Abstract
Introduction:
Ayahuasca is a psychedelic compound of N, N, Dimethyltryptamine (DMT) and harmala alkaloids used for spiritual and medicinal applications in traditional settings. A range of potential psychotherapeutic mechanisms have been proposed for ayahuasca. These are thought to contribute to improvements in various psychiatric conditions including mood disorders and substance dependence. This open label exploratory study explored safety, tolerability, physical, mental health and psychedelic effects of three Acacia based formulations in 9 healthy volunteers with prior use of Ayahuasca.
Method:
Formulations derived from two Acacia species (1mg/kg DMT and 4mg/kg of harmalas) were tested in a cross-over design in 5 adults; a third formulation (ACL-010) was tested in 4 adults at two dosages (1mg/kg DMT and 4mg/kg of harmalas, and then 1.4mg/kg DMT and 5.6mg of harmalas).
Results:
All formulations had a good safety profile. No serious adverse events were reported. Physical examination, vital signs, and pathology revealed no clinically significant changes across the course of the study. The subjective experience of all formulations was generally rated similar to Ayahuasca. Four-week follow-up measures of psychological wellbeing and perceptual effects showed little difference between formulations. The strength and quality of the psychedelic experience elicited with ACL-010 was rated as similar or more beneficial than Ayahuasca.
Discussion:
Our results indicate DMT formulations derived from the Acacia species represent a feasible alternative to traditional Ayahuasca for future clinical trials and possibly clinical contexts. The small sample size and open label design limit generalizability of results.
Clinical trial registration:
https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=384191&isReview=true, identifier ACTRN12622001315707.
Introduction
Traditionally, plant-based formulations consisting of the active constituents N,N -dimethyltryptamine (DMT) and harmala alkaloids have been used for centuries, known more broadly as ‘Ayahuasca’ (1). This traditional plant-based preparation has been used in the Amazon basin for hundreds of years for a range of therapeutic and psycho-spiritual effects (2, 3). Ayahuasca was also adopted as a religious sacrament by several Brazilian syncretic religions in the 1930’s, and these have now expanded internationally to Europe, North America, and Australia (3–6).
Ayahuasca has dramatically increased in popularity since the turn of the millennium, with increasing numbers of tourists visiting the Amazonas in search of therapeutic or spiritual effects (7). Use of the brew outside the Amazon in neo-shamanic ceremonies has also increased in popularity, with various sources of harmala alkaloids and DMT being used across the globe (4, 5, 8). Vast amounts of anecdotal evidence now exist describing the healing effects of the brew, leading researchers to examine the therapeutic potential of DMT-harmala concoctions (9). In Australia, this use extends to native Acacia-based Ayahuasca formulations (to provide the DMT content), which may be used in combination with Banisteriopsis caapi (the Ayahuasca vine) or Peganum harmala (Harmel) to provide the harmala compounds.
The psychoactive compounds of Ayahuasca are regarded as DMT, which is present in traditional brews containing Psychotria viridis or other related species, and three main β-carbolines (harmine, harmaline and tetrahydroharmine) which are found in Banisteriopsis caapi. These β-carbolines are reversible inhibitors of monoamine oxidase (MAOI), while the tetrahydroharmine is also an inhibitor of serotonin reuptake (10). The MAOI function of β-carbolines inhibits DMT degradation in the gastrointestinal system allowing this substance to reach the brain, where it activates serotonergic pathways via 5HT 2A receptor interaction (11). Additionally, research has indicated that harmine could have a central role in Ayahuasca’s anti-addictive effects, including reducing recidivism to alcohol, cocaine and methamphetamine potentially due to MAO-A inhibition, Sigma-1 activity, and neurogenesis promotion (12–14).
A range of potential psychotherapeutic mechanisms have been proposed for ayahuasca, listed below. Combined, these are thought to contribute to improvements in various psychiatric conditions via: Decentering: the ability to observe one’s own thoughts and feelings in a detached, more objective manner (15, 16); Certain mindfulness capabilities: acceptance (non-judgmental and non-reactive processing) and improved observation (17–19); Cognitive flexibility: mental ability to adjust to activity and content (20, 21); Emotional regulation (15); Experiential acceptance (22, 23). While ayahuasca and other classic psychedelics such as psilocybin and LSD share similar effects (e.g. altered perceptual and visual effects and ego dissolution), ayahuasca is more commonly associated with intense emotional catharsis, somatic purging (e.g. vomiting), and vivid visionary experiences potentially involving spiritual themes.
Use of DMT-Harmala formulas have been linked to changes in a range of personality traits. Increases in agreeableness and openness as well as decreases in neuroticism have been observed, with reductions in neuroticism correlating with the subjective intensity of the mystical experience (24, 25). Ayahuasca-induced reductions in grief have been linked to increases in acceptance and the ability to psychologically decenter (26).
Psychedelic agents are however recognized to potentially elicit a range of adverse events (AEs) (27, 28) which are usually transient, including headaches, nausea and possible emesis, anxiety, panic, or agitation, alterations in blood pressure or heart rate, and in rare cases increases in suicidality. Psycho-perceptual changes such as visual/auditory/kinesthetic hallucinations, time distortions, and feelings of awe and transcendent spiritual experiences are considered to not be AEs as such, with data from use in naturalistic settings showing that such mystical experiences are directly related to therapeutic outcomes (29–31).
Recently there has been interest in the development of DMT-harmala preparations as standardized pharmaceutical grade medicines for the clinical treatment of mental health disorders (32). For botanically derived medicines, the use of alternative plant sources of DMT and harmala alkaloids may provide a more scalable option - potentially growing faster and yielding higher concentrations of the active alkaloids. This approach also helps prevent the depletion of Indigenous plant stocks in South America, which is a conservation concern.
The primary purpose of our study was to test the safety, tolerability, and psychedelic effects of three Acacia-based, purified and standardized DMT and harmala alkaloid preparations in healthy volunteers who had experience with Ayahuasca, while also evaluating secondary psychological outcomes. We studied two differing Australian native Acacia spp. (both classified within Acacia section Juliflorae, a taxonomic grouping within the genus Acacia sensu stricto), providing the DMT component, in combination with Peganum harmala which is a prolifically growing shrub in the Middle East and Asia, providing the β-carbolines. The aim was to explore if any differing safety or psychoactive effects occurred between the species (which have slightly different alkaloidal profiles; assayed via HPLC), and if any additional changes occurred from further purifying the active constituents. Our findings will inform a planned Phase 1 pharmacokinetics/pharmacodynamic study, and a randomized controlled trial involving participants with major depressive disorder and alcohol use disorder.
Materials and methods
Trial oversight
The study protocol, Patient Information and Consent Form (PICF), Investigator Brochure, and subsequent amendments were approved by the relevant institutional Human Research Ethics Committee (HREC 118/22). The conduct of this study was in compliance with the approved protocol, and Good Clinical Practice guidelines. The trial was registered with the Australian New Zealand Clinical Trials Register (ACTRN 12622001315707).
In Australia, DMT and harmala alkaloids are prohibited substances [Schedule 9 (S9)] that, by law, may only be used for research purposes. Permits for individual trial participants were granted by Medicines and Poisons Regulation, Department of Health, Victoria, Australia.
This report conforms to the CONSORT reporting guidelines for non-randomized pilot and feasibility studies (33–35).
General study design
This was an exploratory pilot study to test three purified and standardized Australian native Acacia-based formulations of DMT and harmala alkaloids in 9 healthy participants with prior use of oral liquid DMT-harmala preparations, such as ayahuasca. The aim of the study was to provide pilot data on formulation, dose, safety, tolerability and subjective effects of study medication to inform pharmacokinetic studies and a planned Phase 2 study. Figure 1 shows the overall study design and plan for the current study. In Part 1 of the study two formulations of the DMT were studied, derived from different Acacia species, (standardized to 1mg/kg) and harmalas: harmine, harmaline, tetrahydroharmine (standardized collectively to 4mg/kg) formulation (Acacia A + Peganum versus Acacia B + Peganum). Participants were crossed over to experience both formulations (A and B) in an open label manner (9 treatment sessions in total - including one participant withdrawal after the first session). Participants, therapists and researchers were blinded as to the order in which the formulations were administered. In Part 2 of the study, after an interim data analysis, a third formulation was developed, which was derived from Acacia B source in combination with the Peganum component which achieved a purity of >90% DMT and >90% harmala alkaloids (Formulation C; ACL-010). Four participants were given Formulation C at 1mg/kg DMT and 4mg/kg harmalas in the first dosing session, being titrated to 1.4mg/kg DMT and 5.6mg/kg harmalas in the subsequent session (See Figure 1). Treatment sessions were a minimum of seven (7) days apart. In both treatment sessions participants were attended by a therapeutic dyad consisting of a psychiatrist (male) and clinical psychologist (female) both with extensive experience in psychedelic assisted psychotherapy.
Figure 1

Overall study and design plan.
Study setting
Dosing treatment sessions were conducted in specially prepared treatment rooms. Attention was paid to the comfort and aesthetic qualities of the room including the use of subdued lighting, a carefully selected music list, plants and aromatherapy. After dosing, participants were encouraged to lie or sit on the bed, wear eyeshades, and listen to the music list via noise cancelling headphones. They were free to move about the room and could remove headphones and eyeshades if not required.
Primary and secondary outcomes
Primary outcomes
Safety and tolerability was assessed based on Adverse Events (AEs)/Serious Adverse Events (SAEs) post study recruitment; vital sign data including body temperature, heart rate, respiratory rate, and blood pressure during and after the treatment session. Integration Difficulties Scale (36) was used to assess any negative mental health effects of the treatment (1 week post-treatment). Psychedelic effects were assessed using the Mystical Experiences Questionnaire (MEQ) (37).
Secondary outcomes
Acute subjective effects of the psychedelic experience were assessed via the Five Dimensions of Altered States of Consciousness (5D-ASC) scale (38). The modified Short Index of Mystical Orientation (SIMO) (36, 39) measured the intensity of the participant’s acute mystical experience, and an additional single item was added measuring acute extreme fear “Feeling of immense fear…” on a 10-point scale (36). Visual analogue scales (40) acutely assessed mood and anxiety.
To assess four week follow-up mental health effects the following scales were employed: DASS-21 (41), PANAS – SF (42); Kessler-10 (K-10) scale (43). Persisting Effects Questionnaire (PEQ) (44) was employed to assess chronic impact of the treatment. Insomnia Severity Index (ISI) (45) assessed the nature, severity, and impact of insomnia. Temporal Experience of Pleasure Scale (TEPS) (46) measured individual trait dispositions in both anticipatory and consummatory experiences of pleasure. Personal Insights Questionnaire (PIQ) (47) reported the number personal insights experienced. The Integration Difficulties Scale (IDS) (36) assessed integration-related feelings and experiences. The Ayahuasca Preparation and Support Scale (36) rated preparation and support prior and during treatment sessions.
Eligibility criteria
Inclusions
Male or female; aged 25 to 75 years; mental health professionals with an expressed interest in psychedelic assisted psychotherapy; medically and psychiatrically healthy as adjudicated by the investigator based on physical exam and MINI (DSM-5) (48) psychiatric interview; previously tried DMT-harmalas (i.e. Ayahuasca; but not in the last one month); weight between 50kg and 95kg; BMI of 18 to 32; availability of a friend or family member to assist with transport after the active drug session; willing to adhere to dietary requirements prior to the active treatment session including abstinence from alcohol; willing to take adequate contraception measures during the study.
Exclusion
History of psychosis: past or present diagnosis of bipolar disorder, schizophrenia, or schizoaffective disorder; Family history of psychosis: past or present diagnosis of bipolar disorder type 1 in first degree relative, or schizophrenia, or schizoaffective disorder in first or second degree relative; current suicidality or history of suicide attempt; current psychiatric disorder diagnosis; daily/weekly high-risk alcohol use [AUDIT: Alcohol Use Disorders Identification Test (49)]; Use of any psychoactive medication (e.g., a selective serotonin reuptake inhibitor such as paroxetine or citalopram), haloperidol, any medication with Monoamine oxidase activity (such as isocarboxazid, phenelzine, selegiline or tranylcypromine, linezolid, and methylene blue), or any drug that has been indicated as a potential precipitative agent for serotonin syndrome within 28 days prior to study drug administration and through to the end of study; currently taking any other regular medication, including: opioids, antihistamines, anticonvulsants, amphetamines, Kava, and St John’s wort; used an hallucinogen in the month prior to treatment session (a one-month wash-out is acceptable); Use of any recreational drug within the past month (e.g. amphetamines, opioids); smoking/using nicotine; substance/alcohol use disorder; history of Hallucinogen Persisting Perception Disorder (HPPD); serious medical condition e.g., cardiovascular, metabolic, neurological, respiratory, oncological, hematological disorder; serious ECG abnormality; serious abnormal hematology or electrolyte, renal or liver test result (indicated by screening aspartate aminotransferase (AST) or alanine aminotransferase (ALT) ≥2 or total bilirubin ≥1.5 x upper limit of normal (ULN), which remains above these limits if retested) in the previous 12 months (as provided by their GP or SVHM); females who were pregnant, nursing, or trying to become pregnant (pregnancy test provided); not agreeing to fasting from midnight prior to the Dose Day sessions until the afternoon of that treatment day; participation in another clinical study involving investigational study treatment within 30 days or 5 half-lives, whichever was longer, prior to screening.
