- 1School of Health Sciences, Faculty of Health, Arts and Design, Swinburne University of Technology, Melbourne, VIC, Australia
- 2School of Psychology, Faculty of Health, Deakin University, Melbourne, VIC, Australia
Introduction: Breath-hold divers are known for their exceptional breathing control and reduced carbon dioxide (CO2) sensitivity due to training adaptations. In contrast, individuals with panic disorder (PD) often exhibit heightened CO2 sensitivity. This study aimed to explore the potential clinical applications of the diving response (DR), particularly cold facial immersion (CFI), in mitigating panic-related symptoms and cognitions by modulating CO2 sensitivity.
Methods: This study investigated the effects of the CFI task on individuals with PD and a comparison group. Changes in heart rate, respiration rate, and psychological measures were assessed before and after a CO2 challenge to determine whether the CFI task could reduce CO2 sensitivity and panic-related symptoms.
Results: The results did not support the efficacy of the CFI task in reducing physiological markers of CO2 sensitivity—specifically, heart rate and respiration rate—following the CO2 challenge in either the clinical or comparison group, potentially due to the small sample size. However, significant reductions in both physiological and cognitive symptoms of panic were observed in the clinical group following the CFI task.
Discussion: As hypothesized, the CFI task demonstrated anxiolytic effects in individuals with PD by reducing self-reported anxiety and panic symptoms. These findings highlight the potential of the CFI task for clinical application in the treatment of panic disorder, warranting further research with larger samples.
Introduction
Panic disorder (PD) is a debilitating psychiatric condition marked by spontaneous, recurrent panic attacks, affecting approximately 4% of the general population and leading to various personal and socioeconomic challenges (1). Individuals with PD display a thoracic breathing pattern characterized by abnormal variability and irregularity (2). They often experience cardiorespiratory symptoms such as air hunger, dyspnea, rapid breathing, and increased heart rate (3).
Increased carbon dioxide (CO2) sensitivity is a common feature among individuals with PD, and is frequently observed in the respiratory subtype of PD (4). The CO2 hypersensitivity theory suggests that individuals with PD have a lower threshold for detecting CO2 levels (5). This supports the presence of an evolved suffocation alarm system that helps the brain monitor available oxygen (6). For individuals with PD, the 35% CO2 challenge (CO2 challenge), a single-breath inhalation of a gas mixture containing 35% CO2 and oxygen (O2), is commonly used to induce an exaggerated physiological and psychological response that mimics a panic attack. This method helps assess CO2 sensitivity and autonomic reactivity in PD research (6–11). The central and peripheral chemoreflexes are activated during the CO2 challenge, leading to increased ventilation in an effort to remove excess CO2 and stabilize blood pH. This response and the anxiogenic effects of the CO2 challenge may be more sensitive in individuals with PD (12).
Freedivers who practice breath-hold diving, relying on a single deep breath without an external air supply, have been practicing this ancestral form of diving since ancient times (13). Freedivers are a unique group of individuals that have been known for their exceptional breathing control, lower CO2 sensitivity, and pronounced diving response (DR) due to trained effects (14–16). The DR is a physiological reflex that optimizes respiration, allowing humans to endure a lack of oxygen underwater (14). The DR triggers the peripheral chemoreflex which is activated by cold water on the face, leading to vasoconstriction and bradycardia to conserve oxygen. While the DR initially focuses on oxygen conservation, the central chemoreflex is also stimulated and becomes more prominent as CO2 builds up (17). Daily breath-hold training for as little as two weeks has been demonstrated to enhance breath-hold duration and trigger the diving DR more quickly (15). After breath-hold training, apneas of the same length led to less arterial oxygen desaturation, suggesting improved oxygen conservation due to the pronounced effect of the DR, an innate adaptation that can be trained (18). Reduced chemosensitivity to hypercapnia has been observed in groups of freedivers, including synchronized swimmers, underwater hockey players, and trained young competitive swimmers. This suggests that sub-aquatic training involving repetitive breath-holding may lead to desensitization of peripheral chemoreflexes (13).
While both freedivers and individuals with PD exhibit altered CO2 sensitivity, the patterns are opposite. Freedivers have reduced central chemosensitivity and normal peripheral chemosensitivity, enabling prolonged breath-holding. In contrast, those with PD tend to have increased central chemosensitivity, leading to an exaggerated response to CO2 and potentially contributing to panic symptoms (12). In their study, Kyriakoulis et al. (12) discovered that the CO2 challenge triggered anxiety and panic symptoms in clinical participants. In contrast, the cold facial immersion (CFI) task exhibited anxiolytic effects, as evidenced by a reduction in heart rate and a decrease in self-reported symptoms of anxiety and panic in both clinical and comparison groups. The findings of the current study support this hypothesis, revealing that both Clinical and Comparison participants experienced a significant bradycardic effect, with a decrease of approximately 30–35 beats per minute, following the CFI task (12).
This study aimed to investigate whether the cold facial immersion task alters one’s physiological and psychological response to the CO2 challenge. Additionally, the study aims to explore whether the CFI task can serve as an intervention to alleviate and prevent panic symptoms and panic attacks. It is noteworthy to recognize that by activating the DR and lowering heart rate, individuals may experience a reduction in both physiological and cognitive symptoms of panic, as well as a possible decrease in sensitivity to CO2 (12). Furthermore, the rationale for this study appears to follow a coherent progression, as existing literature suggests that individuals with PD exhibit heightened sensitivity to CO2 (19, 20). Additionally, the findings from a previous study by Kyriakoulis et al. (12) demonstrated that the CFI task reduced both physiological and cognitive symptoms of anxiety, hence it was conceptualized that the activation of the DR may be responsible for altering CO2 sensitivity in individuals with PD, a topic that warrants further investigation. In designing this study, the researchers wanted to establish whether trained effects of the CFI task could change individuals’ response to the CO2 challenge.
Consequently, an experimental protocol was developed to explore this further. It was hypothesized that CFI would reduce Clinical and Comparison participants’ sensitivity to CO2 as evidenced by heart rate and respiration rate reductions following the subsequent CO2. It was further hypothesized that participants in both groups would report a reduction in anxiety symptoms following CFI and subsequent CO2 when compared to pre-measures and the CO2 administration without CFI.
This study is the first attempt to examine whether the activation of the DR via CFI may alter CO2 sensitivity in PD individuals. This study aims to further investigate whether CFI has a preventative effect on panic-related symptoms and cognitions. This study examines the effect of CFI in altering CO2 sensitivity in participants with PD.
Method
Participants and sampling
Investigations were carried out with 30 participants: 15 patients with a primary diagnosis of PD with or without agoraphobia (DSM-5, in the Clinical Group, and 15 healthy comparisons in the Comparison Group, who did not meet the criteria for PD or mental illness. Of the 30 participants, 15 were male, and 15 were females. The participants in the Clinical Group had an average age of 36.3 years (SD = 13.8), whilst the participants in the Comparison group had an average age of 33.1 years (SD = 7.7). Both groups comprised 6 males and 9 females. Difficulties with recruitment resulted in the participants not being able to be matched for age and gender and five clinical participants from the preliminary study were invited to join the Clinical Group for this study. Although attempts were made in Study 2 to match participants and to recruit non-university students as comparisons, this was difficult to accomplish. Sample size calculations were not possible as the response to CO2 and CFI has not previously been investigated among persons with PD. Therefore, we recruited as many participants as possible within the time and budget constraints of the research study. The cohort differences are reported in the results section.
Health screening assessments were carried out by a medical doctor (at Swinburne University) to establish medical eligibility to undergo the CO2 challenge. As part of the health screening, all participants completed a demographic and health information form. Demographical data such as age, gender, level of education completed, employment status were included in this form. In addition, the demographical questionnaire included a health screening, height and weight information, as well as fitness and exercise assessment which included a physical activity index. Participants were also asked about their perceived level of comfort with water.
The Mini-International Neuropsychiatric Interview (MINI) is a short structured diagnostic interview used to make diagnoses of Axis I disorders (DSM-IV) and has demonstrated high reliability and validity (21). It was used to screen psychological disorders within the exclusion criteria and identify individuals with PD (DSM-IV). Exclusion criteria for both groups included psychotic disorders, substance abuse, prescription medication, habitual use of benzodiazepines, known allergies to latex, asthma or respiratory problems, cardiovascular problems, hypertension, hypotension, pregnancy, and cerebrovascular problems including epilepsy and organic brain disorder. Finally, other comorbidities to Axis I mental health disorders (DSM-IV) were excluded with the exception of Depression, Generalized Anxiety Disorder and Social Anxiety Disorder, if secondary to PD, along with those with biological relatives with PD.
The Clinical Group was also assessed with the structured clinical interview (SCID-I) for the DSM-IV Axis I Disorders module for PD and Agoraphobia (SCID-I) (22). The SCID-I is a comprehensive structured interview for the diagnosis of psychiatric disorders according to DSM-IV criteria (23). To encourage higher participation rates among PD participants, two movie tickets were offered to each applicant who completed the study.
Physiological measures
A compact physiological monitoring system (Zephyr Bioharness) featuring a chest strap and external multi-recording and monitoring device was used to measure heart rate, posture and respiration rate. Participants were connected to a Zephyr Bioharness and measured throughout the experimental study. Participants’ breath-hold ability was also measured during the experimental phase and included breath-hold ability after maximum exhalation and breath-hold ability following maximum inhalation. All participants undertook the conditions of the experimental study while wearing the chest strap connected to the Zephyr Bioharness, which was connected via Bluetooth to a multirecording and monitoring device (PowerLab). PowerLab version 7.0 data acquisition and analysis software were used as a multirecording device, and recorded data were sampled at a frequency of 60 Hz (60 samples per second). Physiological measures, including heart rate and respiration rate, were recorded at Time 2 and Time 3 of the study.
