- 1Scientific Institute, IRCCS E. Medea, Dipartimento/Unità Operativa Pasian di Prato, Udine, Italy
- 2Swiss Paraplegic Research, Nottwil, Switzerland
- 3Department of Electronics, Information and Bioengineering, Politecnico di Milano, Milan, Italy
- 4Department of Neurosciences and Mental Health, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy
- 5Neuroradiology, Azienda Sanitaria Universitaria Friuli Centrale, ASU FC, Udine, Italy
- 6Psychiatry Unit, Department of Medicine, University of Udine, Udine, Italy
- 7Department of Pathophysiology and Transplantation, University of Milan, Milan, Italy
Background: Patients with depression appear to imagine scenes preferentially from a 3rd person perspective as if they were observers. Methods: By using functional magnetic resonance imaging we asked patients with major depressive disorder (19, MDD) and healthy controls (19, HC) to imagine 1st person perspective state/psychological (vs. action) scenes.
Results: We found that the left superior frontal gyrus (orbital part) and the right putamen were differentially activated by the state/psychological (vs. action) imagery (vs. letter detection) in MDD individuals vs. HC.
Discussion: We suggest that imagery for state/psychological events increased activation in areas involved in self-related processing and personal perspective changes.
1 Introduction
In psychopathology mental imagery is considered both a factor capable of maintaining affective symptomatology and a cognitive skill frequently used in psychological treatments (e.g., 1). Intrusive and unwanted imagery (e.g., 2) is present trans-diagnostically across major psychopathologies (e.g., 3–5). In particular, Holmes et al. (6) have examined mental imagery in depression, addressing its phenomenology, the putative altered mechanisms, and its potential therapeutic applications. Holmes et al. (6) argued that mental imagery alterations in depression involve both a hyperproduction of highly intrusive negative mental images (e.g., 2, 6–8), and an impoverishment of positive mental images (6, 8–12). In addition, depression is related to low mental imagery vividness (13, 14). Interestingly, an additional feature involves the perspective of imagination. Patients with depression appear to use 3rd person perspective imagery as if they were observers (6). In the case of autobiographical memory, 3rd person perspective imagery triggers a reduced emotional impact, as compared with 1st person perspective imagery (e.g., 15, 16). This is quite obvious, as 1st person’s perspective mental imagery triggers the simulation of the corresponding experience, an “as real” emotional experience (5). Contrarily, patients with depression tend to recall 3rd person perspective memories (e.g., 17–20). This strategy could represent a form of avoidance, especially when the content of emotions is negative (19) but also when it is positive (21–23).
While the behavioral pattern of mental imagery in depression has been extensively studied, the understanding of the functional brain correlates of mental emotion imagery in depressed individuals is less explored. Specifically, to the best of our knowledge, there is no functional Magnetic Resonance Imaging (fMRI) study addressing the brain activity underlying emotional imagery in individuals with depression. In one review (24) summarizing functional brain imaging studies of depression it was reported that many studies employed the resting-state paradigm. Out of the included task-based studies, paradigms only involved emotional processing, reward processing, and cognitive control. Similarly, in another review of fMRI studies in depression (25), no single study employing the emotion imagery paradigm was reported.
To fill this gap, we utilized a mental imagery fMRI paradigm in a group of 19 individuals with depression, comparing them to 19 healthy controls. The fMRI task has been previously employed with healthy young (26) and adult (27, 28) participants. Participants were prompted to create a 1st-person perspective mental image of the state/psychological verb’s content and then assess its pleasantness. On the same list of stimuli, participants performed a control task consisting of letter detection. As there is no fMRI study on state/psychological imagery in depressive disorders, we did not formulate hypotheses on which (if any) brain structures might show differential activations in depressed subjects when compared to healthy controls. In Tomasino et al. (27) we showed that the imagery task in healthy controls activated a network of areas involving the middle and superior occipital gyrus and parietal cortex bilaterally, the middle temporal gyrus bilaterally (extending to the right superior temporal gyrus), the left postcentral gyrus (extending to the supramarginal gyrus) and several clusters in the frontal cortex (the left superior frontal gyrus, the left precentral gyrus, the left middle frontal gyrus, the inferior frontal gyrus bilaterally and the right insula). Based on this network it can be hypothesized that activation in the parietal or frontal cortex, which are the most activated regions, may show differential activations in depressed subjects when compared to healthy controls. In addition, based on the available literature on mental imagery in depression cited above, we expected to find hyper-activation in areas related to first-person perspective imagery since patients with depression appear to imagine scenes preferentially from a third-person perspective, and our instructions forced them to imagine in a 1st person perspective.
