Abstract
Background:
Appetite loss is common in major depressive disorder (MDD). However, the psychosocial and biological mechanisms behind appetite loss remain unclear, particularly in the adolescent MDD population. Therefore, this study aimed to examine the links between appetite loss and clinical symptoms as well as inflammatory cytokines levels in this population.
Methods:
Between January and December 2021, this study included 171 depressed adolescents. A range of scales were used to assess the patients’ clinical symptoms, including depression severity, negative life events, insomnia, and alexithymia. Additionally, plasma inflammatory cytokines levels were measured, including interleukin (IL)-1β, IL-6, IL-10, IL-17A and tumor necrosis factor-α (TNF-α).
Results:
The prevalence of appetite loss among adolescents with MDD was as high as 76.0%. Univariate analyses showed that patients with appetite loss had higher scores of the Hamilton Depression Scale (HAMD), interpersonal relationships, study pressure, punishment, sense of loss, the Insomnia Severity Index Scale (ISI) and difficulty identifying feelings, as well as higher levels of Log IL-6 (all p < 0.05) Furthermore, regression analyses revealed that appetite loss was independently associated with HAMD score (OR = 1.158, 95% CI = 1.091-1.229, p < 0.001), punishment score (OR = 1.117, 95% CI = 1.039-1.201, p = 0.003), and Log IL-6 level (OR = 5.041, 95% CI = 1.137-22.344, p = 0.033).
Conclusion:
Adolescents with MDD face an elevated risk of appetite loss, which may correlate with clinical symptoms such as depression severity and negative life events, as well as elevated IL-6 level. Healthcare professionals should target these risk factors, including inflammation, to mitigate appetite loss.
1 Introduction
Major depressive disorder (MDD) is a severe mental disorder projected to become the leading cause of disability globally by 2030 (1). In recent years, driven by social changes and increased academic stress, MDD has emerged as the most common affective disorder among adolescents (2). According to the latest epidemiological survey, the prevalence of MDD among Chinese children and adolescents has been as high as 2.0% (3). Compared to adults, MDD in adolescents is characterized by a longer disease course, more severe symptoms, and higher rates of recurrence and disability (4). In addition, changes in appetite are one of the core symptoms of MDD, with the direction varying according to the subtype. Typically, MDD is associated with appetite loss and weight loss (5). A recent cross-sectional study found that 56.5% of adults with MDD exhibited appetite loss (6). While adolescence is a critical period for the development of eating disorders, adolescents with MDD are more susceptible to a range of maladaptive eating behaviors due to their immature psychocognitive development (7). However, there is a notable lack of studies specifically exploring appetite loss in adolescents with MDD. Co-occurring appetite loss not only poses significant challenges to the treatment of MDD, but also seriously affects patients’ prognosis, growth and development. For example, a secondary analysis of randomized trials found that MDD patients with appetite loss had a poorer treatment response (8). Kitagawa and colleagues also identified appetite loss as a predictor of suicidal ideation and self-harm in adolescents (9). Therefore, understanding the mechanisms underlying appetite loss in adolescents with MDD is crucial for early screening, prevention and optimal treatment in this population.
Previous studies have shown that appetite loss in patients with MDD is associated with sociodemographic, clinical, and biochemical factors. Specifically, it may be linked to sociodemographic factors such as gender, age, and educational level. Additionally, appetite loss is also related to patients’ mental health symptoms. In adolescents with MDD, depressed mood can directly influence eating behaviors. There is evidence that a depressed mood can influence food choices by affecting appetite or altering food availability (10). A recent case-control study found that patients with decreased appetite symptoms also exhibited more severe depressive symptoms (11). Furthermore, research has indicated that stress resulting from negative life events can significantly affect eating behaviors of patients with MDD. For example, a longitudinal study showed that appetite loss was significantly associated with adverse life events (particularly the death of family members and loss of romantic relationships) in depressed adults (12). Also, studies on patients with eating disorders have revealed that both alexithymia and depressive symptoms are more pronounced in individuals with anorexia nervosa than in those with bulimia nervosa (13). Another study found that adolescent anorexia nervosa was also associated with insomnia symptoms (14). However, most of the studies mentioned above have focused on adults with MDD or on eating disorder groups. Therefore, there is a need to explore the psychological factors associated with appetite loss in adolescents with MDD, providing a theoretical basis for early clinical intervention.
Importantly, the combination of MDD with appetite loss may involve complex biological mechanisms. One of the hot topics in recent years is the role of inflammatory cytokines. A study of hospitalized elderly patients showed that appetite loss was significantly associated with elevated levels of several serum inflammatory cytokines, including interleukin (IL)-1β, IL-6, and IL-33 (15). Guo and colleagues found elevated levels of inflammatory cytokines in MDD patients with decreased appetite and suggested that this elevated inflammation might be caused by an abnormal gut microbiota (6). However, there were also studies that came to inconsistent conclusions. Okamoto and colleagues conducted a small-sample cross-sectional study (n = 40) and found that decreased appetite was associated with lower high-sensitivity C-reactive protein (hsCRP) levels in patients with MDD (16). Such inconsistencies may be attributed to the heterogeneity of sample sizes, study populations, and assessment tools. Therefore, additional relevant studies are needed.
This study aimed to preliminarily explore the associations between appetite loss and clinical symptoms (including depression severity, negative life events, insomnia, and alexithymia) as well as inflammatory cytokines levels in adolescents with MDD. And the goal was to provide a theoretical basis for understanding the mechanisms of appetite loss in this population and to inform early clinical interventions.
