Abstract
Introduction:
The prevalence of very low birth weight infants (VLBWIs) continues to rise globally. These infants face increased susceptibility to preterm-related complications and potential long-term chronic health conditions, creating significant psychological and economic burdens for their families. Mothers of VLBWIs demonstrate substantially higher risks of developing post-traumatic stress disorder (PTSD) compared to mothers of term infants. Maternal PTSD can impair psychosocial functioning, compromise therapeutic relationships, and disrupt early mother–infant bonding, with potentially lasting consequences for child development. These findings highlight the critical importance of early PTSD screening and intervention in this vulnerable population.
Methods and analysis:
We enrolled 352 mothers of very low birth weight infants (<1,500 g) admitted to NICUs at three hospitals in Shandong Province between September 2022 and December 2023. We identified PTSD risk factors through multivariable logistic regression and developed predictive models based on these results. Model validation included ROC analysis, calibration curves, and decision curve analysis.
Results:
Among the 352 mothers of preterm infants, the PTSD detection rate was 40.9%. Logistic regression analysis identified six independent risk factors for PTSD in mothers of VLBWIs: history of miscarriage, spousal support, illness uncertainty, neurotic personality traits, negative coping styles, and perceived social support. The nomogram model developed from these factors demonstrated excellent discriminative ability, with an area under the ROC curve of 0.969 (95% CI: 0.953–0.984). Both the Hosmer–Lemeshow goodness-of-fit test (P = 0.956) and calibration curves indicated strong agreement between predicted and observed outcomes.
Discussion:
Understanding the prevalence of PTSD among mothers of extremely low birth weight infants, identifying its independent risk factors, and developing a visual nomogram prediction model are critical for enabling early PTSD detection and facilitating clinical screening of high-risk mothers.
Introduction
Very low birth weight infants (VLBWIs), defined as neonates weighing <1,500 g at birth (1), represent a critical population in neonatal intensive care units (NICUs). Accounting for approximately 20% of NICU admissions (2), their prevalence continues to rise. Compared to term infants, VLBWIs demonstrate significantly higher risks of immediate complications, including thermoregulatory instability, hypoglycemia, and respiratory distress (3), often requiring hospital readmission.
Long-term outcomes may include motor and sensory impairments, neurocognitive deficits, and behavioral disorders (4). These complications contribute to elevated morbidity and mortality rates, increased healthcare expenditures, and prolonged hospitalizations. Furthermore, they impose substantial psychological burdens on parents, particularly predisposing mothers to adverse mental health outcomes including post-traumatic stress disorder.
Post-traumatic stress disorder (PTSD) is a severe psychiatric condition that can develop after exposure to a traumatic event. PTSD is often conceptualized as a single diagnosis without further distinction. However, PTSD presentations vary widely and are classified into several subtypes based on symptom onset and duration. These include acute PTSD (symptoms lasting less than 3 months), chronic PTSD (symptoms persisting for 3 months or longer), delayed-onset PTSD (symptoms emerging ≥6 months after the event), and complex PTSD (stemming from prolonged or repeated trauma). PTSD is clinically diagnosed when the PCL-C score exceeds 38 points following exposure to traumatic events, with symptoms manifesting across three core dimensions: re-experiencing, avoidance/numbing, and hyperarousal (5, 6). Research indicates that 23%–35% of NICU mothers of preterm infants develop PTSD (7), with national studies reporting a prevalence of 33.1%.
Mothers of very low birth weight infants experience significantly greater financial stress compared to mothers of term infants (8). International studies demonstrate that maternal PTSD can lead to maladaptive parenting behaviors, including excessive control tendencies (9, 10) and impaired mother–infant attachment (11). Longitudinal studies reveal that children of mothers with perinatal psychological disorders (including PTSD and depression) exhibit 2–5 times higher rates of behavioral difficulties (12), with increased risks of social withdrawal, anxiety, and peer aggression during school years (13).
These findings underscore the critical importance of early PTSD identification in mothers of very low birth weight infants.
Objectives
-
To investigate the prevalence and identify independent risk factors for PTSD among mothers of ELBWIs
-
To develop and validate a nomogram prediction model for PTSD risk assessment in this population
Preparation
A multidisciplinary research team was assembled, consisting of two neonatologists/gynecologists, one psychiatrist, three nursing specialists, one biostatistician, and three research assistants. All team members underwent standardized training. Through evidence-based literature review and clinical practice integration, the team developed a comprehensive understanding of PTSD in mothers of preterm infants. We conducted multiple rounds of methodological discussions to ensure both the feasibility of the research approach and proper implementation at each study phase.
Participants
This multicenter study will be conducted at several grade III, level A tertiary hospitals in Shandong Province. These study sites were selected based on:
-
High-volume neonatal admissions, ensuring an adequate sample size of preterm infants
-
Established track record of excellent family compliance
-
Comprehensive research infrastructure and data resources
Patient recruitment
This study employed convenience sampling to recruit mothers of ELBWIs (<1,500 g) admitted to NICUs of three tertiary hospitals in Shandong Province (including Shandong Provincial Hospital) between September 2022 and September 2023.
Eligibility criteria
Inclusion criteria
Infant factors: birth weight <1,500 g; gestational age <32 weeks
Maternal factors: willing participation with signed informed consent
Exclusion criteria
Infant factors: hospital discharge outcomes: abandonment, transfer, or mortality
Maternal factors: 1) history of other significant traumatic exposures (e.g., witnessing violent death or serious accidents) and 2) pre-existing psychiatric conditions impairing study participation
Sample size calculation
We determined the minimum required sample size using established logistic regression parameters:
Key parameters:
-
17 candidate predictor variables identified from the literature
-
Minimum of 5–10 events required per variable (conservatively using 5)
-
Estimated PTSD prevalence range: 26%–53% (using th upper bound 53% for robust estimation)
-
20% buffer for potential data attrition
Calculation:
Rationale:
-
The calculation follows Peduzzi et al.’s recommendation for logistic regression studies.
