Abstract
Background:
Chronic obstructive pulmonary disease (COPD) is increasingly prevalent in respiratory medicine, with rising incidence and mortality rates annually. Beyond respiratory implications, it leads to cardiovascular events, osteoporosis, muscle loss, and psychosomatic syndrome, often overlooked yet pivotal in COPD prognosis. Despite this, the relationship between COPD and psychosomatic syndrome remains unclear. This study aimed to explore the correlation between acute exacerbation of chronic obstructive pulmonary disease (AECOPD) in patients and psychosomatic syndrome, alongside peripheral blood inflammatory factors and symptom burden (e.g., modified Medical Research Council [mMRC] and COPD assessment test [CAT]). Identifying high-risk AECOPD patients with psychosomatic syndrome through these markers could enable early intervention and improve prognosis.
Methods:
This observational study recruited 202 AECOPD patients admitted to the Respiratory and Critical Care Medicine Department of Shanghai Jiading Central Hospital from March 1st, 2022 and May 1st, 2024. After obtaining consent, we collected demographic data, blood routine results, albumin, prealbumin, lung function metrics, mMRC, CAT scores, and other parameters. We evaluated psychiatric comorbidities using the revised version of the Diagnostic Criteria for Psychosomatic Research (DCPR-R). Binary logistic regression and ROC curve analyses were employed to assess the correlation between inflammatory factors, symptom burden, and psychosomatic syndrome, as well as their diagnostic and predictive value for psychosomatic syndrome.
Results:
Among 202 AECOPD patients, 144 were in the DCPR (+) group and 58 in the DCPR (-) group. The DCPR (+) group had higher white blood cell counts, neutrophil counts, monocyte counts, interleukin (IL)-6 levels, mMRC scores, and CAT scores than those of the DCPR (-) group, (P < 0.05), Albumin and prealbumin levels were significantly lower in the DCPR (+) group than those in the DCPR (-) group (P < 0.05). There were no significant differences between the two groups in terms of age, sex, marital status, years of education, body mass index, smoking history, drinking history, diabetes, hypertension, PaCO2, PaO2, neutral ratio, lymphocyte count, monocyte ratio, FEV1% pred, and FEV1/FVC% (P > 0.05). Multivariate binary logistic regression analysis identified IL-6, mMRC, and CAT score as independent risk factors for psychosomatic syndrome in AECOPD patients. The odds ratios (OR) and 95% confidence intervals (CI) were: IL-6: OR=1.192 (95% CI: 1.091-1.302), P < 0.001; mMRC: OR=1.922 (95% CI: 1.175-3.144), P = 0.009; CAT: OR=1.149 (95% CI: 1.073-1.231), P < 0.001.
Conclusions:
Peripheral blood IL-6, mMRC, and CAT were independent risk factors for psychiatric comorbidities in AECOPD patients, with good predictive value for psychosomatic integration. Subjective symptoms including cough, phlegm, sleep disturbances, lack of confidence in going out, and difficulty breathing contribute more to psychiatric comorbidities than the objective indicator of FEV1.
1 Background
Chronic obstructive pulmonary disease (COPD) is an inflammatory disease of the airways characterized by progressive airflow limitation. Its main symptoms include cough, sputum production, and progressively worsening breathing difficulties (1). The primary risk factors include smoking, air pollution, and occupational exposure (2). As of 2017, COPD has become the third leading cause of death globally (3). COPD patients experience a prolonged course marked by recurrent exacerbations, slow progression, and gradual deterioration. This condition not only impacts the respiratory system but also heightens the risk of cardiovascular events, osteoporosis, muscle loss, anxiety, depression, cognitive decline, and other systemic and multisystem impairments (4–6). Additionally, the prevalence of psychological disorders is relatively high in COPD patients. According to the meta-analysis conducted by Matte et al. (7) and Zhang et al. (8), the prevalence of depression among COPD patients is reported as 24.6% and 27.1%, respectively, while in non-COPD patients, the prevalence of depression is 11.7% and 10%, respectively. Reportedly, the incidence of cognitive impairment in COPD patients is 17-56.7%, whereas in healthy controls, it is 12-16.7% (9). However, healthcare workers often lack sufficient understanding of COPD, leading to frequent misdiagnosis and improper management, which in turn result in poor health outcomes and prognosis. Rahi et al. pointed out that depression or anxiety consistently increases the risk of adverse outcomes in COPD patients (odds ratio [OR]=1.43; 95% confidence interval [CI]: 1.22-1.68). Specifically, depression increases the risk of death by 1.83 (95% CI: 1.00-3.36), and anxiety heightens the risk of death by 1.27 (95% CI: 1.02-1.58) (10). Anxiety and depression are independent risk factors of death in COPD patients (11), and depression exacerbates their deterioration (12). An increase in hospitalization duration owing to acute exacerbation, decreased lung function, reduced 6-min walking distance, and decreased quality of life has been observed in COPD patients with anxiety or depression (13). Evidently, the relationship among COPD, anxiety, and depression is bidirectional (14). Anxiety and depression have adverse effects on the prognosis of COPD. COPD patients are at an increased risk of anxiety and depression owing to breathing difficulties, partial loss of social function, and economic burden (6). However, the reality is more intricate than commonly perceived.
In COPD, a chronic inflammatory disease, interleukin (IL)-6 and IL-1β (crucial airway inflammatory cytokines) play key roles in disease progression (15). He et al. confirmed that IL-6 accelerates the rate of FEV1 decline in COPD patients who smoke (16). Evidence from both animal and clinical studies suggests that elevated levels of the pro-inflammatory cytokine IL-6 in the peripheral blood or central nervous system play an important role in depression (17). Rizzo et al. induced depression-like behavior in mice through intraventricular injection of recombinant IL-6 (18). Long et al. confirmed a close correlation between serum IL-6 levels and depression in COPD patients (19). Zhang et al.’s study showed that the levels of IL-6, IL-8, IL-10, and tumor necrosis factor-α were significantly higher in the COPD group with depression than in the non-depression group (P < 0.05) (20).
COPD patients who experience both anxiety and depression are at increased risk of acute exacerbations, hospitalizations, and mortality (21, 22). Therefore, respiratory physicians aim to promptly identify COPD patients with psychiatric comorbidities, intervene timely, and improve prognosis. Traditional diagnostic criteria for psychiatric comorbidities are challenging to apply to these patients, often yielding low positivity rates, and many subthreshold mental health conditions are prone to misdiagnosis. The Diagnostic Criteria for Research in Psychology (DCPR) are considered supplementary tools for identifying subthresholds or unclassified psychiatry associated with physical diseases (23, 24). The revised version of the DCPR (DCPR-R) Semi-Structured Interview assesses 14 psychosomatic syndromes: health anxiety, disease phobia, hypochondriasis, thanatophobia, illness denial, persistent somatization, conversion symptoms, anniversary reaction, demoralization, irritable mood, somatic symptoms secondary to a psychiatric disorder, type A behavior, alexithymia, and allostatic overload (25–27).
However, the association between psychosomatic syndrome and pro-inflammatory factors such as IL-6, CRP, and PCT in the peripheral blood of acute exacerbation of chronic obstructive pulmonary disease (AECOPD) patients is still unclear. Considering the promoting role of IL-6 in depression among COPD patients, we hypothesize an intrinsic link between IL-6 and psychosomatic syndromes. Our aim was to identify high-risk COPD patients for psychosomatic syndromes as early through simple IL-6 detection, implement psychological interventions, improve patient prognosis, and alleviate disease burden.
