- 1Department of Psychiatry and Biobehavioral Sciences, University of California, Los Angeles, Los Angeles, CA, United States
- 2Department of Psychology, Marquette University, Milwaukee, WI, United States
- 3Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, Baltimore, MD, United States
- 4Department of Psychiatry and Behavioral Sciences, Stanford University, Stanford, CA, United States
- 5Department of Psychology, Suffolk University, Boston, MA, United States
- 6Department of Psychiatry, Weill Cornell Medicine, New York, NY, United States
- 7Greenwich Anxiety, Greenwich, CT, United States
- 8Pritzker Department of Psychiatry and Behavioral Health, Ann and Robert H. Lurie Children's Hospital of Chicago, Chicago, IL, United States
Background: Sleep disturbance is common in individuals with Tourette’s disorder (TD). Tic symptoms, medication, functional impairment, and psychiatric comorbidity frequently contribute to sleep disturbance in children and adults with TD. However, long-term predictors of sleep disturbance in TD are not known. This study examined longitudinal and cross-sectional predictors of sleep disturbance in a treatment follow-up sample with TD.
Methods: Eighty subjects who completed a 10-week randomized controlled trial of behavior therapy for tics in childhood (Mage = 11.47, SD = 2.42 years) participate in follow-up evaluation on average, 11.17 (SD = 1.25) years after post-treatment assessment (Mage = 22.87, SD = 2.70 years). At post-treatment (10-week) and long-term follow-up, an independent evaluator assessed tic severity and tic-related impairment using the Yale Global Tic Severity Scale. Parents provided demographic and medical history (e.g., tic medication and stimulant medication status) and rated ADHD severity. Children rated anxiety and depression. At follow-up, participants rated anxiety, depression, and ADHD severity, and reported tic and stimulant medication status. Multiple linear regression was performed to examine longitudinal and cross-sectional predictors of sleep disturbance (Pittsburgh Sleep Quality Index) at long-term follow-up.
Results: tic-related impairment (β = .34, p = .014) at post-treatment positively predicted sleep disturbance at follow-up. Chronological age (β = .21, p = .041), anxiety severity (β = .40, p = .001), and ADHD severity (β = .31, p = .010) were positive cross-sectional predictors of sleep disturbance at follow-up.
Conclusion: Results highlight the role of residual tic-related impairment following behavior therapy for tics delivered in childhood in addition to older age, anxiety severity, and ADHD severity in early adulthood in sleep disturbance in a treatment follow-up sample of adults with TD.
1 Introduction
Tourette’s disorder (TD) is a neurological condition marked by abrupt, repeated, involuntary movements (i.e., motor tics) and vocalizations (i.e., vocal tics) present for beyond one year (1). Tics typically first emerge between 4 and 8 years of age, with symptoms peaking in severity around 10 to 12 years, and thereafter waning during adolescence for most (2, 3). Though up to eighty percent of children experience a significant reduction in tic severity to mild levels or lower by early adulthood, twenty percent continue to experience clinically significant symptoms in adulthood - with females more likely to follow this non-remitting course (2, 4, 5). Thus, TD is more prevalent in males than females (ratio of 4:1) in childhood, though the sex ratio is less skewed toward male prevalence in adulthood (6).
TD exacts a significant toll on functioning and quality of life in children and adults, with psychiatric comorbidity exacerbating outcomes (5, 7). Psychiatric comorbidities are present in 85% of individuals with TD; attention-deficit/hyperactivity disorder (ADHD) is the most common co-occurring psychiatric condition, with obsessive-compulsive disorder (OCD), depression, and anxiety disorders also being prevalent (3). Comprehensive behavioral intervention for tics (CBIT) is a first-line intervention for TD (8), though just under half of children (48%) fail to exhibit positive treatment response and it showed limited benefit for co-occurring psychiatric symptoms and psychosocial functioning following acute treatment (9). Alpha-2 adrenergic agonists and antipsychotic medications are also efficacious and commonly prescribed (8), though a common side effect of alpha-2-agonists is sedation (10), and antipsychotic medications are associated with weight gain, sedation, metabolic syndrome, cognitive problems, and extrapyramidal symptoms, and to a lesser extent – insomnia (11, 12). Stimulant medications are frequently administered to address ADHD symptoms in patients with TD as well (13), but are commonly associated with negative side effects, such as reduced appetite, headache, stomach pain, insomnia, and sleep disorder (14, 15).
Sleep disturbance has received limited attention in TD. Sleep problems are prevalent at a rate of 34% in individuals with TD (16). Sleep disturbance per parent report composite (scored from 0 to 14) showed a significant age-related increase of 0.07 points per year among a large clinical cohort of individuals with TD between the ages of 5 and 26 years, suggesting sleep worsening with age (10, 17). Sleep disturbance serves as the linking mechanisms between tic severity and reduced tic-related quality of life (16). Sleep disturbance in individuals with TD is marked by insomnia, parasomnias (e.g., sleep walking, sleep talking), sleep-disordered breathing, and daytime sleepiness at respective prevalence rates of 32%, 27%, 15%, and 12% (16). Tics can also directly impact sleep – with 14% to 23% of individuals with TD reporting tic-related interference in falling asleep (18–21), and 14% to 32% reporting tic occurrence during sleep (22). Polysomnography, the gold standard for sleep measurement, confirms the presence of tics during sleep, though at reduced frequency, intensity, and complexity relative to waking periods (23–26). Common demographic and clinical correlates of sleep disturbance in children with TD include female sex, tic severity, impairment, and symptoms of co-occurring psychiatric conditions, most notably, ADHD, anxiety, and depression (27–32). In the few studies examining sleep in adult-only TD samples, older age, ADHD, overall impairment, and emotion dysregulation were associated with sleep disturbance (21, 33).
However, long-term predictors of sleep disturbance in TD are not known, and studies have not yet examined long-term and cross-sectional predictors of sleep disturbance in adulthood within the same sample. Such knowledge may yield differences in child and adult indicators of adult sleep outcomes, informing the potential utility of targeted interventions aimed at ameliorating sleep disturbance and improving quality of life in children and adults with TD. Therefore, this investigation examined candidate variables, including chronological age, sex, tic medication, stimulant medication, tic severity, tic-related impairment, anxiety severity, depression severity, and ADHD severity as both long-term and cross-sectional predictors of sleep disturbance in a treatment follow-up sample of adolescents and young adults with TD who had participated in a randomized controlled trial examining the efficacy of CBIT in childhood (9, 34). This sample was selected as there are limited longitudinal studies in clinical samples of adults with TD, and few are focused on long-term physical health outcomes (35). This sample allows for analysis of factors implicated in long-term sleep quality. As simpler statistical models offer greater interpretability and generalizability, this investigation used an exploratory variable selection approach for determining final predictors in order to achieve a model with reduced complexity and optimal goodness-of-fit (36).
2 Materials and methods
2.1 Participants
Participants were 80 individuals who completed a long-term follow-up evaluation (34) 11.17 years (SD = 1.25 years) after completion (i.e., 10-week post-treatment timepoint) of the original randomized controlled trial examining the efficacy of CBIT relative to Psychoeducation and supportive therapy [PST]) in 126 children and adolescents (9). See Table 1 and Results for sample characteristics. Among the long-term follow-up participants, 38 were originally randomized to CBIT and 42 were randomized to PST. Among those who received CBIT, 55.6% (n = 21) were classified as treatment responders and 44.7% (n = 17) were non-responders, and among those who received PST, 14.3% (n = 6) were classified as treatment responders and 85.7% (n = 36) were non-treatment responders (See Procedure and Data Analysis section for definition of treatment responder and 34 for more details regarding sample characteristics).