Measures
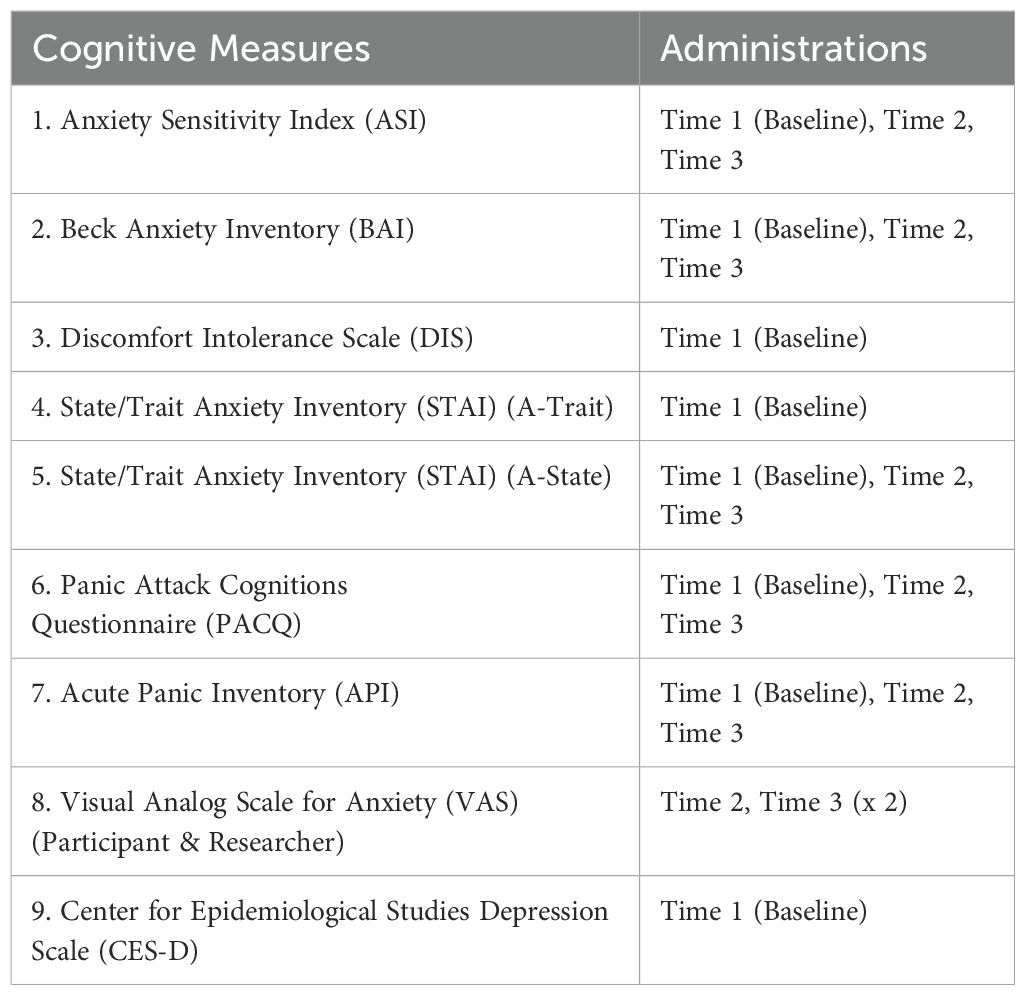
All participants were required to complete a battery of psychological assessments at three time points throughout the study. Table 1 provides a list of the battery of psychological measures and the time points at which the measures were administered throughout the study.
Research design
Participants took part in two experimental challenges at two different time intervals, including the (1) CO2 Challenge, and (2) CFI Task followed by CO2 Challenge. Figure 1 displays the experimental procedure indicating when physiological and cognitive measures were collected as part of this study.
In the CO2 Challenge, participants were instructed to take one maximum inhalation of a 35% CO2 and 65% O2 mixture, hold their breath for 4 seconds, and then exhale. In the CFI Task, participants were instructed to take a deep breath and immerse their face in a tub filled with cold water regulated between 7 ˚C - 12˚C, whilst maintaining the room temperature at a constant 22˚C.
Procedure
The experimental study was held at a clinical office at Swinburne University, which was specifically set up for the research. Experimental conditions were not randomly assigned to the Clinical and Comparison participants, who were required to undertake Time 1 and Time 2 procedures on the same day, either in the morning or afternoon. Participants were scheduled to complete Time 3 tasks on a different day, as ethics approval only allowed for participants to complete one CO2 challenge per day. Participants were asked to refrain from caffeine-containing beverages, including cola and smoking, as well as physical exercise for at least two hours prior to the CO2 challenge. They were also asked to refrain from alcohol from the day prior. Testing was conducted in the morning or the afternoon.
Data cleaning
Prior to statistical analyses, all variables were assessed for the presence of missing data and outliers. The data revealed missing values and hence Little’s Missing Completely at Random (MCAR) test was used to assess whether data were missing at random. Assumptions for the MCAR test were assessed and fulfilled, χ2 (39) = 46.165, DF = 39, p >.005. Missing values were replaced using the expectation-maximization (EM) algorithm for imputation. Prior to conducting statistical analyses, the distributions of all variables were visually inspected to determine if they met the assumption of normality of distribution, a requirement of parametric statistical analyses. In cases where data did not adequately meet the assumptions of normality and could not be transformed to normalize their distributions according to recommended procedures (24), non-parametric analyses were conducted.
Statistical analyses
Prior to conducting parametric analyses, data were inspected to ensure they met the assumptions for such analyses. Physiological data, including respiration rate and heart rate data satisfied the assumptions for parametric analysis. Hence t-tests and ANOVA analyses were employed to investigate the differences between the Clinical and Comparison Groups on the experimental conditions. Examination of normal distribution revealed that scores across all self-report cognitive measures taken at Time 1(Pre-test), at Time 2 (CO2) and Time 3 (CFI followed by CO2), including API, ASI, BAI, PACQ, STAI, VAS-R, and VAS-P did not meet the assumptions of normal distribution for parametric analyses. Hence non-parametric tests were utilized. Spearman rank-order correlation coefficient was used to investigate the relationships between the psychological measures. Friedman’s test was used to examine differences in the cognitive measures collected across the experimental conditions. Demographic information was compared between the Clinical Group and the Comparison Group using chi-squared tests of association and Fishers. The Statistical Package for Social Sciences version 22.0 was used for all analyses.
Results
Overview of analysis
The analysis of this study is presented in three sections. The first section presents the comparison of demographic details between groups and the correlations of the Time 1 (Pre-test) measures. The second section reports an examination of the physiological differences between the Clinical and Comparison Groups at Time 1 and 2. This section also reports the results of mixed ANOVAs, examining the effects of Time 1 and Time 2 tasks on participant’s physiological responses (heart rate and respiration rate). The third section examines the effects of the CO2 challenge and ultimately the effect of CFI on CO2 sensitivity across the anxiety measures. When outcomes were not normally distributed, Friedman tests were used instead of ANOVA tests and Wilcoxon signed–rank tests were used instead of paired t-tests.
Correlations between premeasures
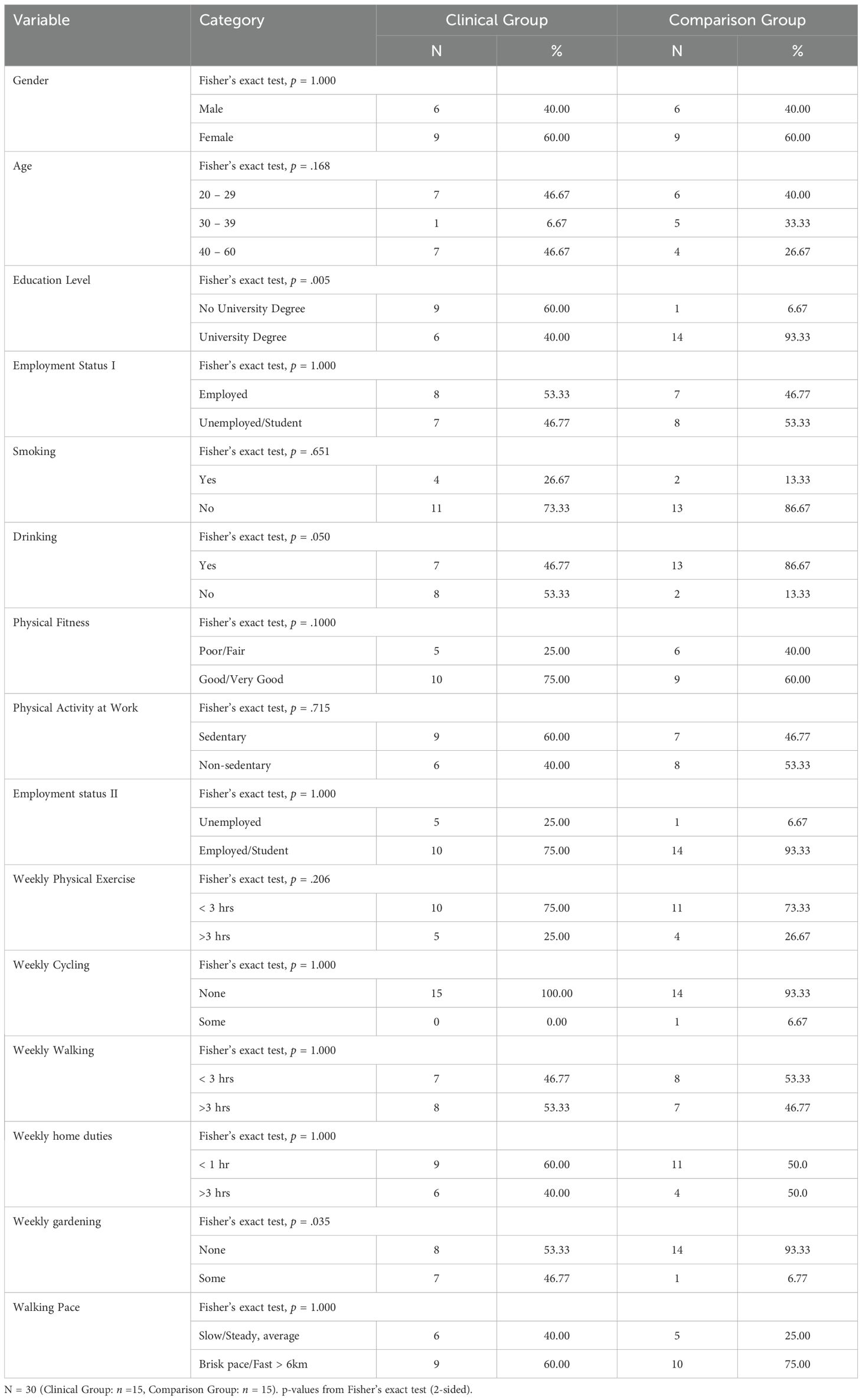
Tables 2 and 3 provide a comparison for the Clinical and Comparison Groups. No significant differences were found. In examining the demographic information in Study 2, the main differences included fewer participants in the Clinical Group who reported drinking alcohol. The mean average age for the Clinical Group (M = 36.33, SD = 13.77), and the Comparison Group (M = 33.13, SD = 7.71) respectively.
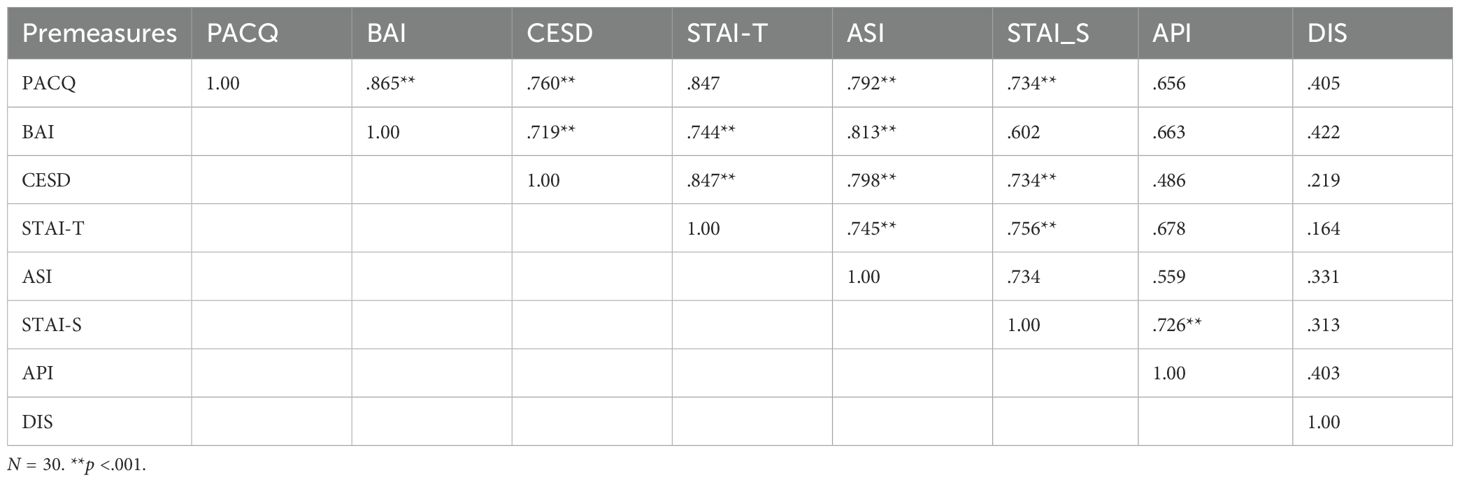
The Spearman rank-order correlation coefficient was used as a non-parametric measure to determine the strength and direction of association that exists between the premeasure assessments. Correlation coefficients between premeasure assessments are presented in Table 4.