-
Using the upper prevalence bound ensures adequate power across potential incidence rates.
-
The 20% attrition buffer accounts for potential missing data or withdrawals.
Final determination: A minimum of 200 mother–infant dyads were required to ensure sufficient power for detecting significant predictors while maintaining statistical reliability.
Measurement tool
1) Participants were assessed for PTSD using standardized diagnostic criteria and questionnaires. PTSD diagnosis was based on the DSM-5 criteria, which require A) exposure to actual or threatened death, serious injury, or sexual violence; and B–E) the presence of one or more intrusive re-experiencing symptoms, one or more avoidance symptoms, two or more negative alterations in cognition or mood, and two or more hyperarousal symptoms, persisting for at least 1 month after the trauma. We administered the PTSD Checklist-Civilian version (PCL-C), a 17-item self-report measure of PTSD symptoms. Each item was rated on a 5-point scale (1 = “not at all” to 5 = “extremely”), yielding a total score from 17 to 85. Following published guidelines, we used a cutoff score of 38 to indicate probable PTSD (14).
The neuroticism subscale of the Five-Factor Inventory (FFI-N) was administered to assess maternal personality traits. This validated 12-item instrument uses a 5-point Likert scale, with higher composite scores (range: 12–60) indicating greater neurotic tendencies. The scale demonstrates excellent reliability in our sample (Cronbach’s α = 0.89). The 2) Perceived Social Support Scale (PSSS) and the Parents’ Perception of Uncertainty in Illness Scale (PPUS) were also administered.
3) In addition to PTSD measures, we systematically recorded perinatal stressors experienced by each participant. These included obstetric complications, delivery factors, and neonatal issues. The choice of these stressors was guided by prior literature identifying such events as risk factors for postpartum PTSD (15–17). Other relevant measures (e.g., demographic and clinical variables) were collected according to standard protocols.
Results
PTSD symptom profiles
Mothers of preterm infants exhibited PTSD symptoms with the following characteristics: total PCL-C scores: from 17 to 74 (mean 38.6 ± 11.26) and symptom cluster scores: 1) re-experiencing: 10.36 ± 4.06, 2) avoidance/numbing: 7.80 ± 2.99, and 3) hyperarousal: 13.60 ± 4.21.
Group comparisons
Univariate analysis identified significant differences (P < 0.05) between PTSD and control groups across four domains:
-
Demographic factors: maternal age, household income
-
Obstetric history: assisted conception, delivery mode, miscarriage history, prior psychiatric diagnosis
-
Neonatal factors: infant birth weight, NICU length of stay
-
Psychosocial measures: illness uncertainty, neuroticism, coping strategies (positive/negative), perceived social supp
The complete statistical results are presented in Table 1.
Table 1
| Sports event | Total sample (n = 352) |
PTSD group (n = 144) |
Non-PTSD group (n = 208) |
Statistical test | P-value |
|---|---|---|---|---|---|
| (A person’s) age | 32 (28, 35) | 33 (29, 36) | 31 (28, 34) | −2.789 | <0.01 |
| Monthly household income | 14.468 | <0.01 | |||
| 4,000 and below | 37 (10.51%) | 16 (4.55%) | 21 (5.97%) | ||
| 4,000–6,000 | 128 (36.36%) | 65 (18.47%) | 63 (17.90%) | ||
| 6,000–8,000 | 99 (28.13%) | 41 (11.65%) | 58 (16.48%) | ||
| 8,000 and above | 88 (25%) | 22 (6.25%) | 66 (18.75%) | ||
| Educational attainment | 5.455 | 0.065 | |||
| High school and below | 131 (37.22%) | 64 (18.18%) | 67 (19.03%) | ||
| College and bachelor’s degree | 213 (60.51%) | 77 (21.88%) | 136 (38.64%) | ||
| Postgraduate and above | 8 (2.27) | 3 (0.85%) | 5 (1.42%) | ||
| Type of pregnancy | 9.735 | <0.01 | |||
| Natural conception | 278 (79%) | 102 (28.98%) | 176 (50%) | ||
| In-vitro fertilization | 74 (21%) | 42 (11.93%) | 32 (9.09%) | ||
| Previous psychiatric history | 5.973 | <0.01 | |||
| Present | 12 (3.41%) | 9 (2.56%) | 3 (0.85%) | ||
| Absent | 340 (96.59%) | 135 (38.35%) | 205 (58.24%) | ||
| Complications during pregnancy | 0.896 | 0.344 | |||
| Present | 180 (51.14%) | 78 (22.16%) | 102 (28.98%) | ||
| Absent | 172 (48.86%) | 66 (18.75%) | 106 (30.11%) | ||
| Mode of delivery | 4.942 | 0.026 | |||
| Natural childbirth | 26 (7.39%) | 16 (4.55%) | 10 (2.84%) | ||
| Cesarean section | 326 (92.61%) | 128 (36.36%) | 198 (56.25%) | ||
| Planned pregnancy | 1.411 | 0.235 | |||
| Present | 257 (73.01%) | 110 (31.25%) | 147 (41.76%) | ||
| Absent | 95 (26.99%) | 34 (9.66%) | 61 (27.33%) | ||
| Complications of prematurity | 0.896 | 0.344 | |||
| Present | 180 (51.14%) | 78 (22.16%) | 102 (28.98%) | ||
| Absent | 172 (48.86%) | 66 (18.75%) | 106 (30.11%) | ||
| Number of abortions | 1 (0, 1) | 1 (0, 2) | 1 (0, 1) | −4.945 | <0.01 |
| Birth weight of premature babies | 1,295 (1,000, 1,450) | 1,085 (900, 1,340) | 1,390 (1,122.5, 1,450) | −7.741 | <0.01 |
| Length of hospitalization | 38 (28, 57) | 45 (30, 63) | 34 (25, 47) | −4.003 | <0.01 |
| Disease uncertainty | 85 (76, 98) | 98 (89, 115) | 77 (47, 85) | −13.119 | <0.01 |
| Uncertainty | 29 (25, 37) | 37 (29, 49) | 27 (24, 32) | −9.493 | <0.01 |
| Sophistication | 24 (21, 28) | 28.7 ± 5.766 | 21 (20, 24) | −10.555 | <0.01 |
| Lack of information | 17 (15, 19) | 19 (16, 20) | 16 (15, 18) | −4.938 | <0.01 |
| Unpredictability | 14 (11, 15) | 15 (14, 17) | 12 (10, 14) | −10.777 | <0.01 |
| Neuroticism | 33 (29, 36) | 35 (33, 39) | 31 (27, 34) | −8.419 | <0.01 |
| Positive ways of responding | 31 (28, 34) | 30 (27, 33) | 32 (29, 34) | −2.575 | <0.01 |
| Negative coping styles | 19 (16, 21) | 20 (18, 22) | 17 (14, 17) | −6.564 | <0.01 |
| Social security (pensions, medical insurance) | 60 (51, 70) | 52 (46, 60) | 68.5 (57, 73) | −9.714 | <0.01 |
Results of one-way analysis of PTSD.
