- 1Medical School, University of Cyprus, Nicosia, Cyprus
- 2Department of Clinical Sciences/Psychiatry, Umeå University, Umeå, Sweden
Introduction: Effective management of suicidality and impulsivity in hospitalized psychiatric patients is vital for improving outcomes and ensuring safety. Psychiatric patients, especially those with schizophrenia, exhibit higher tendencies towards aggressive and suicidal behaviours. This study aims to explore sex-specific associations between lipid profiles, impulsivity, and suicidality among psychiatric inpatients.
Methods: A total of 158 psychiatric inpatients (92 men and 66 women) were assessed using the Barratt Impulsiveness Scale, the Columbia Suicide Severity Rating Scale, and the Karolinska Interpersonal Violence Scale. Serum lipid levels (total cholesterol, LDL, HDL) were obtained from fasting blood samples.
Results: Among men, higher total cholesterol and LDL were positively correlated with impulsivity (LDL and BIS-11 total score: rho = .308, p = .006). In women, higher HDL was associated with lower suicidality (HDL and lifetime suicide attempt frequency: rho = –.374, p = .021). Regression confirmed LDL predicts impulsivity in men (R squared = .265, p = .031), and HDL, LDL, age, and BMI explain 52 percent of suicidality variance in women (R squared = .523, p = .0006).
Conclusion: Elevated LDL may indicate higher impulsivity in men, while low HDL suggests higher suicide risk in women. Lipid monitoring could enhance risk assessment in psychiatric care.
Introduction
Impulsivity and suicidality are major concerns among psychiatric inpatients, and both phenomena have been linked to an elevated risk of harm to oneself or others (1). The factors that contribute to impulsive behaviors and suicidal actions are complex, involving neurobiological, psychological, and social components (2). Recent research has highlighted associations between metabolic indicators, such as lipid levels, and high-risk behaviors, suggesting their potential as biomarkers linked to underlying biological processes (3, 4).
Abnormalities in the lipid profile, particularly involving cholesterol subfractions, have been suggested to correlate with aggression, violent behavior, and self-injurious actions in specific clinical populations (5, 6). Low total cholesterol has been reported in individuals who exhibit severe depressive symptoms, heightened impulsivity, and suicidality (7–11). Several studies suggest that low total cholesterol is related to a higher risk of suicidal behavior, especially in violent or impulsive attempts (12, 13).
In violent suicide attempters, investigations have also revealed reduced platelet serotonin and cholesterol concentrations, hinting at a potential link between serotonergic dysregulation, lipid imbalance, and the propensity for more aggressive forms of self-harm (14–16). These findings are further supported by evidence that low serum cholesterol might co-occur with habitually violent tendencies, as seen in both clinical populations and offender cohorts (17, 18). One explanation points to disruptions in serotonin metabolism, where reduced cholesterol in neuronal membranes may alter the function of serotonin receptors and transporters, potentially exacerbating impulsive or aggressive tendencies (19, 20). This hypothesis is supported by reports of lower serum cholesterol among individuals with a history of violent suicide attempts, as opposed to non-violent attempts (14, 15, 21, 22). Furthermore, large-scale population studies have found that reduced lipid levels might be linked to overall increases in both self-harm and externalizing behaviors (23, 24).
On the other hand, some authors argue that the relationship between low cholesterol and suicidality is neither universally observed nor straightforward, noting that additional factors can influence both lipid levels and psychiatric outcomes (25–27). Factors such as age, psychiatric diagnoses, and comorbid medical conditions may modulate the influence of lipids on behavior (28–30). For example, certain data suggest that higher cholesterol may also be associated with suicidal tendencies in specific subgroups only, adding complexity to the discussion (31, 32).
Sex in particular is emerging as an important factor in psychiatric disorders and their treatment (33, 34). Yet, relatively few studies have examined how sex might influence the relationship between lipid profiles and risk-taking or self-harm behaviors, despite indications of divergent lipid-risk relationships between men and women (15, 35). Biological differences, including hormonal regulation, could affect how lipids are metabolized and how they interact with neural circuits linked to impulsive decision-making (5). Women who attempt suicide have been reported to have lower serum cholesterol levels (36). Hormonal fluctuations in women may make them more susceptible to mood dysregulation, which in turn could intensify suicidal thoughts under certain metabolic conditions (8, 37). Conversely, men might exhibit alternative lipid-mediated pathways that contribute to impulsive or aggressive behavior, potentially leading to higher rates of lethal self-harm (11). Furthermore, lifestyle and psychosocial factors, such as stressors or cultural norms, could potentially differ between men and women, possibly influencing how lipid levels are associated with risk-taking or self-harm behaviors. Although some evidence suggests sex-specific mechanisms (38), further research is needed to clarify these relationships (39–41).
The primary aim of this study is to investigate sex-specific associations between lipid profiles (total cholesterol, LDL, HDL, and triglycerides) and impulsivity and suicidality among psychiatric inpatients. We hypothesize that lipid dysregulation is differentially associated with impulsivity and suicidal behaviors in men and women. Specifically, we predict that elevated LDL cholesterol levels are associated with higher impulsivity primarily among men, whereas lower HDL cholesterol levels correlate with increased suicidality predominantly among women. Our findings, interpreted considering existing literature, seek to provide new insights into the multifaceted roles that lipids may play in psychiatric conditions marked by elevated risk of self-harm or aggression (42, 43).
Methods
Study settings
The study participants were recruited among inpatients at Nicosia General Hospital Psychiatric Clinic and Athalassa Psychiatric hospital in Cyprus between the years 2019-2024. The patients were asked to take part in a study on biological markers focusing on suicidal and violent behavior. The study protocols (Dnr EEBK/EΠ/2019/15) were approved by the Cyprus Bioethics committee, and all patients gave their written informed consent before inclusion in the study.
Subjects
The cohort study consists of 158 patients. There were 92 men with a mean age of 40.3 years (SD: 12.7, range: 19-70) and 66 women, mean age of 41.9 years (SD: 12.7, range: 19-67). To be included in the study, all participants must exhibit a fair capacity to communicate verbally and in writing the Greek language and be 18 years or older. Psychiatric inpatients with diagnoses of mood disorder (depression, bipolar disorder), psychosis spectrum disorder (schizoaffective disorder, schizophrenia, delusional disorder), personality disorders and anxiety related disorders were included in the study.
