- 1Campbell Family Mental Health Research Institute, Centre for Addiction and Mental Health, Toronto, ON, Canada
- 2Adult Neurodevelopment and Geriatric Psychiatry Division, Centre for Addiction and Mental Health, Toronto, ON, Canada
- 3Department of Psychiatry, Temerty Faculty of Medicine, University of Toronto, Toronto, ON, Canada
- 4Temerty Centre for Therapeutic Brain Intervention, Centre for Addiction and Mental Health, Toronto, ON, Canada
- 5Biostatistics Core, Centre for Addiction and Mental Health, Toronto, ON, Canada
- 6College of Public Health, University of South Florida, Tampa, FL, United States
- 7Harquial Centre for Neuromodulation, Sunnybrook Health Sciences Centre, Toronto, ON, Canada
- 8Research and Development, Soterix Medical Inc., Woodbridge, NJ, United States
- 9Department of Psychology, Queen’s University, Kingston, ON, Canada
- 10Department of Medical Biophysics, University of Toronto, Toronto, ON, Canada
- 11Physical Sciences Platform, Sunnybrook Research Institute, Toronto, ON, Canada
- 12Toronto Dementia Research Alliance, University of Toronto, Toronto, ON, Canada
- 13Department of Psychiatry, O’Donnell Brain Institute, University of Texas Southwestern Medical Center, Dallas, TX, United States
Mild Cognitive Impairment (MCI) is a clinical prodromal stage of Alzheimer’s disease. Enhancing executive functions in patients with MCI could optimize cognitive compensatory mechanisms and slow cognitive decline. The prefrontal cortex (PFC) and its connections to the hippocampus support executive functions, including working memory. Transcranial alternating current stimulation (tACS) can modulate these connections by engaging theta-gamma coupling (TGC) and may thereby strengthen working memory. This study, “tACS to engage theta-gamma coupling and enhance working memory in MCI” (tACS-MCI), will assess the feasibility and cognitive effects of EEG and MRI-guided individualized tACS. The stimulation will target the prefrontal and temporal cortices in 20 MCI participants. Participants will be randomized to receive either individualized tACS or sham tACS for 10 days. tACS individualization will involve adjusting the theta frequency, tACS electrode locations, and current intensity for each participant. Cognitive and functional assessments will occur at baseline and post-intervention. We aim to determine: 1) the feasibility of individualized tACS in MCI, including recruitment and retention; 2) whether tACS engages TGC by assessing its increase in response to tACS; and 3) changes in N-back working memory performance following tACS, as well as whether changes in TGC mediate the changes in performance. The tACS-MCI study will employ an EEG and MRI-guided individualized approach to promote synchronization between frontal and temporal cortices, using participant’s unique brain structure and neurophysiology. We aim to assess the feasibility of this novel intervention as a potential approach to more effectively prevent cognitive decline.
Introduction
Dementia is a progressive loss of cognitive and behavioral abilities that significantly impacts quality of life. Alzheimer’s disease (AD) is the most common cause of dementia among older adults (1). However, by the time AD symptoms become evident it may be too late to slow progression of the neurodegeneration. Thus, identifying effective strategies to prevent and slow the progression of AD is crucial.
For prevention and early intervention in AD, we propose this study that is focused on Mild Cognitive Impairment (MCI), i.e., the clinical prodromal stage (2). Specifically, in MCI, the prefrontal cortex (PFC) enables compensatory mechanisms that could delay progression to AD (3, 4) via executive functioning (5, 6). MCI patients with higher executive functions perform better on verbal memory than those with low executive functions (3, 7). MCI patients’ verbal memory performance is also associated with PFC thickness beyond any association with the temporal cortex (3). Impairments in executive function (4, 8) and working memory (9, 10) drive progression from MCI to AD. Thus, interventions that enhance executive function and working memory could prevent or delay progression from MCI to AD.
Executive functions in general and working memory in particular are supported by the PFC and its connections to other parts of the brain, including hippocampus and temporal cortex (11–13). Among these connections, those connecting the PFC to the hippocampus either directly or through intermediary regions are critical (14). The role of the PFC-hippocampus connections has been established in several studies where disconnecting the PFC and the hippocampus in rodents results in working memory deficits (15–20). Working memory refers to the ability to manipulate and maintain items of information within a short period of time (21, 22). In our heuristic model, neuronal assemblies generating gamma oscillations represent these items of information (23). Further, the hippocampus entrains PFC theta oscillations (24, 25), causing synchrony between the hippocampus and PFC. This synchrony drives the order of activation of the neuronal assemblies, and thus, provides the temporal context for the PFC to manipulate information (26–28). Using electroencephalography (EEG), this process results in the coupling of the amplitude of gamma oscillations to the phase of theta oscillations (theta-gamma coupling, TGC) during a working memory task (29, 30).
Preclinical (27, 31) and clinical neurophysiology studies, including those in MCI and AD (30, 32–34) show that modulation of TGC supports executive functions, particularly working memory. A study using intracranial EEG in humans with epilepsy has also shown that frontal and hippocampal activity are coordinated via TGC during working memory (34). Accordingly, our study aims at enhancing TGC to support working memory in MCI. One approach to enhance TGC is using transcranial alternate current stimulation (tACS). tACS has been shown to engage TGC and, through this engagement, enhance working memory in healthy older adults (35). In that study, a high-definition tACS system with an M × N nine-channel configuration was utilized. Target areas included the left PFC and the left temporal cortex. The alternating current was in-phase across the two targeted regions (i.e., 0° relative phase difference) to promote network synchronization. While participant-specific theta frequency was applied for each participant (“frequency-individualized stimulation”), stimulation intensity and electrode montage remained non-individualized and a generic brain MRI was used to optimize electrode placement across participants (“non-individualized optimized stimulation”) (35). However, given anatomical variability across participants specifically in MCI participants with potential brain structural atrophy, this frequency-only individualization may not achieve the required electrical field and sufficient dosing, for each individual target to elicit measurable neurophysiological effects (36–38).
Given the high inter-individual variability in tACS response documented in the literature, we developed a fully individualized approach. Utilizing individual EEG and structural MRI data, we will determine individualized theta frequency for delivering tACS to enhance synchronization between PFC and temporal cortices using individualized electrode montage (i.e., electrode locations and their currents based on each participant’s unique brain MRI) to ensure that the maximum electric field reaches the specified targets (“EEG and MRI-guided individualized stimulation”). We will then deliver this EEG and MRI-guided individualized tACS bilaterally, considering that compensatory mechanisms involve both the left and right PFC (6). There is also evidence of neural modulation by tACS when applied bilaterally (39, 40). We believe that this study will be instrumental in furthering potential preventive strategies for cognitive decline in MCI, as no study to date has assessed the effect of EEG and MRI-guided individualized tACS on TGC and cognitive function in AD or MCI.
Design
Overview
We propose to investigate the feasibility and preliminary effects of EEG and MRI-guided individualized tACS on TGC and working memory in MCI participants. This proof-of-concept study will test whether tACS engage TGC as a target and, in turn, enhance working memory. We aim to (1): determine the feasibility of tACS in older individuals with MCI and examine recruitment and retention (Objective 1) (2); determine TGC engagement in response to tACS by assessing whether TGC increases in response to tACS during a working memory task (Objective 2); and (3) assess change in working memory in response to tACS, and whether changes in TGC mediate changes in working memory performance (Objective 3).
We hypothesize that at least 30% of screened participants will agree and be eligible to receive the intervention they are assigned to (Hypothesis 1a (H1a)); at least 70% of participants will attend at least 80% of their treatment sessions (H1b); participants randomized to tACS will experience higher increase in TGC than those randomized to sham-tACS (H2); participants randomized to tACS will experience more improvement on working memory from baseline following the intervention than those randomized to sham-tACS (H3a); and across all participants, change in TGC will mediate change in working memory performance (H3b).
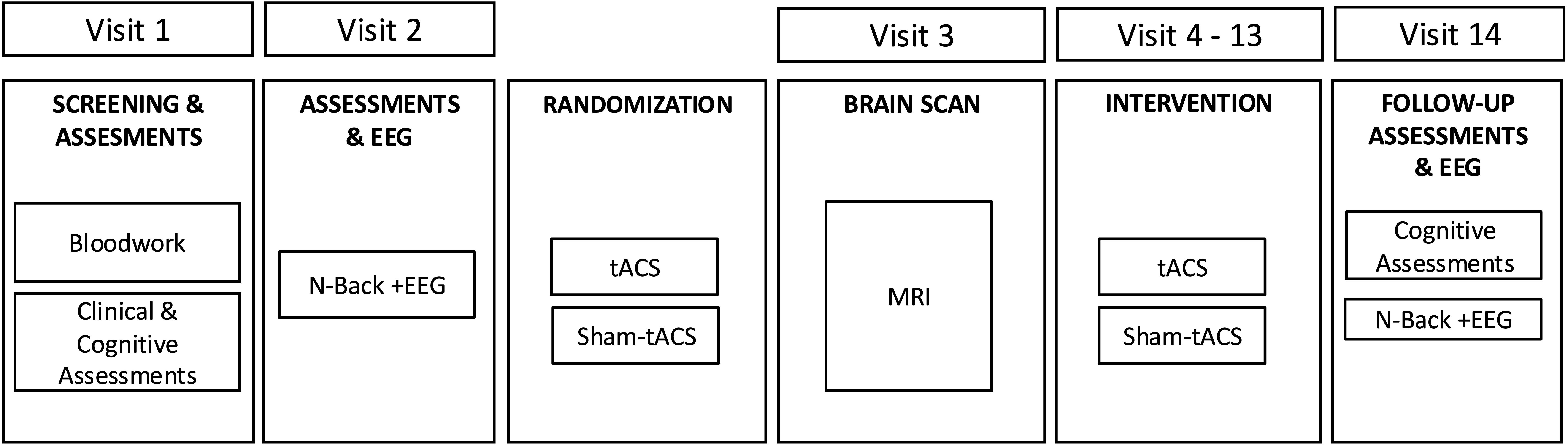
We will randomize 20 MCI participants to receive either EEG and MRI-guided individualized tACS or sham-tACS (1:1). Sequential bilateral tACS or sham-tACS will be delivered to the PFC and temporal cortices. Each participant will receive daily stimulation for 5 days per week for two weeks. Working memory and TGC during working memory performance will be assessed at baseline and after the last intervention session. Working memory will be assessed using the N-back task (41) with 2-back d’ index being the primary measure as justified by our preliminary findings (30, 32, 42). TGC will be assessed using EEG during N-back performance. Figure 1 represents the overall study design.
