- 1Child and Adolescent Neuropsychiatry Unit, Bambino Gesù Children’s Hospital, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS), Rome, Italy
- 2Department of Neuroscience, Università Cattolica del Sacro Cuore, Rome, Italy
- 3Department of Psychiatry, Massachusetts General Hospital, Harvard Medical School, Boston, MA, United States
- 4Department of Life Sciences and Public Health, Università Cattolica del Sacro Cuore, Rome, Italy
Background: Sex differences in psychiatric symptoms among children and adolescents with a major depressive episode (MDE) are less studied than among adults. Previous non-recent studies reported a greater severity in adolescent girls and small differences between sexes in specific symptoms. We aim to explore the differences between male and female patients in the diagnoses, comorbidities, and psychiatric symptoms in a large cohort of pediatric patients referred to a tertiary center for child and adolescent psychiatry.
Methods: We collected cross-sectional data on 382 consecutively referred patients (age 6–18 years; 73.8% female patients) with current MDEs (unipolar or bipolar) thoroughly evaluated with clinician (Children’s Depression Rating Scale-Revised; K-SADS Mania Rating Scale; Columbia Suicide Severity Rating Scale) and self and parent report (Children’s Depression Inventory-2; Multidimensional Anxiety Scale for Children-2; Child Behavior Checklist) standardized measures. Bivariate analyses were followed by a logistic regression model to assess significant predictors of the MDE phenotype of female (vs. male) patients.
Results: Female patients were more likely to show severe MDEs (41.5% vs. 26.0%; p = 0.006), suicidal ideation (63.9% vs. 47.0%; p < 0.001) and behaviors (29.4% vs. 13.0%; p = 0.001), and non-suicidal self-injury (58.5% vs. 27.0%; p < 0.001). Male patients were more frequently diagnosed with bipolar disorder (21% vs. 11%; p = 0.012) and/or comorbid ADHD/behavior disorders (20% vs. 8.9%; p = 0.003). Male patients also had more frequently significant mixed hypo/manic symptoms (17% vs. 7.7%; p = 0.01) and were younger at the onset of the first psychiatric symptom (6.32 vs. 7.75 years; p = 0.003), onset of mood disorder (11.3 vs. 12.5 years; p = 0.005), and evaluation (14.0 vs. 15.2 years; p = 0.001). Several symptoms were significantly and independently associated with female patients diagnosed with a current MDE, including a) excessive weeping (OR 1.53; p < 0.001), b) mood lability (OR 1.50; p = 0.014), c) excessive fatigue (OR 1.38; p = 0.002), d) appetite disturbance (OR 1.28; p = 0.041), and e) attention problems (OR 1.07; p = 0.001). Distractibility (OR 0.55; p = 0.009) and conduct problems (OR 0.93; p = 0.001) were in turn correlated with MDE among male patients.
Discussion: The study confirms that female patients with MDEs exhibit more severe affective symptoms, while male patients present with more externalizing behaviors and comorbidities. We further report more mixed symptoms and bipolar disorder diagnoses in male patients, who also have an earlier onset of psychiatric symptoms. These findings are discussed also considering implications for the diagnosis of pediatric bipolar disorder. A high clinical sensitivity is needed for highlighting subtle mixed and/or atypical features in severe MDEs among girls.
1 Introduction
Depression is a prevalent psychiatric disorder globally and one of the leading causes of both mental and physical disability worldwide according to the World Health Organization (1). The pooled prevalence rate of depressive disorders in children and adolescents in Europe is 1.7% (2). Its burden is even greater in lower-middle-income countries (3). The prevalence of depression before puberty is similar in both sexes (4), and boys and girls diagnosed with major depressive disorder (MDD) exhibit comparable symptoms before puberty (5). During adolescence, the prevalence of depression among female patients doubles, resulting in a 1:2 male-to-female ratio in both adolescents and adults (4). Studies on adult samples report sex differences in depressive symptoms, with more externalizing behaviors among men and more severe depressive symptoms among women (6). Indeed, men are reported to display more likely externalizing behaviors, impulsivity, and risk-taking, which are not included in the DSM-5 criteria for major depressive episode (MDE) and may serve as maladaptive coping strategies (6, 7). Women are more likely to experience more severe depressive symptoms (8), report them more openly, and present with greater appetite disturbances (9). They are also more likely to have comorbid internalizing disorders, such as anxiety and eating disorders (6). The effect sizes of differences in specific symptoms remain small (≤0.2) (6).
Research on sex differences in adolescent depression is less extensive than in adults. In general population samples, female patients have been reported to express higher self-rated depressive symptoms compared to male patients, findings that most studies contextualized within the complex biopsychosocial interactions during adolescent development. Emotional reactivity to interpersonal stressors has been reported to be higher among girls (10), and specific stressors, including the absence of social support, may impact sexes differently (11). According to a theoretical model, female patients have increased affiliative needs during puberty, which makes them more susceptible to interpersonal stressors as a risk factor for the development of depressive symptoms (12).
The literature on adolescents with a confirmed clinical diagnosis of depression is more limited and has shown mixed results. Some authors found no clear gender differences in depressive features in clinically referred adolescents (13, 14); however, other studies showed that female adolescents with depression were more likely to report sadness, depressed mood, self-disappointment, self-blame, feelings of failure, concentration difficulties, fatigue, and health worries, while male adolescents were more likely to report anhedonia and worsened mood in the morning (15) and to display more impulsive and antisocial behaviors (16). Coping strategies may also differ: female adolescents tend to ruminate, while male adolescents more often avoid emotional exploration (17). Female adolescents with depression exhibit greater self-doubt, self-blame, and negative self-evaluation, with a need for external validation (16).
Cultural differences may have an impact on the expression of psychiatric symptoms (18) and may be relevant in interpreting reports on gender differences in depression; however, studies from different cultural contexts report consistent findings. In a sample of adolescents with confirmed DSM-IV MDD from China, girls were found to experience higher levels of depression and anxiety symptoms (19). In a sample of Japanese adolescents, higher emotional expressivity and lower self-efficacy, competitiveness, and risk-taking behaviors were described in female adolescents (20).
Epidemiological studies indicate a higher lifetime incidence (149 vs. 55.3 per 100,000/year) (21) and prevalence (3.3% vs. 2.6%) (22) of bipolar disorder (BD) in female adolescents. Male adolescents more frequently meet the criteria for hypo/manic episodes, while female adolescents report more depressive symptoms, particularly at older ages (23). In a previous study by our group, women with MDEs and mixed features exhibited greater affective symptoms, fatigue, and emotional dysregulation, while men showed more psychomotor activation (24). This remains the only study to date assessing gender differences in mood disorders using measures for both depressive and hypo/manic symptoms, emotional dysregulation, and problematic behaviors.
Research on sex differences in adolescent depression—especially in clinical populations assessed with clinician-rated and multi-informant scales—remains limited, but some evidence suggests that sex-specific factors significantly shape symptoms, with implications for neurobiology and social influences on early-onset mood disorders. Additionally, mixed hypomanic symptoms, often overlooked in standard depression assessments, are common also in children and adolescents with severe early-onset mood disorders and are crucial for evaluating clinical severity (25). Our study examines sex differences in mood disorder diagnoses and psychiatric symptoms in a large clinical population of children and adolescents over nearly a decade, incorporating clinician, parent, and self-report measures.
2 Methods
2.1 Participants
The sample for this study was recruited from a day hospital unit of a specialized psychiatric service for the assessment and treatment of early-onset mood disorders, managed by the Mood Disorders Program team at Bambino Gesù Children’s Hospital in Rome. Subjects were referred to confirm a diagnostic hypothesis of a moderate/severe mood disorder. Referral sources included primary care doctors (pediatricians and general practitioners), secondary care providers (child psychiatrists in national healthcare centers), and the emergency department of Bambino Gesù Children’s Hospital.