Exclusion criteria were inability to give informed consent to participate in the study, ed psychiatrist and diagnosis was established according to DSM-5 criteria. Further information was acquired from the medical file of the patient and the multidisciplinary treatment team of the clinic. Trained clinicians performed and assessed all interviews and ratings.
In this study 43% of the patients fulfilled criteria for having schizophrenia, 24% for mood disorders and 5% for anxiety disorder. The subjects were generally somatically healthy. The non-psychiatric diagnoses of the patients at study inclusion were metabolic syndrome and diabetes, chronic obstructive pulmonary disease, hypertension, glaucoma and hypo/hyperthyroidism.
Serum cholesterol assays
Blood samples were collected under controlled conditions from the antecubital vein after participants had fasted overnight, which helps ensure more accurate measurements by minimizing the influence of recent dietary intake. The samples were then processed at the Laboratory of Clinical Chemistry in the Nicosia General Hospital, where total serum cholesterol, LDL cholesterol, and HDL cholesterol were measured using standard laboratory procedures.
Assessments
The Barratt Impulsiveness Scale (BIS-11) (44, 45) is a 30-item self-report questionnaire designed to evaluate impulsive levels in both clinical and non-clinical populations. Respondents rate each item on a four-point Likert scale, from 1 (Rarely/Never) to 4 (Almost Always/Always), producing total scores that can range from 30 to 120. Higher scores correspond to greater degrees of impulsiveness. The BIS-11 organizes impulsivity into six first-order factors—attention, motor, self-control, cognitive complexity, perseverance, and cognitive instability—and three second-order factors (cognitive, motor, and non-planning impulsiveness). This multidimensional structure helps clinicians and researchers identify specific areas of impulsivity, which can be particularly important for tailoring interventions in conditions where impulsive behavior plays a significant role.
The Columbia–Suicide Severity Rating Scale (C-SSRS) (46, 47) is a standardized measure commonly used in both clinical and research settings to assess the presence and intensity of suicidal ideation, as well as suicidal behaviors. It captures critical details such as the frequency of suicidal thoughts and any history of suicide attempts over a person’s lifetime or within a specified timeframe (e.g., the past three months). By evaluating these factors, clinicians and researchers can identify individuals at higher risk, monitor changes in risk level over time, and make informed decisions about the most appropriate intervention strategies. In this context, data on suicide attempts, lifetime and recent suicidal behavior, and the frequency of suicidal thoughts were specifically analyzed to provide a clearer picture of the individual’s overall risk profile.
The Karolinska Interpersonal Violence scale (KIVS) consists of four subscales, designed to assess the exposure to and expression of interpersonal violence as a child (between 6 and 14 years of age), and as an adult (15 and older), (48). The ratings are based on a semi-structured interview and items refer to serious events during the lifetime. The scoring is between 0 and 5 for all four subscales (49, 50).
Data analysis
To establish the reliability of the tests used, we measured Cronbach’s α for the BIS and C-SSRS scale. We did not use this metric for KIVS as each item is assessed independently. We used Spearman’s rank correlation coefficient (Spearman’s ρ) to investigate relationships among our variables because it does not require data to observe a normal distribution—an important consideration given that most of our groups did not meet normality criteria. After identifying significant correlations, we applied a linear regression model to further explore these associations, using a stepwise approach to systematically introduce additional variables while ensuring the model’s best fit. The assumption of the regression analysis, specifically linearity of the relationship, homoscedasticity using the Breusch-Pagan test, normality of residuals using a Shapiro-Wilk test and the absence of overly influential outliers calculating Cook’s distance for each data point were tested and met. Throughout all analyses, we employed a 95% confidence level (α = 0.95) to determine statistical significance, and we presented the data in graphs as mean ± standard error of the mean (SEM), mean and standard deviation in tables, and Spearman rho values with a heatmap in correlational matrices. This methodological approach helps ensure that our results accurately capture the relationships between variables while accommodating the distributional characteristics of our data. Data analyses were performed using R (51, version 4.2.0), which provided a robust environment for statistical computing. Custom scripts were developed in Python (52, version 3.9) to handle data processing and visualization.
Results
Sample
Our sample consists of 158 inpatients, 92 men and 66 women. In the BIS questionnaire, the sample characteristics were (mean = 68.03, SD = 13.29, range = 41 – 112; mean = 69.01, SD = 13.19, range = 43 – 112; mean = 66.55, SD = 13.47, range = 41–102 for the total sample, men, and women respectively). The Cronbach’s α of the BIS scale was 0.81 (95% CI 0.77 - 0.85).
In C-SSRS, for suicide attempts throughout the lifetime, the sample characteristics were (mean = 1.66, SD = 2.15, range = 0 – 5; mean = 1.32, SD = 2.00, range = 0 – 5; mean = 2.15, SD = 2.27, range = 0–5 for the total sample, men, and women respectively). Accordingly, in suicide attempts in the last 3 months, our sample was as follows (mean = 1.66, SD = 5.00, range = 0 – 50; mean = 1.53, SD = 6.00, range = 0 – 50; mean = 1.85, SD = 3.30, range = 0–16 for the total sample, men, and women respectively). The Cronbach’s α of the C-SSRS scale was 0.9 (95% CI 0.87 - 0.92).
When it came to the KIVS scale, use of violence in adulthood was measured, and the sample characteristics were (mean = 1.22, SD = 1.44, range = 0 – 5; mean = 1.53, SD = 1.51, range = 0 – 5; mean = 0.78, SD = 1.23, range = 0–4 for the total sample, men, and women respectively). In victim of violence in childhood, the sample characteristics were (mean = 2.10, SD = 1.72, range = 0 – 5; mean = 2.21, SD = 1.56, range = 0 – 5; mean = 1.97, SD = 1.92, range = 0–5 for the total sample, men, and women respectively).
For clarity, each value we measured and used in our analyses is presented in Supplementary Table 1 organized by test performed and sex.