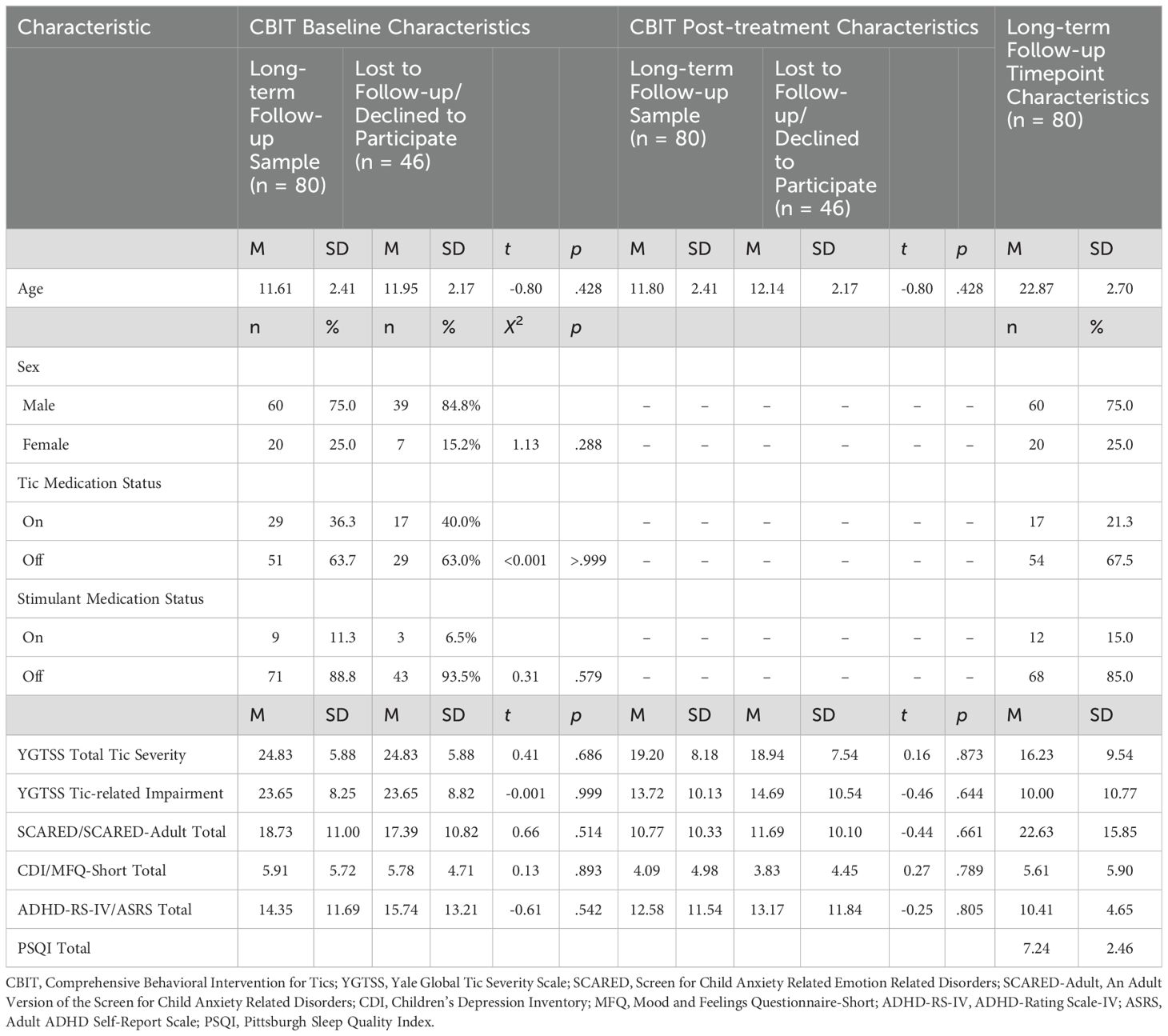
Table 1. Demographic and clinical characteristics at baseline and post-treatment (10-week) for original (n = 126) and follow-up (n = 80) samples, and at long-term follow-up.
Inclusion criteria for the original trial included an age of 9 to 17 years; diagnosis of Tourette’s disorder, chronic motor tic disorder, or chronic vocal tic disorder, moderate or greater severity as measured by a Yale Global Tic Severity Scale (YGTSS; 37) Total score >13 (>9 for chronic motor tic disorder or chronic vocal tic disorder); fluency in English; and an intelligence quotient > 80. Exclusion criteria included the need for immediate treatment or change in current treatment for any of the co-occurring psychiatric disorders allowed at study entry: ADHD, obsessive–compulsive disorder, anxiety disorders, depressive disorders, or oppositional defiant disorder; changes in the dosage or schedule of any psychotropic medications within six weeks of study enrollment or planned changes or initiation during the study; unstable medical condition; current diagnosis of substance abuse/dependence; lifetime diagnosis of pervasive developmental disorder, mania or psychosis; or ≥ 4 sessions of habit reversal training (9). Of the original CBIT sample (n = 126), 30 were lost to follow and 16 declined to participate. See Table 1 for baseline and post-treatment (10-week) demographic and clinical characteristics for participants who completed long-term follow-up (n = 80) and participants who were lost to follow up or declined to participate (n = 46). At follow-up, the sample ranged in age from 16 to 30 years (M = 22.87, SD = 2.70), was predominantly male (n = 60, 75.0%) and of non-Hispanic/Latino (n = 73, 91.2%) ethnicity. Participants endorsed white (n = 69, 86.3%), Black (n = 1, 1.3%), Asian (n = 4, 5.0%), multi-racial (n = 4, 5.0%), and other racial (n = 2, 2.5%) backgrounds. The majority of the sample were single/never married (n = 66, 82.5%) and had attained some college education or higher (n = 57, 71.3%). Half of the sample were employed (n = 40, 50.0%). See Espil et al. (34) for further methodological details.
2.2 Measures
Pittsburgh Sleep Quality Index (PSQI). The PSQI (61), administered at long-term follow-up is a 19-item self-report measure of sleep quality and disturbance over the previous month. Items are summed to produce seven subscale scores (sleep duration, sleep disturbance, sleep latency, daytime dysfunction due to sleepiness, sleep efficiency, overall sleep quality, and needs medication to sleep) are calculated from these items. These subscale scores are summed to yield a global sleep quality score. Higher scores indicate greater sleep disturbance. A PSQI global score greater than 5 is indicative of poor sleep quality (61). Forty-six participants in the long-term follow-up sample (57.5%) were above the cutoff for poor sleep quality. The PSQI has demonstrated acceptable reliability and validity (62–64). The internal consistency for PSQI Total in the present sample is.49.
