Abstract
Background:
Chronic kidney disease (CKD) is associated with various health complications, including sleep disorders. Understanding the relationship between CKD and sleep disturbances is essential for improving patient management and outcomes in this population.
Patients and methods:
This study analyzed data from the National Health and Nutrition Examination Survey (NHANES) across two cycles (2015–2016 and 2017-2018), involving 4758 participants aged 20 years and older. Sleep disorders were evaluated via targeted questions from the Sleep Disorders Questionnaire. Chronic kidney disease (CKD) was classified by low estimated glomerular filtration rate (eGFR) and proteinuria. Logistic regression analyses examined the association between sleep disorders and CKD, adjusting for demographic and lifestyle confounders.
Results:
Among 4,758 participants, 863 (14%) were diagnosed with chronic kidney disease (CKD). Elevated C-reactive protein (CRP) levels and differential leukocyte counts were observed in the CKD cohort. CKD was associated with increased reports of snoring, sleep apnea, and sleep disturbances, along with higher fatigue levels. A significant positive association between CKD and sleep difficulties persisted in adjusted models. Infrequent snoring (1–2 times per week) demonstrated a negative association, whereas excessive daytime sleepiness (16–30 times/month) correlated positively with CKD. Significant associations between sleep disorders and CKD were observed in participants aged <60, with a BMI <28 kg/m², normal blood pressure, and CRP ≥1.8 mg/L. Sleep disorders were found to be correlated with obesity, hypertension, diabetes, CKD, and proteinuria. Notably, CKD patients with sleep difficulties had markedly elevated CRP levels compared to those without sleep issues, while other inflammatory markers were similarly elevated (P < 0.001).
Conclusion:
Patients with chronic kidney disease experience higher rates of sleep issues, highlighting the importance of addressing these problems in CKD management.
Introduction
Chronic kidney disease (CKD) is currently defined based on a variety of assessed variables indicating abnormalities in kidney structure or function, which include glomerular filtration rate (GFR), urine albumin levels, and the duration of damage (1). It is a common disease worldwide. Due to the rising prevalence of diabetes, hypertension, and obesity, along with the increasing issues of population aging, CKD has reached epidemic proportions. Regardless of the underlying causes, CKD progresses slowly, leading to irreversible loss of nephron units, end-stage renal disease, and premature death (2). According to statistics, in 2017, the global prevalence of CKD reached 9.1%, corresponding to approximately 700 million cases (3, 4). The World Health Organization (WHO) estimates that globally, the annual number of deaths directly attributed to chronic kidney disease ranges from 5 to 10 million, exerting significant pressure on social healthcare resources and individual health (5).
It is generally believed that the circadian rhythm system has evolved as a stabilizing advantage for organisms. However, the lifestyle of modern society, characterized by a 24-hour day and a 7-day week, has made circadian rhythm disruptions increasingly common. Circadian rhythm disruption is defined as a misalignment between the endogenous circadian rhythm and the external environment, with even a one-hour deviation being associated with an increased incidence of cardiovascular events (6). Epidemiological studies in this century indicate that shift workers often experience chronic circadian rhythm disturbances, further increasing the prevalence of chronic kidney disease and hypertension in this population (7–9). Prolonged work hours can also lead to circadian rhythm disruption and are significantly related to declines in kidney function. These data suggest a clear vicious cycle between circadian rhythm disturbances and disease (6). Sleep disorders may manifest as a form of circadian rhythm disruption, as symptoms such as difficulty falling asleep, early waking, and excessive daytime sleepiness may occur when the internal biological clock is misaligned with the external environment or expected schedules.
In current medical research, the interplay between CKD and sleep disorders has garnered increasing attention (10–13). Multiple studies have shown that CKD patients often experience varying degrees of problems with sleep quality, manifesting as insomnia (14–16), sleep apnea (17, 18), and excessive daytime fatigue (11, 19–21). This association is not coincidental, but rather involves complex physiological and pathological mechanisms, in which inflammatory processes may play a crucial role. Therefore, a thorough investigation of the inflammatory pathways associated with sleep disorders in the context of CKD, along with their clinical manifestations, not only aids in elucidating the disease progression but also holds significant practical implications for developing effective intervention strategies to improve the quality of life and prognosis for CKD patients.
Subjects and methods
Data source and study population
The data for this study was derived from the National Health and Nutrition Examination Survey (NHANES). NHANES is a cross-sectional survey conducted by the National Center for Health Statistics under the Centers for Disease Control and Prevention (CDC). This survey received approval from the Institutional Review Board of the CDC, and written informed consent was obtained from all participants. A stratified, random, multi-stage probability cluster design was employed to select households that were interviewed, aiming to monitor trends in the health and nutritional status of the non-institutionalized civilian population in the United States. NHANES is a publicly available dataset with ongoing updates. To enhance research accuracy and reduce sampling bias, data from two cycles (2015–2016 and 2017-2018) of NHANES were merged. Participants aged 20 years and older were included in the study (n=8637). Individuals lacking data on serum creatinine and urine albumin-to-creatinine ratio were excluded (n=1387), as were those with missing fasting glucose weight variables (n=5143). After screening, a total of 4758 participants were incorporated into this analysis, of which 14% (n=863) were identified as CKD patients (Figure 1).
Figure 1

Flow chart of patient selection. Source: NHANES, 2015–2016 and 2017–2018 cycles. NHANES, National Health and Nutrition Examination Survey; CKD, Chronic Kidney Disease; ACR, Albumin-to-Creatinine Ratio.
Assessment of sleep disorders
While numerous health and lifestyle factors may contribute to circadian rhythm disturbances, for the purposes of this study, the assessment of sleep disorders was streamlined by utilizing specific items from the “Sleep Disorders Questionnaire.” The following questions were evaluated: “Ever told doctor had trouble sleeping”, “Sleep hours”, “How often do you snore?”, “How often do you snort or stop breathing?”, and “How often feel overly sleepy during day?” “Sleep hours” is categorized into three groups: long sleepers (>9 hours), normal sleepers (6–9 hours), and short sleepers (<6 hours).