Investigational product
All formulations were orally delivered consisting of DMT to harmala alkaloids at a 1:4 ratio, respectively. The 3 principle harmala alkaloids were harmine, harmaline, and tetrahydroharmine, which were provided at a set ratio with relatively low levels of harmaline (the specific DMT to individual harmalas ratio is proprietary information). The dosage is based on the doses used in previous oral Ayahuasca/DMT studies (50–52).
The formulations used in Part 1 of this research (Formulas A and B) were produced at NICM Health Research Institute, Western Sydney University via the following general process:1) Initially a horticulturist confirmed the plant materials (Peganum harmala seeds, and phyllodes and thin stems from two Australian native Acacia spp. [Formula A an Acacia species sourced from a private orchard from Northern New South Wales in Australia; Formula B A.acuminata sourced from a commercial source in Western Australia]. Note that the Formula A species is not disclosed due to concerns over potential wild harvesting; 2) Plant material was dried and milled; 3) Extraction of the plant constituents occurred via a heptane/ethanol extraction, before the application of a proprietary method to create a two final water solution extraction. A pH decrease was also applied to facilitate a higher yield of the active constituents (i.e. DMT and harmala alkaloids), while reducing the level of un-needed constituents; 4) The solute was then evaporated; 5) The dried powder was then encapsulated in a compounding pharmacy based in Melbourne; 6) A sample of capsules was then tested at partner labs to ensure standardization of the constituents and also to confirm the presence of no contaminants or obvious extraneous toxins (e.g. aflatoxins). The alkaloidal levels being ~5% and ~13% DMT for Acacia spp. A and Acacia spp. B (A.acuminata), respectively, and ~48% for the harmalas for the first formulas, with the remaining constituents being other plant constituents which were less than 2% individually (as revealed via HPLC assay).
Formulation C used in Part 2 of the study was developed in concert with CSIRO Australia and manufactured at NICM Health Research Institute, Western Sydney University. Formulation C provided a standardized combination of DMT (>90% purity) and harmala alkaloids (>90% purity) from Acacia acuminata. and Peganum harmala. The plant material was processed and manufactured via a similar process to the first formulations with additional purification steps.
In Part 1 of the study, formulations A and B were administered at a dosage of 1.0.mg/kg of DMT and 4mg/kg of total harmalas. In Part 2 of the study, ALC-010 was administered at 1.0.mg/kg of DMT and 4mg/kg of total harmala in the first dosing session and titrated to 1.4mg//kg of DMT and 5.6mg of total harmala alkaloids in the second dosing session if deemed suitable.
Formulation A was derived from an Eastern Australian Acacia spp. and Peganum harmala. Each capsule contained 18 mg and 5 mg of DMT, along with 108 mg and 22 mg of harmala alkaloids.
Formulation B was derived from Acacia acuminata and Peganum harmala, delivering 22.5 mg and 6.5 mg of DMT per capsule, in addition to 108 mg and 22 mg of harmala alkaloids.
Formulation C (designated as ALC-010) was a purified DMT-harmala extraction derived from Acacia acuminata and Peganum harmala, with each capsule containing 20 mg and 5 mg of DMT, as well as 80 mg and 20 mg of harmala alkaloids.
Standardized DMT doses used in this study were at the upper end of those reported in other clinical studies using oral DMT-harmala preparations. Lower standardized mg/kg doses of DMT (0.3-0.4mg/kg) were reported by Palhano-Fontes et al. (53) and Lanaro et al. (54). Several studies have reported using DMT 1mg/kg (55, 56), while standardized DMT doses of 1.76mg/kg were reported by and Zeifmann et al. (57), and total DMT doses of 96mg-160mg by Sanches et al (58).
Stability data provided by NICM Health Research Institute at Western Sydney University showed that all formulations were stable (within 10% specification deviation) for DMT and harmala constituent levels via post-study HPLC analysis.
Recruitment
Recruitment took place between December 2022 and November 2023. Potential participants were recruited from professional networks, via word of mouth, and self-referral. Twenty-four participants were screened for eligibility by phone. Participants who were deemed to meet broad eligibility criteria attended an in-person screening visit for clinical assessment and informed consent. Reasons for exclusion and subsequent trial enrolment and disposition are shown in Figure 2.
Figure 2
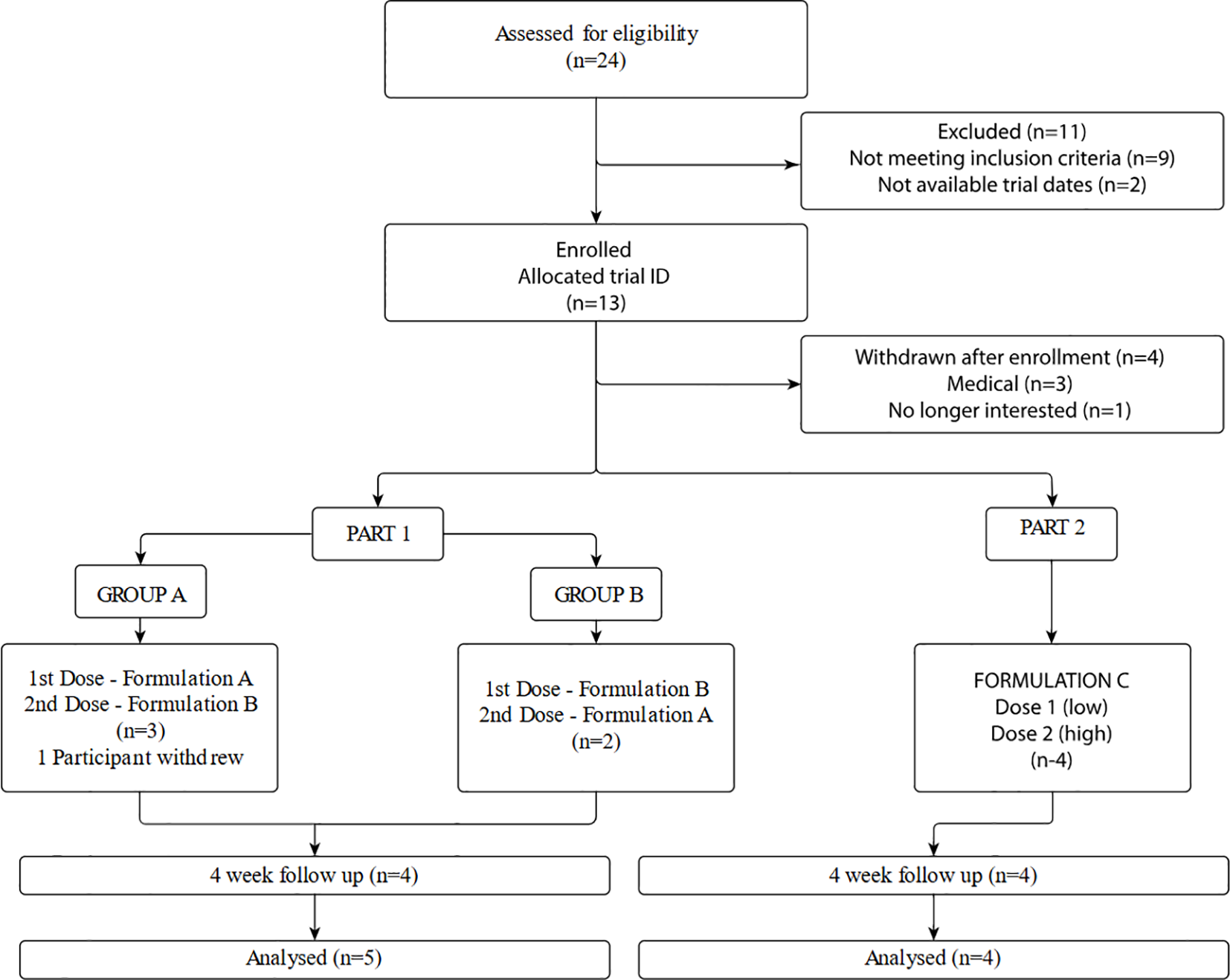
Trial enrolment and disposition.
Trial procedures
Screening and baseline assessment
During the in-person screening process a detailed physical and mental health history, and substance use history was taken including MINI-Plus psychiatric interview, the Alcohol Use Disorders Identification (AUDIT) and a general physical examination. This involved assessment of the general medical exclusion criteria, including blood pressure, heart rate, height, weight, body temperature, an ECG and blood screening (including pregnancy test if female). After review of the Participant Informed Consent Form (PICF) the participant was asked to sign the consent form and scheduled for their preparatory psychotherapy session. Participants were required to self-complete baseline psychometric assessments: Depression, Anxiety and Stress Rating Scale – 21 questions [DASS-21(41)]; Insomnia Severity Index [ISI (45)] – modified; Temporal Experience of Pleasure Scale [TEPS (46)]; Kessler - K10 (43); Positive and Negative Affect Schedule – Short Form [PANAS- SF (42)]. Participants were allocated to receive Formula A or Formula B in the first treatment session, in a cross-over design.
Preparatory session
The preparatory session lasted approximately 90–120 minutes with both treating therapists. During these sessions, the therapists provided psychoeducation to prepare the participant for the dosing session. The participant and therapists discussed what would happen during the session including relaying some of the commonly experienced effects of DMT-harmala medicines and the participants’ expectations for the dosing session. Therapists also inquired about any possible changes in the participant’s health to confirm that the participant met eligibility criteria. Participants were oriented to the optional use of therapeutic touch and given the opportunity to provide informed consent to include touch during their session, having the choice to rescind consent at any time. Participants were also given instructions regarding dietary restrictions and fasting requirements prior to treatment, arrival time, suitable clothing, use of an eye mask and headphones, and the music playlist. Participants were advised that in case of extreme persistent agitation an oral, intravenous, or intramuscular delivered sedative and antipsychotic combination (lorazepam and haloperidol) would be administered (from the psychiatrist or physician attending the session).
Dosing treatment (2 sessions)
On the day of the dosing treatment session the participant arrived approximately 1 hour prior to dose administration (aiming to arrive at 9am). Therapists reviewed procedures for the experimental session with the participant and discussed any participant concerns. Participants were asked to reconfirm consent in writing and confirm that they had fasted from the midnight and had not taken any excluded herbal supplements, contraindicated foods or medications (prescribed or over-the-counter). Participants were required to self-complete a visual analogue scale (VAS) of mood and anxiety and the PANAS. Participants’ blood pressure (BP) and heart rate (HR) were recorded. Approximately 1 hour after arrival participants were provided the oral dose capsules with a glass of water and instructed to take one harmala capsule followed by one DMT capsule in an alternating manner (to ensure a blended consumption). A 15-minute guided body scan and breathing audio track was played after which the music soundtrack commenced.
At least one of the trial therapists remained with the participant throughout the entire session (both therapists remain with the participant for most of the time, with the opportunity for short breaks) and were available for psychological and medical support during that time. This included a non-directive participant led approach to support the participant in exploring whatever psychological experience was emerging.
Cardiovascular monitoring (heart rate and blood pressure) was conducted at 90 minutes and again between 4 hours and 8 hours (based on when the participant’s psychedelic experience had waned; or assessed more regularly if needed), as well as ongoing monitoring of the participant psychiatric state throughout the dose sessions.
The session ended if all medical and psychiatric parameters were acceptable and the participant was alert, ambulatory, and psychologically stable. Prior to leaving at the end of the session, participants completed various measures assessing different aspects of their experience during the session, including: the Five Dimensions of Altered States of Consciousness scale [5D-ASC (38)], Mystical Experience Questionnaire [MEQ (37)] and VAS assessment of mood and anxiety.
The participant support person (close friend or family member) provided transportation to their residence. Therapists remained available to speak with participants for 24 hours after the treatment session via a study mobile phone number.
Post-treatment next day follow-up (2 sessions)
Participants were contacted by phone by the research coordinator the day following each treatment session. They were assessed for any AEs, any use of concomitant medications, and provided a general debrief. The PANAS was emailed to them to complete that day online. Participants were encouraged to use their dream diary. Participants were requested to inform study staff of any emergent AEs or inter-current illnesses. They were also reminded of all study restrictions.
One-week post-treatment follow-up assessments (2 assessments)
One week post-treatment participants were emailed a link for online self completion of the following assessments: VAS; Persisting Effects Questionnaire (PEQ); PANAS; TEPS; K-10; DASS-21; ISI- modified; Personal Insights Questionnaire (PIQ); Integration Difficulties Scale (IDS); Preparation & Support assessment (PIS); The modified Short-Index of Mystical Orientation (SIMO).
Integration (2 sessions)
The integration psychotherapy session was scheduled approximately one week after each dosing session and was conducted either in-person, or via videoconferencing. The participant was encouraged to discuss their insights, feelings and experiences during and after the session. The therapists supported the participant to process any residual psychological distress they were experiencing. The therapists were supportive, validating the participant’s experience, to facilitate emotional processing, allow exploration of content, and consolidate any therapeutic insights gained. Therapists assessed the participant’s mental health and the presence of any remaining reactions during integrative psychotherapy immediately after the dosing session. The participant was reminded that the therapists would be available for support outside the scheduled integration session via phone, telehealth, or in person by arrangement if extra support was required.
Four-week post-treatment follow-up and qualitative interview
Participants were contacted by phone by the research coordinator four weeks post their second treatment session. They were assessed for any emergent AEs, any use of concomitant medications, and a general debrief. Participants were sent an email link to complete Persisting Effects Questionnaire (PEQ); VAS; PANAS; TEPS; K-10; DASS-21; ISI- modified; Personal Insights Questionnaire (PIQ); Integration Difficulties Scale (IDS); Preparation & Support assessment (PIS); open-ended free text items.