A series of t-tests for independent groups was employed to determine if significant differences existed between the clinical and the comparison group on the scores of all psychological measures at the Time 1 (Pre-test) stage. The results of the analysis indicated that there were significant differences (p <.05) across all psychological measures at Time 1 (Pre-test) except for the DIS which was not significant at (p >.05).
Physiological differences at Time 2 (CO2 Challenge) and Time 3 (CFI + CO2 Challenge)
A mixed, ANOVA was conducted to compare the Clinical and Comparison Groups in terms of the effect of the CO2 challenge task and group on participants’ respiration rate. Table 5 shows respiration rate means and standard deviations for all participants before and after the CO2 Challenge (Time 2) and before and after the CFI + CO2 Task (Time 3). In the tables and figures below “b” refers to “before” and “p” for “post”.
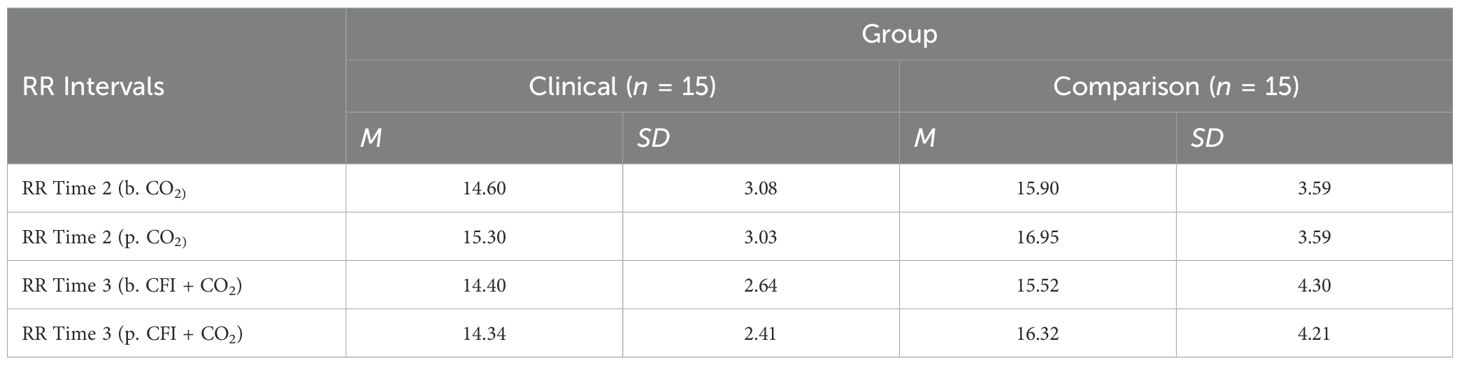
Table 5. Descriptive statistics for respiration rate (RR) before and after time 2 (CO2) and before and after time 3 (CFI + CO2).
There was no significant interaction between the effects of group and CO2 challenge task on participant’s respiration rate, (F = (1, 28) = .706, p = 0.38, η2 =.005). Simple main effects analysis showed that between 30 seconds prior to the CO2 challenge (Time 2) and 60 seconds following the CO2 challenge (Time 3), participants experienced no significant change in respiration rate, (F = (1, 28) = 3.549, p =.070, η2 =.112). There were no significant differences in respiration rates observed between the Clinical and Comparison Groups, (F(1, 30) = 1.704, p = .20, η2 =.057).
A mixed, ANOVA was also conducted to compare the Clinical and Comparison Groups in terms of the effect of the CO2 challenge task and group on participants’ HR. Table 6 shows respiration rate means and standard deviations for all participants before and after the CO2 Challenge (Time 2) and before and after the CFI + CO2 Task (Time 3).
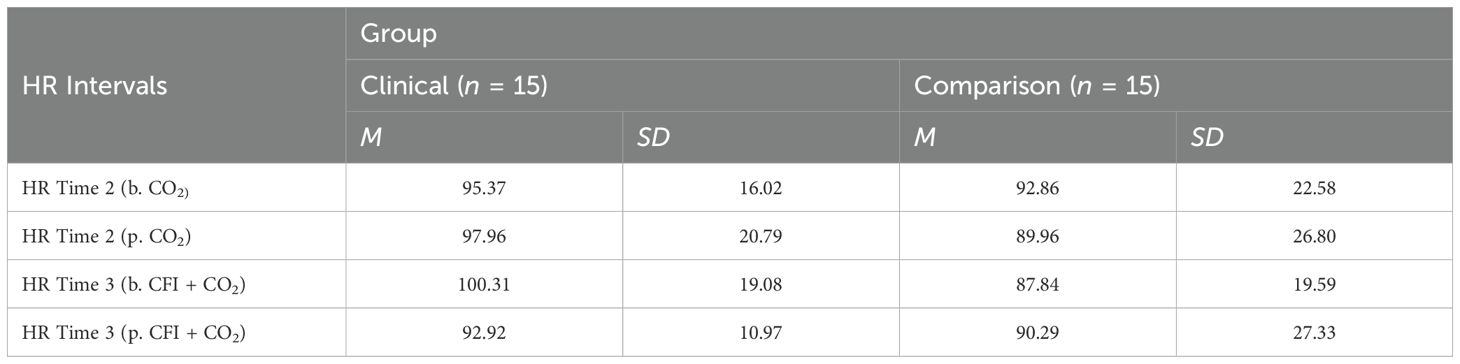
Table 6. Descriptive statistics for heart rate (HR) before and after time 2 (CO2) and after time 3 (CFI + CO2).
Furthermore, no significant interaction was found between the effects of group and CO2 on participant’s HR, (F = (1, 28) =1.028, p =.319, η2=.035). Simple main effects analysis showed that between 30 seconds prior to the CO2 challenge and 60 seconds following the CO2 challenge at Time 2, participants experienced no significant change in heart rate, (F = (1, 28) = .003, p = .955, η2=.000). There were also no significant differences between heart rates observed between the Clinical and Comparison Groups, (F(1, 28) = .489, p = .490, η2=.017).
Further mixed ANOVA analyses were conducted to compare the groups in terms of the effects of CFI, specifically at Time 2 (CO2 challenge) and Time 3 (CFI followed by the CO2 challenge), on participants’ heart rate (HR). A mixed ANOVA was conducted with the Clinical and Comparison Groups to examine the effect of CFI on participants’ heart rate and examine whether differences between groups were observed. There was no significant interaction between the effects of group and CFI on participant’s HR, (F(1, 28) = .074, p = .787, η2=.003). Simple main effects analysis showed that, at Time 3- both at the start and end of the CFI task (prior to the CO2 Challenge) - participants experienced a significant reduction in heart rate, (F(1, 28) = 121.492, p < 0.01, η2 = .813) (see Figure 2). However, no significant differences were observed between the Clinical and Comparison Groups, (F(1, 28) = 2.902, p = .100, η2 =.094).
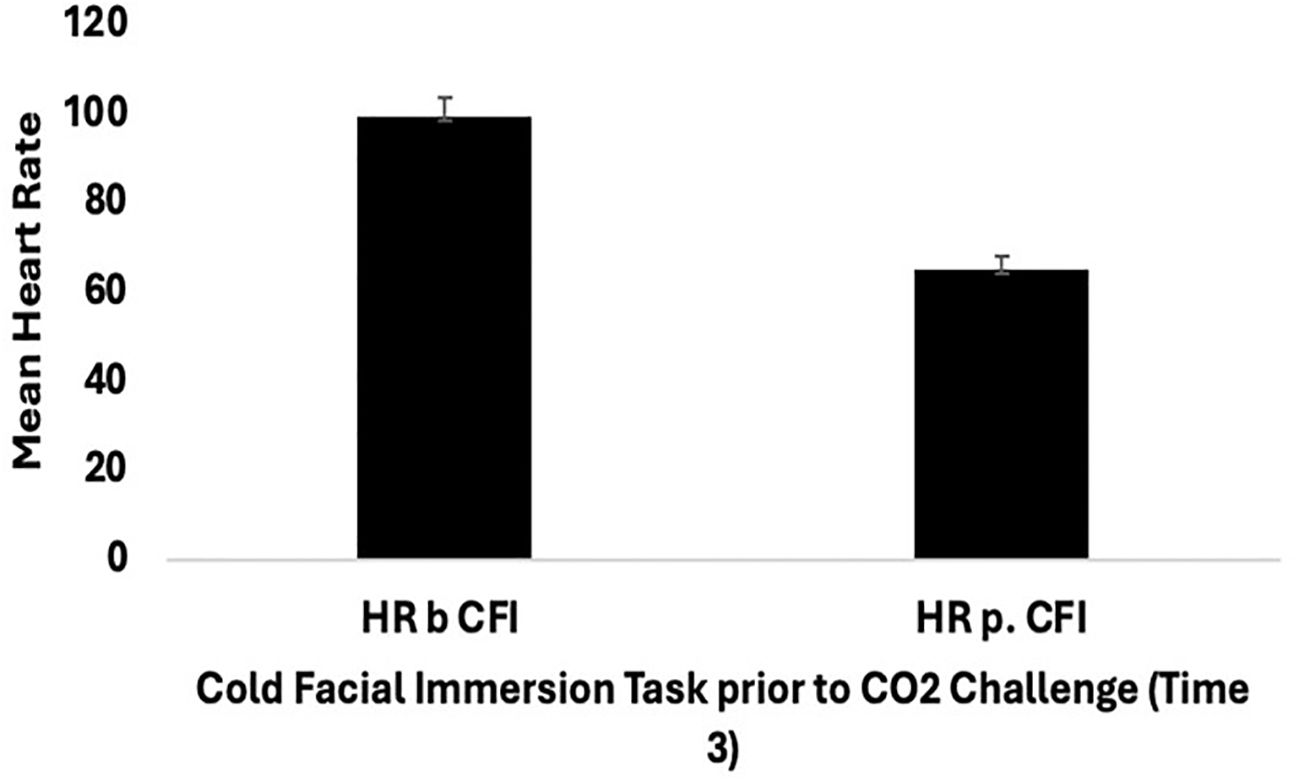
Figure 2. Mean heart rate (SE) for all participants (clinical group n = 15, and comparison group n = 15) pre and post cold facial immersion (CFI) prior to CO2 at Time 3.
Cognitive measures and effect of CFI on CO2 sensitivity
Table 7 provides means for all self-report cognitive measures taken at Time 1 (Pre-measures), Time 2 (CO2), and Time 3 (CFI followed by CO2).