Definition of low eGFR, proteinuria, and chronic kidney disease
The primary outcome of our study was to assess the chronic kidney disease status of the participants, which we defined as either a low estimated glomerular filtration rate (eGFR, ml/min/1.73 m²) or the presence of proteinuria (1). Laboratory data were collected for serum creatinine levels, measured using the Jaffe reaction enzymatic method, for both serum and urine samples. eGFR (22) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation based on demographic data and serum creatinine levels. Participants with an eGFR of less than 60 mL/min/1.73 m² were classified as having low eGFR. Urinary albumin data were also obtained from laboratory measurements, utilizing solid-phase fluorescent immunoassay methods. Proteinuria was defined as a urinary albumin-to-creatinine ratio (UACR) of 30 mg/g or greater. It is important to note that low eGFR and proteinuria can exert independent effects on the body. Therefore, our secondary outcome included the assessment of participants for the presence of either proteinuria or low eGFR.
Demographic variables
Demographic variables were obtained through standardized questionnaires administered during the interviews. The following variables were recorded: age, gender (male and female), race/ethnicity (Mexican American, Other Hispanic, Non-Hispanic White, Non-Hispanic Black, Non-Hispanic Asian, Other/Multiracial), marital status (married, widowed, divorced, separated, never married and living with a partner), Ratio of family income to poverty (PIR), and education level (Less than 9th grade, 9th-11th grade, high school diploma/GED or equivalent, some college or AA degree, college or higher). The PIR was utilized to assess household income. Lifestyle factors included body mass index (BMI), smoking status, diabetes, and hypertension. BMI was evaluated in the Mobile Examination Center (MEC) and categorized into non-obesity (<30 kg/m²) and obesity (≥30 kg/m²). Smoking status was classified into two categories: never smokers and current smokers. Never smokers were defined as individuals who have smoked no more than 100 cigarettes in their lifetime, while current smokers were defined as those who had smoked at least 100 cigarettes. Diabetes was defined based on one of the following criteria: self-reported history of diabetes, currently taking insulin or diabetes medication, fasting glucose level ≥7.0 mmol/L (126 mg/dL), or hemoglobin A1c (HbA1c) level ≥6.5%. Hypertension was defined based on blood pressure measurements and medication use, with categories classified as normal, prehypertension, and hypertension. Normal blood pressure was defined as systolic blood pressure (SBP) <120 mmHg and diastolic blood pressure (DBP) <80 mmHg without the use of antihypertensive medications. Prehypertension was defined as SBP between 120–139 mmHg or DBP between 80–89 mmHg without the use of antihypertensive medications. Participants with SBP ≥140 mmHg, DBP ≥90 mmHg, or those reporting current use of antihypertensive medications were classified as having hypertension. In cases where systolic and diastolic pressures fell into different categories, participants were assigned to the more severe category.
Systemic inflammatory markers
Systemic inflammatory markers were assessed using neutrophil, monocyte, eosinophil, and total white blood cell counts (measured in 10³ cells/µL) alongside C-reactive protein (CRP) levels (measured in mg/L). The white blood cell counts were conducted using the Beckman Coulter DxH-800 Analyzer, while CRP levels were measured using the Roche Cobas 6000 (c501 module).
Statistical analysis
Statistical analyses were conducted using R software (version 4.2.1) following the NHANES data analysis guidelines. The study design accounted for survey design factors, non-response, and post-stratification, assigning sample weights to each participant to consider survey sampling weights. For continuous variables and those following a normal distribution, means and standard deviations were calculated. Descriptive statistics and Chi-square tests were performed to evaluate differences in the distribution of categorical variables. Independent samples t-tests or non-parametric tests were utilized to assess mean differences in continuous variables across groups. Logistic regression analyses were employed to explore the association between CKD and sleep disorders, with odds ratios (ORs) and 95% confidence intervals (CIs) reported to adjust for potential confounders. A two-tailed P-value of less than 0.05 was considered statistically significant. Three distinct logistic regression models examine the association between different types of sleep disorders and CKD status, with each sleep disorder included as an independent variable and CKD status as the dependent variable. In addition to an unadjusted Model 1, Model 2 included gender and race to account for sociodemographic factors, while Model 3 incorporated gender, race, BMI, smoking status, education level, and PIR. Sensitivity analyses were performed on a dataset that had not been adjusted for weight variables. Subgroup analyses were carried out to examine the associations between sleep disorders and CKD, low eGFR, and proteinuria within various subgroups, stratified by age (<60 years and ≥60 years), BMI (<28 kg/m² and ≥28 kg/m²), blood pressure status (hypertension/normal blood pressure/prehypertension), CRP (<1.8 mg/L and ≥1.8 mg/L), and PIR (<2.88 and ≥2.88). Adjustments were made for gender, race, BMI, smoking status, education level, and PIR. Additionally, we employed logistic regression models, treating sleep disorders as independent variables and chronic non-communicable diseases as dependent variables, incorporating multiple covariates such as gender, race, BMI, smoking status, education level, and PIR to examine the association between sleep disorders and chronic non-communicable diseases.
Results
Baseline characteristics of the study population
Among 4,758 participants, 863 (14%) were diagnosed with CKD. The CKD group comprised 57% females and 43% males, with an average age of 65 years compared to 45 years in the non-CKD group. The average BMI was 29 kg/m² in CKD patients and 28 kg/m²in non-CKD individuals. There were also significant differences in marital status, education level, and family poverty level between the two groups. CKD patients exhibited a higher prevalence of diabetes and hypertension. CKD patients had elevated CRP levels. Significant differences in white blood cell counts, monocyte counts, neutrophil counts, and eosinophil counts were observed, with higher counts in CKD patients. In the CKD cohort, the snoring frequency, occurrence of sleep apnea, and reporting of sleep disturbances to a physician were all significantly higher compared to the non-CKD group. Additionally, CKD patients reported a higher frequency of feeling fatigue (Table 1).
