- 1Clinical Trial Center, West China Hospital, Sichuan University, Chengdu, Sichuan, China
- 2West China School of Nursing, Sichuan University, Chengdu, Sichuan, China
- 3Nursing Department, The Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, Zhejiang, China
Background: Kinesiophobia is a significant psychological factor that affects the early ambulation of postoperative patients, and is especially prevalent among postoperative cardiac patients. Nonetheless, few studies have explored this phenomenon in patients following cardiac surgery under cardiopulmonary bypass.
Objectives: The primary objective of this study was to investigate the prevalence of kinesiophobia and its contributing factors in patients after cardiac surgery under cardiopulmonary bypass, to inform the development of targeted intervention strategies to address kinesiophobia, enhance patient motivation and adherence to early activities, and optimize postoperative rehabilitation outcomes.
Methods: A cross-sectional survey was conducted, assessing patients via questionnaires, including the Tampa Scale for Kinesiophobia Heart, Exercise Self-Efficacy Scale, D Personality Scale-14, Pain Catastrophizing Scale, and Family Care Index Questionnaire. The χ2 test, Fisher’s exact test, and Mann-Whitney U test were used for univariate analysis, and binary Logistic stepwise regression analysis was adopted for multivariate analysis. The difference was considered statistically significant at P<0.05.
Results: The study encompassed a total of 293 patients, of whom 45.73% (134 patients) exhibited kinesiophobia. Binary logistic regression identified the following risk factors: being accompanied by a nursing assistant (OR = 9.009, P < 0.001), longer cardiopulmonary bypass duration (OR = 1.005, P < 0.05), delayed first postoperative ambulation (OR = 4.031, P < 0.05), Type D personality (OR = 2.582, P < 0.01), heightened safety concerns (OR = 8.270, P < 0.05), and pain catastrophizing (OR = 4.253, P < 0.001). Conversely, family function and exercise self-efficacy mitigated kinesiophobia (P < 0.05).
Conclusions: Kinesiophobia is highly prevalent and multifactorial in post-cardiopulmonary bypass patients. Healthcare providers should prioritize patients with the above risk profiles, while also establishing financial/emotional support systems, improving patient-family communication, optimizing acute pain management, implementing Enhanced Recovery After Cardiac Surgery protocols, and leveraging technology to design rehabilitation programs. These measures may reduce kinesiophobia and maximize the benefits of early ambulation.
1 Introduction
Cardiovascular disease (CVD) has risen significantly due to population aging and lifestyle changes, driving increased global morbidity and mortality. Over the past three decades, the global prevalence of CVD has increased by 93% (1), affecting hundreds of millions patients, while mortality rates have climbed by approximately 54%, resulting in 17.5 million annual deaths and imposing a substantial burden on global health and economies (1, 2). Cardiac surgery with cardiopulmonary bypass (CPB) has become a cornerstone of CVD treatment, effectively alleviating symptoms, slowing disease progression, and improving survival rates. CPB involves using specialized equipment to temporarily replace heart and lung functions, enabling surgeons to repair or replace valves and vessels during cardiac arrest (3). As techniques advance, surgical success rates have risen, complications have declined, and procedural volumes have increased. Globally, over 2 million cardiac surgeries are performed annually, most requiring CPB support (4).
Despite its benefits, CPB involves complex non-physiological processes, and patients face elevated risks of complications such as atelectasis, delirium, and low cardiac output syndrome (5). Respiratory dysfunction is particularly consequential, driven by CPB-induced systemic inflammation, ischemia-reperfusion injury, and alveolar-capillary damage from non-pulsatile flow and hemodilution. These mechanisms impair pulmonary perfusion, causing ventilation-perfusion mismatch and reduced lung compliance, often manifesting as ARDS, atelectasis, or prolonged mechanical ventilation—the leading morbidity in on-pump surgery (6, 7). Critically, respiratory complications independently predict prolonged ICU stays, higher mortality, and delayed recovery (8, 9), exacerbating perioperative risks and extending hospitalization. Which not only increases the pain of patients, delays their functional recovery but also prolongs their hospital stay. Therefore, promoting the physical rehabilitation of postoperative patients and improving their quality of life have become important goals in treating CVD.
A large amount of clinical data shows that early postoperative ambulation is vital for recovery, enhancing cardiopulmonary endurance, reducing stress responses, shortening hospital stays, and lowering costs (10, 11). However, although early ambulation has numerous benefits, in clinical practice, it has been found that many patients experience kinesiophobia after surgery, resulting in a low participation rate and poor compliance in their early ambulation (12, 13). Kinesiophobia is an exaggerated and irrational fear of physical activity due to concerns about pain or injury, which can lead to avoidance behavior and hinder rehabilitation (14, 15). Kinesiophobia is a psychological stress response that occurs during the disease treatment process. It has been reported that the incidence rate of kinesiophobia in patients after cardiac surgery ranges from 20% to 83%, and largely reduces the enthusiasm and compliance of patients in early rehabilitation (14, 16). Kinesiophobia diminishes participation in early mobilization (17), reduces 6-minute walking distance (18), and compromises rehabilitation outcomes and quality of life (19). For CPB patients, postoperative pain, fatigue, and weakness may exacerbate cognitive biases and psychological insecurity, further hindering ambulation.
Examining kinesiophobia through stress system process theory (15) clarifies how psychological stressors and imbalanced factors disrupt rehabilitation. This study investigates kinesiophobia’ s determinants in post-CPB patients, aiming to inform targeted interventions that address stressors and improve recovery trajectories.
2 Methods
2.1 Design and study population
This is a cross-sectional study that utilized a convenience sampling method to select subjects. Patients with cardiac disease who were admitted to the Cardiovascular Surgery Intensive Care Unit of a hospital between February 2023 and December 2023 were sequentially included as study subjects. The individuals in this study satisfied specific criteria (1) Aged 18–70 years old; (2) Underwent heart or large vessel surgery under CPB for the first time and met postoperative ambulation criteria including consciousness, normal vital signs, blood oxygen saturation ≥ 95%, absence of vasoactive drugs or recent dose increase, pain numerical score(NRS)≤ 4 points, and muscle strength ≥ 4; (3) Possessed normal communication and literacy abilities, could fill in questionnaires, and gave informed consent for voluntary participation in the study. Exclusion criteria included a history of cognitive impairment or mental illness, severe physical activity disorder, or withdrawal from the study.
