Abstract
Objective:
To explore the bidirectional association between dyslipidemia and depression, and the potential predictive role of lipid level changes for depression onset.
Methods:
A systematic search of the Cochrane Library, Web of Science, Embase, and PubMed databases was conducted to identify cohort studies on blood lipids and 5-hydroxytryptamine (5-HT) parameters related to depression, from database inception to May 2024. Data were analyzed using Stata 14.0 software.
Results:
Seven studies were included in the analysis. Serum high-density lipoprotein (HDL) and triglyceride levels are significantly associated with an increased risk of depression (OR = 1.28, 95% CI: 1.07 - 1.53; OR = 1.41, 95% CI: 1.20 - 1.66, P < 0.05). However, depressive symptoms do significantly affect serum HDL, LDL, triglyceride, or total cholesterol levels (ORs = 0.88, 1.05, 1.05, 1.11; 95% CIs: 0.58 - 1.35, 0.88 - 1.24, 0.91 - 1.21, 0.9 - 1.32, P > 0.05).
Conclusion:
Based on the present findings, changes in serum HDL and triglyceride levels are significantly linked to depression incidence. Monitoring these two lipid parameters may aid in early identification of at-risk individuals and enhance the prognosis and quality of life for depressed patients through timely interventions.
Systematic Review Registration:
https://www.crd.york.ac.uk/prospero/, identifier CRD42024542833.
1 Introduction
As a major global public health challenge, depression contributes substantially to the global disease burden (1)— its core symptoms, defined by the Diagnostic and Statistical Manual of Mental Disorders (DSM-5), directly impair individual functioning and societal productivity. These key symptoms include persistent low mood, anhedonia (loss of interest or pleasure in previously enjoyable activities), generalized fatigue, psychomotor retardation or cognitive slowing, difficulty concentrating, and memory decline; In some cases, individuals may experience hallucinations, delusions, or even suicidal tendencies (2, 3). Together, these symptoms often lead to disrupted daily life, reduced work efficiency, and increased demand for healthcare services.
Fundamentally, depression is a multifactorial disease, and its pathogenesis involves three interrelated dimensions: biology (genetics, neurotransmitter imbalances) (4), psychology (chronic stress, negative cognitive patterns) (5), and society (interpersonal problems, socioeconomic status) (6). Despite extensive research, there remains no unified consensus in the academic community on the exact pathogenesis of depression, as its development typically arises from the complex interplay of the above factors rather than a single cause. In recent years, with advances in pathophysiological research, immune-inflammatory responses and metabolic abnormalities have gradually been confirmed as potential core mechanisms underlying the development of depressive disorders (7). Among these, abnormal serotonin (5-HT) levels and dyslipidemia serve as two key biological indicators. The association and interaction of these indicators with depression have become crucial scientific questions that urgently need clarification in the field.
Serotonin is an important neurotransmitter that plays a critical role in numerous physiological processes, including platelet aggregation, pain, sleep, appetite, muscle contraction, mood, and compulsive behaviors (8). Targeting the serotonin system is a key strategy for developing new potential antidepressants. Serotonin and its receptors are distributed in the central nervous system (CNS), peripheral nervous system (PNS), and multiple non-neuronal tissues, such as those in the gut, cardiovascular system, and blood. Serotonin can bind to cell-surface 5-HT1B receptors to regulate various physiological functions (e.g., pain, sleep, mood, and memory), and its levels have been associated with anxiety, depression, and schizophrenia (9). It has been reported that 5-HT2A receptor density is significantly increased in the cortex of postmortem brain tissue from depressed patients and individuals who died by suicide. This increase may be a compensatory response by the brain to reduced serotonin levels in depressed patients, achieved by upregulating 5-HT2A receptor density (10). Long-term administration of some antidepressant drugs downregulates 5-HT2A receptor density and exerts antidepressant effects by modulating subcortical circuits in the forebrain (11). These findings support a link between 5-HT2A receptor regulation and the therapeutic mechanism of these antidepressants; however, evidence for a direct association with depression pathogenesis remains to be supplemented. Vargas MV et al. (12)demonstrated that primary localization of 5-HT2ARs in cortical neurons is intracellular, cellular import of serotonin leads to structural plasticity and antidepressant-like effects.
The traditional monoamine hypothesis posits a link between brain monoamine neurotransmitter levels and depression onset, which laid the foundation for early antidepressant development but is now considered overly simplified—for instance, it cannot explain the delayed efficacy of monoamine-targeting drugs or why many patients fail to respond. Recent studies have updated this view by situating the monoamine system within a multi-mechanism network, with neuroinflammation as a key regulatory node. In depressed patients, elevated pro-inflammatory cytokines (e.g., IL-6, TNF-α) disrupt monoamine homeostasis: they inhibit tryptophan hydroxylase (reducing 5-HT synthesis) and activate indoleamine 2,3-dioxygenase (shifting tryptophan metabolism to neurotoxic metabolites), exacerbating monoamine imbalance (13, 14). Conversely, monoamines (especially 5-HT) modulate glial activation to regulate neuroinflammation, forming a bidirectional loop (15, 16). Notably, 5-HT2A receptors, which regulate monoamine release, may further mediate this ‘inflammation-monoamine’ crosstalk (17), linking their function to both depression pathogenesis and antidepressant mechanisms more precisely.
In the central nervous system, lipids are critical for maintaining the structure, function, and membrane integrity of various cells. Specifically, cholesterol—a key lipid component—modulates cellular signaling pathways and has been implicated in both the mechanism of antidepressant action and the regulation of emotional stability. Since blood cholesterol levels correlate with brain cholesterol levels, a decrease in neuronal membrane lipids can reduce serotonin (5-HT) receptor levels in the membrane, and this reduction is associated with increased risks of depression and suicide (18). This lipid-5-HT axis may further interact with 5-HT-related receptor complexes, which are mechanisms closely linked to major depressive disorder. For instance, Borroto-Escuela DO et al. (19) demonstrated that serotonin heteroreceptor complexes, which mediate signal integration in neurons and astrocytes, are highly relevant to the pathogenesis of major depressive disorder. Moreover, Ambrogini P et al. (20) found that 5-HT1A receptor-FGFR1 heteroreceptor complexes differentially modulate G protein-coupled inwardly rectifying potassium (GIRK) currents in the dorsal hippocampus and dorsal raphe nucleus serotonin neurons between control rats and a genetic depression model. These findings suggest that the function of 5-HT receptor complexes may be altered in the context of depression-related lipid/5-HT dysregulation. Beyond these molecular mechanisms, neuroplasticity changes in key brain regions also contribute to depression pathogenesis, with the hippocampus, prefrontal cortex, and amygdala being core areas involved (21). Notably, these neuroplasticity changes are regulated by multiple systems, including glutamatergic signaling, neurotrophic factors, monoamine neurotransmitters (e.g., 5-HT), and neuroinflammation—hinting at potential crosstalk between lipid/5-HT pathways and neuroplasticity regulation in depression.
