- 1Advanced Science Research Center at The Graduate Center, Neuroscience Initiative, City University of New York, New York, NY, United States
- 2Department of Psychology, Queens College, City University of New York, New York, NY, United States
- 3Department of Psychology, the Graduate Center, City University of New York, New York, NY, United States
- 4Beijing Key Lab of Learning and Cognition, School of Psychology, Capital Normal University, Beijing, China
- 5Graduate School of Health Innovation, Kanagawa University of Human Services, Kawasaki, Japan
- 6Research Centre for Child Mental Development, Hamamatsu University School of Medicine, Hamamatsu, Japan
- 7Department of Psychiatry, Icahn School of Medicine at Mount Sinai, New York, NY, United States
- 8Department of Epidemiology and Biostatistics, Graduate School of Public Health and Health Policy, City University of New York, New York, NY, United States
Introduction: Prenatal maternal stress may predispose a child to alterations in neurodevelopment and future psychopathology. Meanwhile, environmental disasters related to climate change are increasing in severity with significant impacts on physical and mental health. The current study explores the relationships among child behaviors, brain morphometry, and weather-related in-utero stress during Superstorm Sandy (SS).
Methods: Parents completed the Behavioral Assessment System for Children, Second Edition (BASC-2) to quantify the extent of adaptive and clinical (externalizing/internalizing) behaviors at age 5. Magnetic resonance imaging of 9 SS-exposed and 21 non-exposed children at age 8 was used to assess brain volume. We analyzed main effects of in-utero SS exposure on brain volume/behavior and mediation-moderation models of exposure, behaviors and brain volume to determine how the association between exposure and brain volume is influenced by early childhood behavioral phenotypes.
Results: The SS-exposed group had significantly greater externalizing behavioral problems, bilateral amygdala enlargement, and volumetric reduction of the left medial orbitofrontal cortex. While no behavioral phenotype mediated the association of exposure with brain volume, adaptive behaviors, as measured by four subdomains of the BASC-2 (social skills, activities in daily life, functional communication, and adaptivity), moderated the adverse impact of in-utero stress on brain volume later in life.
Discussion: These findings highlight the importance of evaluating the interactive relationships among in-utero stress, behaviors, and neural development of the child to facilitate early identification and intervention for more vulnerable children. Promoting adaptive behaviors in early childhood may minimize the deleterious impact of prenatal stress exposure on subsequent brain development.
1 Introduction
Climate change poses an increasing threat to mental health. The growing frequency and intensity of hurricanes, tropical storms, wildfires, flooding, and droughts are disrupting social and economic stability and disproportionately endangering vulnerable populations such as pregnant women (1, 2). As a result of such environmental crises, prenatal maternal stress (PNMS), which broadly represents various forms of psychological distress during pregnancy (3, 4), has emerged as a target for intervention in recent years (5). Accumulating evidence demonstrates that elevated maternal anxiety and/or depressive symptoms during pregnancy are associated with child and adolescent externalizing behaviors such as elevated hyperactivity, aggression, and attention deficits, and internalizing behaviors such as elevated anxiety, depression, and somatic complaints (4, 6–17). However, more limited research has examined the role of adaptive behaviors on optimal child development (18). Adaptive behaviors refer to the behaviors and/or skill sets that are required for children to meet their basic needs for self-care, decision-making, communicating, and learning. More recent research shows that exposure to PNMS could facilitate adaptive behaviors, thereby acting as an inoculation to later life psychopathological outcomes (19). Various mechanisms and findings support this counterintuitive claim. Ceniceros et al. have shown maternal stress from temporary hospitalization-related relocation during pregnancy to lead to increased resilience in offspring observed from increased activity, decreased anxiety, increased interaction with novel objects, and increased temperamental confidence (20). Bondarenko et al. suggest a biological mechanism whereby PNMS leads to increases in placental and fetal serotonin levels which then mediate increased reactive and adaptive behaviors in offspring in mice studies (21). Several human studies (22–24) have shown that moderate levels of prenatal stress have an inoculation effect on developing infants, for example by being associated with advanced mental and motor skills, thereby promoting resilience (23, 25). However, neither the relationship between weather related in-utero stress and child brain development, nor potential positive influences of adaptive behaviors on promoting resilience among such populations, have been studied extensively.
Investigation of the underlying mechanisms linking maternal experiences during pregnancy to future child psychopathology has implicated dysregulation of the fetal hypothalamic-pituitary-adrenal (HPA)-axis, which in turn affects early neuronal development and leads to an increase in the offspring’s susceptibility to somatic diseases and mental health problems (3, 4). To evaluate the consequences of maternal stress on child neurophysiological development, magnetic resonance imaging (MRI) is often used for quantitative brain phenotyping. As the brain develops from the prenatal period through to infancy and early childhood, the limbic and prefrontal regions are at high risk of growth perturbations as they are most sensitive to stress exposure (26, 27). Further, the limbic and prefrontal regions form a highly interconnected circuit, allowing for coupling effects to spread alterations from one brain region to another, impacting related cognitive processes. For example, the amygdala has been shown to form extensive bidirectional connections with the orbitofrontal cortices, which in turn have been shown to influence emotion regulation and decision making (28, 29). The amygdala is a subcortical brain region functionally responsible for integrating sensory information and processing their emotional saliency. It is traditionally known as the “fight or flight” center of the brain and is involved in regulating the autonomic nervous and endocrine systems (30). Several disorders such as anxiety disorders, social phobias, intermittent explosive disorder, post-traumatic stress disorder, and panic disorders are linked to alterations in the amygdala and its connectivity to other brain regions. Key regions in the prefrontal cortex, such as the medial orbitofrontal cortex, are heavily implicated in regulating emotional behavior by projecting inhibitory connections to the amygdala (31). Connections between these regions develop rapidly in childhood and continue to mature into the third or fourth decade of life (32). Therefore, we focus the current study on the amygdala and medial orbitofrontal cortices.
Prior neuroimaging studies have aimed to pinpoint the structural and functional changes that occur in these brain regions to understand their relationship with behavioral changes among children exposed to prenatal stress (33). Interestingly, elevated stress has been found to be associated with both decreased and increased brain structure size. For example, some studies have reported a smaller amygdala volume among young adults exposed to elevated maternal stress during the first half of pregnancy (34), while others have reported a larger right amygdala in 4.5 year old girls exposed to elevated prenatal maternal depressive symptoms (35). In the frontal lobe, results also appear mixed. Heightened maternal anxiety during mid-gestation has been associated with reductions in gray matter density in the prefrontal cortex among child offspring, aged 6 to 9 (36). Reductions in the thickness of frontal and temporal cortical regions were also seen among child offspring born to mothers with depression (37, 38). In contrast, other studies have found increased cortical thickness in frontal regions to be associated with elevated prenatal maternal cortisol levels (39).
Brain structure has also been examined as a mediator between PNMS and child behaviors. Buss and colleagues reported that increased maternal cortisol levels during early gestation were associated with a larger right amygdala volume among girls at age 7, and amygdala volume was found to partially mediate the association between elevated maternal cortisol and affective problems (40). Similarly, Acosta and colleagues reported that elevated maternal anxiety during the second trimester was associated with a larger left amygdala volume among girls. Importantly, at 4 years of age, the volume of the left amygdala was found to partially mediate the association between maternal anxiety during pregnancy and child behaviors. However, contrary to the results of Buss et al, the partial mediation of a larger amygdala was related to a reduction, not increase, in child behavioral difficulties (41). In addition to the previous findings related to the amygdala, other work has demonstrated that prefrontal cortical thinning mediates the association between prenatal maternal depression and externalizing behaviors at around 8 years of age (42) as well as depressive symptoms during adolescence (43).
Given the complex and dynamic influences of the environment on brain and behavior, these seemingly contradictory findings are not entirely surprising, and may be due to any number of possible confounding and/or causal factors. For example, PNMS is quite general and can be subcategorized according to the source of the stressor, such as weather-related prenatal stress from natural disasters as is the case in the present study. It is also possible that associations between PNMS and neural development differ according to earlier behavioral problems or adaptive skills. However, to our knowledge, no study to date has examined both the possible mediating and moderating effects of behavioral expression earlier in childhood on associations between PNMS and subsequent neural development. While prior studies have examined the pathways from stress to later life behaviors via the indirect mediating path of neural indices, few have examined the predictiveness of behavior on later brain development. Here, we examine the role of clinical and adaptive behaviors both as potential mediators and moderators of brain morphometry as these behaviors may function through distinct developmental pathways: as mediators, these behaviors may explain the mechanisms by which prenatal stress exposure leads to changes in brain structure, while as moderators, the same behavioral patterns may influence the extent to which prenatal stress affects brain development. This dual analytical framework recognizes that both clinical symptoms and adaptive skills can serve as pathways of risk transmission and potential resilience factors.
