- 1School of Acupuncture-Moxibustion and Tuina, Shandong University of Chinese Medicine, Jinan, Shandong, China
- 2Department of Proctology, China-Japan Friendship Hospital, Beijing, China
- 3Department of Acupuncture and Moxibustion, Second Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan, Shandong, China
Background: Male Sexual Dysfunction (MSD), comprising erectile dysfunction (ED), premature ejaculation, and hypoactive sexual desire disorder, exhibits an age-related prevalence affecting 50% of males beyond their fourth decade. Epidemiological studies demonstrate ED prevalence rates of 79.4% in general clinical populations. Beyond physiological manifestations, MSD with comorbid anxiety and depression exerts profound psychosocial impacts, potentially exacerbating pre-existing mental health conditions and impairing interpersonal relationships. Emerging evidence suggests Chinese Herbal Medicine (CHM) may offer therapeutic potential for addressing this clinical intersection. Therefore, this study will perform a systematic review and meta-analysis to assess the efficacy of various CHM interventions for MSD patients with comorbid anxiety and depression.
Methods: This study will systematically search four Chinese databases (China National Knowledge Infrastructure, Wanfang Database, China Biomedical Database, and VIP Database) and four international databases (PubMed, Web of Science, EMBASE, and Cochrane Library). Randomized controlled trials (RCTs) investigating CHM interventions for MSD with comorbid anxiety and depression will be identified. The retrieved studies will undergo rigorous screening and quality assessment using standardized tools.
Results: The findings of this investigation will establish an evidence-based foundation for optimizing CHM therapeutic protocols in managing MSD with comorbid anxiety and depression.
Conclusions: This investigation will synthesize evidence-based data to evaluate therapeutic outcomes of various CHM modalities for MSD with comorbid anxiety and depression. Through comparative effectiveness analyses, the study aims to establish hierarchical treatment recommendations, enabling clinicians to optimize intervention strategies and facilitate personalized treatment selection in clinical practice.
Clinical trial registration: https://www.crd.york.ac.uk/prospero, identifier CRD420250652254
1 Introduction
Male Sexual Dysfunction (MSD) encompasses a range of conditions that impair male sexual function, including erectile dysfunction (ED), premature ejaculation (PE), and hypoactive sexual desire disorder (1). For men, ED and PE are the two most prevalent subtypes of male sexual dysfunction, with clinical presentations typically categorized as primary or secondary (2). ED is defined as the persistent inability of the penis to achieve or maintain an erection sufficient for satisfactory sexual activity. Based on etiological differences, ED can be categorized into three types: psychogenic ED, organic ED, and mixed ED (3, 4). PE can be defined as ejaculation that almost always occurs within approximately one minute of vaginal penetration since the first sexual intercourse (primary premature ejaculation), or a marked reduction in intravaginal latency time (IELT) to less than three minutes, typically due to a previous absence of such issues (secondary premature ejaculation) (5). The prevalence of MSD increases with age; approximately 50% of men over the age of 40 experience varying degrees of sexual dysfunction (6). The Massachusetts Male Aging Study (MMAS) reported that among men aged 40 to 70 in the Boston area, the overall prevalence of ED was 52%, with 50% to 60% of cases attributed to penile vascular dysfunction and 16% to 78% linked to mental health disorders (7–10).The overall incidence of PE is estimated to be between 20% and 30% (11), with 36% to 63% classified as primary premature ejaculation and 16% to 28% as secondary premature ejaculation (12, 13). Beyond its impact on physical health, MSD significantly affects mental well-being and interpersonal relationships, potentially exacerbating psychological issues such as anxiety and depression (14). The pathophysiological mechanisms underlying MSD are multifaceted, encompassing neurological, vascular, endocrine, and psychological factors. ED is commonly associated with inadequate penile blood flow, which may result from cardiovascular conditions such as atherosclerosis, diabetes mellitus, or hypertension (15). Endocrine dysregulation in patients with diabetes not only impairs testosterone synthesis but may also exacerbate ED by compromising vascular endothelial function (16). Research indicates that the prevalence of sexual dysfunction in individuals with anxiety disorders is significantly higher compared to the general population, and elevated anxiety levels are strongly associated with reduced sexual satisfaction (17). Furthermore, lifestyle factors such as smoking, excessive alcohol consumption, and physical inactivity can also adversely affect sexual function (18). Research indicates that sexual dysfunction is closely associated with anxiety and depression, forming a complex interrelationship that often traps patients in a vicious cycle. Anxiety and depression not only exacerbate the symptoms of sexual dysfunction but can also lead to avoidance of sexual activities, thereby further increasing the psychological burden on patients (19, 20). There is a significant association between depression and sexual dysfunction, particularly among male patients, where the prevalence of depression can reach up to 60% or higher (21, 22). Therefore, it is imperative to accord increased attention to anxiety and depression in the context of MSD.