2 Methods
2.1 Study design and participants
This research was conducted as a cross-sectional study. Between January and December 2021, we enrolled adolescents diagnosed with MDD at two medical centers in Hefei, Anhui Province, China: the Chaohu Hospital of Anhui Medical University and the Fourth People’s Hospital of Hefei. Participants were required to meet the following criteria: (1) aged between 12 and 18 years; (2) diagnosed with MDD based on the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5); and (3) willing to actively engage and collaborate throughout the study period. Individuals were excluded if they met any of the following conditions: (1) diagnosed with other mental disorders such as schizophrenia or bipolar disorder according to DSM-5; (2) had major infections, autoimmune diseases, or other significant health issues that could influence the study outcomes; and (3) were currently undergoing treatment with anti-inflammatory drugs.
This study commenced after receiving ethical review and approval from the Ethics Committee of the Chaohu Hospital of Anhui Medical University, under reference number 202009-KYXM-04. All procedures were conducted in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Before enrolling participants, we provided a detailed explanation of the study procedures to both the participants and their parents or legal guardians, and obtained their written informed consent.
2.2 Measuring Instruments
2.2.1 Sociodemographic characteristics
In this study, participants were asked to provide various sociodemographic details through a custom-designed survey. The information gathered included their gender, age, body mass index (BMI), age at onset, duration of illness, and types of antidepressants.
2.2.2 Depressive symptoms
The Hamilton Depression Scale (HAMD) was employed to evaluate the severity of depressive symptoms exhibited by the patients over the past week (17). The HAMD consists of 24 items, with scores ranging from 0 to 76. An elevated total score indicates a greater severity of depressive symptoms. Additionally, patients were categorized into two groups based on item 12 of the HAMD: an appetite loss group (n = 130) and a non-appetite loss group (n = 41) (Figure 1) (6).
Figure 1

Case screening flowchart.
2.2.3 Negative life events
The Adolescent Self-rating Life Events Checklist (ASLEC) was used to assess the occurrence of negative life events in patients (18). The ASLEC includes 27 items, each of which is scored on a six-point scale. These items primarily assess six dimensions: interpersonal relationships, study pressure, punishment, sense of loss, healthy adaptation, and other factors. The higher the score on each dimension, the greater the stress associated with the relevant negative life events. Currently, the ASLEC is widely used among Chinese adolescents (19, 20).
2.2.4 Alexithymia
The Toronto Alexithymia Scale (TAS-20) was used to measure individuals’ difficulties in identifying and expressing feelings (21). The scale comprises three subscales: difficulty identifying feelings, difficulty describing feelings, and externally oriented thinking. The TAS-20 consists of 20 items, each rated on a 5-point scale. The total score ranges from 20 to 100, with a higher total score indicating greater difficulties in emotional expression.
2.2.5 Insomnia symptoms
The Insomnia Severity Index Scale (ISI) was used to measure the intensity of insomnia symptoms reported by participants over the previous two weeks (22, 23). This instrument comprises 7 items related to insomnia, each rated on a 5-point scale ranging from ‘0 = none’ to ‘4 = very severe’. The total ISI score ranges from 0 to 28, with higher scores indicating more severe insomnia symptoms among participants.
2.3 Inflammatory cytokines measurements
All subjects had their fasting blood drawn from the antecubital vein between 6:00 and 8:00 am the following day, after an overnight fast. The collected blood samples were centrifuged at 3000 rpm for 15 minutes, and the resulting plasma was extracted and stored at -80°C until analysis. The concentrations of IL-1β, IL-6, IL-10, IL-17A, and tumor necrosis factor-α (TNF-α) were measured using the Meso QuickPlex SQ120 (Meso Scale Discovery, Rockville, MD, USA). To ensure that the data for these inflammatory cytokines followed a normal distribution, we applied logarithmic transformation to the values prior to conducting statistical analyses.
2.4 Statistical analysis
Statistical analyses were conducted using SPSS 23.0. The Kolmogorov-Smirnov test was used to assess the normality of continuous variables. To ensure that the measurements of inflammatory cytokines conformed to a normal distribution, we referred to previous studies that performed a logarithmic transformation with a base of 10 on the levels of these cytokines to obtain Log (X) values for Log IL-1β, Log IL-6, Log IL-10, Log IL-17A and Log TNF-α (24, 25). These transformed values were used in subsequent statistical analyses. Continuous variables were summarized as mean ± standard deviation (SD) or median (quartiles) [M (P25, P75)], while categorical variables were presented as percentages (%). For univariate analyses, various statistical tests were employed, including independent samples t-tests, Mann-Whitney U-tests, and chi-square tests, to compare differences between groups with and without appetite loss across all variables. In multifactorial analyses, stepwise logistic regression was utilized to identify independent predictors of appetite loss in adolescents with MDD. And we included several confounders, including gender, age, interpersonal relationships, study pressure, sense of loss, difficulty identifying feelings and ISI score, in the analyses to control for their potential effects. All statistical tests were two-tailed, and a p-value of ≤ 0.05 was considered statistically significant.
3 Results
3.1 Comparison of sociodemographic and clinical characteristics of patients in appetite loss and without appetite loss groups
A total of 176 adolescents with MDD were initially recruited for this study. However, due to blood samples from an additional 5 participants not meeting the study criteria, we ended up statistically analyzing data from only 171 patients (Figure 1). The mean age of patients was 15.47 ± 1.44 years, and 72.5% were female. Compared to the group without appetite loss, patients with appetite loss had higher scores of HAMD (t = 6.598, p < 0.001), interpersonal relationships (t = 4.035, p < 0.001), study pressure (t = 2.167, p = 0.032), punishment (t = 1.858, p = 0.002), sense of loss (t = 1.551, p = 0.016), ISI (t = 3.026, p = 0.003) and difficulty identifying feelings (t = 3.181, p = 0.002), as well as higher levels of Log IL-6 (t = 2.154, p = 0.034) (Table 1; Figure 2).