Existing studies are also limited by temporal and etiological ambiguity surrounding the initiation and maintenance of PNMS. Measuring PNMS induced by exposure to isolated weather events, rather than from generalized psychological distress, allows for a less confounded identification of the cause and timeframe of the stressor. As an example, a quasi-experimental design paradigm explored the role of brain volume in the association between PNMS and child behavior by examining PNMS as a consequence of the 1998 Quebec Ice Storm. Among girls, an increase in left amygdala volume was found to partially mediate the positive association between prenatal maternal stress and externalizing behavioral problems. Similar results were found in the right amygdala among boys, prior to adjusting for postnatal factors (44). However, such quasi-experimental studies remain underrepresented in the literature on PNMS.
Here, we capitalize on existing behavioral data from the Stress in Pregnancy (SIP) study, a quasi-experiment with a longitudinal follow-up conducted by our group which focuses on the psychological consequences of exposure to Superstorm Sandy (SS), a large Category 3 hurricane which made landfall in Metropolitan New York in 2012 (45). To date, the SIP study has uncovered a trove of findings relating SS exposure to: clinical and adaptive behaviors (25), psychiatric disorders (46), suicidal ideation during the COVID-19 pandemic (47), changes in the placental transcriptome and infant temperament (48–50), and sex moderation of sympathetic nervous system with exposure (51). However, very little work has been done relating our behavioral findings to specific neuronal indices (52). The current work examines the relationship between PNMS, early childhood behavior, and brain development, with a specific focus on the mediating and moderating roles of early childhood behaviors on the association between in-utero SS exposure and later-life structural brain volumes of the bilateral amygdala and medial orbitofrontal cortex (mOFC). We address the following questions: 1) do SS-exposed children demonstrate different behavioral phenotypes (internalizing, externalizing, and adaptive behaviors) and fronto-limbic brain phenotypes compared to unexposed children?; 2) if so, are the effects of in-utero SS exposure on structural differences in brain development at age 8 directly or indirectly mediated by earlier-life clinical/adaptive behaviors?, and 3) do earlier-life behaviors moderate the relationship between in-utero SS exposure and brain structure? Elucidating the complex interplay of weather-related prenatal stress exposure and behavioral alterations in determining the trajectory of brain development will allow for early identification and intervention to enhance social and emotional resilience in children affected by natural disasters.
2 Materials and methods
2.1 Superstorm sandy exposure
The cohort was categorized into groups based on the timing of Superstorm Sandy exposure. Mothers of exposed children were pregnant at the time when Sandy made landfall, whereas the mothers of unexposed children were pregnant either before or after the storm (45).
2.2 Child behavior assessment
Child behaviors based on the Parent Rating Scale of the Behavioral Assessment System for Children, Second Edition (BASC-2) were reported by mothers for their school age children, at approximately age 5 (mean=4.51, SD=0.77, males/females=4.25/4.61, SD=0.36/0.86). For every item on the questionnaire, the child’s parent is asked to indicate if their child never, sometimes, often, or almost always performs a particular behavior. The BASC-2 is a well-standardized, multidimensional evaluation of the behavior of young children and measures both clinical (externalizing and internalizing problems) and adaptive (social skills, activity of daily living, functional communication, and adaptability) behaviors. Those scores were quantified using standardized T-scores (mean=50, SD=10), according to the BASC-2 system (53). Greater impairment is indicated by higher clinical (externalizing and internalizing) scores and lower adaptive scores (54). Internal consistency was excellent for the composite scales (all above α =.90).
2.3 MRI assessment
Participants were pooled from the Stress in Pregnancy (SIP) Study which has continued to follow children from the prenatal period to early adolescence since study inception in 2009 (45). Thirty children aged 5–11 years old (mean=8.50, SD=1.98, males/female=8.80/8.39, SD=1.54/2.14), participated in this pilot longitudinal study. Of those, 9 (30%) children were exposed (SS+) and 21 (70%) were not exposed to SS (SS-) in-utero. Despite the limitations of the small sample size, this pilot study can still inform future research and interventions given the initial findings and unique focus on adaptive behaviors. There were four siblings in the study (2 in SS+ and 2 in SS- groups). The protocol was approved by the Institutional Review Board at the City University of New York (CUNY). Exclusion criteria for participation in the parent study included HIV infection, maternal psychosis, maternal age <15 years, life-threatening maternal medical complications, and congenital or chromosomal abnormalities in the fetus. Exclusion criteria for MRI participation included metal implants, devices, and/or objects in the body.
2.4 MRI acquisition
All participants were scanned using a Siemens 3 Tesla Prisma MRI Scanner at the CUNY Advanced Science Research Center at approximately 8 years of age, several years after the child behavioral assessment. A mock scanner was used prior to imaging to acclimate children to the MRI environment. Children were taught to practice staying still by balancing a small toy on their nose, and the relationship between movement and good/blurry pictures was explained, similar to the “submarine protocol” (55). 3D high-resolution T1-weighted images were collected for each participant with a magnetization prepared rapid gradient echo (MP-RAGE) protocol with the following parameters: inversion time (TI)/repetition time (TR)/echo time (TE) = 1070/2500/2.9 msec, flip angle = 8.0 degrees, field of view = 256 mm × 256 mm, matrix size = 256 × 256, slice thickness = 1 mm without gap, and number of slices = 176. Real-time motion detection and correction was implemented using Volumetric Navigators (vNav) (56).
2.5 Brain tissue volume analysis
The FreeSurfer pipeline was used to generate cortical and subcortical volumetric measures (57). The skull was stripped from the T1 images and the interface between the white and grey matter was estimated, which was further refined to obtain the thickness of grey matter. Cortical surfaces were inflated and Talairach transformation was performed. The cortex was parcellated into different anatomical regions using the Destrieux atlas. The brain regional volumes were normalized by the total intracranial volume on an individual basis. Analyses focused on the bilateral amygdala and mOFC defined by the atlas, illustrated in Figure 1.
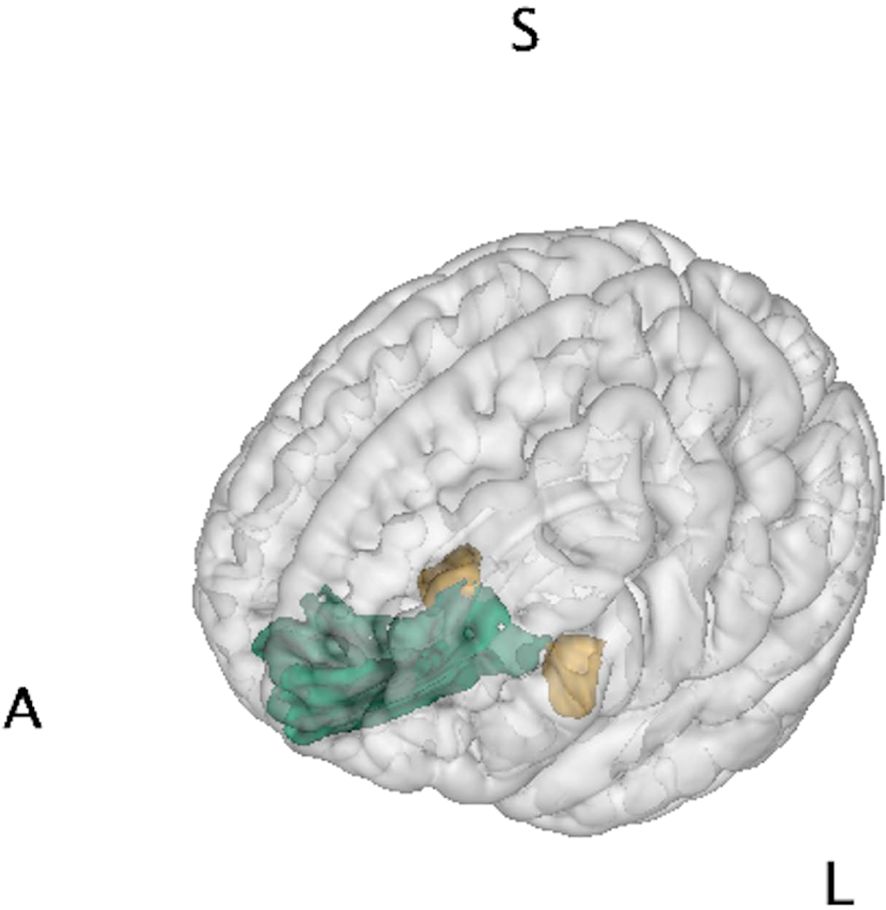
Figure 1. Brain regions of interest. Amygdala (yellow) and medial orbitofrontal cortex (green) were chosen as representative regions of the limbic system for brain-behavior analysis of Superstorm Sandy prenatal exposure. Letters denote orientation: A/S/L, anterior/superior/left.