Currently, the treatment options for MSD primarily include pharmacotherapy, psychotherapeutic interventions, and lifestyle modifications. In drug therapy, phosphodiesterase-5 inhibitors (PDE5Is) represent the first-line therapy for ED, while selective serotonin reuptake inhibitors (SSRIs) are primarily used for managing PE (23, 24). To date, four drugs have been approved by both the FDA and EMA: Sildenafil, Tadalafil, Vardenafil, and Avanafil. The therapeutic efficacy of these agents has been thoroughly validated. PDE5Is enhance NO-mediated vascular smooth muscle relaxation and promote increased blood flow and erection maintenance by inhibiting PDE5 enzyme activity and reducing cGMP degradation in the penile corpus cavernosum. Selective PDE5 inhibitors are effective agents for promoting penile erection, with their primary role being to support erection maintenance rather than directly induce erection onset (23). SSRIs are a class of antidepressants. Clinical observations have revealed their ability to prolong Intravaginal Ejaculation Latency Time (IELT), leading to their widespread use in the treatment of PE. Common SSRIs include dapoxetine hydrochloride, paroxetine, sertraline, and fluoxetine. These agents function by binding to and inhibiting 5-HT reuptake receptors on the presynaptic membrane, thereby increasing the concentration of 5-HT in the synaptic cleft. This process desensitizes 5-HT1A and 5-HT1B receptors, ultimately contributing to the prolongation of IELT and aiding in the management of PE (25, 26). It is worth noting that existing studies have explored the application of PDE5i in the treatment of PE, indicating that PDE5i may exert therapeutic effects through multiple mechanisms. These include alleviating sexual anxiety, reducing sympathetic nerve excitation, and promoting the dilation of smooth muscles in the vas deferens and seminal vesicles, all of which potentially contribute to delaying ejaculation (25, 27). However, while these medications can improve MSD symptoms to some extent, their efficacy in addressing concomitant psychological issues such as anxiety and depression is limited (28). It is worrying that common adverse reactions of SSRI drugs include fatigue, nausea, diarrhea and excessive sweating; while PDE5i drugs may cause discomfort such as headache, indigestion, vision problems and myalgia (26, 29). In addition, due to the high cost of treatment and limited efficacy, many patients eventually choose to discontinue treatment (23, 30). In psychological therapy interventions, a comprehensive analysis and evaluation of the psychological issues present in patients with ED and PE should be performed. By alleviating their inner fears and reducing anxiety and depressive symptoms, patients can be assisted in confronting their sexual dysfunction. The primary goal of psychological therapy is to eliminate fear, anxiety, and depression related to sexual activity while fostering effective communication between patients and their partners. Furthermore, sexual psychological therapy may be combined with behavioral techniques, such as the “start-stop” method and the “squeeze” technique (2, 31). The most critical aspect is the adjustment of lifestyle. Patients should conduct self-health management under medical supervision and maintain long-term treatment adherence. Specifically, establishing regular living habits and adhering to a consistent medication schedule are crucial for effective disease management. Treatment plans should be tailored as much as possible to individual patient needs. For instance, during the early stages of treatment or in cases of severe symptoms, a combination of multiple therapeutic approaches may be warranted (2, 23). Against this backdrop, Chinese Herbal Medicine (CHM) have increasingly garnered attention. Studies have demonstrated their efficacy in improving sexual function and alleviating anxiety and depression, with relatively fewer side effects compared to conventional treatments (16).
CHM has demonstrated significant advantages in treating anxiety and depression among patients with MSD, particularly in improving psychological well-being, enhancing sexual function, and providing robust clinical research evidence. The multi-component and multi-target nature of CHM confers a unique advantage in treating complex diseases, as its ability to simultaneously act on multiple pathological mechanisms enhances therapeutic efficacy (20, 32). Through data mining of medication patterns for male sexual dysfunction, it was revealed that Cistanche, Epimedium, and Lycium barbarum are frequently utilized in clinical practice (33, 34). Specifically, Cistanche enhances sexual function by elevating testosterone levels, exerting antioxidant properties, and combating hypoxia (35). Epimedium significantly boosts plasma testosterone levels and stimulates the proliferation and secretory functions of testicular tissue (36). Additionally, Lycium barbarum exhibits immunomodulatory effects, increases blood testosterone concentrations, and provides a tonic effect (37). Compound traditional Chinese medicine represents another effective therapeutic approach for male sexual dysfunction. Studies have demonstrated that Shugan Yiyang Capsules can significantly upregulate the gene and protein expression levels of the three nitric oxide synthase (NOS) subtypes, as well as enhance the protein expression of cyclic guanosine monophosphate (cGMP), ultimately contributing to the effective improvement of sexual dysfunction (38). CHM prescriptions, such as Bazhen Decoction and Sijunzi Decoction, demonstrate significant efficacy in regulating qi and blood and enhancing physical constitution, while also effectively alleviating symptoms of anxiety and depression, thereby contributing to improved sexual function (17, 39). However, although individual studies have explored the effects of CHM on MSD and associated psychological conditions including anxiety and depression, the research findings remain inconsistent. Particularly in cases involving comorbidities between MSD and mental health disorders such as anxiety and depression, a comprehensive evaluation of the therapeutic efficacy of CHM remains to be established.
