- 1Alzheimer Center Amsterdam, Department of Neurology, Amsterdam University Medical Center (UMC) location Vrije Universiteit Medical Center (VUmc), Vrije Universiteit Amsterdam, Amsterdam, Netherlands
- 2Amsterdam Neuroscience, Neurodegeneration, Amsterdam, Netherlands
- 3Neurochemistry Laboratory, Department of Clinical Chemistry, Amsterdam Neuroscience, Program Neurodegeneration, Amsterdam University Medical Center (UMC), Vrije Universiteit Amsterdam, Amsterdam, Netherlands
- 4Faculty of Behavioural and Movement Sciences, Clinical Developmental Psychology & Clinical Neuropsychology, Vrije Universiteit Amsterdam, Amsterdam, Netherlands
- 5Department of Radiology & Nuclear Medicine, Amsterdam Neuroscience, Vrije Universiteit Amsterdam, Amsterdam University Medical Center (UMC), Amsterdam, Netherlands
- 6Department of Medical Psychology, Amsterdam Public Health Research Institute, University of Amsterdam, Amsterdam University Medical Center (UMC), Amsterdam, Netherlands
- 7Department of Psychiatry, Amsterdam University Medical Center (UMC) location Universiteit Medical Center (VUmc), Amsterdam, Netherlands
Introduction: Depressive/anxiety symptoms are common in subjective cognitive decline (SCD) and may relate to Alzheimer’s pathology, potentially modulated by personality characteristics.
Methods: Depressive/anxiety symptoms were assessed over 4 ± 2 years in 329 SCD (88 amyloid-positive/241 amyloid-negative) using Geriatric Depression Scale-15 (GDS), Center for Epidemiological Studies-Depression (CES-D), and Hospital Anxiety and Depression Scale-Anxiety (HADS-A). Mixed-effects models assessed associations between amyloid status and these symptoms, with neuroticism and somatization as effect-modifiers.
Results: Amyloid status was not directly associated with GDS, CES-D or HADS-A. However, neuroticism modified the association between amyloid status and GDS (p<0.05). In lower neuroticism, amyloid positivity was associated with GDS increase (β:0.10 ± 0.08), but not in higher neuroticism (β:-0.04 ± 0.12). Somatization modified the association between amyloid status and CES-D (p<0.05). In lower somatization, amyloid positivity was associated with CES-D increase (β:0.65 ± 0.23), but not in higher somatization (β:-0.12 ± 0.29).
Discussion: Amyloid-positive individuals with lower neuroticism/somatization increased more in depressive symptoms over time, suggesting a preclinical AD-related depressive phenotype.
1 Introduction
Subjective cognitive decline (SCD) has gained increasing interest as a potential early stage of Alzheimer’s Disease (AD). SCD is defined by self-reported cognitive decline, in the absence of objective cognitive impairment (1). SCD can occur as one of the earliest symptomatic expressions of underlying AD (also recognized as stage 2 AD). More often however, SCD is not related to early neurodegenerative diseases, but rather to personality features, psychiatric disorders, lifestyle factors, or systemic illnesses (2–5).
In addition to the subjective experience of cognitive decline, individuals with SCD frequently exhibit symptoms of depression and anxiety (9–11), which can already compromise daily functioning even at the earliest stages of cognitive decline (12, 13). Previous studies report an association between amyloid pathology and symptoms of depression and anxiety in cognitively unimpaired individuals (14–16). However, findings in individuals with SCD were more inconsistent, with some studies identifying an association between amyloid pathology and symptoms of depression and anxiety, while other studies did not (17–20). The origin of symptoms of depression and anxiety in individuals with SCD is multifaceted and may be influenced by various factors (21). These symptoms may be underpinned by pathophysiological alterations associated with AD, such as the accumulation of beta-amyloid and tau, and could therefore be a direct symptom of AD (22). Alternatively, symptoms of depression and anxiety may be entirely unrelated to underlying AD pathology. Personality characteristics such as neuroticism which involves a tendency towards negative emotions, and somatization, characterized by experiencing psychological distress as somatic symptoms, may influence and exacerbate how individuals with SCD experience symptoms of depression and anxiety (6). Additionally, previous evidence suggests that neuroticism may increase susceptibility to Alzheimer’s disease pathology and its progression, although the nature of this association has not been fully elucidated (7, 8). Given prior evidence that individuals with SCD have higher levels of neuroticism and poorer perceived physical health, we hypothesized that neuroticism and somatization may influence the trajectories of symptoms of depression and anxiety in this population and potentially serve as effect modifiers in the association between amyloid status and symptoms of depression and anxiety (23).
Our aims were to investigate (1) whether amyloid status at baseline is associated with the longitudinal course of symptoms of depression and anxiety in individuals with SCD and (2) the influence of neuroticism and somatization on symptoms of depression and anxiety and their modulating effect on the association between amyloid status and symptoms of depression and anxiety.
2 Methods
2.1 Participants
We included 329 participants with SCD from the Subjective Cognitive Impairment Cohort (SCIENCe), embedded within the Amsterdam Dementia Cohort at the Alzheimer Center Amsterdam, Amsterdam UMC for whom baseline amyloid status had been assessed using cerebrospinal fluid (CSF) or positron emission tomography (PET). SCIENCe participants were recruited from our tertiary memory clinic, where they were primarily referred by their general physician, neurologist or geriatrician. The main inclusion criteria of SCIENCe are (1) a diagnosis of SCD, defined as the subjective experience of cognitive decline, without objective cognitive impairment on neuropsychological testing across all cognitive domains, including memory, language, attention/processing speed, and executive functioning, and (2) an age of 45 years or older. Exclusion criteria were (1) cognitive complaints caused by mild cognitive impairment, dementia or other neurological diseases or major psychiatric disorders as defined by the DSM-5, including individuals with a GDS score above 6 and evidence of a depressive disorder (2) history of alcohol/substance abuse. A full description of the SCIENCe inclusion and exclusion criteria has been published previously (24). All included participants underwent a standardized workup at baseline that included an assessment of their medical history, physical examination, neurological examination, psychological questionnaires, neuropsychiatric questionnaires, neuropsychological assessments, laboratory testing, and cerebrospinal fluid (CSF) and/or amyloid positron emission tomography (PET). During annual follow-up visits, we repeated the neuropsychiatric questionnaires and neuropsychological assessments, and reevaluated diagnoses to determine whether participants had progressed to MCI, AD dementia or other types of dementia (i.e., vascular dementia, dementia with Lewy bodies or frontotemporal dementia). On average, the follow-up time was 4±2 years.