In addition to the neurotransmitter system, lipid disorders in metabolic abnormalities have also been shown to be closely associated with depression. Studies have demonstrated that lipid metabolism disorders—specifically, lower levels of total cholesterol and LDL-C—are linked to an increased risk of suicide in patients with depression, making these lipid abnormalities among the high-risk factors for suicide in this population (22, 23). These reduced lipid levels may serve as potential biomarkers for predicting suicide attempts in patients with depression. The study by Nikolić V et al. helps elucidate a potential biological mechanism connecting dietary fiber intake to lipid profiles, thereby providing mechanistic support for the observed association between low HDL-cholesterol levels and depression (24). Recent advances in biomarker research have also provided new insights into the diagnosis and management of depression. These advances not only emphasize electrophysiological markers of depression (25) and genetic and epigenetic factors that underlie neurobiological and inflammatory pathways (26), but also suggest potential crosstalk between lipid metabolism and these neurobiological and inflammatory pathways in the pathogenesis of depression.
In specific populations, this association exhibits significant gender and age differences—for example, Yang et al. (27) found that women are more likely than men to develop dyslipidemia following antidepressant therapy, characterized by elevated triglyceride and LDL-C levels, with these elevations being more pronounced in women. Interestingly, high HDL-cholesterol levels in middle-aged adults have been associated with an increased risk of depression, and a relationship has been observed between depression and levels of total cholesterol and LDL-C (28). Furthermore, Mehdi SMA et al. confirmed, based on multi-generational depression cohorts and national health and nutrition survey data, that depression is significantly associated with lipid disorders (29). This provides empirical evidence at the population level for the involvement of lipid metabolism—particularly abnormal HDL-cholesterol levels—in the pathological process of depression.
At the mechanistic level, short-term population-based follow-up studies have shown that metabolic and hunger-related hormones (e.g., growth hormone, cortisol, leptin, ghrelin) directly affect serum lipid levels (30),or interact synergistically with immune inflammatory responses to jointly promote the occurrence and aggravation of depressive symptoms. Additionally, the use of antidepressant drugs has also been reported to alter lipid metabolism (31), which further intensifies the interaction between metabolic and hunger-related hormones and immune inflammatory responses. It is worth noting that metabolic syndrome is a pathological state characterized by multiple risk factors, including obesity, hypertension, dyslipidemia, and abnormal glucose metabolism. It promotes the development of cardiovascular and cerebrovascular diseases, as well as diabetes mellitus, and importantly, its association with depression has also been linked to lipid metabolism abnormalities (32–34). Studies have indicated that the association between depression and dyslipidemia is similar in strength and direction across different ages (35). Hence, lipid levels have the potential to be biomarkers for the diagnosis, classification, and prognosis of depression. However, there is still no definitive conclusion about the relationship between depression and dyslipidemia at different ages, and more studies are needed to explore this relationship.
Collectively, a large number of studies have confirmed correlations between lipid levels, 5-HT levels, and depression; however, the association between specific lipid parameters (e.g., HDL, LDL, triglycerides) and 5-HT-related indices and depression risk has rarely been investigated, and few studies have analyzed the bidirectional and causal relationships among these factors. Therefore, this study explored the bidirectional and causal relationships between depression and both serum 5-HT levels and dyslipidemia via a meta-analysis, thereby providing reliable evidence for the prevention and treatment of depression and a theoretical basis for further investigating its pathogenesis.
2 Research data and methods
2.1 Study inclusion and exclusion criteria
Inclusion criteria (1): Studies that measure depression using standardized self-report scales or diagnostic assessments. The scales include the 4-item version of the General Health Questionnaire (GHQ) (36, 37), (score ≥ 4 indicates depressive symptoms), the Center for Epidemiologic Studies Depression Scale (CES-D) (38), (a valid tool with a threshold score of 16 indicating clinically significant depressive symptoms), the 17-item Hamilton Depression Rating Scale (HDRS) (39), (score ≥ 18 reflects moderate-to-severe depression), and the Mini International Neuropsychiatric Interview (MINI) (40), (a structured diagnostic interview for psychiatric disorders, including depression). For diagnostic assessments, studies must use ICD-10 codes or DSM-IV criteria to confirm diagnoses (41) (2). Studies that include exposure factors as serum levels of 5-hydroxytryptamine (5-HT) and lipid parameters (total cholesterol, triglycerides, high-density lipoprotein cholesterol [HDL], low-density lipoprotein cholesterol [LDL], very-low-density lipoprotein cholesterol [VLDL]), with at least one parameter reported per study. Venous blood is collected from participants after an ≥ 12-hour fast. Serum levels of total cholesterol, HDL, triglycerides, and 5-HT are determined via conventional enzymatic methods. Studies must clearly report the units of each parameter (e.g., total cholesterol: mmol/L; serum 5-HT: ng/mL) to ensure accurate data synthesis in the meta-analysis and avoid unit inconsistencies (3). Studies with a longitudinal cohort design (4). Manuscripts published in English.
Exclusion Criteria (1): Studies including subjects with major chronic diseases (e.g., rheumatism, cancer), severe unstable medical conditions (e.g., advanced organ failure), diagnosed mental disorders other than unipolar depression (e.g., bipolar disorder, schizophrenia), or specific populations (e.g., postpartum women), except for studies including patients diagnosed with unipolar depression (2). Studies involving non-human subjects (3). Manuscripts without reported outcome data, and for which authors cannot provide such data (4). Data reported in meetings, abstracts, editorials, or letters (not full-text research articles).
2.2 Study retrieval strategies
We searched the Cochrane Library, Web of Science, Embase, and PubMed databases. Specifically, we searched for cohort studies investigating the association between serum serotonin (5-HT), total cholesterol, triglycerides, high-density lipoprotein cholesterol (HDL), low-density lipoprotein cholesterol (LDL), and very-low-density lipoprotein cholesterol (VLDL) and depression. The search was restricted to English-language publications (including full-text articles and conference proceedings).