Participants
Participants will meet the following inclusion criteria
1) Age 60 years or above, 2) Diagnosis of MCI due to AD using the core clinical criteria by the National Institute on Aging and Alzheimer’s Association for MCI participants (NIA-AA) (43) and ascertained by a study investigator, 3) Objective evidence of single or multi domain MCI using a comprehensive neuropsychological battery, 4) willingness to provide informed consent, 5) ability to read and communicate in English (with corrected vision and hearing, if needed).
Participants will also be excluded if they meet the following exclusion criteria
1) Current use of an acetylcholine esterase inhibitor or memantine, 2) Major Depressive Disorder with active symptoms in the last 3 months, 3) a lifetime diagnosis of bipolar disorder; intellectual disability; or a psychotic disorder, 4) substance use disorder active in the last 3 months, 5) any other DSM-5 (44) diagnosis that may be associated with prefrontal cortical dysfunction as ascertained using the study investigator opinion, 6) current anticonvulsant use due to its impact on brain stimulation induced activity. An exception will be made if they are taking gabapentin or pregabalin AND if the dose had been stable for at least 4 weeks prior to study entry AND if prescribed for chronic pain, 7) current benzodiazepine use of more than what is equivalent to lorazepam 2 mg/day. This is due to their known pro-GABAergic activity and the suppressive effect of GABAergic agents on cortical plasticity, and 8) any contraindication to MRI or contraindication to tACS (e.g., cardiac pacemaker, acoustic device, history of seizures) (45, 46).
Assessments and outcome measures
Baseline assessments
Clinical and cognitive assessments
Participants will undergo the following assessments
Clinical: MINI International Neuropsychiatric Interview (MINI) (47) or Structured Clinical Interview for DSM-5 (SCID) (48) to ascertain eligibility, and the Clinical Dementia Rating Scale (49) (CDR) to assess current functional status. Participants will also be asked to provide results from clinical blood tests undertaken within the previous 6 months or undertake new lab tests prior to commencing the study. The required lab tests will be complete blood count (CBC), Sodium, Potassium, Chloride, Bicarbonate, Urea, Creatinine, Alanine Aminotransferase (ALT), Aspartate Aminotransferase (AST), Alkaline Phosphatase (ALP), Gamma-Glutamyl Transferase (GGT), Cholesterol, High-Density Lipoprotein (HDL), Thyroid-Stimulating Hormone (TSH), and Vitamin B12, as these could be related to aging and cognitive decline.
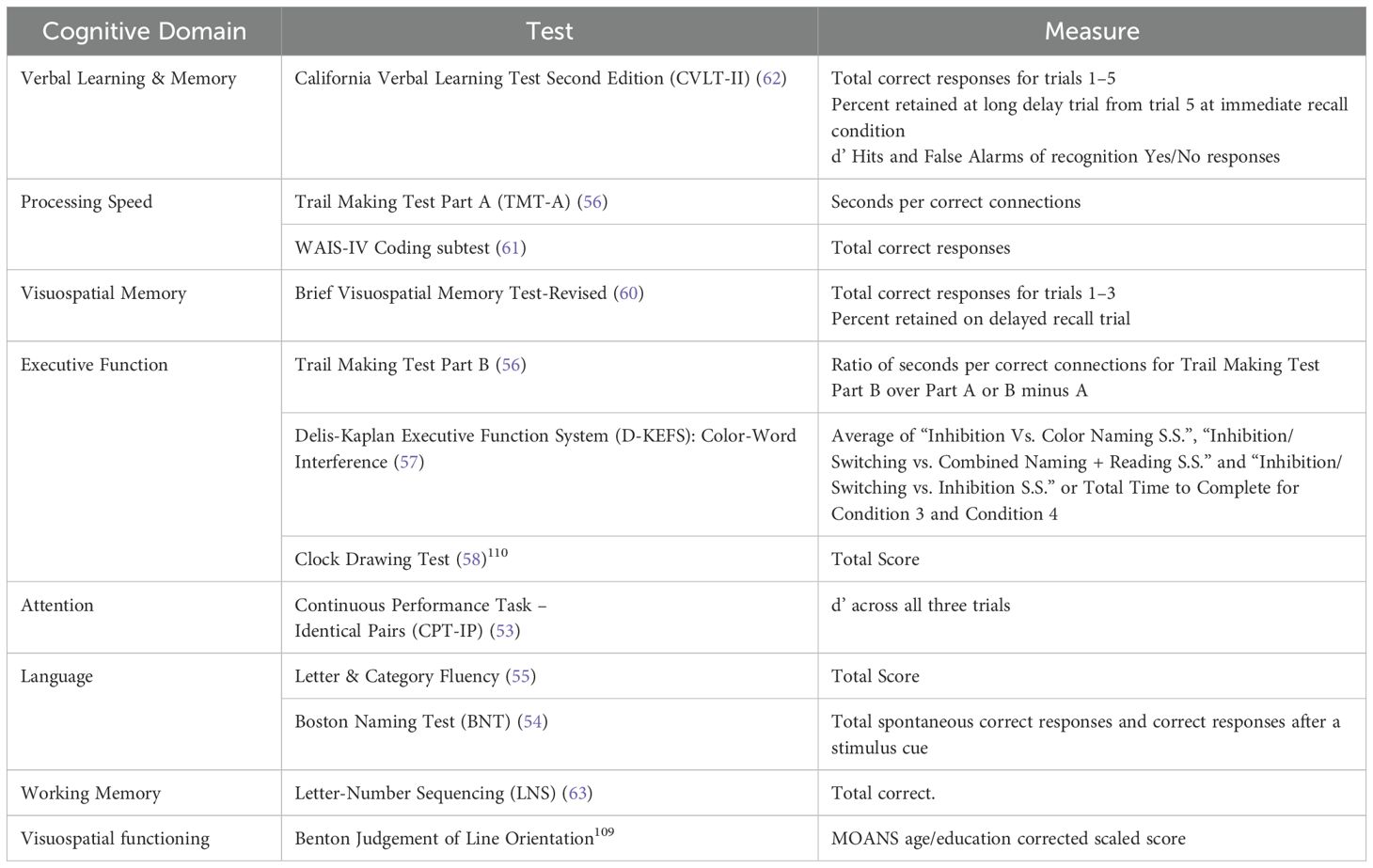
Cognitive (other than N-back): Participants will be tested using the Montreal Cognitive Assessment (MoCA) (50) to ascertain eligibility and the Wide Range Achievement Test-4 (WRAT) Reading Recognition Subtest (51) to estimate premorbid IQ for the interpretation of the cognitive scores. We will also use a neuropsychological battery that is well-established, standardized, reliable, and well-tolerated by older adults with various brain disorders, including MCI as reported in our recent publication (30, 52), and is also used in two ongoing clinical trials in MCI by our team (NCT02386670; NCT04583215). We will assess the following domains with the following tests: Attention (Continuous Performance Task – Identical Pairs (53)); Language (Boston Naming Test (54), Letter & Category Fluency (55)); Executive Function (Trail Making Test Par B (56), Delis-Kaplan Executive Function System (D-KEFS): Color-word interference (57); Clock Drawing (58)); Visuospatial Functioning (Benton Judgement of Line Orientation (59)); Visuospatial Memory (Brief Visuospatial Memory Test-Revised (60)); Processing Speed (Trail Making Test Part A (56), WAIS-IV Coding Subtest (61)); Verbal Learning and Memory (California Verbal Learning Test (62)); and Working Memory (Letter-Number Sequencing (63)). The battery takes about 2 hours to complete and will be administered on Day 1 as part of the baseline assessments. Participants’ performance on the battery will also be used to confirm eligibility and the type of MCI. For each domain, a composite z-score will be generated using performance on each individual test within the domain. The NP tests will be repeated at the follow-up visit to evaluate cognitive performance before and after the brain intervention. Table 1 represents the outcome measures from the neuropsychological battery.
The N-back task: Following the baseline assessments, participants will undergo an N-back-EEG as we have done previously (64). During the N-back the participant is presented continuously on a computer screen with a series of letters, one at a time. For every letter, the participant has to determine whether this specific letter matches (i.e., a target letter) or not (i.e., a non-target letter) the letter that was presented N trials back, with N being 1, 2 or 3 depending on the session (Figure 2A). The N-back task assesses working memory capacity, maintenance and manipulation of information and is sensitive to PFC dysfunction (41). It has good test-retest reliability and minimal practice effects (65), which makes it suitable to assess the effect of the intervention.
