- 1Department of Tieling Mental health Prevention and Control, The Third People’s Hospital of Liaoning, Tieling, China
- 2Department of Ophthalmology, The Fourth People’s Hospital of Shenyang, Shenyang, China
- 3Beijing Anding Hospital, Capital Medical University, Beijing, China
- 4Department of Shenyang mental Health Prevention and Control, Shenyang Mental Health Center, Shenyang, China
- 5Department of First Clinical, Jinzhou Medical University, Jinzhou, China
- 6Center for Psychological Development, China Medical University, Shenyang, China
Background: Postpartum depression (PPD) is an extremely common mood disorder that occurs at any time up to 1 year after delivery. PPD can have a negative impact on the mother and family. This study aimed to determine the prevalence of PPD and factors influencing PPD. Specifically, this study focused on the effects of marital quality and mental well-being on PPD and the mediating roles
Methods: A cross-sectional study was conducted in the Maternity and Child Health Care Hospitals of Liaoning Province in northeast China. The PHQ-9 scale was used to screen for PPD with a score ≥10, indicating a positive result. The Warwick-Edinburgh Mental Health and Marriage Perception scales were used to evaluate the mental well-being and marital quality of parturients.
Results: A total of 1048 participants were included in the study. The prevalence of PPD symptoms was 11.16%. Factors, such as education level (F = 2.63; p < 0.05), co-living status (F = 5.84; p < 0.01), agreement with fetal gender expectations (t = 19.39; p < 0.001), amount of physical activity (F = 17.15; p < 0.001), and knowledge about PPD (t = 3.66; p < 0.001 and t = 5.099; p < 0.001), were all associated with the PPD score and the prevalence. Mental well-being and marital quality were intricately linked to PPD symptoms. Mental well-being influenced PPD via two mediating factors (marital interaction [p < 0.001] and marital conflict [p< 0.001]).
Conclusions: PPD is a significant postpartum issue that is influenced by numerous factors. Early screening of parturients, accurate diagnosis of PPD, and timely intervention are crucial. Targeted interventions addressing risk factors may help to mitigate the incidence of PPD.
1 Introduction
Changes in the physiologic-psychological-social environment are known to occur during the perinatal period (1). Such changes have an impact on the mental well-being of gravidas and parturients, thereby elevating the susceptibility to various mental disorders (2), most notably anxiety and depression (3). Postpartum depression (PPD) is an extremely common mood disorder that occurs at any time up to 1 year after delivery and can have a negative impact on the mother and the family (4). Approximately 1 in 7 women (13%) are affected by PPD. Research suggests that PPD should receive given special attention in the puerperium (5). A 2022 cross-sectional study in Palestine reported a postpartum depression (PPD) prevalence of 33.9% at 7–12 weeks postpartum, with factors such as husband’s education level, primiparity, vacuum use, stressful events during pregnancy, and low social support significantly associated with PPD (6). Another study found PPD prevalence rates of 22.4% at 3 days and 3.2% at 6 months postpartum (7). A multinational study revealed an overall PPD symptom rate of 13.6%, ranging from 2.3% in Syria to 26% in Ghana, with an overall diagnosis rate of 6.2% (8).
Studies have shown that perinatal depression can last a long time, and if not effectively treated, can have a negative impact on maternal health, the family, and the baby (9). Interestingly, most women with perinatal depression refrain from seeking professional assistance (10).
The clinical practice guidelines of the American College of Obstetricians and Gynecologists (ACOG) and Chinese Expert Consensus suggest the need to screen women for mental health issues, including anxiety, depression, stress, suicide, and self-injury, during the perinatal period (11, 12). The recommended screening tools for depressive symptoms include the Patient Health Questionnaire-9 (PHQ-9) and Edinburgh Postpartum Depression Scale (EPDS) (13). Several confirmatory studies have utilized these tools extensively. The purpose of screening is to fulfill the requirements of tertiary prevention by detecting and intervening at the earliest possible time to minimize pain and burden (14). Randomized controlled trials have shown that APP-based cognitive behavioral therapy programs are effective in preventing PPD during the early postpartum period (15).
Several biological, psychological, socioeconomic, and cultural factors have associations with the development of PPD (16), including depression, anxiety insomnia, and stress (16, 17), infant feeding (18), unplanned pregnancy, a first-time mother, a poor mother-in-law relationship, and poor family support (19).
Negative partner or family relationship (20), poor self-esteem, and a miscarriage (21). Mobile phone usage, low birthweight (21), employee status (22), financial conditions (23), negative life events, and neuroticism (24). Robust social support and regular physical exercise serve as protective factors against PPD, a conclusion supported by substantial empirical evidence (25, 26). There are distinctive risk factors that impact PPD symptoms in China. For example, girl infants exhibit a higher prevalence of depressive symptoms compared to boys, which could potentially be attributed to the cultural inclination towards male offspring in China (27, 28). The practice of “zuoyuezi,” also known as “doing the month,” is a distinctive form of postnatal self-care in China. Zuoyuezi has been shown to provide social support and reduce depressive symptoms for mothers receiving care (29), while simultaneously leading to increased conflicts when receiving care from a mother-in-law, resulting in higher levels of depressive symptoms (30, 31).
PPD has been shown to negatively influence the physical and mental well-being of women (32). Partner support and the quality of a couple’s relationship are correlated with PPD (33) and intimate partner violence (34). However, there have been no studies involving the relationship between marital happiness and PPD symptoms. We propose that mental well-being and marital quality influence PPD symptoms through a mediating relationship.
With the social economy advances in China, mental health issues have gained significant attention. Comprehensive planning has been made for the entire population, ranging from severe mental disorders to the establishment of a social psychological service system (35). According to several official government documents, mental health screening for perinatal women is considered one of the key tasks in promoting mental health among specific populations. A specific plan for screening and intervention of women during the perinatal period was formulated in the annual major public health service projects in China. Maternal healthcare institutions and primary healthcare institutions are required to include screening for maternal depression during routine prenatal examinations and postpartum visits. Trained medical personnel or social workers will perform screening and pregnancy and PPD follow-up evaluations. Our research, as one part of the region, received support from this plan.
We performed a cross-sectional survey of parturients to document the prevalence of PPD symptoms. We then examined the associations between marital well-being, positive psychology, and PPD symptoms. Next, we analyzed the mediating role of positive psychology on the effect of marital well-being on PPD.
2 Materials and methods
2.1 Participants
The cross-sectional study was administered from 13 May to 31 December 2023. Convenience sampling was adopted to recruit parturients in four Maternity and Child Health Care Hospitals of Liaoning Province. Inclusion criteria: participants must be 18 years of age or older; within a few days to 12 months postpartum; voluntarily signed the informed consent form. The exclusion criteria were as follows: <18 years of age; declined to participate; multiple pregnancies; a current or prior history of bipolar disorder, schizophrenia, or other mental illnesses; known mental disorders; a major chronic illness; cognitive impairments;visual or auditory processing disorders;incomplete questionnaires; questionnaires with clear logical inconsistencies and obstetric complications (including but not limited to severe preeclampsia/eclampsia, placenta previa, placental abruption, major postpartum infection, intrauterine fetal demise, major congenital anomalies, or newborn weight <1500 grams).
2.2 Procedures
The approval for this study was obtained from the Medical Ethics Committee of Liaoning Mental Health Center and Hospital in Liaoning, China (Approval number: LNMHC2023-TT0001). A cross-sectional survey was performed and online questionnaires were made using an online survey platform (www.wjx.cn). Questionnaires were administered via QR codes placed in the outpatient area of the maternity hospital. Participants completed the questionnaires during waiting periods for follow-up visits or vaccinations, under the supervision of medical staff. The participants completed the questionnaires with unified instruction and affirmed comprehension of the study purpose. Informed consent was obtained from the participants and the validity of the collected questionnaire was checked. The responses were considered invalid for the following reasons: the response time was either too short or too long; the response was repeated or abnormal; the IP address was a duplicate or abnormal; the response consistency test failed; and if the questionnaire did not pass the “false question” test. A total of 1148 valid questionnaires were collected and the effective recovery rate was 84.3%.
2.3 Measurement tools
2.3.1 Associated characteristics survey
Data on associated variables,including 1)demographic:years of marriage, educational level, only child family, monthly income;location, cohabitation, working consistently during pregnancy, postpartum childcare centers? 2)health-related characteristics:smoking,alcohol consumption;exercise; 3)obstetric, current pregnancy, and infant-related characteristics, and their association:planned pregnancy;mode of conception;number of pregnancies;number of deliveries; adverse pregnancy history;high-risk maternity;how many kids is that?fatal sex;postpartum stage;4)psycho and social characteristics:maternal fetal sex expectation,consistency in fetal sex expectation, key family members’ fetal sex expectation, literacy (Know postpartum depression? Know where seeking-help for postpartum depression).