Consequently, the goal of this proposed meta-analysis is to address the existing knowledge gap regarding CHM in managing MSD with comorbid anxiety and depression. By systematically synthesizing evidence from clinical studies, we aim to evaluate CHM’s therapeutic potential and provide a foundational framework for future randomized controlled trials (RCTs), ultimately supporting evidence-based clinical decisions
2 Materials and methods
This study will adhere to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (40, 41). The registration number of the protocol is CRD420250652254 (https://www.crd.york.ac.uk/prospero/).
2.1 Search strategy
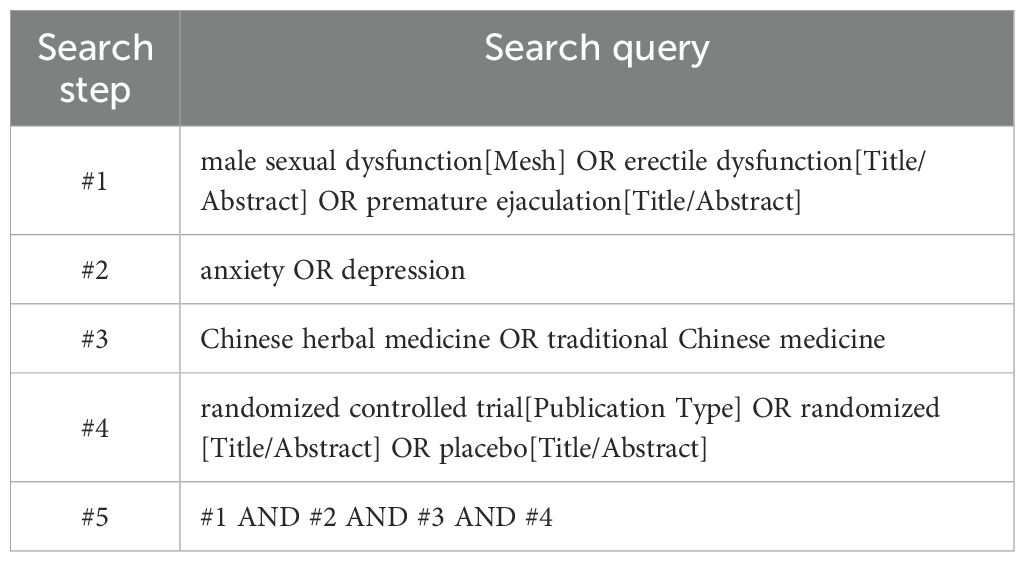
Two researchers comprehensively searched 4 Chinese databases, including China National Knowledge Infrastructure (CNKI), Wanfang Data Knowledge Service Platform, VIP, and CBM, and 4 English databases, including PubMed, Web of Science, EMBASE, and The Cochrane Library. We searched the database for all articles from its inception to 24 February 2025 in Chinese and English, with no geographical restrictions. A combination of subject matter and free terminology is employed to ensure a comprehensive search, regardless of language or type of publication. All databases will be searched to ensure that all relevant articles are identified. The detailed search strategy for the PubMed database is presented in Table 1.
2.2 Inclusion and exclusion criteria
2.2.1 Type of study
Only RCTs were included. However, the studies must be in either English or Chinese. Observational studies, cross-sectional studies, animal studies, and other non-RCT designs were excluded because they are highly susceptible to confounding factors that may compromise the causal inference of CHM efficacy.
2.2.2 Type of participants
Adult male patients diagnosed with MSD and comorbid anxiety and/or depression.
2.2.3 Type of intervention
The experimental group must receive treatment with either single-agent or combination CHM, while the control group may receive placebo, conventional pharmacotherapy, or no intervention.
2.2.4 Type of outcome measures
Outcome measures required at least one assessment of anxiety, depression, or overall symptoms. Primary outcomes included the Self-Rating Anxiety Scale (SAS) (42), the Self-Rating Depression Scale (SDS) (43), the Hamilton Anxiety Rating Scale (HAMA) (44), the Hamilton Depression Rating Scale (HAMD) (45), and the Hospital Anxiety and Depression Scale (HADS) (46). Secondary outcomes pertained to the global alleviation of symptoms (47).
