- 1West China School of Public Health and West China Fourth Hospital, Sichuan University, Chengdu, China
- 2Department of Neurosurgery, Affiliated Hospital of Zunyi Medical University, Zunyi, China
- 3Department of Orthopedics, West China Hospital, Sichuan University, Chengdu, China
Background: Total hip/knee arthroplasty (THA/TKA) has emerged as the gold-standard treatment for end-stage osteoarthritis. However, persistent postoperative pain and surgical trauma often induce psychological distress, complications that are frequently overlooked despite high procedural success rates. Leveraging Post-Traumatic Growth (PTG) theory, this study longitudinally investigated PTG levels. Through multidimensional analysis of predictive factors, we can provide an evidence-based framework for psychological interventions to accelerate rehabilitation.
Methods: This prospective longitudinal study included 160 patients undergoing joint replacement surgery for osteoarthritis (79 hip and 81 knee patients). In addition to collecting demographic and disease-related data, several assessments were conducted at four time points: preoperative day 1 (T1), postoperative day 1 (T2), postoperative month 1 (T3), and postoperative month 3 (T4). The instruments used included the Post-Traumatic Growth Inventory (PTGI), General Self-Efficacy Scale (GSES), Perceived Social Support Scale (PSSS), Medical Coping Modes Questionnaire (MCMQ), Harris Hip Score (HHS), and Knee Society Score (KSS). Univariate analysis and stratified regression were applied to explore the influencing factors.
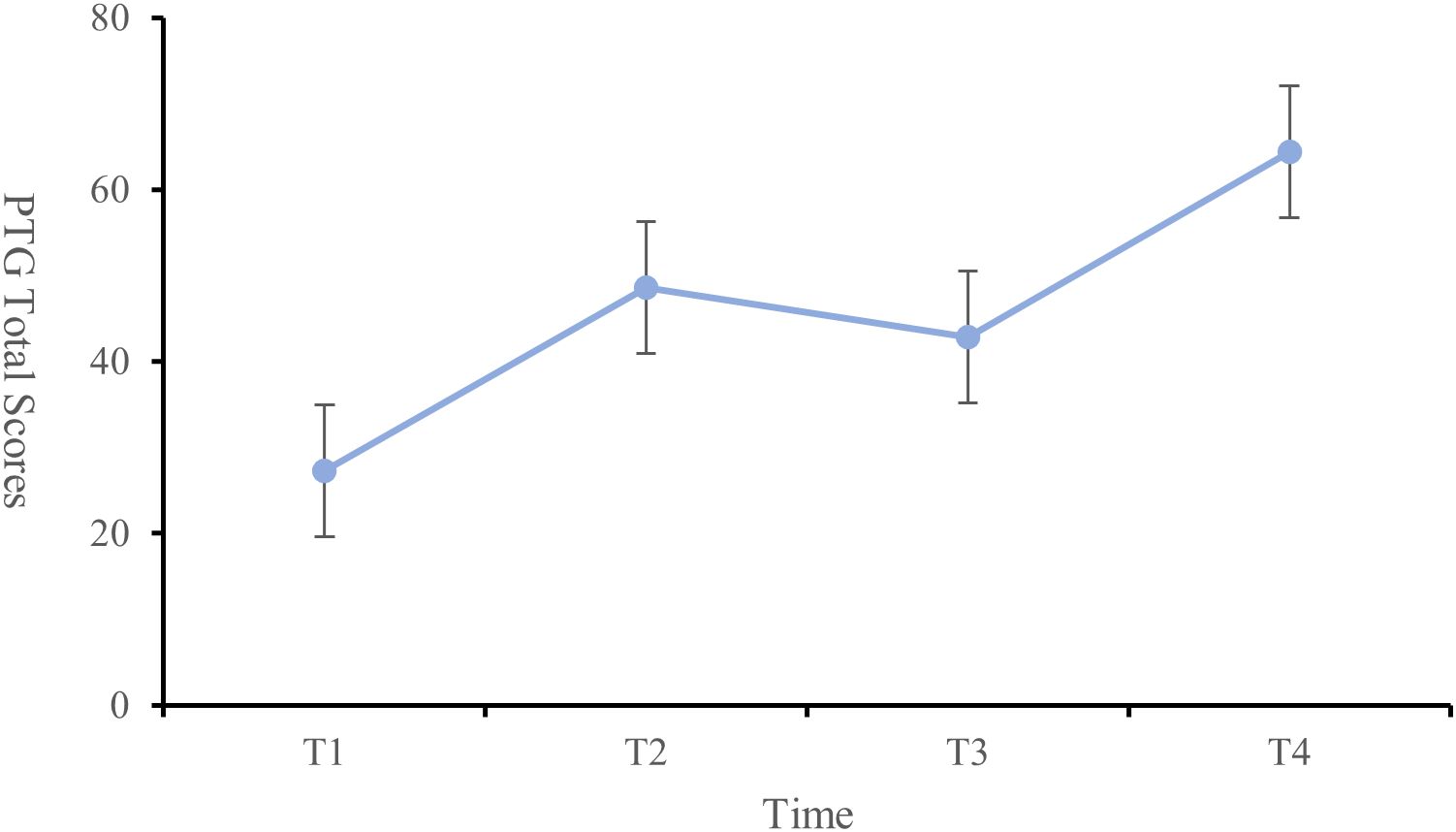
Results: PTG scores showed a statistically significant nonlinear progression characterized by: preoperative day 1 (27.23 ± 12.87), postoperative day 1 (48.61 ± 14.49), postoperative month 1 (42.87 ± 12.72), and postoperative month 3 (64.37 ± 9.42). Housing type (T1, T2, T4), arthritis location (T1), surgical site (T2), complications (T4), comorbidities (T1), and joint function (T1–T4) were significant predictors of PTG (P < 0.05). Self-efficacy was positively correlated with PTG at T1 and T2 (P < 0.05). Coping strategies, such as “facing” (T1, T4), were positively correlated with PTG, while “avoidance” (T1, T2, T4) and “yielding” (T1) showed negative correlations (P < 0.05). Family support (T2, T3) was positively correlated with PTG (P < 0.05).
Conclusions: The PTG scores in THA/TKA patients demonstrated a curvilinear upward trajectory over time, maintaining moderate to low overall levels. Notably, the 1-month postoperative period emerged as a critical window for targeted interventions. Joint function, self-efficacy, proactive coping strategies, and social support significantly enhanced PTG levels, whereas yield-avoidant coping strategies showed negative correlations with PTG scores. Healthcare providers should monitor multidimensional factors influencing PTG variations across different time points, formulating evidence-based health education programs and personalized interventions to accelerate rehabilitation processes.
Introduction
Osteoarthritis (OA) is a common chronic joint disease characterized by degenerative changes in articular cartilage and bone hypertrophy, leading to joint swelling, pain, and functional impairment. Over 500 million people worldwide are affected by OA (1–3). Total hip arthroplasty (THA) and total knee arthroplasty (TKA) have become the preferred treatment options for end-stage OA (3). However, 44.4% of patients scheduled for THA/TKA experience negative emotions prior to surgery, which are often overlooked. During postoperative rehabilitation, issues such as pain and joint maladaptation frequently arise, and more than 20% of TKA patients report dissatisfaction with surgical outcomes. The conventional biomechanical evaluation framework fails to adequately account for this gap in patient satisfaction (4–6).