A 60-minute qualitative interview was conducted approximately 4 weeks after the final treatment session by a post-graduate student with protocol specific and GCP training. Therapists were also interviewed to assess their experiences of conducting the treatment model. Results of the qualitative research will be published separately.
A detailed schedule of procedures and assessments is included as Appendix 1.
Data preparation and analysis
Baseline demographic and background variables are summarized for all participants. For categorical variables, frequencies and percentages are provided. For continuous variables, descriptive statistics including the sample size, mean, median, standard deviation and range, are presented. Continuous variables are summarized descriptively providing, where applicable, the number of participants, mean, standard deviation (SD), median, interquartile range (IQR), minimum (min) and maximum (max). Individual (absolute and change from baseline) and summary blood pressures, heart rate, respiratory rate, and body temperature and oxygen saturation are presented using descriptive statistics including mean, median, and standard deviation and range (min and max) as appropriate. AEs are summarized from each participant’s Adverse Event log by total individual number for each individual type of AE. The AE s on the Adverse Event log and the rating of severity is described. Serious Adverse Events (SAEs) and SAEs, drug medication-related AEs and serious drug-related AEs are also summarized.
Results
Given the small number of participants in this study and the inter-individual variation in responses, application of statistical analyses to the data is not appropriate. Aggregated scores and individual responses are presented where appropriate in tabular and graphic form.
Participant characteristics
Participant characteristics at baseline are shown in Table 1 for all participants who received at least one dose of the trial medication. Participants were five male and four female, aged 32 to 54 years (mean 40.6, SD 7.5 years). All were tertiary educated. Participants had previously used Ayahuasca on average 2.2 times (SD 1.5; range 1–5 times). The mean duration between most recent use and first trial dose was 5.3 years (SD8.2 years; range of 0.2 to 26.4 years).
Table 1
| Characteristic | Category / descriptive statistic | Participants |
|---|---|---|
| Gender | Female / male (n, %) | 4 (44.4) / 5 (55.6) |
| Age (years) | Mean (standard deviation) | 40.6 (7.5) |
| Median (interquartile range) | 39.2 (36.0 – 45.8) | |
| Range (minimum – maximum) | 31.8 – 53.5 | |
| Country of birth | Australia (n, %) | 7 (77.8) |
| Other (South Africa; Singapore) (n, %) | 2 (22.2) | |
| Aboriginality | Non Aboriginal / Torres Strait Islander (n, %) | 9 (100) |
| Highest level of education | Post-graduate (n, %) | 9 (100) |
| Ayahuasca Use History – number of sessions | Mean (standard deviation) | 2.2 (1.5) |
| Median (interquartile range) | 2 (1 – 3) | |
| Range (minimum – maximum) | 1 – 5 | |
| Ayahuasca Use History – date of first use | AEOS_1 | 2016 |
| AEOS_2 | 2016 | |
| AEOS_3 | 2013 | |
| AEOS_6 | Jan-23 | |
| AEOS_7 | Aug-18 | |
| AEOS_9 | Jul-23 | |
| AEOS_10 | 1997 | |
| AEOS_11 | Nov-19 | |
| AEOS_13 | Aug-19 | |
| Ayahuasca Use History – date of most recent use | AEOS_1 | 2018 |
| AEOS_2 | Late 2020 | |
| AEOS_3 | Nov-22 | |
| AEOS_6 | Jan-23 | |
| AEOS_7 | Aug-18 | |
| AEOS_9 | Jul-23 | |
| AEOS_10 | 1997 | |
| AEOS_11 | Nov-19 | |
| AEOS_13 | Aug-19 | |
| Ayahuasca Use History – duration between most recent use (before trial) and first dose of trial (years) | AEOS_1 | 4.7 |
| AEOS_2 | 2.4 | |
| AEOS_3 | 0.5 | |
| AEOS_6 | 0.2 | |
| AEOS_7 | 4.8 | |
| AEOS_9 | 0.3 | |
| AEOS_10 | 26.4 | |
| AEOS_11 | 4 | |
| AEOS_13 | 4.3 | |
| Mean (standard deviation) | 5.3 (8.2) | |
| Median (interquartile range) | 4.0 (0.5 – 4.7) | |
| Range (minimum – maximum) | 0.2 – 26.4 |
Participant characteristics at baseline (n=9).
Primary outcomes
All adverse events/ serious adverse events
All nine participants (100%) who received at least one dose of trial medication reported at least one Adverse Event (AE) from the signing of consent through to the end of the trial.
There were no serious adverse events (SAEs) that occurred during this study.
Of the non-serious adverse events, a total of 71 AEs occurred across the nine participants throughout the study. Three of these events were classified as severe (Flu Type A; Chest infection secondary to Flu; Agitation). Another 16 events were classified as moderate severity (urinary tract infection; insomnia; irritability x 2; panic attack; perceptual disturbance; nightmare; anxiety x 2; depressed mood; delusion; suicidal ideation; aggressive behavior; myalgia; pain; scar). The remaining 52 AEs were classified as mild severity.
Study medication related adverse events
Eight participants reported at least one AE that was considered to be related to the study medication. Of all AEs, 55 in total were considered to be study medication-related (78%, 55/71) (see Table 2). The most frequently occurring study medication-related AEs were adverse physical effects (69%, 38/55), followed by adverse mental health effects (31%, 17/55). The two most common physical AEs were “nausea” (n=11) and “headache” (n=7), while vomiting/retching occurred in 2 out of 9 participants. The most common mental health effect was anxiety (4/17). Of the 17 mental health effects, 11 events occurred when one participant received the higher dose of ACL-010 Formulation C (n=11). The high dose was well-tolerated by the other 3 participants. See Table 2.
Table 2
| Adverse event (preferred term defined in MedDRA*) | Total number (across both formulations) | Participants 1, 3, 6, 7 | Participants 9, 10, 11, 13 | ||
|---|---|---|---|---|---|
| Most recent formulation administered | |||||
| Formula A | Formula B | Formula C (low dose) | Formula C (high dose) | ||
| Number of participants with > 1 event | 8 of 9 (88.9%) | 4 of 5 (80%) | 4 of 4 (100%) | 4 of 4 (100%) | 4 of 4 (100%) |
| Adverse physical effects | 38 | 6 | 10 | 11 | 11 |
| General symptom adverse physical effects | |||||
| Nausea | 11 | 2 | 3 | 3 | 3 |
| Headache | 7 | 1 | 3 | 2 | 1 |
| Vomiting | 3 | . | . | 1 | 2 |
| Stomach cramps1 | 2 | 1 | 1 | . | . |
| Insomnia | 2 | . | 1 | 1 | . |
| Abdominal pain | 1 | . | 1 | . | . |
| Fatigue | 1 | . | 1 | . | . |
| Hunger | 1 | 1 | . | . | . |
| Compensatory sweating | 1 | – | – | 1 | – |
| Tachycardia2 | 1 | – | – | 1 | – |
| Tremor | 1 | – | – | – | 1 |
| Skin abrasions3 | 1 | – | – | – | 1 |
| Pain3 | 1 | – | – | – | 1 |
| Scar3 | 1 | – | – | – | 1 |
| Neurological adverse physical effects | |||||
| Brain fog | 3 | 1 | – | 1 | 1 |
| Paresthesia | 1 | – | – | 1 | – |
| Adverse mental health effects | 17 | 2 | 1 | 3 | 11 |
| Altered perception adverse mental health effects | |||||
| Hallucination, visual4 | 1 | 1 | . | . | . |
| Perceptual disturbance5 | 2 | . | . | 1 | 1 |
| Emotional-cognitive adverse mental health effects | |||||
| Anxiety | 4 | 1 | . | 1 | 2 |
| Irritability | 1 | . | 1 | . | . |
| Panic attack6 | 1 | . | . | . | 1 |
| Nightmare6 | 1 | . | . | . | 1 |
| Depressed mood6 | 1 | . | . | . | 1 |
| Delusion6 | 1 | . | . | . | 1 |
| Suicidal ideation6 | 1 | . | . | . | 1 |
| Agitation6 | 1 | . | . | . | 1 |
| Aggressive behaviour6 | 1 | . | . | . | 1 |
| Arthromyalgical adverse physical effects (MR) | |||||
| Myalgia | 2 | . | . | 1 | 1 |
| Total | 55 | 8 | 11 | 14 | 22 |
Number of study medication-related adverse events (n=55), total numbers and split by most recent study medication formula.
MedDRA, Medical Dictionary for Regulatory Activities.
*1) Therapist described these ‘stomach cramps’ as being different from ‘abdominal pain’.
*2) The event of ‘tachycardia’ was a brief increase in heart rate.
*3) These 3 adverse events were due to the one incident of the participant rubbing their head on carpet, causing a slight skin abrasion, followed by 24 hours of moderate severity pain, followed by a scar whilst the abrasion healed.
*4) Therapist described this Hallucination, visual event as a “closed eye visual”. It occurred 4 days post dose (formula A “Recurrence of visuals from second dose when eyes closed. Not unpleasant, very subtle … this occurred during another altered state exp”.
*5) Two events were classified as a perceptual disturbance. An event of “disembodied”, and a “feeling disconnected or alone”.
*6 ) All events occurred in one participant following dose 2 of formula C (high dose).
Of the 55 AEs considered to be study medication-related, most had a stop date during the trial (n=53), with 2 events ongoing at the end of the trial (skin abrasion, scar). These two events were related and were effectively a “carpet burn” caused from repeated rubbing on the carpet of the treatment room. Thirty-nine AEs stopped the same day as the event had started, and eight the following day, 37 of 55 AEs did not require any medication / intervention with each event resolving itself. The median duration was 4.8 hours with an interquartile range of 1.1 to 12.3 hours. The shortest event lasted 15 minutes and the longest event was 19 days (mild headache which resolved without medication).
Physical examination, vital signs, and pathology
Physical examinations included the following: general appearance, HEENT (head, ears, eyes, nose and throat), skin, cardiovascular system, respiratory system, gastrointestinal system, nervous system, vital signs and other. Any changes in physical condition were noted by the principal investigator and AEs were recorded in the AE log by the study coordinator and trial clinicians. Most participants recorded no changes in general appearance and physical condition across the duration of the study. One participant suffered a facial abrasion during a treatment session which was recorded as an AE.
Vital signs monitoring included body temperature, heart rate, respiratory rate, oxygen saturation and blood pressure. No clinically significant changes were recorded. Vital signs (absolute values, and changes from baseline) are reported in Appendix 2.
There were no significant changes in pathology results for participants over the course of the study. Pathology values are reported in Appendix 3.
Integration difficulties
Integration Difficulties Scale (IDS) showed that participants reported low rates on integration difficulties across all formulations and timepoints with slightly higher average scores for ACL-010 Formulation C (low and high) See Table 3A.
Table 3A
| Integration Difficulties Scale (IDS): is a list of 10 difficult experiences. Participants are asked if these difficulties had increased since their treatment session. Answers included “not at all”, “slightly”, “moderately”, “very much” and “unsure”. | |||||||
|---|---|---|---|---|---|---|---|
| Outcome measure | Descriptive statistic | Product A (n=5) | Product B (n=4) | A/B (n=4) | Product C (low dose) (n=4) | Product C (high dose) (n=4, unless specified) | |
| 1 week post T* | 1 week post T* | 4 week post T* | 1 week post T1 | 1 week post T2 | 4 week post T2 (n=3) | ||
| Count of | Mean (SD) | 9.4 (0.9) | 9.5 (1.0) | 9.5 (0.6) | 8.5 (1.9) | 8.3 (0.5) | 8.7 (0.6) |
| “not at all” | Min – Max | 8 – 10 | 8 – 10 | 9 – 10 | 6 – 10 | 8 – 9 | 8 – 9 |
| Count of “slightly”, “moderately” and “very much” | Mean (SD) | 0.6 (0.9) | 0.5 (1.0) | 0.5 (0.6) | 1.5 (1.9) | 1.8 (0.5) | 1.3 (0.6) |
| Min – Max | 0 – 2 | 0 – 2 | 0 – 1 | 0 – 4 | 1 – 2 | 1 – 2 | |
| Count of | Mean (SD) | 0 | 0 | 0 | 0 | 0 | 0 |
| “very much” | Min – Max | – | – | – | – | – | – |
Integration Difficulties Scale (IDS) 1 and 4 weeks post treatment.
Mystical experience
On the primary psychometric scale outcome measure, the MEQ (given immediately after each psychedelic experience), the mean total MEQ scores were higher (and within the range of Complete Mystical Experience) for ACL-010 Formulation C (low and high) compared to formulas A and B, and on all MEQ subscales except “Positive Mood”. However, it should be noted that the high SD on all scales indicates high inter-individual variability See Table 3B.