Table 1
| Variables | Total sample (n = 171) | MDD with appetite loss (n = 130) | MDD without appetite loss (n = 41) | t/Z/χ2 | p |
|---|---|---|---|---|---|
| Females, n (%) | 124 (72.51) | 99 (76.15) | 25 (60.98) | 3.603 | 0.058 |
| Age (years), mean (SD) | 15.47 (1.44) | 15.35 (1.48) | 15.85 (1.28) | -1.979 | 0.049 |
| BMI (kg/m2), mean (SD) | 21.12 (4.25) | 20.87 (4.18) | 21.95 (4.41) | -1.427 | 0.155 |
| Age at onset (years), mean (SD) | 13.78 (1.76) | 13.68 (1.78) | 14.10 (1.69) | -1.339 | 0.182 |
| Duration of illness (months), median (P25, P75) | 18.00 (12.00, 30.00) | 19.00 (12.00, 31.50) | 16.00 (7.50, 24.00) | 0.553a | 0.920 |
| Antidepressants, n (%) | 0.140 | 0.932 | |||
| None | 56 (32.75) | 43 (33.08) | 13 (31.71) | ||
| SSRIs | 105 (61.40) | 79 (60.77) | 26 (63.41) | ||
| Others | 10 (5.85) | 8 (6.15) | 2 (4.88) | ||
| HAMD score, mean (SD) | 28.63 (8.41) | 30.76 (7.60) | 21.88 (7.26) | 6.598 | <0.001 |
| ASLEC score, mean (SD) | |||||
| Interpersonal relationships | 14.45 (4.89) | 15.26 (4.36) | 11.88 (5.60) | 4.035 | <0.001 |
| Study pressure | 11.86 (4.62) | 12.28 (4.45) | 10.51 (4.94) | 2.167 | 0.032 |
| Punishment | 12.00 (7.00, 16.00) | 13.00 (9.00, 17.00) | 7.00 (3.50, 12.00) | 1.858 | 0.002 |
| Sense of loss | 2.00 (0.00, 5.00) | 3.00 (1.00, 5.00) | 1.00 (0.00, 3.00) | 1.551 | 0.016 |
| Healthy adaptation | 6.79 (3.25) | 6.94 (3.18) | 6.32 (3.44) | 1.069 | 0.286 |
| Other factors | 7.48 (3.73) | 7.78 (3.69) | 6.54 (3.75) | 1.871 | 0.063 |
| TAS-20 score , mean (SD) | |||||
| Difficulty identifying feelings | 26.05 (5.37) | 26.87 (4.75) | 23.44 (6.37) | 3.181 | 0.002 |
| Difficulty describing feelings | 18.38 (3.10) | 18.64 (2.85) | 17.56 (3.72) | 1.955 | 0.052 |
| Externally oriented thinking | 23.50 (3.87) | 23.76 (3.79) | 22.66 (4.04) | 1.599 | 0.112 |
| ISI score, mean (SD) | 13.20 (5.42) | 13.89 (5.00) | 11.02 (6.15) | 3.026 | 0.003 |
| Log IL-1β (pg/mL), mean (SD) | -0.54 (0.44) | -0.54 (0.45) | -0.54 (0.43) | -0.018 | 0.986 |
| Log IL-6 (pg/mL), mean (SD) | 0.28 (0.30) | 0.30 (0.32) | 0.21 (0.21) | 2.154 | 0.034 |
| Log IL-10 (pg/mL), mean (SD) | -0.24 (0.29) | -0.22 (0.30) | -0.30 (0.26) | 1.409 | 0.161 |
| Log IL-17A (pg/mL), mean (SD) | 0.37 (0.33) | 0.38 (0.34) | 0.36 (0.29) | 0.282 | 0.778 |
| Log TNF-α (pg/mL), mean (SD) | 0.08 (0.27) | 0.09 (0.29) | 0.06 (0.20) | 0.657 | 0.512 |
Comparison of sociodemographic and clinical characteristics of patients in appetite loss and without appetite loss groups.
MDD, major depressive disorder; BMI, body mass index; HAMD, hamilton depression scale; ASLEC, adolescent self-rating life events checklist; TAS-20, toronto alexithymia scale; ISI, insomnia severity index scale; IL, interleukin; TNF-α, tumor necrosis factor-α.
Bolded p value: < 0.05; SD, standard deviation.
Mann-Whitney U test.