The search strategy was developed in accordance with the guidelines of The Cochrane Handbook for Systematic Reviews of Interventions. English search terms included “depression”, “triglycerides”, “cholesterol”, “lipids”, “high-density lipoprotein cholesterol”, “low-density lipoprotein cholesterol”, and “serotonin” (the detailed retrieval process was provided in the supplementary material). A combination of MeSH terms (Medical Subject Headings) and free-text terms was used for the search. This study was registered in PROSPERO (International Prospective Register of Systematic Reviews) with the registration number CRD42024542833.
2.3 Study screening and data extraction
Two researchers independently read the articles according to the inclusion and exclusion criteria and then screened them to exclude articles with small sample sizes (n < 50), duplicate reports, or no original data. The information to be extracted included the following (1): Basic information: article title, author(s), publication year, sample size, study population, and population source (2); Study subject details: age, gender, and other demographic characteristics (3); Outcome data: number of participants in the exposed and non-exposed groups, original values of the exposure factors (serum 5-HT and lipid parameters), and reported Odds Ratio (OR) [Relative Risk (RR) was pre-specified as a potential effect size metric for extraction, given its common use in cohort studies. However, no included studies reported RR; we therefore synthesized and presented ORs to ensure accuracy and avoid metric conversion bias] or 95% Confidence Intervals (CI). The two researchers cross-checked the extracted content and entered the extracted data into a standardized database.
2.4 Evaluation of article quality
We used the Newcastle-Ottawa Scale (NOS) to assess the methodological quality of the included studies. The NOS consists of 8 items across 3 dimensions, each focusing on a key aspect of study quality (1): participant selection (4 items) (2); between-group comparability (1 item, maximum score of 2) (3); outcome measure assessment (3 items, 1 point each). The total possible score ranges from 0 to 9. A total score of ≥ 7 was defined as high methodological quality, 4–6 as moderate quality, and ≤ 3 as low quality; the higher the score, the better the methodological quality of the included studies.
2.5 Statistical analysis
Meta-analysis of the associations between serum lipid/(5-HT) levels and depression was performed using Stata 14.0 software. Count data were analyzed using RR or OR as effect sizes, and their 95% CI were reported. “Count data” specifically refers to dichotomous outcome variables quantified by event counts in the included longitudinal studies. These primarily include two core outcomes (1): incident depressive disorder (i.e., counting participants who developed depression during follow-up versus those who did not) and (2) incident dyslipidemia (counting participants who developed dyslipidemia during follow-up versus those who did not). For such event-based dichotomous data, we used RR or OR (with 95% CIs) as effect sizes, which is a standard statistical approach for quantifying associations between exposures and binary outcomes in observational studies.
The I² statistic is a core indicator for assessing heterogeneity among studies, with a range of 0% to 100%. Currently, the most commonly used international standard is the interpretation guidelines for I² values proposed by Higgins et al. (2022) (42). Heterogeneity among studies for each association was tested, with results expressed as P-values and I² values. If P > 0.1 and I² < 50%, this indicates low heterogeneity, and a fixed-effects model is used for analysis; otherwise, a random-effects model is applied. Egger’s test was used to evaluate publication bias among the included studies. P < 0.05 indicates statistically significant publication bias.
3 Results
3.1 Article screening process
A total of 7,274 articles were obtained from the initial screening. We followed a systematic, cascading exclusion-based study screening process and finally included 7 articles. Publications of the included studies spanned from 2008 to 2024.
Of these 7 included studies, four were carried out in France (43–46), one in China (47), one in Canada (48), and one in Tel Aviv, Israel (49). A PRISMA-compliant screening flow chart is shown in Figure 1.
Figure 1

Article screening flowchart.
3.2 Basic characteristics of the included studies
The basic characteristics of the included articles are shown in Table 1, including 7 articles with a total of 17,465 participants. Five studies (43–45, 47, 48) reported the effect of depressive disorders on blood lipids, and two studies (46, 49) reported the effect of blood lipids on the onset of depression. All of the studies were cohort studies, and the quality of the included articles was evaluated by the NOS; the overall scores ranged from 6 to 8, which were relatively good, as shown in Table 2.
Table 1
| Type | Author | Year | Country | Study type | Sample source | Age | Sample size | Male/female | Index | Influence Factor | Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Depression → Lipid | Ancelin ML | 2010 | France | longitudinal study | Montpellier district | 65+ | 1792 | 752/1040 | HDL/LDL/TG/TC | age/education | 7-year follow-up |
| Depression → Lipid | Akbaraly TN | 2011 | France | cohort study | electoral rolls | – | 4446 | – | HDL/TG/TC | Age/sex.etc | 2- and 4-year follow-ups |
| Depression → Lipid | El Asmar K | 2023 | France | Cohort Study | six university psychiatry departments |
42.3 ± 13.0 | 117 | 38/83 | HDL/TG | Age/Sex.etc | 3 and 6 months |
| Depression → Lipid | Khalfan AF | 2023 | Canada | Cohort Study | children’s hospital | 15.0 ± 1.9 | 239 | – | HDL | Sex/age/BMI | – |
| Depression → Lipid | Cheng P | 2024 | China | Cohort Study | the Second Xiangya Hospital of Central South University |
26.4 ± 15.31 | 1429 | 513/943 | HDL/LDL | Region of residence/Thyroid stimulating hormone |
spanning 90, 180, and 365 days |
| Lipid → Depression | Toker S | 2008 | Tel Aviv | logistic regression analysis | healthy employees | 45.2 ± 10.6/46.2 ± 10.1 | 3880 | 2355/1525 | HDL/TG | – | 2 years |
| Lipid → Depression | Akbaraly TN | 2009 | London | cohort study | office staff | 41-61 | 5562 | 3958/1604 | HDL/LDL/TG | Sex/age. etc | After 6 years |
Basic characteristics of the included studies.
Table 2
| Study (cohort) | Representative of the exposed cohort | Selection of non-exposed cohort | Ascertainment of exposure | Outcome not present before study | Comparability | Assessment of outcome | Follow-up long enough | Adequacy of follow up | Quality score |
|---|---|---|---|---|---|---|---|---|---|
| Toker S 2008 |
* | * | * | * | * | * | * | 7 | |
| Akbaraly TN 2009 |
* | * | * | * | * | * | * | 7 | |
| Ancelin ML 2010 |
* | * | * | * | * | * | * | 7 | |
| Akbaraly TN 2011 |
* | * | * | * | * | * | * | * | 8 |
| El Asmar K 2023 |
* | * | * | * | * | * | 6 | ||
| Khalfan AF 2023 |
* | * | * | * | * | * | 6 | ||
| Cheng P 2024 |
* | * | * | * | * | * | 6 |
Quality evaluation of included studies.