2.6 Potential confounders
Child’s age, sex, objective SS challenges, and normative prenatal stress were considered a priori as potential confounders and were included in the models for statistical adjustment. Objective SS challenges were assessed by the Storm32 scale (46, 58) and normative prenatal stress was extracted using latent profile analysis (59) with pregnancy-related anxiety, depression symptoms, anxiety symptoms, perceived stress, and stressful life events. Normative prenatal stress was classified into three categories (low, medium, and high) (46).
2.7 Statistical approach
First, tests of normality on all outcomes in relation to Superstorm Sandy exposure were conducted using QQ plots and Shapiro-Wilk tests. If the assumption of normal distribution was violated, appropriate normalization was applied. Initial diagnostics followed by a series of linear regressions via generalized estimating equations were conducted to determine group differences in behavioral or brain phenotypes and to model the influence of earlier life behaviors as potential moderators/mediators for the relationship between in-utero SS exposure and later life brain structure (Figure 2/Supplementary Information). For all analyses, potential confounders and intrafamilial correlations (i.e., four siblings) were adjusted. Finally, to correct for multiple testing, the Benjamini-Hochberg procedure was followed with a 15% false discovery rate (60) and applied to all analyses.
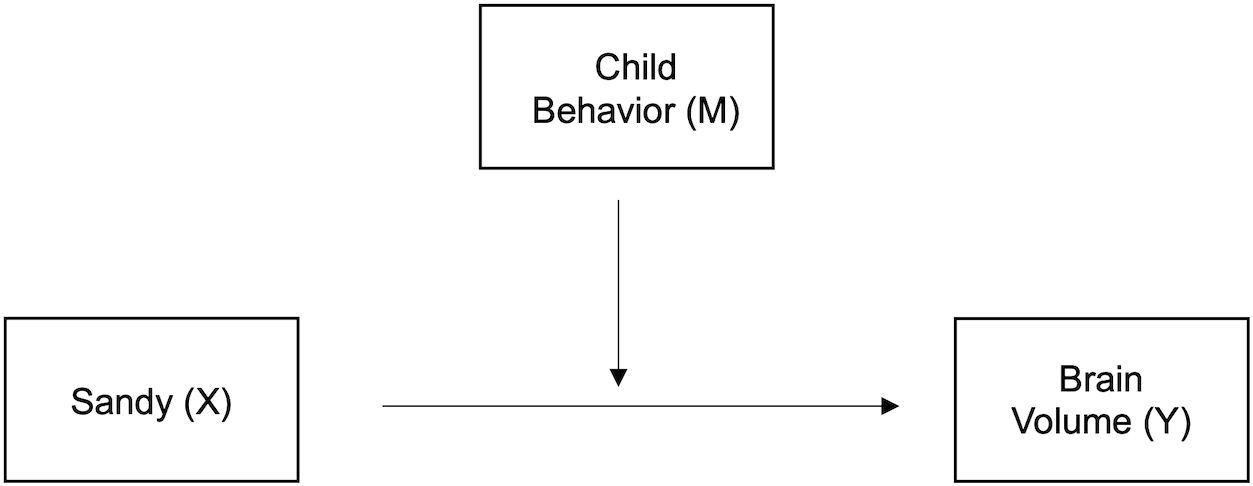
Figure 2. Moderation model. Early-life child behavior (externalizing/internalizing problems and adaptiveness) serves as the moderator, M, between Superstorm Sandy prenatal exposure, antecedent X, and brain volume during preadolescence, outcome Y.
3 Results
3.1 Demographics
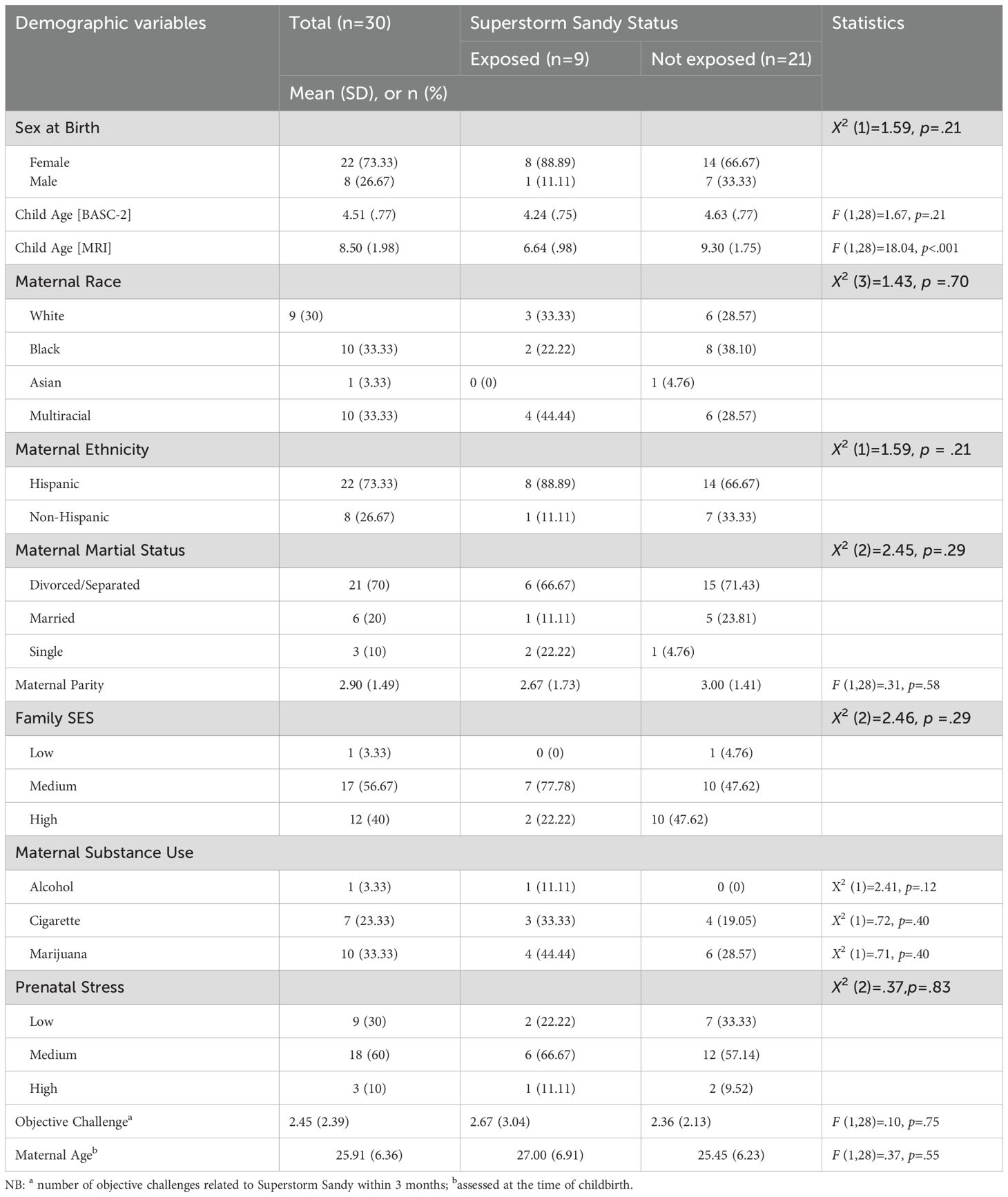
With the exception of child age at MRI assessment, there were no major demographic differences between the SS exposed and unexposed children (Table 1). The age at MRI assessment difference was expected, since to be considered SS exposed the child needed to be born during a very specific time frame.
3.2 Is in-utero SS exposure associated with clinical/adaptive behaviors and amygdala/mOFC brain structural differences during early childhood?