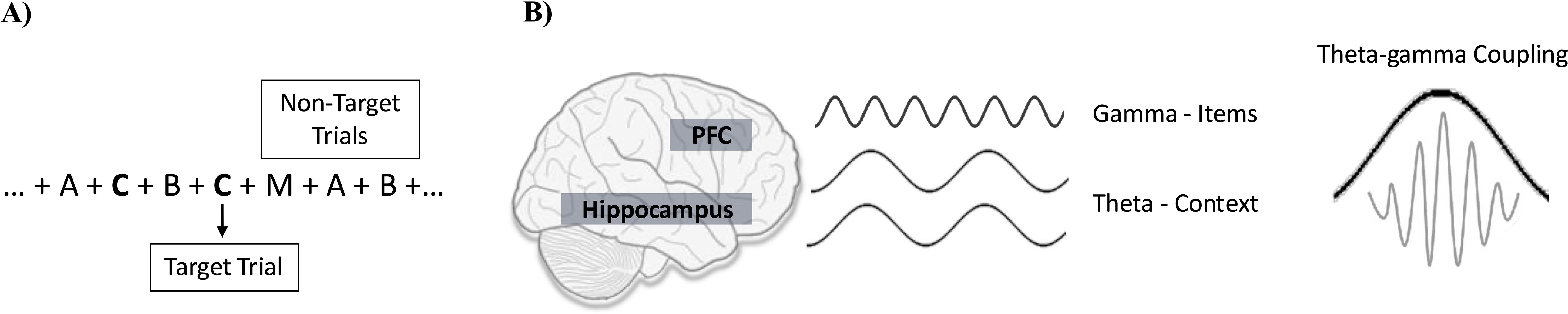
Figure 2. N-back task (2-back) and TGC. (A) 2-back task: To respond correctly, the participant must hold in memory all recent letters which are represented by gamma oscillations in the PFC. In addition, the participant has to hold these letters “in mind” in the correct order. The correct order depends on the N-back condition which determines the time span (1, 2, or 3 letters back) over which the order needs to be maintained. It also depends on the new letter that is presented because every new letter triggers the updating of the order since it needs to be added to the list of the recently presented ones. (B) TGC: During the N-back task, participants are required to recall the sequential order of letters presented on the screen. Each letter is represented by a distinct pattern of neuronal activation within the prefrontal cortex (PFC), resulting in unique gamma oscillations. These neuronal assemblies are linked to a larger assembly spanning PFC-hippocampus connections, which encodes the order of letter presentation. This synchronized activation couples gamma oscillations to specific phases of theta oscillations, known as Theta-Gamma Coupling (TGC). Ultimately, TGC contribute to accurate recollection of letter sequences.
The N-back-EEG will then be repeated after the 10-session course. The primary outcome measures will be based on the 2-back condition as justified by our preliminary findings (30, 32, 42). The N-back accuracy will be assessed using d’, a sensitivity index based on the z scores of the hit and false alarm rates using the following formula: d’ = z(H)- z(FA) where H is the hit rate and FA is the false alarm rate. We will use this index for randomization to active vs sham (see below).
Measuring TGC: EEG data will be collected using a 64-channel Synamps 2 EEG system and the 10–20 montage system while participants complete the N-back task. The reference electrode will be placed posterior to Cz. The sampling rate for collecting EEG data will be 1 kHz and we will apply DC and low pass filter of 100 Hz to the signal. Then we will perform preprocessing using MATLAB (The MathWorks, Inc.) and EEGLAB following established methodologies (29, 30, 32). To calculate TGC, we will first extract theta (4–7 Hz) and gamma (30–50 Hz) oscillations using second-order zero-phase shift filters. We then calculate theta phase and gamma amplitude using Hilbert transform. Since a longer signal ensures the reliability and stability of the TGC value, we will concatenate the epochs to reach to a signal of 5,000 ± 150 ms (29). We will then compute TGC by segmenting theta phases into eighteen 20° intervals and constructing a phase-amplitude distribution function by averaging gamma amplitudes across these intervals. TGC values will then be determined by comparing the observed amplitude distribution against a uniform distribution (29):
Let N denote the quantity of phase bins, where log (N) denotes the entropy of a uniform distribution. P represents the relative amplitude distribution arranged by phase bins, and H (P) indicates the entropy of the P distribution, computed as follows:
Increased coupling correlates with reduced entropy, leading to a higher TGC value. TGC is computed for each electrode, and then averaged across the right and left frontal electrodes (F7/8, F5/F6, F3/4, F1/2, and Fz). For this study, consistent with our previous work, we will use TGC as measured across target trials as target trials require a higher degree of ordering for correct performance, and thus, a higher level of TGC (Figure 2B) (29).
Randomization
Participants will be assigned to one of the two treatment arms: tACS or sham-tACS. Given the importance to balance gender and baseline 2-back performance, and the relatively small sample size of the trial, we will use a dynamic allocation method, known as the covariate-adaptive randomization, to minimize the imbalance across the treatment arms (66). This method is advantageous over the conventional stratified randomization because it attains balance over more covariates with smaller sample size. It does increase the complexity of the design and the subsequent analyses. However, the current literature agrees that the benefit of this method overweighs its limitations in general (66). In detail, when a new participant enters the trial, we will calculate the degree of imbalance for each of the two possible assignments and the one that achieves the least total imbalance will be chosen with a higher probability. If there are assignments of the same least total imbalance, one of them will be chosen randomly. The degree of imbalance of the covariates of interest, gender and baseline 2-back performance, is calculated separately using standardized measure of distance across the two arms and the total imbalance is the sum of the imbalance scores.
Intervention
Individualized transcranial alternating current stimulation
The alternating-current stimulation will be administered using M × N high-definition tACS stimulator (Soterix Medical). tACS will include multiple sintered 12 mm diameter Ag/AgCl electrodes that will be attached to high-definition plastic holders which will be embedded in a cap. The bipolar sinusoidal alternating current will be delivered to the PFC and temporal regions on both sides sequentially, randomly starting on the left or right side. The frequency of stimulation will be at the individually defined theta oscillation determined for each participant.
To fully individualize the approach adopted by Reinhart and Nguyen (35) in calculating theta frequency, we will use individuals’ own MRI alongside EEG. We will use the baseline N-back-EEG at 2-back condition and identify the endogenous theta peak frequency. To calculate the individualized tACS frequency, we conduct source analysis through a series of steps. Initially, after recording MRI data, we transform it to MNI coordinates. Then, we segment the MRI data into five layers: gray matter, white matter, cerebrospinal fluid (CSF), skull, and scalp. We set the conductivity of the segmented tissues and create a head model. This involves setting the conductivity values for gray matter to 0.33, white matter to 0.14, CSF to 1.79, skull to 0.01, and scalp to 0.43 (S/m). Subsequently, we will create a source model based on MNI template grid (35).
To perform source analysis, we apply the Linearly Constrained Minimum Variance (LCMV) method. LCMV estimates the activity of a specific source while concurrently suppressing contributions from other sources and noise (67). The resulting sources are then mapped to the Automated Anatomical Labeling (AAL) atlas, with a specific focus on identifying regions of interest in the superior frontal cortex and superior temporal regions.
In the source space, we will apply spectral decomposition across the 1 to 30 Hz frequency range with 0.1 Hz intervals for each trial. Data will be segmented to trials from -1,400 ms to 3,100 ms relative to letter onset, using the 2-back target and non-target letter as the stimulus. Using Morlet wavelets (constant center frequency ratio = 14 and cycles = 6), we will assess theta band synchronization between frontal and temporal regions. We will calculate phase-locking value in the source space between the left temporal cortex and left PFC, as well as the right temporal cortex and right PFC. We will examine the difference in phase-locking value between 2-back target and non-target trials that were responded to correctly within the time frame of 0 to response-time relative to the onset of the stimulus. We will focus on target trials as they require a higher degree of ordering for accurate performance compared to non-target trials, which rely on synchronization between temporal and frontal regions (29, 35). Within the theta band, we will identify the frequency with the maximum difference in mean phase-locking value between target and non-target trials on each side (left and right) for each participant. This frequency is then utilized as the target stimulation frequency for the corresponding side. Individualized frequency-tuned stimulation is administered with 0.5 Hz resolution, rounding up from 0.3 Hz (35).
During each session, stimulation on each side will last for 30 minutes with a 60-second ramp-up and ramp-down period. After stimulation is completed on one side, the scalp will be wiped clean, and a new cap will be used to minimize the shunting effect caused by excessive gel on the scalp. Sessions will occur 5 days a week for 2 weeks. During the sham-tACS procedure, the device will have a ramp up of 60 seconds to reach better tolerability, followed by an immediate decrease. At the end of the session, the device undergoes another 60-second ramp-up, followed by a ramp-down. This sham stimulation is designed to elicit the tingling sensation commonly experienced with active stimulation (35, 68, 69).
Optimizing the electrode montage
We will use SimNIBS (70, 71) for optimizing and determining the individualized electrode montage. The process begins with creating the head model based on the structural MRI image. Then, we will create a lead field matrix using default EEG cap in SimNIBS. We will optimize the current and location of stimulation electrodes (i.e., electrode montage) to achieve the maximum electrical field in the 3 mm radios spheres centered at targeted frontal and temporal regions of each hemisphere, specifically the superior frontal gyrus dorsolateral (right and left) and superior temporal gyrus (right and left).
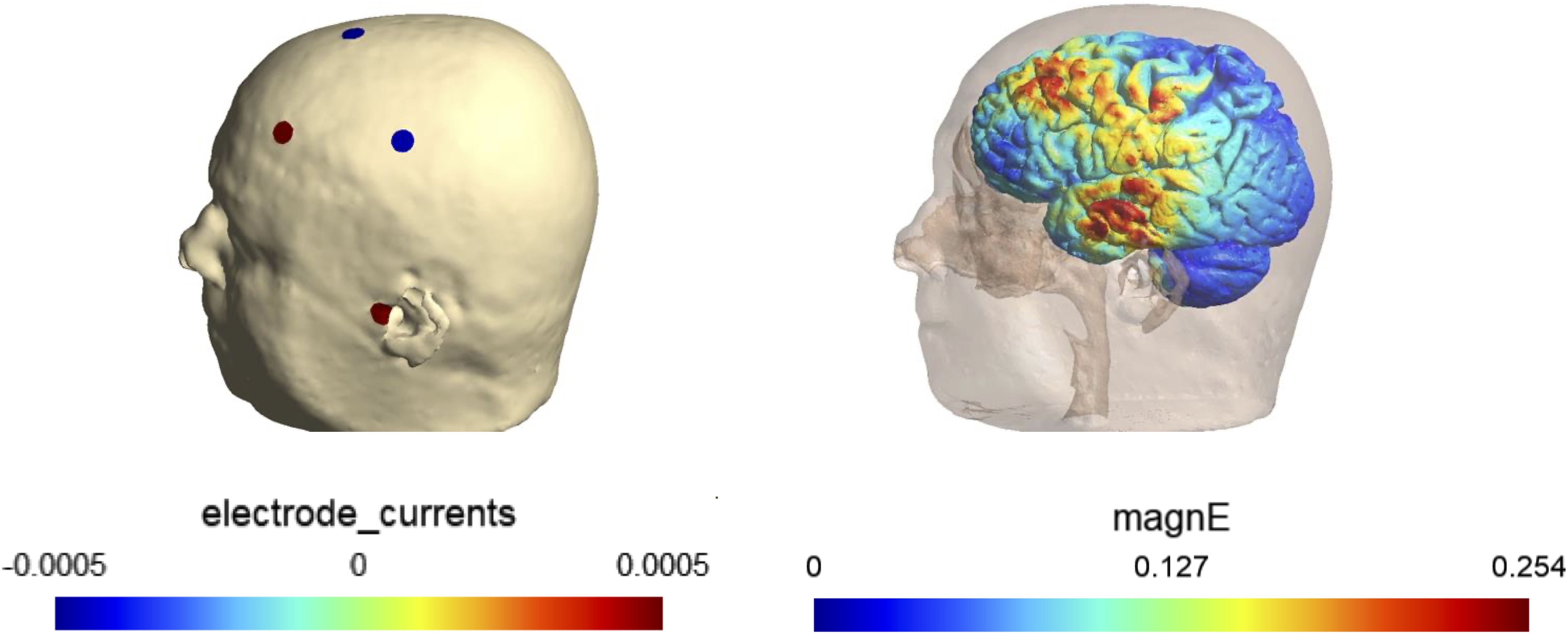
For safety considerations, we will impose constraints on the stimulation parameters. The maximum total current is set to 1 mA (baseline to peak), and the current through any single electrode will not exceed 0.5 mA. Additionally, no more than eight electrodes will be active during the stimulation. The electric field at targets will be calculated using current flow simulation based on the optimized electrode montage. An example of an individualized montage for stimulating left PFC and temporal regions is shown in Figure 3.