Impulsivity, lipid profile and sex differences
Men exhibited a pronounced positive correlation of total cholesterol with BIS Total, BIS Prorating Total, BIS Factor Non-Planning, and BIS Factor Cognitive (ρ = .293, p = .01; ρ = .299, p = .009; ρ = .238, p = .03; and ρ = .301, p = .008, respectively). Similarly, LDL levels were correlated with the same ratings (BIS Total, BIS Prorating Total, BIS Factor Non-Planning, and BIS Factor Cognitive: ρ = .308, p = .006; ρ = .310, p = .006; ρ = .241, p = .026; and ρ = .320, p = .004, respectively). (see Figure 1) Virtually all impulsivity factors were linked to cholesterol and LDL, except for motor impulsivity (BIS Motor: cholesterol, LDL: ρ = .158, p = .109; and ρ = .159, p = .116, respectively). However, there was no correlation between impulsivity measures and HDL in men. In women there was no association between impulsivity scales and total cholesterol, HDL or LDL (BIS total vs total cholesterol, HDL and LDL: ρ = -.285, p = .102; ρ = -.163, p = .343; and ρ = -.136, p = .429, respectively) (see Supplementary Figure 1).
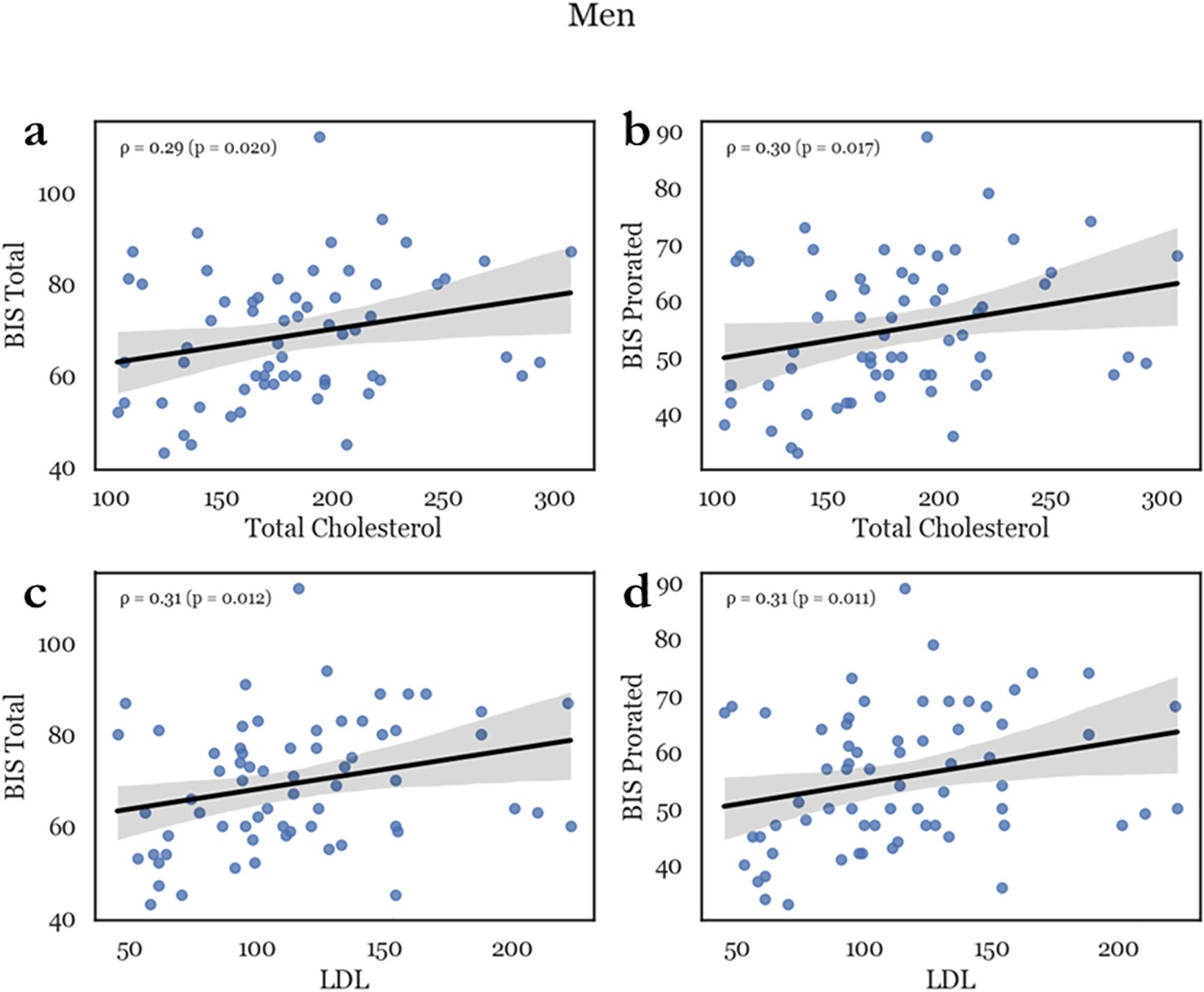
Figure 1. Representative scatterplots depicting the correlation between lipids and impulsivity in men. Total cholesterol (TC) was positively correlated with impulsivity scores such as total BIS score (a) and BIS prorating (b). LDL similarly correlated with total BIS score (c) and prorating (d), with an ρ between 250 and 300 which indicated a medium effect.
Suicidal behavior, lipid profile and sex differences
In men, there was no correlation between suicidality measures and either total cholesterol, HDL or LDL. All correlations are depicted in Supplementary Figure 2.
In women, higher cholesterol was associated with decreased lifetime suicide attempts and lifetime suicide behavior (CSSRS suicide attempts – lifetime, CSSRS suicidal behavior – lifetime: ρ = -.440, p = .01; and ρ = -.322, p = .044, respectively). (see Figure 2) On the other hand, HDL was negatively correlated with most measured suicidality factors (CSSRS suicide attempts – lifetime, CSSRS suicide attempts – 3-month, CSSRS suicidal behavior – lifetime, CSSRS suicidal behavior – 3-month, CSSRS suicidal ideation – 3-month, CSSRS suicidal ideation frequency – lifetime, and CSSRS suicidal ideation frequency – 3-month: ρ = -.374, p = .021; ρ = -.506, p = .002; ρ = -.314, p = .043; ρ = -.459, p = .005; ρ = -.359, p = .024; ρ = -.451, p = .005; and ρ = -.546, p <.001, respectively). Conversely, in women there was no association between suicidality and LDL. (see Supplementary Figure 2).
