- 1Food Addiction Reset LLC, Seattle, WA, United States
- 2Department of Psychiatry and Behavioral Sciences, Medical University of South Carolina, Charleston, SC, United States
Background and objective: Despite their clinical differences, loss of control binge eating (LCBE) is a core feature of all binge-type eating disorders (EDs), including binge eating disorder (BED), bulimia nervosa (BN), and anorexia nervosa binge purge type (AN-BP). The emerging concept of food addiction (FA), or ultra-processed food addiction (UPFA), is also characterized by LCBE. However, LCBE treatment has rejected addiction recovery approaches, especially abstinence or reduced harm through reduced use, to the detriment of patients. Treatment could be more successful if barriers to addiction recovery protocols such as reduced harm and abstinence were addressed.
Hypothesis and theory: The phenomenology and clinical features of binge-type EDs and UPFA overlap considerably, yet they also have distinct clinical features and treatment approaches. Among their commonalities, these conditions share pathophysiological mechanisms. Specifically, available evidence demonstrates that LCBE, regardless of diagnosis, is characterized by alterations in neurobiological systems mediating reward sensitivity, stress reactivity, and cognitive function that are similar to the disturbances found in Ultra-Processed Food Addiction (UPFA), Alcohol Use Disorder (AUD) and other substance use disorders (SUDs). Ultra-processed foods (UPFs) used by patients with LCBE have clearly been shown to have powerful addictive properties. However, the key substance use disorder (SUD) recovery protocols of harm reduction or abstinence from addictive substances are not commonly employed in the treatment of binge-type EDs. The objectives of this paper are to organize evidence that the LCBE characteristic of binge-type EDs and UPFA overlap in many cases and to consider the impact of these findings on treatment protocols, specifically the application of harm reduction and/or abstinence from psychoactive UPFs. This hypothesis can be tested in clinical trials of individuals with LCBE.
Results: Neurobiological studies of individuals with LCBE consistently show signs of addictive alterations, especially hyperactive reward centers, stress reactivity, and cognitive impairment, as well as maladaptive use of UPFs. This is very similar to the results of addictive use of alcohol for which abstinence and harm reduction are demonstratively helpful. However, this approach has not been used in the eating disorders field which may be to the detriment of patients with LCBE.
Discussion: These findings suggest that treatment outcomes for binge-type EDs characterized by LCBE might improve if harm reduction and/or abstinence protocols for recovery from UPFA were applied. A level of support high enough for a severe addiction could improve treatment outcomes for these often recurrent and treatment refractory disorders. Possible rationales for current treatment exclusion or marked reduction of UPF abstinence protocols are offered.
Introduction
Approximately 17% of people worldwide suffer from broadly defined eating disorders (EDs) (1). The great majority of EDs in the general population are binge-type EDs, which are characterized by loss of control binge eating (LCBE), and include binge eating disorder (BED), bulimia nervosa (BN), and anorexia nervosa binge-purge type (AN-BP), as well as subthreshold forms of BN and BED characterized as forms of other feeding and eating disorder (OSFED) (2–5). LCBE is defined by the DSM 5 as consuming a large amount of food in a short period (typically within two hours) while experiencing a subjective sense of inability to stop eating or regulate the type of quantity of food consumed (6). LCBE has been thought to be a core component of objective binge eating. This is in contrast to food addiction which can be characterized by the 11 DSM 5 criteria used for Alcohol Use Disorder (AUD) and other SUD. This is discussed in more detail below. Eating disorders are typically treated with a combination of psychotherapy, medical care, nutritional counseling, and psychotropic medications (7, 8). However, the overall rate of recovery for all ED patients pooled together is only 46% with a mean follow-up interval of 45 months (9).
Over the last several years, evidence has accumulated that supports a diagnosis of addiction to processed or ultra-processed foods (UPFs). Ultra-processed food addiction (UPFA) is also characterized by LCBE and phenomenologically overlaps with diagnoses of all of binge-type EDs (10–22). Further, brain studies of people with obesity show neuroadaptations that parallel those of people with AUD as well as patients with LCBE. These consist of hyperactive reward centers, hyperactive stress pathways, and hypoactive cognitive functions (23). As might be expected, UPFs are shown to be associated with weight gain. In a study of humans in a controlled laboratory setting, Hall at al. found that a diet that contained ultra-processed foods resulted in greater weight gain than a diet of unprocessed foods (24). Processed foods are found to be associated with addictive eating (25). These findings reinforce LaFata and Gearhardt’s analysis of the correlation of the development of obesity with the rise in consumption of UPF (26). The importance of distinguishing between weight status and LCBE or UPFA is illustrated by Wiss et al. who found an increased prevalence of food addiction among an underweight population (29). This reinforces the value of treating based on factors other than weight status. The evidence demonstrates that obesity shows neuro-adaptations in addictive patterns similar to both AUD and binge-type eating disorders.
These overlapping mechanisms suggest a possible framework that could lead to improved results in the treatment of LCBE. Methods consistent with recovery from substance use disorders (SUD) as applied to addictive ultra-processed foods could be effective in the treatment of binge-type EDs. Such approaches could include the use of harm reduction that builds to abstinence under a high level of support consistent with a severe addiction (27). The essence of the concept of harm reduction is to ameliorate adverse consequences of drug use while, at least in the short term, drug use continues (28). Harm reduction is as opposed to abstinence which has the goal of not using the drug at all. These concepts have been adapted to use in recovery from addictive foods.
The reduced harm/abstinence approach is supported by research showing addictive properties for UPFs that are found in binge-type EDs such as sugar (29), high fructose corn syrup (30), flour (31), gluten (32), salt (33), dairy (34), excessive fat (35), caffeine (36), and food additives (37). Research has shown that individuals with LCBE almost exclusively binge on UPFs (38).
The addictive properties of UPFs could explain the addictive neuroadaptations found in populations that eat these processed foods, including populations with binge-type EDs. This approach is supported by numerous similarities in syndromes between people with obesity, who have been shown to be high consumers of UPFs, and people with alcohol/drug addiction. On a macro level, business practices of the tobacco and processed food industries are similar (39). Subsidies for tobacco and for wheat, corn, and sugar keep the prices of the products low enough to make them affordable enough to be used often enough to keep the addiction active (40). The overlap between alcohol/drug and UPFA is consistent, which supports a role for SUD recovery approaches to binge-type EDs, including abstinence and harm reduction strategies.
Our perspective is that individuals diagnosed with a binge-type ED characterized by LCBE are likely experiencing UPFA and are often being treated with protocols that are not consistent with SUD recovery, specifically protocols that exclude abstinence and harm reduction strategies. We argue that this could be the case regardless of etiology. Whether the addiction started from a single origin of repeat exposure to the addictive processed foods found pervasively in western diets, or whether the addiction was exacerbated by a drive to numb trauma, or whether bingeing developed in response to calorie restriction (dieting), the results are the same, i.e. that the brains of people with LCBE diagnoses exhibit the signs of SUD. These findings support the argument that using addiction recovery protocols of reduced harm and abstinence could be valuable regardless of the factors fostering the addiction. As with any SUD recovery program, behavioral habits and the effects of trauma may need to be addressed alongside abstinence or reduced harm.
Limitations
Substance addictions and eating disorders are vast fields. This paper is focused specifically on key similarities of LCBE and UPFA versus dissimilarities in treatment protocols related to processed food use of reduced harm and abstinence. Thus, this paper does not address such issues as the etiology and development of addictive or eating disorders. It does not address the consequences of overconsumption of processed foods with the exception of obesity. It also does not address treatment outside the issue under consideration which is the absence of reduced harm and abstinence from processed foods in treatment of LCBE. For readers who wish to pursue topics outside the scope of this paper, we recommend the following. For in-depth understanding of the neurology of substance use disorders and hedonic eating, we refer readers to Chapters 7 (41) and 8 (42) of Wilcox 2021. For better understanding of other SUD treatment approaches, Val-Laillet et al. would be helpful (43). To better understand the etiology and development of SUD and LCBE, we recommend Kwako et al. (44).
