- 1Department of Social Science of Physical Activity and Health, Institute of Human Movement Science and Health, Faculty of Behavioural and Social Sciences, Chemnitz University of Technology, Chemnitz, Germany
- 2BG Hospital for Occupational Disease Bad Reichenhall, Bad Reichenhall, Germany
Background: Rehabilitation is an effective and feasible approach for post-COVID patients to improve mental health and cognitive complaints. However, knowledge regarding the long-term impact of rehabilitation on neuropsychological health of these patients is lacking.
Objective: This study aims to investigate psychological health, fatigue, and cognitive function 6 and 12 months after inpatient post-COVID rehabilitation of patients, who acquired COVID-19 in the workplace. In addition, group differences in these outcome parameters according to sex, age, acute COVID status, socioeconomic status, profession, and pre-existing diseases will be detected.
Methods: This longitudinal observational study examined the changes in mental and cognitive health of 127 patients with COVID-19 as an occupational disease or work accident. Symptoms of depression and anxiety, fatigue severity, somatic symptom severity, trauma-related symptoms, and cognitive functioning were assessed at the beginning as well as six and 12 months after rehabilitation. Group differences concerning sex, age, acute COVID status, socioeconomic status, occupational status, and existing diseases prior to COVID-19 were also analyzed.
Results: The results showed that the improvements direct after rehabilitation in mental health and fatigue severity could not be maintained six and 12 months after rehabilitation discharge. Contrary, patients’ cognitive function maintained stable during follow-up. Significant group differences were observed regarding age, sex, acute COVID status, socioeconomic status, occupational status, and pre-existing diseases.
Conclusion: This study highlights the importance of the aftercare process and the implementation of adequate and individualized therapeutic interventions such as psychological support and strengthen self-management skills.
The study is registered in the German Clinical Trials Register with the identifier DRKS00022928.
1 Introduction
An infection with SARS-CoV-2 is primarily known as respiratory disease but it can also impact other organs and develop into a multisystem disease. The course of disease is quite unspecific, has a broad symptom range, and can impact patients’ health and quality of life in a long term (1). According to national and international guidelines persistent manifestations and symptoms following an acute SARS-CoV-2 infection that cannot be justified by an alternative diagnosis are classified either into long-COVID (four weeks or longer) or post-COVID (more than 12 weeks) (2, 3). The known transmission ways of SARS-CoV-2 lead to increased risk of infection particularly in workplaces with intensive interpersonal and physical contacts with colleagues, patients and clients (4, 5). Especially healthcare-workers and social professionals showed a higher prevalence of SARS-CoV-2 compared to the general population (6), thus, it is necessary to highlight this vulnerable group in post-COVID research. In Germany, 357.396 cases of COVID-19 have been recognized as occupational diseases. Furthermore, 26.958 recognized cases of COVID-19 have been recorded as work-related accidents (according to the German Social Accident Insurance) (7).
1.1 Post-COVID and psychological symptoms
It is known that post-COVID can manifest in psychological symptoms, which can persist several months after acute SARS-CoV-2 infection (8–10). A systematic review from Marchi et al. (8) conclude, that 27-30% of patients experience sleep disturbances, 16-31% of patients experience symptoms of anxiety, 21-25% symptoms of depression, and 19-22% symptoms of PTSD. These prevalences are in line with the results of a recent systematic review and meta-analysis of Seighali et al. (9) including over 165 studies with 9.923.461 total post-COVID patients, reporting a pooled prevalence of depression and anxiety of 23% and of sleep disorders of 45%. A retrospective cohort study could reveal that the risk of experiencing mental health concerns (major depression, anxiety) is twice as high for COVID-19/Long-COVID survivors than for patients without history of COVID-19 (11). Overall, the authors of the above-mentioned studies also emphasize the high heterogeneity among existing studies in the prevalence of psychiatric symptoms e.g., due to heterogeneous study designs. Hyassat et al. (12) assessed post-COVID symptoms in a population of healthcare-workers with post-COVID and 58.4% of patients reported mood changes due to post-COVID. According to D’Ávila K et al. (13), Fouad et al. (14) and Mendola et al. (15) 8-44% of healthcare-workers are still showing symptoms of depression and anxiety after three to ten months of acute SARS-CoV-2-infection. Longitudinal studies highlight the long mental burden of COVID-19 and post-COVID. Fernandez-de-Las-Penas et al. (16) assessed symptoms of anxiety and depression with the hospital anxiety and depression scale (HADS) five (T1) and 10 (T2) months after hospital discharge in 2000 patients. The scores revealed that the prevalence of symptoms of anxiety decreased from 16.0% to 15.1% from T1 to T2, and symptoms of depression from 18.0% to 13.2%. Until know, there are some studies with follow-up times ≥ 24 months (17–19). In all studies the prevalence of anxiety and depression is decreasing during follow-up. With a prevalence of anxiety within 16.6-38.0% and of depression within 21.8-40.0% after 24 months after COVID-19. These findings emphasize that most patients are experiencing a long-lasting burden of disease with a recovery time of several months or years and long-term therapeutic concepts are needed.
1.2 Post-COVID and fatigue
Post-COVID patients are often reporting symptoms of fatigue (18, 20–22). According to a prospective registry study including 1.022 participants, around 30% of post-COVID patients fulfilled the criteria for ME/CFS after 255 days post infection (21). This prevalence is decreasing to 19.4% after 402 days post-infection. The assessed BFI (Brief Fatigue Inventory) score has a mean value of 5.4 points at visit 1 indicating moderate symptoms of fatigue. According to self-reported symptoms, 83% of patients have symptoms of fatigue. At visit 2 (402 days post infection) the mean BFI value is 5.0 and 73% of patients are reporting symptoms of fatigue. Van Wambeke et al. (18) report higher prevalence of fatigue (90%) 24 months after infection. However, the study sample was quite small with only 45 post-COVID patients. Another study with a median follow-up duration of 26 months since infection did reveal a prevalence of 21% for clinically relevant fatigue assessed by the FACIT Fatigue scale (23). There are only few studies assessing symptoms of fatigue in healthcare-workers. Hyassat et al. (12) assessed fatigue by self-reports as wells as the Fatigue Assessment Scale (FAS). After three months of SARS-CoV-2 infection 18.2% of healthcare-workers are reporting symptoms of fatigue which is decreasing to 10% after 6 months of infection. This prevalence is substantial lower than in the above-mentioned studies which presumably results from the relatively small and young study sample. According to the FAS results, female healthcare-workers are showing higher levels in fatigue than male healthcare-workers. In sum, the reported studies so far illustrate the high prevalences of fatigue which maintain stable over a long period of time. This should be addressed through adequate and patient-centered therapies.
1.3 Post-COVID and cognitive disturbances
Cognitive dysfunction is also one of the most reported symptoms of post-COVID patients (10, 19, 24). The prevalence of cognitive deficits in post-COVID patients is between 4-26% (8). A longitudinal study of Martin et al. (24) compared cognitive performance of post-COVID patients with matched healthy controls. Post-COVID patients showed cognitive slowing compared to control group. Further, there was no improvement in cognitive function after 6 months follow-up. Fernandez-de-Las-Penas et al. (25) examined cognitive function in hospitalized COVID-19 patients over 18 months and predicted recovery curves. While the prevalence of concentration loss and cognitive blurring is decreasing and will probably fall below 1% after two years, the prevalence of memory loss will remain high with over 10% after two years. A prospective study revealed a prevalence of cognitive impairments of 19% among post-COVID patients after 26 months post infection. The authors assume that the prevalence may be even higher as the used assessment tool (Montreal Cognitive Assessment (MoCA)) is not as sensitive as a more detailed cognitive test battery (23). There is also a high prevalence of cognitive complaints within healthcare-workers. After nine months of infection, 70.7% of healthcare-workers were reporting difficulties in concentrating and memory (26). Omar et al. (27) also revealed lower scores in Addenbrooke’s Cognitive Examination III for attention and memory in healthcare-workers with post-COVID compared to healthy healthcare-workers (control group). These findings underscore the importance of recognizing and addressing cognitive deficits in post-COVID patients to support their long-term recovery and health related quality of life.
1.4 Post-COVID and rehabilitation
To date, it is known that rehabilitation is a promising approach to improve post-COVID patients’ mental and cognitive health as well as quality of life (28, 29). A prospective study of Hayden et al. (30) observed improvements in symptoms of depression, anxiety and fatigue direct after rehabilitation discharge. Observations by Rutsch et al. (31) and Kupferschmitt et al. (32) confirm these results. The investigation of Müller et al. (33) revealed significant improvements in neuropsychological health and fatigue in patients who acquired COVID-19 in the workplace after an inpatient rehabilitation. In addition, healthcare-workers showed significantly greater improvements in depressive symptoms than non-healthcare-workers (33). Systematic reviews have also shown a positive effect of rehabilitation on psychological health (34–36). Three to six months after rehabilitation the scores for depression and fatigue decreased slightly but stayed on a higher level than before rehabilitation (30). The scores for anxiety decreased to baseline level (30). According to Gloeckl et al. (37) it is important to differentiate post-COVID patients after their predominant symptom to choose the most appropriate therapy and rehabilitation program. Several guidelines emphasize the importance of an interdisciplinary approach for the treatment and rehabilitation of post-COVID patients, especially in patients with psychological and cognitive ailments (2, 38–40).
To sum up, recent research confirms impaired mental health, symptoms of fatigue and cognitive deficits in post-COVID patients over two years after primary infection. This also applies healthcare-workers, which have a higher risk for getting infected with SRARS-CoV-2. As it is known that rehabilitation is a feasible and comprehensive therapy to improve patients’ mental health and cognitive impairments, there is limited knowledge regarding the long-term impact of rehabilitation on post-COVID in consideration of work-related infections, for example, in healthcare-workers. Longitudinal research data is needed to gain more knowledge about the long-term course of disease and to derive profound insights for an adequate after-care process of post-COVID patients. Thus, the current study investigates psychological health, fatigue, and cognitive function 6 and 12 months after inpatient post-COVID rehabilitation of patients, who acquired COVID-19 in the workplace. In addition, the study aims to detect group differences in these outcome parameters according to sex, age, acute COVID status, socioeconomic status (SES), profession, and pre-existing diseases.