The montage is designed to maximize electric filed in PFC and temporal targets based on the individual’s MRI. The electrode montage is shown on the left and the simulated electric field is shown on the right.
Blinding
This study will be conducted under triple-blind conditions (1): Participants will be blind to what intervention they will receive (active or sham) (2); the research assistant delivering the 10-session course will be blind to group assignment; and (3) the research assistant conducting the N-back-EEG assessments and cognitive assessments at baseline and follow-up will also be blind to group assignment. We will assess the participants’ expectancy of treatment outcome using Stanford Expectations of Treatment Scale (SETS) as it can affect their response to intervention (72). It has been shown that participants’ belief can also affect the intervention outcome (73). To evaluate the integrity of the blinding, we will ask participants, interventionists, assessors, and the Principal Investigator, to guess which intervention the participant received, both after the first intervention session and at the completion of the study.
Analytic strategy and power analysis
Sample size determination
This study is designed to generate pilot data for future studies. Thus, no a priori data is used to calculate the sample size. However, with the proposed sample size, we anticipate reliable estimates of recruitment (15.5% margin of error) and retention rate (17.5% margin of error) (Objective 1). For Objectives 2 and 3, we will have 46% power to detect a large treatment effect (Cohen’s d = 1.0) and the minimum detectable effect size to attain 80% power is 1.5. These values are consistent with the pilot nature of the study. We have taken into account a 20% attrition rate and used a 95% confidence interval or two-tailed tests with 0.05 significance level in the power analysis.
Statistical methods
Variables will be first subjected to descriptive analyses. Non-normal data will be transformed, or analyzed using nonparametric procedures. Following intent-to-treat principles, all randomized participants will be considered in the analyses. For Objective 1, we will primarily conduct descriptive analysis to estimate recruitment rate and retention rate using 95% confidence intervals. For Objectives 2 and 3, linear regression will be used to examine the association between TGC engagement and changes in working memory following EEG and MRI-guided individualized tACS courses. Covariates, including demographics and baseline outcome measures, will be incorporated into the model in sensitivity analyses with the understanding that the proposed sample size does not support complex analysis. Full information maximum likelihood method will be employed to account for potential bias incurred by missing data. All missing cases and their reasons will be documented.
Discussion
In this section, we discuss the rationale behind the key components of our study design.
Rationale for tACS
tACS involves delivering an alternating sinusoidal current of approximately 0.5–1 mA baseline-to-peak at a specific frequency based on the brain oscillation the tACS aims to entrain. A usual tACS session lasts between 20 to 60 min. Research has shown that standard tACS delivered at gamma frequency and to the dorsolateral PFC improve recognition in episodic memory (74) and retrieval (75) in healthy young adults. Standard tACS delivered at the theta frequency and to the temporoparietal regions has also been shown to improve long-term memory recognition in healthy younger adults (76, 77) and associative learning in healthy older adults (78). This evidence underscores the potential of tACS as a powerful tool for modulating cognitive functions through targeted brain stimulation.
Few studies have investigated the effects of tACS in patients with AD or MCI. In one study, 30 individuals with multi-domain amnestic MCI were randomized to either home-based frontal theta tACS (6 Hz, current of 1.5 mA baseline-to-peak) combined with cognitive control training (CCT) or control tACS (1 Hz) for 8 sessions. This study reported high tolerability and adherence, along with improvements in attention in the group receiving theta tACS + CCT compared to the control group (79). Another study examined gamma tACS (40 Hz) on individuals (N=13) with amnestic MCI, targeting 8 electrodes (F7, F8, FT7, FT8, T7, T8, P7, and P8; 10–20 EEG system; total current of 1.6 mA baseline-to-peak) over 5 days in the first week and one day per week for the next 3 weeks while performing cognitive tasks. This study found gamma tACS to be feasible in this population and reported improvement in episodic memory, although no changes were observed in fluid biomarkers. Notably, improvements in episodic memory were positively associated with the induced electric field based on current flow modeling (80). In AD participants, a small pilot study (N=8) showed that home-based multi-channel tACS at 40 Hz (6 electrodes and current from each electrode was below 2.0 mA) targeting the left angular gyrus led to improved memory performance without changes in the MoCA score. This study used current flow modeling to optimize the tACS montage for targeting the left angular gyrus, but it used a standard brain model and the montage was not individualized (“non-individualized optimized stimulation”) (81). A larger randomized double-blind study used gamma tACS (current of 1.5 mA baseline-to-peak) in AD participants (N=60). The study found a significant effect of tACS on Rey Auditory Verbal Learning (RAVLT) and face-name association scores in the active group compared to sham tACS group. Using current flow modeling, this study also demonstrated that the induced electric field was associated with clinical outcomes (82). In one non-controlled, open-label, small pilot study (N = 17), 11 participants with mild-to-moderate dementia received tACS (40Hz, current of 0.75 baseline-to-peak) over the left dorsolateral PFC for 30 min twice a day combined with cognitive training sessions, 2 sessions/day for 5 days/week for 4 weeks (83). Compared to those 6 participants who received only training sessions, these 11 participants experienced lasting improvements in memory as assessed at a 1-month follow-up post intervention.
Despite these promising findings, all studies have utilized the same montage and frequency for all participants, which may not be optimal given structural and functional differences between individuals (38, 84). There is promising evidence that individualized electrode montage informed by participants’ structural data, can enhance the efficacy of tACS by addressing inter-individual variability in its effect (85).
tACS and TGC: No study to date has specifically assessed the effect of standard or individualized tACS on TGC and cognitive function in AD or MCI. However, frequency-individualized tACS has been shown to engage TGC and, through this engagement, to enhance working memory in older healthy adults (35). In this study, as expected, young healthy adults demonstrated better working memory than older adults at baseline (i.e., before tACS). In addition, and unlike older adults, they demonstrated theta synchronization between the left PFC and the left temporal cortex during the working memory task as well as TGC across the left temporal electrodes. In contrast, older adults did not exhibit theta synchronization nor TGC. Following frequency-individualized tACS, older adults’ performance improved to a level that became no different from that of younger adults. Further, PFC-temporal theta phase synchronization improved in older adults. Most importantly, TGC emerged over the left temporal electrodes in older adults and the strength of TGC was predictive of their performance on the working memory task post-tACS (35).