2.2 Amyloid status
We determined amyloid status of all participants using amyloid PET (n=231) or CSF biomarkers (n=98). When both PET and CSF were available, we used PET to determine amyloid status. Amyloid PET scans were conducted using either [18F]florabetapir (n=144), [18F]florabetaben (n=80), [18F]flutemetamol (n=5) or [11C]Pittsburgh compound-B (n=2) radiotracers, and were acquired on the following systems: Gemini TF PET/CT, Ingenuity TF PET/CT, and Ingenuity PET/MRI (Philips Healtcare, Best, The Netherlands). A more detailed description of the PET protocol is provided elsewhere (24, 25). Trained nuclear medicine physicians visually rated the amyloid PET scans as “amyloid positive” or “amyloid negative”. CSF was obtained by performing a lumbar puncture at the L3/L4, L4/L5 or L5/S1 intervertebral space, using an atraumatic 25-gauge needle, which was then collected into a polypropylene tube, centrifuged, aliquoted into 0.5ml and stored at -80°C until further analysis. CSF was analyzed using either Innotest ELISA (n=58, Innogenetics-Fujirebio, Ghent, Belgium) or Elecsys (n=40, Roche Diagnostics GmbH, Mannheim, Germany). Innotest Aβ42 values were adjusted in the CSF biomarker analyses, to account for drift that occurred over the years (26). Amyloid positivity was defined as a drift-corrected concentration of <813 pg/ml for Innotest (26) and as a pTau/Aβ42 ratio of >0.02 for Elecsys (27).
2.3 Symptoms of depression and anxiety
We assessed symptoms of depression using the Geriatric Depression Scale-15 (GDS) and the Center for Epidemiological Studies Depression scale (CES-D), both self-reported questionnaires (28, 29). The GDS consists of 15 items with a total score ranging from 0 to 15, and the CES-D consists of 20 items with a score ranging from 0 to 60, with higher score indicating more severe symptoms of depression for both questionnaires. We assessed symptoms of anxiety using the Hospital Anxiety & Depression Scale – Anxiety subscale (HADS-A) (30). The HADS-A is a self-reported screening tool, which consists of 7 items, rating on a scale from 0 to 3. The total score of the HADS-A ranges from 0 to 21, with higher score indicating more severe symptoms of anxiety. During the study duration, we obtained a total of 1015 GDS datapoints, with a median of 3 measurements per participant. For CES-D and HADS-A, we obtained 1484 and 1485 datapoints, respectively, with a median of 4 measurements per participant.
2.4 Personality characteristics
At baseline, we assessed neuroticism using the Dutch Personality Questionnaire – Neuroticism subscale (DPQ-N) (31). The DPQ-N was shortened from 20 to 15 items, with a total score ranging from 0 to 30, with higher scores indicating a greater level of neuroticism. We assessed somatization using the Four-Dimensional Symptom Questionnaire – Somatization subscale (4DKL-S) (32). The 4DKL-S consists of 16 items, with total score ranging from 0 to 32, with higher score indicating a higher level of somatization.
2.5 Statistical analysis
All statistical analyses were performed in Rstudio version 2022.12.0 (RStudio, Inc., Boston, MA, USA), with packages ‘lme4’ version 1.1–30 and ‘lmtest’ version 0.9-40. To compare differences in demographic characteristics, we used the independent t-test, Mann-Whitney U test or Pearson χ2, depending on the data distribution. First, we used linear mixed models (LMM) with only time as a predictor to examine the trajectory of symptoms of depression and anxiety over time in the total group. Next, we analyzed associations between amyloid status and symptoms of depression and anxiety with models including terms for amyloid status (positive/negative), time and amyloid status*time interaction. Separate models were used with GDS, CES-D and HADS-A as outcome variable. Similarly, we used LMMs to analyze associations between neuroticism and somatization (separate models) and symptoms of depression and anxiety, with terms for each personality characteristic, time and personality characteristic*time. Subsequently, we investigated the potentially modifying effect of neuroticism and somatization, constructing models including terms for amyloid status, time, effect modifier (neuroticism or somatization), and amyloid status*time*effect modifier interaction. When the three-way interaction term (amyloid status*time*effect modifier) was significant (p<0.05), we considered this as evidence of effect modification and used a median split of the effect modifier to visualize the interaction and to perform post hoc analyses stratified by “low” and “high” levels of neuroticism and somatization to explore the nature of the interaction effect.