Figure 2
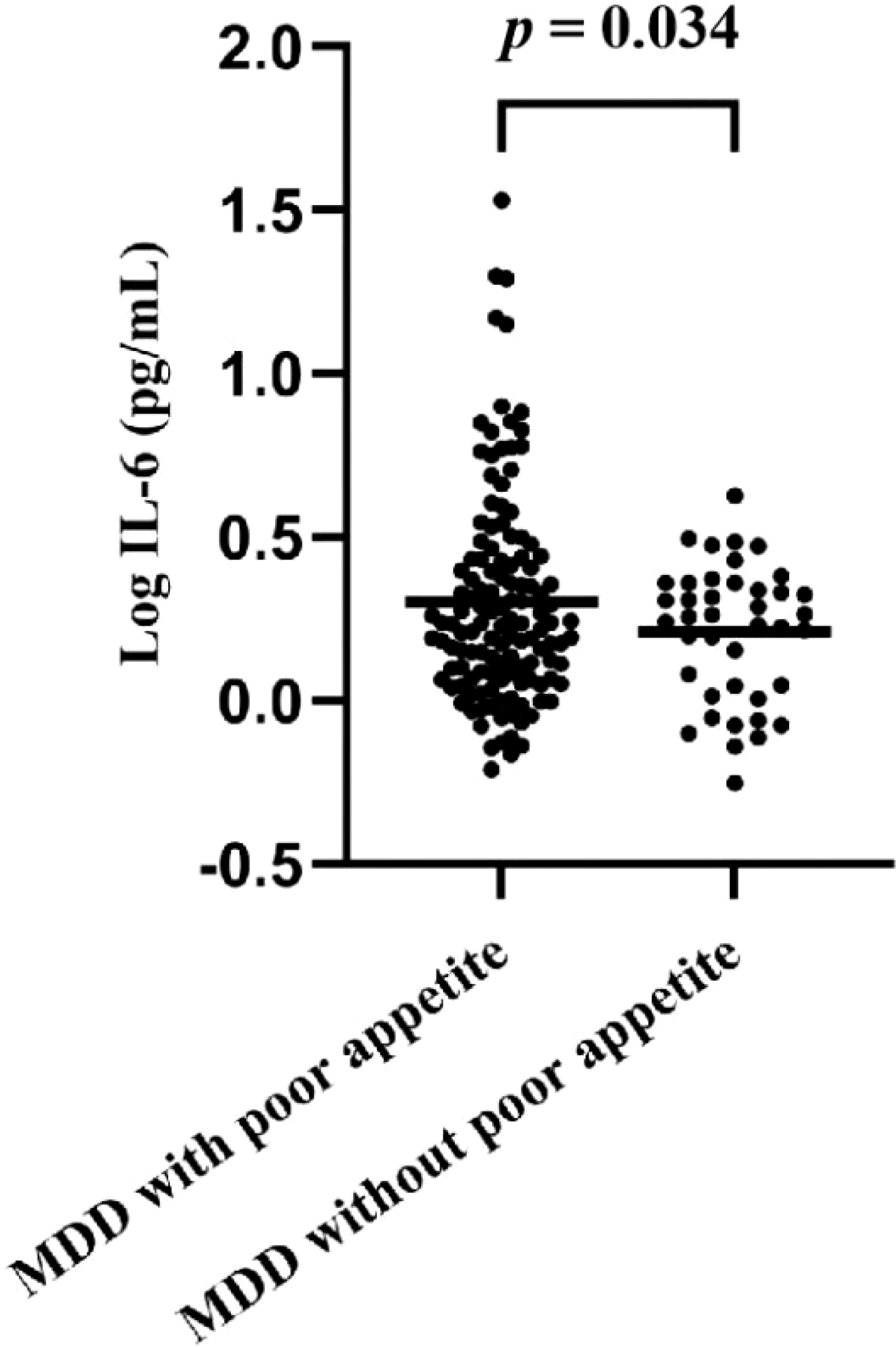
Comparison of IL-6 levels in patients with appetite loss and without appetite loss.
3.2 Independent factors associated with appetite loss by multivariate logistic stepwise regression analysis.
The results of multivariate logistic stepwise regression analysis were summarized in Table 2. The results showed that HAMD score (OR = 1.158, 95% CI = 1.091-1.229, p < 0.001), punishment score (OR = 1.117, 95% CI = 1.039-1.201, p = 0.003), and Log IL-6 level (OR = 5.041, 95% CI = 1.137-22.344, p = 0.033) were independent correlates of appetite loss in adolescents with MDD.
Table 2
| Variables | B | SE | Wald χ2 | OR | 95% CI | p |
|---|---|---|---|---|---|---|
| HAMD score | 0.147 | 0.030 | 23.463 | 1.158 | 1.091 - 1.229 | <0.001 |
| Punishment | 0.111 | 0.037 | 8.983 | 1.117 | 1.039 - 1.201 | 0.003 |
| Log IL-6 | 1.618 | 0.760 | 4.533 | 5.041 | 1.137 - 22.344 | 0.033 |
Independent factors associated with appetite loss by multivariate logistic stepwise regression analysis a.
HAMD, hamilton depression scale; IL, interleukin.
Bolded p value: < 0.05; SE, standard error; OR, odds ratio; CI, confidence interval.
The regression analyses were adjusted for several confounders, including gender, age, interpersonal relationships, study pressure, sense of loss, difficulty identifying feelings and ISI score.
4 Discussion
In our study, the prevalence of appetite loss among adolescents with MDD was 76.0%, significantly higher than that observed in adults with MDD (6). A case-control study showed that vegetative symptoms, including changes in appetite and weight, were more prevalent among adolescents with MDD than among adults (26). Maxwell and colleagues also identified a higher prevalence of appetite loss in adolescents with MDD through a review of empirical studies examining the associations between appetite disturbances and depressive symptoms in children, adolescents, and adults with depression (27). This phenomenon may be related to significant deficits in emotion regulation observed in adolescents with MDD (28). In addition, adolescence is a period of rapid physical and psychological changes, during which adolescents may experience dissatisfaction and anxiety regarding their body image (29). Such anxiety may stem from the internalization of societal aesthetic standards, comparisons with peers, and influences from family and media (30). When adolescents experience discomfort regarding their body size, changes in eating behaviors, such as appetite loss, may occur (31). Thus, decreased appetite in adolescents with MDD warrants high clinical priority.
In terms of clinical symptoms, this study found that patients in the appetite loss group exhibited more severe depressive symptoms. This finding was generally consistent with previous studies (11, 26). Such associations may involve complex psychological and physiological mechanisms. Firstly, regarding psychological mechanisms, depressive moods can reduce adolescents’ interest and pleasure in food, turning eating into a task rather than an enjoyment (32). For example, a survey conducted in China found that individuals with symptoms of depression and anxiety generally experienced impaired pleasure in food, particularly in its sensory experience, and may have aversions and negative expectations (33). Depressed patients often have negative self-perceptions and pessimistic attitudes towards life (34). And they may believe that they do not deserve to enjoy food or that eating does not enhance their emotional state. Regarding physiological mechanisms, depressed patients often exhibit imbalances in neurotransmitters such as serotonin and norepinephrine, which not only affect mood regulation but are also closely related to appetite regulation (35). For instance, reduced serotonin levels can result in decreased appetite (36). Additionally, orexin is an important neuropeptide involved in the regulation of physiological responses such as sleep-wakefulness and ingestion. Research has found that the orexin system becomes dysregulated in MDD, thereby affecting appetite regulation (37). Finally, disturbances of the endocrine system in depressive states, such as abnormally elevated cortisol levels, can also affect appetite and energy metabolism, leading to appetite loss (38).