2 Materials and methods
This research was undertaken at Chemnitz University of Technology, Germany, in collaboration with BG Hospital Bad Reichenhall. It was registered in the German Clinical Trials Register with the identifier DRKS00022928. Approval for the study was granted by the Ethics Committee of the Bavarian State Medical Association (number 21092) and the Ethics Committee of Chemnitz University of Technology (TU Chemnitz, Chemnitz, Germany), Faculty of Behavioural and Social Sciences (number V-427-17-KM-COVID-19-18022021). In the following sections, only pertinent information related to the present research question is presented. A comprehensive overview of the study and the inpatient rehabilitation program is given in the previously published study protocol (41).
2.1 Study design and participants
The longitudinal study design consists of four distinct measurement points at home as well as during the clinical stay: the beginning (T1) and end (T2) of the inpatient rehabilitation period, as well as 6 months (T3) and 12 months (T4) after the end of rehabilitation. Patients were recruited by a study nurse at the BG Hospital Bad Reichenhall after being registered for rehabilitation by the respective accident insurance provider. A majority of the post-COVID patients treated at this facility are employed in the healthcare sector, although other professional groups are also represented. Patients in the post-acute phase of COVID-19, recognized as an occupational disease or work-related accident, were eligible for inclusion if they met specific criteria (being in the post-acute phase without evidence of infectivity, confirmed capability for rehabilitation, and voluntary participation in the study), and provided written informed consent. The current report presents results of the measurement points T3 and T4. Results from the analysis of the measurement point T1 compared to T2 are already published elsewhere (33).
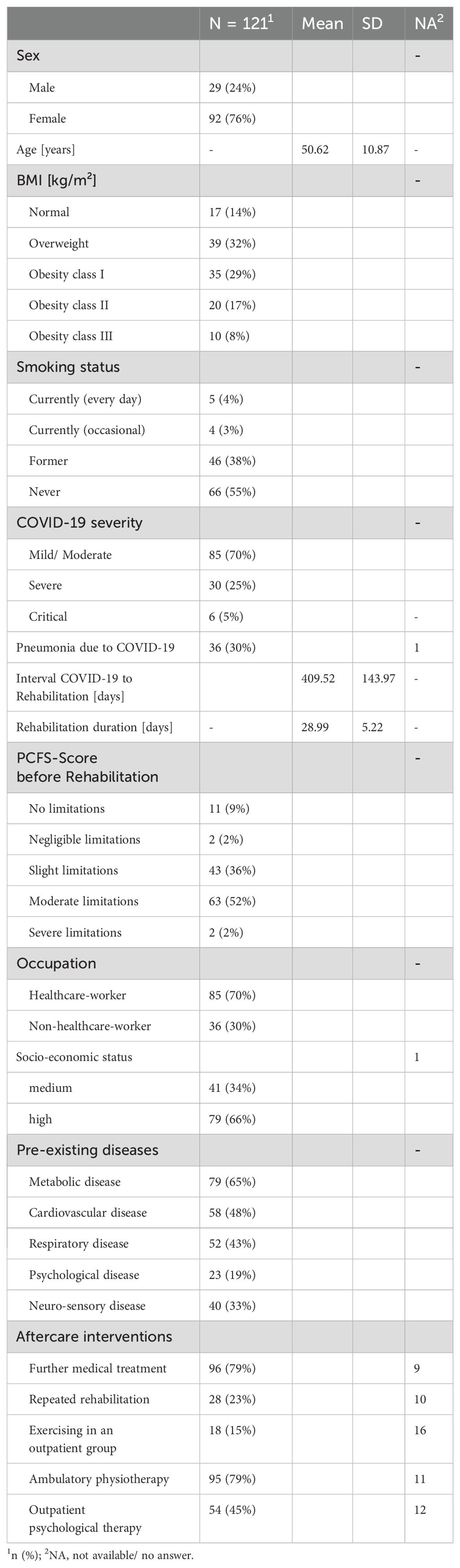
All participants went through an inpatient multidisciplinary post-COVID rehabilitation program at the BG Hospital with a mean duration of M=28.99 (± 5.22) days. In addition to medical treatment and care, patients received in comprehensive physical and psychological treatments by specialists. For detailed information on the inpatient rehabilitation, see Müller et al. (41).
The study included 127 patients at T1. At time point T2, three participants were classified as dropouts, either due to discontinuation of rehabilitation (n=1) or lack of further interest in study participation (n=2). At time point T3, three participants discontinued the study due to lack of interest. At time point T4, two participants discontinued the study without providing reasons, one participant lost interest in participation, one participant experienced a stroke shortly before the T4 assessment, one participant couldn’t complete the questionnaires or travel to the clinic in time, and one participant cited professional reasons for discontinuing participation at T4. Consequently, there are 121 patients in the paired sample T1-T3 and 115 patients in the paired sample for T1-T4. Due to missing values (reasons: e.g., missing questionnaire response) or missing clinical examination, cases for each variable vary between 107-118 for T1-T3 comparison and 98-114 for T1-T4 comparison.
At this point it should be mentioned, that after inpatient rehabilitation at BG Hospital Bad Reichenhall up to the time point T4 participants received the following post-COVID-19 treatments: 79% further medical treatment by the general practitioner, 23% repeated rehabilitation, 45% outpatient psychological therapy, 79% ambulatory physiotherapy, 15% exercise in an outpatient group (see Table 1).
The patients are m=50.62 years (± 10.87) old and n=97 (76%) categorize themselves as female. At T1, 85% are overweight (BMI >25 kg/m2). Six patients are smokers, four patients are occasional smokers, and 49 patients are former smokers. 67 patients did never smoke. Further, 85 patients are working within the healthcare-sector and 36 patients are classified as non-healthcare-workers (e.g., administrative staff, industrial-/building technicians, social education staff, and teachers). According to the SES, 41 patients are reaching a medium SES, and 79 patients are reaching a high SES. None of the patients were in the lowest SES category.
2.2 Measurements
Several socioeconomic and -demographic variables (e.g., age, sex, socio-economic status, education) were obtained via questionnaire based on the German Health Interview and Examination Survey for Adults (DEGS) (42, 43). Pre-existing diseases were assessed using the subscale “Current diseases” of the work ability index (WAI) (44), and were complemented by a semi-standardized interview by a physician during medical anamnesis. Additionally, the Post-COVID-19 Functional Status (PCFS) scale with ranges from 0 (no limitations/symptoms in everyday life) to 4 (severe limitations/symptoms in everyday life was also used (45).
The German version of the Hospital Anxiety and Depression Scale (HADS-D) was used to assess the presence of psychological symptoms. The subscales for depression (HADS-DDepression) and anxiety (HADS-DAnxiety) consist of seven items, respectively. A sum score for each scale was generated (range 0–21) based on a Likert scale from 0 (‘no symptoms’) to 3 (‘severe symptoms’). Scores between 8 and 10 indicated mild symptoms, while scores above 10 indicated moderate to severe symptoms (46, 47). Based on analyses with cardiac patients after rehabilitation, a minimal clinically important difference (MCID) of >1.7 points was reported in each subscale (48). The subjectively perceived status of mental health was reported by participants on a self-generated questionnaire with 10 items on a scale of 0–10 (0 = very bad, 10 = very well).
To measure various physical symptoms and their symptom severity in patients the self-administered Somatic Symptom Disorder - B Criteria Scale (SSD-12 (49)) was used. SSD-12 consists of 12 items that assess the extent and impact of somatic symptoms, and each item is rated on a Likert scale from 0 (never) to 4 (very often), resulting in a total score ranging from 0 to 48. Higher scores indicate greater severity and impact of somatic symptoms and the MCID is defined as a change of 3 points (50).
The International Trauma Questionnaire (ITQ (51)) is a self-reported measure to assess the severity, frequency, and duration of trauma-related symptoms in individuals. The ITQ consists of 6 Items, rated on a Likert scale from 0 (not at all) to 4 (extremely). The calculated total score ranges from 0 to 24. A cut-off score of 19 or above may indicate the presence of PTBS, while higher scores indicate a more severe level of trauma-related symptoms. There is no well-defined MCID for the ITQ in the current literature.
The Brief Fatigue Inventory (BFI) and the Fatigue Impact Scale (FIS) were used to assess symptoms of fatigue. The BFI consists of ten questions concerning fatigue and fatigue-related symptoms to detect fatigue severity. The mean score of all items ranges from 0–10, a higher score indicates a more severe level of fatigue. In addition, depending on the mean score, the following categories can be used: 0 for ‘no fatigue’, 1-3 for `mild fatigue`, 4-6 for `moderate fatigue` and 7-10 for `severe fatigue` (52, 53). The MCID for in BFI score is defined as a change of 1.33 points (54). The FIS includes 40 items with a five-point Likert scale from 0 (‘no problem’) to 4 (‘extreme problem’) assigned to the three subscales (cognitive, physical, and psychosocial functioning). In addition, a sum score is calculated (range 0-160), with higher scores indicating more severe function impairments due to fatigue (55). A study in patients with multiple sclerosis defined a MCID of 10-20 points in FIS sum score (56).
We used three German versions of the MoCA (T1: Version 8.1, T2: version 8.2, T3: version 8.3, T4: version 8.1) to assess global cognitive functioning, e.g., short-term memory, visuospatial abilities, attention, concentration, working memories, language, orientation in space and time, and executive functions. The total sum score, ranging from 0 to 30 points, was calculated in addition to classifying participants into cognitively healthy (26-30 points) and mildly cognitively impaired (20-25 points) individuals (57). The MCID for the MoCA is defined as a change of two points in the total sum score, which was used to assess significant improvements or decline in cognitive abilities (58). Furthermore, the Digit Symbol Substitution Test (DSST) was used to examine various cognitive functions, including sustained attention, visual–spatial skills, response speed, and set-shifting. Participants were given a sheet of paper with rows of symbols and the task of matching each symbol to a number. A legend at the top of the page indicated which symbol matches which digit (1–9). Afterward, the number of correct digit-symbol matches executed in 90 s was recorded (59). There is no well defined MCID of the change in DSST score in a clinical sample. However, Jehu et al. (60) defined a MCID of 3-5 symbols in a population of older adults. The Trail Making Test (TMT) is a neuropsychological assessment tool that assess the individual`s focus, attention, execution function, and cognitive flexibility. There are two parts to the TMT (1): TMT-A, participants have to connect numbers in an ascending order (2), TMT-B, participants have to connect numbers and letters in an alternating way (61). The MCID of TMT-A is 11.7 seconds and 24.4 seconds in cognitive unimpaired individuals (62).