Hypothesis and theory
Although binge-type EDs characterized by LCBE are phenomenologically similar to UPFA, as defined by various versions of the Yale Food Addiction Scale (YFAS), their respective treatments may involve very different approaches, particularly when it comes to the question of nutritional therapy (45–47). This paper focuses on the prominent characteristics of addiction which are also found in binge-type EDs or LCBE: alterations in reward sensitivity, cognitive impairment, and stress responding (48). Most review articles found several of these addictive neuroadaptations in LCBE populations, some of which involved UPFs.
This paper stands on specific studies showing the neurological similarities between LCBE and UPFA. As shown below, the studies are numerous, varied in design, and consistent in results.
Reward alterations
Neuroimaging research shows consistent alterations in reward pathways of individuals with binge-type EDs. Skunde et al. found diminished frontostriatal brain activation in patients with BN vs those without (49). In a review article, Frank found that dopamine pathways were hyporesponsive in bulimia and obesity consistent with downregulation found in drug-addicted populations (50). In a review of 58 brain imaging studies of people with BED, Leenaerts et al. found systematic structural and functional changes in the reward system (51). In a review of animal studies, Blanco-Gandia and colleagues found that palatable foods lead to reward sensitization which can support the development of drug addiction (52). In a second study, these investigators recommended that nutritional patterns be used in treatment of substance use disorders (53). Reward alterations are also consistently found in research on binge eating. In a review article, Hadad and Knackstedt found that BN shared increased dopamine production similarly to drug addiction (54). Lee et al. describes reward sensitivity that is found in both BE and BN regardless of differences in other neuroalterations between the two conditions (55). Simon et al. found that patients with binge-eating and bulimia showed a greater response to food rewards compared to healthy controls. The higher response was related to higher levels of trait food craving, and external eating (56). In a review of animal studies of neurochemical alterations in binge-eating, Avena et al. found that alterations in dopamine (DA), acetylcholine (ACh) and opioid systems in reward-related brain areas occur in response to binge eating of palatable foods (57). In a systematic review of reward sensitivity and eating behavior, Sutton et al. found that reward sensitivity is highly correlated with emotional and binge eating (58). In a review of LCBE populations, cannabinoid systems were dysregulated in a manner consistent with addiction (59). Schag et al. found that reward sensitivity was higher in an obese population with BED than an obese population without (60). Boguzs et al. found that AUD was 1.5 times more likely in a binge-eating population than a control group suggesting reward sensitization in LCBE (61).
These review articles show a consistent pattern of dysregulated reward systems which is characteristic of SUDs such as drug and alcohol addiction as well as addiction to UPFs (62).
Cognitive impairment
Aloi et al. used neurological tests to find lack of attention and poor decision-making in a population with LCBE (63). In a review article of fMRI studies of disinhibited eating, Giddens et al. found that stress deactivated regions implicated in cognitive control (64). These findings are also consistent with findings in drug addiction (65).
Both reward alterations and cognitive impairment
In a review of weight-loss and eating disorder literature, Brassard and Balodis found that LCBE populations demonstrated greater decision-making impairments and greater wanting (or craving) for high-fat sweet foods (66). Giel et al. found that BED represents a distinct phenotype within the obesity spectrum that is characterized by increased impulsivity and reward sensitivity specifically towards food (67). In a narrative review, Hartogsv et al. describe the three alterations most prominent in binge-type EDs as increased reward sensitivity, decreased cognitive control, and altered stress responding (48). Kessler et al. synthesized neuroimaging, neuro cognitive, genetics, and animal studies to find an impulsive/compulsive disorder with altered reward sensitivity and food-related attentional biases which are similar to alterations observed in substance abuse (68). In a review of 100 publications of the phenomenology of neuroendocrine changes, emotional homeostasis factors, and reward circuits published between 2000 and 2021, Milano et al. found that these phenomenon are associated with exposure to highly palatable foods, loss of control, the way we eat, an increase in impulsiveness and the inability to change eating behavior despite the negative consequences related to overweight and obesity (23).
Stress
Stress, trauma and its effects, including the development of Post Traumatic Stress Disorder (PTSD) and related comorbidity, has been shown to be a prominent clinical characteristic associated with both UPFA and LCBE (69–82). Chronic stress administered to animals increases susceptibility to binge-eating and food addiction (83). Similarly, stress and associated negative affect, is one of the most common triggers of binge-eating (84). Sinha found in a review article that UPF stimulate both reward and stress circuitry contributing to cravings, excessive food intake, and weight gain (85).
Consumption of UPFs simultaneously produces a combination of pleasurable reinforcing effects and reinforcing “comforting” effects that in the short term normalize an individual’s responses to stress, yet repetitive and intermittent UPF intake may instead amplify brain stress circuitry and downregulate the brain’s reward pathways in such a way that continued intake becomes obligatory to prevent the development of negative emotional states via negative reinforcement (86). This interplay between stress/trauma, hedonic reward and affective dysregulation helps to explain why patients with LCBE/UPFA have significantly higher psychopathology and lower quality of life (71, 72, 87, 88 ).
Involvement of ultra-processed foods
Senol et al. reviewed research on the dysregulated pathways found in EDs and found that excessive consumption of energy-dense foods alters the brain circuits implicated in reward, decision-making, control, habit formation, and emotions that are central to drug addiction (89). Steward et al., in a review of fMRI studies of people with EDs found heightened responses to food cues and anticipated food receipt occurring with diminished recruitment of cognitive control circuitry which combine to contribute to LCBE of palatable foods (90). Vasiliu et al. found evidence for an overlap between food addiction and EDs to the point of questioning whether they are distinct diagnoses (91). However, the article only mentions pharmaceuticals for therapeutic solutions. In a narrative review, Via et al. connected UPFs with poor impulse control, hyperactivity of reward regions and precedents for subthreshold BED and BED in a population of children and adolescents (92). Vrieze et al. assert that understanding the specific underlying aberrant reward mechanisms in LCBE, associated with different stages of the illness, enables caregivers to focus their treatment more precisely (93).
Comparison of alcohol use disorder, ultra-processed food addiction, and binge-type eating disorders in terms of approaches to abstinence
To compare and summarize the evidence, the Table 1 shows similarities in the characteristics of AUD, UPFA, and LCBE while contrasting their respective approaches to treatment. AUD was chosen for the comparison because it is an established addictive substance that, like UPFs, also has calories and is legal.
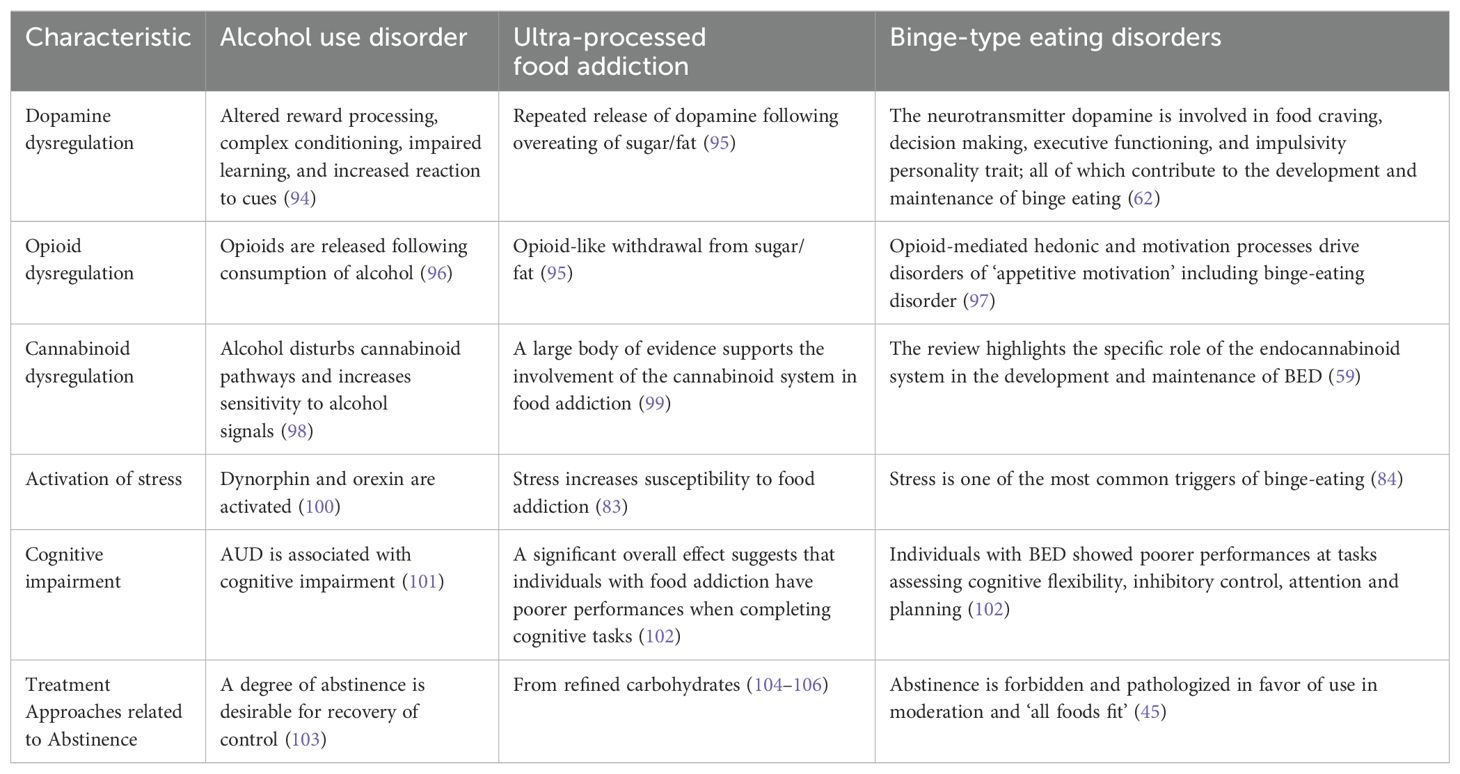
Table 1. Characteristics of alcohol use disorder, ultra-processed food addiction, and binge-type eating disorder vs approaches to abstinence in treatment.