2 Method
2.1 Study participants and consent to participate
AECOPD patients admitted to the Respiratory and Critical Care Medicine Department of Shanghai Jiading Central Hospital (Jiading District Central Hospital Affiliated Shanghai University of Health and Medicine) between March 1st, 2022 and May 1st, 2024 were considered for inclusion in this study. All participants provided written informed consent (As shown in Figure 1).
Figure 1

Research flowchart. DCPR, The Diagnostic Criteria for Psychosomatic Research; mMRC, modified Medical Research Council; CAT, COPD assessment test; AECOPD, Acute exacerbation of Chronic Obstructive Pulmonary Disease; IL-6, Interleukin 6; FEV1, Forced Expiratory Volume in 1 s; FEV1%pred, The percentage of predicted values of FEV1; PaO2, The partial pressure of arterial oxygen; PaCO2, The partial pressure of arterial carbon dioxide.
2.2 Ethical support
This study has been approved by the Research Ethics Committee of Shanghai Jiading Central Hospital (2022K05).
2.2 Inclusion criteria
According to the 2023 Global Initiative for Chronic Obstructive Lung Disease guidelines (28), patients are diagnosed with COPD if they meet the following criteria: (1) age ≥ 40 years old; (2) post-bronchodilator (salbutamol) inhalation, lung function FEV1/FVC<70%; (3) ability to read and write Chinese, and communicate effectively with healthcare providers; (4) no systemic glucocorticoids use in the past 4 weeks; (5) willingness to provide voluntary and signed informed consent.
2.3 Exclusion criteria
(1) Patients receiving long-term oral corticosteroid treatment (≥ 4 weeks); (2) patients with severe liver and kidney dysfunction, hematological malignancies, or other malignant tumors; (3) patients with intellectual disability, confusion, or difficulty writing.
2.4 Flowchart
2.4 Process and tools used
2.4.1 General data
Demographic and sociological data of the patients were collected, including age, sex, marital status, years of education, BMI, smoking history, alcohol consumption, presence of diabetes, and hypertension.
2.4.2 Psychosomatic medicine interviews
Experienced physicians, who had undergone systematic training and were familiar with the diagnostic criteria for psychosomatic medicine, conducted interviews with AECOPD patients included in the study using a semi-structured diagnostic tool for psychosomatic medicine (DCPR-R-SSI), which corresponded to the DCPR-R. The DCPR-R-SSI focuses on the preceding 6–12 months and comprises 79 items answered with yes/no (29). It assesses 14 psychosomatic syndromes across four diagnostic modules (stress, illness behavior, psychological manifestations, and personality). The stress module covers allostatic overload, representing the cumulative effects of stressful experiences. Illness behavior encompasses hypochondriasis, disease phobia, thanatophobia, health anxiety, persistent somatization, conversion symptoms, anniversary reactions, and illness denial. Psychological manifestations include demoralization, irritable mood, and secondary somatic symptoms. The personality module addresses type A behavior and alexithymia (27). Research conducted domestically substantiates the suitability of DCPR for clinical implementation within China (30, 31).
2.4.3 Determination of blood routine tests and levels of IL-6, albumin, prealbumin, PaO2, PaCO2
On the morning of admission, venous blood was collected from patients on an empty stomach using a disposable blood collection vessel was with EDTA as the anticoagulant. A total of 2 ml of blood was collected. Blood routine analysis was conducted using the French ABXPENTRA 120 DF automatic blood analyzer, which employs flow cytometry and impedance spectroscopy. Additionally, 3 ml of venous blood was collected on an empty stomach and centrifuged at 3000 rpm for 10 min. The serum was then collected for further analysis. A Chinese Hotgen fully automated chemiluminescence analyzer C3000 was used to detect IL-6 using a magnetic particle chemiluminescence immunoassay. The Abbott ARCHITECT C16000 fully automated biochemical analyzer was utilized to detect albumin employing the bromocresol green method, and prealbumin was detected using the immunoprojection turbidity method. On the day of admission, 1.5 ml of arterial blood was extracted from patients, and PaO2 and PaCO2 were detected using an ABL80 SC80 blood gas analyzer (Redumit, Denmark).
2.4.4 Pulmonary function measurement
According to the guidelines of the American Thoracic Society and European Respiratory Society, lung function tests were conducted using the JaegerMaster Screen lung function testing system for vital capacity, whole-body plethysmography, and pulmonary diffusion function measurements. The recording personnel were professionally trained physicians. The forced expiratory volume (FEV1/FVC), one-second rate (FEV1/FVC), and percentage of forced expiratory volume in one second to the expected value (FEV1% pred) were recorded.
2.4.5 Modified Medical Research Council
The mMRC scale is a self-rating tool used to measure the degree of disability caused by breathlessness in daily activities, rated on a scale from 0 to 4: 0, no breathlessness except with strenuous exercise; 1, shortness of breath when hurrying on the level or walking up a slight hill; 2, walks slower than people of same age on the level because of breathlessness or has to stop to catch breath when walking at their own pace on the level; 3, stops for breath after walking ∼100m or after few minutes on the level; and 4, too breathless to leave the house, or breathless when dressing or undressing (32).
2.4.6 COPD assessment test
CAT is a questionnaire designed to measure the health status of COPD patients. Eight statements assess the best- and worst-case scenarios for coughing, phlegm, chest tightness, breathlessness when going up hills/stairs, activity limitations at home, confidence in leaving home, sleep, and energy. Each statement is scored from 0 to 5 (best to worst), with the total score ranging from 0 to 40 (33).
2.5 Statistical analysis
This study builds upon prior research and employs G*Power software to determine the appropriate sample size. An effect size of 0.3 was utilized, with a statistical power of 0.8 and a significance level of 0.05. The calculated minimum sample size required was 180 participants. To account for a potential dropout rate of 10%, a total of 202 patients were ultimately recruited.
The experimental data were established in a database using Windows Excel software, and statistical analysis and plotting were performed using SPSS 26.0 and GraphPad Prism software (version 8.0) for data processing. Measurement data that conform to a normal distribution are represented by mean ± standard deviation (X ± S), while non-normal distribution measurement data are expressed as median and quartile range M (P25, P75). Classification data were analyzed using the chi-square test; quantitative data, t-test; and a logistic regression model, causal relationship between the DCPR and independent variables. We evaluated the diagnostic value of IL-6, mMRC, and CAT score in predicting psychosomatic syndrome in AECOPD patients using receiver operating characteristic (ROC) curves and calculated the area under the curve (AUC), Maximum Youden index, optimal threshold, sensitivity, and specificity. In all statistical analyses, a bidirectional 95% effective interval test was used, and a difference of p<0.05 was considered statistically significant.
3 Results
3.1 Demographic sociology and clinical data of AECOPD patients
Excluding three cases of inability to cooperate in completing psychosomatic interviews and four cases of inability to cooperate in completing lung function, 202 AECOPD patients were enrolled. Of which, 98 (48.5%) exhibited illness behavior; 37 (18.3%), allostatic overload; and 43 (21.3%), two or more psychosomatic syndromes (Table 1).