2.2 Sample
In quantitative nursing science studies that involve multiple variables, it is a standard practice to ensure the sample size is at least 10 times the number of variables (20). Given that this study anticipated 18 variables, considering a 20% attrition rate, we ultimately determined that a minimum sample size of 225 cases would be necessary for robust analysis [18 * 10 = 180, 180/(1 - 0.20) = 225].
2.3 Data collection
Given the heterogeneity in participants’ primary diagnoses, disease severity, and surgical procedures, no consensus exists regarding the optimal timing for early postoperative ambulation following cardiac surgery. Consequently, this study determined the timing of questionnaire administration based on functional readiness for ambulation (achievement of mobilization criteria). Informed by rehabilitation literature and clinical consensus, participants were surveyed before their first postoperative ambulation within postoperative days 2-7. Before the survey, researchers explained the survey’s purpose, obtained informed consent, and provided assistance as needed. During the survey, patients filled out the questionnaire independently, and those who were unable to do so were assisted by the researcher according to the patient’s intention. Any doubts about the questionnaire were clarified using standardized language. Each questionnaire took 15–20 minutes to complete and was collected immediately to ensure accuracy.
2.4 Measurements
General information questionnaire included three parts: ① Socio-demographic factors: gender, age, educational level, marital status, family per capita monthly income, medical expense payment method, and companion; ② Clinical factors: previous history of cardiovascular diseases, type of surgery, operative time, CPB duration, pain level, time for the first postoperative ambulation; ③ Psychological factors: degree of safety concern, exercise self-efficacy, type D personality, family function, pain catastrophizing.
The Tampa Scale for Kinesiophobia Heart (TSK-SV Heart) was adapted by Dr. Bäck et al. (21) in 2012. The scale includes 4 dimensions and 17 entries, including fear of injury, fear of activity, avoidance of exercise, and dysfunction. The entries were rated on a 4-point Likert scale, ranging from 1 for “completely disagree” to 4 for “completely agree”, with entries 4, 8, 12, and 16 being reversed scores. The overall score ranged from 17 to 68 points, with a score above 37 indicating kinesiophobia. Higher scores reflect a greater degree of kinesiophobia. The Cronbach’s alpha coefficient was 0.755 in this study.
Exercise Self-Efficacy Scale (ESES) was developed by Kroll et al. (22) to measure individuals’ confidence in adhering to exercise. The scale is a unidimensional scale consisting of 10 items, each of which is rated on a 4-point Likert scale, ranging from 1 for “completely inconsistent” to 4 for “completely consistent.” The total score on the scale was 10 to 40 points, with higher scores indicating greater confidence in exercise adherence. The Cronbach’s alpha coefficient of this scale in this study was 0.884.
Type Personality Scale-14 (DS-14) was compiled by Dutch psychologists Denollet et al. (23) to measure patients’ personality types, consisting of 2 latitudes, negative affect, and social inhibition, with a total of 14 items, which were scored on a Likert 5-point scale, ranging from 0 for “completely inconsistent” to 4 for “completely consistent,” with items 1 and 3 being reverse-scored. The total score for each subscale ranges from 0 to 28 points. A score of “negative affectivity ≥ 10 points and social inhibition ≥ 10 points” indicated a Type D personality. The Cronbach’s alpha coefficient for this scale was 0.889.
The Chinese Version of the Pain Catastrophizing Scale (PCS), was originally compiled by Sullivan et al. (24) in 1995 and translated into Chinese by Yap. Which consists of 13 items across three dimensions: rumination, exaggeration, and helplessness. Each item is scored on a 5-point Likert scale, ranging from 0 for “never” to 4 for “always”. The total score of the scale ranged from 0 to 52, and a total score on ≥38 indicated the presence of pain catastrophizing. In this study, the Cronbach’s α for this scale was 0.975.
The Family APGAR Index Questionnaire, designed by Smilkstein et al. (25) in 1978, evaluates an individual’s satisfaction with family function. The questionnaire measures five dimensions: Adaptation, Partnership, Growth, Affection, and Resolve. Each item is scored on a 3-point Likert scale, with “always” scored as 2, “sometimes” as 1, and “rarely” as 0. The total score ranges from 0 to 10 points, with higher scores indicating better family function. 0 to 3 indicate severe family dysfunction, 4 to 6 indicates moderate dysfunction and 7 to 10 indicates good family function. The Cronbach’s alpha coefficient for this scale was 0.716 in this study.
2.5 Ethical and research approvals
This study has been approved by the Ethics Committee of the hospital (IR20220227). All participants consented to participate in this study and provided informed consent in writing. All methods were carried out according to relevant guidelines and regulations.
2.6 Statistical analysis
Data were meticulously entered into Excel by two specialists from the research team, and statistical analyses were conducted using SPSS 26.0 software. The analysis included a statistical description, where measurement data were expressed as M ± SD and count data as frequency and composition ratio (%). Non-normally distributed measurement data were expressed by median and quartile [P50 (P25, P75)]. For univariate analysis, the chi-square test or Fisher’s exact test was used for the comparison between groups of count data. In this study, all measurement data did not conform to the normal distribution, so the non-parametric test method (Mann-Whitney U test) was used for the comparison between groups. Binary Logistic regression analysis was used for multivariate analysis. P<0.05 indicated that the difference was statistically significant.