Historical data obtained during clinical assessments performed in our outpatient service from June 2013 to March 2022 were extracted from clinical records. We included consecutively referred children and adolescents, aged 6 to 18 years at the time of evaluation. Categorical diagnoses were clinically made and confirmed with the Kiddie-Schedule for Affective Disorders and Schizophrenia for School-aged Children, Present and Lifetime version (K-SADS-PL) (26) following the DSM-5 criteria. We included subjects diagnosed with a current MDE in the context of either an MDD or BD. Subjects diagnosed with persistent depressive disorder with intermittent major depressive episodes, with current MDE, and subjects diagnosed with MDE with mixed symptoms were also included.
We excluded patients with MDE in partial or complete remission at the time of evaluation, those with intellectual disability, autism spectrum disorder, substance-induced mood disorders, or mood disorders due to other medical conditions, as defined by DSM-5. Parents/legal guardians provided written informed consent for anonymous reports of data in aggregate form, in compliance with research ethics. Data collected were stored in individual clinical records. The study has been conducted according to the guidelines established in the Declaration of Helsinki. Considering the retrospective nature of the analysis, the current study did not require the approval of the local ethics committee according to current legislation, but a notification was sent (Comitato Etico Ospedale Pediatrico Bambino Gesù practice number 3380). Data were retrospectively anonymously analyzed in line with personal data protection policies.
2.2 Assessment
All subjects were evaluated over a minimum of three appointments, totaling 9–10 h of clinical assessment. All study participants were assessed by an experienced child and adolescent psychiatrist and an experienced psychologist using the K-SADS-PL (26), a semistructured, clinician-administered diagnostic interview performed with both subjects and their parents or adult legal representatives to assess current and past psychopathological features and psychiatric disorders, including primary mood disorders and comorbidities in juveniles, according to the DSM-5 criteria. Childhood clinical features (syndromal or subsyndromal) preceding the onset of current disorders were systematically assessed and coded both as “traits” if subsyndromal or “disorder” if meeting the criteria for a DSM-5 diagnosis, with particular attention to the time of onset of first mood and non-mood disorder-related psychiatric symptoms (27).
Additionally, depressive and hypo/manic or mixed symptoms were rated by the same experienced clinicians using the Children’s Depression Rating Scale-Revised (CDRS-R) (28) and the K-SADS Mania Rating Scale (KMRS) (29). The CDRS-R is a semistructured interview used to rate depressive symptoms in subjects aged 6–18 years, based on 17 items with a raw score range of 17–120 (sum of the scores of single items, considered positive at scores >30 corresponding to a T-score >55). We analyzed the CDRS-R total score and the single items’ scores (school dysfunctions, difficulties in having fun, difficulties in interpersonal relationships, sleep disorders, appetite disorders, excessive fatigue, psychosomatic complaints, irritability, excessive guilt, low self-esteem, depressive feelings, morbid ideas, suicidal ideation, excessive crying, reduced facial expressions, slow speech, and motor hypoactivity). The KMRS is a structured interview used to rate hypo/manic symptoms in subjects aged 6–18 years, based on 14 items, with a total score range of 1–68 (sum of the scores of 0 to 6 for single items minus 13, considered indicative of significant hypomanic symptoms at scores >12). We analyzed both the total score and the single items’ score (euphoria and expansiveness; irritability and anger; mood lability; reduced need for sleep; crowded thoughts; increased energy; increased activities; motor hyperactivity; grandiosity; rapid or pressured speech; distractibility; impaired judgment; hallucinations; and delusions). Single items’ score of <2 is considered normal, a score of 2–3 is considered borderline, and a score >3 is considered clinically significant (29). Depression severity at the time of evaluation—whether mild, moderate, severe, with or without psychotic symptoms, or in partial/complete remission—was defined based on clinical judgment according to the DSM-5 criteria and CDRS-R cutoffs: a total score of 30 to 44 for mild, 45 to 55 for moderate, 55 or more for severe depression, and 29 or less for remission.
Suicidal ideation and suicidal behaviors were evaluated with the Columbia Suicide Severity Rating Scale (C-SSRS) (30). Suicidal behavior is defined as any self-harming behavior resulting in any kind of damage with non-zero intent to die, declared by the patient or evident from documented circumstances. Non-suicidal self-injury (NSSI) was systematically investigated lifetime and accurately differentiated from suicidal behavior and defined as in section III of DSM-5 (31).
To assess self-reported symptoms, the Children’s Depression Inventory-2 (CDI-2) (32) and the Multidimensional Anxiety Scale for Children-2 (MASC-2) (33) were administered to patients and their parents/caregivers. For each module of CDI-2 and MASC-2, the total T-score was analyzed.
Finally, the Italian version of the Child Behavior Checklist (CBCL) (34) was administered to parents/caregivers to score different behavioral and emotional problems severity. It has strong psychometric properties and is widely used in Italy (35, 36). It provides scores on three behavior rating scales that address internalizing symptoms, externalizing symptoms, and total behavioral problems. Sub-items of these scales include syndromic and DSM-oriented scales.
An overall Deficient Emotional Self-Regulation CBCL profile was calculated by summing scores for attention problems, aggression, and anxious-depressed syndromic scales. This score is reported to be indicative of Deficient Emotional Self-Regulation at scores between 180 and 210 (1 to 2 standard deviations) and as meeting the criteria for a Dysregulation Profile at a >210 (>2 standard deviations) score (37).
2.3 Statistical analysis
Categorical variables were presented as counts and frequency rates, while continuous variables were expressed as means and standard deviations. For scales with missing data, participants were excluded from analyses, and no imputation methods were applied.
Associations between unpaired categorical variables were tested using Pearson’s chi-square test, with Fisher’s exact test applied when more than 20% of cells in the contingency table had expected frequencies <5 (38). Mean differences of continuous variables between the two groups were tested using Student’s t-test for unpaired data.
We performed a logistic regression analysis with female sex as the dependent variable, to assess which of the studied variables best predict the MDE phenotype in female patients, while controlling for other factors. In more detail, our aim was to determine how accurately and independently the most relevant clinician-, self-, and parent-rated measures can distinguish between male and female patients with a current MDE based on psychiatric symptom expression. Of the CDRS-R items, KMRS items, CBCL scales, and MASC-2 and CDI-2 T-scores, variables found significantly different between sexes at previous bivariate analyses were tested as predictors, while female sex was set as the predicted value. Predictor selection was performed using a stepwise backward approach, progressively removing variables with p >0.05. Odds ratios (OR), with their 95% confidence intervals (95% CI), p-values, and Wald test statistics have been presented. All tests were two-tailed. A p-value of 0.05 or less was considered indicative of significance. Analyses were conducted with Microsoft Office 365—Excel and IBM SPSS Statistics V26 software.