Also, we found that a series of negative life events (especially punishment) were significantly associated with appetite loss in adolescents with MDD. Previous studies have well established that various types of negative life events are significantly associated with depression in adolescents (39). Given the strong association between depressive symptoms and appetite loss, negative life events may lead to appetite loss in adolescents through their impact on depressive symptoms. Psychological stress resulting from negative life events may also influence appetite in adolescents with MDD (40). Moreover, negative life events can trigger intense negative emotions such as anxiety, fear and despair (41). These emotions may disrupt normal appetite regulation mechanisms, causing individuals to prioritize coping with emotional stress over satisfying physiological needs (42). For example, the experience of punishment can induce feelings of shame and self-blame in adolescents. This psychological burden can cause discomfort during meals, leading to reduced food intake. Consequently, clinicians should closely monitor the dietary status of adolescents with MDD who have experienced adverse life events.
As well, insomnia and alexithymia might be associated with appetite loss in adolescents with MDD, although regression analyses did not show statistical significance. We speculated that insomnia might lead to physical and psychological fatigue, which could disrupt appetite regulation mechanisms and decrease interest in food. Additionally, insomnia may also lead to an increased stress response in the body, disrupting the normal functioning of the hypothalamic-pituitary-adrenal (HPA) axis and causing appetite loss (43). Most research on the association between alexithymia and appetite has focused on patients with eating disorders. A meta-analysis of 44 studies showed that patients with all types of eating disorders exhibit more pronounced symptoms of alexithymia, particularly in terms of difficulty identifying or describing feelings (44). We speculated that patients with alexithymia might be prone to prolonged negative emotional states due to difficulties in recognizing and expressing emotions, and that these negative emotions might suppress appetite. Of course, more research is needed to confirm this hypothesis.
Of note, patients in the appetite loss group exhibited higher levels of plasma inflammatory cytokines (particularly IL-6) in this study. Previous studies conducted with different populations have yielded similar findings (6, 15, 45, 46). A study of patients with end-stage cancer found that plasma IL-6 levels were positively correlated with appetite loss (45). Another network analysis study also showed that various depressive symptoms, including feelings of worthlessness and poor appetite, were associated with IL-6 in adolescents (46). The factors mediating the associations between inflammatory cytokines and appetite loss are likely multifaceted. In fact, some cytokines (e.g. IL-6 and TNF-α) have anorexic properties themselves and can directly influence appetite regulatory centres (47, 48). Research has also shown that inflammatory cytokines can stimulate the release of leptin, an anorexigenic peptide that suppresses appetite (49, 50). In addition, pro-inflammatory cytokines can suppress appetite by activating the HPA axis and the sympathetic nervous system, resulting in elevated levels of cortisol and norepinephrine (49, 51). Finally, pro-inflammatory cytokines can induce anhedonia, a core symptom of depression directly related to appetite loss (52). Notably, in the present study, IL-6 showed a more significant correlation with appetite loss in adolescents with MDD. This phenomenon may stem from the unique role of IL-6 in appetite regulation. Compared with other cytokines such as TNF-α and IL-1, the role of IL-6 in appetite regulation may be more critical. Firstly, IL-6 is able to pass through the blood-brain barrier (BBB) or act in areas where the BBB is weak (e.g., the posterior hypothalamic region), directly or indirectly affecting the appetite-regulating centers in the hypothalamus (53). Specifically, IL-6 can activate neuropeptides such as corticotropin-releasing hormone (CRF) in the hypothalamus and inhibit the activity of the appetite-stimulating neuropeptide Y (NPY), which leads to a decrease in appetite (54). In addition, although a variety of cytokines are involved in the inflammatory response, IL-6 may play a more critical role in certain specific inflammatory states (e.g., chronic low-grade inflammation) (55). This inflammatory state may be more closely associated with appetite loss in adolescents with MDD. However, relatively few studies have been conducted on the relationship between appetite loss and IL-6 in adolescents with MDD, so it is necessary to further explore this topic in the future to clarify the specific mechanism of IL-6’s role in appetite loss in this special population and to provide a theoretical basis for clinical intervention.
However, there were some limitations in this study: (1) The sample was drawn from only two healthcare organizations, and the sample size was relatively small, which might limit the generalizability of the results. (2) This study utilized a cross-sectional design, which did not support explicit inferences about causality. Therefore, future longitudinal or interventional studies are necessary to further elucidate the causal relationship between appetite loss and inflammatory cytokines in adolescents with MDD. (3) The assessment of appetite loss in this study was based solely on one item of the HAMD scale, which may introduce bias. Therefore, future validation of these findings using a more reliable tool is necessary. (4) Previous studies have shown that antidepressant medications and certain personality traits, such as impulsivity, can also affect appetite (56, 57). Therefore, future research should include additional variables to fully explore the factors influencing appetite loss in adolescents with MDD.