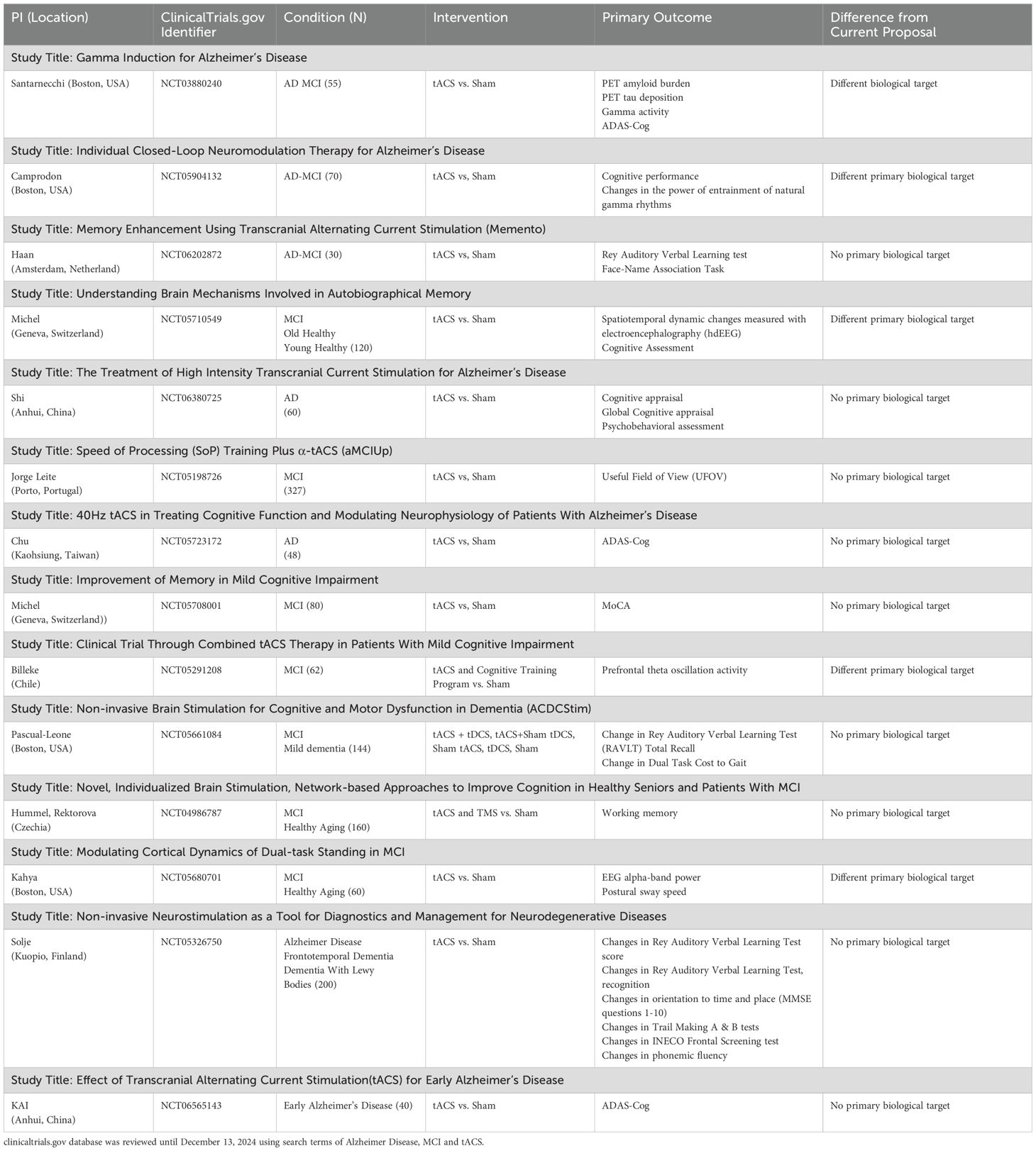
Taken together, the current literature suggests that tACS can enhance cognitive function in both healthy adults and those with AD and MCI. It also suggests that when tACS is individualized to person-specific theta frequency (frequency-individualized stimulation) and target PFC-temporal connections, it can robustly enhance TGC and, in turn, improve working memory. Table 2 summarizes the ongoing tACS studies in MCI and AD populations.
Supporting data for working memory and TGC
Several groups, including ours, have demonstrated that TGC is associated with working memory in healthy (29, 86, 87) and clinical populations, including those with MCI (32). In patients with MCI, we found that TGC during a working memory task is impaired compared to healthy older adults even though these MCI patients were minimally (and not significantly) impaired on performance of the working memory task (32). These findings suggest that TGC in MCI patients is linked to cognitive compensation, which would have to decrease below a specific threshold before behavioral impairment occurs. By enhancing TGC, our goal is to enhance cognitive compensation and, in turn, prevent cognitive decline in patients with MCI.
Further, TGC is not only associated with working memory performance during the execution of the N-back task, but also with performance on other working memory and executive function tasks that require PFC-supported manipulation and context-based ordering of information (30). Importantly, the associations between TGC and performance on these other tasks were present even when TGC and the tasks were administered several weeks apart. This provides evidence for a stable and trait-type relationship between TGC and PFC function (30). We have also demonstrated in a longitudinal study in healthy older individuals that changes in TGC are associated with changes in working memory over a 12-week of follow-up period (88). Finally, we have shown that TGC is associated with working memory performance across different age groups, showing its relevance irrespective of aging (87).
In addition to these cross-sectional and longitudinal studies, intervention studies further support the role of TGC in working memory. In one study, frequency-individualized tACS enhanced TGC in healthy older individuals and, through this enhancement, improved their working memory (35). Another study has shown that using peak-coupled theta–gamma cross-frequency tACS targeting the dorsolateral PFC improves 2-back task in healthy older adults (33). In another study, transcranial direct current stimulation (tDCS) was shown to enhance working memory via TGC enhancement in a group of healthy younger adults (89). Taken together, these studies support that TGC is a promising target to engage for the enhancement of working memory and PFC function, particularly in individuals with MCI.
Support for PFC-hippocampus connections role in working memory and TGC
PFC-hippocampus pathways are well established (14, 90). One key bidirectional connection between the PFC and the hippocampus is via the perirhinal and lateral entorhinal cortices (91, 92). Via this connection, specific item representations (e.g., representations of the new letters during the N-back task) are supported for processing (93, 94). Another key bidirectional connection is via the thalamic nucleus reuniens (Re) (95, 96). The Re is thought to couple the hippocampus and the PFC by synchronizing the two areas (97–99) supporting the transfer of these specific items representations between the hippocampus and PFC for processing. Through these connections, excitatory glutamatergic pyramidal neurons from the hippocampus project and terminate on principle neurons (100, 101) and GABAergic interneurons in the PFC (102, 103). Oscillatory synchrony emerges and the PFC and the hippocampus are coupled, and, in turn, operate as a system in which the PFC receives information that is salient to the current context provided by the hippocampus (104–106).
The hippocampus has been shown to entrain PFC theta oscillations (24, 25),. Over time and as memories become long-term, the interaction between PFC and the hippocampus reverses such as the PFC starts leading the hippocampus in theta oscillations to retrieve long-term memories (107).
In mice, PFC-hippocampus connections have also been shown to support TGC between hippocampal theta and PFC gamma oscillations (31, 108). In one of these studies, these connections have also been shown to support working memory by supporting TGC. Within the mouse PFC, local-field potential recordings demonstrated TGC (31). Further, gamma oscillations within the PFC were coupled to theta oscillations within the hippocampus (31). Interestingly, in a mutant mouse model that demonstrates impairment in working memory, there was an increase in strength of TGC between hippocampal theta and PFC gamma oscillations when these mice performed correctly on the working memory task. By contrast, no changes were observed in local PFC TGC - i.e., TGC between PFC gamma and PFC theta oscillations (31). In addition, firing of PFC neurons was phase-locked to PFC local gamma oscillations that were coupled with hippocampal theta oscillations. These findings strongly support the PFC-hippocampus connection role in mediating a compensatory cognitive mechanism via TGC, i.e., the mechanism that we propose to optimize in our study.
Human studies also support the role of the PFC-hippocampus connections in TGC. In one study, adults with acute depression who received repetitive Transcranial Magnetic Stimulation (rTMS) to the left PFC and experienced reduced clinical symptoms, increased left hippocampal volume, and enhanced TGC over the left central area. Further, changes in TGC were correlated with changes in hippocampal volume (109). Additionally, using intracranial EEG recordings in presurgical epilepsy patients, one study showed an association between TGC within the hippocampus and memory performance (110). Recently, another intracranial EEG study in patients with drug-resistance epilepsy highlighted the role of TGC in coordinating frontal cognitive control and maintenance of information in hippocampus during working memory (34).
Limitations and future directions
There are several areas that warrant further investigation in future work. First, although the use of a sham control condition allows us to assess the feasibility and tolerability of EEG and MRI-guided tACS, it does not fully disentangle the contributions of transcranial versus sensory stimulation. Prior research suggests that gamma tACS, when phase-locked to the trough of theta tACS, does not modulate TGC (111). Building on our findings, future studies focused specifically on underlying mechanisms could incorporate such waveforms to examine their impact on frontal and temporal synchronization and their potential as a control condition to complement or replace sham.
Second, our study employs individualized targeting based on EEG and MRI to optimize specificity and potential efficacy. However, we recognize that in some clinical settings, such methods may not be feasible due to cost or limited access to imaging technologies. Future work could explore whether standardized targeting strategies offer similar benefits to enhance scalability for broader clinical use.
Third, while our stimulation parameters were carefully selected based on modeling, current protocol does not include systematic manipulation of stimulation frequency or anatomical location. Future studies may build on our results by exploring how variations in these parameters influence outcomes.
In conclusion, the overall goal of this study is to use EEG and MRI-guided individualized tACS to strengthen the bidirectional connections between the PFC and the hippocampus. We will stimulate these two regions bilaterally, directly to the PFC and indirectly to the temporal cortices, to optimize their connectivity, enhance TGC, and cognitive performance. The ultimate goal is to enhance overall cognitive performance and mitigate the risk of neurodegenerative processes.
Ethics statement
The studies involving humans were approved by Research Ethics Board - Centre for Addiction and Mental Health. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
MM: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. IP: Writing – original draft, Writing – review & editing. HB: Writing – review & editing. RZ: Methodology, Writing – review & editing. AM: Project administration, Writing – review & editing, Writing – original draft. WW: Methodology, Writing – review & editing. SN: Writing – review & editing. DB: Validation, Writing – review & editing. AD: Resources, Writing – review & editing. CB: Writing – review & editing. BM: Investigation, Project administration, Writing – review & editing. KH: Funding acquisition, Supervision, Writing – review & editing. SK: Supervision, Writing – review & editing. TR: Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work is funded by CAMH Foundation.