Table 3B
| Mystical Experience Questionnaire (MEQ): 6-point rating scale (0 = “none; not at all”, 1 = “so slight cannot decide”, 2 = “slight”, 3 = “Moderate”, 4 = “strong”, and 5 = “extreme (more than ever before in my life)”. The sum of the answers in each subscale are expressed as a percentage of the maximum possible. | |||||
|---|---|---|---|---|---|
| Outcome measure | Descriptive statistic | Product A (n=5) | Product B (n=4) | Product C (low dose) (n=4) | Product C (high dose) (n=4, unless specified) |
| Post T* | Post T* | Post T1 | Post T2 | ||
| 1. Mystical | Mean (SD) | 38.4 (15.7) | 47.7 (35.5) | 62.3 (36.2) | 71 (33.2) |
| Minimum - Maximum | 18.7 – 56.0 | 6.7 – 93.3 | 10.7 – 94.7 | 24.0 – 100 | |
| 2. Positive Mood | Mean (SD) | 46.7 (24.4) | 61.7 (19.3) | 67.5 (40.8) | 70 (34.7) |
| Minimum - Maximum | 23.3 – 80.0 | 40.0 – 86.7 | 6.7 – 93.3 | 20.0 – 100 | |
| 3. Transcendence of Time/Space | Mean (SD) | 44.7 (22.9) | 55 (28.4) | 79.2 (23.8) | 85.0 (13.7) |
| Minimum - Maximum | 16.7 – 70.0 | 13.3 – 76.7 | 46.7 – 100 | 73.3 – 100 | |
| 4. Ineffability | Mean (SD) | 65.3 (17.3) | 60 (49.0) | 76.7 (34.6) | 96.7 (3.9) |
| Minimum - Maximum | 40.0 – 86.7 | 0 – 100 | 26.7 – 100 | 93.3 – 100 | |
| MEQ Total | Mean (SD) | 44 (12.4) | 53.2 (30.9) | 68.2 (33.6) | 76.2 (25.8) |
| Minimum - Maximum | 24.0 – 53.3 | 14.0 – 89.3 | 18.7 – 93.3 | 40.0 – 100 | |
Mystical Experience Questionnaire (MEQ).
T = treatment session
T1 = treatment session 1
T2 = treatment session 2
T* = treatment session (either 1 or 2 depending on the random order of product A / product B)
Pre T = during the treatment session prior to the administration of the study medication
Post T = during the treatment session at the conclusion of the psychedelic experience (a minimum of 4 hours to 8 hours post dose)
SD = 1 standard deviation
Min = minimum
Max = maximum
Secondary acute effects
Tolerability and differential experience
At the end of the treatment session trial participants were asked to compare their experience with the trial medication to past experience with traditional Ayahuasca, specifically the strength of the psychedelic experience, the subjective experience (quality), and how beneficial they found the experience. The strength of psychedelic experience of formulations A and B were generally rated as weaker than previous experience with Ayahuasca whereas low dose ACL-010 was rated as similar, with the higher dose version of this formulation being rated as stronger. The subjective experience (quality) of all formulations was generally rated as similar to previous experience with Ayahuasca. Both dose levels of ACL-010 Formulation C were rated as similar or more beneficial than previous experience with Ayahuasca, while Formulations A and B tended to be as less beneficial than previous experience with Ayahuasca See Figures 3A–C.
Figure 3
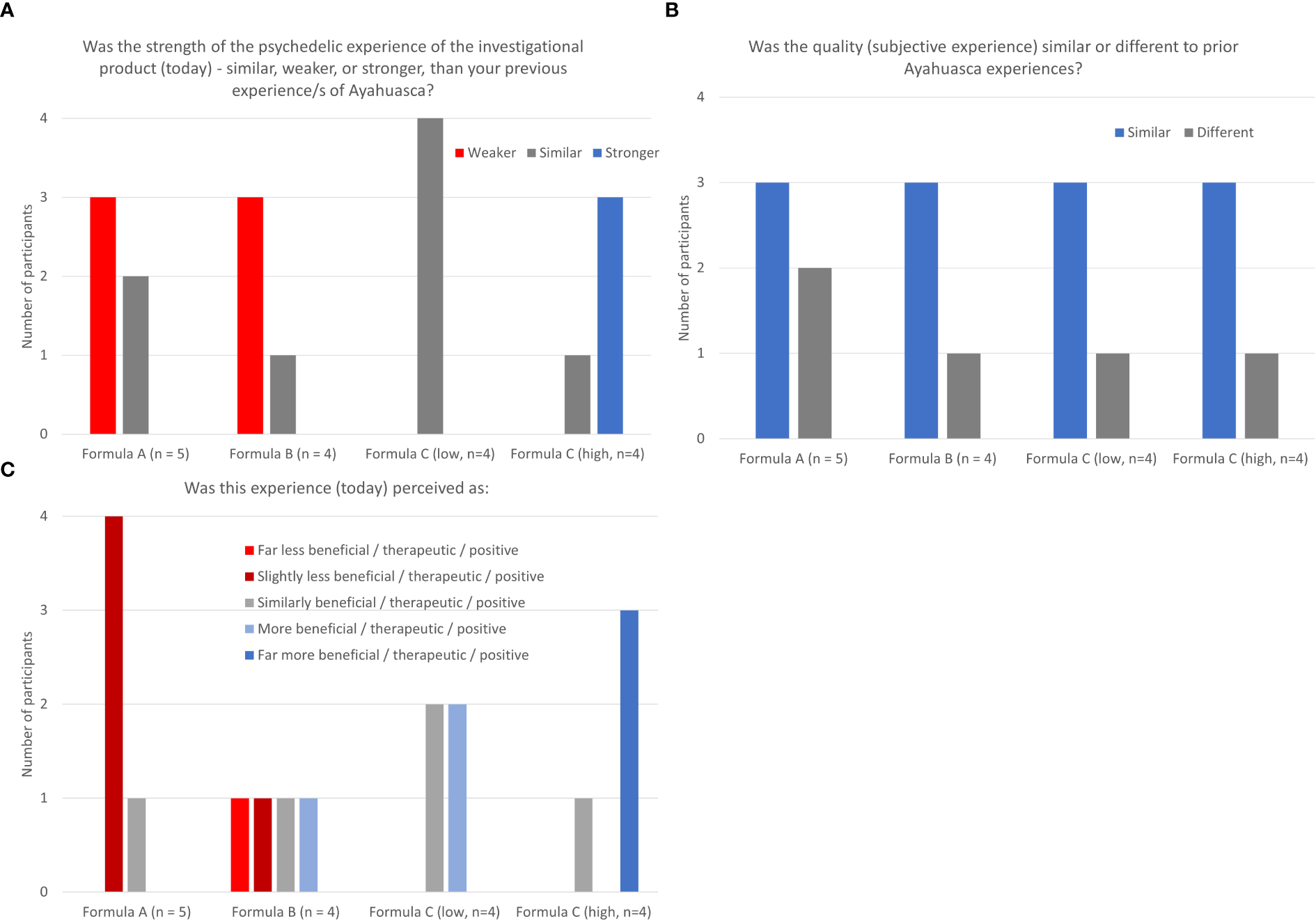
(A–C) Tolerability and differential experience: strength, quality, and benefit of psychedelic experience.
Subjective experience
The modified Short Index of Mystical Orientation scores for ACL-010 Formulation C (high) were higher than those for Formulations A, B and C (low). Item 10 “Feeling of immense fear” was given the lowest possible rating by participants after dosing with formulations A and B, however ACL-010 Formula C (high) was rated more highly, and in particular two participants scored 10 (max) and 8 post ACL-010 Formulation C (high). See Table 3C. On the Five Dimensions of Altered States of Consciousness (5D-ASC) scale there was a trend on the 11 subscales for higher ratings on ACL-010 (Formulation C), particularly at the higher dose, however there was high inter-individual variability and ratings of the 3 formulas was inconsistent. See Table 3D. Both mood and anxiety scores on the VAS were generally relatively low (below 30/100) at each time point but with some inter- and intra-participant variability. Mood scores were generally lower than anxiety scores See Table 3E.
Table 3C
| Short-Index of Mystical Orientation (SIMO): 10 items about the participant’s inclination towards mystical experiences and spiritual connectedness. (Responses for each item: 1 = “not at all”; 10 = “very much”). | |||||
|---|---|---|---|---|---|
| Outcome measure | Descriptive statistic | Product A | Product B | Product C (low dose) | Product C (high dose) |
| (n=5) | (n=4) | (n=4) | (n=4, unless specified) | ||
| Post T* | Post T* | Post T1 | Post T2 | ||
| Sum of Items 1-9 (maximum = 90) | Mean (SD) | 37.2 (13.7) | 41.3 (28.1) | 53.8 (27.0) | 70.8 (20.0) |
| Min – Max | 23 – 58 | 10 – 77 | 31 – 86 | 41 – 84 | |
| Raw score (Item 10: feeling immense fear …) |
Mean (SD) | 1 (0) | 1 (0) | 1.8 (1.0) | 5.8 (3.9) |
| Min - Max | 1 – 1 | 1 – 1 | 1 – 3 | 2 – 10 | |
Acute effect measures: Short-Index of Mystical Orientation (SIMO).
Table 3D
| 5 Dimensions of Altered States of Consciousness (5D-ASC) scale: Eleven subscales (sum of the answers in each subscale, expressed as a percentage of the maximum possible). | |||||
|---|---|---|---|---|---|
| Outcome measure | Descriptive statistic | Product A | Product B | Product C | Product C |
| (n=5) | (n=4) | (low dose) | (high dose) | ||
| (n=4) | (n=4, unless specified) | ||||
| Post T* | Post T* | Post T1 | Post T2 | ||
| 1. Experience of Unity | Mean (SD) | 52.5 (13.2) | 51.0 (33.7) | 50.4 (31.5) | 74.7 (21.8) |
| Min - Max | 35.4 – 71.8 | 11.0 – 93.0 | 17.2 – 89.2 | 42.8 – 91.6 | |
| 2. Spiritual Experience | Mean (SD) | 55.1 (14.0) | 63.6 (36.0) | 57.7 (46.5) | 70.5 (38.4) |
| Min - Max | 31.3 – 68.3 | 18.3 – 96.7 | 8.3 – 100 | 14.3 – 100 | |
| 3. Blissful State | Mean (SD) | 57 (15.6) | 60.7 (18.4) | 41.8 (33.9) | 51.2 (26.3) |
| Min - Max | 33.0 – 75.7 | 37.3 – 79.0 | 2.7 – 84.3 | 22.0 – 85.3 | |
| 4. Insightfulness | Mean (SD) | 54.9 (12.5) | 68.3 (21.8) | 63.8 (32.6) | 69.6 (18.3) |
| Min - Max | 33.0 – 63.7 | 36.7 – 86.7 | 31.7 – 98.3 | 46.7 – 91.0 | |
| 5. Disembodiment | Mean (SD) | 51.7 (30.6) | 54.3 (24.4) | 52.8 (19.6) | 52.8 (16.2) |
| Min - Max | 3.0 – 87.7 | 18.3 – 70.3 | 31.7 – 72.7 | 29.0 – 64.0 | |
| 1. 6. Impaired Control and Cognition | Mean (SD) | 24.6 (23.4) | 26.2 (22.8) | 39.5 (19.6) | 50.8 (11.8) |
| Min - Max | 2.9 – 57.1 | 0 – 49.7 | 18.3 – 59.4 | 40.0 – 67.3 | |
| 7. Anxiety | Mean (SD) | 14.4 (15.2) | 21.5 (21.9) | 34.7 (29.2) | 55.7 (24.8) |
| Min - Max | 3.0 – 41.0 | 0 – 49.0 | 4.0 – 64.2 | 32.2 – 85.3 | |
| 8. Complex Imagery | Mean (SD) | 50.2 (17.4) | 70.6 (25.4) | 79.2 (29.1) | 88.8 (7.9) |
| Min - Max | 40.7 – 76.3 | 37.0 – 95.3 | 36.0 – 98.0 | 78.7 – 95.7 | |
| 9. Elementary Imagery | Mean (SD) | 51.7 (29.3) | 51.7 (46.9) | 79.1 (23.3) | 85.6 (14.7) |
| Min - Max | 27.7 – 92.3 | 0 - 96.7 | 49.0 – 98.0 | 64.3 – 97.0 | |
| 10. Audio-Visual Synesthesia | Mean (SD) | 56.5 (14.8) | 65.2 (32.6) | 84.1 (19.0) | 76.7 (29.3) |
| Min - Max | 31.0 – 67.7 | 19.7 – 89.7 | 57.0 – 100 | 36.0 – 100 | |
| 11. Changed Meaning of Percepts | Mean (SD) | 36.3 (20.1) | 56.7 (40.5) | 61.4 (7.3) | 66.1 (19.2) |
| Min - Max | 6.7 – 56.0 | 0 – 91.3 | 51.0 – 67.7 | 40.0 – 84.7 | |
5 Dimensions of Altered States of Consciousness (5D-ASC) scale.
Table 3E
| Visual Analogue Scale (VAS) - anxiety: “Note how anxious (on average) you felt over the past 24 hours with the slider on the scale”, (minimum (0) = not at all anxious; maximum (100) = extremely anxious). Visual Analogue Scale (VAS) - mood: “Note how your mood was (on average) over the past 24 hours with the slider on the scale”, (minimum (0) = not at all depressed; maximum (100) = extremely depressed). | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcome measure | Descriptive statistic | Product A | Product B | A/B | Product C (low dose) | Product C (high dose) | |||||||||
| (n=5) | (n=4) | (n=4) | (n=4) | (n=4, unless specified) | |||||||||||
| Pre T* | Post T* | 1 week post T* | Pre T* | Post T* | 1 week post T* | 4 week post T* | Pre T1 | Post T1 | 1 week post T1 | Pre T2 | Post T2 | 1 week post T2 | 4 week post T2 | ||
| (n=3) | |||||||||||||||
| Anxiety | Mean SD Min - Max |
16.2 23.6 1 - 58 |
12. 14.9 0 - 37 |
16.8 28.7 1 - 68 |
24.5 33.6 0 - 74 |
7.0 4.5 1 - 11 |
26.3 27.1 3 - 54 |
14.8 12.4 2 - 30 |
32.5 20.0 14 - 61 |
38.8 24.7 11 - 67 |
20 10.9 12 - 36 |
24.3 11.6 15 - 40 |
18.5 11.0 3 - 28 |
12.5 8.5 6 - 25 |
23.3 9.3 13 - 31 |
| Mood | Mean SD Min - Max |
3 2.3 1 - 6 |
4.2 5.6 0 - 14 |
14.4 20.5 0 - 50 |
5.5 6.0 0 - 14 |
2.5 4.4 0 - 9 |
5 4.2 1 - 10 |
8.5 6.7 3 - 18 |
22.8 18.5 6 - 49 |
10 8.6 2 - 22 |
13 10.8 4 - 27 |
13.8 14.5 2 - 33 |
12.3 12.0 2 - 28 |
27.3 34.8 1 - 78 |
24.3 24.6 1 - 50 |
Visual Analogue Scale (VAS) for anxiety and mood.