The theory of post-traumatic growth (PTG) highlights a four-phase pathway—disruption of core beliefs, cognitive-emotional processing, resource regulation, and positive transformation—to explain the psychological mechanisms through which individuals experience positive changes following trauma (7, 8). PTG has been shown to be positively associated with quality of life and rehabilitation adherence in various medical stress contexts, including major surgery, cancer, accidents, and chronic illness (7, 9). In the orthopedic field, relevant studies suggest that PTG contributes to an expanded understanding of life among patients with rheumatoid osteoarthritis and offers practical guidance for promoting health (10). Furthermore, PTG levels have been positively correlated with concurrent quality of life in patients with hip fractures (11). In patients with OA, the chronic pain associated with the disease and the traumatic stress of joint replacement surgery together constitute significant stressors, meeting the PTG model’s criteria for core belief disruption through both prolonged and acute stressors (12–14). During the postoperative rehabilitation process, THA/TKA patients face persistent psychological stress due to pain, discomfort, and unmet high expectations (4, 15). Factors such as self-efficacy and social support facilitate cognitive restructuring and resource regulation (16, 17), theoretically forming a closed-loop pathway from distress and rumination to eventual growth.
For joint diseases, PTG in patients with rheumatoid arthritis enhanced their knowledge of living with the disease and provided practical health-promoting advice (10). Progressive OA leads to chronic pain, functional impairment, surgical trauma, and postoperative rehabilitation discomfort, all of which may trigger anxiety and depression across disease stages (18–20). However, systematic investigations into PTG and its determinants in THA/TKA patients remain scarce, resulting in clinical interventions predominantly focused on functional rehabilitation while lacking evidence-based strategies for psychological growth (6, 21, 22).
This study therefore employs PTG theory to identify modifiable predictors—including coping styles, self-efficacy, and social support—to establish an evidence-based framework for targeted psychological interventions, ultimately enhancing functional recovery and subjective well-being.
Methods
Study design
This study is a prospective longitudinal study conducted using a convenience sampling method. After admission, trained research team members explained the study’s purpose and procedures to the patients and invited them to participate. The study was conducted following the principles of the Declaration of Helsinki and the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE )guidelines, and it was approved by the Ethics Committee of West China Hospital, Sichuan University (Approval No. 1349). All participants signed an informed consent form prior to their involvement in the study. All patients completed a 3-month postoperative follow-up.
Participants
This study was conducted from September 2022 to November 2023 in the Department of Orthopedics at West China Hospital, Sichuan University. A total of 163 patients diagnosed with OA participated in the study, including 81 patients undergoing THA and 82 patients undergoing total knee arthroplasty TKA. The inclusion criteria were as follows: (1) age ≥ 18 years; (2) diagnosis of primary hip or knee OA; (3) undergoing primary unilateral total hip or knee arthroplasty; (4) basic literacy skills and the ability to communicate effectively. Exclusion criteria included patients with severe heart, liver, or kidney dysfunction, malignancy, or those who had experienced other significant events within the past year.
Sample size calculation
The sample size was calculated using PASS 15 software. PTG scores were used as the primary outcome variable. Based on preliminary survey results, the standard deviation was 23, and the allowable error was 4. Using a two-sided test (α = 0.05, β = 0.8), the required sample size was calculated as N = 130. Considering a 20% invalid questionnaire rate, the final required sample size was 163 participants.
Data collection
To ensure the quality of the surveys, the research instruments were assessed and reviewed by an orthopedic expert team, and the researchers were strictly trained before conducting the data collection. The research instruments included:
①Posttraumatic Growth Inventory (PTGI): The total scale had a Cronbach’s α coefficient of 0.874. For its five dimensions (Personal Strength, New Possibilities, Spiritual Change, Relating to Others, and Appreciation of Life), the Cronbach’s α coefficients ranged from 0.611 to 0.796 (23).
②Medical Coping Modes Questionnaire (MCMQ): This scale includes three dimensions—Confrontation, Avoidance, and Resignation—with Cronbach’s α coefficients of 0.64, 0.85, and 0.67, respectively (24).
③The General Self-Efficacy Scale (GSES) is a unidimensional instrument comprising 10 items, rated on a 4-point Likert scale (1–4). The total score ranges from 10 to 40, with higher scores indicating greater self-efficacy. The scale demonstrated good internal consistency, with a Cronbach’s alpha coefficient of 0.87 (25).
④Perceived Social Support Scale (PSSS): The Cronbach’s α coefficients for the overall scale and its three dimensions (Family Support, Friend Support, and Other Support) ranged from 0.813 to 0.840 (26).
⑤Harris Hip Score (HHS): This scale assesses hip joint function, including pain, function, deformity, and range of motion, with a maximum score of 100. Scores are categorized into four levels: excellent (>90), good (80–89), fair (70–79), and poor (<70) (27).
⑥Hospital for Special Surgery Knee Score (HSS): This scale evaluates knee joint function, including six aspects: pain, function, range of motion, muscle strength, flexion deformity, and stability. The total score is 100, categorized into four levels: excellent (>85), good (70–84), fair (60–69), and poor (≤59) (28).
The PTGI, MCMQ, GSES, and PSSS were used to assess PTG (higher scores indicate higher PTG levels), coping strategies (higher scores indicate a stronger tendency toward specific coping strategies), self-efficacy (higher scores indicate a higher sense of self-efficacy), and social support (higher scores indicate higher social support), respectively. Joint function was assessed using the Harris Hip Score (HHS) (27) or Knee Society Score (KSS) (28).
Demographic data collected included sex, age, ethnicity, body mass index (BMI), marital status, education level, occupation, long-term residence, housing types, living arrangements, family income, payment method, and exercise. Disease-related data included arthritis location, disease duration, comorbidities, albumin levels at admission, surgical site, smoking and drinking history, past medical history, blood transfusion (during hospitalization), previous hospitalizations, and postoperative complications.
Demographic data, disease-related data, and the MCMQ were collected on preoperative day 1 (T1); PTGI, GSES, PSSS, and HHS/KSS were collected on T1, postoperative day 1 (T2), postoperative month 1 (T3), and postoperative month 3 (T4). Data from T1 and T2 were collected in the ward, and data from T3 and T4 were collected via telephone follow-up or outpatient visits. T1 was considered the baseline psychological state after prolonged suffering from osteoarthritis, with its score reflecting the potential for growth under the cumulative trauma of chronic illness rather than an immediate reaction to the acute surgical event. Postoperative time points (T2–T4) were directly associated with the surgical experience. Since the majority of pain relief and significant improvements in daily functional ability after THA/TKA occur within 1 to 3 months postoperatively, focusing on this critical rehabilitation period aimed to capture key turning points in the dynamic trajectory of PTG, thereby providing a crucial window of opportunity for early intervention.
Statistical analysis
For continuous data, means and standard deviations were used for normally distributed data, while medians and interquartile ranges were used for non-normally distributed data. Categorical data were expressed as frequencies and percentages. Univariate analyses were performed using the Mann-Whitney U test, independent t-test, one-way analysis of variance, repeated measures analysis of variance, and Spearman correlation analysis. Multivariate analysis was conducted using stratified regression to explore the influence of medical coping strategies, self-efficacy, and social support on PTG scores, after controlling for demographic and disease-related factors. All data analyses were performed using SPSS 26.0 software, with a two-sided significance level of α = 0.05.
Results
All patients completed follow-up at four time points. Initially, 163 patients met the inclusion criteria. However, one patient undergoing TKA was lost to follow-up due to a change in contact details, and two patients undergoing THA withdrew from the study. As expected, there were no statistically significant differences in baseline data between the 160 patients (P > 0.05). The baseline characteristics of the 160 patients are shown in Table 1.