3 Results
3.1 Participants’ demographics and psychiatric diagnoses
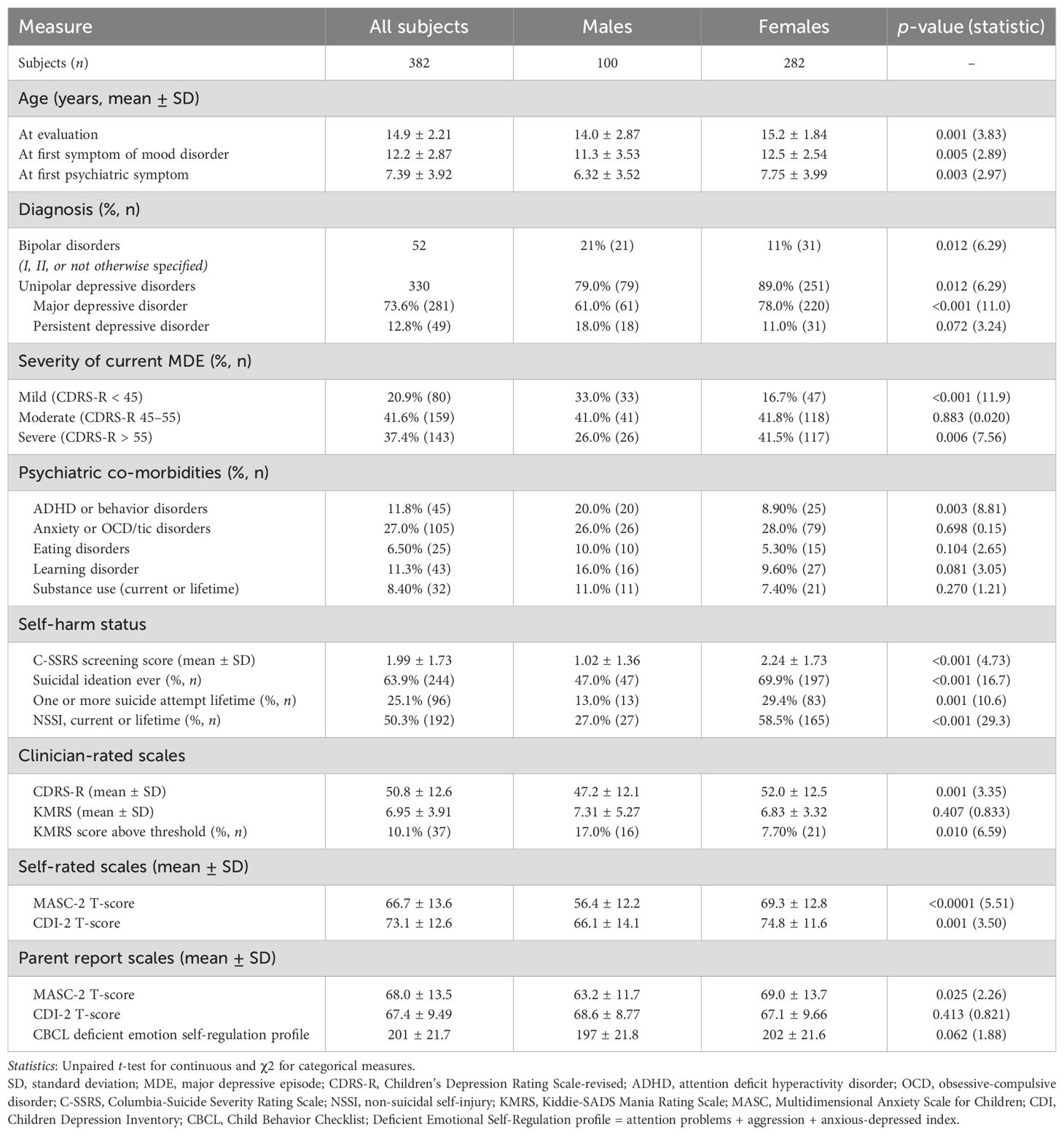
After the application of the exclusion criteria, we retrospectively identified 382 patients diagnosed with current MDE who were eligible for inclusion in the study. Of these participants, 73.8% (N = 282) were female patients and 26.2% (N = 100) were male patients. Male patients were significantly younger at the time of evaluation (14.0 years vs. 15.2 years; p = 0.001). They were also younger when their first psychiatric symptom was reported by parents/caregivers (6.32 years vs. 7.75 years; p = 0.003) and when the first symptoms of their mood disorder were reported (11.3 years vs. 12.5 years; p = 0.005).
Bipolar disorder was diagnosed in 13.6% (N = 52) of the participants. This diagnosis was more common in male participants (21% vs. 11%; p = 0.012). Conversely, MDD diagnosis was significantly more frequent among female participants (78% vs. 61%; p < 0.001).
Thirty-seven percent of the subjects had a severe MDE, with a CDRS-R total score >55. Most patients were classified as having a moderate severity depressive episode (CDRS-R total score between 45 and 55). Severe MDEs were more frequently diagnosed in female than in male participants (41.5% vs. 26.0%; p = 0.006); mild MDEs, in turn, were more frequently diagnosed in male participants (33.0% vs. 16.7%; p < 0.001).
The most frequent comorbidities were anxiety disorders and obsessive-compulsive or tic disorders (27%), followed by ADHD/behavior disorders (11.8%), learning disorders (11.3%), substance abuse (8.4%), and eating disorders (6.5%). Only ADHD/behavior disorders were significantly more prevalent among male participants than female participants (20% vs. 8.9%; p = 0.003). No other significant differences in comorbidities were observed between male and female participants. For further details on participants’ demographics and psychiatric diagnoses, see Table 1.
3.2 Sex differences in suicidal ideation and behaviors
More than 60% of the participants reported having experienced at least one episode of active suicidal ideation during their lifetime. History of active suicidal ideation was more commonly reported by female than male participants (63.9% of female patients vs. 47.0% of male patients; p < 0.001). Almost one-third of female subjects reported a lifetime history of one or more suicide attempts, while male subjects reported suicide attempts significantly less frequently (29.4% vs. 13.0%; p = 0.001). Consistently, female patients scored higher on the C-SSRS screening version (2.24 ± 1.73 vs. 1.02 ± 1.36; t = 4.73; p < 0.001). Non-suicidal self-injury (current or lifetime) was also reported in approximately half of the sample and was more commonly reported by female patients (58.5% vs. 27.0%; t = 29.3; p < 0.001). For further details, see Table 1.