2.3 Data analysis
The data were analyzed using SPSS software (version 29, SPSS Inc., Armonk, NY, USA). Given the non-normal distribution of most parameters, the Wilcoxon signed-rank test was used to compare variables across T1, T3, and T4. Group differences at T1 and over time (Δ=T3-T1, Δ=T4-T1) concerning sex (male vs. female), age (≤50 years vs. >50 years), profession (healthcare services vs. other), acute COVID status (mild/moderate vs. severe/critical), socio-economic status (medium SES vs. high SES), and diseases prior to COVID-19 (cardiovascular disease, respiratory disease, neuro-sensory disease, metabolic disease, mental impairment) were analyzed using the Mann-Whitney U test. Only significant results regarding group differences at each measurement time point are presented in the text. Missing data were noted and are clearly presented in the tables, and p-values <0.05 considered statistically significant. Effect sizes were reported as r, with an effect size of 0.1 representing a ‘small’ effect, 0.3 a ‘medium’ effect, and 0.5 a ‘large’ effect, following the guidelines of Fritz et al. (63).
3 Results
3.1 6 months after rehabilitation
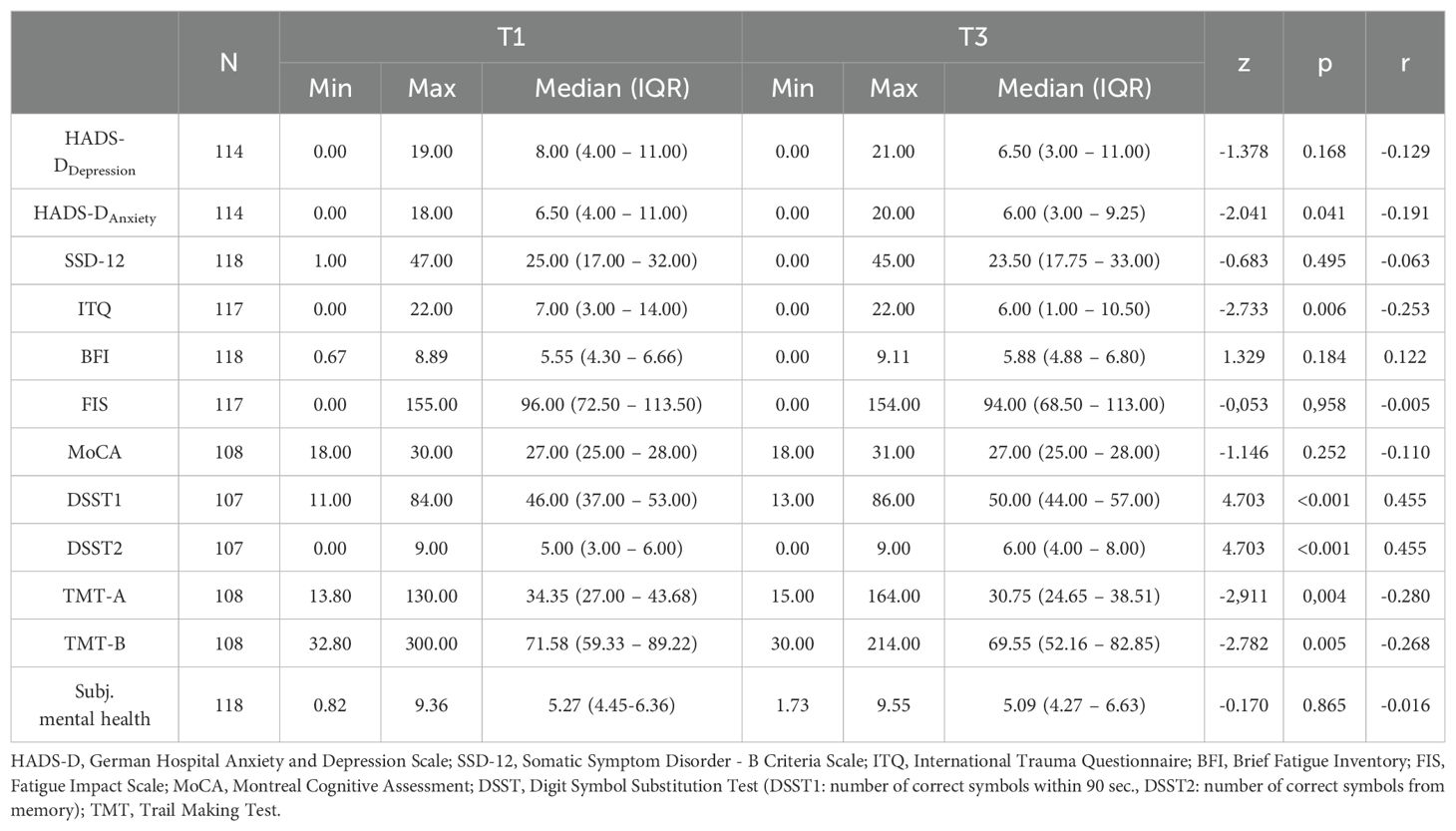
At T1, 23.7% of patients had mild symptoms of depression (HADS-DDepression) and 27.2% showed symptoms of severe depression whereas at T3 17.5% of patients had mild symptoms of depression and 25.5% showed symptoms of severe depression (Supplementary Figure 1). For anxiety (HADS-DAnxiety), 19.3% had mild symptoms and 25.4% had severe symptoms at T1. In comparison, at T3 mild symptoms were found in 14.9% of patients and 18.4% had severe symptoms (Supplementary Figure 2). At T1 mild cognitive impairment (MoCA) was found in 26.9% of patients and at T3 in 31.5% (Supplementary Figure 3). At baseline, 61.0% of patients showed moderate and 23.7% severe symptoms of fatigue according to the BFI score. At T3, the prevalence was almost the same with 61.9% of patients with moderate and 23.7% of patients with severe symptoms in fatigue (Supplementary Figure 4).
Table 2 presents the differences in the mental and cognitive health assessments between T1 and T3. From T1 to T3, a significant improvement in HADS-DAnxiety was recorded (T1: Mdn=6.50, IQR: 4.00-11.00; T3: Mdn=6.00; IQR: 3.00-9.25; p=0.041) with a low effect (r=-0.191). ITQ also showed a significant change between T1 (Mdn=7.00, IQR: 3.00-14.00) and T3 (Mdn=6.00, p=0.006, r=-0.253). A significant change in SSD-12, depression (HADS-DDepression), fatigue (BFI, FIS), and subjective mental health was not recorded between the measurement points T1 and T3 (p>0.05). The patients’ cognitive performance did improve, although not significantly, from T1 to T3 measured by MoCA. However, executive functions in the context of working memory and processing speed improved (DSST1: T1: Mdn=46.00, IQR: 37.00-53.00; T3: Mdn=50.00, IQR: 44.00-57.00; p<0.001), as well as recall performance (DSST2: T1: Mdn=5.00, IQR: 3.00-6.00; T3: Mdn=6.00, IQR: 4.00-8.00; p<0.001) significantly with moderate effect size (r=0.454). TMT-A showed a significant change from T1 (Mdn=34.35, IQR: 27.00-43.68) to T3 (Mdn=30.75, IQR: 24.65-38.51; p=0.004, r=-0.280), as did TMT-B from T1 (Mdn=71.58, IQR: 59.33-89.22) to T3 (Mdn=69.55, IQR: 52.16-82.85; p=0.005, r=-0.268), both with low effect sizes. However, no significant change was observed in the ratio of TMT B/A (p>0.05).
Over time (T1 to T3), female patients (ΔDSST2: Mdn=2.00, IQR: 0.00-3.00) as well as patients with a mild-moderate COVID-19 course (ΔDSST2: Mdn=2.00, IQR: 0-3.00) showed a greater improvement in memory performance (DSST2) than male patients (ΔDSST2: Mdn=0.00, IQR: -1.00-1.75, p=0.003, r=0.292) or those with a severe to critical acute course (ΔDSST2: Mdn=1.00, IQR: -1.00-2.00, p=0.028, r=-0.212). Post-COVID patients working within the healthcare sector improved from T1 to T3 slightly in their depression severity (ΔHADS-DDepression: Mdn=-1, IQR: -3-1) whereas non-healthcare-workers stayed at the same level (ΔHADS-DDepression: Mdn=0, IQR: -1-3, p=0.020, r=0.209). Furthermore, patients with a pre-existing mental impairment in the T1-T3 course showed an improvement in their fatigue severity (ΔBFI: Mdn=-0.41 IQR: -1.13-0.44), while patients without a pre-existing mental impairment worsened in their fatigue severity (ΔBFI: Mdn=0.33, IQR: -0.66-1.30, p=0.014, r=-0.227). TMT-A showed a significant group difference between healthcare-workers (ΔMdn=-4.84; IQR: -11.18-1.86) and non-healthcare-workers (ΔMdn=3.00; IQR: -10.70-9.34; p=0.035; r=0.203). Additionally, patients with a pre-existing psychological disease demonstrated a greater reduction in ITQ (ΔMdn=-4.00; IQR: -8.00-0.50) compared to those without a pre-existing psychological disease (ΔMdn=-1.00, IQR: -3.00-2.00, p=0.020, r=-0.215). For a complete overview of the conducted group analysis regarding the change from T1 to T3 see the corresponding tables (Supplementary Tables A1, A3, A5, A7, A9, A11, A13, A15, A17, A19, A21) in the Supplementary Material.