Conflict of interest
Author AD was employed by the company Soterix Medical Inc. TR has received research support from Brain Canada, Brain and Behavior Research Foundation, BrightFocus Foundation, Canada Foundation for Innovation, Canada Research Chair, Canadian Institutes of Health Research, Centre for Aging and Brain Health Innovation, National Institutes of Health, Ontario Ministry of Health and Long-Term Care, Ontario Ministry of Research and Innovation, and the Weston Brain Institute. TR also received for an investigator-initiated study in-kind equipment support from Newronika, and in-kind research online accounts from Scientific Brain Training Pro, and participated in 2021 and 2022 in an advisory activity for Biogen Canada Inc. Between September 1, 2023 and September 30, 2024, TR was an ex officio member of the Board of Trustees of the Centre for Addiction and Mental Health CAMH in his role as Chair of the Medical Advisory Committee at CAMH. TR maintains a collaborator scientist and a courtesy appointment at CAMH and a Status-Only appointment at the University of Toronto. TR is also an inventor on the United States Provisional Patent No. 17/396,030 that describes cell-based assays and kits for assessing serum cholinergic receptor activity. DB receives research support from the Canadian Institutes of Health Research CIHR, National Institutes of Health – US NIH, Brain Canada Foundation and the Temerty Family through the CAMH Foundation and the Campbell Family Research Institute. He received research support and in-kind equipment support for an investigator-initiated study from Brainsway Ltd. and he was the site principal investigator for three sponsor-initiated studies for Brainsway Ltd. He received in-kind equipment support from Magventure for investigator-initiated studies. He received medication supplies for an investigator-initiated trial from Indivior. He has participated in an advisory board for Janssen. He has participated in an advisory board for Welcony Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Breijyeh Z and Karaman R. Comprehensive review on alzheimer’s disease: causes and treatment. Molecules (Basel Switzerland). (2020) 25(24):5789. doi: 10.3390/molecules25245789
2. Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol. (2014) 13:614–29. doi: 10.1016/S1474-4422(14)70090-0
3. Chang YL, Jacobson MW, Fennema-Notestine C, Hagler DJ, Jennings RG, Dale AM, et al. Level of executive function influences verbal memory in amnestic mild cognitive impairment and predicts prefrontal and posterior cingulate thickness. Cereb Cortex. (2010) 20:1305–13. doi: 10.1093/cercor/bhp192
4. Gomar JJ, Bobes-Bascaran MT, Conejero-Goldberg C, Davies P, Goldberg TE, and Alzheimer’s Dis N. Utility of combinations of biomarkers, cognitive markers, and risk factors to predict conversion from mild cognitive impairment to alzheimer disease in patients in the alzheimer’s disease neuroimaging initiative. Arch Gen Psychiatry. (2011) 68:961–9. doi: 10.1001/archgenpsychiatry.2011.96
5. Gigi A, Babai R, Penker A, Hendler T, and Korczyn AD. Prefrontal compensatory mechanism may enable normal semantic memory performance in mild cognitive impairment (MCI). J Neuroimaging. (2010) 20:163–8. doi: 10.1111/j.1552-6569.2009.00386.x
6. Grady C. BRAIN AGEING The cognitive neuroscience of ageing. Nat Rev Neurosci. (2012) 13:491–505. doi: 10.1038/nrn3256
7. Brooks BL, Weaver LE, and Scialfa CT. Does impaired executive functioning differentially impact verbal memory measures in older adults with suspected dementia? Clin Neuropsychologist. (2006) 20:230–42. doi: 10.1080/13854040590947461
8. Jung YH, Park S, Jang H, Cho SH, Kim SJ, Kim JP, et al. Frontal-executive dysfunction affects dementia conversion in patients with amnestic mild cognitive impairment. Sci Reports. (2020) 10:772. doi: 10.1038/s41598-020-57525-6
9. Gagnon LG and Belleville S. Working memory in mild cognitive impairment and alzheimer’s disease: contribution of forgetting and predictive value of complex span tasks. Neuropsychology. (2011) 25:226–36. doi: 10.1037/a0020919
10. Belleville S, Sylvain-Roy S, de Boysson C, and Ménard M-C. Chapter 23 Characterizing the memory changes in persons with mild cognitive impairment. In: Sossin WS, Lacaille J-C, Castellucci VF, and Belleville S, editors. Progress in brain research, vol. 169 . Elsevier (2008). p. 365–75.
11. Miller EK and Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. (2001) 24:167–202. doi: 10.1146/annurev.neuro.24.1.167
12. Alvarez JA and Emory E. Executive function and the frontal lobes: a meta-analytic review. Neuropsychol review. (2006) 16:17–42. doi: 10.1007/s11065-006-9002-x
13. Salazar RF, Dotson NM, Bressler SL, and Gray CM. Content-specific fronto-parietal synchronization during visual working memory. Sci (New York NY). (2012) 338:1097–100. doi: 10.1126/science.1224000
14. Eichenbaum H. Prefrontal-hippocampal interactions in episodic memory. Nat Rev Neurosci. (2017) 18:547–58. doi: 10.1038/nrn.2017.74
15. Barker GRI, Banks PJ, Scott H, Ralph GS, Mitrophanous KA, Wong LF, et al. Separate elements of episodic memory subserved by distinct hippocampal-prefrontal connections. Nat Neurosci. (2017) 20:242–50. doi: 10.1038/nn.4472
16. Barker GRI, Bird F, Alexander V, and Warburton EC. Recognition memory for objects, place, and temporal order: A disconnection analysis of the role of the medial prefrontal cortex and perirhinal cortex. J Neurosci. (2007) 27:2948–57. doi: 10.1523/JNEUROSCI.5289-06.2007
17. Chao OY, Huston JP, Li JS, Wang AL, and Silva MAD. The medial prefrontal cortexlateral entorhinal cortex circuit is essential for episodic-like memory and associative object-recognition. Hippocampus. (2016) 26:633–45. doi: 10.1002/hipo.22547
18. Floresco SB, Seamans JK, and Phillips AG. Selective roles for hippocampal, prefrontal cortical, and ventral striatal circuits in radial-arm maze tasks with or without a delay. J Neurosci. (1997) 17:1880–90. doi: 10.1523/JNEUROSCI.17-05-01880.1997
19. Hannesson DK, Howland JG, and Phillips AG. Interaction between perirhinal and medial prefrontal cortex is required for temporal order but not recognition memory for objects in rats. J Neurosci. (2004) 24:4596–604. doi: 10.1523/JNEUROSCI.5517-03.2004
20. Wang GW and Cai JX. Disconnection of the hippocampal-prefrontal cortical circuits impairs spatial working memory performance in rats. Behav Brain Res. (2006) 175:329–36. doi: 10.1016/j.bbr.2006.09.002
21. Baddeley A. The episodic buffer: a new component of working memory? Trends Cogn Sci. (2000) 4:417–23. doi: 10.1016/s1364-6613(00)01538-2
23. Roux F, Wibral M, Mohr HM, Singer W, and Uhlhaas PJ. Gamma-band activity in human prefrontal cortex codes for the number of relevant items maintained in working memory. J Neurosci. (2012) 32:12411–20. doi: 10.1523/JNEUROSCI.0421-12.2012
24. Benchenane K, Peyrache A, Khamassi M, Tierney PL, Gioanni Y, Battaglia FP, et al. Coherent theta oscillations and reorganization of spike timing in the hippocampal-prefrontal network upon learning. Neuron. (2010) 66:921–36. doi: 10.1016/j.neuron.2010.05.013
25. O’Neill PK, Gordon JA, and Sigurdsson T. Theta oscillations in the medial prefrontal cortex are modulated by spatial working memory and synchronize with the hippocampus through its ventral subregion. J Neurosci. (2013) 33:14211–24. doi: 10.1523/JNEUROSCI.2378-13.2013
26. Gruber MJ, Hsieh LT, Staresina BP, Elger CE, Fell J, Axmacher N, et al. Theta phase synchronization between the human hippocampus and prefrontal cortex increases during encoding of unexpected information: A case study. J Cognit Neurosci. (2018) 30:1646–56. doi: 10.1162/jocn_a_01302
27. Kidder KS, Miles JT, Baker PM, Hones VI, Gire DH, and Mizumori SJY. A selective role for the mPFC during choice and deliberation, but not spatial memory retention over short delays. Hippocampus. (2021) 31:690–700. doi: 10.1002/hipo.2330
28. Roberts BM, Hsieh L-T, and Ranganath C. Oscillatory activity during maintenance of spatial and temporal information in working memory. Neuropsychologia. (2013) 51:349–57. doi: 10.1016/j.neuropsychologia.2012.10.009
29. Rajji TK, Zomorrodi R, Barr MS, Blumberger DM, Mulsant BH, and Daskalakis ZJ. Ordering information in working memory and modulation of gamma by theta oscillations in humans. Cereb Cortex. (2017) 27:1482–90. doi: 10.1093/cercor/bhv326
30. Brooks H, Goodman MS, Bowie CR, Zomorrodi R, Blumberger DM, Butters MA, et al. Theta-gamma coupling and ordering information: a stable brain-behavior relationship across cognitive tasks and clinical conditions. Neuropsychopharmacology. (2020) 45:2038–47. doi: 10.1038/s41386-020-0759-z
31. Tamura M, Spellman TJ, Rosen AM, Gogos JA, and Gordon JA. Hippocampal-prefrontal theta-gamma coupling during performance of a spatial working memory task. Nat Communications. (2017) 8:2182. doi: 10.1038/s41467-017-02108-9
32. Goodman MS, Kumar S, Zomorrodi R, Ghazala Z, Cheam ASM, Barr MS, et al. Theta-gamma coupling and working memory in alzheimer’s dementia and mild cognitive impairment. Front Aging Neurosci. (2018) 10:101. doi: 10.3389/fnagi.2018.00101
33. Diedrich L, Kolhoff HI, Bergmann C, Bähr M, and Antal A. Boosting working memory in the elderly: driving prefrontal theta–gamma coupling via repeated neuromodulation. GeroScience. (2024) 47:1425–40. doi: 10.1007/s11357-024-01272-3
34. Daume J, Kamiński J, Schjetnan AGP, Salimpour Y, Khan U, Kyzar M, et al. Control of working memory by phase–amplitude coupling of human hippocampal neurons. Nature. (2024) 629:393–401. doi: 10.1038/s41586-024-07309-z
35. Reinhart RMG and Nguyen JA. Working memory revived in older adults by synchronizing rhythmic brain circuits. Nat Neurosci. (2019) 22:820–7. doi: 10.1038/s41593-019-0371-x
36. Wischnewski M, Alekseichuk I, and Opitz A. Neurocognitive, physiological, and biophysical effects of transcranial alternating current stimulation. Trends Cogn Sci. (2023) 27:189–205. doi: 10.1016/j.tics.2022.11.013
37. Minjoli S, Saturnino GB, Blicher JU, Stagg CJ, Siebner HR, Antunes A, et al. The impact of large structural brain changes in chronic stroke patients on the electric field caused by transcranial brain stimulation. NeuroImage: Clinical. (2017) 15:106–17. doi: 10.1016/j.nicl.2017.04.014
38. Evans C, Bachmann C, Lee JSA, Gregoriou E, Ward N, and Bestmann S. Dose-controlled tDCS reduces electric field intensity variability at a cortical target site. Brain Stimulation. (2020) 13:125–36. doi: 10.1016/j.brs.2019.10.004
39. Zoefel B, Allard I, Anil M, and Davis MH. Perception of rhythmic speech is modulated by focal bilateral transcranial alternating current stimulation. J Cogn Neurosci. (2020) 32:226–40. doi: 10.1162/jocn_a_01490
40. Schwab BC, Misselhorn J, and Engel AK. Modulation of large-scale cortical coupling by transcranial alternating current stimulation. Brain Stimulation. (2019) 12:1187–96. doi: 10.1016/j.brs.2019.04.013
41. Owen AM, McMillan KM, Laird AR, and Bullmore E. N-back working memory paradigm: A meta-analysis of normative functional neuroimaging. Hum Brain Mapp. (2005) 25:46–59. doi: 10.1002/hbm.20131
42. Brooks H, Mirjalili M, Wang W, Kumar S, Goodman MS, Zomorrodi R, et al. Assessing the longitudinal relationship between theta-gamma coupling and working memory performance in older adults. Cereb Cortex (New York NY: 1991). (2022) 32:1653–67. doi: 10.1093/cercor/bhab295
43. Jack CR Jr., Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. (2018) 14:535–62. doi: 10.1016/j.jalz.2018.02.018
44. First MB. Diagnostic and statistical manual of mental disorders, 5th edition, and clinical utility. J Nerv Ment Dis. (2013) 201(9):727–9. doi: 10.1097/NMD.0b013e3182a2168a
45. Dill T. Contraindications to magnetic resonance imaging. Heart. (2008) 94:943. doi: 10.1136/hrt.2007.125039
46. San-Juan D, Sarmiento CI, Hernandez-Ruiz A, Elizondo-Zepeda E, Santos-Vázquez G, Reyes-Acevedo G, et al. Transcranial alternating current stimulation: A potential risk for genetic generalized epilepsy patients (Study case). Front Neurol. (2016) 7:213. doi: 10.3389/fneur.2016.00213
47. Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. (1998) 59 Suppl 20:22–33.