2.5 Study selection process and data extraction
Two researchers will independently screen titles and abstracts to confirm whether they meet the inclusion criteria, and will conduct full-text evaluations of all included studies. The following data will be extracted using standardized forms: first author’s name, year of publication, age range of participants, sample size, diagnostic criteria, interventions in the treatment and control groups, duration of treatment, outcome measures, and adverse events. In the event of disagreements, a third researcher will be consulted, and a final decision will be made through discussion. The PRISMA flowchart (Figure 1) will provide a comprehensive overview of the selection process.
2.6 Risk of bias assessment
Two researchers will independently assess the risk of bias for each included randomized controlled trial (RCT) using the Cochrane Handbook-recommended Risk of Bias tool, RoB 2.0 (48). The assessment will cover the following domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, incomplete outcome data, selective reporting, and other potential sources of bias. Based on the assessment, each study will be classified as having a high, low, or unclear risk of bias. In the event of disagreements, a third researcher will be consulted to assist in reaching consensus.
2.7 Statistical analysis
We will perform a meta-analysis using STATA. As each score represents a numeric variable with potentially different measurement scales and scoring criteria, we will extract score differences before and after treatment. The standardized mean difference (SMD) will be selected as the effect measure, with 95% confidence intervals (95% CI) serving as the statistical test intervals for effect estimates. Heterogeneity will be evaluated using the Chi-squared test and I² statistic. If studies demonstrate low heterogeneity (I² < 50%), we will employ a fixed-effect model for meta-analysis. For substantial heterogeneity (I² ≥ 50%), a random-effects model will be utilized, followed by subgroup analyses to investigate heterogeneity sources. When quantitative synthesis is inappropriate, we will provide narrative synthesis of results for publication inclusion. For trials reporting only pre-post values, mean changes will be calculated by subtracting baseline measurements from post-intervention values, with corresponding standard deviations (SD) of change estimated accordingly.
2.8 Subgroup analyses
In cases of significant heterogeneity, planned subgroup analyses will be conducted to explore potential sources of heterogeneity by examining the following factors: Age (<40 vs. ≥40 years), Baseline depression severity (mild [PHQ-9 <10] vs. moderate-severe [PHQ-9 ≥10]), CHM intervention type (single-herb vs. compound formulations; short-term [<8 weeks] vs. long-term [≥8 weeks] regimens). When adequate data exists across subgroups, we will perform quantitative analyses using mixed-effects models to examine subgroup-by-treatment interactions. For subgroups with insufficient data for quantitative synthesis, a structured qualitative synthesis will be implemented, involving within-study comparisons, cross-study pattern analysis, and evidence grading through GRADE criteria, enabling meaningful interpretation without formal meta-analytic combination (49).
2.9 Sensitivity analysis
Should significant heterogeneity persist following subgroup analyses, we will conduct sensitivity analyses to evaluate result stability. The sensitivity analysis protocol will involve systematically re-running the meta-analysis while excluding studies with high risk of bias (RoB ≥ 4 on modified Newcastle-Ottawa Scale) and statistical outliers identified through Galbraith plots. By comparing effect size estimates between primary and sensitivity analyses using Hartung-Knapp adjustment, we will quantify the influence of individual studies on pooled effects. This methodological approach enables assessment of result robustness while clarifying potential heterogeneity drivers through differential exclusion impacts.
2.10 Publication bias
When the meta-analysis includes 10 or more studies, we will evaluate publication bias using Egger’s regression test. The results will be visually complemented with funnel plots to assess potential asymmetry in effect size distribution.
3 Discussion
In recent years, research on the use of traditional CHM for managing anxiety and depression symptoms associated with MSD has garnered increasing attention. CHM demonstrates potential advantages in simultaneously addressing sexual dysfunction and psychological comorbidities through its multi-target mechanisms, contrasting with conventional therapies limited to isolated symptom management. Although systematic reviews inherently rely on existing data rather than recruiting new patients, the publication of a protocol remains methodologically essential. First, pre-registration reduces the risk of unintended deviations from predefined inclusion criteria and statistical methods, thereby minimizing selective reporting bias (41). Second, given that CHM trials often exhibit heterogeneous outcome measures, publishing the protocol in advance ensures transparency in standardizing these endpoints. Finally, considering the ongoing debate regarding methodological standards for CHM reviews (50), this protocol adheres to PRISMA-P guidelines to establish a rigorous precedent for evidence synthesis in integrative psychosexual medicine.