3 Results
3.1 Demographic characteristics
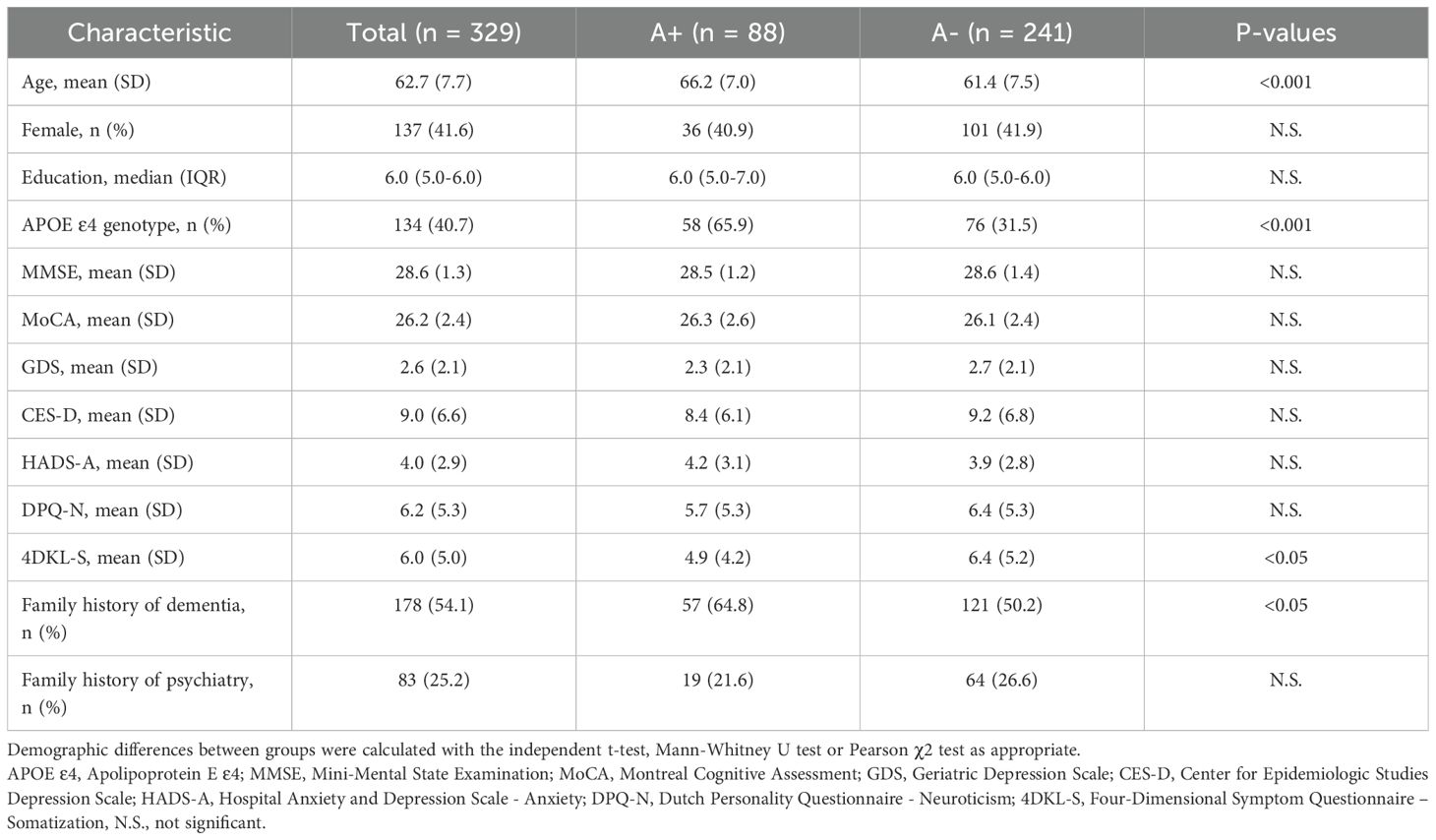
We included 329 participants, of whom 88 (26.7%) were amyloid positive (A+) and 241 (73.3%) were amyloid negative (A-; Table 1). A+ participants (66.2±7.0) were somewhat older than A- participants (61.4±7.5; p<0.001). Somatization (4DKL-S) was lower in the A+ group (4.9±4.2) than in the A- group (6.4±5.2; p<0.05). Distribution of sex, educational level, baseline MMSE, and neuroticism (DPQ-N) did not differ according to amyloid status. At baseline, symptoms of depression and anxiety across the entire group were: GDS 2.6±2.1, CES-D 9.0±6.6, and HADS-A 4.0±2.9. Longitudinally, CES-D slightly increased over time (β±SETime 0.18±0.07; p<0.05), while the scores of GDS and HADS-A remained stable over time (βTime -0.01±0.03, -0.02±0.03). The demographic and clinical characteristics of the included participants are summarized in Table 1.
3.2 Associations between amyloid status and symptoms of depression and anxiety
Univariate LMMs showed no significant associations between amyloid status and GDS, CES-D and HADS-A at baseline or over time (Table 2, Figure 1A). However, we observed a trend towards a more pronounced increase in CES-D over time in A+ than in A- (βTime*Amyloid status 0.32 ± 0.19; p=0.088).
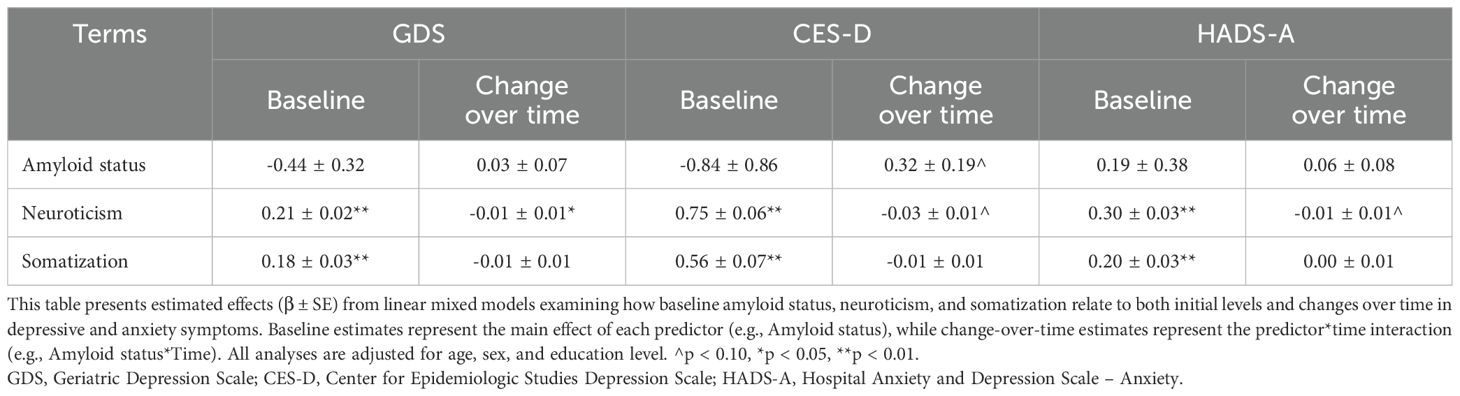
Table 2. Linear mixed models of the association between amyloid status & personality characteristics and symptoms of depression and anxiety.