2 Materials and methods
2.1 Subjects
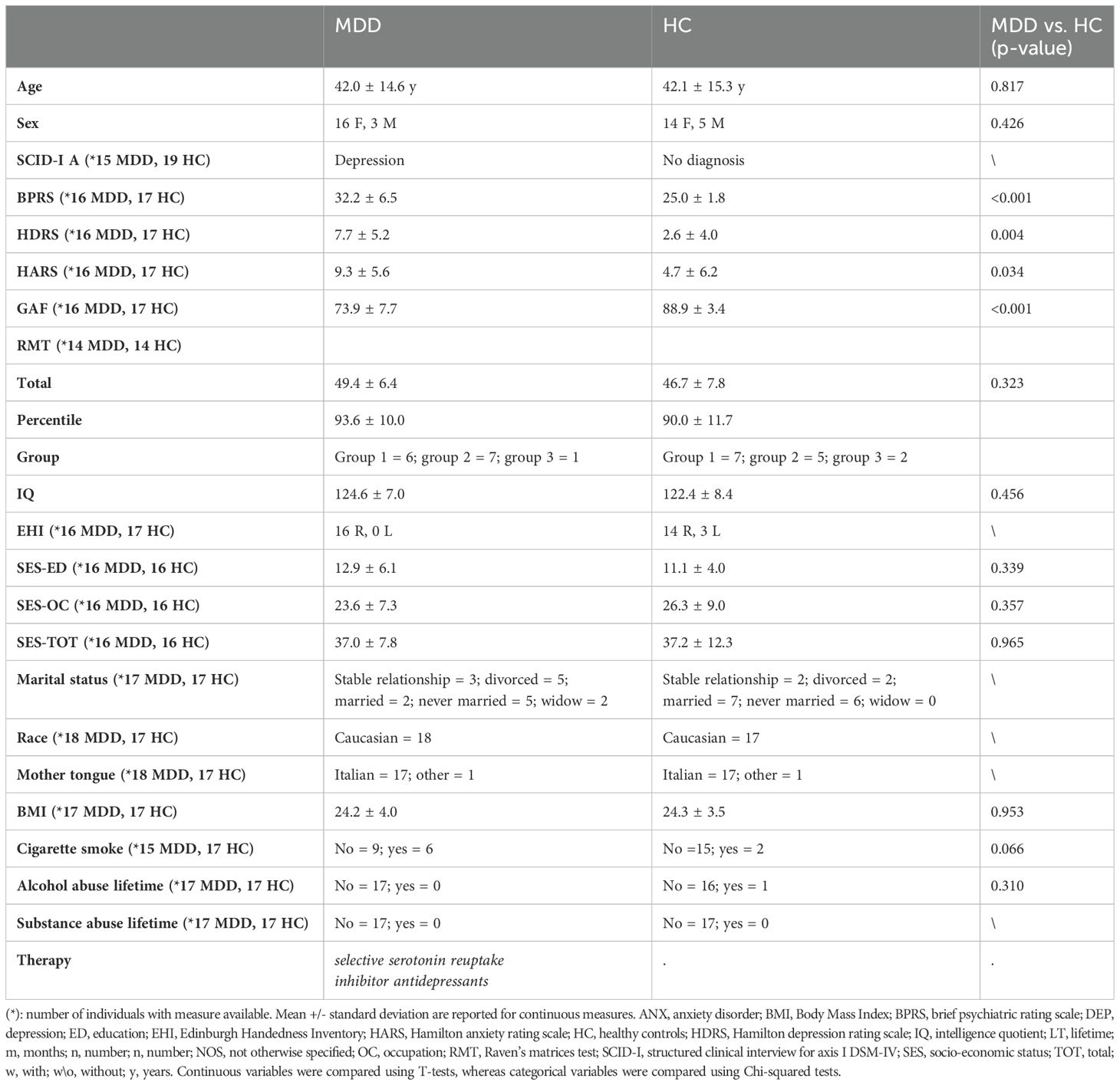
A total of 38 participants were included in the study. The sample was composed of 19 individuals affected by major depressive disorders (MDD, mean age 42 ± 14.6, 16/19 females) and 19 healthy controls (HC, mean age 42.1 ± 15.3, 14/19 females). Exclusion criteria for HC were any diagnosis of psychiatric disorders, history of alcohol or substance abuse, head trauma, neurological or major medical illnesses, and history of psychiatric disorders in first-degree relatives. Exclusion criteria for patients included the presence of other comorbid axis I disorders, neurological or medical disorders with possible effect on brain development, history of traumatic head injury with loss of consciousness and alcohol or substance abuse. The research protocol was approved by the competent Research Ethical Committee of the Research Institute IRCCS E. Medea by the 2013 Fortaleza version of the Helsinki Declaration and subsequent amendments. All participants provided written informed consent to the study.
2.2 Psychopathological assessment
The clinical diagnoses were obtained using the Italian version of the Structured Clinical Interview for Axis I DSM-IV disorders (SCID-I) (29) and confirmed by the clinical evaluation of two expert psychiatrists. For the HC group, a brief non-patient version of the SCID-I was used to confirm the absence of psychiatric axis I disorders. Moreover, the psychopathological assessment included the 24-item Brief Psychiatric Rating Scale (BPRS) (30) and the Hamilton Anxiety and Depression Rating Scales (HARS and HDRS, respectively) (31, 32). The social, occupational, and psychological functioning were also assessed with the Global Assessment of Functioning scale (GAF) (33), while the cognitive performances were assessed by the Raven Matrices Test (RMT) (34). Handedness was determined with the Edinburgh Handedness Inventory (EHI) (35). Refer to Table 1 for detailed information on the socio-demographic and clinical characteristics of the sample.
2.3 Experimental design
2.3.1 Neuroimaging data acquisition
A 3T Philips Achieva scanner (Philips, Best, the Netherlands) equipped with an 8-channel head coil was used to acquire Magnetic Resonance Imaging (MRI) data. Participants’ head movements were limited by using restraining foam pads. The fMRI data (612 volumes) were acquired using a T2*-weighted gradient echo planar imaging (GE-EPI) sequence (repetition time (TR)= 2500 ms, echo time (TE)= 35 ms, flip angle= 90°, 30 axial slices with no gap, 128 × 128 in-plane matrix, voxel size= 1.79 mm × 1.79 mm × 3 mm). In addition, a 3D T1-weighted MPRAGE turbo field echo (TFE) SENSE image (TR= 8.2 ms, TE= 3.76 ms, 190 axial slices with no gap, 240 × 240 in-plane matrix, voxel size= 1 mm3, total scan duration= 8 minutes and 53 seconds) was collected and used for morphological referencing of the fMRI results. Visual stimuli were presented through MRI-compatible VisuaStim Digital goggles (Resonance Technology Inc., Northridge, CA, USA), and subjects’ responses were collected via an MRI-compatible hand Evoke Response Pad (Resonance Technology Inc., Northridge, CA, USA) for the index and middle fingers. All subjects utilized the right hand, which was the dominant one, to respond. Before the fMRI experiment, subjects were trained to perform the task outside the MR environment.