2.3.2 Patient health questionnaire-9 (PHQ-9)
The PHQ-9 is a self-report version based on 9 items from the DSM-IV diagnostic criteria of major depression disorder (36). The PHQ-9 has good reliability and validity in facilitating the diagnosis of depression in the general population (37)and parturients (38, 39). Ren et al. conducted a study of reliability and validity assessment in Chinese samples (37). The ACOG clinical practice guidelines recommend the PHQ-9 as one of the tools to screen parturients for depressive symptoms 8. Each item has a 4-point frequency score ranging from “0” (not at all) to “3” (almost every day). The total score ranges from 0–27. The higher the score, the more severe the depressive symptoms. The scoring ranges for different levels of depression severity are as follows: minimal or no depression (0–4);mild depression (5–9);moderate depression (10-14);moderately severe or severe depression (15-19) Cronbach’s alpha for the internal consistency reliability of the Chinese version of the PHQ-9 was 0.86 for the entire scale (40). The Cronbach’s α coefficient of the PHQ-9 was 0.892 for patients with MDD in psychiatric hospital (41). A score of 10 was the cut-off value for “positive depressive symptoms.”
2.3.3 Warwick-Edinburgh mental health scale
The WEM-WBS was developed to enable monitoring of mental well-being. The WEM-WBS consists of 14 items covering hedonic and eudaimonic aspects of mental health, including positive affect, satisfying interpersonal relationships, and positive functioning. Individuals completing the scale are required to respond to statements that best describes their experience over the past 2 weeks.The Likert scale represents a score for each item ranging from 1 (never) to 5 (all of the time) for a minimum score of 14 and a maximum score of 70. The overall score for the WEM-WBS was calculated by determining the scores for each item with equal weights. A higher WEM-WBS score indicated a higher level of mental well-being (42). The Chinese version of the Warwick–Edinburgh Mental Well-being Scale was used as the reliability and validity basis for the Chinese study, Cronbach’s α = 0.94 and test–retest reliability = 0.83 (43).
2.3.4 Marriage perception scale
Wang et al. developed the MPS, which is based on subjective perceptions of marriage. The MPS is used to assess the quality of an individual’s subjective feelings about marriage as indicators, which cover 3 dimensions, such as “Couple Interaction,” “Family Relationships,” and “Couple Conflict,” with 10, 5, and 5 questions for each factor, respectively. A Likert scale ranging from 1 (completely inconsistent) to 7 (completely consistent) was used. The factor score was calculated by summing up the corresponding item scores as follows: MPS total score = couple interaction + family relationship - couple conflict. A higher MPS total score indicated better marital quality with Cronbach’s α of 0.89 (44).
2.4 Statistics
The data were analyzed using online Social Science Statistics SPSSpro. Descriptive statistics, including frequency, percentage, and mean ± standard deviation, were employed to characterize the data. For comparisons between two groups, independent samples t-tests were utilized for normally distributed data, while the Mann-Whitney U test was applied for non-normally distributed data. In cases involving three or more groups, one-way ANOVA (assuming homogeneity of variance) was conducted for normally distributed data, whereas the Kruskal-Wallis test was used for non-parametric data. Chi-square tests were performed to examine differences in negative and positive symptoms of depression across demographic and psychosocial factors. Pearson correlation analysis was conducted to assess the relationships between PHQ-9 scores and both WEMWBS and MPS scores. Following a homogeneity of variance test, Welch’s ANOVA was selected to evaluate differences in WEMWBS and MPS scores across varying levels of PHQ-9 severity. The statistical analysis to examine the potential mediating effects of WEM-WBS and MPS on postpartum women’s PHQ-9 scores was conducted using a mediation analysis framework.To test this hypothesis, we conducted mediation analyses using WEM-WBS and MPS (including its sub-scales) as independent variables and mediators, respectively. We employed stepwise regression to examine the total effect and potential mediating pathways. Indirect effects were calculated, and their significance was assessed using the Bootstrap method. Based on the presence or absence of significant mediating effects, we constructed a structural equation model (SEM) to delineate the relationships among WEM-WBS, MPS (including sub-scales), and PHQ-9 scores, and tested the overall model fit and significance.The significance level was set at p<0.05.
3 Results
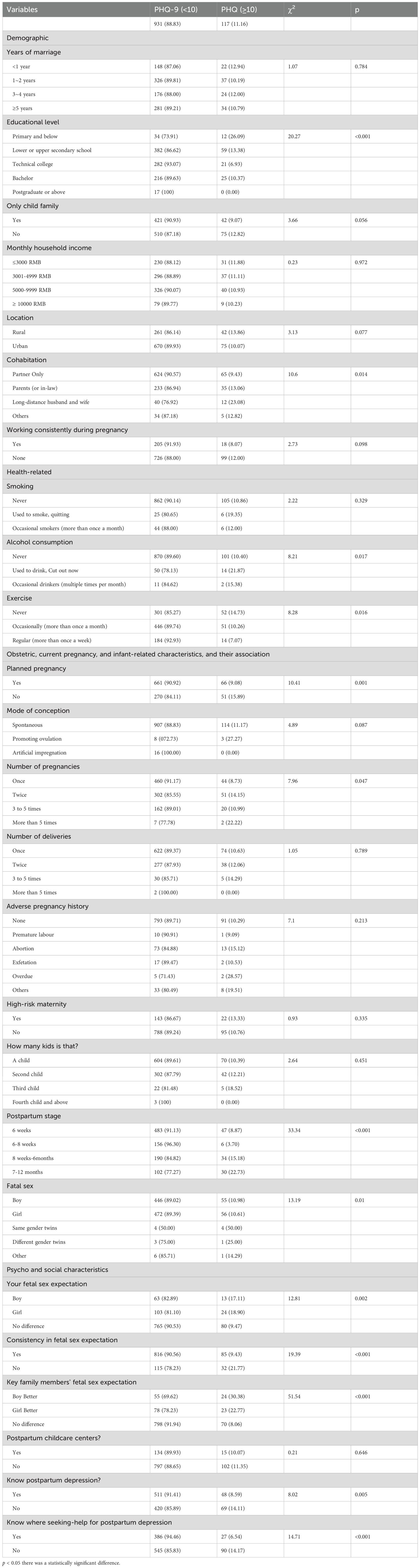
A total of 1148 pregnant women in northeast China were invited to participate in this study and complete the assessment. Overall, 1048 participants completed all assessments and provided effective answers. Participants who did not complete all assessments were regarded as non-responders (n = 100). The associated characteristics of this sample are shown in Table 1. The mean age of the participants was 30.171 years (SD ± 4.957 years). Among the 1048 participants, the prevalence of depression symptoms (PHQ-9 ≥ 10) was 11.15% (n = 117).
3.1 Associated characteristics of women with PPD symptoms
Table 1 summarizes the differences between variables with and without depression symptoms. The chi-square test results presents the demographic, health-related, and obstetric characteristics of women with postpartum depression (PPD) symptoms, categorized by their PHQ-9 scores (PHQ-9 < 10 and PHQ-9 ≥ 10). The chi-square test results of PHQ-9 scores on various variables are presented in Table 1. Women with primary education or below had a significantly higher proportion of PPD symptoms (26.09%) compared to those with higher education levels (χ2 = 20.27,p < 0.001). Women from families with only one child showed a lower proportion of PPD symptoms (9.07%) compared to those with more than one child (12.82%), though the difference was marginally significant (χ2 = 3.66,p > 0.056). Women living with their partners only had a lower proportion of PPD symptoms (9.43%) compared to those living with parents or in-laws (13.06%) or those in long-distance relationships (23.08%) χ2 = 10.60,p < 0.05). Women who never consumed alcohol had a lower proportion of PPD symptoms (10.40%) compared to those who used to drink but quit (21.87%)(χ2 = 8.21,p < 0.05). Women who exercised regularly had a lower proportion of PPD symptoms (7.07%) compared to those who never exercised (14.73%) (χ2 = 8.28,p < 0.05). Women with planned pregnancies had a lower proportion of PPD symptoms (9.08%) compared to those with unplanned pregnancies (15.89%) (χ 2 = 10.41,p < 0.01). Women in the 6–8 weeks postpartum stage had the lowest proportion of PPD symptoms (3.70%), while those in the 7–12 months stage had the highest (22.73%) (χ2 = 33.34,p < 0.001). Women who had no specific expectation for the fetal sex had a lower proportion of PPD symptoms (9.47%) compared to those who expected a boy (17.11%) or a girl (18.90%) (χ2 = 1.81,p < 0.01).Women who knew about postpartum depression had a lower proportion of PPD symptoms (8.59%) compared to those who did not (14.11%) (χ2 = 8.02,p < 0.01).3.2. Sociodemographic characteristics on the WEM-WBS and MPS.