Table 1
| Demographic sociology | |
|---|---|
| Age, (mean (years) ± SD) | 75 ± 8.3 |
| Age range (years) | 40-94 |
| Sex (M/F) (N, %) | 176/26(87.1/12.9) |
| Marital status N (%) | |
| Married | 181 (89.6%) |
| Unmarried | 3 (1.5%) |
| Divorce | 5 (2.5%) |
| Bereave | 13 (6.4%) |
| Education status(mean (years) ± SD) | 5.5 ± 3.2 |
| Clinical | |
| Body mass index, (mean (kg/m2) ± SD) | 22.7 ± 3.9 |
| Smoking use N (%) | |
| Never | 89(44.1%) |
| Quitted | 61(30.2%) |
| Current | 52(25.7%) |
| Alcohol intake N (%) | |
| Never | 143(70.8%) |
| Quitted | 31(15.3%) |
| Current | 28(13.9%) |
| Type 2 diabetes N(%) | 36(17.8%) |
| Hypertension N(%) | 125(61.9%) |
| PaCO2(mean(mmHg)± SD) | 47.8 ± 13.1 |
| PaO2(mean(mmHg)± SD) | 96.4 ± 32.9 |
| White blood cell (109/L), (mean ± SD) | 8.1 ± 3.9 |
| Neutrophil count (109/L), (mean± SD) | 6.3 ± 3.7 |
| Neutrophil ratio (%), (mean ± SD) | 73.8 ± 11.8 |
| Lymphocyte count (109/L), (mean± SD) | 1.1 ± 0.6 |
| Lymphocyte ratio (%), (mean ± SD) | 15.7 ± 9.0 |
| Monocyte count (109/L), (mean± SD) | 0.7 ± 0.4 |
| Monocyte ratio (%), (mean± SD) | 8.6 ± 3.9 |
| IL-6 (mean (pg/ml) ± SD) | 20.9 ± 32.8 |
| Albumin (mean (g/l) ± SD) | 36.1 ± 5.2 |
| Prealbumin (mean (mg/l) ± SD) | 169.2 ± 70.6 |
| FEV1%pred, (mean± SD) | 41.6 ± 17.9 |
| FEV1/FVC (%), (mean± SD) | 53.5 ± 11.7 |
| mMRC (mean± SD) | 2.7 ± 1 |
| CAT-score (mean± SD) | 21.0 ± 8.1 |
| DCPR(+) | 144(71.3%) |
Demographic sociology and clinical data of AECOPD patients.
PaCO2, The partial pressure of arterial carbon dioxide; PaO2, The partial pressure of arterial oxygen; IL-6, Interleukin 6; FEV1, Forced Expiratory Volume in 1s; FEV1%pred, The percentage of predicted values of FEV1; FVC, Forced Vital Capacity (FVC); mMRC, modified Medical Research Council; DCPR, The Diagnostic Criteria for Psychosomatic Research.
3.2 Comparison of demographic, sociological, and clinical data of AECOPD patients between DCPR (+) and DCPR (-) groups
Among 202 AECOPD patients, 144 were in the DCPR (+) and 58 were in the DCPR (-) groups. The DCPR (+) group exhibited higher white blood cell counts, neutrophil counts, monocyte counts, IL-6, mMRC, and CAT scores than those of the DCPR (-) group, with values of ((8.5 ± 4.2) * 109/L vs. (7.2 ± 3.0) * 109/L, P = 0.026), ((6.7 ± 4.0) * 109/L vs. (5.3 ± 2.7) * 109/L, P = 0.005), ((0.7 ± 0.4) * 109/L vs. (0.6 ± 0.3) * 109/L, P = 0.036), ((27.3 ± 36.8) pg/ml vs. (4.7 ± 4.3) pg/ml, P < 0.0001), ((3 ± 0.9) vs. (2 ± 0.9), P < 0.0001), ((23.3 ± 7.5) vs. (15.1 ± 6.5), P < 0.0001). The DCPR (+) group had significantly lower albumin and prealbumin levels than those in the DCPR (-) group (35.6 ± 6.5). 5.6) g/L vs. (37.4 ± 3.9) g/L, P = 0.026), ( (161.6 ± 69.2) mg/L vs. (188.9 ± 71.0) mg/L, P = 0.014) (P < 0.05). Age, sex, marital status, years of education, BMI, smoking history, drinking history, diabetes, hypertension, PaCO2, PaO2, neutral ratio, lymphocyte count, monocyte ratio, FEV1% pred, and FEV1/FVC% were not significantly different between the two groups (P > 0.05; Table 2; Figure 2).
Table 2
| Variable | DCPR 144(+) | DCPR 58(-) | t-test/χ2 | P -values |
|---|---|---|---|---|
| Age (years) | 75.1 ± 7.9 | 75.0 ± 9.2 | t = -0.08 | 0.94 |
| Sex (M/F) | 129/15 | 47/11 | χ2 = 2.7 | 0.1 |
| Marital status N (Married/Unmarried/Divorce/Bereave) | 128/2/4/10 | 53/1/1/3 | χ2 = 0.45 | 0.93 |
| Education status (years) | 5.4 ± 3.1 | 5.6 ± 3.6 | t = 0.32 | 0.75 |
| Body mass index, (kg/m2) | 22.7 ± 3.9 | 22.7 ± 3.9 | t = 0.1 | 0.92 |
| Smoking use N (Never/Quitted/Current) | 60/48/36 | 29/13/16 | χ2 = 2.4 | 0.3 |
| Alcohol intake N (Never/Quitted/Current) | 101/23/20 | 42/8/8 | χ2 = 0.16 | 0.92 |
| Type 2 diabetes N | 27 | 9 | χ2 = 0.23 | 0.59 |
| Hypertension N | 88 | 37 | χ2 = 0.13 | 0.72 |
| PaCO2(mmHg) | 45.6 ± 13.8 | 45.8 ± 11.4 | t = -1.46 | 0.15 |
| PaO2(mmHg) | 96.4 ± 32.3 | 96.4 ± 34.6 | t = -0.001 | 1 |
| White blood cell (109/L) | 8.5 ± 4.2 | 7.2 ± 3.0 | t = -2.2 | 0.026 |
| Neutrophil count (109/L) | 6.7 ± 4.0 | 5.3 ± 2.7 | t = -2.9 | 0.005 |
| Neutrophil ratio (%) | 74.7 ± 11.7 | 71.7 ± 12.0 | t = -1.6 | 0.102 |
| Lymphocyte count (109/L) | 1.0 ± 0.5 | 1.1 ± 0.6 | t = 1.2 | 0.25 |
| Lymphocyte ratio (%) | 14.8 ± 8.9 | 18.1 ± 8.8 | t = 2.4 | 0.017 |
| Monocyte count (109/L) | 0.7 ± 0.4 | 0.6 ± 0.3 | t = -2.1 | 0.036 |
| Monocyte ratio (%) | 8.7 ± 3.6 | 8.4 ± 4.4 | t = -0.49 | 0.63 |
| IL-6 (pg/ml) | 27.3 ± 36.8 | 4.7 ± 4.3 | t = -7.24 | <0.0001 |
| Albumin (g/l) | 35.6 ± 5.6 | 37.4 ± 3.9 | t = 2.2 | 0.026 |
| Prealbumin (mg/l) | 161.6 ± 69.2 | 188.9 ± 71.0 | t = 2.5 | 0.014 |
| FEV1/%pred | 40.0 ± 17.7 | 45.5 ± 18.2 | t = 2.0 | 0.051 |
| FEV1/FVC (%) | 52.5 ± 11.7 | 55.9 ± 11.6 | t = 1.9 | 0.062 |
| mMRC | 3 ± 0.9 | 2 ± 0.9 | t = -6.7 | <0.0001 |
| CAT-score | 23.3 ± 7.5 | 15.1 ± 6.5 | t = -7.3 | <0.0001 |
Comparison of demographic, sociological, and clinical data of AECOPD patients between DCPR (+) and DCPR (-) groups.