3.2 12 months after rehabilitation
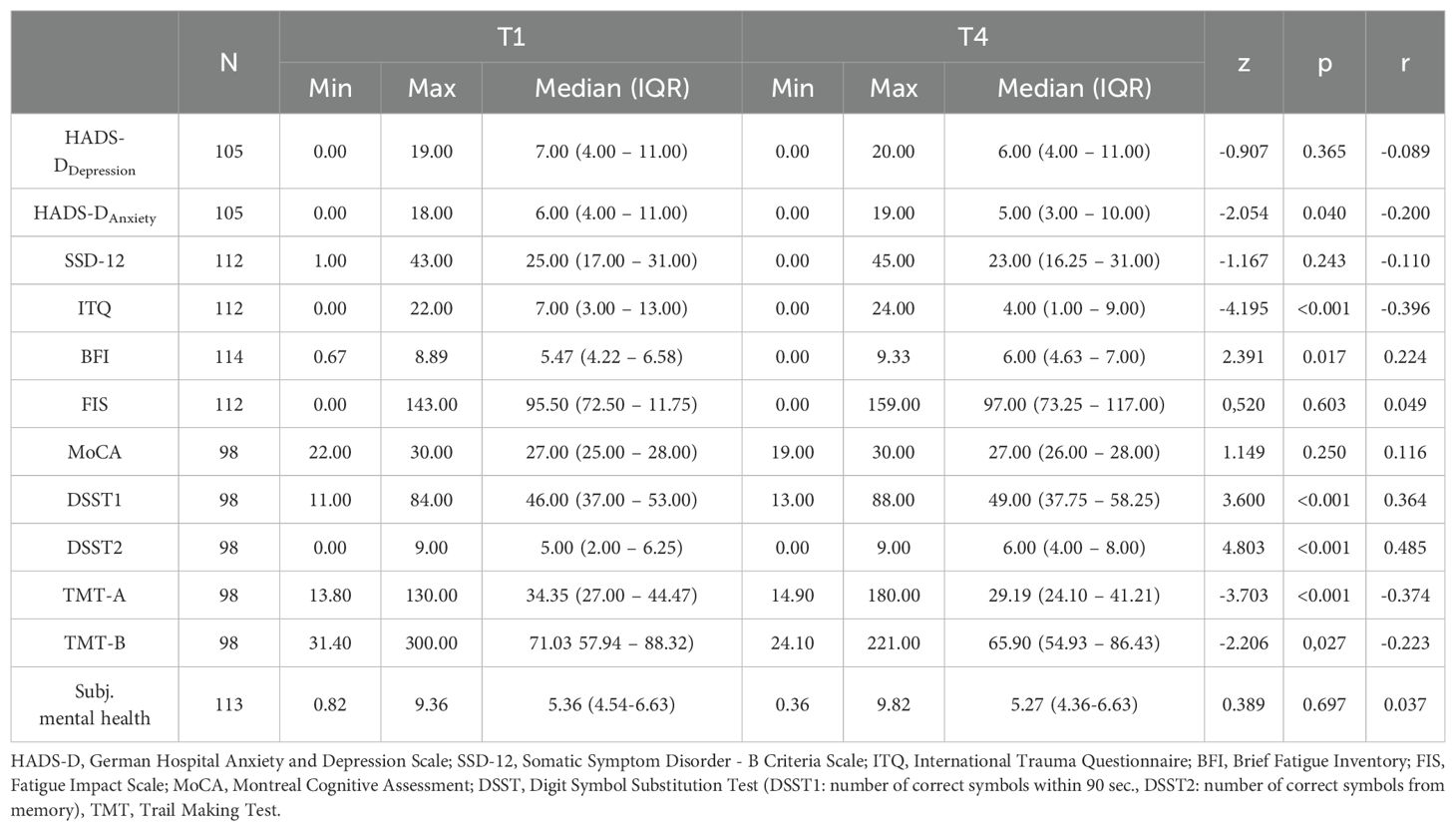
At T4, 12.3% of patients had mild symptoms of depression (HADS-DDepression) and 26.7% showed symptoms of severe depression (Supplementary Figure 5). At T4, 17.1% of patients had mild symptoms and 21.0% had severe symptoms in anxiety (HADS-DAnxiety) (Supplementary Figure 6). 12 months after rehabilitation 21.4% patients showed symptoms of mild cognitive impairment (MoCA) (Supplementary Figure 7). 58.8% of patients showed moderate and 26.3% severe symptoms of fatigue (BFI) (Supplementary Figure 8).
From the beginning of inpatient rehabilitation (T1) to 12 months after rehabilitation discharge (T4), various improvements were achieved within the cognitive and mental health assessment (see Table 3). The HADS-D showed a significant improvement in the patients’ anxiety symptoms (HADS-DAnxiety: T1: Mdn=6.00, IQR: 4.00-11.00; T4: Mdn=5.00; IQR: 3.00-10.00; p=0.040) with a low effect size (r=-0.200). A significant change was observed in the ITQ score between T1 (Mdn=7.00; IQR: 3.00-13.00) and T4 (Mdn=4.00; IQR: 1.00-9.00; p<0.001) with a medium effect size (r=-0.396). Furthermore, the post-COVID patients showed a significant worsening of fatigue (BFI) (T1: Mdn=5.47, IQR: 4.22-6.58; T4: Mdn=6.00, IQR: 4.63-7.00; p=0.017) with a low effect size (r=0.224). The depressive symptoms (HADS-DDepression), SSD-12, FIS, and subjective mental health did not change significantly between T1 and T4 (p>0.05). Within the cognitive performance measures from T1 to T4, significant improvements in executive functions in the context of working memory and processing speed (DSST1: T1: Mdn= 46.00, IQR: 37.00-53.00; T4: Mdn=49.00, IQR: 37.00,75.00-58.25; p<0.001) with moderate effect size (r=0.364), as well as recall performance (DSST2: T1: Mdn=5.00, IQR: 2.00-6.25; T4: Mdn=6.00, IQR: 4.00-8.00; p<0.001) with a medium effect size (r=0.485) were determined. TMT-A showed a significant change from T1 (Mdn=34.35, IQR: 27.00-44.47) to T4 (Mdn=29.19, IQR: 24.10-41.21; p<0.001) with a medium effect size (r=-0.374). Similarly, TMT-B changed significantly from T1 (Mdn=71.03, IQR: 57.94-88.32) to T4 (Mdn=65.90, IQR: 54.93-86.43; p=0.027) with low effect size (r=-0.223).
Younger patients showed a greater improvement from T1 to T4 in cognitive performance (ΔDSST1: Mdn=5.00, IQR: 0.50-12.50) than older patients (ΔDSST1: Mdn=2.00, IQR: -3.00-6.50, p=0.025, r=-0.227), and in terms of sex, female patients were found to improve to a greater extend at T4 (ΔDSST2: Mdn=2.00, IQR: 0.00-3.00) than male patients (ΔDSST2: Mdn=1.00, IQR: -0.25-2.00, p=0.003, r=0.292). Over the course of 12 months, patients with a mild-moderate COVID-19 course showed greater improvements in subjectively perceived mental health (Δ: Mdn=0.14, IQR: -0.66-1.18) as well as in cognitive performance (ΔDSST2: Mdn=2.00, IQR: 0.00-3.00) than patients with severe-critical COVID-19 (Δsubj. mental health: Mdn=-0.64 IQR: -1.55-0.45, p=0.026, r=-0.209; ΔDSST2: Mdn=1.00, IQR: -1.00-2.00, p=0.043, r=-0.204). At T4, there was a significant difference in the change in fatigue severity (BFI score) with regard to socioeconomic status. Post-COVID patients with a medium socioeconomic status worsened to a greater extent (ΔSES: Mdn=0.88, IQR: 0.00-1.27) than post-COVID patients with a high socioeconomic status (ΔSES: Mdn=0.27, IQR: -0.88-1.08, p=0.033, r=-0.201). Patients with and without a pre-existing mental impairment also differed in terms of the change in fatigue severity from T1 to T4. Patients with a pre-existing mental impairment improved significantly in terms of their fatigue severity (ΔBFI: Mdn=-0.55, IQR: -1.38-0.47) from T1 to T4. In contrast, patients without a pre-existing mental impairment showed a significant deterioration in the BFI score over the 12-month follow-up (ΔBFI: Mdn=0.66, IQR: -0.77-1.22, p<0.001, r=-0.320). A significant group difference was observed in the TMT-B test between patient with pre-existing cardiovascular disease (ΔMdn=0.46; IQR: -13.40-16.08) and without cardiovascular disease (ΔMdn=-8.39; IQR: -22.20-4.30p=0.026; r=0.225). Additionally, patients with a psychological disease demonstrated a greater reduction in FIS score (ΔMdn=-10.00; IQR: -20.50-8.00) compared to those without a psychological disease (ΔMdn=2.00, IQR: -10.00-18.00, p=0.026, r=-0.210). Furthermore, patients with a neuro-sensory disease showed less improvement the TMT-B test (ΔMdn=3.62, IQR: -13.22-25.50) compared to those without neuro-sensory disease (ΔMdn=-7.41, IQR: -21.92-4.83, p=0.022, r=0.232). For a complete overview of the conducted group analysis regarding the change from T1 to T4 see the corresponding tables (Supplementary Tables A2, A4, A6, A8, A10, A12, A14, A16, A18, A20, A22) in the Supplementary Material.
4 Discussion
This study is one of the first studies to assess mental health and cognitive function of post-COVID patients six and 12 months after inpatients post-COVID rehabilitation. In general, cognitive function improved after rehabilitation and could be maintained during follow-up. The results of mental health are heterogeneous and depend on the parameters measured.
4.1 Prevalence of psychological symptoms, fatigue, and cognitive impairment
The prevalence of depression and anxiety symptoms in our study showed slight improvement over time. However, at T4, 39.1% of patients still exhibited mild to severe symptoms of depression, and 38.1% had mild to severe symptoms of anxiety. Compared to previous studies, the prevalence of symptoms of depression and anxiety are higher (19, 64). Fatigue remained a persistent and significant issue in our cohort, with 61.0% of patients reporting moderate and 23.7% severe fatigue at T1. These levels remained relatively stable at T3 and T4, indicating that fatigue is a long-lasting symptom for many post-COVID-19 patients in our study. These findings are consistent with other research, which reported prevalence rates of fatigue between 44-60% up to 24 months post-infection (16, 19). Van Wambeke et al. (18) specifically noted that fatigue was the most common symptom, present in more than 90% of post-COVID patients over a two-year follow-up period. This emphasizes the need for targeted interventions to manage fatigue more effectively, as it significantly impacts the quality of life and overall recovery in post-COVID-19 patients. The prevalence of mild cognitive impairment in our study showed a mixed trend, initially increasing from 25.9% at T1 to 30.6% at T3, before decreasing to 19.4% at T4. This suggests that cognitive recovery may take longer and varies among individuals. Our findings align with other research showing that cognitive impairment prevalence can be as high as 50% four months after acute infection (65). Over time, the prevalence tends to decrease, however 24-29% of patients still exhibit cognitive deficits nine months post-infection (66, 67). Long-term studies also indicate that around 20% of patients continue to experience cognitive impairments up to 24 months after the acute infection (64). The existing impairments have a crucial impact on the patient’s return to social and professional life (68, 69). Therefore, long-term treatment strategies are needed to address the high and complex psychological symptom burden of post-COVID patients in an adequate manner.