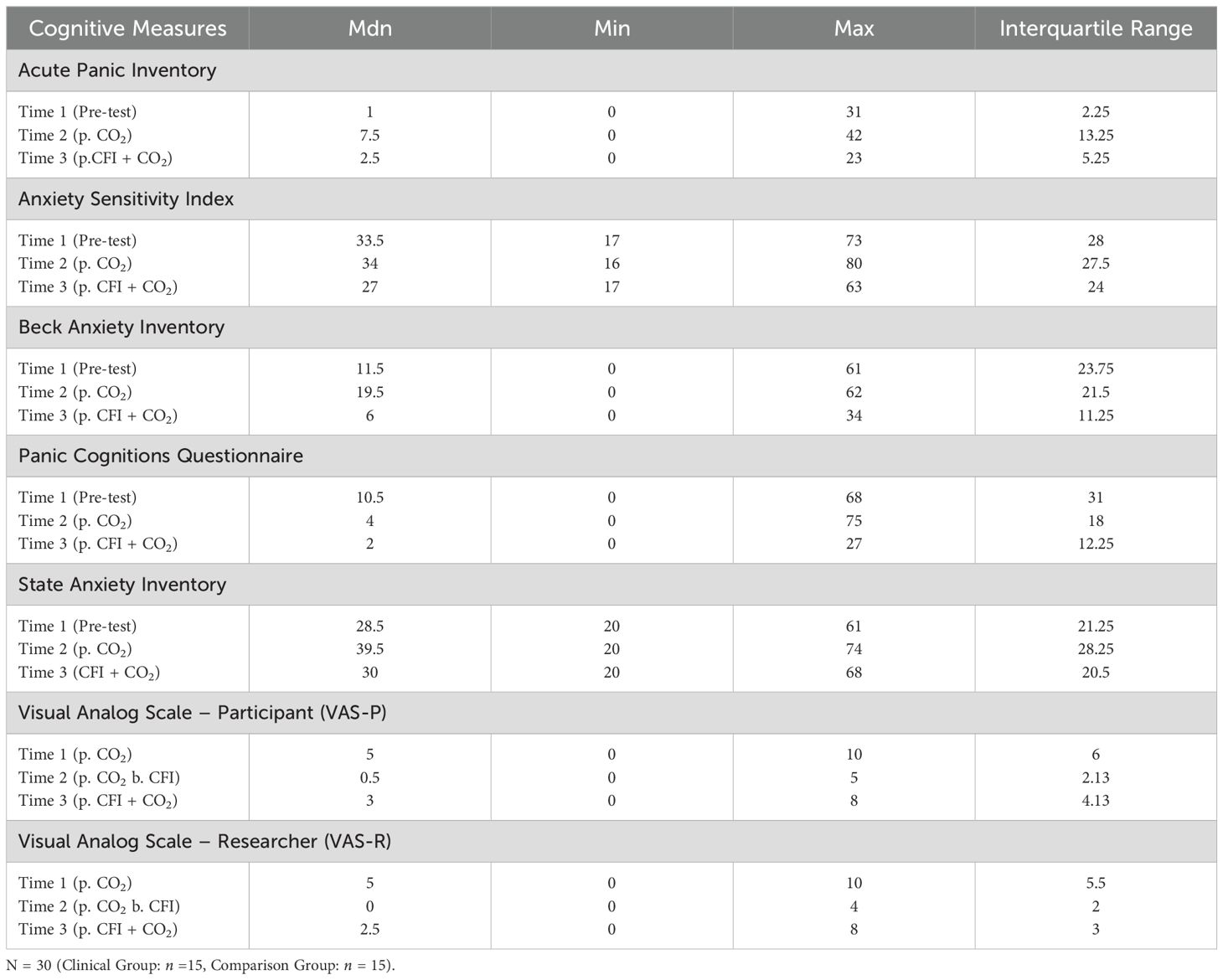
Table 7. Medians, minimum, maximum and interquartile ranges for cognitive measures at Time 1, Time 2 and Time 3.
API ratings were significantly different across the three times, χ2(2) = 28.404, p <.001. Post-hoc analysis with Wilcoxon signed-rank tests was conducted with a Bonferroni correction applied, resulting in a significance level set at Alpha = p < 0.017. The median API ratings were 1.0 at Time 1, 7.5 at Time 2, and 2.5 at Time 3. A significant increase was seen between Time 1 and Time 2, (Z = -4.401, p <.001). A significant decrease was observed between Time 2 and Time 3, (Z = 3.235, p =.001). There were no significant differences between the participants’ baseline API measures and those taken at time 3 following the CO2 challenge, (Z = -.1651, p>.017). Figure 3 displays a box plot of participants’ API scores measured at baseline (Time 1), following the CO2 challenge (Time 2), and after the CFI and CO2 challenge (Time 3).
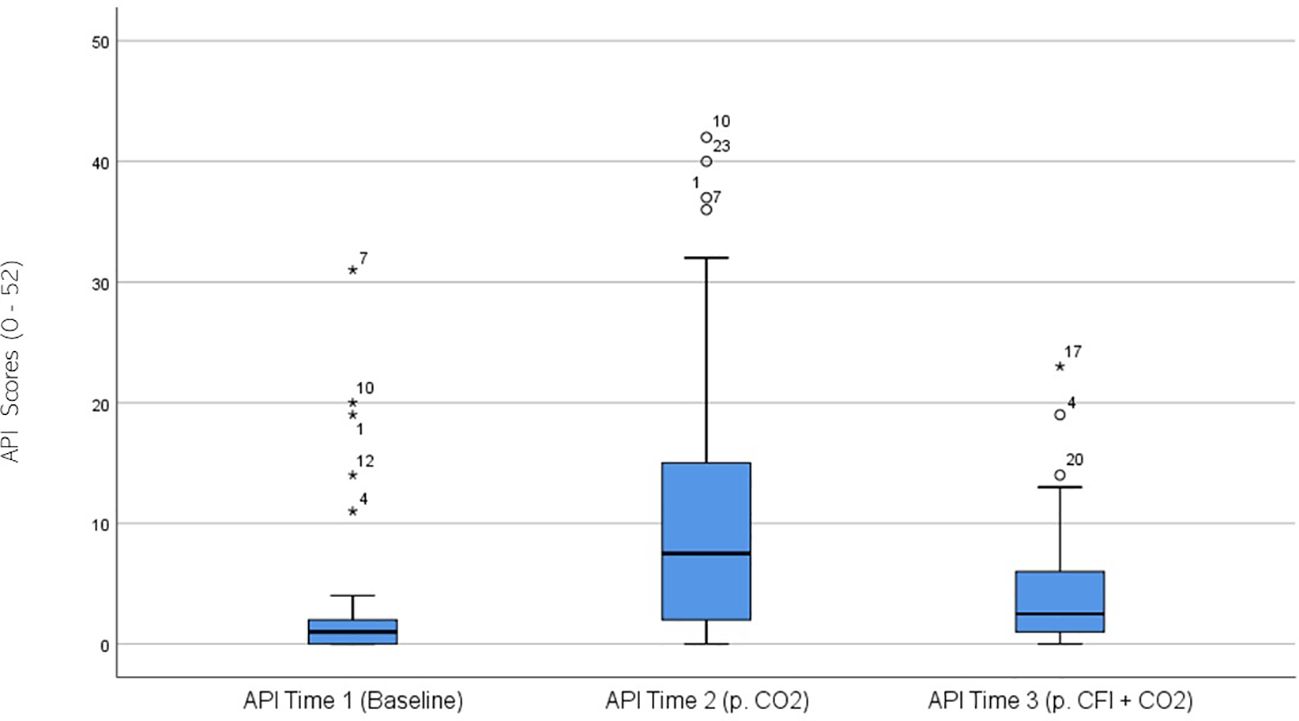
Figure 3. Box plot showing the median and interquartile range (IQR) for Acute Panic Inventory (API) scores in the Clinical and Comparison Groups (N = 30) at Time 1, Time 2, and Time 3. The boxes represent the IQR, with the median indicated by a horizontal line. Whiskers extend to values within 1.5 times the IQR, and asterisks denote significant differences (p <.05).
ASI ratings were significantly different across the three times, χ2(2) = 17.741, p < 0.01. Post-hoc analysis with Wilcoxon signed-rank test was conducted with a Bonferroni correction applied, resulting in a significance level set at Alpha = p <.016. The median ASI ratings were 33.5 at Time 1, 34 at Time 2, and 27 at Time 3. No significant difference was observed between baseline and Time 2, (Z = 0.520, p >.017), whilst a significant decrease was noted between Time 2 and Time 3, (Z = 2.654, p =.008). A significant decrease was also observed between baseline and Time 3, (Z = -3.019 p =.003). Figure 4 displays a box plot of participants’ ASI scores measured at baseline (Time 1), following the CO2 Challenge (Time 2), and after the CFI and CO2 Challenge (Time 3).
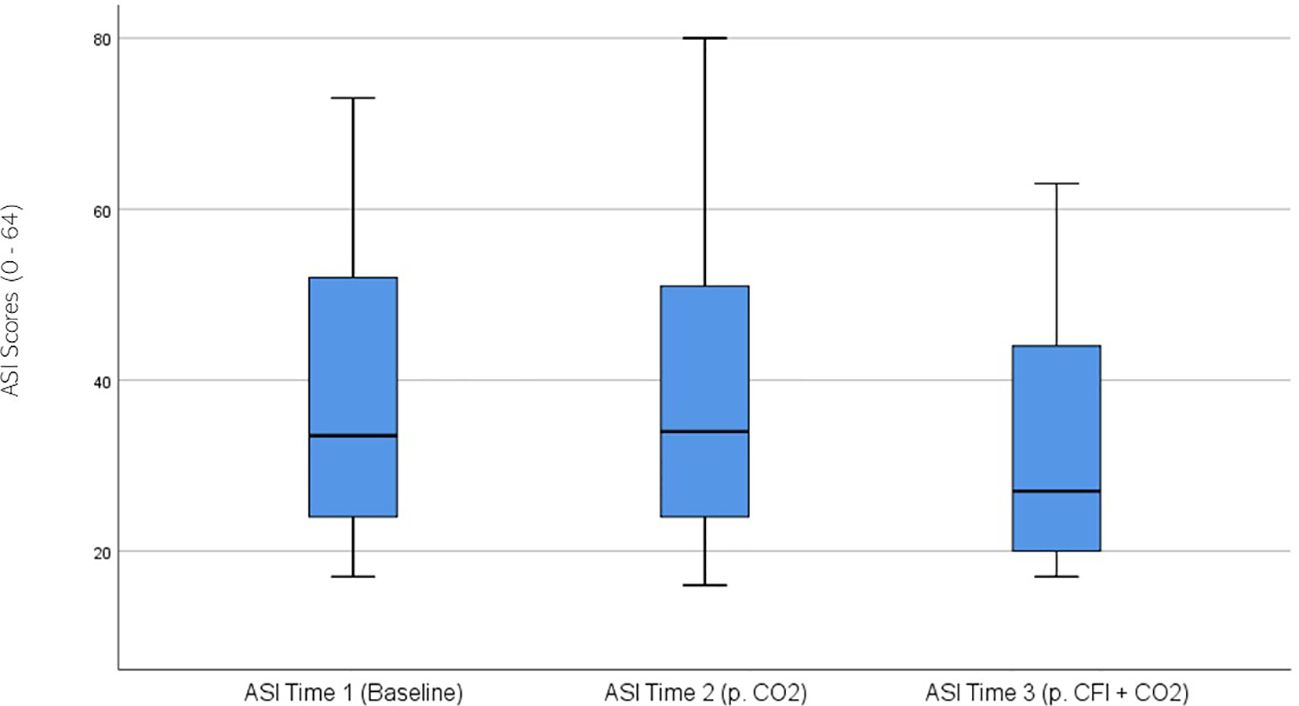
Figure 4. Box plot showing the median and interquartile range (IQR) for Anxiety Sensitivity Index (ASI) scores in the Clinical and Comparison Groups (N = 30) at Time 1, Time 2, and Time 3. The boxes represent the IQR, with the median indicated by a horizontal line. Whiskers extend to values within 1.5 times the IQR, and asterisks denote significant differences (p <.05).