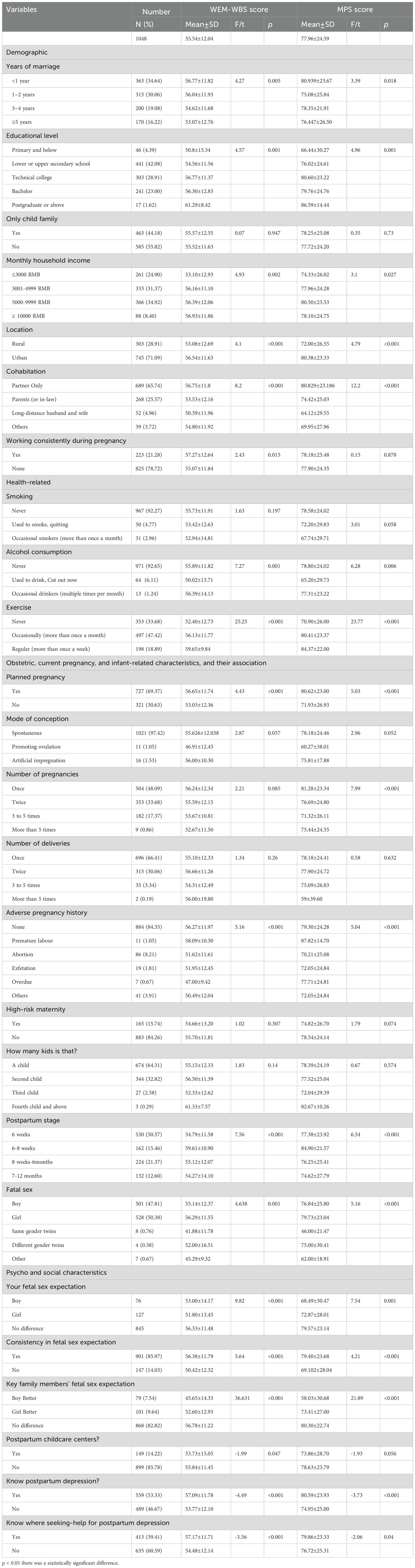
Table 2 examines the association between various demographic and health-related variables and the WEM-WBS (Well-being) and MPS (Marital Satisfaction) scores. The mean score on the WEM-WBS was 55.54 ± 12.041 (total possible score = 70) and the mean score on the MHS was 77.955 ± 24.586 (total possible score = 100).Women married for less than 1 year had higher WEM-WBS scores (56.77 ± 11.82) compared to those married for 5 years or more (53.07 ± 12.76) (F = 4.27, p < 0.01). Women with higher incomes (≥ 10000 RMB) had higher WEM-WBS scores (56.93 ± 11.86) compared to those with lower incomes (≤ 3000 RMB) (53.10 ± 12.93) (F = 4.93, p < 0.01). Urban women had higher WEM-WBS scores (56.54 ± 11.63) compared to rural women (53.08 ± 12.69) (t = 4.1, p < 0.001).Women living with their partners only had higher WEM-WBS scores (56.75 ± 11.8) compared to those living with parents or in-laws (53.53 ± 12.16) (F = 8.2, p < 0.001). Women who exercised regularly had higher WEM-WBS scores (59.65 ± 9.84) compared to those who never exercised (52.40 ± 12.73)(F = 25.25, p < 0.001). Women with planned pregnancies had higher WEM-WBS scores (56.65 ± 11.74) compared to those with unplanned pregnancies (53.03 ± 12.36) (t = 4.43, p < 0.001). Women in the 6–8 weeks postpartum stage had the highest WEM-WBS scores (59.61 ± 10.90), while those in the 7–12 months stage had the lowest (54.27 ± 14.10) (F = 7.56, p < 0.001).
The study examined the associations between various demographic, health-related, obstetric, and psychosocial characteristics with the Maternal Postnatal Scale (MPS) scores. The results are presented in Table 2, highlighting significant differences across multiple variables. Significant differences in MPS scores were observed based on the duration of marriage (F = 3.39, p < 0.05). Participants married for less than one year had higher MPS scores (80.94 ± 23.67) compared to those married for 1–2 years (75.08 ± 25.84), 3–4 years (78.35 ± 21.91), and 5 years or more (76.45 ± 26.50). Educational attainment significantly influenced MPS scores (F = 4.96, p < 0.001). Participants with postgraduate education or above had the highest MPS scores (86.59 ± 14.44), while those with primary education or below had the lowest scores (66.44 ± 30.27). Urban residents had significantly higher MPS scores (80.38 ± 23.33) compared to rural residents (72.00 ± 26.55) (F = 4.79, p < 0.001). Participants living only with their partners had the highest MPS scores (80.83 ± 23.19), while those in long-distance relationships had the lowest scores (64.12 ± 29.55) (F = 12.2, p < 0.001). In health-related characteristic,Non-smokers had higher MPS scores (78.58 ± 24.02) compared to occasional smokers (67.74 ± 29.71) (F = 3.01, p = 0.058). Non-drinkers had significantly higher MPS scores (78.80 ± 24.02) compared to occasional drinkers (65.20 ± 29.73) (F = 6.28, p < 0.001). Regular exercisers had the highest MPS scores (84.37 ± 22.00), while those who never exercised had the lowest scores (70.90 ± 26.00) (F = 23.77, p < 0.001).
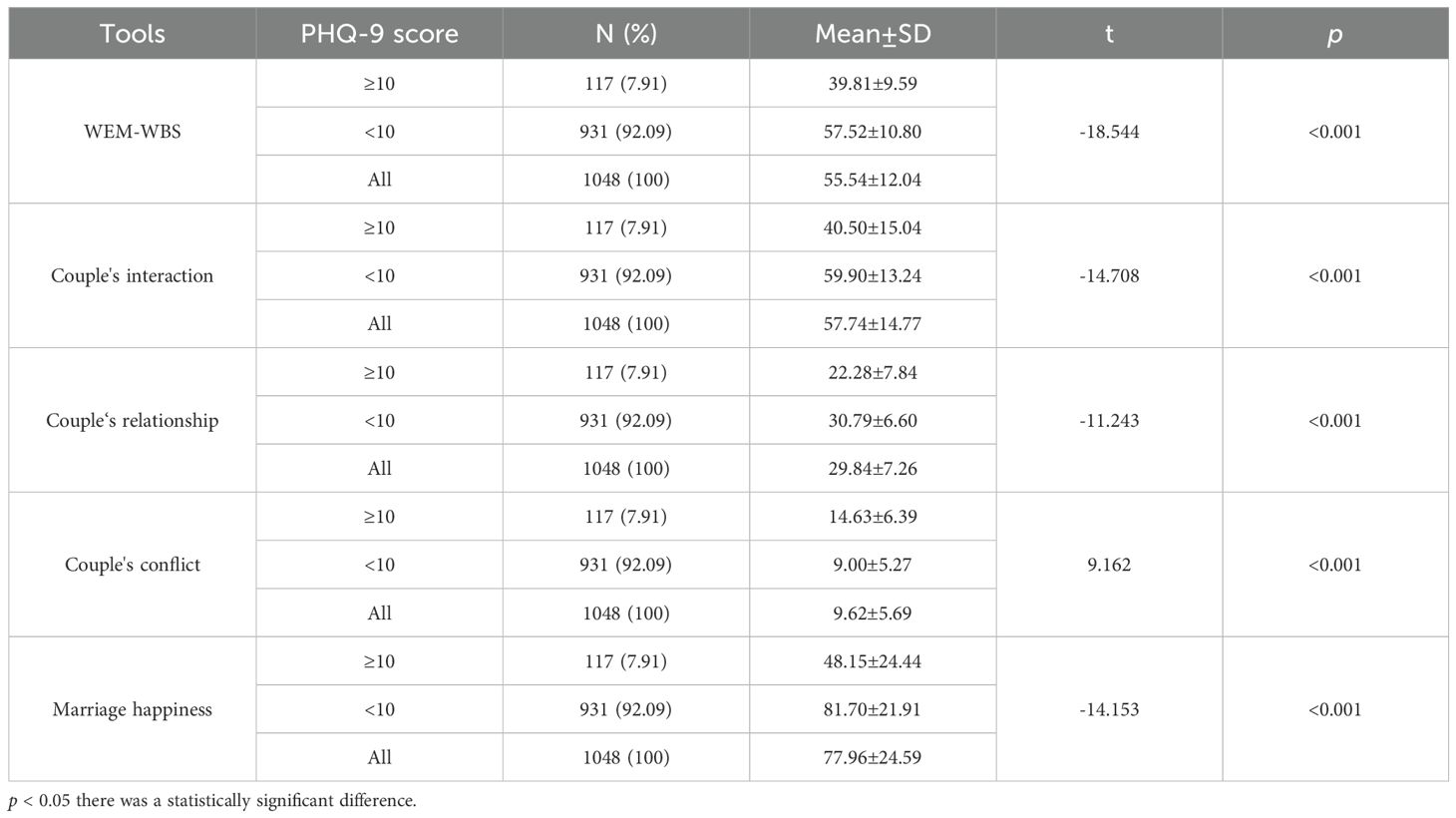
3.3 Postpartum PHQ-9 score and differences on the WEM-WBS and MPS
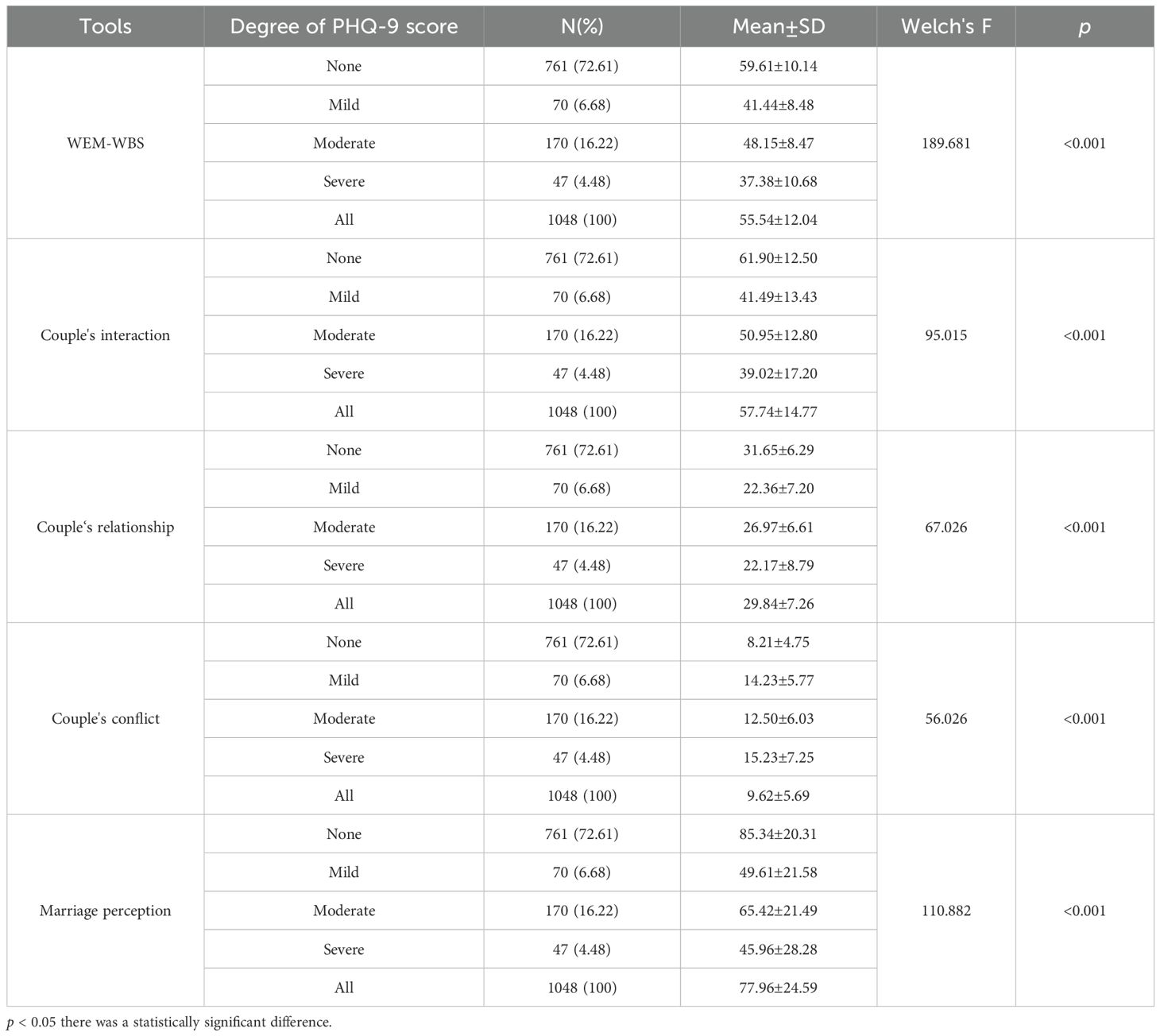