Discussion
The literature consistently shows that populations with LCBE also show the characteristics of addiction to alcohol and to UPFs. These include altered reward functions in dopamine, opioid, and cannabinoid pathways as well as stress sensitivity and cognitive impairment. There is also substantial evidence for the role of excessive consumption of addictive UPFs in LCBE, regardless of diagnosis. However, the treatment of binge-type eating disorders characterized by LCBE, such as BED, typically does not include protocols for abstinence from UPFs that can have the kind of psychoactive properties that can cause these neuro-alterations. In a recent prospective, naturalistic, community-based study of individuals with BED, full remission was elusive, occurring in 46% at 5 years, and relapse was common. Specifically, the median time to remission exceeded 60 months, while the median time to relapse was 30 months. However, the percentage of individuals with BED in the community who received treatment for their eating disorder was not reported (107). Even in patients who have received standard inpatient treatment for BED or BN, approximately one-third still were found to meet criteria for an ED 12 years later (108). Another long-term study of patients with BED who took part in a clinical psychotherapy trial showed that a substantial minority of patients (23-48%) either had not responded or relapsed at 12-year follow-up (109).
This inconsistency between the evidence that UPF could be an important contributor to LCBE versus treatment recommendations for reducing or abstaining from UPFs is also found in descriptions of treatment. For example, Wu et al. write that the current standard of care for BED involves psychotherapy, pharmacotherapy, and the management of comorbid conditions, with nutritional rehabilitation reserved for severe cases of anorexia nervosa. The paper goes on to note that unfortunately, many patients often fail to respond, leaving a concerning treatment gap between the current and requisite treatments for EDs (110). It is notable that recommendations to reduce or abstain from UPFs appears nowhere in the major guidelines for the treatment of EDs, including those developed by dieticians (7, 111). Instead, the myth that “all foods fit” all of the time for all types of EDs has persisted despite the lack of supporting data (45).
Further, calls for future research include many topics but not reduction of nor abstinence from psychoactive UPFs (112). In recommendations for future ED research, Hower at al. describe the need to examine predictors of outcomes, biological/neuropsychological techniques, a focus on severe anorexia, a risk calculator, biological and neurological markers, timepoints during which markers begin to emerge, recovery criteria, standardized assessments, lived experience narratives, timelines for recovery in different areas, nuances of the mentorship role, and social media use in at-risk populations (113).
There is no mention in either the Wu or Hower papers of the role of UPFs in the etiology of binge-type EDs, their use by patients with LCBE, their role in the creation of addictive neuro-alterations and loss of control, nor reduction of, or abstinence from, UPFs as a treatment approach.
The absence of any mention of reduction of or abstinence from UPFs is puzzling because in addition to the evidence for the role of abstinence in the restoration of control in SUD treatment, there is also ample justification for reduction in or elimination of UPFs due to well-established consequences of UPF use (114). There is no mention of the consequences of training patients with LCBE to consume UPFs, such as the effect of UPFs on systemic impairment of cell function (115), epidemics of diet-related diseases including mental illnesses (114), nor the 1.7 million Americans who died from diet-related diseases in 2020 (116).
Gearhardt and DiFeliceantonio describe the dangers of overlooking the addictive properties of UPFs by pointing to missed addictive properties of tobacco and resulting failure to regulate, which led to epidemics of severe consequences (112). They go on to point out that SUD recovery approaches focused on abstinence from UPFs are commonly used on Overeaters Anonymous (OA) 12-step fellowships with approximately 6500 weekly meetings worldwide. Food addiction recovery 12 Step fellowships describe abstinence from various UPFs as an essential prerequisite for reversing loss of control and establishing normal eating (104, 105). While there have not been any controlled trials testing the principles of OA or other 12-step approaches for EDS (117), harm reduction and/or abstinence treatment protocols have begun to be tested in clinical populations (46, 47, 81).
There is strong evidence for the benefits of both abstinence and harm reduction in the treatment of SUDs. Abstinence from alcohol has been shown to result in the best long-term control over drinking (118). Attempts to use alcohol socially have been shown to lead to a return to loss of control. Abstinence for AUD does not necessarily mean that abstinence will work for UPFA. This remains to be seen in on-going research (46). However, the harm reduction approach has been shown to be helpful (81). Thus the “all foods fit” approach used in LCBE appears to run counter to evidence that moderate use of UPFs could lead to loss of control.
What follows are five possible explanations for the inconsistency between SUD symptomology and ‘moderate use’ treatment in LCBE populations.
1. Brewerton and colleagues offer several possible explanations for the inconsistency between SUD and binge-type ED treatment (45). Individualized nutritional treatment plans may be quite challenging to employ and oversee in therapeutic settings. Maintaining a simpler “all foods fit” approach in which staff do not have to individualize nutritional approaches for patients with UPFA can be self-serving in that the clinical work of supporting patients who may be triggered by peers with different nourishment plans can be avoided. In addition, ED treatment programs may not recruit staff with the expertise needed to customize food plan (45). Patients with LCBE are typically treated right alongside patients with AN-R and are given similar food plans that are meant to primarily counter caloric restriction, which has long been thought to be a key driving factor in all EDs ever since the “transdiagnostic” theory of EDs was published (119). Specifically, Fairburn and colleagues stated, “binge eating is largely a product of the particular way that these patients attempt to restrict their eating.” Little of no credence has been given to the hedonic, addicting aspects of UPFs in most ED programs.
2. Advocating abstinence from UPFs could also be framed as a threat to UPF markets. As noted, EDs are estimated to occur in 17% of the worldwide population (1), while global estimates of UPFA are 15-20% (26, 120, 121). However, estimates are that most EDs are undiagnosed (122), as is UPFA, suggesting higher numbers of Americans suffer from LCBE and other types of disordered eating, such as “grazing” (123, 124) and would benefit from UPF abstinence and/or reduction. The UPF industry could be fighting through its dietitians to avoid losing that market.
Further, awareness of relief from diet-related diseases that comes with abstinence from UPFs could spread from LCBE populations to the 83% of Americans with overweight and obesity (125). In this scenario, the UPF market could collapse. Americans eat on average 3600 calories per day (126) of which 73% are ultra-processed (127).
3. A third possible explanation for avoiding abstinence protocols in recovery from LCBE is the fear that withdrawing from a UPF could lead to bingeing. However, it has been shown that it can take years to establish consistent abstinence in recovery from SUD (103). Indeed, Koob and Volkow describe the nature of addicted neurocircuitry that supports the idea that lapsing in early recovery is the rule rather than the exception (128). In alcohol recovery, four years has been shown to be needed to achieve consistent abstinence (129). It has also been shown that restriction does not lead to bingeing (130). Transient bingeing has been shown to occur in early withdrawal from sugar and fat (131), but this early-stage lapsing is time-limited and does not seem to warrant avoiding abstinence or harm reduction protocols altogether.