4 Analysis of results
4.1 Effect of lipid parameters on the onset of depression
Two studies (46, 49) reported the association between serum HDL levels and depression. A fixed-effects model was chosen due to low heterogeneity (I² = 0%, P = 0.669). Meta-analysis based on this model showed that changes in serum HDL levels were associated with an increased risk of depression (OR = 1.28, 95% CI: 1.07–1.53), indicating that individuals with abnormal serum HDL levels had a 28% higher risk of depression compared to those with normal levels. The Z-statistic for the overall effect was 2.738, with P = 0.006 < 0.05, indicating statistically significant results, as shown in Figure 2 (Forest plot of the association between serum HDL levels and depression).
Figure 2

The association between HDL and depression. Each horizontal line segment in the figure represents the 95% confidence interval of the corresponding effect size.
Only one study (46) reported the association between changes in LDL levels and depression. The results showed that changes in LDL levels were not significantly associated with the risk of depression (OR = 1.26, 95% CI: 0.98–1.61; Z = 1.825, P = 0.068 > 0.05).
Two studies (46, 49) reported the association between serum triglyceride levels and depression. Due to low heterogeneity (I² = 0%, P = 0.638), a fixed-effects model was chosen. Meta-analysis based on this model showed that changes in serum triglyceride levels were significantly associated with a higher risk of depression; the pooled overall effect was OR = 1.41, 95% CI: 1.20–1.66 (Z = 4.179, P < 0.001). These statistically significant results are shown in Figure 3 (Forest plot of the association between serum triglyceride levels and depression).
Figure 3

The association between triglycerides and depression.
4.2 Effect of depression on lipid levels
Five studies (43–45, 47, 48) reported the association between depression and serum HDL levels. Due to high heterogeneity (I² = 87.1%, P < 0.001), a random-effects model was chosen; potential sources of heterogeneity will be discussed in the subsequent section. The results showed that depression was not significantly associated with changes in serum HDL levels when comparing depressed individuals with non-depressed individuals (OR = 0.88, 95% CI: 0.58–1.35; Z = -0.578, P = 0.563 > 0.05). See Figure 4 (Forest plot of the association between depression and serum HDL levels) for details.
Figure 4

The effect of depression on HDL.
4.3 Subgroup analysis
Due to high heterogeneity (I² = 87.1%, P < 0.001) in the five studies investigating the association between depressive symptoms and serum HDL levels, we performed a subgroup analysis according to the countries where the studies were carried out. Subgroup-specific heterogeneity and statistical results (OR, 95% CI, Z, P) were as follows: France (43–45) I² = 92.5%, (high heterogeneity), (Z = -1.120, P = 0.263 > 0.05, no statistical significance); Canada (48) Single study, no heterogeneity, (Z = 1.383, P = 0.167 > 0.05, no statistical significance); and China (47) Single study, no heterogeneity, (Z = 2.569, P = 0.010 < 0.05, statistically significant).
We found that in the Chinese study (47), there was a significant negative association between depressive symptoms and serum HDL levels: individuals with depressive symptoms had significantly lower serum HDL levels than non-depressed individuals, and this association was statistically significant. See Figure 5 (Subgroup analysis forest plot for the association between depressive symptoms and serum HDL levels in China) for details.
Figure 5
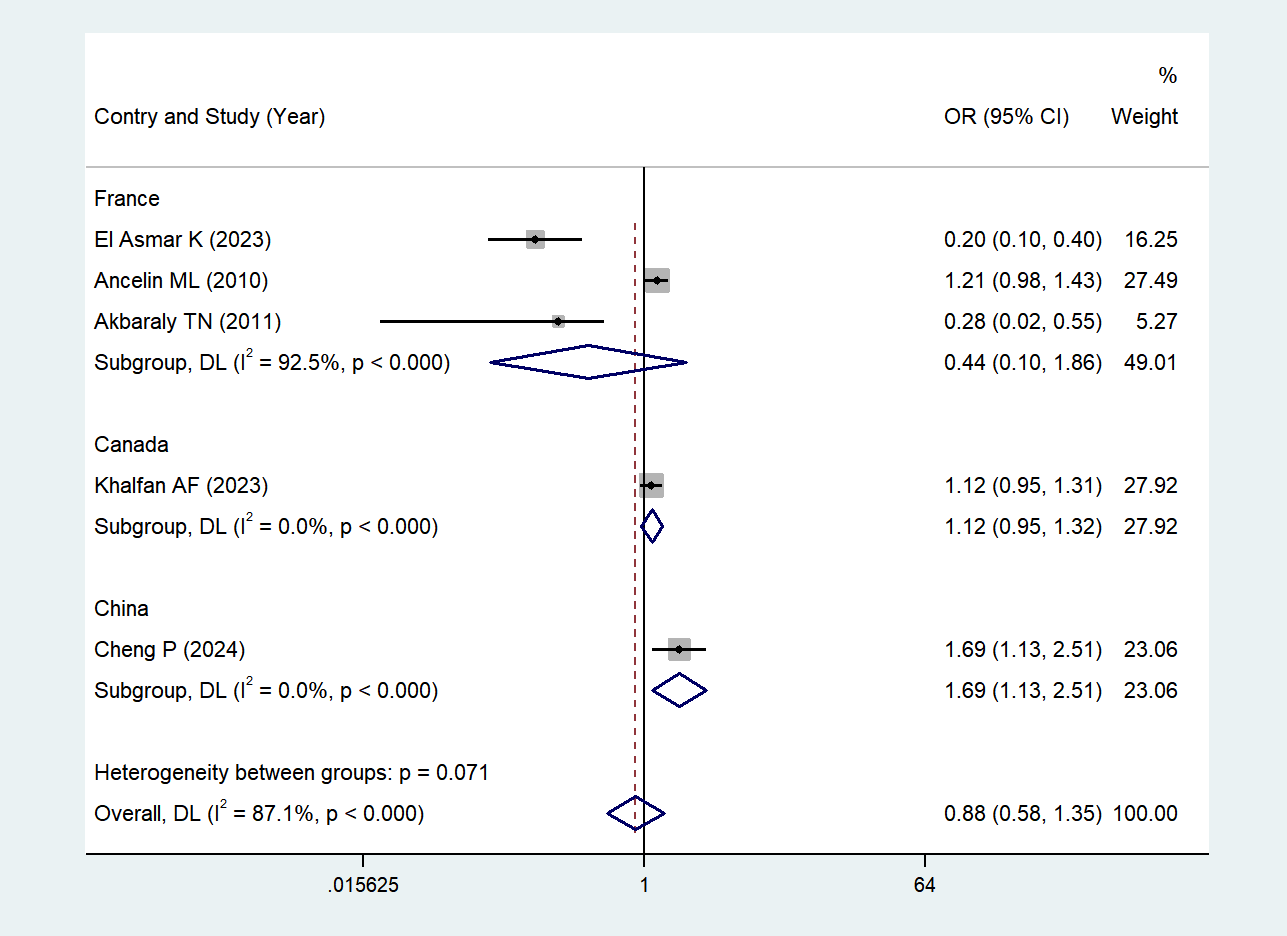
Based on the results of subgroup analyses conducted in countries.