The results also revealed significant variations in the WEM-WBS and MHS scores across different levels of depressive symptoms (Table 3), as well as varying degrees of severity in depressive symptoms (Table 4). Women with PHQ-9 scores ≥ 10 had significantly lower WEM-WBS scores (39.81 ± 9.59) compared to those with PHQ-9 scores < 10 (57.52 ± 10.80) (t = -18.544, p < 0.001). Women with PHQ-9 scores ≥ 10 had significantly lower couple interaction scores (40.50 ± 15.04) compared to those with PHQ-9 scores < 10 (59.90 ± 13.24) ((t = -14.708, p < 0.001). Women with PHQ-9 scores ≥ 10 had significantly lower couple relationship scores (22.28 ± 7.84) compared to those with PHQ-9 scores < 10 (30.79 ± 6.60) ((t = -11.243, p < 0.001). Women with PHQ-9 scores ≥ 10 had significantly higher couple conflict scores (14.63 ± 6.39) compared to those with PHQ-9 scores < 10 (9.00 ± 5.27) ((t = 9.162, p < 0.001). Women with PHQ-9 scores ≥ 10 had significantly lower marriage happiness scores (48.15 ± 24.44) compared to those with PHQ-9 scores < 10 (81.70 ± 21.91) ((t = -14.153, p < 0.001)(Table 3). Specifically, women experiencing depressive symptoms exhibited poorer positive psychological qualities, lower levels of marital happiness, and deteriorated relationships characterized by reduced interaction and increased conflict (Figure 1). Furthermore,Women with severe PHQ-9 scores had the lowest WEM-WBS scores (37.38 ± 10.68), while those with no PHQ-9 symptoms had the highest (59.61 ± 10.14) (F =189.68, p < 0.001).Women with severe PHQ-9 scores had the lowest couple relationship scores (22.17 ± 8.79), while those with no PHQ-9 symptoms had the highest (31.65 ± 6.29) (F = 67.03,p < 0.001). Women with severe PHQ-9 scores had the highest couple conflict scores (15.23 ± 7.25), while those with no PHQ-9 symptoms had the lowest (8.21 ± 4.75) (F = 56.03, p < 0.001).Women with severe PHQ-9 scores had the lowest marriage perception scores (45.96 ± 28.28), while those with no PHQ-9 symptoms had the highest (85.34 ± 20.31) (F =110.88, p < 0.001) (Table 4).

Figure 1. Associations between the PHQ-9 score, WEM-WBS, and MPS. PPD, Postpartum depression; ACOG, American College of Obstetricians and Gynecologists; PHQ-9, Patient Health Questionnaire-9; EPDS, Edinburgh Postpartum Depression Scale; WEM-WBS, Warwick-Edinburgh mental health scale; MPS, Marriage perception scale.
3.4 Mediation analysis of the WEM-WBS score in the MPS score on the depression level
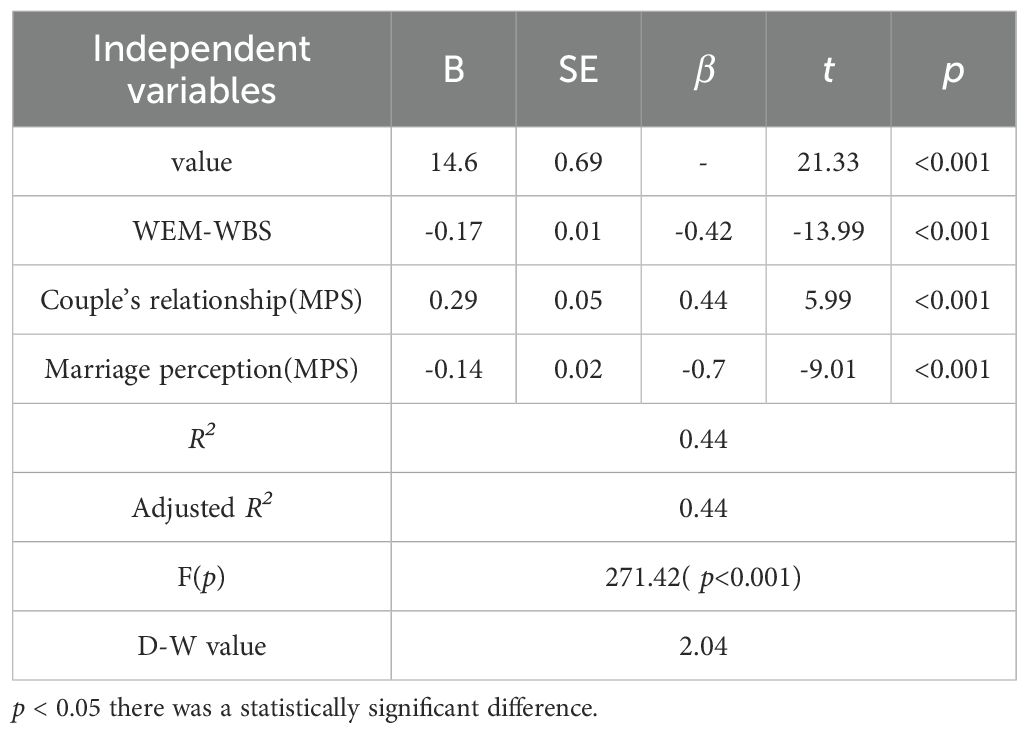
Table 5 presents the results of a stepwise regression analysis examining the predictors of PHQ-9 scores After automatic recognition by the model, three items were included in the final model: the total WEM-WBS score; the marital relationship on the MPS; and the marital happiness score on the MPS. The R2 was 0.44, indicating that these variables explained 44% of variation in PHQ-9 scores(p< 0.001). WEM-WBS scores were negatively associated with PHQ-9 scores (β = -0.42, p < 0.001). Couple’s relationship scores were positively associated with PHQ-9 scores (β = 0.44, p < 0.001).Marriage perception scores were negatively associated with PHQ-9 scores (β = -0.70, p < 0.001).D-W was 2.0.4 generally considered as no significant autocorrelation (Table 5).
