Abstract
Background:
Sarcopenia is the main cause of disability in an aging society and increases the risk of death in older adults. However, the relationship between cognitive function and muscle mass and the underlying mechanisms are not clear. This study aims to investigate the relationship between cognitive function and muscle mass in the older adults.
Methods:
This study was based on the Chinese Longitudinal Healthy Longevity Survey (CLHLS), phase III from 2011 to 2018. We analyzed 2536 participants aged ≥60 years. SPSS 27.0 software was used for data screening and statistical analysis, and MPLUS 8.7 and R4.4.2 software were used to construct cross-lag models and restricted cubic splints.
Results:
In this study, out of 2,536 participants, there were 1,283 males (50.6%) and 1,253 females (49.4%), with an average age of 77.54 ± 8.6 years. Correlation analysis showed that cognitive function was positively correlated with muscle mass in older adults. At all time points (P<0.05). The cross-lag model revealed a one-way prediction effect: The path coefficients of ASMI→MMSE in T1→T2 and T2→T3 were statistically significant in the general population, men and women (P<0.05), and the path coefficients β were all greater than 0. The association of MMSE → ASMI was significant only at the T2 → T3 time point in the overall population (β = 0.010, P < 0.05), and not statistically significant at T1 → T2 and T2 → T3 time points in both males and females (P <0.05). RCS results showed that the association between skeletal muscle mass and cognitive impairment in the total population (Poverall trend <0.05, Pnon-linear <0.05), older men (Poverall trend <0.05, Pnon-linear <0.05) and older women (Poverall trend <0.05, Pnon-linear <0.05) showed a nonlinear increasing trend. It is suggested that ASMI should be maintained at 7.45kg/m2 and 5.68kg/m2 or above in older men and women, respectively.
Conclusion:
Muscle mass had a major predictive effect on cognitive trajectory, especially in females. Maintaining ASMI above gender-specific thresholds may help slow cognitive decline, suggesting that muscle mass can serve as an adjustable biomarker for dementia prevention. Longitudinal studies should verify the validity of these thresholds in different populations.
1 Introduction
As a major challenge to human society in the 21st century, population aging has become an irreversible global trend. It is projected that by 2050, the proportion of individuals aged 65 and above in China will reach 27.9% (1), surpassing the United Nations’ threshold of 7%, signifying China’s transition into a super-aging society. Concurrently, cognitive dysfunction, closely associated with the aging process, has emerged as a significant concern. Cognitive impairment not only severely affects core cognitive domains such as judgment and attention in older adults (2) but also poses a risk of progressing to irreversible dementia (3), thereby becoming a critical factor impacting the healthy quality of life for older adults. Research has confirmed a significant positive correlation between cognitive impairment and aging (4).
Additionally, studies have demonstrated that factors such as education level, obesity status, lifestyle, and vascular-related conditions can influence cognitive function differentiation in older adults (5). Notably, cognitive function is a key biomarker for predicting healthy life expectancy in older adults (6). However, current clinical interventions remain limited to symptom management, lacking effective etiological treatments (7). Therefore, identifying and managing risk factors for cognitive impairment at an early stage holds great practical significance and urgency in the field of older adults’ health.
Sarcopenia, a common and progressive skeletal muscle disease characterized by muscle atrophy and reduced strength and quality (8), significantly impairs physical function and increases the risk of falls, fractures, and mortality in older adults (9), profoundly affecting their quality of life. Consequently, it has garnered considerable attention in both academic and clinical fields. According to the latest definition of sarcopenia (10), low muscle mass is a crucial diagnostic criterion. From a physiological perspective, muscle mass plays an essential role in regulating energy metabolism, influencing insulin sensitivity, and maintaining overall bodily function (11).
Given that muscle mass and cognitive function share neuroendocrine regulatory pathways (such as the insulin signaling pathway) and inflammatory mechanisms (11, 12), numerous studies have explored the complex relationship between muscle mass and cognitive function. Recent epidemiological studies have shown that older adults with lower muscle mass are at higher risk of cognitive decline and neurodegenerative diseases like Alzheimer’s (13–15). These findings suggest an intrinsic link between muscle mass and cognitive function. Mechanistic studies indicate that muscle-derived factors, such as myokines, may be released into the bloodstream to influence neuroplasticity, neurotransmitter regulation, and inflammatory responses in the brain, all of which are closely related to cognitive function (16, 17). Animal models and human trials further support this relationship (18–20).
The current research landscape is marred by several significant gaps. Firstly, the majority of Chinese studies are cross-sectional, which constrains the ability to draw causal conclusions (21, 22). Secondly, longitudinal analyses tend to concentrate on one-way relationships, overlooking the possibility of reverse causality (23). Thirdly, despite ample documentation of gender differences in muscle mass and cognitive function, there is a dearth of research that systematically investigates gender-specific thresholds for muscle mass in terms of cognitive protection (24–26). Finally, existing dose-response analyses frequently hinge on linear assumptions (27), potentially missing out on nonlinear dynamics that are crucial for clinical interventions.
This scholarly endeavor seeks to bridge the existing knowledge gaps by utilizing longitudinal data sourced from the Chinese Longitudinal Healthy Longevity Survey (CLHLS). We have adopted a Cross-Lagged Panel Model (CLPM) to scrutinize the reciprocal temporal dynamics between muscular mass and cognitive faculties, further enhanced by a Restricted Cubic Spline (RCS) analysis to delineate thresholds that are specific to each gender. Our research is guided by a dual purpose: firstly, to ascertain the directional precedence between muscular mass and cognitive deterioration, and secondly, to formulate actionable Appendicular Skeletal Muscle Index (ASMI) thresholds for the prevention of dementia, specifically calibrated for the elderly demographic in China. By amalgamating bidirectional causality with nonlinear dose-response methodologies, this investigation sheds new light on the interplay between muscle and cognition, furnishing data-driven objectives for the formulation of public health initiatives.
The implications of our findings are profound. Should muscular mass emerge as a more robust harbinger of cognitive trajectories, interventions aimed at combating sarcopenia—such as resistance training and nutritional interventions—could be accorded precedence in strategies for dementia prevention. Additionally, the identification of gender-specific thresholds may enable a more nuanced approach to personalized care, effectively addressing the disparities in muscular composition and hormonal profiles observed between elderly males and females.
2 Methods
2.1 Data sources and subjects
The data for this study were sourced from the CLHLS, which encompasses over 500 sites across 23 provinces, municipalities, and autonomous regions in China. The baseline survey was initiated in 1998, with subsequent follow-ups conducted in 2000, 2002, 2005, 2008, 2011, 2014, and 2018. Data usage rights were obtained through the Open Research Data Platform of Peking University. In this study, a total of 9,765 elderly individuals were surveyed in 2011 (T1). Follow-up assessments in 2014 (T2) and 2018 (T3) revealed deaths and dropouts among the elderly participants. After excluding 348 subjects with missing key variable information, a final sample of 2,536 elderly individuals was included in the analysis. See Figure 1 for details.