We further performed a subgroup analysis based on study quality scores: For studies with a score of 6 (45, 48): Z = -0.604, P = 0.546 > 0.05; For studies with a score of 7 (44): Z = 1.977, P = 0.048 < 0.05; For studies with a score of 8 (43): Z = -1.506, P = 0.132 > 0.05. We found that in studies with a score of 7 (44), the association between depressive symptoms and serum HDL levels was stronger than that in non-depressed individuals, with a statistically significant difference. See Figure 6 (Subgroup analyses based on study quality scores) for details.
Figure 6

Subgroup analyses based on study quality scores.
The association between depression and serum LDL levels was reported in 2 studies (44, 47). Due to low heterogeneity (I² = 0%, P = 0.909), a fixed-effects model was chosen. The results showed that the association between depressive disorders and serum LDL levels was not statistically significant when compared with non-depressed individuals (OR = 1.05, 95% CI: 0.88–1.24; Z = 0.505, P = 0.613 > 0.05). See Figure 7.
Figure 7

The effect of depression on LDL.
The association between depressive symptoms and serum triglyceride levels was reported in three studies (43–45). Due to low heterogeneity (I² = 0%, P = 0.609), a fixed-effects model was chosen. The results showed that the association between depressive symptoms and serum triglyceride levels was not statistically significant when compared with non-depressed individuals (OR = 1.05, 95% CI: 0.91–1.21; Z = 0.712, P = 0.476 > 0.05). See Figure 8.
Figure 8

The effect of depressive symptoms on serum triglycerides.
Only one study (44) reported the association between depression and serum total cholesterol levels. The results showed that the association between depressive symptoms and serum total cholesterol levels was not statistically significant when compared with non-depressed individuals (OR = 1.11, 95% CI: 0.90–1.32), with Z = 1.068 and P = 0.285 > 0.05.
4.4 Publication bias analysis
Egger’s test was used to assess publication bias associated with the effect of depressive symptoms on serum HDL levels. The results were visualized using a funnel plot (Figure 9), which showed a relatively symmetric distribution. Egger’s test yielded P = 0.320 > 0.05, collectively indicating no significant publication bias.
Figure 9

Publication bias analysis.
5 Discussion
The main objective of this meta-analysis was to systematically analyze the bidirectional and causal relationships between lipid parameters and depression: specifically, to investigate whether dyslipidemia exacerbates depression severity, whether depression further affects lipid levels, and whether these clinical indicators can serve as biomarkers for assessing depression risk. By analyzing seven included studies, we found that changes in serum HDL and triglyceride levels were significantly associated with an increased risk of depression (HDL: OR = 1.28, 95% CI: 1.07–1.53; triglycerides: OR = 1.41, 95% CI: 1.20–1.66; both P < 0.05). In contrast, compared with non-depressed individuals, depressive disorders were not significantly associated with changes in serum HDL, LDL, triglyceride, or total cholesterol levels (HDL: OR = 0.88, 95% CI: 0.58–1.35; LDL: OR = 1.05, 95% CI: 0.88–1.24; triglycerides: OR = 1.05, 95% CI: 0.91–1.21; total cholesterol: OR = 1.11, 95% CI: 0.90–1.32; all P > 0.05).
In summary, the risk of depression is associated with changes in serum HDL and triglyceride levels, and these lipid indicators can serve as biomarkers for assessing depression risk. The pathogenesis of depression is complex, involving biological, psychological, and social environmental factors. This study focuses on the bidirectional relationship between blood lipids and depression; investigating this association is of great significance for understanding the pathogenesis of depression, formulating prevention strategies, and developing individualized treatment plans for patients.
At the same time, this study can help clinicians evaluate the lipid status of patients with depression, develop targeted and timely treatment plans, and provide guidance for family members on patient care—such as instructing patients to adhere to a diet rich in dietary fiber and low in saturated fat, and engage in moderate-intensity physical activity to manage lipid levels. Ultimately, this may help minimize the exacerbation or recurrence of depression in affected patients through lipid control.
For clinical practice, the following specific recommendations are provided: Clinicians are recommended to perform a four-component lipid profile (total cholesterol, triglycerides, LDL-C, HDL-C) and apolipoprotein A1/B testing in patients with depression during their first visit. This is particularly important for patients with obesity, metabolic syndrome, or those receiving long-term antidepressant therapy—some of which may affect lipid metabolism. For these high-risk patients, lipid testing should be repeated every 3–6 months to dynamically monitor changes in lipid metabolism.
If a depressed patient presents with abnormal lipid parameters and moderate-to-severe depressive symptoms, it is recommended to consult with an endocrinologist first. Based on the consultation advice, psychological intervention, antidepressant treatment, and lipid-lowering intervention can be combined: start with lifestyle modifications; if lipid levels remain abnormal after 3 months, short-term lipid-lowering medications may be initiated—with close monitoring of potential adverse effects and drug-drug interactions with antidepressants.
6 Limitation of the study
Egger’s test results indicated no significant publication bias among the included studies, suggesting that the results of this meta-analysis are relatively reliable—at least in terms of publication bias control. However, several limitations should be acknowledged: First, the included studies were not strictly categorized by key variables, and most lacked explicit subgrouping. This inconsistency may have introduced some selection bias into the results. Second, the scales used to assess depression in the included studies were not standardized, which may have compromised the accuracy of outcome assessment. Third, the number of included studies investigating the association between lipid parameters and depression was relatively small. Additionally, many relevant studies only assessed whether overall lipid levels were abnormal and did not report detailed lipid parameters, so these studies could not be included in this meta-analysis. Furthermore, the search was limited to English-language publications during the literature retrieval process, which may exclude region-specific evidence published in other languages. Therefore, future studies should include non-English literature to deepen our understanding of this association.