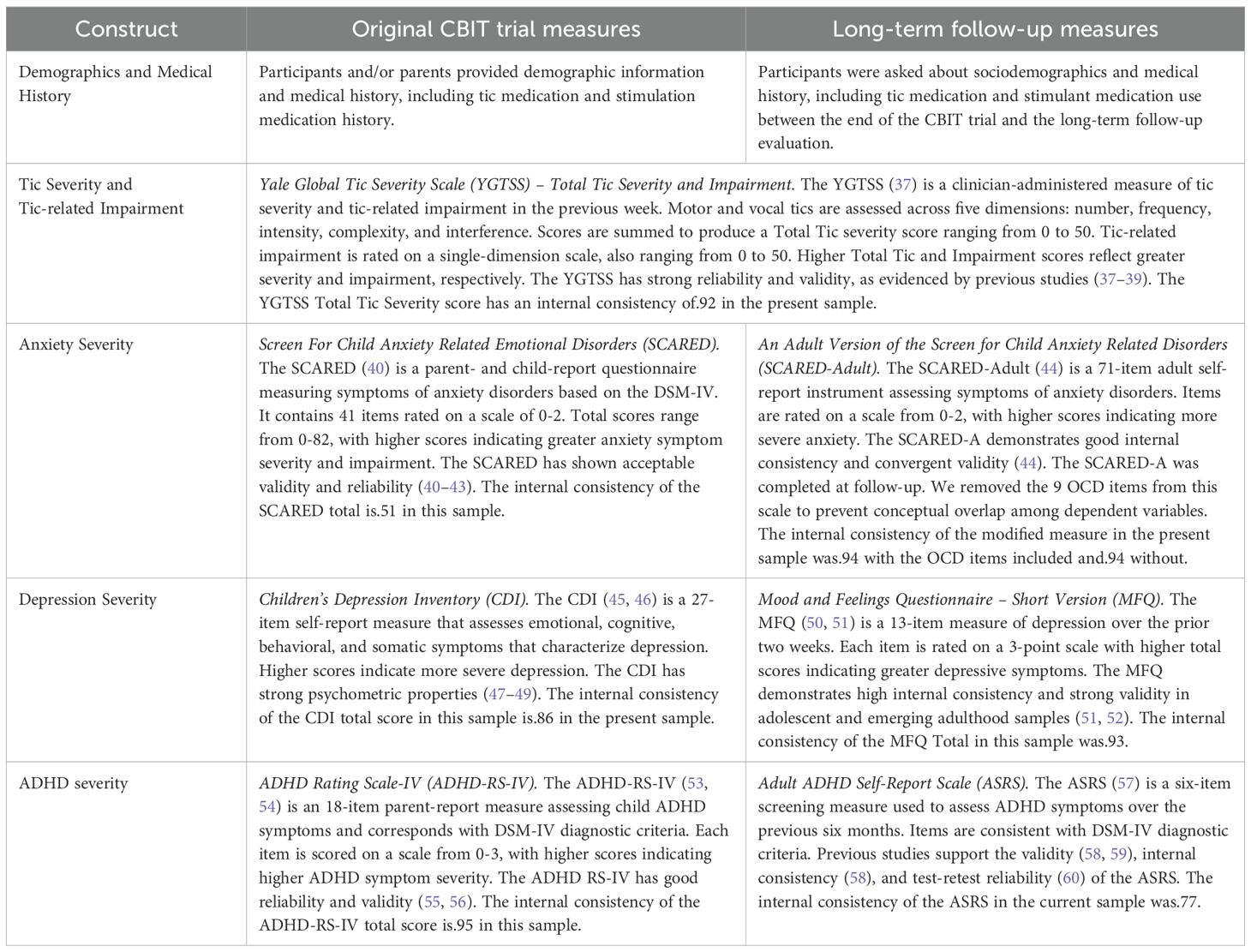
See Table 2 for detailed descriptions of measures evaluating predictor variables. including demographics (i.e., age, sex) and medical history (i.e., tic medication and stimulant medication status), tic severity and tic-related impairment, anxiety severity, depression severity, and ADHD symptom severity.
2.3 Procedure
Original CBIT Trial: Following institutional review board parent permission, child assent, and screening assessment procedures, participants and their parents completed a baseline evaluation for a 10-week randomized, controlled clinical trial evaluating the efficacy of CBIT relative to PST (9). CBIT is an 8-session blended intervention delivered over 10 weeks, involving habit reversal training, function-based assessment and intervention, relaxation training, and behavioral rewards (65). PST is a structured intervention providing supportive therapy and psychoeducation about tics developed to control attention and time (9). Treatment was delivered at three sites (University of California, Los Angeles [n = 45], University of Wisconsin-Milwaukee/Marquette University [n = 40] and Johns Hopkins University [n = 41]). This was followed a post-treatment evaluation during which a trained independent evaluator (IE) assessed tic severity and tic-related impairment (Yale Global Tic Severity Scale; YGTSS; 37) and rated degree of participant improvement in global illness-related functioning using the Clinical Global Impressions-Improvement Scale [CGI-I]; 66). Parents provided demographics and medical history and rated child behavior, including ADHD symptom severity (ADHD Rating Scale-IV; ADHD-RS-IV; 53, 54). Children rated anxiety severity (Screen for Child Anxiety Related Emotional Disorders; SCARED; 40) and depressive symptom severity (Children’s Depression Inventory; CDI; 45, 46).
Long-term Follow-up Assessment: At long-term follow-up assessment, participants from the original trial were recruited from two of the three original CBIT sites: University of California, Los Angeles (n = 32), Marquette University (n = 22), and Weill Cornell University (n = 26) due to the move of the original Johns Hopkins University principal investigator to that university. Adults provided institutional review board-approved informed consent for their participation. The three child participants (aged 16–17 years) provided assent, and their parents provided parent permission. The follow-up evaluation was completed in person or via web-based videoconferencing as needed. Independent evaluators (IEs) with a bachelor’s degree or higher, masked to original treatment assignment, and trained to reliability on clinical interviews (i.e., received didactic training and achieved reliable ratings on two consecutive interview administrations; see 34), evaluated psychiatric diagnosis (Mini International Neuropsychiatric Interview; MINI; 67), tic severity, and tic-related impairment (YGTSS). Participants rated sleep (PSQI Total), anxiety severity (An Adult Version of the Screen for Child Anxiety Related Disorders; SCARED-Adult; 44), depressive symptom severity (Mood and Feelings Questionnaire – Short Version; MFQ; 50), and ADHD symptom severity (Adult ADHD Self-Report Scale; ASRS; 57).
2.4 Data analysis
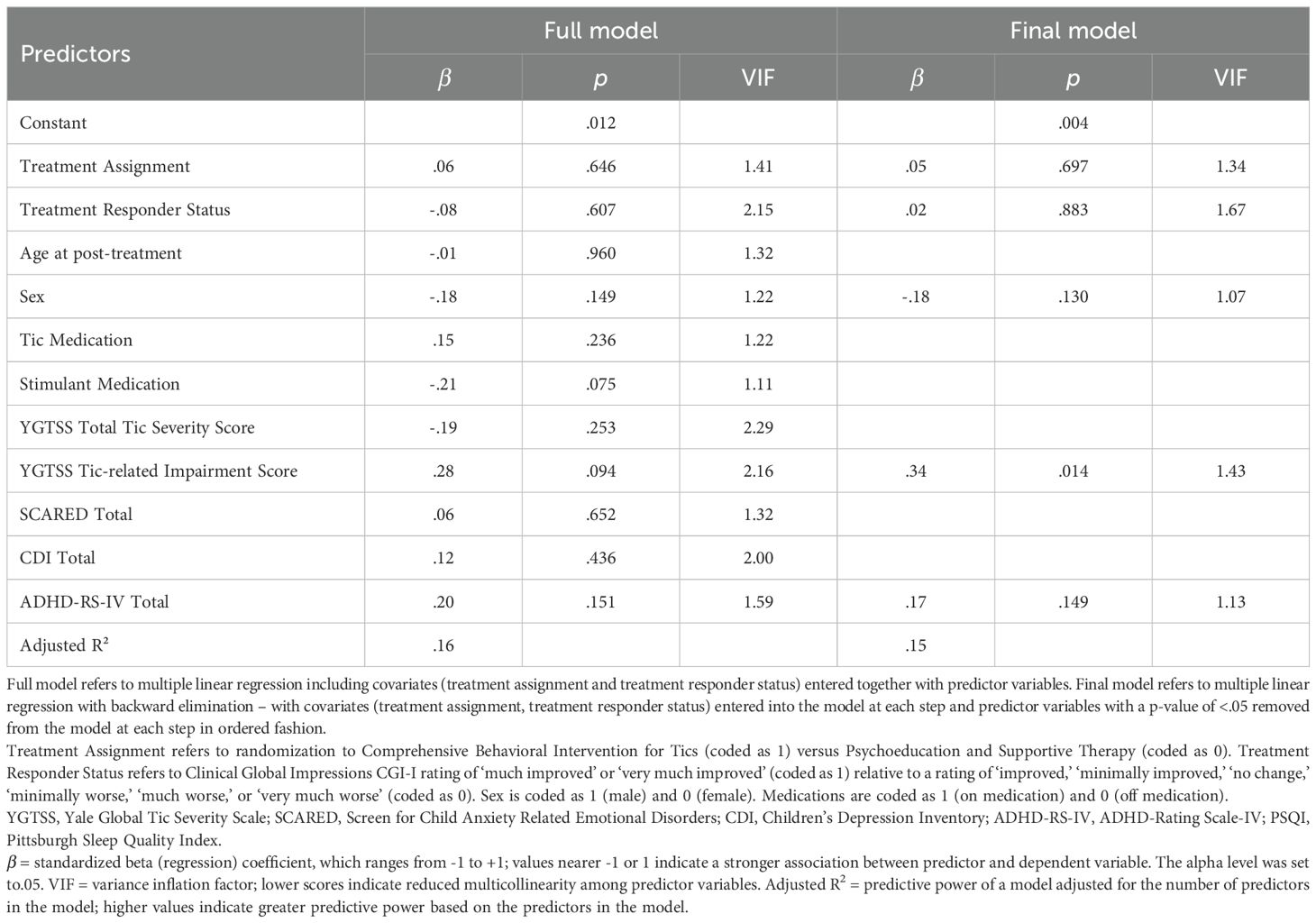