Figure 1

Flowchart of the study population.
2.2 Survey tools
2.2.1 Mini-mental state examination
The dataset incorporates the Chinese MMSE scale to evaluate cognitive performance. This 24-question tool covers aspects such as overall capability, responsiveness, concentration, arithmetic skills, memory, linguistic understanding, and self-regulation. Scores are distributed from 0 to 30, with thresholds for cognitive impairment varying by educational background: ≤17 for the non-literate, ≤20 for those with primary education, and ≤24 for individuals with junior high school education or higher. A higher score indicates superior cognitive health (28).
2.2.2 Muscle mass
Appendicular skeletal muscle index (ASMI) (29), adjusted for height, was used to assess muscle mass. Appendicular skeletal muscle mass (ASM) was obtained by using the anthropometric equation for Chinese adults developed by Xu Wen (30) and other scholars. ASM= 0.193 × weight (kg) + 0.107 × height (cm) -4.157 × gender (male =1, female =2) -0.037 × age (years) -2.631. Previous studies have shown (24) that the ASM calculated by this formula is consistent with the results of dual-energy X-ray absorptiometry, and can be used as an economical and feasible index to evaluate the appendicular skeletal muscle mass. After adjusting height, ASMI can be obtained: ASMI=ASM/height (m)2.
2.2.3 Covariates
The covariates included in this study included age, place of residence, gender, pension status, multimorbidity, smoking, drinking, sports, education level, marital status, Basic activities of daily living(BADL), Instrumental activity of daily living (IADL), and body mass index (BMI). Multimorbidity was defined as the simultaneous presence of two or more chronic diseases (31). BADL was assessed using a six-item scale developed by Katz (32), which has a total score ranging from 6 to 18 points. Lower scores indicate better BADL. IADL was assessed by the scale developed by Lawton (33), the total score ranged from 8 to 24, the lower the score, the better the IADL. BMI= weight/height2 (kg/m2) (34).
2.3 Statistical methods
Statistical analyses were conducted using SPSS 27.0 and Mplus 8.7 software. Quantitative data were categorized into normally distributed and non-normally distributed variables, with the former described by x ± s, and the latter by M(P25, P75). Categorical data were presented as frequencies and percentages. To assess differences in general data between genders, we employed independent sample t-tests, rank sum tests, and chi-square tests. For examining variations in cognitive function and muscle mass among older adults at distinct time points, we utilized repeated measures ANOVA and the Friedman test. Correlations between variables were assessed using Pearson or Spearman correlation coefficients. Cross-lagged regression models were formulated to investigate the temporal causality between cognitive function and muscle mass. To elucidate the dose-response relationship, we applied the restricted cubic spline (RCS) model, selecting the optimal model based on the lowest Akaike information criterion (AIC). In conclusion, we explored the relationship between muscle mass and cognitive dysfunction across different subgroups based on the COX model. The significance level was set at α=0.05.
3 Results
3.1 General information
A total of 2536 participants were included and followed up twice for 7 years. There were 1283 males (50.6%) and 1253 females (49.4%), with an average age of (77.54 ± 8.6) years. Independent sample t-test or x2 test showed that MMSE and ASMI differed significantly between males and females (P<0.05). See Table 1 for details.
Table 1
| Characteristics | Total | Male | Female | P | |
|---|---|---|---|---|---|
| Age (years)a | 77.54 ± 8.6 | 76.49 ± 8.02 | 78.62 ± 9.04 | <0.05 | |
| Place of residence b | City | 350 (13.8) | 168 (13.09) | 182 (14.53) | 0.035 |
| Counties and towns | 763 (30.09) | 378 (29.46) | 385 (30.73) | ||
| Rural | 1423 (56.11) | 737 (57.44) | 686 (54.75) | ||
| Pension status b | With family | 2037 (80.32) | 1079 (84.1) | 958 (76.46) | <0.05 |
| Living alone | 474 (18.69) | 188 (14.65) | 286 (22.83) | ||
| Nursing home | 25 (0.99) | 16 (1.25) | 9 (0.72) | ||
| Multimorbidityb | Yes | 770 (30.36) | 355 (27.67) | 415 (33.12) | <0.05 |
| No | 1766 (69.64) | 928 (72.33) | 838 (66.88) | ||
| Smoking b | Yes | 542 (21.37) | 480 (37.41) | 62 (4.95) | <0.05 |
| No | 1994 (78.63) | 803 (62.59) | 1191 (95.05) | ||
| Drinking b | Yes | 530 (20.9) | 427 (33.28) | 103 (8.22) | <0.05 |
| No | 2006 (79.1) | 856 (66.72) | 1150 (91.78) | ||
| Exercise b | Yes | 1009 (39.79) | 522 (40.69) | 487 (38.87) | 0.349 |
| No | 1527 (60.21) | 761 (59.31) | 766 (61.13) | ||
| Education b | Illiteracy | 1185 (46.73) | 352 (27.44) | 833 (66.48) | <0.05 |
| Elementary school | 963 (37.97) | 628 (48.95) | 335 (26.74) | ||
| Junior high | 348 (13.72) | 270 (21.04) | 78 (6.23) | ||
| Secondary school and high school | 20 (0.79) | 14 (1.09) | 6 (0.48) | ||
| College or above | 20 (0.79) | 19 (1.48) | 1 (0.08) | ||
| Marriage b | Married cohabiting | 1425 (56.19) | 903 (70.38) | 522 (41.66) | <0.05 |
| Married and separated | 63 (2.48) | 35 (2.73) | 28 (2.23) | ||
| Divorce | 6 (0.24) | 5 (0.39) | 1 (0.08) | ||
| Widowed | 1008 (39.75) | 307 (23.93) | 701 (55.95) | ||
| Unmarried | 34 (1.34) | 33 (2.57) | 1 (0.08) | ||
| IADL c | 24 (23, 24) | 24 (24, 24) | 24 (22, 24) | <0.05 | |
| BADL c | 18 (18, 18) | 18 (18, 18) | 18 (18, 18) | <0.05 | |
| BMI (kg/m2 )a | 23.75 ± 4.28 | 23.75 ± 4.28 | 24.18 ± 4.69 | <0.05 | |
| MMSE (T1)a | 29 (28, 30) | 28.37 ± 2.43 | 28.01 ± 2.77 | <0.05 | |
| ASMI (kg/m2 ) (T1) a | 6.8 ± 1.36 | 6.8 ± 1.36 | 6.06 ± 1.42 | <0.05 | |
General information (N=2536).
