- 1School of Acupuncture and Tuina, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 2Department of Rheumatology and Immunology, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, China
Background: Several neuroimaging studies have confirmed that acupuncture can elicit alterations in brain networks and regions associated with depressive disorder (DD). This review provides an overview of the methodologies and results of neuroimaging investigations into the efficacy of acupuncture in treating DD, with the intention of guiding future research objectives.
Methods: Neuroimaging studies of acupuncture for DD being published between February 2, 2014 and February 2, 2024, were gathered from PubMed, Cochrane Library, EMBASE, Web of Science, China National Knowledge Infrastructure, Chongqing VIP Database, WanFang Database, and Chinese Biomedical Literature Database. The methodological quality of the studies was assessed utilizing the Risk of Bias 2.0 and Risk of Bias in Non-Randomized Studies of Interventions tools. Following a qualitative analysis of the studies, relevant information regarding acupuncture interventions and brain imaging data was extracted.
Results: A total of 26 studies met the inclusion criteria. These studies featured a combined sample size of 1138 participants. All studies employed magnetic resonance imaging. Our findings indicate that acupuncture can affect neural activity in the cingulate gyrus, precuneus, insula, prefrontal lobe, etc. The neuroimaging results of most DD patients were correlated with the Hamilton Rating Scale for Depression scores.
Conclusions: The results of the current study indicate that acupuncture treatment may have a regulatory effect on the abnormal functioning of neural regions and networks in individuals diagnosed with DD. These networks are predominantly localized within various brain regions, including the default mode network, limbic system, emotion regulation and cognitive network, reward network, central executive network, salience network, and sensorimotor network. It is essential to conduct additional high-quality and multimodal neuroimaging research to expand upon these findings and elucidate the mechanisms by which acupuncture impacts patients with DD.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42023400557.
1 Introduction
According to the World Health Organization, 300 million individuals have been diagnosed with depressive disorder (DD) (1, 2). Although the general incidence of DD is rising, the increase is particularly severe among those aged 13–18 years (3). The prevalence of DD in this population reportedly ranges between 1.5 and 3, with females surpassing males (4). By 2030, DD is projected to become the leading aggravator of disease-related economic and medical burden to patients, their families, and the communities in which they live (5). DD is characterized by severely diminished motivation, the propensity to anger, limited attention, insomnia or drowsiness, fatigue, self-deprecation, and continual suicidal ideation (6, 7).
At present, antidepressants are still the main clinical treatment for DD (8). Although antidepressants are generally effective, they also have certain limitations, including side effects after long-term use, drug resistance, and recurrence (9, 10). Or even worse, antidepressants do not have a relieving effect and instead exacerbate emotional or physical symptoms in some patients, increasing the risk of comorbidity (11, 12). Therefore, it is necessary to find greener, safer, and more effective alternative treatments to improve efficacy and safety.
An increasing body of evidence indicates that acupuncture, as a highly effective non-pharmacological therapy, can significantly improve both the physical and psychological symptoms of patients with DD (13–15). By stimulating specific acupoints, acupuncture engages in multi-level, multi-target regulation, and has been widely utilized in the treatment of DD (16, 17). However, the precise mechanisms underlying acupuncture’s therapeutic effects on depression remain scientifically unclear. With advances in medical imaging techniques, brain imaging-based neuroimaging technologies have become invaluable tools for exploring the mechanisms of acupuncture in treating depression. Neuroplasticity changes play a crucial role in the pathogenesis of DD (18). Alterations in brain neural circuits are direct contributors to the depressive symptoms observed in DD patients (19). Neuroimaging studies have found that the onset of DD is associated with structural and functional abnormalities in specific brain regions involved in emotions and cognition, including the cingulate cortex, prefrontal cortex, amygdala, hippocampus, insula, and striatum (20–24). In resting-state functional magnetic resonance imaging (fMRI), significant changes in these brain regions and nuclei have been observed in individuals with depression, and acupuncture has been found to influence these alterations (25). These brain regions participate in neural networks such as the default mode network (DMN), limbic system, emotion regulation and cognitive network, reward network, and salience network (SN) (26–28). Notably, alterations within and between the DMN, and SN have been highlighted as key findings in many imaging studies on DD (29). Some research suggests that acupuncture may alleviate DD by enhancing the neuroplasticity of brain circuits (15). Furthermore, studies have confirmed that electroacupuncture can modulate the functional activity of the DMN, sensorimotor network, emotion regulation and cognitive network, limbic system, and visual network, thereby exerting therapeutic effects (30).
While brain imaging has been used to provide visual evidence for the benefits that acupuncture affords patients with DD and has helped to elucidate central regulatory mechanisms underpinning these healthful effects, the studies that have collected this evidence were subject to methodological limitations, including their chosen methods of intervention, brain imaging, and analysis. This systematic review seeks to analyze and assess recent neuroimaging studies on the impact of acupuncture on patients with DD in order to identify specific central targets and brain networks influenced by acupuncture therapy. The findings aim to serve as a valuable resource for further clinical research and practice.
2 Methods
2.1 Registration
This review was performed in accordance with the standards set forth in the Cochrane Handbook for Systematic Reviews of Interventions and followed the Preferred Reporting Items for Systematic Review guidelines (31, 32). The registered study protocol for this systematic review was published in PROSPERO (registration number: CRD42023400557).
2.2 Search strategy
The following eight databases were searched for studies published between 2 February 2014 and 2 February 2024: PubMed, Cochrane Library, EMBASE, Web of Science, China National Knowledge Infrastructure, Chongqing VIP Database, WanFang Database, and Chinese Biomedical Literature Database. We restricted our search to reports published in English or Chinese. The search strategy combined the main keywords: neuroimaging, acupuncture, and depressive disorder. The strategy used to search PubMed is presented in Table 1; this strategy was modified to optimize searches through other databases. To find articles not detected in database searches, the authors (YO and LL) also searched the reference lists of relevant primary and review articles.
2.3 Eligibility criteria
1. Participants: patients that were diagnosed with DD; the search was not restricted according to DD type.
2. Interventions: the acupuncture and moxibustion methods mainly include 1) manual acupuncture (MA); 2) percutaneous acupoint stimulation (transcutaneous electrical acupoint stimulation, TEAS), the TEAS mainly includes electroacupuncture (EA) and percutaneous acupoint electric nerve stimulation (transcutaneous electrical nerve stimulation, TENS) (33).
3. Comparisons: healthy controls (HC), placebo or sham acupuncture.
4. Study design: randomized and non-randomized controlled trials of using acupuncture to treat patients with DD.
5. At least one neuroimaging approach should have been used: structural magnetic resonance imaging (sMRI), functional magnetic resonance imaging (fMRI), functional near-infrared spectroscopy (fNIRS), positron emission tomography (PET), etc.
All neuroimaging clinical studies on the effects of using acupuncture to treat DD were eligible for inclusion. We excluded the experimental models, conference abstracts, case reports, protocols, animal studies, monographs, reviews, and studies lacking neuroimaging data.
2.4 Study selection and data extraction
The data extraction adhered to the Standards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA) guidelines. Details on the acupuncture interventions used in the reviewed studies were collected based on these guidelines. Two investigators (LL and PZ) independently extracted data from the included studies using a self-defined standardized extraction format. The extracted data included the name of the primary investigator, publication year, DD type, patient age, the country in which the study was performed, diagnosis, study design, type of acupuncture intervention, type of control intervention, the sample size of each intervention group, intervention duration, clinical variables, imaging modality, analysis methods, and brain imaging data. Any disagreements were solved by consensus between the two investigators. In the event that a consensus could not be reached, a third investigator (DP) made the final judgment. When information was missing from a study, the corresponding author was contacted if the information necessary for correspondence was available.
2.5 Risk-of-bias assessment
A Microsoft Excel template based on the Cochrane Handbook guidelines (available from https://www.riskofbias.info/) was used to independently assess the risk of bias in the extraction performed by two researchers (DL and YO). The risk of bias 2.0 tool (RoB 2) and the risk of bias in non-randomized studies of interventions tool (ROBINS-I) were used to evaluate the risk of bias of included randomized controlled trials (RCTs) and non-RCTs, respectively (34, 35). If disagreements were not resolved between the two reviewers, a third reviewer (SY) participated in the discussion until a consensus was reached.
2.6 Statistical analysis
Due to significant methodological differences among the included studies, we used descriptive statistical analysis to identify significant acupuncture-induced brain alterations in patients with DD as reported in the literature.
3 Results
3.1 Search results
A total of 817 potentially eligible articles were identified. After removing 165 duplicates and screening the 652 remaining articles, 139 candidate studies were isolated. Review of the full text of each shortlisted study based on the eligibility criteria excluded 113 more studies. Hence, 26 studies were included in this systematic review (25, 36–60) (Figure 1).