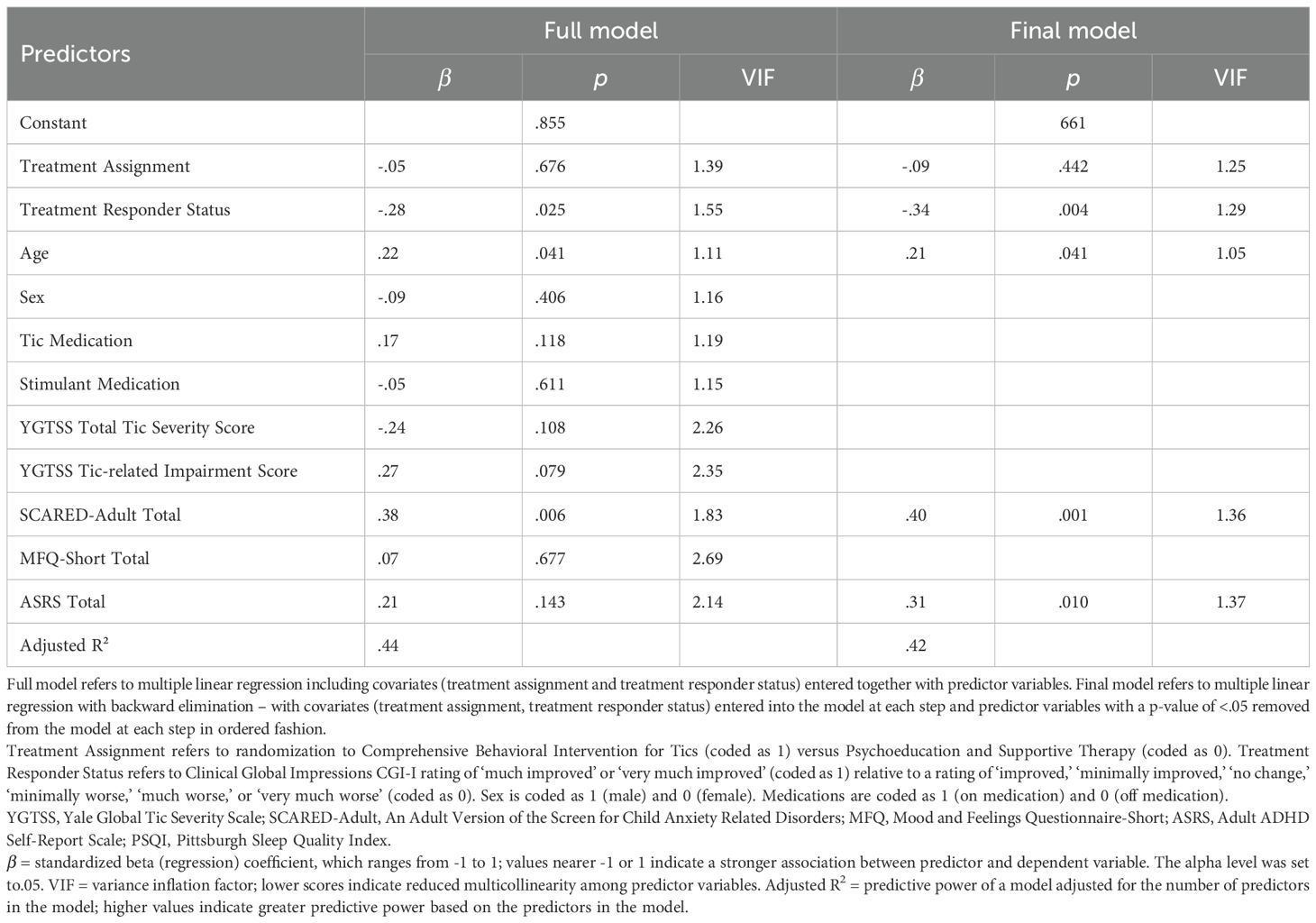
Analyses were conducted using SPSS 28.0. Descriptive statistics, including means and frequencies, are presented to characterize the sample with respect to demographics. Multiple linear regression was performed to examine whether post-treatment (i.e., 10-week timepoint of original CBIT trial) variables (including sex, tic medication status, stimulant medication status, YGTSS Tic Severity Total, YGTSS Impairment, SCARED Total, CDI Total, and ADHD-RS-IV Total) were predictors of the PSQI Total score at long-term follow-up. Multiple linear regression was also performed to examine whether variables measured at long-term follow-up (sex, tic medication status since the trial ended, stimulant medication status since the trial ended, YGTSS Tic Severity Total, YGTSS Tic-related Impairment Total, SCARED-Adult Total, CDI Total, and ADHD-RS-IV Total) were significant predictors of PSQI Total score. In both analyses, treatment assignment (CBIT versus PST), and treatment responder status (i.e., CGI-I rating of ‘much improved’ or ‘very much improved’ relative to a rating of ‘improved,’ ‘minimally improved,’ ‘no change,’ ‘minimally worse,’ ‘much worse,’ or ‘very much worse’) were included as covariates to account for prior tic treatment effects.
Then these analyses were repeated using multiple linear regression with backward elimination. Covariates (treatment assignment, treatment responder status) were entered into the model at each step of the backward elimination process, while predictor variables with a p-value of <.05 were removed from the model at each step in ordered fashion. Model fit was assessed at each step until achieving the final reduced model. Final reduced models were selected based on parsimony (i.e., simplest model that maintains goodness-of-fit) (36). We report adjusted R2, indicating the proportion of variance explained by the model adjusted for the number of predictors in the model. A higher R2 value indicates greater goodness-of-fit based on the predictors in the model (68). The variance-inflation factor (VIF) is reported as a measure of multicollinearity. Lower VIF scores indicate reduced collinearity among predictor variables. VIF scores above 10 indicate substantial multicollinearity among independent variables (69).
3 Results
3.1 Comparison of demographic and clinical characteristics in long-term follow-up sample and those lost-to follow-up or who declined to participate
There were no significant differences in demographic and clinical characteristics (i.e., age, sex, tic medication status, stimulant medication status, YGTSS Total Tic Severity and Tic-related Impairment scores, SCARED Total, CDI Total, ADHD-RS-IV Total) at baseline of the original CBIT trial between participants who completed long-term follow-up and participants who were lost to follow-up or declined to participate (p = >.999 –.288). There were no significant differences in clinical chacteristics at post-treatment (10-week) timepoint of the original CBIT trail between participants who completed long-term follow-up and participants who were lost to follow up or declined to participate (p = .644 –.873). For bivariate correlations among clinical measures at post-treatment (10-week) and long-term follow-up see Supplementary Tables 1, 2.