PTG trajectory analysis
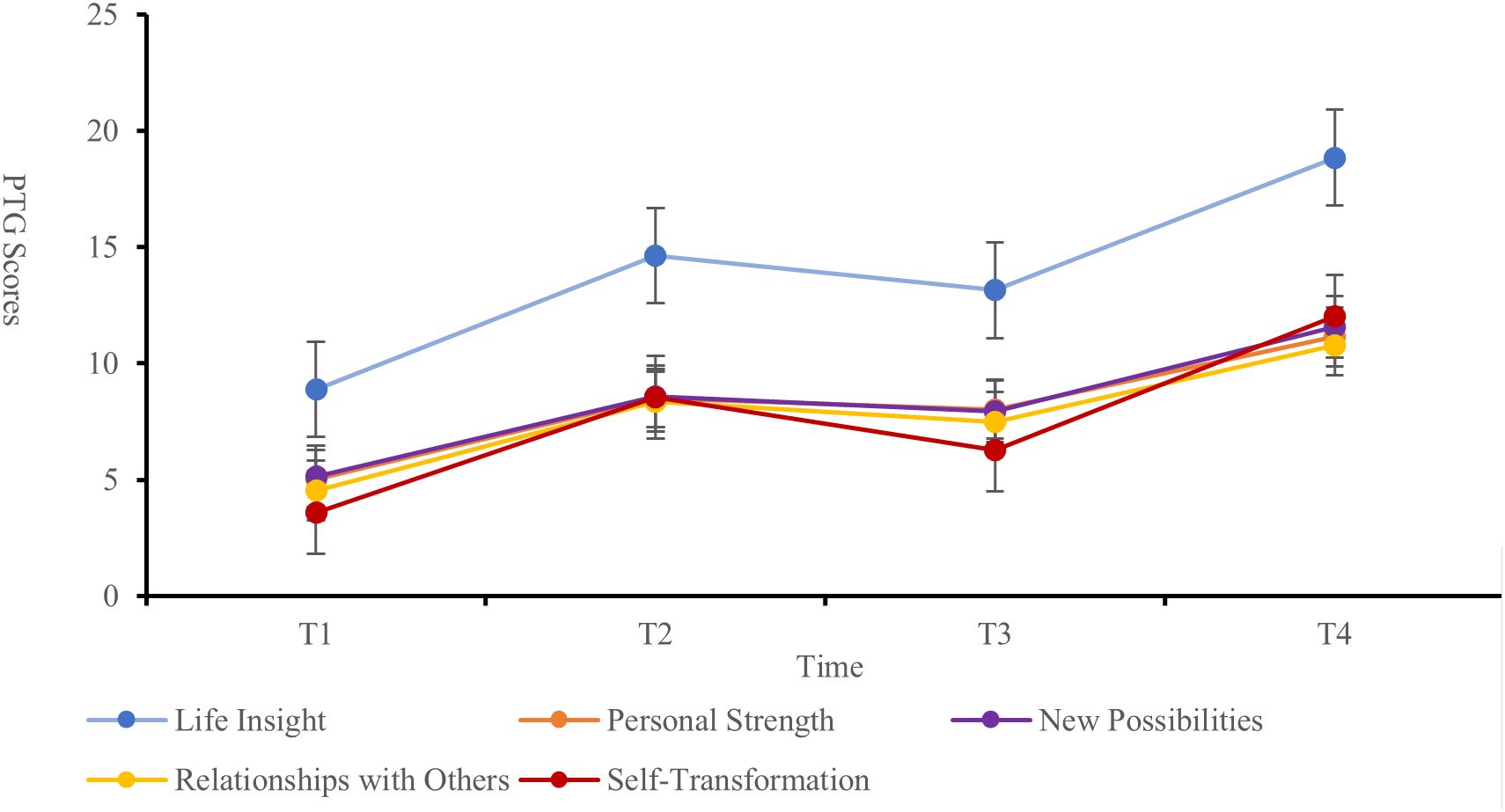
The PTG scores of the patients at each time point were as follows: T1 (27.23 ± 12.87), T2 (48.61 ± 14.49), T3 (42.87 ± 12.72), and T4 (64.37 ± 9.42), indicating an overall moderate to low level of PTG. The changes in PTG scores across dimensions and the total score showed a trend of increasing, then decreasing, and then increasing again (Figure 1). Significant differences were observed in the self-transformation, life realization dimensions, and total PTG score across the four time points (P < 0.001) (Table 2, Figure 2).
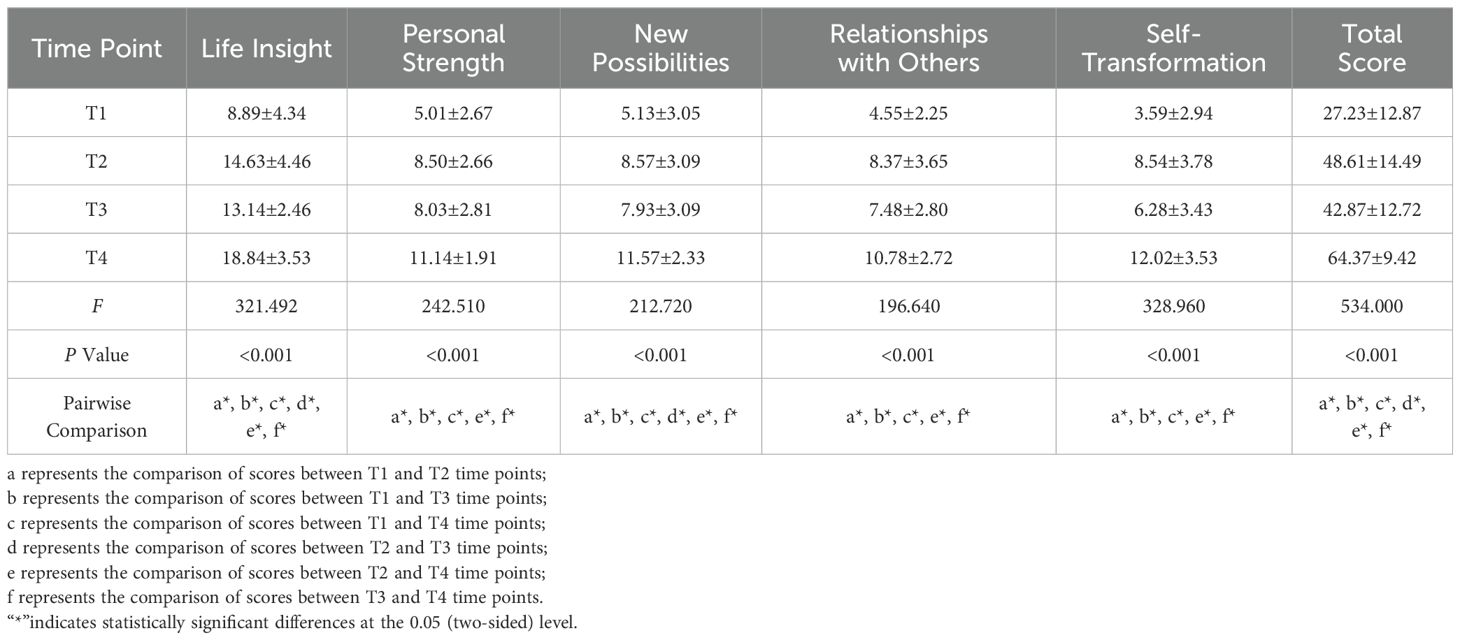
Table 2. Longitudinal analysis of PTG dimensions and total scores at T1 to T4 time points (N=160, ±s).