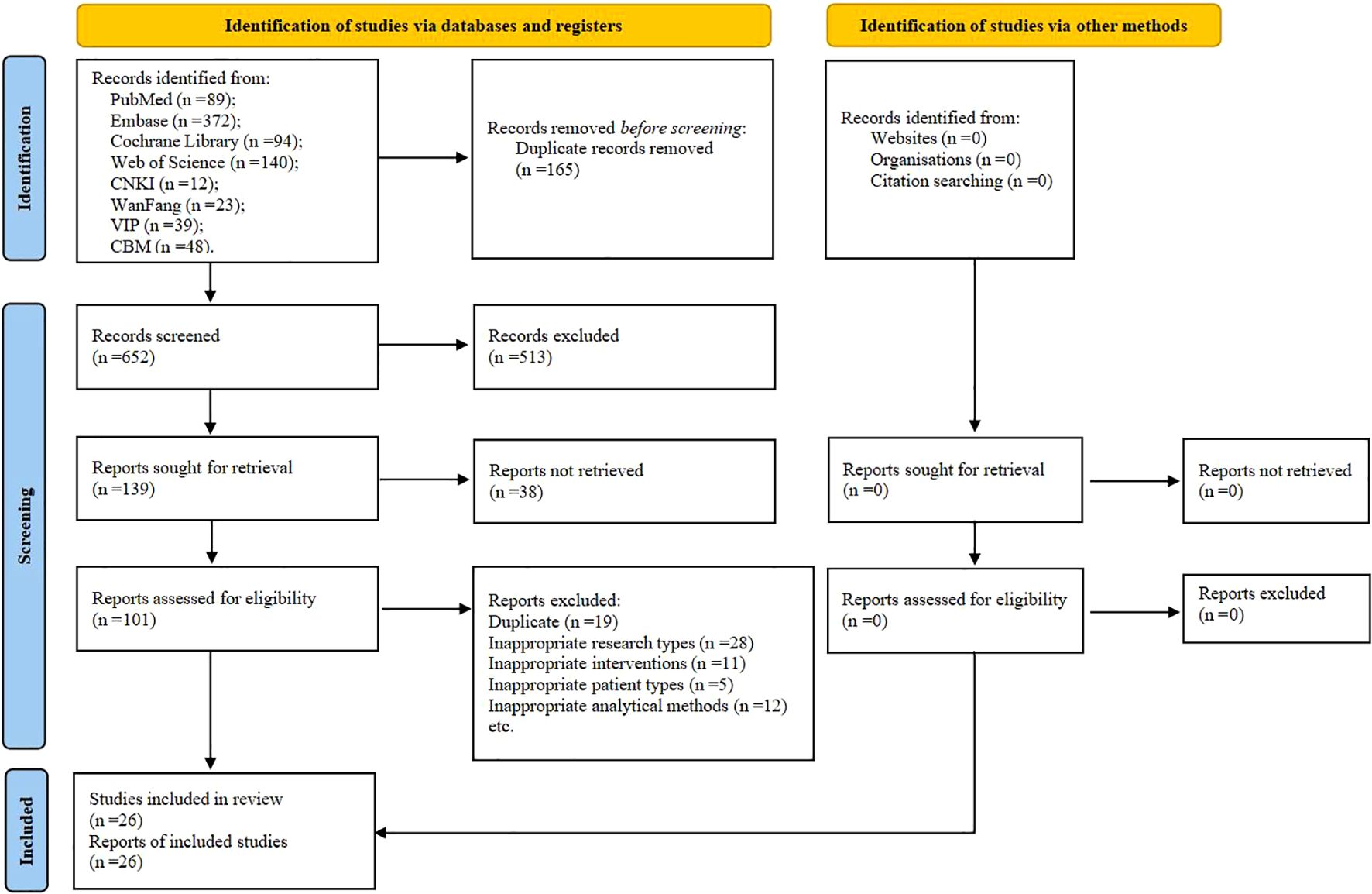
Figure 1. The PRISMA flow chart of selection process. CNKI, China National Knowledge Infrastructure; VIP, Chongqing VIP Database; CBM, Chinese Biomedical Literature Database.
3.2 Study characteristics
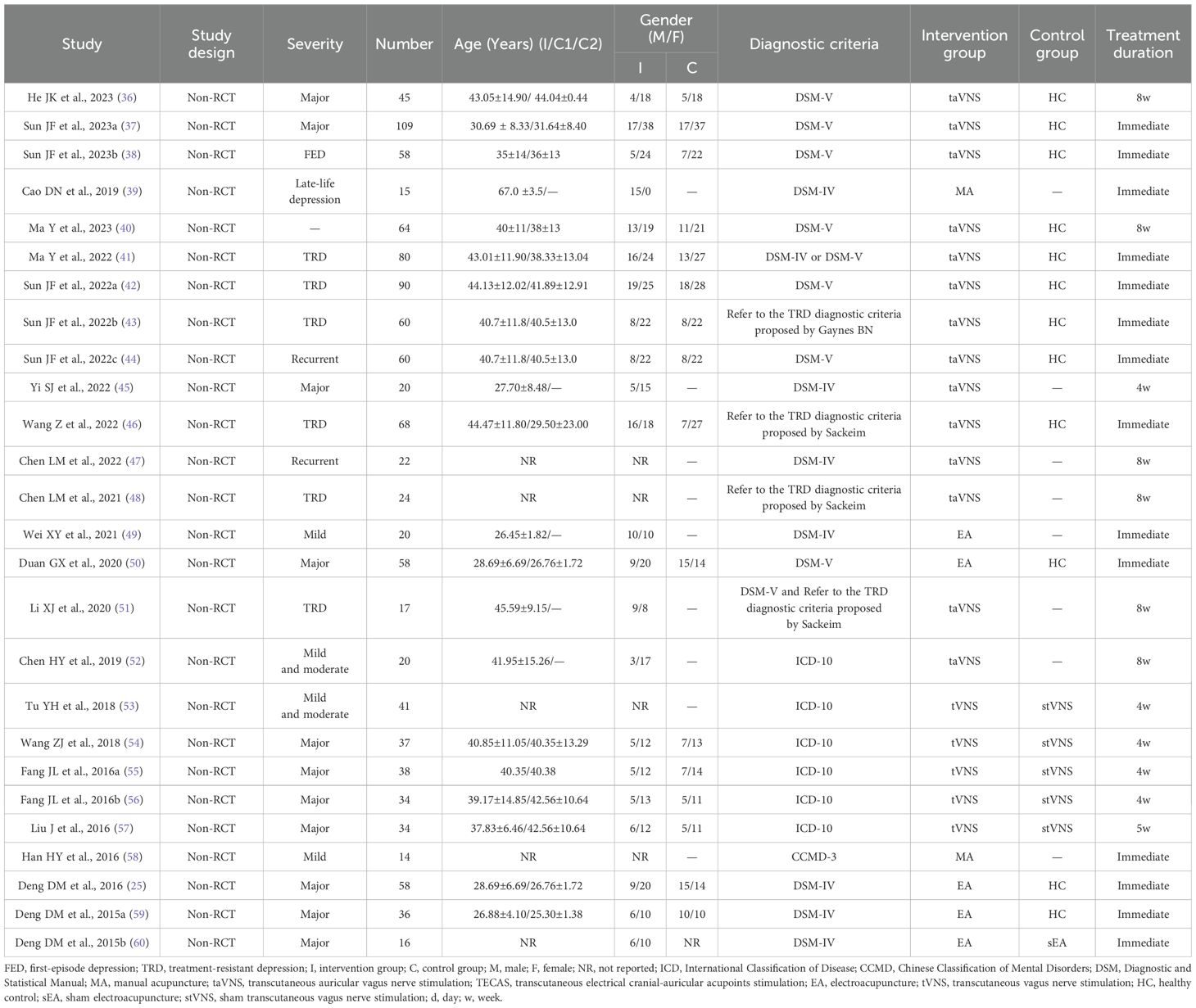
The main characteristics of the included studies are shown in Table 2. The 26 studies (25, 36–60) included 1138 participants, with a total of 742 DD patients and 396 HCs. All studies were non-RCTs and came from China.
Regarding the diagnostic criteria for DD, 16 studies used the DSM-IV or DSM-V criteria (25, 36–42, 44, 45, 47, 49–51, 60), four studies referred to the TRD diagnostic criteria proposed by Sackeim (43, 46, 48, 51), ICD-10 was used in six studies (52–57), and CCMD-3 was used in one study (58).
In terms of DD types, MDD was diagnosed in 11 studies (25, 36, 37, 45, 50, 54–57, 59, 60), first-episode depression was diagnosed in one study (38), late-life depression was diagnosed in one study (39), treatment-resistant depression was diagnosed in six studies (41–43, 46, 48, 51), recurrent depression was diagnosed in two studies (44, 47), mild and/or moderate depression was diagnosed in four studies (49, 52, 53, 58), and the remaining were not specified.
All included studies used at least one of the following acupuncture techniques: transcutaneous auricular vagus nerve stimulation (taVNS) (36–38, 40–48, 51, 52), transcutaneous vagus nerve stimulation (tVNS) (53–57), EA (25, 49, 50, 59, 60), MA (39, 58). 12 studies (25, 36–38, 40–44, 46, 50, 59) used HCs to populate a control group, six (53–57, 60) used sham-acupuncture group as their controls, and the remaining were not specified.
In terms of the selection of stimulation sites, the auricular points for the heart and kidney (38, 40, 43, 44, 46–48, 51, 52), the auricular concha area (36, 37, 41, 42, 45, 53–57), and Baihui (GV20) (25, 49, 50, 59, 60) were selected by most studies. Most studies observed acupuncture retention times of 20–30 min.
3.3 Clinical variables
To evaluate depressive disorder, 17 studies used the Hamilton Rating Scale for Depression (HAMD) series scale. The 17-item HAMD was used in 12 (36–38, 40, 41, 43, 45–48, 51, 52), the 24-item HAMD in four (53, 55–57), and an unspecified version of the HAMD in one (54). Four studies used the 17-item hamilton depression rating scale (HDRS-17) (25, 45, 49, 50). 11 studies evaluated their patients using the Hamilton Anxiety Rating Scale (HAMA). The 14-item HAMA was used in five (36, 40, 43, 46, 53), and the HAMA in six (38, 41, 47, 55–57). Nine studies used the Self-Rating Depression Scale (SDS) and the Self-Rating Anxiety Scale (SAS) (25, 45–47, 50, 53, 55–57). One study used the ruminative response scale (RRS) (47). Two studies used the wisconsin card sorting test (WCST) (36, 40). One study used Visual Analysis Scale (VAS) (49).
