Fischer A, Ismael M, Despotovic V. Voice for health: the use of vocal biomarkers from research to clinical practice. Digit biomark. (2021) 5:78–88. doi: 10.1159/000515346
3. Trevino AC, Quatieri TF, Malyska N. Phonologically-based biomarkers for major depressive disorder. EURASIP J Adv Signal Process. (2011) 2011:42. doi: 10.1186/1687-6180-2011-42
4. Mundt JC, Vogel AP, Feltner DE, Lenderking WR. Vocal acoustic biomarkers of depression severity and treatment response. Biol Psychiatry. (2012) 72:580–7. doi: 10.1016/j.biopsych.2012.03.015
5. Williamson JR, Quatieri TF, Helfer BS, Horwitz R, Yu B, Mehta DD. Vocal biomarkers of depression based on motor incoordination. In: Proceedings of the 3rd ACM international workshop on Audio/visual emotion challenge. Barcelona Spain: ACM. (2013), 41–8. doi: 10.1145/2512530.2512531
6. Huang Z, Epps J, Joachim D. (2020). Exploiting vocal tract coordination using dilated CNNS for depression detection in naturalistic environments, in: ICASSP 2020 - 2020 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP), Barcelona, Spain. pp. 6549–53. IEEE.
7. Huang Z, Epps J, Joachim D, Sethu V. Natural language processing methods for acoustic and landmark event-based features in speech-based depression detection. IEEE J Sel Top Signal Process. (2020) 14:435–48. doi: 10.1109/JSTSP.2019.2949419
8. Taguchi T, Tachikawa H, Nemoto K, Suzuki M, Nagano T, Tachibana R, et al. Major depressive disorder discrimination using vocal acoustic features. J Affect Disord. (2018) 225:214–20. doi: 10.1016/j.jad.2017.08.038
9. Cho G, Yim J, Choi Y, Ko J, Lee SH. Review of machine learning algorithms for diagnosing mental illness. Psychiatry Investig. (2019) 16:262–9. doi: 10.30773/pi.2018.12.21.2
10. Arevian AC, Bone D, Malandrakis N, Martinez VR, Wells KB, Miklowitz DJ, et al. Clinical state tracking in serious mental illness through computational analysis of speech. PloS One. (2020) 15:e0225695. doi: 10.1371/journal.pone.0225695
11. Huang Z, Epps J, Joachim D. Investigation of speech landmark patterns for depression detection. IEEE Trans Affect Comput. (2022) 13:666–79. doi: 10.1109/TAFFC.2019.2944380
12. Zhao Q, Fan HZ, Li YL, Liu L, Wu YX, Zhao YL, et al. Vocal acoustic features as potential biomarkers for identifying/diagnosing depression: A cross-sectional study. Front Psychiatry. (2022) 13:815678. doi: 10.3389/fpsyt.2022.815678
13. Stasak B, Epps J, Goecke R. An investigation of linguistic stress and articulatory vowel characteristics for automatic depression classification. Comput Speech Lang. (2019) 53:140–55. doi: 10.1016/j.csl.2018.08.001
14. Sverdlov O, Curcic J, Hannesdottir K, Gou L, De Luca V, Ambrosetti F, et al. A study of novel exploratory tools, digital technologies, and central nervous system biomarkers to characterize unipolar depression. Front Psychiatry. (2021) 12:640741. doi: 10.3389/fpsyt.2021.640741
15. Chen ZS, Kulkarni P, Galatzer-Levy IR, Bigio B, Nasca C, Zhang Y. Modern views of machine learning for precision psychiatry. Patterns. (2022) 3:100602. doi: 10.1016/j.patter.2022.100602
16. Caldeira C, Chen Y, Chan L, Pham V, Chen Y, Zheng K. Mobile apps for mood tracking: an analysis of features and user reviews. AMIA Annu Symp Proc. (2017) 2017:495.
17. Schueller SM, Neary M, Lai J, Epstein DA. Understanding people’s use of and perspectives on mood-tracking apps: interview study. JMIR Ment Health. (2021) 8:e29368. doi: 10.2196/29368
18. Toles LE, Roy N, Sogg S, Marks KL, Ortiz AJ, Fox AB, et al. Relationships among personality, daily speaking voice use, and phonotrauma in adult female singers. J Speech Lang Hear Res. (2021) 64:4580–98. doi: 10.1044/2021_JSLHR-21-00274
19. Hillman RE, Stepp CE, Van Stan JH, Zañartu M, Mehta DD. An updated theoretical framework for vocal hyperfunction. Am J Speech Lang Pathol. (2020) 29:2254–60. doi: 10.1044/2020_AJSLP-20-00104
20. Goldman SL, Hargrave J, Hillman RE, Holmberg E, Gress C, ASHA Wire, et al. Stress, Anxiety, Somatic Complaints, and Voice Use in Women with Vocal Nodules. Am J Speech Lang Pathol. (2018) 5(1):44–54. doi: 10.1044/1058-0360.0501.44.