CHM exerts therapeutic effects on MSD with comorbid anxiety and depression through multi-target interventions involving neurotransmitter regulation, metabolic modulation, and neuroendocrine-immune integration. CHM formulations restore serotonin (5-HT) and norepinephrine balance by inhibiting monoamine oxidase activity and upregulating synaptic neurotransmitter reuptake. For instance, Chaihu Shugan San, which contains Saikosaponins, has been shown to significantly improve HAMD scores via this mechanism (51–53). Herbs such as Gardenia jasminoides, which contain iridoids, suppress corticosterone overproduction and downregulate glucocorticoid receptor expression in hippocampal neurons, thereby reversing stress-induced dysregulation of the HPA axis (54). Saikosaponins (SSs), the primary active components of Bupleurum scorzonerifolium Willd, have demonstrated significant improvement in depression-like behavior, attenuation of central inflammation, and marked inhibition of neuronal pyroptosis in mice across both in vivo and in vitro models (55). CHM formulas suppress hyperactivity of the hypothalamic-pituitary-adrenal (HPA) axis and reduce proinflammatory cytokines by modulating glucocorticoid receptor expression. For instance, iridoids from Gardenia jasminoides exert anti-inflammatory effects through NF-κB pathway inhibition, thereby restoring neuroendocrine-immune homeostasis (56). Tianmeng Oral Liquid alleviate depressive symptoms by modulating nucleotide, energy, and amino acid metabolism, thereby replenishing ATP and reducing oxidative stress in MSD-related neuronal circuits (57). The multicomponent nature of CHM enables it to simultaneously target neurotransmitter imbalance, inflammation, and vascular dysfunction, thereby distinguishing it from conventional single-pathway antidepressants (56, 58).
However, in the research and clinical application of traditional CHM, several challenges also arise. Firstly, the scientific evidence supporting the efficacy and mechanisms of action of CHM remains relatively limited. While some preliminary studies have demonstrated promising results, large-scale, RCTs are still scarce. Secondly, due to the complex composition of CHM, standardizing dosage and usage methods is critically important. This lack of standardization may compromise the reproducibility and safety of therapeutic outcomes.Therefore, it is of great significance to systematically evaluate the efficacy and safety of CHM in treating MSD accompanied by anxiety and depression, and to explore its integration with traditional treatment regimens.
We will analyze published RCTs to comprehensively assess the therapeutic effects of various CHM formulations, with the aim of identifying optimal administration methods, treatment duration, and dosage regimens. The findings are anticipated to provide evidence-based guidance for clinical practice and establish a theoretical foundation for integrating CHM into comprehensive treatment strategies for sexual dysfunction with psychological comorbidities. However, this systematic review and network meta-analysis may encounter several methodological limitations, including potential publication bias, clinical heterogeneity, and selection bias. Firstly, the predetermined stringent inclusion criteria, although crucial for ensuring methodological rigor, might limit the number of eligible RCTs. Future reviews could consider incorporating high-quality observational studies to investigate the long-term safety and adherence patterns of CHM in real-world settings. Secondly, linguistic restrictions limited to English and Chinese publications could introduce geographical selection bias. Finally, we will focus on the most widely used and representative CHM formulations, which may limit the exploration of less common but potentially effective therapies. Despite these limitations, we anticipate that this study will establish an evidence base for CHM in managing MSD with comorbid anxiety and depression, while offering novel perspectives for psychosexual integrative care.
Author contributions
ZX: Conceptualization, Formal Analysis, Investigation, Methodology, Software, Supervision, Writing – original draft. XZ: Data curation, Validation, Visualization, Writing – review & editing. HJ: Writing – review & editing, Funding acquisition, Supervision. YZ: Writing – review & editing, Project administration, Resources, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by Shandong Provincial Medical Staff Science and Technology Innovation Program (SDYWZGKCJHLH202410); Clinical Research Project of Shandong University of Traditional Chinese Medicine(LCKY202424).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2025.1584306/full#supplementary-material
References
1. Association AP. Diagnostic and Statistical Manual Of Mental Disorders. 5th ed. The United States: American Psychiatric Association Publishing (2013) p. 423–50.