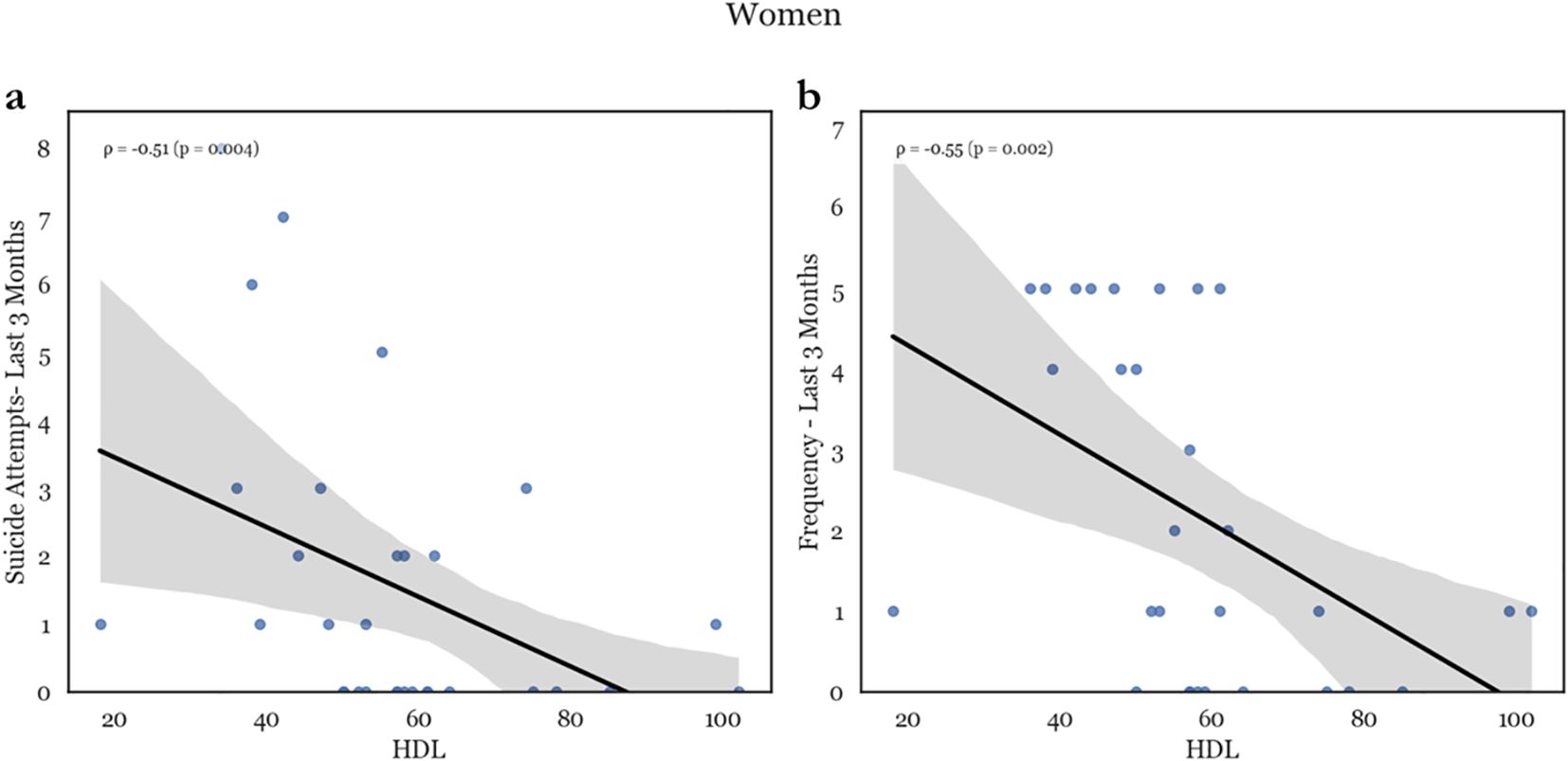
Figure 2. Representative scatterplots of HDL levels with suicide attempts in the past 3 months (a) and frequency of ideation in the past 3 months (b). There is a major negative correlation between HDL levels and both recent attempts as well a recent suicidal ideation.
Violence and lipids in both sexes
In the current dataset there was no statistically significant correlation between lipids and the status of either victim or perpetrator of violence, as measured by KIVS. For men, neither HDL, LDL or total cholesterol was associated with victimhood or use of violence in childhood, or victimhood or use of violence later in adult life. In women, we similarly failed to uncover a statistically significant relationship of the above measures (see Supplementary Figure 3).
However, KIVS significantly correlates with impulsivity in both sexes, and with suicidality in women but in men. Specifically, in men use of violence in childhood as measured in KIVS correlated closely with total impulsivity (BiS total, BiS prorated: ρ = .257, p = .018; and ρ = .260, p = .0.16, respectively) and so did status of victimhood of violence in childhood (BiS total, BiS prorated: ρ = .297, p = .006; and ρ = .261, p = .0.16, respectively). No measures in CSSRS correlate with KIVS values.
In women, impulsivity as measured by BIS is interestingly more closely correlated with use of violence in childhood (BIS total, BIS prorated: ρ = .358, p = .006; and ρ = .383, p = .0.003, respectively) and victimhood of violence in childhood (BIS total, BIS prorated: ρ = .356, p = .006; and ρ = .341, p = .0.009, respectively) as measured by KIVS. However, these very same items of the KIVS scale correlate with current suicide ideation and number of attempts throughout the lifetime (KIVS use of violence in childhood vs CSSRS suicidal ideation – 3-month, CSSRS suicide attempts – lifetime: ρ = .266, p = .047; and ρ = .274, p = .043., respectively, KIVS victimhood of violence in adulthood vs CSSRS suicidal ideation – 3-month, CSSRS suicide attempts – lifetime: ρ = .302, p = .021; and ρ = .340, p = .0.11., respectively, and KIVS victimhood of violence in childhood vs CSSRS suicide attempts – lifetime: ρ = .276, p = .011). This three-way overlap but not a direct link of correlations with lipid profiles over both sexes might point to a deeper causal link that has yet to be uncovered between trauma, impulsivity and suicidality (see Supplementary Figure 3).