2.3.2 fMRI task
The event-related fMRI experiment included a mental imagery (I) task and a letter detection (LD) task. Instructions lasted 5 s (“You will be asked to read a series of words silently and respond to a yes-no question by pressing the corresponding button”). In the I task subjects were explicitly asked to form a mental image of the verb content from a first-person perspective and to determine its pleasantness by answering the question, “Do you like it?”. In the LD task, subjects were explicitly asked to identify a target letter in the word by responding to the question “Search: Is the “S” present?”. Stimuli could be state/psychological verbs (Sta/Psy) or motor verbs (M) for a total of 144 trials. 64% of M and 58% of Sta/Psy trials included the letter “S.”
Stimuli lasted 4 s and were followed by variable inter-trial intervals, with a duration jittered from 3250 to 4000 ms with incremental steps of 250 ms. In addition, 36 null events (i.e., blank screens) perceived as a prolongation of their inter-trial period were randomly interspersed among the event trials to increase the power of estimating the fMRI blood oxygenation level-dependent (BOLD) response (36). To avoid any potential priming effect, trial sequences were pseudo-randomized in the two tasks.
The Sta/Psy and M stimuli were previously validated in a rating study (27). They significantly differed in motor relatedness (t(35)=132.7, p<0.001), imageability (t(35)=19.43, p<0.001), relatedness to a psychological state/emotion (t(35)=-40.24, p <.001), and written frequencies (t(35)=2.25, p<.05). By contrast, they were matched for familiarity (t(35)=-.95,p>.05, n.s.) and length (t(35)=.55, p >.05, n.s.). Regarding the distribution of the Sta/Psy stimuli in the affective space, arousal and valence were collected in a previous study (37) on a sample of three groups of healthy volunteers of different ages. Participants rated arousal and valence on a scale from 1 to 9 on the Self-Assessment Manikin (SAM) (38). For the arousal, they reported the level of arousal triggered by the action or feeling or state described by the verb (score 1-9, 1= very calm; 9= very aroused). As regards the emotional valence, they reported how happy the action or feeling or state described by the verb made them feel (score 1-9, 1= very happy; 9= very unhappy). For the 16-19-year-old participants, the Sta/Psy stimuli had a mean arousal of 4.4 ± 1.5 and a mean valence of 5.5 ± 2.1.
2.3.3 fMRI data analyses
Matlab R2019a (The Mathworks, Inc.) and Statistical Parametric Mapping (SPM12) software (https://www.fil.ion.ucl.ac.uk/spm) (39), its Marsbar toolbox (40), Matlab in-house scripts were used for data analysis and functions from the Statistics and Machine Learning ToolboxTM.
For each subject, the first 6 dummy volumes were excluded from the analyses. To reduce head motion artifacts, we used a least squares approach and a rigid-body transformation to spatially realign the remaining 606 fMRI volumes to the first reference volume. We used 3 mm in translation and 3 degrees in rotation thresholds for exclusion. No participants exceeded this threshold. The mean of the resliced fMRI images was used as a reference for co-registration with the subject’s structural T1-weighted image, which was segmented into different tissue types and used for estimation of the deformation field for spatial normalization into the Montreal Neurological Institute (MNI) space. The realigned fMRI volumes was normalized to the MNI space, spatially resampled them to 2 mm × 2 mm × 2 mm, and smoothed them using a 3D Gaussian kernel filter with 6 mm Full Width at Half Maximum (FWHM).
A voxel-based General Linear Model (GLM) activation analysis was run. The design matrix had four experimental conditions: LD_M, LD_Sta/Psy, I_M, I_Sta/Psy, their time derivative as well as the regressor coding for fixations. The design matrix included the subjects’ movement parameters as confounding regressors. After GLM β coefficient estimation, a subject-level contrast map was extracted for the four experimental conditions using the corresponding β value map.
We entered each subject’s contrast maps in a second-level flexible factorial GLM analysis. The flexible factorial design included subjects, task (I vs. LD), stimulus (Sta/Psy vs. M), and group (MDD vs. HC) as factors, and age and sex as covariates in interaction with the group factor.
After estimating the GLM β coefficients, group effects on task-by-stimuli, task, and stimuli, fMRI activations were assessed through two-sided t-tests on the corresponding linear combination of β values by properly selecting both group and condition regressors. A multiple comparison correction was performed via a cluster-based family-wise error (cFWE) correction, using as voxel-wise primary threshold p=0.001 and as cluster-level threshold p=0.05. The anatomical interpretation of the fMRI results was performed using the SPM Anatomy toolbox (41).
2.3.4 Task performance analyses
A GLM with the participants’ Reaction Times (RTs) and the percentage of the participants’ likability responses for I_Sta/Psy and I_M as dependent variables and diagnosis, age, and sex as independent variables was run. Group differences were extracted via two-sided t-tests on the corresponding β coefficients (p<0.05). Unfortunately, in the MDD group, the responses of six individuals were not recorded due to technical problems and could not be included in the performance analysis.
2.4 Results
2.4.1 Behavioral performance
Both the linear GLM model and the GLM model considering the diagnosis in interaction with age and sex did not report significant differences in RTs or likeability scores between the groups (Tables 2, 3).