3.4 Imaging modality and analysis
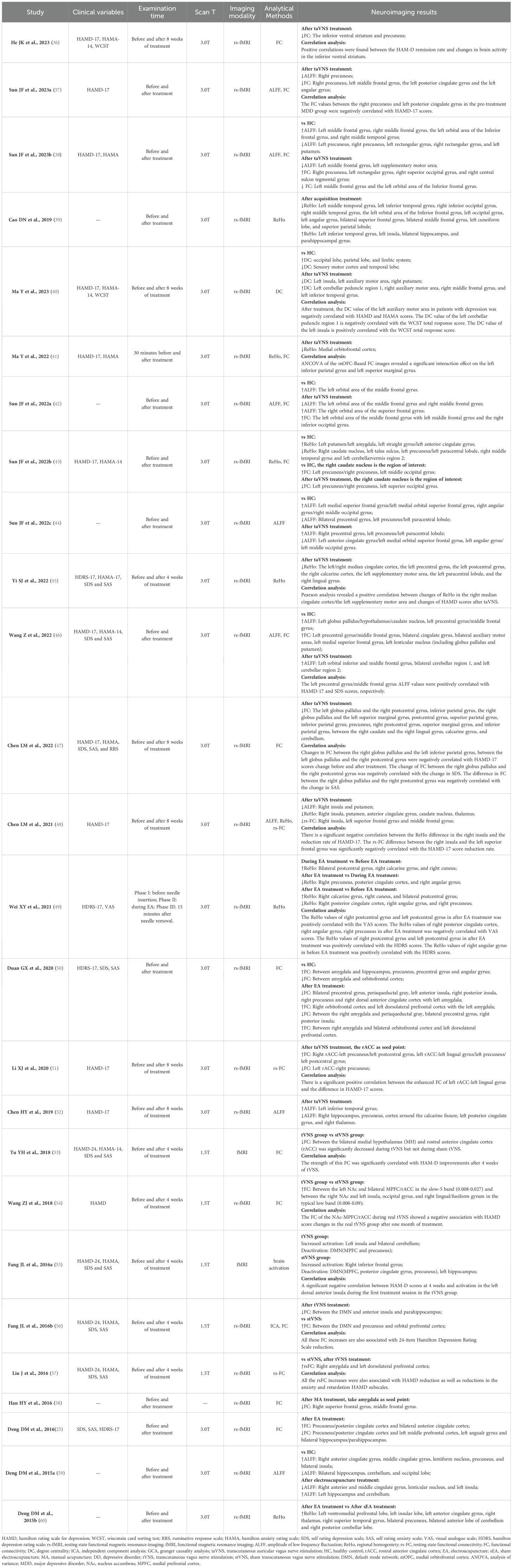
All 26 studies used magnetic resonance imaging (MRI) to explore neural changes following the administration of acupuncture (Table 3). Of the 26 studies that evaluated changes in neuronal activity, 24 used resting state functional magnetic resonance imaging (rs-fMRI) (25, 36–52, 54, 56–60) and two studies applied functional magnetic resonance imaging (fMRI) (53, 55) to evaluate the amplitude of low frequency fluctuation (ALFF), functional connectivity (FC), regional homogeneity (ReHo), resting state functional connectivity (rs-FC), independent component analysis (ICA), degree centrality (DC), and brain activation.
3.5 Results of risk of bias assessment
Of the 26 non-RCTs (25, 36–60), 11 had a “Low” overall risks of bias (38–41, 43, 44, 46, 52, 53, 59, 60). The remaining 15 had “Serious” risks of bias due to participant dropout (25, 36, 37, 42, 45, 47–51, 54–58). A summary of the risk of bias assessment of each included study is presented in Table 4.
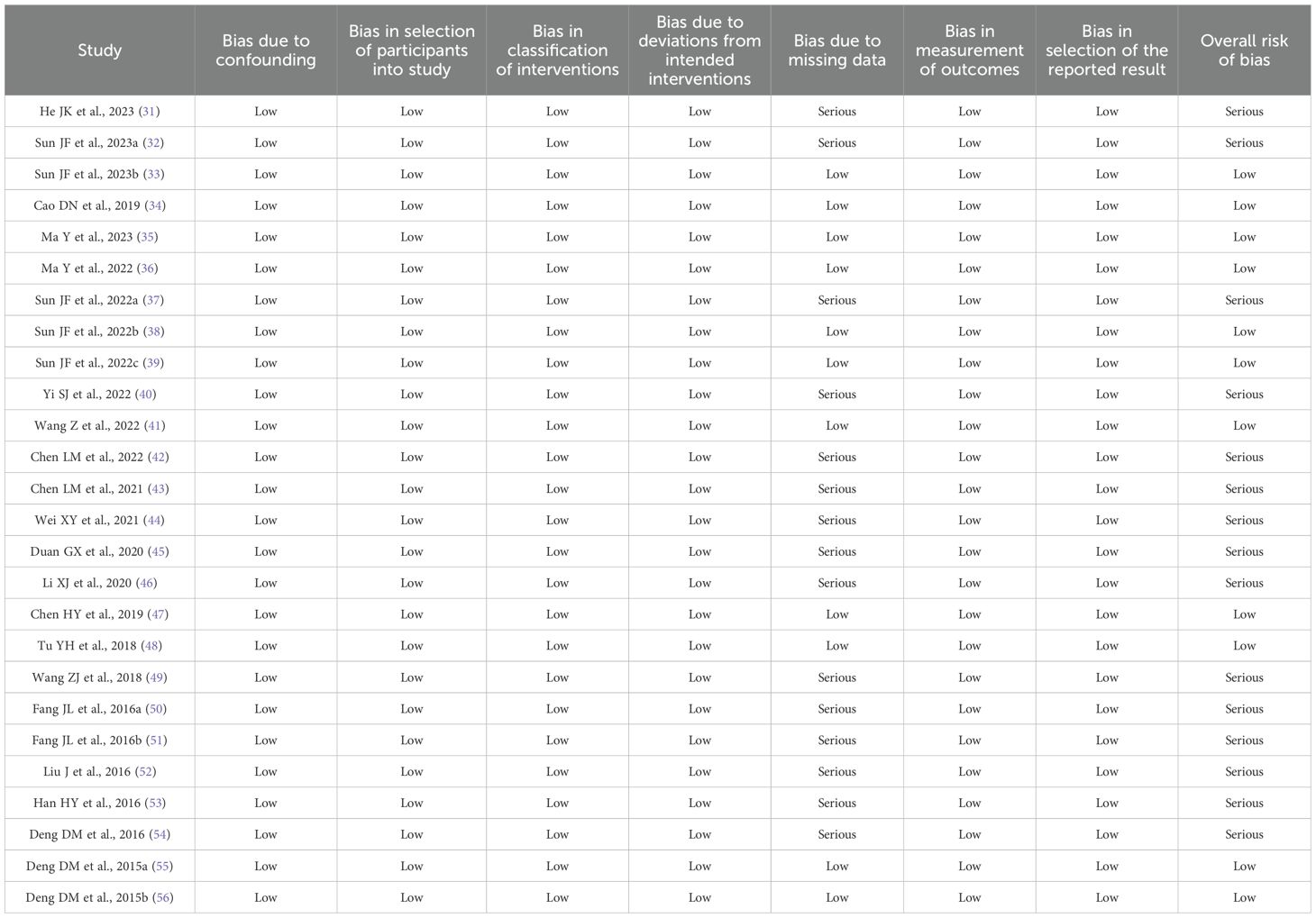
Table 4. The results of risk of bias assessment of non-randomised controlled trials by the ROBINS-I.
3.6 Brain imaging data
3.6.1 Brain alterations of DD
Compared with HCs, patients with DD exhibited aberrant activity in the precuneus (25, 36–38, 43, 44, 50, 59), cingulate gyrus (37, 43, 44, 46, 50, 59), middle frontal gyrus (37, 38, 40, 42, 46), cerebellum (40, 43, 46, 59), and angular gyrus (25, 37, 44, 50).
A correlation analysis was conducted of pre-treatment neural activity associated with DD (37). The FC values between the right precuneus and left posterior cingulate gyrus in the pre-treatment MDD group were negatively correlated with HAMD-17 scores.
3.6.2 Acupuncture-related brain alterations in patients with DD
In the 14 studies using transcutaneous auricular vagus nerve stimulation (taVNS) (36–38, 40–48, 51, 52), the most common neural changes in patients with DD following the intervention were observed in the precuneus (36–38, 43, 44, 47, 51, 52), cingulate gyrus (37, 43, 44, 46, 48, 51, 52), middle frontal gyrus (37, 38, 40, 42, 46, 48), putamen (38, 40, 43, 46, 48), and cerebellum (40, 43, 46, 47).
Of the five studies that used transcutaneous vagus nerve stimulation (tVNS) (53–57), the most frequent neural changes in patients DD patients following the intervention were observed in the prefrontal lobe (54–57), insula (54–56), cingulate gyrus (54, 55), precuneus (55, 56).
In the five studies that employed EA (25, 49, 50, 59, 60), the most common neural changes in patients with DD following the intervention were observed in the precuneus (25, 49, 50, 59, 60), cingulate gyrus (50, 59, 60), insula (50, 59, 60), prefrontal lobe (25, 50, 60), angular gyrus (25, 49, 50), hippocampus (25, 50, 59).
Two studies found that the superior frontal and middle frontal gyri of patients with DD exhibited changes following the administration of MA (39, 58).