Analysis of coping strategies, GSES, and PSSS
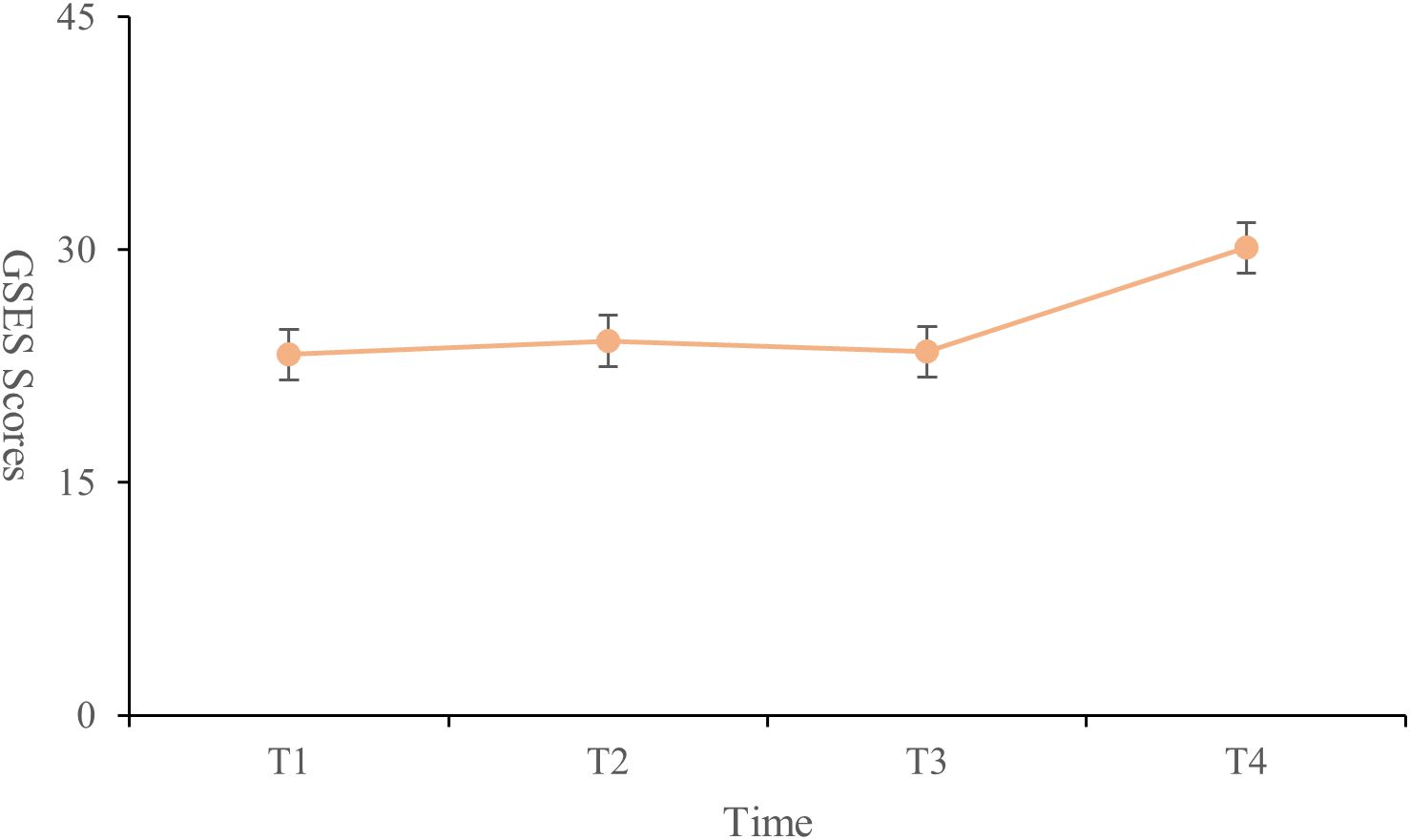
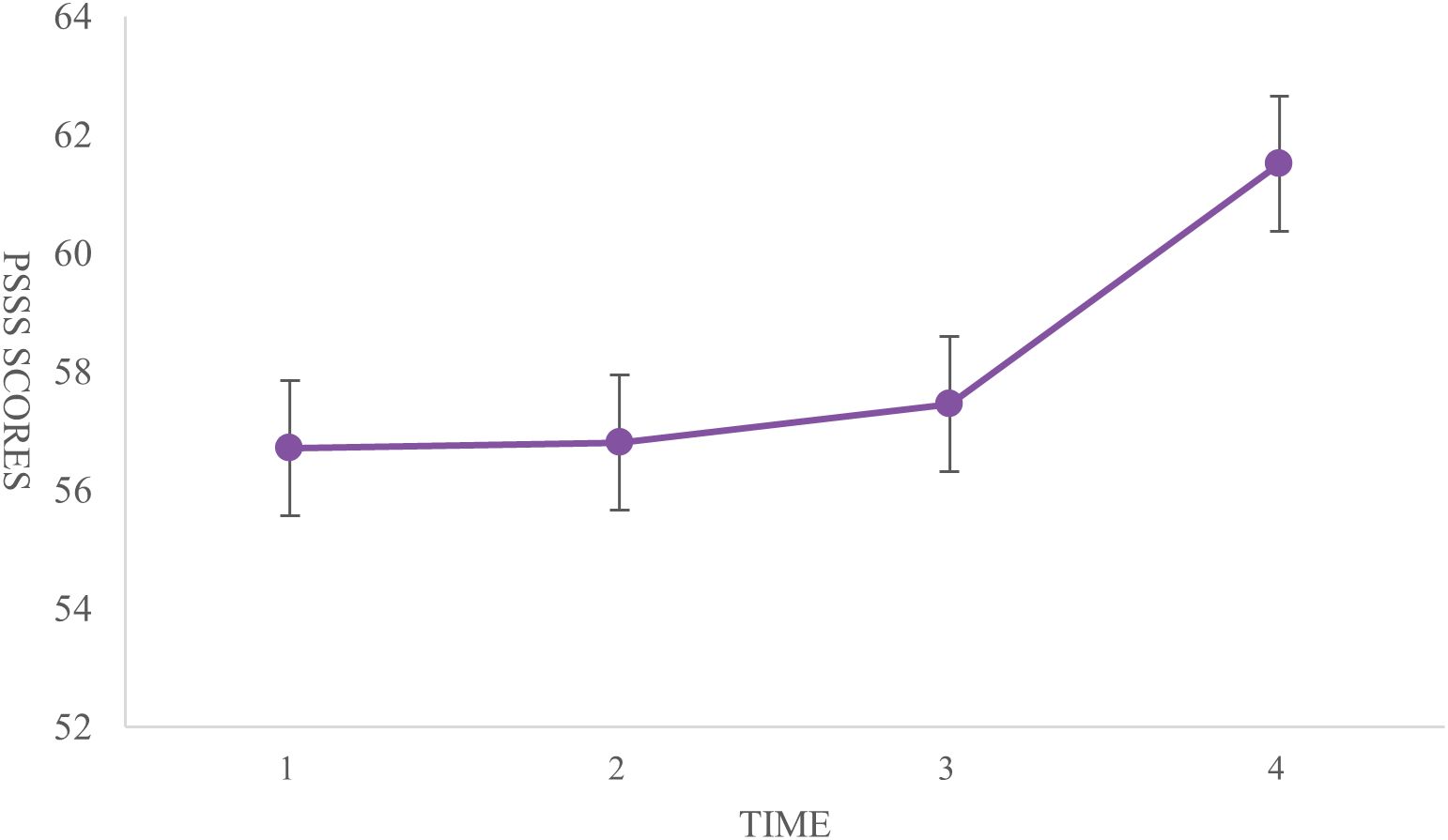
The highest score for medical coping strategies was in the avoidance dimension (18.38 ± 3.14), followed by facing (17.73 ± 3.04) and yielding (10.11 ± 2.13). General self-efficacy scores were lowest at T1 (23.24 ± 5.71) and highest at T4 (30.13 ± 4.36). The scores at T2 (24.13 ± 4.87) were higher than those at T3 (23.43 ± 4.34). Significant differences were found between T1 and T2, T1 and T4, and T2 and T4 (P < 0.001) (Figure 3). The total social support score showed significant differences between T1 and T4, and between T2 and T4 (P < 0.001). Significant differences were observed in the family support dimension across all four time points (P < 0.001). The friend support dimension showed significant differences only between T2 and T4 (P < 0.001). Differences in other support dimensions at T4 were statistically significant when compared to the other time points (P < 0.05) (Table 3, Figure 4).