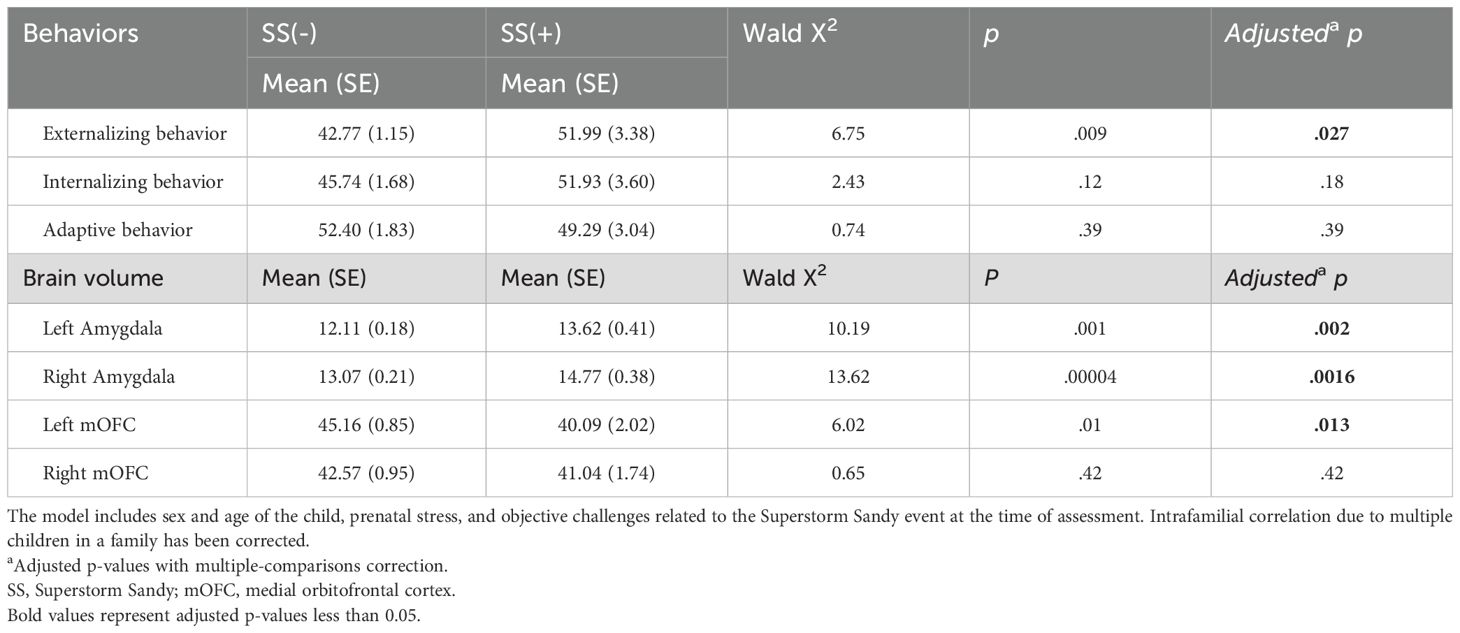
We examined the main effects of in-utero SS exposure on behaviors and brain volume (Figure 3). In-utero SS exposure was significantly associated with greater externalizing behaviors (p = .027), as we have previously found in the larger sample, indicating this subsample is relatively representative of the whole. No significant main effects were found for internalizing (p = .18) or adaptive behaviors (p = .39). In-utero SS exposure was associated with a larger left amygdala (p = .002) and right amygdala (p = .0016) as well as a smaller left mOFC (p = .013). No significant association was found between SS exposure group and the volume of the right mOFC (p = .42). Numerical results are summarized in Table 2.
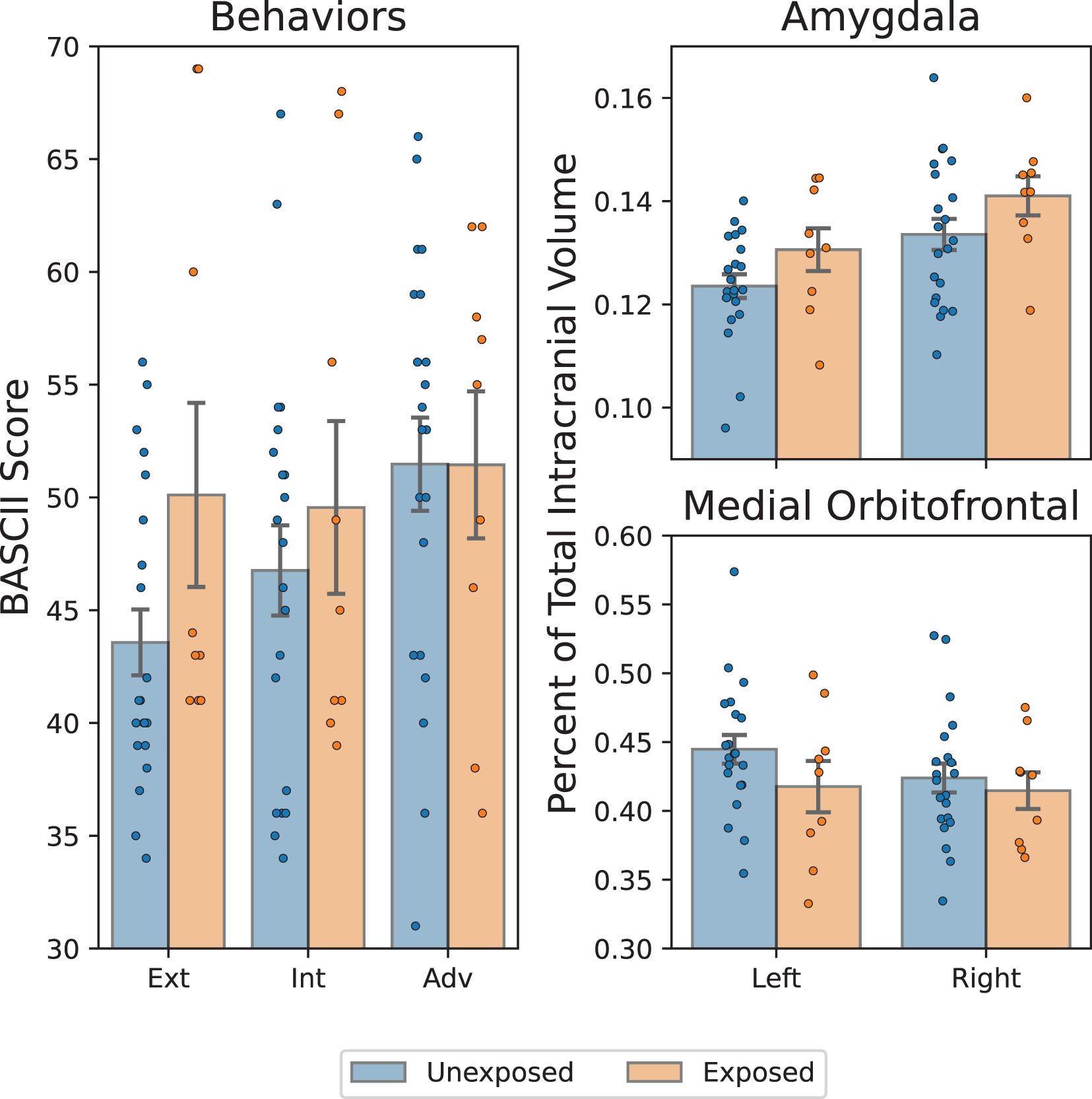
Figure 3. Comparison of in-utero hurricane exposure with childhood brain and behavior outcomes. Left: BASC-2 scores of externalizing/internalizing (ext/int) problems and adaptiveness (adv). Statistically significant greater externalizing problems among exposed group. Top/bottom right: amygdala/medial orbitofrontal cortex volume, expressed as a percentage of total intracranial volume. Significantly greater bilateral amygdala volume and significantly smaller right medial orbitofrontal cortex are present among exposed group: *p < 0.05, **p < 0.002, adjusted.
3.3 Are structural differences in brain development between SS exposed and unexposed children at age 8 directly or indirectly mediated by earlier-life clinical and/or adaptive behaviors?
We observed statistically strong direct effects of SS exposure on all four hypothesized brain structures except the right mOFC. In-utero exposure to SS was associated with increased volume of the amygdala bilaterally (p < 0.0025) and decreased volume of the left mOFC (p < 0.013). However, we observed no statistically significant indirect mediating effects of any of the 3 behavioral measures on the association of SS exposure and either amygdala or mOFC volume. Therefore, while SS exposure significantly impacted regional brain volumes, this relationship was not dependent on the presence of externalizing, internalizing, or adaptive behaviors at age 5. Supplementary Table S1 summarizes the mediation analysis results.
3.4 Do early-life child clinical and/or adaptive behaviors moderate the relationship between in-utero SS exposure and later-life brain structure?