3.2 Post-treatment predictors of sleep disturbance at long-term follow up
In the full model, there were no significant post-treatment (10-week) predictors of PSQI Total at long-term follow up (see Table 3 and Supplementary Table 3). The full model explained 16% of the variance in PSQI Total score at long-term follow-up. In the final model, three predictors were retained, including sex (i.e., female), YGTSS Tic-related Impairment score, and ADHD-RS-IV Total score. Of these, YGTSS Tic-related Impairment score (β = .34) was a statistically significant predictor of PSQI Total score (see Table 3 and Supplementary Table 3). Sex and ADHD-RS-IV Total score were retained in the final model despite p-values >.05 based on parsimony; together, these three variables reflected the simplest model while preserving adjusted R2. The final model explained 15% of the variance in PSQI Total score.
3.3 Cross-sectional predictors of sleep disturbance at long-term follow-up
In the full model, SCARED-Adult Total score (β = .38) was a significant cross-sectional predictor of PSQI Total at long-term follow-up (see Table 4 and Supplementary Table 4). The full model explained 44% of the variance in PSQI Total score at long-term follow-up. Three predictors were retained in the final reduced model; chronological age, SCARED-Adult Total score (β = .40), ASRS Total score (β = .31)) were positive predictors of PSQI Total score at long-term follow-up (See Table 4 and Supplementary Table 4). The final reduced model explained 42% of the variance in PSQI Total score.
4 Discussion
The present study examined both long-term and cross-sectional predictors of sleep disturbance in early adulthood in a treatment-follow-up sample with TD. Findings showed residual tic-related impairment following acute behavior therapy for tics was a significant predictor of sleep disturbance in early adulthood. Older chronological age, in addition to greater anxiety severity and ADHD severity were significant cross-sectional predictors of sleep disturbance in early adulthood.
The association between residual tic-related impairment and sleep disturbance in early adulthood aligns with cross-sectional findings showing greater parent-rated tic-related impairment in children with TD and comorbid sleep disorders relative to TD alone (30) and correlations between reduced tic-related quality of life and greater sleep disturbance (16). In the Li et al. (16) study, greater sleep disturbance was associated with reduced quality of life broadly and across several domains, including cognitive, obsessive-compulsive, physical/activities of daily living, and psychological. As the YGTSS tic-related impairment item used in the present study is global it is unclear which aspects of tic-related impairment may be more strongly associated with sleep disturbance. This finding may suggest the need for more direct intervention targeting tic-related impairment. However, it is important to note that although CBIT failed to show significant positive effects on secondary psychiatric symptoms and psychosocial functioning relative to PST in the acute treatment period, for positive treatment responders, there were positive improvements in these outcomes at 3- and 6-month follow-up.
The cross-sectional association between older chronological age and sleep disturbance in early adulthood aligns with general age-related trends in sleep patterns in the general population and TD samples, which indicate sleep worsens with older age (17, 70, 71; Ricketts et al., 2022). The finding that ADHD symptom severity was a significant predictor of sleep disturbance is consistent with the wealth of studies showing links between ADHD and sleep disturbance in adults, including difficulties falling asleep, nighttime awakenings, reduced sleep quality, and daytime sleepiness (72, 73). Shared neural correlates (i.e., reduced gray matter volumes in the middle frontal and inferior frontal gyri, amygdala, striatum, and insula) are one potential mechanism underlying the association between ADHD and sleep disruption (74). The association found between anxiety and sleep disturbance at follow-up is not surprising due to well-established links between anxiety and sleep disturbance broadly (75) and in individuals with TD (27). Anxiety may increase emotional and physiological arousal impeding sleep and potentially exacerbating tics (76, 77; 78). One potential mechanism linking anxiety and sleep disturbance is the hypothalamic-pituitary-adrenal (HPA) axis, which modulates the stress response system (79). There is also preliminary support for the role of the HPA axis in TD (80).
The present study has notable limitations. The PSQI had low internal consistency in this sample, which could impact the validity of our results. We lack objective measurement of sleep disturbance. Also, the modest sample size might limit the generalizability of these findings to the broader population of individuals with TD. Additionally, as this study includes a follow-up sample stemming from a treatment seeking sample, results may not generalize to the broader population of individuals with TD. Further, this analysis does not address bidirectionality in associations between predictors and sleep disturbance. Moreover, this analysis does not examine change in sleep disturbance across acute behavior therapy for tics (CBIT), limiting conclusions which may be drawn regarding the treatment implications of this research.
In sum, the present study highlights the role of residual tic-related impairment following acute behavior therapy for tics and chronological age, anxiety, and ADHD symptoms on sleep disturbance in early adulthood. Future research should seek to understand directional associations and mechanistic links between TD, demographic and clinical chacteristics, and sleep disturbance. Future prospective trials are also needed to examine the degree to which CBIT improves sleep in a sample selected based on presence of comorbid TD and clinical threshold sleep disturbance using validated subjective and objective sleep measures.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by University of California, Los Angeles, Medical IRB 3 (MIRB3): 00004473. The studies were conducted in accordance with the Declaration of Helsinki and local legislation and institutional requirements. Written informed IRB-approved parent permission, child assent, and consent for participation in these studies was provided by the participants and the participants' legal guardian/next of kin.
Author contributions
MT: Conceptualization, Writing – original draft, Formal Analysis, Writing – review & editing. KB: Writing – review & editing, Writing – original draft. JM: Data curation, Project administration, Writing – review & editing, Writing – original draft. FE: Project administration, Writing – original draft, Funding acquisition, Methodology, Investigation, Data curation, Conceptualization, Writing – review & editing. JTS: Data curation, Writing – review & editing, Investigation, Writing – original draft. JSS: Project administration, Writing – original draft, Writing – review & editing, Investigation. SB: Writing – original draft, Methodology, Investigation, Writing – review & editing. MS: Writing – original draft, Investigation, Writing – review & editing, Funding acquisition, Methodology. JW: Funding acquisition, Writing – review & editing, Writing – original draft, Methodology. DW: Writing – review & editing, Methodology, Investigation, Funding acquisition, Writing – original draft. JP: Investigation, Writing – original draft, Funding acquisition, Methodology, Writing – review & editing. ER: Methodology, Investigation, Conceptualization, Project administration, Writing – review & editing, Supervision, Writing – original draft, Formal Analysis.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was conducted with research support from the Tourette Association of America to Drs. Espil, Specht, Walkup, Piacentini, and Woods, and National Institutes of Mental Health (NIMH) R01MH070802 funding to Dr. Piacentini. The content is the responsibility of the authors and is not necessarily representative of the views of the Tourette Association of America or the National Institutes of Health.
Acknowledgments
We thank Christopher C. Bauer, Silvia Orellana, Madeline Rasch, and Caitlin Lau for their support for study coordination and data collection.