3.3 Sex differences in clinician-rated symptoms
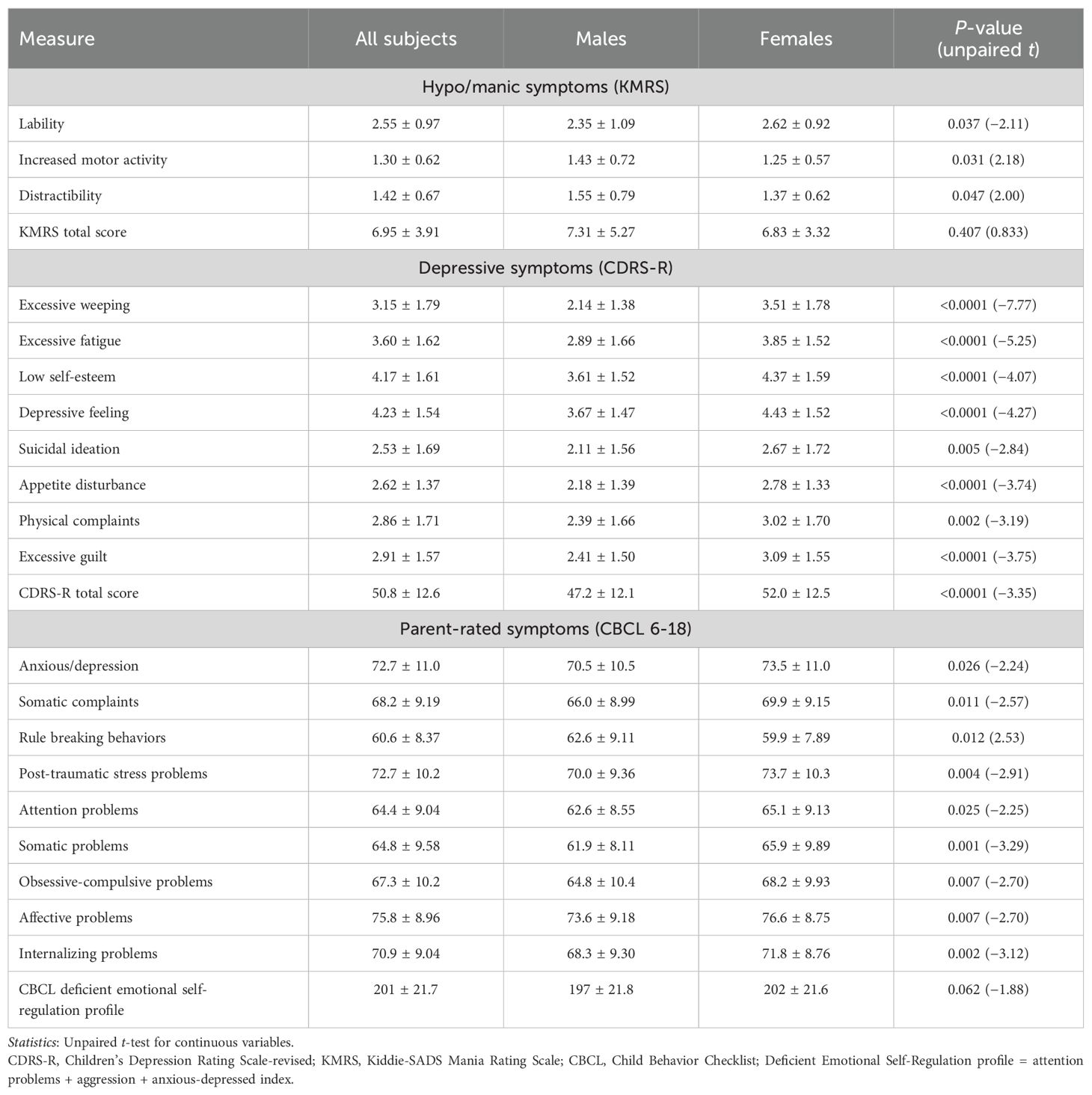
Depression severity, measured by the CDRS-R total score, was significantly higher among female than male patients (52.0 ± 12.5 vs. 47.2 ± 12.1; t = 3.35; p < 0.001; Table 2). Several depressive symptoms were also significantly more severe in female participants compared to male participants, including excessive weeping (3.51 vs. 2.14; p < 0.001), excessive fatigue (3.85 vs. 2.89; p < 0.001), low self-esteem (4.37 vs. 3.61; p < 0.001), depressive feelings (4.43 vs. 3.67; p < 0.001), suicidal ideation (2.67 vs. 2.11; p = 0.005), appetite disturbance (2.78 vs. 2.18; p < 0.001), physical complaints (3.02 vs. 2.39; p = 0.002), and excessive guilt (3.09 vs. 2.41; p < 0.001; Table 2). No depressive symptom was found to be more severe in male participants.
The severity of hypo/manic symptoms, measured by the KMRS total score, was similar in mean values between male and female patients, but significant mixed hypo/manic symptoms (defined by the KMRS total score above the instrument threshold of 12) were identified in more male than in female participants (17% vs. 7.7%; p = 0.010; Table 1). Male patients scored higher than female patients in KMRS increased motor activity (1.43 vs. 1.25; p = 0.031) and KMRS distractibility (1.55 vs. 1.37; p = 0.047), whereas KMRS mood lability was significantly higher in female patients (2.62 vs. 2.35; p = 0.037). For further details on clinician-rated symptoms, see Table 2.
3.4 Sex differences in self-/parent-rated symptoms
Both self-reported and parent-reported anxiety symptoms, measured by the MASC-2 total score, were significantly higher among female than male patients (69.3 vs. 56.4, p < 0.0001 and 69.0 vs. 63.2, p = 0.025, respectively). Self-reported depressive symptoms, measured by the CDI-2 total score, were also significantly higher among female than male patients (74.8 vs. 66.1; p = 0.001; Table 1). Parent reports of depressive symptoms, however, did not show significant differences between groups.
Different internalizing and externalizing parent-rated symptoms and behaviors assessed by the CBCL were different between the sexes. Post-traumatic stress problems (73.7 vs. 70.0; p = 0.004), attention problems (65.1 vs. 62.6; p = 0.025), internalizing problems (71.8 vs. 68.3; p = 0.002), the anxious/depression scale (73.5 vs. 70.5; p = 0.026), the somatic complaints scale (69.9 vs. 66.0; p = 0.011), obsessive-compulsive problems (68.2 vs. 64.8; p = 0.007), and affective problems (76.6 vs. 73.6; p = 0.007) were more severe in female than male patients. Conversely, male participants had significantly higher scores in rule-breaking behaviors compared to female participants (62.6 vs. 59.9; p = 0.012). For further details on self/parent-rated symptoms, see Table 2.
3.5 Logistic regression model
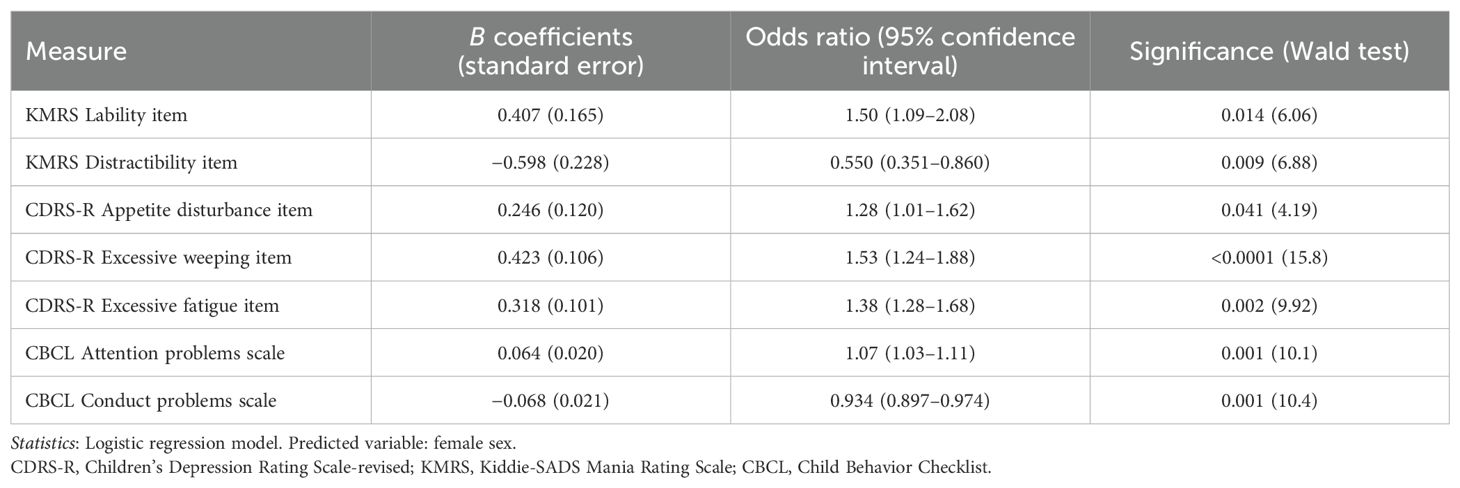
To determine the relative importance of each symptom in differentiating between male and female participants with an MDE, a logistic regression model was developed using items that showed significant differences between sexes in the bivariate analyses. The model accurately predicted 80% of cases, correctly classifying 92.5% of female participants and 46.9% of male participants.
The following factors were significantly and independently associated with female gender: a) CDRS-R excessive weeping item score (OR 1.53; p < 0.001), b) KMRS lability item score (OR 1.50; p = 0.014), c) CDRS-R excessive fatigue item score (OR 1.38; p = 0.002), d) CDRS-R appetite disturbance item score (OR 1.28; p = 0.041), and e) CBCL attention problems scale score (OR 1.07; p = 0.001). Factors significantly and independently associated with male gender included a) KMRS distractibility item score (OR 0.55; p = 0.009) and b) CBCL conduct problems scale score (OR 0.93; p = 0.001). For further details on the regression model, see Table 3.
