- 1School of Nursing, Harin Medical University, Harin, China
- 2Key Laboratory of Human Factors and Ergonomics, State Administration for Market Regulation, China National Institute of Standardization, Beijing, China
- 3Psychiatry Department, The Third People’ Hospital of Daqing, Daqing, China
Background: Non-suicidal self-injury (NSSI), a prevalent psychiatric behavioral problem in adolescents, manifests diverse outcomes including cessation of remission, aggravation, and even progression to suicidal behaviors. It is crucial to track its progression and identify predictors of clinical outcomes in NSSI. The aim of this study was to determine whether the N2 and P3 responses in adolescents with NSSI were defective in the presence of the affective Stroop paradigm and associated with their clinical outcomes.
Methods: Participants were selected from an ongoing longitudinal study designed to predict clinical outcomes of NSSI in adolescents. Twenty-six in the remission group (RG), twenty-nine in the aggravation group (AG), and twenty-seven in the healthy group (HG) completed the affective Stroop task with EEG. Accuracy and reaction times (RTs) served as behavioral indexes, while N2 and P3 amplitude served as electrophysiological indexes; they were analyzed across groups. We used the EEGNet model to predict the NSSI clinical outcomes with EEG component.
Result: No significant main effects of group or affective stimuli or an effect of their interaction were observed on accuracy (p > 0.05). For RTs, there was a significant main effect of group, with slower RTs observed in the AG compared to the HG (p < 0.05). For N2 and P3 amplitude, there were significant main effect of group and affective stimuli and an effect of their interaction. Under neutral stimuli, the N2 amplitude in the AG was significantly larger than that in the RG (p < 0.05) and the HG (p < 0.01), while the P3 amplitude in AG was significantly smaller than that in the HG (p < 0.05), but there was no significant difference between RG and AG(p > 0.05). The EEGnet model demonstrated that N2 amplitudes elicited by neutral stimuli achieved the highest classification accuracy (92.31%) for predicting clinical outcomes of NSSI.
Conclusion: The findings indicate that NSSI is linked to cognitive processing deficits, including impaired control and resource allocation to stimuli. Additionally, N2 amplitudes were shown to reliably predict clinical outcomes in NSSI.
Background
Non-suicidal self-injury (NSSI) refers to deliberate and direct damage to body tissue without suicidal intent (1). While not intended to result in death, evidence suggests that NSSI can predict future suicide attempts and fatalities (2, 3). A comprehensive global meta-analysis of an extensive sample revealed that the lifetime prevalence of non-suicidal self-injury (NSSI) among adolescents is 22.1% (4), with evidence indicating a gradual upward trend. NSSI in this demographic serves as a significant predictor of suicide, substantially elevating the associated risk (5). According to the 2019 Global Burden of Disease data, NSSI ranks as the third leading cause of disability-adjusted life years (DALYs) globally among individuals aged 10–24 years (6). This condition imposes a considerable socio-economic burden and constitutes a critical public health issue that necessitates urgent attention and intervention. The classification of NSSI as an independent category in the DSM-5 underscores its significance and the necessity for further research.
NSSI typically begins in early adolescence and progresses into diverse developmental trajectories over time. The clinical outcome of NSSI in adolescents is usually characterized by several possible developmental paths: cessation of short-term episodes, repeated long-term episodes, and even progression to suicidal behaviors (7). A study involving 13- to 19-year-olds identified two patterns of NSSI frequency: Low Stable and High Persistent (8). Similarly, a year-long study of 3,381 adolescents found two trajectory groups: a remission group (87.4%) and an aggravation group (12.7%) (9). These longitudinal studies highlight the varied developmental pathways of NSSI, emphasizing the importance of tailored prevention and intervention strategies. While identifying trajectories is crucial, determining predictors of clinical outcomes holds even greater importance.
The clinical outcomes of adolescents engaging in NSSI exhibit significant individual variability, influenced by a multitude of factors. Early identification of potential clinical trajectories is crucial for psychiatric professionals, as it enables the timely and effective implementation of tailored treatment and intervention strategies, thereby mitigating the risk of adverse clinical outcomes. Current forecasting methods largely rely on validated scales. From the perspectives of family system theory and cumulative risk, family conflict risk has been identified as a key predictor of NSSI addiction (7). Other studies highlight the roles of interpersonal factors, such as unstable relationships and parental criticism, as predictors of high NSSI fluctuations (9). Personal factors, including impulsive behaviors, depression (9), emotion regulation difficulties, self-compassion deficits (10), psychiatric symptoms, and comorbidities (8), also contribute significantly. However, reliance on subjective self-reports introduces bias and reduces diagnostic accuracy. Objective measurements based on individual characteristics offer a more reliable alternative, presenting a promising avenue for predicting NSSI outcomes with greater precision.
Behavioral findings from various cognitive tasks have provided insights into cognitive control in subgroups of individuals with NSSI. Frequent NSSI has been associated with poorer performance on the Criticism Gambling Task, suggesting that impulsive decision-making in response to criticism may serve as a critical indicator of NSSI progression (11). Additionally, high-severity NSSI groups have been found to exhibit working memory deficits, whereas low-severity groups demonstrate impaired inhibitory control (12). Electroencephalogram (EEG) devices allow for the collection of stimulus-evoked EEGs, or event-related potentials (ERPs), through specifically designed experimental tasks. Previous studies have reported that adolescents with NSSI, compared to HC, exhibit increased N2 amplitudes (13) and decreased P3 amplitudes (14), indicating significant cognitive control dysfunction. Further, Zhao et al. identified that adolescents with NSSI displayed smaller N2 and larger P3 responses during negative emotional face stimuli (15). However, no ERP-based studies currently address clinical outcomes of NSSI, highlighting the potential of investigating neurocognitive factors using ERP methodologies.
ERPs offer high temporal resolution, making them valuable for studying cognitive processes with millisecond precision (16, 17). The N2 component, located in the anterior central brain region, is indicative of conflict processing, with its amplitude reflecting the degree of attention control (18). The P3 component is thought to reflect the individual’s attention or alertness, allocation of attention resources and attention transfer (19). Cognitive processing involves the activation and coordination of multiple brain regions. For instance, the affective Stroop task studied via fMRI has demonstrated activation in regions such as the amygdala, insula, and prefrontal cortex, alongside cognitive networks involving the prefrontal cortex, superior temporal lobe, and insula (20). This study seeks to examine the cognitive processing features of NSSI outcome groups using an affective Stroop paradigm, which is a reliable tool for assessing cognitive functions (21). Given that negative emotions can impair cognitive performance (22, 23), the affective Stroop task is a classical paradigm used in recent years (24, 25). Washburn et al. noted that individuals frequently engaging in NSSI might have a behavioral addiction (26), sharing neurobiological roots with addiction (27). Drug addiction is marked by cue reactivity (28), shown through behavioral and neurobiological methods like ERP. A prior study indicated that adolescents with NSSI might show altered behavioral and brain responses to NSSI cues (29). Therefore, a novel affective Stroop paradigm which contains neutral and NSSI images as stimuli was adapted to evaluate the effects of emotional valence on cognitive tasks across NSSI outcome groups. We used the affective Stroop paradigm to assess how emotional distraction from NSSI-related images impacts cognitive control, as reflected in Stroop performance and ERPs. We hypothesize that adolescents with worsening NSSI would show larger N2 and smaller P3 amplitudes than those in remission or healthy controls. The objective of this study was to identify sensitive electrophysiological markers capable of predicting various clinical outcomes of NSSI, with the intent of offering objective screening tools and therapeutic targets for clinical psychiatric practitioners.
Deep learning has demonstrated significant capability in automatically learning and extracting features from raw data, making it highly applicable to EEG signal analysis (30, 31). Compared to conventional methods, deep learning techniques offer several advantages, including robust feature extraction and representation, end-to-end training, and superior generalization. Among these, EEGNet, a convolutional neural network (CNN) specifically designed for Brain-Computer Interface (BCI) applications (32), has shown exceptional performance in processing task-related EEG datasets. EEGNet is designed for processing EEG signals. It addresses the complexities of non-stationary EEG data with a carefully structured architecture. The model starts with a temporal convolution layer to identify temporal patterns, then uses depthwise convolution to learn spatial filters for each channel, and concludes with separable convolution to combine these filters and extract additional features. These include applications such as P3 visual-evoked potentials (33), movement-related cortical potentials (MRCP) (34), and sensory motor rhythms (SMR) (35). In this study, ERP components such as N2 and P3 were extracted from the affective Stroop dataset for comparative analysis using EEGNet. The secondary objective was to evaluate the accuracy of clinical outcome predictions for NSSI by using these ERP components as input features to the EEGNet model.