3 Results
3.1 Basic participant characteristics
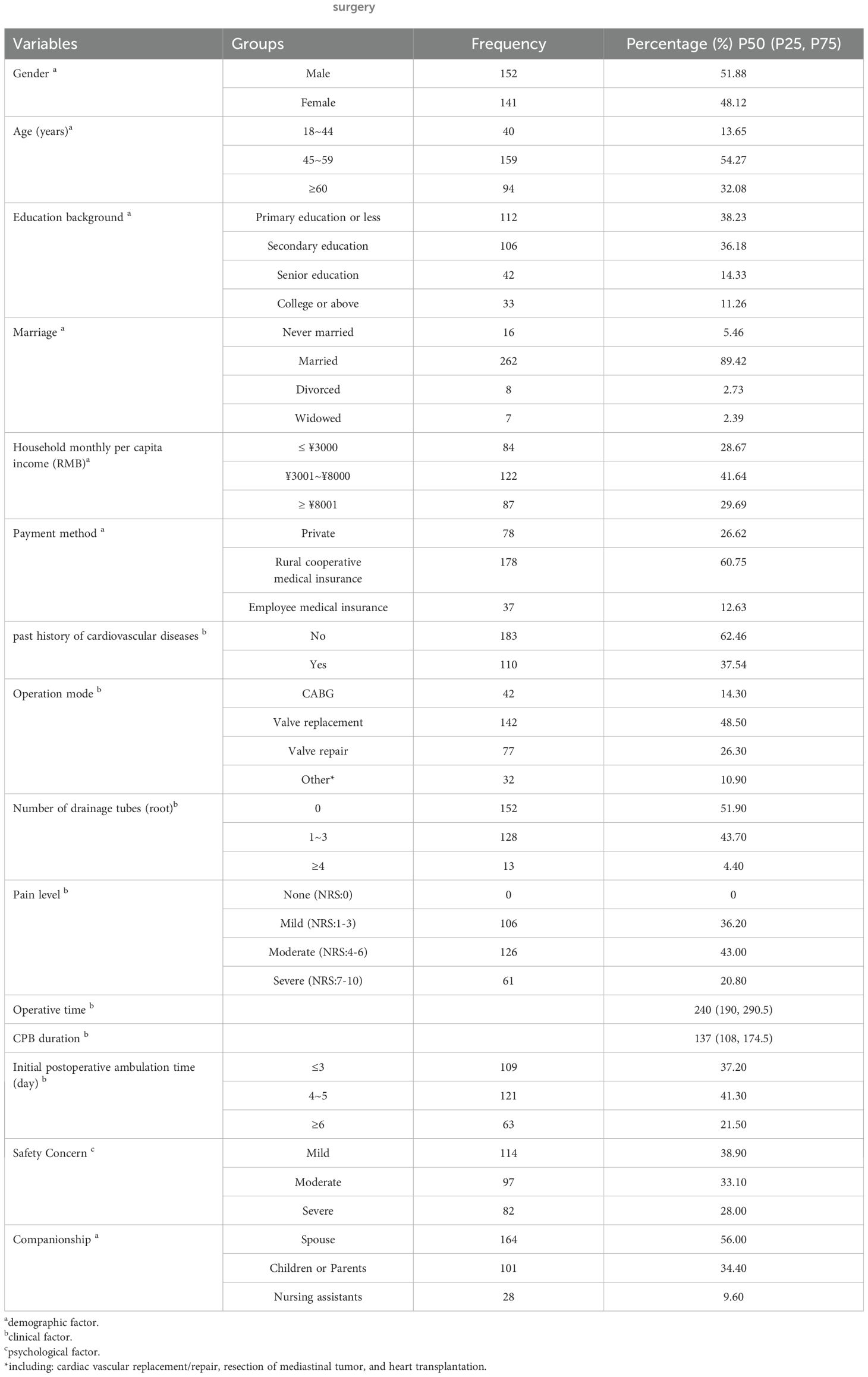
In this study, a total of 300 questionnaires were distributed, and 293 valid questionnaires were collected, resulting in a valid return rate of 97.7%. There was a relatively balanced distribution of male and female patients, 51.88% vs. 48.12% respectively. The age of the study population was 54.32 ± 9.85 years old. Regarding educational background, 38.23% of the patients had only an elementary school education or below. The majority of the participants were married with a percentage of 89.42%. In terms of economic status, 41.64% of the participants’ households reported a per capita monthly income ranging from ¥3,001 to ¥8,000. For medical expense payment, rural cooperative medical care was mainly adopted, with 178 cases (60.75%). Additionally, 62.46% of the participants had no previous history of cardiovascular diseases. Regarding the type of surgery, 48.50% underwent valve replacement surgery. After the operation, more than half of the patients did not have drainage tubes indwelling, while 43.00% reported experiencing moderate postoperative pain. The duration of the operation in our study population was 240 (190, 290.5) minutes, and the time of CPB was 137 (108, 174.5) minutes. The majority of patients (41.03%) began ambulating for the first time between 4–5 days post-surgery. Furthermore, 61.10% of the patients expressed concerns about the safety of getting out of bed. In terms of postoperative accompaniment, 90.40% of the patients were accompanied by family members. The general characteristics of the participants are summarized in Table 1.
3.2 The general characteristics of measurements
The total score of kinesiophobia in 293 patients after cardiac surgery under CPB was 37 (34, 42) points, which was at the cut-off value of kinesiophobia. Among them, 134 patients had a score > 37 points, and the incidence of kinesiophobia in this study population was 45.73%. In addition, the findings showed that the overall exercise self-efficacy of the study population was generally at a moderate level, with 195 (66.60%). 98 (33.45%) patients had pain catastrophizing, 168 (57.34%) patients had type D personality traits, and the vast majority of patients (201 patients, 68.60%) had good family functions.