Secondary four week follow-up effects
On other outcomes, the Depression Anxiety Stress Scale – 21 (DASS-21), the PANAS, and the Kessler-10 (K-10) ratings across all time points and formulations showed little variation. et al., 20 See Tables 4A-C. As detailed in Table 4D, on the PEQ positive responses for all subscales across all formulations were greater than their negative counterparts. For each of the six positive subscales, responses for Formulations B, C (low and high) tended to be higher than Formulation A. ACL-010 Formulation C (low and high) tended to score more highly on each of the additional parameters “How personally meaningful were the experiences”, “the degree to which the experiences were spiritually significant”, “how psychologically challenging were … the experiences”, “how personally psychologically insightful”, “the degree to which the experiences changed the sense of personal well-being or life satisfaction”. Effects on all subscales tended to continue to the 4-week post last dose assessment.
Table 4A
| Depression, Anxiety and Stress Scale (DASS21): Individual responses range from 0 = “did not apply to me at all” to 3 = “most of the time”. For the total of the 7 “depression” items, “normal” depression ranges from 0 – 4. For the total of the 7 “anxiety” items, “normal” anxiety ranges from 0 – 3. For the total of the 7 “stress” items, “normal” stress ranges from 0 to 7. | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcome measure | Descriptive statistic | Product A (n=5) | Product B (n=4) | A/B (n=4) | Product C (low dose) (n=4) | Product C (high dose) (n=4, unless specified) | |||
| Base (n=5) | 1 week post T* | 1 week post T* | 4 week post T* | Base (n=4) | 1 week post T1 | 1 week post T2 | 4 week post T2 (n=3) | ||
| Depression Score |
Mean (SD) Min – Max |
1.2 (1.3) 0 – 3 |
0.8 (0.8) 0 – 2 |
0.5 (0.6) 0 – 1 |
0.8 (1.0) 0 – 2 |
0.8 (1.0) 0 – 2 |
1.0 (1.4) 0 – 3 |
0.5 (1) 0 - 2 |
0.7 (1.2) 0 – 2 |
| Anxiety Score | Mean (SD) Min – Max |
0 - |
0.2 (0.4) 0 – 1 |
0.8 (1.0) 0 – 2 |
0.3 (0.5) 0 – 1 |
0 - |
0.3 (0.5) 0 – 1 |
0.5 (0.6) 0 – 1 |
0.3 (0.6) 0 – 1 |
| Stress Score | Mean (SD) Min – Max |
3.4 (3.0) 0 – 7 |
3.4 (5.3) 0 – 12 |
4.3 (3.0) 1 – 8 |
3.3 (3.8) 0 – 7 |
2.3 (1.3) 1 – 4 |
2.8 (1.0) 2 – 4 |
1.5 (0.6) 1 – 2 |
1.7 (0.6) 1 – 2 |
Four-week follow-up effect measures: Depression, Anxiety and Stress Scale (DASS_21).
Table 4B
| Positive Affect and Negative Affect Scale (PANAS): Positive Affect (PA) items are related to pleasurable engagement with the environment, and the Negative Affect (NA) items reflect general distress (e.g. anger, guilt, anxiety). Each item is rated on a 5-point scale ranging from 1=“very slight, or not at all” to 5=“extremely”. The final score is the sum of the ten items for both the positive and negative affect. | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcome measure | Descriptive statistic | Product A (n=5) | Product B (n=4) | A/B (n=4) | Product C (low dose) (n=4) | Product C (high dose) (n=4, unless specified) | |||||||||||
| Base (n=5) | Pre T* | 1 day Post T* | 1 week post T* | Pre T* | 1 day Post T* | 1 week post T* | 4 week post T* | Base (n=4) | Pre T1 | 1 day post T1 | 1 week post T1 | Pre T2 | 1 day post T2 | 1 week post T2 | 4 week post T2 (n=3) | ||
| Positive Affect (PA) | Mean (SD) Min-Max |
38.4 (4.0) 34 - 43 |
37.4 (2.5) 35 - 40 |
37.5 (3.7) 32 - 40 |
33.2 (7.7) 20 - 40 |
38.5 (5.9) 34 - 47 |
38.3 (2.9) 35 - 42 |
37.8 (1.5) 36 - 39 |
35.8 (9.3) 22 - 42 |
34 (8.9) 24 - 42 |
31.5 (4.7) 27 - 36 |
35.3 (8.6) 23 - 43 |
35.5 (5.8) 30 - 43 |
36 (6.3) 29 - 44 |
n=2* 36.5 (0.7) 36 - 37 |
36.8 (3.3) 33 - 40 |
36.7 (3.5) 33 - 40 |
| Negative Affect (NA) | Mean (SD) Min-Max |
15.0 (4.0) 11 - 19 |
15.0 (5.0) 11 - 23 |
15.6 (5.5) 11 - 24 |
15.6 (6.3) 10 - 25 |
17.0 (8.5) 10 - 29 |
14.8 (4.6) 10 - 20 |
15.0 (4.2) 11 - 20 |
15.8 (4.6) 11 - 21 |
3.3 (1.7) 11 - 15 |
16.3 (4.9) 12 - 21 |
16.8 (6.4) 11 - 26 |
14.0 (3.4) 12 - 19 |
13.8 (2.2) 11 - 16 |
n=2* 24.0 (5.7) 20 - 28 |
13.8 (2.9) 10 - 17 |
14.0 (1.0) 13 - 15 |
Four-week follow-up effect measures: Positive and Negative Affect Scale (PANAS).
Table 4C
| Kessler-10 (K10): 10 items to identify significant levels of psychological distress. Response to individual items range from 1 = “none of the time” to 5 = “all of the time”. | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcome measure | Descriptive statistic | Product A (n=5) | Product B (n=4) | A/B (n=4) | Product C (low dose) (n=4) | Product C (high dose) (n=4, unless specified) | |||
| Baseline (n=5) | 1 week post T* | 1 week post T* | 4 week post T* | Baseline (n=4) | 1 week post T1 | 1 week post T2 | 4 week post T2 (n=3) | ||
| Total (max=50) |
Mean (SD) Min – Max |
13.8 (2.7) 10 – 16 |
14.6 (4.4) 11 – 22 |
13.8 (2.5) 11 – 17 |
13.5 (3.4) 10 – 18 |
13.0 (1.2) 12 – 14 |
13.3 (1.0) 12 – 14 |
13.0 (1.6) 11 – 15 |
12.7 (1.2) 12 – 14 |
Four-week follow-up effect measures: Kessler-10 (K10).
Table 4D
| Persisting Effects Questionnaire (PEQ): 140 questions that assess changes in attitudes, moods, behavior, and spiritual experience. Each question is rated on a 6-point scale (0 = “none, not at all” to 5 = “extreme, more than ever before in your life and stronger than 4”). Each of the 6 subscales have a positive and a negative version. Scores are expressed as the percentage of the maximum possible score for each subscale. | |||||||
|---|---|---|---|---|---|---|---|
| Outcome measure | Descriptive statistic | Product A (n=5) | Product B (n=4) | A/B (n=4) | Product C (low dose) (n=4) | Product C (high dose) (n=4, unless specified) | |
| 1 week post T* | 1 week post T* | 4 week post T* | 1 week post T1 | 1 week post T2 | 4 week post T2 (n=3) | ||
| 1a) Positive attitudes about life | Mean (SD) Minimum - Maximum |
29.2 (10.1) 18.5 – 38.5 |
48.8 (36.2) 0 – 83.1 |
38.8 (25.9) 6.2 – 69.2 |
49.6 (27.0) 18.5 – 78.5 |
66.2 (24.1) 35.4 – 90.8 |
62.6 (14.0) 47.7 – 75.4 |
| 1b) Negative attitudes about life | Mean (SD) Minimum - Maximum |
0.6 (0.8) 0 – 1.5 |
0.4 (0.8) 0 – 1.5 |
1.9 (3.8) 0 – 7.7 |
4.2 (2.3) 1.5 – 6.2 |
7.3 (4.4) 1.5 – 12.3 |
4.1 (5.8) 0 – 10.8 |
| 2a) Positive attitudes about self | Mean (SD) Minimum - Maximum |
24.7 (10.2) 14.5 – 38.2 |
40.9 (33.9) 0 – 80 |
35.5 (21.1) 16.4 – 65.5 |
45.0 (29.6) 5.5 – 74.6 |
65.5 (16.5) 45.5 – 83.6 |
59.4 (6.4) 52.7 – 65.5 |
| 2b) Negative attitudes about self | Mean (SD) Minimum - Maximum |
2.5 (4.7) 0 – 10.9 |
0.9 (1.1) 0 – 1.8 |
2.7 (5.5) 0 – 10.9 |
1.8 (2.6) 0 – 5.5 |
9.1 (2.6) 7.3 – 12.7 |
4.2 (1.0) 3.6 – 5.5 |
| 3a) Positive mood changes | Mean (SD) Minimum - Maximum |
24.9 (7.6) 13.3 – 33.3 |
38.3 (31.5) 0 – 75.6 |
32.8 (20.8) 13.3 – 62.2 |
46.1 (34.4) 0 - 80 |
53.3 (21.1) 22.2 – 68.9 |
51.1 (19.4) 28.9 – 64.4 |
| 3b) Negative mood changes | Mean (SD) Minimum - Maximum |
1.8 (2.4) 0 – 4.4 |
2.2 (4.4) 0 – 8.9 |
3.3 (4.3) 0 – 8.9 |
1.1 (2.2) 0 – 4.4 |
7.2 (6.4) 0 – 15.6 |
1.5 (2.6) 0 – 4.4 |
| 4a) Altruistic / positive social effect | Mean (SD) Minimum - Maximum |
21.8 (12.0) 6.7 – 37.8 |
42.8 (28.5) 0 – 57.8 |
36.7 (22.4) 11.1 – 62.2 |
30.6 (22.0) 0 – 51.1 |
43.4 (24.4) 17.8 – 71.1 |
45.2 (9.0) 35.6 – 53.3 |
| 4b) Antisocial / negative social effect | Mean (SD) Minimum - Maximum |
4.9 (8.5) 0 – 20 |
3.3 (3.9) 0 – 6.7 |
5.0 (8.6) 0 – 17.8 |
1.7 (2.1) 0 – 4.4 |
7.8 (5.9) 0 – 13.3 |
2.2 (2.2) 0 – 4.4 |
| 5a) Positive behavioral changes | Mean (SD) Minimum - Maximum |
24 (8.9) 20 – 40 |
45 (34.2) 0 – 80 |
45 (19.1) 20 – 60 |
40 (46.2) 0 – 80 |
55 (37.9) 0 – 80 |
60 (20.0) 40 – 80 |
| 5b) Negative behavioral changes | Mean (SD) Minimum - Maximum |
0 - |
0 - |
5 (10) 0 – 20 |
5 (10) 0 – 20 |
15.0 (19.1) 0 – 40 |
6.7 (11.5) 0 – 20 |
| 6a) Increased spirituality | Mean (SD) Minimum - Maximum |
16.2 (13.9) 0 – 36.4 |
33.6 (29.7) 0 – 60 |
29.3 (24.6) 0.9 – 59.1 |
34.1 (25.0) 0 – 60 |
41.8 (21.8) 22.7 – 67.3 |
41.2 (19.8) 19.1 – 57.3 |
| 6b) Decreased spirituality | Mean (SD) Minimum - Maximum |
1.1 (1.2) 0 – 2.9 |
0 - |
0.7 (1.4) 0 – 2.9 |
1.0 (1.1) 0 – 1.9 |
2.4 (3.0) 0 – 6.7 |
1.0 (1.0) 0 – 1.9 |
| Additional questions about the experiences during the participant’s last session. I) How personally meaningful were the experiences? (1 = no more than routine, everyday experiences to 8 = the single most meaningful experience of my life); II) Indicate the degree to which the experiences were spiritually significant to you? (1 = no more than routine, everyday experiences to 8 = the single most spiritually significant experience of my life; III) How psychologically challenging were the most psychologically challenging portions of the experiences? (1 = no more than routine, everyday experiences to 8 = the single most difficult or challenging experience of my life); IV) How personally psychologically insightful to you were the experiences? (1 = no more than routine, everyday psychologically insightful experiences to 8 = the single most psychologically insightful experience of my life); V) do you believe that the experiences and your contemplation of that experience have led to change in your current sense of personal well-being or life satisfaction? (-3 = decreased very much, 0 = no change, +3 = increased very much). | |||||||
| I) Personally meaningful | Mean (SD) Minimum - Maximum |
3.6 (1.1) 2 – 5 |
3.8 (2.5) 1 – 7 |
4.0 (1.4) 3 – 6 |
5.8 (1.3) 4 – 7 |
7 (0) 7 – 7 |
6.7 (0.6) 6 – 7 |
| II) Spiritually significant | Mean (SD) Minimum - Maximum |
3.6 (0.9) 3 – 5 |
3.5 (2.1) 1 – 6 |
4.3 (1.9) 3 – 7 |
4.5 (2.6) 1 – 7 |
7 (0) 7 – 7 |
7 (0) 7 – 7 |
| III) Psychologically challenging | Mean (SD) Minimum - Maximum |
1.8 (0.8) 1 – 3 |
2.0 (1.2) 1 – 3 |
3.3 (1.3) 2 – 5 |
4.8 (1.7) 3 – 7 |
6.5 (1.3) 5 – 8 |
6.7 (1.5) 5 - 8 |
| IV) Psychologically insightful | Mean (SD) Minimum - Maximum |
2.8 (1.5) 1 - 5 |
3.8 (2.1) 1 – 6 |
4.0 (1.6) 2 – 6 |
5.3 (1.7) 3 – 7 |
7.3 (0.5) 7 – 8 |
7.0 (1.0) 6 – 8 |
| V) Change in well-being / life satisfaction | Mean (SD) Minimum - Maximum |
0.6 (0.5) 0 – 1 |
1.3 (1.0) 0 – 2 |
1.5 (1.3) 0 – 3 |
1.8 (1.0) 1 – 3 |
2.0 (0.8) 1 – 3 |
2.0 (0) 2 – 2 |
Four-week follow-up effect measures: Persisting Effects Questionnaire.