2. Expert Consensus Writing Group of Branch of Sexology of Traditional Chinese Medicine CSA. Erectile dysfunction and premature ejaculation comorbidity diagnosis and treatment by integrated Traditional Chinese and Western Medicine Chinese Expert Consensus. Chin J Exper Tradit Med Form. (2024) 30:147–53. doi: 10.13422/j.cnki.syfjx.20241093
3. Minhas S, Bettocchi C, Boeri L, Capogrosso P, Carvalho J, Cilesiz NC, et al. European association of urology guidelines on male sexual and reproductive health: 2021 update on male infertility. Eur Urol. (2021) 80:603–20. doi: 10.1016/j.eururo.2021.08.014
4. Salonia A, Bettocchi C, Boeri L, Capogrosso P, Carvalho J, Cilesiz NC, et al. European association of urology guidelines on sexual and reproductive health-2021 update: male sexual dysfunction. Eur Urol. (2021) 80:333–57. doi: 10.1016/j.eururo.2021.06.007
5. Althof SE, Mcmahon CG, Waldinger MD, Serefoglu EC, Shindel AW, Adaikan PG, et al. An update of the international society of sexual medicine’s guidelines for the diagnosis and treatment of premature ejaculation (PE). Sex Med. (2014) 2:60–90. doi: 10.1002/sm2.28
6. Anderson D, Laforge J, Ross MM, Vanlangendonck R, Hasoon J, Viswanath O, et al. Male sexual dysfunction. Health Psychol Res. (2022) 10:37533. doi: 10.52965/001c.37533
7. Dong W, Niu H. Application of penile vascular reconstruction in the treatment of vascular erectile dysfunction. Chin J Hum Sex. (2015) 24:41–3. doi: 10.3969/j.issn.1672-1993.2015.02.014
8. Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, Mckinlay JB. Impotence and its medical and psychosocial correlates: results of the massachusetts male aging study. J Urol. (1994) 151:54–61. doi: 10.1016/s0022-5347(17)34871-1
9. Hatzichristou DG, Hatzimouratidis K, Ioannides E, Yannakoyorgos K, Dimitriadis G, Kalinderis A. Nocturnal penile tumescence and rigidity monitoring in young potent volunteers: reproducibility, evaluation criteria and the effect of sexual intercourse. J Urol. (1998) 159:1921–6. doi: 10.1016/S0022-5347(01)63197-5
10. Lue TF, Giuliano F, Montorsi F, Rosen RC, Andersson K, Althof S, et al. Summary of the recommendations on sexual dysfunctions in men. J Sex Med. (2004) 1:6–23. doi: 10.1111/j.1743-6109.2004.10104.x
11. Gao J, Peng D, Zhang X, Hao Z, Zhou J, Fan S, et al. Prevalence and associated factors of premature ejaculation in the anhui male population in China: evidence-based unified definition of lifelong and acquired premature ejaculation. Sex Med. (2017) 5:e37–43. doi: 10.1016/j.esxm.2016.11.002
12. Zhang X, Gao J, Liu J, Xia L, Yang J, Hao Z, et al. Distribution and factors associated with four premature ejaculation syndromes in outpatients complaining of ejaculating prematurely. J Sex Med. (2013) 10:1603–11. doi: 10.1111/jsm.12123
13. Serefoglu EC, Cimen HI, Atmaca AF, Balbay MD. The distribution of patients who seek treatment for the complaint of ejaculating prematurely according to the four premature ejaculation syndromes. J Sex Med. (2010) 7:810–5. doi: 10.1111/j.1743-6109.2009.01570.x
14. Pitta RM, Kaufmann O, Luz JSN, Ritti-Dias RM, Queiroga LDL, Wolosker N. The association between erectile dysfunction and depression: a cross-sectional study of 21,139 Brazilian men. Einstein (Sao Paulo). (2024) 22:eAO1063. doi: 10.31744/einstein_journal/2024AO1063
15. Flegge LG, Barr A, Craner JR. Sexual functioning among adults with chronic pain: prevalence and association with pain-related outcomes. Pain Med. (2023) 24:197–206. doi: 10.1093/pm/pnac117
16. Liu Y, Jin B. Mechanism of traditional chinese medicine extract in the treatment of diabetic erectile dysfunction. J Ethnopharmacol. (2025) 341:119332. doi: 10.1016/j.jep.2025.119332
17. Strizzi JM, Hald GM, Pavan S, Heymann-Szlachcinska A, Ollgaard M, Winding C, et al. Predictors of sexual dysfunction, associated distress, and sexual satisfaction among male and female patients living with anxiety disorders in Denmark. J Sex Res. (2024), 1–16. doi: 10.1080/00224499.2024.2432608
18. Jia B, Li Z, Zhao D, Fu Q. Research progress on ferroptosis in organic erectile dysfunction. Arch Esp Urol. (2023) 76:746–54. doi: 10.56434/j.arch.esp.urol.20237610.90