3.3 Influencing factors of kinesiophobia in patients after cardiac surgery under CPB
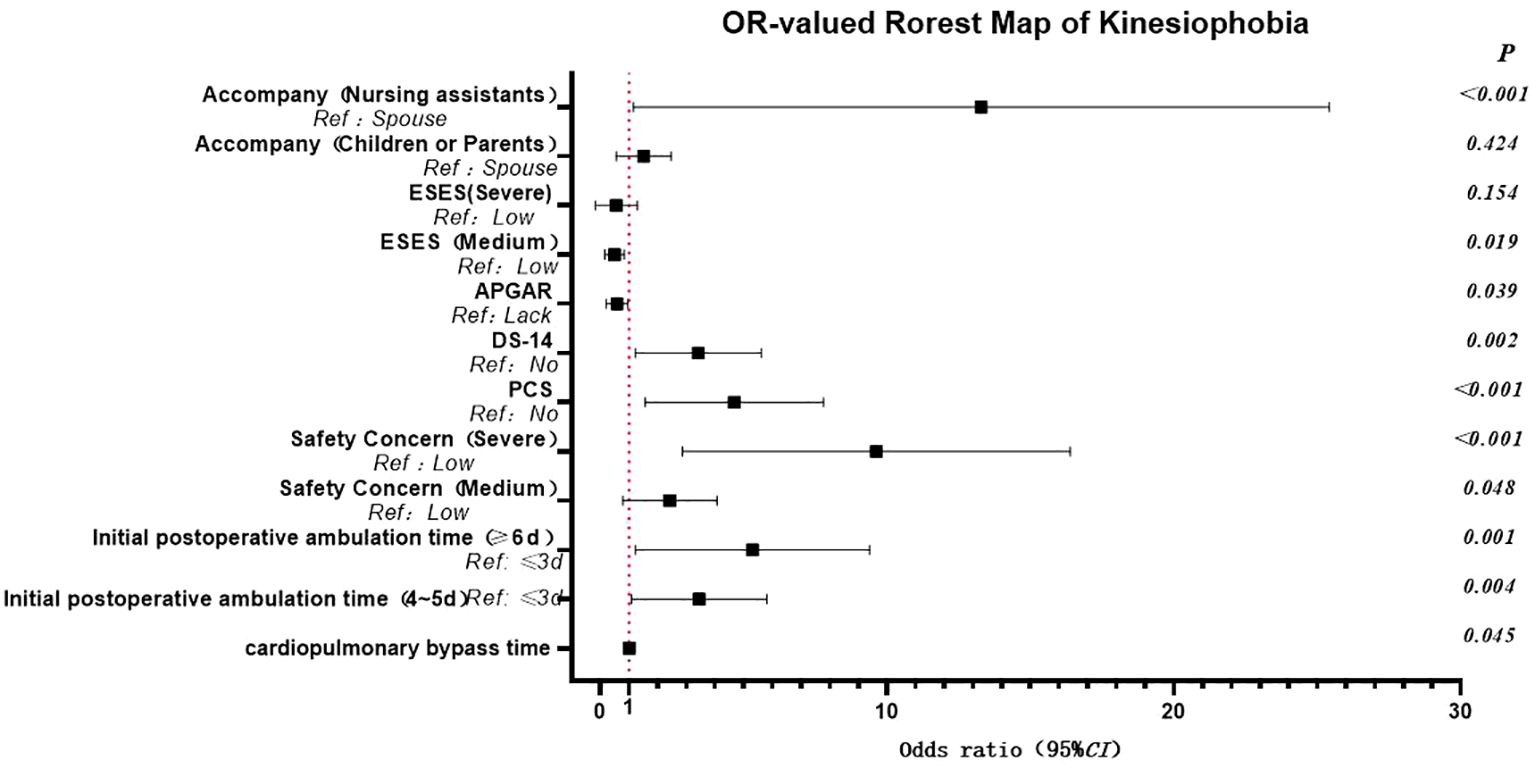
The results of the logistic regression analysis identified several risk factors for kinesiophobia in patients following cardiac surgery under CPB. Including having a companion as a nursing assistant, a longer duration of CPB, a delay in the first ambulation after the operation, type D personality, heightened concerns about safety, and pain catastrophizing. Conversely, good family function emerged as a protective factor. Specifically, patients accompanied by nursing assistants exhibited a significantly higher risk of kinesiophobia, increasing by 8.911 times compared to those accompanied by their spouses. Compared to those with a lack of family functioning, the odds of good family functioning for exercise fear decreased by 0.502 times. The prolongation of the CPB duration during the operation was significantly associated with an increased risk of postoperative kinesiophobia. The timing of the first postoperative ambulation also had a substantial impact on kinesiophobia. Patients who initiated ambulation 4–5 days post-surgery had a 2.899 times higher risk compared to those who began ambulating 2–3 days after surgery. For patients who first ambulated 6–7 days post-surgery, the risk escalated to 4.256 times higher. In addition, compared with non-type D personality patients, Type D personality patients had 2.945 times increased risk of kinesiophobia, aligning with previous research (26). The degree of patients’ safety concern was also closely related to kinesiophobia. Patients with moderate concerns had a 2.058 times higher risk, while those with severe concerns faced 7.366 times increase in risk. Pain catastrophizing was an independent risk factor for kinesiophobia, and the risk of patients with pain catastrophizing was 3.987 times higher than that of those without catastrophizing, this view has been confirmed by previous studies (27, 28). Moreover, exercise self-efficacy was found to have a notable influence on kinesiophobia, as shown in Table 2 and Figure 1.