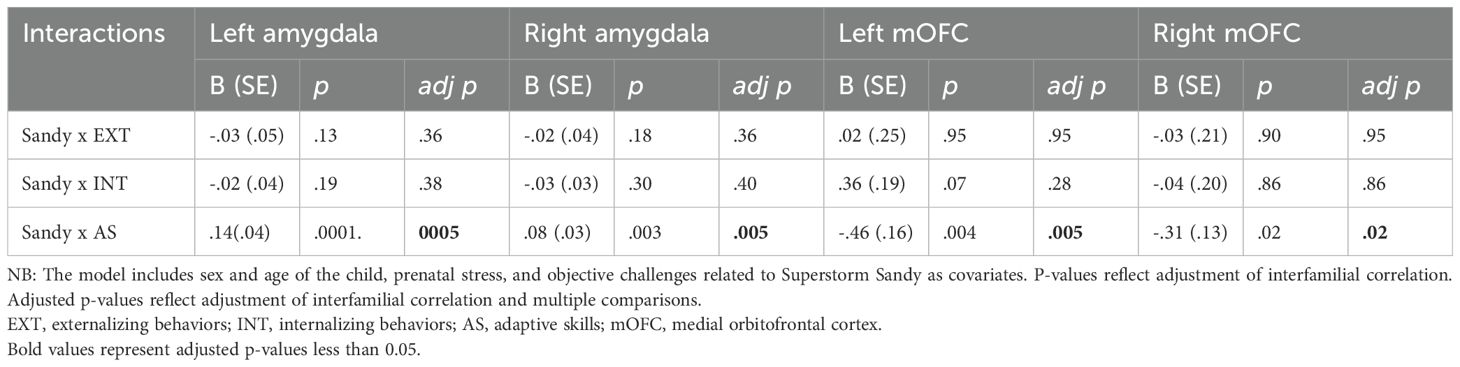
Adaptive behaviors strongly moderated the relationship between SS exposure and all four brain structures we examined. Interestingly, the change in brain volume moderated by adaptive behaviors was opposite in the bilateral amygdalae (increased) compared to the bilateral mOFC (decreased). In stark contrast, neither clinical internalizing nor externalizing behaviors moderated the relationship between exposure and any of the brain structures of interest. Specific details with respect to each brain region are summarized below.
Amygdala: The association between SS exposure and left amygdala volume was moderated by adaptive behaviors (b = .14, p = .0005, i.e., better adaptive behaviors were associated with greater amygdala volume), but not externalizing (b = -.03, p = .36) or internalizing behaviors (b = -.02, p = .38). For the right amygdala, adaptive behaviors moderated the association (b = .08, p = .005, i.e., better adaptive behaviors were associated with greater volume), but not externalizing (b = -.02, p = .36) or internalizing (b = -.03, p = .40) (Table 3).
mOFC: Similarly, the association between SS exposure and left mOFC volume was also moderated by adaptive behaviors (b = -.46, p <.005, however, unlike with the amygdala, adaptive behaviors were associated with smaller left mOFC volume), consistent with the direct effects of SS exposure (i.e., reducing left mOFC volume). In contrast, neither internalizing behaviors (b = .36, p = .28, nor externalizing behaviors (b = .02, p = .95) were significant moderators. The association between SS exposure and right mOFC volume was also moderated by adaptive behaviors (b = -.31, p = .02, i.e., better adaptive behaviors were associated with smaller right mOFC), but not externalizing (b = -.03, p = .95) or internalizing clinical behaviors (b = -.04, p = .86) (Table 3). This indicates a significant moderation effect of adaptive behaviors on right mOFC volume, even though the direct effects of SS exposure were not statistically significant.
4 Discussion
Our primary aims were to answer three central questions: 1) do SS exposed children exhibit marked differences in behavioral and brain phenotypes compared to unexposed children; 2) are effects of SS exposure on brain phenotypes mediated by previous behavioral phenotypes, and 3) do early-life behaviors moderate brain morphometry later in childhood? We have three major findings in parallel with these three primary aims. First, SS in-utero exposure alone predicts greater externalizing behaviors around age 5 as well as larger bilateral amygdala and smaller left mOFC around age 8. Second, none of the earlier life clinical and adaptive behaviors, including externalizing behaviors, mediated the relationship between SS exposure and later life brain structure in the fronto-limbic circuit, although this might have been predicted due to the greater externalizing behaviors observed in the SS-exposed group several years prior to structural brain measurements. Third, adaptive behaviors moderated the relationship between SS exposure and greater bilateral amygdala volume as well as smaller bilateral mOFC, while neither internalizing nor externalizing behaviors moderated any relationship between exposure and the brain structures of interest.
Perhaps counterintuitively, increased or decreased brain volume does not necessarily signal greater or poorer cognitive or behavioral function, respectively (61). Traditionally, the typical model has associated larger amygdala and smaller prefrontal cortices with increased disruptions in emotional regulation during development. This traditional model has been supported by findings from several studies, as examples: 1) an increased amygdala volume was found to underlie an increase in child problem behaviors, particularly anxiety and hypervigilance (i.e., internalizing problems) (33), 2) an enlarged right amygdala in girls has correlated positively with fearfulness in a cohort of healthy children and adolescents (62) and 3) asymmetrically increased amygdala and decreased orbitofrontal cortices have been associated with adolescent, male-specific aggressive behavior (63). While discrepancies in the direction of amygdala volume difference as a response to prenatal stress may be explained by sex differences, this model may oversimplify the interaction between environment, behavior, and brain by neglecting the many nuances and confounds in human development. Not surprisingly, many studies have presented findings that deviate from this traditional model (64). One alternative explanation overlooked in most studies, which we bring to light here, involves the role of resilience in the interplay of stress exposure, emotional regulation, and neurodevelopment.
Importantly, a child’s increased alertness to threat could serve as an adaptive mechanism to promote survival in the child’s stressful postnatal environment (3, 33). Similarly, impulsivity and aggression (i.e., externalizing behaviors) may help children cope better in more stressful postnatal environments. However, in a postnatal environment that is more benign than anticipated, the rise in child behavioral outcomes such as anxiety, impulsivity, and aggression are less adaptive. This stems from a mismatch between the life-threatening prenatal environment and the much less dangerous postnatal environment (3). Our results suggest that SS-exposed children may be more sensitive to perceived environmental threats, which likely contributes to their elevated externalizing phenotype and larger amygdala volume. In a similar vein, reductions in brain volume may not simply indicate poorer functioning, and in this case may be compensatory. The smaller mOFC volumes we observed in the SS cohort may relate to less hesitancy in responding to adverse stimuli, which could assist in situations of acute stress when it is necessary to make abrupt decisions to avoid an immediate perceived danger.
We hypothesize that this combination of increased amygdala and decreased mOFC volumes may facilitate greater resilience among children with prenatal SS exposure, relative to unexposed children. Here, resilience is defined as an adaptive response to serious hardship; it is rooted in evolution-based theories which posit that individuals better respond to stress via effective coping and adaptive strategies. Resilience manifests in demanding situations, including periods of collective adversity, such as the aftermath of a natural disaster (65). While transient activation of the stress system as seen in the manifestation of externalizing and internalizing behaviors may be protective against highly threatening environments, we are not certain if this will be a long-lasting advantage beyond mid-childhood when the cohort’s brain volumes were measured. Severe exposure to stress has been suggested to leave the brain “stuck” in a state of high alert, causing chronic strain. Chronic strain leads to wear and tear on the brain and body later in development, a concept known as allostatic load (26, 61). As such, short-term survival benefits, through elevated vigilance and stress reactivity (i.e., clinical behaviors in this study), may come at a later cost due to the future risk of poor neurodevelopmental outcomes (3). Such long-term costs may include both physical, i.e. increased heart rate and blood pressure, and mental, i.e. risk of developing post-traumatic stress disorder, memory and attention deficits, increased irritability and mood swings.
It is important to recognize, however, that adaptive behaviors are also responses to stress. Obviously, adaptive behaviors act on the allostatic load, but in an opposite direction from the clinical (externalizing and internalizing) behaviors, contributing to greater resilience. Unlike these behaviors, adaptive behaviors may operate differently and affect different or additional parts of the brain. Our finding related to the contrasting role of adaptive behaviors is novel: adaptive behaviors moderate the effects of SS-stress on neural development. It is also important to note that our cross-sectional design does not logically imply a causal link between adaptive behaviors and protection from neurodevelopmental risk. However, since the promotion of adaptive, or resilient, behaviors in the face of traumatic exposures is often neglected, despite its importance in alleviating the impact of stress, it has great public health implications.