21. Alves M, Krüger E, Pillay B, van Lierde K, van der Linde J. The effect of hydration on voice quality in adults: A systematic review. J Voice. (2019) 33:125.e13–125.e28. doi: 10.1016/j.jvoice.2017.10.001
22. Abaza MM, Levy S, Hawkshaw MJ, Sataloff RT. Effects of medications on the voice. Otolaryngol Clin North Am. (2007) 40:1081–90. doi: 10.1016/j.otc.2007.05.010
23. Nemr K, Di Carlos Silva A, Rodrigues DDA, Zenari MS. Medications and adverse voice effects. J Voice. (2018) 32:515.e29–515.e39. doi: 10.1016/j.jvoice.2017.07.009
24. Sinha P, Vandana VP, Lewis NV, Jayaram M, Enderby P. Predictors of effect of atypical antipsychotics on speech. Indian J Psychol Med. (2015) 37:429–33. doi: 10.4103/0253-7176.168586
25. De Boer JN, Voppel AE, Brederoo SG, Wijnen FNK, Sommer IEC. Language disturbances in schizophrenia: the relation with antipsychotic medication. NPJ Schizophr. (2020) 6:24. doi: 10.1038/s41537-020-00114-3
26. Rocha BR, Behlau M. The influence of sleep disorders on voice quality. J Voice. (2018) 32:771.e1–771.e13. doi: 10.1016/j.jvoice.2017.08.009
27. Holmqvist S, Santtila P, Lindström E, Sala E, Simberg S. The association between possible stress markers and vocal symptoms. J Voice. (2013) 27:787.e1–787.e10. doi: 10.1016/j.jvoice.2013.06.012
28. Cohen AS, McGovern JE, Dinzeo TJ, Covington MA. Speech deficits in serious mental illness: A cognitive resource issue? Schizophr Res. (2014) 160:173–9. doi: 10.1016/j.schres.2014.10.032
29. Caligiuri MP, Ellwanger J. Motor and cognitive aspects of motor retardation in depression. J Affect Disord. (2000) 57:83–93. doi: 10.1016/S0165-0327(99)00068-3
30. Breznitz Z, Sherman T. Speech patterning of natural discourse of well and depressed mothers and their young children. Child Dev. (1987) 58:395. doi: 10.2307/1130516
31. Greden JF, Carroll BJ. Decrease in speech pause times with treatment of endogenous depression. Biol Psychiatry. (1980) 15:575–87.
32. Hoffman GMA, Gonze JC, Mendlewicz J. Speech pause time as a method for the evaluation of psychomotor retardation in depressive illness. Br J Psychiatry. (1985) 146:535–8. doi: 10.1192/bjp.146.5.535
33. Alpert M, Pouget ER, Silva RR. Reflections of depression in acoustic measures of the patient’s speech. J Affect Disord. (2001) 66:59–69. doi: 10.1016/S0165-0327(00)00335-9
34. Darby JK, Simmons N, Berger PA. Speech and voice parameters of depression: A pilot study. J Commun Disord. (1984) 17:75–85. doi: 10.1016/0021-9924(84)90013-3
35. Beitchman JH, Brownlie EB, Bao L. Age 31 mental health outcomes of childhood language and speech disorders. J Am Acad Child Adolesc Psychiatry. (2014) 53:1102–10. doi: 10.1016/j.jaac.2014.07.006
36. Beitchman JH, Nair R, Clegg M, Ferguson B, Patel PG. Prevalence of psychiatric disorders in children with speech and language disorders. J Am Acad Child Psychiatry. (1986) 25:528–35. doi: 10.1016/S0002-7138(10)60013-1
37. Cantwell DP, Baker L, Mattison RE. The prevalence of psychiatric disorder in children with speech and language disorder an epidemiologic study. J Am Acad Child Psychiatry. (1979) 18:450–61. doi: 10.1097/00004583-197922000-00004
38. Nusseck M, Immerz A, Spahn C, Echternach M, Richter B. Long-term effects of a voice training program for teachers on vocal and mental health. J Voice. (2021) 35:438–46. doi: 10.1016/j.jvoice.2019.11.016
39. Bauer M, Glenn T, Geddes J, Gitlin M, Grof P, Kessing LV, et al. Smartphones in mental health: a critical review of background issues, current status and future concerns. Int J Bipolar Disord. (2020) 8:2. doi: 10.1186/s40345-019-0164-x
40. Chaudhry UAR, Wahlich C, Fortescue R, Cook DG, Knightly R, Harris T. The effects of step-count monitoring interventions on physical activity: systematic review and meta-analysis of community-based randomised controlled trials in adults. Int J Behav Nutr Phys Act. (2020) 17:129. doi: 10.1186/s12966-020-01020-8
41. Eyben F, Wöllmer M, Schuller B. Opensmile: the munich versatile and fast open-source audio feature extractor. In: Proceedings of the 18th ACM international Conference on Multimedia. Association for Computing Machinery, New York, NY, USA (2010). p. 1459–62. doi: 10.1145/1873951.1874246