3.6.3 Correlation between the neuroimaging results and clinical variables
The neuroimaging findings in most patients with DD were correlated with their HAMD scores. One study found that positive correlations were found between the HAM-D remission rate and changes in brain activity in the inferior ventral striatum (36). Another investigation found that the FC values between the right precuneus and left posterior cingulate gyrus in the pre-treatment MDD group were negatively correlated with HAMD-17 scores (37). Another study found that after treatment, the DC value of the left auxiliary motor area in patients with depression was negatively correlated with HAMD scores (40). A study (45) reported Pearson analysis revealed a positive correlation between changes of ReHo in the right median cingulate cortex/the left supplementary motor area and changes of HAMD scores after taVNS. One study found that the left precentral gyrus/middle frontal gyrus ALFF values were positively correlated with HAMD-17 scores, respectively (46). Another trail found that changes in FC between the right globus pallidus and the left inferior parietal gyrus, between the left globus pallidus and the right postcentral gyrus were negatively correlated with HAMD-17 scores change before and after treatment (47). Another study reported there is a significant negative correlation between the ReHo difference in the right insula and the reduction rate of HAMD-17, the rs-FC difference between the right insula and the left superior frontal gyrus was significantly negatively correlated with the HAMD-17 score reduction rate (48). Another study found that there is a significant positive correlation between the enhanced FC of left rACC-left lingual gyrus and the difference in HAMD-17 scores (51). Another study reported the strength of this FC was significantly correlated with HAM-D improvements after 4 weeks of tVNS (53). Another study found that the FC of the NAc-MPFC/rACC during real tVNS showed a negative association with HAMD score changes in the real tVNS group after one month of treatment (54). A study reported a significant negative correlation between HAM-D scores at 4 weeks and activation in the left dorsal anterior insula during the first treatment session in the tVNS group (55). Another study found that all the rsFC increases were also associated with HAMD reduction as well as reductions in the anxiety and retardation HAMD subscales (57).
In addition, the neuroimaging results of patients with DD were found by several studies to be related to HDRS, HAMA, SDS, SAS, VAS, and the WCST total response scores (Table 3).
3.6.4 Neural regions most affected by acupuncture in patients with DD
Acupuncture-induced brain changes in patients with DD were mainly observed in the cingulate gyrus (25, 37, 40, 43–46, 48–55, 59, 60), precuneus (25, 36–38, 43, 44, 47, 49–52, 55, 56, 59, 60), insula (39, 40, 48, 50, 54–56, 59, 60), prefrontal lobe (25, 41, 50, 54–57, 60), middle frontal gyrus (37–40, 42, 46, 48, 58), cerebellum (40, 43, 46, 47, 55, 59, 60), hippocampus (25, 39, 40, 50, 52, 55, 59), putamen (38, 40, 43, 46, 48, 59), angular gyrus (25, 37, 39, 44, 49, 50), superior frontal gyrus (39, 44, 46, 48, 58), lingual gyrus (45, 47, 51, 54), precentral gyrus (44–46, 50), postcentral gyrus (45, 47, 49, 51), thalamus (40, 48, 52, 60), parahippocampal gyrus (25, 39, 40, 56), and amygdala (40, 43, 50, 57).
4 Discussion
4.1 Study characteristics of the included studies
As far as we know, as the latest and most comprehensive systematic review, this review summarizes the neuroimaging research on acupuncture treatment of DD in the past decade. It included 742 patients with DD and 396 HCs.
In terms of study design, this review evaluated a total of 26 non-RCTs from China, with sample sizes ranging from 14 to 109 individuals per study. None of the studies mentioned randomization and allocation concealment, and only three studies involved blinding (blinding participants) (54, 56, 57). According to the bias risk assessment results of non-RCTs, 15 studies had “Serious” risks of bias due to participant dropout (25, 36, 37, 42, 45, 47–51, 54–58). In addition, RCTs are the gold standard for comparing intervention effects and effectiveness in medical research design (61). Therefore, in order to reduce the risk of bias and enhance the credibility of the study, more careful design is needed in future research on random methods and allocation concealment, blinding, sample size evaluation, and processing of subject dropout data.
In terms of intervention details, the involved acupuncture methods include taVNS, tVNS, EA and MA, which can change the function of brain regions related to DD patients, thus alleviating their mental and physical symptoms. In addition, taVNS, tVNS and EA seem to be the most commonly used acupuncture techniques in the included studies. The auricular points of the heart and kidney, auricular concha, and Baihui (GV20) are the most frequently used stimulation sites. This may be because the surface of the ear is distributed with the afferent vagus nerve, and the surface distribution of the auricular concha or auricular points and the vagus nerve ear branch mostly overlap (62). The anatomical pathway of the vagus nerve is connected to many brain regions related to emotion regulation, such as the amygdala, hippocampus, cingulate gyrus, prefrontal lobe, and insula (63). Therefore, acupuncture stimulation of the auricle or auricular points can activate the vagus nerve pathway, regulate the above brain regions, and alleviate depressive symptoms. Furthermore, vagus nerve stimulation is associated with the neural plasticity of brain networks. Vagus nerve stimulation can enhance the neural plasticity of related brain regions such as the hippocampus to regulate the emotional and cognitive functions of DD (64). In addition, the onset of DD has been associated with reduced levels of monoamine neurotransmitters such as 5-hydroxytryptamine (5-HT) and dopamine (DA) in the central nervous system (65), as well as cytokines such as interleukin-6 (IL-6) and IL-1β (66). Anti-inflammatory cytokines, including IL-10 and IL-4, may exert antidepressant effects (67). Acupuncture stimulation of GV20 can reduce serum concentrations of IL-6 and IL-1β in patients with DD, while increasing the serum levels of IL-10 and IL-4 (68, 69), as well as the expression of 5-HT and DA in the hippocampus and hypothalamus (70). Overall, molecular changes help to attenuate the depressive state. However, the included research lacks the comparison of different acupuncture methods and stimulation sites, so it is impossible to recommend the preferred acupuncture techniques and stimulation sites for clinical practice. We hope that future research can pay attention to this.
In terms of control setting, 12 studies (25, 36–38, 40–44, 46, 50, 59) compared patients with HC and DD, aiming to explore the neuropathological characteristics of DD. It was found that patients with DD exhibited neuroimaging abnormalities in certain brain regions, such as the precuneus, cingulate gyrus, middle frontal gyrus, cerebellum, and angular gyrus. Six trails (53–57, 60) used the sham acupuncture group as a control to exclude any changes caused by perception of receiving real acupuncture. This study found that real acupuncture could activate more relevant brain regions in patients with DD than sham acupuncture, and could increase the correlation with clinical variables.
In terms of the outcome measures, the included studies mainly used the HAMD series of scales to evaluate outcome indicators. The HAMD series of scales have good reliability and validity in the diagnosis of DD, and can be used to evaluate the severity and functional impact of the disease (71). Furthermore, the included studies revealed a significant correlation between HAMD scores and neuroimaging findings in the majority of patients with DD. These findings were observed in various brain regions, including the insula, precentral gyrus, cingulate cortex, precuneus, prefrontal cortex, and middle frontal gyrus. These regions are primarily situated in neural circuits such as the DMN, central executive network (CEN), and reward network. These correlations suggest that acupuncture treatment may regulate brain regions associated with emotional regulation, providing evidence for its therapeutic effects. Additionally, increased functional connectivity in regions related to emotional regulation may help clinicians identify biomarkers that predict treatment response, which could guide personalized treatment plans and enhance the effectiveness of acupuncture in treating depression. Finally, by understanding the underlying neuromechanisms of acupuncture effects, clinicians may be better equipped to use acupuncture as an adjunctive treatment for patients with depression, especially those who are resistant to conventional therapies.
In the imaging techniques mentioned, all of the studies included employed fMRI to reveal the brain activity of depression patients following acupuncture treatment. This preference is likely due to the numerous benefits of fMRI, such as being non-invasive, free from radiation, offering high spatial resolution, and its ability to perform both functional and morphological imaging simultaneously (33). Regarding analytical approaches, the majority of studies utilized ALFF and/or FC. ALFF is a method that reflects spontaneous neural activity at the voxel level and provides insight into the level of intrinsic brain activity during rest (72). FC, based on predefined regions of interest (ROIs) in resting-state fMRI, extracts the BOLD signal time series for each voxel within the ROI and computes the correlation with time series from other brain areas (73). Studies have indicated that combining ALFF and FC offers a more thorough understanding of the central mechanisms underlying acupuncture’s effects in treating depression (37, 74), and it is hoped that future research will focus on this.
4.2 Neuroimaging changes following the treatment of DD with acupuncture
4.2.1 Functional neural abnormalities associated with DD
12 of the reviewed studies reported that patients with DD exhibited altered neural function when compared with HCs. The variations in ReHo, FC, and ALFF were primarily observed across different networks of the brain, including the DMN, CEN, and limbic systems. Brain regions such as the precuneus, amygdala, cingulate gyrus, precentral gyrus, superior frontal gyrus, pallidum, putamen, caudate nucleus, prefrontal cortex, and hippocampus were all found to be affected by DD. These findings agree with previous reports that the most significant neural differences between patients with DD and HCs are mainly distributed in the DMN and limbic system (43, 75). These neuroimaging results may inform potential targets for future studies exploring acupuncture-induced brain alterations in patients with DD.
4.2.2 Acupuncture-related neural changes in patients with DD
Acupuncture-induced changes in the brain regions of individuals with DD were primarily observed in the cingulate gyrus, precuneus, insula, prefrontal lobe, middle frontal gyrus, cerebellum, putamen, angular gyrus, superior frontal gyrus, lingual gyrus, precentral gyrus, postcentral gyrus, thalamus, parahippocampal gyrus, amygdala, and caudate nucleus. Its main neuroimaging results are as shown in the Table 3.
The cingulate gyrus and amygdala belong to the limbic system and are heavily involved in emotional processing, learning, and memory (76). The precuneus plays an important role in the processing of self-related information and situational memory (77). The insula is responsible for the normal coordination of the cognitive and emotional systems and has been implicated in the onset of DD (78). The dorsolateral prefrontal cortex includes the superior frontal and middle frontal gyri, participates in cognitive and control functions, and can contributes to emotional regulation (79–81). The cerebellum is thought to be related to emotional experience, as abnormal cerebellar function can impair the ability to experience positive emotions (82). The putamen and caudate nucleus are important components of the corpus striatum, which functions as the core structure of the reward network. Abnormal corpus striatal function can impair neural reward mechanisms in patients with DD (83). Highly sensitive to stress and a key structure for emotional regulation, the hippocampus is associated with the onset of DD (84). The angular gyrus is responsible for the semantic processing, digital processing, attention, and memory of the human body (85). The thalamus is a relay station for various kinds of sensory information and participates in coordinating memory, emotions, and language (86). The parahippocampal gyrus forms part of a pathway that provides multiple types of perceptual information to the hippocampus. The abnormal functioning of either the parahippocampal gyrus or the hippocampus itself could affect various cognitive functions and thus cause or aggravate negative emotions (87, 88). Functional abnormalities in the precentral and postcentral gyri may induce somatic and depressive symptoms in patients with DD (89).