PaCO2, The partial pressure of arterial carbon dioxide; PaO2, The partial pressure of arterial oxygen; IL-6: Interleukin 6; FEV1, Forced Expiratory Volume in 1s; FEV1%pred, The percentage of predicted values of FEV1; FVC, Forced Vital Capacity (FVC); mMRC, modified Medical Research Council; CAT, COPD assessment test.
Figure 2
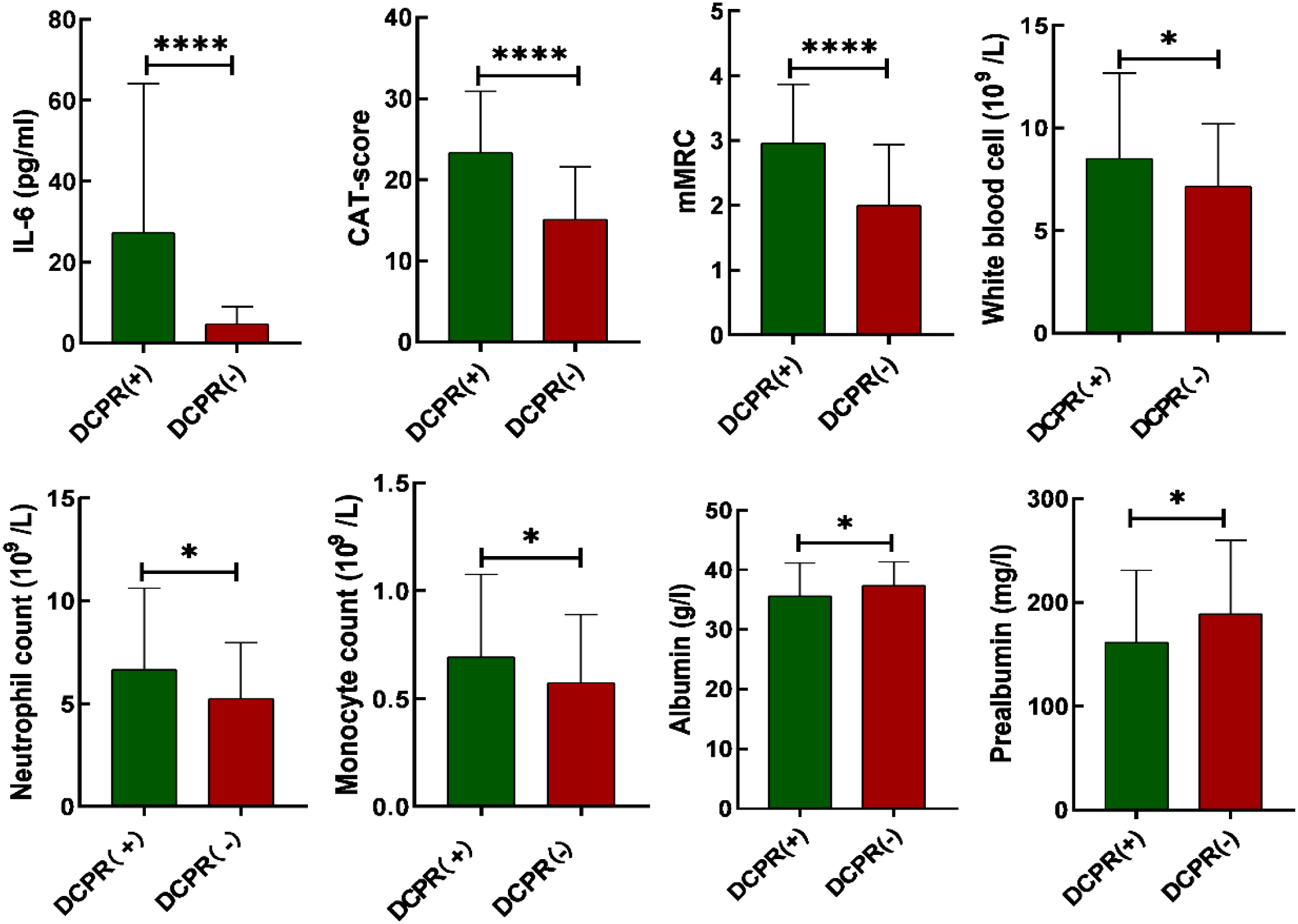
Comparison of bar charts illustrating IL-6, CAT scores, mMRC, white blood cell count, neutrophil count, monocyte count, albumin and prealbumin between patients with DCPR (+) and DCPR (-). DCPR, The Diagnostic Criteria for Psychosomatic Research; IL-6, Interleukin 6; CAT, COPD assessment test; mMRC, modified Medical Research Council; *P < 0.05, ****P < 0.0001.