In the table, data marked with superscript 'a' is represented as mean ± standard deviation, data marked with superscript 'b' is presented in the form of n (%), and data marked with superscript 'c' is displayed as M (P25, P75); ADL, Activity of Daily Living (Instrumental); BADL, Basic Activities of Daily Living; BMI, Body Mass Index; MMSE (T1), Mini-Mental State Examination (baseline score in 2011); ASMI (T1), Appendicular Skeletal Muscle Mass Index (baseline value in 2011).
3.2 Correlation analysis of cognitive function and activities of daily living in older adults
Table 2 shows the status quo of the three measurement time points of MMSE and AMSI of older adults, and the differences in the three scores of the two were statistically significant. Correlation analysis showed that MMSE and AMSI were significantly positively correlated at the same time point (0.289 ≤ r ≤ 0.375, P<0.001), and also at different time points (0.281 ≤ r ≤ 0.369, P<0.05). See Table 3 for details.
Table 2
| Items | Projects | T1 | T2 | T3 | P |
|---|---|---|---|---|---|
| The total | MMSE | 29 (28, 30) | 29 (28, 30)① | 29( 27, 30)①② | < 0.05 |
| ASMI | 6.80 ± 1.36 | 6.79 ± 1.35① | 6.78 ± 1.42①② | < 0.05 | |
| male | MMSE | 30 (28, 30) | 29 (28, 30)① | 29(27, 30)①② | < 0.05 |
| ASMI | 7.53 ± 0.80 | 7.52 ± 0.79① | 7.59 ± 0.86①② | < 0.05 | |
| female | MMSE | 29(27, 30) | 29( 27, 30)① | 29 (25, 30)①② | < 0.05 |
| ASMI | 6.06 ± 1.42 | 6.06 ± 1.40① | 5.95 ± 1.40①② | < 0.05 |
Analysis of differences in cognitive function and muscle mass in older adults at different time points (N=2536).
Compared with T1,①P<0.05; Compared with T2, ②P<0.05; MMSE, Mini-Mental State Examination; ASMI, Appendicular Skeletal Muscle Mass Index. The MMSE is presented using the M(P25, P75), while ASMI is displayed with the mean ± standard deviation.
Table 3
| MMSE(T1) | MMSE(T2) | MMSE(T3) | ASMI(T1) | ASMI(T2) | ASMI(T3) | |
|---|---|---|---|---|---|---|
| MMSE(T1) | 1 | |||||
| MMSE(T2) | 0.320** | 1 | ||||
| MMSE(T3) | 0.324** | 0.379* | 1 | |||
| ASMI(T1) | 0.312** | 0.289** | 0.369** | 1 | ||
| ASMI(T2) | 0.312** | 0.289** | 0.367** | 0.999** | 1 | |
| ASMI(T3) | 0.309** | 0.281** | 0.375** | 0.916** | 0.916** | 1 |
Correlation analysis of MMSE and AMSI at the same and different time points in older adults (N=2536).
*P<0.05, **P<0.01; MMSE, Mini-Mental State Examination; ASMI, Appendicular Skeletal Muscle Mass Index. MMSE(T1), MMSE(T2), MMSE(T3): Mini-Mental State Examination scores at Time Points 1 (2011), 2 (2014), and 3 (2018), respectively. ASMI(T1), ASMI(T2), ASMI(T3): Appendicular Skeletal Muscle Mass Index measurements at Time Points 1 (2011), 2 (2014), and 3 (2018), respectively.
3.3 CLPM analysis of cognitive function and muscle mass in older adults
The Cross-lag model was used to analyze the dynamic association between cognitive function and muscle mass, as well as the direction of causality. After controlling the confounding factors such as age, gender, pension, multimorbidity, smoking, drinking, education, marriage, BADL, IADL, and BMI, the model fitted well (CFI=0.996>0.90, TLI=0.985>0.90, SRMR=0.027<0.05, RMSEA=0.076<0.08). The autoregression effect suggested that cognitive function and muscle mass at the Tn moment were affected by their own Tn-1 level, suggesting that the stability of cognitive function was at a medium-low level, and the stability of muscle mass was at a high level. The path coefficients of MMSE to ASMI from T1 to T2 were not statistically significant (P>0.05), while the path coefficients from T2 to T3 were statistically significant (P<0.05) and the path coefficient β>0, suggesting that when MMSE decreased to a certain extent, the decline of cognitive function could cause the decline of muscle mass. The path coefficients of ASMI→MMSE in T1→T2 and T2→T3 were statistically significant (P<0.05), and the path coefficients β were all greater than 0, suggesting that the decline of muscle mass can cause the decline of cognitive function. The path coefficient of the ASMI→MMSE path direction was significantly greater than that of the MMSE→ ASMI path direction, suggesting that the influence of muscle mass on cognitive function was greater than that of cognitive function on muscle mass. The model results are shown in Figure 2, and the path coefficients are shown in Table 4.