4.2 Changes in mental and cognitive health and fatigue over time
The results of our previous published paper show, that directly after rehabilitation the scores of HADS-DDepression and HADS-DAnxiety improved significantly (33). This is in line with other studies (30, 31) and review-analyses (34, 35). The results suggest a good effectiveness of the implemented multidisciplinary inpatient rehabilitation program even if the missing control group prohibits causal statements. The current investigation demonstrates that six to 12 months after rehabilitation discharge the scores of HADS-DDepression and HADS-DAnxiety are approaching the baseline level (T1). Similar to depression and anxiety, fatigue severity (measured by BFI) is improving after rehabilitation (33). However, the improvements in fatigue are not maintained at T3, and in fact worsen significantly at 12 months follow-up. The long course of the disease with diverse symptoms, the everyday restrictions experienced as a result of the disease, the uncertainty about the further course of disease and recovery, existential worries regarding the return to work (including financial concerns), the patient’s social life and if applicable also the working environment can play a decisive role here, and may even increase psychological stress (70, 71). Furthermore, it is known, that reduced physical capacity is associated with decreased psychological symptoms, a relationship observed in both general contexts and specific conditions such as lung diseases (72, 73). The COVID-19 pandemic has further illuminated the connection between physical and mental health. According to a recent study, post-COVID patients with physical impairments were associated with more cognitive dysfunction (74). Reduced physical capacity can trigger several physiological mechanisms that contribute to increased psychological symptoms in post-COVID patients. These include disruptions in neurotransmitter regulation, impairment of neuroplasticity, immune system dysregulation, microvascular injury, and exacerbation of inflammatory processes (75–77). During rehabilitation, the patients received intensive psychological care. The absence of ongoing psychological support after rehabilitation discharge, combined with the return to everyday life and work, may have contributed to the observed worsening of symptoms. Further, if return to work is not possible due to continued presence of post-COVID condition and the associated reduced work ability, patients may experience financial strain which also negatively impacts their mental health in the long-term. Our findings suggest that patients with medium SES experienced a greater worsening in their BFI scores compared to those with high SES from T1 to T4. This underscores how socioeconomic factors significantly influence the mental health trajectory of post-COVID patients over time. In addition, reduced social activities and the loss of social support as the disease progresses could also be reasons for worsening mental health (78, 79). The results measured by standardised and validated questionnaires (HADS-D and BFI) are supported by the subjective perceived mental health, assessed by a self-generated questionnaire. After rehabilitation, the post-COVID patients perceived an improvement in their mental health (see Müller et al. (33)), but this deteriorated again after six and 12 months analogously to the scores for depression and fatigue. Based on the results, the ITQ scores show significant improvements six and 12 months after rehabilitation, indicating a reduction in post-traumatic stress symptoms among post-COVID patients. This finding aligns with another study that reported a gradual decrease in trauma-related symptoms at a 3- and 6-month follow-up (22). In contrast, the SSD-12 scores do not show significant improvements and remain at a high level, suggesting persistent somatic symptoms. This is consistent with findings from other studies that highlight the ongoing presence of somatic complaints in post-COVID patients and demonstrate an association between baseline SSD-12 scores and persistent symptoms at follow-up (80, 81).
Contrary to mental health, the results of the cognitive function are more positive. The significant improvement in processing speed (DSST1) and memory performance (DSST2), ability to switch between different cognitive tasks (sequencing and attention, TMT-A), and cognitive flexibility (TMT-B) after rehabilitation discharge (T2) could be maintained over six to 12 months follow-up. The change in the DSST1 score even reaches the MCID of 3-5 points. These improvements are substantial compared to normative data, suggesting that while patients still show some impairment compared to healthy controls, their cognitive function has markedly improved over time (82). Comparative studies have reported analogous findings. A recent study showed that the results of the TMT were significantly higher in the post-COVID-19 group compared to healthy people (83). However, the significant improvement in MoCA score at T2 couldn’t be maintained during the 12 months follow-up period. This underlines current findings of Lynch et al. (84), revealing a lack of sensitivity of the MoCA regarding the cognitive complaints of post-COVID patients in their study.
The revealed group differences point out that the pre-existing mental impairment influences the course of fatigue-severity in the mid- and long-term. According to the assessed BFI score, patients with pre-existing mental impairment showed better BFI scores at T3 and T4 compared to T1. Probably these patients get already psychological support before rehabilitation and the transition from rehabilitation to aftercare is more fluent. Since patients without pre-existing mental impairment worsen within their fatigue severity during follow-up, more medical support is needed to counteract this increase. Rehabilitation providers must take this into account and should also encourage patients without history of mental impairment to seek ongoing psychological or medical treatment after rehabilitation to account for their long-lasting fatigue complaints. Regarding cognitive function over time, female post-COVID patients, younger post-COVID patients and patients with mild-moderate COVID-19 showed a higher improvement in cognitive function (DSST2) six and 12 months after rehabilitation. Nevertheless, male post-COVID patients, older post-COVID patients and patients with severe-critical COVID-19 also improved within follow-up regarding cognitive function although not in the same extent. Further, the socioeconomic status influences the course of fatigue severity since patients with a medium SES worsened to a greater extent than patients with a high SES. Financial reserves, better physical and mental working conditions and higher (health-related) human capital (educational level) possibly relieve the patients of the burden of disease in a greater extend. There is research highlighting the influence of a higher SES on health behaviour, morbidity, and access to medical services (85, 86). Therefore, it is necessary to recognize and address the socio-economic factors in health research approaches that impact individuals’ quality of life. However, even the post-COVID patients with a high SES worsen in their fatigue severity 12 months after rehabilitation discharge.
4.3 Recommendations for improving rehabilitation management
General, the results of the follow-up timepoints illustrate the need of adequate and seamless aftercare strategies to maintain the improved mental health and cognitive function after inpatient rehabilitation. The long course of disease in some cases over several years is an additional psychological burden for the post-COVID patients. In addition, post-COVID patients are exposed to stigmatization as the disease is not always taken seriously (87). Stigmatization and stereotyping also affects the course of disease in a negative way and even prevents patients from seeking for psychological assistance (88). Educating the society and health care providers about post-COVID syndrome and providing patients long-term psychological support can contribute to improve mental health. Individual resources and self-management strategies should also be strengthened by psychological interventions within rehabilitation and during the aftercare process. The ability to perceive one’s own health status is an essential component of the implementation of self-management techniques, such as the PACING technique. It is crucial for patients to be able to accurately assess their individual and daily mental, cognitive, and physical resources to maintain their daily activities and tasks within their energy limits. Further, the German S1 guideline Long-/Post-COVID emphasizes the positive effects of sport and exercise programs on mental health and fatigue (2). Thus, patients should be encouraged to participate in exercising in an outpatient group e.g., in rehabilitation sport clubs or other adequate exercise programs after rehabilitation discharge. One exception to this is the group of post-COVID patients diagnosed with ME/CFS. The current guideline does not recommend exercise therapy for ME/CFS patients (89). Nevertheless, Gloeckl et al. (90) propose the implementation of person-oriented exercise therapy, which should be tailored to the severity of post-exertional malaise and the individual’s needs, limitations, and tolerance to exercising. At last, multimodal therapeutic approaches must be utilized in aftercare. Digital and telemedicine solutions for aftercare can be a promising method to improve symptom management in post-COVID patients. Studies highlight that telehealth platforms, such as video consultations and remote monitoring, alongside health apps and teletherapy, provide continuous support and effective symptom control, facilitating early intervention and personalized treatment adjustments (91–93). Reviews further underscore the efficacy of telerehabilitation in managing COVID-19 patients (94). This may enable more specific and individualized measures for the effective management of exercise-related rehabilitation and aftercare processes.
4.4 Limitations
There are some limitations which should be considered when interpreting the results. Due to ethical aspects, it was not possible to implement a control group within our study. Thus, the current findings can’t be fully attributed to inpatient rehabilitation, but they may also be a result of natural recovery. But we agree with Hayden et al. (30) that improvements after rehabilitation are more likely to be a result of rehabilitation than from natural recovery. Further, the study sample is quite small and the selective study population with work-related SARS-CoV-2 infection attending an inpatient rehabilitation at only one clinic has to be mentioned as a potential source of bias in participant recruitment and affecting the representativeness of the study population. However, the gained results seem suitable for comparisons with further post-COVID research data. 70% of included patients are working within the healthcare-sector. Healthcare-workers may have special professional circumstances, in particular during the COVID-19 pandemic. They were reporting higher suicidal ideation, increased stress, and decreased quality of life than other professionals (95). Further, the sample size of the different subgroups sex, age, COVID-19 severity, profession, socioeconomic status, and pre-existing diseases were not well balanced. More women than men were included in the current study sample, which is a result of the high amount of women working within the healthcare sector (96). Moreover, some of the neuropsychological instruments used, such as the MoCA, are screening measures rather than comprehensive neuropsychological assessments (97). This should be considered when interpreting the cognitive outcomes, as screening tools have limitations in detecting mild cognitive impairments (98). While the study follows patients for 12 months, longer follow-up periods (e.g., 24 months or more) would provide a more comprehensive understanding of the long-term effects of rehabilitation. A one-year observation period allows for insights into recovery trajectories; however, post-COVID symptoms have been reported to persist beyond this timeframe in some individuals (99). Extending the follow-up period could help determine whether improvements observed at 12 months remain stable, continue to progress, or potentially regress over time. Additionally, long-term data could provide valuable insights into the need for ongoing interventions. At last, the conducted analyses and group comparisons indicate that other multivariate and intra-individual analysis methods can provide further important insights to account for the complex associations and highly individual post-COVID courses. The mentioned limitations must be considered in comparison with findings from other studies in the context of COVID-19 rehabilitation.
5 Conclusion
The current analysis is one of the first, examining the course of post-COVID patients’ mental health and cognitive performance up to 12 months after inpatient rehabilitation. Previous results indicated an improvement in depressive symptoms and anxiety, fatigue severity and cognitive function direct after inpatient rehabilitation. The missing improvements in our study six- and 12-months follow-up in depression, somatic symptom disorders, and fatigue indicate the importance of the aftercare process and the implementation of adequate therapeutic interventions such as psychological support and strengthen self-management skills. The results could also identify factors influencing the symptom recovery in the mid- and long-term. Nevertheless, future research is needed to evaluate how post-COVID patients after inpatient rehabilitation can be best accompanied and supported in the long course of their disease.