2.4.2 fMRI activations
2.4.2.1 Task-by-stimuli interaction
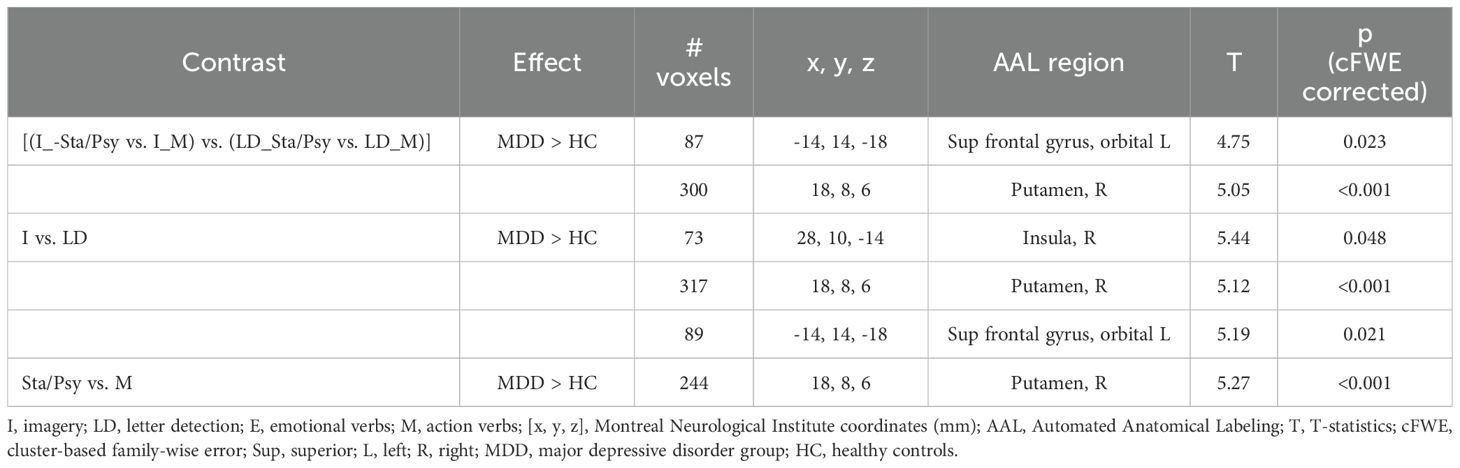
Increased BOLD response to the imagery (vs. letter detection) of emotion (vs. motor) stimuli [(I_Sta/Psy vs. I_M) vs. (LD_Sta/Psy vs. LD_M)] in MDD individuals compared to HC was found in the left superior frontal gyrus, orbital part and in the right putamen (see Table 4; Figure 1).
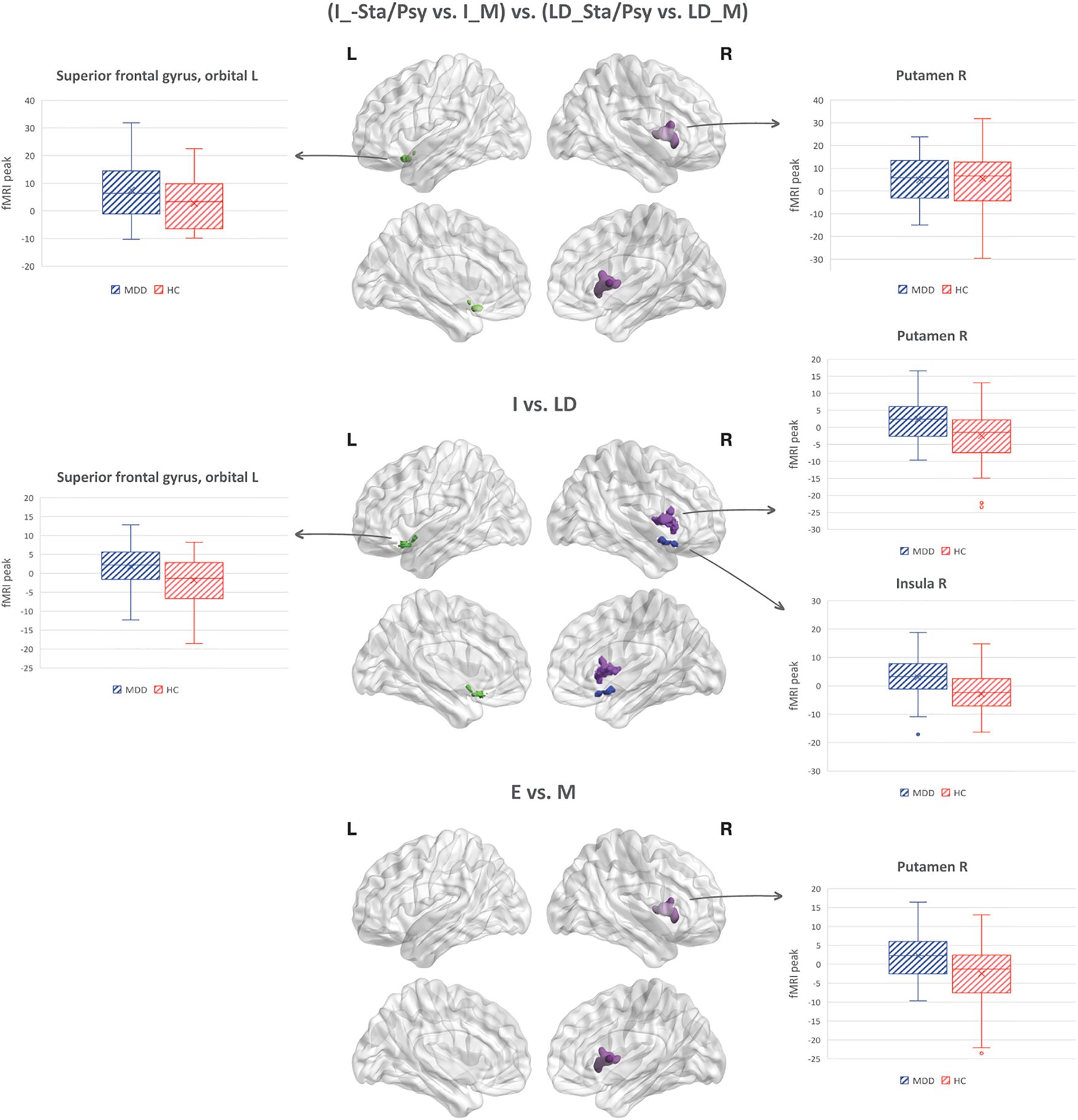
Figure 1. Task-by-stimuli interaction (p<0.05, cFWE corrected) along with the main effect of task and stimuli.