The articles included in this study cover regions of France (n = 4 studies), China (n = 1 study), Canada (n = 1 study), and Tel Aviv, Israel (n = 1 study). This uneven distribution might affect the generalizability of result interpretation. First, interference from regional and dietary cultural differences: The included studies were from France (with a Mediterranean diet pattern, characterized by high intake of unsaturated fats and dietary fiber), China (with an East Asian diet pattern, characterized by high carbohydrate intake and low saturated fat intake), Canada (with a Western diet pattern, high in processed foods and saturated fat), and Tel Aviv, Israel (with a Middle Eastern diet pattern, high in olive oil and spice intake). Varied dietary patterns have regulatory effects on lipid metabolism, and these regional and dietary cultural differences may lead to variations in the magnitude of the association between lipid levels, 5-HT, and depression across studies—for example, the high unsaturated fat intake in the Mediterranean diet may mitigate the negative association between low HDL and depression, resulting in a weaker effect size in French studies (50, 51). Second, the potential influence of genetic background: The included studies covered the Caucasian population (from France, Canada, and Tel Aviv, Israel) and the East Asian population (from China). Different populations vary in the allele frequencies of genes related to lipid metabolism and key rate-limiting enzymes for 5-HT synthesis. These genetic differences may lead to population-specific sensitivity thresholds for the effects of lipids and 5-HT on depression, thereby increasing heterogeneity among studies. Therefore, future research directions should consider the following improvements: use subgroup analysis (stratified by region, population, and dietary pattern) or meta-regression analysis (with “diet type” and “population” as covariates) to quantify the impact of heterogeneity sources on the association effect and improve the accuracy of result interpretation.
Finally, although serum 5-HT was included in our search strategy, we did not identify any studies that met the inclusion criteria. The missing 5-HT data may lead to two limitations: First, although this study revealed the bidirectional association between lipid indicators and depression, the lack of 5-HT data prevented us from conducting mediation analysis to verify whether lipids regulate serum 5-HT levels to indirectly affect the onset of depression—thus failing to clarify the key mediating pathway. Second, there is a potential impact on the applicability of the study results. Some patients with depression may have abnormal serum 5-HT levels independently of lipids (e.g., primary 5-HT synthesis disorder). Due to the lack of this indicator data, this study cannot distinguish subgroup differences between “pure lipid-related depression” (depression with normal serum 5-HT levels) and “depression related to both lipids and 5-HT” (depression with concurrent lipid abnormalities and 5-HT dysregulation). This may reduce the applicability of the study conclusions to patients with comorbid abnormal 5-HT levels and also fails to provide a reference for whether it is necessary to monitor 5-HT in conjunction with lipid indicators to optimize depression risk stratification in clinical practice.
7 Conclusion
In summary, our meta-analysis shows that among serum lipid parameters, lower HDL levels and higher triglyceride levels exhibit a stronger association with depression onset. This relationship suggests that integrating these lipid indices could aid early intervention, potentially improving prognosis and quality of life in depressed patients. Despite this potential, current evidence remains insufficient. Further large-scale, prospective studies—particularly those focusing on clinical utility, determination of cutoff values, and validation in specific populations—are needed before these biomarkers can be widely implemented in clinical practice.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author contributions
XJ: Writing-original draft, Methodology, Software, CK: Writing-original draft, Investigation, YL: Writing-original draft, Investigation, YiY: Writing-original draft, Investigation, FZ: Writing – review & editing, Funding acquisition, Supervision, TG: Writing – review & editing, Funding acquisition, Supervision, YoY: Writing – review & editing, Funding acquisition, Supervision, XL: Writing-original draft, Investigation.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by a series of research grants, including the National Natural Science Foundation Youth Project (82204851), the Shaanxi Provincial Science and Technology Department Youth Project (2022JQ-955), the National Natural Science Foundation of China (82074552, 82474489, 81873387), the Project of the Construction Plan for the Scientific and Technological Innovation Talent System of Shaanxi University of Chinese Medicine (2023-CXTD-02), the Project for Enhancing the Clinical Research and Transformation Capability of Central High-level Traditional Chinese Medicine Hospitals—Special Project for Clinical Evidence-Based Research in Traditional Chinese Medicine (HLCMHPP2023090), the Key Collaborative Research Project of the Innovation Science and Technology Program of China Academy of Chinese Medical Sciences (CI2022C005), the Innovation Science and Technology Program of China Academy of Chinese Medical Sciences (CI2022C004-02), and the Project for Enhancing the Clinical Research and Transformation Capability of Central High-level Traditional Chinese Medicine Hospitals—Special Project for Emergency Prevention and Control of Respiratory Infectious Diseases (HLCMHPP2023091). We are grateful to Professor Wensheng Qi for his support of this project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2025.1498773/full#supplementary-material.
Abbreviations
5-HT, 5-hydroxytryptamine; 5-HT1A R, 5-Hydroxytryptamine 1A Receptor; FGFR1, Fibroblast Growth Factor Receptor 1; GIRK, G protein-coupled inwardly rectifying potassium; HDL, high density lipoprotein; LDL, low density lipoprotein; 95% CI, 95% confidence intervals; OR, Odds ratio; RR, Relative risk; NOS, Newcastle-Ottawa Scale; V-LDL, very low density lipoprotein; PRISMA, Preferred Reporting Items for Systematic Evaluations and Meta-Analysis; CNS, central nervous system; PNS, peripheral nervous system; GHQ, General Health Questionnaire; CES-D, The Center for Epidemiologic Studies Depression Scale; HDRS, The 17-item Hamilton Depression Rating Scale; MINI, The Mini International Neuropsychiatric Interview; PROSPERO, International Prospective Register of Systematic Reviews; MeSH terms, Medical Subject Headings; DSM, Diagnostic and Statistical Manual of Mental Disorders.