4. Another possibility is that there is confusion about what is meant by ‘restricting.’ Restricting calories, i.e. not eating enough and setting up fear of famine (132) is dangerous. Dieting and fasting have been shown to precede the development of eating disorders (133, 134). This is as opposed to restricting use of UPFs which are associated with disease (114). Pathologizing calorie restriction is justified while pathologizing abstinence from UPFs clearly is not.
5. A final possible explanation for why ED practitioners resist abstinence in treatment of binge eating is that abstinence from UPFs may be beyond the capabilities of LCBE treatment as it is presently structured. The severity of the addiction in LCBE patients is routinely missed and therefore the requisite high level of care is not provided. Research suggests that a majority of Americans might be manifesting enough of the DSM-5 SUD criteria to meet the threshold for severe addiction if the criteria for AUD or other SUD were adapted to loss of control over UPFs. Manifestation of six out of the 11 SUD criteria is the threshold for severity (6). A majority of Americans may be manifesting six criteria including 1) unintended use among the 82% of Americans suffering from overweight, obesity, or severe obesity (125), 2) failure to cut back among the 80% of people who regain lost weight (135), 3) cravings shown to increase with higher BMI (136), 4) use in spite of consequences among the 93% of Americans with a diet-related diagnosis (137), 5) tolerance or progression shown in the general population by an increase in percentage of processed food use among American adults from 67% in 2001 to 72% in 2018 (138), and 6) withdrawal among 70% of people with obesity reporting eating in the absence of hunger (withdrawal avoidance) in a two-week period (139).
Thus, when ED practitioners back away from abstinence or harm reduction strategies because of observations of bingeing in response to using such protocols, it raises the possibility that the bingeing could be due to inadequate support. Severe AUD is treated by various levels of support from residential to intensive outpatient to daily 12 Step support, where harm reduction or abstinence skills can be developed in protected environments free from availability and cueing. This level of protection from UPF cues and availability may not be routinely offered in LCBE treatment.
As shown above, reaching the severity threshold suggests that a treatment match for UPFA would be a high level of care. There are additional reasons to believe that a high level of care is indicated for UPFA. Thus, typical LCBE outpatient care may also be inadequate because of the seriousness of complications associated with UPFA. UPFA may be deeply seated because of intense cueing (140), increased sensitivity to cueing (141, 142), polysubstance use patterns (143), the highly addictive properties of sugar (144), very young age of onset (145), and the drive to conform to social circles that eat UPFs (146, 147). In light of this evidence, it is reasonable to expect that it could take years of neuro-conditioning and skill-building to maintain a healthy level of ultra-processed food abstinence. Cue avoidance, stress management, emotional stability, food production, and relationship skills are among the many skills needed to eat differently from mainstream culture while maintaining a stable, non-craving brain.
Currently, abstinence from addictive UPFs is not an accepted modality for treatment of binge-type EDs. Although dietitians may be hampered by their close association with the UPF industry (148, 149), other practitioners such as therapists, doctors, nurses, and nutritionists may be more open to training in managing complex withdrawal and building skills for abstinence. This gap in treatment raises hope for improved outcomes by offering the high level of support needed to achieve abstinence in cases of severe addiction to UPFs.
Conclusion
Binge-type EDs are typically treated with food plans that contain UPFs in spite of evidence that UPFs are addictive and that these patients consistently suffer from addictive neuroadaptations including hyperactive reward, impaired cognitive function, and stress reactivity. Low success rates using an ‘all foods fit’ approach point to the need to consider significantly decreased exposure to, or abstinence from, UPFs in their treatment. Possible explanations are offered for why reduction of or abstinence from UPFs is not used in the treatment of binge-type EDs. Research shows that LCBE within the context of UPFA is typically severe and more complicated which suggests that current levels of support could be too low for success in achieving beneficial long-term abstinence from UPFs.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
JI: Conceptualization, Writing – original draft, Writing – review & editing. TB: Conceptualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
Author JI was employed by Food Addiction Reset LLC.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Galmiche M, Déchelotte P, Lambert G, and Tavolacci MP. Prevalence of eating disorders over the 2000–2018 period: a systematic literature review. Am J Clin Nutr. (2019) 109:1402–13. doi: 10.1093/ajcn/nqy342
2. Duncan AE, Ziobrowski HN, and Nicol G. The prevalence of past 12-month and lifetime DSM-IV eating disorders by BMI category in US men and women. Eur Eat Disord Rev. (2017) 25:165–71. doi: 10.1002/erv.2503
3. Hudson JI, Hiripi E, Pope HG Jr., and Kessler RC. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry. (2007) 61:348–58. doi: 10.1016/j.biopsych.2006.03.040
4. Mohler-Kuo M, Schnyder U, Dermota P, Wei W, and Milos G. The prevalence, correlates, and help-seeking of eating disorders in Switzerland. Psychol Med. (2016) 46:2749–58. doi: 10.1017/s0033291716001136
5. Udo T and Grilo CM. Prevalence and correlates of DSM-5-defined eating disorders in a nationally representative sample of U.S. Adults. Biol Psychiatry. (2018) 84:345–54. doi: 10.1016/j.biopsych.2018.03.014
6. American Psychiatric Association. The Diagnostic and Statistical Manual of Mental Disorders. Fifth Edition Vol. 5. Arlington VA: American Psychiatric Publishing (2013).
7. American_Psychiatric_Association. The American Psychiatric Association Practice Guideline for the Treatment of Patients With Eating Disorders. (Arlington, Virginia, USA: American Psychiatric Association Publishing). (2023). doi: 10.1176/appi.books.9780890424865.
8. Monteleone AM, Pellegrino F, Croatto G, Carfagno M, Hilbert A, Treasure J, et al. Treatment of eating disorders: A systematic meta-review of meta-analyses and network meta-analyses. Neurosci Biobehav Rev. (2022) 142:104857. doi: 10.1016/j.neubiorev.2022.104857
9. Solmi M, Monaco F, Højlund M, Monteleone AM, Trott M, Firth J, et al. Outcomes in people with eating disorders: a transdiagnostic and disorder-specific systematic review, meta-analysis and multivariable meta-regression analysis. World Psychiatry. (2024) 23:124–38. doi: 10.1002/wps.21182
10. Criscuolo M, Cinelli G, Croci I, Chianello I, Caramadre AM, Tozzi AE, et al. Psychopathological profile associated with food addiction symptoms in adolescents with eating disorders. Int J Environ Res Public Health. (2023) 20. doi: 10.3390/ijerph20043014
11. Davis C. Compulsive overeating as an addictive behavior: overlap between food addiction and binge eating disorder. Curr Obes Rep. (2013) 2:171–8. doi: 10.1007/s13679-013-0049-8
12. Davis C and Carter JC. Compulsive overeating as an addiction disorder. A review of theory and evidence. Appetite. (2009) 53:1–8. doi: 10.1016/j.appet.2009.05.018
13. de Vries SK and Meule A. Food addiction and bulimia nervosa: new data based on the yale food addiction scale 2.0. Eur Eat Disord Rev. (2016) 24:518–22. doi: 10.1002/erv.2470
14. Fauconnier M, Rousselet M, Brunault P, Thiabaud E, Lambert S, Rocher B, et al. Food addiction among female patients seeking treatment for an eating disorder: prevalence and associated factors. Nutrients. (2020) 12. doi: 10.3390/nu12061897
15. Gearhardt AN, White MA, Masheb RM, and Grilo CM. An examination of food addiction in a racially diverse sample of obese patients with binge eating disorder in primary care settings. Compr Psychiatry. (2013) 54:500–5. doi: 10.1016/j.comppsych.2012.12.009
16. Gearhardt AN, White MA, Masheb RM, Morgan PT, Crosby RD, and Grilo CM. An examination of the food addiction construct in obese patients with binge eating disorder. Int J Eat Disord. (2012) 45:657–63. doi: 10.1002/eat.20957
17. Granero R, Hilker I, Aguera Z, Jimenez-Murcia S, Sauchelli S, Islam MA, et al. Food addiction in a Spanish sample of eating disorders: DSM-5 diagnostic subtype differentiation and validation data. Eur Eat Disord Rev. (2014) 22:389–96. doi: 10.1002/erv.2311
18. Hilker I, Sanchez I, Steward T, Jimenez-Murcia S, Granero R, Gearhardt AN, et al. Food addiction in bulimia nervosa: clinical correlates and association with response to a brief psychoeducational intervention. Eur Eat Disord Rev. (2016) 24:482–8. doi: 10.1002/erv.2473
19. Meule A. On the prevalence of ‘food addiction’ in persons with bulimia nervosa. Eur Eat Disord Rev. (2024) 32:490–2. doi: 10.1002/erv.3061
20. Meule A, von Rezori V, and Blechert J. Food addiction and bulimia nervosa. Eur Eat Disord Rev. (2014) 22:331–7. doi: 10.1002/erv.2306
21. Novelle MG and Diéguez C. Food addiction and binge eating: lessons learned from animal models. Nutrients. (2018) 10. doi: 10.3390/nu10010071
22. Penzenstadler L, Soares C, Anci E, Molodynski A, and Khazaal Y. Effect of assertive community treatment for patients with substance use disorder: A systematic review. Eur Addict Res. (2019) 25:56–67. doi: 10.1159/000496742
23. Milano W, Carizzone F, De Biasio V, Angela Mercorio M, Francesca Milano M, Saetta B, et al. Neurobiological correlates shared between obesity, BED and food addiction. Endocr Metab Immune Disord Drug Targets. (2023) 23:283–93. doi: 10.2174/1871530322666220627125642
24. Hall KD, Ayuketah A, Brychta R, Cai H, Cassimatis T, Chen KY, et al. Ultra-processed diets cause excess calorie intake and weight gain: an inpatient randomized controlled trial of ad libitum food intake. Cell Metab. (2019) 30:67–77.e63. doi: 10.1016/j.cmet.2019.05.008
25. Schulte EM, Avena NM, and Gearhardt AN. Which foods may be addictive? The roles of processing, fat content, and glycemic load. PloS One. (2015) 10:e0117959. doi: 10.1371/journal.pone.0117959
26. LaFata EM and Gearhardt AN. Ultra-processed food addiction: an epidemic? Psychother Psychosom. (2022) 91:363–72. doi: 10.1159/000527322
27. National Institute of Mental Health. Eating Disorders: About More than Food. Bethesda MD: National Institutes of Health (2021). Available at: https://www.nimh.nih.gov/health/publications/eating-disorders (Accessed January 10, 2025).