Figure 1. Associations between amyloid status & personality characteristics and symptoms of depression and anxiety over time. Figures show symptom trajectories of depression and anxiety over 8 years based on linear mixed models for GDS (left column), CES-D (middle column), and HADS-A (right column), with shaded areas representing 95% confidence intervals. (A–C) show comparisons by amyloid status (red = negative, blue = positive), neuroticism, and somatization (red = low, blue = high), with neuroticism and somatization groups defined by a median split. GDS, Geriatric Depression Scale; CES-D, Center for Epidemiologic Studies Depression Scale; HADS-A, Hospital Anxiety and Depression Scale – Anxiety.
3.2 Associations between personality characteristics and symptoms of depression and anxiety
Higher neuroticism levels were associated with higher baseline GDS, CES-D and HADS-A (βNeuroticism 0.21 ± 0.02; 0.75 ± 0.06; 0.30 ± 0.03; all p<0.001; Table 2, Figure 1B). Longitudinally, higher neuroticism levels were associated with a slight decrease over time in GDS (βTime*Neuroticism -0.01 ± 0.01, p<0.05), while no associations were observed in CES-D and HADS-A over time. Likewise, higher somatization levels were associated with higher baseline GDS, CES-D and HADS-A (βTime*Neuroticism 0.18 ± 0.03; 0.56 ± 0.07; 0.20 ± 0.03, all p<0.001; Figure 1C), but not with change over time in any of the measures.
3.3 Effect modification of the association between amyloid status and symptoms of depression and anxiety
Subsequently, we evaluated whether the associations between amyloid status and symptoms of depression or anxiety over time were modified by neuroticism and somatization. Using three-way interaction models, we found that neuroticism modified the association between amyloid status and change in GDS over time (p<0.05; Table 3, Figure 2A), with a stronger association observed in individuals with low neuroticism levels compared to those with high neuroticism levels. Specifically, amyloid positivity was more strongly associated with an increase in GDS over time compared to amyloid negativity (βTime*Amyloid status 0.10 ± 0.08) in individuals with lower neuroticism, whereas this association was not observed in those with higher neuroticism (βTime*Amyloid status -0.04 ± 0.12). After stratification, we found a similar trend for the association between amyloid status and CES-D over time; the three-way interaction did not reach statistical significance, but its effect size was very similar to the effect size of the interaction term with GDS as outcome (βTime*Amyloid status*Neuroticism -0.04 ± 0.04, p=0.284). Similarly, amyloid positivity was more strongly associated with an increase in CES-D over time compared to amyloid negativity (βTime*Amyloid status 0.50 ± 0.19) in individuals with lower neuroticism, whereas this association was not observed in those with higher neuroticism (βTime*Amyloid status 0.03 ± 0.33).

Table 3. Linear mixed model of the association between amyloid status and symptoms of depression and anxiety with personality characteristics as effect modifier.
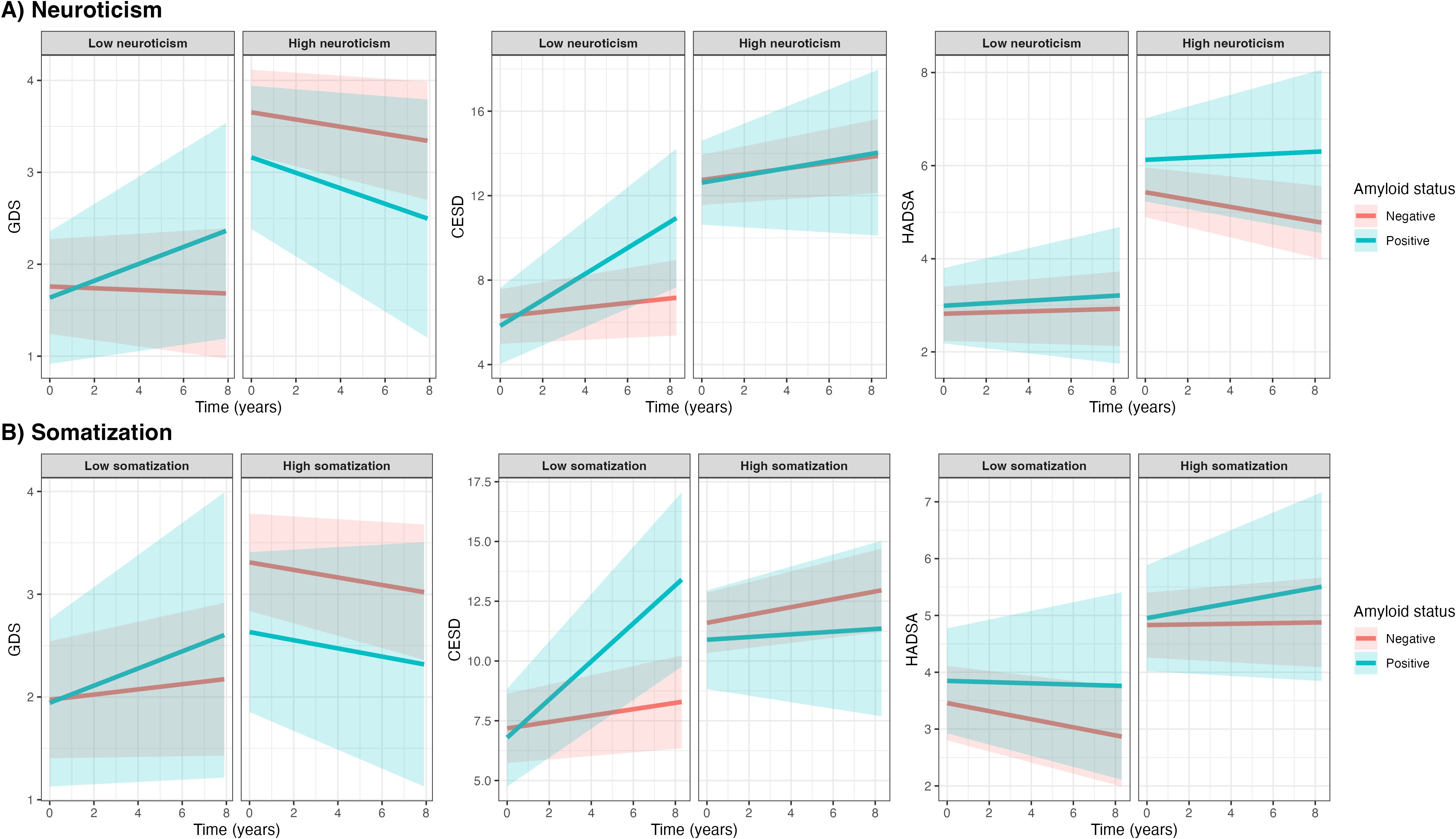
Figure 2. Associations between amyloid status and symptoms of depression and anxiety with personality characteristics as effect modifiers. Figures show symptom trajectories of depression and anxiety over 8 years by amyloid status, based on linear mixed models stratified by low or high neuroticism and somatization (A, B, respectively), using median splits (median neuroticism = 5; median somatization = 5). Shaded areas represent 95% confidence intervals. Diverging patterns between high and low groups suggest that neuroticism and somatization may modulate the association between amyloid status and symptoms of depression and anxiety.
Somatization modified the association between amyloid status and change in CES-D over time (p<0.05; Table 3, Figure 2B), with a stronger association observed in individuals with low somatization levels compared to those with high somatization levels. Amyloid positivity was more strongly associated to an increase in CES-D over time compared to amyloid negativity (βTime*Amyloid status 0.65 ± 0.23) in individuals with low somatization, whereas this association was not observed in those with higher somatization (βTime*Amyloid status -0.12 ± 0.29). The association between amyloid status and HADS-A was not modified by either neuroticism or somatization.
4 Discussion