Table 1
| Characteristic | Overall, N=4758(100%) | No CKD, N=3895(86%) | CKD, N=863(14%) | P-value |
|---|---|---|---|---|
| Sex | 0.036 | |||
| Female | 2,465 (52%) | 2,019 (51%) | 446 (57%) | |
| Male | 2,293 (48%) | 1,876 (49%) | 417 (43%) | |
| Age (year) | 48 (33, 61) | 45 (32, 58) | 65 (52, 76) | <0.001 |
| Race | 0.086 | |||
| Mexican American | 738 (9.0%) | 610 (9.1%) | 128 (8.7%) | |
| Other Hispanic | 559 (6.7%) | 482 (7.0%) | 77 (5.3%) | |
| Non-Hispanic White | 1,601 (63%) | 1,254 (62%) | 347 (65%) | |
| Non-Hispanic Black | 1,030 (11%) | 832 (11%) | 198 (13%) | |
| Non-Hispanic Asian | 616 (5.7%) | 542 (6.0%) | 74 (4.2%) | |
| Other/multiracial | 214 (4.5%) | 175 (4.6%) | 39 (3.7%) | |
| Marital status | <0.001 | |||
| Married | 2,415 (54%) | 2,000 (54%) | 415 (49%) | |
| Widowed | 340 (5.3%) | 199 (3.5%) | 141 (16%) | |
| Divorced | 549 (11%) | 432 (11%) | 117 (13%) | |
| Separated | 167 (2.3%) | 134 (2.2%) | 33 (2.4%) | |
| Never married | 843 (18%) | 743 (19%) | 100 (11%) | |
| Living with partner | 442 (9.8%) | 387 (9.9%) | 55 (8.9%) | |
| Education level | <0.001 | |||
| Less than 9th grade | 477 (5.1%) | 364 (4.7%) | 113 (7.6%) | |
| 9-11th grade | 566 (8.3%) | 442 (7.9%) | 124 (11%) | |
| High school graduate/GED or equivalent | 1,090 (25%) | 861 (24%) | 229 (31%) | |
| Some college or AA degree | 1,463 (31%) | 1,220 (32%) | 243 (29%) | |
| College graduate or above | 1,161 (30%) | 1,008 (32%) | 153 (22%) | |
| BMI (kg/m(2)) | 28 (25, 33) | 28 (24, 33) | 29 (25, 35) | 0.006 |
| Smoked at least 100 cigarettes in life | 2,072 (44%) | 1,642 (44%) | 430 (50%) | 0.018 |
| Ratio of family income to poverty | 2.88 (1.52, 5.00) | 2.97 (1.57, 5.00) | 2.43 (1.41, 4.18) | 0.003 |
| Diabetes | <0.001 | |||
| Yes | 1,078 (16%) | 679 (12%) | 399 (41%) | |
| No | 447 (9.0%) | 381 (8.9%) | 66 (9.4%) | |
| Uncertain | 3,233 (75%) | 2,835 (79%) | 398 (50%) | |
| Doctor told you have diabetes | <0.001 | |||
| Yes | 761 (11%) | 453 (8.1%) | 308 (30%) | |
| No | 3,854 (87%) | 3,330 (90%) | 524 (66%) | |
| Borderline | 140 (2.3%) | 109 (2.0%) | 31 (4.2%) | |
| Taking insulin now | 227 (2.8%) | 103 (1.8%) | 124 (8.7%) | <0.001 |
| Take diabetic pills to lower blood sugar | 622 (44%) | 386 (39%) | 236 (57%) | <0.001 |
| Fasting glucose (mmol/L) | 5.66 (5.27, 6.22) | 5.61 (5.27, 6.11) | 6.11 (5.55, 7.38) | <0.001 |
| Glycohemoglobin (%) | 5.50 (5.20, 5.80) | 5.40 (5.20, 5.70) | 5.90 (5.50, 6.60) | <0.001 |
| Hypertension | <0.001 | |||
| Hypertensive | 2,005 (38%) | 1,386 (32%) | 619 (71%) | |
| Normotensive | 1,428 (37%) | 1,331 (40%) | 97 (15%) | |
| Prehypertensive | 1,073 (25%) | 957 (27%) | 116 (14%) | |
| Systolic (mmHg) | 120 (112, 132) | 118 (110, 130) | 132 (118, 146) | <0.001 |
| Diastolic (mmHg) | 70 (64, 78) | 70 (64, 78) | 70 (62, 78) | 0.9 |
| Taking prescription for hypertension | 1,605 (89%) | 1,078 (87%) | 527 (95%) | 0.003 |
| Albumin creatinine ratio (mg/g) | 7 (4, 12) | 6 (4, 9) | 46 (16, 106) | <0.001 |
| Creatinine (mg/dL) | 0.83 (0.70, 0.97) | 0.82 (0.70, 0.95) | 0.96 (0.73, 1.20) | <0.001 |
| CRP (mg/L) | 1.8 (0.8, 4.3) | 1.7 (0.7, 4.1) | 2.3 (1.0, 5.4) | <0.001 |
| WBC (103cells/uL) | 6.50 (5.50, 7.90) | 6.40 (5.50, 7.80) | 6.90 (5.70, 8.60) | <0.001 |
| Monocyte (103cells/uL) | 0.50 (0.40, 0.60) | 0.50 (0.40, 0.60) | 0.60 (0.50, 0.70) | <0.001 |
| Neutrophils (103cells/uL) | 3.74 (2.90, 4.80) | 3.70 (2.90, 4.70) | 4.20 (3.20, 5.25) | <0.001 |
| Eosinophils (103cells/uL) | 0.20 (0.10, 0.30) | 0.20 (0.10, 0.20) | 0.20 (0.10, 0.30) | 0.002 |
| Sleep hours (hour) | <0.001 | |||
| <6h | 469.00 (7.78%) | 377.00 (7.37%) | 92.00 (10.34%) | |
| >=6h and <=9h | 3,725.00 (82.48%) | 3,110.00 (83.89%) | 615.00 (73.68%) | |
| >9h | 534.00 (9.74%) | 390.00 (8.74%) | 144.00 (15.98%) | |
| How often do you snore | <0.001 | |||
| Never | 1,134 (25%) | 919 (24%) | 215 (29%) | |
| Rarely - 1-2 nights a week | 1,121 (29%) | 971 (30%) | 150 (18%) | |
| Occasionally - 3-4 nights a week | 871 (19%) | 731 (20%) | 140 (18%) | |
| Frequently - 5 or more nights a week | 1,301 (27%) | 1,027 (26%) | 274 (34%) | |
| How often do you snort or stop breathing | <0.001 | |||
| Never | 3,318 (75%) | 2,767 (76%) | 551 (72%) | |
| Rarely - 1-2 nights a week | 602 (13%) | 501 (14%) | 101 (10%) | |
| Occasionally - 3-4 nights a week | 295 (6.4%) | 233 (6.2%) | 62 (7.7%) | |
| Frequently - 5 or more nights a week | 247 (4.8%) | 183 (4.0%) | 64 (9.8%) | |
| Ever told doctor had trouble sleeping | 1,365 (31%) | 1,061 (30%) | 304 (39%) | <0.001 |
| How often feel overly sleepy during day | 0.014 | |||
| Never | 827 (13%) | 704 (13%) | 123 (12%) | |
| Rarely - 1 time a month | 1,115 (23%) | 936 (24%) | 179 (21%) | |
| Sometimes - 2-4 times a month | 1,607 (36%) | 1,327 (37%) | 280 (33%) | |
| Often- 5-15 times a month | 811 (19%) | 637 (19%) | 174 (22%) | |
| Almost always - 16-30 times a month | 393 (7.9%) | 290 (7.3%) | 103 (12%) | |
| Low eGFR | 375 (6.0%) | 0 (0%) | 375 (43%) | <0.001 |
| Albuminuria | 637 (9.8%) | 0 (0%) | 637 (70%) | <0.001 |
| CKD | 863 (14%) | 0 (0%) | 863 (100%) | <0.001 |
Baseline data of study population for participants with and without CKD from the national health and nutrition examination survey, 2015-2016 and 2017-2018 cycles.