While we found early-life adaptive behaviors moderated the association between SS exposure and an enlarged/reduced amygdala/mOFC volume, it is possible that increased adaptive skills and fronto-limbic alterations may leave individuals more sensitive to later life experiences, with the potential to both exacerbate and mitigate the effects of a negative environment. This emphasizes the importance of continuing to assess the same group of children over time to examine the trajectories of brain development and to clarify the complex interplay between behaviors and brain development. It is important to note that alterations found in brain development from early-life adversity may not be permanent. For example, initial enlargements found in the amygdala from early-life adversity may eventually lead the amygdala to become sensitized to future stressors, leading to volume reductions during adulthood (27). More research needs to be conducted to gain a more complete understanding of the short and long-term implications of early-life exposure to adversity on neurobehavioral development and emotion regulation. In the short term, it seems clear from our cohort that an orientation in clinical focus towards identifying and strengthening adaptive behaviors may have a tremendous positive effect.
Several prior studies have suggested potential intervention strategies for targeting adaptive behaviors with a strong focus on children diagnosed with Autism Spectrum Disorder (ASD), a population known to display deficits in adaptive skills (66). These strategies include video modeling for teaching social skills (67), functional communication therapy for reducing severe problem behaviors (68), and cognitive behavioral therapy (69) for increasing daily living skills in children with high-functioning ASD and comorbid anxiety symptoms. While our sample primarily presents with subclinical symptoms, the findings from this research can inform the development of targeted therapies for adaptive behaviors among prenatally stress-exposed children. Approaches that strengthen family relationships, build emotional regulation skills, communication skills, and enhance coping strategies would appear to be particularly relevant based on our results. Additionally, interventions that address both individual and family-level factors that embrace environments promoting adaptive skills and provide children with scaffolding may be most effective for promoting resilience and adaptive functioning. However, these preliminary findings require replication in larger samples before specific clinical recommendations can be made.
4.1 Limitations, strengths, and conclusions
The study is based on a small sample size (N=30). Of these, only 9 children were exposed to Superstorm Sandy, thereby limiting robust generalizations from the statistical comparisons and potentially introducing biases. Thus, findings were limited to group differences and were not sufficiently powered to explore finer subdivisions by sex or trimester-specific exposure. While we controlled for highly pertinent potential confounders (child age and sex) as well as various prenatal confounders, such as normative prenatal stress and severity of SS challenges, there are other possible confounders. For example, although all mothers of the SS exposed group were pregnant and in the New York area during the hurricane, some experienced greater hardship and more stress than others. Another potentially important confounder is maternal mental health, which may have a strong influence on caregiver quality. Since our sample size prevents us from incorporating a wider array of confounders, future work that includes the overall cohort (N=~350 mother-child dyads) will help resolve these limitations. An additional limitation is a potential reporting bias in behavioral measures as they relied on maternal reporting. Also, the false discovery rate threshold choice of 15%, while appropriate for hypothesis-generating research and to reduce Type II error, may limit the definitiveness of our conclusions. Lastly, we do not have brain imaging data at age 5 when behavioral phenotypes were measured. The absence of this data limits confidence with which we can connect brain structure and behavioral phenotype data cross sectionally. Caution is warranted to prevent over-generalization of these findings. Having multiple assessments of the brain imaging and behavioral phenotype data could have helped elucidate the relationship more clearly. Furthermore, additional investigation of functional imaging analysis would further help clarify the possible interplay of these regions within a broader brain network. It is also possible that neural development at age 5 could have moderated the associations between weather-related in-utero stress and later behavioral problems, although it would be naïve to assume that the brain structures of interest did not change in the critical years of development between five and eight. Future studies should include concurrent behavioral assessments with neuroimaging to better understand brain-behavior relationships. Still, it is clear that early behavioral indicators can be used to identify a subsequently measured pattern of altered neural development. The study also has notable strengths. First, the cohort has been followed since birth and behavioral assessments were blinded with respect to brain volume measurements. Second, our findings are robust to adjustment for multiple testing, the intrafamilial correlation due to 4 siblings’ participation, and various pertinent confounders, such as sex and age as well as different sources of prenatal stress, in all analyses. We will also continue to follow these children to gain greater insight into the current findings.
4.2 Future directions
Several key research directions emerge from this pilot study. First, longitudinal follow-up studies are needed to track neurodevelopmental trajectories over time and understand how prenatal stress, adaptive behaviors, and brain development interact across childhood and adolescence. Second, future expansions to larger and more diverse samples will improve reliability and enable sex-specific and trimester-specific analyses that our current sample could not examine. Third, intervention-based research should test whether promoting adaptive behaviors can mitigate prenatal stress effects on brain development, providing clinical value by identifying modifiable protective factors. These directions will guide future research and help translate our preliminary findings into clinically meaningful interventions for at-risk children.
4.3 Conclusion
We measured weather-related in-utero stress using a quasi-experimental design where SS exposure served as a naturally assigned independent variable. This distinguishes our analysis from many other studies, which tend to measure PNMS by more subjective methods, such as self-reported scales. However, the small sample size, particularly the SS exposed group, is a significant limitation and warrants conservative interpretation of the results. It is important to note that our composite adaptive behaviors scale was measured by four subscales that identify the level of child adaptation skills, including skills in daily living activity, social skills, functional communication, and adaptability. As such, it is critical to inform policy makers, educators, and pediatricians of the moderating role that adaptive behaviors can play in potentially ameliorating the deleterious effects of disaster-induced PNMS on amygdala and mOFC volume. In summary, our findings will help inform early identification and intervention strategies that can target specific behavioral phenotypes, especially to promote resilience and to minimize the risk of altered brain development among children exposed to prenatal stress.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Institutional Review Boards at the City University of New York. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
ADS: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Visualization, Writing – original draft, Writing – review & editing. DD: Conceptualization, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. TW: Formal analysis, Software, Writing – review & editing. MSR: Formal analysis, Methodology, Writing – review & editing. MB: Writing – review & editing. JN: Writing – review & editing. YN: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Support for this research was provided by the City University of New York Interdisciplinary Research Grant and National Institute of Mental Health (R01MH131638).
Acknowledgments
The authors would like to acknowledge the Magnetic Resonance Imaging Facility of the CUNY Advanced Science Research Center for instrument use and technical assistance. We would like to acknowledge Wai Man Wang for her contribution in the earlier version of the manuscript John Lopez, Catherine Heitz, Warda Azhar, and Labiba Aziz for their assistance in recruiting our study participants and Kathryn Sie for research coordination.