Insomnia Severity Index (ISI) (45) average total scores across all formulations were within the “no clinically significant insomnia” range (noting there was high variability across all formulations on Sleep Quality scores) See Table 4E.
Table 4E
| Insomnia Severity Index (ISI) + 2 questions: to assess the nature, severity, and impact of insomnia. Responses to the 7 items are based on a 5-point Likert scale. A total score of 0–7 indicates “no clinically significant insomnia”, 8–14 indicates “subthreshold insomnia”, 15–21 indicates “clinical insomnia (moderate severity)”, and 22–28 indicates “clinical insomnia (severe)”. Sleep Quality: “How would you rate your sleep quality in the last week?” Responses ranged from 0 = Highly satisfactory to 4 = not at all satisfactory. Sleep hours: ”How many hours sleep have you had for the past week?” | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcome measure | Descriptive statistic | Product A (n=5) | Product B (n=4) | A/B (n=4) | Product C (low dose) (n=4) | Product C (high dose) (n=4, unless specified) | |||
| Base (n=5) | 1 week post T* | 1 week post T* | 4 week post T* | Base (n=4) | 1 week post T1 | 1 week post T2 | 4 week post T2 (n=3) | ||
| Total | Mean (SD) Min – Max |
5.2 (3.9) 3 – 12 |
5.8 (5.0) 1 – 14 |
6.0 (3.9) 1 – 10 |
5.0 (3.7) 1 – 10 |
2.8 (1.5) 1 – 4 |
5.5 (2.1) 3 – 8 |
4.5 (3.3) 0 – 7 |
3.7 (1.5) 2 – 5 |
| Sleep Quality | Mean (SD) Min – Max |
1.6 (0.9) 1 – 3 |
1.8 (0.8) 1 – 3 |
1.5 (0.6) 1 – 2 |
1.5 (1) 1 – 3 |
1.0 (0) 1 – 1 |
1.8 (1.0) 1 – 3 |
1.3 (0.5) 1 – 2 |
1.3 (0.6) 1 – 2 |
| Sleep hours (past week) |
Mean (SD) Min – Max |
47.8 (2.8) 45 – 51 |
48.0 (5.8) 42 – 56 |
n=3 49.7 (5.7) 45 – 56 |
n=3 49.0 (6.1) 45 – 56 |
49.0 (3.4) 45 – 53 |
46.8 (3.5) 45 – 52 |
n=3 49.7 (0.6) 49 – 50 |
47.3 (2.5) 45 – 50 |
Four-week follow-up effect measures: Insomnia Severity Index (ISI).
On other outcome scales, the TEPS data showed that scores across both subscales and total TEPS scores were relatively high for all formulations and time points See Table 4F. Personal Insights assessed via the PIQ revealed that the average number of insights experienced was lowest for participants 1 week post dose Formulation A, and greatest for participants 4 weeks post dose ACL-010 Formulation C (high) See Table 4G.Finally, on the Ayahuasca Preparation and Support Scale the average of the participant’s responses ranged between 3 (moderately) and 4 (very much). This was consistent across all formulations See Table 4H.
Table4F
| Temporal Experience of Pleasure Scale (TEPS): designed to measure individual trait dispositions in both anticipatory (10 items) and consummatory (8 items) experiences of pleasure. Responses to the 18 items are based on a 6-point Likert scale (1 = “very false” for me to 6 = “very true for me”). Items are averaged in each subscale. Higher scores indicate a stronger tendency to anticipate or experience pleasure. | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcome measure | Descriptive statistic | Product A (n=5) | Product B (n=4) | A/B (n=4) | Product C (low dose) (n=4) | Product C (high dose) (n=4, unless specified) | |||
| Base (n=5) | 1 week post T* | 1 week post T* | 4 week post T* | Base (n=4) | 1 week post T1 | 1 week post T2 | 4 week post T2 (n=3) | ||
| Anticipatory | Mean (SD) Min – Max |
4.9 (0.2) 4.5 – 5.0 |
4.6 (0.9) 3.1 – 5.2 |
4.6 (0.5) 3.8 – 5.0 |
4.9 (0.7) 4.0 – 5.6 |
4.9 (0.3) 4.6 – 5.2 |
5.1 (0.6) 4.3 – 5.6 |
5.0 (0.8) 3.8 – 5.7 |
5.3 (0.6) 4.6 – 5.8 |
| Consummatory | Mean (SD) Min – Max |
5.4 (0.2) 5.1 – 5.8 |
4.9 (1.3) 2.3 – 6.0 |
5.3 (0.4) 5.0 – 6.0 |
5.3 (0.5) 4.8 – 5.9 |
5.1 (0.4) 4.6 – 5.6 |
5.2 (0.6) 4.6 – 6.0 |
5.0 (0.7) 4.4 – 6.0 |
5.0 (0.1) 4.9 – 5.1 |
| Total | Mean (SD) Min – Max |
5.1 (0.2) 4.9 – 5.3 |
4.7 (1.0) 2.9 – 5.4 |
4.9 (0.4) 4.4 – 5.4 |
5.1 (0.6) 4.3 – 5.7 |
5.0 (0.1) 4.9 – 5.1 |
5.2 (0.4) 4.7 – 5.7 |
5.0 (0.7) 4.1 – 5.6 |
5.1 (0.4) 4.7 – 5.5 |
Four-week follow-up effect measures: Temporal Experience of Pleasure Scale (TEPS).
Table 4G
| Personal Insights Questionnaire (PIQ): the summed number of the 7 personal insights items reported. | |||||||
|---|---|---|---|---|---|---|---|
| Outcome measure | Descriptive statistic | Product A (n=5) | Product B (n=4) | A/B (n=4) | Product C (low dose) (n=4) | Product C (high dose) (n=4, unless specified) | |
| 1 week post T* | 1 week post T* | 4 week post T* | 1 week post T1 | 1 week post T2 | 4 week post T2 (n=3) | ||
| Total count | Mean (SD) Min – Max |
3.6 (2.2) 1 – 7 |
3.8 (2.2) 1 – 6 |
4.3 (2.1) 2 – 6 |
4.0 (0.8) 3 – 5 |
4.3 (1.5) 3 – 6 |
4.7 (2.3) 2 – 6 |
Four-week follow-up effect measures: Personal Insights Questionnaire (PIQ).
Table 4H
| Ayahuasca Preparation & Support Scale: The first four questions are based on safety and support. The final two questions are based on preparation. The four-point scale answers were 1 = “not at all”; 2 = “a small amount”; 3 = “moderately”; 4 to “very much”. Items are averaged in each subscale. | |||||||
|---|---|---|---|---|---|---|---|
| Outcome measure | Descriptive statistic | Product A (n=5) | Product B (n=4) | A/B (n=4) | Product C (low dose) (n=4) | Product C (high dose) (n=4, unless specified) | |
| 1 week post T* | 1 week post T* | 4 week post T* | 1 week post T1 | 1 week post T2 | 4 week post T2 (n=3) | ||
| Safety and Support | Mean (SD) Min – Max |
3.6 (0.2) 3.5 – 4.0 |
3.8 (0.2) 3.5 – 4.0 |
3.6 (0.4) 3.0 – 4.0 |
3.9 (0.1) 3.8 – 4.0 |
3.9 (0.3) 3.5 – 4.0 |
3.8 (0) 3.8 – 3.8 |
| Preparation | Mean (SD) Min – Max |
3.7 (0.4) 3.0 – 4.0 |
3.8 (0.5) 3.0 – 4.0 |
3.5 (0.6) 3.0 – 4.0 |
3.9 (0.3) 3.5 – 4.0 |
3.9 (0.3) 3.5 – 4.0 |
3.7 (0.6) 3.0 – 4.0 |
Four-week follow-up effect measures: Ayahuasca Preparation & Support Scale.
Base = baseline session
T = treatment session
T1 = treatment session 1
T2 = treatment session 2
T* = treatment session (either 1 or 2 depending on the random order of product A / product B)
Pre T = during the treatment session prior to the administration of the study medication
Post T = during the treatment session at the conclusion of the psychedelic experience (a minimum of 4 hours to 8 hours post dose)
SD = 1 standard deviation
Min = minimum
Max = maximum
N*=2, only 2 of the 4 participants completed the day 1 post treatment session 2 PANAS
Discussion
Interpretation
The primary objective of this study was to evaluate and compare three different formulations of a DMT/Harmala encapsulated product on a range of safety and efficacy parameters.
All three formulations demonstrated a good safety profile. Physical examination, vital signs monitoring, and pathology results did not yield findings of concern at any timepoint throughout the trial. Most AEs resolved within the treatment session or within 24 hours. There were 71 adverse events recorded, with most considered to be study medication-related. The most frequently occurring study medication-related AEs were adverse physical effects followed by adverse mental health effects. The adverse events reported in this study are consistent with other experimental studies of traditional Ayahuasca in healthy volunteers and clinical populations (59).
Traditional Ayahuasca formulations are known to induce nausea and vomiting, with estimates ranging between 60 – 96% of users (60, 61). Vomiting was infrequently recorded in our participant group (2 of 9 participants) which is a positive outcome from the perspective of suitability of a formulation for use in clinical contexts. In traditional use of ayahuasca, “purging” is considered an integral part of the therapeutic process (62). The reduced gastrointestinal effect observed in this study is potentially due to the oral preparation being provided in dried powder form via capsules, as opposed to the traditional liquid form. It remains to be seen if this lack of emetic action has an impact on the therapeutic potential of our Acacia based formulations in clinical populations.
The majority of mental health events occurred with one participant following ingestion of ACL-010 Formulation C (high dose). This participant experienced a number of emotional-cognitive adverse mental health effects (including transient suicidal ideation) during their psychedelic experience which resolved during the session after interventions administered by the therapists (breathing techniques, reassurance, physical holding and restraint, physical repositioning, and removing hazards to personal safety). Review of the participant’s file and notes from pre-treatment preparation sessions did not reveal any factors that could have predicted this participant’s challenging emotional and psychological experience. This suggests possible high variability in the inter-individual response to this formulation, however as our data is based on only four participants receiving ACL-010 Formulation C, drawing a definitive conclusion is difficult. Rossi et al. (63) discuss other cases where trial participants have had similarly intense and challenging experiences with Ayahuasca which were also resolved during the session without the need for pharmacological intervention. Although these cases are rare and the adverse events are transient, trial staff should be aware that some participants may be prone to these responses.
Reporting of AEs in clinical trials with psychedelics is in itself challenging because the framework for the reporting of AEs does not take account of the possibility that occurrences typically considered as AEs may be a part of the therapeutic process in this context. Separating these different types of events is difficult, as is consistent assessment, classification, and reporting (64). In our reporting we have used the standardized medical terminology for reporting AEs in clinical studies – Medical Dictionary for Regulatory Activities (MedDRA) (65), however we acknowledge that this is somewhat reductive. Extra notes to the AE table provide further clarification and context to some of the reported AEs.
Of particular interest to study investigators was the strength and quality of the psychedelic experience induced by the study formulations. A primary outcome measure of the study was the rating of each formulation on the MEQ which purports to measure the strength of a classic mystical experience (CME). Higher ratings of a mystical type experience have been found to be positively related to changes in well-being after a psychedelic experience. Average scores for both high and low dose ACL-010 Formulation C on the MEQ were higher than Formulations A and B on all subscales and within the range of a CME. In comparison to other studies reporting total MEQ scores associated with ayahuasca consumption, the formulation C high dose average score was marginally to significantly higher (53, 66, 67).
Responses on the Tolerability and Differential Experience scale indicate that strength of psychedelic experience of Formulations A and B were generally rated as weaker than previous experience with Ayahuasca whereas low dose ACL-010 Formulation C was rated as similar and high dose ACL-010 Formulation C as stronger. The subjective experience (quality) of all formulations was generally rated as similar to previous experience with Ayahuasca. Both high and low dose levels of ACL-010 Formulation C were rated as similar or more beneficial than previous experience with Ayahuasca, Formulations A tended to be rated as less beneficial than previous experience with Ayahuasca. Other acute effect measures (5D-ASC, and SIMO) indicated a stronger subjective effect associated with ACL-010 Formulation C (low and high dose).