Continuous variables were reported as means ± SD. Categorical variables were reported as N (%).
BMI, Body Mass Index; CRP, C-Reactive Protein; WBC, White Blood Cell; GED, General Educational Development; AA, Associate of Arts degree; eGFR, estimated Glomerular Filtration Rate; CKD, Chronic Kidney Disease.
Relationship between sleep disorders and CKD
A positive association exists between CKD and reporting sleep difficulties, which remains significant in adjusted models. Compared to individuals with normal sleep duration, both long sleepers (>9 hours) and short sleepers (<6 hours) are associated with a higher incidence of CKD. There is a negative association with infrequent snoring (1–2 times per week), while no significant association is noted for frequent snoring (≥5 times per week). Additionally, less frequent sleep apnea (1–2 times per week) demonstrated no significant association, but a positive association was observed for more frequent instances (≥5 times per week). Lastly, CKD correlates positively with excessive daytime sleepiness occurring 16–30 times per month (Table 2).
Table 2
| Characteristic | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| OR, (95%CI) | P value | OR, (95%CI) | P value | OR, (95%CI) | P value | |
| Ever told doctor had trouble sleeping | ||||||
| No | ||||||
| Yes | 1.05 (1.02,1.09) | 0.002 | 1.05 (1.02,1.08) | 0.005 | 1.04 (1.01,1.08) | 0.025 |
| Sleep hours | ||||||
| >=6h and <=9h | ||||||
| <6h | 1.06 (1.00, 1.12) | 0.037 | 1.06 (1.01, 1.12) | 0.031 | 1.05 (0.98, 1.13) | 0.2 |
| >9h | 1.11 (1.04, 1.18) | 0.003 | 1.11 (1.04, 1.18) | 0.003 | 1.09 (1.02, 1.17) | 0.016 |
| How often do you snore | ||||||
| Never | ||||||
| Rarely - 1-2 nights a week | 0.93 (0.90,0.96) | <0.001 | 0.93 (0.90,0.96) | <0.001 | 0.92 (0.89,0.96) | 0.001 |
| Occasionally - 3-4 nights a week | 0.97 (0.93,1.01) | 0.091 | 0.97 (0.93,1.01) | 0.14 | 0.96 (0.92,1) | 0.042 |
| Frequently - 5 or more nights a week | 1.01 (0.97,1.05) | 0.7 | 1.01 (0.97,1.06) | 0.5 | 0.99 (0.94,1.04) | 0.7 |
| How often do you snort or stop breathing | ||||||
| Never | ||||||
| Rarely - 1-2 nights a week | 0.97 (0.94,1.01) | 0.2 | 0.98 (0.94,1.02) | 0.2 | 0.97 (0.92,1.01) | 0.12 |
| Occasionally - 3-4 nights a week | 1.03 (0.97,1.1) | 0.3 | 1.04 (0.97,1.11) | 0.2 | 1.03 (0.96,1.11) | 0.4 |
| Frequently - 5 or more nights a week | 1.16 (1.04,1.29) | 0.008 | 1.16 (1.04,1.29) | 0.01 | 1.14 (1.02,1.28) | 0.026 |
| How often feel overly sleepy during day | ||||||
| Never | ||||||
| Rarely - 1 time a month | 1.00 (0.95,1.04) | 0.8 | 0.99 (0.95,1.03) | 0.6 | 1.01 (0.96,1.07) | 0.7 |
| Sometimes - 2-4 times a month | 1.00 (0.95,1.05) | >0.9 | 0.99 (0.95,1.04) | 0.8 | 1.01 (0.96,1.07) | 0.7 |
| Often- 5-15 times a month | 1.03 (0.98,1.08) | 0.2 | 1.02 (0.98,1.07) | 0.3 | 1.04 (0.98,1.1) | 0.2 |
| Almost always - 16-30 times a month | 1.08 (1.01,1.16) | 0.022 | 1.07 (1.00,1.15) | 0.042 | 1.07 (0.99,1.16) | 0.089 |
Association between sleep characteristics and chronic kidney disease.
Model 1: No adjust;
Model 2: Adjusted for sex, race;
Model 3: Adjusted for sex, race, BMI, Smoked at least 100 cigarettes in life, Education level, Ratio of family income to poverty.
OR, odds ratio.
Source: NHANES, 2015–2016 and 2017–2018 cycles.
Subgroup analysis of sleep disorders related to CKD, low eGFR, and proteinuria
Significant associations were found between sleep disorders and CKD in participants aged <60 years, BMI <28 kg/m², normal blood pressure, CRP ≥1.8 mg/L, and PIR <2.88. In pre-hypertensive participants, sleep disorders showed a negative association with CKD. Additionally, sleep disorders were significantly associated with low eGFR in those aged <60 years and CRP ≥1.8 mg/L. Furthermore, significant associations with proteinuria were observed in participants aged <60 years, BMI <28 kg/m², CRP ≥1.8 mg/L, and a household income-to-poverty ratio <2.88 (Figure 2).