Figure 2
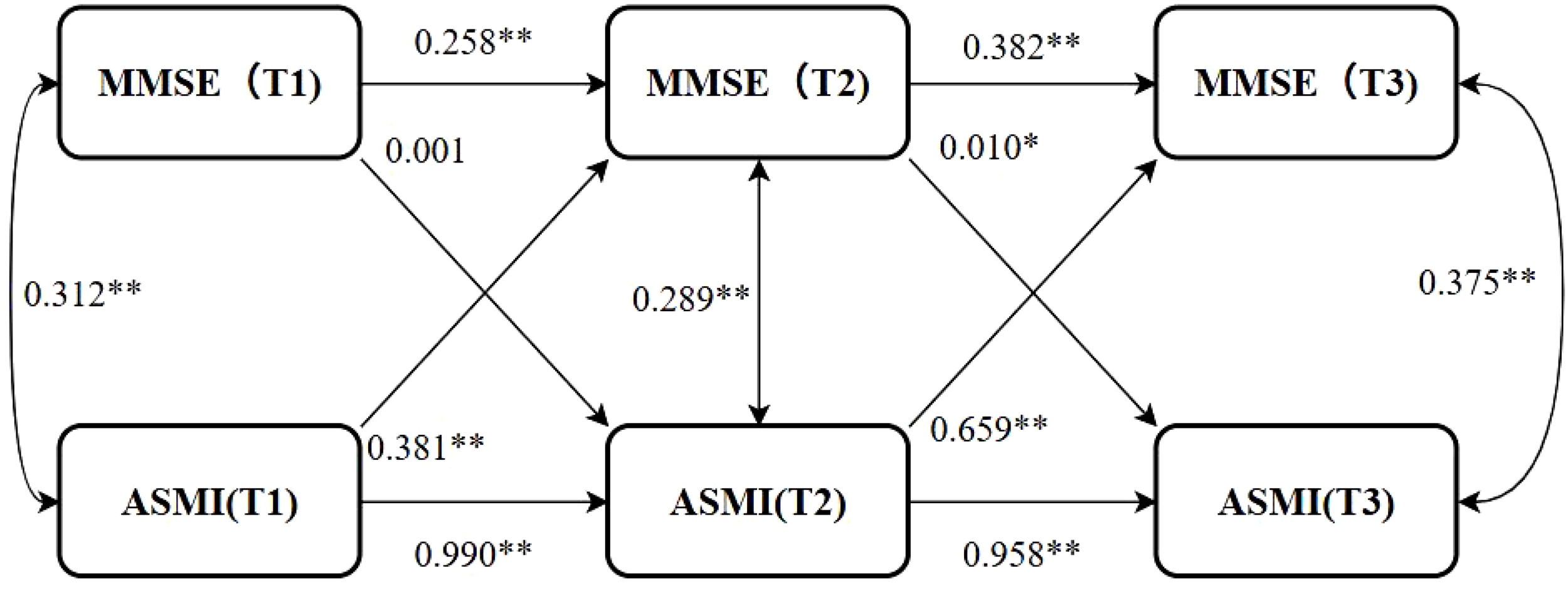
Cross-lag model. *P<0.05, **P<0.01; MMSE(T1), MMSE(T2), MMSE(T3): Mini-Mental State Examination scores at Time Points 1 (2011), 2 (2014), and 3 (2018), respectively. ASMI(T1), ASMI(T2), ASMI(T3): Appendicular Skeletal Muscle Mass Index measurements at Time Points 1 (2011), 2 (2014), and 3 (2018), respectively.
Table 4
| The path | The total | male | female | |||
|---|---|---|---|---|---|---|
| T1→T2 | T2→T3 | T1→T2 | T2→T3 | T1→T2 | T2→T3 | |
| MMSE autoregressive | 0.258** | 0.382** | 0.205** | 0.341** | 0.288** | 0.407** |
| ASMI autoregressive | 0.990** | 0.958** | 0.983** | 0.852** | 0.986** | 0.907** |
| MMSE→ASMI | 0.001 | 0.010* | -0.001 | 0.008 | 0.001 | 0.009 |
| ASMI→MMSE | 0.381** | 0.659** | 0.325** | 0.555** | 0.351** | 0.792** |
Path coefficients (N=2536).
*P<0.05, **P<0.01; The MMSE is presented using the M(P25, P75), while ASMI is displayed with the mean ± standard deviation.
When stratified by gender, the other covariates were the same as those in the general population, and the model fitted well across different genders (Male: CFI = 0.987, TLI=0.955, SRMR=0.046, RMSEA<0.001; Female: CFI=0.998, TLI=0.992, SRMR=0.018, RMSEA=0.042). The results showed that there were gender differences in the causal time sequence relationship between cognitive function and muscle mass in older adults. The autoregressive effect showed that the stability of cognitive function and muscle mass in females was higher than that in males. The path coefficients of ASMI→MMSE in each time period were statistically significant in both men and women (P<0.05), and the path coefficients in women were significantly higher than those in men, suggesting that the effect of muscle mass on cognitive function in women was more significant than that in men. However, in the path direction of MMSE→ASMI, there were no significant differences in the path coefficients for males and females from T1→T2 and from T2→T3 (P>0.05), indicating that cognitive function does not predict muscle mass in stratified specific analyses. The path coefficients are shown in Table 4.
3.4 Dose-response relationship between muscle mass and cognitive impairment
CLPM analysis showed that the effect of muscle mass on cognitive function was greater than that of cognitive function on muscle mass, highlighting the importance of muscle mass in this relationship. The dose-response relationship between muscle mass and cognitive function was further explored. The optimal number of nodes in this model is 3-7. According to the spline regression coefficient and AIC criterion, this study found that in the model related to cognitive dysfunction, AICtotal=6456.58, AICmale=1908.71, AICfemale=3994.858 reached the minimum, and the number of nodes was 7, 5, and 7 respectively. After controlling for confounding factors such as age, gender, elderly care, smoking, drinking, education, marriage, BADL, IADL, and BMI, there was a nonlinear dose-response relationship between muscle mass and cognitive impairment (Poverall trend <0.05, Pnon-linear <0.05). When ASMI < 6.96 kg/m2, HR > 1, it is suggested that muscle mass is a risk factor for cognitive dysfunction in older adults. After stratified analysis, there was a nonlinear dose-response relationship between muscle mass and cognitive dysfunction in older men (Poverall trend <0.05, Pnon-linear <0.05). When ASMI<7.45kg/m2, HR>1, suggests that muscle mass is a risk factor for cognitive dysfunction in older men at this time. There was a nonlinear dose-response relationship between muscle mass and cognitive dysfunction in older women (Poverall trend <0.05, Pnon-linear <0.05). When ASMI <5.68kg/m2, HR >1, indicating that muscle mass was a risk factor for cognitive dysfunction in older women. The model results are shown in Figures 3–5.
Figure 3

Dose-response relationship between skeletal muscle mass and cognitive impairment in the overall older adults population. RCS, Restricted Cubic Spline Model; HR(95%CI), Hazard Ratio (95% Confidence Interval); ASMI, Appendicular Skeletal Muscle Mass Index.
Figure 4

Dose-response relationship between skeletal muscle mass and cognitive dysfunction in male. See Figure 3 for details.