Methods
Participants
Our previous study tracked the trajectories of NSSI in 550 adolescents with NSSI behavior (12–18 years old) across three waves of follow-up a one-year period, each separated by a six-month interval. A total of 502 adolescents completed the entire follow - up survey, and 48 adolescents were lost to follow-up. We tested for differences in gender, age, and baseline NSSI between participants lost to follow-up and those effectively tracked. Results showed no significant differences (p < 0.05), suggesting random sample loss and no impact on research outcomes. All the research subjects in this study were from the community and had no specific disease diagnoses. NSSI was evaluated based on DSM-5 criteria (36) for the past six months, which includes eight specific behaviors: cutting/stabbing, hair pulling, head banging, biting, cauterizing, scratching, hitting, and rubbing until bleeding occurred. The adolescents rated their engagement in each NSSI behavior for the past six-month on a 4-point Likert-type scale (1 = “never,” 2 = “once or twice,” 3 = “three to five times,” and 4 = “six times or more”). If one or more NSSI behaviors were reported, it was classified as an instance of NSSI behavior. Latent class analysis revealed two subgroups remission group (RG) (n = 371, 73.90%), and aggravation group (AG) (n = 131, 26.10%). In RG, participants showed a decreased frequency or medical severity of NSSI episodes from the start to the end of the follow-up. In AG, participants showed an increased frequency or medical severity of NSSI episodes from the start to the end of the follow-up. Participants in RG and AG were recruited from our previous longitudinal study designed to predict clinical outcomes of NSSI in adolescents. The inclusion criteria: φAdolescents met the DSM-5 diagnostic criteria for NSSI, with NSSI occurring at least once in the past year; κNormal visual acuity or corrected visual acuity; ΛRight-handed. Exclusion criteria: φThose with suicidal ideation or behavior in the past year with a structured interview; κPatients with other mental disorders; ΛPatients with a history of traumatic brain injury, epilepsy, etc; μThose who have received drug or psychotherapy in the past year. Healthy group (HG) were healthy adolescents recruited through advertisements. The inclusion criteria: φNo past or current mental disorders as per the Brief International Neuropsychiatric Interview for Children and Adolescents (ICAO-5); κNo history of NSSI behaviors; ΛNormal or corrected visual acuity; μRight-handed. Exclusion criteria: the same as the NSSI group.
Those who met these criteria underwent structured clinical interviews based on DSM-5 criteria conducted by counselors for group assignment confirmation. Participants volunteered for the study, provided informed consent, could withdraw at any time, and were compensated with cash. Ethical approval was obtained from the Ethics Committee of Harbin Medical University Daqing Campus, and the study adhered to the ethical standards outlined in the World Medical Association Declaration of Helsinki.
Task paradigm
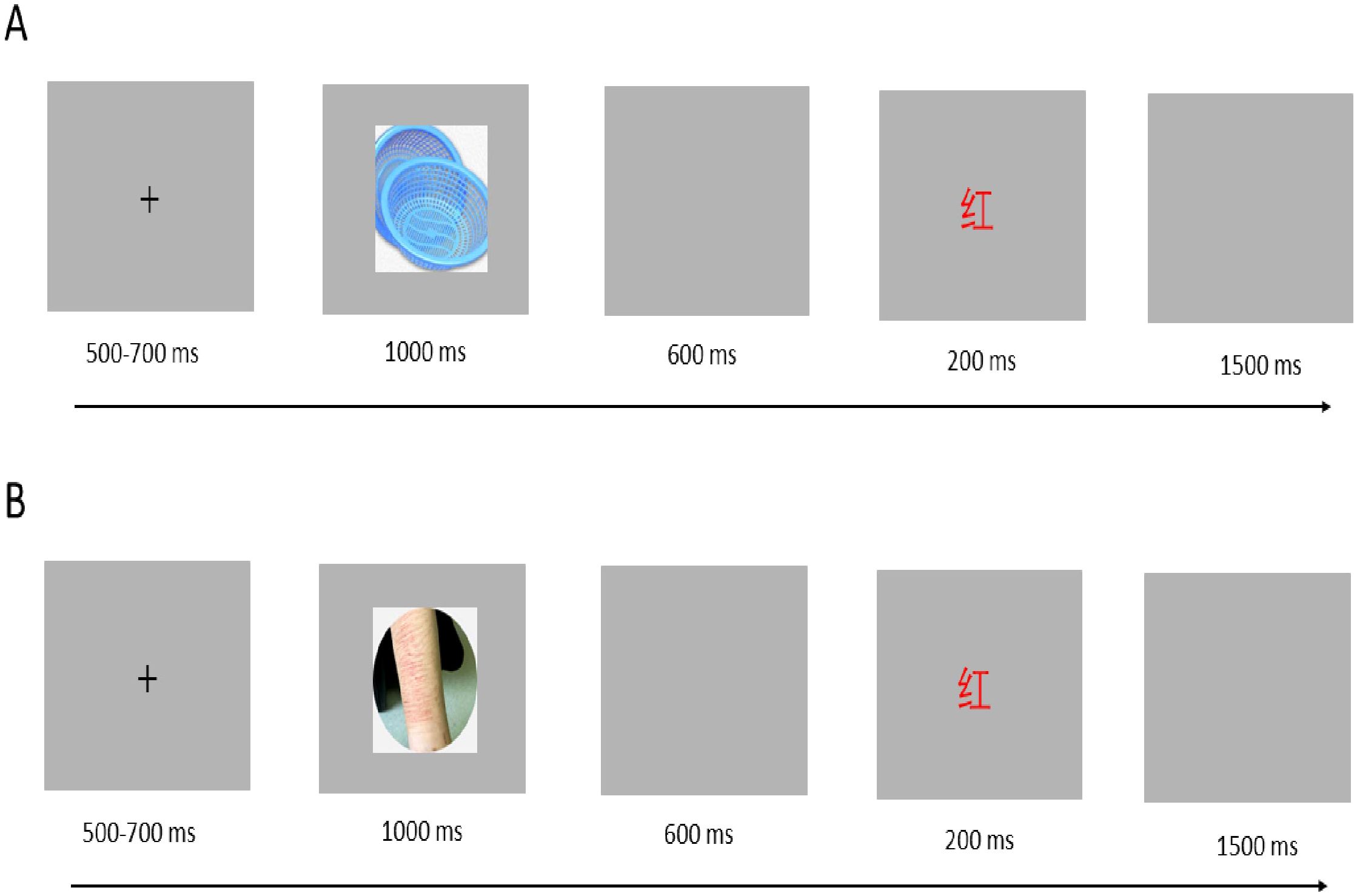
All participants were required to complete the affective Stroop task during EEG recording. The task was created using E-prime 3.0 (see Figure 1). It consisted of two blocks, each containing 120 neutral trials and 120 negative trials. The trials were presented in a pseudo-randomized order within each block. The emotional stimuli included 30 non-NSSI-related neutral pictures selected from the Chinese Affective Picture System (CAPS) (37, 38) and 30 neutral pictures sourced from NSSI images of the participants. We gave participants a detailed NSSI image-selection guideline. It required images to clearly depict study-relevant NSSI acts, restricting behaviors to common ones (e.g., cutting, burning, scratching). We also asked them to avoid overly distracting or complex images for consistent core content across participants. Prior to the main experiment, we pre-tested participant-specific NSSI images with a demographically similar volunteer group. Images were rated on a standardized arousal scale, and those with extreme ratings were excluded. We then selected images for each participant within a narrow arousal range to standardize stimulus arousal.
The two sets of emotional pictures differed significantly in valence [F (1, 58) = 136.51, P < 0.001] but were similar in arousal [F (1, 58) = 5.17, P = 0.21]. The literal stimuli consisted of single words presented in one of four colors (red, yellow, blue, or green). Two experimental conditions were used: the congruent condition, where each color word was displayed in its corresponding color, and the incongruent condition, where each color word was presented in a color other than the one it referred to (e.g., the word “red” displayed in yellow).
Each trial began with a white “+” displayed for 500–700 ms in the center of the screen on a grey background, followed by an emotional picture presented for 1000 ms, then a blank screen for 600 ms, followed by a color word displayed for 200 ms, and a final blank screen for 1500 ms. Participants were required to determine whether the word and color were congruent. If they were congruent, participants pressed the “D” key with their left index finger; if they were incongruent, participants pressed the “J” key with their right index finger. Both response time and accuracy were recorded in this study.
EEG recording and processing
The EEG data were recorded using a Neuroscan ESI 64-channel system, equipped with an Ag/AgCl electrode cap and the extended international 10–20 electrode placement. The system was configured with a bandpass filter of 0.05–100 Hz and a sampling rate of 500 Hz. A left mastoid electrode was used as the online reference, which was later re-referenced offline to the common average (39). Vertical electrooculogram (EOG) was recorded from electrodes placed above and below the left eye, while horizontal EOG was captured from electrodes positioned at the outer canthi. Electrode impedance was kept below 5 kΩ throughout the task.