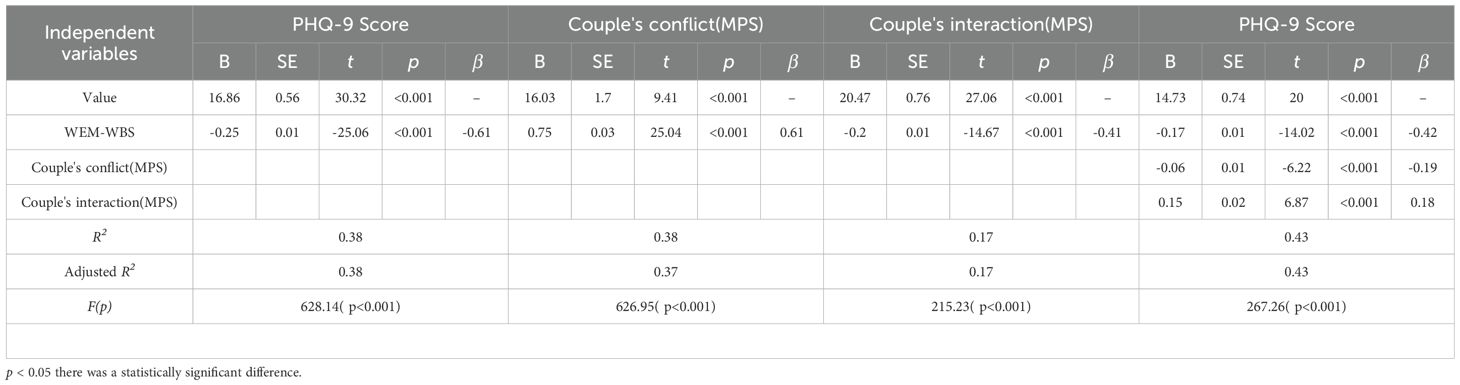
Table 6 presents the mediation analysis results for the relationship between PHQ-9 scores and couple’s conflict (MPS) and interaction (MPS). WEM-WBS scores were negatively associated with PHQ-9 scores (β = -0.61, p < 0.001) and positively associated with couple’s interaction (β = 0.61, p < 0.001). Couple’s conflict was negatively associated with PHQ-9 scores (β = -0.19, p < 0.001). Couple’s interaction was positively associated with PHQ-9 scores (β = 0.18, p < 0.001). The models explained 38% to 43% of the variance in the outcomes (Adjusted R² = 0.37 to 0.43, p < 0.001).
The mediating effects ratios of the MPS couple interaction and MPS couple conflict were 12.12% and 18.73%, respectively.
4 Discussion
In the current study the positive rate of depressive symptoms (PHQ-9 ≥ 10) in parturients was 11.16%. Wang Zy et al.’s 2021 systematic review of global studies revealed that meta-regression analysis indicated a pooled prevalence of PDD at 17.22% (45). This review encompassed 565 articles(prevalence of postpartum depression in the range of 3.5%–63.3%) published before July 2021, thoroughly examining regional and national prevalence differences, variations in assessment tools, and significant risk factors. The latest research findings exhibit significant variability depending on the geographical location and timing of the investigations.A study in Iran showed that the prevalence of PPD was 22.4% and 3.2%. at 3 days and at 6 month after delivery, respectively (7). Amer SA et al.’s multinational study, conducted across six countries - Egypt, Yemen, Iraq, India, Ghana, and Syria - revealed an overall postpartum depression (PPD) prevalence of 13.6%. The lowest prevalence was observed in Syria at 2.3%, while the highest was reported in Ghana at 26% (8). Xiao et al. and Xiaojuan et al. reported positive rates for depressive symptoms of 8.90% (46) and 7.69% in China, respectively (47), which were lower than our findings. A study conducted in Japan reported that the prevalence rates of postpartum depression (PPD) among mothers were 6.5% at 3 days, 7.5% at 3 months, 7.5% at 6 months, and 8.4% at 1 year postpartum, all of which were lower than the corresponding rates observed in our study (48). The distinction lies in the use of EPDS as opposed to our the PHQ-9. A retrospective study published after July 2021, involving 4,619 participants in the United States, revealed that 10.7% of postpartum women scored above 10 on the PHQ-9, indicating symptoms of postpartum depression. This investigation focused on women who had given birth in obstetric hospitals (49). Most studies primarily focus on the prevalence of symptoms measured by standardized scales rather than the prevalence of the clinical diagnosis.Furthermore, the depression diagnosis rate (approximately 9.0%) using the Structured Clinical Interview for DSM Disorders (SCID) was closely aligned with the EPDS at a score ≥10 (50, 51). As a result, measurements using self-report questionnaires will be higher than actual DSM diagnoses.
There are numerous factors that influence symptoms of PPD, including sociodemographic, physical and biological, cultural, and obstetric and pediatric factors (52). In the current study we used a comprehensive survey based on demographic,health-relatedObstetric, current pregnancy, and infant-related, Psycho and social characteristics. The study results revealed that significant variations were noted in the prevalence of depressive symptoms among women with respect to education level, co-habitation, alcohol consumption, exercise routine, number of pregnancies, fetal gender preference, and gender expectations.
The study findings indicated that individuals with lower educational attainment exhibit higher rates of PPD positivity. Few studies have determined the risk of education on PPD symptoms (53–55). However, other studies have shown that PPD symptoms are not related to education level (19, 56, 57).
A study indicated that unplanned pregnancy and first-time motherhood are risk factors for PPD (19). Our findings indicated that unplanned pregnancies exhibit a higher prevalence and associated score. However, this observation does not apply to first-time mothers.
Co-habitation status was shown to be a significant risk factor for PPD symptoms. The findings indicated that couples residing independently exhibited the lowest depressive symptom prevalence (9.87%). Several studies have specifically highlighted living with in-laws as a notable risk factor for PPD symptoms (31, 58). Co-habitants can influence PPD symptoms, which has often been the focus of research. Our study focused solely on the identities of the co-habitants and did not inquire into the depth of the relationships between the study participants and co-habitants. In this study we did not distinguish between parents and parents-in-law.
There was no significant association between newborn gender and the score or prevalence of depressive symptoms in parturients. Rihua et al. reported a higher prevalence of depressive symptoms among women in China who gave birth to girls (12.2%) compared to women who gave birth to boys (24.6%) (27). However, no significant difference was detected in postpartum rates between boys and girls. Interestingly, the presence of twins versus having a singleton did have a notable distinction, suggesting that the stress associated with childrearing may be influenced by the number of children. Studies have demonstrated that parturients who give birth to male infants face an increased susceptibility to depression, with the likelihood of experiencing PPD symptoms increasing by 105% in ACCESS and 424% in PRISM (59). Furthermore, mothers of boys exhibit a higher prevalence of depressive symptoms, which is possibly attributed to pro-inflammatory cytokines (60), reproductive hormone fluctuations (61), and maternal immune activation (62). Our research focuses more on social and cultural factors. This viewpoint will be discussed in detail below. We focused on fetal gender, maternal and familial expectations of fetal gender, as well as various scenarios in which there is consistency between fetal gender and expectations. The findings revealed that the level and prevalence of depressive symptoms in parturients were influenced by disparities between expected and actual fetal gender, as well as differences between expected fetal gender and the expectations held by immediate family members. Given the cultural significance attached to fetal gender in China, coupled with declining birth rates resulting from family planning policies favoring single-child households, it is plausible that gender expectations are linked to depressive symptoms. The current study demonstrated a significant disparity in depressive symptom scores between individuals who had specific gender preferences for their unborn child versus individuals without such preferences (independent of the desired gender). Interestingly, the newborn actual gender did not appear to be directly associated with maternal depressive symptoms. However, incongruence between anticipated and actual newborn gender significantly impacted maternal mental health outcomes. Furthermore, expectant mothers desiring a male offspring exhibited higher levels of depressive symptoms compared to mothers without specific gender preferences. Conversely, individuals who did not have a preference regarding newborn gender displayed lower levels of depressive symptoms. Therefore, we failed to identify any significant correlations among the aforementioned factors. Fetal gender satisfaction is a risk factor for PPD (63). A study conducted in Shenzhen, China also demonstrated that fetal gender and gender expectations have an impact on PPD symptoms (19).
We concur that the impact of factors, such as living arrangements, relationships with in-laws, fetal gender, and gender expectations on PPD symptoms, among women is closely tied to Chinese cultural norms, including the preference for male offspring, the traditional concept of filial piety in which in-laws are often regarded as having greater childrearing experience and thus deserve deference, and the potential for intergenerational conflicts between couples and their parents or in-laws (19). Cigarette smoking (64) and alcohol consumption (8) are recognized as risk factors for the development of PPD. Our findings indicated a significant association between PPD and regular alcohol consumption; no correlation was detected with cigarette smoking. This discrepancy may be attributed to the participants’ awareness of the detrimental effects of cigarette smoking on fetal health, leading to intentional reduction or cessation of smoking and rendering the impact statistically insignificant.
Prenatal and postnatal exercise should serve as a protective factor against PPD. Our findings indicated that exercise habits, including sporadic physical activity, are positively associated with a reduction in the symptoms and prevalence of PPD. These results align with most existing research in this field. Exercise has beneficial effects on the improvement of maternal depression and postpartum interventions may be more effective than antepartum interventions (65). Randomized controlled trials have shown that compared to no exercise, exercise-only interventions reduce the severity of PPD symptoms (66). Higher levels of physical activity during pregnancy are consistently associated with improved postpartum mental health outcomes, including reduced depressive symptoms, lower anxiety, and enhanced overall well-being (67).