The aforementioned brain regions are mainly distributed across the DMN, limbic system, emotional and cognitive regulation network, and reward network. Brain networks such as the CEN, SN, and sensorimotor network (SMN) are also involved in mediating the effects of acupuncture on patients with DD (Figure 2). Comprised of the prefrontal cortex, cingulate gyrus, precuneus, angular gyrus, superior frontal gyrus, and hippocampus, the DMN is responsible for generating and processing introspective thoughts and participates in emotional regulation (90, 91). The emotion regulation and cognitive networks consist of the limbic system, prefrontal cortex, corpus striatum, and thalamus, as well as their connections to the amygdala, hippocampus, prefrontal cortex, caudate nucleus, cingulate gyrus, thalamus, and other brain regions (92). The reward network plays an important role in the pathogenesis of DD (93). Though the cortical-basal ganglia circuit functions as the core of the reward network, the cingulate cortex, prefrontal cortex, and corpus striatum are key structures in the network, and their interaction with the amygdala, hippocampus, and thalamus is essential to regulating the reward network (83). The SN includes the cingulate cortex, insular lobe, and amygdala; all these regions feature strong connections to the limbic system. The CEN is comprised of the prefrontal cortex, cingulate cortex, and precentral gyrus among other brain regions. Both the SN and CEN play important roles in emotion and cognition (94). Composed of the postcentral gyrus, precentral gyrus, and cingulate gyrus, the SMN plays an important role in the onset of somatic and depressive symptoms associated with DD.
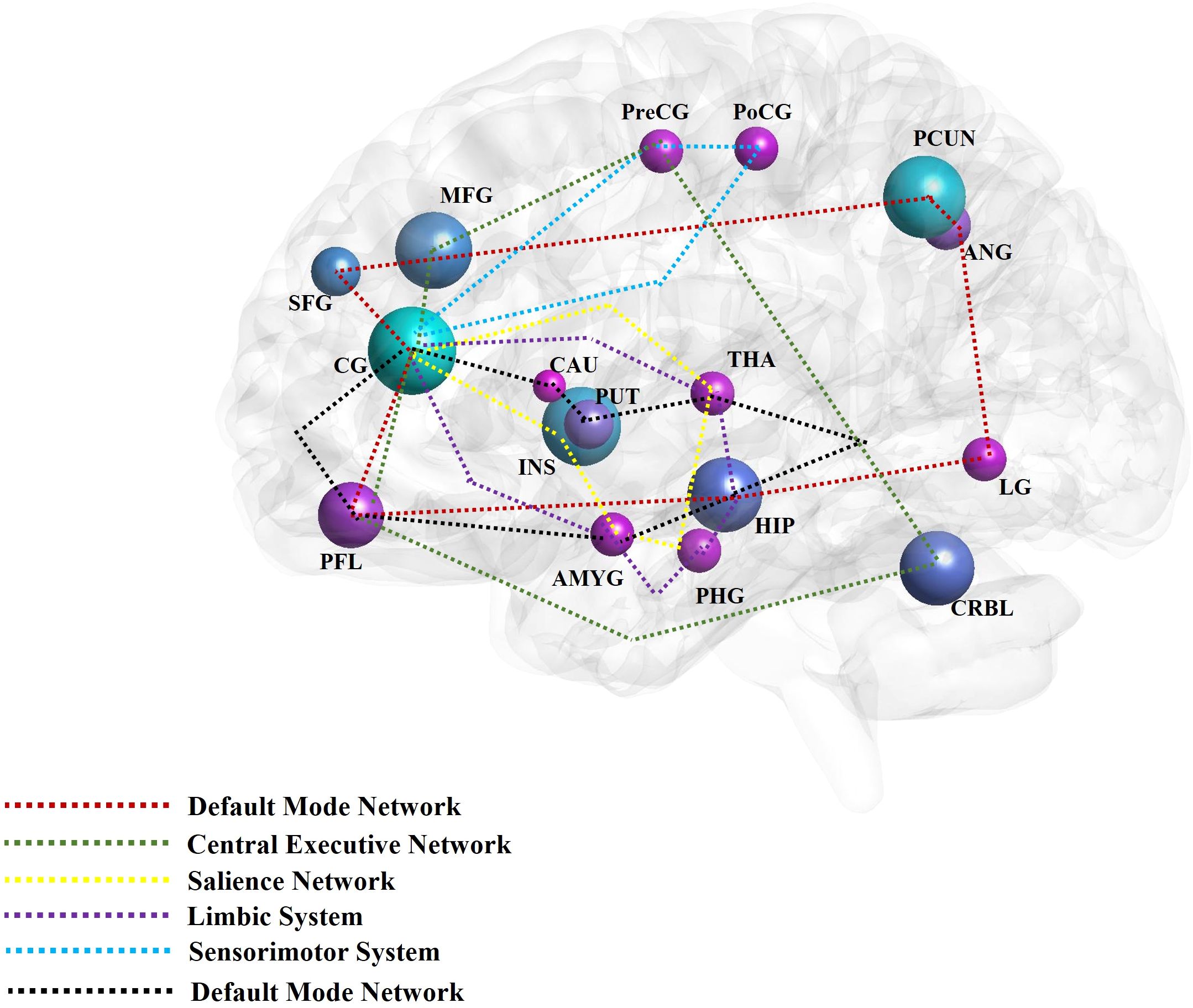
Figure 2. The main findings of acupuncture for depressive disorder by neuroimaging techniques. The high frequent reported brain regions that have been affected by acupuncture for depressive disorder in the included studies were noted with different colors. The different color circuits represent the different pathways or networks. CG, cingulate gyrus; PCUN, precuneus; INS, insula; MFG, middle frontal gyrus; PFL, prefrontal lobe; CRBL, cerebellum; HIP, hippocampus; PUT, putamen; ANG, angular gyrus; SFG, superior frontal gyrus; LG, lingual gyrus; PreCG, precentral gyrus; PoCG, postcentral gyrus; THA, thalamus; PHG, parahippocampus gyrus; AMYG, amygdala; CRBL, cerebellum; CAU, caudate nucleus; MOG, middle occipital gyrus; ORBf, orbitofrontal cortex.
In summary, the alterations in brain activity observed in individuals with DD following acupuncture treatment are marked by collaborative disruptions across various brain networks and regions. Subsequent investigations into the association between acupuncture and DD ought to prioritize elucidating the fundamental mechanisms through which acupuncture may mitigate DD symptoms. These inquiries should emphasize a thorough examination of the aforementioned brain regions and networks, as well as their interconnections. For example, studies have shown that acupuncture can significantly regulate the activity and connectivity of extensive neural networks to alleviate depressive symptoms. This is manifested by a direct or indirect reduction in the connectivity between the posterior DMN and the emotional and reward networks, while enhancing the connections between the anterior and posterior DMN, the anterior DMN and the CEN, and the CEN and the emotional and reward circuits (29).
4.3 Strengths of the present review
Prior systematic reviews have primarily concentrated on assessing the effectiveness and safety of acupuncture as a treatment for DD. Although a systematic review of magnetic resonance imaging studies on acupuncture therapy in depression has been published (95). However, compared to this review, the above study, 1) The search was limited to four pertinent electronic databases, potentially resulting in incomplete data retrieval and the exclusion of some eligible research. 2) The data retrieval timeframe extends until October 20, 2020, with three years having elapsed since that date. It is conceivable that recent neuroimaging studies on the efficacy of acupuncture treatment for DD have not been adequately considered. 3) Acupuncture intervention measures such as taVNS and tVNS are excluded, and incomplete intervention details may also lead to incomplete retrieval. 4) Moreover, it only focuses on the changes of some brain regions’ functions caused by acupuncture, but does not summarize the relevant brain networks, which cannot intuitively present the central mechanism of acupuncture’s effect on depression.
In order to offer a comprehensive and up-to-date examination of the topic, this review will redirect its attention to neuroimaging studies on the efficacy of acupuncture in the treatment of DD. Additionally, this study will provide a detailed analysis of the neural activity alterations induced by acupuncture in individuals with DD. Moreover, it considers the association between these findings and clinical parameters with the aim of establishing a framework for future investigations into the neural mechanisms underlying a specific acupuncture technique for treating DD.
4.4 Limitations
Although this study is the latest and most comprehensive neuroimaging review on acupuncture treatment of DD, the following limitations still exist. Firstly, differences were found between the included studies in their methods of intervention: specifically, in their needle retaining time, intensity, and frequency. As a further example, while most studies discussed the VNS- and EA-induced brain changes in patients with DD, the waveform was mainly administered as a density wave with a frequency and intensity of 4–5/20 Hz and 4–8 mA, respectively. Differences in the parameter settings of electroacupuncture (EA) were noted, with the waveform predominantly administered as a continuous wave at frequencies of 1–2 Hz and intensities of 2–3 mA. These variations may have influenced the outcomes of neuroimaging studies. Furthermore, the utilization of MRI technology, including resting-state functional MRI (rs-fMRI) and functional MRI (fMRI), in the included studies lacked integration across multiple studies, resulting in a reliance on a single imaging modality. Furthermore, the included studies limited their inquiry to single or several independent brain regions or networks related to the effect of acupuncture on patients with DD. The lack of an integrated analysis of brain regions or networks further compromised the identification of a mechanism underlying the treatment’s therapeutic effect. Thirdly, the reviewed studies included far more female patients with DD than male patients accounted. Although the literature suggests that female DD patients are more common than male DD patients due to reproductive events elevating the risk of DD, comparing male and female DD patients in the same study would enhance heterogeneity of the study’s findings. In addition, many of the studies included in the review had relatively small sample sizes, which could reduce the statistical power of the findings and affect the generalizability of the results. Larger sample sizes and more high-quality RCTs are needed to strengthen the evidence for acupuncture treatment of depression. Finally, as the 26 included studies were performed in mainland China, their neuroimaging findings have limited applicability to countries and regions outside China, and their reliability as a reference is thus diminished.