BAI ratings were significantly different across the three times, χ2(2) = 14.131, p = .001. Post-hoc analysis with Wilcoxon signed-rank test was conducted with a Bonferroni correction applied, resulting in a significance level set at Alpha =p <.017. The median BAI ratings were 11.51 at Time 1, 19.5 at Time 2, and 6.0 at Time 3. No significant difference was noted between Time 1 and Time 2 (Z = -1.211, p >.017). A significant decrease was seen between Time 1 and Time 3, (Z = -3.607, p <.001). A significant decrease was observed between Time 1 and Time 3, (Z = -2.849, p = .004). Figure 5 displays a box plot of participants’ BAI scores measured at baseline (Time 1), following the CO2 Challenge (Time 2), and after the CFI and CO2 challenge (Time 3).
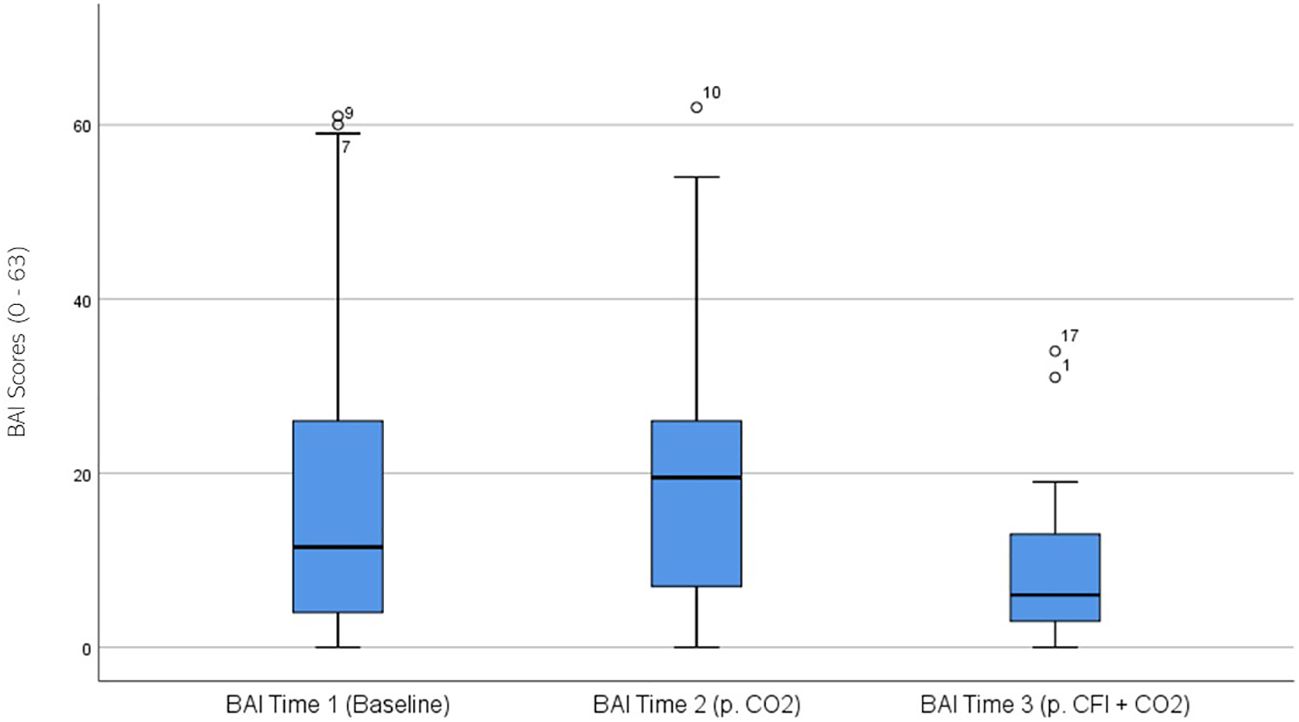
Figure 5. Box plot showing the median and interquartile range (IQR) for Beck Anxiety Inventory (BAI) scores in the Clinical and Comparison Groups (N = 30) at Time 1, Time 2, and Time 3. The boxes represent the IQR, with the median indicated by a horizontal line. Whiskers extend to values within 1.5 times the IQR, and asterisks denote significant differences (p <.05).
PACQ ratings were significantly different across the three times, χ2(2) = 12.896, p = .002. Post-hoc analysis with Wilcoxon signed-rank test was conducted with a Bonferroni correction applied, resulting in a significance level set at Alpha = p <.017. The median PACQ ratings were 10.5 at Time 1, 4.0 at Time 2, and 2.0 at Time 3. No significant differences were observed between baseline and time 2, (Z = 2.234, p >.017), and Time 2 and Time 3 (Z = -2.218, p >.017). A significant decrease was observed between Time 1 and Time 3, (Z = 3.495, p <.001). Figure 6 displays a box plot of participants’ PACQ scores measured at baseline (Time 1), following the CO2 challenge (Time 2), and after the CFI and CO2 challenge (Time 3).
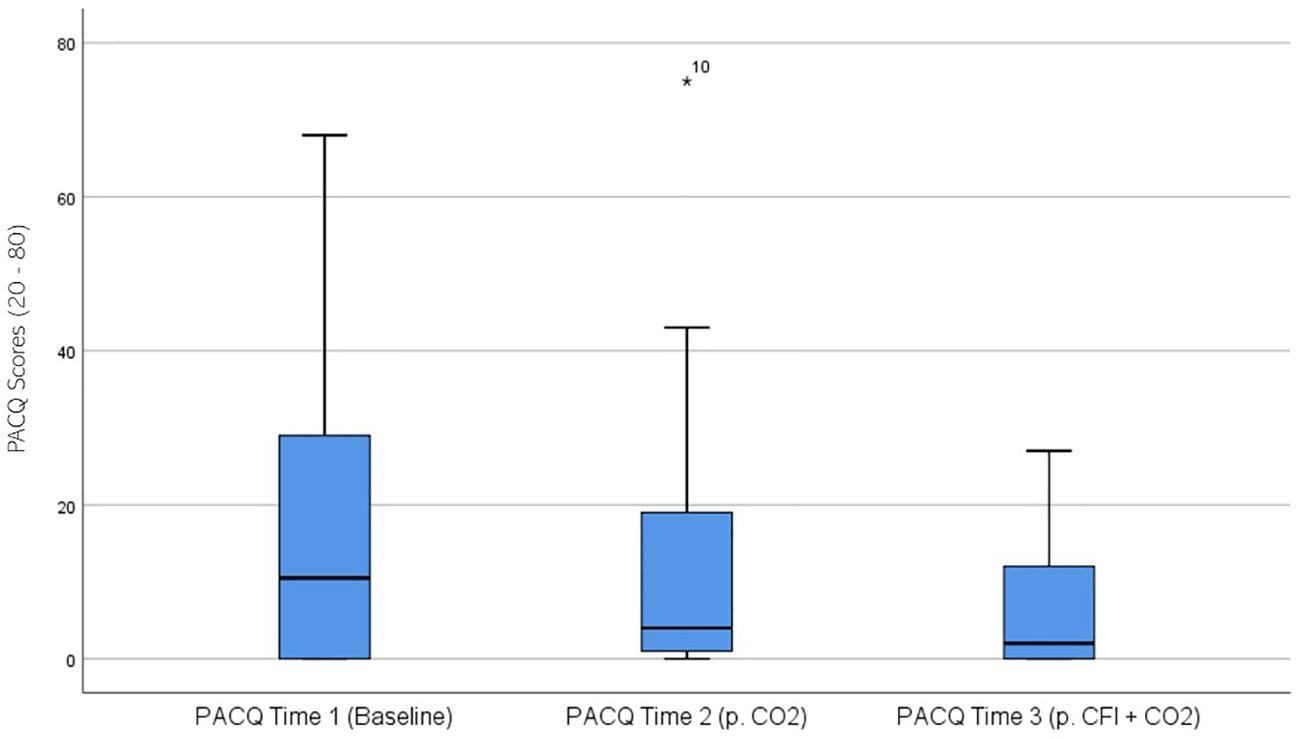
Figure 6. Box plot showing the median and interquartile range (IQR) for Panic Cognitions Questionnaire (PACQ) scores in the Clinical and Comparison Groups (N = 30) at Time 1, Time 2, and Time 3. The boxes represent the IQR, with the median indicated by a horizontal line. Whiskers extend to values within 1.5 times the IQR, and asterisks denote significant differences (p <.05).
STAI ratings were significantly different across the three time periods, χ2(2) = 23.078, p < 0.01. Post hoc analysis with Wilcoxon signed-rank tests was conducted with a Bonferroni correction applied, resulting in a significance level set at Alpha = p <.017. The median STAI ratings were 28.5 at Time 1, 39.5 at Time 2, and 30.0 at Time 3. There was a significant increase between the participants’ baseline STAI measures and those taken at time 2, following the CO2 challenge, (Z = -.4.159, p <.001). A statistically significant decrease was seen between Time 2 and Time 3, (Z = -3.305, p = .001). There was no significant difference between Time 1 and Time 3, (Z = -0.833, p >.017). Figure 7 displays a box plot of participants’ STAI-S scores measured at baseline (Time 1), following the CO2 challenge (Time 2), and after the CFI and CO2 challenge (Time 3).