We used to questions (“Know postpartum depression?” and “Know where to seek help for PPD?”) to determine the correlation between literacy and PPD symptoms. The research findings indicated that individuals with a higher awareness of PPD, as well as an understanding of the need for help and access to resources, exhibit lower depressive symptom scores and a reduced prevalence of depressive symptoms. Several studies have demonstrated that the level of PPD literacy among women in China is relatively low and it is imperative to implement measures to enhance the literacy of parturients (68, 69). Mental health literacy is positively associated with attitudes toward professional psychological help-seeking (70).
The current study investigated the impact of positive mental health and marital well-being on PPD in women by analyzing the correlations and significant differences in positivity and severity of depression. The findings indicated that the depressive symptom score was positively associated with mental well-being. Mental well-being is closely related to mental health symptoms. The Short Warwick-Edinburgh Mental Well-being Scale (SWEMWBS) could be used as an alternative to the PHQ-9 in monitoring and evaluating CMD treatment (71). There is evidence that poor mental well-being is associated with negative feelings, such as psychological stress, low self-esteem, loneliness, and depression (72). Positive psychology (PP) interventions are effective in reducing the level of depression and increasing mental well-being (73). A study conducted during the COVID-19 pandemic showed that deterioration in mental well-being makes the population vulnerable to several psychiatric conditions (74). There has been a paucity of in-depth research examining the relationship between mental well-being and depression, especially among women with PPD, and includes an exploration of potential causal relationships between mental health and PPD symptoms, as well as the role of mediating factors.
Ultimately, the MPS, which was developed for Chinese populations, was used to investigate the impact of marital happiness on PPD symptoms. The findings indicated a positive correlation between PPD symptoms and the overall score of marital happiness, as well as the subscales of marital interaction and marital relationship. Conversely, there was a negative correlation with marital conflict. Significant differences in the prevalence and severity of depressive symptoms were observed across various dimensions of marital happiness and its subscales.
A poor marital relationship, poor living conditions, and a lack of social support (63). Social support from the husband and family is significantly associated with PPD and this relationship may be modulated by fetus gender and the family’s expectations regarding fetal gender (63). A strained marital relationship increases the risk of PPD (75). The mother’s level of family cohesion, marital satisfaction, and parent-child relationship is closely associated with that of her partner. At 6 weeks postpartum, mothers who exhibit higher levels of family cohesion and lower levels of depressive symptoms demonstrate less impaired mother-child relationships (76).Marital satisfaction plays a crucial role in the development of perinatal depression both directly and indirectly through the mechanisms of family support and emotional connection. The greater the family support received by a gravida and parturient, the lower the EPDS score (77). The most significant factors contributing to elevated postpartum EPDS scores were higher prenatal EPDS scores, followed by inadequate communication between partners during pregnancy (the wife feeling unappreciated by her husband), and a lack of support from the husband postpartum (78). Despite the differences in the tools utilized in our study compared to other studies, marital quality, encompassing relationship dynamics, family support, and effective communication between partners, serves as protective factors against PPD. Conversely, poor marital quality, inadequate family support, and suboptimal interactions are identified as risk factors for PPD.
We performed an analysis to examine the impact of co-habitation arrangements on marital quality and the role as a covariate in PPD symptoms. The findings indicated that co-habitation arrangements significantly influenced marital happiness, marital conflict, marital interaction, and family relationships but did not have a combined effect on PPD symptoms. It is noteworthy that the study stands as the sole investigation to have examined the impact of mental well-being on PPD symptoms via the mediation of marital quality. However, delving deeper into the mechanisms underlying this influence or exploring additional relationships presents considerable challenges.
5 Limitations
This study was conducted in northeast China and provides highly valuable insight into the risk factors associated with PPD. Areas, such as literacy, mental well-being, and marital quality, are currently under-researched and further exploration of these related factors is constrained. However, This study did not choose to calculate the sample size, the convenience sampling method used in this study also limited the extrapolation of the results. In this study, we utilized the PHQ-9 questionnaire, although the majority of international studies predominantly employ the Edinburgh Postnatal Depression Scale (EPDS). Despite this difference, the PHQ-9 and EPDS have been shown to exhibit high consistency in their assessments. Self-report measurements can indicate the prevalence of depression symptoms but do not necessarily reflect the prevalence of a major depression disorder. It is widely acknowledged that PPD may be associated with various biological, genetic, hormonal, and other mechanistic changes. However, as a cross-sectional study, we were unable to comprehensively analyze the underlying mechanisms of these factors.
6 Conclusion
The findings of this study indicate a higher prevalence of depressive symptoms among postpartum women, influenced by multiple factors, particularly those associated with positive mental health and subjective marital satisfaction. However, the primary objective of postpartum depression screening is not merely to determine the prevalence of symptoms but to effectively reduce both the incidence of postpartum depression and its impact on affected women. From a tertiary prevention perspective, we recommend enhancing the dissemination of perinatal mental health knowledge to improve mental health literacy and awareness among perinatal women, with targeted health education addressing identified risk factors. Secondly, based on screening outcomes, it is crucial to provide tailored interventions for varying levels of symptom severity, including psychological counseling and psychotherapy. Women with more severe symptoms should be referred to psychiatry for comprehensive evaluation and treatment, which may involve pharmacotherapy, physical therapy, or cognitive-behavioral therapy (CBT). Thirdly, our study revealed that many symptomatic women are reluctant to accept recommended follow-up interventions. Investigating the barriers to help-seeking behavior is essential to identify and address these obstacles. Fourthly, research indicates that positive psychology and marital satisfaction are associated with reduced depressive symptoms. Therefore, promoting positive mental health and family well-being could serve as an effective preventive measure and warrants further investigation. Finally, from a broader healthcare system perspective, there is currently a lack of mental health services in obstetrics and gynecology departments in China. Efforts are underway to establish more psychological clinics within these departments and enhance mental health services. With ongoing policy support, we anticipate significant improvements in the tertiary prevention of perinatal mental health.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving humans were approved by The approval for this study was acquired from the Medical Ethics Committee of Liaoning Mental Health center, an Hospital, Liaoning, China (Approval number: LNMHC2023-TT0001). All participants provided informed consent before enrollment in the present study. All methods were performed in according with relevant guidelines and regulations(declarations of Helsinki). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
WJin: Investigation, Project administration, Writing – review & editing. YJ: Investigation, Project administration, Writing – review & editing, Data curation. SY: Data curation, Investigation, Writing – review & editing. WD: Data curation, Investigation, Software, Writing – original draft. GZ: Investigation, Formal Analysis, Methodology, Writing – review & editing. WJia: Investigation, Supervision, Validation, Writing – review & editing. WY: Supervision, Conceptualization, Project administration, Writing – review & editing. RJ: Conceptualization, Project administration, Formal Analysis, Investigation, Methodology, Resources, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the National Natural Science Foundation of China, No. 32000677,the Young Scholars Program of China Medical University (QGZ-2018094), and Liaoning Provincial Department of Education Basic Research Project for General Programs (Humanities and Social Sciences), JYTMS20230146.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Moya J, Phillips L, Sanford J, Wooton M, Gregg A, and Schuda L. A review of physiological and behavioral changes during pregnancy and lactation: Potential exposure factors and data gaps. J Exposure Sci Environ Epidemiol. (2014) 24:449–58. doi: 10.1038/jes.2013.92