28. Single E. Defining harm reduction. Drug Alcohol Rev. (1995) 14:287–90. doi: 10.1080/09595239500185371
29. Wiss DA, Avena N, and Rada P. Sugar addiction: from evolution to revolution. Front Psychiatry. (2018) 9:545. doi: 10.3389/fpsyt.2018.00545
30. Lustig RH. Fructose: it’s “alcohol without the buzz. Adv Nutr. (2013) 4:226–35. doi: 10.3945/an.112.002998
31. Lennerz B and Lennerz JK. Food addiction, high-glycemic-index carbohydrates, and obesity. Clin Chem. (2018) 64:64–71. doi: 10.1373/clinchem.2017.273532
32. Takahashi M, Fukunaga H, Kaneto H, Fukudome S, and Yoshikawa M. Behavioral and pharmacological studies on gluten exorphin A5, a newly isolated bioactive food protein fragment, in mice. Jpn J Pharmacol. (2000) 84:259–65. doi: 10.1254/jjp.84.259
33. Brown RB. Sodium chloride, migraine and salt withdrawal: controversy and insights. Med Sci (Basel). (2021) 9. doi: 10.3390/medsci9040067
34. Teschemacher H, Koch G, and Brantl V. Milk protein-derived opioid receptor ligands. Biopolymers. (1997) 43:99–117. doi: 10.1002/(SICI)1097-0282(1997)43:2<99::AID-BIP3>3.0.CO;2-V
35. Lee HS, Giunti E, Sabino V, and Cottone P. Consummatory, feeding microstructural, and metabolic effects induced by limiting access to either a high-sucrose or a high-fat diet. Nutrients. (2020) 12. doi: 10.3390/nu12061610
36. Addicott MA. Caffeine use disorder: A review of the evidence and future implications. Curr Addict Rep. (2014) 1:186–92. doi: 10.1007/s40429-014-0024-9
37. Onaolapo AY and Onaolapo OJ. Food additives, food and the concept of ‘food addiction’: Is stimulation of the brain reward circuit by food sufficient to trigger addiction? Pathophysiology. (2018) 25:263–76. doi: 10.1016/j.pathophys.2018.04.002
38. Ayton A, Ibrahim A, Dugan J, Galvin E, and Wright OW. Ultra-processed foods and binge eating: A retrospective observational study. Nutrition. (2021) 84:111023. doi: 10.1016/j.nut.2020.111023
39. Ifland JR and Preuss HG. Focusing the Fight against Food Addiction. In Preuss FG and Bagchi D (Eds.), Dietary Fat, Salt, and Sugar in Dietary Health 1 ed. (Cambridge MA: Elsevier) (2020) pp. 157–170. doi: 10.1016/C2018-0-04143-7
40. Tillotson JE. America’s obesity: conflicting public policies, industrial economic development, and unintended human consequences. Annu Rev Nutr. (2004) 24:617–43. doi: 10.1146/annurev.nutr.24.012003.132434
41. Wilcox CE. Chapter 7. Neurobiology and Cognitive Neuroscience of Substance use Disorders. In: Food Addiction, Obesity, and Disorders of Eating: An Evidence-Based Assessment and Clinical Guide, 1 ed. Cham, Switzerland: Springer (2021). doi: 10.1007/978-3-030-83078-6
42. Wilcox CE. Chapter 8. Neurobiology and Cognitive Neuroscience of Hedonic Eating. In: Food Addiction, Obesity, and Disorders of Eating: An Evidence-Based Assessment and Clinical Guide, 1 ed. Cham, Switzerland: Springer (2021). doi: 10.1007/978-3-030-83078-6
43. Val-Laillet D, Aarts E, Weber B, Ferrari M, Quaresima V, Stoeckel LE, et al. Neuroimaging and neuromodulation approaches to study eating behavior and prevent and treat eating disorders and obesity. NeuroImage Clin. (2015) 8:1–31. doi: 10.1016/j.nicl.2015.03.016
44. Kwako LE, Momenan R, Litten RZ, Koob GF, and Goldman D. Addictions neuroclinical assessment: A neuroscience-based framework for addictive disorders. Biol Psychiatry. (2016) 80:179–89. doi: 10.1016/j.biopsych.2015.10.024
45. Brewerton TD, Dennis K, and Wiss DA. Dismantling the myth of “all foods fit” in eating disorder treatment. J Eat Disord. (2024) 12:60. doi: 10.1186/s40337-024-01017-9
46. Dennis K, Barrera S, Bishop N, Nguyen C, and Brewerton TD. Food addiction screening, diagnosis and treatment: A protocol for residential treatment of eating disorders, substance use disorders and trauma-related psychiatric comorbidity. Nutrients. (2024) 16. doi: 10.3390/nu16132019
47. O’Hea EL, Edwards-Hampton SA, Beall Brown DL, Sonneville KR, Ziedonis DM, and Gearhardt AN. The food addiction clinical treatment (FACT) manual: A harm reduction treatment approach. Behav Sci (Basel). (2024) 14. doi: 10.3390/bs14070557
48. Hartogsveld B, Quaedflieg C, van Ruitenbeek P, and Smeets T. Volume and connectivity differences in brain networks associated with cognitive constructs of binge eating. eNeuro. (2022) 9. doi: 10.1523/eneuro.0080-21.2021
49. Skunde M, Walther S, Simon JJ, Wu M, Bendszus M, Herzog W, et al. Neural signature of behavioural inhibition in women with bulimia nervosa. J Psychiatry Neurosci. (2016) 41:E69–78. doi: 10.1503/jpn.150335
50. Frank GK. Advances from neuroimaging studies in eating disorders. CNS Spectr. (2015) 20:391–400. doi: 10.1017/s1092852915000012
51. Leenaerts N, Jongen D, Ceccarini J, Van Oudenhove L, and Vrieze E. The neurobiological reward system and binge eating: A critical systematic review of neuroimaging studies. Int J Eat Disord. (2022) 55:1421–58. doi: 10.1002/eat.23776
52. Blanco-Gandía MC, Miñarro J, and Rodríguez-Arias M. Common neural mechanisms of palatable food intake and drug abuse: knowledge obtained with animal models. Curr Pharm Des. (2020) 26:2372–84. doi: 10.2174/1381612826666200213123608
53. Blanco-Gandia MC, Montagud-Romero S, and Rodríguez-Arias M. Binge eating and psychostimulant addiction. World J Psychiatry. (2021) 11:517–29. doi: 10.5498/wjp.v11.i9.517
54. Hadad NA and Knackstedt LA. Addicted to palatable foods: comparing the neurobiology of Bulimia Nervosa to that of drug addiction. Psychopharmacol (Berl). (2014) 231:1897–912. doi: 10.1007/s00213-014-3461-1
55. Lee JE, Namkoong K, and Jung YC. Impaired prefrontal cognitive control over interference by food images in binge-eating disorder and bulimia nervosa. Neurosci Lett. (2017) 651:95–101. doi: 10.1016/j.neulet.2017.04.054
56. Simon JJ, Skunde M, Walther S, Bendszus M, Herzog W, and Friederich HC. Neural signature of food reward processing in bulimic-type eating disorders. Soc Cognit Affect Neurosci. (2016) 11:1393–401. doi: 10.1093/scan/nsw049
57. Avena NM and Bocarsly ME. Dysregulation of brain reward systems in eating disorders: neurochemical information from animal models of binge eating, bulimia nervosa, and anorexia nervosa. Neuropharmacology. (2012) 63:87–96. doi: 10.1016/j.neuropharm.2011.11.010