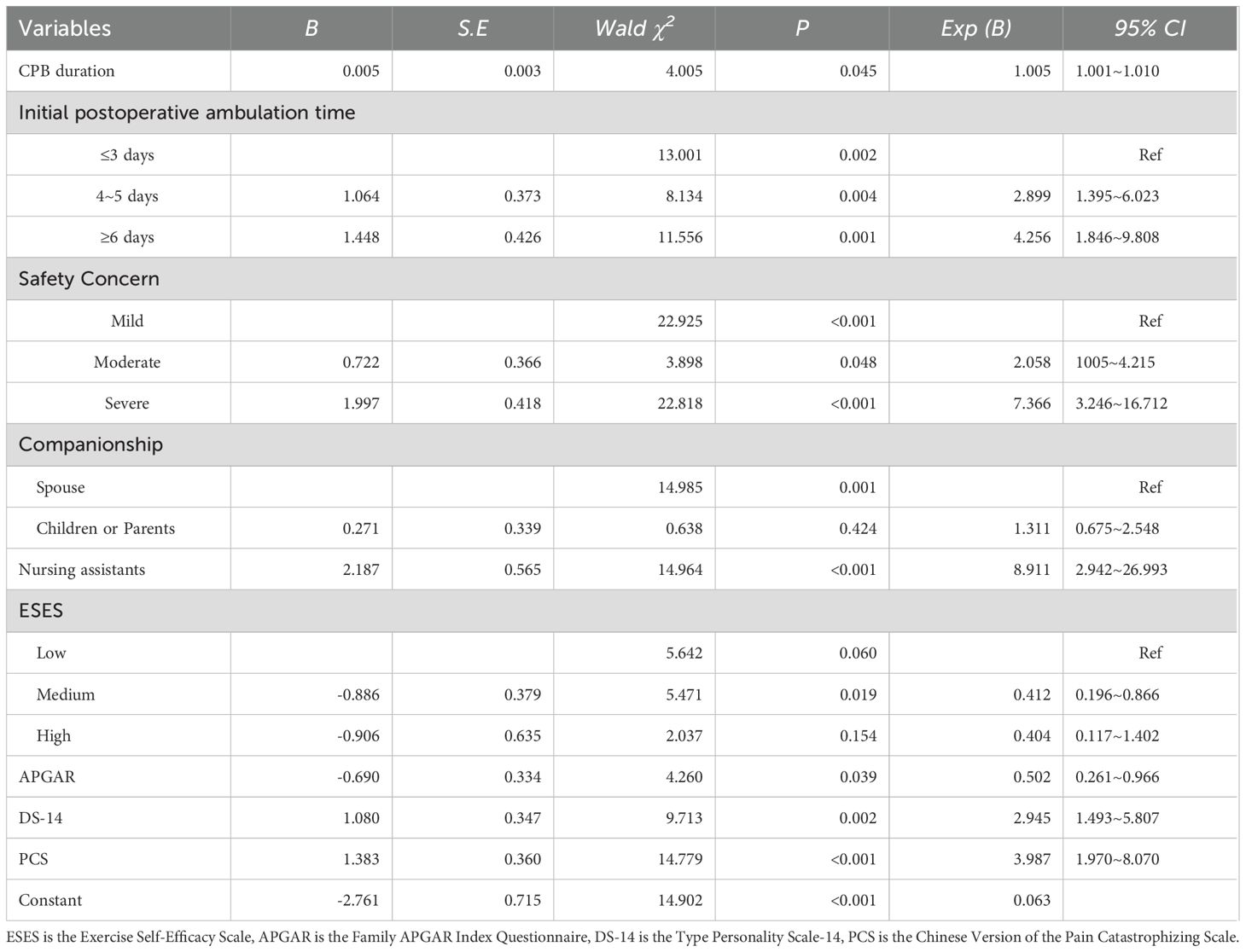
Table 2. Binary Logistic regression analysis of kinesiophobia in patients after cardiac surgery under CPB.
4 Discussions
This investigation revealed that the incidence of kinesiophobia in patients after cardiac surgery under CPB was as high as 45.73%, which was lower than the incidence in postoperative patients undergoing cardiac surgery through either midline sternotomy or thoracotomy (64.5%) (29). The discrepancy may be attributed to the inclusion of critically ill patients, such as those undergoing coronary artery bypass grafting or aortic dissection, in the open-heart surgery cohort. Previous studies reported that the incidence of kinesiophobia in patients after total knee replacement and thoracolumbar spine fracture surgery was 24.4%-38.2% (30, 31) and 31.88% (32) respectively, significantly lower than that after cardiac surgery under CPB. Such differences might stem from the unique characteristics of cardiac surgery under CPB, including hemodynamic instability, the use of larger doses of vasoactive drugs, prolonged mechanical ventilation, and slower recovery of cardiac function. These factors collectively contribute to increased physical and psychological discomfort in patients, resulting in a higher incidence of kinesiophobia. The findings of this study indicated that the factors influencing kinesiophobia were multifaceted, encompassing socio-demographic, clinical, and psychological factors.
Socio-demographic factors mainly manifested in the impact of the companion on kinesiophobia. Patients accompanied by nursing assistants exhibited a significantly higher risk of kinesiophobia compared to those accompanied by their spouses. This may be attributed to the relatively slow recovery of physical and mental functions following CPB surgery, coupled with lower levels of awareness and acceptance of the condition, leading to increased distress. Nursing assistants were primarily responsible for patients’ daily care rather than providing comprehensive, 24-hour monitoring and companionship, and therefore might not fully understand the patients’ true feelings and needs, which could diminish patients’ psychological sense of security and potentially trigger kinesiophobia.
Clinical factors influencing kinesiophobia included the duration of CPB and the timing of first postoperative ambulation. The duration of CPB might increase kinesiophobia risk through several mechanisms. First, it could lead to reperfusion injury, release of inflammatory mediators, and microthrombi formation, potentially causing complications like pulmonary infections, atrial fibrillation, and neurological dysfunction (33–35). These complications could affect cardiac function and lead to cognitive biases about patients’ abilities, inducing kinesiophobia. Second, longer bypass durations indicated more severe conditions and higher surgical complexity (33), increasing patients’ health concerns and fear of exercise-induced harm. Last, given the prolonged disease course and multiple invasive procedures associated with cardiovascular disease, patients were eager to return to normal life and had heightened needs for rehabilitation. Unmet rehabilitation needs after surgery could heighten insecurity and fear.
Delayed postoperative ambulation was also linked to kinesiophobia. This delay might reflect disease severity, with more severe conditions leading to lower activity confidence and increased fear. Patients with delayed ambulation were also more prone to complications like delirium, pulmonary infections, and muscle atrophy, which could increase perceived danger and induce kinesiophobia. Acute postoperative pain significantly impacted kinesiophobia, as cardiac valve surgery often involves sternotomy and multiple drainage tubes, causing pain that lasts up to 4–6 days. Patients starting activities within 3 days experienced less pain and lower kinesiophobia incidence (36). Finally, insufficient nursing care during the ICU transition period could contribute to kinesiophobia (37). While in the ICU, patients received early rehabilitation education and assistance, reducing kinesiophobia risk. However, those transferred to general wards might face limited rehabilitation guidance, feeling neglected and vulnerable, and fearing re-injury from improper exercise.