2. Thapa SB, Mainali A, Schwank SE, and Acharya G. Maternal mental health in the time of the COVID-19 pandemic. Acta Obstetricia Gynecologica Scandinavica. (2020) 99:817–8. doi: 10.1111/aogs.13894
3. Scrandis DA and Scrandis KS. Perinatal depression and anxiety. Nurse practitioner. (2024) 49:29–33. doi: 10.1097/01.NPR.0000000000000188
5. Falah-Hassani K, Shiri R, and Dennis CL. The prevalence of antenatal and postnatal co-morbid anxiety and depression: a meta-analysis. psychol Med. (2017) 47:2041–53. doi: 10.1017/S0033291717000617
6. Wildali D, Nazzal S, Hamshari S, and Belkebir S. Prevalence and risk factors of postpartum depression among women attending primary healthcare centers in northern of West Bank/ Palestine: a cross-sectional study, 2022. BMC Womens Health. (2024) 24:43. doi: 10.1186/s12905-024-02887-6
7. Zakeri MA, Khoram S, Bazmandegan G, Ghaedi-Heidari F, Talebi B, Ramezani N, et al. Postpartum depression and its correlates: a cross-sectional study in southeast Iran. BMC Womens Health. (2022) 22:387. doi: 10.1186/s12905-022-01978-6
8. Amer SA, Zaitoun NA, Abdelsalam HA, Abbas A, Ramadan MS, Ayal HM, et al. Exploring predictors and prevalence of postpartum depression among mothers: Multinational study. BMC Public Health. (2024) 24:1308. doi: 10.1186/s12889-024-18502-0
9. Grigoriadis S, VonderPorten EH, Mamisashvili L, Tomlinson G, Dennis CL, Koren G, et al. The impact of maternal depression during pregnancy on perinatal outcomes: a systematic review and meta-analysis. J Clin Psychiatry. (2013) 74:e321–41. doi: 10.4088/JCP.12r07968
10. Dennis CL and Chung-Lee L. Postpartum depression help-seeking barriers and maternal treatment preferences: a qualitative systematic review. Birth. (2006) 33:323–31. doi: 10.1111/j.1523-536X.2006.00130.x
11. Screening and diagnosis of mental health conditions during pregnancy and postpartum: ACOG clinical practice guideline no. 4. Obstetrics gynecology. (2023) 141:1232–61. doi: 10.1097/AOG.0000000000005200
12. Department of Obstetrics and Gynecology, Chinese Medical Association. Expert consensus on screening and diagnosis of perinatal depression J. Chin J Obstetrics Gynecology. (2021) 56:521–27. doi: 10.3760/cma.j.cn112141-20210115-00022
13. Hawkins SS. Screening and the new treatment for postpartum depression. JOGNN. (2023) 52:429–41. doi: 10.1016/j.jogn.2023.09.007
14. Moore Simas TA, Whelan A, and Byatt N. Postpartum depression-new screening recommendations and treatments. JAMA. (2023) 330:2295–6. doi: 10.1001/jama.2023.21311
15. Qin X, Liu C, Zhu W, Chen Y, and Wang Y. Preventing postpartum depression in the early postpartum period using an app-based cognitive behavioral therapy program: A pilot randomized controlled study. Int J Environ Res Public Health. (2022) 19:16824. doi: 10.3390/ijerph192416824
16. Zhao XH and Zhang ZH. Risk factors for postpartum depression: an evidence-based systematic review of systematic reviews and meta-analyses. Asian J Psychiatr. (2020) 53:102353. doi: 10.1016/j.ajp.2020.102353
17. O’Hara M and McCabe J. Postpartum depression: Current status and future directions. Annu Rev Clin Psychol. (2013) 9:379–407. doi: 10.1146/annurev-clinpsy-050212-185612
18. Radwan H, Fakhry R, Hanach N, Obaid RS, Moez Al Islam EF, Al Marzooqi S, et al. Maternal mental health and infant feeding practices cohort protocol: Methodology and baseline characteristics. Hamdan Med J. (2020) 13:141. doi: 10.4103/HMJ.HMJ_13_20
19. Xiong R, Deng A, Wan B, and Liu Y. Prevalence and factors associated with postpartum depression in women from single-child families. Int J gynaecology obstetrics: Off Organ Int Fed Gynaecology Obstetrics. (2018) 141:194–9. doi: 10.1002/ijgo.12461
20. Banker E and LaCoursiere DY. Postpartum depression: risks, protective factors, and the couple's relationship. Issues Ment Health Nurs. (2014) 35:503–08. doi: 10.3109/01612840.2014.888603
21. Shi P, Ren H, Li H, and Dai Q. Maternal depression and suicide at immediate prenatal and early postpartum periods and psychosocial risk factors. Psychiatry Res. (2018) 261:298–306. doi: 10.1016/j.psychres.2017.12.085
22. Lewis BA, Billing L, Schuver K, Gjerdingen D, Avery M, and Marcus BH. The relationship between employment status and depression symptomatology among women at risk for postpartum depression. Women's Health (London England). (2017) 13:3–9. doi: 10.1007/s00127-022-02372-1
23. Guvenc G, Yesilcinar I, Ozkececi F, Öksuz E, Ozkececi CF, Konukbay D, et al. Anxiety, depression, and knowledge level in postpartum women during the COVID-19 pandemic. Psychiatr Care. (2020) 57:1449–58. doi: 10.1111/ppc.12711
24. Hu N, Luo J, Xiang W, Yang G, Huang T, Guan L, et al. The relationship between postpartum negative life events and postpartum depression: a moderated mediation model of neuroticism and psychological flexibility. BMC Psychiatry. (2024) 24:147. doi: 10.1186/s12888-024-05594-6
25. Nakamura A, van der Waerden J, Melchior M, Bolze C, El-Khoury F, and Pryor L. Physical activity during pregnancy and postpartum depression: Systematic review and meta-analysis. J Affect Disord. (2019) 246:29–41. doi: 10.1016/j.jad.2018.12.009
26. Vargas-Terrones M, Barakat R, Santacruz B, Fernandez-Buhigas I, and Mottola MF. Physical exercise programme during pregnancy decreases perinatal depression risk: a randomised controlled trial. Br J sports Med. (2019) 53:348–53. doi: 10.1136/bjsports-2017-098926
27. Xie RH, He G, Liu A, Bradwejn J, Walker M, and Wen SW. Fetal gender and postpartum depression in a cohort of Chinese women. Soc Sci Med (1982). (2007) 65:680–84. doi: 10.1016/j.socscimed.2007.04.003
28. Xie RH, He G, Koszycki D, Walker M, and Wen SW. Fetal sex social support and postpartum depression. Can J Psychiatry Rev Can Psychiatr. (2009) 54:750–6. doi: 10.1177/070674370905401105
29. Heh SS, Coombes L, and Bartlett H. The association between depressive symptoms and social support in Taiwanese women during the month. Int J Nurs Stud. (2004) 41:573–9. doi: 10.1016/j.ijnurstu.2004.01.003
30. Lee DT, Yip AS, Leung TY, and Chung TK. Ethnoepidemiology of postnatal depression. Prospective multivariate study of sociocultural risk factors in a Chinese population in Hong Kong. Br J Psychiatry. (2004) 184:34–40. doi: 10.1192/bjp.184.1.34
31. Peng K, Zhou L, Liu X, Ouyang M, Gong J, Wang Y, et al. Who is the main caregiver of the mother during the doing-the-month: is there an association with postpartum depression? BMC Psychiatry. (2021) 21:270. doi: 10.1186/s12888-021-03203-4
32. Radzi WM and Jenatabadi HS. Samsudin N. Postpartum depression symptoms in survey-based research: A structural equation analysis. BMC Public Health. (2021) 21:27. doi: 10.1186/s12889-020-09999-2
33. Misri S, Kostaras X, Fox D, and Kostaras D. The impact of partner support in the treatment of postpartum depression. Can J Psychiatry Rev Can Psychiatr. (2000) 45:554–8. doi: 10.1177/070674370004500607
34. Faisal-Cury A, Menezes PR, d'Oliveira AF, Schraiber LB, and Lopes CS. Temporal relationship between intimate partner violence and postpartum depression in a sample of low income women. Matern Child Health J. (2013) 17:1297–303. doi: 10.1007/s10995-012-1127-3
35. Chen R, Zhang W, and Wu X. Mental health policy and implementation from 2009 to 2020 in China. SSM- Ment Health. (2023) 4:100244. doi: 10.1016/S0140-6736(22)01540-9
36. Kroenke K, Spitzer RL, and Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Internal Med. (2001) 16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x
37. Ren Y, Yang H, Browning C, Thomas S, and Liu M. Performance of screening tools in detecting major depressive disorder among patients with coronary heart disease: a systematic review. Med Sci Monit. (2015) 21:646–53. doi: 10.12659/MSM.892537
38. Park SH and Kim JI. Predictive validity of the Edinburgh postnatal depression scale and other tools for screening depression in pregnant and postpartum women: a systematic review and meta-analysis. Arch Gynecol Obstet. (2023) 307:1331 –1345. doi: 10.1007/s00404-022-06525-0
39. Kurtz S, Levine J, and Safyer M. Ask the question: screening for postpartum mood and anxiety disorders in pediatric primary care. Curr Probl Pediatr Adolesc Health Care. (2017) 47:241–53. doi: 10.1016/j.cppeds.2017.08.002
40. Wang W, Bian Q, Zhao Y, Li X, Wang W, Du J, et al. Reliability and validity of the Chinese version of the Patient Health Questionnaire (PHQ-9) in the general population. Gen Hosp Psychiatry. (2014) 36:539–44. doi: 10.1016/j.genhosppsych.2014.05.021
41. Sun Y, Fu Z, Bo Q, Mao Z, Ma X, and Wang C. The reliability and validity of PHQ-9 in patients with major depressive disorder in psychiatric hospital. BMC Psychiatry. (2020) 20:474. doi: 10.1186/s12888-020-02885-6
42. Tennant R, Hiller L, Fishwick R, Platt S, Joseph S, Weich S, et al. The Warwick-Edinburgh Mental Well-being Scale (WEMWBS): development and UK validation. Health Qual Life Outcomes. (2007) 5:63. doi: 10.1186/1477-7525-5-63
43. Dong A, Chen X, Zhu L, Shi L, Cai Y, Shi B, et al. Warwick–edinburgh mental well-being scale–chinese version (C-WEMWBS) database record. APA PsycTests. doi: 10.1037/t63796-000
44. Yuzhong W, Zhongjie W, Lizhai J, Jiangtao W, and Li R. The development of the marriage perception scale(MPS)J. China J Health Psychol. (2009) 1:112–11.