5 Conclusion
There was an elevated risk of appetite loss among adolescents with MDD. The present study suggested that appetite loss might be associated with a range of clinical symptoms (especially depression severity and negative life events) and levels of inflammatory cytokines (especially IL-6). Therefore, to effectively prevent and ameliorate appetite loss in adolescents with MDD, healthcare professionals should actively intervene to address relevant risk factors, such as modulating inflammation levels. Additionally, future research should include more high-quality prospective studies to provide in-depth mechanistic insights into appetite loss in this population.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the Chaohu Hospital of Anhui Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
LL: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Software, Visualization, Writing – original draft, Writing – review & editing. XiZ: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – review & editing. JX: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – review & editing. LZ: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – review & editing. PT: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – review & editing. YT: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – review & editing. HF: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – review & editing. MH: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – review & editing. XinZ: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – review & editing. FG: Conceptualization, Data curation, Investigation, Writing – review & editing. DM: Conceptualization, Data curation, Investigation, Writing – review & editing. LX: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Supervision, Writing – review & editing. HL: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Anhui Health Research Project (No. AHWJ2024Aa10004), the Research Fund Project of Anhui Translational Research Institute (No. 2022zhyx-B01), the Scientifc Research Project of Anhui Higher Education Institutions (No. 2024AH050681) and the National Natural Science Foundation of China (No. 82401798).
Acknowledgments
We sincerely thank all the study participants.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
GBD 2017 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017, 2018. Lancet (London England). (2018) 392:1789–858. doi: 10.1016/S0140-6736(18)32279-7
2
Daly M . Prevalence of depression among adolescents in the U.S. From 2009 to 2019: analysis of trends by sex, race/ethnicity, and income. J Adolesc Health. (2022) 70:496–9. doi: 10.1016/j.jadohealth.2021.08.026
3
Li F Cui Y Li Y Guo L Ke X Liu J et al . Prevalence of mental disorders in school children and adolescents in China: diagnostic data from detailed clinical assessments of 17,524 individuals. J Child Psychol Psychiatry Allied Disciplines. (2022) 63:34–46. doi: 10.1111/jcpp.13445
4
Zisook S Lesser I Stewart JW Wisniewski SR Balasubramani GK Fava M et al . Effect of age at onset on the course of major depressive disorder. Am J Psychiatry. (2007) 164:1539–46. doi: 10.1176/appi.ajp.2007.06101757
5
American Psychiatric Association DSM-5 Task Force . Diagnostic and statistical manual of mental disorders: DSM-5™ (5th ed.). American Psychiatric Publishing, Inc. (2013) p. 591–643. doi: 10.1176/appi.books.9780890425596
6
Guo F Jing L Xu Y Zhang K Li Y Sun N et al . Gut microbiota and inflammatory factor characteristics in major depressive disorder patients with anorexia. BMC Psychiatry. (2024) 24:334. doi: 10.1186/s12888-024-05778-0
7
Mora F Alvarez-Mon MA Fernandez-Rojo S Ortega MA Felix-Alcantara MP Morales-Gil I et al . Psychosocial factors in adolescence and risk of development of eating disorders. Nutrients. (2022) 14:1481. doi: 10.3390/nu14071481
8
Bondar J Caye A Chekroud AM Kieling C . Symptom clusters in adolescent depression and differential response to treatment: a secondary analysis of the Treatment for Adolescents with Depression Study randomised trial. Lancet Psychiatry. (2020) 7:337–43. doi: 10.1016/S2215-0366(20)30060-2
9
Kitagawa Y Ando S Yamasaki S Foo JC Okazaki Y Shimodera S et al . Appetite loss as a potential predictor of suicidal ideation and self-harm in adolescents: A school-based study. Appetite. (2017) 111:7–11. doi: 10.1016/j.appet.2016.12.026
10
Gibson-Smith D Bot M Paans NP Visser M Brouwer I Penninx BW . The role of obesity measures in the development and persistence of major depressive disorder. J Affect Disord. (2016) 198:222–9. doi: 10.1016/j.jad.2016.03.032
11
Kroemer NB Opel N Teckentrup V Li M Grotegerd D Meinert S et al . Functional connectivity of the nucleus accumbens and changes in appetite in patients with depression. JAMA Psychiatry. (2022) 79:993–1003. doi: 10.1001/jamapsychiatry.2022.2464
12
Keller MC Neale MC Kendler KS . Association of different adverse life events with distinct patterns of depressive symptoms. Am J Psychiatry. (2007) 164:1521–622. doi: 10.1176/appi.ajp.2007.06091564
13
Corcos M Guilbaud O Speranza M Paterniti S Loas G Stephan P et al . Alexithymia and depression in eating disorders. Psychiatry Res. (2000) 93:263–6. doi: 10.1016/S0165-1781(00)00109-8
14
Rossi L Silva C Charvin I Da Fonseca D Bat-Pitault F . Sleep and emotionality in adolescents with anorexia nervosa during the Covid-19 pandemic. Eur Eating Disord Review: J Eating Disord Association. (2023) 31:462–73. doi: 10.1002/erv.2971