Conflict of interest
JM has received support from the National Center for Complementary and Integrative Health NCCIH, National Institute for Neurological Disorders and Stroke NINDS, Misophonia Research Fund, and the Tiny Blue Dot Foundation. He receives royalties from Elsevier and serves as a consultant for Emalex Biosciences and Noema Pharma. FE receives research support from the Foundation for OCD Research and past support from the TAA and the American Academy of Neurology AAN. SB has received research support, speaking fees and travel support for speaking engagements from the TAA. SB also receives royalties from Wolters Kluwer. MS has received research support and speaking honoraria from the TAA. JW serves on the advisory board and Speaker’s Bureau of the TAA and receives royalties from Oxford University Press, and Wolters Kluwer. DW has received royalties from Guilford Press, Springer Press, and Oxford University Press. DW has also received speaking fees from the Tourette Association of America. JP has received research support from NIMH, the TLC Foundation for BFRBs, and Pfizer Pharmaceuticals; publication royalties from Guilford Press and Oxford University Press; and travel/speaking honoraria from the TAA, International OCD Foundation, and the TLC Foundation for BFRBs. ER has received honoraria and funding from the Tourette Association of America TAA. She has also received research funding from the National Institute of Mental Health NIMH, TLC Foundation for Body-Focused Repetitive Behaviors BFRBs: BFRB Precision Medicine Initiative, American Academy of Sleep Medicine, and Brain and Behavior Research Foundation. She has received honoraria from the Centers for Disease Control and Prevention and Springer Nature.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The content is the responsibility of the authors and is not necessarily representative of the views of the Tourette Association of America or the National Institutes of Health.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2025.1594347/full#supplementary-material
References
1. American Psychiatric Association. (2022). Diagnostic and statistical manual of mental disorders (5th ed., text rev.). doi: 10.1176/appi.books.9780890425787
2. Bloch MH and Leckman JF. Clinical course of Tourette syndrome. J Psychosomatic Res. (2009) 67:497–501. doi: 10.1016/j.jpsychores.2009.09.002
3. Hirschtritt ME, Lee PC, Pauls DL, Dion Y, Grados MA, Illmann C, et al. Lifetime prevalence, age of risk, and genetic relationships of comorbid psychiatric disorders in Tourette syndrome. JAMA Psychiatry. (2015) 72:325–33. doi: 10.1001/jamapsychiatry.2014.2650
4. Lichter DG and Finnegan SG. Influence of gender on Tourette syndrome beyond adolescence. Eur Psychiatry. (2015) 30:334–40. doi: 10.1016/j.eurpsy.2014.07.003
5. Ricketts EJ, Woods DW, Espil FM, McGuire JF, Stiede JT, Schild J, et al. Childhood predictors of long-term tic severity and tic impairment in Tourette’s disorder. Behav Ther. (2022) 53:1250–64. doi: 10.1016/j.beth.2022.07.002
6. Levine JLS, Szejko N, and Bloch MH. (2019). Meta-analysis: Adulthood prevalence of Tourette syndrome. Prog Neuropsychopharmacol Biol Psychiatry. 95:109675. doi: 10.1016/j.pnpbp.2019.109675
7. Evans J, Seri S, and Cavanna AE. (2016). The effects of Gilles de la Tourette syndrome and other chronic tic disorders on quality of life across the lifespan: A systematic review. Eur Child Adolesc Psychiatry. 25:939–948. doi: 10.1007/s00787-016-0823-8
8. Pringsheim T, Okun MS, Müller-Vahl K, Martino D, Jankovic J, Cavanna AE, et al. Practice guideline recommendations summary: treatment of tics in people with Tourette syndrome and chronic tic disorders. Neurology. (2019) 92:896–906. doi: 10.1212/WNL.0000000000007466
9. Piacentini J, Woods DW, Scahill L, Wilhelm S, Peterson AL, Chang S, et al. Behavior therapy for children with Tourette disorder: a randomized controlled trial. JAMA. (2010) 303:1929–37. doi: 10.1001/jama.2010.607
10. Cothros N and Pringsheim T. Alpha agonists for the treatment of Tourette syndrome. In: International Review of Movement Disorders, vol. 4. Academic Press (2022). p. 251–66. doi: 10.1016/bs.irmvd.2021.12.003
11. Can A, Vermilion J, Mink JW, and Morrison P. Pharmacological treatment of tourette disorder in children. J Child Adolesc Psychopharmacol. (2024) 34:346–52. doi: 10.1089/cap.2023.0026
12. Cavanna AE, Selvini C, Termine C, Luoni C, Eddy CM, and Rickards H. Tolerability profile of aripiprazole in patients with Tourette syndrome. J Psychopharmacol. (2012) 26:891–5. doi: 10.1177/0269881111408462
13. Farhat LC, Behling E, Landeros-Weisenberger A, Macul Ferreira de Barros P, Polanczyk GV, Cortese S, et al. Pharmacological interventions for attention-deficit/hyperactivity disorder in children and adolescents with Tourette disorder: A systematic review and network meta-analysis. J Child Adolesc Psychopharmacol. (2024) 34:373–82. doi: 10.1089/cap.2024.0049
14. Faraone SV, Po MD, Komolova M, and Cortese S. Sleep-associated adverse events during methylphenidate treatment of attention-deficit/hyperactivity disorder: a meta-analysis. J Clin Psychiatry. (2019) 80:18r12210. doi: 10.4088/JCP.18r12210
15. Hennissen L, Bakker MJ, Banaschewski T, Carucci S, Coghill D, Danckaerts M, et al. (2017). Cardiovascular effects of stimulant and non-stimulant medication for children and adolescents with ADHD: A systematic review and meta-analysis. CNS Drugs. 31:199–215. doi: 10.1007/s40263-017-0410-7
16. Li N, Yan J, Xu C, Li Y, and Cui Y. Prevalence and influencing factors of sleep problems in tic disorders: a meta-analysis. World J Biol Psychiatry. (2024) 25:130–40. doi: 10.1080/15622975.2023.2287729
17. Groth C, Mol Debes N, Rask CU, Lange T, and Skov L. Course of Tourette syndrome and comorbidities in a large prospective clinical study. J Am Acad Child Adolesc Psychiatry. (2017) 56:304–12. doi: 10.1016/j.jaac.2017.01.010
18. Champion LM, Fulton WA, and Shady GA. Tourette syndrome and social functioning in a Canadian population. Neurosci Biobehav Rev. (1988) 12:255–7. doi: 10.1016/S0149-7634(88)80054-X
19. Erenberg G. Sleep disorders in Gilles de la Tourette’s syndrome. Neurology. (1985) 35:1397. doi: 10.1212/WNL.35.9.1397-a
20. Jankovic J and Rohaidy H. Motor, behavioral and pharmacologic findings in Tourette's syndrome. Can J Neurological Sci. (1987) 14:541–6. doi: 10.1017/S0317167100038087
21. Ricketts EJ, Montalbano GE, Burgess HJ, McMakin DL, Coles ME, Piacentini J, et al. Sleep and chronotype in adults with persistent tic disorders. J Clin Psychol. (2022) 78:1516–39. doi: 10.1002/jclp.23323
22. Jiménez-Jiménez FJ, Alonso-Navarro H, García-Martín E, and Agúndez JAG. (2020). Sleep disorders in Tourette syndrome. Sleep Med Rev. 53:101335. doi: 10.1016/j.smrv.2020.101335
23. Cohrs S, Rasch T, Altmeyer S, Kinkelbur J, Kostanecka T, Rothenberger A, et al. Decreased sleep quality and increased sleep related movements in patients with Tourette's syndrome. Journal of Neurology. Neurosurg Psychiatry. (2001) 70:192–7. doi: 10.1136/jnnp.70.2.192
24. Fish DR, Sawyers D, Allen PJ, Blackie JD, Lees AJ, and Marsden CD. The effect of sleep on the dyskinetic movements of Parkinson's disease, Gilles de la Tourette syndrome, Huntington's disease, and torsion dystonia. Arch Neurol. (1991) 48:210–4. doi: 10.1001/archneur.1991.00530140106023
25. Glaze DG, Frost JD Jr., and Jankovic J. Sleep in Gilles de la Tourette's syndrome: disorder of arousal. Neurology. (1983) 33:586–6. doi: 10.1212/WNL.33.5.586
26. Silvestri R, De Domenico P, Di Rosa AE, Bramanti P, Serra S, and Di Perri R. The effect of nocturnal physiological sleep on various movement disorders. Movement Disord. (1990) 5:8–14. doi: 10.1002/mds.870050104
27. Lee W-T, Huang H-L, Wong LC, Weng W-C, Vasylenko T, Jong Y-J, et al. Tourette syndrome as an independent risk factor for subsequent sleep disorders in children: A nationwide population-based case-control study. Sleep. (2017) 40:zsw072. doi: 10.1093/sleep/zsw072
28. Modafferi S, Galli F, Menghini D, Seri S, and Vicari S. Sleep, ADHD and neurodevelopmental disorders: A literature review. Eur J Paediatric Neurol. (2016) 20:756–64. doi: 10.1016/j.ejpn.2016.05.003
29. Ricketts EJ, Rozenman M, Choy C, Goldberg HB, Kim JS, Colwell CS, et al. Sleep sufficiency in pediatric and adolescent Tourette's disorder: National Survey of Children's Health. J Dev Behav Pediatr. (2018) 39:72–6. doi: 10.1097/DBP.0000000000000518
30. Ricketts EJ, Wolicki SB, Holbrook JR, Rozenman M, McGuire JF, Charania SN, et al. Clinical characteristics of children with Tourette syndrome with and without sleep disorder. Pediatr Neurol. (2023) 141:18–24. doi: 10.1016/j.pediatrneurol.2022.12.011
31. Storch EA, Milsom V, Lack CW, Pence SL, Geffken GR, Jacob ML, et al. (2009). Sleep-related problems in youth with Tourette’s syndrome and chronic tic disorder. Child Adolesc Ment Health. 14:97–103. doi: 10.1111/j.1475-3588.2008.00497.x
32. Swisher V, Tooker M, Qu C, Burgess HJ, Coles ME, Bennett S, et al. Sleep disorders, sleep medication use, and predictors of sleep disturbance in children with persistent tic disorders. Child Health Care. (2024) 53:23–40. doi: 10.1080/02739615.2023.2175682
33. Tulen JH, Niers T, Vegt R, Cath D, and Hengeveld MW. Sleep quality and motor activity during sleep in adults with ADHD or Tourette’s disorder. Sleep-wake Res Netherlands. (2007) 18:119–22.