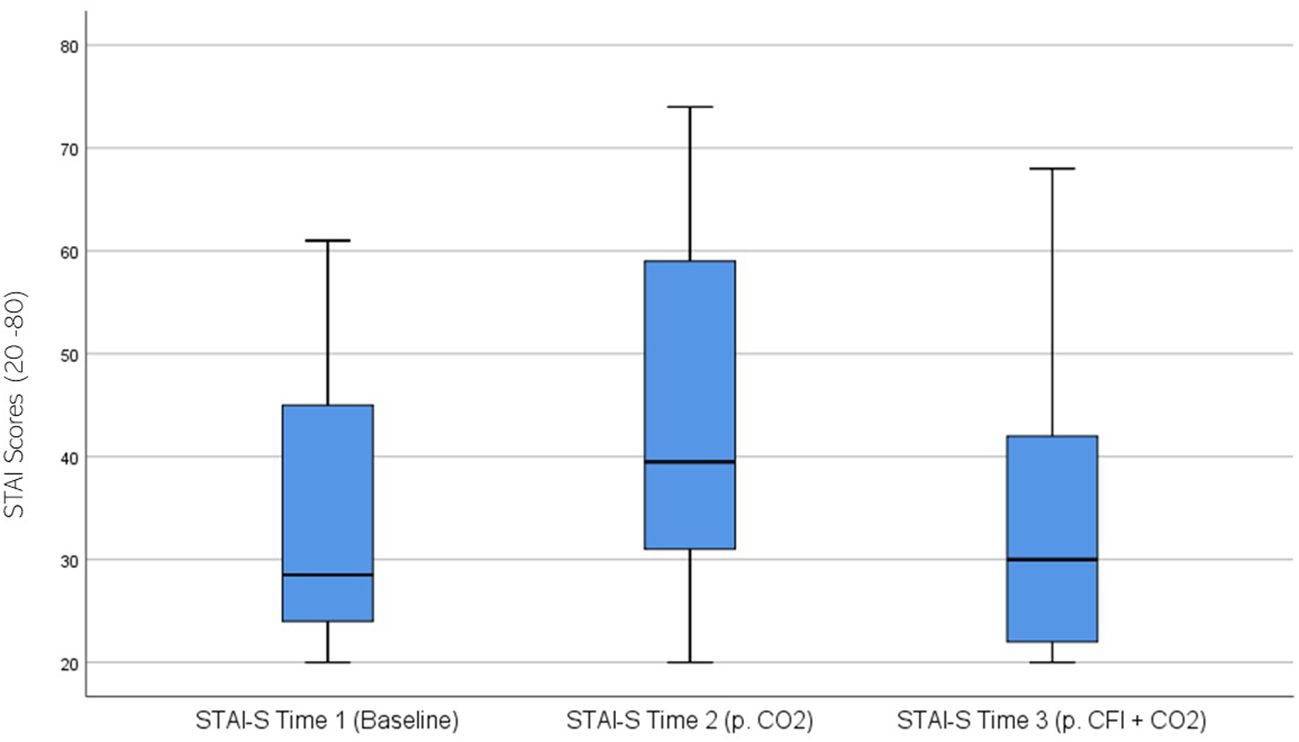
Figure 7. Box plot showing the distribution of State-Trait Anxiety Inventory (STAI) scores for the Clinical and Comparison Groups (N = 30) at Time 1, Time 2, and Time 3. The boxes represent the interquartile range (IQR), with the median indicated by a horizontal line. Whiskers extend to values within 1.5 times the IQR, and asterisks denote significant differences (p <.05).
VAS Participant (VAS-P) ratings were significantly different across the three times, χ2(2) = 28.055, p < 0.01. Post-hoc analysis with Wilcoxon signed-rank test was conducted with a Bonferroni correction applied, resulting in a significance level set at Alpha = p <.017. The median VAS-P ratings were 5.0 at Time 1, 0.5 at Time 2, and 3.0 at Time 3. Significant decreases were observed between baseline and Time 2, (Z = -4.363, p <. 001), and between Time 2 and Time 3, (Z = -3.782 p <. 001). Furthermore, a significant decrease was seen between Time 1 and Time 3, (Z = -2.801, p = .005). Figure 8 displays a box plot of participants’ VAS-P scores measured at baseline (Time 1), following the CO2 challenge (Time 2), and after the CFI and CO2 challenge (Time 3).
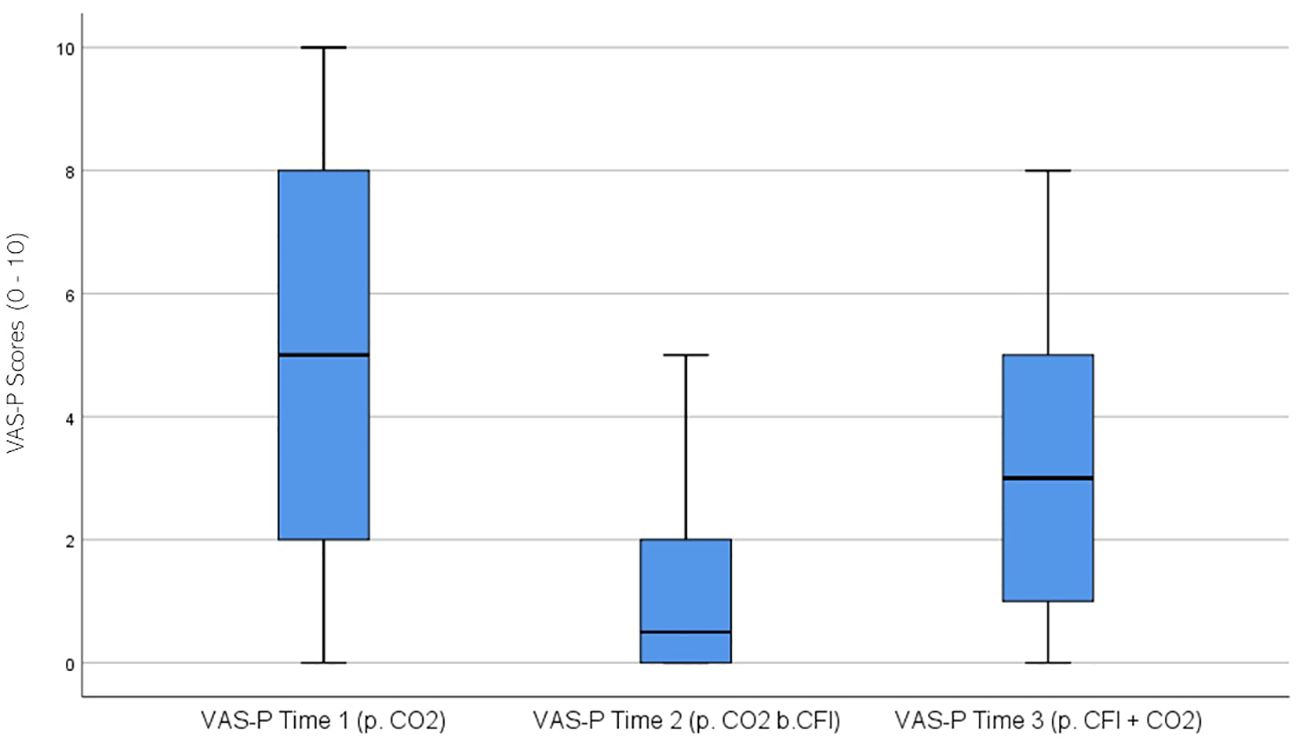
Figure 8. Box plot showing the median and interquartile range (IQR) for Visual Analog Scale – Participant (VAS-P) scores in the Clinical and Comparison Groups (N = 30) at Time 1, Time 2, and Time 3. The boxes represent the IQR, with the median indicated by a horizontal line. Whiskers extend to values within 1.5 times the IQR, and asterisks denote significant differences (p <.05).
VAS Researcher (VAS-R) ratings were significantly different across the three times, χ2(2) = 39.086, p < 0.01. Post-hoc analysis with Wilcoxon signed-rank test was conducted with a Bonferroni correction applied, resulting in a significance level set at Alpha =p <.017. The median VAS-R ratings were 5.0 at Time 1, 0 at Time 2, and 2.5 at Time 3. A significant decrease was seen between baseline and Time 2, (Z = -4.462, p <.001), and between Time 2 and Time 3, (Z = 4.178 p <.001). Furthermore, there was a significant decrease between Time 1 and Time 3, (Z = -3.506, p <.001). Figure 9 displays a box plot of VAS-R scores measured at baseline (Time 1), following the CO2 challenge (Time 2), and after the CFI and CO2 challenge (Time 3).
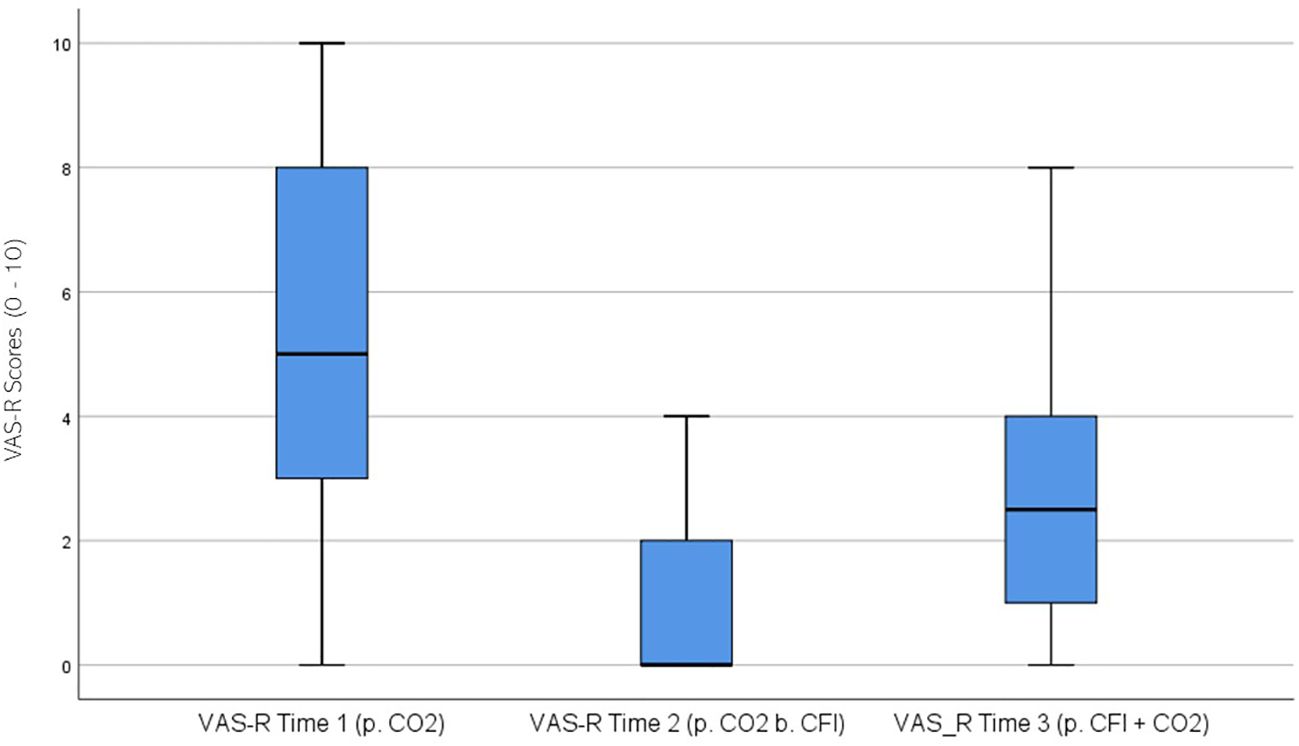
Figure 9. Box plot showing the median and interquartile range (IQR) for Visual Analog Scale – Researcher (VAS-R) scores in the Clinical and Comparison Groups (N = 30) at Time 1, Time 2, and Time 3. The boxes represent the IQR, with the median indicated by a horizontal line. Whiskers extend to values within 1.5 times the IQR, and asterisks denote significant differences (p <.05).
Discussion
The principal findings of this study were that there were no differences in heart rate and respiration rate within or between groups in response to the CO2 challenge. Following the CO2 challenge, the Clinical Group was observed to have more elevated anxiety and panic symptoms, as reported by the anxiety measures in comparison to the Comparison Group. Furthermore, a significant reduction in anxiety symptomatology was observed in both the Clinical and Comparison Groups following the cold facial immersion (CFI). The results of this study are consistent with the finding of the preliminary study (12).