4.5 Outlook
The literature would benefit from neuroimaging studies on the use of acupuncture in treating DD performed outside of China in the future. Such research should strictly follow the Cochrane Handbook, feature superior RCT design, employ a rigorous randomization process, adequately handle dropout data, and ensure excellent methodological quality. It is imperative for future research on the effects of acupuncture on patients with DD to incorporate advanced imaging techniques and analytical methods in order to enhance understanding of the treatment’s mechanisms of action. A multimodal approach should be adopted to investigate the interactions among various brain regions or networks, with equal emphasis on assessing alterations in neural function and structure. Additionally, in order to minimize the influence of bias resulting from small sample sizes, it is advisable to include participant populations of maximal size. The need for a large sample should be balanced against the careful consideration of recruiting patients with homogenous characteristics, including gender classification, to facilitate the exploration of neural mechanisms underlying gender-based differences in acupuncture treatment for DD. In addition, future studies should strengthen longitudinal designs to assess the durability of treatment effects and explore how acupuncture influences brain activity over a longer period, which may provide insights into the mechanisms for sustained benefits. Ultimately, it is desirable to achieve standardization and uniformity in the method of administering acupuncture, such as the parameter settings for VNS and EA, to minimize bias resulting from differences in intervention methods.
5 Conclusion
This review provides evidence that acupuncture induces changes in brain function in distinct neural regions among individuals with Depressive Disorder, encompassing the Default Mode Network (DMN), limbic system, emotional and cognitive regulation network, reward network, Central Executive Network (CEN), Salience Network (SN), and Sensorimotor Network (SMN). Notably, these changes were primarily observed in the cingulate gyrus, precuneus, insula, prefrontal lobe, amygdala, superior frontal gyrus, cerebellum, putamen, caudate nucleus, middle frontal gyrus, hippocampus, angular gyrus, thalamus, parahippocampal gyrus, precentral gyrus, and postcentral gyrus. However, the studies included in this analysis only used MRI neuroimaging technology. Hence, additional randomized controlled trials incorporating high-quality, multimodal designs and enhanced integration of neuroimaging data are necessary to further elucidate the underlying mechanism of acupuncture in the treatment of DD.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author contributions
DL: Conceptualization, Data curation, Formal analysis, Methodology, Software, Writing – original draft. QR: Conceptualization, Data curation, Formal analysis, Software, Writing – original draft. YO: Conceptualization, Formal Analysis, Software, Writing – original draft. LL: Conceptualization, Data curation, Formal analysis, Writing – original draft. DP: Conceptualization, Methodology, Project administration, Writing – review & editing. SY: Conceptualization, Funding acquisition, Methodology, Project administration, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of Sichuan Province (Grant number 2023NSFSC0696), the Project of the Central Guidance for Local Projects (Grant number 2022ZYD0101), and the National Natural Science Foundation of China (Grant numbers 82004487 and 82230127 (Key Program)).
Acknowledgments
We thank Bullet Edits Limited for the linguistic editing and proofreading of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Loula R, Monteiro LHA. An individual-based model for predicting the prevalence of depression. Ecol Complex. (2019) 38:168–72. doi: 10.1016/j.ecocom.2019.03.003
2. Friedrich MJ. Depression is the leading cause of disability around the world. Jama-J Am Med Assoc. (2017) 317:1517. doi: 10.1001/jama.2017.3826
3. Avenevoli S, Swendsen J, He JP, Burstein M, Merikangas KR. Major depression in the national comorbidity survey-adolescent supplement: prevalence, correlates, and treatment. J Am Acad Child Psy. (2015) 54:37–44. doi: 10.1016/j.jaac.2014.10.010
4. Ma F. Diagnostic and statistical manual of mental disorders-5 (DSM-5). In: Encyclopedia of Gerontology and Population Aging. Springer International Publishing, Cham (2022). p. 1414–25. doi: 10.1007/978-3-030-22009-9_419
5. WHO. Depression and other common mental disorders: global health estimates (No. WHO/MSD/MER/2017.2). Geneva: World Health Organization (2017). Available at: https://apps.who.int/iris/handle/10665/254610 (Accessed February 14, 2017).
6. McCarron RM, Shapiro B, Rawles J, Luo J. Depression. Ann Intern Med. (2021) 174:Itc65–itc80. doi: 10.7326/AITC202105180
7. Otte C, Gold SM, Penninx BW, Pariante CM, Etkin A, Fava M, et al. Major depressive disorder. Nat Rev Dis Primers. (2016) 2:16065. doi: 10.1038/nrdp.2016.65
8. Monroe SM, Harkness KL. Major depression and its recurrences: life course matters. Annu Rev Clin Psychol. (2022) 18:329–7. doi: 10.1146/annurevclinpsy-072220-021440
9. Carreno FR, Frazer A. Vagal nerve stimulation for treatmentresistant depression. Neurotherapeutics. (2017) 14:716–27. doi: 10.1007/s13311-017-0537-8
10. Horowitz MA, Taylor D. Tapering of SSRI treatment to mitigate withdrawal symptoms. Lancet Psychiatry. (2019) 6:538–6. doi: 10.1016/S2215-0366(19)30032-X
11. Chung KF, Yu YM, Yeung WF. Correlates of residual fatigue in patients with major depressive disorder: the role of psychotropic medication. J Affect Disord. (2015) 186:192–7. doi: 10.1016/j.jad.2015.07.026
12. Romera I, Pérez V, Quail D, Berggren L, Lenox-Smith A, Gilaberte I. Individual residual symptoms and functional impairment in patients with depression. Psychiatry Res. (2014) 220:258–2. doi: 10.1016/j.psychres.2014.07.042
13. Smith CA, Armour M, Lee MS, Wang LQ, Hay PJ. Acupuncture for depression. Cochrane Database Syst Rev. (2018) 3(3):CD004046. doi: 10.1002/14651858.CD004046
14. Armour M, Smith CA, Wang LQ, Naidoo D, Yang GY, MacPherson H, et al. Acupuncture for depression: a systematic review and meta-analysis. J Clin Med. (2019) 8:E1140. doi: 10.3390/jcm8081140
15. Yang NN, Lin LL, Li YJ, Li HP, Cao Y, Tan CX, et al. Potential mechanisms and clinical effectiveness of acupuncture in depression. Curr Neuropharmacol. (2022) 20:738–0. doi: 10.2174/1570159X19666210609162809
16. Xu G, Lei H, Huang L, Xiao Q, Huang B, Zhou Z, et al. The dose-effect association between acupuncture sessions and its effects on major depressive disorder: A meta-regression of randomized controlled trials. J Affect Disord. (2022) 310:318–27. doi: 10.1016/j.jad.2022.04.155
17. Kim M, Choi EJ, Kwon OJ, Park HJ, Kim AR, Seo BN, et al. Electroacupuncture plus moxibustion for major depressive disorder: A randomized, sham-controlled, pilot clinical trial. Integr Med Res. (2022) 11:100846. doi: 10.1016/j.imr.2022.100846
18. Kandola A, Ashdown-Franks G, Hendrikse J, Sabiston CM, Stubbs B. Physical activity and depression: Towards understanding the antidepressant mechanisms of physical activity. Neurosci Biobehav Rev. (2019) 107:525–39. doi: 10.1016/j.neubiorev.2019.09.040
19. Duman RS. Neurobiology of stress, depression, and rapid acting antidepressants: remodeling synaptic connections. Depress Anxiety. (2014) 31:291–6. doi: 10.1002/da.22227
20. Silbersweig D. Default mode subnetworks, connectivity, depression and its treatment: toward brain-based biomarker development. Biol Psychiatry. (2013) 74:5–6. doi: 10.1016/j.biopsych.2013.05.011
21. Phillips ML, Chase HW, Sheline YI, Etkin A, Almeida JR, Deckersbach T, et al. Identifying predictors, moderators, and mediators of antidepressant response in major depressive disorder: neuroimaging approaches. Am J Psychiatry. (2015) 172:124–38. doi: 10.1176/appi.ajp.2014.14010076
22. Murphy JA, Sarris J, Byrne GJ. A review of the conceptualisation and risk factors associated with treatment-resistant depression. Depression Res Treat. (2017) 2017:4176825. doi: 10.1155/2017/4176825
23. Bennabi D, Aouizerate B, El-Hage W, Doumy O, Moliere F, Courtet P, et al. Risk factors for treatment resistance in unipolar depression: a systematic review. J Affect Disord. (2015) 171:137–41. doi: 10.1016/j.jad.2014.09.020
24. Fife D, Blacketer C, Reps JM, Ryan P. Database studies of treatment-resistant depression should take account of adequate dosing. Prim Care Companion CNS Disord. (2018) 20:18m02274. doi: 10.4088/PCC.18m02274