Collinearity diagnostics
We assessed multicollinearity among predictor variables using two metrics: 1) tolerance values (>0.1) and 2) variance inflation factors (VIFs < 5). Diagnostic results confirmed the absence of multicollinearity concerns across all 13 examined variables, supporting their independent inclusion in subsequent regression analyses.
Multicollinearity assessment
We evaluated potential multicollinearity among predictors using tolerance values and VIFs. All 13 candidate variables demonstrated acceptable ranges (tolerance > 0.1; VIF < 5), indicating no substantial multicollinearity concerns.
Logistic regression analysis
We constructed a binary logistic regression model with maternal PTSD status as the outcome variable. All 13 variables showing significant associations (P < 0.05) in the univariate analyses were entered as predictors after appropriate coding (see Table 2). The final regression model results are presented in Table 3, identifying significant independent risk factors for PTSD development in mothers of preterm infants.
Table 2
| Variable name | Variable assignment |
|---|---|
| X1 Previous family history of mental illness | No = 0; yes = 1 |
| X2 Pregnancy methods | Natural conception = 1; in-vitro fertilization = 2 |
| X3 Mode of delivery | Normal birth = 1; cesarean section = 2 |
| X4 Monthly household income | Below $4,000 = X4 (1) |
| 4,000–6,000 = X4 (2) | |
| 6,000–8,000 = X4 (3) | |
| 8,000 and above = X4 (4) | |
| X5 Age | Original value entry |
| X6 Number of abortions | Original value entry |
| X7 Birth weight | Original value entry |
| X8 Length of stay | Original value entry |
| X9 Disease uncertainty | Original value entry |
| X10 Neurotic personality | Original value entry |
| X11 Positive response approach | Original value entry |
| X12 Negative coping styles | Original value entry |
| X13 Support score | Original value entry |
| Y Post-traumatic stress disorder | No = 0; yes = 1 |
Logistic regression assignment table.
Table 3
| Predictor variable | B | SE | Wald | P | OR | 95% CI |
|---|---|---|---|---|---|---|
| Number of abortions | 0.816 | 0.243 | 11.261 | 0.001 | 2.262 | 1.404–3.644 |
| Birth weight | −0.004 | 0.001 | 16.769 | 0 | 0.996 | 0.995–0.998 |
| Disease uncertainty | 0.136 | 0.02 | 45.789 | 0 | 1.145 | 1.101–1.191 |
| Negative coping styles | 0.265 | 0.073 | 13.118 | 0 | 1.304 | 1.129–1.505 |
| Neuroticism | 0.157 | 0.042 | 13.807 | 0 | 1.171 | 1.077–1.272 |
| Total support score | −0.087 | 0.021 | 16.653 | 0 | 0.917 | 0.879–0.956 |
| Constant | −13.934 | 3.141 | 19.673 | 0 | 0 |
Binary logistic regression analysis for PTSD.
Predictive nomogram development
We developed a clinically applicable nomogram (Figure 1) to assess PTSD risk in mothers of VLBWIs, incorporating all significant predictors identified through our multivariable logistic regression analysis. This visual predictive tool enables individualized risk estimation, immediate clinical assessment, and stratification of high-risk patients.
Figure 1

Individualized nomogram early warning model for PTSD in very low birth weight infants.
Model discrimination performance
The nomogram demonstrated excellent discriminative ability: AUC = 0.969 (95% CI: 0.953–0.984); sensitivity: 93.1%; specificity: 89.4%; optimal cutoff: 0.356 (Youden index = 0.825) (Figure 2).
Figure 2
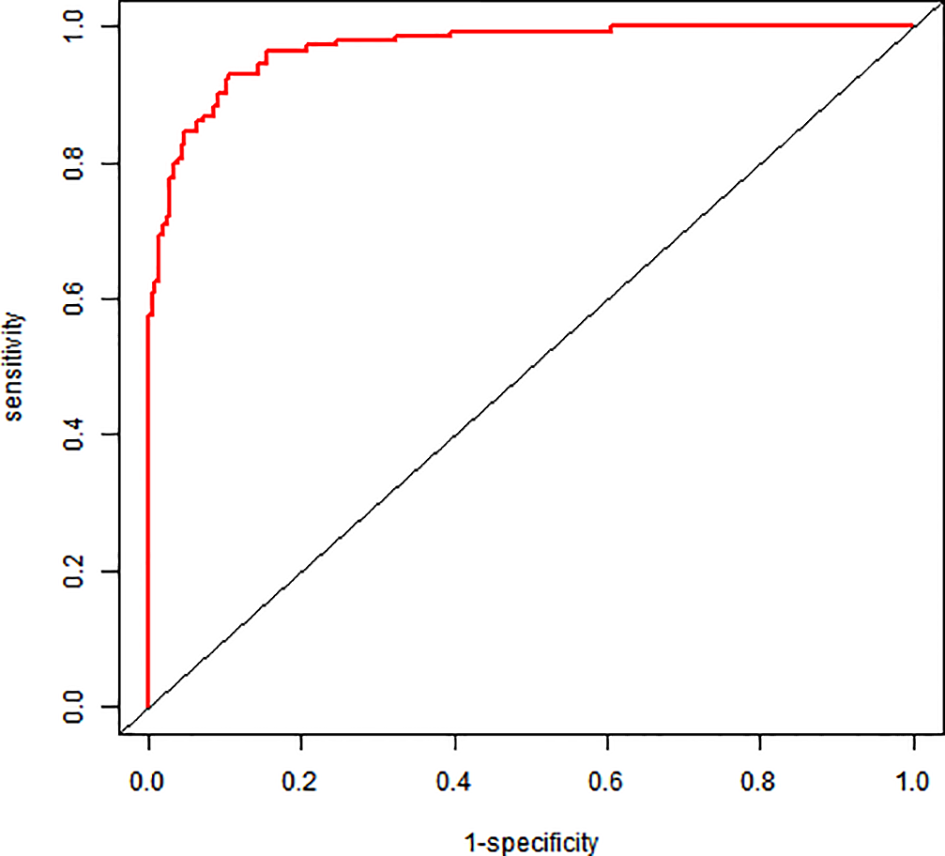
ROC graph for a column-line graph.