The correlation between the signal extracted from the frontal cluster (x=-14 y=14 z=-18) and the behavioral RTs data was not significant (I_E r(13)=.318, I_M r(13)=.411, LD_E r(13)=.262 and LD_M r(13)=.235), nor it was the correlation between the signal extracted from the putamen cluster (x=18 y=8 z=-6) and the behavioral RTs data (I_E r(13)= .696, I_M r(13)= .891, LD_E r(13)= -.603 and LD_M r(13)= .529).
The correlation between the signal extracted from the frontal cluster (x=-14 y=14 z=-18) and the HDRS data was not significant (r(16)=.335, p>.05, n.s.), nor it was the correlation between the signal extracted from the putamen cluster (x=18 y=8 z=-6) and the HDRS data (r(16)=-.151, p>.05, n.s).
2.4.2.2 Main effect of task
<p>Increased BOLD response to I vs. LD in MDD individuals compared to HC was found in the right putamen, right insula, and left orbitofrontal cortex (see Table 4; Figure 1).
2.4.2.3 Main effect of stimuli
Increased BOLD response to Sta/Psy vs. M stimuli in MDD individuals compared to HC was found in the right putamen (see Table 4; Figure 1).
2.5 Discussion
We investigated the neural correlates of emotion (vs. motor) -related imagery in a group of MDD compared to HC. At the behavioral level, MDD and HC performed similarly. However, we considered behavioral results to be poorly informative since they were available only for one-third of the MDD sample. This could, in principle, have affected the behavioral pattern. Thus, we decided not to comment on it further.
By contrast, the imaging dataset was complete. At the neural level, our main result is that imagery (vs. letter detection) of emotion (vs. motor) stimuli in MDD individuals compared to HC activated the left superior frontal gyrus, orbital part, and the right putamen.
As to the activation in the orbitofrontal cortex, neuroimaging literature focused on the neural basis of perspective-taking has extensively shown that this area is directly involved in self-awareness (42), self-related tasks and in perspective changes-related tasks (e.g., 43). For instance, processing sentences focused on the self (vs. another person) activates the orbitofrontal cortex (44–48), so this area has been related to the representation of self-related knowledge. Even more interestingly, for the present study, this area has been associated with tasks requiring changes in perspective, e.g., from a third-person perspective to a first-person perspective (49) and vice versa. We remind that our task instructions explicitly asked participants to imagine the scenes from a first-person perspective; we also reported in the introduction that, according to the literature, MDD appear to imagine scenes preferentially from a third-person perspective, as if they were observers (6). Therefore, the increased activation in the orbitofrontal cortex could be related to a continuous shift in the perspective the MDD participants assume during the task. Activation in the orbitofrontal cortex is found for imagining emotional scenes and not motor scenes. This would be consistent with the view that this area is preferentially involved in emotional perspective-taking, which consists of processing what the characters of a scenario are feeling, in contrast with cognitive perspective-taking, which consists of processing what the characters are thinking (50). Other studies on person perspective-related tasks reported activation in this area for both first-person perspective as compared to third-person perspective (51) and for third as compared to first-person perspective (49). Our results add further evidence showing that when instructed to imagine in a first-person perspective, activation in areas involved in self-other distinction is enhanced: this does not occur a-specifically whenever they imagined scenario, but only when they are engaged in emotion imagery.
As to the cluster of activation localized in the right putamen, we acknowledge that this result is not new, as it has been reported in a previous study of our group (28), in which we addressed the role of age and sex on mental imagery of emotion verbs. In detail, the same fMRI task has been used in the present and in the mentioned study. Activation in the right putamen was found to increase significantly in women, compared to men, for emotional imagery. In line with these results, in the present study, the sample of the MDD group included a majority of women (16 vs. 3 males). The putamen is involved in emotion processing as part of the striatum, a region that receives inputs from the amygdala (52–54), which in turn receives input from prefrontal areas. Our results further confirm the idea that the striatum is involved in imagery, emotion, and language processing (e.g., 55–58) and not simply in action planning and execution. The activation in the right putamen was also related to a general effect of stimuli, as the stimuli’ main contrast, performed on both the I and LD tasks, returned significant results in the right putamen, suggesting that the activation was related to processing Sta/Psy verbs (as compared with the other conditions) in MDD vs controls.
Unfortunately, no significant correlation was found among the behavioral scores, HDRS score and the imaging dataset. This could be due to the small sample size and further studies are planned to test this relation. Another possibility is that the signal extracted from the frontal and the putamen cluster does not reflect the time a participant took to imagine an emotional scene, but other imagery related variables like the imagery vividness for instance. Similarly, it is possible that the signal would better reflect a relation with 1st vs 3rd imagery vividness. Further studies are planned to test this relation.
2.6 Conclusion
Our study adds new and important neuroimaging evidence to the view that patients with depression tend to use a third-person perspective when imagining scenes and events (6). Likely they do so in order to diminish the emotional load (e.g., 15, 16), as the third-person perspective corresponds to imaging scenes as spectators. When forced to use a first-person perspective, as by the present fMRI paradigm, at the neural level, it is possible to detect an increase in activation in areas related to self-referential processing and perspective taking, likely because they are less used to doing so. Future fMRI studies in which we further investigate difference between first-person perspective and 3rd person perspective of fMRI data of MDD individuals are at the focus of future experiments. In the present study we limited our design to a 2x2 design with task (imagery, letter detection) and type of verbs (emotion/motor) as factors, to keep simpler the interpretation of putative interaction terms.