Experimental conditions, equipment, and physiological responses can introduce interference that affects the collected signals. To minimize these effects, preprocessing steps were applied to the EEG data. EOG blink artifacts were corrected using linear regression estimation (40). The EEG signals were band-pass filtered between 0.05 Hz and 30 Hz and segmented into 1000 ms epochs, with a 200 ms pre-stimulus baseline. Specifically, we used an epoch time window from –200 ms pre-stimulus to 1000 ms post-stimulus. Epochs with voltage deviations exceeding ±80 μV were excluded from the ERP analysis. On average, the retained neutral trials in HG, RG, and AG groups were 103 ± 14, 94 ± 21, and 93 ± 19 respectively, with no significant inter-group difference (F =2.496; p = 0.089). The retained negative trials in HG, RG, and AG groups were 96 ± 20, 91 ± 17, and 86 ± 22 respectively, with no significant inter-group difference (F =1.771; p = 0.175). Separate averages for the congruent and incongruent conditions were computed for neutral and negative stimuli, respectively. Incorrect responses may be associated with different cognitive processes, such as error detection and correction, which could confound the analysis of the N2 and P3 components. Only trials with correct responses were included in the calculation of average ERPs.
ERP analysis
Based on ERP components linked to cognition as reported in previous research (41–43), as well as the characteristics of the ERP waveforms observed in the current study, specific ERP components were selected for further analysis. For N2 analysis, frontal- central electrodes were selected. These regions are related to cognitive processes like conflict monitoring and error detection, relevant to our study. The P3 component is thought to reflect widely - distributed attentional and evaluative processes in the frontal network. The amplitudes of the N2 (260–320 ms; FZ, FCZ, PZ, CPZ, PZ) and P3 (320–370 ms; F3, F1, FZ, F2, F4, FC3, FC1, FCZ, FC2, FC4) components were examined. For each ERP component, the mean amplitude within the specified time window was calculated. Our analyses were based on the raw ERP amplitudes for each condition.
Statistical analysis
A repeated-measures analysis of variance (ANOVA) was conducted to assess accuracy, reaction time (RT), and ERP component amplitude across different subgroups. The analysis included a three-way repeated-measures design with group (healthy, declining, and worsening) as the between-subject factor, and emotion (neutral, negative) and congruency (congruent, incongruent) as within-subject factors. Statistical analyses were performed using repeated-measures ANOVAs with Greenhouse-Geisser corrections at a significance level of 0.05. Significant interaction effects were followed by simple effect analysis, with pairwise comparisons adjusted using the Bonferroni method and an alpha value of 0.05 divided by the number of comparisons.
In the model, a 64-channel EEG signal was used as the input, and the group served as the output. The model was trained and tested through a deep convolutional layer followed by a deep separable convolutional layer. This network extracts intrinsic characteristic information from the waveform using convolutional kernels, enabling the prediction of clinical outcomes associated with NSSI. We inputted raw EEG time series data from the Stroop task into the EEGNet model, which includes ERP components like N2 and P3 amplitudes. This allows the model to autonomously identify relevant features and patterns. The datasets were split in two ways: randomly into three subsets-60% for training, 20% for validation, and 20% for testing-ensuring that no participant’s data appeared in more than one subset, and employing 5-fold cross-validation. We added dropout layers to our EEGNet architecture to enhance model robustness and prevent overfitting by randomly setting a portion of input units to zero during training.
Results
General characteristics
Ninety adolescents were recruited from the longitudinal study, with 30 participants in each subgroup (HG, RG, AG). Eight participants were excluded: two for accuracy below 75%, three for z-scores above 3.29, and three due to excessive EEG artifacts in over 25% of trials. The final sample included 82 participants (mean age 15.69 ± 1.73; 49 females): 27 in HG, 26 in RG, and 29 in AG. Using G*Power 3.1 with effect size f=0.25f=0.25, α=0.05α=0.05, and 1−β=0.951−β=0.95, the calculated total sample size was 68, with 23 per group to ensure result robustness. The demographic characteristics of all participants are summarized in Table 1. There were no significant differences in general demographic data among the three groups of participants.
Behavioral performance
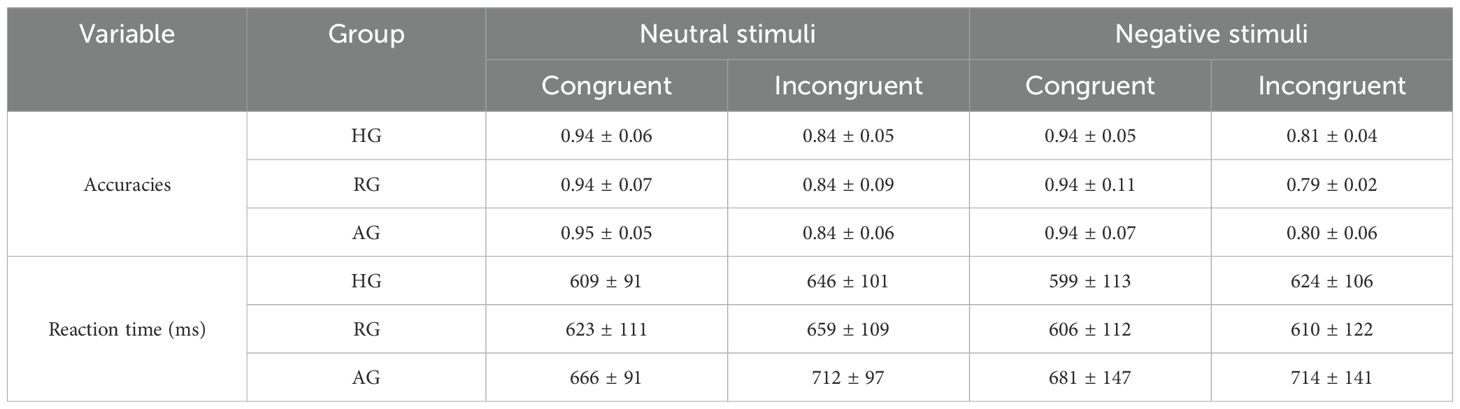
Table 2 presents the accuracy and reaction times (RTs) for the different groups.
The ANOVA of accuracy revealed a significant main effect of emotion type (F (1,79) = 21.494, p < 0.001, η2 = 0.214), indicating that accuracy was higher for neutral stimuli compared to negative stimuli (p < 0.001). The main effect of congruency was also significant (F (1, 79) = 682.573, p < 0.001, η2 = 0.898), showing that accuracy was higher for the congruent condition than for the incongruent condition (p < 0.001). The main effect of group on accuracies was not significant (F (1, 79) = 0.096, p = 0.909, η2 = 0.002) (Figure 2A).
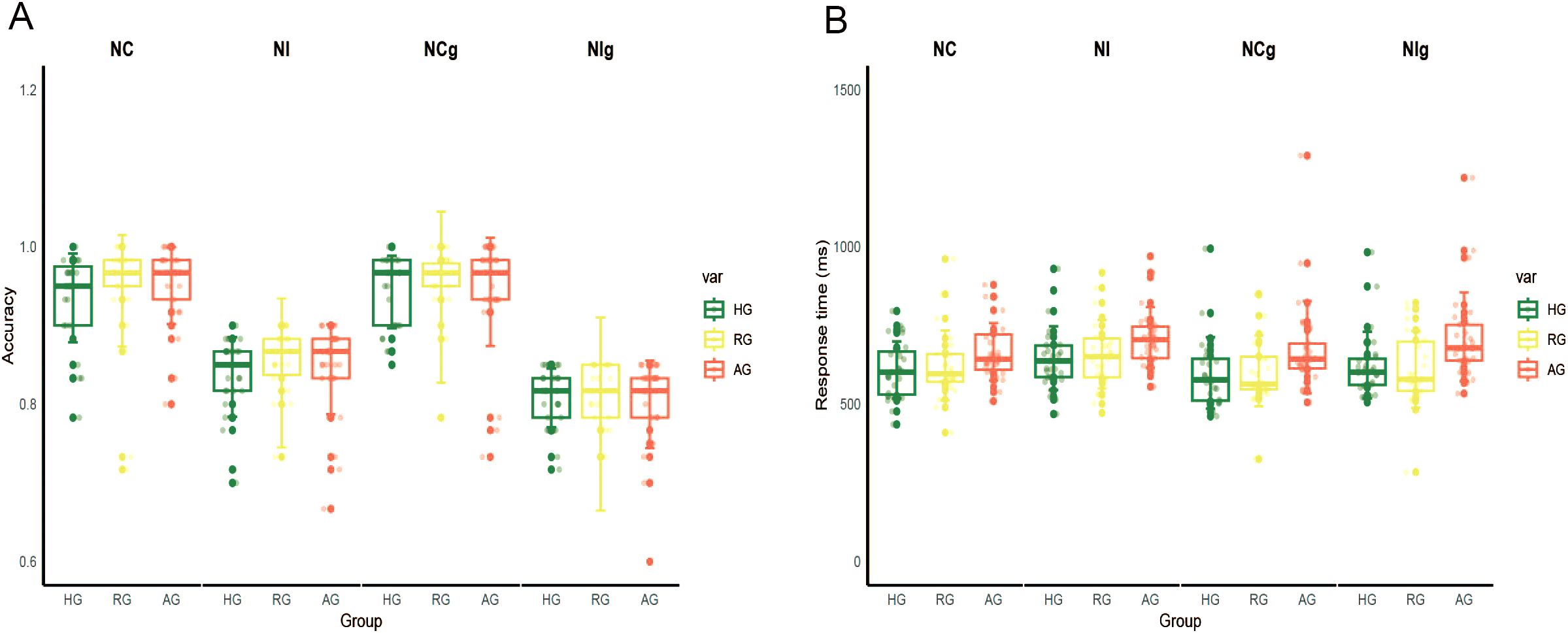
Figure 2. The results of behavior data. (A) The accuracy in the HG, RG and AG. (B) The response time in the HG, RG and AG. NC, congruent in neutral stimuli; NI, incongruent in neutral stimuli; NCg, congruent in negative stimuli; NIg, incongruent in negative stimuli.