References
1
Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. (2020) 396:1204–22. doi: 10.1016/s0140-6736(20)30925-9
2
Liu L Wang H Chen X Zhang Y Zhang H Xie P . Gut microbiota and its metabolites in depression: from pathogenesis to treatment. EBioMedicine. (2023) 90:104527. doi: 10.1016/j.ebiom.2023.104527
3
Lu X Yang D Liang J Xie G Li X Xu C et al . Effectiveness of intervention program on the change of glycaemic control in diabetes with depression patients: A meta-analysis of randomized controlled studies. Prim Care Diabetes. (2021) 15:428–34. doi: 10.1016/j.pcd.2021.01.006
4
Weissman MM Wickramaratne P Gameroff MJ Warner V Pilowsky D Kohad RG et al . Offspring of depressed parents: 30 years later. Am J Psychiatry. (2016) 173:1024–32. doi: 10.1176/appi.ajp.2016.15101327
5
Peters A McEwen BS Friston K . Uncertainty and stress: Why it causes diseases and how it is mastered by the brain. Prog Neurobiol. (2017) 156:164–88. doi: 10.1016/j.pneurobio.2017.05.004
6
Marx W Manger SH Blencowe M Murray G Ho FY Lawn S et al . Clinical guidelines for the use of lifestyle-based mental health care in major depressive disorder: World Federation of Societies for Biological Psychiatry (WFSBP) and Australasian Society of Lifestyle Medicine (ASLM) taskforce. World J Biol Psychiatry. (2023) 24:333–86. doi: 10.1080/15622975.2022.2112074
7
Beurel E Toups M Nemeroff CB . The bidirectional relationship of depression and inflammation: double trouble. Neuron. (2020) 107:234–56. doi: 10.1016/j.neuron.2020.06.002
8
Zhou M Fan Y Xu L Yu Z Wang S Xu H et al . Microbiome and tryptophan metabolomics analysis in adolescent depression: roles of the gut microbiota in the regulation of tryptophan-derived neurotransmitters and behaviors in human and mice. Microbiome. (2023) 11:145. doi: 10.1186/s40168-023-01589-9
9
Bhatt S Devadoss T Manjula SN Rajangam J . 5-HT(3) receptor antagonism a potential therapeutic approach for the treatment of depression and other disorders. Curr Neuropharmacol. (2021) 19:1545–59. doi: 10.2174/1570159x18666201015155816
10
Barnes NM Ahern GP Becamel C Bockaert J Camilleri M Chaumont-Dubel S et al . International union of basic and clinical pharmacology. CX. Classification of receptors for 5-hydroxytryptamine; pharmacology and function. Pharmacol Rev. (2021) 73:310–520. doi: 10.1124/pr.118.015552
11
Carhart-Harris RL Nutt DJ . Serotonin and brain function: a tale of two receptors. J Psychopharmacol. (2017) 31:1091–120. doi: 10.1177/0269881117725915
12
Vargas MV Dunlap LE Dong C Carter SJ Tombari RJ Jami SA et al . Psychedelics promote neuroplasticity through the activation of intracellular 5-HT2A receptors. Science. (2023) 379:700–06. doi: 10.1126/science.adf0435
13
Enache D Pariante CM Mondelli V . Markers of central inflammation in major depressive disorder: A systematic review and meta-analysis of studies examining cerebrospinal fluid, positron emission tomography and post-mortem brain tissue. Brain Behav Immun. (2019) 81:24–40. doi: 10.1016/j.bbi.2019.06.015
14
Frid LM Kessler U Ousdal OT Hammar Å Haavik J Riemer F et al . Neurobiological mechanisms of ECT and TMS treatment in depression: study protocol of a multimodal magnetic resonance investigation. BMC Psychiatry. (2023) 23:791. doi: 10.1186/s12888-023-05239-0
15
Miller AH Raison CL . The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. (2016) 16:22–34. doi: 10.1038/nri.2015.5
16
He Y Han Y Liao X Zou M Wang Y . Biology of cyclooxygenase-2: An application in depression therapeutics. Front Psychiatry. (2022) 13:1037588. doi: 10.3389/fpsyt.2022.1037588
17
Sălcudean A Bodo CR Popovici RA Cozma MM Păcurar M Crăciun RE et al . Neuroinflammation-A crucial factor in the pathophysiology of depression-A comprehensive review. Biomolecules. (2025) 15(4):502. doi: 10.3390/biom15040502
18
Luo J Yang H Song BL . Mechanisms and regulation of cholesterol homeostasis. Nat Rev Mol Cell Biol. (2020) 21:225–45. doi: 10.1038/s41580-019-0190-7
19
Borroto-Escuela DO Ambrogini P Narvaez M Di Liberto V Beggiato S Ferraro L et al . Serotonin heteroreceptor complexes and their integration of signals in neurons and astroglia-relevance for mental diseases. Cells. (2021) 10(8):1902. doi: 10.3390/cells10081902
20
Ambrogini P Lattanzi D Pagliarini M Di Palma M Sartini S Cuppini R et al . 5HT1AR-FGFR1 heteroreceptor complexes differently modulate GIRK currents in the dorsal hippocampus and the dorsal raphe serotonin nucleus of control rats and of a genetic rat model of depression. Int J Mol Sci. (2023) 24(8):7467. doi: 10.3390/ijms24087467
21
Zeng J Wang Z Zhang X Zhao A Qi H Jiang Y et al . Exploring the neuroplasticity hypothesis in depression: The role of traditional Chinese herbal medicine. Phytomedicine. (2025) 143:156927. doi: 10.1016/j.phymed.2025.156927
22
Li H Zhang X Sun Q Zou R Li Z Liu S . Association between serum lipid concentrations and attempted suicide in patients with major depressive disorder: A meta-analysis. PloS One. (2020) 15:e0243847. doi: 10.1371/journal.pone.0243847
23
Ma YJ Wang DF Yuan M Zhang XJ Long J Chen SB et al . The prevalence, metabolic disturbances and clinical correlates of recent suicide attempts in Chinese inpatients with major depressive disorder. BMC Psychiatry. (2019) 19:144. doi: 10.1186/s12888-019-2131-6
24
Nikoli V Simi M Kandi V Dodevska M Titan P Dodig D et al . Pasting properties and the baking functionality of whole-grain wheat flour with different amylose and dietary fibers content. J Food Process Preservation. (2021) e15805. doi: 10.1111/jfpp.15805
25
Milić J . How to design a reliable and practical biomarker: the electrophysiologic coefficient of depressiveness - δEPCD. Biomarkers. (2022) 27:711–14. doi: 10.1080/1354750x.2022.2122565
26
Milic J Jovic S Sapic R . Advancing depression management through biomarker discovery with a focus on genetic and epigenetic aspects: A comprehensive study on neurobiological, neuroendocrine, metabolic, and inflammatory pathways. Genes (Basel). (2025) 16(5):487. doi: 10.3390/genes16050487
27
Yang R Wang L Cao S Chen M Wu CJ Silva F et al . Sex difference in lipid levels in first-diagnosed drug-naïve depression patients: A case-control and 12-weeks follow-up study. World J Biol Psychiatry. (2022) 23:228–35. doi: 10.1080/15622975.2021.1961500