Figure 2
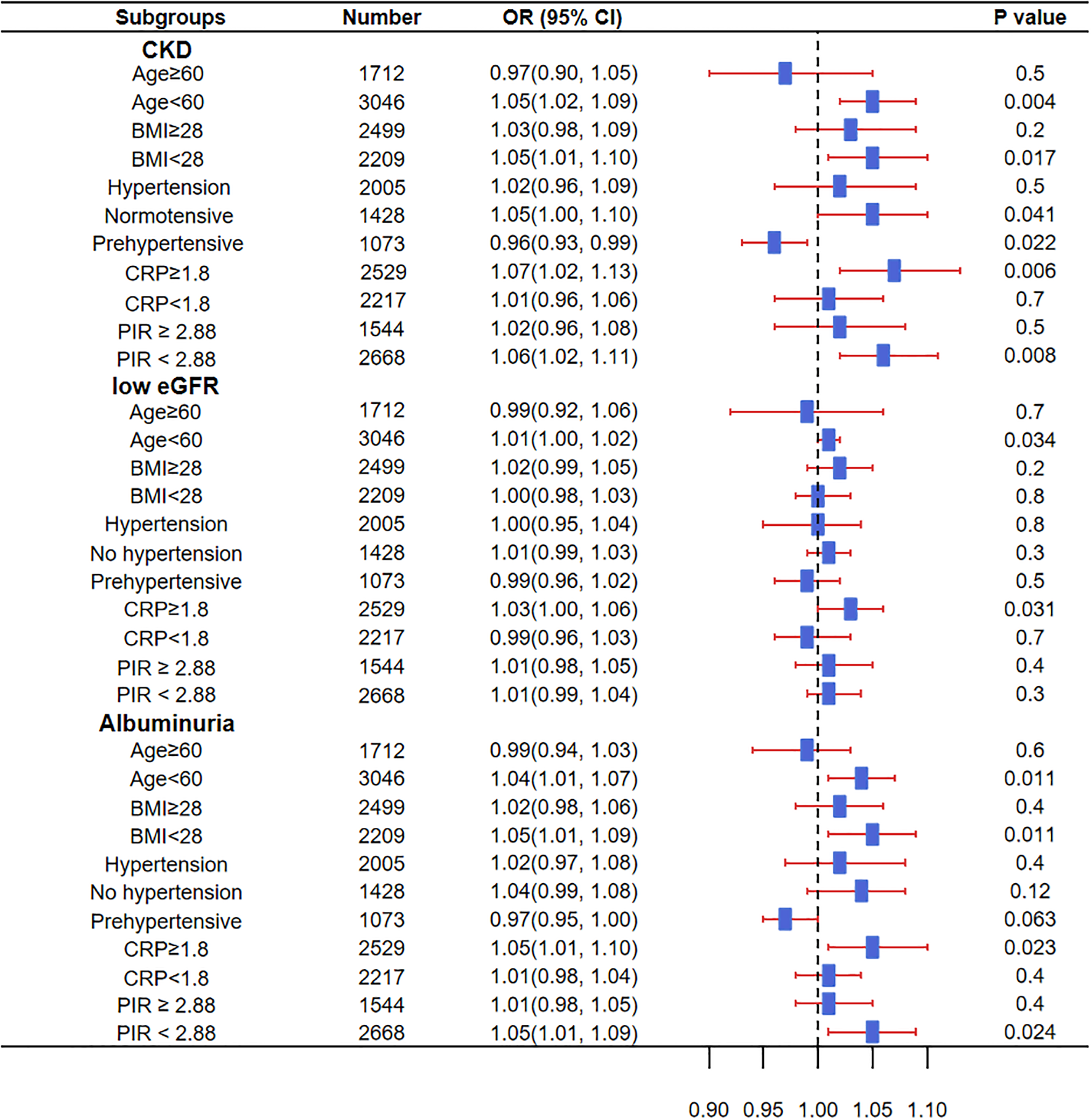
Impact of sleep disorders in different subgroups on the prevalence of CKD, low eGFR, and proteinuria. OR, Odds Ratio; CKD, Chronic Kidney Disease; BMI, Body Mass Index; CRP, C-Reactive Protein; PIR, Ratio of family income to poverty; eGFR, estimated Glomerular Filtration Rate. Source: NHANES, 2015–2016 and 2017–2018 cycles.
Association between sleep disorders and chronic non-communicable diseases
Sleep disorders in adults were found to be correlated with obesity, hypertension, diabetes, CKD, and proteinuria, but no association was observed with low eGFR (Figure 3).
Figure 3

Association between sleep disorders and chronic non-communicable diseases. OR, Odds Ratio; CKD, Chronic Kidney Disease; eGFR, estimated Glomerular Filtration Rate. Source: NHANES, 2015–2016 and 2017–2018 cycles.
Relationship between sleep disorders and blood biomarkers in CKD patients
In CKD patients, those reporting sleep difficulties had a higher average CRP level compared to those without sleep issues. No significant differences were observed in average white blood cell count, monocyte count, neutrophil count, and eosinophil count. In participants with sleep difficulties, CKD patients had a significantly higher CRP level than non-CKD participants. CRP levels, average white blood cell count, monocyte count, and neutrophil count were markedly higher in CKD patients with sleep difficulties than in CKD-free individuals (P < 0.001), while eosinophil count showed no significant difference (Table 3).
Table 3
| Characteristic | CKD and trouble sleeping, N = 304 (5.4%) | Non-CKD and trouble sleeping, N = 1061 (25%) | CKD and non-trouble sleeping, N = 558 (8.5%) | Non-CKD and non-trouble sleeping, N = 2833 (61%) | |||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | P-value | Mean (SD) | P-value | Mean (SD) | P-value | |
| CRP (mg/dL) |
6.7 ± 15.2 | 4.1 ± 6.9 | <0.001 | 4.3 ± 8.2 | 0.002 | 3.5 ± 6.4 | <0.001 |
| WBC (10 |
7.38 ± 2.19 | 7.01 ± 2.3 | 0.046 | 7.33 ± 3.9 | 0.2 | 6.71 ± 1.89 | <0.001 |
| Monocyte (10 |
0.6 ± 0.19 | 0.56 ± 0.18 | 0.057 | 0.59 ± 0.2 | 0.4 | 0.54 ± 0.17 | <0.001 |
| Neutrophils (10 |
4.52 ± 1.72 | 4.13 ± 1.75 | 0.001 | 4.33 ± 1.57 | 0.085 | 3.89 ± 1.5 | <0.001 |
| Eosinophils (10 |
0.23 ± 0.18 | 0.2 ± 0.15 | 0.2 | 0.24 ± 0.18 | 0.6 | 0.2 ± 0.15 | 0.14 |
Association between sleep disorders in CKD patients and blood biomarkers.