The ANOVA of RTs showed a significant main effect of group (F (2,79) = 4.145, p < 0.05, η2 = 0.095), with slower RTs observed in the AG compared to the HG (p < 0.05). The main effect of congruency was also significant (F (1, 79) = 65.734, p < 0.001, η2 = 0.454), indicating that RTs were slower for the incongruent condition compared to the congruent condition (p < 0.001) (Figure 2B).
ERP results
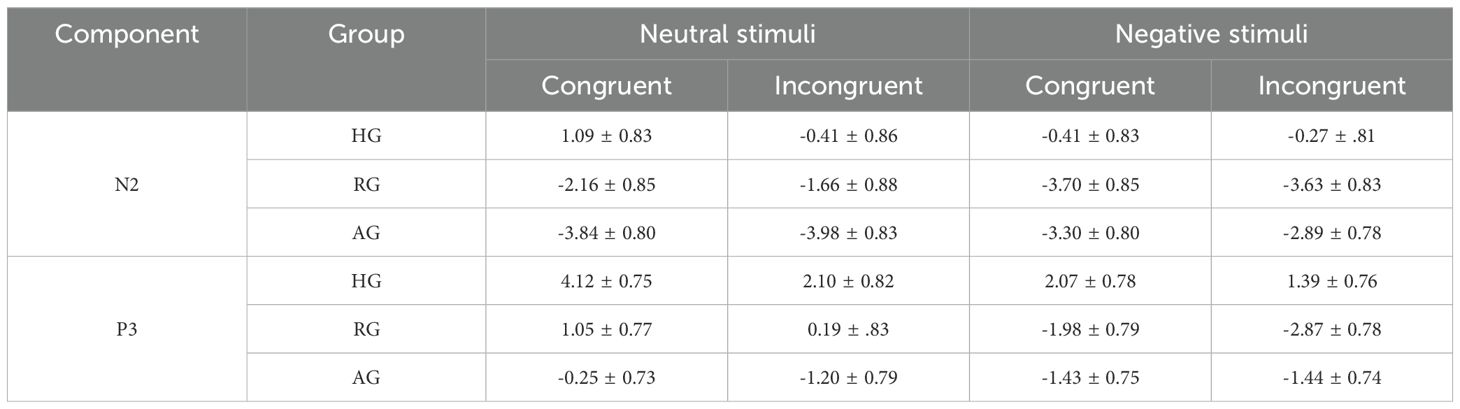
Table 3 presents the average amplitude for each ERP component in the different groups.
N2 amplitude
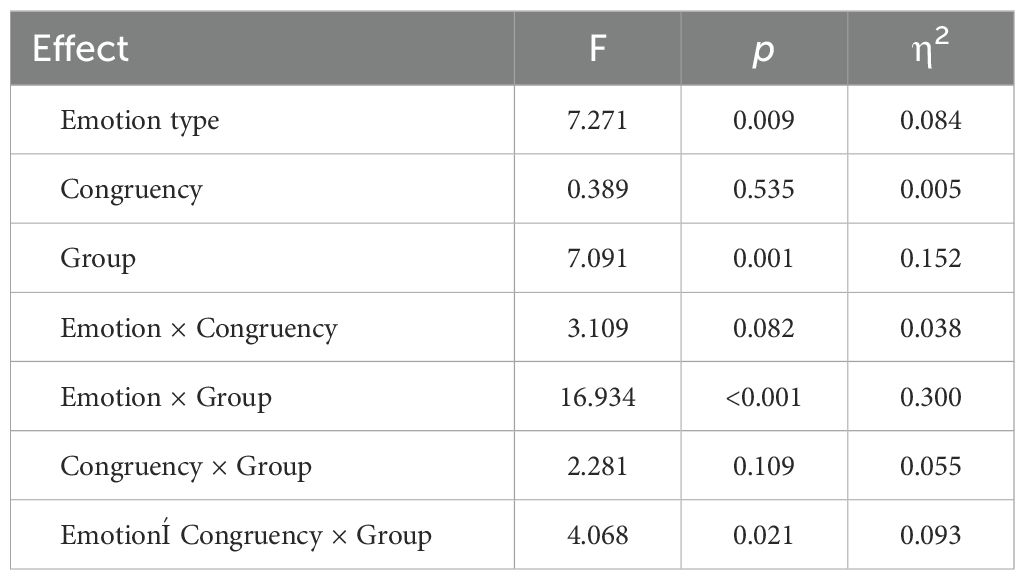
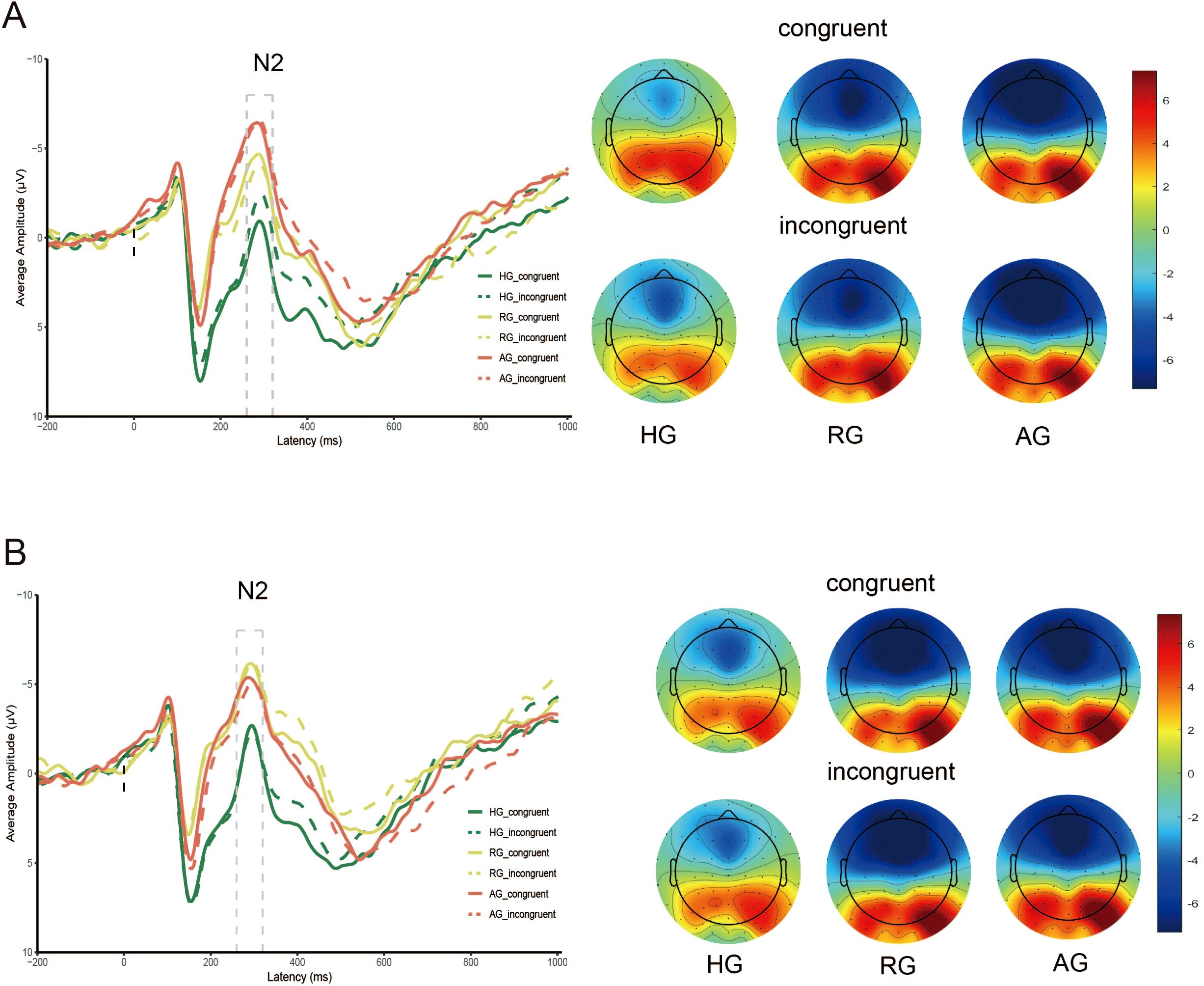
The results, presented in Table 4 and Figure 3, revealed a significant main effect of emotion type (F (1, 79) = 7.271, p < 0.01, η2 = 0.084), where the N2 elicited by negative stimuli was larger than that elicited by neutral stimuli. The main effect of group was also significant (F (2, 79) = 7.091, p < 0.01, η2 = 0.152). Specifically, the N2 in the HG was significantly smaller than in the RG and AG (ps < 0.05). However, there was no significant difference between RG and AG (p = 0.089). The interaction between group and emotion was significant (F (2, 79) = 16.934, p < 0.001, η2 = 0.300). Under neutral stimuli, the N2 in HG was significantly smaller than in the RG (p < 0.05) and AG (p < 0.01), with the RG also being significantly smaller than the AG (p < 0.05). Under negative stimuli, the N2 in HG was significantly smaller than in the RG (p < 0.01) and AG (p < 0.05), and there was no significant difference between RG and AG (p = 0.101).