Regression analyses
To further investigate our findings, we applied linear regression models separately for men and women to validate the previously observed correlations between impulsivity and suicidality.
Subsequently, we constructed a regression model based on the results of our correlational analysis, introducing variables in a stepwise manner to identify the optimal model. The Barratt Impulsiveness Scale (BIS) score served as the dependent variable. The best-fitting model included LDL cholesterol as the sole independent variable. This model was statistically significant (F(1,64) = 4.824, p = 0.031) with an adjusted R² of 0.265, indicating that the linear regression analysis confirmed the results suggested by the correlational analysis.
In the female cohort, we conducted a similar linear regression analysis but were able to include more variables in the model. We began by introducing HDL cholesterol, LDL cholesterol, triglycerides and total cholesterol stepwise with suicidal ideation during the past month as the dependent variable. The objective was to explore the previously observed relationship between HDL cholesterol and suicidality and to develop a model that could explain the relationship between blood lipid concentrations and suicidal behavior. Total cholesterol was excluded as a predictor due to significant collinearity with LDL (VIF = 13.26). The best fit model included the above variables as well as age and BMI as covariates, while triglycerides were excluded from the final analysis as they did not meet the significance threshold (p < 0.05).
The final model was statistically significant (F(4,23) = 5.697, p = 0.006) with an adjusted R² of 0.5225. This model suggests that the above variables together account for approximately 52% of the variance in suicidality among hospitalized women.
Discussion
Understanding the intricate relationship between cholesterol levels, impulsivity, and suicidality in hospitalized patients can provide valuable insights into mental health and inform targeted treatment strategies. Our findings, combined with a growing body of literature, highlight the need to consider not only conventional cardiovascular factors but also how lipid dysregulation may interact with neurobiological and psychosocial variables to influence behavior and mood.
Low-density lipoprotein
A major focus of this investigation was on LDL cholesterol—often referred to as “bad” cholesterol due to its association with cardiovascular risk (53)—and its nuanced role in brain function. Elevated LDL levels have been linked to changes in membrane fluidity and neurotransmitter receptor functioning, particularly in regions implicated in impulse control, such as the prefrontal cortex (54). Higher LDL may, therefore, contribute to heightened impulsivity by disrupting serotoninergic and dopaminergic signaling pathways that regulate mood and behavioral inhibition. This phenomenon may help explain observed links between elevated LDL levels and increased impulsivity, especially among men.
Cholesterol’s potential impact on aggression and the nature of suicidal behavior remains complex (13). Previous studies have suggested that alterations in lipid profiles could be more closely linked to violent rather than non-violent forms of suicide, potentially due to the interplay between aggression and dysregulated neurobiological pathways (55). However, in our sample, we found no significant relationship between LDL (or high-density lipoprotein [HDL]) levels and suicidality in men, given the assumptions involved in a correlation analysis. This suggests that while LDL may contribute to impulsivity, suicidality may depend more on a constellation of factors, including genetic predispositions, psychosocial stressors, or comorbid mental health conditions (56).
High-density lipoprotein
Another critical component of lipid dysregulation relates to HDL cholesterol, often termed “good” cholesterol for its cardiovascular protective effects (57). Beyond heart health, HDL may exert neuroprotective influences by mitigating oxidative stress and inflammation—both implicated in depressive symptoms and suicidal ideation (58). In our findings, lower HDL levels in women were associated with higher suicidality. While the nature of our study precludes causal interpretations, this association raises the possibility that HDL may play a role in pathways related to mood regulation and stress response, warranting further investigation in longitudinal research. Further, inflammation or endocrine dysregulation, which can be exacerbated by abnormal lipid profiles, has been increasingly recognized as a factor in the pathogenesis of both depression and suicidal behavior (59, 60).
The absence of a strong link between cholesterol levels and impulsivity in women in our study and in literature (61) suggests that other mechanisms such as hormonal regulation or genetic predisposition (62) may play a larger role in influencing impulsivity. Estrogen, for instance, is known to affect mood, cognitive function, and potentially aggression and impulsivity (63).
Moreover, it is crucial to consider potential reverse causality when interpreting associations between lipid profiles and suicidality. Individuals experiencing severe mood disorders or suicidal ideation may exhibit changes in appetite and physical activity that result in weight fluctuations and altered serum lipid levels (64). Thus, while dyslipidemia could contribute to suicidality risk in women, it may also be a consequence of mental health changes, emphasizing the need for longitudinal studies to clarify causality.
Strengths and limitations
This study has several strengths, including a well-characterized inpatient psychiatric population and a comprehensive assessment of violence, suicidality, and impulsivity. However, it also has limitations. The cross-sectional design prevents conclusions about causality, making it difficult to determine whether lipid abnormalities directly influence impulsivity and suicidality or are merely associated with them. Additionally, the relatively small sample size, especially for women, limits the ability to detect more subtle effects, increasing the risk of false-negative findings. The focus on hospitalized patients only and the absence of a matched healthy control group and missing data further constrain the interpretation of our results. Additionally, due to the primarily correlational approach of our study, our findings have interpretive barriers as causality and the influence of cofounders cannot be definitely determined. Future studies with larger, well-matched cohorts and multivariate, longitudinal designs are needed to confirm these findings.