25. Deng D, Liao H, Duan G, Liu Y, He Q, Liu H, et al. Modulation of the Default Mode Network in First-Episode, Drug-Naïve Major Depressive Disorder via Acupuncture at Baihui (GV20) Acupoint. Front Hum Neurosci. (2016) 10:230. doi: 10.3389/fnhum.2016.00230
26. Mulders PC, van Eijndhoven PF, Schene AH, Beckmann CF, Tendolkar I. Resting-state functional connectivity in major depressive disorder: A review. Neurosci Biobehav Rev. (2015) 56:330–44. doi: 10.1016/j.neubiorev.2015.07.014
27. Carl H, Walsh E, Eisenlohr-Moul T, Minkel J, Crowther A, Moore T, et al. Sustained anterior cingulate cortex activation during reward processing predicts response to psychotherapy in major depressive disorder. J Affect Disord. (2016) 203:204–12. doi: 10.1016/j.jad.2016.06.005
28. Wagner G, Schachtzabel C, Peikert G, Bär KJ. The neural basis of the abnormal self-referential processing and its impact on cognitive control in depressed patients. Hum Brain Mapp. (2015) 36:2781–94. doi: 10.1002/hbm.22807
29. Liu CH, Yang MH, Zhang GZ, Wang XX, Li B, Li M, et al. Neural networks and the anti-inflammatory effect of transcutaneous auricular vagus nerve stimulation in depression. J Neuroinflammation. (2020) 17:54. doi: 10.1186/s12974-020-01732-5
30. Wang X, Luo P, Zhang L, Sun J, Cao J, Lei Z, et al. Altered functional brain activity in first-episode major depressive disorder treated with electro-acupuncture: A resting-state functional magnetic resonance imaging study. Heliyon. (2024) 10:e29613. doi: 10.1016/j.heliyon.2024.e29613
31. Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. (2019) 10(10):ED000142. doi: 10.1002/14651858.ED000142
32. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
33. Wong KKL, Xu J, Chen C, Ghista D, Zhao H. Functional magnetic resonance imaging providing the brain effect mechanism of acupuncture and moxibustion treatment for depression. Front Neurol. (2023) 14:1151421. doi: 10.3389/fneur.2023.1151421
34. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 366:l4898. doi: 10.1136/bmj.l4898
35. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. (2016) 355:i4919. doi: 10.1136/bmj.i4919
36. He JK, Li SY, Wang Y, Zhao B, Xiao X, Hou XB, et al. Mapping the modulating effect of transcutaneous auricular vagus nerve stimulation on voxel-based analyses in patients with first-episode major depressive disorder: a resting-state functional magnetic resonance imaging study. Braz J Psychiatry. (2023) 45:93–101. doi: 10.47626/1516-4446-2022-2788
37. Sun J, Guo C, Ma Y, Gao S, Luo Y, Chen Q, et al. Immediate modulatory effects of transcutaneous auricular vagus nerve stimulation on the resting state of major depressive disorder. J Affect Disord. (2023) 325:513–21. doi: 10.1016/j.jad.2023.01.035
38. Sun JF, He JK, Ma Y, Wang Z, Guo CL, Luo Y, et al. Immediate brain effects of electroacupuncture at auricular concha in the treatment of first-episode depression using the resting-state fMRI technology. Shanghai J Acupuncture Moxibustion. (2023) 42:477–84. doi: 10.13460/j.issn.1005-0957.2023.05.0477
39. Cao DN, Zhang F, Wang F, Ji YH, Jiao MX, Zhao CY, et al. Changes of ReHo of r-fMRI before and after acupuncture at right Taichong point in elderly patients with depression. Shandong Med J. (2019) 59:9–11,15. doi: 10.3969/j.issn.1002-266X.2019.29.003
40. Ma Y, Guo CL, Sun JF, Gao SS, Luo Y, Chen QY, et al. Effect of transcutaneous auricular vagus nerve stimulation on functional connectivity in the related brain regions of patients with depression based on the resting-state fMRI. Chin Acupuncture Moxibustion. (2023) 43:367–73. doi: 10.13703/j.0255-2930.20221007-k0002
41. Ma Y, Wang Z, He J, Sun J, Guo C, Du Z, et al. Transcutaneous auricular vagus nerve immediate stimulation treatment for treatment-resistant depression: A functional magnetic resonance imaging study. Front Neurol. (2022) 13:931838. doi: 10.3389/fneur.2022.931838
42. Sun J, Ma Y, Du Z, Wang Z, Guo C, Luo Y, et al. Immediate modulation of transcutaneous auricular vagus nerve stimulation in patients with treatment-resistant depression: A resting-state functional magnetic resonance imaging study. Front Psychiatry. (2022) 13:923783. doi: 10.3389/fpsyt.2022.923783
43. Sun JF, Chen LM, He JK, Wang Z, Guo CL, Li XJ, et al. Instant brain effect of transcutaneous auricular vagus nerve stimulation in treating the treatment-resistant depression by resting state functional magnetic resonance imaging. Chin J Traditional Chin Med. (2022) 37:2740–4. doi: 10.3389/fpsyt.2022.923783
44. Sun JF, He JK, Chen LM, Wang Z, Guo CL, Ma Y, et al. Preliminary fMRI study on the immediate brain effect of ear nail electroacupuncture in the treatment of recurrent depression. Chin J Integrated Traditional Western Med Imaging. (2022) 20:11–5. doi: 10.3969/j.issn.1672-0512.2022.01.003
45. Yi S, Wang Z, Yang W, Huang C, Liu P, Chen Y, et al. Neural activity changes in first-episode, drug-naïve patients with major depressive disorder after transcutaneous auricular vagus nerve stimulation treatment: A resting-state fMRI study. Front Neurosci. (2022) 16:1018387. doi: 10.3389/fnins.2022.1018387
46. Wang Z, He JK, Sun JF, Chen LM, Guo CL, Zhang GL, et al. Observation of the immediate brain effect of otoelectric therapy on refractory depression based on resting functional magnetic resonance imaging. Magnetic resonance Imaging. (2022) 13:70–75+97. doi: 10.12015/issn.1674-8034.2022.01.014
47. Chen LM, Sun JF, Guo CL, Li XJ, Wang Z, Hong Y, et al. Preliminary single-arm study of brain effects during transcutaneous auricular vagus nerve stimulation treatment of recurrent depression by resting-state functional magnetic resonance imaging. J Tradit Chin Med. (2022) 42:818–24. doi: 10.19852/j.cnki.jtcm.2022.05.010
48. Chen LM, Li XJ, Xu K, Guo CL, Zhang GL, Han M, et al. Functional MRI-based study on neuromechanism of trans-auricular vagus nerve stimulation for treatment-resistant depression. Acupuncture Res. (2021) 46:869–74. doi: 10.13702/j.1000-0607.20210241
49. Wei XY, Chen H, Guo C, Tan WL, Zhan SH. The instant and sustained effect of electroacupuncture in postgraduate students with depression: an fMRI study. Neuropsychiatr Dis Treat. (2021) 17:873–83. doi: 10.2147/NDT.S307083
50. Duan G, He Q, Pang Y, Chen W, Liao H, Liu H, et al. Altered amygdala resting-state functional connectivity following acupuncture stimulation at BaiHui (GV20) in first-episode drug-Naïve major depressive disorder. Brain Imaging Behav. (2020) 14:2269–80. doi: 10.1007/s11682-019-00178-5
51. Li XJ, Xu K, Fang JL, Hong Y, Chen LM, Gao DQ, et al. Observation of the therapeutic effect of ear nail electroacupuncture on drug-resistant depression and exploration of its resting state fMRI brain mechanism. Magnetic Resonance Imaging. (2020) 11:84–8. doi: 10.12015/issn.1674-8034.2020.02.002
52. Chen HY, Zhao B, Zhang L, Cao JD, Chen D, Zhao PH, et al. Study on the brain effect of ear nail electroacupuncture treatment for mild to moderate depression based on ALFF analysis. World Sci Technol - Modernization Traditional Chin Med. (2019) 21:2272–7. doi: 10.11842/wst.20190620009
53. Tu Y, Fang J, Cao J, Wang Z, Park J, Jorgenson K, et al. A distinct biomarker of continuous transcutaneous vagus nerve stimulation treatment in major depressive disorder. Brain Stimul. (2018) 11:501–8. doi: 10.1016/j.brs.2018.01.006
54. Wang Z, Fang J, Liu J, Rong P, Jorgenson K, Park J, et al. Frequency-dependent functional connectivity of the nucleus accumbens during continuous transcutaneous vagus nerve stimulation in major depressive disorder. J Psychiatr Res. (2018) 102:123–31. doi: 10.1016/j.jpsychires.2017.12.018
55. Fang J, Egorova N, Rong P, Liu J, Hong Y, Fan Y, et al. Early cortical biomarkers of longitudinal transcutaneous vagus nerve stimulation treatment success in depression. NeuroImage Clin. (2016) 14:105–11. doi: 10.1016/j.nicl.2016.12.016
56. Fang J, Rong P, Hong Y, Fan Y, Liu J, Wang H, et al. Transcutaneous vagus nerve stimulation modulates default mode network in major depressive disorder. Biol Psychiatry. (2016) 79:266–73. doi: 10.1016/j.biopsych.2015.03.025
57. Liu J, Fang J, Wang Z, Rong P, Hong Y, Fan Y, et al. Transcutaneous vagus nerve stimulation modulates amygdala functional connectivity in patients with depression. J Affect Disord. (2016) 205:319–26. doi: 10.1016/j.jad.2016.08.003
58. Han HY, Zhang C, Yan B, Tong L, Zheng ZZ, Yang HY, et al. Influence \of acupuncturing at pericardium channel on brain network effects of mild depression patients with amygdale as the seed point. Chin J Traditional Chin Med. (2016) 31:292–5.