Model validation
Internal validation via bootstrap resampling (1,000 iterations) confirmed model robustness: Hosmer–Lemeshow test: χ² = 2.6128, P = 0.956. The calibration curves showed excellent agreement between predicted and observed risks (Figure 3).
Figure 3
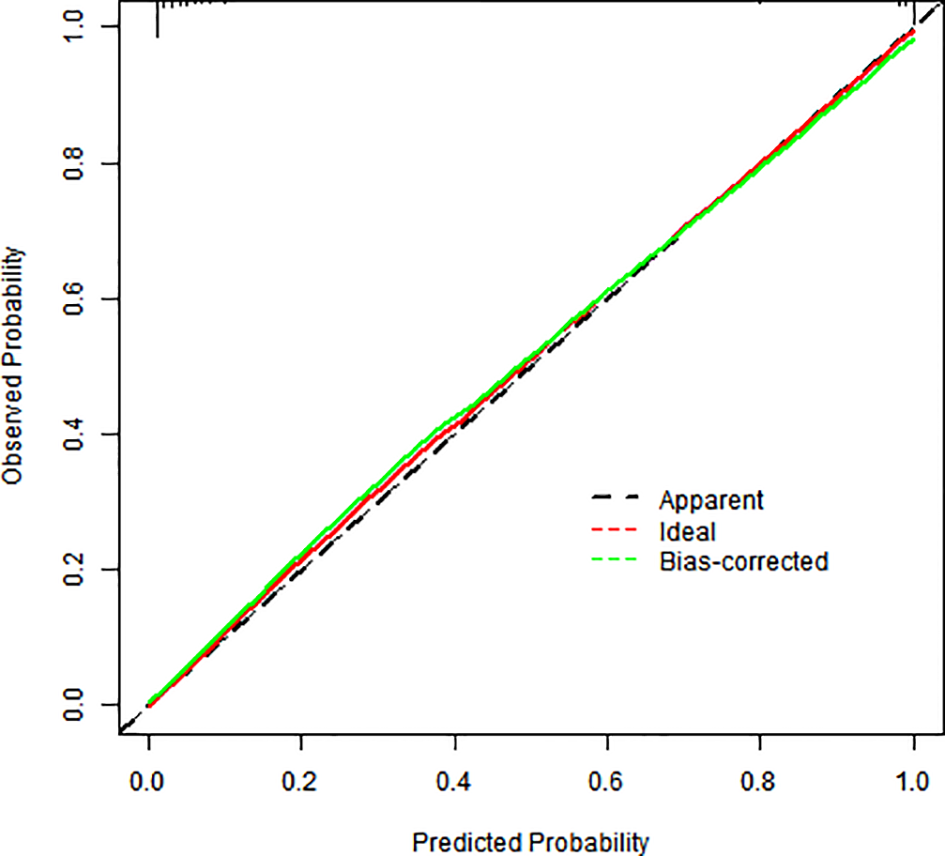
Calibration graph for a column-line graph.
Clinical applicability
The model showed 1) strong predictive accuracy for PTSD risk, 2) excellent clinical utility per decision curve analysis (Figure 4), and 3) significant clinical value across multiple thresholds (Figure 5).
Figure 4
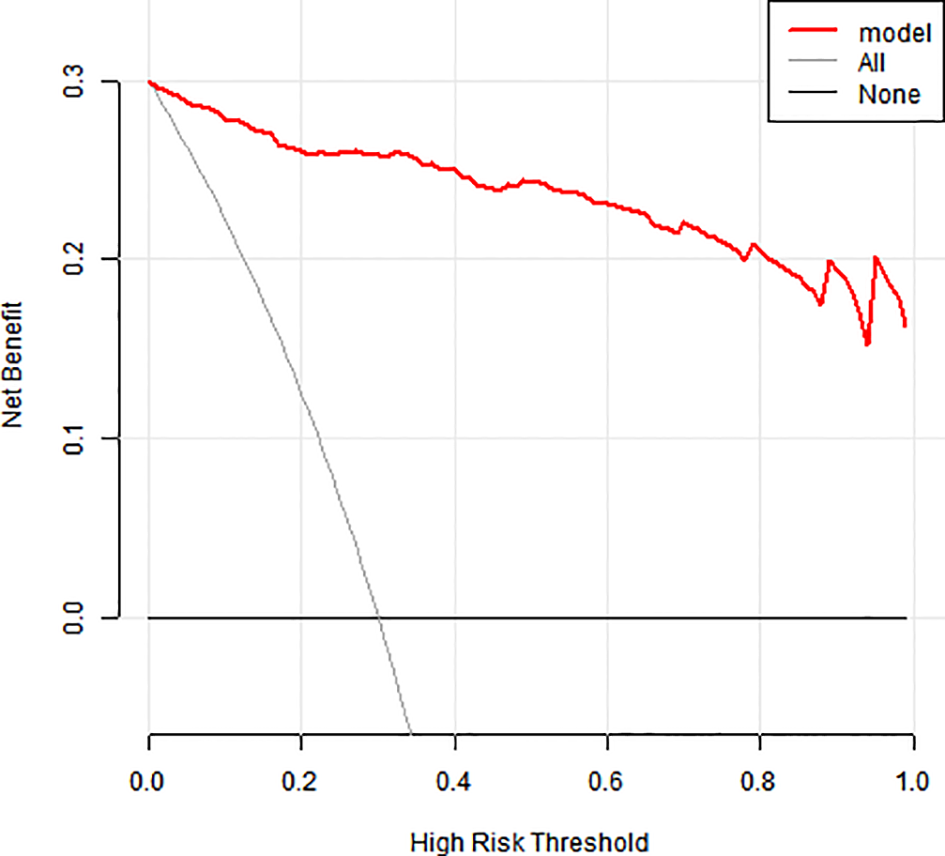
Clinical decision-making curve for a column-line diagram.