The main finding of this longitudinal study is that there was no simple association between amyloid status and symptoms of depression and anxiety over time in individuals with SCD. Neuroticism and somatization modified the association with symptoms of depression, although not for the association with symptoms of anxiety. Individuals with higher levels of neuroticism and somatization exhibited higher baseline levels of symptoms of depression, regardless of amyloid status, suggesting these symptoms may stem from personality characteristics or other factors. In contrast, in those with lower levels of neuroticism and somatization, amyloid positivity was associated with a steeper increase in symptoms of depression over time. These findings suggest a potential preclinical AD-specific trajectory of symptoms of depression that develops independently of traditional psychiatric risk factors such as neuroticism and somatization.
Research into the influence of personality characteristics on the association between amyloid pathology and symptoms of depression and anxiety in individuals with SCD is limited, with a notable scarcity of longitudinal studies examining the progression of symptoms of depression and anxiety over time. We investigated the association between amyloid pathology and symptoms of depression and anxiety, providing a longitudinal analysis in a relatively large group of individuals with SCD with substantial follow-up. Former findings have been inconsistent, suggesting that other factors also play a role in this association. For this reason, we explored the modulating effects of neuroticism and somatization.
A recent systematic review demonstrated that amyloid pathology was associated with more severe symptoms of depression and anxiety in community dwelling elderly (14). However, studies examining the association between AD biomarkers and symptoms of depression and anxiety in individuals with SCD have been more inconsistent, with some studies reporting associations with more severe symptoms of depression (18, 19), while others did not (17, 20). These inconsistent findings could be partially due to most previous studies being cross-sectional and not accounting for personality characteristics, which we specifically considered in our study. Moreover, the unique nature of the SCD population likely contributes to these inconsistencies. Individuals with SCD, who present with cognitive complaints and worries, likely differ from community-dwelling elderly without SCD. This difference may explain that symptoms of depression and anxiety in SCD might be less directly attributable to AD pathology and more influenced by a broader range of effect-modifying factors, such as personality characteristics, stress responses, maladaptive coping strategies, and pervasive worries. One plausible explanation is that individuals with SCD, particularly those who seek help for cognitive complaints at memory clinics, are likely to have more pronounced concerns, which tend to correlate with higher levels of neuroticism and poorer perceived physical health compared to community-dwelling adults (23, 33). Neuroticism and somatization are well-known personality characteristics and risk factors, which lead to higher degree of depression and anxiety (34, 35). Interestingly, while neuroticism and somatization usually give rise to more psychiatric symptoms and comorbidity, we found a relative stronger increase of depressive symptoms over time in individuals with lower neuroticism and somatization and evidence for amyloid pathology. This suggests state-dependent changes related to a preclinical AD profile in absence of common psychiatric risk factors. While this finding needs further investigation, it is possible that amyloid pathology directly contributes to depressive symptoms as one of the earliest AD-related manifestations. Although the underlying mechanisms are not yet fully understood, amyloid accumulation has been proposed to contribute to affective disturbances through inflammatory pathways, monoaminergic system disruption, or neurocircuitry modifications (21, 22). In contrast, individuals with higher levels of neuroticism and somatization consistently exhibited more severe symptoms of depression and anxiety, regardless of their amyloid status, but these symptoms remained more ‘stable’ over time, which likely illustrates a trait characteristic. This suggests that their symptoms of depression and anxiety may be influenced more by their personality characteristics than by amyloid pathology, underscoring the possibility that higher levels of neuroticism and somatization overshadows or obscures the direct effect of amyloid pathology on the manifestation of depressive symptoms.