Conflict of interest
JN was a consultant/advisory board member for Adlon Therapeutics, Cingulate Therapeutics, Corium, Hippo T&C, Ironshore, Lumos, Medice, Mentavi, MindTension, Myriad, Otsuka, Rhodes, Shire/Takeda, Signant Health and Supernus; he received research support from Otsuka, Supernus; honoraria for disease state lectures from Otsuka and Shire, and served as a consultant for the US National Football League.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2025.1481347/full#supplementary-material
References
1. Clemens V, von Hirschhausen E, and Fegert JM. Report of the intergovernmental panel on climate change: implications for the mental health policy of children and adolescents in Europe-a scoping review. Eur Child Adolesc Psychiatry. (2022) 31:701–13. doi: 10.1007/s00787-020-01615-3
2. Zakrison TL, Valdes DM, and Shultz JM. The medical, public health, and emergency response to the impact of 2017 hurricane irma in Cuba. Disaster Med Public Health Prep. (2020) 14:10–7. doi: 10.1017/dmp.2019.71
3. Monk C, Lugo-Candelas C, and Trumpff C. Prenatal developmental origins of future psychopathology: mechanisms and pathways. Annu Rev Clin Psychol. (2019) 15:317–44. doi: 10.1146/annurev-clinpsy-050718-095539
4. Van den Bergh BRH, van den Heuvel MI, Lahti M, Braeken M, de Rooij SR, Entringer S, et al. Prenatal developmental origins of behavior and mental health: The influence of maternal stress in pregnancy. Neurosci Biobehav Rev. (2020) 117:26–64. doi: 10.1016/j.neubiorev.2017.07.003
5. Zietlow AL, Nonnenmacher N, Reck C, Ditzen B, and Muller M. Emotional stress during pregnancy - associations with maternal anxiety disorders, infant cortisol reactivity, and mother-child interaction at pre-school age. Front Psychol. (2019) 10:2179. doi: 10.3389/fpsyg.2019.02179
6. O’Connor TG, Heron J, Golding J, Beveridge M, and Glover V. Maternal antenatal anxiety and children’s behavioural/emotional problems at 4 years. Report from the Avon Longitudinal Study of Parents and Children. Br J Psychiatry. (2002) 180:502–8. doi: 10.1192/bjp.180.6.502
7. Pickles A, Sharp H, Hellier J, and Hill J. Prenatal anxiety, maternal stroking in infancy, and symptoms of emotional and behavioral disorders at 3.5 years. Eur Child Adolesc Psychiatry. (2017) 26:325–34. doi: 10.1007/s00787-016-0886-6
8. Loomans EM, van der Stelt O, van Eijsden M, Gemke RJ, Vrijkotte T, and den Bergh BR. Antenatal maternal anxiety is associated with problem behaviour at age five. Early Hum Dev. (2011) 87:565–70. doi: 10.1016/j.earlhumdev.2011.04.014
9. Cents RA, Diamantopoulou S, Hudziak JJ, Jaddoe VW, Hofman A, Verhulst FC, et al. Trajectories of maternal depressive symptoms predict child problem behaviour: the Generation R study. Psychol Med. (2013) 43:13–25. doi: 10.1017/S0033291712000657
10. Velders FP, Dieleman G, Henrichs J, Jaddoe VW, Hofman A, Verhulst FC, et al. Prenatal and postnatal psychological symptoms of parents and family functioning: the impact on child emotional and behavioural problems. Eur Child Adolesc Psychiatry. (2011) 20:341–50. doi: 10.1007/s00787-011-0178-0
11. Leis JA, Heron J, Stuart EA, and Mendelson T. Associations between maternal mental health and child emotional and behavioral problems: does prenatal mental health matter? J Abnorm Child Psychol. (2014) 42:161–71. doi: 10.1007/s10802-013-9766-4
12. Korhonen M, Luoma I, Salmelin R, and Tamminen T. Maternal depressive symptoms: associations with adolescents’ internalizing and externalizing problems and social competence. Nord J Psychiatry. (2014) 68:323–32. doi: 10.3109/08039488.2013.838804
13. Capron LE, Glover V, Pearson RM, Evans J, O’Connor TG, Stein A, et al. Associations of maternal and paternal antenatal mood with offspring anxiety disorder at age 18 years. J Affect Disord. (2015) 187:20–6. doi: 10.1016/j.jad.2015.08.012
14. Pearson RM, Evans J, Kounali D, Lewis G, Heron J, Ramchandani PG, et al. Maternal depression during pregnancy and the postnatal period: risks and possible mechanisms for offspring depression at age 18 years. JAMA Psychiatry. (2013) 70:1312–9. doi: 10.1001/jamapsychiatry.2013.2163
15. Plant DT, Pariante CM, Sharp D, and Pawlby S. Maternal depression during pregnancy and offspring depression in adulthood: role of child maltreatment. Br J Psychiatry. (2015) 207:213–20. doi: 10.1192/bjp.bp.114.156620
16. Betts KS, Williams GM, Najman JM, and Alati R. Maternal depressive, anxious, and stress symptoms during pregnancy predict internalizing problems in adolescence. Depress Anxiety. (2014) 31:9–18. doi: 10.1002/da.22210
17. Davis EP and Sandman CA. Prenatal psychobiological predictors of anxiety risk in preadolescent children. Psychoneuroendocrinology. (2012) 37:1224–33. doi: 10.1016/j.psyneuen.2011.12.016
18. Edge MD, Ramel W, Drabant EM, Kuo JR, Parker KJ, and Gross JJ. For better or worse? Stress inoculation effects for implicit but not explicit anxiety. Depress Anxiety. (2009) 26:831–7. doi: 10.1002/da.20592
19. Glover V. Annual Research Review: Prenatal stress and the origins of psychopathology: an evolutionary perspective. J Child Psychol Psychiatry. (2011) 52:356–67. doi: 10.1111/j.1469-7610.2011.02371.x
20. Ceniceros LC, Capitanio JP, and Kinnally EL. Prenatal relocation stress enhances resilience under challenge in infant rhesus macaques. Front Behav Neurosci. (2021) 15:641795. doi: 10.3389/fnbeh.2021.641795
21. Bondarenko NS, Voronova SN, Voronezhskaya EE, and Melnikova VI. Prenatal stress and adaptive behavior of offspring: the role of placental serotonin. Dokl Biochem Biophys. (2022) 503:104–7. doi: 10.1134/S160767292202003X
22. Hartman S, Freeman SM, Bales KL, and Belsky J. Prenatal stress as a risk-and an opportunity-factor. Psychol Sci. (2018) 29:572–80. doi: 10.1177/0956797617739983
23. DiPietro JA, Novak MF, Costigan KA, Atella LD, and Reusing SP. Maternal psychological distress during pregnancy in relation to child development at age two. Child Dev. (2006) 77:573–87. doi: 10.1111/j.1467-8624.2006.00891.x
24. Serpeloni F, Radtke KM, Hecker T, Sill J, Vukojevic V, de Assis SG, et al. Does prenatal stress shape postnatal resilience? - an epigenome-wide study on violence and mental health in humans. Front Genet. (2019) 10:269. doi: 10.3389/fgene.2019.00269
25. Nomura Y, Zhang W, and Hurd YL. Stress in pregnancy: Clinical and adaptive behavior of offspring following Superstorm Sandy. Dev Psychopathol. (2022) 34:1249–59. doi: 10.1017/S0954579421000304
26. National Scientific Council on the Developing Child. Connecting the Brain to the Rest of the Body: Early Childhood Development and Lifelong Health Are Deeply Intertwined Working Paper No. 15. Cambridge, MA.:Harvard University (2020). Available online at: www.developingchild.harvard.edu. 11, 7, 2022.
27. Tottenham N. Early adversity and the neotenous human brain. Biol Psychiatry. (2020) 87:350–8. doi: 10.1016/j.biopsych.2019.06.018
28. Kebets V, Favre P, Houenou J, Polosan M, Perroud N, Aubry JM, et al. Fronto-limbic neural variability as a transdiagnostic correlate of emotion dysregulation. Transl Psychiatry. (2021) 11:545. doi: 10.1038/s41398-021-01666-3
29. Maddux JE, Winstead BA, and Ebscohost. Psychopathology: foundations for a contemporary understanding. Fifth. New York: Routledge (2020).