19. Wang YL, Geng LG, He CB, Yuan SY. Chinese herbal medicine combined with tadalafil for erectile dysfunction: a systematic review and meta-analysis. Andrology. (2020) 8:268–76. doi: 10.1111/andr.12696
20. Wu H, Gao Z, Dai D, Liu X, Fang Y, Chen X, et al. Efficacy and safety assessment of traditional chinese medicine for erectile dysfunction: a meta-analysis and trial sequential analysis. Andrology. (2023) 11:1345–67. doi: 10.1111/andr.13420
21. Sibley AA, Shrestha S, Lipovac-Dew M, Kunik ME. Examining depression symptoms with/without coexisting anxiety symptoms in community-dwelling persons with dementia. Am J Alzheimers Dis Other Demen. (2021) 36:1312416379. doi: 10.1177/1533317521990267
22. Zhang W, Nan N, He Y, Zuo H, Song X, Zhang M, et al. Prevalence of depression and anxiety symptoms and their associations with cardiovascular risk factors in coronary patients. Psychol Health Med. (2023) 28:1275–87. doi: 10.1080/13548506.2022.2104885
23. Longoni M, Bertini A, Schifano N, Zaffuto E, Maggio P, Piercarlo R, et al. A review on pharmacological options for the treatment of erectile dysfunction: state of the art and new strategies. Expert Opin Pharmacother. (2023) 24:1375–86. doi: 10.1080/14656566.2023.2221785
24. Liu K, Wang Z, Liu Y, Zhu P, Zhang S, Lu S, et al. An electrophysiological technique to accurately diagnose and treat erectile dysfunction. J Vis Exp. (2022) 189:14. doi: 10.3791/63851
25. Mitsogiannis I, Dellis A, Papatsoris A, Moussa M. An up-to-date overview of the pharmacotherapeutic options for premature ejaculation. Expert Opin Pharmacother. (2022) 23:1043–50. doi: 10.1080/14656566.2022.2035361
26. Waldinger MD. Drug treatment options for premature ejaculation. Expert Opin Pharmacother. (2018) 19:1077–85. doi: 10.1080/14656566.2018.1494725
27. Abu El-Hamd M. Efficacy and safety of daily use of tadalafil in treatment of patients with premature ejaculation: a randomised placebo-controlled clinical trial. Andrologia. (2018) 50:e13005. doi: 10.1111/and.13005
28. Shahrajabian MH. Powerful stress relieving medicinal plants for anger, anxiety, depression, and stress during global pandemic. Recent Pat Biotechnol. (2022) 16:284–310. doi: 10.2174/1872208316666220321102216
29. Carvalheira AA, Pereira NM, Maroco J, Forjaz V. Dropout in the treatment of erectile dysfunction with pde5: a study on predictors and a qualitative analysis of reasons for discontinuation. J Sex Med. (2012) 9:2361–9. doi: 10.1111/j.1743-6109.2012.02787.x
30. Kim S, Lee Y, Seo K, Jung G, Kim T. Reasons and predictive factors for discontinuation of pde-5 inhibitors despite successful intercourse in erectile dysfunction patients. Int J Impot Res. (2014) 26:87–93. doi: 10.1038/ijir.2013.41
31. Zhang Y, Guo J, Zhang C, Zhang G, Yan S, Chen L, et al. Expert consensus on doctor-patient communication in the diagnosis and treatment of erectile dysfunction combined with premature ejaculation. Chin J Androl. (2016) 30:58–62. doi: 10.3969/j.issn.1008-0848.2016.09.014
32. Xu R, Ma J, Zhang X, Liao Z, Fu Y, Lv B. Efficacy of Chinese herbal medicine formula in the treatment of mild to moderate erectile dysfunction: study protocol for a multi-center, randomized, double-blinded, placebo-controlled clinical trial. Int J Gen Med. (2023) 16:5501–13. doi: 10.2147/IJGM.S436347
33. Yang Y, Weng J, Wu Q, Zhao J, Zhu H, Cui C, et al. Analysis of administration rules of chinese patent medicine for impotence based on data mining. New Chin Med. (2023) 55:36–42. doi: 10.13457/j.cnki.jncm.2023.07.006
34. Shi X, Xu H. Research on medication regularities in the premature ejaculation treatment based on data mining from “prepared traditional Chinese medicine formulas. Chin hea car. (2025) 43:27–31. Available online at: https://kns.cnki.net/kcms2/article/abstract?v=KlmjsyJjhUT8GxKcEnoYIHvDz-q6JjZGw3ce7TC74xABQMjnMoMAMJmmMrxJ2EKEzkXv1DO8yy2XmqNorg6uXs9r_hGnWcwUdsCGE7Y_S0hANun0KKhWLF4p-pYk0CqOqwcGWJ3KkIIklrYDHOHJDiXNC5L64ph5JentPjC-YOqxWmvJnvf9YPf_60yLnqw1WuJTk2Zo5YQ=&uniplatform=NZKPT&language=CHS.