Our study is subject to several limitations. First, we excluded all individuals where SCD was primarily explained by psychiatric diseases, including major depression and anxiety disorders. While this allowed us to focus on the association between AD pathology and symptoms of depression and anxiety in a clinically well-defined SCD population, it may limit the generalizability of our findings to the broader older adult population, particularly those with more than subclinical, diagnosed psychiatric disorders. Second, the inclusion of individuals with only subclinical symptoms may have led to an underestimation of the associations between amyloid status and symptoms of depression and anxiety. While we observed moderating effects of neuroticism and somatization, the effect sizes were modest, underscoring the subtle nature of these associations. This may also be partly attributed to sample size limitations or the sensitivity of the measurement instruments, suggesting the need for further investigation in larger and more diverse cohorts. Third, our study did not include a control group of community dwelling elderly without SCD. Therefore, we cannot compare our observed scores on symptoms of depression and anxiety and personality characteristics between individuals with and without SCD. This restricts our ability to fully understand the extent to which these factors are specific to SCD. Nonetheless, the clinical set-up makes our findings a highly relevant for clinical practice.
In conclusion, in individuals with low levels of neuroticism or somatization, amyloid positivity was associated with a greater increase in depressive symptoms over time, suggesting a possible preclinical AD-related depressive profile that exists independently of traditional psychiatric risk factors. In contrast, individuals with high levels of neuroticism or somatization had higher levels of depression regardless of amyloid status, suggesting that their symptoms of depression are more likely to have a different origin, possibly stemming from their personality characteristics or other factors. This underscores the necessity for a personalized approach when assessing and managing symptoms of depression and anxiety in individuals with SCD.
Data availability statement
Data will be shared (anonymized) within the boundaries imposed by the informed consent and data sharing legislation.
Ethics statement
The studies involving humans were approved by Medical Ethical Committee of the VU University Medical Center. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
CT: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Software, Visualization, Writing – original draft, Writing – review & editing. MV: Project administration, Writing – review & editing. L-MS: Project administration, Writing – review & editing. JE: Project administration, Writing – review & editing. IV: Project administration, Resources, Writing – review & editing. SS: Writing – review & editing. SV: Investigation, Writing – review & editing. EV: Writing – review & editing. CET: Conceptualization, Investigation, Supervision, Writing – original draft, Writing – review & editing. WV: Conceptualization, Investigation, Methodology, Resources, Supervision, Writing – original draft, Writing – review & editing. AV: Conceptualization, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Research of Alzheimer Center Amsterdam is part of the neurodegeneration research program of Amsterdam Neuroscience. Alzheimer Center Amsterdam is supported by Stichting Alzheimer Nederland and Stichting Steun Alzheimercentrum Amsterdam. The chair of Wiesje van der Flier is supported by the Pasman stichting. The SCIENCe project receives funding from stichting Dioraphte and from the Noaber foundation.
Conflict of interest
WV reports that her research programs have been funded by ZonMW, NWO, EU-JPND, EU-IHI, Alzheimer Nederland, Hersenstichting, CardioVascular Onderzoek Nederland, HealthHolland, Topsector Life Sciences & Health, Stichting Dioraphte, Gieskes-Strijbis Fonds, Stichting Equilibrio, Edwin Bouw Fonds, Pasman Stichting, Stichting Alzheimer & Neuropsychiatrie Foundation, Philips, Biogen MA Inc., Novartis-NL, Life-MI, AVID, Roche BV, Eli Lilly-NL, Fujifilm, Eisai, and Combinostics. WV holds the Pasman Chair. WV is the recipient of ABOARD, a public-private partnership funded by ZonMW #73305095007 and HealthHolland, Topsector Life Sciences & Health PPP-allowance; #LSHM20106. WV is also the recipient of TAP-dementia, funded by ZonMW #10510032120003, with co-financing from Avid Radiopharmaceuticals and Amprion. All funding is paid to her institution. WV has been an invited speaker at Biogen MA Inc., Danone, Eisai, WebMD Neurology Medscape, Novo Nordisk, Springer Healthcare, and the European Brain Council, with all funding paid to her institution. WV has consulted for Oxford Health Policy Forum CIC, Roche, Biogen MA Inc., and Eisai, with all funding paid to her institution. WV has participated in advisory boards for Biogen MA Inc., Roche, and Eli Lilly and is a member of the steering committee of EVOKE/EVOKE+ Novo Nordisk. She is also a member of the steering committee of PAVE and Think Brain Health. WV was associate editor of Alzheimer Research & Therapy in 2020/2021 and is currently an associate editor at Brain. CET reports that her research is supported by the European Commission through several grants, including the Marie Curie International Training Network grant agreement No. 860197, MIRIADE, TAME, Innovative Medicines Initiatives 3TR Horizon 2020, grant No. 831434, EPND IMI 2 Joint Undertaking