Most four-week follow-up measures showed little difference between the 3 formulations (PANAS, K-10, DASS-21, ISI, TEPS, PIQ, IDS) and little change from baseline or week 1 post-dose values. It is possible that these effects measures are not particularly sensitive in non-clinical populations where baseline levels are quite low. However, scores for ACL-010 Formulation C (high) were considerably higher on a number of positive PEQ subscales (attitudes about life and self, mood, positive behavior, and spirituality) at both one week and 4 weeks post dose 2.
If DMT/Harmala formulations are to be used in clinical and /or research settings it is important to be able to quantify the dose of both substances prior to administration and consistently deliver the known dose over multiple time points. ACL-010 Formulation C used in this study was a highly purified and standardized formulation which allowed more precise quantification of the active ingredients in each capsule. The data tentatively indicates that this formulation delivered superior outcomes in terms of the strength of the psychedelic experience, which has been shown to be predictive of therapeutic effect (30, 31, 68, 69). The ability to produce an encapsulated product of high purity and consistency which can be readily titrated up or down as clinically indicated is a potentially beneficial consideration if the product is to be used in future clinical trials, and eventually in clinical contexts. Furthermore, the stability of traditional Ayahuasca beverages have been studied, and the harmala alkaloid component has been shown to degrade over time at a faster rate than the DMT component (70). Data from our stability studies indicate that these compounds when formulated in a pharmaceutical manner with appropriate excipients may potentially be more stable.
The therapist dyad, consisting of a psychiatrist and psychologist with extensive experience in psychedelic assisted psychotherapy, was a strength of our study, enhancing the value of the preparation and integration sessions, and the safety of trial participants during treatment sessions.
Our study protocol specified 2 treatment sessions with a minimum of 7 days between sessions. A washout period between 7–14 days has been used in a number of pharmacokinetic studies of DMT/harmine formulations (71–73) and given the half-life of the longest compound THH is approximately 6 hours there is no possible pharmacological carry-over effect. Nevertheless, it is possible that the subjective effects of the treatment may have cumulative effects. In fact, traditional ayahuasca ceremonies involve ingestion of the brew over multiple sessions. Treatment protocols for psychedelic assisted therapy are still emerging but typically involve one to three dosing sessions with the interval between sessions guided by both therapist and patient. Going forward, the optimal number of sessions and the interval between treatment sessions is likely to be determined by the mental health condition being treated and individual patient response to treatment.
A final comment is regarding the traditional use of these medicines. In the broader context of use of these compounds, it is important to consider culturally safe and effective treatment models. It is recommended that traditional custodians be ideally involved in protocol design through expert groups. Furthermore, regulatory bodies and sponsors should support this participation while addressing barriers such as cost to improve access and equity in clinical trials and treatments. See 74 for more discussion on this area.
Limitations
This study has a number of limitations which include small sample size, lack of placebo, and the open label trial design. Study participants were healthy volunteers who were all mental health professionals with an interest in psychedelic assisted psychotherapy and previous experience with Ayahuasca. These factors limit the generalizability of results to the general population and clinical populations. Another possible confounder in our study is expectancy bias. One potential approach to minimize the impact of expectancy bias (from pre-existing positive beliefs inflating the treatment effect size) is via the use of the Stanford Expectancy Bias Scale (75) which can either exclude people pre-randomization with high positive treatment expectancy, or which can be used as a moderating covariate in efficacy analyses. Furthermore, it is noted that it is important to monitor the long-term effects of psychedelic administration via post-4-week follow-up assessments.
Our study was designed to test the safety, tolerability, physical, mental health and psychedelic effects of the three formulations in a naturalistic setting. Pharmacokinetics and pharmacodynamics were not assessed. The intrusive nature of procedures for frequent blood and urine sample collection were considered likely to detract from the psychotherapeutic nature and effects of the treatment sessions. A planned Phase 1 pharmacokinetic/pharmacodynamic study will be an integral part of ongoing formulation development prior to a Stage 2 trial.
Conclusion
A unique aspect of the trial medication is that the DMT component of all formulations is derived from Acacia species. As far as the authors are aware, this is the first time a DMT/Harmala formulation derived from Acacia has been tested in a clinical trial. Our results are promising in terms of both the safety profile and subjective effects of the formulations, in particular ACL-010 Formulation C. Based on these preliminary findings, and considering of clinical trial data and the context of traditional dosage, we theories that ACL-010 Formulation C at a dose potentially midway between the low and high doses reported here could be most appropriate for further study. In summary, our results indicate that DMT formulations derived from the Acacia species represent a feasible alternative to the traditional Ayahuasca preparations (for reference, additional comparative data is currently in submission elsewhere). The caveat is the previously acknowledged small sample size, and therefore any conclusions regarding dosage, safety, and efficacy must be verified in an adequately powered randomized placebo-controlled trial.
Statements
Data availability statement
The data for this study are not available for sharing due to the small sample size creating an unacceptable risk of participant re-identification. Requests for access to summary data or aggregated results will be considered upon reasonable request. Requests to access the datasets should be directed to lisa.collins@svha.org.au.
Ethics statement
The studies involving humans were approved by St Vincent's Hospital Melbourne Human Research and Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YB: Conceptualization, Funding acquisition, Methodology, Investigation, Writing – review, & editing. AN: Conceptualization, Investigation, Methodology, Project administration, Writing – original draft, Writing – Review & editing. LC: Data curation, Validation, Formal analysis, Writing – review & editing. MR: Methodology, Conceptualization, Investigation, Writing – review & editing. JD: Methodology, Conceptualization, Investigation, Writing – review & editing. DP: Conceptualization, Funding acquisition, Methodology, Writing – review & editing. JS: Conceptualization, Funding acquisition, Methodology, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was fully funded via by Psychae Institute and Psychae Therapeutics (now known as Neurala Biosciences). The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Acknowledgments
The study participants are gratefully acknowledged. Additionally, Jacob Buckley-Lennox assisted with data management and analysis. Amanda Streitberg coordinated trial medication storage and dispensing. Thanks are also extended to Dr. Mitchell Low and Dr. Damian Hall from NICM Health Research Institute, Dr. Andrew Riches, Dr. Ivan Botella-Martinez, and Dr. Marc McEwan from CSIRO, and Cheryl Chia from Lifecare Compounding Pharmacy for the valued CMC contribution.
Conflict of interest
JS and DP are Directors of Psychae Therapeutics rebranded as Neurala Biosciences and the connected not-for-profit Psychae Institute, which are both involved with psychedelics research and the development of these agents as registered medicines. They are employed and hold equity with Psychae Therapeutics.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2025.1545915/full#supplementary-material
References
1
Ruffell S Netzband N Bird C Young AH Juruena MF . The pharmacological interaction of compounds in ayahuasca: a systematic review. Rev Bras psiquiatria (Sao Paulo Brazil: 1999). (2020) 42:646–56. doi: 10.1590/1516-4446-2020-0884
2
Naranjo P . El ayahuasca en la arqueología ecuatoriana. América indígena. (1986) 46:117–27.
3
Shanon B . The antipodes of the mind: charting the phenomenology of the Ayahuasca experience. Oxford UK: Oxford University Press (2002).
4
Tupper KW . Ayahuasca healing beyond the Amazon: the globalization of a traditional indigenous entheogenic practice. Global Networks. (2009) 9:117–36. doi: 10.1111/j.1471-0374.2009.00245.x
5
Trichter S . Ayahuasca beyond the Amazon: The benefts and risks of a spreading tradition. J Transpersonal Psychol. (2010) 42:131–48.
6
Lowell JT Adams PC . The routes of a plant: ayahuasca and the global networks of Santo Daime. Soc Cultural Geogr. (2017) 18:137–57. doi: 10.1080/14649365.2016.1161818
7
Peluso D . Global Ayahuasca: an entrepreneurial ecosystem. (2016), 203–21. doi: 10.4324/9781315551425
8
Gearin AK . ‘Whatever you want to believe’: kaleidoscopic individualism and ayahuasca healing in Australia. Aust J Anthropology. (2015) 26:442–55. doi: 10.1111/taja.12143
9
Winkelman M . Drug tourism or spiritual healing? Ayahuasca seekers in Amazonia. J psychoactive Drugs. (2005) 37:209–18. doi: 10.1080/02791072.2005.10399803
10
Palhano-Fontes F Alchieri JC Oliveira JPM Soares BL Hallak JEC Galvao-Coelho N et al . The therapeutic potentials of ayahuasca in the treatment of depression. (2013), 23–39. doi: 10.1007/978-3-642-40426-9_2
11
Araujo AM Carvalho F Bastos Mde L Pinho PG M . The hallucinogenic world of tryptamines: an updated review. Arch Toxicol. (2015) 89:1151–73. doi: 10.1007/s00204-015-1513-x
12
Aricioglu-Kartal F Kayır H Tayfun Uzbay I . Effects of harman and harmine on naloxone-precipitated withdrawal syndrome in morphine-dependent rats. Life Sci. (2003) 73:2363–71. doi: 10.1016/S0024-3205(03)00647-7
13
Brierley DI Davidson C . Developments in harmine pharmacology - Implications for ayahuasca use and drug-dependence treatment. Prog Neuropsychopharmacol Biol. (2012) 39:263–72. doi: 10.1016/j.pnpbp.2012.06.001
14
Owaisat S Raffa RB Rawls SM . In vivo comparison of harmine efficacy against psychostimulants: Preferential inhibition of the cocaine response through a glutamatergic mechanism. Neurosci Lett. (2012) 525:12–6. doi: 10.1016/j.neulet.2012.07.052
15
Domínguez-Clavé E Soler J Elices M Pascual JC Álvarez E de la Fuente Revenga M et al . Ayahuasca: Pharmacology, neuroscience and therapeutic potential. Brain Res Bull. (2016) 126:89–101. doi: 10.1016/j.brainresbull.2016.03.002
16
Franquesa A Sainz-Cort A Gandy S Soler J Alcázar-Córcoles MÁ Bouso JC . Psychological variables implied in the therapeutic effect of ayahuasca: A contextual approach. Psychiatry Res. (2018) 264:334–9. doi: 10.1016/j.psychres.2018.04.012
17
Soler J Elices M Franquesa A Barker S Friedlander P Feilding A et al . Exploring the therapeutic potential of Ayahuasca: acute intake increases mindfulness-related capacities. Psychopharmacology. (2016) 233:823–9. doi: 10.1007/s00213-015-4162-0
18
Thomas G Lucas P Capler NR Tupper KW Martin G . Ayahuasca-assisted therapy for addiction: results from a preliminary observational study in Canada. Curr Drug Abuse Rev. (2013) 6:30–42. doi: 10.2174/15733998113099990003
19
Uthaug M Van Oorsouw K Kuypers K Van Boxtel M Broers N Mason N et al . Sub-acute and long-term effects of ayahuasca on affect and cognitive thinking style and their association with ego dissolution. Psychopharmacol (Berl). (2018) 235:2979–89. doi: 10.1007/s00213-018-4988-3
20
Murphy-Beiner A Soar K . Ayahuasca’s ‘afterglow’: Improved mindfulness and cognitive flexibility in ayahuasca drinkers. Psychopharmacology. (2020) 237:1161–9. doi: 10.1007/s00213-019-05445-3
21
Stemme A Deco G Busch A . The neuronal dynamics underlying cognitive flexibility in set shifting tasks. J Comput Neurosci. (2008) 23:313–31. doi: 10.1007/s10827-007-0034-x
22
González D Cantillo J Pérez I Farré M Feilding A Obiols JE et al . Therapeutic potential of ayahuasca in grief: a prospective, observational study. Psychopharmacol (Berl). (2020) 1-12:1171–82. doi: 10.1007/s00213-019-05446-2
23
Soler J Elices M Dominguez-Clavé E Pascual JC Feilding A Navarro-Gil M et al . Four weekly ayahuasca sessions lead to increases in “acceptance” capacities: a comparison study with a standard 8-week mindfulness training program. Front Pharmacol. (2018) 9:224. doi: 10.3389/fphar.2018.00224
24
Netzband N Ruffell S Linton S Tsang W Wolff T . Modulatory effects of ayahuasca on personality structure in a traditional framework. Psychopharmacology. (2020) 237:3161–71. doi: 10.1007/s00213-020-05601-0
25
Weiss B Miller JD Carter NT Keith Campbell W . Examining changes in personality following shamanic ceremonial use of ayahuasca. Sci Rep. (2021) 11:1–15. doi: 10.1038/s41598-021-84746-0
26
Gonzalez D Cantillo J Perez I Carvalho M Aronovich A Farre M et al . The shipibo ceremonial use of ayahuasca to promote well-being: an observational study. (2021) 12(1059):. doi: 10.3389/fphar.2021.623923
27
Hinkle JT Graziosi M Nayak SM Yaden DB . Adverse events in studies of classic psychedelics: A systematic review and meta-analysis. JAMA Psychiatry. (2024) 81(12):1225–35. doi: 10.1001/jamapsychiatry.2024.2546
28
White E Kennedy T Ruffell S Perkins D Sarris J . Ayahuasca and dimethyltryptamine adverse events and toxicity analysis: A systematic thematic review. Int J Toxicol. (2024) 43:327–39. doi: 10.1177/10915818241230916
29
Garcia-Romeu A Davis AK Erowid F Erowid E Griffiths RR Johnson MW . Cessation and reduction in alcohol consumption and misuse after psychedelic use. J Psychopharmacology. (2019) 33:1088–101. doi: 10.1177/0269881119845793
30
Romeo B Fauvel B Dejean S Strika L Amirouche A Verroust V et al . Impact of a naturalistic psychedelic experience on smoking: a retrospective survey. J Psychoactive Drugs. (2023) 55:640–9. doi: 10.1080/02791072.2023.2227171
31
Romeo B Kervadec E Fauvel B Strika-Bruneau L Amirouche A Verroust V et al . Significant psychedelic experiences evaluated for mystical characteristics Associated with Cannabis Use reduction and psychological flexibility improvement: a naturalistic cross-sectional retrospective survey. J Psychoactive Drugs. (2024), 1–12. doi: 10.1080/02791072.2024.2375720
32
Aicher HD Mueller MJ Dornbierer DA Suay D Elsner C Wicki I et al . Potential therapeutic effects of an ayahuasca-inspired N,N-DMT and harmine formulation: a controlled trial in healthy subjects. Front Psychiatry. (2023) 14:1302559. doi: 10.3389/fpsyt.2023.1302559
33
Schulz KF Altman DG Moher D For The CONSORT Group . CONSORT 2010 Statement: updated guidelines for reporting parallel group randomized trials.