4 Discussion
We identified significant sex differences in psychopathology in a large cohort of pediatric patients with current MDEs. Female patients exhibited higher clinician- and self-rated depressive symptoms, while male patients showed a greater prevalence of mixed hypomanic symptoms, comorbid ADHD or disruptive behavior disorders, and pediatric BD diagnoses.
Female MDEs were characterized by greater clinician-rated emotional lability, excessive weeping, fatigue, appetite disturbances, and parent-reported attention problems. In contrast, male participants displayed more distractibility and conduct problems, as reported by both clinicians and parents. These were the most discriminant sex differences.
Female participants also had more severe—but less discriminant—depressive feelings, low self-esteem, excessive guilt, somatic complaints, internalizing problems, post-traumatic stress, and obsessive-compulsive symptoms (as reported by parents). Male participants, while exhibiting greater motor activity, showed fewer distinguishing internalizing symptoms. Additionally, female participants reported higher levels of anxiety, suicidal ideation, suicide attempts, and NSSI.
4.1 Differences in depressive symptoms
Depressive symptoms were more severe in female participants, as assessed by both clinicians using the CDRS-R and patients using the CDI-2. However, parent-rated depressive symptoms (CDI-2 parent-report module) did not differ between the sexes. Discrepancies between self- and parent-reported measures of depression are well-documented, with self-report generally recommended as the primary source for assessing depressive symptoms in pediatric patients (39). Some studies suggest that nearly half of parents struggle to distinguish between normal mood swings and signs of depression (40), highlighting the challenges in parental recognition of depressive symptoms. However, it must be acknowledged that some authors have questioned the wording of the CDI instrument and the use of discriminative memory required, which can pose difficulties for younger children (41). Given this, we primarily relied on the clinician-rated CDRS-R for our assessment of depression severity. Furthermore, the consistency of results between the CDI-2 self-report module and the CDRS-R strengthens our findings pointing toward a meaningful difference between sexes in both the CDRS-R and the CDI-2 self-report module, while the CDI-2 parent-report module appears less sensitive to detecting these differences.
Higher depressive symptom severity in female participants is in line with previous findings (15) reporting small but significant sex differences in adolescent depression severity and among specific symptoms, including guilt, body image dissatisfaction, concentration problems, depressed mood, fatigue, and health concerns. Our more homogeneous sample, characterized by greater severity and evaluation across a broad range of psychiatric symptoms, allowed us to identify additional differences in affective symptoms, also including mixed features. Additionally, our findings showed greater consistency across raters, and our regression model demonstrated improved fit and classification accuracy.
The higher severity of depressive symptoms in female patients with early-onset mood disorders can be understood within a complex biopsychosocial framework. Several vulnerability factors for depression—such as genetic predispositions, pubertal timing, affective temperament, and cognitive response styles—differ by sex (42). Adolescence brings psychological and physical changes that influence self-perception, interpersonal experiences, and mental health outcomes within a socio-environmental context. Cultural, social, and familial factors are significant, as gender-specific social pressures affect male and female adolescents differently. Girls may also experience a difference in the incidence of adverse childhood events, which we did not investigate, but could partly explain the observed differences (43). Beyond exposure to stressors, the attribution of significance to life events and coping responses differs by sex (44), with relational problems among peers and rumination as a stress-coping strategy more prominent in female subjects (45). This may contribute to the greater expression of core depressive symptoms in female respondents. Recent findings also highlight the role of neurobiological factors in sex-specific susceptibility to depression. Studies of adolescents with depression link gender differences to variations in the hypothalamic–pituitary–adrenal axis response to stress (46). Increased stress reactivity during late puberty and reduced cortisol secretion in early puberty have been identified as risk factors for depression in female adolescents (47). Sex-specific neuroimmune-endocrine factors involved in stress responses and depression have been identified (48), as well as structural nervous system differences observable via magnetic resonance imaging (49). These findings underscore the interplay between biological, psychological, and social factors in explaining the higher depressive symptom severity in female subjects.
Sex differences in appetite disturbances cannot be attributed to differing rates of comorbid eating disorders, which are rare in our sample. This reflects our unit’s referral process, where patients with prominent eating symptoms are directed to a specialized eating disorder unit. Consequently, our homogeneous sample strengthens the study by ensuring that observed differences reflect sex-specific mood disorder expression.
Additionally, clinician- and parent-rated somatic complaints were significantly associated with MDEs in female patients, consistent with previous research (50). We propose that excessive fatigue, appetite disturbances, and somatization in female patients may represent a subset of atypical depressive symptoms. This aligns with studies in adults showing a higher prevalence of atypical MDEs in women (51). However, atypical depression remains understudied in pediatric age.
4.2 Differences in mixed symptoms and bipolar disorder diagnosis
To our knowledge, sex differences in subthreshold mixed hypomanic symptoms have not been previously studied in children and adolescents with MDE. Our earlier study assessed fewer adolescents with full-threshold mixed symptoms per the DSM-5 criteria (24). In the present study, mixed features (subthreshold or threshold) were systematically rated using the KMRS in a larger population diagnosed with an MDE. Significant mixed hypomanic features (KMRS total score at threshold) were present in ~10% of the sample and were more frequent in male patients. Male participants were also twice as likely to be diagnosed with pediatric BD at their first psychiatric evaluation for MDE. Prior pediatric studies reported no sex differences in pediatric BD diagnosis (23), but in earlier samples of children diagnosed before age 12, 85% were male patients (52). Consistent with our findings, adult BD cohorts show that female respondents more often experience depressive episodes at onset (53), more depressive episodes throughout illness (54), and mixed features during hypomania or mania (55). Adolescent BD cohorts tend to show hypomanic or manic episodes more frequently in male patients (23). Furthermore, in children and adolescents with severe early-onset mood disorders, conversion from MDD to BD is common during follow-up, affecting up to 45% of cases (56). The DSM-5 criteria limit BD diagnosis at first assessment, as follow-up often reveals additional affective episodes meeting the BD criteria over time. Thus, BD diagnoses at first evaluation in our sample may reflect only a subset of cases where the current MDE is neurobiologically linked to BD. This higher rate of early BD diagnosis in male patients suggests that the DSM-5 criteria more readily recognize BD in male patients, who typically present with manic polarity and distinct episodes, compared to female participants, who more often present with severe MDE and persistent depressive symptoms. Additionally, male patients exhibit more recognizable DSM-5 hypo/manic features in mixed states, as noted in our previous work (24).
Consistent with earlier findings (24), male participants in this study more frequently reached the KMRS threshold, while subthreshold mixed hypomanic features were similarly represented in both sexes, with sex differences in mixed symptom expression. Female participants showed more severe emotional lability, whereas male participants exhibited greater motor activity and distractibility, reflecting a pattern where male patients express more “outer” psychomotor symptoms and female patients display more “inner” emotional lability. While motor activation and distractibility align with DSM-5 mania and mixed features criteria, emotional lability does not. Given the prognostic importance of mixed hypomanic symptoms in adolescents with MDE, even when subthreshold (57), we stress the need for high clinical sensitivity in assessing such symptoms in female patients. Future research should include longitudinal studies to verify these findings and explore the utility of alternative tools, such as the Koukopoulos Mixed Depression Rating Scale (58), which may better capture “inner excitation” symptoms and enhance diagnostic sensitivity beyond current DSM-5 criteria.
We observed that the first psychiatric symptoms emerged over a year earlier in boys than in girls. This aligns with the higher incidence of comorbid ADHD in male participants and is consistent with previous findings on psychiatric comorbidities in mood disorders and the well-documented BD-ADHD comorbidity in pediatric populations (27, 37). These findings highlight the importance of assessing potential neurodevelopmental disorder traits in patients with early-onset mood disorders.