Figure 5
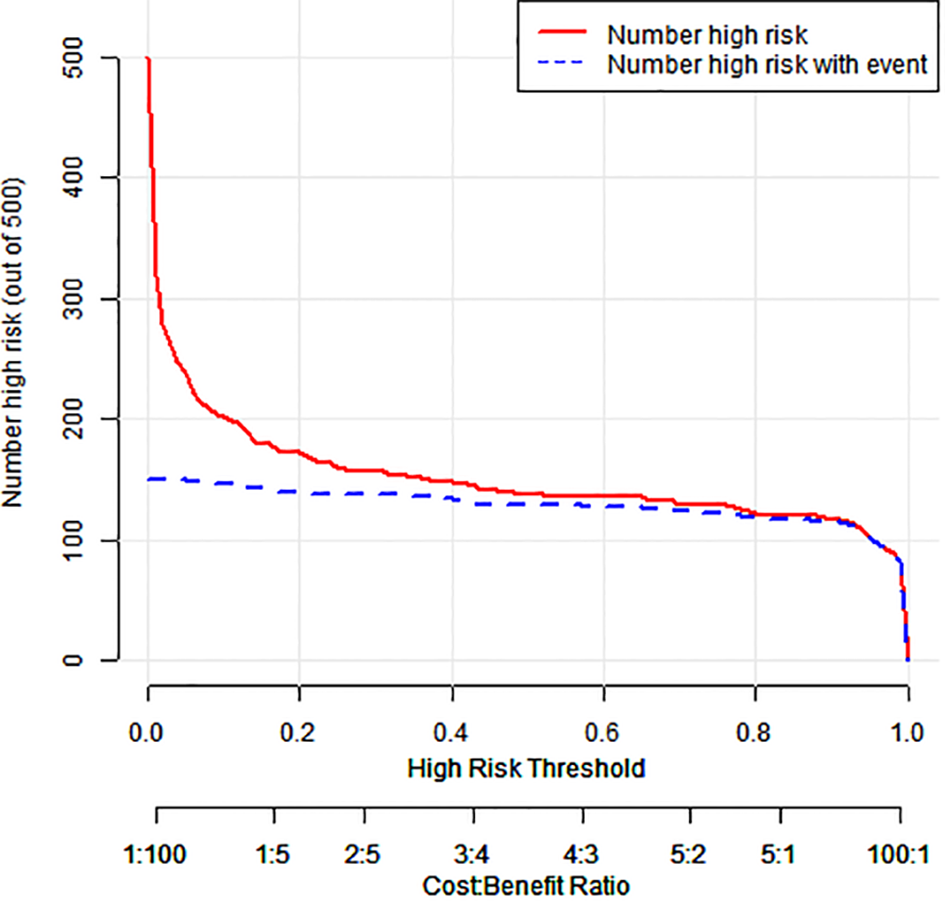
Clinical impact curve for a column-line diagram.
Discussion
Our findings indicate that neurotic personality traits represent a significant risk factor for PTSD among mothers of preterm infants, with higher neuroticism scores correlating with increased PTSD susceptibility. This aligns with the findings of Mattson (18), showing that neuroticism serves as both a psychological vulnerability factor for stress-related disorders and one of the strongest predictors of PTSD, characterized by negative emotionality and affective instability. Individuals with high neuroticism exhibit heightened emotional reactivity to stressors, demonstrating greater negative affect volatility and predisposition to anxiety/impulsivity compared to the general population. Consequently, early targeted psychological interventions by healthcare providers could enhance stress resilience in these mothers, potentially mitigating PTSD incidence through improved coping capacity and emotional regulation.
Existing literature (19) demonstrates that prior pregnancy loss (including spontaneous abortion, stillbirth, and/or induced abortion) significantly increases preterm mothers’ risk of postpartum depression, anxiety, and PTSD. This finding aligns with the study of Aftyka (20), showing significant positive correlations between pregnancy/abortion frequency and maternal distress. Studies indicate that pregnancy loss exacerbates psychological burden during subsequent pregnancies, with emotional outcomes being moderated by maternal age, education level, and abortion frequency. Notably, the incidence of adverse emotional states shows a dose–response relationship with the number of prior abortions. Our findings demonstrate that birth weight significantly impacts infant outcomes, consistent with the results reported by Chang (21). Parents of low birth weight infants, particularly very low/extremely low birth weight infants, exhibit higher PTSD symptom prevalence compared to parents of normal birth weight preterm infants. Very low birth weight infants are more susceptible to prematurity-related complications, including sepsis, bronchopulmonary dysplasia, intraventricular hemorrhage, and necrotizing enterocolitis. Preterm birth and low birth weight may adversely affect neurodevelopmental and psychological outcomes, with failure to achieve catch-up growth potentially increasing risks for adult-onset conditions like diabetes and cardiovascular disease (22, 23). Furthermore, prolonged NICU stays resulting in mother–infant separation, combined with maternal inexperience in breastfeeding and post-discharge feeding anxieties (e.g., aspiration and cyanosis concerns), may exacerbate psychological distress and increase PTSD vulnerability.