28
Shin HY Kang G Kang HJ Kim SW Shin IS Yoon JS et al . Relationships between high-density lipoprotein cholesterol and depressive symptoms: Findings of the Korean National Health and Nutrition Examination Survey (KNHANES). Psychiatry Res. (2016) 241:172–4. doi: 10.1016/j.psychres.2016.05.003
29
Mehdi SMA Costa AP Svob C Pan L Dartora WJ Talati A et al . Depression and cognition are associated with lipid dysregulation in both a multigenerational study of depression and the National Health and Nutrition Examination Survey. Transl Psychiatry. (2024) 14:142. doi: 10.1038/s41398-024-02847-6
30
Broussard J Brady MJ . The impact of sleep disturbances on adipocyte function and lipid metabolism. Best Pract Res Clin Endocrinol Metab. (2010) 24:763–73. doi: 10.1016/j.beem.2010.08.007
31
Parekh A Smeeth D Milner Y Thure S . The role of lipid biomarkers in major depression. Healthcare (Basel). (2017) 5(1):5. doi: 10.3390/healthcare5010005
32
Bot M Milaneschi Y Al-Shehri T Amin N Garmaeva S Onderwater GLJ et al . Metabolomics profile in depression: A pooled analysis of 230 metabolic markers in 5283 cases with depression and 10,145 controls. Biol Psychiatry. (2020) 87:409–18. doi: 10.1016/j.biopsych.2019.08.016
33
Furuyashiki T Akiyama S Kitaoka S . Roles of multiple lipid mediators in stress and depression. Int Immunol. (2019) 31:579–87. doi: 10.1093/intimm/dxz023
34
Wei YG Cai DB Liu J Liu RX Wang SB Tang YQ et al . Cholesterol and triglyceride levels in first-episode patients with major depressive disorder: A meta-analysis of case-control studies. J Affect Disord. (2020) 266:465–72. doi: 10.1016/j.jad.2020.01.114
35
Chuang CS Yang TY Muo CH Su HL Sung FC Kao CH . Hyperlipidemia, statin use and the risk of developing depression: a nationwide retrospective cohort study. Gen Hosp Psychiatry. (2014) 36:497–501. doi: 10.1016/j.genhosppsych.2014.05.008
36
Spitzer RL Williams JB Kroenke K Linzer M deGruy FV 3rd Hahn SR et al . Utility of a new procedure for diagnosing mental disorders in primary care. The PRIME-MD 1000 study. Jama. (1994) 272:1749–56. doi: 10.1001/jama.1994.03520220043029
37
Spitzer RL Kroenke K Williams JB . Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. Jama. (1999) 282:1737–44. doi: 10.1001/jama.282.18.1737
38
Radloff LS . The CES-D scale A self-report depression scale for research in the general population. Appl psychol Measurement. (1977) 1:385–401. doi: 10.1177/014662167700100306
39
Hamilton M . A rating scale for depression. J Neurol Neurosurg Psychiatry. (1960) 23:56–62. doi: 10.1136/jnnp.23.1.56
40
Sheehan DV Lecrubier Y Sheehan KH Amorim P Janavs J Weiller E et al . The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. (1998) 59 Suppl 20:22–33.
41
Ritchie K Artero S Beluche I Ancelin ML Mann A Dupuy AM et al . Prevalence of DSM-IV psychiatric disorder in the French elderly population. Br J Psychiatry. (2004) 184:147–52. doi: 10.1192/bjp.184.2.147
42
Higgins JP Thompson SG Deeks JJ Altman DG . Measuring inconsistency in meta-analyses. Bmj. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
43
Akbaraly TN Ancelin ML Jaussent I Ritchie C Barberger-Gateau P Dufouil C et al . Metabolic syndrome and onset of depressive symptoms in the elderly: findings from the three-city study. Diabetes Care. (2011) 34:904–9. doi: 10.2337/dc10-1644
44
Ancelin ML Carrière I Boulenger JP Malafosse A Stewart R Cristol JP et al . Gender and genotype modulation of the association between lipid levels and depressive symptomatology in community-dwelling elderly (the ESPRIT study). Biol Psychiatry. (2010) 68:125–32. doi: 10.1016/j.biopsych.2010.04.011
45
El Asmar K Annan NB Khoury R Colle R Martin S Ghoul TE et al . Non-overweight depressed patients who respond to antidepressant treatment have a higher risk of later metabolic syndrome: findings from the METADAP cohort. Psychol Med. (2023) 53:6560–69. doi: 10.1017/s0033291722003919
46
Akbaraly TN Kivimäki M Brunner EJ Chandola T Marmot MG Singh-Manoux A et al . Association between metabolic syndrome and depressive symptoms in middle-aged adults: results from the Whitehall II study. Diabetes Care. (2009) 32:499–504. doi: 10.2337/dc08-1358
47
Cheng P Wang L Zhao G Li W . Dynamic risk factors of psychiatric readmission for major depressive disorder: A longitudinal study on patients treated with mono-antidepressant. Psychiatry Res. (2024) 333:115750. doi: 10.1016/j.psychres.2024.115750
48
Khalfan AF Campisi SC Lo RF McCrindle BW Korczak DJ . The association between adolescent depression and dyslipidemia. J Affect Disord. (2023) 338:239–45. doi: 10.1016/j.jad.2023.06.017
49
Toker S Shirom A Melamed S . Depression and the metabolic syndrome: gender-dependent associations. Depress Anxiety. (2008) 25:661–9. doi: 10.1002/da.20379
50
Achour Y Lucas G Iceta S Boucekine M Rahmati M Berk M et al . Dietary patterns and major depression: results from 15,262 participants (International ALIMENTAL study). Nutrients. (2025) 17(9):1583. doi: 10.3390/nu17091583
51
Lassale C Batty GD Baghdadli A Jacka F Sánchez-Villegas A Kivimäki M et al . Healthy dietary indices and risk of depressive outcomes: a systematic review and meta-analysis of observational studies. Mol Psychiatry. (2019) 24:965–86. doi: 10.1038/s41380-018-0237-8
Summary
Keywords
depressive disorder, bidirectional relationship, serum 5-HT, dyslipidemia, a systematic, meta-analysis
Citation
Jin X, Kang C, Lu Y, Yang Y, Zhou F, Gao T, Liu X and Yan Y (2025) Exploring the bidirectional relationship between depressive disorder and dyslipidemia: a systematic review and meta-analysis. Front. Psychiatry 16:1498773. doi: 10.3389/fpsyt.2025.1498773
Received
20 September 2024
Accepted
13 October 2025
Published
10 December 2025
Volume
16 - 2025
Edited by
Mark M. Rasenick, University of Illinois Chicago, United States
Reviewed by
Shinsuke Hidese, Teikyo University, Japan
Jelena Milic, Institute of Public Health of Serbia “Dr Milan Jovanovic Batut”, Serbia
Updates
Copyright
© 2025 Jin, Kang, Lu, Yang, Zhou, Gao, Liu and Yan.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feng Zhou, zhoufeng_brain@163.com; Tao Gao, gtpeach@163.com; Yongmei Yan, 13609216551@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.