3.3 Multivariate binary logistic regression analysis of risk factors for DCPR in AECOPD patients
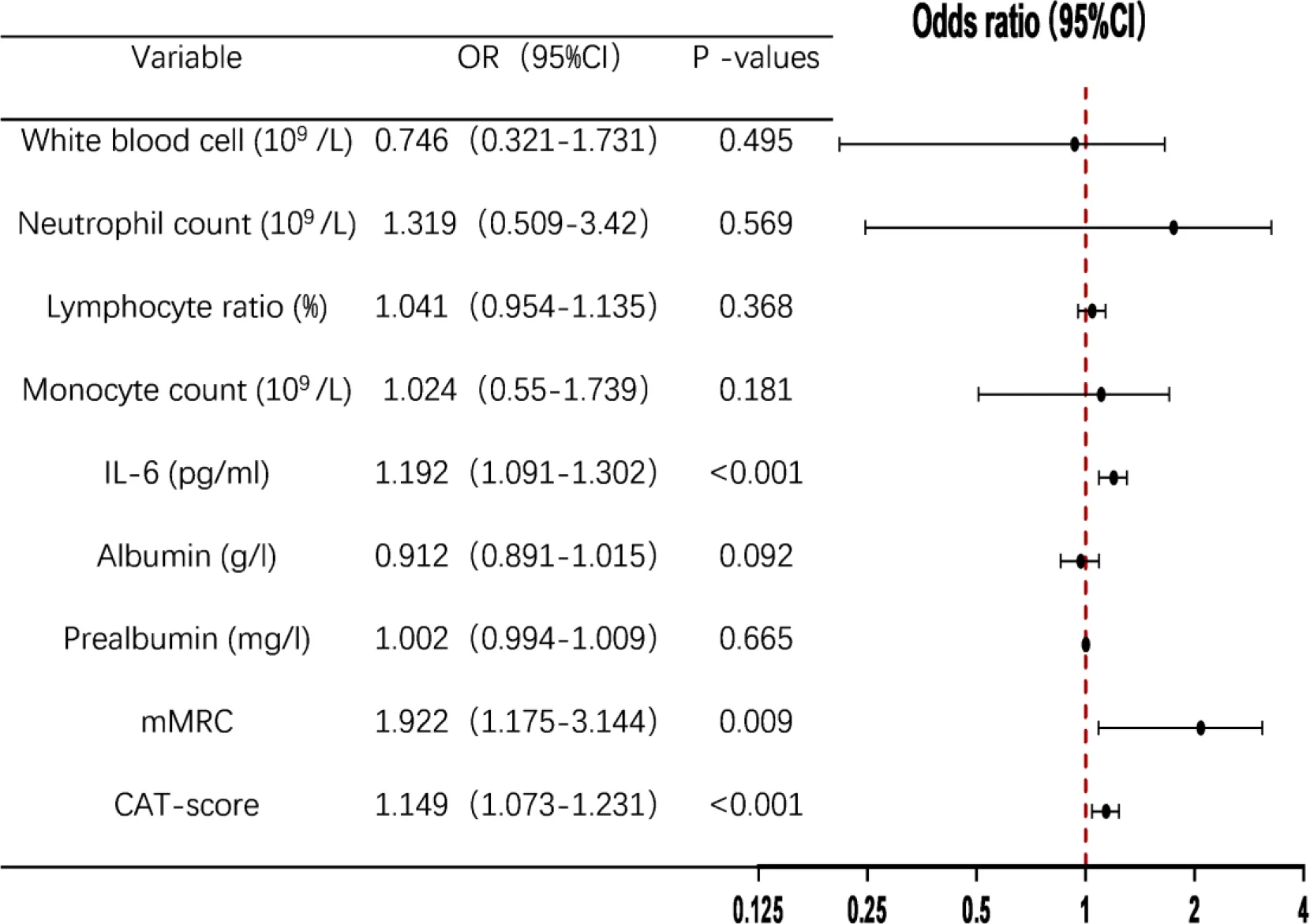
The 202 AECOPD patients were divided into DCPR (+) and DCPR (-) groups, with psychosomatic syndrome as the binary dependent variable and white blood cell count, neutrophil count, lymphocyte count, monocyte percentage, IL-6, albumin, prealbumin, mMRC, and CAT scores as independent variables. A multivariate binary logistic regression analysis was conducted, and the results showed that IL-6, mMRC, and CAT score were independent risk factors for psychosomatic syndrome in AECOPD patients, with OR=1.192 (95% CI: 1.091-1.302), P < 0.001), OR=1.922 (95% CI: 1.175-3.144), P = 0.009), OR=1.149 (95% CI: 1.073-1.231), P < 0.001), as shown in Table 3.
Table 3
|
Multivariate binary logistic regression analysis of risk factors for DCPR.
IL-6, Interleukin 6; mMRC, modified Medical Research Council; CAT, COPD assessment test; OR, Odds Ratio; CI, Confidence Interval.
3.4 Analysis of predictive factors for psychosomatic syndrome in AECOPD
Multivariate binary logistic regression analysis showed that IL-6, mMRC, and CAT scores were predictive factors for psychosomatic syndrome, with good sensitivity and specificity, as shown in Figure 3; Table 4.
Figure 3

ROC curves for predicting DCPR with IL-6, mMRC, and CAT. ROC, Receiver Operating Characteristic Curve; IL-6, Interleukin 6; mMRC, modified Medical Research Council; CAT, COPD assessment test.
Table 4
| Characteristic | AUC(95%CI) | Maximum youden index | Optimal critical value | Sensitivity% | Specific% |
|---|---|---|---|---|---|
| IL-6 mMRC CAT-score |
0.834(0.778-0.89) 0.755(0.683-0.827) 0.789(0.722-0.856) |
0.575 0.374 0.443 |
7.9 2.5 17.5 |
68.1 66.7 73.6 |
89.5 70.7 70.7 |
Analysis of ROC curves for IL-6, mMRC, and CAT scores in predicting DCPR.
ROC, Receiver Operating Characteristic Curve; IL-6, Interleukin 6; mMRC, modified Medical Research Council; CAT, COPD assessment test; AUC, Area Under Curve; CI, Confidence Interval.
4 Discussion
Research has confirmed that psychiatric comorbidities are prevalent in AECOPD patients. Among the 202 AECOPD patients enrolled in this study, more than half (144 cases, 71.3%) had psychosomatic syndromes. Specifically, 98 (48.5%) exhibited illness behavior; 37 (18.3%), allostatic overload; and 43 (21.3%), two or more psychosomatic syndromes. The incidence rates can vary depending on the interview skills of clinical doctors and the specific evaluation tools. Through this observational study, we concluded that there is an inherent connection between AECOPD and psychosomatic syndromes, mainly manifested through immune responses triggered by inflammatory factors and symptom burden experienced by these patients. However, to date, the literature on risk factors, such as inflammatory markers and symptom burden, is still limited, which is addressed by our study. We investigated the correlation between AECOPD patients with psychosomatic syndrome and inflammatory factors such as IL-6, white blood cell count, and neutrophil count. We also examined symptoms such as mMRC and CAT scores and their assessed their predictive value for psychosomatic syndrome.
The pathophysiology of COPD and its psychiatric comorbidities are complex. In recent years, many studies have confirmed that inflammatory factors, such as IL-6, white blood cell count, and CRP, play a more significant role in psychiatric comorbidities (34). IL-6 is a pro-inflammatory cytokine that represents the level of inflammatory response in the body. Peripheral pro-inflammatory cytokines enter the central nervous system through humoral, neural, and cellular pathways. Inflammatory factors entering the brain supposedly mainly affect it through the following pathways, leading to psychiatric comorbidities. 1. IL-6 intensifies the stress response by modulating the hypothalamic-pituitary-adrenal (HPA) axis. Specifically, IL-6 directly stimulates the release of corticotropin-releasing hormone (CRH) from neurons located in the paraventricular nucleus (PVN) of the hypothalamus, which in turn facilitates the secretion of adrenocorticotropic hormone (ACTH) from the pituitary gland and cortisol from the adrenal glands (35). Prolonged elevated levels of cortisol subsequently diminish the expression of brain-derived neurotrophic factor (BDNF), resulting in the inhibition and atrophy of the hippocampal nervous system, thereby impairing cognitive and emotional regulatory functions (36, 37). 2. IL-6 Disrupts Tryptophan Metabolism and Neurotransmitter Synthesis, Impacting Emotional Regulation Pathways. IL-6 promotes the expression of indoleamine 2,3-dioxygenase (IDO), which shifts tryptophan metabolism towards the kynurenine (KYN) pathway rather than serotonin (5-HT) synthesis, consequently reducing synaptic 5-HT levels. Furthermore, IL-6 impedes glutamate uptake by astrocytes while activating NMDA receptors, resulting in excitotoxic damage to postsynaptic neurons (38). 3. Central interleukin-6 (IL-6) plays a pivotal role in the activation of microglia, thereby initiating a neuroinflammatory cascade. Upon activation, microglia secrete cytokines, including tumor necrosis factor-alpha (TNF-α) and interleukin-1 beta (IL-1β), which serve to further amplify inflammatory signaling and disrupt synaptic plasticity (39). Concurrently, IL-6 enhances the production of reactive oxygen species (ROS) via the NADPH oxidase pathway, culminating in mitochondrial dysfunction and neuronal apoptosis (40). Evidence from animal models indicates that IL-6 overexpression can inhibit the proliferation of neural stem cells within the dentate gyrus and adversely affect the capacity for emotional recovery (41).