Psychological factors included family function, Type D personality, degree of safety concern, and pain catastrophizing. Family function was crucial for patients. It not only provided key resources for adapting to environmental changes but also enhanced patients’ confidence in overcoming the disease. For patients after cardiac surgery under CPB, sternum damage and slow wound healing made them prone to cognitive biases and kinesiophobia. Patients lacking family care and support often showed negative attitudes of avoiding and refusing exercise. Conversely, family companionship and support could help patients better understand the disease and actively cope with postoperative discomfort, thus effectively overcoming kinesiophobia.
The relationship between Type D personality traits and kinesiophobia could be understood from both socio-psychological and biological perspectives. Socio-psychologically, individuals with Type D personality often experience insecurity, anxiety, depression, and pessimism (38), and the accumulation of these emotions leads to increased psychological distress and induced kinesiophobia. Additionally, these patients had weaker social skills, limited disease knowledge, and insufficient understanding of exercise benefits, often restricting activity due to fears that improper exercise might affect recovery. Biologically, Type D personality, as a chronic stressor, impacts stress responses and contributes to coronary heart disease through mechanisms like hormone secretion, inflammation, and vascular endothelial damage, thereby increasing discomfort and leading to kinesiophobia (39).
The degree of patients’ safety concerns was directly proportional to their risk of kinesiophobia, primarily manifesting as fear of falling. This fear might be attributed to postoperative discomforts like fatigue and nausea, which impair physical function and balance, reducing self-control and triggering kinesiophobia. Additionally, the complexity of CPB surgery and potentially severe complications, such as massive bleeding and acute renal failure, necessitate ICU monitoring. The ICU hospitalization experience might lead patients to underestimate their functional abilities and cause them to overly restrict their activities (40).
Pain catastrophizing was an independent risk factor for kinesiophobia because, according to the Fear-Avoidance Model (41), patients equated pain with injury, resulting in intensification of postoperative pain and triggering a psychological stress response that generated fear and avoidance of activities. Xu et al. (28) found that pain catastrophizing had a direct positive effect on the aggravation of kinesiophobia in patients after total knee arthroplasty. Additionally, pain catastrophizing was a key psychological factor in pain perception, and postoperative pain was common, affecting more than 80% of patients, with 20% to 56% experiencing moderate to severe pain (42). Large incisions and prolonged cardiac surgery released inflammatory factors, worsening pain catastrophizing and increasing kinesiophobia risk. Meta-analyses showed that reducing pain catastrophizing and pain levels could effectively reduce kinesiophobia (43).
5 Conclusions
This study revealed a high incidence of kinesiophobia in post-cardiac surgery patients under CPB, influenced by complex factors including companionship, family function, bypass duration, time to first ambulation, Type D personality, safety concerns, and pain catastrophizing. To mitigate kinesiophobia and enhance early activity compliance, we recommend: enhancing clinical focus by training healthcare staff on psychological assessment and integrating routine kinesiophobia screening for timely intervention; establishing robust support systems through improved medical insurance to alleviate financial burden and family encouragement for emotional support; implementing the Enhanced Recovery After Cardiac Surgery (ERACS) program, prioritizing multimodal interventions such as optimized pain management, early extubation, and structured activity; and leveraging emerging technologies like cognitive-behavioral therapy, exposure therapy, pain neuroscience education, and virtual reality to deliver individualized rehabilitation plans and overcome resource limitations.
6 Strengths and limitations
While research on kinesiophobia in cardiovascular disease has predominantly focused on patients with coronary artery disease and heart failure, few studies have addressed this phenomenon in patients recovering from cardiac surgery. These postoperative patients face a heightened risk of exercise fear due to surgical trauma and wound pain. Therefore, this study investigated the status and characteristics of kinesiophobia specifically in cardiac surgery patients who underwent CPB. Starting from the perspective of the role of stress, this study took the theoretical model of the stress system process as the theoretical basis, combined with the patient-specific indicators of postoperative cardiac surgery under CPB, and clarified the main influencing factors of kinesiophobia in this population.
Nevertheless, there are some limitations in this study. Firstly, this was a cross-sectional study and could not observe the changing trend of patients’ kinesiophobia at different time points. Longitudinal studies should be carried out in the future to explore the development trajectory of kinesiophobia in patients after cardiac surgery under CPB. Secondly, the research participants were only from a single research center, which is a small sample size and has a certain bias. In the future, multi-center and large-sample studies need to be carried out to increase the credibility and stability of the research results. Finally, the questionnaires used in this study relied solely on patient self-reporting, which introduces inherent subjectivity and lacks robust objective data support (e.g., ASA scale, APACHE II score, frailty, high BMI, and low cardiac function). Therefore, incorporating more objective indicators in future research would be valuable to identify more specific and comprehensive influencing factors.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the ethics committee of Human Research in the Second Affiliated Hospital of Zhejiang University School of Medicine/The Second Affiliated Hospital of Zhejiang University School of Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