48. First MB and Gibbon M. The structured clinical interview for DSM-IV axis I disorders (SCID-I) and the structured clinical interview for DSM-IV axis II disorders (SCID-II). In: Comprehensive handbook of psychological assessment, Vol 2: Personality assessment. John Wiley & Sons, Inc, Hoboken, NJ, US (2004). p. 134–43.
49. Morris JC. The clinical dementia rating (CDR) - current versiona nd scoring rules. Neurology. (1993) 43:2412–4. doi: 10.1212/wnl.43.11.2412-a
50. Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatrics Society. (2005) 53:695–9. doi: 10.1111/j.1532-5415.2005.53221.x
51. Wilkinson GS and Robertson GJ. Wide range achievement test - fourth edition. Lutz, FL: Psychological Assessment Resources (2006).
52. Rajji TK, Bowie CR, Herrmann N, Pollock BG, Lanctôt KL, Kumar S, et al. Slowing cognitive decline in major depressive disorder and mild cognitive impairment: A randomized clinical trial. JAMA Psychiatry. (2024) 82:12–21. doi: 10.1001/jamapsychiatry.2024.3241
53. Cornblatt BA, Risch NJ, Faris G, Friedman D, and Erlenmeyerkimling L. The continuous performance-test, identical pairs version (Cpt-ip).1. New findings about sustained attention in normal-families. Psychiatry Res. (1988) 26:223–38. doi: 10.1016/0165-1781(88)90076-5
54. Kaplan E, Goodglass H, Weintraub S, and Goodglass H. Boston naming test. Philadelphia: Lea & Febiger (1983).
55. Strauss E, Sherman EMS, and Spreen O. A compendium of neuropsychological tests: administration, norms, and commentary. New York: Oxford University Press (2006).
56. Reitan RM and Wolfson D. The Halstead-Reitan neuropsychological test battery: theory and clinical interpretation. Tucson, AZ: Neuropsychology Press (1985).
57. Delis DC, Kramer JH, Kaplan E, and Holdnack J. Reliability and validity of the Delis-Kaplan Executive Function System: An update. J Int Neuropsychol Society. (2004) 10:301–3. doi: 10.1017/S1355617704102191
58. Rouleau I, Salmon DP, Butters N, Kennedy C, and McGuire K. Quantitative and qualitative analyses of clock drawings in alzheimers and huntingtons-disease. Brain Cognition. (1992) 18:70–87. doi: 10.1016/0278-2626(92)90112-Y
59. Benton AL, Varney NR, and Hamsher KD. Visuospatial judgment: A clinical test. Arch Neurology. (1978) 35:364–7. doi: 10.1001/archneur.1978.00500300038006
60. Benedict RHB. Brief visuospatial memory test–revised. Professional manual. Lutz, PL: PAR Inc (1997).
61. Wechsler D. Wechsler adult intelligence scale WAIS-IV; technical and interpretive manual. San Antonio: Pearson (2008).
62. Delis DC, Kramer JH, Kaplan E, and Ober BA. California verbal learning test - second edition - adult version. New York: The Psychological Corporation (2000).
63. Psychological C. WAIS-III, Weschler Adult Intelligence Scale, third edition: WMS-III, Weschler Memory Scale, third edition: technical manual. San Antonio: Psychological Corp., Harcourt, Brace & Co (1997).
64. Kumar S, Zomorrodi R, Ghazala Z, Goodman MS, Blumberger DM, Cheam A, et al. Extent of dorsolateral prefrontal cortex plasticity and its association with working memory in patients with alzheimer disease. JAMA Psychiatry. (2017) 74:1266–74. doi: 10.1001/jamapsychiatry.2017.3292
65. Hockey A and Geffen G. The concurrent validity and test-retest reliability of a visuospatial working memory task. Intelligence. (2004) 32:591–605. doi: 10.1016/j.intell.2004.07.009
66. Lin Y, Zhu M, and Su Z. The pursuit of balance: An overview of covariate-adaptive randomization techniques in clinical trials. Contemp Clin Trials. (2015) 45:21–5. doi: 10.1016/j.cct.2015.07.011
67. Van Veen BD, van Drongelen W, Yuchtman M, and Suzuki A. Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Trans bio-medical engineering. (1997) 44:867–80. doi: 10.1109/10.623056
68. Reinhart RMG. Disruption and rescue of interareal theta phase coupling and adaptive behavior. Proc Natl Acad Sci United States America. (2017) 114:11542–7. doi: 10.1073/pnas.1710257114
69. Reinhart RMG, Cosman JD, Fukuda K, and Woodman GF. Using transcranial direct-current stimulation (tDCS) to understand cognitive processing. Attention Perception Psychophysics. (2017) 79:3–23. doi: 10.3758/s13414-016-1224-2
70. Thielscher A, Antunes A, and Saturnino GB eds. (2015). Field modeling for transcranial magnetic stimulation: A useful tool to understand the physiological effects of TMS?, in: 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), .
71. Saturnino GB, Siebner HR, Thielscher A, and Madsen KH. Accessibility of cortical regions to focal TES: Dependence on spatial position, safety, and practical constraints. NeuroImage. (2019) 203:116183. doi: 10.1016/j.neuroimage.2019.116183
72. Younger J, Gandhi V, Hubbard E, and Mackey S. Development of the Stanford Expectations of Treatment Scale (SETS): A tool for measuring patient outcome expectancy in clinical trials. Clin Trials. (2012) 9:767–76. doi: 10.1177/1740774512465064
73. Fassi L, Hochman S, Daskalakis ZJ, Blumberger DM, and Kadosh RC. The importance of individual beliefs in assessing treatment efficacy: insights from neurostimulation studies. eLife Sciences Publications, Ltd (2023).
74. Nomura T, Asao A, and Kumasaka A. Transcranial alternating current stimulation over the prefrontal cortex enhances episodic memory recognition. Exp Brain Res. (2019) 237:1709–15. doi: 10.1007/s00221-019-05543-w
75. Javadi AH, Glen JC, Halkiopoulos S, Schulz M, and Spiers HJ. Oscillatory reinstatement enhances declarative memory. J Neurosci. (2017) 37:9939–44. doi: 10.1523/JNEUROSCI.0265-17.2017
76. Lang S, Gan LS, Alrazi T, and Monchi O. Theta band high definition transcranial alternating current stimulation, but not transcranial direct current stimulation, improves associative memory performance. Sci Rep. (2019) 9:8562. doi: 10.1038/s41598-019-44680-8
77. Alekseichuk I, Turi Z, Veit S, and Paulus W. Model-driven neuromodulation of the right posterior region promotes encoding of long-term memories. Brain Stimul. (2020) 13:474–83. doi: 10.1016/j.brs.2019.12.019
78. Antonenko D, Faxel M, Grittner U, Lavidor M, and Floel A. Effects of transcranial alternating current stimulation on cognitive functions in healthy young and older adults. Neural Plast. (2016) 2016:13. doi: 10.1155/2016/4274127
79. Jones KT, Ostrand AE, Gazzaley A, and Zanto TP. Enhancing cognitive control in amnestic mild cognitive impairment via at-home non-invasive neuromodulation in a randomized trial. Sci Reports. (2023) 13:7435. doi: 10.1038/s41598-023-34582-1
80. Jones KT, Gallen CL, Ostrand AE, Rojas JC, Wais P, Rini J, et al. Gamma neuromodulation improves episodic memory and its associated network in amnestic mild cognitive impairment: a pilot study. Neurobiol Aging. (2023) 129:72–88. doi: 10.1016/j.neurobiolaging.2023.04.005
81. Cappon D, Fox R, den Boer T, Yu W, LaGanke N, Cattaneo G, et al. Tele-supervised home-based transcranial alternating current stimulation (tACS) for Alzheimer’s disease: a pilot study. Front. Hum. Neurosci. (2023) 17:1168673. doi: 10.3389/fnhum.2023.1168673
82. Benussi A, Cantoni V, Grassi M, Brechet L, Michel CM, Datta A, et al. Increasing brain gamma activity improves episodic memory and restores cholinergic dysfunction in alzheimer’s disease. Ann Neurol. (2022) 92:322–34. doi: 10.1002/ana.26411
83. Kehler L, Francisco CO, Uehara MA, and Moussavi Z. (2020). The effect of transcranial alternating current stimulation (tACS) on cognitive function in older adults with dementia, in: 42nd Annual International Conferences of the Ieee Engineering in Medicine and Biology Society: Enabling Innovative Technologies for Global Healthcare Embc’20. IEEE Engineering in Medicine and Biology Society Conference Proceedings, . pp. 3649–53.