According to Mishel’s uncertainty in illness theory (24), illness-related uncertainty impairs the ability of mothers of preterm infants to seek and process medical information, ultimately affecting maternal and infant health outcomes. In this study, the mother of a preterm infant scored 85 on the illness uncertainty scale, significantly higher than the threshold of 70, indicating a high level of uncertainty. Illness uncertainty is a risk factor for PTSD in these mothers, consistent with the findings of Gorsky. Among the four uncertainty dimensions, the highest average scores were in unpredictability and lack of information. Preterm infants with lower birth weights, more complex conditions, and greater complications heighten maternal uncertainty. Since all preterm infants in this study weighed <1,500 g, their mothers scored particularly high in unpredictability. The primary source of uncertainty stems from inadequate provider–patient communication: the elevated scores in the information deficiency dimension may reflect insufficient maternal health literacy.
This study found that mothers of preterm infants perceived stronger support from family [median score: 22 (IQR 18–25)] and friends [19 (16–24)] compared to other sources [e.g., relatives, colleagues; 19 (16–23)], suggesting that family and friends provide more substantial psychological support (25, 26), demonstrating that social support acts as a buffer between psychosocial stress and health outcomes, reducing the risk of PTSD. Given that very low birth weight infants typically require prolonged hospitalization and incur higher medical costs, robust social support becomes particularly crucial. Insufficient social support may elevate cortisol secretion and heighten biological stress sensitivity, whereas adequate support mitigates trauma perception (26, 27) and alleviates maternal psychological distress. Based on these findings, we propose the following strategies to reduce PTSD incidence among mothers of preterm infants: 1) family-level interventions: family members and friends—especially spouses—should provide consistent companionship, emotional support, and encouragement; 2) individual-level actions: mothers should recognize the value of social support, engage in community activities, and proactively seek help from their social networks to manage emotional distress; and 3) healthcare provider role: clinicians should screen for maternal distress early and facilitate peer support groups where mothers can share experiences, fostering confidence in their infant’s recovery and preventing PTSD through shared coping strategies.
Coping style is a critical determinant of outcomes following stressful events. Mothers of preterm infants experience chronic stress due to multiple factors: disease complexity, prolonged hospitalization, uncertain prognosis, restricted NICU access, high medical costs, and fear of unknown outcomes. Those who adopt negative coping strategies often develop anxiety, depression, and other adverse emotional states, which hinder their own recovery. Research indicates that positive coping strategies enhance psychological resilience and confidence when facing traumatic events, promoting both mental and physical health (28, 29). Therefore, healthcare providers should regularly assess mothers’ coping styles, offer disease-specific information and emotional support to families, and minimize ineffective coping behaviors.
Postpartum PTSD frequently presents with psychiatric comorbidities, particularly major depressive disorder and anxiety disorders. Existing literature demonstrates substantial comorbidity, with one study finding 90.4% of postpartum women with PTSD concurrently meeting the diagnostic criteria for major depression (30). Anxiety symptom elevation similarly demonstrates a high co-occurrence with PTSD in the perinatal period. A methodological limitation of our study was the absence of validated depression or anxiety assessment tools. This precluded both the quantification of comorbidity prevalence and statistical adjustment for these confounding factors. Future investigations should incorporate standardized measures (e.g., Edinburgh Postnatal Depression Scale, GAD-7) to better characterize these comorbidities and their interactions with PTSD.
PTSD in the postpartum period commonly co-occurs with other mental health conditions, especially depression and anxiety. Although our study focused on PTSD symptoms, comorbidity was high. For instance, one study reported that 90.4% of women with postpartum PTSD also met the criteria for major depression (31, 32). Similarly, elevated anxiety symptoms often accompany PTSD in new mothers. Our data collection did not include validated instruments for postpartum depression or anxiety. The absence of these measures limits our ability to quantify comorbidity rates or adjust for their influence. Future studies should administer standardized depression and anxiety scales to examine this overlap with PTSD.
Our scope was limited to PTSD as defined by symptoms persisting at least 1 month postpartum. We deliberately did not assess acute trauma reactions or grief-specific responses. Acute stress disorder (ASD) refers to PTSD-like symptoms occurring within the first month after trauma (33, 34). By requiring a 1-month interval, our protocol excluded cases of ASD. It is possible that some women experienced significant acute distress immediately postpartum that either resolved or progressed to full PTSD. Future studies might track early post-traumatic symptoms to predict later PTSD. Similarly, we did not specifically measure perinatal grief reactions (such as following miscarriage or stillbirth), which involved trauma and loss but were beyond our study’s focus.
Potential confounders and effect modifiers were not all accounted for in our analysis. In particular, social support and coping style can significantly influence PTSD risk. Adequate social support during and after childbirth generally protects against PTSD, whereas low support elevates risk (35, 36). Similarly, adaptive coping strategies mitigate trauma impact, while maladaptive coping (e.g., avoidance) can exacerbate symptoms (37). We did not measure these psychosocial factors, so differences in support or coping between groups could confound our observed associations.
Postpartum depression and anxiety are highly prevalent among mothers with PTSD. Due to the absence of depression and anxiety screening tools in this study, we acknowledge this as a limitation and recommend inclusion in future research to fully evaluate comorbidity effects.
Our study evaluated PTSD symptoms at a follow-up point beyond 1 month postpartum and did not focus on acute stress reaction (within days) or acute PTSD (within 1 month). Furthermore, perinatal grief—typically linked to fetal or neonatal loss—was not assessed, as our cohort involved surviving infants. However, emotional responses related to threatened loss or severe neonatal complications may mimic grief processes, suggesting a need for future investigation (38).