58. Sutton CA, L’Insalata AM, and Fazzino TL. Reward sensitivity, eating behavior, and obesity-related outcomes: A systematic review. Physiol Behav. (2022) 252:113843. doi: 10.1016/j.physbeh.2022.113843
59. Bourdy R and Befort K. The role of the endocannabinoid system in binge eating disorder. Int J Mol Sci. (2023) 24. doi: 10.3390/ijms24119574
60. Schag K, Schönleber J, Teufel M, Zipfel S, and Giel KE. Food-related impulsivity in obesity and binge eating disorder–a systematic review. Obes Rev. (2013) 14:477–95. doi: 10.1111/obr.12017
61. Bogusz K, Kopera M, Jakubczyk A, Trucco EM, Kucharska K, Walenda A, et al. Prevalence of alcohol use disorder among individuals who binge eat: a systematic review and meta-analysis. Addiction. (2021) 116:18–31. doi: 10.1111/add.15155
62. Yu Y, Miller R, and Groth SW. A literature review of dopamine in binge eating. J Eat Disord. (2022) 10:11. doi: 10.1186/s40337-022-00531-y
63. Aloi M, Liuzza MT, Rania M, Carbone EA, de Filippis R, Gearhardt AN, et al. Using latent class analysis to identify different clinical profiles according to food addiction symptoms in obesity with and without binge eating disorder. J Behav Addict. (2024) 13:262–75. doi: 10.1556/2006.2023.00074
64. Giddens E, Noy B, Steward T, and Verdejo-García A. The influence of stress on the neural underpinnings of disinhibited eating: a systematic review and future directions for research. Rev Endocr Metab Disord. (2023) 24:713–34. doi: 10.1007/s11154-023-09814-4
65. Koob GF. Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology. (2009) 56 Suppl 1:18–31. doi: 10.1016/j.neuropharm.2008.07.043
66. Brassard SL and Balodis IM. A review of effort-based decision-making in eating and weight disorders. Prog Neuropsychopharmacol Biol Psychiatry. (2021) 110:110333. doi: 10.1016/j.pnpbp.2021.110333
67. Giel KE, Teufel M, Junne F, Zipfel S, and Schag K. Food-related impulsivity in obesity and binge eating disorder-A systematic update of the evidence. Nutrients. (2017) 9. doi: 10.3390/nu9111170
68. Kessler RM, Hutson PH, Herman BK, and Potenza MN. The neurobiological basis of binge-eating disorder. Neurosci Biobehav Rev. (2016) 63:223–38. doi: 10.1016/j.neubiorev.2016.01.013
69. Brewerton TD. Eating disorders, trauma, and comorbidity: focus on PTSD. Eat Disord. (2007) 15:285–304. doi: 10.1080/10640260701454311
70. Brewerton TD. Posttraumatic stress disorder and disordered eating: food addiction as self-medication. J Womens Health (Larchmt). (2011) 20:1133–4. doi: 10.1089/jwh.2011.3050
71. Brewerton TD. Food addiction as a proxy for eating disorder and obesity severity, trauma history, PTSD symptoms, and comorbidity. Eat Weight Disord. (2017) 22:241–7. doi: 10.1007/s40519-016-0355-8
72. Brewerton TD. Mechanisms by which adverse childhood experiences, other traumas and PTSD influence the health and well-being of individuals with eating disorders throughout the life span. J Eat Disord. (2022) 10:162. doi: 10.1186/s40337-022-00696-6
73. Brewerton TD, Gavidia I, Suro G, and Perlman MM. Eating disorder onset during childhood is associated with higher trauma dose, provisional PTSD, and severity of illness in residential treatment. Eur Eat Disord Rev. (2022) 30:267–77. doi: 10.1002/erv.2892
74. Dansky BS, Brewerton TD, Kilpatrick DG, and O’Neil PM. The National Women’s Study: relationship of victimization and posttraumatic stress disorder to bulimia nervosa. Int J Eat Disord. (1997) 21:213–28. doi: 10.1002/(SICI)1098-108X(199704)21:3<213::AID-EAT2>3.0.CO;2-N
75. Hardy R, Fani N, Jovanovic T, and Michopoulos V. Food addiction and substance addiction in women: Common clinical characteristics. Appetite. (2018) 120:367–73. doi: 10.1016/j.appet.2017.09.026
76. Hoover LV, Yu HP, Duval ER, and Gearhardt AN. Childhood trauma and food addiction: The role of emotion regulation difficulties and gender differences. Appetite. (2022) 177:106137. doi: 10.1016/j.appet.2022.106137
77. Mason SM, Flint AJ, Roberts AL, Agnew-Blais J, Koenen KC, and Rich-Edwards JW. Posttraumatic stress disorder symptoms and food addiction in women by timing and type of trauma exposure. JAMA Psychiatry. (2014) 71:1271–8. doi: 10.1001/jamapsychiatry.2014.1208
78. Mitchell KS and Wolf EJ. PTSD, food addiction, and disordered eating in a sample of primarily older veterans: The mediating role of emotion regulation. Psychiatry Res. (2016) 243:23–9. doi: 10.1016/j.psychres.2016.06.013
79. Siegel SE, Ranney RM, Masheb RM, Huggins J, and Maguen S. Associations between posttraumatic stress disorder and eating disorder symptoms among women veterans. Eat Behav. (2024) 52:101851. doi: 10.1016/j.eatbeh.2024.101851
80. Stojek MM, Lipka J, Maples-Keller JM, Rauch SAM, Black K, Michopoulos V, et al. Investigating sex differences in rates and correlates of food addiction status in women and men with PTSD. Nutrients. (2021) 13. doi: 10.3390/nu13061840
81. Unwin J, Delon C, Giæver H, Kennedy C, Painschab M, Sandin F, et al. Low carbohydrate and psychoeducational programs show promise for the treatment of ultra-processed food addiction. Front Psychiatry. (2022) 13:1005523. doi: 10.3389/fpsyt.2022.1005523
82. Wiss DA and LaFata EM. Structural equation modeling of adverse childhood experiences, ultra-processed food intake, and symptoms of post-traumatic stress disorder, ultra-processed food addiction, and eating disorder among adults seeking nutrition counseling in Los Angeles, CA. Appetite. (2025) 208:107938. doi: 10.1016/j.appet.2025.107938
83. Wei NL, Quan ZF, Zhao T, Yu XD, Xie Q, Zeng J, et al. Chronic stress increases susceptibility to food addiction by increasing the levels of DR2 and MOR in the nucleus accumbens. Neuropsychiatr Dis Treat. (2019) 15:1211–29. doi: 10.2147/ndt.s204818
84. Naish KR, Laliberte M, MacKillop J, and Balodis IM. Systematic review of the effects of acute stress in binge eating disorder. Eur J Neurosci. (2019) 50:2415–29. doi: 10.1111/ejn.14110
85. Sinha R. Role of addiction and stress neurobiology on food intake and obesity. Biol Psychol. (2018) 131:5–13. doi: 10.1016/j.biopsycho.2017.05.001
86. Parylak SL, Koob GF, and Zorrilla EP. The dark side of food addiction. Physiol Behav. (2011) 104(1):149–156. doi: 10.1016/j.physbeh.2011.04.063
87. Brewerton TD, Perlman MM, Gavidia I, Suro G, Genet J, and Bunnell DW. The association of traumatic events and posttraumatic stress disorder with greater eating disorder and comorbid symptom severity in residential eating disorder treatment centers. Int J Eat Disord. (2020) 53(12):2061–2066. doi: 10.1002/eat.23401