It was expected that the CFI task would reduce CO2 sensitivity, as evidenced by heart rate and respiration rate reductions following the subsequent CO2 challenge in Clinical and Comparison participants. The findings did not provide support for this hypothesis as there was no significant difference found in participants’ heart rate and respiration rate between Time 2 (CO2 challenge) and Time 3 (CFI and CO2). However, the results examining the effects of the CFI task (prior to the administration of CO2) indicate a significant reduction in heart rate for both Clinical and Comparison participants. The clinical group had a larger reduction in heart rate between Time 2 and Time 3 compared to the comparison group suggesting that the CFI may be a useful intervention for the treatment of PD. In line with the findings of Kyriakoulis et al. (12), these results indicate that CFI has anxiolytic effects. However, despite its powerful response, it cannot be inferred that one single administration of CFI can alter one’s sensitivity to CO2.
The study found that there was a significant reduction noted between Time 1 (baseline pre-measures) and Time 3 (CFI followed by CO2) on the following anxiety measures Anxiety Sensitivity Index (ASI), State and Trait Anxiety Inventory (STAI), Panic Attack Cognitions Questionnaire (PACQ), Beck Anxiety Inventory (BAI), Visual Analog Scale completed by the participant (VAS-P) and researcher (VAS-R), with the exception of Acute Panic Inventory (API) for the both the Clinical and Comparison Groups. Hence, the CFI task led to broad improvements in anxiety-related symptoms, especially in cognitive, emotional, and perceived anxiety. Furthermore, significant decreases in anxiety symptoms were reported from Time 2 (CO2) to Time 3 (CFI + CO2) on the following measures; API, ASI, BAI, STAI, VAS-R, and VAS-P, with the exception of the PACQ which was not found to change significantly between Time 2 and 3. A possible explanation for this finding is that Comparison Group participants did not experience panic cognitions in response to the CO2 challenge. As non-parametric analyses were used to assess the effects of the CFI on CO2, this did not allow for a comparison between groups across the different time points. Whilst the Comparison Group had minimal symptoms overall in response to the CO2 challenge, they did report a reduction in symptoms and calming effects in response to the CFI task. The Clinical Group reported a decrease in anxiety symptoms, following exposure to the CFI task on all anxiety measures including the ASI, API, STAI, PACQ, BAI, VAS-R and VAS-P. Hence frequent exposure to the CFI tasks and to breath-holding may be able to reduce CO2 sensitivity in individuals with PD over time.
Practicing breath-holding and activating the DR through freediving and CFI regularly has been known to have trained effects, subsequently reducing CO2 sensitivity (14, 15). Reports in the literature have suggested that a blunted hypercapnic response and prolonged breath-hold times have been associated with the benefits of trained effects and repeated apneas (25–29). Goosens et al. (30) in their study found that experienced divers displayed a decreased behavioral response to CO2 as compared to clinical participants with PD and healthy comparisons. This finding is also consistent with the research of Earing et al. (31), who found that experienced divers possess a lower ventilatory response to CO2 suggesting a strong adaptation of central CO2 sensitivity. Through breath-holding training, the body of a freediver adapts to prolonged periods of low oxygen (hypoxia) and high CO2 (hypercapnia). This leads to reduced sensitivity to CO2, allowing better control during deep dives where oxygen and CO2 levels fluctuate. Over time, the body becomes accustomed to higher CO2 levels without triggering the typical urge to breathe.
Whilst the current study was unable to identify whether one single administration of the CFI task can alter CO2 sensitivity, it appears promising as an intervention given that it was able to significantly reduce self-reported anxiety symptoms and reduced heart rate for both Clinical and Comparison participants.
Study limitations
Limitations of this study included recruitment challenges, and the extensive list of exclusion criteria for individuals to be eligible to participate in the study, which limited the sample size and matching of participants. As a result of recruitment challenges, researchers invited five participants from a preliminary study (12) who had expressed interest in participating in future research in this area. In order to ameliorate recruitment challenges participants with intermittent use of benzodiazepines, anxiolytics and painkillers were allowed to participate in the current study and we extended the age from 55 years of age to 60 years of age, in an attempt to recruit more participants.
Despite efforts to match participants and recruit non-university students as comparisons, achieving this proved challenging. Therefore, the results may not accurately reflect a typical population. Some participants reported challenges, such as disliking the taste of CO2 gas, feeling anxious during the task, and struggling to inhale deeply. Although every effort was made, a few participants were unable to hold their breath for the required four seconds. Given that this is required for the test to be considered a valid, participants must inhale at least 80% of their vital capacity of CO2 to experience the full effects of the CO2 challenge (8).
A further limitation is that both the CO2 challenge and the CFI task elicit a brief response. A drawback to this may be that participants may not accurately report what they experience as by the time they complete the self–report anxiety measures minutes later, their symptoms may have dissipated. In the current study, cold facial immersion bradycardia reached a peak within 20 to 30 seconds, which is consistent with previous research (25, 32). Reports in the literature suggest that lower water temperatures (0˚C - 10˚C) lead to an increase in minute ventilation and a more pronounced bradycardic response compared to warmer water (32, 33). The effectiveness and extent of the activation of the DR can vary based on factors such as water temperature and the duration of immersion (34). However, acute panic responses were less responsive to change, indicating a potential need for more targeted treatment in that area.
Other methodological considerations for future studies investigating the CFI and the DR include improving the provocation of the CO2 challenge, the continuous data acquisition during the experimental design, and matching participants for age and gender, as well as including the VAS-P and VAS-R measures at baseline before any condition as a means of comparison.
Considerations for future research
Research has established a clear link between respiratory disorders and PD (35–41). Future studies should investigate the differences between respiratory and the non-respiratory clinical groups to assess how each responds to the provocation methods (i.e., breath-hold tasks, 35% CO2 challenge and CFI task). Specifically, future research is needed to investigate the time course for the development of the trained effects, determining how often and how many apnea exposures are required (26). This area of inquiry will help clarify benefits of breath-hold training and CFI as treatments for PD. Understanding the physiological adaptations of apnea training and freediving techniques, such as the activation of the DR, may also contribute to the development of innovative treatments for panic and anxiety symptoms (14).
Implications and future directions
This study opens several avenues for future research on the role of the DR and breath-hold training in managing PD. Given that individuals with PD often exhibit heightened sensitivity to CO2 and autonomic dysfunction, exploring the effects of controlled breath-hold tasks, like CFI, on both physiological and psychological symptoms could inform novel therapeutic interventions. Specifically, future research should investigate the long-term effects of CFI practice and other apnea-based techniques on anxiety sensitivity and panic symptoms (42, 43). Understanding how the DR contributes to anxiety regulation may also help integrate breath-hold exercises into cognitive-behavioral therapy (CBT) for PD, leveraging DR adaptations to address anxiety sensitivity and maladaptive beliefs linked to panic-related issues (42, 43).
While freediving techniques have shown promise in regulating autonomic function, the optimal frequency and duration of breath-hold tasks needed to produce lasting benefits remain unclear. Future studies should systematically examine different apnea exposure protocols to determine their effects on vagal tone, heart rate variability, and emotion regulation (33, 44). Additionally, identifying individual differences in response to apnea training could help determine which subgroups of PD patients may benefit most from such interventions.
Beyond the therapeutic potential of breath-hold training, the CFI task itself could serve as a valuable diagnostic and intervention tool for assessing physiological and psychological responses to CO2 challenges. By activating the DR, CFI may reduce heart rate and alleviate physiological and cognitive symptoms of panic, potentially decreasing CO2 sensitivity. Clinicians could use CFI to observe changes in heart rate, respiratory rate, and self-reported anxiety symptoms, gaining insights into a patient’s sensitivity to physiological arousal and their susceptibility to panic attacks. Moreover, heart rate variability (HRV) could be a useful biomarker for studying potential changes in panic and anxiety symptoms following CFI and breath hold training. Future research should explore how CFI could be integrated into psychoeducation or as part of a behavioral experiment, to help individuals challenge and correct maladaptive beliefs about bodily sensations associated with panic. Previous research indicates that addressing anxiety sensitivity can help reduce panic attacks and implementing behavioral experiments that activate the DR could help patients confront feared sensations and reframe their interpretations of physiological symptoms (45). Barlow et al. (46–48) highlighted the efficacy of cognitive restructuring and behavioral experiments in significantly reducing anxiety symptoms, further supporting the potential of DR-based techniques in treatment.
While this study has focused on the implications of DR activation and CFI for PD, future research should investigate the potential benefits of DR activation and CFI for other anxiety-related disorders, such as generalized anxiety disorder or social anxiety disorder, where heightened sensitivity to bodily sensations is a common feature. This could expand the application of these techniques beyond PD and into other areas of anxiety treatment.
Conclusion
This study found that the CFI task effectively reduced anxiety and panic symptoms triggered by the CO2 challenge. Although, there were no significant differences between the Clinical and Comparison Groups, the findings suggest that the CFI task may still be beneficial for individuals with PD. One of the most distressing symptoms for those with PD is tachycardia (increased heart rate), which can amplify feelings of anxiety and panic. By activating the DR through cold exposure, it is possible to reduce the heart rate, which in turn may help alleviate these symptoms. The reduction in heart rate is thought to contribute to an anxiolytic effect on the autonomic nervous system, helping to counter the physiological arousal that often accompanies anxiety and panic. Therefore, the activation of the DR, which is easily achieved through methods like CFI and cold moisture (e.g., ice packs), could serve as an accessible and effective treatment for panic disorder and other anxiety disorders s. Whilst these findings are promising, further research is needed to explore the long-term effects and optimal use of DR activation in clinical settings. Specifically, investigating the precise mechanisms, the frequency of its application, and its broader therapeutic potential would be critical for advancing its role in anxiety treatment. anxiolytic effects of DR activation.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Swinburne University Human Research Ethics Committee (SUHREC). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
PK: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. CC: Data curation, Formal analysis, Methodology, Resources, Software, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Guan X and Cao P. Brain mechanisms underlying panic attack and panic disorder. Neurosci Bull. (2024) 40:795–814. doi: 10.1007/s12264-023-01088-9
2. Papp LA, Martinez JM, Klein DF, Coplan JD, and Gorman JM. Rebreathing tests in panic disorder. Biol Psychiatry. (1995) 38:240–5. doi: 10.1016/0006-3223(94)00296-F
3. Papp LA, Klein DF, and Gorman JM. Carbon dioxide hypersensitivity, hyperventilation, and panic disorder. Am J Psychiatry. (1993) 150:1149–57. doi: 10.1176/ajp.150.8.1149
4. Freire RC and Nardi AE. Panic disorder and the respiratory system: clinical subtype and challenge tests. Braz J Psychiatry. (2012) 34:S32–52. doi: 10.1016/S1516-4446(12)70053-3
5. Gorman JM, Askanazi J, Liebowitz MR, Fyer AJ, Stein J, Kinney JM, et al. Response to hyperventilation in a group of patients with panic disorder. Am J Psychiatry. (1984) 141:857–61. doi: 10.1176/ajp.141.7.857
6. Klein DF. False suffocation alarms, spontaneous panics, and related conditions. Arch Gen Psychiatry. (1993) 50:306–17. doi: 10.1001/archpsyc.1993.01820160076009
7. Fishman SM, Carr DB, Beckett A, and Rosenbaum JF. Hypercapneic ventilatory response in patients with panic disorder before and after alprazolam treatment and in pre- and postmenstrual women. J Psychiatry Res. (1994) 28:165–70. doi: 10.1016/0022-3956(94)90027-2
8. Griez E and Van Den Hout MA. Effects of carbon dioxide-oxygen inhalations on subjective anxiety and some neurovegetative parameters. J Behav Ther Exp Psychiatry. (1982) 13:27–32. doi: 10.1016/0005-7916(82)90032-5
9. Nardi AE, Valença AM, Nascimento I, Mezzasalma MA, and Zin WA. Double-blind acute clonazepam vs. placebo in carbon dioxide-induced panic attacks. Psychiatry Res. (2000) 94:179–84. doi: 10.1016/S0165-1781(00)00135-9
10. Nardi AE, Freire RC, and Zin WA. Panic disorder and control of breathing. Respir Physiol Neurobiol. (2009) 167:133–43. doi: 10.1016/j.resp.2008.07.011
11. Perna G, Battaglia M, Garberi A, Arancio C, Bertani A, and Bellodi L. Carbon dioxide/oxygen challenge test in panic disorder. Psychiatry Res. (1994) 52:159–71. doi: 10.1016/0165-1781(94)90085-X
12. Kyriakoulis P, Kyrios M, Nardi AE, Freire RC, and Schier M. The implications of the diving response in reducing panic symptoms. Front Psychiatry. (2021) 12:2157. doi: 10.3389/fpsyt.2021.784884
13. Tetzlaff K and Muth CM. Physiological challenges and adaptations in competitive freediving. Dtsch Z Sportmed. (2024) 75:207–15. doi: 10.5960/dzsm.2024.613
14. Kyriakoulis P. The cardio-respiratory mechanisms involved in the diving response adaptation and breath-hold training effects in freedivers. J Cardiol Res Rev Rep. (2024) 5(3):1–12. doi: 10.47363/JCRRR/2024(5)195. SRC/JCRRR-218.