The interaction between group, emotion, and congruency was significant (F (2, 79) = 4.068, p < 0.05, η2 = 0.093). Under neutral stimuli, the N2 in HG was smaller than in RG (p < 0.01) and AG (p < 0.001) for both congruent and incongruent conditions, and the N2 in RG was also smaller than in AG for the congruent condition (p < 0.01). Under negative stimuli, the N2 in HG was smaller than in RG (p < 0.001) and AG (p < 0.01) for both congruent and incongruent conditions, and there was no significant difference between RG and AG for both congruent and incongruent conditions (p = 0.199; p = 0.084).
P3 amplitude
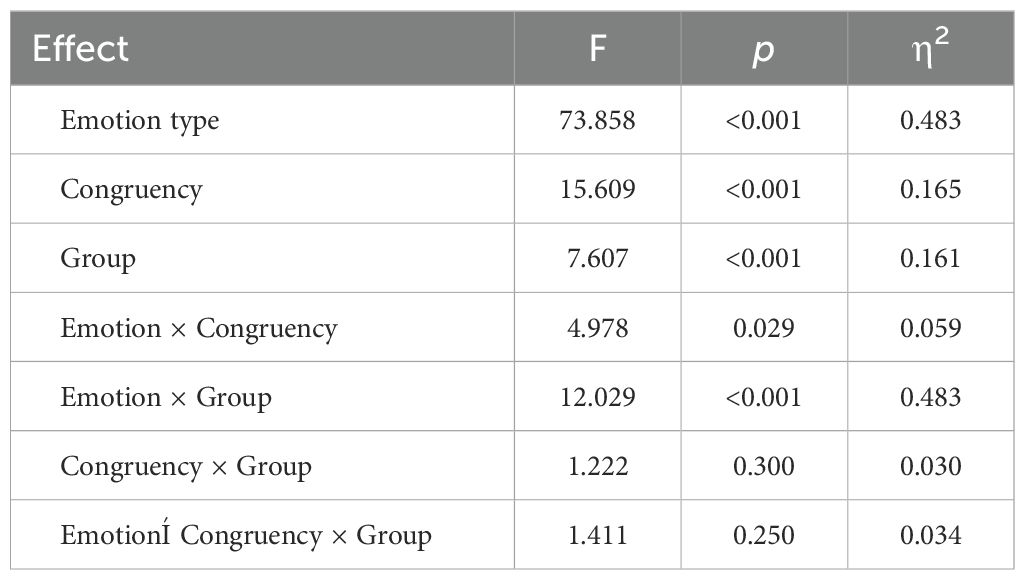
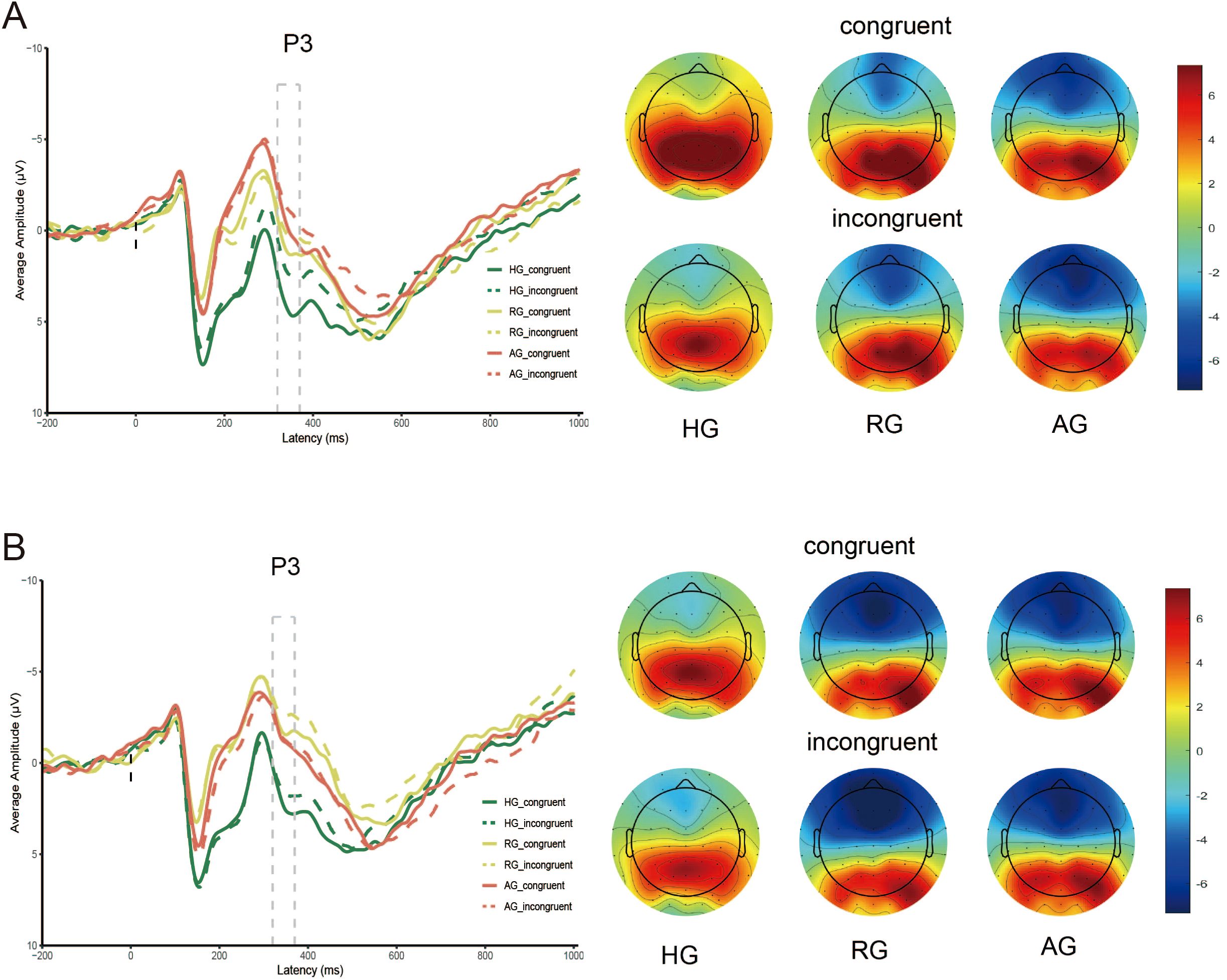
As shown in Table 5 and Figure 4, the main effect of emotion type was significant (F (1, 79) = 73.858, p < 0.001, η2 = 0.483), with the P3 elicited by neutral stimuli being larger than that elicited by negative stimuli. The main effect of group was also significant (F (2, 79) = 7.607, p < 0.001, η2 = 0.161), where the P3 in HG was significantly larger than in RG (p < 0.01) and AG (p < 0.01). However, there was no significant difference between RG and AG (p = 0.158). The interaction between group and emotion was significant (F (2, 79) = 12.029, p < 0.001, η2 = 0.233). Under neutral stimuli, the P3 in HG was significantly larger than in the AG (p < 0.01), but there was no significant difference between RG and AG (p = 0.053) and between HG and RG (p = 0.066). Under negative stimuli, the P3 in HG was significantly larger than in the RG (p < 0.001), but there was no significant difference between RG and AG (p = 0.101) and HG and AG (p = 0.128).
The main effect of congruency was significant (F (1, 79) = 15.609, p < 0.001, η2 = 0.165), with the P3 elicited by the congruent condition being significantly larger than that elicited by the incongruent condition (p < 0.001).
No significant interaction effect between group, emotion, and congruency was observed (F (2, 79) = 1.411, p = 0.250, η2 = 0.034). Simple effects analysis revealed that, under neutral stimuli, the P3 elicited by the congruent condition in HG was larger than in AG (p < 0.001). Under negative stimuli, the P3 in HG were larger than in RG (ps < 0.01) for both congruent and incongruent conditions.