34. Espil FM, Woods DW, Specht MW, Bennett SM, Walkup JT, Ricketts EJ, et al. Long-term outcomes of behavior therapy for youth with Tourette disorder. J Am Acad Child Adolesc Psychiatry. (2022) 61:764–71. doi: 10.1016/j.jaac.2021.08.022
35. Isaacs DA, Bonnet K, Eckland MR, Markowitz K, Pena M, and Schlundt DG. Perspectives from adults with Tourette syndrome on research priorities and registry development: A focus group study. Neuropsychiatr Dis Treat. (2024) 20:257–69. doi: 10.2147/NDT.S442131
36. Chowdhury MZI and Turin TC. Variable selection strategies and its importance in clinical prediction modelling. Family Med Community Health. (2020) 8:e000262. doi: 10.1136/fmch-2019-000262
37. Leckman JF, Riddle MA, Hardin MT, Ort SI, Swartz KL, Stevenson J, et al. The Yale Global Tic Severity Scale: Initial testing of a clinician-rated scale of tic severity. J Am Acad Child Adolesc Psychiatry. (1989) 28:566–73. doi: 10.1097/00004583-198907000-00015
38. McGuire JF, Piacentini J, Storch EA, Murphy TK, Ricketts EJ, Woods DW, et al. A multicenter examination and strategic revisions of the Yale Global Tic Severity Scale. Neurology. (2018) 90:e1711–9. doi: 10.1212/WNL.0000000000005474
39. Storch EA, Murphy TK, Geffken GR, Sajid M, Allen P, Roberti JW, et al. Reliability and validity of the yale global tic severity scale. psychol Assess. (2005) 17:486–91. doi: 10.1037/1040-3590.17.4.486
40. Birmaher B, Khetarpal S, Brent D, Cully M, Balach L, Kaufman J, et al. The Screen for Child Anxiety Related Emotional Disorders (SCARED): Scale construction and psychometric characteristics. J Am Acad Child Adolesc Psychiatry. (1997) 36:545–53. doi: 10.1097/00004583-199704000-00018
41. Birmaher B, Brent DA, Chiappetta L, Bridge J, Monga S, and Baugher M. Psychometric properties of the Screen for Child Anxiety Related Emotional Disorders (SCARED): A replication study. J Am Acad Child Adolesc Psychiatry. (1999) 38:1230–6. doi: 10.1097/00004583-199910000-00011
42. Muris P, Mayer B, Bartelds E, Tierney S, and Bogie N. The revised version of the Screen for Child Anxiety Related Emotional Disorders (SCARED-R): Treatment sensitivity in an early intervention trial for childhood anxiety disorders. Br J Clin Psychol. (2001) 40:323–36. doi: 10.1348/014466501163724
43. Torp NC, Dahl K, Skarphedinsson G, Compton S, Thomsen PH, Weidle B, et al. Predictors associated with improved cognitive-behavioral therapy outcome in pediatric obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. (2015) 54:200–207.e201. doi: 10.1016/j.jaac.2014.12.007
44. Van Steensel FJA and Bögels SM. An adult version of the screen for child anxiety related emotional disorders (SCARED-A). Netherlands J Psychol. (2014) 68:81–7.
46. Kovacs M. Children’s Depression Inventory: Technical manual. New York, NY: Multi-Health Systems Inc (1992).
47. Finch AJ Jr., Saylor CF, Edwards GL, and McIntosh JA. Children's Depression Inventory: Reliability over repeated administrations. J Clin Child Psychol. (1987) 16:339–41. doi: 10.1207/s15374424jccp1604_7
48. Saylor CF, Finch AJ, Spirito A, and Bennett B. The Children's Depression Inventory: A systematic evaluation of psychometric properties. J Consulting Clin Psychol. (1984) 52:955–67. doi: 10.1037/0022-006X.52.6.955
49. Timbremont B, Braet C, and Dreessen L. Assessing depression in youth: Relation Between the Children's Depression Inventory and a Structured Interview. J Clin Child Adolesc Psychol. (2004) 33:149–57. doi: 10.1207/S15374424JCCP3301_14
50. Angold A, Costello EJ, Messer SC, and Pickles A. Development of a short questionnaire for use in epidemiological studies of depression. J Methods Psychiatr Res. (1995) 5:237–49.
51. Eyre O, Jones RB, Agha SS, Wootton RE, Thapar AK, Stergiakouli E, et al. Validation of the short Mood and Feelings Questionnaire in young adulthood. J Affect Disord. (2021) 294:883–8. doi: 10.1016/j.jad.2021.07.090
52. Turner N, Joinson C, Peters TJ, Wiles N, and Lewis G. Validity of the Short Mood and Feelings Questionnaire in late adolescence. psychol Assess. (2014) 26:752. doi: 10.1037/a0036572
53. DuPaul GJ, Power TJ, Anastopoulos AD, and Reid R. ADHD rating scale-IV: Checklists, Norms, and Clinical Interpretation. Guilford Publications (1998).