4.3 Differences in suicidal ideation and behavior
Our findings confirm the higher prevalence of suicidal ideation and attempts in female patients compared to male patients, consistent with extant literature (15, 24, 59). This aligns with the “gender paradox” in suicidal behavior, where women are more likely to attempt suicide, while men have higher rates of suicide completion and often use more lethal methods (59). Explanations for this phenomenon often point to greater impulsivity and higher rates of substance abuse in men (60).
A recent review of adolescents highlights this differential pattern, identifying both common and sex-specific risk factors for suicide attempts. Female-specific risk factors include eating disorders, PTSD, BD, dating violence victimization, depressive symptoms, interpersonal problems, and previous abortion. Male-specific factors include disruptive behavior, hopelessness, parental separation/divorce, peer suicide, and access to means (61). Similarly, a cohort of Chinese college students found suicide-related outcomes linked to depressive symptoms in female students and anger in male students (62). In our study, suicidal ideation and attempts were more frequent in female participants and correlated with their higher burden of depression severity, further supporting the interplay between depression and suicidal behavior in female subjects.
4.4 Differences in externalizing and internalizing comorbidities
Analysis of parent-reported symptoms in the CBCL revealed notable sex differences: female subjects scored higher on internalizing symptoms, while male subjects scored higher on conduct problems. This aligns with the findings in adults with unipolar depression (6) and BD (63), where women are reported to experience comorbid anxiety and post-traumatic symptoms more frequently than men, while men show higher rates of conduct and substance use disorders. Researchers have noted that female participants are more likely to manage emotional problems with internalizing strategies, whereas male participants often adopt externalizing “escaping behaviors” (64).
Of note, no significant sex difference in substance use was observed in our sample, likely due to the study’s focus on early-phase juvenile-onset mood disorders. Such differences may become evident in adults during more chronic phases of these disorders.
Finally, female respondents reported higher levels of self- and parent-rated anxiety symptoms than male respondents, consistent with previous studies in adults (65) and adolescents (66).
4.5 Strengths and limitations
This study enriches the literature by providing a detailed, multidimensional analysis of sex differences in severe early-onset mood disorders, a topic rarely explored in comparable populations. A key strength is the use of a single-center sample of referred early-onset mood disorders, ensuring population homogeneity and consistent structured assessments for all participants. However, several limitations should be noted. First, the high severity of depressive episodes in this clinically referred sample may limit the generalizability of the findings to broader clinical populations. Second, while the CDRS-R and KMRS are internationally recognized tools widely used in Italy (24, 27, 67), their validity and reliability in Italian adolescents have not been formally addressed. Reliability analysis in this sample showed acceptable internal consistencies (Cronbach’s alpha: 0.669 for KMRS, 0.796 for CDRS-R). The lower KMRS consistency may reflect the uneven distribution of hypo/manic symptoms, as significant symptoms were confined to participants with subthreshold mixed features, with many participants with a low score of all KMRS items. Third, the study did not assess adverse childhood events, which may vary by sex and influence the findings.
4.6 Conclusion
Our study identifies a moderately distinct pattern of sex differences in depressive symptoms among children and adolescents with early-onset mood disorders, partially mirroring adult findings. Consistent with adult literature and limited adolescent studies, we confirm that female participants with MDEs experience more severe depressive symptoms, anxiety, and suicidal behaviors, while male participants more frequently exhibit disruptive behaviors. Moreover, our study highlights sex-based differences in mixed hypomanic symptoms, pediatric BD diagnoses, and early childhood clinical features preceding mood disorder onset.
These findings should be understood within a framework where the interplay of biological, psychological, and social factors begins in childhood and continues into adolescence and adulthood. This interaction shapes sex-specific depression development and intersects with diagnostic systems that often overlook sex and gender differences in key clinical presentations. These observations highlight the importance of integrating a sex- and gender-informed perspective in research, diagnosis, and treatment to enhance outcomes for adolescents with mood disorders.
Data availability statement
The datasets presented in this article are not publicly available because of the nature of the research, due to ethical reasons. Further inquiries can be directed to the corresponding author. Requests to access the datasets should be directed to Z2l1bGlhLnNlcnJhQG9wYmcubmV0.
Ethics statement
The study was conducted in accordance with the Declaration of Helsinki and in accordance with Italian legal and ethical requirements for clinical data. Ethical review and approval were waived for this study due to the retrospective nature of the analysis. The current study did not require the approval of the local ethics committee according to current legislation, but a notification was sent. Data were retrospectively analyzed in line with personal data protection policies.
Author contributions
MA: Conceptualization, Formal Analysis, Funding acquisition, Writing – review & editing. EA: Data curation, Writing – original draft. GD: Data curation, Writing – review & editing. CL: Data curation, Writing – original draft. CG: Data curation, Methodology, Writing – review & editing. MT: Data curation, Investigation, Methodology, Writing – review & editing. MI: Data curation, Investigation, Methodology, Writing – review & editing. GM: Data curation, Investigation, Methodology, Writing – review & editing. SV: Conceptualization, Supervision, Writing – review & editing. GS: Conceptualization, Funding acquisition, Methodology, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by a grant (grant GR-2018-12367476) from the Italian Ministry of Health (to GS) and a grant (grant 202405 progetto 5 per mille 2024) from Ospedale Pediatrico Bambino Gesù to MA. This work was supported also by the Italian Ministry of Health with Current Research funds. Funders had no involvement with the design, analysis, or reporting of the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. WHO (World Health Organ). Depression. Fact sheet. Geneva: WHO (2023). Available at: https://www.who.int/news-room/fact-sheets/detail/depression. (Accessed August 1, 2024)
2. Sacco R, Camilleri N, Eberhardt J, Umla-Runge K, Newbury-Birch D. A systematic review and meta-analysis on the prevalence of mental disorders among children and adolescents in Europe. Eur Child Adolesc Psychiatry. (2024) 33:2877–94. doi: 10.1007/s00787-022-02131-2
3. Lu B, Lin L, Su X. Global burden of depression or depressive symptoms in children and adolescents: A systematic review and meta-analysis. J Affect Disord. (2024) 354:553–62. doi: 10.1016/j.jad.2024.03.074