88. Nunes-Neto PR, Kohler CA, Schuch FB, Quevedo J, Solmi M, Murru A, et al. Psychometric properties of the modified Yale Food Addiction Scale 2.0 in a large Brazilian sample. Braz J Psych. (2018) 40(4):444–448. doi: 10.1590/1516-4446-2017-2432
89. Senol E and Mohammad H. Current perspectives on brain circuits involved in food addiction-like behaviors. J Neural Transm (Vienna). (2024) 21:275–90. doi: 10.1007/s00702-023-02732-4
90. Steward T, Menchon JM, Jiménez-Murcia S, Soriano-Mas C, and Fernandez-Aranda F. Neural network alterations across eating disorders: A narrative review of fMRI studies. Curr Neuropharmacol. (2018) 16:1150–63. doi: 10.2174/1570159x15666171017111532
91. Vasiliu O. Current status of evidence for a new diagnosis: food addiction-A literature review. Front Psychiatry. (2021) 12:824936. doi: 10.3389/fpsyt.2021.824936
92. Via E and Contreras-Rodríguez O. Binge-eating precursors in children and adolescents: neurodevelopment, and the potential contribution of ultra-processed foods. Nutrients. (2023) 15. doi: 10.3390/nu15132994
93. Vrieze E and Leenaerts N. Neuronal activity and reward processing in relation to binge eating. Curr Opin Psychiatry. (2023) 36:443–8. doi: 10.1097/yco.0000000000000895
94. Beck A, Ebrahimi C, Rosenthal A, Charlet K, and Heinz A. The dopamine system in mediating alcohol effects in humans. Curr Top Behav Neurosci. (2023) 64:65–87. doi: 10.1007/7854_2022_415
95. Avena NM, Bocarsly ME, and Hoebel BG. Animal models of sugar and fat bingeing: relationship to food addiction and increased body weight. Methods Mol Biol. (2012) 829:351–65. doi: 10.1007/978-1-61779-458-2_23
96. Margolis EB, Moulton MG, Lambeth PS, and O’Meara MJ. The life and times of endogenous opioid peptides: Updated understanding of synthesis, spatiotemporal dynamics, and the clinical impact in alcohol use disorder. Neuropharmacology. (2023) 225:109376. doi: 10.1016/j.neuropharm.2022.109376
97. Nathan PJ and Bullmore ET. From taste hedonics to motivational drive: central μ-opioid receptors and binge-eating behaviour. Int J Neuropsychopharmacol. (2009) 12:995–1008. doi: 10.1017/s146114570900039x
98. Serrano A and Natividad LA. Alcohol-endocannabinoid interactions: implications for addiction-related behavioral processes. Alcohol Res. (2022) 42:9. doi: 10.35946/arcr.v42.1.09
99. D’Addario C, Micioni Di Bonaventura MV, Pucci M, Romano A, Gaetani S, Ciccocioppo R, et al. Endocannabinoid signaling and food addiction. Neurosci Biobehav Rev. (2014) 47:203–24. doi: 10.1016/j.neubiorev.2014.08.008
100. Anderson RI, Moorman DE, and Becker HC. Contribution of dynorphin and orexin neuropeptide systems to the motivational effects of alcohol. Handb Exp Pharmacol. (2018) 248:473–503. doi: 10.1007/164_2018_100
101. Wang G, Li DY, Vance DE, and Li W. Alcohol use disorder as a risk factor for cognitive impairment. J Alzheimers Dis. (2023) 94:899–907. doi: 10.3233/jad-230181
102. Iceta S, Rodrigue C, Legendre M, Daoust J, Flaudias V, Michaud A, et al. Cognitive function in binge eating disorder and food addiction: A systematic review and three-level meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. (2021) 111:110400. doi: 10.1016/j.pnpbp.2021.110400
103. Kelly JF, Greene MC, and Bergman BG. Beyond abstinence: changes in indices of quality of life with time in recovery in a nationally representative sample of U.S. Adults. Alcohol Clin Exp Res. (2018) 42:770–80. doi: 10.1111/acer.13604
104. Food Addicts Anonymous. Food Addicts Anonymous. Port St. Lucie, FL: Food Addicts Anonymous, Inc (2010).
105. Food Addicts in Recovery Anonymous. Food Addicts in Recovery Anonymous. Woburn, MA: Food Addicts in Recovery Anonymous (2013).
106. Rostanzo E, Marchetti M, Casini I, and Aloisi AM. Very-low-calorie ketogenic diet: A potential treatment for binge eating and food addiction symptoms in women. A pilot study. Int J Environ Res Public Health. (2021) 18. doi: 10.3390/ijerph182312802
107. Javaras KN, Franco VF, Ren B, Bulik CM, Crow SJ, McElroy SL, et al. The natural course of binge-eating disorder: findings from a prospective, community-based study of adults. Psychol Med. (2024) 54:1–11. doi: 10.1017/S0033291724000977
108. Fichter MM, Quadflieg N, and Hedlund S. Long-term course of binge eating disorder and bulimia nervosa: relevance for nosology and diagnostic criteria. Int J Eat Disord. (2008) 41:577–86. doi: 10.1002/eat.20539
109. Hilbert A, Bishop ME, Stein RI, Tanofsky-Kraff M, Swenson AK, Welch RR, et al. Long-term efficacy of psychological treatments for binge eating disorder. Br J Psychiatry. (2012) 200:232–7. doi: 10.1192/bjp.bp.110.089664
110. Wu K, Lo YT, Cavaleri J, Bergosh M, Ipe J, Briggs RG, et al. Neuromodulation of eating disorders: A review of underlying neural network activity and neuromodulatory treatments. Brain Sci. (2024) 14. doi: 10.3390/brainsci14030200
111. Ozier AD, Henry BW, and American Dietetic A. Position of the American Dietetic Association: nutrition intervention in the treatment of eating disorders. J Am Diet Assoc. (2011) 111:1236–41. doi: 10.1016/j.jada.2011.06.016
112. Gearhardt AN and DiFeliceantonio AG. Highly processed foods can be considered addictive substances based on established scientific criteria. Addiction. (2023) 118:589–98. doi: 10.1111/add.16065
113. Hower H, LaMarre A, Bachner-Melman R, Harrop EN, McGilley B, and Kenny TE. Conceptualizing eating disorder recovery research: Current perspectives and future research directions. J Eat Disord. (2022) 10:165. doi: 10.1186/s40337-022-00678-8
114. Lane MM, Gamage E, Du S, Ashtree DN, McGuinness AJ, Gauci S, et al. Ultra-processed food exposure and adverse health outcomes: umbrella review of epidemiological meta-analyses. Bmj. (2024) 384:e077310. doi: 10.1136/bmj-2023-077310
115. Lustig RH. Chapter 7: the “Diseases” that aren’t diseases. In: Metabolical: The Lure and the Lies of Processed Food, Nutrition, and Modern Medicine. New York, NY: Harper Collins (2021). p. 103–22.
116. Center for Disease Control. Mortality in the United States 2021. Atlanta: Centers for Disease Control and Prevention (CDC), National Center for Health Statistics (2022).