MY: Methodology, Writing – original draft, Writing – review & editing. QM: Data curation, Writing – original draft. XC: Data curation, Writing – original draft. YS: Investigation, Writing – original draft. QL: Investigation, Writing – original draft. ML: Investigation, Writing – original draft. HH: Methodology, Writing – original draft. JS: Writing – original draft, Conceptualization, Resources.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We deeply appreciate the contributions of the participants and those who made the study hospitals’ research possible.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2025.1584789/full#supplementary-material
References
1. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J Am Coll Cardiol. (2020) 76:2982–3021. doi: 10.1016/j.jacc.2020.11.010
2. Masaebi F, Salehi M, Kazemi M, Vahabi N, Azizmohammad Looha M, Zayeri F, et al. Trend analysis of disability adjusted life years due to cardiovascular diseases: results from the global burden of disease study 2019. BMC Public Health. (2021) 21:1268. doi: 10.1186/s12889-021-11348-w
3. Featherstone PJ and Ball CM. The origins of cardiopulmonary bypass. Anaesth Intensive Care. (2018) 46:351–3. doi: 10.1177/0310057X1804600401
4. Zilla P, Yacoub M, Zühlke L, Beyersdorf F, Sliwa K, Khubulava G, et al. Global unmet needs in cardiac surgery. Glob Heart. (2018) 13:293–303. doi: 10.1016/j.gheart.2018.08.002
5. Zhou R and Liu B. Analysis of adverse events of extracorporeal circulation in West China Hospital. Chin J Extracorporeal Circ. (2014) 12:198–200. doi: 10.13498/j.cnki.chin.j.ecc.2014.04.02
6. Liu J, Li X, Xie W, Wang Y, Xu Z, Bai YX, et al. Risk factors and short-term outcomes of postoperative pulmonary complications in elderly patients after cardiopulmonary bypass. Clin Interv Aging. (2024) 19:31–9. doi: 10.2147/CIA.S439601
7. Nteliopoulos G, Nikolakopoulou Z, Chow BHN, Corless R, Nguyen B, and Dimarakis I. Lung injury following cardiopulmonary bypass: a clinical update. Expert Rev Cardiovasc Ther. (2022) 20:871–80. doi: 10.1080/14779072.2022.2149492
8. Hu J, Liu Y, Huang L, Song M, and Zhu G. Association between cardiopulmonary bypass time and mortality among patients with acute respiratory distress syndrome after cardiac surgery. BMC Cardiovasc Disord. (2023) 23:622. doi: 10.1186/s12872-023-03664-3
9. Bartz RR, Ferreira RG, Schroder JN, Davies J, Liu WW, Camara A, et al. Prolonged pulmonary support after cardiac surgery: incidence, risk factors and outcomes: a retrospective cohort study. J Crit Care. (2015) 30:940–4. doi: 10.1016/j.jcrc.2015.04.125
10. Kanejima Y, Shimogai T, Kitamura M, Ishihara K, and Izawa KP. Effect of early mobilization on physical function in patients after cardiac surgery: A systematic review and meta-analysis. Int J Environ Res Public Health. (2020) 17:7091. doi: 10.3390/ijerph17197091
11. Sibilitz KL, Anayo L, and Taylor RS. Cochrane corner: Exercise-based cardiac rehabilitation for adults after heart valve surgery. Heart. (2021) 107:1935–7. doi: 10.1136/heartjnl-2021-319628
12. Castelino T, Fiore JF Jr, Niculiseanu P, Landry T, Augustin B, Feldman LS, et al. The effect of early mobilization protocols on postoperative outcomes following abdominal and thoracic surgery: A systematic review. Surgery. (2016) 159:991–1003. doi: 10.1016/j.surg.2015.11.029
13. Zhang Z, Pack Q, Squires RW, Lopez-Jimenez F, Yu L, and Thomas RJ. Availability and characteristics of cardiac rehabilitation programmes in China. Heart Asia. (2016) 8:9–12. doi: 10.1136/heartasia-2016-010758
14. Zochios V, Klein AA, and Gao F. Protective invasive ventilation in cardiac surgery: A systematic review with a focus on acute lung injury in adult cardiac surgical patients. J Cardiothorac Vsc Anesth. (2018) 32:1922–36. doi: 10.1053/j.jvca.2017.10.031
15. Kori S H M R P and Todd DD. Kinisophobia: A new view of chronic pain behavior. Pain manage. (1990) 1:35–43.
16. Lethem J, Slade PD, Troup JD, and Bentley G. Outline of a Fear-Avoidance Model of exaggerated pain perception–I. Behav Res Ther. (1983) 21:401–8. doi: 10.1016/0005-7967(83)90009-8
17. Bäck M, Cider Å, Herlitz J, Lundberg M, and Jansson B. Kinesiophobia mediates the influences on attendance at exercise-based cardiac rehabilitation in patients with coronary artery disease. Physiother Theory Pract. (2016) 32:571–80. doi: 10.1080/09593985.2016.1229828
18. Doury-Panchout F, Metivier JC, and Fouquet B. Kinesiophobia negatively influences recovery of joint function following total knee arthroplasty. Eur J Phys Rehabil Med. (2014) 51:155–61.
19. Dąbek J, Knapik A, Gallert-Kopyto W, Brzęk AM, Piotrkowicz J, and Gąsior Z. Fear of movement (kinesiophobia) - an underestimated problem in Polish patients at various stages of coronary artery disease. Ann Agric Environ Med. (2020) 27:56–60. doi: 10.26444/aaem/106143
20. Ertefaie A, Small DS, and Rosenbaum PR. Quantitative evaluation of the trade-off of strengthened instruments and sample size in observational studies. J Am Stat Assoc. (2018) 113:1122–34. doi: 10.1080/01621459.2017.1305275
21. Bäck M, Jansson B, Cider A, Herlitz J, and Lundberg M. Validation of a questionnaire to detect kinesiophobia (fear of movement) in patients with coronary artery disease. J Rehabil Med. (2012) 44:363. doi: 10.2340/16501977-0942
22. Kroll T, Kehn M, Ho P, and Groah S. The SCI Exercise Self-Efficacy Scale (ESES): development and psychometric properties. Int J Behav Nutr Phys Activity. (2007) 4:34. doi: 10.1186/1479-5868-4-34
23. Denollet J. DS14: standard assessment of negative affectivity, social inhibition, and Type D personality. Psychosomatic Med. (2005) 67:89–97. doi: 10.1097/01.psy.0000149256.81953.49
24. Yap J, Lau J, Chen P, Gin T, Wong T, Chan I, et al. Validation of the Chinese Pain Catastrophizing Scale (HK-PCS) in patients with chronic pain. Pain Med. (2008) 9:186–95. doi: 10.1111/j.1526-4637.2007.00307.x
25. Smilkstein G, Ashworth C, and Dan M. Validity and reliability of the family APGAR as a test of family function. J Fam Pract. (1982) 15:303–11.