In cases where chronic obstructive pulmonary disease (COPD) is accompanied by hypoxemia, persistent hypoxia upregulates interleukin-6 (IL-6) expression via the hypoxia-inducible factor 1-alpha (HIF-1α) pathway, concurrently exacerbating oxidative stress and mitochondrial impairment within cerebral tissues (42). During acute exacerbations of COPD, there is a marked escalation in pulmonary inflammation, resulting in a rapid surge in IL-6 levels that surpasses the protective threshold of the blood-brain barrier, thereby precipitating central nervous system inflammation (37, 43). IL-6 contributes to the pathogenesis of psychiatric disorders by influencing the hypothalamic-pituitary-adrenal (HPA) axis, altering the metabolism of monoamine neurotransmitters, and promoting neuroinflammatory processes. Current research on psychiatric inflammation suggests that immune system disorders caused by infections promote psychiatric comorbidities (35). Stroll et al. found that elevated plasma IL-6 levels are associated with depressive symptoms in COPD patients and that systemic inflammation may play an important bidirectional role in COPD-related depression (44). Chan et al. confirmed that systemic inflammation leads to an increase in monocytes, neutrophils, and lymphocytes in the blood, thereby upregulating pro-inflammatory processes in immune-related peripheral blood, which contributes to the development of psychiatric comorbidities (45); this was confirmed in the present study. We analyzed the laboratory data of the DCPR (+) and DCPR (-) groups and found that the white blood cell count, neutrophil count, monocyte count, and IL-6 levels in the DCPR (+) group were higher than those in the DCPR (-) group: ((8.5 ± 4.2) * 109/L vs. (7.2 ± 3.0) * 109/L, P = 0.026), ((6.7 ± 4.0) * 109/L vs. (5.3 ± 2.7) * 109/L, P = 0.005), ((0.7 ± 3.0) * 109/L, P = 0.026), (0.4) * 109/L vs. (0.6 ± 0.3) * 109/L, P = 0.036), ((27.3 ± 36.8) pg/ml vs. (4.7 ± 4.3) pg/ml, P = 0.005), and the difference was statistically significant (<0.05). The percentage of lymphocytes in the DCPR (+) group was lower than that in the DCPR (-) group: ((14.8 ± 8.9) % vs. (18.1 ± 8.8) %, P = 0.017), and the difference was statistically significant (P < 0.05; Table 2, Figure 2). After adjusting for confounding factors such as sex, age, comorbidities, smoking, and alcohol consumption, IL-6 was ultimately identified as an independent risk factor for psychosomatic syndrome (OR 1.192, 95% CI 1.091-1.302, P < 0.001) and an important predictor (AUC = 0.834; 95% CI 0.778-0.89), with a sensitivity of 68.1% and a specificity of 89.5% (Tables 3 and 4, and Figure 3). However, actually, comorbidities such as type 2 diabetes (T2DM) and hypertension may worsen psychosomatic syndromes through IL-6 and other inflammatory markers (46, 47). This influence will be further studied in the future research.
Inflammation not only plays a crucial role in the comorbidity of psychosomatic syndrome in COPD but also contributes to decreased quality of life also and increased symptom burden, thereby promoting the occurrence of psychiatric comorbidities (48). The symptoms of COPD are primarily characterized by breathing difficulties, and the clinical evaluation of the severity of these difficulties in patients is conducted using the mMRC scale (32). Quality of life mainly includes coughing and sputum production, ability to perform household chores, confidence in going out, sleep, and energy status. CAT is used to clinically evaluate the degree of impact on quality of life (33). A study by Wu et al. confirmed that psychiatric comorbidities in COPD patients are closely related to their subjective symptoms, such as difficulty in breathing, coughing, phlegm, and sleep, and there is no significant correlation with objective indicators such as FEV1 (48). Poor subjective emotional feelings of patients lead to a higher incidence of psychiatric comorbidities in COPD (6). Long (49) et al. found that the CAT score was an independent risk factor and a significant predictive factor for AECOPD patients with mental illness (AUC=0.790, 95% CI 0.740-0.834). Increasing evidence shows that the burden of symptoms (including cough, expectoration, wheezing, and chest tightness) has a significant adverse impact on health status, quality of life, and daily activities, leading to an increase in the incidence rate of psychosomatic syndrome and a worse prognosis of the disease (50). Our study confirmed that the mMRC and CAT scores of the DCPR (+) group were higher than those of the DCPR (-) group ((3 ± 0.9) vs. (2 ± 0.9), P < 0.001), ((23.3 ± 7.5) vs. (15.1 ± 6.5), P < 0.001), and the differences were statistically significant (P < 0.05), while there was no statistically significant difference in FEV1% pred between the two groups (Table 2, Figure 2). Multivariate binary logistic regression showed that the mMRC and CAT scores were independent risk factors for psychosomatic syndrome ((OR 1.922, 95% CI 1.175-3.144), (OR 1.149, 95% CI 1.073-1.231)) and important predictive factors ((AUC=0.755, 95% CI 0.683-0.827) (AUC=0.789, 95% CI 0.722-0.856)), respectively (Tables 3 and 4, Figure 3). This finding is consistent with those of the previous studies.
The levels of albumin and prealbumin in the DCPR (+) group were lower than those in the DCPR (-) group: (35.6 ± 5.6) g/L vs. (37.4 ± 3.9) g/L, P = 0.026), ((161.6 ± 69.2) mg/L vs. (188.9 ± 71.0) mg/L, P = 0.014), with statistically significant difference (Table 2; P < 0.05). White matter is an indirect indicator of inflammation, and its synthesis in the liver is inhibited in presence of systemic inflammation (51). Albumin and prealbumin are not used to evaluate nutritional status but rather represent the level of inflammation in the body. Therefore, a decrease in albumin and prealbumin levels indicates occurrence and development of psychosomatic syndromes.
The present study had several limitations. 1.The sample utilized in this study was derived from a single center at Jiading Central Hospital in Shanghai. To enhance the generalizability of the findings, future research should incorporate multi-center and multi-population studies. 2.This investigation employs a cross-sectional observational design, which allows for the identification of correlations between IL-6 levels, symptom burden (as measured by CAT/mMRC), and psychosomatic syndrome, but does not permit the establishment of causal relationships. Therefore, further validation through longitudinal cohort studies or intervention trials is warranted in future research endeavors.