84. Kasten FH, Duecker K, Maack MC, Meiser A, and Herrmann CS. Integrating electric field modeling and neuroimaging to explain inter-individual variability of tACS effects. Nat Communications. (2019) 10:5427. doi: 10.1038/s41467-019-13417-6
85. Cabral-Calderin Y, van Hinsberg D, Thielscher A, and Henry MJ. Behavioral entrainment to rhythmic auditory stimulation can be modulated by tACS depending on the electrical stimulation field properties. eLife. (2024) 12:RP87820. doi: 10.7554/eLife.87820.3.sa3
86. Canolty RT and Knight RT. The functional role of cross-frequency coupling. Trends Cognit Sci. (2010) 14:506–15. doi: 10.1016/j.tics.2010.09.001
87. Mirjalili M, Zomorrodi R, Daskalakis ZJ, Hill SL, Kumar S, Blumberger DM, et al. Cognitive control, interference inhibition, and ordering of information during working memory in younger and older healthy adults. GeroScience. (2022) 44:2291–303. doi: 10.1007/s11357-022-00577-5
88. Brooks H, Mirjalili M, Wang W, Kumar S, Goodman MS, Zomorrodi R, et al. Assessing the longitudinal relationship between theta-gamma coupling and working memory performance in older adults cereb cortex. (2021).
89. Jones KT, Johnson EL, and Berryhill ME. Frontoparietal theta-gamma interactions track working memory enhancement with training and tDCS. Neuroimage. (2020) 211:116615. doi: 10.1016/j.neuroimage.2020.116615
90. Zangbar HS, Ghadiri T, Vafaee MS, Kalan AE, Fallahi S, Ghorbani M, et al. Theta oscillations through hippocampal/prefrontal pathway: importance in cognitive performances. Brain Connect. (2020) 10:157–69. doi: 10.1089/brain.2019.0733
91. Apergis-Schoute J, Pinto A, and Pare D. Ultrastructural organization of medial prefrontal inputs to the rhinal cortices. Eur J Neurosci. (2006) 24:135–44. doi: 10.1111/j.1460-9568.2006.04894.x
92. Burwell RD, Witter MP, and Amaral DG. Perirhinal and postrhinal cortices of the rat: A review of the neuroanatomical literature and comparison with findings from the monkey brain. Hippocampus. (1995) 5:390–408. doi: 10.1002/hipo.450050503
93. Igarashi KM, Lu L, Colgin LL, Moser MB, and Moser EI. Coordination of entorhinal-hippocampal ensemble activity during associative learning. Nature. (2014) 510:143–+. doi: 10.1038/nature13162
94. Keene CS, Bladon J, McKenzie S, Liu CD, O’Keefe J, and Eichenbaum H. Complementary functional organization of neuronal activity patterns in the perirhinal, lateral entorhinal, and medial entorhinal cortices. J Neurosci. (2016) 36:3660–75. doi: 10.1523/JNEUROSCI.4368-15.2016
95. DollemanVanderWeel MJ and Witter MP. Projections from the nucleus reuniens thalami to the entorhinal cortex, hippocampal field CA1, and the subiculum in the rat arise from different populations of neurons. J Comp Neurol. (1996) 364:637–50. doi: 10.1002/(SICI)1096-9861(19960122)364:4<637::AID-CNE3>3.0.CO;2-4
96. Vertes RP, Hoover WB, Szgeti-Buck K, and Leranth C. Nucleus reuniens of the midline thalamus: Link between the medial prefrontal cortex and the hippocampus. Brain Res Bull. (2007) 71:601–9. doi: 10.1016/j.brainresbull.2006.12.002
97. Ketz NA, Jensen O, and O’Reilly RC. Thalamic pathways underlying prefrontal cortex-medial temporal lobe oscillatory interactions. Trends Neurosci. (2015) 38:3–12. doi: 10.1016/j.tins.2014.09.007
98. Cassel JC, de Vasconcelos AP, Loureiro M, Cholvin T, Dalrymple-Alford JC, and Vertes RP. The reuniens and rhomboid nuclei: Neuroanatomy, electrophysiological characteristics and behavioral implications. Prog Neurobiol. (2013) 111:34–52. doi: 10.1016/j.pneurobio.2013.08.006
99. Mitchell AS, Sherman SM, Sommer MA, Mair RG, Vertes RP, and Chudasama Y. Advances in understanding mechanisms of thalamic relays in cognition and behavior. J Neurosci. (2014) 34:15340–6. doi: 10.1523/JNEUROSCI.3289-14.2014
100. Carr DB and Sesack SR. Hippocampal afferents to the rat prefrontal cortex: Synaptic targets and relation to dopamine terminals. J Comp Neurol. (1996) 369:1–15. doi: 10.1002/(SICI)1096-9861(19960520)369:1<1::AID-CNE1>3.0.CO;2-7
101. Jay TM, Thierry AM, Wiklund L, and Glowinski J. Excitatory amino-acid pathway from the hippocampus to the prefrontal cortex - Contribution of AMPA receptors in hippocampo-prefrontal cortex transmission. Eur J Neurosci. (1992) 4:1285–95. doi: 10.1111/j.1460-9568.1992.tb00154.x
102. Gabbott P, Headlam A, and Busby S. Morphological evidence that CA1 hippocampal afferents monosynaptically innervate PV-containing neurons and NADPH-diaphorase reactive cells in the medial prefrontal cortex (areas 25/32) of the rat. Brain Res. (2002) 946:314–22. doi: 10.1016/S0006-8993(02)02487-3
103. Tierney PL, Degenetais E, Thierry AM, Glowinski J, and Gioanni Y. Influence of the hippocampus on interneurons of the rat prefrontal cortex. Eur J Neurosci. (2004) 20:514–24. doi: 10.1111/j.1460-9568.2004.03501.x
104. Kjelstrup KB, Solstad T, Brun VH, Hafting T, Leutgeb S, Witter MP, et al. Finite scale of spatial representation in the hippocampus. Science. (2008) 321:140–3. doi: 10.1126/science.1157086
105. Royer S, Sirota A, Patel J, and Buzsaki G. Distinct representations and theta dynamics in dorsal and ventral hippocampus. J Neurosci. (2010) 30:1777–87. doi: 10.1523/JNEUROSCI.4681-09.2010
106. Komorowski RW, Garcia CG, Wilson A, Hattori S, Howard MW, and Eichenbaum H. Ventral hippocampal neurons are shaped by experience to represent behaviorally relevant contexts. J Neurosci. (2013) 33:8079–87. doi: 10.1523/JNEUROSCI.5458-12.2013
107. Wirt RA and Hyman JM. ACC theta improves hippocampal contextual processing during remote recall. Cell Rep. (2019) 27:2313–+. doi: 10.1016/j.celrep.2019.04.080
108. Sirota A, Montgomery S, Fujisawa S, Isomura Y, Zugaro M, and Buzsaki G. Entrainment of neocortical neurons and gamma oscillations by the hippocampal theta rhythm. Neuron. (2008) 60:683–97. doi: 10.1016/j.neuron.2008.09.014
109. Noda Y, Zomorrodi R, Daskalakis ZJ, Blumberger DM, and Nakamura M. Enhanced theta-gamma coupling associated with hippocampal volume increase following high-frequency left prefrontal repetitive transcranial magnetic stimulation in patients with major depression. Int J Psychophysiol. (2018) 133:169–74. doi: 10.1016/j.ijpsycho.2018.07.004
110. Vivekananda U, Bush D, Bisby JA, Baxendale S, Rodionov R, Diehl B, et al. Theta power and theta-gamma coupling support long-term spatial memory retrieval. Hippocampus. (2021) 31:213–20. doi: 10.1002/hipo.23284
Keywords: mild cognitive impairment, theta-gamma coupling, individualization, tACS, working memory
Citation: Mirjalili M, Palamarchuk IS, Brooks H, Zomorrodi R, Melichercik A, Wang W, Nestor SM, Blumberger DM, Datta A, Bowie C, Mdawar B, Hynynen K, Kumar S and Rajji TK (2025) Individualized frequency and montage tACS to engage theta-gamma coupling and enhance working memory in mild cognitive impairment. Front. Psychiatry 16:1565881. doi: 10.3389/fpsyt.2025.1565881
Received: 23 January 2025; Accepted: 12 May 2025;
Published: 02 June 2025.
Edited by:
Surjo R. Soekadar, Charité University Medicine Berlin, GermanyReviewed by:
Arun Sasidharan, National Institute of Mental Health and Neurosciences, IndiaNicole R. Nissim, Mayo Clinic Florida, United States
David Haslacher, Charité University Medicine Berlin, Germany
Copyright © 2025 Mirjalili, Palamarchuk, Brooks, Zomorrodi, Melichercik, Wang, Nestor, Blumberger, Datta, Bowie, Mdawar, Hynynen, Kumar and Rajji. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tarek K. Rajji, dGFyZWsucmFqamlAdXRzb3V0aHdlc3Rlcm4uZWR1
 Mina Mirjalili
Mina Mirjalili Iryna S. Palamarchuk
Iryna S. Palamarchuk Heather Brooks
Heather Brooks Reza Zomorrodi
Reza Zomorrodi Ashley Melichercik2
Ashley Melichercik2 Wei Wang
Wei Wang Abhishek Datta
Abhishek Datta Christopher Bowie
Christopher Bowie Bernadette Mdawar
Bernadette Mdawar Kullervo Hynynen
Kullervo Hynynen Sanjeev Kumar
Sanjeev Kumar Tarek K. Rajji
Tarek K. Rajji