34
Eldridge SM Chan CL Campbell MJ Bond CM Hopewell S Thabane L et al . CONSORT 2010 statement: extension to randomized pilot and feasibility trials. bmj. (2016) 355:355. doi: 10.1136/bmj.i5239
35
Lancaster GA Thabane L . Guidelines for reporting non-randomized pilot and feasibility studies. Pilot Feasibility Stud. (2019) 5:114. doi: 10.1186/s40814-019-0499-1
36
Perkins D Schubert V Simonová H Tófoli LH Bouso JC Horák M et al . Influence of context and setting on the mental health and wellbeing outcomes of ayahuasca drinkers: results of a large international survey. Front Pharmacol. (2021) 12. doi: 10.3389/fphar.2021.623979
37
MacLean KA Leoutsakos JMS Johnson MW Griffiths RR . Factor analysis of the mystical experience questionnaire: A study of experiences occasioned by the hallucinogen psilocybin. J Sci study religion. (2012) 51:721–37. doi: 10.1111/j.1468-5906.2012.01685.x
38
Dittrich A . The standardized psychometric assessment of altered states of consciousness (ASCs) in humans. Pharmacopsychiatry. (1998) 31 Suppl 2:80–4. doi: 10.1055/s-2007-979351
39
Francis F Louden S . ). A short index of mystical experience (SIMO): A study among roman catholic priests. Pastoral Psychol. (2004) 53:49–51. doi: 10.1023/B:PASP.0000039325.40451.65
40
Hayes MHS Patterson DG . Experimental development of the graphic rating method. Psychol Bull. (1921) 18:98–9.
41
Lovibond PF Lovibond SH . The structure of negative emotional states: A comparison of the depression of the depression anxiety stress scale (DASS) with the beck depression and anxiety inventories. Behav Res Ther. (1995) 33:335–43. doi: 10.1016/0005-7967(94)00075-U
42
Crawford JR Henry JD . The Positive and Negative Affect Schedule (PANAS): Construct validity, measurement properties and normative data in a large non-clinical sample. Br J Clin Psychol. (2004) 43:245–65. doi: 10.1348/0144665031752934
43
Kessler RC Barker PR Colpe LJ Epstein JF Gfroerer JC Hiripi E et al . Screening for serious mental illness in the general population. Arch Gen Psychiatry. (2003) 60:184–9. doi: 10.1001/archpsyc.60.2.184
44
Griffiths RR Richards WA McCann U Jesse R . Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance. Psychopharmacol (Berl). (2006) 187:268–83. doi: 10.1007/s00213-006-0457-5
45
Bastien CH Vallières A Morin CM . Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. (2001) 2:297–307. doi: 10.1016/S1389-9457(00)00065-4
46
Gard DE Gard MG Kring AM John OP . Anticipatory and consummatory components of the experience of pleasure: A scale development study. J Res Pers. (2006) 40:1086–102. doi: 10.1016/j.jrp.2005.11.001
47
Perkins D Opaleye E Bouso JC Tófoli L Sarris J Schubert V et al . Insights, life changes and lifestyle changes reported by individuals consuming ayahuasca in naturalistic settings: nature, frequency and associations with mental health and wellbeing. Psychoactives. (2023) 2:256–86. doi: 10.3390/psychoactives2040017
48
Sheehan D Janavs J Harnett-Sheehan K Sheehan M Gray C Lecrubier Y et al . MINI international neuropsychiatric interview 7.0. 0 for DSM-V. Tampa, FL: University of South Florida (2010).
49
Babor TF Higgins-Biddle JC Saunders JB Monteiro MG World Health Organization World Health Organization . AUDIT: the alcohol use disorders identification test: guidelines for use in primary health care (No. WHO/MSD/MSB/01.6 a). Geneva: World Health Organization (2001).
50
Riba J Rodriguez-Fornells A Urbano G Morte A Antonijoan R Montero M et al . Subjective effects and tolerability of the South American psychoactive beverage Ayahuasca in healthy volunteers. Psychopharmacol (Berl). (2001) 154:85–95. doi: 10.1007/s002130000606
51
Riba J Anderer P Jane F Saletu B Barbanoj MJ . Effects of the South American psychoactive beverage ayahuasca on regional brain electrical activity in humans: a functional neuroimaging study using low-resolution electromagnetic tomography. Neuropsychobiology. (2004) 50:89–101. doi: 10.1159/000077946
52
Dos Santos RG Osório FL Rocha JM Rossi GN Bouso JC Rodrigues LS et al . Ayahuasca improves self-perception of speech performance in subjects with social anxiety disorder: A pilot, proof-of-concept, randomized, placebo-controlled trial. J Clin Psychopharmacol. (2021) 41:540–50. doi: 10.1097/JCP.0000000000001428
53
Palhano-Fontes F Barreto D Onias H Andrade KC Novaes MM Pessoa JA et al . Rapid antidepressant effects of the psychedelic ayahuasca in treatment-resistant depression: a randomized placebo-controlled trial. psychol Med. (2018) 49:1–9. doi: 10.1017/S0033291718001356
54
Lanaro R Mello SM Cunha KF Silveira G Correa-Neto NF Hyslop S et al . Kinetic profile of N,N-dimethyltryptamine and [beta]-carbolines in saliva and serum after oral administration of ayahuasca in a religious context. Drug Testing Anal. (2021) 13:664. doi: 10.1002/dta.2955
55
Riba J Romero S Grasa E Mena E Carrió I Barbanoj M . Increased frontal and paralimbic activation following ayahuasca, the pan-amazonian inebriant. Psychopharmacology. (2006) 186:93–8. doi: 10.1007/s00213-006-0358-7
56
dos Santos R Grasa E Valle M Ballester M Bouso J Nomdedéu J et al . Pharmacology of ayahuasca administered in two repeated doses. sychopharmacology. (2012) 219:1039–53. doi: 10.1007/s00213-011-2434-x
57
Zeifman RJ Singhal N Dos Santos RG Sanches RF d. L. Osório F Hallak JEC et al . Rapid and sustained decreases in suicidality following a single dose of ayahuasca among individuals with recurrent major depressive disorder: results from an open-label trial. Psychopharmacology. (2021) 238:453–9. doi: 10.1007/s00213-020-05692-9
58
Sanches RF Osório FDL Dos Santos RG Macedo LRH Maia-de-Oliveira JP Wichert-Ana L et al . Antidepressant effects of a single dose of ayahuasca in patients with recurrent depression: A SPECT study. J Of Clin Psychopharmacol. (2016) 36:77–81. doi: 10.1097/JCP.0000000000000436
59
Dos Santos RG Hallak JEC . Ayahuasca: pharmacology, safety, and therapeutic effects. CNS spectrums. (2025) 30. doi: 10.1017/S109285292400213X
60
Durante Í Dos Santos RG Bouso JC Hallak JE . Risk assessment of ayahuasca use in a religious context: self-reported risk factors and adverse effects. Braz J Psychiatry. (2021) 43:362–9. doi: 10.1590/1516-4446-2020-0913
61
Bouso JC Andión Ó Sarris JJ Scheidegger M Tófoli LF Opaleye ES et al . Adverse effects of ayahuasca: Results from the Global Ayahuasca Survey. PloS Glob Public Health Nov. (2022) 16:2(11). doi: 10.1371/journal.pgph.0000438
62
Fotiou E Gearin AK . Purging and the body in the therapeutic use of ayahuasca. Soc Sci Med. (2019) 239:112532. doi: 10.1016/j.socscimed.2019.112532
63
Rossi GN Dias ICDS Baker G Bouso Saiz JC Dursun SM Hallak JE et al . Ayahuasca, a potentially rapid acting antidepressant: Focus on safety and tolerability. Expert Opin Drug Saf. (2022) 21:789–801. doi: 10.1080/14740338.2022.2054988
64
Breeksema JJ Kuin BW Kamphuis J van den Brink W Vermetten E Schoevers RA . Adverse events in clinical treatments with serotonergic psychedelics and MDMA: A mixed-methods systematic review. J Psychopharmacology. (2022) 36:1100–17. doi: 10.1177/02698811221116926
65
Brown EG Wood L Wood S . The medical dictionary for regulatory activities (MedDRA). Drug Saf. (1999) 20:109–17. doi: 10.2165/00002018-199920020-00002
66
Perkins D Pagni BA Sarris J Barbosa PCR Chenhall R . Changes in mental health, wellbeing and personality following ayahuasca consumption: Results of a naturalistic longitudinal study. Front Pharmacol. (2022) 13. doi: 10.3389/fphar.2022.884703
67
Strickland JC Garcia-Romeu A Johnson MW . The mystical experience questionnaire 4-item and challenging experience questionnaire 7-item. Psychedelic Med (New Rochelle). (2024) 2:33–43. doi: 10.1089/psymed.2023.0046
68
Roseman L Nutt DJ Carhart-Harris RL . Quality of acute psychedelic experience predicts therapeutic efficacy of psilocybin for treatment-resistant depression. Front Pharmacol. (2018) 8:974. doi: 10.3389/fphar.2017.00974
69
Ko K Knight G Rucker JJ Cleare AJ . Psychedelics, mystical experience, and therapeutic efficacy: A systematic review. Front Psychiatry. (2022) 13:917199. doi: 10.3389/fpsyt.2022.917199
70
de Oliveira Silveira G dos Santos RG Lourenço FR Rossi GN Hallak JEC Yonamine M . Stability evaluation of DMT and harmala alkaloids in ayahuasca tea samples. Molecules 25 no. (2020) 9:2072. doi: 10.3390/molecules25092072
71
Dornbierer DA Marten L Mueller J Aicher HD Mueller MJ Boxler M et al . Overcoming the clinical challenges of traditional ayahuasca: a first-in-human trial exploring novel routes of administration of N, N-Dimethyltryptamine and harmine. Front Pharmacol. (2023) 14:1246892. doi: 10.3389/fphar.2023.1246892
72
Egger K Redondo JJ Müller J Dornbierer J Smallridge J Aicher HD et al . Examining the pharmacokinetic and pharmacodynamic interaction of N, N-dimethyltryptamine and harmine in healthy volunteers: α factorial dose-escalation study. Biomedicine Pharmacotherapy. (2025) 184:117908. doi: 10.1016/j.biopha.2025.117908
73
Mueller MJ Aicher HD Dornbierer DA Marten L Suay D Meling D et al . Pharmacokinetics and pharmacodynamics of an innovative psychedelic N, N-dimethyltryptamine/harmine formulation in healthy participants: a randomized controlled trial. Int J Neuropsychopharmacol. (2025) 28:pyaf001. doi: 10.1093/ijnp/pyaf001
74
Sebben B Stone J Sarris J Perkins D Mallie K Barnett S et al . Psychedelic medicine and cultural responsiveness: A call for Aboriginal and Torres Strait Islander engagement in Australian clinical trials and practice. Aust New Z J Public Health. (2024) 48:100200. doi: 10.1016/j.anzjph.2024.100200
75
Younger J Gandhi V Hubbard E Mackey S . Development of the Stanford Expectations of Treatment Scale (SETS): a tool for measuring patient outcome expectancy in clinical trials. Clin Trials. (2012) 9:767–76. doi: 10.1177/1740774512465064
Summary
Keywords
ayahuasca, psychedelics, plant medicine, mental health, depression, anxiety, ethnobotany
Citation
Bonomo YA, Norman AF, Collins L, Ross M, Dwyer J, Perkins D and Sarris J (2025) DMT and harmala alkaloids: an exploratory study of oral Acacia based formulations in healthy volunteers. Front. Psychiatry 16:1545915. doi: 10.3389/fpsyt.2025.1545915
Received
16 December 2024
Accepted
09 June 2025
Published
15 August 2025
Volume
16 - 2025
Edited by
Jonathan Brett, University of New South Wales, Australia
Reviewed by
Davide Arillotta, University of Hertfordshire, United Kingdom
Milan Scheidegger, Psychiatric University Hospital Zurich, Switzerland
Updates
Copyright
© 2025 Bonomo, Norman, Collins, Ross, Dwyer, Perkins and Sarris.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amanda F. Norman, amanda.norman@svha.org.au
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.