CKD, Chronic Kidney Disease. SD, standard deviation; CRP, C-Reactive Protein; WBC, White Blood Cell;
Source: NHANES, 2015–2016 and 2017–2018 cycles.
Discussion
The ICSD-3 diagnostic criteria for sleep disorders systematically analyze 241 distinct diagnostic criteria and categorize them into nine types, including clinical manifestations, objective markers, distress, and disability (23). Sleep disorders encompass a wide range of conditions, from obstructive sleep apnea to rapid eye movement sleep behavior disorder. In the diagnosis of sleep disorders, doctors can initially determine the presence of a sleep disorder by thoroughly understanding the patient’s medical history, including sleep patterns, daytime dysfunction, and related symptoms. Traditional polysomnography is considered the gold standard for diagnosing sleep disorders. It can record multiple physiological signals, including electroencephalography, electrooculography, and electromyography, thereby providing a comprehensive assessment of the various stages and quality of sleep (24). Self-assessment scales and questionnaire tools play an important role in the preliminary screening and assessment of sleep disorders. These tools typically include standardized scales, such as the Pittsburgh Sleep Quality Index and the Epworth Sleepiness Scale, which can help doctors quickly evaluate patients’ sleep quality and daytime sleepiness levels. The data in this article comes from the NHANES database, with participants being a large civilian population. The assessment of sleep disorders in this study is based on questionnaire surveys. Although lacking the precision of polysomnography or activity monitors, this method provides a cost-effective screening approach, which is particularly valuable in large-scale observational studies.
CKD and sleep disorders influence each other. Patients with CKD often have hyperphosphatemia and hypocalcemia, and these electrolyte changes may lead to increased neural excitability, thereby affecting the normal sleep cycle (21). The accumulation of toxins in the body of patients with uremia can have a negative impact on the central nervous system, leading to issues such as insomnia, daytime sleepiness, and sleep apnea (25). Uremia may also cause inflammatory responses in the central nervous system, which are considered an important physiological mechanism leading to sleep disorders (26). Sleep disorders in CKD patients are also associated with an increased risk of cardiovascular disease. There is a bidirectional relationship between cardiovascular disease and CKD, where cardiovascular issues may further exacerbate the severity of sleep disorders, creating a vicious cycle. Common symptoms of sleep apnea in CKD patients are closely related to the occurrence of cardiovascular events (10). Sleep disorders such as insomnia and circadian rhythm disturbances lead to reduced or disrupted secretion of melatonin (27, 28). Melatonin has antioxidant, anti-inflammatory, and anti-fibrotic effects, and its deficiency can exacerbate oxidative stress and fibrosis in the kidneys (29, 30). Sleep disorders lead to sustained sympathetic nervous system hyperactivity and parasympathetic nervous system inhibition. The sympathetic nervous system is crucial for maintaining renal function homeostasis. Excessive sympathetic nerve activity can cause functional and morphological changes in renal physiology and structure, potentially resulting in kidney damage and the progression of chronic kidney disease. Muscle sympathetic nerve activity and norepinephrine show a significant negative correlation with renal function indicators (31, 32). Parasympathetic inhibition weakens anti-inflammatory functions, such as the cholinergic anti-inflammatory pathway, exacerbating renal inflammation (33, 34). These pathophysiological mechanisms involve bidirectional pathways. The data in this article comes from the NHANES database and is a cross-sectional study, which cannot determine the temporal sequence between CKD and sleep disorders. Although reverse causation cannot be ruled out, statistical methods have been employed to control for key confounding factors. Future assessments are needed to conduct longitudinal studies and interventional trials focusing on sleep treatment in patients with CKD.
The main innovation of our research lies in the comprehensive assessment of the association between various sleep disorders and the chronic kidney disease using the NHANES dataset, which provides a robust and representative sample of the U.S. population. Previous studies have primarily focused on specific sleep disorders, such as sleep apnea or insomnia, within chronic kidney disease populations, often limited to small cohorts or specific subgroups (35, 36). Our research integrates various sleep disorders, including sleep duration, snoring frequency, sleep apnea, and daytime excessive sleepiness, to examine their association with chronic kidney disease. Furthermore, our study is innovative in adjusting for multiple confounding variables, including demographic factors, lifestyle behaviors, and systemic inflammation markers. This comprehensive approach allows us to gain a more detailed understanding of the associations between various sleep disorders and chronic kidney disease. The research indicates that sleep apnea is associated with the progression of chronic kidney disease. Our findings reveal that intermittent sleep apnea and excessive sleepiness significantly associated with chronic kidney disease. In addition, we provided key insights using logistic regression and subgroup analysis, revealing how these associations vary across different demographic and health characteristics. We found interesting phenomena, particularly that younger individuals and those with lower BMI or higher CRP levels are especially susceptible to the adverse effects of sleep disorders on kidney function. This level of detail enhances the clinical relevance of our findings, suggesting targeted interventions for specific high-risk populations. Clinicians should consider incorporating sleep assessments into the routine care plans for patients with chronic kidney disease to identify and manage these disorders early.
This study has several limitations that should be acknowledged. First, the cross-sectional design of the NHANES data prevents establishing a causal relationship between sleep disorders and CKD. Although we can identify associations, we cannot infer causality from this study design. Second, relying on self-reported data for sleep disorders and other lifestyle factors introduces potential recall bias and misclassification. Participants might not accurately recall or report their sleep patterns and other health behaviors, which can impact the reliability of the findings. Furthermore, the wide heterogeneity among sleep disorders, ranging from obstructive sleep apnea to REM sleep behavior disorder, each with distinct pathophysiological mechanisms, could also impact the interpretability and robustness of the observed associations. Moreover, the absence of objective measures, such as actigraphy or polysomnography, further constrains the accuracy of sleep disorder diagnoses. CKD was treated as a binary outcome, without considering disease progression or severity. This approach may overlook potential differences in the association between sleep disorders and CKD across various stages of disease severity. Lastly, while the study controlled for a variety of potential confounders, residual confounding cannot be entirely ruled out. Future research should aim to use longitudinal designs to better assess causality, incorporate objective measures of sleep disorders, and ensure a more representative sample to enhance the generalizability of the findings.