5 Conclusions
Our study indicates that elevated levels of peripheral blood leukocytes, neutrophils, lymphocytes, and IL-6, along with decreased monocytes, in AECOPD patients are associated with the development of psychiatric comorbidities. Moreover, subjective symptoms such as cough, phlegm, sleep disturbances, confidence in going out, and difficulty breathing in AECOPD patients contribute more to psychiatric comorbidities than objective indicators such as FEV1.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This study has been approved by the Research Ethics Committee of Shanghai Jiading Central Hospital (2022K05). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
WZ: Data curation, Formal Analysis, Writing – original draft. YC: Data curation, Investigation, Writing – review & editing. LS: Data curation, Software, Writing – review & editing. CT: Data curation, Formal Analysis, Writing – review & editing. YY: Software, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the Chronic Obstructive Pulmonary Disease (COPD) Screening, Diagnosis and Treatment, and Network-based Management – Clinical Specialty Capacity Enhancement Training Project (Hospital-level, grant number JZXLCZK-2024-06), and Jiading District Health Commission of Shanghai for a study investigating the diversity of airway microbiota in patients with frequent acute exacerbations of chronic obstructive pulmonary disease (COPD) (grant number 2021-QN-3).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AECOPD, Acute exacerbation of Chronic Obstructive Pulmonary Disease; DCPR, The Diagnostic Criteria for Psychosomatic Research; mMRC, modified Medical Research Council; CAT, COPD assessment test; IL-6, Interleukin 6; FEV1, Forced Expiratory Volume in 1 s; FEV1%pred, The percentage of predicted values of FEV1; FVC, Forced Vital Capacity; PaO2, The partial pressure of arterial oxygen; PaCO2, The partial pressure of arterial carbon dioxide.
References
1
Singh D Agusti A Anzueto A Barnes PJ Bourbeau J Celli BR et al . Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease: the GOLD science committee report 2019. Eur Respir J. (2019) 53:1900164. doi: 10.1183/13993003.00164-2019
2
Yang IA Jenkins CR Salvi SS . Chronic obstructive pulmonary disease in never-smokers: risk factors, pathogenesis, and implications for prevention and treatment. Lancet Respir Med. (2022) 10:497–511. doi: 10.1016/S2213-2600(21)00506-3
3
GBD Chronic Respiratory Disease Collaborators . Prevalence and attributable health burden of chronic respiratory diseases, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir Med. (2020) 8:585–96. doi: 10.1016/S2213-2600(20)30105-3
4
Malerba M Nardin M Radaeli A Montuschi P Carpagnano GE Clini E . The potential role of endothelial dysfunction and platelet activation in the development of thrombotic risk in COPD patients. Expert Rev Hematol. (2017) 10:821–32. doi: 10.1080/17474086.2017.1353416
5
Horne BD Hegewald MJ Crim C Rea S Bair TL Blagev DP . The summit score stratifies mortality and morbidity in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. (2020) 15:1741–50. doi: 10.2147/COPD.S254437
6
Volpato E Toniolo S Pagnini F Banfi P . The relationship between anxiety, depression and treatment adherence in chronic obstructive pulmonary disease: A systematic review. Int J Chron Obstruct Pulmon Dis. (2021) 16:2001–21. doi: 10.2147/COPD.S313841
7
Matte DL Pizzichini MM Hoepers AT Diaz AP Karloh M Dias M et al . Prevalence of depression in COPD: A systematic review and meta-analysis of controlled studies. Respir Med. (2016) 117:154–61. doi: 10.1016/j.rmed.2016.06.006
8
Zhang MW Ho RC Cheung MW Fu E Mak A . Prevalence of depressive symptoms in patients with chronic obstructive pulmonary disease: a systematic review, meta-analysis and meta-regression. Gen Hosp Psychiatry. (2011) 33:217–23. doi: 10.1016/j.genhosppsych.2011.03.009
9
Pelgrim CE Peterson JD Gosker HR Schols AMWJ van Helvoort A Garssen J et al . Psychological co-morbidities in COPD: Targeting systemic inflammation, a benefit for both? Eur J Pharmacol. (2019) 842:99–110. doi: 10.1016/j.ejphar.2018.10.001
10
Rahi MS Thilagar B Balaji S Prabhakaran SY Mudgal M Rajoo S et al . The impact of anxiety and depression in chronic obstructive pulmonary disease. Adv Respir Med. (2023) 91:123–34. doi: 10.3390/arm91020011
11
Martínez-Gestoso S García-Sanz MT Carreira JM Salgado FJ Calvo-Álvarez U Doval-Oubiña L et al . Impact of anxiety and depression on the prognosis of copd exacerbations. BMC Pulm Med. (2022) 22:169. doi: 10.1186/s12890-022-01934-y
12
García Sanz MT González Barcala FJ . COPD is more than just lung function: let’s not forget depression. Arch Bronconeumol (Engl Ed). (2021) 57:519–20. doi: 10.1016/j.arbres.2021.03.013
13
Li Z Liu S Wang L Smith L . Mind-body exercise for anxiety and depression in COPD patients: A systematic review and meta-analysis. Int J Environ Res Public Health. (2019) 17:22. doi: 10.3390/ijerph17010022
14
Ng TP Niti M Tan WC Cao Z Ong KC Eng P . Depressive symptoms and chronic obstructive pulmonary disease: effect on mortality, hospital readmission, symptom burden, functional status, and quality of life. Arch Intern Med. (2007) 167:60–7. doi: 10.1001/archinte.167.1.60
15
Xie ZK Huang QP Huang J Xie ZF . Association between the IL1B, IL1RN polymorphisms and COPD risk: a meta-analysis. Sci Rep. (2014) 4:6202. doi: 10.1038/srep06202
16
He JQ Foreman MG Shumansky K Zhang X Akhabir L Sin DD et al . Associations of IL6 polymorphisms with lung function decline and COPD. Thorax. (2009) 64:698–704. doi: 10.1136/thx.2008.111278
17
Ting EY Yang AC Tsai SJ . Role of interleukin-6 in depressive disorder. Int J Mol Sci. (2020) 21:2194. doi: 10.3390/ijms21062194
18
Sukoff Rizzo SJ Neal SJ Hughes ZA Beyna M Rosenzweig-Lipson S Moss SJ et al . Evidence for sustained elevation of IL-6 in the CNS as a key contributor of depressive-like phenotypes. Transl Psychiatry. (2012) 2:e199. doi: 10.1038/tp.2012.120
19
Long J Xu P Chen J Liao J Sun D Xiang Z et al . Inflammation and comorbidities of chronic obstructive pulmonary disease: The cytokines put on a mask! Cytokine. (2023) 172:156404. doi: 10.1016/j.cyto.2023.156404
20
Zhang T Wang G Li Q Yan P Sun J Jin Y . Relationship between serum Th1/Th2 imbalance and depression in elderly patients with COPD and its clinical implications. Technol Health Care. (2023) 31:2047–58. doi: 10.3233/THC-230665
21
Pooler A Beech R . Examining the relationship between anxiety and depression and exacerbations of COPD which result in hospital admission: a systematic review. Int J Chron Obstruct Pulmon Dis. (2014) 9:315–30. doi: 10.2147/COPD.S53255
22
Abrams TE Vaughan-Sarrazin M van der Weg MW . Acute exacerbations of chronic obstructive pulmonary disease and the effect of existing psychiatric comorbidity on subsequent mortality. Psychosomatics. (2011) 52:441–9. doi: 10.1016/j.psym.2011.03.005