Data availability statement
The raw data supporting the conclusions of this article will be made available from the corresponding author upon justified request.
Ethics statement
The studies involving humans were approved by Ethics Committee of the Bavarian State Medical Association (number 21092) and the Ethics Committee of the Chemnitz University of Technology, Faculty of Behavioral and Social Sciences (number V-427-17-KM-COVID-19-18022021). The participants provided their written informed consent to participate in this study.
Author contributions
KM: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. IP: Data curation, Formal analysis, Investigation, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. MO: Data curation, Formal analysis, Investigation, Software, Validation, Writing – review & editing. RC-W: Investigation, Methodology, Resources, Writing – review & editing. MS: Conceptualization, Investigation, Methodology, Resources, Writing – review & editing. TS: Investigation, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by German Social Accident Insurance (Deutsche Gesetzliche Unfallversicherung e.V., DGUV), grant number FF-FB 0326. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication. The publication of this article was funded by Chemnitz University of Technology.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2025.1460097/full#supplementary-material
References
1. Feldt T, Guggemos W, Heim K, Kobbe R, Lübbert C, Mikolajewska A, et al. Hinweise zu Erkennung, Diagnostik und Therapie von Patienten mit COVID-19: Robert Koch-Institut (2023). https://edoc.rki.de/bitstream/handle/176904/6511.26/Diagnose-und-Therapie-Hinweise_Covid-19_STAKOB_U24_FINAL_ONLINESTELLUNG_clean_20230208.pdf;jsessionid=5B6F6E9BF46E0A9161CEB462666DC1D0?sequence=12. (Accessed March 4, 2025).
2. Koczulla AR, Ankermann T, Behrends U, Berlit P, Brinkmann FUF, et al. S1-Leitlinie “Long/Post-COVID” (2024). Available online at: https://register.awmf.org/assets/guidelines/020-027l_S1_Long-Post-Covid_2024-06.pdf (Accessed June 30, 2025).
3. Sivan M, Taylor S. Nice guideline on long COVID. BMJ BRIT Med J. (2020) 371:m4983. doi: 10.1136/bmj.m4938
4. Burdorf A, Porru F, Rugulies R. The COVID-19 pandemic: one year later - an occupational perspective. Scand J Work Environ Health. (2021) 47:245–7. doi: 10.5271/sjweh.3956
5. Khalatbari-Soltani S, Cumming RG, Delpierre C, Kelly-Irving M. Importance of collecting data on socioeconomic determinants from the early stage of the COVID-19 outbreak onwards. J Epidemiol Community Health. (2020) 74:jech–2020-21429. doi: 10.1136/jech-2020-214297
6. Gómez-Ochoa SA, Franco OH, Rojas LZ, Raguindin PF, Roa-Díaz ZM, Wyssmann BM, et al. COVID-19 in health-care workers: A living systematic review and meta-analysis of prevalence, risk factors, clinical characteristics, and outcomes. Am J Epidemiol. (2021) 190:161–75. doi: 10.1093/aje/kwaa191
7. DGUV. Berufskrankheiten und Arbeitsunfälle im Zusammenhang mit COVID-19: DGUV (2024). Available online at: https://www.dguv.de/medien/inhalt/mediencenter/hintergrund/covid/dguv_zahlen_covid.pdf (Accessed June 24, 2024).
8. Marchi M, Grenzi P, Serafini V, Capoccia F, Rossi F, Marrino P, et al. Psychiatric symptoms in long-COVID patients: A systematic review. Front Psychiatry. (2023) 14:1138389. doi: 10.3389/fpsyt.2023.1138389
9. Seighali N, Abdollahi A, Shafiee A, Amini MJ, Teymouri Athar MM, Safari O, et al. The global prevalence of depression, anxiety, and sleep disorder among patients coping with post COVID-19 syndrome (Long COVID): A systematic review and meta-analysis. BMC Psychiatry. (2024) 24:105. doi: 10.1186/s12888-023-05481-6
10. Taquet M, Skorniewska Z, Zetterberg H, Geddes JR, Mummery CJ, Chalmers JD, et al. Post-acute COVID-19 neuropsychiatric symptoms are not associated with ongoing nervous system injury. Brain Commun. (2024) 6:fcad357. doi: 10.1093/braincomms/fcad357
11. Zhang Y, Chinchilli VM, Ssentongo P, Ba DM. Association of long COVID with mental health disorders: A retrospective cohort study using real-world data from the USA. BMJ Open. (2024) 14:e079267. doi: 10.1136/bmjopen-2023-079267
12. Hyassat D, El-Khateeb M, Dahbour A, Shunnaq S, Naji D, Bani Ata E, et al. Post-COVID-19 syndrome among healthcare workers in Jordan. East Mediterr Health J. (2023) 29:247–53. doi: 10.26719/emhj.23.029
13. D’Ávila KG, Monaiar LR, Dantas LDP, Freitas AA, Rodrigues MM, Bonamigo RR, et al. Decrease in health-related quality of life and post-COVID-19 syndrome in healthcare workers after sars-cov-2 infection: A cohort study. J Occup Environ Med. (2022) 65(1):e1–3. doi: 10.1097/jom.0000000000002727
14. Fouad MM, Zawilla NH, Maged LA. Work performance among healthcare workers with post COVID-19 syndrome and its relation to antibody response. Infection. (2022) 51:83949. doi: 10.1007/s15010-022-01942-4
15. Mendola M, Leoni M, Cozzi Y, Manzari A, Tonelli F, Metruccio F, et al. Long-term COVID symptoms, work ability and fitness to work in healthcare workers hospitalized for sars-cov-2 infection. Med Lav. (2022) 113:e2022040. doi: 10.23749/mdl.v113i5.13377
16. Fernandez-de-Las-Penas C, Martin-Guerrero JD, Cancela-Cilleruelo I, Moro-Lopez-Menchero P, Rodriguez-Jimenez J, Pellicer-Valero OJ. Trajectory curves of post-COVID anxiety/depressive symptoms and sleep quality in previously hospitalized COVID-19 survivors: the long-COVID-exp-cm multicenter study. Psychol Med. (2023) 53:4298–9. doi: 10.1017/S003329172200006X
17. Guillen-Burgos HF, Galvez-Florez JF, Moreno-Lopez S, Gonzalez I, Guillen M, Anaya JM. Factors associated with mental health outcomes after COVID-19: A 24-month follow-up longitudinal study. Gen Hosp Psychiatry. (2023) 84:241–9. doi: 10.1016/j.genhosppsych.2023.08.009
18. Van Wambeke E, Bezler C, Kasprowicz AM, Charles AL, Andres E, Geny B. Two-years follow-up of symptoms and return to work in complex post-COVID-19 patients. J Clin Med. (2023) 12(3):741. doi: 10.3390/jcm12030741
19. Wahlgren C, Forsberg G, Divanoglou A, Ostholm Balkhed A, Niward K, Berg S, et al. Two-year follow-up of patients with post-COVID-19 condition in Sweden: A prospective cohort study. Lancet Reg Health Eur. (2023) 28:100595. doi: 10.1016/j.lanepe.2023.100595
20. Barker-Davies RM, O’Sullivan O, Holdsworth DA, Ladlow P, Houston A, Chamley R, et al. How long is long-COVID? Symptomatic improvement between 12 and 18 months in a prospective cohort study. BMJ Mil Health. (2023) 171(2):126–33. doi: 10.1136/military-2023-002500
21. Reuken PA, Besteher B, Finke K, Fischer A, Holl A, Katzer K, et al. Longterm course of neuropsychological symptoms and me/cfs after sars-cov-2-infection: A prospective registry study. Eur Arch Psychiatry Clin Neurosci. (2023) 274(8):1903–10. doi: 10.1007/s00406-023-01661-3
22. Steinmetz A, Gross S, Lehnert K, Lucker P, Friedrich N, Nauck M, et al. Longitudinal clinical features of post-COVID-19 patients-symptoms, fatigue and physical function at 3- and 6-month follow-up. J Clin Med. (2023) 12(12):3966. doi: 10.3390/jcm12123966
23. Hartung TJ, Bahmer T, Chaplinskaya-Sobol I, Deckert J, Endres M, Franzpotter K, et al. Predictors of non-recovery from fatigue and cognitive deficits after COVID-19: A prospective, longitudinal, population-based study. EClinicalMedicine. (2024) 69:102456. doi: 10.1016/j.eclinm.2024.102456
24. Martin EM, Srowig A, Utech I, Schrenk S, Kattlun F, Radscheidt M, et al. Persistent cognitive slowing in post-COVID patients: longitudinal study over 6 months. J Neurol. (2023) 271:46–58. doi: 10.1007/s00415-023-12069-3
25. Fernandez-de-Las-Penas C, Cancela-Cilleruelo I, Rodriguez-Jimenez J, Arias-Navalon JA, Martin-Guerrero JD, Pellicer-Valero OJ, et al. Trajectory of post-COVID brain fog, memory loss, and concentration loss in previously hospitalized COVID-19 survivors: the long-COVID-exp multicenter study. Front Hum Neurosci. (2023) 17:1259660. doi: 10.3389/fnhum.2023.1259660
26. Peters C, Dulon M, Westermann C, Kozak A, Nienhaus A. Long-term effects of COVID-19 on workers in health and social services in Germany. Int J Env Res Public Health. (2022) 19:6983. doi: 10.3390/ijerph19126983
27. Omar AKA-E, Dahesh SMA, Ellakwa DE-S, Gomaa MK, Abdulsamad B, Hanafy R, et al. Cognitive impairment in health care workers recovering from COVID-19 infection: A cross-sectional comparative study. Middle East Curr Psychiatr. (2022) 29(1):79. doi: 10.1186/s43045-022-00245-6
28. Ahmed I, Mustafaoglu R, Yeldan I, Yasaci Z, Erhan B. Effect of pulmonary rehabilitation approaches on dyspnea, exercise capacity, fatigue, lung functions, and quality of life in patients with COVID-19: A systematic review and meta-analysis. Arch Phys Med Rehabil. (2022) 103:2051–62. doi: 10.1016/j.apmr.2022.06.007
29. Chen H, Shi H, Liu X, Sun T, Wu J, Liu Z. Effect of pulmonary rehabilitation for patients with post-COVID-19: A systematic review and meta-analysis. Front Med. (2022) 9:837420. doi: 10.3389/fmed.2022.837420
30. Hayden MC, Schuler M, Limbach M, Schwarzl G, Stenzel N, Nowak D, et al. Patient-reported outcomes (Pros) 3 and 6 months after pulmonary rehabilitation following COVID-19. Rehabil (Stuttg). (2023) 62:349–58. doi: 10.1055/a-2134-2142
31. Rutsch M, Frommhold J, Buhr-Schinner H, Gross T, Schuller PO, Deck R. Pneumologische Rehabilitation bei Long COVID – Gesundheitliche Veränderungen am Ende der statioären Rehabilitationsmaßnahme. Rehabil (Stuttg). (2023) 62:359–68. doi: 10.1055/a-1964-7401
32. Kupferschmitt A, Langheim E, Tuter H, Etzrodt F, Loew TH, Köllner V. First results from post-COVID inpatient rehabilitation. Front Rehabil Sci. (2023) 3:1093871. doi: 10.3389/fresc.2022.1093871
33. Müller K, Poppele I, Ottiger M, Zwingmann K, Berger I, Thomas A, et al. Impact of rehabilitation on physical and neuropsychological health of patients who acquired COVID-19 in the workplace. Int J Env Res Public Health. (2023) 20(2):1468. doi: 10.3390/ijerph20021468
34. Al-Mhanna SB, Mohamed M, Noor NM, Afolabi HA, Irekeola AA, Bello KE, et al. Effectiveness of pulmonary rehabilitation among COVID-19 patients: A systematic review and meta-analysis. Healthcare (Basel). (2022) 10:2130. doi: 10.3390/healthcare10112130
35. Ceban F, Ling S, Lui LMW, Lee Y, Gill H, Teopiz KM, et al. Fatigue and cognitive impairment in post-COVID-19 syndrome: A systematic review and meta-analysis. Brain Behav Immun. (2022) 101:93–135. doi: 10.1016/j.bbi.2021.12.020
36. Nopp S, Moik F, Klok FA, Gattinger D, Petrovic M, Vonbank K, et al. Outpatient pulmonary rehabilitation in patients with long COVID improves exercise capacity, functional status, dyspnea, fatigue, and quality of life. Respiration. (2022) 101:593–601. doi: 10.1159/000522118
37. Gloeckl R, Leitl D, Schneeberger T, Jarosch I, Koczulla AR. Rehabilitative interventions in patients with persistent post COVID-19 symptoms-a review of recent advances and future perspectives. Eur Arch Psychiatry Clin Neurosci. (2023) 274:1819–28. doi: 10.1007/s00406-023-01631-9
38. Weinbrenner S, Asgari S, de Masi A, Gehring M, Hessel A, Hofmann M, et al. Eckpunktepapier für die Medizinische Rehabilitation bei Post-COVID-Syndrom. https://www.deutsche-rentenversicherung.de/SharedDocs/Downloads/DE/Experten/infos_reha_einrichtungen/eckpunkte-reha-post-covid-syndrom-10-2023.html: Deutsche Rentenversicherung. (2023).