35. Li Z, Li J, Li Y, Guo L, Xu P, Du H, et al. The role of cistanches herba and its ingredients in improving reproductive outcomes: a comprehensive review. Phytomedicine. (2024) 129:155681. doi: 10.1016/j.phymed.2024.155681
36. Szabo R, Racz CP, Dulf FV. Bioavailability improvement strategies for icariin and its derivates: a review. Int J Mol Sci. (2022) 23:12–7. doi: 10.3390/ijms23147519
37. Zhang M, Yue K, Jiang J, Chen H. Research progress on pharmacological effects of lycii fructus and its active ingredients. Drug Eval Res. (2023) 46:1611–9. doi: 10.7501/j.issn.1674-6376.2023.07.027
38. Wang J, Wang Q, Liu B, Li D, Yuan Z, Zhang H. A Chinese herbal formula, shuganyiyang capsule, improves erectile function in male rats by modulating Nos-CGMP mediators. Urology. (2012) 79:241. doi: 10.1016/j.urology.2011.08.026
39. Feng Y, Shi T, Fu Y, Lv B. Traditional Chinese medicine to prevent and treat diabetic erectile dysfunction. Front Pharmacol. (2022) 13:956173. doi: 10.3389/fphar.2022.956173
40. Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. (2021) 372:n160. doi: 10.1136/bmj.n160
41. Page MJ, Mckenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The prisma 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
42. Zung WW. A rating instrument for anxiety disorders. Psychosomatics. (1971) 12:371–9. doi: 10.1016/S0033-3182(71)71479-0
43. Zung WW. A self-rating depression scale. Arch Gen Psychiatry. (1965) 12:63–70. doi: 10.1001/archpsyc.1965.01720310065008
44. Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. (1959) 32:50–5. doi: 10.1111/j.2044-8341.1959.tb00467.x
45. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. (1960) 23:56–62. doi: 10.1136/jnnp.23.1.56
46. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. (1983) 67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x
47. Xiaoyu Z. Guidelines for Clinical Research of New Chinese Medicines (Trial). 05 ed. Beijing: China Medical Science and Technology Press (2002). p. 402.
48. Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 366:l4898. doi: 10.1136/bmj.l4898
49. Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. Grade guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. (2011) 64:401–6. doi: 10.1016/j.jclinepi.2010.07.015
50. Luo S, Long Y, Xiao W, Wang X, Chen R, Guo Q, et al. Risk of bias assessments and reporting quality of systematic reviews and randomized controlled trials examining acupuncture for depression: an overview and meta-epidemiology study. J Evid Based Med. (2020) 13:25–33. doi: 10.1111/jebm.12372
51. Yang L, Di YM, Shergis JL, Li Y, Zhang AL, Lu C, et al. A systematic review of acupuncture and chinese herbal medicine for postpartum depression. Complement Ther Clin Pract. (2018) 33:85–92. doi: 10.1016/j.ctcp.2018.08.006
52. Feng D, Tang T, Lin X, Yang Z, Yang S, Xia Z, et al. Nine Traditional Chinese herbal formulas for the treatment of depression: an ethnopharmacology, phytochemistry, and pharmacology review. Neuropsychiatr Dis Treat. (2016) 12:2387–402. doi: 10.2147/NDT.S114560
53. Li D, Li X, Duan J, Cai W. Wuling capsule promotes hippocampal neurogenesis by improving expression of connexin 43 in rats exposed to chronic unpredictable mild stress. Zhong Xi Yi Jie He Xue Bao. (2010) 8:662–9. doi: 10.3736/jcim20100710
54. Sun G, Shih J, Chiou S, Hong C, Lu S, Pao L. Chinese herbal medicines promote hippocampal neuroproliferation, reduce stress hormone levels, inhibit apoptosis, and improve behavior in chronically stressed mice. J Ethnopharmacol. (2016) 193:159–68. doi: 10.1016/j.jep.2016.07.025
55. Bi Y, Li M, Wang Y, Yao J, Wang Y, Wang S, et al. Saikosaponins from bupleurum scorzonerifolium willd. Alleviates microglial pyroptosis in depression by binding and inhibiting P2x7 expression. Phytomedicine. (2025) 136:156240. doi: 10.1016/j.phymed.2024.156240
56. Li C, Huang B, Zhang Y. Chinese herbal medicine for the treatment of depression: effects on the neuroendocrine-immune network. Pharmaceuticals (Basel). (2021) 14:65. doi: 10.3390/ph14010065
57. Xie Z, Zhang Z, Xie C, Guo Y. Sexual function of depression in men with combination of tianmeng oral liquid and anti-depressant. Chin Tradit Her Dru. (2018) 49:2620–3. doi: 10.7501/j.issn.0253-2670.2018.11.020
Keywords: male sexual dysfunction, chinese herbal medicine, anxiety, depression, systematic review, psychosexual health
Citation: Xie Z, Zhang X, Jia H and Zhang Y (2025) Efficacy of Chinese herbal medicine in the treatment of anxiety and depression in male sexual dysfunction: a systematic review and meta-analysis protocol. Front. Psychiatry 16:1584306. doi: 10.3389/fpsyt.2025.1584306
Received: 03 March 2025; Accepted: 09 April 2025;
Published: 02 May 2025.
Edited by:
Luca Steardo jr, University Magna Graecia of Catanzaro, ItalyReviewed by:
Hatice Ayça Kaloğlu, Ankara Etlik City Hospital, TürkiyeMattia Longoni, Vita-Salute San Raffaele University, Italy
Copyright © 2025 Xie, Zhang, Jia and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongling Jia, amlhaGwxOTY5QDE2My5jb20=; Yongchen Zhang, emhhbmd5YzU4QHNpbmEuY29t
 Zhaozhan Xie
Zhaozhan Xie Xuecheng Zhang2
Xuecheng Zhang2