Prediction results on the EEGNet
We identified N2 and P3 components during neutral and negative stimulation using the EEGNet model to predict NSSI clinical outcomes. Under neutral stimuli, the ERP components achieved peak accuracies of 92.31% for N2 and 76.60% for P3, while under negative stimuli, the accuracies were 71.16% for N2 and 49.57% for P3. These results indicate that N2 performs best under neutral stimuli, with all components showing reduced accuracy under negative stimuli.
Discussion
This study explored cognitive control in adolescents with varying NSSI clinical outcomes using an affective Stroop task, supporting the role of cognitive control as a predictor of NSSI and its clinical outcomes. Specifically, compared with the healthy group, NSSI groups had higher RTs, N2 and P3 amplitude, indicating that attention control and attention resource allocation in adolescents with NSSI were impaired. Following the introduction of emotional stimuli, the N2 amplitude demonstrated a significant impact in forecasting the clinical outcomes of NSSI. The N2 elicited by neutral stimuli exhibited significant differences between the RG and AG groups, with the AG displaying a larger N2 response than the RG. This suggests that the N2 component serves as a more sensitive indicator for distinguishing the clinical outcomes of NSSI.
Our research utilized neutral and negative images with distinct valence differences as stimuli in the affective Stroop task. The results indicated that negative stimuli impaired cognitive performance relative to neutral stimuli, leading to slower RTs and lower accuracy. This suggests that negative emotions should be closely monitored in individuals with NSSI to prevent adverse outcomes related to impaired cognitive response (44). The RG and AG groups exhibited slower RTs compared to the HG, suggesting lower processing efficiency and impaired cognitive control. Participants with required more time to perform conflict detection and task-related activities. No significant differences in accuracy were observed between groups, indicating that NSSI primarily affected processing efficiency rather than task effectiveness, although this finding requires further confirmation due to the small sample size.
Negative stimuli evoked greater N2 amplitudes than neutral stimuli, demonstrating that negative stimuli can rapidly and automatically capture attention and utilize attentional resources. Yuan et al. reported that individuals display heightened sensitivity to negative stimuli, processing them at an earlier stage (45). The results showed a significant difference in N2 amplitudes among the HG, RG, and AG, with the amplitude exhibiting a gradient change pattern across these groups. These findings indicate that participants in RG and AG, particularly AG, exhibit abnormalities in cognitive control mechanisms. Hanslmayr et al. observed that individuals responded to increased conflict levels by temporarily enhancing cortical responses to task-relevant information, which improved attention control performance instead of suppressing task-irrelevant information (46). Participants in RG and AG were not required to inhibit or overcome interference caused by affective stimuli. This absence of inhibition enhanced attention to target stimuli, resulting in larger N2 components. In contrast, HG displayed superior attention control, allowing effective inhibition of task-irrelevant information and successful interference management during task completion (47). Under neutral stimuli, participants in AG exhibited more pronounced N2 amplitude deficits compared to RG, with significant deficits in incongruent N2 amplitude relative to HG. While negative stimuli evoke strong emotions and neural variability, neutral stimuli may better predict emotion-independent cognitive control. The N2 amplitude differences in RG and AG under neutral stimuli likely indicate a baseline cognitive control deficit, unaffected by emotional arousal. Our findings suggest that N2 amplitude variations can distinguish between RG and AG, highlighting differences in non-emotional conflict management. Further research is needed to explore cognitive control deficits and their effects on the groups’ functioning. A larger N2 amplitude generally indicates heightened conflict monitoring or greater cognitive- control difficulty under emotional interference. In our study, AG adolescents might have hyper-reactive conflict detection. Their brains rapidly detect conflicts upon emotional stimulation, resulting in a larger N2, implying more effort in conflict monitoring than others. These results suggest that N2 amplitude serves as a sensitive marker for distinguishing HG from NSSI groups, as well as between RG and AG, providing an effective measure for predicting NSSI outcomes.
The P3 amplitude were markedly smaller under negative stimuli or incongruent conditions, aligning with findings from previous studies (48). The P3 component is widely utilized as a biomarker in research on attention resource allocation associated with emotion and cognition (19). It is instrumental in assessing inhibitory processes, such as in the emotional signal stop task, where larger P3 amplitudes signify stronger inhibitory control (49). During conflict processing, suppressing responses to sad faces has been shown to reduce P3 amplitude (50). In the present study, the decreased P3 amplitude indicates that cognitive resources are heavily consumed by negative stimuli or conflicts during the Stroop task. Additionally, the P3 exhibited a gradient downward trend across the HG, RG, and AG, suggesting a progressive decline in the brain’s resource utilization efficiency in the NSSI group (51). A smaller P3 in adolescents implies less attentional allocation to task stimuli, likely due to emotional stimulus interference. Stimulus processing may divert attention, reducing task engagement. It may also signal impaired inhibitory control: limited cognitive control hampers focus by failing to suppress irrelevancies, consistent with poorer stimulus processing. Under neutral stimuli, AG participants demonstrated more severe P3 amplitude deficits than HG in congruent conditions. Similarly, RG participants displayed significant P3 amplitude deficits under negative stimuli, indicating notable cognitive control impairments compared to HG. These results highlight the importance of continued monitoring for RG participants, even though their NSSI behaviors are alleviated.
Findings support that adolescents with persistent NSSI have trouble mobilizing neural resources for inhibitory control when emotionally triggered. A larger N2 suggests conflict-monitoring struggles, while a smaller P3 indicates less attentional allocation and impaired inhibition. Together, these neural manifestations suggest that persistent self-injurers are more likely to resist the urge to injure and show more severe cognitive control deficits. Worth pointing out, Zhao et al. discovered that adolescents in the NSSI group displayed a smaller N2 and larger P3 in a two-choice oddball task with negative emotional stimuli (15). Similarly, Zhou et al. found that the NSSI group showed a larger P3 when exposed to self-injury cues in an oddball paradigm (52). These findings differ from the results of this study. Our findings might differ because of: 1) task variations that could elicit unique neural responses; 2) differences in sample age, gender, and NSSI severity; 3) our emphasis on outcome subgroups. Analyzing the aggravation group against others may uncover distinct NSSI-related neural mechanisms, enhancing field understanding.
Notably, AG participants showed an intermediate pattern under negative stimuli, with N2 and P3 amplitudes positioned between those of HG and RG. This may suggest that negative images become desensitized for AG participants due to frequent NSSI behaviors, resulting in reduced vigilance compared to RG (53). However, AG participants are “desensitized” to negative stimuli lacks direct evidence. In future research, measure skin conductance (a recognized arousal index) during negative stimulus presentation. Its changes, reflecting emotional responses, can provide objective data on AG participants’ reduced arousal, supporting the desensitization hypothesis. Alternatively, AG participants may experience more pronounced cognitive dysfunction, requiring additional resources to process negative stimuli, thereby impairing Stroop task performance (49, 54). Although the precise mechanisms remain unclear, these findings provide valuable insights into potential developmental trajectories.
In the RG and AG subgroups, a significant difference was observed in N2 amplitude but not in P3 amplitude, suggesting that AG participants experience greater difficulties with attention control over stimuli rather than resource allocation. This finding highlights impaired attention control as a central factor in the cognitive dysfunction associated with NSSI outcomes, corroborating earlier studies (55, 56). Clinicians can utilize the EEG as a neurophysiological marker to identify adolescents with ongoing NSSI behaviors, aiding in risk stratification to determine who needs more intensive intervention. The EEGnet model used in this study applies depthwise separable convolutional layers to optimize EEG feature extraction, encompassing spatial filtering and filter bank construction. Notably, the analysis demonstrated that N2 amplitudes elicited by neutral stimuli achieved the highest classification accuracy (92.31%) for predicting clinical outcomes of NSSI among the ERP components evaluated. The model’s high accuracy indicated a strong signal in the neural data that distinguishes outcome groups, supporting the ANOVA analysis results. These findings establish N2 amplitudes as reliable indicators for identifying clinical outcomes of NSSI, contributing to early detection and intervention strategies. This study suggests that N2 amplitude could serve as a biomarker for predicting NSSI outcomes, offering a potential avenue for developing interventions to reduce addiction risk. Improving attention control by addressing deficits in the N2 amplitude may effectively mitigate the risk of addiction in individuals with NSSI. The model demonstrated high accuracy, but this is a preliminary result from a small sample. Further research with larger groups is needed to confirm its generalizability. Given all that, cognitive control deficits are likely a central feature in adolescents with ongoing NSSI. Therefore, therapies aimed at enhancing executive function and emotion regulation, such as cognitive-behavioral therapies focused on impulse control and emotional processing, may aid in managing NSSI. Moreover, specialized cognitive training programs could be designed to tackle these deficits, though more studies are required to confirm their efficacy.