39. Marshall-Andon T, Walsh S, Berger-Gillam T, Pari AAA. Systematic review of post-COVID-19 syndrome rehabilitation guidelines. Integr Healthc J. (2022) 4:e000100. doi: 10.1136/ihj-2021-000100
40. WHO. Clinical management of COVID-19: living guideline (2023). Available online at: https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2023.1 (Accessed March 15, 2024).
41. Müller K, Zwingmann K, Auerswald T, Berger I, Thomas A, Schultz AL, et al. Rehabilitation and return-to-work of patients acquiring COVID-19 in the workplace: A study protocol for an observational cohort study. Front Rehabil Sci. (2022) 2:754468. doi: 10.3389/fresc.2021.754468
42. Lampert T, Kroll L, Müters S, Stolzenberg H. Messung des sozioökonomischen Status in der Studie zur Gesundheit Erwachsener in Deutschland (DEGS1). Bundesgesundheitsbl. (2013) 56:631–6. doi: 10.1007/s00103-012-1663-4
43. Scheidt-Nave C, Kamtsiuris P, Gößwald A, Hölling H, Lange M, Busch MA, et al. German health interview and examination survey for adults (DEGS)-design, objectives and implementation of the first data collection wave. BMC Public Health. (2012) 12:1–16. doi: 10.1186/1471-2458-12-730
44. Hasselhorn H-M, Freude G. Der Work-Ability-Index: Ein Leitfaden. Bremerhaven: Wirtschaftsverlag NW (2007). p. 54.
45. Klok FA, Boon GJ, Barco S, Endres M, Geelhoed JM, Knauss S, et al. The post-COVID-19 functional status scale: A tool to measure functional status over time after COVID-19. Eur Respir J. (2020) 56(1):2001494. doi: 10.1183/13993003.01494-2020
46. Hermann-Lingen C, Buss U, Snaith R. Hads-D Hospital Anxiety and Depression Scale-German Version. 4. ed. Bern: Hogrefe (2018). p. 72.
47. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. (1983) 67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x
48. Lemay KR, Tulloch HE, Pipe AL, Reed JL. Establishing the minimal clinically important difference for the hospital anxiety and depression scale in patients with cardiovascular disease. J Cardiopulm Rehabil Prev. (2019) 39:E6–e11. doi: 10.1097/hcr.0000000000000379
49. Toussaint A, Löwe B, Brähler E, Jordan P. The somatic symptom disorder-B criteria scale (Ssd-12): factorial structure, validity and population-based norms. J Psychosom Res. (2017) 97:9–17. doi: 10.1016/j.jpsychores.2017.03.017
50. Volz F, Lahmann C, Wolf K, Fung C, Shah MJ, Lutzen N, et al. More than a headache-somatic and mental symptom burden in spontaneous intracranial hypotension before and after surgical treatment. Front Neurol. (2024) 15:1421579. doi: 10.3389/fneur.2024.1421579
51. Cloitre M, Shevlin M, Brewin CR, Bisson JI, Roberts NP, Maercker A, et al. The international trauma questionnaire: development of a self-report measure of ICD-11 ptsd and complex ptsd. Acta Psychiatr Scand. (2018) 138:536–46. doi: 10.1111/acps.12956
52. Mendoza TR, Wang XS, Cleeland CS, Morrissey M, Johnson BA, Wendt JK, et al. The rapid assessment of fatigue severity in cancer patients: use of the brief fatigue inventory. Cancer. (1999) 85:1186–96. doi: 10.1002/(sici)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.co;2-n
53. Radbruch L, Sabatowski R, Elsner F, Everts J, Mendoza T, Cleeland C. Validation of the german version of the brief fatigue inventory. J Pain Symptom Manage. (2003) 25:449–58. doi: 10.1016/s0885-3924(03)00073-3
54. Gunn HJ, Zaniletti I, Breen WG, Leavitt T, Bogan A, Mahajan A, et al. Establishing the minimal clinically important difference of the brief fatigue inventory for brain or CNS cancer patients undergoing radiotherapy. Neuro-Oncology Pract. (2024) 11:633–9. doi: 10.1093/nop/npae034
55. Fisk JD, Ritvo PG, Ross L, Haase DA, Marrie TJ, Schlech WF. Measuring the functional impact of fatigue: initial validation of the fatigue impact scale. Clin Infect Dis. (1994) 18:79–83. doi: 10.1093/clinids/18.Supplement_1.S79
56. Rendas-Baum R, Yang M, Cattelin F, Wallenstein GV, Fisk JD. A novel approach to estimate the minimally important difference for the fatigue impact scale in multiple sclerosis patients. Qual Life Res. (2010) 19:1349–58. doi: 10.1007/s11136-010-9704-7
57. Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The montreal cognitive assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc. (2005) 53:695–9. doi: 10.1111/j.1532-5415.2005.53221.x
58. Wong GKC, Mak JSY, Wong A, Zheng VZY, Poon WS, Abrigo J, et al. Minimum clinically important difference of montreal cognitive assessment in aneurysmal subarachnoid hemorrhage patients. J Clin Neurosci. (2017) 46:41–4. doi: 10.1016/j.jocn.2017.08.039
59. Jaeger J. Digit symbol substitution test: the case for sensitivity over specificity in neuropsychological testing. J Clin Psychopharmacol. (2018) 38:513–9. doi: 10.1097/JCP.0000000000000941
60. Jehu DA, Davis JC, Madden K, Parmar N, Liu-Ambrose T. Minimal clinically important difference of executive function performance in older adults who fall: A secondary analysis of a randomized controlled trial. Gerontology. (2021) 68:771–9. doi: 10.1159/000518939
61. Reitan RM, Wolfson D. Category test and trail making test as measures of frontal lobe functions. Clin Neuropsychologist. (1995) 9:50–6. doi: 10.1080/13854049508402057
62. Borland E, Edgar C, Stomrud E, Cullen N, Hansson O, Palmqvist S. Clinically relevant changes for cognitive outcomes in preclinical and prodromal cognitive stages. Neurology. (2022) 99:e1142–e53. doi: 10.1212/WNL.0000000000200817
63. Fritz CO, Morris PE, Richler JJ. Effect size estimates: current use, calculations, and interpretation. J Exp Psychol. (2012) 141:2–18. doi: 10.1037/a0024338
64. Morioka S, Tsuzuki S, Maruki T, Terada M, Miyazato Y, Kutsuna S, et al. Epidemiology of post-COVID conditions beyond 1 year: A cross-sectional study. Public Health. (2023) 216:39–44. doi: 10.1016/j.puhe.2023.01.008
65. Crivelli L, Palmer K, Calandri I, Guekht A, Beghi E, Carroll W, et al. Changes in cognitive functioning after COVID-19: A systematic review and meta-analysis. Alzheimers Dement. (2022) 18:1047–66. doi: 10.1002/alz.12644
66. Dressing A, Bormann T, Blazhenets G, Schroeter N, Walter LI, Thurow J, et al. Neuropsychologic profiles and cerebral glucose metabolism in neurocognitive long COVID syndrome. J Nucl Med. (2022) 63:1058–63. doi: 10.2967/jnumed.121.262677
67. Hartung TJ, Neumann C, Bahmer T, Chaplinskaya-Sobol I, Endres M, Geritz J, et al. Fatigue and cognitive impairment after COVID-19: A prospective multicentre study. eClinicalMedicine. (2022) 53:101651. doi: 10.1016/j.eclinm.2022.101651
68. Delgado-Alonso C, Cuevas C, Oliver-Mas S, Diez-Cirarda M, Delgado-Alvarez A, Gil-Moreno MJ, et al. Fatigue and cognitive dysfunction are associated with occupational status in post-COVID syndrome. Int J Env Res Public Health. (2022) 19(20). doi: 10.3390/ijerph192013368
69. Pauwels S, Boets I, Polli A, Mylle G, De Raeve H, Godderis L. Return to Work after Long COVID: Evidence at 8th March 2021. (2021). https://www.hse.gov.uk/research/assets/docs/return-to-work-after-long-covid.pdf (Accessed March 4, 2025).