15
Pourhassan M Babel N Sieske L Westhoff TH Wirth R . Inflammatory cytokines and appetite in older hospitalized patients. Appetite. (2021) 166:105470. doi: 10.1016/j.appet.2021.105470
16
Okamoto N Hoshikawa T Ikenouchi A Yoshimura R . Comparison of psychiatric symptoms between patients with major depression with higher and lower levels of high-sensitivity C-reactive protein in the serum: a preliminary study. Ther Adv In Psychopharmacology. (2021) 11:20451253211060228. doi: 10.1177/20451253211060228
17
Hamilton M . A rating scale for depression. J Neurology Neurosurgery Psychiatry. (1960) 23:56–62. doi: 10.1136/jnnp.23.1.56
18
Liu XC Liu LQ Yang J Cai FX Wang AZ Sun LM et al . Establishment and reliability and validity test of adolescent life events scale. Shangdong Psychiatry. (1997) 01:15–9. doi: 10.16128/j.cnki.1005-3611.1997.01.011
19
Hua Y Jiang W He Y Zheng X Huang C Guo L et al . Associations of recent stressful life events with anxiety symptoms among Chinese adolescents with a consideration of family functioning. Eur J Psychotraumatology. (2024) 15:2337577. doi: 10.1080/20008066.2024.2337577
20
Yang SL Tan CX Li J Zhang J Chen YP Li YF et al . Negative life events and aggression among Chinese rural left-behind adolescents: do self-esteem and resilience mediate the relationship? BMC Psychiatry. (2023) 23:167. doi: 10.1186/s12888-023-04587-1
21
Bagby RM Parker JD Taylor GJ . The twenty-item Toronto Alexithymia Scale–I. Item selection and cross-validation of the factor structure. J Psychosomatic Res. (1994) 38:23–32. doi: 10.1016/0022-3999(94)90005-1
22
Bastien CH Vallières A Morin CM . Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Medicine. (2001) 2:297–307. doi: 10.1016/s1389-9457(00)00065-4
23
Chung KF Kan KKK Yeung WF . Assessing insomnia in adolescents: comparison of Insomnia Severity Index, Athens Insomnia Scale and Sleep Quality Index. Sleep Medicine. (2011) 12:463–70. doi: 10.1016/j.sleep.2010.09.019
24
Duivis HE de Jonge P Penninx BW Na BY Cohen BE Whooley MA . Depressive symptoms, health behaviors, and subsequent inflammation in patients with coronary heart disease: prospective findings from the heart and soul study. Am J Psychiatry. (2011) 168:913–20. doi: 10.1176/appi.ajp.2011.10081163
25
Quidé Y Bortolasci CC Spolding B Kidnapillai S Watkeys OJ Cohen-Woods S et al . Association between childhood trauma exposure and pro-inflammatory cytokines in schizophrenia and bipolar-I disorder. psychol Medicine. (2019) 49:2736–44. doi: 10.1017/S0033291718003690
26
Rice F Riglin L Lomax T Souter E Potter R Smith DJ et al . Adolescent and adult differences in major depression symptom profiles. J Affect Disord. (2019) 243:175–81. doi: 10.1016/j.jad.2018.09.015
27
Maxwell MA Cole DA . Weight change and appetite disturbance as symptoms of adolescent depression: toward an integrative biopsychosocial model. Clin Psychol Review. (2009) 29:260–73. doi: 10.1016/j.cpr.2009.01.007
28
Zsigo C Sfärlea A Lingl C Piechaczek C Schulte-Körne G Feldmann L et al . Emotion regulation deficits in adolescent girls with major depression, anorexia nervosa and comorbid major depression and anorexia nervosa. Child Psychiatry Hum Dev. (2023) 54:1476–88. doi: 10.1007/s10578-022-01353-4
29
Vannucci A Ohannessian CM . Body image dissatisfaction and anxiety trajectories during adolescence. J Clin Child Adolesc Psychol. (2018) 53. 47:785–95. doi: 10.1080/15374416.2017.1390755
30
de Vries DA Peter J de Graaf H Nikken P . Adolescents’ Social network site use, peer appearance-related feedback, and body dissatisfaction: testing a mediation model. J Youth Adolescence. (2016) 45:211–24. doi: 10.1007/s10964-015-0266-4
31
Pehlivan MJ Okada M Miskovic-Wheatley J Barakat S Touyz S Simpson SJ et al . Eating disorder risk among Australian youth starting a diet in the community. Appetite. (2024) 203:107685. doi: 10.1016/j.appet.2024.107685
32
Ekinci GN Sanlier N . The relationship between nutrition and depression in the life process: A mini-review. Exp Gerontology. (2023) 172:112072. doi: 10.1016/j.exger.2022.112072
33
Hyldelund NB Byrne DV Chan RCK Andersen BV . The relationship between social anhedonia and perceived pleasure from food-an exploratory investigation on a consumer segment with depression and anxiety. Foods (Basel Switzerland). (2022) 11:3659. doi: 10.3390/foods11223659
34
Harrison P Lawrence AJ Wang S Liu S Xie G Yang X et al . The psychopathology of worthlessness in depression. Front In Psychiatry. (2022) 13:818542. doi: 10.3389/fpsyt.2022.818542
35
Albert PR Blier P . Does serotonin matter in depression? J Psychiatry Neuroscience: Jpn. (2023) 48:E400–3. doi: 10.1503/jpn.230130
36
Lam DD Garfield AS Marston OJ Shaw J Heisler LK . Brain serotonin system in the coordination of food intake and body weight. Pharmacology Biochemistry Behavior. (2010) 97:84–91. doi: 10.1016/j.pbb.2010.09.003
37
Hsu JW Chen LC Bai YM Huang KL Tsai SJ Su TP et al . Appetite hormone dysregulation, body mass index, and emotional dysregulation in nonobese adolescents with first-episode schizophrenia, bipolar disorder, and major depressive disorder: a cross-sectional association study. CNS Spectrums. (2023) 28:629–36. doi: 10.1017/S1092852923000081
38