42. Boersma P, Weenink D. Praat: doing phonetics by computer(2023). Available online at: http://www.praat.org.
43. Quatieri TF, Malyska N. Vocal-source biomarkers for depression: a link to psychomotor activity. In: Interspeech 2012. ISCA (2012). p. 1059–62. Available at: https://www.isca-speech.org/archive/interspeech_2012/quatieri12_interspeech.html.
44. Ozdas A, Shiavi RG, Silverman SE, Silverman MK, Wilkes DM. Investigation of vocal jitter and glottal flow spectrum as possible cues for depression and near-term suicidal risk. IEEE Trans BioMed Eng. Thirteenth annual conference of the international speech communication association. (2004) 51:1530–40. doi: 10.1109/TBME.2004.827544
45. Kiss G, Vicsi K. Mono- and multi-lingual depression prediction based on speech processing. Int J Speech Technol. (2017) 20:919–35. doi: 10.1007/s10772-017-9455-8
46. Horwitz R, Quatieri TF, Helfer BS, Yu B, Williamson JR, Mundt J. (2013). On the relative importance of vocal source, system, and prosody in human depression, in: 2013 IEEE International Conference on Body Sensor Networks, Cambridge, MA, USA. pp. 1–6. IEEE. Available at: http://ieeexplore.ieee.org/document/6575522/.
47. Mundt JC, Snyder PJ, Cannizzaro MS, Chappie K, Geralts DS. Voice acoustic measures of depression severity and treatment response collected via interactive voice response (IVR) technology. J Neurolinguistics. (2007) 20:50–64. doi: 10.1016/j.jneuroling.2006.04.001
48. Cannizzaro M, Harel B, Reilly N, Chappell P, Snyder PJ. Voice acoustical measurement of the severity of major depression. Brain Cogn. (2004) 56:30–5. doi: 10.1016/j.bandc.2004.05.003
49. Kuny St, Stassen HH. Speaking behavior and voice sound characteristics in depressive patients during recovery. J Psychiatr Res. (1993) 27:289–307. doi: 10.1016/0022-3956(93)90040-9
50. Vlasenko B, Sagha H, Cummins N, Schuller B. Implementing gender-dependent vowel-level analysis for boosting speech-based depression recognition. In: Interspeech 2017. ISCA (2017). p. 3266–70. Available at: https://www.isca-speech.org/archive/interspeech_2017/vlasenko17_interspeech.html.
51. Scherer S, Morency LP, Gratch J, Pestian J. (2015). Reduced vowel space is a robust indicator of psychological distress: A cross-corpus analysis, in: 2015 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP), South Brisbane, Queensland, Australia. pp. 4789–93. IEEE. Available at: http://ieeexplore.ieee.org/document/7178880/.
52. Themistocleous C, Eckerström M, Kokkinakis D. Voice quality and speech fluency distinguish individuals with Mild Cognitive Impairment from Healthy Controls. PloS One. (2020) 15:e0236009. doi: 10.1371/journal.pone.0236009
53. Yu B, Quatieri TF, Williamson JR, Mundt JC. Cognitive impairment prediction in the elderly based on vocal biomarkers. In: Interspeech 2015. ISCA (2015). p. 3734–8. Available at: https://www.isca-speech.org/archive/interspeech_2015/yu15b_interspeech.html.