Conclusion
CKD patients had a higher prevalence of sleep difficulties reported to physicians, higher CRP levels, and increased counts of white blood cells, monocytes, neutrophils, and eosinophils compared to non-CKD participants. CKD is positively associated with both short and long sleep durations, frequent sleep apnea (≥5 times per week), and excessive daytime sleepiness (16–30 times per month), while infrequent snoring (1–2 times per week) shows a negative association, and less frequent sleep disturbances generally lack significant associations. Subgroup analysis revealed significant associations between sleep disorders and CKD in specific populations.
Statements
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: The data can be directly accessed through the NHANES website: NHANES Data.
Ethics statement
The study was performed in accordance with the ethical principles outlined in the Declaration of Helsinki. All procedures involving human participants were reviewed and approved by the Institutional Review Board of the National Center for Health Statistics. Written informed consent was obtained from all individuals prior to their participation.
Author contributions
HX: Conceptualization, Data curation, Writing – original draft. KL: Data curation, Methodology, Writing – review & editing. GH: Formal analysis, Funding acquisition, Writing – review & editing. XZ: Funding acquisition, Investigation, Methodology, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by: Medical research Fund of Shenzhen Medical Academy of Research and Translation (C2301004); Shenzhen Fund for Guangdong Provincial High-level Clinical Key Specialties (SZGSP001); Shenzhen key Laboratory of Kidney Diseases (ZDSYS201504301616234); Scientific Research and Cultivation Fund of the Affiliated Shunde Hospital of Jinan University (No:202401009); Medical Joint Fund of Jinan University (No: YXJC2022004); Foshan Self-Funded Scientific and Technological Innovation Projects (No:2420001003634).
Acknowledgments
We would like to express our sincere gratitude to Liu Kai for his invaluable assistance with data statistics. Additionally, we acknowledge the financial support provided by Shenzhen People’s Hospital and the Affiliated Shunde Hospital of Jinan University, which made this research possible.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CKD, Chronic kidney disease; eGFR, estimated glomerular filtration rate; WHO, World Health Organization; NHANES, National Health and Nutrition Examination Survey; CDC, National Center for Health Statistics under the Centers for Disease Control and Prevention; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; UACR, urinary albumin-to-creatinine ratio; PIR, Ratio of family income to poverty; BMI, body mass index; MEC, Mobile Examination Center; SBP, systolic blood pressure; DBP, diastolic blood pressure; CRP, C-reactive protein; ORs, odds ratios; CIs, confidence intervals.
References
1
Glassock RJ Warnock DG Delanaye P . The global burden of chronic kidney disease: estimates, variability and pitfalls. Nat Rev Nephrology. (2017) 13:104–14. doi: 10.1038/nrneph.2016.163
2
Ruiz-Ortega M Rayego-Mateos S Lamas S Ortiz A Rodrigues-Diez RR . Targeting the progression of chronic kidney disease. Nat Rev Nephrology. (2020) 16:269–88. doi: 10.1038/s41581-019-0248-y
3
Chronic Kidney Disease Collaboration GBD . Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet (London England). (2020) 395:709–33. doi: 10.1016/S0140-6736(20)30045-3
4
Cockwell P Fisher LA . The global burden of chronic kidney disease. Lancet (London England). (2020) 395:662–4. doi: 10.1016/S0140-6736(19)32977-0
5
Luyckx VA Tonelli M Stanifer JW . The global burden of kidney disease and the sustainable development goals. Bull World Health Organ. (2018) 96:414–22d. doi: 10.2471/BLT.17.206441
6
Mohandas R Douma LG ScIndia Y Gumz ML . Circadian rhythms and renal pathophysiology. J Clin Invest. (2022) 132e148277. doi: 10.1172/JCI148277
7
Uhm JY Kim HR Kang GH Choi YG Park TH Kim SY et al . The association between shift work and chronic kidney disease in manual labor workers using data from the Korea National Health and Nutrition Examination Survey (KNHANES 2011-2014). Ann Occup Environ Med. (2018) 30:69. doi: 10.1186/s40557-018-0279-z
8
Rahim A McIsaac MA Aronson KJ Smith PM Tranmer JE . The associations of shift work, sleep quality, and incidence of hypertension in ontario adults: A population-based study. Can J Cardiol. (2021) 37:513–8. doi: 10.1016/j.cjca.2020.09.003
9
Uwumiro F Nebuwa C Nwevo CO Okpujie V Osemwota O Obi ES et al . Cardiovascular event predictors in hospitalized chronic kidney disease (CKD) patients: A nationwide inpatient sample analysis. Cureus. (2023) 15:e47912. doi: 10.7759/cureus.47912
10
Lyons OD . Sleep disorders in chronic kidney disease. Nat Rev Nephrology. (2024) 20:690–700. doi: 10.1038/s41581-024-00848-8
11
Kalantar-Zadeh K Lockwood MB Rhee CM Tantisattamo E Andreoli S Balducci A et al . Patient-centred approaches for the management of unpleasant symptoms in kidney disease. Nat Rev Nephrology. (2022) 18:185–98. doi: 10.1038/s41581-021-00518-z
12
Natale P Ruospo M Saglimbene VM Palmer SC Strippoli GF . Interventions for improving sleep quality in people with chronic kidney disease. . Cochrane Database systematic Rev. (2019) 5:Cd012625. doi: 10.1002/14651858.CD012625.pub2
13
Stabouli S Papadimitriou E Printza N Dotis J Papachristou F . Sleep disorders in pediatric chronic kidney disease patients. Pediatr Nephrol (Berlin Germany). (2016) 31:1221–9. doi: 10.1007/s00467-015-3237-9
14
Parajuli S Tiwari R Clark DF Mandelbrot DA Djamali A Casey K . Sleep disorders: Serious threats among kidney transplant recipients. Transplant Rev (Orlando Fla). (2019) 33:9–16. doi: 10.1016/j.trre.2018.09.002
15