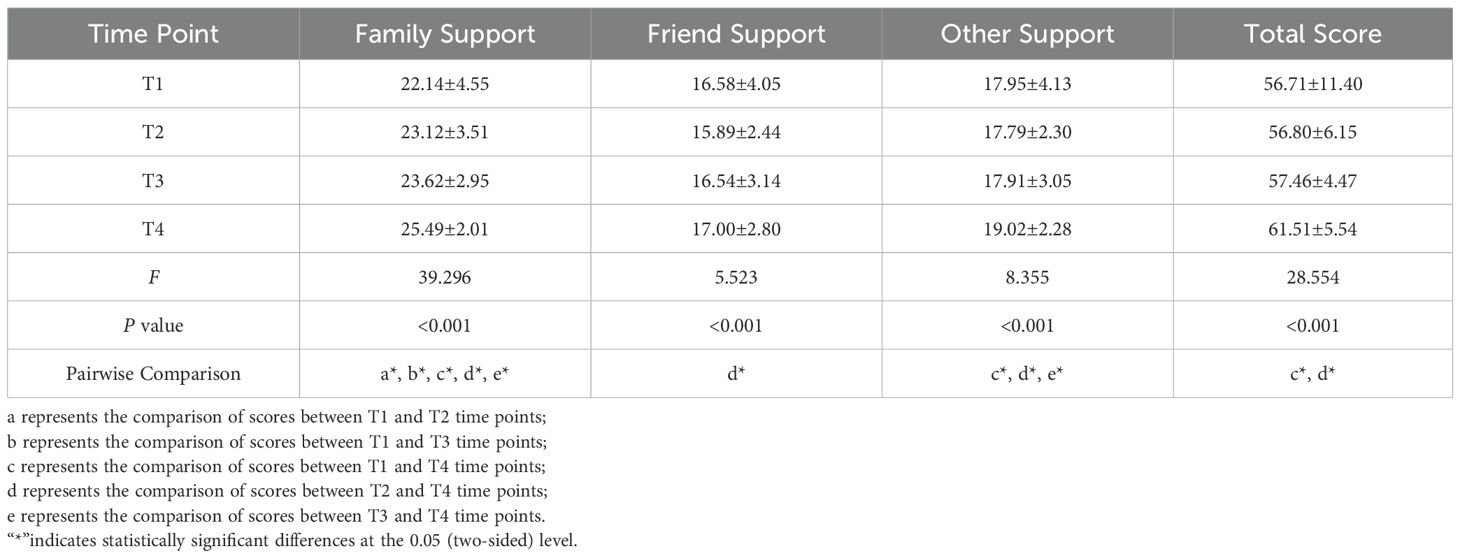
Table 3. Longitudinal changes in social support dimensions and total dcores at T1 to T4 (n=160, points, ±s).
Stratified analysis
Compared with living alone, patients living with a spouse at T1 (P < 0.001) or with children at T1 (P < 0.05) had lower PTG scores. At T2, patients living with a spouse had higher PTG scores (P < 0.05). At T4, patients living with children had higher PTG scores (P < 0.05) (Table 4). The arthritis location, comorbidities, and joint function at T1 influenced PTG levels (P < 0.05). surgical site and joint function influenced PTG levels at T2 (P < 0.05), while joint function influenced PTG levels at T3. At T4, joint function and comorbidities influenced PTG levels (P < 0.05; Table 4).
Self-efficacy was positively correlated with PTG scores at both T1 and T2 (P < 0.001; Table 4). For coping strategies, in the facing dimension, scores at T1 (P < 0.001) and T4 (P < 0.01) were positively correlated with PTG scores. In the avoidance dimension, T1, T2, and T4 scores were negatively correlated with PTG scores (P < 0.01). The yielding dimension score at T1 was negatively correlated with PTG scores (P < 0.01; Table 4). For social support, in the social support domain, family support at T2 and T3 was positively correlated with PTG scores (P < 0.05; Table 4).
Discussion
This study revealed a curvilinear trajectory of PTG among patients undergoing THA or TKA, characterized by a sharp increase immediately after surgery, a decline at one month postoperatively, and a subsequent rise at three months. Overall, PTG levels remained moderate to low. The initial surge in PTG immediately post-surgery may be attributed to effective pain management and intensive care, which activate patients’ acute positive reappraisal responses; this sense of growth often precedes actual functional recovery. At one month, the combination of intensified rehabilitation efforts and persistent residual pain may result in a gap between expectations and reality, while the reduction in medical and social support resources contributes to a decline in PTG, reflecting cognitive instability during psychological restructuring. By three months postoperatively, with improved joint function and enhanced self-efficacy, patients are more likely to complete the reconstruction of trauma-related meaning and establish a more stable and internalized form of positive psychological adaptation. This trend aligns with the curvilinear development pattern proposed in recent PTG research, emphasizing that PTG is not a linear accumulation but a dynamic process involving emotional peaks, cognitive restructuring, and adaptive integration (29, 30). These trajectory characteristics deepen our understanding of the psychological recovery mechanisms in THA/TKA patients and suggest that the first postoperative month may serve as a critical window for interventions aimed at transitioning PTG from short-term adaptation to long-term growth. Strengthening social support and external guidance for cognitive reframing during this period may enhance the stability and clinical significance of PTG.