59. Deng DM, Liao H, Duan GX, He QC, Pang Y, Liu HM, et al. The changed amplitude of low frequency fluctuation of functional brain areas induced by acupuncture at Baihui in major depressive disorder patients. J Clin Radiol. (2015) 34:884–8. doi: 10.13437/j.cnki.jcr.2015.06.007
60. Deng DM, Duan GX, Liao H, Pang Y, He QC, Liu HM, et al. Detection of brain functional areas activated by acupuncture at Baihui point in patients with severe depression using the ReHo method. Chin Med Imaging Technol. (2015) 31:683–7. doi: 10.13929/j.1003-3289.2015.05.011
61. Egbewale BE. Common design concepts in randomized controlled trials. Niger Med J. (2020) 61:51–4. doi: 10.4103/nmj.NMJ_112_19
62. Wang Y, Zhan G, Cai Z, Jiao B, Zhao Y, Li S, et al. Vagus nerve stimulation in brain diseases: Therapeutic applications and biological mechanisms. Neurosci Biobehav Rev. (2021) 127:37–53. doi: 10.1016/j.neubiorev.2021.04.018
63. Butt MF, Albusoda A, Farmer AD, Aziz Q. The anatomical basis for transcutaneous auricular vagus nerve stimulation. J Anat. (2020) 236:588–611. doi: 10.1111/joa.13122
64. Kamel LY, Xiong W, Gott BM, Kumar A, Conway CR. Vagus nerve stimulation: An update on a novel treatment for treatment-resistant depression. J Neurol Sci. (2022) 434:120171. doi: 10.1016/j.jns.2022.120171
65. Zemlan FP, Garver DL. Depression and antidepressant therapy: receptor dynamics. Prog Neuropsychopharmacol Biol Psychiatry. (1990) 14:503–23. doi: 10.1016/0278-5846(90)90004-z
66. Beurel E, Toups M, Nemeroff CB. The bidirectional relationship of depression and inflammation: double trouble. Neuron. (2020) 107:234–56. doi: 10.1016/j.neuron.2020.06.002
67. Köhler CA, Freitas TH, Maes M, de Andrade NQ, Liu CS, Fernandes BS, et al. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatr Scand. (2017) 135:373–87. doi: 10.1111/acps.12698
68. Liu Y, Feng H, Mo YL, Liu WJ, Song MF, Wang SD, et al. Study on the synergistic effect of acupuncture on SSRI antidepressants in treating depression and regulating TH1/TH2 balance. Chin Arch Traditional Chin Med. (2014) 32:1927–9. doi: 10.13193/j.issn.1673-7717.2014.08.040
69. Sun H, Zhao H, Zhang J, Bao F, Wei J, Wang DH, et al. Effect of acupuncture at Baihui and Zusanli on serum inflammatory cytokines in patients with depression. Chin Acupuncture Moxibustion. (2010) 30:195–9. doi: 10.13703/j.0255-2930.2010.03.021
70. Lou R, Zhang H, Chen HD, Huang KQ, Li HZ, Li JW, et al. The effect of Baihui acupoint long needle retention method on central 5-HT and DA levels in chronic stress depression rats. Chin Arch Traditional Chin Med. (2014) 32:1702–4. doi: 10.13193/j.issn.1673-7717.2014.07.054
71. Chen Y, Lyu D, Wang F, Huang Q, Yang W, Zhang M, et al. Efficacy of adjunctive intensive transcranial direct current stimulation of different cortices in treatment-resistant depression: a study protocol for a randomized double-blinded sham-controlled trial. BMC Psychiatry. (2022) 22:802. doi: 10.1186/s12888-022-04465-2
72. Zang YF, He Y, Zhu CZ, Cao QJ, Sui MQ, Liang M, et al. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. (2006) 29:83–91. doi: 10.1016/j.braindev.2006.07.002
73. Fingelkurts AA, Fingelkurts AA, Kähkönen S. Functional connectivity in the brain–is it an elusive concept? Neurosci Biobehav Rev. (2005) 28:827–36. doi: 10.1016/j.neubiorev.2004.10.009
74. Zhao B, Bi Y, Li L, Zhang J, Hong Y, Zhang L, et al. The instant spontaneous neuronal activity modulation of transcutaneous auricular vagus nerve stimulation on patients with primary insomnia. Front Neurosci. (2020) 14:205. doi: 10.3389/fnins.2020.00205
75. Irmen F, Horn A, Mosley P, Perry A, Petry-Schmelzer JN, Dafsari HS, et al. Left prefrontal connectivity links subthalamic stimulation with depressive symptoms. Ann Neurol. (2020) 87:962–75. doi: 10.1002/ana.25734
76. Chen L, Wang Y, Niu C, Zhong S, Hu H, Chen P, et al. Common and distinct abnormal frontal-limbic system structural and functional patterns in patients with major depression and bipolar disorder. NeuroImage Clin. (2018) 20:42–50. doi: 10.1016/j.nicl.2018.07.002
77. Xiao H, Yuan M, Li H, Li S, Du Y, Wang M, et al. Functional connectivity of the hippocampus in predicting early antidepressant efficacy in patients with major depressive disorder. J Affect Disord. (2021) 291:315–21. doi: 10.1016/j.jad.2021.05.013
78. Zhu L, Wen CM, Wu Z, Wu Q, Chang LH. Research progress on the relationship between changes in brain insula morphology and function and depression. Shandong Med J. (2017) 57:97–100. doi: 10.3969/j.issn.1002-266X.2017.43.031
79. Zald DH. Orbital versus dorsolateral prefrontal cortex: anatomical insights into content versus process differentiation models of the prefrontal cortex. Ann New York Acad Sci. (2007) 1121:395–406. doi: 10.1196/annals.1401.012
80. Koenigs M, Grafman J. The functional neuroanatomy of depression: distinct roles for ventromedial and dorsolateral prefrontal cortex. Behav Brain Res. (2009) 201:239–43. doi: 10.1016/j.bbr.2009.03.004
81. Schmaal L, Hibar DP, Sämann PG, Hall GB, Baune BT, Jahanshad N, et al. Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol Psychiatry. (2017) 22:900–9. doi: 10.1038/mp.2016.60
82. Adamaszek M, D’Agata F, Ferrucci R, Habas C, Keulen S, Kirkby KC, et al. Consensus paper: cerebellum and emotion. Cerebellum. (2017) 16:552–76. doi: 10.1007/s12311-016-0815-8
83. Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. (2010) 35:4–26. doi: 10.1038/npp.2009.129
84. Gulyaeva NV. Functional neurochemistry of the ventral and dorsal hippocampus: stress, depression, dementia and remote hippocampal damage. Neurochem Res. (2019) 44:1306–22. doi: 10.1007/s11064-018-2662-0
85. Seghier ML. The angular gyrus: multiple functions and multiple subdivisions. Neuroscientist. (2013) 19:43–61. doi: 10.1177/1073858412440596
86. Li Y, Peng C, Wang W, Li S, Li YC, Yang N. Changes in the expression of nerve growth factors and their receptors in the hippocampus and thalamus of post-stroke depression rats. Chin J Geriatric Cardiovasc Disease. (2015) 17:1305–9. doi: 10.3969/j.issn.1009-0126.2015.12.022
87. Bu Q, Li K, Liu DT, Jia XQ, Pei XJ, Jia R, et al. Microstructural changes in the parahippocampal gyrus of patients with cerebrovascular disease and depression. Chin J Clin Physicians. (2021) 49:1316–8. doi: 10.3969/j.issn.2095-8552.2021.11.017
88. Burwell RD. The parahippocampal region: corticocortical connectivity. Ann N Y Acad Sci. (2000) 911:25–42. doi: 10.1111/j.1749-6632.2000.tb06717.x
89. Yan CG, Chen X, Li L, Castellanos FX, Bai TJ, Bo QJ, et al. Reduced default mode network functional connectivity in patients with recurrent major depressive disorder. Proc Natl Acad Sci U.S.A. (2019) 116:9078–83. doi: 10.1073/pnas.1900390116
90. Andrews-Hanna JR, Smallwood J, Spreng RN. The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Ann N Y Acad Sci. (2014) 1316:29–52. doi: 10.1111/nyas.12360
91. Liu P, Tu H, Zhang A, Yang C, Liu Z, Lei L, et al. Brain functional alterations in MDD patients with somatic symptoms: A resting-state fMRI study. J Affect Disord. (2021) 295:788–96. doi: 10.1016/j.jad.2021.08.143
92. Bracht T, Federspiel A, Schnell S, Horn H, Höfle O, Wiest R, et al. Cortico-cortical white matter motor pathway microstructure is related to psychomotor retardation in major depressive disorder. PLoS One. (2012) 7:e52238. doi: 10.1371/journal.pone.0052238
93. Ng TH, Alloy LB, Smith DV. Meta-analysis of reward processing in major depressive disorder reveals distinct abnormalities within the reward circuit. Transl Psychiatry. (2019) 9:293. doi: 10.1038/s41398-019-0644-x
94. Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. (2007) 27:2349–56. doi: 10.1523/JNEUROSCI.5587-06.2007
Keywords: acupuncture, depressive disorder, neuroimaging, brain network, systematic review
Citation: Lin D, Ren Q, Ou Y, Li L, Peng D and Yang S (2025) Neuroimaging studies of acupuncture for depressive disorder: a systematic review of published papers from 2014 to 2024. Front. Psychiatry 16:1536660. doi: 10.3389/fpsyt.2025.1536660
Received: 29 November 2024; Accepted: 14 April 2025;
Published: 15 May 2025.
Edited by:
Haoran Dou, Sichuan Normal University, ChinaReviewed by:
Francesco Monaco, Azienda Sanitaria Locale Salerno, ItalyAnnarita Vignapiano, Department of Mental Health, Italy
Jin Xian, Affiliated Hospital of Shandong University of Traditional Chinese Medicine, China
Copyright © 2025 Lin, Ren, Ou, Li, Peng and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dezhong Peng, MTU4MjgwNTU2OThAMTYzLmNvbQ==; Sha Yang, eWFuZ3NoYUBjZHV0Y20uZWR1LmNu
†These authors share first authorship
 Dezhi Lin
Dezhi Lin Qiang Ren
Qiang Ren Yangxu Ou
Yangxu Ou Longlong Li
Longlong Li Dezhong Peng
Dezhong Peng Sha Yang
Sha Yang