JU, grant No. 101034344, JPND bPRIDE, CCAD, and the European Partnership on Metrology, co-financed by the European Union’s Horizon Europe Research and Innovation Programme and Participating States 22HLT07 NEuroBioStand. CET is also supported by the CANTATE project funded by the Alzheimer Drug Discovery Foundation, the Alzheimer Association, the Michael J. Fox Foundation, Health Holland, the Dutch Research Council ZonMW, the Alzheimer Drug Discovery Foundation, The Selfridges Group Foundation, and Alzheimer Netherlands. CET is the recipient of ABOARD, a public-private partnership funded by ZonMW #73305095007 and Health~Holland, Topsector Life Sciences & Health PPP-allowance; #LSHM20106, and TAP-dementia, a project funded by ZonMW #10510032120003 in the context of the Dutch National Dementia Strategy. CET has research contracts with Acumen, ADx Neurosciences, AC-Immune, Alamar, Aribio, Axon Neurosciences, Beckman-Coulter, BioConnect, Bioorchestra, Brainstorm Therapeutics, Celgene, Cognition Therapeutics, EIP Pharma, Eisai, Eli Lilly, Fujirebio, Instant Nano Biosensors, Novo Nordisk, Olink, PeopleBio, Quanterix, Roche, Toyama, and Vivoryon. CET is the editor-in-chief of Alzheimer Research & Therapy and serves on the editorial boards of Molecular Neurodegeneration, Neurology: Neuroimmunology & Neuroinflammation, and Medidact Neurologie/Springer. CET also serves on committees defining guidelines for cognitive disturbances and acute neurology in the Netherlands. CET has had consultancy and speaker contracts with Aribio, Biogen, Beckman-Coulter, Cognition Therapeutics, Eli Lilly, Merck, Novo Nordisk, Olink, Roche, and Veravas.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Jessen F, Amariglio RE, van Boxtel M, Breteler M, Ceccaldi M, Chételat G, et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement. (2014) 10:844–52. doi: 10.1016/j.jalz.2014.01.001
2. Janssen O, Jansen WJ, Vos SJB, Boada M, Parnetti L, Gabryelewicz T, et al. Characteristics of subjective cognitive decline associated with amyloid positivity. Alzheimers Dement. (2022) 18:1832–45. doi: 10.1002/alz.12512
3. Merema MR, Speelman CP, Foster JK, and Kaczmarek EA. Neuroticism (Not depressive symptoms) predicts memory complaints in some community-dwelling older adults. Am J Geriatr Psychiatry. (2013) 21:729–36. doi: 10.1016/j.jagp.2013.01.059
4. Mewton L, Sachdev P, Anderson T, Sunderland M, and Andrews G. Demographic, clinical, and lifestyle correlates of subjective memory complaints in the Australian population. Am J Geriatr Psychiatry. (2014) 22:1222–32. doi: 10.1016/j.jagp.2013.04.004
5. Jack CR Jr., Andrews JS, Beach TG, Buracchio T, Dunn B, Graf A, et al. Revised criteria for diagnosis and staging of Alzheimer’s disease: Alzheimer’s association workgroup. Alzheimers Dement. (2024) 20:5143–69. doi: 10.1002/alz.13859
6. Aschwanden D, Sutin AR, Ledermann T, Luchetti M, Stephan Y, Sesker AA, et al. Subjective cognitive decline: is a resilient personality protective against progression to objective cognitive impairment? Findings from two community-based cohort studies. J Alzheimers Dis. (2022) 89:87–105. doi: 10.3233/jad-220319
7. Terracciano A, Luchetti M, Stephan Y, Löckenhoff CE, Ledermann T, and Sutin AR. Changes in personality before and during cognitive impairment. J Am Med Dir Assoc. (2023) 24:1465–70.e1. doi: 10.1016/j.jamda.2023.05.011
8. Terracciano A, Bilgel M, Aschwanden D, Luchetti M, Stephan Y, Moghekar AR, et al. Personality associations with amyloid and tau: results from the baltimore longitudinal study of aging and meta-analysis. Biol Psychiatry. (2022) 91:359–69. doi: 10.1016/j.biopsych.2021.08.021
9. Eikelboom WS, van den Berg E, Singleton EH, Baart SJ, Coesmans M, Leeuwis AE, et al. Neuropsychiatric and cognitive symptoms across the Alzheimer disease clinical spectrum: cross-sectional and longitudinal associations. Neurology. (2021) 97:e1276–e87. doi: 10.1212/wnl.0000000000012598
10. Masters MC, Morris JC, and Roe CM. Noncognitive” Symptoms of early alzheimer disease. Neurology. (2015) 84:617. doi: 10.1212/WNL.0000000000001238
11. Jenkins A, Tree J, and Tales A. Distinct profile differences in subjective cognitive decline in the general public are associated with metacognition, negative affective symptoms, neuroticism, stress, and poor quality of life. J Alzheimer’s Dis. (2021) 80:1231–42. doi: 10.3233/JAD-200882
12. Giannouli V and Tsolaki M. Mild Alzheimer disease, financial capacity, and the role of depression: eyes wide shut? Alzheimer Dis Assoc Disord. (2021) 35:360–2. doi: 10.1097/wad.0000000000000427
13. Giannouli V, Stamovlasis D, and Tsolaki M. Longitudinal study of depression on amnestic mild cognitive impairment and financial capacity. Clin Gerontol. (2022) 45:708–14. doi: 10.1080/07317115.2021.2017377
14. Ng KP, Chiew H, Rosa-Neto P, Kandiah N, Ismail Z, and Gauthier S. Associations of at(N) biomarkers with neuropsychiatric symptoms in preclinical Alzheimer’s disease and cognitively unimpaired individuals. Transl Neurodegener. (2021) 10:11. doi: 10.1186/s40035-021-00236-3
15. Krell-Roesch J, Lowe VJ, Neureiter J, Pink A, Roberts RO, Mielke MM, et al. Depressive and anxiety symptoms and cortical amyloid deposition among cognitively normal elderly persons: the mayo clinic study of aging. Int Psychogeriatr. (2018) 30:245–51. doi: 10.1017/s1041610217002368
16. Lussier FZ, Pascoal TA, Chamoun M, Therriault J, Tissot C, Savard M, et al. Mild behavioral impairment is associated with B-amyloid but not tau or neurodegeneration in cognitively intact elderly individuals. Alzheimers Dement. (2020) 16:192–9. doi: 10.1002/alz.12007