117. von Ranson KM and Robinson KE. Who is providing what type of psychotherapy to eating disorder clients? A survey. Int J Eat Disord. (2006) 39:27–34. doi: 10.1002/eat.20201
118. MacKenzie N, Smith DJ, Lawrie SM, and McCartney D. Substance use, risk behaviours and well-being after admission to a quasi-residential abstinence-based rehabilitation programme: 4-year follow-up. BJPsych Open. (2023) 9:e52. doi: 10.1192/bjo.2023.23
119. Fairburn CG, Cooper Z, and Shafran R. Cognitive behaviour therapy for eating disorders: a “transdiagnostic” theory and treatment. Behav Res Ther. (2003) 41:509–28. doi: 10.1016/s0005-7967(02)00088-8
120. LaFata EM, Allison KC, Audrain-McGovern J, and Forman EM. Ultra-processed food addiction: A research update. Curr Obes Rep. (2024) 13:214–23. doi: 10.1007/s13679-024-00569-w
121. Praxedes DRS, Silva-Junior AE, Macena ML, Oliveira AD, Cardoso KS, Nunes LO, et al. Prevalence of food addiction determined by the Yale Food Addiction Scale and associated factors: A systematic review with meta-analysis. Eur Eat Disord Rev. (2022) 30:85–95. doi: 10.1002/erv.2878
122. Bryant E, Spielman K, Le A, Marks P, Touyz S, and Maguire S. Screening, assessment and diagnosis in the eating disorders: findings from a rapid review. J Eat Disord. (2022) 10:78. doi: 10.1186/s40337-022-00597-8
123. Bonder R and Davis C. Associations between food addiction and substance-use disorders: A critical overview of their overlapping patterns of consumption. Curr Addict Rep. (2022) 9:326–33. doi: 10.1007/s40429-022-00443-6
124. Bonder R, Kuk JL, Ardern CI, Wharton S, Kamran E, and Davis C. Grazing and food addiction: Associations between varied patterns of overconsumption and addictive-like eating. Appetite. (2024) 204:107768. doi: 10.1016/j.appet.2024.107768
125. Hales CM, Carroll MD, Fryar CD, and Ogden CL. Prevalence of obesity and severe obesity among adults: United States 2017-2018. NCHS Data Brief. (2020) 360:1–8.
126. Food and Agriculture Organization of the United Nations. Food Balances, (2010-) (2020). Available online at: https://www.fao.org/faostat/en/data/FBS (Accessed January 20, 2025).
127. Monteiro CA, Cannon G, Moubarac JC, Levy RB, Louzada MLC, and Jaime PC. The UN Decade of Nutrition, the NOVA food classification and the trouble with ultra-processing. Public Health Nutr. (2018) 21:5–17. doi: 10.1017/s1368980017000234
128. Koob GF and Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. (2016) 3:760–73. doi: 10.1016/s2215-0366(16)00104-8
129. Vaillant GE. The Natural History of Alcoholism Revisited. Cambridge, MA: Harvard University Press (1995).
130. Bartholomay J, Schaefer LM, Forester G, Crosby RD, Peterson CB, Crow SJ, et al. Evaluating dietary restriction as a maintaining factor in binge-eating disorder. Int J Eat Disord. (2024) 57:1172–80. doi: 10.1002/eat.24094
131. Parnarouskis L, Leventhal AM, Ferguson SG, and Gearhardt AN. Withdrawal: A key consideration in evaluating whether highly processed foods are addictive. Obes Rev. (2022) 23:e13507. doi: 10.1111/obr.13507
132. Boggiano MM, Turan B, Maldonado CR, Oswald KD, and Shuman ES. Secretive food concocting in binge eating: test of a famine hypothesis. Int J Eat Disord. (2013) 46:212–25. doi: 10.1002/eat.22077
133. Stice E and Agras WS. Subtyping bulimic women along dietary restraint and negative affect dimensions. J Consult Clin Psychol. (1999) 67:460–9. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10450616.
134. Stice E, Davis K, Miller NP, and Marti CN. Fasting increases risk for onset of binge eating and bulimic pathology: a 5-year prospective study. J Abnorm Psychol. (2008) 117:941–6. doi: 10.1037/a0013644
135. Knell G, Li Q, Pettee Gabriel K, and Shuval K. Long-term weight loss and metabolic health in adults concerned with maintaining or losing weight: findings from NHANES. Mayo Clin Proc. (2018) 93:1611–6. doi: 10.1016/j.mayocp.2018.04.018
136. Taetzsch A, Roberts SB, Gilhooly CH, Lichtenstein AH, Krauss AJ, Bukhari A, et al. Food cravings: Associations with dietary intake and metabolic health. Appetite. (2020) 152:104711. doi: 10.1016/j.appet.2020.104711
137. O’Hearn M, Lauren BN, Wong JB, Kim DD, and Mozaffarian D. Trends and disparities in cardiometabolic health among U.S. Adults 1999-2018. J Am Coll Cardiol. (2022) 80:138–51. doi: 10.1016/j.jacc.2022.04.046
138. Juul F, Parekh N, Martinez-Steele E, Monteiro CA, and Chang VW. Ultra-processed food consumption among US adults from 2001 to 2018. Am J Clin Nutr. (2022) 115:211–21. doi: 10.1093/ajcn/nqab305
139. Goldschmidt AB, Crosby RD, Cao L, Pearson CM, Utzinger LM, Pacanowski CR, et al. Contextual factors associated with eating in the absence of hunger among adults with obesity. Eat Behav. (2017) 26:33–9. doi: 10.1016/j.eatbeh.2017.01.005
140. Lifshitz F and Lifshitz JZ. Globesity: the root causes of the obesity epidemic in the USA and now worldwide. Pediatr Endocrinol Rev. (2014) 12:17–34.
141. Cohen DA. Neurophysiological pathways to obesity: below awareness and beyond individual control. Diabetes. (2008) 57:1768–73. doi: 10.2337/db08-0163
142. Ghobadi-Azbari P, Mahdavifar Khayati R, and Ekhtiari H. Habituation or sensitization of brain response to food cues: Temporal dynamic analysis in an functional magnetic resonance imaging study. Front Hum Neurosci. (2023) 17:1076711. doi: 10.3389/fnhum.2023.1076711
143. Connor JP, Gullo MJ, White A, and Kelly AB. Polysubstance use: diagnostic challenges, patterns of use and health. Curr Opin Psychiatry. (2014) 27:269–75. doi: 10.1097/yco.0000000000000069
144. Madsen HB and Ahmed SH. Drug versus sweet reward: greater attraction to and preference for sweet versus drug cues. Addict Biol. (2015) 20:433–44. doi: 10.1111/adb.12134
145. Filgueiras AR, Pires de Almeida VB, Koch Nogueira PC, Alvares Domene SM, Eduardo da Silva C, Sesso R, et al. Exploring the consumption of ultra-processed foods and its association with food addiction in overweight children. Appetite. (2019) 135:137–45. doi: 10.1016/j.appet.2018.11.005
146. Bahr DB, Browning RC, Wyatt HR, and Hill JO. Exploiting social networks to mitigate the obesity epidemic. Obes (Silver Spring). (2009) 17:723–8. doi: 10.1038/oby.2008.615
147. Christakis NA and Fowler JH. The spread of obesity in a large social network over 32 years. N Engl J Med. (2007) 357:370–9. doi: 10.1056/NEJMsa066082
148. Rowe S, Alexander N, Clydesdale FM, Applebaum RS, Atkinson S, Black RM, et al. Funding food science and nutrition research: financial conflicts and scientific integrity. J Nutr. (2009) 139:1051–3. doi: 10.3945/jn.109.105668
Keywords: food addiction, binge eating, ultra-processed food, eating disorders, loss of control, bulimia, anorexia, purging
Citation: Ifland J and Brewerton TD (2025) Binge-type eating disorders and ultra-processed food addiction: phenomenology, pathophysiology and treatment implications. Front. Psychiatry 16:1584891. doi: 10.3389/fpsyt.2025.1584891
Received: 27 February 2025; Accepted: 14 May 2025;
Published: 20 June 2025.
Edited by:
Jen Unwin, The Collaborative Health Community, United KingdomReviewed by:
Vera I. Tarman, University of Toronto, CanadaClaire Wilcox, Mind Research Network (MRN), United States
Copyright © 2025 Ifland and Brewerton. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joan Ifland, am9hbmlmbGFuZEByZW1pc3Npb25vcHRpbWlzdGljLmNvbQ==
 Joan Ifland
Joan Ifland Timothy D. Brewerton
Timothy D. Brewerton