In this study, social support and living arrangements significantly influenced patients’ PTG scores, with living arrangements showing varied effects at different time points. Although the PSSS measures relatively stable perceived social support, the event of joint replacement may prompt patients to reassess their support systems. At postoperative time points (T2–T4), surgery as a stressor may lead to renewed evaluation of the support network. At T2 (postoperative day 1), when pain peaks, perceived family support may be reinforced through caregiving behaviors. This study found statistically significant differences in perceived family support across all four time points, while friend support showed significant differences only between T2 and T4, suggesting differentiated roles of support sources during rehabilitation. These findings highlight the importance of stage-specific psychological interventions. Previous research has found that among patients living alone after total joint arthroplasty, the 90-day readmission rate was only 2.1%, showing no significant difference compared to those living with others (31). For hip fracture patients, longitudinal studies revealed that functional support (e.g., assistance with daily care) was more effective in promoting walking recovery than structural support (e.g., number of cohabitants) (31). Increased perceived levels of social support were associated with reduced stress responses to surgery and rehabilitation exercises in THA/TKA patients, leading to better adherence to recovery programs. Therefore, for patients discharged after THA/TKA, the key consideration may be whether there is a supportive environment for functional recovery, rather than living arrangements alone. When developing rehabilitation plans for patients with different living arrangements, it is recommended to comprehensively consider the degree of family support, accessibility of social resources, and the patient’s baseline functional status (31). For example, wearable remote monitoring devices and AI-assisted training have been shown to be as effective as clinical supervision (32). For patients living alone, the safety of home rehabilitation depends on preoperative functional status and appropriate modifications to the home environment, such as installing handrails or adjusting furniture height to reduce fall risk (31, 32).
Patients with comorbidities are often associated with more severe symptoms of disease (33, 34). In this study, the main comorbidities included hypertension (67.8%), diabetes (17.2%), and insomnia (13.7%). Patients with comorbidities at T1 had lower PTG levels. In surgical patients, clinically significant depressive symptoms were independently correlated with increased blood pressure when patients were aware of their hypertension (35). Diabetes is associated with a higher incidence of perioperative complications, such as the need for blood transfusions, pneumonia, delayed discharge, surgical site infections, and in-hospital mortality (36). Insomnia increased the risk of depression, inflammatory diseases, and infectious diseases, and led to an increase in all-cause mortality (37). Rigorous preoperative screening and intervention for comorbidities can help reduce surgical risks and alleviate patients’ psychological burden. Postoperative tracking revealed 1 case of wound infection and 1 case of dislocation at T3, and 5 cases of joint stiffness at T4. In addition to the effects of comorbidities, patients with postoperative complications showed lower PTG levels at T4. In addition to providing postoperative rehabilitation guidance, future research should continue to evaluate the effectiveness of continuous nursing interventions in reducing the incidence of complications.
At T1, patients with more OA-affected joints had lower PTG levels. The ongoing decline in joint function leads to decreased self-care ability, making patients more susceptible to negative psychological states such as anxiety and depression (6, 38, 39). In this study, joint function scores were positively correlated with PTG levels at all time points; better joint function was associated with higher PTG levels. However, at T2, TKA patients had lower PTG levels compared to THA patients. Previous studies have shown that pain resulting from joint function rehabilitation exercises is more pronounced in TKA patients compared to THA patients. As pain was a predictor of anxiety and depression, it contributed to lower levels of PTG in these patients (40–42). Personalized assessments led by physical therapists, integrating pain coping techniques with psychological strategies, are essential. For instance, dual-task training that combines physical exercise with cognitive engagement has been shown to break the conditioned response of pain during movement (43). Additionally, graded exposure therapy, which gradually expands patients’ functional activity thresholds, may significantly reduce the negative experiences associated with pain (44).
Coping styles play an important role in maintaining individuals’ psychological well-being and overall health. Positive coping strategies encourage individuals to adopt proactive approaches, thereby promoting both physical and psychological recovery (45, 46). Active coping behaviors are significantly positively correlated with rehabilitation adherence (30). However, patients undergoing THA/TKA often face various barriers to activity participation. For example, avoidance strategies may delay early mobilization and increase the risk of deep vein thrombosis, while submissive coping may lead to dependence on analgesics, hindering the recovery process (45, 47). In this study, preoperative medical coping scores consistently influenced PTG scores at various postoperative time points, suggesting the importance of assessing patients’ coping styles before surgery. Interventions should promote positive coping strategies, such as prehabilitation and cognitive behavioral therapy, which can involve progressive muscle relaxation and attentional redirection. Cognitive restructuring has been proven effective in reducing catastrophizing tendencies like pain-related fear and in building alternative, positive cognitions to better manage postoperative rehabilitation challenges (21, 48, 49).
General Self-Efficacy refers to an individual’s belief in coping with various stresses or challenges (50). Individuals with high self-efficacy not only set higher goals but also persistently strive to achieve them (51, 52). In our study, the trajectory of patients’ self-efficacy across four time points showed a positively correlated upward trend with PTG scores, with significant positive correlations at T1 and T2. This suggests that enhancing self-efficacy may be an important approach to promoting PTG and facilitating recovery. Strategies to improve self-efficacy may include developing structured educational programs that integrate preoperative and postoperative modular courses, covering pain management, rehabilitation training, and psychological adjustment (52, 53). Additionally, based on social cognitive theory, establishing a collaborative model between families and hospitals, such as involving family members in the development of rehabilitation plans, can further enhance patients’ self-efficacy (52).
Limitations
This study has several limitations. First, the study participants were all from a single hospital where postoperative continuity services are provided, and all patients received standardized follow-up care. Therefore, the representativeness of the results may be limited. Future studies should include patients from hospitals of different levels and compare PTG levels between patients receiving continuity nursing services and those under traditional care models. Secondly, the data collection in this study was limited to 3 months post-surgery, a time when joint function generally stabilizes but has not yet reached the point where patients forget about the joint. Therefore, more long-term follow-up studies are needed to understand the patients’ PTG levels in the long term. Thirdly, although preoperative PTG is considered a baseline for growth related to chronic illness, the PTGI is inherently more suited to assessing responses to acute traumatic events. To address this, the present study employed hierarchical regression, controlling for joint function scores, to isolate the influence of psychological transformation mechanisms, such as coping strategies and self-efficacy, from physical recovery. Future studies should incorporate mixed methods (e.g., interviews) to triangulate and validate preoperative PTG experiences. Finally, we did not systematically collect chronic disease control indicators (e.g., HbA1c, blood pressure control rate), which may have led to an underestimation of the inhibitory effects of poorly controlled conditions on PTG. Future research should incorporate objective medical indicators to strengthen this analysis.