Conclusion
Our findings highlight the complex relationship between lipid metabolism, impulsivity, and suicidality, with distinct sex differences. Elevated LDL cholesterol may serve as a potential marker of impulsivity in men, while lower HDL levels could indicate higher suicide risk in women. These insights suggest that clinicians should consider sex-specific risk assessments, including lipid profile monitoring, lifestyle modifications, and targeted pharmacological interventions. Further research is needed to clarify the biological, genetic, and psychosocial mechanisms linking lipid dysregulation to suicidal and aggressive behaviors in diverse psychiatric populations.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Cyprus National Bioethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
EB: Writing – original draft, Conceptualization, Data curation, Investigation, Writing – review & editing. VK: Software, Writing – review & editing, Investigation, Writing – original draft, Formal analysis, Visualization, Data curation, Methodology, Validation. AC: Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2025.1595783/full#supplementary-material
References
1. McHugh CM, Chun Lee RS, Hermens DF, Corderoy A, Large M, and Hickie IB. Impulsivity in the self-harm and suicidal behavior of young people: A systematic review and meta-analysis. J Psychiatr Res. (2019) 116:51–60. doi: 10.1016/j.jpsychires.2019.05.012
2. Dempsey RC, Dodd AL, Gooding PA, and Jones SH. The types of psychosocial factors associated with suicidality outcomes for people living with bipolar disorder: A scoping review. Int J Environ Res Public Health. (2024) 21(5):525. doi: 10.3390/ijerph21050525
3. Devine DP. The neuropathology of Self-Injurious Behavior: Studies using animal models. Brain Res. (2024) 1844:149172. doi: 10.1016/j.brainres.2024.149172
4. Raji H, Dinesh S, and Sharma S. Inside the impulsive brain: a narrative review on the role of neurobiological, hormonal and genetic factors influencing impulsivity in psychiatric disorders. Egyptian J Neurol Psychiatry Neurosurgery. (2025) 61:4. doi: 10.1186/s41983-024-00930-9
5. Diaz-Sastre C, Baca-Garcia E, Perez-Rodriguez MM, Garcia-Resa E, Ceverino A, Saiz-Ruiz J, et al. Low plasma cholesterol levels in suicidal males: a gender- and body mass index matched case–control study of suicide attempters and nonattempters. Prog Neuropsychopharmacol Biol Psychiatry. (2007) 31:901–5. doi: 10.1016/j.pnpbp.2007.02.004
6. Jones BDM, Farooqui S, Kloiber S, Husain MO, Mulsant BH, and Husain MI. Targeting metabolic dysfunction for the treatment of mood disorders: review of the evidence. Life (Basel). (2021) 11(8):819. doi: 10.3390/life11080819
7. Bartoli F, Di Brita C, Crocamo C, Clerici M, and Carra G. Lipid profile and suicide attempt in bipolar disorder: A meta-analysis of published and unpublished data. Prog Neuropsychopharmacol Biol Psychiatry. (2017) 79:90–5. doi: 10.1016/j.pnpbp.2017.06.008
8. Messaoud A, Mensi R, Mrad A, Mhalla A, Azizi I, Amemou B, et al. Is low total cholesterol levels associated with suicide attempt in depressive patients? Ann Gen Psychiatry. (2017) 16:20. doi: 10.1186/s12991-017-0144-4
9. Sen P, Adewusi D, Blakemore AI, and Kumari V. How do lipids influence risk of violence, self-harm and suicidality in people with psychosis? A systematic review. Aust N Z J Psychiatry. (2022) 56:451–88. doi: 10.1177/00048674211025608
10. Troisi A. Cholesterol in coronary heart disease and psychiatric disorders: same or opposite effects on morbidity risk? Neurosci Biobehav Rev. (2009) 33:125–32. doi: 10.1016/j.neubiorev.2008.09.003
11. Wu S, Ding Y, Wu F, Xie G, Hou J, and Mao P. Serum lipid levels and suicidality: a meta-analysis of 65 epidemiological studies. J Psychiatry Neurosci. (2016) 41:56–69. doi: 10.1503/jpn.150079
12. Maes M, Zhou B, Jirakran K, Vasupanrajit A, Boonchaya-Anant P, Tunvirachaisakul C, et al. Towards a major methodological shift in depression research by assessing continuous scores of recurrence of illness, lifetime and current suicidal behaviors and phenome features. J Affect Disord. (2024) 350:728–40. doi: 10.1016/j.jad.2024.01.150
13. Tomson-Johanson K and Harro J. Low cholesterol, impulsivity and violence revisited. Curr Opin Endocrinol Diabetes Obes. (2018) 25:103–7. doi: 10.1097/MED.0000000000000395
14. Alvarez JC, Cremniter D, Gluck N, Quintin P, Leboyer M, Therond P, et al. Low serum cholesterol in violent but not in non-violent suicide attempters. Psychiatry Res. (2000) 95:103–8. doi: 10.1016/S0165-1781(00)00171-2
15. Atmaca M, Kuloglu M, Tezcan E, and Ustundag B. Serum leptin and cholesterol values in violent and non-violent suicide attempters. Psychiatry Res. (2008) 158:87–91. doi: 10.1016/j.psychres.2003.05.002
16. Montiel F, Karanassios G, Streb J, Dudeck M, and Fritz M. The relationship between serum cholesterol, triglycerides, and self-reported appetitive and reactive aggression, as well as violent crimes in male forensic patients with substance use disorder. Psychiatry Int. (2024) 5:823–30. doi: 10.3390/psychiatryint5040056
17. Douglas J and Nasrallah HA. Low high-density lipoprotein and psychopathology: A review. Ann Clin Psychiatry. (2019) 31:209–13.