70. Mulfinger N, Lampl J, Dinkel A, Weidner K, Beutel ME, Jarczok MN, et al. Psychische Belastungen durch Epidemien bei Beschäftigten im Gesundheitswesen und Implikationen für die Bewältigung der Corona-Krise: eine Literaturübersicht. Z Psychosom Med Psychother. (2020) 66:220–42. doi: 10.13109/zptm.2020.66.issue-3
71. Schumann M, Marschall J, Hildebrandt S, Nolting H. Gesundheitsreport 2022. In: Storm A, editor. Beiträge zur Gesundheitsökonomie und Versorgungsforschung (Band 39). medhochzwei-Verlag, Heidelberg (2022). p. 186.
72. Schuch FB, Vancampfort D, Firth J, Rosenbaum S, Ward PB, Silva ES, et al. Physical activity and incident depression: A meta-analysis of prospective cohort studies. Am J Psychiatry. (2018) 175:631–48. doi: 10.1176/appi.ajp.2018.17111194
73. Yohannes AM, Willgoss TG, Baldwin RC, Connolly MJ. Depression and anxiety in chronic heart failure and chronic obstructive pulmonary disease: prevalence, relevance, clinical implications and management principles. Int J Geriatr Psychiatry. (2010) 25:1209–21. doi: 10.1002/gps.2463
74. Gunnarsson DV, Miskowiak KW, Pedersen JK, Hansen H, Podlekareva D, Johnsen S, et al. Physical function and association with cognitive function in patients in a post-COVID-19 clinic-a cross-sectional study. Int J Env Res Public Health. (2023) 20(10). doi: 10.3390/ijerph20105866
75. Barlow P, Thow AM. Neoliberal discourse, actor power, and the politics of nutrition policy: A qualitative analysis of informal challenges to nutrition labelling regulations at the world trade organization, 2007-2019. Soc Sci Med. (2021) 273:113761. doi: 10.1016/j.socscimed.2021.113761
76. Carvalho Â, Ferreira G, Seixas D, Guimarães-Teixeira C, Henrique R, Monteiro FJ, et al. Emerging lab-on-a-chip approaches for liquid biopsy in lung cancer: Status in CTCs and ctDNA research and clinical validation. Cancers. (2021) 13(9). doi: 10.3390/cancers13092101
77. Keane L, Antignano I, Riechers SP, Zollinger R, Dumas AA, Offermann N, et al. mTOR-dependent translation amplifies microglia priming in aging mice. J Clin Invest. (2021) 131(1):e132727. doi: 10.1172/jci132727
78. Aksoy O, Wu AF, Aksoy S, Rivas C. Social support and mental well-being among people with and without chronic illness during the COVID-19 pandemic: Evidence from the Longitudinal UCL Covid Survey. BMC Psychol. (2024) 12:136. doi: 10.1186/s40359-024-01596-x
79. Wickramaratne PJ, Yangchen T, Lepow L, Patra BG, Glicksburg B, Talati A, et al. Social connectedness as a determinant of mental health: A scoping review. PloS One. (2022) 17:e0275004. doi: 10.1371/journal.pone.0275004
80. Kachaner A, Lemogne C, Dave J, Ranque B, de Broucker T, Meppiel E. Somatic symptom disorder in patients with post-COVID-19 neurological symptoms: A preliminary report from the somatic study (Somatic symptom disorder triggered by COVID-19). J Neurol Neurosurg Psychiatry. (2022) 93:1174–80. doi: 10.1136/jnnp-2021-327899
81. Pignon B, Matta J, Wiernik E, Toussaint A, Loewe B, Robineau O, et al. Psychological burden associated with incident persistent symptoms and their evolution during the COVID-19 pandemic: A prospective population-based study. BMJ Ment Health. (2024) 27(1):e300907. doi: 10.1136/bmjment-2023-300907
82. Ouvrard C, Berr C, Meillon C, Ribet C, Goldberg M, Zins M, et al. Norms for standard neuropsychological tests from the french constances cohort. Eur J Neurol. (2019) 26:786–93. doi: 10.1111/ene.13890
83. Rahimi F, Saadat M, Hessam M, Ravanbakhsh M, Monjezi S. Post-COVID-19 physical and cognitive impairments and associations with quality of life: A cross-sectional study. Front Sports Act Living. (2024) 6:1246585. doi: 10.3389/fspor.2024.1246585
84. Lynch S, Ferrando SJ, Dornbush R, Shahar S, Smiley A, Klepacz L. Screening for brain fog: is the montreal cognitive assessment an effective screening tool for neurocognitive complaints post-COVID-19? Gen Hosp Psychiatry. (2022) 78:80–6. doi: 10.1016/j.genhosppsych.2022.07.013
85. Mackenbach JP, Stirbu I, Roskam A-J, Schaap MM, Menvielle G, Leinsalu M, et al. Socioeconomic inequalities in health in 22 european countries. New Engl J Med. (2008) 23(358):2468–81. doi: 10.1056/NEJMsa0707519
86. Stringhini S, Carmeli C, Jokela M, Avendano M, Muennig P, Guida F, et al. Socioeconomic status and the 25 X 25 risk factors as determinants of premature mortality: A multicohort study and meta-analysis of 1.7 million men and women. Lancet. (2017) 389:1229–37. doi: 10.1016/S0140-6736(16)32380-7
87. Pantelic M, Ziauddeen N, Boyes M, O’Hara ME, Hastie C, Alwan NA. Long COVID stigma: estimating burden and validating scale in a UK-based sample. PloS One. (2022) 17:e0277317. doi: 10.1371/journal.pone.0277317
88. Schomerus G, Riedel-Heller S. Focus on the stigma of mental disease. Nervenarzt. (2020) 91:777–8. doi: 10.1007/s00115-020-00964-3
89. Baum E, Lindner N, Andreas S, Behrends U, Scheibenbogen C, Chritsmann T, et al. S3-Leitlinie Müdigkeit. (2022). https://register.awmf.org/assets/guidelines/053-002l_S3_Muedigkeit_2023-01_01.pdf: AWMF (Accessed March 24, 2025).
90. Gloeckl R, Zwick RH, Furlinger U, Schneeberger T, Leitl D, Jarosch I, et al. Practical recommendations for exercise training in patients with long COVID with or without post-exertional malaise: A best practice proposal. Sports Med Open. (2024) 10:47. doi: 10.1186/s40798-024-00695-8
91. Estebanez-Pérez MJ, Martín-Valero R, Vinolo-Gil MJ, Pastora-Bernal JM. Effectiveness of digital physiotherapy practice compared to usual care in long COVID patients: A systematic review. Healthcare (Basel). (2023) 11(13):1970. doi: 10.3390/healthcare11131970
92. Ndwabe H, Basu A, Mohammed J. Post pandemic analysis on comprehensive utilization of telehealth and telemedicine. Clin eHealth. (2024) 7:5–14. doi: 10.1016/j.ceh.2023.12.002
93. Pinnock H, Murphie P, Vogiatzis I, Poberezhets V. Telemedicine and virtual respiratory care in the era of COVID-19. ERJ Open Res. (2022) 8(3):00111. doi: 10.1183/23120541.00111-2022
94. Huang J, Fan Y, Zhao K, Yang C, Zhao Z, Chen Y, et al. Do patients with and survivors of COVID-19 benefit from telerehabilitation? A meta-analysis of randomized controlled trials. Front Public Health. (2022) 10:954754. doi: 10.3389/fpubh.2022.954754
95. Bond AE, Wagler K, Anestis MD. Essential workers: past month suicidal ideation and COVID-19 stress. J Clin Psychol. (2021) 77:2849–59. doi: 10.1002/jclp.23276
96. WHO. Delivered by Women, Led by Men: A Gender and Equity Analysis of the Global Health and Social Workforce. Geneva: World Health Organization (2019).
97. Coen RF, Robertson DA, Kenny RA, King-Kallimanis BL. Strengths and limitations of the MoCA for assessing cognitive functioning: findings from a large representative sample of irish older adults. J Geriatr Psychiatry Neurol. (2016) 29:18–24. doi: 10.1177/0891988715598236
98. Cersonsky TEK, Mechery S, Carper MM, Thompson L, Lee A, Alber J, et al. Using the montreal cognitive assessment to identify individuals with subtle cognitive decline. Neuropsychology. (2022) 36:373–83. doi: 10.1037/neu0000820
99. Peter RS, Nieters A, Gopel S, Merle U, Steinacker JM, Deibert P, et al. Persistent symptoms and clinical findings in adults with post-acute sequelae of COVID-19/post-COVID-19 syndrome in the second year after acute infection: A population-based, nested case-control study. PloS Med. (2025) 22:e1004511. doi: 10.1371/journal.pmed.1004511
Keywords: work-related COVID-19, post-COVID, mental health, depression, cognitive health, rehabilitation, long-term outcomes
Citation: Müller K, Poppele I, Ottiger M, Weber R-C, Stegbauer M and Schlesinger T (2025) Course of neuropsychological health in post-COVID patients differs 6 and 12 months after inpatient rehabilitation. Front. Psychiatry 16:1460097. doi: 10.3389/fpsyt.2025.1460097
Received: 05 July 2024; Accepted: 12 March 2025;
Published: 25 April 2025.
Edited by:
Maria Chiara Maccarone, University of Padua, ItalyReviewed by:
João Carlos Alchieri, Federal University of Rio Grande do Norte, BrazilEduardo Fernández-Jiménez, Hospital Infantil La Paz, Spain
Copyright © 2025 Müller, Poppele, Ottiger, Weber, Stegbauer and Schlesinger. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Katrin Müller, a2F0cmluLm11ZWxsZXJAaHN3LnR1LWNoZW1uaXR6LmRl
 Katrin Müller
Katrin Müller Iris Poppele
Iris Poppele Marcel Ottiger
Marcel Ottiger Rainer-Christian Weber2
Rainer-Christian Weber2 Michael Stegbauer
Michael Stegbauer Torsten Schlesinger
Torsten Schlesinger