4. Weller EB, Kloos A, Kang J, Weller RA. Depression in children and adolescents: does gender make a difference? Curr Psychiatry Rep. (2006) 8:108–14. doi: 10.1007/s11920-006-0007-1
5. Sørensen MJ, Nissen JB, Mors O, Thomsen PH. Age and gender differences in depressive symptomatology and comorbidity: an incident sample of psychiatrically admitted children. J Affect Disord. (2005) 84:85–91. doi: 10.1016/j.jad.2004.09.003
6. Cavanagh A, Wilson CJ, Kavanagh DJ, Caputi P. Differences in the expression of symptoms in men versus women with depression: A systematic review and meta-analysis. Harv Rev Psychiatry. (2017) 25:29–38. doi: 10.1097/HRP.0000000000000128
7. Streb J, Ruppel E, Möller-Leimkühler AM, Büsselmann M, Franke I, Dudeck M. Gender-specific differences in depressive behavior among forensic psychiatric patients. Front Psychol. (2021) 12:639191. doi: 10.3389/fpsyg.2021.639191
8. Scheibe S, Preuschhof C, Cristi C, Bagby RM. Are there gender differences in major depression and its response to antidepressants? J Affect Disord. (2003) 75:223–35. doi: 10.1016/s0165-0327(02)00050-2
9. Carter JD, Joyce PR, Mulder RT, Luty SE, McKenzie J. Gender differences in the presentation of depressed outpatients: a comparison of descriptive variables. J Affect Disord. (2000) 61:59–67. doi: 10.1016/s0165-0327(00)00151-8
10. Hankin BL, Mermelstein R, Roesch L. Sex differences in adolescent depression: stress exposure and reactivity models. Child Dev. (2007) 78:279–95. doi: 10.1111/j.1467-8624.2007.00997.x
11. Marcotte D, Alain M, Gosselin MJ. Gender differences in adolescent depression: gender-typed characteristics or problem-solving skills deficits? Sex Roles. (1999) 41:31–48. doi: 10.1023/A:1018833607815
12. Cyranowski JM, Frank E, Young E, Shear MK. Adolescent onset of the gender difference in lifetime rates of major depression: a theoretical model. Arch Gen Psychiatry. (2000) 57:21–7. doi: 10.1001/archpsyc.57.1.21
13. Kovacs M. Gender and the course of major depressive disorder through adolescence in clinically referred youngsters. J Am Acad Child Adolesc Psychiatry. (2001) 40:1079–85. doi: 10.1097/00004583-200109000-00017
14. Masi G, Favilla L, Mucci M, Poli P, Romano R. Depressive symptoms in children and adolescents with dysthymic disorder. Psychopathology. (2001) 34:29–35. doi: 10.1159/000049277
15. Bennett DS, Ambrosini PJ, Kudes D, Metz C, Rabinovich H. Gender differences in adolescent depression: do symptoms differ for boys and girls? J Affect Disord. (2005) 89:35–44. doi: 10.1016/j.jad.2005.05.020
16. Calvete E, Cardenoso O. Gender differences in cognitive vulnerability to depression and behavior problems in adolescents. J Abnorm Child Psychol. (2005) 33:179–92. doi: 10.1007/s10802-005-1826-y
17. Hankin BL, Abramson LY. Development of gender differences in depression: description and possible explanations. Ann Med. (1999) 31:372–9. doi: 10.3109/07853899908998794
18. Birtel MD, Mitchell BL. Cross-cultural differences in depression between White British and South Asians: Causal attributions, stigma by association, discriminatory potential. Psychol Psychother. (2023) 96:101–16. doi: 10.1111/papt.12428
19. Sun Y, Zhong Y, Sun W, Chu L, Long J, Fan XW. More prevalent and more severe: gender differences of depressive symptoms in Chinese adolescents. Front Public Health. (2023) 11:1167234. doi: 10.3389/fpubh.2023.1167234
20. Takakura M, Sakihara S. Gender differences in the association between psychosocial factors and depressive symptoms in Japanese junior high school students. J Epidemiol. (2000) 10:383–91. doi: 10.2188/jea.10.383
21. Merikangas KR, He JP, Burstein M, Swanson SA, Avenevoli S, Cui L, et al. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication–Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry. (2010) 49:980–9. doi: 10.1016/j.jaac.2010.05.017
22. Andersson P, Jokinen J, Jarbin H, Lundberg J, Desai Boström AE. Association of bipolar disorder diagnosis with suicide mortality rates in adolescents in Sweden. JAMA Psychiatry. (2023) 8):796–802. doi: 10.1001/jamapsychiatry.2023.1390
23. Duax JM, Youngstrom EA, Calabrese JR, Findling RL. Sex differences in pediatric bipolar disorder. J Clin Psychiatry. (2007) 68:1565–73. doi: 10.4088/jcp.v68n1016
24. Apicella M, Serra G, Iannoni ME, Trasolini M, Maglio G, Andracchio E, et al. Gender differences in the psychopathology of mixed depression in adolescents with a major depressive episode. Curr Neuropharmacol. (2023) 21:1343–54. doi: 10.2174/1570159X20666221012113458
25. Serra G, Iannoni ME, Trasolini M, Maglio G, Frattini C, Casini MP, et al. Characteristics associated with depression severity in 270 juveniles in a major depressive episode. Brain Sci. (2021) 11:440. doi: 10.3390/brainsci11040440
26. Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. (1997) 36:980–8. doi: 10.1097/00004583-199707000-00021
27. Guidetti C, Serra G, Apicella M, Andracchio E, Iannoni ME, Trasolini M, et al. Childhood clinical features preceding the onset of bipolar versus major depressive disorders during adolescence. J Atten Disord. (2024) 28:648–63. doi: 10.1177/10870547231225819
28. Poznanski EO, Grossman JA, Buchsbaum Y, Banegas M, Freeman L, Gibbons R. Preliminary studies of the reliability and validity of the children’s depression rating scale. J Am Acad Child Psychiatry. (1984) 23:191–7. doi: 10.1097/00004583-198403000-00011
29. Axelson D, Birmaher BJ, Brent D, Wassick S, Hoover C, Bridge J, et al. A preliminary study of the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children mania rating scale for children and adolescents. J Child Adolesc Psychopharmacol. (2003) 13:463–70. doi: 10.1089/104454603322724850
30. Posner K, Brown GK, Stanley B, Brent DA, Yershova KV, Oquendo MA, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. (2011) 168:1266–77. doi: 10.1176/appi.ajp.2011.10111704
31. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Fifth Edition. Washington, DC: American Psychiatric Association (2013). (DSM-5).
32. Kovacs M. Children’s depression inventory 2nd edition: Technical manual. In: Multi-health system Toronto, ON, Canada (2011).
33. March JS. Multidimensional anxiety scale for children. In: MultiHealth systems, 2nd ed. MASC 2, Toronto, Canada (2012). doi: 10.1037/t05050-000
34. Achenbach TM, Rescorla LA. Manual for the ASEBA school- age forms & profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, and Families (2001).
35. Pace CS, Muzi S, Frigerio A, Morganti W, Bianchi V, Rogier G. Twenty years of emotional-behavioral problems of community adolescents living in Italy measured through the Achenbach system of empirically based assessment (ASEBA): a systematic review and meta-analysis. Front Psychiatry. (2023) 14:1161917. doi: 10.3389/fpsyt.2023.1161917
36. Poli P, Sbrana B, Marcheschi M, Masi G. Self-reported depressive symptoms in a school sample of Italian children and adolescents. Child Psychiatry Hum Dev. (2003) 33:209–26. doi: 10.1023/a:1021404613832
37. Biederman J, Spencer T, Lomedico A, Day H, Petty CR, Faraone SV. Deficient emotional self-regulation and pediatric attention deficit hyperactivity disorder: a family risk analysis. Psychol Med. (2012) 42:639–46. doi: 10.1017/S0033291711001644
38. Kim HY. Statistical notes for clinical researchers: Chi-squared test and Fisher’s exact test. Restor Dent Endod. (2017) 42:152–5. doi: 10.5395/rde.2017.42.2.152
39. Martel MM, Markon K, Smith GT. Research Review: Multi-informant integration in child and adolescent psychopathology diagnosis. J Child Psychol Psychiatry. (2017) 58:116–28. doi: 10.1111/jcpp.12611