17. Auning E, Selnes P, Grambaite R, Šaltytė Benth J, Haram A, Løvli Stav A, et al. Neurobiological correlates of depressive symptoms in people with subjective and mild cognitive impairment. Acta Psychiatr Scand. (2015) 131:139–47. doi: 10.1111/acps.12352
18. Kleineidam L, Wagner M, Guski J, Wolfsgruber S, Miebach L, Bickel H, et al. Disentangling the relationship of subjective cognitive decline and depressive symptoms in the development of cognitive decline and dementia. Alzheimers Dement. (2023) 19:2056–68. doi: 10.1002/alz.12785
19. Moulinet I, Touron E, Mézenge F, Dautricourt S, de la Sayette V, Vivien D, et al. Depressive symptoms have distinct relationships with neuroimaging biomarkers across the Alzheimer’s clinical continuum. Front Aging Neurosci. (2022) 14:899158. doi: 10.3389/fnagi.2022.899158
20. Zapater-Fajarí M, Diaz-Galvan P, Cedres N, Rydberg Sterner T, Rydén L, Sacuiu S, et al. Biomarkers of Alzheimer’s disease and cerebrovascular disease in relation to depressive symptomatology in individuals with subjective cognitive decline. J Gerontol A Biol Sci Med Sci. (2023) 79:glad216. doi: 10.1093/gerona/glad216
21. Geda YE, Schneider LS, Gitlin LN, Miller DS, Smith GS, Bell J, et al. Neuropsychiatric symptoms in Alzheimer’s disease: past progress and anticipation of the future. Alzheimers Dement. (2013) 9:602–8. doi: 10.1016/j.jalz.2012.12.001
22. Chen Y, Dang M, and Zhang Z. Brain mechanisms underlying neuropsychiatric symptoms in Alzheimer’s disease: A systematic review of symptom-general and -specific lesion patterns. Mol Neurodegener. (2021) 16:38. doi: 10.1186/s13024-021-00456-1
23. Jenkins A, Tree JJ, Thornton IM, and Tales A. Subjective cognitive impairment in 55-65-year-old adults is associated with negative affective symptoms, neuroticism, and poor quality of life. J Alzheimers Dis. (2019) 67:1367–78. doi: 10.3233/jad-180810
24. Slot RER, Verfaillie SCJ, Overbeek JM, Timmers T, Wesselman LMP, Teunissen CE, et al. Subjective cognitive impairment cohort (Science): study design and first results. Alzheimer’s Res Ther. (2018) 10:76. doi: 10.1186/s13195-018-0390-y
25. Ebenau JL, Timmers T, Wesselman LMP, Verberk IMW, Verfaillie SCJ, Slot RER, et al. Atn classification and clinical progression in subjective cognitive decline. Neurology. (2020) 95:e46. doi: 10.1212/WNL.0000000000009724
26. Tijms BM, Willemse EAJ, Zwan MD, Mulder SD, Visser PJ, van Berckel BNM, et al. Unbiased approach to counteract upward drift in cerebrospinal fluid amyloid-B 1–42 analysis results. Clin Chem. (2018) 64:576–85. doi: 10.1373/clinchem.2017.281055
27. Willemse EAJ, Tijms BM, van Berckel BNM, Le Bastard N, van der Flier WM, Scheltens P, et al. Comparing csf amyloid-beta biomarker ratios for two automated immunoassays, elecsys and lumipulse, with amyloid pet status. Alzheimers Dement (Amst). (2021) 13:e12182. doi: 10.1002/dad2.12182
28. Radloff LS. The ces-D scale: A self-report depression scale for research in the general population. Appl psychol Measure. (1977) 1:385–401. doi: 10.1177/014662167700100306
29. Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, et al. Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatr Res. (1982) 17:37–49. doi: 10.1016/0022-3956(82)90033-4
30. Zigmond AS and Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. (1983) 67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x
31. Luteijn F, Starren J, and van Dijk H. Manual for the Dutch personality questionnaire (Handleiding bij de npv). (1985).
32. Terluin B, van Marwijk HW, Adèr HJ, de Vet HC, Penninx BW, Hermens ML, et al. The four-dimensional symptom questionnaire (4dsq): A validation study of a multidimensional self-report questionnaire to assess distress, depression, anxiety and somatization. BMC Psychiatry. (2006) 6:34. doi: 10.1186/1471-244x-6-34
33. Steinberg SI, Negash S, Sammel MD, Bogner H, Harel BT, Livney MG, et al. Subjective memory complaints, cognitive performance, and psychological factors in healthy older adults. Am J Alzheimers Dis Other Demen. (2013) 28:776–83. doi: 10.1177/1533317513504817
34. Dijkstra-Kersten SMA, Sitnikova K, van Marwijk HWJ, Gerrits MMJG, van der Wouden JC, Penninx BWJH, et al. Somatisation as a risk factor for incident depression and anxiety. J Psychosomatic Res. (2015) 79:614–9. doi: 10.1016/j.jpsychores.2015.07.007
Keywords: Alzheimer’s disease, depression, anxiety, subjective cognitive decline, neuroticism, somatization
Citation: Trieu C, van Leeuwenstijn MSSA, Schlüter L-M, Ebenau JL, Verberk IMW, Sikkes SAM, Verfaillie SCJ, van de Giessen E, Teunissen CE, van der Flier WM and van Harten AC (2025) Longitudinal associations between amyloid and symptoms of depression and anxiety in subjective cognitive decline: the impact of personality characteristics. Front. Psychiatry 16:1572174. doi: 10.3389/fpsyt.2025.1572174
Received: 06 February 2025; Accepted: 20 May 2025;
Published: 10 June 2025.
Edited by:
Huan Yang, Central South University, ChinaReviewed by:
Vaitsa Giannouli, Aristotle University of Thessaloniki, GreeceHui Zhao, Nanjing Drum Tower Hospital, China
Copyright © 2025 Trieu, van Leeuwenstijn, Schlüter, Ebenau, Verberk, Sikkes, Verfaillie, van de Giessen, Teunissen, van der Flier and van Harten. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Calvin Trieu, Yy50cmlldUBhbXN0ZXJkYW11bWMubmw=
 Calvin Trieu
Calvin Trieu Mardou S. S. A. van Leeuwenstijn1,2
Mardou S. S. A. van Leeuwenstijn1,2 Inge M. W. Verberk
Inge M. W. Verberk Sietske A. M. Sikkes
Sietske A. M. Sikkes Sander C. J. Verfaillie
Sander C. J. Verfaillie Charlotte E. Teunissen
Charlotte E. Teunissen