Figure 5
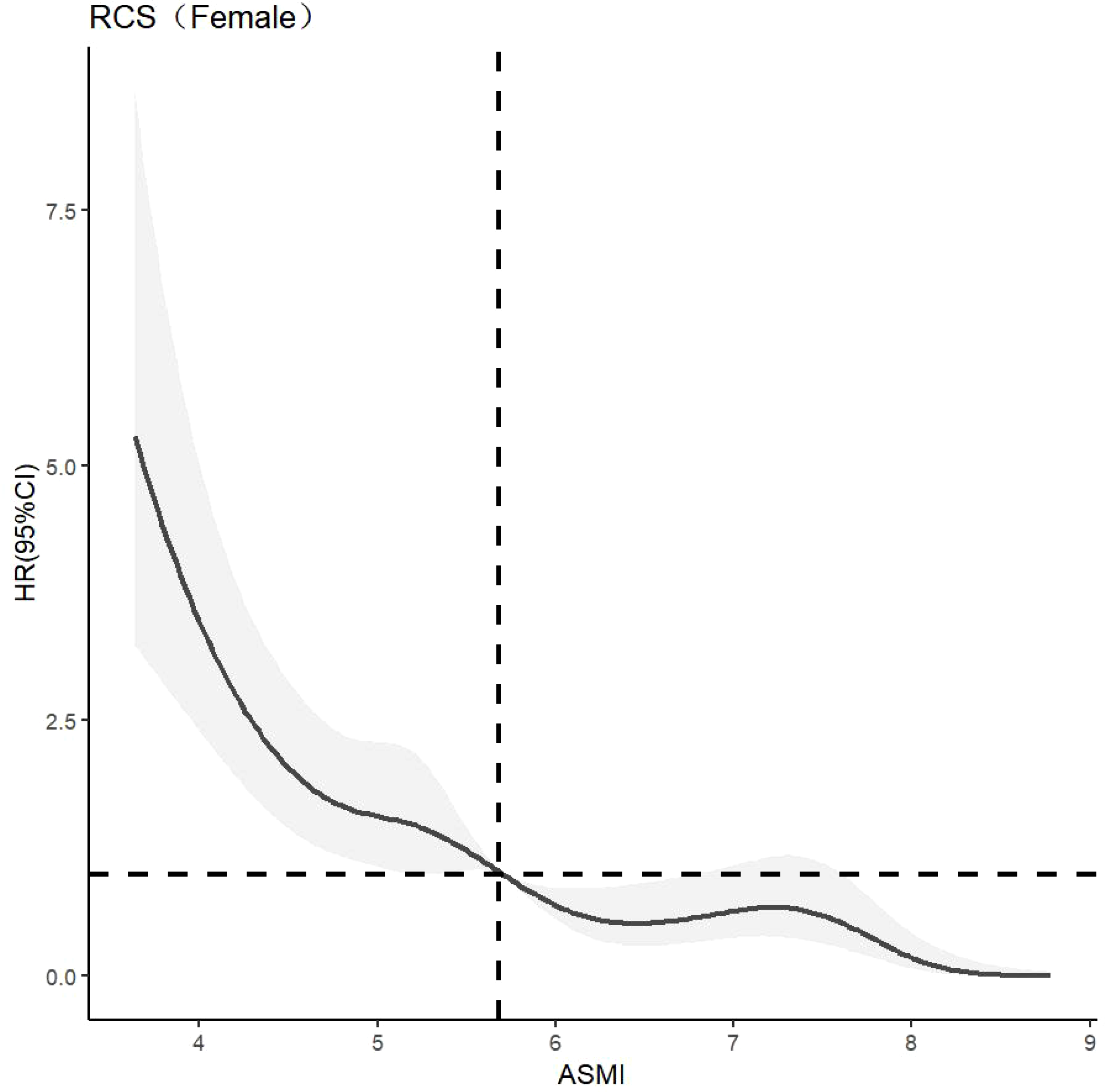
Dose-response relationship between skeletal muscle mass and cognitive dysfunction in female. See Figure 3 for details.
3.5 Subgroup analysis
As age advances, the relationship between sarcopenia and cognitive impairment in the elderly becomes increasingly evident, prompting this study to perform age-stratified subgroup analyses. This methodological choice bolsters the research’s credibility and persuasiveness while mitigating the risk of biases. The subgroup analyses, employing the COX regression model, demonstrated a correlation between decreased muscle mass and the onset of cognitive dysfunction in specific elderly subgroups. Interaction tests further exposed notable variations in the link between muscle mass and cognitive function across different age, gender and education(Pfor interaction <0.05). Notably, the 60-69 age cohort exhibited the most robust association (HR=0.44, 95%CI:0.32~0.61), with females exhibiting a more pronounced protective effect (HR=0.48, 95%CI:0.44~0.53) than males (HR=0.62, 95%CI:0.55~0.71). Additionally, individuals with lower levels of education displayed a consistent protective effect (HR=0.51-0.59, all P<0.05.), suggesting that the influence of muscle mass on cognitive health is multifaceted and influenced by a range of demographic factors.The detailed results can be found in Figure 6.
Figure 6
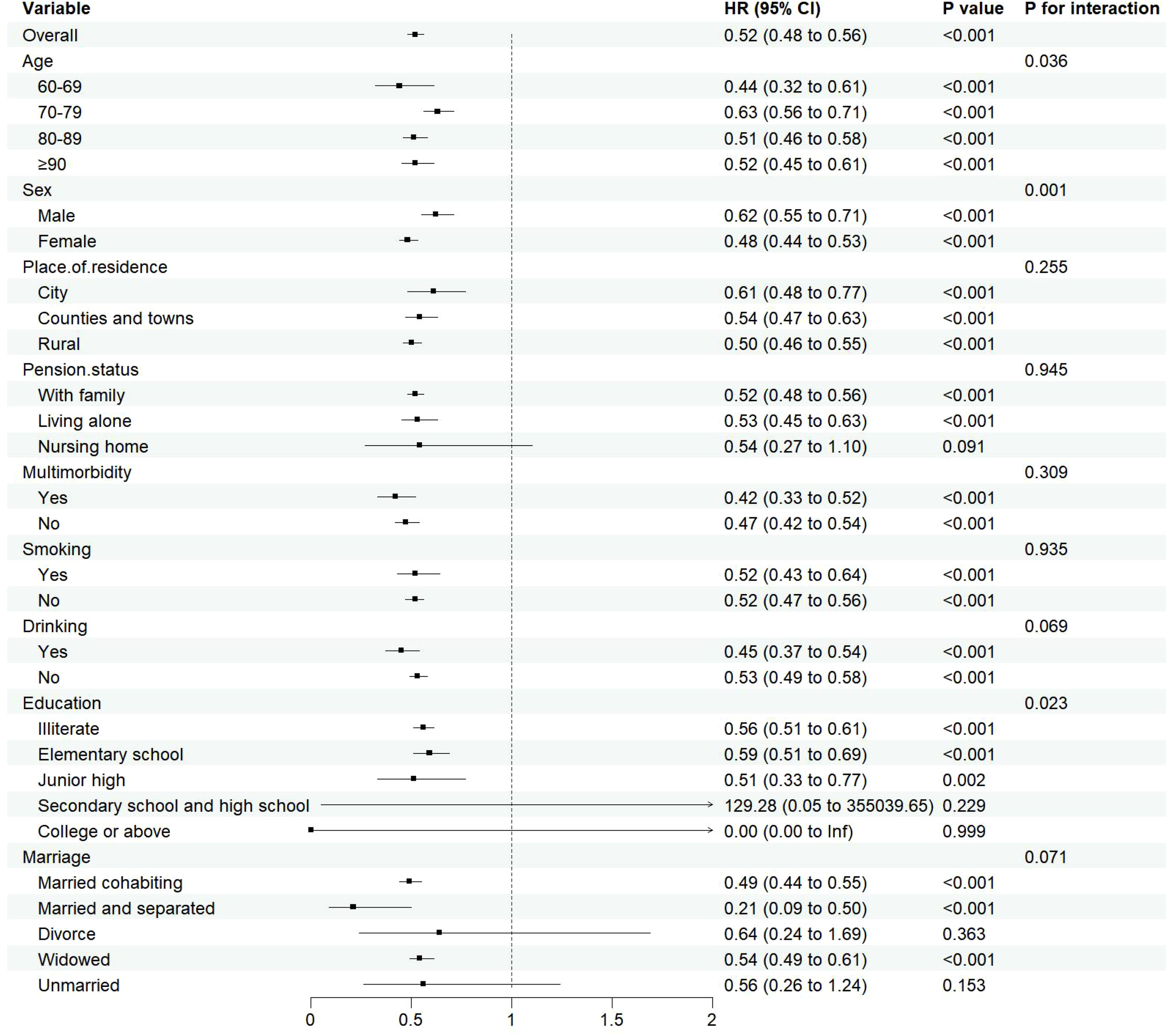
Subgroup analysis results.