15. Konstantinidou S and Chairopoulou C. Physiological adaptations of apnea-conditioned athletes and their implications for synchronized swimmer’s performance. Arch Sports Med. (2017) 1:20–30. doi: 10.36959/987/225
16. Christoforidi V, Koutlianos N, Deligiannis P, Kouidi E, and Deligiannis A. Heart rate variability in free diving athletes. Clin Physiol Funct Imaging. (2012) 32:162–6. doi: 10.1111/j.1475-097X.2011.01070.x
17. González AA, Rodríguez FA, and Peña J. Chemoreflex control as the cornerstone in immersion water sports. Front Physiol. (2021) 12:681531. doi: 10.3389/fphys.2021.681531
18. Engan H, Richardson MX, Lodin-Sundström A, Beekvelt M, and Schagatay E. Effects of two weeks of daily apnea training on diving response, spleen contraction, and erythropoiesis in novel subjects. Scandinavian J Med Sci Sports. (2013) 23:340–8. doi: 10.1111/j.1600-0838.2011.01391.x
19. Gorman JM, Kent JM, Sullivan GM, and Coplan JD. Neuroanatomical hypothesis of panic disorder, revised. Am J Psychiatry. (2000) 157:493–505. doi: 10.1176/appi.ajp.157.4.493
20. Klein DF. False suffocation alarms, spontaneous panics, and related conditions: An integrative hypothesis. Arch Gen Psychiatry. (1993) 50:306. doi: 10.1001/archpsyc.1993.01820160076009
21. Sheehan DV, Lecrubier Y, Harnett Sheehan K, Janavs J, Weiller E, Keskiner A, et al. The validity of the Mini International Neuropsychiatric Interview (MINI) according to the SCID-P and its reliability. Eur Psychiatr. (1997) 12:232–41. doi: 10.1016/S0924-9338(97)83297-X
22. First MB, Gibbon M, Spitzer RL, and Williams JBW. User’s Guide for the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I, Version 2.0). New York, NY: New York State Psychiatric Institute (1995).
23. First MB, Spitzer RL, Gibbon M, and Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders: Patient Edition, SCID-I/P. New York, NY: New York State Psychiatric Institute (1996). doi: 10.1037/t07827-000
25. Andersson JP and Schagatay E. Repeated apneas do not affect the hypercapnic ventilatory response in the short term. Eur J Appl Physiol. (2009) 105:569–74. doi: 10.1007/s00421-008-0936-y
26. Walterspacher S, Scholz T, Tetzlaff K, and Sorichter S. Breath-hold diving: Respiratory function on the longer term. Med Sci Sports Exercise. (2011) 43:1214–9. doi: 10.1249/MSS.0b013e31820a4e0c
27. Roecker K, Metzger J, Scholz T, Tetzlaff K, Sorichter S, and Walterspacher S. Modified ventilatory response characteristics to exercise in breath-hold divers. Int J Sports Physiol Perform. (2014) 9:757–65. doi: 10.1123/ijspp.2013-0308
28. Grassi M, Caldirola D, Di Chiaro NV, Riva A, Daccò S, Pompili M, et al. Are respiratory abnormalities specific for panic disorder? A meta-analysis. Neuropsychobiology. (2014) 70:52–60. doi: 10.1159/000364830
29. Foster GE and Steel AW. The human diving response, its function, and its control. Scandinavian J Med Sci Sports. (2005) 15:3–12. doi: 10.1111/j.1600-0838.2005.00440.x
30. Goossens L, Leibold N, Peeters R, Esquivel G, Knuts I, Backes W, et al. Brainstem response to hypercapnia: A symptom provocation study into the pathophysiology of panic disorder. J Psychopharmacol. (2014) 28:449–56. doi: 10.1177/026988111452736331
31. Earing CMN, McKeon DJ, and Kubis HP. Divers revisited: the ventilatory response to carbon dioxide in experienced scuba divers. Respir Med. (2014) 108:758–65. doi: 10.1016/j.rmed.2014.02.010
32. Baranova TI, Berlov DN, and Yanvareva IN. Changes in cerebral blood flow on performance of a diving reaction in humans. Neurosci Behav Physiol. (2016) 46:36–41. doi: 10.1007/s11055-015-0195-4
33. Schagatay E, van Kampen M, Emanuelsson S, and Holm B. Effects of physical and apnea training on apneic time and the diving response in humans. Eur J Appl Physiol. (2000) 82:161–9. doi: 10.1007/s004210050668
34. Hess HW, Schlader ZJ, Russo LN, Clemency BM, and Hostler D. Cold water submersion attenuates post-submersion aerobic performance and orthostatic tolerance irrespective of partial rehydration with water. Undersea Hyperbaric Med. (2019) 46:7–16. doi: 10.22462/01.03.2019.2
35. Biber B and Alkin T. Panic disorder subtypes: Differential responses to CO2 challenge. Am J Psychiatry. (1999) 156:739–44. doi: 10.1176/ajp.156.5.739
36. Nardi AE, Nascimento I, Valença AM, Lopes FL, Zin WA, Mezzasalma MA, et al. A breath-holding challenge in panic disorder patients, their healthy first-degree relatives, and normal controls. Respir Physiol Neurobiol. (2002) 133:43–7. doi: 10.1016/S1569-9048(02)00149-0
37. Nardi AE, Valença AM, Lopes FL, Nascimento I, Veras AB, Freire RC, et al. Psychopathological profile of 35% CO2 challenge test–induced panic attacks: a comparison with spontaneous panic attacks. Compr Psychiatry. (2006) 47:209–14. doi: 10.1016/j.comppsych.2005.07.007
38. Nardi AE, Valença AM, Mezzasalma MA, Lopes FL, Nascimento I, Veras AB, et al. 35% Carbon dioxide and breath-holding challenge tests in panic disorder: A comparison with spontaneous panic attacks. Depression Anxiety. (2006) 23:236–44. doi: 10.1002/da.20165
39. Freire RC, Lopes FL, Valença AM, Nascimento I, Veras AB, Mezzasalma MA, et al. Panic disorder respiratory subtype: a comparison between responses to hyperventilation and CO2 challenge tests. Psychiatry Res. (2008) 157:307–10. doi: 10.1016/j.psychres.2007.07.015
40. Papp LA, Goetz R, Cole R, Klein DF, and Jordan F. Hypersensitivity to carbon dioxide in panic disorder. Am J Psychiatry. (1989) 146:779. doi: 10.1176/ajp.146.6.779
41. Valenca AM, Nardi AE, Nascimento I, Zin WA, and Versiani M. Respiratory panic disorder subtype and sensitivity to the carbon dioxide challenge test. Braz J Med Biol Res. (2002) 35:783–8. doi: 10.1590/S0100-879X2002000700004
42. Bouton ME, Mineka S, and Barlow DH. A modern learning theory perspective on the etiology of panic diorder. psychol Rev. (2001) 108:4–32. doi: 10.1037/0033-295X.108.1.4
43. Dixon LJ, Sy JT, Kemp JJ, and Deacon BJ. Does anxiety sensitivity cause panic symptoms? An experimental investigation. J Exp Psychopathol. (2013) 4:jep–027512. doi: 10.5127/jep.027512
44. Park G and Thayer JF. From the heart to the mind: Cardiac vagal tone modulates top-down and bottom-up visual perception and attention to emotional stimuli. Front Psychol. (2014) 5:278. doi: 10.3389/fpsyg.2014.00278
45. Gallagher MW, Payne LA, White KS, Shear KM, and Woods SW. Mechanisms of change in cognitive behavioral therapy for panic disorder: The unique effects of self-efficacy and anxiety sensitivity. Behav Res Ther. (2013) 51:767–77. doi: 10.1016/j.brat.2013.09.001
46. Barlow DH. Anxiety and its disorders: The nature and treatment of anxiety and panic. New York: Guildford Press (1988).
47. Barlow DH. Anxiety and its disorders: The nature and treatment of anxiety and panic. New York: Guilford press (2002).
Keywords: panic disorder, anxiety, diving response, cold facial immersion, carbon dioxide sensitivity
Citation: Kyriakoulis P and Caballero CL (2025) The implications of the diving response in altering carbon dioxide sensitivity as measured by changes in heart rate, respiration rate and psychological measures in panic disorder patients. Front. Psychiatry 16:1533019. doi: 10.3389/fpsyt.2025.1533019
Received: 22 November 2024; Accepted: 09 May 2025;
Published: 02 June 2025.
Edited by:
Steffen Schulz, Charité – Universitätsmedizin Berlin, GermanyReviewed by:
James Sackett, Cornerstone University, United StatesAmanda G. Harp, Centering Space, PLLC, United States
Pauliina A. Ahti, University of Jyväskylä, Finland
Copyright © 2025 Kyriakoulis and Caballero. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peter Kyriakoulis, cGV0ZXJAcG9zaXRpdmVwc3ljaG9sb2d5Lm5ldC5hdQ==; Catherine Lissette Caballero, Y2F0aHkuY2FiYWxsZXJvQGRlYWtpbi5lZHUuYXU=
 Peter Kyriakoulis
Peter Kyriakoulis Catherine Lissette Caballero
Catherine Lissette Caballero