45. Wang Z, Liu J, Shuai H, Cai Z, Fu X, Liu Y, et al. Correction: Mapping global prevalence of depression among postpartum women. Transl Psychiatry. (2021) 11:640. doi: 10.1038/s41398-021-01692-1
46. Xiao Y, Cui Y, Li F, Zeng W, Rozelle S, Shi C, et al. The mediating effect of family support in the relationship between socio-economic status and postpartum depressive symptoms. BMC Public Health. (2024) 24:3374. doi: 10.1186/s12889-024-20849-3
47. Su X, Zhang Y, Chen M, Wang H, and Liu G. Influencing factors and risk prediction modeling of maternal postpartum depression: a cross-sectional study in Chinese puerperal women of sitting the month. Front Psychiatry. (2023) 14:1252789. doi: 10.3389/fpsyt.2023.1252789
48. Yamakawa Y, Maruta M, Higuchi Y, Tokunaga A, Iwanaga R, Honda S, et al. Factors influencing postpartum depression among Japanese parents: A prospective longitudinal study. Neuropsychopharmacol Rep. (2023) 43:213–21. doi: 10.1002/npr2.12326
49. Sidebottom AC, Vacquier M, LaRusso E, Schulte AK, and Nickel A. Prenatal and postpartum depression diagnosis in a large health system: prevalence and disparities. Ann Med. (2023) 55:2281507. doi: 10.1080/07853890.2023.2281507
50. Lyubenova A, Neupane D, Levis B, Wu Y, Sun Y, He C, et al. Depression prevalence based on the Edinburgh Postnatal Depression Scale compared to Structured Clinical Interview for DSM DIsorders classification: Systematic review and individual participant data meta-analysis. Int J Methods Psychiatr Res. (2021) 30:e1860. doi: 10.1002/mpr.1860
51. Levis B, McMillan D, Sun Y, He C, Rice DB, Krishnan A, et al. Comparison of major depression diagnostic classification probability using the SCID CIDI and MINI diagnostic interviews among women in pregnancy or postpartum: An individual participant data meta-analysis. Int J Methods Psychiatr Res. (2019) 28:e1803. doi: 10.1002/mpr.1803
52. Norhayati MN, Hazlina NH, Asrenee AR, and Emilin WM. Magnitude and risk factors for postpartum symptoms: a literature review. J Affect Disord. (2015) 175:34–52. doi: 10.1016/j.jad.2014.12.041
53. Alshikh Ahmad H, Alkhatib A, and Luo J. Prevalence and risk factors of postpartum depression in the Middle East: a systematic review and meta-analysis. BMC pregnancy childbirth. (2021) 21:542. doi: 10.1186/s12884-021-04016-9
54. Chien LY, Tai CJ, Ko YL, Huang CH, and Sheu SJ. Adherence to ‘doing-the month’ practices is associated with fewer physical and depressive symptoms among postpartum women in Taiwan. Res Nurs Health. (2006) 29:374–83. doi: 10.1002/nur.20154
55. Moya E, Mzembe G, Mwambinga M, Truwah Z, Harding R, Ataide R, et al. Prevalence of early postpartum depression and associated risk factors among selected women in southern Malawi: a nested observational study. BMC pregnancy childbirth. (2023) 23:229. doi: 10.1186/s12884-023-05501-z
56. He J, Li Y, Chen L, and Zhang Y. Corrigendum: Non-biological factors associated with postpartum depression among women in Shenzhen: a case-control study. Front Public Health. (2024) 12:1500641. doi: 10.3389/fpubh.2024.1500641
57. Liang P, Wang Y, Shi S, Liu Y, and Xiong R. Prevalence and factors associated with postpartum depression during the COVID-19 pandemic among women in Guangzhou, China: a cross-sectional study. BMC Psychiatry. (2020) 20:557. doi: 10.1186/s12888-020-02969-3
58. Wang YY, Li H, Wang YJ, Wang H, Zhang YR, Gong L, et al. Living with parents or with parents-in-law and postpartum depression: a preliminary investigation in China. J Affect Disord. (2017) 218:335–8. doi: 10.1016/j.jad.2017.04.052
59. Cowell W, Colicino E, Askowitz T, Nentin F, and Wright RJ. Fetal sex and maternal postpartum depressive symptoms: findings from two prospective pregnancy cohorts. Biol sex Dif. (2021) 12:6. doi: 10.1186/s13293-020-00348-x
60. Enninga EA, Nevala WK, Creedon DJ, Markovic SN, and Holtan SG. Fetal sex based differences in maternal hormones, angiogenic factors, and immune mediators during pregnancy and the postpartum period. Am J Reprod Immunol. (2015) 73:251–62. doi: 10.1111/aji.12303
61. Schiller CE, Meltzer-Brody S, and Rubinow DR. The role of reproductive hormones in postpartum depression. CNS Spectr. (2015) 20:48–59. doi: 10.1017/S1092852914000480
62. Yonkers KA, Vigod S, and Ross LE. Diagnosis, pathophysiology, and management of mood disorders in pregnant and postpartum women. Obstet Gynecol. (2011) 117:961–77. doi: 10.1097/AOG.0b013e31821187a7
63. Qi W, Zhao F, Liu Y, Li Q, and Hu J. Psychosocial risk factors for postpartum depression in Chinese women: a meta-analysis. BMC pregnancy childbirth. (2021) 21:174. doi: 10.1186/s12884-021-03657-0
64. Leung BM, Letourneau NL, Giesbrecht GF, Ntanda H, Hart M, and APrON Team. Predictors of postpartum depression in partnered mothers and fathers from a longitudinal cohort. Community Ment Health J. (2017) 53:420–31. doi: 10.1007/s10597-016-0060-0
65. Yu H, Mu Q, Lv X, Chen S, and He H. Effects of an exercise intervention on maternal depression, anxiety, and fatigue: a systematic review and meta-analysis. Front Psychol. (2024) 15:1473710. doi: 10.3389/fpsyg.2024.1473710
66. Deprato A, Ruchat SM, Ali MU, Cai C, Forte M, Gierc M, et al. Impact of postpartum physical activity on maternal depression and anxiety: a systematic review and meta-analysis. Br J Sports Med. (2024), 59(8), 550–561 108478. doi: 10.1136/bjsports-2024-108478
67. Hicks LE, Graf MD, and Yeo S. Prenatal exercise and its effects on postpartum mental health: systematic review and meta-analysis. Arch women's Ment Health. (2024). doi: 10.1007/s00737-024-01525-2
68. Huang W, Li G, Wang D, Qu H, Tian M, and Wang Y. Postpartum depression literacy in Chinese perinatal women: a cross-sectional study. Front Psychiatry. (2023) 14:1117332. doi: 10.3389/fpsyt.2023.1117332
69. Li K, Lu J, Pang Y, Zheng X, Liu R, Ren M, et al. Maternal postpartum depression literacy subtypes: A latent profile analysis. Heliyon. (2023) 9:e20957. doi: 10.1016/j.heliyon.2023.e20957
70. Jones A. Postpartum help-seeking: the role of stigma and mental health literacy. Maternal Child Health J. (2022) 26:1030–7. doi: 10.1007/s10995-022-03399-1
71. Shah N, Cader M, Andrews B, McCabe R, and Stewart-Brown SL. Short Warwick-Edinburgh Mental Well-being Scale (SWEMWBS): performance in a clinical sample in relation to PHQ-9 and GAD-7. Health Qual Life outcomes. (2021) 19:260. doi: 10.1186/s12955-021-01882-x
72. Kraiss JT, Klooster PM, Moskowitz JT, and Bohlmeijer ET. The relationship between emotion regulation and well-being in patients with mental disorders: A meta-analysis. Compr Psychiatry. (2020) 102:152189. doi: 10.1016/j.comppsych.2020.152189
73. Kaya R and Tanrıverdi D. The effect on mental well-being, life attitude and depression levels of positive psychology program applied to patients diagnosed with depression. Curr Psychol (New Brunswick N.J.). (2023), 1–13. doi: 10.1007/s12144-023-04244-6
74. Sarkar S, Kaur T, Ranjan P, Sahu A, and Kumari A. Tools for the evaluation of the psychological impact of COVID-19: A practical guide for Family physicians and Primary Care Practitioners. J Family Med Prim Care. (2021) 10:1503–7. doi: 10.4103/jfmpc.jfmpc_2107_20
75. Glavin K, Smith L, and Sørum. Prevalence of postpartum depression in two municipalities in Norway. Scandinavian J caring Sci. (2009) 23:705–10. doi: 10.1111/j.1471-6712.2008.00667.x
76. Ngai FW and Xie YJ. Psychosocial factors and parent-infant bonding. J perinatal neonatal Nurs. (2023) 37:303–9. doi: 10.1097/JPN.0000000000000743
77. Keles E, Bilge Y, Kumru P, Celik Z, and Cokeliler I. Association between perceived social support, marital satisfaction, differentiation of self and perinatal depression. Northern Clinics Istanbul. (2023) 10:181–8. doi: 10.14744/nci.2023.79923
Keywords: postpartum depression, mental well-being, marital quality, mental health, China
Citation: Jinhuan W, Jie Y, Ying S, Dongzhuo W, Zhongli G, Jiayue W, Yan W and Jintao R (2025) Associations among mental well-being, marital quality, and maternal depressive symptoms in China: a cross - sectional study and mediated analysis. Front. Psychiatry 16:1551588. doi: 10.3389/fpsyt.2025.1551588
Received: 26 December 2024; Accepted: 22 April 2025;
Published: 23 May 2025.
Edited by:
Xiao Yang, Sichuan University, ChinaReviewed by:
Mohammad Ali Zakeri, Rafsanjan University of Medical Sciences, IranIsidora S Vujcic, University of Belgrade, Serbia
Copyright © 2025 Jinhuan, Jie, Ying, Dongzhuo, Zhongli, Jiayue, Yan and Jintao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ren Jintao, bmV1cm9wc3kyMDEzQDEyNi5jb20=
†These authors have contributed equally to this work and share first authorship
 Wang Jinhuan1†
Wang Jinhuan1† Wang Yan
Wang Yan Ren Jintao
Ren Jintao