Conclusion
PTG in hip and knee arthroplasty patients follows a dynamic trajectory—initial rise, decline at one month, and subsequent increase. The first postoperative month represents a key intervention window. Higher PTG is associated with better joint function, self-efficacy, positive coping, and social support, while avoidance and yield coping correlate negatively. As influencing factors vary over time, targeted education and interventions are needed to enhance PTG and support recovery.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of West China Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YX: Conceptualization, Formal analysis, Methodology, Project administration, Software, Writing – original draft, Writing – review & editing. ZF: Conceptualization, Writing – review & editing, Data curation, Formal analysis, Investigation, Methodology, Supervision, Writing – original draft. PL: Data curation, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing, Software. JC: Writing – review & editing, Formal analysis, Project administration, Validation. LZ: Formal analysis, Validation, Writing – review & editing, Investigation, Software. YL: Software, Writing – review & editing, Funding acquisition, Methodology, Resources. NN: Funding acquisition, Resources, Writing – review & editing, Conceptualization, Project administration. ZZ: Funding acquisition, Project administration, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Mahmoudian A, Lohmander LS, Mobasheri A, Englund M, and Luyten FP. Early-stage symptomatic osteoarthritis of the knee - time for action. Nat Rev Rheumatol. (2021) 17:621–32. doi: 10.1038/s41584-021-00673-4
2. Turkiewicz A, Petersson IF, Bjork J, Hawker G, Dahlberg LE, Lohmander LS, et al. Current and future impact of osteoarthritis on health care: a population-based study with projections to year 2032. Osteoarthritis Cartilage. (2014) 22:1826–32. doi: 10.1016/j.joca.2014.07.015
4. de Beer J, Petruccelli D, Adili A, Piccirillo L, Wismer D, and Winemaker M. Patient perspective survey of total hip vs total knee arthroplasty surgery. J Arthroplasty. (2012) 27:865–9 e1-5. doi: 10.1016/j.arth.2011.12.031
5. Lutzner C, Postler A, Beyer F, Kirschner S, and Lutzner J. Fulfillment of expectations influence patient satisfaction 5 years after total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. (2019) 27:2061–70. doi: 10.1007/s00167-018-5320-9
6. Springer BD and Sotile WM. The psychology of total joint arthroplasty. J Arthroplasty. (2020) 35:S46–9. doi: 10.1016/j.arth.2020.01.002
7. Groarke A, Curtis R, Groarke JM, Hogan MJ, Gibbons A, and Kerin M. Post-traumatic growth in breast cancer: how and when do distress and stress contribute? Psychooncology. (2017) 26:967–74. doi: 10.1002/pon.4243
8. Jayawickreme E, Infurna FJ, Alajak K, Blackie LER, Chopik WJ, Chung JM, et al. Post-traumatic growth as positive personality change: Challenges, opportunities, and recommendations. J Pers. (2021) 89:145–65. doi: 10.1111/jopy.12591
9. Ayache RA, Chabrol H, Kendall-Tackett K, and Goutaudier N. Posttraumatic growth and depreciation in people with chronic pain: A profile analysis. Psychological trauma : theory, research, practice and policy. (2021) 13:149–56. doi: 10.1037/tra0000969
10. Sato M, Yamazaki Y, Sakita M, and Bryce TJ. Benefit-finding among people with rheumatoid arthritis in Japan. Nurs Health Sci Mar. (2008) 10:51–8. doi: 10.1111/j.1442-2018.2007.00372.x
11. Li N, Zhou ZY, Yang YH, Liu H, and Lu YF. Correlation between post-traumatic growth, post-traumatic stress disorder, and quality of life in patients with hip fracture. Qilu Nurs J. (2018) 24:94–6.
12. Zhuo M. Neural mechanisms underlying anxiety-chronic pain interactions. Trends Neurosci. (2016) 39:136–45. doi: 10.1016/j.tins.2016.01.006
13. Blackburn-Munro G and Blackburn-Munro RE. Chronic pain, chronic stress and depression: coincidence or consequence? J Neuroendocrinol. (2001) 13:1009–23. doi: 10.1046/j.0007-1331.2001.00727.x
14. Giannoudis PV, Dinopoulos H, Chalidis B, and Hall GM. Surgical stress response. Injury. (2006) 37 Suppl 5:S3–9. doi: 10.1016/S0020-1383(07)70005-0
15. Husain A and Lee GC. Establishing realistic patient expectations following total knee arthroplasty. J Am Acad Orthop Surg. (2015) 23:707–13. doi: 10.5435/JAAOS-D-14-00049
16. Wylde V, Kunutsor SK, Lenguerrand E, Jackson J, Blom AW, and Beswick AD. Is social support associated with patient-reported outcomes after joint replacement? A systematic review and meta-analysis. Lancet Rheumatol. (2019) 1:e174–86. doi: 10.1016/S2665-9913(19)30050-5
17. Fuchs S, Schwettmann L, Katzenberger B, Paulus A, Holzapfel BM, Biebl JT, et al. Association of self-efficacy, risk attitudes, and time preferences with health-related quality of life and functioning after total hip or knee replacement - Results of the MobilE-TRA 2 cohort. Health Qual Life Outcomes. (2025) 23:44. doi: 10.1186/s12955-025-02374-y
18. Anakwe RE, Jenkins PJ, and Moran M. Predicting dissatisfaction after total hip arthroplasty: A study of 850 patients. J Arthroplasty. (2011) 26:209–13. doi: 10.1016/j.arth.2010.03.013
19. Nizar N, Mahomed MHL, Cook EF, Daltroy LH, Fortin PR, Fossel AH, et al. The importance of patient expectations in predicting functional outcomes after total joint arthroplasty. J Rheumatol. (2002) 29:1273–9.
20. Santaguida PL, Hawker GA, Hudak PL, Glazier R, Mahomed NN, Kreder HJ, et al. Patient characteristics affecting the prognosis of total hip and knee joint arthroplasty: a systematic review. J Can chir. (2008) 51:428–36.
21. Bay S, Kuster L, McLean N, Byrnes M, and Kuster MS. A systematic review of psychological interventions in total hip and knee arthroplasty. BMC Musculoskelet Disord. (2018) 19:201. doi: 10.1186/s12891-018-2121-8
22. Ayers DC, Zheng H, and Franklin PD. Integrating patient-reported outcomes into orthopaedic clinical practice: proof of concept from FORCE-TJR. Clin Orthop Relat Res. (2013) 471:3419–25. doi: 10.1007/s11999-013-3143-z
23. Smith SG and Cook SL. Are reports of posttraumatic growth positively biased?. J Trauma Stress. (2004) 17:353–58. doi: 10.1023/B:JOTS.0000038485.38771.c6
24. Atema V, van Leeuwen M, Oldenburg HS, Retél V, van Beurden M, Hunter MS, et al. Design of a randomized controlled trial of Internet-based cognitive behavioral therapy for treatment-induced menopausal symptoms in breast cancer survivors. BMC cancer. (2016) 16:920. doi: 10.1186/s12885-016-2946-1
25. Cheung S-K and Sun SYK. Assessment of optimistic self-beliefs: further validation of the chinese version of the general self-efficacy scale. psychol Rep. (1999) 85:1221–4. doi: 10.2466/pr0.1999.85.3f.1221
26. Gill DP, Blunt W, Bartol C, Pulford RW, De Cruz A, Simmavong PK, et al. HealtheSteps™ Study Protocol: a pragmatic randomized controlled trial promoting active living and healthy lifestyles in at-risk Canadian adults delivered in primary care and community-based clinics. BMC Public Health. (2017) 17:173. doi: 10.1186/s12889-017-4047-8
27. Smith MV, Klein SE, Clohisy JC, Baca GR, Brophy RH, and Wright RW. Lower extremity-specific measures of disability and outcomes in orthopaedic surgery. J Bone Joint Surgery. (2012) 94:468–77. doi: 10.2106/jbjs.J.01822