23
Schröder A Fink P . The proposed diagnosis of somatic symptom disorders in DSM-V: two steps forward and one step backward? J Psychosom Res. (2010) 68:95–6; author reply 99-100. doi: 10.1016/j.jpsychores.2009.06.013
24
Porcelli P Guidi J . The clinical utility of the diagnostic criteria for psychosomatic research: A review of studies. Psychother Psychosom. (2015) 84:265–72. doi: 10.1159/000430788
25
Altamura M Porcelli P Balzotti A Massaro CR Bellomo A . Influence of DCPR syndromes in the psychosocial functioning of patients with major depressive and bipolar disorders. Psychother Psychosom. (2015) 84:387–8. doi: 10.1159/000437148
26
Altamura M D’Andrea G Angelini E Tortorelli FMP Balzotti A Porcelli P et al . Psychosomatic syndromes are associated with IL-6 pro-inflammatory cytokine in heart failure patients. PloS One. (2022) 17:e0265282. doi: 10.1371/journal.pone.0265282
27
Fava GA Cosci F Sonino N . Current psychosomatic practice. Psychother Psychosom. (2017) 86:13–30. doi: 10.1159/000448856
28
Agustí A Celli BR Criner GJ Halpin D Anzueto A Barnes P et al . Global initiative for chronic obstructive lung disease 2023 report: GOLD executive summary. Eur Respir J. (2023) 61:2300239. doi: 10.1183/13993003.00239-2023
29
Mansueto G Romanazzo S Cosci F . Clinical utility of the Diagnostic Criteria for Psychosomatic Research for a comprehensive assessment of the elderly. Clin Psychol Psychother. (2022) 29:1963–71. doi: 10.1002/cpp.2766
30
Liao S Chen I Tu C Hsu C Ma H Lee M et al . Subsyndromal psychosomatic concepts and personality traits in community adults. Compr Psychiatry. (2017) 75:110–6. doi: 10.1016/j.comppsych.2017.03.002
31
Xu W Jiang W Ding R Tao H Wang Y Tang Y et al . Study of rates and factors associated to psychosomatic syndromes assessed using the diagnostic criteria for psychosomatic research across different clinical settings. Psychother Psychosom. (2024) 93:386–96. doi: 10.1159/000541404
32
Bestall JC Paul EA Garrod R Garnham R Jones PW Wedzicha JA . Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. (1999) 54:581–6. doi: 10.1136/thx.54.7.581
33
Jones PW Harding G Berry P Wiklund I Chen WH Kline Leidy N . Development and first validation of the COPD Assessment Test. Eur Respir J. (2009) 34:648–54. doi: 10.1183/09031936.00102509
34
Mohammadi S Keshteli AH Saneei P Afshar H Esmaillzadeh A Adibi P . The relationship between linoleic acid intake and psychological disorders in adults. Front Nutr. (2022) 9:841282. doi: 10.3389/fnut.2022.841282
35
Miller AH Raison CL . The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. (2016) 16:22–34. doi: 10.1038/nri.2015.5
36
Mondelli V Pariante CM Navari S Aas M D’Albenzio A Di Forti M et al . Higher cortisol levels are associated with smaller left hippocampal volume in first-episode psychosis. Schizophr Res. (2010) 119:75–8. doi: 10.1016/j.schres.2009.12.021
37
Chesnokova V Pechnick RN Wawrowsky K . Chronic peripheral inflammation, hippocampal neurogenesis, and behavior. Brain Behav Immun. (2016) 58:1–8. doi: 10.1016/j.bbi.2016.01.017
38
Felger JC Treadway MT . Inflammation effects on motivation and motor activity: role of dopamine. Neuropsychopharmacology. (2017) 42:216–41. doi: 10.1038/npp.2016.143
39
Yirmiya R Rimmerman N Reshef R . Depression as a microglial disease. Trends Neurosci. (2015) 38:637–58. doi: 10.1016/j.tins.2015.08.001
40
Beurel E Toups M Nemeroff CB . The bidirectional relationship of depression and inflammation: double trouble. Neuron. (2020) 107:234–56. doi: 10.1016/j.neuron.2020.06.002
41
Zunszain PA Anacker C Cattaneo A Choudhury S Musaelyan K Myint AM et al . Interleukin-1β: a new regulator of the kynurenine pathway affecting human hippocampal neurogenesis. Neuropsychopharmacology. (2012) 37:939–49. doi: 10.1038/npp.2011.277
42
Suresh MV Balijepalli S Solanki S Aktay S Choudhary K Shah YM et al . Hypoxia-inducible factor 1α and its role in lung injury: adaptive or maladaptive. Inflammation. (2023) 46:491–508. doi: 10.1007/s10753-022-01769-z
43
Hurst JR Perera WR Wilkinson TM Donaldson GC Wedzicha JA . Systemic and upper and lower airway inflammation at exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. (2006) 173:71–8. doi: 10.1164/rccm.200505-704OC
44
Strollo HC Nouraie SM Hoth KF Riley CM Karoleski C Zhang Y et al . Association of systemic inflammation with depressive symptoms in individuals with COPD. Int J Chron Obstruct Pulmon Dis. (2021) 16:2515–22. doi: 10.2147/COPD.S322144
45
Chan KL Poller WC Swirski FK Russo SJ . Central regulation of stress-evoked peripheral immune responses. Nat Rev Neurosci. (2023) 24:591–604. doi: 10.1038/s41583-023-00729-2
46
Corlateanu A Covantev S Scutaru E Rusu D Botnaru V Corlateanu O et al . COPD and comorbidities in the republic of Moldova. Eurasian J Pulmonol. (2022) 24:9–17. doi: 10.14744/ejop_78_21
47
Negro Dal RW Bonadiman L Turco P . Prevalence of different comorbidities in COPD patients by gender and GOLD stage. Multidiscip Respir Med. (2015) 10:24. doi: 10.1186/s40248-015-0023-2
48
Wu D Zhao X Huang D Dai Z Chen M Li D et al . Outcomes associated with comorbid anxiety and depression among patients with stable COPD: A patient registry study in China. J Affect Disord. (2022) 313:77–83. doi: 10.1016/j.jad.2022.06.059
49
Long J Ouyang Y Duan H Xiang Z Ma H Ju M et al . Multiple factor analysis of depression and/or anxiety in patients with acute exacerbation chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. (2020) 15:1449–64. doi: 10.2147/COPD.S245842
50
Miravitlles M Ribera A . Understanding the impact of symptoms on the burden of COPD. Respir Res. (2017) 18:67. doi: 10.1186/s12931-017-0548-3
51
McFarland DC Applebaum AJ Bengtsen E Alici Y Breitbart W Miller AH et al . Potential use of albumin and neutrophil-to-lymphocyte ratio to guide the evaluation and treatment of cancer-related depression and anxiety. Psychooncology. (2022) 31:306–15. doi: 10.1002/pon.5811
Summary
Keywords
COPD, IL-6, MMRC, cat, DCPR
Citation
Zhang W, Cui Y, Sun L, Tu C and Yu Y (2025) Correlation between serum inflammatory factors and psychosomatic syndrome in patients with chronic obstructive pulmonary disease: an observational study. Front. Psychiatry 16:1586399. doi: 10.3389/fpsyt.2025.1586399
Received
02 March 2025
Accepted
18 June 2025
Published
10 July 2025
Volume
16 - 2025
Edited by
Lamyae Benzakour, University of Geneva, Switzerland
Reviewed by
Serghei Covantsev, National Medical Research Treatment and Rehabilitation Centre of the Ministry of Health of Russia, Russia
Kasper Sipowicz, The Maria Grzegorzewska University, Poland
Updates
Copyright
© 2025 Zhang, Cui, Sun, Tu and Yu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanfang Yu, wm2779@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.