Geng T Li X Ma H Heianza Y Qi L . Adherence to a healthy sleep pattern and risk of chronic kidney disease: the UK biobank study. Mayo Clinic Proc. (2022) 97:68–77. doi: 10.1016/j.mayocp.2021.08.028
16
Lee KM Kim JS Hwang S Cho NJ Park S Gil HW et al . The higher the CKD stage, the higher the psychological stress in patients with CKD during COVID-19 pandemic. J Clin Med. (2022) 11:4776. doi: 10.3390/jcm11164776
17
Umbro I Fabiani V Fabiani M Angelico F Del Ben M . A systematic review on the association between obstructive sleep apnea and chronic kidney disease. Sleep Med Rev. (2020) 53:101337. doi: 10.1016/j.smrv.2020.101337
18
Jhamb M Ran X Abdalla H Roumelioti ME Hou S Davis H et al . Association of sleep apnea with mortality in patients with advanced kidney disease. Clin J Am Soc Nephrology: CJASN. (2020) 15:182–90. doi: 10.2215/CJN.07880719
19
Beaudin AE Raneri JK Ahmed S Hirsch Allen AJ Nocon A Gomes T et al . Association of insomnia and short sleep duration, alone or with comorbid obstructive sleep apnea, and the risk of chronic kidney disease. Sleep. (2022) 45:zsac088. doi: 10.1093/sleep/zsac088
20
Li J Huang Z Hou J Sawyer AM Wu Z Cai J et al . Sleep and CKD in chinese adults: A cross-sectional study. Clin J Am Soc Nephrology: CJASN. (2017) 12:885–92. doi: 10.2215/CJN.09270816
21
De Santo RM Di Iorio BR . History of sleep disorders in chronic kidney disease: first approach. Exp Clin transplantation: Off J Middle East Soc Organ Transplantation. (2023) 21:115–20. doi: 10.6002/ect.IAHNCongress.27
22
Pottel H Björk J Courbebaisse M Couzi L Ebert N Eriksen BO et al . Development and validation of a modified full age spectrum creatinine-based equation to estimate glomerular filtration rate: A cross-sectional analysis of pooled data. Ann Internal Med. (2021) 174:183–91. doi: 10.7326/M20-4366
23
Gauld C Lopez R Geoffroy PA Morin CM Guichard K Giroux É et al . A systematic analysis of ICSD-3 diagnostic criteria and proposal for further structured iteration. Sleep Med Rev. (2021) 58:101439. doi: 10.1016/j.smrv.2021.101439
24
Xu S Li Y Ye J Han D . Sleep medicine in China: current clinical practice. J Clin sleep medicine: JCSM: Off Publ Am Acad Sleep Med. (2023) 19:2125–31. doi: 10.5664/jcsm.10784
25
Diaz S Abad K Patel SR Unruh ML . Emerging treatments for insomnia, sleep apnea, and restless leg syndrome among dialysis patients. Semin nephrology. (2021) 41:526–33. doi: 10.1016/j.semnephrol.2021.10.005
26
Hui L Benca R . The bidirectional relationship between obstructive sleep apnea and chronic kidney disease. J stroke cerebrovascular diseases: Off J Natl Stroke Assoc. (2021) 30:105652. doi: 10.1016/j.jstrokecerebrovasdis.2021.105652
27
Pandi-Perumal SR Srinivasan V Spence DW Cardinali DP . Role of the melatonin system in the control of sleep: therapeutic implications. CNS Drugs. (2007) 21:995–1018. doi: 10.2165/00023210-200721120-00004
28
Spiegelhalder K Nissen C Riemann D . Clinical sleep-wake disorders II: focus on insomnia and circadian rhythm sleep disorders. Handb Exp Pharmacol. (2019) 253:261–76. doi: 10.1007/164_2017_40
29
Movahhed SMM . Possible benefits of exogenous melatonin for individuals on dialysis: a narrative review on potential mechanisms and clinical implications. Naunyn-Schmiedeberg’s Arch Pharmacol. (2021) 394:1599–611. doi: 10.1007/s00210-021-02099-x
30
El Agaty SM Khedr S Mostafa DKM Wanis NA Abou-Bakr DA . Protective role of melatonin against diclofenac-induced acute kidney injury. Life Sci. (2024) 353:122936. doi: 10.1016/j.lfs.2024.122936
31
Kusirisin P Apaijai N Noppakun K Kuanprasert S Chattipakorn SC Chattipakorn N . Protective effects of melatonin on kidney function against contrast media-induced kidney damage in patients with chronic kidney disease: A prospective, randomized, double-blinded, placebo-controlled trial. J pineal Res. (2025) 77:e70031. doi: 10.1111/jpi.70031
32
Asghar MS Ahsan MN Jawed R Rasheed U Ali Naqvi SA Hassan M et al . A comparative study on the use of alprazolam and melatonin for sleep disturbances in hemodialysis patients. Cureus. (2020) 12:e11754. doi: 10.7759/cureus.11754
33
Fairley AS Mathis KW . Cholinergic agonists reduce blood pressure in a mouse model of systemic lupus erythematosus. Physiol Rep. (2017) 5:e13213. doi: 10.14814/phy2.13213
34
Pham GS Wang LA Mathis KW . Pharmacological potentiation of the efferent vagus nerve attenuates blood pressure and renal injury in a murine model of systemic lupus erythematosus. Am J Physiol Regulatory Integr Comp Physiol. (2018) 315:R1261–r71. doi: 10.1152/ajpregu.00362.2017
35
Maung SC El Sara A Chapman C Cohen D Cukor D . Sleep disorders and chronic kidney disease. World J nephrology. (2016) 5:224–32. doi: 10.5527/wjn.v5.i3.224
36
Koh JH Lim CYJ Yam KJM Yeo BSY Ng ACW Loh SRH et al . Bidirectional association of sleep disorders with chronic kidney disease: a systematic review and meta-analysis. Clin Kidney J. (2024) 17:sfae279. doi: 10.1093/ckj/sfae279
Summary
Keywords
sleep disorders, chronic kidney disease, NHANES, inflammatory markers, chronic non-communicable diseases
Citation
Xiao H, Liu K, Hong G and Zhang X (2025) Association between self-reported sleep disorders and prevalence of chronic kidney disease in a nationally representative sample of U.S. adults. Front. Psychiatry 16:1570723. doi: 10.3389/fpsyt.2025.1570723
Received
04 February 2025
Accepted
29 October 2025
Published
18 November 2025
Volume
16 - 2025
Edited by
Daniele Corbo, University of Brescia, Italy
Reviewed by
Nicolas Padilla-Raygoza, Institute of Public Health of the State of Guanajuato (ISAPEG), Mexico
Hotma Rumahorbo, Bandung Health Polytechnic, Indonesia
Updates
Copyright
© 2025 Xiao, Liu, Hong and Zhang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinzhou Zhang, zhang.xinzhou@szhospital.com; Guobao Hong, hongguobao101@hotmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.