Several potential confounders and effect modifiers—such as maternal age, socioeconomic status, obstetric history, and social support—may influence PTSD risk. While our regression model controlled for many of these variables, unmeasured confounding may remain. We also note that social support and coping styles may interact with stress exposure to modify PTSD risk.
Conclusion
Through rigorous methodology, we developed and validated a clinically useful nomogram for predicting PTSD risk in mothers of ELBWIs. This practical scoring tool enables clinicians to identify high-risk mothers efficiently and to optimize their clinical management. The model provides valuable early warning capabilities and enhances screening for high-risk cases, representing an important advancement in maternal mental healthcare.
Research innovations
-
Utilizing a prospective design, this study provides a comprehensive systematic evaluation of risk factors for PTSD in mothers of VLBWIs, synthesizing domestic and international evidence to facilitate early identification of high-risk populations.
-
This represents the first application of a nomogram for visual risk prediction of PTSD in mothers of VLBWIs, offering clinicians an intuitive, efficient tool for individualized risk assessment.
Study limitations
-
The study’s relatively small sample size and single-region hospital recruitment may limit the generalizability of the findings.
-
Reliance on self-reported questionnaire data introduces potential reporting biases, including possible overreporting of symptoms.
-
The nomogram requires external validation in diverse populations to confirm its clinical utility.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by The Ethics Committee of Shandong First Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
LL: Writing – original draft, Data curation, Formal Analysis, Investigation, Visualization, Writing – review & editing. YX: Data curation, Formal Analysis, Writing – review & editing. XJ: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Berseth CL Bisquera JA Paje VU . Prolonging small feeding volumes early in life decreases the incidence of necrotizing enterocolitis in very low birth weight infants. Pediatrics. (2003) 111:529–34. doi: 10.1542/peds.111.3.529
2
Alhamad M Kumar A Chaaban H Wickline KM Ho T . Platelets and immature neutrophils in preterm infants with feeding intolerance. Am J Perinatol. (2021) 38:1150–7. doi: 10.1055/s-0040-1710555
3
van der Kolk BA Pelcovitz D Roth S Mandel FS McFarlane A Herman JL . Dissociation, somatization, and affect dysregulation: the complexity of adaptation of trauma. Am J Psychiatry. (1996) 153:83–93. doi: 10.1176/ajp.153.7.83
4
Buck GM Msall ME Schisterman EF Lyon NR Rogers BT . Extreme prematurity and school outcomes. Paediatr Perinat Epidemiol. (2000) 14. doi: 10.1046/j.1365-3016.2000.00276.x
5
Feeley N Zelkowitz P Cormier C Charbonneau L Lacroix A Papageorgiou A et al . Posttraumatic stress among mothers of very low birthweight infants at 6 months after discharge from the neonatal intensive care unit. Appl Nurs Res. (2011) 24:114–7. doi: 10.1016/j.apnr.2009.04.004
6
Singer LT Fulton S Kirchner HL Eisengart S Lewis B Short E et al . Parenting very low birth weight children at school age: maternal stress and coping. J Pediatr. (2007) .151:463–9. doi: 10.1016/j.jpeds.2007.04.012
7
Bosquet EM Egeland B Carlson E Blood E Wright RJ . Mother-infant attachment and the intergenerational transmission of posttraumatic stress disorder. Dev Psychopathol. (2014) 26:41–65. doi: 10.1017/S0954579413000515
8
Barch DM Rogers C . Maternal depression and child development: clues to causal mechanisms from potential confounders. Am J Psychiatry. (2019) . 176:680–2. doi: 10.1176/appi.ajp.2019.19070707
9
Goodman SH Rouse MH Connell AM Broth MR Hall CM Heyward D et al . Maternal depression and child psychopathology: a meta-analytic review. Clin Child Fam Psychol Rev. (2011) . 14:1–27. doi: 10.1007/s10567-010-0080-1
10
Mattson E James L Engdahl B . Personality factors and their impact on PTSD and post-traumatic growth is mediated by coping style among OIF/OEF veterans. Mil Med. (2018) 183:e475–80. doi: 10.1093/milmed/usx201
11
Giannandrea SA Cerulli C Anson E Chaudron LH . Increased risk for postpartum psychiatric disorders among women with past pregnancy loss. J Womens Health (Larchmt). (2013) .22:760–8. doi: 10.1089/jwh.2012.4011
12
Wereszczak J Miles MS Holditch-Davis D . Maternal recall of the neonatal intensive care unit. Neonatal Netw. (1997) 16:33–40.
13
Horbar JD Carpenter JH Badger GJ Kenny MJ Soll RF Morrow KA et al . Mortality and neonatal morbidity among infants 501 to 1500 grams from 2000 to 2009. Pediatrics. (2012) 129:1019–26. doi: 10.1542/peds.2011-3028
14
Weathers FW Litz BT Herman DS Huska JA Keane TM . The PTSD Checklist (PCL): Reliability, validity, and diagnostic utility. San Antonio, TX: Presented at: Annual Convention of the International Society for Traumatic Stress Studies (1993).
15
Cloitre M Garvert DW Weiss B Carlson EB Bryant RA . Distinguishing PTSD, complex PTSD, and borderline personality disorder: A latent class analysis. Eur J Psychotraumatol. (2014) 5:25097. doi: 10.3402/ejpt.v5.25097
16
Grekin R O’Hara MW . Prevalence and risk factors of postpartum posttraumatic stress disorder: A meta-analysis. Clin Psychol Rev. (2014) 34:389–401. doi: 10.1016/j.cpr.2014.05.003
17
Seng JS Sperlich M Low LK . Mental health, demographic, and risk behavior profiles of pregnant survivors of childhood and adult abuse. J Midwifery Womens Health. (2008) 53:511–21. doi: 10.1016/j.jmwh.2008.04.001
18
Mishel MH . Reconceptualization of the uncertainty in illness theory. Image J Nurs Sch. (1990) 22:256–62. doi: 10.1111/j.1547-5069.1990.tb00225.x
19
Vignato J Georges JM Bush RA Connelly CD . Post-traumatic stress disorder in the perinatal period: A concept analysis. J Clin Nurs. (2017) 26:3859–68. doi: 10.1111/jocn.13800
20
Aftyka A Rybojad B Rozalska-Walaszek I Rzonca P Humeniuk E . Post-traumatic stress disorder in parents of children hospitalized in the neonatal intensive care unit (NICU): medical and demographic risk factors. Psychiatr Danub. (2014) 26:347–52.