28. Wang C, Zhang C, Liu DL, Tong WW, He CR, Huang X, et al. Simplified Chinese version of hip and knee replacement expectations surveys in patients with osteoarthritis and ankylosing spondylitis: cross-cultural adaptation, validation and reliability. BMC Musculoskeletal Disord. (2018) 19:1–10. doi: 10.1186/s12891-018-2129-0
29. Paunescu AC, Kvaskoff M, Delpierre C, Delrieu L, Jacob G, Pannard M, et al. The influence of locus of control, coping strategies and time perspective on post-traumatic growth in survivors with primary breast cancer. BMC Psychol. (2025) 13:42. doi: 10.1186/s40359-025-02353-4
30. Kazemi-Sufi S, Bagheri A, Mazhari SA, Farhadi B, Alizadeh-Otaghvar H, Zaboli Mahdiabadi M, et al. Post-traumatic growth and its explanatory factors in burn patients: A systematic review. Int Wound J. (2024) 21:e70066. doi: 10.1111/iwj.70066
31. Fleischman AN, Austin MS, Purtill JJ, Parvizi J, and Hozack WJ. Patients living alone can be safely discharged directly home after total joint arthroplasty: A prospective cohort study. J Bone Joint Surg Am. (2018) 100:99–106. doi: 10.2106/JBJS.17.00067
32. Naylor JM, Hart A, Harris IA, and Lewin AM. Variation in rehabilitation setting after uncomplicated total knee or hip arthroplasty: a call for evidence-based guidelines. BMC Musculoskelet Disord. (2019) 20:214. doi: 10.1186/s12891-019-2570-8
33. Saha S, Lim CCW, Cannon DL, Burton L, Bremner M, Cosgrove P, et al. Co-morbidity between mood and anxiety disorders: A systematic review and meta-analysis. Depression Anxiety. (2020) 38:286–306. doi: 10.1002/da.23113
34. Kok XLF, Newton JT, Jones EM, and Cunningham SJ. Social support and pre-operative anxiety in patients undergoing elective surgical procedures: A systematic review and meta-analysis. J Health Psychol. (2022) 28:309–27. doi: 10.1177/13591053221116969
35. Rantanen AT, Korkeila JJA, Löyttyniemi ES, Saxén UKM, and Korhonen PE. Awareness of hypertension and depressive symptoms: a cross-sectional study in a primary care population. Scandinavian J Primary Health Care. (2018) 36:323–8. doi: 10.1080/02813432.2018.1499588
36. Browne JA, Cook C, Pietrobon R, Bethel MA, and Richardson WJ. Diabetes and early postoperative outcomes following lumbar fusion. Spine. (2007) 32:2214–9. doi: 10.1097/BRS.0b013e31814b1bc0
37. Irwin MR. Sleep and inflammation: partners in sickness and in health. Nat Rev Immunol. (2019) 19:702–15. doi: 10.1038/s41577-019-0190-z
38. Gudmundsson P, Nakonezny PA, Lin J, Owhonda R, Richard H, and Wells J. Functional improvement in hip pathology is related to improvement in anxiety, depression, and pain catastrophizing: an intricate link between physical and mental well-being. BMC Musculoskeletal Disord. (2021) 22:1–9. doi: 10.1186/s12891-021-04001-5
39. Jones AR, Al-Naseer S, Bodger O, James ETR, and Davies AP. Does pre-operative anxiety and/or depression affect patient outcome after primary knee replacement arthroplasty? Knee. (2018) 25:1238–46. doi: 10.1016/j.knee.2018.07.011
40. Salisbury C, O’Cathain A, Thomas C, Edwards L, Gaunt D, Dixon P, et al. Telehealth for patients at high risk of cardiovascular disease: pragmatic randomised controlled trial. Journal article. BMJ (Clinical Res ed). (2016) 353:i2647. doi: 10.1136/bmj.i2647
41. Salmon P, Hall GM, Peerbhoy D, Shenkin A, and Parker C. Recovery from hip and knee arthroplasty: Patients’ perspective on pain, function, quality of life, and well-being up to 6 months postoperatively. Arch Phys Med Rehabil. (2001) 82:360–6. doi: 10.1053/apmr.2001.21522
42. Bourne RB, Chesworth B, Davis A, Mahomed N, and Charron K. Comparing patient outcomes after THA and TKA: is there a difference? Clin Orthop Relat Res. (2010) 468:542–6. doi: 10.1007/s11999-009-1046-9
43. Parks MJ, Slater JS, Rothman AJ, and Nelson CL. Interpersonal communication and smoking cessation in the context of an incentive-based program: survey evidence from a telehealth intervention in a low-income population. J Health communication. (2016) 21:125–33. doi: 10.1080/10810730.2015.1039677
44. Jurewicz A, Gasiorowska A, Leźnicka K, Pawlak M, Sochacka M, Machoy-Mokrzyńska A, et al. Individual factors modifying postoperative pain management in elective total hip and total knee replacement surgery. Life (Basel). (2024) 14(2):211. doi: 10.3390/life14020211
45. Gil-Gonzalez I, Perez-San-Gregorio MA, Funuyet-Salas J, Conrad R, and Martin-Rodriguez A. Significance of post-traumatic growth and mental health for coping in multiple sclerosis caregivers. Healthcare (Basel). (2023) 11(10):1390. doi: 10.3390/healthcare11101390
46. Wang Y, Huang C, Zhang H, Cai Y, Shen Z, Hu X, et al. Correlation among psychological resilience, social support, and coping style in patients with complicated hepatolithiasis. Front Behav Neurosci. (2022) 16:939512. doi: 10.3389/fnbeh.2022.939512
47. Smith TO, Dainty JR, and MacGregor AJ. Changes in social isolation and loneliness following total hip and knee arthroplasty: longitudinal analysis of the English Longitudinal Study of Ageing (ELSA) cohort. Osteoarthritis Cartilage. (2017) 25:1414–9. doi: 10.1016/j.joca.2017.04.003
48. Sun JN, Chen W, Zhang Y, Zhang Y, Feng S, and Chen XY. Does cognitive behavioral education reduce pain and improve joint function in patients after total knee arthroplasty? A randomized controlled trial. Int Orthop. (2020) 44:2027–35. doi: 10.1007/s00264-020-04767-8
49. Konnyu KJ, Thoma LM, Cao W, Aaron RK, Panagiotou OA, Bhuma MR, et al. Prehabilitation for total knee or total hip arthroplasty: A systematic review. Am J Phys Med Rehabil. (2023) 102:1–10. doi: 10.1097/PHM.0000000000002006
50. Schwarzer R, Bäßler J, Kwiatek P, Schröder K, and Zhang JX. The assessment of optimistic self-beliefs:Comparison of the german, spanish, and chinese versions of the general self-efficacy scale. Int Assoc Appl Psychol. (1997) 46:69–8. doi: 10.1111/j.1464-0597.1997.tb01096.x
51. Koring M, Richert J, Lippke S, Parschau L, Reuter T, and Schwarzer R. Synergistic effects of planning and self-efficacy on physical activity. Health Educ Behav. (2012) 39:152–8. doi: 10.1177/1090198111417621
52. Meng Y, Deng B, Liang X, Li J, Li L, Ou J, et al. Effectiveness of self-efficacy-enhancing interventions on rehabilitation following total hip replacement: a randomized controlled trial with six-month follow-up. J Orthop Surg Res. (2022) 17:225. doi: 10.1186/s13018-022-03116-2
Keywords: hip arthroplasty, knee arthroplasty, post-traumatic growth, influencing factors, social support, self-efficacy, coping strategy
Citation: Xie Y, Fu Z, Li P, Chen J, Zhang L, Liu Y, Ning N and Zhou Z (2025) Post-traumatic growth trajectories and influencing factors in patients undergoing hip and knee arthroplasty: a prospective longitudinal study. Front. Psychiatry 16:1556829. doi: 10.3389/fpsyt.2025.1556829
Received: 07 January 2025; Accepted: 07 July 2025;
Published: 04 August 2025.
Edited by:
Lamyae Benzakour, University of Geneva, SwitzerlandReviewed by:
Jiawei Xu, Harvard Medical School, United StatesGiuseppe Basile, Marche Polytechnic University, Italy
Copyright © 2025 Xie, Fu, Li, Chen, Zhang, Liu, Ning and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ning Ning, bmluZ25pbmc2NDA1QDE2My5jb20=
†These authors share first authorship
 Yan Xie
Yan Xie Zhongmin Fu
Zhongmin Fu Peifang Li3
Peifang Li3 Ning Ning
Ning Ning Zongke Zhou
Zongke Zhou