18. Peer N, Abrahams N, and Kengne AP. A systematic review of the associations of adult sexual abuse in women with cardiovascular diseases and selected risk factors. Glob Heart. (2020) 15:65. doi: 10.5334/gh.760
19. Fiedorowicz JG and Coryell WH. Cholesterol and suicide attempts: a prospective study of depressed inpatients. Psychiatry Res. (2007) 152:11–20. doi: 10.1016/j.psychres.2006.09.003
20. Garland MR, Hallahan B, McNamara M, Carney PH, Grimes H, Hibbeln JR, et al. Lipids and essential fatty acids in patients presenting with self-harm. Br J Psychiatry. (2007) 190:112–7. doi: 10.1192/bjp.bp.105.019562
21. Perez-Rodriguez MM, Baca-Garcia E, Diaz-Sastre C, Garcia-Resa E, Ceverino A, Saiz-Ruiz J, et al. Low ser cholesterol may be associated with suicide attempt history. J Clin. (2008) 69:1920–7. doi: 10.4088/JCP.v69n1210
22. Virkkunen M. Serum cholesterol levels in homicidal offenders. A low cholesterol is connected with habitually violent tendency under the influence of alcohol. Neuropsychobiology. (1983) 10:65–9. doi: 10.1159/000117987
23. Golomb BA, Stattin H, and Mednick S. Low cholesterol and violent crime. J Psychiatr Res. (2000) 34:301–9. doi: 10.1016/S0022-3956(00)00024-8
24. Zhou S, Zhao K, Shi X, Sun H, Du S, Miao X, et al. Serum lipid levels and suicide attempts within 2 weeks in patients with major depressive disorder: is there a relationship? Front Psychiatry. (2021) 12:676040. doi: 10.3389/fpsyt.2021.676040
25. Capuzzi E, Bartoli F, Crocamo C, Malerba MR, Clerici M, and Carra G. Recent suicide attempts and serum lipid profile in subjects with mental disorders: a cross-sectional study. Psychiatry Res. (2018) 270:611–5. doi: 10.1016/j.psychiatrres.2018.10.050
26. Ma YJ, Zhou YJ, Wang DF, Li Y, Wang DM, Liu TQ, et al. Association of Lipid Profile and Suicide Attempts in a large sample of first episode drug-naive patients with major depressive disorder. Front Psychol. (2020) 11:543632. doi: 10.3389/fpsyt.2020.543632
27. Xu P, Fan C, Yan M, Liu J, and Zhang X. Remnant cholesterol and suicide attempts in untreated first-episode major depressive disorder. Front Psychiatry. (2025) 16:1493509. doi: 10.3389/fpsyt.2025.1493509
28. Choi W, Kang HJ, Kim JW, Kim HK, Kang HC, Lee JY, et al. Age-specific associations between serum cholesterol levels and suicidal behaviors in patients with depressive disorders: A naturalistic prospective observational cohort study. Front Psychiatry. (2023) 14:1095579. doi: 10.3389/fpsyt.2023.1095579
29. Kaplan JR, Muldoon MF, Manuck SB, and Mann JJ. Assessing the observed relationship between low cholesterol and violence-related mortality. Implications for suicide risk. Ann N Y Acad Sci. (1997) 836:57–80. doi: 10.1111/j.1749-6632.1997.tb52355.x
30. Vevera J, Zukov I, Morcinek T, and Papezová H. Cholesterol concentrations in violent and non-violent women suicide attempters. Eur Psychiatry. (2003) 18:23–7. doi: 10.1016/s0924-9338(02)00011-1
31. Qing G, Deng W, Zhou Y, Zheng L, Wang Y, and Wei B. The association between non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio (NHHR) and suicidal ideation in adults: a population-based study in the United States. Lipids Health Dis. (2024) 23:17. doi: 10.1186/s12944-024-02012-4
32. Tanskanen A, Vartiainen E, Tuomilehto J, Viinamäki H, Lehtonen J, and Puska P. High serum cholesterol and risk of suicide. Am J Psychiatry. (2000) 157:648–50. doi: 10.1176/appi.ajp.157.4.648
33. Kafetzopoulos V, Kokras N, Katsaitis F, Sousa N, Leite-Almeida H, Sotiropoulos I, et al. Prefrontal cortex-nucleus reuniens-hippocampus network exhibits sex-differentiated responses to stress and antidepressant treatment in rats. Psychopharmacol (Berl). (2024). doi: 10.1007/s00213-024-06667-w
34. Seeman MV. Sex differences in schizophrenia relevant to clinical care. Expert Rev Neurother. (2021) 21:443–53. doi: 10.1080/14737175.2021.1898947
35. Alvarez JC, Creminter D, Lesiuer P, Gregorie A, Gilton A, Macquin-Mavies L, et al. Low blood cholesterol and low platelet serotonin levels in violent suicide attempts. Biol Psychiatry. (1999) 45:1066–9. doi: 10.1016/S0006-3223(98)00160-7
36. Park CHK, Kim D, Kim B, Rhee SJ, Cho SJ, and Ahn YM. Serum lipids as predictive markers for death by suicide. Psychiatry Res. (2024) 335:115837. doi: 10.1016/j.psychres.2024.115837
37. Ruljancic N, Mihanovic M, and Cepelak I. Thrombocyte serotonin and serum cholesterol concentration in suicidal and non-suicidal depressed patients. Prog Neuro-Psychopharmacol Biol Psychiatry. (2011) 35:1261–7. doi: 10.1016/j.pnpbp.2011.02.007
38. Li H, Zhang X, Sun Q, Zou R, Li Z, and Liu S. Association between serum lipid concentrations and attempted suicide in patients with major depressive disorder: a meta-analysis. PloS One. (2020) 15:e0243847. doi: 10.1371/journal.pone.0243847
39. Guo J, Wang L, Zhao X, Wang D, and Zhang X. Sex difference in association between suicide attempts and lipid profile in first-episode and drug naive patients with major depressive disorder. J Psychiatr Res. (2024) 172:24–33. doi: 10.1016/j.jpsychires.2024.02.026
40. Herceg D, Mimica N, Herceg M, and Puljic K. Aggression in women with schizophrenia is associated with lower HDL cholesterol levels. Int J Mol Sci. (2022) 23(19):11858. doi: 10.3390/ijms231911858
41. Tomson-Johanson K, Kaart T, Kiivet RA, Veidebaum T, and Harro J. Low cholesterol levels in children predict impulsivity in young adulthood. Acta Neuropsychiatr. (2020) 32:196–205. doi: 10.1017/neu.2019.48
42. Capuzzi E, Caldiroli A, Capellazzi M, Tagliabue I, Auxilia A, Ghilardi G, et al. Exploring the role of serum lipid profile and neutrophil-to-lymphocyte ratio in violent suicide attempters: a cross sectional study. CNS Spectr. (2020) 27:362–8. doi: 10.1017/S1092852920002199
43. Kunugi H, Takei N, Aoki H, and Nanko S. Low serum cholesterol in suicide attempters. Biol Psychiatry. (1997) 1:196–200. doi: 10.1016/S0006-3223(95)00672-9
44. Patton JH, Stanford MS, and Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. (1995) 51:768–74. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1
45. Stanford MS, Mathias CW, Dougherty DM, Lake SL, Anderson NE, and Patton JH. Fifty years of the Barratt Impulsiveness Scale: An update and review. Pers Individ Dif. (2009) 47:385–95. doi: 10.1016/j.paid.2009.04.008
46. Mundt JC, Greist JH, Jefferson JW, Federico M, Mann JJ, and Posner K. Prediction of suicidal behavior in clinical research by lifetime suicidal ideation and behavior ascertained by the electronic Columbia-Suicide Severity Rating Scale. J Clin Psychiatry. (2013) 74:887–93. doi: 10.4088/JCP.13m08398
47. Posner K, Brown GK, Stanley B, Brent DA, Yershova KV, Oquendo MA, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. (2011) 168:1266–77. doi: 10.1176/appi.ajp.2011.10111704
48. Jokinen J, Nordström AL, and Nordström P. Cholesterol, CSF 5-HIAA, violence and intent in suicidal men. Psychiatry Res. (2010) 178(1):217–220.