2.7 Limitation
Unfortunately, we do not have a behavioral counterpart of the fMRI pattern, as reaction times and likeability data were available only for 2/3 of the sample. This lack of behavioral information is a main limitation of the study. It leaves open the question of whether we would have detected (if the dataset were complete) some informative effects at the behavioral level, strengthening our fMRI results. A further limitation of the study is the lack of control in the fMRI analysis of the putative effect of clinical treatments, such as medication or psychotherapy. We acknowledge that in future studies, it is recommended that the impact of clinical treatment is taken into account when determining inclusion criteria. We acknowledge that the sample size of the two groups was small. Moreover, nearly one-third of the important behavioral data in the patient group was missing. Nonetheless we remark that the subjects in the study were in fact very homogeneous with accurate diagnoses and no comorbidities.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Research Ethical Committee of the Research Institute IRCCS E. Medea. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
BT: Conceptualization, Funding acquisition, Investigation, Writing – original draft, Writing – review & editing. MP: Formal Analysis, Writing – review & editing. EM: Data curation, Formal Analysis, Writing – review & editing. CB: Investigation, Writing – review & editing. SD: Writing – review & editing. MB: Writing – review & editing. PB: Conceptualization, Funding acquisition, Investigation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Italian Ministry of Health (Ricerca Corrente 2025) to BT and CB. EM was supported by the European Union – NextGeneration EU (PRIN 2022 PNRR, grant n. P20229MFRC). PB was partially supported by grants from the Italian Ministry of Education and Research - MUR (‘Dipartimenti di Eccellenza’ Programme 2023–27 - Dept. of Pathophysiology and Transplantation, Università degli Studi di Milano), the Italian Ministry of Health (Hub Life Science- Diagnostica Avanzata, HLS-DA, PNC-E3-2022-23683266– CUP: C43C22001630001 / MI-0117; Ricerca Corrente 2025; RF-2019-12371349), the Fondazione Cariplo (Made In Family, grant number 2019–3416), and the ERANET Neuron JTC 2023 (ERP-2023-23684211 - ERP-2023-Neuron-ResilNet).
Acknowledgments
We would like to thank the volunteers and our colleagues from the MRI staff for their technical services.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Clark IA, James EL, Iyadurai L, and Holmes EA. Mental imagery in psychopathology: from the lab to the clinic. In: Clinical Perspectives on Autobiographical Memory. New York, NY: Cambridge University Press (2015). p. 133.
2. Brewin CR, Gregory JD, Lipton M, and Burgess N. Intrusive images in psychological disorders: characteristics, neural mechanisms, and treatment implications. Psychol Rev. (2010) 117:210–32. doi: 10.1037/a0018113
3. Pearson DG, Deeprose C, Wallace-Hadrill SMA, Heyes SB, and Holmes EA. Assessing mental imagery in clinical psychology: a review of imagery measures and a guiding framework. Clin Psychol Rev. (2013) 33:1–23. doi: 10.1016/j.cpr.2012.09.001
4. Pearson J, Naselaris T, Holmes EA, and Kosslyn SM. Mental imagery: functional mechanisms and clinical applications. Trends Cognit Sci. (2015) 19:590–602. doi: 10.1016/j.tics.2015.08.003
5. Ji JL, Kavanagh DJ, Holmes EA, MacLeod C, and Di Simplicio M. Mental imagery in psychiatry: conceptual & clinical implications. CNS Spectrums. (2019) 24:114–26. doi: 10.1017/S1092852918001487
6. Holmes EA, Blackwell SE, Burnett Heyes S, Renner F, and Raes F. Mental imagery in depression: phenomenology, potential mechanisms, and treatment implications. Annu Rev Clin Psychol. (2016) 12:249–80. doi: 10.1146/annurev-clinpsy-021815-092925
7. Holmes EA and Mathews A. Mental imagery and emotion: a special relationship? Emotion. (2005) 5:489–97. doi: 10.1037/1528-3542.5.4.489
8. Weßlau C and Steil R. Visual mental imagery in psychopathology — implications for the maintenance and treatment of depression. Clin Psychol Rev. (2014) 34:273–81. doi: 10.1016/j.cpr.2014.03.001
9. Stöber J. Worry, problem elaboration and suppression of imagery: the role of concreteness. Behav Res Ther. (1998) 36:751–6. doi: 10.1016/S0005-7967(98)00027-8
10. Anderson RJ and Evans GL. Mental time travel in dysphoria: Differences in the content and subjective experience of past and future episodes. Conscious Cogn. (2015) 37:237–48. doi: 10.1016/j.concog.2014.05.006
11. Szőllősi Á, Pajkossy P, and Racsmány M. Depressive symptoms are associated with the phenomenal characteristics of imagined positive and negative future events. Appl Cogn Psychol. (2015) 29:762–7. doi: 10.1002/acp.v29.5
12. Weßlau C, Cloos M, Höfling V, and Steil R. Visual mental imagery and symptoms of depression - results from a large-scale web-based study. BMC Psychiatry. (2015) 15:308. doi: 10.1186/s12888-015-0689-1
13. Holmes EA, Lang TJ, Moulds ML, and Steele AM. Prospective and positive mental imagery deficits in dysphoria. Behav Res Ther. (2008) 46:976–81. doi: 10.1016/j.brat.2008.04.009