40. Mott Poll Report. Recognizing Youth Depression at Home and School. In: Rivista National Poll on Children’s Health del C.S. Mott Children’s Hospital (Michigan Medicine), vol. 35. Ipsos Public Affairs, LLC (2019). Available at: https://mottpoll.org/reports/recognizing-youth-depression-home-and-school. (Accessed August 1, 2024)
41. Carnevale T. An integrative review of adolescent depression screening instruments: applicability for use by school nurses. J Child Adolesc Psychiatr Nurs. (2010) 24:51–7. doi: 10.1111/j.1744-6171.2010.00256.x
42. Hyde JS, Mezulis AH, Abramson LY. The ABCs of depression: Integrating affective, biological, and cognitive models to explain the emergence of the gender difference in depression. psychol Review;. (2008) 115:291–313. doi: 10.1037/0033-295X.115.2.291
43. de Souza AF, Máximo RO, de Oliveira DC, Ramírez PC, Luiz MM, Delinocente MLB, et al. Gender differences in the association between adverse events in childhood or adolescence and the risk of premature mortality. Sci Rep. (2022) 12:19118. doi: 10.1038/s41598-022-23443-y
44. Salk RH, Petersen JL, Abramson LY, Hyde JS. The contemporary face of gender differences and similarities in depression throughout adolescence: Development and chronicity. J Affect Disord. (2016) 205:28–35. doi: 10.1016/j.jad.2016.03.071
45. Aldao A, Nolen-Hoeksema S, Schweizer S. Emotion-regulation strategies across psychopathology: A meta-analytic review. Clin Psychol Rev. (2010) 30:217–37. doi: 10.1016/j.cpr.2009.11.004
46. Heck AL, Handa RJ. Sex differences in the hypothalamic-pituitary-adrenal axis’ response to stress: an important role for gonadal hormones. Neuropsychopharmacology. (2019) 44:45–58. doi: 10.1038/s41386-018-0167-9
47. Colich NL, Kircanski K, Foland-Ross LC, Gotlib IH. HPA-axis reactivity interacts with stage of pubertal development to predict the onset of depression. Psychoneuroendocrinology. (2015) 55:94–101. doi: 10.1016/j.psyneuen.2015.02.004
48. Bekhbat M, Howell PA, Rowson SA, Kelly SD, Tansey MG, Neigh GN. Chronic adolescent stress sex-specifically alters central and peripheral neuro-immune reactivity in rats. Brain Behav Immun. (2019) 76:248–57. doi: 10.1016/j.bbi.2018.12.005
49. Hu X, Zhang L, Liang K, Cao L, Liu J, Li H, et al. Sex-specific alterations of cortical morphometry in treatment-naïve patients with major depressive disorder. Neuropsychopharmacology. (2022) 47:2002–9. doi: 10.1038/s41386-021-01252-7
50. Wenzel A, Steer RA, Beck AT. Are there any gender differences in frequency of self-reported somatic symptoms of depression? J Affect Disord. (2005) 89:177–81. doi: 10.1016/j.jad.2005.06.009
51. Smith DJ, Kyle S, Forty L, Cooper C, Walters J, Russell E, et al. Differences in depressive symptom profile between males and females. J Affect Disord. (2008) 108:279–84. doi: 10.1016/j.jad.2007.10.001
52. Wozniak J, Biederman J, Kiely K, Ablon JS, Faraone SV, Mundy E, et al. Mania-like symptoms suggestive of childhood-onset bipolar disorder in clinically referred children. J Am Acad Child Adolesc Psychiatry. (1995) 34:867–76. doi: 10.1097/00004583-199507000-00010
53. Viguera AC, Baldessarini RJ, Tondo L. Response to lithium maintenance treatment in bipolar disorders: Comparison of women and men. Bipolar Disord. (2001) 3:245–52. doi: 10.1034/j.1399-5618.2001.30503.x
54. Altshuler LL, Kupka RW, Hellemann G, Frye MA, Sugar CA, McElroy SL, et al. Gender and depressive symptoms in 711 patients with bipolar disorder evaluated prospectively in the Stanley Foundation bipolar treatment outcome network. Am J Psychiatry. (2010) 167:708–15. doi: 10.1176/appi.ajp.2009.09010105
55. Sherazi R, McKeon P, McDonough M, Daly I, Kennedy N. What’s new? The clinical epidemiology of bipolar I disorder. Harv. Rev Psychiatry. (2006) 14:273–84. doi: 10.1080/10673220601070047
56. Faedda GL, Marangoni C, Serra G, Salvatore P, Sani G, Vázquez GH, et al. Precursors of bipolar disorders: a systematic literature review of prospective studies. J Clin Psychiatry. (2015) 76:614–24. doi: 10.4088/JCP.13r08900
57. Maalouf FT, Porta G, Vitiello B, Emslie G, Mayes T, Clarke G, et al. Do sub-syndromal manic symptoms influence outcome in treatment resistant depression in adolescents? A latent class analysis from the TORDIA study. J Affect Disord. (2012) 138:86–95. doi: 10.1016/j.jad.2011.12.021
58. Sani G, Vöhringer PA, Barroilhet SA, Koukopoulos AE, Ghaemi SN. The Koukopoulos Mixed Depression Rating Scale (KMDRS): An International Mood Network (IMN) validation study of a new mixed mood rating scale. J Affect Disord. (2018) 232:9–16. doi: 10.1016/j.jad.2018.01.025
59. Nock MK, Green JG, Hwang I, McLaughlin KA, Sampson NA, Zaslavsky AM, et al. Prevalence, correlates, and treatment of lifetime suicidal behavior among adolescents: results from the National Comorbidity Survey Replication Adolescent Supplement. JAMA Psychiatry. (2013) 3):300–10. doi: 10.1001/2013.jamapsychiatry.55
60. Canetto SS. Meanings of gender and suicidal behavior during adolescence. Suicide Life Threat Behav. (1997) 27(4):339–51.
61. Miranda-Mendizabal A, Castellví P, Parés-Badell O, Alayo I, Almenara J, Alonso I, et al. Gender differences in suicidal behavior in adolescents and young adults: systematic review and meta-analysis of longitudinal studies. Int J Public Health. (2019) 64:265–83. doi: 10.1007/s00038-018-1196-1
62. Xuan L, Hua S, Lin L, Jianli Y. Gender differences in the predictive effect of depression and aggression on suicide risk among first-year college students. J Affect Disord. (2023) 327:1–6. doi: 10.1016/j.jad.2023.01.123
63. Diflorio A, Jones I. Is sex important? Gender differences in bipolar disorder. Int Rev Psychiatry. (2010) 22:437–52. doi: 10.3109/09540261.2010.514601
65. Sloan DM, Kornstein SG. Gender differences in depression and response to antidepressant treatment. Psychiatr Clin North Am. (2003) 26:581–94. doi: 10.1016/s0193-953x(03)00044-3
66. Muris P, Merckelbach H, Ollendick T, King N, Bogie N. Three traditional and three new childhood anxiety questionnaires: their reliability and validity in a normal adolescent sample. Behav Res Ther. (2002) 40:753–72. doi: 10.1016/s0005-7967(01)00056-0
67. Serra G, Apicella M, Andracchio E, Della Santa G, Lanza C, Trasolini M, et al. Factors associated with high parent- and youth-rated irritability score in early-onset mood disorders: A cross-sectional study with the affective reactivity index (ARI). Brain Sci. (2024) 14:611. doi: 10.3390/brainsci14060611
Keywords: depression, bipolar, adolescent, sex, gender, comorbidities, neurodevelopment, psychopathology
Citation: Apicella M, Andracchio E, Della Santa G, Lanza C, Guidetti C, Trasolini M, Iannoni ME, Maglio G, Vicari S and Serra G (2025) Sex differences in pediatric major depressive episodes: a cross-sectional study on psychiatric symptoms in early-onset mood disorders. Front. Psychiatry 16:1503794. doi: 10.3389/fpsyt.2025.1503794
Received: 29 September 2024; Accepted: 24 March 2025;
Published: 25 April 2025.
Edited by:
Xiaolei Li, University of Pennsylvania, United StatesReviewed by:
Zhang Haoling, University of Science Malaysia (USM), MalaysiaKathleen McDeavitt, Baylor College of Medicine, United States
Nimran Kaur, IQVIA, India
Copyright © 2025 Apicella, Andracchio, Della Santa, Lanza, Guidetti, Trasolini, Iannoni, Maglio, Vicari and Serra. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Massimo Apicella, bWFzc2ltby5hcGljZWxsYUBvcGJnLm5ldA==; Giulia Serra, Z2l1bGlhLnNlcnJhQG9wYmcubmV0
†These authors have contributed equally to this work and share first authorship
 Massimo Apicella
Massimo Apicella Elisa Andracchio1†
Elisa Andracchio1† Maria Elena Iannoni
Maria Elena Iannoni Stefano Vicari
Stefano Vicari Giulia Serra
Giulia Serra