4 Discussion
The present study, conducted as a longitudinal investigation, revealed a bidirectional relationship between muscle mass and cognitive function. Specifically, muscle mass can predict changes in cognitive function, while cognitive dysfunction at certain stages can reciprocally predict changes in muscle mass. Moreover, the influence of muscle mass on cognitive function appears to be more pronounced, particularly evident in women.
The results suggest that cognitive function and muscle mass are interpredictive in older adults. Previous systematic reviews and meta-analyses, such as those by Gao Qianqian, have identified cognitive impairment as a risk factor for sarcopenia (22). This study refines this causal inference through its longitudinal design, observing for the first time the predictive role of cognitive function on muscle mass in early stages. Cognitive decline in older adults is associated with reduced orientation and memory. When this decline reaches a critical level, it leads to diminished daily living abilities, decreased physical activity, and inadequate nutritional intake, ultimately resulting in reduced muscle mass (22). Consistent with our findings, Beeri reported in a U.S.-based longitudinal study that muscle mass loss predicted cognitive impairment (23). This may be due to decreased muscle mass increasing the incidence of adverse clinical outcomes like falls, fractures, and physical disability, which limit activity and reduce social participation, further exacerbating cognitive impairment (35).
Additionally, our study found that the impact of muscle mass on cognitive function was more significant than the reverse effect. This may be because muscle activity directly influences basic physiological processes of brain health and function. Muscle activity stimulates the production of nerve growth factors, improves blood circulation, increases oxygen and nutrient supply to the brain, and affects hormone levels such as testosterone and growth hormone, thereby promoting brain health and neuroplasticity (13, 36, 37), which in turn influences cognitive function.
General information indicates that men typically exhibit higher levels of cognitive function than women, consistent with Sivaniya’s study (24). Some scholars (25) consider the female gender a risk factor for cognitive dysfunction, possibly due to post-menopausal reductions in estrogen and related hormones, as well as differences in brain structure, leading to lower cognitive function in women compared to men. Similarly, muscle mass is generally higher in men than in women, reflecting gender-based differences in body composition. Women typically have a higher body fat percentage and lower muscle mass than men (26). Additionally, older women have longer life expectancies but may experience lower cognitive function and muscle mass due to the cumulative effects of less physical exercise, poorer health status, and broader differences in social and family roles (38).
Due to these gender differences, stratified analysis revealed that cognitive function was more significantly affected by muscle mass in women than in men, consistent with Lee (39). These differences are supported by gender-specific physiological factors, where gender hormones influence sarcopenia and hippocampal plasticity, activation, and morphology. Estradiol not only regulates muscle satellite cell proliferation (e.g., by promoting MyoD expression) through ERα receptors (40) but also enhances synaptic plasticity in the central nervous system via the BDNF-TrkB pathway (41), which is more active in females, playing a key role in gender differences.
Further analysis identified a nonlinear dose-response relationship between muscle mass and the risk of cognitive impairment. As muscle mass decreases, the risk of cognitive impairment increases nonlinearly. The study suggests maintaining ASMI above 6.96 kg/m² in older adults, 7.45 kg/m² in older men, and 5.68 kg/m² in older women. This aligns with the Asian Consensus for the Diagnosis of Sarcopenia (7.0 kg/m² for men and 5.4 kg/m² for women) (22), indicating that these values can serve as targets for cognitive protective interventions. Demura’s study (27) also found similar results but used only linear and logistic regression, which did not fully capture the dynamic change trajectory of muscle mass and its impact on cognitive function. The recommended value of ASMI is higher in men than in women, which may be related to the difference in body composition between men and women. Fat mass is generally higher in women than in men, and the initial muscle mass is also higher in men than in women (26). This finding is consistent with the gender differences that Xu Wen (30) considered when developing the anthropometric equation.
Subgroup analysis in this study has uncovered the heterogeneity in the protective association between muscle mass and cognitive function across different groups, suggesting that the underlying mechanisms may involve the interplay of biological and social factors. Age-stratified analysis reveals that the strongest association is found in the 60-69 age group, possibly linked to a critical biological window during the transition from middle to old age: at this stage, mitochondrial function in skeletal muscle has not yet significantly declined (42), and neurotrophic factors secreted by muscles remain at high levels (43), both of which synergistically enhance neural plasticity. After the age of 70, the association weakens, potentially due to the exacerbation of aging-related systemic inflammation and insulin resistance, pathological processes that can disrupt the muscle-brain metabolic coupling (44). Gender differences show that women exhibit a stronger protective effect, which has been previously discussed. Regarding education level, the protective effect is stable in those with low education, but in the highly educated subgroup, the sample size is severely insufficient (only 0.79%), leading to statistical overfitting in the Cox proportional hazards model and rendering these results statistically insignificant. This insufficiency in sample size may lead to extreme hazard ratios that do not accurately reflect the actual situation.
The strength of this study is that the cross-lagged regression model based on a nationally representative sample of the CLHLS database was used for a longitudinal study to continuously observe the changes in muscle mass and cognitive function, thereby more precisely revealing the causal temporal relationship between them. In addition, this study further explored the dose-response relationship between muscle mass and cognitive function to avoid the limitations caused by categorical data and make the effect of muscle mass on cognitive function clearer. This study also has limitations. Firstly, although a standardized scale was used to assess cognitive function, it may still be affected by environmental interference and recall bias. Secondly, the use of relative skeletal muscle mass index based on weight, height, weight, and age may be insufficient to assess muscle mass, such as difficulty in taking into account individual differences. Therefore, it is necessary to further explore the related indicators that affect muscle mass in the future to fully reveal the biological mechanism and dynamic relationship between muscle mass and cognitive function in older adults.