30. Simic G, Tkalcic M, Vukic V, Mulc D, Spanic E, Sagud M, et al. Understanding emotions: origins and roles of the amygdala. Biomolecules. (2021) 11(6):823. doi: 10.3390/biom11060823
31. Kessler R, Schmitt S, Sauder T, Stein F, Yüksel D, Grotegerd D, et al. Long-term neuroanatomical consequences of childhood maltreatment: reduced amygdala inhibition by medial prefrontal cortex. Front Syst Neurosci. (2020) 14:2020. doi: 10.3389/fnsys.2020.00028
32. Von Der Heide RJ, Skipper LM, Klobusicky E, and Olson IR. Dissecting the uncinate fasciculus: disorders, controversies and a hypothesis. Brain. (2013) 136:1692–707. doi: 10.1093/brain/awt094
33. Lautarescu A, Craig MC, and Glover V. Prenatal stress: Effects on fetal and child brain development. Int Rev Neurobiol. (2020) 150:17–40. doi: 10.1016/bs.irn.2019.11.002
34. Mareckova K, Miles A, Liao Z, Andryskova L, Brazdil M, Paus T, et al. Prenatal stress and its association with amygdala-related structural covariance patterns in youth. NeuroImage Clin. (2022) 34:102976. doi: 10.1016/j.nicl.2022.102976
35. Wen DJ, Poh JS, Ni SN, Chong YS, Chen H, Kwek K, et al. Influences of prenatal and postnatal maternal depression on amygdala volume and microstructure in young children. Transl Psychiatry. (2017) 7:e1103. doi: 10.1038/tp.2017.74
36. Buss C, Davis EP, Muftuler LT, Head K, and Sandman CA. High pregnancy anxiety during mid-gestation is associated with decreased gray matter density in 6-9-year-old children. Psychoneuroendocrinology. (2010) 35:141–53. doi: 10.1016/j.psyneuen.2009.07.010
37. Lebel C, Walton M, Letourneau N, Giesbrecht GF, Kaplan BJ, and Dewey D. Prepartum and postpartum maternal depressive symptoms are related to children’s brain structure in preschool. Biol Psychiatry. (2016) 80:859–68. doi: 10.1016/j.biopsych.2015.12.004
38. El Marroun H, Tiemeier H, Muetzel RL, Thijssen S, van der Knaap NJ, Jaddoe VW, et al. Prenatal exposure to maternal and paternal depressive symptoms and brain morphology: A population-based prospective neuroimaging study in young children. Depress Anxiety. (2016) 33:658–66. doi: 10.1002/da.22524
39. Davis EP, Head K, Buss C, and Sandman CA. Prenatal maternal cortisol concentrations predict neurodevelopment in middle childhood. Psychoneuroendocrinology. (2017) 75:56–63. doi: 10.1016/j.psyneuen.2016.10.005
40. Buss C, Davis EP, Shahbaba B, Pruessner JC, Head K, and Sandman CA. Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proc Natl Acad Sci U S A. (2012) 109:E1312–1319. doi: 10.1073/pnas.1201295109
41. Acosta H, Tuulari JJ, Scheinin NM, Hashempour N, Rajasilta O, Lavonius TI, et al. Maternal pregnancy-related anxiety is associated with sexually dimorphic alterations in amygdala volume in 4-year-old children. Front Behav Neurosci. (2019) 13:175. doi: 10.3389/fnbeh.2019.00175
42. Sandman CA, Buss C, Head K, and Davis EP. Fetal exposure to maternal depressive symptoms is associated with cortical thickness in late childhood. Biol Psychiatry. (2015) 77:324–34. doi: 10.1016/j.biopsych.2014.06.025
43. Davis EP, Hankin BL, Glynn LM, Head K, Kim DJ, and Sandman CA. Prenatal maternal stress, child cortical thickness, and adolescent depressive symptoms. Child Dev. (2020) 91:e432–50. doi: 10.1111/cdev.13252
44. Jones SL, Dufoix R, Laplante DP, Elgbeili G, Patel R, Chakravarty MM, et al. Larger amygdala volume mediates the association between prenatal maternal stress and higher levels of externalizing behaviors: sex specific effects in project ice storm. Front Hum Neurosci. (2019) 13:144. doi: 10.3389/fnhum.2019.00144
45. Finik J and Nomura Y. Cohort profile: stress in pregnancy (SIP) study. Int J Epidemiol. (2017) 46:1388–1388k. doi: 10.1093/ije/dyw264
46. Nomura Y, Newcorn JH, Ginalis C, Heitz C, Zaki J, Khan F, et al. Prenatal exposure to a natural disaster and early development of psychiatric disorders during the preschool years: stress in pregnancy study. J Child Psychol Psychiatry. (2022) 46(7):1080–91. doi: 10.1111/jcpp.13698
47. Sie SY, Maharaj R, Lopez J, DeIngeniis D, Monir F, and Nomura Y. A-270 synergistic relationship of prenatal hurricane sandy exposure and postnatal suicidal ideation on child fear during the COVID-19 pandemic. Arch Clin Neuropsychol. (2022) 37:1420–1. doi: 10.1093/arclin/acac060.270
48. Buthmann J, Huang D, Casaccia P, O’Neill S, Nomura Y, and Liu J. Prenatal exposure to a climate-related disaster results in changes of the placental transcriptome and infant temperament. Front Genet. (2022) 13:887619. doi: 10.3389/fgene.2022.887619
49. Nomura Y, Rompala G, Pritchett L, Aushev V, Chen J, and Hurd YL. Natural disaster stress during pregnancy is linked to reprogramming of the placenta transcriptome in relation to anxiety and stress hormones in young offspring. Mol Psychiatry. (2021) 26:6520–30. doi: 10.1038/s41380-021-01123-z
50. Zhang W, Ham J, Li Q, Deyssenroth MA, Lambertini L, Huang Y, et al. Moderate prenatal stress may buffer the impact of Superstorm Sandy on placental genes: Stress in Pregnancy (SIP) Study. PloS One. (2020) 15:e0226605. doi: 10.1371/journal.pone.0226605
51. Buthmann J, Finik J, Bedoya A, and Nomura Y. The children of superstorm sandy: sex moderates link between electrodermal reactivity and prenatal storm exposure. Biol Psychiatry. (2020) 87:S448. doi: 10.1016/j.biopsych.2020.02.1141
52. Demirci GM, Delngeniis D, Wong WM, Shereen AD, Nomura Y, and Tsai CL. Superstorm Sandy exposure in utero is associated with neurobehavioral phenotypes and brain structure alterations in childhood: A machine learning approach. Front Neurosci. (2023) 17:1113927. doi: 10.3389/fnins.2023.1113927
54. Reynolds CR. Behavior assessment system for children. In: The Corsini Encyclopedia of Psychology (2010) Hoboken:NJ and Wiley.
55. Theys C, Wouters J, and Ghesquiere P. Diffusion tensor imaging and resting-state functional MRI-scanning in 5- and 6-year-old children: training protocol and motion assessment. PloS One. (2014) 9:e94019. doi: 10.1371/journal.pone.0094019
56. Tisdall MD, Hess AT, Reuter M, Meintjes EM, Fischl B, and van der Kouwe AJ. Volumetric navigators for prospective motion correction and selective reacquisition in neuroanatomical MRI. Magn Reson Med. (2012) 68:389–99. doi: 10.1002/mrm.23228
57. Dale AM, Fischl B, and Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. (1999) 9:179–94. doi: 10.1006/nimg.1998.0395
58. King S and Laplante DP. The effects of prenatal maternal stress on children’s cognitive development: Project Ice Storm. Stress. (2005) 8:35–45. doi: 10.1080/10253890500108391
59. Tein JY, Coxe S, and Cham H. Statistical power to detect the correct number of classes in latent profile analysis. Struct Equ Modeling. (2013) 20:640–57. doi: 10.1080/10705511.2013.824781
60. Benjamini Y and Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc Ser B (Methodol). (1995) 57:289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x
61. McEwen BS and Gianaros PJ. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann N Y Acad Sci. (2010) 1186:190–222. doi: 10.1111/j.1749-6632.2009.05331.x
62. van der Plas EA, Boes AD, Wemmie JA, Tranel D, and Nopoulos P. Amygdala volume correlates positively with fearfulness in normal healthy girls. Soc Cognit Affect Neurosci. (2010) 5:424–31. doi: 10.1093/scan/nsq009
63. Whittle S, Yap MB, Yucel M, Fornito A, Simmons JG, Barrett A, et al. Prefrontal and amygdala volumes are related to adolescents’ affective behaviors during parent-adolescent interactions. Proc Natl Acad Sci U S A. (2008) 105:3652–7. doi: 10.1073/pnas.0709815105
64. Hanson JL and Nacewicz BM. Amygdala allostasis and early life adversity: considering excitotoxicity and inescapability in the sequelae of stress. Front Hum Neurosci. (2021) 15:624705. doi: 10.3389/fnhum.2021.624705
65. Greene RR. Resilience as effective functional capacity: an ecological-stress model. J Hum Behav Soc Environment. (2014) 24:937–50. doi: 10.1080/10911359.2014.921589
66. Tamm L, Day HA, and Duncan A. Comparison of adaptive functioning measures in adolescents with autism spectrum disorder without intellectual disability. J Autism Dev Disord. (2022) 52:1247–56. doi: 10.1007/s10803-021-05013-9
67. Wang P and Spillane A. Evidence-based social skills interventions for children with autism: A meta-analysis. Educ Training Dev Disabilities. (2009) 44:318–42, http://www.jstor.org/stable/24233478.
68. Tiger JH, Hanley GP, and Bruzek J. Functional communication training: a review and practical guide. Behav Anal Pract. (2008) 1:16–23. doi: 10.1007/BF03391716
Keywords: natural disaster, prenatal stress, MRI, limbic, brain, behavior
Citation: Shereen AD, DeIngeniis D, Wu T, Rahman MS, Blum M, Newcorn JH and Nomura Y (2025) Behavioral moderators of In-utero superstorm sandy exposure and fronto-limbic cortical development—potential role of adaptiveness in clinical intervention strategies, a pilot study. Front. Psychiatry 16:1481347. doi: 10.3389/fpsyt.2025.1481347
Received: 15 August 2024; Accepted: 24 June 2025;
Published: 17 July 2025.
Edited by:
Sarah Nazzari, University of Pavia, ItalyReviewed by:
Alessandra Provera, University of Bologna, ItalyMaria Spinelli, University of Studies G. d’Annunzio Chieti and Pescara, Italy
Copyright © 2025 Shereen, DeIngeniis, Wu, Rahman, Blum, Newcorn and Nomura. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: A. Duke Shereen, YXNoZXJlZW5AZ2MuY3VueS5lZHU=
 A. Duke Shereen
A. Duke Shereen Donato DeIngeniis
Donato DeIngeniis Tingting Wu
Tingting Wu Md. Shafiur Rahman
Md. Shafiur Rahman Melissa Blum
Melissa Blum Jeffrey H. Newcorn
Jeffrey H. Newcorn Yoko Nomura
Yoko Nomura