Behavioral data showed no accuracy difference between groups but slower RTs in the AG group, hinting at latent processing disparities despite similar correct-response performance. ERP results, however, revealed significant N2/P3 amplitude differences, indicating distinct task-related neural processing. EEG metrics (N2, P3) directly reflect neural activity during information processing. They can detect neural changes not shown in behavior. Although ERPs have shown differences between adolescents with and without NSSI, using EEG in clinical settings is difficult due to technical and resource constraints. However, these insights enhance our understanding of NSSI mechanisms and could guide future research, like larger studies or trials, to explore if therapeutic interventions improving cognitive control can reduce NSSI occurrence and severity. This study has several limitations. Sample size: small subgroup samples limit analysis. A larger sample would increase confidence and enable in-depth studies of comorbidities or sex differences, as the current one may miss nuanced effects. Population: all participants were Chinese adolescents from one region. Cultural factors impact NSSI. Results may not be global without further studies; future research should include diverse cultures. Follow-up: One-year follow-up may be insufficient for NSSI trajectories as behavior can change. A longer follow-up with larger samples to validate N2 as a stable biomarker and explore its utility in guiding interventions (e.g., cognitive training targeting attention control) is needed for long-term NSSI understanding. Potential confounds: depression, anxiety, medication use and comorbidities during follow-up may affect NSSI outcomes. Future studies should control these variables. Task specificity: the affective Stroop paradigm may not cover all inhibitory control. Different tasks/measures may vary results. Future studies should use multimodal approaches.
Conclusions
This study employed a novel affective Stroop task to examine the neurocognitive mechanisms underlying different NSSI outcomes and to predict these outcomes using ERP components in adolescents. The findings demonstrated that NSSI is associated with abnormal cognitive processing, characterized by impaired attention control and resource allocation. Notably, N2 amplitudes emerged as reliable indicators for identifying clinical outcomes of NSSI. These results provide an objective foundation for identifying NSSI, predicting its developmental progression, and formulating early intervention strategies. To advance this research, we propose: validating these neural markers in a larger, diverse adolescent group with NSSI for better generalizability; conducting long-term studies to evaluate the stability and predictive value of ERP differences for NSSI outcomes; and exploring whether therapy can alter ERP differences to improve treatment understanding and interventions.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Harbin Medical University Daqing Campus. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
FY: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Writing – original draft. FS: Formal analysis, Methodology, Writing – original draft. WJ: Data curation, Investigation, Writing – review & editing. SH: Data curation, Investigation, Writing – review & editing. BW: Data curation, Investigation, Writing – original draft. NY: Investigation, Writing – original draft. JC: Conceptualization, Formal analysis, Methodology, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by the National Natural Science Foundation of China (Grant No. 72204065).
Acknowledgments
The authors thank all the students and schools who participated in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Nock MK. Self-injury. Annu Rev Clin Psychol. (2010) 6:339–63. doi: 10.1146/annurev.clinpsy.121208.131258
2. Ribeiro JD, Franklin JC, Fox KR, Bentley KH, Kleiman EM, Chang BP, et al. Self-injurious thoughts and behaviors as risk factors for future suicide ideation, attempts, and death: a meta-analysis of longitudinal studies. Psychol Med. (2016) 46:225–36. doi: 10.1017/s0033291715001804
3. Wolff JC, Thompson E, Thomas SA, Nesi J, Bettis AH, Ransford B, et al. Emotion dysregulation and non-suicidal self-injury: A systematic review and meta-analysis. Eur Psychiatry. (2019) 59:25–36. doi: 10.1016/j.eurpsy.2019.03.004
4. Lim KS, Wong CH, McIntyre RS, Wang J, Zhang Z, Tran BX, et al. Global lifetime and 12-month prevalence of suicidal behavior, deliberate self-harm and non-suicidal self-injury in children and adolescents between 1989 and 2018: A meta-analysis. Int J Environ Res Public Health. (2019) 16:4581. doi: 10.3390/ijerph16224581
5. Liu RT, Walsh RFL, Sheehan AE, Cheek SM, and Sanzari CM. Prevalence and correlates of suicide and nonsuicidal self-injury in children: A systematic review and meta-analysis. JAMA Psychiatry. (2022) 79:718–26. doi: 10.1001/jamapsychiatry.2022.1256
6. GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. (2020) 396:1204–22. doi: 10.1016/s0140-6736(20)30925-9
7. Li L and Yang H. Heterogeneity in adolescents’ Non-suicidal self-injury behaviour trajectories based on the group-based trajectory model and a decision tree analysis of family-related determinants. Psychol Res Behav Manage. (2023) 16:3359–71. doi: 10.2147/prbm.S427090
8. Mason GE, Auerbach RP, and Stewart JG. Predicting the trajectory of non-suicidal self-injury among adolescents. J Child Psychol Psychiatry. (2025) 66:189–201. doi: 10.1111/jcpp.14046
9. Wang B, You J, Lin MP, Xu S, and Leung F. Developmental trajectories of nonsuicidal self-injury in adolescence and intrapersonal/interpersonal risk factors. J Res Adolesc. (2017) 27:392–406. doi: 10.1111/jora.12273
10. Farrell BCT, Ewing L, and Hamza CA. Examining trajectories of nonsuicidal self-injury across the first year of university. J Affect Disord. (2024) 367:202–9. doi: 10.1016/j.jad.2024.09.003
11. Allen KJD, Fox KR, Schatten HT, and Hooley JM. Frequency of nonsuicidal self-injury is associated with impulsive decision-making during criticism. Psychiatry Res. (2019) 271:68–75. doi: 10.1016/j.psychres.2018.11.022
12. Fikke LT, Melinder A, and Landrø NI. Executive functions are impaired in adolescents engaging in non-suicidal self-injury. Psychol Med. (2011) 41:601–10. doi: 10.1017/s0033291710001030
13. Wen Y, Zhang X, Xu Y, Qiao D, Guo S, Sun N, et al. Cognitive impairment in adolescent major depressive disorder with nonsuicidal self-injury: evidence based on multi-indicator ERPs. Front Hum Neurosci. (2021) 15:637407. doi: 10.3389/fnhum.2021.637407
14. Liu H, Wen Y, Liang X, Xu Y, Qiao D, Yang C, et al. Prefrontal cortex neural activity predicts reduction of non-suicidal self-injury in adolescents with major depressive disorder: An event related potential study. Front Neurosci. (2022) 16:972870. doi: 10.3389/fnins.2022.972870
15. Zhao L, Zhou D, Ma L, Hu J, Chen R, He X, et al. Changes in emotion-related EEG components and brain lateralization response to negative emotions in adolescents with nonsuicidal self-injury: An ERP study. Behav Brain Res. (2023) 445:114324. doi: 10.1016/j.bbr.2023.114324
16. Li F, Chen B, Li H, Zhang T, Wang F, Jiang Y, et al. The time-varying networks in P300: A task-evoked EEG study. IEEE Trans Neural Syst Rehabil Eng. (2016) 24:725–33. doi: 10.1109/tnsre.2016.2523678
17. Zinchenko A, Kanske P, Obermeier C, Schröger E, and Kotz SA. Emotion and goal-directed behavior: ERP evidence on cognitive and emotional conflict. Soc Cognit Affect Neurosci. (2015) 10:1577–87. doi: 10.1093/scan/nsv050
18. Heidlmayr K, Kihlstedt M, and Isel F. A review on the electroencephalography markers of Stroop executive control processes. Brain Cogn. (2020) 146:105637. doi: 10.1016/j.bandc.2020.105637
19. Vanlessen N, Rossi V, De Raedt R, and Pourtois G. Positive emotion broadens attention focus through decreased position-specific spatial encoding in early visual cortex: evidence from ERPs. Cognit Affect Behav Neurosci. (2013) 13:60–79. doi: 10.3758/s13415-012-0130-x
20. Raschle NM, Fehlbaum LV, Menks WM, Euler F, Sterzer P, and Stadler C. Investigating the neural correlates of emotion-cognition interaction using an affective stroop task. Front Psychol. (2017) 8:1489. doi: 10.3389/fpsyg.2017.01489
21. Scarpina F and Tagini S. The stroop color and word test. Front Psychol. (2017) 8:557. doi: 10.3389/fpsyg.2017.00557