5 Conclusion
Based on the analysis of longitudinal data from the CLHLS cohort, this study revealed a dynamic bidirectional association between muscle mass (ASMI) and cognitive function (MMSE) in older adults, with significant gender heterogeneity. The results of this study suggest that improving muscle mass through reasonable dietary intervention and increasing physical exercise may have a positive effect on cognitive function in older adults, which provides a relevant reference for cognitive intervention strategies in older adults.
Statements
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://opendata.pku.edu.cn/dataverse/CHADS.
Ethics statement
The studies involving humans were approved by Research Ethics Committees of Duke University and Peking University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YS: Conceptualization, Writing – original draft. YZ: Conceptualization, Writing – original draft. XY: Methodology, Writing – original draft. JY: Methodology, Writing – review & editing. SW: Methodology, Writing – review & editing. YH: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Zhang C Liu Y Zeng L Luo X Fan G Shi H et al . Combined associations of cognitive impairment and psychological resilience with all-cause mortality in community-dwelling older adults. J Affect Disord. (2024) 351:962–70. doi: 10.1016/j.jad.2024.02.015
2
Morozova A Zorkina Y Abramova O Pavlova O Pavlov K Soloveva K et al . Neurobiological highlights of cognitive impairment in psychiatric disorders. Int J Mol Sci. (2022) 23:1217. doi: 10.3390/ijms23031217
3
Amini N Ibn Hach M Lapauw L Dupont J Vercauteren L Verschueren S et al . Meta-analysis on the interrelationship between sarcopenia and mild cognitive impairment, Alzheimer’s disease and other forms of dementia. J cachexia sarcopenia Muscle. (2024) 15:1240–53. doi: 10.1002/jcsm.13485
4
Plassman BL Langa KM McCammon RJ Fisher GG Potter GG Burke JR et al . Incidence of dementia and cognitive impairment, not dementia in the United States. Ann Neurol. (2011) 70:418–26. doi: 10.1002/ana.22362
5
Kivipelto M Ngandu T Laatikainen T Winblad B Soininen H Tuomilehto J . Risk score for the prediction of dementia risk in 20 years among middle aged people: a longitudinal, population-based study. Lancet Neurol. (2006) 5:735–41. doi: 10.1016/S1474-4422(06)70537-3
6
Bezdicek O Červenková M Georgi H Schmand B Hladká A Rulseh A et al . Long-term cognitive trajectory and activities of daily living in healthy aging. Clin neuropsychologist. (2021) 35:1381–97. doi: 10.1080/13854046.2020.1745895
7
Pérez Palmer N Trejo Ortega B Joshi P . Cognitive impairment in older adults: epidemiology, diagnosis, and treatment. Psychiatr Clinics North America. (2022) 45:639–61. doi: 10.1016/j.psc.2022.07.010
8
Cruz-Jentoft AJ Bahat G Bauer J Boirie Y Bruyère O Cederholm T et al . & Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2 (2019). Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. (2019) 48:16–31. doi: 10.1093/ageing/afy169
9
Cruz-Jentoft AJ Sayer AA . Sarcopenia. Lancet (London England). (2019) 393:2636–46. doi: 10.1016/S0140-6736(19)31138-9
10
Sayer AA Cruz-Jentoft A . Sarcopenia definition, diagnosis and treatment: consensus is growing. Age Ageing. (2022) 51:afac220. doi: 10.1093/ageing/afac220
11
Kim G Kim JH . Impact of skeletal muscle mass on metabolic health. Endocrinol Metab (Seoul Korea). (2020) 35:1–6. doi: 10.3803/EnM.2020.35.1.1
12
Kim M Won CW . Sarcopenia is associated with cognitive impairment mainly due to slow gait speed: results from the Korean frailty and aging cohort study (KFACS). Int J Environ Res Public Health. (2019) 16:1491. doi: 10.3390/ijerph16091491
13
Huang XT Lv X Jiang H . The weight-adjusted-waist index and cognitive impairment among U.S. older adults: a population-based study. Front Endocrinol. (2023) 14:1276212. doi: 10.3389/fendo.2023.1276212
14
Sui SX Balanta-Melo J Pasco JA Plotkin LI . Musculoskeletal deficits and cognitive impairment: epidemiological evidence and biological mechanisms. Curr osteoporosis Rep. (2022) 20:260–72. doi: 10.1007/s11914-022-00736-9
15
Nourhashémi F Andrieu S Gillette-Guyonnet S Reynish E Albarède JL Grandjean H et al . Is there a relationship between fat-free soft tissue mass and low cognitive function? Results from a study of 7,105 women. J Am Geriatrics Soc. (2002) 50:1796–801. doi: 10.1046/j.1532-5415.2002.50507.x
16
Yang Y Xiao M Leng L Jiang S Feng L Pan G et al . A systematic review and meta-analysis of the prevalence and correlation of mild cognitive impairment in sarcopenia. J cachexia sarcopenia Muscle. (2023) 14:45–56. doi: 10.1002/jcsm.13143
17
Ragusa FS Veronese N Vernuccio L Dominguez LJ Smith L Bolzetta F et al . Mild cognitive impairment predicts the onset of Sarcopenia: a longitudinal analysis from the English Longitudinal Study on Ageing. Aging Clin Exp Res. (2024) 36:129. doi: 10.1007/s40520-024-02781-z
18
Yoon DH Kang D Kim HJ Kim JS Song HS Song W . Effect of elastic band-based high-speed power training on cognitive function, physical performance and muscle strength in older women with mild cognitive impairment. Geriatrics gerontology Int. (2017) 17:765–72. doi: 10.1111/ggi.12784
19
Moon HY Becke A Berron D Becker B Sah N Benoni G et al . Running-induced systemic cathepsin B secretion is associated with memory function. Cell Metab. (2016) 24:332–40. doi: 10.1016/j.cmet.2016.05.025
20
Hook G Reinheckel T Ni J Wu Z Kindy M Peters C et al . Cathepsin B gene knockout improves behavioral deficits and reduces pathology in models of neurologic disorders. Pharmacol Rev. (2022) 74:600–29. doi: 10.1124/pharmrev.121.000527
21
Hu Y Peng W Ren R Wang Y Wang G . Sarcopenia and mild cognitive impairment among elderly adults: The first longitudinal evidence from CHARLS. J cachexia sarcopenia Muscle. (2022) 13:2944–52. doi: 10.1002/jcsm.13081