14. Morina N, Deeprose C, Pusowski C, Schmid M, and Holmes EA. Prospective mental imagery in patients with major depressive disorder or anxiety disorders. J Anxiety Disord. (2011) 25:1032–7. doi: 10.1016/j.janxdis.2011.06.012
15. McIsaac HK and Eich E. Vantage point in episodic memory. Psychon Bull Rev. (2002) 9:146–50. doi: 10.3758/bf03196271
16. Nigro G and Neisser U. Point of view in personal memories. Cogn Psychol. (1983) 15:467–82. doi: 10.1016/0010-0285(83)90016-6
17. Kuyken W and Howell R. Facets of autobiographical memory in adolescents with major depressive disorder and never-depressed controls. Cogn Emotion. (2006) 20:466–87. doi: 10.1080/02699930500342639
18. Kuyken W and Moulds ML. Remembering as an observer: How is autobiographical memory retrieval vantage perspective linked to depression? Memory. (2009) 17:624–34. doi: 10.1080/09658210902984526
19. Williams AD and Moulds ML. Cognitive avoidance of intrusive memories: recall vantage perspective and associations with depression. Behav Res Ther. (2007) 45:1141–53. doi: 10.1016/j.brat.2006.09.005
20. Williams AD and Moulds ML. Manipulating recall vantage perspective of intrusive memories in dysphoria. Memory. (2008) 16:742–50. doi: 10.1080/09658210802290453
21. Lemogne C, Piolino P, Friszer S, Claret A, Girault N, Jouvent R, et al. Episodic autobiographical memory in depression: Specificity, autonoetic consciousness, and self-perspective. Conscious Cogn. (2006) 15:258–68. doi: 10.1016/j.concog.2005.07.005
22. Bergouignan L, Lemogne C, Foucher A, Longin E, Vistoli D, Allilaire JF, et al. Field perspective deficit for positive memories characterizes autobiographical memory in euthymic depressed patients. Behav Res Ther. (2008) 46:322–33. doi: 10.1016/j.brat.2007.12.007
23. Nelis S, Debeer E, Holmes EA, and Raes F. Dysphoric students show higher use of the observer perspective in their retrieval of positive versus negative autobiographical memories. Memory (Hove England). (2013) 21:423–30. doi: 10.1080/09658211.2012.730530
24. Kerestes R, Davey CG, Stephanou K, Whittle S, and Harrison BJ. Functional brain imaging studies of youth depression: a systematic review. NeuroImage Clin. (2013) 4:209–31. doi: 10.1016/j.nicl.2013.11.009
25. Pilmeyer J, Huijbers W, Lamerichs R, Jansen JFA, Breeuwer M, and Zinger S. Functional MRI in major depressive disorder: A review of findings, limitations, and future prospects. J Neuroimaging. (2022) 32:582–95. doi: 10.1111/jon.13011
26. Tomasino B, Nobile M, Re M, Bellina M, Garzitto M, Arrigoni F, et al. The mental simulation of state/psychological verbs in the adolescent brain: An fMRI study. Brain Cogn. (2018) 123:34–46. doi: 10.1016/j.bandc.2018.02.010
27. Tomasino B, Fabbro F, and Brambilla P. How do conceptual representations interact with processing demands: An fMRI study on action- and abstract-related words. Brain Res. (2014) 1591:38–52. doi: 10.1016/j.brainres.2014.10.008
28. Tomasino B, Maggioni E, Bonivento C, Nobile M, D’Agostini S, Arrigoni F, et al. Effects of age and gender on neural correlates of emotion imagery. Hum Brain Map. (2022) 43(13):4116–27. doi: 10.1002/hbm.25906. Advance online publication.
29. Spitzer RL, Williams JB, Gibbon M, and First MB. The Structured Clinical Interview for DSM-III-R (SCID). I: History, rationale, and description. Arch Gen Psychiatry. (1992) 49:624–9. doi: 10.1001/archpsyc.1992.01820080032005
30. Ventura J, Nuechterlein KH, Subotnik KL, Gutkind D, and Gilbert EA. Symptom dimensions in recent-onset schizophrenia and mania: a principal components analysis of the 24-item Brief Psychiatric Rating Scale. Psychiatry Res. (2000) 97:129–35. doi: 10.1016/S0165-1781(00)00228-6
31. Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. (1959) 32:50–5. doi: 10.1111/j.2044-8341.1959.tb00467.x
32. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. (1960) 23:56–62. doi: 10.1136/jnnp.23.1.56
33. Endicott J, Spitzer RL, Fleiss JL, and Cohen J. The global assessment scale. A procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry. (1976) 33:766–71. doi: 10.1001/archpsyc.1976.01770060086012
34. Raven J. The Raven’s progressive matrices: change and stability over culture and time. Cognit Psychol. (2000) 41:1–48. doi: 10.1006/cogp.1999.0735
35. Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. (1971) 9:97–113. doi: 10.1016/0028-3932(71)90067-4
36. Dale AM and Buckner RL. Selective averaging of rapidly presented individual trials using fMRI. Hum Brain Mapp. (1997) 5:329–40. doi: 10.1002/(SICI)1097-0193(1997)5:5<329::AID-HBM1>3.0.CO;2-5
37. Bonivento C, Tomasino B, Garzitto M, Piccin S, Fabbro F, and Brambilla P. Age-dependent changes of thinking about verbs. Front Behav Neurosci. (2017) 11:40. doi: 10.3389/fnbeh.2017.00040
38. Bradley MM and Lang PJ. Fearfulness and affective evaluations of pictures. Motivation Emotion. (1999) 23:1–13. doi: 10.1023/A:1021375216854
39. Friston KJ. Statistical parametric mapping. In: Kötter R, editor. Neuroscience Databases. Boston, MA, Springer (2003).