54. Toth L, Hoffmann I, Gosztolya G, Vincze V, Szatloczki G, Banreti Z, et al. A speech recognition-based solution for the automatic detection of mild cognitive impairment from spontaneous speech. Curr Alzheimer Res. (2018) 15:130–8. doi: 10.2174/1567205014666171121114930
55. Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig L, et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. Radiology. (2015) 277:826–32. doi: 10.1148/radiol.2015151516
56. Regier DA, Kuhl EA, Kupfer DJ. The DSM-5: Classification and criteria changes. World Psychiatry. (2013) 12:92–8. doi: 10.1002/wps.20050
57. Sullivan EV, Pfefferbaum A. Alcohol and the nervous system. (Handbook of clinical neurology) 2014 Edinburgh London New York: Elsevier.
58. Moreira TDC, Gadenz C, Figueiró LR, Capobianco DM, Cunha K, Ferigolo M, et al. Uso de substâncias psicoativas, alterações vocais e qualidade de vida em usuários de drogas lícitas e ilícitas. Rev CEFAC. (2015) 17:374–84. doi: 10.1590/1982-021620156714
59. SurveyLex. (2020). Available at: surveylex.com.
60. Ball K, MacPherson C, Hurowitz G, Settles B, DeVeaugh-Geiss J, Weir S, et al. M3 checklist and SF-12 correlation study. Best Practices in Mental Health.. (2015)11(1):83–9.
61. Gaynes BN, DeVeaugh-Geiss J, Weir S, Gu H, MacPherson C, Schulberg HC, et al. Feasibility and diagnostic validity of the M-3 checklist: A brief, self-rated screen for depressive, bipolar, anxiety, and post-traumatic stress disorders in primary care. Ann Fam Med. (2010) 8:160–9. doi: 10.1370/afm.1092
62. Smith DJ, Kyle S, Forty L, Cooper C, Walters J, Russell E, et al. Differences in depressive symptom profile between males and females. J Affect Disord. (2008) 108:279–84. doi: 10.1016/j.jad.2007.10.001
63. Lin D, Nazreen T, Rutowski T, Lu Y, Harati A, Shriberg E, et al. Feasibility of a machine learning-based smartphone application in detecting depression and anxiety in a generally senior population. Front Psychol. (2022) 13:811517. doi: 10.3389/fpsyg.2022.811517
64. Karam ZN, Provost EM, Singh S, Montgomery J, Archer C, Harrington G, et al. (2014). Ecologically valid long-term mood monitoring of individuals with bipolar disorder using speech, in: 2014 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP), Florence, Italy. pp. 4858–62. IEEE. Available at: http://ieeexplore.ieee.org/document/6854525/.
65. Amagai S, Pila S, Kaat AJ, Nowinski CJ, Gershon RC. Challenges in participant engagement and retention using mobile health apps: literature review. J Med Internet Res. (2022) 24:e35120. doi: 10.2196/35120
66. Pratap A, Neto EC, Snyder P, Stepnowsky C, Elhadad N, Grant D, et al. Indicators of retention in remote digital health studies: a cross-study evaluation of 100,000 participants. NPJ Digit Med. (2020) 3:21. doi: 10.1038/s41746-020-0224-8
67. Borghouts J, Eikey E, Mark G, De Leon C, Schueller SM, Schneider M, et al. Barriers to and facilitators of user engagement with digital mental health interventions: systematic review. J Med Internet Res. (2021) 23:e24387. doi: 10.2196/24387
68. Mental Health - Household Pulse Survey - COVID-19. (2023). Available online at: https://www.cdc.gov/nchs/covid19/pulse/mental-health.htm.
Keywords: mental fitness, digital health, mHealth, user engagement, transdiagnostic assessment, longitudinal observation, fitness tracking, vocal biomarkers
Citation: Larsen E, Murton O, Song X, Joachim D, Watts D, Kapczinski F, Venesky L and Hurowitz G (2024) Validating the efficacy and value proposition of mental fitness vocal biomarkers in a psychiatric population: prospective cohort study. Front. Psychiatry 15:1342835. doi: 10.3389/fpsyt.2024.1342835
Received: 22 November 2023; Accepted: 14 February 2024;
Published: 05 March 2024.
Edited by:
Ward Van Breda, VU Amsterdam, NetherlandsReviewed by:
Claire R. van Genugten, VU Amsterdam, NetherlandsYael Bensoussan, University of Southern California, United States
Copyright © 2024 Larsen, Murton, Song, Joachim, Watts, Kapczinski, Venesky and Hurowitz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Erik Larsen, ZWxhcnNlbkBzb25kZWhlYWx0aC5jb20=
 Erik Larsen
Erik Larsen Olivia Murton1
Olivia Murton1 Dale Joachim
Dale Joachim Flavio Kapczinski
Flavio Kapczinski