49. Chatzittofis A, Nordstrom P, Uvnas-Moberg K, Asberg M, and Jokinen J. CSF and plasma oxytocin levels in suicide attempters, the role of childhood trauma and revictimization. Neuro Endocrinol Lett. (2014) 35:213–7.
50. Haglund A, Lindh AU, Lysell H, Renberg ES, Jokinen J, Waern M, et al. Interpersonal violence and the prediction of short-term risk of repeat suicide attempt. Sci Rep. (2016) 6:36892. doi: 10.1038/srep36892
51. R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. (2022). Available at: https://www.R-project.org/.
52. Van Rossum G and Drake FL. Python 3 Reference Manual. CreateSpace Independent Publishing Platform (2009).
53. Das P and Ingole N. Lipoproteins and their effects on the cardiovascular system. Cureus. (2023) 15:e48865. doi: 10.7759/cureus.48865
54. Yates JR. Aberrant glutamatergic systems underlying impulsive behaviors: Insights from clinical and preclinical research. Prog Neuropsychopharmacol Biol Psychiatry. (2024) 135:111107. doi: 10.1016/j.pnpbp.2024.111107
55. Boston PF, Dursun SM, and Reveley MA. Cholesterol and mental disorder. Br J Psychiatry. (1996) 169:682–9. doi: 10.1192/bjp.169.6.682
56. Deisenhammer EA, Kramer-Reinstadler K, Liensberger D, Kemmler G, Hinterhuber H, and Fleischhacker WW. No evidence for an association between serum cholesterol and the course of depression and suicidality. Psychiatry Res. (2004) 121:253–61. doi: 10.1016/j.psychres.2003.09.007
57. Khan SH, Asif N, Ijaz A, Manzoor SM, Niazi NK, and Fazal N. Status of non-HDL-cholesterol and LDL-cholesterol among subjects with and without metabolic syndrome. J Pak Med Assoc. (2018) 68:554–8.
58. Zhang X, Liu H, Mi C, Mao J, Zhang D, and Wei H. Association between suicidal ideation and oxidative balance score (OBS): National Health and Nutrition Examination Survey (NHANES) 2005-2018. J Affect Disord. (2025) 370:328–36. doi: 10.1016/j.jad.2024.11.010
59. Fatemian H, Moslemi H, Hosseini Y, and Moshfeghinia R. C-reactive protein (CRP) level in depressed patients with suicidal behavior: A systematic review and meta-analysis. J Affect Disord. (2024) 366:423–33. doi: 10.1016/j.jad.2024.08.135
60. Hasan G, Balcioglu YH, and Solmaz M. The role of impulsive and aggressive traits, albumin and thyroid functions in recent suicide attempters: an investigation with a transdiagnostic approach. Neurochemical J. (2022) 16:491–7. doi: 10.1134/S1819712422040079
61. Hjell G, Morch-Johnsen L, Holst R, Tesli N, Bell C, Lunding SH, et al. Disentangling the relationship between cholesterol, aggression, and impulsivity in severe mental disorders. Brain Behav. (2020) 10:e01751. doi: 10.1002/brb3.1751
62. Schwartz JA, Rowland MW, and Beaver KM. A genetically informed test of cholesterol levels and self-control, depressive Symptoms, antisocial behavior, and neuroticism. J Affect Disord. (2014) 164:139–47. doi: 10.1016/j.jad.2014.04.015
63. Grigoriadis S and Seeman MV. The role of estrogen in schizophrenia: implications for schizophrenia practice guidelines for women. Can J Psychiatry. (2002) 47:437–42. doi: 10.1177/070674370204700504
Keywords: impulsivity, suicidality, suicide, HDL, LDL, lipids, inpatients, sex differences
Citation: Bella E, Kafetzopoulos V and Chatzittofis A (2025) Sex differences of the lipid profile, impulsivity and suicidality in psychiatric inpatients. Front. Psychiatry 16:1595783. doi: 10.3389/fpsyt.2025.1595783
Received: 18 March 2025; Accepted: 14 April 2025;
Published: 19 May 2025.
Edited by:
Yasin Hasan Balcioglu, Bakirkoy Prof Mazhar Osman Training and Research Hospital for Psychiatry, Neurology, and Neurosurgery, TürkiyeReviewed by:
Hasan Gökçay, University of Health Sciences, TürkiyeUğur Takım, Erzurum Regional Research and Training Hospital, Türkiye
Copyright © 2025 Bella, Kafetzopoulos and Chatzittofis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andreas Chatzittofis, YW5kcmVhcy5jaGF0eml0dG9maXNAdW11LnNl
 Evanthia Bella1
Evanthia Bella1 Vasilios Kafetzopoulos
Vasilios Kafetzopoulos Andreas Chatzittofis
Andreas Chatzittofis