26. Vogel M, Riediger C, Krippl M, Frommer J, Lohmann C, and Illiger S. Negative affect, type D personality, quality of life, and dysfunctional outcomes of total knee arthroplasty. Pain Res Manag. (2019) 2019:6393101. doi: 10.1155/2019/6393101
27. Duport A, Pelletier R, Martel M, and Léonard G. The influence of kinesiophobia and pain catastrophizing on pain-induced corticomotor modulation in healthy participants: A cross sectional study. Neurophysiol Clin. (2022) 52:375–83. doi: 10.1016/j.neucli.2022.08.001
28. Song XM, Ma SH, Peng YT, Zhu YF, An LJ, Li SX, et al. Analysis of influencing factors of exercise fear in patients with coronary heart disease based on structural equation model. J Nurs Sci. (2022) 37:64–7. doi: 10.3870/j.issn.1001-4152.2022.12.064
29. Zheng DL, Ji YX, Guo RF, Mao YJ, Guo SP, et al. Correlation analysis of kinesiophobia and chronic pain self-efficacy in patients after cardiac thoracotomy. Chin Gen Pract Nurs. (2020) 18:2335–8. doi: 10.3870/10.12104/j.issn.1674-4748.2020.19.008
30. Cai L, Liu Y, Xu H, Xu Q, Wang Y, and Lyu P. Incidence and risk factors of kinesiophobia after total knee arthroplasty in Zhengzhou, China: A cross-sectional study. J Arthroplasty. (2018) 33:2858–62. doi: 10.1016/j.arth.2018.04.028
31. Xu HP, Zhang YG, Liu YJ, Guo YR, Cai LB, Zhang HJ, et al. Study on the influencing factors of kinesiophobia in patients after total knee arthroplasty. Chin J Nurs. (2021) 56:1460–5. doi: 10.3761/j.issn.0254-1769.2021.10.003
32. An YL, Sun Q, Guo X, Li HP, Han YR, Lei H, et al. Investigation of the causes and influencing factors of kinesiophobia in patients after thoracolumbar fracture surgery. Chin J Health Statistics. (2021) 38:581–4. doi: 10.3969/j.issn.1002-3674.2021.04.027
33. Xu S, Liu J, Li L, Wu Z, Li J, Liu Y, et al. Cardiopulmonary bypass time is an independent risk factor for acute kidney injury in emergent thoracic aortic surgery: a retrospective cohort study. J Cardiothorac Surg. (2019) 14:90. doi: 10.1186/s13019-019-0907-x
34. Zheng J, Xu SD, Zhang YC, Zhu K, Gao HQ, Zhang K, et al. Association between cardiopulmonary bypass time and 90-day post-operative mortality in patients undergoing arch replacement with the frozen elephant trunk. Chin Med J. (2019) 132:2325–32. doi: 10.1097/CM9.0000000000000443
35. Smulter N, Lingehall HC, Gustafson Y, Olofsson B, Engström KG, Appelblad M, et al. Disturbances in oxygen balance during cardiopulmonary bypass: A risk factor for postoperative delirium. J Cardiothorac Vasc Anesth. (2018) 32:684–90. doi: 10.1053/j.jvca.2017.08.035
36. Shen A. Study on infusion stability and pharmacoeconomics of patient-controlled analgesia pump. Zhegnjiang:Zhejiang University (2018). Available at: https://www.cnki.net/.
37. Herling SF, Brix H, Andersen L, Jensen LD, Handesten R, Knudsen H, et al. Patient and spouses experiences with transition from intensive care unit to hospital ward - qualitative study. Scand J Caring Sci. (2020) 34:206–14. doi: 10.1111/scs.12722
38. Ma H. The relationship between type D personality, disease perception, self-efficacy and self-management in patients with coronary heart disease PCI. Shangdong:Qingdao University (2019). Available at: https://www.cnki.net/.
39. Kupper N and Denollet J. Type D personality as a risk factor in coronary heart disease: a review of current evidence. Curr Cardiol Rep. (2018) 20:104. doi: 10.1007/s11886-018-1048-x
40. Leon-Llamas JL, Murillo-Garcia A, Villafaina S, Domínguez-Muñoz FJ, Morenas J, and Gusi N. Relationship between kinesiophobia and mobility, impact of the disease, and fear of falling in women with and without fibromyalgia: A cross-sectional study. Int J Environ Res Public Health. (2022) 19:8257. doi: 10.3390/ijerph19148257
41. Crombez G, Eccleston C, Van Damme S, Vlaeyen JW, and Karoly P. Fear-avoidance model of chronic pain: the next generation. Clin J Pain. (2012) 28:475–83. doi: 10.1097/AJP.0b013e3182385392
42. Mi X, Zou B, Rashidi P, Baharloo R, Fillingim RB, and Wallace MR. Effects of patient and surgery characteristics on persistent postoperative pain: A mediation analysis. Clin J Pain. (2021) 37:803–11. doi: 10.1097/AJP.0000000000000979
43. Kamonseki DH, Christenson P, Rezvanifar SC, and Calixtre LB. Effects of manual therapy on fear avoidance, kinesiophobia and pain catastrophizing in individuals with chronic musculoskeletal pain: Systematic review and meta-analysis. Musculoskelet Sci Pract. (2021) 51:102311. doi: 10.1016/j.msksp.2020.102311
Keywords: cardiopulmonary bypass, cardiac surgery, kinesiophobia, associated factors, postoperative rehabilitation
Citation: You M, Mou Q, Cao X, Luo M, Song Y, Li Q, Hu H and Song J (2025) Kinesiophobia and associated factors among patients after cardiac surgery under cardiopulmonary bypass: a cross-sectional study. Front. Psychiatry 16:1584789. doi: 10.3389/fpsyt.2025.1584789
Received: 27 February 2025; Accepted: 23 June 2025;
Published: 14 July 2025.
Edited by:
Saraswati Dhungana, Institute of Medicine, NepalCopyright © 2025 You, Mou, Cao, Luo, Song, Li, Hu and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qianqian Mou, bXVxaWFuMTAyNEAxMjYuY29t
 Min You
Min You Qianqian Mou1,2*
Qianqian Mou1,2*