Tichomirowa MA Keck ME Schneider HJ Paez-Pereda M Renner U Holsboer F et al . Endocrine disturbances in depression. J Endocrinological Investigation. (2005) 28:89–99. doi: 10.1007/BF03345535
39
Stikkelbroek Y Bodden DHM Kleinjan M Reijnders M van Baar AL . Adolescent depression and negative life events, the mediating role of cognitive emotion regulation. PloS One. (2016) 11:e0161062. doi: 10.1371/journal.pone.0161062
40
Torres SJ Nowson CA . Relationship between stress, eating behavior, and obesity. Nutr (Burbank Los Angeles County Calif.). (2007) 23:887–94. doi: 10.1016/j.nut.2007.08.008
41
Matthews TA Shao H Forster M Kim I . Associations of adverse childhood experiences with depression and anxiety among children in the United States: Racial and ethnic disparities in mental health. J Affect Disord. (2024) 362:645–51. doi: 10.1016/j.jad.2024.07.121
42
Meule A . The psychology of food cravings: the role of food deprivation. Curr Nutr Reports. (2020) 9:251–7. doi: 10.1007/s13668-020-00326-0
43
Yuksel D Kiss O Prouty D Arra N Volpe L Baker FC et al . Stress, hypothalamic pituitary adrenal axis activity and autonomic nervous system function in adolescents with insomnia. Int J Psychophysiol. (2023) 187:43–53. doi: 10.1016/j.ijpsycho.2023.02.006
44
Westwood H Kerr-Gaffney J Stahl D Tchanturia K . Alexithymia in eating disorders: Systematic review and meta-analyses of studies using the Toronto Alexithymia Scale. J Psychosomatic Res. (2017) 99:66–81. doi: 10.1016/j.jpsychores.2017.06.007
45
Inagaki M Akechi T Okuyama T Sugawara Y Kinoshita H Shima Y et al . Associations of interleukin-6 with vegetative but not affective depressive symptoms in terminally ill cancer patients. Supportive Care In Cancer. (2013) 21:2097–106. doi: 10.1007/s00520-013-1767-x
46
Manfro PH Anselmi L Barros F Gonçalves H Murray J Oliveira IO et al . Youth depression and inflammation: Cross-sectional network analyses of C-Reactive protein, interleukin-6 and symptoms in a population-based sample. J Psychiatr Res. (2022) 150:197–201. doi: 10.1016/j.jpsychires.2022.03.065
47
Bernardoni F Tam F Poitz DM Hellerhoff I Arold D Geisler D et al . Effect of serum concentrations of IL-6 and TNF-α on brain structure in anorexia nervosa: a combined cross-sectional and longitudinal study. Neuropsychopharmacology. (2024) 49:1509–17. doi: 10.1038/s41386-024-01836-z
48
Solmi M Veronese N Favaro A Santonastaso P Manzato E Sergi G et al . Inflammatory cytokines and anorexia nervosa: A meta-analysis of cross-sectional and longitudinal studies. Psychoneuroendocrinology. (2015) 51:237–52. doi: 10.1016/j.psyneuen.2014.09.031
49
Andréasson A Arborelius L Erlanson-Albertsson C Lekander M . A putative role for cytokines in the impaired appetite in depression. Brain Behavior Immunity. (2007) 21:147–52. doi: 10.1016/j.bbi.2006.08.002
50
Casado ME Collado-Pérez R Frago LM Barrios V . Recent advances in the knowledge of the mechanisms of leptin physiology and actions in neurological and metabolic pathologies. Int J Mol Sci. (2023) 24:1422. doi: 10.3390/ijms24021422
51
Schorr M Miller KK . The endocrine manifestations of anorexia nervosa: mechanisms and management. Nat Rev Endocrinology. (2017) 13:174–86. doi: 10.1038/nrendo.2016.175
52
Lucido MJ Bekhbat M Goldsmith DR Treadway MT Haroon E Felger JC et al . Aiding and abetting anhedonia: impact of inflammation on the brain and pharmacological implications. Pharmacol Rev. (2021) 73:1084–117. doi: 10.1124/pharmrev.120.000043
53
Sun Q van de Lisdonk D Ferrer M Gegenhuber B Wu M Park Y et al . Area postrema neurons mediate interleukin-6 function in cancer cachexia. Nat Communications. (2024) 15:4682. doi: 10.1038/s41467-024-48971-1
54
Timper K Brüning JC . Hypothalamic circuits regulating appetite and energy homeostasis: pathways to obesity. Dis Models Mechanisms. (2017) 10:679–89. doi: 10.1242/dmm.026609
55
Hunter CA Jones SA . IL-6 as a keystone cytokine in health and disease. Nat Immunol. (2015) 16:448–57. doi: 10.1038/ni.3153
56
Gill H Gill B El-Halabi S Chen-Li D Lipsitz O Rosenblat JD et al . Antidepressant medications and weight change: A narrative review. Obesity (Silver Spring Md.). (2020) 28:2064–72. doi: 10.1002/oby.22969
57
Lundahl A Wahlstrom LC Christ CC Stoltenberg SF . Gender differences in the relationship between impulsivity and disordered eating behaviors and attitudes. Eating Behaviors. (2015) 18:120–4. doi: 10.1016/j.eatbeh.2015.05.004
Summary
Keywords
appetite loss, clinical features, inflammatory cytokines, adolescents, major depressive disorder
Citation
Liu L, Zhang X, Xue J, Zhao L, Tang P, Tian Y, Fan H, Hao M, Zhao X, Geng F, Mo D, Xia L and Liu H (2025) Associations between appetite loss and clinical features as well as inflammatory cytokines in adolescents with major depressive disorder. Front. Psychiatry 16:1583060. doi: 10.3389/fpsyt.2025.1583060
Received
27 February 2025
Accepted
17 April 2025
Published
14 May 2025
Volume
16 - 2025
Edited by
Luca Steardo Jr, University Magna Graecia of Catanzaro, Italy
Reviewed by
Andrés Treviño-Alvarez, Universidad Autonoma de Nuevo Leon, Mexico
Adriano Alberti, Planalto Catarinense University, Brazil
Updates
Copyright
© 2025 Liu, Zhang, Xue, Zhao, Tang, Tian, Fan, Hao, Zhao, Geng, Mo, Xia and Liu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huanzhong Liu, huanzhongliu@ahmu.edu.cn; Lei Xia, xialei@ahmu.edu.cn
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.