21
Chang HP Chen JY Huang YH Yeh CJ Huang JY Su P et al . Factors associated with post-traumatic symptoms in mothers of preterm infants. Arch Psychiatr Nurs. (2016) . 30:96–101. doi: 10.1016/j.apnu.2015.08.019
22
Ayers S Bond R Bertullies S Wijma K . The aetiology of post-traumatic stress following childbirth: a meta-analysis and theoretical framework. Psychol Med. (2016) . 46:1121–34. doi: 10.1017/S0033291715002706
23
Petit AC Eutrope J Thierry A Bednarek N Aupetit L Saad S et al . Mother’s Emotional and Posttraumatic Reactions after a Preterm Birth: The Mother-Infant Interaction Is at Stake 12 Months after Birth. PLoS One. (2016) .11:e151091. doi: 10.1371/journal.pone.0151091
24
Ranstam J Cook JA Collins GS . Clinical prediction models. Br J Surg. (2016) 103:1886. doi: 10.1002/bjs.10242
25
Shaw RJ Lilo EA Storfer-Isser A Ball MB Proud MS Vierhaus NS et al . Screening for symptoms of postpartum traumatic stress in a sample of mothers with preterm infants. Issues Ment Health Nurs. (2014) . 35:198–207. doi: 10.3109/01612840.2013.853332
26
Shuman DW . The diagnostic and statistical manual of mental disorders in the courts. Bull Am Acad Psychiatry Law. (1989) 17:25–32.
27
Lawrence JW Fauerbach JA . Personality, coping, chronic stress, social support and PTSD symptoms among adult burn survivors: a path analysis. J Burn Care Rehabil. (2003) . 24:63–72, 62. doi: 10.1097/00004630-200301000-00016
28
Lefkovics E Rigo JJ Kovacs I Talaber J Szita B Kecskemeti A et al . Effect of maternal depression and anxiety on mother’s perception of child and the protective role of social support. J Reprod Infant Psychol. (2018) 36:434–48. doi: 10.1080/02646838.2018.1475726
29
Malin KJ Johnson TS Brown RL Leuthner J Malnory M White-Traut R et al . Uncertainty and perinatal post-traumatic stress disorder in the neonatal intensive care unit. Res Nurs Health. (2022) .45:717–32. doi: 10.1002/nur.22261
30
Rodriguez DC Ceriani CJ Abarca P Edwards E Barrueco L Lesta P et al . Chronic post-traumatic stress in mothers of very low birth weight preterm infants born before 32 weeks of gestation. Arch Argent Pediatr. (2020) .118:306–12. doi: 10.5546/aap.2020.eng.306
31
Garthus-Niegel S Ayers S von Soest T Torgersen L Eberhard-Gran M . The impact of postpartum posttraumatic stress disorder on partner relationship and child development: A population-based cohort study. J Affect. Disord. (2015) 182:180–6. doi: 10.1016/j.jad.2015.04.033
32
Verreault N Da Costa D Marchand A Ireland K Dritsa M Khalifé S . Rates and risk factors associated with postpartum posttraumatic stress disorder symptoms. Arch Womens Ment Health. (2012) 15:423–33. doi: 10.1007/s00737-012-0302-0
33
Ruiming W Jie S Xiaoyu B Yubing W Lei M . Validity and reliability of the Chinese version of the PTSD Checklist–Civilian Version (PCL-C) in earthquake survivors. BMC Psychiatry. (2007) 7:17. doi: 10.1186/1471-244X-7-17
34
Horsch A Gilbert L Luyten P . The role of maternal attachment in perinatal posttraumatic stress disorder. J Affect. Disord. (2015) 179:148–56. doi: 10.1016/j.jad.2015.03.010
35
Razurel C Bruchon-Schweitzer M Dupanloup A Irion O Epiney M . Stressful events, social support and coping strategies of primiparous women during the postpartum period: A qualitative study. Midwifery. (2011) 27:237–42. doi: 10.1016/j.midw.2009.06.005
36
Taylor SE . Social support: A review. In: FriedmanHS, editor. The Oxford Handbook of Health Psychology. Oxford University Press, New York (2011). p. 189–214.
37
Ford E Ayers S . Support during birth interacts with prior trauma and birth intervention to predict postnatal posttraumatic stress symptoms. Psychol Health. (2009) 24:1239–56. doi: 10.1080/08870440802582610
38
Gorski PA Ruchala PL Moro T . The relationship between maternal uncertainty and psychological distress in mothers of infants in the NICU. Adv Neonatal Care. (2020) 20:123–30. doi: 10.1097/ANC.0000000000000715
Summary
Keywords
very low birth weight infants, mothers, post-traumatic stress disorder, PTSD, nomogram, risk prediction, neonatal intensive care
Citation
Lu L, Xu Y and Jin X (2025) Construction of a visual nomogram prediction model for post-traumatic stress disorder in mothers of very low birth weight infants. Front. Psychiatry 16:1550267. doi: 10.3389/fpsyt.2025.1550267
Received
04 January 2025
Accepted
23 June 2025
Published
16 July 2025
Volume
16 - 2025
Edited by
Tao-Hsin Tung, Taizhou Hospital of Zhejiang Province Affiliated to Wenzhou Medical University, China
Reviewed by
Darpan Kaur, Mahatma Gandhi Missions Medical College and Hospital, India
Suchetha S. Rao, Manipal Academy of Higher Education, India
Updates
Copyright
© 2025 Lu, Xu and Jin.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yujiao Xu, 17862664453@163.com; Xiaolong Jin, jinxiaolong1977@sina.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.