22
Gao Q Hu K Yan C Zhao B Mei F Chen F et al . Associated factors of sarcopenia in community-dwelling older adults: A systematic review and meta-analysis. Nutrients. (2021) 13:4291. doi: 10.3390/nu13124291
23
Beeri MS Leugrans SE Delbono O Bennett DA Buchman AS . Sarcopenia is associated with incident Alzheimer’s dementia, mild cognitive impairment, and cognitive decline. J Am Geriatrics Soc. (2021) 69:1826–35. doi: 10.1111/jgs.17206
24
Subramaniapillai S Almey A Natasha Rajah M Einstein G . Sex and gender differences in cognitive and brain reserve: Implications for Alzheimer’s disease in women. Front neuroendocrinology. (2021) 60:100879. doi: 10.1016/j.yfrne.2020.100879
25
Jia L Du Y Chu L Zhang Z Li F Lyu D et al . Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: a cross-sectional study. Lancet Public Health. (2020) 5:e661–71. doi: 10.1016/S2468-2667(20)30185-7
26
Alodhayani AA . Sex-specific differences in the prevalence of sarcopenia among pre-frail community-dwelling older adults in Saudi Arabia. Saudi J Biol Sci. (2021) 28:4005–9. doi: 10.1016/j.sjbs.2021.04.010
27
Demura T Okuno T Miwa T Iritani O Nakano H Yamamoto J et al . Sarcopenia and decline in appendicular skeletal muscle mass are associated with hypoperfusion in key hubs of central autonomic network on 3DSRT in older adults with progression of normal cognition to Alzheimer’s disease. Geriatrics gerontology Int. (2023) 23:16–24. doi: 10.1111/ggi.14515
28
Ma W Wu B Gao X Zhong R . Association between frailty and cognitive function in older Chinese people: A moderated mediation of social relationships and depressive symptoms. J Affect Disord. (2022) 316:223–32. doi: 10.1016/j.jad.2022.08.032
29
Chen LK Woo J Assantachai P Auyeung TW Chou MY Iijima K et al . Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Directors Assoc. (2020) 21:300–307.e2. doi: 10.1016/j.jamda.2019.12.012
30
Wen X Wang M Jiang CM Zhang YM . Anthropometric equation for estimation of appendicular skeletal muscle mass in Chinese adults. Asia Pacific J Clin Nutr. (2011) 20:551–6.
31
Sun X Liu X Wang X Pang C Yin Z Zang S . Association between residential proximity to major roadways and chronic multimorbidity among Chinese older adults: a nationwide cross-sectional study. BMC geriatrics. (2024) 24:111. doi: 10.1186/s12877-024-04712-z
32
Katz S Ford AB Moskowitz RW Jackson BA Jaffe MW . Studies of illness in the aged. The index of adl: a standardized measure of biological and psychosocial function. JAMA. (1963) 185:914–9. doi: 10.1001/jama.1963.03060120024016
33
Lawton MP Brody EM . Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. (1969) 9:179–86. doi: 10.1093/geront/9.3_Part_1.179
34
Lv Y Zhang Y Li X Gao X Ren Y Deng L et al . Body mass index, waist circumference, and mortality in subjects older than 80 years: a Mendelian randomization study. Eur Heart J. (2024) 45:2145–54. doi: 10.1093/eurheartj/ehae206
35
Cunningham C O’ Sullivan R Caserotti P Tully MA . Consequences of physical inactivity in older adults: A systematic review of reviews and meta-analyses. Scandinavian J Med Sci sports. (2020) 30:816–27. doi: 10.1111/sms.13616
36
Yang J Jiang F Yang M Chen Z . Sarcopenia and nervous system disorders. J Neurol. (2022) 269:5787–97. doi: 10.1007/s00415-022-11268-8
37
Liu C Wong PY Chow SKH Cheung WH Wong RMY . Does the regulation of skeletal muscle influence cognitive function? A scoping review of pre-clinical evidence. J orthopaedic translation. (2022) 38:76–83. doi: 10.1016/j.jot.2022.10.001
38
Chaoping P Cen W Kelifa MO Xuyang L Wang P . Gender disparity in disability among Chinese oldest-old: Age and cohort trends. J Women Aging. (2023) 35:243–58. doi: 10.1080/08952841.2022.2031711
39
Lee HJ Choi JY Hong D Kim D Min JY Min KB . Sex differences in the association between sarcopenia and mild cognitive impairment in the older Korean population. BMC geriatrics. (2023) 23:332. doi: 10.1186/s12877-023-03911-4
40
Geraci A Calvani R Ferri E Marzetti E Arosio B Cesari M . Sarcopenia and menopause: the role of estradiol. Front Endocrinol. (2021) 12:682012. doi: 10.3389/fendo.2021.682012
41
Maggio M Lauretani F Ceda GP . Sex hormones and sarcopenia in older persons. Curr Opin Clin Nutr Metab Care. (2013) 16:3–13. doi: 10.1097/MCO.0b013e32835b6044
42
Xu J Wan CS Ktoris K Reijnierse EM Maier AB . Sarcopenia is associated with mortality in adults: A systematic review and meta-analysis. Gerontology. (2022) 68:361–76. doi: 10.1159/000517099
43
Sleiman SF Henry J Al-Haddad R El Hayek L Abou Haidar E Stringer T et al . Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body β-hydroxybutyrate. eLife. (2016) 5:e15092. doi: 10.7554/eLife.15092
44
Komleva Y Chernykh A Lopatina O Gorina Y Lokteva I Salmina A et al . Inflamm-aging and brain insulin resistance: new insights and role of life-style strategies on cognitive and social determinants in aging and neurodegeneration. Front Neurosci. (2021) 14:618395. doi: 10.3389/fnins.2020.618395
Summary
Keywords
older adults, cognitive function, muscle mass, cross lag, restricted cubic splines
Citation
Shi Y, Zhang Y, Yang X, Yang J, Wang S and Hong Y (2025) The relationship between cognitive function and muscle mass in older adults: a longitudinal study based on CLHLS. Front. Psychiatry 16:1595625. doi: 10.3389/fpsyt.2025.1595625
Received
18 March 2025
Accepted
19 May 2025
Published
03 June 2025
Volume
16 - 2025
Edited by
Chi Shen, Xi’an Jiaotong University, China
Reviewed by
Mati Pääsuke, University of Tartu, Estonia
Jingxian Sun, Nanjing University of Chinese Medicine, China
Updates
Copyright
© 2025 Shi, Zhang, Yang, Yang, Wang and Hong.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: YanFang Hong, 15858621957@163.com
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.