54. Faries DE, Yalcin I, Harder D, and Heiligenstein JH. Validation of the ADHD rating scale as a clinician administered and scored instrument. J Attention Disord. (2001) 5:107–15. doi: 10.1177/108705470100500204
55. Erbilgin Gün S and Kilincaslan A. Quality of life among children and adolescents with Tourette disorder and comorbid ADHD: A clinical controlled study. J Attention Disord. (2019) 23:817–27. doi: 10.1177/1087054718772158
56. Zhang S, Faries DE, Vowles M, and Michelson D. ADHD rating scale IV: Psychometric properties from a multinational study as clinician-administered instrument. Int J Methods Psychiatr Res. (2005) 14:186–201. doi: 10.1002/mpr.7
57. Kessler RC, Adler L, Ames M, Demler O, Faraone S, Hiripi E, et al. (2005). The World Health Organization Adult ADHD Self-Report Scale (ASRS): A short screening scale for use in the general population. Psychol Med. 35:245–256. doi: 10.1017/S0033291704002892
58. Adler LA, Spencer T, Faraone SV, Kessler RC, Howes MJ, Biederman J, et al. Validity of pilot Adult ADHD Self-Report Scale (ASRS) to rate adult ADHD symptoms. Ann Clin Psychiatry. (2006) 18:145–8. doi: 10.1080/10401230600801077
59. Kessler RC, Adler LA, Gruber MJ, Sarawate CA, Spencer T, and Van Brunt DL. Validity of the World Health Organization Adult ADHD Self-Report Scale (ASRS) Screener in a representative sample of health plan members. Int J Methods Psychiatr Res. (2007) 16:52–65. doi: 10.1002/mpr.208
60. Silverstein MJ, Alperin S, Faraone SV, Kessler RC, and Adler LA. Test–retest reliability of the adult ADHD Self-Report Scale (ASRS) v1. 1 Screener in non-ADHD controls from a primary care physician practice. Family Pract. (2018) 35:336–41. doi: 10.1093/fampra/cmx115
61. Buysse DJ, Reynolds CF, Monk TH, Berman SR, and Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. (1989) 28:193–213. doi: 10.1016/0165-1781(89)90047-4
62. Buysse DJ, Hall ML, Strollo PJ, Kamarck TW, Owens J, Lee L, et al. Relationships between the Pittsburgh Sleep Quality Index (PSQI), Epworth Sleepiness Scale (ESS), and clinical/polysomnographic measures in a community sample. J Clin Sleep Med. (2008) 4:563–71. doi: 10.5664/jcsm.27351
63. Grandner MA, Kripke DF, Yoon I-Y, and Youngstedt SD. Criterion validity of the Pittsburgh Sleep Quality Index: Investigation in a non-clinical sample. Sleep Biol Rhythms. (2006) 4:129–36. doi: 10.1111/j.1479-8425.2006.00207.x
64. Mollayeva T, Thurairajah P, Burton K, Mollayeva S, Shapiro CM, and Colantonio A. The Pittsburgh Sleep Quality Index as a screening tool for sleep dysfunction in clinical and non-clinical samples: A systematic review and meta-analysis. Sleep Med Rev. (2016) 25:52–73. doi: 10.1016/j.smrv.2015.01.009
65. Woods DW, Piacentini J, Change SW, Deckersbach T, Ginsburg GS, Peterson AL, et al. Managing Tourette syndrome. A behavioral intervention for children and adults. Therapist Guide. New York, NY: Oxford University Press (2008). doi: 10.1093/med:psych/9780195341287.001.0001
66. Guy W. Clinical global impressions. In: Guy W, editor. ECDBU assessment manual for psychopharmacology. National Institute of Mental Health, Rockville, MD (1976). p. 218–22.
67. Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. (1998) 59:22–33.
68. Austin PC and Steyerberg EW. The number of subjects per variable required in linear regression analyses. J Clin Epidemiol. (2015) 68:627–36. doi: 10.1016/j.jclinepi.2014.12.014
69. Mela CF and Kopalle PK. The impact of collinearity on regression analysis: the asymmetric effect of negative and positive correlations. Appl Econ. (2002) 34:667–77. doi: 10.1080/00036840110058482
70. Hysing M, Harvey AG, Bøe T, Heradstveit O, Vedaa Ø., and Sivertsen B. Trajectories of sleep problems from adolescence to adulthood. Linking two population-based studies from Norway. Sleep Med. (2020) 75:411–7. doi: 10.1016/j.sleep.2020.08.035
71. Park H, Chiang JJ, Irwin MR, Bower JE, McCreath H, and Fuligni AJ. Developmental trends in sleep during adolescents' transition to young adulthood. Sleep Med. (2019) 60:202–10. doi: 10.1016/j.sleep.2019.04.007
72. Díaz-Román A, Mitchell R, and Cortese S. Sleep in adults with ADHD: systematic review and meta-analysis of subjective and objective studies. Neurosci Biobehav Rev. (2018) 89:61–71. doi: 10.1016/j.neubiorev.2018.02.014
73. Voinescu BI, Szentagotai A, and David D. Sleep disturbance, circadian preference and symptoms of adult attention deficit hyperactivity disorder (ADHD). J Neural Transm. (2012) 119:1195–204. doi: 10.1007/s00702-012-0862-3
74. Shen C, Luo Q, Chamberlain SR, Morgan S, Romero-Garcia R, Du J, et al. (2020). What is the link between attention-deficit/hyperactivity disorder and sleep disturbance? A multimodal examination of longitudinal relationships and brain structure using large-scale population-based cohorts. Biol Psychiatry. 88:459–469. doi: 10.1016/j.biopsych.2020.03.010
75. Cox RC and Olatunji BO. A systematic review of sleep disturbance in anxiety and related disorders. J Anxiety Disord. (2016) 37:104–15. doi: 10.1016/j.janxdis.2016.01.001
76. Brandt V, Essing J, Jakubovski E, and Müller-Vahl K. Premonitory urge and tic severity, comorbidities, and quality of life in chronic tic disorders. Movement Disord Clin Pract. (2023) 10:922–32. doi: 10.1002/mdc3.13742
77. Ludlow AK, Anderson S, Hedderly T, and Gutierrez R. The relationship between anxiety and tic severity in children and adolescents with Tic disorders: a scoping review. Curr Psychol. (2024) 41:21808–29. doi: 10.1007/s12144-024-05975-w
78. Ricketts EJ, Swisher V, Greene DJ, Silverman D, Nofzinger EA, and Colwell CS. Sleep disturbance in Tourette's disorder: Potential underlying mechanisms. Curr Sleep Med Rep. (2023) 9:10–22. doi: 10.1007/s40675-022-00242-5
79. Dieleman GC, Huizink AC, Tulen JH, Utens EM, Creemers HE, van der Ende J, et al. Alterations in HPA-axis and autonomic nervous system functioning in childhood anxiety disorders point to a chronic stress hypothesis. Psychoneuroendocrinology. (2015) 51:135–50. doi: 10.1016/j.psyneuen.2014.09.002
Keywords: tics and Tourette syndrome, psychiatric comorbidity, impairment, sleep, medication
Citation: Tooker MS, Barber KE, McGuire JF, Espil FM, Stiede JT, Schild JS, Bennett SM, Specht MW, Walkup JT, Woods DW, Piacentini J and Ricketts EJ (2025) Longitudinal and cross-sectional predictors of sleep disturbance in a treatment follow-up sample with Tourette’s disorder. Front. Psychiatry 16:1594347. doi: 10.3389/fpsyt.2025.1594347
Received: 15 March 2025; Accepted: 28 October 2025;
Published: 26 November 2025.
Edited by:
Jean Marc Guile, University of Picardie Jules Verne, FranceReviewed by:
Francesca Felicia Operto, University of Salerno, ItalyGraham Reid, Western University, Canada
Copyright © 2025 Tooker, Barber, McGuire, Espil, Stiede, Schild, Bennett, Specht, Walkup, Woods, Piacentini and Ricketts. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Emily J. Ricketts, ZXJpY2tldHRzQG1lZG5ldC51Y2xhLmVkdQ==
 Maya S. Tooker1
Maya S. Tooker1 Kathryn E. Barber
Kathryn E. Barber Joseph F. McGuire
Joseph F. McGuire Flint M. Espil
Flint M. Espil Matthew W. Specht
Matthew W. Specht John T. Walkup
John T. Walkup John Piacentini
John Piacentini Emily J. Ricketts
Emily J. Ricketts