40. Brett M, Anton JL, Valabregue R, and Poline JB. Region of interest analysis using an SPM toolbox [abstract]. In: Presented at the 8th International Conference on Functional Mapping of the Human Brain, June 2-6, Sendai, Japan. Available on CD-ROM in NeuroImage. 16, abstract 497 (2002).
41. Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage. (2005) 25:1325–35. doi: 10.1016/j.neuroimage.2004.12.034
42. Beer J, John O, Scabini D, and Knight R. Orbitofrontal cortex and social behavior: integrating self-monitoring and emotion-cognition interactions. J Cognit Neurosci. (2006) 18:871. doi: 10.1162/jocn.2006.18.6.871
43. Northoff G and Bermpohl F. Cortical midline structures and the self. Trends in Cognitive Sciences.. (2004) 8(3)102–7. doi: 10.1016/j.tics.2004.01.004
44. D’Argembeau A, Ruby P, Collette F, Degueldre C, Balteau E, Luxen A, et al. Distinct regions of the medial prefrontal cortex are associated with self-referential processing and perspective taking. J Cognit Neurosci. (2007) 19:935–44. doi: 10.1162/jocn.2007.19.6.935
45. D’Argembeau A, Collette F, van der Linden M, Laureys S, Del Fiore G, Degueldre C, et al. Self-referential reflective activity and its relationship with rest: a PET study. Neuroimage. (2005) 25:616–24. doi: 10.1016/j.neuroimage.2004.11.048
46. Farb NA, Segal ZV, Mayberg H, Bean J, McKeon D, Fatima Z, et al. Attending to the present: mindfulness meditation reveals distinct neural modes of self-reference. Soc Cognit Affect Neurosci. (2007) 2:313–22. doi: 10.1093/scan/nsm030
47. Moran JM, Macrae CN, Heatherton TF, Wyland CL, and Kelley WM. Neuroanatomical evidence for distinct cognitive and affective components of self. J Cognit Neurosci. (2006) 18:1586–94. doi: 10.1162/jocn.2006.18.9.1586
48. D’Argembeau A. On the role of the ventromedial prefrontal cortex in self-processing: the valuation hypothesis. Front Hum Neurosci. (2013) 7:372. doi: 10.3389/fnhum.2013.00372
49. Ruby P and Decety J. What you believe versus what you think they believe: a neuroimaging study of conceptual perspective-taking. Eur J Neurosci. (2003) 17:2475–80. doi: 10.1046/j.1460-9568.2003.02673.x
50. Hynes CA, Baird AA, and Grafton ST. Differential role of the orbital frontal lobe in emotional versus cognitive perspective-taking. Neuropsychologia. (2006) 44:374–83. doi: 10.1016/j.neuropsychologia.2005.06.011
51. David N, Bewernick BH, Cohen MX, Newen A, Lux S, Fink GR, et al. Neural representations of self versus other: visual-spatial perspective taking and agency in a virtual ball-tossing game. J Cognit Neurosci. (2006) 18:898–910. doi: 10.1162/jocn.2006.18.6.898
52. Alexander GE, DeLong MR, and Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. (1986) 9:357–81. doi: 10.1146/annurev.ne.09.030186.002041
53. Middleton FA and Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Brain Res Rev. (2000) 31:236–50. doi: 10.1016/s0165-0173(99)00040-5
54. Pennartz CM, Ito R, Verschure PF, Battaglia FP, and Robbins TW. The hippocampal-striatal axis in learning, prediction and goal-directed behavior. Trends Neurosci. (2011) 34:548–59. doi: 10.1016/j.tins.2011.08.001
55. Viñas-Guasch N and Wu YJ. The role of the putamen in language: a meta-analytic connectivity modeling study. Brain Struct Funct. (2017) 222:3991–4004. doi: 10.1007/s00429-017-1450-y
56. Makary MM, Eun S, and Park K. Greater corticostriatal activation associated with facial motor imagery compared with motor execution: a functional MRI study. Neuroreport. (2017) 28:610–7. doi: 10.1097/WNR.0000000000000809
57. Radke S, Hoffstaedter F, Loffler L, Kogler L, Schneider F, Blechert J, et al. Imaging the up’s and down’s of emotion regulation in lifetime depression. Brain Imaging Behav. (2018) 12:156–67. doi: 10.1007/s11682-017-9682-2
Keywords: state/psychological stimuli, emotion, imagery, depression, fMRI
Citation: Tomasino B, Piani MC, Maggioni E, Bonivento C, D’Agostini S, Balestrieri M and Brambilla P (2025) First person state/psychological imagery modulates the orbital frontal gyrus and the putamen in individuals with depression. Front. Psychiatry 16:1583482. doi: 10.3389/fpsyt.2025.1583482
Received: 25 February 2025; Accepted: 07 April 2025;
Published: 19 May 2025.
Edited by:
Ali Saffet Gonul, Ege University, TürkiyeReviewed by:
Yuanshu Chen, University of Electronic Science and Technology of China, ChinaDemin Gao, Hong Kong Baptist University, Hong Kong SAR, China
Copyright © 2025 Tomasino, Piani, Maggioni, Bonivento, D’Agostini, Balestrieri and Brambilla. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Barbara Tomasino, YmFyYmFyYS50b21hc2lub0BnbWFpbC5jb20=;; Paolo Brambilla, cGFvbG8uYnJhbWJpbGxhQHBvbGljbGluaWNvLm1pLml0
 Barbara Tomasino
Barbara Tomasino Maria Chiara Piani
Maria Chiara Piani Eleonora Maggioni
Eleonora Maggioni Carolina Bonivento
Carolina Bonivento Serena D’Agostini
Serena D’Agostini Matteo Balestrieri6
Matteo Balestrieri6 Paolo Brambilla
Paolo Brambilla