22. Uher R, Brooks SJ, Bartholdy S, Tchanturia K, and Campbell IC. Increasing cognitive load reduces interference from masked appetitive and aversive but not neutral stimuli. PloS One. (2014) 9:e94417. doi: 10.1371/journal.pone.0094417
23. Chen L, Lui S, Wu QZ, Zhang W, Zhou D, Chen HF, et al. Impact of acute stress on human brain microstructure: An MR diffusion study of earthquake survivors. Hum Brain Mapp. (2013) 34:367–73. doi: 10.1002/hbm.21438
24. Flaisch T, Steinhauser M, and Schupp HT. Adaptive cognitive control attenuates the late positive potential to emotional distractors. Neuroimage. (2019) 200:51–8. doi: 10.1016/j.neuroimage.2019.06.040
25. Dragan WU, Dragan WU, Sokoowski A, Sokoowski A, Folkierska-Ukowska M, and Folkierska-Ukowska M. Temperament and neural activation during the affective Stroop task: A functional connectivity study. Pers Individ Dif. (2022) 186:111385–. doi: 10.1016/j.paid.2021.111385
26. Washburn JJ, Juzwin KR, Styer DM, and Aldridge D. Measuring the urge to self-injure: preliminary data from a clinical sample. Psychiatry Res. (2010) 178:540–4. doi: 10.1016/j.psychres.2010.05.018
27. Luijten M, Machielsen MW, Veltman DJ, Hester R, de Haan L, and Franken IH. Systematic review of ERP and fMRI studies investigating inhibitory control and error processing in people with substance dependence and behavioural addictions. J Psychiatry Neurosci. (2014) 39:149–69. doi: 10.1503/jpn.130052
28. Drummond DC. What does cue-reactivity have to offer clinical research? Addiction. (2000) 95 Suppl 2:S129–44. doi: 10.1080/09652140050111708
29. Burke TA, Allen KJD, Carpenter RW, Siegel DM, Kautz MM, Liu RT, et al. Emotional response inhibition to self-harm stimuli interacts with momentary negative affect to predict nonsuicidal self-injury urges. Behav Res Ther. (2021) 142:103865. doi: 10.1016/j.brat.2021.103865
30. Ho CSH, Wang J, Tay GWN, Ho R, Husain SF, Chiang SK, et al. Interpretable deep learning model for major depressive disorder assessment based on functional near-infrared spectroscopy. Asian J Psychiatr. (2024) 92:103901. doi: 10.1016/j.ajp.2023.103901
31. Tian X, Zhu L, Zhang M, Wang S, Lu Y, Xu X, et al. Social anxiety prediction based on ERP features: A deep learning approach. J Affect Disord. (2024) 367:545–53. doi: 10.1016/j.jad.2024.09.006
32. Lawhern VJ, Solon AJ, Waytowich NR, Gordon SM, Hung CP, and Lance BJ. EEGNet: a compact convolutional neural network for EEG-based brain-computer interfaces. J Neural Eng. (2018) 15:056013. doi: 10.1088/1741-2552/aace8c
33. Polich J. Updating P300: an integrative theory of P3a and P3b. Clin Neurophysiol. (2007) 118:2128–48. doi: 10.1016/j.clinph.2007.04.019
34. Gordon SM, Lawhern V, Passaro AD, and McDowell K. Informed decomposition of electroencephalographic data. J Neurosci Methods. (2015) 256:41–55. doi: 10.1016/j.jneumeth.2015.08.019
35. Lotte F. Signal processing approaches to minimize or suppress calibration time in oscillatory activity-based brain–computer interfaces. Proc IEEE. (2015) 103:871–90. doi: 10.1109/JPROC.2015.2404941
36. American Psychiatric Association K. Diagnostic and statistical manual of mental disorders: DSM-5. Vol. 5 Vol. 5. . Washington, DC: American psychiatric association (2013).
37. Wang YM, Chen J, and Han BY. The effects of cognitive reappraisal and expressive suppression on memory of emotional pictures. Front Psychol. (2017) 8:1921. doi: 10.3389/fpsyg.2017.01921
38. Yuan JJ, Yang JM, Meng XX, Yu FQ, and Li H. The valence strength of negative stimuli modulates visual novelty processing: electrophysiological evidence from an event-related potential study. Neuroscience. (2008) 157:524–31. doi: 10.1016/j.neuroscience.2008.09.023
39. Keil A, Debener S, Gratton G, Junghöfer M, Kappenman ES, Luck SJ, et al. Committee report: publication guidelines and recommendations for studies using electroencephalography and magnetoencephalography. Psychophysiology. (2014) 51:1–21. doi: 10.1111/psyp.12147
40. Semlitsch HV, Anderer P, Schuster P, and Presslich O. A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology. (1986) 23:695–703. doi: 10.1111/j.1469-8986.1986.tb00696.x
41. Wang X, Shangguan C, and Lu J. Time course of emotion effects during emotion-label and emotion-laden word processing. Neurosci Lett. (2019) 699:1–7. doi: 10.1016/j.neulet.2019.01.028
42. Folstein JR and Van Petten C. Influence of cognitive control and mismatch on the N2 component of the ERP: a review. Psychophysiology. (2008) 45:152–70. doi: 10.1111/j.1469-8986.2007.00602.x
43. Baetens K, der Cruyssen LV, Achtziger A, Vandekerckhove M, and Van Overwalle F. N400 and LPP in spontaneous trait inferences. Brain Res. (2011) 1418:83–92. doi: 10.1016/j.brainres.2011.08.067
44. Hart SJ, Green SR, Casp M, and Belger A. Emotional priming effects during Stroop task performance. Neuroimage. (2010) 49:2662–70. doi: 10.1016/j.neuroimage.2009.10.076
45. Yuan J, Zhang Q, Chen A, Li H, Wang Q, Zhuang Z, et al. Are we sensitive to valence differences in emotionally negative stimuli? Electrophysiological evidence from an ERP study. Neuropsychologia. (2007) 45:2764–71. doi: 10.1016/j.neuropsychologia.2007.04.018
46. Hanslmayr S, Pastötter B, Bäuml KH, Gruber S, Wimber M, and Klimesch W. The electrophysiological dynamics of interference during the Stroop task. J Cognit Neurosci. (2008) 20:215–25. doi: 10.1162/jocn.2008.20020
47. Xinyu G, Yunyun L, Qin Z, Lixia C, and Ping W. Weakening of recognition memory caused by task-irrelevant emotional encoding context can be modulated by individuals’ inhibitory control. Pers Individ Dif. (2018) 134:201–9. doi: 10.1016/j.paid.2018.06.012
48. Yang K, Zeng Y, Tong L, Hu Y, Zhang R, Li Z, et al. Extremely negative emotion interferes with cognition: Evidence from ERPs and time-varying brain network. J Neurosci Methods. (2023) 396:109922. doi: 10.1016/j.jneumeth.2023.109922
49. Senderecka M. Emotional enhancement of error detection-The role of perceptual processing and inhibition monitoring in failed auditory stop trials. Cognit Affect Behav Neurosci. (2018) 18:1–20. doi: 10.3758/s13415-017-0546-4
50. Yu F, Zhou X, Qing W, Li D, Li J, Chen X, et al. Decreased response inhibition to sad faces during explicit and implicit tasks in females with depression: Evidence from an event-related potential study. Psychiatry Res Neuroimaging. (2017) 259:42–53. doi: 10.1016/j.pscychresns.2016.10.013
51. Duncan CC, Kosmidis MH, and Mirsky AF. Closed head injury-related information processing deficits: an event-related potential analysis. Int J Psychophysiol. (2005) 58:133–57. doi: 10.1016/j.ijpsycho.2005.05.011
52. Zhou DD, Zhao L, Ma LL, Hu JH, Chen R, Jiang ZH, et al. Altered neural reactivity in adolescents with nonsuicidal self-injury during exposure to self-injury related cues: electrophysiological evidence from a two-choice oddball paradigm. Front Psychiatry. (2022) 13:827480. doi: 10.3389/fpsyt.2022.827480
53. Baker A, Mystkowski J, Culver N, Yi R, Mortazavi A, and Craske MG. Does habituation matter? Emotional processing theory and exposure therapy for acrophobia. Behav Res Ther. (2010) 48:1139–43. doi: 10.1016/j.brat.2010.07.009
54. Ahumada-Méndez F, Lucero B, Avenanti A, Saracini C, Muñoz-Quezada MT, Cortés-Rivera C, et al. Affective modulation of cognitive control: A systematic review of EEG studies. Physiol Behav. (2022) 249:113743. doi: 10.1016/j.physbeh.2022.113743
55. Liu J, Wang H, Xing S, and Liu X. Sensitivity to reward and punishment in adolescents with repetitive non-suicidal self-injury: The role of inhibitory control. Int J Clin Health Psychol. (2024) 24:100456. doi: 10.1016/j.ijchp.2024.100456
Keywords: non-suicidal self-injury, EEG, developmental outcomes, EEGNET, ERP, cognitive control, adolescent
Citation: Yin F, Si F, Jiang W, Huo S, Wang B, Yang N and Cao J (2025) N2 and P3 responses in adolescents with non-suicidal self-injury and their association with clinical outcomes: a cohort follow-up study based on the affective stroop paradigm. Front. Psychiatry 16:1564049. doi: 10.3389/fpsyt.2025.1564049
Received: 21 January 2025; Accepted: 09 June 2025;
Published: 24 June 2025.
Edited by:
Eduardo Fernández-Jiménez, European University of Madrid, SpainReviewed by:
Abdulhakim Al-Ezzi, Huntington Medical Research Institutes, United StatesRahima Dahlan, Universiti Putra Malaysia, Malaysia
Copyright © 2025 Yin, Si, Jiang, Huo, Wang, Yang and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianqin Cao, Y2pxaG11QDE2My5jb20=
 Fei Yin
Fei Yin Feng Si
Feng Si Wenlong Jiang3
Wenlong Jiang3